Note: Descriptions are shown in the official language in which they were submitted.
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
ALLOY-BASED ANODE STRUCTURES FOR ALUMINIUM PRODUCTION
Field of the Invention
This invention relates to alloy-based oxygen-
evolving anodes for the electrowinning of aluminium having
an improved design for increasing their lifetime, cells
using them and a method of producing aluminium with such
anodes.
Backaround Art
The technology for the production of aluminium by
the electrolysis of alumina, dissolved in molten cryolite,
at temperatures around 950°C is more than one hundred years
old and still uses carbon anodes and cathodes.
Using metal anodes in aluminium electrowinning cells
would drastically improve the aluminium process by reducing
pollution and the cost of aluminium production.
Several attempts have been made in order to develop
non-carbon anodes for aluminium electrowinning cells,
resistant to chemical attacks of the bath and by the cell
environment, and with an electrochemical active surface for
the oxidation of oxygen ions to atomic and molecular gaseous
oxygen and having a low dissolution rate. However, all
attempts have failed mainly due to the anode materials which
had a low electrical conductivity and caused unacceptable
contamination of the aluminium produced. Many patents have
been filed on non-carbon anodes but none has found
commercial acceptance, also because of economical reasons.
US Patent 4,614,569 (Duruz/Derivaz/Debely/Adorian)
describes metal anodes for aluminium electrowinning coated
with a protective coating of cerium oxyfluoride, formed in-
situ in the cell or pre-applied, this coating being
maintained during electrolysis by the addition of small
amounts of a cerium compound to the molten cryolite
electrolyte so as to protect the surface of the anode from
the electrolyte attack.
Several designs for oxygen-evolving anodes for
aluminium electrowinning cells were proposed in the
following documents. US Patent 4,681,671 (Duruz) discloses
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
_ 2 _
vertical anode plates or blades operated in low temperature
aluminium electrowinning cells. US Patent 5,310,476
(Sekhar/de Nora) discloses oxygen-evolving anodes consisting
of roof-like assembled pairs of anode plates. US Patent
5,362,366 (de Nora/Sekhar) describes non-consumable anode
shapes including roof-like assembled pairs of anode plates.
US Patent 5,368,702 (de Nora) discloses vertical tubular or
frustoconical oxygen-evolving anodes for multimonopolar
aluminium cells. US Patent 5,683,559 (de Nora) describes an
aluminium electrowinning cell with oxygen-evolving bent
anode plates which are aligned in a roof-Like configuration
facing correspondingly shaped cathodes. US Patent 5,'725,744
(de Nora/Duruz) discloses vertical oxygen-evolving anode
plates, preferably porous or reticulated, in a
multimonopolar cell arrangement for aluminium electrowinning
cells operating at reduced temperature.
W000/40781 and W000/40782 (both de Nora) both
disclose aluminium production anodes with a series of
parallel spaced-apart elongated anode members which are
electrochemically active for the oxidation of oxygen.
Various anode members with different cross-sections are
disclosed in these applications, in particular anode members
with a tapered upper part and a flat electrochemically
active bottom surface as shown in Figure 5 of W000/40781 as
well as in Figures 3 and 13 of W000/40782.
Summary of the Invention
The present invention relates to improved anode
designs, in particular those disclosed in WO00/40781 and
WO00/40782 mentioned above. The anode member designs of the
present invention are specially adapted to promote gas
release and/or electrolyte circulation through the anode and
increase the lifetime of the anode that is made from an
alloy comprising an electrically conductive inert structural
metal, such as nickel and/or cobalt, and an active
diffusable metal, such as iron, that diffuses to the
electrochemically active anode surface where it is oxidised
for maintaining the electrochemically active surface.
Thus, the invention provides a long-lasting metal-
based oxygen-evolving anode for the electrowinning of
aluminium from alumina dissolved in a molten electrolyte.
This anode has a plurality of electrochemically active anode
members. Each anode member comprises a bottom part which has
a substantially constant width over its height and which is
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 3 -
extended upwardly by a tapered top part for guiding a
circulation of electrolyte thereon. The bottom part of each
anode member is made of a metal alloy with a substantially
flat oxide bottom surface which is electrochemically active
for the oxidation of oxygen.
The metal alloy of the bottom part of each anode
member comprises an electrically conductive inert structural
metal and an active diffusable metal that during
electrolysis slowly diffuses to the electrochemically active
bottom surface where it is oxidised for maintaining the
electrochemically active bottom surface and slowly dissolves
into the molten electrolyte. This bottom part forms a long-
lasting supply of the active metal diffusable to the
electrochemically active bottom surface.
For instance, the inert structural metal is nickel
and/or cobalt. The active diffusable metal may be iron, the
electrochemically active bottom surface being iron oxide
based. Before use, the inert structural metal/active
diffusable metal atomic ratio can be up to or even above 1,
in particular from 1 to 4.
Usually, the metal alloy of the bottom part
comprises the inert structural metal and the active
diffusable metal in a total amount of at least 65 weight%,
in particular at least 80 weight%, preferably at least 90
weight% of the alloy. For example, the metal alloy of the
bottom part further comprises at least one metal selected
from chromium, copper, silicon, titanium, tantalum,
tungsten, vanadium, zirconium, scandium, yttrium,
molybdenum, manganese, niobium, cerium and ytterbium in a
total amount of up to 10 weight% of the alloy. Furthermore,
the metal alloy of the bottom part may comprise at least one
catalyst selected from iridium, palladium, platinum,
rhodium, ruthenium, tin or zinc metals, Mischmetals and
their oxides and metals of the Lanthanide series and their
oxides as well as mixtures and compounds thereof, in a total
amount of up to 5 weight o of the alloy. The metal alloy of
the bottom part can comprise aluminium in an amount less
than 20 weight%, in particular less than 10 weight%,
preferably from 1 to 6 weight% of the alloy.
Examples of suitable metal alloys for the bottom
part and conditioning are described in greater detail in
WO00/06803 (Duruz/de Nora/Crottaz), WO00/06804 (Crottaz/
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 4 -
Duruz), W001/42534 (de Nora/Duruz), W001/42536 (Duruz/
Nguyen/de Nora) and PCT/IB02/01241 (Nguyen/de Nora).
In. one embodiment, the anode is covered with a
protective layer made of one or more cerium compounds, in
particular cerium oxyfluoride. Such coatings and cell
operation therewith are disclosed in. US Patents 4,614,569
(Duruz/Derivaz/Debely/Adorian), 4,680,094 (Duruz), 4,683,037
(Duruz) and 4,966,674 (Bannochie/Sherriff). These coatings
reduce the dissolution of the oxidised diffusable metal, in
particular iron, and thus reduce the required diffusion of
the diffusable metal to the electrochemically active bottom
surface thereby extending the lifetime of the anode.
The diffusion rate of the diffusable metal at the
operating Conditions can be adjusted by an appropriate
addition of one or more additives to the alloy of the anode
bottom part as disclosed in PCT/IB02/01241 (Nguyen/de Nora).
Usually, the width of the bottom part is of the same
order as the size of the height of the bottom part. For
example, the height of the bottom part is in the range of
about half to twice the size of the width of the bottom
part.
The height of the reservoir-forming bottom part is
usually at least several millimetres, typically from 5 to
mm, in particular from 10 to 15 mm. Such a reservoir has
25 the capacity to provide an additional anode lifetime of 50
to 1000, for instance an additional lifetime of 5'000 to
10'000 hours to an anode member that has a lifetime of
10'000 hours without a reservoir-forming bottom part, in
particular when the anode member has a composition and is
operated under conditions exemplified in PCT/IB02/01241 or
PCT/IB02/01952 (both in the name of Nguyen/de Nora).
The tapered top part of the or each anode member may
have one or more upwardly converging inclined surfaces with
a substantially constant slope, i.e. generally triangular or
trapezoidal in cross-section. The top part may have a
generally curved cross-section, in particular generally
elliptic or semi-circular. The cross-section may be
symmetric or asymmetric as explained below.
The height of the tapered top part may be greater
than half the size of the width of the anode member but
preferably not greater than twice the size of the width of
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 5 -
the anode member. The surface of the tapered top part may
have an average slope in the range of 30 and 75 deg, in
particular 45 to 60 deg, to the horizontal.
During use, the tapered top part permits an improved
up-flow of electrolyte from the electrochemically active
surface by delimiting an electrolyte up-flow path with a
gradually increasing section that reduces or prevents the
formation of flow-inhibiting turbulences adjacent andlor
above the anode members in the electrolyte.
The overall height of the anode member is usually of
the same order as its width, for instance from half to three
times, in particular from equal to twice, the width.
Usually, the electrochemically active anode members
are spaced. apart, usually parallel to one another and
preferably with their electrochemically active bottom
surfaces in a generally coplanar arrangement. In most
embodiments, each anode member is elongated and has a
substantially constant cross-section along its length. The
anode members may be straight or arched or circular.
Alternatively, the anode members may have a generally
circular or quadratic or other polygonal base.
The spacing between the anode members should be
sufficient to permit a flow of electrolyte and gas, in
particular an up-flow driven by anodically released gas,
between them. The spacing between the anode members can be
of the same oxder as the height of the reservoir-forming
bottom part of each anode member, for instance between half
to twice the height of the bottom part. Usually, the spacing
between two anode members is greater than 10 mm. To avoid
substantial reduction of the overall surface area of the
electrochemically active anode surfaces, the anode members
should not be spaced by more than 20 mm, preferably 15 mm.
Preferably, the dimensions of the anode members and
spacing between them are adapted to the hydrodynamic
conditions during use in the molten electrolyte.
These spaced apart anode members can be connected
through one or more electrically conductive connecting
cross-members which may be embedded in the tapered top part
of the anode members. A plurality of such connecting cross-
members may be connected together through one or more
electrically conductive connecting transverse members.
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 6 -
Usually, the anode comprises a vertical current feeder which
is mechanically and electrically connected to the or one of
the above connecting members and which is connectable to a
positive bus bar.
Furthermore, the anode may comprise one or more
electrolyte guide members for guiding an electrolyte flow
from and/or to the electrochemically active bottom
surface(s), for example as disclosed in WO00/40781 (de
Nora ) .
The shape of the tapered top part may be adapted for
the down-flow of alumina-rich electrolyte or for the up-flow
of alumina-depleted electrolyte. For instance, an anode
member, in particular with a top part having an asymmetric
cross-section, may be designed for a down-flow of
electrolyte on one side and an up-flow of electrolyte on the
other side of the tapered top part. In other words, the
shape of the tapered top part can be arranged to promote an
up-flow of electrolyte over one side of the top part and a
down-flow of electrolyte over the other side of the top
part.
The invention also relates to a cell for the
electrowinning of aluminium from alumina, comprising at
least one of the above described oxygen-evolving anodes
facing a cathode in a molten electrolyte.
Suitable cell features are disclosed in US Patent
6,258,246 (Duruz/de Nora), W000/63463 (de Nora), WO00/63464
(de Nora/Berclaz), W001/31086 (de Nora/Duruz), W001/42168
(de Nora/Duruz), W001/42531 (Nguyen/Duruz/de Nora) and
PCT/IB02/00670 (de Nora).
Another aspect of the invention relates to a method
of electrowinning aluminium. The method comprises passing an
electrolysis current in a molten electrolyte containing
dissolved alumina between a cathode and at least one of the
above described oxygen-evolving anodes to evolve oxygen on
the anodes) and produce aluminium on the cathode.
A protective layer of one or more cerium compounds,
in particular cerium oxyfluoride, may be deposited and/or
maintained on the anode by the presence of cerium species in
the molten electrolyte, as disclosed in the abovementioned
US Patents 4,614,569, 4,680,094, 4,683,037 and 4,966,674.
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
The molten electrolyte, usually a cryolite-based
molten electrolyte, may be at a temperature in the range of
700° to 1000°C, in particular from 830° to 930° or
940°C.
Preferably, the electrolyte is saturated or nearly saturated
with dissolved alumina to reduce the solubility of the metal
alloy of the bottom part of the oxygen-evolving anode(s).
A further inventive aspect concerns a metal-based
anode for an aluminium electrowinning cell. The anode
comprises a metal-based structure having an anode surface
which is active for the anodic evolution of oxygen and which
is arranged to be placed in the cell substantially parallel
to a facing cathode. The metallic structure has a series of
parallel anode members, each anode member comprising a
tapered top part and an electrochemically active oxygen-
evolving bottom surface below and integral with the tapered
top part. The electrochemically active bottom surfaces of
the metal-based structure are in a generally coplanar
arrangement to form the active anode surface. The anode
members are spaced laterally to form longitudinal flow
through openings for the flow of electrolyte.
The tapered top part of at least one anode member
has an asymmetric cross-section adapted for an electrolyte
up-flow on a first face of the tapered top part and for an
electrolyte down-flow on a second face of the tapered top
part. The first face delimits an up-flow through opening and
the second face delimits a down-flow through opening.
In other~words, the shape of the tapered top part is
arranged to promote an up-flow of electrolyte over one face
of the top part and a down-flow of electrolyte over the
other face of the top part.
As mentioned above, at least one anode member may
comprise a bottom part which has a substantially constant
width over its height and which is extended upwardly by the
tapered top part, the bottom part laeing made of a metal
alloy with a substantially flat oxide bottom surface which
forms said electrochemically active surface.
Such a metal alloy can comprise an electrically
conductive inert structural metal and an active diffusable
metal that during electrolysis slowly diffuses to the
electrochemically active bottom surface where it is oxidised
for maintaining the electrochemically active bottom surface
and slowly dissolves into the molten electrolyte. Such a
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
_ g _
bottom part forms a long-lasting supply of the active metal
diffusable to the electrochemically active bottom surface.
The bottom part can include any of the corresponding
abovementioned features, in particular the features relating
to the composition, shape and dimensions of the bottom part.
The electrochemically active bottom surface of at
least one anode member can be joined to opposite bottom ends
of the tapered top part of the anode member. In this case,
the bottom surface can be generally planar or curved, in
particular convex.
A pair of adjacent anode members can have their
tapered top parts upwardly converging. Usually, the first
faces of the pair of anode members delimit an up-flow
through opening between the anode members of the pair and
the second faces of the pair of anode members delimit two
down-flow through openings on opposite sides of the pair of
anode members. The first faces of the pair of anode members
can be vertical or upwardly converging and the second faces
of the pair of anode members can be upwardly converging.
At least one of these first face and second face can
be generally planar and at least one of them can be curved,
in particular convex. Various combinations of such shapes
are described below.
As mentioned above, the height of the anode member
is usually of the same order as its width, for instance from
half to three times, in particular from equal to twice, the
width. The first and second faces of the tapered top part
may have an average slope in the range of 30 and 75 deg, in
particular 45 to 60 deg, to the horizontal.
In most embodiments, each anode member is elongated
and has a substantially constant cross-section along its
length. The anode members may be straight or arched or
circular. Alternatively, the anode members may have a
generally circular or quadratic or other polygonal base.
The average spacing between the anode members can be
of the same order as the height of the anode member bottom
part of each anode member, for instance from a quarter to
twice the height of the anode member. Usually, the average
spacing between two anode members is greater than about 5 to
10 mm. To avoid substantial reduction of the overall surface
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 9 -
area of the electrochemically active anode surfaces, active
bottom surfaces of the anode members should not be spaced by
more than about 20 to 30 mm.
Suitable anode materials for making the anode
members are disclosed above. Further anode materials are
disclosed in US Patents 6,077,415 (Duruz/de Nora), 6,113,758
(de Nora/Duruz), 6,248,227 (de Nora/Duruz), 6,372,099
(Duruz/de Nora) and W000/40783 (de Nora/Duruz). Suitable
electrochemically active anode coatings that can be
maintained in-situ are disclosed in US Patents 4,614,569
(Duruz/Derivaz/Debely/Adorian), 4,680,094 (Duruz), 4,683,037
(Duruz), 4,966,674 (Bannochie/Sheriff), 6,372,099 (Duruz/de
Nora) and PCT/IB02/01169 (de Nora/Nguyen), further suitable
electrochemically active coating are for example disclosed
in US Patents 6,103,090 (de Nora), 6,361,681 (de Nora/
Duruz), 6,365,01.8 (de Nora) and W099/36594 (de Nora/Duruz).
The invention also relates to an aluminium
production cell comprising an anode as described above and
to a method of electrowinning aluminium with such an anode.
The method of electrowinning aluminium comprises
passing an electrolysis current in a molten electrolyte
containing dissolved alumina between the anode a facing
cathode to evolve oxygen anodically and produce aluminium
cathodically. The anodically evolved oxygen drives an up-
flow of alumina-depleted electrolyte over the first faces of
the anode members of the anode, which up-flow promotes a
down-flow of alumina-rich electrolyte over the second faces
of the anode members of the anode.
Suitable additional features relating to the cell
and its operation are disclosed above.
Brief Descri~ation of the Drawings
The invention will now be described by way of
example with reference to the schematic drawings, wherein:
- Figures 1a and 1b show respectively a side
elevation and a plan view of an anode according to the
invention;
- Figures 2a and 2b show respectively a side
elevation and a plan view of another anode according to the
invention;
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 10 -
- Figure 3 shows an aluminium electrowinning cell.
operating with anodes according to the invention fitted with
electrolyte guide members;
- Figures 4, 5 and 6 are schematic views of parts of
aluminium electrowinning cells operating with anodes
according to the invention, Figure 4 illustrating
electrolyte circulation;
Figure 7 is a cross section of another anode
according to the invention with electrolyte guide members
only one of which is shown;
- Figure 8 shows a plan view of half of an assembly
of several electrolyte guide members like the one shown in
Figure 7;
- Figure 9 is a plan view of the anode shown
Figure 13 with half of an assembly of electrolyte guide
members as shown in Figure 8;
- Figure 10 is a plan view of a variation of the
anode of Figure 9; and
- Figures 11 to 14 are schematic views of parts of
aluminium electrowinning cells operating with anodes having
anode members with an asymmetric cross section.
Detailed Description
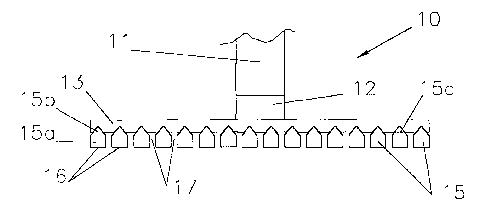
Figures 1a and 1b schematically show an anode 10 for
the electrowinning of aluminium according to the invention.
The anode 10 comprises a vertical current feeder 11
for connecting the anode to a positive bus bar, a transverse
member 12 and a pair of connecting cross-members 13 for
connecting a series of elongated straight anode members 15.
In accordance with the invention, the anode members
15 have a bottom part 15a which has a substantially
rectangular cross-section with a constant width over its
height and which is extended upwardly by a tapered top part
15b with a generally triangular cross-section. Each anode
member 15 has a flat electrochemically active lower oxide
surface 16 where oxygen is anodically evolved during cell
operation.
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 11 -
The anode members 15, in particular their bottom
parts 15a, are made of an alloy comprising nickel and/or
cobalt as electrically conductive inert structural metals)
and iron as an active diffusable~ metal that during
electrolysis slowly diffuses to the electrochemically active
bottom surface where it is oxidised for maintaining the
electrochemically active bottom surface and slowly dissolves
into the molten electrolyte.
The anode members 15 are in the form of parallel
rods in a coplanar arrangement, laterally spaced apart from
one another by inter-member gaps 17. The inter-member gaps
17 constitute flow-through openings for the circulation of
electrolyte and the escape of anodically-evolved gas
released at the electrochemically active surfaces 16.
The anode members 15 are connected by the pair of
connecting cross-members 13 which are in turn connected
together by the transverse member 12 on which the vertical
current feeder 11 is mounted. The current feeder 11, the
transverse member 12, the connecting cross-members 13 and
the anode members 15 are mechanically secured together by
welding, rivets or other means.
Each anode member 15 has two flats 15c at the
appropriate location in the tapered top part 15b for
securing the cross-members 13 thereon. For simplicity, only
one flat 15c is indicated in Fig. 1a
As described above, the electrochemically active
surface 16 of the anode members 15 can be iron-oxide based
in particular as described in greater detail in WO00/06803,
WO00/06804, W001/42534, W001/42536 and PCT/IB02/01241
mentioned above. Also, the anode may be covered with a
coating of one or more cerium compounds in particular cerium
oxyfluoride as for example disclosed in US Patents
4,614,569, 4,680,094, 4,683,037 and 4,966,6'74 also mentioned
above.
The transverse member 12 and the connecting cross-
members 13 are so designed and positioned over the anode
members 15 to provide a substantially even current
distribution through the anode members 15 to their
electrochemically active surfaces 16. The current feeder 11,
the transverse member 12 and the connecting cross-members 13
do not need to be electrochemically active and their surface
may passivate when exposed to electrolyte. However they
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 12 -
should be electrically well conductive to avoid unnecessary
voltage drops and should not substantially dissolve in
electrolyte.
When the anode members 15 and the transverse members
12 are exposed to different thermal expansion, each anode
member 15 may be made into two (or more where appropriate)
separate "short" anode members. The "short" anode members
should be longitudinally spaced apart when the thermal
expansion of the anode members 15 is greater than the
thermal expansion of the transverse members 12.
Alternatively, it may be advantageous in some cases,
in particular to enhance the uniformity of the current
distribution, to have more than two connecting cross-members
13 and/or a plurality of transverse members 12.
Also, it is not necessary for the two connecting
cross-members 13 to be perpendicular to the anode members 15
in a parallel configuration as shown in Figures 1a & 1b. The
connecting cross-members 13 may be in an X configuration in
which each connecting member 13 extends from one corner to
the opposite corner of a rectangular or square anode
structure, a vertical current feeder 11 being connected to
the intersection of the connecting members 13.
Figures 2a and 2b in which the same reference
numerals designate the same elements, schematically show a
variation of the anode 10 shown in Figures 1a and 1b.
Instead of having connecting cross-members 13, a
transverse member 12 and a current feeder 11 for
mechanically and electrically connecting the anode members
15 to a positive bus bar as illustrated in Figures 1a and
1b, the anode 10 shown in Figures 2a and 2b comprises a pair
of cast or profiled support members 14 fulfilling the same
function. Each cast support member 14 comprises a lower
horizontally extending foot 14a for electrically and
mechanically connecting the anode members 15, a stem 14b for
connecting the anode 10 to a positive bus bar and a pair of
lateral reinforcement flanges 14c between the horizontally
extending foot 14a and stem 14b.
The anode members 15 may be secured by force-fitting
or welding the horizontally extending foot 14a on the flats
15c of the anode members 15. As an alternative, the shape of
the anode members 15 and corresponding receiving slots in
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 13 -
the horizontally extending foot 14a may be such as to allow
only longitudinal movements of the anode members. For
instance the anode members 15 and the horizontally extending
foot 14a may be connected by dovetail joints.
Figure 3 in which the same numeral references
designate the same elements, shows an aluminium
electrowinning cell according to the invention having a
series of anodes 10 which are similar to those shown in
Figures 1a and 1b, immersed in an electrolyte 30. The anodes
10 face a cathode cell bottom 20 connected to a negative
bulbar by current conductor bars 21. The cathode cell bottom
is made of conductive material such as graphite or other
carbonaceous material coated with an aluminium-wettable
refractory cathodic coating 22 on which aluminium 35 is
15 produced and from which it drains or on which it forms a
shallow pool, a deep pool or a stabilised pool. The molten
produced aluminium 35 is spaced apart from the facing anodes
10 by an inter-electrode gap.
Pairs of anodes 10 are connected to a positive bus
20 bar through a primary vertical current feeder 11' and a
horizontal current distributor 11" connected at both of its
ends to an anode 10 through a secondary vertical current
distributor 11"'.
The secondary vertical current distributor 11"' is
mounted on the anode structure 12,13,15, on a transverse
member 12 which is in turn connected to a pair of connecting
cross-members 13 for connecting a series of anode members
15 . The current feeders 11' , 11 ", 11 "', the transverse member
12, the connecting cross-members 13 and the anode members 15
are mechanically secured together by welding, rivets or
other means.
The anode members 15 have an electrochemically
active lower surface 16 on which during cell operation
oxygen is anodically evolved. The anode members 15 are in
the form of parallel rods in a foraminate coplanar
arrangement, laterally spaced apart from one another by
inter-member gaps 17. The inter-member gaps 17 constitute
flow-through openings for the circulation of electrolyte and
the escape of anodically-evolved gas from the
electrochemically active surfaces 16.
The iron oxide surface may extend over all immersed
parts 11 "',12,13,15 of the anode 10, in particular over the
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 14 -
immersed part of the secondary vertical current distributor
11 "' which is preferably covered with iron oxide at least up
to 10 cm above the surface of the electrolyte 30.
The immersed but inactive parts of the anode 10 may
be further coated with zinc oxide. However, when parts of
the anode 10 are covered with zinc oxide, the concentration
of dissolved alumina in the electrolyte 30 should be
maintained at or close to saturation to prevent excessive
dissolution of zinc oxide in the electrolyte 30.
The core of the inactive anode components
11', 11 ", 11 "', 12, 13 is preferably highly conductive and can
be made of copper protected with successive layers of
nickel, chromium, nickel, copper and optionally a further
layer of nickel.
The anodes 10 are further fitted with means for
enhancing dissolution of fed alumina in the form of
electrolyte guide members 5 formed of parallel spaced-apart
inclined baffles 5 located above and adjacent to the
foraminate anode structure 12,13,15. The baffles 5 provide
upper downwardly converging surfaces 6 and lower upwardly
converging surfaces 7 that deflect gaseous oxygen which is
anodically produced below the electrochemically active
surface 16 of the anode members 15 and which escapes between
the inter-member gaps 17 through the foraminate anode
structure 12,13,15. The oxygen released above the baffles 5
promotes dissolution of alumina fed into the electrolyte 30
above the downwardly converging surfaces 6.
The aluminium-wettable cathodic coating 22 of the
cell shown in Figure 3 can advantageously be a slurry-
applied refractory hard metal coating as disclosed in
W001/42531 (Nguyen/Duruz/de Nora), W001/42168 (de
Nora/Duruz), W001/42531 (Nguyen/Duruz/de Nora) and
PCT/IB02/01932 (Nguyen/de Nora).
The cell also comprises sidewalk 25 of carbonaceous
or other material. The sidewalls 25 are coated/impregnated
above the surface of the electrolyte 30 with a boron or a
phosphate protective coating/impregnation 26 as described in
US Patent 5,486,278 (Manganiello/Duruz/Bello).
Below the surface of the electrolyte 30 the
sidewalk 25 are coated with a highly aluminium-wettable
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 15 -
coating 23, for example as disclosed in W001/42531,
W001/42168 and PCT/IB02/01932 mentioned above, so that
molten aluminium 35 driven by capillarity and magneto-
hydrodynamic forces covers and protects the sidewalls 25
from the electrolyte 35. The aluminium-wettable coating 23
extends from the aluminium-wettable cathodic coating 22 over
the surface of connecting corner prisms 28 up the sidewalls
25 at least to the surface of the electrolyte 30. The
aluminium-wettable side coating 23 may be advantageously
made of an applied and dried and/or heat treated slurry of
particulate TiB2 in colloidal silica which is highly
aluminium-wettable.
The sidewalk 25 and cathode bottom 20 may also be
shielded. from the electrolyte 30 by an aluminium-wettable
openly porous lining (not shown), as disclosed in
PCT/IB02/00668, PCT/IB02/00670, PCT/IB02/01883 and
PCT/IB02/01884 (all in the name of de Nora) filled with
molten aluminium.
Alternatively, above and below the surface of the
electrolyte 30, the sidewalls 25 may be covered with a zinc-
based coating, such as a zinc-oxide coating optionally with
alumina or a zinc aluminate coating. When a zinc-based
coating is used to coat sidewalls 25 or anodes 10 as
described above, the concentration of dissolved alumina in
the molten electrolyte 30 should be maintained at of close
to saturation to substantially prevent dissolution of such a
coating.
In a further alternative, the cell may be operated
with a conventional frozen electrolyte ledge covering and
protecting the sidewalk 25.
During cell operation, alumina is fed to the
electrolyte 30 all over the baffles 5 and the metallic anode
structure 12,13,15. The fed alumina is dissolved and
distributed from the bottom end of the converging surfaces 6
into the inter-electrode gap through the inter-member gaps
17 and around edges of the metallic anode structure
12,13,15, i.e. between neighbouring pairs of anodes 10 or
between peripheral anodes 10 and sidewalk 25. By passing an
electric current between anodes 10 and facing cathode cell
bottom 20 oxygen is evolved on the electrochemically active
anode surfaces 16 and aluminium is produced which is
incorporated into the cathodic molten aluminium 35. The
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 16 -
oxygen evolved from the active surfaces 16 escapes through
the inter-member gaps 17 and is deflected by the upwardly
converging surfaces 7 of baffles 5. The oxygen escapes from
the uppermost ends of the upwardly converging surfaces 7
enhancing dissolution of the alumina fed over the downwardly
converging surfaces 6.
The aluminium electrowinning cells partly shown in
Figures 4, 5 and 6 in which the same numeral references
designate the same elements, are similar to the aluminium
electrowinning cell shown in Figure 3.
In Figure 4 the guide members are inclined baffles 5
as shown in Figure 3. In this example the uppermost end of
each baffle 5 is located just above mid-height between the
surface of the electrolyte 30 and the transverse connecting
members 13.
Also shown in Fig. 4, an electrolyte circulation 31
is generated by the escape of gas released from the active
surfaces 16 of the anode members 15 between the inter-member
gaps 17 and which is deflected by the upward converging
surfaces 7 of the baffles 5 confining the gas and the
electrolyte flow between their uppermost edges. From the
uppermost edges of the baffles 5, the anodically evolved gas
escapes towards the surface of the electrolyte 30, whereas
the electrolyte circulation 31 flows down through the
downward converging surfaces 6, through the inter-member
gaps and around edges of the metallic anode structure
12,13,15 to compensate the depression created by the
anodically released gas below the active surfaces 17 of the
anode members 15. The electrolyte circulation 31 draws down
into the inter-electrode gap dissolving alumina particles 32
which are fed above the downward converging surfaces 6.
Figure 5 shows part of an aluminium electrowinning
cell operating with an anode 10 according to the invention
having electrochemically active members 15 with a rounded
tapered upper part 15b having a semi-circular cross-section.
The anode 10 is covered with baffles 5 operating as
electrolyte guide members like those shown in cell of Figure
4 but whose surfaces are only partly converging. The lower
sections 4 of the baffles 5 are vertical and parallel to one
another, whereas their upper sections have upward and
downward converging surfaces 6,7. The uppermost end of the
baffles 5 are located below but close to the surface of the
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 17 -
electrolyte 30 to increase the turbulence at the electrolyte
surface caused by the release of anodically evolved gas.
Figure 6 shows a variation of the anode members
baffles shown in Figure 5, wherein the anode members 15 have
a rounded tapered upper part 15b with an elliptic cross-
section and the baffles 5 have their parallel vertical
sections 4 located above their converging surfaces 6,7.
By guiding and confining anodically-evolved oxygen
towards the surface of the electrolyte 30 with baffles or
other confinement means as shown in Figures 5 and 6 and as
further described in WO00/40781 (de Nora), oxygen is
released so close to the surface as to created turbulences
above the downwardly converging surfaces 6, promoting
dissolution of alumina fed thereabove.
It is understood that the electrolyte confinement
members 5 shown in Figures 3, 4, 5 and 6 can either be
elongated baffles, or instead consist of a series of
vertical chimneys of funnels of circular or polygonal cross-
section, for instance as described below.
Figures 7 and 9 where the same numeral references
designate the same elements, illustrate an anode 10' having
a circular bottom, the anode 10' being shown in cross-
section in Figure 7 and from above in Figure 9. On the right
hand side of Figures 7 and 9 the anode 10' is shown with
electrolyte guide members 5' according to the invention. The
electrolyte guide members 5' represented in Figure 9 are
shown separately in Figure 8.
The anode 10 ' shown in Figures 7 and 9 has several
concentric circular anode members 15. The anode members 15
are laterally spaced apart from one another by inter-member
gaps 17 and connected together by radial connecting cross-
members in the form of flanges 13 which join an outer ring
13'. The outer ring 13' extends vertically from the
outermost anode members 15, as shown in Figure 7, to form
with the radial flanges 13 a wheel-like structure 13,13',
shown. in Figure 9, which secures the anode members 15 to a
central anode current feeder 11.
As shown in Figure 7, the innermost circular anode
member 15 partly merges with the current feeder 12, with
ducts 18 extending between the innermost circular anode
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 18 -
member 15 and the current feeder 11 to permit the escape of
oxygen produced underneath the central current feeder 11.
Each electrolyte guide member 5' is in the general
shape of a funnel having a wide bottom opening 9 for
receiving anodically produced oxygen and a narrow top
opening 8 where the oxygen is released to promote
dissolution of alumina fed above the electrolyte guide
member 5'. The inner surface 7 of the electrolyte guide
member 5' is arranged to canalise and promote an upward
electrolyte flow driven by anodically produced oxygen. The
outer surface 6 of the electrolyte guide member 5' is
arranged to promote dissolution of alumina fed thereabove
and guide alumina-rich electrolyte down to the inter
electrode gap, the electrolyte flowing mainly around the
foraminate structure.
As shown in Figures 8 and 9, the electrolyte guide
members 5' are in a circular arrangement, only half of the
arrangement being shown. The electrolyte guide members 5'
are laterally secured to one another by attachments 3 and so
arranged to be held above the anode members 15, the
attachments 3 being for example placed on the flanges 13 as
shown in Figure 9 or secured as required. Each electrolyte
guide member 5' is positioned in. a circular sector defined
by two neighbouring radial flanges 13 and an arc of the
outer ring 13' as shown in Figure 9.
The arrangement of the electrolyte guide members 5'
and the anode 10' can be moulded as units. This offers the
advantage of avoiding mechanical joints and the risk of
altering the properties of the materials of the electrolyte
guide members 5' or the anode 10' by welding.
Figure 10 where the same numeral references
designate the same elements, illustrates a square anode 10'
as a variation of the round anode 10' of Figures 7 and 9.
The anode 10' of Figure 10 has generally rectangular
concentric parallel anode members 15 with rounded corners.
The anode 10' shown in Figure 10 can be fitted with
electrolyte guide members similar to those of Figures 7 to 9
but in a corresponding rectangular arrangement.
Figures 11 to 14 in which the same reference
numerals designate the same elements, show anodes 10
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 19 -
according to the invention having anode members 15,15' which
are asymmetric in vertical cross-section. The anode members
15,15' are arranged in pairs with their tapered upper parts
15b upwardly converging. More specifically, the tapered
upper parts 15b have faces 15d',15e' for guiding an up-flow
of alumina-depleted electrolyte indicated by arrows 31' in
an up-flow through opening 17' and faces 15d ",15e " for
guiding a down-flow of alumina-rich electrolyte indicated by
arrows 31 " in a down-flow through opening 17" between
adjacent pairs of anode members 15,15' and around the
outermost anode members 15,15' of the anodes 10.
In Figures 11 and 13 faces 15d',15d" are planar and
inclined, whereas in Figure 12 these faces 15e',15e " are
convex. This applies also to the second pair of anode
members 15 starting from the left of Fig. 14. The remaining
anode members 15 shown in Fig. 14 have one planar face 15d'
and one convex face 15e ".
On the left-hand side of Figs l1 and 12, each anode
member 15 comprises a bottom part 15a which has a constant
width over its height and which is extended upwardly by the
tapered top part 15b that is integral with the bottom part
15a. The bottom part 15a is made of a metal alloy with a
substantially flat oxide bottom surface which forms the
electrochemically active surface 16. The metal alloy can
comprise an electrically conductive inert structural metal
and an active diffusable metal that during electrolysis
slowly diffuses to the electrochemically active bottom
surface 16 where it is oxidised for maintaining the
electrochemically active bottom surface and slowly dissolves
into the molten electrolyte 30. According to the invention,
the bottom part forms a long-lasting supply of the active
metal diffusable to the electrochemically active bottom
surface 16.
On the right-hand side of Figs. 11 and 12, the
electrochemically active bottom surface 16 of each anode
member 15' is joined to opposite bottom ends of the tapered
top part of the anode member 15'.
Such an anode member design can also be appropriate
when the anode members are made of materials that are
inhibited from dissolving in the molten electrolyte 30 under
CA 02450071 2003-12-08
WO 03/006716 PCT/IB02/02732
- 20 -
the cell operating conditions, for example when the anodes
are coated with an in-situ maintained cerium oxyfluoride-
based coating as disclosed in US Patents 4,614,569
(DuruzlDerivaz/Debely/Adorian), 4,680,094 (Duruz), 4,683,037
(Duruz), 4,966,674 (Bannochie/Sheriff), 6,372,099 (Duruz/de
Nora) and PCT/IB02/01169 (de Nora/Nguyen), or when the
anodes are covered with another electrochemically active
coating as for example disclosed in US Patents 6,103,090 (de
Nora), 6,362,681 (de Nora/Duruz), 6,365,018 (de Nora) and
W099/36594 (de Nora/Duruz). Further suitable anode materials
are disclosed in US Patents 6,077,415 (Duruz/de Nora),
6,113,758 (de Nora/Duruz), 6,248,227 (de Nora/Duruz),
6,372,099 (Duruz/de Nora) and WO00/40783 (de Nora/Duruz).
Figures 13 and 14 show further anodes 10 with anode
members 15 illustrating different asymmetric profiles
(cross-sections). The anode members 15 have a bottom part
15a which has a constant width over its height and which is
extended upwardly by a tapered top part 15b.
In Figs. 13 and 14 the anode members 15 have
vertical planar faces 15d' (except the second pair of anode
members 15 starting from the left of Fig. 14 whose faces
15e' are convex) for guiding an up-flow of electrolyte 30
(indicated by arrows 31' ) . The inclined faces 15d",15e" for
guiding a down-flow of electrolyte 30 (indicated by arrows
31"), are planar in Fig. 13 and convex in Fig. 14.
On the left-hand side of Figs. 13 and 14 the bottom
part 15a of each anode member 15 extends vertically below
the tapered top parts 15b, whereas on the right-hand. side of
Figs. 13 and 14, the bottom part 15a of each anode member 15
extends below the tapered top parts 15b along an inclined
direction in continuation of faces 15d ",15e".
In variations of the anode .members 15,15' shown in
Figs. 11 to 14, some or all faces 15d',15d ",15e',15e " can be
made concave.