Note: Descriptions are shown in the official language in which they were submitted.
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
INTEGRATED UREA MANUFACTURING PLANTS AND PROCESSES
CROSS REFERENCE TO RELATED APPLICATION
This is a continuation-in-part of U.S. Patent Application Serial No.
09/376,709 filed 08/17/99, titled "Processes for the Production of
Hydrocarbons,
Power and Carbon Dioxide from Carbon-Containing Materials.
FIELD OF THE INVENTION
Syngas generators such as reformers and gasifiers of hydrocarbon fluids
and solid carbonaceous materials and Fischer Tropsch (FT) units for primarily
for
creating liquid hydrocarbons from syngas are combined to create an integrated
plant for providing one or more of urea, ammonia, carbon dioxide, electric
power,
and even sulfur when dealing with sulfur-containing raw material.
BACKGROUND OF THE INVENTION
Our modern civilization cannot be sustained without burning carbonaceous
materials for primarily motive and electrical power within the foreseeable
future.
The-carbon--dioxide (C02) generated _by such_burning_may be contri_buting_ to
the _
gradual increase of the planet's temperature since 1900. This is occurring
because C02 permits the sun's energy to pass through the atmosphere but traps
the longer wavelength energy radiated by the earth into the atmosphere.
The integrated plants and processes of this invention can help reduce the
amount of C02 currently vented into the air through the production of the
various
1
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
products later discussed in the description of the manufacturing plant flow
diagrams. Further, the plants of this invention produce substantial energy
savings by balancing exothermic and endothermic reactors as discussed below.
A variety of reformers and gasifiers are known. Thus, U.S. Pat. 5,611,947
to J. S. Vavruska, U.S. Pat. 5,993,761 to Plotr and Albin Czernichowski et al
and
U.S. Patent 6,153,852 to A. F. Blutke et al all teach plasma reformers useful
in
constructing the integrated facilities used in the process of this invention.
Likewise, Charles B. Benham et al, U.S. Patent 5,621,155, utilize reformers to
provide feed streams to Fischer Tropsch reactors utilizing iron-based
catalysts.
U.S. patent application S/N 09/376/709, filed 08/17/99 by Mark S. Bohn et al
teaches that hydrocarbons and electric power can be manufactured at a plant
using the Fischer-Tropsch (FT) reactors. It also suggests that urea can be
produced but no suggestion is given as to how to manufacture the urea or the
practicality of such a course of action.
The mentioned references deal with economic niches where tax
incentives, regulatory penalties and other incentives must combine with other
factors to make the processes commercial. A continuing increase in world
temperatures or a more firm tie-in between the C02 in the atmosphere and
increasing world climate temperatures could quickly result in such incentives.
The plants can be of particular utility when sited at remote locations where
there
is a large surplus of natural gas, petroleum, coal or other carbonaceous
materials
which are presently unrecoverable because of transportation costs, etc.
2
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
Increasing regulatory demands have limited, and, in some instances
extinguished, the petroleum producers' and refiners' ability to flare waste
gases.
Further, there are often limitations on the amounts and kinds of other wastes
that
can be disposed of locally without harm to the environment, e.g., at an
offshore
crude oil producing platform. The multi-product plants of this invention
provide a
mechanism for packaging the various unit processes required for the
utilization of
this invention in a manner that the resulting plants can be utilized to supply
electricity for a platform, eliminate the need for flares, convert the waste
gases
and liquids normally flared into liquid hydrocarbons, ammonia and/or urea
while
substantially eliminating local C02 emissions. Solid commercial products can
also be produced for agriculture, e.g., sulfur and urea prills. Such self-
contained
plants provide trade-offs; for offshore petroleum and/or natural gas
platforms,
which can improve their economic life span. This is particularly true where
the
deposits being recovered are sour or include some C02 production.
The unit processes of this invention are each individually well known and
are commercially proven. However, the joining of these unit processes as
taught
herein provides a utility for environmental and other purposes that has
heretofore
been unforeseen.
SUMMARY OF THE INVENTION
3
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
Ammonia, carbon dioxide, hydrocarbons, electric power and urea are
producible as products by the reaction of oxygen, water and a carbon source in
a
syngas generator to produce a syngas, utilizing a water gas shift mechanism to
provide C02, reacting the syngas in an FT reactor to produce FT hydrocarbons
and hydrogen, reacting the hydrogen with nitrogen from the air separation
oxygen
plant to form ammonia, then reacting the C02 and ammonia to form urea.
Electric power can be produced by combustion of hydrogen in a gas turbine used
to drive an electricity generator and/or utilizing steam formed during syngas
production to drive a steam turbine which, in turn, drives an electricity
generator.
Sulfur and various heavy metals can be recovered when sulfur or metal-
containing carbon sources are utilized. As noted, a number of the compounds,
an element and electric power produced in the manufacture of ammonia can be
"packaged" for commercial sale.
BRIEF DESCRIPTION OF THE FIGURES
The Figures illustrate the favorable economics and ease of interaction
which can be obtained through a combination of several well known unit
processes and for manufacturing a variety of materials, all of which can be
provided in amounts suitable for commercial sales if suitable raw materials
are
available.
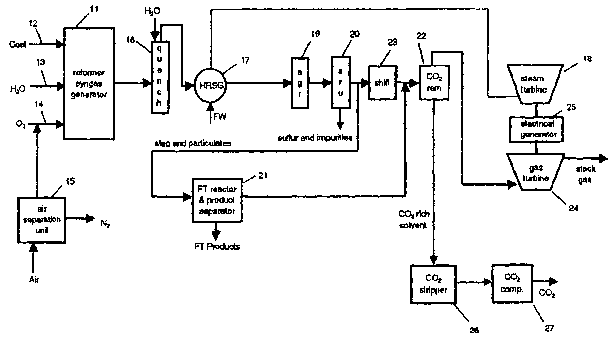
Figure 1 depicts a syngas producing unit and an FT unit combined to
provide liquid hydrocarbons and electric power.
4
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
Figure 2a utilizes the basic plan of Figure 1 and delineates the minimal
equipment and indicates the valuing and other plumbing changes needed to
convert the unit of Figure 1 into an ammonia manufacturing plant utilizing
hydrocarbon gases from a substantially solid carbonaceous feed such as coal
and petroleum refining residues.
Figure 2b depicts the added equipment needed to convert the ammonia
produced in the plant of Figure 2a into a urea plant.
Figure 3a and 3b teach an alternate exemplary plant layout for
manufacturing urea utilizing natural gas as the syngas feedstoclc.
DETAILED DESCRIPTION OF THE DRAWINGS
In the coal gasification/FT/power plant of Figure 1, crushed coal, water
(H2~), preferably as steam, and oxygen (02) are introduced into the syngas
generator 11 through piping 12, 13 and 14, respectively. The oxygen is
preferably from a cryogenic air separation unit 15. However, pressure swing
absorption can also be utilized. Either can provide nitrogen (N2) for an
ammonia
;N-H3)-plant--(not---shown): The hot -gases ar-e- exhausted_ fr.~m. tha
syngas.
generator 11 at temperatures of 2400°F to 2700 °F and are cooled
in one or more
water-cooled quench units 16 to remove slag and other minerals. The cooled
syngas and soluble impurities are piped into a heat recovery steam generator
(HRSG) 17 which is used to heat the feed water (FW) to steam of a desired
temperature, e.g., 230°F to 600°F, and provide steam to power
steam turbine 18;
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
the acid gas removal unit (AGR) 19 and the sulfur removal unit (SRU) 20. The
syngas is piped to the acid gas removal unit (AGR) 19 to remove bulk sulfur
from
the syngas generator 11 output. The resulting gas is then passed through
sulfur
removal unit (SRU) 20 to remove trace quantities of sulfur. Preferably, the
SRU
20 uses a zinc oxide-based catalyst and is run at temperatures of 600°F
to 725°F.
with a linear velocity of 4-10 ft/sec.
To the extent needed, the gaseous treated stream from the SRU is then
piped to the FT reactor and product separation unit 21 to obtain the liquid FT
hydrocarbon products. The FT reactor and product separator 21 tail gas is
piped
to remove carbon dioxide via C02 removal unit 22. A second portion of the
desulfurized syngas is piped to a water gas shift reactor 23, preferably
designed
for use with a high temperature iron/chrome catalyst. The tail gas stream from
the FT reactor and product separation unit 21 is combined with the output of
the
shift reactor 23 and passed through C02 removal units) 22. Combustible
components from the C02 removal units) 22 are fed to the gas turbine 24 which
is used to drive a coupled electricity generator 25. Likewise, the steam
turbine 18
can be used to drive the electrical generators) 25. The stack gases of the gas
turbine 24 are returned to heat recovery steam generator (HRSG) 17.
The C02 absorbed in the C02 removal unit 22 is desorbed in the CO~
stripper unit 26 and compressed by C02 compressors) 27 for tank or other
storage, preferably at pressures above 135 atm or recycled as needed.
In Figure 2a, the equipment differs from that of Figure 1 only to the extent
that the non-C02 output of the C02 removal unit 22 is passed through a
hydrogen
6
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
(H2) removal unit 28 and the recovered hydrogen is piped to an ammonia
converter 38 (Fig. 2b). The non- HZ output of the H2 removal unit (HRU) 28 is
piped to the gas turbine 24 as fuel. Preferably the H2 removal unit 28
utilizes a
membrane separator. Such units are manufactured by Monsanto Company,
located at St. Louis, Missouri, USA or Air Liquide located at Paris, France.
In Figure 2b, the continuation of the flow chart of Figure 2a, the C02 from
stripper unit 26 (Fig. 2a) is compressed by C02 compressor 27 and introduced
into urea synthesizer 29 which operates at 330°F to 375°F and
2000 to 3000
psig. The urea synthesized in the urea synthesizer 29 is pumped to the urea
purification unit 31 to reduce the water and other impurities. The urea is
then
prilled or formulated into aqueous urea or anhydrous prills for sale.
Hydrogen (H2) from the hydrogen removal unit 28 (Fig. 2a) is passed
through hydrogen compressor 32 and combined with nitrogen (N2) from air
separation unit 15 (See Fig. 1) and the mixture is passed to heater 33 to
raise the
temperature to about 500°F and introduced into methanator 34 which
operates at
500 °F to 600°F utilizing, preferably, a 27-35% nickel oxide
catalyst.
The methanator utilizes a catalyst which is delivered as nickel oxide on
alumina and reduced to nickel on site for operation. A variety of suppliers
market
the catalyst. The methanator operates at temperatures between about
500°F to
550°F at the inlet and pressures between about 275 to about 375 psig.
The
methanator 34 product stream is passed through cooler 35 into ammonia/syngas
compressor 36. The compressed product stream is cooled in exchanger 37 and
is fed to NH3 converter 38. The resulting ammonia stream returns to the heat
7
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
exchanger 37 through line 39. The cooled effluent is further cooled in
exchanger
41 and still further cooled with a cold stream from ammonia refrigeration unit
42
before passing to separator 43. The condensed ammonia from ammonia
separator 43 is then passed through pump 44, piped into a urea synthesizer 29,
dried for prilling or made into aqueous solutions of desired concentrations.
In Figure 3a, the liquid oxygen (02) from air separation unit 40 is passed
through cryogenic pump 45, heater 46 and introduced into mixing zone 47. The
natural gas is compressed to about 200 to about 500 psia in compressor 48,
heated in exchanger 49 and run through a sulfur removal unit 51 to "sweeten"
the
raw gas and then into another heater 52 before entering the mixing zone 47.
The
natural gas and oxygen are converted into syngas in syngas generator 53 and
cooled in exchanger 54 prior to treatment in C02 removal unit 55.
The absorbed C02 is stripped in stripper 56 prior to recycling to the urea
synthesizer 29 (Fig. 3b). The syngas stream passes through heater 57 before
entering FT reactor 58. The resulting products are introduced into product
separator 59 to provide a liquid hydrocarbon stream with pentane or greater
fractions, a stream of aqueous oxygenated hydrocarbons which is pumped from
about 200 to about 500 psia by pump 60, and reheated in heat exchangers 61
and 52 prior to entering mixing zone 47. A tail gas stream also flows to
mixing
zone 47 via compressor 62 while the C02 is sent to a C02 compressor 63 (Fig.
3b). The flow diagram of Figure 3b shows C02 from the C02 stripper 56 passing
through compressor 63 into urea synthesizer 64 and thence through a urea
purification unit 65. The FT tail gas stream then passes a pressure swing
8
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
absorber 66 to remove H2. A hydrogen-lean fraction is used as fuel while the
remainder is mixed with nitrogen (N2) from air separation unit 40, piped to
heater
67 and thence to methanator 68 which removes the remaining traces of carbon
oxides. The product stream from the methanator is piped into cooler 69 and
then
into ammonia syngas compressor 71. The compressor 71 product is cooled in
exchanger 72 and introduced into ammonia converter 73. The ammonia from the
converter 73 is fed to the exchanger 72, cooled in exchanger 74 by ammonia
refrigeration unit 75. The ammonia stream from exchanger 74 passes through
ammonia separator 76. A portion of the effluent from separator 76 is recycled
to
the ammonia syngas compressor 71 and the remainder is used as fuel. The
purified ammonia is passed through pump 77 and mixed with compressed C02
before introduction to urea synthesizer 64. The urea produced is then passed
through purifier 65 and readied for use or sale.
EXAMPLES
Example 1 Coal Gasification to FT Liquids. Electrical Power and COa.
Example 1 is a computer simulation based on the flow sheet of Figure 1.
5500 tpd Pittsburgh #8 coal is gasified with 3328 tpd water and 5156 tpd
oxygen.
The coal is 74.16% carbon. After quenching and cleaning, a portion of the
syngas is sent to an FT reactor. The remainder of the syngas is shifted to
convert as much of the CO to C02 as is possible. This shifted stream is
combined with the FT reactor tail gas. C02 is removed from the combined
9
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
stream and compressed for commercial usage or sequestration. The C02-free
gas is sent to the gas turbine to produce power.
The flow sheet takes advantage of the water-gas shift activity of an iron-
based FT catalyst in converting much of tie carbon in the feed coal to C02.
This
catalyst is discussed in U.S. Patent 5,504,118 issued to Charles B. Benham et
al.
A computer simulation utilizing the equipment of this flow sheet of 5500 ton
per
day of coal produces 6000 barrels per day of FT liquids, 400 MW net electrical
power, and 10515 ton per day of sequesterable C02. Only 9% of the feed carbon
is in the stack gas.
Example 2. Coal Gasification to FT Liquids. Electrical Power and Urea.
Example 2 is based on the flow sheet of Figures 2a and 2b. This flow
sheet builds on the flow sheet of Example 1 by reacting the sequestered CO2
with
ammonia to produce urea. To make the required ammonia, nitrogen from the air
separation unit is reacted with hydrogen removed from the gas stream prior to
power generation. This demonstrates the synergies possible with the iron-based
FT catalyst.
Using these two flow sheets, a computer simulation based on 5500 ton per
day coal produces 6000 barrels per day FT liquids, 223 MW net electrical power
and 4230 ton per day urea. Carbon in the stack gas is the same as in Example
1. The difference is that the sequestered C02 has been used to produce urea.
Example 3. Natural Gas to FT Liquids and Urea.
This example is based on figures 3a and 3b and the use of a sour natural
gas. The natural gas is reformed with oxygen in an autothermal reformer. After
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
cooling the syngas, C02 is removed and sent to the urea plant. The syngas is
then sent to an FT reactor. Most of the FT tail gas is recycled to the
autothermal
reactor. The rest is used in the ammonia plant. Ammonia and C02 are removed
from the syngas and piped to the urea plant.
In this flow sheet, a computer simulation shows that 100 MMSCFD natural
gas produces 10,170 barrels per day FT liquids and 275 ton per day urea. Note
that virtually all of the feed carbon ends up in the FT liquids and the urea.
Simulation of the coal gasifier was based on synthesis gas composition
given in Table 1 of "Syngas Production from Various Carbonaceous feedstocks",
Texaco Gasification Process for Solid Feedstocks, Texaco Development
Corporation, 1993. Simulation of the Fischer-Tropsch reactor was based on
Rentech's iron-based catalyst (U.S. Pat. 5,504,118).
GENERAL TEACHING OF THE INVENTION
The obvious benefits of utilizing the unit operations and processes of this
invention include:
1. With respect to Figures 1, 2a and 2b, there is an unexpected benefit
from shifting the use of the coal gasifier operation to convert the usually
desired
CO to COz production. It enables the heat values of the syngas to
simultaneously produce electrical power and sequester CO2. The use of iron-
based FT catalysts to form C02 from CO allows some of the feed carbon to be
sequestered as C02. When the sequestered CO2 is reacted with hydrogen
recovered from the syngas production, the FT tail gas and nitrogen from the
air
11
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
separation unit, a synergistic benefit is obtained via the production of urea.
Further, when C02 from a natural gas feed, H2 obtained from the FT tail gas
and
the nitrogen obtained from the air separation unit are reacted as shown, urea
can
be produced rather than having to vent the C02 to the atmosphere.
Feedstocks include both natural gas and low value industrial materials,
e.g., coal and refinery bottoms having a hydrogen to carbon atom ratio of
about
1. Feedstocks can, however, have higher ratios, e.g., natural gas with a ratio
approaching 4:1. Many of these materials will include contaminants which must
be removed, e.g., sulfur, arsenic and silicaceous materials which are removed
during the course of the syngas manufacturing steps as slag or sulfur
compounds.
The syngas produced can be contaminated with carbon dioxide and
unwanted impurities such as chlorine, chlorides and other toxic materials
which
must be safely removed and stored.
For the purposes of this invention, iron-based FT catalysts are preferred
because they produce C02 via their water gas shift activity. In general, the
reactors, materials of construction and processes are well known to those
skilled
in the refining and Fischer Tropsch utilizing industries. The assembly of the
reactors taught form the basis of the claimed chemical processing units
sequenced in the invention. The sequenced chemical processes, catalysts,
temperatures, concentrations and other stated parameters form the basis of the
chemical process claims. It is to be understood that the order of the chemical
processing units and the process steps and conditions described in Figures and
12
CA 02461685 2004-03-25
WO 03/027062 PCT/USO1/30017
the discussion thereof can be varied and the variations are intended to fall
within
the claims as taught in the description and Figures.
13