Note: Descriptions are shown in the official language in which they were submitted.
CA 02449186 2003-12-05
DESCRIPTION
Field of the invention
The present invention pertains to the field of immunology, more particularly
to the field
of generating immune regulatory DC. Said DC are a useful treatment for
treatment of a
variety of disorders associated by over-activation or pathological inhibition
of immune
responses.
Background
It is known in the art that immune therapy offers exciting prospects for
patients with
cancer, autoimmunity and transplanted organs. Recently many researches have
investigated the practical utility of dendritic cells (DC) as tools for immune
modulation.
The ability of DC to act both as augmenters and inhibitors of immune response
has
prompted investigation into their therapeutic use in experimental models and
clinically.
A shortcoming of DC-therapeutics is the present inability to gene-specifically
modify the
DC in an effective manner. It is the object of the present invention to
disclose novel
methods of manipulating DC, either through endowing stimulatory or inhibitory
capacities. This is accomplished through silencing of immune regulatory genes,
co-
transfection with immune stimulatory genes, multiple targeting of synergistic
targets, and
cell-specific transfection.
Control of Immunity by DC
Stimulation and control of T cell [1], B cell [2], NK cell [3, 4] arid NKT
cell [5] function
is co-ordinated directly and indirectly by the dendritic cell (DC). Acting as
the most
potent of all antigen presenting cells (APC), the DC is uniquely able to
influence the
immune response through possessing 3 broadly defined molecular signals: 1)
Direct
molecules for stimulation of the T cell receptor TCR. These include MHC I, MHC
II,
and CD 1 d for stimulation of the conserved NKT cell TCR [6]. 2) Membrane-
bound
costimulatory signals (ie CD40, CD80/86 and OX-40L) [7]. 3) Soluble
stimulatory
CA 02449186 2003-12-05
2
molecules (ie IL-12, LIGHT) [8]. Additionally, the biology of the DC is
uniquely formed
for its ability to activate T cell responses. Generally immature DC are found
in the
periphery, constantly patrolling for foreign antigens. Immature DC are highly
phagocytic, but possess low T cell activatory activity. Upon recognition of
various
foreign entities, DC mature, upregulate expression of lymph node homing
receptors, and
migrate into T cell-rich areas for stimulation of immunity [9]. Phagocytosed
material is
stored inside DC endosomes and upon activation, the pre-formed endosomes are
rapidly
exported to the cell surface where the MHC II-Ag complexes activate T cells
[9]. On the
other hand, the ability of the immature DC to constantly phagocytose self
antigens, leads
to its ability to generated tolerance to "self' and thus prevent autoimmunity
[10].
The ability of DC to control whether a stimulatory or inhibitory response will
follow antigenic immunization depends of which of the 3 signals described
above are
present, and in what concentration they are present. For example, we have
previously
generated tolerogenic DC (Tol-DC) through the inhibition of the IKK-(3 pathway
using
LF-15015, an analogue of the immune-suppressive drug deoxyspergualin [11].
These
Tol-DC are inhibitors of T cell activation, inhibitors of costimulated T
cells, and induce
production of T regulatory cells. Interestingly, the expression of MHC II,
CD40, CD$6
and IL-12 was suppressed. Supporting the idea that Tol-DC possess less of the
Signals
1,2 or 3 comes from experiments with KLH-pulsed DC from CD40-knockout which
induced antigen-specific T regulatory (Treg) cells in vivo [12]. On the other
hand, DC
transfected to expression high levels of one or more of the 3-Signals can be
used for
stimulating potent immune responses against viruses, bacteria, or cancer-
antigens [13].
Such stimulatory DC are particularly beneficial in clinical circumstances
where a
predisposition exists for weakened immune response such as cancer, in which DC
vaccination upregulates beneficial Thl immunity [14].
Generation of Stimulatory DC Through Various Manipulations
The fact that DC are potent stimulators of immunity has prompted their use for
treatment
of conditions that require activation of T cells such as cancer. The inherent
immunogenicity of DC can be upregulated by stimulation of these cells through
various
CA 02449186 2003-12-05
receptors, such as the Toll-like receptor (TLR) family. Ligands of these
receptors such as
long double-stranded RNA (TLR-3) [15], unmethylated cpg motifs (TLR-9) [16],
and
imiquimod (TLR-?) [17] are successful for stimulation. Host factors, however,
play
certain roles that can inhibit ability of DC to stimulate immune response. For
example,
melanoma is known to secrete high amounts of vascular endothelial growth
factor
(VEGF) that inhibits DC maturation through blocking NF-kB activation [18].
Alternatively, prostate cancer secretes a soluble DC-apoptosis inducing factor
[19]. In
order to generate more potent DC, investigators have transfected DC with
antigenic
mRNA [20], IL-12 gene [21], flt-3L gene [22], yr GM-CSF plasmid [23]. These
have
produced increased immune stimulatory potency of the DC, however further work
needs
to be performed for optimization. Consequently, a more detailed understanding
of the
receptor-ligand interactions associated with inhibition of DC will assist in
developing
modified DC that are successful for usage in these conditions. A potential
modification
of the DC for use in cancer would be blocking the receptors associated with
immune
suppressive signals, such as the IL-10 [24] or VEGF receptors [25].
Generation of Stimulatory DC Through Various Manipulations
An immature DC is classified phenotyically as having low expression of Signal
1,2, and
3, and functionally as a poor stimulatory of mixed lymphocyte reaction (MLR)
[26].
Typically, immature DC are known to be tolerogenic, inhibiting immune response
through providing weak Signal 1,2 and/or 3 [27]. While immature DC are highly
phagocytic and can be pulsed with specific antigens for a "tolerogenic
vaccine", a
concern is that these DC will mature upon in vivo administration. Generation
of
maturation-resistant DC was reported by Lutz et al through culture in low dose
GM-CSF
in absence of IL-4 [28]. Further studies have generated immature DC through
culture
with inhibitory cytokines such as IL-10 [29] and TGF-(3 [30], although
maturation-
resistance was not evaluated. Recent studies have noted that Tol-DC and
immature DC
may not be exactly the same. For example Sato et al demonstrated that for
maximum
tolerogenicity, DC must be raised under conditions that inhibit Signal 2 and
3, while
stimulation with LPS/TNF-alpha is needed for induction of high level of Signal
1 [31 ].
CA 02449186 2003-12-05
4
In light of this, it will be important to specifically be able to silence
certain immune
stimulatory genes, while at the same time not alter the basal immune
inhibitory genes
found in the DC.
DC can either be transfected with certain molecules, or endogenous signals
from the DC
can be inhibited by either pharmaceutical, genetic, or cell-culture
techniques. There are a
multitude of reports describing gene-transfection of DC. We ourselves have
used FasL-
transfected DC for the induction of donor-reactive apoptosis [32]. Others have
reported
that augmenting the levels of immune inhibitory cytokines through transfection
with IL-4
[33], IL-10 [34] or TGF-(3 [35], allows the DC to inhibit graft rejection, or
to protect from
autoimmune diseases.
Gene Manipulation of DC
Antisense oligonucleotides (AO) are sequences of DNA designed to block target
genes
by annealing with target mRNA, forming a RNA-DNA duplex, which is recognized
and
cleaved by the enzyme RNase H [36]. AO was the first therapeutic modality to
offer the
hope of gene-specific suppression. Disadvantages of the original AO technology
included susceptibility of the nucleotides to intracellular, and in vivo
degradation.
Overcoming this problem through the use of morpholino and phosphothiorate
backbones
has led to widespread interested in AO therapies [37, 38]. Unfortunately, the
problems of
non-specific suppression, and longevity of suppressing effect remain
significant
drawbacks that impede the wide-spread clinical use of AO as drugs. In fact, a
recent
Phase III trial of AO for colitis has demonstrated no significant benefit
[39].
Despite these drawbacks, the ability of AO to specifically inhibit genes of
interest has
stimulated the interest of immunologists. Due to the importance of cytokines
in
controlling immune functions, immunomodulation using AO to cytokines has been
proposed [40]. An interesting early experiment targeted the T cell stimulatory
cytokine
IL-2 in the context of allograft rejection. Using osmotic pumps to deliver AO
to IL-2, Qu
et al have increased allograft survival by blocking IL-2 production in murine
cardiac
CA 02449186 2003-12-05
S
allograft recipients [41]. Targeting other genes important for immune function
has also
been performed. Blocking expression of the LPS co-receptor CD14 using AO,
increased
survival in murine models of scepticemia [42). By targeting expression of
intercellular
adhesion molecule-1 with AO, Toda et al reduced leukocyte-induced damage to
ischemic
lungs [43].
As DC are the most potent immune regulatory cell, application of AO to this
cell is of
particular interest. DC transfection with AO has been reported using either
electroporation or liposomal methods. Interestingly, one of the first gene
targets using
AO on DC was the peptide transporter, Tr~anspo~t Associated Frotein (TAP).
Inhibition
of this protein on DC was able to endow the cells with more potent antigen
presenting
function [44]. Following this finding, targeting of the MHC invariant chain
with AO was
also performed. Through facilitating less competition for the MHC binding
groove, DC
with suppressed invariant chain where able to prime immune responses better
than
control DC [45]. Another AO approach to generating DC with heightened immune
stimulating capability was performed by suppressing the inhibitory cytokine IL-
10. It is
known that during DC differentiation, IL-10 acts in a negative autocrine
manner to
regulate the maturation, and T cell-stimulating capacity of DC. Taking this
into account,
Igietseme et al compared the T cell-activating function of wild-type DC, DC
from IL-10
knockout mice, and DC treated with AO to IL-10. The knockout and the AO-
suppressed
IL-10 DC possessed greater T cell stimulating function, and also invoked the
generation
of a Thl phenotype (ie high IFN-y, low IL-4) [46].
Inhibitory DC would have practical applications for treatment of transplant
rejection and
autoimrnunity. To this end, inhibition of the T cell costimulatory molecules
CD80 and
CD86 was reported by AO. DC in which either CD80 or CD86 was suppressed
possessed inhibited allostimulatoxy activity, induced high IL-4, low IFN-g
production
from T cells, increased the percentage of apoptotic T cells in culture, and
inhibited the
generation of CD8 CTL in vivo [47). Importantly, the administration of AO
manipulated
DC to cardiac recipients was able to prolong allograft survival, although only
modestly
[47]. Targeting of other DC-bound immune stimulatory molecules was also
performed.
CA 02449186 2003-12-05
6
Gorczynski et al demonstrated that suppressing expression of MD-1 on DC
resulted in
inhibition of allostimulatory activity, Thl>Th2 cytokine switch and
prolongation of
allograft survival [48]. These effects were dependent on ability of suppressed
MD-1 to
increase expression of the DC inhibitory molecules OX-2. In addition to this,
AO
inhibition of the novel costimulatory molecule B7H3 on DC has resulted in DC
with
similar inhibitory functions as described above [49J. The ability to induce
immune
modulation through suppressing DC genes suggests a novel and practical method
of
altering immune function. The recent observation that administration of
manipulated DC
can not only inhibit the generation of immune response, but can also inhibit a
T cell
response after initiation, suggests the practicality of DC immunotherapy [12].
However,
the fact that AO possess temporally limited effects provides the concern that
DC may
start to re-express the immune stimulatory genes after being placed in vivo.
In such a
situation, the administered DC may actually serve the counter-purpose of being
immune
stimulatory. Although AO are theoretically promising, clinical applications
have not
been beneficial. Additionally, several problems are intrinsic to AO
therapeutics: 1. Large
quantities are needed for effects; 2. Lack of specificity in some cases [S0,
51] and; 3.
Poor transfection into target cells [S0, 51]. For example, in the study cited
above using
IL-2 specific AO to block graft rejection, a very high dose of AO was needed
to be
administered using continuous intravenous osmotic pump in order to achieve a
modest
graft survival benefit over untreated controls [41]. Similarly, although AO
have entered
Phase III clinical trials, there was no significant difference over placebo
[52]. For these
reasons, novel methods therapeutically applicable gene-specific silencing are
desired.
RNA interference (RNAi)
RNAi is a process by which a double-stranded RNA (dsRNA) selectively
inactivates
homologous mRNA transcripts. The initial suggestion that dsRNA may possess
such a
gene silencing effect came from work in Petunias in which overexpression of
the gene
responsible for purple pigmentation actually caused the flower to lose their
endogenous
color [53]. This phenomenon was termed co-suppression since both the inserted
gene
transcript and the endogenous transcript Were suppressed. In 1998, Fire et~ al
injected C.
CA 02449186 2003-12-05
elegans with RNA in sense, antisense and the combination of both in order to
suppress
expression of several functional genes. Surprisingly, injection of the
combined sense and
antisense RNA led to more potent suppression of gene expression than sense or
antisense
used individually. Inhibition of gene expression was so potent that
approximately 1-3
molecules of duplexed RNA per cell were effective at knocking down gene
expression.
Interestingly, suppression of gene expression would migrate from cell to cell
and would
even be passed from one generation of cells to another. This seminal paper was
the first
to describe RNAi [54]. One problem present at the initial description of RNAi,
and
subsequent papers following, was that in order to induce RNAi, long pieces 200-
800 base
pairs, of dsRNA had to be used. This is impractical for therapeutic uses due
to the
sensitivity of long RNA to cleavage by RNAses found in the plasma and
intracellularly.
In addition, long pieces of dsRNA induce a panic response in eukaryotic cells,
part of
which includes nonspecific inhibition of gene transcription but production of
interferon-a
[55]. In 2001, it was demonstrated that subsequent to entry of long dsRNA
duplex into
the cytoplasm, a ribonuclease III type enzymatic activity cleaves the duplex
into smaller,
21-23 base-pairs which are active in blocking endogenous gene expression.
These small
pieces of RNA, termed small interfering RNA (siRNA) are capable of blocking
gene
expression in mammalian cells without triggering the nonspecific panic
response [56].
Therefore, there are 2 methods of inducing RNAi, the naturally occurring
method that
takes place when viral or long double-stranded RNA enters the cell. Upon
crossing the
membrane, the dsRNA is recognized by: 1) 2'S' OS, an enzyme that turns on an
enzymatic cascade leading to inhibition of protein synthesis, 2) Activation of
the protein
kinase R (PKR) which also results in non-specific shut-down of cellular
activity, and 3)
DICER, a nuclease cuts the dsRNA into 21-23 base-pairs that are active in
blocking
endogenous gene expression (Figure lA). This method of gene-silencing is not
advantageous for research or experimental purposes due to the non-specificity
of effects.
However, theoretically it is conceivable that administration of long dsRNA
targeting
cancer immune suppressive genes would have the two-fold effect of non-
specifically
blocking tumor proliferation, as well as silencing the immune suppressive
genes. The
other method of inducing RNAi is through administration of pre-formed,
synthetic
CA 02449186 2003-12-05
siRNA of 21-23 nucleotide base-pairs. This approach only targets the
endogenous RNA
transcript and does not possess indiscriminate inhibitory effects (Figure 1B).
Several recent studies have demonstrated the utility and practicality of siRNA
mediated
gene silencing for blocking expression of disease-associated genes in vitro.
Novina et al
demonstrated inhibition of HIV entry and replication using siRNA specific for
CD4 and
gag, respectively [57]. Suppression of human papilloma virus gene expression
in tissue
biopsies from women with cervical carcinoma was reported using siRNA specific
fox the
E6 and E7 genes [58). Furthermore, induction of leukemic cell line apoptosis
and
complete inhibition of bcr-abl expression was achieved using siRNA [59]. The
first
report of siRNA used in animal models is from McCaffrey et al who suppressed
expression of luciferase in mice by administration of siRNA using a
hydrodynamic
transfection method [60]. A subsequent study using HeLa cells xenografted on
nude
mice compared efficacy of gene suppression between AO and siRNA. Consistent
with in
vitro suggestions, in vivo siRNA administration resulted in a more potent and
longer
lasting suppression of gene expression than obtained with AO [61].
Silencing gene expression through siRNA is superior to conventional gene or
antibody
blocking approaches due to the following: 1) Blocking efficacy is more potent
[61]; 2)
Targeting gene expression is more specific [62]; 3) Inhibitory effects can be
pass for
multiple generations [63]; 4) In vitYO transfection efficacy is higher and can
be expressed
in a stable manner [64]; 5) In vivo use is more practical and safer due to
lower
concentration needed and no neutralizing antibody; 6) Tissue or cell specific
gene
targeting is possible using specific promoter vector [65, 66] or specific
antibody
conjugated liposome; 7) Simultaneously targeting multiple genes or multiple
exons
silencing is possible for increasing efficacy [67].
DESCRIPTION OF THE INVENTION
The disclosed invention teaches methodologies for manipulation of DC in order
to
stimulate or inhibit immune responses. One embodiment of the present invention
is
CA 02449186 2003-12-05
9
generation of an inhibitory regulatory DC (iREG-DC) population that is
suppressive to T
cell responses. More specifically, the invention teaches that iREG-DC can be
produced
through selective silencing of a single or multiple immune stimulatory genes
using the
technique of RNAi. Additionally, the inhibitory capacity of iREG-DC can be
further
augmented through transfectian with genes known to inhibit immune responses.
Genes
encoding molecules that both stimulate and inhibit ability of the DC to
activate T cells
are well known and described in the art. The advantage of generating iREG-DC
resides
in their ability to act as an antigen-specific activator of T cells when the
iREG-DC is
pulsed with antigen. Delivery of antigens into iREG-DC can be performed before
or
subsequent to gene silencing and/or transfection. Methods of introducing
antigens to DC
include but are not limited to co-culture with antigenic proteins, peptides,
or mRNA
encoding the antigen of interest. An alternative approach for delivery of
antigen includes
transfection of iREG-DC with a plasmid encoding the antigenic protein or
derivatives
thereof. Ability of iREG-DC to inhibit immune responses can be assessed both
in vitro
and based on in vivo efficacy. During production of iREG-DC parameters such as
ability
to induce Treg formation, ability to inhibit MLR, and capacity for inducing a
Thl>Th2
shift in T cell cytokine profile can be used to determine the regulatory
abilities of iREG-
DC.
Generation of iREG-DC can be initiated with a bone marrow culture for
production of
bone marrow-derived DC. Said cultures have been extensively described in the
art. One
example of generating BM-DC involves: l) Extraction of bone marrow from femurs
and
tibia of given mice followed by purification of mononuclear cells through
either density
gradient methods such as Ficoll, or lysis of erthrocytes using a hypotonic
lysis buffer
solution; 2) Said mononuclear cells are then cultured in a media suitable for
sustaining
cellular viability. Suitable culture media include RPMI, DMEM, Opti-MEM
supplemented with fetal calf serum or the AIM-V which does not require serum
supplementation. In order to induce proliferation of the monocytic progenitor
cells
granulocyte-monocyte colony stimulating factor is added to the culture media
at a
concentration between 5 ng/ml to 100 ng/ml, but preferably, 10 ng/ml.
Interleukin-4 is
simultaneously added to the culture in order to suppress macrophage
overgrowth. After
CA 02449186 2003-12-05
5-12 days, of culture, but preferable 7-days, a population of cells arises
that possesses a
high concentration of DC as witnessed by CD1 lc expression; 3) The generated
DC
population is then transfected with siRNA in order to silence immune
stimulatory genes.
The siRNA may be delivered in the form of free oligonucleotides, siRNA-
expression
cassettes, siRNA-expression plasmids, or siRNA-carrying viral vectors.
Suitable targets
for silencing include gene encoding adhesion molecules, membrane-bound immune
stimulatory molecules, cytokines, and immune-stimulatory transcription factors
such as
NF-kB, STAT4, T-bet, and GATA-3; 4) In order to increase the efficacy of
suppression
by iREG-DC simultaneous cotransfection, or transfection subsequent to gene
silencing
may be performed with genes encoding immune suppressive molecules, said
molecule
may include TGF-b, IL-10, thrombospondin, indolamine-dioxygenase, or galectin-
3; 5)
Antigen-specificity of iREG-DC can be endowed through pulsing said cells with
antigens of interest. The pulsing procedure could comprise of addition of
exogenous
antigens in the form of proteins, peptides, or aggregated peptides.
Alternatively, genetic
pulsing of iREG-DC can be performed through transfecting said cells using mRNA
encoding the antigen of interest, or a plasmid that once internalized will
transcribe the
given antigen. Advantages of genetic pulsing include the preferential
localization of the
antigens in the "intracellular" pathway of antigen presentation. Antigens
entered through
this route preferentially become expressed on MHC I and target CD8+ T cells.
The
pulsing procedure can be performed both before and/or after the
silencing/transfection
step of gene manipulation described. In some circumstances it may be
advantageous to
add antigens to the DC preparation early in the culture time since immature DC
possess a
higher endocytic rate compared to mature ones. Once iREG-DC are generated,
they can
be used to treat a variety of diseases associated with immune hyperactivation.
It will be obvious to one skilled in the art that many variations of the above
iREG-DC
generating approach can be performed without departing from the scope, or
spirit of the
invention disclosed. For example, DC progenitors can be purified from a
variety of
sources besides the bone marrow. For clinical use, DC are generally obtained
from
monocytic cultures whose starting source is monocytes obtained from peripheral
blood
CA 02449186 2003-12-05
11
mononuclear cells that are subsequently cultured in IL-4 and GM-CSF. Other
methods of
generating DC include addition of calcium ionophore or the peptide EPI.b
recently.
Using the example of multiple sclerosis (MS) as a prototypic autoimmune
disease, iR.EG-
DC can be used as a cellular therapy by pulsing said cells with antigenic
targets important
in MS. It is known both in clinical MS, and in animal models that myelin basic
protein
(MBP) acts as an autoantigen. Clinical trials attempting to tolerize
recipients to MBP
have shown some success in ameliorating disease progression, however effects
are
transient and inconsistent. Administration to the patient a composition of MBP-
pulsed
iREG-DC can be a useful method of specifically inactivating pathogenic T
cells, while at
the same time inducing production of T-regulatory cells that would prevent
reoccurrence
of autoimmune attack. Such an approach to treatment of autoimmunity is more
beneficial
than commonly used approaches such as immune suppressants that non-
specifically
inhibit all T cell activity, leading to increased susceptibility to pathogens
and neoplasms.
Another embodiment of the invention involves generation of iREG-DC in vivo. It
is
known that a variety of adjuvants such as Complete Freund's Adjuvant (CFA)
possess
the ability to initiated macrophage and dendritic cell homing and uptake of
exogenous
entities. Based on our findings that siRNA can be uptaken by DC in absence of
specialized transfection reagents, we have admixed CFA with siRNA for immune
stimulatory gene with the antigen we sought to modify. The mixture of CFA,
siRNA,
and antigen subsequently initiate an immune regulatory process that results in
the
generation of T regulatory cells and inhibition of antigen-specific response.
Such a
tolerance-inducing vaccine or "ToleroVax" can be used for inhibiting immune
responses
to a variety of autoimmune disease including but not limited to: rheumatoid
arthritis,
Stevens-Johnson syndrome, juvenile rheumatoid arthritis, psoriatic arthritis,
allergies,
psoriasis, leprosy reversal reactions, erythema nodosum leprosum, autoimmune
uveitis,
multiple sclerosis, allergic encephalomyelitis, systemic lupus erythematosus,
acute
necrotizing hemorrhagic encephalopathy, idiopathic bilateral progressive
sensorineural
hearing loss, aplastic anemia, pure red cell anemia, idiopathic
thrombocytopenia,
polychondritis, scleroderma, Wegener's granulomatosis, chronic active
hepatitis,
CA 02449186 2003-12-05
12
myasthenia gravis, idiopathic spree, lichen planes, Crohn's disease, Graves
ophthalmopathy, sarcoidosis, contact dermatitis primary biliary cirrhosis,
primary
juvenile diabetes, dry eye associated with Sjogren's syndrome, uveitis
posterior, and
interstitial lung fibrosis.
Another embodiment of the invention is preparation of immune stimulatory DC
termed
sREG-DC. Using the culture procedures described above for generation of iREG-
DC,
sREG-DC are produced by silencing immune suppressive genes and/or transfection
with
immune stimulatory genes. Similar to the preparation of i~EG-DC, sIREG-DC can
be
pulsed with a variety of antigens in order to increase the magnitude and
specificity of
immune responses. sREG-DC are of great benefit for viral infections such as
HIV where
it is preferential to induce responses in a polyvalent fashion due to the
great variability of
antigens on the viral surface. Immunological depression in neoplasia results
in an
inability to mount successful responses. In addition, the ability of cancers
to suppress the
antigen-presenting compartment of the immune response often makes conventional
vaccination strategies unsuccessful.
Examples
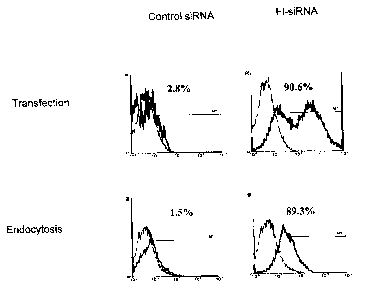
1. Incorporation of siIZNA into DC by endocytosis
DC were cultured from bone marrow progenitors in the presence of GM-CSF and IL-
4. 1
x 106 day-7 cultured DC were liposomely transfected with unlabeled (control
siRlVA), or
fluorescein labeled (Fl-siRNA) siRNA specific for luciferase sequence at
indicated
concentration (upper panel). Alternately, these siRNAs were added to DC
culture without
transfection reagent at day 5. DC were activated with LPSlTNFa on day 8 and
the
transfection efficacy assessed by flow cytametry on day 9. The data on Figure
1.
indicates that DC can be efficiently (80-90%) transfected by phagocytosis.
2. Silencing of DC by siRNA-containing organ storage solution
DC were cultured for 4 days in 6-well plate. SEC-GAPDH was added in the
culture
without transfection reagents. DC were collected at indicated times and
stained with anti-
CA 02449186 2003-12-05
13
GAPDH mAb. The expression of GAPDH was analyzed using flow cytometry and
compared with control DC (bolded lines) and silenced DC (fine lines) (Figure
2).
Incorporation of fluorescent (Fl) siRNA into perfused kidney. Fl-siRNA
targeting
luciferase was admixed with Ringer's lactated solution at a concentration of
100 nMol.
A total of 4 ml of the said admixture was flushed through a kidney from an
anaesthetized
BALB/c mouse. Subsequently the kidney was clamped for half an hour.
Fluorescence
was detected by confocal microscopy. A significant increase in green staining
was
observed in the perfused kidney (siPerfuseTM Treated) compared to controls
(Figure 3).
Silencing of GAPDH in a perfused kidney model. siRNA targeting GAPDH was
designed and was admixed with Ringer's lactated solution at a concentration of
100
nMol. A total of 4 ml of the said admixture was flushed through a kidney from
an
anaesthetized BALB/c mouse. Subsequently the kidney was clamped for half an
hour.
GAPDH expression was detected by immunohistochemistry. A significant decrease
in
GAPDH staining was observed in the perfused kidney compared to control (Figure
4).
3. Mufti-gene silencing in DC
pSilencer-IL12 and pSilencer-GAPDH were co-transfected to 7-day cultured DC by
GenePorter. 48 hours after gene silencing, the control siRN.A transfected DC
(control-
DC, upper panel) and siRNA transfected DC (lower panel) were staining with
anti-mouse
IL-12 and GAPDH (bolded lines) respectively. The isotype controls are shown as
broken lines (Figure 5).
4. Gene-silencing of MHC II
A siRNA construct derived from SEQ 1D NO 2, specifically targeting MHC II
alpha
chain was inserted into the commercially available pSilencer and added to
marine BM-
DC culture at the concentration of lug/ml at day 4. On Day 7 cells were
activated with
LPS and TNF at a concentration of 10 ng/ml. MHC II expression was detected by
flow
cytometry on 24-48 hours later. A profound inhibition of MHC II expression was
observed in the siRNA-pSilencer treated groups (Figure 6).
CA 02449186 2003-12-05
14
5. Gene-silencing of IL-12 p35
A siRNA construct derived from SEQ ID NO 1, specifically targeting IL-12p35
was
inserted into the commercially available pSilencer and added to murine BM-DC
culture
at the concentration of lug/ml at day 4. On Day 7 cells were activated with
LPS and
TNF at a concentration of 10 nglml. IL-12 expression was detected by flow
cytometry on
24-48 hours later using intracellular staining. A profound inhibition of IL-12
expression
was observed in the siRNA-pSilencer treated groups (Figure 7).
6. Gene-silencing of CD40 using SEC
SEC were generated as PCR products consisting of a hairpin siRNA template
flanked by
promoter and terminator sequences. Once the SEC is transfected into cells, the
hairpin
siRNA is expressed from the PCR product and leads to gene silencing. siRNA
expression cassette kit (Ambion Inc, Texas USA) was used to generate SEC
targeting
CD40. Briefly, 4 targets of 21 nucleotide long were selected from cDNA
(GeneBank).
The active sequences are designated SEQ ID NOS 3-6. 3 PCR reactions were
performed
to generate SEC. The first reaction formed half of the hairpin siRNA (sense),
the second
reaction made the precursor SEC (second half of the siRNA hairpin), the last
reaction
added the terminator and restriction sites for cloning to the SEC. CD40-
SEC(four
different target sites: SEQ 1D NOS 3-6) were added into DC culture at 150ng/ml
at day6
followed by transfection of 300ng SEC at day 7. DC were activated with LPS
with a
period of 20 hours. FAGS was performed at day 8. Varying degrees of CD40
inhibition
were seen by flow cytometry (Figure 8).
7. Gene-silencing of IL-12 using SEC
SEC were generated as PCR products consisting of a hairpin siRNA template
flanked by
promoter and terminator sequences. Once the SEC is transfected into cells, the
hairpin
siRNA is expressed from the PCR product and leads to gene silencing. siRNA
expression cassette kit (Ambion Inc, Texas USA) was used to generate SEC
targeting IL-
12. Briefly, 4 targets of 21 nucleotide long were selected from cDNA
(GeneBank). The
CA 02449186 2003-12-05
active sequences are designated SEQ 1D NOS 15-18. 3 PCR reactions were
performed to
generate SEC. The first reaction formed half of the hairpin siRNA (sense), the
second
reaction made the precursor SEC (second half of the siRNA hairpin), the last
reaction
added the terminator and restriction sites for cloning to the SEC. IL-12
SEC(four
different target sites: SEQ ID NOS 15-18) were added into DC culture at
150ng/ml at day
6 followed by transfection of 304ng SEC at day 7. DC were activated with LPS
with a
period of 20 hours. FACS was performed at day 8. Varying degrees of IL-12
inhibition
were seen by flow cytometry (Figure 9).
8. pInterference Plasmid
siRNA sequence of a variety of specificities can be inserted in our
plnterference plasmid
(Figure 10) for expansion and utilization in mammalian cells. Mun I and Hindi
III
restriction sites serve as the area for integrating SEC DNA that was described
in SEQ ID
NOS 3-18. The pInterference plasmid was used to deliver SEC DNA targeting SEQ
ID
4, which specifically inhibits expression of CD40. Treatment with
plnterference
containing mismatched DNA did not modify expression of CD40 (Figure 1 la). In
contrast plnterference containing the CD40-specific SEQ ID 4 was able to
markedly
reduce CD40 expression (Figure l lb).
9. Inhibition of ReIB expression
siRNA targeting ReIB specific SEC ID NOS 19-22 was administered to DC using
GeneSilence reagent. Assessment of RelB expression was performed using RT-PCR.
A
23%, 69%, 95%, and 15% reduction in ReIB mRNA was observed compared to control
cells using SEC ID 19, 20, 21, and 22, respectively
Sequence Listings
Sequence ID: 1
CA 02449186 2003-12-05
16
SEQUENCE CHARACTERISTICS: IL-12 sense construct
LENGTH: 65
TYPE: DNA
ORGANISM: Synthetic
gatcccgCCTGCTGAAGACCACAGATttcaagagaATCTGTGGTCTTCAGCAGGttttttgga
as
Sequence m: 2
SEQUENCE CHARACTERISTICS: MHC II alpha Sense construct
LENGTH: 64
TYPE: DNA
ORGANISM: Synthetic
gatcccGACGACATTGAGGCCGACCttcaagagaGGTCGGCCTCAATGTCGTCttttttggaa
a
Sequence ID: 3
SEQUENCE CHARACTERISTICS: CD40(1), Sense SEM Template
LENGTH: 55
TYPE. DNA
ORGANISM: Synthetic
ACACTACACAAATGTTCCACTGGGCTGAGAACCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 4
SEQUENCE CHARACTERISTICS: CD40(2), Sense SEM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
CCTCTACACAAAAGGTACAGACAGTGTCTGACCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 5
SEQUENCE CHARACTERISTICS: CD40(3), Sense SEM Template
CA 02449186 2003-12-05
17
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
AAACTACACAAATTTCTGTAGGACCTCCAAGCCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 6
SEQUENCE CHARACTERISTICS: CD40(4), Sease SEA Template
LENGTH: 54
TYPE: DNA
ORGANISM: Synthetic
GTGCTACACAAACACTGAGATGCGACTCTCTCGGTGTTTCGTCCTTTCCACAA
G
Sequence ID: 7
SEQUENCE CHARACTERISTICS: CD80(1), Sense SEM Template
LENGTH: 54
TYPE: DNA
ORGANISM: Synthetic
CTCCTACACAAAGAGCCTTGGACATGGAAACCGGTGTTTCGTCCTTTCCACAA
G
Sequence II?: 8
SEQUENCE CHARACTERISTICS: CD80(2), Sense SEM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
GAGCTACACAAACTCATCTTCATGAGGAGAGCCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 9
SEQUENCE CHARACTERISTICS: CD80(3), Sense SEM Template
LENGTH: 54
TYPE: DNA
CA 02449186 2003-12-05
18
ORGANISM: Synthetic
AGACTACACAAATCTTATACTCGGGCCACACCGGTGTTTCGTCCTTTCCACAA
G
Sequence m: 10
SEQUENCE CHARACTERISTICS: CD80(4), Sense SEM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
GTTCTACACAAAAACCAAGAGAAGCGAGGCTCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 11
SEQUENCE CHARACTERISTICS: CD86(1), Sense SEM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
CTGCTACACAAACAGCTCACTCAGGCTTATGCCGGTGTTTCGTCCTTTCCACA
AG
Sequence lD: 12
SEQUENCE CHARACTERISTICS: CD86(2). Sense SEM Template
LENGTH: 54
TYPE: DNA
ORGANISM: Synthetic
AATCTACACAAAATTGATCCTGTGGGTGGCTCGGTGTTTCGTCCTTTCCACAA
G
Sequence ID: 13
SEQUENCE CHARACTERISTICS: CD86(3), Sense SEM Template
LENGTH: 55
CA 02449186 2003-12-05
19
TYPE: DNA
ORGANISM: Synthetic
TTCCTACACAAAGAATGAAAGAGAGAGGCTGCCGGTGTTTCGTCCTTTCCAC
AAG
Sequence 117: 14
SEQUENCE CHARACTERISTICS: CD86(4), Sense SEM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
ATCCTACACAAAGATAGTCTCTCTGTCAGCGCCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 15
SEQUENCE CHARACTERISTICS: II.-12(1) Sense, S8M Template
LENGTH: 54
TYPE: DNA
ORGANISM: Synthetic
AGACTACACAAATCTGTGGTCTTCAGCAGGTCGGTGTTTCGTCCTTTCCACAA
G
Sequence ID: 16
SEQUENCE CHARACTERISTICS: IL-12(2) Sense, SFM Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
ACCCTACACAAAGGTCATCATCAAAGACGTCCGGTGGTTCGTCCTTTCCACA
AG
Sequence 1D: 1?
SEQUENCE CHARACTERISTICS: IL-12(3) Sense, SFM Template
LENGTH: 55
TYPE: DNA
CA 02449186 2003-12-05
ORGANISM: Synthetic
ATCCTACACAAAGATCTGCTGATGGTTGTGACCGGTGTTTCGTCCTTTCCACA
AG
Sequence 117: 18
SEQUENCE CHARACTERISTICS: IL-12(4) Sens a SEbt Template
LENGTH: 55
TYPE: DNA
ORGANISM: Synthetic
GCTCTACACAA.AAGCAGGATGCAGAGCTTCACCGGTGTTTCGTCCTTTCCACA
AG
Sequence ID: 19
SEQUENCE CHARACTERISTICS: RelB Targetl, sense
LENGTH: 19
TYPE: DNA
ORGANISM: Mouse
GACCATAGATGAATTGGAA
Sequence ID: 20
SEQUENCE CHARACTERISTICS: RelB Target2, sense
LENGTH: 19
TYPE: DNA
ORGANISM: Mouse
GGACATATCCGTGGTGTTC
Sequence 117: 21
SEQUENCE CHARACTERISTICS: RelB Target3, sense
LENGTH: 19
TYPE: DNA
CA 02449186 2003-12-05
21
ORGANISM: Mouse
CATCGGAGCTGCGGATTTG
Sequence ID: 22
SEQUENCE CHARACTERISTICS: ReIB Target4, sense
LENGTH: 19
TYPE: DNA
ORGANISM: Synthetic
GCAGATCGCCATTGTGTTC
Sequence 117: 23
SEQUENCE CHARACTERISTICS: CD40 (target a)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AATTCTCAGC:CCAG'I'GGAAC~A.
Sequence ID: 24
SEQUENCE CHARACTERISTICS: CD40 (target b)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AATCAGACACTGTCTGTACCT
Sequence ID: 25
SEQUENCE CHARACTERISTICS: CD40 (target c)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AACTTGC'rAGGTCCTACACiAAA
Sequence ID: 26
SEQUENCE CHARACTERISTICS: CD40 (target d)
LENGTH: 21
TYPE: DNA
CA 02449186 2003-12-05
22
ORGANISM: Mouse
AAAGAGACrTCGCA'I'C'CCACi I'G
Sequence ID: 27
SEQUENCE CHARACTERISTICS: CD80 (target a)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAGTTTCCATGTCCAAGGt~'TC.
Sequence ID: 28
SEQUENCE CHARACTERISTICS: CD80 (target b)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAC:TCTCC~TCATGAAGATGAG
Sequence ID: 29
SEQUENCE CHARACTERISTICS: CD80 {target c)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAG'I'G'I"GGCC:C CiAG' I:'A'a'A A GA
Sequence ff~: 30
SEQUENCE CHARACTERISTICS: CD80 (target d)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAAGCCTC:GC:TTCTCTTGGTTA
Sequence ID: 31
SEQUENCE CHARACTERISTICS: CD86 (target a)
LENGTH: 21
CA 02449186 2003-12-05
23
TYPE: DNA
ORGANISM: Mouse
AACATAAGCCTGAGTGAGCTG
Sequence ID: 32
SEQUENCE CHARACTERISTICS: CD86 (target b)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAACiCt:AC.'CC;AC'AGGA'l:'CAA'1:'
Sequence lD: 33
SEQUENCE CHARACTERTSTICS: CD86 (target c)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AACAGCCTC1~C'1'C'~1"I"fCA'1'~l'C
Sequence ID: 34
SEQUENCE CHARACTERISTICS: CD86 (target d)
LENGTH: 21
TYPE: DNA
ORGANISM: Mouse
AAt;GCTGACAGAGAGACTATC
References:
1. Platsoucas, C.D., et al., Immune responses to human tumors: development of
tumor vaccines. Anticancer Res, 2003. 23(3A): p. 1969-96.
2. MacLennan, I. and C. Vinuesa, Dendritic cells, BAFF, and APRIL: innate
players
in adaptive antibody responses. Immunity, 2002. 17(3): p. 235-8.
3. Mailliard, R.B., et al., Dendritic cells mediate NK cell help for Thl and
CTL
responses: two-signal requirement for the induction of NK cell helper
function. J
Immunol, 2003. 171(5): p. 2366-73.
4. Moretta, A., Natural killer cells and dendritic cells: rendezvous in abused
tissues.
Nat Rev Immunol, 2002. 2(12): p. 957-64.
CA 02449186 2003-12-05
24
5. Nishimura, T., et al., The interface between innate and acquired immunity:
glycolipid antigen presentation by CDI d-expressing dendritic cells to NKT
cells
induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int
Immunol, 2000. 12(7): p. 987-94.
6. Chun, T., et al., CD~d-expressing dendritic cells but not thymic epithelial
cells
can mediate negative selection of NKT cells. J Exp Med, 2003. 197(7): p. 907-
18.
7. Kufer, P., et al., Minimal costimulatory requirements for T cell priming
and THI
differentiation: activation of naive human T lymphocytes by tumor cells armed
with bifunctional antibody constructs. Cancer Immun, 2001. 1: p. 10.
8. Morel, Y., et al., The TNFsuperfamily members LIGHT and CDI54 (CD40
ligand) costimulate induction of dendritic cell maturation and elicit specifzc
CTL
activity. J Immunol, 2001. 167(5): p. 2479-86.
9. Turley, S.J., et al., Transport of peptide-MHC class II complexes in
developing
dendritic cells. Science, 2000. 288(5465): p. 522-7.
10. Steinman, R.M., et al., The induction of tolerance by dendritic cells that
have
captured apoptotic cells. J Exp Med, 2000. 191(3): p. 411-6.
11. Yang, J., et al., LFI S-0195 generates tolerogenic dendritic cells by
suppression of
NF kappaB signaling through inhibition of IKK activity. 3 Leukoc Biol, 2003.
74(3): p. 438-47.
12. Martin, E., et al., Antigen-specific suppression of a primed immune
response by
dendritic cells mediated by regulatory T cells secreting interleukin-10.
Immunity,
2003. 18(1): p. 155-67.
13. Ribas, A., et al., Cancer immunotherapy using gene-modified dendritic
cells. Curr
Gene Ther, 2002. 2(1): p. 57-78.
14. Svane, LM., et al., Clinical application of dendritic cells in cancer
vaccination
therapy. Apmis, 2003. 111(7-8): p. 818-34.
15. Matsumoto, M., et al., Subcellular localization of toll-like receptor 3 in
human
dendritic cells. J Immunol, 2003. 171(6): p. 3154-62.
16. Krieg, A.M., CpG motifs in bacterial DNA and their immune effects. Annu
Rev
Immunol, 2002. 20: p. 709-60.
17. Stanley, M.A., Imiquimod and the imidazoquinolones: mechanism of action
and
therapeutic potential. Clin Exp Dermatol, 2002. 27(7): p. 571-7.
18. Oyama, T., et al., Vascular endothelial growth factor affects dendritic
cell
maturation through the inhibition of nuclear factor-kappa B activation in
hemopoietic progenitor cells. J Immunol, 1998. 160(3): p. 1224-32.
19. Pirtskhalaishvili, G., et al., Transduction of dendritic cells with Bcl xL
increases
their resistance to prostate cancer-induced apoptosis and antitumor effect in
mice. J Immunol, 2000. 165(4): p. 1956-64.
20. Nair, S. and D. Boczkowski, RNA-transfected dendritic cells. Expert Rev
Vaccines, 2002. 1(4): p. 507-13.
21. Satoh, Y., et al., Local administration of IL-12-transfected dendritic
cells induces
antitumor immune responses to colon adenocarcinoma in the liver in mice. J Exp
Ther Oncol, 2002. 2(6): p. 337-49.
22. Liu, Y., et al., Dendritic cells engineered to express the Flt3 ligand
stimulate type
I immune response, and induce enhanced cytoxic T and natural killer cell
cytotoxicities and antitumor immunity. J Gene Med, 2003. 5(8): p. 668-80.
CA 02449186 2003-12-05
23. Botella, R., et al., Inhibition of marine melanoma growth by granulocyte-
macrophage colony stimulating factor gene transfection is not haplotype
specific.
Melanoma Res, 1998. 8(3): p. 245-54.
24. Yang, A.S. and E.C. Lattime, Tumor-induced interleukin 10 suppresses the
ability
of splenic dendritic cells to stimulate CD4 and CD8 T cell responses. Cancer
Res,
2003. 63(9): p. 2150-7.
25. Takahashi, A., et al., Correlation of vascular endothelial growth factor-C
expression with tumor-infiltrating dendritic cells in gastric cancer.
Oncology,
2002. 62(2): p. 121-7.
26. Ichim, T.E., R. Zhong, and W.P. Min, Prevention of allograft rejection by
in vitro
generated tolerogenic dendritic cells. Transpl Immunol, 2003. 11(3-4): p. 295-
306.
27. Mahnke, K., et al., Immature, but not inactive: the tolerogenic function
of
immature dendritic cells. Immunol Cell Biol, 2002. 80(5): p. 477-83.
28. Lutz, M.B., et al., Immature dendritic cells generated with low doses of
GM CSF
in the absence of IL-4 are maturation resistant and prolong allograft survival
in
vivo. Eur J Immunol, 2000. 30(7): p. 1813-22.
29. Muller, G., et al., Interleukin-10-treated dendritic cells modulate immune
responses of naive and sensitized T cells in vivo. J Invest Dermatol, 2002.
119(4):
p. 836-41.
30. Strobl, H. and W. Knapp, TGF betal regulation of dendritic cells. Microbes
Infect, 1999. 1(15): p. 1283-90.
31. Sato, K., et al., Regulatory dendritic cells protect mice from marine
acute graft-
versus-host disease and leukemia relapse. Immunity, 2003. 18(3): p. 367-79.
32. Min, W.P., et al., Dendritic cells genetically engineered to express Fas
ligand
induce donor-specific hyporesponsiveness and prolong allograft survival. J
Immunol, 2000. 164(1): p. 161-7.
33. Kim, S.H., et al., Effective treatment of established marine collagen-
induced
arthritis by systemic administration of dendritic cells genetically modified
to
express IL-4. J Immunol, 2001. 166(5): p. 3499-505.
34. Takayama, T., H. Tahara, and A.W. Thomson, Transduction of dendritic cell
progenitors with a retroviral vector encoding viral interleukin-10 and
enhanced
green fluorescent protein allows purification of potentially tolerogenic
antigen-
presenting cells. Transplantation, 1999. 68(12): p. 1903-9.
35. Lee, W.C., et al., Enhancement of dendritic cell tolerogenicity by genetic
modification using adenoviral vectors encoding cDNA for TGF beta 1. Transplant
Proc, 1999. 31(1-2): p. 1195.
36. Wang, L., et al., Progress in the delivery of therapeutic
oligonucleotides:
organlcellular distribution and targeted delivery of oligonucleotides in vivo.
Antisense Nucleic Acid Drug Dev, 2003. 13(3): p. 169-89.
37. Heasman, J., Morpholino oligos: making sense of antisense? Dev Biol, 2002.
243(2): p. 209-14.
38. Corey, D.R. and J.M. Abrams, Morpholino antisense oligonucleotides: tools
for
investigating vertebrate development. Genome Biol, 2001. 2(5): p.
REVIEWS1015.
CA 02449186 2003-12-05
26
39. Gewirtz, A.T. and S. Sitaraman, Alicaforsen. Isis Pharmaceuticals. Curr
Opin
Investig Drugs, 2001. 2(10): p. 1401-6.
40. Lefebvre d'Hellencourt, C., L. Diaw, and M. Guenounou, Immunomodulation by
cytokine antisense oligonucleotides. Eur Cytokine Netw, 1995. 6(1): p. 7-19.
41. Qu, X., et al., Selective inhibition of IL-2 gene expression by IL-2
antisense
oligonucleotides blocks heart allograft rejection. Transplantation, 2001.
72(5): p.
915-23.
42. Furusako, S., et al., Protection of mice from LPS-induced shock by CD14
antisense oligonucleotide. Acta Med Okayama, 2001. SS(2): p. 105-15.
43. Toda, K., et al., Antisense intercellular adhesion molecule-1 (ICAM I)
oligodeoxyribonucleotide delivered during organ preservation inhibits
posttransplant ICAM I expression and reduces primary lung isograft failure.
Circ
Res, 2000. 86(2): p. 166-74.
44. along, C., M. Morse, and S.K. Nair, Induction of primary, human antigen-
specific
cytotoxic T lymphocytes in vitro using dendritic cells pulsed with peptides. J
Immunother, 1998. 21(1): p. 32-40.
45. Zhao, Y., et al., Inhibition of invariant chain expression in dendritic
cells
presenting endogenous antigens stimulates CD4+ T cell responses and tumor
immunity. Blood, 2003.
46. Igietseme, J.U., et al., Suppression of endogenous IL-10 gene expression
in
dendritic cells enhances antigen presentation for specific Thl induction:
potential
for cellular vaccine development. J Immunol, 2000. 1.64(8): p. 4212-9.
47. Liang, X., et al., Administration of dendritic cells transduced with
antisense
oligodeoxyribonucleotides targeting CD80 or CD86 prolongs allograft survival.
Transplantation, 2003. 76(4): p. 721-9.
48. Gorczynski, R.M., et al., Regulation of gene expression of murine MD-1
regulates
subsequent T cell activation and cytokine production. J Immunol, 2000. 16S(4):
p.
1925-32.
49. Chapoval, A.L, et al., B7-H3: a costimulatory molecule for T cell
activation and
IFN gamma production. Nat Immunol, 2001. 2(3): p. 269-74.
50. Merdan, T., et al., Prospects for cationic polymers in gene and
oligonucleotide
therapy against cancer. Adv Drug Deliv Rev, 2002. S4(5): p. 715.
51. Gewirtz, A.M., Oligonucleotide therapeutics: clothing the emperor. Curr
Opin
Mol Ther, 1999. 1(3): p. 297-306.
52. Yacyshyn, B.R., et al., Double blind, placebo controlled trial of the
remission
inducing and steroid sparing properties of an ICAM I antisense
oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent
Crohn's disease. Gut, 2002. Sl(1): p. 30-6.
53. Jorgensen, R.A., et al., Chalcone synthase cosuppression phenotypes in
petunia
flowers: comparison of sense vs. antisense constructs and single-copy vs.
complex
T DNA sequences. Plant Mol Biol, 1996. 31(5): p. 957-73.
54. Fire, A., et al., Potent and specific genetic interference by double-
stranded RNA
in Caenorhabditis elegans. Nature, 1998. 391(6669): p. 806-11.
55. Proud, C.G., PKR: a hew name and new roles. Trends Biochem Sci, 1995.
20(6):
p. 241-6.
CA 02449186 2003-12-05
27
56. Elbashir, S.M., et al., Duplexes of 21-nucleotide RNAs mediate RNA
interference
in cultured mammalian cells. Nature, 2001. 411(6836): p. 494-8.
57. Novina, C.D., et al., siRNA-directed inhibition of HIY~I infection. Nat
Med, 2002.
8(7): p. 681-6.
58. Jiang, M. and J. Milner, Selective silencing of viral gene expression in
HPT
positive human cervical carcinoma cells treated with siRNA, a primer of RNA
interference. Oncogene, 2002. 21(39): p. 6041-8.
59. Wilda, M., et al., Killing of leukemic cells with a BCRlABL fusion gene by
RNA
interference (RNAi). Oncogene, 2002. 21(37): p. 5716-24.
60. McCaffrey, A.P., et al., RNA interference in adult mice. Nature, 2002.
418(6893):
p. 38-9.
61. Bertrand, J., et al., Comparison of antisense oligonucleotides and siRNAs
in cell
culture and in vivo. Biochem Biophys Res Common, 2002. 296(4): p. 1000.
62. Celotto, A.M. and B.R. Graveley, Exon-specific RNAi: a tool for dissecting
the
functional relevance of alternative splicing. Rna, 2002. 8(6): p. 718-24.
63. Grishok, A., H. Tabara, and C.C. Mello, Genetic requirements for
inheritance of
RNAi in C. elegans. Science, 2000. 287(5462): p. 2494-7.
64. Brummelkamp, T.R., R. Bernards, and R. Agami, A system for stable
expression
of short interfering RNAs in mammalian cells. Science, 2002. 296(5567): p. 550-
3.
65. Paul, C.P., et al., Effective expression of small interfering RNA in human
cells.
Nat Biotechnol, 2002. 20(5): p. 505-8.
56. Devroe, E. and P.A. Silver, Retrovirus-delivered siRNA. BMC Biotechnol,
2002.
2(1): p. 15.
67. Yang, D., et al., Short RNA duplexes produced by hydrolysis with
Escherichia coli
RNase III mediate effective RNA interference in mammalian cells. Proc Natl
Acad
Sci U S A, 2002. 99(15): p. 9942-7.