Note: Descriptions are shown in the official language in which they were submitted.
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
TITLE: STENT-BASED DELIVERY OF STATINS TO PREVENT RESTENOSIS
RELATZONSHTP TO OTHER APPLICATIONS
This application claims the benefit of copending U.S.
Provisional Patent Application No. 60/286,519, filed April 27;
2001, the entire disclosure of which is incorporated herein by
reference.
BACKGROUND OF THE INVENTION
Field of the Invention:
This invention relates to the prevention of restenosis of
arteries after angioplasty and more particularly to the use of
a stmt platform which is coated with a composition including a
statin compound, the effect of which is to prevent such
restenosis.
Brief Description of the Prior Art:
Coronary angioplasty has become an important method of
treating narrowed (stenotic) arteries supplying the heart or
the legs. Although the initial success rate of coronary
angioplasty for opening obstructed coronary arteries exceeds
950, restenosis occurs at the site of angioplasty in 25-50% of
patients within six months, regardless of the type of
angioplasty procedure used. Although the use of stem s has
appreciably reduced the'rate of stenosis, even with this
treatment strategy restenosis occurs in 5 to 200 of patients.
Importantly, when restenosis occurs within a stmt, the chance
that restenosis will recur is very high. Thus, the problem of
restenosis is still formidable, despite recent advances in
reducing its incidence.
1
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
Two primary mechanisms appear to be involved in the
development of restenosis. First, recoil of the vessel wall
(negative remodeling) leads to gradual narrowing of the vessel
lumen. Second, an exaggerated healing response of medial
and/or adventitial smooth muscle cells (SMCs) to vascular
injury involves the excessive proliferation of SMCs and the
migration of SMCs to the subintima, where they continue to
proliferate and begin to secrete extracellular matrix. These
processes involving SMCs cause the neointimal mass to expand
and gradually encroach upon the coronary lumen. Ultimately the
expanding lesion narrows the vessel, increases the resistance
to blood flow, and causes ischemic symptoms. In the absence of
stenting, both remodeling and an expanding neointima contribute
to restenosis. When stems are deployed, negative vascular
remodeling is prevented and restenosis occurs only as.a result
of the expanding neointimal mass. Given these pathophysiologic
mechanisms, the problem of controlling restenosis occurring
with stmt deployment becomes largely the problem of
controlling the development of the neointimal mass.
Many attempts have been made to prevent the development of
restenosis, and with the notable exception of brachytherapy,
many such attempts have been reported to be successful in
inhibiting neointima development in various experimental
models. However, almost invariably their translation to
clinical~interventions has been without success. These
strategies have included the oral administration of drugs,
their systemic administration, and~their local delivery.
Local Delivery:
Therapeutic strategies began to focus on local delivery as
it became apparent that high concentrations of active agent
were needed at the target site. It would be very unlikely that
such high concentrations could be achieved by any other
approach than local delivery. Unfortunately, despite years of
2
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
development and testing, the consensus is that catheter
delivery systems are too inefficient to provide a high
probability of success. Only one percent or less of the
delivered product appears to persist for any period of time in
the vessel wall.
The concept that drugs could be incorporated into stmt
coatings has become popularized, with mixed results. Most
studies have shown no effect. However, preliminary encouraging
results using stems having a coating impregnated with either
Taxol~ or its derivatives, or rapamycin, have been reported at
several international meetings.
The success of these drugs is based on the cellular and
molecular effects on microtubular modulation of the response of
cells to mitogens and cytokines, and proteins controlling
progress of cells through the cell-cycle.
Proteins controlling progress of cells through the cell-
cycle: SMCs within the vessel wall are normally in a quiescent
state. Immediately after injury, however, early response genes
are expressed and the cells enter the cell cycle, wherein their
replication is tightly regulated by an array of cell cycle
regulatory proteins acting conjointly and in sequence at
various points of the cycle. These regulatory proteins include
cyclin-dependent kinases (cdc2 and cdk2), which phosphorylate
critical regulatory proteins, and which interact with cyclin-
dependent kinase inhibitors, such as p16, p21, and p27kip1.
Changes in the levels of these inhibitors exert marked effects
on cell cycle progression, through inhibition of critical
phosphorylation reactions.
One of the proteins involved in cell cycle progression
that is regulated by phosphorylation is the tumor suppressor
protein retinoblastoma protein (Rb). In the hypophosphorylated
state Rb complexes with DNA binding and gene activating
proteins, such as E2F, thereby exerting an inhibiting effect on
3
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
cell cycle progression in Go/mid Gl. Upon phosphorylation, the
Rb/E2F complex dissociates, freeing E2F to bind to its DNA
binding sites and consequently stimulate the transcription of
genes inducing progression to the S phase of the cell cycle.
Rapamycin, a macrolide antibiotic, is a potent inhibitor
of cell proliferation. Tt has recently been shown in a pig
coronary artery injury model to significantly reduce the
neointimal response to injury. The mechanism of action of
rapamycin almost certainly largely derives from its ability to
interfere with cell cycling. Thus, down-regulation of p27kipi
by mitogens is blocked by rapamycin. Consistent with this
activity, in the porcine injury model, rapamycin administration
was associated with increased p27 levels and inhibition of Rb
phosphorylation within the vessel wall. The most likely
relevant molecular mechanisms are as follows: After binding to
its cytosolic receptor, FKBP12, rapamycin increases p27,
reduces cdc2 and cdk2 activity, and inhibits Rb
phosphorylation, thereby inhibiting release of E2F.
Eliminating E2F activity blocks the E2F-mediated transcription
of the broad array of genes that contribute to cell cycle
progression.
Microtubular modulation of the response of cells to
mitogens and cytokines: The microtubular system has been shown
to modulate the response of cells to various mitogens and
cytokines through activation of transmembrane signaling
cascades. Targets of these pathways include activation of
kinases, including mitogen-activated protein kinase activity,
changes associated with microtubular depolymerization. The
microtubules have also shown to play a part in the changes in
SMCs that lead to their contributing to the restenosis lesion.
Paclitaxel favors stabilization of microtubule assembly,
forming numerous disorganized microtubules within the
cytoplasm, and thereby inhibiting many of the microtubular
4
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
mediated cell signaling cascades cited above, including
inhibition of cell division, predominantly in the Go/G1 and G2/M
phases of the cell cycle. Importantly, paclitaxel in
biologically relevant concentrations does not appear to induce
apoptosis. Taxol~ inhibited, in vitro, both platelet-derived
growth factor-stimulated SMC migration and SMC proliferation,
and in vivo, inhibited neointimal accumulation in the rat
carotid artery injury model.
The disappointing results of most strategies designed to
inhibit restenosis, whether administered systemically or
locally via catheter, and the initial promising results of
xagents administered with a stmt-based delivery platform,
emphasize the continued need to develop new agents to prevent
restenosis, with an emphasis on delivering high local levels of
the agent. It appears that the most promising strategy to
achieve the latter goal is to deliver a potent anti-restenosis
agent via a stmt-based delivery system. A stmt-based
delivery system is disclosed in copending International Patent
Application No. PCT/US01/45755, filed on December 07, 2001,
designating the United States, the entire disclosure of which
is incorporated herein by reference. That application
disclosed a method of preventing restenosis using a stmt
coated with DNA coding for gene products with anti-restenosis
activity or cells containing such DNA. However, that
application did not disclose using a stmt coated with
particular small molecules capable of exercising anti-
restenosis activity on SMCs.
Accordingly, a need has continued to exist for additional
methods of preventing restenosis using a stmt coated with a
substance having anti-restenosis activity on the cells of a
blood vessel that has been treated by an angioplastic
procedure.
5
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
SUMMARY OF THE INVENTION
An advance in the treatment of restenosis after
angioplasty has been achieved by this invention wherein a stmt
implanted in the treated artery is coated with a composition
incorporating a statin compound (or DNA or other vector
containing DNA encoding a molecule with statin-like activity).
Alternatively, the statin may be incorporated into a matrix
which is supported by the coating on the stmt structure.
Accordingly, it is an object of the invention to provide a
method for preventing or alleviating restenosis of an artery
after angioplasty.
A further object is to provide a stent for implantation
into an artery after angioplasty that is coated with a
composition comprising a a source of a statin compound capable
of delivering high local concentrations of the statin.
A further object is to provide a stmt for implantation
into an artery after angioplasty that is coated with a
composition comprising a very high percentage by weight of a
statin compound.
Further objects will be apparent from the description of
the invention which follows.
BRIEF DESCRIPTION OF THE DRAWINGS
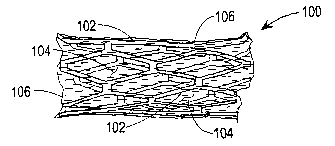
Figure 1 illustrates an uncoated or bare stmt of the type
implanted in an artery after angioplasty to inhibit restenosis.
Figure 2 is a schematic illustration of the stmt of
Figure 1 coated with a composition containing a statin.
Figure 3 is a schematic illustration of an enlarged cross-
section of a strut of the coated stent of Figure 2, taken along
the line 3-3 in Figure 2.
6
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
Figure 4 is a schematic illustration of the stmt of
Figure Z coated with a collagen gel that fills the areas
between the struts and releasably contains a statin compound.
S DETAILED DESCRIPTION OF THE INVENTION AND PREFERRED EMBODIMENTS
The invention comprises 1) a delivery system comprising a
stmt and a stent coating that is impregnated with a selected
statin (or DNA or other vector containing DNA encoding a
molecule with statin-like activity), and, correspondingly, 2)
contacting the arterial wall adjacent the stmt with a high
dosage of a statin (or DNA or other vector containing DNA
encoding a molecule with statin-like activity), thus inhibiting
restenosis of the scent-treated artery. The strategy described
herein has the benefits of substantially reducing the incidence
1S of restenosis with minimal incidence of untoward complications,
a result that has not been achieved by other anti-restenosis
strategies whose results have been limited or, as with
radiation therapy, carry unknown future risks.
The statin compounds useful in the method of the invention
are natural and/or synthetic compounds that are known to have
the physiological effect of lowering serum cholesterol levels
in human patients. The class of statin compounds is well-known
to those skilled in the art. Such compounds are typically
mevalonate derivatives that limit cholesterol biosynthesis by
2S inhibiting the enzyme 3-hydroxy-3-methylglutaryl coenzyme A
reductase (HMG-CoA reductase). Statins useful in the method of
the invention include, but are not limited to, lovastatin,
pravastatin, simvastatin, atorvastatin, fluvastatin,
cerivastatin, and the like. Other molecules having statin-like
activity may also be used in the method and coated stmt of the
invention. It is also according to the invention to
incorporate into the stmt coating DNA or a DNA-containing
vector capable of transfecting target cells (smooth muscle
7
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
cells or other cells) and coding for the production of statins
or statin-like compounds within those cells. Consequently, the
invention includes a method of preventing restenosis using a
stmt coated with a source of a statin compund that provides a
dose of a statin compund to the SMCs of the blood vessel wall,
either directly, by releasing a statin to act on the SMCs, or
indirectly, by causing cells of the vessel wall adjacent to the
stent to produce a statin compound.
According to this invention, the delivery systems utilized
in contacting the arterial wall with a composition including a
statin, may take several forms.
In a first embodiment, the delivery system comprises a
scent covered by a composition including the selected statin,
which adheres to the surface of the stmt, thereby facilitating
the delivery of the statin within the injured vessel wall, or
to cells that are migrating from the media and/or adventitia to
form the neointima. The coating can be formed from any
material that can cover the surface of the stmt and that has
the above characteristics. One such candidate coating has been
created by the Photolink~ process of the SurModics Company
(Eden Prairie, MN).
V~Tithin the first embodiment or strategy of the invention,
two alternatives may be used:
1. A statin is incorporated into the stmt coating, which
covers the stmt struts but not intervening spaces.
2. The stent coating acts as a support scaffolding for the
binding of collagen, or a similarly appropriate polymer, to
the stmt. The collagen or polymer will provide a matrix
for the statin that will allow complete coverage of the
vessel wall. This interation of the invention would be
particularly appropriate for vein grafts, which have no side
branches. Hence, the complete coverage for the vessel wall
will not result in side branch occlusion. The completely
8
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
covered stmt will facilitate two important features of the
invention.
a. It will provide efficient contact between the
statin and all of that part of the vessel in which
the stent is deployed so that a greater percentage of
the cells within the vessel wall will be in tight
apposition to the statin.
b. It will provide a collagen or polymer barrier to
cells migrating from the media or adventitia on their
way to form the expanding neointima.
Figure 1 illustrates the bare stent 100 without coating.
The stmt comprises struts 102 having interstices or openings
104 between them.
Figure 2 illustrates the stmt 100 with a coating 106
releasably incorporating a statin compound. The coating 106
covers the metal struts 102 but not the intervening spaces 104.
Figure 3 is a greatly enlarged view of a cross-section of
a portion of the stent 100 of Figure 2, taken along the
line 3-3 in Figure 2, showing the coated strut 102 and coating
106.
Figure 4 illustrates the stmt 100 of Figure 1 provided
with a coating of collagen 110 releasably containing a statin
compound. The stent 100 serves as a scaffold for supporting
the collagen gel 110 and statin compound incorporated into it.
The coating of the collagen gel 110 supported by the stent 100
covers not only the metal struts 102 (which cover only 15-20%
of the arterial wall over which the stent extends), but also
the intervening spaces or interstices 104, providing total
coverage of the arterial wall.
Those skilled in the art will recognize that besides
collagen other polymeric matrices capable of suspending the
statins on the stmt struts themselves, or of filling the
interstices between the struts of the stmt other coatings can
9
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
be used, provided that they exhibit the necessary compatibility
with the statin and permit release of the active agents to the
adjacent artery wall or to cells migrating through the matrix.
The properties of many such natural or synthetic polymeric
matrices are well known or can be determined without undue
experimentation to determine their suitability for use in the
stmt of this invention.
As previously stated, according to the invention, a statin
(or DNA or other vector containing DNA encoding a molecule with
statin-like activity) will be incorporated into a stmt
coating. The stmt coating will consist of a substance that
adheres to the stent, and which will incorporate the statin
molecule without damaging it. It is expected that the platform
system will facilitate delivery of the molecule to the cells
within the injured vessel wall (or to,the cells that are
migrating from the media and/or adventitia to form the
neointima), and is applied to the injured vessel wall at the
time of angioplasty and stmt implantation. This can be
performed in any artery or interposed vein (such as, but not
limited to, a saphenous vein graft to a coronary artery) that
is obstructed and thereby impairs blood flow to the target
tissue (whether it be heart or leg). The invention will employ
any coating that can be attached to a stent and that has the
above characteristics, and any molecule related chemically or
functionally to a statin or DNA or other vector containing DNA
encoding a molecule with statin-like activity. One such
candidate coating has been created by the Photolink~ process of
SurModics. Oral formulations of many different statin
molecules have been clinically tested; any of these, or those
still being developed or that will be developed, are candidate
molecules for this invention.
The intimate and prolonged contact between the injured
vessel wall and a statin (or DNA or other vector containing DNA
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
encoding a molecule with statin-like activity) that is
contained within the stmt coating and released therefrom, will
lead to high local levels of the statin. This will exert the
desired therapeutic effects on the cells contained within the
vessel wall, such as, but not limited to, inhibition of smooth
muscle cell (SMC) proliferation or migration and induction of
SMC apoptosis.
These in vitro effects have in vivo parallels. Statins
have antiproliferative effects on SMCs in acute vascular injury
in nonatherosclerotic, normocholesterolemic rats and rabbits,
and when administered orally to rats significantly reduce
neointimal formation both after simple arterial injury and,
importantly, after arterial stenting. This activity is
completely reversed by simultaneous local administration of
mevalonate which, as indicated above, supports a role of
protein prenylation inhibition in these statin-induced actions.
The effect of statins on the development of restenosis and
clinical outcome after coronary scent implantation has been
assessed, but only in a retrospective study. Statin therapy
was associated with a significant reduction in repeat target
vessel revasculari~ation procedures during ~-month follow-up.
Minimal lumen diameter was significantly larger, late lumen
loss was significantly less, and net gain significantly
increased in patients receiving statin therapy. Dichotomous
angiographic restenosis (>_ 50%) rates were significantly lower,
with 25o in the statin group compared with 38% in the no-statin
group.
Importance of high dose on anti-restenotic effect: One of
the findings of the study (referred to above) is of particular
relevance to the current invention, which in a preferred
embodiment comprises administering high concentrations of a
selected statin to the potential restenotic site by stmt-based
delivery. Thus, it was found that whereas a low oral dose of
11
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
the statin reduced cholesterol and.decreased neointimal
response to injury, a higher dose, despite no further effect on
lipid lowering, resulted in a highly significant reduction in
neointima formation.
Also, as indicated above, the replication of SMC
replication is tightly regulated by cell cycle regulatory
proteins including cyclin-dependent kinases (cdc2 and cdk2),
which move cells through the cell cycle, and cyclin-dependent
kinase inhibitors (such as p16, p21, and p27k1P1), which inhibit
progression of cells through the cell cycle. Thus, Rapamycin,
one of the few pharmacologic agents tested for which there are
encouraging (albeit preliminary) clinical results suggesting a
beneficial effect in limiting restenosis, is believed to act
largely by its ability to block the down-regulation of p27kip1
induced by various mitogens. This effect is accompanied by
increased p27 levels, which inhibit Rb phosphorylation and
thereby inhibit the release of Rb-bound E2F, a transcription
factor responsible for stimulating the expression of a broad
array of genes leading to cell cycle progression.
The small GTPases: The statins also exert molecular
effects on cell-cycling proteins, but their effects are
targeted to the small GTPases (ras, rho, etc), which are
upstream effectors of the cell cycle regulatory proteins. Rho
mediates cell cycle progression by down-regulating the
expression of the cdk inhibitor p27kipl~ thereby leading to
increased activity of cdk2 and hyperphosphorylation of Rb,
which consequently causes release of E2F and E2F-mediated cell
cycle progression. Post translational isoprenylation of these
small GTPases is critical to their function by leading to their
translocation to the cell membrane.
Cellular effects of.statins that modulate proliferation
and apoptosis independent of cholesterol-lowering effects:
Statins competitively inhibit HMG-CoA reductase and thereby
12
CA 02445524 2003-10-27
WO 02/087472 PCT/US02/12120
reduce cellular levels of mevalonate, precursor of the
isoprenoids. Isoprenoids cause the prenylation of the small
GTPases noted above. HMG-CoA reductase inhibitors decrease rho
geranylgeranylation and membrane translocalization, thereby
preventing down-regulation of p27k'~pl expression, which leads to
increased activity of cdk2 and hyperphosphorylation of Rb.
This consequently inhibits release of E2F and E2F-mediated cell
cycle progression, thereby inhibiting SMC proliferation. These
cellular effects also may be linked to the demonstrated
induction of SMC programmed cell death (apoptosis) and
inhibition of SMC migration caused by the statins. These pro-
apoptotic and anti-proliferative and migratory effects are
fully reversed by mevalonate, supporting a role of protein
prenylation inhibition in these statin-induced actions. Such
activities, if affecting SMCs located in the injured vessel
wall, would reduce neointimal growth of developing restenotic
lesions.
The invention, having now been fully described, should be
understood that it may be embodied in other specific forms or
variations without departing from its spirit or essential
characteristics. Accordingly, the embodiments described above
are to be considered in all respects as illustrative and not
restrictive, the scope of the invention being indicated by the
appended claims rather than the foregoing description, and all
changes which come within the meaning and range of equivalency
of the claims are intended to be embraced therein.
13