Note: Descriptions are shown in the official language in which they were submitted.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
1
Arran;~ement of anode for utilisation in an electrolysis cell
The present invention relates to an arrangement of anode for utilisation in an
electrolysis
cell. More specific it relates to improvements of anodes useful for retrofit
of existing
electrolysis cells, in which the anodes remains inert during operation.
Prior art:
Aluminium is presently produced by electrolysis of an aluminium containing
compound
dissolved in a molten electrolyte, and the electrowinning process is performed
in cells of
conventional Hall-Heroult design. These electrolysis cells are equipped with
horizontally
aligned electrodes, where the electrically conductive anodes and cathodes of
today's cells
are made from carbon materials. The electrolyte is based on a mixture of
sodium fluoride
and aluminium fluoride, with smaller additions of alkaline and alkaline earth
fluorides.
The electrowinning process takes place as the current passed through the
electrolyte from
the anode to the cathode causes the electrical discharge of aluminium
containing ions at the
cathode, producing molten aluminium, and the formation of carbon dioxide at
the anode.
During production of aluminium metal in accordance with the Hall-Heroult
principles,
carbon based anodes are used. The carbon anodes are consumed in the
electrolytic
process, through reactions in which the carbon material in the anodes combine
with the,
oxygen in the added alumina feed stock to form carbon dioxide gas. The
currently used
process displays several shortcomings and weaknesses, but it is still the only
industrial
process for aluminium production. The environmental impact from the Hall-
Heroult
process is unwanted due to production of pollutant greenhouse gases like COz
and CO in
addition to the so-called PFC gases (CFa, CzF6, etc.). The traditional
aluminium production
cells also utilise carbon materials as the electrically conductive cathode.
Since carbon is
not wetted by molten aluminium, it is necessary to maintain a deep pool of
molten alumin-
ium metal above the carbon cathode, and it is in fact the surface of the
aluminium pool that
is the "true" cathode in the present cells.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
2
The environmental impact from electrolytic aluminium production could be
reduced if
inert (or dimensionally stable) anodes were utilised. If the process could be
operated
without consumable anodes, i.e. using inert anodes, oxygen gas would be
evolved at the
anode in stead of carbon dioxide gas. As demonstrated by Keniry (Keniry, J.:
"The
economics of inert anodes and wettable cathodes for aluminium reduction
cells", JOM, pp.
43-47, May 2001), also possible operational cost savings imply that the
retrofit of conven-
tional Hall-Heroult electrolysis cells remain an attractive option if one
could retain to the
highest possible extent the cell superstructure, cathode shell, bus-bar system
and other cell
features of the present technology, in order to minimise the cost of the
retrofitting.
Over the times, numerous material technical solutions aimed at solving the
problems
related to inert anodes have been suggested, however, to the present day none
of which
have proven commercially feasible.
Field of invention:
The present invention relates to an improved anode design mainly for retrofit
of Hall-
Heroult cells, where the anode of a principally inert material is fabricated
in a specific
manner to overcome one of the most important obstacles of utilisation of inert
anodes in
retrofit of Hall-Heroult cells; The purity of the produced aluminium metal. A
reduction in
the contamination of anode components in the produced aluminium metal can be
achieved
by increasing the electroactive surface of the anode, i.e. increasing the
cathodic current
density with respect to the anodic current density in the electrolysis cell.
This feature can
be obtained by optimising the shape of the anode surface and the overall anode
structure.
Inert anodes utilised in existing Hall-Heroult cells have to satisfy several
demands. The
most important demand is to contribute to the production of commercial purity
aluminium
metal, as pointed out by Thonstad and Olsen (Thonstad, J. and Olsen, E.: "Cell
operation
and metal purity challenges for the use of inert anodes", JOM, pp. 36-38, May
2001),
without the need for new, costly purification processes. This requirement put
demands on
the electrochemical integrity of the inert anode material under the prevailing
circumstances
in the electrolyte. Additionally , however, also the design and/or electrode
design can be
utilised to contribute to maintain acceptable metal purities in retrofitted
Hall-Heroult cells.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
3
The electrolyte (bath) in the aluminium electrolysis cell can for all
practical purposes be
considered to be saturated with inert anode components as dissolved oxides.
The accumu-
lation of anode material elements in the aluminium produced is then governed
by the mass
transfer coefficient for the species from the bath to the aluminium metal
pool. A major
drawback of inert anode retrofit of Hall-Heroult cells is that there are
limited possibilities
for reducing the large area of the metal pool cathode exposed to the
electrolyte, without
costly rebuilds of the cell (i.e. drained cell concepts). Hence, optional ways
of reducing the
metal contamination should be sought after, and one seductive possibility is
to increase the
electroactive surface of the anode.
During electrolysis alumina containing species diffuse towards the anode and
are
discharged. In a thin layer (diffusion layer) toward the anode, the alumina
concentration is
different from the bulk electrolyte due to this discharge. By increasing the
anodic current
density the alumina concentration will decrease in the diffusion layer, due to
the discharge
rate at the anode being higher than the diffusion rate of the alumina species
into the diffu-
sion layer. Hence, the solubility of anode species (as oxides) will increase
in the layer
compared to the bulk electrolyte. It is well known that the solubility of
inert anode
material components, as oxides, decrease as the alumina concentration in the
electrolyte
increase. Diffusion of anode species from the layer close to the anode surface
and into the
bulk electrolyte will lead to precipitation of anode species in the bulk
electrolyte due to
super-saturation, and consequently a destruction of the inert anode material.
However, by
increasing the anode surface area, the anodic current density will decrease
(if the current
load is maintained unchanged) and as a result, the alumina concentration in
the diffusion
layer will increase. This will reduce the solubility of inert anode species
(as oxides) in the
diffusion layer and also reduce the concentration of these species in the bulk
electrolyte.
As a result, the contamination of the produced aluminium metal by anode
material compo-
nents will be reduced and a commercial quality aluminium can be produced with
inert
anodes. This approach will also increase the durability of the oxide-ceramic
(or metals or
cermets) inert anodes in the electrolysis cells.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
4
However, since the reduction of the metal pool surface area is not practically
feasible
during retrofit of existing Hall-Heroult cells, the angle of attack will be to
increase the
anode surface area. This is amongst others described in US 4,392,925,
4,396,481,
4,450,061, 5,203,971, 5,279,715 and 5,938,914 and in GB 2 076 021. Increased
anode
surface area is amongst others described in US 4,707,239 and 5,286,359 in
addition to NO
176189 and 308141.
NO 176189 involves a novel cell design for an aluminium electrolysis cell
involving the
use of a horizontal, wetted cathode and several vertically aligned inert
anodes. The
purpose of the novel cell design is to increase the total anode surface area
by inserting
several vertical, planar anodes above the cathode, but maintained within the
outlined outer
circumference of the cathode, so that a low anodic current density can be
maintained. The
low anodic current density is necessary to operate the low temperature cell to
prevent
formation of fluorine containing species due to the low solubility of alumina
in the
suggested electrolyte. Such an electrolyte is not feasible to use in existing
Hall-Heroult
cells with retrofitted inert anodes.
US 4,707,239 describes an electrode assembly for production of lead from a
chloride based
electrolyte. In the proposed assembly, the anodes (and cathodes) are designed
with saw
tooth pattern and spacers to maintain stable ACD and the anodes are also
equipped with
holes for gas release. The purpose of the patented increased electrode area is
to decrease
voltage and energy requirements, increase metal production, increase effective
inter
electrode electrolyte area, enhance rapid gas removal, and reduce the overall
metal produc-
tion costs. The proposed anode design will have limited benefits in a
retrofitted Hall-
Heroult cell with inert anodes and a horizontal metal pool introducing
variations in the
effective ACD, without substantial changes made to the anode (electrical)
properties).
NO 308141 relates to the insertion of shapes (contours) on the cathode surface
to "in situ"
produce a rounding of the anode surface. The patent is based on the shapes
(contours)
being placed on the cathode of an Hall-Heroult cell, in which the cathodes are
at least
partially operated under drained conditions. This means that no horizontal
metal pool is
present as a continous surface across the whole cathode panel area. The "in
situ"
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
formation of the rounded anodes for enhanced gas release and reduced cell
voltage is based
on the use of carbon consumable anodes, and is as such not applicable to
retrofit of exist-
ing Hall-Heroult cells with inert anodes, maintaining a horizontal metal pool
in the cell.
US 5,286,359 concerns the use of pyramid shaped anodes and cathodes in
existing Hall-
Heroult cells. Both electrode types are made from inert materials and the cell
is operated
at low ACDs with a metal pool located below the active cathode surfaces. The
invention
obtains increased anode and cathode surface area, although the proposed anode
design
would most likely operate at increased anodic current densities if deployed in
a retrofitted
cell with a horizontal metal pool due to the relative high electrical
conductivity of the
electrolyte.
Detailed description of the present invention:
The present invention relates to an arrangement of anode for utilisation in an
electrolysis
cell. More specific it relates to improvements of anodes useful for retrofit
of existing Hall-
Heroult electrolysis cells, in which the anodes remains inert during
operation. The
proposed anode design takes into consideration the increase of the anode
electroactive
surface area in order to obtain one or more of the features listed below,
whereof the two
main features is:
- Reduced contamination of the produced aluminium metal in the cell by
increasing the
anode to cathode surface area. Reduced contamination in produced metal will
lead to
reduced dissolution of anode material in the electrolyte, and as such
contribute to
prolong the lifetime of the anodes by maintaining its structural integrity.
Anodic current density can be kept lower than in existing cells, or be
maintained at the
same level through an amperage increase.
Other features obtained by the invention, and as also pointed out in US
4,392,925,
4,396,481, 4,450,061, 4,707,239, 5,203,971, 5,279,715, 5,286,359 and
5,938,914, in NO
176189 and 308141, as well as in GB 2 076 021, are:
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
6
- Possibilities for reduced voltage and energy requirements during aluminium
production.
- Possible increase in metal production through increase in effective inter
electrode
electrolyte area.
- Enhanced (and rapid) gas removal and there through reduced voltage drops.
The combined result of these effects will represent possible reductions in the
overall
production cost of aluminium metal.
Based on the desire to accomplish these features, an invention with respect to
the design of
the anode surface has been proposed in order to enhance the electroactive
surface area of
the anode. Advantages as mentioned above and further improvements can be
achieved in
accordance with the present invention as defined in the accompanying claims.
The invention is in the following described by examples and figures, where:
Figure 1: shows a first design of an anode surface with increased surface
area,
Figure 2: shows a second proposed design of an anode surface with increased
surface
area,
Figure 3: shows a third possible design of an anode surface with increased
surface area,
Figure 4: shows a fourth possible design of an anode surface with increased
surface area,
Table 1: presents a comparison of different anode surface areas with a mainly
horizontal
underside with an extent of 700x1000 mm2 with respect to alternative anode
surface design modifications.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
7
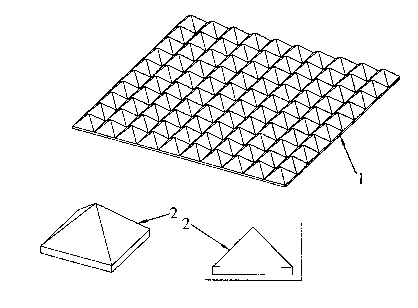
In figure 1 there is shown an anode surface design (1), in which the surface
area is
increased through the introduction (forming, shaping) of a series of pyramidal
elements
(2).
In figure 2 is shown another an anode surface design (10), in which the
surface area is
increased through the introduction (forming, shaping) of a series of (upward)
protruding
elements (11) with a pyramidal shape and rounded tops. To illustrate the
design of the
elements, a separate element (12) is also shown in perspective in the figure.
In figure 3 is shown a third possible design of an anode surface (20), in
which the surface
area is increased through the introduction (forming, shaping) of a series of
(upward)
protruding elements (21). To illustrate the design of the elements, a separate
element (22)
is also shown in perspective in the figure. As can be seen from the figure,
this particular
element is designed with a plurality of recesses/ steps (23, 24, 25, 26) that
will actively
contribute to the increase of the anode surface area.
In figure 4 there is illustrated a fourth possible design of an anode surface
(30), in which
the surface area is increased through the introduction (forming, shaping) of a
series of
(upward) protruding elements (31 ). The figure shows the anode surface
increasing
measures applied in the length wise direction, although it may be applied both
length wise
and crosswise. To illustrate the design of the elements, a separate element
(32) is also
shown in perspective in the figure. As can be seen from the figure, this
particular element
is designed with first a series of waves defined by a sinus function (33).
Thereafter, a
second series of sinus waves (34) are superimposed on the first, creating what
is called a
double sinus function. This design will actively contribute to the increase of
the anode
surface area.
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
8
Table 1 presents the effect on the anode surface area increase as a function
of anode
surface design changes. From the calculations in Table 1 it is clear that if
the anode
surface for instance is formed to a sinus-like shape, the anode surface area
is considerably
increased. By imposing the sinus function in two dimensions, the overall anode
surface
area does not increase if the amplitude and frequency is the same in both
directions.
However, by superimposing a second sinus function on the first one, where the
superim-
posed sinus function has shorter wave length and a shorter amplitude, the
surface area will
increase even more. A sketch of this "double sinus" function is provided in
figure 4. As
indicated in Table 1, the double sinus function can increase the surface area
of the anode
by 240%. This corresponds to a (theoretical) current increase from 200 kA to
480 kA and
yet maintaining the anodic current density of the retrofitted cell.
The described shapes/designs of the anode surfaces given above, as well as
shown in
Figures 1 through 4 and Table 1, represents only a few of the possible
modifications to
obtain the desired increase in anode surface area. Other embodiments of the
proposed
designs may also be used.
It should be understood that the anode may be designed so that its electrical
conductivity in
the outer layers) is of the same order of magnitude as in the electrolyte.
This can for
instance be done by its construction based upon the conductivity of the
material composi-
tion in the outer layer(s).
Table 1: Effect of surface design modifications on anode surface area.
Reference is a
horizontal anode with a flat underside (700x1000 mm2), and the table express
the percent
increase in anode surface area by introducing groves, saw tooth, rows of peaks
and valleys,
CA 02445676 2003-10-24
WO 02/088432 PCT/N002/00157
9
etc. on the electroactive anode surface,
Surface pattern Extent Dimensions Surface
area
Horizontal, flat 100%
Horizontal, jaggedwidth 50 mm, Lengthwise 108%
height 10 mm
Horizontal, jaggedwidth 25 mm, Lengthwise 108%
height 5 mm
Horizontal, jaggedwidth 50 mm, Length and crosswise108%
height 10 mm
Horizontal, jaggedwidth 25 mm, Length and crosswise108%
height 5 mm
Horizontal, sinus radii (1) 5 Lengthwise 168%
mm
Horizontal, sinus radii ( 1) Lengthwise 171
3 mm %
Horizontal, sinus radii (1) 5 Length and crosswise168%
mm
Horizontal, sinus radii ( 1 ) Length and crosswise171
3 mm %
Horizontal, doubleradii (1) 5 Length and crosswise240%
sinus mm
radii (2) 1
mm
Horizontal, sinus radii (1) 5 Length and crosswise177%
mm
w/protuberance radii (2) 1
mm