Note: Descriptions are shown in the official language in which they were submitted.
CA 02477136 2004-08-20
1
DyStar Textilfarben GmbH & Co. Deutschland KG Dr. KUN DYS 2002/D 503/NP1
DYE MIXTURES OF FIBER-REACTIVE AZO DYES AND PREPARATION AND USE
THEREOF
This invention relates to the technical field of fiber-reactive azo dyes.
Mixed fiber-reactive azo dyes and their use for dyeing hydroxyl- and
carboxamido-
containing material in black shades are known for example from the documents
US 5,445,654, US 5,611,821, KR 94-2560, Sho 58-160362 and EP-A-0 870 807.
However, they have certain application defects, such as for example an overly
large dependence of the color yield on varying dyeing parameters in the dyeing
process or an insufficient or unlevel color build-up on cotton (good color
build-up
results from the ability of a dye to provide a proportionally stronger dyeing
when
used in higher concentrations in the dyebath), or an excessive salt dependence
of
the dyeings. Consequences of these defects can be poor reproducibilities for
the
dyeings that are obtainable. The documents WO 98/42784, WO 98/42785, WO
93/18224 and US 5,330,539 disclose dyes and dye mixtures which can be dyed in
the presence of small amounts of salt, but which provide only very weak
dyeings
in the absence of salt.
Since it is commercially as well as environmentally necessary to reduce the
salt
content of dyeing effluent, there is a need for reactive dyes which provide
dyeings
of high color strength in the presence of small amounts of salt or even in the
absence of electrolyte salts.
The present invention, then, provides dye mixtures which provide dyeings of
high
color strength in the presence of only very low levels or even in the absence
of
electrolyte salts.
The invention accordingly provides dye mixtures comprising one or more, such
as
two or three, preferably 1 or 2, dyes of the hereinbelow indicated and defined
general
formula (I)
CA 02477136 2004-08-20
2
D~-N=N N=N-D2
~S~OM
O O
(I),
one or more, such as two or three, preferably 1 or 2, dyes of the hereinbelow
indicated and defined general formula (II)
D3
i
~H N~~N H
~~N-R
O, S ~ / / S,,O
y ~OM
MO O O
n
and, optionally, as further components for blending or shading, one or more,
such as
two or three, preferably 1 or 2, dyes having, for example, the hereinbelow
indicated
and defined general formulae (Illa) - (II If)
~R ~R32
a HN 5 HN
s
D wN-N \ D ~N-N \ N-N~D
/ NH2 / NH2
O;S;O O;S;O
OM OM
OH NH2
,O
(Illa)
(Illb)
CA 02477136 2004-08-20
D~N;N R33
HO N~N D\ R34
N=N \ R35
I HO N'
O
S 02Z R36
(Illc) (Illd)
R37
OH M03S
N
N
N \ ~ %N ~ ~ ~ ~ N// ~ \iN
,N a N
R3' . S03M HO
S02Z
Z02S
(Ille)
R3s H
N~Z3
- N\,N ~
R3s
I~
m (SO3M)P
(Illf)
where:
D' and D2 are each a group of the general formula (1 )
z
0
R~--N
i
A R3
X~
(1 )
- CA 02477136 2004-08-20
4
where
R' is hydrogen, (C~-C4)-alkyl, aryl or a substituted aryl radical;
R2 and R3 are independently hydrogen, (C~-Ca)-alkyl, (C~-C4)-alkoxy,
hydroxyl, sulfo, carboxyl, amido or halogen; and
A is a phenylene group of the general formula (2)
Ra R5
(2)
where
R4 and R5 are independently hydrogen, (C~-C4)-alkyl, (C~-C4)-
alkoxy, hydroxyl, sulfo, carboxyl, amido or halogen; or
a naphthylene group of the general formula (3)
R6
R' (3)
where
R6 and R' are independently hydrogen, (C~-C4)-alkyl, (C~-C4)-
alkoxy, hydroxyl, sulfo, carboxyl, amido or halogen; or
a polymethylene group of the general formula (4)
-(CR$R9)k- (4)
where
k is an integer greater than 1 and
R$ and R9 are independently hydrogen, (C,-C4)-alkyl, (C~-C4)-
alkoxy, hydroxyl, cyano, amido, halogen or aryl; and
CA 02477136 2004-08-20
_, 5
X' is hydrogen or a group of the formula -S02-Z; or
are each a phenyl group of the general formula (5)
R,~ R"
Xz (5)
where
R'° and R" are independently hydrogen, (C~-C4)-alkyl, (C~-C4)-alkoxy,
hydroxyl, sulfo, carboxyl, amido or halogen; and
X2 has one of the meanings of X';
or are each a naphthyl group of the general formula (6)
R~z
R~3 X3
where
R'2 and R'3 are independently hydrogen, (C~-C4)-alkyl, (C~-C4)-alkoxy,
hydroxyl, sulfo, carboxyl, amido or halogen; and
X3 has one of the meanings of X';
Z is -CH=CH2, -CH2CH2Z' or hydroxyl,
where
Z' is hydroxyl or an alkali-eliminable group; and
M is hydrogen, an alkali metal or one equivalent of an alkaline earth metal;
D3 has one of the meanings of D' or D2 or is a group of the general formula
(7)
CA 02477136 2004-08-20
6
Z21
R23 N R21
R22
S03M
(7)
where
R21 and R22 independently have one of the meanings of R2 and R3;
R23 is hydrogen, (C1- C4)-alkyl, unsubstituted or (C1-C4)-alkyl-, (C1-C4)-
alkoxy-, sulfo-, halogen- or carboxyl-substituted phenyl; and
Z21 is a fiber-reactive group of the general formula (8) or (9) or (10)
U1 O
V ~ Q1 N~ Q2 ~ N~ CI
N N N ~N l /~
U2 ~N CI
(8) (9) (10)
where
V is fluorine or chlorine;
U1 and U2 are independently fluorine, chlorine or hydrogen;
and
Q1 and Q2 are independently chlorine, fluorine, cyanamido, hydroxyl,
(C1-C6)-alkoxy, phenoxy, sulfophenoxy, mercapto, (C1-C6)-
alkylmercapto, pyridino, carboxypyridino, carbamoylpyridino
or a group of the general formula (11 ) or (12)
R2. R3'
-N
-N
W-S02Z .R4.
(11) (12)
where
R2' is hydrogen or (C1-C6)-alkyl, sulfo-(C1-C6)-alkyl,
- CA 02477136 2004-08-20
7
or phenyl which is unsubstituted or substituted by
(C~-C4)-alkyl, (C~-C4)-alkoxy, sulfo, halogen,
carboxyl, acetamido or ureido;
R3' and R4' independently have one of the meanings of R2',
or combine to form a cyclic ring system of the formula
-(CH2)~- , where j is 4 or 5, or alternatively -(CH2)2-E-
(CH2)2-, where E is oxygen, sulfur, sulfo, -NR5'-, where
R5' is equal to (C~-Cs)-alkyl, or they are a group of the
general formula (13)
R24 R25
N+ R2s
* B_
(13)
where
R2a, R25 and R2s are each (C~-C4)-alkyl or (C~-Ca)-
hydroxyalkyl; and
B- is the equivalent of an anion, such as
hydrogensulfate, sulfate, fluoride, chloride,
bromide, dihydrogenphosphate,
hydrogenphosphate, phosphate, hydroxide or
acetate;
W is phenylene which is unsubstituted or substituted by 1
or 2 substituents, such as (C~-C4)-alkyl, (C,-C4)-alkoxy,
carboxyl, sulfo, chlorine, bromine, or is (C~-C4)-
alkylene-arylene or (C2-Cs)-alkylene, which can be
interrupted by oxygen, sulfur, sulfo, amino, carbonyl,
carboxamido, or is phenylene-CONH-phenylene, which
is unsubstituted or substituted by (C~-C4)-aakyl, (C~-C4)-
' CA 02477136 2004-08-20
8
alkoxy, hydroxyl, sulfo, carboxyl, amido, ureido or
halogen, or is naphthylene, which is unsubstituted or
substituted by one or two sulfo groups; and
Z is as defined above;
R is hydrogen, (C~-C4)-alkyl or phenyl which is unsubstituted or substituted
by
(C~-C4)-alkyl, (C~-C4)-alkoxy, sulfo, halogen, carboxyl, acetamido or ureido;
or
is a group of the formula (14)
CH2 - S03M (14),
where M is as defined above; and
n is 1 or 2,
D4, D5, D6, D' and D$ have one of the meanings of D', D2 or D3, and,
if R3~ is not a group of the general formula (8) or (9), D4, and
also D5 or D6 and D8, contain at least one fiber-reactive group
of the formula -S02Z or Z2~;
R3' is hydrogen, acetyl, carbamoyl, sulfomethyl or
a group of the general formula (8) or (9),
R32 is hydrogen or sulfomethyl,
R33 is methyl, carboxyl or carboxyalkyl, with C~-C4-alkyl,
R34 is hydrogen or methyl,
R35 is hydrogen, cyano, carbamoyl, carboxyl or
sulfomethyl,
R36 is methyl, ethyl or f~-sulfoethyl,
R3' is methyl, carboxyl or carboxyalkyl, with C~-C4-alkyl,
R3$ is acetamido, ureido or methyl,
R39 is hydrogen, methyl or methoxy,
m is0or1,
p is 1, 2 or 3, and
CA 02477136 2004-08-20
9
Z3 has one of the meanings of Z2'
In the general formula (I) at least one of D' and D2 is a group of the general
formula
( 1 );
when A is a group of the general formula (4), R' is aryl or substituted aryl;
and
the reactive dye of the general formula (I) contains at least one -SOz-Z
group.
The dyes of the general formula (II) contain independently of one another at
least
one fiber-reactive group of the formula -S02-Z or Z2'.
The individual symbols in the general formulae above and below can have
identical
or different meanings under their definition, irrespective of whether the
symbols bear
the same or a different designation.
(C~-Cq.)-Alkyl R may be straight-chain or branched and is in particular
methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl or tert-butyl. Methyl and
ethyl are
preferred. The same logic applies to (C~-Cq.)-alkoxy groups.
Aryl R is in particular phenyl. Substituted aryl R~ is in particular phenyl
substituted by
one, two or three independent groups selected from the group consisting of
(C~-Cq.)-alkyl, (C~-C4)-alkoxy, hydroxyl, sulfo, carboxyl, amido and halogen.
Halogen R is in particular fluorine, chlorine or bromine, and fluorine and
chlorine are
preferred.
Alkali-eliminable Z~ in the ~i-position of the ethyl group of Z includes for
example
halogen atoms, such as chlorine and bromine, ester groups of organic
carboxylic and
sulfonic acids, as of alkylcarboxylic acids, substituted or unsubstituted
benzenecarboxylic acids and substituted or unsubstituted benzenesulfonic
acids,
such as alkanoyloxy of 2 to 5 carbon atoms, especially acetyloxy, benzoyloxy,
sulfobenzoyloxy, phenylsulfonyloxy and toluylsulfonyloxy, also acidic ester
groups of
inorganic acids, as of phosphoric acid, sulfuric acid and thiosulfuric acid
(phosphato,
CA 02477136 2004-08-20
sulfato and thiosulfato groups), similarly dialkylamino groups having alkyl
groups of 1
to 4 carbon atoms in each case, such as dimethylamino and diethylamino.
Z is preferably vinyl, ~-chloroethyl and particularly preferably (3-
sulfatoethyl.
5
The groups "sulfo", "carboxyl", "thiosulfato", "phosphato" and "sulfato"
include not
only their acid form but also their salt form. Accordingly, sulfo groups are
groups
conforming to the general formula -S03M, thiosulfato groups are groups
conforming
to the general formula -S-SOgM, carboxyl groups are groups conforming to the
10 general formula -COOM, phosphato groups are groups conforming to the
general
formula -OP03M2 and sulfato groups are groups conforming to the general
formula
-OS03M, in each of which M is as defined above.
The dyes of the general formula (I) to (III) may possess different fiber-
reactive groups
-S02Z within the meaning of Z. More particularly, the fiber-reactive groups -
S02Z
may be on the one hand vinylsulfonyl groups and on the other -CH2CH2Z~ groups,
preferably ~3-sulfatoethylsulfonyl groups. If the dyes of the general formula
(I) to (III)
contain vinylsulfonyl groups in some instances, then the fraction of the
respective dye
with the vinylsulfonyl group is up to about 30 mol%, based on the respective
amount
of total dye.
Alkali M is in particular lithium, sodium or potassium. M is preferably
hydrogen or
sodium.
R~ to R~3 are each preferably hydrogen and R6, R7, R~2 and R~3 are each
preferably sulfo as well.
When A is phenylene and X~ is -S02Z, the S02Z group is preferably disposed
meta
or para relative to the nitrogen atom. In the group of the general formula (1
), the
carboxamide group is preferably disposed para or meta relative to the diazo
group.
When A is naphthylene, the bond leading to the nitrogen atom is preferably
attached
to the naphthalene nucleus in the ~3-position. When D~ or D2 is a group of the
general
CA 02477136 2004-08-20
11
formula (6), then the bond which leads to the diazo group is preferably
attached to
the naphthalene nucleus in the ~-position.
When D~ or D2 is a group of the general formula (5) and X2 is -S02Z, then the
S02Z group is preferably disposed meta or para relative to the diazo group.
Examples of substituents A are in particular 1,2-phenylene, 1,3-phenylene,
1,4-phenylene, 2-chloro-1,4-phenylene, 2-chloro-1,5-phenylene, 2-bromo-
1,4-phenylene, 2-sulfo-1,4-phenylene, 2-sulfo-1,5-phenylene, 2-methoxy-
1,5-phenylene, 2-ethoxy-1,5-phenylene, 2,5-dimethoxy-1,4-phenylene, 2-methoxy-
5-methyl-1,4-phenylene, 2-methyl-1,4-phenylene, 2,6-naphthylene, 2,8-
naphthylene,
1-sulfo-2,6-naphthylene, 6-sulfo-2,8-naphthylene or 1,2-ethylene and 1,3-
propylene.
A is particularly preferably 1,3-phenylene, 1,4-phenylene, 2-sulfo-1,4-
phenylene,
2-methoxy-1,5-phenylene, 2,5-dimethoxy-1,4-phenylene, 2-methoxy-5-methyl-
1,4-phenylene or 1,2-ethylene and 1,3-propylene, and in the case of the two
last-
mentioned alkylene groups R~ is preferably phenyl or 2-sulfophenyl.
k is preferably 2 or 3.
Examples of groups D~ to D8 of the general formulae (5) and (6) are 2-(~i-
sulfato-
ethylsulfonyl)phenyl, 3-(~3-sulfatoethylsulfonyl)phenyl, 4-((3-
sulfatoethylsulfonyl)-
phenyl, 2-carboxy-5-((3-sulfatoethylsulfonyl)phenyl, 2-chloro-4-((3-
sulfatoethyl-
sulfonyl)phenyl, 2-chloro-5-((3-sulfatoethylsulfonyl)phenyl, 2-bromo-4-(~3-
sulfato-
ethylsulfonyl)phenyl, 2-sulfo-4-((3-sulfatoethylsulfonyl)phenyl, 2-sulfo-5-(~i-
sulfato-
ethylsulfonyl)phenyl, 2-methoxy-5-(~3-sulfatoethylsulfonyl)phenyl, 2-ethoxy-
5-((3-sulfatoethylsulfonyl)phenyl, 2,5-dimethoxy-4-(~3-
sulfatoethylsulfonyl)phenyl,
2-methoxy-5-methyl-4-(~-sulfatoethylsulfonyl)phenyl, 2-methyl-4-(~-
sulfatoethyl-
sulfonyl)phenyl, 2- or 3- or 4-(~3-thiosulfatoethylsulfonyl)phenyl, 2-methoxy-
5-(~3-thiosulfatoethylsulfonyl)phenyl, 2-sulfo-4-(~3-
phosphatoethylsulfonyl)phenyl, 2- or
3- or 4-vinylsulfonylphenyl, 2-sulfo-4-vinylsulfonylphenyl, 2-chloro-4-(~3-
chloro-
ethylsulfonyl)phenyl, 2-chloro-5-(~i-chloroethylsulfonyl)phenyl, 3- or
CA 02477136 2004-08-20
12
4-((i-acetoxyethylsulfonyl)phenyl, 6- or 8-(~3-sulfatoethylsulfonyl)naphth-2-
yl,
6-(~i-sulfatoethylsulfonyl)-1-sulfonaphth-2-yl and 8-((3-sulfatoethylsulfonyl)-
6-sulfo-
naphth-2-yl, preferably 3-((i-sulfatoethylsulfonyl)phenyl, 4-((3-
sulfatoethylsulfonyl)-
phenyl, 2-sulfo-4-((3-sulfatoethylsulfonyl)phenyl, 2-methoxy-5-((3-
sulfatoethylsulfonyl)-
phenyl, 2,5-dimethoxy-4-(~-sulfatoethylsulfonyl)phenyl, 2-methoxy-5-methyl-
4-(~-sulfatoethylsulfonyl)phenyl and 3- or 4-vinylsulfonylphenyl.
In the general formula (II), R is preferably hydrogen or a group of the
formula
(14), M being as defined above.
In the general formula (II), D3 is preferably 2-(f~-
sulfatoethylsulfonyl)phenyl,
3-(f3-sulfatoethylsulfonyl)phenyl, 4-(f3-sulfatoethylsulfonyl)phenyl, 2-sulfo-
4-(f3-sulfatoethylsulfonyl)phenyl, 2-methoxy-5-(f3-
sulfatoethylsulfonyl)phenyl,
2,5-dimethoxy-4-(f5-sulfatoethylsulfonyl)phenyl, 2-methoxy-5-methyl-
4-(f3-sulfatoethylsulfonyl)phenyl, 6-(f3-sulfatoethylsulfonyl)-1-sulfonaphth-2-
yl, 2-,
3- or 4-vinylsulfonylphenyl, 1-sulfo-4-(2,4-difluoropyrimidin-6-yl)amino-2-
phenyl,
1-sulfo-4-(4,6-difluoropyrimidin-2-yl)amino-2-phenyl, 2-sulfo-4-(2,4-
difluoropyrimidin-6-yl)amino-1-phenyl or 2-sulfo-4-(4,6-difluoropyrimidin-2-
yl)amino-1-phenyl.
In the general formula (II), where D3 is a group of the general formula (6),
the
bond leading to the diazo group is preferably attached in f3-position to the
naphthalene nucleus of (6).
In the general formula (7), R2' to R23 are each preferably hydrogen and R2~
and
R22 are preferably sulfo as well.
In the general formulae (11 ) and (12), R2' to R4' are each preferably
hydrogen or
methyl, R2' is preferably phenyl as well and R3' and R4' are each preferably 2-
sulfoethyl, 2-, 3- or 4-sulfophenyl, 3- or 4-trimethylammoniophenyl sulfate, 3-
or 4-
trimethylammoniophenyl chloride, or R3' and R4' combine to form a cyclic ring
system which preferably conforms to the formula -(CH2)2-O-(CH2)2-.
CA 02477136 2004-08-20
13
W is preferably 1,3-phenylene, 1,4-phenylene, 2-sulfo-1,4-phenylene, 2-methoxy-
1,5-phenylene, 2,5-dimethoxy-1,4-phenylene, 2-methoxy-5-methyl-1,4-phenylene,
1,2-ethylene, 1,3-propylene.
Examples of the groups Q' and Q2 in the general formula (9) are independently
of
one another fluorine, chlorine, hydroxyl, methoxy, ethoxy, phenoxy,
3-sulfophenoxy, 4-sulfophenoxy, methylmercapto, cyanamido, amino,
methylamino, ethylamino, morpholino, piperidino, phenylamino,
methylphenylamino, 2-sulfophenylamino, 3-sulfophenylamino,
4-sulfophenylamino, 2,4-disulfophenylamino, 2,5-disulfophenylamino,
3-trimethylammoniumphenylamino, 4-trimethylammoniumphenylamino,
2-sulfoethylamino, N-methyl-2-sulfoethylamino, pyridino, 3-carboxypyridino,
4-carboxypyridino, 3-carbamoylpyridino, 4-carbamoylpyridino, 2-(2-
sulfatoethylsulfonyl)phenylamino, 3-(2-sulfatoethylsulfonyl)phenylamino, 4-(2-
sulfatoethylsulfonyl)phenylamino, N-ethyl-3-(2-
sulfatoethylsulfonyl)phenylamino,
N-ethyl-4-(2-sulfatoethylsulfonyl)phenylamino, 2-carboxy-5-(2-
sulfatoethylsulfonyl)phenylamino), 2-chloro-4-(2-
suffatoethylsulfonyl)phenylamino,
2-chloro-5-(2-sulfatoethylsulfonyl)phenylamino, 2-bromo-4-(2-
sulfatoethylsulfonyl)phenylamino, 2-sulfo-4-(2-
sulfatoethylsulfonyl)phenylamino,
2-sulfo-5-(2-sulfatoethylsulfonyl)phenylamino, 2-methoxy-5-(2-
sulfatoethylsulfonyl)phenylamino, 2,5-dimethoxy-4-(2-
sulfatoethylsulfonyl)phenyl-
amino, 2-methoxy-5-methyl-4-(2-sulfatoethylsulfonyl)phenylamino, 2-methyl-4-(2-
sulfatoethylsulfonyl)phenylamino, 2-(vinylsulfonyl)phenylamino, 3-
(vinylsulfonyl)-
phenylamino, 4-(vinylsulfonyl)phenylamino, N-ethyl-3-
(vinylsulfonyl)phenylamino,
N-ethyl-4-(vinylsulfonyl)phenylamino, 6-(2-sulfatoethylsulfonyl)naphth-2-
ylamino,
8-(2-sulfatoethylsulfonyl)naphth-2-yiamino, 8-(2-sulfatoethyfsulfonyl)-6-
sulfonaphth-2-ylamino, 3-(2-(2-
sulfatoethylsulfonyl)ethylcarbamoyl)phenylamino,
4-(2-(2-sulfatoethylsulfonyl)ethylcarbamoyl)phenylamino, 3-(2-
(vinylsulfonyl)ethyl-
carbamoyl)phenylamino, 4-(2-(vinylsulfonyl)ethylcarbamoyl)phenylamino, 4-(N-
methyl-2-(2-sulfatoethylsulfonyl)ethylcarbamoyl)phenylamino, 4-(N-phenyl-2-(2-
sulfatoethylsulfonyl)ethylcarbamoyl)phenylamino, 4-(3-(2-sulfatoethylsulfonyl)-
phenylcarbamoyl)phenylamino, 4-(4-(2-sulfatoethylsulfonyl)phenylcarbamoyl)-
phenylamino, 3-(3-(2-sulfatoethylsulfonyl)phenylcarbamoyl)phenylamino, 3-(4-(2-
sulfatoethylsulfonyl)phenylcarbamoyl)phenylamino, 3-(2-Sulfatoethylsulfonyl)-
CA 02477136 2004-08-20
14
propylamino, N-methyl-N-(2-(2-sulfatoethylsulfonyl)ethyl)amino, N-phenyl-N-(2-
(2-
sulfatoethylsulfonyl)ethyl)amino, N-phenyl-N-(3-(2-
sulfatoethylsulfonyl)propyl)-
amino.
Preferably the groups Q' and Q2 in the general formula (9) independently of
one
another are fluorine, chlorine, cyanamido, morpholino, 2-sulfophenylamino,
3-sulfophenylamino, 4-sulfophenylamino, 3-trimethylammoniumphenylamino,
4-trimethylammoniumphenylamino, N-methyl-2-sulfoethylamino,
3-carboxypyridino, 4-carboxypyridino, 3-carbamoylpyridino, 4-
carbamoylpyridino,
3-(2-sulfatoethylsulfonyl)phenylamino, 4-(2-sulfatoethylsulfonyl)phenylamino,
3-(vinylsulfonyl)phenylamino, 4-(vinylsulfonyl)phenylamino, 4-(3-(2-
sulfatoethylsulfonyl)phenylcarbamoyl)phenylamino, 4-(4-(2-
sulfatoethylsulfonyl)-
phenylcarbamoyl)phenylamino, 3-(3-(2-sulfatoethylsulfonyl)phenylcarbamoyl)-
phenylamino, 3-(4-(2-sulfatoethylsulfonyl)phenylcarbamoyl)phenylamino,
N-methyl-N-(2-(2-sulfatoethylsulfonyl)ethyl)amino, N-phenyl-N-(2-(2-
sulfatoethylsulfonyl)ethyl)amino.
With particular preference the groups Q~ and Q2 in the general formula (9)
independently of one another are fluoro, chloro, cyanamido, morpholino, 2-
sulfophenylamino, 3-sulfophenylamino, 4-sulfophenylamino, 3-
trimethylammoniumphenylamino, 4-trimethylammoniumphenylamino, 3-(2-
sulfatoethylsulfonyl)phenylamino, 4-(2-sulfatoethylsulfonyl)phenylamino, 3-
(vinylsulfonyl)phenylamino, 4-(vinylsulfonyl)-phenylamino, N-methyl-N-(2-(2-
sulfatoethylsulfonyl)ethyl)amino, N-phenyl-N-(2-(2-
sulfatoethylsulfonyl)ethyl)amino.
In the general formula (13) the radicals R24 to R26 are each preferably methyl
or
ethyl.
Anion B- is preferably sulfate or chloride.
In the general formula (13), the quaternary ammonium group is preferably meta
or
para to the free bond on the benzene nucleus.
Examples of the group Z2' are 2,4-difluoropyrimidin-6-yl, 4,6-
difluoropyrimidin-2-yl,
5-chloro-2,4-difluoropyrimidin-6-yl, 5-chloro-4,6-difluoropyrimidin-2-yl, 4,5-
difluoro-
CA 02477136 2004-08-20
5
pyrimidin-6-yl, 5-chloro-4-fluoropyrimidin-6-yl, 2,4,5-trichloropyrimidin-6-
yl, 4,5-
dichloropyrimidin-6-yl, 2,4-dichloropyrimidin-6-yl, 4-fluoropyrimidin-6-yl, 4-
chloro-
pyrimidin-6-yl, or a group of the general formula (9) with the above-indicated
examples for Q' and Q2 or 2,3-dichloroquinoxaline-6-carbonyl.
Z2' is preferably 2,4-difluoropyrimidin-6-yl, 4,6-difluoropyrimidin-2-yl, 5-
chloro-2,4-
difluoropyrimidin-6-yl, 5-chloro-4,6-difluoropyrimidin-2-yl or a group of the
general
formula (9) with the above-indicated preferred groups for Q' and Q2.
10 With particular preference Z2' is 2,4-difluoropyrimidin-6-yl, 5-chloro-2,4-
20
difluoropyrimidin-6-yl or a group of the general formula (9) with the above-
indicated particularly preferred groups Q' and Q2.
Preferred mixtures contain one or more dyes of the general formula (la)
' -O
R A MO/SO
I O OM
S02Z (la)
OH NH2
O N=N ~ ~ N=N , S02Z
w
-N O_-
and one or more dyes of the general formula (Ila)
D3
i
OH N~~N H
N-R
O~S / /
~ 11
MO O (Ila)
plus if desired one or more dyes of the general formulae (Illa) to (Illf).
CA 02477136 2004-08-20
16
In the general formulae (la) and (Ila), M, A, R', Z, D3 and R are each as
defined
above.
In the general formula (la), it is particularly preferable for A to be
phenylene and Z to
be vinyl or ~3-sulfatoethyl.
In the general formula (la), it is most preferable for A to be phenylene, R~
to be
hydrogen and Z to be vinyl or ~-sulfatoethyl.
The dye mixtures according to the invention contain bisazo dyes of the general
formula (I) in an amount of 30 to 95% by weight and preferably 50 to 90% by
weight and monoazo dyes of the general formula (II) in an amount of 5 to 70%
by
weight and preferably 10 to 50% by weight, and dyes of the general formulae
(Illa)
to (Illf) in an amount of 0 to 40% by weight, preferably 5 to 25% by weight.
Optionally, the dye mixtures according to the invention may also contain one
or
more monoazo dyes of the general formulae (15) or (16) in an amount of up to
10% by weight, preferably up to 5% by weight,
OH NH2 /D2 NH2 OH
N=N ~ ~ N=N
M03S S03M M30S ~ ~ ~S03M
(15) (16)
where M and D2 are each as defined above. It is particularly preferable for D2
to
be 4-(2-sulfatoethylsulfonyl)phenyl or 4-vinylsulfonylphenyl.
Dyes of the general formula (I) are known from EP-A1046677, the dyes of the
general formula (II) are known in part from WO 9610610, WO 9725377,
WO 9947608 and EP-A 922735.
Dyes of the general formulae (15) and (16) are obtainable via standard
synthetic
methods or are in some instances formed during the synthesis of dyes of the
general formula (I). They are customarily used as shading components.
CA 02477136 2004-08-20
17
The dye mixtures according to the invention can be present as a preparation in
solid
or liquid (dissolved) form. In solid form, they contain, to the extent
necessary, the
electrolyte salts customary in the case of water-soluble and especially fiber-
reactive
dyes, such as sodium chloride, potassium chloride and sodium sulfate, and may
further contain the auxiliaries customary in commercial dyes, such as buffer
substances capable of setting a pH in aqueous solution between 3 and 7, such
as
sodium acetate, sodium citrate, sodium borate, sodium bicarbonate, sodium
dihydrogenphosphate and disodium hydrogenphosphate, dyeing auxiliaries,
dustproofing agents and small amounts of siccatives; when they are present in
a
liquid, aqueous solution (including a content of thickeners of the type
customary in
print pastes), they may also contain substances which ensure a long life for
these
preparations, for example mold preventatives.
In solid form, the dye mixtures according to the invention are generally
present as
powders or granules which contain electrolyte salt and which will hereinbelow
generally be referred to as a preparation with or without one or more of the
abovementioned auxiliaries. In the preparations, the dye mixture is present at
10 to
90% by weight, based on the preparation containing it. The buffer substances
are
generally present in a total amount of up to 5% by weight, based on the
preparation.
When the dye mixtures according to the invention are present in an aqueous
solution,
the total dye content of these aqueous solutions is up to about 50% by weight,
for
example between 5 and 50% by weight, the electrolyte salt content of these
aqueous
solutions preferably being below 10% by weight, based on the aqueous solution;
the
aqueous solutions (liquid preparations) can contain the aforementioned buffer
substances in an amount which is generally up to 5% by weight and preferably
up to
2% by weight.
The dye mixtures according to the invention are preparable in a conventional
manner, as by mechanically mixing the individual dyes, whether in the form of
their
dye powders or granules or their as-synthesized solutions or in the form of
aqueous
solutions of the individual dyes generally, which may additionally contain
customary
auxiliaries, or by conventional diazotization and coupling of suitable
mixtures of diazo
and coupling components in the desired amount ratios.
CA 02477136 2004-08-20
18
For example, when the diazo components with the groups D2 and D3 in the
general
formulae (I) and (II) possess the same meaning (D2 = D3), an amine of the
general
formula (17)
DZ - NHZ (17),
where D2 is as defined above can be diazotized in a conventional manner and
the
resulting diazonium compound then reacted in aqueous medium at a pH below 3
with
a mixture of 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid or 4-amino-5-
hydroxynaphthalene-1,7-disulfonic acid and an optionally N-substituted 6-amino-
4-
hydroxynaphthalene-2-sulfonic acid or 3-amino-5-hydroxynaphthalene-2,7-
disulfonic
acid to give a mixture of two red monoazo dyes of the formulae (15) and (II).
Subsequently an amine of the general formula (18)
D' - NHZ (18),
where D2 is as defined above is diazotized in a conventional manner and the
resulting diazonium compound is then coupled in a second stage with the
mixture of
the monoazo dyes of the general formulae (15) and (II) obtained in the first
stage,
coupling taking place at a pH of between 3 and 7.5, to give a mixture of the
dyes of
the general formulae (I) and (II).
Alternatively, where the diazo components with the groups D~ and D3 in the
general
formulae (I) and (II) possess the same meaning (D' = D3), the dye mixture of
the
invention can be prepared by conventionally diazotizing an amine of the
general
formula (18), where D' is as defined in claim 1, and then reacting the
resulting
diazonium compound of an aqueous solution or suspension of a mixture of
defined
proportion of a red monoazo dye of the general formula (15) and an optionally
substituted 6-amino-4-hydroxynaphthalene-2-sulfonic acid, first with a pH
below 3 to
give a mixture of two red monoazo dyes of the general formulae (15) and (II),
and,
when acidic coupling is at an end, by raising the pH to 4 - 7.5, carrying out
the
second coupling of the monoazo dye of the general formula (15), giving a
mixture of
the two dyes of the general formulae (I) and (II).
The red monoazo dyes of the general formula (15) can be prepared by
conventionally
diazotizing an amine of the general formula (17), where D2 is as defined in
claim 1,
and then coupling the resulting diazonium compound in an aqueous medium at a
pH
CA 02477136 2004-08-20
19
below 2 with 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid and/or 4-amino-5-
hydroxynaphthalene-1,7-disulfonic acid in a first stage.
The dye mixture of the invention is isolated in a conventional manner, where
appropriate following the addition of one or more, such as two or three,
yellow
components of the general formulae (I Ila) to (Illf), in solid form or as an
aqueous
solution, by salting out for example with sodium chloride or potassium
chloride or by
spray drying.
Additionally, the as-synthesized solutions of the dye mixtures of the general
formula
(I) and (II) can be used directly as a liquid preparation for dyeing, where
appropriate
following addition of one or more, such as two or three, yellow components of
the
general formulae (Il la) to (Illf) in solid form or as aqueous solutions and
also, where
appropriate, addition of a buffer substance and, where appropriate, after
concentration or dilution.
Dye mixtures which as well as ~-chloroethylsulfonyl or ~3-
thiosulfatoethylsulfonyl or ~-
sulfatoethylsulfonyl groups also contain vinylsulfonyl groups as reactive
radicals can
be synthesized not only starting from appropriately substituted
vinylsulfonylanilines or
naphthylamines but also by reaction of a dye mixture where Z is ~-chloroethyl,
~i-
thiosulfatoethyl or (3-sulfatoethyl with 'an amount of alkali required for the
desired
fraction and converting the ~i-substituted ethylsulfonyl groups mentioned into
vinylsulfonyl groups. This conversion is effected in a manner familiar to one
skilled in
the art.
The dye mixtures according to the invention have useful application
properties. They
are used for dyeing or printing hydroxyl- and/or carboxamido-containing
materials, for
example in the form of sheetlike structures, such as paper and leather or of
films, for
example composed of polyamide, or in bulk, as for example polyamide and
polyurethane, but especially for dyeing and printing these materials in fiber
form.
The present invention thus also provides for the use of the dye mixtures
according to
the invention for dyeing or printing these materials, or rather processes for
dyeing or
CA 02477136 2004-08-20
printing these materials in a conventional manner, by using a dye mixture
according
to the invention or its individual components (dyes) individually together as
a
colorant. The materials are preferably employed in the form of fiber
materials,
especially in the form of textile fibers, such as woven fabrics or yarns, as
in the form
5 of hanks or wound packages.
Hydroxyl-containing materials are those of natural or synthetic origin, for
example
cellulose fiber materials or their regenerated products and polyvinyl
alcohols.
Cellulose fiber materials are preferably cotton, but also other vegetable
fibers, such
10 as linen, hemp, jute and ramie fibers; regenerated cellulose fibers are for
example
staple viscose and filament viscose and also chemically modified cellulose
fibers,
such as aminated cellulose fibers or fibers as described for example in WO
96/37641
and WO 96/37642 and also in EP 0 538 785 and EP 0 692 559.
15 Carboxamido-containing materials are for example synthetic and natural
polyamides
and polyurethanes, especially in the form of fibers, for example wool and
other
animal hairs, silk, leather, nylon-6,6, nylon-6, nylon-11 and nylon-4.
The dye mixtures according to the invention can be applied to and fixed on the
20 substrates mentioned, especially the fiber materials mentioned, by the
application
techniques known for water-soluble dyes and especially for fiber-reactive
dyes. For
instance, on cellulose fibers they produce by the exhaust method from a long
liquor
and also from a short liquor, for example in a liquor to goods ratio of 5 : 1
to 100 : 1,
preferably 6 : 1 to 30 : 1, using various acid-binding agents and optionally
neutral
salts as far as necessary, such as sodium chloride or sodium sulfate, dyeings
having
very good color yields. Application is preferably from an aqueous bath at
temperatures between 40 and 105°C, optionally at a temperature of up to
130°C
under superatmospheric pressure, but preferably at 30 to 95°C,
especially 45 to
65°C, in the presence or absence of customary dyeing auxiliaries. One
possible
procedure here is to introduce the material into the warm bath and to
gradually heat
the bath to the desired dyeing temperature and complete the dyeing process at
that
temperature. The neutral salts which accelerate the exhaustion of the dyes may
also
if desired only be added to the bath after the actual dyeing temperature has
been
reached.
CA 02477136 2004-08-20
21
Padding processes likewise provide excellent color yields and a very good
color
build-up on cellulose fibers, the dyes being fixable in a conventional manner
by
hatching at room temperature or elevated temperature, for example at up to
about
60°C, or in a continuous manner, for example by means of a pad-dry-pad
steam
process, by steaming or using dry heat.
Similarly, the customary printing processes for cellulose fibers, which can be
carried
out in one step, for example by printing with a print paste containing sodium
bicarbonate or some other acid-binding agent and by subsequent steaming at 100
to
103°C, or in two steps, for example by printing with a neutral to weak
acidic print
color and then fixing either by passing the printed material through hot
electrolyte-
containing alkaline bath or by overpadding with an alkaline electrolyte-
containing
padding liquor and subsequent hatching of the alkali-overpadded material or
subsequent steaming or subsequent dry heat treatment of the alkali-overpadded
material, produce strong prints with well-defined contours and a clear white
ground.
The outcome of the prints is little affected, if at all, by variations in the
fixing
conditions.
When fixing by means of dry heat in accordance with the customary thermofix
processes, hot air at 120 to 200°C is .used. In addition to the
customary steam at 101
to 103°C, it is also possible to use superheated steam and high-
pressure steam at
temperatures of up to 160°C.
The acid-binding agents which effect the fixation of the dyes of the dye
mixtures
according to the invention on the cellulose fibers are for example water-
soluble basic
salts of alkali metals and likewise alkaline earth metals of inorganic or
organic acids
or compounds which liberate alkali in the heat, and also alkali metal
silicates.
Especially suitable are the alkali metal hydroxides and alkali metal salts of
weak to
medium inorganic or organic acids, the preferred alkali metal compounds being
the
sodium and potassium compounds. Such acid-binding agents are for example
sodium hydroxide, potassium hydroxide, sodium carbonate, sodium bicarbonate,
potassium carbonate, sodium formate, sodium dihydrogenphosphate, disodium
hydrogenphosphate, sodium trichloroacetate, trisodium phosphate or~waterglass
or
CA 02477136 2004-08-20
22
mixtures thereof, for example mixtures of aqueous sodium hydroxide solution
and
waterglass.
The dye mixtures according to the invention are notable for outstanding color
strength when applied to the cellulose fiber materials by dyeing or printing
in the
presence of no or very small amounts of alkali or alkaline earth metal
compounds.
For instance, no electrolyte salt is required for a shallow depth of shade,
not more
than 5 g/l of electrolyte salt is required for a medium depth of shade and not
more
than 10 gll of electrolyte salt is required for deep shades.
According to the invention, a shallow depth of shade refers to the use of 2%
by
weight of dye based on the substrate to be dyed, a medium depth of shade
refers to
the use of 2 to 4% by weight based on the substrate to be dyed and a deep
shade
refers to the use of 4 to 10% by weight of dye based on the substrate to be
dyed.
The dyeings and prints obtainable with the dye mixtures according to the
invention
possess bright shades; more particularly, the dyeings and prints on cellulose
fiber
materials possess good lightfastness and especially good wetfastnesses, such
as
fastness to washing, milling, water, seawater, crossdyeing and acidic and
alkaline
perspiration, also good fastness to pleating, hotpressing and rubbing.
Furthermore,
the cellulose dyeings obtained following the customary aftertreatment of
rinsing to
remove unfixed dye portions exhibit excellent wetfastnesses, in particular
since
unfixed dye portions are easily washed off because of their good solubility in
cold
water.
Furthermore, the dye mixtures according to the invention can also be used for
the
fiber-reactive dyeing of wool. Moreover, wool which has been given a
nonfelting or
low-felting finish (cf. for example H. Rath, Lehrbuch der Textilchemie,
Springer-
Verlag, 3rd edition (1972), pages 295-299, especially finished by the
Hercosett
process (page 298); J. Soc. Dyers and Colourists 1972, 93-99, and 1975, 33-
44), can ,
be dyed to very good fastness properties. The process of dyeing on wool is
here
carried out in a conventional manner from an acidic medium. For instance,
acetic
acid and/or ammonium sulfate or acetic acid and ammonium acetate or sodium
acetate can be added to the dyebath to obtain the desired pH. To obtain a
dyeing of
CA 02477136 2004-08-20
23
acceptable levelness, it is advisable to add a customary leveling agent, for
example a
leveling agent based on a reaction product of cyanuric chloride with three
times the
molar amount of an aminobenzenesulfonic acid and/or of an
aminonaphthalenesulfonic acid or on the basis of a reaction product of for
example
stearylamine with ethylene oxide. For instance, the dye mixture according to
the
invention is preferably subjected to the exhaust process initially from an
acidic
dyebath having a pH of about 3.5 to 5.5 under pH control and the pH is then,
toward
the end of the dyeing time, shifted into the neutral and optionally weakly
alkaline
range up to a pH of 8.5 to bring about, especially for very deep dyeings, the
full
reactive bond between the dyes of the dye mixtures according to the invention
and
the fiber. At the same time, the dye portion not reactively bound is removed.
The procedure described herein also applies to the production of dyeings on
fiber
materials composed of other natural polyamides or of synthetic polyamides and
polyurethanes. In general, the material to be dyed is introduced into the bath
at a
temperature of about 40°C, agitated therein for some time, the dyebath
is then
adjusted to the desired weakly acidic, preferably weakly acetic acid, pH and
the
actual dyeing is carried out at a temperature between 60 and 98°C.
However, the
dyeings can also be carried out at the boil or in sealed dyeing apparatus at
temperatures of up to 106°C. Since the water solubility of the dye
mixtures according
to the invention is very good, they can also be used with advantage in
customary
continuous dyeing processes. The color strength of the dye mixtures according
to the
invention is very high.
The dye mixtures according to the invention dye the materials mentioned,
preferably
fiber materials, in navy to jet black shades having good fastness properties.
The examples hereinbelow serve to illustrate the invention. Parts and
percentages
are by weight, unless otherwise stated. Parts by weight relate to parts by
volume as
the kilogram relative to the liter. The compounds described in the examples in
terms
of a formula are indicated in the form of the sodium salts, since they are
generally
prepared and isolated in the form of their salts, preferably sodium or
potassium salts,
and used for dyeing in the form of their salts. The starting compounds
described in
the examples hereinbelow can be used in the synthesis in the form of the free
acid or
CA 02477136 2004-08-20
24
likewise in the form of their salts, preferably alkali metal salts, such as
sodium or
potassium salts.
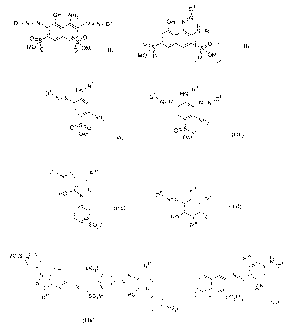
Example 1
70 parts of an electrolyte-containing dye powder containing the navy disazo
dye
of the formula (IA)
O
O~~ ~O
HN I \ I \ S~OS03Na
N OH NHz N
\ N / \ N
:0 I
Na03SO~S~ Na03S \ / S03Na
(IA)
in a 70% fraction, 20 parts of an electrolyte-containing dye powder containing
the
orange-red monoazo dye of the formula (IIA)
F
N i _N
F' v 'NH
Na03S / OH ~ I
\ I N-N \
I S03Na
NH2
(IIA)
in a 75% fraction and 10 parts of an electrolyte-containing dye powder
containing the
yellow disazo dye of the formula (II If-1 )
CA 02477136 2004-08-20
S03Na / N N~ F
/ / N'N \ ~ CI ~ ~N
Na0 S \ ~ ~ HN O F
3
(Illf-1 ) CH3
in a 70% fraction are mechanically mixed with each other.
5 The resulting dye mixture according to the invention provides jet black
dyeings and
prints, on cotton for example, under the dyeing conditions customary for
reactive
dyes.
10 Example 2
70 parts of an electrolyte-containing dye powder containing the navy disazo
dye of
the formula (IA) in a 70% fraction, 20 parts of an electrolyte-containing dye
powder
containing the red monoazo dye of the formula (IIB)
F
O ~S O
/ N i 'N
J~ J\
Na03S0 ~H N NH
/
~S03Na
(IIB) N
OH N
\ \ NHZ
/ /
15 Na03S
in an 80% fraction and 10 parts of an electrolyte-containing dye powder
containing
the yellow disazo dye of the formula (Illc-1 )
CA 02477136 2004-08-20
26
0
HN
/ NON COONa
O
/ //
S=O
HO NON
OS03Na
(II Ic-1 ) Sp3Na
in a 70% fraction are dissolved in 500 parts of water and the resulting dye
solution is
adjusted to pH 5.5-6.5. Evaporation or spray drying of this dye solution
provides a
dye mixture which provides jet black dyeings and prints on cotton under the
dyeing
conditions customary for reactive dyes.
Example 3
a) 281 parts of 4-(~i-sulfatoethylsulfonyl)aniline are suspended in 650 parts
of ice-
water and 180 parts of 30% hydrochloric acid and diazotized by dropwise
addition of
173 parts of 40% sodium nitrite solution. 223 parts of 4-amino-5-
hydroxynaphthalene-2,7-disulfonic acid and 72 parts of 6-amino-4-
hydroxynaphthalene-2-sulfonic acid are added and coupled in a first step at pH
1
- 1 .8 at below 20°C to form a mixture of two red monoazo dyes
conforming to
the formulae (15-1 ) and (IIC). The stated pH range is set and maintained
during
the coupling reaction by addition of a total of about 150 parts of sodium
bicarbonate.
o ,o
's'
\ ~OS03Na
O\~ ~ O
OH NHz N / Na03SO~S ~ ~ HO / I S03Na
\ N ~N,N \
Na03S \ / S03Na H N
z
(15-1 ) (IIC)
CA 02477136 2004-08-20
27
b) In a second, separate reaction vessel, 280 parts of 4-amino-N-(3-((f3-
sulfatoethyl)-sulfonyl)phenyl)benzamide are suspended in 1600 parts of ice-
water, adjusted to pH 6.5 - 7 with about 35 parts of sodium carbonate and
admixed with 122 parts of 40% sodium nitrite solution. This suspension is
added dropwise to a mixture of 450 parts of ice, 350 parts of ice-water and
255
parts of concentrated hydrochloric acid. After subsequent stirring at 5-
10°C for 2
hours, the excess nitrite is reduced with amidosulfonic acid and the resulting
diazo suspension is pumped into the mixture of red monoazo dyes of a).
Subsequently, at below 25°C, the reaction mixture is adjusted to a pH
of 5 - 6 with
sodium carbonate and, after the end of the coupling reaction, 110 parts of the
yellow
monoazo dye of the formula (Ills-1 ) are added in the form of an aqueous
solution to
this reaction mixture, and the resulting 73 : 16 : 10 mixture of the dyes
(IB), (IIC)
and (Illa-1 ) is isolated by spray drying.
Alternatively, the dye solution obtained can also be buffered at pH 5.5 - 6 by
addition
of a phosphate buffer and be adjusted by further dilution or concentration to
provide a
liquid brand of defined strength.
The resulting dye mixture according to the invention dyes cotton in black
shades.
0
o,,
HN \ \ S~OS03Na
Na03S0 I / I /
/ N OH NHZ N
\ I N / \ N
O S,,O \ I /
Na03S S03Na
(IB)
O\~ ~O
S
Na03S0~ ~ I NHZ
\ N%N \
I/
-NHZ
(Ills-1 ) S03Na
Example 4
CA 02477136 2004-08-20
28
a) 178 parts of 4-(f3-sulfatoethylsulfonyl)aniline are suspended in 420 parts
of
ice-water and 1 15 parts of 30% hydrochloric acid and diazotized by dropwise
addition of 1 10 parts of 40% sodium nitrite solution. 201 parts of 4-amino-5-
hydroxynaphthalene-2,7-disulfonic acid are added and coupled in a first step
at
pH 1 to 1 .3 at below 20°C to form a red monoazo dye of formula (15-1
). The
stated pH range is set and maintained during the coupling reaction by addition
of
a total of about 90 parts of sodium bicarbonate.
b) In a second, separate reaction vessel, 353 parts of 4-amino-N-(3-1113-
sulfatoethyl)-sulfonyl)phenyl)benzamide are suspended in 1950 parts of ice-
water, adjusted to pH 6.5 - 7 with about 65 parts of sodium carbonate and
admixed with 154 parts of 40% sodium nitrite solution. This suspension is
added dropwise to a mixture of 550 parts of ice, 450 parts of ice-water and
320
parts of concentrated hydrochloric acid. After subsequent stirring at 5-
10°C for
2 hours, the excess nitrite is reduced with amidosulfonic acid and the
resulting
diazo suspension is pumped into the solution of the red monoazo dye of a).
This
reaction mixture is then admixed with 80 parts of 3-amino-5-
hydroxynapthalene=.
2,7-disulfonic acid.
Subsequently, at below 25°C, the pH is first adjusted to 1 .5 - 2.5
using sodium
bicarbonate and maintained, with acidic coupling to 3-amino-5-
hydroxynapthalene-2,7-disulfonic acid producing a red monoazo dye of the
formula (IID). After the end of acidic coupling a pH of 5 - 6 is a stablished
by
adding sodium carbonate and the reaction mixtured obtained after the end of
the
second coupling reaction of the monoazo dye of the formula (15-1 ) is admixed
with 100 parts of the yellow disazo dye of the formula (Ille-1 ).
The resulting 70 : 20 : 10 mixture of the dyes (IB), (IID) and (Ille-1 ) is
isolated
by spray drying.
The resulting dye mixture according to the invention dyes cotton in black
shades.
CA 02477136 2004-08-20
29
0
Na03SO~S \ \ I N / HO / S03Na
O~ .O H \ I N \
N I
(IID)
HZN
S03Na
O
~S,O
Na03S0-'
OH Na03S CHs
N N
N \ ~ N N ~ ~ ~ ~ N// ~ \~N
N
CH S03Na HO
3
~OS03Na
(Ille-1)
O;S
O
Example 5
406 parts of 4-amino-N-(3-((f3-sulfatoethyl)sulfonyl)phenyl)benzamide are
suspended in 2250 parts of ice-water, the suspension is adjusted to a pH of
6.5
- 7 with about 75 parts of sodium car bonate and 177 parts of 40% sodium
nitrite solution are added. This suspension is added dropwise to a mixture of
630
parts of ice, 520 parts of ice-water and 370 parts of concentrated
hydrochloric
acid. After subsequent stirring at 5-10°C for 2 hours, the excess
nitrite is
reduced with amidosulfonic acid and the resulting diazo suspension is pumped
into an aqueous solution of 426 parts of the red monoazo dye of the formula
(15-1 ), prepared as described in example 4a). This reaction mixture is then
admixed wth 80 parts of 3-amino-5-hydroxynaphthalene-2,7-disulfonic acid and
38 parts of 5-hydroxy-1-(4-sulfophenyl)-1 H-pyrazole-3-carboxylic acid.
Subsequently, at below 25°C, the pH is first adjusted to 1 .5 - 2.5
with sodium
bicarbonate and maintained, with acidic coupling producing a red monoazo dye
of the formula (IID). After the end of acidic coupling sodium carbonate is
added
to set a pH of 5 - 6 and the 70 : 20 : 10 mixture of the dyes (IB), (IID) and
(Illc-1 ) formed after the end of the second coupling reaction is isolated by
spray
drying. '
CA 02477136 2004-08-20
The resulting dye mixture according to the invention dyes cotton in black
shades.
5 Example 6
350 parts of 4-amino-N-(4-((f3-sulfatoethyl)sulfonyl)phenyl)benzamide are
suspended in 2000 parts of ice-water, the pH of the suspension is adjusted to
6.5 - 7 using about 44 parts of sodium carbonate, and 152 parts of 40%
sodium nitrite solution are added. This suspension is added dropwise to a
10 mixture of 560 parts of ice, 440 parts of ice-water and 320 parts of
concentrated hydrochloric acid. After subsequent stirring at 5-10°C for
2 hours,
the excess nitrite is reduced with amidosulfonic acid and the resulting diazo
suspension is pumped into an aqueous mixture of 474 parts of the red monoazo
dye (15-1 ) and 173 parts of the red monoazo dye of the formula (IIC),
prepared
15 in analogy to example 3a). This reaction mixture is admixed with 44 parts
of 4-
(5-hydroxy-3-methylpyrazol-1-yl)benzenesulfonic acid and subsequently adjusted
to a pH of 5 - 6 at below 25°C with sodium carbonate. After the end of
the coupling .,
reaction the resultant 73 : 16 : 1 1 mixture of the three dyes (IA), (IIC) and
(Illc-2)
is isolated by spray drying.
0
HN
N,N CH3
,o HO NON
Na03SO~s O
(Ills-2)
S03Na
Alternatively, the dye solution obtained can also be buffered at pH 5.5 - 6 by
addition of a phosphate buffer and adjuted by further dilution or further
concentration to provide a liquid brand of defined strength.
CA 02477136 2004-08-20
31
The resulting dye mixture according to the invention dyes cotton in black
shades.
Example 7
a) 281 parts of 4-(~i-sulfatoethylsulfonyl)aniline are suspended in 650 parts
of ice-
water and 180 parts of 30% hydrochloric acid and diazotized by dropwise
addition of
173 parts of 40% sodium nitrite solution. 174 parts of 4-amino-5-
hydroxynaphthalene-2,7-disulfonic acid, 87 parts of 6-amino-4-
hydroxynaphthalene-2-sulfonic acid and 17 parts of 2,4-diaminobenzenesulfonic
acid are added and coupled in a first step at pH 1 - 1.8 at below 20 °
C to form
a mixture of three monoazo dyes conforming to the formulae (15-1 ), (IIC) and
(Illa-1 ). The stated pH range is set and maintained during the coupling
reaction
by addition of solid sodium bicarbonate.
b) In a second, separate reaction vessel, 255 parts of 4-amino-N-(3-((f3-
sulfatoethyl)-sulfonyl)phenyl)benzamide are suspended in 1500 parts of ice-
water, adjusted to pH 6.5 - 7 with about 32 parts of sodium carbonate and
admixed with 1 1 1 parts of 40% sodium nitrite solution. This suspension is
added dropwise to a mixture of 410 parts of ice, 320 parts of ice-water and
235
parts of concentrated hydrochloric acid. After subsequent stirring at 5-
10°C for 2
hours, the excess nitrite is reduced with amidosulfonic acid and the resulting
diazo suspension is pumped into the mixture of the three monoazo dyes of a1.
Subsequently, at below 25°C, the pH is adjusted to 5 - 6 using sodium
carbonate
and the 67 : 23 : 10 mixture of the dyes (IB), (IIC) and (Illb-1 ) obtained
after the
end of the second coupling reaction is isolated by spray drying.
The resulting dye mixture according to the invention dyes cotton in black
shades.
CA 02477136 2004-08-20
32
O
o,,S O
~NH
OS03Na
Na03S0 N NH2 N
N ~ N ~ ~ S
NHZ O O
(Illb-1 )
S03Na
Examples $ to 159
The table examples hereinbelow describe further inventive mixtures of the dyes
of the general formulae (I) to (III), each recited in the form of the sodium
salts.
The mixing ratios are indicated in percent by weight. The dye mixtures provide
black dyeings, on cotton for example, by the dyeing methods customary for
reactive dyes.
CA 02477136 2004-08-20
M O O In
O O M pp
O .. .. .. ..
c'''o I~ O N t~
Cfl I~~ f~ CD
3
0 0
U Z o~ o,
f0 ~N .N
_ O O o' / ~ / ~ z
= 2 \ ~ ~ o
f" t v m
z=z z ~ z=z z ~
w
0
j z-z f' o t" - o f z ~n
O 1, O
Q \ ~ N t1J Z-Z Z=Z O Z
p N
y O'' ~ / D O' ~ / ~ O\ ~ ~ = U
O . O ~ O -V
O O
O
0
Z
Z
Z Z Z Z ,
p w o 0
O
M '" cn
\ ~ \ z ~ \ z
\ \ \ o _ \ o
p \ / 0 0 0
z z z z z = z z
Z ~ = 2 = U-O Z
Z _ Z
O -
O \ ~ O~O Z O \ ~ O , O \ ~ O O
N ~N v O ~ " O ~ -U
O
o p o 0
0
O >. 0 0 0 0
O z z z z
_O
Q
ca
X
N
O
+-. O
C
O
w ..-
O c
U O
U m
O
Q Q Q Q
+~ D
_X
d
O
W a o0 Q~ O
CA 02477136 2004-08-20
O O N
.- ~ r-
O O Ld 00
N N .- .-
O .. ..
o CO O tt~ O
n
z
z o
z~~
0
\Z / ~ N =Z ~ O U ~ Z-V
m
U ~, _ ~ ~ = O
N
O o z U I \ o o N z
ci? v ~ z/
/z ~'
U Z
O .r ~~ p Z _
CJ7 ~ in U_ '~ H N ~ ~ 2I
O _
° ~ ~ I ~ ~, y
O Z U
o" r°n / ~ .... p z
O z z
'° z z Z.
z
ON ~ ~ O
M
m
z ~ - p
O
O o z z
II = z z z
7 U_O Z S Z O O ZI Z O . Z S
stn U O y Z2 J ZS
O ~ ~= O; '. Z ..
p U~Z IZ ti~Z
O ~Z~
Z Z U
_(0
O
C
O
Q Q Q Q
D
a
,
N M d-
x
um-
CA 02477136 2004-08-20
In d' N O
O O O O
N N N N
o .. . ..
cep Lf~ Cfl 00 O
O O n
_\ ,o z z
N ~ 4
0 O
/ \ _ ~=o
0
0
-z xz
z/ \ c3 ~z Y
~z z/ \~'~ ,... z°z
xz z z
co O xz~ z
0
\ / f~ \ / ~Y' o
O ~ T' i
~i O z
Z N Z ~ ~ Z'Z
\ / Z \ /
O I
O
\ / \ / . r-
N N
N N Y-
R Z R
m
In z z Z z
M o 0
/ \ z '°
/_\ \ \ - o \ ~ \ - o
0 0 \ / ~ o o \ / '~
- ~x
'°_ '°= z z z z= z z
_cp Z z z z z z
o II z o II = o II z o II
O N Z cn Z U Z ~ Z
2
U = _
,I
\ / _ \ / =m Z~U \ / _ _ \ /
C -
_ Z \/ ~ a \/ ~ ~ \/
z- z_ z_ z
Z~ ~ Z~ Z-( U~ Z
O ~ Z u.
_co
v
O
C
O
4-
O
Q Q Q
N
a
E
n
x
w r- r- r-
CA 02477136 2004-08-20
O ~ O O
r- .- r- .-
in O N p
~- N N N
O . . .. ..
c"o tn tt~ 00 O
OC n CO (D I~
x"
z
0 o f,
v / z--~
~ z ~'
O , \
C
Zx
(77 Z O
N
y- z
z / \ N;o v'- v=
x y
D o
m ,~ z
z z o
O O °'
z / \
_\
_ o
m ~~ ~ \ z \ zx z z
Z m0 ~ O~n m0 ~ z x
_ O
Z= Z Z= O _ m Z= Z Z
O
c0 O z zx z z ~ ~ ~ Z = \ /
,~ ~ ~ Z =
o,
E O _ __
0 0
a o~ v
O
~z= ~ xz / ~ wn zx v ~ ~ ~ °
Z-\ '. ~Z \ / o O~ ~ Z-\ ,,O
S~Z /Z Z/_Z~2
O
D L1_ U 2 ~ Z
_f0
L
O
v-
C
N
O
Q Q Q Q
D
a>
Q
X O ~- N M
tL N N N N
CA 02477136 2004-08-20
O O O
r r r
a0 try O
r r N
O .. .. ..
cep N LSD O
z
0
N 2~
/Z=
2~
~~ /\Z Z
Z _ 2
O
\ ~ N
0 Z=Z
V
\ / ch
o m
,N
..
O U ~ "°,
0
D z'
m
z
m is O
Z
z z z v~
O
O
o / \
,, m - ~ - \
z z
O \ ~ O, \ ~ m
~~O ~O \ / O;~ O ~ ~ O p" z i
z z ~ \
_ z= z zx O,
O II
z z o %n \ /
O zx ~ / - O zx - Z \ / z Zx
r \ ~I\l
zx ~ / \ zx z
z z
Z I Z / ~ N Z Z2
2 -~ / _
N Z~ = Z O
'~ \ /
D U a
_O
O
O
v-
C
O
Q Q Q
0
_O
Q
X
u~ N N N
CA 02477136 2004-08-20
O O O
M I'~ N
N
O .. .. ..
cYo I'~ M 00
n n O
z
0
0
o,
\ x
z=z z ,~
_ z
x o
E NJ \ /
o z=z
'"' v
C ' \ /
o ,.
~N
p'. n n
o e- r
1 ' 1
N N N
Z
N
Z
Z 2 O
p ~
7 O U v~
m
\ Z
O
O
-_ O ZO= ~ 2x Z Z ~O z 2
t0 O O II O ~ Z = cn Z
vi z
O 'n O ~ ~ Z
L ~ U
\ ~ Z Q'n ~ ~ ~ z
Z
zx O \ ~ Z= ~ Z= a
z O Z O ~ Z
C O~ ~Z~ o ~Z~\ /Z
x \ ~ O ~ O Z ~ ~ Z
7
O
C
m
O
a Q Q Q
D
a>
Q
X
m N N N
CA 02477136 2004-08-20
O
O O
N N
O .. ..
~ca''o O N
t\
c9
7
O
C
a~
_ _
o N ~''~
a>
D
A
z z
0 0
C~ / \ / \
\
z _
0 0
z= z z
o ,~~ o
- p ? z zx ° z
- o II _
Z C o.
,, o o,
o ~ ~_ \ /
o° \ / z \
o -
"- \ /
Z~ i \
'z o
a~ ~ \
O
2
O 2
7
L
C
Q
Q
X Q c-
tL M M
CA 02477136 2004-08-20
Examples 32 - 57
Repetition of Examples 1, 2 and 8 - 31 using dye (IB) instead of dye (IA).
Examples 58 - 83
5 Repetition of Examples 1, 2 and 8 - 31 using dye (IC) instead of dye 11A):
o ,o
\ / ,S~OS03Na
/ N OH NHz N \
Na03SO~S~ O N / \ N
O~ ~O \
Na03S S03Na
(IC)
Examples 84 - 109
Repetition of Examples 1, 2 and 8 - 31 using dye (ID) instead of dye (IA):
o .o
\ / , S ~OS03Na
H
/ N / N OH NHZ N \
\ ( O N / \ N
,O
Na03SO~s~ Na03S \ / S03Na
Examples 1 10 - 135
Repetition of Examples 1, 2 and 8 - 31 using dye (IE) instead of dye (IA):
/ ~ ° o .o
'N \ / 1 S~OSO Na
3
~ N OH NH N
I 'O ii 2 ii
Na03SO~S~ N / I \ N
Na03S \ / S03Na
(IE)
CA 02477136 2004-08-20
O
r-
O O O O r- O O O ..
.. .. .. .. .. . .. ..
tn N 00 O Cfl CO tn I~ o ..
= ~ N r- N ~- ~- ~- ~- '+~
o .. .. .. .. .. .. .. .. m N
In d0 N O M ~fi In M
n O n ~ ~ n
E
'° o
E
o a~
O O M
c
N ~- .- r. M
0
O ~ U ~ N cB ~~. '~ v~-
p
m c~
z ac
O --
d' z
O
x
z z
w
z
4 ~ O
C ~ ~ ~ O
m
'f- O
O O
U C'7 U L7 U C~ U C~ >. _ ~ ~ n''o
p p o
M
N N
a a
m cv
x x
w ~ w
o ~ o
c E c E
o ~ o
o ~ o
U ~ U
(B y- (p
O N O
>. Q m U U D O u~ w ~ >. Q
+~.. p
X_ X_
a .~ a
E
M M ~ M ct ~t ~t d' ~ X d'
D W ~ ~- ~- ~ ~ r- r- c- Q ~,_, ~-
Ln
CA 02477136 2004-08-20
N O O O O
r- r- ~- r-
Cfl O I'~ 00 O
.- N r' e- N
o .. .. .. .. ..
N O M N O
n
o
C
a~
r r ~ ~ e-
O
O U c9 v~- ~+- N
D
m m
z
O
O ~
N 'n z
\ - o \
_ \ - z o \ / '~ \ / Q
= = o
m o \ / ° n = ~ x
z z
= z z~ _ Z ' n
x z
z
o z a \ / z
\ / o
o~ \ / o; cno 0 o Z z o
o ~-o / \ o 0
. z / \
a~ z \ / -~~ ~'=o U C7
o- " n
0 0 0
Z
0
0
;o
_ \ /
co z=z
o = ._
o \ /
C
Z=Z N
07 p O
Y- ~ Z
/
U ~ '~ lL
o ,o
Q
LSD Cfl I~ 00
X d' d' ~' d'
W
CA 02477136 2004-08-20
O O N O M
M ~ ~ M
o .. .. .. .. .. ..
In M O CO O
o= ~ ~ ~ n n
_co
O
C
N
Q) _ _ _
o N ~ M N
M
o ~ U ~ ~+- c0 ~+-
D
M
co
0
w-
c
0
O _ _ _ _ _
U C~ U C~ U C'7
0
o.. ~0 0
/ \ ~ ,o
Z / \~ / \~ o
0 0 0 o z
x
z = z o
\ / o \ / Z \ / ~ z
z-z ~n O z-z w
co z=z ~n
Z / \ ~ _ / \ Z / \
_ z _ _
o ° \ / V o \ / ° \ /
Z.Z ~ Z Z-_Z N Z 2-__Z f/~
O ~ O _ O O
o ° \ / _ ~ \ / z ° \ /
~/~~O CrJ ~/'~~p Z ~/N~O
O O O O
_O
Q
O ~ N M dw7
L7
X
W c- r- ~- e- ~ c-
CA 02477136 2004-08-20
O O O O
I~ ti7 00 O
e- ~ N
O .. .. .. ..
M ~ N O
n
i
0
O N
O in
~,N
/ \
z=z
O ~ ~ ~ -_z
/ \ ug ~ / \
G
O z_z z
o z=z
3 / \ 0 3 Ln
o,
I N O 1
N ~ ~ ~ O~Z- ~O
l OIIN'~ _ \ l
_(0
>_
O
4-
N
'I' ~ ~
~ Z
j" ~ Q ~ Q
D
_O
d
t9
X
UJ
O
+J
07
O O
O O
U O
U
t0 4-
O
a m LL C'J C7
0
X_
a.
N ~ CO I~ 00 M
LL~ LO Lf~ I~
W O
' CA 02477136 2004-08-20
Use example 1
2 parts of a dye obtained according to example 1 - 7 are dissolved in 999
parts of
water and 5 parts of sodium carbonate, 0,7 part of sodium hydroxide (in the
form of a
32.5% aqueous solution) and optionally 1 part of a wetting agent are added.
This
dyebath is entered with 100 g of a cotton fabric. The temperature of the
dyebath is
first maintained at 25°C for 10 minutes, then raised over 30 minutes to
the final
temperature (40-60°C) and maintained at that level for a further 60-90
minutes.
Thereafter, the dyed material is rinsed initially with tap water for 2 minutes
and then
with ion-free water for 5 minutes. The dyed material is neutralized at
40°C in
1000 parts of an aqueous solution containing 1 part of 50% acetic acid for
10 minutes. It is subsequently rinsed with ion-free water at 70°C and
thereafter
soaped off at the boil with a detergent for 15 minutes, rinsed once more and
dried.
This gives a strong navy to gray dyeing having very good fastness properties.
Use example 2
4 parts of a dye obtained according to example 1 - 7 and 5 parts of sodium
chloride
are dissolved in 999 parts of water, 5 parts of sodium carbonate, 0.7 part of
sodium
hydroxide (in the form of a 32.5% aqueous solution) and optionally 1 part of a
wetting
agent are added. This dyebath is entered with 100 g of a cotton fabric. The
rest of the
processing is as indicated in use example 1. This gives a strong navy to black
dyeing
having very good fastness properties.
Use example 3
8 parts of a dye obtained according to example 1 - 7 and 10 parts of sodium
chloride
are dissolved in 997 parts of water, 5 parts of sodium carbonate, 1.3 parts of
sodium
hydroxide (in the form of a 32.5% aqueous solution) and optionally 1 part of a
wetting
agent are added. This dyebath is entered with 100 g of a cotton fabric. The
rest of the
processing is as indicated in use example 1. This gives a jet black dyeing
having very
good fastness properties.