Note: Descriptions are shown in the official language in which they were submitted.
CA 02469625 2004-06-07
WO 03/049738 1 PCT/EP02/13240
Description
Use of pyridine-2,4-dicarboxylic acid diamides and of pyrimidine-4,6-
dicarboxylic
acid diamides for selective collagenase inhibition
The invention relates to the use of pyridine-2,4-dicarboxylic acid diamides
and
pyrimidine-4,6-dicarboxylic diamides for selectively inhibiting collagenase
(MMP 13).
The pyridine-2,4-dicarboxylic acid diamides and pyrimidine-4,6-dicarboxylic
acid
diamides can therefore be employed for treating degenerative diseases of the
joints.
It is known that pyrimidine-4,6-dicarboxylic acid diamides and 2,4-substituted
pyridine-N-oxides inhibit the enzymes proline hydroxylase and lysine
hydroxylase
and thereby bring about an inhibition of collagen biosynthesis by influencing
the
collagen-specific hydroxylation reaction (EP 0418797; EP 0463592). This
inhibition
of collagen biosynthesis results in the formation of a nonfunctional,
underhydroxylated collagen molecule which can only be released by the cells
into
the extracellular space in small quantity. In addition, the underhydroxylated
collagen
cannot be incorporated into the collagen matrix and is very easily broken down
proteolytically. As a consequence of these effects, the overall quantity of
extracellularly deposited collagen decreases.
In diseases such as osteoarthritis and rheumatism, the joint is destroyed,
due, in
particular, to the proteolytic degradation of collagen by collagenases.
Collagenases
belong to the metalloproteinase (MP) or matrix metalloproteinase (MMP)
superfamily. MMPs cleave collagen, laminin, proteoglycans, elastin or gelatin
under
physiological conditions and therefore play an important role in bone and
connective
tissue. A large number of different inhibitors of the MMPs or the collagenases
are
known (EP 0 606 046; W094/28889). The known inhibitors of the MMPs frequently
suffer from the disadvantage that they lack the specificity of inhibiting only
one class
of the MMPs. As a result, most MMP inhibitors inhibit several MMPs at the same
time because the catalytic domains of the MMPs exhibit similar structures. As
a
consequence, the inhibitors act, in an undesirable manner, on many enzymes,
including those which have a vital function (Massova I., et al., The FASEB
Journal
(1998) 12, 1075-1095).
CA 02469625 2004-06-07
2
In an endeavor to find active compounds for treating connective tissue
diseases, it
has now been found that the compounds which are employed in accordance with
the
invention are powerful inhibitors of matrix metalloproteinase 13 whereas they
are
essentially inactive in the case of MMPs 3 and 8.
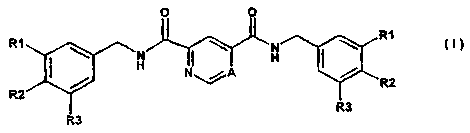
The invention therefore relates to the use of compounds of the formula I
0 0
RI R1
N N I \ (~)
I H
/ H\%A H
R2 \
R2
R3
R3
and/or all stereoisomeric forms of the compound of the formula I and/or
mixtures of
these forms in any ratio, and/or a physiologically tolerated salt of the
compound of
formula I, for producing a pharmaceutical for the prophylaxis and therapy of
diseases
whose course involves an increased activity of matrix metalloproteinase 13,
where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. halogen,
3. -(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once, twice
or three times by halogen,
4. -O-(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once,
twice or three times by halogen,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
7. -N(R5)(R6), in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl or
4. -S02-(C1-C4)-alkyl,
CA 02469625 2004-06-07
3
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl,
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2,
R2 is 1. hydrogen atom,
2. halogen,
3. -O-(C1-C4)-alkyl,
4. -(C1-C4)-alkyl,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
7. -N(R5)(R6), in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl or
4. -S02-(C1-C4)-alkyl,
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl,
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a 5- or 6-membered ring which is aromatic or saturated and
contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur,
and
the other radical R1 or R3 has the abovementioned meaning of 1. to 11.
The invention furthermore relates to the use of compounds of the formula I
where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. halogen,
CA 02469625 2004-06-07
4
3. -(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once, twice
or three times by halogen,
4. -O-(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once,
twice or three times by halogen,
R2 is 1. hydrogen atom,
2. halogen,
3. -O-(C1-C4)-alkyl, or
4. -(C1-C4)-alkyl, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a 5- or 6-membered ring which is aromatic or saturated and
contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur,
and
the other radical R1 or R3 has the abovementioned meaning of 1. to 4.
The invention furthermore relates to the use of compounds of the formula I
where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. chlorine,
3. fluorine,
4. trifluoromethyl,
5. methoxy,
6. methyl,
7. -C(O)-OH,
8. -C(O)-O-CH3,
9. -CN,
10. -NH2,
11. -NH-C(O)-CH3,
12. -NH-S02-CH3,
13. -N-(CH3)2,
14. -S02-NH2,
15. -OH,
CA 02469625 2004-06-07
16. -O-CH2-(CHF2),
17. -S-CH3,
18. -S(O)-CH3,
19. -S(O)2-CH3 or
5 20. bromine,
R2 is 1. hydrogen atom,
2. chlorine,
3. fluorine,
4. methoxy,
5. methyl,
6. bromine,
7. -C(O)-OH,
8. -C(O)-O-CH3,
9. -CN,
10. -NH2,
11. -NH-C(O)-CH3,
12. -NH-S02-CH3,
13. -N-(CH3)2,
14. -S02-NH2,
15. -OH,
16. -O-CH2-(CHF2),
17. -S-CH39
18. -S(O)-CH3, or
19. -S(O)2-CH3, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a dioxolane, dihydrofuran or furan ring, and
the other radical R1 or R3 has the abovementioned meaning of 1. to 20.
The invention furthermore relates to the use of compounds of the formula I
where
A is a carbon atom or nitrogen atom,
CA 02469625 2004-06-07
6
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. chlorine,
3. fluorine,
4. trifluoromethyl,
5. methoxy, or
6. methyl,
R2 is 1. hydrogen atom,
2. chlorine,
3. fluorine,
4. methoxy, or
5. methyl, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a dioxolane, dihydrofuran or furan ring.
The invention furthermore relates to compounds of the formula I,
0 0
R9 N R9 (l~
N H
Rz
R3
R3
and/or all the stereoisomeric forms of the compound of the formula I, and/or
mixtures
of these forms in any ratio, and/or a physiologically tolerated salt of the
compound of
the formula I, where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. halogen,
3. -(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once, twice
or three times by halogen,
4. -O-(C1-C4)-alkyl, in which alkyl is unsubstituted or. substituted once,
twice or three times by halogen,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
CA 02469625 2004-06-07
7
7. -N(R5)(R6), in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl or
4. -S02-(C1-C4}-alkyl,
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl,
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2,
R2 is 1. hydrogen atom,
2. halogen,
3. -O-(C1-C4)-alkyl,
4. -(C1-C4)-alkyl,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
7. -N(R5)(R6), in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl or
4. -S02-(C1-C4)-alkyl,
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl,
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a 5- or 6-membered ring which is aromatic or saturated and
contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur,
and
the other radical R1 or R3 has the abovementioned meaning of 1. to 11.
with the exception of the case where
CA 02469625 2004-06-07
8
a) the radicals R1, R2 and R3 are all simultaneously hydrogen atom, or
b) all the radicals R1, R2 and R3 are, independently of each other, only
hydrogen atom, halogen, nitro, -(C1-C4)-alkyl or -(C1-C4)-alkoxy.
The invention furthermore relates to compounds of the formula I where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl, in which alkyl is substituted once, twice or three times
by halogen,
3. -O-(C1-C4)-alkyl, in which alkyl is substituted once, twice or three times
by halogen,
4. -OH,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
7. -N(R5)R6, in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl or
4. -S02-(C1-C4)-alkyl,
8. -S-(C1-C4)-alkyl,
9. -S(O)-(C1-C4)-alkyl, or
10. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2,
in which R1, R2 and R3, are not simultaneously hydrogen atom, and
R2 is 1. hydrogen atom,
2. halogen,
3. -O-(C1-C4)-alkyl,
4. -(C1-C4)-alkyl,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
CA 02469625 2004-06-07
9
7. -N(R5)R6, in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. (C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl, or
4. -S02-(C1-C4)-alkyl,
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl, or
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or -NH2, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a 5- or 6-membered ring which is aromatic or saturated and
contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur,
and the other radical R1 or R3 is
1. hydrogen atom,
2. halogen,
3. -(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once, twice
or three times by halogen,
4. -O-(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once,
twice or three times by halogen,
5. -C(O)-O-R4, in which R4 is hydrogen atom or -(C1-C4)-alkyl,
6. -CN,
7. -N(R5)R6, in which R5 and R6 are identical or different and are,
independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl,
3. -C(O)-(C1-C4)-alkyl, or
4. -S02-(C1-C4)-alkyl,
8. -OH,
9. -S-(C1-C4)-alkyl,
10. -S(O)-(C1-C4)-alkyl, or
11. -S(O)2-R7, in which R7 is -(C1-C4)-alkyl, -OH or-NH2.
CA 02469625 2004-06-07
The invention furthermore relates to compounds of the formula I, where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
5 I. hydrogen atom,
2. chlorine,
3. fluorine,
4. trifluoromethyl,
5. methoxy,
10 6. methyl,
7. -C(O)-OH,
8. -C(O)-O-CH3,
9. -CN,
10. -NH2,
11. -NH-C(O)-CH3,
12. -NH-S02-CH3,
13. -N-(CH3)2,
14. -S02-NH2,
15. -OH,
16. -O-CH2-(CHF2),
17. -S-CH3,
18. -S(O)-CH3,
19. -S(O)2-CH3 or
20. bromine,
in which R1, R2 and R3 are not simultaneously hydrogen atom, and
R2 is 1. hydrogen atom,
2. chlorine,
3. fluorine,
4. methoxy,
5. methyl,
6. bromine,
7. -C(O)-OH,
8. -C(O)-O-CH3,
CA 02469625 2004-06-07
11
9. -CN,
10. -NH2,
11. -NH-C(O)-CH3,
12. -NH-S02-CH3,
13. -N-(CH3)2,
14. -S02-NH2,
15. -OH,
16. -O-CH2-(CHF2),
17. -S-CH3,
18. -S(O)-CH3, or
19. -S(O)2-CH3, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a dioxolane, dihydrofuran or furan ring, and
the other radical R1 or R3 has the abovementioned meaning of 1. to 20..
The invention furthermore relates to the compounds of the formula I,
and/or all the stereoisomeric forms of the compound of the formula I, and/or
mixtures
of these forms in any ratio, and/or a physiologically tolerated salt of the
compound of
the formula I, where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom,
2. -(C1-C4)-alkyl, in which alkyl is substituted once, twice or three times
by halogen, or
3. -O-(C1-C4)-alkyl, in which alkyl is substituted once, twice or three times
by halogen,
in which R1, R2 and R3 are not simultaneously hydrogen atom, and
R2 is 1. hydrogen atom,
2. halogen,
3. -O-(C1-C4)-alkyl, or
CA 02469625 2004-06-07
12
4. -(C1-C4)-alkyl, or
R1 and R2 or R2 and R3 form, together with the carbon atoms to which they are
in
each case bonded, a 5- or 6-membered ring which is aromatic or saturated and
contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur,
and the other radical R1 or R3 is
1. hydrogen atom,
2. halogen,
3. -(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once, twice
or three times by halogen, or
4. -O-(C1-C4)-alkyl, in which alkyl is unsubstituted or substituted once,
twice or three times by halogen.
The invention furthermore relates to compounds of the formula I where
A is a carbon atom or nitrogen atom,
R1 and R3 are identical or different and are, independently of each other,
1. hydrogen atom, or
2. trifluoromethyl,
in which R1, R2 and R3 are not simultaneously hydrogen atom, and
R2 is 1. hydrogen atom,
2. chlorine,
3. fluorine,
4. methoxy or
5. methyl, or
R1 and R2 or R2 and R3 form, together with the nitrogen atoms to which they
are in
each case bonded, a dioxolane, dihydrofuran or furan ring, and
the other radical R1 or R3 is
1. hydrogen atom,
2. chlorine,
3. fluorine,
4. trifluoromethyl,
5. methoxy, or
6. methyl.
CA 02469625 2004-06-07
13
The term "halogen" is understood as meaning fluorine, chlorine, bromine or
iodine.
The term "(C1-C4)-alkyl" is understood as meaning hydrocarbon radicals whose
carbon chain is straight-chain or branched and contains 1 to 4 carbon atoms,
for
example, methyl, ethyl, propyl, i-propyl, butyl or tertiary butyl.
The term " R1 and R2 or R2 and R3 form, together with the carbon atoms to
which
the are in each case bonded, a 5- or 6-membered ring which is aromatic or
saturated
and contains zero, one or two heteroatoms from the series oxygen, nitrogen or
sulfur" is understood as meaning radicals which can be derived from dioxolane,
pyrrole, pyrrolidine, pyridine, piperidine, tetrahydropyridine, pyrazole,
imidazole,
pyrazoline, imidazoline, pyrazolidine, imidazolidine, pyridazine, pyrimidine,
pyrazine,
piperazine, pyran, furan, dihydrofuran, tetrahydrofuran, oxazole, isoxazole, 2-
isoxazoline, isoxazolidine, morpholine, oxothiolane, thiopyran, thiazole,
isothiazole,
2-isothiazoline, isothiazolidine or thiomorpholine.
The compounds of the formula I can be prepared, for example, by reacting a
compound of the formula II
0
II
-Y
C-Y
N II
0
a) with a compound of the formula III
R9
112- NI-12 III
R3
where R1, R2 and R3 have the meanings given in formula I and Y is halogen,
hydroxy or C1-C4-alkoxy or, together with the carbonyl group, forms an active
ester or a mixed anhydride, with a compound of the formula I being formed
and the reaction products being converted, where appropriate, into their
physiologically tolerated salts, or
b) reacting a compound of the formula II with a compound of the formula III to
give a compound of the formula IV.
CA 02469625 2004-06-07
14
O O
R9 Y (IV)
N
H N.~A
RZ
R3
where R1, R2 and R3 have the meanings given in formula I and Y is halogen,
hydroxy or C1-C4-alkoxy or, together with the carbonyl group, forms an active
ester or a mixed anhydride, and purifying the compound of the formula IV,
where appropriate, and then converting it, with a compound of the formula III,
into a compound of the formula I.
In that which follows, the preparation of compounds according to formula I and
the
preparation of the starting substances which are required for this purpose,
insofar as
10' they are not commercially available, are described in more detail.
The compounds according to the invention are prepared in the simplest manner
by
mixing together the two components, i.e. the pyrimidine or pyridine derivative
according to formula (II) and the amine according to formula (III) in
equimolar
quantities or in up to about a 5-fold excess of I I I and reacting them at
temperatures
of between -30 C and 150 C, preferably at from 20 C up to 100 C, until the
reaction
has come to an end. When the compound of the formula IV is being prepared, the
amine according to (III) is admixed up to an equimolar quantity of the
compound of
the formula III and reacted as above. The completion of the reaction can be
determined, for example, with the aid of thin layer chromatography or HPLC-MS.
A
variant of this process is that the reaction is carried out in a suitable
solvent, such as
diethyl ether, dimethoxyethane or tetrahydrofuran, chlorinated hydrocarbons
such as
methylene chloride, chloroform or tri- or tetrachioroethylene, benzene or
toluene, or
else polar solvents such as dimethylformamide, acetone or dimethyl sulfoxide.
In this
case, too, it is possible to use an excess of amine according to formula
(III), which
excess can amount to up to about 5-fold quantities. The reaction temperatures
in this
case are between room temperature and the boiling point of the solvent, with
temperatures in the range from room temperature up to 130 C being particularly
preferred.
CA 02469625 2004-06-07
The reaction can also take place by way of a mixed anhydride such as ethyl
chloroformate or by way of an active ester such as paranitrophenyl ester (Y =
CICH2-COO or N02-C6H4-O). Corresponding methods are described in the
literature.
5
Where appropriate, the reaction can also take place in the presence of bases.
Examples of suitable additional bases are carbonates or hydrogen carbonates
such
as sodium carbonate or potassium carbonate or sodium hydrogen carbonate or
potassium hydrogen carbonate, or tertiary amines, such as triethylamine,
10 tributylamine or ethyldiisopropylamine, or heterocyclic amines, such as N-
alkyl-
morpholine, pyridine, quinoline or dialkylanilines.
Where appropriate, the products, in particular the compound of the formula IV,
can
be worked up, for example, by extraction or chromatography, for example
through
15 silica gel. The isolated product can be recrystallized and, where
appropriate,
converted into a physiologically tolerated salt using a suitable acid.
Examples of
suitable acids which come into consideration are:
mineral acids, such as hydrochloric acid and hydrobromic acid and also
sulfuric acid,
phosphoric acid, nitric acid or perchloric acid, or organic acids, such as
formic acid,
acetic acid, propionic acid, succinic acid, glycolic acid, lactic acid, malic
acid, tartaric
acid, citric acid, maleic acid, fumaric acid, phenylacetic acid, benzoic acid,
methanesulfonic acid, toluenesulfonic acid, oxalic acid, 4-aminobenzoic acid,
naphthalene-1,5-disulfonic acid or ascorbic acid.
Insofar as they are not commercially available, the starting compounds of the
formula (III) can be readily synthesized (e.g. Organikum, Organisch Chemisches
Grundpraktikum [Organikum, Basic Practical Course in Organic Chemistry], 15th
edtn., VEB Deutscher Verlag der Wissenschaften [VEB German Publishing
Company for the Sciences], 1976; an overview of the various options can be
found in
the methods index, p. 822).
The starting compounds of the formula (II) can be obtained, for example, by
converting pyrimidine-4,6-dicarboxylic acid or pyridine-2,4-dicarboxylic acid,
respectively, into the corresponding pyrimidine-4,6-dicarbonyl halide, or,
CA 02469625 2004-06-07
16
respectively, pyridine-2,4-dicarbonyl halide, preferably chloride (using
methods
known from the literature), preferably in the presence of a catalyst such as
d i methylform amid e. This acid halide can then be reacted, for example,
either with a
suitable alcohol, e.g. paranitrobenzyl alcohol, to give the corresponding
active ester
or else with lower alcohols, such as methanol or ethanol, to give the
corresponding
esters. The pyrimidine-4,G-dicarboxylic acid can also initially be converted,
in the
added presence of a suitable carboxylic acid or of a carboxylic ester, such as
ethyl
chloroformate, into a mixed anhydride, which is then reacted with the amines
of the
compound of the formulae (III) and (IV) to give the products according to the
invention. An appropriate method is also described in the literature.
The pyrimidine-4,6-dicarboxylic acid is prepared using methods known from the
literature, for example by oxidizing 4,6-dimethylpyrimidine, which, for its
part, can be
obtained, for example, by catalytically hydrogenating commercially obtainable
2-mercapto-4,6-d imethylpyrimidine.
Insofar as compounds of the formula I permit diastereoisomeric or enantiomeric
forms, and accrue as their mixtures in connection with the chosen synthesis,
the
separation into the pure stereoisomers is achieved either by chromatography on
an
optionally chiral support material or, insofar as the racemic compound of the
formula I is capable of sulfonation, by fractional crystallization of the
diastereomeric
salts which are formed using an optically active base or acid as auxiliary
substance.
Examples of suitable chiral stationary phases for the thin-layer-
chromatographic or
column-chromatographic separation of enantiomers are modified silica gel
supports
(what are termed Pirkle phases) and also high molecular weight carbohydrates
such
as triacetylcelIulose. Gas-chromatographic methods on chiral stationary phases
can
also be used for analytical purposes following appropriate derivatization, as
known to
the skilled person. In order to separate the racemic carboxylic acids into
their
enantiomers, the differently soluble diastereomeric salts are formed using an
optically active base which can as a rule be obtained commercially, such as (-
)-
nicotine, (+)- and (-)-phenylethylamine, quinine bases, L-lysine or L- and D-
arginine,
the more difficulty soluble component is then isolated as a solid, the more
readily
soluble diastereomer is separated off from the mother liquor, and the pure
enantiomers are then isolated from the diastereomeric salts which had been
CA 02469625 2004-06-07
17
obtained in this way. The racemic compounds of the formula I which contain a
basic
group such as an amino group can in principle be converted into the pure
enantiomers in the same way using optically active acids, such as (+)-camphor-
10-
sulfonic acid, D- and L-tartaric acid, D- and L-lactic acid and also (+) and (-
)-
mandelic acid. Chiral compounds which contain alcohol or amine functions can
also
be converted into the corresponding esters or amides using appropriately
activated
or optionally N-protected enantiomerically pure amino acids or, conversely,
chiral
carboxylic acids can be converted into the amides using carboxy-protected
enantiomerically pure amino acids or into the corresponding chiral esters
using
enantiomerically pure hydroxycarboxylic acids such as lactic acid. The
chirality of the
amino acid radical or alcohol radical which has been introduced in
enantiomerically
pure form can then be used for separating the isomers by means of separating
the
diastereomers, which are now present, by crystallization or chromatography on
suitable stationary phases and, after that, once again eliminating the
entrained chiral
molecular moiety using suitable methods.
Acidic or basic products of the compound of the formula I can be present in
the form
of their salts or in free form. Preference is given to pharmacologically
tolerated salts,
for example alkali metal salts or alkaline earth metal salts or
hydrochlorides,
hydrobromides, sulfates, hemisulfates, all the possible phosphates, and also
salts of
the amino acids, natural bases or carboxylic acids.
Physiologically tolerated salts are prepared in a manner known per se from
compounds of the formula I, including their stereoisomeric forms, which are
capable
of salt formation. The carboxylic acids and hydroxamine acids form stable
alkali
metal salts, alkaline earth metal salts or, where appropriate, substituted
ammonium
salts with basic reagents such as hydroxides, carbonates, hydrogen carbonates,
alcoholates and ammonia or organic bases, for example trimethylamine or
triethylamine, ethanolamine or triethanolamine, or else basic amino acids, for
example lysine, ornithine or arginine. Insofar as the compounds of the formula
I
possess basic groups, stable acid addition salts can also be prepared using
strong
acids. Both inorganic and organic acids, such as hydrochloric acid,
hydrobromic
acid, sulfuric acid, phosphoric acid, methanesulfonic acid, benzenesulfonic
acid, p-
toluenesulfonic acid, 4-bromobenzenesulfonic acid, cyclohexylamidosulfonic
acid,
CA 02469625 2004-06-07
18
trifluoromethylsulfonic acid, acetic acid, oxalic acid, tartaric acid,
succinic acid or
trifluoroacetic acid, are suitable for this purpose.
Because of their pharmacological properties, the compounds of the formula I
are
suitable for the prophylaxis and therapy of all those diseases whose course
involves
an increased activity of matrix metalloproteinase 13.
These diseases include degenerative joint diseases such as osteoarthroses,
spondyloses, chondrolysis following joint trauma or a relatively long period
of joint
immobilization following injuries to the meniscus or patella or the tearing of
a
ligament. In addition, they also include diseases of the connective tissue
such as
collagenoses, periodontal diseases, wound healing disturbances and chronic
diseases of the locomotor system, such as inflammatory, immunologically or
metabolism-determined acute and chronic arthritides, arthropathies, myalgias
and
disturbances of bone metabolism or forms of cancer such as breast cancer.
The pharmaceuticals according to the invention can be administered by
subcutaneous, intraarticular, intraperitoneal or intravenous injection.
Intraarticular
injection is preferred. It is also possible to administer them rectally,
orally, by
inhalation or transdermally.
The invention also relates to a process for producing a pharmaceutical, in
which
process at least one compound of the formula I is brought, together with a
pharmaceutically suitable and physiologically tolerated excipient and, where
appropriate, further suitable active compounds, additives or auxiliary
substances,
into a suitable form for administration.
The compounds of the formula I are mixed with the additives which are suitable
for
this purpose, such as carrier substances, stabilizers or inert diluents and
brought,
using the customary methods, into suitable administration forms, such as
tablets,
sugar-coated tablets, hard gelatin capsules, aqueous alcoholic or oily
suspensions or
aqueous or oily solutions. Examples of inert carrier substances which can be
used
are gum arabic, magnesium oxide, magnesium carbonate, potassium phosphate,
lactose, glucose or starch, in particular corn starch. In this connection, the
CA 02469625 2004-06-07
19
preparation can also be effected as dry granules or wet granules. Examples of
suitable oily carrier substances or solvents are vegetable or animal oils,
such as
sunflower oil or cod liver oil.
For the purpose of subcutaneous, intraarticular, intraperitoneal or
intravenous
administration, the active compounds are, if desired, brought into solution,
suspension or emulsion using the substances which are suitable for this
purpose,
such as solubilizers, emulsifiers or other auxiliary substances. Examples of
suitable
solvents are physiological sodium chloride solution or alcohols, for example
ethanol,
propanol or glycerol, and, in addition, sugar solutions, such as glucose or
mannitol
solutions, or else a mixture of the different solvents which have been
mentioned.
In addition, use is made of customary adjuvants, such as carrier substances,
disintegrants, binders, coating agents, swelling agents, glidants, lubricants,
flavorants, sweeteners and solubilizers. Frequently employed auxiliary
substances
which may be mentioned are magnesium carbonate, titanium dioxide, lactose,
mannitol and other sugars, talc, milk protein, gelatin, starch, cellulose and
its
derivatives, animal and vegetable oils, such as cod liver oil, sunflower oil,
peanut oil
or sesame oil, polyethylene glycol and solvents such as sterile water and
monohydric or polyhydric alcohols, such as glycerol.
The compounds of formula I are preferably produced and administered as
pharmaceutical preparations in dosage units, with each unit containing, as the
active
constituent, a particular dose of the compound of the formula I. For this
purpose,
they can be administered orally in doses of from 0.01 mg/kg/day to 25.0
mg/kg/day,
preferably of from 0.01 mg/kg/day to 5.0 mg/kg/day, or parenterally in doses
of from
0.001 mg/kg/day to 5 mg/kg/day, preferably of from 0.001 mg/kg/day to
2.5 mg/kg/day. The dose can also be increased in severe cases. However,
relatively
small doses suffice in many cases. These figures relate to an adult weighing
about
75 kg.
CA 02469625 2004-06-07
The invention is explained below with the aid of examples.
Examplel :
5 Pyrimidine-4,6-dicarboxylic acid dibenzylamide (Formula I: R1 = R2 = H)
1.7 g of pyrimidine-4,6-dicarboxylic acid are suspended in 20 ml of toluene
and 2.4 g
of thionyl chloride and 0.2 ml of dimethylformamide are added. The mixture is
heated
to reflux until it is no longer possible to observe any gas evolution (about 3
hours
10 (h)). About 5 ml of solvent are distilled off and the mixture is then
cooled down to
from 0 C to 10 C and 2.7 g of benzylamine, dissolved in 10 ml of toluene, are
added.
The solution is slowly heated to room temperature, then stirred at room
temperature
for 12 hours and evaporated down to dryness. The residue is taken up in 50 ml
of
methylene chloride and the solution is extracted 3 times by shaking with
saturated
15 sodium hydrogen carbonate solution; the organic phase is washed with water,
dried
with magnesium sulfate and evaporated.
The solid is recrystallized from diisopropyl ether.
Yield: 2.1 g; m.p.: 131 C to 132 C.
20 Example 2:
Pyrimidine-4,6-dicarboxylic acid bis(3-chloro-4-fluorobenzylamide) (Formula I:
R1 = Cl; R2 = F)
NON SOCI2 NN 11 HO OH C1 Y~/y CI
0 0 0 O
CI NH2
F
H NN H
CH2CI2, NEt3 CI N I/ N / CI
0 0
200 mg (1.2 mmol) of pyrimidine-4,6-dicarboxylic acid were suspended in 0.3 ml
(4.1 mmol) of thionyl chloride. This mixture was heated at 85 C for 2 h while
being
stirred. After it had been cooled down to room temperature, 2 ml of absolute
CA 02469625 2004-06-07
21
dichloromethane were added. The suspension was cooled down to 0 C and 0.33 ml
(2.4 mmol) of triethylamine was added. 861 mg (5.4 mmol) of 3-chloro-4-
fluorobenzylamine were added while stirring vigorously. The mixture was then
stirred
for a further 15 minutes. It was then diluted with 10 ml of dichloromethane
after
which 10 ml of water were added. After 5 minutes, the mixture was transferred
into a
separating funnel and the phases were separated. The organic phase was
extracted
twice with saturated sodium chloride solution and then dried over magnesium
sulfate. After filtration, the filtrate was concentrated under reduced
pressure and the
residue which was obtained in this way was dissolved in ethyl acetate. The
product
was crystallized from the solution by adding heptane. Beige-colored flakes
were
obtained and were dried under reduced pressure. Yield: 263 mg (49%)
The following compounds were prepared in analogy with example 2.
Table 1:
Example Structure MS (ESI+)
3 N^N Zcl 415.13
N I / N 0 0
4 346.27
N
N I / N ,,.,,o
0 CIH O
5 H3C N^N CH3 375.26
N / N
0 O
6 515.21
~nN a-~~ 0 N / N 0
F_~F 0 0 F~-F
F F
CA 02469625 2004-06-07
22
7 F F 382.21
\ I N I / N \
O 0
0yOH
H
8 Aa 374.25
H3C CH3
0 0
9 p 406.31
H3e N \ / CH3
N N \
0 0
OyOH
H
F F 419.22
f~N
F \ N N
0 0
11 / N nN / 483.24
F \ I N '/ N \~ F
F F
F 0 0 F
12 N / 414.15
CI CI
0 CIH 0
13 F N^N F 560.18
F F \ N N F (M+MeCN)
,,~ r"
F 0 0 F F
CA 02469625 2004-06-07
23
14 N/\N 383.17
\ ~ N yl~/y
F F
0 0
15 F N^N F 383.15
0 0
16 F N"N / F 419.02
\ I N I/ N \ I
1-1 F F
0 0
17 CH3 CH3 407.23
0 / I ~ ~ 1 0
N Y/ N
0 0
18 N^N 375.13
N N
H3C CH3
0 0
19 / N"N 415.06
\ I N I N
CI a
0 0
20 N'N 435.22
Y~~y
0
0 0
CA 02469625 2004-06-07
24
21 0,, 0 N N^N N 0 X0H 407.30
YUZ \ I
0 0
22 0 N/~N 431.06
\ N I / N \
0 0
23 F / N^N / F 411.25
H3C CH3
\ N yl--~--If N \
0 0
Example 24 Dimethyl pyrimidine-4,6-dicarboxylate
g (0.059 mol) of pyrimidine-4,6-dicarboxylic acid were suspended in 1.4 I of
methanol, after which 10.93 ml (0.356 mol) of concentrated hydrochloric acid
were
5 added and the mixture was stirred at reflux (65 C) for 3 hours (h). The
reaction
mixture was concentrated under reduced pressure after which the residue was
taken
up once again in methanol; the mixture was filtered and the resulting solution
was
concentrated.
Yield 11.02 g (94.4%) MS (ES+): m/e = 197.20
2.55 g (0.01299 mol) of the resulting compound dimethyl pyrimidine-4,6-
dicarboxylate were dissolved in 100 ml of dimethylformamide (DMF), after which
1.42 ml (0.01299 mol) of benzylamine were added and the mixture was heated to
50 C. After 4 h, the solution is concentrated under reduced pressure. The
residue is
chromatographed through a 500 ml silica gel column using heptane/ethyl acetate
(1:1). Fractions containing the compound methyl 6-benzylcarbamoylpyrimidine-4-
carboxylate were concentrated.
Yield: 1.268 g (36%) MS (ES+): m/e = 272.20
CA 02469625 2010-01-07
200 mg (0.737 mmol) of the resulting compound methyl 6-benzylcarbamoyl-
pyrimidine-4-carboxylate were dissolved in 4 ml of DMF, after which 225.98 mg
(1.29
mmol) of 3-trifluoromethylbenzylamine were added and the mixture was stirred
at
50 C for 1 day. After that, the solution was concentrated under reduced
pressure.
5 The residue was purified by means of preparative HPLC (water/acetonitrile
gradient,
Purospher RP18 ). Fractions containing pyrimidine-4,6-dicarboxylic acid 4-
benzyl-
amide-6-(3-trifluoromethylbenzylamide) were concentrated under reduced
pressure
and freeze-dried. Yield: 240 mg (79%) MS (ES+): m/e = 415.27
10 The following compounds were prepared in an analogous manner:
0 0
N I \ RIO
H N \% N
Table 2
Example R10 radical MS (ES+): m/e
25 3-fluorobenzylamine 365.23
26 4-fluorobenzylamine 365.23
27 3,4-difluorobenzylamine 383.27
28 4-methoxybenzylamine 377.28
29 3-methylbenzylamine 361.28
3-chlorobenzylamine 381.23
15 The following examples were prepared in analogy with the examples 1 and 30:
Table 3:
Example Structure MS ES+ : m/e
31~.S.o
. o .
N I i a ~c~-ti 470.22 ES+
0 0
CA 02469625 2004-06-07
26
32 FL N~ i~ 0
0 K,
484.12 ES+
33
roc
o 0
378.15 ES+
34 0
F OH
F
NN
466.13 ES+
35 F \ 4 inN N / I O-,
0 0
H-a
429.17 ES+
36 ~~S0
N"~N F
N ~ / N \
0 0 456,13 ES+
37
H ~^N N -OH,
0 0 455.10 ES+
38
G
N / O
C)
417.11 ES+
CA 02469625 2004-06-07
27
39
N"N
N I N \ I 0
Ftc 0 Ct~ 409.28 ES+
40 F !
NIA N
Cl ` N / N \ F
o H-Cl O 417.11 ES+
41 F
O
/ - N /
\ N / N
Cl
O 0
441.25
42
0 ~ N^N
\ I N I/ N \. I CI
0 0 423.26
43 F
N`N / O
C N I / N \
O 0 421.29 E5+
44 N F N I/~N N F
,,R,,~ I Br
H3C
o 0 477.15 ES+
45 F
N N
Cl
0 H-Cl 0 399.20 ES+
46 F
Nl N / I
/ N \ CI
y-z y -
o H-Cl 0 417.16 ES+
47 l/~ N / I F
CI N / F
o H-Cl o 435.14 ES+
CA 02469625 2004-06-07
28
48
N N
N
N I / \ I CH3
0 403.31 ES+
49 0 / I hN / F
N N F
I
0 0 425.30 ES+
F / N ' N /
N N
H3C
0 0 379.29 ES+
51
(/ I
H3C / N \ CH3
0 0 393.33 ES+
52
F aZ-~ F
H3C, N k/ N ICH3
O O
0 0 443.28 ES+
53 O N/~N
\ I N ~ / N \ F =
F
O O F
457.20 ES+
54
N'~N
N N
F CH
3
0 0
H-CI 379.19 ES+
NN / I
F \ N / N \ CI
0 O
H-Cl 399.14.ES+
56 F N cl \ I N I/ N \ I F
F
0
H-ci 0 F 467.17 ES+
CA 02469625 2004-06-07
29
57
N N
\' N I/ N F
F
0 H-Cl 0 F 433.24 ES+
58
N^N
N I / N CI
0 0
H-CI 399.18 ES+
59
N N
N i / N \ I
CH3
0 H-Cl 0 379.10 ES+
60 F ^ F'
N N
\ I N
I / N F
Y
0 0
H -01 401.05 ES+
61
/ F
0 H-Cl 0 F 415.27 ES+
62
O\ N +/ N \ I O
cH, 0 0 CH3 406.26 ES+
63
H2N \ N ( / N \ NH2
0 0 377.32 ES+
64 CF6 CH,
S / N-" ~N
I / N
0 0 439.21 ES+
CA 02469625 2004-06-07
65 F` /F F` /F
"~N 0
N N
a o 479.18 ES+
66 N\~ N
1, I N / N
0 0 397.21 ES+
1
F 0 0 F 383.27 ES+
Pharmacological examples
Determining the enzymic activity of the catalytic domain of human collagenase-
3
(MMP-13).
5
This protein is obtained as an inactive proenzyme from INVITEK, Berlin
(catalog No.
30 100 803). Activation of the proenzyme:
2 parts by volume of proenzyme are incubated with 1 part by volume of APMA
solution at 37 C for 1.5 hours. The APMA solution is prepared from a 10 mmol/I
10 solution of p-aminophenylmercuric acetate in 0.1 mmol/I NaOH by diluting it
with 3
parts by volume of Tris/HCI buffer, pH 7.5 (see below). The pH is adjusted to
between 7.0 and 7.5 by adding 1 mmol/I HCI. After the enzyme has been
activated, it
is diluted with the Tris/HCI buffer down to a concentration of 1.67 g/ml.
15 In order to measure the enzyme activity, 10 ,u1 of enzyme solution are
incubated for
15 minutes with 10 l of a buffered 3% (v/v) solution of dimethyl sulfoxide
(reaction 1). In order to measure the enzyme inhibitor activity, 10 NI of
enzyme
solution are incubated with 10p1 of a buffered 3% (v/v) solution of dimethyl
sulfoxide
which contains the enzyme inhibitor (reaction 2).
Both in reaction 1 and in reaction 2, the enzyme reaction is monitored by
fluorescence spectroscopy (328 nm (extinction)/393 nm (emission)) after 10 p1
of a
CA 02469625 2010-01-07
31
3% (v/v) aqueous solution of dimethyl sulfoxide containing 0.75 mmol of the
substrate/I have been added.
The enzyme activity is presented as increase in extinction/minute.
The inhibitor effect is calculated as percentage inhibition in accordance with
the
following formula:
% inhibition = 100 - [(increase in extinction/minute in reaction 2) /
(increase in
extinction/minute in reaction 1) x 100].
The IC50, i.e. the concentration of inhibitor which is required for a 50%
inhibition of
the enzyme activity, is determined graphically by plotting the percentage
inhibitions
at different inhibitor concentrations.
The buffer solution contains 0.05%Brij (Sigma, Deisenhofen, Germany) and also
0.1 mol of Tris/HCI/I, 0.1 mol of NaCI/l and 0.01 mol of CaCl2/I (pH=7.5).
The enzyme solution contains 1.67 Ng of the enzyme domain/mi.
The substrate solution contains 0.75 mmol of the fluorogenic substrate (7-
methoxy-
coumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2'.4'-dinitrophenyl)-L-2,3-
diaminopropionyl-
Ala-Arg-NH2/I (Bachem, Heidelberg, Germany).
The following table 4 shows the results.
Table 4:
Example IC50 MMP13 (nM) Example IC50 MMP13 (nM) Example IC50 MMP13 (nM)
1 400 10 300 19 57
2 23 11 300 20 14
3 5600 12 260 21 10
4 3400 13 210 22 9
5 2000 14 200 23 8
6 700 15 190
7 620 16 105
8 400 17 80
9 320 18 72
CA 02469625 2004-06-07
32
Comparative example
The compound pyrimidine-4,6-dicarboxylic acid diethylamide was prepared as
described in EP 0418797. A value of 90 000 nM was obtained when the IC50 value
for the inhibition of human collagenase -3 (MMP-1 3) was determined as
described in
the above example. This compound therefore has practically no inhibitory
effect on
MMP 13.
Determining the enzymic activity of the catalytic domains of human neutrophil
collagenase (MMP-8) and human stromelysin (MMP-3).
The enzymes human neutrophil collagenase and human stromelysin were prepared
as active catalytic domains as described in Weithmann et al Inflamm Res, 46
(1997),
pages 246-252 carried out. The measurement of the activity of the enzymes, and
the
determination of the inhibitory effect of inhbitors on the activity of the
enzymes, were
also carried out as described in that publication.
The compounds according to the abovementioned examples 1 to 23 in each case
exhibited IC50 values of more than 100 000 nM when determining human
neutrophil
collagenase and human stromelysin. These compounds therefore have practically
no
inhibitory effect on MMP 3 and MMP 8.