Note: Descriptions are shown in the official language in which they were submitted.
CA 02473995 2004-07-29
METHOD AND APPARATUS FOR CONTINUOUS FLOW REDUCTION
OF MICROBIAL ACTIVITY IN A LIQUID PRODUCT
USING PRESSURIZED CARBON DIOXIDE
FIELD OF THE INVENTION
g This invention relates to a method and ~ipparatus
for the processing of liquids to reduce microbial
and/or enzymatic activity therein and, more
particularly, to the use of pressurized carbon dioxide
to achieve reductions of microbial a.nd/or enzymatic
activity.
REFERENCE TO RELATED APPLICATION
This application is a division of Canadian Patent
Application No. 2,280,240 filed Auguat 10, 1999.
BACKGROUND OF THE INVENTION
There are many methods for improving the shelf
life of liquid products such as orange juice, apple
juice, milk, latex paints, peanut butter, soup; etc.
Commercially, thermal methods such as
pasteurization are the predominant methods used to
improve the shelf life of liquid foods. Ultra-high
pressure treatment is also used for liquid foods, but
much less frequently.
In high pressure treatment facilities, fluids
containing microbial contamination are pressurized
hydrostatically to kill the majority of the bacteria.
In such systems, .pressures are created which equal or
exceed 30,000 psia. Such hydrostatic treatment,
however, does not destroy enzymes, is unsafe because of
the very high pressures, is a lengthy process, is batch
rather than continuous, and is expeazsive due to the
high capital costs of the required equipment.
Other methods for shelf-life extension of liquids
include nuclear irradiation, ultra-violet exposure and
CA 02473995 2004-07-29
D-20, 671
- 2 -
application of microwaves. These treatments are
expensive and not widely used commercially at present.
High pressure homogenization has been used to
increase the shelf life of orange juice and other
single-strength citrus juices as described in U.S.
Patent 5,232,726 to Clark et al. It is disclosed that
a citrus juice being processed is subjected to a high
pressure of about 15,000 psia, with the result being a
significant reduction in biological activity in the
l0 juice.
Carbon dioxide has been used to inactivate enzymes
in food and reduce microbial populations in fruit
juices as described in U.S. Patent 5,393,547 to Balaban
et al. Balaban et al. describe a.method for
inactivating enzymes in liquid food products wherein
the food is exposed to pressurized carbon diaxi.de
which, in turn, produces a carbonic acid solution with
a pH that is sufficiently low to irreversibly
inactivate enzymes in the liquid food. The Balaban et
al. method is indicated as being applicable to either
batch mode or continuous flow made processing of food. ' "'''
Balaban et al. further indicate that supercritical
carbon dioxide is introduced at a rate sufficient to
allow enough thereof to dissolve in the food to
inactivate the enzymes. After enzymatic inacaivation,
the food flows to a section where pressure is reduced
and the released carbon dioxide may be recycled for
repeat usage.
U.S. Patent 5,704,276 to Osajima et al. describes
a method for continuous deactivation of enzymes in
liquid foodstuffs; using a supercritical form of carbon
dioxide. Osajima et al. indicate that the density of
CA 02473995 2004-07-29
D-20, 671
- 3 -
the supercritical fluid is less than that of the liquid
food and that the supercritical carbon dioxide is
injected continuously into the liquid food and is
separated therefrom in a later stage of the process.
Osajima et al. also indicate that their process
deodorizes the liquid food and removes volatile
components.
Arreola et al. in "Effect of Supercritical Carbon
Dioxide on Microbial Populations in Single Strength
Orange Juice", Journal of Food Quality, iTOlume 14
(1991), pp. 275-284, describe th.e effect of
supercritical carbon dioxide on microbial populations
in orange juice. Using a batch process, Arreola et al.
concluded that high pressure carbon dioxide treatment
resulted in microbial reduction in single strength
orange juice, even at low temperatures. Further, they
conclude that a combination of high pressure, and shear
forces to which the orange juice is subjected during
depressurization and lower pH due to temporary
formation of carbonic acid may have further inhibitory
effects on the normal flora within orange juice. ~~~~<~
During the processing described in this paper, the
minimum temperature utilized was 35°C.
Tt is an object of this invention to provide an
improved method and apparatus for reducing microbial
and/or enzymatic activity in liquid products.
Tt is a further object of this invention to
provide a method and apparatus for reducing microbial
and/or enzymatic activity in liquid products using
pressurized carbon dioxide, wherein the processing
temperature to which the liquid is subjected does not
CA 02473995 2004-07-29
D-20, 671
- 4 -
deleteriously affect the liquid products.
It is yet another object of this invention to
provide a continuous flow method and apparatus, for
reducing microbial and/or enzymatic activity in liquid
products using pressurized carbon dioxide.
SUMiKARY OF THE INVENTION
A continuous method using a pressurized flow of
carbon dioxide is described for the reduction of
IO microorganisms present in the liquid product and/or the
inactivation of one or more enzymes in a pressurized
flow of the liquid product. The pressure in the flow
regions are maintained at a level which is sufficient
to keep the carbon dioxide in dense phase, but at a
temperature which does not freeze the liquid product.
The pressurized mixture of the carbon dioxide and
liquid flows through a reaction zone for a sufficient
time to reduce harmful microorganisms and inactivate
undesirable enzymes and then enters a plurality of
expansion stages wherein the pressure of the mixture
flow is decreased sr~fficientiy to ai i o:~r the separation
of carbon dioxide from the liquid product. Heat is
applied in at least some of the expansion stages to
prevent a cooling of the mixture flow to the freezing
point of the liquid product. Heat may be applied to
prevent the freezing of the liquid product to control
the temperature so that it does not exceed a
temperature at which deleterious effects are
experienced. (Freezing and excessive high temperature
can have negative effects .on the juice quality.
Temperatures over 40°C begin to degrade the product.)
CA 02473995 2004-07-29
D-20, 671
- 5 -
The present invention is contemplated for use with
any fluid that may be transported through a conduit,
including for example, beverage products such as juices
and milk, semi-liquid foods such as mayonnaise, salad
dressings, soup and cottage cheese, and other fluids
such as paint and sterile injectibles.
BRIEF DESCRIPTION OF THE DRAWING
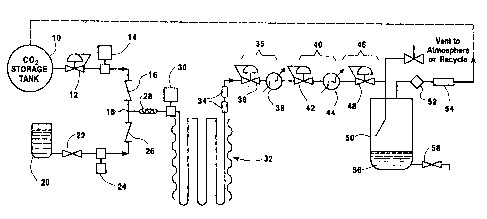
The figure is a schematic flow diagram of
to apparatus which performs the method of the invention.
DETAILED DESCRIPTION OF THE TNVENTION
Referring to the figure, pressurized carbon
dioxide is fed from carbon dioxide supply l0 through
optional pressure regulator 12 to a pump 14 which
increases the pressure of the carbon dioxide flow and
then feeds it through a check valve 16 to a.junc.ture
18. The carbon dioxide is pressurized at pump 14 to
prevent any boiling of the dense phase carbon dioxide
during later stages of the process.
I~~ similar fasha ozA; liquid product is fed from a
liquid product feed tank 20 through ~ valve 22 to a
pump 24. Pump 24 raises the feed pressure of the
liquid product to the same level as that of the dense
phase carbon dioxide exiting from pump l4. The
pressurized liquid product feed passes through check
valve 26 to juncture l8 where it combines with the
pressurized flow of carbon dioxide. The mixture of the
liquid product and carbon dioxide then passes to an in-
line mixer 28 which essentially comprises a heavily .
baffled conduit that thoroughly mixes the carbon
dioxide and liquid product streams. Of course, other
CA 02473995 2004-07-29
D-20, 671
- 6 -
mixers may be employed which achieve a desired level of
liquid product/carbon dioxide mixing. The liquid
mixture exits from in-line mixer 28 and is further
pressurized by the action of pump 30 to a process
pressure.
Depending upon the specific liquid product feed,
the process pressure will vary accordingly. It is
preferred that the process pressure be within the range
of 300 psia to 20,000 psia. If orange juice is being
processed as a liquid food, a preferred range of
pressure is about 1750 psia to about 2200 psi.a.
Once the liquid mixture exits from pump 30, it
enters a reaction zone 32 that is of suitable size and
length to provide sufficient contact (or residence)
time for the carbon dioxide and liquid product to
interact in a manner which reduces microorganisms
and/or inactivates undesirable enzyr~ies present in the
liquid product. The selected residence time will
depend on the liquid product to be processed and its
flowrate, as well as the size and length of 'the
reaction zone. It is preferred that the reaction zone
residence time is in the range of about 1.0 to .about
15.0 minutes.
For example, for processing orange juice, at a
flowrate of 20-200 ml/min in a reaction zone having a
. length of about 20 feet and tubing size of about 7.9mm
inner diameter (L.D.), the preferred residence time is
about l.5 to 13.0 minutes, and more preferably about
3.0 minutes of residence time.
As the liquid mixture stream exits from reaction
zone 32, it enters one or more interaction chambers 39
(optional) wherein high shear forces are applied which
CA 02473995 2004-07-29
D-20, 671
_ 7 _
enable a rupture of microbial cell walls in the liquid
mixture. Such action enables a further reduction of
the microbial populations in the liquid mixture. High
shear interaction chambers that are suitable for
inclusion in this process are manufactured by the
Microfluidics International Corp., Newton,
Massachusetts.
At this stage, the pressurized carbon
dioxide/liquid product mixture must be depressurized in
such a fashion as to avoid freezing the liquid product
(due to the Joule-Thompson cooling effect of the
expansion of the carbon dioxide). If-_ the pressure is
lowered to ambient in one or two stages, very large
heat exchange or application of supplemental heat is
required. If too much heat is added to the mixture,
damage will occur to the liquid product, either in its
flavor characteristics or its compos~.tion. Also,
important volatiles such as flavor components may be
carried away. Accordingly, it has been found that
substantial care must be taken during the .
depressurization action to maintain the liq;~ia mixture
within two boundaries. The lower boundary is the
freezing point of the liquid mixture and the upper
boundary point is the maximum temperature to which the
liquid product can be subjected, without damage to the
product.
In the case of orange juice, the maximum
temperature is about 60°C and the minimum temperature
is about 0°C. Accordingly, when choosing a pressure
reduction scheme, a pressure/enthalpy chart for carbon
dioxide is followed to determine the optimum pressure
CA 02473995 2004-07-29
D-20, 671
_ g _
and heating temperature needed for plural pressure
reduction stages, while keeping (in this example)' the
orange juice at a temperature between that which will
injure its flavor and its freezing point. Tt has been
determined that at .least two stages of depressurization
are required, but it is preferred that there be at
least three stages.
Returning to the figure, the first
depressurization stage includes a pressure control
device 36, such as a back pressure regulator, followed
by a heat exchanger 38. Assuming that the liquid
product being processed is orange juice and that the
process pressure within reaction zone 32 and
interaction chamber 34 is about 2,000 psia, a first
depressurization stage 35 reduces the pressure of the.
liquid mixture to approximately 600 psig and applies
sufficient heat through heat exchanger 38 to maintain
the liquid mixture at about 30°C.
A second depressurization stage 40 includes a
pressure control device 42 and heat exchanger. 44 which,
in combination, reduce the pressure of the liquid
mixture to about 250 psia and maintains its temperature
at approximately 30°C. A final stage depressurizer 46
includes only a pressure control device 48 to reduce
the pressure of the liquid mixture to the point where
the dense phase carbon dioxide will vaporize and may be
separated from the liquid products while minimizing
loss of important volatile components. In the
embodiment shown in the figure, no heat exchanger is
required subsequent to pressure control device 48,
however, one may be provided, if required, to maintain
CA 02473995 2004-07-29
D-20, 671
_ g
the liquid mixture within the required temperature
range.
As the liquid mixture exits from pressure: control
device 48, it enters a liquid product/carbon dioxide
separator vessel 50 or other collection device at
reduced pressure. There, the carbon dioxide vapor
separates from the liquid product, is captured and
passed through a filter 52, flow meter 54 (if desired)
and is either vented to atmosphere or is passed through
to a pressurization stage (not shown) for recycling.back
to carbon dioxide supply 10. The liquid product poo l
56 may then be drained through valve 58 for subsequent
processing and/or use.
It is to be understood, that the continuous
process method shown iri the figure is made practical by
the multiple depressurization stages which enable the
liquid mixture to be maintained within the
aforementioned temperature boundaries. As a result, a
continuous process for reduction of microbial and/or
enzymatic activity is achieved while overcoming the
principal problem of. the prior art, i.e., batch
processing which is an uneconomic and undesired
processing procedure in a commercial environment.
If the carbon dioxide gas is to be recycled, it is
preferred that it be passed through a coalescing filter
to remove droplets of the processed liquid product.
Thereafter, the gas is recondensed to the liquid state
by passage through a condensing heat exchanger.
Further, to assure removal of the dissolved carbon
dioxide in the processed liquid product, a liquid
product/carbon dioxide separator downstream from
separator tank 50 may include means for deaeration.
CA 02473995 2004-07-29
D-20, 671
- 10 -
The resultant gas, remaining after processing, may
carry additional valuable aromas and/or flavors. To
recover or remove such aromas or flavors, a method such
as condensation or absorption may be utilized.
It should be understood that the foregoing
description is only illustrative of the invention.
Various alternatives and modifications can be devised
by those skilled .in the art without departing from the
invention.