Note: Descriptions are shown in the official language in which they were submitted.
CA 02469988 2010-02-26
78703-36
CARBON DIOXIDE FOAMED FLUIDS
Technical Field of the invention
[00011 This invention relates generally to viscous fluids foamed or energized
with carbon
dioxide and to methods of their use.
Background of the Invention
[00021 Foamed fluids are used in many applications, for example as fracturing
fluids in
the oil field. Although such fluids are commonly called foams, when the second
phase is
CO2 the fluids are more like an emulsion of water and supercritical CO2 under
most
application conditions. Furthermore, when the fraction of the non-aqueous
phase (called
the "foam quality" and sometimes abbreviated here as FQ) is less than about
54%, such
fluids are commonly called "energized". When we say "foamed" we include any
quality,
including an emulsion; when we say "energized' we mean a quality of less than
about
54%. CO2 "foamed" fracturing fluids are often preferred over N2 (or other gas)
foamed
fracturing fluids under certain circumstances. For instance, CO2 provides
additional
cleanup energy (relative to N2) when it is gasified as the pressure drops
after a treatment
is completed: Also, for deeper and hotter wells, CO2 foamed fluids require
less
horsepower due to their higher density than N2 foamed fluids of equal foam
quality.
Therefore N2 fluids are rarely pumped over about 180 F (about 82 C), and CO2
fluids
are routinely used up to about 240 F (about 116 C) and higher.
1
CA 02469988 2010-02-26
78703-36
[00031 It is a desirable feature to have a fracturing fluid compatible with
CO2.
Surfactant-based fluids ("VES" or "viscoelastic surfactant" fluids) provide
many benefits
over conventional polymer fluids when used in fracturing (and for other uses),
but most
VES fluids are not very compatible with CO2. Not to be limited by theory, but
it is
believed that this is because the solvency of supercritical CO2 disrupts the
micellar
structure (for example but not limited to a worm-like micelle structure) that
is essential
for fluid viscosity. It would be advantageous to make VES/CO2 foamed fluids
stable for
longer times and/or at higher temperatures.
Summary of the Invention
[0004] -One embodiment of the invention is a fluid composition containing a
viscoelastic
surfactant and a synergistic co-surfactant. In another embodiment the
composition also
contains water; the viscoelastic surfactant is present in a concentration of
from about 0.3
to about 10 %; the synergistic co-surfactant is present in a concentration of
from about
0.008 to about 4 %; and the ratio of surfactant to synergistic co-surfactant
is from about
5:1 to about 15:1. In another embodiment the fluid composition is foamed or
energized
with carbon dioxide in a separate phase, for example with a foam quality of
from about
30 to about 80%. The viscoelastic surfactant and the synergistic co-surfactant
co-
operatively stabilize the carbon dioxide-water interface and the synergistic
co-surfactant
improves the compatibility of the viscoelastic surfactant with the carbon
dioxide.
2
CA 02469988 2010-02-26
78703-36
[0004.1] In one embodiment of the invention, there is provided a fluid
composition comprising a viscoelastic surfactant, carbon dioxide in a separate
phase and a synergistic co-surfactant that co-operatively stabilizes the
carbon
dioxide-water interface, said synergistic co-surfactant being selected from a
copolymer having regions of polypropylene oxide and regions of vinyl alcohol
vinyl
acetate, further comprising water, wherein said viscoelastic surfactant is
present in
a concentration of from about 0.3 to about 10%, and said synergistic co-
surfactant
is present in a concentration of from about 0.008 to about 4%, wherein the
foam
quality is from about 55% to about 80%.
[0005] In yet another embodiment, the viscoelastic surfactant is a
zwitterionic surfactant that has the formula:
RCONH-(CH2)a(CH2CH2O)m(CH2)b-N+(CH3)2-
(CH2)a'(CH2CH2O)m'(CH2)b'COO"
in which R is an alkyl group that contains from about 17 to about 23 carbon
atoms
which may be branched or straight chained and which may be saturated or
unsaturated; a, b, a', and b' are each from 0 to 10 and m and m' are each from
0
to 13, provided that both m and m' are not equal to 0; a and b are each 1 or 2
if m
is not 0 and (a+b) is from 2 to 10 if m is 0; a' and b' are each I or 2 when
m' is not
0 and (a'+b') is from 1 to 5 if m' is 0;
2a
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
(m + m') is from 1 to 14; and CH2CH20 may also be OCH2C'H2. As an example, the
surfactant is a betaine, for further example oleylamidopropyl betaine or
erucylamidopropyl betaine.
[00061 In yet another embodiment the synergistic co-surfactant is quaternary
amine
surfactant in which the cation is
4
N+
2
3
or
R -NT
+0
in which R1, R2, R3, and R4 are each alkyl, alkenyl, arylalkenyl, or
hydroxyalkyl, each
having from 1 to about 22 carbon atoms and being saturated or unsaturated and
branched
or straight chained, and Rl may also be alkylaminoalkyl and alkylar nidoalkyl.
The
synergistic co-surfactant may also be an ethoxylated anionic surfactant,
having the
3
CA 02469988 2010-02-26
78703-36
general formula R - (CH2CH2-O)õ-COO-M+ in which R is an alkyl chain having
from about 6 to about 22 carbon atoms, that can be straight chained or
branched, and
saturated or unsaturated and n has a value of from about 0 to about 30. The
synergistic
co-surfactant can also contain a mixture of either or both of the amine cation
types
shown, or a mixture of the ethoxylated anions, or a mixture of the amines and
the anions.
The synergistic co-surfactant may also be an oligomer or polymer of the
monomeric
examples above or of other materials such as polymers that have carbon dioxide-
phillic
region or regions and a hydrophilic region or regions, such as polypropylene
oxide and
vinyl alcohol vinyl acetate copolymer.
[0007] In yet another embodiment, the surfactant is BET-E-40 and the
synergistic co-
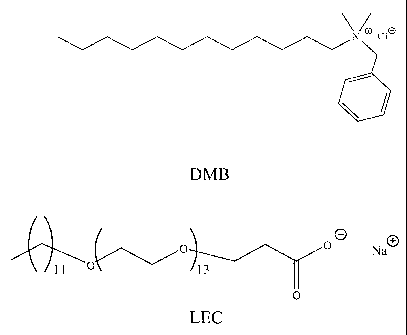
surfactant is C12 alkyl dimethyl benzyl ammonium chloride, sodium laureth-13
carboxylate or a mixture of the two.
[0008] A further embodiment is a method of increasing the compatibility of an
aqueous
fluid, containing the surfactants described above, with carbon dioxide which
includes
adding a sufficient amount of the synergistic co-surfactant.
[0009] Among the uses of these fluids is a method of treating a subterranean
formation
penetrated by a wellbore, or of treating the wellbore itself, by injecting the
carbon dioxide
foamed fluid into formation or wellbore. Zirconium oxychloride may be used as
a clay
stabilizing agent in these uses.
Brief Description of the Drawings
[0010] Figure 1 shows representative examples of suitable synergistic co-
surfactants.
[0011] Figure 2 shows temperature profiles of unfoamed and CO2 foamed fluids
made
without a synergistic co-surfactant.
[0012) Figure 3 shows viscosity temperature profiles for unfoamed fluids
having varying
ratios of synergistic co-surfactant to surfactant.
*Trade-mark
4
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
[0013] Figure 4 shows viscosity temperature profiles of unfoamed fluids made
with
various concentrations of a fixed ratio of synergistic co-surfactant to
surfactant.
[0014] Figure 5 shows viscosity temperature profiles of unfoamed and CO2
foamed
fluids made with a synergistic co-surfactant and a surfactant.
[0015] Figure 6 shows the viscosities of a CO2 foamed fluid made with a
synergistic co-
surfactant and a surfactant at different foam qualities and temperatures.
[0016] Figure 7 shows the effect of foam quality and temperature ort the
viscosity of CO2
foamed fluids made with a synergistic co-surfactant and a surfactant,
[0017] Figure 8 shows the foam rheology of a CO2 foamed fluid containing two
synergistic co-surfactants.
[0018] Figure 9 compares the foam rheologies of CO2 fumed fluids made with a
synergistic co-surfactant and a surfactant and with a polymer-based fluid.
Detailed Description of the Invention
[0019] VES/CO2 foamed fluids will be described primarily in terms of their use
as
fracturing fluids, although the invention should not be limited to fracturing
fluids and
fracturing. CO2 fractures target primarily low pressure or depleted wells. For
such wells,
there is often not enough reservoir pressure to push the fracture fluid back
to the surface
after a job. In order to improve cleanup of any well, fracture fluids may be
energized or
foamed to compensate for low pressure; the gas included in the fluid system
flows back
to the well as the pressure is released.
[0020] N2 and CO2 are most commonly used as foaming gases. CO2 foamed fluids
have
several advantages over N2 foamed fluids. They have a lower hydraulic
horsepower
requirement. CO2 has a higher molecular weight than N2 and has a higher
boiling point
so it is more easily liquefied. At typical fracturing conditions, CO2 is in
the liquid or
supercritical state; these are denser states than the gas state. Therefore a
fracturing fluid
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
foamed with CO2 can create more hydrostatic pressure than one foamed with N2.
In fact,
N2 foamed fluids are so light that when the temperature is above about 180 F
(about 82
C), the horsepower requirements for pumping them becomes economically
unfavorable,
and treating equipment pressure requirements become unfeasible. Furthermore,
CO2 has
a higher solubility in aqueous media which makes it easier to pump higher foam
quality
fluids.
[00211 CO2 also generates more energy down hole. Since the CO2 pumped down
hole is
in a liquid or supercritical phase, when the pressure is released or the
temperature is
increased, the CO2 gasifies. A small amount of liquid or supercritical CO2
occupies
much larger volumes when it turns into a gas. It creates a higher pressure-
gradient for
flow back per unit loading of CO2.
[00221 CO2 also gives better cleanup, i.e. causes less formation damage. Since
CO2 is
more soluble in aqueous fluids and easier to liquefy, higher foam quality
fluids can easily
be achieved. This reduces the total liquid load for each fracture job. With
less loading,
the likelihood of formation damage is reduced. Furthermore, because of the
high
solubility of CO2 in aqueous fluids, the fluid leaked out into the formation
always
contains some dissolved CO2. When this portion of the CO2 gasifies, it helps
to clean up
the fluid in the formation farther away from the wellbore. Finally, many
unfoamed or
N2- foamed polymer-based fluids are basic, but the pH of CO2 fluids is
normally about 4
because of the high concentration of dissolved CO2. This acidic environment
limits the
swelling of some formation clays.
[00231 Traditionally, linear and crosslinked polymers have dominated the
market for
viscosifiers for CO2 foams. However, foams based on viscoelastic surfactants
(VES's)
have good proppant transport properties, excellent retained permeability, are
simple and
robust to prepare and use, and all the components except the water (and any
salt added to
the water to make a brine) and CO2 can optionally be premixed in a single
concentrate.
6
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
[00241 When a fracturing fluid is mixed with CO2 and pumped down hole, the
surface
treating pressures are usually a few thousand psi (a few tens of MPa) (the
pressure is
higher at bottom hole due to the hydrostatic pressure of the fluid inside the
tubulars).
Under these conditions, CO2 is in a liquid state below 88 F (31 C). When the
temperature is above 88 F (31 C) and the pressure is lower than about 90,000
psi (about
600 MPa), the CO2 is in a supercritical state. Thus CO2 fracturing fluids are
normally
supercritical. Supercritical CO2 has unique chemical and physical properties
that are
used to advantage in embodiments of the present invention. In this state, it
has the
solvating power of a liquid and the diffusivity of a gas.
[00251 It has now been found that certain chemical compounds can be added to
VES
fluids to take advantage of synergistic effects and make them more compatible
with CO2.
That is, certain VES fluids that are not compatible or are not very compatible
with CO2
can be made compatible, or more compatible, by the addition of these
materials, that we
will call "synergistic co-surfactants" here. The "improved compatibility" may
be
demonstrated for a given surfactant concentration either by formation and
maintenance of
a foam under conditions at which the foam could not otherwise have been formed
or
maintained (malting the VES/CO2 system compatible where otherwise it was not);
or by
either higher viscosity of the foamed fluid at a given temperature, longer
foam life at a
given temperature, or a higher temperature at which useful fluid viscosity can
be
generated or maintained for a useful time (making the VES/C02 system. more
compatible
than it would have been without the synergistic co-surfactant material).
[00261 It should be repeated that, in addition, the VES/CO2 systems containing
synergistic co-surfactants of embodiments of the invention demonstrate
enhanced
performance (relative to analogous fluids foamed with nitrogen) when they are
energized
with CO2. The fluid performance can be adjusted with the fraction of C02 in
the system.
Up to about 80% C02, when the synergistic co-surfactant is present an increase
in the
fraction of CO2 results in higher viscosities and better foam stabilities
compared to
unfoamed or N2-foamed fluids, provided that the CO2 is supercritical.
7
CA 02469988 2010-02-26
78703-36
[00271 Suitable surfactants have a relatively good ability to form
viscoelastic aqueous
gels and have a relatively good affinity for C02- Suitable synergistic co-
surfactants have
shorter hydrophobic chains and a greater affinity for C02 than the
surfactants. Head
groups for the synergistic co-surfactants that have a suitable affinity for
CO2 include
carboxyl or carboxylate groups, fluorine-containing groups, silicon-containing
groups,
and quaternary ammonium groups. Synergistic co-surfactants may also be
oligomers and
polymers, for example oligomers or polymers of polypropylene oxide, and co-
oligomers
or copolymers of polyvinyl alcohol with polyvinyl acetate. Such oligomers and
polymers
have a region or regions that are preferably attracted to carbon dioxide
relative to water
and a region or regions that are preferably attracted to water relative to
carbon dioxide.
Short chain amphiphiles may also be used. The combination of surfactant and
synergistic
co-surfactant stabilizes the C02/H2O interface.
[00281 Several types of zwitterionic surfactants have been found to be
particularly useful
in forming stable foamed VES/CO2 systems when the synergistic co-surfactants
described above are used. In general, suitable zwitterionic surfactants have
the formula:
RCONH-(CH2)a(CH2CH2O)n,(CH2)b-N+(CH3)2-(CH2)a=(CH2CH2O)m=(CH2)b'COO
[00291 in which R is an alkyl group that contains from about 17 to about 23
carbon atoms
which may be branched or straight chained and which may be saturated or
unsaturated; a,
b, a', and b' are each from 0 to about 10 and m and m' are each from 0 to
'about 13; a and
b are each I or 2 if in is not 0 and (a + b) is from 2 to about 10 if in is 0;
a' and b' are
each I or 2 when m' is not 0 and (a' + b') is from 1 to about 5 if m is 0; (m
+ m') is from
0 to about 14; and CH2CH2O may also be OCH2CH2. Preferred surfactants are
betaines.
[00301 Two examples of betaines are, respectively, BET-O-30 and BET-E-40. The
VES
surfactant in BET-O-30 is shown below; one chemical name is oleylamidopropyl
betaine. .
It is designated BET-O-30 because as obtained from the supplier (Rhodia, Inc.
Cranbury,
New Jersey, U. S. A.) it is called Mirataine BET-O-30; it contains an oleyl
acid amide
group (including a C17H33 alkene tail group) and is supplied as about 30%
active
surfactant; the remainder is substantially water, sodium chloride, glycerol
and propane-
*Trade-mark
8
CA 02469988 2010-02-26
78703-36
1,2-diol. An analogous material, BET-E-40, is also available from Rhodia and
contains
an erucic acid amide group (including a C21H41 alkene tail group) and is about
40%
active ingredient, with the remainder being substantially water, sodium
chloride, and
isopropanol. (This material is termed "formulation 1" in the experiments
described
below.) The surfactant in BET-E-40 is also shown below; one chemical name is
erucylamidopropyl betaine. BET surfactants, and others that are suitable, are
described
in U. S. Patent No. 6,258,859.
[00311 Certain co-surfactants may be useful in extending the brine tolerance,
to increase
the gel strength, and to reduce the shear sensitivity of VES fluids, in
particular for BET-
O-type surfactants. An example given in U. S. Patent No. 6,258,859 is sodium
dodecylbenzene sulfonate (SDBS, shown below). VES's may be used with or
without
this type of co-surfactant, for example those having a SDBS-like structure
having a
saturated or unsaturated, branched or straight-chained C6 to C16 chain;
further examples
of this type of co-surfactant are those having a saturated or unsaturated,
branched or
straight-chained C8 to C16 chain. Other suitable examples of this type of co-
surfactant,
especially for BET-O-30, are certain chelating agents such as trisodium
hydroxyethylethylenediamine triacetate. Note that this type of co-surfactant
is not the
same type of co-surfactant as the "synergistic co-surfactants" of embodiments
of the
current invention.
[00321 Experiments described below used BET-E-40 although other VES's may be
used.
Although experiments have not been performed, it is believed that mixtures of
BET-E-40
with other surfactants (including both betaines, such as BET-O-30, and other
types) form
foamed VES/CO2 systems that are more stable than foamed VES/CO2 systems made
with
the other surfactants but without the addition of BET-E-40. Such mixtures are
within the
scope of embodiments of the present invention.
9
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
H H3C O
IV+ . (CH2)p
C17H33 N
\(CH2 n `CH3
O
Surfactant in BET-O-30 (when n = 3 and p = 1)
H H3C\~.(CH2) p
C 2 1 (CH2 n \CH;s
O
Surfactant in BET-E-40 (when n = 3 and p = 1)
S03
(CH2)xCH3
SDBS (when x = 11 and the counter ion is Na)
[00331 Betaines that are suitable include those in which the alkene side chain
(tail group)
contains 17 - 23 carbon atoms (not counting the carbonyl carbon atom) which
may be
branched or straight chained and which may be saturated or unsaturated, n = 2 -
10, and p
= 1 - 5, and mixtures of these compounds. As a further example, suitable
betaines
include those in which the alkene side chain contains 17 - 21 carbon atoms
(not counting
the carbonyl carbon atom) which may be branched or straight chained and which
may be
saturated or unsaturated, n = 3 - 5, and p = 1 - 3, and mixtures of these
compounds.
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
[00341 Examples of two classes of compounds that are suitable synergistic co-
surfactants
for creating or improving the compatibility and thus performance of VES/CO2
systems
follow. The first class is certain quaternary amine surfactants having either
of the generic
formulas for the cation:
R4
\ '111MR,
2
TI/ 3
or
0
RI-N+
in which R1, R2, R3, and R4 can be alkyl, alkenyl, arylalkenyl, and
hydroxyalkyl having
from I to about 22 carbon atoms and can be saturated or unsaturated and
branched or
straight chained. RI can also be alkylaminoalkyl and alkylarn.idoalkyl. The
anion may be
inorganic (such as Cl , and Er) and organic (such as acetate and other organic
acid
groups). An example of such a synergistic co-surfactant is an alkyl dimethyl
benzyl
ammonium chloride having an alkyl group that is saturated and straight-chained
and has
about 8 to 16 carbon atoms, or having an alkyl group that is a mixture of
saturated and
straight-chained alkyl groups having about 8 to 1.6 carbon atoms. One example
of such
11
CA 02469988 2010-02-26
78703-36
compounds is C12 alkyl dimethyl benzyl ammonium chloride, obtained from
Rhodia, Inc.
Cranbury, New Jersey, U. S. A. under the trade name Alkaquat DMB 80, which
will be
called "DMB" here
[00351 The second class is ethoxylated anionic surfactants of the general
formula:
- +
R - (CH2CH2-O),,-CH2CH2000 M
having an alkyl chain R of from about 6 to about 30 carbon atoms, that can be
straight
chained or branched, and saturated or unsaturated, and a value of n from 0 to
about 20.
The cation can be inorganic (for example K+, Na+, and Cs+) and organic (for
example
quaternary amine). An example is sodium laureth-13 carboxylate, sold by
Rhodia, Inc.
Cranbury, New Jersey, U. S. A. under the trade name Miranate LEC-80, and
hereinafter
referred to as "LEC". LEC has a C12 straight chained alkyl group, 13 ethoxy
groups and
a sodium cation. As received,- Miranate LEC-80 is about 79% active ingredient
and also
contains mixed alcohol and water.
[00361 Mixtures of more than one member of either class or of one or more
members of
each of the two classes of synergistic co-surfactants may also be used. A
typical foamed
VES fluid has about 0.3 to. about 10 weight percent VES (expressed as active
surfactant
in the aqueous phase of the foamed fluid, not as as-received surfactant), and
about 0.008
to about 4 weight percent synergistic co-surfactant (expressed as synergistic
co-surfactant
in the aqueous phase of the foamed fluid). In another example, concentrations
are about
0.6 to about 1.6 weight percent VES, and about 0.08 to about 0.4 weight
percent
synergistic co-surfactant. The ratio of surfactant to synergistic co-
surfactant is typically
from about 5:1 to about 15:1, for example from about 7.5:1 to about 10:1. The
volume
percent CO2 ("foam quality") is from about 30 to about 85 percent, for example
from
about 60 to about 75 percent.
[00371 Representative examples of suitable synergistic co-surfactants are
shown in
Figure 1, in which DMB is C12 alkyl dimethyl benzyl ammonium chloride, and LEC
is
sodium laureth-13 carboxylate. Oligomers and polymers of these materials may
also be
used.
*Trade-mark .
12
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. at.
Express Mail No. ER 975518748 US
[00381 The water that can be used may be any water, such as municipal water,
lake or
stream water, sea water or brine. For optimal performance, VES/CO2 foamed
fluids
made with BET-E-40 should contain about 2% KCI. Suitable concentrations of any
added materials, such as salts, acids, mutual solvents, alcohols, and others,
may readily
be determined by simple experiments such as those describe below.
[0039] The new VES/CO2 systems are used in the same way as other energized or
foamed systems are used, for example in the oilfield in such operations as
fracturing, and
in other industrial uses. No special equipment or procedures are needed. The
major
difference is the inclusion of the synergistic co-surfactant, and in
adjustments to the
component concentrations or to the job designs that may be required as a
result of the
better performance. Normally the synergistic co-surfactant is premixed with
the VES
surfactant in a concentrate that is then added to water, before or after other
components
that are typically used in such formulations (like iron controllers, biocides,
foaming
agents, corrosion inhibitors, proppants, clay control agents, and others).
Optionally some
of these other components may also be included in the concentrate. The
synergistic co-
surfactant may, however, be added premixed with any other component or
components,
for example as a concentrate, or may be added to the fluid separately.
[0040] In addition to fracturing, these fluids may be used in many other
oilfield
applications such as but not limited to acidizing, acid fracturing, gravel
packing,
diversion, and well cleanout. They may of course also be used in treatments of
other
types of wells such as but not limited to wells for the production of
hydrocarbon gas,
distillate, helium, carbon dioxide, water, and other materials, and in
injection wells for
enhanced recovery, storage, or disposal.
[0041] Another use is for equilibrium-controlled controlled release of acid
for acidizing.
For example, in a reaction like that of HCl with CaCO3, CO2 is generated;
having a high
concentration of CO2 in the system drives the equilibrium reaction backward,
therefore
suppresses the reaction rate (retards the reaction). When this is well
controlled, the
reaction rate can be tuned by varying the CO2 partial pressure. This feature
is used for
example to improve efficiency, to control fluid placement, and to control
wormholing.
13
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
Other industrial uses of CO2 foams that can be improved include, by non-
limiting
example, use as a super solvent for difficult-to-dissolve compounds, use as a
removable
solvent template to generate uniformly structured porous materials, use for
blending two
immiscible polymers, and use in creating controlled-release coatings for
drugs.
[0042] In oilfield operations it is very common to use clay control agents
such as
potassium chloride or tetramethyl ammonium chloride. It is known that
zirconium
oxychloride is an excellent clay stabilizer that can be very inexpensive to
use because it
can be used at very low concentrations. However, it can only very rarely be
used with
most polymer based viscous fluids because zirconium is a crosslinker for such
polymers
as polysaccharides. Zirconium oxychloride can be used as a clay control agent
in fluids
and methods of embodiments of the invention, provided that it does not
interfere with the
ability of the specific synergistic co-surfactant/VES system to form a
viscoelastic foamed
fluid. This should be tested in the laboratory before use.
Experimental:
[0043] Unfoamed fluids were prepared using a Waring blender equipped with a
Variac.
Unfoamed fluid viscosities were measured with a Fann 50 viscometer according
to API
standards. Unfoamed fluid dynamic rheology and low-shear rheology was measured
with
a Bohlin Rheometer using a bob and cup geometry. CO2 foamed fluid viscosities
were
measured with a Chandler Foam Rheometer.
[0044] To make a typical unfoamed fluid, 200 ml of tap water was added to a 1L
Waring
blender cup. To this fluid, 4 g (2%) of KC1 salt was added and dissolved, and
7 ml
(3.5%) of a VES concentrate was then added with gentle stirring. The fluid was
blended
with a Variac set to 30% to 40% of the full speed for 5 minutes, then with the
Variac set
to 60% for 40 seconds.
[0045] Surfactant concentrates from which VES fluids were made are called
formulations. Formulation 1 contained only as-received betaine surfactant BET-
E-40.
When fluids made with 3% formulation 1 were foamed with CO2, the fluid
viscosities
14
CA 02469988 2010-02-26
78703-36
remained low (-50 cP) as the temperature was increased until the temperature
was above
about 200 OF (about 93 C). Note that the foam qualities increased during such
a test as
the temperature was raised; foam quality increases also contributed to the
fluid viscosity
increases observed here. The fluid viscosity then remained above 100 cP until
the
temperature reached about 270 OF (about 132 C). In order for a fluid to be
useful as a
fracturing fluid at about 200 OF (about 93 C), it should have good viscosity
below that
temperature, so that it can carry proppant before it reaches high temperature.
Therefore
formulation I alone is unsatisfactory for making good VES/CO2 fluids for
fracturing.
[00461 Formulation 2 was as-received betaine surfactant BET-E-40 containing
about 1%
of DAXAD 17, a low molecular weight sodium polynaphthalene sulfonate available
from
Hampshire Chemical Corporation, Nashua, NH, USA. As shown in Figure 2, a CO2
fluid
made with 6% formulation 2 did not give good viscosity throughout the whole
temperature range studied. The viscosity increased with temperature up to
about 130 OF
(about 54 C) but then decreased with further increasing temperature; it
decreased to less
than about 50 cP as the temperature increased to above no more than about 240
OF (about
116 C). However, the foam did not look stable and drainage defoaming could be
seen
during the test when the foam was observed in a high temperature cell;
drainage de-
foaming was clearly visible after the fluid was isolated in the cell for a few
minutes.
Formulation 2 is therefore considered insufficiently compatible with CO2.
[00471 The DAXAD had been added to formulation 1 to decrease the shear
recovery time
of the fluid (not shown) but it also decreased the viscosity of the CO2 foamed
fluid. We
have found that a small amount of the proper synergistic co-surfactant in a
VES system
acts as a shear-recovery additive as well as an agent to maintain and even
improve the
viscosity and foam stability. An example of such a synergistic co-surfactant
is the
cationic surfactant Alkaquat DMB 80. Not to be limited by theory, but it is
believed that
the addition of the DMB changed the distribution of the surfactants on the
C02/water
interface. The resulting VES fluid was compatible with CO2 and was
particularly
suitable as a fracture fluid when the foam quality was from about 60% to about
80%.
Fluid viscosity could be maintained above 100 cP from room temperature up to
at least
*Trade-mark
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. at.
Express Mail No. ER 975518748 US
about 250 F (about 121 C). It is estimated that the DMB extended the useful
temperature of the fluid by at least about 50 F (about 38 C). The
concentrations of VES
used in these experiments were about half the concentrations sometimes used in
unfoamed fracturing fluids. If the higher concentrations are used in foamed
fluids
containing DMB the fluids are expected to perform well at temperatures
approaching
about 300 F (about 149 C). The addition of DMB may in some cases impair the
unfoamed fluid viscosity at high temperatures, but such a fluid when foamed
with CO2
will none-the-less have an extended temperature stability range.
[00481 For fluids made with BET-E-40 and DMB, it was found that there was a
close
relationship between the performance of an unfoamed fluid and that of a CO2
foamed
fluid. (A satisfactory unfoamed fluid means the fluid has both satisfactory
viscosity and
satisfactory viscoelasticity. Fluids made with the synergistic co-surfactants
of
embodiments of the invention are not only viscous but viscoelastic.) As a
result of this
relationship it was assumed that if the viscosity of an unfoamed fluid was
satisfactory, the
CO2 foamed fluid viscosity and stability would also be satisfactory. This
relationship
greatly simplified the developmental process. Most screening tests were done
using tests
of unfoamed fluids. Foamed fluids were used only for spot checks during the
screening.
Therefore, most of the results presented below were obtained with unfoamed
fluids.
[00491 Fluids were prepared having varying ratios of DMB to formulation 1 to
find the
ratio giving the optimal viscosity profile and optimal shear recovery time
under these
conditions. Shown in Figure 3 are the viscosity temperature profiles of these
tests with
fluids containing 3% formulation 1 and 2% KCl. It is clear that the ratio
affected the
viscosity performance. More DMB usually boosted the low-temperature viscosity
(and
shortened the shear recovery time as well) but usually deleteriously affected
the high-
temperature performance. The results were spot checked with a foam rheometer
(data not
shown). In balancing all the properties, a DMB/formulation 1 volume ratio of
0.123 was
chosen for more thorough testing. This material, termed formulation 3,
contained 89% of
formulation 1 and 11 % of the Alkaquat DMB 80.
16
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
[0050] The viscosity vs. temperature behavior of fluids having different KCI
concentrations (3.37% formulation 3 for all samples) was investigated. It was
found that
2% KC1 was an excellent concentration for this particular fluid to show good
viscosity
over the entire temperature range of 75 to 250 F (24 to 121 C). It was also
found that
the fluid performance was satisfactory up to a KCl concentration of about 4%.
This gives
some room for jobs when a higher clay stabilizer concentration is needed. It
should also
be kept in mind that the performance is related to both the DMB/formulation 1
ratio and
the KCl concentration. If a very high KCl concentration were needed for a CO2
fracturing fluid, the DMB/formulation 1 ratio may be adjusted to re-optimize
the
performance by simple viscosity measurements.
[0051] Mixing water contaminant type and concentration vary greatly on
location, so
determination of the effects of common contaminants on fluids made with any
VES/synergistic co-surfactant combination could be important. The common
contaminants sulfate, bicarbonate, iron ion, calcium and magnesium were
investigated
with fluids made from 3.37% formulation 3 and 2% KCI. The highest
concentrations
selected for the tests were normally higher than those that are commonly seen
in the field.
[0052] With increasing concentrations of K2S04 up to 4000 pprn (much higher
than
normally seen in formation water), there was almost no effect on the unfoamed
fluid
viscosity profile. Sodium bicarbonate (up to 2000 ppm) had almost no effect on
these
fluids made with formulation 3. Up to 500 ppm, ferric ion showed no effect on
unfoamed
fluids made with formulation 3.
[0053] Examination of several fluids having varying calcium concentrations
showed that
when the calcium concentration was 100 to 500 ppm, there was a slight
detrimental effect
on the fluid viscosity, but the effect virtually disappeared when the calcium
concentration
was 1000 ppm or higher. Magnesium behaves like calcium under most conditions.
[0054] When 500 ppm calcium was added to a fluid made with formulation 3 and
the
fluid was foamed with CO2, the foamed fluid showed insignificant differences
in
rheology compared to the same fluid without calcium added. It is believed that
the CO2
dissolved in the solution will overwhelm the levels of calcium or magnesium
that would
17
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
be found in frac water. All the calcium ions (or magnesium ions) had been
converted to
calcium (magnesium) carbonate or bicarbonate. Converting to carbonate would
cause the
calcium to precipitate out of the solution while converting to bicarbonate
would have a
minimal impact on the fluid rheology. Therefore no effect was found. This
might not be
true for every surfactant/synergistic co-surfactant combination at high
calcium or
magnesium concentrations and should be tested for each fluid.
100551 Foamed fluids actually contain only a small fraction of the aqueous
phase,
especially when the foam quality is high. This alone limits the amount of
fluid left in the
formation after a treatment and thus minimizes the damage that could be done.
During
the flow back stage, when the surface pressure is reduced, the gas in the
foamed fluid
flows back and at the same time carries the aqueous fluid back to the surface.
For N2
foam, there is not much of the dissolved gas in the fluid; if a certain amount
of aqueous
phase leaks out into the formation, it may not be carried back out of the
formation by the
amount of gas that can be formed. As mentioned earlier, foamed VES/CO2 fluids,
such
as fluids made with synergistic co-surfactants, are more like emulsions than
like fluids
foamed with a gas. Therefore, any fluid leaked off into the formation
inherently contains
a substantial amount of supercritical CO2 as emulsion droplets. This provides
deeper
cleanup of the formation since the liquid droplets in an emulsion have less of
a tendency
to be separated from the aqueous phase of the system during leak off than does
the gas
phase of a N2 foam. Furthermore, CO2 has a much higher solubility in an
aqueous fluid
than does N2, so even if there is no CO2 present as emulsion droplets, the
fluid leaked out
into the formation always has a certain amount of dissolved CO2. When the
pressure is
released, or the temperature is increased, the CO2 gasifies from any aqueous
phase that
has leaked off and helps to bring it back. The cleanup is thus usually much
better than
cleanup of N2 foamed fluids. In most cases, for these reasons, no breaker is
needed.
Furthermore, since CO2 increases the viscosity when it is dissolved in VES
fluids, for
hydraulic fracturing the unfoamed fluid viscosity is designed to be low when
the CO2 in
the fluid has been depleted and when the fluid has warmed up to the formation
18
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. at.
Express Mail No. ER 975518748 US
temperature; thus the fluid viscosity is low when most of the CO2 has flowed
back and
this facilitates cleanup.
[0056J However, in some cases, it is still desired to have a breaker to help
thin the fluid
to facilitate the flowback. Breakers for fluids made with formulation 3 have
been
investigated. Examples of suitable breakers for fluids made with formulation 3
are NISB
(non-ionic surfactant blend) and EPNS (encapsulated polynaphthalenesulfonate).
NISB
is approximately 10% butan-l-o1, 25% 2-butoxyethanol, 15% water, 8.5% of a
mixture of
linear and branched Ctl alcohol ethoxylates having about 3 ethoxy units, 39 %
of linear
and branched C, 1 alcohol ethoxylates having about 8 ethoxy units, and 2.5 %
undecanol.
EPNS is approximately 7-8% sodium 2-naphthalenesulfonic acid, 2-8% water, 4-
10%
sodium sulfate, and 55-65% sodium polynaphthalenesulfonate, all encapsulated
in 15-
25% of a vinylidene chloride/methacrylate copolymer.
[00571 The effect of NISB on fluids made with formulation 3 was studied over
the
temperature range of 75 to 250 F (24 to 121 C). The fluid viscosity was
decreased
substantially by 0.5% NISB (especially above about 130 F (about 54 C)) and
almost
completely above about 175 F (about 80 C) and was decreased completely by 1%
NISB
above about 150 F (about 66 C). The fluid low shear viscosity was affected
even more.
As one example of its use, NISB is pumped as an aqueous solution as part of
the pre-pad
stage of a fracturing treatment. It leaks off into the formation; when it
flows back after
the job and contacts the VES/CO2 fluid, the fluid is broken.
[00581 EPNS is an encapsulated breaker. The encapsulated material breaks the
VES
fluid by disrupting the electrolyte balance of the surfactant. EPNS is, for
example,
pumped as part of the slurry stage in a fracture treatment and is thus placed
with the
proppant in the fracture. When the fracture closes, the closure stress crushes
the coating
of the EPNS and releases the encapsulated breaker material. Unfoatned fluids
made with
formulation 3 were broken by this encapsulated breaker. Depending upon the
surfactant
concentration, we have found that about 7 to about 10 ppt (about 0.8 to about
1.2 g/L) is a
suitable concentration of EPNS. To confirm this, we tested foamed broken fluid
with the
foam rheometer. When an unfoamed fluid was broken with 10 ppt (1.2 g/L) of
EPNS and
19
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
then foamed, that foamed fluid had much lower viscosity than one made without
prior
treatment with crushed EPNS. In addition, the foam half life of the broken
fluid was also
much shorter, which further ensures the ease with which the broken fluid is
cleaned up.
100591 Since the concentrate may need to be stored or pumped at low
temperature under
certain conditions, its low temperature stability and pumpability are
important. The fluid
may also be stored for a certain period of time at high temperatures, so its
long term high
temperature stability was investigated. Investigation of formulation 3
surfactant
concentrate viscosity as a function of temperature showed that it is still
very pumpable
even at about 20 OF (about -7 C).
[00601 Aging tests of formulation 3 concentrate were done at low and high
temperatures
to mimic cold and hot storage environments. Although the temperature of about
150 OF
(about 66 C) that was picked to examine high temperature conditions is higher
than
realistic, surfactant concentrate decomposition (if any) was accelerated at
this
temperature. An aging test for a comparatively short time at about 150 OF
(about 66 C)
showed whether the chemicals in the surfactant concentrate were stable for
much longer
times at field conditions. The tests were done by storing the surfactant
concentrates in a
water bath at about 150 OF (about 66 C) and in a freezer at about 20 OF
(about -7 C).
At designated times, portions of the surfactant concentrates were removed and
aqueous
fluid samples were prepared from them using 3.37% concentrate in, water
containing 2%
KCI. The rest of the surfactant concentrate stayed in the hot or cold
environment for
further aging. Unfoamed fluids made with hot or cold aged formulation 3 were
than
tested with the Fann 50 rheometer and the viscosities were compared to the
viscosities of
fluids made with un-aged concentrate. Neither aging at high temperature nor at
low
temperature for 3 months had any detrimental effect on the performance of
formulation 3
surfactant concentrate.
[00611 The effect of the concentration of formulation 3 was investigated.
Shown in
Figure 4 is the viscosity temperature profile of unfoamed fluids made with
formulation 3
in 2% KC1. Increasing the formulation 3 concentration increased the fluid
viscosity over
most of the temperature range. Above about 230 OF (about 110 C), the fluids
had very
little viscosity and increasing the formulation 3 concentration. did not help.
As shown in
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
Figure 5, the viscosity profile of an unfoamed fluid made with 4% of
formulation 3 in 2%
KCl was compared to the same fluid foamed with CO2 at 70% and 75% foam
quality.
The CO2 foamed fluid viscosities were higher than the unfoamed fluid viscosity
over
almost the entire temperature range studied. Furthermore, foaming with CO2
extended
the fluid temperature upper limit by more than about 50 IF (more than about 28
C).
[0062] The effects of CO2 foam quality were investigated in more detail. It
was found
that CO2 foamed fluid viscosities were directly related to foam quality. For
CO2 foamed
fluids made with formulation 3 in 2% KC1 to have viscosities greater than
about 100 cP at
100 s' shear rate, the foam quality generally had to be greater than about
60%. For
example, Figure 6 shows the viscosities of CO2 foamed fluid made with 4% of
formulation 3 in 2% KCl at different foam qualities at 100 IF (about 38 C)
and at 200 OF
(about 93 C). It can be seen that at the higher temperature the viscosity
increased
dramatically when the foam quality was at least about 60%. At the lower
temperature
viscosity increased with foam quality but the effect was not as great. This
foam quality
reduced the liquid fluid (hydrostatic) load and provided good foam fluid
viscosity. Foam
qualities too high (i.e. above about 80%) tended to make the foam texture
difficult to
control and there is the possibility of inverting the internal and external
phase of the
foamed fluid. If inversion happens, the viscosity of the foamed fluid could
drop to very
low values.
[0063] Figure 7 shows the viscosities of CO2 foamed fluid made with 4.5 % of
formulation 3 in 2% KC1 at different foam qualities at temperatures ranging
from 90 IF
(about 32 C) to 250 IF (about 121 C). At 90 OF (about 32 C), the
viscosities were all
about the same at foam qualities from 55 to 75%. At higher temperatures,
higher foam
qualities clearly gave higher viscosities. At this formulation 3
concentration, for each
foam quality there was a temperature above which the foam viscosity started to
decrease,
for example about 200 IF (about 93 C) for 75% foam quality, about 150 IF
(about 66
C) for 70% foam quality, and about 90 OF (about 32 C) for lower foam
qualities.
Experiments not shown indicated that the behavior was similar at formulation 3
concentrations down to at least as low as about 2.5%, except that the
viscosities were
21
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
lower for any combination of temperature and foam quality and that at lower
formulation
3 concentrations the effects of varying foam quality were less pronounced but
still
significant.
[0064] Experiments (not shown) have indicated that foams using the suitable
formulations discussed here are usually generated satisfactorily during normal
field
mixing and pumping procedures. However, a foam generator may be used if
desired.
[0065] Experiments (not shown) have indicated that the fluids of the invention
are
compatible with fibers used, for example as cleanout aids or to inhibit
proppant flowback
and sand and particle migration.
[0066] Figure 8 gives the foam rheology results with a fluid that contained 3%
formulation 1, 0.19% DMB, 0.03% LEC, and 0.2% tetramethyl ammonium chloride.
In
Figure 8 the times are given in the format x:yy:zz, that stands for x hours,
yy minutes,
and zz seconds. It can be seen this fluid containing a combination of
synergistic co-
surfactants provided a viscous foam stable to at least about 207 OF (about 97
C). It is
believed that stable viscous foams would have been obtained at higher
temperatures but
the experiments were not done. On the figure, the sets of higher viscosities
represent
reductions of the shear rates from 100 to 75, 50, and 25 sec . These results
were
obtained on a Chandler foam rheometer in a 0.1750 or 0.2055 inch (0.445 or
0.522 cm)
inside diameter capillary at a volumetric flow rate of 0.02 to 0.09 in3/sec
(0.33 to 1.47
cm3/sec). The test was performed by continuously circulating the fluid in the
capillary
loop at different temperatures. The viscosity values were calculated from the
pressure
drops within a segment of the foam loop.
[0067] The carbon dioxide foamed VES fluids of embodiments of the invention
are
comparable in viscosity to carbon dioxidefoamed polymer fluids that are
currently in use
for fracturing. Figure 9 shows a comparison of the fluid viscosities at about
150 OF
(about 66 C) of formulation 4 (4% formulation 1 and 0.5% DMB in 2% KC1) and
formulation 5 (a fluid commonly used in foamed fracturing, containing the
equivalent of
40 pounds of guar per thousand gallons of fluid (about 4.8 g/L), made by
adding to tap
water 0.9 volume percent of a 50/50 weight percent mixture of guar in diesel,
0.2 volume
22
CA 02469988 2004-06-04
Patent Application
Docket Number 56.0740
Inventors: Chen, et. al.
Express Mail No. ER 975518748 US
percent tetramethyl ammonium chloride, 0.025 volume percent of a bactericide,
0.1
volume percent coco dimethyl ammonium chloride, and 0.6 volume percent of an
approximately 25-50 weight percent aqueous mixture of C6-Clo alcohol
ethoxysulfate).
The foam quality of the VES fluids was about 70% and the foam quality of the
polymer
fluid was about 65%. The VES fluid performance was very comparable to that of
the
polymer.
23