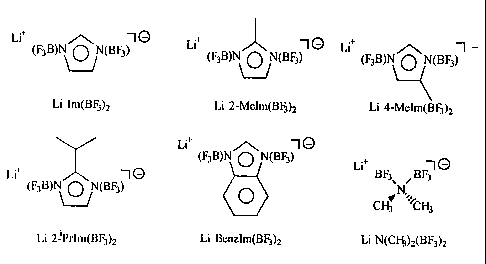Note: Descriptions are shown in the official language in which they were submitted.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
1
NON-AQUEOUS ELECTROLYTES FOR LITHIUM ELECTROCHEMICAL CELLS
Field of the Invention
This invention relates to non-aqueous electric current producing
electrochemical
cells in general and more particularly to both primary and secondary lithium
cells employing
non-aqueous electrolytes containing a new class of lithium salts which are
highly ionically
conductive and which exhibit good thermal stability.
Background of the Invention
One attractive class of modern high energy density rechargeable cells is the
Lithium-ion (Li-ion) cell. The principle components of a Li-ion cell are
graphitic carbon
anode, for example, natural or artificial graphite, a typical example being
mesocarbon
microbead (MCMB) carbon, a lithiated transition metal oxide cathode such as
LiCoOz, and a
highly conductive electrolyte solution. The electrolyte provides mobility to
the Li ions,
which are transported from the anode to the cathode, and vice versa, during
discharge and
charge of the battery. The electrolyte in a Li-ion cell is composed of a
lithium salt that is
dissolved in a nonaqueous solvent such as an organic carbonate(s). To a large
extent, the salt
used in the electrolyte of the cell governs the overall performance of the
cell and the salt must
~ 0 therefore meet certain requirements. In terms of performance, a salt must
have high
conductivity, high thermal stability, and electrochemical stability above the
potential of the
fully charged cell (4.1 V vs. Li in cells employing carbon anode materials),
and be nontoxic
and safe.
Unfortunately, no salts adequately meet all the cost, performance, and safety
2 5 requirements imposed by the industry. The most common salt in use today is
LiPF6, which is
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
2
added to organic carbonate solvent mixtures to form the electrolyte solution.
This salt has
excellent conductivity and electrochemical stability in these solvents but is
expensive. In
addition, this salt is limited to an operational temperature range of -
40°C to +50°C. The
LiPF6 is thermally unstable and is believed to decompose at temperatures above
60°C
according Equation 1 below.
(Equation 1) LiPF6 ~ LiF+PF
5
In addition, both LiPF6 and PFS are susceptible to hydrolysis and, as a
result, they will react with
any moisture in the electrolyte according to Equations 2 and 3 to form HF.
(Equation 2) LiPF6 + HZO ~ pOF3 + 2 HF + LiF
(Equation 3) PFS + H20 ~ pOF3 + 2 HF
The HF and PFS can catalyze the decomposition of the solvents, react with the
electrodes
to increase the electrode/electrolyte interfacial impedance, and corrode the
current collectors. Other
lithium salts based on perfluorinated inorganic anions with the general
formula LiMFX, have been
extensively studied. The order of conductivity of these salts is LiSbFg >
LiAsF6~ LiPFg > LiBF4.
15However, each of these salts has either poor electrochemical stability
(LiSbF6), toxicity (L,iAsF6), or
poor cycling e~ciency (LiBF4).
The recent development of several organic anions, some of which have high
conductivities, has overcome some of the performance problems with the
inorganic anions. The
most promising group of these anions is that based on fluorinated sulfonyl
ligands. The Li salt of
2 0N(SOZCF3)2 , for example, is highly conductive and thermally stable to
360°C. However, it has
been reported to corrode aluminum at high potentials which is a problem for
cells employing
aluminum current collectors. Other related salts being investigated include
LiC(S02CF3)3 and
those obtained by the substitution of various fluorinated organic groups (R)
on LiN(SOZR)Z. While
these anions have promising performance characteristics, they are expensive
and the need for an
2 5 inexpensive salt remains unsatisfied.
U.S. Patent No. 6,022,643 issued to Hung S. Lee et al. on February 8, 2000,
assigned to
Brookhaven National Laboratory, discloses that the addition of a three-
coordinate boron compound
to a lithium salt in organic carbonate solutions dramatically increases the
conductivity of the lithium
salt. The lithium salts, LiF, CF3COZLi, and C2FSC02Li, were combined with
various
3 0 organofluorine boron based compounds. The patentees referred to the three
coordinate boron based
compounds as "anion receptors" because they would seek a fourth ligand from
the salt anion, thus
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
3
increasing the conductance and Li transference number. While these solutions
are conductive and
electrochemically stable over the necessary potential range, they require the
use of an expensive
Lewis acid in a 1:1 ratio with the lithium salt, which increases the cost of
the electrolyte.
U.S. Patent No. 6,395,671 issued to Robert E. LaPointe, assigned to The Dow
Chemical Company, discloses that the addition of two Lewis acids to a
monoanionic species with
two Lewis basic sites yields an anion that is only very weakly Lewis basic.
Potassium and
ammonium salts of these anions were prepared, and the ammonium salts were used
in the
preparation of olefin polymerization catalysts, which requires that the anion
be dissociated from
cation. The dissociation of the anion from the cation (ie. low degree of ion-
pairing) is also
important in achieving a highly conductive lithium salt. However, the
synthetic routes to the salts
shown below in Equations 4 and 5 do not include a synthetic route to a lithium
salt.
(Equation 4)
N~NH+ 2 M(is)s + ~~R~ toh ~ ~ ~~ ((CSFs)3~NO N~(~s)s) O
~R2
(Equation 5)
N~ NK + 2 M(~FS)3 toluene K ((t~Fs)3M)N~ N(M(~FS)3)
(M = B, Al)
Summary of the Invention
According to the present invention, a non-aqueous electric current producing
2 0 electrochemical cell is provided comprising an anode and a cathode, an
ionically permeable
separator interposed between the anode and the cathode, and a non-aqueous
electrolyte comprising
an ionically conducting salt in a non-aqueous medium, the ionically conducting
salt corresponding
to the formula:
2 5 wherein:
~(Z*(J*);~*)~
M is a lithium atom,
Z* is an anion group containing two or more Lewis basic sites and comprising
less than
50 atoms not including hydrogen atoms,
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
4
J* independently each occurance is a Lewis acid coordinated to at least one
Lewis
basic site of Z*, and optionally two or more such J* groups may be joined
together
in a moiety having multiple Lewis acidic functionality,
X* independently each occurrence is selected from the group consisting of H,
Cl-Ca
alkyl, alkoxide, halide and mixtures thereof,
j is an integer from 2 to 12, and
x, , is an integer from 0 to 4
The present invention is based on the unexpected discovery that anions similar
to those
investigated by LaPointe, supra, for use specifically as catalyst activators,
but coupled in this case
with a lithium based cation make excellent candidates for use as the sonically
conducting salt in a
lithium cell electrolyte. The lithium salt used in the non-aqueous electrolyte
according to the
present invention is prepared from the combination of an anion having a 1-
charge that has
multiple Lewis basic sites and a suffcient quantity of a Lewis acid such that
all the Lewis basic
sites of the anion are complexed. The salt may be incorporated within a non-
aqueous liquid
medium such as, for example, an organic solvent. The salt may also be employed
with various
polymers and gels as the non-aqueous medium. The non-aqueous cell electrolyte
of the present
invention is useful in both primary and secondary lithium cells. The cell
electrolyte is compatible
with other cell components and generally exhibits good conductivity and
thermal stability. The
2 0 electrolyte is fixrthermore relatively easy to prepare and inexpensive to
use in typical lithium cells.
Brief Description of the Drawings
In the accompanying drawing:
Figure 1 illustrates the chemical structure of a number of lithium salts used
in the
2 5 preparation of electrolytes and cells according to the present invention.
The abbreviations listed
below each structure correspond to the abbreviations used in the detailed
description and examples
herein.
Figure 2 is an x-ray structure of LiBenzIm (BF3)Z. Two LiBenzIm (BF3)Z ~2
ethylene
carbonate molecules are present in the structure shown with each related by
symmetry. Atoms are
3 O labeled as F=fluorine, C= carbon, B=boron, Li=lithium, N--nitrogen,
O=oxygen with a numeric
suffix to distinguish atoms that are not related by symmetry. Hydrogen atoms
are omitted for
clarity.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
Figure 3 is a plot of the charge and discharge capacity of a 7 Ah Li-ion
battery prepared
and tested according to the present invention.
Detailed Description of the Invention
5 It has been discovered in accordance with the present invention that non-
aqueous,
primary and secondary, electric current producing electrochemical cells having
good performance
characteristics can be prepared at relatively low costs by employing a novel
class of conductive
lithium salts in various non-aqueous mediums as the cell electrolyte. The
novel class of
conductive lithium salts correspond to the general formula:
~(Z*(J*>;(X*)~J
wherein:
M is a lithium atom,
Z* is an anion group containing two or more Lewis basic sites and comprising
less than
50 atoms not including hydrogen atoms,
J* independently each occurance is a Lewis acid coordinated to at least one
Lewis
basic site of Z*, and optionally two or more such J* groups may be joined
together
in a moiety having multiple Lewis acidic functionality,
X* independently each occurrence is selected from the group consisting of H,
Cl-Cd
2 0 alkyl, alkoxide, halide and mixtures thereof,
j is an integer from 2 to 12, and
x is an integer from 0 to 4.
Z* can be any anionic moiety having a 1- overall charge and containing two or
more
2 5 Lewis basic sites. Preferably, the Lewis base sites are on different atoms
of a polyatomic anionic
moiety. Desirably, such Lewis basic sites are relatively sterically accessible
to the Lewis acid, J*.
Preferably the Lewis basic sites are on nitrogen atoms or carbon atoms.
Examples of suitable Z*
anions include cyanide, azide, amide, amidinide, substituted amidinide,
dicyanamide, imidazolide,
substituted imidazolide, imidazolinide, substituted imidazolinide,
benzoimida~olide, substituted
3 0 benzoimidazolide, tricyanomethide, tetracyanoborate, puride, squarate,
1,2,3-triazolide, substituted
1,2,3-triazolide, 1,2,4-triazolide, substituted 1,2,4-triazolide,
pyrimidinide, substituted
pyrimidinide, tetraimidazoylborate, substituted tetraimidazoylborate,
tris(imidazoyl)fluoroborate,
substituted tris(imidazoyl)fluoroborate, bis(imidazoyl)diffuoroborate,
substituted
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
6
bis(imidazoyl)difluoroborate anions and mixtures thereof, wherein each
substituent, if present, is
is selected from the group consisting of a halo, hydrocarbyl, halohydrocarbyl,
silyl,
silylhydrocarbyl, a halocarbyl group of up to 20 atoms not counting hydrogen
and mixtures
thereof, and further wherein two substituents, if present, together form a
saturated or unsaturated
ring system. Preferred Z* groups are imidazolide, 2-methylimidazolide, 4-
methylimidazolide,
benzoimidazolide, and dimethylamide.
Coordinated to the Lewis base sites of the anion are from 2 to 12 Lewis acids,
J*, two
or more of which may be joined together in a moiety having multiple Lewis acid
functionality.
Preferably, from 2 to 4 J* groups having from 3 to 100 atoms are present.
Preferred Lewis acids
are those having a formula selected from the group consisting of
R1
O-B
1R-B O (Rl)2-M Ar (Rl)-M Ar M'''-
O
X1)3 M* ~ Rl , (Rl)2-M Art' , (Rl)-M ~~ 2, and M~
as well as mixtures thereof
wherein:
M* is aluminum or boron;
Rl independently each occurrence is a compound selected from the group
consisting of
a halide, alkyl, aryl, alkoxide, aryloxide, dialkylamido, halogenated alkyl,
halogenated aryl, halogenated alkoxide, halogenated aryl oxide and mixtures
thereof, said Rl having up to twenty carbon atoms, and
Arf'-Arm in combination is independently, a divalent aromatic group of 6 to 20
carbon atoms.
Highly preferred Lewis acids are BR13 and ALR13 wherein R1 independently each
occurrence is selected from the group consisting of a halogen, alkoxide,
fluorinated alkoxide,
halogenated alkyl, halogenated aryl and mixtures thereof, Rl having up to 20
carbon atoms. In a
more highly preferred embodiment, Rl is a fluorine atom.
2 5 The foregoing lithium salts (illustrated by those having imidazolide,
substituted
imidazolide, benzoimidazolide, substituted benzoimidazolide, and amide) may be
depicted below
as follows:
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
7
R
Li+ ~ -IO R
(J*)N O N(J*) Li+ * ~ * ~ O Li J; ~~J* ~ O
(J )N O N(J ) N
R,~ ~R'
R' R"
R' R"
wherein:
Li is lithium,
R, R', and R" are hydrogen or hydrocarbyl group,
and J~' is a Lewis acid, for example, BF3, B(OCH3)3, B(C6F5)3, or
B(OCH(CF3)a)s.
Examples of the highly preferred lithium salts include lithium salts of
bis(trifluorborane)imidazolide, bis(trifluorborane)-2-methylimidazolide,
bis(trifluorborane)-4-
methylimidazolide, bis(trifluorborane)-2-isopropylimidazolide,
bis(trifluorborane)benzimidazolide,
bis(trifluorborane)dimethylamide, bis(trifluoroborane)diisopropylamide,
l0bis(trimethoxyborane)imidazolide, bis(trimethoxyborane)-2-methylimida~olide,
bis(trimethoxyborane)-4-methylimidazolide, bis(trimethoxyborane)-2-
isopropylimidazolide,
bis(trimethoxyborane)benzimidazolide, bis(trimethoxyborane)dimethylamide,
bis(trimethoxyborane)diisopropylamide.
The compounds may be prepared by a condensation reaction between the lithium
salt of
l5the anion Z* and a Lewis acid, J*, or its Lewis acid base adduct such as an
etherate. For example,
contacting imidazole, or substituted imida~ole, with a lithium alkyl such as n-
BuLi will yield
lithium imidazolide, or substituted lithium imidazolide. The lithium
imidazolide may then be
contacted with a Lewis acid, J*, or its Lewis base adduct to yield the desired
lithium salt.
Preferably, the reaction is performed in non-aqueous and non-protic solvents.
Electrolytes may be
2 0 prepared by dissolving the lithium salt into an organic solvent, a
polymer, or a gel.
As shown in Figure 2, Li [Benzlmm(BF3)2] (Ben~Im = benzimida~olide), ie. a
lithium salt
of the [BenzIrn(BF3)2]' anion, the benzimidazolide anion appears to be
complexed at both of the
Lewis basic nitrogen atoms by Lewis acidic BF3. Without being bound by any
theory, it is believed
that each Li cation is bonded to a fluorine atom from one BF3 group of two
[BenzIrn(BF3)z]' anions.
2 5 The lithium cation appears to be further bonded to the carbonyl oxygen
atom of two ethylene
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
8
carbonate molecules which cocrystallized with the compound. In order to
maintain
electroneutrality, there is one lithium ration per anion.
It has been discovered that these compounds, when added to an appropriate
solvent,
form a useful electrolyte for lithium and Li-ion batteries. Suitable solvents
include non-aqueous
5liquid polar solvents such as organic carbonates including ethylene
carbonate, dimethyl carbonate
ethylinethyl carbonate, diethyl carbonate and mixtures thereof. Other solvents
which may be in a
mixture with organic carbonates are organic ethers, lactones, such as gama-
butyrolactone, formates,
esters, sulfones, nitrites, and oxazolidinones which are used in primary and
secondary Li batteries.
Electrolytes prepared from these salts have been found to be highly conductive
and
electrochemically stable over the operating range of a lithium and Li-ion
cell. Furthermore, cells
prepared with these electrolytes have low capacity fade over several cycles
demonstrating long
cycle life.
Without being bound by any theory, it is believed that these compounds have
high
conductivity because there is a high degree of separation of the ions in the
electrolyte. Separation
of the anions from the rations is necessary for the formation of charged
species in solution, thus
allowing the transfer of the rations from the anode to the cathode during
discharge and from the
cathode to the anode during charge. Increasing the fraction of the rations
that are separated from
the anions relative to those that are ion-paired to the anion should increase
the overall conductivity
of the electrolyte thereby increasing the rate capability and cathode
utilization of an electrochemical
2 0 cell. These compounds have a high degree of separation between the ration
and the anion because
the anions are very weakly basic, which will allow the solvent, a stronger
Lewis base, to bond to
and effectively solvate the lithium ration, thus separating the anion from the
ration. The Lewis
basicity of the anion is minimized by proper choice of a Lewis base, Z*, and
Lewis acid, J*. In
general, it is preferable to have Lewis base sites that are on different atoms
of a polyatomic anionic
2 5 moiety so that the charge is delocalized over a large portion of the anion
so that there is less
electrostatic interactions between the anion and ration. It is also preferable
that the Lewis acid J*
be strongly Lewis acidic and incorporate highly electronegative atoms because
this will allow it to
form a strong complex to the Lewis base and have high electrochemical
stability. Furthermore, it is
sometimes advantageous to keep the salt to a low mass, as high mass salts can
lead to viscous
3 0 solutions, thus reducing the conductivity. Therefore, low mass Lewis acids
such as BF3 are
preferred although higher mass Lewis acids such as B(C6F5)3 and B(OCH(CF3)a)s
are not excluded
from this invention.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
The new materials may be used in primary cells, which have an anode and
cathode as
components of the cell. Typical anode materials which may be used in primary
cells are lithium,
lithium alloys, lithium carbon intercalated compounds, lithium graphite
intercalation compounds,
lithium metal oxide intercalation compounds, and mixtures thereof. The cathode
in a primary cell
is typically composed of a transition metal oxide, a transition metal
chalcogenide, a
poly(carbondisulfide) polymer, an organo-disulfide redox polymer, a
polyaniline, an
organodisulfide/polyaniline composite and an oxychloride. Examples of
materials that may be
used as a cathode in a primary cell include SOz, CuO, CuS, AgzCr04, I2, PbI2,
PbS, SOCIZ, VZOSa
Mo03, or Mn02, or poly(carbon manofiuoride), (CF)n. Typically, organic
solvents such as
acetonitrile and propylene carbonate and inorganic solvents, such as thionyl
chloride are used in
primary cells.
The compounds have been found to be useful in secondary (rechargeable) cells.
A
secondary lithium or lithium-ion battery must have a cathode and anode, one of
which has lithium
incorporated into it. The anode for these cells is capable of reversibly
incorporating lithium metal.
15Examples of these materials include lithium metal, lithium alloys, lithium-
carbon or lithium-
graphite intercalation compounds, lithium metal oxide intercalation compounds
such as LiXW02 or
LMoOz or a lithium metal sulfide such as LiTiSz. The cathode material must
also be capable of
reversibly incorporating lithium metal. Suitable cathode materials include
transition metal oxides
and transition metal chalogenides, examples of which are LiNio.gCoo,20z,
LiZ,5V6013, Li1.2V20s,
2 OLiCoOz, LiNi02, LiMna04, LiMn02, Li3NbSe3, LiTiS2, and LiMoSz.
In assembling the cell of the present invention, the cathode is typically
fabricated by
depositing a slurry of the cathode material, a electrically conductive inert
material, the binder, and a
liquid carrier on the cathode current collector, and then evaporating the
carrier to leave a coherent
mass in electrical contact with the current collector.
2 5 In assembling a cell of the present invention, the anode can similarly be
fabricated by
depositing slurry of the highly graphitic carbonaceous anode material, the
electrically conductive
inert material, the binder, and a liquid carrier on the anode current
collector, and then evaporating
the carrier to leave a coherent mass in electrical contact with the current
collector.
The cathode assembly is then combined with the anode assembly with the porous
non-
3 0 conducting separator sandwiched between these two assemblies. Suitable
porous non-conducting
separator materials include microporous polyethylene film and a porous glass
membrane, for
example. The preferred way of constructing high voltage rechargeable cells is
to make them with
the cathode in the discharged state because the material is stable in air. In
a Li-ion cell employing a
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
carbonaceous anode material, this material is also in a discharged state
during cell assembly. The
layered assembly is then wound around a metal post which may serve as terminal
for the cell.
Alternatively, several of these layers maybe assembled together to form a
prismatic cell. After
assembly of the electrode materials in the cell, the electrolyte solution in
which the salt is dissolved
5 is added. The cell container is then capped.
The electrolyte solution includes a lithium salt dissolved in the electrolyte
solvent.
Suitable electrolyte solvents include non-aqueous liquid polar solvents such
as ethylene carbonate,
dimethyl carbonate, ethylmethyl carbonate, diethyl carbonate, and mixtures
thereof. Other solvents
are organic carbonates, lactones, formates, esters, sulfones, nitrites, and
oxazolidinones.
10 There are several types of polymer electrolytes that may be useful in
electrochemical
cells of the present invention. One type consists of lithium salts dissolved
in linear polyethers such
as polyethylene oxide which may have branched or comb shaped polymers which
have flexible
inorganic backbones such as (-P--N-)n or (-Si0-)". Polymer electrolytes may be
further modified by
addition of additives such as plasticizicers such as organic carbonates.
Gelled electrolytes are another type of electrolyte that is usefi~l for the
electrochemical
cells of this invention. Gelled electrolytes include a solution of a lithium
salt in a liquid organic
solvent and a supporting matrix of a polymer such as poly(acrylonitrile) (PAID
or poly(vinylidene
fluoride-hexafluoro-propylene) (PVDF-HFP) copolymer. Solvent mixtures such as
binary or
ternary mixtures of organic carbonates can also be used as liquid solvents in
gelled electrolytes.
2 0 Experimental
All preparations and physical measurements were carried out with rigorous
exclusion of
air and water. Schlenk and glovebox techniques were employed with purified
argon used as an
inert gas when required. All reagents and solvents were reagent grade or
better. Imidazole,
benzimidazole, 2-methylimidazole, 4-methylimidazole, 2-isopropylimidazole, and
lithium
2 5 dimethylamide were all purchased from Aldrich and used as received. Boron
trifiuoride diethyl
etherate were both purchased from Alfa Aesar and used as received. The
following solvents were
dried by distillation from the indicated drying agent: dichloromethane (PZOS),
toluene (Na), and
acetone (4 ~ molecular sieves). Ethylinethyl carbonate (<30 ppm Ha0), ethylene
carbonate (<30
ppm H20), diethyl carbonate (<15 ppm HZO, and dimethyl carbonate (<15 ppm HZO)
were
3 0 purchased from EM Science and used as received.
NMR spectra were recorded using a BRLJKER AC 250 or a JEOL GSX 400 MHz
NMR spectrometer. Chemical shifts (8) are relative to Si(CH3)4 (~ = 0 for 1H
NMR) and CFC13 (~
- 0 for 19F NMR). Negative- and positive-ion electrospray mass spectra were
performed on a
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
11
Micromass Quattro II with cone voltages ranging from 15 to 70 V. Ten ~,L were
injected into a
Rheodyne injector with a acetonitrile flow.
Conductivities of one molar (1 M) salt solutions (except for lithium
bis(trifluoroborane)benzimidazolide which was 0.5 M) at varying temperatures
in ethylene
carbonate (EC)/ethylinethylmethyl carbonate (EMC) mixture were measured using
a Metrohm 712
conductivity meter. The cell assembly was an Orion 018010 or a Metrohm 712
conductivity cell,
both of which have platinized platinum electrodes with cell constants of about
1 cW 1. Cells were
filled and sealed inside a glovebox under an argon atmosphere. The measurement
temperatures
were controlled to within 1°C using a Tenney Environmental temperature
chamber. The EC/EMC
(1:3 by weight) solvent mixture is representative of the solvents used in
commercial Li-ion
batteries.
Test cells were made which employed a 1 M electrolyte solution of LiIm(BF3)Z
in a
1:1:1 EC:DMC:DEC solvent mixture (by weight). Cathodes comprised a mixture of
a transition
metal oxide powder, a carbonaceous conductive dilutant, and polyvinylidene
fluoride (PVDF)
15binder that was coated uniformly onto aluminum foil. The transition metal
oxide used was
LiNio,BCoo,2O2. The anode was comprised of lithium metal or a carbonaceous
powder, a
carbonaceous conductive dilutant, and PVDF binder that was coated onto copper
foil. Setela°
microporous polyethylene film was used as a separator to prevent electrical
contact between the
anode and cathode electrodes. Other separator materials that may be used
include porous glass
2 0 membranes, for example. Cells made with lithium metal for the anode were
made in a button cell
configuration with a few drops of the electrolyte and the separator sandwiched
between the lithium
and the cathode material. A Li-ion cell was constructed using MCMB carbon for
the active anode
material and LiNio.BCoo,ZOa as the transition metal oxide for the cathode. The
electrolyte was added
to the cell inside the glovebox. The theoretical capacity was 7.65 ampere-hour
(Ah). The cell was
2 5hermetically sealed inside a stainless steel can after formation cycles
were completed.
Example 1
Lithium imidazolide (LiC3H3N2)
A slurry of imidazole (5.00 g, 73.5 mmol) in toluene (50 mL) was treated with
28 mL of
3 0 a 2.65 M n-BuLi (74.2 mmol) solution in hexanes. This solution mixture was
then refluxed for
three days during which time the slurry became an off white color. The slurry
was then filtered
over a medium glass flit and the solid was washed with two 10 mL portions of
toluene and then
dried under vacuum to yield an off white powder. Yield: 5.40 g, 99.4%.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
12
Example 2
Lithium bis(trifluoroborane)imidazolide (Li(BF3)2C3H3N2)
A slurry of lithium imidazolide (5.00 g, 67:6 mmol) in CHZC12 (100 mL) was
treated
with BF3(Et20) (19.6 mL, 154 mmol) and the mixture was refiuxed for five days
during which time
the slurry became yellow. The solid was then dried under vacuum to yield an
off white solid.
Yield: 13.77 g, 97.1 %. The solid was then dissolved in 40 mL of ethylmethyl
carbonate and
filtered. Dichloromethane was added to this filtrate and a precipitate formed.
This precipitate was
collected and dried under vacuum at 60°C. Yield: 8.63 g, 61%.
1H NMR (acetone-d6) 8 7.87 (singlet, 1H), 7.08 (singlet, 2H)
1019F NMR (acetone-d6) 8 -147.5 (quartet, JB_F = 13 Hz)
Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
C3H3NZBZF6 203. Found m/z 203 [(M-Li)]-.
Example 3
lSLithium 2-methylimidazolide (LiC4H5Nz)
A slurry of 2-methylimidazole (4.00 g, 48.7 mmol) in toluene (50 mL) at
0°C was
treated with 17.4 mL of a 2.8 M n-BuLi (48.7 mmol) solution in hexanes. This
solution mixture
was then refiuxed for one day during which time the slurry became an off white
color. The slurry
was then filtered over a medium glass frit and dried to give an off white
solid. Yield: 4.295 g,
2 0100%.
Example 4
Lithium bis(trifluoroborane)-2-methylimidazolide (Li(BF3)zC4H5N2)
A slurry of lithium 2-methylimidazolide (4.00 g, 67.6 mmol) in CHZCl2 (70 mL)
at 0°C
25was treated with BF3(Et20) (11.7 mL, 93.2 mmol) and the mixture was refluxed
for three days
during which time the slurry became yellow. The solid was then dried under
vacuum to yield an
off white solid. Yield: 9.76 g, 96.0 %. The solid was then dissolved in about
15 mL of dimethyl
carbonate and filtered. Dichloromethane was added to this filtrate and a
precipitate formed. This
precipitate was collected and dried under vacuum.
3 0 Yield: 7.34 g, 72.2%
1H NMR (acetone-d6) 8 6.93 (singlet, 2H), 2.50 (singlet, 3H)
19F NMR (acetone-d6) 8 -146.0 (quartet, JB_F = 14 Hz)
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
13
Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
C4HSN2BZF6 217. Found m/z 217 [(M-Li)]-.
Example 5
Lithium 4-methylimidazolide (LiC4H5N2)
A slurry of 4-methylimidazole (4.00 g, 48.7 mmol) in toluene (50 mL) at
0°C was
treated with 17.4 mL of a 2.8 M n-BuLi (48.7 mmol) solution in hexanes. This
solution mixture
was then refluxed for one day during which time the slurry became an off white
color. The slurry
was then filtered over a medium glass frit and dried to give an off white
solid.
lOYield: 4.365 g, 102%.
Example 6
Lithium bis(tritluoroborane)-4-methylimidazolide (Li(BF3)ZC4HSNz)
A slurry of lithium 2-methylimidazolide (4.00 g, 67.6 mmol) in CHZCIz (70 mL)
at 0°C
15was treated with BF3(EtZQ) (11.7 mL, 93.2 mmol) and the mixture was refluxed
for three days
during which time the slurry became yellow. The solid was then dried under
vacuum to yield an
off white solid. Yield: 9.10 g, 89.6%. The solid was then dissolved in about
15 mL of dimethyl
carbonate and filtered. Dichloromethane was added to this filtrate and a
precipitate formed. This
precipitate was collected and dried under vacuum.
2 OYield: 6.80 g, 66.9%
1H NMR (acetone-d6) 8 7.77 (singlet, 1H), 86.79 (singlet, 1H), 3.71 (singlet,
3H)
19F NMR (acetone-d6) 8 -146.6 (quartet, JB_F = 14 Hz, 3F), 8 -148.0 (quartet,
JB_F = 14 Hz, 3F)
Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
C4HSNZBZF6 217. Found m/z 217 [(M-Li)]-.
25 -
Example 7
Lithium 2-isopropylimidazolide (LiC6H9Nz)
A slurry of 2-isopropylimidazole (4.00 g, 36.3 mmol) in toluene (40 mL) at -
78°C was
treated with 17.4 mL of a 2.8 M n-BuLi (48.7 mmol) solution in hexanes. This
solution mixture
3 0 was then refluxed for one day during which time the slurry became orange.
The slurry was then
filtered over a medium glass fi-it and dried to give a white solid.
Yield: 4.32 g, 102%.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
14
Example 8
Lithium bis(trifluoroborane)-2-isopropylimidazolide (Li(BF3)zCgH9Nz)
A slurry of lithium 2-methylimidazolide (4.00 g, 34.4 mmol) in CHzCIz (100 mL)
at 0°C
was treated with BF3(EtzO) (11.7 mL, 93.2 mmol) and the mixture was refluxed
for three days
during which time the slurry became yellow. The solid was then dried under
vacuum to yield an
off white solid. The solid was then dissolved in about 10 mL of dimethyl
carbonate and filtered.
Dichloromethane was added to this filtrate and a precipitate formed. This
precipitate was collected
and dried under vacuum.
Yield: 6.44 g, 58.6%
101H NMR (acetone-d6) 8 6.96 (singlet, 2H), b3.78 (septet, JH_H = 7 Hz, 1H),
3.71 (doublet, 7 Hz, 6H)
19F NMR (acetone-d6) 8 -143.2 (quartet, JB_F =14 Hz)
Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
C4HSNzBzF6 245. Found m/z 245 [(M-Li)]-.
Example 9
Lithium benzimidazolide (LiC~HSNz)
A slurry of benzimidazole (8.50 g, 36.3 mmol) in toluene (40 mL) at 0°C
was treated
with 25.8 mL of a 2.8 M n-BuLi (72.2 mmol) solution in hexanes. This solution
mixture was then
refluxed for one day during which time the slurry became off white. The slurry
was then filtered
2 0 over a medium glass flit and dried to give a white solid.
Yield: 8.629 g, 96.7%.
Example 10
Lithium bis(trifluoroborane)benzimidazolide (Li(BF3)zC~H5N2)
2 5 A slurry of lithium benzimidazolide (8.25 g, 66.42 mmol) in CHzCIz (100
mL) was
treated with BF3(EtzO) (17.5 mL, 138.1 mmol) and the mixture was refluxed for
three days during
which time the slurry became gray. The solid was then dried under vacuum to
yield an off white
solid. Yield: 16.14 g, 93.5%. The solid was then dissolved in a 1:3 ethylene
carbonate:
ethylinethyl carbonate and recrystallized.
3 0 Yield: 13.93 g, 48.2% when the two ethylene carbonate molecules are
accounted for in the crystal
lattice.
1H NMR (acetone-dg) ~ 8.35 (singlet, 1H), b 7.83 (multiplet, 2H), 7.37
(multiplet, 2H)
y NMR (acetone-d6) ~ -146.3 (quartet, JB_F =14 Hz) spectrum
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
C~HSN2BZF6 253. Found mlz 253 [(M-Li)]-. The x-ray structure of LiBenzIm(BF3)z
is shown in
Figure 2.
5 Example 11
Lithium bis(trifluoroborane)dimethylamide (LiN(CH3)z(BF3)z)
A slurry of lithium dimethylamide ( 1.3 67 g, 26. 80 mmol) in toluene ( 100
mL) at -78 ° C
was treated with BF3(EtzO) ( 17.5 mL, 13 8.1 mmol) dropwise through an
addition funnel. On
warming, the solution became bright white. The mixture was then refluxed for
three days during
10 which time the slurry became off white. The slurry was then filtered and
the solid was then dried
under vacuum to yield an off white solid.
Yield: 4.26 g, 85.2°1°.
1H NMR (acetone-d6) 8 2.25 (singlet, 1H)
y. NMR (acetone-d6) 8 -156.9 (quartet, JB_F = 17 Hz) spectrum
15Low resolution mass spectrum (Negative ion electrospray, acetone solution)
Calculated for
CzHs~zFs 180. Found m/~ 180 [(M-Li)]-.
Example 12
Conductivity Studies
2 0 In this example, the ionic conductivity of electrolyte solutions
containing various lithium
salts of the present invention. Table 1 below lists the ionic conductivity
data for 1 M salt solutions
(except for lithium bis(trifluoroborane)benzimidazolide which was 0.5 M) in a
1:3 EC:EMC
solvent mixture at various temperatures.
Table 1
Ionic Conductivity of 1.0 Ma Lithium Salts in 1:3 EC:EMC
Temperature (°C)
-40 -25 -10 5 20 35 50 65 80
Salt Conductivity (mS/cm)
3 0 Li Im(BF3)z (Ex. 2) 0.7 1.5 2.5 3.7 5.1 6.5 7.9 9.3 10.6
Li 2-Melm(BF3)z (Ex.4) 0.5 1.2 2.1 3.2 4.4 5.7 7.0 8.3 9.6
Li 4-MeIm(BF3)z (Ex. 6) 0.6 1.2 2.1 3.1 4.2 5.4 6.6 7.7 8.8
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
16
Li 2-'PrIm(BF3)Za (Ex. 8) 0.2 0.6 1.2 2.0 2.9 4.1 5.2 6.3 7.5
Li BenzIm(BF3)Z (Ex. 10) 0.4 1.0 1.8 2.8 3.8 4.9 6.0 7.0 7.8
Li N(CH~)~(BF~)2 (Ex. 11) 0.4 0.8 1.3 1.9 2.5 3.2 3.9 4.5 5.8
aThe electrolyte solution containing Li Ben~Im(BF3)Z (Ex. 10) was only 0.5 M.
Example 13
Lithium Batteries with Transition Metal Oxide for Cathode
This example demonstrates that the salts may be used in a lithium battery and
compatibility of the salts with a transition metal oxide. A button cell with
lithium metal as anode
and LiNio.BCoo,zOz as the active cathode material was prepared inside the
glovebox. Between the
two electrodes was placed the separator and 60 ~,L of a 1 M solution of the
salt (except
LiBenzIm(BF3)z (Ex. 10) which was 0.5 M) in 1:3 EC:EMC (by weight). The cells
were charged
and discharged at the C/7 rate from 3.0 to 4.2 V. The capacity of the
LiNio.gCoo.2O2 m mAh/g for
cells prepared with these salts is shown in Table 2 below,
Table 2
Capacity of Lithium LiNio.BCoo,202 Button Cells Using 1.0 Ma Lithium Salts in
1:3 EC:EMC for
Electrolyte
lsc lsc 2na 2na Stn Sin
Charge Discharge Charge Discharge Charge Discharge
Salt LiNi~.RCo~.2~2 Capacity (mAh/g)
Li Im(BF3)Z (Ex. 2) 209.5 183.3 191.6 183.2 188.1 186.0
2 5 Li 2-MeIm(BF3)2 (Ex. 4) 208.8 180.8 196.3 185.8 190.4 183.0
Li 4-MeIm(BF3)2 (Ex. 6) 199.7 172.3 191.3 171.5 184.6 163.4
Li BenzIm(BF3)za (Ex. 10) 194.$ 169.5 176.7 171.8 172.2 165.8
Li N(CH~)z(BF3)Z (Ex. 11) 207.3 180.6 186.5 182.3 190.7 183.9
aThe electrolyte solution containing Li BenzIm(BF3)z (Ex. 10) was only 0.5 M.
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
17
Example 14
Lithium Batteries with MCMB Carbon for Cathode
This example demonstrates that the salts may be used in a lithium battery and
compatibility of the salts with MCMB carbon, which is a common carbonaceous
material used as
the active anode material in Li-ion batteries. A button cell with lithium
metal as anode and MCMB
carbon as the active cathode material was prepared inside the glovebox.
Between the two
electrodes was placed the separator and 60 ~,L of a 1 M solution of the salt
(except LiBenzlm(BF3)a
(Ex. 10) which was 0.5 M) in 1:3 EC:EMC (by weight). The cells were charged
and discharged at
the C/7 rate from 0.01 to 3.0 V. The capacity of the MCMB carbon in mAh/g for
cells prepared
with these salts is shown in Table 2 below.
Table 3
Capacity of Lithium~MCMB Carbon Button Cells Using 1.0 Ma Lithium Salts in 1:3
EC:EMC
for Electrolyte
lsc lsr 2na 2na S~n Scn
_ Discharge Charge Discharge Charge Discharge Charge
Salt MCMB Carbon Capacity (mAh/g)
Li Im(BF3)Z (Ex. 2) 206.6 185.1 222.1 221.1 263.1 262.7
2 0 Li 2-Melm(BF3)Z (Ex. 4) 289.6 256.3 272.2 270.1 267.0 267.0
Li 4-MeIm(BF3)2 (Ex. 6) 280.7 246.8 271.3 268.8 279.3 278.8
Li 2-'FrIm(BF3)2 (Ex. 8) 123.3 97.5 185.6 182.5 250.2 249.6
Li BenzIm(BF3)2a (Ex. 10) 54.8 35.2 62.3 58.3 128.5 127.1
Li N(CH.~)2(BF~) _ (Ex. 11) 172.4 141.7 217.7 206.4 155.8 153.1
2 5aThe electrolyte solution containing Li BenzIm(BF3)Z (Ex. 10) was only 0.5
M.
Example 15
Lithium-ion Battery
This example demonstrates that the salt may be used in a lithium-ion battery
employing
3 0 a carbonaceous material and transition metal oxide as the active materials
in the anode and cathode
electrodes, respectively. The active anode material was MCMB carbon and the
active cathode
CA 02466350 2004-05-06
WO 03/043102 PCT/US02/35785
18
material was LiNio.BCoo.20z, which were each coated onto copper and aluminum
foil, respectively.
A lithium-ion cell with a nominal capacity of 7 Ah was constructed using these
electrode materials
and separated by a microporous polyethylene sheet. These materials were
assembled and placed
into a stainless steel can. The electrolyte, a 1 M solution of LiIm(BF3)2 (Ex.
2) in 1:1:1
EC:DMC:DMC, was added to the can and the cell was then put on formation, which
consisted of
one cycle of a charge and discharge at C/20 rate followed by two cycles at the
C/10 rate. During
this time gas was allowed to escape through a mineral oil bubbler. After the
formation cycles were
complete, the cell was hermetically sealed and cycled at the C/5 rate for 50
cycles. The charge and
discharge capacity of the cycles after formation is shown in Figure 3.
Those skilled in the art will appreciate that numerous changes and
modifications may be
made to the preferred embodiments of the invention and that such changes and
modifications may
be made with out departing from the spirit of the invention. It is therefore
intended that the
appended claims cover all such equivalent variations as fall within the true
spirit of the invention.
20
30