Note: Descriptions are shown in the official language in which they were submitted.
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
DEGP PROTEASE: CLEAVAGE SITE IDENTIFICATION AND PROTEOLYSIS OF A
NATURAL TARGET IN E. COLT
BACKGROUND OF THE INVENTION
Pathogenic Gram-negative bacteria cause a number of pathological
conditions such as bacteraemia, bacteria-related diarrhea, meningitis, and
urinary
tract infection, including pyelonephritis, cystitis, and urethritis.
Urinary tract infections (UTIs)are a major cause of morbidifiy in females.
Despite the overall importance of urinary tract infections, few efforts have
been
directed toward the development of novel strategies for treatment or
preventions of
these diseases. Currently, conventional antibiotics (e.g., penicillins,
cephalosporins,
aminoglycosides, sulfonamides, tetracyclines, nitrofurantoin, and nalidixic
acid) are
employed to treat these infections. It is expected that emerging antibiotic
resistance
will become a significant obstacle to treating these infections. In fact,
multiple
antibiotic resistance in uropathogens has already been detected and is
increasing.
Estimates of the annual cost of evaluation and treatment of women with UTIs
exceed one billion dollars. Further, approximately a quarter of the 4 billion
dollar
annual cost associated with nosocomial infections is a consequence of UTIs.
Among Gram-negative bacterial culprits of UTIs, Escherichia coli predominates.
The ability of Gram negative bacteria (e.g., E. coli, Haemophilus influenzae,
Salmonella enteridifis, Salmonella typhimurium, Bordetella pertussis, Yersinia
pestis,
Yersinia enterocolifica, helicobacter pylori, and Klebsiella pneumoniae) to
adhere to
a variety of epithelial tissues is an important factor in infectability. As
one example,
E. coli adheres to epithelial cells despite a unidirectional flushing effect
of urine from
the kidneys.
The initiation and persistence of many bacterial infections such as those
described above is thought to require the presentation of adhesins on the
surface of
_I_
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
the microbe in accessible configurations which promote binding events that
dictate
whether extracellular colonization, internalization, or other cellular
responses will
occur. Adhesins are often components of the long, thin filamentous,
heteropolymeric protein appendages known as pili, fimbriae, or fibrillae. The
bacterial attachment event is often the result of a stereochemical fit between
an
adhesin frequently located at the pilus tip and specific receptor
architectures on host
cells, often comprising carbohydrate structures in membrane-associated
glyoconjugates.
Uropathogenic strains of E. coli express P pili that bind to receptors present
in
uroepithelial cells. Adhesive P pili are virulence determinants associated
with
pyelonephritic strains of E. coli. At least eleven genes are involved in the
biosynthesis and expression of functional P pili; the DNA sequence of the
entire pap
gene cluster has been determined. P pili are composite heteropolymeric fibers
consisting of flexible adhesive fibrillae joined end to end to pilus rods. The
pilus rod
is composed of repeating PapA protein subunits arranged in a right-handed
helical
cylinder. Tip fibrillae which extend from the distal ends of each pilus rod
were found
to be composed mostly of repeating subunits of PapE arranged in an open
helical
conformation. The PapG adhesin was localized to the distal ends of the top
fibrillae,
a location which is assumed to maximize its ability to recognize glycolipid
receptors
on eukaryotic cells. Two minor pilus components, PapF and PapK, are
specialized
adaptor proteins found in the top fibrillum. PapF links the adhesin moiety to
the
fibrillum while PapK joins the fibrillum to the pilus rod. The composite
architecture of
the P pilus fibre reveals the strategy used by uropathogenic E. coli to
present the
PapG adhesin to eukaryotic receptors. The rigid PapA rod extends the adhesin
away from interference caused by LPS and other components at the bacterial
cell
surface while the flexible fibrillum allows PapG steric fredom to recognize
and bind
to a digalactoside moiety on the uroepithelium.
-2-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
The DegP (HtrA, Protease Do) protease is a multifunctional protein essential
for the removal of misfolded and aggregated proteins in the periplasm of E,
coli.
DegP is one of several dozen proteases in E, coli and is known to have
homologues
in virtually all Gram-negative bacteria, cyanobacteria, mycobacteria, as well
as in
higher order organisms: yeast and man (fallen and Wren, Mol. Microbiol.
26(2):209-
221 (1997)). DegP homologues have recently been described in a number of Gram-
positive bacteria, including Enterococcus faecalis, Lactococcus lactis,
Streptococcus
pneumoniae, S, gordonii, S. pyogenes, S. mufans, Lactobacillus helveticus,
Bacillus
subtilis, and Staphylococcus aureus (2 homologues) (Jones et al., Infecf. &
Immun.
69(9):5538-5545 (2001 ); Noone et al., J. Bacteriol. 182:1592-1599 (2000);
Poquet et
al., Mol. Microbiol. 35:1042-1051 (2000); Smeds et al., J. Bacteriol. 180:6148-
6153
(1998)). There are also two homologues of DegP, DegQ and DegS, in E. coli
(Kolmar et al., J. Bacteriol. 178:5925-5929 (1996); Waller and Sauer, J.
Bacteriol.
178:1146-1153 (1996)). Kolmar et al. (1996) have suggested that DegQ possesses
similar specificity to DegP. DegP has been rediscovered several times as is
revealed by the nomenclature (Miller, "Protein Degradation and Proteolytic
Modification" in Escherichia coli and Salmonella: Cellular and Molecular
Bioloay,
F.C. Niehardt, ed., ASM Press, Washington DC, pp. 938-954 (1996); fallen and
Wren, 1997; Seol et al., Biochem. Biophys. Res. Comm. 176:730-736 (1991 ). The
DegP (deg,radation) nomenclature refers to the initial mapping of a mutation
in E.
coli that allowed accumulation of unstable fusion proteins in the periplasm
(Strauch
and Beckwith, Proc. Nafl. Acad. Sci. USA 85:1576-1580 (1988), Strauch et al.,
J.
Bacteriol. _ _171:2689-2696 (1989)). The Htr (h_eat shock regulated)
designation
indicates that a transposon insertion in the same gene resulted in a
temperature
sensitive growth phenotype (Lipinska et al., Nucl. Acids Res. 16:10053-10066
(1988)). Lastly, DegP was also designated protease "Do" again as a mutation
that
conferred a temperature sensitive growth phenotype in E. coli (Seol et al.,
1991 ).
-3-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
The "DegP designation will be used throughout this specification when
referring to
the E. coli protein.
Purified DegP exhibited functional protease activity in in vitro assays using
casein as a substrate, although its activity on this substrate was weak
(Lipinska et
al., J. Bacteriol. 172:1791-1797 (1990)). Lipinska et al. (Lipinska et al.,
1990)
demonstrated that the activity on casein was inhibitable by diisopropyl
fluorophosphate and not by any other known protease inhibitors, suggesting
that
DegP contains an active site serine residue. Interestingly, DegP is not
inhibited by
the classic serine protease inhibitor, phenylmethylsulfonylfluoride (PMSF),
suggesting differences in the mode of action of DegP (Kolmar, "DegP or
Protease
DO CLAN SA" in Handbook of Proteoh is Enzymes, Barrett et al., eds., Academic
Press, Great Britain (1998), Lipinska et al. (1990)). Site-directed
mutagenesis at
serine 210 and histidine 105, two components of the serine protease catalytic
triad,
compromised Deg function in vitro and in vivo; i.e., strains carrying serine
210 or
histidine 105 mutant derivatives were sensitive for growth at elevated
temperatures
(Skorko-Glenek et al., Gene 163:47-52 (1995)). A recent study has shown that
DegP functions as both a chaperone and a protease; at low temperatures
(28°C) the
chaperone function predominates and after a shift to high temperature
(45°C) the
protease function is activated (Spiess et al., Cell 97:339-347 (1999)).
Synthesis of
DegP is controlled by eE, the so-called "stress" sigma factor that controls
genes
essential for survival in the face of extracellular stress (Rains et al., J.
Bacteriol.
175(8):5009-5021 (1993); Rouviere et al., EMBO J. 14:1032-1042 (1995)). This
response is regulated, in part, by the CpxA/CpxR two-component regulatory
system
(Danese and Silhavy, Genes & Develop. 11:1183-1193 (1997); Danese et al.,
Genes & Develop. 9:387-398 (1995)). Recently, Jones et al. (Jones et al., EMBO
J.
16:6394-6406 (1997)) demonstrated that expression of pilin subunits in the
absence
of the cognate periplasmic chaperone (such conditions result in pilin subunit
aggregation) resulted in activation of the degP promoter.
-4-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
The first identified in vivo _target for DegP was colicin A lysis protein
(Cal)
(Cavard et al., J. Bacteriol. 171:6316-6322 (1989)). DegP was found to clip
the
acylated precursor form of Cal into two fragments. Mature Cal also accumulated
to
higher levels in degP mutant strains (Cavard et al., 1989). A second family of
DegP
targets identified were bacterial pilins. The K88 and K99 pilin subunits were
found to
accumulate to higher levels in degP mutant strains (Bakker et al., Mol.
Microbiol.
5(4):875-886 (1991 )). A more detailed study of this phenomenon demonstrated
that
P pilins, specifically PapA and PapG, are substrates for the DegP protease
(Jones
ef al., 1997). The H. influenzae non-pilus adhesin proteins HMW1 and HMW2 were
also found to be in vivo substrates for DegP (St. Geme III and Grass, Mol.
Microbiol. 27:617-630 (1998)). In addition, Spiess et al. (Spiess et al.,
1999)
recently demonstrated that the MaIS protein of E, coli was fully degraded in
vitro by
DegP.
In the last several years, a significant body of data has accumulated
demonstrating that DegP is a virulence factor for several pathogenic
organisms. In
Salmonella typhinurium, Salmonella typhi, Brucella abortus, Brucella
melitensis and
Yersinia enterocolitica htrA nulls were found to reduce or abolish virulence
(Elzer et
al., Res. Veterin. Sci. 60:48-50 (1996a), Elzer et al., Infect. Immun. 64:4838-
4841
(1996b); Johnson et al., Mol. Microbiol. 5:401-407 (1991 ); Li et al., Infect.
Immun.
64:2088-2094 (1996); Phillips et al., Res. Veterin. Sci. 63:165-167 (1997);
Tacket et
al., Infect. Immun. 65:452-456 (1997)). The htrA null mutants were found to be
more
sensitive to oxidative stress and killing by immune cells. Moreover, an htrA
lesion
was found to be useful in attenuating both Salmonella typhi (Tacket et al.,
1997;
Tacket et al., Infect. Immun. 68(3):1196-1201 (2000)) and Salmonella
typhimurium
(Roberts et al., Infect. Immun. 68(10):6041-6043 (2000)) for implementation as
vaccine strains. Boucher et al. (Boucher et al., J. Bacteriol. 178:511-523
(1996))
demonstrated that Pseudomonas aeruginosa conversion to mucoidy, the so-called
cystic fibrosis phenotype, involved two htrA homologues. In a recent report,
-5-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
Pederson et al. (Pederson et al., Infect. Immun. 69(4):2569-2579 (2001 ))
demonstrated that and htrA null mutant in Legionella pneumophilia was
attentuated
for virulence. Recently, degP was insertionally inactivated in Streptococcus
pyogenes and found to result in temperature and oxidative sensitivity as well
as
compromising virulence in a mouse model (Jones et al., 2001 ).
Early in vivo data suggested that pilin subunits of the so-called chaperone-
usher assembly pathway were DegP substrates (Bakker et al., 1991 ). Expression
of
pilin subunit proteins in the absence of the cognate-chaperone (PapD) resulted
in
failure to accumulate subunits in the periplasm of wild-type (DegP+) bacteria
and
degP mutant strains were found to accumulate higher levels of pilin subunit in
the
periplasm (Bakker et al., 1991; Hultgren et al., "Bacterial Adhesins and Their
Assembly" in Escherichia coli and Salmonella: Cellular and Molecular Bioloay,
F.C.
Niehardt, ed., ASM Press, Washington DC, pp. 2730-2756 (1996); Hultgren et
al.,
Annu. Rev. Microbiol. 45:383-415 (1991 ); Jones et al., 1997). Both the
toxicity and
accumulation was suppressed by complementation with papD, encoding the
periplasmic chaperone, or degP (Jones et al., 1997). Previously, we developed
a
scheme for purification of the PapA subunit of the P pilus and demonstrated
that this
protein is a natural substrate that is efficiently cleaved by the DegP
protease. In the
current invention, we also identify three cleavage sites within the PapA
sequence
and characterized the determinants essential for proteolytic cleavage of one
of the
peptide substrates.
SUMMARY OF THE INVENTION
The present invention provides for methods of treatment and/or prophylaxis of
diseases caused by pilus-forming bacteria by modulating DegP protease
activity.
The present invention provides for the identification of DegP cleavage sites
on PapA
pilin subunit and methods for identifying substances which modulate DegP
activity.
The present invention provides for polypeptides identified which are cleavable
-6-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
substrates for DegP. The present invention provides for the identification of
a
polypeptide which enhances DegP protease activity.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 Expression and purification of PapA-6H4A.
1A. Accumulation of PapA-6H4A in the periplasm is dependent on the PapD
chaperone. Periplasmic fractions prepared from KS474/pCL101/pHJ9203
were analyzed following IPTG (1 mM) induction of PapA-6H4A alone (lane 1)
or co-induction of both PapA-6H4A and PapD (lane 2). Cultures were
induced for 60 minutes at mid-logarithmic growth. Periplasm was fractionated
on SDS-PAGE and stained with Coomassie blue.
1 B. Purification of PapZ-6H4A-PapD complex from the periplasm. Periplasm
from double induced cultures was applied to Talon metal affinity resin
(Clontech). The pre-Talon, unbound fraction and first three washed (20 mM
Tris, pH=8, 100 mM NaCI) are shown in lanes 2-6, respectively. Talon resin
containing bound PapD-PapA-6H4A complex was treated with 8 M urea and
washed to remove PapD (lanes 7-9). The bound PapA-6H4A was then
eluted with 0.1 M imidazole, 8 M urea (lanes 10-12). In order to remove the
trace of PapD contaminating the PapA-6H4A (lanes 10-12), the material in
the three elution fractions was pooled, dialyzed, and reapplied to Talon resin
in 20 mM Tris, pH=8, 8 M urea, following extensive washes, PapA-6H4A was
eluted with 0.1 M imidazole, 8 M urea (lane 13). Lane 1 contains molecular
weight markers.
1 C. Reconstitution of PapD-PapA-6H4A complex. Denatured PapA-6H4A
(lane 2) was applied to Talon metal affinity resin and diluted into a solution
containing purified PapD chaperone. The resin was washed and the
chaperone-subunit complex eluted with 0.1 M imidazole (lane 3). Lane 1
contains purified native PapD PapA-6H4A comples for reference.
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
1 D. DegP protease binding and cleavage of PapA-6H4A. Denatured PapA-
6H4A was lined to metal affinity resin and incubated for 60 minutes at room
temperature with control periplasm (KS272/pACYC184, lane 1 ) or periplasm
enriched with DegP protease (KS272/pKS17, lane 2). Following three
washes, bound protein was eluted with 0.1 M imidazole. Three novel bands
are apparent following exposure of PapA-6H4A to DegP-enriched periplasm.
The ~48 kDa band (lane 2) was identified by amino terminal sequence
analysis as the DegP protease while the approximately 12 kDa band (lane 2)
has the expected amino terminus for PapA-6H4A. The third band, which runs
above the indicated 14 kDa molecular weight marker, has yet to be identified.
Lane 3 contains molecular weight markers. In A-D, the sizes of molecular
weight markers are indicated.
Figure 2. Toxicity Assays.
2A. PapA toxicity suppression by PapD. Ks474/pCL101/pHJ9203 (papD)
was induced with 0.3 mM IPTG to induce synthesis of PapA-6H4A at the
onset of growth in the presence (~) or absence (1) of PapD. PapD was
induced with 4% arabinose at the onset of culture growth. The A6oo of each
culture was monitored throughout growth.
2B. PapA toxicity suppression by DegP. KS474/pCL101/pKSl7 (degP) (~)
and KS474/pCL101/pACYC184 (vector control) (1) were induced with 0.3
mM IPTG at the onset of growth. The A6oo of each culture was monitored
throughout growth
Figure 3. Purification of DegP.
3A. Cation exchange fractionation. Periplasm prepared from 30 grams of
cells was applied to a 5 mL HiTrap SP column and eluted with a linear salt
gradient. The starting material and flow-through fraction are shown in lanes 2
and 3, respectively. The relevant portion of the elution gradient is shown in
lanes 4-8. DegP eluted at approximately 100 mM NaCI.
_g_
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
3B. HIC butyl fractionation. Peak fractions from the cation exchange
fractionation were pooled, brought to 0.5 M ammonium sulfate and applied to
a HiTrp HIC butyl column. The flow-through fraction is shown in lane 2 and a
portion of the gradient elution is shown in lanes 3-8. DegP eluted in
approximately 0.3 M NaCI and is shown in lanes 4-8. The small arrows
indicated truncated forms of DegP, all of which were identified by amino-
terminal sequencing (unpublished data). In both A and B, lane 1 contains
high molecular weight markers.
Figure 4. In vitro Protease Assay.
Pooled peak HIC butyl fractions from three separate DegP purification runs
(A, B, C) were checked for general protease activity on a commercial casein
substrate. The EnzChek assay kit uses a sensitive fluorescein labeled casein
substrate that is internally quenched until cleaved. The BODIPY-casein FL
signal is read following excitation at 485 nm and monitoring 530 nm emission.
Peak fractions containing DegP were incubated with the substrate according
to manufacturer's instructions and after one hour were read in a plate
fluorometer. Trypsin was used as a control for the assay.
Figure 5. In vitro DegP Cleavage Assay.
5A. Reduced and carboxymethylated PapA-6H4A (PapA-6H4A-rcm) was
mixed with DegP in 20 mM Tris, pH=8, and incubated overnight at 45°C.
The
reactions were resolved on SDS-PAGE, transferred to PVDF membrane and
developed with a polyclonal antibody raised against whole P pili. PapA-
6H4A-rcm (lanes 2 & 3, 0.25 ,ug, lanes 4 & 5, 0.5 ,ug) was incubated in the
presence (lanes 3 & 5) and absence (lanes 2 &4) of approximately 50 and
100 fold molar excess of DegP (lanes 3 & 5, respectively). The large
arrowhead indicates a PapA aggregate in lane 4. Densitometric
quantification of the cleaved PapA-6H4A band indicated that DegP cleaved
84% of the input protein (compare lanes 2 & 3) and nearly 100% of the
-9-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
aggregate band and 49% of the monomer in lane 5. Lane 1 contains DegP
alone as a control. Some cross-reactivity between PapA antisera and DegP
was seen in the blot.
5B. Addition of divalent cation, MgCl2, enhances DegP cleavage of PapA.
Reduced and carboxymethylated PapA-6H4A (20 ,ug) was mixed with DegP
(20 ,ug) in 20 mM Tris, pH=8, 5 mM MgCIZ and incubated for 4 hours at
45°C.
The reactions, containing DegP alone (lane 1 ), PapA-6H4A-rcm alone (lane
3) and DegP + PapA-6H4A-rcm (lane 2) were resolved on SDS-PAGE and
stained with Coomassie brilliant blue. Nearly 50% of the input PapA-6H4A-
rcm was cleaved during the 4-hour incubation.
Figure 6. DegP Cleavage of PapA-6H4A Substrate Is Inhibited by
Chelating Agent and Stimulated by Addition of a Non-Cleavable Peptide,
SPCJ-1.
DegP (5,ug) was incubated with carboxymethylated PapA-6H4A (40 ,ug) in 20
mM Tris, pH=8, 5 mM MgCl2 in the presence of 200 ,uM SPCJ-1. EDTA (10
mM) was added to the reactions loaded in lanes 7-10. Aliquots were taken at
the indicated time points and the reaction stopped by the addition of SDS-
loading buffer and incubation on ice. The reactions were resolved on SDS-
PAGE and stained with Coomassie brilliant blue. The ~12 kDa cleavage
product (lanes 3-5) seen in earlier cleavage assays (Fig. 1 D) is indicated by
the arrowhead. In excess of 50% of input PapA-6H4A was degraded in 60
minutes and 90% at the four hour time point.
Figure 7. Identification of the DegP Protease Cleavage Site in PapA.
SPOT synthesis technology was used to construct two overlapping (3
residues) peptide libraries, 7-mer and 12-mer, of the PapA sequence. The
peptides were synthesized linked to a continuous cellulose membrane
(ProteaseSpots) and had a fluorescent tag, aminobenzoic acid (Abz) at the
amino-terminus. The PepSpots were assayed in a 96-well format with 0.28
-10-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
mg/ml DegP in 20 mM P04, pH=7.5, 5 mM MgCl2. The data presented are
the liberated fluorescence (excitation - 325 nm, emission - 420 nm) following
28 hours incubation. The data show relative levels of cleavage for each of
the peptides in the 7-mer (Fig. 7A) and 12-mer (Fig. 7B) libraries.
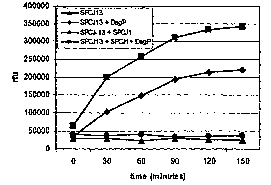
Figure 8. In vitro Peptide Cleavage Assay.
8A. SPCJ-12 (8 pM) was incubated with, 5 ,ug (~), and without (1) DegP in
reaction buffer (25 mM HEPES, pH=8, 5 mM CaCl2~ for the indicated times.
8B. SPCJ-13 (~, ~) and SPCJ-14 (1, D) (10 pM) were incubated with, 5 ,ug
(~,1), and without, ( 0, D), DegP in reaction buffer for the indicated times.
8C. Inhibitory activity of SPCJ-14 was demonstrated by pre-incubating DegP,
5 pg, with 10 pM (~) or 100 pM (~) SPCJ-14 for 30 minutes and then adding
10 pM SPCJ-13 and continuing the incubation for 60 minutes.
8D. SPCJ-13 was incubated with, 5 ,ug (~, ~), and without, (~, 1), DegP in
reaction buffer. To test the stimulatory effect of SPCJ-1, 200 ,uM peptide was
added (~, ~) at the onset of incubation. Assays were monitored (excitation -
340 nm, emission - 420 nm) at the indicated times in a Victory V plate reader
Figure 9. Mapping DegP Cleavage Site in Substrate Peptides.
9A. RP-HPLC was employed to resolve products of a scaled-up (5 ml)
cleavage reaction. The peak (actually two peaks - see insert), at 45.72 ml,
that showed absorbance at 215 nm, 340 nm, and 420 nm was lyophilized to
dryness and the substituents identified by MALDI TOF Mass Spectroscopy.
9B. The single peak contained two species resulting from the cleavage of the
peptide. The first species had a m/z of 480.59 corresponding to VK-
DAP(Dnp)-NH2 (SEQ ID NO.: 1 ) while the other species had a m/z of 708.99
corresponding to Abz-HYTAV (SEQ ID NO.: 2). This analysis was repeated
using the SPCJ-12 peptide (12-mer) and mapped the same cleavage site
(data not shown).
-11-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
In general, terms in the present application are used consistent with the
manner in which those terms are understood in the art.
By "modulate" is meant methods, conditions, or agents which enhance,
increase, inhibit, or decrease the wild-type activity of an enzyme and the
like.
By " modulated activity" is meant any activity, condition, disease or
phenotype
which is modulated by a protein. This modulation may take place by e.g., by
direct
agonistic or antagonistic effect as, for example, through inhibition,
activation,
binding, or release of substrate, modification either chemically or
structurally, or by
direct or indirect interaction which may involve additional factors. This
change may
be an increase/decrease in catalytic activity and/or binding to substrates.
By "DegP" is meant a multifunctional protein essential for the removal of
misfolded and aggregated proteins in the periplasm of E, coli. DegP also
refers to
HtrA and Protease Do in the current invention. "DegP homologue" refers to
other
proteases, such as DegQ and DegS, which are other E. coli. proteins with high
homology to DegP. Although the Examples refer to DegP of E, coli, the current
invention is not limited to this organism and is intended to encompass other
Gram-
negative homologues of DegP, chlamydia, and certain Gram-positive bacteria
possessing DegP homologues.
The current invention provides for a method for identifying a substance that
modulates the protease activity of DegP or a DegP homologue by adding said
substance to DegP or a DegP homologue in the presence of a cleavable
substrate,
and detecting enhancement or inhibition of the cleavage of said cleavable
substrate,
thereby determining whether said substance modulates said protease activity.
In a preferred embodiment, the cleavable substance used in this method is a
polypeptide selected from the group consisting of HYTAVVKKSSAV (SEQ ID NO:
3), HYTAVVK (SEQ ID NO: 4), LDIELVNCDITA (SEQ ID NO: 5), ELVNCDI (SEQ ID
-12-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
NO: 6), KLAFTGPIVNGH (SEQ ID NO: 7), FTGPIVNGHSDE (SEQ ID NO: 8),
TLKDGENVLHYT (SEQ ID NO: 9), DGENVLH (SEQ ID NO: 10), NVLHYTA (SEQ
ID NO: 11), and NGHSDEL (SEQ ID NO: 12), which are PapA pilin subunit peptides
identified as cleaved by DegP.
In a preferred embodiment the DegP homologue is DegS or DegQ.
In another preferred embodiment, the substance of this method enhances
protease activity. In another preferred embodiment, the substance inhibits
protease
activity. In a particularly preferred embodiment, the substance modulates DegP
activity.
The current invention provides for a method for treatment or prophylaxis of
disease caused by pilus-forming bacteria, comprising preventing, inhibiting,
or
enhancing the protease activity of DegP or a DegP homologue. It will be
apparent
to one skilled in the art from the foregoing Background section and the
Example
below, that DegP is a virulence factor which is responsible for the
degradation of
denatured or aggregated proteins from the periplasmic space. Therefore,
inhibition
of DegP protease activity may lead to toxic buildup of pilin subunits in the
periplasmic space, which may lead to increased morbidity and/or mortality or
decreased infectiveness of a bacterium. However, enhancement of DegP protease
activity may also have deleterious consequences for a bacterium, such as
depletion
of necessary pilin subunits. Thus, either enhancement or inhibition of DegP
protease activity may reduce the pathogenicity of pilus-forming bacteria.
In the current invention, cleavage sites on the PapA pilin subunit have been
identified which are efficiently cleaved by DegP protease. Accordingly, the
current
invention provides for isolated polypeptides comprising the amino acid
sequences
HYTAVVKKSSAV (SEQ ID NO: 3), HYTAVVK (SEQ ID NO: 4), LDIELVNCDITA
(SEQ ID NO: 5), ELVNCDI (SEQ ID NO: 6), KLAFTGPIVNGH (SEQ ID NO: 7),
FTGPIVNGHSDE (SEQ ID NO: 8), TLKDGENVLHYT (SEQ ID NO: 9), DGENVLH
(SEQ ID NO: 10), NVLHYTA (SEQ ID NO: 11 ), and NGHSDEL (SEQ ID NO: 12).
-13-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
These sequences were identified in assays performed in the current invention
as
DegP protease sites on the PapA pilin subunit. It will be appreciated by one
skilled
in the art that the peptides of the current invention may be modified, e.g.,
with
aminobenzoic acid and/or diaminopropionamide dinitrophenyl, and still perform
according to the current invention. Any modified peptides may be tested, e.g.,
in the
assays detailed in the Example, to determine whether such modification affects
the
activity of the peptide in modulating DegP protease activity. It was observed,
as is
noted in the Example below, that in most cases, cleavable substrates of DegP
identified contained paired hydrophobic residues.
The amino acid compounds of the invention are polypeptides which are
partially defined in terms of amino acid residues of designated classes.
Polypeptide
homologs would include conservative amino acid substitutions within the amino
acid
classes described below. Amino acid residues can be generally sub-classified
into
four major subclasses as follows:
Acidic: The residue has a negative charge due to loss of H+ ion at
physiological pH, and the residue is attracted by aqueous solution so as to
seek the
surface positions in the conformation of a peptide in which it is contained
when the
peptide is in aqueous medium, at physiological pH.
Basic: The residue has a positive charge due to association with H+ ion at
physiological pH, and the residue is attracted by aqueous solution so as to
seek the
surface positions in the conformation of a peptide in which it is contained
when the
peptide is in aqueous medium at physiological pH.
Neutral/non-polar: The residues are not charged at physiological pH, but the
residue is repelled by aqueous solution so as to seek the inner position in
the
conformation of a peptide in which it is contained when the peptide is in
aqueous
medium. These residues are also designated "hydrophobic."
Neutral~olar: The residues are not charged at physiological pH, but the
residue is attracted by aqueous solution so as to seek the outer positions in
the
-14-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
conformation of a peptide in which it is contained when the peptide is in
aqueous
medium.
It is understood, of course, that in a statistical collection of individual
residue
molecules some molecules will be charged, and some not, and there will be an
attraction for or repulsion from an aqueous medium to a greater or lesser
extent. To
fit the definition of "charged", a significant percentage (at least
approximately 25%)
of the individual molecules are charged at physiological pH. The degree of
attraction or repulsion required for classification as polar or nonpolar is
arbitrary and,
therefore, amino acids specifically contemplated by the invention have been
classified as one or the other. Most amino acids not specifically named can be
classified on the basis of known behavior.
Amino acid residues can be further subclassified as cyclic or noncyclic, and
aromatic or non-aromatic, self-explanatory classifications with respect to the
side
chain substituent groups of the residues, and as small or large. The residue
is
considered small if it contains a total of 4 carbon atoms or less, inclusive
of the
carboxyl carbon. Small residues are, of course, always nonaromatic.
The secondary amino acid proline, although technically within the group
neutral/nonpolar/large/cyclic and nonaromatic, is a special case due to its
known
effects on the secondary conformation of peptide chains, and is not,
therefore,
included in this defined group.
Other amino acid substitutions can also be included in peptide compounds
within the scope of the invention and can be classified within this general
scheme
according to their structure.
All of the compounds of the invention may be in the form of the
pharmaceutically acceptable salts or esters. Salts may be, for example, Na+,
K+,
Ca+~, Mg+? and the like; the esters are generally those of alcohols of 1-6
carbons.
The current invention provides for a method for modulating the protease
activity of DegP or a DegP homologue comprising adding one or more substances
-15-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
selected from the group consisting of a non-cleavable substrate or a cleavable
substrate to DegP or a DegP homologue in the presence of a cleavable substrate
in
an amount sufficient to modulate said protease activity.
In a preferred embodiment, the DegP homologue is DegQ or DegS. In
another preferred embodiment, protease activity is inhibited. In another
embodiment, the modulating substance is a cleavable substrate. In a
particularly
preferred embodiment, the cleavable substrate is a polypeptide selected from
the
group consisting of HYTAVVKKSSAV (SEQ ID NO: 3), HYTAVVK (SEQ ID NO: 4),
LDIELVNCDITA (SEQ ID NO: 5), ELVNCDI (SEQ ID NO: 6), KLAFTGPIVNGH
(SEQ ID NO: 7), FTGPIVNGHSDE (SEQ ID NO: 8), TLKDGENVLHYT (SEQ ID NO:
9), DGENVLH (SEQ ID NO: 10), NVLHYTA (SEQ ID NO: 11 ), and NGHSDEL (SEQ
ID NO: 12). In another preferred embodiment, protease activity is enhanced. In
a
particularly preferred embodiment, said substance is a polypeptide having
amino
acid sequence KSMCMKLSFS (SEQ ID NO: 13).
The present invention provides for a composition of matter, comprising a
substance which modulates the protease activity of DegP or a DegP homologue,
and a carrier therefor. This composition of matter may have diagnostic or
pharmaceutical use. For example, small molecule inhibitors of DegP or DegP
homologue function identified by an assay of the current invention can be used
as
novel anti-infectives. Further, small peptide molecules such as those
identified in
the present invention as cleavage sites can be used as fusion protein
"linkers."
Such linkers stabilize proteins which are difficult to produce for
overexpression and
purification in E. coli. DegP or a DegP homologue can then be used to cleave
the
fusion protein during purification.
In a preferred embodiment, the substance of the composition of matter
comprises a polypeptide comprising an amino acid sequence selected from the
group consisting of HYTAVVKKSSAV (SEQ ID NO: 3), HYTAVVK (SEQ ID NO: 4),
LDIELVNCDITA (SEQ ID NO: 5), ELVNCDI (SEQ ID NO: 6), KLAFTGPIVNGH
-16-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
(SEQ ID NO: 7), FTGPIVNGHSDE (SEQ ID NO: 8), TLKDGENVLHYT (SEQ ID NO:
9) , DGENVLH (SEQ ID NO: 10), NVLHYTA (SEQ ID NO: 11 ), and NGHSDEL (SEQ
ID NO: 12). In another preferred embodiment, the composition of matter
comprises
a polypeptide comprising amino acid sequence KSMCMKLSFS (SEQ ID NO: 13). In
another embodiment, the composition of matter further comprises at least one
antibacterial agent, wherein said agent is selected from the group consisting
of
penicillins, cephalosporins, aminoglycosides, sulfonamides, tetracyclines,
chloramphenicol, polymixins, antimycobacterial drugs, and urinary antiseptics.
Substances that are assayed by the above disclosed methods can be
randomly selected or rationally selected or designed. As used herein,
substance is
said to be randomly selected when the agent is chosen randomly without
considering the specific sequences involved in the association of DegP alone
or with
its associated substrates, etc. An example of randomly selected agents is the
use
of a chemical library or a peptide combinatorial library, or a growth broth of
an
organism.
The substances of the present invention can be, as examples, peptides, small
molecules, vitamin derivatives, as well as carbohydrates. A skilled artisan
can
readily recognize that there is no limit as to the structural nature of the
agents of the
present invention.
The peptide substances of the invention can be prepared using standard solid
phase (or solution phase) peptide synthesis methods, as is known in the art.
In
addition, the DNA encoding these peptides may be synthesized using
commercially
available oligonucleotide synthesis instrumentation and produced recombinantly
using standard recombinant production systems. The production of polypeptides
using solid phase peptide synthesis is necessitated if non-nucleic acid-
encoded
amino acids are to be included.
-17-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
EXAMPLE
The following example is intended to demonstrate preferred embodiments of
the invention and is not considered to be limiting.
Identification of Deg~P Cleavage Sites and Proteolysis of PaaA
and Enhancement of DegiP Protease Activity
Materials and Methods
Strains and Genetic Constructs
DHSa (Hanahan, J. Mol. 8iol. 166:557-580 (1983)) and INVaF' (Invitrogen,
Carlsbad, CA) were used in cloning steps. KS272 (MC1000 [F-~(ara-leu)7697 galE
galK DIacX74 rpsL (strr)] was used for expression of DegP (Strauch et al., J.
8acteriol.
171:2689-2696 (1989)). KS474 (KS272 degP::kan) was used for production of
PapA-6H4A. pKS17 (Strauch et al., (1989)), a gift of T. Silhavy, was used for
overexpression and purification of DegP. PCR cloning of papA was performed as
previously described (Morrison and Desrosiers, BioTechniques 14(3):454-457
(1993)) using primers designed to insert six histidine codons immediately
downstream of the PapA leader processing site. The mutated papA gene was
amplified to include BamHl and EcoRl restriction sites to facilitate cloning
into
pMMB566 (Furste et al., Gene 48(1 ):119-131 (1986)) creating pCL100. pCL101
was constructed in the same fashion using primers designed to insert six
histidine
codons and two alanine codons downstream of the leader processing site of the
papA gene. The PapD expression plasmid, pHJ9203, was described previously
(Jones et al., EM80 J. 16:6394-6406 (1997)).
PapA-6H4A Expression and Purification
KS474/pCL101/pHJ9203 was grown in Luria-Bertani (LB) broth (Difco,
Becton-Dickinson, Sparks, MD) in the presence of 100 pg/ml ampicillin, 50
pg/ml
-18-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
kanamycin, 100 pg/ml spectinomycin until A6oo=0.8, at which time IPTG was
added
to 1.0 mM and arabinose was added to 0.5%. The culture was induced for 90
minutes, the bacteria harvested and periplasm prepared from approximately 25
grams (wet weight) of cells as previously described (Jones et al., 1997). The
periplasm was dialyzed against 20 mM Tris, pH=8.5, and applied to Talon metal
affinity beads in batch as described by the manufacturer (Clontech, Palo Alto,
CA).
Following incubation for 60 minutes at RT and three washed with 20 mM Tris,
pH=8,
100 mM NaCI the bound complex was eluted with 0.1 M imidazole,.or
alternatively,
the chaperone-subunit complex was denatured by resuspension in 20 mM Tris,
pH=8.0, 100 mM NaCI, 6.0 M guanidine-HCI (or 8.0 M urea) and incubation at
45°
for 60 minutes. Guanidine-HCI was found to work best in our hands for
disruption of
the chaperone-subunit complex. The chaperone was removed by washing in buffer
and denaturant and PapA-6H4A eluted from the Talon resin with 0.1 M imidazole.
For efficient cleavage by DegP the PapA-6H4A was subjected to reduction and
carboxymethylation. The guanidine-HCI-denatured PapA-6H4A was reduced in a
buffer consisting of 400 mM Tris, pH=8.5, 5.0 mM EDTA, 0.11 M 2-
mercaptoethanol
and incubated at 4°C for two hours. The reduced protein was
carboxymethylated by
incubation with 0.21 M iodoacetic acid for one hour at 4°C. The protein
was then
dialyzed into 20 mM Tris, pH=8.5, for use in cleavage assays.
Purification of DeaiP Protease
DegP was purified from whole periplasm, which was prepared as
previously described (Jones et al., 1997). Approximately 30 grams (wet weight)
of
cells were used to prepare periplasm. Following dialysis against 33 mM MES,
33mM HEPES, 33 mM acetate, pH=5.9, the periplasm was applied to a HiTrap S
cation exchange column (Amersham-Pharmacia Biotech, Uppsala, Sweden). DegP
eluted at approximately 100 mM NaCI in a linear salt gradient. Peak protein
fractions were adjusted to 0.5 M ammonium sulfate and applied to an HIC butyl
-19-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
column (Amersham-Pharmacia). DegP eluted from the hydrophobic column at
approximately 0.3 M ammonium sulfate. All six bands appearing in the peak
fractions eluting from the butyl column were identified as DegP species by
amino-
terminal sequence anaylsis.
Whole Protein Cleavage Assav
Proteolysis assays were performed in a buffer consisting of 20 mM Tris,
pH=8, at 45°C for 30 minutes to 15 hours. 5 mM MgCh or CaCh was added
to later
assays and to verify dependence on divalent cations, EDTA was added at 10 mM.
Where indicated, the assay was supplemented (200 ,uM) with the non-cleavable
peptide, SPCJ1, having the sequence KSMCMKLSFS, which is derived from the
carboxy-terminus of the PapG adhesin.
Peptide Scanningi (PepSpotsTM)
Overlapping peptide scanning libraries of PapA sequence in a 7-mer and 12-
mer format and shifted by 3 amino acid residues were prepared by the SPOT-
synthesis technique, conjugated to aminobenzoic acid at the amino-terminus,
linked
(carboxyl-terminus) to continuous cellulose membranes (ProteaseSpotsT"") and
assayed by Jerini AG (Berlin, Germany). Purified DegP was prepared as
described
above and assayed (0.28 mg/ml) in a 96-well microplate format using 20 mM
phosphate buffer, pH=7, 5 mM MgCh. At 1, 4, and 28 hours, aliquots were
removed
from the assay plate and liberated fluorescence quantified (excitation - 325
nm,
emission - 420 nm).
Peptide Cleavage Assav
The following three peptides were used in the fluorescence-proximity peptide
cleavage assay:
SPCJ-12 - Abz-HYTAVVKKSSAV-DAP(Dnp)-NHZ (SEQ ID NO: 14)
-20-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
SPCJ-13 - Abz-HYTAVVK-DAP(Dnp)-NH2 (SEQ ID NO: 15)
SPCJ-14 - Abz-HYTASSK-DAP(Dnp)-NH2 (SEQ ID NO: 16)
where Abz, the fluorescent group, is aminobenzoic acid and DAP(Dnp)-NH2, the
quench group, is diaminopropionamide dinitrophenyl. The peptides were used in
the
assay at concentrations ranging from 200 pM to 1 nM. DegP was added into the
assay at concentrations ranging from 0.5 to 5 pg. The assay buffer consisted
of
25mM HEPES, pH=7.5, 5 mM CaCl2. The cleavage reactions were incubated at
45°C and read in a Wallac Victor~V 1420 Multilabel HTS Counter (Wallac
Oy, Turku,
Finland) at 30-minute intervals. Fluorescence detection utilized excitation at
340 nm
and emission was monitored at 420 nm.
Cleavage Site Mappings
Purification of peptide and products of cleavage reaction was performed on a
C18 5p ST 4.6/250 Sephasil column (Amersham-Pharmacia Biotech) with a gradient
to 100% acetonitrile/0.05% trifluoroacetic acid. Lyophilized cleavage
reactions (5
ml) were resuspended in 2% acetonitrile/0.065% trifluoroacetic acid for
application to
the C18 column. Fractions containing peptide and cleavage products were
lyophilized to dryness and mass determined by MALDI TOF Mass Spectroscopy.
MALDI analysis was conducted using 5 mg/ml solution of the matrix ?-cyano-4-
hydroxycinnamic acid (HCCA) in 0.1 % trifluoroacetic acid, 33% acetonitrile.
Analyte
was mixed with the matrix at a ratio of 1:3. Spectra were produced using a
custom-
built MALDI-TOF mass spectrometer.
Other Methods
Proteins were prepared for sequencing by transfer to PVDF membrane as
previously described (Dodson et al., Proc. Natl. Acad. Sci. USA 90:3670-3674
(1993); Slonim et al., EM80 J. 11 (13):4747-4756 (1992)) and delivered to
Midwest
Analytical Inc. (St. Louis, MO) for amino terminal sequence determination. SDS-
-21-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
PAGE and Western blot analysis were performed as previously described (Dodson
et al., 1992; Slonim et al., 1992). Toxicity assays were performed as
previously
described (Jones et al., 1997). The EnzChek Assay Kit (Molecular Probes,
Eugene,
OR) was used according to manufacturer's instructions and read using a Tecan
SpectrFluor Plus (Research Triangle Park, NC) with the appropriate filter
sets.
Results
Construction and Purification of PapA-6H4A
A significant obstacle to the study of chaperone-mediated pilus biogenesis is
the inability to purify pilin subunits in the absence of the periplasmic
chaperone.
This can be overcome, in part, by inactivation of the DegP protease (Bakker et
al.,
Mol. Microbiol. 5(4):875-886 (1991 )). However, pilin subunits from the P
(Pap) pilus
were found to be highly toxic when expressed, absent the chaperone, in KS474
(degP::kan)(Jones et al., 1997). By taking advantage of metal-affinity
chromatography under denaturing conditions and the addition of an affinity tag
to the
amino-terminus of PapA, the pilin subunit was produced and purified. Using the
method of Morrison & Desrosiers (1993), a sequence encoding a polyhistidine
affinity tag (6-his tag) was inserted into the papA gene. The 6-his tag was
positioned immediately downstream of the leader-processing site so that the
leader-
processed protein would contain an exposed amino-terminal 6-his tag for
affinity
purification. The fusion protein was cloned into pMMB66 placing it under
control of
the IPTG inducible Pta~ promoter (Furste et al., 1986). The initial construct,
PapA-
6H, was not appropriately processed or transported to the periplasm (data not
shown). We postulated that the 6-his affinity tag was sterically blocking
either leader
processing or association with the Sec membrane transport machinery. This
block
was circumvented by the addition of two alanine residues, resulting in a total
of four
alanine residues, positioned to separate the 6-his tag from the leader-
processing
site in PapA. PapA-6H4A expressed from this construct, pCL101, was processed
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
appropriately and transported to the periplasm (Figure 1 ). Moreover, PapA-
6H4A
was dependent on an interaction with the periplasmic chaperone, PapD, for
stability
in the periplasm (Figure 1A). As with the wildtype PapA, the PapA-6H4A protein
was toxic when induced in the absence of the PapD chaperone (Jones et al.,
1997).
The toxicity is manifested in the failure of bacteria to grow following
induction of the
pilin subunit (Figure 2). Earlier studies showed that "unchaperoned" subunits
resided in the inner-membrane fraction and formed insoluble aggregates (Jones
et
al., 1997). The toxicity of PapA-6H4A was suppressed by co-expression of papD
(Figure 2A) or degP (Figure 2B) in traps.
In order to purify sufficient PapA for the planned experiments, PapA-6H4A
was synthesized in the presence of the PapD chaperone in KS474
(degP::kan)(Jones et al., 1997). The PapD-PapA complex was purified from whole
periplasm using metal affinity chromatography (MAC) in batch (Figure 1 B). In
order
to separate the chaperone and the PapA-6H4A subunit the bead-bound complex
was denatured with 6.0 M guanidine HCI. Following washes with 10 mM Tris,
pH=8,
plus denaturant to remove the chaperone, pure PapA-6H4A was eluted with 0.1 M
imidazole (Figure 1 B). To verify that the denatured PapA-6H4A was a suitable
binding partner for PapD, the chaperone-subunit complex was reconstituted and
purified on MAC (Figure 1 C). Denatured PapA-6H4A was applied to the metal
affinity resin and washed, although the denaturant was not removed. The bead-
bound protein was then diluted into either PapD-enriched periplasm (data not
shown) or purified PapD (Figure 1 C). The chaperone-subunit complex was washed
and eluted from the affinity resin with 0.1 M imidazole (Figure 1 C, lane 3).
In order to test the denatured PapA-6H4A protein as a substrate for DegP
protease, the resin-bound, denatured PapA-6H4A was diluted into DegP-enriched
periplasm or control periplasm and incubated for 60 minutes at room
temperature.
Bound proteins were eluted with 0.1 M imidazole and analyzed by Coomassie Blue
staining and amino-terminal sequencing of the eluted products (Figure 1 D).
Two
-23-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
novel bands were identified. The ~48 kDa band was identified as the DegP
protease. An ~12kDa band (PapA-NHS), that appears only following treatment
with
DegP-enriched periplasm, was identified as an amino-terminal fragment of PapA.
This data defines an interaction between PapA and DegP that resulted in
cleavage
of PapA.
Purification of Deg~P Protease
DegP was purified from KS272/pKS17 (Strauch et al., 1989) following
overnight (15-20 hours) growth to saturation, which was sufficient to induce
high-
level expression of DegP. Whole periplasm prepared from six liters of culture
was
fractionated by cation exchange chromatography on SP sepharose (HiTrap SP,
Amersham-Pharmacia Biotech)(Figure 3A) followed by hydrophobic interaction
chromatography on butyl sepharose (HiTrap butyl, Amersham-Pharmacia
Biotech)(Figure 3B). This two-step purification process resulted in
approximately
98% pure DegP protease. The "contaminating bands" (small arrows) seen on SDS-
PAGE analysis shown in Figure 3 were identified as DegP truncates by amino-
terminal sequence analysis and presumably result from autocatalytic cleavage
(data
not shown). Incubation of the purified preparations of DegP at 45°C
resulted in
accumulation of the truncate bands (data not shown).
Assay of Protease Activity
A commercially-available protease assay, EnzChek (Molecular Probes,
Eugene, OR) was used to test cleavage activity of the DegP preparations on a
casein substrate. DegP activity on casein was previously described by Lipinska
et
al. (J. Bacteriol. 172:1791-1797 (1990)). Peak cleavage on the EnzChek
substrate
followed the elution profile of DegP through both chromatography steps (data
not
shown). Figure 4 shows a titration of purified DegP protease and trypsin on
the
casein substrate.
-24-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
Soluble PapA Cleavage Assay
Previous studies of DegP cleavage activity indicate that the preferred
substrates were denatured, aggregated, or unfolded proteins (Kim et al., J.
Mol. Biol.
295(5):1363-1374 (1999); Kolmar et al., J. Bacteriol. 178:5925-5929 (1996);
Pallen
and Wren, Mol. Microbiol. 26(2):209-221 (1997)). To provide DegP with a
suitable
substrate for cleavage in a soluble format, PapA-6H4A was denatured with 6 M
guanidine-HCI, reduced and carboxymethylated (PapA-6H4A-rcm) and dialyzed into
20 mM Tris, pH=8.5. PapA-6H4A-rcm was mixed with DegP and incubated at
45°C
overnight and the resulting reaction products resolved on SDS-PAGE and further
visualized by Western blot using antisera prepared against whole Pap pili
(Figure 5).
As seen in Figure 5A lane 4, PapA-6H4A-rcm forms polymers (or aggregates) that
are stable in SDS, in addition to the 21 IcDa monomer. Both forms of PapA-6H4A-
rcm are sensitive to degradation by DegP protease (Figure 5A, lanes 3 and 5).
Previous reports have described several different buffer systems suitable for
monitoring DegP proteolytic activity (Kim et al., 1999; Kolmar et al., 1996;
Lipinska
et al., 1990; Spiess et al., Cell 97:339-347 (1999)). Divalent cation (Mg2+)
was
reported to be required in some systems (Kim et al., 1999). Therefore, we
tested
the effect of MgCl2, MnCl2, and CaCl2 on DegP cleavage of PapA-6H4A-rcm
(Figure
5B and data not shown). Addition of 5 mM MgCl2 (Figure 5B), MnCh, or CaCl2
(data
not shown) stimulated DegP cleavage of PapA-6H4A-rcm resulting in nearly 50%
of
the input protein being degraded in a four-hour incubation (Figure 5B).
Moreover,
addition of EDTA to the cleavage reaction inhibits cleavage activity (Figure
6,
compare lanes 3-5 and 7-10).
Activation of DegIP bar a Carboxyl-Terminal Pilin Subunit Peptide
The DegP protease has two so-called PDZ domains (Post-synaptic density
95, Discs-large, ZO-1 ) located downstream of the catalytic serine residue
(fallen &
Wren, 1997). PDZ domains have been implicated in both substrate recognition
and
-25-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
protein multimerization in a number of proteins (Levchenko et al., Cell 91:939-
947
(1997); Sassoon et al., Mol. Microbiol. 33:583-589 (1999); Songyang et al.,
Science
275:73-77 (1997)). Levchenko et al. (1997) recently demonstrated that the CIpX
chaperone and regulatory subunit of the CIpXP protease machine utilizes PDZ
domains in substrate recognition. The PDZ domains specifically recognize
disordered peptide sequences found at the carboxyl-terminus of target proteins
(Levchenko et al., 1997; Songyang et al., 1997). The carboxyl-terminus of
pilin
subunits is a highly conserved motif important for interaction with
neighboring
subunits in the ultimate structure of the pilus as well as a recognition motif
for the
periplasmic chaperone (Bullitt et al., Proc. Natl. Acad. Sci. USA 93:12890-
12895
(1996); Choudhury et al., Science 285(5430):1061-1066 (1999); Hultgren et
al.,~
Bacterial Adhesins and Their Assembly in Escherichia coli and Salmonella:
Cellular
and Molecular Bioloay, pages 2730-2756, F.C. Niehardt, ed., ASM Press,
Washington, DC (1996); Sauer et al., Science 285(5430):1058-1061 (1999)). The
carboxyl-terminal motif has some homology to the motif reportedly recognized
by
PDZ domains (Levchenko et al., 1997; Songyang et al., 1997). We hypothesized
that titatraion of the carboxyl-terminal peptide from the PapG adherence
protein
(SPCJ-1 - KSMCMKLSFS) (SEQ ID NO: 13) into the DegP cleavage reaction would
inhibit cleavage of the PapA-6H4A-rcm protein through competition for the
substrate
binding site in the PDZ domain. Much to our surprise, addition of SPCJ-1 to
the
cleavage reaction enhanced degradation of the PapA-6H4A-rcm (Figure 6). In the
absence of SPCJ-1 50% cleavage of the PapA protein required a four-hour
digestion
at 45°C (Figure 5B and data not shown), whereas addition of the
activating peptide
resulted in 50% cleavage in 60 minutes and nearly 90% cleavage at four hours
in a
reaction containing four-fold less enzyme and twice as much substrate (Figure
6,
lanes 2-5). As expected, cleavage of PapA-6H4A-rcm was inhibited (7% cleavage
in 1 hour) in the presence of 10 mM EDTA (Figure 6, lanes 7-10).
-26-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
Identification of Deaf Recoanition Sites Within PaaA by Peatide
Scannina
Utilizing SPOT synthesis technology (Jerini AG, Berlin, Germany), two
overlapping peptide scanning libraries of the complete PapA amino acid
sequence
were constructed and assayed to identify 7-mer and 12-mer peptides that were
suitable substrates for DegP proteolysis (Figure 7). The peptides carried an
aminobenzoic acid (Abz) fluorescent tag at the amino terminus and were
synthesized linked to a cellulose membrane. In both the 7-mer and the 12-mer
ProteaseSpotT"" scans, 3 regions of PapA were identified as having a sequence
cleavable by DegP as represented by release of fluorescent counts from the
cellulose bound peptides. Interestingly, the cleavable regions identified by
this
analysis all lie within or proximal to ~i-strands and are in highly conserved
regions of
pilin subunit proteins (see Figure 4 in Soto and Hultgren, J. Bacteriol.
181:1059-
1071 (1999)).
Peptide Cleavage Assay
In order to establish a soluble peptide cleavage assay to monitor DegP
protease activity, the most efficiently cleaved region, represented by the
sequence -
HYTAVVKKSSAV (SEQ ID NO: 3)- was used as a model substrate and a peptide,
SPCJ-12, prepared for use in a fluorescent-quench detection assay. SPCJ-12 was
prepared with an aminobenzoic acid (Abz) group, the fluorescent moiety, on the
amino-terminus and a diaminopropionamide dinitrophenyl (DAP(Dnp)-NH2) group,
the quench moiety, on the carboxyl terminus. As shown in Figure 8A, DegP
cleaved
SPCJ-12 in a time-dependent fashion. To further narrow the recognition
sequence
and define the determinants of cleavage, two additional peptide reagents, SPCJ-
13
and SPCJ-14, were prepared. SPCJ-13 has the sequence HYTAVVK (SEQ ID NO:
4) (the first 7 residues of SPCJ-12), whereas SPCJ-14 has the sequence HYTASSK
(SEQ ID NO: 17). The double serine replacement was utilized to test the
-27-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
essentiality of the paired hydrophobic residues in SPCJ-13. As shown in Figure
8B,
DegP cleaved SPCJ-13 in a time-dependent fashion, however, SPCJ-14 was not
cleaved. In order to gain insight into the failure of DegP to cleave the
double serine
replacement peptide, we set up an inhibition assay to see if SPCJ-14 could
block
cleavage of SPCJ-13. Such a result would suggest that DegP still recognized
SPCJ-14 but could not cleave the peptide. As shown in Figure 8C, when added in
10-fold molar excess, SPCJ-14 blocked cleavage of SPCJ-13. Lastly, addition of
the non-cleavable peptide SPCJ-1, to the peptide cleavage assay enhanced
cleavage of SPCJ-13 (Figure 8D).
In order to map the precise cleavage sites in SPCJ-12 and SPCJ-13, the
reaction products of the cleavage reactions were resolved on Reverse-Phase
HPLC
using a C18 Sephasil column (Figure 9A). Untreated SPCJ-13 had a retention
volume of 53.06 mls (data not shown), whereas after cleavage a single new peak
was seen with a retention volume of 45.72 mls and the 53.06 mls peak was
greatly
diminished (Figure 9A). The mass of the material in the new peak was
determined
by MALDI-TOF Mass Spectroscopy analysis. The new peak contained two species
having masses (480.59 = VK-DAP(Dnp)-NH2; 708.99 = ABZ-HYTAV) consistent with
cleavage between the paired valine residues in the peptide (Figure 9B). As a
confirmation, the cleavage site in SPCJ-12 was also mapped by MALDI MS and
again it was demonstrated that DegP cleaved between the paired valine residues
(data not shown).
Discussion
The degradation and clearance of misfolded and/or misassembled proteins in
the cytoplasm and periplasm is essential for vigorous growth of bacteria
(Gottesman, Ann. Rev. Genet. 30:465-506 (1996); Laskowska et al., Mol.
Microbiol.
22:555-571 (1996); Pallen & Wren, 1997). In the periplasm of E. coli and most
Gram-negative organisms this task falls to the DegP protease (fallen & Wren,
-28-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
1997). Absent a functional copy of degP, bacteria fail to grow at high
temperature
(Lipinska et al., 1990; Pallen & Wren, 1997). Moreover, recent data shows that
in
addition to compromising growth at high temperature, degP null mutants are
more
sensitive to oxidative stress and are avirulent (Boucher et al., J. Bacteriol.
178:511-
523 (1996); Elzer et al., Res. Vet. Sci. 60:48-50 (1996a); Elzer et al.,
Infect. &
Immun. 64:4838-4841 (1996b); Li et al., Infect. & Immun. 64:2088-2094 (1996);
Pallen & Wren, 1997; Pederson et al., Infect. & Immun. 69(4):2569-2579
(2001 );Phillips et al., Res. Vet. Sci. 63:165-167 (1997); Roberts et al.,
Infect. &
Immun. 68(10):6041-6043 (2000); Tacket et al., Infect. & Immun. 65:452-456
(1997);
Tacket et al., Infect. & Immun. 68(3):1196-1201 (2000)). The current model
suggests
that the oxidative response of the host to an invading pathogen results in
oxidative
denaturation of proteins in the periplasm. The inability of a degP mutant
strain to
clear or refold denatured protein places a sufficient burden on the pathogen
resulting in a reduction in virulence in several recently described animal
models of
infection (Boucher et al., 1996; Elzer et al., 1996a; Elzer et al., 1996b; Li
et al., 1996;
Pallen & Wren, 1997; Pederson et al., 2001;Phillips et al., 1997; Roberts et
al.,
2000; Tacket et al., 1997; Tacket et al., 2000).
In keeping with this model, the preferred substrate for DegP appears to be
proteins that are globally or transiently denatured, supporting the hypothesis
that the
role in vivo is to remove misfolded or denatured proteins from the periplasm
(Kolmar
et al., 1996). Kolmar et al. (Kolmar et al., 1996) demonstrated that DegP
would
cleave slow folding mutants of A repressor and Arc repressor and that the
cleavage
site P1 residue was a hyrdophobic residue, most often a valine. These data
support
the hypothesis, since the hydrophobic cleavage site would only be available in
denatured targets. Therefore, the recognition site for DegP substrates would
be
exposed when the proteins went "off pathway" or were denatured due to an
environmental insult. Although an attractive model, this study was hampered by
the
fact that neither protein used is a periplasmic protein. In support of this
finding,
-29-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
however, Laskowska et al. (Laskowska et al., 1996) demonstrated in vitro that
purified DegP protein would degrade thermally aggregated proteins fractionated
from E. coli extracts. Moreover, they showed that the DnaJ chaperone would
antagonize DegP degradation; i.e., the chaperone would aid in refolding the
proteins
such that they were no longer targets for degradation by DegP.
We have clearly demonstrated that the PapA pilin, from the pyelonephritis-
associated pills (Pap) is an in vitro target for DegP. Using an amino-terminal
poly-
histidine affinity tag coupled with denaturing metal affinity chromatography,
sufFicient
PapA-6H4A could be purified away from the periplasmic chaperone, PapD, for
implementation in a soluble cleavage assay (Figure 1 ). DegP protease was
purified
by standard chromatographic means (Figure 3) and showed proteolytic activity
on
both a casein substrate as well as denatured, reduced, and carboxymethylated
PapA-6H4A (Figures 4 and 5). DegP cleavage activity on PapA-6H4A that had not
been reduced and carboxymethylated was minimal by comparison to its activity
on
PapA-6H4A-rcm (data not shown). However, defining the precise "folded state"
of
PapA absent complex formation with PapD or assembly into the pilus is a
difficult
proposition. Recent co-crystallization studies have revealed that pilin
subunits have
an incomplete Immunoglobulin fold due to the absence of a 7t" carboxyl-
terminal
strand needed to complete a ~i-sheet in the native fold (Choudhury et al.,
1999; Sauer
et al., 1999). During pilus biogenesis, this missing strand is provided by
either the
periplasmic chaperone or the neighboring pilin subunit through donor strand
complementation or donor strand exchange, respectively (Choudhury et al.,
1999;
Sauer et al., 1999).
An amino-terminal truncate of the cleaved PapA was identified using the
denatured substrate on the affinity resin as well as in the soluble cleavage
assay,
suggesting that cleavage occurred near the middle of the protein (Figures 1 D
and 6).
To further define the specificity of DegP cleavage and to map the cleavage
sites
within PapA an overlapping peptide scan was utilized (ProteaseSpotT"", Jerini
AG,
-30-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
Berlin, Germany)(Reineke et al., High Throughput Screening Assay for the
Identification of Protease Substrates in Peptides 2000: Proceedings of the
26tn
European Peptide Sy,Lposium, J. Martinet & J.A. Fehrentz, eds. (2000)). Both
scanning libraries (7-mer and 12-mer, Figure 7) identified 3 regions of PapA
that had
enhanced cleavage by DegP in the solid-phase assay. The scanning results were
verified for the carboxyl-terminal most peptide sequence, SPCJ-12
(HYTAVVKKSSAV) (SEQ ID NO: 3) and the shorter version, SPCJ-13 (HYTAVVK)
(SEQ ID NO: 4) in a soluble cleavage assay utilizing a fluorescence-quench
assay
system (Figure 8). The specificity of cleavage of this sequence was further
defined
using the control peptide, SPCJ-14 (HYTASSK) (SEQ ID NO: 17), which challenged
the protease to cleave the peptide absent the paired valine residues. The lack
of
cleavage of SPCJ-14 is in agreement with previously reported studies from the
Sauer laboratory that showed that the P1 residue of model DegP substrates was
most often a valine residue (Kolmar et al., 1996). Of the other sequences
identified
by the peptide scanning, LDIELVNCDITA (SEQ ID NO: 5), ELVNCDI (SEQ ID NO:
6), KLAFTGPIVNGH (SEQ. ID NO: 7), FTGPIVNGHSDE (SEQ ID NO: 8),
TLKDGENVLHYT (SEQ ID NO: 9), DGENVLH (SEQ ID NO: 10), and NVLHYTA
(SEQ ID NO: 11 ) also have paired hydrophobic residues, however, the NGHSDEL
sequence does not follow this pattern. Clearly, peptide structure, in addition
to
sequence, has a role to play in recognition by DegP as can be seem in several
instances in which "neighboring" peptides which shared sequence were not
equivalently cleaved in the solid-phase assay (Figure 7).
The PapD cleavage fragment (--12 kDa) identified in the initial solid-phase
cleavage assay (Figure 1 D) was identified as an amino-terminal fragment of
PapA
by amino-terriiinal sequencing. This fragment again resulted in the solution
phase
assay (Figure 6) and the identity of the cleavage product determined by amino-
terminal sequencing. This cleavage product most likely results from cleavage
at one
-31-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
of the upstream sites (LDIELVNCDITA) (SEQ ID NO: 5), (FTGPIVNGHSDE) (SEQ
ID NO: 8).
In terms of substrate recognition, Sonyang et al. (Songyang et al., 1997)
recently defined a carboxyl-terminal motif in target proteins recognized by
PDZ
domains. Considering this finding, PDZ domains in bacterial proteases may
recognize a sequence that signals a misfolded state of the protein that
differs from
the protease cleavage site. The most striking feature of the motif identified
by
Songyang et al. is a conserved residue (threonine, serine, or tyrosine) at the
-3
position (>60% of sequences identified) and a highly conserved (>80%)
hydrophobic
carboxyl terminal residue (valine, isoleucine, or leucine). Interestingly, an
alignment
of the carboxyl terminus of 44 pilin subunits revealed that the -3 position
was often a
serine, threonine, or tyrosine (~50%)(Soto and Hultgren, 1999). Bacterial
pili, such
as Pap pili, assembled by the chaperone-usher assembly pathway were recently
divided into two familes based on structural and sequence features: the so-
called
FGS and FGL subfamilies (Hung et al., 1996). In the FGL subfamily, the
conservation at the -3 position (serine, threonine, or tyrosine) is greater
than 80%.
Our initial results with SPCJ-1 (KSMCMICLSFS) (SEQ ID NO: 13), which conforms
to
the carboxyl-terminal 10 residues of PapG, indicate that the PDZ domains in
DegP
play a role in substrate recognition through an interaction with the carboxyl-
terminal
sequence. Addition of SPCJ-1 and not an irrelevant peptide (data not shown)
enhanced the cleavage activity of the protease (Figures 5, 6, & 8D). As
described in
detail in two recent papers (Choudhury et al., 1999; Sauer et al., 1999) and
supported by earlier genetic data (Bullitt et al., 1996; Hultgren et al.,
1996; Kuehn et
al., 1993; Slonim et al., 1992; Soto and Hultgren, 1999; Xu et al., 1995) the
carboxyl-terminus of pilin subunits is an integral component of the
recognition site
for chaperone-subunit complex formation. As discussed briefly above, the lack
of
the carboxyl-terminal 7t" strand of the immunoglobulin fold results in a deep
groove
on the surface of the pilin that exposes the hydrophobic core. One edge of
this
-32-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
groove is lined by the carboxyl-terminal ~i-strand of the pilin (the F strand)
(see
(Choudhury et al., 1999; Sauer et al., 1999) for a complete discussion).
During
donor strand complementation, the chaperone donates its G strand to complete
the
immunoglobulin fold of the pilin. The donated G strand completes the
hydrophobic
core of the pilin through interactions with both sides of the groove. Both
genetic
data and molecular modeling support the model that the carboxyl-terminus of
pilin
subunits is part of the pilin-pilin interface that drives pilus assembly
(Bullitt et al.,
1996; Hultgren et al., 1996; Choudhury et al., 1999; Sauer et al., 1999).
Therefore,
this motif would rarely be exposed to the solvent. The exposure of this motif,
in the
event of misfolding or misassembly, would signal the need for either a
chaperone to
catalyze refolding or a protease to degrade the protein.
These studies illustrate the mechanism by which the major periplasmic
protease, DegP, cleaves misfolded pilin subunits and may relate to the general
proteolytic pathway for DegP substrates. DegP's role in the pathogenesis of
both
Gram-negative and Gram-positive pathogens provides the impetus to develop
assays suitable for high-throughput screening and the identification of small
molecule inhibitors of this important virulence target. The described studies
pave
the way for the development of a novel class of anti-infectives developed
against the
DegP protease.
All cited patents and publications referred to in this specification are
herein
incorporated by reference in their entirety.
-33-
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
SEQUENCE LISTING
<110> Jones, C. Hal
Dexter, Paul L.
Evans, Amy K.
Hruby, Dennis E.
<120> DEGP Protease: Cleavage Site Identification and
Proteolysis of a Natural Target in E. Coli
<l30> 016921-169
<150> US 60/330,855
<151> 2001-11-OZ
<160> 17
<170> FastSEQ for Windows Version 4.0
<210> 1
<211> 2
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<221> MOD_RES '
<222> 2
<223> amino acid 2 is attached to a quench group
(DAP(Dnp)-NH2) diaminopropionamide dinitrophenyl
<400> 1
Val Lys
1
<210> 2
<211> 5
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<221> MOD RES
<222> 1
<223> amino acid l is attached to a fluorescent group
(Abz) aminobenzoic,acid
<400> 2
His Tyr Thr Ala Val
1 5'
Page 1
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
<210> 3
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 3
His Tyr Thr Ala Val Val Lys Lys Ser Ser Ala Val
1 5 10
<210> 4
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 4
His Tyr Thr Ala Val Val Lys
1 5
<210> 5
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 5
Leu Asp Ile Glu Leu Val Asn Cys Asp Ile Thr Ala
1 5 10
<210> 6
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 6 .
Glu Leu Val Asn Cys Asp Ile ,
<210> 7 -
<211> 12
<212> PRT
Page 2
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
<213> Artificial Sequence
<220>
<223> synthetic
<400> 7
Lys Leu Ala Phe Thr Gly Pro Ile Val Asn Gly His
1 5 10
<210> 8
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic '
<400> 8
Phe Thr Gly Pro Ile Val Asn Gly His Ser Asp Glu
1 5 10
<210> 9
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 9
Thr Leu Lys Asp Gly Glu Asn Val Leu His Tyr Thr
1 5 10
<210> 10
<211> 7
<212> PRT
<213> Artificial Sequence
<220> ,
<223> synthetic
<400> 10
Asp Gly Glu Asn Val Leu His
1 5
<210> 11
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
Page 3
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
<223> synthetic
<400> 11
Asn Val Leu His Tyr Thr Ala
1 5
<210> 12
<211> 7
<212> PRT
<2l3> Artificial Sequence
<220>
<223> synthetic
<400> 12
Asn Gly His Ser Asp Glu Leu
1 5
<210> 13
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 13
Lys Ser Met Cys Met Lys Leu Ser Phe Ser
10
<210> 14
<211> 12
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<221> MOD RES
<222> 1 -
<223> amino acid 1 is attached to a fluorescent group
(Abz) aminobenzoic acid
<221> MOD_RES
<222> 12
<223> amino acid 12 is attached to a quench group
v(DAP(Dnp)-NH2) diaminopropionamide dinitrophenyl
<400> 14
His Tyr Thr Ala Val Val Lys Lys Ser Ser Ala Val
1 5 l0
Page 4
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
<210> 15
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<221> MOD_RES
<222> 1
<223> amino acid 1 is attached to a fluorescent group
(Abz) aminobenzoic acid
<221> MOD_RES
<222> 7
<223> amino acid 7 is attached to a quench group
(DAP(Dnp)-NH2) diaminopropionamide dinitrophenyl
<400> 15
His Tyr Thr Ala Val Val Lys
1 5
<210> 16
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<221> MOD_RES
<222> 1 '
<223> amino acid 1 is attached to a fluorescent group
(Abz) aminobenzoic acid
<221> MOD_RES
<222> 7 '
<223> amino acid 7 is attached to a quench group
(DAP(Dnp)-NH2) diaminopropionamide dinitrophenyl
<400> 16
His Tyr Thr Ala Ser Ser Lys
1 5
<210> 17
<211> 7
<212> PRT
<213> Artificial Sequence
<220>
<223> synthetic
<400> 17
Page 5
CA 02465265 2004-04-29
WO 03/064448 PCT/US02/35009
016921-169.ST25
His Tyr Thr Ala Ser Ser Lys
1 5
Page 6