Note: Descriptions are shown in the official language in which they were submitted.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
LUBRICANT BLEND COMPOSITION
FIELD OF INVENTION
[0001] The present invention relates to lubricant fluid blends especially
suitable as base stocks for lubricant compositions. More particularly the
inventive relates to lubricant fluid blends based on hydroprocessed oils and
copolymers made from ethylene with one or more alpha-olefins.
BACKGROUND OF INVENTION
[0002] Most lubricant base stocks, including most of API Group I to Group
IV fluids, have viscosities at 100°C in the range of about 4 to about 6
cS. When
these base stocks are used to formulate different viscosity grade lubricants
it is
necessary to blend them with high viscosity base stocks. Currently, the
readily
available high viscosity base stocks include bright stock, high viscosity poly-
alphaolefin (PAOs) and polyisobutylene (PIB).
[0003] Bright stock and PIB have poor viscosity indicies (Vis) and poor low
temperature properties and hence their potential to improve blend properties
is
limited. This is especially true when blended with low viscosity hydro-
processed Group II, Group III fluids or isomerate tubes derived from Fischer-
Tropsch wax, which usually have VIs close to or greater than 100. Experience
has shown that when Group II, Group III or Fischer-Tropsch wax isomerate
fluids are blended with polyisobutylene (PIB) or bright stock, on many
occasions, the resulting blends have even lower VIs than the starting Group II
or
Group III fluids.
[0004] High viscosity PAOs have excellent viscometrics and low temperature
properties; however, they are more expensive than PIB or bright stock. More-
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-2-
over, the availability of PAOs is limited to some extent due to the limited
supply
of the linear alpha olefins, such as 1-decene, used in preparing them.
[0005] There is a need, therefore, for fluid lubricant base stocks having good
viscometrics, low temperature properties and shear stability that can be made
from readily available material.
[0006] Accordingly, one object of the present invention is to provide a blend
of lubricant fluids having improved viscometrics when compared to blends
containing PIB, bright stock or PAOs.
[0007] Another object is to provide lubricant fluid blends having improved
shear stability when compared to blends containing PIB, bright stock or PAOs.
[0008] Other objects and advantages will become apparent upon reading the
specification which follows:
SUMMARY OF INVENTION
[0009] Simply stated, the present invention is directed toward a fluid blend
suitable for use as a lube basestock comprising two major components: (A) a
polymer made from ethylene with one or more alpha-olefins and containing not
more than 50 wt% ethylene, the copolymer having a number average molecular
weight from up 400 to 10,000 and having a molecular weight distribution
(MWD) < 3 and (B) a polyalpha olefin or hydroprocessed oil having a VI greater
than 80.
[0010] In another embodiment a lubricating composition is provided compris-
ing the fluid blend and a lubricant additive package.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-3-
BRIEF DESCRIPTION OF DRAWINGS
[0011] Figures 1 to 4 graphically compare the viscosity of lubricant base
stock blends prepared from the copolymers of the invention with viscosities of
blends employing polyisobutylene or bright stock.
DETAILED DESCRIPTION OF INVENTION
[0012] One major component, component A, in the fluid blend of the present
invention is a copolymer made from ethylene with one or more alpha-olefins.
Consequently, as used herein, the term copolymer encompass polymers contain-
ing 2, 3 or more different monomer moieties. The copolymers in the blend of
the invention have a number average molecular weight of from 400 to 10,000
and a MWD < 3. Importantly, the copolymer contains not more than 50 wt%
ethylene. The alpha-olefin moiety of the copolymer will be derived from at
least
one or more C3, Cq. or higher alpha olefins.
[0013] Accordingly, suitable alpha-olefinic monomers include those
represented by the formula H2C = CHR1 wherein R1 is a straight or branched
chain alkyl radical comprising 1 to 18 carbon atoms and preferably 1 to 10
carbon atoms. When R1 is a branched chain, the branch is preferred to be at
least two carbons away from the double bond.
[0014] The copolymers are prepared by copolymerizing a feed containing
ethylene and one or more alpha olefins in the weight ratio of 60:40 to about
5:95
in the presence of a metallocene catalyst system.
[0015] Metallocene catalyst systems are well known in the art and mention is
made of U.S. Patent 5,859,159, incorporated herein by reference, for a descrip-
tion of metallocene catalysts systems useful for producing the polymers from
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-4-
ethylene and one or more alpha-olefins suitable for the lubricant fluid blends
of
the present invention.
[0016] The polymer is produced by polymerizing a reaction mixture of
ethylene and at least one additional alpha-olefin monomer in the presence of a
metallocene catalyst system, preferably in solution. Optionally, hydrogen may
be added to regulate the degree of polymerization or molecular weight, and to
reduce the amount of unsaturation in the product. In such situations the
amount
of hydrogen typically will be 0.1 mole% to 50 mole% based on the amount of
ethylene.
[0017] Any known solvent effective for such polymerization can be used.
For example, suitable solvents include hydrocarbon solvent such as aliphatic,
cycloaliphatic and aromatic hydrocarbons. The preferred solvents are propane,
isobutane, pentane, isopentane, hexane, isohexane, heptane, isoheptane,
Norpar,
Isopar, benzene, toluene, xylene, alkylaromatic-containing solvents, or
mixture
of these solvents.
[0018] The polymerization reaction may be carried out in a continuous
manner, such as in a continuous flow stirred tank reactor where feed is
continuously introduced into the reactors and product removed therefrom.
Alternatively, the polymerization may be conducted in a batch reactor, prefer-
ably equipped with adequate agitation, to which the catalyst, solvent, and
monomers are added to the reaction and left to polymerize therein for a time
sufficient to produce the desired product.
[0019] Typical polymerization temperature for producing the copolymers
useful herein are in the range of about 0°C to about 300°C and
preferably 25°C
to 250°C at pressures of about 15 to 1500 psig, and preferably 50 to
1000 psig.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-5-
[0020] The conditions under which the polymerization is conducted will
determine the degree of unsaturation in the resulting copolymer. As is known
in
the art, the degree of unsaturation of a polymer can be measured by bromine
number. In the present invention it is preferred that the copolymer have a
bromine number below 2 and more preferably in the range of 0 to 1.
[0021] In those instances where the product copolymer has a high degree of
unsaturation, such as when the copolymer product has a viscosity less than
about
1000 cSt at 100°C, the copolymer preferably is hydrogenated to provide
a final
product having a bromine number below 2. The hydrogenation may be carried
out in a batch mode or in continuous stir tank or in a continuous fixed bed
operation, using typical hydrogenation catalysts. Examples of the
hydrogenation
catalysts are nickel on kieselguhr catalyst, Raney Nickel catalyst, many
commercial hydro-treating catalyst, such as nickel, cobalt, molybdenum or
tungsten on silica, silica-alumina, alumina, zirconium support, etc., or
supported
Group VIIIB metals, such as platinum, palladium, ruthenium and rhodium. The
hydrogenation conditions may range from room temperature to 300°C with
hydrogen pressure from atmospheric pressure to 2000 psi for long enough
residence time to reduce most or all of the unsaturation. The unsaturation
degree
can be measured by bromine number of iodine index. Preferably the bromine
number of the finished product should be below 2. The lower the bromine
number the better the oxidative stability. More preferably, the reaction
temperature, pressure, residence time, catalyst loading all will be adjusted
to
achieve 0-1 bromine number.
[0022] In instances where the polymerization conditions favor the formation
of copolymers having a very low degree of unsaturation, hydrogenation of the
copolymer is not necessary and the copolymer can be used directly in forming
the lubricant blend.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-6-
[0023] The other major component, component B, in the fluid blend of the
present invention is a polyalpha olefin or a hydroprocessed oil having a VI
greater than 80. Examples of such oils are Group II and III oils, Fischer-
Tropsch
wax isomerates (as disclosed in US 6090989, US 6080301 or US 6008164) and
Group IV synthetic polyalpha olefin fluids.
[0024] The amounts of ethylene-a-olefin copolymer and hydroprocessed oils
in the blends of fluid the present invention are not critical and will depend
on the
intended use of the blend. In general the amount of ethylene a-olefin
copolymer
will constitute from about 1 to about 95 wt% of the blend. Generally, it is
prefer
to be from 5 to 80%. If too small amount of the polymer is used, the blend
will
not have sufficient viscometrics. On the other hand, if too much of the
polymer .
is used, it maybe more costly or the blend viscosity may be too high for
practical
use.
[0025] The fluid blends of the present invention can be combined with
selected lubricant additives to provide lubricant compositions.
[0026] The additives listed below are typically used in such amounts so as to
provide their normal attendant functions. Typical amounts for individual
components are also set forth below.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
Broad Wt% Preferred Wt%
Viscosity Index Improver 1 - 12 1 - 4
Corrosion Inhibitor 0.01 - 3 0.01 - 1.5
Oxidation Inhibitor 0.01 - 5 0.01 - 1.5
Dispersant 0.1 - 10 0.1 - 5
Lube Oil Flow Improver 0.01 - 2 0.01 - 1.5
Detergents and Rust Inhibitors0.01 - 6 0.01 - 3
Pour Point Depressant 0.01 - 1.5 0.01 - 1.5
Antifoaming Agents 0.01 - 0.1 0.001 - 0.01
Antiwear Agents 0.001 - 5 0.001 - 2
Extreme Pressure Additives0.001 - 5 0.001 - 2
Seal Swellant 0.1 - 8 0.1 - 4
Friction Modifiers 0.01 - 3 0.01 - 1.5
Fluid Blend of Invention _> 80% _> 80%
[0027] When other additives are employed, it may be desirable, although not
necessary, to prepare additive concentrates comprising concentrated solutions
or
dispersions of the dispersant, together with one or more of the other
additives to
form an additive mixture, referred to herein as an additive package whereby
several additives can be added simultaneously to the base stock to form the
lubricating oil composition. Dissolution of the additive concentrate into the
lubricating oil may be facilitated by solvents and by mixing accompanied with
mild heating, but this is not essential. The concentrate or additive-package
will
typically be formulated to contain the dispersant additive and optional
additional
additives in proper amounts to provide the desired concentration in the final
formulation when the additive package is combined with a predetermined
amount of the fluid blend of the invention.
[0028) All of the weight percents expressed herein (unless otherwise
indicated) are based on active ingredient (A.L) content of the additive,
and/or
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
_g_
upon the total weight of any additive-package, or formulation which will be
the
sum of the A.I. weight of each additive plus the weight of total oil or
diluent.
[0029] The composition of the invention may also include a co-base stock to
enhance lubricant performance or to improve additive solubility in the
basestock.
Typically co-basestocks are selected from polar fluids useful as lubricants.
[0030] Examples of these fluids include many types of esters, alkyl-
aromatics, and oil-soluble polyalkylene glycols. Typical esters used in
lubricant
formulations include polyol esters, adipate esters, sibacate esters, phthalate
esters, sterates, etc. Typical alkylaromatics used in Tube formulation include
alkylated naphthalenes, alkylbenzenes, alkyltoluenes, detergent alkylate
bottoms,
etc. Typical oil-soluble polyalkylene glycols include poly-propylene oxides,
poly-butylene oxides, etc. Such fluids may be used in amounts of about 1 wt%
to about 60 wt% although amounts of about 1 wt% to about 10 wt% are
preferred.
[0031] The present invention is further illustrated by the examples which
follow.
EXAMPLES
Example 1
[0032] 1-butene was charged at 100 ml/hour and ethylene was charged at 16
gram/hour to a 600 ml autoclave containing a catalyst solution of 20 mg
zirconocene dichloride, 0.4 gram methylaluminoxane and 50 gram toluene, and
cooled in an ice water bath. The feeds were discontinued after four hours.
After
12 hours of reaction at room temperature or below, the reaction was quenched
with water and alumina. The catalyst and any solid was removed by filtration.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-9-
The viscous liquid product was isolated in 90% yield by distillation at
140°C/0.1
millitorr for 2 hours to remove any light end. This liquid product was further
hydrogenated at 200°C, 1000 psi HZ pressure using 2 wt% nickel on
Kieselguhr
catalyst for 4 hours. The hydrogenated copolymer product had the following
properties: 100°C Kv = 45.8 cS, 40°C Kv = 548.0 cS, VI = 136,
pour point =
-36°C. This polymer contains 28.6 wt% ethylene as measured by C 13-NMR.
Example 2
[0033] Similar to Example 1, except ethylene was added at 20 grams per
hour. The distilled liquid yield = 92%. The hydrogenated product had the
following properties: 100°C Kv = 161.3 cS, 40°C Kv = 2072.8 cS,
VI = 190,
pour point = -25°C. This polymer contains 38.7 wt% ethylene as measured
by
C13-NMR. The Mn of this polymer is 2280 and MWD is 2.66.
Example 3
[0034] This polymer was prepared in a continuous mode of operation. In this
reaction, polymer grade ethylene, polymer grade 1-butene and polymer grade
iso-butane solvent were charged into a 200 gallon reactor after purification
through molecular sieve and treatment by injecting 50 ppm tri-t-butylaluminum.
The feed rates for ethylene, 1-butene and iso-butane were 12, 120 and 180
lb/hour, respectively. A catalyst solution, containing 5 x 10 6 g-mole/liter
of
dimethylsilylbis (4,5,6,7 tetrahydro-indenyl) zirconium dichloride and methyl-
aluminoxane of 1/400 Zr/Al molar ratio in toluene, was charged into the
reactor
at 13.5 ml/minute. The reactor temperature was maintained 89.4°C and
95.6°C,
pressure 237-261 psi and average residence time 2 hours. The crude reaction
product was withdrawn form the reactor continuously and washed with 0.4 wt%
sodium hydroxide solution followed with a water wash. A viscous liquid
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-10-
product was obtained by devolitalization to remove iso-butane solvent, light
stripping at 66°C/5 psig followed by deep stripping at 140°C/1
millitorr. The
residual viscous liquid was then hydro-finished at 200°C, 800-1200 psi
H2
pressure with 2 wt% Ni-on-Kieselguhr catalyst for eight hours. The hydro-
genated product contains 34 wt% ethylene content and had the following
properties: 100°C Kv = 114.0 cS, 40°C Kv = 1946.5 cS, VI = 145
and pour
point = -24°C. This polymer has Mn of 2374 and MWD of 1.88.
Example 4
[0035] This polymer was prepared in a similar manner as in Example 3,
except that the feed rates for ethylene, 1-butene and isobutane were 58, 120
and
283 lb/hour, and the reaction temperature was between 98.3°C and
101.1°C,
pressure 290-300 psi and average residence time 1 hour. After hydrofinishing,
the lube base stock contained 44 wt% ethylene and had the following
properties:
100°C Kv = 149.9 cS, 40°C Kv = 2418.4 cS, VI = 164 and pour
point = -24°C.
This polymer has Mn of 2660 and MWD of 1.76.
Example 5
[0036] This polymer was prepared in a similar manner as in Example 3,
except that the feed contained 40 wt% 1-butene, 11 wt% ethylene and 49 wt%
isobutane, the reaction temperature was 71 °C, and average residence
time 1
hour. After hydrofinishing, the hydrogenated product contained 19 wt%
ethylene and had the following properties: 100°C Kv = 1894 cS,
40°C Kv =
42608 cS, VI = 278 and pour point = -1°C. This polymer has Mn of 5491
and
MWD of 2.80.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-11-
Example 6
[0037] This polymer was prepared in a similar manner as in Example 3,
except that the feed contained 40 wt% 1-butene, 35 wt% ethylene and 25 wt%
isobutane, the reaction temperature was 93.3°C, and average residence
time
approximately 1 hour. After hydrofinishing, the tube base stock contained 44.5
wt% ethylene and had the following properties: 100°C Kv = 1493 cS,
40°C Kv
= 49073 cS, VI = 230 and pour point = 5°C. This polymer has Mn of 5664
and
MWD of 2.76.
Example 7
[0038] A series of blends were prepared using copolymers of the invention
and a hydroprocessed Group III or a Group II base stock. For comparative
purposes additional blends of the Group III and Group II basestocks were
prepared using the blending fluids shown in Table 1.
TABLE 1
Blending Fluid100C Kv, cS 40C Kv, VI Pour Point,
cS C
PIB H50 '~ 117 3442 104 - 15
PIB H300~ 663 25099 117 2
Bright Stock 32 474 96 - 7
100 cS PAO~ 100 1250 170 - 23
O PIB H50 and H300 are trade names for polyisobutylene sold by BP Chemical
Co. BP North America (chemicals), 150 W Warrenville Rd., N-3, Naperville,
IL 60563 USA.
The 100 cS PAO is available from ExxonMobil Chemical Co at Edison, NJ.
(0039] The properties of the blends made from the Group III basestocks with
the copolymers of Example 3, PIB H50 and bright stock were determined and
are shown in Table 2.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
- 12 -
b0
U
.a; , ~ O yO p ~ N d'
Cn ~ N ~ 00 ~ ~ ~ 01
U ~
H
'~ ON M M N
U
N ~ ~ 0 ~ ~ ~ ~ N
t 0
~ 0
U ~ N , ~ . M ~ N
N
W
0
O
U
~ ~ 0 ~ O O
c wt 0 M
0
N o M v'i O~ ~ WO ,_ d wi G1
",,
W O
a
~o
H
c
b ~
0 0 ~, o o ,~ 0 0
O C~ N ~ Ov N ~ pv N O
...
,..,
w
b M M M U U U
C!~C/~
U ~ ~ ~ G~7 L~ C .~ ~'.,
rI
O DC YG DC Q~,,Clr_
A'
.d
N M W v0 I~ oo Cn
c
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-13-
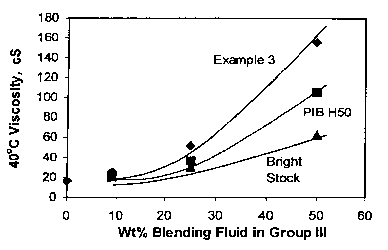
[0040] Although the Example 3 polymer and PIB H50 both have the similar
100°C viscosities, the blends from Example 3 have higher 100°C
and 40°C
viscosities than PIB at same weight percent (Figures 1 and 2). The thickening
efficiency for Example 3 is also higher than PIB. These data demonstrated that
the Example 3 sample have better viscosity boosting effect than PIB of compar-
able viscosity. Furthermore, the lube base fluids made from Example 3 and
Group III base stocks have higher VI at similar 100°C viscosity, as
shown in
Figure 3. Similar trends were observed when compared to the blends with bright
stock.
[0041] The properties of blends made from the Group III base stock with the
copolymer of Example 2, Example 4 and PIB H300 were determined and are
shown in Table 3.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
- 14 -
bn
~.,
U
'~ ~ ~ ~ M ~ ~ N 01 ~
~U
H
~ Ov '~ht~ ~n a\ ~n ~ M
~ t~ I~ 00 ~O l~ I~ ~ V'7M
U
N ~ ~ 0 '-'
O v0 v~ d; Q\ 0
O 0
U ~ N ~ ~ N v~'~ N .
0 1
O
d.
U
00 I~ ~O ~ O~
N ~ M N M ~ M
0
M
O
O
r~
G4
~
b O
~
~ O ,_,O O ,~ O O ,_,O O
U
_ O Gv N ~ Ov N ~ p~ N O
...,
b
w
N N N ~t ~' ~ p 0 0
x x x
x
U ~
U cd c~ cd c~ cd
(%~
O ~ N M d' V7 ~C ~ o0
~'
z
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-15-
[0042] Although Examples 2 and 4 fluids both have much lower 100°C
viscosities than PIB H300 (161 cS and 150 cS vs. 663 cS), the blends from
Example 2 and 4 fluids have higher viscosities than those from PIB H300. At
the same weight percent of blend stock, the thickening efficiencies of Example
2
and 4 fluids are higher than PIB H300. These data demonstrate that Example 2
and 4 fluids have better viscosity-boosting effect than PIB. Also, the VI of
the
blends from Example 3 and 5 fluids are higher than those from PIB H300
(Figure 4).
[0043] The properties of blends prepared form the Group III base stock with
the Example 5 and 6 fluids were determined and are shown in Table 4.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
- 16 -
U
U ~ O M O ~ C~
~~
U ' N N M
U
xw
~ V~ ~ Q~ ~ t~ 00
U
O OMO
V ~ N N ~ ~ N 'Od.
0
O
U
~ I~ ~ WO ~ N
U cri Wo c W wi o0
f
0
W o
W
E1 ,
0
0 0 0 0 0 0
~ N ~ ~ N
r.,
b
w
x
U
O v7 ~n v7 ~O ~O ~O
b
i
~ ~ ~
a ~ ~ W
w
w w w w w
as
.b
ON N N N N
"~
z
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-17-
[0044] As can be seen the blends have a VI that is higher than the Group III
base stock alone.
[0045] Blends were prepared from a Group II basestock with the Example 3
and 4 fluids and with PIB H50. The details and properties of the blends are
given in Table 5.
TABLE 5
Blend Blending 100C Kv, 40C Kv,
Number Fluid Wt% cS cS VI
25 PIB H50 9.1 10.62 90.96 99
26 PIB H50 25.0 14.65 147.06 98
27 PIB H50 50.0 24.93 342.48 94
28 Exam le 3 9.1 12.01 101.03 109
29 Exam le 3 25.0 18.93 179.30 119
30 Exam le 3 50.0 36.01 415.09 129
31 Exam le 4 9.1 12.51 97.88 122
32 Exam le 4 25.0 20.41 188.71 126
33 Exam le 4 50.0 40.25 413.78 147
[0046] As can be seen, the blends from Example 2 and 3 fluids had higher
viscosities and VIs then blends with PIB.
Example 8
[0047] A series of blends of ISO 32 viscosity grade were prepared from the
Group III base stock, Example 3 and 4 fluids, PIB PAO and bright stock. The
blend viscosities, thickening efficiency and shear stability (ASTM Test D
5621)
were determined and are shown in Table 6.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
- 18 -
~
U
, O
U
v
N O O N
U
V r--~,-- m.-~~ ,--i
w
~,
s
O O N M o
o.
O O O
~ ~ N
O ~
U .--~N ~ C WT
~
C M M ~ N N
/I
,~
M ~ N
O I~ O
U O O
U N ~
,--~ ,-~ov
M M ~ N N
H
due-oMO d:
U
O ~O ~O ~ ~O
O
s
M M M O~
M ~t M O N
di ~ U ~
w . . ,
r
~
w w w ~ w
~.
M M M M M
'~
z
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-19-
[0048] As can be seen, the blending fluids of this invention (Blends 34 to 36)
have comparable thickening efficiency as the best comparative example (Blend
38). At this comparable thickening efficiency, the copolymer blend of the
inven-
tion (Blend 34 to 36) has better shear stability than that of the PIB blend
37.
[0049] Similarly, a blend (blend no 38) is prepared using the Example 2 fluid,
which has a much broader MWD (2.66) than the Example 3 and 4 polymers.
The polymer again has excellent thickening efficiency (Table 6), better than
PIB
H300. However, this polymer still has better shear stability than PIB when
tested in the D5621 method.
[0050] Data in Table 6 further demonstrated that the blends containing
polymers from ethylene-alpha-olefins with narrower molecular weight
distribution have better shear stability. Blends 34 to 36 were prepared using
polymers with MWD of 1.75 to 2.01. They have slightly better shear stability
(0.2% viscosity loss) than the blend prepared by using polymer with MWD of
2.66 (blend 38 with 1.3% viscosity loss). Therefore, we conclude that blends
containing polymer made from ethylene and alpha-olefins with narrower MWD
are more desirable than blends made from ethylene and alpha-olefins with
broader MWD.
(0051] Table 7 compares the shear stability of the blends made with Example
and Example 6 (blend 39 and 40) versus a blend made with commercial
sample, Viscoplex 8-219 (available from RohMax USA, Inc) of comparable
thickening efficiency in a Group III base stock. As the data showed that
blends
39 and 40 have much better shear stability with only 1.3 and 1.6% viscosity
loss
as compared to the comparative blend 41 with 6% viscosity loss.
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-20-
TABLE 7
Shear Stability Comparison of Example 5 and 6
Polymers with Comparative Blends
Blend 100C 40C %
No. Blending Kv, Kv, Shear Shear Thickening
Fluid Wt% cS cS Viscosit Loss Efficienc
39 Exam le 6.8 6.68 30.91 30.42 1.6 211
5
40 Exam le 6.3 6.99 32.22 31.81 1.3 249
6
41 Viscoplex 6 6.36 32.68 30.69 6.1 167
8-219 (b)
Example 9
[0052] In another set of experiments, ethylene alpha-olefins copolymers were
prepared similar to Example 3 except using different amounts of ethylene in
the
feed. The polymers when blended with Group III base stocks are clear and
bright and have excellent viscometrics as shown in Table 8. These example
demonstrated that even with high ethylene content (44 wt%) and MWD of 2.3,
blends of excellent properties can be obtained.
TABLE 8
Blend Properties of Group III base stocks
with ethylene alpha-olefins of high ethylene contents
Wt% C2H4 Mn Wt% in 100C 40C
in blend bY Group vis, vis,
III
stock GPC MWD base stockcS cS VI Appearance
40.6 6667 2.23 5 7.59 36.35 184 clear
44.0 ~ 5050 2.3 5 ~ 6.59 32.73 181 clear
~ ~ ~ ~ ~
CA 02477331 2004-08-25
WO 03/076555 PCT/US03/06558
-21-
Comparative Example
[0053] Following the procedure of Example 3, except using higher ethylene
feed rate, a copolymer sample containing 50.8 wt% ethylene was prepared. This
polymer has Mn of 2386, which is comparable to example 3. However, it has
broader MWD of 2.81, instead of 1.88 as the Example 3 polymer.
[0054] This polymer with high ethylene content and broad MWD was found
to be not as good as that of Examples 1 to 7. When blended with same Group III
base stock used in the blend of the examples, the resulting blend was very
cloudy and the blend would not be used as high performance base stock.
Furthermore, when 20% of this comparative polymer was blended with Group
III base stock, the blend had only 124 VI, whereas a similar blend with
Example
3 polymer has VI of 167 or 158, as shown in Table 8.
TABLE 9
Comparison of blend properties
Blending Wt% Blending 100C 40C VI
Blend Stock Fluid in GroupKv, Kv,
Number Fluid III cS cS
Grou III - - 0.0 3.98 16.70 140
2 Exam le 25.0 9.41 51.78 167
3
3 Exam le 50.0 20.92 155.74 158
3
Comparative Comparative20 18.07 150.14. 124
blend of er