Note: Descriptions are shown in the official language in which they were submitted.
CA 02459622 2013-02-28
COMPOSITIONS AND METHODS FOR RESTORING SENSITIVITY TO
TREATMENT WITH HER2 ANTAGONISTS
BACKGROUND OF THE INVENTION
[0001j Approximately 25-30% of breast cancer patients overexpress the
proto-oncoprotein and cell surface receptor c-erbB2 (human epidermal growth
factor receptor 2 protein), also known as HER2/neu. Overexpression of the c-
erbB2 oncogene has been linked to poor outcome and decreased survival for
patients. The HER-2/neu proto-oncogene is overexpressed in 20-30% of
metastatic breast cancers, and is associated with decreased survival and
increased recurrence of breast cancer. HER-2/neu is also overexpressed in
other
cancers types including endometrial cancer, kidney cancer, gastric cancer, and
prostate cancer. Presently, the most common form of treatment for these
patients
is the use of the humanized monoclonal antibody Trastuzumab, also known as
HerceptinO.
[0002] HerceptinC) is a recombinant DNA-derived humanized
monoclonal antibody that selectively binds with high affinity (Kd = 5 nM) to
the
extracellular domain of c-erbB2 in a cell-based assay. See Science
1985;230:1132-9
and Cancer Res 1993;53:4960-70. Hercepting is an IgG1 kappa antibody that
binds to HER2 and contains human framework regions with the complementarity-
determining regions of a murine antibody (4D5). Id. However, only 25% of the
patients treated with Herceptin or any other antibody to c-erbB2/HER2 are
responsive to this therapy. Several models have been postulated to explain
resistance to treatment with c-erbB2/HER2 antibodies.
1
CA 02459622 2004-02-27
BRIEF SUMMARY OF THE INVENTION
C000s] The present invention is based in part on the discovery that an
autocrine growth factor, PC-Cell Derived Growth Factor ("PCDGF"), confers
resistance to the antineoplastic effects of c-erbB2/HER2 ("HER2") antagonists.
Preferred embodiments of this invention are directed to therapeutic
compositions
and methods for restoring growth inhibition sensitivity to tumor cells
resistant to
the antineoplastic effects of HER2 antagonists by administering a PCDGF
antagonist in an amount effective to restore growth inhibition sensitivity to
HER2
antagonists. Another embodiment of the invention provides therapeutic
compositions and methods for inhibiting tumor cell growth comprising
administering a PCDGF antagonist and a HER2 antagonist in an amount effective
to inhibit tumor cell growth.
[0004.: The invention also provides preferred compositions comprising
a HER2 antagonist and a PCDGF antagonist. In another embodiment, the
invention provides a pharmaceutical composition comprising a HER2 antagonist,
a PCDGF antagonist, and a pharmaceutically-acceptable carrier (e.g., water,
saline, Ringer's solution, dextrose solution, and human serum albumin).
[0005j Further embodiments of the invention provide methods of
determining whether a patient is resistant to the antineoplastic effects of
HER2
antagonists, comprising obtaining a biological sample containing cells from a
patient; detecting PCDGF in the biological sample; and determining the amount
of PCDGF in said sample wherein the amount of PCDGF is indicative of
resistance to the antineoplastic effects of HER2 antagonists.
BRIEF DESCRIPTION OF THE DRAWINGS
[mos] FIG. 1 summarizes pathological studies in paraffin embedded
human breast cancer biopsies. PCDGF and erbB2 staining were monitored by
2
= CA 02459622 2004-02-27
immunohistochemisty. Thus, overexpression of PCDGF in cells overexpressing
erbB2 renders the cells HER2 antagonist resistant. It is known that 25% of
patients with tumors that overexpress erbB2 will be responsive to HER2
antagonist therapy.
[0007: FIG. 2 shows erbB2 levels in MCF7 cells (breast cancer
cells)
transfected with erbB2.
[0008] FIG. 3 shows PCDGF expression in MCF7 cells transfected
with
erbB2.
E0009] FIG. 4 shows PCDGF protein levels in conditioned media of
cells transfected with both PCDGF and erbB2 (D.c2) and erbB2 alone (erbB2.c1).
[0010] FIG. 5 shows that PCDGF stimulates erbB2 phosphorylation
in
erbB2 overexpressing cells.
Coon] FIG. 6 shows that PCDGF stimulates erbB2 phosphorylation
in
other breast cancer cells that are known to overexpress erbB2 such as BT474
and
SKBR3 (see FIG. 12).
Coo12j FIG. 7 shows the dose response of PCDGF stimulation of
erbB2
in BT474 cells.
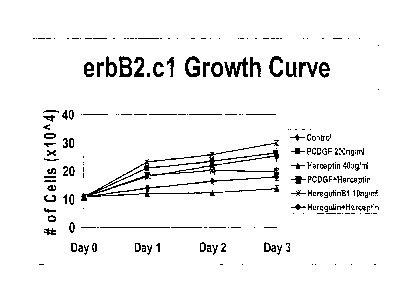
0013] FIG. 8 shows the long term growth of erbB2 cells in
response to
Herceptin0. Herceptin0 inhibits proliferation of erbB2 overexpressing cells as
it
is a monoclonal antibody that neutralizes erbB2 and inhibits proliferation in
erbB2 overexpressing breast cancer cells.
3
CA 02459622 2014-10-24
E001 4j FIG. 9 shows that when PCDGF is overexpressed in the erbB2
overexpressing cells (e.g., D.c2 cells), Hercep tin no longer inhibits the
proliferation of the breast cancer cells.
[00153 FIG. 10 shows the same results as FIG. 9 using thymidine
incorporation as a measure of cell growth.
[0016] FIG. 11 shows that PCDGF overexpression prevents Herceptin0
inhibition of breast cancer cells in a soft agar (tumorigenesis) assay in
erbB2
overexpressing cells. These results demonstrate that overexpression of PCDGF
confers Herceptin r resistance in erbB2 overexpressing cells.
[0017] FIG. 12 shows that PCDGF stimulates erbB2 phosphorylation in
SKBR3 cells. Overexpression of PCDGF confers Herceptin0 resistance in SKBR3
cells overexpressing erbB2.
[0017A] FIG. 13 shows stable transfectants according to the present
invention.
DETAILED DESCRIPTION OF THE INVENTION
Coots] PCDGF is an 88 kDa autocrine. growth factor characterized in
our laboratory and shown to be overexpressed in and induce tumorigenesis of a
wide variety of human and animal tumor cells (e.g., neuroblastoma,
glioblastoma, astrocytoma, sarcomas, and cancers of the prostate, blood,
cerebrospinal fluid, liver, kidney, breast, head and neck, pharynx, thyroid,
pancreas, stomach, colon, colorectal, uterus, cervix, bone, bone marrow,
testes,
brain, neural tissue, ovary, skin, and lung). See, e.g., U.S. Patent Number
6,309,826.
[00193 PCDGF also confers resistance to the antineoplastic effects
HER2 antagonists (e.g., HerceptinO, HER2 kinase inhibitors) on tumor cells. As
described in U.S. Patent Number 6,309,826, overexpression of PCDGF leads to
uncontrolled cell growth and increased tumorigenesis. The degree of PCDGF
4
CA 02459622 2004-02-27
overexpression directly correlates with the degree of cellular tumorigenicity.
Cells overexpressing PCDGF do not require external signals to maintain
uncontrolled cell growth. Loss of regulated cell growth, such as a loss in
responsiveness to insulin and/or estrogen, leads to increased malignancy and
excessive unregulated cell growth. Development of methods and compositions
that interfere with the tumorigenic activity of PCDGF is therefore of great
interest
for the treatment of cancer.
E0020] HER2 antagonist therapy (e.g., but not limited to, Herceptin0
and HER2 kinase inhibitors) is useful for treatment of patients with
metastatic
breast cancer, including patients whose tumors overexpress the HER2 protein
and who have received one or more chemotherapy regimens for their metastatic
disease. Herceptin0, in particular, also is approved for combination therapy
with paclitaxel for treatment of patients with metastatic breast cancer whose
tumors overexpress the HER2 protein and who have not received chemotherapy
for their metastatic disease. However, only 25% of the patients treated with
Herceptin0 or any other antibody to HER2 are responsive to such therapy.
[002 1] PCDGF antagonists, such as anti-PCDGF antibodies, interfere
with the biological activity of PCDGF (e.g., tumorigenic activity) by binding
PCDGF directly and preventing PCDGF from transmitting cell growth signals to
a target cell (e.g., breast cancer cell). An anti-PCDGF antibody may bind the
active site of PCDGF (e.g., the PCDGF receptor binding site) and prevent PCDGF
from binding to its receptor. Alternatively, anti-PCDGF antibodies may bind to
a
site on PCDGF other than the active site, alter the conformation of the active
site,
and thus render PCDGF incapable of binding to its receptor. Anti-PCDGF
antibodies include PCDGF neutralizing antibodies. "Neutralizing" antibodies
have the ability to inhibit or block the normal biological activity of PCDGF,
CA 02459622 2004-02-27
including PCDGF's ability to stimulate cell proliferation, increase cell
survival,
block apoptosis, or induce tumor growth in animals and in humans. PCDGF
antagonists also include nucleic acids (antisense, siRNA etc.) and anti-PCDGF
receptor antibodies. PCDGF confers resistance to the antineoplastic effects of
HER2 antagonists. Immunohistochemistry studies in paraffin embedded human
breast cancer biopsies showed that PCDGF was highly expressed in 25% of c-
erbB2 positive (+3) invasive ductal carcinomas.
[0022] Preferred embodiments of the invention are directed to methods
of restoring growth inhibition sensitivity to tumor cells resistant to the
antineoplastic effects of HER2 antagonists by administering a PCDGF antagonist
to a human patient in an amount effective to restore the patient's growth
inhibition sensitivity to HER2 antagonists.
[00233 The term "HER2 antagonist" refers to any molecule (e.g.,
protein, peptide, small molecule, nucleic acid, antisense, or siRNA) that is
capable of binding, interfering with, or inhibiting the activity of HER2 or
any
analogs or derivatives of HER2 that retain the neoplastic properties of HER2
(e.g., but not limited to HerceptinC) and HER2 kinase inhibitors).
[0024] In one embodiment, a HER2 antagonist includes a molecule that
can target or selectively bind to HER2 and, for example, deliver a toxin or
other
compound or molecule to kill a cell or inhibit cell growth. For example, a
HER2
antibody can be coupled to a toxin or chemotherapeutic agent that is delivered
to
a tumor cell after the antibody binds to HER2. HER2 antagonists also include
molecules (e.g., peptides, small molecules, antisense molecules, and siRNA)
that
modulate the biological activity of molecules that regulate the activity of
HER2.
A HER2 antagonist can be an antibody that recruits an immune response, e.g.,
through ADCC (antibody dependent cell cytotoxicity).
6
= CA 02459622 2004-02-27
[0025] The term "PCDGF antagonist" refers to any molecule
(e.g.,
protein, peptide, small molecule, nucleic acid, antisense, or siRNA) that is
capable of selectively binding, interfering with, or inhibiting the biological
activity of PCDGF including, but not limited to, the HER2 antagonist
resistance-
conferring activity of PCDGF or any analogs or derivatives of PCDGF that
retain
the properties of PCDGF. In one embodiment, a PCDGF antagonist includes
PCDGF receptor antibodies that can target or selectively bind to the PCDGF
receptor and, for example, deliver a toxin or other compound or molecule to
kill
a cell or inhibit cell growth.
[0026] The invention also provides pharmaceutical compositions
containing one or more HER2 antagonists and one or more PCDGF antagonists.
Patients receiving both HER2 and PCDGF antagonists will benefit from the
antineoplastic effects of each antagonist in that the PCDGF antagonist can
restore
the HER2 antagonist sensitivity of patients resistant to HER therapy.
Pharmaceutical compositions comprising a HER2 antagonist, a PCDGF
antagonist, and an acceptable pharmaceutical carrier (such as water, saline,
Ringer's solution, dextrose solution, and human serum albumin) are also
provided.
[0027] Preferred embodiments of the invention also include
methods of
determining whether a patient is resistant to the antineoplastic effects of
HER2
antagonist therapy by obtaining a biological sample containing cells from the
patient; detecting PCDGF in the biological sample; and determining the amount
of PCDGF in the sample wherein the amount of PCDGF is indicative of resistance
to the antineoplastic effects of HER2 antagonist therapy.
[0028D The term antibody herein includes but is not limited to
human
and non-human polyclonal antibodies, human and non-human monoclonal
7
CA 02459622 2004-02-27
antibodies (mAbs), chimeric antibodies, anti-idiotypic antibodies (anti-IdAb),
neutralizing antibodies, non-neutralizing antibodies, and humanized
antibodies.
Polyclonal antibodies are heterogeneous populations of antibody molecules
derived either from sera of animals immunized with an antigen or from chicken
eggs. Monoclonal antibodies ("mAbs") are substantially homogeneous
populations of antibodies to specific antigens. mAbs may be obtained by any
suitable method. Such antibodies may be of any immunological class including
IgG, IgM, IgE, IgA, IgD and any subclass thereof. The term antibody is also
meant to include both intact molecules as well as fragments thereof such as,
for
example, Fab and F(ab')2, which are capable of binding to the antigen. Fab and
F(ab')2 fragments lack the Fc fragment of intact antibody, clear more rapidly
from the circulation, and may have less non-specific tissue binding than an
intact
antibody. Such fragments are typically produced by proteolytic cleavage, using
enzymes such as papain (to generate Fab fragments) and pepsin (to generate
F(ab')2 fragments).
[0029] In yet another embodiment, a subject's cells (e.g., tumor
cells)
are removed from the body, transfected with a polynucleotide encoding a HER2
antagonist and a PCDGF antagonist, and injected at the site of the tumor.
Expression of the polynucleotides encoding the HER2 antagonist and PCDGF
antagonist localizes the HER2 and PCDGF antagonists at the tumor site. A
subject's cells (e.g., tumor cells) can also be directly transfected in the
body (e.g.,
in situ) with a construct containing a nucleic acid encoding a HER2 antagonist
and/or a PCDGF antagonist. Expression of the nucleic acid results in
production
of the HER2 antagonist and/or PCDGF antagonist inside the transfected cell.
[ooso] For in vivo applications, PCDGF antagonists, and HER2
antagonists can be provided to a subject by a variety of administration routes
and
8
CA 02459622 2004-02-27
dosage forms. A subject, preferably a human subject, suffering from a
neoplastic
condition, including but not limited to breast cancer, or other disease
condition
associated with increased HER2 and/or PCDGF expression, is treated
consecutively or simultaneously with an HER2 antagonist and a PCDGF
antagonist. In one embodiment, the HER2 antagonist and PCDGF antagonist are
co-administered. In another embodiment, the HER2 antagonist and PCDGF
antagonist are sequentially administered, preferably the level of PCDGF
ascertained and, if elevated, the PCDGF antagonist being administered first.
[ow ij Treatment with a PCDGF antagonist can precede, follow, or be
conducted concurrently with treatment with a HER2 antagonist. Treatment with
a PCDGF antagonist may precede or follow treatment with a HER2 antagonist by
intervals ranging from minutes to weeks. In another embodiment, a PCDGF
antagonist and a HER2 antagonist are administered in a way to ensure that a
prolonged period of time does not elapse between the time of administration of
each agent. For example, each antagonist can be administered to a patient
within
seconds, minutes, or hours of the other antagonist.
[0032] In a preferred embodiment, treatment with a PCDGF antagonist
increases c-erbB2 phosphorylation by at least 10%, more preferably by at least
50%. In a further preferred embodiment, treatment with a PCDGF antagonist
increases HER2 sensitivity by at least two-fold, more preferably by at least
three-
fold.
[0033: The antagonists of the present invention may be administered
by any means that achieves their intended purpose. For example, antibody
administration may be by various routes including but not limited to
subcutaneous, intravenous, intradermal, intramuscular, intraperitoneal, and
oral.
Parenteral administration can be by bolus injection or by gradual perfusion
over
9
CA 02459622 2013-02-28
time. Preparations for parenteral administration include sterile aqueous or
non-
aqueous solutions, suspensions and emulsions, which may contain auxiliary
agents or excipients. Pharmaceutical compositions such as tablets and capsules
can also be prepared. It is understood that the dosage will be dependent upon
the age, sex and weight of the recipient, kind of concurrent treatment, if
any,
frequency of treatment and the nature of the effect desired.
0034] In another embodiment of the invention, a HER2 antagonist in
combination with a PCDGF antagonist and/or chemotherapy (e.g., paclitaxel,
cisplatin (CDDP), carboplatin, procarbazine, mechlorethamine,
cyclophosphamide, camp tothecin, ifosfamide, melphalan, chlorambucil,
busulfan, nitrosurea, dactinomycin, daunorubicin, doxorubicin, bleomycin,
plicomycin, mitomycin, etoposide (VP16), tamoxifen, raloxifene, estrogen
receptor binding agents, TaxolTm, gemcitabien, navelbine, farnesyl-protein,
tansferase inhibitors, transplatinum, 5-fluorouracil, vincristin, vinblastin
andmethotrexate, or any analog or derivative or variant of the foregoing) can
be
used to treat metastatic breast cancer or cancers of a variety of tissues
(e.g.,
neuroblastoma, glioblastoma, astrocytoma, sarcomas, and cancers of the
prostate,
blood, liver, kidney, breast, head and neck, pharynx, thyroid, pancreas,
stomach,
colon, colorectal, uterus, cervix, bone, bone marrow, testes, brain, neural
tissue,
ovary, skin, and lung). Combination therapy can be administered independently
of diagnostic evaluation.
[0035] In a preferred modality of the invention, a breast cancer patient
(e.g., having metastatic breast cancer and overexpressing HER2) is given HER2
antagonist treatment with, for example, Herceptin. Although, the patient
exhibits some degree of tumor growth inhibition, her progress declines, and
she
becomes resistant to HER2 antagonist therapy. A biopsy or serum sample is
CA 02459622 2004-02-27
conducted and reveals that the patient has elevated levels of PCDGF. In
another
embodiment, the patient's PCDGF levels are determined prior to beginning
HER2 antagonist therapy. The patient is then treated with a PCDGF antagonist
alone, or co-administration of PCDGF antagonist and HER2 antagonist, to
restore
sensitivity to HER2 antagonist therapy. Following treatment with PCDGF
antagonist, the patient is again responsive to HER2 antagonist therapy. The
PCDGF level of the patient is periodically monitored, for example, weekly or
monthly throughout the HER2 therapy and a PCDGF antagonist provided to
again restore HER2 sensitivity if needed. Alternatively, the patient can
continue
to receive co-administration of PCDGF antagonist and HER2 antagonist.
C0036] The ranges of effective doses provided below are not intended
to limit the invention and merely represent illustrative dose ranges. However
the
most preferred dosage will be tailored to the individual subject as is
understood
and determinable by one of ordinary skill in the art given the teachings
herein.
The total dose required for each treatment may be administered by multiple
doses or in a single dose. In one embodiment, effective amounts of each of a
HER2 antibody and a PCDGF antibody are from about 0.01 ng to about 500
pg/m1 and preferably from about 10 ng to about 100 n/ml. An HER2 antagonist
and a PCDGF antagonist may be administered alone, administered together, or
in conjunction with other therapeutics. In another embodiment, the amount of
each antagonist administered will typically be in the range of about 0.1 to
about
mg/kg of patient weight, so long as the HER2 and PCDGF antagonists are
administered to the patient in therapeutically effective amounts (i.e.,
amounts
that eliminate and/or reduce the patient's tumor burden or restore sensitivity
to
the antineoplastic effects of any HER2 antagonists).
11
CA 02459622 2004-02-27
[0037] A preferred treatment regimen comprises co-administration of
an effective amount of a HER2 antagonist and a PCDGF antagonist over a period
of one or several weeks and including between about one week and six months.
In another embodiment, a HER2 antagonist can be provided in an initial dose of
4 mg/ kg followed by 2 mg/kg intravenous (i.v.) weekly; or dose of 8 mg/kg
initial dose followed by 4 mg/kg i.v. weekly. Treatment with a PCDGF
antagonist can be provided, for example, in an initial dose of 4 mg/ kg
followed
by 2 mg/kg intravenous (i.v.) weekly; or a dose of 8 mg/kg initial dose
followed
by 4 mg/kg i.v. weekly. Additional examples of regimens for cancer treatment
that can be used for treatment with a HER2 antagonist, a PCDGF antagonist,
and/or chemotherapeutic agents are disclosed in the following articles, hereby
incorporated by reference in their entirety: Hamid, 0.1 Am Pharm Assoc, 2004
Jan-Feb;44(1):52-8; Kubo et al., Anticancer Res. 2003 Nov-Dec; 23(6a):4443-9;
Slamon et al., N Engl J Med. 2001;344:783-792.; Baselga et al., Cancer Res
1998;58:2825-2831; and Jones, et al., Ann Oncol. 2003 Dec;14(12):1697-704.
EXAMPLE 1
PCDGF CONFERS RESISTANCE TO THE ANTINEOPLASTIC EFFECTS OF HER2
ANTAGONIST THERAPY
[0038] To investigate the role of PCDGF in HER2 antagonist
resistance
of c-erbB2 overexpressing cells, MCF-7 breast cancer cells were stably
transfected
with c-erbB2 cDNA. A c-erbB2 overexpressing clone (erbB2.c1) was selected, and
used for the subsequent stable transfection with PCDGF cDNA. Clones
overexpressing both c-erbB2 and PCDGF were obtained and further examined in
comparison to single erbB2 overexpressing cells. We show in the appended
Figures that dual-overexpressing clones (D.c2) (i.e., clones overexpressing
both
PCDGF and erbB2) were HER2 antagonist resistant with respect to their ability
to
proliferate in vitro, whereas the erbB2.c1 cells were sensitive to Herceptin0
12
CA 02459622 2004-02-27
treatment. These HerceptinC)-sensitive erbB2.c1 cells also had a growth
advantage when treated with PCDGF. Moreover PCDGF stimulates c-erbB2
phosphorylation in these cells. We also show that the dual expressing D.c2
cells
were more resistant than the erbB2.c1 cells to the antiestrogen ICI 182,780.
Our
findings demonstrate that PCDGF confers HerceptinC) resistance in c-erbB2
overexpressing tumors. See Figures. Blocking PCDGF action would be
beneficial for patients undergoing HER2 antagonist treatment by reversing the
initial resistance.
13
CA 02459622 2014-10-24
EXAMPLE 2
[0039] The increase of the endogenous levels of PCDGF in erbB2-
overexpressing human breast cancer cells provides Herceptin resistance to
these cells.
Stable transfections were carried out by the Calcium phosphate method. Clones
were
established in the presence of Zeocin and/or G418. Cell proliferation assays:
Cells
were plated in DMEM/F12 (+5% FBS). 24 hrs. later, cells were washed, and fresh
PFMEM was added along with the various treatments. Cells were counted for the
next
three days using a haemocytometer. Thymidine incorporation assays: Cells were
plated in DMEM/F12 (+5% FBS). 48 hrs. later, cells were treated with various
drugs.
48 hrs. later, ltiCi of 3H-Thymidine was added to each well. 24 hrs. later,
cell lysates
were collected, and levels of 3H-Thymidine were measured by liquid
scintillation
counter. Activation of erbB2: Cells were plated in DMEM/F12 (+5% FBS). 48 hrs.
later, PFMEM was added. 24 hrs. later, various treatments were added. Samples
were
lysed with RIPA buffer, and separated on a SDS-PAGE gel, and transferred onto
a
PVDF membrane. Membranes were blocked, and incubated with anti-phospho-erbB2,
followed by incubation with secondary antibody. Proteins were detected with
ECL.
14