Note: Descriptions are shown in the official language in which they were submitted.
Boehringer Ingelheim Pharma KG 1 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
80371 fftlatest
Bicyclic heterocyclic compounds, pharmaceutical compositions
containing these compounds, their use and process for preparing them
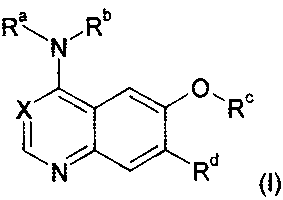
The present invention relates to bicyclic heterocyclic groups of general
formula
R-, N,Rb
0,R
X
N Rd
(I),
the tautomers, the stereoisomers, the mixtures and the salts thereof,
particularly the physiologically acceptable salts thereof with inorganic or
organic acids or bases which have valuable pharmacological properties,
particularly an inhibitory effect on signal transduction mediated by tyrosine
kinases, the use thereof for treating diseases, particularly tumoral diseases,
as well as benign prostate hyperplasia (BPH), diseases of the lungs and
respiratory tract, and the preparation thereof.
In the above general formula I
Ra denotes a hydrogen atom or a C1-4-alkyl group,
Rb denotes a phenyl or 1-phenylethyl group, wherein the phenyl nucleus is
substituted in each case by the groups R1 to R3, while
R1 and R2, which may be identical or different, in each case denote a
hydrogen, fluorine, chlorine, bromine or iodine atom,
a C1 -alkyl, hydroxy, Ci_4-alkoxy, C2_3-alkenyl or C2_3-alkynyl group,
an aryl, aryloxy, arylmethyl or arylmethoxy group,
Boehringer Ingelheim Pharma KG 2 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a heteroaryl, heteroaryloxy, heteroarylmethyl or heteroarylmethoxy
group,
a methyl or methoxy group substituted by 1 to 3 fluorine atoms or
a cyano, nitro or amino group, and
R3 denotes a hydrogen, fluorine, chlorine or bromine atom or
a methyl or trifluoromethyl group,
Rc denotes a cyclobutyl, cyclopentyl or cyclohexyl group which is substituted
in each case by a group R4-N-R5, while
R4 denotes a hydrogen atom or a C1.3-alkyl group and
R5 denotes a hydrogen atom or a C1-3-alkyl group,
an aminocarbonyl-Cl-3-alkyl, C1.3-alkylaminocarbonyl-C1-3-alkyl, di-
(C1_3-alkyl)aminocarbonyl-C1_3-alkyl, pyrrolidin-1-ylcarbonyl-C1_3-alkyl,
piperidin-1-ylcarbonyl-C1.3-alkyl, homopiperidin-1-ylcarbonyl-C1-3-alkyl,
morpholin-4-ylcarbonyl-C1-3-alkyl, homomorpholin-4-ylcarbonyl-
C1_3-alkyl, piperazin-1-ylcarbonyl-C1-3-alkyl, 4-C1.3-alkyl-piperazin-1-
ylcarbonyl-C1_3-alkyl, homopiperazin-l-ylcarbonyl-C1.3-alkyl or a 4-
C1_3-alkyl-homopiperazin-1-ylcarbonyl-C1_3-alkyl group,
a hydroxy-C2_4-alkyl, C1_3-alkyloxy-C2_4-alkyl, C1.4-alkyloxy-
carbonylamino-C2_4-alkyl, amino-C2.4-alkyl, C1.3-alkylamino-C2_4-alkyl,
di-(C1-3-alkyl)amino-C2_4-alkyl, C1.3-alkylcarbonylamino-C2_4-alkyl,
aminocarbonylamino-C2.4-alkyl, C1_3-alkylaminocarbonylamino-
C2_4-alkyl, di-(C1-3-alkyl)amino-carbonylamino-C2-4-alkyl, pyrrolidin-1-
ylcarbonylamino-C2.4-alkyl, piperidin-1-ylcarbonylamino-C2.4-alkyl,
morpholin-4-ylcarbonylamino-C2.4-alkyl, C1.3-alkylsulphonyl-C2-4-alkyl or
Boehringer Ingelheim Pharma KG 3 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a C1_3-alkylsulphonylamino-C2-4-alkyl group,
a (2-oxo-pyrrolidin-1-yl)-C2_4-alkyl, (2-oxopiperidin-1-yl)-C2-4-alkyl, (3-
oxo-morpholin-4-yl)-C2.4-alkyl, (2-oxo-imidazolidin-1-yl)-C2-a-alkyl, (2-
oxo-3-C1_3-alkyl-imidazolidin-1-yl)-C2-4-alkyl, (2-oxo-
hexahydropyrimidin-1-yl)-C2-4-alkyl or a (2-oxo-3-C1.3-alkyl-
hexahydropyrimidin-1-yl)-C2-4-alkyl group,
a C1.4-alkylsulphonyl, chloro-C1_4-alkylsulphonyl, bromo-
C1_4-alkylsulphonyl, amino-C1 -alkylsulphonyl, C1.3-alkylamino-
C1.4-alkylsulphonyl, di-(C1.3-alkyl)amino-C1-4-alkylsulphonyl, (pyrrolidin-
1-yl)-C1.4-alkylsulphonyl, (piperidin-1-yl)-C1-4-alkylsulphonyl,
(homopiperidin-1-yl)-C1.4-alkylsulphonyl, (morpholin-4-yi)-C1_4-alkyl-
sulphonyl, (homomorpholin-4-yi)-C1-4-alkylsulphonyl, (piperazin-1-yl)-
C1-4-alkylsulphonyl, (4-C1_3-alkyl-piperazin-1-yl)-C1-4-alkylsulphonyl,
(homopiperazin-1-yl)-C1-4-alkylsulphonyl or a (4-C1-3-alkyl-
homopiperazin-1-yl)-C1-4-alkylsulphonyl group,
a C1_4-alkyloxycarbonyl group,
a formyl, C1-4-alkyl-carbonyl, C1.3-alkyloxy-Cl.4-alkyl-carbonyl,
tetra hydrofuranylcarbonyl, tetrahydropyranylcarbonyl, amino-C1_4-alkyl-
carbonyl, C1.3-alkylamino-C1_4-alkyl-carbonyl, di-(C1-3-alkyl)amino-
C1_4-alkyl-carbonyl, pyrrolidin-1-yl-C1 -alkyl-carbonyl, piperidin-1-yl-
C1-4-alkyl-carbonyl, (homopiperidin-1-yl)-C1-4-alkyl-carbonyl, morpholin-
4-yI-C1_4-alkyl-carbonyl, (homomorpholin-4-yl)-C1.4-alkyl-carbonyl,
(piperazin-1-yl)-C1-4-alkyl-carbonyl, (4-C1.3-alkyl-piperazin-1-yl)-
C1_4-alkyl-carbonyl, (homopiperazin-1-yl)-C1-4-alkyl-carbonyl, (4-
C1_3-alkyl-homopiperazin-1-yl)-C1_4-alkyl-carbonyl or a
C1_3-alkylsulphonyl-C1_4-alkyl-carbonyl group,
a cyano, aminocarbonyl, C1-3-alkyl-aminocarbonyl, di-(C1.3-alkyl)amino-
carbonyl, (C1.3-alkyloxy-C2-a-alkyl)aminocarbonyl, N-(C1_3-alkyl)-N-(C1_3-
alkyloxy-C2_4-alkyl)aminocarbonyl, arylaminocarbonyl, pyrrolidin-1-
Boehringer Ingelheim Pharma KG 4 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
ylcarbonyl, piperidin-1-ylcarbonyl, homopiperidin-1-ylcarbonyl,
morpholin-4-ylcarbonyl, homomorpholin-4-ylcarbonyl, 2-oxa-5-aza-
bicyclo[2.2.1 ]hept-5-ylcarbonyl, 3-oxa-8-aza-bicyclo[3.2.1 ]oct-8-
ylcarbonyl, 8-oxa-3-aza-bicyclo[3.2.1 ]oct-3-ylcarbonyl, piperazin-1-
ylcarbonyl, 4-C1_3-alkyl-piperazin-1-ylcarbonyl, homopiperazin-1-
ylcarbonyl, 4-C1_3-alkyl-homopiperazin-1-ylcarbonyl, aminosulphonyl,
C1_3-alkyl-aminosulphonyl, di-(C1.3-alkyl)amino-sulphonyl, pyrrolidin-1-
yl-sulphonyl, piperidin-l-ylsulphonyl, homopiperidin-1-ylsulphonyl,
morpholin-4-ylsulphonyl, homomorpholin-4-ylsulphonyl, piperazin-1-
ylsuiphonyl, 4-C1_3-alkyl-piperazin-1-ylsulphonyl, homopiperazin-1-
ylsulphonyl or a 4-C1_3-alkyl-homopiperazin-1-ylsulphonyl group,
a cyclobutyl, cyclopentyl or cyclohexyl group which is substituted in each
case
by a group R6, where
R6 denotes a 2-oxo-pyrrolidin-1-yi, 2-oxopiperidin-1-yl, 3-oxo-
morpholin-4-yl, 2-oxo-imidazolidin-1-yl, 2-oxo-3-C1-3-alkyl-imidazolidin-
1-yl, 2-oxo-hexahydropyrimidin-1 -yl or a 2-oxo-3-C1_3-alkyl-
hexahydropyrimidin-1-yl group,
an azetidin-3-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
a pyrrolidin-3-yl group which is substituted in the 1 position by the group
R5,
while R5 is as hereinbefore defined,
a piperidin-3-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
a piperidin-4-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
or a tetra hyd rofuran-3-yl, tetra hyd ro pyra n-3-yl or tetrahydropyran-4-yl
group,
Boehringer Ingelheim Pharma KG 5 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rd denotes a hydrogen atom or a fluorine, chlorine or bromine atom,
a hydroxy group,
a C1-4-alkyloxy group,
a methoxy group substituted by 1 to 3 fluorine atoms,
an ethyloxy group substituted by 1 to 5 fluorine atoms,
a C2-4-alkyloxy group which is substituted by a group R6 or R7, while
R6 is as hereinbefore defined and
R7 denotes a hydroxy, C1-3-alkyloxy, C3-6-cycloalkyloxy, amino,
C1-3-alkylamino, di-(C1-3-alkyl)amino, bis-(2-methoxyethyl)-amino,
pyrrolidin-1-yl, piperidin-1-yl, homopiperidin-1-yl, morpholin-4-yl,
homomorpholin-4-yl, 2-oxa-5-aza-bicyclo[2.2.1 ]hept-5-yl, 3-oxa-8-aza-
bicyclo[3.2.1 ]oct-8-yl, 8-oxa-3-aza-bicyclo[3.2.1 ]oct-3-yl, piperazin-1-yl,
4-C1.3-alkyl-piperazin-1-yl, homopiperazin-1-yl or C1-3-alkyl-
homopiperazin-1-yl group, or
a formylamino, C1-4-alkylcarbonylamino, C1-3-alkyloxy-C1-3-alkyl-
carbonylamino, C1-4-alkyloxycarbonylamino, aminocarbonylamino, C1-3-
alkylaminocarbonylamino, di-(C1-3-alkyl)aminocarbonylamino,
pyrrolidin-1-ylcarbonylamino, piperidin-l-ylcarbonylamino, piperazin-1-
ylcarbonylamino, 4-C1-3-alkyl-piperazin-1-ylcarbonylamino, morpholin-
4-ylcarbonylamino or a C1.4-alkylsulphonylamino group,
a C3-7-cycloalkyloxy or C3-7-cycloalkyl-Cl-4-alkyloxy group,
a tetra hyd rofu ra n-3-yl oxy, tetra hyd ropyra n -3-yloxy or tetra hyd
ropyran-4-yloxy
group,
Boehringer Ingelheim Pharma KG 6 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a tetra hyd rofu ra nyl-C 1 -4-a lkyloxy or tetra hydropyranyl-C1 -alkyloxy
group,
a C1-4-alkoxy group which is substituted by a pyrrolidinyl, piperidinyl or
homopiperidinyl group substituted in the 1 position by the group R8, while
R8 denotes a hydrogen atom or a C1_3-alkyl group,
or a C1.4-alkoxy groin, ~ which is substituted by a morpholinyl group
substituted
in the 4 position by the group R8, while R8 is as hereinbefore defined, and
X denotes a methyne group substituted by a cyano group or a nitrogen atom,
and
by the aryl groups mentioned in the definition of the above groups is meant in
each case a phenyl group which is mono- or disubstituted by R9, while the
substituents may be identical or different and
R9 denotes a hydrogen atom, a fluorine, chlorine, bromine or iodine
atom or a C1_3-alkyl, hydroxy, C1.3-alkyloxy, difluoromethyl,
trifluoromethyl, difluoromethoxy, trifluoromethoxy or cyano group,
by the heteroaryl groups mentioned in the definition of the above groups is
meant a pyridyl, pyridazinyl, pyrimidinyl or pyrazinyl group, while the
abovementioned heteroaryl groups are each mono- or disubstituted by the
group R9, while the substituents may be identical or different and R9 is as
hereinbefore defined, and
the abovementioned pyrrolidinyl, piperidinyl, piperazinyl and morpholinyl
groups may be substituted in each case by one or two C1.3-alkyl groups, and
unless otherwise stated, the abovementioned alkyl groups may be straight-
chained or branched,
with the proviso that the compound 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-
Boehringer Ingelheim Pharma KG 7 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
tetra hyd rofu ra n-3-yloxy)-7-hyd roxy-q u in azo I i ne is excluded.
Preferred compounds of the above general formula I are those wherein
Ra denotes a hydrogen atom,
Rb denotes a phenyl group substituted by the groups R' to R3, while
R1 denotes a hydrogen, fluorine, chlorine or bromine atom,
a methyl, trifluoromethyl or ethynyl group,
a phenyloxy or phenylmethoxy group, while the phenyl moiety of the
abovementioned groups is optionally substituted by a fluorine or
chlorine atom, or
a pyridyloxy or pyridinylmethoxy group, while the pyridinyl moiety of the
abovementioned groups is optionally substituted by a methyl or
trifluoromethyl group,
R2 denotes a hydrogen, fluorine or chlorine atom or a methyl group and
R3 denotes a hydrogen atom,
R` denotes a cyclopentyl group which is substituted in the 3 position by a
group R4-N-R5, while
R4 denotes a hydrogen atom or a C1-3-alkyl group and
R5 denotes a hydrogen atom or a C1_3-alkyl group,
an aminocarbonyl-C1-3-alkyl, C1_3-alkylaminocarbonyl-C1_3-alkyl, di-
(C1-3-alkyl)aminocarbonyl-C1.3-alkyl, pyrrolidin-1-ylcarbonyl-C1-3-alkyl,
piperidin-1-ylcarbonyl-C1_3-alkyl, piperazin-1-ylcarbonyl-C1_3-alkyl,
Boehringer Ingelheim Pharma KG 8 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
4-C1_3-alkyl-piperazin-1-yl-carbonyl-C1_3-alkyl or morpholin-4-ylcarbonyl-
C1_3-alkyl group,
a hydroxy-C2-alkyl, C1_3-alkyloxy-C2.4-alkyl, C1_4-alkyloxy-
carbonylamino-C24-alkyl, amino-C2-4-alkyl, C1_3-alkylamino-C2.4-alkyl,
di-(C1.3-alkyl)amino-C2.4-alkyl, C1_3-alkylcarbonylamino-C2-4-alkyl,
aminocarbonylamino-C2-4-alkyl, C1_3-alkylaminocarbonylamino-
C2_4-alkyl, di-(C1.3-alkyl)amino-carbonylamino-C2-4-alkyl, morpholin-4-
ylcarbonylamino-C2_4-alkyl, C1-3-alkylsulphonyI-C2_4-alkyl or
C1_3-alkylsulphonylamino-C2_4-alkyl group,
a (2-oxo-pyrrolidin-1-yl)-C24-alkyl, (2-oxopiperidin-1-yl)-C2-4-alkyl, (3-
oxo-morpholin-4-yl)-C24-alkyl, (2-oxo-imidazolidin-1-yl)-C2_4-alkyl, (2-
oxo-3-methyl-imidazolidin-1-yl)-C2.4-alkyl, (2-oxo-hexahydropyrimidin-1-
yI)-C2-4-alkyl or (2-oxo-3-methyl-hexahydropyrimidin-1-yl)-C2-4-alkyl
group,
a C1-3-alkylsulphonyI, chloro-C2.4-alkylsulphonyI, bromo-
C2.4-alkylsulphonyI, amino-C2-4-alkylsulphonyI, C1.3-alkylamino-
C2.-alkylsulphonyI, di-(C1_3-alkyl)amino-C2_4-alkylsulphonyI, (pyrrolidin-
1-yI)-C2-a-alkylsulphonyI, (piperidin-1-yl)-C2_4-alkylsulphonyI or
(morpholin-4-yl)-C2-4-alkylsulphonyI group,
a C1_4-alkyloxy-carbonyl group,
a formyl, C1_3-alkyl-carbonyl, C1_3-alkyloxy-C1_3-alkyl-carbonyl,
tetrahydrofuranylcarbonyl, tetrahydropyranylcarbonyl, amino-C1.3-alkyl-
carbonyl, C1_3-alkylamino-C1_3-alkyl-carbonyl, di-(C1_3-alkyl)amino-
C1_3-alkyl-carbonyl, pyrrolidin-1-yI-C1_3-alkyl-carbonyl, piperidin-1-yi-
C1.3-alkyl-carbonyl, piperazin-1-yl-C1_3-alkyl-carbonyl, 4-C1_3-alkyl-
piperazin-1-yl-C1.3-alkyl-carbonyl, morpholin-4-yl-C1.3-alkyl-carbonyl or
a C1_3-alkylsulphonyI-C1.3-alkyl-carbonyl group,
a cyano, aminocarbonyl, C1_3-alkyl-aminocarbonyl, di-(C1_3-alkyl)amino-
Boehringer Ingelheim Pharma KG 9 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
carbonyl, (C1.3-alkyloxy-C2-4-alkyl)aminocarbonyl, N-(C1.3-alkyl)-N-
(C1-3-alkyloxy-C2-4-alkyl)aminocarbonyl, phenylaminocarbonyl,
pyrrolidin-l-ylcarbonyl, piperidin-l-ylcarbonyl, morpholin-4-ylcarbonyl,
C1-3-alkyl-morpholin-4-ylcarbonyl, di-(C1_3-alkyl)morpholin-4-ylcarbonyl,
homomorpholin-4-ylcarbonyl, 2-oxa-5-aza-bicyclo[2.2.1 ]hept-5-
ylcarbonyl, 3-oxa-8-aza-bicyclo[3.2.1]oct-8-ylcarbonyl, 8-oxa-3-aza-
bicyclo[3.2.1 ]oct-3-ylcarbonyl, piperazin-1-ylcarbonyl, 4-(C1_3-alkyl)-
piperazin-1-ylcarbonyl, aminosulphonyl, C1.3-alkyl-aminosulphonyl, di-
(C1-3-alkyl)amino-sulphonyl, pyrrolidin-l-yl-sulphonyl, piperidin-l-
ylsulphonyl or a morpholin-4-ylsulphonyl group, or
a cyclopentyl group which is substituted in the 3 position by a group R6,
while
R6 denotes a 2-oxo-pyrrolidin-1-yl, 2-oxopiperidin-1-yl, 3-oxo-
morpholin-4-yl, 2-oxo-imidazolidin-1-yl, 2-oxo-3-methyl-imidazolidin-1-
yl, 2-oxo-hexahydropyrimidin-1-yl or a 2-oxo-3-methyl-
hexahydropyrimidin-1-yl group,
a cyclohexyl group which is substituted in the 3 position or in the 4 position
by
a group R4-N-R5, while R4 and R5 are as hereinbefore defined,
a cyclohexyl group which is substituted in the 3 position or in the 4 position
by
a group R6, while R6 is as hereinbefore defined,
a pyrrolidin-3-yl group which is substituted in the 1 position by the group
R5,
while R5 is as hereinbefore defined,
a piperidin-3-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
a piperidin-4-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined, or
a tetrahydrofuran-3-yl, tetra hydropyran-3-yi or tetra hyd ropyra n -4-yl
group,
Boehringer Ingelheim Pharma KG 10 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rd denotes a hydrogen atom,
a C1_3-alkyloxy group,
a methoxy group which is substituted by one to three fluorine atoms,
an ethyloxy group which is substituted in the 2 position by a group R6 or R7,
while R6 is as hereinbefore defined and
R7 denotes a hydroxy, C1_3-alkyloxy, amino, C1_3-alkylamino, di-
(C1.3-alkyl)amino, bis-(2-methoxyethyl)-amino, pyrrolidin-1-yl, piperidin-
1-yl, morpholin-4-yl, homomorpholin-4-yl, 2-oxa-5-aza-
bicyclo[2.2.1 ]hept-5-yl, 3-oxa-8-aza-bicyclo[3.2.1 ]oct-8-yl, 8-oxa-3-aza-
bicyclo[3.2. 1 ]oct-3-yl, piperazin- 1 -yl or a 4-C1_3-alkyl-piperazin-1-yl
group, or
a formylamino, C1-4-alkylcarbonylamino, C1_3-alkyloxy-C1_3-alkyl-
carbonylamino, C1_4-alkyloxycarbonylamino, aminocarbonylamino, C1_3-
alkylaminocarbonylamino, di-(C1.3-alkyl)aminocarbonylamino,
pyrrolidin-1-ylcarbonylamino, piperidin-1-ylcarbonylamino, piperazin-1-
ylcarbonylamino, 4-C1-3-alkyl-piperazin-1-ylcarbonylamino- morpholin-
4-ylcarbonylamino or a C1-4-alkylsulphonylamino group,
a propyloxy group which is substituted in the 3 position by a group R6 or R7,
while R6 and R7 are as hereinbefore defined, or
a butyloxy group which is substituted in the 4 position by a group R6 or R7,
while R6 and R7 are as hereinbefore defined, and
X denotes a nitrogen atom,
while, unless stated otherwise, the abovementioned alkyl groups may be
straight-chained or branched,
Boehringer Ingelheim Pharma KG 11 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
their tautomers, their stereoisomers, their mixtures and their salts.
Particularly preferred compounds of the above general formula I are those
wherein
Ra denotes a hydrogen atom,
Rb denotes a 3-ethynylphenyl, 3-bromophenyl, 3,4-difluorophenyl or 3-chioro-
4-fluoro-phenyl group,
a 3-chloro-4-benzyloxy-phenyl, 3-chloro-4-[(3-fluoro-benzyl)oxy]-phenyl, 4-
(pyridin-3-yloxy)-phenyl, 4-[(6-methyl-pyridin-3-yl)oxy]-phenyl, 3-methyl-4-
(pyridin-3-yloxy)-phenyl, 3-methyl-4-[(6-methyl-pyridin-3-yl)oxy]-phenyl, 3-
chloro-4-(pyridin-3-yloxy)-phenyl or 3-chloro-4-[(6-methyl-pyridin-3-yl)oxy]-
phenyl group,
Rc denotes a cyclohexyl group which is substituted in the 3 position or in the
4
position by a group R4-N-R5, while
R4 denotes a hydrogen atom, a methyl or ethyl group and
R5 denotes a hydrogen atom, a methyl, aminocarbonylmethyl,
methylaminocarbonylmethyl, dimethylaminocarbonylmethyl, pyrrolidin-
1-ylcarbonylmethyl, piperidin-1-ylcarbonylmethyl, piperazin-l -
ylcarbonylmethyl, 4-methylpiperazin-1-ylcarbonylmethyl, morpholin-4-
ylcarbonylmethyl, 2-(morpholin-4-yl-carbonyl)ethyl or 3-(morpholin-4-yl-
carbonyl)propyl group,
an ethyl, propyl, 2-hydroxyethyl, 3-hydroxypropyl, 2-methoxyethyl, 3-
methoxypropyl, 2-(butyloxycarbonylamino)-ethyl, 2-aminoethyl, 3-
aminopropyl, 2-(acetylamino)ethyl, 3-(acetylamino)propyl, 2-
(ethylcarbonylamino)ethyl, 3-(ethylcarbonylamino)propyl, 2-
(propylcarbonylamino)ethyl, 3-(propylcarbonylamino)propyl, 2-
Boehringer Ingelheim Pharma KG 12 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(ethylaminocarbonylamino)ethyl, 3-(ethylaminocarbonylamino)propyl,
2-(dimethylaminocarbonylamino)ethyl, 3-
(dimethylaminocarbonylamino)propyl, 2-(morpholin-4-
ylcarbonylamino)ethyl, 3-(morpholin-4-ylcarbonylamino)propyl, 2-
(methylsulphonyl)ethyl, 3-(methylsulphonyl)propyl, 2-(methylsulphonyl-
amino)ethyl or a 3-(methylsulphonylamino)propyl group,
a 2-(2-oxo-pyrrolidin-1-yI)ethyl, 2-(2-oxopiperidin-1-yi)ethyl, 2-(3-oxo-
morpholin-4-yl)ethyl, 2-(2-oxo-imidazolidin-1-yl)ethyl, 2-(2-oxo-3-
methyl-imidazolidin-1-yl)ethyl, 2-(2-oxo-hexahydropyrimidin-1-yl)ethyl
or a 2-(2-oxo-3-methyl-hexahydropyrimidin-1-yl)ethyl group,
a 3-(2-oxo-pyrrolidin-1-yl)propyl, 3-(2-oxopiperidin-1-yI)propyl, 3-(3-oxo-
morpholin-4-yl)propyl, 3-(2-oxo-imidazolidin-1-yI)propyl, 3-(2-oxo-3-
methyl-imidazolidin-1-yI)propyl, 3-(2-oxo-hexahydropyrimidin-1-
yl)propyl or a 3-(2-oxo-3-methyl-hexahydropyrimidin-1-yI)propyl group,
a methylsulphonyl, ethylsulphonyl, 3-chloropropylsulphonyl, 2-
(morpholin-4-yl)-ethylsulphonyl or a 3-(morpholin-4-yl)-propylsulphonyl
group,
a propyloxycarbonyl or butyloxycarbonyl group,
a formyl, acetyl, ethylcarbonyl, propylcarbonyl, methoxyacetyl, (2-
methoxyethyl)carbonyl, (3-methoxypropyl)carbonyl, tetrahydrofuran-2-
ylcarbonyl, tetrahydropyran-4-ylcarbonyl, aminoacetyl,
methylaminoacetyl, dimethylaminoacetyl, morpholin-4-ylacetyl, [2-
(morpholin-4-yl)ethyl]carbonyl, [3-(morpholin-4-yl)propyl]carbonyl or a
methylsulphonylacetyl group,
a cyano, aminocarbonyl, methylaminocarbonyl, dimethylaminocarbonyl,
ethylaminocarbonyl, diethylaminocarbonyl, propylaminocarbonyl, (2-
methoxyethyl)aminocarbonyl, N-methyl-N-(2-methoxyethyl)-
aminocarbonyl, (3-methoxypropyl)aminocarbonyl, N-methyl-N-(3-
Boehringer Ingelheim Pharma KG 13 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
methoxypropyl)-aminocarbonyl, phenylaminocarbonyl, pyrrolidin-1-
ylcarbonyl, piperidin-l-ylcarbonyl, morpholin-4-ylcarbonyl, 2-
methylmorpholin-4-ylcarbonyl, 2,6-dimethylmorpholin-4-ylcarbonyl,
homomorpholin-4-ylcarbonyl, 2-oxa-5-aza-bicyclo[2.2.1 ]hept-5-
ylcarbonyl, 3-oxa-8-aza-bicyclo[3.2.1 ]oct-8-ylcarbonyl, 8-oxa-3-aza-
bicyclo[3.2.1 ]oct-3-ylcarbonyl, 4-methylpiperazin-1-ylcarbonyl,
aminosulphonyl, methylaminosulphonyl, dimethylaminosulphonyl or a
morpholin-4-ylsulphonyl group,
a cyclohexyl group which is substituted in the 3 position or in the 4 position
by
a group R6, while
R6 denotes a 2-oxo-pyrrolidin-1-yl, 2-oxopiperidin-1-yl, 3-oxo-
morpholin-4-yl, 2-oxo-imidazolidin-1-yl, 2-oxo-3-methyl-imidazolidin-1-
yl, 2-oxo-hexahydropyrimidin-1-yl or a 2-oxo-3-methyl-
hexahydropyrimidin-1-yl group,
a pyrrolidin-3-yl group which is substituted in the 1 position by the group
R5,
while R5 is as hereinbefore defined,
a piperidin-3-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
a piperidin-4-yl group which is substituted in the 1 position by the group R5,
while R5 is as hereinbefore defined,
a tetrahydrofuran-3-yl, tetrahydropyran-3-yl or tetrahydropyran-4-yl group,
Rd denotes a hydrogen atom,
a methoxy, difluoromethoxy or ethyloxy group,
an ethyloxy group which is substituted in the 2 position by a group R6 or R7,
while R6 is as hereinbefore defined and
Boehringer Ingelheim Pharma KG 14 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
R7 denotes a hydroxy, methoxy, ethoxy, amino, dimethylamino,
diethylamino, bis-(2-methoxyethyl)-amino, pyrrolidin-l-yl, piperidin-1-yl,
morpholin-4-yl, homomorpholin-4-yl, 2-oxa-5-aza-bicyclo[2.2.1]hept-5-
yl, 3-oxa-8-aza-bicyclo[3.2.1 ]oct-8-yl, 8-oxa-3-aza-bicyclo[3.2.1 ]oct-3-
yl, piperazin-l-yl, 4-methylpiperazin-1 -yl or 4-ethylpiperazin-1 -yl group,
or
an acetylamino, ethylcarbonylamino, propylcarbonylamino,
butylcarbonylamino, methoxyacetylamino, butyloxycarbonylamino,
ethylaminocarbonylamino, dimethylaminocarbonylamino, pyrrolidin-1-
ylcarbonylamino, piperidin-l-ylcarbonylamino, morpholin-4-
ylcarbonylamino, methylsulphonylamino, ethylsuiphonylamino or
butylsulphonylamino group,
a propyloxy group which is substituted in the 3 position by a group R6 or R7,
while R6 and R7 are as hereinbefore defined, or
a butyloxy group which is substituted in the 4 position by a group R6 or R7,
while R6 and R7 are as hereinbefore defined, and
X denotes a nitrogen atom,
while, unless stated otherwise, the abovementioned alkyl groups may be
straight-chained or branched,
their tautomers, their stereoisomers, their mixtures and their salts.
Most particularly preferred compounds of general formula I are those wherein
R8 denotes a hydrogen atom,
Rb denotes a 3-bromophenyl, 3,4-difluorophenyl, 3-chloro-4-fluoro-phenyl or a
3-ethynylphenyl group, or
Boehringer Ingelheim Pharma KG 15 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a 3-chloro-4-benzyloxy-phenyl, 3-chloro-4-[(3-fluorbenzyl)oxy]-phenyl, 4-
(pyridin-3-yloxy)-phenyl, 4-[(6-methyl-pyridin-3-yl)oxy]-phenyl, 3-methyl-4-
(pyridin-3-yloxy)-phenyl, 3-methyl-4-[(6-methyl-pyridin-3-yl)oxy]-phenyl, 3-
chloro-4-(pyridin-3-yloxy)-phenyl or 3-chloro-4-[(6-methyl-pyridin-3-yl)oxy]-
phenyl group,
R denotes a cyclohexyl group which is substituted in the 3 position by an
amino, acetylamino, tert.-butyloxycarbonylamino or methylsuiphonylamino
group,
a cyclohexyl group which is substituted in the 4 position by an amino,
methylamino, ethylamino, dimethylamino, aminocarbonylmethylamino,
methylaminocarbonylmethylamino, dimethylaminocarbonylmethylamino,
morpholin-4-ylcarbonylmethylamino, [3-(morpholin-4-ylcarbonyl)propyl]amino,
[2-(methylsulphonyl)ethyl]amino, [3-(methylsulphonyl)propyl]amino or [2-
(methylsulphonylamino)ethyl]amino group,
a cyclohexyl group which is substituted in the 4 position by a [2-(2-oxo-
pyrrolidin-1-yl)ethyl]amino, [2-(2-oxopiperidin-1-yl)ethyl]amino, [2-(2-oxo-
imidazolidin-1-yl)ethyl]amino, [2-(2-oxo-3-methyl-imidazolidin-1-
yl)ethyl]amino,
[2-(2-oxo-hexahydropyrimidin-1-yl)ethyl]amino or [2-(2-oxo-3-methyl-
hexahydropyrimidin-1-yi)ethyl]amino group,
a cyclohexyl group which is substituted in the 4 position by a [3-(2-oxo-
pyrrolidin-1-yl)propyl]amino, [3-(2-oxopiperidin-1-yl)propyl]amino, [3-(2-oxo-
imidazolidin-1-yl)propyl]amino, [3-(2-oxo-3-methyl-imidazolidin-1-
yl)propyl]amino, [3-(2-oxo-hexahydropyrimidin-1 -yl)propyl]amino or [3-(2-oxo-
3-methyl-hexahydropyrimidin-1-yl)propyl]amino group,
a cyclohexyl group which is substituted in the 4 position by an acetylamino, N-
(acetyl)-methylamino, aminomethylcarbonylamino,
methylaminomethylcarbonylamino, dimethylaminomethylcarbonylamino,
morpholin-4-ylmethylcarbonylamino, methoxyacetylamino, N-(methoxyacetyl)-
Boehringer Ingelheim Pharma KG 16 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
methylamino, tetrahydropyran-4-ylcarbonylamino, N-(tetrahydropyran-4-
ylcarbonyl)-methylamino, tert.-butyloxycarbonylamino, N-(tert.-
butyloxycarbonyl)-methylamino, aminocarbonylamino,
methylaminocarbonylamino, N-(ethylaminocarbonyl)-methylamino,
dimethylaminocarbonylamino, N-(dimethylaminocarbonyl)-methylamino, N-
(piperidin-1-ylcarbonyl)-methylamino, morpholin-4-ylcarbonylamino, N-
(morpholin-4-ylcarbonyl)-methylamino or N-(4-methylpiperazin-1-ylcarbonyl)-
methylamino group,
a cyclohexyl group which is substituted in the 4 position by a 2-oxo-
pyrrolidin-
1-yl, 2-oxopiperidin-1-yl, 3-oxo-morpholin-4-yl, 2-oxo-imidazolidin-1-yl, 2-
oxo-
3-methyl-imidazolidin-1-yl, 2-oxo-hexahydropyrimidin-1-yl or a 2-oxo-3-
methyl-hexahydropyrimidin-1-yl group,
a cyclohexyl group which is substituted in the 4 position by a
methylsulphonylamino, N-(methylsulphonyl)-methylamino,
ethylsulphonylamino, N-(ethylsulphonyl)-methylamino,
dimethylaminosulphonylamino, N-(dimethylaminosulphonyl)-methylamino,
morpholin-4-ylsulphonylamino, N-(morpholin-4-ylsulphonyl)-methylamino- 3-
chloropropylsulphonylamino, [2-(morpholin-4-yl)-ethyl]sulphonylamino or [3-
(morpholin-4-yl)-propyl]sulphonylamino- group,
a pyrrolidin-3-yl group,
a pyrrolidin-3-yl group which is substituted in the 1 position by a methyl,
acetyl, methoxyacetyl, tert.-butyloxycarbonyl, morpholin-4-ylcarbonyl or
methylsulphonyl group,
a piperidin-3-yl group,
a piperidin-3-yl group which is substituted in the 1 position by a methyl,
acetyl,
methoxyacetyl, tert.-butyloxycarbonyl, morpholin-4-ylcarbonyl or
methylsulphonyl group,
Boehringer Ingelheim Pharma KG 17 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a piperidin-4-yl group which is substituted in the 1 position by a methyl,
ethyl,
propyl, isopropyl, 2-hydroxyethyl, 2-methoxyethyl, 3-methoxypropyl, 2-
(methylsulphonyl)-ethyl, 3-(methylsulphonyl)-propyl, 2-(tert.-
butyloxycarbonylamino)-ethyl, 2-aminoethyl, 2-(acetylamino)-ethyl, 2-
(ethylcarbonylamino)-ethyl, 2-(propylcarbonylamino)-ethyl, 2-
(ethylaminocarbonylamino)-ethyl, 2-(dimethylaminocarbonylamino)-ethyl, 2-
(morpholin-4-ylcarbonylamino)-ethyl, 3-(acetylamino)-propyl, 3-
(ethylcarbonylamino)-propyl, 3-(propylcarbonylamino)-propyl, 3-
(ethylaminocarbonylamino)-propyl, 3-(dimethylaminocarbonylamino)-propyl,
3-(morpholin-4-ylcarbonylamino)-propyl, 2-(methylsulphonylamino)-ethyl, 3-
(methylsulphonylamino)-propyl, (aminocarbonyl)methyl,
(methylaminocarbonyl)methyl, (dimethylaminocarbonyl)methyl, (pyrrolidin-1-
ylcarbonyl)methyl, (morpholin-4-ylcarbonyl)methyl, 2-(morpholin-4-
ylcarbonyl)-ethyl or 3-(morpholin-4-ylcarbonyl)-propyl group,
a piperidin-4-yl group which is substituted in the 1 position by a 2-(2-oxo-
pyrrolidin-1-yl)-ethyl, 2-(2-oxopiperidin-1-yl)-ethyl, 2-(3-oxomorpholin-4-yl)-
ethyl, 2-(2-oxo-imidazolidin-1-yl)-ethyl, 2-(2-oxo-3-methyl-imidazolidin-1-yl)-
ethyl, 2-(2-oxo-hexahydropyrimidin-1-yl)-ethyl or 2-(2-oxo-3-methyl-
hexahydropyrimidin-1-yl)-ethyl group,
a piperidin-4-yl group which is substituted in the 1 position by a 3-(2-oxo-
pyrrolidin-1-yl)-propyl, 3-(2-oxopiperidin-1-yl)-propyl, 3-(3-oxomorpholin-4-
yl)-
propyl, 3-(2-oxo-imidazolidin-1-yl)-propyl, 3-(2-oxo-3-methyl-imidazolidin-1-
yl)-
propyl, 3-(2-oxo-hexahydropyrimidin-1-yl)-propyl or 3-(2-oxo-3-methyl-
hexahydropyrimidin-1-yl)-propyl group,
a piperidin-4-yl group which is substituted in the 1 position by a formyl,
acetyl,
methoxyacetyl, (2-methoxyethyl)carbonyl, (3-methoxypropyl)carbonyl,
methylsulphonylacetyl, aminoacetyl, methylaminoacetyl,
(dimethylamino)acetyl, (morpholin-4-yl)acetyl, [2-(morpholin-4-yl)-
ethyl]carbonyl, [3-(morpholin-4-yl)-propyl]carbonyl, tetrahydrofuran-2-
ylcarbonyl or tetrahydropyran-4-ylcarbonyl group,
Boehringer Ingelheim Pharma KG 18 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a piperidin-4-yl group which is substituted in the 1 position by a cyano,
aminocarbonyl, methylaminocarbonyl, ethylaminocarbonyl, (2-
methoxyethyl)aminocarbonyl, N-methyl-N-(2-methoxyethyl)-aminocarbonyl,
(3-methoxypropyl)aminocarbonyl, N-methyl-N-(3-methoxypropyl)-
aminocarbonyl, isopropylaminocarbonyl, phenylaminocarbonyl,
dimethylaminocarbonyl, diethylaminocarbonyl, pyrrolidin-1-ylcarbonyl,
piperidin-1-ylcarbonyl, morpholin-4-ylcarbonyl, 2-methylmorpholin-4-
ylcarbonyl, 2,6-dimethylmorpholin-4-ylcarbonyl, homomorpholin-4-ylcarbonyl,
2-oxa-5-aza-bicyclo[2.2.1 ]kept-5-ylcarbonyl, 3-oxa-8-aza-bicyclo[3.2.1 ]oct-8-
ylcarbonyl, 8-oxa-3-aza-bicyclo[3.2.1 ]oct-3-ylcarbonyl, 4-methylpiperazin-1-
ylcarbonyl, isopropyloxycarbonyl or tert.-butyloxycarbonyl group,
a piperidin-4-yl group which is substituted in the 1 position by a
methylsuiphonyl, ethylsulphonyl, [2-(morpholin-4-yl)-ethyl]sulphonyl, [3-
(morpholin-4-yl)-propyl]sulphonyl, aminosuiphonyl, methylaminosulphonyl,
dimethylaminosuiphonyl or morpholin-4-ylsulphonyl group, or
a tetrahydrofuran-3-yl, tetra hyd ropyra n-3-yl or tetra hyd ro pyra n-4-yl
group,
Rd denotes a hydrogen atom,
a methoxy, difluoromethoxy or ethyloxy group,
a 2-(morpholin-4-yl)ethyloxy, 3-(morpholin-4-yi)propyloxy or 4-(morpholin-4-
yl)butyloxy group,
a 3-(dimethylamino)propyloxy, 3-(d iethylamino)propyloxy, 3-[bis-(2-
methoxyethyl)-amino]propyloxy, 3-(piperazin-1-yl)propyloxy, 3-(4-
methylpiperazin-1-yl)propyloxy or 3-(4-ethylpiperazin-1-yl)propyloxy group,
a 3-(homomorpholin-4-yl)-propyloxy, 3-(2-oxa-5-aza-bicyclo[2.2.1 ]hept-5-yl)-
propyloxy, 3-(3-oxa-8-aza-bicyclo[3.2.1]oct-8-yl)-propyloxy or 3-(8-oxa-3-aza-
bicyclo[3.2.1 ]oct-3-yl)-propyloxy group,
Boehringer Ingelheim Pharma KG 19 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
a 2-(2-oxo-pyrrolidin-1-yl)-ethyloxy, 2-(2-oxopiperidin-1-yl)-ethyloxy, 2-(3-
oxomorpholin-4-yi)-ethyloxy, 2-(2-oxo-imidazolidin-1-yl)-ethyloxy, 2-(2-oxo-3-
methyl-imidazolidin-l -yl)-ethyloxy, 2-(2-oxo-hexahydropyrimidin-1-yl)-
ethyloxy
or 2-(2-oxo-3-methyl-hexahydropyrimidin-1-yl)-ethyloxy group,
a 3-(2-oxo-pyrrolidin-1-yl)-propyloxy, 3-(2-oxopiperidin-1-yl)-propyloxy, 3-(3-
oxomorpholin-4-yl)-propyloxy, 3-(2-oxo-imidazolidin-1-yl)-propyloxy, 3-(2-oxo-
3-methyl-imidazolidin-1-yl)-propyloxy, 3-(2-oxo-hexahydropyrimidin-1-yl)-
propyloxy or 3-(2-oxo-3-methyl-hexahydropyrimidin-1-yl)-propyloxy group,
a 2-(methoxy)-ethyloxy, 2-(tert.-butyloxycarbonylamino)-ethyloxy, 2-(amino)-
ethyloxy, 2-(acetylamino)-ethyloxy, 2-(ethylcarbonylamino)-ethyloxy, 2-
(propylcarbonylamino)-ethyloxy, 2-(isobutylcarbonylamino)-ethyloxy, 2-
(methoxyacetylamino)-ethyloxy, 2-(ethylaminocarbonylamino)-ethyloxy, 2-
(dimethylaminocarbonylamino)-ethyloxy, 2-(pyrrolidin-1-ylcarbonylamino)-
ethyloxy, 2-(piperidin-1 -ylcarbonylamino)-ethyloxy, 2-(morpholin-4-
ylcarbonylamino)-ethyloxy, 2-(methylsulphonylamino)-ethyloxy group, 2-
(ethylsulphonylamino)-ethyloxy or 2-(butylsulphonylamino)-ethyloxy group, or
a 3-(tert.-butyloxycarbonylamino)-propyloxy, 3-(amino)-propyloxy, 3-
(acetylamino)-propyloxy or 3-(methylsulphonylamino)-propyloxy group,
and
X denotes a nitrogen atom,
their tautomers, their stereoisomers, their mixtures and their salts.
Particularly preferred compounds of general formula I are those wherein
Ra denotes a hydrogen atom,
Rb preferably denotes a 3-chloro-4-fluoro-phenyl group or also a 3-
ethynyiphenyl group,
Boehringer Ingelheim Pharma KG 20 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rc denotes a cyclohexyl group which is substituted in the 3 position by an
amino, acetylamino, tert.-butyloxycarbonylamino or methylsulphonylamino
group,
a cyclohexyl group which is substituted in the 4 position by an amino,
methylamino, dimethylamino, acetylamino, N-(acetyl)-methylamino,
methoxyacetylamino, N-(methoxyacetyl)-methylamino, tetrahydropyran-4-
ylcarbonylamino, N-(tetrahydropyran-4-ylcarbonyl)-methylamino, tert.-
butyloxycarbonylamino, N-(tert.-butyloxycarbonyl)-methylamino, N-
(ethylaminocarbonyl)-methylamino, dimethylaminocarbonylamino, N-
(d imethylaminocarbonyl)-methylamino, N-(piperidin-1-ylcarbonyl)-
methylamino, morpholin-4-ylcarbonylamino, N-(morpholin-4-ylcarbonyl)-
methylamino, N-(4-methylpiperazin-1-ylcarbonyl)-methylamino,
methylsulphonylamino, N-(methylsulphonyl)-methylamino,
ethylsulphonylamino, N-(ethylsulphonyl)-methylamino,
dimethylaminosulphonylamino, N-(dimethylaminosulphonyl)-methylamino,
morpholin-4-ylsulphonylamino, N-(morpholin-4-ylsulphonyl)-methylamino, 3-
chloropropylsuiphonylamino, or [3-(morpholin-4-yi)-propyl]sulphonylamino
group,
a pyrrolidin-3-yl group,
a pyrrolidin-3-yl group which is substituted in the 1 position by a tert.-
butyloxycarbonyl or methylsulphonyl group,
a piperidin-3-yl group,
a piperidin-3-yl group which is substituted in the 1 position by a tert.-
butyloxycarbonyl or methylsulphonyl group,
a piperidin-4-yl group,
a piperidin-4-yl group which is substituted in the 1 position by a methyl,
Boehringer Ingelheim Pharma KG 21 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(aminocarbonyl)methyl, (d imethylaminocarbonyl)methyl, (morpholin-4-
ylcarbonyl)methyl, 2-(tert.-butyloxycarbonylamino)ethyl, 2-aminoethyl, 2-
(acetylamino)ethyl, 2-(methylsulphonylamino)ethyl, cyano, acetyl,
methoxyacetyl, (dimethylamino)acetyl, (morpholin-4-yl)acetyl,
tetrahydropyran-4-ylcarbonyl, ethylaminocarbonyl, isopropylaminocarbonyl,
phenylaminocarbonyl, dimethylaminocarbonyl, diethylaminocarbonyl,
pyrrolidin-1-ylcarbonyl, piperidin-1-ylcarbonyl, morpholin-4-ylcarbonyl, 2-
methylmorpholin-4-ylcarbonyl, 2,6-dimethylmorpholin-4-ylcarbonyl,
homomorpholin-4-ylcarbonyl, 4-methylpiperazin-1-ylcarbonyl,
isopropyloxycarbonyl, tert.-butyloxycarbonyl, methylsulphonyl,
dimethylaminosulphonyl or morpholin-4-ylsulphonyl group, or
a tetrahydrofuran-3-yl, tetra hyd ropyra n-3-yl or tetra h yd ropyra n-4-yl
group,
Rd denotes a hydrogen atom,
a methoxy or ethyloxy group,
a 2-(morpholin-4-yl)ethyloxy, 3-(morpholin-4-yl)propyloxy or 4-(morpholin-4-
yl)butyloxy group,
a 2-(3-methyl-2-oxo-hexahydropyrimidin-1-yl)-ethyloxy group,
a 2-(methoxy)-ethyloxy, 2-(tert.-butyloxycarbonylamino)-ethyloxy, 2-amino-
ethyloxy, 2-(acetylamino)-ethyloxy or 2-(methylsulphonylamino)-ethyloxy
group or
a 3-(tert.-butyloxycarbonylamino)-propyloxy, 3-amino-propyloxy, 3-
(acetylamino)-propyloxy or 3-(methylsulphonylamino)-propyloxy group,
and
X denotes a nitrogen atom,
Boehringer Ingelheim Pharma KG 22 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
their tautomers, their stereoisomers, their mixtures and their salts.
Of the bicyclic heterocyclic groups of general formula I as described above as
well as the sub-groups specified as being preferred, particularly preferred,
most particularly preferred and especially preferred, special mention should
be made of those compounds wherein
(a) R denotes a cyclohexyl group substituted in the 4 position,
(b) Rc denotes a pyrrolidin-3-yl group optionally substituted in the 1
position,
(c) Rc denotes a piperidin-3-yl group optionally substituted in the 1
position,
(d) Rc denotes a piperidin-4-yl group optionally substituted in the 1
position,
(e) Rc denotes a tetrahydrofuran-3-yl group,
(f) Rcdenotes a tetrahydropyran-3-yl group, or
(g) R` denotes a tetrahydropyran-4-yl group,
while Ra, Rb, Rd and X in each case are as hereinbefore defined.
The following are mentioned as examples of particularly preferred compounds
of general formula I:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-
meth oxy-q u i n azol i ne,
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
methoxy-quinazoline,
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((R)-tetrahydrofuran-3-yloxy)-7-
methoxy-quinazoline,
Boehringer Ingelheim Pharma KG 23 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexane-1-
yloxy)-7-methoxy-quinazoline,
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methanesulphonylamino-
cyclohexane-1-yloxy)-7-methoxy-quinazoline,
(6) 4-[(3-chIoro-4-f1 uoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-
quinazoline,
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-
yloxy)-7-methoxy-quinazoline,
(8) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{[3-(morpholin-4-yl)-
propyl]sulphonylamino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline,
(9) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-
methoxy-quinazoline,
(10) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-{[3-(morpholin-4-yl)-
propyl]sulphonylamino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline,
(11) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-
methoxy-quinazoline,
(12) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yI)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline,
(13) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(methoxymethyl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline,
(14) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-
methoxy-quinazoline,
Boehringer Ingelheim Pharma KG 24 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(15) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)sulphonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline,
(16) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-acetylamino-ethyl)-
piperidin-4-yloxy]-7-methoxy-quinazoline,
(17) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-
[(dimethylamino)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline,
(18) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline,
(19) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(morpholin-4-
yl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline,
(20) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
acetylamino-ethoxy)-quinazoline,
(21) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methanesulphonylamino-ethoxy)-quinazoline and
(22) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methoxy-ethoxy)-quinazoline,
as well as their salts.
The compounds of general formula I may be prepared for example by the
following methods:
a) reacting a compound of general formula
Boehringer Ingelheim Pharma KG 25 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
RNRb
O-H
N Rd
,(II)
wherein
Ra, Rb, Rd and X are as hereinbefore defined, with a compound of general
formula
Z' - Rc ,(III)
wherein
Rc is as hereinbefore defined and Z' denotes a leaving group such as a
halogen atom, e.g. a chlorine or bromine atom, a suiphonyloxy group such as
a methanesuiphonyloxy or p-toluenesulphonyloxy group or a hydroxy group.
With a compound of general formula III wherein Z' denotes a hydroxy group,
the reaction is carried out in the presence of a dehydrating agent, preferably
in the presence of of a phosphine and an azodicarboxylic acid derivative such
as e.g. triphenylphosphine/diethyl azodicarboxylate, conveniently in a solvent
such as methylene chloride, acetonitrile, tetrahydrofuran, dioxane, toluene or
ethylenglycoldiethylether at temperatures between -50 and 150 C, but
preferably at temperatures between -20 and 80 C.
b) In order to prepare compounds of general formula I wherein Rd denotes
one of the optionally substituted alkyloxy groups mentioned hereinbefore:
reacting a compound of general formula
Boehringer Ingelheim Pharma KG 26 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
b
R\N,R
O,R
X
N O-H
,(IV)
wherein Ra, Rb, R and X are as hereinbefore defined, with a compound of
general formula
Z2 - Rd, 'M
wherein Rd' denotes a C1_4-alkyl group, a methyl group substituted by 1 to 3
fluorine atoms, an ethyl group substituted by 1 to 5 fluorine atoms, a C2-4-
alkyl
group substituted by a group R6 or R7, where R6 and R7 are as hereinbefore
defined, a C1-a-alkyl group which is substituted by a pyrrolidinyl,
piperidinyl or
homopiperidinyl group substituted in the 1 position by the group R8, or a
C1_4-alkyl group which is substituted by a morpholinyl group substituted in
the
4 position by the group R8, while R8 in each case is as hereinbefore defined,
and
Z2 denotes a leaving group such as a halogen atom, an alkylsulphonyloxy,
arylsulphonyloxy or a hydroxy group.
If the leaving group is a halogen atom such as a chlorine, bromine or iodine
atom or an alkylsulphonyloxy or arylsulphonyloxy group such as the
methanesulphonyloxy or p-toluenesulphonyloxy group, the reaction is
preferably carried out in the presence of an organic or inorganic base such as
potassium carbonate, sodium hydride or N-ethyl-diisopropylamine. If the
leaving group is a hydroxy group, the reaction is carried out in the presence
of
a dehydrating agent, preferably in the presence of a phosphine and an
azodicarboxylic acid derivative such as e.g. triphenylphosphine/diethyl
azodicarboxylate.
c) In order to prepare compounds of general formula I wherein Rd denotes
Boehringer Ingelheim Pharma KG 27 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
one of the abovementioned alkyloxy groups which is substituted by an
optionally substituted amino, alkylamino or dialkylamino group or by an
optionally substituted heterocyclic group bound via an iminonitrogen atom:
reacting a compound of general formula
Ra\N~ Rb
O
X Rc
ki" N O-(CH2)2-4-Z3 (VI),
wherein Ra, Rb, Rc and X are as hereinbefore defined and Z3 denotes a
leaving group such as a halogen atom, e.g. a chlorine or bromine atom or a
sulphonyloxy group such as a methanesulphonyloxy or p-toluenesulphonyloxy
group,
with ammonia, a corresponding, optionally substituted alkylamine,
dialkylamine or an imino compound or the suitable salts or derivatives
thereof,
such as morpholine, for example.
d) In order to prepare compounds of general formula I wherein Rd denotes a
hydroxy group:
Cleaving a protecting group from a compound of general formula
RaRb
X O Rc
IRd" (VII),
wherein Ra, Rb, Rc and X are as hereinbefore defined and Rdõ denotes a
group which may be converted into a hydroxy group, for example an
Boehringer Ingelheim Pharma KG 28 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
optionally substituted benzyloxy group, a trimethylsilyloxy, acetyloxy,
benzoyloxy, methoxy, ethoxy, tert-butoxy or trityloxy group.
The protecting group is cleaved for example by hydrolysis in an aqueous
solvent, e.g. in water, isopropanol/water, acetic acid/water,
tetrahydrofuran/water or dioxan/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an
alkali metal base such as sodium hydroxide or potassium hydroxide or
aprotically, e.g. in the presence of iodotrimethylsilane, at temperatures
between 0 and 120 C, preferably at temperatures between 10 and 100 C.
However, a benzyl or methoxybenzyl group is cleaved, for example,
hydrogenolytically, e.g. with hydrogen in the presence of a catalyst such as
palladium/charcoal in a suitable solvent such as methanol, ethanol, ethyl
acetate or glacial acetic acid, optionally with the addition of an acid such
as
hydrochloric acid at temperatures between 0 and 100 C, but preferably at
ambient temperatures between 20 and 60 C, and at a hydrogen pressure of 1
to 7 bar, but preferably 3 to 5 bar. A 2,4-dimethoxybenzyl group, however, is
preferably cleaved in trifluoroacetic acid in the presence of anisole.
A tert.butyl or benzyl group is cleaved for example by treating with an acid
such as trifluoroacetic acid, hydrochloric acid or hydrobromic acid or by
treating with iodotrimethylsilane, optionally using a solvent such as
methylene
chloride, dioxan, methanol or diethyl ether.
e) In order to prepare compounds of general formula I wherein Rc contains a
-NH- group:
cleaving a protecting group from a compound of general formula
Ra Rb
X RC
N Rd
(VIII),
Boehringer Ingelheim Pharma KG 29 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
wherein Ra, Rb, Rd and X are as hereinbefore defined and R ' has the
meanings given for R hereinbefore, with the proviso that R contains a
protected nitrogen atom.
Conventional protecting groups for an amino, alkylamino or imino group are
for example the formyl, acetyl, trifluoroacetyl, ethoxycarbonyl,
tert.-butoxycarbonyl, benzyloxycarbonyl, benzyl, methoxybenzyl or
2,4-dimethoxybenzyl group, while for the amino group the phthalyl group is an
additional possibility.
The protecting group is cleaved for example by hydrolysis in an aqueous
solvent, e.g. in water, isopropanol/water, acetic acid/water,
tetrahydrofuran/water or dioxan/water, in the presence of an acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the presence
of an
alkali metal base such as sodium hydroxide or potassium hydroxide or
aprotically, e.g. in the presence of iodotrimethylsilane, at temperatures
between 0 and 120 C, preferably at temperatures between 10 and 100 C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is cleaved, for
example hydrogenolytically, e.g. with hydrogen in the presence of a catalyst
such as palladium/charcoal in a suitable solvent such as methanol, ethanol,
ethyl acetate or glacial acetic acid, optionally with the addition of an acid
such
as hydrochloric acid at temperatures between 0 and 100 C, but preferably at
ambient temperatures between 20 and 60 C, and at a hydrogen pressure of 1
to 7 bar, but preferably 3 to 5 bar. A 2,4-dimethoxybenzyl group, however, is
preferably cleaved in trifluoroacetic acid in the presence of anisole.
A tert.butyl or tert.butyloxycarbonyl group is preferably cleaved by treating
with
an acid such as trifluoroacetic acid or hydrochloric acid or by treating with
iodotrimethylsilane optionally using a solvent such as methylene chloride,
dioxan, methanol or diethyl ether.
Boehringer Ingelheim Pharma KG 30 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
A trifluoroacetyl group is preferably cleaved by treating with an acid such as
hydrochloric acid, optionally in the presence of a solvent such as acetic acid
at
temperatures between 50 and 120 C or by treating with sodium hydroxide
solution, optionally in the presence of a solvent such as tetrahydrofuran at
temperatures between 0 and 50 C.
A phthalyl group is preferably cleaved in the presence of hydrazine or a
primary amine such as methylamine, ethylamine or n-butylamine in a solvent
such as methanol, ethanol, isopropanol, toluene/water or dioxane at
temperatures between 20 and 50 C.
f) In order to prepare compounds of general formula I wherein R` contains an
alkyl group substituted by an optionally substituted amino, alkylamino or
dialkyamino group or by an optionally substituted heterocyclic group bound
via a nitrogen atom:
reacting a compound of general formula
R \N,Rb
X ~, O
Rcõ Z3
N Rd (IX),
wherein Ra, Rb, Rd and X are as hereinbefore defined, Z3 denotes a leaving
group, for example a halogen atom such as a chlorine or bromine atom, or a
sulphonyloxy group such as a methanesulphonyloxy or p-toluenesulphonyloxy
group, and Rc" has the meanings given for Rc hereinbefore with the proviso
that a hydrogen atom bound to an aliphatic carbon atom is replaced by the
group Z3,
with ammonia, a corresponding, optionally substituted alkylamine,
dialkylamine or an imino compound or the appropriate salts or derivatives
thereof, such as morpholine, for example.
Boehringer Ingelheim Pharma KG 31 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
If according to the invention a compound of general formula I is obtained
which contains an amino, alkylamino or imino group, this may be converted by
acylation, cyanation or sulphonylation into a corresponding acyl, cyano or
sulphonyl compound of general formula I, the acylating agents being for
example isocyanate, carbamoyl chloride, carboxylic acid halide, carboxylic
acid anhydride and carboxylic acids with activating agents such as N,N'-
carbonyldiimidazole, N,N'-dicyclohexylcarbodiimide or O-(benzotriazol-1-yl)-
N,N,N'N'-tetramethyluronium-tetrafluoroborate, the sulphonylating agents
being sulphonyl halides and the cyanating agents being chlorine or
bromocyanogen, and/or
if a compound of general formula I is obtained which contains an amino,
alkylamino or imino group, this may be converted by alkylation or reductive
alkylation into a corresponding alkyl compound of general formula I and/or
if a compound of general formula I is obtained which contains a chloro-
CI_4-alkylsulphonyl or bromo-C1_4-alkylsulphonyl group, this may be converted
by reaction with an amine into a corresponding amino-Ci-4-alkylsulphonyl
compound
and/or
if a compound of general formula I is obtained which contains a tert.-
butyloxycarbonylamino, N-alkyl-N-(tert.-butyloxycarbonyl)amino or a N-tert.-
butyloxycarbonylimino group, this may be converted into a corresponding
amino, alkylamino or imino compound of general formula I by treatment with
an acid such as hydrochloric acid or trifluoroacetic acid.
In the reactions described hereinbefore, any reactive groups present such as
hydroxy, carboxy or imino groups may be protected during the reaction by
conventional protecting groups which are cleaved again after the reaction.
For example, a protecting group for a hydroxy group may be a trimethylsilyl,
acetyl, trityl, benzyl or tetrahydropyranyl group.
Boehringer Ingelheim Pharma KG 32 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Protecting groups for an amino, alkylamino or imino group may be a formyl,
acetyl, trifluoroacetyl, ethoxycarbonyl, tert.butoxycarbonyl,
benzyloxycarbonyl,
benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group, for example.
Any protecting group used is optionally subsequently cleaved for example by
hydrolysis in an aqueous solvent, e.g. in water, isopropanol/water, acetic
acid/water, tetrahydrofuran/water or dioxan/water, in the presence of an acid
such as trifluoroacetic acid, hydrochloric acid or sulphuric acid or in the
presence of an alkali metal base such as sodium hydroxide or potassium
hydroxide or aprotically, e.g. in the presence of iodotrimethylsilane, at
temperatures between 0 and 120 C, preferably at temperatures between 10
and 100 C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is cleaved, for
example, hydrogenolytically, e.g. with hydrogen in the presence of a catalyst
such as palladium/charcoal in a suitable solvent such as methanol, ethanol,
ethyl acetate or glacial acetic acid, optionally with the addition of an acid
such
as hydrochloric acid at temperatures between 0 and 100 C, but preferably at
ambient temperatures between 20 and 60 C, and at a hydrogen pressure of 1
to 7 bar, but preferably 3 to 5 bar. A 2,4-dimethoxybenzyl group, however, is
preferably cleaved in trifluoroacetic acid in the presence of anisole.
A tert.butyl or tert.butyloxycarbonyl group is preferably cleaved by treating
with an acid such as trifluoroacetic acid or hydrochloric acid or by treating
with
iodotrimethylsilane optionally using a solvent such as methylene chloride,
dioxan, methanol or diethyl ether.
A trifluoroacetyl group is preferably cleaved by treating with an acid such as
hydrochloric acid, optionally in the presence of a solvent such as acetic acid
at temperatures between 50 and 120 C or by treating with sodium hydroxide
solution, optionally in the presence of a solvent such as tetrahydrofuran at
temperatures between 0 and 50 C.
Boehringer Ingelheim Pharma KG 33 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Moreover, the compounds of general formula I obtained may be resolved into
their enantiomers and/or diastereomers, as mentioned hereinbefore. Thus, for
example, cis/trans mixtures may be resolved into their cis and trans isomers,
and compounds with at least one optically active carbon atom may be
separated into their enantiomers.
Thus, for example, the cis/trans mixtures may be resolved by chromatography
into the cis and trans isomers thereof, the compounds of general formula I
obtained which occur as racemates may be separated by methods known per
se (cf. Allinger N. L. and Eliel E. L. in "Topics in Stereochemistry", Vol. 6,
Wiley Interscience, 1971) into their optical antipodes and compounds of
general formula I with at least 2 asymmetric carbon atoms may be resolved
into their diastereomers on the basis of their physical-chemical differences
using methods known per se, e.g. by chromatography and/or fractional
crystallisation, and, if these compounds are obtained in racemic form, they
may subsequently be resolved into the enantiomers as mentioned above.
The enantiomers are preferably separated by column separation on chiral
phases or by recrystallisation from an optically active solvent or by reacting
with an optically active substance which forms salts or derivatives such as
e.g. esters or amides with the racemic compound, particularly acids and the
activated derivatives or alcohols thereof, and separating the diastereomeric
mixture of salts or derivatives thus obtained, e.g. on the basis of their
differences in solubility, whilst the free antipodes may be released from the
pure diastereomeric salts or derivatives by the action of suitable agents.
Optically active acids in common use are e.g. the D- and L-forms of tartaric
acid or dibenzoyltartaric acid, di-o-tolyltartaric acid, malic acid, mandelic
acid,
camphorsulphonic acid, glutamic acid, aspartic acid or quinic acid. An
optically active alcohol may be for example (+) or (-)-menthol and an
optically
active acyl group in amides, for example, may be a (+)-or
(-)-menthyloxycarbonyl.
Furthermore, the compounds of formula I may be converted into the salts
thereof, particularly for pharmaceutical use into the physiologically
acceptable
Boehringer Ingelheim Pharma KG 34 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
salts with inorganic or organic acids. Acids which may be used for this
purpose include for example hydrochloric acid, hydrobromic acid, sulphuric
acid, methanesuiphonic acid, phosphoric acid, fumaric acid, succinic acid,
lactic acid, citric acid, tartaric acid or maleic acid.
The compounds of general formulae II to I used as starting materials are
known from the literature in some cases or may be obtained by methods
known from the literature (cf. Examples I to XXII) or the methods described
hereinbefore, optionally with the additional use of protecting groups (e.g.
compounds of formula IV or VII and VIII).
As already mentioned hereinbefore, the compounds of general formula I
according to the invention and the physiologically acceptable salts thereof
have valuable pharmacological properties, particularly an inhibiting effect on
signal transduction mediated by the Epidermal Growth Factor receptor
(EGF-R), whilst this may be achieved for example by inhibiting ligand
bonding, receptor dimerisation or tyrosine kinase itself. It is also possible
that
the transmission of signals to components located further down is blocked.
The biological properties of the new compounds were investigated as follows:
The inhibition of human EGF-receptor kinase was determined using the cyto-
plasmic tyrosine kinase domain (methionine 664 to alanine 1186 based on the
sequence published in Nature 309 (1984), 418). For this the protein was
expressed in Sf9 insect cells as GST fusion protein using the Baculovirus
expression system.
The enzyme activity was measured in the presence or absence of the test
compounds in serial dilutions. The polymer pEY (4:1) obtained from SIGMA
was used as the substrate. Biotinylated pEY (bio-pEY) was added as the
tracer substrate. 100 pI of reaction solution contained 10 pl of the inhibitor
in
50% DMSO, 20 pl of the substrate solution (200 mM HEPES pH 7.4, 50 mM
magnesium acetate, 2.5 mg/ml poly(EY), 5 pg/ml bio-pEY) and 20 pl of
enzyme preparation. The enzyme reaction was started by the addition of 50 pl
Boehringer Ingelheim Pharma KG 35 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
of a 100 pM ATP solution in 10 mM of magnesium chloride. The dilution of the
enzyme preparation was adjusted so that the incorporation of phosphate in
the bio-pEY was linear in terms of time and quantity of enzyme. The enzyme
preparation was diluted in 20 mM HEPES pH 7.4, 1 mM EDTA, 130 mM
common salt, 0.05% Triton X-100, 1 mM DTT and 10% glycerol.
The enzyme assays were carried out at ambient temperature over a period of
30 minutes and ended by the addition of 50 pl of a stopping solution (250 mM
EDTA in 20 mM HEPES pH 7.4). 100 pl were placed on a streptavidine-
coated microtitre plate and incubated for 60 minutes at ambient temperature.
Then the plate was washed with 200 pl of a wash solution (50 mM Tris, 0.05%
Tween 20). After the addition of 100 pl of an HRPO-labelled anti-PY antibody
(PY20H Anti-PTyr:HRP made by Transduction Laboratories, 250 ng/ml) the
preparation was incubated for 60 minutes. Then the microtitre plate was
washed three times with 200 pl of wash solution. The samples were then
combined with 100 pl of a TMB-peroxidase solution (A:B = 1:1, Kirkegaard
Perry Laboratories). After 10 minutes the reaction was stopped. The extinction
was measured at OD45onm with an ELISA reader. All the results were
measured three times.
The data were adapted by iterative calculation using an analytical pogramme
for sigmoidal curves (Graph Pad Prism Version 3.0) with a variable Hill pitch.
All the iterative data produced had a correlation coefficient of more than 0.9
and the upper and lower values of the curves showed a spread of at least a
factor of 5. The active substance concentration which inhibits the activity of
EGF receptor kinase by 50% (IC50) was derived from the curves.
Boehringer Ingelheim Pharma KG 36 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The following results were obtained:
Compound Inhibition of EGF-
(Example Nr.) receptor kinase
IC50 [nM]
1 0.13
1(1) 0.12
1(2) 2
1(3) 1.1
1(4) 0.6
1(5) 0.6
1(6) 0.69
1(7) 1.6
2 4.5
2(1) 0.16
2(2) 0.22
3 0.9
3(1) 0.14
3(2) 0.22
3(7) 0.7
3(8) 0.6
3(9) 0.2
3(11) 0.1
3(15) 1
3(16) 1
3(17) 0.3
3(18) 0.4
3(20) 1
3(21) 0.4
4 0.41
4(1) 0.16
7(5) 1
Boehringer Ingelheim Pharma KG 37 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The compounds of general formula I according to the invention thus inhibit
signal transduction by tyrosine kinases, as demonstrated by the example of
the human EGF receptor, and are therefore useful for treating
pathophysiological processes caused by hyperfunction of tyrosine kinases.
These are e.g. benign or malignant tumours, particularly tumours of epithelial
and neuroepithelial origin, metastasisation and the abnormal proliferation of
vascular endothelial cells (neoangiogenesis).
The compounds according to the invention are also useful for preventing and
treating diseases of the airways and lungs which are accompanied by
increased or altered production of mucus caused by stimulation by tyrosine
kinases, e.g. in inflammatory diseases of the airways such as chronic
bronchitis, chronic obstructive bronchitis, asthma, bronchiectasis, allergic
or
non-allergic rhinitis or sinusitis, cystic fibrosis, al-antitrypsin
deficiency, or
coughs, pulmonary emphysema, pulmonary fibrosis and hyperreactive
airways.
The compounds are also suitable for treating diseases of the gastrointestinal
tract and bile duct and gall bladder which are associated with disrupted
activity of the tyrosine kinases, such as may be found e.g. in chronic
inflammatory changes such as cholecystitis, Crohn's disease, ulcerative
colitis, and ulcers in the gastrointestinal tract or such as may occur in
diseases of the gastrointestinal tract which are associated with increased
secretions, such as Menetrier's disease, secreting adenomas and protein loss
syndrome.
In addition, the compounds of general formula I and the physiologically
acceptable salts thereof may be used to treat other diseases caused by
abnormal function of tyrosine kinases, such as e.g. epidermal
hyperproliferation (psoriasis), benign prostate hyperplasia (BPH),
inflammatory processes, diseases of the immune system, hyperproliferation of
haematopoietic cells, the treatment of nasal polyps, etc.
Boehringer Ingelheim Pharma KG 38 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
By reason of their biological properties the compounds according to the
invention may be used on their own or in conjunction with other
pharmacologically active compounds, for example in tumour therapy, in
monotherapy or in conjunction with other anti-tumour therapeutic agents, for
example in combination with topoisomerase inhibitors (e.g. etoposide), mitosis
inhibitors (e.g. vinblastine), compounds which interact with nucleic acids
(e.g.
cis-platin, cyclophosphamide, adriamycin), hormone antagonists (e.g.
tamoxifen), inhibitors of metabolic processes (e.g. 5-FU etc.), cytokines
(e.g.
interferons), antibodies, etc. For treating respiratory tract diseases, these
compounds may be used on their own or in conjunction with other therapeutic
agents for the airways, such as substances with a secretolytic (e.g. ambroxol,
N-acetylcysteine) , broncholytic (e.g. tiotropium or ipratropium or fenoterol,
salmeterol, salbutamol) and/or anti-inflammatory activity (e.g. theophylline
or
glucocorticoids). For treating diseases in the region of the gastrointestinal
tract, these compounds may also be administered on their own or in
conjunction with substances having an effect on motility or secretion. These
combinations may be administered either simultaneously or sequentially.
These compounds may be administered either on their own or in conjunction
with other active substances by intravenous, subcutaneous, intramuscular,
intraperitoneal or intranasal route, by inhalation or transdermally or orally,
whilst aerosol formulations are particularly suitable for inhalation.
For pharmaceutical use the compounds according to the invention are
generally used for warm-blooded vertebrates, particularly humans, in doses of
0.01 -100 mg/kg of body weight, preferably 0.1-15 mg/kg. For administration
they are formulated with one or more conventional inert carriers and/or
diluents, e.g. with corn starch, lactose, glucose, microcrystalline cellulose,
magnesium stearate, polyvinylpyrrolidone, citric acid, tartaric acid, water,
water/ethanol, water/glycerol, water/sorbitol, water/polyethylene glycol,
propylene glycol, stearyl alcohol, ca rboxymethylcel I u lose or fatty
substances
such as hard fat or suitable mixtures thereof in conventional galenic
preparations such as plain or coated tablets, capsules, powders, suspensions,
solutions, sprays or suppositories.
Boehringer Ingelheim Pharma KG 39 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The following Examples are intended to illustrate the present invention
without
restricting it:
Preparation of the starting compounds:
Example I
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-benzyloxy-
quinazoline-hydrochloride
A mixture of 10.84 g 4-chloro-6-(tetrahydropyran-4-yloxy)-7-benzyloxy-
quinazoline and 4.50 g 3-chloro-4-fluoranilin in 300 ml isopropanol is
refluxed
for four hours and then left to stand overnight at ambient temperature. The
precipitate formed is suction filtered, washed with isopropanol and stirred
with
150 ml of methanol. The suspension is stirred for another half hour at ambient
temperature and then suction filtered. The filter cake is washed repeatedly
with methanol and dried.
Yield: 9.07 g (60 % of theory)
Rf value: 0.27 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI-): m/z = 478, 480 [M-H]-
The following compounds are obtained analogously to Example I:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-
benzyloxy-q uinazoline-hyd rochloride
Rf value: 0.34 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 466, 468 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-piperidin-4-
yloxy)-
quinazoline-hydrochloride
Rf value: 0.17 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 469, 471 [M+H]+
CA 02476008 2004-10-28
25771-944
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-piperidin-4-
yloxy)-
7-acetoxy-quinazoline-hydrochloride
Rf value: 0.70 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 527, 529 [M+H]+
(4) 4-[(3-ethynyl-phenyl)amino]-6-acetoxy-7-methoxy-quinazoline
Rf value: 0.59 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 334 [M+H]+
Example ii
4-chloro-6-(tetrahydropyran-4-yloxy)-7-benzyloxy-quinazoline
Boehringer Ingelheim Pharma KG 41 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Prepared by reacting 6-(tetrahydropyran-4-yloxy)-7-benzyloxy-3H-
quinazoline-4-on with thionyl chloride in the presence of N,N-
dimethylformamide in acetonitrile at reflux temperature.
Rf value: 0.90 (silica gel, ethyl acetate/methanol = 9:1)
The following compounds are obtained analogously to Example II:
(1) 4-chloro-6-((S)-tetrahydrofuran-3-yloxy)-7-benzyloxy-quinazoline
Rf value: 0.85 (silica gel, ethyl acetate/methanol = 9:1)
(2) 4-chloro-6-(1-trifluoroacetyl-piperidin-4-yloxy)-quinazoline
Rf value: 0.92 (silica gel, ethyl acetate)
(3) 4-chloro-6-(1-trifluoroacetyl-piperidin-4-yloxy)-7-acetoxy-quinazoline
Example III
6-(tetrahyd ropyran-4-yloxy)-7-benzyloxy-3H-quinazoline-4-on
A mixture of 15.08 g 2-amino-4-benzyloxy-5-(tetrahydropyran-4-yloxy)-
benzoic acid and 14.40 g formamidine acetate in 250 ml of absolute ethanol is
refluxed overnight. The cooled reaction mixture is combined with 250 ml of
water. The precipitate formed is suction filtered and dried at 70 C in the
drying cupboard.
Yield: 10.00 g (65 % of theory)
Rf value: 0.40 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 353 [M+H]'
The following compounds are obtained analogously to Example III:
(1) 6-((S)-tetrahydrofuran-3-yloxy)-7-benzyloxy-3H-quinazolin-4-one
Rf value: 0.60 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Boehringer Ingelheim Pharma KG 42 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 339 [M+H]+
(2) 6-[1 -(tert. -b utyloxycarbonyl)-p ipe rid in -4-yloxy]-3H-q u in azol i n-
4-o ne
Rf value: 0.48 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 346 [M+H]+
(3) 6-[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-hydroxy-3H-quinazolin-
4-one
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 362 [M+H]+
Example IV
2-amino-4-benzyloxy-5-(tetrahydropyran-4-yloxy)-benzoic acid
16.40 g 2-nitro-4-benzyloxy-5-(tetrahydropyran-4-yloxy)-benzoic acid are
hydrogenated in the presence of 1.64 g Raney nickel in 800 ml of methanol at
55 C, until the calculated amount of hydrogen has been taken up. The
catalyst is filtered off and the filtrate evaporated down, whereupon the
desired
product crystallises out.
Yield: 15.08 g (100 % of theory)
Rf value: 0.60 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
The following compounds are obtained analogously to Example IV:
(1) benzyl 2-amino-4-benzyloxy-5-((S)-tetrahydrofuran-3-yloxy)-benzoate
Rf value: 0.70 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 420 [M+H]+
(2) 2-amino-5-[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-benzoic acid
Rf value: 0.43 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 337 [M+H]+
Boehringer Ingelheim Pharma KG 43 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(3) 2-amino-4-hydroxy-5-[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-
benzoic acid
Rf value: 0.23 (silica gel, methylene chloride/methanol/acetic acid = 90:10:1)
Example V
2-nitro-4-benzyloxy-5-(tetrahydropyran-4-yloxy)-benzoic acid
Prepared by saponification of benzyl 2-nitro-4-benzyloxy-5-(tetrahydropyran-
4-yloxy)-benzoate with 1 N sodium hydroxide solution in methanol at ambient
temperature.
Rf value: 0.20 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI{'): m/z = 374 [M+H]+
Example VI
Benzyl 2-nitro-4-benzyloxy-5-(tetrahydro-pyran-4-yloxy)-benzoate
42.60 g potassium-tert.-butoxide are added to 38 ml of tetrahydrofuran-4-ol in
228 ml N,N-dimethylformamide while cooling with an ice bath. The mixture is
stirred for one hour at ambient temperature, then 22.90 g 6-nitro-
benzo[1,3]dioxol-5-carboxylic acid are added. After 1.5 hours the reaction is
complete according to thin layer chromatography and 28.94 ml of
benzylbromide are added dropwise while cooling with an ice bath. The
reaction mixture is stirred overnight at ambient temperature, combined with
100 ml 10% citric acid and stirred for another day at ambient temperature.
Then the reaction mixture is evaporated down in vacuo at 60 C and added to
800 ml ice water. The aqueous phase is extracted with ethyl acetate and the
combined extracts are washed with water and saturated sodium chloride
solution, dried over magnesium sulphate and evaporated.
The residue is stirred with diethyl ether, while 2-nitro-4-benzyloxy-5-
(tetrahydropyran-4-yloxy)-benzoic acid crystallises out as a by-product. This
is
filtered off and the filtrate is evaporated down. The main product remaining
is
benzyl 2-nitro-4-benzyloxy-5-(tetrahydro-pyran-4-yloxy)-benzoate, which is
Boehringer Ingelheim Pharma KG 44 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
saponified without any further purification to form carboxylic acid (see
Example V).
The following compounds are obtained analogously to Example VI:
(1) benzyl 2-nitro-4-benzyloxy-5-((S)-tetrahydrofuran-3-yloxy)-benzoate
Rf value: 0.75 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 450 [M+H]+
(2) 2-n itro-4-hyd roxy-5-[1 -(tert. -b utyloxyca rbonyl)-p i pe rid i n-4-
yloxy]-be nzo ic
acid
No reaction is carried out with benzyl bromide.
Rf value: 0.40 (silica gel, methylene chloride/methanol/acetic acid = 90:10:1)
Mass spectrum (ESI-): m/z = 381 [M-H]-
Example VII
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[2-(tert.-butyloxycarbonylamino)-
ethyl]-piperidin-4-yloxy}-7-methoxy-quinazoline
A mixture of 410 mg 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-
7-methoxy-quinazoline-dihydrochloride, 240 mg N-(tert.-butyloxycarbonyl)-2-
bromo-ethylamine and 360 mg potassium carbonate in 5 ml N,N-
dimethylformamide is stirred overnight at ambient temperature. Then a further
80 mg of N-(tert.-butyloxycarbonyl)-2-bromo-ethylamine are added and the
reaction mixture is stirred for a further four hours at ambient temperature.
For
working up it is diluted with water and extracted with ethyl acetate. The
combined organic phases are washed with saturated sodium chloride
solution, dried over magnesium sulphate and evaporated. The residue is
chromatographed through a silica gel column with ethyl acetate/methanol
(95:5 to 90:1) as eluant.
Yield: 370 mg (79 % of theory)
Rf value: 0.33 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Boehringer Ingelheim Pharma KG 45 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI"): m/z = 544, 546 [M-H]-
The following compound is obtained analogously to Example VII:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[2-(tert.-
butyloxycarbonylamino)-ethyl]-piperidin-4-yloxy}-quinazoline
Rf value: 0.38 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 516, 518 [M+H]+
Example VIII
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-
quinazoline-dihydrochloride
Prepared by treating 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(tert.-
butyloxycarbonyl)-piperidin-4-yloxy]-7-methoxy-quinazoline with concentrated
hydrochloric acid in dioxane at ambient temperature.
Rf value: 0.53 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 403, 405 [M+H]+
The following compounds are obtained analogously to Analog Example VIII:
(1) 6-(piperidin-4-yloxy)-3H-quinazolin-4-one x 2 trifluoroacetic acid
Carried out with trifluoroacetic acid in methylene chloride.
Mass spectrum (ESI+): m/z = 246 [M+H]+
(2) 6-(piperidin-4-yloxy)-7-hydroxy-3H-quinazolin-4-one
Carried out with trifluoroacetic acid in methylene chloride.
Rf value: 0.60 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 262 [M+H]+
Boehringer Ingelheim Pharma KG 46 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example IX
4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1 -(tert.-butyloxycarbonyl)-piperidin-4-
yloxy]-7-methoxy-quinazoline
A solution of 7.80 ml diethyl azodicarboxylate in 100 ml methylene chloride is
added dropwise to a mixture of 10.00 g 4-[(3-chloro-4-fluoro-phenyl)amino]-6-
hydroxy-7-methoxy-quinazoline and 9.40 g 1-(tert.-butyloxycarbonyl)-4-
hydroxy-piperidine and 12.40 g triphenylphosphine in 400 ml methylene
chloride at ambient temperature. The suspension is stirred for three days at
ambient temperature and then suction filtered. The filtrate is evaporated and
chromatographed through a silica gel column with methylene
chloride/methanol (98:2 auf 95:5) as eluant. The crude product obtained is
combined with diisopropylether, stirred overnight therein, suction filtered
and
dried.
Yield: 5.34 g (34 % of theory)
Rf value: 0.46 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 503, 505 [M+H]+
Example X
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahyd ropyran-4-yloxy)-7-(4-bromo-
butyloxy)-quinazoline
A mixture of 500 mg 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-
yloxy)-7-hydroxy-quinazoline, 165 pl 1-bromo-4-chloro-propane and 360 mg
potassium carbonate in 5 ml N,N-dimethylformamide is stirred overnight at
80 C. For working up the reaction mixture is diluted with water and extracted
with ethyl acetate. The combined organic phases are washed with saturated
sodium chloride solution, dried over magnesium sulphate and evaporated.
The crude product is further reacted without any more purification.
Yield: 650 mg (97 % of theory)
The following compounds are obtained analogously to Example X:
Boehringer Ingelheim Pharma KG 47 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-(4-
bromo-butyloxy)-quinazoline
Rf value: 0.84 (silica gel, ethyl acetate/methanol = 9:1)
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-piperidin-4-
yloxy)-
7-ethoxy-quinazoline
Mass spectrum (ESI+): m/z = 513, 515 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-piperidin-4-
yloxy)-
7-(2-methoxy-ethoxy)-quinazoline
Rf value: 0.38 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 543, 545 [M+H]+
Example Xl
1-(2-hydroxy-ethyl)-3-methyl-tetrahydropyrimidin-2-on
Prepared by hydrogenolytically cleaving 1-(2-benzyloxy-ethyl)-3-methyl-
tetrahydropy rimidin-2-one in the presence of palladium on activated charcoal
in methanol at ambient temperature.
Rf value: 0.23 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 159 [M+H]+
Example XII
1-(2-benzyloxy-ethyl)-3-methyl-tetrahydropyrimidin-2-on
Prepared by reacting 1-(2-benzyloxy-ethyl)-tetrahydropyrimidin-2-one with
methyl iodide in the presence of potassium-tert.-butoxide in N,N-
dimethylformamide at ambient temperature.
Rf value: 0.62 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): mlz = 249 [M+H]+
Boehringer Ingelheim Pharma KG 48 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example XIII
1 -(2-benzyloxy-ethyl)-tetrahyd ropyri mid in-2-on
Prepared by treating 1-(2-benzyloxy-ethyl)-3-(3-chloro-propyl)-urea with
potassium-tert.-butoxide in N,N-dimethylformamide at ambient temperature.
Rf value: 0.42 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 235 [M+H]+
Example XIV
1-(2-benzyloxy-ethyl)-tetrahydropyrimidin-2-one
Prepared by reacting 2-benzyloxy-ethylamine with 3-chloro-propyl-isocyanate
in tetrahydrofuran.
Rf value: 0.73 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 271, 273 [M+H]+
Example XV
3-(tert.-butyloxycarbonylamino)-cyclohexanol
Prepared by reacting 3-amino-cyclohexanol with di-tert.butyl pyrocarbonate in
the presence of triethylamine in a mixture of dioxan/water (2:1) at 50 C.
Rf value: 0.34 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI"): m/z = 214 [M-H]-
The following compounds are obtained analogously to Example XV:
(1) cis-4-[N-(tert.-butyloxycarbonyl)-N-methyl-amino]-cyclohexanol
The reaction takes place in methanol.
Rf value: 0.70 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 230 [M+H]+
IAdI I I UU A V I
6-(1-trifluoroacetyl-piperidin-4-yloxy)-3H-quinazolin-4-one
Boehringer Ingeiheim Pharma KG 49 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example XVI
6-(1 -trifl u oroacetyl-p ipe rid i n-4-yloxy)-3H-q u in azol i n-4-one
Prepared by reacting 6-(piperidin-4-yloxy)-3H-quinazolin-4-one x 2
trifluoroacetic acid with trifluoroacetic acid anhydride in the presence of
triethylamine in tetrahydrofuran.
Rf value: 0.48 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 342 [M+H]+
The following compounds are obtained analogously to Example XVI:
(1) 6-(1-trifluoroacetyl-piperidin-4-yloxy)-7-hydroxy-3H-quinazolin-4-one
Carried out with methyl trifluoroacetate in the presence of Honig base in
methanol.
Rf value: 0.80 (silica gel, methylene chloride/methanol = 4:1)
Mass spectrum (ESI+): m/z = 358 [M+H]+
Example XVII
2-nitro-5-[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-benzoic acid
21.00 g potassium-tert.-butoxide are added batchwise to 25.14 g 1-(tert.-
butyloxycarbonyl)-piperidin-4-oI in 120 ml N,N-dimethylformamide while
cooling with an ice bath, while the temperature is kept below 10 C. The
mixture is stirred for a further 30 minutes while cooling with an ice bath,
then
11.60 g of 5-fluoro-2-nitro-benzoic acid are added. After another three hours
the reaction mixture is poured onto water, adjusted to pH 1 with conc.
hydrochloric acid and extracted with ethyl acetate. The combined organic
phases are washed with dilute citric acid solution, dried over magnesium
sulphate and evaporated. The residue is triturated with diethyl ether, suction
filtered and dried. More product crystallises out of the filtrate after
standing for
some time, and this is also suction filtered and dried.
Yield: 9.58 g (42 % of theory)
Boehringer Ingelheim Pharma KG 50 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rf value: 0.43 (silica gel, methylene chloride/methanol/acetic acid = 90:10:1)
Mass spectrum (ESI+): m/z = 367[M+H]+
Example XVIII
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-bromacetyl-piperidin-4-yloxy)-
quinazoline and 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-chloracetyl-piperidin-
4-yloxy)-quinazoline
Prepared by reacting 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-
quinazoline with bromoacetic acid chloride in the presence of Hunig base in
tetrahydrofuran at ambient temperature. A mixture of the bromine and chlorine
compounds is obtained.
Rf value: 0.43 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 493, 495, 497 [M1 +H]+ and 449, 451, 453
[M2+H]+
The following compounds are obtained analogously to Example XVIII:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-chloracetyl-piperidin-4-yloxy)-7-
methoxy-quinazoline
The reaction takes place with chloroacetyl chloride.
Rf value: 0.59 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI-): m/z = 477, 479, 481 [M-H]-
Example XIX
1 -methyl-3-[([1,4]oxazepan-4-yl)carbonyl]-3H-imidazol-1 -ium-iodide
Prepared by reacting 3-[([1,4]oxazepan-4-yl)carbonyl]-3H-imidazole with
methyl iodide in acetonitrile at ambient temperature. The crude product is
reacted further without any more purification.
The following compounds are obtained analogously to Example XIX:
CA 02476008 2004-10-28
25771-944
51
(1) 1-methyl-3-[(cis-2,6-dimethyl-morpholin-4-yl)carbonyl]-3H-imidazol-1-
ium-iodide
Rf value: 0.12 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
(2) 1-methyl-3-[(2-methyl-morpholin-4-yl)carbonyl]-3H-imidazol-1-ium-iodide
Rf value: 0.02 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Example XX
3-[([1,4]oxazepan-4-yl)carbonyl]-3H-imidazole
Prepared by reacting [1,4]oxazepan with N,N'-carbonyldiimidazole in the
presence of triethylamine in tetrahydrofuran at ambient temperature.
Rf value: 0.30 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 196 [M+H]+
CA 02476008 2004-10-28
25771-944
52
The following compounds are obtained analogously to Example XX:
(1) 3-[(cis-2,6-dimethyl-morpholin-4-yl)carbonyl]-3H-imidazole
Rf value: 0.46 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
(2) 3-[(2-methyl-morpholin-4-yl)carbonyl]-3H-imidazole
Rf value: 0.43 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Example XXI
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-piperidin-4-yloxy)-7-
hydroxy-quinazoline
Prepared by treating 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1 -trifluoroacetyl-
p i perid in-4-yloxy)-7-acetoxy-q u in azol i ne-hyd roch lo ride with
saturated sodium
hydrogen carbonate solution in methanol at ambient temperature. In addition
to the desired product, some 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-
4-yloxy)-7-hydroxy-quinazoline is also isolated as a by-product.
Rf value: 0.20 (silica gel, methylene chloride/methanol = 20:1)
CA 02476008 2004-10-28
25771-944
53
Mass spectrum (ESI'): m/z = 483, 485 [M-H]-
The following compounds are obtained analogously to Example XXI:
(1) 4-[(3-ethynyl-phenyl)amino]-6-hydroxy-7-methoxy-quinazoline
Carried out with 40 % sodium hydroxide solution in ethanol.
Rf value: 0.32 (silica gel, ethyl acetate)
Mass spectrum (ESIi'): m/z = 292 [M+H]+
Example XXII
6-(1-trifl u oroacetyl-pi pe rid i n-4-yloxy)-7-acetoxy-3H-q u inazo li n-4-
one
Prepared by reacting 6-(1-trifluoroacetyl-piperidin-4-yloxy)-7-hydroxy-3H-
quinazolin-4-one with acetic anhydride in pyridine at 80 C.
Rf value: 0.60 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 400 [M+H]+
CA 02476008 2004-10-28
25771-944
54
Preparation of the end compounds:
Example 1
4-[(3-ch loro-4-fluoro-phenyl)amino]-6-((S)-tetrahyd rofuran-3-yloxy)-7-
methoxy-quinazoline
ci
F
NH
N
kN O
CH3
300 mg of 4-[(3-chloro-4-fluoro-phenyl)amino]-6-hydroxy-7-methoxy-
quinazoline in 6 ml acetonitrile are combined with 114 pl (R)-3-hydroxy-
tetrahydrofuran and 370 mg triphenyiphosphine. Then 234 pI diethyl
azodicarboxylate are added and the reaction mixture is stirred overnight at
ambient temperature. For working up the reaction mixture is filtered and the
filtrate evaporated down in vacuo. The crude product is purified by
chromatography over a silica gel column with ethyl acetate/methanol (95:5) as
eluant.
Yield: 53 mg (15 % of theory)
melting point: 178 C
Mass spectrum (ESI+): m/z = 390, 392 [M+H]+
The following compounds are obtained analogously to Example 1:
Boehringer Ingelheim Pharma KG 55 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
methoxy-quinazoline
cl
F
NH
N
O
N O I
CH3
Rf value: 0.54 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 404, 406 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(tert.-
butyloxycarbonylamino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
cl
F
NH
\ O
N
O NH
CH3
O O
H3C
H3C CH3
Rf value: 0.70 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 517, 519 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((R)-tetrahydrofuran-3-yloxy)-7-
methoxy-quinazoline
Cl
F
NH
N` O
O
1
CH3
Boehringer Ingelheim Pharma KG 56 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rf value: 0.64 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 390, 392 [M+H]+
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(tert.-
butyloxycarbonylamino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
Cl
F
NH
N
NH
N O CH3
Q 'It" 0
H3C>
H3C CH3
Rf value: 0.65 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 517, 519 [M+H]+
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(tert.-butyloxycarbonyl)-
piperidin-4-yloxy]-7-methoxy-quinazoline
cl
F I:t~NH
N O
N O N ~O
I
CH3 O CH3
H3C CH3
melting point: 184 C
Mass spectrum (ESI+): m/z = 503, 505 [M+H]+
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-3-yloxy)-7-
methoxy-quinazoline
Boehringer Ingelheim Pharma KG 57 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Cl
F
NH
N \ xo
1
CH3
Rf value: 0.52 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 404, 406 [M+H]+
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-
methoxy-quinazoline
F
CI NH
I`
`N\OO NCH3
I
CH3
melting point: 218 C
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(8) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[(S)-1-(tert.-butyloxycarbonyl)-
pyrrolidin-3-yloxy]-7-methoxy-quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
Rf value: 0.51 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(9) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[ 1-(tert.-butyloxycarbonyl)-
piperidin-3-yloxy]-7-methoxy-quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
Rf value: 0.56 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI"): m/z = 501, 503 [M-H]-
Boehringer Ingelheim Pharma KG 58 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(10) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-[2-
(3-methyl-2-oxo-hexahydropyrimidin-1-yl)-ethoxy]-quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
melting point: 235 C
Mass spectrum (ESI+): m/z = 516, 518 [M+H]+
(11) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[3-(tert.-butyloxycarbonylamino)-
cyclohexan-1-yloxy]-7-methoxy-quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
Rf value: 0.68 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI-): m/z = 515, 517 [M-H]"(12) 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-{cis-4-[N-(tert.-butyloxycarbonyl)-
N-methyl-amino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
Rf value: 0.37 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 531, 533 [M+H]+
(13) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[N-(tert.-
butyloxycarbonyl)-N-methyl-amino]-cyclohexan-1-yloxy}-7-methoxy-
quinazoline
Carried out with diisopropyl azodicarboxylate in methylene chloride.
melting point: 231 C
Mass spectrum (ESI+): m/z = 531, 533 [M+H]+
Example 2
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-amino-cyclohexan-1 -yloxy)-7-
methoxy-quinazoline x trifluoroacetic acid
Boehringer Ingelheim Pharma KG 59 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
F /
Cl ~ NH
O
INII \ \
N O NHZ
CH3
Prepared by treating 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(tert.-
butyloxycarbonylamino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline with
trifluoroacetic acid in methylene chloride at ambient temperature.
melting point: 221 C
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
The following compounds are obtained analogously to Example 2:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-amino-cyclohexan-1-
yloxy)-7-methoxy-quinazoline
F /
CI \ NH
\
N O
kN / O NI-12
CH3
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-
quinazoline x trifluoroacetic acid
F /
CI NH
\ \
N 0
NH
N O
1
CH3
melting point: 232 C
Boehringer Ingelheim Pharma KG 60 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 403, 405 [M+H]+
Example 3
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methanesulphonylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline
F
CI \ NH
N \ \ O O
I II
N O H Ise CH3
CH3
Prepared by reacting 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-amino-
cyclohexan-l-yloxy)-7-methoxy-quinazoline x trifluoroacetic acid with
methanesulphonic acid chloride in the presence of Hunig base in
tetrahydrofuran at ambient temperature.
Rf value: 0.77 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
40:10:1)
Mass spectrum (ESI+): mlz = 495, 497 [M+H]+
The following compounds are obtained analogously to Example 3:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methanesulphonylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline
F
CI NH
N O O
N OI "N S(CH3
CH3
Rf value: 0.20 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 495, 497 [M+H]+
Boehringer Ingelheim Pharma KG 61 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-
yloxy)-7-methoxy-quinazoline
F /
CI \ NH
N O --ON
N O "S
CH3 O CH3
Rf value: 0.59 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 481, 483 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[(3-chloro-
propyl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
F /
CI NH
N \ O O
II
~N O NS
I H O
CH3
The reaction takes place with 3-chioropropansuiphonyl chloride.
Rf value: 0.79 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI"): m/z = 555, 557, 559 [M-H]-
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(3-chloro-
propyl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
F /
CI NH
IN \ \ O O
N O H ~~
CH3
Boehringer Ingelheim Pharma KG 62 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The reaction takes place with 3-chloropropanesuiphonyl chloride.
Rf value: 0.42 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 557, 559, 561 [M+H]+
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methylcarbonyl-piperidin-4-
yloxy)-7-methoxy-quinazoline
F
CI NH
N \ `\ O
O
N O NY
I
CH3 CH3
The reaction takes place with acetic anhydride.
melting point: 216 C
Mass spectrum (ESI+): m/z = 445, 447 [M+H]+
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(dimethylamino)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
F
CI NH
N
O
kN ao
I NY
CH3 H3C,N,~ CH3
The reaction takes place with N,N-dimethylcarbamoylchloride.
Rf value: 0.28 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 474, 476 [M+H]+
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
Boehringer Ingelheim Pharma KG 63 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
F /
CI \ NH
N \ \ O
O N\ JO
1 `~
CH3 LIN)
O
The reaction takes place with (morpholin-4-yl)carbonylchloride in
acetonitrile.
Rf value: 0.37 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 516, 518 [M+H]+
(8) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(methoxymethyl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
F /
CI NH
o
N
N O N O
CH3
1
CH3
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.80 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 475, 477 [M+H]+
(9) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-7-
methoxy-quinazoline
F /
CI NH
O
IIII
Dr' \
N O
CH3
Boehringer Ingelheim Pharma KG 64 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The reaction takes place with bromocyanogen in methylene chloride.
Rf value: 0.40 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 428, 430 [M+H]+
(10) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(dimethylamino)sulphonyl]-
piperid in-4-yloxy}-7-methoxy-quinazoline
F
CI NH
O
N
NS~O
N O ~
1 0 1
CH3 H3C'~N,_ CH3
The reaction takes place with N,N-dimethylsulphamoylchloride in acetonitrile.
Rf value: 0.24 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 510, 512 [M+H]+
(11) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)sulphonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
F
CI \ NH
NIIII O
0-"ON O
N ~S
CH3 O WN
O
The reaction takes place with (morpholin-4-yl)sulphonyl chloride in
acetonitrile.
Rf value: 0.29 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Boehringer Ingelheim Pharma KG 65 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 552, 554 [M+H]+
(12) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-3-
yloxy)-7-methoxy-quinazoline
Rf value: 0.33 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 481, 483 [M+H]+
(13) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-1-methanesulphonyl-
pyrrolidin-3-yloxy)-7-methoxy-quinazoline
melting point: 249 C
Mass spectrum (ESI+): m/z = 467, 469 [M+H]+
(14) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-methanesulphonylamino-
ethyl)-piperidin-4-yloxy]-7-methoxy-quinazoline
Rf value: 0.49 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 524, 526 [M+H]+
(15) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-acetylamino-ethyl)-piperidin-
4-yloxy]-7-methoxy-quinazoline
The reaction takes place with acetic anhydride.
Rf value: 0.51 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 488, 490 [M+H]+
(16) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-
[(dimethylamino)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-
quinazoline
The reaction takes place with N,N-dimethylsulphamoylchloride in acetonitrile.
Rf value: 0.69 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 524, 526 [M+H]+
Boehringer Ingelheim Pharma KG 66 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(17) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride in
acetonitrile.
Rf value: 0.38 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
(18) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(morpholin-4-
yl)sulphonylamino]-cyclohexan-l-yloxy}-7-methoxy-quinazoline
The reaction takes place with (morpholin-4-yl)sulphonyl chloride in
acetonitrile.
melting point: 237 C
Mass spectrum (ESI-): m/z = 564, 566 (M-H]-
(19) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(3-methanesulphonylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline
Rf value: 0.66 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI-): m/z = 493, 495 (M-H]-
(20) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
acetylamino-ethoxy)-quinazoline
The reaction takes place with acetylchloride in acetonitrile.
melting point: 224 C
Mass spectrum (ESI+): m/z = 475, 477 [M+H]+
(21) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methanesulphonylamino-ethoxy)-quinazoline
melting point: 227 C
Mass spectrum (ESI+): m/z = 511, 513 [M+H]+
(22) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-3-acetylamino-cyclohexan-1-
yloxy)-7-methoxy-quinazoline
The reaction takes place with acetylchloride in acetonitrile. Cis- and trans-
isomer are separated by chromatography over a silica gel column.
Rf value: 0.43 (silica gel, methylene chloride/methanol/conc. aqueous
Boehringer Ingelheim Pharma KG 67 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459, 461 [M+H]+
(23) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-3-acetylamino-cyclohexan-
1-yloxy)-7-methoxy-quinazoline
The reaction takes place with acetylchloride in acetonitrile. Cis- and trans-
isomer are separated by chromatography over a silica gel column.
Rf value: 0.49 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 459, 461 [M+H]+
(24) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(3-
acetylamino-propyloxy)-quinazoline
The reaction takes place with acetylchloride.
melting point: 225 C
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(25) 4-[(3-chloro-4-fl uoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(3-
methanesulphonylamino-propyloxy)-quinazoline
melting point: 222 C
Mass spectrum (ESI+): m/z = 525, 527 [M+H]+
(26) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methanesulphonyl-piperidin-4-
yloxy)-quinazoline
Rf value: 0.44 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 451, 453 [M+H]+
(27) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-
piperidin-4-yloxy}-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride in
acetonitrile.
Rf value: 0.40 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 486, 488 [M+H]+
(28) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-
Boehringer Ingelheim Pharma KG 68 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
quinazoline
The reaction takes place with acetic anhydride.
Rf value: 0.50 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 415, 417 [M+H]+
(29) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(dimethylamino)carbonyl]-
piperidin-4-yloxy}-quinazoline
The reaction takes place with N,N-dimethylcarbamoylchloride.
Rf value: 0.47 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 444, 446 [M+H]+
(30) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-acetylamino-cyclohexan-
1-yloxy}-7-methoxy-quinazoline
The reaction takes place with acetic anhydride.
Rf value: 0.50 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 459, 461 [M+H]+
(31) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-
[(dimethylamino)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-
quinazoline
The reaction takes place with N,N-dimethylcarbamoylchloride.
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 488, 490 [M+H]+
(32) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(2-methoxy-acetylamino)-
cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(33) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-methoxy-acetyl)-piperidin-4-
yloxy]-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.41 (silica gel, methylene chloride/methanol = 9:1)
Boehringer Ingelheim Pharma KG 69 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 445, 447 [M+H]+
(34) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-isopropyloxycarbonyl-piperidin-
4-yloxy)-7-methoxy-quinazoline
The reaction takes place with isopropyl chloroformate.
Rf value: 0.67 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
98:2:1)
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(35) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-cyano-piperidin-4-yloxy)-
quinazoline
The reaction takes place with bromocyanogen in methylene chloride.
Rf value: 0.49 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI"): m/z = 396, 398 [M-H]-
(36) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(dimethylamino)sulphonyl]-
piperidin-4-yloxy}-quinazoline
The reaction takes place with N,N-dimethylsulphamoylchloride in acetonitrile.
Rf value: 0.34 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 480, 482 [M+H]+
(37) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)sulphonyl]-
piperidin-4-yloxy}-quinazoline
The reaction takes place with (morpholin-4-yl)sulphonyl chloride in
acetonitrile.
Rf value: 0.15 (silica gel, cyclohexane/ethyl acetate = 1:1)
Mass spectrum (ESI+): m/z = 522, 524 [M+H]+
(38) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-acetylamino-ethyl)-piperidin-
4-yloxy]-quinazoline
The reaction takes place with acetic anhydride in acetonitrile.
melting point: 221 C
Mass spectrum (ESI+): m/z = 458, 460 [M+H]+
Boehringer Ingelheim Pharma KG 70 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(39) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(diethylamino)carbonyl]-
piperid in-4-yloxy}-7-methoxy-quinazoline
The reaction takes place with N,N-diethylcarbamoylchloride.
Rf value: 0.40 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:1)
Mass spectrum (ESI+): m/z = 502, 504 [M+H]+
(40) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(piperidin-1-yl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
The reaction takes place with (piperidin-1-yl)carbonylchloride.
Rf value: 0.51 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:1)
Mass spectrum (ESI-): m/z = 512, 514 [M-H]-
(41) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(pyrrolidin-l-yl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
The reaction takes place with (pyrrolidin-1 -yl)carbonylchloride.
melting point: 237 C
Mass spectrum (ESI+): m/z = 500, 502 [M+H]+
(42) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(4-methyl-piperazin-1-
yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline
The reaction takes place with (4-methyl-piperazin-1-yl)carbonylchloride-
hydrochloride.
Rf value: 0.28 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI-): m/z = 527, 529 [M-H]-
(43) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-methanesulphonyl-N-
methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place in methylene chloride.
Rf value: 0.71 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 509, 511 [M+H]+
Boehringer Ingelheim Pharma KG 71 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(44) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-acetyl-N-methyl-amino)-
cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place with acetic anhydride.
melting point: 234 C
Mass spectrum (ESI+): m/z = 473, 475 [M+H]+
(45) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[N-(2-methoxy-acetyl)-N-
methyl-amino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.40 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 503, 505 [M+H]+
(46) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-dimethylaminocarbonyl-
N-methyl-amino)-cyclohexan-l-yloxy]-7-methoxy-quinazoline
The reaction takes place with N,N-dimethylcarbamoylchloride.
Rf value: 0.51 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 502, 504 [M+H]+
(47) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(morpholin-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-
quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.50 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 544, 546 [M+H]+
(48) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(morpholin-4-
yl)sulphonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-
quinazoline
The reaction takes place with (morpholin-4-yl)sulphonyl chloride in
acetonitrile.
Rf value: 0.24 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 580, 582 [M+H]+
Boehringer Ingelheim Pharma KG 72 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(49) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(N-dimethylaminosulphonyl-
N-methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place with N,N-dimethylsulphamoylchloride in acetonitrile.
Rf value: 0.53 (silica gel, ethyl acetate)
Mass spectrum (ESI+): m/z = 538, 540 [M+H]+
(50) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-ethanesulphonylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline
The reaction takes place with ethanesulphonic acid chloride in methylene
chloride.
Rf value: 0.41 (silica gel, ethyl acetate)
Mass spectrum (ESI+): mlz = 509, 511 [M+H]+
(51) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-
piperidin-4-yloxy}-7-ethoxy-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.48 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
(52) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-meth anesulphonyl-piperidin-4-
yloxy)-7-ethoxy-quinazoline
Rf value: 0.50 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 495, 497 [M+H]+
(53) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-methoxy-acetyl)-piperidin-4-
yloxy]-7-ethoxy-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.40 (silica gel, methylene chloride/methanol = 20:1)
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(54) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-meth anesulphonyl-piperidin-4-
yloxy)-7-(2-methoxy-ethoxy)-quinazoline
Rf value: 0.47 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 525, 527 [M+H]+
Boehringer Ingelheim Pharma KG 73 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(55) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-
piperidi n-4-yloxy}-7-(2-methoxy-ethoxy)-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.48 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 560, 562 [M+H]+
(56) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-methoxy-acetyl)-piperidin-4-
yloxy]-7-(2-methoxy-ethoxy)-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.48 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 519, 521 [M+H]+
(57) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-acetylamino-cyclohexan-1-
yloxy)-7-methoxy-quinazoline
The reaction takes place with acetic anhydride.
melting point: 281 C
Mass spectrum (ESI+): m/z = 459, 461 [M+H]+
(58) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[cis-4-(2-methoxy-acetylamino)-
cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place with methoxyacetic acid chloride.
melting point: 264 C
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(59) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(piperidin-1-
yl)carbonyl]-
N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline
The reaction takes place with (piperidin-1-yl)carbonylchloride.
melting point: 253 C
Mass spectrum (ESI+): m/z = 542, 544 [M+H]+
(60) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(4-methyl-piperazin-1-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-
quinazoline
Boehringer Ingelheim Pharma KG 74 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The reaction takes place with (4-methyl-piperazin-1-yl)carbonylchloride-
hydrochloride.
melting point: 262 C
Mass spectrum (ESI+): m/z = 557, 559 [M+H]+
(61) 4-[(3-chIoro-4-fluoro-phenyl)amino]-6-[cis-4-(N-ethanesulphonyl-N-
methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
The reaction takes place with ethanesulphonic acid chloride in methylene
chloride.
Rf value: 0.19 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 523, 525 [M+H]+
(62) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[(morpholin-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.33 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
(63) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{cis-4-[(morpholin-4-
yl)sulphonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
The reaction takes place with (morpholin-4-yl)sulphonyl chloride in
acetonitrile.
Rf value: 0.81 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 566, 568 [M+H]+
(64) 4-[(3-ethynyl-phenyl)amino]-6-(1-acetyl-piperidin-4-yloxy)-7-methoxy-
quinazoline
The reaction takes place with acetic anhydride.
Rf value: 0.30 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 417 [M+H]+
CA 02476008 2004-10-28
25771-944
(65) 4-[(3-ethynyl-phenyl)amino]-6-[1-(2-methoxy-acetyl)-piperidin-4-yloxy]-7-
methoxy-quinazoline
The reaction takes place with methoxyacetic acid chloride.
Rf value: 0.37 (silica gel, methylene chloride/methanoUconc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447 [M+H]+
(66) 4-[(3-ethynyl-phenyl)amino]-6-(1-methanesulphonyl-pipe(din-4-yloxy)-7-
methoxy-quinazoline
Rf value: 0.59 (silica gel, ethyl acetate/methanoUconc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 453 [M+H]+
(67) 4-[(3-ethynyl-phenyl)amino]-6-{1-[(morpholin-4-yl)carbonyl]-piperidin-4-
yloxy)-7-methoxy-quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.43 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 488 [M+H]+
(68) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[trans-4-(N-methanesulphonyl-N-
methyl-amino)-cyclohexan-1-yloxy]-7-methoxy-quinazoline
Rf value: 0.50 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 509, 511 [M+H]+
(69) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-{N-[(morpholin-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-
quinazoline
The reaction takes place with (morpholin-4-yl)carbonylchloride.
Rf value: 0.54 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 544, 546 [M+H]+
CA 02476008 2004-10-28
25771-944
76
Example 4
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{[3-(morpholin-4-yl)-
propyl]sulphonylamino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline
F
CI \ NH
N O rO
N O N
I H O
CH3
23 pl of morpholine are added to 60 mg of 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-{cis-4-[(3-chloro-propyl)sulphonylamino]-cyclohexan-1-
yloxy}-7-methoxy-quinazoline in 2 ml acetonitrile and the reaction mixture is
refluxed overnight. For working up the mixture is taken up in ethyl acetate
and
washed with water. The organic phase is dried over magnesium sulphate and
evaporated down. The crude product is purified through a silica gel column
with methylene chloride/methanol (9:1) as eluant.
Yield: 18 mg (27% of theory)
Rf value: 0.36 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 608, 610 [M+H]+
The following compounds are obtained analogously to Example 4:
Boehringer Ingelheim Pharma KG 77 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-{[3-(morpholin-4-yl)-
propyl]sulphonylamino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline
F
Cl NH
O
li \ \ )O
N C H/ O N
CH3
Rf value: 0.16 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 608, 610 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-[4-
(morpholin-4-yl)-butyloxy]-quinazoline
Carried out in the presence of sodium carbonate and sodium iodide in N-
methylpyrrolidone at 100 C.
Rf value: 0.18 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
40:10:0.5)
Mass spectrum (ESI+): m/z = 531, 533 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-[4-
(morpholin-4-yl)-butyloxy]-quinazoline
Carried out in the presence of sodium carbonate and sodium iodide in N-
methylpyrrolidone at 100 C.
Rf value: 0.32 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
80:20:1)
Mass spectrum (ESI+): m/z = 517, 519 [M+H]+
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)acetyl]-
piperidin-4-yloxy}-quinazoline
Carried out in the presence of Honig base in tetrahydrofuran at ambient
temperature.
Boehringer Ingelheim Pharma KG 78 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Rf value: 0.30 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 500, 502 [M+H]+
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-dimethylaminoacetyl-piperidin-
4-yloxy)-quinazoline
Carried out in the presence of Hunig base in tetrahydrofuran at ambient
temperature.
Rf value: 0.11 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 458, 460 [M+H]+
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-dimethyI aminoacetyl-piperidin-
4-yloxy)-7-methoxy-quinazoline
Carried out in the presence of Hunig base in tetrahydrofuran at ambient
temperature.
Rf value: 0.19 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 488, 490 [M+H]+
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-yl)acetyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
Carried out in the presence of Hunig base in tetrahydrofuran at ambient
temperature.
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
Example 5
4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-pyrrolidin-3-yloxy)-7-methoxy-
quinazoline-dihyd rochloride
A solution of 370 mg 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[(S)-1-(tert.-
butyloxy-carbonyl)-pyrrolidin-3-yloxy]-7-methoxy-quinazoline in 5 ml dioxane
is combined with 0.32 ml concentrated hydrochloric acid and stirred overnight
at ambient temperature. The precipitate formed is suction filtered and washed
Boehringer Ingelheim Pharma KG 79 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
with copious amounts of dioxane. The crude product is dissolved in a little
methanol and re-precipitated by the addition of the same amount of ethyl
acetate. The white solid thus obtained is suction filtered and dried.
Yield: 200 mg (57 % of theory)
melting point: 281 C
Mass spectrum (ESI+): m/z = 389, 391 [M+H]+
The following compounds are obtained analogously to Example 5:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-3-yloxy)-7-methoxy-
quinazoline-dihydrochloride
melting point: 263 C
Mass spectrum (ESI+): m/z = 403, 505 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-amino-ethyl)-piperidin-4-
yloxy]-7-methoxy-quinazoline-dihydrochloride
melting point: 277 C
Mass spectrum (ESI+): m/z = 446, 448 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(3-amino-cyclohexan-1-yloxy)-7-
methoxy-quinazoline-dihydrochloride
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
amino-ethoxy)-quinazoline-dihydrochloride
Carried out with isopropanolic hydrochloric acid (5-6 M) in methylene
chloride.
Rf value: 0.58 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 433, 435 [M+H]+
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(3-
amino-propyloxy)-quinazoline-dihydrochloride
Carried out with isopropanolic hydrochloric acid (5-6 M) in methylene
chloride.
Rf value: 0.44 (Reversed phase ready-made TLC plate (E. Merck),
CA 02476008 2004-10-28
25771-944
methanol/5% aqueous sodium chloride solution = 7:3)
Mass spectrum (ESI+): m/z = 447, 449 [M+H]+
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-[1-(2-amino-ethyl)-piperidin-4-
yloxy]-quinazoline-dihydrochloride
Rf value: 0.50 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 416, 418 [M+H]+
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-methylamino-cyclohexan-1-
yloxy)-7-methoxy-quinazoline-dihydrochloride
Carried out with isopropanolic hydrochloric acid (5-6 M) in methylene
chloride.
Rf value: 0.35 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 431, 433 [M+H]+
(8) 4-[(3-ethynyl-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-quinazoline-
dihydrochloride
Carried out with isopropanolic hydrochloric acid (5-6 M) in methylene
chloride.
Rf value: 0.50 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 375 [M+H]+
(9) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-methylamino-cyclohexan-
1-yloxy)-7-methoxy-quinazoline-dihydrochloride
melting point: 251 C
Mass spectrum (ESI+): m/z = 431, 433 [M+H]+
CA 02476008 2004-10-28
25771-944
81
Example 6
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-hydroxy-
quinazoline
A mixture of 9.00 g 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-
yloxy)-7-benzyloxy-quinazoline-hydrochloride and 50 ml trifluoroacetic acid is
heated to 100 C for 1.5 hours. Then the reaction mixture is evaporated and
the residue is taken up in 10 ml acetonitrile. This solution is added dropwise
to
100 ml saturated sodium hydrogen carbonate solution with vigorous stirring.
After 1.5 hours the precipitate formed is suction filtered and washed several
times with water. The crude product is stirred with diethyl ether, suction
filtered and dried.
Yield: 5.90 g (87 % of theory)
Rf value: 0.21 (silica gel, ethyl acetate)
Boehringer Ingelheim Pharma KG 82 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 390, 392 [M+H]+
The following compounds are obtained analogously to Example 6:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-
hydroxy-quinazoline
Rf value: 0.44 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 376, 378 [M+H]+
Example 7
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-[3-
(morpholin-4-yl)-propyloxy]-quinazoline
A mixture of 300 mg 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-
yloxy)-7-hydroxy-quinazoline, 130 mg 3-(morpholin-4-yl)-propylchloride and
530 mg potassium carbonate in 5 ml N,N-dimethylformamide is stirred
overnight at 80 C. For working up the reaction mixture is diluted with 25 ml
of
water and extracted with ethyl acetate. The combined organic phases are
washed with saturated sodium chloride solution, dried over magnesium
sulphate and evaporated. The residue is stirred with diethyl ether, suction
filtered and dried.
Yield: 250 mg (63 % of theory)
melting point: 205 C
Mass spectrum (ESI+): m/z = 517, 519 [M+H]+
The following compounds are obtained analogously to Example 7:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-[2-
(morpholin-4-yl)-ethoxy]-quinazoline
Rf value: 0.33 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
40:10:0.5)
Mass spectrum (ESI+): m/z = 503, 505 [M+H]+
Boehringer Ingelheim Pharma KG 83 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-
ethoxy-quinazoline
Rf value: 0.76 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 418, 420 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-[3-
(morpholin-4-yl)-propyloxy]-quinazoline
Rf value: 0.20 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI-): m/z = 501, 503[M-H]-
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-((S)-tetrahydrofuran-3-yloxy)-7-[2-
(morpholin-4-yl)-ethoxy]-quinazoline
Rf value: 0.19 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 489, 491 [M+H]+
(5) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-(2-
methoxy-ethoxy)-quinazoline
Rf value: 0.57 (silica gel, methylene chloride /methanol = 9:1)
Mass spectrum (ESI+): m/z = 448, 450 [M+H]+
(6) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-[2-
(tert.-butyloxycarbonylamino)-ethoxy]-quinazoline
Rf value: 0.64 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:0.1)
Mass spectrum (ESI+): m/z = 533, 535 [M+H]+
(7) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(tetrahydropyran-4-yloxy)-7-[3-
(tert.-butyloxycarbonylamino)-propyloxy]-quinazoline
Rf value: 0.74 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:0.1)
Mass spectrum (ESI+): m/z = 547, 549 [M+H]+
Boehringer Ingelheim Pharma KG 84 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example 8
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-quinazoline
A solution of 4.55 g 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-trifluoroacetyl-
piperidin-4-yloxy)-quinazoline-hydrochloride in 35 ml methanol is combined
with 13 ml (3 N) sodium hydroxide solution and stirred for about half an hour
at ambient temperature. For working up the reaction mixture is diluted with
water and extracted with ethyl acetate. The combined organic phases are
washed with saturated sodium chloride solution, dried over magnesium
sulphate and evaporated. The residue is stirred with diethyl ether, suction
filtered and dried.
Yield: 3.00 g (89 % of theory)
Rf value: 0.48 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 373, 375 [M+H]+
The following compounds are obtained analogously to Example 8:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-ethoxy-
quinazoline
Rf value: 0.20 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 417, 419 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-(2-methoxy-
ethoxy)-quinazoline
Rf value: 0.10 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 447, 449 [M+H]+
Example 9
Boehringer Ingelheim Pharma KG 85 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(ethylamino)carbonyl]-piperidin-4-
yloxy}-7-methoxy-quinazoline
Prepared by reacting 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-
7-methoxy-quinazoline with ethyl isocyanate in tetrahydrofuran at ambient
temperature.
Rf value: 0.53 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 474, 476 [M+H]+
The following compounds are obtained analogously to Example 9:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(isopropylamino)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
melting point: 236 C
Mass spectrum (ESI"): m/z = 486, 488 [M-H]-
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(phenylamino)carbonyl]-
piperidin-4-yloxy}-7-methoxy-quinazoline
Rf value: 0.70 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
95:5:0.1)
Mass spectrum (ESI+): m/z = 522, 524 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(ethylamino)carbonyl]-N-
methyl-amino}-cyclohexan-1-yloxy)-7-methoxy-quinazoline
Rf value: 0.38 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 502, 504 [M+H]+
Example 10
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(dimethylamino)carbonylmethyl]-
piperidin-4-yloxy}-quinazoline
Prepared by reacting 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-
quinazoline with 2-chloro-N,N-dimethylacetamide in the presence of
CA 02476008 2004-10-28
25771-944
86
potassium carbonate in N,N-dimethylformamide at ambient temperature.
Rf value: 0.24 (silica gel, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 458, 460 [M+H]+
The following compounds are obtained analogously to Example 10:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-
yl)carbonylmethyl]-piperid in-4-yloxy}-quinazoline
Rf value: 0.42 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 500, 502 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-aminocarbonylmethyl-piperidin-
4-yloxy)-7-methoxy-quinazoline
melting point: 251 C
Mass spectrum (ESI+): m/z = 460, 462 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-
[(dimethylamino)carbonylmethyl]-piperidin-4-yloxy}-7-methoxy-
quinazoline
melting point: 233 C
Mass spectrum (ESI+): m/z = 488, 490 [M+H]+
(4) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(morpholin-4-
yl)carbonylmethyl]-piperid in-4-yloxy}-7-methoxy-quinazoline
melting point: 245 C
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
CA 02476008 2004-10-28
25771-944
87
Example 11
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(tetrahydropyran-4-yl)carbonyl]-
p i perid i n-4-yloxy}-7-methoxy-q u i n azo line
90 mg 1-hydroxy-1 H-benzotriazole and 250 mg 2-(1 H-benzotriazol-1-yl)-
1,1,3,3-tetramethyluronium-tetrafluoroborate are added to a mixture of 300
mg 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-yloxy)-7-methoxy-
quinazoline-dihydrochloride, 82 mg tetrahydropyran-4-carboxylic acid and
0.54 ml Honig base in 5 ml N,N-dimethylformamide. The reaction mixture is
stirred overnight at ambient temperature. For working up it is combined with
25 ml ethyl acetate and washed with water, 10% potassium carbonate
solution and saturated sodium chloride solution. The organic phase is dried
over magnesium sulphate and evaporated. The residue is stirred with a little
ethyl acetate, suction filtered and dried.
Yield: 250 mg (77 % of theory)
Rf value: 0.43 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 515, 517 [M+H]'
The following compounds are obtained analogously to Example 11:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{trans-4-[(tetrahydropyran-4-
yl)carbonylamino]-cyclohexan-1-yloxy}-7-methoxy-quinazoline
Rf value: 0.44 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 529, 531 [M+H]+
CA 02476008 2004-10-28
25771-944
88
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(cis-4-{N-[(tetrahydropyran-4-
yl)carbonyl]-N-methyl-amino}-cyclohexan-l-yloxy)-7-methoxy-
quinazoline
Rf value: 0.31 (silica gel, ethyl acetate/methanol = 9:1)
Mass spectrum (ESI+): m/z = 543, 545 [M+H]+
Example 12
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[([1,4]oxazepan-4-yl)carbonyl]-
piperidin-4-yloxy}-7-methoxy-q u inazoline
4-[(3-ch loro-4-fluoro-phenyl)amino]-6-(piperid in-4-yloxy)-7-methoxy-
quinazoline-dihydrochloride and 1.05 ml triethylamine are added to 900 mg of
1-methyl-3-[([1,4]oxazepan-4-yl)carbonyl]-3H-imidazol-1-ium-iodide in 10 ml
methylene chloride. The yellowish suspension is stirred for about 24 hours at
ambient temperature. For working up the reaction mixture is combined with 50
ml methylene chloride and extracted with water as well as 10% citric acid. The
organic phase is washed with saturated sodium chloride solution, dried over
magnesium sulphate and evaporated down. The residue is chromatographed
CA 02476008 2004-10-28
25771-944
89
through a silica gel column with methylene chloride/methanol/conc. ammonia
as eluant. The desired product is stirred with diethyl ether, suction filtered
and
dried.
Yield: 800 mg (80 % of theory)
Rf value: 0.30 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
The following compounds are obtained analogously to Example 12:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(cis-2,6-dimethyl-morpholin-4-
yl)carbonyl]-piperidin-4-yloxy}-7-methoxy-quinazoline
Rf value: 0.41 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 544, 546 [M+H]+
(2) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[(2-methyl-morpholin-4-
yl)carbonyl]-piperid in-4-yloxy}-7-methoxy-quinazoline
Rf value: 0.50 (silica gel, ethyl acetate/methanol/conc. aqueous ammonia =
90:10:1)
Mass spectrum (ESI+): m/z = 530, 532 [M+H]+
Boehringer Ingelheim Pharma KG 90 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Mass spectrum (ESI+): m/z = 532, 534 [M+H]+
Example 13
4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-ethoxy-
quinazoline
35 pl 37 % aqueous formalin solution and 110 mg of sodium
triacetoxyborohydride are added to 175 mg 4-[(3-chloro-4-fluoro-
phenyl)amino]-6-(piperidin-4-yloxy)-7-ethoxy-quinazoline in 1 ml of
tetrahydrofuran. The reaction mixture is stirred for about four hours at
ambient
temperature. For working up 5 ml saturated sodium hydrogen carbonate
solution are added and the mixture is stirred thoroughly. Then 20 ml ethyl
acetate are added and the aqueous phase is separated off. The organic
phase is washed with water and saturated sodium chloride solution, dried
over magnesium sulphate and evaporated. The residue is stirred with
diisopropylether, suction filtered and dried.
Yield: 144 mg (80 % of theory)
Rf value: 0.80 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 60:10:1)
Mass spectrum (ESI+): m/z = 431, 433 [M+H]+
The following compounds are obtained analogously to Example 13:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(1-methyl-piperid in-4-yloxy)-7(2-
methoxy-ethoxy)-quinazoline
Rf value: 0.85 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 60:10:1)
Mass spectrum (ESI+): m/z = 461, 463 [M+H]+
(2) 4-[(3-ethynyl-phenyl)amino]-6-(1-methyl-piperidin-4-yloxy)-7-methoxy-
quinazoline-hydrochloride
Rf value: 0.26 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
CA 02476008 2004-10-28
25771-944
91
Mass spectrum (ESI+): m/z = 389 [M+H]+
(3) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(trans-4-dimethylamino-
cyclohexan-1-yloxy)-7-methoxy-quinazoline
Rf value: 0.80 (aluminium oxide, methylene chloride/methanol = 9:1)
Mass spectrum (ESI+): m/z = 445, 447 [M+H]+
Example 14
4-[(3-ethynyl-phenyl)amino]-6-[1-(tert.-butyloxycarbonyl)-piperidin-4-yloxy]-7-
methoxy-quinazoline
A mixture of 3.00 g 4-[(3-ethynyl-phenyl)amino]-6-hydroxy-7-methoxy-
quinazoline, 4.50 g 1-(tert.-butyloxycarbonyl)-4-(p-toluolsulphonyloxy)-
piperidin and 2.90 g potassium carbonate in 30 ml N,N-dimethylformamide is
CA 02476008 2004-10-28
25771-944
92
stirred for two days at 60 C. For working up the mixture is combined with 200
ml ethyl acetate and extracted with water. The organic phase is washed with
saturated sodium chloride solution, dried over magnesium sulphate and
evaporated. The crude product is purified over a silica gel column with
methylene chloride/methanol/conc. ammonia as eluant.
Yield: 3.25 g (67 % of theory)
Rf value: 0.25 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 95:5:1)
Mass spectrum (ESI+): m/z = 475 [M+H]+
The following compounds are obtained analogously to Example 14:
(1) 4-[(3-ethynyl-phenyl)amino]-6-(tetrahydropyran-4-yloxy]-7-methoxy-
quinazoline
Rf value: 0.40 (silica gel, methylene chloride/methanol/conc. aqueous
ammonia = 90:10:1)
Mass spectrum (ESI+): m/z = 376 [M+H]+
CA 02476008 2004-10-28
25771-944
93
Example 15
4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[2-(tert.-butyloxycarbonylamino)-
ethyl]-piperidin-4-yloxy}-7-methoxy-quinazoline
A mixture of 410 mg of 4-[(3-chloro-4-fluoro-phenyl)amino]-6-(piperidin-4-
yloxy)-7-methoxy-quinazoline-dihydrochloride, 240 mg of N-(tert.-
butyloxycarbonyl)-2-bromo-ethylamine and 360 mg of potassium carbonate in
ml N,N-dimethylformamide is stirred overnight at ambient temperature. Then
another 80 mg N-(tert.-butyloxycarbonyl)-2-bromo-ethylamine are added and
the reaction mixture is stirred for a further four hours at ambient
temperature.
For working up it is diluted with water and extracted with ethyl acetate. The
combined organic phases are washed with saturated sodium chloride
solution, dried over magnesium sulphate and evaporated. The residue is
CA 02476008 2004-10-28
25771-944
94
chromatographed through a silica gel column with ethyl acetate/methanol
(95:5 to 90:1) as eluant.
Yield: 370 mg (79 % of theory)
Rf value: 0.33 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI-): m/z = 544, 546 [M-H]-
The following compound is obtained analogously to Example 15:
(1) 4-[(3-chloro-4-fluoro-phenyl)amino]-6-{1-[2-(tert.-
b utyloxycarbonylamino)-ethyl]-piperid in-4-yloxy}-q uinazoline
Rf value: 0.38 (Reversed phase ready-made TLC plate (E. Merck),
acetonitrile/water/ trifluoroacetic acid = 50:50:1)
Mass spectrum (ESI+): m/z = 516, 518 [M+H]+
The following compounds may also be prepared analogously to the foregoing
Examples and other methods known from the literature:
Boehringer Ingelheim Pharma KG 95 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
( 1) F C)
CI \ I N
O
I
O~UCY
(2) F
1\
CI N 9
\ \ O
O
CI \ I N
N L O
00,
CI \ I N
N \ O
N
(5) F I `r )
CI \ 1 N
\ \ O
N
N OZO
CI \ I N Y
N \ \ O
~N O"~Nj-j
(7) F 7
CI \ I N
\ a00""'N
N F
(8) CI \ I ~ C)
N
N( \ \ O I^
Boehringer Ingelheim Pharma KG 96 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
CI I N
O
N ON"'
(10) F C)
CI aN
N L \ O
N O
(11) ` ~I C1
CI \ N
N~I L O
N ON~\/O\
O\
(12) F O~
CI \ N 9
O
N ~ O'er
N
(13) I)\ N C)
CI
O
N O------
ON"
(14) J ~ IN `l'
('
CI \'
O
ON,-,,
(15) )O C)
CI ~ N
O
N( L
CI I N
(16) J
N
N O~\N ,N
Boehringer Ingeiheim Pharma KG 97 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(17)
CI \ N
N O
N ON' `
(18) I ~ ~ 90
CI \ N N O~-N N
0
CI \ N
(19) F
N Oi\
O
(20) F aN
CI N 0
(21) F 1 0
CI \ I N
\ \ O
N II
0
CI a N
N
(22) I 7
O IN
N O-~-N~
(23) I
CI \ N
N
k'N\oO
"N6
(24) F C )
CI \ N
~N ~ ON
Boehringer Ingelheim Pharma KG 98 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
CI \ I N
(25) F /
N \ \ O
Q N / O^-~-N 00
(26) F /
CI \ I N
\
(N N O"`""'N
O
(27) CI \ N Y
i \ O
N ON 00
(28) F /
CI \ N
N \
/ \O
OQC/\
N
0
(29) F /
CI \ I N
O
N \ \
N 0 N~ N\/
00
(30) F /
N
CI~
Nl \ :X,
ON If N~
T 00
(31) F /
CI \ I N
O
N \, \
N Nom/
(32) F /
CI \ N
N \ O` ^ O
Boehringer Ingelheim Pharma KG 99 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(33) F all
CI N
N \ O` ~ O
(34) F
CI \ N
N
OO
(35) F /
CI \ N
\ p
I
~ / Q Nom./'\~~\
N 0
(36) F /
CI \ N
N
O
O
N N
O
(37) F /
CI \ N
N O vNv N
(38) F /
CI \ N
N ON v 'N
I
(39) F /
CI \ N
N O` ^NJN/
7`vl
(40) F /
CI \ N
N-,~~, I'll O"ONJ
o
Boehringer Ingelheim Pharma KG 100 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(41) F /
CI \ N
~---
/ O-ON 'N
0
(42) F /
CI \ I N
I=N
(43) F / i
Cl \ N /\
NN O~`7 1N- Nlp
I` ~ I /jj N
(44) F /
CI \ N
N
\/ I (45) F aN
CO
LN N
(46) F / I
CI \ N
\ O\^ O~-N /
N / n 7lvlN\/\~N
N
(47) F /
N
CI \ I
N \
N OQ N""N'tN
(48) F / I
Cl \ N R
N / 0 ON~"N N/
Li
Boehringer Ingelheim Pharma KG 101 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(49) F / I
CI \ N
N'-!'!" c~o~\~N
V
(50) F /
CI \ N
N(I
N / n N~,~~N
(51) F /
CI \ N
`N O\ /\ N
(52) F /
CI \ I N
N O\ ^ N
~O
(53) F /
CI \ N
LO
N N N
00
(54) F /
CI \ N \J/~~'
i / O\/ QQ
N~,,N,
N \
0
(55) F / I
Cl \ N
' \ O` ^
0
(56) F /
CI \ I N
N ~.a
NyN
0
Boehringer Ingelheim Pharma KG 102 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(57) F /
CI' 'N
II` / q N
Nõ \ O \/
N
0
(58) F /
CI \ I N
~lN
N O` ^ ~ N
0
(59) F /
CI \ N
\
~N Oq NN
0
(60) F /
CI \ N
N-,,; \ O` ^ N
qI
(61) F /
CI \ N
`ry QO(N
(62) F /
CI \ I N
lvlN
(63) F /
CI \ I N
ry~\ \ O~, gp
N / N'illl\
1 O
(64) F /
CI \ I N
N OO---\N
Boehringer Ingelheim Pharma KG 103 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(65) F /
CI \ N
O v _N
(66) F
CI N
N N
(67) F /
CI/ v 'N
N
I
(68) F /
CI \ N
NN
L2
(69) F /
CI \ N
N O~N~
1 ,N
(70) F /
CI \ N
O, O
N N" N
(71) F /
CI \ N
N O
\
i\ / N N
(72) F /
CI \ N
O
N \
kN / Q N'N
Boehringer Ingelheim Pharma KG 104 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(73) F
CI \ ! N
N N
(74) F !!
CI' O 'N
O
N \ \
N~ N" -N' (75) F
CI \ I N
O
\ \
N N" _
~N~ N~
(76) F
CI \ ! N
N O^yN
N N 0
(77) F
CI \ ! N
O
N NN,
0
(78) F
CI \ ! N
O
N N-
Y tt N~
OII
(79) F
CI \ I N
II`
N O ~`N 0
(80) F
CI \ ! N
N'~ N\/
0
Boehringer Ingelheim Pharma KG 105 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(81) F
CI N
N
(82) F
CI \ N
N / OON ~,N
I
(83) F \ I/
CI N
N N O-aN~'
(84) F /
CI \ N
N O aN~ I.
(85) F /
CI \ N
~N / o-aN~/NJ
(86) F /
N
CI \
N \ O 0,
/
N
(87) F /
CI \ N
NII \\O
N / N
I I
O
(88) F /
CI \ I N
\ \
N
I
I0
Boehringer Ingelheim Pharma KG 106 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(89) F /
CI \ ( N
N
I
(90) F aN
C~(91) F /
CI \ N
II\ ^/
N, O V\N 0 N
(92) F /
CI \ N
INI \ O ON
~N,:T
D
(93) F /
CI \ I N
Nõ N O--aN------\N O
(94) F aN
CN \ \
N / OQ N---~-N" _N-
(95) F /
CI \ N
N Q N""~ N
(96) F /
CI ~ N
0
ryIIl
`N / N""N
Boehringer Ingelheim Pharma KG 107 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(97) 0
N)"
N O^~~ l
(98)
/ \ I N
O
N 0"~ N0O
(99) Br \ N 9
N \ \
,N O""-"-"N
L0
(100)
~ o
CI \ I N Y
N N
(101)
F \
CI \ I N 9
N O' '~
0
(102) I N O/ i N O
`N o'er`
O
(103) C )
N \ N ~J
N O~~N~
Boehringer Ingelheim Pharma KG 108 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(104) C
N N
N O"'N
O
(105) /I
N \ N
N
N 0------N
O
(106) )01N Y
C
NI \ \ O ^^
~N /
(107) F aN
CI N
(108) F
CI \ N
O
NIIII \ \
N N 00
-ll
(109) F n',
CI N
O
o
N
(110) F /
CI \ N
O
N\
(111) F /I
CI \ N
7I \
-0-ON
N Y
i
0
Boehringer Ingelheim Pharma KG 109 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(112) F a--l.,
CI' ~Or OyO
O
(113) F /
CI \ N
ON
N
-N
(114) F /
CI \ N
N 0"'a
N N
(115) F aN
CO'CN
(116) F /
CI \ N
N~ i j 0.,,
N 0 NI
(117) F /
Cl \ N
N % O ^ O~
N O N/\i \))
(118) F /
CI \ N
NI O. O
N O aN -,
(119) F /
CI \' N
N 0
~--
~, O N/-NA
Boehringer Ingelheim Pharma KG 110 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(120)
CI \ N
N a--- O"'aO
N O N"~'N 0 N
(121) F aN
CNN O
~N-
N O
(122) F /
CI \ N
N - O
I
N O~
(123) F
CI N
O 0
NI \ \ N
~N / O
I
(124) F o
CI \ N \
~,(N
N O N \~
N O
(125) F
CI \ N
II \ \ O
~N / oN CO
I
(126) F aN
CINI
`N O N
0
Boehringer Ingelheim Pharma KG 111 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(127) /
CI \ I N
kN oN~ O\
101
(128) F
CI \ N
N \ 0'-ON
N O O
IOI
(129) F'I
CI \ N
NI-\ xoo
0
(130) F
CI \ N
O
N
N 0 NN~
O 00
(131) F n::,
CI N
N~I \ \ O
`N~ / n NuN~~~O~
T O
(132) F
CI \ N
N 0
I
N\ 0 N 0
(133) F
CI \ N
N( O
Nu N,
N O
O
Boehringer Ingelheim Pharma KG 112 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(134) F /
CI \ N
lI \ \
O
\ N N
N u
/
1 O
(135) F aN
CINII \
O
`N -ON
(136) F /
CI \ N
O
N Ny
O
(137) F aN
CIN O O
- / N N
N i T
O
(138) F / I
CI \ N
N, N / 0O-ON N!! O
(139) F /
CI \ N
Nl \
xoE
F'j, F
Boehringer Ingelheim Pharma KG 113 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(140) F / 1
CI \ N
O ro
)ao N`~
0
1
1
(141) F
CI \ N
0
N \ 0
-N\ O ON V v N~
O
(142) F /
CI \ N
NlI \ ao
Q~N=~/`\
(143) F
CI N
0--ON
Gao I I
(144) F /
CI \ N
\ O
N / O N" O\
(145) F
CI \ N
NC'-,-
N), O (146) F /
CI \ N
\ O
~N ON'-'-'N
Boehringer Ingelheim Pharma KG 114 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(147) F
CI
N - O Ou
NN ON~/~NN/\
(148) F
CI \ N
O O
O N - ~N 0 N
(149) F
CI N
N O
kN / N~N 0
N
(150) F aN
CNN', N
(151) F /
CI \ N
O
N
~N O
0
(152) F /
CI \ N
N)
NI ~/
O N~/N II
0
(153) F /
CI \ N
Nl
\N /O ON,.,~N~ N~~
101
Boehringer Ingelheim Pharma KG 115 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(154) F
CI
N O
\N / O N/NuN
O
(156) F
CI \ N
NI 0
l J
N,N O
\N / O
U
O
(157) F /
CI NH
NII ~ Na O-ON,,,~N
O
(158) F /
CI NH
O
INIII ~ O
NN
N'
O
(159) F /
CI \ NH
N~ a O
O
N_,-,,_,N
N O O
(160) F /
CI \ N
NlI ~ \ o
\N / ON , ~N /
0
Boehringer Ingelheim Pharma KG 116 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(161) / 1
F \ 0
CI// N
0
\ \
N
0
(162)
F/~\ ~
CI N
xo
(163)
N /~\ I N
\ 0
e \ O
N / N
(164) ~
N CI 1 N
INI xo
(165) / I
N CI \ NH
NIIII \ \
N / ON
(166) F / I
CI N
\ \
N / O
(167) F / I
CI \ NH 0
N \ \
N / O-0
Boehringer Ingelheim Pharma KG 117 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(168) F
CI N 0
~N / O v
(169) F /
CI \ I N 0
NlI ao-O N `~N O O
(170) /
CI I \ I N
N
~N
,N N
O
(171) F
CI ~ N
NlI O
C-\N
N
O
(172) F /
CI \ N
N O rO
N
O
(173) F /
CI a N
N \ \ O
NyN
\N / 0,-,,
0
Boehringer Ingelheim Pharma KG 118 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(174) F / I
Cl N
N 0
O
N O--"--N
O
(175)
CI \ I N
N 0
II
O
(176) F /
CI \ N
N O
IN Y
O
(178) F /
CI N L O
0~/N
O
(179) F i l o
CI \ N
NIIII " O
~N / Oil/N~Oi
0
(180) F C
N O
CI \ N Y
IO
IN O~\/N
O
Boehringer Ingelheim Pharma KG 119 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example No. Structure
(181) F I
CI
eN- \ O
O~/N OS~/\
I I
O
(182) /
CI \ I N
N \ ao,--,N O
'NUN
IO
(183)
CI \ I N
Nl
N / ~\/NuN\
IOI
(184) F / Cl ~ N
INI \ a N 0,--,,,N No
y
O
(185) F /
CI \ N
\
N O
,N / ^,,,Ny
O
(186) F /
CI \ NH
N O
0,-,--,,N y
Boehringer Ingelheim Pharma KG 120 Case 111317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example 16
Coated tablets containing 75 mg of active substance
1 tablet core contains:
active substance 75.0 mg
calcium phosphate 93.0 mg
corn starch 35.5 mg
polyvinylpyrrolidone 10.0 mg
hydroxypropylmethylcellulose 15.0 mg
magnesium stearate 1.5 mq
230.0 mg
Preparation:
The active substance is mixed with calcium phosphate, corn starch, polyvin-
ylpyrrolidone, hydroxypropylmethylcellulose and half the specified amount of
magnesium stearate. Blanks 13 mm in diameter are produced in a tablet-
making machine and these are then rubbed through a screen with a mesh size
of 1.5 mm using a suitable machine and mixed with the rest of the magnesium
stearate. This granulate is compressed in a tablet-making machine to form
tablets of the desired shape.
Weight of core: 230 mg
die: 9 mm, convex
The tablet cores thus produced are coated with a film consisting essentially
of
hydroxypropylmethylcellulose. The finished film-coated tablets are polished
with beeswax.
Weight of coated tablet: 245 mg.
Boehringer Ingelheim Pharma KG 121 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example 17
Tablets containing 100 mg of active substance
Composition:
1 tablet contains:
active substance 100.0 mg
lactose 80.0 mg
corn starch 34.0 mg
polyvinylpyrrolidone 4.0 mg
magnesium stearate 2.0 mg
220.0 mg
Method of Preparation:
The active substance, lactose and starch are mixed together and uniformly
moistened with an aqueous solution of the polyvinylpyrrolidone. After the
moist
composition has been screened (2.0 mm mesh size) and dried in a rack-type
drier at 50 C it is screened again (1.5 mm mesh size) and the lubricant is
added. The finished mixture is compressed to form tablets.
Weight of tablet: 220 mg
Diameter: 10 mm, biplanar, facetted on both sides and notched on one
side.
Example 18
Tablets containing 150 mg of active substance
Composition:
1 tablet contains:
active substance 50.0 mg
powdered lactose 89.0 mg
corn starch 40.0 mg
colloidal silica 10.0 mg
polyvinylpyrrolidone 10.0 mg
magnesium stearate 1.0 mg
300.0 mg
Boehringer Ingeiheim Pharma KG 122 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Preparation:
The active substance mixed with lactose, corn starch and silica is moistened
with a 20% aqueous polyvinylpyrrolidone solution and passed through a
screen with a mesh size of 1.5 mm. The granules, dried at 45 C, are passed
through the same screen again and mixed with the specified amount of
magnesium stearate. Tablets are pressed from the mixture.
Weight of tablet: 300 mg
die: 10 mm, flat
Example 19
Hard gelatine capsules containing 150 mg of active substance
1 capsule contains:
active substance 50.0 mg
corn starch (dried) approx. 80.0 mg
lactose (powdered) approx. 87.0 mg
magnesium stearate 3.0 mg
approx. 420.0 mg
Preparation:
The active substance is mixed with the excipients, passed through a screen
with a mesh size of 0.75 mm and homogeneously mixed using a suitable
apparatus. The finished mixture is packed into size 1 hard gelatine capsules.
Capsule filling: approx. 320 mg
Capsule shell: size I hard gelatine capsule.
Boehringer Ingelheim Pharma KG 123 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Example 20
Suppositories containing 150 mg of active substance
1 suppository contains:
active substance 150.0 mg
polyethyleneglycol 1500 550.0 mg
polyethyleneglycol 6000 460.0 mg
polyoxyethylene sorbitan monostearate 840.0 mg
2000.0 mg
Preparation:
After the suppository mass has been melted the active substance is
homogeneously distributed therein and the melt is poured into chilled moulds.
Example 21
Suspension containing 50 mg of active substance
100 ml of suspension contain:
active substance 1.00 g
carboxymethylcellulose-Na-salt 0.10 g
methyl p-hydroxybenzoate 0.05 g
propyl p-hydroxybenzoate 0.01 g
glucose 10.00 g
glycerol 5.00 g
70% sorbitol solution 20.00 g
flavouring 0.30 g
dist. water 100 ml
Preparation:
Boehringer Ingelheim Pharma KG 124 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
The distilled water is heated to 70 C. The methyl and propyl
p-hydroxybenzoates together with the glycerol and sodium salt of
carboxymethylcelIulose are dissolved therein with stirring. The solution is
cooled to ambient temperature and the active substance is added and
homogeneously dispersed therein with stirring. After the sugar, the sorbitol
solution and the flavouring have been added and dissolved, the suspension is
evacuated with stirring to eliminate air.
ml of suspension contain 50 mg of active substance.
Example 22
Ampoules containing 10 mg active substance
Composition:
active substance 10.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water 2.0 ml
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI,
made isotonic with common salt, filtered sterile and transferred into 2 ml
ampoules.
Example 23
Ampoules containing 50 mg of active substance
Composition:
active substance 50.0 mg
0.01 N hydrochloric acid q.s.
double-distilled water 10.0 ml
Boehringer Ingelheim Pharma KG 125 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
Preparation:
The active substance is dissolved in the necessary amount of 0.01 N HCI,
made isotonic with common salt, filtered sterile and transferred into 10 ml
ampoules.
Example 24
Capsules for powder inhalation containing 5 mg of active substance
1 capsule contains:
active substance 5.0 mg
lactose for inhalation 15.0 mg
20.0 mg
Preparation:
The active substance is mixed with lactose for inhalation. The mixture is
packed into capsules in a capsule-making machine (weight of the empty
capsule approx. 50 mg).
weight of capsule: 70.0 mg
size of capsule = 3
Example 25
Inhalable solution for hand-held nebulisers containing 2.5 mg active
substance
1 spray contains:
active substance 2.500 mg
benzalkonium chloride 0.001 mg
1 N hydrochloric acid q.s.
Boehringer Ingelheim Pharma KG 126 Case 1/1317-Ro
D-55216 INGELHEIM CA 02476008 2004-08-11 Foreign filing text
ethanol/water (50/50) 15.000 mg
Preparation:
The active substance and benzalkonium chloride are dissolved in
ethanol/water (50/50). The pH of the solution is adjusted with 1 N
hydrochloric
acid. The resulting solution is filtered and transferred into suitable
containers
for use in hand-held nebulisers (cartridges).
Contents of the container: 4.5 g