Note: Descriptions are shown in the official language in which they were submitted.
CA 02475622 2004-07-23
..
a ,.
-1-
METHOD AND APPARAT1JS FOR MEASURING CHEMICAL COMPOUNDS
USING SCATTERED LIGHT SPECTROSCOPY
The present invention relates to measuring chemical compounds using scattered
light spectroscopy. More specifically this invention pertains to measuring
absorbing and
non-absorbing chennical compounds using scattered light spectroscopy.
BACKGROUND OF THE I1~1VENTION
There is a need for the development of instruments and associated methods for
rapid analysis of blood and bodily fluids without application of chemical
reagents.
Preferably, such methods and instrumentation for medical diagnosis would
involve
non-invasive spectroscopic methods. To date the success of non-invasive
spectroscopic methods is limited, with Oxy-meters being an exception.
Current spectroscopic methods for non-invasive analysis of bodily fluids is
performed using the long wavelength part of the near infrared spectrum
(1,b00nm to
2,550nrn - LNIR) [1-6] or middle infrared spectrum (2,550nm to 11,OOOnm- MIR)
[6]. Radiation in these spectral ranges usually can be measured with either
InGaAs or
lead sulfde detectors. However, radiation in the l,&00nm to 2,550nm, or
2,550nm to
11,000nm ranges is known to have shallow tissue penetration. Therefore
potential for
the application of these wavelengths for in-vivo medical analysis is very
limited.
Radiation with shorter wavelengths has deeper tissue penetration. However,
the application of infrared spectroscopy to the analysis of certain analytes
in bodily
fluids, for example electrolytes, is lacking due to a lack suitable features
in the
infrared absorbance spectrum of these electrolytes. Detection of electrolytes
can be
CA 02475622 2004-07-23
_2_
partially resolved using several methods, for example flame spectrophotometry
(for
potassium and sodium) or by chemical binding of electrolytes to some other
species,
whose spectral properties are modified in the process,.and these modified
substances
are used as indicators of the original substance concentration using ordinary
spectrophotometry, [7,8].
All substances are able to leave their imprint on electromagnetic radiation
through specimen specific, spectrally dependent absorbance, and also through
specimen specific, wavelength dependent variations of propagation affected by
refractive index. While the first imprint is widely used for spectaal
identification of
samples, and for analysis of their chemical composition, the second imprint
for
purposes of chemical analysis is practically non-existent mainly because of
lack of
suitable instrumentation and associated methods.
US 5,529,065 provides a method and apparatus for measuring internal
information of a scattering medium using a light source having specific
wavelengths
and two or more detectors. The detectors are positioned on the surface of the
scattering medium and receive light scattered by the medium and emitted from a
light
source of specific wavelength.
WO 00/ 20843 teaches a method and apparatus for determining optical
parameters of turbid media. The apparatus includes the use of a probe
containing both
transmitting and receiving optics for delivering light to the media, and
receiving back-
scattered light from the media. This apparatus and method are directed to
measuring
spatially-resolved reflectance of the turbid media.
WO 02/40971 discloses an apparatus for determining optical parameters of
turbid media. The apparatus comprises a plurality of spatial and angular
detectors that
resolve spatially diffuse Light, and angular resolved diffuse Light,
respectively, from
the sample. These detectors are located at different positions around the
sample.
CA 02475622 2004-07-23
-3-
US 6,075,610 teaches a method and apparatus for determining optical
parameters of a sample. The apparatus comprises a plurality of detectors
positioned
around the surface of the sample used to obtain measured values of the sample.
The
mean of the measured values is used to determine a reference value indicative
of an
internal property of the sample.
It is an object of the invention to overcome disadvantages of the prior art.
The above object is met by the combinations of features of the main claims,
the
sub-claims disclose further advantageous embodiments of the invention.
CA 02475622 2004-07-23
SUMMARY OF TIC INVENTION
T'he present invention relates to measuring chemical compounds using scattered
light spectroscopy. More specifically this invention pertains to measuring
absorbing and
non-absorbing chemical compounds using scattered light spectroscopy.
According to the present invention there is provided a method of determining
the
concentration of a compound of interest in a sample using scattered light
spectroscopy
comprising,
i) providing a scattered light spectrometer comprising an algorithm developed
for
the compound of interest;
ii) introducing radiation of about 585nm to about 1635nm to the sample;
iii) measuring collected radiation after interaction with the sample;
iv) determining the concentration of the compound of interest using the
algorithm.
The present invention pertains to the method described above, wherein the
compound of interest does not exhibit a measurable variability in absorbance
within the
wavelengths of about 585nm to about 1635nm, and where the refractive index of
the
compoand of interest changes in a wavelength specific manner over at least a
portion of
the wavelengths of about 585nrn to about 1635nm.
Furthermore, the invention is directed to the method described above, wherein
the
step of measuring (step (iii)) involves measuring both scattered and absorbed
radiation.
The compound of interest may be selected from the group consisting of protein,
albumin,
bilirubin, creatine, cholesterol, triglycerides, glucose, urea, intralipid,
chloride,
potassium, sodium, phosphorous, calcium, magnesium, manganese, iron, sulphur,
zinc,
aluminium, silicon, copper, nickel, arsenic, nitrogen, fluorine, lithium,
selenium,
bromine, cadmium iodine, mercury, gold, or other ion or compound that exhibits
the
property of a refractive index that changes with wavelength, and the sample is
a body
part, a liquid sample, or a gas sample.
CA 02475622 2004-07-23
- 5
The present invention also provides an apparatus for determining the
concentration of a compound of interest in a sample using scattered light
spectroscopy
comprising,
- a radiation source that emits radiation from about 585nm to about 1635nm,
- a first optical transmission element for receiving, transmitting and
directing
the radiation from the radiation source to a sample holder that comprises a
sample, the
first optical transmission element having a first and second end, the first
end
positioned to receive the radiation produced by the radiation source, the
second end
for scattering the radiation leaving the first optical transmission element to
produce
scattered radiation, and for directing the scattered radiation to the sample
holder;
- the sample holder comprising two or more than two windows;
- a second optical transmission element for receiving the scattered radiation
after interaction with the sample, and for directing the scattered radiation
to one or
more than one scattered radiation processing system;
-the one or more than one scattered radiation processing system comprising a
diffraction grating, and a radiation detection system comprising one or more
than one
algorithms for determining the concentration of the compound of interest.
The present invention further embraces the apparatus described above,
wherein the radiation detection system comprises a first and second set of
lenses, the
first set of lenses focusing the scattered radiation through .a slit, and the
second set of
lenses, positioned to receive the scattered radiation after passing through
the slit, and
comprising the diffraction grating, placed between the lenses in the second
set of
lenses. Furthermore, the second optical transmission element may be bifurcated
and
splits the scattered radiation into a first and a second scatta~ed radiation
beam path.
The first scattered radiation beam path, a$er passing through the second set
of lenses
may be directed onto a photo diode array capable of detecting radiation from
about
585nm to about 1635nm, and wherein the second scattered radiation beam path,
after
passing through the second set of lenses may be directed onto a photo diode
array
capable of detecting radiation from about 900nm to about 1635nm.
CA 02475622 2004-07-23
-6-
The present invention pertains to the apparatus a described above, wherein the
diffraction grating is a volume diffraction grating.
The present invention also provides a method of determining the concentration
of
a compound of interest in a sample comprising,
i) introducing scattered radiation to the sample using an apparatus comprising
a) a radiation source that emits radiation from about 585nm to about 1635nn~
b) a first optical transmission element for receiving, transmitting and
directing
the radiation from the radiation source to a sample holder that comprises a
sample, the
first optical transmission element having a first and second end, the first
end
positioned to receive the radiation produced by the radiation source, the
second end
for scattering the radiation leaving the first optical transmission element to
produce
scattered radiation, and for directing the. scattered radiation to the sample
holder;
c) the sample holder comprising two or more than two windows;
d) a second optical transmission element for receiving the scattered radiation
after interaction with the sample, and for directing the scattered radiation
to one or
more than one scattered radiation processing system;
e) the one or more than one scattered radiation processing system comprising a
diffraction grating, and a radiation detection system comprising one or more
than one
algorithms for determining the concentration of the compound of interest
ii) measuring radiation collected after interaction with the sample;
iii) determining the concentration of the compound of interest.
The present invention provides a spectrophotometer, which is sensitive to
spectral variation of the absorption, and also spectral variation of
refractive index. As
a result, this spectrophotometer can be used for spectrophotometric
measurements of
chemical components that are characterized as not demonstrating a measurable
variability of absorbance, but whose refractive index changes with wavelength
in a
substance specific manner. Applicability of this spectrophotometer was tested
on
liduid samples in the form of different matrices including samples prepared by
mixing
sera extracted from different animals. Good measurability was obtained for a
range of
CA 02475622 2004-07-23
- 7 -
compounds in serum.
This summary of the invention does not necessarily describe all necessary
features
of the invention but that the invention may also reside in a sub-combination
of the
described features.
CA 02475622 2004-07-23
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the invention will become more apparent from the
following description in which reference is made to the appended drawings
wherein:
Figure 1 shows a schematic of operation principles of radiation scattering
system
Figure 2. shows a schematic of a spectrometer.
Figure 3 shows prediction of several compounds in water detected using
radiation
scattered spectroscopy. Figure 3a shows prediction of albumin in water with
intralipid and glucose. Figure 3b shows prediction of intralipid in water with
albumin and glucose. Figure 3c shows prediction of glucose in water with
albumin
and intralipid.
Figure 4 shows prediction of glucose in water using scattered light
spectroscopy.
Figure 4a shows prediction of glucose in water with albumin and intralipid on
the
worst batch of 100 samples. Figure 4b shows prediction of glucose in water
with
albumin and intralipid on the best batch of 100 samples
Figure 5 shows correlations between compounds in water, in the presence or
absence
of intralipid, using scattered light spectroscopy. Figure Sa shows correlation
between
concentrations of chemical components in the water samples not containing
intralipid.
Figure Sb shows correlation between concentrations of chemical components in
the
water samples containing intralipid
Figure 6 shows prediction of several compounds is water, in the absence of
intralipid
using scattered light spectroscopy. Figure 6a shows prediction of glucose in
water
and other analytes without intraiipid. Figure 6b shows rediction of albumin in
water
and other analytes without intralipid. Figure 6c shows prediction of sodium in
water
and other analytes without intralipid. Figure 6d shows prediction of chloride
in water
and other analytes without intralipid. Figure 6e shows prediction of
phosphoraus in
CA 02475622 2004-07-23
-9-
water and other analytes without intralipid. Figure 6f shows prediction of
potassium
in water and other analytes without intralipid.
Figure 7 shows prediction of several compounds is water, in the presence of
intralipid
using scattered light spectroscopy. Figure 7a shows prediction of glucose in
water
and other analytes with intralipid. Figure 7b shows prediction of albumin in
water
and other analytes with intralipid. Figure 7c shows prediction of sodium in
water and
other analytes with intralipid. Figure 7d shows prediction of chloride in
water and
other analytes with intralipid. Figure 7e shows prediction of phosphorous in
water
and other analytes with intralipid. Figure 7f shows prediction of potassium in
water
and other analytes with intralipid. Figure 7g shows prediction of intralipid
in water
with other analytes.
Figure 8 shows prediction of several compounds in animal serum using scattered
light spectroscopy. Figure 8a shows prediction of glucose in animal serum.
Figure
8b shows prediction of albumin in animal serum. Figure 8c shows prediction of
cholesterol. Figure 8d shows prediction of proteins. Figure 8eshows prediction
of
triglycerides. Figure 8f shows prediction of urea. Figure 8g shows prediction
of
sodium. Figure 8h shows prediction of potassium. Figure 8i shows prediction of
chlorides. Figure 8j shows prediction of carbon dioxide. Figure 81~ shows
prediction
of phosphorous. Figure 81 shows prediction of creatinine. Figure 8m shows
prediction of bilirubin.
Figure 9 shows prediction of several other compounds in animal serum using
scattered light spectroscopy. Figure 9a shows prediction of magnesium. Figure
9b
shows prediction of HDL .
CA 02475622 2004-07-23
-10-
DESCRIPTION OF PREFERRED EMBODIMENT
The present invention relates to measuring chemical compounds using scattered
light spectroscopy. More specifically this invention pertains to measuring
absorbirg and
non-absorbing chemical compounds using scattered Light spectroscopy.
The following description is of a preferred embodiment by way of example only
and without limitation to the combination of features necessary for carrying
the invention
into effect.
The present invention provides a spectrophotometer, which is sensitive to the
spectral variation of refractive index of a compound in a sample. This
invention also
provides a method for determining the concentration of compound of interest in
a
sample. The spectrophotometer can be used for spectrophotometric measurements
of
chemical components that are characterized as not demonstrating a measurable
variability of absorbance, but whose refractive index changes with wavelength
in a
substance specific manner. The spectroscopic system, and a method for using
this
spectroscopic system, may be used for spectral characterization of radiation
transported through a sample, either biological or non-biological samples, and
over a
spectral range from about 585nm to about 1635nm.
The spectrophotometer, used for determining the concentration of a compound
of interest in a sample comprises,
- a radiation source that emits radiation from about 585nrn to about 1d35nm,
- a first optical transmission element for receiving, transmitting and
directing
the radiation from the radiation source to a sample holder that comprises a
sample, the
first optical transmission element having a first and second end, the first
end
positioned to receive the radiation produced by the radiation source, the
second end
for scattering the radiation leaving the first optical transmission element to
produce
scattered radiation, and for directing the scattered radiation to the sample
holder;
- the sample holder comprising two or more than twowindows;
CA 02475622 2004-07-23
-11-
- a second optical transmission element for receiving the scattered radiation
after interaction with the sample, and for directing the scattered radiation
to one or
more than one scattered radiation processing system;
the one or more than one scattered radiation processing system comprising a
diffraction grating, a radiation detection system, and comprising one or more
than one
algorithm for determining the concentration of the compound of interest.
Also provided is a method of determining the concentration of a compound of
interest in a sample. The method comprising,
i) introducing scattered radiation to the sample using an apparatus comprising
a) a radiation source that emits radiation from about 585nm to about 1635nm,
b) a first optical transmission element for receiving, transmitting and
directing
the radiation from the radiation source to a sample holder that comprises a
sample, the
first optical transmission element having a first and second end, the first
end
positioned to receive the radiation produced by the radiation source, the
second end
for scattering the radiation leaving the first optical transmission element to
produce
scattered radiation, and for directing the scattered radiation to the sample
holder;
c) the sample holder comprising two or more than two windows;
d) a second optical transmission element for receiving the scattered radiation
after interaction with the sample, and for directing the scattered radiation
to one or
more than one scattered radiation processing system;
e) the one or more than one scattered radiation processing system comprising a
diffraction grating, a radiation detection system, and comprising one or more
than one
algorithm for determining the concentration of the compound of interest;
ii) measuring radiation collected after interaction with the sample;
iii) determining the concentration of the compound of interest using the one
or
more than one algorithm.
The compound of interest may be characterized as not exhibiting a measurable
variability in absorbance within the wavelengths of about 585nm to about
1635nm. In
this case the refractive index of the compound of interest changes in a
wavelength
CA 02475622 2004-07-23
-12-
specific manner over at least a portion of thewavelengths of about 585nm to
about
1635nm. However, spectral variation of the absorption, associated with the
compound of interest may also be used if any spectral variation is available.
By compounds or chemical components that are characterized as not
demonstrating a measurable variability of absorbance, it is meant that the
absorbance
of the compound or component does not exhibit variability over a spectral
range from
about 585nm to about 1635nm. There may be an absorbance associated with the
compound, but the associate absorbance does not change in in~nsity to any .
significant degree, or the absorbance is otherwise not measurable.
By a refractive index that changes with wavelength in a substance specific
manner, it is meant that one or more than one of the wavelengths of the
radiation
comprise a change in refractive index as a result of interacting with a
compound in a
sample.
Physical Background
In the simplest case of a plane electromagnetic wave [9,10] the electrical
component E(Z~t), which is mostly responsible for a signal registered by photo
detectors, measured in direction of wave propagation at the distance ~ from
the origin
at some moment t , can be expressed mathematically in the form:
~ (Z~t) = E~ei(cp + kz -Wit) (1)~
where:
E~, is the amplitude of vibrations of the electrical vector;
~ , is the phase of vibration at the origin of the coordinate system at the
moment;
t=a~
Z, is the distance from the origin in the direction of the wave propagation;
~, is the circular frequency ofvibrations; and
CA 02475622 2004-07-23
-13-
k, is the complex propagation constant, consisting of real, ~ , and imaginary
parts,
a
k~m
k = kRe + ikIm = ~x _ -2~(n + i -~'-Q-a ) (2)
4~
where:
1C' , is the complex index of refraction;
~,~ , the wavelength of the wave in vacuum;
Yt , the index of refraction; and
Gl,' , the absorption coefficient.
The real part of propagation constant, k Re ' thus is related to refractive
index yl
kRe _= (cv )~ - 2~ ~ (3)
a ~o
and its imaginary part, ~ , to the absorption coefficient, GL , of the
radiation in the
m
medium:
~rx
m 2
In the absence of scattering, intensity, I (Z) , of a radiation beam at a
distance, Z,
averaged over the time significantly longer than vibration period of the
electromagnetic field, can be found by calculation of the squared module of
electrical
component in expression (1):) ~(Z) 12 i ~(Z)~* (Z) , which leads to relation:
I (Z) = I (0)e_aZ (5)
with j ( 0 ) being an initial intensity of the beam at origin of the
coordinate system.
The above expression (5) clearly shows that in absence of scattering, the
CA 02475622 2004-07-23
-14-
intensity of the radiation beam, which is directly measurable with radiation
detectors,
depends on the imaginary part of complex refractive index only (the absorbance
coefficient), and does not depend on its real part i.e. on the refractive
index. Hence,
only substances, which absorb radiation can be identified from attenuation
measurement.
In physical media both the refractive index and the absorption coefficient
take
different values for radiation with different frequencies (wavelengths or wave
numbers) [9-10]. This relationship is a result of the atomic and molecular
structure of
matter, due to the dynamic redistribution of electrical charges in atoms or in
molecules enforced by an imposed electromagnetic field. According to nuclear
theory, atoms consist of a positively charged nucleus surrounded by a cloud of
electrons with coincident centers of gravity, resulting in an electrically
neutral atom.
Molecules consist of a set of electrically interacting atoms, which, as a
result of
interaction, produce a certain joint distribution of positive and negative
charges. in
space. According to quantum mechanics theory, there are limits on the variety
of
spatial distributions, which both charges can take in space. This gives rise
to particular
spatial structures (usually called states but for clarity, referenced here as
nodes, by
association with vibration nodes) that can be permanently maintained in a
state of
equilibrium. In an equilibrium state, the centers of gravity of both positive
and
negative charges can be coincident, producing a neutral particle, or be
spatially
separated resulting in a polarized dipole-like structure. Since spatial
distribution of
charges for each node is different, relative potential energy of the charges
in each
node also differs and the transition from one node to another either requires
energy,
which has to be delivered from outside when particle is transferred from a
node with
lower potential energy to anode with higher potential energy, or energy
is~released
When a transition occurs in the opposite direction. One of several possible
mechanisms for such a transition includes an energy exchange with an external
electromagnetic field from which energy either can be taken, in the act of
absorption,
or released, in the form of a radiated photon. Once the particles (atoms or
molecules)
reach a state of equilibrium, the charges may perform certain periodical
vibrations
CA 02475622 2004-07-23
-15-
around the center of equilibrium. This state of equilibrium defines physical
properties
of externally non-disturbed matter.
The situation changes when an external, periodically changing electromagnetic
field is applied. The field distuxbs the fine equilibrium between the charges
and
enforces their periodical spatial redistribution, resulting on average in an
additional
polarization effect, for example the creation of a mean electrical dipole. The
mean
dipole value for each particle (atom or molecule) depends on vibration
frequency of
incident electromagnetic wave and other factors such as mass, charge, spatial
distribution of charges in space (node) and so on. The mean dipole value
therefore
depends on molecular and atomic structure of the particles under consideration
and
their physical state. Hence each kind of particle, each in a different state
(node)
produces a different, sample-spedfic, and node-specific, impact on the
electromagnetic field. The strength of this impact can be additionally
influenced by
the presence and state of other particles in neighborhood. The dipoles
interact with an
incident electromagnetic wave affecting its propagation, and the strength of
this
impact depends on all these parameters, which determine thevalue of the
dipole.
The total effect of the induced dipoles on an electromagnetic wave is
proportional to number of dipoles per unit volume interacting with the wave,
hence is
dependent on the spatial density of each kind of particle in each state
present in the
sample. Their impact on the propagation velocity of the electromagnetic wave
is
expressed by the refractive index of the medium. The refractive index
represents the
collective impact of each type particle in all possible states per unit volume
on the
propagation velocity of the wave. Hence, on a macroscopic scale, the impact is
expressed as a sum normalized to unit volume of all partial contributions of
each type
of particle for all possible states in the medium under consideration. The
contribution
for a single type of particle in a single state can be expressed
mathematically in terms
of a complex function, resembling that used for the damped harmonic oscillator
in the
presence of an external harmonic excitation. The total effect represented
byrefractive
index is obtained by summation of contributions of each type of particle in
all
CA 02475622 2004-07-23
-16-
possible states contained in unit volume.
The final complex refractive index is a surn of contributions made by all
substances and its squared value 1~ 2 can be expressed as:
z
x2 c~ =1+ Nlel far
~ 2 ~~ 2 _i .
l 0 Jr ~r y .1 r Jr (6)
where:
l , is the number of different component present in the sample;
NI , the number of atoms or molecules of the given substance per unit volume;
a , the charge of electron or ion in a molecule;
1
yy~l, the mass of l -th component;
g0, the permittivity of free space; ,
~ , , the j-th resonant frequency for l th particle;
.~l
.~ the sample specific oscillator strength for, ~ the resonant frequency;
~Jr .Ir
the sample specific frictional canstant for the ~ frequency.
~Jr .Ir
The above expression implies that both the absorption coefficient and the
refractive
index are sample specific, and dependent on the frequency of radiation {or
equivalently said, dependent on the waveiength or wave number). Since both the
absorption coefficient and the refractive index demonstrate strong frequency
dependent behavior, they may be used for spectral analysis.
The imaginary part, which expresses absorption of radiation in a medium, can
be measured with relative ease and. is widely used in spectroscopy for
analysis of the
chemical composition of substances. ~iowever, the spectral dependence of the
CA 02475622 2004-07-23
- 17-
refractive index on wavelength of radiation is seldom used for this purpose.
The measurement of the spectral dependence of absorbance, which is directly
related to the extinction coefficient, consists in comparing the intensity of
the
radiation incident on the sample with that affected by the sample. Compound-
specific
spectral variation of absorption is expressed by Beer's law, and dependence of
absorption on.the concentration ofthe compound is commonly used in
spectroscopic
analysis. Conversely, spectral dependence of the refractive index is more
difficult to
measure, since refractive index variations influence only the radiation group
velocity,
and the propagation direction in a medium. Refractive index variations do not
normally affect the intensity of radiation. Furthermore, there is no simple
relation
between changes in one of these two parameters (i.e. radiation group velocity,
and
propagation direction in a medium) and concentration, as is the case with
Beer's law.
However, with an appropriate signal, multivariate regression may be used to
find correlations between refractive index information and a corresponding
concentration of an analyte. With this approach, measurement of the
concentration of
an analyte is determined using information contained in the spectrum of
radiation that
is reflected or back scattered by the sample. This approach is possible when
the
registered signals depend not only on the absorption coefficient but also on
refractive
index of the sample.
Reflected and backscattered signal are very weak, especially if they are taken
from boundary layer between two media of comparable refractive index, such as
the
boundary between a cuvette wall and liquid inside the cuvette. The signal may
be
increased, for example by increasing the multiple total internal reflection
from a
boundary surface, to a degree sufficient to measure the concentration of
components,
that are otherwise not measurable with standard spectroscopic methods, because
of an
insufficient absorption signal.
There are two physical properties, which can be used to transform information
CA 02475622 2004-07-23
-lg-
related to refractive index into measurable intensity variations. One consists
of an
apparent distance change when a remote object is observed through a medium
whose
refractive index varies. The second consists of a dependence of the refraction
angle of
radiation on the refractive index of a prism. Both properties can be used b
translate
variability of the refractive index into intensity variability associated with
scattered
light interacting with a sample.
When non-collimated scattered radiation is transmitted through a scattering or
non-scattering media, the intensity of radiation collected by an optical
system with a
limited field of view and a limited numerical aperture (i.e. a limited angular
collecting
capability) strongly depends on the refractive index of the medium. As a
result, the
intensity of radiation transported through this media is both absorption and
refractive
index dependent, and contains more information about sample than spectral
dependence of absorption alone. This capability can be demonstrated with
reference to
the apparatus shown in Figure 1. In this apparatus, a collimated beam of
radiation
(BR; 20), is produced by an illumination optical system (IOS; 10). The beam
illuminates a strongly light scattering diffuser (D1; 40), which may
simultaneously
acts as a window for cuvette (C; 30), containing a sample, S, with a
refractive index,
y~ : The cuvette is closed with another diffuser (D2; 50). Due to the
refraction of light
in the medium, the refractive index of the sample influences the optical
distance
between the diffusers. The optical distance decreases proportionally to the
refractive
index of the medium between the diffusers.
For an infinitely large incident beam and an infinitely large La~mbertian
diffuser, the radiation intensity on the second diffuser would not depend on
the
refractive index of the medium between diffusers, and the radiation collecting
system
(RCS; 60), which delivers radiation to the detector (PD; 70), would provide
the same
signal regardless of refractive index of the substance between them. If these
conditions are not fulfilled, as is the case in a typical system, the
radiation intensity on
the second diffuser (50) depends on the optical distance from the first
diffuser (40),
and hence on the refractive index of the medium between diffusers, and the
amount of
CA 02475622 2004-07-23
-19-
radiation captured by the radiation collecting system {60) and delivered to
the
photodetector (?0) depends on refractive index of the medium between
diffusers. The
signal is also affected by absorption of the sample medium, therefore the
measured
signal contains information related to both factors: absorption and refractive
index of
the sample medium placed between diffusers.
The situation becomes more complex, when the sample medium between
diffusers {40, 50) contains scattering centers in the form of snail, suspended
particles.
In this case, the scattering efficiency of suspended particles depends on the
ratio of
their size, to the wavelength of the interacting radiation, and on relative
refractive
index of the particles in relation to the surrounding medium. Therefore, these
particles contribute additional information on refractive index of the medium.
As is
readily apparent, the scattering of radiation by the first diffuser (40) and
by particles
suspended in the medium, different photons may travel different distances
before they
reach the second diffuser (50), and eventually the detector (?0). The chance
that a
photon is absorbed varies depending on traveling path, which may also depend
on the
refractive index.
Variation of the refractive index on the intensity of the collected radiation,
can
be further enhanced by using a wedge shaped sample holder (30) and a wedge
shaped
sample. However, rectangular or circular shaped sample holders, and sample,
may
also be used as required. A wedge shaped sample introduces variability due to
optical
distance variation, and also due to variability in the refraction of radiation
{hence the
capability to collect radiation with different wavelengths) that also becomes
dependent on the refractive index, and takes full advantage of changes caused
by
refractive index variability. The variability of the refractive index,
obtained using a
wedge-shaped sample, can be then registered by the detecting system (80). The
registered signal also depends on properties of beam forming system, optical
properties of the diffusers and the collecting capability of the light
collecting system.
Therefore, contrary to ordinary spectrometers, the results obtained with
radiation
scattering samples are typically instrument dependent. As aresult, the simple
relation
CA 02475622 2004-07-23
-20-
between the absorbance of a chemical compound to the concentration of chemical
compound in sample, is not readily obtained.
However, as described in more detail below, a suitable calibration model can
be developed using techniques of multivariate regression, for example but not
limited
to, partial least squares regression (PLSR, PCAR), supervised neural network
or other
techniques that would be known to one of skill in the art, for samples of a
given shape
and volume. Furthermore, substances, which do not posses characteristic
absorption
bands may still be measured, due to compound specific dependence of refractive
index on wavelength.
Therefore, the present invention provides a spectrophotometer, which is
sensitive to spectral variation of the absorption, and also spectral variation
of
refractive index of a sample. The spectrophotometer can be used for
spectrophotometric measurements of chemical components that are characterized
as
not demonstrating a measurable variability of absorbance, but whose refractive
index
changes with wavelength in a substance specific manner. The spectroscopic
system,
and a method for using this spectroscopic system, was developed for spectral
characterization of radiation transported through a sample, for example but
not limited
to biological and non-biological samples including a body part, for example
but not
limited to a human finger, an ear lobe and the like. A spectral range from
about
585nm to about 1635nm may be used for spectral characterization of the sample.
Therefore, the present invention provides a method of determining the
concentration of a compound of interest in a sample using scattered light
spectroscopy
comprising,
i) providing a scattered light spectrometer comprising analgorithm developed
for
the compound of interest;
ii) introducing radiation of about 585nrn to about 1635 nm to the sample;
iii) measuring collected radiation after interaction with the sample;
iv) determining the concentration of the compound of interest using the
algorithm.
CA 02475622 2004-07-23
-21-
Furthermore, the compound of interest may be selected from the group
consisting of
protein, albumin, bilxrubin, creatine, cholesterol, triglycerides, glucose,
urea, intralipid,
chloride, potassium, sodium; phosphorous, calcium, magnesium, manganese, iron,
sulphur, zinc, aluminium, silicon, copper, nickel, arsenic, nitrogen,
fluorine, lithium,
selenium, bromine, cadmium iodine, mercury, gold, or other ion or compound
that
exhibits the property of a refractive index that changes with wavelength. The
sample
may be a gas or liquid sample, or a body part, for example but not limited to
a finger, ear
lobe or other body part where radiation may be passed through.
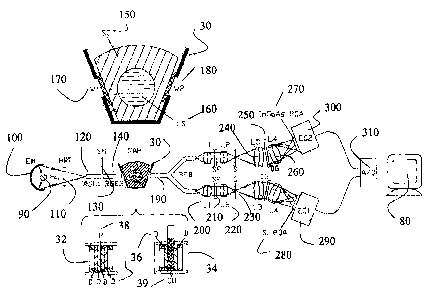
Description of the measurement system.
With reference to Figure 2 there is shown a system for the spectral
characterization of radiation scattering samples. Visible and infrared
radiation
produced by a suitable light source, for example a halogen (HL; 90}, xeon,
metal
halide, fluorescent lamp is collected by means of an elliptical mirror (EM;
100) and
optionally passes through a heat rejection filter (HRF; 110), before it enters
a radiation
delivering optical system, also referred to as a first optical transrriission
element. The
optical transmission element may consist of one or two radiation guiding
elements
(R.GE 1; 120; and RGE2; 140), with a shutter (SH; 130} placed between them.
However, the shutter may be placed elsewhere, as desired. The shutter (130) is
able to
block radiation completely from entering a sample holder (SAH; 30) through the
second radiation-guiding element (RGE2; 140). The radiation guiding elements
(the
first optical transmission element) may be any suitable element for
transmitting
radiation, for example but not limited to one or mare than one fiber optic
bundles, or
radiation guiding rods, for example but not limited to fused silica rods. In
this
arrangement, no additional beam-forming elements such as lenses or mirrors
need to
be used, however, beam- forming elements may be used if desired. To further
increase production of random scattered radiation, the end face of the second
guiding
element (140) acts as an artificial, spatially extended radiation source
producing
within its emission cone randomly scattered radiation, which enters the sample
holder
(30).
CA 02475622 2004-07-23
-22-
The sample holder (30) may be of any shape for holding a liquid or solid
sample, it may be a receptor adapted to receive a body part, as is known in
the art, for
example but not limited to US 5,631,758; which is incorporated herein by
reference.
A non-limiting example of a sample holder, and an enlarged view of the holder
(30),
adapted for receiving a finger and having the cross-sectional shape is shown
in Figure
2. The body of the sample holder (30) may be made of a nontransparent material
with
two holes, each enclosed by windows (Wl; 170, and W2; 180), to deliver
radiation to,
and collect it from, the sample (150,160) within the holder (30). The windows
may
also be modified to act as diffusers, as indicated above. The sample may be a
body
part, for example but not limited to a finger, an. ear lobe, a body part where
radiation
may be passed through, or an artificial member (150, 170). A non-limiting
example of
an artificial member is disclosed in W~ 01/115597 (which is incorporated
herein by
reference). The artificial member has a sample container {SC, 150) with an
axial bore
containing a liquid sample (LS, 160), or other desired medium. The sample
container
(150) is made of highly scattering, for example, but not limited to PTFE.
For reference measurements the sample container is replaced by a thermally
stable radiation attenuator (32), consisting of a set of diffusers (D; 36)
and, placed
between them, a metallic mesh with an array of fine holes (M, 38). The density
and
size of the holes in the mesh {38), as well as the number of diffusers (36) in
the
attenuator are selected to obtain optical characteristics comparable to the
sample and
to achieve the attenuation optimal for the best signal to noise, S/N, ratio of
the
reference measurement. A~ alternative sample holder (34) contains at least one
diffuser (D, 36) on each side of standard spectrometric cuvette or vial (CU,
39).
Furthermore, highly mixed divergent radiation delivered to the sample holder
(30) by
the radiation delivering optical system is additionally scattered either by
the sample
surface, for example skin in the case of a body part, the radiation scattering
container
(150, 34) or diffusers (e.g. 170, 180, or 36), before entering the sample. The
sample
itself may also contain radiation scattering centers.
CA 02475622 2004-07-23
- 23 -
The radiation transported through the sample is further scattered on exit, by
a
similar set of components (e.g. skin, diffusing walls, diffusing windows or
diffuser),
before directing the scattered radiation to a scattered radiation processing
system.
The scattered radiation processing system may comprise a second oprical
transmission
element (190), a set of first lenses (200, 220), optionally a shaping filter
(210), a slit
(230), a second set of lenses (240, 260), a diffiaction grating (250), one or
more than
one detector (e.g. 2?0, 280), optionally one or more than one camera (e.g.
290, 300),
and a computer for processing the received data (310, 80).
After leaving the sample, the scattered radiation enters a second optical
transmission element, for example but not limited to a bifurcated fiber optic
bundle
(BFB, 190) whose radiation collecting end is placed as close to the sample
holder (30)
as possible to secure e~cient collection of light and to reduce or eliminate a
need for
additional radiation collecting elements such as lenses or mirrors. The bundle
(190)
collects radiation from the sample holder (30) and, if desired, divided, for
example
into two or more than two parts. However, the scattered radiation processing
system
may comprise one detection system, and the second optical transmission element
may
be undivided, delivering the scattered radiation to one detection system. The
number
of optical transmission elements the scattered radiation may be divided into
can be
modified as desired depending upon the application and the number of detector
systems used in the scattered radiation processing system. In the present
example,
which is not to be considered limiting in any manner, the radiation is
delivered to two
separate spectrophotometers. Also, in the present example, the
spectrophotometers of
the scattered radiation processing system are array-based. However, other
spectrophotometers, as would be known to one of skill in the art, may be used
in place
of an array-based system.
In the present example, one spectrophotometer {EC1, 290) contains a silicon
photodiode array (Si PDA, 280) and covers spectral range from about 585nm to
about
1180nm with resolution of about l4nm, while the other spectrophotometer (EC2,
300)
contains an InGaAs photodiode array (InGaA PDA, 270) and covers spectral range
CA 02475622 2004-07-23
-24-
from about 900nm to about 1635nm'with resolution of about ll.Snm. Variations
in
the type of arrays used, the spectral range of the array, and the resolution
of the array,
as can be determined by one of skill in the art are, and these variations are
considered
to be included within the scope of the present inventi~n. Both
spectrophotometers
may otherwise be similar.
If a bifurcated bundle (190, the second optical transmission element) is used
in
the scattered radiation processing system, then radiation from the each leg of
the
bifurcated fiber optic bundle (190) is captured by lens (L1, 200) located at a
distance
equal to the focal length of the lens. For example, which is not be considered
limiting, the f# close to 2.2 may be used for L 1 (200). Radiation then
optionally
passes through a closely placed, long wavelength transmission-shaping filter
(SF,
210) of the scattered radiation processing system. Filter (210) eliminates
second order
of di~action of grating (DG, 250) by blocking short wavelength radiation in
the
working range of the spectrophotometer. When the sample is placed in the
sample
holder, filter (210) equalizes the response of the system across the spectral
range of
the spectrometer. The shaping filter (210) for example, may be designed to
equalize
response of the spectrophotometer for samples with optical characteristics of
the
human finger. This secures a uniform dynamic range for all wavelengths of the
spectrophotometer in the presence of the sample. A second lens (L2, 220) is
placed as
close to the filter (210) as possible, and captures the radiation transmitted
through the
filter (210) and creates an image of the fber optic bundle end in a slit plane
(S, 230).
The width of the slit (230) assists in determining resolution of the
spectrophotometer.
The radiation transmitted through the slit (230) is captured by another lens
(L3, 240)
of similar f# but longer focal length as lens L1 (200). Lens L3 (240) is
placed at the
distance equal to the focal length of the lens.
The beam of radiation from lens L3 (240), is directed, and passes through, a
high efficient volume holographic transmission grating (DG, 250). The
diffraction
grating may be any suitable high efficiency grating, for example but not
limited to that
disclosed in WO 01/37014 (which is incorporated herein by reference). However,
an
CA 02475622 2004-07-23
- 25 -
alternate device may be used to decompose the radiation and obtain its
corresponding
spectrum, for example but not limited to a prism. The radiation dispersed by
the
diffraction grating is collected by another lens (L4, 260) that is similar to
L3 (240),
and is focused to create spectrally dispersed images on the slit on a
photosensitive
surface of a suitable detector, fox example a linear photodetector array,
either Si PDA
(280) for the first spectrophotometer, or InGaAs PDA (270) for the second
spectrophotometer. In both cases the resolution of spectrophotometers is far
from the
diffracted limited for the applied optics and is determined by the slit width
used in
spectrometers as can readily be determined by one of skill in the art. Non-
limiting
examples of slit widths to be used in the system presently described are about
0.3mm
and about 0.2mm for the spectrometers with silicon (280) and InGaAs arrays
(270),
respectively.
The arrays may be cooled, for example a thermoelectrically cooled, 256
element arrays, with O.OSmm wide pixels, produced by Hlamamatsu. A silicon
detector array may be used in the spectrophotometer predestined to work in the
about
585nm to about 1180nm range, while a InGaAs array may be applied in the
spectrophotometer for about 900nm to about 1635nm spectral range. The signals
captured by the arrays are extracted using standard electronic cameras (ECI,
290; and
EC2, 300), for example but not limited to an electronic camera obtained from
Hamamatsu, and digitized using an AID converter, for example but not limited
to a
National Instrument I6bits AID converter. Data is then stored in a computer
memory
for further processing. Further processing may include development of a
calibration
algorithm as is known in the art, for example using MATLAB.
Software tools used for developing calibration algorithms comprises of the
following: MathlabTM used to create programs for smoothing absorbances and
derivative of absorbances; StafViewTM used to create algorithms by "step-wise
multiple linear regression." PirouetteTM may be used to create calibration
algorithms
by Partial Least Squares (PLS) or Principal Component Analysis (PCA). It will
be
appreciated however that other software tools may also be used. It will also
be
CA 02475622 2004-07-23
-26-
appreciated that any statistical technique may be used, for example, which
should not
be considered limiting in any way, simple linear regression, multiple linear
regression,
and multivariate data analysis. Examples of multivariate data analysis, which
should
not be considered limiting in any way, are Principal Component Analysis (PCA),
Principal Component Regression (PCR), Partial Least Squares regression (PLS),
and
Neural networks.
The above-described spectrophotornetric system, was used for the analysis of
solutions comprising various analytes in water and animal serum. A first
example
included the analysis of concentration of glucose, albumin and intralipid in
water, to
verify the measurability of non-correlated analytes in the presence of a
radiation
scattering media. Other determinations were performed to demonstrate the
usability
of the instrument for the measurement of various analytes, including these
without
clear absorbance bands within applied spectral range.
As described in more detail in the examples below, scattered light
transmission spectrometry in long wavelength visible and short wavelength NIR
parts
of the spectrum (far example but not limited to 580nm to 1380nm) may be used
to
measure physiologically important chemical components, including those
normally
considered immeasurable with direct spectroscopic methods. lVieasurement of
these
compounds may be carried out in various matrices, for example but not limited
to
animal serum, biological fluids or a non-biological sample, and without any
chemical
treatment of the sample. Other chemical components, not disclosed in the
examples,
can also be measured with the apparatus described herein. Non-limiting
examples of
such chemical components include, but are not limited to protein, albumin,
bilirubin,
creative, cholesterol, triglycerides, glucose, urea, intralipid, chloride,
potassium,
sodium, phosphorous; calcium, magnesium, inangane~, iron, sulphur, zinc,
aluminium, silicon, copper, nickel, arsenic, nitrogen, fluorine, lithium,
selenium,
bromine, cadmium iodine, mercury, gold, or other ion that exhibits the
property of a
refractive index that changes with wavelength, or other compound that exhibits
the
property of a refractive index that changes with wavelength
CA 02475622 2004-07-23
-27-
Furthermore, measurement precision can be further improved, as required, by
optimizing the apparatus described herein so that a large number of serum
analytes
may be analyzed. Since the present method does not require the use of
additional,
consumable reagents, expense associated with each analysis is less than that
of present
multifunctional analyzers used in medical laboratories.
Therefore, the present invention provides a spectrophotometer, which can be
used
for spectrophotometric measurements of chemical components that are
characterized as
not demonstrating a measurable variability of absorbance, but whose refractive
index
changes with wavelength in a substance specific manner. The apparatus may be
used for
determining the concentration of a compound of interest in a sample using
scattered light
spectroscopy. The apparatus comprising,
- a radiation source that emits radiation from about 538nm to about 1635nm,
- a first optical transmission element for receiving, transmitting and
directing
the radiation from the radiation source to a sample holder that comprises a
sample, the
first optical transmission element having a first and second end, the first
end
positioned to receive the radiation produced by the radiation source, the
second end
for scattering the radiation lea~ring the f rst optical transmission element
to produce
scattered radiation, and for directing the scattered radiation to the sample
holder;
- the sample holder comprising two or more than two windows;
- a second optical transmission element for receiving the scattered radiation
after interaction with the sample, and for directing the scattered radiation
to one or
more than one scattered radiation processing system;
-the one or more than one scattered radiation processing system comprising a
diffraction grating, a radiation detection system, and comprising one or more
than one
algorithm for determining the concentration of the compound of interest.
The above description is not intended to limit the claimed invention in any
manner, furthermore, the discussed combination of features might not be
absolutely
necessary for the inventive solution.
CA 02475622 2004-07-23
-28-
The present invention will be further illustrated in the following examples.
However it is to be understood that these examples are for illustrative
purposes only, and
should not be used to limit the scope of the present invention in any manner.
Examples
Description of the measurement system.
The measurement system is as described above, using custom built
spectrophotometers. The first spectrophotometer contains a silicon photodiode
array
and covers spectral range from 585nm to 1180nm (280) with resolution about
l4nm,
while the second one contains an InGaAs photodiode array (270) and covers
spectral
range from 900nm to 1635nm with resolution about 11.Snm. Slit width used in
spectrometers is 0.3mm and 0.2mm, for the spectrometers with silicon and
InGaAs
arrays, respectively. Both arrays are thermoelectrically cooled, 256 elements
arrays
(Hamamatsu), with 0.05mm wide pixels. The signals captured by the arrays are
extracted using standard electronic cameras (Hamamatsu), and digitized with a
standard National Instrument l6bits A/,D converter, and stored in a computer
memory
for further processing.
Example 1: Measurement technique
Two kinds of data were collected during each measurement process: a
reference and sample measurement. 1~ or reference measurements, a stable
diffusing
reference member (32), consisting of volume diffusers (36, Figure 2) and mesh
radiation attenuator (38), whose radiation transporting capability is as close
as
possible to that of the sample, is placed in the sample holder (30) for
reference
measurement. The shutter (140) is then opened, and the system takes
measurements of
the signal at all pixels, identifies the pixel with the largest signal and
sets integration
CA 02475622 2004-07-23
-29-
time in such a way that reading at this pixel reaches approximately 85% of the
possible maximum. Having the integration time defined, the system collects a
number
of spectra, which can be averaged to improve, signal to noise ratio (SNR).
After the
collection of the reference signal is completed, the shutter blocks the
optical path of
the radiation and a set of reference dark measurements with the same
integration time
as reference signal is collected. For sample measurements, the diffuse
reference
attenuator (32) is then replaced with a sample (150), and the process of
signal reading,
determining integratian time, light and dark signals collecting is repeated
for the
signal affected by the sample.
The mean dark signals are subtracted from corresponding mean light signals
and, similar to an absorbance calculation, a negative logarithm of ratio of
radiation
transported through the sample to that transported through the reference
member is
calculated at each wavelength. The value calculated in such a way is further
referenced as a relative diffuse absorbance. The relative diffuse absorbance,
tagether
with the data on the concentration of all chemical compounds, gives the
starting point
for evaluation of the instrument applicability for measurements of non-
absorbing
samples.
Since the relative diffuse absorbance depends not only on optical properties
of
the sample and reference member, but also on collecting capabilities of the
optical
system, a simple relation between the relative diffuse absorbance and
concentration of
the compound in the sample cannot be established. Rather, a relationship
between the
relative diffuse absorbance and concentration of the compound in the sample
can be
modeled for each instrument applying techniques of multivariate regression [11-
13],
as described below.
A large number of samples (several hundred in some cases) with statistically
non-correlated concentrations distribution of all analytes were prepared and
measured.
This number depends on complexity of the samples, variability range of all non-
correlated components in matrix, system noise and required precision, so that
a
CA 02475622 2004-07-23
-30-
distribution of concentration across the variability range is obtained.
Naturally
available samples were normally distributed in terms of their analyte
concentration. A
calibration sample set was created by spiking some samples with selected
analytes,
and mixing some samples. Care was taken to destroy any possible correlation
between concentrations of different analytes in the samples used for model
development. As well, care was made to insure that there were no correlations
in
sample order.
The concentration of the compounds of interest in the samples was determined
(calculated from mixing proportions of samples with known concentrations of
the
compounds in the base samples, and, by standard analytical measurements of the
concentration in the final sample). In the case of discrepancy between values
obtained
with these methods, a spectrum of the sample was collected and used to
identify
sources of discrepancy between measurements, and not for model development and
verification.
After sufficient number of samples with known concentration of analytes of
interest had been produced and spectra had been collected, the obtained data
set was
analyzed to identify evident outliers. Remaining samples were divided into two
subsets containing comparable numbers of samples, using an interleaved
division. In
this method, the spectrum of every second sample was assigned to a separate
subset,
producing two statistically independent subsets, so that the two interleaved
set did not
have sample repeats in common. ~ne interleaved set is used for model
development
using PLSR method, while the second interleaved set is used for model
verification.
The performance difference between both sample sets was statistically
negligible.
Example 2: Measurement of glucose, albumin and intralipid in water
The first test was performed using a mixture of three analytes: glucose,
intralipid; and albumin, in non-correlated quantities, in water. The glucose
variability
range extended from Ommol/L to 5~mmol/L, the albumin range extended from
4g/dl.
CA 02475622 2004-07-23
-31-
to 8gIdL (with a few points in 3g/dL to 4g/dL range) and the intralipid range
extended
from 0.4g/dL to 0.8g/dL. A small amount of preservative was added to prevent
sample deterioration in time between its production and characterization was
carried
out with traditional analytical method. The concentrations of each analyte in
each
sample within their respective variability ranges were selected accordingly to
the
numbers produced by a random number generator, being a part of MATLAB software
environment, and samples were produced by weighing and mixing of particular
analytes accordingly to generated numbers in quantities proportional to the
intended
volume of the final solution. Initially these quantities were dissolved in
limited
volume of steam distilled water and after dissolving, steam distilled water
was
replenished to produce predetermined volume of the solution.
After samples were produced, concentration of each analyte was
independently verified using standard analytical methods and correlation
between
concentrations of all three analyzes were recalculated. In the produced set of
samples
the correlation coefficient between glucose and albumin concentrations was
0.03 ~,
between glucose and intralipid 0.053 and between albumin and intralipid 0.035.
The
coefficients are close to values observed for two sets of random numbers
containing
similar number of elements varying in similar ranges.
Samples were kept in a refrigerator for cooling and storage. The first
spectrum
was collected shortly after the sample was taken from the refrigerator, and
four more
spectra immediately ore after another (each spectrum consisting of a number of
averaged raw measurements at a fixed integration time). One measurement (light
and
dark readings for reference and sample) lasted about two minutes, with a total
measurement.time for a given set of 5 measurements lasting about ten minutes.
During this time the temperature of samples increased from that of
refrigerator (about
4°C) to room temperature (about 20-24°C), or higher, due to
sample heating by both
environment and the radiation used for testing. All spectra are to be
considered as
taken at random temperatures. The spectra of the same sample were treated as a
single block, and to avoid prediction on itself, the block was assigned to the
same
CA 02475622 2004-07-23
-32-
selection for model development and prediction. Errors in predictions, for
spectra
taken at different temperatures, were not correlated to the measurement order.
Therefore, the models developed do not make use of temperature dependent
features
to a signif cant degree. The presented results are mean predicted values of
all five
measurements taken during the rise the sample temperature.
Altogether over 500 samples were prepared. Five spectral measurements were
taken for each sample at variable temperatures, resulting in a total of 2500
measurements. Spectra obtained were visually tested for evident outliers and
after
elimination of bad measurements, 499 samples (2495 measurements) were selected
far analysis. The samples and associated measurements were divided into five
consecutive batches (four containing 100 and one containing 99 samples}. To
avoid
prediction on itself all spectra of the same sample were treated as a single
block and
always assigned to the same batch. The blocks of spectra of a given selection
(batch or
all samples together) were divided into two independent subsets of comparable
size
applying either random pickup or interleaved selection, as described before.
Standard PLSR procedures were applied to one of these subsets to build three
sLparate sets of prediction models (one set for each compound}. These models
were
then used to predict the concentrations in the second subset of respective
spectral
measurement and the standard error of prediction (SEP) was calculated for each
subset. The models providing the smallest SEP were selected for performance
comparison of models build on sets with different numbers of elements. From
this
comparison it was concluded that setseontaining 100 samples divided into two
equal
subsets for model development and prediction are sufficient for preliminary
evaluation of measurability of particular compounds with instrumentation
described
above.
The above described process was repeated on the same subsets in the reversed
order (second subset used for model development and first for model
selection).
CA 02475622 2004-07-23
- 33 -
The results from this analysis are shown in Figures 3a to 3c (Figure 3a for
albumin, Figure 3b for intralipid and Figure 3c for glucose), in a form of
scatter plots.
Each scatter plot shows the relation between reference value and value
predicted on
independent samples, together with basic statistical information, which
includes the
correlation coefficient, r, between predicted and reference values, the slope
of the
linear regression line for these values, the intercept of the linear
regression line with
the ordinate and mean percentage error of prediction. These figures also show
the
identity line (line with slope equal to 1), the linear regression line for
predicted versus
reference values (ideally it should coincidence with the identity Line) and
20% relative
error limit lines. Error was found to be very small in comparison to the mean
measured value, the intercept was found to be close to 0 and the slope was
found to be
close to 1, demonstrating that all three analytes were measured using the
apparatus
described herein.
Taking into account that these analyt~s are randomly mixed it is unlikely that
any, of them, especially glucose, whose volume concentration is much smaller
than
other components, is measured through a water replacement signal, which would
be of
limited value owing to its lack of specificity. It is clear that the
components can~be
measured in selected variability ranges with precision sufficient for majority
applications.
These results were obtained using relatively large set of data (about 250
samples far model development and comparable amount for model selection),
resulting in long evaluation period. Therefore a test was performed to reduce
the data
set of I00 samples. Sample size reduction had the largest effect on glucose
measurability, and two extreme cases, the worst and the best, are shown in
Figures 4a
and 4b, respectively. Comparison of results presented in Figures 4a and 4b,
with
Figure 3c indicates that the difference in performance of models built on I00
or 500
samples is within the. statistical error.
Ezample 3: Measurement of glucose, albumin, sodium, potassium,
CA 02475622 2004-07-23
r
-34-
chloride and phosphorous without and with intralipid, in water
The procedures developed in Example 2 were used for the measurability test
of six different analytes: glucose, albumin, sodium, potassium, chloride, and
phosphorous, all present in solution of distilled water in the absence and
presence of
intralipid as a radiation scattering factor. Glucose and albumin were u~d to
test
system performance, while the other components (excluding intralipid), which
do not
possess clear absorbance spectra in applied spectral range, were used for
testing
applicability of the method and instrumentation for concentration measurements
of
non absorbing substances.
Each of these substances had different molar concentration ranges, and their
concentrations were intentionally combined in a way reducing possible
correlations
between them. The role of intralipid was to introduce scattering into the
samples.
Correlation coefficients for water solutions of the selected six components
without intralipid, and with intraiipid, as well as correlation of their
concentrations to
sample number, are shown in Figures 5a and Sb, respectively. The results
presented
in Figures Sa and Sb demonstrate that the correlation between sample number
and
concentrations of ail analyzes is comparable to that expected for correlations
of similar
size of data sets containing the series of ordered natural numbers and equal
size set of
random numbers, and that the predictability for measurable analyzes is not a
result of
spurious correlation.
Prediction results from the measurement of the compounds, for samples
prepared in the absence of intraiipid are shown in Figures 6a to 6f, and
Figures 7a to
7g, for samples prepared in intralipid. Figure 7g shows predictability of
intralipid.
'These results presented in Figures 6a to 6d, and 7a to 7d, demonstrate that
scattered light spectroscopy may be used to measure physiologically important
analytes that do not possess specific absorbance bands in working range of the
.". ,_ . ... _,... ~,~,w. ,..~ _~.,. ~~~"-x~~z.~w~.~:,,,~<~~..,.~.,~,~., w.
.~..... .. ..._. .___. _..
CA 02475622 2004-07-23
0
y ,
- 35 -
spectrometric system. Furthermore, scattered light spectroscopy may be used to
measure analytes over physiologically important ranges of their concentration.
The analytes potassium and phosphate, which do not exhibit predictability in
water (Figures 6e and 6fj, show some predictability in a solution with
intralipid
(Figures 7e and 7t]. Without wishing to be bound by theory, this result may be
explained as a result of their low molar concentrations used, and that for
given optical
path the concentration of each of these analytes was not sufficient to produce
a
recognizable signal. In the presence of intralipid, the effective optical path
is
increased, and these compounds exhibit a refractive index dependent signature.
Adding intralipid increases the mean relative prediction error of analytes
which possess absorption bands in applied spectral range, glucose and albumin.
For
components that exhibit their presence by light scattering, the error
increase.is not as
evident.
Example 4: Measurement of a range of compounds in animal serum
Nine samples of serum, from horse, goat, sheep, chicken, pig, and bovine, two
adult cows and two newly barn calves, were obtained from Sigma-Aldrich. These
samples were characterized using a J&J Vitros 250 instrument, and the
concentration
of the the following analytes: albumin, chloride, cholesterol, carbon dioxide,
creatinine, glucose, potassium, sodium, total bilirubin, total protein
(including
albumin), triglyceride, and urea, determined. Small quantities of each sample
were
also extracted for spectral characterization, and the remaining sera used for
the
production of one hundred samples.
The sample set was by mixing basic sera in different proportions and spiking
with selected analytes to expand their variability range to fill the extremes
expected in
human physiology. Five analyzes were used for spiking: albumin, creatinine,
glucose,
intralipid and urea. The sera used for spiking had the following
concentrations of the
CA 02475622 2004-07-23
-36-
selected compounds: albumin 20g/dL; creatinine 100mmo1/L; glucose 300mmoI/L;
urea 2,Smo1/L and a 20% standard intralipid solution. Intralipid was used to
modify
concentration of triglycerides and to modify scattering properties of the
samples. High
concentration of additives was used so that only a small amount of spiking
solution
was required to adjust the final concentration of the selected component in
the sample,
thereby having a minimal impact on concentrations of other components,
existing in
the spiked serum. Final concentration of a particular analyte in a sample was
determined by calculation, and verified using a YSI Model 2300 Scat Plus, for
glucose
measurements, and a Vitros DT60II with DTE II module for glucose and other
analytes.
Two batches of serum were prepared for a first measurement. The first batch
contained one hundred samples prepared by mixing and spiking as described
above.
After collecting and analyzing the spectra from the fast hundred of the
samples and
analyzed, a second batch of samples was prepared. The original, non-modified
samples of nine animal sera described above, were used as samples of the
second
batch for model prediction. However, these samples were not used for productDn
of
calibration model. The remaining 294 samples (403 in total) were produced
using six
different sera (horse, sheep and four bovine: three cows and one newly born
calf] and
spiked with albumin, creatinine, glucose, ir~tralipid, urea, sodium (in form
of NaO~
and potassium (in form of KH2P04), as described above.
The prepared samples were stored in a refrigerator, from which they were
removed just before measurement. The measurement protocol was as described in
Example 2. As required, the data were either treated as one large set or
divided in
different subsets, each subset containing all measurements of the same sample
and
alternatively used either to build models or to select the model providing the
best
performance using PLSR procedures. The comparable size of these subsets
allowed
for comparison of the models build on each of them.
CA 02475622 2004-07-23
-37-
The first analysis was performed using one hundred samples of the first run
(each sample consisting of five replicate measurements), and divided into two
interleaved subsets for model development and model selection. For all
selected
analytes up to fifty models were generated each model corresponding to the
number
of latent variables used. Performance of each model was evaluated, giving rise
to a
range of models that demonstrated the ability to predict with mean relative
errors well
below 20% and with a slope of the best fitting regression line within 15% from
the
slope of the identity line.
The scatter plots of prediction using models built on a complete set of 403
samples (divided into two of comparable sized subsets for model development
and
model evaluation for ail thirteen components, for which calibration data were
available, are presented in Figures 8a to 8m.
Caood predictability was observed with potassium (Figure 8h) over a
concentration variability range that was close to that used in experiment with
water
solution (Example 3). Similarly to that observed with potassium, models were
also
developed that predicted concentrations of phosphorous (Figure 8k).
The results were confirmed in separate tests. In addition to the majority of
analytes analyzed during first test the measurability of two new components,
magnesium and I-il)L was successfully verified, and corresponding scatter
plots are
produced (Figures 9a and 9b).
The results shown in Figures 8h and 8k demonstrate that potassium and
phosphate, in serum, are measurable using scatter light spectroscopy.
Existence of many components in the serum gives rise to a refractive index,
which
is significantly higher than that of water, resulting in higher sensitivity of
the system to
refractive index associated with analyzes of interest.
CA 02475622 2004-07-23
J
v 1
-38-
All citations are herein incorporated by reference.
The present invention has been described with regard to preferred embodiments.
However, it will be obvious to persons skilled in the art that a number of
variations and
modifications can be made without departing from the scope of the invention as
described herein.
Z. References
[1] R. Jensen, I. Lugan, E. Peuchant, "Biological analysis without reagent.
Myth or reality? Application to determination of serum total lipids". Bull Soc
Pharm.
Bordeaux 1986, 125, 43-52
[2] J. W. Hall and A. Pollard; 'mtear-Infrared Spectrophotometry: A New
Dimension in Clinical Chemistry", Clin. Chem., 38, (1992), 1623-1631
[3] J. W. Hall and A. Pollard, "Near-Infrared Spectroscopic Determination of
Serum Total Proteins, Albumin, Globulins, and Urea", Clin. Biochem., 26,
(1993),
483-490
[4] K. H. Hazen, M. A. Arnold and G. W. Small, "Measurement of glucose
and other analytes in undiluted human serum with near-infrared transmission
spectroscopy", Anal. Chim. Acta, 371, (1998), 255-267
[5] P. A, da Costa Filho and R. J. Poppi, "Determination of triglycides in
human plasma using near-infrared spectroscopy and multivariate calibration
methods", Anal. Chim. Acta, 446, (2001), 39-47
[6] R. A. Shaw and H. H. Mantsch, "Infrared Spectroscopy in Clinical and
Diagnostic Analysis", Encyclopedia of Analytical Chemistry, Edited by R. A.
Meyers,
John Wiley & Sons Ltd, Chichester,.2000, 1-19
[7] M. G. Scott, J. W. Heusel, V. A. LeGrys and O. Siggaard-Andersen,
Chapter 31 "Electorlytes and Blood Gases" in Tietz Textbook of Clinical
Chemistry,
3~ edition, W.B. Saunders Company, Philadelphia, (1999)
[8] L. A. Kaplan, A. J. Pesce and S. C. Kazmierczak, "Clinical Chemistry:
Theory, analysis and correlation" 3~° edition, Mosbay, St Louis,
(1996)
[9] M, Born and E. Wolf, "Principles of Optics", 6~' edition, Pergamon Press,
CA 02475622 2004-07-23
-39-
Oxford, (1980).
[10] M. V. Klein, "Optics", John Willey and Sons, new York, (1970)
[1 l] "Chemometrics: Mathematics and Statistics in Chemistry", B. R.
Kowalski, Ed., NATO ASI Series C: Mathematical and Physical Sciences Vol. 138,
D. Reidel Publishing Company, Dordrecht, Boston, Lancaster, 1983
[12] H. Martens and T. Naes; "Multivariate Calibration", John Wiley & Sons
Ltd, Chichester, 1989
[I3] D. A. Burns and E. W. Ciurczak, "Handbook of Near-Infrared Analysis",
Marcel Dekker Inc., New York, Basel, Hong Kong, 1992