Note: Descriptions are shown in the official language in which they were submitted.
CA 02462706 2004-04-27
. 60557-4723(D)
_1_
MULTI-ARM BLOCK COPOLYMER,
AND PRESSURE SENSITIVE ADHESIVE AND TAPE
EMPLOYING A MULTI-ARM ELASTOMERIC
BLOCK COPOLYMER
This is a divisional application of Canadian
application No. 2,122,303.
FIELD OF THE INVENTION
The inventions of this divisional application and
the parent are directed to multi-arm copolymers, pressure
sensitive adhesives formulated from the multi-arm
elastomeric block copolymers, and tape prepared from the
adhesives.
The invention of the parent application is
directed towards a multi-arm block copolymer having the
general structure (A-B)mY(C-D)n wherein A, B, C and D are
polymer segments, Y is a residue of a multifunctional
coupling agent, m and n are the number of arms and both are
greater than 0, and the sum of m plus n is at least 3. The
parent application also describes a pressure sensitive
adhesive composition comprising the above multi-arm block;.
copolymer. The present divisional application is directed
to a pressure sensitive adhesive composition comprising a
mufti-arm elastomeric block copolymer having the general
structure (A'-B)mY wherein A' and B are polymer segments, Y
is a residue of a multifunctional coupling agent and m ins
the number of arms and is at least 3, and to a film and a
tape comprising the mufti-arm elastomeric block copolymer.
CA 02462706 2004-04-27
60557-4723(D)
-la-
BACKGROUND
Pressure sensitive adhesives that are appropriate for use in removable
tape applications require a fourfold balance of peel, tack, adhesion, and
resistance to low stress peel. Especially important is the balance between
adhesion and resistance to low stress .peel.
Masking tapes provide one example of a removable tape. Masking tapes
are often used in the automotive industry to mask surfaces during painting.
Typically, a masking .tape is applied to a surface, exposed to elevated ,
temperature andlor a chemical environment that often includes organic solvent,
and removed when the tape user has finished the task. The tape must be easy
to apply, stay in place without lifting or curling under conditions of high '
temperature and chemical environment; and remove cleanly and easily without
breaking, damaging the surface, or leaving adhesive residue.
Pressure sensitive adhesives based on non-thermoplastic hydrocarbon
elastomers such as natural rubber may be readily formulated to provide an
adhesive that meets the requirements of a masking tape. The dominant .means
of processing such adhesives involves dissolving the.elastomer and other
adhesive components in a hydrocarbon solvent, coating the solution onto a~
backing, and drying the coated product to remove the solvent. These solvent-
based processes have become increasingly undesirable., however, because of the
environmental and safety considerations associated with the use of solvents.
CA 02462706 2004-04-27
-2-
Environmental and safety considerations have led to accelerated interest
in the use of hot melt extrusion coating of adhesive compositions. The
elastomers typically employed in this technique are "thermoplastic" elastomers
of the block copolymer type, including, for example, styrene-isoprene block
copolymers.
Although adhesives based on thermoplastic elastomers eliminate the need
for solvent-based processing, their adhesive properties are different than
those
of adhesives based on non-thermoplastic elastomers, and they are not suitable
for some applications. For example, conventional star monoalkenylarene/
conjugated dime block copolymers (such as star styrene-isoprene-styrene (SIS)
block copolymers) are generally not suitable for the formulation of removable
adhesives. When formulated to give adhesion in the range desirable for a
removable tape, the adhesive lacks the strength to prevent lifting, and the
peel
and unwind are often not smooth. When formulated to have sufficient strength
to prevent lifting, the adhesion is too high, and the tape is difficult to
remove.
Patent literature discloses a wide variety of block copolymer structures
which are useful in the formulation of adhesives. For example, St. Clair (U.S.
Patent 4,391,949) discloses and claims a block copolymer having the structure
(A-B)x Y(C)Z wherein A is a poly(monoalkenyl) block, B and C are
poly(conjugated diene) blocks, Y is a residue of multifunctional coupling
agent,
and x plus z is greater than 6. St. Clair (U.S. Patent 4,444,93) discloses and
claims an adhesive composition comprising the block copolymer described in
U.S. Patent 4,391,949. Hansen (U.S. Patent 4,133,731) discloses and claims
that the block copolymer in a pressure sensitive adhesive can be chemically
crosslinked by including a multifunctional acrylate or methacrylate
crosslinking
agent in the pressure sensitive adhesive formulation and exposing the adhesive
to high energy radiation such as electron beam or ultraviolet radiation.
Erickson (U.S. Patent 5,104,921) discloses a cured adhesive composition
prepared by high energy ionizing radiation initiated curing of a polymer
composition comprising an alkenylarene/conjugated dime block copolymer and
an oligomer such that the unsaturation index of the composition is minimized.
CA 02462706 2004-04-27
-3-
The radiation initiated curing of the adhesive is accomplished without
requiring
the aid of a coupling agent to promote crosslinking of the block copolyrrier
during exposure to the radiation. Heinz (U.S. Patent 4,430,417) discloses a
photopolymerizable mixture of one or more block copolymers which are solely
elastomeric and comprise two or more elastomeric polymer blocks having a
glass transition temperature (1'g) of from -20°C to 15°C, linked
by one or more
elastomeric polymer blocks having a glass transition temperature of below
-20°C. Lau (U.S. Patent 4,780,367) discloses a pressure sensitive
adhesive
composition comprising a star block copolymer having the general structure
(A-B)°C wherein A is a terminal polymeric block consisting essentially
of
polymerized monovinyl aromatic monomer having 8 to 18 carbon atoms and
selected from the group consisting of styrene and alkylated styrene; B
represents a polymeric block consisting essentially of polymerized conjugated
dime monomer having 4 to 12 carbon atoms; C represents the residue of a
IS polyvinyl aromatic compound providing a nucleus which links together the
arms
of the star block copolymer;, and the number of arms, represented by n, is at
least I2.
St. Clair (U.S. Patent 4,556,464) discloses pressure-sensitive adhesive,
made with ABA star block copolymers wherein the endblock (1) is a random
copolymer of a monoalkenylarene and a conjugated diene and (2) has a Tg
between 19°C and 100°C. The copolymer endblock includes a diene
in order
to introduce a reactive site into the endblock for crosslinking. St. Clair
shor~rs
that tack loss can be improved by adding a crosslinking additive that is
mainlly
compatible with the endblo<:k phase to preferentially promote crosslinking in
the
endblock over the midblock.
The present invention provides a novel mufti-arm block copolymer,
particularly useful in the formulation of a pressure sensitive adhesive. The
adhesive of the invention can be hot. melt extrusion coated to provide a
variety
of different types of tapes, especially removable tapes having sufficient
resistance to low stress peel while maintaining moderate peel adhesion. Such
tapes may be used as masking tapes, packaging tapes, medical tapes and
CA 02462706 2004-04-27
60557-4723 (D)
_4_
autoclave indicator tapes. Additionally, the pressure sensitive adhesive may
be
used to make adhesive-backed protective sheeting, labels, and facestock.
SUMMARY OF THE INVENTION
In accordance with one aspect of the invention
of the parent application, there is provided
a novel mufti-arm block copalymer, and both a novel pressure=sensitive
adhesive and a novel tape made therewith. The novel. mufti-airn block
copolymer has the general stricture
(A-B)mY(C-D)" ~ (Formula I)
wherein A, B, C and D are polymer segments; Y is a residue.of a
multifunctional coupling agent; m is the number of A-B polymer segment aTins;
n is the number of C-D polymer segment arms; m and n are both greater than
0; and the .sum of m plus n is at least 3.~ Segment A comprises a random --
copolymer of a monoalkenylarene and a conjugated diene; B and C may be the
, ~ - same or different and comprise either a homopolymer~of a conjugated
diene or ,
a polymer of two or more conjugated dienes; D comprises either a
homopolymer of a monoalkenylarene or a copolymer of a monoalkenylarene
and a conjugated diene. The glass transition temperature Tg of D is greater
than the Tg of A. The total weight percent of monoalkenylarene in the block
copolymer is 40% or less. Preferably the novel mufti-arm block copolymer is
elasiomeric.
The pressure sensitive adhesive based upon the Fonrnzl.a I block
copolymer comprises 100 parts by weight of the novel mufti-arrt~, elastomeric
block copolymer, about 20 to about 300 parts by weight. of a taekifying~resin,
0
to about 50 parts by weight of,a crosslinking agent, and 0 to about 200 parts-
by
weight of a plasticizer.
According to one aspect of the invention of the present divisional
application,
there is provided a pressure-sensitive adhesive composition based upon a mufti-
arm
elastomerie block copolymer of the general structure
(A'-B)," Y ~ ~ (Formula II)
CA 02462706 2004-04-27
60557-4723(D)
-5-
In this embodiment the pressure sensitive adhesive
comprises 100 parts by weight of the Formula II copolymer,
about 20 to about 300 parts by weight of a tackifying resin,
0 to about 50 parts by weight of a crosslinking agent, and 0
to about 200 parts by weight of a plasticizer. In the
(A°-B)mY structure, A' and B are polymer segments, Y is a
residue of a multifunctional coupling agent, and m
represents the number of arms and is at least three.
Segment A° comprises a random copolymer of a
monoalkenylarene and a conjugated diene, wherein the Tg of
A' is less than 19°C, and B comprises either a homopolymer
of a conjugated diene or polymer of two or more conjugated
dienes.
According to another aspect o:~ the invention of
the present divisional application, there is provided a film
of a pressure-sensitive adhesive composition as described
herein.
According to still another aspect of the invention
of the present divisional application, there is provided a
tape comprising a pressure-sensitive adhesive composition as
described herein.
Applicants have discovered the surprising result
that by controlling the Tg of the A or ,A' segment of the
mufti-arm block copolymer to a sufficiently low level, a
pressure sensitive adhesive tape having a good balance of
resistance to low stress peel and peel adhesion is achieved.
Accordingly, the adhesive of the present invention, and tape
made from it, (1) resist lifting under light loads and (2)
maintain moderate peel adhesion thereby remaining easy to
remove. In addition, the adhesive may be chemically
crosslinked to achieve high temperature cohesive strength
, CA 02462706 2004-04-27
'' 60557-4723(D)
-5a-
and solvent resistance without significantly altering
characteristics (1) and (2) .
The tape of the invention comprises a layer of the
pressure sensitive adhesive on one or both surfaces of a
carrier. The carrier may comprise paper, polymer or any
other suitable material. The carrier may also comprise <~
release surface so that the adhesive coated thereon may be
utilized as a transfer tape. The tape is especially useful
for masking tape applications but may also be useful in
other applications including packaging tapes, and autocl<~ve
indicator tapes. The adhesive may also be used to provide
tape that withstands a. variety of temperatures and chemical
environments.
BRIEF DESCRIPTION OF THE DRAWINGS
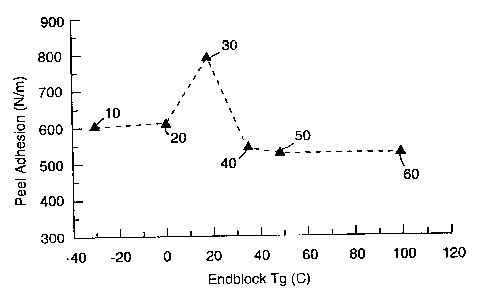
Fig. 1 is a plot of Peel Adhesion versus Endblock
Tg of the mufti-arm block copolymer.
CA 02462706 2004-04-27
Fig. 2 is a plot of the Log of Low Stress Peel versus Endblock Tg of
the multi-arm block copolymer.
DETAILED DESCRIPTION
The general structure of the novel block copolymer of the present
invention is (A-B)mY(C-D)a, wherein polymer segments A and D are endblocks
and polymer segments B and C are midblocks. All polymer segments may be
present as separate phases except in the special case where B and C.comprise
the same homopolymer. Each of the arms of the block copolymer are
represented by the segments (A-B)- and (C-D)- which are also referred to as .
diblocks when not connected to Y. The number of arms which comprise the
block copolymer are indicated by m and n. The residue of a multifunctional
coupling agent, Y, enables the attachment of the arms to form a radial or star
configuration.
The A segment of the block copolymer comprises a random copolymer
of a monoalkenylarene and a conjugated diene. The term °'random" means
that
the A segment comprises segments of monoalkenylarene and conjugated diene.
in no prescribed order. The number average molecular weight (Mn) of each ,A
segment is preferably between about 5,000 and 200,000.
The B and C segments comprise either a homopolymer of a conjugated
diene or a polymer of two or more conjugated dienes. The Mn of each B and
C segment is preferably between about 5,000 and 200,000 and more preferably
between about 10,000 and 100,000. The D segment comprises either a
homopolymer of a monoalkenylarene or a copolymer of a monoalkenylarene
and conjugated dime, and the molecular weight of D is preferably between
about 5,000 and 100,000. The conjugated dimes in segments B, C, and D may
be the same or different monomers.
In addition, the Tg of D is greater than the Tg of A. Preferably the D
block has a Tg greater than 30°C, more preferably greater than
80°C. The 'Tg
of A is preferably less than 30°C. The Tg of A is controlled by
adjusting the
ratio of monoaikenylarene and conjugated diene which comprise the A block.
CA 02462706 2004-04-27
The Tg of an endblock may be determined experimentally using
differential scanning calorimetry (DSC) as measured by ASTM Test Method
D34I8-82 using a Perkin-Elmer 7 series thermal analysis system at a headn,g
rate of 20°C/min. The Fox Equation may be used to predict the Tg of the
S endblock based on monomer feed ratios. The Fox Equation has the generaY
form of:
1!'fg = ~(M;/Tg;]
where M; is the mass fraction of component i and Te; is the glass transition
temperature of component i (L.H. Sperling, Introduction of Phi sm'ca1 PPolymer
Sci nce, John Wiley & Sons (1986). The Fox Equation assumes a single
phase.
The monoalkenylarene useful in the present invention preferably has
8-18 carbon atoms within its molecular structure. Examples of useful
monoalkenylarenes include styrene, alpha-methyl styrene, vinyltoluene;
vinylpyridine, ethylstyrene, t-butylstyrene, isopropylstyrene,
dimeth~lstyrene,
and other alkylated styrenes as well as mixtures of the same. Styrene is the
preferred monoalkenylarene. The conjugated diene useful in the present
invention preferably has from 4 to i 2 carbon atoms within its molecular
structure. Examples of useful conjugated dienes include isoprene, butadiene,
ethylbutadiene, phenylbutadiene, piperylene, dimethyl butadiene, hexadiene,
ethylhexadiene, and mixtures of the same. Butadiene and isoprene are
preferred conjugated dienes, and of the two, isoprene is most preferred.
The block copolymer may be prepared by conventional block copolymer
anionic polymerization technology. The random copolymer endblock (A) may
be made by contacting a monoalkenylarene with an initiator in the presence of
an inert diluent and adding increments of a conjugated diene to form a living
polymer having the simplified structure A-M, where A represents the
essentially random copolymer and M represents a Group I metal such as Na, K
and Li.
Organomonolithium compounds are.useful initiators in the process
described above. These compounds are represented by the structure RLi where
CA 02462706 2004-04-27
.8.
R is an aliphatic, cycloaliphatic, or aromatic radical. Examples include ethyl
lithium, n-propyl lithium, isopropyl lithium, n-butyl lithium, sec-butyl
lithium,
terioctyl lithium, n-decyl lithium, phenyl lithium, 2-napthyl lithium,
4-butylphenyl lithium, 4-phenylbutyl lithium, cyclohexyl lithium, and the
like.
The inert diluent may be aromatic, naphthenic or paraffinic hydrocarbon.
Specific examples of useful inert diluents include n-pentane, n-hexane,
isooctane, cyclohexane, toluene, benzene, xylene and the like.
In this case where a block copolymer having the structure
(A-B)mY(C-D)~ is being formed, the living polymer A-M may be contacted with
a conjugated dime to form living polymer having the general structure A-B-r~i.
The living polymer is then coupled with arms (D-C-M) using a multifunctional
coupling agent to form the Finked block copolymer. Since this coupling
reaction may not always go to completion, there may also be some unlinked
diblock, (A-B) or (C-D), present in the polymer mass. The amount of unlinked
diblock will vary with the coupling efficiency of the linking reaction.
The multifunctional coupling agents suitable for the invention may be
any of the poIyalkenyl coupling agents or other materials known to have
functional groups which can react with carbanions of the living polymer to
form
linked polymers. Examples of suitable multifunctional coupling agents include
silicon halides, polyepoxides, polyisocyanates, polyketones, polyanhydrides,
dicarboxylic acid esters. Suitable polyalkenyl coupling agents may be
aliphatic,
aromatic or heterocyclic. Examples of aliphatic polyalkenyl coupling agents
include the polyvinyl and polyalkyl acetylenes, diacetylenes, phosphates and
phosphites, dimethacrylates such as ethylene dimethacrylate, and the like.
Examples of suitable heterocyclic polyalkenyl coupling agents include divinyl
pyridine, divinyl thiophene, and the like. Examples of suitable aromatic
alkenyl coupling agents, which are preferred in the present invention, include
polyvinyl benzene, polyvinyl toluene, polyvinyl xylene, polyvinyl anthracene,
polyvinyl naphthalene, divinyl durene and the like. Suitable polyvinyl groups
3D include divinyl, trivinyl and tetravinyl. Divinylbenzene (DVB) is the
preferred
CA 02462706 2004-04-27
-9-
coupling agent in the present invention, and may include o-divinylbenzene,
m-divinylbenzene, p-divinylbenzene, and mixtures of the same.
The novel mufti-arm block copolymer described above and having the
general structure (A-B)mY(C-D)p is especially useful in providing a pressure
sensitive adhesive. A related mufti-arm elastomeric block copolymer having the
general structure (A'-B),"Y is also useful in providing a pressure sensitive
adhesive. In the (A'-B)mY structure, A' and B are polymer segments as
described above with reference to Formula I with the added proviso that the
'Tg
of A' is less than 19°C. Y is a residue of a multifunctional coupling
agent;
and m is the number of arms and is at least three.
Various formulating ingredients are known for the preparation of
adhesives from block copolymers of Formulae I and II. The -formulating
ingredients may include tackifying resins and plasticixers, which perform a
variety of functions in the formulation of adhesives. The block copolymer
itself
is normally not sufficiently tacky to function as an adhesive. Thus, it is
often
necessary to add a tackifying resin or combination of resins to increase the
tack. At least one tacltifying resin must be compatible with the midblock of
the
block copolymer, but it may also be compatible with at least one of the
endblock polymer segments. In the present invention, solid or hard tackifying
resins that are compatible with the midblock are generally preferred.
Tackifiers or tacltifying resins generally refer to materials which are
miscible with the midblock in the block copolymer, have a number average
molecular weight {Mn) of 10,00(? grams per mol {g/mol) or less, a softening
point above 70°C as detea~mined using a ring and ball apparatus, and a
Tg of
-30°C or more as measured by differential scanning calorimetry (DSC).
Suitable tackifying resins may include rosin and rosin derivatives,
polyterpenes,
coumarone indenes, hydrogenated resins and hydrocarbon resins, for example:
alpha pinene-based resins, beta pinene-based resins, limonene-based resins,
piperylene-based hydrocarbon resins, esters of rosins, polyterpene and
aromatic
modified polyterpene resins, aromatic modified piperylene-based hydrocarbon
resins, aromatic modified dicyclopentadiene-based hydrocarbon resins and
CA 02462706 2004-04-27
~10°
aromatic modified di-terpene and tri-terpene resins. Preferably a tackifying
resin is present in the pressure sensitive adhesive of the present invention
in an
amount of from about 20 to about 300, (more preferably from about S0 to 200)
parts by weight per 100 parts by weight of the block copolymer.
Plasticizers may also be used in the adhesive formulation to,provide
wetting action and/or viscosity control. These plasticizers are well known in
the art and may include hydrocarbon ails, liquid or soft tackifiers, including
liquid hydrocarbon resins, liquid polyterpenes, liquid rosin esters, liquid
polystyrene resins and the like, elastomer oligomers, waxes, and mixtures of
oils and hard tackifiers. A plasticizer may be present in the pressure
sensitive
adhesive of the present invention in an amount of from 0 to about 200 parts by
weight per 100 parts by weight of the block copolymer.
The adhesive of the present invention may also be crosslinked. In
general, crosslinking improves the solvent resistance and high temperature
IS cohesive strength of the adhesive. Various crosslinking agents such as
crosslinking promoters and reactive curatives may be employed to facilitate
crosslinking of the adhesive. These agents are known to those of skill in the
art
and may be used in combination with heat, ultraviolet radiation or electron
beam radiation to effectuate crosslinking. A crosslinking agent may be present
in the pressure sensitive adhesive of the present invention in an amount of
from
0 to about 50 parts by weight per 100 parts of copolymer:
The compositions of this invention may be modified with supplementary
materials including pigments, fillers; and the like as well as stabilizers and
oxidation inhibitors.
The adhesive compositions of the present invention may be applied to
the substrate from a solution of up to about 40% weight solids of the
ingredients in a solvent such as toluene, the solvent being removed by
evaporation prior to crossIinking by exposure to the radiation. Alternatively,
the ingredients may be mixed in a solvent, the mixture may be emulsified and
the solvent evaporated, and the adhesive may be applied to the substrate as
50-60% weight solids water-based emulsion, the water being removed by
CA 02462706 2004-04-27
~Z~~
evaporation prior to crosslinking. Adhesives of the present invention may also
be applied to the substrate as 100% solids hot melt adhesives. For example, an
extruder may be used to mix the adhesive, feed a coating die and apply the
adhesive to the substrate.
A preferred use of the pressure-sensitive adhesive of the invention is in
the preparation of a novel pressure-sensitive tape. The tape comprises a layer
of the adhesive composition of the present invention coated on at least a
portion
of at least one major surface of a carrier. The carrier may be a plastic film,
paper, foamed polymer or any other suitable material, and the tape may include
various other layers or coatings, such as primers, release coatings and the
like,
which are used in the manufacture of pressure-sensitive adhesive tapes.
EXAMPLE 1
A star block copolymer having the general structure (A-B)mY(C-D)" was
prepared. The A block of the star block copolymer of this example comprised
a copolymer of styrene and isoprene, while the B and C blocks comprised
homopolyrners of polyisoprene, and the D block comprised a homopolymer of
polystyrene. The star block copolymer was prepared as follows:
Reactor 1 was charged with dry cyclohexane (100 ml), sec-butyllithium
(0.24 mmol) and purified styrene (6.0 g) under argon protection using a
syringe. The polymerization of the living styrene endblock was allowed to
proceed at 40°C for at least one hour to achieve a molecular weight of
25,000 g/mol. Reactor 2 was charged with dry cyclohexane (300 ml) and
sec-butyllithium (0.97 mmol). Purified styrene (8.4 g) was added to reactor 2,
followed by incremental introduction of isoprene (20.7 g) to form living
styrene-isoprene random copolymer endblock. The contents in the reactor 2
were allowed to polymerize for a minimum of one hour at ~0-b0°C after
the
last charge of the monomers. Reactor 3 was charged with dry cyclohexane
(1500 ml) and purified isoprene (84.9 g). The living styrene endblock solution
in reactor 1 and the styrenelisoprene copolymer endblock solution in reactor 2
were then transferred into polymerization reactor 3 through a cannula under
CA 02462706 2004-04-27
-12-
argon protection. The polyisoprene midblock was then allowed to polymerize
for a minimum of two hours at 50-60°C. The living diblock copolymer
arms
thus formed were linked to a star structure through a coupling reaction with
dry
divinylbenzene (DVB, 12.1 mmol). A bright dark red color developed after the
addition of DVB. The coupling reaction was allowed to complete at 50.-
60°C
for 12 hours. The reaction was terminated with 1 ml isopropanol (previously
purged with argon) followed by the addition of 1-2% (based on polymer
weight) octadecyl-3, 5-di-tart-butyl-4-hydroxyhydrocinnamate (Irganox'~ 1076)
antioxidant. The star block copolymer was precipitated into isopropanol and
dried in a vacuum oven ac 50°C for three days.
The Mn of the endblock, diblock and star block copolymers were
determined as follows:
The endblock, diblock and star block copolymers were characterized
using a Hewlett-Packard Model 1082B size exclusion chromatograph equipped
with two bimodal Zorbax PSM Kits (two columns at 60-S A and two columns
at 1000-S A). Individual endblock, diblock and star block copolymer samples
were dissolved in filtered, AR grade tetrahydrofuran (available from
Mallinckrodt Chemical Co., Paris, KY) and passed through columns at a rate of
0.5 ml per minute at 40°C. The refractive indices of the samples were
measured using a Hewlett-Packard Model 1037A differential refractometer
detector and compared against the calibration curves obtained using
polystyrene
standards. All molecular weight averages are polystyrene equivalent molecular
weights and are summarized in Table 1. The average number of arms on each
star was calculated directly from the SEC measurements without considering
any branching factor, and these also are summarized in Table 1.
The Tg in °C of the midblocks and endblocks were measured by
differential scanning calorimetry (DSC) according to ASTM Test Method
D3418-82 using a Perkin-Elmer 7 series thermal analysis system at a heating
rate of 20°Clmin. The measured values are included in Table 1. The Fox
Equation was also used to calculate the theoretical Tg of each endblock based
on monomer feed ratios. In this calculation, a Tg of 373°K was used for
CA 02462706 2004-04-27
-13-
polystyrene and a Tg of 213°K was used for polyisoprene. The calculated
values are included in parentheses in Table 1.
Table 1
Mn Tg (C)
X c~~
10-3
c~
Polymer ~I of Arms Endblock
# ~"~
S/IS (S/1)-Star Mid-BlockS/I S
1 39 35237 1, ? -59 -21 2(
613 (-30) 100)
(a) Measured by SEC using polystyrene standards.
(b) Calculated from SEC measurements, not considering the branching
factor.
(c) Measured by DSC and the values in parentheses were calculated using
Fox equation.
EXAMPLE 2
Five separate star block copolymers were prepared. Three of the five
star block copolymers illustrate the invention while two of them are for
comparative purposes. All f ve star block copolymers of this example have the
general structure (A'-B)nY wherein block A' comprises a copolymer of styrene
and isoprene and block B comprises a homopolymer of isoprene. The block
copolymers were prepared as follows:
Polymerization reactions were conducted in single neck round bottom
flasks equipped with Rotoflo'"' stopcocks and magnetic stirring bars. All
transfers of solvents, monomers and coupling agents into the flasks were
conducted through the stopcock either under high vacuum conditions or argon
atmosphere.
A first reactor vessel was charged with dry.Analytical Reagent (AR)
grade cyclohexane (available from Mallinckrodt Chemicals Co., Paris, K7~').
Next, 12 wt % sec-butyllithium in cyclohexane (available from Lithium
Corporation of America, Bemmemer City, NC) was added to the vessel.
Following that addition, 99% pure styrene (available from Aldrich Chemical
Company, Milwaukee; WI) was added to the vessel. 99 % . pure isoprene
CA 02462706 2004-04-27
-14-
(available from Goodyear Tire & Rubber Co., Akron, OlH) was then added to
the vessel in 3-5 ml increments. All quantities of the above-mentioned
chemicals are given in Table 2.
CA 02462706 2004-04-27
60557-4723 (D)
-15-
a
y. . . ~ :~;
~
~. U ~ ..
U
' ~
~
v~ ~ o o ~ ~ n
a o
o
0
...
-. sc ~ 0 0
s s v s s
N ~ ~ ~ Q
t O
O
U
a0,1 c~ O yt ~ O
r,
M
N ~ tr1et
N
U N
p ~ ~ ~ V7 ~ N ~
~
~ ~ ~7 ~ C N
~
U
n
E
N
V
,..a
N
~ N O o O O
O
.-. ._:..~,~ r.;
. .-.N
~c > >
4. ......
U
E N . st
M
' p, p,
,>
O
Q'' O O
U t3
CA 02462706 2004-04-27
-16-
After the last addition of isoprene, the contents of the reaction vessel were
allowed to polymerize at 50-60°C (122-140°F) for a minimum of
one hour to form a
random styrenelisoprene [S/I] copolymer endblock. The resulting living styrene-
isoprene endblock solution was then transferred through a cannula, under argon
atmosphere, into a second reactor vessel charged with dry AR grade cyclohexane
and
99% pure isoprene as indicated in Table 1. The contents were allowed to
polymerize
for a minimum of two hours at 50-60°C (122-140°F) to form a
styrenelisoprene-
isoprene ((S/I)-I] diblock copolymer. 1.3 grams of dry divinylbenzene (DVB)
(available from Dow Chemical Company, Midland; MI) was then added to the
vessel
and allowed to react for about I2 hours at 50-60°C (I31-149°F)
to link the living
diblock arms into a star structure. A few drops of AR grade isopropyl alcohol
(available from Mallinckrodt Chemicals Co., Paris, KY) were introduced into
the
reactor vessel to terminate the reaction, and I-2 % (based on polymer weight)
of
Irganox~" 1076 antioxidant (octadecyl-3,5,-di-tert-butyl-4-
hydroxyhydrocinnamate,
available from Ciba-Geigy Corp. , Hawthorne; NY), was added to stabilize the
polymer. The star block copolymer was precipitated into AR grade isopropanol
and
dried in a vacuum oven at 50°C for three days.
The Mn of the endblock (S/I), diblock ((SII)-I) and star block copolymer were
determined as described in Example 1 and are given in Table 3. The average
number
of arms and the Tg of the midblocks and endblocks were also determined as in
Example I and are given in Table 3.
CA 02462706 2004-04-27
17
o .-. .~
M :. O o
~ o
~
~ v ~ ~ ~
,O ~ ~ C
'C C7 ~'~'
O
..
.
U ~
e ~
_
on .se
E- ~
o
~ ~
n n , . . ~ ~n
w c
o,p
_ cd
g ~
,D U
E o V
~ ~D ~ 00 ~
Ov
a ~o
.c 3
N
N C ~ ~ ~
~
.D~ ~' 0 ~
~' 0 a
. ~
O ~ ~
O .~ ..,
.
~ N
~ ~
O ~.. H
>, ~.. t~ .
""'
~' ~ .-.o t~ ~n ~ a
~t ~ ~ ~ ~ ~ .~ ~' ~ ,~
n ~ ~ ...
C
.~ W ~
U vy >,
N N N , j ~'" .a 'fl
N ~G
H
U
N ~U~z
> >
....
N c'7 ~ ~ r~ r~ w r~
t!' V ~ '
v ~
,v
O O
U U ,
CA 02462706 2004-04-27
'18'
EXAMPLE 3
A star block copolymer having the general structure (A'-B~,Y wherein block A'
comprises styrene and block B comprises isoprene was prepared for comparative
purposes. The block copolymer was prepared as follows:
Reactor 1 was charged with dry cyclohexane (200 ml), sec-butyllithium (I.0
mmol) and purified styrene (12:0 g) under argon protection using a syringe.
The
polymerizatian of the living styrene end block was allowed to complete at 40'C
for a
minimum of one hour. Reactor 2 was charged with dry cyclohexane (1500 ml) and
purified isoprene (100 g). The living styrene endblock in Reactor 1 was then
transferred into polymerization Reactor 2 through a cannula under argon
protection.
The polyisoprene midblock was alltiwed to polymerize for a minimum of two
hours at
50-60'C: The living diblock copolymer arms thus formed were linked to a star
structure through a coupling reaction with divinylbenzene (DVB, 12.1 mmol). A
bright dark red color developed after the addition of DVB. The coupling
reaction was
allowed to complete at 50-60' C for 12 hours. The reaction was terminated with
1 ml
isopropanol; previously purged with argon, followed by the addition of 1-2%
(based on
polymer weight) octadecyl-3, 5-di-tert-butyl-4-hydroxyhydro-cinnarnate
(Irganox"'
1076) antioxidant. The star block copolymer was precipitated into isopropanol
and
dried in a vacuum oven for three days.
The Mn of the endblock was determined as in Example 1 and is given in ?'able
4. The design targets for the Mn of the diblock and star as well as the number
of arms
are also given in Table 4. The Tg of the midblock and endblock are also given
in
Table 4.
CA 02462706 2004-04-27
_19_
Table 4
Mn X # Tg ('
I0-3 ~ C) .~>
~'~
Arms
Polymer # End-BlackDi-Block Star '~ Mid-BlockEnd-Block
Comparative 13 140 1,109 8 -64 92
3
(a) Measured by SEC using polystyrene standards.
(b) Calculated from SEC measurements, not considering, the
branching factor.
(c) Measured by DSC.
EXAMPLE 4
Each of the star block copolymers described in Examples 1-3 above was
formulated into different adhesive compositions.
1S The block copolymers were combined with Wingtack~" Extra tackifier resin; a
Cs hydrocarbon modified with alpha methyl styrene (available from Goodyear
Tire &
Rubber Co., Chicago, IL) and Zonarez'r" A-25, a low molecular weight alpha
pinene
resin having a ring and ball softening point of 25°C (available from
Arizona Chemical
Co., Panama City, FL). The amount of each resin component used in the adhesive
formulations is given in Table 5 as parts by weight per 100 parts block
copolyrner
(PPh)~
The resulting compositions were weighed dry and dissolved in toluene too give
35% solids by weight solution. The solutions were separately knife coated onto
38.1
micrometer (1.5 mil) thick biaxially oriented polyethylene terephthalate (PET)
film at a
coating weight of 41.94 g/m2 (10 grains/24 inz). The coatings were dried for
three
minutes at room temperature (22°C or 72°F), followed by 2
minutes at 180°F (82°C)
in a convection oven. The coatings were then . removed from the oven and
covered
with a silicone coated release liner. Two samples of each formulation were
made, and
one sample of each was irradiated with electron beam radiation using an
Electrocurtain
CB-300 electron beam system (available from Energy Sciences, inc., Wilmington,
MA). Before irradiation, .the liner was removed, and the adhesive was
irradiated at
12S kV at a dose of 6 MRads. The liner was then replaced. The low stress peel
CA 02462706 2004-04-27
-20-
properties of each adhesive formulation were then measured. These measurements
were conducted in a controlled environment testing room maintained at
70°F (21 °C)'
and 5086 relative humidity,
To measure adhesion, the tapes were conditioned in the controlled. environment
S for 24 hours and analyzed on a SintecH .6 computerized system for material
testing,
according to standard tape method PSTC-1, Peel Adhesion for Single Coated
Tapes
180° Angle. The tape was removed at an angle of 180 degrees at a rate
of 30.5
cm/min (12 in/min). A load cell linked to a computer was used to estimate the
value
reported in Table 5.
' To measure resistance to low stress peel, 19.0Smm (.7S inch) by 10I .6mm (4
inch) samples of each tape were conditioned for 24 hours in a controlled
environment.
After conditioning, a sample was applied to a diacetone alcohol washed 101:6mm
(4 inch) brightly annealed, highly polished 304 stainless steel test panel
using four
passes of a 2 kg (4.5 1b) rubber-faced roller. A static load of 200 grams was
attached .
to the tape at an angle of 90 degrees, and the time, it took for the load to
drop was
measured in minutes. The test results given in Table S represent the average
of two
duplicate tests.
The mode of failure for each the peel adhesion and resistance to low stress
peel
test are,also indicated in Table S for each tape. "C" indicates cohesive
failure,
meaning that the adhesive left a visible residue on the test panel substrate.
"A"
indicates adhesive failure wherein the adhesive left no visible residue on the
test panel
substrate. "I" indicates adhesive failure at the adhesiveObacking iriterface
wherein the
adhesive pulled away from the backing and remained on the test panel. "X"
indicates
that the value was extrapolated.
CA 02462706 2004-04-27
21
U U ~ a U U a ~ U U a U U- ~
n. u,,
N
M
C O ...M M N ~ ....,'....~ ~
M M
.~, ~ ~ ~ ~ N O O O O~
N N 0 ~ ~
~ ~ ~ N ~ ~
a a a a U U < a a U < a a a a .
.
M 00 1n !~ N M N O I~ O M
....~p ~ ~ ~ V'1N ~L7: ~D M M ...,
b z M ,..,N ...a v a
t"~ O ~ ~ ~ h
a M V~ V
O O O O o y O ~ d~ ~O I~ I'~rd'-~
~
et V'1O t' ' ~ ~ 'f')M ~ N V1 00 N M
M ~D M' '~ a a M V1 M ' d' h ~ . h
a ~l7
~ .
N M O M O M O M O M . M O c<1O M
G O
M O M ~ ch O M O craO M O M Q M
C M ~'7M V7 ~ 1P1M V1 ~ ~ . In f ~ M
~ M 7
.~
H
O O W t7 O O ~O ~O O O ~G ~D O O ~D
E" ~"'~E""f"'E" ~."f" E"'~' E"'f" E-'. E.,
W W W W W W W W W W W ~ W E-"
W
_ W
t~i.pr p, p, p. 0.G. 0. p, p. 0. G. . p: CL~
. ~.
a..
a~
,.. .....- r-.tV N N N M M M M ~t Q' et
'
.-.
CA 02462706 2004-04-27
60557-4723 (D)
-22 -
V Q a a d Q d a a. a d d a
...
C 00 ~ M 00 CM O~ ~D 00 vi
'' 0 D p N N et e0
~"
~ O o0 M .-.0 ~D ~ ....p
M G
.
I
~
'8 a a a 4 a a .~ a Q a a Q a
~
'~
tt N N V'~.--~O ~ O ~O O~ 00 N ~O vi
00 tn cri~ fh 00 1n 00 h ~ N . ~
00 ....-..V"1d' M ~1 M N ~ f tn fa
~ 01 N
a ~ et vD e! ~ eh d' '~ h N M ~1 ~
00
.r .
G o o a o 0 0 0 0 r~
n -~o? c
~ ~ M V~'7~h ~ d0'et d0'et N l'~~M ef''G
~ . O
~ . .
~ M t'~ f''~ M M M C O ~ .
~C
., O M O M O M C? M O M O O t~ O
V'7 N ~"~ V1 ~ ~'1 h
f' c' r1 aG
~ ~ U
~
3
3
U
M M M l'~ M M ~
~ ~ Q Q ~ f O E
b M t"~ M M I M 1~ M y ~ p
4 M M M c'~1j ch M r~ . ~
~ '
~ ~ ~
'r E;E
~a
,
~ ~
~ w
E
~
~
o v ~
y ,
o o a va .r,o o vo c o o ~o ~ ~ ~ .~ .~
a~
.
Ii . a .~
x ~ ~
~. H ~- H H H ~. H r~ H H H
W ~
W W W W W W W W W W W
~ i1.G. C5.G, CL Q. 0. p. ~L t1.p, p, pr E ~ ~
(~
w
e~
N N N N c~ M M c~1~ c~ ~ .~
N N C7 U d~l03 U ~ W b1 ~ U ~ '. ~ ~
> > > > > > a ' > > > > ; .c
>
aJ ~i.~y ~ ~.vr~a=1.Jj.11J ~~ a~r~a ~. 'N ....
C'Ctd tc~etS!CScd ed c~ ~ ~ U
G t
T ~y to td !C~ ~S Cd
p 0. C. 0. C1.fs.C t3.C~.C1.t1.Cs.Q
a E E E E E E ' E E E E E '~
E
. I!
C~ U U U 'U U U U U U U U
CA 02462706 2004-04-27
23
RESULTS
One of the primary goals of this series of experiments was td identify a
pressure sensitive adhesive tape having a sufficient balance of adhesion and
resistance to low stress peel. Only tapes of the present invention (made from
polymers 1-5) showed both high resistance to low stress peel while maintaining
moderate adhesion. None of the comparatives, on the other hand, showed
acceptable resistance to low stress peel.
Applicants have discovered that this balance of properties is achieved by
controlling the Tg of the endblock of the block copolymer to a suffciently low
level. This is further illustrated in Figures 1 and 2 which show Peel Adhesion
and the Log of Low Stress Peel as a function of Endblock Tg respectively. The
graphs plot adhesion and low stress peel values for adhesives prepared from
polymers 2, 3, 4, comparative 1, comparative 2 and comparative 3. Polymers
2, 3 and 4 each of have an endblock Tg of less than room temperature. The
y comparative polymers each have an endblock Tg of greater than room,
temperature. Each of the adhesive compositions consisted of 100 parts by
weight of the polymer, 50 parts by weight of Wingtack~" Extra and 50 parts by
weight Zonarez'"' A-25. The compositions were applied to a biaxially oriented
PET backing, dried and irradiated as described above in this Example 4. Iv the
Figures, reference numerals 10, 2U, 30, 40, 50 and 60 refer to adhesives
employing polymers 2, 3; 4, comparative 1, comparative 2 and comparative 3
respectively.
The figures show that tapes made from polymer having .endblock Tg
values less than room teinperature'exhibit acceptable resistance to low stress
peel values while maintaining moderate adhesion. Above an endblock Tg value
of 30°C, the balance of properties is lost as the resistance to low
stress peel
drops off markedly. Accordingly, the adhesive of the present invention, and
tape made from it, (1) resist lifting under light loads and (2) maintain
moderate .
peel adhesion thereby remaining easy to remove.