Note: Descriptions are shown in the official language in which they were submitted.
CA 02449467 2003-11-14
METHOD FOR THIOSiTLFATE LEACHING OF
PRECIOUS 1METAL-CONTAINING MATERIALS
FIELD OF THE INVENTION
The present invention is directed generally to the recovery of precious metals
from precious metal-containing material and specifically to the recovery of
precious
metals from precious metal-containing material using thiosulfate lixiviants.
BACKGROUND OF THE INVENTION
A traditional technique for recovering precious metal(s) from precious metal-
containing ore is by leaching the material with a cyanide lixiviant. As used
herein, a
"precious metat" refers to gold, silver, and the platinum group metals (e.g.,
platinum,
palladium, ruthenium, rhodium, osmium, and iridium). Wny countries are placing
severe
limitations on the use of cyanide due to the deleterious effects of cyanide on
the
environment. Incidents of fish and other wildlife having been killed hy the
leakage of
cyaavide into waterways have been reported. The limitations being placed on
cyanide use
have increased substantially the cost of extracting precious metal(s) from
ore, thereby
decreasing precious metal reserves in many countries. Cyanide is also unable
to recover
precious mtals such as gold from refractory ores without a pretreatrnent step.
"Refractory ores" refer to those ores that do not respond well to conventional
cyanide
r.... .
. . .. . . . . ~~a
CA 02449467 2003-11-14
leaching. Examples of refractory ores include sulfidic ores (where at least
some of the
precious metals are locked up in the sulfide matrix), carrbonaceous ores
(where the
precious metal complex dissoived in the lixiviant adsorbs onto carbonaceous
matter in the
ores), and ores that are both sulfidic and carbonaceous.
Thiosulfate has been actively considered as a repiacement for cyanide.
Thiosulfate is relatively inexpensive and is far less hatTnful t.o the
environment than
cyanide. Thiosulfate has also been shown to be effective in recovering
precious metals
from pretreated refractory preg-robbing carbonaceous ores and sulfidic ores.
As used
herein, "preg-robbing" is any material that interacts with (e.g., adsorbs or
binds) precious
metals after dissolution by a lixiviant, thereby interfering with precious
metal extraction,
and "carbonaceous material" is any material that includes one or more carbon-
containing
compounds, such as, but not limited to, humic acid, graphite, bitumins and
asphaltic
compounds.
Where gold is the precious metal, thiosulfate leaching techniques have
typically
relied on the use of copper ions to catalyze and accelerate the oxidation of
gold, ammonia
to facilitate the formation and stabilization of cupric ammine ions and/or a
pH at pH 9 or
above to maintain a region of stability where both the cupric ammine and gold
thiosulfate
complexes are stable.
It is well known in the art that the catalytic effect of copper and annmonia
in
conventional thiosulfate leaching of gold is described by the following
sequence of
reactions.
-2 -
~--, .
CA 02449467 2003-11-14
Formation of the cupric amrnine complex:
Cu'' +4NH, -~ Cu(NH,),' {~)
Oxidation of gold by cupric ammine, gold complexation as the gold-thiosulfate
anion,
and reduction of cupric ammine to cuprous thiosulfate:
A u + Cu(NH3)4+ + 5S203 --~ Au(S2C3)2 + Cu(S2U3)3 + 4NH3 (2)
Oxidation of the cuprous thiosulfate back to cupric ammine with oxygen:
Cu(S201)3 +4NH3+Y4 02+A H20 -4 Cu(Nfi'3)4++35203 +OH- {3)
Summing equations (2) and (3) yields the overall thiosulfate leach reaction
for gold:
.Au + 2S2 03 - + y 02 + H2U -~ Au(S2 0s ) ~ + UH (4)
It can be seen from the above equations that copper and ammonia act as
catalysts
in that they are neither produced nor consumed in the overall leach reaction.
Copper and ammonia can be a source of problems. Rapid oxidation of thiosuifate
by cupric ammine to form polythionates occurs,leading to excessive degradation
and Ioss
of thiosulfate:
2Cr,,<NH)~+ +8S2q --* 2Cu(S203)3 +S4~ +8NH (5)
Oxidative degradation of thiosulfates by molecular oxygen to polythionates and
sulfates
is accelerated markedly in the presence of copper ions and/or mnmonia.
Molecular
oxygen conversion to thiosulfates is believed to occur according to sequence
of reactions
that involve the formation of intermediate polytluonates (polythionates can be
2-
represented by 'sn O6 , where n= 2-6):
-3 -
~.,..
CA 02449467 2003-11-14
Tetrathionate formation: 2S203 +/ 02 + H2O -* S406 + 20H- (6)
Trithionate formation: 3S406 + x 02 + H20 4 4S306 ~+ 2H+ (7)
Sulfite formation: S306 + j/ 02 + 2H2 0 -+ 3S03 + 4H+ (g)
Sulfate formation: 2S03 + 02 -~ 2SO4 - (9)
Overall: S2 03 + 202 + H20 -~ 2SO4 + 2Ht (10)
Not only can the degradation of thiosulfate lead to increased reagent costs
but also it has
been discovered that excessive levels of sulfate can cause decreased gold
recoveries.
While not wishing to be bound by any theory, it is believed that excessive
levels of
sulfates can lead to unacceptable rates of thiosulfate degradation and levels
of instability
in the thiosulfa.te lixiviant. Additionally, ammonia gas can be released into
the
atmosphere when atmospheric leaching is performed. The loss of amrnonia by
volatilization occurs readily, particularly in unsealed gas-sparged reactors
and heaps
operatsng at pH greater than 9.2, leading to excessive amrnonia consumption:
NH4 + OH -a NHJ(ag) + H2O -> NH3cg> + H20 (11)
SUMIYIA.'RY OF THE IlWENTION
These and other needs have been addressed by the methodologies and systems of
the present invention. The methodologies can recover precious metals, such as
gold and
silver, from a variety of matexials, including refractory carbonaceous or
sulfidic ores,
double refractory ores (e.g., ores containing both sulfide-locked gold and
carbonaceous
preg-robbing matter), oxide ores, nonrefractory sulfidic ores, and ores also
containing
-4 -
~.,,...._ ,
_ _ .. ..._._~.
CA 02449467 2003-11-14
copper minerals and other materials derived from such omes (e.g.,
concentrates, tailings,
etc.).
In one embodiment, a process for recovering a precious metal from a preccious
metal-containing material, includes the steps of:
(a) providing a heap of the preci.ous metal-containing material; and
(b) passing a thiosulfate lixiviant and molecular oxygen through the heap to
form a pregnant leach solution comprising dissolved prex:ious metals. As used
herein, a
"hea.p" refers to any self-supporting body of particulate material, including,
without
litnitation, a particulate-containing heap, vat, and dump. The molecular
oxygen is at a
pressure greater than ambient atmospheric pressure before introduction into
the heap.
Preferably, the dissolved molecular oxygen content of the lixiviant ranges
from about 1 to
about 50 mg/L, and more preferably from about 3 to about 40 mg/L. Molecular
oxygen
can avoid the need for high Ievels of copper and ammonia in the lixiviant as
catalysts
without compromising precious metal recoveries. Preferably, the lixiviant
comprises no
more than about 20 mg/L dissolved copper.
The thiosulfate lixiviant can be derived from anv suitable form(s) of
thiosuifate,
such as sodium thiosulfate, calcium thiosulfate, potassium thiosulfate and/or
ammonium
thiosulfate.
The precious metal can be recovered from the pregnant leach solution by any
suitable technique. By way of example, the precious metal can be recovered by
resin
adsorbtion methods such as resin-in-pulp, resin-in solution, and resin-in-
leach or by
solvent extraction, cementation, electrolysis, precipitation, and/or
combinations of two or
more of these techniques.
-5 -
r._..
.., ti. _... ~... .,,~
~
CA 02449467 2003-11-14
As will be appreciated, heap leaching can typically be perfortned at lower
capital
and operating costs than tank leaching and can yield similar precious metal
recoveries.
Recoveries of precious metals by both processes can. be at least about 70% and
sometimes at least about 800/o, without the need for high levels of copper in
the
thiosulfate lixiviant. Surprisingly, when ammoniurn thiosulfate is used the
presence of
ammonium in the thiosulfate lixiviant does not necessarily cause the release
of significant
arnounts of ammonia gas, notwithstanding the countercurrent circulation of a
molecular
oxygen-containing gas through the heap. This is so because the pH of the
lixiviant is
preferably maintained at a pH of no more than about pH 9. :Cn this manner, the
free
ammonia content of the lixiviant can be maintained at no more than about 2,000
ppm.
Reducing or eliminating the need to have copper ions and/or ammonia present in
the leach by effective use of molecular oxygen as the oxidant can provide
significant
multiple benefits. First, the cost of having to add coppe,~r and ammonia
reagents to the
process can be reduced significantly or eliminated. Second, environmental
concerns
relating to the presence of potentially harmful amounts of copper and ammonia
in the
tailings or other waste streams generated by the process can be xnitigated.
Third, the near-
absence or complete absence of copper and ammonia in the leach can provide for
a much
more reliable and robust leaching process, yielding more stable leachates,
able to operate
over a wider pH and oxidation-reduction potential (ORP) range than is possible
with
conventional thiosulfate leaching. The latter process must operate in the
relatively
narrow window of pH and ORP where both the cupric ammine complex and the gold
thiosulfate complex co-exist. Finally, the near-absence or complete absence of
copper
-6 -
~- ,
,
CA 02449467 2003-11-14
and ammonia in the leach can reduce or eli.rninate entirely a host of
deleterious side
reactions that consume thiosulfate and are otherwise difficult or impossible
to prevent.
Preferably, the thiosulfate lixiviant is at least substantially free of
sulfite during
the leaching step. The elimination or near elimination of sulfrte from the
thiosulfate
S leach can have advantages. Sulfite can depress the rate of dissolution of
precious metal
from the precious metal-containing material by reducing significantly the
oxidation
reduction potential (ORP) of the leach solution or lixiviant. As will be
appreciated, the
rate of oxidation of the gold (and therefore the rate of dissolution of the
gold) is directly
dependent on the OR.P.
In yet another embodiment, a process for recovering a precious metal from a
carbonaceous preeious metal-containing material is provided that includes the
steps of:
(a) contacting a thiosulfate lixiviant with a precious metal-containing
material to
form a pregnant leach solution, the pregnant leach solution comprising a
dissolved
precious metal, thiosulfate, polythionate, and sulfate; and
(b) maintaining a dissolved sulfa.te concentration in the pregnant leach
solution of
no more than about 100 g/L.
Sulfates are commonly in the lixiviant due to the degradation of thiosulfate.
The
pre.sence of sulfate has been found to decrease precious metal recoveries,
which is
believed to be due to the increased instability of thiosulfate in the presence
of sulfate.
Higher levels of sulfates are believed to cause a more rapid rate of
degradation of
thiosulfate into polythionates and, ultimately, sulfate. As will be
appreciated, sulfate
removal can be effected by numerous techniques, including precipitation,
membrane
filtration, solvent extraction, and ion exchange.
-7 -
r-- ,
ii
CA 02449467 2003-11-14
In a preferred process configuration, the dissolved sulfate is precipitated
using
calcium. The calcium is typically introduced into the lixiviant as calciurn
carbonate,
calcium chloride, calcium nitrate, calcium oxide, calcium thiosulfate, calcium
hydroxide,
and mixtures thereof.
In yet another embodiment, the pregnant leach solution from a thiosulfate
leaching step is contacted, after the leaching step, with a reagent to convert
at least about
50% and typically at least most of polythionates (particularly trithionate and
tetrathionate) into thiosulfate and elemental sulfur and precipitate dissolved
precious
metals (and dissolved transition metals) followed by conversion of the
elemental sulfur
into thiosulfate. The reagent or reductant can be an,y suitable reactant to
convert
polythionates into thiosulfate, with any sulfide, and/or polysulfide (i.e., a
compound
containing one or a mixture of polymeric ion(s) SX2-, where x= 2-6, such as
disulfide,
trisulfide, tetrasulfide, pentasulfide and hexasulfide) being particularly
preferred. A
sulfite reagent can also be used for thiosulfa.te regeneration but is
generally effective only
in converting polythionates of the forni S,06 ', where x= 4 to 6, to
thiosulfa.te.
The elemental sulfur is converted into thiosulfate by contacting the product
of the
sulfide precipitation step with a sulfite reagent. The sulf te reagent can be
any form of
sulfite, with a bisulfite being preferred. The conversion of the elemental
sulfur into
thiosulfate can lead to lower thiosulfate reagent costs carnpared to a process
in which the
elemental sulfar is discarded and can control effectively the form and amount
of sulfur at
differing locations in the process.
-8 -
r---=. .
....__ ..._ ,. ...~.,~ ~ ~ . .. .. ~ . m~~~,.~ . ~~
. ,~
CA 02449467 2003-11-14
The sulfide, bisulfide, and/or polysulfide can be compounded with any cation,
with Group IA and IIA elements of the Periodic Table, anamonium, and hydrogen
being
preferred.
In yet another embodiment, a process for recovering a precious metal from a
carbonaceous precious metal-containing material is provided in which a
carbonaceous
precious metal-containing material is contacted with a thiosulfate-containing
lixiviant.
The lixiviant contains a blinding agent. While not wishing to be bound by any
theory, it
is believed that the precious metal thiosulfa.te oomplex may be unstable under
certain
conditions and that the precious metal can be stripped iiom the thiosulfate-
containing
solution by a number of substances commonly encountered in precious metal-
containing
materials. The substances or preg robbing materials typically absorb, adsorb
or
precipitate the precious metal. Such preg-robbing materials include
carbonaceous
materials, pyrite-containing materials, chacopyrite and iron oxides.
Surprisingly and
unexpectedly, blinding agents may be used in the thiosulfate lixiviant to
prevent or inhibit
preg robbing of the precious metal by the preg robbing material. The blinding
agent itself
absorbs or adsorbs (in preference to the precious metal) or otherwise
neutralizes (such as
by chemical reaction) the preg robbing sites on the material. The blinding
agent
preferably includes one or more of hydrocarbons, alcohols, estera, aldehydes,
surfactants,
lauryl sulfonates, phosphates, and metal salts.
BRIEF DESCRIPTION OF THE DFiAWINGS
Fig. 1 is a flow schematic of a first embodiment of the present invention;
Fig. 2 is a flow schematic of second embodiment of the present invention;
-9 -
CA 02449467 2003-11-14
Fig. 3 is a plot of gold extraction in percent (vertical axis) against time
(horizontal
axis) with unagglomerated and agglomerated precious metal-containing ore; and
Fig. 4 is a plot of gold extraction in percent (vertical axis) against applied
solution
amount (horizontal axis) with and without heap aeration.
DETAILED DESCRIPTIO14
The present invention provides an improved thiosulfate leaching process for
the
recovery of precious metals from precious metal-bearing material. The precious
metal(s)
can be associated with nonprecious metals, such as base xnetals, e.g., copper,
nickel, and
cobalt. The precious metal-bearing material includes ore, concentrates,
tailings, recycled
industrial matter, spoil, or waste and mixtures thereof. The invention is
particularly
effective for recovering precious metals, particularly gold, from refractory
carbonaceous
material.
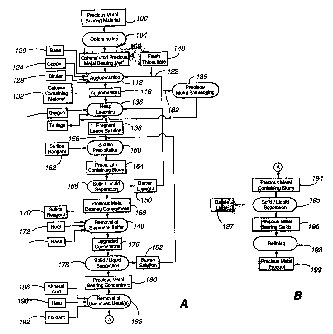
FigareslA and B are collectively a flow chart according to a first embodiment
of
the present invention. The process of the flow chart is particularly effective
in recovering
gold from sulfidic and carbonaceous material and oxide material and mixtures
thereof.
Referring to Figure lA, a precious metal-bearing material 100 is comminuted
104, such as by wet and/or dry erushing and optionally wet and/or dry
grinding, to form a
comminuted precious metal-bearing materiai 108. Comminution 104 typically
reduces
the particle size of the material 100 sufficiently to libeTate the gold-
bearing rninerals.
Typically, the comminuted precious metal-bearing material 108 is comminuted to
a P80
size of from about 2 inches to about 1/4 inch.
-10 -
r-......
;~ .
CA 02449467 2003-11-14
To provide desired levels of heap porosity and penneability, the comminuted
precious metal bearing material 108 is agglornerated 112 by known techniques
to form
agglomerate.s 116. Clne or more of a base 120, the thiosulfate lixiviant 122,
copper 124, a
binder 128, and a calcium-containing material 132 may be contacted with the
comminuted material 108 immediately before or during agglomeration 112 to
assist in
agglomerate formation and "jump start" the leaching process of step 136. In
other words,
the base 120, thiosulfate lixiviant 122, copper 124, and b:inder 128 are
incorporated into
the aggiomerate matrix.
The base 120 can be any suitable base material effective to adjust the pH of
the
thiosulfate lixiviant to desired levels. Preferably, the pH of the
tlziosulfate lixiviant is no
more than about pH 10, more preferably no more than about pH 9, and even more
preferably ranges from about pH 8 to about 9. Preferred bases include alkali
or alkaline
earth metal oxides, carbonates, hydroxides, cement, amm.onia, and mixtures
thereof. To
realize these operating pHs, the amount of base (lime) incorporated into the
agglomeratess
of a typical ore ranges from about 0.1 to about 10 kg/tonne of comminuted
precious
metal bearing material 108. The base 120 is typically introduced in powdered
form to
the comminuted precious metal-bearing material 108 during agglomeration 112.
The copper 124, which is optional, can be in any suitable form that is soluble
in
the thiosulfate lixiviant. Preferably, the copper 124, when added, is the form
of a copper
sulfate, copper oxide, copper nitrate, copper chloride, and mixtures thereof.
Sufficient
copper may be added to catalyze the leaching reaction when necessary to
realize desired
rates of preclous metal recavery. When added, the preferred mass ratio of the
copper ion
to thiosulfate ion is preferably from about 1:10 to about 1:1000. Typically,
the copper is
-11 -
~.. .
CA 02449467 2003-11-14
added in the form of copper sulfate in an amount ranging from about 1 to about
100
g/tonne of comminuted precious metal-bearing material 108. Preferably, no
copper is
added but rather oxidation is effected by raising the lixiviant's dissolved
molecular
oxygen content above naturally occurring levels.
The binder 128, which is also optional, can be any suitable binder capable of
producing robust agglomerates. Possible binders incl-ude a commercially
available
cohesivity agent such as NALCO 9704, cement, lime, and other long chain
polymers,
water, and mixtures theTeof. The preferred binder 128 is a cohesivity agent,
which along
with the tlliosulfate lixiviant 122, can provide highly robust agglomerates.
The amount
of binder 128 employed typically ranges from about 0.1 to about 10 kg/tonne of
comminuted precious metal-bearing material 108. The binder 128 is typically
added to
the comminuted precious metal-bearing material as a free flowing particulate
or a liquid
before or during agglomeration.
The calcium-containing material 132 controls the concentration of sulfates in
the
various process solutions described below. The calcium-containing material 132
is in a
form that is soluble in the thiosulfate lixiviant so as to provide calcium
ions to react with
sulfate ions to form and precipitate gypsum (CaSOa). Because the gypsum
precipitates in
the heap removal of gypsum by filtration or other means is not required.
Preferred
calcium-containing materials include lime (Ca0), calcium carbonates, calcium
nitra.tes,
calcium chlorides, calccium hydroxides, calcium thiosulfate, and mixtures
thereof, with
lime being particularly preferred. Lime is able to perform not only sulfate
control but
also pH control, thereby potentially rendering the base 120 unnecessary. The
arnount of
calcium-containing material is preferably sufficient to rnaintain a maximum
dissolved
-12 -
r---.. ,
,
CA 02449467 2003-11-14
sulfate ion concentration in the pregnant leach solution 138 of about 150 g/L,
more
prefera.bly of about 100 g/L, and even more preferably oiF about 50 g/L, and
even more
preferably of about 30 g/L. The amount of calcium-containing material
therefore
depends on the rate of degea.dation of the thiosulfate into sulfate between
cycles of
thiosulfate regeneration in steps 140 and 144 (discussed below). This can be
quantified
by measuring the current sulfate concentration at one or more selected points
in the
process and adding sufficient calcium to reduce the concentration to desired
levels.
Typically, the amount of calcium is at least about 0.1 kg, more typically at
least about 1
kg, and even more typically ranges from about 0.1 to about 5 kg/tonne of
comminuted
precious metal bearing material 108. The calcium-containing material 132 is
typically
added to the comminuted precious metal bearing material 108 as a free flowing
particulate material or slurry or liquid before or during agglomeration. As
will be
appreciated, the amount of calcium includes native or natuirally occurring
calcium already
present in the material 108.
As will be appreciated, metals other than calcium may be used to effect
sulfate
precipitation. Examples of other metals include lead and barium. These other
metals are
generally not preferred for purposes of cost and environmental considerations.
In a preferred process configuration, the calcium-containing material 132 is a
mixture of calcium compounds, with a mixture of lime and calcium carbonate
being
particularly preferred.
Finally, the thiosulfate lixiviant 122 is contacted with the comminuted
precious
metal bearing materia1108 before or during agglomeration 112. The thiosulfate
lixiviant
122 is made by rechargiag the conditioned recycle solution 144 (discussed
below) with
-13
r--- ,
CA 02449467 2003-11-14
fresh thiosulfate 148. As discussed below, the conditioned recycle solution
144 is the
product of conditioning the recycled barren lixiviant 150. The optimum
solution
thiosulfate concentration to maintain during heap leaching 136 and therefore
the optimum
solution thiosulfate concentration in the thiosulfate lixiviant 122 will
depend on the
nature of the material being leached, but will preferably range from about
0.005 to about
2 molar (M), more preferably about 0.02 to about 0.5 M, iand even more
preferably from
about 0.05 to about 0.2 M. T'he source of the fresh thiosulfate 148 can be any
available
thiosulfate-containing compound, such as sodiutn thiosulfa.te, potassium
thiosulfate,
calcium thiosulfate, ammonium thiosulfate, or any other thiosulfate-containing
material
or thiosulfate precursor. Alternatively, thiosulfate can be generated in situ
or in a
separate step by reaction of elemental sulfur with a source of hydroxyl ions,
in
accordance with the following reaction:
2(x + 1)S + 60H- -+ S203 + 2S2
x + 3H2O (12)
where x= 3-6, or by rea.ction of bisulfide with bisulfite:
2HS- + 4HS03 -* 3S203 + 3H2O (13)
or by reaction of elemental sulfur with sulfite:
S + S03 _ -* S203 _
(14)
As will be appreciated, to produce a stractured agglomerate cement (not shown)
may be added during agglomeration. The cement is added in particulate form
before or
during agglomeration and is thereby incorpora.ted into the agglomerate. When
used, the
amount of cement typically ranges frorn about 1 to about 50 kg/tonne of
comminuted
precious metal-bearing material 108.
-14 -
~
õ,
i
CA 02449467 2003-11-14
The size of the agglomerates 116 depends, of course, on the heap desagn.
Typically, it is preferred that the P8Q size of the particlesJagglomerates
formed into the
heap is at least about 150 m, more preferably at least about 500 m, and even
more
preferably at least about 1,000 m.
As an alternative to agglomeration, it is possible to provide desired levels
of heap
porosity and permeability by comminuting the precious metal-containing
material to a
desired size range. In that event, the base 120, copper 124, and calcium
conditioning
material 132 is incorporated into the heap during heap constniction (or
stacking). In
other words, these materials may be contacted with the comminuted precious
metal-
bearing material 108 on the stacking conveyor belts or in, the haulage
compartment of a
haulage vehicle which will dump the material 108 onto the heap pad. The
various
rnaterials may be located uniformly throughout the heap or in a zone of the
heap, such as
at the bottom, middle or top. When agglomeration is not performed, the
comminuted
material 108 has a preferred P84 size of at least about 150 pm, more
preferably at least
about 500 m, and even mre preferably at least about 1,000 m. This size range
is
realized by removing fine particles (particles preferably having a size of
less than about
150 m and more preferably of less than about 500 m) from the comminuted
material
108, by suitable screens, filters, and the like, prior to materia.l placement
on the leach pad.
Thiosulfate lixiviant can be contacted with the particles of material as the
particles are
being placed on the heap.
In step 136, the agglomerates 116 are leached to form a pregnant leach
solution
138 containing dissolved precious metals solubilized from the precious metal-
bearing
material 108. The extraction of precious metals in the leaching step 136 is
relatively
-15 -
aCA 02449467 2003-11-14
high, particularly for carbonaceous ores. Typically, at least about 50%, rnore
typically at
least about 70%, and even more typically at least about 800/o of the precious
metal in the
precfous metal-containing material 108 is extracted or solubilized into the
pregnant
solution 138. The concentra.tion of the dissoived precious metal in the
pregnant solution
138 typically ranges from about 0.05 to about 100 ppm and more typically from
about 0.1
to about 20 ppm.
Before leaching can commence, the heap must be formed on a leach pad. The pad
typically includes a liquid impervious liner, which is placed beneath the
heap, to collect
the pregnant leach solution 138 and prevent the pregnant leach solution 138
from being
lost to the surrounding environment. The height of each iift of the heap is
typically from
about 4 to about 8 m and of the heap itself can be up ta 100 m. Rather than
moving the
heap after thiosulfate leaching is completed (which is done in a dynamic heap
configuration), further heaps or lifts can be built on top of exhausted
heap(s) (which is
done in a static heap configuration).
During heap constnzetion, a network of aerating pipes can be located in a
lower
portion of the heap to force an oxygen-containing gas 154 through the heap
during
thiosulfate leaching. The pipes can be perforated so as to provide a
substantially uniform
dispersion of the gas throughout the heap. The oxygen-containing gas 154 is
typically
forced through the pipes using a single- or multi-stage compressor, blower,
fan, or other
mechanical device. When the oxygen-containing gas 154 is pressurized and
foraed
through the pipes, it typically has a pressure greater than the ambient
atmospheric
pressure, more typically of at least about 1 inch H20, and even more typically
of at least
about 30 inches H20 greater tha:n the ambient atmospheric pressure.
Preferably, at least a
-16 -
,--- - ,
CA 02449467 2003-11-14
stoichiometric amount of molecular oxygen (relative to the amount of precious
metal in
the precious metal-containing material) is deliberately introduced into the
heap during
leaching 136. More preferably, at least about 0.5 kg of molecular oxygen and
even rnore
preferably from about 1 to about 10 kg of molecular oxygen is introduced into
the heap
during leaching for each ton of material to be leached in the heap.
Preferably, at least
about 2 and more preferably from about 4 to about 40 m3 of oxygen-containing
gas is
introduced into the heap for each cubic meter of lixiviant applied to the
heap.
Using gold as an example, the thiosulfate leaching of precious metal-bearing
material in the presence of molecular oxygen can be illustrated by the
following reaction:
Au+ 2S203 + 7' 2 + XH2 --> Au(S2 3)2- + H- (15)
The oxygen-containing gas may include atmospheric air, or it may include
relatively pure
(95%+) oxygen such as that produced from any commercially available oxygen
plant, or
it may include any other available source of oxygen.
To control evolution of ammonia gas during forced air introduction into the
heap,
the pH of the thiosulfate lixiviant 122 and recirculated pregnant leach
solution 138 are
controlled. Preferably, the pH of the thiosulfate lixiviant and solution 138
are maintained
(when introduced into the heap and during passage through the heap) at a pH of
no more
than about pH 9, more preferably of no more than about pH 8.75, and even more
preferably of from about pH 6.5 to about pH 8.75. Alternatively, the
concentration of
free ammonia can be maintained below levels sufficient to result in evolution
of
significant amounts of ammonia gas. In some configurations, the concentration
of free
amrnonia in the thiosulfate lixiviant applied to the top of the heap is
maintained at a level
of no more than about 2000 ppm, more preferably no more than about 1000 ppm,
and
-17 -
~..
~---
CA 02449467 2003-11-14
even more preferably no more than about 500 ppm. This can be realized, for
example, by
using sodium thiosulfate alone as the lixiviant or using a mixture of ammonium
and
sodium thiosulfate.
The pH can be controlled by using suitable (acid or base) buffering agents to
produce the desired change in pH. In one configuration, carbonaceous
compounds, such
as calcium carbonates, (in addition to the base 120 incorporated in the
agglomerates 116)
are added to the lixiviant 122 and solution 138 before or after application to
the heap
and/or to the heap itself. The carbon component in the buffering agent has
been found
under suitable conditions to perform, at most, only a minimal degree of preg
robbing.
Typical consumption of carbonates in this configuration is in the range of
about 0.5 to
about 101b/ton of material in the heap.
To apply the thiosulfate lixiviant to the heap, a number of techniques can be
employed. For example, spray systems (such as spray nozzles), drip and/or
trickle
systems (such as drip emitters and perforated pipes), injection holes in the
heap, and
irrigation ditches on top of the heap can be used to apply the iixiviant. The
preferred
lixiviant distribution system preferably applies the lixiviant at least
substantially
uniformly throughout the heap. In a preferred configuration, the applied
lixiviant flows
countercurrently through the heap relative to the flow of the oxygen-
containing gas.
Typically, the lixiviant flows from the top to the bottom of the heap while
the gas flows
from the bottom to the top of the heap. In a preferred configuration, at least
about 0.5
and even more preferably from about 1 to about 10t solution /t ore of
lixiviant is applied
to the top of the heap from start-to-finish of heap leaching. In this
configuration the
-18 -
r---. .
.u..,:,.,,_ ..,.,,p,,,, ..s,.,vTi'dck' .'Sv.fiR.... ` ~~ . ."ve':.'i m^."___._
_ . . _. . ... . ._. . . . . . . ..w-...e.....,re...Q.
..,..,.,..:.~..+......... . ...n. ..-e.~,.__~_..~_.
CA 02449467 2003-11-14
lixiviant is applied for at least about 0.5 and even more preferably from
about 1 to 48
rnonths from the start to finish of heal leaching.
In one configuration, the lixiviant is sparged with the oxygen-containing gas
before application to the heap or the gas is otherwise contacted with the
lixiviant before
application to the heap (such as by in-line mixing) to cause the lixiviant to
have a
heightened dissolved molecular oxygen content. Additional gas may be
deliberately
introduced into the heap separately from the lixiviant, if desired. The
dissolved
molecular oxygen content of the lixiviant preferably is at least about 1 mg/L,
more
preferably is at least about 3 mg/L, even more preferably ranges from about 3
to about 40
mg/L, and even mre preferably ranges from about 3 to about 15 mg/L.
In one configuration, the dissolved molecular oxygen content is realized by
adding chemicals, such as a peroxide, that break down to generate molecular
oxygen in
the heap.
After construction of the heap, the thiosulfate lixiviant 122 is applied to
the top of
the heap while the oxygen-containing gas is introduced to the bottom of the
heap. The
pregnant leach solution 138 is collected frorn the base of the heap. A portion
of the
pregnant leach solution 138 is recycled to the top of the heap. The recycle
rate is
sufficient to provide an application ra.te of the lixiviant to the top of the
heap of frorn
about 0.5 and more prefera'bly from about 2 to about 24 LIh/m2 of top surface
area for the
heap. During recycle, at least a portion 156 (typically at least about 5 vol%
and more
typically from about 50 to about 100 vol.%) of the pregnant leach solution 138
is
removed and subjected to further processing to effect precious metal recovery.
At least
-19 -
CA 02449467 2003-11-14
most of the precious metal in the material 108 is solubilized by the lixiviant
and,
therefore, dissolved in the pregnant leach solution 138.
The first processing step 160 is sulfide precipitation of the dissolved
precious
metals using a sulfide reagent 162 to form a precipitate-containing slurry
164. Sulfide
precipitation not only preaipitates the precious metal but also precipitates
transition
metals, such as copper, and regenerates the thiosulfate by converting
polythionates into
thiosulfate. By way of example, a sulfide-containing reagent can reduce the
polythionates
back to thiosulfate, as shown by the following reactions:
2S406 + S2 + 32H2(?-3 ~S203 + 3H+ (16)
5306 + S2+ -4 2S203
(17)
Any sulfide reagent that releases sulfide ions on dissolution will suffice,
such as a sulfide,
bisulfide, or polysulfide. Examples of preferred reagents include ammonium
sulfide,
sodium bisulfide, NaHS, sodium sulfide, Na2S, or hydrogen sulfide gas, H2S.
Sulfide precipitation 160 is typically conducted under anerobic or oxygen-
depleted conditions, as noted above. Such conditions can be realized and
maintained by
de-aerating the pregnant leach solution 138 with a vacuum, inert or oxygen-
deficient gas
bubbling or sparging through the solution 138, maintaining a blanket of a
noble gas in the
atmosphere over the solution 138, and/or allowing the solution 138 to stand
dormant for a
sufficient period of time for the dissolved oxygen level to decrease to
desired levels.
Preferably, the solution 138 contains no more than about 1 ppm dissolved
molecular
oxygen and more preferably less than about 0.2 ppm dissolved molecular oxygen
concentration.
-20 -
r._._
CA 02449467 2003-11-14
In one process configuration, the oxygen-depleted atmosphere is inert. As used
herein, "inert" refers to any gas which is at least substantially free of
oxidants, such as
molecular oxygen, that can cause thiosulfate to be converted into a
polythionate. For
example, an "inert" gas would include a reducing gas. T`ypically, the inert
atmosphere
will include at least about 85 vol % of an innert gas, preferably nitrogen
gas, and no more
than about 5 vol % oxidants, such as oxygen gas, that can cause thiosulfate
conversion
into a polythionate. The molecular nitrogen can be a byproduct of the oxygen
plant that
is employed in the leaching step to provide oxygen gas.
While not wishing to be bound by any theory, it is believed that sparging is
more
effective than an inert atmosphere without sparging in controlling
polythionate and
sulfate production. Sparging appears to inhibit molecular oxygen ingress into
the
soiution, even where the reactor is open to the ambient atmosphere, because of
the
outflow of inert gas from the surface of the solution.
Preferably, sufficient sulfide is added to the pregnant leach solution 138 to
precipitate at least most of the dissolved precious and transition metal(s) as
sulfides and
to convert at least most of the polythionates to thiosulfate, m re preferably
to precipitate
at least about 99% of the precious and transition metals and convert at least
about 90% of
the polythionates to thiosulfate, effectively regenerating the thiosulfate
lixiviant.
Typically, the amount of sulfide reagent contacted with the solution 138 is at
least about
100 to about 150 10 of the stoichiometric amount required to convert at least
substantially
all of the polythionates in the solution 138 into thiosulfates. This amount is
generally
sufficient to precipitate at least most of the precious and transition metals.
Typically, at
-21 -
~--.._.
õ
CA 02449467 2003-11-14
least about 50%, more typically at least most, and even more typically from
about 80 to
about 95% of the polythionates are converted into thiosulfates in step 160.
While not wishing to be bound by any theory, it is believed that the rnost
likely
composition of the precipitate is the metallic precious rnetal and/or a
precious metal
sulfide, such as Au2S. Maximum precipitation of gold and regeneration of
thiosulfate is
accomplished by adding at least a stoichiometric amount of sulfide reagent 162
(relative
to the dissolved precious metal and polythionate concentrations) to reduce the
solution
ORP to at least about 50 mV (SHE). The effectiveness of the conversion causes
significantly less thiosulfate reagent to be consumed during the process than
for
conventional thiosulfate leaching processes.
The pH of the pregnant leach solution 138 is adjusted if necessary to about pH
5.5-12, more preferably about pH 7-11, even more preferably about pH 8-10
using a
suitable basic reagent such as sodium hydroxide before or during contact of
the solution
with the sulfide reagent 162. The temperature of the solution 162 is
preferably
maintained in the range of about 5 to 40 C, and more preferably at ambient
temperature,
about 20 C. The retention tune is about 5 minutes to about 2 hours, more
preferably
about 15 minutes to about 1 hour.
The precious metal precipitation step 160 can be carried out in any suitabiy
agitated reactor or plurality of agitated reactors.
The precipitate-containing slurry 164 is subjected to liquid/solid separation
166 to
separate the precious metal-bearing precipitates or concentrate 168 containing
at least
most of the precious metal(s) in the slurry 164 from the barren lixiviant 150
containing at
least most of the thiosulfate in the slurry 164. The solid/liquid separation
can be effected
-22 -
r---- ,
CA 02449467 2003-11-14
by any suitable method such as filtration, counter current decantation
("CCD"), and the
like. As will be appreciated, CCD performs liquid/solid separation, provides
water
balancing in the circuit, and prevents buiid up of impurities in the leach
circuit by
removing a portion of the leach solution with the solids.
The barren thiosulfate lixiviant 150 can be recombined with the recycled
pregnant
leach solution and returned to the top of the heap. The barren lixiviant 150
will typically
contain no rnore than about 0.01 ppm precious metals or 1% of the precious
metal(s) in
the pregnant leach solution 138.
The concentrate 168, which is typically in the form of a sludge or slurry,
contains
a substantial amount of elemental sulfur along with various precious metal
sulfides and
non-precious metal sulfides (such as copper sulfides, mercury sulfides, and
nickel
sulfides). The elemental sulfur concentration in the concentrate is typically
at least about
50 wt.% and more typically from about 55 wt. / to about 99.9 wt.%. The
concentrate
168 typically further include from about 0:01 to about 10 wt.% precious rnetal
sulfides
and from about 0.01 to about 10 wt.% non-precious metal sulfides.
The elemental sulfur is removed from the precious metal-bearing concentrate
168
in step 140, and the precious metal concentration of the concentrate 168
significantly
upgraded. fihis is performed by contacting the concentrate 168 with a source
of sulfite
under at least substantially non-oxidizing conditions (or in the presence of
an inert gas
atmosphere) to convert the elemental sulfiur into thiosulfate. As shown in the
Figure, the
concentrate 168 is contacted with a sulfite reagent 170, heat 172, and a base
174 in a
suitable reactor.
-23 -
,.~... ~
. . ... . . a?` ::+4?r'wd'.t~'~~1 .. .. ....... . .. . ... .. .. . . ..,.....
..... .' . .. . . . .... .....A..,....,~:....vm.rm.,_~aa.+amrow.+.a.. . . .
.. . . . . . .. . . . re . .xw.~.
() I
CA 02449467 2003-11-14
The sulfite reagent 170 can be any sulfite-containing compound, such as
ammonium bisulfite, sodium sulfite, sodium bisulfite, and potassium bisulfxte,
with a
bisulfite such as ammonium bisulfite being preferred. For ammonium bisulfite,
the
chemical reaction is believed to proceed in accordance with equation 14 above.
The amount of sulfite reagent 170 used in step 140 depends on the elemental
sulfur content of the concentrate 168. Typically, the amount of sulfite
reagent is at least
the stoichiometric amount, and more typically at least about 120% of the
stoichiometric
amount, required to convert the present elemental sulfar to thiosulfate. For
ammonium
bisulfite as the sulfite reagent 170, the amount of reagent used is typically
at least about 2
kg reagent/kg of present elemental sulfur and more typically ranges from about
3 to about
5 kg reagent/kg of present elemental sulfur.
For the reaction to proceed to completion, the pH of the concentrate 168 is
carefully controlled. The preferred pH is at least about pH 6, more preferably
at least
about pH 7, and even more preferably ranges from about pH 7.5 to about pH 10.
Because
bisulfite wili produce an acidic pH when reacted with elemental sulfur, it is
important to
contact the base 174 with the concentrate 168. The base 174 can be any basic
cornpound,
such as carbonates, oxides, hydroxides, arnmonia gas, with ammonia gas and/or
sodium
carbonate being preferred for reasons of cost.
The temperature of the concentrate 168 during step 140 is preferably at least
about 70 C, and more preferably ranges from about 90 to about 100 C.
The residence time of the concentrate 168 in the reactor is preferably at
least
about 1 minute and more preferably ranges from about 10 to about 20 minutes.
-24 -
~-----..
CA 02449467 2003-11-14
The reactor can be configured as a batch or continuous reactor and as a single-
or
multi-compartment vessel. Preferably, the reactor has from one to six
compartments.
The reactor typically agitates the various components for better reaction
kinetics.
The atmosphere of the reactor is preferably anaerobic to limit the oxidation
of
sulfite and ensure that the precious metal precipitates in the concentrate 168
are not
dissolved. The atmosphere can be realized and maintained by de-aerating the
concentrate
168 with a vacuum, inert or oxygen-deficient gas bubbling or sparging through
the
concentrate 168, maintaining a blanket of nitrogen in the atmosphere over the
concentrate
168, and/or allowing the concentrate 168 to stand dormant for a sufficient
period of time
for the dissolved oxygen level to decrease to desired levels. Preferably, the
concentrate
168 contains no more than about 1 ppm dissolved molecular oxygen and more
preferably
less than about 0.2 ppm dissolved molecular oxygen concentration.
The upgraded concentrate 176 outputted by step 140 comprises the precious and
non-precious precious metal precipitates, thiosulfate, elemental sulfur, and
sulffite
reagent. The upgraded concentrate 176 is a slurry having a liquid component
that
contains predominantly thiosulfate and a solid component that contanns
predominantly
the precious and non-precious metal precipitates. Typically, at least about
50% and more
typically at least about 9011/o of the elemental sulfur is converted into
thiosulfate. The
concentration of the precious metal precipitates in the upgraded concentrate
176 typically
ranges from about 0.1 to about 75 wt.% of the upgraded concentrate 176 and the
concentration of the elemental sulfur from about 0.1 to about 50 wt.% of the
upgraded
concentrate 176.
_25 _
CA 02449467 2003-11-14
In step 178, the upgraded concentrate 176 is subjected to further liquid/solid
separation by any of the techniques noted above to produce precious metal-
bearing solids
180 containing at least most of the precious metal content and a barren
solution 152
containing at least most of the thiosulfate of the upgraded concentrate 176.
The preferred
separation technique is settling and f ltration.
Due to the removal of elemental sulfur, the precious metal concentration in
the
precious metal-bearing solids 180 is substantially higher t.han that in the
upgraded
concentrate 176. Typically, the precious metal concentration in the solids 180
is from
about 500 to 20,000% of the precious metal concentration in the upgraded
concentrate
176.
The barren solution 152 is recycled to the sulfide precipitation step 160.
A minor portion (e.g., from about 2 to about 20 vol%) of the barren lixiviant
150
or bleed stream 182 may have to be bled to tailings to control the buildup of
impurities,
such as soluble sulfate and metallic impurities. Prior to discharge to
tailings the bleed
stream 182 of the lixiviant 150 is directed to the precious metal sca.venging
step 186 to
recover any precious metals remaining in solution that vvere not recovered
previously.
Precious metal scavenging can be accomplished, by any suitable gold recovery
technique
such as by passing the bleed solution 182 through a column containing a strong
base resin
to adsorb the precious metal. While not wishing to be bound by any theory,
precipitated
precious metal can be redissolved due to a trace amount of molecular oxygen in
the
solution and incomplete reduction of polythionates in the solution. Because
the amount
of polythionates in the bleed is negligible, a resin-in-column recovery
technique will have
an excellent ability to load any remaining dissolved precious metal.
-26 -
~ _. .
CA 02449467 2003-11-14
Twming now to the finthher treatment of the precious metal-bearing solids 180,
the
solids 180 are contacted in step 193 with a mineral acid 188, heat 190, and an
oxidant
192 to remove any undesired non-precious metal(s) and form a precious metal-
containing
slurry 194. Examples of such undesired non-precious metal(s) include mercury
in the
form of inercuric sulfide, copper in the form of copper sulfide, and other
transition metal
sulfides. The mineral acid and/or oxidant solubilize at least most of the
mercury or base
metal(s) in the liquid phase and leave at least most of the precious metals in
the solid
phase.
The mineral acid 188 can be any suitable acid, including nitric acid,
hydrochloric
acid, (hydro) sulfuric acid, and mixtures thereof, with nitric acid being
preferred. The
preferrred acid concentration is frorn about 1 to about 50 wt.%.
The oxidant 192 can be any suitable material, such as oxygen, nitric acid,
peroxides, and mixtures thereof, with nitric acid being preferred. The
preferred oxidant
concentration ranges firom about 1 to about 50 wt.%.
The preferred temperature of the solids during step 193 is greater than about
50 C
and more preferably ranges from about 90 to about 100_C.
The residence time of the solids 180 in step 193 preferably ranges from about
10
to about 480 minutes.
The precious metal-containing slurry 194 is subjected to liquid/solid
separation
195 by any of the techniques noted above to form a barren liquid 197 and
precious metal
bearing solids 196. At least about 100/o of the non-precious metals originally
in the
pregnant leach solution 160 are contained in the barren liquid 197, and at
least about 50%
of the precious metals originally in the solution 160 are in the precious
metal bearing
-27 -
i:! I
CA 02449467 2003-11-14
solids 196. The banen liquid 197 may be treated by known techniques to recover
desired
non-precious or base metals and/or discarded.
The precious metal-bearing solids 196 are subjected to refining 198 by known
techniques to produce a precious metal product 199 of high purity.
A second embodiment of the present invention will now be discussed with
reference to Figures 2A and B. The embodiment employs tank leaching rather
than heap
leaching to recover precious metals. Like-numbered elements in Figures 2A and
B on the
one hand and Figures lA and B on the other are the same. Different numbered
elements
are discussed below.
The precious metal-bearing material 100 is comminuted in step 200 to produce a
comminuted precious metal-bearing materia1204. The materia1204 is comminuted
to a
size sufficient to enable the solids to be suspended in an agitated vessel and
to allow for
the efficient leaching of the precious metals. Preferably, wet grinding is
employed with
the recycled thiosulfate leach solution 144 and water being used as the liquid
component
in the slurry. In that event, the slurried material 204 typically contains
from about 0.05 to
about 0.2 M thiosulfates and from about 0.0005 to about 0.025 m polytluonates.
The fully
comminuted material parkicle size is preferably at least smaller than 80%
passing about
48 mesh (300 microns), more preferably 80% passing about 100 mesh (150
microns), and
most preferably 80% passing about 200 mesh (75 microns). The typical solids
content of
the slurred material 204 ranges from about 20 to about 30 wt.%. As wi11 be
appreciated,
other techniques can be used to comminute the material to the desired particle
size(s). By
way of illustration, blasting can be used alone with or without crushing and
grinding and
crushing and grinding can be used alone with or without another comminution
technique.
-28 -
r,.._. . ~
il I ~
CA 02449467 2009-07-27
The slurried comminuted precious metal-bearing material 204 is then thickened
208 to adjust the pulp density to a value suitable for leaching. The ideal
leach pulp
density will vary according to the type of material being leached. Typically,
the pulp
density ranges from about 20 to about 50% solids by weight, but could be as
low as about
1% or as high as about 60%. Thickening 208 will generally not be required if
the desired
pulp density (after wet comminution or formation of the comminuted material
into a
slurry) is less than about 20%.
The thickener overflow solution 212 is recycled back to the comminution step
200
in the event that wet grinding is employed. Otherwise, the overflow solution
212 is
returned to the optional slurry formation step (not shown).
Fresh makeup thiosulfate is added, as necessary, at any suitable location(s),
such
as to the slurried material 204 during comminution 200 and/or in the thickener
208, to the
thickened slurry 216 or overflow solution 212, to leaching 220 and/or to the
recycle
solution.
The thickened slurry 216 is subjected to leaching 220 in the presence of
oxygen
and thiosulfate. In one process configuration, leaching is conducted in the
presence of an
oxygen-enriched atmosphere at atmospheric pressure, or at a pressure above
atmospheric
pressure using an oxygen-containing gas to reduce or eliminate the need for
the presence
of copper and/or ammonia in the leach. The increased oxygen partial pressure
in the
leaching step 220 increases the rate of the reaction in Equation 15 in the
absence or near
absence of copper and ammonia. To accomplish this goal, the oxygen-containing
gas
may include atmospheric air, or it may include relatively pure (95%+) oxygen
such as
that produced from any commercially available oxygen plant, or it may include
any other
29
. . ,. . _
- a:
CA 02449467 2003-11-14
available source of oxygen. The desired oxygen partial pressure (PO2)
maintained during
leaching will depend on the material being leached, but it will be at least
higher than that
provided under normal ambient conditions by air at the elevation the process
is applied.
Thus, if t,he process is practiced at sea level for example the oxygen partial
pressure will
be in excess of about 3 pounds per square inch absolute pressure (psia) to as
high as
about 500 psia, preferably from about 10 to about 115 psia, and most
preferably from
about 15 to about 65 psia. The total operating pressure is the sum of the
molecular
oxygen partial pressure and the water vapor pressure at the temperature
employed in the
leaching step 132, or preferably ranges from about 15 to about 600 psia and
rnore
preferably from about 15 to about 130 psia.
The leaching temperature will be dictated by the type of material being
leached.
The temperature will vary typically from about 5 C to about 150 C, preferably
from
about 20 to about 100 C, and most preferably from about 40 to about 800C.
Higher
temperatures accelerate the leaching of precious metals but also accelerate
the
degradation of thiosulfate. If required, a source of makeup heat such as steam
is added to
the leach reactors to maintain the desired temperature.
The leaching retention time is dependent on the material being leached and the
temperature, and will range from about 1 hour to 96 hours, preferably from
about 2 to
about 16 hours, and most preferably from about 4 to about S hours.
In one process configuration, the absence or substantial absence of copper
and/or
ammonia in the leach greatly simplifies the process. Elimination or near-
elimination of
ammonia and copper from the leach provides the advantage of ailowing for a
consistently
high and reproducible precious metal extraction over a broader pH range than
was
-30 -
r-- , i,l I
CA 02449467 2009-07-27
previously possible with the other thiosulfate leaching processes. Preferably,
the (added
and/or total solution) copper concentration is no more than about 20 ppm, more
preferably no more than about 15 ppm, and even more preferably no more than
about 10
ppm while the (added and/or total solution) ammonia concentrafion is no more
than about
0.05 M, more preferably no more than about 0.03 M, and even more preferably no
more
than about 0.01 M. In this process configuration, leaching can be operated at
about pH 7-
12, preferably about pH 8-11, more preferably about pH 8-10, and even more
preferably
at a pH less than pH 9. The oxidation-reduction potential (ORP) preferably
ranges from
about 100 to about 350 mV and more preferably from about 150 to about 300 mV
(vs. the
standard hydrogen electrode (SHE)).
The leaching step 220 may be conducted in a batch or continuous basis but
continuous operation is preferred. Continuous leaching is carried out in a
multiple series
of one or more reactors that are agitated sufficiently to maintain the solids
in suspension.
Agitation may be accomplished by mechanical, pneumatic or other means. In a
preferred
configuration, gassing impellers are employed, such as those disclosed in U.S.
Patent No.
6,183,706 and copending U.S. Patent No. 6,368,381, filed April 27, 2000. Such
impellers can significantly enhance the amount of dissolved molecular oxygen
in the
leach solution. Leaching may also be carried out in a multi-compartment
autoclave
containing one or more compartments, (with 4 to 6 compartments being
preferred)
similar in design to the autoclaves used to pressure oxidize sulfide-bearing
ores or
concentrates. However, owing to the non-acidic, moderate temperaturre,
relatively mild
conditions employed in the present invention, the autoclave materials of
construction are
much less expensive than
31
CA 02449467 2003-11-14
those found to be necessary when oxidizing sulfide minerals. The latter
autoclaves are
normally constructed of a steel shell fitted with a lead liner and refractory
brick liner and
containing metallic components constructed of titaniurn or other expensive
corrosion-
resistant alloys. The leach reactors and contained metallic cornponents
employed by the
present invention can be simply constructed of stainless steel and do not
require lead or
brick liners.
The pregnant slurry 224 is subjected to solidlliquid separation 22$ by any of
the
techniques set forth above, with the solid fraction forming tailings 236 and
the liquid
fraction forming the pregnant leach solution 13$.
The remaining steps are as described with reference to the corresponding
numbered step in Figures lA and B.
In any of the above processes or in other processes using thiosulfate as a
lixiviant,
the use of a blinding agent may improve metal recoveries. While not wishing to
be
bound by any theory, it is believed that the precious metal thiosulfate
complex may be
unstable under certain conditions, including those set forth above, and that
the precious
metal can be stripped from the thiosulfate-containing solution by a number of
substances
commonly encountered in precious metal-containing materials. The substances or
preg
robbing materials typioally absorb, adsorb or precipitate the precious metal.
Such preg-
robbing materials include carbonaceous materials, pyrite-containi.ng
materials,
chacopyrite and iron oxides. Surprisingly and unexpectedly, blinding agents
may be used
in the thiosulfate lixiviant to prevent or inhibit preg robbing of the
precious metal by the
preg robbing material. The blinding agent itself absorbs or adsorbs (in
preference to the
precious metal) or otherwise neutralizes (such as by chemical reaction) the
preg robbing
-32 -
~..._.
. .w;=wnun.mmrc.:.:n'Hk:- . . , ,'iY,4~t...... .. . .. . ... ~fi ... . . .
.... . . . .. . ..~..--.....e..õ...a..,,.. ewww..,.,k...,~.,...._-._._.-
,_.._........_.õ~....~,~.,.-__,......._.....-._._.........-.._~....._.._.
,
CA 02449467 2003-11-14
sites on the material. Suitable blinding agents include one or more of
hydrocarbon-
containing substances, such as aliphatic or cyclic hydrocarbons, preferably
petroleum
products (e.g., kerosene, diesel fuel, and gasoline), alcohols, esters, or
aldehydes;
surfactants such as detergents, sodium lauryl sulfonate, or organic
phosphates; guar gum;
starch, a cellulose such as a carboxy methyl cellulose; and reactive metal
salts such as
lead, mercury, cadmium, tin, and silver salts. In such situations, the
thiosulfate lixiviant
144 and 148, in any of the leaching processes discussed above, typically
includes at least
about 0.1 mg/L, more typically at least about 1 mg/L, and even more typically
from about
2 to about 200 mg/L of the blinding agent.
While not wishing to be bound by any theory, other agents may also be suitable
as
blinding agents. Preferably, the agents do not destabilize thiosulfate in
solution. Agents
which act as oxidation catalysts, can destabilize thiosulfate in solution. By
way of
example, copper salts under certain conditions are not preferred as a blinding
agent as
copper salts under these conditions can catalyze thiosulfate decomposition.
To facilitate extraction of gold frorn sulfidic and/or carbonaceous materials,
the
thiosulfate leach step in any of the above processes can be preceded by one or
more
pretreatment steps to destroy or neutralize the carbon-cont.aining and/or
sulfidic minerals.
By way of example, the intermediate steps can include one or more of
biooxidation or
chemical oxidation to oxidize suifides, ultrafine grinding to liberate
occluded precious
metals, conventional roasting to destroy carbon- and/or sulfide-containing
minerals,
and/or microwave roasting.
-33 -
~ I
...r.rr..-. _ ~.õ,... _ . .semc: =sm.:;c. . . . . . . . , .s.?gc~. .. . .
=~rax=s~n.~.., . ~.,........>...,w.,n..o..,..., ~õ.....,...M,...:.-,r.-
,,:~.,.,.,...,,.-...a,,. . . ,_._.-.d..e,..e,_._.s___
. . . . , . ~ II ~
CA 02449467 2003-11-14
EXPERIMENTAL
A sulfar sludge contained 0.02 to 0.7 wM/o gold and greater than 85% elemental
sulfur. To replicate step 140 of Figure lA, the sludge was treated with sodium
sulfite or
ammonium bisulfite. To maintain the pH levels identified in the examples, a
base was
sometimes added.
In the examples below, "sludge" refers to the solid material (or the precious
metal bearing concentrate 168) produced by adding sulfide to the pregnant
thiosulfa.te
leach liquor, and "residue" refers to the solid product (or precious metal-
bearing solids
180) from step 140.
EXAMPLE 1
The gold grade increased from 0.6 wt% in the sludge to 25 wt% in the residue
when using as low as 25% excess sodium sulfite (Na2S03) (as defined by the
excess of
reagent applied above the stoichiometric requirement for 100% conversion of
the
elemental sulfur content of the sludge) at 100 C for 70 to 120 minutes. The
gold grade of
the residue was 42 times larger than the gold grade of the sludge. As 1ow as
8% of the
original gold content of the sludge redissolved. Greater than 99% of the
sulfur content of
the sludge was converted primarily to thiosulfate. The sulfur content
decreased firom 87
wt% in the sludge to 16 wt'/o in the residue. The pH of the thiosulfa.te-rich
solution
remained above pH 9 without the nced to add a base.
EXAMPLE 2
The gold grade increased from 0.02 wt% in the sludge to 1.8 wt% in the residue
when using as low as 31 % excess ammonium bisulfite (NHaHS03) at 100 C for 22
-34 -
,
<;t
CA 02449467 2003-11-14
minutes. 'i'he gold grade of the residue was 90 times larger than the goid
grade of the
sludge. The pH of the thiosulfate-rich solution was maintained in the range
between pH
9.5 to pH 10 by using ammonia gas as a base. Greater than 99% of the sulfur
content of
the sludge was converted primarily to thiosulfate. The sulfur content
decreased from 95
wt% in the sludge to 60 wt% in the residue.
EXAMPLE 3
The gold grade increased from 0.6 wt% in the sludge to 25.6 wt% in the residue
when using as low as 36% excess ammonium bisulfite (NH4HS03) at 100 C for 60
minutes. The gold grade of the residue was 44 times larger than the gold grade
of the
sludge. Six percent of the original gold content of the sludge redissolved.
Greater than
99% of the sulfur content of the sludge was converted primarily to
thiosulfate. The sulfur
content decreased from 99 wt% in the sludge to 21 wt% in the residue. The pH
of the
thiosulfate-rich solution was maintained between pH 7.8 to pH 8.8 by using
sodium
carbonate (Na2C03) as a base.
These examples demonstrate that the gold grade of the residue after treatment
is
increased over the gold grade of the feed material. The gold grade of the
residue after
treatment incxeases by the saane factor independently of the reagent used or
the sulfur
grad.e of the feed material.
EXAMPLE 4
In Figure 3 the gold extraction from two lazge crib tests are shown. For both
tests,
the cn'bs that were used had a square cross-section that was 8 ft by 8 ft and
ore was
stacked into these cribs to a height of 20 ft. The ore for both tests was a
carbonaceous
preg-robbing gold ore.
-35 -
.
CA 02449467 2003-11-14
Both cribs were irrigated with a solution that contained ammonium thiosulfate
at a
concentration of 10-15 gIL. The irrigation rate varied during the test for
both tests, but
was between 0.00125 and 0.0025 gpm/R . The aeration rate for both tests was
kept at
0,002 scfm/ft2.
For the unagglomerated crib, the ore was crushed to -2" and then placed in the
crib. For the agglomerated crib, ore wae crashed to 2", and then was mixed in
a rotating
drum for approximately 5 minutes with a solution of 15 g/L ammonium
thiosulfate. This
solution was added to the ore in an amount to produ.ce a visually good
agglomerate, but
arnounted to approximately 5% ofthe ore mass added.
As Figure 3 shows, the gold extraction, when the ore is agglomerated using
ammonium thiosulfate, is significantly better than when no agglomerating
medium is
used.
EXAMPLE 5
In Tables 1 and 2, two column tests are shown - one aerated and one not. In
both
tests, carbonaceous preg-robbing gold ore was used. This ore was placed in 10
in. x 8 ft.
columns and was irrigated at 0.005 gpm/e for the unaerated colunm and 0,0025
gprn/ft2
for the aerated column. The irrigation rate was changed for the aerated column
to ensure
that air, applied to the bottom of the column, could contact all of the ore.
These tables clearly show that when air is applied to a column, the dissolved
oxygen level and Oxidation-lteduction Potential or ORP both increase. This
results in an
increase in gold extraction.
-36 -
CA 02449467 2003-11-14
Table 1: Extraction, OR.P (mV vs. Ag/AgCI) and dissolved Oz content (D02) as a
function of solution applied for a 10 in, column with no air addition.
Sol. Applied ORP D02 Extraction
t mV m
0.0 0%
0.2 34 1.8 2%
0.3 -1 1.6 11%
0.3 -63 0.3 17%
0.4 -65 0.3 22%
0.7 -69 0.3 32%
1.0 -46 0.6 39%
1.3 -33 1.7 43%
1.6 -95 0.9 46%
1.9 -50 1.3 48%
2.3 -76 1.4 50%
2.5 -68 1.3 51 %
2.9 -83 1.2 52%
Table 2: Extraction, ORP (mV vs. Ag/AgCl) and D02 as a function of solution
applied
for a 10 in. column with air added at 0.007 scfin/fl .
Sol. Applied ORP D02 Extraction
(kl t mV MR/L
0.0 0%
0.1 30 5.1 0%
0.2 16 4.5 4%
0.2 16 4.3 12%
0.3 19 4.0 32%
0.5 12 3.4 52%
0.6 13 3.5 60%
0.8 10 5.4 65%
0.9 8 4.2 67%
1.1 1 3.3 69%
1.2 2 4.8 70%
1.4 2 4.8 71%
-37 -
r..... ,
..~~ . --~.~~ ~. ,- - ~ . . .,~ .. , _ ._ ._. ... ... __ ~. .,...-~.~.~
N....._....~n___.___._.. ~.,,M.
,:
ft
CA 02449467 2003-11-14
` 1.5 I -1 1 5.4 I 71% I
EXAMPLE 6
Fig. 4 shows the gold recovery from two columns. Again, in both tests,
carbonaceous preg robbing goid ore was used. This ore was placed in 10 in. x
20 ft.
columns and was irrigated at 0.005 gpm/ft2 for column 2 and 0.0025 gpm/W for
column
1.
Fig. 4 shows that for these tests, before an application ratio of 0.6,
recovery is
independent of application rate. Afler this time, air was introduced to column
1 at a rate
of 0.007 scfm/ft . As this figare shows, the application of air at this rate
resulted in the
gold extraction increasing significantly as compared to #he unaerated test.
A number of variations and modifications of the invention can be used. It
would
be possible to provide for some features of the invention without providing
others.
By way of example, any source of sulfar species with an oxidation state less
than
+2 may be used in any of the above process steps to convert polythionates to
thiosulfate.
The regeneration phase of the conditioning step 182 can be performed in a
variety of
locations. For example, regeneration phase can be performed in the recycle
loop before
or after fresh thiosulfate 148 addition and before comminution 200, between
comminution 200 and thickening 208, in the thickener, and/or immediately
before or
during leaching 220.
Fresh thiosulfate 148 can also be added in a number o iocations. For example,
fresh thiosulfate 148 can be added in any of the locations referred to
previously for the
regeneration phase and can be added after or during regeneration as noted
above or in a
separate tank or location.
-38 -
~-=_ ,
CA 02449467 2003-11-14
The present invention is not limited to the process configurations of Figs. 1
and 2.
For example, steps 140, 193, and 180 may be omitted from the depicted process
configurations. Other process steps may be substituted for the depicted
process steps.
For example, the precious metals may be recovered by techniques other than
sulfide
precipitation in step 160. Such techniques include resin in pulp,
electrowinning,
cementation, ion exchange resins, cyanidation, direct refining, solvent
extraction, and the
like.
The processes to remove precious metals by sulfide precipitation followed by
thiosulfate production are not limited to precious metals. The processes can
be employed
with non-precious metals as well.
Sulfates may be controlled by methods other than precipitation. Sulfates may
be
removed by membrane filtration, solvent extraction, and ion exchange.
Sulfates can be removed by adding calcium to a side stream of the thiosulfate
lixiviant or other process eftluent foilowed by liquid/solid separa.tion to
remove the
precipitated gypsum from the lixiviant. This is shown by the optional use of
the precious
metal scavenging step. Calcium can be placed in the heap separate from the
precious
metal-bearing material 108. Tlus is particularly attractive where
agglomeration is not
employed.
The present invention, in various embodiments, includes components, methods,
processes, systems and/or apparatus substantially as depicted and descri bed
herein,
including various embodiments, subcombinations, and subsets thereof. Those of
slcill in
the art will understand how to make and use the present invention after
understanding the
present disclosure. The present invention, in various embodiments, includes
providing
-39 -
r._...... ,
CA 02449467 2009-07-27
devices and processes in the absence of items not depicted and/or described
herein or in
various embodiments hereof, including in the absence of such items as may have
been
used in previous devices or processes, e.g., for improving performance,
achieving ease
and\or reducing cost of implementation.
The foregoing discussion of the invention has been presented for purposes of
illustration and description. The foregoing is not intended to limit the
invention to the
form or forms disclosed herein. In the foregoing Detailed Description for
example,
various features of the invention are grouped together in one or more
embodiments for
the purpose of streamlining the disclosure. This method of disclosure is not
to be
interpreted as reflecting an intention that the claimed invention requires
more features
than are expressly recited in each claim. Rather, as the following claims
reflect,
inventive aspects lie in less than all features of a single foregoing
disclosed embodiment.
Moreover though the description of the invention has included description of
one
or more embodiments and certain variations and modifications, other variations
and
modifications are within the scope of the invention, e.g., as may be within
the skill and
knowledge of those in the art, after understanding the present disclosure. It
is intended to
obtain rights which include alternative embodiments to the extent permitted,
including
alternate, interchangeable and/or equivalent structures, functions, ranges or
steps to those
claimed, whether or not such alternate, interchangeable and/or equivalent
structures,
functions, ranges or steps are disclosed herein, and without intending to
publicly dedicate
any patentable subject matter.