Note: Descriptions are shown in the official language in which they were submitted.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
Substituted alkylaminopyridazinone derivatives, process for
the preparation thereof and pharmaceutical composition
containing the same
Field of the invention
The. invention relates to substituted alkylaminopyridazinone
derivatives, process for the preparation thereof and
pharmaceutical composition containing the same.
Background of the invention
Anxiety is a major CNS symptom accompanied by many
psychiatric disorders, medical and surgical conditions and stress
situations. Benzodiazepines such as diazepam,
chlordiazepoxide, and alprazolam etc. are the most commonly
used agents in the various ariXiety=disorders=However= sedative=
and amnestic side effects are a major disadvantage of these
drugs especially in disorders affecting active, working
populations. Moreover, withdrawal symptoms may occur
following suspension of benzodiazepine administration after
long term therapy. Therefore, finding of an effective
anxiolytic/antistress compound without such undesirable side
effects, low addictive potential and good safety features still
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
2
remains one of the most challenging aims of CNS
pharmacology research these days.
Piperazinylalkylamino-3(2H)-pyridazinone derivatives having
blood pressure lowering effect and being suitable for the
treatment of heart failure and peripheral circulatory disturbances
are known from EP-A No. 372 305.
Summary of the invention
The object of the invention is to provide new substituted
alkylaminopyridazinone derivatives having useful
pharmaceutical properties and free of disturbing side-effects.
The above object is achieved with the aid of the present
invention.
According to the present invention there are provided new
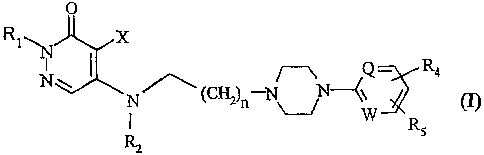
alkylaminopyridazinone derivatives of the general Formula
O
R1~N X
N w ~ /~ Q - R4
N ~ ~C~.)n N~ W (I~
Rs
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
3
(wherein
Rl is hydrogen or alkyl having 1-4 carbon atoms;
X is hydrogen or halogen;
R2 is hydrogen or alkyl having 1-4 carbon atoms;
n is 1, 2 or 3;
Q and W independently from each other stand for N= or -CH=;
and
R4 and RS independently from each other represent hydrogen,
halogen, trifluoromethyl or alkoxy having 1-4 carbon atoms)
and pharmaceutically acceptable acid addition salts thereof.
Description of the preferred embodiments
A preferable subgroup of the invention compounds of the
general Formula I are those derivatives, in which
Rl is hydrogen;
X-is -Hydrogen;-
n is 1;
Q and W independently from each other stand for N= or -CH=;
and
R4 and RS independently from each other represent hydrogen,
chlorine, fluorine, trifluoromethyl or methoxy
and pharmaceutically acceptable acid addition salts thereof.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
4
The following compounds of the general Formula I possess
particularly preferable pharmaceutical properties:
5- { 2- [4-(methoxytrifluoromethylphenyl)-pip erazine-1-yl]-
ethylamino}-2H pyridazine-3-one;
5-{2-[4-(2-fluorophenyl)piperazine-Z-yl]ethyl-amino}-2H
pyridazine-3-one;
5-{2-[4-phenylpiperazine-1-yl]ethylamino}-2H pyridazine-3-
one;
5-[2-(4-pyridin-2-ylpiperazine-1-yl)ethylamino]-2H pyridazine-
3-one;
5-[2-(4-pyrimidin-2-ylpiperazine-1-yl)ethylamino]-ZH
pyridazine-3-one;
5-{2-[4-(3-chlorophenyl)piperazine-1-yl]ethyl-amino}-2H-
pyridazine-3-one;
5- { 2- [4-(4-fluorophenyl)piperazine-1-yl] ethyl-amino }-2H-
pyridazine-3-one
and-pharW aceutically acceptabh acid-addition-salts.-thereof
The definition of the terms used throughout the body of the
specification and the claims is as folloWS:
The term "halogen" means the fluorine, chlorine, bromine and
iodine atoms, and is preferably chlorine.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
The term "alkyl group having I-4 carbon atoms" relates to
straight or branched chained alkyl groups, e.g. methyl, ethyl, n-
propyl, isopropyl, n-butyl, sec-butyl, isobutyl or tent-butyl etc.
The term "alkoxy" relates to straight or branched chained
alkoxy groups, e.g. methoxy, ethoxy, isopropoxy or n-buthoxy,
preferably methoxy.
The term "leaving group" relates to halogen (e.g. chlorine,
bromine) or alkylsulfonyloxy groups (e.g. methylsulfonyloxy)
or arylsulfonyloxy groups (e.g. benzylsulfonyloxy, p-toluene-
sulfonyloxy).
The term "pharmaceutically suitable acid addition salt" of the
substituted alkylaminopyridazinone derivatives of the general
Formula I, relates to non-toxic acid addition salts of the
compounds-formed with=inorganic-acids-suchas--hydrochloric-
acid, hydrobromic acid, sulfuric acid, phosphoric acid etc. or
organic acids such as formic acid, acetic acid, malefic acid,
fumaric acid, lactic acid, tartaric acid, succinic acid, citric acid,
benzenesulfonic acid, p-toluenesulfonic acid, methanesulfonic
acid etc.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
6
According to a further feature of the present invention there is
provided a process for the preparation of compounds of the
general Formula I
(wherein
Rl is hydrogen or alkyl having 1-4 carbon atoms;
X is hydrogen or halogen;
R2 is hydrogen or alkyl having 1-4 carbon atoms;
n is l, 2 or 3;
Q and W independently from each other stand for N= or -CH=;
and
R4 and RS independently from each other represent hydrogen,
halogen, trifluoromethyl or alkoxy having 1-4 carbon atoms)
and pharmaceutically acceptable acid addition salts thereof
which comprises
a) reacting a compound of the general Formula
O
Rl\ X
N
N w N~(CH2) ~ L 1
n
Rz
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
7
(wherein
L1 is leaving group and Rl, R2, X and n are as stated above)
with an amine of the general Formula
Q _ R4
(III)
W R
s
(wherein Q, W, R4 and RS are as stated above); or
b) reacting a compound of the general Formula
O
Rl\ X
Ij I (IV)
N~
Y
(wherein Y is halogen and R1 and X are as stated above) with a
compound of the general Formula
Q _ Rø
~ ~ (CH2~N~ W
W Rs
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
g
(wherein R2, n, Q, W, R4 and RS are as stated above);
and, if desired, subjecting a compound of the general Formula I,
wherein X stands for halogen, to catalytic dehalogenation to
obtain a compound of the general Formula I or its hydrochloride
salt, wherein X is hydrogen; and/or
converting a compound of the general Formula thus obtained
into a pharmaceutically acceptable acid addition salt thereof or
setting free a compound of the general Formula I from an acid
addition salt.
In case of processes (a) and (b) of the invention, the reactions
are carried out in a manner similar to the known analogous
processes, see e.g. March, J.: Advanced Organic Chemistry,
Reactions, Mechanism and Structure, 4~' Edition, John Wiley
and S ons, New York, 1992.
Wlieri-a=substituted=alkylaminapyridazinone derivative=of the=
Formula I, wherein X stands for halogen, preferably chlorine, is
subjected to catalytic hydrogenation, then dehalogenation
proceeds and the corresponding substituted
alkylaminopyridazinone derivative of the Formula I or its
hydrochloride salt, wherein X stands for a hydrogen atom is
formed.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
9
The catalytic hydrogenation is carried out in an analogous
manner as the processes described in the literature [e.g. March,
i
J.: Advanced Organic Chemistry, Reactions,, Mechanism and
Structure, 4~' Edition, John Wiley and Sons, New York, 1992].
As the hydrogen source, for example, hydrogen gas, hydrazine,
hydrazine hydrate, formic acid, a trialkylammonium formate or
an alkali metal formate can be used. The catalyst is suitably
palladium, platinum oxide, Raney nickel etc. The reaction can
be performed in the presence or absence of an acid binding
agent. As the acid binding agent, an inorganic base such as
sodium hydroxide or an organic base such as hydrazine, triethyl
amine, diisopropyl ethyl amine etc. can be used. The reaction
can be carried out in an indifferent erotic or aprotic solvent or a
mixture thereof. The erotic solvent is, for example, an alkanol,
water or mixtures thereof, the aprotic solvent is suitably
dioxane, tetrahydrofurane or dichloromethane. In general, the
reaction terizperature is=0=150°C~ preferabl-y 20--1=00-°C-:
The preparation of the acid addition salt from the free base of
the general Formula I and the liberation of the base from the
acid addition salt are carried out in a manner known peg se.
The alkylaminopyridazinone derivatives of the general Formula
II used as the starting compounds can be prepared by the
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
process described in the International Patent Application No.
PCT/HU98/00054; (WO 99/64402) .
The amines of the general Formula III are partly known
compounds. The novel ones can be prepared in an analogous
way [Pollaxd et al., J.Am.Chem.Soc., 56, 2199 (1934)].
The dihalopyridazinone derivatives of the general Formula IV
are partly known. The novel compounds can be prepared by
using the methods known from the literature [Homer et al., J.
Chem. Soc., 1948, 2194].
The compounds of the general Formula V can be prepared from
the corresponding amine of the general Formula III in a manner
known per se [Shigenaga, S. et al., Arch. Pharm., 329(I), 3-10
(1996); Janssens, F. et al., J. Med. Chem., 28(12), 1934-1943
(I985)He xiao Shu--et a~l:;~ioorg:-lVled:-Chem:--Lett:,--7(l=8),-
2399-2402 (1997)].
The pharmacological effect of the substituted
alkylaminopyridazinone derivatives of the general Formula I
was studied in the following test.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
11
Methods and Results
Effects on blood pressure
The experiments were performed in conscious, freely moving,
male Wistar rats using a radiotelemetry system (Data Sciences
International, St. Paul, Minnesota, USA). Prior to treatments
rats were implanted with transmitters (type: TL11M2-C50-
PXT) that permitted continuos monitoring of arterial blood
pressure. Under sterile surgical conditions, catheter of the
transmitter was introduced into the abdominal aorta for
measurement of arterial blood pressure and the transmitter was
sutured to the abdominal wall of animals anaesthetised with
pentobarbital-Na (60 mg/kg, i.p.; Nembutal inj. Phylaxia-
Sanofi, Budapest). After surgery the animals were treated with
an antibiotic (1 ml/kg i.m. Tardomyocel comp. inj. ad us. vet.,
B ayes =AGE =Lever-kusen-,--Germany).=-A=7-day-postop erative-
recovery period was allowed. Radio signals emitted by the
transmitters were detected by RLA1000 type receivers placed
under each animal's cage. The data were collected, saved and
evaluated using the Dataquest IV. software from Data Sciences.
The computer was set to sample the parameters for 10 seconds
in every second minute.
The test substances or vehicle (methyl cellulose 0.4% w/v) were
administered orally by gavage in a volume of 1 ml/kg at about
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
12
a.m. The effects of test items were measured for 6 hours.
The effect of each compound was compared with that caused by
vehicle treatment using two-way analysis of variance for
repeated measures with Scheffe's post hoc test.
Data obtained are shown in the Table 1. None of the compounds
examined reduced blood pressure of the test animals.
Table 1
Effects of different test items or vehicle on mean arterial blood
pressure for 6 hours after treatment in conscious rats
Example Mean Results
blood of
pressure
after after statistical
treatment treatment evaluation
with with
placebo test
compound
mmH mmH
_ __ Mean S.E. Mean .E.
_ ,_ S
__
7 92.6 3.3 92.7 3.2 N.S.
S.E. = Standard Error (of the mean); N.S. = Not significant
statistically (when compared to placebo)
According to data presented here the compounds of present
invention have no effect on blood pressure, indicating the lack
of antihypertensive potential.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
13
Anxiolytic effect
l~ogel lick conflict
Experiments were performed in a PC operated system
(LIIK.OSYS, Experimetria, Hungary) consisted of 8 test
chambers (20 cm x 20 cm x 20 cm Plexiglas boxes) each of
which was equipped with a water fountain system mounted at
appropriate height on the wall of the chamber and metal grid
floor for delivering electric shocks. 160-180 g male Wistar rats
(N=8) were deprived of drinking water 48 h and fasted for 24 h
prior to test. Test and reference compounds or vehicle were
administered ihtrape~itoneally, 30 min prior to test. All
procedures were carried out in a quiet, air-conditioned room
between 07:30 and 13:00 h at an ambient temperature of 23 ~2
oC. At the beginning of the test the animals were placed in the
test chamber where they had free access to drinking water for a
30 s grace period. After that, electric shocks (600 ~,A, 0.6 s)
were=applied=through=the-drinking--spout:_following =every-20=
licks during the 5 min test period (Vogel et al, 1971). Number
of punished licks were recorded and stored by an IBM
compatible computer. Means ~ SEM of numbers of tolerated
shocks were calculated in each group, statistical analysis of data
was performed by one way~ANOVA followed by Duncan's test
(STATISTICA). The results obtained are shown in Table 2.
Diazepam [7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-
benzodiazepine-2-one] was used as the reference substance.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
14
Table 2
Vogel's drinking conflict test
Compound (Example No.) MED in mg/kg ip.
1 20.0
2 20.0
..~._.._.___,._~_...__..._._._.._._.___.___.._......__._.___..._..._.____...__
3 S.0
4 10.0
._ ~..___._..__. 20.0
7 20.0
Diazepam 5.0
The data of Table 2 indicate that the substituted
alkylaminopyridazinone derivatives of the general Formula I
have significant anxiolytic effect equivalent to that of diazepam.
Elevated plus-maze test in rats
Tests have been performed as described by Pellow and co-
workers [J. Neurosci. Methods, I4, 149 (1985)x. A wooden
cross, 1 S cm wide with 100 cm long arms was used for the
experiments. The sides and ends of two opposite arms of the
cross were equipped with 40 cm high walls, however, the arms
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
were open to the 15x15 cm central area (closed arms). The two
other opposite arms were not encircled by walls (open arms).
Male Sprague-Dawley rats weighing 200-220 g were used for
the experiments. The animals were placed in the central area of
the equipment 60 min after treatment and the following four
parameters have been observed for the 5 min test time:
- time spent in the open arms,
- time spent in the closed arms,
- number of entries into the open arms,
- number of entries into the closed arms.
The effect was expressed as percent increase in either the time
(measured in sec) spent in the open arms or number of entries
into the open arms. MEDs (minimal effective dose) were
determined for each compound regarding the time spent in the
-open=arms..
The results obtained are summarized in Table 3. Buspirone [8-
~4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-
azaspiro[4,5]decane-7,9-dione] was used as the reference
substance.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
16
Table 3
Elevated plus-maze test in rats
Compound (Example No.) MEI) in mg/kg po.
3 0.1
Buspirone 3.0
From Table 3 it is evident that the substituted
alkylaminopyridazinone derivatives of the general Formula I
have outstanding anxiolytic activity in the above test,
considerably exceeding the efficacy of the reference substance.
Sedative effect
Inhibition of spontaneous motor activity
The-effect on spontaneousW iotor-activity was-investigated=
according to Borsy and co-workers [Borsy, J. et al, Arch. Int.
Pharmacodyn., 124, 180-190 (1960)] in a ten channel Dews
instrument, with 1-1 animal in each channel. Animals were
placed into the instrument 60 min after per os treatment with
either vehicle or test compound, and interruptions of infrared
beams were recorded for 30 min. From these data, 50 per cent
inhibitory doses (IDSO)have been determined by regression
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
17
analysis. The results obtained are shown in Table 4. Diazepam
was used as the reference substance.
Table 4
Inhibition of spontaneous motility in mouse
Compound (Example No.) IDso in mg/kg po.
2 21.0 I
3 ~ 60.0
>100.0
Diazepam 7.0
In contrast to the diazepam used as the reference substance, the
tested substituted alkylaminopyridazinone derivatives of the
general Formula I display sedative effect only in a relatively
high dose.
Based on the above test data, the substituted
alkylaminopyridazinone derivatives of the general Formula I are
effective in the treatment of various clinical patterns connected
with anxiety. In case of certain compounds, the anxiolytic
potential exceeds by several orders of magnitude the effect of
the marketed reference substances (diazepam, buspirone).
Sedative side-effect appears only in a dose that is multiple of
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
18
the one needed to produce the-expected therapeutical effect.
This means that the substituted alkylaminopyridazinone
derivatives of the general Formula I do not have sedative, life
quality deteriorating side-effects which are characteristic of
benzodiazepines.
Effect of cognition and memory
Male Wistar rats weighing 200-220g were used. The animals
were obtained from Charles River Co. They were kept in a room
with normal 12-12 h light-dark cycle (light on: 06:00) at relative
humidity of 60~10 %.
The experiment was performed in a five-channel "step
through"-type passive avoidance learning apparatus. The
equipment consisted of two adjacent Plexi-glass boxes of
20x20x16 cm. One of them was made of regular transparent
Plexi-glass and the other one was made of black, non-
transparent Plexi-glass. The boxes were connected with a 7.5x8
cm passage way, equipped with a computer-controlled
guillotine-door. The passage of the rats through the door was
detected by infrared photocells arranged in two parallel lines in
the opening of the passage way. The door was automatically
closed when the animals passed through. The dark compartment
was equipped with stainless steel grid floor through which
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
19
electric foot shocks could have been delivered to the animals. A
W light bulb was installed above the passage way in the light
compartment.
The experiment was performed on two consecutive days, in two
sessions which were 24 h apart from each other.
On Day 1 (Acquisition) the animals acquired information about
the situation (grid floor shock in the dark compartment), on Day
2 (Retention) they recalled the acquired information to avoid
punishment ("if I go into the dark I will be punished, so I stay
outside in the light").
1?r~,~ ~ (Acquisi~ioh)
The individually numbered animals were placed into the light
compartment of the equipment. After 30 s the guillotine door
was=opened-and-the=rats=could__freely_pass to.the=dark=
(considered as safe) compartment. Step through latency was
automatically determined. (Step=trough latency is the time
period spanning from door opening to the time when the animal
passed into the dark compartment.) The door was closed then,
and the timer was automatically stopped. An electric foot shock
of 1.2 mA lasting 2.5 s was applied to the animal through the
grid floor 3 s after the door has been closed, except for rats in
the absolute control group (no shock + vehicle treated). Test
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
animals were removed from the dark compartment immediately
after foot shock has been delivered. The function of the absolute
control group was to show that shocked animals will remember
the unpleasant foot shock as revealed by increased latency time
when compared to absolute control. That is the essence of
acquisition.
1)ay 2 (Retentiofz)
After 24 h, the animals were placed again in the light
compartment of the test apparatus and step-through latency was
measured as described at Acquisition day, except that no foot
shock was applied to the animals in any group on the second
day. A maximum of 180 s time interval was available for the
rats to pass into the dark compartment. The animals were
removed from the light compartment if they did not pass to the
dark compartment within the 180 s test period.
The investigators surprisingly found that the invention
compounds significantly increased step-through latency into the
dark compartment of the passive avoidance apparatus after Day
2 administration of the compound (Fig 1).
It is shown on Fig 1 that in absolute control group(no shock,
untreated), step-trough latency was approximately the same on
both experimental days (meaning that there was nothing to
recall and avoid on the second day).
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
21
In the shocked, vehicle-treated control group the unavoidable
1.2 mA foot shock resulted in a significantly increased step-
through latency on Day 2 when compared to absolute control.
The experimental animals recalled the annoying experience
(foot shock) in the dark, therefore, they pass into the dark
compartment with a significantly longer time (increased
latency).
In the treated groups this augmented latency has been further
increased after the Day 2 treatment indicating that the retention
of memory has been improved.
These surprising effects are not evident since anxiolytic
compounds (i.e. diazepam) have a deleterious effect on
memory.
From therapeutic point of view the advantageous effect of
compounds of general Formula I on learning and memory
signifies that the compounds could be appropriate for treating
and/or preventing diseases or conditions accompanysng=diseases-
wherein learning or memory functions are suffering a loss or
there is a possibility to suffering a loss. Such diseases are, but
not limited to - as mentioned earlier - Alzheimer's disease,
Korsakoff syndrome, Huntington's disease, Parkinson's disease
and mental decline due to ageing processes, impairment of the
cognitive functions or to exposure to toxic substances as well.
Summary
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
The compounds of general Formula I possess surprisingly
considerable anxiolytic properties without sedative side effects
in their anxiolytic dose range. In addition to the anxiolytic
efficacy, the compounds of general Formula I have
advantageous effects on cognition and memory. According to
our studies the compounds of general Formula I surprisingly
have no antihypertensive potential.
The compounds of the invention and pharmaceutically suitable
acid addition salts thereof can be used as active ingredients in
pharmaceutical compositions.
Furthermore, the invention relates to a pharmaceutical
composition comprising a substituted alkylaminopyridazinone
derivatives of the general Formula I or a pharmaceutically
suitable acid addition salt thereof undone or moreco~ventional_
carriers.
The pharmaceutical composition of the invention contains, in
general, 0.1 to 95 per cent by weight, preferably 1 to 50 per cent
by weight, suitably 5 to 30 per cent by weight of the active
ingredient.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
23
The pharmaceutical composition of the invention is suitable for
peroral, parenteral, rectal or transdermal administration or for
local treatment, and can be solid or liquid.
The solid pharmaceutical compositions suitable for peroral
administration may be powders, capsules, tablets, film-coated
tablets, microcapsules etc., and can comprise binding agents
such as gelatine, sorbitol, polyvinyl-pyrrolidone) etc.; filling
agents such as lactose, glucose, starch, calcium phosphate etc.;
auxiliary substances for tabletting such as magnesium stearate,
talc, polyethylene glycol), silica etc.; wetting agents such as
sodium laurylsulfate etc. as the carrier.
The liquid pharmaceutical compositions suitable for peroral
administration may be solutions, suspensions or emulsions and
can comprise e.g. suspending agents such as gelatine,
carboxyxnethylcellulose_etc.; _emulsi _fiers such-asaorbitane-
monooleate etc.; solvents such as water, oils, glycerol,
propylene glycol, ethanol etc.; preservatives such as methyl p-
hydroxybenzoate etc. as the carrier.
Pharmaceutical compositions suitable for parenteral
administration consist of sterile solutions of the active
ingredient, in general.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
24
Dosage forms listed above as well as other dosage forms are
known per se, see e.g. Remington's Pharmaceutical Sciences,
18~' Edition, Mack Publishing Co., Easton, LTSA (1990).
The pharmaceutical composition contains dosage unit, in
general. A typical dose for adult patients amounts to 0.1 to 1000
mg of the compound of the general Formula I or a
pharmaceutically suitable acid addition salt thereof calculated
for 1 kg body weight, daily. The daily dose can be administered
in one or more portions. The actual dosage depends on many
factors and is determined by the doctor.
The pharmaceutical composition is prepared by admixing a
compound of the general Formula I or a pharmaceutically
suitable acid addition salt thereof to one or more carrier(s), and
converting the mixture obtained to a pharmaceutical
comp -osition=in=a_manner-_knownpe~=se Useful =methods-are-
known from the literature, e.g. Remington's Pharmaceutical
Sciences mentioned above.
According to a further feature of the present invention there is
provided the use of compounds of the general Formula I or a
pharmaceutically acceptable acid addition salt thereof as
pharmaceutical active ingredient.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
According to a preferred embodiment of the above feature of
the invention there is provided the use of compounds of the
general Formula I or pharmaceutically acceptable acid addition
salts thereof as anxiolytic and cognition enhancing
pharmaceutically active ingredients.
According to a still further feature of the present invention there
is provided a method of anxiolytic and cognition enhancing
treatment of anxiolytic conditions which comprises
administering to the person in need of such treatment a
pharmaceutically effective amount of a compound of the
general Formula I according to claim 1 or a pharmaceutically
acceptable acid addition salt thereof.
Further details of the present invention are to be found in the
following Examples without limiting the scope of protection to
=said-=Examples.-
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
EXAMPLES
Example 1
Preparation of 5-~2-[4-(methoxytrifluoromethylphenyl)-
piperazine-1-yl]-ethylamino}-2H pyridazine-3-one
trihydrochloride
3.7 g (0.0086 moles) of 4-chloro-5- f 2-[4-(methoxy-
trifluoromethyl-phenyl)p iperazine-1-yl] ethylamino } -H
pyridazine-3-one, 370 cm3 of methanol, 3.2 cm3 (0,018 moles)
of diisopropyl-ethylamine and 3.7 g of palladium on carbon
catalyst consisting of 8% of Pd, 28% of C and 64% of H20 are
transferred into an autoclave. The reaction mixture is stirred at
room temperature and under a hydrogen pressure of I O atm for
4 hours. Then, the excess hydrogen is let out from the autoclave,
~IZe=r-eaction=mixture-is--heated=to-reflux-temperature.and stirred-
at this temperature for 5 minutes, then filtered while hot, and the
catalyst is washed three times using 33 cm3 of a 1:1 mixture of
methanol and dichloromethane each time. The combined
filtrates are evaporated under reduced pressure, and the residue
is subjected to chromatography over a silica gel column using a
I9:1 mixture of chloroform and methanol as the eluent. The
fractions containing the product are evaporated, the residue is
dissolved in a mixture of ethyl acetate and diethyl ether, and, to
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
27
the solution obtained, ether containing hydrogen chloride are
added, drop by drop. The crystals that precipitate are stirred for
half an hour under cooling with ice water, then filtered and
washed with diethyl ether. The product is dried at 80 °C over
phosphorus pentoxide iu vacuo for 3 hours.
Thus, 1.84 g (54 %) of the title compound are obtained. M.p.:
23 8-240 °C
Analysis: for C18H25C13F3NSO2 (506.79)
calculated: C 42.66 %, H 4.97 %, N 13.82 %, Cl 20.99 %;
found: C 42.53 %, H S.OI %, N 13.63 %, Cl 20.69 %.
IR (KBr): 3294, 2340, 1630, 1330, 1 I 15:
1H-NMR (DMSO-d6,1400): I3.23 (b, 1H), 11.49 (b, 1H), 8.43
:(b,-1H); 79_0=_(b_s,-1H), 7=.40 (d,-J 8.5-Hz,.1I3)~ 7_,18 (d,-J 8.7
Hz, 1H), 7.15 (s, 1H), 6.05 (bs, 1H), 3.89 (s, 3H), 3.13-3.75 (m,
12H).
~3C-NMR (DMSO-d6, i400): 162.14, 154.8I, 150.30, 139.98,
134.04, 124.68 (q, J=271.6 Hz), 121.51 (q, J=31.7 Hz), 120.92
(q), 114.81 (q), 112.22, 93.60, 56.13, 53.09, 51.30, 46.69,
36.49.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
28
Example 2
Preparation of 5- f 2-[4-(2-fluorophenyl)piperazine-1-yl]ethyl-
amino}=2H pyridazine-3-one
2.5 g (0.0071 moles) of 4-chloro-5- f 2-[4-(2-
fluorophenyl)piperazine-1-yl]ethylamino~-2H pyridazine-3-one,
400 cm3 of a 9:1 mixture of methanol and distilled water and
2.5 g of palladium on carbon catalyst consisting of 8% of Pd,
28% of C and 64% of HBO are weighed into an apparatus of
1000 cm3 volume equipped with a reflux condenser connected
to a bubbling device.' 1.4 cm3 of hydrazine hydrate are added,
drop by drop, to the reaction mixture that is then stirred at reflux
temperature for 1 hour. The mixture is filtered while hot, and
the catalyst is washed three times using 33 cm3 of a 1:1 mixture
of methanol and dichloromethane. The combined filtrates are
evaporated, and the residue is dissolved in 25 cm3 of a 8:1
mixture-of ethanol=and=water-under:_h_eating,-the= 5olutiori~is_
filtered, and the filtrate is evaporated to the fifth of the original
volume. After cooling, the crystals separated are stirred for
further half an hour under cooling with ice water, then filtered
and washed with diethyl ether. The product is dried at 40°C over
phosphorus pentoxide in vacuo for 3 hours.
Thus, 1.91 g (84..8 %) of the title compound are obtained.
M.p.: 103-105 °C.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
29
Analysis: for Cl6HaoFNsO (317.37)
calculated: C 60.55 %, H 6.35 %, N 22.07 %;
found: C 60.25 %, H 6.34 %, N 21.89 %.
IR (KBr): 3272, 3122, 1633, 1550.
1H-NMR (DMSO-d6,1400): 11.87 (bs, 1H), 8.35 (d, J=4.8 Hz,
2H), 7.48 (d, J=2.6 Hz, 1H), 6.85 (bt, J=5.1 Hz, 1H), 6.62 (t,
J=4.7 Hz, 1H), 5.40 (~s, 1H), 3.73 (m, 4H), 3.14 (~q, J=6.0 Hz,
2H), 2.50 (m)
13C-NMR (DMSO-d6, 1400): 162.42, 161.38, 158.08, 149.44,
131.73, 110.28, 94.38, 55.85, 52.71, 43.40, 39.16.
Example 3
Preparationof 5--_{2-[4-phenylpiperazine-.1---y-1]ethylamino} _2H-.
pyridazine-3-one
3.3 g (0.01 moles) of 4-chloro-5-[2-(4-phenylpiperazine-1-
yl)ethylamino]-2H pyridazine-3-one, 500 cm3 of a 9:1 mixture
of methanol and distilled water and 3.3 g of palladium on
carbon catalyst consisting of 8% of Pd, 28% of C and 64% of
H20 are weighed into an apparatus of 1000 cm3 volume
equipped with a reflux condenser connected to a bubbling
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
device. 2 cm3 of hydrazine hydrate are added, drop by drop, to
the reaction mixture that is then stirred at reflux temperature for
3 hours. The mixture is filtered while hot, and the catalyst is
washed three times using 33 cm3 of a 1:l mixture of methanol
and dichloromethane. The combined filtrates are evaporated,
and the residue is dissolved in 15 cm3 of a 8:1 mixture of
ethanol and water under heating, the solution is filtered, and the
filtrate is evaporated to the fourth of the original volume. After
cooling, the crystals separated are stirred for further half an hour
under cooling with ice water, then filtered and washed with
diethyl ether. The product is dried at 60 °C over phosphorus
pentoxide in ~acuo for 3 hours.
Thus, 2.18 g (72.9 %) of the title compound are obtained. M.p.:
147-149°C.
Analysis: for-:=-C=1-gH~,oFN50 (3=17.3-7)
calculated: C 64.19 %, H 7.07 %, N 2339 %;
found: C 63.74 %, H 7.09 %, N 22.89 %.
IR (KBr): 3484, 3318, 1638, 1600.
1H-NMR (DMSO-d6,1400): 11.98 (bs, 1H), 7.52 (d, J=2.3 Hz,
1H), 7.21 (~t, J=7.8 Hz, 2H), 6.91 (~d, J=8.5 Hz, 2H), 6.90 (bt,
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
31
J=5.1 Hz, 1H), 6.77 (~t, J=7.2 Hz, 1H), 5.44 (d, J=2.3 Hz, 1H),
3.14 (m, 6H), 2.56 (m, 6H).
13C-NMR (DMSO-d6,1400): 162.46, 151.20, 149.4-7, 131.76,
129.11, 118.97, 115.52, 94.37, 55.87, 52.93, 48.30; 39.25.
Example 4
Preparation of 5-j2-(4-pyridin-2-ylpiperazine-1-yl)ethylamino]-
2H pyridazine-3-one
3.2 g (0.0096 moles) of 4-chloro-5-[2-(4-pyridin-2-
ylpiperazine-1-yl)-ethylamino]-2H pyridazine-3-one, 500 cm3
of a 9:1 mixture of methanol and distilled water and 3.2 g of
palladium on carbon catalyst consisting of 8% of Pd, 28% of C
and 64% of H20 axe weighed into an apparatus of 1000 cm3
volume equipped with a reflux condenser connected to a
bubbling=demce:-1=-.6=cm3 of-hydrazine=hydrate-are added, drop
by drop, to the reaction mixture that is then stirred at reflux
temperature for 2 hours. The mixture is filtered while hot, and
the catalyst is washed three times using 33 cm3 of a 1: I mixture
of methanol and dichloromethane. The combined filtrates are
evaporated, and the residue is dissolved in 30 cm3 of a 8:1
mixture of ethanol and water under heating, the solution is
filtered, and the filtrate is evaporated to the fifth of the original
volume. After cooling, the crystals separated are stirred for
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
32
further half an hour under cooling with ice water, then filtered
and washed with diethyl ether. The product is dried at 80 °C
over phosphorus pentoxide in vcccuo for 3 hours.
Thus, 2.44 g (85.0 %) of the title compound are obtained. M.p.:
150-152°C.
IR (I~Br): 3444, 1605, 1340, 1167.
1H-NMR (CDC13, g200): 11.91 (bs, 1H), 8.11 (m, 1H), 7.51 (m
Hz, 2H), 6.95 (b, 1H), 6.85 (m, 1H), 6.65 (m, 1H), 5.44 (s, 1H),
3.52 (m, 4H), 3.22 (m, 4H), 2.63 (m, 4H).
Example 5
Preparation of 5-[2-(4-pyrimidin-2-ylpiperazine-1-
yl)ethylamino]-2H pyridazine-3-one
3.4 g (0.01 moles) of 4-chloro-5-[2-(4-pyrimidin-2-
ylpiperazine-1-yl)-ethylamino]-2H pyridazine-3-one, 500 cm3
of a 9:1 mixture of methanol and distilled water and 3.4 g of
palladium on carbon catalyst consisting of 8% of Pd, 28% of C
and 64% of H20 are weighed into an apparatus of 1000 cm3
volume equipped with a reflux condenser connected to a
bubbling device. 1.7 cm3 of hydrazine hydrate are added, drop
by drop, to the reaction mixture that is then stirred at reflux
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
33
temperature for 1.5 hours. The mixture is filtered while hot, and
the catalyst is washed three times using 3 3 cm3 of a 1:1 mixture
of methanol and dichloromethane. The combined filtrates are
evaporated, and the residue is dissolved in 28 cm3 of a 8:1
mixture of ethanol and water under heating, the solution is
filtered, and the filtrate is evaporated to the fifth of the original
volume. After cooling, the crystals separated are stirred for
further half an hour under cooling with ice water, then filtered
and washed with diethyl ether. The product is dried at 80 °C
over phosphorus pentoxide zn vacuo for 3 hours.
Thus, 2.19 g (72.5 %) of the title compound axe obtained. M.p.:
199-201°C.
Analysis: for Cl4HmN70 (301.35)
calculated: C 55.80 %, H 6.36 %, N 32.54 %;
found - --C-=55 71%,-=:H_6 33-_%, - N 32.41 %.
I~ (I~Br): 3272, 3122, 1633, 1550.
1H-NMR (DMSO-d6, i400): 11.87 (bs, 1H), 8.35 (d, J=4.8 Hz,
ZH), 7.48 (d, J=2.6 Hz, 1H), 6.85 (bt, J=5.1 Hz, 1H), 6.62 (t,
J=4.7 Hz, 1H), 5.40 (~s, 1H), 3.73 (m, 4H), 3.14 (~q, J=6.0 Hz,
2H), 2.50 (m).
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
34
z3C-NMR (DMSO-d6, i400): 162.42, 161.38, 158.08, 149.44,
131.73, 110.28, 94.38, 55.85, 52.71, 43.40, 39.16.
Example 6
Preparation of 5- f 2-[4-(3-chlorophenyl)piperazine-1-yl]ethyl-
amino}-2H-pyridazine-3-one
A mixture of 1.96 g (0.01 moles) of 1-(3-chlorophenyl)-
piperazine, 8 cm3 of dimethylformarnide, 4.5 cm3 (0.014 moles)
of triethylamine, 0.2 g of potassium iodide and 1.9 g (0.009
moles) of 5-(2-chloroethylamino)-2H pyridazine-3-one
hydrochloride is stirred at reflux temperature for 2 hours. To the
reaction mixture, a solution of 3.0 g of sodium hydrogen
carbonate in 40 cm3 of water is added, drop by drop. Due to the
presence of the water, oil separates. The water is decanted from
the oil, and 10 cm3 of dichloro-methane are added to the
residue: The=crystals-separatingunder stirring-_are filtered. The -
crude product is dissolved in methanol at reflux temperature
under stirring, treated with charcoal, filtered, and the filtrate is
evaporated to the fifth of the original volume. The residue is
stirred under cooling with ice water, and the precipitated
crystals axe filtered.
Thus, I .21 g (40.5 %) of the title compound are obtained. M.p.:
187-189°C.
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
3S
Analysis: for Cl6HaoC1N50 (333.82)
calculated: C S7.S7 %, H 6.04 %, Cl 10.62 %, N 20.98 %;
found: C 57.09 %, H 6.0S %, Cl 10.38 %, N 22.68 %.
IR (KBr): 3410, 3277, 1624, 1599, 1240.
iH-NMR (DMSO-d6,1400): 11.93 (bs, 1H), 7.20 (~t, J=8.2 Hz,
1 H), 6. 89 (m, 1 H), 6, 87 (bt, J=S .2 Hz, 1 H), 6.7 8 (m, 1 H), 5.42
(d, J=2. S Hz, 1 H), 3 .18 (m, 6H), 2. S4 (m, 6H).
13C-NMR (DMSO-d6, i400): 162.38, 152.40, 149.42, 134.01,
131.70, 130.57, 118.17, 114.65, 113.78, 94.37, SS.80, 52.71,
47.74, 39.22.
Example 7
Preparation of S-{2-[4-(4-fluorophenyl)piperazine-1-yl]ethyl-
=amino-2H--pyridazine-3 -one _
3.S 1 g (0.01 moles) of 4-chloro-S-{2-[4-(4-
fluorophenyl)piperazine-1-yl]ethylamino)-2H pyridazine-3-one,
3S0 cm3 of a 9:1 mixture of methanol and distilled water, 0.44 g
(0.011 moles) of sodium hydroxide and 3.S g of palladium on
carbon catalyst consisting of 8% of Pd, 28% of C and 64% of
H20 are transferred into an autoclave. The reaction mixture is
stirred at room temperature under a hydrogen pressure of 10 atm
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
36
for 3 hours. Then, the excess hydrogen is let out from the
autoclave, the reaction mixture is heated to reflux temperature
and stirred at this temperature for S minutes, then filtered while
hot, and the catalyst is washed three times using 50 cm3 of a 1:1
mixture of methanol and dichloro methane each time. The
combined filtrates are evaporated to a volume of 20 cm3, the
solution obtained is stirred for half an hour under cooling with
ice water, the crystals obtained are filtered and washed with 10
cm3 of cooled methanol.
Thus, 2.98 g (81.7 %) of the title compound are obtained. M..p.:
96-98°C.
Analysis: for: C~6H2oFN50 (317.37)
calculated: H 6.35 %, N 22.07 %;
found: H 6.30 %, N 21.64 %.
IR (KBr) 3433, 3243, 3081, 1618, 1507, 1226.
1H-NMR (DMSO-d6, i400): 11.95 (bs, 1H), 7.52 (d, J=2.5 Hz,
1H), 7.02 (~t, J=8.9 Hz, 2H), 6.94 (dd, J1=4.7 Hz, J2=9.3 Hz,
2H), 6.90 (bt, J=5.3 Hz, 1 H), 5.43 (d, J=2.5 Hz, 1 H), ~3 .15 (~q,
J=6.0 Hz, 2H), 3.08 (m, 4H), 2.56 (m, 6H).
CA 02461467 2004-03-24
WO 03/027078 PCT/HU02/00097
37
13C-NMR (DMSO-d6,1400): 162.42, 156.16 (d, J=235.4 Hz),
149.45, 148.10 (d, J=1.9 Hz), 131.72, 117.23 (J=7.6 Hz),
115.42 (d, J=21.7 Hz), 94.35, 55.82, 52.91, 49.09, 39.25.