Note: Descriptions are shown in the official language in which they were submitted.
CA 02449834 2009-06-15
28976-237
1
Description
Herbicidal Compositions Comprising Benzoylpyrazoles and Safener
The invention is in the technical field of the crop protection products, in
particular
herbicide/antidote combinations (active ingredient/safener combinations) which
are
suitable for use against competing harmful plants in crops of useful plants.
A large number of herbicidal active ingredients are known as inhibitors of the
enzyme p-hydroxyphenylpyruvate dioxygenase (HPPD). Only recently, more such
active ingredients were disclosed for example in WO 99/58509.
As is the case with many other herbicidal active ingredients, these HPPD
inhibitors
too are not always sufficiently well tolerated by (i.e. not sufficiently
selective in) some
important crop plants such as maize, rice or cereals, so that their use is
very limited.
They can therefore not be employed in some crops, or only at such low
application
rates that the desired broad herbicidal activity against harmful plants is not
ensured.
Specifically, many of the abovementioned herbicides cannot be employed as
fully
selective herbicides against harmful plants in maize, rice, cereals, sugar
cane and
some other crops.
To overcome these disadvantages, it is known to employ herbicidal active
ingredients in combination with what is known as a safener or antidote. Thus,
for
example, WO 99/66795 describes various combinations of a large number of HPPD
inhibitors with a multiplicity of safeners.
A safener is understood as meaning a compound which compensates for, or
reduces, the phytotoxic properties of a herbicide with regard to useful
plants, without
substantially reducing the herbicidal activity against harmful plants.
Finding a safener for a specific groups of herbicides remains a difficult task
since the
mechanisms by which a safener reduces the harmful action of herbicides are not
CA 02449834 2009-06-15
28976-237
2
known in detail. The fact that a compound in combination with a specific
herbicide
acts as safener allows therefore no conclusions as to whether such a compound
also
has a safener action with other groups of herbicides. Thus, it has emerged
when
safeners were used for protecting the useful plants from herbicide damage that
the
safeners may still exhibit certain disadvantages in many cases. These include:
= the safener reduces the activity of the herbicide against the harmful
plants,
= the useful-plant protecting properties are insufficient,
= the spectrum of the useful plants in which the safener/herbicide is to be
employed is not sufficiently wide in combination with a given herbicide,
a given safener cannot be combined with a sufficiently large number of
herbicides.
It was an object of the present invention to provide further combinations of
herbicides
from the group of the HPPD inhibitors with safeners which are suitable for
increasing
the selectivity of these herbicides with regard to important crop plants.
CA 02449834 2009-06-15
28976-237
2a
Summary of Invention
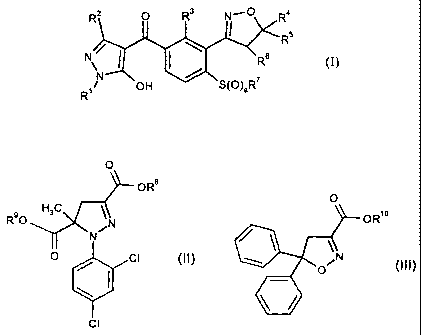
The present invention provides a herbicidal composition comprising
A) a herbicidally active amount of one or more herbicide compounds of the
formula (I),
2 O R3 N-O R4
(I)
N / ( \ 6 R 5
N
N OH S(O)aR7
R1
in which the symbols and indices have the following meanings:
R1 is (C1-C6)-alkyl;
R2 is hydrogen or (C1-C6)-alkyl;
R3 is hydrogen, a halogen atom, P-C6)-alkyl, (C1-C6)-haloalkyl, P-C6)-alkoxy,
(Ci-C6)-haloalkoxy, (Ci-C6)-alkylthio, (Ci-C6)-alkylsulfinyl or (Ci-C6)-
alkylsulfonyl;
R4, R5, R6 independently of one another are hydrogen or (C1-C6)-alkyl;
R7 is (C1-C6)-alkyl;
a is 0, 1 or 2;
and
B) an antidote-effective amount of one or more safener compounds of the
formula (II) or (III), a stereoisomer thereof or a salt thereof conventionally
used in
agriculture
CA 02449834 2009-06-15
28976-237
2b
O O
OR' OR10
9 ;H3 C V\N
N
RO "I O Cl (II) (III)
Cl
in which R8, R9 and R10 independently of one another are hydrogen or
(C1-C4)-alkyl.
The present invention further provides a method of controlling
harmful plants in a crop of plants, which comprises applying a herbicidally
active
amount of the herbicidal composition described herein to the harmful plants,
the
crop plants, crop plant seeds or the area on which the crop plants grow.
CA 02449834 2009-06-15
28976-237
2c
There have now been found novel combinations of specific herbicides from the
group of the HPPD inhibitors, specifically from the group of the
benzoylpyrazoles,
which have selected substituents attached in the 3-position of the benzoyl
moiety,
with some selected safeners which increase the selectivity of these herbicides
with
regard to important crop plants.
The invention therefore relates to a herbicidally active composition
comprising
A) a herbicidally active amount are of one or more compounds of the formula
(I),
R2 O R3 i O R4
R5
\ (I)
l~6
S(O)8R7
R
in which the symbols and indices have the following meanings:
R1 is (C1-C6)-alkyl;
R2 is hydrogen or (C1-C6)-alkyl;
CA 02449834 2003-12-05
3
R3 is hydrogen., halogen, (C1-C6)-alkyl, (C1-C6)-haloalkyl, (C1-C6)-alkoxy,
(C1-C6)-
haloalkoxy, (C1-C6)-alkylthio, (C1-C6)-alkylsulfinyl or (C1-C6)-alkylsulfonyl;
R4, R5, R6 are hydrogen or (C1-C6)-alkyl;
R7 is (C1-C6)-alkyl;
a is 0, 1 or 2;
and
B) an antidote-effective amount of one or more compounds of the formula (II)
or
(III)
0
ORB
0
H3C \ 10
R9O N OR
N 100,
~ , \
0 CI (II) -~ OWN (Ili)
CI
in which the symbols have the following meanings::
R8, R9, R10 independently of one another are hydrogen or (C1-C4)-alkyl,
including the stereoisomers and the salts conventionally used in agriculture.
Herbicidally active amount for the purposes of the invention refers to an
amount of
one or more herbicides suitable for having an adverse effect on plant growth.
Antidote-effective amount for the purposes of the invention refers to an
amount of
one or more safeners suitable for at least partially counteracting the
phytotoxic effect
of the herbicide or herbicide mixture on a useful plant.
Unless specifically otherwise defined, the definitions given hereinbelow
generally
apply to the radicals in the formulae of (I) to (III) and the subsequent
formulae.
CA 02449834 2003-12-05
4
The radicals alkyl, alkoxy, haloalkyl, haloalkoxy and alkylthio and the
corresponding
unsaturated and/or substituted radicals in the carbon skeleton can be in each
case
straight-chain or branched. Alkyl radicals, also in the composite meanings
such as
alkoxy, haloalkyl and the like, preferably have 1 to 4 carbon atoms and are,
for
example, methyl, ethyl, n- or i-propyl, n-, i-, t- or 2-butyl. "(C1-C4)-alkyl"
is the
abbreviation for alkyl having 1 to 4 carbon atoms; the same applies
analogously to
other general definitions of radicals with bracketed ranges of the possible
number of
carbon atoms.
Halogen is fluorine, chlorine, bromine or iodine. Haloalkyl is alkyl, alkenyl
or alkynyl
which is partially or fully substituted by halogen, preferably by fluorine,
chlorine,
and/or bromine, in particular by fluorine or chlorine, for example CF3, CHF2,
CH2F,
CF2CF3, CH2FCCIFH, CC13, CHCI2, CH2CH2CI. Haloalkoxy is, for example, OCF3,
OCHF2, OCH2F, OCF2CF3, OCH2CF3 and OCH2CH2CI. The same applies
analogously to other halogen-substituted radicals.
Formulae (I) to (III) also encompass all stereoisomers which have the same
topological linkage of the atoms, and their mixtures. Such compounds contain
one or
more asymmetric carbon atoms or else double bonds which are not specified
individually in the general formula. The possible stereoisomers which are
defined by
their specific spatial shape such as enantiomers, diasteriomers, Z- and E-
isomers
can be obtained from stereoisomer mixtures by customary methods or else be
prepared by stereoselective reactions in combination with the use of
stereochemically pure starting materials.
Suitable herbicidal active ingredients are in accordance with the invention
those
compounds of the formula (I) which cannot be employed on their own, or which
can
not be employed optimally on their own, in crops of useful plants such as
cereal
crops, rice or maize since they cause too much damage to the crop plants.
Herbicides of the formula (I) are disclosed for example in WO 99/65314 and
WO 99/58509.
CA 02449834 2009-06-15
28976-237
- The publications cited contain detailed information on preparation processes
and
starting materials.
5 The compounds of the formula (II) are known for example from WO 91/07874 and
the literature cited therein and can be prepared by, or analogously to, the
methods
described therein. The compounds of the formula (III) are known from WO
95/07897
and literature cited therein and can be prepared by, or analogously to, the
methods
described therein.
The publications cited contain detailed information on preparation processes
and
starting materials.
For the purposes of the present application, the term "herbicidal compositions
" and
"herbicide/safener combinations" are to be considered as equal.
Preferred herbicidal compositions are those which comprise compounds of the
formula (1), in which the symbols and indices have the following meanings:
R1 is (C1-C6)-alkyl;
R2 is hydrogen or (C1-C6)-alkyl;
R3 is halogen or (C1-C6)-alkyl;
R4, R5, R6 are hydrogen, (C1-C6)-alkyl;
R7 is (C,-C6)-alkyl;
a is 0, 1 or 2.
Also preferred are herbicidal compositions comprising safeners of the formula
(II)
and/or (III), in which R8, R9 and R10 independently of one another are
hydrogen or
(C1-C2)-alkyl.
Especially preferred are herbicidal compositions comprising compounds of the
formula (I), in which R3 is chlorine or methyl.
CA 02449834 2003-12-05
6
Also especially preferred are herbicidal compositions comprising compounds of
the
formula (I) in which a is 2.
The compounds mentioned herein as safeners (antidotes) reduce or compensate
for
phytotoxic effects which may occur when using the herbicidal active
ingredients of
the formula (I) in crops of useful plants without essentially adversely
affecting the
efficacy of the herbicidal active ingredients against harmful plants. Thus,
the field of
application of conventional crop protection agents can be widened considerably
and
extended to, for example, crops such as wheat, barley, rice and maize in which
the
use of the herbicides has previously not been possible or only with
limitations, that is
to say at low dosages with a narrow spectrum of action.
Herbicidal active ingredients and the safeners mentioned can be applied
together (as
readymix or by the tank mix method) or sequentially in any desired sequence.
The
weight ratio of safener to herbicidal active ingredient may vary within wide
limits and
is preferably in the range of from 1:100 to 100:1, in particular from 1:10 to
10:1. The
optimum amount of herbicidal active ingredient and safener depend in each case
on
the type of the herbicidal active ingredient used or on the safener used and
on the
nature of the plant stock to be treated and can be determined in each
individual case
by simple routine preliminary experiments.
Main fields of application for the use of the combinations according to the
invention
are especially maize and cereal crops such as, for example, wheat, rye,
barley, oats,
rice, sorghum, but also cotton and soybean, preferably cereals, rice and
maize.
Depending on their properties, the safeners employed in accordance with the
invention may be used for pretreating the seed of the crop plant (seed
dressing) or
introduced into the seed furrows prior to sowing or used together with the
herbicide
before or after emergence of the plants. Pre-emergence treatment includes not
only
the treatment of the area under cultivation before sowing, but also the
treatment of
the sown soil which does not yet sustain vegetation. Preferred is the
application
CA 02449834 2003-12-05
7
together with the herbicide. Tank mixes or readymixes may be employed for this
purpose.
The safener application rates required may vary within wide limits, depending
on the
indication and the herbicidal active ingredient used; they are, as a rule, in
the range
of from 0.001 to 5 kg, preferably from 0.005 to 0.5 kg, of active ingredient
per
hectare.
The present invention therefore also relates to a method of protecting crop
plants
from phytotoxic side effects of herbicides of the formula (I), which comprises
applying an antidote-effective amount of a compound of the formula (II) and/or
(III)
before, after or simultaneously with the herbicidal active ingredient A of the
formula
(I) to the plants, plant seeds or the area under cultivation.
The herbicide/safener combination according to the invention may also be
employed
for controlling harmful plants in crops of known genetically modified plants
or
genetically modified plants yet to be developed. As a rule, the transgenic
plants are
distinguished by particularly advantageous properties, for example by
resistances to
certain crop protection agents, resistances to plant diseases or to causative
agents
of plant diseases such as specific insects or microorganisms such as fungi,
bacteria
or viruses. Other particular properties concern for example the harvested crop
with
regard to quantity, quality, storability, composition and specific
constituents. Thus,
transgenic plants are known with an increased starch content or a modified
starch
quality, or those with a different fatty acid composition of the harvested
crop.
Preferred is the use of the combinations according to the invention in
economically
important transgenic crops of useful plants and ornamentals, for example of
cereals
such as wheat, barley, rye, oats, sorghum and millet, rice, cassava and maize,
or
else crops of sugar beet, cotton, soybean, oilseed rape, potato, tomato, pea
and
other vegetables. -
CA 02449834 2003-12-05
8
When the combinations according to the invention are used in transgenic crops,
effects in addition to the effects to be observed against harmful plants in
other crops
are frequently found, which are specific for application in the particular
transgenic
crop, for example a modified or specifically widened weed spectrum which can
be
controlled, modified application rates which may be employed for the
application,
preferably good combinability with the herbicides to which the transgenic crop
is
resistant, and an effect on growth and yield of the transgenic crop plants.
The invention thus also relates to the use of the combination according to the
invention for controlling harmful plants in transgenic crop plants.
The safeners of the formulae (II) and (III) and their combinations with one or
more of
the abovementioned herbicidal active ingredients of the formula (I) can be
formulated
in various ways, depending on the prevailing biological and/or chemico-
physical
parameters. Suitable possibilities of formulation are, for example,
wettable powders (WP), emulsifiable concentrates (EC), water-soluble powders
(SP), water-soluble concentrates (SL), concentrated emulsions (BW) such as oil-
in-
water and water-in-oil emulsions, sprayable solutions or emulsions, capsule
suspensions (CS), oil- or water-based dispersions (SC), suspoemulsions,
suspension concentrates, dust (DP), oil-miscible solutions (OL), seed-dressing
products, granules (GR) in the form of microgranules, spray granules, coated
granules and absorption granules, granules for soil application or
broadcasting,
water-soluble granules (SG), water-dispersible granules (WG), ULV
formulations,
microcapsules and waxes.
These individual formulation types are known in principle and are described,
for
example, in: Winnacker-Kuchler, "Chemische Technologie" [Chemical
engineering],
Volume 7, C. Hauser Verlag Munich, 4th Ed., 1986; Wade van Valkenburg,
"Pesticide Formulations", Marcel Dekker N.Y., 1973; K. Martens, "Spray Drying
Handbook", 3rd Ed. 1979, G. Goodwin Ltd. London.
CA 02449834 2003-12-05
9
The formulation auxiliaries which may be required, such as inert materials,
surfactants, solvents and further additives are likewise known and described,
for
example, in: "Handbook of Insecticide Dust Diluents and Carriers", 2nd Ed.,
Darland
Books, Caldwell N.J., H.v. Olphen, "Introduction to Clay Colloid Chemistry";
2nd Ed.,
J. Wiley & Sons, N.Y.; C. Marsden, "Solvents Guide"; 2nd Ed., Interscience,
N.Y.
1963; McCutcheon's "Detergents and Emulsifiers Annual", MC Publ. Corp.,
Ridgewood N.J.; Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chem.
Publ. Co. Inc., N.Y. 1964; Schonfeldt, "Grenzflachenaktive Athylenoxidaddukte"
[Surface-active ethylene oxide adducts], Wiss. Verlagsgesell., Stuttgart 1976;
Winnacker-Kuchler, "Chemische Technologie", Volume 7, C. Hauser Verlag Munich,
4th Ed. 1986.
Based on these formulations, combinations with other crop protectants such as
insecticides, acaricides, herbicides, fungicides, and with safeners,
fertilizers and/or
growth regulators may also be prepared, for example in the form of a readymix
or a
tank mix.
Wettable powders are preparations which are uniformly dispersible in water and
which, besides the active ingredient, additionally comprise ionic and/or
nonionic
surfactants (wetters, dispersants), for example polyoxethylated alkyiphenols,
polyoxethylated fatty alcohols, polyoxethylated fatty amides, fatty alcohol
polyglycol
ether sulfates, alkanesulfonates, alkylbenzenesulfonates, sodium
lignosulfonates,
sodium 2,2'-dinaphthylmethane-6,6'-disulfonate, sodium dibutylnaphthalene-
sulfonate, or else sodium oleoylmethyltaurinate, in addition to a diluent or
inert
substance. To prepare the wettable powders, the herbicidal active ingredients
are
ground finely, for example in customary apparatuses such as hammer mills,
blower
mills and air-jet mills, and simultaneously or subsequently mixed with the
formulation
auxiliaries.
Emulsifiable concentrates are prepared for example by dissolving the active
ingredient in an organic solvent, for example butanol, cyclohexanone, DMF or
else
high-boiling hydrocarbons such as saturated or unsaturated aliphatic or
alicyclic
CA 02449834 2003-12-05
substances, aromatic substances or mixtures of these organic solvents with
addition
of one or more ionic and/or nonionic surfactants (emulsifiers). The following
are
examples of emulsifiers which may be used: calcium alkylarylsulfonates such as
calcium dodecylbenzenesulfonate, or nonionic emulsifiers such as fatty acid
5 polyglycol esters, alkylaryl polyglycol ethers, fatty alcohol polyglycol
ethers,
propylene oxide/ethylene oxide condensates, alkyl polyethers, sorbitan esters
such
as, for example, sorbitan fatty acid esters or polyoxyethylene sorbitan esters
such as
polyoxyethylene sorbitan fatty acid esters. Dusts are obtained in general by
grinding
the active ingredients with finely divided solid materials, for example talc,
natural
10 clays such as kaolin, bentonite and pyrophyllite, or diatomaceous earth.
Suspension concentrates may be water- or oil-based. They can be prepared for
example by wet-milling by means of commercially available beat mills, if
appropriate
with addition of surfactants as, for example, have already been listed above
in the
case of the other formulation types.
Emulsions, for example oil-in-water emulsions (EW), can be prepared for
example by
means of stirrers, colloid mills and/or static mixers using aqueous organic
solvents
and, if appropriate, surfactants as, for example, have already been listed
above in
the case of the other formulation types.
Granules can be produced either by spraying the active ingredient onto
absorptive
granulated inert material or by applying active ingredient concentrates to the
surface
of carriers such as sand, kaolinite or of granulated inert material by means
of
binders, for example polyvinyl alcohol, sodium polyacrylate or else mineral
oils.
Suitable active ingredients may also be granulated in the manner which is
conventional for the production of fertilizer granules, if desired as a
mixture with
fertilizers.
As a rule, water-dispersible granules are prepared by the customary method
such as
spray-drying, fluidized-bed granulation, disk granulation, mixing by means of
high-
speed mixers, and extrusion without solid inert material.
CA 02449834 2003-12-05
11
To prepare disk, fluidized-bed, extruder and spray granules, see, for example,
methods in "Spray-Drying Handbook" 3rd ed. 1979, G. Goodwin Ltd., London;
J.E. Browning, "Agglomeration", Chemical and Engineering 1967, pages 147 et
seq.;
"Perry's Chemical Engineer's Handbook", 5th Ed., McGraw-Hill, New York 1973,
pp.
8-57.
For further details on the formulation of crop protection agents see, for
example,
G.C. Klingman, "Weed Control as a Science", John Wiley and Sons, Inc., New
York,
1961, pages 81-96 and J.D. Freyer, S.A. Evans, "Weed Control Handbook", 5th
Ed.,
Blackwell Scientific Publications, Oxford, 1968, pages 101-103.
As a rule, the agrochemical preparations comprise from 0.1 to 99% by weight,
in
particular from 0.1 to 95% by weight, of active ingredients of the formula
(II) and/or
(III) or of the herbicide/antidote active ingredient mixture (I) and
(11)/(111) and from 1 to
99.9% by weight, in particular from 5 to 99.8% by weight, of a solid or liquid
additive
and from 0 to 25% by weight, in particular from 0.1 to 25% by weight, of a
surfactant.
In wettable powders, the active ingredient concentration is, for example, from
approximately 10 to 90% by weight, the remainder to 100% by weight being
composed of customary formulation components. In the case of emulsifiable
concentrates, the concentration amounts to approximately 1 to 80% by weight.
Formulations in the form of dusts comprise from approximately 1 to 20% by
weight of
active ingredients, sprayable solutions from approximately 0.2 to 20% by
weight of
active ingredients. In the case of granules such as water-dispersible
granules, the
active ingredient content depends partly on whether the active compound is in
liquid
or solid form. As a rule, the active compound content in the water-dispersible
granules is between 10 and 90% by weight.
In addition, the active ingredient formulations mentioned comprise, if
appropriate, the
adhesives, welters, dispersants, emulsifiers, penetrants, preservatives,
antifreeze
agents, solvents, fillers, carriers, colorants, antifoams, evaporation
inhibitors, pH
regulators and viscosity regulators which are conventional in each case.
CA 02449834 2003-12-05
12
The necessary application rates of the herbicides of the formula (I) varies
with the
external conditions such as, inter alia, temperature, humidity and the type of
the
herbicide used. It can be varied within wide limits, for example between 0.001
and
10.0 kg/ha or more of active substance, but it is preferably between 0.005 and
5 kg/ha.
The examples which follow illustrate the invention:
A Formulation examples
a) A dust is obtained by mixing 10 parts by weight of a compound of the
formula
(II) and/or (III) or of an active ingredient mixture of a herbicidal active
ingredient of the formula (I) and a safener of the formula (II) and/or (III)
and 90
parts by weight of talc as inert substance and comminuting the mixture in a
hammer mill.
b) A wettable powder with is readily dispersible in water is obtained by
mixing 25
parts by weight of a compound of the formula (II) and/or (III) or of an active
ingredient mixture of a herbicidal active ingredient of the formula (I) and a
safener of the formula (II) and/or (III), 64 parts by weight of kaolin-
containing
quartz as inert material, 10 parts by weight of potassium lignosulfonate and 1
part by weight of sodium oleoylmethyltaurinate as wetter and dispersant and
grinding the mixture in a pinned-disk mill.
c) A dispersion concentrate which is readily dispersible in water is obtained
by
mixing 20 parts by weight of a compound of the formula (II) and/or (III) or of
an
active ingredient mixture of a herbicidal active ingredient of the formula (I)
and
a safener of the formula (II) and/or (III), 6 parts by weight of alkylphenol
polyglycol ether ( Triton X 207), 3 parts by weight of isotridecanol
polyglycol
ether (8 EO) and 71 parts by weight of paraffinic mineral oil (boiling range
for
example approx.. 255 to above 277 C) and grinding the mixture in a bowl mill
to a fineness of below 5 microns.
CA 02449834 2003-12-05
13
d) An emulsifiable concentrate is obtained from 15 parts by weight of a
compound of the formula (II) and/or (ill) or of an active ingredient mixture
of a
herbicidal active ingredient of the formula (I) and a safener of the formula
(II)
and/or (III), 75 parts by weight of cyclohexanone as solvent and 10 parts by
weight of oxethylated nonylphenol as emulsifier.
e) Water-dispersible granules are obtained by mixing
75 parts by weight of a compound of the formula (II) and/or (III) or of an
active ingredient mixture of a herbicidal active
ingredient of the formula (I) and a safener of the
formula (II) and/or (III),
10 of calcium lignosulfonate,
5 of sodium lauryl sulfate,
3 of polyvinyl alcohol and
7 91 of kaolin,
grinding the mixture on a pinned-disk mill and granulating the powder in a
fluidized bed by spraying on water as granulation liquid.
f) Water-dispersable granules are also obtained by homogenizing and
precomminuting
parts by weight of a compound of the formula (II) and/or (III) or of an
active ingredient mixture of a herbicidal active
ingredient of the formula (I) and a safener of the
formula (II) and/or (III),
25 5 of sodium 2,2'-dinaphthylmethane-6,6'-disulfonate,
2 10 of sodium oleoylmethyltaurinate,
1 of polyvinyl alcohol,
17 of calcium carbonate and
50 of water
in a colloid mill, subsequently milling the mixture in a beat mill and
atomizing
and drying the resulting suspension in a spray tower by means of a single-
substance nozzle.
CA 02449834 2003-12-05
14
B Biological examples
In the experiments which follow, compositions according to the invention
comprising
safeners S1, S2 and herbicides HI and H2 were employed.
H3C COP
CI I\
COP CI N C02Et
O-N
S1 S2
O CI I-0 O CH3 I-0
N\ f I \ N\ ~ I \
OH SO2CH3 % OH SO2CH3
H3C H3C
H1 H2
Post-emergence experiments:
Seeds of useful plants are placed in soil in the open and covered with soil.
At the
two-leaf stage, the plants are treated with the herbicides formulated as
emulsifiable
concentrates or dust and, for comparison purposes, with herbicides and
safeners in
the form of aqueous dispersions or suspensions or emulsions at an application
rate
of 300 to 800 I of water per ha (converted) at various dosages. The damage to
the
useful plants was scored visually 14 or 21 days after the treatment. The
results of
Examples B1 to B4 demonstrate that the damage in the useful plants was reduced
considerably by using the herbicidal compositions comprising herbicide and
safener
in comparison with using the herbicide only. Depending on the rate of
application,
the species of the useful plant and the type of the composition according to
the
invention, the damage is reduced by up to 100% in comparison with using the
CA 02449834 2003-12-05
herbicide only. The dosage is shown in grams of active substance per hectare
(g a.i/ha).
Example B1, Reduction of damage in maize, 14 days post-treatment
Dosage [g a.i/ha] Dosage [g a.i/ha) Damage reduction
Safener S1 Herbicide H1
50 50 -100%
5 Example B2, Reduction of damage in wheat, 14 days post-treatment
Dosage [g a.i/ha] Dosage [g a.i/ha] Damage reduction
Safener S2 Herbicide H1
150 150 -57%
Example B3, Reduction of damage in maize, 21 days post-treatment
Dosage [g a.i/haj Dosage [g a.i/ha] Damage reduction
Safener S1 Herbicide H1
150 150 -93%
Example B4, Reduction of damage in maize, 21 days post-treatment
Dosage [g a.i/ha] Dosage [g a.i/ha] Damage reduction
Safener S1 Herbicide H2
150 150 -92%