Note: Descriptions are shown in the official language in which they were submitted.
CA 02465698 2004-05-14
EXTENDED RELEASE FORMULATIONS
OF ERYTHROMYCIN DERIVATIVES
Technical Field
The present invention relates to pharmaceutical compositions of
erythromycin derivatives with an extended release of an active compound in
the gastrointestinal environment. More particularly, it relates to
pharmaceutical
compositions of clarithromycin which are ingested daily as a single oral
administration.
This Application is a Divisional of Canadian Patent Application Serial
Number 2,325,541, filed March 6, 1998.
Background Of The Invention
Erythromycin and its derivatives are known for their antibacterial
activity against a number of organisms or activity in a number of indications
and are typically administered as immediate release (IR) compositions, two or
three times a day, for a regimen of 10 to 14 days. These compounds have a
bitter taste. In particular, the 6-O-methoxyerythromycin A (clarithromycin)
has
a bitter metallic taste which can result in poor compliance of the regimen or
selection of another, possibly less effective, therapeutic agent.
One approach to improve the possible non-compliance with the regimen
has been to develop controlled release solid preparations containing these
erythromycin derivatives in an alginate matrix comprising a water-soluble
alginate and a complex salt of alginic acid, having one cation that yields a
soluble alginate salt and another cation that alone yields an insoluble
alginate
salt. These formulations are described in U. S. Patent 4,842,866, issued June
27, 1989. However, in-vivo animal studies showed that reproducibly
bioavailable controlled release formulation were not possible using alginates
or
any other monolithic hydrogel tablets.
CA 02465698 2004-05-14
To overcome some of the problems associated with the formulations
described in U.S. Patent 4,842,866, improved controlled release formulations
for poorly soluble basic drugs such as erythromycin derivatives including
clarithromycin, have been developed and are described in Canadian Patent
Application Serial No. 2,202,714, filed November 1 S, 1996. The formulations
described in the patent application comprise a poorly soluble basic drug and
citric acid in an alginate matrix. The formulations are administered once a
day
and are directed towards increasing the bioavailability of the active
ingredient
so that it is bioequivalent with the current immediate release, twice-a-day
compositions. However, these controlled release compositions do not purport
to minimize the adverse effects related to gastrointestinal (GI) disorders
including nausea and vomiting and a phenomenon described as taste
perversion.
One approach to address taste perversion has been to develop acceptable
palatable liquid oral dosage forms of these drugs as described in U. S. Patent
4,808,411, issued February 28, 1989. However, these formulations are
administered twice-a-day for a period of 10 to 14 days and do not address the
frequency and duration of the administration regimen, or the adverse effects
related to GI disorders. Therefore, there still exists a need for developing a
pharmaceutical composition which minimizes the adverse effects described
above and provides a degree of drug plasma concentration control which is
equivalent to or better than the (IR) tablet or liquid formulations currently
used.
Summary Of The Invention
In accordance with the present invention there is provided an extended
release pharmaceutical composition comprising an erythromycin derivative and
a pharmaceutically acceptable polymer, the composition having an improved
CA 02465698 2004-05-14
taste profile as compared to an immediate release composition of the
erythromycin
derivative.
It has been discovered that the extended release (ER) formulations of the
present invention and described herein and which comprise a pharmaceutically
acceptable polymer, provide extended release clarithromycin in vivo when given
once
daily. Maximum concentrations (Cmax) of clarithromycin in plasma are
statistically
significantly lower than the IR formulation given twice daily, and area under
the
plasma concentration-time curve (AUC) and the minimum plasma concentration are
maintained over 24 hours. In contrast, for the controlled release formulations
described in the aforementioned Canadian Patent Application, Serial No.
2,202,714,
filed November 25, 1996, the CmaX values are not statistically significantly
different
from those for the IR formulation. And while the AUCO-24 is maintained, the
Cm~~ is
statistically significantly lower for the controlled-release formulations
relative to the
IR formulation.
The compositions of the invention have surprisingly a two- to three-fold
reduction in incidence rates for taste perversion compared to the IR
formulation.
The present disclosure also describes a pharmaceutical composition for
extended release of an erythromycin derivative in the gastrointestinal
environment,
comprising an erythromycin derivative and a pharmaceutically acceptable
polymer, so
that when ingested orally, the composition induces statistically significantly
lower
mean fluctuation index in the plasma than an immediate release composition of
the
erythromycin derivative while maintaining bioavailability substantially
equivalent to
that of the immediate release composition of the erythromycin derivative.
The present disclosure also describes a pharmaceutical composition for
extended release of an erythromycin derivative in the gastrointestinal
environment,
comprising an erythromycin derivative and a pharmaceutically
-3-
CA 02465698 2004-05-14
acceptable polymer, so that upon oral ingestion, maximum peak concentrations
of the erythromycin derivative are statistically significantly lower than
those
produced by an immediate release pharmaceutical composition, and an area
under the concentration-time curve and the minimum plasma concentration are
substantially equivalent to that of the immediate release pharmaceutical
composition.
The present disclosure also describes a method of using an extended
release, pharmaceutical composition comprising an erythromycin derivative
and a pharmaceutically acceptable polymer, comprising administering the
composition in an effective amount for the treatment of bacterial infection in
a
mammal, whereby an area under the concentration-time curve equivalent to
that for an immediate release pharmaceutical composition of the erythromycin
derivative is maintained.
Brief Description Of The Drawings
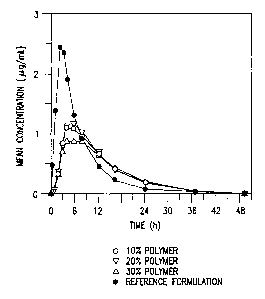
Figure 1 illustrates the mean in vivo plasma concentration-time profiles
following single dose of three S00 mg ER tablets containing clarithromycin and
10%, 20% or 30%, respectively, by weight of hydroxypropylmethylcellulose K
100 LV, as compared to that of the reference 500 mg IR clarithromycin tablet.
Figure 2 illustrates the mean in vivo plasma concentration-time profiles
following multiple doses of each of the two ER tablets containing 10% or 20%,
respectively, of hydroxypropylmethyl cellulose K 100 LV as compared to the
reference IR tablet. The dosage forms included two 500 mg ER tablets given
once daily or one IR 500 mg clarithromycin every 12 hours, respectively,
administered for three days with food.
Figure 3 illustrates the mean in vivo plasma concentration-time profiles
following multiple doses of clarithromycin one-daily 1000 mg (not an example
of the invention) and IR 500 mg twice-a-day.
CA 02465698 2004-05-14
Detailed Descr~tion Of The Invention
"500 mg or 1000 mg" as used herein, means the strength of tablet
composition containing 500 mg darithromycin, or the dose administered as
2x500 mg of clarithromycin, respectively.
5 "C~,aX" as used herein, means maximum plasma concentration of the
erythromycin derivative, produced by the ingestion of the composition of the
invention or the IR comparator.
"C,~,;n" as used herein, means minimum plasma concentration of the
erythromycin derivative, produced by the ingestion of the composition of the
to invention or the IR comparator.
"Cav~~~ as used herein, means the average concentration within the 24-
hour interval.
"T~x" as used herein, means time to the maximum observed plasma
concentration.
~5 "AUC" as used herein, means area under the plasma concentration-time
curve, as calculated by the trapezoidal rule over the complete 24-hour
interval
for all the formulations.
"Degree of Fluctuation (DFL)" as used herein, is expressed as:
DFL=(C~-Cmin)/Cavg.
20 "Erythromycin derivative" as used herein, means erythromycin having
no substituent groups, or having conventional substituent groups, in organic
synthesis, in place of a hydrogen atom of the hydroxy groups and/or a methyl
group of the 3'-dimethylamino group, which is prepared according to the
conventional manner.
25 "Pharmaceutically acceptable" as used herein, means those compounds
which are, within the scope of sound medical judgment, suitable for use in
contact with the tissues of humans and lower animals without undue toxicity,
irritation, allergic response, and the like, in keeping with a reasonable
benefit/risk ratio, and effective for their intended use in the chemotherapy
and
3o prophylaxis of antimicrobial infections.
"Adverse effects" as used herein, means those physiological effects to
various systems in the body such as cardiovascular systems, nervous system,
digestive system, and body as a whole, which cause pain and discomfort to the
individual subject.
35 "Taste perversion" as used herein, means the perception of a bitter
metallic taste normally associated with the erythromycin derivatives,
particularly, with clarithromycin.
- 5 -
CA 02465698 2004-05-14
The pllam~aceutical compositions of the invention described herein comprise a
pharmaceutically active compound and a pharmaceutically acceptable polymer.
The
pharmaceutically active compound is an erythromycin derivative. Preferably,
the
erythromycin derivative is 6-O-methoxy erythromycin A, known as
clarithromycin.
The amount of the erythromycin derivative varies, from about 45% to about 60%
by
weight of the composition. Preferably, the composition comprises about 50% by
weight of the erythromycin derivative.
The pharmaceutically acceptable polymer may be a water-soluble hydrophilic
polymer selected from the group consisting of polyvinylpyrrolidine,
hydroxypropyl
cellulose, hydroxypropylmethyl cellulose, methyl cellulose, vinyl acetate/
crotonic
acid copolymers, methacrylic acid copolymers, malefic anhydride/methyl vinyl
ether
copolymers and derivatives and mixtures thereof. Preferably, the polymer is
selected
from hydroxypropyl cellulose, hydroxypropylmethyl cellulose, and methyl
cellulose.
More preferably, the polymer is hydroxypropylmethyl cellulose. Most
preferably, the
polymer is a low viscosity hydroxypropyl-methyl cellulose with viscosity
ranging
from about 50 cps to about 200 cps. The most preferred low viscosity polymer
is a
hydroxypropylmethyl cellulose with a viscosity of about 100 cps, commercially
available under the Trade-mark Methocel K 100 LV from The Dow Chemical
Company.
-6-
CA 02465698 2004-05-14
The amount of the polymer in the composition generally varies from about S%
to about SO% by weight of the composition. Preferably, the amount of polymers
varies from about 10% to about 3S% by weight of the composition. Most
preferably,
the amount of polymer varies from about 10% to about 30% by weight of the
composition.
The composition of the invention may further comprise pharmaceutically
acceptable excipients and/or fillers and extenders, such as lactose, starches,
glucose,
sucrose, mannitol, and silicic acid, lubricants such as talc, calcium
stearate,
magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, and
mixtures
thereof.
The amount of the lubricants generally varies from about O.S% to about 10%
by weight of the composition. Preferably, the lubricants used are magnesium
stearate
and talc in the total amounts ranging from about 1.0% to about 4.0% by weight
of the
composition. The amount of fillers and extenders varies from about 10% to
about
40% by weight of the composition.
A particularly preferred composition for the extended release of the active
compound therefrom comprises:
CA 02465698 2004-05-14
about 500 mg of clarithromycin; and
from 100 to 300 mg of Methocel K 100 LV.
The formulations are generally prepared by dry blending the polymer,
filler, erythromycin derivative, and other excipients followed by granulating
5 the mixture using water until proper granulation is obtained. The
granulation
is done by methods known in the art. The wet granules are dried in a fluid bed
dryer, sifted and ground to appropriate size. Lubricating agents are mixed
with
the dried granulation to obtain the final formulation.
The compositions of the invention can be administered orally in the
i0 form of tablets, pills, or suspensions. The tablets can be prepared by
techniques
known in the art and contain a therapeutically useful amount of erythromycin
derivative and such excipients as are necessary to form the tablet by such
techniques. Tablets and pills can additionally be prepared with enteric
coatings
and other release-controlling coatings for the purpose of light protection,
and
~5 swallowability. The coating may be colored with a pharmaceutically accepted
dye. The amount of dye and other excipients in the coating liquid may vary
and will not impact the performance of the extended release tablets. The
coating liquid generally comprises film forming polymers such as hydroxy-
propyl cellulose, hydroxypropylmethyl cellulose, cellulose ester or ether, an
20 acrylic polymer or a mixture of polymers. The coating solution is generally
an
aqueous solution further comprising propylene glycol, sorbitan monoleate,
sorbic acid, fillers such as titanium dioxide, a pharmaceutically acceptable
dye.
Liquid dosage forms for oral administration may include
pharmaceutically acceptable emulsions, microemulsions, solutions,
25 suspensions, syrups and elixirs containing inert diluents commonly used in
the art such as water. Such compositions may also comprise adjuvants, such as
wetting agents; emulsifying and suspending agents; and sweetening, flavoring
and perfuming agents.
The daily dose of the composition of this invention administered to a
30 host in single dose can be in the amounts from 500 mg to 1000 mg once-a-day
for five to fourteen days.
Pharmacokinetic Study
The bioavailability study for the formulations of the invention can be
35 done by administering the ER formulation in a tablet form to healthy
subjects
and measuring the levels of erythromycin derivative in the plasma at different
time intervals over a period of twenty four hours.
_ g _
CA 02465698 2004-05-14
Plasma samples are assayed for erythromycin derivative at BAS
Analytics (West Lafayette, Indiana) using a validated high-performance liquid
chromatographic procedure similar to that described in the literature. See for
example, Chu S-Y, et aL, "Simultaneous determination of clarithromycin and
14(R)-hydroxyclarithromycin in plasma and urine using high-performance
liquid chromatography with electrochemical detection", J. Chromatog., 571, pp
199-208 (1991).
Adverse Effects and Taste Profile
Adverse effects including those related to the digestive system, nervous
system, respiratory system and special senses, including taste perversion, are
measured by dosing subjects with multiple doses of 1000 mg of ER and IR
tablets per day, respectively. The adverse effects are monitored, reported
spontaneously by subjects and recorded on case report forms for the skudy
database.
The invention will be understood more clearly from the following
Examples, which are given solely by way of illustration and serve to provide a
clear understanding of the invention and to illustrate its different
embodiments as well as its various advantages.
Examples
Example 1
Preparation of Formulation
MethocelTM (K 100 LV) available from The Dow Chemical Company was
loaded into a mixer, and dry blended with clarithromycin. The mixture was
granulated using water until proper granulation was obtained. The
granulation was then dried, sifted and ground to appropriate size.
Talc and magnesium stearate were screened and blended with dry
3o granulation. The granulation was then loaded into hopper and compressed
into tablets. The tablets were then coated with an aqueous coating.
Three different formulations A, B, and C were prepared according to the
general method described above. The compositions of three different tablet
formulations are given below in Table 1.
_ g _
CA 02465698 2004-05-14
Table 1
Ingredient A B C
mg/tabletmg/tablet mg/tablet
Water (USP, urified)Q.S. Q.S. Q.S.
Clarithromycin 500.00 500.00 500.00
Methocel K 100 LV 200.00 100.00 300.00
Premium
CR Grade*
Lactose, monohydrate260.00 360.00 160.00
Talc, USP 30.00 30.00 30.00
Magnesium Stearate 10.00 10.00 10.00
'Available from The Uow Chemical Company
Example 2
Pharmacokinetic Study of the Extended Release Formulation
The bioavailability study to determine the concentration-time plasma profile
was done on healthy subjects. The study was conducted as a Phase I, single-
dose,
open, randomized, four-period, balanced cross-over study described below.
Single-Dose Study
Twenty-four (24) healthy adult subjects were enrolled and 23 completed all
phases of the study. For the 23 subjects who completed all phases of the study
(12
males, 11 females), the mean age was 29 years (range: 19 to 49 years), the
mean
weight was 69.0 kg (range: 51.5 to 85 kg) and the mean height was 172 cm
(range:
157 to 192 cm).
Clarithromycin 500 mg extended release tablets corresponding to the
formulations A, B, and C of Example 1 and the 500 mg IR clarithromycin tablet
(Reference Formulation), currently sold by Abbott Laboratories under the Trade-
mark
BIAXINTM, were administered to the 23 healthy subjects.
The study was conducted according to a single-dose, open-label, randomized
four-period crossover design in which each subject received a single 500 mg
dose of
clarithromycin during each 30 minute period after starting breakfast. Wash-out
periods of one week separated the doses.
-10-
CA 02465698 2004-05-14
Seven (7) ml blood samples were collected prior to dosing (0 hour) and at 0.5,
1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, 16.0, 24.0, 36.0 and 48.0 hours after each
dose.
Plasma samples were assayed for clarithromycin at BAS Analytics (West
Lafayette,
Indiana) using a validated high performance liquid chromatographic procedure.
Phannacokinetic Anal
Values for clarithromycin pharmacokinetic parameters, including observed
C",~x, T",~X, and AUCo.~, were calculated using standard noncompartmental
methods.
The mean plasma concentration-time profiles for the single-dose study are
illustrated in Fig. 1.
Fig. I illustrates that all the three formulations of the invention are
substantially equivalent in extended release of clarithromycin over a period
of 24
hours.
Table II summarizes the pharmacokinetic results obtained after single-dosing
in the above study.
Table II
Formulation Cmax (pg~ml) Tmax (h) AUCp.
(lt ~h/mL)
A 1.19t0.60* S.Of 1.7* 15.0~6.5*
B 1.33 t 0.70*# 5.5 f 2.4* 15.1 f6.5*
C 1.01 f 0.48* 5.5 ~ 2.2* 14.8 f7.5*
Reference 2.57 t 0.70 2.2 ~ 0.5 17.7 t 5.6
Tablet
* Statistically significantly different from the IR reference tablet
# Statistically significantly different from Formulations A and C in analysis
of logarithms
-11-
CA 02465698 2004-05-14
statistical Analyses
For Cn,ax, AUCp_~, Tr"ax, and the logarithms of Cr,,ax. and AUCp..~~ an
analysis of variance (ANOVA) was performed with sequence, subject nested
within sequence, period and formulation as the sources of variation. Effects
for
subjects were random and all other effects were fixed. Within the framework
of ANOVA, the formulations were compared pairwise, with each test at a
significance level of 0.05. Also within the framework of the ANOVA for the
logarithm of AUCp_~, bioequivalence of the ER formulations to the IR
reference formulation was assessed using the two one-sided tests procedure via
90% confidence intervals. The confidence intervals were obtained by
exponetiating the endpoints of the confidence intervals for the difference of
logarithm means.
Point estimates of relative bioavailability and 90% confidence intervals
for the two one-sided tests procedure from analysis of log-transformed AUCp_
are set forth in Table III below.
Table III
Relative Bioavailability
Formulation ComparisonPoint Estimate 90% Confidence
Interval
A vs Reference 0.815 0.737 - 0.902
B vs Reference 0.835 0.755 - 0.925
C vs Reference 0.787 0.711 - 0.871
The AUCp_~ central values were lower for the three ER formulations
than for the reference IR tablet. The lower Cmax values and the later Tn.,ax
values suggest that all the ER formulations with varying weight percent of
polymer, provide extended-release of clarithromycin in vivo.
2o The lower AUCp-~ values for the ER formulations may suggest that for
a single 500 mg dose administered under nonfasting conditions, the extent of
absorption of ciarithromycin was reduced relative ko that of the reference IR
tablet.
_ ,
CA 02465698 2004-05-14
Multiple-Dose Study
Twenty-four (24) healthy adult subjects were enrolled and 23 completed
all phases of the study. Of the 23 who completed the study (19 males, 4
females), the mean age was 30 years (range: 20 to 47 years), the mean weight
5 was 72 kg (range: 51 to 87 kg) and the mean height was 176 cm (range: 159 to
189.5 cm).
The clarithromycin dosage forms included 500 mg ER tablets of Example
1 containing 10% or 20% by weight of K 100 LV, respectively, and a reference
500 mg IR tablet (BIAXIN).
to The study was conducted according to a single- and multiple-dose, open-
label, randomized three-period crossover design.
Regimen A
A single 1000 mg dose of ER formulation A tablets (two 500 mg tablets)
15 was administered in the morning on Day 1. Beginning on Day 3, a multiple
dose regimen of 1000 mg clarithromycin (two 500 mg tablets) was administered
each morning for three days (Days 3-5).
Regimen B
2o A single 1000 mg dose of ER formulation B tablets (two 500 mg tablets)
was administered in the morning on Day 1. Beginning on Day 3, a multiple
dose regimen of 1000 mg clarithromycin (two 500 mg tablets) was administered
each morning for three days (Days 3-5).
z5 Regimen C
A single 500 mg dose of IR tablet (BIAXIN) was administered in the
morning on Day 1. Beginning on Day 3, a multiple dose regimen of 500 mg
reference tablet BIAXIN was administered every twelve hours for three days.
Each morning dose was administered thirty minutes after breakfast.
3o Every evening dose was administered thirty minutes after starting the
evening
snack.
Wash-out periods of at least one week separated the last dose in a period
and the first dose in the following period.
Seven (7) ml blood samples were collected before dosing on Day 1 (0 hr)
35 and at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, 16.0, 24.0, 36.0, and 48.0
hour after dosing.
For Regimen C, the 12 hour sample was collected within 5 minutes before the
evening dose on Day 5. Plasma harvested from each blood sample was divided
CA 02465698 2004-05-14
into two parts: approximately 5 mL for bioassay and the remainder of the
sample for high performance liquid chromatographic (HPLC) assay. Plasma
samples were assayed for clarithromycin at BAS Analytics (West Lafayette,
Indiana) using a validated high performance liquid chromatographic
procedure.
Pharmacokinetic Analyses
Pharmacokinetic parameter estimates were calculated using
noncompartmental methods. For the Day 1 data, the parameters estimated
included Cmax, Tmax, AUCp_o, or AUCp~g, and tl~z. For the Day 5 data, the
parameters estimated included Cmax. Tmax, Cmin. AUCo-24, and DFL.
Statistical Analyses
No statistical analyses were performed on the bioassay data. Analyses of
i5 variance (ANOVA) were performed for Day 1 and Day 5 pharmacokinetic
variables with effects for regimen, period, sequence, and subject nested
within
sequence. The Cmax and AUCp_,o values for Regimen C were normalized to a
1000 mg dose. For the Day 1 and Day 5 AUC and C,.r,axvalues and for the Day 5
DFL values for both analytes, logarithmic transformation was employed. Each
of the Regimens A and B were compared to the reference Regimen C at a
significance level of 0.05. Within the framework of the ANOVAs for the Day 5
AUC values, equivalence of the ER formulations of the invention to the IR
reference tablet was assessed using the two one-sided tests procedure via 90%
confidence intervals.
The mean plasma concentration-time profiles for the multiple-dose
study are illustrated in Fig. 2.
Table IV summarizes (mean ~ SD) of the Day 5 pharmacokinetic
parameter estimates for the clarithromycin in the ER and IR formulations.
1A _
CA 02465698 2004-05-14
Table IV
FormulationCmax Cmin Tmax AUC~24 Fluctuation
t~g/ml) (Itg/ml)(h) tL.ig~hlmLOIndex
p 2.4510.69"0.700.378.614.439.612.8 1.110.31"t
2.66 0.67 6.91 40.2 1.24 0.37"
0.87" t 0.39 3.3" 13.8
IR 3.210.78 0.780.291.910.640.811.8 1.470.26
Reference
Statistically significantly different from the reference IR formulation.
t Statistically significantly different from Regimen B.
Point estimates of the relative bioavailability and 90% confidence
intervals for the two one-sided tests procedures of Day 5 AUCo-24 are set
forth
in Table V below. The results presented are for logarithmic-transformed
clarithromycin AUC~24 values.
Table V
Relative Bioavailability
Formulation ComparisonPoint Estimate 90% Confidence Interval
A vs Reference 0.964 0.893 - 1.039
B vs Reference 0.970 0.899 - 1.046
to For this multiple dose study under nonfasting conditions, both the 10%
and 20% polymer ER formulations were bioequivalent to the reference IR
tablet with respect to the AUCo-24. The significantly lower Cmax central
values
and later Tmax values suggest that both the formulations provide extended
release of clarithromycin in vivo. The significantly lower DFLs indicate that
t5 plasma concentrations fluctuate less for the ER tablet regimens than for
the IR
tablet regimen. Additionally, the significantly lower DFL for Regimen A
compared to Regimen B indicates that plasma concentrations from the 20%
polymer fluctuate less than those from the 10% polymer tablet.
S -
CA 02465698 2004-05-14
Adverse Effects
The adverse effects, including taste perversion (taste profile), were
studied for the multiple-dose regimes described above.
Multiple-Dose Studv
The formulations A and B of Example 1 (500 mg tablets) and the IR
BIAXIN (reference) 500 mg tablet were administered to healthy subjects in a
multiple-dose regimen as described above.
lu Formulations Of The Invention
A single dose (2 X 500 mg) of the formulations A and 8 of Example 1,
was administered to the subjects, followed by a 48 hour wash-out period.
Multiple dosing in the morning with the 2 X 500 mg regimen, once-a-day,
followed the washout for the next three days.
Reference
A single dose of 500 mg IR BIAXIN tablet was administered to the
subjects, followed by a 48 hour wash-out period. Multiple dosing with the 500
mg tablet, twice-a-day followed the washout for three days.
2o The adverse effects to the body as a whole, cardiovascular system,
digestive system, nervous system, respiratory system, skin and appendages, and
special senses were measured by monitoring the subjects at regular time
intervals. Subjects who reported the same COSTART term more than once
were counted only once for that COSTART term.
The results of the adverse effects are set forth in Table VI below.
CA 02465698 2004-05-14
Table VI
DOSING REGIMEN
BODY SYSTEM A ~ Reference
COSTART TERM (Nm 24) ~m ~) ~m ~)
Percent of
Total Subjects
Overall 9 (37.5%) 10 (43.5%) 11 (47.8%)
Body As A Whole6 (25.096) 3 (13.0%) 1 (4.30)
Asthenia 2 (8.3%) 1 (4.3%) 0 (0.0%)
Chills 0 (0.0%1 1 (4.3%a) 0 (0.0%)
Headache 2 (8.3%) 2 (8.76) 0 (0.0~)
Neck Rigidity 1 (4.2%) 0 (0.0%) 0 (0.0%)
Pain 2 (8.3%) 0 (0.0!0) 1 (4.3%)
Cardiovascular 1 (4.296) 0 (0.0%) 0 (0.0%)
System
Hypertension 1 (4.2%) U (0.0%) 0 (U.0%)
Digestive System4 (16.7%) 4 (17.4%) 4 (1.~.4%)
~
Abdominal Pain 1 (4.2Jo) 0 (0.0%) 0 (0.0%)
~
Constipation 0 (0.0%) 0 (0.0ro) 2 (8.7~)
Diarrhea 2 (8.3ro) 3 (13.0%) 7 (4.396)
Dyspepsia 2 (8.396? 2 (8.7%) 1 (4.3%)
Flatulence 0 (0.096) 1 (4.3k) 0 (0.0~)
Nausea 0 (0.06) 0 (0.0%) 1 (4.3%)
Nervous System 0 (0.0h) 1 (4.3%a) 2 (8.7k)
Depersonalization0 (0.0~O) 1 (4.3%) 0 (0.096)
Hypesthesia 0 (0.0%) 1 (4.3%) 1 (4.3%)
lnsonvtia 0 (0.096) 7 (4.3k) 0 (0.06)
Somnolence 0 (0.0~) 0 (0Ø6) 1 (4.396)
Respiratory 1 (4.296) 1 (4.3.6) 3 (13.096)
System
Cough Increased1 (4.2%) 0 (0.06) 0 (0.096)
Hiccup 0 (0Ø6) 0 (0.0%) 1 (4.396)
Pharyngitis 0 (0.0~) 1 (4.3%) 2 (8.7h)
Rhinitis 1 (4.2.6) 1 (4.3~) 0 (0.096)
_ » _
CA 02465698 2004-05-14
Table VI Continued
DOSWG REGIMEN
BODY SYSTEM A 8 Reference
I
COSTART TERM W m 24) (Nm 23) (Nm 23)
Percent of'1'otal
Subjects
Skin and Appendages0 (0.0%) 2 (8.7~) 2 (8.7%)
Rash 0 (0.0%) 1 (4.3~) 1 (4.3%)
Skin Disorder 0 (0.0%) 1 (4.3%) 2 (8.?%)
Special Senses 3 (12.5%) 3 (73.0do) 6 (26.1%a)
Eye Disorder 0 (0.0%) 1 (4.3%) 0 (0.0%)
Taste Perversion3 (12.5%) 2 (8.7%) 6 (26.1%)
It is evident from the above Table VI that the adverse effects to the
digestive, nervous and respiratory systems normally associated with BIAXIrI
are reduced with the ER tablets. The taste perversion with the formulations of
the invention is significantly reduced. It is reasonably believed that the
reduced adverse effects, particularly taste perversion, would lead to better
compliance and a higher incidence of completion of the prescribed treatment
regimen.
Comparative Example 3
The results of a corriparative pharmacokinetic study of the controlled
release formulation A of the aforementioned Canadian Patent Application,
Serial No. 2,202,714, filed November 25, 1996, as compared with the IR
(BIAXIN) are set forth in Table VII below.
- 18 -
CA 02465698 2004-05-14
Table VII
Clarithromycin Clarithromycin 909b Confidence
500 Interval
PK-Parameter 1000 mg Point
mg BID
Once-Daily Reference Estimator
(Formulation (BIAX1N)
A)
Unit Mean'S.D.b Mean'S.D
b
AUCp.24(ltg'h/ml)27.29810.08628.25610.77097.4 86.9-109-2
Cmax (~tglml)2.4320.905 2.7010.785 89.0 78.2 -
1013
Trnax ( h ) 5.2171.858 2.0430.706
~
Cue, (ttg/mp 0.4690.292 0597 0.241 71.7 60.0 -
85.7
DFL 1.8000.572 1.9000.616
a arithmetic means
b standard deviation
c defined as the ratio of the geometric means of test vs. reference
formulation
The mean DFL values for the controlled release formulation and for the
IR are substantially equal in value as can be seen in the above Table. c.f.
1.800 ~
0.572 (for controlled release) with 1.900 ~ 0.616 (IR).
5 The mean DFL for the composition of the invention is statistically lower
than the IR in vivo profile. The lower DFL indicates that the ER formulations
of the invention provide less variable clarithromycin concentrations
throughout the day than the IR and the sustained release compositions.