Note: Descriptions are shown in the official language in which they were submitted.
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
GAS MICROSPHERE LIPOSOME COMPOSITES
FIELD OF THE INVENTION
The present invention provides a formulation that includes a gas microsphere
liposome composite (MSLC) suspended in a medium. The gas microsphere liposome
composite includes a gas-filled microsphere; at least one of a lipid and a
surfactant adsorbed
onto the surface of the gas-filled microsphere; and liquid-filled liposomes
attached to the lipid
or surfactant. The outer surface of the liquid-filled liposomes can
incorporate a targeting
ligand (i.e., diagnostic agent targeting moiety) for directed delivery of the
MSLCs for
selective imaging of receptors, enzymes, mRNA and other relevant biological
targets.
Additionally, the liquid-filled liposomes can include one'or more drugs (e.g.,
therapeutic
agents and/or diagnostic agents) in the internal volume of the liquid-filled
liposomes. As
such, the therapeutic agent or diagnostic agent can be selectively delivered
to an organ or site
of pathology for localized delivery. Accelerated drug release can be
stimulated by the
application of acoustic energy at the site of pathology where the targeted
MSLCs bind,
thereby providing locally high concentrations of therapeutic agent or
diagnostic agent in a
selective fashion.
BACKGROUND OF THE INVENTION
Ultrasound imaging is useful for imaging structures in the body of a patient
(e.g., mammal) so as to aid in diagnosis and therapy. During ultrasound
imaging, an
ultrasonic scanner can be used to generate and receive sound waves. The
ultrasonic scanner
is placed on the body surface overlying the area to be imaged, and the sound
waves generated
by the scanner are directed toward the area to be imaged. The scanner then
detects sound
waves reflected from the underlying area and translates the data into images.
The acoustic
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
properties (e.g., density) of each structure in the body will typically depend
upon the velocity
of the transmissions and the density of the structure. Changes in acoustic
properties will be
most prominent at the interface between different substances (i.e., at the
interface between
solids, liquids and gases). Thus, when ultrasonic energy is directed at an
area that includes
interfaces between different substances, the different acoustic properties of
the substances
will cause different reflection characteristics. Because the quality of the
resulting ultrasound
image is enhanced by having an interface between different structures, it
would be useful to
increase the difference in acoustic properties between different structures
and to enhance the
quality of the image generated during ultrasound imaging.
One method that can affect the quality of ultrasound imaging is the
introduction of contrast agents into the vasculature of the body to act as
ultrasound contrast
agents. When the contrast agents are injected into and perfuse the
microvasculature, clearer
images may be produced. The agents act as sound wave reflectors, effectively
enhancing the
interface between the vasculature and other structures.
Liquid and solid contrast agents containing entrapped gas are well known in
the art. See, e.g., U.S. Patent No. 4,235,871; U.S. Patent No. 4,265,251; U.S.
Patent No.
4,442,843; U.S. Patent No. 4,533,254; U.S. Patent No. 4,572,203; U.S. Patent
No. 4,657,756;
U.S. Patent No. 4,681,199; U.S. Patent No. 5,088,499; U.S. Patent No.
5,147,631; U.S. Patent
No. 5,228,446; U.S. Patent No. 5,271,928; U.S. Patent No. 5,380,519; U.S.
Patent No.
5,413,774; U.S. Patent No. 5,527,521; U.S. Patent No. 5,531,980; U.S. Patent
No. 5,547,656;
U.S. Patent No. 5,558,094; U.S. Patent No. 5,573,751; U.S. Patent No.
5,585,112; U.S. Patent
No. 5,620,689; U.S. Patent No. 5,715,824; U.S. Patent No. 5,769,080; EP 0 122
624; EP 0
727 225; WO 96/40285; and WO 99/65467. The microbubbles provided by these
contrast
agents act as sound wave reflectors due to the acoustic differences between
the gas
microbubble and surrounding liquid.
Feinstein, U.S. Pat. No. 4,572,203, discloses "microbubbles" of about 6-20
microns in diameter, produced by sonication of certain viscous solutions, for
use as
ultrasound contrast agents. Feinstein also discloses solid or semi-solid metal-
containing
2
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
microparticles, such as glass or graphite, not containing trapped air, small
enough to pass
through capillaries, as ultrasound contrast agents. Also disclosed are
microspheres formed
from an amino acid polymer matrix, such as albumin, with magnetic particles,
such as
magnetite (Fe304) embedded therein.
Tickner, U.S. Pat. No. 4,265,251, discloses the use of certain saccharide
composition "microbubble" particles with a hollow gas-filled interior space as
ultrasound
enhancing agents.
Rasor et al., U.S. Pat. No. 4,442,843, U.S. Pat. No. 4,657,756, and U.S. Pat.
No. 4,681,119, illustrate aggregates of microparticles (of 1-50 micron
diameter) of a solid
material, which are soluble in blood, containing gas in the voids between the
particles, or with
gas adsorbed on the surface of the particle, or containing gas as an integral
part of the internal
structure of the particle, for use in ultrasound imaging. The following solid
materials are
used: various saccharides, NaCI, sodium citrate, sodium acetate, sodium
tartrate, CaCl2 and
A1C13.
Hilmann et al., EP0122624, contains microparticles that include a solid
surface-active substance, including various organic lipophilic compounds, with
enclosed air,
for use as ultrasound contrast agents. Also disclosed is the combination of
particles of the
surface-active material and particles of a non-surface active material, such
as sodium
chloride, sodium citrate, sodium acetate, sodium tartrate, and various
saccharides.
Glajch et al, U.S. Patent No. 5,147,631, discloses porous particles of an
inorganic material that include an entrapped gas or liquid. The materials
disclosed include
monomeric or polymeric borates, monomeric or polymeric aluminas, monomeric or
polymeric carbonates, monomeric or polymeric silicas, monomeric or polymeric
phosphates;
and pharmaceutically acceptable organic or inorganic cationic salts thereof.
Unger disclosed perfluorocarbon gas-filled microspheres (U.S. Patent No.
5,547,656 and U.S. Patent No. 5,527,521) for diagnostic imaging purposes and
gas-filled and
gaseous-precursor-filled liposome compositions, or methods for making or using
these
contrast agents (U.S. Patent No. 5,228,446, U.S. Patent No. 5,585,112, U.S.
Patent No.
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
5,769,080 and U.S. Patent No. 5,715,824)) for general and diagnostic
ultrasound imaging
purposes.
Unger, U.S. Patent No. 5,088,499, discloses the preparation of gas filled
liposomes and their use as ultrasound contrast agents. These include materials
that contain
gases, gaseous precursors, which can be activated by pH, temperature, or
pressure, and other
solid and liquid contrast agents.
In the case of the materials disclosed by Unger herein above, the liposomal
membrane of the encapsulated gas bubble is described as the well-known
unilamellar or
mufti-lamellar head-to-tail structure of amphiphilic lipid membranes such as
phospholipids
(see, Figure 1). As such, the Unger compositions are classical liposomes in
which the liquid-
filled interior is replaced by a gas.
Quay has disclosed methods of use of free gas microbubbles of low Q-factor
(low diffusivity) as ultrasound contrast agents (U.S. Patent No. 5,573,751 and
U.S. Patent No.
5,558,094). In these cases Quay discloses free gas microbubbles of various low
diffusivity
gases, without any disclosure of structure or composition of these
microbubbles.
Schneider, U.S. Patent No. 5,271,928, U.S. Patent No. 5,380,519 and U.S.
Patent No. 5,531,980, disclosed microbubble suspensions, which are hollow
spheres or
globules of finely divided gas and are stabilized by tensides or surfactants.
In the case of Schneider microbubble patents ('928, '519 and '980), the
ultrasound contrast agent is disclosed as being composed of microbubbles
devoid of a material
boundary layer around the gas microbubble. According to Schneider, these
microbubbles "are
only bounded by an evanescent envelope"(U.S. Patent No. 5,531,980, column 1).
The Schneider microbubble disclosures described above ('928, '519 and '980)
are directed to methods of making microbubble-based ultrasound contrast agents
without
reference to preferred composition/structure of the microbubbles themselves.
Schneider, U.S. Patent No. 5,413,774, discloses microvesicles having a
liposomal material boundary layer, which further contain within the vesicle a
low solubility
gas, as the microsphere-based ultrasound contrast agents. However, no
description of the
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
composition or structure of the microballoons is provided; rather, methods of
making a
contrast agent based on these microvesicles or microballoons is described
utilizing selected
low solubility gases.
The contrast agents described above are proposed for general ultrasound
contrast imaging of the vasculature and especially for heart imaging.
The imaging of specific organs, systems, or other areas of the body, would be
useful for diagnosing a variety of specific disease states. Examples of this
include the
specific imaging of tumors, blood clots, and areas of infection in a directed
manner. Quay, et
al., European Patent Application EP727225 illustrates the use of compositions
including a
cell adhesion molecule (CAM) ligand which is incorporated into a desired
molecule to form a
conjugate. The CAM is incorporated in a surfactant or albumin carrier and also
comprises a
chemical with sufficiently high vapor pressure to be a gas at body
temperature.
Unger (WO 96/40285) describes targeted gas-containing liposomes which can
be targeted to specific tissues in the body for diagnostic imaging or for
delivery of bioactive
agents. These targeted materials are comprised of a gas, lipid and targeting
ligand.
All of these materials include a suspension or emulsion of gas microspheres
(alternatively referred to as microbubbles) which are either: 1 ) free
microbubbles (i.e., do not
have a fixed material envelope at the microbubble surface) stabilized by
surfactants in
solution which cause a reduction in surface tension at the gas-liquid
interface, or 2) true
vesicles with a material boundary layer which stabilizes the gas microspheres
as a suspension
in the liquid medium. One of the practical difficulties with all of these
materials is that gas
microbubbles in the relevant, acoustically-active, size range of ~O.S~,m to
10~,m in diameter,
have a density different from that of the aqueous media in which they are
suspended.
Therefore, these microspheres have a natural tendency to rapidly separate out
(i.e., the
microbubble suspensions become heterogenous). This necessitates the rapid use
of the
contrast material after mixing before separation of the microspheres occurs.
In the case of gas microspheres used as platforms for drug delivery (see,
Unger
WO 96/40285 and Quay EP0727225), the materials incorporate the therapeutic
moiety at the
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
surface of the gas microsphere through chemical or physical absorption on the
boundary layer
of lipid or polymer. The practical difficulty with these materials is that
limited quantities of
the therapeutic agent may be absorbed or bound to the surface material
surrounding the gas
microsphere.
Allen et al. (U.S. Pat No. 5,620,689) disclose a method of treating a neoplasm
of B-cells or T-cells utilizing a liposome encapsulated chemotherapeutic agent
with a
biodirecting group on the surface of the liposome attached via a polyethylene
glycol coating
on the liposome. See et al., WO 99/65467, disclose a method of making drug
filled
liposomes of less than 200nm in diameter. These disclosures are representative
of a large
class of similar liposome drug delivery disclosures in the literature, all of
which comprise
liquid-filled liposomes alone without a gas microsphere component in the form
of the MSLC
compositions provided herein.
Notwithstanding the use of such contrast agents described above, the
ultrasound image produced, for example, of the myocardial tissue, can be of
relatively poor
quality, highly variable and not quantifiable. The overall diagnostic results
to date have been
somewhat disappointing. As such, the need still exists for improved agents
useful in
ultrasound imaging which will enhance the quality of ultrasound images by
improving the
contrast between the vascular spaces and tissues in a body. Such contrast
agents should have
excellent and stable acoustic response properties when in dilute aqueous
suspensions.
Additionally, the contrast agents should exhibit minimal microsphere flotation
and separation.
There has been, and continues to be, a need for ultrasound imaging agents
which enhance the quality and clarity of ultrasound images by improving the
delineation of
vascular space and tissues in the human body. In addition, improvements in the
control of
drug delivery to the sites of pathology are needed for many drugs which
exhibit high toxicity
to normal tissues and a resultant poor therapeutic index.
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
SUMMARY OF THE INVENTION
The present invention provides a formulation for contrast enhancement of
ultrasound imaging and for ultrasound (i.e., acoustically) stimulated drug
release. The
formulation provides stable gas microsphere (i.e., finely divided gas bubbles)
suspensions
with excellent and stable acoustic response properties when in dilute aqueous
suspensions.
The formulation can deliver a higher level of active drug per gas-filled
microsphere to a given
tissue, relative to known formulations, thereby achieving the intended
therapeutic benefit of
high local concentrations of drug or gene in the region of pathology. The
formulation has
good ultrasound scattering properties, which causes a selective increase in
the ultrasound
backscatter signal within the vascular space. The increase in the ultrasound
backscatter signal
within the vascular space improves the contrast relative to the surrounding
solid tissue.
Additionally, the formulation exhibits minimal microsphere flotation and
separation.
The present invention provides a formulation that includes a gas microsphere
liposome composite (MSLC) suspended in a medium. The gas microsphere liposome
composite includes a gas-filled microsphere; at least one of a lipid and a
surfactant adsorbed
onto the surface of the gas-filled microsphere; and liquid-filled liposomes
attached to the lipid
or surfactant.
The present invention also provides a method of ultrasound imaging in a
patient (e.g., mammal) in need of such ultrasound imaging. The method includes
administering to the patient (e.g., mammal) an effective amount of a
formulation of the
present invention; allowing a sufficient period of time for the circulation of
the gas-filled
microsphere composite to reach the targeted area; and performing ultrasound
imaging on the
patient (e.g., mammal).
The present invention also provides a method of treating heart disease,
inflammation, infection, cancer or thromboembolic disease in a patient (e.g.,
mammal) in
need of such treatment. The method includes administering to the patient
(e.g., mammal) an
effective amount of a formulation of the present invention, wherein one or
more of the liquid-
filled liposomes independently includes a therapeutic agent; allowing a
sufficient period of
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
time for the circulation of the gas microsphere composite to the targeted
area; and applying
ultrasound energy to the targeted area in the patient (e.g., mammal)
sufficient to cause the
therapeutic agent to be released from the microsphere liposome composite at
the region of
pathology.
The present invention also provides a method for preparing a formulation of
the present invention. The method includes contacting a suspension of
liposomes in a
aqueous solution including at least one of a surfactant and a lipid; and
mixing the suspension
with a gas that has a solubility of less than about 1.0% (v/v) in water at
25°C and 1 atm,
sufficient to provide the formulation.
The present invention also provides a method for preparing a formulation of
the present invention. The method includes contacting a suspension of
liposomes in a
aqueous solution including at least one therapeutic agent and at least one
surfactant or lipid;
and mixing the aqueous liposome suspension with a gas that has a solubility of
less than
about 1.0% (v/v) in water at 25°C and 1 atm, sufficient to provide the
formulation.
The present invention also provides a kit for the preparation of a formulation
of the present invention. The kit includes a container that includes an
aqueous solution,
wherein the aqueous solution includes at least one of a surfactant and a
lipid, and liquid-filled
liposomes; and a means for introducing a gas that has a solubility of less
than about 1.0%
(v/v) in water at 25°C and 1 atm into the aqueous solution.
The present invention also provides the use of a formulation of the present
invention for the manufacture of a medicament for treating heart disease,
inflammation,
infection, cancer or thromboembolic disease in a patient (e.g., mammal) in
need of such
treatment. The formulation includes a gas microsphere liposome composite
suspended in a
medium, wherein the gas microsphere liposome composite includes: a gas-filled
microsphere;
at least one of a lipid and a surfactant adsorbed onto the surface of the gas-
filled microsphere;
and liquid-filled liposomes attached to the lipid or surfactant.
The present invention also provides the use of a formulation of the present
invention for the manufacture of a medicament for ultrasound imaging in a
patient (e.g.,
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
mammal) in need of such ultrasound imaging. The formulation includes a gas
microsphere
liposome composite suspended in a medium, wherein the gas microsphere liposome
composite includes: a gas-filled microsphere; at least one of a lipid and a
surfactant adsorbed
onto the surface of the gas-filled microsphere; and liquid-filled liposomes
attached to the lipid
or surfactant.
The present invention also provides the use of a formulation of the present
invention for the manufacture of a medicament for diagnostic imaging in a
patient (e.g.,
mammal) in need of such diagnostic imaging. The formulation includes a gas
microsphere
liposome composite suspended in a medium, wherein the gas microsphere liposome
composite includes: a gas-filled microsphere; at least one of a lipid and a
surfactant adsorbed
onto the surface of the gas-filled microsphere; and liquid-filled liposomes
attached to the lipid
or surfactant.
BRIEF DESCRIPTION OF THE FIGURES
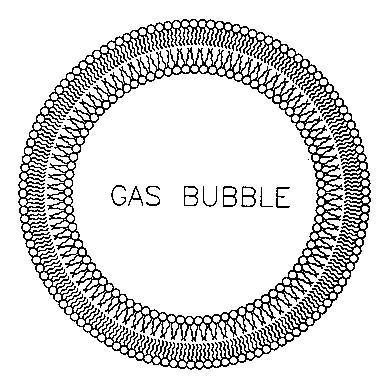
FIG. 1 illustrates a gas-filled liposome.
FIG. 2 illustrates a monolayer gas microsphere liposome composite (MSLC)
of the present invention.
FIG. 3 illustrates a multilayer gas microsphere liposome composite (MSLC) of
the present invention.
DETAILED DESCRIPTION OF THE INVENTION
Referring to Figures 2 and 3, the present invention provides a gas microsphere
liposome composite (MSLC) (1) dispersed in an aqueous medium (2). The gas
microsphere
liposome composite (MSLC) (1) includes of a gas-filled microsphere (3) of a
suitable inert
gas (4). A lipid (5) and/or surfactant (6) is adsorbed on the surface (12) of
the gas-filled
microsphere (3). Liquid-filled liposomes (LFLs) (7) are attached to the lipid
(5) and/or
surfactant (6). The LFLs (7) can include a therapeutic agent (8) or diagnostic
agent (9) in the
liquid interior (10) of the LFLs (7). In addition, a targeting moiety (11) can
be attached to the
surface (13) of the LFLs (7).
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
As used herein, "gas microsphere liposome composite (MSLC)" (1) refers to a
gas-filled microsphere (3) having at least one of a lipid (5) and a surfactant
(6) adsorbed onto
the outer surface (12) of the gas-filled microsphere (3) and also having
liquid-filled liposomes
(7) attached to the lipid (5) or surfactant (6).
As used herein, "surfactant" (6) refers to any material, ionic or non-ionic,
which produces a reduction in interfacial tension in a solution. The term
surfactant (6)
includes both amphiphilic molecules less than about 1,000 molecular weight and
polymers
which are capable of reducing interfacial tension between a gas-filled
microsphere (3) and the
surrounding aqueous medium (2).
As used herein, "liquid filled liposome (LFL)" (7) refers to liposomes that
contain a liquid interior (10) (i.e., a liquid in the internal volume). The
liquid filled liposomes
(7) can be unilamellar (14), bilameller (15), or multilamellar (16). The
liquid filled liposomes
(7) are typically attached to the adsorbed liquid or surfactant (6) in a
continuous fashion.
Each of the liquid filled liposomes (7) can independently contain a
therapeutic agent (8) or
diagnostic agent (9) in the liquid interior (10) of the liquid filled liposome
(7). Additionally,
each of the liquid filled liposomes (7) can independently contain a high
affinity, targeting
moiety (11) attached to the surface (13) of the liquid filled liposome (7).
As used herein, "continuous" or "contiguous", with respect to the liquid-
filled
liposomes (7) attached to the lipid (5) or surfactant (6) coated gas-filled
microsphere (3)
surface, refers to a significant portion (e.g., at least about 50%) of the
outer surface (12) of the
gas-filled microsphere (3) being covered with liquid-filled liposomes (7).
As used herein, "targeting moiety", refers to a biocompatible organic
molecule, biocompatible inorganic molecule, protein, peptide, peptidomimetic,
polysaccharide or other molecule having a high affinity for a receptor,
enzyme, mRNA or
DNA. The biocompatible organic molecule, biocompatible inorganic molecule,
protein,
peptide, peptidomimetic, polysaccharide or other molecule is altered in its
expression at a site
of pathology in-vivo relative to the surrounding normal tissue. Additionally,
this targeting
moiety is principally bound or attached to the surface of the liquid-filled
liposomes (7).
to
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
As used herein, "high affinity" refers to a binding affinity of less than
about
lam when expressed as the dissociation constant, Kd, for the interaction of a
single targeting
moiety and the biological target (e.g., receptor, enzyme, mRNA, or DNA).
As used herein, "patient" refers to one who is suffering from a given disease
or
disorder and is in need of treatment for the specified disease or disorder.
Suitable patients
include, e.g., animals. Suitable animals include, e.g., mammals. Suitable
mammals include,
e.g., humans.
As used herein, "treating" or "treatment" refers to the treatment of a disease
or
disorder in a patient and includes: (i) preventing the disease or disorder
from occurring in a
patient, in particular when such patient is predisposed to the disease or
disorder but has not
yet been diagnosed as having it; (ii) inhibiting the disease or disorder,
i.e., arresting its
development; and/or (iii) relieving the disease or disorder, i.e., causing the
regression of the
disease or disorder.
Gas-Filled Microsphere
As used herein, a "gas-filled microsphere" is a microbubble suspended in a
medium wherein the microbubble has a nominal spherical shape above about the
freezing
point of the medium and below about the boiling point of the medium and above
about 0 atm
pressure and below about 5 atm pressure (e.g., standard temperature and
pressure).
As illustrated in Figure 2 and Figure 3, the gas microsphere liposome
composite (1) (MSLC) includes a gas-filled microsphere (3). The gas-filled
microsphere (3)
is typically acoustically active. The gas-filled microsphere (3) typically has
a solubility of
less than about 1.0% (v/v) in water at 25 °C and 1 atm. Additionally,
the gas-filled
microsphere (3) typically has an average diameter of about 0.1 ~m to about
10~,m. Preferably,
the gas-filled microsphere (3) will have an average diameter of about O.S~m to
about lOp,m.
The gas-filled microsphere (3) will typically include one or more suitable
inert
gases (4). Suitable inert gases (4) of the present invention are well known in
the field of
ultrasound contrast agents. Suitable inert gases (4) useful in the present
invention are
11
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
disclosed, e.g., in Unger, et al., (U.S. Patent No. 5,547,656; U.S. Patent No.
5,527,521; U.S.
Patent No. 5,228,446; U.S. Patent No. 5,585,112; U.S. Patent No. 5,769,080;
and U.S. Patent
No. 5,715,824), Quay, et al., (U.S. Patent No. 5,573,751 and U.S. Patent No.
5,558,094) and
Schneider (U.S. Patent No. 5,271,928; U.S. Patent No. 5,380,519; and U.S.
Patent No.
5,531,980). These may include both gases and gaseous precursors (i.e., liquids
which
undergo a transition to the gas phase under reduced pressure or elevated
temperature).
Preferred inert gases (4) are of low solubility in blood, are non-reactive,
non-metabolizable
and/or are non-toxic in patients (e.g., mammals). Suitable inert gases (4)
useful in the present
invention include, e.g., perfluorocarbon gases (e.g. (CZ-C6)
perfluorocarbons), perfluoroether
gases, Nitrogen, and noble gases (e.g., Helium, Argon, and Neon).
Gas Micros~here Liposome Composite (MSLC)
The gas microsphere liposome composite (1) includes a gas-filled microsphere
(3); at least one of a lipid (5) and a surfactant (6) adsorbed onto the outer
surface (12) of the
gas-filled microsphere (3); and liquid-filled liposomes (7) attached to the
lipid (5) or
surfactant (6). The gas microsphere liposome composite (1) (MSLC) will
typically have a
mean diameter of about 0.1 ~,m to about 10 p,m. Preferably, the gas
microsphere liposome
composite (1) will have a mean diameter of about 0.2 pm to about 4 pm. The gas
microsphere
liposome composite (1) will typically have a density of about 0.90 to about
1.10 of the density
of the medium (2). The gas microsphere liposome composite (1) (MSLC) can exist
as an
aggregate of two or more gas microsphere liposome composites (1). The
aggregate will
typically have a diameter of about 1 p,m to about 100 ~,m.
Lipid and Surfactant
As illustrated in Figure 2 and Figure 3, the gas microsphere liposome
composite (MSLC) (1) includes at least one of a lipid (5) and surfactant (6)
adsorbed onto the
outer surface (12) of the gas-filled microsphere (3). The lipid (5) or
surfactant (6) can exist as
a mono-molecular layer, a bi-molecular layer, or a multi-molecular layer on
the outer surface
12
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
(12) of the gas-filled microsphere (3). The surfactant (6) rapidly adsorbs to
the outer surface
(12) of the gas-filled microspheres (3) and thereby reduces the surface
tension of the low
solubility inert gas (4) or gases. Additionally, the surfactant (6) acts as an
interface to which
the LFLs (7) may adhere.
The surfactant (6) can be any suitable non-ionic surfactant, cationic
surfactant,
or anionic surfactant. Suitable non-ionic surfactants include, e.g.,
polyethylene glycol,
polypropylene glycol, polyvinylpyrollidone, polyvinylalcohol, cellulose,
gelatin, xanthan
gum, pectin, and dextran. Suitable cationic surfactants include, e.g.,
tetraalkyl ammonium,
tetraalkyl phosphonium, or suitable salts thereof. Suitable cationic
surfactants include, e.g.,
tetrahexyl ammonium, tetradecyl ammonium, tetrabutyl ammonium, tetrahexyl
phosphonium,
tetradecyl phosphonium, tetrabutyl phosphonium, tetraphenyl phosphonium, and
suitable salts
thereof. Suitable anionic surfactants include, e.g., alkyl sulfonate, alkyl
carboxylate, and
suitable salts thereof. Suitable anionic surfactants include, e.g., dodecyl
sulfate, palmityl
sulfate, dodecyl carboxylate, palmityl carboxylate, and suitable salts
thereof.
Suitable lipids (5) include, e.g., phospholipids, glycolipids, triglycerides
and
fatty acids. Suitable phospholipids include, e.g., dipalmitoylphosphatidyl
choline chloride,
dimyristoylphosphatidyl choline, dilauryoylphosphatidyl choline, and
dioleoylphosphatidyl
choline.
Liquid-Filled Liposomes (LFLs)
As illustrated in Figure 2 and Figure 3, the gas microsphere liposome
composite (1) (MSLC) includes liquid-filled liposomes (7) (LFLs) attached to
the lipid (5) or
surfactant (6). The presence of liquid-filled liposomes (7) stabilizes the
surfactant-
encapsulated or lipid-encapsulated gas-filled microsphere (3). One or more of
the liquid-
filled liposomes (7) will typically include liquid from the medium of
suspension (2) (i.e.,
medium (2)). Preferably, each of the liquid-filled liposomes (7) will
typically include liquid
from the medium of suspension (2). The presence of liquid from the medium of
suspension
(2) in the liquid interior (10) (e.g., the interval volume) of the liquid-
filled liposomes (7)
13
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
provides for microsphere compositions that have densities which are close
(e.g., within about
20%) to that of the medium of suspension (2), thereby minimizing microsphere
flotation
and/or separation.
The LFLs (7) can contain one or more drugs (e.g., therapeutic agents (8)
and/or
diagnostic agents (9)) in the liquid-filled internal volume. Because the LFLs
(7) are attached
to the surfactant-coated or lipid-coated gas-filled microsphere (3), the LFLs
(7) can burst
upon ultrasound stimulation of the internal gas thereby releasing the one or
more drugs (e.g.,
therapeutic agents (8) andlor diagnostic agents (9)) in a diseased organ or
tissue. The liquid-
filled liposomes (7), however, have limited acoustic activity by themselves.
The LFLs (7) attach and stabilize the surfactant-encapsulated or lipid-
encapsulated gas-filled microsphere (3). This provides for MSLCs (1) which
have densities
that are close (e.g., within about 20%) to that of the medium of suspension
(2), thereby
minimizing gas microsphere flotation and/or separation. This also provides for
gas
microsphere suspensions that are relatively uniform in size distribution
(e.g., about l~,m to
about 5 Vim) over a reasonable period of time after preparation (e.g., up to
about 30 minutes).
The liquid-filled liposomes (7) typically occupy greater than about 50% of the
microsphere surface area. The liquid-filled liposomes (7) are also typically
attached to the
adsorbed lipid (5) or surfactant (6) in an essentially continuous fashion.
This orientation
provides outstanding buoyancy properties for the MSLCs (1), which provides
relatively stable
suspensions with excellent and reproducible acoustic response properties when
in dilute
aqueous suspensions.
The size of the LFLs (7) is relatively important. The liquid-filled liposomes
(7) should preferably have diameters that are less than about 10% of the
diameter of the gas-
filled microsphere (3) diameter. The range of greatest interest for most in
vivo ultrasound
imaging or drug delivery agents are MSLCs (1) that have an overall diameter
between about 1
pm and about S Vim, and are made from liquid-filled liposomes (7) of less than
100nm in
diameter. Larger liquid-filled liposomes (7) (e.g., greater than about 0.2 ~,m
in diameter)
create MSLCs (1) of overall diameter which exceed the diameter of the
capillary vessels in
14
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
the body. This would create a hazardous situation with respect to capillary
plugging, as well
as the consequent biological toxicity associated with blocking the
microcirculation of blood
to tissues. Therefore, it is highly preferred to utilize LFLs (7) of less than
about 100nm in
diameter to create MSLCs (1) of the proper dimensions for safe use in living
patients (e.g.,
mammals). Each of the liquid-filled liposomes (7) typically have a diameter of
about lOnm to
about 200nm. Preferably, each of the liquid-filled liposomes (7) will have a
diameter of
about 20nm to about 100nm. In addition, each of the liquid-filled liposomes
(7) will typically
have a diameter that is less than about 10% of the diameter of the gas-filled
microsphere (3).
As illustrated in Figure 2 and Figure 3, one or more of the LFLs (7) may
include one or more suitable drugs (e.g., therapeutic agents (9) and/or
diagnostic agents (9))
in the liquid-filled internal volume. Each of the liquid-filled liposomes (7)
may
independently include one or more drugs (e.g., therapeutic agents (8) and/or
diagnostic agents
(9)) in the liquid interior (10) of the liquid-filled liposomes (7). The LFLs
(7), when attached
to the surfactant-coated or lipid-coated gas-filled microsphere (3) surface,
can be burst upon
ultrasound stimulation and release the one or more therapeutic drugs (e.g.,
therapeutic agents
(8)) in a diseased organ or tissue in a localized and concentrated fashion.
High energy
ultrasound is generally capable of causing the gas-filled microsphere (3) to
expand and
contract rapidly, which eventually leads to gas bubble rupture. The ultrasound
energy
captured by the gas-filled microsphere (3) will cause the MSLC (1) to fragment
and rupture,
in turn, releasing the one or more drugs (e.g., therapeutic agents (8))
contained in the interior
of the LFLs (7) attached to the surface of the MSLC (1).
Suitable classes of therapeutic agents (8) include, e.g., anticoagulants,
thrombolytics, antineoplastic agents, and anti-inflammatory agents. Suitable
specific
therapeutic agents (8) are disclosed, e.g., in (PCT/US99/13682), and include,
e.g.,
doxorubicin, cyclophosphamide, adriamycin, methotrexate, gemcitabine,
navelbine, cisplatin,
tissue plasminogen activator, integrelin, roxifiban, methotrexate and enbrel.
In a preferred
embodiment of the present invention for ultrasound stimulated drug release,
the MSLCs (1)
include both high affinity targeting moieties (11) and therapeutic drugs
(e.g., therapeutic
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
agents (8)) in the solution of the LFLs (7), in order to maximize the
therapeutic index and the
quantity of drug delivered per gas microbubble.
Suitable classes of diagnostic agents (9) include, e.g., X-ray contrast agents
and MRI contrast agents. Suitable specific diagnostic agents (9) include,
e.g., non-ionic
iodinated X-ray contrast agents, ionic iodinated X-ray contrast agents,
gadolinium containing
MRI contrast agents, iron containing MRI contrast agents, and manganese
containing MRI
contrast agents.
The use of diagnostic agents (9) in the LFLs will allow both ultrasound image
enhancement (e.g., back scatter) and X-ray or MRI image enhancement to be
achieved with
one MSLC composition.
For targeted delivery of one or more drugs (e.g., therapeutic agents (8)
and/or
diagnostic agents (9)) to a selected pathological condition, the LFLs (7) of
the present
invention can be derivatized with a high affinity, targeting moiety (11) that
is covalently
linked or adsorbed onto the surface (13) of the LFLs (7). As such, the liquid-
filled liposomes
(7) may typically have one or more suitable high affinity, targeting moieties
(11) attached to
the surface (13) of the liquid-filled liposomes (7). This provides LFLs (7)
that are capable of
providing ultrasound contrast enhancement to sites of pathology in vivo. This
is
accomplished by providing ligands on the LFLs (7) that have high affinity for
receptors,
enzymes, mRNA, or DNA which are overexpressed or altered in dysfunctional
cells at sites of
diseases. Alternatively, these targeting moieties (11) attached to the LFLs
(7) can bind to
normal tissue receptors for the selective imaging of normal tissues, in
contrast to the absence
of acoustic enhancement of the adjacent diseased tissue which lacks the
receptor being
targeted by the LFLs (7). One or more of the LFLs (7) may include, in the
interior liquid
medium (10), one or more suitable diagnostic agent (9) from the medium of
suspension (2).
Suitable high affinity targeting moieties (11) which can be incorporated onto
the surface (13) of the LFLs (7) for directing the MSLC (1) to specific sites
of pathology have
been disclosed previously. See, e.g., Unger (PCT/L1S96/09938), Allen (U.S.
Patent No.
5,620,689) and Quay (EP 0727225), which provide many examples of the
biological targeting
16
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
moieties that can be incorporated into surfactant (6) or lipid (5) components
of directed
ultrasound imaging agents or drug delivery compositions. Among these are tumor
specific
antibodies, receptor-specific peptides and peptidomimetics such as cell
adhesion molecules
and the like.
Suitable specific targeting moieties (11) include, e.g., 1,2-dipalmitoyl-sn-
glycero-3-phosphoethanolamine-cyclo(Arg-Gly-Asp-D-Phe-Lys)-dodecanoate; DPPE-
PEG34oo-cyclo(Arg-Gly-Asp-D-Phe-Lys)-dodecanoate; 1-(1,2-Dipalmitoyl-sn-
glycero-3-
phosphoethanolamino)-a,c~dicarbonyl PEG3aoo-2-{ [7-(N-
hydroxycarbamoyl)(3S,6R,7S)-4-
aza-6-(2-methylpropyl)-11-oxa-5-oxobicyclo[ 10.2.2]hexadeca-1 ( 15),12( 16),13-
trim-3-
yl]carbonylamino}-N-(3-aminopropyl)acetamide; and 1-(1,2-Dipalmitoyl-sn-
glycero-3-
phosphoethanolamino)-a,c~dicarbonyl PEG3aoo-[7-(N-hydroxycarbamoyl)(3S,6R,7S)-
4-aza-
6-(2-methylpropyl)-11-oxa-5-oxobicyclo[ 10.2.2]hexadeca-1 ( 15),12( 16),13-
trim-3-yl]-N- { [4-
(aminomethyl)phenyl] methyl } carboxamide.
The liquid-filled liposomes (7) used in the present invention are well known
in
the art. Among the preferred materials for making liposomes for use in the
present invention
are phospholipids which can be cationic, anionic or zwitterionic in nature,
and may be used in
admixtures. Many sources exist on the composition and preparation of
liposomes. For
example, see New (R.R.C. New, editor, Liposomes, a practical approach , Oxford
University
Press, Oxford, UK, 1990), Tyrrell ("New Aspects of Liposomes", D. A. Tyrrell,
T. D. Heath,
C. M. Coney & B. E. Ryman, Biochimica & Biophysica Acta, 457 (1976), 259-302),
Schneider (US Patent No. 4,224,179), Woodle (MC Woodle and D. Papahadjopoulos,
Methods in Enzymology 171, 193, 1989). In particular, Papahajopoulos (US
Patent No
4,235,871) has described methods for forming LFLs including therapeutic
agents.
Control of MSLC Mean Size Distribution and Stability
The size and stability of the MSLCs can be controlled through several
parameters, e.g., concentration of the lipid in solution; the diameter of the
LFLs; the
17
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
molecular weight of the polymer surfactant, e.g., polyethylene glycol (PEG),
included in the
composition; and the concentration of the polymer used.
1. The concentration of the phospholipids in solution
Varying the lipid concentration will control the size distribution and
stability
of the liquid filled liposome and, this in turn, will adjust the size of the
MSLC which is
formed and stabilized in suspension. The mean size of the stabilized MSLCs in
suspension is
directly proportional to the concentration and size of the initial LFLs. Since
the number and
size of the LFLs is dependent on the amount of lipid (e.g., phospholipid)
available, the initial
lipid concentration will directly effect the number and size of the MSLC
distribution which is
stabilized in suspension.
2. The diameter of the LFL
Independent of the lipid (e.g., phospholipid) concentration, the size of the
MSLCs in suspension can be varied by changing the LFL size through physical
means. The
LFL size can be varied by methods such as extrusion or ultrasonication, which
are well-
known in the science of liposomes (see, e.g., R.R.C. New, editor, Liposomes,
a~ractical
~proach, Oxford University Press, Oxford, UK, 1990). As described previously,
the
variation of LFL size will result in varying MSLCs size distributions (i.e.,
smaller LFLs in the
size range of less than about 100nm will produce smaller MSLCs in the range of
less than
about 10~m).
3. The molecular weight of surfactant used in the composition
The molecular weight of polymeric surfactant (ionic or non-ionic) in the
preparation can be used to affect the mean diameter of the MSLCs formed. For
example, a
higher molecular weight of polyethylene glycol (PEG), either covalently bound
to other
components/lipids of the composition or added as free PEG in solution, can be
used to
stabilize larger sized gaseous microbubble-containing MSLCs once gas is
introduced into the
18
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
system. For example, by varying the molecular weight of the PEG from 500 to
10,000, the
MSLC diameter can be adjusted.
4. Concentration of the polymer
The size of the MSLC can be controlled by changing the concentration of the
polymer surfactant in the liposomal suspension. An increase in polymer
concentration in the
composition typically results in an increase in the mean size and/or
concentration of the
MSLCs in suspension.
Preparation of Gas Microsphere Liposome Composites
The gas microsphere liposome composites (MSLCs) described herein can be
prepared by mixing a gas of low aqueous solubility with an aqueous solution
containing a
surfactant and liquid-filled liposomes in suspension. This can be accomplished
by
mechanical mixing, ultrasonication or high velocity injection of the gas into
the liquid
containing the surfactant and LFLs.
To form the initial LFLs, phospholipids can be suspended in a bulk aqueous
solution, which can further include a surface active material, as well as non-
aqueous
components, such as glycerol or propylene glycol, or suspending aids such as
polysaccharides, proteins or synthetic polymers, provided such components are
parenterally
acceptable (i.e., non-toxic). Methods for preparing the LFLs used in the
current invention for
preparation of MSLCs have been described previously by Woodle (M.C. Woodle and
D.
Papahadjopoulos, Methods in Enzymolo~y 171, 193, 1989).
If biotargeting of the MSLC is desired, then the LFLs can have a high affinity
targeting moiety covalently bound or adsorbed to the surface of the liquid-
filled liposome.
The targeting moieties can be adsorbed to the surface of the MSLC or, more
preferably,
covalently attached to the LFL as a phospholipid ester or attached to a PEG
component of the
MSLC (see Allen U.S. Patent No. 5,620,689). In the case of MSLCs for
ultrasound
stimulated drug release, the LFLs can be prepared to include a therapeutic
agent in the interior
19
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
liquid volume of the liposomes by preparing the liposomes in a surfactant-
containing,
aqueous medium including the drug, followed by mixing or sonicating the medium
with a
suitable inert gas.
The control of the LFL diameter in the size range of less than about 100nm is
important for forming and stabilizing MSLCs of the desired size range (e.g.,
greater than
about 0.5 ~m and less than aboutl0 ~,m in diameter) for ultrasound imaging and
ultrasound-
stimulated drug release. Methods for controlling the size of LFLs have been
described in the
literature (see, e.g., R.R.C. New, editor, Liposomes, a practical approach,
Oxford University
Press, Oxford, UK, 1990, pp. 36-85). Microfluidization techniques for making
LFLs of the
desired size are particularly effective as described in Cook, et al., (U.S.
Patent No.
4,533,254).
Proof of Structure
To demonstrate the existence of this novel structure (termed gas microsphere
liposome composite (MSLC)), the liposome system described in Example 1 was
prepared and
analyzed using four techniques, Optical Microscopy, Transmission Electron
Microscopy,
Fluorescence Probing and Soft X-ray Microscopy. These techniques provide
information on
the macrostructure (greater than about 1 ~.m in size), microstructure ( 10 nm
to ~ 1000nm), and
the microenvironment of the chemical system (at the molecular level).
Optical Microscony
Optical Microscopy allows the determination of the size and shape of an object
in the micron range. Therefore, a MSLC composition with a diameter in the
range of about 1
to about 10 pm is visible using a 1000X microscope, and will have a magnified
size of about
1 to about 10 mm in diameter. Optical Microscopy was performed to show that
the MSLCs
are spherical in shape, and are present in the size range of about 1 to about
10 arm in diameter.
After preparation of the MSLC suspension (for example, as described in
Example 1), about 0.5 mL is slowly withdrawn from the vial using a syringe (B-
D 5 cc
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
syringe and precision guide 22 1/2 G needle; 0.70 mm x 40 mm). The sample was
placed on
a Hanging Drop slide ( 18 mm diameter; 0.5 mm deep) and covered with a cover
slide. Then
a drop of microscope oil was placed on the cover slide. The sample was
examined with an
Olympus BHA-P Microscope equipped with lOX eyepiece and Oil Immersion
Achromatic
100X Objective, which gave an overall magnification of 1000X. The resulting
picture
showed spherical objects that range in size from less than 1 ~.m to greater
than 10 ~.m. The
gas filled MSLCs of greater than approximately 2 ~,m in diameter appear to be
aggregates of
the smaller-sized primary MSLC units.
Electron Microscopy
The presence of the LFLs on the surface of the MSLCs was demonstrated
using transmission electron microscopy (TEM). Transmission electron microscopy
uses an
electron beam to illuminate a specimen. The electron beam is operated at high
vacuum, and
can magnify up to a 1,000,000X. Both the high vacuum and the electron beam can
be
damaging to the systems being studied. Therefore, in order for many samples to
be
examined, they must be thin, dry and usually contain a contrast stain.
One technique for examining liposome structures is negative staining.
Negative staining enhances the image of a structure by surrounding or
embedding the
specimen in an electron dense material. The sample is examined under TEM using
Phosphotungstic acid (PTA) as the stain, before mixing of the gas and aqueous
system
containing the surfactant-liposome mixture as well as after mixing to
demonstrate formation
of the MSLC.
For the surfactant-LFL system prior to mixing with the gas (the "unactivated"
sample), six drops of the preactivated system were added to 1 ml of 0.3% PTA
stain and
shaken gently. The mixture was left to stand for 5 minutes undisturbed, and
then one drop of
the mixture was applied to the grid plate. The grid was air dried on a piece
of filter paper for
30 minutes, before it was transferred to the grid carrying case for the TEM
study.
21
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
For the MSLC sample (after mixing with the gas) one drop was added to 1 mL
of 0.3% PTA stain and mixed gently. Then a drop of the solution was applied to
the grid.
The excess solution was removed by wicking and air-drying.
TEM pictures show that the composition, prior to mixing with a
perfluorocarbon gas, contains liposomes of about SOnm to about 100nm. The TEM
pictures
of the post-mixing MSLC suspension (after mixing with a perfluorocarbon gas)
show MSLCs
of about 300 nm to about 1000nm, which include a gas-filled microsphere void
with a lipid or
surfactant shell having liposome units of about SOnm to about 100nm along the
surface.
Fluorescence Analysis
Fluorescent probe experiments were used to study the general chemical
properties of a liposome system. A fluorescent probe is a fluorophore,
typically pyrene, that
localizes within a specific region of a liposome and responds to a photon of
energy by
producing a fluorescence emission. This emission can be used to determine the
microenvironment (micropolarity) and localized concentration of the
fluorophore in the
system.
For this experiment, pyrene was injected into vials of control medium
(solution without surfactant or liquid-filled liposomes), vials of surfactant
and liquid-filled
liposomes (prior to mixing with a perfluorocarbon gas) and into vials
containing MSLCs in
suspension (after high speed mixing of the composition with perfluoropropane)
to compare
the pyrene fluorescence spectra. The control medium that was used consisted of
a mixture of
80% sodium chloride solution (9% NaCI), 10% propylene glycol and 10% glycerol.
The
MSLC suspension was prepared as described in Example 1.
The results of the study showed that the microenvironmental polarity of pyrene
in the control medium was consistent with the pyrene being dissolved in a
purely aqueous
environment. The microenvironment polarity of the pyrene in the
surfactant/liquid-filled
liposome system (prior to gas mixing) was consistent with the pyrene being
dissolved in the
lipid membrane of the LFLs. Following high speed mechanical mixing of the
22
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
perfluorocarbon gas with the surfactant/liquid-filled liposome system the
local concentration
of the pyrene was shown to increase in a manner consistent with the presence
of a liposome
aggregate system as such as the MSLC structure observed in the TEM experiment.
Soft X-ray Microscony
Soft X-rays are X-rays with an energy of about 100 to about 1000 eV. These
energies are well matched to K shell absorption edges of low Z atoms like
carbon and oxygen,
or L shell edges of atoms like calcium. The wavelength of these X-rays is in
the 1 to 10 nm
range, whereas those of visible light are 350-700 nm. This makes very high
resolution
imaging possible. Soft X-ray microscopy provides high resolution while
avoiding sample
destruction; the X-rays have negligible effects on the sample.
MSLC suspensions were studied using this Soft X-ray microscopy technique.
The sample was prepared between two silicon nitride membranes. The membranes
have a
thickness of 100nm and a size of 3mm x 3mm in a 9mm x 9mm silicon frame of 200
microns
thickness. After mounting one membrane on each side of the wet cell, a syringe
was used to
put a very small droplet (less than about 5 pL, but not a defined volume) of
the MSLC
material on one of the membranes. For these experiments there is no dilution
or pretreatment
of the sample. Next, the two parts of the wet cell were placed together and
tightened with
screws. The layer thickness of the sample between the two membranes was
checked with a
visible light microscope. If the layer thickness was not appropriate the
screws were adjusted
to obtain the right thickness. A small droplet of water was placed into the
reservoir slot of the
wet cell to prevent evaporation of the sample. The reservoir slot was sealed
with a small
piece of tape and then the wet cell was mounted in the microscope.
The results from the Soft X-ray microscopy showed that the system after high
speed mixing with perfluoropropane gas contained MSLCs of about 300nm to about
SOOnm
having liquid-filled liposome units of about SOnm to about 100nm along
essentially the entire
boundary surface.
23
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
The MSLCs can be used as general purpose ultrasound contrast agents for
diagnostic ultrasound use. They can also be modified to contain biological
targeting moieties
bound or adsorbed to the liquid filled liposomes on the surface of the MSLC to
provide
selective localization of the MSLCs in the body. Biologically-targeted MSLCs
are useful for
targeted contrast ultrasound imaging of specific disease processes. In
addition, these
biologically-targeted MSLCs can be used for localized delivery of drugs, which
are
encapsulated within the liquid-filled liposomes and are released upon exposure
of the MSLCs
to ultrasound energy in vivo.
The formulation may be administered intravenously or intraperitoneally by
infusion or injection. Solutions of the formulation can be prepared in water,
optionally mixed
with a nontoxic surfactant. Dispersions can also be prepared in aqueous
solutions containing
glycerol, liquid polyethylene glycols, or other suitable parenteral diluents.
The pharmaceutical dosage forms suitable for injection or infusion can include
sterile aqueous solutions or dispersions or sterile powders comprising the
formulation which
are adapted for the extemporaneous preparation of sterile injectable or
infusible solutions or
dispersions, optionally encapsulated in liposomes. In all cases, the ultimate
dosage form
should be sterile, fluid and stable under the conditions of manufacture and
storage. The
liquid carrier or vehicle is a pharmaceutically acceptable diluent such as a
mixture of water,
ethanol, a polyol (for example, glycerol, propylene glycol, liquid
polyethylene glycols), and
the like. The proper fluidity can be maintained, for example, by the formation
of liposomes,
by the maintenance of the required particle size in the case of dispersions or
by the use of
surfactants. In many cases, it will be preferable to include isotonic agents,
for example,
sugars, buffers or sodium chloride. Prolonged suspension of the injectable
compositions can
be brought about by the use of agents such as gelatin, cellulose, polyvinyl
pyrollidone or
similar suspension aids.
Sterile injectable solutions are prepared by incorporating the required
ingredients enumerated above, followed by filter sterilization. When employing
sterile
powders for the preparation of sterile injectable solutions, the preferred
methods of
24
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
preparation are vacuum drying and the freeze drying techniques, which yield a
powder of the
active ingredients) plus any additional desired ingredient present in the
previously sterile-
filtered solutions.
The microsphere liposome composites (MSLCs) are injected into a patient or
human as a suspension containing approximately 103 to 109 microsphere liposome
composites in a principally aqueous medium. After allowing sufficient time for
the MSLCs
to circulate throughout the body, an ultrasound imaging machine (such as is
routinely used in
clinical practice) is used to image or (with higher energies or repeated
insonation pulses)
disrupt the MSLCs to release a therapeutic drug at the site of disease or in
an organ of
interest, e.g., the heart, or in tumors, or at sites of inflammation.
The ability of a formulation of the invention to act as a contrast imaging
agent
can be determined using pharmacological models which are well known in the
field. For
example, see Villanueva et al. (Villanueva, F.S., Glasheen, W.P., Sklenar, J.,
Kaul, S.
Circulation, 88, 596-604 (1993)).
The ability of a formulation of the invention to act as a therapeutic agent
can
be determined using pharmacological models which are well known in the field.
For
example, see Unger (PCT/LTS961/09938) (W096/40285).
The invention will now be illustrated by the following non-limiting Examples.
Preparation of General Purpose Diagnostic MSLC Contrast Agent
Example 1
A saline glycerol solution ( 100 ml) was prepared including glycerol ( 10 ml)
and NaCI (680 ~ 2 mg) in water (to a final volume of 100 ml). DPPC
(dipalmitoyl
phosphatidyl choline) (40.0 mg), MPEG500 DPPE (dipalmitoyl phosphatidyl
ethanolamine)
(30.0 mg), and DPPA (4.5 mg) were mixed with propylene glycol ( 10 ml) in a
100 ml
volumetric flask, which was placed in a hot water bath (70oC) and sonicated
for 15 minutes
until the solution cleared. The saline/glycerol solution was then added to
bring the mixture to
final volume of 100 ml, and the suspension was mixed well. The suspension (1.6
ml) was
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
transferred into a 2 ml borosilicate glass vial. The headspace was purged with
perfluoropropane gas, and the vial was stoppered and sealed. The stopper was
West Gray V
50 lyo 13 mm, 4416/50 elastomeric formulation. The seal was a flip off
aluminum seal. The
vial containing the lipid suspension was shaken for 45 seconds using the IONOS
Ionomix~.
After shaking, the suspension became milky white.
Preparation of Biologically-Targeted Diagnostic MSLC Material
Examples 2 and 3 describe the synthesis of ultrasound contrast agents of the
present invention comprising targeting moieties for tumor neovasculature that
are av[33
antagonists.
Example 2
Part A. Synthesis of 1 2-dipalmitoyl-sn-g~cero-3-phosphoethanolamine-cyclo(Ar -
~Gl~p-
D-Phe-Lvs)-Dodecanoate Coniu~ate
NH
H2N
HN
O
O O N H HN
O_ ~O-O O~N~ NH HH
R H O ~"~.,~ ~ O
O~ 10f ~ J9 N~COOH
O~ O
O
Disuccinimidyl dodecanoate (0.424 g, 1 mmol); 1,2-dipalmitoyl-sn-glycero-3-
phosphoethanolamine (DPPE) (1.489 g, 1 mmol); and cyclo(Arg-Gly-Asp-D-Phe-Lys)
TFA
salt (0.831 g, 1 mmol) (see U.S. Serial No. 09/281,474 for synthesis of this
cyclic peptide
targeting moiety, which method is herein incorporated by reference) are
dissolved in
chloroform (25 ml) while stirring (5 min). Sodium carbonate ( 1 mmol) and
sodium sulfate ( 1
26
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
mmol) are added and the solution is stirred at room temperature under nitrogen
(18 h).
Chloroform is removed in vacuo and the title compound is purified from the
crude product
mixture by preparative HPLC or recrystallization.
Part B. Preparation of Contrast Agent Composition
The 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-cyclo(Arg-Gly-Asp-
D-Phe-Lys)-dodecanoate conjugate is mixed with three other lipids--1,2-
dipalmitoyl-sn-
glycero-3-phosphotidic acid; 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine;
and N-
(methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-
phosphatidylethanolamine--in relative amounts of 2 : 4 : 54 : 40 by weight. An
aqueous
suspension containing this lipid mixture (1 mg/mL), sodium chloride (7 mg/mL),
glycerin
(0.1 mL/mL), and propylene glycol (0.1 mL/mL), at pH 6-7, is then prepared in
a 2 cc glass
vial. The air in the vial is evacuated and replaced with perfluoropropane, and
the vial is
sealed. The suspension is agitated in the sealed vial in a dental amalgamator
for 30-45 sec. to
form a milky white solution, which is suitable for use as an ultrasound
contrast agent for
imaging angiogenic vessels.
Example 3
Part A. Preparation of w-amino-PEG34oo-cyclo(Ar -g-Gly-Asp-D-Phe-Lys):
NH
H2N
HN
O
O NH HN
O
O HN
NH
H NCO N ~ N~COOH
~~H O _ O
~ Ph
27
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
Triethylamine (3 mmol) is added to a solution of N-Boc-PEG34pp-sucinimidyl
ester ( 1 mmol) and cyclo(Arg-Gly-Asp-D-Phe-Lys) ( 1 mmol) in
dimethylformamide (DMF)
(25 mL). The reaction mixture is stirred under nitrogen at room temperature
overnight, and
the solvent is removed in vacuo. The crude product is dissolved in
trifluoroacetic
acid/dichloromethane (1:1 vol/vol) and stirred for 4 h. The volatiles are
removed and the title
compound is isolated as the TFA salt via trituration in diethyl ether.
Part B. Preparation of DPPE-PEG34pp-cyclo(Arg Gly-Asp-D-Phe-Lys)-Dodecanoate
Conjugate:
NH
HpN
HN
/ \O
O NH HN
14 O
O H O O NH HN
O O'I~O~N~N~O O N O~N~COOH
OH O 9 H ~ ~~H _ O
O ~ Ph
~O
Disuccinimidyl dodecanoate (1 mmol), 1,2-dipalmitoyl-sn-glycero-3-
phosphoethanolamine (DPPE) ( 1 mmol), and c~-amino-PEG34oo-cyclo(Arg-Gly-Asp-D-
Phe-
Lys) TFA salt (1 mmol) are dissolved in chloroform (25 ml) while stirring for
5 min. Sodium
carbonate (1 mmol) and sodium sulfate (1 mmol) are added and the solution is
stirred at room
temperature under nitrogen for 18 h. DMF is removed in vacuo and the title
compound is
purified from the crude product mixture by either preparative HPLC or
recrystallization.
Part C. Preparation of the Contrast Agent Composition:
The DPPE-PEG34oo-cYclo(Arg-Gly-Asp-D-Phe-Lys)-Dodecanoate conjugate
is mixed with three other lipids--1,2-dipalmitoyl-sn-glycero-3-phosphotidic
acid; 1,2-
dipalmitoyl-sn-glycero-3-phosphatidylcholine; and N-(methoxypolyethylene
glycol 5000
carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine--in relative
amounts of 1
28
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
6 : 54 : 41 by weight. An aqueous suspension, containing this lipid mixture (1
mg/mL),
sodium chloride (7 mg/mL), glycerin (0.1 mL/mL), and propylene glycol (0.1
mL/mL), at pH
6-7, is then prepared in a 2 cc glass vial. The air in the vial is evacuated
and replaced with
perfluoropropane, and the vial is sealed. The suspension is agitated in the
sealed vial in a
dental amalgamator for 30-45 sec. to form a milky white suspension, which is
suitable for use
as an ultrasound contrast agent.
The following examples, Examples 4 and 5, describe the synthesis of
ultrasound contrast agents of the present invention comprised of targeting
moieties for matrix
metalloproteinase inhibitors. These materials are useful for targeting the
MSLCs to the sites
of extracellular matrix degradation, which are present in tumors,
atherosclerotic plaques, and
cardiac tissue degeneration in CHF (Congestive Heart Failure). These
compositions are
useful for localizing the acoustically active MSLCs to sites of disease for
the selective
ultrasound imaging of these pathologies. Alternatively, as described in
Examples 8 and 9,
compositions may be prepared with therapeutic agents in the interior of the
LFLs attached to
the MSLCs, which are useful for ultrasound stimulated drug release at a
specific site of
disease.
Example 4
Svnthesis of 1-(1,2-Dipalmitovl-sn-~lvcero-3-phosphoethanolamino)-a,~
dicarbonylPEG3400-2-1 f7-(N-hydrox~arbamoyl)(3S,6R,7S)-4-aza-6-(2-meth~propyl)-
11-
oxa-5-oxobicvclof 10.2.2~hexadeca-1 ( 1 S),12( 16),13-trim-3-yllcarbonylamino
1-N-(3-
aminopropyl)acetamide conygatete:
o , o
O 15
O H O OII/ ~ O~~ OH
HOHN O H O N~H~H~O~H~O.P~O O
-77 O O
29
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
To a solution of succinimidyl ester DSPE-PEG-NHS ester (Shearwater
Polymers, Huntsville, Alabama) (1 mmol) in chloroform (25 ml) is added 2-{ [7-
(N-
hydroxycarbamoyl) (3S,6R,7S)-4-aza-6-(2-methylpropyl)-11-oxa-5-
oxobicyclo[ 10.2.2]hexadeca-1 ( 15),12( 16),13-trim-3-yl]carbonylamino }-N-(3-
aminopropyl)acetamide TFA salt (1 mmol) (see U.S. Serial No. 60/182,627 for
synthesis of
this targeting moiety). Sodium carbonate ( 1 mmol) and sodium sulfate ( 1
mmol) are added
and the solution stirred at room temperature under nitrogen for 18 h. The
solvent is removed
in vacuo and the title compound is purified from the crude product mixture by
preparative
HPLC.
Preparation of Contrast Agent Composition:
The 1-(1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamino)-a,c~
dicarbonylPEG3400-2-{ [7-(N-hydroxycarbamoyl)(3S,6R,7S)-4-aza-6-(2-
methylpropyl)-11-
oxa-5-oxobicyclo[ 10.2.2]hexadeca-1 ( 15),12( 16),13-trim-3-yl]carbonylamino }-
N-(3-
aminopropyl)acetamide conjugate is mixed with three other phospholipids--1,2-
dipalmitoyl-
sn-glycero-3-phosphotidic acid; 1,2-dipalmitoyl-sn-glycero-3-
phosphatidylcholine; and N-
(methoxypolyethylene glycol 5000 carbamoyl)-1,2-dipalmitoyl-sn-glycero-3-
phosphatidylethanolamine--in a ratio of 1 : 6 : 54 : 41 by weight. An aqueous
suspension,
containing this lipid mixture ( 1 mg/mL), sodium chloride (7 mg/mL), glycerin
(0.1 mL/mL),
and propylene glycol (0.1 mL/mL), at pH 6-7, is prepared in a 2 cc glass vial.
The air in the
vial is evacuated and replaced with perfluorobutane, and the vial is sealed.
The suspension is
agitated in the sealed vial in a dental amalgamator for 30-45 sec to form a
milky white
suspension of the MSLCs targeted to matrix metalloproteinases. The suspension
is suitable
for use as an ultrasound contrast agent.
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
Example 5
~nthesis of 1-( 1 2-Dipalmitoyl-sn-glycero-3-phosphoethanolamino)-a,c~
dicarbonylPEG3400-~7-(N-hxdroxycarbam~l)(3S 6R 7S)-4-aza-6-(2-methylpropyl)-11-
oxa-
5-oxobicyclof 10.2.21hexadeca-1(15).12(16),13-trim-3-yll-N-( f4-
(aminometh~phenyllmeth~lcarboxamide conjugate:
p ~ 'O 15
O O OH
O ~ N~O~N~O.P~O O
HOHN - N N ~ I H -77 H O O \
To a solution of succinimidyl ester DSPE-PEG-NHS ester (Shearwater
Polymers, Huntsville, Alabama) ( 1 mmol) in chloroform (25 ml), is added [7-(N-
hydroxycarbamoyl)(3S,6R,7S)-4-aza-6-(2-methylpropyl)-11-oxa-5-
oxobicyclo[ 10.2.2]hexadeca-1 ( 15),12( 16),13-trim-3-yl]-N-{ [4-
(aminomethyl)phenyl]methyl}carboxamide TFA salt (1 mmol) (see U.S. Serial No.
60/182,627 for the synthesis of this MMP targeting moiety). Sodium carbonate
(1 mmol) and
sodium sulfate (1 mmol) are added and the solution is stirred at room
temperature under
nitrogen for 18 h. The solvent is removed in vacuo and the title compound is
purified from
the crude product mixture by preparative HPLC.
Preparation of Contrast Agent Composition:
1-( 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamino)-a,c~
dicarbonylPEG3400-[7-(N-hydroxycarbamoyl)(3S,6R,7S)-4-aza-6-(2-methylpropyl)-
11-oxa-
5-oxobicyclo [ 10.2.2] hexadeca-1 ( 15),12( 16),13-trim-3-yl]-N- { [4-
(aminomethyl)phenyl]methyl}carboxamide conjugate is mixed with three other
phospholipids--1,2-dipalmitoyl-sn-glycero-3-phosphotidic acid; 1,2-dipalmitoyl-
sn-glycero-3-
phosphatidylcholine; and N-(methoxypolyethylene glycol 5000 carbamoyl)-1,2-
dipalmitoyl-
sn-glycero-3-phosphatidylethanolamine--in relative amounts of 1 : 6 : 54 : 41
by weight. An
31
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
aqueous suspension ( 1.6 ml), containing this lipid mixture ( 1 mg/mL), sodium
chloride
(7 mg/mL), glycerin (0.1 mL/mL), and propylene glycol (0.1 mL/mL), at pH 6-7,
is prepared
in a 2 cc glass vial. The air in the vial is evacuated and replaced with
perfluorobutane, and
the vial is sealed. The suspension is agitated in the sealed vial in a dental
amalgamator for
30-45 sec to form a milky white suspension of the MSLCs targeted to matrix
metalloproteinases. The suspension is suitable for use as an ultrasound
contrast agent.
Preparation of Biologically-Targeted Therapeutic MSLC Materials
Example 6
To the phospholipid contrast agent composition in Example 3 is added
doxorubicin ( 100-200 mg/ml). One to two milliliters is transferred to a vial.
The air in the
vial is evacuated and replaced with perfluorobutane, and the vial is sealed.
The vial is
agitated in a dental amalgamator for 30-45 sec to form a milky white MSLC
suspension for
therapeutic use.
Example 7
To the phospholipid contrast agent composition in Example 4 is added
cyclophosphamide (100-200 mg/ml). One to two milliliters is transferred to a
vial. The air in
the vial is evacuated and replaced with perfluorobutane, and the vial is
sealed. The vial is
agitated in a dental amalgamator for 30-45 sec to form a milky white MSLC
suspension for
therapeutic use.
Example 8
To the phospholipid contrast agent composition in Example 5 is added
cyclophosphamide (100-200 mg/ml). One to two milliliters is transferred to a
vial. The air in
32
CA 02456988 2004-02-12
WO 03/015831 PCT/USO1/25685
the vial is evacuated and replaced with perfluorobutane and the vial is
sealed. The vial is
agitated in a dental amalgamator for 30-45 sec to form a milky white MSLC
suspension for
ultrasonically-activated therapeutic use.
Example 9
To the phospholipid contrast agent composition in Example 5 is added tissue
plasminogen activator (10-100 mg/ml). One to two milliliters is transferred to
a vial. The air
in the vial is evacuated and replaced with perfluorobutane and the vial is
sealed. The vial is
agitated in a dental amalgamator for 30-45 sec to form a milky white MSLC
suspension for
therapeutic use.
Example 10
Following injection into a living patient (e.g., mammal) and allowing
sufficient time for the targeted MSLCs to localize at or near the site of
disease, the diagnostic
ultrasound scan may be acquired, or, in the case of therapeutic agent
delivery, ultrasound
energy of sufficient energy to disrupt the MSLCs and release the drug at the
targeted site may
be applied by either repeated pulsation or by application of very high power
single pulses of
ultrasound energy.
All publications, patents, and patent documents are incorporated by reference
herein, as though individually incorporated by reference. The invention has
been described
with reference to various specific and preferred embodiments and techniques.
However, it
should be understood that many variations and modifications may be made while
remaining
within the spirit and scope of the invention.
33