Note: Descriptions are shown in the official language in which they were submitted.
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
STEEL MAKING MATERIAL RECYCLING SYSTEM
Technical Field
The present invention relates to steel processing materials, methods of
preparing such materials and methods of manufacturing steel using such
materials.
The materials and methods of the invention allow the use or recycling of iron-
bearing
by-product material in the steel industry.
Background of the Invention
In the steel industry, especially when melting scrap steel in an electric arc
furnace, solid waste material, commonly referred to as Furnace Exhaust
Material
(FEM), from the post combustion exhaust chamber is generated. Typically, an
exhaust system is used to direct this material to a bag house. The FEM
typically is
very high in iron (Fe) content. Some of this material, called post combustion
material
(PCM), comprises particles that are too heavy or too large to be exhausted to
the bag
house. Such material can be gravity fed from the combustion chamber to a drop
out
box or similar arrangement. Thus, FEM is generated from the post combustion
1 S chamber drop out box as PCM or is evacuated on to the bag house as bag
house dust.
The iron content from either location is typically about 40% by weight.
However, the
iron content can vary from about 20% to about 75% by weight. These materials
can
also have about 15-25% by weight moisture, about 20% by weight of material
similar
in content to the slag foaming material currently added to the furnace, and up
to about
5% by weight of other metals and oxides. The slag foaming material can include
calcium and magnesium oxides, iron, carbon and/or manganese.
The slag foaming materials are originally introduced into the steel making
process to develop a foamy slag that, among other things, creates a chemical
environment in a heat of steel where the exchange of oxygen and other unwanted
materials in the steel can occur. However, due to the extreme temperatures,
various
chemical reactions, and the necessary environmental exhausting of furnace
gases,
some of the slag foaming materials are undesirably exhausted into the flume or
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
exhaust chamber. Similarly, some of the iron in the steel and in the slag can
also be
exhausted into the chamber. These materials typically agglomerate or otherwise
combine to create dust or larger particles within the exhaust chamber.
The combustion chamber or the post combustion chamber duct work of a steel
manufacturing assemblies are often water cooled. Water from leaks, sprays or
any
other source may travel by gravity through the post combustion chamber and wet
the
post combustion material. Post combustion material removed from the drop out
box
is typically stored in an outside yard for further disposition. Either in the
drop out box
or in the yard, the PCM can absorb a great deal of moisture from the
atmosphere, rain
or other sources. The moisture content of wet PCM is usually significantly
above 2%
and usually is greater than 6% and, more typically, is about 15-20%, all by
weight.
However, some processes may avoid the moisture pickup thus delivering a dry
PCM,
containing less than about 2% by weight moisture content.
Typically, PCM undergoes an expensive secondary reclamation processes to
recover the heavy metals or is sent to landfills for disposal. The use of
secondary
reclamation processes to recover the heavy metals are generally very
expensive.
Currently such processes require expensive equipment, extensive handling of
the
material, and the use of chemical additives. After processing, the material
may still
not be desirable in many applications. U. S. Patent No. 5,738,694, to Ford,
dislcoses
an example of the secondary processing of similar material. Ford discloses
iron rich
material waste products, such as electric arc furnace dust, formed with an
organic
binder into discrete shapes, such as briquettes and/or other solid shapes. The
shapes
can then be used in iron and steel making processes and may allow recovery of
the
iron and heavy metals values in the waste product.
Some manufacturers have found it more economical to send the PCM to
landfills. The cost of reclaiming the heavy metals can be much greater than
the cost
of landfilling and the decrease in steel quality and life of the furnace is
too great to
justify merely reintroducing the PCM back into the process.
Other iron-bearing waste materials may be generated throughout the steel
making process. As previously discussed, bag house dust is continually made in
the
steel making process. Steel makers continue to struggle with cost effective
means of
2
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
processing, selling or otherwise eliminating bag house dust. The secondary
processes
in steel making also create a significant amount of iron-bearing waste
materials,
including for example, scale generated at the caster or rolling mill. Other
sources of
iron wastes include iron fines generated by the recovery of rolling solution
in a cold
rolling mill, the cleaning of steel in a galvanizing line or other
cleaning/finishing
processes. A further source of iron waste is a high purity iron oxide
recovered from
spent pickle liquor of a pickling process. All of these sources of by-product
iron
materials create a dilemma for the steel maker in dealing with disposal of
these
materials.
3
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
Summary of the Invention
In one embodiment, the invention relates to steel processing materials. The
steel processing materials comprise a dried post combustion material (PCM) and
a
slag foaming material.
In another embodiment, the invention is directed to a method of preparing the
steel processing material. The methods comprise recovering PCM from a steel
making process and drying the PCM. In a further embodiment, the methods of
preparing the steel processing material comprise recovering dry PCM from a
steel
making process and mixing the PCM with a slag foaming material.
In another embodiment, the invention is directed to methods of manufacturing
steel. The methods comprise melting a first heat of steel, whereon the heat
has a
liquid steel portion and a foamy slag portion. The melting generates PCM. The
PCM
is dried and added into a second heat of steel.
In yet another embodiment of the current invention a steel processing material
comprises a recycled material and a slag foaming material.
Advantages and novel features of the present invention will become further
apparent to those skilled in the art from the following detailed description,
which
simply illustrates various modes and examples contemplated for carrying out
the
invention. As can be realized, the invention is capable of other different
aspects, all
without departing from the invention. Accordingly, the drawings and
descriptions are
illustrative in nature and not restrictive.
Brief Description of the Drawings
While the specification concludes with claims particularly pointing out and
distinctly claiming the present invention, it is believed that the same can be
better
understood from the following description, taken in conjunction with the
accompanying drawing, in which:
Fig. 1 illustrates a schematic view of an exemplary embodiment of a PCM
reclamation facility in accordance with the present invention.
4
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
Detailed Description of Exemplary Embodiments
Reference can now be made in detail to various exemplary embodiments of
the invention, some of which are also illustrated in the accompanying drawing.
Throughout the specification and claims, all parts, fractions and percentages
are by
weight unless otherwise specified.
Solid waste material such as Furnace Exhaust Material (FEM) is generated by
the steel making process. The current invention contemplates removing some of
the
moisture content and/or otherwise recycling FEM material back into the
process. The
FEM is typically generated as particles collected from a drop out box, known
as Post
Combustion Material (PCM), or dust from the bag house, as described above.
Different plants or operations in the steel industry may use different terms
other than
drop out box particles or bag house dust, however, the term "furnace exhaust
material" as used in this invention should be understood to cover any iron-
bearing
I S material from the exhaust of a steel making furnace. Such furnaces may
include a
basic oxygen furnace, an electric arc furnace, a degasser, or any similar
furnace
creating solid material from the exhaust chamber. The recycled steel making
material
as used in the current invention further includes iron-bearing solid waste
materials
such as iron fines, scale, iron oxide from pickle liquor, or other similar
steel making
materials as known to those skilled in the art.
If PCM is directly reintroduced back into the steel making process, several
problems may occur, for example, the moisture is broken down into its
elemental
components (HZ and OZ). Excess hydrogen in the steel can decrease the
castability
and increase porosity of the steel. The increased oxygen both increases
melting time,
requiring more energy for heat, and produces "dirty" steel. Reactions from
both the
hydrogen and oxygen can also be detrimental to the life of the furnace.
Additional
processing costs may also be incurred, for example, by increased cost and time
at a
treatment facility such as a ladle furnace. Further, the moisture alone can
cause safety
concerns if the PCM is submerged in liquid steel because the expansion of the
moisture, from water to steam, can cause an explosion.
5
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
Reintroducing the PCM back into the process may also cause the foamy slag
characteristics of the furnace to be changed because the moisture of the PCM
decreases the effectiveness of the foamy slag. The chemical reactions between
the
steel and slag may be decreased and poor coverage of the steel by the foamy
slag may
occur. Nitrogen pickup may also increase as poor coverage of the foamy slag
allows
air to contact the liquid steel.
In one embodiment of the current invention, wet PCM, typically at about 15 to
25% by weight water content, is obtained from the steel making furnace, for
example
in particle form from a drop out box. The wet PCM is dried to remove at least
a
portion of the moisture. For example, in one embodiment, the PCM is air dried
to
about 6-I S% by weight water content. The PCM can be sorted to facilitate
further
drying, other processing, or subsequent use of the material. In one embodiment
the
sorting is accomplished by screening to obtain one or more fractions of
desired
average particle size. In a further embodiment, the PCM is sorted to obtain a
fraction
having a maximum particle size, for example of about 1 inch (2.5 cm), more
specifically of about 3/4 inch (2 cm), even more specifically of about S/16
inch (0.8
cm). The sorted material can then be subjected to further processing, and in
one
embodiment is dried further to about 2% by weight water content. The PCM, now
referred to as dried PCM, can be reintroduced into the steel making process.
For
example, the dried PCM can be added by charging buckets, direct charging, or
otherwise reintroduced into the steel making process using techniques known to
those
skilled in the art.
The further drying may be achieved using any apparatus or method known in
the art. For example, the drying may be conducted using a rotary dryer, common
in
the steel industry, or using a screw auger dryer. The screw auger dryer can
heat the
PCM by, for example, induction heaters, gas-fired heaters or other such
heating
systems. Use of a screw auger dryer can be beneficial in that an auger is
relatively
inexpensive as compared with a rotary dryer, the screw auger dryer can be
installed in
a relatively small space, and installation time for a screw auger dryer can be
a few
weeks compared to several months for a rotary dryer. A screw auger with an
induction drier or other type of electric operated drier may also be more
6
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
environmentally friendly as compared with a rotary drier such as one requiring
natural
gas or fuel oil or one having a fluid bed. The induction dryer typically does
not need
preheat time, does not give off hazardous gasses such as NOX, and allows for
tighter
temperature control. The tighter the temperature control, the less the
likelihood of
gases evolving from the material being dried. Thus, the screw auger drier may
also be
useful where environmental conditions need tight control, such as where an
increase
in gasses are objectionable and/or may complicate permitting issues.
However, the rotary drier may operate more efficiently. If time and space are
not critical, a rotary drier could be advantageous.
The sorting step preceding the mechanical drying may vary according to the
type of dryer, the material processed, i.e., the degree or type of
agglomeration or
otherwise fused properties of the material, and/or the contamination of the
material.
Contamination may occur, for example, where large pieces of scrap mix with the
PCM because scrap and PCM are o$en stored adjacent one another. Such scrap
could
damage a dryer or limit further use of the PCM. In one embodiment using a
rotary
dryer, the sorting ahead of a rotary dryer may only need to be to a particle
size of
about 3 inches (8 cm), or the sorting may even be eliminated. However, an
embodiment using an auger may require screening or sorting, for example, to a
maximum of about 3/4 of an inch (2 cm) particle size or less. Other
embodiments are
contemplated wherein no screening step is required due to the inherent small
particle
size and lack of contamination.
In some steel making processes, the PCM may remain dry throughout
generation and recovery. However, even without the moisture content problem,
adding the PCM back into the steel making process may be difficult. For
example,
injecting PCM may be difficult because of the limited size of an injection gun
compared to the size of some PCM particles and other scrap metal which may
tend to
become mixed with the PCM. Also, injected PCM may displace slag foaming
materials inhibiting necessary chemical reaction between the steel and the
slag.
Additional embodiments may include PCM that has not absorbed moisture
and is below 2% moisture content in the drop box. Such "dry PCM" does not need
to
7
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
undergo a further drying process and may be screened and/or mixed with slag
foaming materials for injection into the steel making process, as will be
discussed.
According to another embodiment of the current invention, the dried PCM can
be sorted further. This may include screening to give a size that will not
block or clog
an injection gun as is commonly used to add slag foaming material in an
electric arc
furnace. This screening may be to about 5/16 of an inch (0.8 cm), i.e., to the
size of
the slag foaming material. Once the PCM has been sized to about 5/16 of an
inch (0.8
cm), it can proceed, for example, via a bucket elevator, into a first PCM
container
such as a silo. Once in a first container, the PCM can be discharged into a
second
container such as a super sack or a truck. The PCM can be mixed concurrently
with
the slag foaming material to make a modified slag foaming material. The
modified
slag foaming material can be added into the top of an arc furnace, usually by
an
injection gun, to create a foamy slag on the top of the molten bath of steel.
In one embodiment of the current invention, the modified slag foaming
material is injected after slag has foamed on a heat of steel. Such an
embodiment
typically has an environment that is hot and oxygen rich enough to cause the
generally endothermic materials in the PCM to become exothermic, thus
generating
heat and reducing power usage. The oxygen may create energy, for example, by
oxidizing some of the iron in the PCM. The high temperature may also melt the
iron
from the PCM. Further, both the carbon from the slag foaming material and
other
metals in the PCM may reduce the oxidized iron, further allowing recovery of
the iron
into the liquid steel. Such oxidation and reduction reactions are known to
those
skilled in the art and may be reviewed by the Gibb's free energy equations and
diagrams. An example of generating heat and reducing power will be discussed
later.
A typical slag foaming material may consist of about 90% coal and about 10%
dolomitic stone. In one embodiment of the current invention, a modified slag
foaming material, that is, a slag foaming material with PCM added, may
comprise
about 10 to 20% PCM, about 70 to 80% coal and about 8 to 12% dolomitic stone.
However, according to the principles of the current invention, a modified slag
foaming material may comprise from about 0% up to about 30% by weight PCM, and
behave efficiently in the steel making process.
8
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
In additional embodiments, other slag foaming materials such as any other
carbon and/or low sulfur products, and/or materials including calcium and
magnesium
oxides, iron, carbon, and manganese, as known to those skilled in the art, may
be
mixed with the PCM.
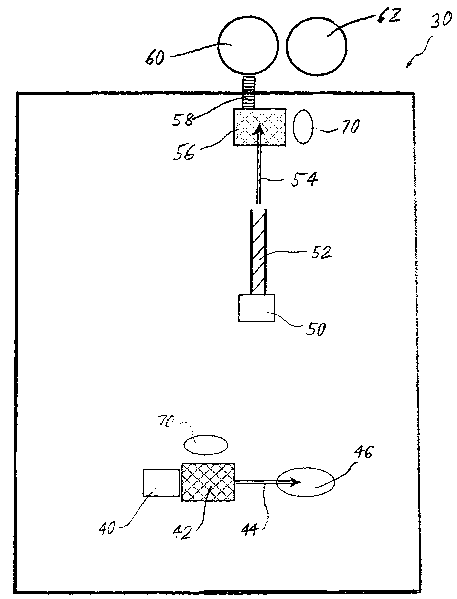
Fig. 1 illustrates one exemplary embodiment of a facility 30 for the
processing
of the PCM in accordance with the invention. The facility 30 includes a first
receiving hopper 40 for loading of PCM generated by the steel making process.
The
material can be processed from the first receiving hopper 40 to a first screen
42. In
one embodiment, the first screen 42 comprises a 5' by 7' (150 x 200 cm) double
decked scalping screen. The first screen 42 screens the PCM to obtain a
fraction
having a desired maximum particle size, for example, of about 3/4 inch (2 cm).
The
screened PCM fraction of the desired size is delivered via a discharge
conveyer 44 to
a first screen fraction or stockpile 46. Material too large to be screened by
the first
screen 42 may be stockpiled, for example, in a screened "overs" stockpile 70,
or
I S otherwise processed to reduce its size, or discarded. Material from the
first screened
fraction stockpile 46 is transported, for example by a front end loader, a
conveyor or
the like to a second receiving hopper 50. The PCM is next fed from the second
receiving hopper 50 to screw auger 52. The auger 52 can be a heated,
dewatering
auger in certain embodiments. For example, the auger may include induction
heaters
to heat the PCM and evaporate the water content of the material. In one
embodiment,
the PCM is heated to reduce the water content to less than about 2%. In other
embodiments of the current invention the auger 52 can be replaced by a
conventional
rotary dryer, or any other dryer effective to reduce the water content of the
PCM.
Exiting the auger 52, the material is transported by a feed conveyor 54 to a
second screen 56. In one embodiment, the second screen 56 comprises a 4' by 8'
(120 x 240 cm) single deck scalping screen. The second screen 56 screens the
PCM
to obtain a fraction having a maximum particle size about 1/4 inch (0.5 cm).
The
screened PCM fraction is transported, for example, by a bucket elevator 58, to
a first
storage silo 60. In the embodiment of Fig. 1, a second storage silo 62 is
adjacent the
storage silo 60. The second storage silo 62 may contain any of a variety of
slag
foaming materials such as anthracitic coal, coke, or any other carbon and/or
any other
low sulfur product known to those skilled in the art for use in a steel making
process.
9
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
The slag foaming material may additionally include materials such as dolomite
or
spar. Further, the two storage silos can have a single load out spout (not
shown). The
single load out spout may allow for mixing of the two materials concurrent
with
addition of the materials to a container such as a transport truck.
Other sources of high iron-bearing steel waste material may be similarly
recycled. Such materials include bag house dust, scale and iron fines. In
alternative
embodiments, dry, high iron-bearing steel waste materials may all be stored in
a
single silo, in combinations of silos, or each in individual silos. In one
embodiment
of the current invention, the bag house dust is stored in a storage silo
similar to the
PCM. The bag house dust is mixed directly with the slag foaming material. A
single
load out spout may also allow the mixing of the bag house dust with the slag
foaming
material concurrently as the materials are added to a transport truck. Since
bag house
dust typically has a moisture content that is less than 2%, a drying process
is typically
not necessary. Also, because bag house dust is usually small in size, less
than 5/16
inch (0.8 cm), and is typically clean or free of other (larger) contaminants,
it may not
need to be sorted. However, should the bag house dust have a high moisture
content
greater than about 2%, or be agglomerated in particle size too large to
inject, the
drying and/or screening process, as described above for the PCM, may also be
used.
Scale, as generated 'from steel processing, such as caster scale or mill
scale,
may be treated similarly. However, since such scale may have a higher
concentration
of iron oxide content, the concentration of scale to slag foaming material may
be
adjusted. Also, scale is typically high in moisture content. High moisture
content
scale may be dried as described above with respect to the PCM. That is, the
scale
may be dried, for example, by a rotary dryer or screw auger dryer to about 2%
by
weight or less water content. Further, the scale may be screened before and
after
drying as needed to reach the previously discussed particle sizes. Upon
injection, the
scale contained in the modified slag foaming material and the high temperature
slag
could possibly become exothermic because the Fe305 will be oxidized to Fez03.
Other iron-bearing materials, such as those generated by the steel cold
finishing processes, may also be mixed with slag foaming material to provide a
steel
processing material. For example, the iron fines recovered from cold mill
rolling
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
solution or temper mill rolling solution or from cleaning processes, such as a
cleaning
process in galvanizing line, may also be used. Again, these materials may be
wet or
of sufficiently large size that drying and/or screening may be necessary.
Drying,
screening, and/or mixing processes as discussed above may be employed. These
materials are typical of high Fe content and may behave similar to PCM in that
the
oxidation of the iron is an exothermic reaction. Also, a relatively high
purity iron
oxide may be recovered from spent pickle liquor. This material, though
possibly
already dried by a roaster, may become wet or otherwise increase to moisture
content.
This material, too, may be screened, dried, and/or mixed according to the
methods
previously discussed for use with the PCM. Depending on the particular iron
oxide
materials available an exothermic reaction with the high temperature slag
could occur.
An exemplary comparison of batch recovered a charging PCM in charge
buckets, drying and mixing PCM with slag foaming materials will now be
discussed.
Approximately 1,000 pounds (450 kg) of PCM is batch charged into a 200 ton
(180,000 kg) heat of steel. Nitrogen increases in the steel by 15 parts per
million
(PPM). Further, when PCM is directly charged in the bucket, the energy per
scrap
ton (KWH/ton or J/kg) increases by about 37 KWH/ton (147,000 J/kg). However,
when the PCM is dried and mixed with slag foaming material in an amount of
about
95% by weight slag foaming material and 5% by weight PCM, an increase in KWH
is
not seen and the KWH actually appears to decrease. This may be due to
oxidation of
iron and manganese. Also, there was no increased nitrogen in the steel and the
Fe0
weight percent in the slag did not increase.
In this example, the dried and mixed PCM contains about 45% by weight of
iron and about 1.7% by weight of manganese. This equates to about 144 pounds
(65
kg) of iron and 5.4 pounds (2.5 kg) of manganese. One hundred forty-four
pounds
(65 kg) of iron, when oxidized during the melting process from approximately
76° F
to 2,900° F (24° C to 1600° C) would create about 85
kilowatt hours (300 mega-
joules), if reacted 100% to completion. However, at only 50% reaction, 43
kilowatt
hours (150 mega joules) would be produced. Similarly, 5.4 pounds (2.5 kg) of
manganese reacted with oxygen from 76° F to 2,900° F (24°
C to 1600° C) generates
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
about 5 KWH (18 mega joules), when reacted completely or 2.5 KWH (75 mega-
joules) when reacted 50%.
In the exemplary comparison, batch charging recovered PCM increases both
power usage and the time necessary to melt a heat. These factors, along with
possible
decreased quality in steel, make recharging PCM directly very expensive,
especially
when considering that newly recovered PCM may increase power usage by
approximately 8%. Alternatively, the dried and mixed PCM may decrease power
usage by approximately 10%.
Additional embodiments of the current invention are directed to methods of
manufacturing steel. In one embodiment of this method, a first heat of steel
is melted.
A slag foaming material may also be added to the heat. A liquid steel portion
and a
foamy slag portion are developed. The melting of the heat evolves both some of
the
steel and the foamy slag as furnace exhaust materials. The furnace exhaust
materials
may be exhausted toward a bag house. Some of the materials (PCM) may be too
heavy or large or may be washed away from the exhaust by a water stream and
may
not be exhausted to the bag house. A drop out box is typically provided to
accumulate these materials.
In an embodiment of this method, the PCM may become wet from leaks,
sprays, rain or any other source inside or external to the exhaust duct or to
the drop
out box. Thus, the PCM may need drying in accordance with the methods
discussed
above. Drying may be achieved by a screw auger, a rotary dryer, or the like.
In other
alternative embodiments, the PCM may not be wet, having a moisture content
less
than about 2% by weight, and may proceed directly to further processing steps.
A method of sorting the PCM before drying, as previously discussed, may be
used to properly size the particles for the drying process. A method of
further sorting
before storing, mixing, or injecting of the PCM may be used as previously
discussed.
Once dried and sorted as needed, the PCM is added into a second heat of steel.
The PCM may be added by injection with an injection gun or mixed with another
material such as a slag foaming material and then injected or added in batch.
As PCM undergoes the process of multiple iterations of being generated,
recovered and added back into the steel making process, a build up in the PCM
of
12
CA 02459112 2004-02-27
WO 03/008651 PCT/US02/23205
heavy metals such as zinc and lead may occur. In one embodiment made in
accordance with the current invention, a limit or set point, such as 0.0010%
by weight
of lead, is set for heavy metal concentration in the PCM. Once the limit is
met, the
PCM is removed from the iterative process. For example, a clean steel producer
may
generate PCM that has heavy metals well below a threshold as set by regulation
or the
producer. Each time PCM is added back into a heat and recovered again, the
concentration of these heavy metals increases. As the concentration of heavy
metals
in the PCM increases to the set point, typically less than the threshold, the
PCM may
be removed from the iterative process and sent to a reclamation process. The
more
concentrated heavy metals may offset the cost of reclamation, should improve
the
efficiency of the reclamation process and may reduce the steel makers need to
land fill
the PCM. However, in an additional embodiment, the PCM with a high
concentration
of heavy metals may be sent to a landfill. Similar embodiments may be
maintained
for bag house dust of any other iron bearing material.
Some of the beneficial characteristics of this invention may include increased
liquid steel yield, decreased energy cost, decreased land fill requirements,
and
decreased shipping and handling of waste material. Thus the invention both
decreases
cost for the steel industry and improves the environment.
Having shown and described the preferred embodiments of the present
invention, further adaptations to the post combustion material recycling
system of the
present invention as described herein can be accomplished by appropriate
modifications by one of ordinary skill in the art without departing from the
scope of
the present invention. Several of these potential modifications and
alternatives have
been mentioned, and others can be apparent to those skilled in the art. For
example,
while exemplary embodiments of the inventive system and process have been
discussed for illustrative purposes, it should be understood that the elements
described
can be constantly updated and approved by technological advances. Similarly,
as
described, the process of this invention could be applied with any steel
processing
waste material substantially bearing iron. Accordingly, the scope of the
present
invention should be considered in terms of the following claims and is
understood not
to be limited to the details of structure, operation or process steps as shown
and
described in this specification and drawing.
13