Note: Descriptions are shown in the official language in which they were submitted.
CA 02459012 2004-02-26
CRYSTALLINE FORM Z OF RABEPRAZOLE SODIUM AND PROCESS FOR
PREPARATION THEREOF
CROSS-REFERENCE TO RELATED APPLICATION
This application claims priority of Indian Patent Application No. 156/MAS/2003
filed on February 28, 2003, the disclosure of which is incorporated herein by
reference in
its entirety.
BACKGROUND OF THE INVENTION
Rabeprazole sodium, 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-
methyl]sulfonyl]-lH-benzi.midazole sodium is an inhibitor of the gastric
proton pump. It
belongs to a class of antisecretory compounds that do not exhibit
anticholinergic or
histamine HZ-receptor antagonist properties, but suppress gastric acid
secretion by
inhibiting the gastric H+, K'ATPase at the secretory surface of the gastric
parietal cell.
Rabeprazole blocks the final step of gastric acid secretion.
SUMMARY OF THE INVENTION
In accordance with one aspect, the invention provides a new crystalline Form Z
of
rabeprazole sodium. Preferably, crystalline Form Z of rabeprazole sodium has
an X-ray
diffraction pattern, expressed in terms of 2 theta angles, that includes four
or more peaks
selected from the group consisting of 4.69 f0.09, 9.0710.09, 9.4210.09, 1
1.2510.09,
14.7110.09, 16.240.09, 17.260.09, 18.520.()9, 18.52f0.09, 19.320.09,
19.630.09,
19.9210.09, 20.8010_09, 21.480.09, 23.070.09, 24.810.09, 25.7010.09,
27.4710.09,
30.0110.09, 30.650.09, 33.370.09, and 36.950.09. Various embodiments and
variants are also provided. r
CA 02459012 2004-02-26
In accordance with another aspect, the invention provides a composition that
includes rabeprazole sodium in a solid form, wherein at least 8U °io by
weight of the solid
rabeprazole sodium is the crystalline Form Z of rabeprazole sodium. Various
embodiments and variants are also provided.
In accordance with yet other aspects, the invention provides a process for
preparing the crystalline Form Z of rabeprazole sodium, and a pharmaceutical
composition that includes the crystalline Form Z of rabeprazole sodium and one
or more
pharmaceutically acceptable carriers or diluents. The pharmaceutical
composition may
also include one or more additional active ingredients. Preferably, the
pharmaceutical
composition is in a solid dosage form for oral administration, such as a
tablet.
The invention also relates to a method of preventing or treating a disease
that is
associated with excess gastric acid secretion, comprising administering to a
patient in
need of such treatment an effective amount of crystalline Form Z of
rabeprazole sodium.
BRIEF DESCRIPTION OF THE ACCOMPANYING DRAWINGS
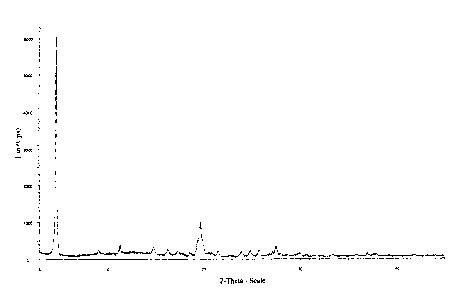
Figure 1 shows an example of an X-ray power diffractogram of the crystalline
Fotm Z of rabeprazole sodium.
Figure 2 shows a example of a differential scanning calorimetry thermogram of
the crystalline Form Z of ziprasidone sodium.
DETAILED DESCRIPTION OF THE INVENTION
Unless defined otherwise, all technical and scientific ternis used herein have
the
same meaning as commonly understood by one of ordinary skill in the art, to
which this
invention belongs. Although any methods and materials similar or eduivalent to
those
CA 02459012 2004-02-26
described herein can be used in the practice or testing of the present
invention, the
preferred methods and materials are described.
Unless stated to the contrary, any use of the words such as "including,"
"containing," "comprising," "having" and the like, means "including without
limitation"
and shall not be construed to limit any general statement that it follows to
the specific or
similar items or matters immediately following it. Embodiments of the
invention are not
mutually exclusive, but may be implemented in various combinations. The
described
embodiments of the invention and the disclosed examples are given for the
purpose of
illustration rather than limitation of the invention as set forth the appended
claims.
For purposes of the present invention, the following terms are defned below.
A "compound" is a chemical substance that includes molecules of the same
chemical structure.
"Pharniaceutically acceptable" means that which is useful in preparing a
pharmaceutical composition that is generally non-toxic and is not biologically
undesirable and includes that which is acceptable for veterinary use and/or
human
pharmaceutical use.
The term "composition" includes, but is not limited to, a powder, a
suspension, an
emulsion and/or mixtures thereof. The term composition is intended to
encompass a
product containing the specified ingredients in the specified amounts, as well
as any
product, which results, directly or indirectly, from combination of the
specified
ingredients in the specified amounts. A "composition" may contain a single
compound
or a mixture of compounds.
3
CA 02459012 2004-02-26
The term "pharniaeeutical composition" is intended to encompass a product
comprising the active ingredient(s), pharmaceutically acceptable excipients
that make up
the carrier, as well as any product which results, directly or indirectly,
from combination,
complexation or aggregation of any two or more of the ingredients, or from
dissociation
of one or more of the ingredients, or from other types of reactions or
interactions of one
or more of the ingredients. Accordingly, the pharmaceuttcai compositions of
the present
invention encompass any composition made by admixing the active ingredient,
additional
active ingredient(s), and pharmaceutically acceptable excipients.
The terns "excipient" means a component of a pharmaceutical product that is
not
the active ingredient, such as filler, diluent, carrier, and so on. The
excipients that are
useful in preparing a pharmaceutical composition are preferably generally
safe, non-toxic
and neither biologically nor otherwise undesirable, and are acceptable for
veterinary use
as well as human pharmaceutical use. "A pharmaceutically acceptable excipient"
as used
in the specification and claims includes both one and more than one such
excipient.
When refez-ring to a chemical reaction, the terms "treating", "contacting" and
"reacting" are used interchangeably herein and refer to adding or mixing two
or more
reagents under appropriate conditions to produce the indicated and/or the
desired product.
It should be appreciated that the reaction, which produces the indicated
and/or the desired
product, may not necessarily result directly from the combination of two
reagents, which
were initially added, i.e., there may be one or more intermediates which are
produced in
the mixture which ultimately leads to the formation of the indicated andior
the desired
product. Also, the tezm "isolating" is used to indicate separation of the
compound being
isolated regardless of the purity of the isolated compound from any unwanted
substance
4
CA 02459012 2004-02-26
which presents with the compound as a mixture. Thus, degree of the purity of
the
isolated or separated compound does not affect the status of "isolating".
The terns "substantially free oF' in reference to a composition, as used
herein,
means that said substance cannot be detected in the composition by methods
known to
those skilled in the art at the time of the filing of this application.
Rabeprazole sodium has the chemical structure:
O~OCH3
CH3 Na
S ~N ~ w
N O N
The preparation of rabeprazole is known in the art. For example, processes for
the preparation of rabeprazole and its sodium salt are disclosed in U.S.
Patent No.
5,045,552, the entire content of which is incorporated herein by reference.
The disclosed
process for preparing rabeprazole involves oxidation of 2-[ {4-(3-
methoxypropoxy)-3-
methylpyridine-2-yl}methylthio]-1H-benzimidazole with m-chloroperbenzoic acid
to
afford rabeprazole base. The base is then converted to rabeprazole sodium salt
in
aqueous sodium hydroxide solution. Rabeprazole sodium prepared by the process
of
U.S. Patent No. 5,045,552 is characterized as a white crystal, which is
precipitated from
ether solvent. The melting point of the disclosed rabeprazole sodium is 140-
141"C.'.
Three different types of crystalline forms of rabeprazoie sodium have been
previously reported. One is disclosed in Japanese patent 2001-39975, wherein
the ..
CA 02459012 2004-02-26
disclosed crystalline form is designated as Crystal II. The X-ray
diffractogram peaks for
Crystal II is also disclosed and reproduced in the following Table 1:
Table 1
Form II
Intensity
2 9() (Illo
19.52 100
17.20 41
26.60 28
20.92 18
18.04 _ 17
~24.76 __ 13
_ 21.20 12
14.22 10
17.60 10
~i-- 25.00 10
29.40 10
___ 28.76 9
27.56 _____
7
27.76 7
12.54
13.20 5
__ 24.38 5
28.50 ___ 5
C 34.04 5
13.80 _
- 4
22.64 4
24.16 4
30.00 4
12.82 3
31.62 3
11.84 2
25.92 2
34.92 2
8.88
I 9.64 1
The process for preparing Crystal II as disclosed in the Japanese patent
includes
crystallization of amorphous rabeprazole sodium or acetone complex of
rabeprazole
sodium in ethyl acetate, isopropyl acetate, isobutyl acetate, ethyl
propionate, isobutyl
propionate or ethyl butyrate.
fi
CA 02459012 2004-02-26
In addition to Crystal II form ofrabeprazole sodium, two other crystalline
forms
of rabeprazole sodium were previously disclosed in WO 03/082858 Al,
incorporated
herein by reference, wherein the two crystalline forms of rabeprazole sodium
were
designated as Forms X and Y respectively. The X-ray diffractograms peaks of
the Fornns
X and Y are shown in Table 2:
Table 2
Form X _ Form
Y
Intensity Intensity
2 E3() (cps) 2 0() (cps)
5.13 _ 1184 ___ 5_.61__ 1635
__ 6_.6_06_ 22_5 _ __1_9.4_42__ 546
'20.01 2_15 ___18.816~ 329
23.4_69 193 _ 7.725 285
_ 169 ___7_.2_07242
8.569 ~
12.92_3 __ _ 154 __ __9.6_46_
~ ~~ 135 ~ 10.3_52235
20.53_9 _ ' ~ 16.899_ 219
22.17_7 _131 ' 186
_ 24.8_1 _ _ ___ 162
~ _ 24.943
_125
~ 10.565 125 __ 16.418_104
12.16_1 _ __ 116 14.546 103_
~
9.353 113 _ 11._23177
18.173 99.3_
.
17.309 85.3 -_
_ 81.3 _
14_.8_64
24.494 _ 75.3
16.372 69.9
14_.414 60.2
7.244 57.5
I 19.072 56.2
The differential scanning calorimetry thertnogram of crystalline Form X
exhibits
a significant endo-exo pattern at 154.62 °C and 214.65°C and
that of crystalline Form Y
shows peaks at 182.61 °C and 215.57 °C. The melting points of
the Forms X and Y were
also measured by the capillary method respectively as 140-150 °C and
160-170 °C.
It is known that polymorphic fornis of the same drug may have substantial
differences in certain pharmaceutically important properties such as
dissolution
7
CA 02459012 2004-02-26
characteristics and bioavailability as well as stability of the drug.
Furthermore, difference
crystalline forms may have different particle size, hardness and glass
transition
temperature. Thus, one crystalline forni may provide significant advantages
over other
crystalline forms of the same drug in solid dosage form manufacture process
such as
accurate measurement of the active ingredients, easier f ltration, or improved
stability,
including light and thermal stability, during granulation or storage.
Furthermore, a
particular process suitable for one crystalline form may also provide drug
manufacturers
several advantages such as economically or environmentally suitable solvents
or process,
or higher purity or yield of the desired product.
Thus, according to one aspect, the present invention provides a novel
crystalline
form of rabeprazole sodium, which is different from the Crystal II, the FOI'nl
X or the
Form Y . The specific crystalline form obtained by the inventors is designated
as Form Z.
Crystalline Form Z may be prepared directly from unpurified rabeprazole sodium
or
purified amorphous or other crystalline forms of rabeprazole sodium. For
example,
rabeprazole sodium may be prepared by reacting rabeprazole with sodium
hydroxide in
methanol. Rabeprazole sodium prepared by the reaction can be used for the
preparation
process of crystalline Form Z without vigorous purification steps. Also,
amorphous forni
or other crystalline forms of rabeprazole sodium such as Form II, X or Y can
be
conveniently converted to crystalline Forni Z by the process described below.
Regardless
of whether unpurified rabeprazole sodium or amorphous or other crystalline
forms of
rabeprazole sodium is used as the starting material, a general process for
crystalline Form
Z may involve providing rabeprazole sodium, which is not crystalline Form Z,
in an
aromatic hydrocarbon solvents; refluxing the reaction mixture azeotropically;
cooling the
CA 02459012 2004-02-26
reftuxed reaction mixture. The aromatic hydrocarbon solvent is preferably
toluene,
xylenes or mixtures thereof and may be used in an amount so that the amount
ratio (w!v)
between rabeprazole sodium and the aromatic hydrocarbon solvent is preferably
between
about 1:3 and about 1:20, more preferably between about 1:3 and about 1:10,
even more
preferably 1:4. The reflex duration can vary depending upon the particular
amount ratio
or the total amount of rabeprazole and the solvents adopted for the process.
Preferably,
the reflex is continued for about 1-10 hours, more preferably for about 2-8,
even more
preferably about 2-6.
Conventional methods for isolating solids from liquid media such as filtration
can
be used to isolate crystalline Form Z of rabeprazoie sodium prepared by the
above
process.
The crystalline Form Z of rabeprazole sodium produced by this process was
characterized by an X-ray powder diffraction pattern, as for example shown in
Figure l,
and the characteristic 2 theta values (in degrees) and intensities of the
identified peaks in
the X-ray diffractograms are shown in Table 3:
Table 3
Form
Z
IntensityIntensity
2 B() (cps) (illo)
'
4.694 5710 100
19.320 829 14.5
__ 19.626368 _ 6.4
18.522 297 5.2
_ _ 3.5
11.254 200
14.712 181 32
27.470 179 3.1
9.070 147 2.6
16.241 141 2.5
24.814 __1_43 2.5
25.702 1 2.4
38
20.802 _ __
122 2.1
9
CA 02459012 2004-02-26
23.073 110 _ 1.9
__17.26494 1.6
18.522 87.9 1.5
~
19.920 8_4.6 1.5
_ 61 __ 1.1
_ 36.950 __
~
30.009 56.5 _
1.0
33.365 49 __ 0.9
9.417 42.1 0.7
21.477 4_0.2 _ 0.7
30.653 39 0.7
The X-ray diffractogram was measured on Broker Axe, DS advance Power X-ray
Diffractometer with Cu K alpha-1 Radiation source.
It should be kept in mind that slight variations in the observed 2 theta
angles
values are expected based on the specific diffractometer employed, the analyst
and the
sample preparation technique. More variation is expected for the relative peak
intensities.
Identification of the exact crystalline fornl of a compound should be based
primarily on
obsen~ed 2 theta angles with lesser importance attributed to relative peak
intensities. The
peaks reported herein are listed in order of their peak intensities. Thus, the
first listed
peak has stronger intensity than the second listed peak in the pattern. The 2
theta
diffraction angles and corresponding d-spacing values account for positions of
various
peaks in the X-ray powder diffraction pattern. D-spacing values are calculated
with
observed 2 theta angles and copper K(a 1 ) wavelength using the Bragg equation
well
known to those of skill in the art.
Thus, some margin of error may be present in each of the 2 theta angle
assignments reported herein. The assigned margin of error in the 2 theta
angles for the
crystalline Form Z of rabeprazole is approximately ~ 0.009 for each of the
peak
assignments. In view of the assigned margin of error, in a preferred variant,
the
crystalline form of rabeprazole may be characterized by an X-ray diffraction
pattern,
CA 02459012 2004-02-26
expressed in terms of 2 theta angles, that includes four or more peaks
selected from the
group consisting of 4.69 X0.09, 9.0710.09, ~)_42~Ø09, 11.250.09, 14_71
X0.09,
16.240.09, 17.260.09, 18.520.09, 18.5210.09, 19.320.09, 19.6310.09, 19.920.09,
20.8010.09, 21 _480.09, 23.0710.09, 24.8110.09, 25.7010_09, 27.470.09, 30.01
10.09,
30.6510.09, 33.370.09, and 36.950.09.
Since some margin of error is possible in the assignment of 2 theta angles and
d-
spacings, the preferred method of comparing X-ray powder diffraction patterns
in order
to identify a particular crystalline form is to overlay the X-ray powder
diffraction pattern
of the unknown form over the X-ray powder diffraction pattern of a known form.
For
example, one skilled in the art can overlay an X-ray powder diffraction
pattern of an
unidentified crystalline form of rabeprazole obtained using the methods
described herein,
over FIG. 1 and readily determine whether the X-ray diffraction pattern of the
unidentified form is substantially the same as the X-ray powder diffraction
pattern of the
crystalline form of this invention. If the X-ray powder diffraction pattern is
substantially
the same as FIG. 1, the previously unknown crystalline form of rabeprazole can
be
readily and accurately identified as the crystalline form of this invention.
The crystalline forms of rabeprazole sodium may also be characterized by
differential scanning calorimetry (DSC). The DSC thermogram of crystalline
Form Z of
rabeprazole sodium obtained by the inventors is shown in Figure 2. It exhibits
a
significant endo-exo pattern with identified peaks around 106. °C and
228.8 °C. The
melting point of the crystalline Form Z was also measured by the capillary
method and
was determined to be 224-230 °C.
CA 02459012 2004-02-26
The invention also relates to a composition containing solid rabeprazole
sodium
of v~~hich at least 80%, by total weight of the solid rabeprazole sodium in
the composition,
is crystalline Form Z. In the more preferred form of this composition, the
solid
rabeprazole sodium is suitable for use as active ingredient in formulating
pharmaceutical
products. The remainder of the solid rabeprazole sodium in the composition,
i.e., 20% or
less of the total weight of rabeprazole sodium, may be amorphous form or
crystalline
Form Il, X or Y of rabeprazole sodium or mixtures thereof. In an embodiment of
the
invention, the composition may comprise at least 90°,% of crystalline
Form Z of
rabeprazole sodium with respect to total weight of the solid rabeprazole
sodium in the
composition. In another embodiment of the invention, the composition may
comprise at
least 9S'~o of crystalline Form Z of rabeprazole sodium with respect to total
weight of the
solid rabeprazole sodium in the composition. In yet another embodiment of the
invention,
the composition is substantially free of any forms of rabeprazole sodium other
than its
crystalline Form Z.
X-ray diffraction provides a convenient and practical means for quantitative
determination of the relative amounts of crystalline and/or amorphous forms in
a solid
mixture. X-ray diffraction is adaptable to quantitative applications because
the intensities
of the diffraction peaks of a given compound in a mixture are proportional to
the fraction
of the corresponding powder in the mixture. The percent composition of
crystalline
rabeprazole sodium in an unknown composition can be determined. Preferably,
the
measurements are made on solid powder rabeprazole sodium. The X-ray powder
diffraction patterns of an unknown composition can be compared to known
quantitative
standards containing pure crystalline forms of rabeprazole sodimm to identify
the percent
12
CA 02459012 2004-02-26
ratio of a particular crystalline form. This is done by comparing the relative
intensities of
the peaks from the diffraction pattern of the unknown solid powder composition
with a
calibration curve derived from the X-ray diffraction patterns of pure known
samples. The
curve can be calibrated based on the X-ray powder diffraction pattern for the
strongest
peak from a pure sample of crystalline forms of rabeprazole sodium. The
calibration
curve may be created in a manner known to those of skill in the art. For
example, five or
more artificial mixtures of crystalline forms of rabeprazole sodium, at
different amounts,
may be prepared. In a non-limiting example, such mixtures may contain, 2%, 5%,
7%,
8°,%, and I O% of rabeprazole sodium for each crystalline form. Then, X-
ray diffraction
patterns are obtained for each artificial mixture using standard X-ray
diffraction
techniques. Slight variations in peak positions, if any, may be accounted for
by adjusting
the location of the peak to be measured. The intensities of the selected
characteristic
peaks) for each of the artificial mixtures are then plotted against the known
weight
percentages of the crystalline form. The resulting plot is a calibration curve
that allows
determination of the amount of the crystalline forms of rabeprazole sodium in
an
unknown sample. For the unknown mixture of crystalline and amorphous forms of
rabeprazole sodium, the intensities of the selected characteristic peaks) in
the mixture,
relative to an intensity of this peak in a calibration mixture, may be used to
determine the
percentage of the given crystalline form in the composition, with the
remainder
determined to be the amorphous material.
Pharmaceutical compositions comprising crystalline Form Z of rabeprazole can
be formulated with one or more pharmaceutically acceptable carriers, also
known as
excipients, which ordinarily lack pharmaceutical activity, but have various
useful
13
CA 02459012 2004-02-26
properties which may, for example, enhance the stability, sterility,
bioavailability, and
ease of formulation of a pharmaceutical composition. 'these carriers are
pharmaceutically acceptable, meaning that they are not harmful to humans or
animals
when taken appropriately and are compatible with the other ingredients in a
given
formulation. The carriers may be solid, semi-solid, or liquid, and may be
formulated with
the compound in bulk. The resulting mixture may be manufactured in the form of
a unit-
dose formulation (i.e., a physically discrete unit containing a specific
amount of active
ingredient) such as a tablet or capsule.
The pharmaceutical compositions may include, in addition to a compound of this
invention, one or more active pharmaceutical compounds in order to achieve
synergistic
effects. For example, U.S. Patent 6,303,644, incoporated by reference,
discloses use of~ a
proton pump inhibitors as combination with an antimicrobial compound to avoid
or
reduce side effects caused by the antimierobial compound. Similarly, L1.S.
Patent
6,117,868, incorporated by reference, discloses treatment of infectious
gastrointestinal
ulcer disease or infectious gastritis disease by administering an
antimicrobial medicament
in combination with a proton pump inhibitor. Thus, the additional
pharmaceutical
compound to be added to the pharmaceutical composition of the present
invention may
includes, for example, antibacterial or antimicrobial compounds such as
penicillins
including benzylpenicillin, phenoxymethylpenicillin, propicillin, azidicillin,
dicioxaeillin,
flucloxacillin, oxacillin, amoxicillin, bacampicillin, ampicillin,
meziocillin, piperacillin,
or aziocillin; cephalosporins including cefadroxil, cefaclor, cefalexin,
cefalexim,
cefuroxim, cefetamet, cefadroxil, ceftibuten, cefpodoxim, cefotetan,
cefazolin,
cefoperazon, cefiizoxim, ceftaxim, ceftazidim, cefamandol, cefepim, cefoxitin,
14
CA 02459012 2004-02-26
cefodizim, cefsulodin, ceftriaxon, cefotiam, or cefmenoxim; aztreonam;
loracarbef;
meropenem; sulbactam; tetracyclines including tetracycline, oxytetracycline,
minocycline, or doxycycline; aminoglycosides including tobramycin, gentamicin,
neomycin, streptomycin, amikacin, netilmicin, paromomycin or spectinomycin;
ampherlcols including chloramphenicol or thiamphenicol; lincomycins;
clindamycin;
lincomycin; erythromycin; clarithromycin; spiramycin; roxithromycin;
azithromycin;
collstin; polymixin B; teioplanin; vancomycin; norfloxacin; cinoxacin;
ciprofloxacin;
pipemidic acid; enoxacin; nalidixie acid; pefloxacin; fieroxacin; ofloxacin;
metronidazole; fomycin; fucidic acid; taurolidine; taurultam; and mixtures
thereof.
Generally, the pharn~aceutical compositions of the invention may be prepared
by
uniformly admixing the active ingredient with liquid or solid carriers and
then shaping
the product into the desired forn~. The pharmaceutical compositions may be in
the form
of suspensions, solutions, elixirs, aerosols, or solid dosage forn~s. Because
of their ease
of administration, tablets and capsules represent the most advantageous oral
dosage unit
form, in which case solid pharmaceutical carriers are employed.
A preferred oral solid preparation is a tablet. A tablet may be prepared by
direct
compression, wet granulation, or molding, of the active ingredients) with a
can-ier and
other excipients in a manner known to those skilled in the art. Compressed
tablets may
be prepared by compressing in a suitable machine the active ingredient in a
free-flowing
form such as powder or granules, optionally mixed with a binder, lubricant,
inert diluent,
surface active agent or dispersing agent. Molded tablets may be made on a
suitable
machine. A mixture of the powdered compound moistened with an inert liquid
diluent is
suitable in the case of oral solid dosage forms (e.g., powders, capsules, and
tablets). If
CA 02459012 2004-02-26
desired, tablets may be coated by standard techniques. The compounds of this
invention
may be formulated into typical disintegrating tablets, or into controlled or
extended
release dosage fom~s.
The pharmaceutical compositions of the invention are contemplated in various
forn~ulations suitable for various modes of administration, including but not
limited to
inhalation, oral, rectal, parenteral (including subcutaneous, intradennal,
intramuscular,
intravenous), implantable, intravaginal and transdennal administration. The
most
suitable route of administration in any given case depends on the duration of
the subject's
condition, the length of treatment desired, the nature and severity of the
condition being
treated, and the particular formulation that is being used. The fornmlations
may be in
bulk or in unit dosage form.
The amount of active ingredient included in a unit dosage form depends on the
type of fornmlation that is formulated. A pharmaceutical composition of the
invention
will generally comprise about 0.1 % by weight to about 99% by weight of active
ingredient, preferably about 1% by weight to 50% by weight for oral
administration and
about 0.2% by weight to about 20% by weight for parenteral administration.
Formulations suitable for oral administration include capsules (hard and
soft),
cachets, lozenges, syrups, suppositories, and tablets, each containing a pre-
determined
amount of the active compound; as a powder or granules; as a solution or a
suspension in
an aqueous or non-aqueous liquid; or as an oil-in-water or water-in-oil
emulsion. Such
formulations may be prepared by any suitable method of pharmacy that includes
the step
of bringing into association the active compound and a suitable carrier or
carriers. The
amount of active ingredient per unit dosage of solid fonmulations may be as
described in
16
CA 02459012 2004-02-26
prior art for preparations of rabeprazole sodium. For liquid oral
fornmlations, a
preferable amount is from about 2% by weight to about 20% by weight. Suitable
carriers
include but are not limited to fillers, binders, lubricants, inert diluents,
surface
active/dispersing agents, flavorants, antioxidants, bulking and granulating
agents,
adsorbents, preservatives, emulsifiers, suspending and wetting agents,
glidants,
disintegrants, buffers and pH-adjusting agents, and colorants. Examples of
carriers
include celluloses, modified celluloses, cyclodextrins, starches, oils,
polyols, sugar
alcohols and sugars, and others. For liquid formulations sugar, sugar
alcohols, ethanol,
water, glycerol, and poyalkylene glycols are particularly suitable, and may
also be used in
solid formulations. Cyclodextrins may be particularly useful for increasing
bioavailability. Formulations for oral administration may optionally include
enteric
coatings known in the art to prevent degradation of the formulation in the
stomach and
provide release of the drug in the small intestine.
Fornmlations suitable for buccal or sub-lingual administration include
lozenges
comprising the active compound in a flavored base, usually sucrose and acacia
or
tragacanth, although other agents are also suitable, and pastilles comprising
the
compound in an inert base such as gelatin and glycerin or sucrose and acacia.
Formulations suitable for rectal administration are preferably presented as
unit
dose suppositories. These may be prepared by admixing the active compound with
one
or more conventional solid carriers, e.g., cocoa butter, and then shaping the
resulting
mixture.
In another aspect, the invention also provides methods of preventing or
treating a
disease that is associated with excess gastric acid secretion, comprising
administering to a
17
CA 02459012 2004-02-26
patient in need of such treatment an effective amount of crystalline Form Z of
rabeprazole sodium. In particular, for example, the method of the present
invention can
be used in healing or maintenance of erosive or ulcerative Gastrocsophageal
Reflux
Disease (GERD), treatment of symptomatic Gastroesophageal Reflux Disease,
healing of
Duodenal Ulcers, helicobacter pylori eradication to reduce the risk of
Duodenal Ulcer
recurrence, and treatment of pathological hypersecretory conditions, including
Zollinger-
Eliison Syndrome.
The effective amount (i.e., dosage) of active compound for treatment will vary
depending on the route of administration, the condition being treated, its
severity, and
duration, and the state and age of the subject. A skilled physician will
monitor the
progress of the subject and will adjust the dosage accordingly, depending on
whether the
goal is to eliminate, alleviate, or prevent a given condition. Generally, the
dosage should
be considered in proportion to the subject's weight, T'he daily dose of
particular
formulations of active compound may be divided among one or several unit dose
administrations. For example therapeutic administration about fifteen to
thirty minutes
before main meals is preferable (i.e. three times daily), although
administration of the
active compounds may be carried out prophylactically, and may be maintained
for
prolonged periods of time. One skilled in the ari will take such factors into
account when
determining dosage. Unit dosage of active ingredient may range preferably from
about
I mg to about 100 mg, more preferably from about 10 mg to about 50 mg.
The invention is further described by reference to the following examples
which
set forth in detail the preparation of compounds and compositions of the
present
invention, as well as their utility. It will be apparent to those skilled in
the art, that many
lb
CA 02459012 2004-02-26
modifications, both to materials, and methods, tray be practiced without
departing from
the purpose and interest of this invention. The examples that follow are not
intended to
limit the scope of the invention as described hereinabove or as claimed below.
Reference Example 1:
Preaaration of 2-[~~4-(3-methoxy~ronoxv)-3-meth~~l-2~vridinyl]-
methyl~sulfinyll-1H-
benzimidazole (Rabeprazole)
2-[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl thin]-1H-benzimidazole
(Prepared as per example 90 of the Patent No. 5,045,552) (100 grams, 0.29
moles) was
added to a mixture of chloroforni (9500 ml) and dimethylsulphoxide (200 ml)
and the
reaction mixture was cooled to -10 °C,' to -15 °C'. 3-
Chloroperbenzoic acid (60 grams, 0.24
moles) was dissolved in chloroform (500m1), and the chloroform solution was
then added
to the above solution at --10 °C to -15 °C over about 1- 2
hours, and the reaction mixture
vas maintained at the same temperature for 30 minutes. Then, 12.8 % (w/v)
aqueous
sodium hydroxide solution (500 ml) was added to the reaction mixture. The pH
of the
reaction mixture was adjusted to 9.5 to 10.0 with acetic acid, which formed a
biphasic
system. The organic layer was separated and then extracted with 1.(i % (w/v)
aqueous
sodium hydroxide solution (500 ml). The sodium hydroxide extract was diluted
with a
mixture of chloroform ( 140 ml) and methanol ( 100 ml). Then the pH of the
mass was
again adjusted to 9.5 to 10.0 m.~ith acetic acid, and the organic layer was
separated again.
To the separated organic layer was added tert_ butyl methyl ether (440 ml)_
The reaction
mixture was stirred for about 1-2 hours at a temperature of ()-5 °C and
was subjected to
filtration. The residue was dissolved in a mixture of 110 % (w/v) aqueous
sodium
hydroxide solution (100 ml) and methanol (65 ml). The pH of the reaction
solution was
19
CA 02459012 2004-02-26
adjusted to 9.0 to 9.S with acetic acid at 10-15 °C and was stirred for
additional 2 hours,
followed by filtration. The wet material is then dissolved in dichloromethane
(130 ml),
and the water layer separated where after solution was added to tent. butyl
methyl ether
(2C0 ml), stirred at a temperature of 0-~ °C for 1-2 hours. The
precipitated solid was
filtered and dried to give 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-
methyl]
sulfinyl]-1 H-benzimidazole.
Reference ExamTle 2
Preparation of amorphous form of 2-[[j4-(3-methoxypropox~)-3-meth~p ry idinylJ-
methyll sulfinyll-lf~-benzimidazole sodium (rabeprazole sodium)
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-1H-
benzimidazole
jobtained as per reference example 1 ) (50.0 grams, 0.139 moles) was dissolved
in a
mixture of sodium hydroxide (7.S grams, 0.187 moles) and methanol (100.0 ml),
and the
solution was stirred at ambient temperature 25-3S °C'. The reaction
solution is filtered
through hi-flow and washed with methanol (50_0 ml). Then, the solvent of the
filtrate
was distilled off under reduced pressure. The reaction mass was cooled to
ambient
temperature, and petroleum ether (400.0 ml) was then added to the residual
mass, which
was then stirred at 2S-30 °C for about 1-2 hours. The precipitated
solid was filtered and
washed with petroleum ether (100.0 ml) and dried at 50-60 °C for 12
hours to afford the
desired amorphous form of rabeprazole sodium { Weight: 50.4 grams, 94.9%).
Reference Example 3
Preparation of CrKstalline form X of 2-[[[4-(3-methoxYpropoxy)-3-meth~pyridin
meth~~sulfnvl)-1H benzimidazole sodium (Rabe~razole sodium)
2 ()
CA 02459012 2004-02-26
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-1H-
benzimidazole
(obtained by reference example 1 ) (50.0 grams, 0.139 moles) was dissolved in
a mixture
of sodium hydroxide (7.5 grams, 0.187 moles) and methanol ( 100.0 ml) and
stirred at
ambient temperature 25-35"C. The reaction solution was filtered through hi-
flow and
washed with methanol (50.0 ml). Then the solvent of the filtrate was distilled
off under
reduced pressure. The reaction mass was cooled to ambient temperature.
Dichloromethane (100.0 ml) was added to the reaction mixture, which was then
distilled
to remove traces of methanol. Dichloromethane (~0.0 ml) and petroleum ether
(100.0 ml)
was then added to the residual mass, which was then stirred at 25-30
°C." for about 6-8
hours. Additional petroleum ether ( 150 ml) was added to the reaction mixture,
which
was stirred at 25-30 °C for I-2 hours. The precipitated solid was
fltered and washed
with petroleum ether (100.0 ml) and dried at 50-60°C for 12 hours to
afford the desired
form X of rabeprazole sodium (Weight: 50.4 grams, 94.9'%).
Reference example 4
Preparation of Crystalline form-Y of 2-[j[4-(3-methox~rpropox~r)-3-methyl-2-
pyridinvll-
meth~rll sulfin~~lH-benzimi~iazole sodium (rabeprazole sodium)
2-[j[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-1H
benzimidazole
(obtained as per reference example 1 ) (750.0 grams, 2.089 moles) was
dissolved in a
mixture of sodium hydroxide ( 112.5 grams, 2.8125 moles) and methanol ( 1500.0
ml),
and the resulting solution was stirred at ambient temperature 25-35°C.
The reaction
solution was f ltered through hi-flow and washed with methanol (750.0 ml). The
solvent
of the filtrate was distilled off completely under reduced pressure. The
reaction mass was
cooled to ambient temperature, and dichloromethane ( I 500.0 ml) was added to
the
21
CA 02459012 2004-02-26
reaction mass. The solvent was distilled again to remove traces of methanol.
The
reaction mass was cooled to ambient temperature, and n-butanol (375.0 ml) and
tertiary
butyl methyl ether (6.0 L) was added to the residual mass which is stirred at
25-30 °C for
Eo-8 hours. The reaction mixture was further cooled to 5-15 °C and then
stirred for
another 3-5 hours. The solid then precipitated was filtered and washed with
tertiary butyl
methyl ether (1500.0 ml) and dried at 50-60 °C for 7 hours to afford
the desired
crystalline Form Y of rabeprazole sodium (Weight: 725.0 grams, 91.1 %).
Example 1
Preparation of Crystalline form Z of 2-j(~~ ~-methoxy~ro~poxv -~-methvl;2-
pyridin,~~ll-
methyl] sulfinyll-1H-benzimidazole sodium (Rabeprazole sodiuml from 2-((~4~3-
methoxvpropox_y)-3-methyl-2 ~yridin~ll~metlyll sulfinyll-1H benzimida- zole
_(rabe~razolel
2-(((4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-1H-
benzimidazole
(obtained by reference. example 1 ) (50.0 grams, 0.139 moles) was dissolved in
a mixture
of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml), and the
resulted
solution was stirred at ambient temperature 25-35°C for about one hour.
The reaction
solution was f ltered through hi-flow and washed with methanol (50.0 ml). Then
the
solvent of the filtrate was distilled off under reduced pressure. The reaction
mass is
cooled to ambient temperature, to which toluene (200.0 mI) was added. The
residual
mass was then retluxed for about 2-~ hours. After cooling the reaction
solution, the
precipitated solid was filtered, washed with toluene ( 100.0 ml) and dried at
90-100 °C for
12 hours to afford the desired form Z of rabeprazole sodium (Weight: 50.4
grams,
94.9%).
22
CA 02459012 2004-02-26
Example 2
fre~aration of C~stalline form Z of 2-~[[4=,_(3-methoxv_propoxyy-3-meth,-2=,p
r~i~inyl~
methvll sulfinyll-1H benzimidazole sodium ~rabeprazole sodium
Crystalline Form X of rabeprazole Sodium (50.0 grams, 0.131 moles) was
charged in toluene (200.0 ml) and the mixture was refluxed for 8-10 hours. The
reaction
mixture was then cooled to 25-35°C. The precipitated solid was filtered
off, washed with
toluene (100.0 ml) and dried at 90-100 °C for 6-8 hours to afford the
desired form Z of
Rabeprazole sodium (Weight: 45 grams, 90%).
Example 3
Pharmaceutical composition of rabeprazole sodium crystalline Form Z
Rabeprazole sodium crystalline Forth Z, precipitated eakium carbonate, corn
starch, lactose, and hydroxypropylcellulose are mixed together. Water is added
to the
mixture, which is then kneaded. The kneaded mixture is dried in vacuum at 40
°C for 16
hours, ground in a mortar and passed through a 16-mesh sieve to give granules.
To this is
added magnesium stearate, and the resultant mixture is made up into tablets.
Composition per tablet
Rabeprazole crystalline Form Z 2U mg
Precipitated calcium carbonate 50 mg
Corn Starch 50 mg
Lactose 73.4 mg
Hydroxypropylcellulose () mg
Magnesium Stearate O.U~ ml t
Total 200.0 mg
23