Note: Descriptions are shown in the official language in which they were submitted.
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
TISSUE BIOPSY APPARATUS
BACKGROUND
Technical Field
This application relates to a surgical apparatus and more particularly to a
surgical
biopsy apparatus for resetting and removing tissue through the apparatus.
Background of Related Art
Biopsy is the excision of a small section of tissue from a patient for
diagnosis of
malignancy or other diseases. For cancerous tissue and many other types of
diseases,
early diagnosis and tissue removal is critical since early detection increases
the chances
of successful treatment and survival.
Numerous devices are currently available for performing biopsies of tissue,
such
as breast tissue or liver tissue. These devices function to dissect a portion
of the tissue
and remove it from the body for pathology to determine whether the tissue is
malignant.
The most invasive procedure is referred to as open excisional 'biopsy. In this
procedure, large tissue samples are surgically removed through a large
incision, requiring
long patient recovery times, risking disfigurement, e.g. of the breast in
breast biopsy, and
resulting in increased pain, scarring and morbidity.
In an attempt to overcome the disadvantages of open surgery, more minimally
invasive instruments have been developed. One minimally invasive approach
utilizes a
percutaneous instrument referred to as a fine needle biopsy instrument. In
this
instrument, a needle and syringe are inserted directly through the tissue,
into the taxget
tissue, e.g. the lump, to remove sample cells for pathology. For a breast
biopsy, the
instrument is inserted directly into the breast; for liver biopsy the
instrument is inserted
directly through the abdomen. One disadvantage of this technique is that
numerous cell
samples are required to be taken from the tissue to obtain a sufficient mass
for testing,
thereby requiring numerous needle sticks, increasing the time required for the
procedure,
and possible requiring re-localization by imaging of the lesion. Another
disadvantage is
that careful locational tracking of the tissue cells, which is required for
accurate analysis,
can be compromised. Also, with these devices there is a greater potential for
false
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
negatives due to the small sized specimens being removed without removal of
sufficient
surrounding areas of healthy tissue for comparison. Hemostasis during a biopsy
is also
an issue.
Another type of minimally invasive device is referred to as core needle
biopsy.
This device has a spring actuated cutter a.nd removes a larger specimen than
the fine
needle biopsy instruments. The specimen is suctioned into a side window in the
needle
and then back through the proximal end of the needle. Although larger than
fine needle
biopsy instruments, these needles are still relatively small, e.g. 2 mm in
diameter. Since
typically removal of between five and twenty tissue cores of 2mm in diameter
and 20 mm
in length is required for accurate pathology, five to twenty needle sticks
into the patient
of this 2mm diameter needle is required. These devices also have the
disadvantage that
the spring force cutting action may displace malignant cells into the adj
acent normal
tissue or into the track along the path of entry. Thus, as the needle comes
out, cancerous
tissue can potentially be withdrawn. Also, the amount of false negatives can
be high
because of inadequate removal of surrounding healthy tissue. Like fine needle
biopsy,
success and accuracy of the procedure is skill dependent because the device
must be
maneuvered to various positions and these different positions accurately
tracked.
Some percutaneous devices enable multiple specimens to be removed with a
single needle stick. The specimens are removed from the proximal end of the
needle by a
vacuum. However, the device has a window formed in the sidewall to receive the
tissue
for resection by a cutter. Since only the tissue received within this lateral
window is cut,
the amount of tissue that can be removed and cut is limited. Therefore, the
device must
be rotated and maneuvered so different tissue sections can enter the window
and be
resected. This manipulation is not only time consuming and skill dependent,
but detracts
from the accuracy of tracking the tissue, thereby reducing the diagnostic
accuracy.
To remove larger specimens of tissue utilizing this lateral window approach,
the
device would have to be made significantly larger. However, if made too large,
then the
procedure becomes more invasive and starts to resemble an open surgical
procedure with
the attendant disadvantages enumerated above. The larger instrument can cause
additional bleeding because of the large incision and requires closure of a
larger incision,
thereby increasing scarring, lengthening patient recovery time, and adding to
the cost,
2
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
time and complexity of the procedure. Additionally, if the needle is too large
than a large
amount of tissue will be removed in the path from the skin surface entry point
to the
interior of the target tissue where the lesion is located. If pathology
indicates the lesion is
benign, then a large tissue mass would have been unnecessarily removed,
resulting in
more pain, a larger scar, and possible disfigurement. The disfigurement issue
is more
pronounced with procedures such as breast biopsy.
It would therefore be advantageous to provide a surgical biopsy device which
is
easy to use, reduces surgical procedure time, reduces bleeding and can
minimally
invasively remove large tissue samples sufficiently intact to improve the
accuracy of
pathology.
SUMMARY
The present invention overcomes the disadvantages and deficiencies of the
prior
art by providing a surgical apparatus for removing a portion of tissue
comprising an
elongated body having a distal edge and a cutting member fixedly mounted to
the
elongated body, having an opening therethrough, and extending distally of the
distal edge
of the elongated body member. The cutting member has an exposed conductive
distal
edge forming an electrosurgical cutting surface for applying electrical energy
to tissue.
The cutting ring resects a tubular region of tissue as the apparatus is
advanced through
tissue and the resected tissue extends through an opening in the cutting
member for
containment within the elongated body.
In another embodiment of the present invention the cutting member is
collapsible
and has a closed loop conductive surface movable from a first loop
configuration to a
second smaller loop configuration. An exposed conductive distal edge of the
cutting
member forms an electrosurgical cutting surface for applying electrical energy
to tissue.
The cutting member resects a tubular region of tissue as the apparatus is
advanced
through tissue and the resected tissue extends through an opening in the
cutting member
for containment within the elongated body. The cutting member is movable to
the
second loop configuration to further sever the tissue.
The elongated body member is preferably flexible and preferably comprises an
inner tube and an outer tube wherein at least a portion of the cutting member
is
sandwiched between the inner and outer tubes. The cutting member is preferably
3
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
electrically connected to an RF generator to apply RF energy to the tissue.
The cut tissue
is preferably stored in an interior lumen of the inner tube. The cutting
member can be
substantially circular or elliptical in cross-sectional shape and can
substantially conform
to the cross-sectional shape of the elongated body.
The inner tube may have a reduced diameter region at a distal portion and the
cutting member can be mounted on the reduced diameter region.
In one embodiment, the elongated member of the apparatus is dimensioned for
insertion through a working channel of an endoscope. In other embodiments, the
elongated member is inserted laparascopically through a trocar, intraluminally
through a
catheter, or directly through the skin (percutaneously).
An obturator can be positioned within the elongated member which is extendable
distally from the elongated member past the distal edge of the cutting ring to
penetrate
tissue. The obturator is preferably spring biased to a protected retracted
position such
that a sharp tip of the obturator is positioned proximally of the distal edge
of the cutting
ring. The obturator is also preferably removable from the apparatus.
The present invention also provides a surgical tissue biopsy system comprising
an
endoscope having a channel formed therein for receiving a surgical instrument,
and a
tissue biopsy apparatus insertable through the channel of the endoscope. The
biopsy
apparatus is connectable to a generator for supplying RF energy and has an
annular
cutting member fixedly mounted at a distal end thereof. The cutting member has
a
distally exposed conductive cutting surface for cutting tissue as the
apparatus is advanced
and the cutting member is energized to apply radiofrequency energy to the
tissue. In one
embodiment the cutting member is collapsible.
The cutting member is a preferably in the form of a cylindrical ring having a
distal edge protruding from an elongated body member of the apparatus.
Preferably, the
elongated member comprises an outer member and an inner member positioned
within
the outer member wherein the inner member has a lumen dimensioned to receive
tissue
cut by the cutting member.
The present invention also provides a surgical apparatus for removing a
portion of
tissue comprising an elongated body having a distal edge and a cutting member
mounted
to the elongated body, having an opening therethrough, and extending distally
of the
4
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
distal edge of the elongated body member. The cutting member has an exposed
conductive distal edge forming a closed loop electrosurgical cutting surface
for applying
electrical energy to tissue. The cutting member resects a tubular region of
tissue as the
apparatus is advanced through tissue and the resected tissue extends through
the opening
in the cutting member for containment within the elongated body.
The closed loop is preferably substantially circular in configuration. A
cutting
wire can be provided, movable with respect to the elongated body, and having a
wire
loop movable from an open position to a closed position to sever tissue. In
one
embodiment the wire loop applies RF energy to sever the tissue.
The present invention also provides a method of taking a tissue biopsy
comprising:
providing an apparatus having an elongated member and an annular cutting
member with a distally exposed conductive cutting surface fixedly mounted to
the
elongated member and extending past the elongated member;
introducing the apparatus into the body;
applying radiofrequency energy to the cutting member; and
advancing the apparatus so the exposed cutting surface of the cutting member
contacts and severs the target tissue and enables the severed tissue to be
captured within
the interior of the elongated member.
In one embodiment, the method further comprises the step of inserting the
apparatus through a working channel in an endoscope. In this embodiment, the
apparatus
can be introduced transjugularly or transanally into the body. In another
embodiment, the
step of introducing the apparatus into the body comprises the step of
introducing the
apparatus percutaneously into the body.
The method of introducing the apparatus into the body may also include the
step
of distally advancing an obturator to penetrate tissue. The method may also
further
comprise the step of removing the tissue from the interior of the elongated
member after
the procedure by advancing the obturator within the elongated member to eject
the tissue.
In one embodiment, the step of introducing the apparatus into the body
comprises
the step of introducing the apparatus into breast tissue for severing and
removing a lesion
in the breast. In another embodiment, the step of introducing the apparatus
comprises the
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
step of advancing the apparatus through the esophagus and stomach wall into
the liver or
kidney.
The present invention also provides a method of removing a large tissue sample
comprising:
providing an apparatus having a ring-shaped electrode mounted thereto and
extending distally from the apparatus;
applying radiofrequency energy to the electrode; and
advancing the apparatus through the tissue so the electrode severs the tissue
as it
is advanced to remove an elongated solid tubular tissue region.
In one embodiment, the method includes applying radiofrequency energy to the
cutting member in a first loop configuration and collapsing the cutting member
to a
second smaller loop configuration. The step of collapsing the cutting member
preferably
comprises pulling the member proximally to reduce the size of the cutting
loop.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred'embodiment(s) of the present disclosure are described herein with
reference to the drawings wherein:
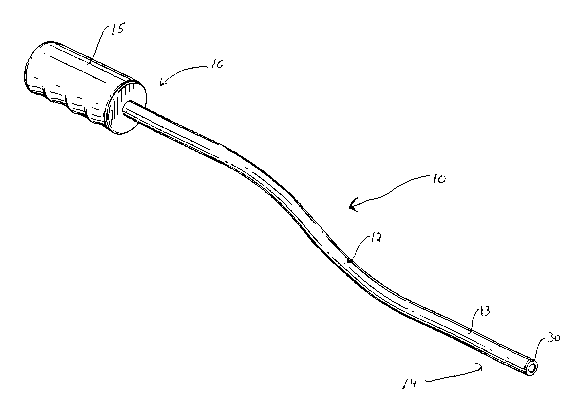
Figure 1 is a perspective view of a first embodiment of the flexible tissue
biopsy
apparatus of the present invention;
Figure 1A is an enlarged perspective view of the distal portion of the biopsy
apparatus of Figure l;
Figure 2 is an exploded perspective view of the distal portion of the biopsy
apparatus of Figure 1 and further schematically showing the electrosurgical
system for
energizing the apparatus;
Figure 3 is a cross-sectional view taken along lines 3-3 of Figure 1A;
Figure 4 is a perspective view of one insertion method of the biopsy apparatus
of
Figure 1 wherein it is introduced laparascopically (through the abdominal
wall), the
Figure showing the biopsy apparatus and the obturator outside the flexible
introducer
sheath;
Figure 5 is an enlarged cross-sectional view of a distal portion of the biopsy
apparatus of Figure 1 showing the obturator positioned within the apparatus
and in an
extended position to penetrate tissue;
6
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
Figure 6 is a side view showing the apparatus of Figure 1 with the obturator
positioned within the biopsy apparatus inside the introducer sheath and in the
extended
position to penetrate into the abdominal cavity;
Figure 6A is view similar to Figure 6 except showing the obturator being
withdrawn from the biopsy apparatus and the biopsy apparatus advanced distally
from the
flexible introducer sheath for resection of tissue from the liver;
Figure 6B is a cross-sectional view of a distal region of the biopsy apparatus
of
Figure 1 showing the apparatus advanced through tissue for removal of a
tubular tissue
section and containment of the tissue section within the lumen of the
apparatus;
Figure 7 is a perspective view of an alternate insertion method of the tissue
biopsy
apparatus of the present invention wherein the biopsy apparatus is inserted
through a
working channel of an endoscope which is shown penetrating the abdominal wall;
Figure 8 is a side view of another insertion method of the tissue biopsy
apparatus
of the present invention wherein the apparatus is inserted transjugularly into
the stomach
through a working channel of an endoscope;
Figure 9 is a perspective view of another application of the tissue biopsy
apparatus of the present invention shown being inserted percutaneously into
breast tissue
with the obturator in the extended position to penetrate tissue;
Figure 10 is a perspective view of the distal portion of an alternate
embodiment of
the tissue biopsy apparatus of the present invention having a cutting wire;
Figure 11 is a cross-sectional view taken along lines 11-11 of Figure 10;
Figure 12 is a perspective view similar to Figure 10 except showing the
cutting
wire loop being closed to sever tissue;
Figure 13 is a perspective view of the distal portion of another alternate
embodiment of the tissue biopsy apparatus of the present invention having a
collapsible
cutting ring;
Figure 14 is a cross-sectional view taken along lines 14-14 of Figure 13; and
Figure 15 is a perspective view similar to Figure 13 except showing the
collapsible ring being closed to sever tissue.
7
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Referring now in detail to the drawings where like reference numerals identify
similar or like components throughout the several views, Figure 1 illustrates
a first
embodiment of the tissue biopsy apparatus of the present invention. The tissue
biopsy
apparatus is designed to resect tissue by application of radiofrequency (RF)
energy and
store the resected tissue section intact in a lumen of the apparatus. The
apparatus 10
achieves this by a distally positioned stationary cutting ring 30 which
resects tissue as the
apparatus is advanced through tissue, progressively removing a continuous
cylindrical
section of tissue. The tissue sample can then be removed from the lumen and
sent to
pathology, with the continuous intact specimen increasing the accuracy of the
testing and
diagnosis of the tissue for malignancy or other diseases. The alternate
embodiment
depicted in Figure 13 utilizes a collapsible cutting wire loop instead of the
fixed cutting
ring to resect a cylindrical tissue section in a similar fashion.
The apparatus 10 with the RF cutting ring has application in laparascopic
approaches, endoscopic approaches both transanally and transjugularly such as
through a
bronchoscope, percutaneous approaches directly through the skin, and
intraluminal
approaches where it can penetrate the vessel wall from the inside to access an
organ or
other target tissue. Several of these approaches are illustrated in the
drawings and
described below.
Additionally, it may be desirable that the cutting ring be energized before
the
cutting ring is in contact with the tissue to be sampled. This facilitates
cutting as it can
prevent the tissue from potentially initially sinking the current. Therefore,
a catheter or
sheath can be provided in which the biopsy apparatus is rernovably positioned
so that the
power can be turned on to energize the apparatus while the cutting ring is
shielded
(retracted) within the catheter (sheath), followed by advancement into the
target tissue.
This is also discussed in more detail below.
The tissue biopsy apparatus of the present invention can be flexible to
facilitate
manipulation and navigation through the patient's body, or alternatively can
be rigid if
being used, for example, in certain laparascopic approaches.
Turning now to the details of the apparatus and with particular reference to
Figures 1-3, biopsy apparatus 10 has a flexible elongated body 12 composed of
a hollow
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
outer sleeve 13 and a hollow inner tube 20 fixedly mounted therein. Positioned
at the
proximal portion of apparatus 10, extending proximally of elongated tubular
body 12, is a
handle grip 15 for grasping by the surgeon. Fixedly positioned at the distal
end portion
14 of apparatus 10 is cutting ring 30 which forms a stationary electrode for
transfer of RF
energy to the tissue which when energized, enables the apparatus to advance
through
tissue, resecting the tissue section as it is advanced and capturing the
resected tissue
within the lumen 25 of inner tube 20. Distal edge 26 of outer sleeve 13 is
preferably
tapered along surface 29, forming a beveled edge to facilitate passage through
tissue. The
distal end of inner tube 20 can also be beveled at surface 21 as shown.
Inner tube 20 has a reduced diameter portion (waist) 22 forming a shoulder 24.
Cutting ring 30 is fixedly mounted on the outer surface of waist 22 in
abutment with
shoulder 24 by compression fitting, insert molding, or other attachment means.
The
cutting ring 30, as shown, is cylindrically shaped in a closed loop and is
hollow forming
an opening 34 for resected tissue. The cutting ring 30 has a distal edge 32
that extends
slightly distal of the distal edge 26 of outer sleeve 13 and the distal edge
27 of inner tube
20. Thus, only the distal edge 32 and the surface slightly proximal of the
edge 32,
designated as area 35, is exposed to the tissue. Consequently, when RF power
is applied,
only the annular exposed surface 35 and annular edge 32 of the cutting ring 30
applies RF
energy to cut and cauterize the tissue.
A conductive wire 28 extends from cutting ring 30 through axial channel 26
formed throughout the length of inner tube 20, extending proximally from the
apparatus
for connection to an electrosurgical generator. That is, wire 28 electrically
connects
cutting ring 30 to RF generator box "A", shown schematically, which is plugged
into an
AC power supply, or alternatively is battery powered. Conventional ground
plate G is
also shown schematically and is electrically connected to generator A and
functions as a
return electrode as in conventional monopolar systems.
As explained above, the distal edge 32 of cutting ring 30 protrudes distally
from
the distal edges 26, 27 of outer sleeve 13 and inner tube 20, respectively, so
it is exposed
to body tissue. As the apparatus 10 is energized by the RF generator and
advanced
through tissue, the exposed edge 32 and exposed adjacent surface 35 cut and
cauterize
the tissue, forming a cylindrical resected tissue section (circular in cross
section). As the
9
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
cutting ring 30 advances, the resected solid tubular section passes through
opening 34 in
cutting ring 30 and through lumen 25 of inner tube 20 where it is stored
therein for
subsequent removal for pathology.
To ensure energy flow through wire 28 to cutting ring 20, inner tube 20 is
composed of a non-conductive material. This insulates wire 28. Additionally,
outer
sleeve 13 is composed of a non-conductive material, although alternatively, it
can be
composed of a conductive material with the portions of the sleeve in contact
with and
adjacent cutting ring 30 composed of a non-conductive material or a non-
conductive
coating. If inner tube 20 is composed of conductive material, wire 28 is
contained in an
insulated sleeve and the portions of the inner tube 20 in contact with cutting
ring 30 are
electrically non-conductive.
It should be appreciated that the apparatus 10 can be inserted with the aid of
an
obturator, such as obturator 50 of Figures 5 and 6. The obturator is
positioned within the
lumen 25 of inner tube 20 and has a sharp penetrating tip such as a trocar tip
52 shown in
Figure 6. Alternatively, the obturator can have a blunt tip as in obturator 60
of Figure
6A. Obturator 50 is preferably spring biased proximally and is thumb actuated
by the
user. The user would press the thumb button (not shown) at the proximal end of
the
obturator, forcing the obturator distally from the inner tube 20 to expose the
penetrating
tip 52 from its retracted position where it is shielded within the inner tube
20 to an
extended position where the penetrating tip 52 extends distally from the inner
tube 20 and
extends past the distal edge 32 of the cutting ring 30. With the obturator in
this position,
the apparatus 10 is forced through tissue to the target site. Once at the
target site, the
thumb button is released, allowing the obturator to return to its retracted
position,
enabling the penetrating tip 52 to retract within the confines of inner tube
20. This is
further described below.
Turning now to the method of use of the apparatus, the apparatus of the
present
invention can be inserted in a variety or ways to treat different regions of
the body. For
example, the apparatus can be inserted percutaneously, i.e. directly through
the skin into
the target tissue. The apparatus can also be inserted laparascopically where
it is inserted
through a trocar extending through the abdominal wall. Further, the apparatus
can be
inserted endoscopically, through a working channel of an endoscope that is
inserted into
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
the patient either transanally or transjugularly. Still further, the apparatus
can be inserted
transluminally through a vessel.
An example of the percutaneous approach is illustrated in Figures 4-6B. An
incision is made through the stomach and a flexible introducer sheath 40 along
with the
apparatus 10 and obturator 50 are inserted therethrough, penetrating the
stomach wall
into the abdominal cavity as shown in Figure 4. The cavity can be insuffflated
in certain
applications. Figure 4 shows the sheath 40, apparatus 10 and obturator 50
separated for
convenience, although they would be inserted as a unit in this embodiment.
More specifically, obturator 50 has a penetrating tip which is spring biased
to the
retracted position. Thus the tip 52 is normally shielded within the obturator
cannula. For
insertion, the obturator 50, which is positioned within the internal lumen 25
of inner tube
20, is actuated to advance the tip 52 out of the cannula and distally of the
inner tube 20
and introducer sheath 40. This actuation can be achieved by pressing a thumb
button on
the proximal end or by other conventional means. With the tip 52 in the
exposed
position, the sheath 40 and apparatus 10 therein can be advanced through the
tissue as
shown in Figures 6 and 6A. Once the sheath is in position, the surgeon
releases the
thumb button, causing the tip 52 to automatically retract to a shielded
position, and the
obturator is withdrawn. Alternatively, the apparatus and obturator can be
advanced
together with respect to the introducer sheath 40. That is, the sheath would
remain in its
position, and the tip of the obturator exposed to advance through additional
tissue to
deliver the apparatus 10 to the target tissue. Once at the target tissue, the
obturator would
be removed from the apparatus and the introducer sheath 40.
Once the obturator 50 is withdrawn, the apparatus 10 is then energized to
apply
RF energy to cutting ring 30 via connecting wire 28 to resect the tissue. The
RF energy
both cuts and coagulates the tissue, and cores through the tissue as the
apparatus 10 and
cutting ring 30 are advanced, minimizing peripheral burn. As can be
appreciated, the
cutting ring 30 resects a tubular section of tissue, having a cross-sectional
dimension
substantially corresponding to the internal diameter of the cutting ring 30.
Either small
bites of tissue can be taken, with the power repeatedly turned on and off, or
the power
can be left on, taking a continuous elongated tubular section of tissue. The
resected
tissue is contained within the internal lumen 25 of inner tube 20, with the
cross sectional
11
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
dimension substantially corresponding to the diameter of the lumen 25. The
apparatus is
then removed from the patient. If desired, the obturator can be reinserted to
force the
tissue samples) distally out of lumen 25 of inner tube 20 for testing of the
sample.
Note that Figure 6A shows as an alternative a blunt tipped obturator 60 which
would be inserted in the same manner as obturator 50, except it would bluntly
dissect the
tissue during penetration. It should be appreciated that the obturator can be
biased in a
variety of ways and can be actuated by mechanisms other than a thumb button.
The
obturator can also be configured so that the apparatus is biased with respect
to the
obturator. Also, the obturator can alternatively be automatically retractable
within the
inner tube after initial tissue penetration.
The obturator can be used at the end of the procedure to eject the tissue
sample
from the lumen in the inner tube. In this version, the obturator would remain
withdrawn
inside the apparatus and the user would then press the thumb button so the
distal end of
the obturator contacts the tissue sample and forces it from inner tube 20.
Suction can also
be used to remove the sample when the instrument is inside or outside the
patient.
In an alternate insertion method, the introducer sheath 40 can be in the form
of a
cannula portion of a conventional trocar which has a removable obturator (not
shown)
with a penetrating tip initially inserted with the cannula to penetrate
tissue, and then
removed, leaving the cannula (sheath) in place. In this embodiment, the
introducer
sheath 40 is placed first, and then obturator 50 and apparatus 10 are
introduced as a unit
through the introducer sheath 40. The obturator tip 52 is then extendable from
the inner
tube 20 to enable the apparatus to be advanced to the desired tissue site.
An alternative embodiment of the apparatus of the present invention is
depicted in
Figure 7. Apparatus 100 is dimensioned for insertion through an endoscope
channel
which is dimensioned to receive a surgical instrument therethrough and is
commonly
referred to as a working channel. Endoscope 200 can be placed through a trocar
210,
which is preferably flexible, for endoscopic or laparascopic surgical
applications, such as
for laparascopic liver biopsy. Optics 202, 204 illuminate the surgical site
and provide .
images for viewing the site. The apparatus 100 as shown is inserted through
the skin and
stomach wall to access the liver. RF energy is applied to the distal cutting
ring 130
extending distally from the outer sleeve and inner tube in the same manner as
described
12
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
above. The resected tissue from the liver is stored within the inner tube and
subsequently
removed for pathology. The endoscope utilized can be flexible or alternatively
can be a
rigid endoscope.
A sheath 102 can optionally be provided, surrounding at least a distal portion
of
the apparatus 100 and also positioned within the working channel of the
endoscope 200.
The apparatus 100 and sheath 102 can be extended through the working channel
distally
of the endoscope with the distal cutting edge remaining shielded within the
sheath 102.
The cutting ring 130 can then be energized within the sheath 102, prior to
contact with
tissue, and then advanced from sheath 102 into tissue once energized.
An obturator can be removably positioned within the apparatus. In this way,
the
apparatus and obturator can be advanced distally of the endoscope, and the
obturator
extended to penetrate tissue to advance the cutting ring to the desired site.
Figure 8 illustrates another application of the apparatus of the present
invention.
The flexible apparatus is inserted transjugularly through a conventional
flexible
endoscope 300. As shown, the apparatus 250 extends through a working channel
of the
endoscope, which extends through the patient's mouth and throat, down through
the
esophagus and stomach wall and into the bowel to access the liver or the
kidney.
Apparatus 250 contains an identical annular cutting ring as apparatus 10 and
is energized
and advanced in the same manner as described above. A sheath similar to the
aforedescribed sheath 102 could optionally be utilized. The apparatus can also
include an
obturator which could be used to enable the apparatus to penetrate the stomach
wall to
access the desired tissue, e.g. the liver.
The obturator could also be used if the apparatus is used intraluminally in
that
obturator would penetrate the vessel wall to allow the cutting ring to access
and energize
the tissue outside the vessel. Alternatively, the RF energy can be used to
penetrate the
vessel wall.
Figure 9 illustrates removal of breast tissue utilizing the apparatus of
Figure 1.
Apparatus 10 is inserted percutaneously with the aid of obturator 50 through
the breast
tissue to access the lesion. RF energy is applied to the cutting ring 30 to
resect a portion
of the lesion and contain it within the internal lumen for withdrawal and
pathology in the
manner described above.
13
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
Figure 10 illustrates an alternate tissue biopsy apparatus of the present
invention.
Apparatus 10' is identical to apparatus 10 described above except for the
provision of a
cutting wire 70 and groove 77 formed in the inner tube 20'. Common parts
between
apparatus 10' and apparatus 10 are identified with the same numerals, except
for the
prime designation for apparatus 10'. Cutting wire 70 has a loop 72 which is
seated
within groove 77 and extends through a channel in the inner tube 20', with the
proximal
end exiting the proximal end of the apparatus 10'. After the tissue section
has been cored
by cutting ring 30' as the apparatus is advanced through tissue in the manner
described
above, the tissue section can be severed by pulling wire 70 proximally in the
direction of
the arrow (Fig. 11) to thereby close the loop (Figure 12) around the tissue.
Figure 12
shows the loop 72 in the open and in the partially closed position. As shown,
wire loop
72 is preferably positioned proximal of cutting ring 30'. Loop 72 can have a
cutting edge
to mechanically sever the tissue or alternately be connected to a RF source to
electrically
sever the tissue. Apparatus 10' can be used in the same surgical applications
as described
with respect to apparatus 10.
Figures 13-15 illustrate an alternate embodiment of the tissue biopsy
apparatus of
the present invention which instead of a stationary cutting ring, utilizes a
collapsible
cutting wire. Apparatus 400 has a tube or sleeve 413, preferably flexible,
having an
annular groove 417 formed at a distal end for receiving the cutting member
430. (Only
the distal end of the apparatus is shown.) Alternatively, an outer and inner
tube could be
utilized, with the cutting member mounted therebetween.
Cutting member 430 forms an electrode for transfer of RF energy to tissue and
is
positioned at the distalmost end of the apparatus, extending past the
distalmost edge 415
of sleeve 413 similar to the way ring 30 extends past the distal edges of
sleeve 13 and
inner tube 20 of apparatus 10 of Figure 1. However, unlike ring 30, cutting
member 430
is in the form of a movable cutting wire with a looped end seated within
groove 417.
Cutting member 430 is movable between an expanded position wherein the loop
432
forms a first loop having a first diameter substantially corresponding to the
diameter of
the sleeve 413 (see Figs. 13 and 14) and a collapsed position wherein the loop
432 forms
a second loop having a second diameter smaller than the first diameter.
14
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
Conductive wire portion 428 is preferably integral with loop 432 and
electrically
connects cutting loop 432 to a RF energy source to apply RF energy to the
tissue. Portion
428 extends in the channel formed between wall portions 421 and 429, exiting
the
proximal end of the instrument.
The apparatus 400 is advanced with the loop in the enlarged position and RF
energy is applied to the tissue via the loop to core through tissue in the
manner described
above with respect to apparatus 10 and 10'. After coring, the proximal portion
of the
cutting wire is pulled proximally in the direction of the arrow of Figure 14,
tightening the
loop around the tissue (see the arrow in Figure 15). This tension pulls loops
432 out of
groove 417 and collapses loop 432 to sever the end of the tissue, thereby
separating the
tubular tissue section from the patient to fully capture it within the lumen
of the tube 413.
Thus, the RF energy applied through loop 432 resects the distal end of the
tissue section.
Note that Figure 15 shows the loop 432 in the partially collapsed position, it
being
understood that fuller closure would be required to sever the tissue. The wire
loop can be
energized before the loop is in contact with the tissue to be sampled by
containment
within a sheath as described above with respect to apparatus 10
Apparatus 400 can be used for the same surgical applications and the same
methods of insertion as described herein with respect to apparatus 10.
As can be appreciated, the biopsy apparatus of the present invention can
remove a
relatively large area of tissue, and can remove a continuous solid cylindrical
strip of
tissue which is intact to improve the accuracy of pathology. The strip can be
continuous if
the RF energy is constantly applied during the resecting process, or, if RF
energy is
applied intermittently, could be a series of discrete adjacent cylindrical
portions which
also facilitates pathology.
The cutting ring (and wire loop) is preferably the same cross-sectional shape
as
the cross-sectional shape of the cannula, thereby conforming to its cross-
sectional
dimensions. Such preferred embodiment enables a tissue section to be severed
substantially equal to the diameter of the cannula, thereby striking a balance
between a
larger incision if the cutting ring or loop exceeded the cannula diameter and
a smaller
tissue biopsied if the cutting ring or loop is smaller than the cannula.
Obviously,
however, although not necessarily optimal, a smaller or larger cutting ring or
loop
CA 02455067 2004-O1-23
WO 03/020136 PCT/US02/26264
relative to the cannula could be utilized. Additionally, although the cutting
ring or loop is
shown as circular in cross-section, it is also contemplated that the cutting
ring or loop
could be oval or elliptical in cross-sectional shape to form a closed loop to
encircle the
tissue. Other shapes are also contemplated.
The apparatus of the present invention can be used fox biopsy of different
types of
tissue, including but not limited to breast tissue, liver, pancreas, brain,
polyps, kidneys
and lymph nodes.
If not used with an endoscope, the apparatus can be used with various known
imaging techniques such as tomographic, stereotactic and ultrasonic imaging.
While the above description contains many specifics, those specifics should
not
be construed as limitations on the scope of the disclosure, but merely as
exemplifications
of preferred embodiments thereof. Those skilled in the art will envision many
other
possible variations that are within the scope and spirit of the disclosure as
defined by the
claims appended hereto.
16