Note: Descriptions are shown in the official language in which they were submitted.
CA 02459672 2008-04-14
~ . .
1
Title: ORGANOSILICON CONTAINING COMPOSITIONS FOR
ENHANCING HYDROCARBON PRODUCTION AND METHOD OF USING
THE SAME
Field of the invention
This invention relates generally to methods and compositions for modifying
the permeability of subterranean formations. In particular, this invention
relates to methods and compositions for selectively reducing the production of
water from subterranean formations; the composition being a water control
treatment fluid containing a relative permeability modifier (RPM)
macromolecule and an organosilicon compound.
Background of the invention
Production of water and aqueous fluids from oil and gas wells is a common
phenomenon which poses a variety of problems. For example, water
production typically reduces the amount of oil and/or gas that may be
ultimately recovered from a well since the water takes the place of other
fluids
that may flow or be lifted from the well. Thus, water production from oil and
gas wells causes significant economic drawbacks. High water rates cause a
reduction in well productivity and increase in operating expenditures.
Furthermore, operating costs associated with disposal of produced water in
an environmentally safe manner typically increase with the volume of
produced water, thus increasing the threshold amount of hydrocarbons that
must be produced in order to continue economical production of the well.
U.S. Patent No. 6,228,812 discloses a chemical treatment that selectively
reduces water production. Such treatments employ relative permeability
modifiers (RPMs). The use of RPMs offer several advantages. For instance,
the use of RPMs reduces costs since the chemicals are used in limited
quantities and the treatment does not require zonal isolation. In addition,
the
use of RPMs entails low risk since the polymer reduces the water permeability
without affecting oil permeability. Further, RPMs are simple to apply and do
not require expensive equipment, such as rigs, for their application.
CA 02459672 2004-03-05
-2-
However, even the most superior RPMs are not certain to impart long-lasting
effectiveness, nor exhibit a high degree of water flow resistance relative to
oil
flow, especially when formation permeability rises above 1 Darcy. New RPM
systems are needed for higher permeability applications.
Summary of the invention
Compositions useful for selective permeability modification of
subterranean formations to reduce or substantially eliminate the amount of
water produced from oil andlor gas wells comprise a relative permeability
modifier (RPM) macromolecule capable of impeding the production of water
and an organosilicon compound. The compositions of the invention reduce or
eliminate the production of water in an oil or gas well without substantially
affecting the production of hydrocarbons.
Suitable as the RPM are homopolymers and copolymers of acrylamide,
optionally having been sulfonated or quaternized, polyvinylalcohol,
polysiloxane, or a hydrophilic polymer selected from natural gums and
chemically modified derivatives thereof.
In a preferred embodiment, the organosiilicon compound is of the
formula:
R
R2 1 R (1)
IR3
wherein R is a halogen, hydrogen, or an amine radical which can be
substituted with hydrogen, organic radicals, or silyl groups, R, is hydrogen,
an
amine, or an organic radical having from 1 to 50 carbon atoms, and R2 and R3
are hydrogen or the same or different halogens, alkyl, alkenyl, aryl or amines
having 1 to 50 carbon atoms; or
CA 02459672 2004-03-05
-3-
R
R5 1 OR7 (I~~
R6
wherein R4, R5 and R6 are independently selected from hydrogen, amine,
halogen, alkoxide, and organic radicals having from 1 to 50 carbon atoms,
provided not all of R4, R5 and R6 are hydrogen, and R7 is an organic radical
having from 1 to 50 carbon atoms.
The organosilicon compound increases flow resistance and is believed
to attach to the RPM poiymer as well as to the mineral surfaces of the
formation. As a result, the effective RPM permeability application range is
significantly extended with the novel compositions.
The compositions of the invention are designed to partition both onto
reservoir rock and into reservoir brines. Such behavior results in significant
reduction of permeability in water-rich environments.
Advantageously, the disclosed method and compositions are relatively
non-damaging to oil permeability, for example, in oil saturated sandstone
while exhibiting the ability to decrease water permeability substantially in
water saturated zones. Therefore, the disclosed compositions may be applied
successfully to a productive zone without the necessity of mechanical
isolation in the wellbore. It will be understood with benefit of this
disclosure
that mechanical isolation, such as isolation of a vvater producing section or
perforations, may be employed if so desired, however, such measures may
add significant costs to a water control treatment. Consequently, treatments
utilizing the disclosed method and compositions without mechanical isolation
are considerably less expensive than conventional methods which require
such measures.
In one respect, disclosed is a method for treating a subterranean
formation, including introducing an aqueous composition into the formation
wherein the concentration of RPM in the aqueous composition is between
from about 100 to about 80,000 ppm, preferably from about 500 to about
CA 02459672 2004-03-05
-4-
10,000 ppm, and the concentration of organosilicon compound is between
from about 500 to about 20,000 ppm. While not intending to be bound to any
theory, it is believed that the organosilicon compound is capable of binding
both to the RPM as well as formation substrate minerals in the well.
The invention has particular applicability in those situations where the
formation permeability in the oil or gas well is between from about 0.1 to
about
8,000 md. Further, the formation substrate minerals may include quartz, clay,
shale, silt, chert, zeolite, or a combination thereof.
In the practice of this method, the composition is a water control
treatment fluid which may optionally be a stimulation fluid. The water control
treatment fluid may be introduced into the subterranean formation prior to,
together with, or following a hydraulic fracturing or stimulation fluid
treatment.
In one embodiment, the composition of the invention may be used to
contact the subterranean formation and substantially reduce permeability to
water within the formation without substantially reducing permeability to oil
within the formation. In another embodiment, the composition of the invention
may be used to contact the subterranean formation so that it has a post-
treatment resistance factor, for water of greater than or equal to about 5 and
a
post-treatment resistance factor for oil of less than 2, as measured across a
Berea core, such as about 2.5 cm diameter by about 4 cm long and having a
permeability to nitrogen of about 1000 md, each of the water and oil
resistance factors being measured at stable larriinar flow rate at constant
pressure.
Brief description of the drawings
In order to more fully understand the drawings referred to herein, a
brief description of each drawing is presented, in which.
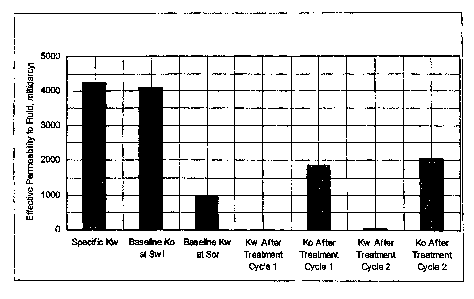
FIG. 1 illustrates results obtained by use of a water treatment fluid
containing 3% RPM and 0.5% of an aqueous solution containing
approximately 50% organosilicon compound in a high permeability Berea
core. As used herein all percentages are weight percentages unless
otherwise noted.
CA 02459672 2004-03-05
-5-
FIG. 2 illustrates results obtained by use of a water treatment fluid
containing 4% RPM and 0.3% of an aqueous solution containing
approximately 50% organosilicon compound in a high permeability Berea
core.
FIG. 3 illustrates results obtained by use of a water treatment fluid
containing 5% RPM and 0.5% of an aqueous solution containing
approximately 50% organosilicon compound in a high permeability Berea
core.
Each of the figures demonstrates that treatment of an oil or gas well
with the aqueous system of the invention significantly reduces water flow
relative to oil flow in very high permeability cores.
Detailed description of the invention
The aqueous compositions of the invention described herein may be
utilized in well treatment methods to selectively reduce the permeability of a
subterranean formation to water, while at the same time leaving the
permeability of the formation to oil virtually unchanged. In a preferred
embodiment, the post-treatment resistance factor for water is greater than or
equal to 5.0, preferably in excess of 9 or more. Furthermore, the disclosed
compositions, when introduced into a formation, tend to exhibit a high
resistance to removal from water bearing areas of the formation over time.
The aqueous compositions of the invention contain a relative
permeability modifier (RPM) and an organosilicon compound.
The RPM for use in the invention is any polymer that can impede the
production of water and which provides suitable attachment, such as grafting,
sites for the organosilicon compound. Most often the RPM is hydrophilic
having the ability to remain hydrated in thia formation waters and
simultaneously having an affinity to adsorb onto the solid formation material.
Such RPMs typically have weight average molecular weights ranging from
about 50,000 to about 20,000,000 g/mole, preferably from about 100,000 to
about 5,000,000 g/mole, most preferably from about 250,000 to about
2,000,000 g/mole.
CA 02459672 2008-04-14
6
In addition to the molecular weight, the RPMs must also have specific
sites that allow interaction with the organosilicon compound. Most often,
interaction of the RPM polymeric material and the silicon-containing organic
compound occurs with any oxygen containing pendent group on the polymeric
material, particularly the hydroxyl group. However, many of the silicon-based
agents are multifunctional having additional functional groups attached to the
silicon-based agent. In most cases, these additional groups are generally non-
oxygen-bearing groups, but could also interact with specific sites on the RPM.
The additional functional groups on the silicon-containing organic compound
include amines, isocyanates, amides, thio-based and phosphorus-based
groups. These additional functional groups can also interact with the specific
sites of the RPM. For example, amine functional groups on the silicon-
containing organic compound can interact with polymers having carboxylic
acid groups or aldehyde groups to form either amides or Schiff bases.
Another example is silicon-based agents having isocyanate or isothiocyanate
functional groups that can interact with amine- or alcohol-based RPMs to
produce urethane type linkages.
Any RPM that offers an attachment site for the organosilicon
compound will provide, to some degree, a favorable response to impede
water production and thus be sufficient as the RPM. Suitable RPMs include
those referenced in U.S. Patent Nos. 5,735,349; 6,169,058; and 6,228,812.
Suitable RPMs include copolymers of hydrophilic monomers and a
second monomer. Hydrophilic monomers may include both ionic and
nonionic monomers. The term "nonionic monomer' refers to monomers that
do not ionize in aqueous solution at neutral pH. In addition, an anionic
monomer, such as salts of acrylates, may be used in conjunction with a
cationic monomer. Examples of suitable nonionic hydrophillic monomers
include, but are not limited to acrylamide, (meth)acrylamide, N-vinyl
pyrrolidone, N-vinyl formamide and N-vinylacetamide. Ionic monomers may
be either anionic or cationic. Examples of anionic monomers include, but are
not limited to, alkaline salts of acrylic acid, ammonium or alkali salts of
CA 02459672 2004-03-05
-7-
acrylamidomethylpropane sulfonic acid ("AMPS"), acrylic acid, (meth)acrylic
acid, maleic acid, itaconic acid, styrene sulfonic acid, and vinyl sulfonic
acid
(or its ammonium or alkali metal salts). Examples of suitable cationic
monomers include, but are not limited to, dimethyldiallyl ammonium chloride
and quaternary ammonium salt derivatives from acrylamide or acrylic acid
such as acrylamidoethyltrimethyl ammonium chloride. Suitable as the second
monomer are N-vinylformamide, N-methylacetamide, N,N-diallylacetamide,
methylenebisacrylamide or a mixture thereof.
Preferred polymers applicable for use in the invention as the RPM
include homopolymers, copolymers and terpolymers based on acrylamide,
particularly those that are sulfonated or quaternized for solubility in high
saline
formation brines. In a preferred mode, such acrylamide copolymers may
contain other components such as acrylic acid or (meth)acrylic acid, or a salt
t h e r e o f , dimethyldiallylammonium chloride,
a c r y I a m i d o e t h y I t r i m e t h y I a m m o n i u m chIoride,
methacrylamidoethyltrimethylammonium chloride,
acrylamidomethylpropanesulfonic acid (AMPS), N-vinyl pyrrolidone, N-vinyl
formamide, N-vinyl acetamide, N-vinylmethylacetamide,
acrylamidoethyltrimethylammonium chloride, vinyl sulfonic acid, maleic acid,
itaconic acid, styrene sulfonic acid, vinylsulfonic acid,
methylenebisacrylamide
and vinylphosphonic acid and sulfonate monomers thereof.
RPMs may further include homopolymers or copolymers which include
the foilowing monomeric units: acrylic acid, (meth)acrylic acid,
dimethyldiallylammonium chloride as well as
a c r y I a m i d o e t h y I t r i m e t h y I a m m o n i u m chIoride,
methacryIamidoethyltrimethyIammci n i u m chloride,
acrylamidomethylpropanesulfonic acid (AMPS), N-vinyl pyrolidone, N-vinyl
formamide, N-vinyl acetamide, N-vinylmethylacetamide, acrylamido
ethyltrimethylammonium chloride, maleic acid, itaconic acid, styrene sulfonic
acid, vinylsulfonic acid and vinylphosphonic acid and sulfonate monomers,
i.e., those monomers containing SO3 pendant or functional groups and salts
thereof, such as those derived with sodium or potassium, or quaternary
CA 02459672 2004-03-05
-8-
ammonium salts. The chloride counter ion referenced above may also be
substituted, for example, with any other halogen, sulfate, or phosphate. Other
suitable monomeric units include dimethyldiallyl ammonium sulfate,
methacrylamido propyl trimethyl ammonium bromide, and methacrylmaido
propyl trimethyl ammonium bromide.
For example, in one embodiment of the invention, the RPM may
include at least one nonionic vinylamide monomer of the formula:
CH2 =C(R)--C(O)N(R')2 (1)
where R and R' independently represent a hydrogen, methyl, ethyl or propyl
moiety. In a second embodiment, the RPM may further include at least one
monomer containing anionic moieties of the formula:
CH2=CHC(O)X (II)
where X represents a moiety containing a carboxylic acid or salt of that acid
or
a moiety containing a salt of a sulfonic acid or the salt of a sulfuric acid.
Lastly, synthetic polymers based on vinyl acetate to produce
polyvinylalcohol (PVA) are also applicable as are polysiloxanes or silicones.
The most preferred polymers are PVA having degrees of hydrolysis between
from about 50% to about 100% and polyacrylamides as described in US
patent 6,228,812 B1 and 5,379,841.
In general the silicones are polymers containing the following units:
organo
~i~-
~
organo
of molecular weight sufficient to afford a viscosity suitable for use in well
treatment methods known to those of skill in the art. Generally, the
polysiloxanes for use as the RPM have a maximum molecular weight of about
20,000 to about 30,000 or an n value from 2 to about 500, though higher
molecular weights may be formed in situ.
CA 02459672 2004-03-05
-9-
Preferred polysiloxanes include polysiYoxane polyalkyl polyether
copolymers. The preferred organo group is a mixture of hydrocarbon such as
alkyl and alkoxide and most preferably being methyl and methoxide or
ethoxide. Inclusive of preferred polysiloxanes are those of the formula:
organo o3rgano organo
I I I .
+organo-ziit7 :;tO- -&-targano
orrgano orgm
r~
Suitable hydrophilic polymers further include natural gums such as
guar, carrageenan, gum Arabic, gum ghatti, karaya, tragacanth, pectin,
starch, locust bean gum, scieroglucan, tamarind and xanthan gums and any
chemically modified derivatives of these gums including derivatives of
cellulose such as the pendent derivatives hydroxyethyl, hydroxypropyl,
hydroxypropylcarboxymethyl, hydroxyethylcarboxymethyl, carboxymethyl or
methyl.
The organosilicon compounds for use in the aqueous compositions are
generally capable of binding both to the RPM as well as to formation substrate
minerals including quartz, clay, chert, shale, silt, zeolite or a combination
thereof. '
Suitable water-soluble organosilicon comipounds for the invention
include, without limitation, amino silanes such as 3-aminopropyltriethoxy
silane and N-2-aminoethyl-3-aminopropyltrimethoxy silane, and vinyl silane
compounds such as vinyl tris-(2-methoxyethoxy) silane. However, as
discussed by M. R. Rosen, "From Treating Solution to Filler Surface and
Beyond. The Life History of a Silane Coupling Agent," Journal of Coatings
Technology, Vol. 50, No. 644, pages 70-82 (1978), many organosilane
compounds are water-soluble for prolonged periods of time after they
hydrolyze to form silanols, and temperatures can serve to aid the hydrolysis.
For purposes of the present invention, then, compounds which form water-
soluble silanols by hydrolysis will be considered as equivalent to the
originally
CA 02459672 2004-03-05
-10-
water-soluble organosilicon compounds. Such organosilicon compounds
include organosilane halides and organosilane alkoxides.
Among the organosilanes especially suitable for use in this invention
are those organosilane halides of the formula:
R.I
I
R~-~Si~-.~
i
R3
wherein X is a halogen, R, is an organic radical having from 1 to 50 carbon
atoms, and R2 and R3 are the same or different halogens as X or organic
radicals of Ri. Preferably, X is a halogen selected from the group consisting
of chlorine, bromine and iodine with chlorine beirig preferred, R, is an
alkyl,
alkenyl, alkoxide or aryl group having from I to 18 carbon atoms and R2 and
R3 are the same or different halogens, or alkyl, alkenyl, alkoxide or aryl
group
having from 1 to 18 carbon atoms.
Suitable specific organosilane halides include
methyldiethylchlorosilane, dimethyldichlorosilane, methyltrichlorosilane,
dimethyldibromosilane, diethyldiiodosilane, dipropyidichlorosilane,
dipropyldibromosilane, butyltrichlorosilarie, phenyltribromosilane,
diphenyidichiorosilane, tolyltribromosilane, methylphenyldichlorosilane,
propyldimethoxychlorosilane and the like.
Among the organosilane alkoxides suitable for use in this invention are
those having the formula:
~
I
R5`Si-OR"7
I
R6
CA 02459672 2008-04-14
11
wherein R4, R5, and R6 are independently selected from hydrogen and
organic radicals having from 1 to 50 carbon atoms, provided not all of R4, R5,
and R6 are hydrogen, and R7 is an organic radical having from 1 to 50 carbon
atoms. Preferably, R4, R5, and R6 are independently selected from hydrogen,
amine, alkyl, alkenyl, aryl, and carbhydryloxy groups having from 1 to 18
carbon atoms, with at least one of the R4, R5, and R6 groups not being
hydrogen, and R7 is selected from amine, alkyl, alkenyl, and aryl groups
having from 1 to 18 carbon atoms. When R4, R5, and R6 are carbhydryloxy
groups, alkoxy groups are preferred.
Suitable specific organosilane alkoxides include methyltriethoxysilane,
d i methyid iethoxys i lane, methyltrimethoxysilane, divinyldimethoxysilane,
divinyldi-2-methoxyethoxy silane, di(3-glycidoxypropyl) dimethoxysilane,
vinyltriethoxysilane, vinyltris-2-methoxyethoxysilane, 3-
glycidoxypropyltrimethoxysilane, 3-methacryloxypropyltrimethoxysilane, 2-
(3,4-epoxycyclohexyl) ethyltrimethoxysilane, N-2-aminoethyl-3-
propylmethyidimethoxysilane, N-2-aminoethyl-3-propyltrimethoxysilane, N-2-
aminoethyl-3-aminopropyltrimethoxysilane, 3-aminopropyltriethoxysilane,
tetraethoxysilane and the like.
The presence of the amine function appears to result in a stronger
adsorption of the silane on the formation rock. The resultant polymer renders
the treated portion of the formation less oil wet than when a non-amine-
containing silane is employed. Thus, in subsequent production of oil through
the formation, less oil is retained by the formation and more of the oil is
produced.
For purposes of brevity and clarity, the terms "amine," "alkyl," "alkenyl,"
"aryl," and "carbhydryloxy" have been used above to describe substituents of
organosilanes and alkoxides of organosilanes which are useful in the practice
of the invention. It is to be understood that these substituents may
themselves
be substituted or unsubstituted and that each, except for aryl species, may be
branched or unbranched.
Such organosilicon compounds are disclosed in U.S. Patent No.
4,580,633 and 4,708,207.
CA 02459672 2004-03-05
-12-
The weight ratio of RPM macromolecule to organosilicon compound in
the aqueous composition is generally from about 3:200 to about 20:4. The
weight percentage of the RPM and organosilicon compound composite in the
aqueous composition is generally from about 0.01 to about 25 weight percent.
For instance, where the RPM macromolecule is PVA, the concentration ratio
in parts per million of PVA RPM macromolecule to silicon in the organosilicon
compound in the aqueous composition is generally from about 20,000:80 to
about 200,000:40,000, preferably from about 50,000:800 to about
100,000:4,000. The weight percentage of the PVA RPM and silicon in the
organosilicon compound composite in the aqueous composition is generally
from about 2.0% to 24.00%, preferably from 5.0% to 10.5%, weight
percentage. The concentration ratio in parts per miliion of polyacrylamide
RPM macromolecule to silicon in the organosilicori compound in the aqueous
composition is generally from about 100:80 to about 6,000:40,000, preferably
from about 900:800 to about 3,000:4,000. The weight percentage of the
polyacryiamide RPM and silicon in the organosilicon compound composite in
the aqueous composition is generally from about 0.02% to 4.60%, preferably
from 0.17% to 0.70%, weight percent.
In one embodiment, a subterranean formation may be treated using the
disclosed aqueous composition by introducing the aqueous composition of the
invention into the formation through a wellbore. Such a water control
treatment fluid may be formulated with the aqueous composition and an
aqueous base fluid. The weight percentage of aqueous composition being
the RPM macrornolecule and organosilane together in the composition of the
invention is generally about 0.01 to about 15.0 weight percent. As set forth
in
Example 1, the amount of combined RPM macroniolecule and organosilicon
compound in the aqueous composition of the invention may be between from
about 0.02% to about 4.60%, preferably from about 0.17% to 0.70%, weight
percentage.
With benefit of this disclosure, an aqueous base fluid may be any
aqueous-base fluid suitable for well treatments known in the art including,
but
not limited to, fresh water, acidified water having pH range from 1.0 to 3.0,
CA 02459672 2004-03-05
-13-
brine, sea water, synthetic brine (such as 2% KCI), produced formation water
etc.
If so desired, optional mutual solvents may also be used with the
aqueous composition of the invention. Mutual solvents, among other things,
may act to remove hydrocarbons adhering to formation material. In this
regard, any mutual solvent suitable for solubilizing hydrocarbons may be
employed including, but not limited to, terpenes (such as limonene), C3 to C9
alcohols, glycol-ether (such as ethylene glycol monobutyl ether, "EGMBE"), or
mixtures thereof.
It will be understood with benefit of the present disclosure that other
additives known in the art for use in stimulation aind well treatments may be
employed in the practice of the disclosed method if so desired. For example,
surfactants, thickeners, diversion agents, pH buffers, etc. may be used. In
one
embodiment, internal diverting materials may be employed if desired.
Examples of suitable diverting agents include, but are not limited to, viscous
water external emulsions, and are known to those of skill in the art. In one
embodiment, an aqueous composition may be added to a salt solution, such
as a 2% salt solution, wherein the salt is preferably potassium chloride.
The disclosed aqueous compositions may be used as the only
component in an aqueous water control treatment fluid or may be combined
with other components of stimulation fluid or other well treatment fluid (such
as hydraulic fracturing fluids, acid fluids, surfactant squeeze treatment
fluids,
etc.).
It will also be understood with benefit of this disclosure that the
disclosed aqueous composition may be mixed with an aqueous base fluid to
form a "spearhead" fluid to precede the introduction of a stimulation fluid or
other well treatment fluid. This may be done, for example, to achieve
diversion
of a stimulation fluid into hydrocarbon bearing areas of the formation by
virtue
of the copolymer's deleterious effect on permeability to water in water
bearing
areas of the formation. Alternatively, or additionally, the aqueous
composition
may follow such a well treatment fluid and/or be combined with the body of
such a well treatment fluid, or used in any combination thereof. In any case,
CA 02459672 2008-04-14
14
the introduction of the aqueous composition into a subterranean formation in
conjunction with a well treatment, such as a stimulation treatment, may be
used to advantageously place the composition in a position to reduce
production of water following the stimulation treatment. Examples of
procedural details for use of water control materials in conjunction with well
treatments may be found in U.S. Pat. No. 6,169,058.
Whether utilized as part of a stand-alone water control treatment fluid,
employed in conjunction with another type of well treatment such as a
stimulation treatment, or otherwise introduced into a well, the disclosed
aqueous composition may be present in any concentration suitable for
controlling water production in a subterranean formation. However, in one
embodiment, one or more of the disclosed RPMs and organosilicon
compounds are present in the treatment fluid at a total concentration of from
about 500 ppm to about 10,000 ppm polymer, alternatively from about 1000
ppm to about 5,000 ppm polymer, based on the total weight of the water
control treatment fluid.
To reduce injection pressures during injection of a well treatment fluid,
the potassium chloride may be added to the aqueous solution and the pH
reduced to a low value, for example to about 1, just prior to introduction of
the
treatment fluid into a wellbore. Using this optional procedure helps minimize
injection pressure and ensure the extent of penetration of the aqueous
composition into the formation. The pH of a well treatment fluid may be
lowered by the addition of any acidic material suitable for decreasing pH of
the fluid to less than about 3, and alternatively between about 1 and about 3.
Suitable acidic materials for this purpose include, but are not limited to,
hydrochloric acid, formic acid and acetic acid, etc. VVith benefit of this
disclosure, those of skill in the art will understand that addition of acidic
material and adjustment of pH may be varied as desired according to
treatment fluid characteristics and formation temperature conditions in order
to optimize polymer retention and water control.
CA 02459672 2004-03-05
-15-
The aqueous composition may be batch prepared or prepared by
continuous mix processes. For example, the water control treatment fluid may
be first prepared in total, and then injected or otherwise introduced into a
subterranean formation. This is referred to as a "batch mixing" process. In
another embodiment, a water control treatment fluid may be prepared by
continuous mix processes, wherein the treatmerit fluid components are mixed
together while the fluid is simultaneously introduced into the wellbore.
Once a treatment fluid is prepared (either by batch or continuous
mixing), the water control treatment fluid is introduced into the subterranean
formation in any amount suitable for contacting a portion of a reservoir
matrix
of flow pathways. By "introduced" it is meant that a fluid may be pumped,
injected, poured, released, displaced, spotted, circulated or otherwise placed
within a well, wellbore, and/or formation using any suitable manner known in
the art. In one embodiment, an amount of treatment fluid sufficient to treat
the
entire height of the producing interval having a radius of from about 3 to
about
10 foot from the wellbore may be employed, however greater or lesser
amounts are also possible.
When employed in conjunction with a non-fracture treatment water
control treatment fluid, introduction rates for either batch or continuous
mixed
water control treatment fluids are typically held below flow rates that would
cause pressures to exceed those necessary to fracture the formation being
treated. In this regard, flow rates may be adjusted during treatment fluid
introduction to ensure that pressures are maintained below those necessary
for fracturing.
When used in conjunction with well treatments such as stimulation
treatments, treatment fluid introduction flow rates typically depend on the
nature of the treatment being performed. For example, in the case of a matrix
acid treatment the disclosed copolymer compositions may be included in a
"spearhead" fluid ahead of the acid treatment, in the acid treatment, or
following the acid treatment (or in any combination of steps before, in, or
after
the acid treatment), and are typically introduced at a rate below the flow
rate
necessary to fracture the formation in a manner similar to the rate employed
CA 02459672 2004-03-05
-16-
for a water control treatment fluid injected alone. When used in conjunction
with a hydraulic fracture treatment, fluid introduction rates (whether
utilized as
a spearhead, in the fracture treatment fluid, or both) are typically above
rates
that cause pressures to exceed those necessary to fracture a formation.
Whether employed as a stand-alone fluid or in a stimulation fluid (such as an
acid fluid or hydraulic fracture fluid), similar concentrations of copolymer
compositions are typically employed.
In one water control treatment embodiment for treating a subterranean
formation in a production well, the well may be shut-in from about 6 to about
48 hours after introduction of a water control treatment fluid in order to
allow
maximum anchoring and retention of the aqueous composition. Following
such a shut-in period, the well may be placed back on production. In another
water control treatment embodiment for treating an injection well, a water
control treatment fluid may be injected in a mariner similar to that described
for treatment of a production well, with the exception that the injection well
is
not typically shut-in after injecting the treatment fluid, but is instead
placed
back on injection immediately. In this embodiment, the aqueous composition
is expected to ultimately improve the water sweep efficiency in the reservoir
by reducing water channeling from the injector to surrounding producing wells.
Such a condition may be the case, for example, in injection wells where water
channeiing is suspected to be occurring through high permeability streaks in
the formation strata penetrated by the injection well.
With benefit of the present disclosure, it will be understood that the
disclosed aqueous composition when placed in a subterranean formation may
induce an artificial pressure barrier and, in the case of the treatment of
vertical
coning problems, may be placed beyond the wellbore to an area beyond that
influenced by the critical draw down pressure responsible for vertical water
migration.
Although the disclosed method and compositions may be employed as
a water control treatment at any time in the producing life of a production
well
or the injection life of an injection well, it may be desirable to perform
such
treatment as soon as a coning or channeling problem (or potential coning or
CA 02459672 2004-03-05
-17-
channeling problem) is identified, rather than waiting to the point where
coning
or channeling becomes severe.
In a preferred embodiment, permeability to water in a subterranean
formation may advantageously be reduced without substantially reducing
permeability to oil in the formation. In this regard, the measure of reduction
of
permeability of a subterranean formation to a given fluid may be expressed as
the resistance factor, Rf. For example, the quotient of permeability to water
at
irreducible oil saturation prior to treatment (Kw;) ito the permeability to
water at
irreducible oil saturation after treatment (Kwf) is defined herein as the
resistance factor, Rf for water. In this regardõ the disclosed methods and
compositions are capable of achieving a water resistance factor, Rf, of
greater
than or equal to about 5, preferably greater thari 8 or 9, measured at laminar
flow rates of about 0.05 to 10.0 ml/min across a 2.5 cm diameter core.
Similarly, the quotient of permeability to oil at irreducible water
saturation before treatment (Kfl;) to permeability to oil at irreducible water
saturation after treatment (Kof) is defined herein as the resistance factor,
Rf ,
for oil. Advantageously, the disclosed method and compositions may be used
to obtain an oil resistance factor, Rf, of from about I to about 2,
alternatively
from about 1 to about 1.5, and alternatively of less than about 2 at flow
rates
of about 0.05 to 6.0 ml/min across a 2.5 cm diameter core, at the same time
the above-described water resistance factors are achieved.
Use of the aqueous compositions of the invention is applicable in high
permeability producing wells previously not considered by RPM-containing
compositions of the prior art. In a preferred embodiment, the aqueous
compositions of the invention are used with a systematic approach consisting
of proper pre-flushes and post-flushes. In a preferred embodiment, wells to
be treated are produced from multi-layered sandstone formations with one or
more layers that are still saturated with hydrocarbon. Otherwise distinct
water
and hydrocarbon production within the production interval(s) is desirable.
Preferably, no cross-flow between layers exists.
The aqueous compositions of the inventiori have particular applicability
in those instances where the formation permeability is between from about 0.1
CA 02459672 2004-03-05
-18-
to about 8,000 md. In high permeability (>1 to 1.5 Darcy) formations,
optimum treatment results have been obtainedõ Core flow test results show
effectiveness at a permeability as high as 7.0 Darcy under high rate flow
conditions.
If the RPM treatment is placed in homogeneous zones producing both
water and hydrocarbon (fractional flow), both water and hydrocarbon
permeability may be decreased significantly. Ideally, resistance to water flow
will substantially exceed resistance to hydrocarbon (oil or gas) flow,
following
RPM treatment.
RPM treatment is ideally designed for radial penetration of 10 ft.
However, as a practical matter, adequate treatment design may be for radial
penetration of 5 to 8 ft. The RPM treatment can be bullheaded. However, it
may be preferable to place the treatment through coiled tubing, especially in
longer intervals.
The following examples will illustrate the practice of the present
invention in its preferred embodiment. Other ernbodiments within the scope
of the claims herein will be apparent to one skilled in the art from
consideration of the specification and practice of the invention as disclosed
herein. It is intended that the specification, together with the example, be
considered exemplary only, with the scope and spirit of the invention being
indicated by the claims which follow.
EXAMPLES
The Examples illustrate that the compositions of the invention are
highly effective in sandstone formations having absolute permeabilities to
brine of 1.5 to 7.0 Darcy in that water flow relative to oil flow is
significantly
reduced in such high permeability sandstone cores. Unless specified to the
contrary, all percentages herein refer to weight percentages.
Example 1.
The treatment fluid used in this test contains an RPM macromolecule
concentrate containing 15% N-vinylformamide, 30% AMPS, 54.9% acrylamide
CA 02459672 2004-03-05
-19-
and at most, about 0.1 % methylenebisacrylamide packaged as a 3% polymer
solution in 1% sodium chloride and the polymer has a Fikentcher K value of
about 250 prior to the addition of salt. The treating fluid was prepared by
diluting 3% (wt) of the RPM macromolecule concentrate in 2% aqueous
potassium chloride and adding 0.5% (wt) orqanosilane binding agent, 3-
aminopropyltriethoxysilane. The polymer solution is mixed well prior to use in
the test.
Core flow tests were conducted on IBerea core plug cylinders,
measuring I in diameter and 3 inches in length, having N2 permeabilities of
1000 md. The core plugs were evacuated with air and then saturated with 2%
aqueous solution of potassium chloride (KCI). The core was then installed in
a core holder. Approximately 200 psi back pressure was applied at the exit
end and approximately 1,000 psi confining stress (overburden pressure) was
applied around the entire cylinder. The confinirig stress pressure simulates
stress in the downhole formation. After these pressures are applied and set,
the temperature was elevated to 150 F. (simulation of the reservoir
temperature). Sequential flows of water and oil were injected through the
core as discussed in the paragraph below. The water composition was 2%
KC1 and the oil was a 50% (wt.) White Mineral Oil in lsopar LTM (Exxon). Each
fluid was injected and pumped at a constant rate of between 0.3 ml/min to 5
mI/min. while measuring pressure drop along the length of the core. After
obtaining a stable pressure differential, permeabilities were calculated using
Darcy's equation for laminar flow through a cylindrical core:
k = Q=m-UDP=A
where
k permeability to liquid, Darcies
Q rate of flow, mi/sec
A = Area, cm2
m = viscosity, centipoises
L = length, cm
DP=pressure differential, atm.
CA 02459672 2004-03-05
-20-
The water composition was first injected in a production direction. This
simulates the production of water from the formation into the wellbore. The
specific permeability to water (brine), kw, md (absolute), is tabulated in
Column III of Table I. After introduction of the White Mineral Oil in Isopar L
solution, the effective permeability of the oil at residual oil saturation was
then
calculated, represented as ko, md (before) in Ccilumn IV of Table I. The water
composition was then injected in the production direction. Effective
permeability to water at residual oil saturation, represented as kw, md
(before)
in Column V of Table I, was then calculated. This is somewhat lower than first
water measurement solution since this simulates water flowing through a
previously oil saturated formation. Approximately 10 pore volumes of the
treatment fluid (the solution of Example 1 in the water composition) was then
injected in the reverse (injection) direction at a constant rate of 1 ml/min.
(This
simulates injection from the wellbore perforation into the formation.) A pore
volume is the volume of fluid that the core can hold at complete fluid
saturation. Shut-in of the treatment fluid at test temperature and confining
pressure was allowed to occur for the designated shut-in period. The water
solution was then injected in the production direction at a constant rate of 1
ml/min while collecting produced fluids and monitoring differential pressure.
Flow was continued until a stable differential pressure was obtained. The
effective permeability to water following treatment was then calculated,
represented as kw, md (after) in Column 6 of Table I. ISOPAR-L was then
injected in the production direction at a constant rate of <10 ml/min. The
effective permeability to oil following treatment, represented as ko, md
(after)
in Column VI of Table 1 was then calculated.
Specific core flow tests were conducted ori the water completion fluids
of Example 1 at various levels of permeability, as high as 7.0 Darcy, under
high rate flow conditions as follows:
The water composition was first injected in a production direction. This
simulates the production of water from the formation into the wellbore. The
specific permeability to water (brine), kw, md (absolute), is tabulated in
Column III of Table I. After introduction of the VWhite Mineral Oil in Isopar
L
CA 02459672 2004-03-05
-21-
solution, the effective permeability of the oil at residual oil saturation was
then
calculated, represented as ko, md (before) in Column IV of Table I. The water
composition was then injected in the production direction. Effective
permeability to water at residual oil saturation, represented as kw, md
(before)
in Column V of Table l, was then calculated. This is somewhat lower than first
water measurement solution since this simulates water flowing through a
previously oil saturated formation. Approximately 10 pore volumes of the
treatment fluid (the solution of Example 1 in the water composition) was then
injected in the reverse (injection) direction at a constant rate of 1 mI/min.
(This
simulates injection from the wellbore perforation into the formation.) A pore
volume is the volume of fluid that the core can hold at complete fluid
saturation. Shut-in of the treatment fluid at test temperature and confining
pressure was allowed to occur for the designated shut-in period. The water
solution was then injected in the production direction at a constant rate of 1
ml/min while collecting produced fluids and monitoring differential pressure.
Flow was continued until a stable differential pressure was obtained. The
effective permeability to water following treatment was then calculated,
represented as kw, md (after) in Column 6 of T'able I. ISOPAR-L was then
injected in the production direction at a constant rate of <10 ml/min. The
effective permeability to oil following treatment, represented as ko, md
(after)
in Column VI of Table I was then calculated.
Specific core flow tests were conducted on the water completion fluids
of Example 1 at various levels of permeability, as high as 7.0 Darcy, under
high rate flow conditions as follows:
Run 1(Comparative). 6% RPM macromolecule concentrate in the
treating fluid (without organosilane agent) - 4.5 D core
Run 2: 6% RPM macromolecule concentrate in the treating fluid
with organosilane - 1.5 D core
Run 3: 6% RPM macromolecule concentrate in the treating fluid
with organosilane - 1.7 D core
Run 4: 6% RPM macromolecule concentrate in the
treating fluid with organosilane - 7.0 D core
CA 02459672 2004-03-05
-22-
Run 5: 6% RPM macromolecule concentrate in the treating fluid
with organosilane - 5.0 D core
The test results are summarized in Table 1. To screen effectiveness in
reducing relative permeability to water only, oil permeabilities were not
measured in Runs 1-4. Once the effectiveness of the Water Control treating
fluid system was determined, the effect on oil permeability was measured
with Run 5. Post-treatment shut-in time, concentrations of the Water
Completion Fluid and effects of buffering the system (from pH > 9 to between
7 and 8) were also varied to maximize treatment effectiveness in reducing
permeability to water while maintaining adequate oil permeability.
Table I
1. 11. Ill. IV. V. vi. Vii. Vllf.
Run Fluid kw, md ko, kw, kw, ko, Rfw I RTo
No. Tested (absolu md md md md
te) (befor (befor (after) (after)
e) e)
1 6% RPM 4480 --- 4480 1014 - 4.4 /-
Concentrate
2 6% RPM 1500 --- 1500 80 ---- 18.8/--
Concentrate and
Organosilane
3(l) 6% RPM 1700 ---- 1700 180 ---- 9.4 /-
Concentrate and
Organosilane
4 6% RPM 7000 4500 870 151 2255 5.8 / 2.0
Concentrate and
Organosilane
5(2) 6% RPM 4953 ---- 4953 170 ---- 29.1 /--
Concentrate and
Organosilane
(buffered)
1. Permeability = 400 md after 24-hour shut-in; reduced to 180 md after 72
hours.
2. Permeability = 170 md after 72-hour shut-in; increased to 225 md after 84
hours.
All Runs were conducted at 150 F and post-treatment shut-in time of 72 hours
- unless noted.
CA 02459672 2004-03-05
-23-
System pH buffered to 7.4 (to increase long-term treatment solution
stability).
Runs 1 through 5 were evaluations of 6% Water Control treating Fluid
containing 1% by volume of the organosilane , 3-aminopropyltriethoxysilane
solution). Tests resulted in no less than 76% reduction in permeability to
water in any case. Run 4 illustrates the effect of Water Completion Fluid on
the relative permeability to oil, as well as to water. After a 72-hour shut-in
period, an 83% reduction in permeability to water was followed by a 50%
return permeability to oil. Return oil permeability reached a point at which
the
value seemed to stabilize and the test was haNted. Continuation of the flow
stage might have resulted in even higher return values.
In Run 4, the oil flow stage permeability continued to increase with time
until it reached a point where the values seemed to stabilize. Continuing oil
flow might have possibly increased the value over a longer time period.
Reduction in oil permeability was similar to the reduction in brine
permeability,
indicating excess binding agent relative to Water Control treating Fluid
concentration in this specific test case. Initial oil permeability was nearly
twice
the absolute brine permeability - anomalous among the Berea cores used by
both laboratories in this study. Typically, initial oil permeability is less
than
absolute brine permeability.
Run 5 was also performed using a buffered Water Control treating
Fluid system. A low pH buffer was used to reduce the final pH of the system
from over 9 to between 7 and 8. Lowering the pH of the system resulted in a
higher reduction in permeability to water when comparing test results where
core permeability, treatment concentration and shut-in time were relatively
similar. Runs 4 and 5 were used for this comparison. Absolute permeabilities
were 7000 and 4953 md respectively. After the 72-hour shut-in time, the
buffered system (Run 5) resulted in a 97% reduction in permeability to water
compared to 83% for the non-buffered system.
In each case, permeabilities to water were measured, and the
resistance factor to water was calculated. In those tests in which oil pre-
and
CA 02459672 2008-04-14
24
post-treatment oil permeabilities were measured, resistance factor to oil was
also calculated. Resistance factors (Rf) are calculated as follows:
Rf Water = kw (BT) T kw (AT)
Rf Oil = ko (BT) T ko (AT)
where kw = permeability to water (brine) at residual oil saturation
ko = permeability to oil at residual water (brine) saturation
BT = Before Treatment with RPM
AT = After Treatment with RPM
High Rf Water (> 5-10) relative to Rf Oil (< 2-2.5) is desirable. If water
permeability is completely shut off (kw (AT) = 0), then Rf Water =V.
Results of these tests indicates that the Water Treatment Fluid of Example 1
effectively reduced relative permeability to water in high permeability
sandstones.
Example 2.
Core flow tests were conducted on Berea core plug cylinders,
measuring 1 in diameter and 3 inches in length, having N2 permeabilities of
1000 md. The core plugs were evacuated with air and then saturated with a
simulated formation brine comprising a mixture of 2% potassium chloride
(KCI), 5% sodium chloride (NaCI) and 1% calcium chloride (CaC12) Each
sample was installed in a specially designed core holder, with a pressure tap
at 1 inch from the injection face, which was located in an air bath oven. In
addition, approximately 200 psi back pressure was applied at the exit end and
approximately 1,000 psi confining stress (overburden pressure) was applied
around the entire cylinder. The temperature was then elevated to 150 F. and
the test brine was then injected in the production direction at a constant
rate
(<10 mI/min) while the produced fluids were collected and differential
pressure
versus time was monitored. Specific permeability to brine was then calculated
for each section of the core (Section 1 being one inch penetration and Section
2 being the remainder of the core). An oil blend of a 50:50 weight mixture of
ISOPAR-L:Chevron Superla White Oil was then injected in the production
direction at stepwise increasing rates while produced fluids were collected
CA 02459672 2004-03-05
-25-
and differential pressure was monitored until an equilibrium permeability was
established at each rate level. Effective permeability to oil at initial water
saturation versus injection pressure data was calculated for each section of
the core. Test brine was then injected in the production direction at stepwise
increasing rates while collecting produced fluids and monitoring differential
pressure and elapsed time until an equilibrium permeability was established at
each rate level. Effective permeability to water at residual oil saturation
versus injection pressure data was calculated for each section of the core.
Approximateiy 10 pore volumes of the treatment fluid of Example 1 was
injected in the injection direction at a constant rate of 0.3 mllmin while
produced fluids were collected and differential pressure was monitored. The
sample was then shut in with the treatment fluid in place for 24 hours. The
test
brine was then injected in the production direction at stepwise increasing
rates
while the produced fluids were collected and differential pressure was
monitored until an equilibrium permeability was established at each rate
level.
Effective permeability to water at residual oil saturation versus injection
pressure data was calculated for each section of the core. An oil blend of
50:50 weight mixture of ISOPAR-L:Chevron Superla White Oil was then
injected in the production direction at stepvvise increasing rates while
produced fluids were collected and differential pressure was monitored until
an equilibrium permeability was established at each rate level. Effective
permeability to oil at initial water saturation versus injection pressure data
were calculated for each section of the core. Additional test brine was then
injected in the production direction, differential pressure monitored,
effective
permeability calculated and oil blend injected in the production direction and
effective permeability to oil at initial water saturation versus injection
pressure
data was calculated for each section of the core. Return permeability to water
and oil data for each section for each cycle was ttien calculated.
Results of these core flow screening tests indicated that the
composition of the invention effectively reduced relative permeability to
water
in high permeability sandstones. The results confirm that the Water Control
treating Fluid of the invention was most effective under the test conditions
of
CA 02459672 2004-03-05
-26-
1500 F and over 2-3 Darcy permeability. Treatment effectiveness was
sufficiently retained as flow differential pressure was increased -
unprecedented in cores with greater than Darcy permeability.
Three tests with high permeability Berea cores were undertaken.
Results are summarized in FIGs. 1, 2 and 3. In each test, permeabilities to
oil
and water were measured in two core sections. The first section (wellbore)
was 1" penetration distance, and the second section (formation) was the
remaining core length. Cores were typically about 4" long. Permeabilities
were measured at stepwise increasing rates. Core section permeabilities
were measured at rates corresponding to 30-40 psi/ft. Core section
permeabilities measured at the highest rate for each flow step are graphically
reported.
Under these tests, the Water Control treating Fluid system of Example
1 did not contain buffer to reduce pH to between 7 and 8. Such buffer may
increase effectiveness further, as indicated in Example 2.
Test 1. In Test 1, a low concentration Water Completion Fluid system of
Example 1 was evaluated (containing 3% Water Control treating Fluid, 0.5%
organosilane, 3-aminopropyltriethoxysilane solution in aqueous 2% KCi
solution . The treatment was effective in significantly decreasing the
relative
permeability to water in both sections of the Berea core - nearly completely
shutting off water flow. The substantial reduction in permeability was
retained
during the second cycle injection, indicating binding agent effectiveness. The
effective permeability to oil was reduced to about 40-45% of the original
permeability. This translates to an Rf Oil value of about 1.7. Flow
performance following the second oil cycle was similar; also showing an
increase in oil permeability. The results are reported in FIG. 1.
Test 2. In Test 2, a 4% Water Control treating Fluid of Example 1 containing
approximately 0.3% of organosilane, 3-aminopropyltriethoxysilane solution
was employed. The treatment did not reduce relative permeability to water in
the sections of the core (overall 41 percent of initial value) - apparently
due to
the reduced level of binding agent used. However, this degree of permeability
reduction (Rf Water -2.5) was retained during the second cycle injection -
CA 02459672 2004-03-05
-27-
which would not be expected with Water Control treating Fluid alone at this
high permeability level. The effective permeability to oil was reduced, but to
acceptable levels of 63.4 percent and 66.1 percent initial value overall after
cycles 1 and 2, respectively (Rf Oil -1.5).
Test 3. In Test 3, a 5% Water Control treating Fluid system of
Example 1 was tested. The binding agent concentration was increased to the
level used in Test 1, following the results of Test 2. The 5% Water Control
treating Fluid treatment reduced relative permeability to water significantly
(overall 17.5 percent of initial value). More importantly, the degree of
reduction in brine permeability was retained during the second cycle
injection.
The effective permeability to oil was only modestly reduced in this case (to
74.8 percent of initial value in both flow cycles).
In summary, the Water Control treating Fluid of the invention is
effective in reducing water flow relative to oil in very high permeability
sandstone (>1.5 Darcy) - extending the previous estimated practical
permeability application range of Water Control treating Fluids without
organosilanes.
Results of flow testing in high permeability Berea cores indicate that
treatment with the inventive Water Completicin Fluid system significantly
reduced water flow relative to oil flow - and niaintained effectiveness with
repeated flow cycles - indicating binding agent effectiveness.
From the foregoing, it will be observed that niumerous variations and
modifications may be effected without departing from the true spirit and scope
of the novel concepts of the invention.