Note: Descriptions are shown in the official language in which they were submitted.
CA 02463663 2004-04-27
WO 99/64095 ' PCTIUS99/13420
d i A
=
1 Inhalation device with acoustic eontrol
~ Field of the Invention
3 The present invention relates generally to the field of metering,
4 packaging and deliverv of pharmaceuticals and drugs. Particular utilitv for
the present invention is found in the area of facilitating metering and
6 packaging of drv powder medications and/or inhalation of powdered
' medications, although other utilities are contemplated, including other
medicament applications
8 Related Art
9 Certain diseases of the respiratory tract are kno~~,rn to respond to
treatment by the direct application of therapeutic agents. As these agents are
11, most readilv available in dry powdered form, their application is most
12 convenientlv accomplished by inhaling the powdered material through the
13 nose or mouth. This powdered form results in the better utilization of the
14 medicament in that the drug is deposited exactly at the site desired and
where
its action may be required; hence, very minute doses.ot the dnig are.often
16 equally as efficacious as larger doses administered by other means, with a
17 consequent marked reduction in the incidence of undesired side effects and
18 medicament cost. Alternatively, the drug in this form mav be used for
19 tFeatment of diseases other than those of the respirator~= svstem. ~Ihen~he
'
drug is deposited on the veiti= large surface areas of the iungs, it may be
very
21 rapidIzT absorbed into the blood stream; hence, this method of application
22 niav take the place of administration by injection, tablet, or other
conventioiial
23 means.
24 It is the opinion of the pharmaceutical industry that the bioavailability
of the drug is optimum when the drug particles delivered to the respiratory
26 tract are between 1.to 5 microns in size: When the drug particles need to
be in
27 this size range the dry powder delivery system needs_ to address a number
of
28 issues:
29 (1) Small size particles develop an electrostatic charge on thernselves
during manufacturing and storage. This causes the particles to agglomerate
1
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
I or aggregate, resulting in clusters of particles which have an effective
size
2 greater than 5 microns. The probabilitv of these large clusters making it to
the
3 deep lungs then decreases. This in turn results in a lower percentage of the
'
4 packaged drug being available to the patient for absorption.
(2) The amount of active drug that needs to be delivered to the patient
6 may be of the order of 10s of micrograms. For example, albuterol, in the
case
7 of a drug used in asthma, this is usuallv 25 to 50 micrograms. Current
8 manufacturing equipment -can effectively deliver aliquots of drugs in
9 milligram dose range with acceptable accuracy. So the standard practice is
to
mix the active drug with a filler or bulking agent such as lactose, This
11 additive also makes the drug "easy to flow". This filler is also called a
carrier
12 since the drug particles also stick to 'these particles through
electrostatic or
13 chemical bonds. These carrier particles are very much larger than the drug
-14 particles in size. The ability of the dry powder inhaler to separate drug
from
tlle carrier is an important performance parameter in the effectiveness of the
16 design.
17 (3) Active drug particles with sizes greater than 5 microns will be
1$ deposited either in the mouth or throat. This introduces another level of
19 uncertainty since the bioavaiiability and absorption of the drug in these $
locations is different from the lungs. Dry powder inhalers need to minimize
21 the drug deposited in these locations to reduce the uncertainty associated
22 with the bioax=ailability of the drug.
23 Prior art dry powder inhalers (DPIs) usually have a means for
24 introducing the drug (active drug plus carrier) into a high velocity air
stream.
The high velocitv air-stream is used as the primary mechanism for breaking
26 up the cluster of micronized particles or separating the drug particles
from
27 the carrier. Several inhalation devices useful for dispensing this powder
form
28 of inedicament are krtown in the prior art. For example, in U.S.
Patent.Nos.
29 3,507,277; 3,518,992; 3,635,219; 3,795,244; and 3,807,400, inhalation
devices are .
2
CA 02463663 2004-04-27
. ~ =
.. 1 ' T .._... ...~ '... . ,3~
WO 99/64095 PCT/US99113420
1 disclosed having means for piercing of a capsule containing a powdered
2 medicament, xvhich upon inhalation is drawn out of the pierced capsule and
3 into the user's mouth. Several of these patents disclose propeller means,
4 which upon inhalation aid in dispensing the powder out of the capsule, so
that it is not necessary to rely solely on the inhaled air to suction powder
from
6 the capsule. For example, in U.S. Patent No. 2,517,48?, a device is
disclosed
7 having a powder containing capsule placed in a lower chamber before
8 inhalation, where it is pierced by manual depression of a piercing pin by
the
9 user. After piercing, inhalation is begun and the capsule is drawn into an
upper chamber of the device where it moves about in all directions to cause a
11 dispensing of powder through the pierced holes and into the inhaled air
12 stream. U.S. Patent No. 3,831,606 discloses an inhalation device having
13 multiple piercing pins, propeller means, and a self-contained power source
14 for operating the propeller means via external manual manipulation, so that
upon inhalation the propeller means aids in dispensing the powder into the
16 stream of inhaled air. See also U.S. Patent No. 5,458,135.
17 These prior art devices present several problems and possess several
18 disadvantages which are remedied by the inhalation devices of the present
19 invention For instance, these prior ai-t devices require that the user
exert
considerable effort in inhalation to etfect dispensing or Nvithdrawal of
powder
21 from a pierced capsule into the inhaled air stream. With these prior art
22 devices, suction of powder through the pierced holes in the capsule caused
by
23 inhalation generally does not withdraw all or even most of the powder out
of
24 the capsule, thus causing a waste of the medicament. Also, such prior art
devices result in uncontrolled amounts or clumps of powdered material being
26 inhaled into the user's mouth, rather than a constant inhalation of
controlled
27 amounts of finely dispersed powder.
28 The above description of the prior art is taken largely from U.S. Pat.
29 No. 3,948,264 to Wilke et al, who disclose a device for facilitating
inhalation of
3
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 a powdered medication that includes a body portion having primary and
2 secondary air inlet channels and an outlet channel. The secondary inlet
3 channel provides an enclosure for a capsule containing the powdered
4 medication and the outlet channel is formed as a mouthpiece protruding from
the body. A capsule piercing structure is provided, which upon rotation puts
6 one or more holes in the capsule so that upon vibration of the capsule by an
7 electro-mechanical vibrator, the powdered drug may be released from the
8 capsule. The piercing means disclosed in Wilke et al includes three radially
9 mounted, spring-biased piercing needles mounted in a trochoidal chamber.
Upon hand rotation of the chamber,-simultaneous inward radial motion of the
11 needles pierces the capsule. Further rotation of the chamber allows the
12 needles to be retracted bv their spring mountings to their original
positions to
13 withdraw the needles from the capsule. The electromechanical vibrator
14 includes, at its innermost end, a vibrating plunger rod ivhich projects
into the
intersection of the inlet channel and the outlet channel. Connected to the
16 plunger rod is a mechanical solenoid buzzer for energizing the rod to
vibrate.
17 The buzzer is powered bv a high energy electric cell and is activated by an
18 external button switch. According to Wilke et al, upon inhalation through
19 outlet channel 3 and concurrent pressing of switch lOd to activate the
electrornechanical vibrating means 10, air is sucked through inlet channels 4
21 and 12 and the air stream through the secondary inlet channel 4 raises the
22 capsule up against the vibrating plunger rod 10a. The capsule is thus
vibrated
23 rapidlv with powder being tluidized and dispensed from the pierced holes
24 therein. (This technique is commonly used in manufacturing for dispensing
powder through a hopper where the hopper is vibrated to fluidize the
26 powder and move it through the hopper outlet. The pierced holes in the
27 capsule represent the hopper outlet.) The air stream through inlet channel
4 =
28 and 12 aids in withdrawal of powder from the capsule and carries this
29 powder through the outlet channel 3 to the mouth of the user." (Wilke et
al, .
4
CA 02463663 2004-04-27
WO 99/64095 ' PCT/US99/13420
1 column 3, lines 45-55). Wilke et al further discloses that the
electromechanical
2 vibrator means may be placed at a right angle to the inlet chamber and that
3 the amplitude and frequency of vibration may be altered to regulate
4 dispensing characteristics of the inhaler.
Tluts, as noted above, the vibrator in Wilke et al's disclosed inhaler is
6 an electromechanical device consisting of a rod driven bv a solenoid buzzer.
7 (This electromechanical means mav be a motor driving a cam [Col. 4, Line
8 40])_ A disadvantage of the inhaler implementation as disclosed by Wilke is
9 the relatively large mechanical movement required of the rod to effectively
vibrate the capsule. The large movement of the rod, usually around 100s of
11 microns, is necessary due to the elasticity of the capsule walls and
inertia of
12 the drug and capsule.
13 Moreox-er, solenoid buzzers typically have operating frequencies less
14 than 5 Khz. This operating frequency tends to be noisy and therefore is
not.
desirable when incorporated into a dry powder inhaler from a patient's
16 perspective. A ttirther disadvantage of the electrochemicalactuators of
Wilke
17 is the requirement for a high energy source (Wilke et al, Col. 3, line 38),
thus
18 requiring a lar'e battery source or frequent changes of the battery pack
for
19 portable units. Both these features are not desirable from a patient safetv
and
"ease of use" standpoint.
21 The inhaler of Wilke et al is primarily intended to reduce the amount of
22 powder left behind in the capsule relative to other inhalers cited in the
patent
23 disclosure. (Wilke et al, Col. 4, lines 59-68; Col. 5, lines 1-48).
However, Wilke
24 et al does not address the need to deaggregate the powder into particle
sizes
or groups less than 6 microns in size as is required for effective delivery of
the
26 medication to the lungs; rather Wilke et al, like the prior art inhalers
continues
27 to rely on the air stream velocitv to deaggregate the powder ejected into
the
28 air stream, into particle sizes suitable for delivery to the lungs.
5
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 Another prior art inhalation device is disclosed in Burns et al U.S.
2 Patent No. 5,284,133. In this device, a liquid medication is atomized by an
3 ultrasonic device such as a piezo element (Burns et al, Col. 10,1ines 36-
51). A
4 stream of air, usually at a high velocity, or a propellant then carries the
atomized particles to the patient. The energy required to atomize the liquid
6 medication in the nebulizer is prohibitively high, making-this approach for
7 the delivery or drugs to the lungs only feasible as a desk top unit.
The.high
8 voltage requirements to drive the piezo, to produce the necessary mechanical
9 displacements. also severely effects the weight and size of the device. It
is
also not obvious that the nebulizer operating principles can be applied to the
11 dry powder inhalers for delivery or powder medicatiori to the lungs.
12 The prior art devices therefore have a number of disadvantages which
13 inakes them less than desirable for the delivery of dry powder to the
lungs.
14 Some of these disadvantages. are:
= The performance of the prior art inhalers depends on the flowrate
16 generated by the user. Lower flowrate does not result in the
17 powder being totally deaggregated and hence adversely affects the
18 dose delivered to the patient.
19 = Inconsistencv in the bioavailabilitv of the drugs irom dose-to-dose
because of lack of consistency in the deaggregation process.
21 = Lar' e energ,\- requirements for driving the electromechanical based
22 inhalers which increases the size of the devices rnaking them
23 unsuitable for portable use.
24 In our prior U.S. Patent No. 5,694,920, issued December 9,1997, we
provide an inhaler that utilizes vibration to facilitate suspension of powder
26 into a gas that overcomes the aforesaid and other disadvantages and
27 drawbacks of the above prior art. More particularly, the inhaler of our
28 aforesaid patent includes a piezoelectric vibrator for vibrating the
powder. A
29 controller is provided for controlling supply (i.e., amplitude and/or
6
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 frequency) of actuating electricity to the vibrator so as to cause vibration
of
2 the powder that is adapted to optimally suspend at least a portion of the
3 powder into the gas. As described in our aforesaid patent, the controller
may
4 include a user-actuable control for permitting the user to select the
vibration
frequencies and/or amplitudes for optimally suspending in the gas the type
6 of powder currently being used in the inhaler. The user-actuable control is
7 pre-calibrated with the controller to cause the controller to adjust the
8 frequenc,v and/or amplitude of actuating electricity supplied to the
vibrator
9 to be that necessary for vibrating the type of powder selected by the user-
actuable control in such a way as to optimally suspend at least a portion of
the
11 powder into the gas. The user-actuable control mav include selection
12 gradations in terms of the average size of the powder particles to be
13 suspended iri the gas, and/or in terms of desired vibration frequencies and
14 amplitudes. Vibration frequency would be adjusted to at least about 12 KHz,
in order to optimally suspend such commonly used powdered medications in
16 the gas. Of course, vibration frequency and amplitude may be adjusted to
17 optimize suspension of the powdered inedication being used.
18 An electrostatic field that is established across the air stream, whereby
19 bv controlling the strength of the electrostatic field primarily onlv
particle
sizes of interest are introduced into the air stream, while larger size
particles
21 are left behind in the container. This reduces the inconsistency v
associated
22 with the bioavailabilitv of the drug because of the large particles being
23 deposited into the mouth or throat as is common with devices described in
prior art.
24 Summary of the Invention
The present invention provides an improvement over prior art
26 inhalation devices such as described in our aforesaid L.S. Patent No.
27 5,694,920. In one einbodiment of the invention, the inhaler contains two or
28 more vibrator means or piezoelectric elements so that different drugs, i.e.
of
29 different particle size, may be delivered from the same inhaler.
7
CA 02463663 2004-04-27
WO 99/64095 PCTIUS99/13420
I In yet another embodiment of the invention, the piezoelectric elements
2 are switched between two. or more set frequencies, or frequencies swept so
as
3 to avoid potentially setting up standing waves in the powder.
4 In yet another embodiment of the invention, the inhaler includes
electronic circuitry for recording and/or controlling one or more functions
6 such as dose counting, patient compliance monitoring, and patient
7 compliance reminders. Also, the inhaler may be programmed according to a
8 delivery protocol, i.e. to alter the quantity of drug delivered over time.
If
9 desired, the inhaler also mav include an environmental sensor and knockout
control, for example, to deactivate the inhaler in the event it is
inadvertently
11 exposed to too high a temperature, a clock to deactivate the inhaler in the
12 event its shelf life is exceeded, and/or a security/safety lock-out.
13 Still yet another embodiment of the present invention provides an air
14 flow sensor for controlling various components of an inhalation device.
Included in the preferred embodiment is an acoustic controller, the acoustic
16 controller including an acoustic element to sense air flow around the
element
17 and for producing signals representative of a frequency and amplitude of
the
18 air flow, the signals being used to control (e.g., activated, deactivate,
apply
19 incremental voltage, etc.) certain components of the inhalation device.
Preferably, acoustic element is a microphone element or pressure
21 transducer positioned within the air passage of an inhalation device,
(e.g., a
22 dry powder inhaler) that produces signals in response to the inhalation air
23 flow, these signals are used to control certain components of the inhaler,
e.g.,
24 a high frequency vibrator, an electrostatic plate, timer, counter, etc.
Also
preferably, these signals are used to activate/control certain components of
26 the inhalation device to maxiunize the inhalation effectiveness to obtain
27 maximum patient benefif from medicament.
28 Thus, the present invention provides a fully automated inhalation
29 device, that is breath activated, that perm:, optimal utilization of the
8
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 particular medication. For example, acoustic.signals can be used to trigger
2 the high frequency vibrator and electrostatic plate onlN? when the patient
has
3 achieved optimum (e.g., maximum) inhalation effort, thereby ensuring that
4 the full (proper) dosage of medicament properly enter the patient's
respiratory system. Alternatively, these signals (breath-activated signals)
can
6 be used to progressively apply increasing power to, or, sequentially
' activate/deactivate the various components of the inhalation device to
S aclueve optimal inhalation dosage.
9 The present invention also relates to the packaging of dry powders and
particularly to the metering and packaging of precise quantities of
11 pharmaceuticals and dnigs for medical uses.
12 The certification of new pharmaceuticals is a lengthy and costly process
13 involving animal studies followed by chemical trails to establish both
efficacy
14 and safety. Because a~pharmaceutical's characteristics may be affected by
changes in manufacturing and/or packaging, the approval process liznits the
16 approval to a particular manufacturing and packaging process.
17 In our earlier U.S. Patent 5,699;649, granted December 23, 1997, we
lS describe a method and apparah.ts for packaging microgram quantities of fine
19 powders such as pharinaceuticals using electrostatic phototechnolog,y }
techniques. IvTore particulariy, as described in our aforesaid US. Patent
21 5,699,649, the ability of powders to acquire an electrical charge
22 advantageouslv is utilized for precisely measuring exact microgram
23 quantities of the powder, whereupon these exact microgram quantities are
24 then placed in individual containers, and the containers sealed.
Electrostatic charge has been employed to attract a given quantity of
26 powder to a surface. An example of this is the laser printer or the
electrostatic
27 copy devices where a drum is charged and toner particles are attracted and
28 held in position by the charge. The charge on the drum is neutralized by
the
29 attracted toner powder, thus limiting the amount of toner in accordance
with
9
CA 02463663 2004-04-27
WO 99/64095 PCTIUS99/13420
1 the charge image on the drum. The charge on these printer drums is then
2 transferred to a sheet of paper or other carrier to give a final image. In
our.
3 U.S. Patent 5,699,649, the same electrostatic charge technology is emploved
4 for transferring a predetermined amount of a finely powdered
pharmaceutical or drug to a carrier or an intermediate such as a drum,
6 carrving a charge of predetermined intensity and area, rotating the charged
7 drum surface; carrying the predetermined amount of powdered
8 pharmaceutical or drug on its surface, to a transfer station where the
charge is
9 overcome and the dry powder is transferred to a package which is then
sealed. In lieu of a drum, a belt, or other movable surface is charged to a
11 given potential in a localized area.
12 When a given anlount of a powdered pharmaceutical or drug is to be
13 packaged, the charge and. area ;of charge can be detei=mined experimentally
14 for each dose of pharmaceutical or drug and each particle size
distribution.
This can be done by controlling either the charged area for a given charge
16 density or the total elec.trostatic.charge on any individual charged area.
These
17 conditions can be adjusted to provide essentiallv the exact desired amount
of
18 the particular pharmaceutical or drug to be transferred at the transfer
station.
19 In the present invention, the electrostatic charge technology described
in our aroresaid U.S. Patent 5,699,649 is adopted to be used for measuring and
21 packaging unit doses of a pharmaceutical.or drug in a readilv ingestible
form,
22 i.e. as a tablet or capsule. The technology thus described permits
reproducible
23 precise measurement a.nd packaging of a pharmaceutical or drug, and which
24 may be scaled from laboratory to pilot plant to full scale production
without
the need for recertification.
26 Still other features and advantages of the present invention may be
27 seen from the following detailed description, taken in connection with the
28 attached drawings, wherein like numerals depict like parts, and wherein:
29 Brief Description of Drawings
Figure 1 is a perspective view of an inhaler of the prior art;
CA 02463663 2004-04-27
WO 99/64095 - PCT/US99/13420
1 Figure 2 is a rear plane view of the inhaler shown in Figure 1;
2 Figure 3 is a longitudinal cross-sectional schematic view of the inhaler
3 of Figure 1;
4 Figure 4 is a functional block diagram of the vibration control system
of one embodiment of Figure 1;
6 Figure 5 is a functional block diagram of the vibration control system
7 of another embodiment ot the invention;
8 Figures 6-10 are function block diagrams of the N-ibration control
9 system in accordance with still yet ot her embodiments of the invention; and
Figure 11 is a view, similar to Fig-Ltre 3 of vet another embodiment of
11 the invention.
12 Figure 12 is a cross-sectional view of a typical inhalation device and the
13 acoustic controller of the present invention;
14 Figure 13 is an expanded cross-sectional view of Figure 12;
Figure 14 is a functional block diagram of a preferred embodiment of
16 tlie acoustic controller of the present invention;
17 Figure 15 shows a schematic representation of the attraction of
18 negatively charged powder particles to a support having a positive charge
on
19 the surface thereof;
Figure 16 shows a block diagram of the various steps involved in
21 practicing the invention;
22 Figure 17 is a schematic representation of one form of drum type
23 electrostatic device for transferring given small quantities of powdered
drugs
24 from an electrostatic attraction station, where a given quantity of
powdered
drug is attracted to and neutralizes a given charge on the drum, and a
26 subsequent transfer station where the drug is transferred from the drum to
a
27 package therefor;
28 Figures 18 and 19 are schematic functional representations of preferred
29 components employed in the Fig. 17 type of apparatus;
I1
CA 02463663 2004-04-27
WO 99/64095 PCT/[1S99/13420
1 Figure 20 shows a different system wherein separate carriers, having
2 micronized drug particles eiectrostatically attached to their surface, are
used
3 to carry the drug to the charged transfer surface;
4 Figures 21 and 22 show methods of aerosolizing the powdered drug
and ionizing the drug to give it a specific charge;
6 Figure 23 shows a graph illustrating the percentage of suspended
7 particles as a function'of time and size, permitting creation of a suspended
8 particle stream of any given desired size distribution;
9 Figure 24 shows another embodiment of applying the aerosolized drug
to a drum carrring charge "image";
11 Figure 23 illustrates an ion projection system for creating the charge
12 "image" on a dielectric surface;
13 Figure 26 is a view similar to Fig. 16, and illustrating an alternative
14 embodiment of the invention;
Figure 27 is a view similar to Fig. 16, and illustrating another
16 alternative embodiment of the invention;
17 Figure 28 is a view similar to Fig. 16, and illustrating yet another
18 alternative einbodiinent of the invention;-and
19 Figure 29 is a view similar to Fig. 16, and illustrating still yet another
alternative embodiment of the invention.
21 Figures 1-3 illustrate an embodiment 10 of inhaler made in accordance
22 with our aforesaid U.S. Patent No. 5,694,920. Inhaler 10 includes a hard
23 plastic or metal housing 18 having a generally L-shaped longitudinal cross-
24 section. Housing 18 includes four air flow openings 20, 28, 30, and 32.
Inhaler
10 includes a inain air flow passage 26 which extends the length of the
26 housing 18 from the front 22 (at opening 20) to the rear 2=1 thereof (at
opening
27 28) and has a generally square-shaped transverse cross-section, so as to
perrnit
28 air flow therethrough (denoted by arrow F in Figure 1).
12
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 Secondary air conduit 31 is generally L-shaped and runs longitudinally
2 from opening 30 in the rear 24 surface of the housing 18 to main passage 26.
3 One-way flow valve 50 is mounted to the inner surface 33 of the main passage
4 26 via a conventional spring-biased hinge mechanism (not shown), which is
adapted to cause the valve 50 to completely block air flow S through the
6 coriduit 31 to the main passage 26 when the pressure of the air flow F in
the
? main passage 26 is below a predetermined threshold indicative of inhalation
8 through the passage 26 by a user.
9 Powder dispensing chamber 54 is formed in housing 18 for holding a
capsule 34 of powder medication to be inhaled. Housing 18 includes a
11 moveable panel portion 32 in the rear 24 for permitting the capsule 34 to
be
12 introduced into the chamber 54 and placed on the seating 52 of vibration
13 means 36 betiveen guiding means 60A, 60B. Preferablv, means 36 comprises a
14 hard plastic or metallic protective shell 37 enclosing a piezoelectric
vibrator
90. (Figure 4). Preferably, vibrator 90 is mechanically coupled through the
16 shell 37 via a disk (not shown) to the drug cartridge 34, so as to permit
17 maximum vibratory energy to be transmitted from the vibrator 90 through
18 the shell 37 to the cartridge 34. Guiding means 60A, 60B includes two
19 surfaces which slant downwardly toward the seating 52 so as to permit easy
introduction and retention of the capsule on the seating 52 in the chamber 51.
21 Removable panel 32 includes another air inlet 34 for permitting additional
air
22 flow S2 from the chamber 51 through conduit 61 into conduit 31 during
23 inhalation bt- the user. Preferabiy, panel 32 and housing 18 include
24 conventional mating mounting means (not shown) for permitting the panel 32
to be removablv resecurable to the housing by the user between introduction
26 of fresh (i.e., completely full) capsules and removal of spent (i.e.,
empty)
27 capsules.
28 Inhaler 10 also includes a conventional miniature air stream velocity or
29 pressure sensor 40 mounted on the inner surface of the conduit 26 so as to
13
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 sense the speed and/or pressure of the air stream F. Preferably, sensor 40
2 comprises a conventional spring-loaded flapper-yield switch which generates
3 electronic signals indicative of the speed and/-or pressure of the air
stream F
4 in the conduit 26, and transmits those signals via electrical connection 42
to
electronic control circuitry 48 contained in housing 18 for controlling
6 actuation of the vibrator means based upon those signals.
7 Preterablv, the control circuitrv 48 is embodied as an application
8 specific integrated circuit chip and/or some other type of very highly
9 integrated circuit chip. Alternatively, control circuitnf 48 may take the
form
of a microprocessor, or discrete electrical and electronic components. As will
11 be described more fully below, the control circuitry v 48 determines the
12 amplitude and frequency of actuating power to be supplied from
13 conventional power source 46 (e.g., one or more D.C. batteries) to the
14 piezoelectric vibrator to thereby control vibration of the vibrator. The
actuating power is supplied to the piezoelectric element 90 via electrical
16 connection 44 between the vibrator and the circuitry 48.
17 Piezoelectric element 90 is made of a material that has a high-
18 frequency, and preferably, ultrasonic resonant vibratory trequency (e.g.,
19 about 15 to 100 MHz), and is caused to vibrate with a particular frequency
and amplitude depending upon the frequency and/or amplitude of excitation
21 electricity applied to the piezoelectric element 90. Examples of materials
that
22 can be used to comprise the piezoelectric element 90 include quartz and
23 polycrystalline ceramic materials (e.g., barium titanate and lead zirconate
24 titanate). Advantageously, by vibrating the piezoelectric element 90 at
ultrasonic frequencies, the noise associated with vibrating the piezoelectric
26 element 90 at lower (i.e., non-ultrasonic) frequencies can be avoided.
27 Turning specifically to Figure 4, the various functional components
28 and operation of the control circuitry 48 will now be described. As will be
29 understood by those skilled in the art, although the functional components
14
CA 02463663 2004-04-27
WO 99/64095 PCT/US99l13420
1 shown in Figure 4 are directed to an analog realization of the control
circuitry
2 48, the components of Figure 4 could be appropriately modified to realize
3 control circuitry 48 in a digital embodiment without departing from this
4 embodiment 10 of the present invention.
Control circuitry 48 preferably includes actuation controller 70 and
6 vibratorv feedback control svstem 72. Actuation controiler 70 corn.prises a
7 conventional switching mechanism for permitting act ,~ating power to be
8 supplied from the power source 46 to the control system 72 depending upon
9 the signals supplied to it from sensor 40 and the state of the power switch
12.
In other words, controller 70 permits actuating power to be supplied from the
11 source 46 to the system 72 when the sliding indicator bar 14 of switch 12
is set
12 to the "ON" position in channel track 16 and the inhalation sensor 40
supplies
13 signals to the controller 70 that indicate that the inhalation is occurring
14 through the main passage 26. However, controller 70 does not permit
actuating power to flow from the source to the system 72 when either the
16 switch 12 is set to "OFF" or the signals supplied to the controller 70 from
the
17 sensor 40 indicate that inhalation is not taking place through the conduit
26.
18 When controller 70 first permits actuating power to be supplied from
19 the source 46 to the feedback control svstem 72, the svstem 72 enters an
initialization state wherein controllable means for supplying a predetermined
21 frequency and amplitude of actuating electricity 74 is caused to generate
22 control signals for causing conventional pump circuit SO to generate an
initial
23 desired frequency and amplitude of actuating electricitv based upon stored
24 values thereof stored in the initialization memory means 82. i'referablv,
means 74 comprises conventional frequency sweep generator and frequency
26 generator means 76 and 78, respectively. The signals generated by means 74
27 are then supplied to charge pump circuit 80 to cause circuit 80 to supply
the
28 piezoelectric element 90 with actuating electricity specified by the
signals.
CA 02463663 2004-04-27
D
WO 99164095 PCT/US99/13420
1 Preferably, the initial frequency and amplitude of actuating electricity
2 supplied to the piezoelectric element 90 is pre-calibrated to cause the
3 piezoelectric element 90 to vibrate at its resonance frequency when no
4 powder cartridge or powder is placed on the means 36. As will be
appreciated by tliose skilled in the art, maximum transfer of vibratory power
6 from the piezoelectric element to the powder in the container 34 takes place
7 when the piezoelectric elen-ient vibrates at its resonant frequency. It has
been
8 found that this results in maximum de-aggregation andsuspension of the
9 powder from the container 34 into the air to be inhaled by the user:
However,
when the container 34 or powder is placed on the vibrator means 36, the
11 weight and volume of the powder container, and the weig ht, volume, and
12 particular size of the powder to be suspended by the piezoelectric element
can
.13 change the vibration characteristics of the piezoelectric element, and
caiuse the
14 piezoelectric element to vibrate at other than its resonant frequency: This
can
result in reduced vibratory energy transfer to the powder from the
16 piezoelectric element, and.thereby, lessen the efficiency of the
piezoelectric
17 element in de-aggregating and suspending the powder in the air inhaled bv
18 the user.
19 The feedback control svstem 72 overcomes this problem. In.contral.
system 72, after tlie initial frequency and amplitude of actuating electricitv
are
21 supplied to the piezoelectric element 90, the frequency generating means 74
22 svstematically generates control signals indicative of many different
23 amplitudes and frequencies of electricity for being supplied to the
24 piezoelectric element 90 by the circuit 80. As the generating means 74
"cycles
through" the different frequencies and amplitudes, the instantaneous power
26 transfer characteristics of the piezoelectric element 90 for each of these
27 different frequencies and amplitudes are determined by the detector 88,
28 which transrnits this iilformation to the peak power detector 86. Peak
29 detector 86 analyzes the instantaneous power transfer. characteristics of
the .
16
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
I piezoelectric element 90 and signals the sample and hold feedback controller
2 84 when the power transfer characteristics are at local maxima. The
controller
3 84 correlates these local maxima with the frequencies and amplitudes
4 commanded bz the generator 74 to be supplied to the piezoelectric element
90.
6 After the freqttency generator 74 has finished its sweep through the
7 frequencies and amp~tudes of power supplied to the piezoelectric element 90,
8 the controller 84 causes the generator 74 to cycle through the frequencies
and
9 amplitudes of power that resulted in local maxima, and then determines
which of these frequencies and amplitudes results in optimal power transfer
11 characteristics through the piezoelectric element 90.
1? CompleHng the controller 72 is a clock 500 which is tripped when
13 actuating electricitv is first supplied to the piezoelectr-ic element 90.
Clock 500
14 includes a counter which prevents a second activation of the piezoelectric
element for a preset period of time. Thus, overuse and overdosin.g bv the
16 patient are prevented.
17 In operation of embodiment 10, the.drug-containing package 34 is
18 punctured and inserted -onto the surface 52 of vibrator 36 in chamber 51 in
the
19 maruier described previouslv.; The power switch 12 is placed in the "ON"
position and the user inhales air through the rnnduit 26, air flow F is
21 generated throuc-h conduit 26. This causes one-way valve 50 to deflect to
22 admit air flow S through opening 30 into conduit 26, and also causes air
flow
23 S2 through opening 3-4 and chamber 51 into conduit 26. The inhalation of
air
24 stream F is sensed by sensor 40 and is signaled .to actuation controller
70,
which causes power to be supplied to the controller 72. The controller 72 then
26 adjusts the amplitude and frequency of actuating power supplied to the
27 piezoelectric element until they are optimized for the best possible de-
28 aggregation and suspension of the powder P from the capsule into the air
29 stream F via air flows S and S2.
17
CA 02463663 2004-04-27
. _~ t . . =WO 99/64095 PCT/US99113420
I Figure 5 illustrates another embodiment of the invention. Figtire 5 is
2 similar to Figure 4, except the clock 500 is replaced with a counter 502
which
3 counts the number of doses delivered by the device. Counter 502 is connected
4 to a display 504 cvhich displays the number of dbses delivered, or,
optionally,
the number o'r doses remaining.
6 Figure 6 illustrates yet another embodiment of the invention. The
7 Figure 6 embodirnent zs similar to the Figure 4 embodiment, except the clock
8 500 is replaced by an internal monitor which contains a record of inhaler
use.
9 Completing the Figure 6 embodiment is a hatch 510 through which a.'
physician mar access, read and/or download the data from monitor 508,
11 whereby to determine patient compliance.
12 Figure. - illustrates yet another embodiment of the invention. The
13 Figure 7 embodiment is similar to-the Figure 4 embodiment except in .the
14 Figure 7 embodiment, clock 500 counts time for the purpose of reminding a
patient to use the inhaler. Thus, clock 500 is connected to a tone generator
514.
16. Figure 8 illustrates yet another embodiment of.the invention. The
17 Figure 8 embodiment is sirnilar to the Figiue 4 embodiment, except that it
18 includes a. clock or.counter 516 which sends a signal to controller 84 to
alter..
19 the activation time, i.e. to a shorter or longer period, whereby to alter
the
20. quantity of drug, delivered; e.g..to, increase or decrease dosage over
time. _
21 Alternatively, clock 516 may be pxogrammed to disable the inhaler once a
22 certain date is passed, i.e. so as to avoid possible use of out-of-date
drugs.
23 Figure 9 illustrates yet another embodiment of the 'invention. Figure 9
24 is similar to Figure 4, except the counter or clock 500 is replaced with a
temperature sensor 518. Certain medications are heat sensitive, and may be
26 deactivated, or,rendered potentially dangerous if exposed to high
27 temperatures, tor example, as might occur if the inhaler is left in an
28 automobile on a sunny day. Temperature monitor 518 will deactivate
29 controller 72 in the event a preset temperature is reached. If desired,
monitor
18
CA 02463663 2004-04-27
WO 99164095 ~ PCT/US99/13420
1 518 also could include a displav warning the patient that a preset
temperature
2 has been reached.
3 The Figure 10 embodiment is similar to the Figure 9 embodiment
4 except in the Figure 10 embodiment, the temperature sensor is replaced with
a"key" such as, for example, a three button kevboard bv which the user's pin
6 code must be entered in order to activate the device. This tivill prevent,
for
7 example, use of the irihaler by someone other than the intended patient, and
8 would prevent, for example, controlled or dangerous drugs from being used
9 by children. For ease of use, kev 520 mav permit the patient (or druggist)
to
program a specific pin code for the intended user.
11 Referrin-, to Figure 11, which illustrates vet another embodiment of the
12 invention, in which two piezoelectric vibrators 90A, 90B, are located side-
by-
13 side within the inhaler shell. In this embodiment, piezoelectric elements
90
14 are designed to.vibrate at different amplitudes and frequencies, i.e. so
that, for
example, two different drugs advantageously may be dispersed
16 simultaneously from the same inhaler, without compromising performance or
17 either drug. This permits delivery of two drugs which. ivhile active
together,
18 mav not readily be stored together. For example, an asthma inhaler mav be
19 provided containing both a bronchodilator, such as albuterol, and a.steroid
which may require different peizo settings. The Figure 11 embodiment
21 includes a pre-calibrated controller 112 which includes a first and a
second
22 pre-calibrated frequencv/amplitude control signal generator 110A,110B,
23 which supplies control signals to pump circuit A and pump circuit B,
24 respectively. Of course, the pre-calibrated controller 112 may be replaced
with a pair of feedback controllers similar to that shown in Figure 4.
26 Referring to Figures 12 and 13, a cross-sectional view of an airflow
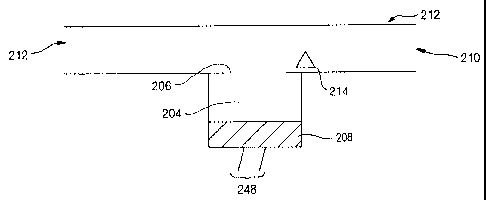
27 passage 212 of an inhalation device 202 is depicted. It should be noted at
the
28 outset that the airflow passage 212 depicted in Figure 12 is a generalized
29 airflow passage of a typical inhalation device, such as those discussed
above..
19
CA 02463663 2004-04-27
WO 99/64095 111 PCT/US99/13420
I However, the present invention is intended to be adapted to any inhalation
2 device, regardless of the particular geometry of the airflow passage. At its
3 most basic level, the present invention operates by providing an air flow
4 sensor 208 to detect air flow turbulence around the sensor 208 (i.e.,
inspiratory
air flow rate of a user of the irihaler) and to control various components of
the
6 inhalation device 202, as a function of the amplitude and/or frequency of
the
7 detected airfloNv turbulence, as described below.
8 As shown in Figure 12, air 110 (or other fluid) enters the airflow
9 passageway 212, typically by the respiratory activity of a patient inhaling
on
the device 202. As air 210 flows through the passage 212, a portion thereof
11 flows through the opening 206 in the passage 202 into a cavitv 204. Placed
12 within the cavitv 204 is an air flow sensing device 208. Preferablv, the
air flow
13 sensing device 208 is an acoustic sensing device, e.g. a microphone. Also
14 preferably, n-iicrophone 208 is adapted to produce appropriate signals 248
in
response to the airtlow detected within the cavity 204. The amplitude and
16 frequency of the airflow within the cavity 204 is a function of the airflow
rate
17 210 within the air passage 212 of the device 202. Thus, output signals 248
18 from the microphone 208 will vary in both frequency and amplitude as a
19 function of air flow rate within the cavitv (which is a function of f7ow
rate
within the passage 212), and thus, can be used to control various components
21 of the inhaler 202 as a function of frequency and/or amplitude, as
described
22 below. Those skilled in the art will appreciate that the shape of the
cavity 204
23 and the size of the opening 206 are chosen in accordance the particular
24 geometry of the air passage 212, the air flow rate 210 through the passage
212,
and/or the frequency response and/or sensitivity of the microphone 208; and
26 all such variations are within the scope of the present invention.
Preferably,
27 as noted above, the shape of the cavity 204 and the size of the opening 206
are
28 chosen to permit at least a portion of the air within the passage 202 to
enter
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
I the cavity 204 with sufficient amplitude to induce a response from the
2 microphone 20S.
3 Referring now to Figure 13, an expanded cross-sectional view of an
4 embodiment of the air flow sensor (described with reference to Figure 12,
above) in a drv powder inhaler, such as disclosed in U.S. Patent 5,694,920.
6 Depicted in Fi~Ture 13 are the components of a typicalfy dry powder inhaler
7 202. A mouthpiece 246 is provided for a user (i.e., patient) to inhale on
the
8 device 202. A high-frequency vibratory nnechanism 228 (e.g., piezoelectric
9 element, ultrasonic acoustic transducer, or other electro/meehanical
vibratory
mechanism, etc.) is provided to vibrate a container 220 (e.g., blister
capsule) of
11 dry powdered medicament 250 to suspend particles of the medicament into
12 the air passage 212. To furtlzer aid the suspension of particles, an
electrostatic
13 potential plate 226 can be provided to draw particles of a certain charge
(i.e.,a
14 charge opposite to that of the electrostatic plate 226) into the air stream
210.
In this embodiment, a portion 210' of the air 210 drawn into the air passage
16 212 is induced into the cavity 204, to be detected by the microphone
element
17 208. Upon detection of airflow, the microphone element produces output
18 signals 248 proportional in amplitude and frequency of the air flow rate
19 within the air passage 212. T11e output signals 248 are used to control
either
the high-frequency vibrator 228 and/or the electrostatic plate 226, or other
21 components of the inhaler, ati described below.
22 Figure 14 is a block diagram representation of the acoustic control
23 system of the present invention for a dry powder inhaier. As described
above,
24 the microphone elenlent 208 produces signals 248 in response to detected
airflow 210'. These signals are processed by an amplitude/frequency
26 processor 230 to condition the signals 248 and to determine the amplitude
27 and/or frequency of the output signals 248. The amplitude/frequency
28 processor produces output signals 248' to control the high-frequency
vibrator
29 and/or electrostatic plate. To that end, outpLat signals 248 are input into
a
21
CA 02463663 2004-04-27
WO 99164095 PCT/US99/13420
1 comparator circuit 240 and/or 232 and compared with a reference threshold
2 signal 242 and / or 234, respectivelv.
3 It should be understood that signals 248 and 24S' are indicative of the
4 airflow rate 210, described above. The present invention is intended to be
controllable as a function of frequency and/or amplitude of signals 248, thus,
6 amplitude/frequency processor can be adapted to condition the signals 248 in
7 terms of anlplitude or frequency are both. High frequency vibrator threshold
8 242 produces a signal ?52 which represents the minimum voltage and/or
9 frequency required to activate the high frequency vibrator controller 244
(which, in turn. activates the high frequency vibrator 2-16). Comparator 240
11 compares signai 252 with signal 248' and if the signals have equal
amplitude
12 and/or frequencv (within some predetermined error margin), comparator
13 activates the high frequencv vibrator controller 244, which activates and
14 directly controls the high frequency vibrator 226. Similarly, electrostatic
plate
deflector controller 236 is activated by an equal match of signals 248' and
254
16 by the comparator 232. Electrostatic plate detector threshold 234 produces
17 signal 254 which represents the minimum voltage and / or frequency required
18 to activate the electrostatic plate 226.
19 Inspiratorv capacity processor 238 is provided to compute the peak
inspiratory flow 210 (represented by signals 248 and 248') of the patient.
21 Although not shown in the drawings, this information can be used to adjust
22 the threshold signals of the high frequency vibrator threshold 242 and/or
23 electrostatic plate detector threshold 234. Of course, to accomplish this,
the
24 high frequencr vibrator threshold 242 and/or electrostatic plate detector
threshold 234 must be progralnmable, as is known in the art. In this way, the
26 microphone 20S can be progranuned to trigger the various components of the
27 inhaler to adjust for varying inspiration flow rates from patient-to-
patient or
28 individually. Thus, for example; the inspirator control scheme of the
present
29 invention can be self-adjusting to account for a patient's decrease in
22
CA 02463663 2004-04-27
WO 99/64095 PCT/US99113420
1 inspiratory flow rate caused by, for example, decreased lung capacity.
2 Alternatively, the processor 238 can be modified to seauentially turn on the
3 various components herein described (e.g., vibrator, electrostatic plate,
etc.) at
4 optimal inhalation times (e.g., peak inhalation effort). Thus, for example,
the
processor 238 can be modified to activate the vibrator at a time just prior to
6 the user's peak inhalation effort, then to activate the electrostatic plate
7 subsequently, thereby inducing the medicament into the airstream at a time
8 that produces optimal respiratory absorption of the medicament. Moreover,
9 processor 238 can be adapted with appropriate memo;y to track a patient's
inspiratorv flow rate which can be used to adjust the poxvdered medicament
11 250 to achieve maximum medication benefit.
12 Manv modifications, alternatives and equivalents are possible. For
13 example, Processor 230, threshold signal generators 23-1 and 242,
comparators
14 242 and 232 and can be any known digital (e.g., microprocessor) or anaiog
circuitry and/or associated software to accomplish the functionality described
16 herein. Although the various components described in Figure 14 have been
17 described in a modular fashion, those skilled in the art will recognize
that
18 each of these components can be discrete off-the-shelf or custom
cornponents,
19 or can be included in a single, unified system.
The present can be modified by permitting the microphone signals 248
21 and 248' to directly control activation of the high frequency vibrator 228
22 and/or electrostatic plate 226, thereby bypassing the comparators 240
and/or
23 232. In this way, microphone 208 can be adapted to activate these
24 components in a binary fashion that is not dependent upon flow rate. Also,
it
will be understood to those skilled in the art that the thresholding circuits
242
26 and 234, the amplitude/frequency processor 230 and the inspiratory
capacitor
27 processor 238 can be adapted to permit user (patient) control and user-
28 definable presets (i.e., minimum flow rate for activation, etc).
23
CA 02463663 2004-04-27
_.~ -._.. ~
WO 99/64095 PCT/US99/13420
1 In addition, comparators 240 and 232 can be adapted to permit
2 generation of activation signals based differing signal strengths and/or
3 frequency. Thus, for example, the high frequency vibrator can be adapted to
4 activate only when a signal frequency of 1Khz is achieved, while the
electrostatic plate will only activate when a signal strength of 35mV. is
6 obtained.
7 Other modifica-tions are also possible. For example, the microphone
8 208 can be positioned directly on the inner wall of the airflow passage 212
of
9 the device 202, instead of within the cavity 204. Also, as shown in Figure
12, a
turbulence generator 214 can be provided to generator air turbulence within
11 the air passage 212. This modification, for example, can be used in an
12 inhalation device that would otherwise not permit a portion 210' of the air
210
13 to enter the cavity 20=1. In addition, instead of a microphone 208, the
acoustic
14 eleinent can be any known fluid pressure transducer (e.g., air pressure
transducer) that will output appropriate signals as a function of fluid
pressure
16 (amplitude) and/or frequency. Accordingly, the present invention can be
17 appropriately modified to operate in any fluid medium (other than air), to
18 provide automatic acoustic control.
19 Still other modifications are possible. For example, although not=
shown in the drawings, the present invention can be provided with a timer
21 that is controlled by signals 248'.. The timer can be appropriately
modified to
22 control a schedule of when the device may be activated, to avoid, for
example,
23 an overdose. Thus, for example, the timer may be modified to only permit
24 activation of the components of the device at certain times of the day.
Moreover, the timer may be appropriately modified to permit downloading
26 of data related to usage (e.g., time of day used, dosage of medicament,
27 inhalation effort, etc.). This data can be particularly relevant for
clinical trials
28 where it is important to track the recommended dosage and times of
29 medication. Of course, the previous description could be accomplished with.
24
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 a counter, or the like, tl-iat simply counts the amount of times that the
device
2 has been used.
3 Although the present invention has been directed to an acoustic control
4 scheme for a dry powder inhaler 202, the present invention is not so
limited.
On the contrary, the present invention is intended to be adapted for any
6 inhalation device that would_require a control mechanism (such as described
7 herein) based breath (inhalation) detection. For example, an anesthetic
device
8 could be modified with the breath sensor and controller as provided herein
to
9 monitor and control the amount of anesthetic a patient receives.
Additionally, the acoustic sensing element can be used to measure peak
11 inspir.atory and;' or expiratory flow of a particular patient, and record
this
12 information for downloading and analysis.
13 Referring to Figure 15 there is illustrated a chamber 314 containing
14 aerosolized dry powder particles of a pharmaceutical or drug 310. These
particles 310 are suspended in air and cariy a charge, for example a negative
16 charge. Also in the chamber is a support surface 312 having a charge
opposite
17 to that on the particles. The support surface 312 will attract a number of
18 charged particles 310 sufficient to neutralize the charge on the surface of
the
19 support 31L. This support surface is one that can hold a discrete
electrical
charge on its surface, such as insulating material, e.g. plastic or a
21 semiconductor inaterial, such as selenium, used in the photocopy industry.
22 T1te actual amount of pharmaceutical or drug powder transferred to
23 the carrier sheet is a function of the mass-to-charge ratio of the powdered
2-4 particles. If one assumes surface charge saturation, the amount of charge
carried by the particles is directly related to the surface area. For
spheriodal
26 particles, the charge varies as the square of the radius and the mass
varies as
27 the cube. Thus, the amount of charged particles picked up by a given
portion
28 of the surface of the charge carrier will be a function the total charge on
the
29 carrier. Thus, c,-ith a given surface charge density on the carrier, the
amount
CA 02463663 2004-04-27
WO 99164095 PCT/US99/73420
1 of pharmaceutical or drug powder picked up is directly proportional to the
2 charged area. Thus, for doubling the amount of pharmaceutical or drug
3 powder to be picked up, and thus the dose amount, the area on which charge
4 is placed can be doubled. This can be used as a basic method to control the
amount of powder to be picked by the carrier. Thus, for any particular
6 powder or particle size distributionof powder, the exact area and amount of
7 charge needed can be experimentally determined.
8 Referring now to Figure 16, there is a schematic tlow diagram of the
9 various items of equipment needed to perform in the total process from
powder supply to packaged pharmaceutical or drug, i.e. in capsule form,
11 containing a specified amount of pharmaceutical or drug powder in the
12 package. At 316 is indicated the pharmaceutical or drug powder supply
13 which is fed into a device 318 for creating an aerosol of the powder. Next
the
14 powder particles are ionized at 320. As will be indicated later, a number
of
these steps and pieces of equipment can be combined. At 324 is indicated a
16 carrier surface capable of maintaining a space charge on its surface. This
can
17 be a plastic belt, for example, or a selenium drum of the type used in
Xerox''"'
18 photocopiers. This carrier surface 324 is passed through a charging station
19 325 where a predetermined electrostatic charge 325A (an eiectrostatic
"image") is created on a predetermined area of the transfer surface. This
21 charged surface 325A then passes through a step 326 wherein powder is
22 deposited on the carrier surface in a sufficient amount 326A to neutralize
the
23 charge carried by the carrier surface. Thereafter, the carrier surface,
carrying
24 the predetermined amount 326A of powder on its surface, is passed to a
powder discharging device 330 which discharges the powder 326A from the
26 surface 324 into the open end of a capsule 329, %=hich capsule is carried
on a
27 convevor belt 328. A carrier 324 and convepor belt 328 are indexed and
28 synchronized in a predeterrnined manner so that the electrostatic "image"
29 aligns directly over the open end of capsule 329 and powder discharging
26
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 device 330 during the discharge sequence. At that time powder discharging
2 device 330 is activated whereupon the predetermined amount 326A of
3 powder is released from surface 325A, and falls into capsule 329. The
capsule
4 329 containing its charge of powder 326A, then passes through a capsule
sealing step 332 wherein the capsule is capped.
6 As mentioned previously in discussing Figure 15, the carrier surface
7 with the electrostatic charge carries a known amount of charge on its
surface
8 and the polarity of this charge is opposite to that of the powder particles
9 suspended in the chamber. The charged particles migrate to the charged
surface because of the attraction by the opposite nature of the charges. This
11 migration of the particles continues until the charge on the carrier
surface is
12 neutralized.
13 The actual amount of powder mass transferred to the carrier surface is
14 a function of the mass-to-charge ratio of the charged particles. Although
it is
difficult to achieve a linear relationship between the mass and the actual
16 charge, it is possible to establish a fixed relationship bettiveen the
surface area
17 of the powder particles and the charge the powder particle is carrying at
18 charge saturation. However, the surface area of a mixed group of powder
19 particles of different sizes and shapes can be extremely t- difficult to
calc~ilate 20 mathematicallv, particularly when the shapes are irregular,
(e.g. non-
21 spherical, microcrvstalline, etc.) As mentioned earlier, the simplest
method
22 of determining the amount and area of charge to attract a given weight of
23 particles is to estimate the correct area and charge and then apply the
24 estimated charge to the estimated area on the carrier surface 324 and
expose
this selectively charged area to a mass of.powder which has been ionized in
26 the ionizing step. The amount of powder deposited can then be readily
27 measured at the. discharge step. Thereafter, either the size of the charged
area
28 or the amount of charne applied to the area at the charging station 325 can
be
29 adjusted upwardly or downwardly to provide the correct amount of charge,.
27
CA 02463663 2004-04-27
_..~ ;
_ ~ _.. . .
= WO 99164095 PCT/US99/13420
I both in area and charge intensity, for picking up a desired weight of
2 oppositely charged powder.
3 ReÃerring now to Figures 17, 18 and 19, one preferred apparatus for
4 accomplishing the invention is illustrated schematicallv in Figure 17, with
details of the components thereof being shown in Figures 18 and 19. The
6 charge carrying surface is illustrated as a photo sensitive drum 324A which
7 rotates between the charge "image" exposure 325 which creates a charge
8 "image" 325A on the surface of the drum 324A. (see Figure 18) This "image"
9 exposure can be a light source e.g., a laser beam (or other controllable
photon
source), which is capable of creating an electrostatic "image" 325A on the
11 surface of the drum of a desired size and, charge density.. The charge
"image"
12 325A is then rotated to the image development station containing an ionized
13 cloud of drug powder which is attracted to the charge "image" 325 to
14 neutralize charge in the "image", thus, forming a powder "image" 326A
containing a predetermined amount of powder. (see Figures 18 and 19) This
16 powder "image" 326A is rotated to a drug transfer station 330 where it is
17 released into the open ended capsule 329 carried on belt 328. This transfer
to
18 the capsules 329 is accomplished, in one preferred embodiment, by the use
of
19 high voltage plate 356 (see Figure 19) which overcomes the attraction of
the
charged "image" 325A on the surface of the drum, thus releasing the powder
21 "image" 326A into the capsule 329. The pocket containing the predetermined
22 quantity of drug is then passed through the capsule capping step 332.
23 Figure 20 shows another embodiment of the invention wherein the
24 micronized drug particles 310 are carried on tlie surface of discrete
carriers
360 which can be, for example; small plastic beads, for example. When these
26 plastic beads are contacted with an image 325A, the micronized particles
310
27 are transferred to the charge "image" 325A on the surface of the drum 324A
28 from the discrete carriers 360. To accomplish this, the positive charge on
the
28
CA 02463663 2004-04-27
WO 99/64095 PCT/US99I13420
1 image 325A should be higher than the positive charge on the surface of the
2 individual carriers 360.
3 Figures 21 and 22 show additional details of means for both handling.
4 drugs and providing aerosolization and ionization to provide a suspended
stream of fine drug powders having a predetermined size and charge. In
6 Figures 21 and 22, elements 316A, 318A and 320A and 316B, 318B and 320B
7 correspond to the equi-valent elements in Figures 16, 17 and 18.
8 Since repeatability is important for drug metering it is necessary to
9 effectivelv address the issue.of charge-to-mass variation with particle
size.
One method of over-coming this problem is to control the particle size
11 distribution in the drug powder. Figure 22 shows one implementation to
12 achieve this control of particle size. The voltage on the electrostatic
deflector
13 is adjusted to control the particle sizes to be suspended in the holding
14 chamber for delivery to the ionization chamber. Once the desired particle
sizes are suspended they are drawn into the ionization chamber to ensure
16 surface charge saturation on the particles. T1zis will give a known charge
to
17 the mass ratio.
18 Figure 21 shows an alternative means for controlling the size
19 distribution. A high velocity air stream is used to deaggregate the powder.
The deaggregated powder is then contained in holding chamber 318A. The
21 purpose of the holding chamber is to allow .the larger size particles to
settle,
22 thereby producing a .favorable particle size distribution. The particle
size
23 distribution is a function of the holding time as shown in Fig. 23. The
24 suspended particles are then ionized and exposed to the charge image as
shotim at 326 in Figure 17.
26 Fig. 23 shows the percentage of particles sizes suspended in a holding
27 chamber as a function of time. Such a chamber may be provided with a slow
28 upward flowing air current to maintain the aerosol suspension. As can be
29 seen, the percentage of suspended particles is very largely determined by
29
CA 02463663 2004-04-27
'~_~ ._.......'~ ~ e
WO 99/64095 PCT/US99/13420
1 particle size. Through experiment one can select a time slot that will give
the
2 desired particle size distribution for any particle drug dosage.
Additionally,
3 or in place of settling time, one or more filters can be used for obtaining
a
4 given particle size range.
Fig. 24 is similar to Figure 18 except that the lmage Development
6 Station 326A u1 this figure is replaced with a stationan= electrode 326B and
an
7 air passageway 350 for carrying the aerosolized powder. The rotating drum
8 has a dielectric or photoreceptor surface 324 on to which is deposited the
9 latent image. As an example the aerosolization chamber would be similar to
that shown in Figure 21. The metering chamber in Figure 21 is then the air-
11 passageway 323 between the dielectric surface 324 and the stationary
12 electrode 326B. The undeposited powder then exits at the right side of this
13 air-passagewat- to be collected for later use or recirculated back into the
.
14 aerosolization chamber.
Figure 23 above shows an ion projection print head where an ion beam
16 is used to produce a charge "image" on a dielectric surface. The corona
wire
17 352 has a high aoltage applied to it which causes the air to breakdown and
18 produces the ions 352A necessary for the operation of the ion projection
19 printers. The remainder of the ion projection print head includes the usual
control'electrode 354, screen electrode 356 and insulator 358. The relative
21 potential that is applied to the control and screen electrodes then
regulates the
22 amount of ions 325C that will be metered and deposited on to the dielectric
23 surface 324 these ions being deposited on the surface to form the latent
image
24 325A. Both the intensity and size of the ion beam can be adjusted as will
be
apparent to one of ordinary skill in the art. The advantage of tl-iis system
is
26 that it does not require a photosensitive surface and can therefore be
rugged
27 making it suitable for the manufacturing environment.
28 The invention is susceptible to modification. For example, the
29 invention advantageously may be employed to form tablets each containing a
CA 02463663 2004-04-27
WO 99/64095 PCT/US99/13420
1 precise amount of pharmaceutical or drug. Figure 26 is similar to Figure 16.
2 However, in the Figure 26 embodiment, tablet binder material 360 is
3 deposited in wells 362 of belt 364 at a first depositing station 366. The
belt 364
4 carrying the partially filled wells 362 is then passed into powder
discharging
device 330, where the belt is indexed, as before, in coordination with carrier
6 surface 324. The predetermined amount 326A of powder is then discharged
7 from surface 324 into well 362, whereupon the belt is then moved to a well
8 filling station 368 where the wells 362 are filled. Well filling station 368
may
9 include a doctor blade (not shown) or the like, for topping the wells 362.
Thereafter the filled wells pass through a tablet hardening station 370
wherein
11 the tablets are foimed into unitary masses iii known manner.
12 Figure 27 illustrates another alternative embodiment of the invention,
13 in which the surface of the drum 324 bearing the charged "image" 325A is
14 passed through a powder bath or fluidized bed 380 containing the powder
particles. As before, the powder particles will stick only to the charged area
16 on the surface of the drum.
17 Referring to Figure 28, in yet another embodiment of the invention, a
18 transport belt 382 carries a plurality of spaced edible wafers 384 or the
like
19 upon which the predetermined amount of powder 386 may then be
discharged orito the individual wafers.
21 Referring to Figure 29, in yet another embodiment of the invention,
22 transport belt 382 carries tape or sheet 388 formed of an edible substrate
such
23 as starch. The powder particles 390 are deposited uniforrnly on the sheet
388,
24 which is then stripped from the belt 382, and cut into specific sizes to
determine the dose.
26 As can be seen from the foregoing description, the present invention
27 perrnits metering and packaging of dry powder pharmaceuticals and drugs,
28 in a highly precise, reproducible manner. Moreover, the invention readilv
29 may be scaled from laboratory, i.e desk top size, to pilot plant to full
scale
31
CA 02463663 2004-04-27
. , ,
WO 99/64095 PC'P/IJS99/13420
1 production capacity by simply changing size and/or handling speed. Since all
2 units operate according to identical processes, the drug used for clinical
trials
3 would have the same manufacturing process as in full scale production.
4 Thus, production certification may be simplified.
Another advantage of the present invention is that the system may be
6 employed to meter and deposit different drugs and/or different dosages by
7 simply changing the "image". Alternatively, dosages may be changed, e.g.
8 larger doses made, by advancing the belt in a step-wise manner so that two
or
9 more printed "dots" or a printed line may be deposited at one site on the
belt.
The belt may then be advanced, and the process continued. Still other
11 modifications are possible. For example, the invention advantageously may
12 be used for "printing" diagnostic reagents or the like on a carrier or
substrate.
13 Still other modifications and variations of the invention described
14 herein may be made and are intended to come within the scope of the
appended ciaims.
32