Note: Descriptions are shown in the official language in which they were submitted.
CA 02469084 2004-06-22
WO 99/13883 PC'fNS98/19.l02 _
-1-
. DESCRIPTION
ANDROGEN AS A MALE CONTRACEPTIVE AND
N011'-CONTRACEPTIVE ANDROGEN REPLACEMEr'T
Tec nical Field
The present invention relates to the fields of medicine and
pharmaceutical science in providing androgen replacement methods of male
contraception and devices useful in accordance with those methods.
Background Art
Male contraception has been enigmatic. Mechanical devices offer
reduced sensation and inconvenience. Vr'hile they may be effective in reducing
the
transmission of sexually transmitted diseases, they may also be a source of
fiiction
in a relationship. Moreover, such contraceptive methods are not without a
significant incidence of failure.
For these and other reasons, chemical methods, such as birth control
pills for women, have been a long sought after objective. However, chemical
protocols for men have been. plagued by their own problems. For example, the
continuous administration of luteinizing hormone releasing hormone (L~ and
its analogs have been proposed as an effective way of suppressing
spermatogenesis.
This is accomplished by blocking gonadotropin secretion. Implants that deliver
LHRH and its analogs therefore represent a potential male contraceptive.
However, the loss of gonadotropins results in diminished secretion of
testosterone
which, amongst. other things, provides a diminished libido and potential loss
of
sexual function. Therefore, along with the administration of LHRH, or another
sterilant, the art recognizes the need for the co-administration of an
androgen. See
U.S. Patent No. 5,733,565. An androben, often testosterone, is supplied to
help
bolster the body's testosterone level and sustain normal sexual function. This
sort
of androgen supplementation, however, brings with it many problems of its own.
Testosterone is expensive and relatively large quantities need to be
administrated,
usually by injection, on a daily basis. Testosterone is also Sa-reduced to DHT
(Dihydrotestosterone) which is itself a very potent agent. It can over-
stimulate
prostate growth, potentially leading to complications such as BPH (benign
prostate
hypoplasia).
CA 02469084 2004-06-22
WO 99/13883 PCTNS98/19402
-2-
In view of the foregoing, there remains a need for an effective
method of male- contraception with a minimum of health risks and further
complications. That need is satisfied by the present invention.
Sutnmar~r c~f the Invention
The present invention is based on, amongst other things, the
discovery that an androgen can be used alone as an effective contraceptive;
not
merely as an androgen supplement. By using an androgen as a contraceptive, one
is
able to eliminate the need for combination therapies using androgens and
sterilants
simplifying contraception and lowering costs dramatically. Moreover, the
androgens used in accordance with the present invention not only act as
contraceptives, but also maintain male sexual function and desire, without the
side
effects and adversities realized by the administration of 5oc-reducible
androgens
such as testosterone.
In accordance with a preferred embodiment, the present invention
relates to methods of male contraception which include administering to a non-
sterile male subject a predetermined amount of an androgen which is sufficient
to
render that male subject reversibly sterile. If the dosage forms used in the
methods
of the present invention are for daily dosing, then sufficient androgen must
be
provided for that day. If the dosage form is active over a plurality of days,
then
sufficient androgen must be provided for the entire period. Stated another
way, the
amount of androgen administered must be sufficient to render the subject
sterile
each day over the predetermined period of time.
The androgen, when provided in the amounts contemplated will
result in blood levels of luteinizing hormone (LH) and follicle stimulating
hormone
?5 (FSH) of 2.5 International units/litre ("IU/I.") or less and blood levels
of
testosterone (T) of 10 nmol/L or less Preferably, the androgen is a non 5a-
reducible androgen, such as a 7a.-modified androgen. More preferably, the
androger~ when provided in the amounts contemplated,.will result in blood
levels of
LH and FSH of 2.0 IU/L or less and blood levels of T of 4nmoUL or less. Most
preferably, the androgen when provided in the amounts contemplated, will
result in
blood levels of LH and FSH of 1.0 IU/L or less, and blood levels of T of 3
nmol/L
or less. Assuming that the androgen used is MENT, a steady-state blood level
of
CA 02469084 2004-06-22
WO 99/13883 PCT/US98/19402
-3-
more than 1.0 nmolllitre ("nmoUL") should result. Preferably, blood levels of
MENT of 1.5 nmoUL and even more preferably of 2 nmoUL or more will result.
If the goal is to provide contraception over a period of six months,
then a sufficient amount of the androgen as described above must be supplied
to the
subject each day so as to ensure contraceptive efficacy each day for six
months.
That means that the blood levels should, at Least generally, .be within the
specified
limits for LH, FSH and testosterone as discussed above. Of course, day-to-day
variation is to be expected based on the subject, the mode of administration,
and the
like. However, on balance, the subject should receive enough androgen,
preferably
each day, to ensure that over the six month period the subject is, for all
practical
purposes, incapable of reproduction.
Dosage forms which contain a sufficient quantity of the specified
androgens are also contemplated. These dosage forms, unlike those that have
gone
before, will provide sufficient androgen to be contraeeptiveiy effective
without also
causing certain side effects such as, for example, overstimulating the
prostate. The
dosage forms contemplated include, without limitation, the androgen and a
pharmaceutically acceptable carrier.
With the understanding that androgens can be used alone as a
contraceptive also comes a new understanding of the use of androgens in
androgen
replacement therapy. There are certainly instances when one would wish to
bolster
or augment a subject's androgen level to provide a certain therapeutic effect,
i_e.,
enhanced sexual drive, v~~ithout also providing contraception. Therefore, the
present invention also relates to methods of androgen replacement therapy
which
comprise the administration of androgen to a male subject in an amount which
is
sufficient to treat the subject's condition without being contraceptively
effective.
The androgen, when provided in the amounts contemplated in accordance with
this
aspect of the present invention, will result in blood levels of LH and FSH of
greater
than 2.5 IL/L and blood levels of testosterone of greater than 10 nmoUliter.
Preferably, the androgen is a non-Sa-reducible androgen such as a 7a-modified-
androgen. This means that the amount of androgen provided is less than that
necessary to provide effective contraception, yet sufficient to provide some
level of
CA 02469084 2004-06-22
-4-
other therapeutic effect. Dosage forms useful in accordance with this aspect
of the invention
are also contemplated.
In a broad aspect, then, the present invention relates to a method of male
contraception comprising the step of administering to a non-sterile male
subject a
predetermined amount of an androgen which is sufficient to render said male
subject reversibly
sterile for a predetermined period of time and which provides said male
subject with blood
levels of LH and FSH of 2.5 IU/L or less and testosterone of 10 nmol/liter or
less.
In another broad aspect, the present invention relates to a method of male
contraception comprising the step of administering to a non-sterile male
subject a
predetermined amount of a non-Sa-reducible androgen which is sufficient to
render said male
subject reversibly sterile for a predetermined period of time and which
provides said male
subject with blood levels of LH and FSH of 2.5 IU/L or less and testosterone
of 10 nmol/liter
or less.
In a further broad aspect, the present invention relates to a male
contraceptive
comprising at least a predetermined amount of an androgen which is sufficient
to render a
male subject reversibly sterile for a predetermined period of time and which
provides said
male subject with blood levels of LH and FSH of 2.5 IU/L or less and
testosterone of 10
nmol/liter or less and a pharmaceutically acceptable carrier.
In yet another broad aspect, the present invention relates to a male
contraceptive
comprising a predetermined amount of a non-Sa-reducible androgen which is
sufficient to
render a male subject reversibly sterile for at least one day and a
pharmaceutically acceptable
carrier wherein said androgen is sufficient to maintain blood levels of LH and
FSH above 2.5
IU/L and testosterone about 10 nmol/liter.
CA 02469084 2004-06-22
-4a-
Brief Description of the Drawings
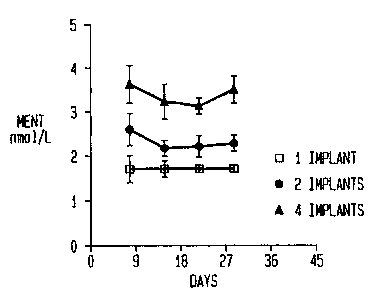
Figure 1 - is a graph of the steady-state blood levels of MENT in
subjects with either one, two.or four MENT Ac implants. Values shown are means
~ SE. Data from three clinics are presented. Figure la (Clinic 1), Figure lb
(Clinic
2) and Figure lc (Clinic 3).
Figure 2 - is a graph of the effect of MENT Ac implants on serum
levels of LH. Values shown are means ~ SE.
Figure 3 - is a graph of the effect of MENT Ac implants on serum
levels of FSH. Values shown are means ~ SE.
Figure 4 - is a graph of the effect of MENT Ac implants on serum
levels of T. Values shown are means ~ SE.
Figure 5 - is a graph of the effect of MENT Ac implants on serum
levels of DHT. Values shown are means ~ SE.
Figure 6 - is a graph of the "average" daily in vivo release of MENT
Ac from either one, two or four MENT Ac implants recovered from subjects.
Values are means f SE. Number shown in each bar graph indicates sample size.
Figure 7 - is a graph of the effect of MENT by intramuscular
injection (im) on serum levels of LH in a first mufti-dose study. Values shown
are
means ~ SE.
Figure 8 - is a graph of the effect of MENT by intramuscular
injection (im) on serum levels of FSH in a first mufti-dose study. Values
shown are
means ~ SE.
Figure 9 - is a graph of the effect of MENT by intramuscular
injection (im) on serum levels of T, in a first mufti-dose study. Values shown
are
means ~ SE.
Figure 10 - is a graph of the effect of MENT by intramuscular
injection on serum levels of LH in a second mufti-dose study. Values shown are
means t SE.
CA 02469084 2004-06-22
WO 99/13883 PCTNS98119402
-5-
Figure 11 - is a graph of the effect of MENT by intramuscular
injection on serum levels of FSH in a second multi-dose study. Values shown
are
means ~ SE.
Figure 12 - is a graph of the cffect of MENT by intramuscular
injection on serum levels of T in a second mufti-dose study. Values shown are
means ~ SE.
Best Mode of Carrvine Out Invention
Before discussing the invention in detail, some definitions are
appropriate. A "male subject," in accordance with the present invention, is a
male
mammal. Preferably, however, a male subject is a human male. All dosages
discussed herein relate to an "average" sized human male. However, dosages can
be scaled accordingly, using known principals of pharmacy, to accommodate
other
species of mammal.
A "non-sterile" male subject is a male subject capable of producing a
sufficient quantity of viable sperm which, under the right conditions, can
lead to~ '.
reproduction. A "sterile male" or "sterile male subject" is a male who does
not
produce sperm, does not produce sperm in sufficient quantity, or does not
produce
sufficient viable sperm so as to make reproduction difficult and preferably
impossible. While a non-sterile man normally produces about 100 to 300 million
sperm per milliliter of ejaculate, clinically sterile males generally produce
less than
about 3 million sperm per milliliter of ejaculate or less. While it is still
possible,
even at 3 million sperm per milliliter of ejaculate, to fertilize an egg;
statistically
speaking, the chances of that happening are greatly reduced. Indeed, this
level has
been acknowledged as "clinical sterility." See "W.H.O. Laboratory Manual for
the
Examination of Human Semcn and Sperm - Cervical Mucus Interaction," 3d Ed.
Cambridge Univ. Press, 1992.
It is a goal of one aspect of the present invention to provide
reversible sterility. "Reversible," in the context of the present invention,
means that
once the methods of the present invention are discontinued, a male subject
previously undergoing treatment wiU be returned to a condition which will
permit
reproduction under normal conditions. At the very least, the androgen will not
present a continued obstacle to reproduction after therapy is discontinued.
CA 02469084 2004-06-22
WO 99113883 PC'T/US98/19402
-6-
Reversible does not necessarily mean instantaneous. Full potency may not be
restored for days or even weeks after treatment is discontinued. -
"Androgen," in accordance with the present invention, is any natural
or synthetic male sex hormone including analogs and salts thereof which are
pharmaceutically acceptable and which, when administered in accordance with
the
present invention, provide contraceptive efficacy. Contraceptive efficacy in -
accordance with the present invention can be defined by the level of sperm
produced as defined above in terms of sterility. However, preferably,
contraceptive
efficacy and sterility are defined herein by the blood levels of certain
compounds
normally found in the circulating blood of male subjects. The androgen, when
provided in the amounts contemplated, will result in blood levels of LH and
FSH of
2.S IU/L or less and blood levels of testosterone of 10 nmoUliter or less.
More
preferably, the androgen, when provided in the amounts contemplated, will
result in
blood levels of LH and FSH of 2.0 lU/L or less and blood levels of
testosterone of
1S 5 nmoUliter or less and even more preferably blood levels of LH and FSH of
1.S ~ ~~
IU/L or less and blood levels of testosterone of 4 nmoVliter or less. Most
preferably, the androgen, when provided in the amounts contemplated, will
result in
blood levels of LH and FSH of 1.0 IU/L or less and blood levels of
testosterone of
3 nmoUliter or less.
Preferably, the androgen used is a non-Sa-reducible androgen.
Testosterone is excluded by this definition as it is a Sec-reducible androgen,
and as
such, can produce higher levels of adverse side effects than equivalent
potencies of
other androgens as described. Its use would also not lower serum testosterone
levels. Non-Sa-reducible androgens include, without limitation, 7a-modified-
2S androgens. Examples of these include 7a-alkyl-androgens such as 7a-methyl-
14-
dehydro-19-nortestosterone (CDB-868B), 7a-methyl-17a(3-propionyloxy-D-
homoestra-4, 16, dien-3-one (CDB 2322A) and 7oc-methyl-19-nortestosterone
(I~~N'f) and their pharmaceutically acceptable salts. See Kumar et al., "The
Biological Activity of 7a-h4ethyl-19-Nortestosterone Is Not Amplified in Mate
Reproductive Tract as is That of Testosterone," Endocrinology, Vol. 130, No.
6,
pgs. 3677-3683 (1992). The preferred androgen is MENT, its acetate, MENT Ac
and related compounds.
CA 02469084 2004-06-22
~ ' -
. Other androgen compounds useful in the method of the invention are
testosterone derivatives having a non~hydrogen substituent in the 6ac or 7a
position. As used in the application, the term testosterone derivatives
encompasses
compounds having the basic four ring structure of testosterone, optionally
modified
at the 3, 5, 9,11,17 or 19 positions. Eicamples of such compounds include: .
7-a -methyl testosterone,
7-ac-methyl-11~ -hydroxytestosterone,
7-a ,17-d'tmethyltestosterone,
7-a ,17-dimethyl-11 ~i -hydroxytestosterone,
7-ac ,17-dimethyl-t9-nortestosterone, .
7-a ,17-dimethyl-11 ~i -hydroxy-19-nortestosterone,
6-a -methyl testosterone,
6-a -methyl-19-nortestosterone,
6-ac -methyl-11 ~i -hydroxytestosterone, ..
6-a ,I7-dimethyltestosterone,
6-a ,17-dimethyl-11 j3 -hydroxytestosterone,
6-a ,17-dimethyl-19-nortestosterone, and
6-a ,17-dimethyl-llp -hydroxy-19-nortestosterone
2p The 7~-methyl testosterone compounds for use in the invention can be
prepared as described in U.S. Pat. No. 3,341,557. Synthesis of the other
compound
identified herein have also been described in the literature.
Preferably, the androgens used should have a minimum of side
effects. One method of determining the degree of side effects exhibited by a
particular androgen is by measuring its ability to stunulate the prostate.
Compounds such as testosterone can overstimulate the prostate. MEN'T, however,
stimulates the prostate to a much lower degree, particularly in the potency
ranges
contemplated as part of the present invention. Indeed, as shown in Figure 5,
levels
of the metabolite DHT actually decreased along with the levels of LH, FSH and
testosterone since, by use of the present invention, the bodies production of
testosterone is reduced.
CA 02469084 2004-06-22
WO 99/13883 PCT/US98/19402
_$_
The "predetermined amount" or "amount" of androgen as used in
accordance with the present invention can vary widely, depending on a number
of
factors known to practitioners of the medical and pharmaceutical sciences. The
predetermined amount can depend on factors such as the size, weight, hormone
level and age of the subject, the subject's sperm count, the type of androgen
used,
the possibility of interactions with other therapies and the type of dosage
form used.
For example, more androgen may need to be administered to a subject
transdermally to arrive at the appropriate blood level when compared to the IV
administration of the same androgen. In addition, the properties of a salt
form of an
androgen may affect the transdermal qualities of a drug when compared to other
forms of the same androgen.
Also important to a determination of the amount of androgen to be
delivered each day is the objective. More androgen is needed for contraception
than non-contraceptive androgen replacement. Because of the wide variation in
these factors, factors which will vary from dosage form to dosage form, from .
androgen to androgen and from subject to subject, it is far more convenient to
describe the amount of androgen useful in terms of the amount of a particular
androgen which is necessary to produce certain levels of various chemicals in
the
subject, on average, each day of treatment. As previously discussed, when the
objective is male contraception, the amount contemplated, will result in blood
levels
of LH and FSH of 2.5 TU/L or less and blood levels of testosterone of 10
nmol/liter
or less. At these levels, on the average, a person has been provided with an
amount
of androgen necessary to provide a contraceptive effect each day, i.e., over a
24
hour period. If non-contraceptive androgen replacement is the object, then
blood
levels of LH, FSH, and testosterone should be higher than 2.5 IU/L and 10
nmoUliter respectively.
A "predeternvned time" in accordance with the present invention is
preferably at least a plurality of days and preferably, at least seven days.
Even more
preferably, the amount of androgen administered will be sufficient to provide
contraceptive efficacy each day over a 30 day period of time and most
preferably
over at least about 180 days. Thus, when a device is to be used to provide
contraception over a predetermined time of about 180 days, for example, the
device
CA 02469084 2004-06-22
WO 99/13883 PCTNS98/19a02
-9-
must comprise sufficient androgen to provide the desired blood levels of LH,
FSH
and testosterone each day over that period of time. Of course, it may take 30
or
more days before the administration of androgen at the levels described herein
is
eontraceptively effective.
Us'mg MEhIT Ac as. a more specific example, an implantable rod, as
described in the examples, which is capable of releasing about S00 micrograms
of
MEl~fT Ac over about 24 hours, and which contains sufficient MENT Ac to
provide
about 500 micrograms of MENT each day for about 28 days, (see Figure 6) can
produce daily blood levels of LH which average below about 2 IU/L, levels of
FSH
which average just above about 2 IUIL and levels of testosterone of about 5.0
nmoUliter. See Figures 2-4 which respectively show the blood levels of LH, FSH
and testosterone resulting from the administration of various daily amounts of
MENT Ac. The "open squares" connected by a line represent the blood levels
resulting from the use of a single implant as described above. As shown in
Figure 1,
senrm levels of MENT of between I .0 and 2.0 nmoUL also resulted from the use
of .
a single implant. The use of two such implants simultaneously, (Figures 2-4
and the
"closed circles" connected by a line) or the use of a single implant which
could
introduce about 1,000 micrograms of MENT Ac into a subject's blood each day as
described can produce daily blood levels of LH which average less than 0.5
ILT/L,
levels of FSH which average just above less than 0.5 IU/L and levels of
testosterone
of about 1.0 nmollliter. Serum levels of 1~ZENT were, on average, between
about
2.0 and about 2.5 nmol/L. See Figure 1.
As demonstrated in Figures 2-4, the use of additional MENT Ac
from implanted, continuous release rods, over 1000 micrograms per day, did not
~5 result in significantly lower levels of LH, FSH or testosterone. The
simultaneous
use of four 500 microgram per day implants resulted in levels of LH, FSH and
testosterone which were very comparable to those obtained at the 1000
microgram
per day level. That means that for MENT/NiENT Ac, the amount of androgen
which should be administered to a subject each day for the entire length of
treatment should vary from between about 200 to about 2000 micrograms. More
preferably, the amount of h'~NT administered each day will range from between
about 400 and about 1500 micrograms and even more preferably, the amount of
CA 02469084 2004-06-22
WO 99/13883 PCTNS98I19402 .
- 10-
MENT administered each day will range from between about 500 to about 1000
micrograms per day.
As Example 2 demonstrates, the rate of administration may also be
important in detenmining the amount of androgen which needs to be
administered.
An implant can administer 1000 micrograms of MENT Ac at roughly a constant
rate over the course of 24 hours each day. However, when an equal dose of
MENT, 1 mg, was administered by i.m. injection, the results, at least in terms
of
testosterone, were inadequate for contraceptive purposes. While the fiill 1 mg
would make it to the blood stream, additional MENT was necessary to ensure
that
throughout a 24 hour period, the steady state blood levels are maintained
sufficiently high, i.e. at a constant serum level comparable to that obtained
by
dosing the same amount using an implant.
As shown in Figures 9 and 12, for example, the use of 4 mg of
MENT by once-a-day, i.m. injection is sufficient to ensure that some level of
effective contraception is obtained. Without wishing to be bound by any
particular
theory, it is believed that at that level, for example, blood levels of MENT
remain
sufficiently high throughout the day to provide effective contraception
roughly
equivalent to that which is obtained by the use of 500 microgram MENT Ac
implants as illustrated in Figures 2 through 4. Therefore, the term
"bioavailable
amount" as used herein means an amount sufficient so as to provide minimum
steady state blood levels of LH, FSH, and testosterone falling within the
levels
discussed herein and effective to provide contraception. The amount of drug
provided in a once-a-day, non-sustained release system may need to be
significantly
higher than needed for constant release dosage forms to provide comparable
minimum steady state drug levels comparable to that obtainable by an implant
or
other long-term constant release systems in accordance with the present
invention
throughout a 24 hour day cycle.
The amounts of other androgens necessary to provide the desired
blood levels of LH, FSH and testosterone will vary as previously described.
However, the amounts can be simply determined by administering a fixed amount
of
an androgen and measuring the resulting blood levels of LH, FSH and
testosterone
using conventional, commercially known techniques. Once the blood levels of
these
CA 02469084 2004-06-22
WO 99/13883 PCTIUS98/19~03
-11
compounds have been determined, the amount of androgen administered can be
adjusted accordingly, either higher or lower, to provide the desired blood
levels of
LH, FSH and testosterone.
LH, FSH and testosterone are more than mere markers indicating
something about the behavior and bioavailability of the androgen. They are
each
important in sperm production. LH stimulates Lydig cells to release
testosterone.
Testosterone is important for spermatogenesis. FSH stimulates germ cells. If
germ
cells are present, but insufficient testosterone is available, spermatogenesis
is
depressed. The reverse is also true. In accordance with the present invention,
sperm production is compromised in two ways. Since FSH is reduced, so too are
germ cells. Moreover, the reduction is LH and testosterone inhibits
spermatogenesis. Yet, sexual function is maintained and potential side effects
reduced or eliminated.
This underscores the importance of using androgens other than
1 S testosterone. The use of testosterone is actually counterproductive as it
elevates ~'
blood testosterone levels and may help support spermatogenesis of any
available
germ cells.
The methods of the present invention can be practiced using
conventional dosage forms and techniques. First, one must select the type of
therapy desired, either contraception or non-contraceptive androgen
replacement.
To a large degree, the objective, the physical condition of the patient, and
the
dosage form will dictate the amount of androgen. Generally, more androgen is
needed for contraceptive methods, less for non-contraceptive androgen
replacement. The androgen selected W It also be important in determining the
dose
as wilt the route of administration. Once the objective, the androgen and the
desired route are selected, the androgen can be administered in conventional
fortttulations and blood tests can be ,given to confirm the resulting blood
levels of
androgen, LH, FSH and testosterone. 1f the levels need to be adjusted, then
the
amount of androgen can be changed, another androgen can be selected or another
dosage form can be employed.
For example, if a subject is to be given 500 micrograms of MENT
per day via a once-a-day transdermal patch, a new patch must be provided and
CA 02469084 2004-06-22
WO 99113883 PCTNS98l19402
- i2 -
applied to the skin of the subject at about the same time each day. The patch
may
contain an excess of MEhIT. But the amount that is bioavailable is 500
micrograms. There may also be an initial burst of androgen when the patch is
first
applied, but the rate of delivery will adjust to a more constant rate and the
average
amount which is bioavailable should fall within the ranges described. If the
steady
state blood levels are, on average over the day, too low, i.e., the blood
levels of LH -
and FSH are above 2.5 IU/L and the level of testosterone is above 10
nmoVtiter, (or
above some lower level as described depending on the needs of the subject and
the
objective of the doctor), several options are open. Subsequent doses
administered
by a transdermal patch can be increased, the dosage form can be changed to,
for
example, a subcutaneous implant andlor a more potent androgen, one which is
compatible with the pharmaceutical carrier and preferably non-Sa-reducible,
can be
used. What is important is the long term maintenance of blood levels of LH,
FSH
and testosterone below certain limits. Therefore, when using MENT transdermal
patches for that purpose, what is important is that minimum steady state blood
.
levels of the androgen be comparable to those which result from the use of
MENT
or MENT Ac containing subdermal implants as discussed herein and as described
in
the examples.
Dosage forms which contain a sufficient quantity of the correct
androgen are also contemplated. These dosage forms, unlike those that have
gone
before, wilt provide sufficient androgen to be contraceptively effective
without
significant side effects. 'The dosage forms contemplated include, without
limitation,
the androgen and a pharmaceutically acceptable carrier.
Pharmaceutically acceptable carriers, in the case of the present
invention, can include, W thout limitation, chemical formulations such as
creams,
salves, lotions, liquids, gets injectable liquids, liquids capable of IV or IP
administration, capsules and/or tablets capable of delivering androgen over an
immediate period or over an extended period of hours or days, as well as
devices
such as transdermal patches, pumps and implants. Pumps and implants can be
subcutaneous or implantable elsewhere and can include both errodable and
diffusion-based devices, as well as any other functionally equivalent devices
known
CA 02469084 2004-06-22
-13-
in the art. These can all be made and administered in accordance with
procedures
well known in the industry.
Intravenous or injectable dosage forms can be comprised of any
suitable liquid carrier which can dissolve or su~ciently emulsify the androgen
to
allow for safe administration. ORen these formulations can also include
viscosity
modifiers, surfactants, preservatives sotubifizing agents, diluents and
additives
useful for rendering the solutions isotonic. Other drugs or pharmaceuticals
can also
be administered along with the androgen from the same dosage form.
Topical dosage forms can include androgen formulated in a lotion,
cream, salve, paste or gel. Any such topical vehicle known in the art may be
used . .
so long as it is capable of administering a suitable quantity of androgen in
accordance with the present invention. Often these formulations can also
include
viscosity modifiers, surfactants, preservatives, solubilizing agents,
diluents,
permeation enhancers, colors, fragrances and skin care additives like
conditioners
and humectants. Other drugs or. pharmaceuticals can also be administered along
with the androgen from the same dosage form. These topical formulations can
include buecal dosage forms as well.
In accordance with the present invention, androgen can also be
administered through oral dosase forms such as liquids, oral suspensions,
elixirs,
syrups, capsules, caplets or tablets Any formulation which can administer the
dosage form orally is contemplated including immediate release and sustained
release dosage forms. Often these formulations can also include viscosity
modifiers,
surfactants, preservatives solubilizing agents, nucrocapsules, microparticles,
granules, diluents, binders, fillers, lubricants, colors, flavors and the Gke.
Other
drugs or pharmaceuticals can also be administered along with the androgen from
the
same dosage form.
Finally, in a preferred aspect of the present invention, an implant or
pump may be used. Implants include implantable subcutaneous devices such as
those
disclosed in U.S. Patent No. 5,733,565. However, in accordance with the
present
invention, the implant (or implants) are designed to provide sufficient
androgen to
pmvide contraception. Therefore, when MENT Ac is used, for example, the
implants will
CA 02469084 2004-06-22
WO 99/13883 PCTNS98/19402
-14-
be capable of providing at least about 500 micrograms per day, each day for at
least
30 days and preferably about 180 days. Preferably, the amount of MENT Ac
administered each day would. not be greater than about 2000 micrograms per day
and more preferably no more than 1000 micrograms. If MENT Ac were used in
androgen replacement implants where contraception was not desired, these same
implants would generally provide less than about 500 micrograms of MENT each
day. While implants of this type are preferred, any similar devices designed
to
remain in the body and administer, in as nearly a uniform manner as possible,
an
amount of, in this case, an androgen as described herein may be used. These
implants can be produced and administered as known in the art. It should be
noted
that MENT Ac is readily hydrolyzed to MENT and it has been found that MENT
Ac is superior in terms of bioavailability in certain subcutaneous implants as
discussed in the examples. MENT may be preferred in other implants and MENT is
preferred for transdermal dosage forms.
It will be appreciated that techniques for the admitustration of ~~
various drugs, including such structurally similar compounds as steroids, are
well
known in the art. Therefore, it is unnecessary to describe in detail
administration
details for oral, topical, IV and implantation techniques. Any conventional
technique or dosage form is suitable provided that it can reliably administer
the
correct amount of the androgens in question. Knowing the objective
(contraception
or non-contraceptive androgen replacement), the types of androgens useful in
accordance with the present invention, the blood levels of various compounds
desired consistent with that objective and methods of determining the blood
levels
of important compounds as indicated will allow for one to practice the
invention
with any dosage form or dosing technique.
EXAh'IPGE 1
Forty-five healthy, normal male subjects were selected for a study of
the contraceptive efl"ect of androgens alone. Subjects were treated over the
course
of four weeks, during which extensive blood work-ups were undertaken. The
subjects were divided into three groups of 15 each. Group A received one
subcutaneous implant, Group B received two subcutaneous implants and Group C
received four subcutaneous implants. Implants remained in place for 28
consecutive
CA 02469084 2004-06-22
WO 99/I3883 PCTNS98I19.I03
~ 15-
days. The implants were manufactured by the Center for Biomedical Research and
the Population Council of New York, 1230 York Avenue, New York, NY 10021
and each implant had a length of 4.4 cm, a drug load per implant of 112 ~ 4 mg
of
NLENT Ac and a release rate of approximately 500 micrograms per implant per
day,
based on an in vivo study.
The procedure for production of the implants involved three basic .
steps. In brief, cores were prepared containing 60% w/w of MENT Ac and 40%
wlw of Ethylene Vinyl Acetate copolymer (EVA); 25% wlw of VA content. The
cores were then encased in EVA (9% VA content) and the ends of the tubing were
sealed with melted EVA (25% VA content).
A. Preparation of cores containing 60% of MENT Ac
1.5 g of EVA pellets (25% VA) was weighed into 18 ml of
methylene chloride and dissolved overnight. 2.225 g of h~NT Ac was weighed
and added to the solution of EVA in methytene~ chloride and Vortexed for 3
minutes
to mix. The methylene chloride was evaporated under vacuum, at room
temperature, for 2 hours. 2.3 g of the resulting solid dispersion was
obtained. This
solid dispersion was filled into a stainless steel syringe and heated to
120°C for
S minutes rising the thermostatically controlled heating jacket for the
syringe. The
solid dispersion was then e~~truded by pressing the syringe, using the small
laboratory press, into a brass mold W th grooves of 2.33 mm diameter and 4.S
crn
length. The mold was opened and the cores cut into 4 cm pieces. The cores were
weighed individually and any core deviating from the mean weight by ~ 7.5%
were
discarded. Cores were taken at random and tested for NIENT Ac content using
known methods. The mean value must not differ from the expected Values by more
35 than ~ 7.5°/o and none of the individual values may differ from the
mean by more
than 10%.
B. Encasement of the core rods by EVA tubing
EVA (9°ro VA) tubing (about 2.55 mm in diameter) was cut into
S cm lengths and soaked in methylene chloride for about 1-3 minutes. Each of
the
4 cm core rods was introduced into one of the soaked 5 cm pieces of EVA tubing
lea«ng about O.S cm empty on both ends and left overnight to get rid of any
residual methylene chloride.
CA 02469084 2004-06-22
WO 99/13883 PCTNS98/19402
- 16-
C. Sealing of the two ends of the filled tubing
About 2 g of EVA (25% VA) was heated in the stainless steel
syringe equipped with the thermostatically controlled heating jacket for 5
minutes at
120°C. The melted EVA was then injected into both ends of each filled
tubing.
The implants were heat sealed at 70°C for 10 minutes to enhance the
adherence
between the inside core and the outside tubing and to ensure complete removal
of
any residual methylene chloride.
The total duration of the study was 42 days. Each man included in
the study was assessed through a medical history and a complete general
physical
examination and a determination of general eligibility. Subjects were tested
on a
weekly basis for six consecutive weeks at approximately the same time each
day.
Day 1 was the date of the subdermal implant's insertion and Day 29 was
removal.
On the last day of the study, Day 43, subjects received a general physical
examination, as well as an additional battery of blood tests. Blood samples
for
hormone immuno assays MENT (7mL), FSH, LH and testosterone (5 mL for the
last 3 hormones together) were obtained during pre-admission and each
subsequent
visit.
Figure 2 illustrates the effect of MENT Ac implants on mean serum
LH concentration. By the first post implant visit on day 8, levels of LH had
already
dropped significantly. In fact, subjects to whom 2 or 4 implants (500
nucrograms
each) were administered had nearly undetectable levels of LH. By suppressing
LH,
testosterone production is suppressed. This is demonstrated in Figure 4 which
illustrates the effect of MENT Ac implants on the mean serum T concentration
(testosterone). Again, subjects to whom two or four implants had been
administered exhibited very low testosterone levels. Subjects who received a
single
implant still showed much reduced levels of testosterone. A reduction in
testosterone reduces or eliminates spermatogenesis. However, because androgen
is
being supplied, sexual function is generally maintained.
Finally, Figure 3 illustrates the effect of MENT Ac implants on the
mean serum FSH concentration. The use of the androgen containing implants had
a
significant impact on the serum FSH concentration in subjects. FSH stimulates
germ ceU production. If germ cells are present, but testosterone is
insufficient, then
CA 02469084 2004-06-22
WO 99/13883 PCTIUS98/19403
- 1'l -
'spermatogenesis cannot take place and sperm cells will not be produced.
Similarly,
if FSH concentrations are low enough, germ cells will not be produced and
spermatogenesis cannot take place irrespective of testosterone levels. Without
wishing to be bound by any theory of operation, by the use of androgen, in
accordance with the present invention, sperm production can be controlled by
effectively reducing both effective testosterone levels and germ cell
production.
Two multiple injection studies were undertaken in which groups of
four normal, non-sterile men each received 1, 2 or 4 mg of MENT daily by intra-
muscular (i.m.) injection for six consecutive days (24 men, 13 in each study).
The
formulation in each injection included the appropriate amount of MENT
suspended
in 27.6 mg/mL of polyethylene glycol, 1.8 mglmL of Tween 80 and 8.3 mg/mL of
sodium chloride. Serum FSH, LH and testosterone levels were measured daily.
The results are illustrated in Figures 7-12.
In both studies, all three doses of MEI~'~'T caused a gradual decrease
in serum gonadotropins and testosterone levels which reached their lowest
levels by
the end of treatment at six days. The values returned to the normal range by
day
15-30 of the study period. V'ith reference to Figure 8 (open boxes connected
by a
line = 1 mg, closed boxes connected by a line = 2 mg, open circles connected
by a
line = 4 mg) a dose of at least 4 mg was required to obtain serum levels of
FSH
below two international units ("IU") per liter by day 6. This must be compared
with
the results obtained by the use of 1000 pg (1 mg) of the same androgen
delivered
by a subcutaneous implant as illustrated in Figures Z-4 and Example 1.
Therefore,
considerably more of this same androgen ~~as required to obtain certain
depressed
serum levels of LH, FSH and testosterone in males when the androgen was
administered through a once-a-day infra-muscular injection when compared to
continuous release implants Indeed, depending upon the level of LH, FSH and
testosterone desirable by a particular health care professional, a dose
significantly in
excess of 4 mg may be necessary for this type of dosage form. Figure 7
illustrates
the level of serum LH achieved in the first study and Figure 9 illustrates the
level of
serum testosterone achieved. Figures 10-12 illustrate the levels of LH, FSH'
and
CA 02469084 2004-06-22
WO 99/13883 PCTNS98I19~03 _
-18-
testosterone realized by the same protocol at the second testing site. Both
the data
and the overall trends of both studies are largely the same.
Industrial A~pli~a~ilitv
The present invention relates to the medical and pharmaceutical
industries and, in particular, to the preparation and use of certain compounds
and
devices for contraception and androgen replacement, as well as the dosage
forms
themselves.