Note: Descriptions are shown in the official language in which they were submitted.
CA 02453123 2007-10-22
Specification
INTERMEDIATE FOR CONDENSED BENZAZEPINE DERIVATIVES
TECHNICAL FIELD
This invention relates to novel aromatic and
heterocyclic ring-condensed benzazepine derivatives which
are useful as arginine vasopressin antagonists, to salts
thereof, to pharmaceutical preparations which contain
these compounds as an active ingredient and to
intermediates which are useful for the synthesis of these
compounds.
BACKGROUND ART
Arginine vasopressin (AVP) is a peptide which
consists of 9 amino acid residues and is synthesized and
secreted in the hypothalamo-neurohypophyseal system. As
antagonists of the arginine vasopressin, peptide type
compounds and non-peptide type compounds have been
synthesized. For example, a compound disclosed in JP-A-2-
32098 is known as the peptide type compound (the term "JP-
A" as used herein means an "unexamined published Japanese
patent application"). On the other hand, 2,3,4,5-
tetrahydro-IH-benzazepine derivatives represented by the
following general formula have been disclosed in EP-A-
0514667 and JP-A-5-132466 as non-peptide type vasopressin
antagonists.
- 1 - '
i ~
CA 02453123 2004-01-20
R' R
Q 1V
R' I
C0
R '
R3
(As for symbols in the above formula, see aforementioned
patent publications.)
Also, International Patent Publication No.
91/05549 disclosing the compound represnted by the
following general formula, and 2,3,4,5-tetrahydro-lH-
benzodiazepine derivatives and 2,3,4,5-tetrahydro-lH-l-
benzazepine derivatives disclosed in JP-A-4-154765 are
known.
W
N
R' 4
C=
R2
:R 3
(As for symbols in the above formula, see aforementioned
patent publications.)
Although various studies have been made as
described above, creation of novel arginine vasopressin
- 2 -
CA 02453123 2004-01-20
antagonists having more excellent profiles is still now an
important clinical object.
On the other hand, almost no compound is known as
a compound having a nitrogen-containing aromatic 5-
membered ring-condensed benzazepine skeleton, which is the
basic structure of the compound of the present invention,
and only processes for the synthesis of a few compounds
having such a ring structure have been reported in J.
Chem. Soc., Perkin Trans. 1 (1978) No. 8, 862-70 and Orc{.
Prep. Proced. Int., 25 (5), 602-6 (1993), but their
structures are clearly different from the structure of the
compound of the present invention. In addition, use of
these compounds as pharmaceutical preparati ns have not
been known.
DISCLOSURE OF THE INVENTION
The inventors of the present invention have
conducted extensive studies on compounds having arginine
vasopressin antagonism and accomplished the present
invention based on the finding that a novel aromatic and
heterocyclic ring-condensed benzazepine derivative
represented by the following general formula (I) shows
unexpectedly excellent arginine vasopressin antagonism.
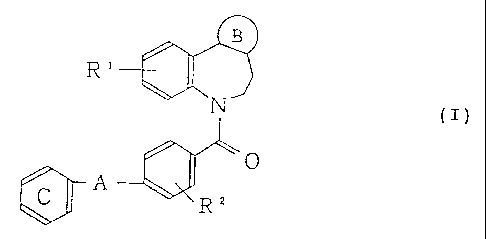
Accordingly, the present invention relates to a
nitrogen-containing aromatic 5-membered ring-condensed
_ 8 -
CA 02453123 2004-01-20
benzazepine derivative represented by the following
formula (I) and a salt thereof.
B
R'
N (~)
0
A
R2
(Symbols in the formula have the following meanings;
ring B: a nitrogen-containing aromatic 5-membered ring
having at least 1 nitrogen atom and optionally one oxygen
or sulfur atom, which may optionally have substituent(s),
R1, R2: these may be the same or different from each other
and each represents a hydrogen atom, a halogen atom, a
lower alkyl group, an amino group which may optionally be
substituted by lower alkyl group(s), a lower alkoxy group,
A: a single bond; a group represented by the formula
NHCO-(CR3R4)n ,
n: 0 or an integer of from 1 to 3,
R3, R4: these may be the same or different from each other
and each represents a hydrogen atom, a lower alkyl group
(provided that R3 and R4 may together form a lower
alkylene group having 2 to 7 carbon atoms), and
ring C: a benzene ring which may optionally have
substituent(s).)
_ 4 _
CA 02453123 2004-01-20
Further, the particularly preferable compound is
the nitrogen-containing aromatic 5-membered ring-condensed
benzazepine derivative (I) or a salt thereof wherein
i) the ring B is a ring represented by the formula:
XZ
z '*'~
X 9-Z3
(symbols in the formula have the following meanings;
X1, X3: one of them is a group represented by the formula
=N-, and the other is a group represented by the formula
-NR5-, -0-, -S- or =N-,
X2: a group represented by the formula =CR6-, -0-, -S- or
=N-,
R5: a.hydrogen atom, a lower alkyl group, and
R6:
a) a hydrogen atom,
b) a lower alkyl, lower alkenyl or lower=alkynyl
group, which is-unsubstituted or substituted by the
following groups,
an amino group; a mono or di lower alkylamino
group; a lower alkanoylamino group substituted by
an amino group or a mono or di lower alkylamino
group; a protected amino group; a 1-pyrrolidinyl
group; a piperidino group; a morpholino group; a
1-piperazi.nyl, 1-imidazolidinyl, 1-homopiperazinyl
_ 5 _
CA 02453123 2004-01-20
or 1-pyrazolidinyl group, which may optionally be
substituted by a lower alkyl group at the nitrogen
atom of the ring; a guanidino group; an amidino
group; a hydroxyl group; a lower alkoxyl group; a
cyano group; a carbamoyl group; a carboxyl group;
a lower alkoxycarbonyl group; a lower alkanoyloxy
group; or a phenyl, imidazolyl, pyridyl,
pyrazinyl, pyrimidinyl, pyridazinyl, pyrazolyl,
pyrrolyl, tetrazolyl, triazolyl, thiazolyl or
oxazolyl group, which may optionally be
substituted by a lower alkyl group, a halogen
atom, a lower alkoxyl group, an amino group, a
mono or di lower alkylamino group, a hydroxyl
group or a carboxyl group,
c) a cycloalkyl group having 3 to 8 carbon atoms,
d) an amino group; an amino group mono- or di-
substituted by a lower alkyl group, a lower alkenyl group,
a lower alkynyl group or a lower alkanoyl group (these
groups may further be substituted by an amino group; a
mono or di lower alkylamino group; a 1-pyrrolidinyl group;
a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
which may optionally be substituted by a lower alkyl group
at the nitrogen atom of the ring); a 1-pyrrolidinyl group;
a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
- 6 -
CA 02453123 2004-01-20
which may optionally be substituted by a lower alkyl
group,
e) a guanidino group, an amidino group, or
f) a hydroxyl group, a lower alkoxyl group, a
mercapto group, a lower alkylthio group), and
ii) the ring C is a benzene ring which may optionally
have 1 to 5 substituents respectively selected from
a) a lower alkyl, lower alkenyl or lower alkynyl
group, which may optionally be substituted by a halogen
atom or a hydroxyl group,
b) a lower alkoxy group which may optionally be
substituted by a halogen atom, a cyano group, a hydroxyl
group, a carboxyl group, a lower alkoxycarbonyl group, a
lower alkanoyl group, a lower alkanoyloxy group, a
carbamoyl group, a lower alkylaminoca,rbonyl group or a
phthalimido group; a hydroxyl group; a mercapto group; or
a lower alkylthio group,
c) a halogen atom; a cyano group,
d) a carboxyl group; a lower alkoxycarbonyl group;
a lower alkanoyl group; a lower alkanoyloxy group; a
carbamoyl group; a lower alkylaminocarbonyl group,
e) an amino group; a mono or di lower alkylamino
group; a lower alkanoylamino group; a 1-pyrrolidinyl
group; a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
-
- 7
CA 02453123 2004-01-20
which may optionally be substituted by a lower alkyl group
at the nitrogen atom of the ring,
f) a cycloalkyl group,
g) a phenyl group which may optionally be
substituted by a lower alkyl group, a lower alkenyl group,
a lower alkynyl group, a halogen atom, a lower alkoxy
group, an amino group, a mono or di lower alkylamino
group, a hydroxyl group or a carboxyl group, and
h) an imidazolyl, triazolyl, tetrazolyl, pyrrolyl,
pyridyl, pyrazinyl or pyrimidinyl group, which may
optionally be substituted by a lower alkyl group, a
cycloalkyl group or a phenyl group.
The present invention also relates to a
pharmaceutical composition, especially an arginine
vasopressin antagonist, which contains the above nitrogen-
containing aromatic 5-membered ring-condensed benzazepine
derivative or a salt thereof as an active ingredient.
Moreover, the present invention also relates to
(biphenyl-2-ylcarboxamide)benzoic acid which is useful as
an intermediate for the synthesis of the above nitrogen-
containing aromatic 5-membered ring-condensed benzazepine
derivative.
Chemical structure of the compound of the present
invention is characterized in that its basic structure is
a nitrogen-containing aromatic 5-membered ring-condensed
benzazepine ring to which a substituted or unsubstituted
- ~ -
CA 02453123 2004-01-20
biphenylcarbonyl group, a substituted or unsubstituted
benzoylaminobenzoyl group or a substituted or
unsubstituted phenylalkanoylaminobenzoyl group has been
linked. The compound of the present invention having such
a basic structure has excellent arginine vasopressin
antagonism, is excellent in oral absorption and shows
prvper prolonged action because of its stability to
metabolism in the living body.
The following describes the compound of the
present invention in detail.
With regard to the nitrogen-containing aromatic 5-
membered ring moiety of the="nitrogen-containing aromatic
5-membered ring having at least 1 nitrogen atom and
optionally one oxygen or sulfur atom, which may optionally
have substituent(s)" as the ring B of the compound of the
present invention represented by the formula (I), a
pyrrole ring, a pyrazole ring, an imidazole ring, a
triazole ring, an isoxazole ring, an oxazole ring, an
isothiazole ring, a thiazole ring, an oxadiazole ring, a
thiadiazole ring and the like may be exemplified. Each of
these rings may optionally have substituent(s) which will
be described later and is condensed with a benzazepine
ring through its adjacent two ring-forming atoms.
Particularly, as the nitrogen-containing aromatic
5-membered ring moiety of the ring B, a nitrogen-
containing aromatic 5-membered ring represented by
- 9 -
CA 02453123 2004-01-20
N~NH HN 'N N0 p
iS JN
H
~i
EN~ N~ ~N NH
0 S
Ni ~N NTi ~N 0~ N N~ s
SNN~0
or is preferable, a nitrogen-containing aromatic 5-membered
ring represented by
N4_~_ NH EN--~N
N 15~ o aN N 155_~ S S~N
or
is more preferable, and a nitrogen-containing aromatic 5-
membered ring represented by
- 10 -
CA 02453123 2004-01-20
N~hH iiN~~N N NS
or
~
is most preferable.
In these rings, a hydrogen atom on the ring-
forming carbon or nitrogen atom may optionally be a
substituent described in the following.
The substituent to be located on the nitrogen-
containing aromatic 5-membered ring of the ring B or on
the benzene ring of the ring C may be selected from those
which are conventionally used in the art as substituents
on aromatic heterocyclic rings or a benzene ring. The
nitrogen-containing aromatic 5-membered ring of the ring B
may optionally have 1 to 2 substituents, and the benzene
ring of the ring C may optionally=have 1 to 5 (preferably
1 to 3) substituents. Preferably, the substituent on the
benzene ring of the ring C may be located at the o (ortho)
position. Examples of these substituents include a
substituted or unsubstituted alkyl, alkenyl or alkynyl
group, a substituted or unsubstituted cycloalkyl or
cycloalkenyl group, a substituted or unsubstituted aryl
group and a substituted or unsubstituted saturated or
unsaturated heterocyclic group, as well as a halogen atom,
a hydroxyl group, an alkoxyl group, a substituted alkoxyl
group, an alkenyloxy group, an alkynyloxy group, a
cycloalkyloxy group, a cycloalkenyloxy group, an aryloxy
- 11 -
CA 02453123 2004-01-20
group, an aralkyloxy group, an aralkenyloxy or
aralkynyloxy group, a mercapto group, an alkylthio group,
an alkenylthio group, an alkynylthio group, a
cycloalkylthio group, a cycloalkenylthio group, an
arylthio group, an aralkylthio group, an aralkenylthio or
aralkynylthio group, an alkoxycarbonyl group, an
alkenyloxycarbonyl group, an alkynyloxycarbonyl group, a
cycloalkyloxycarbonyl group, a cycloalkenyloxycarbonyl
group, an aryloxycarbonyl group, an aralkyloxycarbonyl
group, an aralkenyloxycarbonyl or aralkynyloxycarbonyl
group, an alkylaminocarbonyl group, an aliphatic or
aromatic acyl or acyloxy group, a carbamoyl group, a
carboxyl group, a sulfone group, an oxo group, a thioxo
group, a cyano group, a nitro group, an amino group, a
mono- or di-substituted amino group, a guanidino group, an
amidino group and a substituted or unsubstituted imino
group. In addition to these groups, a divalent group
which is substituted or not substituted, may contain
hetero atoms (for example, 1 to 3 nitrogen, oxygen and/or
sulfur atoms), and forms a condensed ring with the benzene
ring through its-binding to the adjacent carbon atoms of
the benzene ring, such as a lower alkylene group, a lower
alkenylene group, a lower alkynylene group or a lower
alkylenedioxy group,- may be used as the substituent for
the benzene ring.
- 12 -
CA 02453123 2004-01-20
Examples of the substituents of "substituted alkyl
group", "substituted alkenyl group" and " substituted
alkynyl group" as the aforementioned substituents of the
nitrogen-containing aromatic 5-membered ring or the
benzene ring include a cycloalkyl group, a cycloalkenyl
group, a substituted or unsubstituted aryl group, a
substituted or unsubstituted saturated or unsaturated
heterocyclic group, a halogen atom, a hydroxyl group, an
alkoxyl group, an alkenyloxy group, an alkynyloxy group, a
cycloalkyloxy group, a cycloalkenyloxy group, an aryloxy
group, an aralkyloxy group, an aralkenyloxy or
aralkynyloxy group, a mercapto group, an alkylthio group,
an alkenylthio group, an alkynylthio group, a
cycloalkylthio group, a cycloalkenylthio group, an
arylthio group, an aralkylthio group, an aralkenylthio or
aralkynylthio group, an alkoxycarbonyl group, an
alkenyloxycarbonyl group, an alkynyloxycarbonyl group, a
cycloalkyloxycarbonyl group, a cycloalkenyloxycarbonyl
group, an aryloxycarbonyl group, an aralkyloxycarbonyl
group, an aralkenyloxycarbonyl or aralkynyloxycarbonyl
group, an alkylaminocarbonyl group, an aliphatic or
aromatic acyl or acyloxy group, a carboxyl group, a
suifone group, an oxo group, a thioxo group, a carbamoyl
group, a cyano group, a nitro group, an amino group, a
mono- or di-substituted amino group, a protected amino
- 13 -
CA 02453123 2004-01-20
group, a guanidino group, an amidino group and a
substituted or unsubstituted imino group.
Examples of the substituents of the aforementioned
"substituted alkoxy group" include a halogen atom, a cyano
group, a hydroxyl group, a carboxyl group, a lower
alkoxycarbonyl group, a lower alkanoyl group, a lower
alkanoyloxy group, a carbamoyl group, a lower
alkylaminocarbonyl group, a phthalimido group and the
like.
Examples of the substituents of the "substituted
cycloalkyl or cycloalkenyl group" include a lower alkyl
group, a lower alkenyl group, a lower alkynyl group, a
lower alkoxy group, a lower alkanoyl group, a lower
alkanoyloxy group, a lower alkoxycarbonyl group, an amino
group, a mono or di lower alkylamino group, a hydroxyl
group, a carboxyl group, a carbamoyl group and the like.
Examples of the substituents of the "substituted
aryl group" include a lower alkyl group, a lower alkenyl
group, a lower alkynyl group, a halogen atom, a lower
alkoxyl group, an amino group, a mono or di lower
alkylamino group, a hydroxyl group, a carboxyl group and
the like.
The "substituted saturated or unsaturated
heterocyclic group" may preferably be a nitrogen-
containing heterocyclic ring, more preferably a nitrogen-
containing aromatic 5- or 6-membered ring (most preferably
- 14 -
CA 02453123 2004-01-20
an imidazolyl group, a pyridyl group, a pyrazinyl group, a
pyrimidinyl group, a pyridazinyl group, a pyrazolyl group,
a pyrrolyl group, a tetrazolyl group, a triazolyl group, a
thiazolyl group or an oxazolyl group) and a nitrogen-
containing saturated 4- to 7-membered ring (most
preferably a pyrrolidinyl group, a piperidyl group, a
morpholinyl group, a piperazinyl group, an imidazolidinyl
group, a homopiperazinyl group or a pyrazolidinyl group).
Examples of their substituents include a lower alkyl
group, a cycloalkyl group, a-phenyl group, a halogen atom,
a lower alkoxyl group, an amino group, a mono or di lower
alkylamino group, a hydroxyl group, a carboxyl group and
the like.
Examples of the substituents of the "mono- or di-
substituted amino group" include a lower alkyl group, a
lower alkenyl group, a lower alkynyl group, a lower
alkanoyl group and the like, and these groups may
optionally be further substituted by the following groups:
an amino group; a mono or di lower alkylamino
group; a 1-pyrrolidinyl group; a piperidino group;
a morpholino group; and a 1-piperazinyl, 1-
imidazolidinyl or 1-homopiperazinyl group which
may optionally be substituted by a lower alkyl
group at the nitrogen atom of the ring.
- 15 -
CA 02453123 2004-01-20
Examples of the substituents of the "substituted
imino group" include an alkyl group, an aryl group, an
aralkyl group and the like.
Of the aforementioned substituents on the
nitrogen-containing aromatic 5-membered ring of the ring B
or the benzene ring of the ring C, substituents to be
located on carbon atoms of the ring B may preferably be
a) a lower alkyl, lower alkenyl or lower alkynyl
group, which is unsubstituted or substituted by the
following groups,
an amino group; a mono or di lower alkylamino
group; a lower alkanoylamino group substituted by
an amino group or a mono or di lower alkylamino
group; a protected amino group; a 1-pyrrolidinyl
group; a piperidino group; a morpholino group; a
1-piperazinyl, 1-imidazolidinyl, 1-homopiperazinyl
or 1-pyrazolidinyl group, which may optionally be
substituted by a lower alkyl group at the nitrogen
atom of the ring; a guanidino group; an amidino
group; a hydroxyl group; a lower alkoxyl group; a
cyano group; a carbamoyl group; a carboxyl group;
a lower alkoxycarbonyl group; a lower alkanoyloxy
group; and a phenyl, imidazolyl, pyridyl,
pyrazinyl, pyrimidinyi.; pyridazinyl, pyrazolyl,
pyrrolyl, tetrazolyl, triazolyl, thiazolyl or
oxazolyl group, which may optionally be
- 16 -
CA 02453123 2004-01-20
substituted by a lower alkyl group, a halogen
atom, a lower alkoxyl group, an amino group, a
mono or di lower alkylamino group, a hydroxyl
group or a carboxyl group,
b) a cycloalkyl group having 3 to 8 carbon atoms,
c) an amino group; an amino group mono- or di-
substituted by a lower alkyl group, a lower alkenyl group,
a lower alkynyl group or a lower alkanoyl group (these
groups may further be substituted by an amino group; a
mono or di lower alkylamino group; a 1-pyrrolidinyl group;
a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
which may optionally be substituted by a lower alkyl group
at the nitrogen atom of the ring); a 1-pyrrolidinyl group;
a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
which may optionally be substituted by a lower alkyl group
at the nitrogen atom of the ring,
d) a guanidino group, an amidino group, or
e) a hydroxyl group, a lower alkoxyl group, a
mercapto group, a lower alkylthio group,
more preferably
a) a lower alkyl group which is unsubstituted or
substituted by the following groups,
an amino group; a mono or di lower alkylamino
group; a lower alkanoylamino group substituted by
- 17 -
CA 02453123 2004-01-20
an amino group or a mono or di lower alkylamino
group; a 1-pyrrolidinyl group; a piperidino group;
a morpholino group; a 1-piperazinyl group which
may optionally be substituted by a lower alkyl
group at the nitrogen atom of the ring; a
guanidino group; an amidino group; or a phenyl,
imidazolyl, pyridyl, pyrazinyl, pyrimidinyl,
pyridazinyl, pyrazolyl, pyrrolyl, tetrazolyl or
triazolyl group, which may optionally be
substituted by a lower alkyl group,
b) a cycloalkyl group having 3 to 8 carbon atoms,
c) an amino group; an amino group mono- or di-
substituted by a lower alkyl group or a lower alkanoyl
group (these groups may further be substituted by an amino
group or a mono or di lower alkylamino group), or
d) a guanidino group, an amidino group,
most preferably
a) a lower alkyl group which is unsubstituted or
substituted by the following groups,
an amino group; a mono or di lower alkylamino
group; a morpholino group; an imidazolyl group
which may optionally be substituted by a phenyl
group or a lower alkyl group; or a pyridyl group,
b) a cyclopropyl group,
_
_ 18
CA 02453123 2004-01-20
c) an amino group; a dimethylamino-substituted
lower alkylamino group; or an amino lower alkanoylamino
group, or
d) a guanidino group.
A lower alkyl group is particularly preferred as
the substituent on the nitrogen atom of the ring B.
5ubstituents to be located on the benzene ring of
the ring C may preferably be
a) a lower alkyl, lower alkenyl or lower alkynyl
group, which may optionally be substituted by a halogen
atom or a hydroxyl group,
b) a lower alkoxy group which may optionally be
substituted by a halogen atom, a cyano group, a hydroxyl
group, a carboxyl group, a lower alkoxycarbonyl group, a
lower alkanoyl group, a lower alkanoyloxy group, a
carbamoyl group, a lower alkylaminocarbonyl group or a
phthalimido group; a hydroxyl group; a mercapto group; or
a lower alkylthio group,
c) a halogen atom; a cyano group,
d) a carboxyl group; a lower alkoxycarbonyl
group; a lower alkanoyl group; a lower alkanoyloxy group;
a carbamoyl group; a lower alkylaminocarbonyl group;
e) an amino group; a mono or di lower alkylamino
group; a lower alkanoylamino group; a 1-pyzrolidinyl
group; a piperidino group; a morpholino group; or a 1-
piperazinyl, 1-imidazolidinyl or 1-homopiperazinyl group
- 19 -
CA 02453123 2004-01-20
which may optionally be substituted by a lower alkyl group
at the nitrogen atom of the ring,
f) a cycloalkyl group,
g) a phenyl group which may optionally be
substituted by a lower alkyl group, a lower alkenyl group,
a lower alkynyl group, a halogen atom, a lower alkoxy
group, an amino group, a mono or di lower alkylaminc
group, a hydroxyl group or a carboxyl group, or
h) an imidazolyl, triazolyl, tetrazolyl,
pyrrolyl, pyridyl, pyrazinyl or pyrimidinyl group, which
may optionally be substituted by a lower alkyl group, a
cycloalkyl group or a phenyl group,
more preferably
a lower alkyl group; a lower alkoxy group; a
hydroxyl group; a halogen atom; a cycloalkyl
group; a phenyl group which may optionally be
substituted by a lower alkyl group, a lower
alkenyl group, a lower alkynyl group, a halogen
atom, a lower alkoxy group, an amino group, a mono
or di lower alkylamino group, a hydroxyl group or
a carboxyl group; or an imidazolyl, triazolyl,
tetrazolyl or pyrrolyl group, which may optionally
be substituted by a lower alkyl group,
most preferably an unsubstituted phenyl group or a phenyl
group substituted by a lower alkyl group.
_
_ 20
CA 02453123 2004-01-20
Unless otherwise noted, the term "lower" as used
in the definition of the general formula of the present
invention means a straight or branched carbon chain having
1 to 6 carbon atoms.
Examples of the "alkyl group" include straight- or
branched-chain alkyl groups, preferably a lower alkyl
group. Illustrative examples of the "lower alkyl group"
include alkyl groups each having 1 to 6 carbon atoms, such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-
butyl, tert-butyl, pentyl, isopentyl, neopentyl, tert-
pentyl, 1-methylbutyl, 2-methylbutyl-, 1,2-dimethylpropyl,
hexyl, isohexyl, 1-methylpentyl, 2-methylpentyl, 3-
methylpentyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-
dimethylbutyl, 1,3-dimethylbutyl, 2,3-dimethylbutyl, 3,3-
dimethylbutyl, 1-ethylbutyl, 2-ethylbutyl, 1,1,2-
trimethylpropyl, 1,2,2-trimethylpropyl, 1-ethyl-l-
methylpropyl, 1-ethyl-2-methylpropyl and the like, of
which methyl and ethyl groups are preferred.
Examples of the "alkenyl group" include straight-
or branched-chain alkenyl groups, preferably a lower
alkenyl group. Illustrative examples of the "lower
alkenyl group" include alkenyl groups each having 2 to 6
carbon atoms, such as vinyl, allyl, 1-propenyl,
isopropenyl, 1-butenyl, 2-butenyl, 3-butenyl, 2-methyl-l-
propenyl, 2-methylallyl, 1-methyl-l-propenyl, 1-
methylallyl, 1,1-dimethylvinyl, 1-pentenyl, 2-pentenyl, 3-
-
2 1
CA 02453123 2004-01-20
pentenyl, 4-pentenyl, 3-methyl-l-butenyl, 3-methyl-2-
butenyl, 3-methyl-3-butenyl, 2-methyl-l-butenyl, 2-methyl-
2-butenyl, 2-methyl-3-butenyl, 1-methyl-l-butenyl, 1-
methyl-2-butenyl, 1-methyl-3-butenyl, 1,1-dimethylallyl,
1,2-dimethyl-l-propenyl, 1,2-dimethyl-2-propenyl, 1-ethyl-
1-propenyl, 1-ethyl-2-propenyl, 1-hexenyl, 2-hexenyl, 3-
hexenyl, 4-hexenyl, 5-hexenyl, 1,1-dimethyl-l-butenyl,
1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl, 3,3-
dimethyl-l-butenyl, 1-methyl-l-pentenyl, 1-methyl-2-
pentenyl, 1-methyl-3-pentenyl, 1-methyl-4-pentenyl, 4-
methyl-l-pentenyl, 4-methyl-2-pentenyl, 4-methyl-3-
pentenyl and the like.
Examples of the "alkynyl group" include straight-
or branched-chain alkynyl groups, preferably a lower
alkynyl group. Illustrative examples of the "lower
alkynyl group" include straight- or branched-chain alkynyl
groups each having 2 to 6 carbon atoms, such as ethynyl,
1-propynyl, 2-propynyl, 1-butynyl, 2-butynyl, 3-butynyl,
1-methyl-2-propynyl, 1-pentynyl, 2-pentynyl, 3-pentynyl,
4-pentynyl, 3-methyl-l-butynyl, 2-methyl-3-butynyl, 1-
methyl-2-butynyl, 1-methyl-3-butynyl, 1,1-dimethyl-2-
propynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl, 4-hexynyl, 5-
hexynyl and the like.
The "cycloalkyl group" or "cycloalkenyl group" are
preferably cycloalkyl or cycloalkenyl groups having 3 to 8
carbon atoms; such as dyclopropyl, cyclobutyl,
- 22 -
CA 02453123 2004-01-20
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl,
cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl,
cycloheptenyl, cyclooctenyl and the like, of which
cyclohexyl and cyclohexenyl groups are respectively
preferred.
The "aryl group" is preferably an aryl group
having 6 to 14 carbon atoms, such as phenyl, biphenyl,
naphthyl, anthryl, phenanthryl and the like, of which
phenyl and naphthyl groups are preferred and phenyl group
is particularly preferred.
Examples of the "alkoxy group" include straight-
or branched-chain alkoxy groups, preferably a lower alkoxy
group. The "lower alkoxyl group" is preferably a lower
alkoxyl group having the aforementioned lower alkyl group
as its alkyl moiety, and examples of the "lower alkoxyl
group" include methoxy, ethoxy, propoxy, isopropoxy,
butoxy, isobutoxy, sec-butoxy, tert-butoxy, pentyloxy
(amyloxy), isopentyloxy, tert-pentyloxy, neopentyloxy, 2-
methylbutoxy, 1,2-dimethylpropoxy, 1-ethylpropoxy,
hexyloxy and the like, of which methoxy and isopropoxy
groups, especially a methoxy group, are preferred.
Examples of the "alkanoyl group" include straight-
or branched-chain alkanoyl groups, preferably a lower
alkanoyl group. Illustrative examples of the "lower
alkanoyl group" include lower acyl groups each having 1 to
6 carbon atoms derived from saturated aliphatic carboxylic
- 23 -
CA 02453123 2004-01-20
acids, such as formyl, acetyl, propionyl, bytylyl,
isobutylyl, valeryl, isovaleryl, pivaloyl, hexanoyl and
the like.
The "alkanoyloxy group" is preferably a group
containing the aforementioned lower alkanoyl group as its
alkanoyl moiety, such as acetoxy, propionyloxy and the
like.
The "alkanoylamino group" is preferably a group
containing the aforementioned lower alkanoyl group as its
alkanoyl moiety, such as acetamide, propionylamino and the
like.
Examples of the "halogen atom" include fluorine,
chlorine, bromine and iodine.
The term "mono or di lower alkylamino group" means
an amino group mono- or di-substituted by the
aforementioned lower alkyl group, its illustrative
examples including mono lower alkylamino groups such as
methylamino, ethylamino, propylamino, isopropylamino,
butylamino, isobutylamino, sec-butylamino, tert-
butylamino, pentyl(amyl)amino, isopentylamino,
neopentylamino, tert-pentylamino, hexylamino and the like
and symmetric or asymmetric di lower alkylamino groups
such as dimethylamino, diethylamino, dipropylamino,
diisopropylamino, dibutylamino, dis.sobutylamino,
ethylmethylamino, methylpropylamino and the like.
- 24 -
CA 02453123 2004-01-20
The "aralkyl group", 'aralkenyl. group" or
"aralkynyl group" is preferably an aralkyl, aralkenyl or
aralkynyl group which is composed of the aforementioned
aryl moiety (especially a phenyl or naphthyl group) and a
lower alkyl, lower alkenyl or lower alkynyl moiety.
The "alkenyloxy group", "alkynyloxy group",
"cycloalkyloxy group", "cycloalkenyloxy group", "aryloxy
group", "aralkyloxy group", 'aralkenyloxy group" or
"aralkynyloxy group" and "alkylthio group", "alkenylthio
group", "alkynylthio group", "cycloalkylthio group",
"cycloalkenylthio group", "arylthio group", "aralkylthio
group " , 'aralkenylthio group" or "aralkynylthio group" are
preferably those groups having a lower hydrocarbon chain
as the respective hydrocarbon group moiety and, if the
"alkenyloxy group" is taken as an example, the "alkenyloxy
group" is preferably a lower alkenyloxy group having the
aforementioned lower alkenyl group as its alkenyl moiety.
The "alkoxycarbonyl group" is preferably a lower
alkoxycarbonyl group having the aforementioned lower alkyl
group as its alkyl moiety, which is formed by the
esterification of a straight- or branched-chain alcohol
having 1 to 6 carbon atoms with carbonyl group, such as
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl,
sec-butoxycarbonyl, tert-butoxycarbonyl,
pentyloxycarbonyl, isopentyloxycarbonyl,
-
_ 25
CA 02453123 2004-01-20
neopentyloxycarbonyl, tert-pentyloxycarbonyl,
hexyloxycarbonyl or the like.
In the same manner, "alkenyloxycarbonyl group",
"alkynyloxycarbonyl group", "cycloalkyloxycarbonyl group",
"cycloalkenyloxycarbonyl group", "aryloxycarbonyl group",
"aralkyloxycarbonyl group", "aralkenyloxycarbonyl group",
'aralkynyloxycarbonyl group" or "alkylaminocarbonyl group"
is preferably such a group that, if the
"alkenyloxycarbonyl group" is taken as an example, a lower
alkenyloxycarbonyl group having the aforementioned lower
alkenyl group as its alkenyl moiety.
The "aliphatic acyl group" is preferably a lower
acyl group derived from a saturated or unsaturated lower
fatty acid, and the aforementioned lower alkanoyl group
may be preferable. Illustrative examples of "aromatic
acyl group" include benzoyl, toluoyl, salicyl, naphthoyl,
phthaloyl and the like group. The "acyloxy group" is a
group which contains the aforementioned lower alkanoyl or
aromatic acyl group as its acyl moiety, with its preferred
examples including acetoxy, benzoyloxy and the like.
Illustrative examples of the "protected amino
group" include amino groups-each of which being protected
with an aliphatic or aromatic acyl group, a carbamoyl
group, a carbamide group, a phthaloyl group, or the like.
The "lower alkylene group" is a straight or
branched divalent carbon chain having 1 to 7 carbon atoms,
- 26
-
CA 02453123 2004-01-20
with its illustrative examples including methylene,
ethylene, propylene, tetramethylene, 2-methyltrimethylene,
1-ethylethylene, pentamethylene, 1,2-diethylethylene,
hexamethylene and the like.
The "lower alkenylene group" is a straight or
branched divalent carbon chain having 2 to 7 carbon atoms,
with its illustrative examples including vinylvne,
propenylene, 2-propenylene, 1-methylvinylene, 2-
methylvinylene, butenylene, 2-butenylene, 3-butenylene, 1-
methylpropenylene, l-methyl-2-propenylene, 2-pentenylene,
1-methyl-l-butenylene, 2-hexenylene and the like.
The "lower alkynylene group" is a straight or
branched divalent carbon chain having 2 to 7 carbon atoms,
with its illustrative examples including ethynylene, 2-
propynylene, 2-butynylene, 3-butynylene, 1-methyl-2-
propynylene, 2-pentynylene, 2-hexynylene and the like.
The "dimethylamino-substituted lower alkylamino
group" is an amino group which is mono-substituted by the
aforementioned lower alkyl group that is further
substituted by dimethylamino group(s).
The "amino lower alkanoylamino group" is an amino
group which is mono-substituted by the aforementioned
lower alkanoyl group that is further substituted by amino
group(s).
The salt of the compound of the present invention
is an acid addition salt with an inorganic or organic acid
- 27
CA 02453123 2004-01-20
or a salt with an inorganic or organic base, and a
pharmaceutically acceptable salt is preferable.
Illustrative examples of such salts include: an acid
addition salt with a mineral acid such as hydrochloric
acid, hydrobromic acid, hydroiodic acid, sulfuric acid,
nitric acid, phosphoric acid or the like, an organic acid
such as formic acid, aceti,- acid, propionic acid, oxalic
acid, malonic acid, succinic acid, fumaric acid, maleic
acid, lactic acid, malic acid, tartaric acid, citric acid,
methanesulfonic acid, ethanesulfonic acid or the like or
an acidic amino acid such as aspartic acid, glutamic acid
or the like; and a salt with an inorganic base such as
sodium, potassium, magnesium, calcium, aluminium or the
like, an organic base such as methylamine, ethylamine,
ethanolamine or the like or a basic amino acid such as
lysine, ornithine or the like. Also useful are quaternary
ammonium salts. Illustrative examples of quaternary
ammonium salts include a lower alkyl halide, a lower alkyl
trifurate, a lower alkyl tosylate, a benzyl halide and the
like, preferably methyl iodide, benzyl chloride and the
like.
The compound of the general formula (I) may form
optical isomers due to an asymmetric carbon atom or
geometrical isomers due to a double bond or a cyclohexane
ring. Mixtures and separated forms of various isomers
including such geometrical isomers and optical isomers are
- 28 -
CA 02453123 2004-01-20
also included in the scope of the present invention. Also
included in the present invention are hydrates, solvates,
tautomers and the like of the compound of general formula
(I). Some of the compounds of the present invention show
polymorphism and all types of polymorphism of the
inventive compound are also included in the present
invention.
(Production Process)
The compound of the present invention and salts
thereof can be produced by various synthetic techniques
making use of the characteristics of its basic skeleton or
the type of substituents. In that case, it may be
effective from the viewpoint of production techniques to
substitute an amino group, a carbonyl group, a hydroxyl
group.and a mercapto group of an intermediate or the
compound of the present invention with appropriate
protective groups, namely functional groups which can
easily be converted into an amino group, a carbonyl group,
a hydroxyl group and a mercapto group. Protective groups
disclosed, for instance, by Greene and Wuts in "Protective
Groups in Organic Synthesis, 2nd ed." may optionally be
used in accordance with the reaction conditions. in
addition to these groups, hydroxymethylene group (CH-OH)
is also a functional group which can easily be converted
into a carbonyl group, and such a functional group can
also be used as the protective group for a carbonyl group.
_
+ 29
CA 02453123 2004-01-20
The following describes typical examples of the
process for the production of the compound of the present
invention.
First process (amidation A)
COOH B
,
' R
A Rz
N
C H
(ITIj (IV)
or a reactive derivative thereof or a salt thereof
B
If necessary, removal Rt ~ of the protecti.ve group j
0
A RZ
(I)
(In the above formulae, RI, R2, A. ring B and ring C have
the same respective meanings as described in the
foregoing.)
The compound (I) of the present invention can be
produced by subjecting the substituted benzoic acid
represented by the formula (III) which may optionally be
protected, or a reactive derivative thereof, and the 5-
-
3 0
CA 02453123 2004-01-20
membered nitrogen-containing aromatic and heterocyclic
ring-condensed benzazepine derivative represented by the
formula (IV) which may optionally be protected, or a salt
thereof, to amidation in the usual way and by, if
necessary, removing the protective group.
Examples of the reactive derivative of the
compound (III) include: its usual esters such as methyl
ester, ethyl ester, isobutyl ester, tert-butyl ester and
the like; its acid halides such as acid chloride, acid
bromide and the like; its acid azides; its active esters
obtained by allowing it to react with a phenolic compound
such as p-nitrophenol or an N-hydroxylamine compound such
as 1-hydroxysuccinimide, 1-hydroxybenzotriazole or the
like; its symmetric acid anhydrides; and its mixed acid
anhydrides including organic acid-type mixed acid
anhydrides obtained by allowing it to react with
halocarboxylic acid alkyl esters such as alkylcarbonic
acid halides or pivaloyl halides and phosphoric acid-type
mixed acid anhydrides obtained by allowing it to react
with diphenylphosphoryl chloride or N-methylmorpholine.
Also, when the compound (III) is allowed to react
as a free acid, as an active ester without isolation, or
the like, it is desirable to use a condensing agent such
as dicyclohexylcarbodiimide, carbonyldiimidazole,
diphenylphosphorylamide, diethylphosphoryl cyanide, 1-
-
3 1
CA 02453123 2004-01-20
ethyl-3-(3-dimethylaminopropyl)carbodiirnide hydrochloride
or the like.
The reaction may be carried out generally in an
inert organic solvent selected, for example, from
halogenated hydrocarbons such as dichloromethane,
dichloroethane, chloroform and the like, aromatic
hydrocarbons such as benzene, toluene, xylene and the
like, ethers such as ether, tetrahydrofuran and the like,
esters such as ethyl acetate and the like, N,N-
dimethylformamide and dimethylsulfoxide depending on the
used r_eactive derivative, condensing agent and the like,
and at a cooling temperature or at a temperature-of from
cooling temperature to room temperature or from room
temperature to heating temperature depending on the
reactive derivative used.
In order to effect smooth progress of the
reaction, it may sometimes be advantageous to use the
compound (III) in an excess amount or carry out the
reaction in the presence of a base such as N-
methylmorpholine, trimethylamine, triethylamine, N,N-
dimethylaniline, pyridine, 4-(N,N-dimethylamino)pyridine,
picoline, lutidine or the like. Pyridine can be used also
as a solvent.
The reaction may be effected preferably in the
absence of a mercapto group and reactive amino, carboxy,
hydroxy and the like groups, but the product of interest
-
- 32
CA 02453123 2004-01-20
can be obtained by carrying out the reaction after
introducing protective groups and removing the protective
groups after completion of the reaction.
Method for the removal of protective groups varies
depending on the type of the protective group used.
For example, when the protective group for an
amino group is a substituted or unsubstituted
benzyloxycarbonyl group or the like, catalytic reduction
may be effective and, in some cases, acid treatment with
hydrobromic acid/acetic acid, hydrobromic
acid/trifluoroacetic acid, hydrofluoric acid and the like.
In the case of other urethane type protective groups such
as tert-butoxycarbonyl group and the like, it is
advantageous to employ acid treatment with hydrobromic
acid/acetic acid, trifluoroacetic acid, hydrochloric acid,
hydrochloric acid/acetic acid, hydrochloric acid/dioxane
and the like.
When the protective group for an amino group is
the group which forms a phthalimido group together with
the nitrogen atom of the amino group, a primary amino
group can be formed through the removal of the phthaloyl
group by its treatment with hydrazines such as hydrazine,
methylhydrazine, ethylhydrazine and the like, ammonia or
primary amines such as methylamine, ethylamine,
propylamine and the like.
_ 33 -
CA 02453123 2004-01-20
The protective groups for a carboxyl group can
easily be removed by saponification when the protective
group is methyl and ethyl groups; by catalytic reduction
or saponification when the protective group is a benzyl
group and various substituted benzyl groups; by the
aforementioned acid treatment when the protective group is
tert-butyl group; and by contact with water when the
protective group is a trimethylsilyl group.
In the case of protective groups for a mercapto
group and a hydroxyl group, they can be removed in most
cases by the sodium/liquid ammonia treatment or the
hydrofluoric acid treatment, certain types of the
protective groups (for example, O-benzyl, 0-
benzyloxycarbonyl and S-p-nitrobenzyl) can be removed by
catalytic reduction, and acyl-type protective groups can
be removed by their hydrolysis in the presence of an acid
or an alkali.
These treatments can be carried out in the usual
way.
In this connection, the starting compounds (III)
and (IV) can easily be obtained by the aforementioned
amidation reaction or a cyclization reaction which will be
described later.
- 34 -
CA 02453123 2004-01-20
Second process (amidation B)
Rt ) - t_. O V dl cIJV(CR3
R
N
O
H2 N RZ
(V) (VI)
or a reactive derivative thereof or a salt thereof
B
If necessary, removal R1
of the protective group
N
0
(CR' R')n CONH RZ
rc
(Ia)
(In the above formulae, Rl, R2, R3, R4, n, ring B and ring
C have the same respective meanings as described in the
foregoing.)
The compound (Ia) as one of the compounds of the
present invention, in which A is -(CR3R9)n-CONH-, can be
produced by subjecting the corresponding carboxylic acid
(V) which may optionally have a protective group, or a
reacti.ve derivative thereof, and the corresponding amine
(VI) which may optionally have a protective group, or a
_ 35
CA 02453123 2004-01-20
salt thereof, to amidation reaction in the usual way and
by, if necessary, removing the protective group.
Types of the reactive derivatives, reaction
conditions, removal of protective groups and the like are
the same with the first process and the reaction can be
effected by the similar way.
In this connection, the starting compound (VI) can
easily be obtained by the aforementioned amidation
reaction or a cyclization reaction which will be described
later.
Third process (amidation C) A' _ NH 2
B'
R'
R8-COOR + N
0
(vzz) A R2
C (Ib)
or a reactive derivative thereof or a salt thereof
A' -NHCO-RB
B'
If necessary, removal
of the protective group R'
1',
0
A RZ
C
(Ic)
_ 36 -
CA 02453123 2004-01-20
(In the above formulae, R1, Ra, ring C and A have the same
respective meanings as described in the foregoing, and
ring B' is the same as ring B except that one hydrogen
atom or substituent is removed, R8 is a lower alkyl group
which may optionally be substituted by an amino or mono or
di lower alkylamino group that may optionally have a
protective group, and A1 is a single bond or a lower
alkylene group.)
The compound (Ic) as one of the compounds of the
present invention, in which a substituted or unsubstituted
lower alkanoylamino group is located on the 5-membered
ring, can be produced by subjecting the corresponding
carboxylic acid (VII) which may optionally have a
protective group, or a reactive derivative thereof, and
the corresponding amine (Ib) which may optionally have a
protective group, or a salt thereof, to amidation reaction
in the usual way and by, if necessary, removing the
protective group.
Types of the reactive derivatives, reaction
conditions, removal of protective groups and the like are
the same with the first process and the reaction can be
effected by the similar way.
In addition, a compound in which a substituted or
unsubstituted aminocarbonyl group is located on the 5-
membered ring or another compound in which -Ni3CO- or
_ 37 -
'CA 02453123 2004-01-20
..w, a=t:9 =.r' , t ' 1 .~ + r:y.,'.
4 =!'~ ~v e .
_ ! :1-.r, t .1 : ='i4: . - ..7 . b F..
,= jt ., .
'+I~. = ;.
Ja.'r
:4;. .
-t~ . - - -
i;_i- - ~,, ~:: ' .-,=.
. n i =.v :,'_ rF .
Y.,s ~ t~,'=~.
. . a Y'= = 1= -
-= .. a . .. . . . ' - . ' '..'' -CONH- is located on the ring C.can also be
produced in
the same manner as .in the fi'r.st:. process.
Pourth process (cyclization)
Y' Y' Y'
R' Y
+ R9C
~NH2
0
A RZ
(~~I) (IX)
_ R9
If necessary, removal of the protective group 21~ x .3
Rl-i-
N
0
A R
c
(id)
(In the above formulae, Ri, R2, ring C, A, Xl and X3 have
the same respective meanings as described in the
foregoing, and one of Y' and Y2, and Y3 and Y4 form an oxo
group-(-0) in combination and the other are a halogen atom
--
-
' 38
CA 02453123 2004-01-20
X halogen
and a hydrogen atom , R9 is a hydrogen
S~A
atom or asubstituent, and Z is a group represented by =NH,
=0 or =S.)
A compound as one of the compounds of the present
invention, in which an imidazole ring, an oxazole ring or
a thiazole ring is condensed, can be produced by allowing
the corresponding haloketone (VIII) which may optionally
have a protective group to react with corresponding
amidines, guanidines, amides, ureas, thioamides or
thioureas represented by formula (IX) and by, if
necessary, removing the protective group.
In this reaction, corresponz3ing thioamide and
thiourea, amidine and guanidine or carboxilic acid amide
and urea derivative may sometimes form a salt with acid.
In order to accelerate the reaction, the reaction niay be
carried out in the presence of an inorganic base such as
sodium hydroxide, potassium hydroxide, sodium carbonate,
potassium carbonate, sodium bicarbonate, potassium
bicarbonate or the like or a salt of a weak acid with a
strong base or an organic base such as pyridine,
diisopropylethylamine, l,5-diazabicyclo[4.3.0]non-5-ene or
the like. The reaction may preferably be carried out in
an inert solvent which includes alcohol solvents such as
methyl alcohol, ethyl alcohol, isopropyl alcohol and the
like, ether solvents such as ether, tetrahydrofuran,
- 39 -
CA 02453123 2004-01-20
dioxane and the like, acetonitrile, dimethylformamide and
dimethylsulfoxide, and at a temperature of from room
temperature to reflux temperature of the solvent used. If
necessary, the reaction may be carried out under a
pressure.
In this instance, oxazoles may sometimes be formed
when amidines or guanidins are used in the reaction. In
that case, imidazoles can be obtained as the main product
by carrying out the reaction in an atmosphere of ammonia
gas in the presence of ammonium carbonate, ammonium
acetate, formamide or the like.
The starting compound (VIII) to be used in this
reaction can be produced, as shown in the following
reaction formula, by subjecting p-substituted benzoic acid
(X) which may optionally have a protective group, or a
reactive derivative thereof, and a benzazepine derivative
(XI) which may optionally have a protective group, or a
salt thereof, to amidation reaction in the same manner as
in the first process and by allowing the resulting product
to react with a halogenation agent and, if necessary,
removing the protective group at any step. In this
connection, a compound in which A of the p-substituted
benzoic acid (X) is -(GR3R4)-C NH- can be produced by
subjecting the corresponding carboxylic acid (XIII) or a
reactive derivative thereof and the corresponding p-
_ 4 -
CA 02453123 2004-01-20
aminobenzoic acid (XIV) to amidation reaction in the same
manner as in the first process.
(CR3 R' )n-COOH
C (XIII)
COOH
(XIY)
H2 N RZ
COOH
(X)
C
Y5 Y jY'
R i \ y 8
N Jy (XI)
H
Y ' Y ~Y'
-y a Halogenation agent (VIII)
R
-C:~ N
0
A Rz
(XII)
(In the above formulae, R1, R2, R3, R4, ring C and A have
the same respective meanings as described in the
- 41 -
CA 02453123 2004-01-20
foregoing, and one of Y5 and Y6, and Y7 and Y8 form an oxo
group in combination and the other are both hydrogen
H
atoms a)
H
Types of the reactive derivatives, reaction
conditions, removal of protective groups and the like in
the first step amidation reaction are the same with the
first process.
With regard to the halogenation reagent to be used
in the halogenation step, any agent conventionally used
for the halogenation of saturated cyclic ketones may be
used, but preferably a metal reagent such as copper(II)
halide (e.g., copper(II) bromide, copper(II) chloride or
the like), or a perbromide of pyridine, a-pyrroli.done,
quaternary ammonium, dioxane or the like, such as dioxane
dibromide, phenyltrimethylammonium tribromide, pyridinium
hydrobromide perbromide, pyrrolidone hydrotribromide or
the like, as well as a halogen itself such as chlorine,
bromine or the like or a hydrohalogenic acid such as
hydrochloric acid, hydrobromic acid or the like.
Using a metal reagent or a perbromide, the
reaction of the compound (XIi) with this halogenation
reagent is advantageously carried out.generally in an
inert solvent selected, for example, from halogenated
hydrocarbons such as dichloromethane, chloroform, carbon
tetrachloride and the like ether solvents such as ether,
- 42 -
CA 02453123 2004-01-20
tetrahydrofuran, dioxane and the like, alcohol solvents
such as methyl alcohol, ethyl alcohol and the like,
aromatic hydrocarbon solvents such as benzene, toluene,
xylene and the like, acetic acid, ethyl acetate, water or
a mixed solvent thereof, and at room temperature or with
heating, if necessary in the presence of a small amount of
catalyst such as a hydrogen halide or the like.
The compound of interest can also be obtained by
allowing the compound (XII) to react with a halogen itself
as the halogenation agent in an inert solvent such as a
halogenated hydrocarbon (e.g., dichloromethane,
chloroform, carbon tetrachloride and the like) and
ethylene glycol, acetic acid and the like, or by allowing
the compound (XII) to react with a hydrohalogenic acid as
the halogenation agent in its acidic solution or in a
basic solution such as a sodium hydroxide aqueous
solution. In that case, the reaction may be carried out
at a temperature in the range of preferably from.-3D C to
reflux temperature of the solvent used.
Although a process for the synthesis of a compound
in which an imidazole ring, an oxazole ring or a thiazole
ring is condensed has been described in the above, a
compound in which an oxadiazole ring, a thiadiazole ring
or a triazole ring is condensed can be produced by a
conventional process shown by the following reaction
formula.
- 43 -
CA 02453123 2004-01-20
0
R1
N
. ~
A R'
(XV)
jNR2 OH
NOH NOH
R1
A Rz
C 11
(XVI)
/H2 0 H2 S
/0,, '-,S' ,
N Q N N Q N
R' R1
N N
p
A "R2 a R
C C
(Ie) (If)
+
w 44
CA 02453123 2004-01-20 -= -
=a''w=.Y : ~.'_ =.1'~- _ ,~w =!'= _ =x. 1~; -
~ ~ =~ ~~ .
..'+ :=~ '~'
_ _ = ~~ ~. '
=~:
;~~ : =~:/
_ ~s-~ ~~.P-'=
:'c~
..E; .;..
- ~ 3i% :,= -=
:v,~ "._~=".>
.. .4= .
~..'~s'~r~=f .}~. ''ri . .
Gr'== )
. ~. 'i~=
- ~Y ~~ ~='De '=4e ,'Mr:.
. ~: a;== ''~V ..R. ~w . .};~' .ary
~ ' = _ = '=. "~= ,~~= .. .. .'e'':.. .ly~r =,y~i.! ~ =
= ' . . ~ = . ' _ . . . = . = _ .- . . , .. - ~ !r'=' = .
. , . . = . , .. . .. . ' . ~ ~ . ~ . = . ~. _ = , _ . =
O Y
R 7
N
O
A R= CVI I I-a)
l N H 3
0 NH2 0 % IV
R' ~r 11 ~
R'
N N
O _ NaNO2 0
A R2 A~ Rz
C
CXVIII) Cxlx) "
i Y
NH;,
CH3 COONH:
0 Q N
NH< S H
N
O
A ~Rz
C
CIg)
~
- . ~. .
-
- 45
CA 02453123 2004-01-20
v
H
NNI
~ 0 S i~ R1 ~
R ' ~'~/ \
N N N
0 0
R ' -P' R 2
C
{I}~) CI i)
~ 46
CA 02453123 2004-01-20
(In the above formulae, R1, R2, ring C and A have the same
respective meanings as described in the foregoing, and Y9
is a halogen atom.)
That is, the compound (Ie) in which 1,2,5=
oxadiazole ring is condensed and the compound (If) in
which 1,2,5-thiadiazole ring is condensed can be produced
by allowing a benzazepinedione derivative to react with
hydroxylamine hydrochloride in the presence of a base such
as sodium acetate or the like to obtain the dioxime
compound (XVI) and dehydrating the resulting compound with
heating in the presence of a dehydrating agent or treating
the compound with hydrogen sulfide. Each reaction step
can be effected by conventional means.
On the other hand, the compound (Ig) in which
1,2,3-oxadiazole ring is condensed can be produced by
treating the compound (VIIIa) with ammonia and treating-
the resulting compound (XVIII) with a diazotation agent
such as sodium nitrite. That is, the compound (Ig) is in
the equilibrium state with the diazo compound (XIX).
Also, the compound (Ih) in which 1,2,3-oxadiazole ring is
condensed and the compound (Ii) in which 1,2,3-triazole
ring is condensed can be produced by allowing the diazo
compound (XIX), or the compound (Ig), to react with
ammonium hydrosulfide or with ammonia and ammonium
acetate. Each of these reaction steps can be effected by
conventional means.
- 47 -
CA 02453123 2004-01-20
The starting compound (XV) can easily be obtained
in the same manner as the aforementioned amidation method
for the production of compound (XII) from compound (XI),
and the other starting compound (VIII-a) can easily be
obtained by the method described in the foregoing.
When a haloketone compound having different
positions for an oxo group and a halogen atom is used as
the starting compound instead of the compound (VIII-a),
compounds in which 1,2,3-oxadiazole ring and 1,2,3-
thiadiazole ring are condensed at different positions can
be produced.
- 48 -
CA 02453123 2004-01-20
. 4'. ~ = . A'. . '~.
J=' Q..
8 =
y=,r.J. . .4 vb' A m'c..h a4... j.
.X ~Y= :r r
'.a . . ' t, = ,:,'. :.v+' c '.5.=.:.= .i'... =t'='.=r. :e7 -n .":.
i
Fifth process {rnutuall conversion of subst~~tuents on the
-=arcrmati-c carbon rilig }
R,
'xx
~T Hti\R ) ' 0
F a l~
a tI ~)
B
Rj
\v~!~
---- ~
R1 o R i t O
N A
~ ,
{I k)
(In the above formulae, R$, R2, ring B and A have the same
respective meanings as described in the foregoing, and
ring C' is the same with the ring C except that one
hydrogen atom or substituent is removed, Rlo and R" may
be the same or different from each oti-ier and each
represents a hydrogen atom, a lower alkyl group, a
- 49 -
CA 02453123 2004-01-20
protective group or an amidino group, provided that R10
and R" may be combined with the adjacent nitrogen atom to
form a hetero ring which may optionally be substituted.)
A compound of the present invention in which its
aromatic carbon ring has a substituent can be produced by
selecting the corresponding starting compound and
repeating the aforementioned process but, when the
substituent on the aromatic carbon ring contains a
characteristic functional group, it can be produced by
mutual conversion such as substituent introduction or
substitution on the aromatic carbon ring.
For example, the compound (Ik) which contains at
least one amine-type substituent as a substituent on the
ring C can also be produced by allowing the fluorine
compound (Ij) which has -CO- or -C = N on the adjacent
position when A is a single bond or -CONH- to react with
ammonia, a corresponding amine, a corresponding cyclic
imine or guanidine.
Conventional N-alkylation method can be applied to
this process. That is, although the reaction progresses
in the absence of solvent, the reaction may be carried out
generally in an inert organic solvent selected, for
example, from dimethylformamide, dimethylsulfoxide,
aromatic hydrocarbons such as benzene, toluene, xylene and
the like, halogenated hydrocarbons such as
dichloromethane, dichloroethane, chloroform and the like
_ 50
CA 02453123 2004-01-20
and alcohols such as methyl alcohol, ethyl alcohol,
isopropyl alcohol and the like. In order to effect smooth
progress of the reaction, it may sometimes be advantageous
to carry out the reaction in the presence of an inorganic
base such as sodium hydride, potassium carbonate, sodium
carbonate or the like. This reaction is generally carried
out at room temperature, with heating or at reflux
temperature.
This conversion method to form an amine-type
substituent on the aromatic carbon ring can also be
applied to the case in which conversion into an amine-type
substituent as R2 is carried out.
_ 51 _
CA 02453123 2004-01-20
Sixth process (mutual conversion of substituents on the
hetero ring)
A 2 _ y d t
B v
, IO
N\R (XX)
N R
0
A R'
C
(I ~)
R,O..
A ~
~ \Rõ
~
- ~ i
0
a ~~z
tIm)
(In the above formulae, RI, R2, ring B', A, ring C, R10
and R11 have the same respective meanings as described in
the foregoing, and A2 is a single bond or a lower alkylene
group and Y11 is a halogen atom, an organic sulfonic acid
residue or, when A is a single bond, an alkoxy or
alkylthio group.
_ 52
CA 02453123 2004-01-20
Mutual conversion of substituents on the 5-
membered hetero ring can be made more easily than the case
of the aromatic ring. For example, the compound (Im)
which contains at least one amine-type substituent on its
hetero ring can be produced by allowing the corresponding
halide or sulfonate or, when A is a single bond, ether or
the thioether compound (Il) to zeact with an amine
compound (XX).
Examples of the organic sulfonic acid residue
include alkanesulfonic acid residues such as
methanesulfonyloxy group, ethanesulfonyloxy group and the
like and aromatic sulfonic acid residues such as
benzenesulfonyloxy group, toluenesulfonyloxy group
(especially p) and the like.
The reaction can be effected by almost the same
manner as in the case of the fifth process.
In this instance, the mutual conversion into amine
substituent on the hetero ring can be used as a process in
which an N-substituted compound is produced by allowing an
imino nitrogen-containing hetero ring-consended compound
to react with the corresponding halide or sulfonate such
as a lower alkyl halide or a lower alkyl sulfonate.
Other processes
Although only amidation, cyclization and amine-
type substituent introduction have been described in the
foregoing, the compound of the present invention can be
- 53 -
CA 02453123 2004-01-20
synthesized by various conventional means because the
inventive compound contains various characteristic
functional groups.
For example, a compound having a carboxyl group
can be produced by hydrolyzing its corresponding ester; an
ester compound can be produced by esterificating its
corresponding carboxylic acid; alcohol, phenol, mercaptan
and thiophenol compounds can be produced by hydrolyzing
ether and thioether compounds; and ether and thioether
compounds can be produced by allowing corresponding
alcohol, phenol, mercaptan and thiophenol compounds to
react with the corresponding halides such as alkyl
halides.
The reaction products obtained by the above
processes are isolated and purified in the form of free
compounds, salts thereof, hydrates thereof or various
solvates thereof. Salts can be produced by usual salt
forming reactions.
Isolation and purification are carried out by
applying usual chemical operations such as extraction,
concentration, distillation, crystallization, filtration,
recrystallization and various types of chromatography.
As described in the foregoing, isomers such as
racemates, optically active substances, diastereoisomers
and the like are present alone or as a mixture with
respect to the compound of the present invention. Racemic
_ 54
CA 02453123 2004-01-20
compound can be made into stereochemically pure isomer by
the use of a proper starting compound or by means of
conventional racemic resolution (for example, a method in
which a racemic compound is made into a diastereoisomer
salt with a usual optically active acid (tartaric acid or
the like) and then subjected to optical resolution).
Also, a mixture of diastereoisomers can be separated by
conventional means such as fractional crystallization,
chromatography and the like.
INDUSTRIAL APPLICABILITY
Compounds of the present invention and salts
thereof show excellent antagonism on arginine vasopressin
Vi and/or V2 receptor. That is, the compounds of the
present invention include a compound which shows strong
antagonism on both Vl and V2 receptors, a compound which
selectively shows excellent antagonism on Vl receptor and
a compound which selectively shows excellent antagonism on
V2 receptor.
Particularly preferred is the compound which shows
strong antagonism on both VI and V2 receptors.
The compounds of the present invention are
excellent in oral absorption and show proper prolonged
action because of its stability to metabolism in the
living body.
_
_ 55
CA 02453123 2004-01-20
In consequence, on the basis of these functions,
the compounds of the present invention show water diuresis
action, urea excretion enhancing action, factor VIII
secretion inhibiting action, vasodilation action, cardiac
function accelerating action, mesangial cell contraction
inhibiting action, mesangial cell proliferation inhibiting
action, liver gluconeogenesis inhibiting action, platelet
aggregation inhibiting action, aldosterone secretion
inhibiting action, endotheline production inhibiting
action, central blood pressure controlling action, renin
secretion controlling action, memory controlling action,
thermoregulation action, prostaglandin production
controlling action and the like, and are useful as
characteristic water diuretics, urea excretion enhancers,
vasodilators, hypotensive agents, agents used to treat
heart failure and renal failure and blood coagulation
inhibitors, and are effective for the prevention and
treatment of heart failure, hyponatremia, syndrome of
inappropriate vasopressin secretion (SIADH), hypertension,
renal diseases (nephrosis, nephritis, diabetic
nephropathy, chronic or acute renal failure), edema, brain
edema, ascites, hepatic cirrhosis, hypokalemia, water
metabolism disorder, diabetes, various ischemic diseases,
cerebrovasculardisease, cyclothymic failure, gastric
ulcer, nausea, vomiting, syncope, renal function disorder
_ 56
CA 02453123 2004-01-20
and the like and for the alleviation of sequelae of
cerebral infarction, intracerebral bleeding and the like.
Usefulness of the compounds of the present
invention was confirmed by the following tests.
(1) Vi receptor binding assay
A rat liver membrane sample was prepared in
accordance with the method of Nakamura et al. (J. Biol.
Chem., 258, 9283 (1983)), and [3H]-Arg-vasopressin (2 nM,
specific activity = 75.8 Ci/mmol), 70 ug of the membrane
sample and each drug to be tested (10 $ to 10-4 M) were
incubated at 30 C for 3_0 minutes in 250 ul of 100 mM Tris-
HC1 buffer (pH 8.0) containing 5 mM magnesium chloride,
1 mM ethylenediaminetetraacetic acid (EDTA) and 0.1%
bovine serum albumin (BSA). Thereafter, the incubation
solution was sucked off using a cell harvester and free
ligand and excess buffer were removed by passing the
reaction mixture through a glass filter (GF/B), thereby
trapping receptor-bound labeled ligand on the glass
filter. The glass filter was taken out, thoroughly dried
and then mixed with a liquid scintillation cocktail, and
the amount of the membrane-bound j3H]-vasopressin was
measured using a liquid scintillation counter to calculate
the inhibition ratio by the following formula.
Inhibition ratio (%) =100 - C1 - Bi X 100
C - Bi
-
_ 57
CA 02453123 2004-01-20
C1: amount of [3H]-vasopressin bound to the membrane in
the coexistence of known amount of each drug to be
tested and [3H]-vasopressin
Co: amount of [3H]-vasopressin bound to the membrane when
the drug to be tested was not added
B1: amount of [3H]-vasopressin bound to the membrane in
the presence of excess vasopressin (10-6 M)
Concentration of the drug to be tested which gives
50% inhibition ratio by the above calculation was defined
as IC5p and used in the following formula to calculate the
binding affinity of nonradioactive ligand, namely the
dissociation constant (Ki).
Ki = IC50
1 + [L]/KD
[L]: concentration of radioactive ligand
KD: dissociation constant calculated from Scatchard plot
Negative logarithm of the thus calculated value
was used as pKi value. The results are shown in Table 1.
(2) V2 receptor binding assay
A rabbit renal medulla membrane sample was
prepared in accordance with the method of Campbell et al.
(J. Biol. Chem., 247, 6167 (1972)), and [3H]-Arg-
vasopressin (2 nM, specific activity = 75.8 Ci/mmol), 100
ug of the membrane sample and each drug to be tested (10-8
to 10~4 M) were subjected to the assay in the same manner
as the case of the aforementioned V1 receptor binding
-
_ 58
CA 02453123 2004-01-20
assay and the pKi values were calculated in the same
manner. The results are shown in Table 1.
Compounds of the present invention show excellent
arginine vasopressin antagonism. For example, the
compounds of Examples 17, 18(2), 20, 21, 23 and 37 showed
excellent antagonisms on both VI and V2 receptors, which
were markedly strong even in comparison with a V2 receptor
antagonist compound OPC-31260 and a Vi receptor antagonist
compound OPC-21268 which are under development as arginine
vasopressin antagonists (cf. Table 1).
-
- 59
CA 02453123 2004-01-20
Table 1
Antagonism on arginine vasopressin Vl and V2 receptors
Binding activity Binding activity
on arginine on arginine
vasopressin vasopressin
Example No. Vl receptor (pki) V2 receptor (pki)
1 8.33 7.21
2 8.82 8.25
4 8.36 8.69
6 7.95 8.62
8 7.74 8.25
8.61 8.59
12 8.52 8.01
8.91 8.93
17 9.04 9.11
18(1) 8.37 8.59
18(2) 9.05 8.83
9.18 9.04
21 8.74 8.42
22 8.11 8.07
23 8.91 8.98
24 7.77 8.64
27 8.21 7.23
37 9.49 9.30
38 8.24 7.31
Comparative 6.71 8.01
compound (1)*
Comparative 7.85 4.29
compound (2)**
60 -
CA 02453123 2004-01-20
* OPC-31260 (WO 9105549, compound of Example 408,
hydrochloride)
~Me
N M e
N
Me C?
L-"' N
H HCl
** OPC-2126.8 (EP 0382185, compound of Example 141)
N
N
O
CH3--CO+.fiH-(CH2 ) 3-0
(3) Vi antagonism in conscious rats (oral administration)
Vl antagonism was examined using male Wister rats
(body weight, 300 to 320 g) each of which has been
subjected, 2 to 3 days before the test, to cannulation
into the left carotid for the measurement of blood
pfessure and into the left jugular for the administration
of arginine vasopressin (AVP). Blood pressure was
measured under no anesthesia from the carotid cannula via
a pressure transducer. Each compound to be tested was
- 61 -
CA 02453123 2004-01-20
suspended in 0.5% methylcellulose aqueous solution and
orally administered in a dose of 1 or 10 mg/kg.
Increase in the diastolic blood pressure caused by
the intravenous administration of 30 mU/kg of AVP before
the administration of a compound to be tested was defined
as 100%, and increase in the blood pressure caused by the
intravenous administration of 30 mU/kg of AVP was measured
periodically during a period of from 30 minutes after the
test compound administration to 8 hours after the test
compound administration to calculate the inhibition ratio
of pressure increase by the test compound, namely VI
antagonism of the test compound.
Pressure increase by AVP was repressed to 50% or
below during a period of from 30 minutes after the test
sample administration to 6 hours after the test compound
administration by the administration of 1 mg/kg of each of
the compounds of Examples 18(2), 21 and 23, thus showing
prolonged action of the inventive compounds. On the other
hand, oral administration of OPC-21268 in a dose of
mg/kg which was ten times larger than the dose of these
inventive compounds was effective in repressing.the
pressure increase by AVP to 50% or lower level but during
a period of only from 30 minutes to 1 hour after the
administration, and the pressure increase by AVP returned
to the 100% level 4 hours after the administration, thus
showing disappearance of the Vl antagonism.
_ 62 -
CA 02453123 2004-01-20
On the basis of the above results, it was
confirmed that the V1 antagonism of the compounds of the
present invention by their oral admini.stration into
conscious rats is strong and long-acting in comparison
with OPC-21268.
(4) V2 antagonism (water -diuresi.s ).i.n conscious rats
(oral administration)
Each compound to be tested was suspended in 0.5%
methylcellulose aqueous solution and orally administered
in a dose of 3 mg/kg to male Wister rats (body weight., 270
to 300 g) which had been subjected to fasting with nca
water for 16 to 20 hours. Using a metabolic cage, urine
samples were collected just after the administration of
each test sample and until 4 hours after the
administration to measure the amount of urine.
In the test group in which each of the compounds
of Examples 18(2), 20, 21 and 23 was administered, the
amount of urine collected during a period of from just
after the administration to 2 hours after the
administration was 47 to 95 times larger than that in the
solvent-administered group, and the amount of urine
collected during a period of from 2 hours to 4 hours after
the administration was 8 to 10 times larger than that in
the solvent-administered group, thus showing prolonged
water diuresis enhancing effect. On the other hand, in
the OPC-31260-administered group, the amount of urine
- 63 -
CA 02453123 2004-01-20
collected during a period of from just after the
administration to 2 hours after the administration was 11
times larger than that in the solvent-administered group,
but the amount of urine collected during a period of from
2 hours to 4 hours after the administration was almost the
same as that in the solvent-administered group, thus
showing disappearance of the water diuresis enhancing
effect.
On the basis of the above results, it was
confirmed that the water diuresis enhancing effect of the
compounds of the present invention by their oral
administration into conscious rats is strong and long--
acting in comparison with OPC-31260.
A pharmaceutical composition which contains as its
active ingredient one or more of the compounds of the
general formula (I) and pharmaceutically acceptable salts
thereof is made into various dosage forms such as tablets,
powders, fine granules, granules, capsules, pills,
solutions, injections, suppositories, ointments, plasters
and the like, making use of conventionally used
pharmaceutical carriers, excipients and other additives,
and administered orally or parenterally.
Clinical dose of the compound of the present
invention to human may optionally be decided taking
symptoms, weight, age, sex and the like of each patierit
into consideration, but it may generally be 0.1 to 50C1 mg
-
- 64
CA 02453123 2004-01-20
per adult per day in the case of oral administration, and
the daily dose may be used in one portion or divided
portions. Since the dose varies under various conditions,
sufficient effects may be obtained in some cases with
smaller dose than the above range.
As solid compositions for oral administration
according to the present invention, tablets, powders,
granules and the like may be used. In such solid
compositions, one or more of active ingredient(s) may be
mixed with at least one inert diluent such as lactose,
mannitol, glucose, hydroxypropylcellulose, fine
crystalline cellulose, starch, polyvinyl pyrrolidone or
magnesium aluminate metasilicate. In accordance with the
conventional way, the composition may contain other
additives than the inert diluent, which include a
lubricant such as magnesium stearate,, a disintegrating
agent such as fibrin calcium glycolate, a stabilizing
agent such as lactose and a solubilizing agent or a
solution adjuvant such as glutamic acid or aspartic acid.
If necessary, tablets or pills may be coated with a film
of gastric or enteric substance such as sucrose, gelatin,
hydroxypropylcellulose, hydroxypropylmethylcellulose
phthalate or the like.
Liquid conipositions for use in oral administration
include pharmaceutically acceptable emulsions, solutions,
suspensions, syrups, elixirs and the like which contain
_ 65
CA 02453123 2004-01-20
conventionally used inert diluents such as purified water
and ethanol. In addition to the inert diluents, such
compositions may also contain adjuvarits such as a
solubilizing agent or a solution adjuvant, a moistening
agent, a suspending agent and the like, as well as a
sweetening agent, a flavoring agent, an aromatic agent and
an antiseptic agen'c.
Injections for use in parenteral administration
include aseptic aqueous or non-aqueous solutions,
suspensions and emulsions. Examples of diluent for use in
aqueous solutions and suspensions include distilled water
for injection use and physiological saline. Examples of
non-aqueous diluent for use in solutions and suspensions
include plant oils such as propylene glycol, polyethylene
glycol, olive oil and the like, alcohols such as ethanol
and the like and Polysorbate 80 (trade name). Such
compositions may also contain additives such as a tonicity
agent, an antiseptic agent, a moistening agent, an
emulsifying agent, a dispersing agent, a stabilizing agent
(lactose for example), a solubilizing agent or a solution
adjuvant and the like. These compositions are sterilized
by bacterial filtration through a bacteria-retaining
filter, bactericide blending or irradiation.
Alternatively, an aseptically produced solid composition
may be used by dissolving it in sterile water or a sterile
injection solvent prior to its use.
- 66
-
CA 02453123 2004-01-20
BEST MODE FOR CARRYING OUT THE INVENTION
Thus, the compounds of the present invention and
their production processes have been described which will
be further illustrated in detail with reference to the
following examples. The present invention, however, is
not limited by these examples. Since some of the starting
compounds of the prcsent invention are novel compounds,
examples of their production processes are shown as
Reference Examples.
Reference Example 1
A 3.32 g portion of 2,3,4,5-tetrahydro-lH-1-
benzazepin-5-one and 4.31 ml of triethylamine were
dissolved in 33 ml of dichloromethane and, with stirring
on an ice bath, 4.59 g of p-nitrobenzoyl chloride was
added to the resulting solution. The reaction solution
was stirred at room temperature for additional 60 minutes.
The reaction solution was then mixed with a saturated
sodium bicarbonate aqueous solution and subjected to phase
separation. The dichloromethane layer was separated and
washed with a-1 N hydrochloric acid aqueous solution and a
saturated sodium chloride aqueous solution once for each.
The thus washed layer was dried over anhydrous magnesium
sulfate and then concentrated under a reduced pressure.
The thus obtained residue was recrystallized from methyl
alcohol to obtain 5.68 g of 1-(4-nitrobenzoyl)-2,3,4,5-
tetrahydro-lH-l-benzazepin-5-one.
- 67 -
CA 02453123 2004-01-20
Physicochemical properties
1H-NMR (6 ppm in CDC13, TMS internal standard):
2.17 (2H, :rn), 2.90 (total 3H), 4.1 (1H), 6.7
(1H, m), 7.2 - 7.55 (total 4H), 7.78 - 8.15
(total 3H).
MS ( FAB ): 311 ( M~ + 1).
Reference Example 2
A 19.2 g portion of l-(4-nitrobenzoyl)-2,3,4,.5-
tetrahydro-lH-1-benzazepin-5-one was dissolved in air,;ixed
solvent consisting of 200 ml of dimethylformamide and
100 ml of methyl alcohol, and 3 ml of Raney nickel was
added to the resulting solution to carry out hydrogenation
at normal pressure.. After completion of the hydrogen
absorption, the reaction solution was filtered and
concentrated. The thus obtained residue was dissolved in
dichloromethane and then washed with a saturated sodium
bicarbonate aqueous solution. The resulting
dichloromethane layer was dried over anhydrous magnesium
sulfate and then concentrated under a reduced pressure.
The thus obtained residue was recrystallized from methyl
alcohol to obtain 15.5 g of l-(4-aminobenzoyl)-2,3,4,5-
tetrahydro-lH-l-benzazepin-5-one.
-
- 68
CA 02453123 2004-01-20
Physicochemical properties
1H-NMR (6 ppm in CDC13, TMS internal standard):
2.15 (2H, m), 2.90 (2H, m), 4.05 (2H), 6.45
(2H, d), 6.77 (lH, m), 7.0 -- 7.35 (total4H), 7.88
(1H, m).
MS ( FAB ): 281 (M+ + 1).
Reference Example 3
With stirring at -15 C, 2.25 ml of oxalyl chloride
and a catalytically effective amount of N,N-
dimethylformamide were added to a solution which had been
prepared by dissolving 3.4 g of o-phenylbenzoic acid in
34 ml of dichloromethane, and the resulting mixture was
warmed up to room temperature spending 2 hours and stirred
for additional 2 hours. The reaction solution was
concentrated under a reduced pressure and subjected to
azeotropic treatment three times with dichloromethan.e.
The thus obtained residue was dissolved in 34 ml of
dichloromethane and, with stirring on an ice bath, the
resulting solution was dropwise added to 40 ml of a
dichioromethane solution containing 4.0 g of 1-(4-
aminobenzoyl)=-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one and
3.0 ml of triethylamine. The reaction solution was warmed
up to room temperature and the stirring was continued for
120 minutes. The resulting reaction solution was mixed
with a saturated sodium bicarbonate aqueous solution, and
subjected to phase separation. The dichlorornethane layer
- 69 -
CA 02453123 2004-01-20
was separated, dried over magnesium sulfate and then
concentrated. The thus obtained residue was
recrystallized from toluene to obtain 5.82 g of 2-phenyl-
4'-[(5-oxo-2,3,4,5-tetrahydro-lH-l-benzazepin-l-
yl)carbonyl]benzanilide.
Physicochemical properties
1H-NMR (S ppm in CDC13, TMS internal standard):
2.23 (2H, m), 2.87 (2H, m), 4.1 (2H), 6.75
(1H, m), 6.8 - 7.7 (total 15H), 7.85 (1H, m).
MS (FAB): 461 (M+ + 1).
Reference Example 4
Using o-(4-methylphenyl)benzoic acid and 1-(4-
aminobenzoyl)-2,3,4,5-tetrahydro-lH-1-benzazepin-5-one as
starting materials, the procedure of Reference Example 3
was repeated to obtain 2-(4-methylphenyl)-4-[(5-oxo-
2,3,4,5-tetrah.ydro-lH-1-benzazepin-1
yl)carbonyllbenzanilide.
Physicochemical properties
1H-NMR (6 ppm in CDC13, TMS internal standard):
2.18 (2H, m), 2.35 (3H, s), 2.88 (2H, m), 4.1
(2H), 6.72 (1H, m), 6.85 - 7.7 (total 13H), 7.85
(2H).
MS (FAB): 475 (M+ + 1).
Example 1
After dissolving 500 mg of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-
- 70 -
CA 02453123 2004-01-20
yl)carbonyl]benzanilide in a mixed solvent consisting of
15 ml of chloroform and 1.5 ml of ethyl acetate, the
resulting solution was mixed with 560 mg of copper(II)
bromide and subjected to 3 hours of heating under reflux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration and the resulting filtrate was
washed with a saturated sodium bicarbonate aqueous
solution. The resulting organic layer was dried over
anhydrous magnesium sulfate, concentrated under a reduced
pressure and then evaporated to dryness using a vacuum
pump. The thus obtained solid substance was dissolved in
12 ml of ethyl alcohol, and the resulting solution was
mixed with 100 mg of thiourea and subjected to 3 hours of
heating under reflux. During the reflux, colorless
crystals were precipitated. After cooling the reaction
solution on an ice bath, crystals were collected by
filtration and washed with a small volume of ethyl alcohol
to obtain 540 mg of 4 -[(2-amino-5,6-dihydro-4H-
thiazolo[5,4-d][l]benzazepin-5-yl)carbonyl]-2-
phenylbenzanilide hydrobromate.
Physicochemical properties
Melting point: >250 C
_ 71 -
CA 02453123 2004-01-20
Elemental analysis data (C31H24N4 O2S-HBr)
C(%) HM N(%) SM Br(%)
Ca1c.: 62.31 4.22 9.38 5.37 13.37
Found: 62.39 4.42 9.18 5.21 13.51
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
2.8 - 3.4 (total 3H), 5.0 (1H), 6.6 - 7.8
(total. 16H), 8.16 (1H, m), 10.27 (1H, s).
MS (FAB): 517 (M+ + 1).
Example 2
After dissolving 500 mg of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]benzanilide in a mixed solvent consisting of
15 ml of chloroform and 1.5 ml of ethyl acetate, the
resulting solution was mixed with 560 mg of copper(I:[)
bromide and subjected to 3 hours of heating under reflux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials we:re
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in a mixed solvent
consisting of 10 ml of 2-propyl alcohol and 2 ml of methyl
alcohol, and the resulting solution was mixed with 155 mg
of guanylthiourea and subjected to 6 hours of heating
-72-
CA 02453123 2004-01-20
under reflux. During the reflux, colorless crystals were
precipitated. After cooling the reaction solution on an
ice bath, crystals were collected by filtration and washed
with a small volume of cold 2-propyl alcohol. The thus
washed crystals were recrystallized from methyl alcohol to
obtain 452 mg of 4'-[(2-guanidino-5,6-dihydro-4H-
thiazolo[5,4-dJ[lJbenzazepin-6-yl}carbonylJ-2-
phenylbenzanilide hydrobromate.
Physicochemical properties
Melting point: >250 C
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
2.9 - 3.5 (total 3H), 4.95 (1H), 6.7 - 7.8
(total 16F[), 8.18 (total 5H), 10.32 (1H, s).
MS (FAB): 559 (M} + 1).
Example 3
The reaction of Example 1 was repeated except that
470 mg of 2-(4-methylphenyl)-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-l-yl)carbonyl]benzanilide was
used as the starting material, the resulting reaction
solution was concentrated and the thus obtained residue
was subjected to phase separation using ethyl acetate and
a sodium bicarbonate aqueous solution. The ethyl acetate
layer was separated, dried over magnesium sulfate and then
concentrated. The thus obtained residue was
recrystallized from ethyl acetate to obtain 358 mg of 4'-
_ 73 -
CA 02453123 2004-01-20
[(2-amino-5,6-dihydro-4H-thiazoloj5,4-d][1]benzazepin-6-
yl)carbonyl)-2-(4-methylphenyl)benzanilide.
Physicochemical properties
Melting point: 161 - 163 C
Elemental analysis data (C32H25N402S)
C(%) H(g) N(%) S(g)
Calc.. 72.43 4.94 10.56 6.04
Found: 72.32 4.85 10.52 5.78
1H-NMR (8 ppm in DMSO-d6, TMS internal standard):
2.27 (3H, s), 3.07 (2H), 5.0 (1H), 6.72 (1H, m),
6.8 - 7.7 (total 14H), 8.18 (1H, m), 10.29
(1H, s).
MS ( FAB ): 531 (M-' + 1) .
Example 4
Using 400 mg of 2-(4-methylphenyl)-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-l-benzazepin-l-=
yl)carbonyl]benzanilide as a starting material, the
procedure of Example 2 was repeated to obtain 392 xng of
4'-[(2-guanidino-5,6-dihydro-=4H-thiazolo[5,4-
d][13benzazepin-6-yl)carbonyl]-2-(4-
methylphenyl)benzanilide hydrobromate.
Physicochemical properties
Melting point: >230 C
- 74 -
CA 02453123 2004-01-20
Elemental analysis data (C33H28N602S-HBr)
C(%) H(%) N(%-) S(%) Br($)
Calc.: 60.64 4.47 12.86 4.91 12.2:3
Found: 60 . 35 4.49 12.72 4.73 12.08
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
2.27 (3H, s), 3.30 (total 3H), 6.7 - 7.8
(total 15H), 7.92 (total 4H), 8.22 (1H, m), 10.29
(lH, s).
MS (FAB): 573 (M+ + 1).
Example 5
After dissolving 400 mg of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-benzazepin-l-yl)carbonyl]benzanilide
in a mixed-solvent: consisting of 15 ml of chloroform and
2 ml of ethyl acetate, the resulting solution was mixed
with 390 mg of copper(II) bromide and subjected to 3 hours
of heating under reflux with vigorous stirring. After
cooling down the reaction solution to room temperature,
insoluble materials were removed by filtration. The
resulting filtrate was washed with a saturated sodium
bicarbonate aqueous solution. The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using a vacuum pump. The thus obtained solid substance
was dissolved in 20 ml of 2-propyl alcohol, and the
resulting solution was mixed with 372 mg of 4-
imidazolylthioaceyamide hydrochloride and subjected to
-,75-
CA 02453123 2004-01-20
24 hours of heating under reflux. After cooling down the
reaction solution to room temperature, the solvent was
distilled off and the resulting residue was mixed with
chloroform and a saturated sodium bicarbonate aqueous
solution to separate the resulting organic layer which was
subsequently washed with water and a saturated sodium
chloride aqueous solution, dried over anhydrous magnesium
sulfate and then subjected to removal of the solvent by
distillation under reduced pressure. The thus obtairied
residue was subjected to silica gel column chromatography
and elution was carried out with chloroform-methyl alcohol
(25:1). The resulting eluate in chloroform was mixed with
ml of 4 N hydrochloric acid-ethyl acetate and the
solvent was removed by distillation, and the thus obtained
residue was recrystallized from ethyl alcohol-diethyl
ether to obtain 262 mg of 4'-j(2-(4-imidazolylmethyl)-5,6-
dihydro-4H-thiazolo[5,4-d][l]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide=2HC1.
Physicochemical properties
Melting point: 192 - 195 C
Elemental analysis data (C35H27N5O2S-2HCl=1.5H2O)
C(%) H(A) N(%) S(%) Cl(%)
Calc.: 61.67 4.73 10.27 4.70 10.40
Found: 61.82 4.37 10.27 4.79 10.31)
_ 76 -
CA 02453123 2004-01-20
1H-NMR (8 ppm in DMSO-d6, TMS internal standard):
3.04 (lH, m), 3.37 (2H, m), 4.56 (2E, s), 5.00
(1H, m), 6.78 (1H, d), 6.90 (2H, d), 7.08 (1H, t),
7.25 - 7.69 (total 14H), 8.29 (1H, d), 10.35
(1H, s), 14.59 (1H, s).
MS (FAB): 582 (M+ + 1).
Example 6
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-1-yl)carbonyl]benzanilide and
262 mg of 4-(2-methylimidazolyl)thioacetamide
hydrochloride, the procedure of Example 5 was repeated to
obtain 263 mg of 4'-[[2-[4-(2-methylimidazolyl)methyl]-
5,6-dihydro-4H-th-iazolo[5,4-d][1]benzazepin-6-
yl)carbonyl]-2-phenylbenzanilide=2HC1.
Physicochemical properties
Melting point: 197 - 2000C
Elemental analysis data (C36H291V5O2S=2HCl--1.5H2O)
C(%) H(%) N(%) S(%) Cl(%)
Caic.: 62.97 4.82 10.20 4.67 10.33
Found: 62.75 4.62 10.24 4.73 9.99
1H-NMR (6 ppm in DMSO-d 6, TMS internal standard):
2.56 (3H, s), 3.05 (1H, m), 3.36 (2H, m), 4.48
(2H, s), 5.00 (1H, m), 6.79 (1H, d), 6.90 (2H, d),
7.09 (1H, t), 7.25 - 7.58 (total 13H), 8.33
(1H, d), 10.34 (1H, s), 14.20 (1H, s).
MS (FAB): 596 (M} + 1).
- 77 -
CA 02453123 2004-01-20
Example 7
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide and
370 mg of 2-pyridy:Lthioacetamide hydrochloride, the
procedure of Example 5 was repeated, and the resulting
free base was recrystallized from chioroform-diethyl ether
to obtain 300 ing of 4'-[[2-(2-pyridylmethyl)-5,6-dihydro-
4H-thiazolo[5,4-d][1]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide.
Physi.cochemica:l properties
Melting point: 215 - 218 C
Elemental analysis data (C37H28N402S)
CM HM N(%) S(%)
Calc.: 74.98 4.76 9.45 5.41
Found: 74.69 4.68 9.32 5.39
1H-NMR (6 ppm in CDC13, TMS internal standard):
3.10 (2H, m), 3.49 (1H, m), 4.56 (2H, s), 5.17
(1H, dd), 6.66 (1H, d), 6.85 (1H, d), 6.96 - 7.10
(5H, m), 7.22 - 7.49 (total 8H), 7.46 (1H, t)-
7.53 (1H, t), 7.61 (1H, t), 7.86 (1H, d), 8.42
(1H, d), 8.63 (1H, d).
MS (FAB): 593 (M} + 1).
Example 8
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide and
400 mg of 3-pyridylthioacetamide hydrochloride, the
- 78 -
CA 02453123 2004-01-20
.. ,~5' ' T=
S= -
..Y. =t.='::L, '!'!i~' ii'( .ri-'..{ =f+i= ==y;~!:=w
r~:wi~ :~f.=. :~=tffrr=J7{"t:.= '=-t=+.w'": 'f'.y=
- ' - L. - :Y= =y.= _ '-ti= _ .~ : }'
' ~ s6 =2f:-~, =s.=.
: y-w iõ~,= . i ,~= .
=:s'. si t ~I:. .i===t =+:.
;:.yn . 'rt.=5.,...'
- .:~=' =' .{..
. =}'.i ..:y .:~: er=.
. . . 'r:T . . . ..
, . . . = =r, '. . ,
procedure of Example 5 was repeated. to obtain 100, mg of
4 ' [ I 2- ( 3-pyri-dyl~iethy.l ) -5 , 6-dihydro-4H-th3azol.ol 5, 4 -"
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide
hydrochloride as an amorphous solid.
Physicochemical properties
1H-NMR (6 ppm in UMSO-ds, TMS internal standard):
3.03 (1E, m), 3.29 (2H, m), 4.66 (2H, s), 4.99
(IH, d), 6.78 (IH, d), 6.89 (2H, d), 7.08 (IH, t),
7.25 - 7.58 (total 12H), 8.03 (IH, t), 8.25
(IH, d), 8.60 (1H, d), 8.85 (1H, d), 9.04 (111, s),
10.32 (1H,- s).
MS (FAB): 593 (M~ + 1).
Example 9
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide and
337 mg of 4-morpholynobutanethioamide hydrochloriae, the
procedure of Example 5 was repeated and the resulting
residue was recrystallized from methyl alcohol-diethyl
ether to obtain 360 mg of 4'--[[2-(3-morpholynopropyl)-5,6-
dihydro-4H-thiazolo[5,4-d][1]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: 215 - 218 C
- . s. _,.
79 -
CA 02453123 2004-01-20 '
.~: .r= ~r =e~.: '.~.
s4 =..'~: = ,~e',
!. :.rM ~t c=::= tit~.
i. =r":. -.z 'l:~ , ri''e. ~~' _y ~{,
, 4'4: , =,s., .. F~~ .~'.e . i . 'I .. 4 Y.,.
.y.: =-_'. ..~.C'e 't = . = 7 .
._aõ c:. ..r= 'ti=." i',A.o ~':= -rP:.ii=.. .C.~n''~
t.: e;=.'- ....- .-. Mõ=i.'= .:a :rti=.. _ ai':.
= S- - . =-~,~ . = ~ q. j = _ = w.r.', =.=t-.. . = :i' =t,.=:,C.'a
Elemental analysis data (C38N36N403S-2HC1-1.6H20)
C(%) H(-~i) = N(.A S(%')= - C
Calc::- 62.48 5.68 7.67 4.39 9.71
Found: 62.13 5.59 7.45 4.38 9.16
1H-NMR (S ppm in DMSO-ds, TMS internal standard):
2.27 (2E, m), 3.06 - 3.39 (total 9H), 3.45
(2H, m), 3.85 (1H, rn), 3.85 (2H, t), 3.95 (2H, m),
5.00 (1H, m), 6.79 (1H, d), 6.90 (2H, d), 7.08
(1H, t), 7.25 - 7.57 (total 12H), 8.35 (1H, ci),
10.34 (1H, s).
MS (FAB) : 629 (M+ + 1).
Example 10
Using 400 mg of 2-phenyl-4'-((5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilideand
300 mg of dimethyl.aminoethylthiourea hydrochloride and
using ethyl alcohol as the.reaction solvent, the procedure
of Example 5 was repeated and the resulting residue was
recrystallized from ethyl acetate-diethyl ether to ohtain
300 mg of 4'-[(2-dimethylaminoethylaraino-5,6-dihydro-4H-
thiazolo[5,4-d][l]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide-2EC1.
Physicochemical properties
Melting point: 187 - 190 C
A=
- 80 -
CA 02453123 2006-11-01
2167673
Elemental analysis data (C35H33N5021S -2HC1-3H20)
C(%) H(%) N(%) S(%) Cl(%)
Calc.: 58.82 5.78 9.80 4.49 9.92
Found: 58.60 5.40 9.73 4.53 9.51
'H-NMR (S ppm in DMSO-d6, TMS internal standard):
2.85 (6H, s), 3.02 (2H, m), 3.19 (1H, m), 3.37
(2H, t), 3.76 (2H, m), 4.97 (1H, m), 6.74 (1H, d),
6.93 (2H, d), 7.04 (1H, t), 7.24 - 7.58 (total
12H), 8.24 (1H, d), 10.35 (1H, s), 10.59 (1H, s).
MS (FAB): 514 (M+ + 1).
Example 11
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-l-yl)carbonyl]benzanilide and
204 mg of dimethylaminothioacetamide hydrochloride, the
procedure of Example 5 was repeated to obtain 167 mg of
4'-[(2-dimethylamino-5,6-dihydro-4H--thiazolo[5,4-
d][1]benzazepin-6-yl.)carbonyl]-2-phenylbenzanilide
hydrochloride as an amorphous solid.
Physicochemical properties
'H-NMR (6 ppm in DMSO-d6, TMS internal standard):
3.04 (1H, m), 3.12 (6H, s), 3.29 (2H, d), 4.96
(1H, m), 6.7-3 (1H, d), 6.92 (2H, d), 7.04 (1H, t),
7.24 - 7.58 (total 12H), 8.24 (1H, d), 10.33
(1H, s).
MS (FAB) : 545 (iK+ + 1).
- 81 -
CA 02453123 2004-01-20
W ~, " h J ~ ' t c T
.'S~:=:,f. i ;' c ?=-~=" x.. . r t = =.~== . a. , = i . , :,
Example 12
Using 4.00 mg of 2-phenyl=4'--[j5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l---yl)carbonyl3benzanilide and-
285 . mg of dimethylaminobutanethioamide hydrochloride, the
procedure of Example 5 was repeated to obtain 212 mg of
4'-[[2-(3-dimethylaminopropyl)-5,6-dihydro-4H-
thiazolo[5,4-d][1]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide hydrochloride as an amorphous solid.
Physicochemical properties
1H-NMR Q ppm in DMSO-d6, TMS internal standard):
2.19 (2H, m), 2.79 (6H, s), 3.10 (3H, m), 3.18
(2H, t), 3.27 (2H, m), 5.04 (1H, m), 6.77 (1H, d),
6.90 (2H, d), 7.08 (?H, t), 7.25 -7.58 (total
12H), 8.35 (1H, d), 10.33 (1H, s).
MS (FAB): 587 (M+ + 1).
Exainple 13
Using 400 mg of 2-phenyl-4 -[(5-oxo-2,3,4,5-
tetrahydro-lH--l-benzazepin-l-yl)carbonyl]benzanilide and
185 mg of 2-carboxypropanethioamide, the procedure of:
Example 5 was repeated and the resulting free base was
recrystallized from methyl alcohol-diethyl ether to obtain
186 mg of 4'-[(2-methyl-5,6-dihydro-4H-thiazo3.o[5,4-
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide.
Physicochemical properties
Melting point:, 165 - 168 C
- ~= ..
- 82 -
CA 02453123 2004-01-20
Elemental analysis data (C32H25N302S 0.4H20)
C(%) E(%) N(%) S(%)
Calc.L 73.52 4.97 8.04 6.13
Found: 73.35 5.08 7.56 5.88
1H-NMR (d ppm in DMSO-d6, TMS internal standard):
2.75 (3H, s), 3.07 - 3.19 (2H, m), 3.55 (1H, m),
5.20 (1H, m), 6.65 (1H, d), 6.85 (2H, d), 6.96 -
6.99 (3H, m), 7.01 - 7.85 (total 9H), 8.38
(1H, d), 8.39 (1H, d).
MS (FAB): 516 (M+ + 1).
Example 14
(1) After dissolving 461 mg of 2-phenyl-4'-1(5-
oxo-2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyllbenzanilide in a mixed solvent consisting of
14 ml of chloroform and 1.4 ml of ethyl acetate, the
resulting solution was mixed with 560 mg of copper(II)
bromide and subjected to 3 hours of heating under reflux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in 12 ml of 2-
propyl alcohol, and the resulting solution was mixed with
- 83 -
CA 02453123 2004-01-20
220 mg of phthalimidothioacetamide and subjected to 6
hours of heating under reflux. During the reflux,
colorless crystals were precipitated. After cooling the
reaction solution on an ice bath, crystals were collected
by filtration and washed with a small volume of cold 2-
propyl alcohol to obtain 410 mg of 4'-[(2-
phthalimidomethyl-5,6-dihydro-4H-thiazolo[5,4-
d]jl]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide.
Physicochemical properties
1H-NMR (6 ppm in CDC13. TMS internal standard):
2.8 -- 3.8 (total 3H), 5.21 (2H, s), 6.64 (1H, dd),
6.75 - 8.1 (total 19H), 8.40 (1H, dd).
MS ( FAB ): 661 (M+ + 1).
(2) After suspending 390 mg of 4'-j(2-
phthalimidomethyl-5,6-dihydro-4H-thiazolo[5,4-
d][l]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide in 20
ml of methyl alcohol, the resulting suspension was mixed
with 1.2 ml of a mixed solvent consisting of 40 weight
parts of methylamine and 60 weight parts of methyl alcohol
and stirred overnight at room temperature. The reaction
solution was concentrated and the thus obtained residue
was purified by silica gel column chromatography
(chloroform-methyl alcohol = 20:1). The thus obtained
solid substance was dissolved in 3.5 ml of methyl alcohol,
and the resulting solution was mixed with a 4 N
hydrochloric acid-ethyl acetate solution and then with
- 84 -
CA 02453123 2004-01-20
r 7. N=.
==a=r ="1. . . . . . ..S";..
acetonitrile to effect formation of 3DreciDitate. The thus
forrried precipitate was collected. by i=iltration and washed:
with a small volume of acetonitrile"to obtain 200'mcr of
4 -[(2-aminomethyl-5,6-dihydro-4H-th=lazolo[5,4-
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide
hydrochloride.
Physicochemical properties
HPLC purity: >96%; ODS-80TM (Tosoh)
1H-NMR (d ppm in DMSO-d5, TMS internal standard):
2.51 (1H, m), 3.09 (1H, m), 3.36 (total 2H), 4.47
(2H, s), 5.02 (1H), 6.85 (2H), 7.11 (1H, t), 7.2 -
7.7 (total 13H), 7.9 (1H), 8.45 (1H, d), 8.81
(2H), 10.35 (1Hy s).
MS ( FAB ) a 5 31 (M+ + 1) .
Example 15
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide and
300 mg of 3-phthaiimidopropanethioami.de, the procedure of
Example 14 was repeated to obtain 135 mg of 4'-[(2-
aminoethyl-5,6-dihydro-4H-thiazolo[5,4-d][1]benzazepin-6-
yl)carbonyl]-2-phenylbenzanilide hydrochloride.
Physicochemical properties
HPLC purity: >91%; ODS-80TM (Tosoh)
- ~= _.
-
_ 85
CA 02453123 2004-01-20
' i pz 'J='h .Y ' _ - .='
' . . - . , . , . . t'. .
1H-NIKR (6 ppm in DMSO-dfi, TMS internal standard):
3:05. (lH,* m), 3.40 - 3.37- (tota:l 6H), -5.01
(1H, m), 6.77 (1H, d), 6.91: (2H, d), 7.09 (1H, t),
7.25 - 7.58 (total 12H), 8.14 (iH, br), 8.38
(1H, d), 10.33 (1H, s).
MS (FflB) : 545 (M} + 1) .
Example 16
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-be.nzazepin-l-yl)carbonyl]benzanilide and
376 mg of 4_Phthalimidebutanethioamide, the procedure of
Example 14 was repeated to obtain, using ethyl alcohol-
ethyl acetate as a recrystallization solvent, 193 mg of
4'-[(3-aminopropyl--5,6-dihydro-4H-thiazolo[5,4-
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide
hydrochloride.
Physicochemical properties
Melting point: 185 - 188 C
Elemental analysis data (C34H30 N4O2S-HCl H2O)
C(%) H(%) N(%-) SM Cl(%)
Calc.: 62.50 5.29 8.41 6.39 7.90
Found: 62.27 5.09 8.51 5.17 8.15
1H-NMR (S ppm in DMSO-ds, TMS internal standard):
2.09 (2H, m), 2.97 (2H, m), 3.05 (1H, m), 3.10
(1H, t), 3.34 (2H, m), 5.01 (1H, m), 6.77 (1H, d),
6.89 (2H, d), 7.08 (1H, t), 7.26 - 7.58 (total
12H), 7.99 (2H, br), 8.33 (lH, d), 10.33 (1H, s).
- . ~= -_
_ 86 -
CA 02453123 2004-01-20
MS (FAB) : 559 (M} + 1).
Example 17
After dissolving 176 mg of t-
butoxycarbonylglycine, 205 mg of 1-hydroxybenztriazole and
0.15 ml of N-methylmorpholine in 3.5 ml of
dichloromethane, 192 mg of 1-ethyl-3--(3-
dimethylaminopropyl)carbodiimide hydrochloride was added
to the resulting solution with stirring on an ice bath,
and the mixture was warmed up to room temperature and
stirred for 60 minutes. To this reaction solution, again
cooled on an ice bath, was added dropwise 4 ml of
dichloromethane in which 400 mg of the 4'-[(2-amino-5,6-
dihydro-4H=thiazolo[5,4-d][1]benzazepin-6-yl)carbonylJ-2-
phenylbenzanilide hydrobromide described in Example 1 and
0.103 ml of triethylamine had been dissolved, followed by
overnight stirring at room temperature. The reaction
solution was mixed with water, stirred for 60 minutes and
then subjected to phase separation. The dichloromethane
layer was separated, washed with a saturated sodium
bicarbonate aqueous solution and a saturated sodium
chloride aqueous solution once for each and then dried
over anhydrous magriesium sulfate. After removing the
solvent by distillation, the thus obtained residue was
suspended in 3 ml of methyl alcohol. With cooling on an
ice bath, the suspension was mixed with 4.4 ml of 4 N
hydrochloric acid-dioxane and stirred for 3 hours.
-
_ 87
CA 02453123 2004-01-20
Thereafter, the reaction solution was concentrated and the
thus obtained residue was recrystallized from 2-propyl
alcohol to obtain 250 mg of 4'-[(2-gl.ycylamino-5,f-
dihydro-4H-thiazolo[5,4-d][1]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide 2-propylalcohol hydrochloride.
Physicochemical properties
Melting point: >230 C
Elemental analysis data (C33H27N5 3S-HCl C3H80)
CM H(%) N(%) SW Cl(%)
Calc.: 64.51 5.41 10.45 4.78 5.29
Found: 64.35 5.19 10.20 4.80 5.10
'H-NMR (6 ppm in DMS -d6, TMS internal standard):
1.04 '(6H, d), 3.80 (1H, Ta), 5.05 (1H), 6.7 - 7.8
(total 16H), 8.24 (1H, dd), 1Ø30 (1H, s).
MS (FAB) : 574 (M+ -+, 1).
Example 18
After dissolving 500 mg of 2-,phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro--1H-1-benzazepin-l-
yl)carbonyl]benzana..lide in a mixed solvent consisting of
15 ml of chloroforni and 1.5 ml of ethyl acetate, the
resulting solution was mixed with 560 mg of copper(II)
bromide and subjected to 3 hours of heating under ref:tux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
_ 88 -
CA 02453123 2004-01-20
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in 10 ml of
acetonitrile, and the resulting solution was mixed with
750 mg of potassium carbonate and 510 mg of acetoamidine
hydrochloride and subjected to 90 minutes of heating under
reflux with vigorous stirring. After cooling down the
reaction solution to room temperature, insoluble materials
were removed by filtration and then the solvent was
distilled off under a reduced pressure. The resulting
residue was dissolved in chloroform, and the resulting
solution was washed with water and dried over anhydrous
magnesium sulfate. After distilling off the solvent, the
thus obtained residue was purified by silica gel column
chromatography (chloroform-methyl alcohol = 20:1) to
obtain, in the order of elution, 4'-[(2-methyl-5,6-
dihydro-4H-oxazolo[4,5-d]jl]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide and 4'-[(2-methyl-1,4,5,6-tetrahydro-
imidazo[4,5-d][l]benzazepin-6-=yl)carbonyl]-2-
phenylbenzanilide.
4'-[(2-Methyl-5,6-dihydro-4H-oxazolo[4,5-
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide was
recrystallized from ethyl acetate to obtain 40 mg of
crystals (Example 18(1)).
_
_ 89
CA 02453123 2004-01-20
4'-[(2-Methyl-1,4,5,6-tetrahydroimidazo[4,5--
d][l]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide was
dissolved in 5 ml of ethyl alcohol, the resulting solution
was mixed with 0.19 ml of 4 N hydrochloric acid-ethyl
acetate and cooled on an ice bath and then the thus
precipitated crystals were collected by filtration and
washed with a small volume of ethyl alcohol to obtain
220 mg of 4'-[(2-methyl-1,4,5,6-tetrahydroimidazo[4,5-
d][1]benzazepin-6-yl)carbonyl]-2-phenylbenzanilide
hydrochloride (Example 18(2)).
Physicochemical properties
4'-[(2-Methyl-5,6-dihydro-4H-oxazolo[4,5-d][l]benzazepin-
6-y1)carbonyl]-2-phenybenzanilide
Melting point: 234-236 C
1H-NMR (S ppm in CDC13, TMS internal standard):
2.57 (3H, s), 2.90 (2H, m), 3.27 (1H, m), 5.17
(1H, m), 6.66 (1H, d), 6.8 - 7.0 (total 6H), 7.23
(1H), 7.3 - 7.6 (total 8H), 7.7 - 7.9 (total 2H).
MS (FAB): 500 (M} + 1)
(CI) : 499 (M+).
High Resolution MS (FAB): Found 500. 200597
Calc. 500. 197417
Rational formula C32H25N3O3
4'-[(2-Methyl-1,4,5,6-tetrahydroimidazo[4,5-
d][l,benzazepin-6-yl)carbonyl]-2-phenylbenzanilide
hydrochloride
_ 90 -
CA 02453123 2004-01-20
Melting point: >230 C
1H-NMR (8 ppm in DMSO-d6, TMS internal standard):
2.70 (3B, s), 2.99 (1E, t), 3.17 (2H, m), 4.99
(1H, m), 6.8 - 7.0 (total 3H), 7.14 (1H, t), 7.2 -
7.7 (total 12H), 8.02 (1H, d), 10.31 (1H, s), 14.6
(total 2H).
MS (FAB): 499 (M+ ~ 1)
(Cl) : 498 (M+).
High Resolution MS (FAB): Found 499. 215808
Calc. 499. 213401
Rational formula C32H26N402
Example 19
After dissolving 800 mg of 2-=(4-methylphenyl)-4'-
[(5-oxo-2,3,4,6-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]benzani.lide in a mixed solvent consisting of
24 ml of chloroforr;l and 2.4 ml of ethyl acetate, the
resulting solution was mixed with 560 mg of copper(II)
bromide and subjected to 3 hours of heating under retlux
with vigorous stirring. After cooling down the react:ion
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in 16 ml of
-91-
CA 02453123 2004-01-20
acetonitrile, and the resulting solution was mixed with
1.17 g of potassium carbonate and 800 mg of acetoamidine
hydrochloride and subjected to 120 minutes of heating
under reflux with vigorous stirring. After cooling down
the reaction solution to room temperature, insoluble
materials were removed by filtration and then the solvent
was distilled off unde, a reduced pressure. The resulting
residue was dissolved in chloroform, and the resulting
solution was washed with water and dried over anhydrous
magnesium sulfate. After distilling off the solvent, the
thus obtained =residue was purified by silica'gel column
chromatography (chloroform-methyl alcohol = 30:1) to
obtain, in the order of elution, 2-(4-methylphenyl)-4'-
[(2-methyl-5,6-dihydro-4H-oxazolo[4,5-d][1]benzazepin-6-
yl)carbonyl]benzanilide (Example 19(1)) and 2-(4-
methylphenyl)-4'-[;(2-methyl-1,4,5,6-tetrahydro-
imidazo[4,5-d][l]benzazepin-6-yl)carbonyl]benzanilide.
2-(4-Methylphenyl)-4'-[(2-methyl-1,4,5,6-
tetrahydroimidazoj4,5-d][1]benzazepin-6-
yl)carbonyl]benzanilide was dissolved in 10 ml of ethyl
alcohol, the resulting solution was mixed with 0.37 ml of
4 N hydrochloric acid-ethyl acetate and cooled on an ice
bath and then the thus precipitated crystals were
collected by filtration and washed with a small volume of
ethyl alcohol to obtain 500 mg of 2-(4-methylphenyl)-4'-
-92-
CA 02453123 2004-01-20
[(2-methyl-1,4,5,6--tetrahydroimidazo[4,5-d][l]benzazepin-
6-yl)carbonyl]benzanilide hydrochloride (Example 19(2)).
Physicochemical properties
2-(4-Methylphenyl)--4'-[(2-methyl-1,4,5,6-tetrahydro-
imidazo[4,5-d][1]benzazepin-6-yl)carbonyl]benzani.lide
hydrochloride
MelLing point: 220 - 223 C
IH-NMR (S ppm in DMSO-d6, TMS internal standard):
2.25 (3H, s), 2.67 (3H, s), 3.02 (1H, m), 3.16
(2H, m), 4.99 (1H, m), 6.8 - 7.0 (total 3H), 7.15
(total 3H), 7.2 - 7.6 (total 9H), 8.04 (1H, d),
10.33 (1H, s),-14.6 (total 2H).
MS (FAB): 513 (M+ + 1)
Example 20
After dissolving 400 mg of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-l-benzazepin-l-
yl)carbonyl]benzanilide in a mixed solvent consisting of
15 ml of chloroform and 2 ml of ethyl acetate, the
resulting solution was mixed with 390 mg of copper(II)
bromide and subjected to 3 hours of heating under reflux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
-93-
CA 02453123 2004-01-20
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in 20 ml of
acetonitrile, and the resulting solution was mixed with
1.1 g of potassium carbonate and 371 mg of
ethylcarbamidine carbonate and subjected to 1 hour of
heating under reflux with vigorous stirring. After
filtration of the reaction solution, solvent in the
resulting filtrate was distilled off, and the resulting
residue was mixed with a saturated sodium bicarbonate
aqueous solution and chloroform to separate the organic
layer which was subsequently washed with water and a
saturated sodium chloride aqueous solution and dried over
anhydrous magnesium sulfate. After distilling off the
solvent under a reduced pressure, the thus obtained
residue was subjected to silica gel column chromatography
and eluted with a niixed solvent of chloroform and methyl
alcohol (20:1). The resulting eluate was mixed with '5 ml
of 4 N hydrochloric acid-ethyl acetate and cooled on an
ice bath, and the thus precipitated crystals were
collected by filtration and subjected to recrystallization
using ethyl alcohol as a recrystallization solvent,
thereby obtaining 248 mg of 4'-j(2-ethyl-1,4,5,6-
tetrahydroimidazo[4,5-d](1]benzazepin.-6-yl)carbonyl]-2-
phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: >230 C
- 94 -
CA 02453123 2004-01-20
Elemental analysis data (C33H28N402-HC1-1.6H20)
C($) H(%) N(%) Cl(%)
Calc.: 68.59 5.62 9.69 6.13
Found: 68.28 5.54 9.62 6.48
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
1.38 (3H, t), 2.99 (1H, t), 3.08 (2H, q), 3.1.2
(2H, m), 4.98 (1H, m), 6.76 (1H, d), 6.93 (2H, d),
7.14 (1H, t), 7.26 - 7.58 (total 12H), 8.13
(1H, d), 10.31 (1H, s), 14.70 (1H, br).
MS (FAB) : 513 (M+ + 1).
Example 21
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-1-yl)carbonyljbenzanilide,
597 mg of propylcarbamidine carbonate and-1.2 g of
potassium carbonate, the procedure of Example 20 was
repeated to obtain, using ethyl acetate-ethyl alcohol as a
recrystallization solvent, 243 mg of 4'-[(2-propyl-
1,4,5,6-tetrahydroimidazo(4,5-d][lJbenzazepin-6-
yl)carbonyl)-2-phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: >230 C
Elemental analysis data (C34H3ON402'HC1y2H2O)
r(%) H(%) N(%) C1(%)
Calc.: 68.16 5.89 9.35 5.92
Found: 68.86 5.61 9.62 6.00
-95-
CA 02453123 2004-01-20
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
1.00 (3H, t), 1.80 (2H, q), 2.99 (3H, m), 3.56
(2H, m), 4.99 (1H, m), 6.86 (1H, d), 6.93 (2H, d),
7.13 (1H, t), 7.23 - 7.58 (total 12H), 8.08
(1H, d), 110.32 (1H, s), 14.60 (1H, br).
MS (FAB): 527 (M} + 1).
Example 22
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-l-yl)carbonyl]benzanilide,
576 mg of benzylcarbamidine carbonate and 740 mg of
potassium carbonate, the procedure of Example 20 was
repeated to obtain, using ethyl acetate-ethyl alcohol as a
recrystallization solvent, 225 mg of 4'-[(2-benzyl-
1,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl)carbonyl]-2-phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: >230 C
Elemental analysis data (C38H3DN402=HC1 1..5Ha0)
C(g) HM N(%) Cl(%)
Calc.: 71.52 5.37 8.78 5.56
Found: 71.55 5.22 8.82 5.59
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
2.97 (1H, in), 3.09 (2H, m), 3.41 (2H, s), 4.96
(1H, m), 6.86 - 7.58 (total 22H), 8.14 (1H, d.),
10.32 (1H, s), 15.00 (1H, br).
MS ( FAB ): 575 ( M+ + 1).
-
- 96
CA 02453123 2004-01-20
Example 23
Using 400 mg of 2-phenyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide, 585
mg of cyclopropylcarbamidine carbonate and 750 mg of
potassium carbonate, the procedure of Example 20 was
repeated to obtain, using ethyl acetate-ethyl alcohol as a
recrystallization solvent, 276 mg of 4'-[(2-cyclopropyl-
1,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl)carbonyl]-2-phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: >230 C
Elemental analysis data (C34H28N402-HC:I=1.5H2O)
C(%) H($) N(%) - Cl(%)
Calc.: 69.44 5.48 9.53 6.03
Found: 69.10 5.39 9.42 6.15
'H-NMR (d ppm in DMSO-d6, TMS internal standard):
1.28 - 1.37 (total 4H), 1.99 (1H, m), 2.96 (1H,
m), 3.09 (11H, m), 4.96 (1H, ni), 6.83 (1H, d), 6.94
(2H, d), 7.12 (1H, t), 7.21 - 7.58 (total 12H),
8.17 (1H, d), 10.33 (1H, s), 14.60 (1H, br).
MS (FAB) : 525 (M+ + 1).
Reference Example 5
Using o-met.hylbenzoic acid and 1-(4-aminobenzoyl)-
2,3,4,5-tetrahydro-lH-l-benzazepin-5-one as starting
materials, the procedure of Reference Example 3 was
- 97 -
CA 02453123 2004-01-20
repeated to obtain 2-methyl-4'-[(5-oxo-2,3,4,5-tetrahydro-
1H-1-benzazepin-1-yl)carbonyl]benzanilide.
Physicochemical properties
1H-NMR (d ppm in CL>C13, TMS internal standard):
2.47 (3H, s), 2.90 (2H, m), 4.1 (2H), 6.8 (1H, m),
7.1 - 7.7 (total 10H), 7.82 (2H).
MS (EI): 398 (M+).
Reference Examples 6 to 11
The following compounds were synthesized in the
same manner as described in Reference Example 5.
Reference Example 6
2-Isopropyl.-4'-[(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin-1-yl)carbonyl]benzanilide
Reference Example 7
2-Methoxy-4'-[(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin--l-yl)carbonyl]benzanilide
Reference Example 8
2-Ethoxy-4'-[(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin-1-yl)carbonyl]benzanilide
Reference Example 9
2-Isopropyloxy-4'-[(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin-1-yl)carbonyl]benzanilide
Reference Example 10
2-Methyl-4'-[(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin-l-yl)carbonyl]phenylacetoanilide
_ 98 _
CA 02453123 2004-01-20
Reference Example 11
2-Methoxy-4'-j(5-oxo-2,3,4,5-tetrahydro-lH-1=-
benzazepin-l-yl)carbonyl]phenylacetoanilide
Reference Example 12
A 1.67 g portion of 2'-methoxybiphen-4-
ylcarboxylic acid was dissolved in 17 ml of
dichloromethane, C'..95 ml of oxalyl chloride and a
catalytically effective amount of dimethylformamide were
added to the resulting solution with cooling on an ice
bath and then the resulting mixture was warmed up to room
temperature. When completion of foaming was confirmed,
the reaction solution was concentrated under a reduced
pressure and subjected to azeotropic treatment with
toluene twice. The thus obtained residue was dissolved in
8.4 ml of dichloromethane and, with cooling on an ice
bath, the resulting solution was dropwise added to a
solution obtained by dissolving 1.0 g of 5-oxo-2,3,4,5-
tetrahydro-lH-l-ben.zazepine and 1.53 ml of triethylamine
in 10 ml of dichloromethane. The reaction solution was
warmed up to room temperature and the stirring was
continued for 1 hour. The resulting reaction solution was
mixed with water and subjected to phase separation to
separate dichloromethane layer which was subsequently
washed with 0.5 N hydrochloric acid and a saturated sodium
bicarbonate aqueous solution and dried over anhydrous
magnesium sulfate. After removing the solvent by
_ 99 _
CA 02453123 2004-01-20
distillation, the thus obtained residue was crystallized
from toluene to obtain 1.65 g of 1-(2 -methoxybiphen-4-
ylcarbonyl)-5-oxo-2,3,4,5-tetrahydro--lH-l-benzazepine as
crude crystals.
Physicochemical properties
1H-NMR (6 ppm in CDC13, TMS internal standard):
2.17 (2E, -m), 2.93 (2H, rri), 3.75 (3H, s), 6."7 -
7.7 (total 8H), 7.79 (1H, d)õ 7.89 (2H), 8.2 (IH,
d).
MS (EI): 371 (M+).
Example 24
After dissolving 2.0 g of 2-methyl-4 -[(5-oxo-
2,3,4,5-tetrahydro-lH-benzazepin-l-yl)carbonyl)benzanilide
in a mixed solvent consisting of 30 ml of chloroform and 3
ml of ethyl acetate, the resulting solution was mixed with
2.47 g of copper(II) bromide and subjected to 3 hours of
heating under reflux with vigorous stirring. After
cooling down the reaction solution to room temperature,
insoluble materials were removed by filtration. The
resulting filtrate was washed with a saturated sodium
bicarbonate aqueous solution. The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using a vacuum pump. The thus obtained solid substance
was dissolved in 80 ml of chloroform, and the resulting
solution was mixed with 2.37 g of acetamidine
- 100
-
CA 02453123 2004-01-20
hydrochloride and 4.86 g of potassium carbonate and
subjected to 20 hours of heating under reflux in a stream
of argon. The resulting reaction solution was mixed with
water and subjected to phase separation to separate the
organic layer which was subsequently dried over anhydrous
magnesium sulfate. After removing the solvent by
distillation under a reduced pressure, the thus obtained
residue was crystallized from toluene to obtain 1.41 g of
2-methyl-4'-[(2-methyl-1,4,5,6-tetrahydroimidazo[4,5-
d][l]benzazepin-6-yl)carbonyl]benzanilide. A 1.0 g
portion of this compound was dissolved in 10 ml of ethyl
alcohol, mixed with 0.86 ml of 4 N hydrochloric acid-ethyl
acetate and recrystallized to obtain 860 mg of 2-methyl-
4'-[(2-methyl-1,4,5,6-tetrahydroimidazo[4,5-
d][1]benzazepin-6-yl)carbonyl]benzanilide hydrochloride.
Physicochemical properties
Melting point: >230 C
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
2.33 (3H, s), 2.70 (3H, s), 3.00 (2H, t), 5.0
(1H, m), 6.99 (2H, d), 7.14 (1H, t), 7.27 (1H, t),
8.17 (1H, d), 10.40 (1H, s), 14.9 (1H, br).
MS (FAB): 437 (M} + 1).
Example 25
Using 2.0 g of 2-:methoxy-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-l-yl)carbonyl]benzanilide, 890
mg of crude crystals were obtained by repeating the
- 101 -
CA 02453123 2004-01-20
procedure of Example 24, and 360 mg of 2-methoxy-4'-[(2-
methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepin-6-
yl)carbonyl)benzanilide hydrochloride was obtained from
400 mg of the thus obtained crystals
Physicochemical properties
Melting point: >210 C
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
2.69 (3H, s), 3.00 (1H, t), 3.85 (3H, s), 5.01
(1H, m), 6.88 (1H, d), 7.36 (1H, t), 7.48 (1Hr t),
8.14 (1H, d), 10.20 (1H, s), 14.83 (1H, br).
MS (FAB): 453 (M+ + 1).
Example 26
Using 2.0 g of 2-ethoxy-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazep:in-1-yl)carbonyl]benzanilide, 927
mg of.crude crystals were obtained by repeating the
procedure of Example 24, and 465 mg of 2-ethoxy-4'-[(2-
methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepin-6-
yl)carbonyl]benzanilide hydrochloride was obtained from
500 mg of the thus obtained crystals.
Physicochemical properties
Melting point: >220 C
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
1.344 (3H, t), 2..70 (3H, s), 3.00 (1H, t), 4.16
(3H, q), 5.02 (1H, m), 6.88 (1H, d), 7.03 (3H,' m),
7.13 (1H, t), 7.-:35 (1H, t), 7.46 (1H, t), 7.54
- 102 -
CA 02453123 2004-01-20
(1H, d), 8.18 (1H, d), 10.19 (1H, s), 14.86
(1H, br).
MS (FAB): 467 (M} + 1).
Example 27
A 410 mg portion of bromine dissolved in 2 ml of
chloroform was dropwise added gradually (spending about 60
minutes) to 20 ml of chloroform solution containing 1.0 g
of 2-isopropoxy-4'-j(5-oxo-2,3,4,5-tetrahydro-lH-1-
benzazepin-l-yl)carbonyl)benzanilide at room temperature.
When disappearance of the color of bromine was confirmed,
the reaction solution was washed with a saturated sodium
bicarbonate aqueous solution. The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using.a vacuum pump. The thus obtained solid substance
was dissolved in 40 ml of chloroform, and the resulting
solution was mixed with 1.10 g of acetamidine
hydrochloride and 2.25 g of potassium carbonate and
subjected.to 20 hours of heating under reflux in a stream
of argon. The resulting reaction solution was mixed with
water and stirred to collect precipitated solid substance
by filtration, and the thus collected compound was
suspended in 20 ml of ethyl alcohol, mixed with 0.58 ml of
4 N hydrochloric acid-ethyl acetate and recrystallized to
obtain 600 mg of 2-isopropoxy-4'-j(2-methyl-1,4,5,6-
- 103 -
CA 02453123 2004-01-20
tetrahydroimidazoj4,5-d][l]benzazepin-6-
yl)carbonyl)benzanilide hydrochloride.
Physicochemical properties
Melting point: >300 C
1H-NMR (8 ppm in pMSO-d6,. TMS internal standard):
1.30 (6H, d), 2.68 (3H, s), 3.02 (1H, t), 4.72
(1H, q), 5.0 (1H, m), 6.89 (1H, d), 7.37 (1I1, t),
7.65 (1H, d), 8.10 (1H, d), 10.18 (1H, s), 14.7
(1H, br).
MS ( FAB ): 481 (M+ + 1).
Example 28
A 1.32 g portion of bromine dissolved in 6.6 ml of
chloroform was dropwise added gradually (spending about 60
minutes) to 36 ml of chloroform solution containing 3.55 g
of 4'-j(5-oxo-2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]-2-isopropoxybenzanilide at room temperature.
When disappearance of the color of bromine was confirmed,
the reaction solution was washed with a saturated sodium
bicarbonate aqueous solution. 'The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using a vacuum pump. The thus obtained solid substance
was dissolved in 40 ml of chloroform, and the resulting
solution was mixed with 5.0 g_of cyclopropylcarbamidine
hydrochloride and 8.02 g of potassium carbonate and
subjected to 20 hours of heating under reflux in a stream
- 104 -
CA 02453123 2004-01-20
of argon. The resulting reaction solution was mixed with
water to effect phase separation, and the separated
organic layer was dried over anhydrous magnesium sulfate.
After removing the solvent by distillation under a reduced
pressure, the thus obtained residue was crystallized from
toluene to obtain 2.96 g of 4'-[(2-cyclopropyl-l,4,5,6-
tetrahydroimidazo[4,5-d][l]benzav2pin-6-yl)carbonyl]-2-
isopropoxybenzanilide. A 1.08 g portion of this compound
was dissolved in 20 ml of ethyl alcohol, mixed with 0.8 ml
of 4 N hydrochloric acid-ethyl acetate and recrystallized
to obtain 916 mg of 4'-[(2-cyclopropyl-1,4,5,6-
tetrahydroimidazo[4,5-d][1]benzazepin-6-yl)carbonyl]-2-
isopropoxybenzanilide hydrochloride.
Physicochemical properties
Melting point: >210 C
1H-NMR (6 ppm in DMSO-d5, TMS internal standard):
around 1.36 (total lOH), 2.98 (1H, t), 3.46
(1H, br), 4.72 (:1H, q), 5.0 (1H, m), 6.87 (1Hr d),
7.37 (1H- t), 7.66 (1H, d), 8.17 (1H, d), 10.18
(1E, s), 14.4 (1H, br).
MS (FAB): 507 (M+ + 1).
Example 29
Using 5.0 g of 2--fluoro-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepin-1-yl)carbonyl]benzanilide, 4.76
g of crude crystals were obtained by repeating the
procedure of Example 24, and 1.02 g of 2-fluoro-4'-j(2-
- 105 -
CA 02453123 2004-01-20
methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1)benzazepin-6-
yl)carbonyl]benzanilide hydrochloride was obtained from
1.0 g of the thus obtained crystals.
Physicochemical properties
Melting point: >270 C
'H-NMR (d ppm in DMSO-d6, TMS internal standard):
2.70 ~3H, s), 3.01 (1H, t), 5.02 (1H, m), 6.87
(1H, d), 7.02 (2H:, m), 7.14 (1H, t), 8.18 (1H, d),
10.55 (1H, s), 14.8 (1H, br).
MS (FAB): 440 (M+ + 1).
Example 30
With cooling on an ice bath, a 793 mg portion of
phenyltrimethylammonium tribromide was added to 20 ml of
tetrahydrofuran solution containing 1.0 g of 4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-yl)carbonyl]-2-
isopropylbenzanilide, and the mixture was warmed up to
room temperature. Filtration was carried out when
disappearance of the color of bromine was confirmed after
about 60 minutes. The filtered material was washed with
tetrahydrofuran, and the filtrates were combined and
concentrated. The thus obtained residue was dissolved in
chloroform, washed with a sodium bicarbonate aqueous
solution and then dried over anhydrous magnesium sulfate.
After distilling off the solvent, the residue was further
evaporated to dryness using a vacuum pump. The thus
obtained solid substance was dissolved in 40 ml of
- 106 -
CA 02453123 2004-01-20
chloroform, and the resulting solution was mixed with 1.11
g of acetamidine hydrochloride and 2.26 g of potassium
carbonate and subjected to 20 hours of heating under
reflux in a stream of argon. The resulting reaction
solution was mixed with water to effect phase separation,
and the organic layer was separated and dried over
anhydrous magnesium sulfate. After removing the solvent
by distillation under a reduced pressure, t'he thus
obtained residue was crystallized from toluene to obtain
640 mg of 2-isopropoxy-4'-[(2-methyl-1,4,5,6-
tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl)carbonyl]benzanzlide. A 563 mg portion of this
compound was dissolved in 5.5 ml of ethyl alcohol, mixed
with 0.45 ml of 4 N hydrochloric acid-ethyl acetate and
recrystallized to obtain 400 mg of 2-isopropyl-4'-[(2-
methyl-1,4,5,6-tetrahydroimidazo[4,5-dl[l]benzazepin-6-
yl)carbonyl)benzanilide hydrochloride.
Physicochemical properties
Melting point: 251 to 253 C
3H-NMR (d ppm in DMSO-d6, TMS internal standard):
1.18 (6H, t), 3.00 (1H, t), 3.38 (2H, br), q), 5.0
(1H, m) 6.89 (1H, d), 7.16 (1H, t), 7.55 (2H, d),
8.11 (1H, d), 10.47 (1H, s), 14.7 (1H, br).
MS (FAB): 465 (M} + 1).
107 -
CA 02453123 2004-01-20
Example 31
Using 2.0 g of 2-methoxy-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]phenylacetan~-lide, 1.19 g of crude crystals
were obtained by repeating the procedure of Example 30,
and 1.25 g of 2-methoxy-4'-[(2-methyl-1,4,5,6-
tetrahydroimidazo(4,5-d][1]benzazepin-6-
yl)carbonyl]phenylacetani.lide hydrochloride was obtained
from 1.19 g of the thus obtained crystals.
Physicochemical properties
Melting point: >200 C
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
2.68 (3H, s), 2.98 (1H, t), 3.60 (2H, s), 3.73
(3H, s), 5.0 (1H, m), 7.12 (1H, t), 8.10 (1H, d),
10.26 (1H, s), 14.7 (2H, br).
MS (FAB): 467 (M+ + 1).
Example 32
Using 2.0 g of 2-methyl-4'-[(5-oxo-2,3,4,5-
tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]phenylacetanilide, 1.26 g of crude crystals
were obtained by repeating the procedure of Example 30,
and 898 mg of 2-methyl-4'-[(2-methyl-1,4,5,6-
tetrahydroimidazo[4,5-d][l]benzazepin-6-
y1)carbonyl]phenylacetanilide hydrochloride was obtained
from 1.2 g of the thus obtained crystals.
- 108 -
CA 02453123 2004-01-20
Physicochemical properties
Melting point: 201 to 203 C
1H-NMR (d ppm in DMSO-db, TMS internal standard):
2.25 (3H, s), 2.68 (3H, s), 2.98 (1H, t), 3.66
(2H, s), 5.0 (1H, m), 6.90 (1H, d), 7.34 (1H, t),
8.09 (1H, d), 10.44 (1H, s), 14.7 (2H, br).
MS (FAB): 451 (M+ + 1).
Example 33
A 3 ml portion of a chloroform solution containing
300 mg of bromine was dropwise added gradually (spending
about 60 minutes) at room temperature to 700 mg of 1-(2'-
methoxybiphenyl-4-ylcarbonyl)-5-oxo-2,3,4,5-tetrahydro-lH-
1-benzazepine dissolved in 0.7 ml of chloroform. When
disappearance of the color of bromine was confirmed, the
reaction solution was washed with a saturated sodium
bicarbonate aqueous solution. The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using a vacuum pump. The thus obtained solid substance
was dissolved in 28 ml of chloroform, and the resulting
solution was mixed with 714 mg of acetamidine
hydrochloride and 1.46 g of potassium carbonate and
subjected_to 20 hours of heating under reflux in a stream
of argon. The resulting reaction solution was mixed with
water to effect phase separation, and the organic layer
was separated and dried over anhydrous magnesium sulfate.
- 109 -
CA 02453123 2004-01-20
After distilling off the solvent, the thus obtained
residue was purified by silica gel column chromatography
(chloroform-methyl alcohol = 20:1) to obtain, in the order
of elution, 210 mg (glassy solid) of 6-[(2'-methoxy-4-
biphenylyl)carbonyl]-2-methyl-5,6-dihydro-4H-oxazolo[4,5-
d][l]benzazepine (Example 33(1)) and 390 mg (glassy solid)
of 6-[(2'-methoxy-4-bi.phenylyl)carbonyl]-2-methyl-1,4,5,6-
tetrahydroimidazo[4,5-d][1]benzazepine.
6-[(2'-Methoxy-4-biphenylyl)carbonyl]-2-methyl-
1,4,5,6-tetrahydroimidazo[4,5-d][l)benzazepine was
dissolved in 4.8 ml of ethyl alcohol, the solution was
mixed with 0.44 ml of 4 N hydrochloric acid-ethyl acetate
and cooled on an ice bath to effect crystal formation, and
then the thus formed crystals were collected by filtration
and washed with a small volume of ethyl alcohol to obtain
260 mg of 6-[(2'-methoxy--4-biphenylyl)carbonyl]-2-methyl-
1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine
hydrochloride (Example 33(2)).
Physicochemical properties
6-[(2'-methoxy-4-biphenylyl)carbony]-2-methyl-5,6-dihydro-
4H-oxazolo[4,5-d][l]benzazepine
'H-NMR (a ppm in CDC13, TMS internal standard):
2.57 (3H, s), 3.73 (3H, s), 5.22 (1H, m), 6.78
(1H, dd), 7.82 (1H, dd).
MS (EI): 410 (M+).
- 110 -
CA 02453123 2004-01-20
6-[(2'-methoxy-4-biphenylyl)carbony]-2-methyl-1,4,5,6-
tetrahydroimidazo[4,5-d][1]benzazepine hydrochloride
Melting point: >240 C
'H-NMR (d ppm in DMSO-d6, TMS internal standard):
2.69 (3H, s), 3.03 (1H, t), 3.70 (3H, S), 5.02
(1H, m), 6.9 - 7.4 (total 11H), 8.12 (1H, d), 14.7
(total 2H).
MS ET: 409 (M+)
Example 34
A 1.0 g portion of the crude crystals of 2-fluoro-
4'-[(2-methyl-1,4,5,5-tetrahydroimidazo[5,4-
d]jl]benzazepin-6-yl)carbonyl]benzanilide obtained in
Example 29 and 1.1 g of 2-ethylimidazole were dissolved in
ml of dimethyl sulfoxide and stirred for 24 hours at
120 C. The reaction solution was added to water and
extracted twice with chloroform. The chloroform layers
were combined, washed with a saturated sodium chloride
aqueous solution and then dried over anhydrous magnesium
sulfate. After removing the solvent by distillation, the
thus obtained residue was purified by silica gel column
chromatography using a solvent system of chloroform-methyl
alcohol-28% aqueous ammonia (10:1:0.1) to obtain 1.02 g of
a glassy solid. This compound was dissolved in 20 ml of
ethyl alcohol, mixed with 1.42 ml of 4 N hydrochloric
acid-ethyl acetate and then concentrated. The thus
obtained residue was made into an amorphous powder using
- 111 -
CA 02453123 2004-01-20
= ~ =; õ~. -: ;;;> . _ . .
'6= P . _ i- :=i~ .. js* "' 'y . =
. = . _ _ ~, t _ - . ~. -~ ,
. = =. < . ' ~=: ti.s..e:- .~:6= .*:
. . . . . . . . ' - ... . . - . . . . . . . : . . . . ''_, - =.YI'= ' .i ., y
. = . = i.r
isopropyl alcohol and then collected by filtration:to
obtain 460 mg of 2-(2-ethyl-lH-imidazol-1-yl)-4 ={2-.
methyl-T,4,5,6-tetrahydroimidazo[4,5-d)(1Jbenzazepin-6-
yl)carbonyl]benzanilide-2HC1.
Physicochemical properties
'H-NNiR (S ppm in DMSO-d6, TMS internal standard):
2.70 (3H, s), 3.01 (1H, t), 5.02 (1H, m), 7.12
(1H, t), 8.24 (111, d), 10.93 (1H, s).
MS (FAB): 517 (M} + 1)
Reference Example 13
A 5.46 g portion of 3-phthalimidopropionitrile was
dissolved in 35 ml of dry chloroform, 1.76 ml of dry
ethanol was added to the solution and then hydrochloric
acid gas was bubbled for 30 minutes into the resulting
mixture with cooling on an ice bath, followed by 12 hours
of stirring. The reaction solution was mixed with ether,
the thus formed precipitate was collected by filtration
and dissolved in 150 ml of ethanol and then the resulting
solution was mixed with :3 gof ammonium carbonate and
stirred at room temperature for 24 hours. By distilling
off the solvent from the reaction solution, 5.5 g of
3-phthalimidopropionamidine 1/2 carbonate was
obtained.
Physicochemical properties
MASS (PAB): 218 (M} + 1)
- s _..
- 112 -
"CA 02453123 2004-01-20.
. - . . " 1'. . .-9 .
. = .. .,."~ = ==f.'l ~
\. '~l s . . .. . . ='. .
= _ . _ ,_ _ =~~ ..~r.. if=r Z' . . :T=y _ =y.
.. . . . . . ..: . . = . . . " . . . ' .. ~.y~ , ,. =~~ ' =~t:, .. r~r.'.
Reference Example 14
Using 2.963 g of 4-phthalimidobutylonitrile as a
starting rnaierial, the procedure of Reference Example 13
was repeated to obtain 3.162 g of 4-
phthalimidobutanarnidine 1/2 carbonate.
Phvsicochemical properties
MS (FAB): 232 (M+ + 1)
Reference Example 15
Using 4.472 g of 5-phthalimidovaleronitrile as a
starting material, the procedure of Reference Example 13
was repeated to obtain 4.364 g of 5-
phthalimidopentanamidine 1/2 carbonate.
Physicochemical properties
MS (FAB): 245 (M' + 1)
Reference Example 16
After dissolving 3.03 g of 2-phenyl-42-[(5=oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]benzani.lide in a mixed solvent consisting of
120 ml of chloroform and 15.ml of ethyl acetate, the
resulting solution was mixed with 2.95 g of copper(II)
bromide and subjected to 3 hours of heating under reflux
with vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
- 113 -
" : CA 02453123 2004-01-20 -
.. ,. ' . . . '._ 'g' w.= . , , t . .. . .. = tf . . .. .. . . .; . ' .. ' ~
't.
sulfate,, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump.' A 500 mg
portion of the thus obtained foam-like substance was
dissolved in 150 ml of chloroform, and the resulting
solution was mixed with 900 mg of potassium carbonate and
1.3 g of 3-phthalimidopropionamidine 1/2 carbonate
and obtained in Reference Example 13, and subjected to 16
hours of heating under reflux. After cooling down the
reaction solution to room temperature, insoluble materials
were removed by filtration. The resulting filtrate was
mixed with a saturated sodium bicarbonate aqueous solution
and the organic layer was separated. The resulting
organic layer was washed with water and a saturated sodium
chloride solution, and dried over anhydrous magnesium
sulfate. After distilling off the solvent under a reduced
pressure, the thus obtained=iesidue was subjected to
silica gel column chromatography to obtain 221 mg of 4'-
II2-(2-phthalimidoethyl)--1.4,5,6-tetrahydroimidazoj4,5-
d]jl]benzazepin-6-yl]carbonyl]-2-phenylbenzan.ilide from
chloroform-methyl alcohol (50:1) eluate.
Physicochemical properties
MS ( FAB ): 658 (M+ + 1)
Reference Example 17
After dissolving 3.03 g of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]benzanilide in a mixed solvent consisting of
-
- 114
CA 02453123 2004-01-20
120 ml of chloroform and 15 ml of ethyl acetate, the
resulting solution was mixed with 2.95 g of copper bromide
and subjected to 3 hours of heating under reflux with
vigorous stirring. After cooling down the reaction
solution to room temperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbcnate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. Using a 500 mg
portion of the thus obtained foam-like substance and 1.758
g of 4-phthalimidobutanamidine 1/2 carbonate
obtained in Reference Example 14 as starting materials,
the similar procedure as in Reference Example 16 was
repeated to obtain 389 mg of 4'-[{2-(3-phthalimidopropyl)-
1,4,5,6-tetrahydroimidazo[4,5-d][2jbenzazepin-6-
yl]carbonyl]-2-phenylbenzanilide.
Physicochemical properties
MS (FAB): 672 (M} + 1)
Reference Example 18
After dissolving 3.03 g of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl)benzanilide in a mixed solvent consisting of
120 ml of chloroform anci. 15 ml of ethyl acetate, the
resulting solution was mixed with 2.95 g of copper bromide
and subjected to 3 hours of heating under reflux with
- . s- .
- 115 -
CA 02453123 2004-01-20
vigorous stirring. Afte cooling down the reaction
solution to room ternperature, insoluble materials were
removed by filtration. The resulting filtrate was washed
with a saturated sodium bicarbonate aqueous solution. The
resulting organic layer was dried over anhydrous magnesium
sulfate, concentrated under a reduced pressure and then
evaporated to dryness using a vacuum pump. Using a 500 mg
portion of the thus obtained foam-like substance and 1.424
g of S-phthalimido pentanamidine 1/2 carbonate
obtained in Reference Example 15 as starting materials,
the similar procedure as in Reference Example 16 was
repeated to obtain 316 mg of 4'-[[2-(4-phthalimidobutyl)-
1,4,5,6-tetrahydroimidazo[4,5-dJ[lJbenzazepin-6-
yllcarbonyl)-2-phenylbenzanilide.
Physicochemical properties
MS ( FAB ): 686 (M+ + 1)
Reference Example 19
In a stream of argon, 60% sodium hydride was
dissolved in 10 ml of tetrahydrofuran, and the solution
was mixed with 2.0 g of benzyl cyanide, stirred for 1 hour
at room temperature, further mixed with 3.69 g of 1,4-
dibromobutane and again stirred for 16 hours at room
temperature. The reaction mixture was mixed with water
and ethyl acetate, and the resulting organic layer was
separated, washed with a saturated sodium chloride aqueous
solution and then dried_over anhydrous magnesium sulfate.
s.
- 116 -
CA 02453123 2004-01-20
After removing the solvent by distillation under a reduced
pressure, the thus obtained residue was subjected to
silica gel column chromatography, and the resulting hexane
eluate was mixed with 45 ml of sulfuric acid and subjected
to 24 hours of heating under reflux. After cooling down
to room temperature, the reaction solution was mixed with
ice water and ethyl acetate to separate water layer which
was subsequently mixed with concentrated hydrochloric acid
and ethyl acetate, and the resulting organic layer was
separated, washed with water and a saturated sodium
chloride aqueous solution and then dried over anhydrous
magnesium sulfate. By removing the solvent by
distillation under a reduced pressure, 978 mg of
1-phenylcyclopentanecarboxylic acid was obtained.
Physicochemical properties
1H-NMR (6 ppm in CDC13, TMS internal standard):
1.84 - 2.08 (m, 8H), 7.21 - 7.45 (m, 4H)
MS (EI) : 190 (M})
Reference Example 20
Using 2.0 g of benzyl_ cyanide and 3.9 g of 1,5-
dibromopentane, the procedure of Reference Example 19 was
repeated to obtain 980 mg of 1-phenylcyciohexanecarboxylic
acid.
Physicochemical properties
IH-NMR (b ppm in CDC13, TMS internal standard):
1.26 - 1.87 (m, 10H), 7.22 - 7.52 (m, 4H)
r.
- 117 -
CA 02453123 2004-01-20
MS (EI): 204 (M})
Reference Example 21
In 20 ml of dichloromethane, a 978 mg portion of
1-phenyicyciopentanecarboxylic acid obtained in Reference
Example 19 was mixed with 0_7 ml of oxazyl chloride and
stirred for 1 hour on an ice bath. After distilling off
the reaction solvent, the thus obtained residue was
dissolved in 10 ml of dic:hloromethane and added to a 20 ml
dichloromethane solution containing 1.24 g of 1-(4-
aminobenzoyl)-2,3,4,5-tetrahydro-lH-1-benzazepin-5-one and
0.72 ml of triethylamine, and the mixture was stirred for
3 hours at room temperature. The resulting reaction
solution was mixed with a saturated sodium carbonate
aqueous solution to separate the organic layer which was
subsequently washed with water and a saturated sodium
chloride aqueous soluti.on and dried over anhydrous
magnesium sulfate. After removing the solvent by
distillation under a reduced pressure, the thus obtained
residue was subjected to silica gel column chromatography
to obtain 759 mg of 1-[4-(1-phenylcyclopentan-l-
yl)aminobenzoyl]-5-oxo-2,3,4,5-1H-l-benzazepine from the
chloroform-methyl alcohol (50:1) eluate.
Physicochemical properties
MS (FAB) : 453 (M} + 1)
118 -
CA 02453123 2004-01-20
Reference Example 22
Using 980 mg of 1-phenylcycIohexanecarboxylic acid
and 1.2 g of 1-(4-aminobenzoyl)-2,3,4,5-tetrahydro-lH-l-
benzazepin-5-one as starting materials, the procedure of
Reference Example 21 was repeated to obtain 1.453 g of 1-
[4-(1-phenylcyclohexan -1-yl)aminobenzoyl]-5-oxo-2,3,4,5-
1H-1-ben-7azepine.
Physicochemical properties
MS (FAB): 467 (M} } 1)
Reference Example 23
After dissolving 2.966 g of 1-(4-nitrobenzoyl)-
2,3,4,5-tetrahydro-lH-l-benzazepin-5-one in a mixed
solvent consisting of 925 ml of chloroform and 9.2 ml of
ethyl acetate, the resulting solution was mixed with 5.34
g of copper bromide and subjected to 2 hours of heating
under reflux with vigorous stirring. After-cooling down
the reaction solution to room temperature, insoluble
materials were removed by filtration and the resulting
filtrate was washed with a saturated sodium bicarbonate
aqueous solution. The resulting organic layer was dried
over anhydrous magnesium sulfate, concentrated under a
reduced pressure and then evaporated to dryness using a
vacuum pump. The thus obtained solid substance was
dissolved in 250 ml of chloroform, and the resulting
solution was mixed with 10.5 g of potassium carbonate and
5.12 g of acetamidine hydrochloride and subjected to 20
_ õ ~ _- 119 -
CA 02453123 2004-01-20
hours of heating.under reflux. After cooling down the
reaction solution to room temperature, insoluble materials
were removed by filtration and the resulting filtrate was
washed with a saturated sodium bicarbonate aqueous
solution, water and a saturated sodium chloride aqueous
solution and then dried over anhydrous magnesium sulfate.
Aftar removing the solvent by distillation under a reduced
pressure, the thus obtained residue was subjected to
silica gel column chromatography to obtain 2.077 g of 6-
(4-nitrobenzoyl)-2-methyl-1,4,5,6-tetrahydroimidazo[4,5-
d][l]benzazepine from the chloroform-methyl alcohol (30:1)
eluate.
Physicochemical properties
MS (FAB): 349 (M+ + 1)
Reference Example 24
In a stream of argon, 144 mg of 60% sodium hydride
was suspended in a small volume of N,N-dimethylformamide
to which, with cooling on an ice bath, was then added
dropwise a solution prepared by dissolving 500 mg of 6-(4-
nitrobenzoyl)-2-methyl-l,4,5,6-tetrahydroimidazo[4,5-
d][i]benzazepine in 20 ml of N,N-dimethylformamide. After
1 hour of stirring at room temperature, the reaction
solution was mixed with 0.11 ml of methyl iodide and
stirred for 24 hours at room temperature. The reaction
solution was mixed with water and chloroform, and the
resulting organic layer was separated, washed with a
- 120 -
CA 02453123 2004-01-20
saturated sodium chloride aqueous solution and then dried
over anhydrous magnesium sulfate. After removing the
solvent by distillation under a reduced pressure, the thus
obtained residue was subjected to silica gel column
chromatography to obtain 351 mg of 6--(4-nitrobenzoyl)-2,3-
dimethyl-3,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepine
from the chloroforrn-methyl alcohol (30:1) eluate.
Physicochemical properties
iH-NMR (S ppm in CI)C13, TMS internal standard):
2.37 (3H, s), 2.85 - 2.90 (1H, m), 3.12 (1H, m),
3.36 - 3.51 (1H, m), 3.59 (3H, s), 5.14 - 5.17
(1E, dd), 6.57 (1H, d), 6.83 (1H, t), 7.22 - 7.26
(3H, m), 7.92 (2H, d), 7.26 (1H, d)
MS ( FAB ): 303 ( M} + 1)
Reference Example 25
A 1.421 g portion of 6-(4-nitrobenzoyl)-2,3-
dimethyl-3,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepi.ne
was dissolved in 50 ml of methyl alcohol, and the solution
was mixed with 300 mg of palladium-carbon and subjected to
hydrogenation under normal pressure. After completion of
the hydrogen absorption, the reaction mixture was
subjected to filtration and the resulting filtrate was
concentrated to obtain 571 mg of 6-(4-aminobenzoyl)-2,3-
dimethyl-3,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine.
Physicochemical properties
MS ( FAB ): 333 (M+ + 1)
- 121 -
CA 02453123 2004-01-20
Example 35
A 392 mg portion of 4'-[[2-(2-phthalimidoethyl)-
1,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl]carbonyl]-2-phenylbenzanilide obtained in Reference
Example 16 was dissolved in 10 ml of methyl alcohol, and
the resulting solution was mixed with 10 ml of a
methylamine-methyl alcohol solution and stirred at room
temperature for 4 hours. The reaction solution was m:ixed
with chloroform and 1 N hydrochloric acid to separate
water layer which was then mixed with chloroform and
neutralized with 1 N sodium hydroxide to separate organic
layer. The organic layer was washed with water and a
saturated sodium chloride aqueous solution, dried over
anhydrous magnesium sulfate and then subjected to solvent
removal by distillation under a reduced pressure. The
thus obtained residue was dissolved iri a small volume of
ethyl acetate and mixed with 4 N hydrochloric acid-eth;yl
acetate, and the thus formed precipitate was washed with
ethyl alcohol to obtain 70 mg of 4'-[[2-(2-aminoethyl)-
1,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl]carbonyl]-2-phenylbenzanilide 2HC1 as an aanorphous
solid.
- 122
-
CA 02453123 2004-01-20
Physicochemical properties
'H-NMR (8 ppm in DMSO-d6, TMS internal standard):
1.44 - 1.64 (m, 3H), 2.06 - 2.11 (m, 2H), 2.26 -
2.30 (m, 2H), 4.96 (m, 1H), 6.86 - 7.58
(total 17H), 8.14 (d, 1H), 15.0 (br, 1H)
MS (FAB) : 528 (M+ + 1)
Example 36
Using 389 mg of 4'-[[2-(3-phthalimidopropyl)--
1,4,5,6-tetrahydroimidazo[4,5-d][l]benzazepin-6-
yl}carbonyl]-2-phenylbenzanilxde obtained in Reference
Example 17 as a starting material, the procedure of
Example 35 was repeated and the product was recrystallized
from ethyl acetate-ethyl alcohol to obtain 90 mg of 4'-
[j2-(3-aminopropyl)-1,4,5,6-tetrahydroimidazo[4,5-
d][l]benzazepin-6-yl]carbonyl]-2-phenylbenzanilide-2HC1.
Physicochemical properties.
Melting point: 220 to 2230C
Elemental analysis data (C34H31N5O2 2HC1-3H2O)
C($) H($) N(%) C3(%)
Calc.: 60.79 5.88 10.37 10.60
Found: 60.51 5.76 9.94 10.30
1H-NMR (6 ppm in DMSO-d6, TMS internal standard):
1.44 - 1.64 (3H, m), 2.14 - 2.17 (2H, m), 3.40 -
3.45 (4H, m), 4.96 (111, m),' 6.82 - 7.54
(total 17I3), 8.14 (1H, d), 15.0 (1H, br)
MS (FAB): 542 (M+ + 1) .
- 123 -
CA 02453123 2004-01-20
Example 37
Using 316 mg of 4'-[[2-(4-phthali.midobutyl)-
1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepin-6-
yl]carbonyl]-2-phenylbenzanilide obtained in Referenc,e
Example 18 as a starting material, the procedure of
Example 35 was repeated to obtain 136 mg of 4'-[[2-(4-
aminobutyl)-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepin-
6-yl]carbonyl]-2-phenylbenzanilide=2HC1 as an amorphous
powder.
Physicochemical properties
HPLC purity: >90% (TOSOH ODS-80T)
1H-NMR (6 ppm in DMSO-d6, TMS interna:l standard):
1.20 - 1.26 (2H, m), 1.44 - 1.64 (3H, m), 2.14 -
2.17 (2H, m), 3.40 - 3.43 (4H, br), 4.99 (1H, m),
6.86 - 7.58 (total 17H), 8.14 (1H, d), 15.0
(1H, br)
MS (FAB): 556 (M} + 1).
Example 38
After dissolving 726 mg of 1-[4-(l-
phenylcyclopentan-l-yl)carboxamidobenzoyl]-5-oxo-2,3,4,5-
1H-1-benzazepine obtained in Reference Example 21 in a
mixed solvent consisting of 35 ml of chloroform and 4 ml
of ethyl acetate, the resulting solution was mixed with
717 mg of copper bromide and subjected to .1 hour of
heating under reflux with vigorous stirring. After
cooling down the reaction solution to room temperature,
- 124 -
CA 02453123 2004-01-20
insoluble materials were removed by filtration and the
resulting filtrate was washed with a saturated sodium
bicarbonate aqueous solution. The resulting organic layer
was dried over anhydrous magnesium sulfate, concentrated
under a reduced pressure and then evaporated to dryness
using a vacuum pump. The thus obtained solid substance
was dissolved in 50 ml of chloroform, and the resulting
solution was mixed with 1.6 g of potassium carbonate and
780 mg of acetamidine hydrochloride and subjected to 20
hours of heating under reflux. After cooling down the
reaction solution to room temperature, insoluble materials
were removed by filtration and the resulting filtrate was
washed with a saturated sodium bicarbonate aqueous
solution, water and a saturated sodium chloride aqueous
solution, and then dried over anhydrous magnesium sulfate.
After removing the solvent by distillation under a reduced
pressure, the thus obtained residue was subjected to
silica gel column chromatography, the chloroform-methyl
alcohol (30:1) eluate was mixed, in ethyl acetate, with 4
N hydrochloric acid-ethyl acetate and then the residue
obtained after removal of the solvent by distillation was
recrystallized from ethyl alcohol to obtain 181 mg of N-
[4-[(2-methyl-1,4,J,5-tetrahydroimidazo[4,5-
d][1]benzazepin-6- -
yl)carbonyl]phenylcyclopentanecarboxamido hydrochloride.
- 125 -
CA 02453123 2004-01-20
Physicochemical properties
Melting point: 213 to 216 C
Elemental analysis data (C31H3aN.o2-HCl-2.5H20)
CM H(%) N(%) Cl(%)
Calc.: 65.08 6.34 9.79 6.20
Found: 65.09 5.98 9.73 6.28
IH-NMR (6 ppm in DMSO-d6, TMS internal star;3ard) :
1.54 - 1.64 (8H, m), 1.90 - 2.00 (1H, m), 3.68
(3H, s), 2.97 - 3.12, (2H, m), 4.99 (1H, m), 6.82
- 7.41 (total 13H), 8.08 (1H, d), 14.6 (1H, br)
MS (FAB): 491 (M+ + 1).
Example 39
Using 1.38 g of l-[4-(l-phenylcyclo hexan-l-
yl)carboxamidobenzoyl]-5-oxo-2,3,4,5-lH-l-benzazepine,
1.32 g of copper bromide and 1.4 g of acetamidine
hydrochloride as s:.arting materials, the procedure of
Example 38 was repeated to obtain 877 mg of N-[4-[(2-
methyl-1,4,5,6--tetrahydroimidazo[4,5-d][1]benzazepin-6-
yl)carbonyllphenyl]-1-phenylcyclohexanecarboxamido
hydrochloride.
Physicochemical properties
Melting point: 222 to 225 C
Elemental analysis data (C32E32N402-HC:1-1.4H20)
C(%) H(%)- N(%) C1(%)
Calc.: 67.87 6.37 9.89 6.26
Found: 67.53 6.76 9.64 6.21
.. . x. - 126 -
CA 02453123 2004-01-20
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
1.27 - 1.73 (10H, m), 1.90 - 2.00 (1H, m), 3õ68
(3H, s), 2.97 - 3.12 (2H, m), 4.99 (1H, m), 6.82 -
7.41 (total 13H), 8.08 (1H, d), 14.6 (1H, br)
MS ( FAB ): 505 (M+ + 1).
Example 40
A 512 mg portion of o-phenylbenzoic acid was
dissolved in 30 ml of dichloromethane and, with cooling on
an ice bath, the resulting solution was mixed with 0.45 ml
of oxazyl chloride and stirred for 1 hour. After
distilling off the reaction solvent under a reduced
pressure, the thus obtained residue was dissolved in 10 ml
of dichloromethane and, with stirring on an ice bath,
added dropwise to a 30 ml dichloromethane solution
containing 571 mg of 6-(4-aminobenzoyi)-2,3-dimethyl-
3,4,3,6-tetrahydroiinidazo[4,5-d][1]benzazepine and 0.72 ml
of triethylamine. After warming up to room temperature,
the reaction solution was stirred for 6 hours. The
resulting reaction solution was mixed with a saturated
sodium bicarbonate aqueous solution to separate the
organic layer which was subsequently washed with water and
a saturated sodium chloride aqueous solution and dried
over anhydrous magnesium sulfate. After removing the
solvent by distillation under a reduced pressure, the thus
obtained residue was subjected to silica gel column
chromatography, the resulting chloroform-methyl alcohol
- 127 -
CA 02453123 2004-01-20
(30:1) eluate was mixed with 4 N hydrochloric acid-ethyl
acetate and then the residue obtained after removal of the
solvent by distillation was recrystallized from ethyl
alcohol-diethyl ether to obtain 230 mg of 4'-[(2,3-
dimethyl-3,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepin-6-
yl)carbonyl]-2-phenylbenzanilide hydrochloride.
Physicochemical properties
Melting point: 195 to 198 C
Elemental analysis data (C33H28N4O2 1.1HClv2.8H2O)
C($) H(%) N(%) Cl(%)
Calc.: 65.71 5.80 9.29 6.47
Found: 65.73 5.61 9.82 6.96
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
2.37 (3H, s), 2.85 - 2.90 (1H, m), 3.12 (1H, m),
3.36 - 3.51 (1H, m), 3.59 (3H, s), 5.14 - 5.17
(1H, br), 6.72 - 7.57 (total 17H), 8.02 (1H, d),
MS (FAB): 513 (M{ + 1).
Reference Example 26
A 3.0 g portion of o-phenylbenzoic acid was
dissolved in 15 ml of methylene chloride and, with cooling
on an ice bath, a catalytically effective amount of
dimethylformamide and 1.98 g of thionyl chloride were
added to the solution. After gradually warming up to room
temperature, the reaction mixture was stirred for 1 hour
at the same temperature and then the solvent was distilled
off under a reduced pressure. The resulting residue was
- 128 -
CA 02453123 2004-01-20
mixed with 15 ml of benzene and again concentrated under a
reduced pressure. The thus obtained oily material was
dissolved in 20 ml of acetone and, with cooling on an ice
bath, mixed with 2.08 g of p-aminobenzoic acid and 2.02 g
of N,N-dimethylaniline, followed by gradual warming up to
room temperature. After 1.5 hours of stirring at the same
temperature, the reaction solution was mixed with 20 ml or
water to collect the precipitate by filtration. By drying
under a reduced pressure, 4.52 g of 4-(biphen-2-
ylcarboxyamide)benzoic acid was obtained in the form of
white crystalline powder.
Physicochemical properties
NMR (d ppm, DMSO-d., TMS internal standard):
7.28 - 7.61 (9H), 7.66 (2H, d), 7.86 (2H, d),
10.57 (1H, s)
MS (EI): 317 (M+)
Reference Example 27
A 500 mg portion of 4-(biphen-2-
ylcarboxyamide)benzoic acid was dissolved in 5 ml of
methylene chloride and, with cooling on an ice bath, a
catalytically effective amount of dimethylformamide and
220 mg of oxalyl chloride were added to the solution.
After gradually warming up to room temperature, the
reaction mixture was stirred for 1.5 hours at the same
temperature and then the solvent was distilled off under a
reduced pressure. The resulting residue was mixed with 10
- 129 -
CA 02453123 2004-01-20
ml of benzene and again concentrated under a reduced
pressure. The thus obtained oily material was dissolved
in 5 ml of methylene chloride to obtain an acid chloride
solution.
With cooling on an ice bath, the thus prepared
acid chloride solution was added to 2.5 ml of a methylene
chloride solution containing 254 mg G' 5-oxo-2,3,4,5-
tetrahydro-lH-l-benzazepine and 149 mg of pyridine. After
gradually warming up to room temperature, the reaction
mixture was stirred for about 2 hours at the same
temperature. The resulting reaction solution was mixed
with 5 ml of methylene chloride and=l0 ml of water to
separate organic layer which was subsequently washed with
ml of dilute hydrochloric acid and 10 ml of 5% sodium
carbonate aqueous solution. After concentrating the
organic layer under a reduced pressure, the thus obtained
amorphous powder was subjected to silica gel column
chromatography (eluent: methylene chloride-ethyl acetate
= 6:1) to collect fractions containing the compound of_
interest, and then the solvent was reinoved from the
fractions by distillation to obtain 530 mg of 2-phenyl-4'-
[(5-oxo-2,3,4,5-tetrahydro-lH-1-benzazepin-l-
yl)carbonyl]benzanilide in the form of amorphous powder.
Physicochemical properties -
IH-NMR (S ppm in CDC13, TMS internal standard):
2.19 (2H, rcÃ.), 2.86 (2H, m), 4.03 (2H), 6.69
- 130 -
CA 02453123 2004-01-20
6.8 - 7.6 (15H), 7.85 (1H, m)
Example 41
After dissolving 2.7 g of 2-phenyl-4'-[(5-oxo-
2,3,4,5-tetrahydro-lH-l-benzazepin-l--
yl)carbonyl]benzanilide in 40 ml of chloroform, the
resulting solution was mixed with 1.92 g of pyridinium
hydrobromide perblomide and stirred at 40 C for 60
minutes. After cooling down to room temperature, the
reaction solution was washed twice with water and then
dried over anhydrous magnesium sulfate. After distilling
off the solvent, the thus obtained residue was dissolved
in 120 ml of chloroform, and-the resulting solution was
mixed with 2.7 g of acetamidine hydrochloride and 5.52 g
of potassium carbonate and subjected to 20 hours of
heating under reflux in a stream of argon. The resulting
reaction solution was mixed with water and subjected to
phase separation to separate the chloroform layer which
was subsequently dz-ied over anhydrous magnesium sulfate.
After removing the solvent by distillation, the thus
obtained residue was recrystallized from methyl alcohol to
obtain 2.09 g of 4'-[t2-methyl-1,4,5,6-
tetrahydroimidazo[4,5-d][l]benzazepin-6-yl)carbonyl]-2-
phenylbenzanilide. This compound was crystallized from
31.5 ml of ethyl alcohol and 27.2 ml of 1 N hydrochloric
acid to obtain crude crystals (B crystal) of 4'-[(2-
methyl-1,4,5,6-tetrahydrolmldazo[4,5-d][l]benzazepin-6-
- 131 -
CA 02453123 2004-01-20
yl)carbonyl]-2-phenylbenzanilide hydrochloride. These
crystals were suspended in 45 ml of acetonitrile, heated
for 30 minutes under reflux, cooled down, collected by
filtration and then dried to obtain crude crystals (y
crystal). Thereafter, they were suspended in 26 ml of
ethyl alcohol, heated for 30 minutes under reflux, cooled
down, collected by filtration and then dried to obtain 1.6
g of 4'-[(2-methyl-1,4,5,5-tetrahydroimidazo[4,5-
d][1]benzazepin-6-y1)carbonyl)-2-phenylben.zanilide
hydrochloride in the form of crystals (a crystal).
Physicochemical properties (a crystal)
Melting point: >300 C
1H-NMR (S ppm in DMSO-d6, TMS internal standard):
2.66 (3H, s), 3.00 (1H, t), 4.99 (1H, m), 6.89
(2H), 7.14 (1H, t), 8.02 (1H, d), 10.31 (1H, s),
14.6 (1H, br)
MS (EI): 498 (M})
- 132 -
CA 02453123 2004-01-20
Formulation Examples
Injections
Composition
Formulation 1 Inventive compound 1.5 mg
Lactic acid 0.2 mg
Lactose 200 mg
Distilled water 2.0 ml in total
for injection use
Formulation 2 Inventive compound 1.5 mg
Lactic acid 0.2 mg
Glycerol 52 mg
Distilled water 2.0 ml in total
for injection use
About 300 ml of distilled water for injection use
containing 0.75 g of the inventive compound and 0.1 g of
lactlic acid was mixed with about 500 ml of distilled water
for injection use containing 100 g of lactose (or 26 g of
glycerol), and the mixture was stirred. Contents in the
resulting mixture was dissolved by heating the mixture at
50 C. After cooling down to-room temperature, total
volume of the solution was adjusted to 1,000 ml. The thus
prepared solution was filtered through a membrane filter,
dispensed and sealed into ampoules in 2 ml portions and
then sterilized to obtain injections each ampoule
containing 1.5 mg of the inventive compound.
- 133 -
CA 02453123 2004-01-20
Tablets
Composition
[Tablet]
Inventive compound 5.0 mg
Lactose 73.2
Corn starch 18.3
Hydroxypropylcellulose 3.0
Magnesium stearate 0.5
Subtotal 100 mg
[Coat]
Hydroxypropyl
methylcellulose 2910 2.5 mg
Polyethylene glycol 6000 0.5
Talc 0.7
Titanium oxide 0.3
Subtotal 4 mg
Total 104 mg
A 25 g portion of the inventive compound was mixed
with 366 g of lactose and pulverized using Sample Mill
(manufactured by Hosokawa Micron). After uniformly mixing
391 g of the thus pulverized mixture with 91.5 g of corn
starch in a fluidized granulation coating machine
(manufactured by Okawara Mfg.)-, 150 g of 10%
hydroxypropylcellulose aqueous solution was sprayed on the
mixture to effect granulation. After drying, the thus
-134-
CA 02453123 2004-01-20
prepared granules were passed through a 24 mesh screen,
mixed with 2.5 g of magnesium stearate and then made into
tablets, each weighing 100 mg, by a rotary tabletting
machine (manufactured by Hata Tekko-sho) using a
pestle/mortar system of 6.5 mm~ x 7.8 R. Using a coating
apparatus (manufactured by Freund Sangyo), 154 g of an
aqueous coating solution containing 12.5 g of
hydroxypropylcellulose, 2.5 g of polyethylene glycol 6000,
3.5 g of talc and 1.5 g of titanium oxide was sprayed on
the thus prepared tablets to obtain film coated tablets
each having 4 mg of coated film and containing 5.0 mg of
the inventive compound.
135 -
CA 02453123 2004-01-20
The compounds prepared in Reference Examples 1 to
27 and Examples 1 to 41 have the structures shown below.
Table 2
Re~erence
Example Chemical Formula
Np.
0
~ N
0
02 N
0
2 N
O
HZ N
0
3
0
N
H
0
CH3 aN
4
0 0
N
H
- 136 -
CA 02453123 2004-01-20
Table 3
Reference
Example L"}lE,'Y111ca1. .F rIIltlI_<3
No.
0
aN
CH3
0 0
N
H
0
~7 N
o Cr13 CH3
O 0
N
H
0
OCN
7 ~
C H3 0 0 O
H
0
~
CDC
N.
8
Cz Hs 0 0 0
H_
137 -
CA 02453123 2004-01-20
Table 4
Reference
Example Chemical Formula
No_
0
C HS CH (:tN
-"-D
9 'Ca"~ 0 O 0
Or~ N
H
0
C)~ND
1 0
O 0
N
CH3 H
0
OCN)
1 1 ~
O
N
CH3 O H
0
1 2
CH3 0 0
- 138
-
CA 02453123 2004-01-20
Table 5
Reference
~XamPie Chemical Formula
No.
0 NH
~ ~ = 1/2 HZC 03
13 NNHz
0
0
aN NH2 H 0,
1 4 N
NH
0
0 NH
x\
1 5 (/ N NH2
= 1/2 H,C 0,
0
0 N0
1 6
HN N
OON
~ 0 0
N
H
- 139 -
CA 02453123 2004-01-20
Table 6
Reference
EX mple Chemical Formula
No.
O
0
HN N
1 7
O~N
o o
N
H
0 o
:l 8
HN N
CN
0 0
N
H
1 9
IC 2 11
- 140
-
CA 02453123 2004-01-20
Table 7
Reference Example ChOIl11.C31. FOZZRlll.a
No. f
2 4
Co2H
2 1
~
N
H
O
2,2
H
CH3
HN N
23 0[7N~O
Oz N
141 -
CA 02453123 2004-01-20
Table 8
Reference
Examnle Chemical Formula
No.
CH3
N N-- CH,
O2 N
C H 3
N N-CH3
2 5 MN
O
H2 N
C 0 0 H
2 0 0
N
H
0
CC-N)
2 7
0
.,i 0
r ~
~
142 -
CA 02453123 2004-01-20
Table 9
EXanrp1e Chemical Formula
Noo
NH~
N'~
S
1
N
HBr
9G C}
~
N <~~
H
NH
HNNHZ
N
N
H B r
G G
N
H
I,1Ha
N -A
S
3 CH;
N
0 0
N
H
- 143 -
CA 02453123 2004-01-20
Table 10
Exaniple
No Chemical Formula
NH
HNNHZ
N
'IS
~ CH3
cc'
H B ?"
[) 0
N
H
N
N'~'
H
N
2HC1
N O
H
~~=~ , N C H
N H
6
N
2 H C I
C)
N
9-0
H - 144 -
CA 02453123 2004-01-20
Table 11.
ixamale
~o. Chemical Formula
N .~ N
Y 0 ~0
U H
N
N f
8
HC1
90 0
_~FN
0
L
H C I
a
N
H
- 145 -
CA 02453123 2004-01-20
Table 12
E"ample Chemical Formula
No.
3
HNN C H
~
N CH3
N =2HC1
0 0
N
H
CH,
N
CH
N
,
1 1
N
HCI
p 0
N
H
CHa
N
CH3
12 N
0 HC1
0 0
N
H
- 146 -
CA 02453123 2004-01-20
Table 13
Ekamle Chemical Formula
No.
CH3
N
N
1 3 ~
0 0
N
H
IT NH_
N
i s
1 4
N
(30 L~ () - HCI
H
NHZ
__j
---~
N
s
C" --~
N HC1
0
N
H
- 147 -
CA 02453123 2006-11-01
2167673
Table 14
Example Chemical Formula
No.
NNHz
16 HC1
0
I--
H
0
H N NH:
N
1 7 ' ~~N
0
=HC1
H (CMzCH(OH)
- 148 -
CA 02453123 2004-01-20
Table 15
E"arrtple Chemical Foxmula
No.
C1) CH,
04
1
N
9" 0 0
N
H
1 8
CL~ l.i H 3
H N A
N
N =-HC l
0
N
H
- 149 -
CA 02453123 2004-01-20
Table 16
Example Chemical Formula
No.
CH~
0 A
CH3
N
o
N
H
1 9
(2)
.~
~.. s
HN4
N
CH3
UN HCl
0
N
H
- 150
-
CA 02453123 2004-01-20
Table 17
EXample Chemical Formu!la
No.
CzHs
HN
N
N =HCl
0 0
N
H
n - C 3H,
HN -~\
N
21
N = H C I
0 0
N
H
H N
N HCI
22 N
H
Y 0 0
N
H
-
151
CA 02453123 2004-01-20
Table 18
Example Chemical Forznula
No.
H N
N HCI
2 3
N
99Q
N
H
CH3
HN
N = H C 1
2 4
CC" H3 C o 0
N
H
CH,
HN ---~
N H C 1
N
CH3 0 0
N
H
- 152
-
CA 02453123 2004-01-20
Table 19
Exaniple Chemical Foxmula
No.
CH3
HN~
N HC1
26
N
C Z H 5 0 0 0
N
H
CH3
HN
N HC1
? (
CH3 N
H 3 C O 0 0
i
- N
H
HN
N HC I
28 C H 3 N
H3 C 0 0 C
N
H
- 153 -
CA 02453123 2004-01-20
Table 20
Example Chemical Formula
No.
CH,
HN
N HCI
2 9
N
F O 0
N
H
CH3
HN --~\
N -HCI
N
CH3 CH3
O CI
N
H
CH3
HN --~
N H C I
31 N
0
N O
H
0 C H,
-
- 154
CA 02453123 2004-01-20
Table 21
E"arnple Chemical Formula
No.
C / H 3
HN~
N =HC1
3 2
N
O,~
N
H
CH3
c, H 3
o
N
1V
~,
C H3 0 C)
3
CH3
HN
N HCI
N
C H 3 0 0
- 155 -
CA 02453123 2004-01-20
Table 22
Example Chemical Formula
No.
CH3
HN
N 2 HC )
34 oc ",-,
N
C ~_~/~~
N N
Z H 5 \ 0 0
,
H
NT
H N NHZ
IV
N 2HC1
Qoo
N
H
H N~~~~ N H 2
N
36
OC'7~ 2 H C 1
0 O
CD N
H
- 156 -
CA 02453123 2004-01-20
Table 23
Example Chemical Formula
No.
N H2
HN~
N
3 7 N 7~ ? H C 1
~
0 ~ O
N
H
CHs
~/
HN! \\
~ N
38
N H C 1
0 0
I I
N
CH3
HN iV .
39 CCN---- HCI
O C)
N
H
- 157
-
CA 02453123 2004-01-20
Table 24
Exarriple Chemical Formula
No.
CH3
~
4 0 ~v-cH3
HC
C>
H
N
C 11 3
/
HN -~(
HCI
' 4 1
N
Cl
H
- 158 -
CA 02453123 2004-01-20
Examples 42 to 95
According to the processes described in the
specification, compounds having the structures shown below
are prepared.
Examples 42 to 49
R
N
N
0
N ~
H
Table 25
No:~ R R' No ~ R R'
42 -(CN2)z,VH2 4-CH3 46 ~NN-CH3 4-C1I3
43 -(CH_)31NH2 4-CHa 47 0 J,NHZ 4-CH3
H
N p
44 CH3 4- CH3 48 ~-'~''"~ NH2 H
N
H
45 N~N-CH3 H 49 0 4- CH3
NH 2
- 159 -
CA 02453123 2004-01-20
Examples 50 to 63
R
s~
N
N
o o
H
Table 26
No, R R" No ~ R ~ R N
~0 NH2 -OiPr 57 NH Afe -~~N~
~ NH N;i z N
Nr1
Y
51 NH
~N~HNH' -0i~r 58 ~NH---11-1 \;2 N)
N
0 0
52 ~ NHN H2 - Oi r 19---- N.--~~ NH= Et ~
H N
53 0 0
\ NH'J~--N~O - OiPr 60 ~ NH'Jl''-NIO Ht -'~N)
54 0 0
N
~NH~~ ~ 0 61 ~NH~~N
D Et ~N
55 0 0
~ NH ~~- NN-CH3 62 ~ NH Et
~~- -CH, ~ N
N
56 0
~'~
\ NH J~- Nr N-CH3 OH3 63 ~9e Et -/<
N
!
------------------
- 160 -
CA 02453123 2004-01-20
Lxamnles 64 to 75
R
0
N
0 C)
N
H
Table 27
No, R R 1\10 R ( ~' I
6-~ - CH2NH . H 70 -- NN-CH3
x
, - NH
65 - (CHz)llN-;-iz }71 H
H
NH
66 -(CH2)3NH2 s-1 72 N CH3
H
67 -(CI1z)AH2 4-CH3 73 NH2 fl
H
N
68 ~7- CH, H 74 0 ~;IZ
1
fi~u 4 _ CF3
~ fl
~3 ! 0
69 H 75 NN, H
~ - H
- .~ _.
- 161 -
CA 02453123 2004-01-20
Examples 76 to 93
R
~0
N
R"
0
;j
N
H
Table 28
No R Rõ INo R Rõ
76 ~ - (CH2)2NtI2
-OiPr 86 Me Me -,"
N
77 N ~ - OiPr
1~-~ 87 ~{ e E t-~
78 -(Ciiz)2-N 0 -OiPr h
79 -(CHZ)z-NO --~~~ 88 E# N~
801 NHZ OiPr !
0 89 Me Et N
81 NH2 -OiPr
H
0 N.
82 , NH -OiPr 90 NN/0 Et N)
-~lri N H~ H ~--/ 1
r
4 0
83 \ NH0 - OiPr 91 N
N''~~ N Et N)
H ti
84 0 s-~ 92 0 ~ Et
NH NN-CH, - OiPr ~'J'-- i1N~{e N
0 0 N
85 \ NH~ ~N-CH, --~~ 93 \ NHNH~ Et
- 162
-
CA 02453123 2004-01-20
Examples 94 to 103
R
~
N
~- ~
_ R 0
N 0
H
Table 29
NoI R R' No R R'
94 H H 99 - NH? _ H
95 1 1 il3 100 - 1'H2 csi.113
96 H 101 - N(CHa)2 H
97 - C H ZNii 2 H 102 0c NIH2 H
H
98 ~N 0 H 103
~
~--~ H ~x~ H
- 163
-
CA 02453123 2004-01-20
Examples 104 to 113
R
/
N
N - R
N
o ~ o
N ior
H
Table 30
No R I Rj R" No R R5 1 Rõ
10? -(CH2)2NH2 - H - OiPr 109 -NHCO(CH2)2NH2 - OiPr
H
105 -(CH2)2-N0 -H - OiPr 110 - DH -H - OiPr
10n -CH3 -CH3 --~ 111 -OH -H 4~
107 -CH3 - OiPr 112 - OCH3 -H
100 -NHZ -H -OiPr 113 -SCZH5 -H
-
- 164