Note: Descriptions are shown in the official language in which they were submitted.
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
ELECTROPOLISHING ASSEMBLY AND METHODS FOR
ELECTROPOLISHING CONDUCTIVE LAYERS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority of earlier filed provisional
applications U.S. Application No. 60/332,417, entitled ELECTROPOLISHING
ASSEMBLY, filed on November 13, 2001, and U.S. Application No. 60/372,567,
entitled
METHOD AND APPARATUS FOR ELECTROPOLISHING METAL FILM ON
SUBSTRATE, filed on April 14, 2002, the entire contents of which are
incorporated herein
by reference.
BACKGROUND OF THE INVENTION
1. Field:
[0002] This invention relates generally to semiconductor processing apparatus,
and
more particularly to electropolishing apparatus for electropolishing
conductive layers on
semiconductor devices.
2. Description of the Related Art:
[0003] Semiconductor devices are manufactured or fabricated on semiconductor
wafers using a number of different processing steps to create transistor and
interconnection
elements. To electrically connect transistor terminals associated with the
semiconductor
wafer, conductive (e.g., metal) trenches, vias, and the like are formed in
dielectric materials
as part of the semiconductor device. The trenches and vias couple electrical
signals and
power between transistors, internal circuit of the semiconductor devices, and
circuits
external to the semiconductor device.
[0004] In forming the interconnection elements the semiconductor wafer may
undergo, for example, masking, etching, and deposition processes to form the
desired
electronic circuitry of the semiconductor devices. In particular, multiple
masking and
etching steps can be performed to form a pattern of recessed areas in a
dielectric layer on a
semiconductor wafer that serve as trenches and vias for the interconnections.
A deposition
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
process may then be performed to deposit a metal layer over the semiconductor
wafer
thereby depositing metal both in the trenches and vias and also on the non-
recessed areas of
the semiconductor wafer. To isolate the interconnections, such as patterned
trenches and
vias, the metal deposited on the non-recessed areas of the semiconductor wafer
is removed.
[0005] Conventional methods of removing the metal film deposited on the non-
recessed areas of the dielectric layer on the semiconductor wafer include, for
example,
chemical mechanical polishing (CMP). CMP methods are widely used in the
semiconductor industry to polish and planarize the metal layer within the
trenches and vias
with the non-recessed areas of the dielectric layer to form interconnection
lines.
(0006] In a CMP process, a wafer assembly is positioned on a CMP pad located
on
a platen or web. The wafer assembly includes a substrate having one or more
layers and/or
features, such as interconnection elements formed in a dielectric layer. A
force is then
applied to press the wafer assembly against the CMP pad. The CMP pad and the
substrate
assembly are moved against and relative to one another while applying the
force to polish
and planarize the surface of the wafer. A polishing solution, often referred
to as polishing
slurry, is dispensed on the CMP pad to facilitate the polishing. The polishing
slurry
typically contains an abrasive and is chemically reactive to selectively
remove from the
wafer the unwanted material, for example, a metal layer, more rapidly than
other materials,
for example, a dielectric material.
[0007] CMP methods, however, can have several deleterious effects on the
underlying semiconductor structure because of the relatively strong mechanical
forces
involved. For example, as interconnection geometries move to 0.13 microns and
below,
there can exist a large difference between the mechanical properties of the
conductive
materials, for example copper and the low k films used in typical damascene
processes.
For instance, the Young Modulus of a low k dielectric film may be greater than
10 orders
of magnitude lower than that of copper. Consequently, the relatively strong
mechanical
force applied on the dielectric films and copper in a CMP process, among other
things, can
cause stress related defects on the semiconductor structure that include
delamination,
dishing, erosion, film lifting, scratching, or the like.
2
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0008] New processing techniques are therefore desired. For example a metal
layer
may be removed or etched from a wafer using an electropolishing process. In
general, in
an electropolishing process the portion of the wafer to be polished is
immersed within an
electrolyte fluid solution and an electric charge is then applied to the
wafer. These
conditions result in copper being removed or polished from the wafer.
BRIEF SUMMARY OF THE INVENTION
[0009] In one aspect of the present invention, an exemplary apparatus and
method
are provided for electropolishing a conductive film on a wafer. One exemplary
apparatus
includes a wafer chuck for holding a wafer, an actuator for rotating the wafer
chuck, a
nozzle configured to electropolish the wafer, and a shroud positioned around
the edge of
the wafer. One exemplary method of electropolishing a conductive film on a
wafer
includes rotating a wafer chuck with sufficient speed such that electrolyte
fluid incident
upon the wafer flows on the surface of the wafer towards the edge of the
wafer.
[0010] The present invention is better understood upon consideration of the
detailed
description below in conjunction with the accompanying drawings and claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0011] Figs. lA and 1B are cross-sectional and top views respectively of an
exemplary semiconductor processing apparatus including a shroud;
[0012] Figs. 1C, 1D, and lE are cross-sectional views of exemplary nozzles of
a
semiconductor processing apparatus;
[0013] Fig. 2 is a cross-sectional view of exemplary nozzles of a
semiconductor
process apparatus;
[0014] Fig. 3 is a cross-sectional view of exemplary nozzles of a
semiconductor
process apparatus;
3
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0015] Fig. 4 is a cross-sectional view of exemplary nozzles and a block of a
semiconductor process apparatus;
[0016] Figs. SA through SH illustrate cross-sectional views of various
exemplary
nozzle shapes and configurations;
[0017] Figs. 6A and 6B illustrate cross-sectional and top views of an
exemplary
nozzle structure;
[0018] Figs. 6C through 6I illustrate top views of various exemplary nozzles
structures;
[0019] Fig. 7 illustrates a cross-sectional view of an exemplary semiconductor
processing apparatus including a conductive member;
[0020] Figs. 8A and 8B illustrate cross-sectional views of an exemplary
semiconductor processing apparatus including a conductive member;
[0021] Fig. 8C illustrates an exploded view of an exemplary wafer chuck
assembly
including a conductive member;
[0022] Figs. 9A and 9B illustrate cross-sectional views of exemplary
semiconductor
processing apparatus including a conductive member;
[0023] Figs. l0A and l OB illustrate cross-sectional views of exemplary
semiconductor processing apparatus including one and two optical sensors
respectively;
[0024] Figs. 11A and 11B illustrate a top view and cross-sectional view of an
exemplary semiconductor processing apparatus;
[0025] Fig. 12 illustrates a cross-sectional view of an exemplary
semiconductor
processing apparatus;
4
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0026] Figs. 13A-13E illustrate an exemplary electropolishing assembly with a
multiple rotary assembly;
[0027] Figs. 14A and 14B illustrate cross-sectional views of exemplary
multiple
rotary nozzle assemblies;
[0028] Fig. 14C illustrates an exemplary process for electropolishing a
conductive
layer on a wafer;
[0029] Fig. 15 illustrates a cross-sectional view of an electropolishing
chamber with
an exemplary multiple linearly movable nozzle assembly; and
[0030] Figs. 16A-16E illustrate exemplary views of an electropolishing
apparatus
with a linearly movable and multiple rotary nozzles.
DETAILED DESCRIPTION
(0031] In order to provide a more thorough understanding of the present
invention,
the following description sets forth numerous specific details, such as
specific materials,
parameters, and the like. It should be recognized, however, that the
description is not
intended as a limitation on the scope of the present invention, but is instead
provided to
enable a better description of the exemplary embodiments.
[0032] I. Exemplary Electropolishing Apparatus:
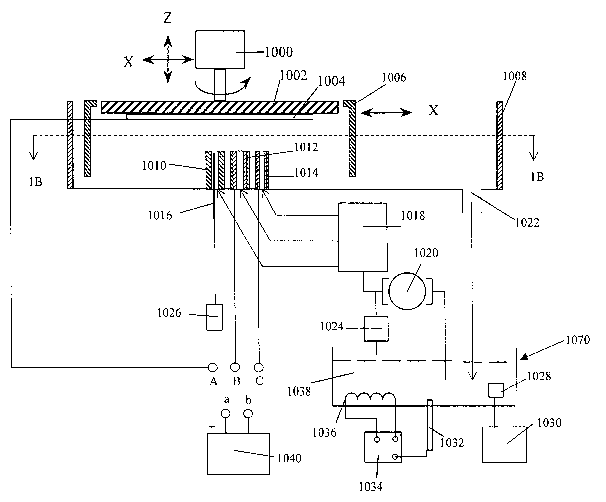
[0033] Figs. lA and 1B illustrate a cross-sectional and top view of an
exemplary
wafer electropolishing apparatus that may be used to polish wafer 1004.
Broadly speaking,
the exemplary electropolishing apparatus operates by directing a stream of
electrolyte fluid
towards a metal film on a wafer while an electric charge is applied to the
wafer. The
electric charge and electrolyte fluid cause metal ions in the metal film to
dissolve in the
electrolyte fluid. The current density of the electrolyte fluid and
concentration of metal
ions in the electrolyte fluid determine, at least in part, the rate of
polishing. Thus, by
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
controlling the current density, the electrolyte solution concentrations, and
the like, the
electropolishing apparatus may precisely polish metal layers disposed on
semiconductor
wafers.
[0034] As shown in Fig. lA, the electropolishing apparatus may include chuck
1002, actuator 1000, and polishing receptacle 1008. Polishing receptacle 1008
can be
formed from any material electrically insulated and resistant to acid and
corrosion, such as
polytetrafluoroethylene (commercially known as TEFLON), Polyvinyl Chloride
(PVC),
PolyVinylindene Fluoride (PVDF), Polypropylene, and the like. Preferably,
polishing
receptacle 1008 can be formed from PVDF. It should be recognized, however,
that
polishing receptacle 1008 can be formed of different materials depending on
the
application.
[0035] As shown in Fig. lA, electrolyte fluid 1038 can flow into polishing
receptacle 1008 through nozzles 1010, 1012, and/or 1014. More particularly,
pump 1020
pumps electrolyte fluid 1038 from electrolyte fluid reservoir 1070 past return
valve 1024 to
pass filter 1018. Pass filter may include Liquid Mass Flow Controllers (LMFCs)
that can
control the amount and rate of electrolyte fluid 1038 delivered to nozzles
1010, 1012, and
1014. Additionally, pass filter 1018 can filter contaminants from electrolyte
fluid 1038 in
order to reduce the amount of contaminants that could possibly enter polishing
receptacle
1008 through nozzles 1010, 1012, or 1014 and possibly degrade the
electropolishing
process or clog the LMFCs if used. In this manner, contaminants are prevented
from
entering polishing receptacle 1008 and/or from clogging the LMFCs. In the
present
example, pass filter 1018 suitably removes particles larger than about 0.05 to
about 0.1
micrometers. It should be recognized, however, that various filtering systems
could be
used depending on the particular application. Additionally, although filtering
contaminants
is advantageous, pass filter 1018 can be omitted from:the wafer polishing
assembly in some
applications.
[0036] Electrolyte fluid 1038 can include any convenient electropolishing
fluid,
such as phosphoric acid, and the like. Preferably, electrolyte fluid 1038
includes
orthophosphoric acid (H3P04) having a concentration between about 60 percent
by weight
and about 85 percent by weight, and more preferably about 76 percent by
weight.
6
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
Additionally, electrolyte fluid 1038 preferably includes glycol at about 10 to
40 percent by
weight, with the remainder including water and H3P04 acid having about 1
percent
aluminum metal (against weight of the acid). However, the concentration and
composition
of electrolyte fluid 1038 can vary depending on the particular application
[0037] Pump 1020 can include any suitable hydraulic pump, such as a
centrifugal
pump, a diaphragm pump, a bellow pump, and the like. Additionally, pump 1020
can be
resistant to acid, corrosion, and contamination. Although one pump 1020 is
shown, it
should be recognized that any number of pumps 1020 could be used. For example,
a
separate pump might be used for each nozzle 1010, 1012, and 1014.
Additionally,
electrolyte fluid 1038 can flow into polishing receptacle 1008 through nozzles
1010, 1012,
and 1014 without pump 1020 in some applications. For example, electrolyte
fluid 1038 can
be maintained at a pressure within electrolyte fluid reservoir 1070.
Alternatively, the
supply lines between electrolyte fluid reservoir 1070 and nozzles 1010, 1012,
and 1014 can
be maintained at a pressure.
[0038] LMFCs can include any convenient mass flow controller, which is further
preferably resistant to acid, corrosion, and contamination. Additionally,
LMFCs deliver
electrolyte fluid 1038 at set flow rates to nozzles 1010, 1012, and 1014.
Additionally,
LMFCs may suitably deliver electrolyte fluid 1038 at flow rates proportionate
to the cross-
section area of nozzles 1010, 1012, and 1014. For example, if nozzle 1012 is
larger in
diameter than nozzle 1014, then it might be advantageous for LMFCs to deliver
electrolyte
fluid 1038 at a greater flow rate to nozzle 1012. In the present exemplary
embodiment,
LMFCs are preferably configured to deliver electrolyte fluid 1038 at a flow
rate between
0.5 liters per minute and 40 liters per minute depending on the nozzle sizes,
distance
between the nozzle and the wafer, and the like.
[0039] Fluid reservoir 1070 may further include a heat exchanger 1036,
cooler/heater 1034, and a temperature sensor 1032 for controlling the
temperature of
electrolyte fluid 1038 within fluid reservoir 1070. Further, one or more
electrodes 1028
may be included in reservoir 1070 and coupled to power supply 1030. Applying
electric
charges to electrodes 1028 removes metal ions from the electrolyte fluid 1038
thereby
7
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
adjusting the metal ion concentration of electrolyte fluid 1038. An opposite
charge may be
applied to electrode 1028 to add metal ions to electrolyte fluid 1038.
[0040] The exemplary wafer polisher assembly further includes electrodes
disposed
within nozzles 1012 and 1014. As will be described in greater detail below,
although the
present exemplary embodiment includes two nozzles with two electrodes therein,
any
number of nozzles and electrodes per nozzle, whether fewer or greater than
two, may be
used. In general, increasing the surface area of the electrodes within a
nozzle increases the
current density and the electropolishing rate across the profile of the stream
of electrolyte
fluid 103 8.
[0041] As shown in Figs. 1D and lE, nozzles 1012 and 1014 include electrodes
1056 and 1060, respectively. Electrodes 1056 and 1060 may include any
electrically
conducting material, such as copper, stainless steel, Tatanium (Ta), Titanium
(Ti), TaN,
TiN, lead, platinum, and the like.
[0042] During the electropolishing process, some of the metal ions, which
migrate
out of metal layer on wafer 1004, may accumulate on electrodes 1056 and 1060.
As will be
described in greater detail below, the metal accumulation or plating may be
removed in a
depleting process. For example, when electrodes 1056 and 1060 are charged
positively and
wafer 1002 is charged negatively, wafer 1004 is electroplated rather than
electropolished.
In this and similar manners, the metal plated on electrodes 1056 and 1060 may
be removed,
i.e., depleted. Alternatively, electrodes 1056 and 1060 can be suitably
replaced at any
appropriate time. For example, electrodes 1056 and 1060 could be replaced
after
processing about 100 wafers.
[0043] In some examples, the metal layer may include copper. Accordingly,
during
the electropolishing process, some of the copper ions from the metal layer
being polished
migrate to electroplate electrodes 1056 and 1060. If, however, electrodes 1056
and 1060
include copper, electrodes 1056 and 1060 may dissolve during the depleting
process and
become deformed. Therefore, in some examples, it is desirable that electrodes
1056 and
1060 include materials that are resistant to being dissolved during the
depleting process.
For example, electrodes 1056 and 1060 may include platinum or platinum alloys.
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
Alternatively, electrodes 1056 and 1060 may include titanium suitably coated
with a layer
of platinum, for example, with a thickness of about 50 microns to about 400
microns.
[0044] In the present exemplary apparatus, wafer chuck 1002 suitably holds and
positions wafer 1004 within or above receptacle 1008. More particularly, wafer
1004 is
suitably positioned opposite nozzles 1010, 1012, and 1014 and within shroud
1006. Shroud
1006 may optionally be included around wafer 1004 to prevent splashing and the
like as
will be described in greater detail below.
[0045] After wafer 1004 is suitably positioned within polishing receptacle
1008,
electrodes 1056 and 1060 are electrically charged by power supply 1040.
Additionally,
wafer 1004 is electrically charged by power supply 1040. Alternatively, more
than one
power supply may be used to charge electrodes 1056 and 1060 and wafer 1004.
When
appropriately charged and electrolyte fluid 1038 flows between the electrodes
1056 and
1060 within nozzles 1012 and 1014 and the surface of wafer 1004, an electrical
circuit is
formed. More particularly, electrodes 1056 and 1060 are electrically charged
to have
negative electric potential in comparison to wafer 1004. In response to this
negative
electric potential at electrodes 1056 and 1060, metal ions migrate away from
wafer 1004
into electrolyte fluid 1038, thus electropolishing wafer 1004. When the
polarity of the
circuit is reversed, however, metal ions migrate toward wafer 1004, thus
electroplating
wafer 1004.
[0046] Additionally, as shown in Figs. lA and 1C, nozzle 1010 includes
injection
nozzle 1052 and end-point detector 1016. During the electropolishing process,
injection
nozzle 1052 can be configured to supply electrolyte fluid 1038 and end-point
detector 1016
can be configured to detect the thickness of the metal layer on wafer 1004.
End-point
detector 1016 can include various sensors, such as ultrasonic sensors, optical
reflection
sensors, electromagnetic sensors such as Eddy-current sensors, and the like.
Electrolyte
fluid 1038 supplied by injection nozzle 1052 can act as a medium through which
the end-
point detector 1016 emits signals and measures the metal film thickness. Using
electrolyte
fluid 1038 as a single medium to transmit signals increases the accuracy of
the
measurements taken by end-point detector 1016 because electrolyte fluid 1038
provides a
single phase. In contrast, if injection nozzle 1052 does not provide
electrolyte fluid 1038,
9
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
emissions and measurements from end-point detector may pass through various
other
media, such as air and the like, before passing through electrolyte fluid 1038
that is applied
to wafer 1004 by nozzle 1012 or nozzle 1014. As will be described below,
having updated
or real-time characteristics of electrolyte fluid 1038 that may change over
time may also
increase the accuracy of the end-point measurement. Further, although one
nozzle 1010 is
shown having an end-point detector 1016, any number of nozzles having any
number of
end-point detectors may be used.
[0047] As further shown in Fig. lA, actuator 1000 can rotate chuck 1002 and
wafer
1004 about the z-axis. Furthermore, in some applications, actuator 1000 can
move chuck
1002 and wafer 1004 along the x-direction, while nozzles 1010, 1012, and 1014
remain
stationary. In other applications, nozzles 1010, 1012, and 1014 can move along
the x-
direction, while chuck 1002 and wafer 1004 remain stationary along the x-
direction. In yet
other applications, actuator 1000 can move chuck 1002 and wafer 1004 along the
x-
direction, while nozzles 1010, 1012, and 1014 also move along the x-direction.
[0048] Furthermore, the electroplating apparatus may be oriented in
alternative
fashions. For example, nozzles 1010, 1012, and 1014 may be positioned above
wafer
1004, such that electrolyte fluid is directed downward towards wafer 1004.
Additionally,
wafer 1004 may be oriented vertically with nozzles 1010, 1012, and 1014
directing
electrolyte fluid towards wafer 1004.
[0049] For additional discussions of exemplary wafer electropolishing
apparatus,
see U.S. Patent Serial No. 6,395,152, entitled METHODS AND APPARATUS FOR
ELECTROPOLISHING METAL INTERCONNECTIONS ON SEMICONDUCTOR
DEVICES, filed on July 2, 1999, which is incorporated in its entirety herein
by reference.
Furthermore, for additional discussions of exemplary end-point detectors, see
U.S. Patent
Application No. 6,447,688, entitled METHODS AND APPARATUS FOR END-POINT
DETECTION, filed on May 12, 2000, which is incorporated in its entirety herein
by
reference.
[0050] II. Electrolyte Fluid Splash Protection
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0051] An exemplary electropolishing method includes rotating wafer 1004 while
electrolyte fluid 1038 is directed to the surface of wafer 1004. Wafer 1004 is
rotated at a
rate sufficient to create centrifugal forces that cause incident electrolyte
fluid 1038 to flow
across the surface of wafer 1004 toward the edge of wafer 1004. Preferably,
the electrolyte
fluid 103 8 flows to the edge of the wafer 1004 before falling from the
surface. By directing
the flow across the surface of wafer 1004 the fluid can be prevented from
falling from the
wafer surface and disrupting the stream of electrolyte fluid 1038 or forming a
continuous
column of electrolyte fluid in receptacle 1008. The process, however, may
cause
electrolyte fluid to splash within the receptacle and escape the apparatus or
disrupt the
stream of electrolyte fluid. Therefore, an exemplary electropolishing
apparatus includes a
shroud 1006 positioned around wafer 1004 to diminish or prevent the liquid
that has been
acted upon by the centrifugal forces from splashing within receptacle 1008 or
escaping
from receptacle 1008.
[0052] Figs. 1 A and 1 B illustrate shroud 1006 configured to surround wafer
1004
and chuck 1002. As shown in Fig. lA, nozzle 1012 can supply a stream of
electrolyte fluid
to the surface of wafer 1004. In order to polish a metal film on wafer 1004
more
uniformly, wafer 1004 can be rotated in a manner to cause electrolyte fluid
1038 to flow
across wafer 1004 to the exposed portion of chuck 1002 without allowing the
electrolyte
fluid to fall from the surface of wafer 1004 into polishing receptacle 1008.
Any electrolyte
fluid that falls from wafer 1004 and forms a continuous column of electrolyte
fluid between
wafer 1004 and polishing receptacle 1008 may cause over-polishing of wafer
1004 where
the column is formed. The additional polishing may lead to uneven and
unpredictable
polishing rates of the metal layer.
[0053] Additionally, any electrolyte fluid that falls from wafer 1004 or
splashes
within receptacle 1008 can disturb the stream of electrolyte fluid supplied by
nozzle 1012.
The shape, or more specifically the profile of the stream of electrolyte fluid
1038, in turn
affects the current density and the polishing rate of the electropolishing
apparatus. It is
therefore desirable to have electrolyte fluid 1038 flow along the surface of
wafer 1004
towards the edge of wafer 1004 and away from the stream of electrolyte fluid
1038 directed
at wafer 1004.
11
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0054] Wafer 1004 can be rotated at an appropriate rotational speed, depending
on
the viscosity of the electrolyte fluid used, to cause the electrolyte fluid to
flow across wafer
1004 towards the edge of wafer 1004 or to the exposed portion of chuck 1002.
The
rotational speed should be such that the electrolyte fluid 1038 may flow
across wafer 1004
without falling from the surface of wafer 1004 and forming a continuous column
or
interfere with the stream of electrolyte fluid 1038. In particular, the lower
the viscosity of
the electrolyte fluid, the higher the centrifugal acceleration needed. For
instance, for 85%
phosphoric acid, the centrifugal acceleration can be chosen above about 1.5
meterlsec2. In
one exemplary method, a 300 mm diameter wafer is rotated within a range of
about 100
rotations-per-minute (rpm) to about 2,000 rpm or more, and preferably, in a
range of about
1,500 rpm to about 2,000 rpm.
[0055] Typically, nozzle 1012 or 1014 will scan the entire surface of the
wafer
1004 to more uniformly polish wafer 1004. Wafer 1004 can be rotated to create
a constant
centrifugal acceleration on the incident electrolyte fluid 1038 when nozzle
1012 is scanning
different portions of wafer 1004. For example, the centrifugal acceleration is
directly
proportional to the radial distance from the center of the wafer and the
square of the
rotational speed. Therefore, the speed at which wafer 1004 is rotated may be
decreased
when nozzle 1012 or 1014 is polishing portions of wafer 1004 near the edge of
wafer 1004,
i.e., a large radius, and increased when polishing portions near the center of
wafer 1004,
i.e., a small radius.
[0056] Typically, when electrolyte fluid is supplied to wafer 1004 in the
manner
described above, electrolyte fluid may flow towards the edge of wafer 1004 and
past the
edge of wafer 1004 toward the wall of polishing receptacle 1008. Without
shroud 1006,
the electrolyte fluid 1038 may contact the wall of polishing receptacle 1008
and splash
within receptacle 1008 thereby disrupting the stream of electrolyte fluid 1038
or escape
from polishing receptacle 1008.
[0057] As illustrated in Figs. lA and 1B, shroud 1006 may be placed around
wafer
1004 and chuck 1002 to decrease or prevent the electrolyte fluid 1038 from
splashing
within or escaping from polishing receptacle 1008. Furthermore, shroud 1006
can move
together in the x-direction with chuck 1002 and actuator 1000 during the
polishing process.
12
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
In particular, shroud 1006 can be attached to chuck 1002 and/or actuator 1000
with a
mechanical attachment, joint, and the like. Alternatively, another actuator
that
synchronizes the movement of shroud 1006 with chuck 1002 and actuator 1000 may
drive
shroud 1006 separately. Shroud 1006 may also be rotated in unison or otherwise
with
chuck 1002.
[0058] Shroud 1006 can be formed in any suitable shape, such as a circle,
polygon,
and the like. Preferably shroud 1006 is shaped to decrease the splashing of
the electrolyte
fluid 1038 after it flows from wafer 1004 and contain the electrolyte fluid
1038 within
receptacle 1008. The gap between chuck 1002 and shroud 1006 can be, for
example, in the
range of about 1 mm to about 10 mm, and preferably about 5 mm. Additionally,
as shown
in Fig. lA, the cross section of the side-wall of shroud 1006 can be formed in
the shape of
an L in order to prevent electrolyte fluid from splashing above shroud 1006 or
chuck 1002.
However, the cross-section of shroud 1006 can have various other shapes. For
instance, the
sidewall of shroud 1006, i.e., the vertical portion of the L-shape, can be
formed in other
shapes such as a C-shape and the like. Further, shroud 1006 may be tapered in
or out to
diminish splashing and the like. Shroud 1006 may also extend farther above or
below
wafer 1004 and chuck 1002 than is shown in Fig. lA.
[0059] Shroud 1006 can be made from plastics, ceramics, and the like, or
anticorrosive metals or alloys such as Tantalum, Titanium, stainless steel in
the 300 series,
and the like. Additionally, shroud 1006 can be coated with electrolyte fluid-
resistant
materials such as Teflon, and the like.
(0060] It should be recognized, however, that the method of electropolishing
described does not require that electrolyte fluid 1038 flow past the edge of
the wafer and be
incident upon shroud 1006. The problems of electrolyte fluid 1038 forming a
continuous
column with the receptacle 1008 and splashing within or out of receptacle 1008
may be
decreased or prevented without electrolyte fluid flowing completely across
wafer 1004.
For example, merely by rotating wafer 1004 such that electrolyte fluid flows
along a
portion of the surface of wafer 1004 toward the edge of wafer 1004 before
falling from
wafer 1004 may diminish or prevent the undesirable effects.
[0061] III. Reduction of Edge Over-Polishing
13
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0062] In another aspect, an electropolishing method and apparatus for
reducing
over-polishing at or near the edges of a wafer is described. Typically, a
portion of the
metal layer at or near the edge of a wafer is polished faster than the
portions of the metal
layer on other areas of the wafer. An electrode connected to the edge of a
wafer may
increase the current density within electrolyte fluid near the edge region of
the wafer
resulting in an increased polishing rate. In general, the higher current
density and polishing
rate near the edges of the wafer may be reduced by absorbing a portion of the
current
density through the electrolyte fluid with a conductive member such as a ring
or the like
placed at or near the edge of the wafer. The current density near the edges
may also be
adjusted by charging the conductive member to vary the amount of current that
is absorbed
thereby controlling the current density to a greater degree.
[0063] With reference, to Fig. 7, an exemplary apparatus and method are shown
for
reducing edge over polishing. A stream of electrolyte fluid 7080 is applied to
wafer 7004
from nozzle 7054. Wafer 7004 is rotated at a sufficient rotational speed to
form a thin layer
of electrolyte fluid 7081 that can polish a metal layer on wafer 7004.
Typically, if an
electrode is connected to the edge of wafer 7004 the metal layer at or near
the edge of
wafer 7004 is polished by the thin layer of electrolyte fluid 7081 faster than
the metal on
other areas of wafer 7004. Accordingly, the metal layer at or near the edge of
wafer 7004
can become over polished.
[0064] Chuck 7002 includes a conductive member 7114 that can reduce the amount
of over-polishing at or near the edges of wafer 7004. For example, both wafer
7004 and
conductive member 7114 can be connected to power supply 7110 and charged such
that a
portion of the polishing current in the thin layer of electrolyte fluid 7081
is absorbed by
conductive member 7114. By absorbing a portion of the polishing current,
conductive
member 7114 can reduce the polishing rate of the metal layer at or near the
edges of wafer
7004 and reduce or prevent the over-polishing.
[0065] Conductive member 7114 may include a single ring positioned near or at
the
edge of wafer 7004. Alternatively conductive member may include two or more
sections
that are arranged at or near the edge of wafer 7004. Conductive member 7114
can include
14
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
metals or alloys such as Tantalum, Titanium, stainless steel, and the like, as
well as other
conductive materials suitable for contact with electrolyte fluid 7081.
[0066] Further, wafer 7004 may be positioned between wafer chuck 7002 and
conductive member 7114 as shown in Fig. 7. For example, a robot arm or the
like, may
position wafer 7004 adjacent wafer chuck 7002 or between wafer chuck 7002 and
conductive member 7114. Wafer chuck 7002 and conductive member 7114 may then
be
brought together or closed to hold wafer 7004 therebetween. The exemplary
assembly may
therefore include additional elements, such as holders or positioners to align
and hold wafer
chuck 7002 and conductive member 7114 together as well as insulative members
between
conductive member 7114 and contacts made to charge wafer 7004.
[0067] It should be understood that the exemplary apparatus depicted in Fig. 7
may
also include other features such as those shown in Fig. lA, but have been
omitted to
illustrate the specific example. For instance, shroud 1006 (Fig. lA, 1B) may
be used with
the exemplary apparatus as well as various pumps, nozzles, filters, and the
like.
[0068] Fig. 8A illustrates another exemplary electropolishing apparatus useful
for
reducing the polishing rate near the edges of a wafer. A chuck 8002 with a
conductive
member 8114 is illustrated that may reduce the amount of over-polishing at or
near the
edges of wafer 8004. Fig. 8A is similar to Fig. 7 except that conductive
member 8114 is
separated from wafer 8004 by a spacer element 8118. Spacer element 8118
includes, for
example, an o-ring. Spacer element 8118 may further be formed of a material
that is
electrically insulative and further resistant to acid and corrosion, such as
ceramic,
polytetrafluoroethylene (commercially known as TEFLON), Polyvinyl Chloride
(PVC),
PolyVinylindene Fluoride (PVDF), Polypropylene, silicon rubber, Viton rubber,
and the
like. Conductive member 8114 is coupled to power supply 8112, and a second
conductive
member or electrode, such as spring member 8119 is coupled to power supply
8110. As
shown, current flowing through conductive member 8114 can be adjusted or
controlled by
power supply 8112 in order to control the polishing rate of the metal layer at
or near the
edge of wafer 8004. Generally, as the amount of current absorbed by bottom
chuck 8114
increases, the polishing rate of the metal layer at or near the edge of wafer
8004 decreases.
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0069] Power supply 8112 can be a DC power supply, an AC power supply
synchronized with main polishing power supply 8110, and the like. An AC power
supply
can also include a forward pulse power supply, and a forward and backward
power supply.
Furthermore, power supply 8112 can be operated at a constant current mode, a
constant
voltage mode, or a combination of constant current and constant voltage mode,
wherein a
constant current mode is applied during a portion of the polishing time and a
constant
voltage mode is applied during the other portion of the polishing time. One
could also
replace the power supply 8112 with a variable resistor thereby applying a
varying charge to
conductive member 8114 (See Fig. 9A, for example). Further, a variable
resistor may be
included between both conductive member 8114 and spring member 8119.
[0070] Conductive member 8114 may similarly include metals or alloys such as
Tantalum, Titanium, stainless steel, and the like as well as other conductive
materials.
Further, conductive member 8114 may include one or more sections positioned
near or at
the edge of wafer 8004.
[0071] Therefore, in this exemplary electropolishing apparatus, the electric
charge
applied to the wafer 8004 through spring member 8119 and conductive member
8114 can
be independently controlled by power supplies 8110 and 8112 respectively. This
allows for
greater control of the current density near the edge region of wafer 8004 to
control and
reduce over-polishing of the edge region.
[0072] Fig. 8B illustrates an enlarged view of the configuration and
connections
made with conductive member 8114 and wafer 8004 of Fig. 8A. In particular,
conductive
member 8114 is charged by power supply 8112 and is spaced from wafer 8002 by
spacer
element 8118. Wafer 8004 is charged separately by power supply 8110 that is
coupled to
spring member 8119 positioned around the edge of wafer 8004. Spring member
8119
provides a charge to wafer 8004 that is more uniformly distributed around
wafer 8004 than,
for example, several electrodes positioned around the edge of wafer 8004. An
insulative
member 8121 may be positioned between conductive member 8114 and spring member
8119 when separate charges are applied to conductive member 8114 and spring
member
8119. Spring member 8119 may be formed as a coil spring formed in a ring (see,
e.g., Fig.
8C), however, other cross-sectional profiles, such as an elliptical cross-
sectional profile are
16
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
possible. Further, any number of coil springs may be used depending on the
application.
Spring member can be formed from any convenient electrically conducting
material, such
as stainless steel, spring steel, titanium, and the like. Spring member 8119
may also be
formed of corrosion-resistant material or coated with a corrosion resistant
material such as
platinum, TiN, TaN, and the like.
[0073] The number of contact points formed between wafer 8004 and the power
supply can be varied by varying the number of coils in spring member 8119. In
this
manner, the electric charge applied to wafer 8004 may be more evenly
distributed around
the outer perimeter of wafer 8004. For example, for a 200-millimeter wafer, an
electric
charge having about 1 to about 10 amperes is typically applied. Configuring
spring
member 8119 to create approximately 1,000 contact points with wafer 8004
reduced the
electric charge to about 1 to about 10 mini-amperes per contact point.
[0074] It should be recognized, however, that the wafer 8004 may also be
charged
by one or more electrical contacts. Further, any means to distribute
electrical charge
around wafer 8004 may advantageously be used.
[0075] When conductive member 8114 is separated from wafer 8004 by spacer
element 8118 shorting may result if spring member 8119 is exposed to the
electrolyte fluid.
The shorting of spring member 8119 may reduce the uniformity of the polishing
rate near
the edge portions of wafer 8004. Therefore, in one example, spacer element
8118 serves as
a seal to isolate spring member 8119 from the electrolyte fluid. Spacer
element 8118 may
be formed of anti-corrosive material, such as Viton (fluorocarbon) rubber,
silicone rubber,
and the like. Further, spacer element 8118 may have various shapes and
configuration
depending on the particular application.
[0076] Fig. 8C illustrates an exploded view of an exemplary wafer chuck holder
for
use with the exemplary electropolishing apparatus useful for reducing the
polishing rate
near the edges of a wafer. The exemplary wafer chuck includes a body with a
base section
8002 in an upper portion of the body and a conductive member 8114, where wafer
8004 is
held between base section 8002 and conductive member 8114 of the body. The
wafer
chuck may fiuther include a top holder (not shown) to clamp or otherwise hold
wafer 8004
and the assembly together. In addition to the first conductive member 8114,
the wafer
17
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
chuck includes a second conductive member, such as spring member 8119, to
apply a
charge to wafer 8004. In some examples, the wafer chuck may further include
insulator
member 8121 and spacer member 8118 disposed between base section 8002 and
conductive member 8114 included in a lower portion of the body. It should be
recognized,
however, that in some examples spring member 8119 and spacer member 8118 may
be
omitted, for example, as illustrated in Fig. 7. In an instance where spring
member 8119 is
omitted, an electrode or the like may be included as a second conductive
member to apply a
charge to wafer 8004.
[0077] In the present example, spring member 8119 is disposed between wafer
8004 and spacer member 8118. When pressure is applied to hold conductive
member 8114
and base section 8002 together, spring member 8119 conforms to maintain
electrical
contact to wafer 8004 (see Fig. 8B). Further, spacer member 8118 conforms
between
conductive member 8114 and wafer 8004 to form a seal that protects spring
member 8119
from electrolyte fluid and provides electrical insulation between spring
member 8119 and
conductive member 8118 if desired.
[0078] Semiconductor wafers are typically substantially circular in shape.
Accordingly, the various components of the wafer chuck are depicted as having
substantially circular shapes. It should be recognized, however, that the
various
components of the wafer chuck may include various shapes depending on the
particular
application and/or shape of the wafer. For example, a semiconductor wafer may
have a
truncated shape for which the components of the wafer chuck conform with.
[0079] Other exemplary configuration of a wafer chuck assembly for holding and
applying a charge to a wafer suitable in the apparatus and methods described
can be found
in U.S. Patent Serial No. 6,248,222 entitled METHODS AND APPARATUS FOR
HOLDING AND POSITIONING SEMICONDUCTOR WORKPIECES DURING
ELECTROPOLISH1NG AND/OR ELECTROPLATING OF THE WORKPIECES, issued
on June 19, 2001, and incorporated herein by reference in its entirety.
[0080] Fig. 9A illustrates another exemplary electropolishing apparatus useful
for
reducing the polishing rate near the edges of a wafer. In particular, wafer
chuck 9002
includes conductive member 9114 that may reduce the amount of over-polishing
at or near
18
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
the edges of wafer 9004 as described above. Fig. 9A is similar to Fig. 8A,
except that
conductive member 9114 includes an insulating ring 9115 and a conducting ring
9116
formed in insulating ring 9115. Insulating ring 9115 can include non-corrosive
insulting
materials such as plastics, ceramics, and the like. Conducting ring 9116 can
include metals
or alloys such as Platinum, Tantalum, Titanium, stainless steel, and the like.
Conducting
ring 9116 may be connected to power supply 9110 through variable resistor 9112
or the
like. Additionally, spacer element 9118, for example, an o-ring or the like,
can be placed
between conductive member 9114 and wafer 9004 to prevent electrolyte fluid
from
contacting the portion of wafer 9004 that is connected through one or more
electrodes to
power supply 9110. Further, a spring member or the like (not shown) may also
be included
to more uniformly distribute the electrical charge to the wafer 9004.
[0081] The exemplary apparatus of Fig. 9A allows for a smaller amount of
conductive material to be used with conductive member 9114. This allows the
apparatus to
be cheaper, lighter, and consume less power during operation. Further, the
smaller surface
area of conductive member 9114, compared to conductive member 8114 (Figs. 8A,
8B)
may allow for greater control of the current density in the edge region of
wafer 8004.
Further, the configuration of Fig. 9A (and Fig. 7) may be advantageously used
with those
of Figs. 7 and 8A through 8C.
[0082] Fig. 9B illustrates an enlarged view of another example of an
electropolishing apparatus. This example is similar to Fig. 9A, except that
the conductive
member 9114 includes an insulative member 9121 formed on the lower portion of
conductive member 9114, i.e., the side opposite of wafer 9004. Further, the
configuration
of the wafer assembly is such that metal layer 9005 on wafer 9004 is charged
near the edge
through a conductive spacer element 9118.
(0083] Thus, as seen in Fig. 9B, as electrolyte fluid 9080 is directed near
the edge
of wafer 9004 a portion of the current h flows to the metal layer 9005 and a
second portion
of the current I2 flows to conductive member 9114. Insulative member 9121
formed on the
lower portion of conductive member 9114 serves to reduce current I2 and
increase current
h flowing to the metal layer 9005. The relative thickness of insulative member
9121 and
conductive member 9114 may therefore be adjusted to adjust currents h and Ia
accordingly.
19
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0084] IV. Method for Electropolishing a Fragmented Metal Layer on a Wafer
[0085] A metal layer formed over a wafer may become fragmented during an
electropolishing process. For example, there may become one or more
discontinuous
regions of metal on the surface of the wafer. When this occurs some fragments
of the metal
layer may become isolated from the edges of the wafer where the electrodes are
located. In
such instances, traditional electropolishing methods are unable to efficiently
polish these
fragmented sections because the electrodes do not charge the fragmented metal
layers. In
one exemplary method, by rotating a wafer with a conductive member disposed
around the
fragmented portions of the metal layer at sufficient rotational speeds, a thin
layer of
electrolyte fluid may be formed over the fragmented portions and in contact
with the
conductive member. The thin layer of electrolyte fluid and conductive ring
allow the
fragmented portions to be electropolished.
[0086] As shown in Figs. 11A and 11B, metal layer 11150 has become fragmented,
for example, during a polishing process. The fragments of metal layer 11150
are not
connected to or located at the edges of wafer 11004 where an electrode (not
shown) is
connected to power supply 11110. Because the fragments of metal layer 11150
are not
located at the edge of wafer 11004 or connected to these edges by metal,
electric current
cannot be conducted through the fragments to an electrode at the edge of wafer
11004.
Thus, traditional polishing methods, such as submersing the wafer in a
polishing bath, and
the like are generally ineffective in polishing these fragments.
[0087] Fragments of metal layer 11150 of the metal layer, for example, may
include
the exposed portions of a barrier layer that remain on the non-trenched
portions of a
semiconductor device after a copper layer is polished away. Further, fragments
of metal
layer 11150 may be a result of uneven polishing or over-polishing in edge
regions, for
example.
[0088] With reference to Fig. 11B an exemplary electropolishing apparatus is
illustrated for electropolishing fragments of metal layer 11150 on wafer
11004. The system
includes chuck 11002, actuator 11000, stationary nozzle 11054, and power
supply 11110.
As stationary nozzle 11054 applies a stream of electrolyte fluid 11080 to
wafer 11004,
actuator 11000 can rotate chuck 11002 such that electrolyte fluid 11080 flows
across the
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
surface of wafer 11004, as described above, and forms a thin layer 11081
extending over
the fragmented portions of metal layer 11150. For example, wafer chuck 11002
can be
rotated at a rate in the range of about 100 rpm to about 2000 rpm, and
preferably about
1500 rpm for a 300 mm diameter wafer. Thin layer 11081 provides a path across
the
fragments of metal layer 11150 to conduct current between the stream of
electrolyte fluid
11080 and the conductive member 11114 of chuck 11002. This current allows the
apparatus to electrically polish isolated fragments of metal layer 11150 on
wafer 11004.
[0089] Additionally, the exemplary apparatus depicted in Figure 11B may be a
part
of a larger electropolishing assembly such as the one depicted in Fig. lA. For
example, a
shroud 1006 (Fig. 1) may be included to prevent splashing, uneven polishing,
or disruption
of the polishing stream of electrolyte fluid 1038. Further, various exemplary
embodiments
of conductive member 11114 described in regard to the reduction of edge
polishing may be
used with the apparatus of Fig. 11B.
[0090] Fig. 12 shows another system that can be used to electropolish
fragments of
metal layer on wafer 12004. Fig. 12 is similar to Fig. 11, except that
actuators 12180 and
12182 can move nozzle 12054 along the x-direction while actuator 12000 rotates
chuck
12002 in a stationary location.
[0091] Although Figs. 11B and 12 show systems in which either the chuck or the
nozzle moves along the x-direction, it should be recognized that both the
chuck and the
nozzle can be moved in varying directions depending on the particular
application.
[0092] V. Metal Concentration Measurement and End Detection Control
[0093] One factor in achieving more consistent and acceptable polishing
quality of
wafers in a mass production environment is controlling the concentration of
metal in the
supply of electrolyte fluid used to polish the wafers. When the concentration
of metal in
the supply of electrolyte fluid reaches a certain value, the electrolyte fluid
can become very
active even when no current is applied. This may cause, for example, chemical
etching or
corrosion of the wafers during the post-electropolishing process. Therefore,
it is desirable
to monitor the concentration of metal in the electrolyte fluid during a
process run and make
adjustments on a real-time basis as desired.
21
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0094] Further, end-point detection sensors typically use optical detectors
that
measure through the electrolyte fluid. The measurements therefore depend, at
least in part,
on the optical characteristics of the electrolyte fluid. The optical
characteristics of the
electrolyte fluid, however, may change over time depending on the
concentration of the
metal dissolved in the electrolyte fluid as well as on other factors, such as
contaminant
particles, hydrogen gas bubble formation within the electrolyte fluid, and the
like. Thus, as
the optical characteristics of the electrolyte fluid changes during a process
run, the
measurements from the end-point detector can be adjusted accordingly to
increase the
accuracy of the end-point detection measurements.
(0095] Fig. l0A depicts an exemplary system that may be used to measure the
concentration of metal in a supply of electrolyte fluid 10038, such as
electrolyte fluid
reservoir 1070 (Fig. lA), and the like. The exemplary system includes fiber
probe 10102,
fiber optical sensor 10104, and reflector 10100. Fiber probe 10102 and
reflector 10100 can
be immersed in electrolyte fluid 10038, and fiber probe 10102 can be
positioned with
respect to reflector 10100 in a manner that allows light emitted from fiber
probe 10102 to
be reflected by reflector 1 O 100 back to fiber probe 10102 with a maximum
light intensity.
For instance, fiber probe 10102 can be positioned to emit light in a direction
perpendicular
to the surface of reflector 10102, as shown in Fig. 10A.
[0096] Additionally, the distance H between reflector 10100 and fiber probe
10102
may effect the accuracy of the measurement of the concentration of metal in
the electrolyte
fluid. Accordingly, distance H can be chosen such that the intensity of light
received by
optical sensor 10104 reaches a maximum value when the metal concentration
reaches a
minimum concentration in the supply of electrolyte fluid 10038. It should be
recognized
that other paths between a optical sensor 10104 and reflector 10100 may be
chosen,
including a path with multiple paths and multiple reflections depending on the
application
and desired path length. The fiber probe 10102 may also be placed exterior to
the fluid
reservoir with a path traversing a portion of the electrolyte fluid 1038.
Further, an optical
sensor positioned to detect a light intensity that is received by optical
sensor 10104 may
replace reflector 10100.
22
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[0097] Generally, the color of the electrolyte fluid depends on the type and
concentration of metal ions that are dissolved in the electrolyte fluid. For
example, copper
ions in Phosphoric acid (H3P04) have a blue color. Additionally, the intensity
of light
passing through the electrolyte fluid can decay depending on the color of the
electrolyte
fluid. In general, as the concentration of metal ions in the electrolyte fluid
increases, the
decay of light intensity increases.
[0098] For the system depicted in Fig. 10A, the relationship between the
concentration of metal in the electrolyte fluid and the decay of light
intensity can be
tabulated for a particular metal and electrolyte fluid used with the system as
follows:
Metal Concentration (wt %) Light Intensity Decay
0 Y1
0.2 Y2
0.4 Y3
0.6 Y4
0.8 YS
1.0 Y6
[0099] This tabulated information can be stored in computer 10105. TJsing the
tabulated information, the computer can automatically calculate the
concentration of metal
in the electrolyte fluid based on the intensity of light detected by optical
sensor 10104, by
using interpolation, rounding, or other approximation methods. Although
certain values are
listed in the above table for Metal Concentration (wt %), any values can be
used, and any
number of values can be used.
[00100] The color of light emitted by fiber probe 10102 can be chosen to
increase
the sensitivity of the measurements detected by optical sensor 10104. In
particular, the
color of light emitted by fiber probe 10102 can be different from the color of
the metal ions
in the electrolyte fluid supply in order to increase the sensitivity to the
particular metal ions.
For example, for copper ions in a supply of phosphoric acid, emitting red
light provides a
higher sensitivity to copper ion concentration than emitting green light, and
emitting green
23
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
light provides a higher sensitivity than emitting blue light. However, for any
color of metal
ions in an electrolyte fluid, white light may be emitted.
[00101] Fig. l0A also depicts another aspect of an exemplary system described
above that can be used to remove metal ions from the supply of electrolyte
fluid 10038.
The system further includes two electrodes 10028 and 10029, and power supply
10030.
When optical sensor 10104 measures that the metal ion concentration in the
supply of
electrolyte fluid 10038 has reached a first pre-set value, computer 10105 can
instruct power
supply 10030 to apply voltage to electrodes 10028 and 10029 in order to remove
metal ions
from the electrolyte fluid supply. When voltage is applied to electrodes 10028
and 10029,
metal ions from the supply of electrolyte fluid 10038 begin plating onto
electrode 10029.
When optical sensor 10104 detects that the metal ion concentration has fallen
below a
second pre-set value, computer 10105 can instruct power supply 10030 to stop
applying
voltage to electrodes 10028 and 10029 in order to stop the removal of metal
ions from the
supply of electrolyte fluid 10038. In this manner, the concentration of metal
ions in the
supply of electrolyte fluid 10038 can be maintained between the first and
second pre-set
values, for example, during an electropolishing process.
[00102] The concentration values of the metal ions in electrolyte fluid 10038
may
also be used to aid end-point detector 1016 (Fig. lA, 1B). End-point detector
1016 may be
used to determine the thickness of the metal layer on wafer 1004. The
information may be
used by the electropolishing apparatus to determine when to continue or
discontinue an
electropolishing process on a particular area of wafer 1004. It may also be
used to
determine a suitable polishing rate. End-point detector 1016 may include
various sensors,
such as ultrasonic sensors, optical sensors, electromagnetic sensors, and the
like. Using the
electrolyte fluid 1038 as a medium to transmit signals and take measurements
increases the
accuracy of the measurement because medium interfaces, for example, air to
electrolyte
fluid 1038, do not have to be considered. If properties of the electrolyte
fluid 1038 that
may affect the sensors change, however, the measurements may not be accurate
over time.
Therefore, the end-point detector measurements may be improved by taking into
consideration the changing properties of electrolyte fluid 1038.
24
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00103] Fig. l OB shows another exemplary system for monitoring the optical
characteristics of the electrolyte fluid, which may be used, for example, to
adjust an end-
point detector measurement. Fig. 10B is similar to Fig. l0A except that a
second optical
sensor 10204 and optical fiber 10202 are included. Optical sensor 10104,
optical fiber
10102 and reflector 10100 operate in a similar manner as described in
reference to Fig.
10A. The second optical sensor 10204 and optical fiber 10202 also operate
similar to
optical sensor 10104 and optical fiber 10102; however, optical sensor 10204
and optical
fiber 10202 measure other optical characteristics of the electrolyte fluid.
For example,
during an electropolishing process, hydrogen bubbles often form on the
electrodes. The
bubbles may adversely affect the end-point detector by diffracting and
decreasing the
intensity of the measurement beams in the electrolyte fluid. The decrease in
intensity may
affect the measurements of metal ion concentration, but, by using multiple
detectors
sensitive to different characteristics the metal ion concentration can be
accurately
determined.
[00104] In the example of determining the optical characteristics of the
electrolyte
fluid due to bubbles, the color of light emitted by fiber probe 10202 can
again be chosen to
increase the sensitivity of the measurements detected by optical sensor 10204.
In this
instance, the color of light emitted by fiber probe 10202 can be chosen as the
same color of
the metal ions in the electrolyte fluid supply in order to increase
sensitivity to the bubbles
and decrease the sensitivity to the metal ions. For example, for copper in a
supply of
phosphoric acid, emitting blue light provides a higher sensitivity to bubbles
and lower
sensitivity to copper ions than emitting white light, and emitting white light
provides a
higher sensitivity to bubbles and a lower sensitivity to copper ions than
emitting red light.
[00105] Additionally, the intensity of red light from fiber probe 10102 will
also be
reduced due to any bubbles in electrolyte fluid such that the measurement of
copper ion
concentration will be inaccurate. The second optical sensor 10204, however,
will indicate
the portion of the decrease in intensity due primarily to the bubbles and not
the copper ion
concentration because the sensitivity of fiber probe 10202 was chosen to be
insensitive to
the copper ion concentration. The decrease in intensity of the red light may
therefore be
determined by considering the portion due to bubbles determined by the second
optical
sensor 10204. Further, the end-point detector 1010 (Fig. lA) will be able to
retrieve the
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
optical characteristics of the electrolyte fluid from computer 10105 and make
accurate
measurements of the metal thickness on wafer 1004 (Fig. lA). Thus, second
optical sensor
10204 may increase the accuracy of both the end-point detector measurements
and the
metal ion concentration measurements.
[00106] It should be recognized that any number of sensors may be used to
measure
various properties of the electrolyte fluid. The various properties, for
example, the optical
properties and the like, may then be stored and used to adjust or determine
end-point
detector measurements and the like.
[00107] VI. Nozzle Configurations
[00108] According to another aspect, an exemplary method and apparatus for
electropolishing a metal film on a wafer includes using multiple sized nozzles
with
different polishing rates. In general, a large nozzle allows for higher
polishing rates of a
metal film, for example, copper, formed on a wafer and a small nozzle produces
lower
polishing rates. A large nozzle can therefore be used as a rough polish of the
metal layer
followed by using a small nozzle to more precisely control the
electropolishing process.
Multiple nozzles are therefore advantageous for more precisely polishing
different regions
of a wafer. However, because of the limited space within a clean room, for
example, an
apparatus with multiple nozzles is desirably compact. An exemplary apparatus
with a
number of nozzles configured on a rotating nozzle holder therefore allows for
the use of
multiple nozzles in a compact space.
[00109] Figs. 13A, 13B, 13C, 13D, and 13E illustrate an exemplary
electropolishing
assembly including a multiple rotary nozzle assembly. Figs. 13A through 13E
are similar
to Figs. lA through lE, except for the addition of rotary nozzle 1012 with
multiple nozzles
positioned adj acent optical end-point detector 1016, and Eddy-current
thickness/end-point
detector 1009. As indicated by the arrow in Fig. lA, rotary nozzle 2012 may
rotate and
position different sized and/or shaped nozzles 1014 to direct a stream of
electrolyte fluid
1038 to the wafer 1004. Thus, pump 1018 only directs electrolyte fluid 1038 to
nozzle
1010 of the end detector 1016 and a single nozzle 1014, whereas in Fig. 1 A
electrolyte
fluid 1038 is directed to each individual nozzle used therein.
26
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00110] End-point detector 1009 may operate to measure the thickness of a
metal
film formed on wafer 1004. Detector 1009 may measure the thickness of the
metal film
prior, during, and after an electropolishing process. In one exemplary method,
end-point
detector 1009 is used to determine the thickness of the metal film over the
entire wafer
1004 prior to electropolishing, using for example, an eddy current end-point
detector. The
metal film thickness may then be used to control the local polishing rate for
various
positions on wafer 1004 by controlling the current density and/or stream
profile. The
distance between the end-point detector 1009 and wafer 1004 is, for example,
in the range
of about 5 to about 1000 microns. The film thickness over the entire wafer may
be
determined by rotating wafer 1004 and moving chuck 1002 in a horizontal
direction while
simultaneously allowing the end-point detector 1009 to scan the entire surface
of wafer
1004. It should be understood, however, that alternatively end detector 1009
could scan a
stationary wafer 1004.
[00111] Rotary nozzle 2012 may then rotate to select a desired nozzle 1014
depending on the portion of wafer 1004 that is being polished, the metal film
thickness and
the like. For example, in areas where metal layer is thick a larger nozzle may
be used, and
in areas where metal layer is thin a small nozzle may be used. Including
numerous nozzles
of varying sizes and profiles within a simple compact electropolish assembly
that may be
quickly and easily interchanged thus enhances the precision of the polishing.
[00112] With reference to Fig. 14A a cross-sectional view of an exemplary
multiple
rotary nozzle holder 2012 is shown. Rotary nozzle holder 2012 holds nozzles
2014.
Driving means 2070 rotates the rotary nozzle 2012 through driving joint 2068
for
positioning a new nozzle to direct a stream of electrolyte fluid. An o-ring
2066 seals
driving joint 2068, for example. The driving means 2070 may be a stepper
motor,
servomotor, pneumatic (compressed gas or liquid) driving rotation means, and
the like.
Nozzles 2014 in rotary nozzle holder 2012 include electrodes 2056 that may be
electrically
coupled to outside power supply 1040 (Fig. 13A) through electrical current
feed-throughs
2062. Rotary nozzle holder 2012 rests on plate 2084, which is sealed with
chamber 1008
by o-ring 2072 and bolt 2074.
27
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00113] Nozzle holder 2012 may be made of plastics such as PVC, PVD, TEFLON,
polypropylene, and the like or coated with a material that is generally
insulative and non-
corrosive. Nozzles 2014 may be made of tantalum, titanium, platinum, stainless
steel, and
the like.
[00114] Figure 14C illustrates an exemplary process for electropolishing a
metal film
from a wafer 1002 using the apparatus of Figure 14A. In block 1 the metal film
thickness
profile is determined, for example, by end-point detector 1009 translating in
the x-direction
as the wafer 1004 is rotated as described above. In block 2, the metal film
can be polished
initially with a high polishing rate using a large nozzle 2014. Following the
high polishing
rate the nozzle holder 2012 may be rotated to use a lower polishing rate using
a small
nozzle 2014 in block 3. After the initial polishing in block 1 andlor block 2
the remaining
metal thickness profile may be deternlined in block 4 using end-point detector
1009, for
example, an eddy current end-point detector, optical end-point detector, or
the like. Based
on the remaining metal thickness profile determined in block 4, the polishing
current may
be adjusted or tuned in block 5 to polish thick film locations at a higher
rate, polish thin
film locations at a low rate, and stop the polish at zero film thickness
locations. The
polishing current may be tuned, for example, by using different nozzles 2014
and/or
varying the charge supplied by the power supply. In block 6, measurements of
the
thickness profile are repeated, i.e., block 4. If the thickness of the metal
layer reaches a
pre-set value the polishing process may be stopped. If, however, the thickness
of the metal
has not reached a pre-set value, then block 5 may be repeated until a desired
thickness is
reached.
[00115] It should be recognized that numerous modifications and variations may
be
made to the process described with reference to Figure 14C. Further, numerous
other
processes may be used in conjunction with the exemplary apparatus of Fig. 14A.
[00116] With reference to Fig. 14B, another exemplary multiple rotary nozzle
assembly is illustrated. The rotary nozzle assembly shown in Fig. 14B is
similar to that
shown in Fig. 14A except that driving joint 2068 is replaced by magnetically
coupled joints
2078 and 2082. An advantage of using magnetically coupled joints 2078 and 2082
is that
driving joint 2078 does not make a direct connection to nozzle holder 2012 and
o-ring 2066
28
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
of Fig. 14A may be omitted. This reduces the possible occurrence of
electrolyte fluid 1038
leaking through to driving joint 2068. It should be recognized therefore that
various
methods of coupling driving joint 2068 to rotary nozzle 2012 are possible.
[00117] With reference to Fig. 15, an exemplary linearly movable multiple
nozzle
assembly is illustrated. The multiple linearly movable nozzle assembly
operates similar to
the rotary nozzle 2012 of Figs. 13A through 13E except that the nozzles move
in a linear
motion as opposed to rotational motion. The multiple linear movable nozzle
assembly
includes nozzle 3054, nozzle 3222, and nozzle 3226 including electrodes 3056,
3220, and
3224 respectively. The three nozzles 3054, 3222, and 3226 may be configured to
have
different profiles, for example, different diameters, and therefore may
provide different
polishing rates.
(00118] Nozzles 3054, 3222, and 3226 are movable in a horizontal direction,
i.e., x-
direction, through nozzle holder 3180 and moving guide 3182. Electrodes 3056,
3220, and
3224 are further connected with power supply 3110 through electrical feed-
throughs (not
shown). Electrolyte fluid 3080 is supplied to nozzles 3054, 3222, and 3226
through nozzle
holder 3180. As described with reference to Fig. 14C, different sized nozzles
3054, 3222,
and 3226 may be used interchangeably during an electropolishing process to
remove a
metal film disposed on wafer 1004. In general, a large nozzle may be used to
polish the
metal film at a higher polish rate when the metal film is thick, and a small
nozzle may be
used to polish the metal film at a lower polish rate if the metal film is
thin, or a small
amount of metal is desired to be removed.
[00119] Figs. 16A through 16E illustrate an exemplary electropolishing
assembly
including a multiple rotary nozzle assembly. Figs. 16A through 16E are similar
to Figs.
13A through 13E, except for the addition of a linear movable base 4180 and
moving guide
4182 with rotary nozzles 4012 and 4014 mounted thereon.
[00120] In particular, rotary multiple nozzles 4014, optical end-point
detector 4016,
and eddy current thickness/end-point detector 4060 are mounted on linearly
movable base
4180. The linearly movable base member may be moved in a horizontal direction,
i.e., x-
direction, along moving guide 4182. This assembly allows for multiple nozzles
to be
included within a compact space.
29
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00121] The structure and operation of the multiple nozzles 4014 is similar to
those
shown in Figs 14A and 14B, however, structures such as the rotating driving
means,
driving joint, electrical current feed-through, and electrolyte feed-throughs
have been
omitted for illustrative purposes.
[00122] VII. Nozzle Self Cleaning Processes
[00123] According to another aspect, an exemplary process for self cleaning
electropolishing nozzles is described. During a typical electropolishing
process metal
dissolved in the electrolyte fluid may become plated on the nozzle electrodes.
The plated
metal may restrict or deform the opening of the nozzle thereby altering the
shape and/or
direction of the stream of electrolyte fluid. Changing the shape of the stream
may change
the current density of the stream and consequently alter the polishing rate of
the
electropolishing apparatus. The nozzles may be deplated, or cleaned, by
applying a reverse
voltage to the nozzles that cause metal ions to dissolve back into the
electrolyte solution.
For example, the metal may be plated to another nozzle, a sacrificial
material, or the like.
[00124] With reference again to Figs. lA through lE, metal from a metal layer
that
is polished from wafer 1004 becomes dissolved in electrolyte fluid 1038 and
may result in
a portion of the dissolved metal becoming plated onto nozzle electrodes 1056
and/or 1060.
In order to remove the metal from nozzle electrodes 1056 and/or 1060, a
reverse voltage
can be applied to nozzle electrodes 1056 and/or 1060. Either a DC or an AC
power supply
can be used to apply the reverse voltage. In one exemplary process, a reverse
voltage is
applied to dissolve the metal buildup into the electrolyte fluid. In another
exemplary
process, a reverse voltage is applied to plate the metal buildup onto a
disposable wafer. In
yet another example, a reverse voltage is applied to plate the metal buildup
onto a block.
[00125] A. Dissolving Metal Buildup into Electrolyte fluid Using a DC Power
Supply
[00126] With reference to Fig. lA, metal buildup on nozzle 1012 can be
polished off
and dissolved into electrolyte fluid 1038 using a DC power supply. More
particularly, lead
C can be connected to lead b and lead B can be connected to lead a, such that
nozzle
electrode 1056 (Figs. 1B-lE) acts as an anode and nozzle electrode 1060 acts
as a cathode.
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
Electrolyte fluid 1038 can be supplied through nozzles 1012 and 1014 in order
to form an
electrical circuit between electrode 1056 and 1060 that allows metal buildup
on nozzle
1012 to be removed from nozzle 1012 and dissolved into electrolyte fluid 1038.
A portion
of the metal dissolved into electrolyte fluid 1038 may become plated onto
nozzle 1014.
[00127] Although it appears that the process simply moves the metal from one
nozzle and plates it to another, the majority of the metal removed from nozzle
1012
remains dissolved in electrolyte fluid 1038. The metal concentration in the
electrolyte fluid
1038 for an exemplary electropolishing process is typically low, for example,
less that 3%
by weight, such that the electropolishing is driven by electrically charging
electrodes 1012
and 1014 and not by the chemistry of the electrolyte solution 1038. Thus, the
amount of
metal polished from nozzle 1012 is greater than the amount of metal plated to
nozzle 1014.
For example, ten metal ions might be removed from nozzle 1012 for every one
metal ion
plated to nozzle 1014 resulting in the majority of the metal ions being
dissolved into the
electrolyte fluid 1038.
[00128] With continued reference to Fig. lA, the process can be reversed such
that
metal buildup on nozzle 1014 is polished off and dissolved into electrolyte
fluid 1038 using
a DC power supply. More particularly, lead B can be connected to lead b and
lead C can
be connected to lead a, such that nozzle electrode 1060 (Figs. 1B-lE) acts as
an anode and
nozzle electrode 1056 acts as a cathode. Electrolyte fluid 1038 can be
supplied through
nozzles 1012 and 1014 in order to form an electrical circuit that allows metal
buildup on
nozzle 1014 to be removed from nozzle 1014 and dissolved into electrolyte
fluid 1038.
Again, a portion of the metal dissolved into electrolyte fluid 1038 can become
plated onto
nozzle 1012.
[00129] By repeating this process, i.e., reversing the voltage for each nozzle
used in
the apparatus, the nozzles can be cleaned. In one exemplary process, the
nozzles are
quickly cleaned by electropolishing successive wafers by first deplating
nozzle 1012 and
plating nozzle 1014, followed by deplating nozzle 1014 and plating 1012. Both
nozzles are
effectively cleaned because, as discussed, most of the metal is dissolved in
the electrolyte
fluid 1038 as opposed to being plated to the opposing nozzle.
31
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00130] Figs. 2 and 3 show two exemplary configurations of nozzles 1012 and
1014
during a cleaning process. Nozzles 1012 and 1014 are positioned near each
other and
electrolyte fluid is allowed to flow through nozzle 1012 and 1014 and form a
film or path
of electrolyte fluid therebetween. When nozzles 1012 and 1014 are placed
closer together,
as shown in Fig. 3, the two films or paths of electrolyte fluid 1080 flowing
between nozzle
1012 and 1014 can join to form a single path. This single path reduces the
length of the
electrical circuit, thereby increasing the efficiency of the metal buildup
removal process. It
should be recognized of course that the exemplary method may also be employed
with
more than two nozzles.
[00131] B. Plating Metal Buildup onto Wafer Using a DC Power Supply
[00132] With reference to Fig. lA, metal buildup on nozzle 1012 can be
polished off
and plated onto wafer 1004 using a DC power supply according to another
exemplary
process. More particularly, lead A can be connected to lead b and lead B can
be connected
to lead a, such that wafer 1004 acts as a cathode and nozzle electrode 1056
(Figs. 1B-lE)
acts as an anode. Electrolyte fluid 1038 can be supplied through nozzle 1012
to wafer 1004
in order to form an electrical circuit allowing metal buildup on nozzle 1012
to be plated
onto wafer 1004. Wafer 1004 can be discarded after the metal buildup on nozzle
1012 is
removed.
[00133] Similarly, with reference to Fig. lA, metal buildup on nozzle 1014 can
be
polished off and plated onto wafer 1004 using a DC power supply. More
particularly, lead
A can be connected to lead b and lead C can be connected to lead a, such that
wafer 1004
acts as a cathode and nozzle electrode 1060 (Figs. 1B-lE) acts as an anode.
Electrolyte
fluid 1038 can be supplied through nozzle 1014 to wafer 1004 in order to form
an electrical
circuit that allows metal buildup on nozzle 1014 to be plated onto wafer 1004.
Nozzle
1012, or other nozzles in the electropolishing apparatus, may be cleaned in
parallel or in
series with nozzle 1014. Wafer 1004 can be discarded after the metal buildup
on nozzle
1014 is removed.
[00134] C. Plating Metal Buildup onto a Block Using a DC Power Supply
32
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00135] With reference to Fig. 4, metal buildup 1057 on nozzle 1012 can be
polished
off and plated onto block 1082 using a DC power supply according to another
exemplary
process. More particularly, lead B can be connected to lead a and lead D can
be connected
to lead b, such that block 1082 acts as a cathode and nozzle electrode 1056
acts as an
anode. Electrolyte fluid 1038 (Fig. 1) can be supplied through nozzle 1012 and
allowed to
contact block 1082 in order to form an electrical circuit through electrolyte
fluid 1038
allowing metal buildup on nozzle 1012 to be plated onto block 1082. Block 1082
can be
discarded after the metal buildup on nozzle 1012 is removed, or when otherwise
convenient.
[00136] Similarly,.with reference to Fig. 4, metal buildup 1057 on nozzle 1014
can
be polished off and plated onto block 1082 using a DC power supply. More
particularly,
lead C can be connected to lead a and lead D can be connected to lead b, such
that block
1082 acts as a cathode and nozzle electrode 1060 acts as an anode. Electrolyte
fluid 1038
(Fig. 1) can be supplied through nozzle 1014 and allowed to contact block 1082
in order to
form an electrical circuit that can allow metal buildup on nozzle 1014 to be
plated onto
block 1082. Block 1082 can be discarded after the metal buildup on nozzle 1014
is
removed, or when otherwise convenient. Further, electrode 1056 and 1060 may be
cleaned
in series or in parallel.
[00137] D. Removing Metal Buildup Using an AC Power Supply
[00138] In another exemplary nozzle cleaning process, an AC power supply, as
opposed to a DC power supply, may be used with any of the above
configurations.to
remove metal buildup from nozzles 1012 and 1014. In particular, an AC power
supply is
used to dissolve the metal buildup into the electrolyte fluid, plate the metal
buildup onto a
wafer to be discarded, or plate the metal buildup onto a block or other
sacrificial material.
[00139] Removing metal buildup from the nozzles with an AC power supply
becomes more efficient as the concentration of the metal in the electrolyte
fluid decreases.
Accordingly, the metal concentration can be typically in the range of about
0.1 % to about
5% wt, and preferably less than about 0.5% wt during the removal process.
[00140] VIII. Nozzle Shapes
33
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00141] In any of the exemplary embodiments described, nozzles of various
shapes
may be advantageously employed. Different types of nozzles, for example,
different sizes,
profiles, cross-sectional shapes, and the like, provide different polishing
characteristics and
may be advantageously used depending on the particular application. For
example, as seen
in Fig. 1 B, the exemplary electropolishing apparatus may include two
different sized
nozzles 1012 and 1014 that may be used to electropolish different sections of
wafer 1004.
[00142] Additionally, Figs. SA through SH illustrate various exemplary nozzles
having a variety of shapes and configurations. The shape of a nozzle, for
example, the
channel and the distal end, can change the profile of the electrolyte fluid
flowing from the
nozzle, the current density within the stream of electrolyte fluid, and the
like. Figs. SA
through SE illustrate various nozzle configurations and shapes that include
insulator 5054
and an electrode 5056. Figs. SF through SH illustrate nozzles without an
insulator. Several
of the nozzle configurations have a curved electrode 5056 near the opening.
The curved
electrode 5056 prevents an electrical peak at a sharp point in the electrode,
which helps
produce a more uniform current density in the stream of electrolyte fluid.
Fig. SH depicts a
nozzle including electrode 5056 and rod 5058 located near the center of the
nozzle to
increase the surface area of the electrode and create a more uniform current
density.
[00143] For each of the above-described nozzles, electrode 5056 can include a
metal
or alloy such as Tantalum, Titanium, stainless steel, and the like.
Additionally, insulator
5054 can include plastics such as PVC, PVD, Teflon, and the like, or ceramics
such as
A1203, Zr02, SiOa, and the like. Accordingly, because metals and alloys are
typically
easier to form into various shapes than plastics and ceramics, nozzles having
electrodes
with curved or tapered shapes and insulators with straight shapes can be less
expensive to
manufacture than other shapes. Furthermore, nozzles having only an electrode
5056, such
as those shown in Figs. SF, SG, and SH, can have a simpler design and more
surface area.
Additionally, the nozzle shown in Fig. SH includes rod 5058 as part of
electrode 5056,
which provides the electrode with more surface area and may distribute more
uniformly the
electric potential across electrolyte fluid 1038 (Fig. lA) flowing from the
nozzle. A more
uniformly distributed electric potential leads to more uniform
electropolishing of wafer
1004.
34
CA 02464423 2004-04-21
WO 03/042433 PCT/US02/36567
[00144] Figs. 6A and 6B illustrates another exemplary nozzle having insulator
6054,
electrode 6056, and conductive inner structure 6086. Inner structure 6086
includes a metal
or alloy such as Tantalum, Titanium, stainless steel, and the like.
Additionally, inner
structure 6086 includes multiple channels that can increase the surface area
of the
electrode, and can distribute the electric potential across nozzle 6056 more
uniformly. The
size of the channels can be in the range of about 0.1 mm to about 10 mm,
depending on the
diameter of the nozzle and the particular application. Preferably, the size of
each of the
channels can be about one tenth of the nozzle diameter.
[00145] The channels can be formed in various cross-sectional shapes, such as
those
shown in Figs. 6B to 6I. For example, channels can be square, fiber-shaped,
straight slots,
metal rods, waved slots, rectangular, honeycomb, and the like. In addition,
although
particular cross-sectional shapes are shown in Figs. 6B to 6I, the channels
can be formed in
any cross-sectional shape, such as a triangle, polygon, ellipse, and the like.
[00146] The above detailed description is provided to illustrate exemplary
embodiments and is not intended to be limiting. It will be apparent to those
skilled in the
art that numerous modifications and variations within the scope of the present
invention are
possible. For example, the different exemplary electropolishing apparatuses,
for example,
the shroud, the conductive member, the various nozzles, end-point detectors,
and the like
may be used together in a single assembly or may be used separately to enhance
a
conventional electropolishing apparatus. Accordingly, the present invention is
defined by
the appended claims and should not be limited by the description herein.