Note: Descriptions are shown in the official language in which they were submitted.
CA 02463214 2004-04-02
Medical Device with Track and Method of Use
Field of the Invention
The present invention relates to a medical device, and more particularly to a
medical device that can be advanced along a track located within a lumen of a
patient's
body.
B ackground
A physician typically accesses and visualizes tissue within a patient's
gastrointestinal (GI) tract with a long, flexible endoscope. For the upper GI,
a physician
may insert a gastroscope into the sedated patient's mouth to examine and treat
tissue in
the esophagus, stomach, and proximal duodenum. For the lower GI, a physician
may
insert a colonoscope through the sedated patient's anus to examine the rectum
and colon.
Some endoscopes have a working channel, typically about 2.5-3.5mm in diameter,
extending from a port in the handpiece to the distal tip of the flexible
shaft. A physician
may insert medical instruments into the working channel to help diagnose or
treat tissues
within the patient. Physicians commonly take tissue biopsies from the mucosal
lining of
the GI tract using a flexible, biopsy forceps through the working channel of
the
endoscope.
Insertion of a flexible endoscope, especially into the colon, is usually a
very time-
consuming and uncomfortable procedure for the patient, even when sedated with
drugs.
A physician often needs several minutes to push a flexible endoscope through
the
convoluted sigmoid, descending, transverse, and ascending portions of the
colon. The
physician may diagnose and/or treat tissues within the colon either during
insertion or
removal of the endoscope. Often the flexible endoscope "loops" within the
colon, such as
at the sigmoid colon or at the splenic flexure of the colon, so that it
becomes difficult to
further advance the endoscope along the colon. When a loop is fot _____ aied,
the force exerted
to push the scope stretches the mesentery and causes pain for the patient.
Depending on
the anatomy of the patient and the skill of the physician in manipulating the
flexible
endoscope, some portions of the colon may be unexamined, thus increasing the
risk of
undiagnosed disease.
Given Engineering LTD, Yoqneam, Israel, sells a device in the U.S. called the
M2ATM Swallowable Imaging Capsule. The device contains a tiny video camera,
battery,
1
CA 02463214 2004-04-02
and transmitter. It is propelled through the gastrointestinal tract by natural
peristalsis. The
device is currently used for diagnostic purposes and passes through the
intestinal tract
with a velocity determined by the natural, peristaltic action of the patient's
body. World
Publication No. WO 0108548A1 filed by C. Mosse, et al. describes a self-
propelling
device adapted to travel through a passage having walls containing contractile
tissue. The
applicants disclose that the device is particularly useful as an enteroscope
and may also
carry objects such as feeding tubes, guide wires, physiological sensors or
conventional
endoscopes within the gut. A summary of other alternatives to push endoscopy
can be
found in "Technical Advances and Experimental Devices for Enteroscopy" by C.
Mosse,
et al, published in Gastrointestinal Endoscopy Clinics of North America,
Volume 9,
Number 1, January 1999: pp. 145-161.
Scientists and engineers continue to seek improved methods and devices for
accessing, diagnosing and/or treating tissue within body lumens, including the
GI tract.
Summary of the Invention
Applicant has recognized the desirability of a low cost, potentially
disposable
medical device which may provide physicians with a desirable alternative to
using a
conventional, reusable, flexible endoscope. Eliminating the need for the
operator to make
constant adjustment of the articulation controls of an endoscope may reduce
the skill
required to intubate the device, allowing operators other than physicians to
use the
device. This is advantageous because gastroenterologists currently do not have
the
capacity to handle all of the patients that need colonoscopies, so equipment
that enables
other staff, such as nurses, to help with the procedure could increase the
capacity and
allow gastroenterologists to treat more patients.
In one embodiment, the present invention provides a medical device comprising
an elongate flexible member which can be advanced through a body lumen on a
track.
The track can have a loop portion which is advanced distally of the flexible
member, and
the member is then advanced along the track. The flexible member can include
visualization means, light means, and channels for fluids (gas or liquid) and
instruments.
The present invention also provides a method of moving a medical apparatus
through a patient's body, such as through the GI tract. The method can
comprise the steps
of positioning a portion of the medical device within the body lumen;
advancing a length
of track distally of the medical device; and advancing the medical device on
the track to
2
CA 02463214 2011-09-07
move distally within the lumen. The medical device can be advanced distally
while
simultaneously retracting a portion of the track proximally.
The invention can be used to assist in diagnosis and treatment of tissue,
including
the placement of medical instruments (including without limitation balloons,
dilators, tissue
graspers, tissue cutting devices, tissue stapling devices, tissue staining or
treatment devices,
vessel ligation devices, and tissue ablation devices) at a desired tissue
site.
The present invention also provides a medical device for use in a patient's
body
comprising a flexible member positionable within a body lumen, the flexible
member
comprising a passageway therethrough; and a track disposed in the passageway
and
extending distally of the flexible member, wherein a portion of the track
extending distally
of the flexible member comprises a loop.
The present invention also provides a method of moving a medical device
through a
patient's body comprising the steps of positioning a portion of the medical
device within
the body lumen; advancing a length of track distally of the medical device
without
advancing a track end distally of the medical device; and advancing the
medical device
along the track to move the medical device distally within the lumen.
The present invention also provides use of a medical device for insertion into
and
advancement along a body lumen of a patient, the insertion and advancement
comprising
the steps of positioning a portion of the medical device within the body
lumen; advancing a
length of track distally of the medical device; and advancing the medical
device on the
track to move distally within the lumen while simultaneously retracting a
portion, of the
track proximally.
The present invention also provides use of a medical device for insertion into
and
advancement along a body lumen of a patient, the insertion and advancement
comprising
the steps of positioning a portion of the medical device within the body
lumen; advancing a
length of track distally of the medical device without advancing a track end
distally of the
medical device; and advancing the medical device along the track to move the
medical
device distally within the lumen.
3
CA 02463214 2011-09-07
More particularly, there is disclosed an endoscope for the gastrointestinal
tract,
comprising: a) a flexible elongate member comprising a proximal end and a
distal end, the
flexible elongate member further comprising a guide tube having an opening
adjacent the
distal end projecting at an oblique angle relative to the longitudinal axis of
the flexible
elongate member; b) a visualization device and light source positioned
adjacent the distal
end of the flexible elongate member; c) a working channel extending between
the proximal
and distal ends of the flexible elongate member adapted to receive medical
instruments, the
working channel comprising an opening adjacent the distal end of the flexible
elongate
member; and d) a guide wire slidably received by the guide tube, the guide
wire positioned
between the proximal and distal ends of the flexible elongate member and
extending
distally relative to the flexible elongate member to form a loop disposed
distally relative to
the flexible elongate member, the guide wire being longitudinally moveable
relative to the
flexible elongate member to vary the size of the loop.
Brief Description of the Drawings
While the novel features of the invention are set forth with particularity in
the
appended claims, the invention in all its embodiments may be more fully
understood with
3a
CA 02463214 2004-04-02
reference to the following description and accompanying drawings, wherein FIG.
1 is a
schematic view of a medical device is accordance with a specific embodiment of
the
invention;
FIG. 2 is a side sectional view of the medical device shown in FIG. 1;
FIG. 3 is a cross sectional view of a portion of a gastrointestinal tract
showing the
medical device placed relative to anatomical milestones including a sigmoid, a
descending colon, a left splenic flexure, a transverse colon, a hepatic
flexure, an
ascending colon, and a cecum;
FIG. 4 is a longitudinal section view, enlarged, of the medical device of FIG.
1;
FIG. 5 is a cross sectional view of the medical device taken at line 5-5 of
FIG. 1;
FIG. 6 is a cross-sectional view, enlarged, from FIG. 5, of an umbilicus
component
of the medical device;
FIG. 7 is an isometric view, transparent, of a capsule portion of the medical
device
shown in FIG. 4;
FIG. 8 is a cross-sectional view of the capsule taken at line 8-8 of FIG. 7;
FIG. 9 is a sectional view of the capsule taken at line 9-9 of FIG. 8;
FIG. 10 is a cross-sectional view of the capsule taken at line 10-10 of FIG.
9;
FIG. 11 is a schematic illustration of a medical device according to another
embodiment of the present invention, showing a flexible, elongate member
extending
from a hand piece of the device, and a loop track extending from a distal end
of the
elongate member;
FIG. 12 is a cross-sectional view of the medical device, taken along section
12-12
in FIG. 11, showing a distal end of a flexible elongate member;
FIGS. 13A-E are sectional views of a portion of a colon, showing the
advancement
of the medical device shown in Figure 11 through a portion of the colon;
FIG. 14 provides a schematic view of a handpiece having motors for advancing
and
retracting a track;
4
CA 02463214 2004-04-02
FIG. 15 provides a schematic illustration of a flexible member of a medical
device
in accordance with an embodiment of the invention, showing a single guide tube
and
having an end of the track fixed at or near a distal end of the flexible
member;
FIG. 16 is a cross-sectional end view of the flexible member, taken along
section
16-16 in FIG. 15;
FIG. 17 is a schematic illustration of a flexible member of a medical device
in
accordance with another embodiment of the invention, showing two sets of
guidewires
and two track loop portions; and
FIG. 18 is a cross-sectional end view of the flexible member, taken along
section
18-18 in FIG. 17.
Detailed Description of the Invention
By way of example, the present invention is illustrated and described for
application in the colon of the lower GI tract of a human patient. However,
the present
invention is applicable for use in the body lumens of other hollow organs in
humans and
in other mammals.
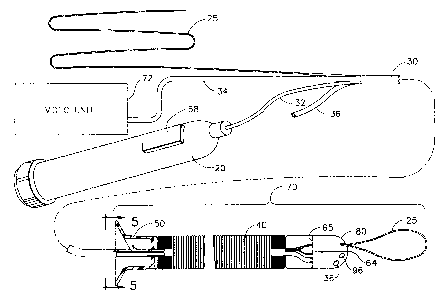
FIG. 1 shows a medical device 70. The medical device 70 can include a movable
apparatus, such as a capsule 80 shaped and sized for movement through a body
lumen, a
compressible sleeve 40, a fixing plate 50, an umbilicus 30, a cable 25, a
video unit 72,
and a handpiece 20.
Capsule 80 generally has a leading end 64 that is smooth for atraumatic
passage
through a tortuous path of a gastrointestinal (GI) tract, such as a colon. In
one
embodiment of capsule 80, leading end 64 is hemispherical and a trailing end
65 is flat to
accept the contents contained in umbilicus 30. Other shapes of capsule 80 are
possible,
such as but not limited to tapered, cylindrical, ovoid, or egg-shaped
configurations, to
facilitate navigation through the colon.
Compressible sleeve 40 can extend from trailing end 65 of capsule 80 to fixing
plate 50. Fixing plate 50 can be anchored to the patient with adhesive. Other
methods of
attachment to the patient include, but are not limited to glue, tape, or a
CA 02463214 2004-04-02
=
close-fitting wrap. Suture or staples may also be used, but are less desirable
because
of the pain involved in their placement or removal. In applications related to
the
lower GI tract, fixing plate 50 can extend into the patient's anus. A secure
attachment of plate 50 to the patients body or other fixture is desirable so
that fixing
plate 50 provides an anchor, thereby enhancing movement of capsule 80 deeper
into the colon.
The proximal portion of umbilicus 30 can extend outside the body and can be
connected to equipment, including video unit 72 and handpiece 20: The distal
portion of umbilicus 30 can be connected to trailing end 65 of capsule 80
inside the
colon. Umbilicus 30 can extend through openings in plate 50 and sleeve 40, and
umbilicus 30 can slide through the openings relative to plate 50 and sleeve
40.
Umbilicus 30 is preferably made from a lightweight, flexible, plastic,
multilumen
tube. For example, umbilicus 30 may have four lumens: a 3mm lumen for a
working
channel 36, a 3mm diameter lumen for the wiring assembly 34, a 5mm diameter
lumen to receive a drive cable 32, and a 3nun lumen to receive cable 25. Many
other sizes and combinations of lumens are possible. Umbilicus 30 may also
comprise separate thin-wall, flexible plastic tubes that are bundled together
with
straps, shrink-wrap, or the like.
Cable 25 can provide a track on which capsule 80 is supported and propelled.
Cable 25 may be constructed in numerous shapes, including a braided strand of
fibers, a coated wire, a flat band, or may have a constant cross sectional
shape
including circular, triangular, or rectangular. Cable 25 may include a
periodic and/or
non periodic pattern of features that assist in traction, such as teeth,
holes, or
grooves. Cable 25 may be made from any suitable material, including without
limitation one or more metals including steel, nitinol, aluminum, or titanium,
and
have diameters including, but not limited to, 0.5nun to 2.5nun.
One suitable material for use as a cable 25 is a guidwire having a nitinol
core with a diameter of about 0.021 inch to about 0.025 inch surrounded by a
stainless steel wire coil having a diameter of about 0.008 inch. The overall
diameter
can be between about 0.037 inch and about 0.041 inch, and the stainless steel
coil
may be soldered or otherwise attached at about 50 cm intervals to hold the
stainless
steel coil in place relative to the nitinol core. Another suitable material
for use as a
6
CA 02463214 2004-04-02
cable 25 is a guidwire marketed as Elite Protector T14 Elite 480 wire guide
available
from Wilson-Cook Medical, Inc. of Winston Salem, NC, and having a diameter of
about 0.035 inch.
A proximal portion of cable 25 extends outside the body, so that an operator
can handle it. Cable 25 is fed through umbilicus 30, though capsule 80, to
form a
cable loop 54 ahead (distally) of capsule 80. As described below, cable loop
54 can
be used to navigate around the tortuous path of the colon, eliminating the
need for
the operator to make constant adjustment of the articulation controls of an
endoscope, thus reducing the skill required to intubate the device. As
alternatives to
cable 25, other suitable track configurations can be used, including without
limitation flexible rails, chains, slides, and belts.
Still referring to FIG. 1, video unit 72 supplies power to a lighting device
96
(FIG. 9), and processes video images taken by a visualization device 95 (FIG.
9) in
= capsule 80 as it moves through the colon so that the operator is able to
view the
inside surface of the lumen. Lighting device 96 may include a bulb or LED
(Light
= Emitting Diode) contained within capsule 80, or include a fiberoptic, a
light pipe, or
a lens of a light source contained in video unit 72. One example of a bulb
that could
be located in capsule 80 is Xenon #724 from Carley Lamps (Torrance, CA).
Visualization device 95 may be a CMOS (Complementary Metallic Oxide
Semiconductor) or CCD (Charged Coupled Device) camera, either of which are
= commercially available in sizes adaptable to use in capsule 80. For
example, a
CMOS chip such as #0V7620 from Onuiivision Technologies (Sunnyvale, CA)
could be used. Wiring assembly 34 transfers signals between video unit 72 and
lighting device 96 and between video unit 72 and visualization device 95. =
=
Handpiece 20 provides a motion control 58 to activate the propulsion of
capsule 80 along cable 25. Capsule 80 can be propelled along cable 25 in any
=
suitable manner. In one embodiment, handpiece 20 contains a motor and operably
controls a flexible drive cable 32, which is constructed to transmit torque,
to operate
a propulsion mechanism 44 (FIG. 7) located inside capsule 80 to move medical
device 70 further into the colon. In one embodiment, motion control 58 has a
forward and reverse setting to change the rotation of a motor within handpiece
20 so
that capsule 80 moves in a forward and backward direction along cable 25.
7
CA 02463214 2004-04-02
The proximal portion of working channel 36 extends out of the body to a
location near handpiece=20, so that the operator can pass medical instruments
in and
out of the colon numerous times. The distal portion of working channel 36
extends
through capsule 80 to an opening in the outer surface of the leading edge 64
of =
5 capsule 80. Medical instruments can be inserted into the proximal end of
working
channel 36 and be directed through working channel to the opening in the outer
surface of the capsule 80 without removing the capsule 80 from the body lumen.
Accordingly, the operator can access lumen tissue adjacent the capsule 80 with
the
medical instruments as the capsule is moved through the lumen. Medical
10 instruments which can be directed through a working channel include
without
limitation tissue graspers, staplers, cutters, clip appliers., tissue ablation
devices,
= tissue staining devices, and devices for dispensing pharmaceutical
agents.
FIG. 2 shows medical device 70 of FIG. 1 including a cable spool 74 outside =
the body, and cable loop 54 ahead (distal) of capsule 80. Cable spool 74
stores a
15 proximal portion of cable 25, and may be used to unwind an additional
length of =
cable 25 through umbilicus 30 and a sliding channel 90, to increase the size
of cable
= loop 54 ahead of capsule 80 in the colon.
Cable loop 54 is formed ahead of capsule 80 from a middle portion of cable
=
25. One end of cable loop 54 is formed by cable extending distally outward
from a =
20 sliding channel 90, and the other end (the return end) of cable loop 54
is formed by
cable extending proximally into an opening in the outer surface of the capsule
80,
where the cable is fed though (and is engaged by) a gripping channel 91 in the
capsule 80. The cable extends from the gripping channel 91 proximally through
compressible sleeve 40 (outside of umbilicus 30) to cable anchor 52. This
=
= 25 arrangement allows an operator to feed an additional portion of cable
25 through
= sliding channel 90 to increase the size of cable loop 54 (other end of
loop is held by
gripping channel 91). As cable loop 54 increases in size, it "unfurls" inside
the
lumen directly ahead of capsule 80, and generally conforms to bends or curves
in the
lumen, thereby laying a track along andlor distal to the bend on which to
propel the =
= 30 capsule. This arrangement of cable loop 54 can be advantageous in
simplifying the
process of navigating the colon. The operator can simply add length to the
loop
portion of the cable to negotiate bends and turns in the GI tract, rather than
trying to
manipulate the end of a guide tube or guide wire through the three dimensional
8
CA 02463214 2004-04-02
curvature of the lumen. The operator then uses motion control 58 (FIG. 1) to
=
advance capsule 80 in a forward direction along the track (the cable loop 54)
to
move the capsule 80 through the bend in colon.
FIG..3 shows medical device 70 positioned in the colon. Cable loop 54 i
introduced first, with capsule 80, compressible sleeve 40, and fixing plate 50
trailing
behind it. Fixing plate 50 can then be securely affixed to the anus or other
suitable
location with adhesive or by other means, creating an anchor point relative to
the
patient.
Cable loop 54 is shown deployed around a bend in the sigmoid colon 100.
The operator monitors progress of capsule 80 and cable loop 54 by viewing
video
unit 72 (FIG. 1), which displays images taken by visualization device 95 (FIG.
9).
When cable loop 54 reaches a sufficient orientation to navigate a bend in the
colon,
capsule 80 is propelled a short distance along cable 25 by propulsion
mechanism 44
(FIG. 7) under control of the operator who activates motion control 58 (FIG.
1).
This process shortens the length of cable loop 54 ahead (distal) of capsule
80.
= To further advance capsule 80 deeper into the colon, the operator slides
more
= of the proximal portion of cable 25 through umbilicus 30 and sliding
channel 90 to
again increase the size of cable loop 54 ahead of capsule 80. This procedure
lays
additional track through additional bends that are deeper in the colon, such
as the left
splenic flexure 112 or the hepatic flexure 110. The operator continues to
slide
cable 25 and activate motion control 58 (FIG. 1), in sequence, to
incrementally
move capsule 80 through the descending colon 102, transverse colon 104, and
ascending colon 106 to cecum 108.
As capsule 80 advances along cable 25,compressible sleeve 40 begins to
uncompress (increase in length) so that a smooth, uninterrupted surface is
maintained from fixing plate 50 to capsule 80.
FIG. 4 is a detailed view of medical device 70 generally comprising capsule
80, compressible sleeve 40, fixing plate 50, and umbilicus 30. In this
embodiment,
capsule 80 is comprised of three sections (a 1st section 77, a rlsection 78,
and a 3rd
section 79) for assembly and contains propulsion mechanism 44 (FIG. 7). Other
embodiments with a different arrangement or number of sections, or other
locations
of propulsion mechanism 44 are possible. Visualization device 95 (FIG. 9) and
=
9
CA 02463214 2004-04-02
lighting device 96 (FIG. 9), located near leading end 64 of capsule 80,
communicate
with video unit 72 through wiring assembly 34 to allow visualization of the
inside of
the lumen in the vicinity of capsule 80. Working channel 36 allows an operator
to
repeatedly pass medical instruments in and out of the patient to perform
treatment in
the vicinity of capsule 80, without removing capsule 80 from the body lumen.
Compressible sleeve 40 can perform at least two functions. First,
compressible sleeve can provide a smooth, uninterrupted, flexible connection
between fixing plate 50 and capsule 80 as it advances deeper into the colon,
to '
thereby assist in protecting the body lumen from damage as medical device 70
navigates the colon. Additionally, compressible sleeve 40 can act to radially
confine
a portion of cable 25 located between gripping channel 91 and cable anchor 52
to
assist in the propulsion of capsule 80 in a forward direction deeper into the
colon.
By radially confining a portion of cable 25 between gripping channel 91 and
anchor
52, the sleeve 40 can assist in preventing a secondary loop from forming in
cable 25
between capsule 80 and fixing plate 50 (prevents formation of a cable loop
behind
(proximal) of capsule 80). Compressible sleeve 40 may be made from any
suitable
material, including without limitation ePTFE (expanded
polytetrafluoroethylene), or
= other suitable flexible material that stretch or otherwise increase in
length to
accommodate the increased distance between the anchor 52 and the capsule 80 as
the
capsule moves deeper into the GI tract.
Propulsion mechanism 44 uses a portion of cable 25 inside gripping channel
91 to propel capsule 80 further into the colon. As motion control 58 (FIG. 1)
is
activated, propulsion mechanism 44 moves a portion of cable 25, initially
comprising cable loop 54, back through gripping channel 91 to a position
between
capsule 80 and fixing plate 50. Therefore, the length of cable 25 between
capsule 80
and fixing plate 50 increases. Because cable 25 is anchored to the patient by
fixing
plate 50 and radially confined by compressible sleeve 40, cable 25 supplies an
axial
force to counteract a traction force applied by propulsion mechanism 44,
resulting in
capsule 80 being propelled further into the colon.
The location of propulsion mechanism 44 inside capsule 80 is advantageous
because it locally propels capsule 80 a short distance from a position already
within
the colon. This decreases the forces needed to push an entire length of
endoscope or
other long flexible extension through the tortuous colon. However, other
CA 02463214 2004-04-02
mechanisms or locations for mechanisms may be used to accomplish the
propulsion. '
For example, propulsion mechanism 44 can be positioned anywhere that allows
the
length of cable 25 between fixing plate 50 and capsule 80 to vary in length,
including a separate pod between capsule 80 and fixing plate 50, a separate
housing
attached to fixing plate 50, or contained within a portion of fixing plate 50.
= FIG. 5 is a cross section of medical device 70 taken at line 5-5 of
FIG. 1, =
showing one embodiment of fixing plate 50 having a relatively large diameter
sized
=for securing it to a patient's anus. Cable anchor 52 is shown as a rigid
attaclunent to
fixing plate 50, so that the distal portion of cable 25 does not move relative
to fixing
= 10 plate 50. Centering attaclunent 56 holds umbilicus 30 in the center of
fixing plate 50 =
for alignment through the anus into the colon.
FIG. 6 shows a detailed view of the cross section of umbilicus 30 from FTG.
5, including a lumen for cable 25, a lumen for wiring assembly 34, a lumen for
drive
cable 32, and working channel 36. FIG. 6 indicates the relative positions and
sizes
=
15 of these lumens and elements in this embodiment of umbilicus
30. Numerous other =
= sizes and arrangements are possible. For example, additional working
channels
could be added, working channel 36 could be sized larger to allow for passage
of
= larger instruments, or the lumen for drive cable 32 could be smaller. In
general, it is
= advantageous to have a small diameter and lightweight umbilicus 30 so
that capsule
20 80 has as little drag as possible when advancing through
the colon.
FIG. 7 is an isometric view of one embodiment of compressible sleeve 40
= and capsule 80 including sliding channel 90, gripping channel 91, working
channel
= 36, and propulsion mechanism 44 including a first miter gear 82, a second
miter gear
83, a pulley 86, and a pulley grip 87. This illustration shows the relative
positions
= 25 of these elements in three-dimensional space.
Propulsion mechanism 44 works by changing the length of cable 25 between
capsule 80 and fixing plate 50, which has been secured to the patient's body.
In this
manner, capsule 80 can move deeper into the colon when this length of cable 25
increases, and moves backward out of the colon when this length decreases. In
this
30
embodiment, propulsion mechanism 44 comprises a gear system described
below
= contained within capsule 80, but other locations and systems are possible
FIG. 8 is a cross sectional view of capsule 80 taken at line 8-8 of FIG. 7,
showing an arrangement of gears in this embodiment of propulsion mechanism 44
= 11
CA 02463214 2004-04-02
=
(FIG. 7). The distal portion of drive cable 32 passes through trailing end 65
of =
capsule 80 and coaxially connects to first miter gear 82. Drive cable 32 is
constructed to transmit torque from handpiece 20 to first miter gear 82, so
that when
the operator activates motion control 58 (FIG. 1), first miter gear 82 rotates
around
= 5 an axis collinear with drive cable 32. =
In the embodiment shown, miter gears 82 and 83 are supported in the capsule
80 (such as by a suitable bearing or bushing) for rotation about their
respective axes
of rotation, which are generally perpendicular to one another. The teeth of
first miter
= gear 82 and second miter gear 83 are each cut at a 45-degree angle, so
that rotational
= 10 motion around the axis of drive cable 32 is converted to rotation
around another axis
90 degrees to the first. Therefore, when the operator activates motion control
58,
first miter gear 82= rotates about its axis of rotation, and transmits torque
to second
miter gear 83, causing gear 83 to rotate about its axis of rotation.
= Pulley 86 is coaxially coupled to second miter gear 83, and pully 86 is
= 15 supported for rotation about the axis of rotation of miter gear 83.
When second
miter gear 83 rotates, pulley 86 rotates with gear 83 around its axis of
rotation. A
portion of cable 25 contained within gripping channel 91 is in contact with
pulley
86. Gripping channel 91 and pulley grip 87 act in concert to prevent slippage
and
apply a traction force from pulley 86 to cable 25, as pulley 86 rotates. In a
fashion
20 similar to a train wheel propelling a locomotive along a railroad track,
pulley 86
propels capsule 80 along cable 25. The result of this motion increases the
length of
cable 25 between capsule 80 and fixing plate 50 to propel capsule 80 further
into the
= colon.
FIG. 9 is a sectional view of capsule 80 taken at line 9-9 of FIG. 8. It shows
25 the relative positions of visualization device 95, lighting device 96,
cable 25, and
pulley 86 within capsule 80. In this embodiment, wiring assembly 34 divides
into
two bundles before it passes through trailing end 65 = of capsule 80. One
bundle =
communicates with lighting device 96, and the other bundle communicates with
visualization device 95. Lighting device 96 shines light to illuminate the
region of
=30 the lumen in the vicinity of capsule 80. Visualization device 95
transmits images
taken at this location back through wiring assernbly 34 to video unit 72 for
the
= operator to view. =
12
CA 02463214 2004-04-02
FIG. 10 is a cross sectional view of capsule 80 taken at line 10-10 of FIG. 9.
As shown in this view gripping channel 91 is positioned and aligned so as to
direct
cable 25 into the pulley grip 86, and pulley grip 86 holds cable 25 in contact
with
pulley 86. Sliding channel 90 is also shown in a position within the GI tract
which is
free of obstructions (e.g. sharp curves or bends in the colon) so that the
operator can
=
slide cable 25 in a forward direction to increase the size of cable
loop 54 (FIG. 2) =
ahead of capsule 80. This embodiment shows wiring assembly 34 split into two
bundles, one bundle on either side of first miter gear 82. One of the bundles
= connects to visualization device 95, and the other bundle connects to
illuminating
device 96.
Generally, medical device 70 is propelled through the colon under control of
the operator for examination and treatment of sites within the lumen. Medical
device 70 is placed into a patient's colon through the anus. Fixing plate 50
is affixed
to the patient at this location. The operator advances a proximal portion of
cable 25
through umbilicus 30 and sliding channel 90 to increase the size of cable loop
54
ahead of capsule 80. As described above, this process provides a path around
the
tortuous bends of the colon for capsule 80 to follow. =
While viewing video unit 72, the operator sees the inside of the lumen in the
vicinity of capsule 80. Motion control 58 of handpiece 20 is activated to
advance
capsule 80 along cable 25, moving it deeper into the colon. To further advance
capsule 80, the operator again feeds cable 25 to further increase the size of
cable
loop 54, and again activates motion control 58. These steps are repeated until
capsule 80 reaches a depth deemed sufficient by an operator, which is cecum
108 in
many cases. At any time during the procedure, the operator may introduce and
remove medical instruments through working channel 36 to treat a site in the
patient.
Medical device 70 is therefore useful for diagnosis as well as therapy.
Figure 11 illustrates a medical device 210 according to another embodiment
of the present invention. Medical device 210 includes an elongate, flexible
member
= 230 extending distally from a handpiece 220. Flexible member 230 can be
attached,
directly or indirectly, to handpiece 220, and can be in the form of an
umbilicus. By
= "flexible" it is meant that the member 230 has sufficient bending
flexibility to allow
= the member 230 to be inserted into and advanced along a body lumen, such
as the GI
= 13
CA 02463214 2004-04-02
tract, without trauma to the patient. Member 230 is elongate in the sense that
it has a
length sufficient to permit a proximal end 232 of the member 230 associated
with
handpiece 220 to be positioned outside the body, or near the entrance to the
body
lumen, while a distal end 234 of the member 230 is advanced into the body
lumen.
In one embodiment, the flexible member 230 can have a length of at least about
36
inches, and more particularly for use in the colon, a length of at least about
100
inches. Flexible member 230 can have an outer diameter of between about 0.1
and
about 1.0 inches to be positionable and advancable within the GI tract. In one
embodiment, flexible member 230 can be in the form of a catheter, or have a
catheter-like configuration, and can have an outer diameter of between about 4-
6
mm, more particularly about 5 mm.
Flexible member 230 can include an outer sheath 236 which extends along
substantially the full length of flexible member 230. A suitable sheath can be
made
of a thin, flexible polymeric film or other suitable flexible material. One
suitable
sheath material is porous teflon tubing (PTFE) having a thickness of about
0.02
inches. A suitable material is manufactured by International Polymer
Engineering of
= Tempe Arizona.
In Figure 11, a portion of the sheath 236 at the distal end 234 Of the
flexible
member 230 is shown in phantom to reveal internal features of the flexible
member
230 and components associated with the distal end 234 of flexible member 230.
Flexible member 230 includes a track guide for receiving a track 250 upon
which the
flexible member 230 can be advanced. In Figure 11 the track guide is shown in
the
form of two track guide tubes 242 and 244. Track 250 is received in track
guide
tubes 242 and 244, and can slide within tubes 242 and 244. Track guide tubes
are
disposed within the sheath 236, and can extend from proximal guide tube ends
associated with the handpiece 220 to distal ends associated with the distal
end of
flexible member 230. In Figure 11, the distal ends of the track guide tubes
are
shown cut at a bevel angle to accommodate the track 250 extending from the
flexible
member 230 at an angle with respect to the longitudinal axis of the flexible
member
230.
Track guide tubes 242 and 244 can be joined, directly or indirectly, in any
suitable manner (e.g. with adhesive, elastic bands, ultrasonic bonding) to the
sheath
236 and/or to handpiece 220 so that the tubes 242, 244, the sheath 236, and
the
14
CA 02463214 2004-04-02
-
handpiece 220 move together. Track guide tubes 242 and 244 can also fit
tightly in
sheath 236 and can be fixed to sheath 236 at either end by heat shrink tubing
(not
shown).
A visualization device and a light source can also be associated with the
5 distal end of the flexible member 230. In Figure 11 and Figure 12, an
optical fiber
320 extends from a light source 324, through handpiece 220 and through
flexible
member 230 to terminate at the distal end of flexible member 230. Optical
fiber 320
carries light from light source 324 to illuminate lumen tissue adjacent the
distal end
of flexible member 230. A camera 420 and associated camera optics 424 can be
10 disposed at the distal end of the flexible member 230. A camera can
include built in
= optics and electronics, and can include CCD or CMOS capability. A
suitable camera
is an MVC-Snake-1 camera manufactured by Micro Video Products having a self
= contained CCD camera with built in optics and electronics. Alternatively,
a CMOS
camera such as one manufactured by Welch Allyn of Schenectady New York could
15 be used. Signal cable 426 extends proximally from camera 420 through
flexible
member 230 and handpiece 220 to provide a signal to a monitor 428 or other
suitable receiver/recorder.
Flexible member230 can also include various channels/passageways for
conveying gases, liquids, or working devices from a point outside the patient
to the
= 20 tissue adjacent the distal end 234 of flexible member 230. Referring
to Figures 11
and 12, a vacuum tube 600 can extend through flexible member 230 and handpiece
220 to be in communication with a vacuum source 620 for providing vacuum to
the
distal end of flexible member 230. Likewise, a fluid tube 700 can extend
through
member 230 and handpiece 220 to a supply 720 of fluid ( e.g water, saline
solution, =
25 lubricating fluids). Figure 12. also shows the opening of a working
channel tube 800
at the distal end of flexible member 230. Working channel tube 800 can extend
through member 230 and handpiece 800 to receive a medical instrument 900. For
instance, once the distal end of flexible member 230 is positioned at a
desired =
location in the body lumen, a medical instrument 900, such as one having a
forceps
30 end 902 as shown in Figure 11, can be introduced through channel tube
800 to
access tissue adjacent the distal end of flexible member 230. In Figure 11 and
12, a
band 500 is shown holding the optical fiber 320 and tubes 600, 700, and 800
against
camera 420.
= 15
CA 02463214 2004-04-02
Track 250 can extend the length of flexible member 230. In Figure 11, track
250
is shown to include first and second ends 252 and 254, and a loop portion 256
disposed
along the track intermediate the first and second ends 252 and 254. Loop
portion 256 is
disposed distally of the distal end of flexible member 230. First and second
ends 252 and
254 can extend proximally from the handpiece 220. If desired, the ends of the
track
extending proximally from the handpiece 220 can be wound, coiled, or otherwise
supported on a suitable spool or receiver to prevent tangling of the ends 252
and 254. The
track 250 extends in a generally continuous manner distally of the flexible
member 230
(there are no track ends in the lumen distal of the flexible member 230).
First and second ends can be manually manipulated by an operator's hands to
advance either end 252 or 254 toward handpiece 220 to advance the track 250
through
flexible member 230, to thereby enlarge the loop portion 256. Alternatively,
the ends 252
and 254 can be associated with a control unit 260 as shown schematically in
Figure 11. In
an embodiment shown in Figure 14, handpiece 220 can include two switches 2210
and
2220 for independently controlling reversible motors 2240 and 2260, which can
be
mounted within handpiece 220. The motor speed and/or torque can be controlled
by
control unit 260, and the motors can include a coupling mechanism which
applies
frictional force to the track 250 to drive the track ends forward (distally
toward flexible
member 230) or backward (proximally away from flexible member 230) depending
on
the position of switches.
In the embodiments shown, track 250 is a single piece which extends from first
end 252, passes through handpiece 220 and extends distally through guide tube
242 to
exit guide tube 242 near the distal end 234 of flexible member 230. Track 250
extends
from the bevel cut distal end of guide tube 242 to extend around loop portion
256 and
enters the bevel cut distal end of guide tube 244 on substantially the
apposite side of
flexible member 230 from which track 250 exits guide tube 242. The loop
portion 256
comprises a smooth, rounded arc or other curve in which the track 250 turns
through
(subtends) an angle of at least about 90 degrees, more particularly at least
about 180
degrees. In Figure 11 of the drawings, the track 250 turns through an angle of
more than
180 degrees in loop portion 256. Track 250 extends proximally through guide
tube 244
and back through handpiece 220 to
16
CA 02463214 2004-04-02
second end 252. In Figure 11, a portion of guide tube 244 is shown cut away to
illustrate track 250 extending through the guide tube 244.
In order to advance the flexible member 230 (and its associated components)
into the body lumen, the distal end of the flexible member 230 can be
positioned in
5 the entrance of the lumen. One (or both) track ends 252 and 254 can be
advanced
toward handpiece 220 (e.g. by pushing gently on the track ends), while the
handpiece
220 and flexible member 230 are held stationary. Advancing either end of the
track
250 causes the loop portion 256 to increase in length and the
track'to"unfurl"as it
advances out of the confines of the distal end of flexible member to advance
within
10 the lumen while following the curvature of the lumen. Once the loop
portion 256
= has been advanced distally from the flexible member 230 (as can be
viewed with =
camera 420), the flexible member 230 can be advanced distally along the= track
250.
Flexible member 230 (and associated camera 420 and optical fiber 320) are
advanced further into the body lumen by pushing distally on the handpiece 220,
and
15 simultaneously pulling proximally on either track end 252 or track end
254. Without
being limited by theory, it is believed that by pushing the handpiece 220 and
attached flexible member 230, while at the same time pulling back (proximally)
on
= one of the track ends 252 and 254, the force required to advance flexible
member
230 through the lumen (e.g. GI tract) is reduced. In particular, in one
embodiment,
= 20 the handpiece 220 can be pushed distally while pulling back on or, but
not both,
ends 252/254.
Figures 13A-E illustrate schematically how the track 250 can be advanced in
= the colon, and how the flexible member 230 can then be advanced along the
track to
position the distal end of the flexible member 230 at a desired location in
the colon. .
25 In Figure 13A, the track 250 is shown in a relatively retracted
configuration after
= initial insertion of the medical device into the GI tract. In Figures 13B-
13D, the =
track is advanced distally through the flexible member 230 (by pushing one or
both
the ends 252 and 254 toward handpiece 220). Advancing track 250 through
= handpiece 220 and member 230 enlarges loop portion 256, and unfurls loop
portion
30 256. In Figure 13D the loop 256 is shown unfurled to a position
associated with the
beginning of the transverse colon. The flexible member 230 and associated
camera
and light source can then be advanced to the transverse colon, as shown in
Figure
= 13E, by pushing the handpiece/flexible member 230 distally into the GI
tract, while
17
CA 02463214 2004-04-02
simultaneously pulling back (proximally) on one of the ends 252 and 254 of the
track 250. In Figure 13E the loop portion 256 has retracted proximally in the
colon
relative to its position in Figure 13D as a result of pulling back on one of
the ends 252/
254. The steps shown in sequence in Figures 13B-E can be repeated, as desired,
to
position the distal end of the flexible member 230 at a desired location.
Track 250 can be a guide wire having a generally round cross section. One
suitable material for use as track 250 is a guide wire having a nitinol core
with a diameter
of about 0.021 inch to about 0.025 inch surrounded by a stainless steel wire
coil having a
diameter of about 0.008 inch. The overall diameter can be between about 0.037
inch and
about 0.041 inch, and the stainless steel coil may be soldered or otherwise
attached at
about 50 cm intervals to hold the stainless steel coil in place relative to
the nitinol core.
Another suitable material for use as a track 250 is a guide wire marketed as
Elite
ProtectorTM Elite 480 wire guide available from Wilson-Cook Medical, Inc. of
Winston
Salem, NC, and having a diameter of about 0.035 inch. The track can have
length of
about 15 feet or more, depending on the length of lumen being investigated.
Track 250 slides within track guide tubes 242 and 244. Track guide tubes 242
and
244 can be faulted of a low friction material, or be treated to have a low
friction coating.
In one embodiment, tubes 242 and 244 can be reinforced TeflonTm tubing to
provide low
friction interface with track 250. The tubing can be a wire reinforced
TeflonTm tube, such
as is manufactured by International Polymer Engineering of Tempe Arizona. The
outer
diameter can be less than or equal to about 0.10" and the wall thickness can
be about
0.016".
While the track guide in Figure 11 is shown as a tube, it will be understood
that
other guide geometries can be employed, including without limitation channels,
rails, and
grooved surfaces, as guides for supporting and guiding track 250. In yet
another
embodiment, the interior diameter of the sheath 236 can be adapted to provide
a track
guide.
While two guide tubes are illustrated in Figure 11, in an alternative
embodiment,
a single guide tube could be used (e.g. guide tube 242), and the track 250
could extend
from a first end (located outside the patient) through handpiece 220 and guide
tube 242,
extend around loop portion 256, and then re-enter guide
18
CA 02463214 2004-04-02
tube 242. Alternatively, the track 250 could extend from a first end located
outside
the patient, through the handpiece 220 and guide tube 242, extend around the
loop
portion 256, and have a second end fixed at or near the distal end of the
flexible
member 230 (in which case a single track end would extend outside the patient,
and
this track end would be advanced toward handpiece 220 to increase loop portion
256).
The camera and light source located at the distal end of flexible member 230
can be built into the flexible member 230, so as not to be removed:
Alternatively,
the camera and light source could be a sealed assembly which is releasably
attached
to the flexible member 230 (such as by threaded attachment, snap ring
attachment,
bayonet style attachment, and the like). In one embodiment, the flexible
member
230 (with associated guide tubes, fluid tubes, and working channel) can be
disposable, and the camera and optics can be reusable.
Figure 15 and Figure 16 (an end view taken at section 15-15) illustrate an
embodiment having a single guide tube 242, along with a camera unit and light
source in self contained unit 420. In Figure 15, a portion of sheath 236 is
shown cut -
away. The track 250 extends from guide tube 242, turns through loop portion
256,
and is attached at the distal end of flexible member 230, such as by being
fixed to an ==
end piece 285 positioned at the distal end of flexible member 230.
Figures 17 and 18 illustrate an embodiment comprising two tracks 250A and
250B, and corresponding track loop portions 256A and 256B which are disposed
in
generally perpendicular planes. In Figure 17, the sheath 236 is shown in
phantom to
=
reveal guide tracks 242A, 242B, and 244B (guide track 244A not visible), as
well as
optical fiber 320 and camera unit 420. In the embodiment shown in Figures 17
and
18, optical fiber 320 can pass through an aperture hole in a band 500, and
band 500
= can hold optical fiber 320 adjacent camera 420. Other tubes passageways
(e.g. 600,
700, 800 not shown in Figures 17 and 18) can also be supported in apertures in
band
500. Track retainer ring 257 is provided to hold and/or space tracks 250A and
250B
in a desired position. Track retainer ring 257 can include apertures spaced
circumferentially at 90 degree intervals around ring 257 (apertures not shown)
through which tracks 250A and 250B can slide. Accordingly, track retainer ring
257
can support track loop portion 256A in a first plane, and loop portion 256B in
a
second plane which is generally perpendicular to the first plane. Spokes 259
can be
19
CA 02463214 2004-04-02
used to support retainer ring 257 on band 500. If desired, sheath 236 can be
attached
to retainer ring 257 if tracks 250A and 250B are supported to slide through
ring 257
(eg. if tracks 250A and 250B pass through apertures in ring 257.)
In yet another embodiment, track 250 can comprise a wire or other suitable
track piece having no distinct ends (i.e. no ends 252/254), but instead may
comprise
a smooth, uninterrupted track having a closed configuration (e.g. race track,
oval,
etc.) with a loop portion of the closed configuration track extending through
the
= flexible member 230 to extend distally of the distal end 234 of the
flexible member
230, and another loop portion of the closed configuration extending proximally
of
the handpiece 220. In such an embodiment, the loop portion extending
proximally
of the handpiece 220 can be manipulated by hand or by a controller to advance
the
loop portion distal of the flexible member 230 within the body lumen.
In each of the embodiments, it will be.understood that one or more seals may
be provided, as desired, to restrict gas or liquid flow through or around the
flexible
member 230, such as from .a point outside the patient to a point within the
lumen,
especially if there is a desire to provide vacuum at the distal end of
flexible member
230, or to otherwise isolate conditions in the lumen from conditions outside
the
lumen. For instance, with reference to Figures 11 and 12, it may desirable to
provide
a seal in association with guide tracks 242 and 244, and in channel 800 to
prevent air
from passing through the guide tracks or the channel 800. Seals for channels
can be
in the form of a small, flexible silicone rubber boot having an aperture
through
which a track 250 or instrument 900 can pass. Similarly, a flexible cuff or
collar can
be positioned over the flexible member 230 and can provide a seal between the
flexible member 230 and the portion of the patient's body adjacent the opening
to .
the patient's lumen. Lubricating gels and other lubricating products can also
be
utilized to enhance or provide sealing.
While various embodiments of the present invention have been disclosed, it
will be obvious to those skilled in the art that such embodiments are provided
by
way of example only. The present invention may be provided in kit form with
other
medical devices, including medical devices useful in the working channel, and
the
kit elements can be pre-sterlized and packaged in a sealed container or
envelope to
prevent contamination. The present invention may be provided as a single use
CA 02463214 2004-04-02
dispostple device, or alternatively, may be constxucted for multiple uses.
Further,
each element or component of the present invention may be alternatively
described
as a means for performing the function or functions performed by the element
or
component. Numerous variations, changes, and substitutions will now occur to
those skilled in the art without departing from the invention. Accordingly, it
is
intended that the invention be limited only by the spirit and scope of the
appended
claims.
21