Note: Descriptions are shown in the official language in which they were submitted.
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
OSMOTIC DELIVERY DEVICE HAVING A TWO-WAY VALVE AND
DYNAMICALLY SELF-ADJUSTING 'FLOW CH-ANNEL
BACKGROUND OF THE INVENT10N
s
Field of the Invention
[0001] The present invention pertains to osmoticall~y controlled
implantable delivery devices, and more particularly to a delivery device
having
a two-way miniature valve and dynamically self-adjusting flow channel for the
io regulation of back-diffusion and fluid delivery rate in an osmotically
driven
delivery system.
Description of the Related Art
[0002] Controlled delivery of beneficial agents, such as drugs, in the
medical and the veterinary fields has been accomplished by a variety of
Is methods, including implantable delivery devices, such as implantable
osmotic
delivery devices. Osmotic delivery systems are very reliable in delivering a
beneficial agent over an extended period of time, called an administration
period. In general, osmotic delivery systems operate by imbibing fluid from an
outside environment and releasing controlled amounts of beneficial agent
2o from the delivery system.
[0003] Representative examples of various types of delivery devices are
disclosed in U.S. Patent Nos. 3,987,790; 4,865,845; 5,059,423; 5,112,614;
5,137,727; 5,213,809; 5,234,692; 5,234,693; 5,308,348; 5,413,572;
5,540;665; 5,728,396; 5,985,305; and 5,221,278, all of which are incorporated
2s herein by reference. All of the above patents generally include some type
of
capsule having walls, or portions of walls (for example, semi-permeable
membranes) that selectively pass water into the interior of the capsule. The
absorption of water by a water-attracting agent contained within the capsule
creates an osmotic pressure within the capsule, which then causes a
3o beneficial agent within the capsule to be expelled. Alternatively, the
water-
attracting agent may be the beneficial agent being delivered to the patient.
However, in most cases, a separate agent is used specifically for its ability
to
draw water into the capsule.
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
[0004] When a separate osmotic agent is used, the osmotic agent may
be separated from the beneficial agent within the capsule by a movable
dividing member such as a piston. The structure of the capsule is generally
rigid such that as the osmotic agent takes in water and expands, the capsule
s itself does not expand. As the osmotic agent expands, the agent causes the
movable dividing member to move, discharging the beneficial agent through
an orifice or exit passage of the capsule. The beneficial agent is discharged
through the exit passage at the same volumetric rate that water combines with
the osmotic agent through the semi-permeable walls of the capsule.
io [0005] In some known implantable delivery devices, the orifice or exit
passage of the capsule is permanently open and thus allows for unimpeded
discharge of the beneficial agent. This results in a direct fluid
communication
between the beneficial agent and water in the surrounding tissue of the
patient. Thus, back diffusion of the water into the beneficial agent reservoir
is may result. One way in which back diffusion of water has been reduced is to
provide a long orifice or exit passage thtat can be in a variety of shapes,
such
as straight or spiral.
[0006] In other known implantable delivery devices, the orifice or exit
passage of the capsule is covered with a stretchable or elastic member or
20 ~ band to reduce back diffusion of water into the beneficial agent
reservoir. The
stretchable or elastic band allows discharge of the beneficial agent once a
threshold pressure has been overcome. The stretchable or elastic member or
band closes the orifice when the pressure in the device is less than the
threshold pressure. However, in these types of devices there is little or no
2s control of the pressure that can build up as the device adjusts to changes
in
temperature and/or internal or external pressure.
[0007] In still other known implantable delivery devices, the orifice or exit
passage. is at least partially made of a stretchable or elastic material that
acts
to reduce back diffusion of water into the beneficial agent reservoir. This
3o stretchable or elastic material deforms once a threshold pressure has been
achieved in the device to allow discharge of the beneficial agent. The
stretchable orifice material closes when the internal pressure in the device
is
less than the threshold pressure. However, in these types of devices there is
2
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
little or no control of the pressure that can build up as the device adjusts
to
changes in temperature and/or internal or external pressure.
[0008] In general, osmotic delivery systems rely on the flow of interstitial
body fluid across a rate-limiting membrane (also known as a semi-permeable
membrane) to drive the osmotic agent expansion that in turn drives the
delivery or discharge of the beneficial agent. During the period immediately
following implantation, this interstitial fluid may also diffuse into the
beneficial
agent via a beneficial agent delivery channel (also known as an orifice or
exit
passage). Such diffusion is undesirable because it results in an uncontrolled
io . dilution of the beneficial agent formulation.
[0009] In those prior known designs which attempt to limit or prevent
back diffusion without covering the orifice or exit passage, one limitation
has
been that a relatively long diffusion path is required to prevent or impede
back
diffusion of the fluid into the beneficial agent compartment. The long
orifice,
is diffusion path, or exit channel in these known designs has been formed by
molding intricate detail into plastic or by machining highly toleranced
surfaces
into metal. These approaches are costly to manufacture and occupy a
relatively large volume, causing the implant to have an increased size.
[00010] A further drawback of known implantable delivery devices is that
2o these devices do not compensate for variations in temperature and internal
pressure that can cause the implantable delivery device to deliver beneficial
agent temporarily at high or low rates. Typically, an implantable, osmotically
driven delivery system will have been stored at ambient room temperature
(approximately 20 to 22°C) prior to implantation into a patient..
Within a few
2s hours following implantation, the system will subsequently come to thermal
equilibrium with the patient (approximately 37°C). This increase in
temperature may cause the beneficial agent formulation within the implantable
device to expand, which may result in a pressurization of the system and a
rapid, short-duration delivery of beneficial agent often referred to as a
start-up
30 "burst". This burst lis typically followed by a short period of somewhat
low
agent delivery (typically lasting from less than one day to 5 days) during
which
time the osmotic pressure is increasing to a degree equal to that of the
piston
friction. As the internal pressure of the implantable device increases, the
rate
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
of beneficial agent delivery will rise until it obtains a steady state. Since
it is
the purpose of an osmotic delivery system to deliver a defined concentration
of beneficial agent at a fixed rate, both the start-up "burst" and the
subsequent
"lag" in delivery are undesirable.
s [00011] A further aspect of an implantable, osmotically-driven beneficial
agent delivery system is that it is subject to external pressure or
temperature
changes (e.g., scuba diving, a hot bath, or temperature cycling during
shipping) which may, in turn, result in transient spikes in the beneficial
agent
delivery profile.
io [00012] It is possible with the current designs to develop high enough
pressures within the implantable osmotic delivery device that one or more of
the implant components fails or is expelled. In an effort to reduce the
possibility of.component failure or expulsion, previous designs have provided
grooves in the reservoir walls and/or ribs in the semi-permeable membrane or
is holes in the wail of the device which are open if a component of the device
moves out of position. These approaches add cost to the device by requiring
additional machining to the part designs.
[00013] Accordingly, it is an objective of the present invention to minimize
the start-up "burst" by containing the beneficial agent with a spring-loaded
2o valve until the internal, osmotic-induced pressure is great enough to
overcome the applied spring force, thereby opening the valve and allowing
controlled release of the agent. It is also an objective of this invention
that the
post-start-up "lag" in beneficial agent delivery be minimized or eliminated as
a
further result of the elimination of the initial agent "burst". A further
2s ~ consequence of this minimization of start-up "burst" and post start-
up."lag" is
that the system may achieve the desired steady-state performance
significantly sooner than in known implantable agent delivery devices. .
[00014] Another objective of the present invention is to provide for the
elimination of back diffusion in a relatively inexpensive manner and without
3o requiring a relatively large or long orifice, diffusion path, or exit
channel.
[00015] An additional objective of the present invention is to provide an
implantable osmotic delivery device capable of containing the total osmotic
4
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
pressure that can develop within the device without requiring relatively
expensive and sophisticated fluid flow bypass pathways.
SUMMARY OF THE INVENTION
s [00016] In accordance with the present invention, an implantable drug
delivery system for use in mammals (preferably in humans) includes a
capsule having an impermeable outer layer. The capsule has a beneficial
agent delivery end and a fluid uptake end that are spaced apart from each .
other, but not necessarily located on opposite ends of said capsule. In
to addition, the capsule comprises a reservoir containing a beneficial agent;
a
movable dividing member separating the reservoir from the osmotic engine;
and an osmotic engine. The delivery system includes a means for controlling
beneficial agent flow through the beneficial agent delivery end that
substantially prevents flow of beneficial agent out of the capsule when
i5 pressure within the capsule is above an upper predetermined pressure and
prevents flow of fluid into the capsule through the beneficial agent delivery
end when the pressure within the capsule is below a lower predetermined
pressure. However, beneficial agent is substantially allowed to flow out of
the
capsule through the beneficial agent delivery end when the pressure within
2o the capsule is between the lower and upper predetermined pressures.
[00017] In accordance with another aspect of the present invention, a
device for dynamically regulating the flow of a beneficial agent from a
pressurized beneficial agent delivery system includes a hollow body having a
lower port and an upper port. The device also includes a means for
2s controlling flow of interstitial fluid through the hollow body when
pressure by
the beneficial agent acting upon the means for controlling flow is below a
lower predetermined pressure. The device also includes a means for
controlling beneficial agent flo~iv out of the device when the pressure in the
device is above an upper predetermined pressure. The beneficial agent is
3o substantially allowed to flow out of the device when the pressure in the
device
is between the lower and upper predetermined pressures.
[00018] According to a further aspect of the Invention, a method' of
variably controlling the delivery of a beneficial agent frorri an implantable
drug
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
delivery system includes the steps of providing a capsule having a beneficial
agent delivery end and..a fluid uptake end, an agent reservoir containing a
beneficial agent, an uptake reservoir containing a fluid attracting agent,.and
a
movable dividing member between the agent reservoir and the uptake
s reservoir. The beneficial agent reservoir and the uptake reservoir are
positioned adjacent the beneficial agent delivery end and the fluid uptake
end,
respectively. The method also includes the step of substantially preventing
the flow of fluid into the capsule when a pressure within the capsule is below
a
lower predetermined pressure and flow of beneficial agent out of the capsule
to when a pressure within the capsule is above an upper predetermined
pressure. The method still further includes the step of variably controlling
the
flow of the beneficial agent out of the capsule when the pressure is between
the lower and upper predetermined pressures.
[00019] In accordance with yet another aspect of the invention, a method
is of variably controlling the delivery of a beneficial agent from an
implantable
osmotically driven delivery system includes the steps of displacing a movable
closing member of a valve assembly with respect to a lower port via
application of fluid pressure thereon from a beneficial agent reservoir to
thereby create an opening between the closing member and the lower port.
2o The method also includes the steps of increasing the size of the opening
via
increased pressure from the beneficial agent reservoir and allowing a
beneficial agent from the beneficial agent reservoir to pass through the lower
port and through the valve assembly. The method further includes the step of
variably controlling the beneficial agent flow through the valve assembly such
2s that the beneficial agent flow is directly proportional to the pressure
applied by
the beneficial agent against the movable closing member until the pressure
approaches a predetermined maximum pressure, at which time the beneficial
agent flow becomes more restricted as the pressure increases.
[00020] The present invention provides the advantage of substantially
3o preventing back diffusion during the start-up phase by causing the spring-
loaded.valve to be closed during this time, effectively preventing fluid
communication between the beneficial agent and interstitial fluids until the
system is sufficiently pressurized, and the beneficial agent pumping at a
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
sufficient rate, to disallow diffusion by the body fluid into the beneficial
agent
reservoir.
[00021] The present invention also provides the advantage of eliminating
the need for a relatively long orifice, diffusion path or exit channel to
prevent
s back diffusion in an implantable osmotically driven delivery device.
[00022] In addition, the present invention provides an implantable
osmotically driven delivery device which has the capability to withstand and
contain the full system osmotic pressure, an especially critical consideration
with any highly potent beneficial agent, without requiring relatively
expensive
to and .sophisticated fluid flow bypass pathways. .
[00023] Furthermore, the present invention eliminates the need for and
cost of a separate fluid bypass pathway.
BRIEF DESCRIPTION OF THE DRAWINGS
Is [00024] The invention will be described in greater detail with reference to
the accompanying drawings in which like elements bear like reference'
numerals, and wherein:
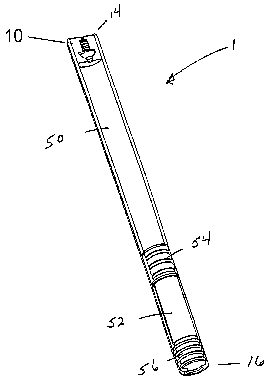
[00025] FIG. 1 is a cross-sectional side view of an osmotic agent delivery
device including a two-way valve and dynamically 'self-adjusting flow channel
2o in a normal condition;
[00026] FIG. 2a is a cross-sectional side view of an upper section of an
osmotic agent delivery device including a two-way valve and dynamically self-
adjusting flow channel, in which the closing member has been axially
displaced; ,
2s [00027] FIG: 2b is a cut away view of the valve shown in FIG. 2, showing
the elongated cylindrical stem 48.
[00028] FIG. 3 is a cross-sectional side view of an upper section of an
osmotic agent delivery device including a two-way valve and dynamically self-
adjusting flow channel, in which the closing member has been axially
3o displaced to a greater extent than that shown in FIG. 2;
[00029] FIG. 4 is a cross-sectional side view of an upper section of an
osmotic agent delivery device including a two-way valve and dynamically self
7
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
adjusting flow channel, in which an upper chamber thereof is substantially
closed off; and
[00030] FIG. 5 is a cross-sectional side view of a two-way and
dynamically self-adjusting flow channel, according to a second embodiment of
s the invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[00031] The present invention relates to a pressure activated, two-vvay
valve and self-adjusting flow channel for use in regulating fluid flow in an
io implantable osmotically-driven beneficial agent delivery system. The
components of the two-way valve and self-adjusting flow channel are
designed to substantially prevent the passage ofi interstitial fluid
therethrough
when the pressure within a beneficial agent reservoir is below a lower
predetermined pressure and passage of beneficial agent therethrough when.
Is the pressure within a beneficial agent reservoir is above an upper
predetermined pressure. This is accomplished by the narrowing of the fluid
flow channel when the pressure within the beneficial agent reservoir is either
below a lower predetermined pressure or above a higher predetermined
pressure. At exceptionally high pressure, the valve can be designed to close
2o altogether at the orifice end, or it can provide a minimal leak path so
that a
maximum fluid flow is never exceeded. At zero or very low pressures, the
valve will close completely or provide a minimal leak path at the beneficial
agent reservoir end, thereby substantially isolating the agent formulation
from
external fluid infiltration and eliminating diffusion of external fluid into
the
2s beneficial agent formulation. While these performance criteria can be
achieved with various discrete components (e.g., relief valve, flow
restrictor,
check valve), this invention combines all the desired performance with a
single, simple, and low cost mechanism.
[00032] While it is impractical to expect that external effects, such as
3o external pressure or temperature changes on the delivery system may be
regulated or eliminated, the present invention minimizes the error
contribution ,
of them by requiring a significant increase in the overall pressure at which
the
system still dispenses beneficial agent. By forcing the system to pump or
8
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
deliver beneficial agent at higher pressures, there is a reduction in overall
variability of the pumping or delivery rate. As an example, a 0.01 psi (pounds
per square inch) pressure increase will contribute substantially more error to
a
system dispensing at a nominal pressure ofØ10 psi than a 0.10 psi increase
s will to a system dispensing at 3 psi (10% vs. 3%).
[00033] FIG. 1 illustrates an implantable .osmotically driven beneficial
.agent delivery system 1 having a capsule 2. The capsule 2 has an
impermeable outer layer and includes a beneficial agent reservoir 50 and an
osmotic agent reservoir 52. The beneficial agent delivery system 1 also
to preferably includes a movable piston 54 positioned between the beneficial
agent reservoir 50 and the osmotic agent reservoir 52. A fluid permeable
membrane 56 is provided at the fluid uptake end 16 of the beneficial agent
delivery system 1. The fluid permeable membrane 56 can be any suitable
membrane or combination of membranes in a shape that can adequately
is . control the amount of fluid entering into the capsule, 2. Additionally,
the
membrane 56 should also be selected to prevent the compositions within the
capsule 2 from passing out of the capsule. A valve assembly 10 is provided
at the beneficial agent delivery end 14 of the capsule.
[00034] The capsule 2 must be sufficiently strong to ensure that it will not
zo leak, crack, break or distort so as to expel its beneficial agent contents
under
stresses it would be subjected to during use while being impermeable. In
particular, it should be designed to withstand the maximum osmotic pressure
that could be generated by the water-swellable osmotic agent in reservoir 52.
Capsule 2 must also be chemically inert and biocompatible, that is, it must be
zs non-reactive with the beneficial agent formulation as well as the body.
Suitable materials generally comprise a non-reactive polymer or a
biocompatible metal or alloy. The polymers include acrylonitrile polymers
such as acrylonitrile-butadiene-styrene terpolymer, and the like; halogenated
polymers such as polytetrafluoroethylene, polychlorotrifluoroethylene,
3o copolymer tertrfluoroethylene andhexafluoropropylene; polyimide;
polysulfone; polycarbonate; polyethylene; polypropylene; polyvinylchloride-
acrylic copolymer; polycarbonafe-acrylonitrile-butadiene-styrene; polystyrene;
and the like. The water vapor transmission rate through compositions useful
9
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
for forming the reservoir are reported in J. Pharm. Sci,. Vol. 29, pp. 1634-37
(1970); Ind. Eng. Chem., Vol. 45, pp. 2296-2306 (1953); Materials
Engineering, Vol. 5, pp. 38-45 (1972); Ann. Book ofASTM Stds., Vol. 8.02,
pp. 208-211 and pp. 584-587 (1984); and Ind. and Eng. CJ~em., Vol. 49, pp.
s 1933-1936 (1957). The polymers are known in the Handbook of Common
Polymers by Scott and Roff, CRC Press, Cleveland Rubber Co., Cleveland,
Ohio. Metallic materials useful in the invention include stainless steel,
titanium, platinum, tantalum, gold and their alloys as well as gold-plated
ferrous alloys, platinum-plated ferrous alloys, cobalt-chromium alloys and
to titanium nitride coated stainless steel. A reservoir made from titanium or
a
titanium alloy having greater than 60%, often greater than 85% titanium is
particularly preferred for most size-critical applications.
[00035] The osmotic agent reservoir 52 may contain any suitable osmotic
agent, examples of which include, but are not limited to, a non-volatile water
.
is soluble osmagent, an osmopolymer which swells on contact with water, or a
mixture of the two. Representative osmagents or osmopolymers are
described, for example, in U.S. Patents 5,413,572 and 6,270,787, which are
hereby incorporated by reference. Osmotic, agents, such as sodium chloride
with appropriate lubricants, binders, and viscosity modifying agents, such as
2o sodium carboxymethylcellulose or sodium polyacrylate can be prepared in
various forms. Sodium chloride in tablet form is a preferred water swellable
agent as described, for example, in U.S. Patent No. 5,728,396, which is
hereby incorporated by reference. The osmotic agent should be capable of
generating between 0 and 5200 psi.
2s [00036] Materials for the fluid permeable membrane 56 are those that are
semipermeable and that can conform to the shape of the reservoir upon
wetting and makes a water tight seal with the rigid surface of the reservoir.
The semipermeable membrane expands as it hydrates when placed in a fluid
environment so that a seal is generated between the mating surfaces of the
so membrane and the reservoir. The polymeric materials from which the
membrane may be made vary based on. the pumping rates and device
configuration requirements and include but are not limited to plasticized
cellulosic materials, enhanced polymethylmethacrylate such as
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
hydroxyethylmethacrylate (HEMA) and elastomeric materials such as
polyurethanes and polyamides, polyether-polyamide copolymers,
thermoplastic copolyesters and the like. Further semipermeable compositions
are described in U.S. Patents 5,413,572 and 6,270,787, which are hereby
s incorporated by reference.
[00037] The movable dividing member 54 can be of any shape that
isolates the water-swellable agent from the beneficial agent formulation,
including, but not limited to a sheet or a piston. The movable dividing member
isolates the water-swellable agent in chamber 52 from the beneficial agent
io formulation in chamber 50 and must be capable of sealably moving under
pressure within capsule 2. The movable dividing member 54 is preferably
made of a material that is of lower durometer than the capsule 2 and that will
deform to fit the interior of the capsule to provide a fluid-tight
compression'
seal with the capsule 2. The materials from which the movable dividing
is member or piston is made are preferably elastomeric materials that are
impermeable and include but are not limited to polypropylene, rubbers such
as EPDM, silicone rubber, butyl rubber, and the like, fluoro elastomers,
perfluoro elastomers, and thermoplastic elastomers such as. plasticized
polyvinylchloride, polyurethanes, Santoprene~, C-Flex~ TPE, a styrene-
2o ethylene-butylene-styrene copolymer (Consolidated Polymer Technologies
Inc.) and the like. The movable dividing member may be of a compression-
loaded design.
[00038] Implantable drug delivery devices of this invention are useful to
deliver a wide variety of active agents. .These agents include but are not
2s limited to pharmacologically active peptides and proteins, genes and gene
products, other gene therapy agents, and other small molecules. The
polypeptides may include but are not limited to. growth hormone, somatotropin
analogues, somatomedin-C, Gonadotropic releasing hormone, follicle
stimulating hormone, luteinizing hormone, LHRH, LHRH analogues such as .
30 leuprolide, nafarelin and goserelin, LHRH agonists and antagonists, growth
hormone releasing factor, calcitonin, colchicine, gonadotropins such as
chorionic gonadotropin, oxtocin, octreotide, somatotropin pluss an amino acid,
vaspressin, adrenocorticotrophic hormone, epidermal growth factor, prolactin,
11
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
somatostatin, somatotropin plus a protein, cosyntropin, lypressin,
polypeptides such as thyrotropin releasing hormone, throid stimulation
hormone, secretin, pancreozymin, enkephalin, glucagons, endrocrine agents .
secreted internally and distributed by way of the bloodstream, and the like.
s Further agents that may be delivered include a~antitrypsin, factor VIII,
factor
IX and other coagulation factors, insulin and other peptide hormones, adrenal
cortical stimulating hormone, thyroid stimulating hormone and other pituitary
hormones, interferon iricluding but not limited to a, ~3, and 8,
erythropoietin,
growth factors such GCSF, GMCSF, insulin-like growth factor 1, tissue
io plasminogen activator, CD4, dDAVP, interleukin-1 receptor antagonist, tumor
necrosis factor, pancreatic enzymes, lactase, cytokines, interleukin 2, tumor
.
necrosis-factor receptor, tumor suppresser proteins, cytotoxic proteins, and
recombinant antibodies and antibody fragments, and the like.
[00039] - The above agents are useful for the treatment of a variety of
is conditions including but not limited to hemophilia and other blood
disorders,
growth disorders, diabetes, leukemia, hepatitis, renal failure, HIV infection;
hereditary diseases such as cerbrosidase deficiency and adenosine
deaminase deficiency, hypertension, septic shock, autoimmune diseases
such as multiple sclerosis, Graves disease, systemic lupus erythematosus
zo and rheumatoid arthritis, shock and wasting disorders, cystic fibrosis,
lactose
intolerance, Crohn's diseased, inflammatory bowel disease, gastrointestinal
and other cancers.
[00040] The active agents may be anhydrous or aqueous solutions,
suspensions or complexes with pharmaceutically acceptable vehicles or
2s carriers such that a flowable formulation is produced that may be stored
for
long periods on the shelf or under refrigeration, as well as stored in an
implanted delivery system. The formulations may include pharmaceutically
acceptable carriers and additional inert ingredients. The active agents may
be in various forms, such as uncharged molecules, components of molecular
3o complexes or pharmacologically acceptable salts. Also, simple derivatives
of
the agents (such as prodrugs, ethers, esters, amides, etc.) which are easily
hydrolyzed by body pH, enzymes, etc., can be employed.
12
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
[00041] Valve body 30 and 32 is preferably made of titanium, steel, and
their alloys, thermoplastics including polyetherether ketone (PEEK) or liquid
crystal polymers (LCP) and the like. More preferably valve body 30 and 32 is
made of a liquid crystal polymer.
s [00042] Spring 24 is preferably made of spring steels including stainless
steel or berillium/copper or injection molded polymer or plastic. The spring
material should provide dimensionality while having a wire thickness that can
be manufactured and inserted into the valve. More preferably spring 24 is
made of stainless steel for a fine wire spring or a suitable plastic for a
thicker
io wire spring. The profile of spring 24 may be round, square, or any other
appropriate shape. Spring 24 provides the fluid path from reservoir 50
through upper port 22.
[00043] Stem 46 and guide post 48 may be made of the same materials
as valve body 30 and 32, or elastomeric materials such as fluoro elastomers,
is perfluoro elastomers, thermoplastice elastorriers such as C-Flex~ or
Santoprene~, hard plastics, or the like. Stem 46 and guide post 48 are
preferably made.of thermoplastic elastomers, or perfluoro e~lastomers, or hard
plastic.
[00044] In operation, fluid from the exterior of the capsule 2 passes
2o through the membrane 56 and into the capsule. Some of the fluid is then
absorbed by the osmotic agent in reservoir 52, thereby causing the osmotic
agent to swell. As the osmotic agent swells, the increased volume thereof
causes the piston 54 to push the beneficial agent housed in the beneficial
agent reservoir 50 to be dispensed through the valve assembly 10 and into
2s the patient's body. However, the beneficial agent is only dispensed through
the valve assembly 10 when the pressure within the capsule 2'is greater than
a lower predetermined pressure. The mechanics of the valve assembly 10
will be described in greater detail below with reference to FIGS. 1-5.
[00045] As can be seen in FIGS: 1 and 2, the valve assembly 10 includes
3o a valve body 12 containing a plurality of intercorinected fluid chambers 60
and
70. The valve assembly should have a height measurement larger than the
diameter measurement. In other words, the ratio of the height to width of the
valve assembly should be greater than 1/1. The-height to width ratio of the
13
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
valve assembly should be less than 115. Preferably the height to width ratio
of
the valve assembly is between 1/1. at~d 1/2. The valve assembly preferably
has a diameter of about 1 to about 10 mm, more preferably about 3 to about 6
mm. The valve assembly preferably has a height of about 5 to about 10 mm.
s~ [00046] The valve body 12 preferably includes two identical halves 30
and 32. The valve assembly 10 further includes a lower port 20 and an upper
port 22. A lower fluid chamber 60 is positioned adjacent to and in fluid
communication with the lower port 20. An upper fluid, chamber 70 is
positioned between and in fluid communication with the upper port 22 and the
to lower fluid chamber 60.
[00047] The lower fluid chamber 60 includes a first surFace 62.having a
conical frustum shape and a second surface 64 having a cylindrical shape.
The diameter of the lowermost portion of the first surface 62 is smaller than
the diameter of the lower port 20. The. diameter of the uppermost portion of
is the first surface 62 is substantially the same as the diameter of the
second
surface 64. The lower fluid chamber 60 also includes a third surface 66 that
is
substantially perpendicular to the second surface 64.
[00048] A passageway 74, formed at the intersection of the third surface
66 and the upper fluid chamber 70, is provided between the upper and lower
20 . 60 fluid chambers. The diameter of the upper port 22 is substantially
smaller
than the diameter of-the upper fluid chamber 70 and a thus top surface 72
(also substantially perpendicular to 64) is provided therebetween.
[00049] As illustrated in FIG. 2, the valve assembly 10 contains a
movable closing member 40 having a~ cylindrical seal 44 and a conical frustum
2s 42 attached to an elongated cylindrical stem 46 (shown more clearly in FIG.
2b) and a guide post 48. Stem 46 is slightly smaller in diameter than spring
24. Guide post 48 should have a diameter slightly smaller than the diarrieter
of upper port 22. The movable closing member 40 also includes a
substantially flat upper surface 90. The closing member 40 and the cylindrical
3o stem 46 may be fabricated as a single piece, preferably by molding, or they
may be separately fabricated and attached in any known manner.
Additionally, the stem 46 may be fabricated with a threaded end that is
14
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
configured to mate with a threaded opening provided on the upper surface 90
of the closing member .40.
[00050]~ The movable closing member 40 can be moved from a
lowermost position substantially adjacent the first surface 62 to an uppermost
position substantially adjacent the third surface 66. The conical frustum 42
of
the closing member 40 is shaped to substantially mate with the first surface
62 when the closing member is in the lowermost position. Additionally, the
upper surface 90 of the closing member 40 is also shaped to substantially ,
cover the third surface 66 of the lower fluid chamber 60 when the closing
io member is in the uppermost position. When the movable closing member 40
is in either of the above described positions, the flow of berieficial agent
from
the beneficial agent reservoir 50 through the valve assembly 12 is
substantially impeded.
[00051] A spring 24 is provided around the cylindrical stem 46 and
is between the top surface 72 and the upper surface 90. The spring 24 is
preferably a helical compression spring and is shown as such in FIG. 1.
However, it is to be understood that any other~suitable spring may be used in
place of the helical compression spring.
[00052] At zero or low pressures (0.5 to 10 psi, for example), such as can
2o be expected during storage or initial pump startup, the spring 24 maintains
the
closing member 40 in a position to substantially prohibit fluid flow in either
direction of the valve assembly 12. Cylindrical seal 44 prevents fluid flow
across the lower port 20 such that there is substantially no fluid
communication between any beneficial agent contained within the beneficial
2s agent reservoir 50 and, once implanted, interstitial fluid present at the
upper
port 22. Further, as can be seen in FIG. 2, movable closing member 40 is
designed to travel through some predetermined axial displacement while still
maintaining a seal at lower port 22. This occurs because the cylindrical seal
44 has a height that is greater than the height of the lower port 20. This
3o feature allows the valve assembly 10 to contain the increased agent
formulation volume that results from thermal expansion upon implantation
without the startup burst that occurs in many devices.
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
(00053] The pressure necessary to either keep valve 10 in the closed
position (as illustrated in FIGS. 2 and 4) or in an open position (as
illustrated
in FIG. 3) is dependent, for example, on the viscosity of the beneficial agent
formulation; the desired rate at which the beneficial agent formulation is
s delivered from the system; the spring constant of spring 24; and/or the
amount of room spring 24 takes up in valve 10.
(00054] The psi (pounds per square inch) range from low to high .
pressure (from valve open to valve closed) needs to be very narrow, but could
be anywhere in the range of about 0.1 to about 2000 psi. Preferably the
io range is about 0.5 to about 100 psi.
(00055] The valve assembly 10.is fabricated by positioning the helical
compression spring 24 over the cylindrical stem 46 of the movable closing
member 40. The assembly 10 is subsequently captured between the two
valve body halves 30 and 32 with the conical frustum 42 and cylindrical seal
is 44 oriented to engage the first surface 62 of lower fluid chamber 60 and
the
lower port 20, respectively. The resulting assembly will cause the spring 24
to
be under a compressive load, forcing the conical frustum 42 to seal against
the first surface 62 at lower fluid chamber 60. Consequently, the valve
assembly 10 is normally closed to~fluid flow at the lower port 20. The body
2o halves 30 and 32 can be sealed together in any of a number of ways known in
the art. For example, using adhesives, ultrasonic welding, or mechanical
mating.
(00056] FIG 3 illustrates the valve operation once the fluid pressure at the
lower port 20 exceeds the minimal value (for example, about 5 psi) as would
2s be the case for normal operation. In this case, the movable closing member
40 will be displaced axially upward toward the upper port 22, creating an
opening at the lower port 20 and allowing the beneficial agent from the agent
reservoir 50 to be pumped through the lower port 20 then through,
successively, fluid chambers 60 and 70, and finally exiting upper port 22. The
3o cross-sectional area of the opening, and thus the fluid flow, is directly
proportional to the pressure applied by the fluid against the movable closing
member 40 until such time as the pressure begins to approach some
predetermined maximum value. In this case, the valve action is reversed as
16
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
the upper surface 90 of the closing member 40 approaches the third surface
66 of the lower fluid chamber 60.
[00057] The spring 24 defines a spiral fluid flow path through the upper
fluid chamber 70. The spring 24 compresses as the movable closing member
s 40 is forced upward by the flowing agent. Consequently, as the fluid
pressure
inside the beneficial agent reservoir 50 and the chamber 60 increases, the
lower port 20 opens more fully while the fluid flow path progressively
narrows,
thus becoming more restrictive. Normal flow will cause a balance between
the opposing forces of the spring and the fluid pressure while low fluid flow
will
io typically be completely impeded by the compression spring 24 causing lower
port 20 to be closed by the movable closing member 40. On the other hand,
high fluid flow will typically be substantially impeded by the upper surface
of
the movable closing member 40 substantially closing off the opening 74.
Compression of spring 24 reduces the flow path between lower fluid chamber
is 60 and upper port 22.
[00058] FIG. 4 shows the valve condition when a maximum pressure is
reached (for example, about 20 psi). The movable closing member 40 has
been driven in FIG. 4 to its uppermost position, forcing the movable member
against third surface 66 of Lower fluid chamber 60. This both limits the
travel
20 of the movable closing member 40 and either closes fluid communication
between lower fluid chamber 60 and upper fluid chamber 70, or, in the
preferred embodiment, limits the fluid flow around the movable closing
member 40 to some predetermined minimal amount via a small fluid bypass
channel around movably closing member 40. As the pressure is relieved, the
2s fluid path increases in cross-sectional area at the upper surface-66,
thereby
again allowing increased fluid flow. In this manner, fluid flow is
continuously
regulated to compensate for pressure and temperature variations, which
would otherwise cause sub-optimal performance.
[00059] The above detailed description refers to a particular embodiment
30 of the present invention. However, it should be obvious from the above
disclosure that a broad range of materials, fabrication technologies, and
alternative embodiments can be readily achieved. One further embodiment of
this invention includes a separate small fluid bypass channel that can be
17
CA 02466816 2004-05-20
WO 03/045352 PCT/US02/37465
formed by either a small hole through movable closing member 40 or a notch
formed in one edge of movable closing member 40.
[00060] As an example, FIG. 5 illustrates another preferred embodiment
of the present invention, in which, a valve assembly 80 can be fabricated as a
s silicon microstructure or molded in thermoplastic. As seen in FIG. 5, the
valve
assembly 80 includes a single chambered valve body 81 having an integrally
formed cantilever spring arm 82 in place of the compression spring described
hereinabove. The cantilever spring arm may be made of. metal (such as those
described above for valve body 30 and 32) or a thermoplastic. Additionally,
io the movable closing member 86 is in the form of a spheroid and is attached
to
the free end of the cantilever spring arm 82. The movable closing member 86
may be made of a metal or metal alloy (such as those described above for
valve body 30 and 32), a thermoplastic, or an elastomer. The upper and
lower ports of this embodiment do not have to have the same. diameter, as
is long as movable closing member 86 closes off the vertical portion of the
upper
or lower port when pressure is either lower or higher than a predetermined
pressure. However, other shapes may also be used for the closing member.
86. One potential benefit is that this embodiment presents an integral
structure rather than an assembly and discrete components. Still another
2o benefit is its extremely small overall size.
[00061] Furthermore, while the above description has described
application to an osmotically-driven agent delivery system in particular, it
should be obvious that the present invention may be applied to any
pressurized fluid delivery system.
2s [00062] ,The above-described exemplary embodiments are intended to be
illustrative in all respects, rather than restrictive, of the present
invention.
Thus, the present invention is capable of many variations and detailed
implementation that can be derived from the description contained herein by a
person skilled in the art. All such variations and modifications are
considered
30 to be within the scope and the spirit of the present invention as defined
by the
following claims.
18