Note: Descriptions are shown in the official language in which they were submitted.
CA 02453589 2004-O1-12
WO 031029813 1 PCTIEP02/10681
Test Strips for the Detection of Prion Proteins
The invention relates to a test strip according to the generic part of Claim 1
and a holder allowing
several test strips to be introduced into different sample containers.
Test strips are being used for the detection of a multitude of different
analytes in liquid or
homogenized samples. Tn general, the test strips are provided with at least
one defined region, in
which a detection reagent for a certain analyte is immobilized.
Usually, a lower section of the test strips is contacted with the sample. The
defined detection
region resides either in the lower section of the test strip and is then
directly wetted by the sample.
According to another option, the detection region is provided farther up on
the test strip, though
this requires that the strip is made from a liquid-conducting material and the
sample liquid can flow
from the contact section to the detection region under the effect of capillary
action.
Whether or not a test is positive or negative may be observed e.g. by means of
a color change in the
detection region.
Usually, test. strips are used especially in detection applications that are
particularly well-suited for
standardization and/or which can be performed at home by otherwise untrained
individuals, etc.
Known examples include e.g. pregnancy tests or test strips far the monitoring
of renal function.
CA 02453589 2004-O1-12
WO 03/029813 2 PCTIEP02/10681
In contrast, the detection or prion proteins requires extensive preparations
the performance of
which is restricted at this time to highly specialized personnel working in
safety laboratories. The
detection reaction itself' which is usually performed separately for each
sample, is associated with
relatively little effort as compared to the sample preparation, which is the
reason why
standardization and simplification have not been seriously considered at this
time.
On the other hand, considering the growing number of tests, e.g. on slaughter
animals, it cannot be
excluded that standardization of the reaction for the detection of prion
proteins may well afford
substantial savings in terms of both Iabor and costs.
Thus, it is the task of the present invention to provide a device allowing for
substantially simpler
detection of pzions in liquid, liquefied or homogenized samples than possible
at this time. It is
another task of the present invention to provide a device allowing for
particularly simple
simultaneous measurement of several samples.
The tasks, as stated, are solved by a test strip with the characterizing
features of Claim 1 and a test
strip with the characterizing features of Claim 9.
Similar to known test strips for other analytes, the test strip according to
the invention comprises a
lower section which can be contacted with a liquid or homogenized sample,
whereby, according to
the invention, a first defined region is provided, in which antibodies or
other detection reagents
binding to the prion protein are immobilized. Suitable antibodies are lrnown
to the expert in this
field from the publication by "Korth, C. et aL, Nature 1997, vol. 390, pages
74-77".
According to Claim 1, the test strip is made from absorbent material, in
particular from
nitrocellulose or polyvinylidene fluoride (PVDl~, so that the transport of
liquid between the lower
section, which can be contacted with the sample, and the first defined region
is possible.
In a common test with the test strip according to the invention, a detection
reagent, e.g. antibodies,
is added to the sample initially in order to form, in the presence of a
marker, a detectable complex
CA 02453589 2004-O1-12
WO 031029813 3 PCT/EP02/1Ob81
with the prior protein. Markers such as radioactive isotopes, fluorescent
substances, chromophores
absorbing UV or visible light can be used for detection. In the latter case,
such markers may
comprise e.g. dyed polymer beads, such as latex beads, gold particles,
liposomes, and dye particles
or similar substances. As a matter of principle, all detectable markers which
bind or can bind
actively or passively to antibodies or other detection reagents are suitable
for this purpose. For
simplicity, only examples of antibodies bound to dyed markers shall be
described in the following.
Subsequently, the test strip is contacted with the sample and the sample
liquid migrates across the
first defined region, in which the detection reagents immobilized therein bind
to the prior protein
and colored marker attached thereto contained in the sample. This leads to a
color change in the
first defined region, which may be detected, e.g. by eye.
Suitable detection reagents include e.g. antibodies, aptamers or other means
specifically
recognizing prior proteins.
in order to check whether or not the detection reaction proceeded properly,
the test strip may be
provided with one or several other defined regions, in which control reagents
are inunobilized.
In this context, the test strip according to a preferred embodiment comprises
a second defined
region, in which reagents capable of bindiuag the colored reagent-marker
complex in the sample are
immobilized. This allows to check whether the reagent-marker complex was truly
added to the
sample and/or whether the concentrations are correct, etc. The control
reagents used for this
purpose may comprise especially an antibody or a functional reagent, which
bind to the colored
reagent-marker complex only, if the complex is explicitly capable of binding
to the prior protein.
in particular, recombinant prior protein may be used as the control reagent.
Another preferred embodiment concerns test strips for use in PrPs' detection
reactions. Priors
proteins are known to exist in two different isoforms denoted PrP' and PrPs'.
PrP' is the isoform of
a normal mammalian protein, whereas PrPs' is an anomalous, pathological
isofontn.
CA 02453589 2004-O1-12
WO 031029813 4 PCT/EP02/10681
Currently, it is being presumed that PrP~ is specific for priors diseases.
Common tests and
detection procedures are based on the presumption that PrPs~ is a marker of
disease and thus test
for the presence of this molecule in samples.
However, this procedure is associated with the problem that samples from
infected sources usually
do not contain PrPs~ exclusively, but also PrP~. Thus, the detection procedure
must differentiate
between the commonly present PrP° and the possibly present PrPs~.
Currently, this differentiation is afforded by digesting the sample with a
protease making use of the
fact that the PrP~ form is completely digestible, whereas only an N-terminal
section of the PrPs'
form is protease-sensitive, while a section denoted PrP 27-30 is not digested.
Thus, in a sgecific PrPs' assay, antibodies specifically binding to PrP 27-30
are immobilized in the
first defined region of a test strip according to the invention, although it
cannot be excluded that a
corresponding region of the PrP° form would be bound upon incomplete
digestion.
in order to be sure in this xegard, another preferred embodiment may provide
the test strip
according to the invention with a third defined region, in which control
reagents are immobilized
which specifically recognize and bind to the N-terminus of the PrP protein. If
the digestion
proceeds to completion, the N-terminal region of PrP should be no longer
detectable. In contrast, a
color change in this region indicates the presence of residual intact
PrP° in the sample which means
that the digestion was incomplete. Suitable control reagents include e.g. the
antibodies known from
the publication of "Barry, R.A. et al.; J. Immunol. 1988, vol. i40, pages 1188-
1193".
This arrangement provides for particularly simple means for checking whether
or not the digestion
was complete simultaneous to the detection of prions, whereas state-of the-art
ELISA procedures
required a separate test for this purpose.
Moreover, the test strip according to the invention may be provided with a
region denoted as
"waste pad" to take up the liquid that flowed through the strip. This region
may be provided e.g.
CA 02453589 2004-O1-12
WO 03/029813 5 PCTlEP02/10681
with an absorbent mat or a fleece or blotting paper or similar means. The
shape and depth of the
test strip may be designed in such a way that a small chromatography column is
formed which
allows far the separation and detection of PrP even in large volumes.
The test strips with waste pads according to the invention as described above
are pxeferably made
from absoxbent material to allow the liquid to flow from the sample to the,
possibly separate,
detection and control regions.
Obviously, it is also conceivable to use test strips not made of absorbent
material. In this case, the
defined detection and, possible, control regions would have to be provided on
the test strip in such
a way that they could be directly wetted with the sample or the liquid would
have to be moved
actively (e.g. by means of suction/aspirataon or centrifugation).
Aside from the tests strips mentioned above, the invention comprises a device
for the simultaneous
testing of multiple samples.
Usually, multiple samples are simultaneously processed by use of microtiter
plates or by means of
other formats combining several sample containers into a composite system with
a defined
geometrical arrangement.
Thus, the preferred device accordung to the invention comprises a holder, in
which several test
strips are arranged and taken up in an oriented fashion in such a way that
their lower sections can
be introduced simultaneously in one of the sample containers each of the
composite system used.
Conceivable is therefore a strip-shaped holder, in which the test strips are
fixed parallel to each
other and at a certain distance from each other with the distance
corresponding to the distance of
the sample containers in the row of a microtiter plate.
CA 02453589 2004-O1-12
WO 031029813 6 PCT/EP02/I068I
Another embodiment of the invention provides a holder which takes up all test
strips required for a
certain format simultaneously. Conceivable is for instance a holder for a
microtiter plate, in which
as many test strips are taken up as con:esponds to the number of sample
container wells.
In a particularly preferred embodiment, a device of this kind might comprise
e.g. a frame, in which
one stripe-shaped holder with a corresponding number of test strips can be
introduced for each row
of the microtiter plate. To read the results, the holders can be removed
successively from the frame,
which faciliates the analysis.
In the following, the invention is illustrated in detail by means of several
figures depicting the
different embodiments. Additional figures show the results of tests, in which
the test strips
according to the invention were used.
As such
Fig. 1 shows a test strip;
Fig. 2 shows an embodiment of the holder, in which several test strips are
attached parallel to
each other to form a row;
Fig. 3 shows a frame in the common microtiter plate format, in which the
holders shown in
Fig. 2 can be placed;
Fig. 4 shows a frame completely fitted with holders according to Fig. 2;
Fig. S shows the results of a test, in which several test strips attached in a
holder such as the
one shown in Fig. 2 were used for the detection of recombinant bovine prior
protein at
various concentrations;
Fig. 6 shows the results of a test with several test strips for the detection
of cellular prior
protein in txansgenic mice and wild types;
Fig. 7 shows the results of a test with two test strips for the detection of
disease-specific,
protease-resistant prior protein, and
Fig. 8 shows the results of a test with three test strips for checking the
digestion conditions of
BSE homogenate.
CA 02453589 2004-O1-12
WO 03JOZ9813 7 PCTIEP02J10681
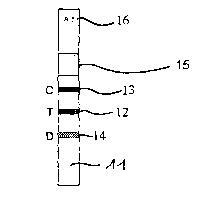
Fig. 1 shows a test strip, 10, for the detection of prion proteins. The test
strip comprises a lower
section, l 1, which can be contacted with a homogenized ar liquid sample.
Moreover, several defined regions, 12,13, and 14 are provided on test strip
10, each of which
contains detection or control reagents. These reagents may be applied to test
strip 10 for instance
by spraying.
Test strop 10 consists of absorbent material, e.g. nitrocellulose. Sample
liquid contacting test strip
in section 11 is aspirated through the test strip along regions 14, I2, 13 to
waste pad I5, which
takes up the liquid after its flow through the test strip. An identification,
16, is provided at the
upper end of the test strip to indicate e.g. the coordinates of the sample in
a microtiter plate.
As mentioned above, different reagents are fixed in the defined regions, 12,
13, and 14. It is
mandatory for any test strip to contain reagents which recognize any prion
protein that may be
present in the sample. In the case shown, these reagents are specific
antibodies against the prion
protein and the reagents are contained in defined region 12.
Region 13 contains control reagents allowing the concentration and/or presence
of the colored
detection reagent-marker complex mentioned above to be checked in the sample.
And lastly, region 14 contains reagents allowing the digestion of prion
proteins to be checked.
Fig. 2 shows a device, 20, in which multiple samples can be analyzed
simultaneously. Device 20
comprises a holder, 21, in which test strips 10, 10', etc., are taken up in a
parallel arrangement with
their lower sections, l l, pointing downwards. The mutual distance between
test strips 10, 10' is
selected in such a way that it corresponds to the usual distance of wells in a
micmtiter plate. Using
holder 21, samples in the wells of a row of a microtiter plate can be checked
simultaneously.
CA 02453589 2004-O1-12
WO 03/029813 8 PG'TIEP02l10681
Perforations 22 facilitating the separation of individual test strips 10, 10'
may be provided between
the individual test strips, 10, 10' on holder 21 which takes the shape of a
strip in the case shown.
It is conceivable to extend this format to the entire microtiter plate.
In this context, Fig. 3 shows frame 30, whose base is selected in such a way
that the frame can be
placed on a conventional microtiter plate by means of an adapter, 32, so that
the plate is completely
covered by the frame. In the area of the upper edges, 30, which extend in a
longitudinal direction,
mutually opposite pairs of slits, 31, 31', are provided, into which one
holder, 21, each with test
strips 10, 10' can be placed.
The number of opposite pairs of slits 31, 31', corresponds to the number of
rows in a microtiter
plate so that one holder 21 each can be introduced per row of the mierotiter
plate.
Holder 30 in its fully assembled state is shown in Fig. 4, in which adapter 32
is not shown.
The test strips shown each comprise a defined region, 12, 13, 14, containing
the different detection
and control reagents. It is self evident that the invention also considers
embodiments, in which
several, rather than one, defined regions are provided on the test strip for
each reagent, i.e. two or
more defined regions each bearing detection reagents capable of detecting
priors protein present in
the sample, or two or more regions containing reagents for checking the
digestion, etc.
Fig. 5 shows the results of a test with several test strips for the detection
of recombinant bovine
priors pmtein (RecBoPrP) at various concentrations. The test strips were
incubated with various
starting concentrations of RecBoPrP in accordance with the methods presented
above. The sample
dilution was in the range from 1 : 1 to 1 : 32. The respective dilution is
indicated in the figure
below the test strip. As a blank control, a test strip was incubated with a
sample containing no
RecBoPrP.
CA 02453589 2004-O1-12
WO 03/029813 9 PCT/EP02/10681
Whereas region 13 of the test strips responding to the presence of the
detection reagent-marker
complex in the sample is constantly colored, region I2 of the test strips
recognizing PrP 27-30
shows decreasing color intensity corresponding to the decreasing concentration
of RecBoPrP. The
difference in the color intensity of region 12 of the test strips over the
concentration range (32-fold)
is easily detectable by eye. Therefore, the test strips can also be used for
semi-quantitative
detection of RecBoPrP.
Fig. 6 shows three rows, 60, 60', and 60", with several test strips each after
incubation with urine
from four transgenic or wildtype mice.
As before, the test strips comprise region 12 recognizing PrP 27-30 and region
13 responding to the
presence of the detection reagent-marker complex in the sample.
The test strips of row 60 were incubated with urine from four wiidtype mice
(WT1-WT4)
producing normal quantities of priors protein which resulted in substantial
coloring of region 12 of
the test strips.
The test strips of row 60' were incubated with urine from four transgenic mice
(Tg201 - Tg20 4)
producing strongly elevated quantities of priors pmtein. This leads to region
12 of the test strips
being even more strongly colored.
The test strips of row 60" were incubated with urine from four transgenic mice
(Ptnp% I
Prnp%4} producing no priors protein. Consequently, region I2 of the test
strips is not colored.
An abbreviation above each test strip in the figuxe allows the identification
of the mouse to which
the test strip corresponds.
It is evident from this figure that the test strips described above
specifically recognize the priors
protein even when it is present in a complex environment (urine).
CA 02453589 2004-O1-12
WO 031029813 1a PCT/EP0211068i
Fig. 7 shows test strips A and B used to analyze protease-treated brain
homogenate from a healthy
cow versus a cow afflicted by BSE.
As mentioned above, the non-infectious isofonn of the priors protein (PrP') is
completely digested
by protease treatment, whereas the infectious isoform (PrPs°) is only
partially digested so that a
domain denoted PrP 27-30 remains.
As before, test strips A and B comprise region 12 recognizing PrP 27-30 and
region 13 responding
to the presence of the detection reagent-marker complex in the sample.
Test strip A was incubated with protease-treated brain homogenate of a healthy
cow. Region 12 of
the test strip remains non-colored, since PrP° was digested to
completion.
Test strip B was incubated with protease-treated brain homogenate of a cow
afflicted by BSE. It is
evident that region 12 of the test strip is colored. This means that PrP 27-30
is present despite
protease treatment indicating that the sample contained PrPs~ prior to the
digestion.
Therefore, a strip test of the type shown herein is well-suited for rapid BSE
screening of bovine
brain samples.
Fig. 8 shows test strips A, B, and C, after incubation with different
homogenates which had been
digested to different degrees.
Aside from regions 12 recognizing PrP 27-30 and regions 13 responding to the
presence of the
detection reagent-marker complex in the sample, test strips A, B, and C
comprise regions 14 which
can bind to the N-terminal region of the priors protein and thus recognize
undigested or
incompletely digested priors protein exclusively, but not digested priors
protein lacking its N-
terminal region.
CA 02453589 2004-O1-12
WO 03/029813 11 PCTIEP02110681
Test strip A was incubated with completely digested, protease-treated brain
homogenate of a cow
afflicted by BSE. Region 12 is colored since the homogenate contains PrP 27-
30, whereas region
14 remains non-colored, because the complete digestion leads to the absence of
N-terminal regions.
Test strip B was incubated with completely digested, protease-treated brain
homogenate of a
healthy cow. This sample contains neither PrP 27-30 nor N-terminal regions
resulting in regions 12
and 14 remaining non-colored.
Test strip C was incubated with incompletely digested, protease-treated brain
homogenate of a
healthy cow. Region 12 of this strip is colored, because the poor digestion
causes the homogenate
to still contain the PrP 27-30 domain, which is also present in PrP'. Region
14 is colored because
there are still some N-terminal regions present. If there were no region I4 in
the test strip, it would
not have been possible in this case to safely differentiate whether the
coloration of region 12 may
be an indication of the presence of PrP 27-30 in a positive sample or instead
be related to
incomplete digestion of normal priors protein.