Note: Descriptions are shown in the official language in which they were submitted.
CA 02454928 2004-O1-08
METHOD FOR STORING HYDROGEN IN AN HYBRID FORM
FIELD OF THE INVENTION
The present invention relates to a method for storing hydrogen in a hybrid
form. More
specifically, it relates to a method for storing hydrogen in two different
forms within a single
tank.
The invention also relates to tanks hereinafter called "hybrid tanks", which
are specially
adapted for carrying out the above method when the hydrogen is stored in
liquid and solid forms
and when the hydrogen is stored in solid and gaseous forms, respectively.
BACKGROUND OF THE INVENTION
Methods for storing hydrogen can be classified in three main categories:
(A) gaseous storage in high pressure tanks;
(B) liquid storage in cryogenic tanks; and
(C) solid storage in tanks containing materials that absorb (in volume) or
adsorb (on surface) hydrogen.
The last category listed above as category (C) is the one that makes use of
metal hydride
storage tanks.
Each of the above categories has advantages and disadvantages that are
summarized in
the following Table l:
CA 02454928 2004-O1-08
-Cj ,Y.~"'"~ ,~ ~ N ~ N
v~ '~ .'..'
U t~.~U 4; , ,_~. ,~
~ b-0 ,.fl
_
U O ,.r".~ = 4~r O "O U O.
~ cue
O c~i~ ~ p O U U ,
o O ~
,
O .~ y..,,__, ~, U
cd a" "O C
~
U ~ 7 ~ v"~~.,U
~ ~ ~ ~' U
U U N ~A ~ r..
~ cn
O ~
~" ~ (-' :N ~ L U . U O O
cd p .i~ N 7.U-,,~ -~ w'
'''" N ~
, " U y 0 b0 ~..~.~..-~ .
~ ~ v y,U
~ ~
_ _ " O
.
O :'_'c3 ' N .~ N ~
U N : N
. y ~ ,.~ cd r-, O
' U ~ ~' :~ O ~ . '..~.
s, cG
~A O ,:~ ~ ~ f , ~ N ~ Y U
Q7 U , r
~ ~ U
.. ~ U U "'~ U ~ ~ U .
.. ~ "~ N
>, ' ,~ ~ '~ c_" ~ 3
.
.ir4-~ ~ J ~ O GG U . ~ U r-w
' '''"' O
~
o ~ o a ~ on ;_ ~, ~ 3 ~ ~ o
~
j cU~ v> O ~ cG 4-i ~A _ ~ ~ N v
J, > U ~ ~
,..,Yr, :-~ U U ~ O U~' _.., J
~ 'C~O~N~
.
_
~ U N . ' ~ +' S3. O C
\ ~ ~ O ~J
'
U S~, c:_:~ D O dj ~
_ V a O ~ . 4 cd ~ ~ ~
~i ~ 0
.
N c N O -i ~ ~ bn
n ~ ~ O O c~
~ ~ cG U ~
'
~ ~ ~ ~ O ~., w ~ ~ S1, ..O ~
U ,- .- '~ cn "'' ,
, U ~.. _
~ :C ''-' '~
J
"C3 ~ cn ~ . in c~ cn ~.,~ ~ _
~ b ,-p
~
~ c~ O ~ O ~ ~ U .~
r~ ~ O O
S' > ~ _ U ~ ~ O c:..~
N N
., ' ~ 4, ~ O ~ c'"' r...,O ~ ~ Y O
~ SC r- ~ "O
a~ on o O Y
,
o ~o~ ~ ~ ~ ~~, 30~ ~~o~
. ' ~ ~ ~ c'.''" U O ,~, O ~ a~
~ :...~y.. "b :~ ij a~
~
s t3 . : ~ =' ~, ' ~,= ~' ~ ~ 3
o ~ . ~ ~ ~., ~_ n
-
~ .n
.~ ~o ~o ~ ., .x~.~ ~.~ >,o,=
N cU b0 ~~ v~ O ' O
O
L U ~ O ~ . U O N
~ ~ .~ ~ U '.~
:.-0
J
c3 e~ C/~U x r-r1.U . .
~O S~ ts. ~ .~ .
~.. ~ c~ ,
. ~
i
~ C/~
~
~ -.
. .
.
.~
O
~ r..~ U ~ N U 4. cn ..O
v~
G7 ~ O c~ cC
r C ~ U _O _O y,:, c~G
U cd
p~ r-' v~ U ~ O O ~ ~ . ~ .
~ >
U .fl ~ 7 O ~ V ;~ V
~
H w ~ ~ ~ ~ ~ U ~ ~ O
4.. W . ~'~" ~ U
~'
"
x U
a ~ ~ .. U N
3 ~ ~
o
a~ . i ' , 'o ~ 4
bn . , C:-.
cn n U U U y.. .
~
~ O ~ ~ U L", ~
'"-'
~ ri
O ; U ~ ~' ;G J,
~ ~
_'~ ,>, U U U
O
'O U cJ ~ '
~ U U :
.r w ~ N ~ t :
' c3 4:;
~ 'O > p ~ ;~ U ' .
Q cn ~
~c,~4
7U O .~
_ O O., Ss.
c~ U .
U ~ ~ cn cn ~ cn cn
>' ~ . ~
~
L ' ~ '_' +~ O ' cd U U
cr3
, = O ~ p ~ U
U ~ ~ .~
~ N
c
t . ~ ,~ Y
: 3 ~n
.
~
Y
V ~
~ a> ~ U . ,~
~ ~ r, ,.~
_r. ~ X ~ ;;n
. ~
~
~ W 3'~ ~ o v
W o v
o
~
a ,..~ 0
0
,
.d ~ b
~
.
o .~ ~ ~ o o
U
b0
O
C~ b.
'
_ 0
'-' U x x o
o
~ ~ _ v~
>
CA 02454928 2004-O1-08
By way of example, in the case of a method for storing hydrogen in a gaseous
form
(category A), a tank of one (1) liter will contain the following amounts of
hydrogen at the
various pressures indicated in Table II:
TABLE II
Gaseous storage
Hydrogen pressure Amount of hydrogen within one
liter
3,600 psig (248 bar) 0.0177 kg
5,000 psig (345 bar) 0.0233 kg
8,000 psig (550 bar) 0.0334 kg
10,000 psig (690 bar) 0.0392 kg
15,000 psig (1,035 bar) ~ 0.0512 kg
In the case of a method for storing hydrogen in a liquid form (category B), a
tardy of one
(l ) liter will contain 0.0708 kg of hydrogen since the density of liquid
hydrogen at -252.8°C.
(that is at the conventional boiling point of hydrogen) is equal to 0.0708
kg/I.
Last of all, in the case of a method for storing hydrogen in a solid form with
a metal
hydride (category C), a tank of one (l) liter containing a hydride of fomula
AB; such as
La?vi;H~ (density; 6.59 kg/1, hydrogen storage capacity of about 1.4%),
occupying the complete
volume of the tank, will contain 0.0923 kg of hydrogen. That is ahmost twice
the amount of
hydrogen stored in a gaseous form in a tank of one liter at 15,000 psig.
The results of this comparative example are given in Table III:
TABLE III
Comparison of the storage capacity of the thru basic methods for storing
hydrogen
Method Amount of hydrogen stored within
tank of
one liter
(A) Gaseous storage at 15,000 psig0.0512 kg
(1,035 bar)
at ambient temperature
(B) Liquid storage at -252.8C (1 0.0708 kg
bar)
(C) Solid storage in a hydride 0.0923 kg
of LaNiS
(10 bar) at ambient temperature
CA 02454928 2004-O1-08
-4-
Of course, in the case of the method for storing hydrogen in a liquid form
(category B),
there is always some gaseous hydrogen in equilibrium with the liquid because
of some
evaporation of the latter. Also, in the case of the method for storing
hydrogen in a solid form
with a metal hydride (category C), operating at low pressure (10 bar), there
is some gaseous
hvdrogen because the hydride never occupies all the space in the tank.
Moreover, in the case of
the method for storing hydrogen in a gaseous form at a very high pressure
(category A), there is
always some hydrogen that is adsorbed (such adsorbed hydrogen is also called
"solid hydrogen"
according to the above terminology) onto the internal walls of the tank.
Therefore, in each
method listed hereinabove (gaseous, liquid and solid), there is always a small
amount of
hydrogen that is stored according to another method of storage.
By way of example, the maximum percentage of hydrogen that may come from
another
method of storage in the case of a tank of one liter containing a metal
hydride powder (Lal~Ti;H~,)
is evaluated. Assuming that the powder is not compacted and, therefore,
occupies about half of
the volume of the tank (about 0.5 liter), considering also that the density of
LaNi;H~ is equal to
6.59 kg/1, and further assuming that the gaseous hydrogen within the tank
(about 0.5 liter) is at a
pressure of 10 bar, the amount of hydrogen that is not solid within the tank
of one liter is
reported in Table IV:
TABLE IV
"Gaseous" hydrogen (10 bar) "Solid" hydrogen Total amount of hydrogen
0.00041 kg (0.9%) 0.0462 kg (99.1%) 0.0466 kg (100%)
This example clearly shows that, for a.ny given method of storage, there can
usually be
1% of hydrogen stored in a different form. However, in all cases, this amount
will always be
lower than S% by weight.
It has alieady been suggested that there could be some advantages in combining
different
means for storing hydrogen within a single tank.
CA 02454928 2004-O1-08
By way of example, U.S. Pat. No. 5,906,792 discloses that there are advantages
when one
combines a low temperature metal hydride with a high temperature metal hydride
in contact with
each other within the same tank. When such a mixture is used for an internal
combustion engine,
the low temperature metal hydride allows cold starting of the engine by
providing the hydrogen
at the start up. When the engine is hot, the heat that is generated effects
desorption of hydrogen
from the high temperature metal hydride.
Similarly, international laid-open patent application No. WO 01/16021
discloses that
there are some advantages in combining solid storage in the volume
(absorption) with solid
storage on the surface (adsorption) in nanoparticles of a hydride in order to
improve, inter alia,
the hydrogen absorption and desorption kinetics.
U.S. Pat. No. 5,872,074 also discloses that the hydrogen sorption kinetics can
be
improved when use is made of a hydride having high specific surface.
Independently of the above, it is also known that the method (C) for storing
hydrogen in a
solid four usually has a response time (loading and unloading) much slower
than the method (A}
for storing hydrogen in a gaseous form and slower than the method (B) for
storing hydrogen in a
liquid form.
Actually, at least 15 minutes, and sometimes more than 1 hour, is required to
fill up a
hydride storage tank. In spite of this drawback, the method for storing
hydrogen in a solid form
has the highest capacity of storage per volume unit (see Table III).
It is known that some technical applications require a response time much
faster than one
minute.
For example, in UPS systems (uninterruptible power supply} using fuel cells
fed with
hydrogen, a response time of about one hundred milliseconds is usually
required. Of course, a
hydrogen storing tank using metal hydride cannot satisfy this particular
requirement. However,
in such a case, use could be made of a tank in which hydrogen is stored in a
gaseous form at high
pressure.
CA 02454928 2004-O1-08
-6-
Similarly, in hydrogen operated vehicles, there are different types of
transitory periods,
such as:
(i) short duration accelerations which usually require a response time of
about one
hundred milliseconds from the propulsion system; and
(ii) power increases when the vehicle is climbing up a hill, which may last a
few
minutes.
In hybrid vehicles which make use of a fuel cell and batteries, the very short
accelerations
(lasting for a few seconds) are powered by the batteries, whereas the
transitory periods of a
longer duration (a few minutes) may depend on gaseous hydrogen for fuel. On
the other hand,
the average power, which is about 20 KW for a typical vehicle, may easily be
accommodated by
a metal hydride tank. The energy contained in the batteries of such a vehicle
usually represents
about 1% of the energy on board. Therefore, one needs an amount of hydrogen
greater than 1%
to function as an energy source during the transitory periods.
In summary, in view of the above, it is obvious that there presently is a need
for a method
for storing hydrogen which would combine the advantages of the different
methods listed
hereinabove.
SUMMARY OF THE INVENTION
The present invention provides a new method for storing hydrogen which
combines the
advantages of at least two of the above mentioned methods for storing
hydrogen, namely the
methods for storing hydrogen in a gaseous form, in a liquid form and in a
solid form.
The present invention provides a single tank, hereinafter referred to as a
"hybrid tank for
storing hydrogen", for storing hydrogen using at least two of the above-
mentioned methods,
namely:
(A) the method for storing hydrogen in a gaseous form
(B) the method for storing hydrogen in a liquid form; and
CA 02454928 2004-O1-08
(C) the method for storing hydrogen in a solid form (i.e. solid state
hydrogen),
in the space defined by the tank or on the surface of the tank wall.
The only condition is that each of the above methods is used for storing at
least 5% by
weight of the total amount of hydrogen within the tank.
Therefore, the invention as claimed is directed to a method for storing
hydrogen in an
hybrid form, which comprises the step of combining and using within a single
tank at least two
hydrogen storage means selected from the group consisting of:
a) means for storing hydrogen in a gaseous form
b) means for storing hydrogen in a liquid form; and
c) means for storing hydrogen in a solid foam by absorption or adsorption,
with the proviso that each of the storing means that are used, is configured
to store at
least 5% by weight of the total amount of hydrogen stored within the tank.
The means mentioned hereinabove for storing hydrogen in different forms are
those
commonly used for carrying out each of the above mentioned methods. They are
very
conventional and need not be further described in detail. The only requirement
is that they be
combined within the same tank, and that each of the means be used for storing
at least 5% by
weight of the hydrogen.
Another aspect of the present invention is to provide a hybrid tank for
storing hydrogen
in both liquid and solid forms, comprising two concentric containers, one of
the containers,
hereinafter called the "inner" container, is located within the other one,
which is hereinafter
called the "outer container", the containers being separated by an insulating
sleeve for
maintaining the inner container at low temperature. The inner container is
used for storing
hydrogen in a liquid form. The outer container is in direct communication with
the inner
container and contains a metal hydride for storing hydrogen in a solid form.
A further aspect of the present invention is to provide a hybrid tank for
storing hydrogen
in both solid and gaseous forms, comprising:
CA 02454928 2004-O1-08
a container having a metallic liner or inner wall covered with a polymeric
outer shell, said
container being devised to store hydrogen in gaseous form at a higher pressure
and to receive and
store a metal hydride in order to store hydrogen in solid foam;
at least one heat pipe mounted within the container to allow circulation of a
heat carrying
fluid; and
a heat exchanger located within the container in order to ensure thermal
connection
between said at least one heat pipe and the hydride.
W another broad aspect, the present invention provides a hydrogen storage
container
containing at least an hydrogen storage composition and hydrogen, the hydrogen
including solid
state hydrogen and gaseous hydrogen, the hydrogen storage composition
including at least a
portion of the solid state hydrogen and having an high equilibrium plateau
pressure, wherein the
solid state hydrogen defines at least 5% by weight of the total weight o.f the
contained hydrogen,
and wherein the gaseous hydrogen has a pressure greater than the high
equilibrium plateau
pressure and defines at least S% by weight of the total weight of the
contained hydrogen.
In a further broad aspect, the present invention provides a system for
converting chemical
energy stored in hydrogen into mechanical energy comprising a hydrogen storage
container
defining a storage space containing at least an hydrogen storage composition
and hydrogen, the
hydrogen including solid state hydrogen and gaseous hydrogen, the hydrogen
storage
composition including at least a portion of tile solid state hydrogen and
having an lvgh
equilibrium plateau pressure, wherein the gaseous hydrogen has a pressure
greater than the high
equilibrium plateau pressure, and an engine fluidly coupled to the container
for receiving the
gaseous hydrogen, the engine being configured to effect conversion of the
chemical energy
stored in gaseous hydrogen delivered from the container to the engine into
mechanical energy.
In another further broad aspect, the present invention provides a system for
converting
chemical energy stored in hydrogen into mechanical energy comprising a
hydrogen storage
container containing at least an hydrogen storage composition and hydrogen,
the hydrogen
including solid state hydrogen and gaseous hydrogen, the hydrogen storage
composition
including at least a portion of the solid state hydrogen and having an
equilibrium desorption
CA 02454928 2004-O1-08
- 9 -
plateau pressure at 20°C of greater than 40 bar, wherein the gaseous
hydrogen has a pressure
greater than the equilibrium desorption plateau pressure of the hydrogen
storage composition,
and a fuel cell fluidly coupled to the container for receiving the gaseous
hydrogen.
In yet another further broad aspect, the present invention provides a system
for
converting chemical energy stored in hydrogen into mechanical energy
comprising a hydrogen
storage container containing at least an hydrogen storage composition and
hydrogen, the
hydrogen including solid state hydrogen and gaseous hydrogen, the hydrogen
storage
composition including at least a portion of the solid state hydrogen and
having an equilibrium
desorption plateau pressure at 20°C of greater than 40 bar, wherein the
gaseous hydrogen has a
pressure greater than the equilibrium desorption plateau pressure of the
hydrogen storage
composition, and a vehicular engine fluidly coupled to the container for
receiving the gaseous
hydrogen.
In yet a further broad aspect, the present invention provides a method of
effecting
hydrogenation of a hydrogen storage composition disposed in a container space
defined by a
hydrogen storage container configured for containing at least hydrogen and the
hydrogen storage
composition, the hydrogen storage composition having an high equilibrium
plateau pressure.,
comprising the step of flowing gaseous hydrogen into the container space so as
to effect
hydrogenation of the hydrogen storage composition at least until the hydrogen
storage
composition includes solid state hydrogen and the solid state hydrogen defines
at least S~% by
weight of the total weight of hydrogen disposed within the container space,
and so as to effect
filling of the container space with the gaseous hydrogen at least until the
gaseous hydrogen
disposed within the container space defines at least 5% by weight of the total
weight of the
hydrogen disposed within the container space.
In one aspect, the gaseous hydrogen defines at least 15% by weight of the
total weight of
the contained hydrogen.
In another aspect, the gaseous hydrogen defines at least 19% by weight of the
total
weight of the contained hydrogen.
CA 02454928 2004-O1-08
-10-
In another aspect, the gaseous hydrogen defines at least 28°l°
by weight of the total
weight of the contained hydrogen.
In yet another aspect, the gaseous hydrogen defines at least 50% by weight of
the total
weight of the contained hydrogen.
In yet a further aspect, the gaseous hydrogen has a pressure of at least 248
bars.
In yet another aspect, the gaseous hydrogen has a pressure of at least 345
bars.
In another aspect, the gaseous hydrogen has a pressure of at least 690 bars.
In a further aspect, the hydrogen storage composition has an equilibrium
desorption
plateau pressure at 20°C of greater than 40 bars, and the gaseous
hydrogen has a pressure greater
than the equilibrium desorption plateau pressure.
In a further aspect, the hydrogen storage material is a metalliferous
material.
In yet another aspect, the metalliferous material is a metal hydride.
In yet another aspect, the metal hydride is in particulate form.
In a further aspect, the hydrogen storage composition has an equilibrium
desoiption
plateau pressure al 20°C of greater than 80 bars, and the gaseous
hydrogen has a pressure greater
than the equilibrium desorption plateau pressure.
In yet a further aspect, the hydrogen storage composition has an equilibrium
desorption
plateau pressure at 20°C of less than 120 bars.
In a further aspect, the gaseous hydrogen defines at least 50% by weight of
the total
weight of the contained hydrogen and has a pressure of at least 345 bars.
BRIEF DESCRIPTION OF THE DRAWINGS
This invention will be better understood by reference to the following
detailed description
of the invention in conjunction with the following drawings, in which:
CA 02454928 2004-O1-08
-Il-
Figure 1 is a diagram illustrating the equilibrium plateau of an hydride
contemplated fox
use in a hybrid gas-solid storage tank disclosed in example 1;
Figure 2 is a schematic cross-sectional view of the hybrid liquid-solid
storage tank
disclosed in example 2;
Figure 3 is a diagram illustrating the equilibrium plateau of an hydride
contemplated for
use in the hybrid gas-solid storage tank disclosed in example 3;
Figure 4 is a schematic cross-sectional view of the hybrid gas-solid storage
tank disclosed
in example 3;
Figures 5 and 6 are diagrams giving the equilibrium plateau of several
hydrides as a
function of the temperature and indicating which one could be used in the
hybrid gas-solid
storage tank disclosed in examples 1 and 3; and
Figure 7 is a diagram illustrating the relationship between reaction heat
(desorption heat
or absorption heat) and equilibrium plateau pressure (absorption or
desorption).
DETAILED DESCRIPTION
EXAMPLE 1: Hybrid storage Tank for Storing Hydrogen in Gas and Solid Fonns
For purposes of illustrating a hybrid storage tank of the present invention, a
hydrogen
storage tank having a volume of 1 liter can be provided and filled with a
powder of nanopauticles
of a hydride of LaNi; having an average diameter of 5 nanometers, The powder
would occupy
50% by volume of the tank, (i.e. 0.5 liters), since it would not be compacted.
The number of
atoms on the surface of these nanoparticles would represent about 28% of the
total amount of
atoms within each particle considering a layer of 0.4 to 0.5 nanometer on the
surface of each
nanoparticle. The tank could then been filled up with gaseous hydrogen at
different pressures
ranging from 10 bar (typical pressure of use of the metal hydride tanks) to
700 bars (typical
pressure used in high pressure gaseous tanks). It is assumed that the amount
of hydrogen in the
volume and at the surface of the metal hydride corresponds to H/M=1
(H=hydrogen, M=metal),
which is typical for most metal hydrides. Under these conditions, the amounts
of hydrogen that
CA 02454928 2004-O1-08
-12-
would be associated to the two different means of storage, have been
calculated and are reported
in Table V hereinafter:
TABLE V
Hydrogen Hydrogen % Hydrogen % Hydrogen % Total amount
in
pressure gaseous phase bound inserted of hydrogen
within the (kg) comiected Wltlllll (kg)
t0 the
tank the surface hydride
of
the hydride
I 0 bar 0.0004 1 0.0142 28 0.0365 71 0.0511
*
150psi
248 bar 0.0089 l~ 0.0142 24 0.0365 61 0.0596
3600psi
345 bar 0.0117 19 0.0142 23 0.0365 58 0.0624
SOOOpsi
690 bar 0.0196 28 0.0142 20 0.0365 52 0.0703
10000psi
It is worth noting that in the first case reported in Table V, that is when
the pressure is
150 psi (10 bar), the amount of hydrogen in gaseous phase represents about 1%
of the total
amount. This example is illustrative of what is presently obtained in
conventional metal hydride
tanks and is therefore outside the scope of the present invention. However, in
the tluree other
cases reported hereinabove, where the pressures are of 3,600 psi, 5,000 psi
and 10,000 psi, the
amounts of hydrogen in gaseous phase represents about 15%, 19% and 28%
respectively of the
total amount of hydrogen within the tank. This is much higher than the limit
of 5% as indicated
hereinabove.
The taut disclosed in example 1 is illustrative of a tank that can be used in
a "back up"
system based on a fuel cell or a hydrogen source generator. In the case of a
failure of the electric
supply, the hydrogen in the gaseous phase will initially supply the fuel cell
or the generator while
such fuel cell or generator will slowly warm up. The pressure within the tank
will be reduced.
When the pressure reaches the equilibrium plateau of the hydride, that is
about 2 bars for an ABS
alloy at room temperature, there will be almost no more hydrogen in the
gaseous phase. Then,
the hydride will take over by supplying hydrogen to the fuel cell or the
generator.
CA 02454928 2004-O1-08
-13-
It is worth noting that, in this example, the equilibrium desorption plateau
pressure of a
hydride of LaNiS which is a conventional low temperature metal hydride at the
operating
temperature (typically ranging between 0 to 100°C), is slightly higher
than the pressure of
hydrogen required at the inlet of the fuel cell, which is typically about 2
bars. If the tank contains
50% by volume of hydride and the balance is occupied with gaseous hydrogen at
690 bars
(10,000 psi), the situation will correspond to that of the diagram given in
Figure 1.
Under such circumstances, during operation of the system, the hydrogen will
first be
supplied by the gaseous phase. Then, when the amount of hydrogen and,
concomitantly, the gas
pressure become low, the hydride will take over by providing hydrogen to the
system. The
pressure within the tank will then be kept at the level of the desorption
plateau of the hydride.
The kinetics of the system will therefore be quite high at the begiiming
(response time of the
gaseous system) and thereafter relatively low (response time of the hydride
system).
There are also other advantages in using such a hybrid method combining gas
and solid
storage, particularly:
a) refilling of the tank is canied out in a short time as compared to
conventional metal hydride tanks;
b) the design of the heat transfer components of the tank is simplified; and
c) the high storage capacity by volume of the metal hydride.
EXAMPLE 2: Hybrid Tauc for Storing Hydrogen in Liquid and Solid Fonns
An hybrid tank 1 for storing hydrogen having a total volume of one liter
comprises two
concentric containers 3,5 (see FIG. 2). The inner container 3 has a volume of
0.8 liter whereas
the outer container 5 has a volume of 0.2 liter. An insulating sleeve 7 is
positioned between the
inner and the outer containers 3,5 to keep the Timer container 3 at low
temperature.
When in use, the Timer container 3 of the tank 1 is filled up with liquid
hydrogen. The
inner container 3 would contain about 0.0708 kg/1x0.8 liter =0.0566 kg of
hydrogen. The outer
container 5 is then filled with a powder of a metal hydride of the type
LaNiSH~ so as to occupy
about 50% of the volume, that is about 0.1 liter. Therefore, the outer
container 5 would contain
CA 02454928 2004-O1-08
-14-
6.59 kg/1x0.1 liter x1.4% =0.0092 kg of hydrogen. The total amount of hydrogen
stored within
the tank 1 would be equal to 0.0658 kg (14% in the outer tank and 86% in the
inner tank).
As compared to a conventional tank for storing hydrogen in a liquid form, the
tank
disclosed in example 2 has the advantage of having essentially no loss of
hydrogen over a period
that may exceed two weeks. Indeed, the problem with any conventional liquid
hydrogen storage
tank is that the hydrogen evaporates (boil off). Up to 1% of the amount of
liquid hydrogen can
evaporate each day from a conventional tank (1% x 0.0566 kg=0.0006 kg/day). In
the hybrid
tank disclosed in example 2, the boil-off hydrogen is absorbed by the metal
hydride (disposed in
the periphery of the inner container) up to its maximum capacity (that is
0.0092 kg/0.0006
kg/day =15 days).
It is worth noting that the idea of using metal hydrides for "catching"
evaporated
hydrogen from a liquid hydrogen storage tank has already been suggested, but
by means of two
separate systems that must be interrelated, connected and independently
controlled. In this
regard, one can refer to U.S. Pat. No. 5,728,483. In contrast, in the present
invention, these two
different means for storing hydrogen are combined within a single tank and
therefore operate in a
simpler manner.
EhAMPLE 3: Hybrid Tank for Storing Hydrogen in Gas-Solid Form For Use in a
System Having Transitory Periods
In the tank disclosed in example 1, use of LaNisH~ is contemplated as the
hydride. This
compound is known to have a low equilibrium plateau pressure (viz. lower than
40 bars). Use
could also be made of other hydride, with a low equilibrium plateau pressure,
such as NaAlH4 or
MgHz.
According to the invention, it is also possible to use also a hydrogen storage
composition
having an equilibrium plateau that is much higher at relative temperatures
(typically ranging
between 0°C and 100°C.) than the equilibrium plateau of the
conventional hydrides (typically
CA 02454928 2004-O1-08
-15-
ranging between 1 to 10 bars at these temperatures). Such an high equilibrium
plateau is 40 bars
or higher . In one embodiment, the hydrogen storage composition has an
equilibrium desorption
plateau pressure at 20°C greater than 40 bars. Examples of such
hydrogen storage compositions
include the following dehydrogenated metalliferous materials which, upon
hydrogenation,
become metal hydrides having an equilibrium desorption plateau pressure
greater than 40 bars at
20°C: Tio.9>Zro.oiCrMn, TiCrI.asMno.~s, TiCrl.sMno.>, Tii.zCri.9Mno.i,
Tio.9;Zro.o;Cr~.zMno.s,
Tio.~~Zro.osCri.?Mno.~Coo.?, Tio.~~Zro.osCr~.zMno.~sVo.os.
An example of a low temperature hydride which has an equilibrium plateau at
room
temperature much higher than 100 bars is a hydride of TiCr~.s (see FIG. 6).
There are also
medium temperature hydrides with equilibrium plateau at high pressures, such
as hydrides of
TiMn~_y, Hf~Cu, Zr~Pd, TiCu3 or Vo.ss$ Cro.~as which can be of interest for
this kind of application
(see FIGS. S and 6).
Preferably, the hydrogen storage composition has an equilibrium desoiption
plateau
pressure ~,reater than 80 bars at 20°C. An example of such hydrogen
storage compositions of
this type include metalliferous materials which, upon hydrogenation, become
metal hydrides
having an equilibrium desorption plateau pressure greater than 80 bars at
20°C, include: TiCrl.s~
TiCr,.rSMno.~S, TiCrMn, and LiAIH~. Use of a hydrogen storage composition
having an
equilibrium desorption plateau pressure at 20°C of greater than 40
bars, and even more
preferably of 80 bars, mitigates or eliminates the need for heat transfer
components to facilitate
heat transfer within a hydrogen storage tank.
There are several reasons why use of such high equilibrium plateau pressure
hydrogen
storage compositions in a hydrogen storage container reduce the need for heat
transfer
components. These include:
1. Desired hydrogen desorption rates achieved at lower rate of heat input;
2. Lower reaction heat during hydrogen absorption;
3. Lower reaction heat during hydrogen desorption; and
CA 02454928 2004-O1-08
-16-
4. Superior heat conductivity of high pressure hydrogen being absorbed or
desorbing from
the hydrogen storage composition.
When hydrogen storage compositions having high equilibrium plateau pressures
are used,
the driving force for hydrogen desorption is relatively higher than with lower
equilibrium plateau
pressure compositions, and acceptable rates of hydrogen desorption can be
achieved with
relatively slower heat input. The rate of hydrogen desorption from a hydrogen
storage
composition is a function of, amongst other things, the differential pressure
driving force (i.e.,
the driving force) defined by the difference between the actual hydrogen gas
pressure in the
hydrogen storage container and the desorption plateau pressure of the hydrogen
storage
composition. Gaseous hydrogen in the tank must exist at a sufficiently high
pressure (>2 bars) in
order to supply hydrogen at a satisfactory rate to a downstream operation
(such as a fuel cell or
an internal combustion engine). For hydrogen storage compositions having a
lower equilibrium
desolption plateau pressure, the driving force is lower than for hydrogen
storage compositions
having a higher equilibrium desorption plateau pressure. This means that
faster heat input is
required to effect adequate hydrogen desorption rates for lower equilibrium
plateau pressure
hydrogen storage compositions than for higher equilibrium plateau pressure
hydrogen storage
compositions. As a consequence, the need for heat transfer components to
facilitate the
necessary heat input 15 IlOt as critical for the higher equilibrium plateau
pressure hydrogen
storage compositions.
Faster hydrogen desolption is also not as critical for hybrid containers using
high
equilibrium plateau pressure hydrogen storage compositions for the reason that
adequate
amounts of gaseous hydrogen are more likely to be present in the container
wThile hydrogen is
desorbing from the hydrogen storage composition, relative to a hybrid
container using a lower
equilibrium plateau pressure hydrogen storage composition. For the high
equilibrium plateau
pressure hydrogen storage composition case, hydrogen desorbs at a relatively
high pressure.
When such hydrogen is being desorbed, there is a relatively significant (in
comparison to the low
equilibrium plateau pressure hydrogen storage composition case) amount of
gaseous hydrogen in
the container. Because there is a relatively significant amount of gaseous
hydrogen in the
container while the hydrogen is being desorbed from the hydrogen storage
composition, it is not
as critical to effect fast desorption of hydrogen from the hydrogen storage
composition, as
CA 02454928 2004-O1-08
-17-
adequate gaseous hydrogen can be supplied from the gaseous hydrogen already
present in the
container. In this respect, the gaseous hydrogen provides a "buffer" time
before the rate of
hydrogen desorption becomes more critical to the supply of gaseous hydrogen
from the
container.
The second and third reasons why the need for heat transfer components is
reduced when
high equilibrium plateau pressure hydrogen storage compositions are used are
based upon the
fact that the hydrogen absorption and desorption phenomena are characterized
by lower reaction
heats (relative to lower equilibrium plateau pressure hydrogen storage
compositions). This is
confirmed thermodynamically by the Van't Hoff equation:
ln(Peq) _ ~ _ ~S
RT R
For example, the heat of formation (ie: absorption of hydrogen) for a hydrogen
storage
composition having an equilibrium absorption plateau pressure of 1 bar at 300k
is 34kJ/mol.H~.
In contrast, the heat of formation for a hydrogen storage composition having
an equilibrium
absorption plateau pressure of 80 bars is 20 KJ/mol.H~, which is only 60% of
the reaction heat
of the lower plateau pressure composition.
Referring to the second reason, relative to an hydrogen storage composition
having a
lower equilibrium plateau pressure, (such as 40 bars at 20°C), an
hydrogen storage composition
having an equilibrium plateau pressure at 20°C of greater than 80 bars
releases less heat energy
during hydrogen absorption. Release of heat energy is a potential concern as
temperatures could
escalate, increasing the equilibrium absorption plateau pressure, and thereby
requiring a higher
gaseous hydrogen pressure to effect absorption of hydrogen by the hydrogen
storage composition
when it is desired to forni the hydrogenated state of the hydrogen storage
composition. To
mitigate against requiring a higher gaseous hydrogen pressure to effect the
hydrogen absorption,
heat transfer components are typically provided in the tank to effect removal
of the heat energy
during hydrogen absorption. W this respect, heat transfer components are less
likely required (or
not required to the same extent) for systems having an hydrogen storage
compositions with an
equilibrium desorption plateau pressure at 20°C greater than 80 bars
than for systems having an
CA 02454928 2004-O1-08
-18-
hydrogen storage composition with a lower equilibrium desorption plateau
pressure (for
example, 40 bars at 20°C).
Referring to the third reason, heat transfer components are also required to a
lesser degree
in systems using metal hydrides having a high equilibrium plateau pressure
(such as greater than
80 bar at 20°C), for the reason that the heat of desorption is less for
higher equilibrium plateau
pressure compositions. Hydrogen desorption is an endothermic reaction,
requiring an input of
heat energy. The delivery of heat energy is less critical for a hydrogen
storage composition
having an equilibrium desorption plateau pressure, at 20°C, of greater
than 80 bar, relative to an
hydrogen storage composition having a lower equilibrium desorption plateau
pressure (for
example, 40 bar at 20°C). This is because heat input is less critical
for hydrogen desorption of
higher equilibrium plateau pressure hydrogen storage compositions. In this
respect, heat transfer
components are more critical for systems using lower equilibrium desorption
plateau pressure
hydrogen storage compositions.
A further reason why the need for heat transfer components is mitigated or
eliminated in
the case of a system v~~ith an hydrogen storage composition having high
equilibrium plateau
pressures is because of the superior heat transfer characteristics of gaseous
hydrogen at higher
pressures. Hydrogen being absorbed by or desorbed from a high equilibrimn
plateau pressure
composition has a higher pressure than hydrogen being absorbed by or desorbed
from a low
equilibrium plateau pressure composition. This means that heat transfer
characteristics during
hydrogen absoiptiondesorption for containers having high equilibrium plateau
pressure
compositions are superior to those for containers having lower equilibrilun
plateau pressure
compositions. This factor further reduces the reliance on heat transfer
components for containers
having high equilibrium plateau pressure hydrogen storage compositions.
The reduction or elimination of heat transfer components improves the
gravimetric
storage capacity of the hydrogen storage container. By such reduction or
elimination, a large
volume of the container becomes accessible for hydrogen storage, thereby
improving gravimetric
storage capacity.
Preferably, towards reducing or eliminating heat transfer components while
concomitantly optimizing gravimetric storage capacity, the present invention
provides a
CA 02454928 2004-O1-08
-19-
hydrogen storage container containing at least an hydrogen storage composition
and hydrogen,
the hydrogen including solid state hydrogen and gaseous hydrogen, wherein the
gaseous
hydrogen defines at least 50% by weight of the total weight of the contained
hydrogen and has a
pressure of at least 345 bars. The hydrogen storage composition includes at
least a portion of the
solid state hydrogen and has an high equilibrium desorption plateau pressure.
The solid state
hydrogen defines at least 5% by weight of the total weight of the contained
hydrogen.
More preferably, the hydrogen storage composition has an equilibrium
desorption plateau
pressure greater than 80 bars at 20°C and less than 120 bars at
20°C. As discussed above, heat of
desorption decreases as equilibrium desoiption plateau pressure increases.
However, above an
equilibrium plateau pressure of 80 bars, the reduction in the heat becomes
less significant with
increasing desorption plateau pressure. This is because of the logarithmic
relationship between
the heat of desorption and equilibrium desorption plateau pressure, as
governed by the above-
mentioned Van't Hoff equation (see Figure 7).
As equilibrium desorption plateau pressure increases, so does the equilibrium
absorption
plateau pressure for a given hydrogen storage composition. In the extreme, the
pressure required
to charge the hydrogen storage composition may become challenging when the
equilibrimn
desorption plateau pressure is above 150 bars. Hydrogen absorption is an
exothermic reaction.
Generation of heat energy during hydrogen absorption increases the temperature
of the hydrogen
storage composition. As the temperature of the hydrogen storage composition
increases, the
equilibrium absorption plateau pressure also increases. As a result, unless
the generated heat is
being transferred away at a sufficient rate from the hydrogen storage
composition, the hydrogen
storage composition becomes more difficult to charge with hydrogen (i.e.
higher pressure
hydrogen is required to effect absorption of hydrogen by the hydrogen storage
composition). To
mitigate this refuelling problem, heat transfer means must be provided to
effect fast heat transfer
from the hydrogen storage composition to control the increase in temperature.
Alternatively,
high pressure hydrogen gas must be provided to effect charging of the hydrogen
storage
composition. In the extreme, charging of a hydrogen storage composition with a
relatively high
equilibrium absorption plateau pressure may become impractical due to
technology challenges
which must be overcome to effect the desired heat transfer or the supply of
hydrogen at a desired
high pressure. For example, where the equilibrium absorption plateau pressure
is 150 bars at
CA 02454928 2004-O1-08
-20-
20°C, heat generated by the hydrogen storage composition may result in
an increase in
temperature of the hydrogen storage composition such that the corresponding
equilibrium
absorption plateau pressure increases to well above 350 bars. Given the
current technology, it is
desirable to maintain the charging pressure between about 350-400 bars,
although it is possible
to exceed this. 1n this respect, it is preferable that the equilibrium
desorption plateau pressure is
120 bars.
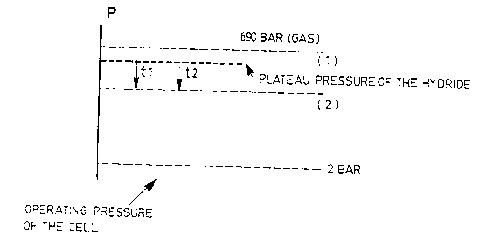
When there is a need for hydrogen, the gaseous system of the storage tank will
permit to
accommodate such a request with a very short response time (tl) of about one
second (for
example in the case of a car that accelerates). When the pressure within the
tank drops and
changes from a value (1) to a value (2) (see FIG. 3), the hydride will
regenerate the gaseous
system with a lower response time (t2) of a few minutes, until the next
acceleration.
It is understood, however, that a "hybrid" hydrogen storage container
containing
pressurized gaseous hydrogen and a high equilibrium plateau pressure hydrogen
storage
composition (such as those described above) is not limited to use in systems
having transitory
periods. Rather, such containers are useful for supplying gaseous hydrogen for
any application
requiring a source of gaseous hydrogen, including fuel cells, internal
combustion engines, and
hydrogen compressors.
This hybrid method makes it possible to substantially simplify the structural
components
required for heat transfer in order to induce the desoiption from the hydride
or absorption
thereby. Moreover, this hybrid storage method mitigates the problem of
refilling hydrides such
as LiAlH4 by requiring filling of the tart with relatively high pressure gas.
As to the kind of
hydrides that can be used, reference can be made to FIG. 5 (hydrides of the
ABS type) and FIG. 6
(hydrides of the ABA type) enclosed herewith.
As an example of the way this method could be earned out, reference can be
made to
FIG. 4 which shows a hybrid tank 11 for storing hydrogen in both solid and
gaseous form. The
tank 11 comprises a container having a metallic liner or Timer wall 15 covered
with a polymeric
outer shell 13. This type of container is conventional and commonly used for
storing hydrogen in
gaseous form at high pressure. It is preferably cylindrical in shape and
provided with an axial
opening 17. The liner 15 is usually made of aluminium whereas its outer shell
is made of a
CA 02454928 2004-O1-08
-21 -
composite material reinforced with carbon fibers. W practice, the container of
the hybrid tank 11
is intended to be used for storing hydrogen in gaseous form at a pressure
usually higher than 40
bar and simultaneously to receive and store a metal hydride in order to store
hydrogen in solid
form as well.
At least one beat pipe 19 is mounted within the container to allow the
circulation of a
heat carrying fluid within the container 11. As shown, the tank 11 preferably
comprises only one
heat pipe 19 which is inserted into the container through the opening 17 and
extends axially
within the same. The tank 11 further comprises a heat exchanger located within
the container to
ensure thermal connection between the heat pipe 19 and the hydride. This heat
exchanger
preferably consists of at least one metallic grid, or a porous metallic
stmcture or fibers 21 which
extends transversally within the container and is in direct contact with the
axial heat pipe 19, the
metal liner wall 15 of the container, and the hydride stored within the same.
The use of such a system of heat pipe and heat exchanger to operate a metal
hydride is
already laiown (see, for example, U.S. Pat. No. 6,015,041). W the present
case, one aspect of the
invention resides in the incorporation of such a system into a tank used so
far only for storing
hydrogen in a gaseous foam at high pressure in order to benefit from the
advantages of both
technologies simultaneously.
It will be understood, of course, that modifications can be made to the
embodiments of
the invention described herein without departing from the scope and purview of
the invention as
defined by the appended claims.