Note: Descriptions are shown in the official language in which they were submitted.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
A cartridge for liquid insulin
The technical field of the invention:
The invention relates to ampoules or cartridges for insulin delivery systems.
Such cartridges
are commonly shaped as a glass tube being at one end closed by a piston, which
may be
pressed into the tube to expel the content of the tube at the other end of the
tube. This other
end is formed as a bottleneck, the outer end of which may be pierced by an
injection needle
or a catheter through which the content is expelled.
Description of related art:
Glass cartridges as described in the preamble of claim 1 are widely known for
various me-
dicament delivery systems. They are especially used for insulin delivery
systems, and are
usually supplied pre-filled with either 1,5 ml of insulin or 3,0 ml of
insulin. A 1,5 ml cartridge
usually has an inside diameter around 6,85 mm and a 3,0 ml cartridge usually
has an inside
diameter around 9,25 mm. These known cartridges are pre-filled with insulin
having a con-
centration on 100 International Units (1U) pr. ml. A 1,5 ml cartridge
therefore contains 150 IU
and a 3,0 ml cartridge contains 300 IU.
The typical diabetes patient will require a certain amount of insulin either
injected or infused
into their body every day. Some patients need as much as 100 IU per day, in
which case the
3,0 ml cartridge is recommended. The patient loads the cartridge into either
an injection sys-
tem or a pump system and injects or infuses the insulin into their body at a
prescribed rate,
either through an injection needle or through a catheter inserted into their
body. Once the
cartridge is empty it is disposed of and a new cartridge is loaded into the
delivery system.
Glass is the most preferred material for cartridges containing insulin, since
glass are both
chemically and biologically inert so that insulin can be stored within the
glass cartridge with-
out reactions occurring between the liquid insulin and the glass material.
Glass has the addi-
tional advantage that it can be thermally sterilized. Glass cartridges are
produced from long
glass tubes, which are cut up into smaller tubes, one end of which is melted
so that a small
opening remains. The opposite open end of the tubes is provided with a movable
piston,
which are usually manufactured from rubber or plastic.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
2
Cartridges made from glass however has the disadvantage that the inside
diameter is vari-
able due to the manufacturing process. The inside diameter of glass
cartridges, varies by 0,1
mm at an average inside diameter of about 10 mm. However, finer tolerances are
available
by pre sorting the glass cartridges or by tightened monitoring of the glass
process. The fol-
lowing table shows the tolerances typically available today:
Inside diameter7 7,5 8 9,25 10 11 12
Tolerances 0,05 0,05 0,06 0,06 0,08 0,09 0,1
+/-
These tolerances of the cartridge are a major problem for the dose accuracy of
the injected
or infused insulin. The dose accuracy of an insulin delivery system is the
subject of the ISO
standard 11608-1. This standard prescribes that a dose in the range 0 to 20 IU
most have an
accuracy of +/- 1 IU, i.e. a nominal dose of 20 IU most contain between 19 to
21 IU. The ISO
standard allows a tolerance of doses smaller than 20 IU to be within +/- 1 IU,
while the toler-
ances of a dose exceeding 20 IU most be within +/- 5 %. The most difficult
part of the stan-
dard to meet is therefore typically the demand for accuracy on +/- 1 IU for a
dose of 20 IU.
A large diameter combined with a large tolerance provides large variations in
the cross sec-
tion area of the glass cartridge, which will result in large tolerances in the
volume delivered
by the insulin delivery system. This is however not a major problem when the
insulin has a
concentration on only 100 IU per ml.
Description of the invention:
It is a constant aim for manufactures of insulin delivery systems to minimise
their systems. A
delivery system however always has to include a cartridge. Manufactures
therefore have a
great demand for smaller and more compact cartridges. Never the less no one
wants to
compromise the number of International Units contained in the cartridge. One
way of solving
this Gordian knot is by increasing the concentration of the insulin contained
in the cartridge.
By increasing the concentration up to 200 IU pr ml, a 1,5 ml cartridge is able
of containing
300 I U.
With the before mentioned large variations in the cross section area of the
glass cartridges it
will apparently be increasingly difficult to meet the ISO 11608-1 standard
when using a liquid
U200 insulin.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
3
In the table in figure 1, the displacement accuracy is calculated for
different dimensions of
glass cartridges. The two first columns show the different dimensions and
tolerances of the
inner diameter of various cartridges. The nominal and maximal cross section
areas of the
cartridges are calculated in column 3 and 4. Column 5 recites the insulin
concentration.
The distance the front wall of the piston most be moved forward inside the
cartridge in order
to expel 1 IU of insulin is calculated in column 6. This movement is
calculated on basis of the
nominal diameter using the following formula:
V
H -_ ______
~ . R2
V : Volume
R : Radius
H : Displacement
One IU of liquid U200 insulin has the volume of 0,005 ml, the front wall of
the piston in e.g. a
cartridge having a nominal inside diameter of 9,25 mm most therefore be
displaced by 0,074
mm in order to expel one IU.
Column 7 indicates how much the tolerances in the cross section area influence
the dose
accuracy. It can be seen that for a cartridge with a nominal diameter of 9,25
mm, +/- 0,260 IU
of the tolerance on +/- 1 IU given in the ISO standard are consumed by the
tolerance of the
cross section area of the cartridge. The tolerance is calculated by extracting
the minimum
cross section area from the maximum cross section area shown in column 4, and
multiplying
this difference with the one unit displacement shown in column 6 and with the
insulin concen-
tration. The remaining +/- 0,740 IU shown in column 8 are then available for
the imprecision
of the insulin delivery system, including the slack of interface to the
cartridge.
The remaining part of the tolerance listed in column 8, is in column 9
expressed in millime-
ters by multiplying the displacement needed for expelling 1 IU with the
remaining tolerance
available for the imprecision of the insulin delivery system.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
4
Once again reciting the numerals for a cartridge having a nominal inside
diameter of 9,25
mm, the front wall of the piston most be moved a distance equal to 20 times
0,074 mm i.e.
1,488 mm +/- 0,055 mm (from 1,433 to 1,543 mm) in order to deliver a dose of
20 IU within a
tolerance of +/- 1 IU.
The volume expelled within these tolerances can be calculated using the
following formula:
V(IU) = CS ~ D ~ IC
V(IU) : Volume expressed in IU
CS : Cross section area of the cartridge (_ ~~ RZ)
D : Displacement of piston
IC : Insulin concentration
The following table show the expelled volume measured in International Units
for a cartridge
having a nominal diameter of 9,25 mm and tolerances of +/- 0,06 mm, i.e. an
inside diameter
between 9,19 mm and 9,31 mm, equaling a cross section area of the volumen of
the car-
tridge between 66,33 mm2 and 68,08 mm2, when the cartridge is used in an
insulin delivery
system which can move the piston forward with a tolerance of +/- 0,055 mm. The
forward
movement hence being in the range 1,433 mm to 1,543 mm.
1,433 mm 1,488 mm 1,543 mm
66,33 mm2 19,01 IU 19,74 IU 20,47 IU
67,20 mm2 19,26 IU 19,99 IU 20,74 IU
68,08 mm2 19,51 IU 20,26 IU 21,00 IU
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
The mechanical insulin delivery systems available today all have quit a large
imprecision due
to the mechanical system. The leading injection devices has a displacement
accuracy
around +/- 0,083, meaning that the front wall of the piston can only be moved
forward within
tolerances of approximately +/- 0,083 mm. When using U200 insulin the maximum
allowable
inner diameter of the cartridge must, according to the table shown in figure
1, be smaller than
a nominal 7,5 mm cartridge in order to meet the demands set up in the ISO
11608-1 stan-
dard. Cartridges having an inside diameter larger than 7,5 mm all needs a
displacement ac-
curacy smaller than +/- 0,083 mm in order to meet the ISO standard. They are
therefore not
suitable for use in ordinary insulin pen systems.
Insulin pumps for pump treatment of diabetes usually have a more precise
mechanism than
mechanical injection devices due to the presence of a motor mechanism, which
is also the
case for motor driven injection devices. It is however not necessary with a
precise mecha-
nism in insulin pumps due to the presence of a continuous insulin delivery
profile.
In recent years mechanical precision injection devices has been developed
which has a
higher degree of accuracy than the known injection devices. In fact these new
injection de-
vices are able of moving the piston forward within tolerances of approximately
+/- 0,055 mm.
It has therefore shown that cartridges containing liquid 0200 insulin and
having a diameter
from the lower tolerance limit of a nominal 7,5 mm cartridge i.e. 7,45 mm to
the upper toler-
ance limit of a nominal 9,25 mm cartridge i.e. 9,31 can be used both in pumps
and in preci-
sion injection devices having tolerances within +l- 0,055 mm to +/- 0,083 mm,
without dis-
pensing from the requirements of ISO 11608-1.
These requirements are fulfilled with a cartridge according to claim 1.
A glass cartridge having an inside diameter between 7,45 and 9,31 mm will,
when filled with
a liquid 0200 insulin, be able to fulfil the ISO 11608-1 standard when the
cartridge is being
used in a precision insulin delivery system having a displacement accuracy of
the mecha-
nism advancing the piston within the range from 0,055 mm to 0,083 mm.
Glass cartridges with an inside diameter in the claimed range can therefore be
used both in
insulin pump systems and in insulin injection systems.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
6
When operating in the lower range of the inside diameters specified in claim 1
it is ensured
that, such as disclosed in claim 2, the cartridge can be made very slim and
with very narrow
tolerances, leaving maximum tolerances for the imprecision of the insulin
delivery system.
A glass cartridge having a nominal inside diameter of 7,5 mm needs a length of
the stroke
zone of approximately 34 mm in order to contain a volume of approximately 1,5
ml. When
containing 1,5 ml of a liquid U200 insulin, the total amount of International
Unit is 300 IU,
which for most patient will be sufficient for three days of treatment. When
using the cartridge
in a pump system, the pump is usually connected to the body of the user
through a catheter.
Due to inflammation of the skin at the site where the catheter is inserted, it
is normally rec-
ommended to change the catheter and the site approximately every third day. A
cartridge
containing insulin for minimum three days are therefore to be preferred.
Due to the size of the connecting zone and the piston zone, the overall length
of such a car-
tridge will be approximately 52 mm.
When operating in the upper range of the inside diameters specified in claim 1
it is ensured
that, such as disclosed in claim 5, the cartridge can be made very short and
still leave suffi-
cient tolerances for the imprecision of the insulin delivery system. A glass
cartridge having a
nominal inside diameter of 9,25 mm needs a length of the stroke zone of
approximately 23
mm in order to contain a volume of approximately 1,5 ml, resulting in an
overall length of ap-
proximately 44 mm.
Brief Description of the Drawings:
The invention will be explained more fully below in connection with a
preferred embodiment
and with reference to the drawings in which:
Figure 1 Shows a table of the displacement accuracy for different cartridge
designs
Figure 2 Shows a glass cartridge including needle penetration according to
the invention.
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
7
The figures are schematic and simplified for clarity, and they just show
details, which are es-
sential to the understanding of the invention, while other details are left
out. Throughout, the
same reference numerals are used for identical or corresponding parts.
Detailed Description of Embodiment:
A table of the displacement accuracy for different cartridge designs is shown
in figure 1.
Glass cartridges containing a liquid U200 insulin and having a nominal
diameter between 7,5
mm and 9,25 mm leaves room for a displacement accuracy between +/- 0,083 and
+/- 0,055
mm, which is needed if the cartridge shall be used both for injection devices
and for pump
systems and fulfil the ISO 11608-1 standard. It can also be seen from the
table in figure 1
that the displacement accuracy of an insulin delivery system using a cartridge
having a
nominal diameter of 9,25 mm must be 0,084 mm when the cartridge contains a
liquid U100
insulin.
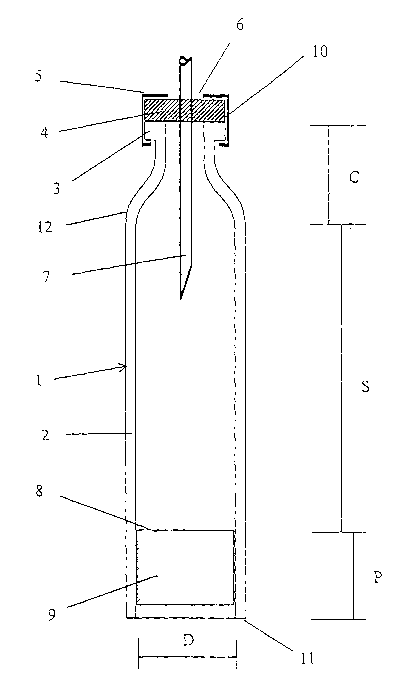
Referring to figure 2 it may be convenient to define that, the term "distal
end" of the cartridge
1 is meant to refer to the end carrying the conduit 7 through which the
insulin is expelled,
whereas the term "proximal end" is meant to refer to the opposite end carrying
the piston 9.
A cartridge 1 comprising a cylindrical wall 2 is disclosed in figure 2. The
cylindrical wall 2 is at
the distal end 10 of the cartridge terminated in a neck part ending in a
circumferential flange
3 against which a piercable and flexible membrane 4 is held sealingly by a
metal cap 5. At a
central part of the membrane 4 the metal cap 5 has an opening 6 through which
the mem
brane 4 is exposed. A hollow conduit 7, such as an injection needle or a
catheter can be
stuck through the membrane 4 to communicate with the inner space of the
cartridge 1 in
which the liquid insulin is stored between the membrane 4 and a front wall 8
of a piston 9
which fits into the cartridge 1.
The piston 9 is usually made from a suitable rubber material, such that it is
tightly sealed
against the inside of the cylindrical wall 2. The inside diameter of the glass
cartridge is indi-
Gated with D in figure 1.
The cartridge 1 is divided into three different zones. The first zone is the
connecting zone C,
which extends from the distal end 10 of the cartridge 1 to the shoulder 12.
Due to the re-
duced diameter of the cylindrical wall 2 of the cartridge 1 on the part of the
cylindrical wall 2
CA 02457943 2004-02-18
WO 03/017914 PCT/DK02/00553
8
lying between the distal end 10 of the cartridge 1 and the shoulder 12, the
piston 9 cannot be
moved beyond the shoulder 12 and into the neck part area of the cartridge 1.
The insulin
contained in the neck part of the cartridge 1 can therefore not be pressed out
of the cartridge,
and will hence be disposed of when the cartridge 1 is discarded.
The second zone is the stroke zone S, which extends from the shoulder 12 to
the front wall 8
of the piston 9. Only the insulin contained in the stroke zone can be utilized
for injection or
infusion.
The third zone is the piston zone P, which extends from the proximal end 11 of
the cartridge
1 to the front wall 8 of the piston 9. This piston zone P holds the piston 9
and is therefore not
available for the insulin contained in the cartridge 1.
The liquid insulin captured between the front wall 8 of the piston 9 and the
flexible membrane
4 and within the inside diameter D of the cylindrical wall 2 will be pressed
out through the hol-
low conduit 7, which at the not shown other end is inserted into the person in
need for insulin,
when the piston 9 is moved forward inside the cartridge 1.
Some preferred embodiments have been shown in the foregoing, but it should be
stressed
that the invention is not limited to these, but may be embodied in other ways
within the sub-
ject matter defined in the following claims.