Note: Descriptions are shown in the official language in which they were submitted.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
_ Z _
Materials Fabrication Method and Apparatus
The present invention relates to a method and an
apparatus for fabricating materials, and in particular for
fabricating superconducting materials.
Technical Backaround
There are strong commercial reasons driving the
development of superconducting materials having high
superconducting parameters, such as critical current
densi y, J~,._critioal temperature,.T~, and upper critical
1o field, H~2. These materials find applications in, for
example, Nuclear Magnetic Resonance ,(NMR) and Nuclear
Magnetic Imaging (NMI) magnets and also in cryogen-free
magnets.
The microstructional requirements for such materials
are very demanding and many approaches and many alloys or
compounds have been developed to address the problems of
improving materials performance, including mechanical and
electrical performance for example, and ease and cost of
fabrication.
2o An important superconductor material is Nb3A1 which
forms an A15 superconducting phase and, by way of
illustration, methods for fabricating this material
include the following.
The fabrication processes can be considered in three
groups; low-temperature, high temperature and
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
transformation processing. In each case, an A15 Nb3Al
strand is processed by first making the final-size strand,
with the constituents subdivided, and then heat-treating
it to form the A15 phase.
Zow Temperature Processincr
Zow temperature (<1000C) processes ensure that the
grain size of Nb3Al does not become too coarse because the
Nb/A1 constituents directly react with diffusion to
suppress Nb3A1 grain growth. But, at low temperatures,
2o there is a deviation from A15 stoichiometry, thus
affecting high-field properties, especial J~. Low
temperature processes include the following.
Jelly-roll (JR) - Alternate foils of Nb and Al are
wound onto a copper rod and inserted into holes drilled in
a copper matrix before drawing to form the final-size
strand.
Rod-in-tube, (RIT) - An alloy rod is inserted into a
Nb tube and drawn down. A triple stacking operation gives
the desired 100nm core diameter to match that of the A1
layer.
Clad chip extrusion, (CCE) - A three-layered clad
foil, of Al/Nb/A1, is cut into square chips, and then
filled into a can in order. to be extruded.
Powder metallurgy, (PM) - A mixture of hydride-
dehydride Nb powder and Al powder are put in a copper tube
can so that they can be extruded and drawn into a
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 3 -
monofilament wire. A bundle of these wires will give an
Al layer thickness of 100nm.
Despite differences in cross sectional area, J~
versus magnetic field curves are very similar for all of
these processes. JR may have a slight advantage for
producing long piece strands.
Hicrh Temperature Processing
At low temperatures the Nb~A1 phase will not be
completely stoichiometric. However, at high temperatures
(>1800C), a diffusion reaction of Nb/Al composites with
laser or electron-beam irradiation allows stoichiometric
A15 phase formation. Annealing at ~700C improves long
range order, and so better T~ and H~Z. Unfortunately, high
temperatures will cause very coarse grains in the
conductor, thereby destroying low field properties.
Transformation Processing
This process, discovered in the last few years,
involves a combination of rapid quenching and annealing.
The Nb/A1 composite is quenched from 1900C to form a bcc
2o supersaturated solid solution of Nb(A1)ss and then
transformation annealed below 1000C. This process will
produce Nb3A1 that is highly stoichiometric and has a fine
grain structure, and therefore the J~ will be high at both
low and high fields. The most common method of
transformation processing is known as the rapid-heating,
quenching and transformation method (RHQT), which produces
lengths of conductors a few hundred metres long.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 4 -
Summary of the 2nvention
The invention provides a method and an apparatus for
fabricating materials as defined in the appended
independent claims. Preferred or advantageous features of
the invention are set out in dependent sub-claims.
The invention uses a process for extracting metals
and alloys from solid compounds by direct electrochemical
reduction, or electrodecomposition, in molten salt, known
as the Fray-Farthing-Chen Cambridge process (FFC), as one
of a series ofvsteps to fabricate a material. The FFC
.process is described in the present applicant's earlier
International patent application PCT/GB99/01781 which is
incorporated herein by reference. The FFC process allows
the treatment of a solid material, which may be a compound
between a metal (or semi-metal) and a substance (such as
an anionic species), or a solid solution of the substance
in the metal, by electrodecomposition in a molten salt to
remove the substance from the solid material. On
completion of the process, the solid material has been
2o converted to the metal. Alternatively, if the solid
material comprises more than one metal, being for example
a mixture of metal compounds, or a mixture of a metal and
a metal compound, or comprises a solid solution of metal
compounds, then on completion of the process an alloy or
intermetallic compound of the metals is formed.
The product of the FFC process is typically porous
and, in the method of the present invention, is then
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 5 -
infiltrated with an element, metal or alloy, typically as
a liquid, to form a material which can be used or further
processed to fabricate a product.
' In a preferred embodiment, the invention may be
particularly efficacious for fabricating superconductors.
For example, if the FFC process is performed on a preform
comprising a mixture of powdered Nb205 and TiOz, a porous
sample of NbTi alloy is produced. This can then be
infiltrated with molten A1 to form a material which can be
1o further processed, for example by deformation and heat
treatment, to form a high-performance superconductor,
advantageously at lower cost than for conventional
methods. In an alternative embodiment, the FFC process is
performed on a preform comprising a mixture of powdered Nb
and Sn oxides. A Nb3Sn superconductor can then be
fabricated.
Thus, the invention may advantageously provide a
method having four steps~as follows for fabricating
Nb-based superconductors:
1) electrochemical reduction. of the Nb-based
compounds,
2) infiltration by A1-based alloys (or any other
elements or alloys to form intermetallics or
artificial pinning centres [APCs]),
3) deformation, and
4) reactive formation of an intermetallic layer
followed by insulation processing.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 6 -
Of course, the method of the invention envisages any
suitable starting material or materials and not only Nb
and A1. In addition it should be noted that the invention
relates particularly to steps 1 and 2 of the list above
and that steps 3 and 4 may be replaced by any appropriate
superconductor fabrication techniques.
Tt should also be noted that the invention is not
limited to the field of superconductor fabrication but
relates primarily to the technique of infiltrating a
1o porous material formed by the FFC process. The FFC
process is very flexible and can produce a wide range of
metals, semi-metals, alloys and intermetallic compounds,
including materials which are difficult to fabricate in
other ways. The additional novel step of infiltrating a
product of the FFC process, which is typically porous,
with a metal or other material may advantageously allow
the fabrication of a wide variety of novel and useful
materials compositions and microstructures.
The infiltration step may be carried out ex-situ or,
2o preferably, in-situ. The FFC process can produce a porous
alloy_or intermetallic immersed in a molten salt. In the
in-situ process, the molten salt is contained in a bath
which also contains the molten material for infiltration.
The infiltration material will usually be denser than the
salt, in which case the salt will float on the
infiltration material. After the FFC process is
completed, the porous sample can then move directly from
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
the salt into the infiltration material, which can
displace the molten salt and infiltrate the porous sample.
If the infiltration material is less dense than the
salt then it will float on the salt but as long as there
is contact at an interface between the two, the porous
sample can move directly from the salt to the infiltration
material, advantageously avoiding contact with any other
substances.
Tn an alternative implementation of in-situ
1o infiltration, the porous sample may be immersed in the
infiltration material by moving the interface between the
salt and the infiltration medium rather than by moving the
porous sample. For example, after completion of the FFC
process the bath containing the salt may be flooded with
infiltration material to displace the salt, or where the
bath contains both salt and infiltration material, the
bath may be moved, rather than the porous sample.
In ex-situ infiltration the molten infiltration
material is held in a separate bath from the molten salt
2o and the porous sample moves from one bath to the other for
infiltration. If this is done in an oxidising atmosphere,
disadvantageous oxidation of the porous sample may occur.
An inert atmosphere may be used to alleviate this problem
but nevertheless contamination of the porous sample may be
more likely than with the in-situ method.
In an alternative implementation of the ex-situ
method, following completion of the FFC process the porous
sample is withdrawn from the molten salt and allowed to
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
_ g _
cool, preferably in an inert atmosphere or in vacuum above
the molten salt. As the sample is withdrawn, much or all
of the salt within pores in the sample is retained and
then solidifies. The sample is then transferred to a pool
of molten infiltration material, where it is immersed and
the salt melts and is displaced by the infiltration
material to infiltrate the sample. This implementation
has the advantage that the solidified salt in the pores of
the sample during transfer to the immersion material helps
1o to protect the sample surface from contamination or
oxidation.
In various embodiments of the invention, where the
infiltration material wets the FFC product better than the
molten salt, it may advantageously substantially entirely
displace the salt from the porous FFC product. In
general, molten salts wet metals relatively poorly and so,
where the FFC product is metallic and the infiltration
material is also metallic, the infiltration material will
tend to wet the FFC product more strongly than the molten
2o salt. In other embodiments of both the in-situ and
ex-situ methods, provision may be made to enhance
infiltration and to ensure that the infiltration material
fills the pores in the porous sample, displacing the
molten salt as much as possible. One method for this is
to pump the molten salt out of the porous sample after or
as it is immersed in the infiltration material. A second
method, which may be combined with the first, is to
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
_ g _
vibrate or agitate the porous sample or the infiltration
material, for example by using an ultrasonic transducer.
Specific Embodiments and Best Mode of the Invention
Specific embodiments of the invention will now be
described by way of example, with reference to the
drawings, in which;
Figure 1 illustrates an electrolytic cell for
carrying out the FFC process
Figure 2 illustrates the infiltration and subsequent
1o steps in a first method embodying the invention;
Figure 3 is a micrograph of a sample of porous Nb
alloy following the FFC process;
Figure 4 is a micrograph of a sample of Nb alloy
following infiltration;
Figure 5 is a~micrograph of a Nb-A1-Ge(X) wire
following mechanical reduction and diffusion treatment;
Figure 6 is a plot of AC susceptibility against
temperature for Nb and NbTi rods embodying the invention;
Figure 7 is a plot of AC susceptibility against
2o temperature for reduced Nb205-Sn02 rods embodying the
invention;
Figure 8 illustrates a cell for in-situ infiltration
according to an embodiment of the invention; and
Figure 9 illustrates a second stage in the in-situ
infiltration method using the cell of figure 8; and
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 10 -
Figure 10 is an element distribution plot for an
infiltrated pellet of niobium oxide.
The electrochemical reduction route of the FFC
process may advantageously be a much easier, quicker and
s cheaper way to extract many metals and alloys than
established metallurgical routes. A schematic of such a
process is presented in figure 1.
Figure 1 shows an apparatus for making the binary
alloy NbTi. It comprises a cell 2 containing molten
1o CaCl~ 4. A graphite anode 6 and a rod-shaped preform 8 of
mixed Nb2O5 and Ti02 are immersed in the salt. The preform
is supported on and electronically connected to a Kanthal
wire 10. The preform is made by mixing powdered Nb.,05 and
TiO~ in the desired proportion, slip casting and
15 optionally partially sintering the mixture.
Other techniques rnay also be applied to the
fabrication of the preform. For example a polymer binder
may be added to improve the slip-casting process and the
polymer then burned off. Alternatively a prefabricated
20 polymer matrix may be used to make the preform. In this
case the polymer matrix is infiltrated with metal oxide
powders and then the polymer is burned off. These
techniques advantageously form porous preforms, which can
be easily treated by electrodecomposition in the FFC
25 process.
In the FFC process, the preform of mixed Nbz05,Ti0~
powders is made the cathode in the molten CaCl2, whose
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- z~ -
cation can form a more stable oxide, CaO, than Nb205 and
Ti02. The oxygen in the Nbz05 and Ti02 mixture is thus
ionised and dissolves in the salt, leaving Niobium-
Titanium alloy metal of the desired composition behind.
The extraction of Nb, NbTi, and Nb3Sn metals and alloys
frorn oxides using this process has been carried out on a
laboratory scale, as has the extraction of many other
metals and alloys from their compounds.
After electrochemical reduction, the final product of
1o the FFC process in the embodiment is a porous, rod-shaped,
metal lic sponge of NbTi alloy, as shown in figure 3.
Rapid oxidation of the Nb-based porous rod normally takes
place after its removal from the chloride bath and may
have a detrimental effect on its surface quality and any
subsequent processing. Therefore a different approach is
proposed to provide better infiltration conditions, using
either in-situ or ex-situ infiltration as described below.
Porosity
The degree of porosity of the final percolative
2o network of the Nb-based alloy sponge~depends on the
density of the oxide preform and on the initial
preparation technique of the prefabricated oxide. For
example, preforms which are sintered show a significant
shrinkage (increased density) and greatly increased
strength in comparison with those prepared by slip casting
only.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 12 -
Materials Considerations in Superconductor Fabrication
Because the metallic product of the electrochemical
reduction (for NbTi or other materials) is soft and
porous, without structural defects or secondary phases, it
can not be regarded as a high critical current density,
J~, superconducting material.
Such semi-finished product has to be upgraded by
introduction of, for example, Al or Sn alloys for the
reactive diffusion formation of the intermetallic phase in
1o the case of A15 intermetallic superconductors, and/or by
introduction of Artificial Pinning Centres (APC) as in the
case of NbTi.
Tnfiltration of a porous sponge of an alloy such as
Nb-Ti-X or Nb-A1-Ge-Z by Al or A1 alloy material, where X
and Z represent additional metals, has its advantages
because for example NbTiTa ternary alloys have recently
been explored as a high-field avenue for APC materials.
The infiltration route is adopted in order to obtain a
relatively ductile material that can be mechanically
2o deformed into a fine wire, containing an interconnected
network of superconducting filaments of the order of
1 micrometre in diameter. Additional advantages of the
infiltration process are due to the fact that the
composition of the infiltrant (A1-Ge or A1-Si alloys and
eutectics) can be readily controlled. The A1-Ge system
forms a low melting point eutectic (424C) at the
composition of 70o Al-30o Ge. The inherent brittleness of
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 13 -
the Al-Ge eutectic when solidified requires particular
attention to the temperature of the infiltrating bath, the
temperature difference between the porous niobium or alloy
rod and the bath, and the rate of infiltration. The
-formability of the Al-Ge eutectic, entrapped and
solidified within the pore volume of the rod, during form
rolling or wire drawing can be significantly improved
through the application of superplasticity principles.
Superplastic behaviour requires a fine duplex
1o microstructure that is stable at the deformation
temperature.
The invention may thus advantageously provide new
techniques that allow manufacture of~complex compositional
superconducting alloy rods by ex-situ and in-situ
infiltration processes.
Ex-situ infiltration
The ex-situ infiltration process is conducted in
vacuum or an inert gas container in such a manner that as
many as possibl.e.of.the pores in the sample are filled as
2o completely as possible with molten metal or alloy. This
maximises the volume fraction of superconductor realisable
from a given amount of infiltrated alloy. A schematic
representation of an embodiment of the process following
the fabrication of the FFC process alloy sponge is shown
in figure 2, which shows the steps of infiltration 50,
here in a Sn/Ga/Al infiltrant bath 52, cladding 54,
mechanical reduction 56 and diffusion processing 58 in a
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 14 -
furnace 60. Figure 2 relates to (Nb, X)3(Sn, A1, Z) wire
processing, using porous (Nb,X) rod fabricated using the
FFC process, for example.
In the embodiment of figure 1 described above, a NbTi
alloy was formed by direct electrochemical reduction to
form an alloy sponge, or rod-shaped sample. Its
microstructure is shown in cross-section in figure 3. For
infiltration, the Nb-based alloy rod is immersed in a bath
of molten Sn or Al-based alloy maintained at a temperature
above melting. Zower temperatures are preferred in order
to prevent the extensive, very often rapid, formation of
brittle intermediate phases which could impair the
ductility of the composite infiltrated material. The
microstructure of the infiltrated alloy sponge is shown in
figure 4.
Taking into account that the final products of
reducing Nb-oxide-based oxide mixtures in the FFC process
typically have pore sizes in the range of 2-20~tm, special
care should be taken to ensure that the Nb-based sponge'
2o surface is as clean and pure as possible to enable
complete infiltration of the porous rod, efficient wetting
by the infiltrating metal or alloy such as Sn, Al etc.
and finally minimisation of superconductor filament damage
caused by the formation of hard Nb~05 (or even of more
complex insulating compounds) on the sponge surface during
removal of the metallic Nb-based rod from the chloride
bath. In some circumstances the oxygen content in the Nb
can reach 2o-3at.o, which is about the solubility limit at
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- Z5 -
the extrusion temperature used later in processing.
Oxygen adsorbed at the surface of the particles in the
sponge may also diffuse into the Nb, increasing its
microhardness to 3500 MNnl2. The plastic deformation of
the composite may then not be uniform because of severe
solution hardening of the Nb, mainly. due to interstitial
oxygen. A successful co-deformation of Nb and Sn
particles requires sufficient reduction of the oxygen
content in the Nb. If the oxygen content in the Nb is
to reduced to 0.lato the microhardness of the Nb matrix drops
to ~1200MNiri2, which is about the value of the surrounding
Cu matrix in which the Nb material is typically
subsequently encased and which is used for cryostability.
Three alternative purification methods have been
tested as follows: (1) because the oxygen solubility in Nb
decreases with temperature, part of the oxygen can be
precipitated in the form of oxides during an annealing
treatment at 600-700C. This treatment has indeed proved
to be beneficial but the deformability of the Nb particles
2o may still be insufficient; (2) a reduction treatment of
the porous Nb-based composite in an H2 or CH4 atmosphere
may improve the deformability of the Nb; (3) an additional
component Q can be added to the Nb205-Q powder mixture,
which has a larger binding enthalpy for oxygen than Nb
does (390 kJ/g-atom 0: interstitial solution). A variety
of additives Q can be used as the skilled person would
appreciate.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 16 -
In summary therefore, ex-situ infiltration can be
used to fabricate superconductors but care needs to be
taken to avoid deleterious oxide formation, which may
require additional processing steps and add to the
complexity of the method.
A variant of ex-situ infiltration aims to address
these concerns. In this embodiment, after completion of
the FFC process the porous metal or alloy sponge is
withdrawn from the molten salt using a control and
1o positioning system until it is held in an inert atmosphere
or in vacuo above or near the salt bath. If wetting of
the metal by the salt is sufficient, the pores in the
sponge remain filled with molten salt, and the sponge can
be cooled to solidify the salt. The sponge surface is
thus protected against contamination or oxidation by the
presence of the solid salt and can be transferred to an
infiltration bath without damage. On immersion in the
infiltration bath, the salt melts and is displaced by the
immersion material.
2o Molten salt can also be more effectively retained in
the porous metal by lowering the temperature of the salt
bath, prior to removal of the metal, to close to the
melting point of the salt.
In-situ Infiltration
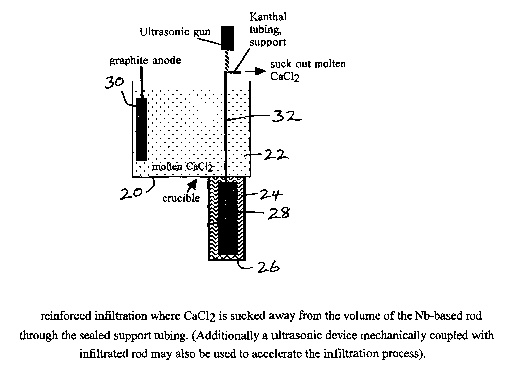
Figures 8 and 9 illustrate the technique of in-situ
infiltration. A cell 20 contains molten salt 22 floating
above a molten metal alloy 24 held in an extended lower
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- i~ -
portion 26 of the cell. As in the embodiment previously
discussed for fabricating a NbTi based superconductor to
carry out the FFC process, a NbzOs/TiO2 preform 28 and a
graphite anode 30 are immersed in the molten salt, which
is CaCl2. The preform is supported on a tubular Kanthal
support 32.
Following use of the FFC process to reduce the
preform to a porous alloy sample or rod, which in this
embodiment is a Nb-based alloy rod, in-situ infiltration
of the porous rod is carried. out by lowering it from the
molten salt directly into the molten metal,(in the
embodiment, molten Al) beneath, as shown in figure 9. The
rod is lowered by a control and positioning system coupled
to the Kanthal support. This in-situ process is
advantageous because there is no direct contact of the Nb
with oxygen before infiltration, and so the metal surface
on infiltration is oxide free. During infiltration molten
CaCl2 is displaced from the sample, or sponge, by the
molten metal. With these materials, effective
2o infiltration may be expected due to the better wetting of
the Nb by the A1 than by the CaCl2.
Tn further embodiments, various methods may be used
to encourage the full metal infiltration of the porous
Nb-based rod. One of these is to pump out or suck out the
molten CaCl~ from the rod. This can be achieved by
pumping the salt through the tubular Kanthal support shown
in figures 8 and 9. In the case of the hollow support and
rod, sucking will be very effective and any excess of the
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 18 -
molten metal within the core of the sample or in the
Kanthal support can be easily removed after infiltration
and replaced with internal cryogenically stabilising
composite such as Cu with a protective Ta diffusion
barrier. In a second method an ultrasonic device
mechanically coupled to the rod or its support, or to the
bath of infiltration material, may also be used to
accelerate the infiltration process.
In-situ infiltration of the Nb-based porous rod
1o should advantageously minimise many of the negative
effects related to.the ex-situ process mentioned above, .
and in particular the risk of surface contamination of the
sample before infiltration.
Post-Infiltration Processing
After the infiltration process, rods would be
machined to the desired shape and inserted in subsequent
tubes 62 to serve as a diffusion barrier and for
electrical and thermal stabilisation. This is the
cladding step 54 of figure 2. Although an elevated
2o temperature during the infiltration stage may produce some
A15 phase, it may be desired to subject the infiltrated
rod or tape to a substantial reduction in thickness by
cold rolling 56 prior to the final diffusion formation 58
of the intermetallic A15 layers in the conductor.
Microstructure Control
An important aspect of superconductor fabrication is
microstructure control. Use of the FFC process as a step
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 19 -
in fabrication enables an element of control through
control of the particle size of the powder used to make
the preform, densification of the preform through
sintering, and the temperature and other electrolysis
parameters at which the electrodecomposition is performed.
Advantages of the Method for Superconductor Fabrication
Using the FFC process for reducing solid oxides to
metals and alloys of pre-defined alloy composition and
infiltrating these materials in our opinion opens new
opportunities for the manufacture of the highest quality
low temperature superconductors, not.only the most
saleable ones but also those best intermetallic ones which
are difficult to manufacture eg A15 intermetallic
conductors such as (Nb,X)3(Sn,Z) and Nb3(Al,Ge)
characterised by the highest J~, B~2~ Tc values may be
produced for a fraction of the cost of the currently
available superconductors. Examples of the measured
performance of various superconductors embodying the
invention are shown in figures 6 and 7. The ex-situ and
2o in-situ infiltration processes can be applied to the
niobium-titanium, niobium-tin and niobium-aluminium
systems as described but in general these techniques can
be used to infiltrate any metals or materials manufactured
by the FFC process, whether for superconducting
applications or other purposes.
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 20 -
Additional Example of Infiltration for Materials
Fabrication
The following specific example describes the
aluminium infiltration of a porous, partially metallised,
Nb pellet produced by the FFC process.
Preparation of the cell for in-situ infiltration
About 100g of aluminium shot was put into a
cylindrical crucible made, in different tests, from either
alumina or graphite (internal diameter and height were
50mm and 90mm respectively). The crucible was then filled
to the rim with dry powders of CaCl2 and NaCl in the
eutectic ratio, and placed in an Inconel tube reactor that
was sealed, flushed with argon and heated in a furnace to
950°C. It was observed that both aluminium and the salt
mixture had melted before the temperature reached 800°C,
with the salt floating on top of the molten aluminium.
Thus, the cell comprised a layer of a molten eutectic
mixture of CaCl~ and NaCl floating on a layer of
molten Al.
Preparation of the Nb Pellet
A cathode preform of Nb205 was prepared by pressing
oxide powder into a small cylindrical pellet (l0mm
diameter, l0mm height, approximately 1.5g mass) which was
then sintered at 1000°C for 2 hours. After sintering the
preform gained a reasonable strength and had a porosity of
about 40500, depending on parameters including starting
material parameters and pressing pressure. A hole (1.5mm
diameter) was drilled through the sintered pellet which
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 21 -
was then threaded onto Kanthal wire. This assembled
cathode was placed in the molten eutectic mixture of CaCl~
and NaCl at 950°C. FFC electrodecomposition was carried
out with a graphite rod anode at 3.1V under argon for a
relatively short time (between 1 and 10 hours) so that
only the surface region of the pellet was reduced to metal
while the central part remained in the oxide phase, i.e.
the oxide pellet was partially metallised.
Infiltration of the Pellet
1o After termination of the electrodecomposition, the
temperature of the furnace was lowered to 690°C. The
cathode was then immediately lowered into the molten
aluminium underneath the molten salt. After a very brief
infiltration/reaction time (a few seconds), the cathode
was removed from the crucible, cooled first in the upper
region of the Inconel reactor, and then removed from the
reactor and further cooled in air. It was seen that the
pellet was completely covered in aluminium.
The pellet was broken into two halves, and the cross
2o section examined by SEM (scanning electron microscopy) and
EDX(energy-dispersive x-ray analysis). It was observed
that the pellet contained two different phases. The outer
layer of the pellet was about 400 micrometres in thickness
and relatively dense, but the central part was porous.
EDX analysis revealed that, as shown in Figure 10, the
outer layer was composed mainly of niobium and aluminium
with about 20ato oxygen, but the central part was of
CA 02463396 2004-04-08
WO 03/031665 PCT/GB02/04603
- 22 -
niobium and calcium with 58at% oxygen (Nb205 contains
7lato oxygen). The calcium content was also much lower in
the outer layer than in the central part. These results
indicate that aluminium had infiltrated into the outer
layer of the pellet, which had been metallised by
electrodecomposition.