Note: Descriptions are shown in the official language in which they were submitted.
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
FLEXIBLE COATINGS FOR ELASTOMER SUBSTRATES
FIELD OF THE INVENTION
[0001 ] The present invention relates to weatherable coatings applied on
exterior
surfaces of flexible substrate articles, particularly elastomeric or rubbery
articles or
substrates containing such materials. In addition to providing protective film
properties, the coatings reduce heat buildup by directing heat away from the
article
(emissive). The coatings can be applied to an elastomeric substrate either
before or
after the substrate has been vulcanized.
BACKGROUND OF THE INVENTION
[0002] Engineered elastomeric products are designed to flex and bend, distort
and
recover, and/or dampen forces including absorbing torque or vibration
repeatedly
during their service life and are utilized in numerous industrial
applications. For
example, elastomeric materials are utilized in the manufacture of tires,
hoses, seals,
mountings such as engine mounts, dampers and insulating devices, and are
designed to exhibit hysteretic losses, and withstand heat, to name a few
design
aspects. These and other articles shaped into myriad articles have many
established uses such as industrial machines and parts for vehicles. Many
elastomer products come into contact with heat from a variety of sources, such
as
from internal combustion engines. Recent increases in operating temperatures,
and
reduction of the size of vehicular engine compartments give rise to closer
proximity
between heat sources and such molded parts as rubber hoses, plastic housings,
belts, various mounts, shrouds, seals, grommets, washers, spacers, covers, and
housings, etc.. Some of these articles are heat vulcanized, others are room
temperature vulcanized and still others are cured in a different manner and
exhibit
characteristic flexing, elongation, rubbery elasticity, as thermoplastics or
thermoset
materials.
[0003] All polymeric materials degrade on account of exposure to heat, light,
oxygen, ozone solvents, oils, and/or fuels. Elastomeric materials, and
especially
natural and/or synthetic vulcanized rubbers are particularly known to degrade
when
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
exposed to these agents, and there is a continuing search within industry to
provide
elastomer articles that are resistant to such degradative elements.
[0004] U. S. Pat. No. 6,022,626 discloses coatings suitable for covering
engine
mounts to protect the rubber substrate from oxygen, ozone and/or UV light,
especially when reaching temperatures of 220°F/104 °C, or more.
The coatings
taught provide a polymer barrier from chemical or UV intrusion. In exposure to
hot
environments, the polymers taught in U.S. Pat. No. 6, 022,626 may provide an
initial
barrier against oxygen, ozone and UV radiation but lack durability to repeated
flexure
over long periods of time. Once adhesion fails or the coating is breached by
cracks,
degradative effects resume. Such coatings as taught in U.S. '626 also do not
provide emissive properties and do not deflect heat.
[0005] U.S. Pat. No. 5,314,741 to Roberts, et. AI. entitled "Rubber Article
Having
Protective Coating" relates to polymeric articles which are coated with
hydrogenated
synthetic rubbers or polymers obtained by hydrogenating an unsaturated polymer
which is a polymer of 1,3-butadiene and optionally one or more
monoethylenically
unsaturated polymers.
[0006] Conventional polymeric stabilizers, UV absorbers and the like are used
for
the rubber articles coated thereon, yet improved aging properties are desired
even in
light of more harsh operating conditions.
[0007] Achieving sufficient permanent adhesion to the underlying rubber which
experiences repeated flexure or extension over long-term service life is
further
needing improvement.
[0008] Alkyd, urethane, and enamel metallic paint finishes are well known for
providing sparkled metallic effects, are widely used as on automotive bodies.
The
substrates are mainly metal or rigid plastic parts where flexure is limited or
the paints
are expected to crack if impacted severely. Speckled- effect metallic coatings
are
commonly provided on metal body panels, whereby 1 % or less metallic pigments
are
interspersed with coloring pigments, and overcoated with clear finish.
Likewise,
aluminized spray paints have been provided for applying to furniture, metal
articles
and the like, however the film forming materials utilized, cure to form a
coating of
very limited elongation, and would be unsuitable as coatings on flexible
substrates
2
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
such as engineered rubber articles due to flex cracking and loss of adhesion
not long
after placing the coating in service. Metal flake effect paints provide visual
aesthetics for appearance parts but do not provide heat emissive properties to
any
extent useful for extending the useful long term service of engineered rubber
products under hot environments.
[0009] One method of rendering elastomeric materials resistant to corrosive
materials is to apply a protective coating to the elastomeric material.
Various
corrosion-resistant coatings previously utilized for both flexible substrates
(e.g.,
elastomeric substrates) and rigid substrates (e.g., steel, stainless steel,
aluminum or
plastic) include polyurethanes, polysulfides and fluorocarbon elastomers. When
applied to rigid substrates, traditional corrosion-resistant coatings such as
fluorocarbon elastomers have been found to provide excellent resistance to oil
and
fuel. However, when applied to flexible elastomeric substrates comprising
natural
rubber and/or diene-type elastomers and mixtures, the fluorocarbon elastomers
suffer from poor fatigue resistance, poor low temperature characteristics, and
poor
adhesion to these substrates.
[0010] Low molecular weight polyolefin or polyisoolefin based elastomers
containing
a low level of chemically bound functionality such as an hydroxyl or an amine
bearing group are known for incorporation into urethane foams. Such elastomers
can be blended with and cured by an unblocked or blocked polyisocyanate. For
example, U.S. Pat. No. 4,939,184 discloses the preparation of flexible
polyurethane
foams made by reacting a low molecular weight polyisobutylene having two or
three
terminal hydroxy groups with a polyisocyanate in the presence of a blowing
agent.
[0011 ] U.S. Pat. No. 4,136,219 to Odam relates to two methods or processes
for
applying polyurethane paint to vulcanized rubber parts.
[0012] U.S. Pat. No. 4,670, 496 discloses tire sidewall striping paint as a
coloring
indicia of any color, such as a dye, and preferably metallic particles are
disposed in a
solution that contains unvulcanized diene rubbers) and rubber vulcanization
accelerator. Crosslinkable silicone and/or modified EPDM may also be disposed
in
the solution. The accelerator is essential for scavenging sulfur from the
vulcanized
rubber substrate to provide auto-vulcanizing of the coating rubber. In order
to
3
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
provide adequate adhesion for long term service as a coating for rubber
articles, a
diene polymer containing more than 10% residual unsaturation after curing will
necessarily undergo degradation and embrittlement and will fail long before
the
underlying substrate fails.
[0013] Diisocyanate containing free isocyanate groups has also been previously
proposed for curing copolymers of isobutylene and modified styrene containing
tertiary aminoalcohol groups in EPA 325 997. EPA 325 997 discloses
diisocyanate
curing of polymers having a molecular weight of 700 to 200,000, and
exemplifies
blends of up to about 30,000 weight average molecular weight (Mw) and about
8,600
number average MW (Mn), as measured by gel permeation chromatography.
[0014] A variety of bulk isocyanate-cured rubbers and mastics have been
disclosed
in the 50's and 60's. Isocyanate reactive functional groups present in the
elastomer
readily cure with NCO groups of the diisocyanate. As an example, U.S. Pat. No.
6,087,454 discloses a process to produce a cured bulk elastomer comprising
combining an elastomeric polymer, having an MW of 60,000 or more and
containing
hydroxyl and/or amine functional groups with a blocked polyisocyanate at a
temperature below the temperature that will unblock the isocyanate. The
mixture is
cured by heating it to a temperature above the temperature that will unblock
the
polyisocyanate. This reaction can be effected at room temperature by the use
of
unblocked isocyanates. Low molecular weight polyisobutylene containing hydroxy
functional groups are cured with a polyisocyanate in the presence of a blowing
agent
as is disclosed in U.S. Pat. No. 4,939,184.
[0015] U.S. Pat. No 4,774,288 discloses a hydrogenated copolymer of a
conjugated
diene and an a,[i--unsaturated nitrite containing an active phenol-
formaldehyde resin
vulcanization system. The disclosure is directed to the bulk vulcanizate,
which is
characterized as having good compression set properties and a good resistance
to
oils and good resistance to oxidative attack in air at elevated temperature
aging
under oxidizing conditions, however no mention is made suggesting coatings
could
be formed on flexible elastomeric substrates such as natural rubber and
polybutadiene which might provide useful properties.
4
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0016] U.S. Patent 5,314,955 discloses a coating composition consisting of (a)
a
hydrogenated acrylonitrile-butadiene copolymer, (b) a phenolic resin, (c) a
curing
component, and (d) a solvent. This coating solves many of the problems of
adhesion
to rubber substrates combined with fatigue resistance and fuel resistance. One
of
the drawbacks of this coating composition is that it requires a high
temperature bake
to cure the coating and to promote adhesion to adjacent metal surfaces. A high
temperature baking conditions even for a coating requires heat soaking of the
entire
article to be coated. Some parts such as helicopter rotor bearings would be
damaged by a high temperature bake, therefore coatings such as taught in '955
are
not practical to apply. The high temperature bake is also costly in production
since it
adds a time delay and additional handling of the parts. There still exists a
need for
improved protective coatings for flexible elastomeric substrates comprising
typical
natural rubber and/or diene-type elastomers that are resistant to fatigue over
a broad
temperature range, and that exhibit effective adhesion to the substrate, and
that can
be cured at room temperature if this is a limiting factor in coating an
article.
[0017] IJ.S. Pat. No. 6,156,379 discloses a conventional base-coat-clear coat
paint
on metal surfaces, containing metal flakes in the base coat. The novel
distinction is
based on bright pigments derived from finely divided vapor-deposited metal.
The
metallic coating composition is applied over a base coating layer and a clear
topcoating layer is applied over the metallic coating layer. A metallic
coating
composition is defined to consist essentially of the bright pigments and the
solvent,
meaning that coating composition either contains no ingredient other than the
flake
pigments and solvent, or a small amount of resin or additive such that the
pigment
weight concentration if 95% or higher. Binders such as acrylic, polyamide,
vinyl
chloride copolymers, urethane and polyesters are suggested. Such binders are
not
recognized as suitable for coating on flexible substrates as these can not
exhibit
100% elongation, and will fail from flex-cracking and adhesion loss after
placing in
service.
[0018] U. S. Patent 5,314,741 discloses a coating composition including a
latex of
highly saturated polymer such as hydrogenated nitrite rubber, highly saturated
styrene/butadiene copolymer, hydrogenated polybutadiene, or hydrogenated
s
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
styrene/vinyl pyridine/butadiene terpolymer. The coating is applied to a
substrate
and cured in place to yield a desired coated article reportedly resistant to
ozone,
oxygen, and UV light. Suitable curatives taught are zinc-sulfur cure packages.
Elevated temperatures are necessary to affect curing of these coatings.
Moreover,
conventional vulcanizing systems high in sulfur content and low vulcanization
accelerator content, or semi-efficient vulcanizing system having a moderate
dosage
of sulfur and vulcanizates accelerator known to the expert, and described e.g.
in W.
Hofmann, Kautschuk-Technologie, Genter Verlag, Stuttgart, 1980 p. 64 and 254-
255
have several drawbacks. Conventional vulcanizing coatings result in
vulcanizates
with good resistance to dynamic stresses (flex life) are very sensitive to
aging and
reversion. Semi-efficient vulcanizing systems usually give vulcanizates which
have a
less of a resistance to dynamic stresses (flex life), but, in return, they are
somewhat
more stable to aging and reversion (cf. R. N. Datta and W. F. Helt, Rubber
World,
August 1997, p. 24, et seq.)
[0019] It has been observed by the present inventors that coatings based on
highly
saturated elastomers utilizing vulcanizing chemistry suffer from loss of
adhesion to
substrates such as blends of natural rubber and diene elastomers widely used
in
rubber articles in the aforementioned articles, especially on automotive
tires, hoses
and the like. A need still exists for an improved elastomeric protective
coating for
flexible elastomeric substrates which provide improved adhesion to the surface
of
elastomers, and improved flex-resistance as well as thermal emissive
properties
enabling the reduction of heat transferred to the underlying polymer
substrate. The
level of stress from heat under long-term service in engineered products is
time and
temperature dependant. Any reduction in absorbed heat and any increase in the
release of heat within the elastomer can significantly extend the service/
performance life of the product. It would be industrially important to
decrease the
rate of heat absorption, and increase the rate of heat dissipation of
engineered
elastomer products in order to extend the useful working life of these
articles.
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
SUMMARY OF THE INVENTION
[0020] One embodiment according to the invention is directed to a non-emissive
coating composition of the invention which is resistant to long-term fatigue
and
temperature variability and provides for excellent adhesion to flexible
elastomeric
substrates. The coating cures at room temperature. The coatings comprise a
film
former polymer (Tg < 0 °C) containing less than 10% ethylenic
unsaturation before
curing. In a preferred embodiment, the coating composition of the invention
comprises (A) a carboxylated hydrogenated acrylonitrile-butadiene copolymer,
(X-
HNBR) (B) a curing component which contains at least one isocyanate group and
a
group which forms crosslinks, and (C) a solvent. The coatings exhibit cured
elongation of at least 100% and remain bonded to the substrate after long-term
weathering. The preferred coating composition comprises 3 to 30 weight percent
of
solids of (a) a carboxylated hydrogenated copolymer comprising a repeating
units
from a conjugated diene, an unsaturated nitrite, and a carboxyl monomer and
(b) a
curing component containing at least one isocyanate group and another group
which
forms crosslinks, and (C) a solvent.
[0021] In another aspect, there is a method for coating a substrate comprising
applying the aforementioned solvent-based coating to the surface of a
vulcanized
rubber substrate which is bonded to metal, drying the coating and allowing the
dried
coating to cure at ambient conditions, optionally with application of heat. It
is
preferred to provide the coating also onto the portion of exposed metal around
the
periphery of the elastomer. The present invention provides coatings for
elastomer-
metal composites with excellent adhesion to the elastomer substrate,
resistance to
corrosive materials and resistance to flex- fatigue over a wide temperature
range.
[0022] A further coating embodiment is an opaque, metal-filled emissive
elastomeric
coating, devoid of rubber accelerator and curable at ambient temperature are
provided. The coatings are in two parts which are mixed together at the time
of
application to the substrate. The first part comprises a flexible film-forming
polymer
exhibiting a Tg of less than 0°C and incorporated therein or thereon a
functional
group which is reactive to an active hydrogen containing curing agent, or the
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
functional group is an active hydrogen-bearing group, and a liquid carrier.
The
second or another part comprises a curing agent component containing either an
active hydrogen bearing group and a crosslinking group, or the curing agent
component contains a group reactive with active hydrogen and a crosslinking
group,
and a carrier liquid and (a) from 10 to 100 parts by weight per 100 parts by
weight of
film forming elastomer of thermally conductive metal particles having a
particle size
average of from 2 to 10 ~,m or (b) from 20 to 150 parts by weight of thermal
conductive particles having an average particle size of 20 to 60 microns.
BRIEF DESCRIPTION OF THE DRAWINGS
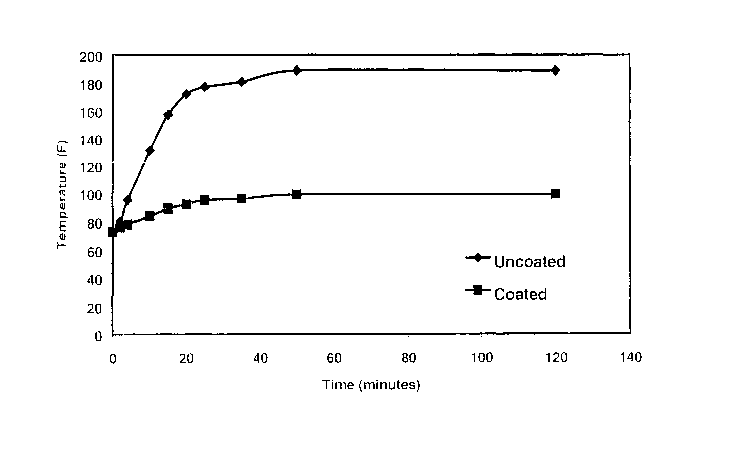
[0023] FIG. 1 is a plot of internal temperature versus time for a coated
versus
uncoated rubber block exposed to an infrared heat source over 120 minutes.
[0024] FIG. 2 is a graphical representation of the effect of a 0.001' (0.00040
cm)
thermal conductive coating applied to natural rubber on internal heat build
under
radiant heat at 0, 10 and 20 phr of a thermal conducting pigment.
[0025] FIG. 3 is a graphical representation of the effect of a 0.001' (0.00040
cm)
thermal conductive coating applied to natural rubber on internal heat build
under
radiant heat at 0, 10 and 20 phr of a thermal conducting pigment.
[0026] FIG. 4 is a graphical representation of the effect of a 0.001' (0.00040
cm)
thermal conductive coating applied to natural rubber on internal heat build
under
radiant heat at 0, 20 and 50 phr thermal conducting pigment.
[0027] FIG. 5 is a graphical representation of the effect of a 0.001' (0.00040
cm)
thermal conductive coating applied to natural rubber on internal heat build
under
radiant heat at 0, 20 and 50 phr thermal conducting pigment.
[0028] FIG. 6 is a graphical representation of the effect on the internal
temperature
of natural rubber blocks coated using three different thermal conductive
coatings
versus an uncoated block under radiant heat after 10 minutes.
s
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
DETAILED DESCRIPTION OF THE INVENTION
[0029] The coatings disclosed herein cure at ambient conditions and are
resistance
to solvents and fuels, and have ozone resistance. The coatings comprise film
forming polymer and a specified amount of particulate metal filler. The film
former
provides a film that is has at least 90% light transmission in the cured
state, and
contains no more than about 90% unsaturation after curing. The 90+% light
transmissive film forming matrix provides low loss of heat reflectivity and
thermal
transfer properties from the reflective metal particulate filler.
[0030] The coating results in reflection of significant heat from the
underlying
conductive particles of the coating, while the coating adheres permanently and
is
resistant to stress or environmental cracking or embrittlement. Such coatings
durably bond to molded rubber, TPE and plastic goods, such as pneumatic tires,
non-pneumatic tires, hoses, belts, mounts, shrouds, deflector panels, and the
like,
especially where used near hot bodies, like engine blocks or other industrial
components emitting heat. The cured coatings are mar and scuff-resistant.
[0031 ] The coatings cure under ambient conditions after coating on flexible
substrates to typical dry film thickness (DFT) of from about 0.5 to 20 mils
(12.7 ~,m -
508 ~,m). The coating is applied in liquid form using an aqueous or organic
carrier
depending on the selected cure agent and film former as a solution
substantially
devoid of water, or an aqueous dispersion. Faster curing can be obtained at
elevated heat conditions, with or without photonic energy, depending on
availability
of curing conditions available. An advantage of the present invention having
ambient cure is that a final assembled engineered rubber product with a
significant
thermal mass need not be heated to effect cure of the coating. The cured
physical
properties of the metal-filled coating films include resistance to flex-
fatigue over a
broad operating temperature range (-40°C - 150°C), resistance to
degradation on
long-term exposure to high temperatures and ozone and include excellent
adhesion
to flexible elastomeric substrates. The coating composition after curing at
room
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
temperature exhibits more than about 50% elongation without distorting (full
recovery), and more typically elongate up to 100%, 200% or 300% without
adhesion
loss, cracking, distortion or separation from the underlying flexing of the
elastomer
substrate. The heat reflective surface maintains its integrity to repeated
flexing and
the thermally conductive particles remain intact to provide a heat-emissive
surface.
[0032] The coating compositions contain at least one film former polymer or
prepolymer which contains functional groups as cure sites for a curing agent
without
the use of vulcanization chemicals. A curing agent is utilized typically from
5 to 100
(phr) parts per 100 parts of film former polymer. The thermal conductive metal
particles are specified hereinbelow in amounts on a weight basis depending
upon
the average size of the metallic particles.
[0033] Examples of useful film forming polymers that contain active hydrogen
functional groups are disclosed herein. Polymers containing functional groups
which
are reactive with active hydrogen containing cure agents are also disclosed.
Film
forming polymers suitable herein include a-olefin elastomers, conjugated diene
elastomers, hydrogenated diene elastomers, fluoroelastomers, ethylene-
carboxylate, ethylene-propylene-diene elastomers, functionalized ethylene-
vinyl
acetate, SB-diblock, SBS- and SIBS-triblock copolymers and hydrogenated
versions
thereof, acrylic rubber, and polyurethanes are adaptable for use herein.
Functional
groups can be provided in the film former by comonomers in the polymerizate,
or by
post-polymerization methods known in the art by conventional means. The
chemical
crosslinks between the curing agent and film forming polymer are an essential
feature of the invention for ambient curing, substrate adhesion and
durability.
[0034] In a preferred embodiment, the coating composition of the invention
comprises a functionalized hydrogenated acrylonitrile-butadiene copolymer (A)
(functionalized HNBR), a curing agent (B) which contains at least one
isocyanate
group, preferably a polyisocyanate, or isocyanate-functional prepolymer, or
isocyanato silane, or at least one multifunctional compound, oligomer,
prepolymer
having an isocyanate group and a group which forms crosslinks, and (C) an
organic
solvent. It is an important aspect of the present invention that the solvent
of the
io
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
coating composition can be either water based or hydrocarbon based. Aqueous
coatings are provided which contain reduced levels of volatile organic
compound
(VOC).
[0035] The coatings of the present invention are applied to elastomer
substrates
either prior or subsequent to vulcanization of the elastomer substrate. In one
aspect,
the present invention sets forth a method for coating a substrate comprising
applying
the coating to a surface of an unvulcanized rubber substrate and drying the
coating
at ambient or elevated temperatures, thereby curing the coating.
[0036] In another invention aspect, a method for coating a substrate is
provided and
comprises a step of applying the coating to the surface of a vulcanized rubber
substrate which itself may optionally be bonded to a metal component, drying
the
coating and allowing the dried coating to cure at ambient conditions,
optionally with
application of heat, light or radiation. When necessary, it is preferred to
provide the
coating also onto the portion of exposed metal around the periphery of the
elastomer.
[0037] The present invention provides exterior coatings for shaped or molded
polymeric articles such as elastomeric materials and elastomer-metal
composites
with excellent adhesion to the elastomer substrate, resistance to corrosive
materials,
resistance to heat build-up, and resistance to flex- fatigue over a wide
temperature
range.
[0038] The coating is formed by a mixture of two liquid parts at the time of
application to the substrate. Part A contains a liquid solution or dispersion
of a
functionalized polymer, and part B contains a liquid curing agent. When the
parts
are combined, the ambient temperature curable embodiments have a typical pot
life
of 30 minutes to one hour. The curable coating mixture of parts A and B
contain
from 2 to 20% solids content. The viscosity can be controlled depending on the
selected components and is less than 20,000 cps (Brookfield) such that the
coating
can be sprayed, brushed or dipped.
11
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
POLYMER FUNCTIONALI~ING METHODS
[0039] Functionalized elastomer film-formers used herein can be provided by
several
routes, such as by copolymerization and in various methods to modify film
forming
polymers by incorporation of functional groups to the polymer after
polymerization.
The term "functionalized" means that an active hydrogen-bearing moiety as part
of
an ethylenic unsaturated comonomer is copolymerized or, an active hydrogen
bearing compound is graft-linked, post-polymerization, The comonomer or
grafted
compound becomes covalently bonded to the polymer structure, and provides a
group capable of reacting with an ambient temperature curing agent.
[0040] The film former is prepared using conventional approaches for
incorporation
of an active hydrogen-bearing functional group on polymerized non-functional
elastomer such as by converting a functional group-bearing compound into a
suitable functional group precursor or the direct incorporation of a suitable
precursor
radical as may be accomplished when the elastomer is in solution or in the
molten
state via the "Ene" reaction, whereby an allylic hydrogen transfer to an
enophile
followed by coupling between two unsaturated termini occurs, or via free-
radical
addition across a carbon-carbon double bond in the molten state or in a dilute
solution with solvent. When the polymer is in the molten state, however, means
capable of imparting high mechanical shear, such as an extruder, will be used
to
effect the desired reaction to incorporate the functional group to be
converted or to
directly incorporate a suitable precursor radical. When the functional group
to be
converted to a suitable precursor or the precursor radical incorporated
directly is
incorporated via techniques such as metallation followed by reaction with a
suitable
electrophile, on the other hand, incorporation will, preferably, be
accomplished with
the polymer in solution.
[0041 ] Of the several methods available for incorporation of a functional
group or
functional group precursor, those methods tending to incorporate'a single
function
group or functional group precursor unit at each site of incorporation with
minimal
coupling of the elastomer polymer such as the Ene reaction and the method
involving metallation followed by reaction with an electrophile are preferred.
When a
functional group to be converted to a suitable precursor is incorporated into
the
12
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
elastomer, conversion of the functional group to the precursor radical will
also,
generally, be accomplished with the polymer in solution. In general, any of
the
solvents known to be useful for preparing such elastomer polymers in solution
may
be used to effect these reactions or conversions.
[0042] A variety of post-polymerization functionalization techniques are known
which
provide heretofore non-functional addition polymers with coupled crosslinking
cure
sites for use in the present invention. Hydroxyl groups are useful functional
groups
for effecting the crosslinking reactions with curing agents used herein. U.S.
Pat. No.
4,118,427 discloses hydroxyl-containing curable liquid hydrocarbon prepolymers
by
ozonizing a high molecular weight saturated hydrocarbon polymer such as
polyisobutylene or ethylene-propylene rubber, followed by reducing the
ozonized
material; e.g., by using reducing agents such as diisobutyl aluminum hydride,
to form
the above-noted hydroxyl-containing liquid prepolymers having a substantially
lower
molecular weight than the parent polymer
(A) FUNCTIONALIZED COMONOMERS
[0043] The curable film forming polymer employed herein can be formed by
copolymerization of elastomer-forming monomers together with functionalized
comonomers or by reaction of a polymer with a functional group containing
monomer
or reactive compound. The incorporated reactive group subsequently cures the
polymer by reaction of the curing component as described herein. The curing
method utilizes reactions of a crosslinking agent with an active hydrogen-
bearing
functional group or active hydrogen reactive group which crosslinks with the
corresponding reactive functional group on the copolymer or pendant on the
copolymer. It is convenient to introduce a functional group bearing comonomer
during polymerization of the film former polymer, as is conventionally
practiced. The
various approaches of free radical addition copolymerization, anionic addition
polymerization, free-radical graftlinking, metathesis grafting, and hydrolytic
grafting
are known in the art. The functional group containing polymers, or copolymers
include polymers characterized by their major constituents, such as a-olefin
13
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
elastomers, diene elastomers, hydrogenated diene elastomers, functionalized
fluoroelastomers, crosslinkable a-olefin copolymer elastomers, functionalized
acrylate or methacrylate acrylate copolymers, and ethylene-carboxylates, etc..
[0044] Preferred examples of rubbery copolymer elastomers include but are not
limited to anionic polymerized olefinic elastomers. Examples of anionic
polymerized
olefinic rubbers include ethylene-propylene rubber, ethylene-propylene-diene
monomer rubber, polyisobutylene, or "butyl rubber", or any other polymer of
isoolefin
optionally copolymerized with conjugated diene (such as isoprene), optionally
containing up to 30 wt. % or an a,[i-ethylenic unsaturated nitrite and/or
styrenic
comonomer (such as styrene and/or alkyl substituted styrene), and the like.
Particularly preferred elastomers include isobutylene-isoprene copolymer,
isobutylene-para methylstyrene copolymer and the like.
[0045] A suitable pendant active hydrogen functional group is provided by
methods
for forming amine-functionalized ethylene propylene diene monomer rubber
(EPDM)
by the process described in U.S. Pat. No. 4,987,200. Likewise higher molecular
weight isobutylene copolymers functionalized with hydroxyl groups can be
produced
using the process described in EPA 325 997. Furthermore any commercially
available halogenated isobutylene based polymer containing a low level of
halogen
typically 0.5 to 2.0 mole % can be combined with an alkylamine or an amino
alcohol
to produce the amine or the hydroxyl functional group respectively.
[0046] Functionalized elastomers having an weight average molecular weight of
1000 up to 200,000 and containing hydroxyl and/or amine functional groups are
known. Hydroxy terminated polyisobutylene are conventionally prepared by
introducing hydroxy groups into the terminal positions of cationically
polymerized
isobutylene by dehydrochlorinating, hydroborating and oxidizing chloro-
terminal
polyisobutylene. Chloro terminated polyisobutylenes obtained by cationically
polymerizing an isobutylene monomer are known. See Faust and Kennedy in,
"Living
Carbocationic Polymerization: III. Demonstration of the Living Polymerization
of
Isobutylene," Polym. Bull. 15:317-23 (1986), disclosing living carbocationic
14
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
polymerization of isobutylene and quenching the living recipe with methanol
and
other reagents such as amines.
[0047] Living polymerization methods are described in U.S. Pat. Nos.
5,350,819;
5,169,914; and 4,910,321 are preferred techniques to form the film forming
polymer.
General conditions under which living polymerizations can be achieved, for
example
using isobutylene include: (1 ) an initiator such as a tertiary alkyl halide,
tertiary alkyl
ether, tertiary alkyl ester, or the like; (2) a Lewis acid co-initiator which
typically
comprises a halide of titanium, boron or aluminum; (3) a proton scavenger
and/or
electron donor; (4) a solvent whose dielectric constant is selected
considering the
choice of the Lewis acid and the monomer in accord with known cationic
polymerization systems and monomer.
Terminal Functional Polymers.
[0048] Active hydrogen groups or groups reactive with active hydrogen groups
can
be incorporated at the terminus of film former polymers which are useful
herein.
U.S. Pat. No. 5,448,100 discloses sulfonated telechelic polyisobtuylene
prepared by
the "inifer" (initiator-transfer agents) initiated carbocationic
polymerization of
isobutylene with Lewis acid to form polymer, followed end-quenching with
acetyl
sulfate and precipitation by steam stripping or with methanol, ethanol,
isopropyl
alcohol, or acetone. The polymerization preferably occurs in a chlorinated
solvent,
most preferably in a mixture of solvents, such as methylene chloride, methyl
chloride, or an aliphatic or alicyclic compound containing five to ten carbon
atoms.
The Lewis acid can be, for example, boron trichloride or titanium
tetrachloride, or
other metal halide (including tin tetrachloride, aluminum chloride, or an
alkyl
aluminum). End-quenching preferably occurs at a temperature between -90
° to 0
°C, and most preferably at the polymerization temperature or at the
decomposition
temperature of the complex . The molar ratio of polyisobutylene to acetyl
sulfate is
preferably 1:1 or greater.
[0049] A film former polymer such as polyisobutylene can contain terminal
silane
groups bearing a hydroxy and/or alkoxy group. These can be obtained by a known
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
route of dehydrohalogenating a polyisobutylene polymer that contains tertiary
carbon-chlorine groups, followed by an addition reaction with an ethylenic
unsaturated silane. For example, chlorobutyl rubber having tertiary carbon-
chlorine
bonds can be reacted with allyltrimethylsilane to give a polyisobutylene
having an
unsaturated group then reacted under addition conditions with platinum
catalyst
using a hydrosilane compound of the general formula
R2
3-a
H S~ Xa
wherein R2 is a hydrogen atom, an alkyl group containing 1 to 20 carbon atoms,
an
aryl group containing 6 to 20 carbon atoms, an arylalkyl group containing 7 to
20
carbon atoms or a triorganosiloxy group of the formula (R')3 Si0-- (in which
each R'
independently represents a hydrogen atom or a substituted or unsubstituted
hydrocarbon group containing 1 to 20 carbon atoms), each X independently
represents a hydroxyl group or well-known hydrolyzable group, a is 0, 1, 2 or
3.
Alternatively a polymeric hydrosilane-terminal siloxane can be used.
Known hydrosilane compounds include halogenated silanes such as
trichlorosilane,
methyldichlorosilane, dimethylchlorosilane, phenyldichlorosilane;
alkoxysilanes such
as trimethoxysilane, triethoxysilane, methyldiethoxysilane,
methyldimethoxysilane,
phenyldimethoxysilane, etc.; acyloxysilanes such as methyldiacetoxysilane,
phenyldiacetoxysilane, etc.; and ketoximate silanes such as
bis(dimethylketoximate)methylsilane, bis(cyclohexylketoximate) methylsilane,
etc.
processes are described, for example, in Japanese Kokoku Publication Hei-4-
69659,
Japanese Kokoku Publication Hei-7-108928, Japanese Kokai Publication Sho-63-
254149, Japanese Kokai Publication Sho-64-22904, and Japanese Patent
Publication 2539445.
Functionalized Hydrogenated Diene Elastomers
[0050] Functionalized hydrogenated diene copolymers suitable for use herein as
the
film forming polymer are solvent soluble polymers preferably of a molecular
weight of
about 50,000 and higher, more typically 200,000 to 500,000, and contain no
more
than 10% conjugated diene segments by weight. These polymers are distinguished
from liquid, functionalized oligomers, such as reactive terminal-group
functional liquid
16
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
polymers, e.g., ATBN and CTBN. The unsaturated functionalized polymer for
preparing the hydrogenated coating polymer comprises broadly, from 50 to 85
percent by weight of conjugated diene monomer units, 5 percent to 50 percent
by
weight one or more non-conjugated, ethylenically unsaturated monomer units,
and 1
to 20 percent by weight of a functional comonomer or graft-linked compound
bearing
a reactive crosslinking site. The preferred conjugated diene monomer units are
derived from 1,3-butadiene monomer, and the non-conjugated ethylenically
unsaturated monomer units are derived from one or more ethylenically
unsaturated
monomers selected from unsaturated acrylic esters, methacrylic esters,
nitrites such
as acrylonitrile and methacrylonitrile, and monovinyl aromatic hydrocarbons
such as
styrene and alkylstyrenes, and vinylidene comonomers. Divinyl aromatic
hydrocarbons such as divinyl benzene, dialkenyl aromatics such as
diisopropenyl
benzene are preferably absent. Other comonomers include alkyl (meth) acrylates
such as methyl acrylate, methyl methacrylate, ethyl acrylate, butyl acrylate,
2-
ethylhexyl acrylate or methacrylate, vinyl pyridine, and vinyl esters such as
vinyl
acetate. The preferred functional comonomers are selected from unsaturated
carboxylic acids and esters thereof such as acrylic acid, methacrylic acid,
crotonic
acid, itaconic acid, and malefic acid. The glass transition temperature of
functionalized diene elastomer film formers must not exceed -10°C , and
preferably
is less than -25°C in order to provide acceptable flex-cracking/ flex-
fatigue resistance
in the thermal conductive particle filled coating.
[0051 ] Carboxyl end groups can be formed on diene elastomer high polymers
containing -C-CH=CH-C- type unsaturation by a chain scission methods in which
a
rubber ozonide is formed, and aldehyde end groups are oxidized to carboxyl
groups
using peroxide or peracid. Alternatively hydroxyl end groups on the rubber
ozonide
can be formed by reductive techniques by catalytic hydrogenation or by
reducing
agents like metal hydrides or borohydrides, and the like. See for example
British
Patent No. 884,448. Likewise, U. S. Pat. No. 4,118,427 discloses liquid
hydroxyl-
containing curable liquid hydrocarbon prepolymers by ozonizing a high
molecular
weight saturated hydrocarbon polymer such as polyisobutylene or ethylene-
propylene rubber, followed by reducing the ozonized material; e.g., by using
reducing agents, preferably diisobutyl aluminum hydride, to form the above-
noted
i7
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
hydroxyl-containing liquid prepolymers of lower molecular weight than the
parent
polymer.
[0052] Modification of a film-forming polymer by incorporation of
mercaptoalcohol or
mercaptocarboxylate grafting compounds yield useful film formers in the
present
invention. Suitable hydroxymercaptans and/or mercaptocarboxylic acid esters
containing hydroxyl. HS-R-OH compounds include those where R is a linear,
branched or cyclic C1 -C36 alkyl group which can optionally be substituted by
up to 6
further hydroxyl groups or can be interrupted by nitrogen, oxygen or sulfur
atoms.
Mercaptocaboxylates such as HS-(CHR2)n-(C(O)OR30H)m wherein R2 is hydrogen
or a C1 -C6 alkyl group, R3 is a linear, branched or cyclic C2 -C36 alkyl
group which
can optionally be substituted by up to 6 further hydroxyl groups or can be
interrupted
by nitrogen, oxygen or sulfur atoms, preferably -OH is primary, n is an
integer from 1
to 5 and m is an integer from 1 to 2 are suitable.
[0053] Preferred hydroxymercaptans are mercaptoethanol, 1-mercapto-3-propanol,
1-mercapto-4-butanol, a-mercapto-c~-hydroxyoligoethylene oxides, e.g., a-
mercapto-
c~-hydroxyoctaethylene glycol, or the corresponding ethylene oxide/propylene
oxide
copolyethers. Mercapto-ethanol and a-mercapto-w-hydroxyoligoethylene oxides
are
preferred. Preferred mercaptocarboxylic acid esters containing hydroxyl groups
are
esters of mercaptoacetic acid, mercaptopropionic acid and mercaptobutyric acid
with
ethylene glycol, propylene glycol, butylene glycol, diethylene glycol,
triethylene
glycol, tetraethylene glycol, octaethylene glycol, dipropylene glycol,
tripropylene
glycol, tetrapropylene glycol and N-methyldiethanolamine. The corresponding
esters
of mercaptoacetic acid and 3-mercaptopropionic acid are particularly
preferred.
Suitable types of elastomer film former base polymers reacted with the
mercapto
compound include polymers of containing isobutylene, chloroprene,
polybutadiene,
isobutylene/isoprene, butadiene/acrylonitrile, butadiene-acrylate copolymers,
S-B
copolymers, butadiene-vinylidene chloride-acrylate type copolymers provided
the
degree of unsaturation is 10% or less. Methods for incorporation of mercapto
compounds are described in U. S. Patent 6,252,008 incorporated herein by
reference and suitable for use as the functional film former polymer herein.
The
rubber contains in the region of 0.1 to 5 wt.% of bonded hydroxyl groups. The
18
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
molecular weight of the solution polymerized diene rubber containing hydroxyl
groups incorporated according to the method of U.S. 6,252,008 should lie in a
range
that dilute solutions of 5 tol5% solids can be obtained and be sprayable,
brushable
or dippable, such as from 10,000 to 200,000 M" (gel permeation
chromatogragphy).
[0054] There are other known approaches for incorporating OH groups into the
suitable film forming polymers used herein, such as by addition reactions with
formaldehyde, reaction with carbon monoxide followed by hydrogenation, and
hydroboration followed by hydrolysis. Copolymerization using silanes
containing an
ethylenic unsaturated group is a suitable method. Representative silane
comonomers include vinylsilane or allylsilane having a reactive silicon group.
Examples which may be mentioned include vinyltrichlorosilane,
vinylmethyldichlorosilane, vinyldimethylchlorosilane,
vinyldimethylmethoxysilane,
divinyldichlorosilane, divinyldimethoxysilane, allyltrichlorosilane,
allylmethyldichlorosilane, allyldimethylchlorosilane,
allyldimethylmethoxysilane,
diallyldichlorosilane, diallyldimethoxysilane, 'y-
methacryloyloxypropyltrimethoxysilane,
and 'y-methacryloyloxypropylmethyldimethoxysilane.
[0055] The functionalized diene elastomer will be described as follows with
respect
to a nitrite copolymer as the most preferred film former embodiment of the
present
invention. A functionalized butadiene acrylonitrile copolymer offers
beneficial film
characteristics such as low temperature flexibility, oil, fuel and solvent
resistance as
well as good abrasion and water-resistant qualities.
[0056] The present invention is most preferredly carried out with a
functionalized
hydrogenated nitrite rubber (HNBR). The functionalization of HNBR with
reactive
functionality provides methods for crosslinking the coating composition and
obtaining
the essential level of adhesion to the elastomer substrates. Without adequate
adhesion to the elastomer substrate, coatings exhibit premature flex-cracking
and/or
delamination . The functional groups for HNBR can be generally classified as
containing active hydrogen groups, ethylenic unsaturated groups or
hydrolyzable
groups.
19
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0057] Curing of the HNBR can be effected through the addition of crosslinking
components mentioned herein, by exposure to moisture, heat (infra-red,
thermal), by
UV radiation, or by e-beam radiation. Depending on the reactive functionality
incorporated into the diene copolymer. Some functionalized HNBR embodiments
mentioned herein below are self-curing without added crosslinker, and all can
be are
cured with suitable crosslinking components added to the functionalized HNBR
such
as but not limited to dinitrosobenzene, ZnO, gamma-POM, phenolic resoles,
multifunctional amine, polyisocyanates, polyacrylates, dicyandiamide ,
dicarboximides, and formaldehyde (or UF, MF) resins.
[0058] A functionalized HNBR can be prepared by a variety of ways known in the
art. Functional groups can be incorporated by the use of functional-group-
containing
comonomers, or by the use of graft-linkable, functional-group-bearing
compounds,
and by functionalization of NBR using metathesis, followed by hydrogenation of
the
modified NBR to give functionalized HBNR or reaction of NBR with methylolated
phenols followed by hydrogenation of the modified NBR to give functionalized
HBNR.
[0059] Functionalized HNBR containing active-hydrogen bearing functional
groups
are preferred crosslinkable film formers in the curable emissive coating
composition.
The presence of unsaturated groups (i.e., vinyl and disubstituted olefins,
nitrites) in
the NBR provides reactive sites in which reactive functionality may be
attached and
used for further crosslinking, post-polymer functionalization, and grafting
reactions.
These reactive sites can be modified through either catalytic or non-catalytic
chemistries. Such modification can introduce any number of active-hydrogen
functional groups such as epoxides by epoxidation of olefinic sites. Epoxides
are
readily converted to other functional groups through ring-opening reactions.
For
example, glycols are produced by ring-opening with base, glycol ethers with
alkoxides or phenoxides, alcohols with carbanions or hydrides. In addition,
epoxides
serve as crosslinkable sites using chemistry available to one skilled in the
art. Many
other functional groups may be introduced by reaction of the backbone olefins:
hydroformylation (aldhehydes, alcohols, carboxylic acids), hydrocarboxylation
(carboxylic acids), hydroesterification (esters), hydrosilylation (silanes),
hydroamination (amines), halogenation (halogens), chlorosulfonylation
(chlorine,
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
sulfonic acids), hydroboration (boranes, alcohols, amines). Examples of such
transformations have been reviewed by Tremont (McGrath, M.P.; Sall, E.D.;
Tremont, S.J. "Functionalization of Polymers by Metal-Mediated Processes,"
Chem.
Rev. 1995, 95, 381 ). The nitrite group of NBR elastomers also can be
converted to
an amide by reaction with alcohols in an acid catalyzed process and to
carboxylic
acids through hydrolysis.
\ ~ \
CN
Reactive Site ~
[0060] Crosslinking can be effected through the addition of a crosslinking
component, moisture, thermal, UV radiation, or e-beam radiation. Depending on
the
reactive functionality attached to HNBR and its intended use, suitable
crosslinking
components can be added to the functionalized HNBR such as dinitrosobenzene,
ZnO, gamma-POM, resoles, multifunctional amine, isocyanates, acrylates, and
dicyandiamide. Particularly preferred crosslinking components are those
components known in the art for obtaining good bonds to elastomeric articles.
These components include DNB, ZnO, and QDO and can be added to enhance the
adhesion of the functionalized HNBR to a wide variety of elastomeric
materials.
[0061 ] The reactive functionality incorporated onto the diene elastomer,
includes, as
non-limiting examples, phenolic OH, aliphatic OH, amine, isocyanate, epoxy,
acrylate, silyl ethers, silyl chlorides, anhydrides, maleimides, and Diets-
Alder
dieneophiles among the aforementioned functional groups.
[0062] The appropriate curing components and aids for the curing reactions are
well-known in the prior literature and patents in the adhesive and coating
area for
curing the R.F. of this invention. For example, when the functional group on
the
polymer is phenol, then isocyanate, dicarboximide, formaldehyde source, and
resoles are suitable curing agents that are useful for crosslinking the phenol-
functionalized HNBR. Likewise, amine functionalized HNBR can be crosslinked
21
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
using isocyanate or dicarboximide, formaldehyde source, and resoles, as
examples.
Epoxy functionalized HNBR can be crosslinked and cured with appropriate amines
and dicyandiamide components, as is known in the art of Epoxy adhesive and
coatings. Isocyanate functionalized HNBR is of particular interest because it
can be
crosslinked or cured by moisture or by the addition of other curative agents
such as
amine or polyols. Incorporation of the isocyanate as part of the HNBR is
particularly
desirable because it reduces that amount of free monomeric and therefore
volatile
isocyanate and its reported health and safety issues. A latent isocyanate
functionalized HNBR can be prepared by reaction of an amine functionalized
HNBR
(or NBR) with a diaryl carbonate to give a urethane functionalized HNBR (or
NBR).
Thermal cracking of the urethane forms the isocyanate functionalized HNBR (or
NBR) (For example, see: Kothandaraman, K.; Nasar, A.S. "The Thermal
Dissociation of Phenol - Blocked Toluene Diisocyanate Crosslinkers", J.M.S. -
Pure
Applied Chem. 1995, A32, 1009; Wicks, D.A.; Wicks, Z.W. "Blocked Isocyanates
III:
Part A. Mechanisms and Chemistry", Progress in Organic Coatings 1999, 36, 148;
Mohanty, S.; Krishnamurti, N. "Synthesis and Thermal Deblocking of Blocked
Diisocyanate Adducts," Eur. Polym. J. 1998, 34, 77). Maleimide functionalized
HNBR can be crosslinked either by the addition of a free radical initiator or
by
Michael addition reactions. Maleimides are known crosslinking agents. Acrylate
functionalized HNBR are capable of both free radical, UV and e-beam curing.
Anhydride functionality can be cured using amines and components described in
the
art for anhydride-epoxy adhesives. Silyl ether and chlorosilanes can be
utilized in
moisture-cured embodiments at room temperature. Diets-Alder adducts are self-
curing or by the addition of a metathesis type catalyst.
[0063] Exemplary detail of the aforementioned graft methods for incorporating
functional groups on a film forming elastomer is the melt processing of molten
film
forming elastomer with a polyfunctional graftlinkable material such as
polyfunctional
acrylate, maleated polybutadiene, and metal salts of difunctional acrylates.
For
example, an olefin elastomer such as EPDM can be masticated on a two roll
mill,
with 5 parts of an acid scavenger such as zinc oxide, 1 part stearic acid, an
antioxidant and a peroxide followed by addition of 5 to 10 parts of a multi-
ethylenic
unsaturated compound such as trimethylolpropane triacrylate, maleated liquid
polybutadiene, or zinc diacrylate to the flux roll.
22
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0064] Functionalized HNBR can be prepared by metathesis, followed by
hydrogenation of the modified NBR to give functionalized HNBR and (2) the
reaction
of NBR with methylolated phenols followed by hydrogenation of the modified NBR
to
give functionalized HBNR.
[0065] A novel method for incorporating a reactive pendant functional group,
such
as a carboxy, anhydride, hydroxy functionality is provided on a NBR elastomer
as
follows:
Direct functionalization of any suitable unsaturated film former polymer
usable
herein, and especially NBR, and is accomplished through the use of olefin
metathesis chemistry. Here, the olefin C=C double bonds are reacted with a
catalyst
and a monomer. The olefin metathesis catalyst must be capable of catalyzing
metathesis reactions in the presence of nitrite functional groups. The monomer
can
be any cycloolefin, olefin, or a,w-diene that is capable of undergoing an
olefin
metathesis reaction (e.g., ring-opening metathesis polymerization [ROMP],
cross-
metathesis, ring-opening-cross-metathesis, and acyclic diene metathesis
polymerization [ADMET]). These monomers are derivatized with groups bearing
functionality (e.g., carboxylic acids, amides, esters, anhydrides, epoxy,
isocyanate,
silyl, halogens, Diets-Alder diene and dienophiles, etc.) to provide cure
sites for
secondary crosslinking reactions of the cured film or to give new properties
to the
polymer. Kinetically, the metathesis catalyst will likely attack the vinyl C=C
bonds
first, however, their low levels in the HNBR copolymer may make attack at the
backbone C=C double bond competitive. Such attack on the backbone unsaturation
will likely cause a drop in molecular weight of the NBR, but the extent of
such a
process can be minimized by using high NBR-to-catalyst levels. After reduction
of
the modified NBR using for example the aforementioned catalytic hydrogenation
methods, a reactive modified HNBR polymer is obtained. The polymer can be
crosslinked using moisture, a selected curing agent, or an external energy
source
(UV or e-beam). One particular preferred advantage of metathesis catalysis is
that it
provides a unique means of introducing reactive functionality into NBR under
mild
conditions in water or in solvent. So even NBR latex can be modified with
reactive
functionality without de-stabilizing the latex through metathesis catalyst.
This feature
23
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
allows the functionalization of a variety of commercially well-known NBR
polymers, in
solution or as aqueous dispersions, and latexes (water-based polymerizate),
followed by hydrogenation to yield functionalized HNBR.
Hydrogenated Protic Group terminated Diene polymers.
[0066] Hydrogenated hydroxy or carboxy terminated diene polymers, alone, or in
blends with different high molecular weight (10,000 Mn and above) film forming
polymers are also suitable as a curable film former used in the emissive
coating of
the present invention. Substantially saturated polyhydroxylated polydiene
polymers
are known and commercially available. These represent anionic polymerized
conjugated diene hydrocarbons, such as butadiene or isoprene, with lithium
initiators, and terminated with OH groups. The process steps are known as
described in U.S. Pat. Nos. 4,039,593; Re. 27,145; and 5,376,745, all of which
are
hereby incorporated by reference for their disclosure of preparing
polyhydroxylated
polydiene polymers. Such polymers have been made with di-lithium initiator,
such as
the compound formed by reaction of two moles of sec-butyllithium with one mole
of
diisopropylbenzene. Such a polymerization of butadiene has been performed in a
solvent composed of 90% by weight cyclohexane and 10% by weight diethyl ether.
The molar ratio of di-initiator to monomer determines the molecular weight of
the
polymer. The polymer is capped with two moles of ethylene oxide and terminated
with two moles of methanol to produce the dihydroxy polybutadiene. The
hydroxylated polydiene polymer is hydrogenated where substantially all of the
carbon-to-carbon double bonds become saturated. Hydrogenation has been
performed by those skilled in the art by established processes including
hydrogenation in the presence of such catalysts as Raney Nickel, noble metals
such
as platinum and the like, soluble transition metal catalysts and titanium
catalysts as
in U.S. Pat. No. 5,039,755. Suitable polyhydroxylated polydienes are those
available
from Shell Chemical Company in the U.S.A. under the trade designation of
KRATON
LIQUID~ POLYMERS, HPVM 2200 series products, and from ATOCHEMIE under
the PoIyBD~ mark. The high molecular weight polymers suitable in blends with
the
hydrogenated hydroxyl butadiene polymers are not limited, and include for
example
the aforementioned' carboxy modified chlorinated polyethylene, chlorinated
24
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
polyethylene, polymers of epichlorohydrin, ethylene-acrylic copolymers, SBR,
SBS,
nitrite rubber (NBR), SIBS, EPDM, EPM, polyacrylates, halogenated
polyisobutylene, and polypropylene oxide, among others mentioned herein, and
known. The weight proportion of lipuid hydrogenated polybutadiene polyol to
high
molecular weight film former is limited such that the percent of unsaturation
in the
combination is less than 10% overall. Therefore where mixtures of the
hydrogenated
polydiene polyol are made with unsaturated high polymers such as SBR, NBR, and
the like, the proportion of unsaturated polymer will be limited to maintain
the overall
degree of saturation of at least 90%. Modified chlorinated polyolefins can
include
those modified with an acid or anhydride group. Some examples of modified
chlorinated polyolefins are described in U.S. Pat. Nos. 4,997,882 (column 1,
line 26
to column 4, line 63); 5,319,032 (column 1, line 53 to column 2, line 68); and
5,397,602 (column 1, line 53 to column 2, line 68), hereby incorporated by
reference.
The chlorinated polyolefins preferably have a chlorine content of from about
10 to 40
weight percent, more preferably from about 10 to 30 weight percent based on
the
weight of starting polyolefin. One suitable example of a modified chlorinated
polyolefin is the modified chlorinated polyolefin that has a chlorine content
of from
about 10 to about 30 weight percent based on the weight of polyolefin, which
is not
neutralized with an amine, and has an acid value in the range of about 50 to
about
100.
Hydrogenated Block Copolymers
[0067] Suitable film formers adaptable according the invention are
hydrogenated
styrene-butadiene-styrene block copolymers, hydrogenated styrene-isoprene-
styrene block copolymers, which are modified according to methods disclosed
herein
above, adapted for chlorinated polyethylene, and elsewhere provide cure
functionality on the block copolymer for interaction with the curing agent.
Some
elastomeric block copolymers containing carboxyl groups are available
commercially. Those block copolymers which contain unsaturation can be
hydrogenated according to known hydrogenated methods, including methods
referenced herein.
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Phenol Functional Elastomer
[0068] Functionalization of HNBR with phenol functionality can be carried out
by the
combination of a methylolated phenol and the NBR, followed by hydrogenation of
the
phenol-modified NBR intermediate. Methylolated phenols can form covalent bonds
with NBR and NBR copolymers by a variety of chemical reactions as reported in
the
literature [A. Knop and L. Pilato, "Phenolic Resins Chemistry and Applications
and
Performance" Springer-Verlag, New York 1985, Chapter 19 pg 288-297].
[0069] Various known isocyanate-reactive functional groups can be incorporated
in
a functionalized elastomer film-forming polymer. The aforementioned carboxy-
functional, hydroxy-functional and amine functional elastomers are most
readily
adaptable. Functional comonomers, like carboxy-functional comonomers are
readily
adaptable to form a copolymer of carboxylated hydrogenated nitrite rubber. For
the
purposes of the present invention, the functionalized hydrogenated nitrite
rubber can
be defined as a polymer comprising at least one diene monomer, nitrite
monomer,
and a functional group-bearing compound such as a comonomer or a graftlinking
compound containing a functional group or a combination thereof. When the
abbreviation HNBR is utilized herein, it is to be understood that the term
refers to
rubbers which can include diene monomer other than 1,3 butadiene, and
comonomers other than acrylonitrile, unless specifically stated. It is also
important to
note that additional monomers can be polymerized along with or grafted to the
diene
monomer to form the functionalized HNBR. The additional monomers can, for
example, provide at least one functional group to facilitate crosslinking.
[0070] Functionalization of HNBR with phenolic functionality can be carried
out with
the unsaturated un-hydrogenated polymer, or a partially hydrogenated XHNBR
polymer (80-97% hydrogenation level) by addition of methylol phenol or ether
derivative under heat and optionally catalyzed by suitable Lewis acid .
Preferably an
ether blocking group is provided on the methylol phenol compound, facilitating
ease
of post reaction hydrogenation. Addition can be through the nitrite or
carboxyl
groups by ester formation, or by way of the aforementioned addition at allylic
sites.
Preferably a metathesis reaction of an ethylenic unsaturated compound bearing
a
phenol group can be done in solvent or water. Alternatively, an olefinic
bearing
26
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
methylolated phenyl ether or phenol can be metathesized with NBR, followed by
hydrogenation. The phenol functionalized NBR is subsequently hydrogenated. A
methylolation reaction can be undertaken using a phenol functional NBR or HNBR
with formaldehyde to generate a methylolated phenol functionality in the NBR,
or
with HNBR. Methylolated phenols can form covalent bonds with NBR and NBR
copolymers by a variety of chemical reactions as reported in the literature.
See, A.
Knop and L. Pilato, "Phenolic Resins Chemistry and Applications and
Performance"
Springer-Verlag, New York 1985, Chapter 19, pg. 288-297. The following
structural
diagrams illustrate functionalizing with a representative phenolic bearing
compound.
H H H H OH OH
+ ~ \
H~\HJ ~ H C-NJ n
R
OH OH H ~CH~
~N=C
-~ O
R1-4 N
HO
R1-4
OH OH H ~ iH~
\ O= C
O
C
R1 4 HO \\O
HO
R1'4
OH OH ~H- iH~%'~
\ H2C
OH
14
R1
27
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0071 ] While it is possible to combine any methylolated phenol with NBR, mono-
methylolated phenols are especially preferred. The combination of Mono-
methylolated phenols with NBR polymers yields phenol functionalized-NBR
products
which are stable. After hydrogenation of the phenol-modified NBR according to
known procedures in the art (e.g. cat. hydrogenation), a stable phenol-
modified
HNBR copolymer is obtained. The phenol-functionalized HNBR copolymer can be
crosslinked with a variety of well-known crosslinkers for phenolic resins
including
those selected from dicarboximides, isocyanate, and formaldehyde source
(paraformaldehyde, gamma-POM, hexamethylene amine, phenolic resoles or
etherified phenols).
[0072] Methylolated phenol functionalized nitrite rubber (NBR) or hydrogenated
versions (HBNR) can be prepared by procedures known in the art. The phenol
functionalized NBR/HNBR can be prepared by either the mono-methylolated phenol
or by metathesis involving unsaturated monomer with the unsaturated NBR. The
methylolated phenol functionalized NBR/HBNR prepared by metathesis utilizes a
methylolated phenolic monomer with NBR. These materials are useful not only as
coatings in accordance with the present invention, but also as components of
elastomer-to-metal adhesives, autodepositing materials, RFL dips, and reactive
tougheners (e.g. epoxy adhesives) taking advantage of their unique curing,
film-
forming, metal adhesion and compatibility properties. Methylolated phenol
functionalized NBR/HNBR are capable of self-curing (i.e. without an external
curing
agent). Methylolated phenol functionalized NBR/HNBRderivatives are capable of
curing with other coating components, such as phenolic novolaks, active
hydrogen
reactive or active hydrogen containing crosslinkers and rubber/elastomer
toughening
agents. Methylolated phenol functional HNBR can be used with known vulcanizing
agents for rubber. The vulcanization reaction is based on the formation of
either a
quinone methide or a benzylic carbenium that is generated by the thermal or
catalytic activation of the methylolated phenols. The quinone methide
intermediate
reacts by abstraction of allylic hydrogen. Alternatively, methylolated phenols
can
generate reactive benzyl carbenium ions under acidic catalyzed conditions
which will
react with unsaturated polymers in the substrate.
28
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0073] When the reactive functional group on the HNBR is phenol, then
isocyanate,
dicarboximide, formaldehyde source, and resole curing agents are useful for
crosslinking the phenol-functionalized HNBR to the elastomer substrate.
Likewise,
amine-functionalized HNBR can be crosslinked using isocyanate or
dicarboximide, a
formaldehyde source, and/or resoles, as examples. Epoxy-functionalized HNBR
can
be crosslinked and cured with known curing agents, e.g., amines, amidoamines,
and/or dicyandiamide, well known in the art of Epoxy adhesives.
[0074] Isocyanate functionalized HNBR can be crosslinked or cured by moisture
or
by the addition of other curative agents such as amine or polyols.
Incorporation of
the isocyanate as part of the HNBR is particularly desirable because it
reduces that
amount of free monomeric and therefore volatile isocyanate and its reported
health
and safety issues. Maleimide functionalized HNBR can be crosslinked either by
the
addition of a free radical initiator or by Michael addition reactions.
Ethylenic
unsaturated acrylate-functionalized HNBR is capable of both free radical, UV
and e-
beam curing. Anhydride functional HNBR can be cured using amines and
components described in the art for anhydride-epoxy adhesives. Silyl ether and
chlorides are moisture curing. Diets-Alder adducts are self-curing or by the
addition
of known metathesis catalysts.
[0075] To provide the ethylenically unsaturated nitrite-conjugated diene
rubber with
at least 90% saturation, the nitrite rubber is hydrogenated by conventional
means.
Generally any of the numerous known processes for hydrogenation can be
utilized,
including but not limited to, solution hydrogenation and oxidation/reduction
hydrogenation. The hydrogenation serves to saturate at least 90% of the
unsaturated bonds of the rubber. When the degree of saturation is less than
90%,
the rubber's heat resistance is low, The more preferred degree of saturation
of the
rubber is 95-99.99%.
[0076] The preferred conjugated diene monomers useful for preparing the
carboxylated acrylonitrile-butadiene copolymers which are further hydrogenated
can
be any of the well-known conjugated dienes including dienes having from about
4 to
about 10 carbon atoms, such as, but not limited to, 1,3-butadiene; 2-methyl-
1,3-
29
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
butadiene, 2,3-dimethyl-1,3-butadiene; 1,3-pentadiene; 1,3-hexadiene; 2,4-
hexadiene; 1,3-heptadiene; piperylene; and isoprene, with 1,3-butadiene
presently
being preferred.
[0077] The unsaturated nitrite monomers copolymerized to form a carboxylated
acrylonitrile-diene copolymer typically correspond to the following formula:
ACC CN
A
wherein each A is hydrogen or a hydrocarbyl group having from 1 to about 10
carbon
atoms. Examples of A groups include alkyl and cycloalkyl, such as methyl,
ethyl,
isopropyl, t-butyl, octyl, decyl, cyclopentyl, cyclohexyl, etc., and aryls
such as phenyl,
tolyl, xylyl, ethylphenyl, t-butylphenyl, etc. Acrylonitrile and
methacrylonitrile are the
presently preferred unsaturated nitrites.
[0078] The HNBR of the present invention also includes functional group
containing
monomers which are polymerized into the backbone of the HNBR, or functional
group containing compounds which have been grafted to the HNBR, or a
combination thereof.
[0079] Carboxyl group containing monomers are optionally utilized in the film-
forming
elastomer used in the present invention. Carboxyl groups can be provided by
a,[i-
unsaturated monocarboxylic acid monomers with 3 to about 5 C-atoms such as
acrylic acid, methacrylic acid and crotonic acid and/or other known carboxyl
group-
containing monomers such as, but not limited to cc,[3-unsaturated dicarboxylic
acids
with 4 to about 5 or about 6 C-atoms, e.g., malefic acid, fumaric acid,
citraconic acid
and itaconic acid, and anhydrides of these. The bound unsaturated carboxylic
acid
may be present in an amount of from about 1 to about 10 weight percent of the
copolymer, with this amount displacing a corresponding amount of the
conjugated
diolefin. Preferably, the monomer is an unsaturated mono- or di-carboxylic
acid
derivative (e.g., esters, amides and the like). Functions of the carboxyl
group-
containing monomers include serving as a crosslinking site and enhancing
adhesion.
[0080] Additional, other functional comonomers can be copolymerized into the
backbone of the HNBR copolymer. Examples of the functional ethylenically
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
unsaturated monomers which are copolymerizable with the nitrite monomers and
the
conjugated diene monomers are: hydrazidyl-group containing ethylenic
unsaturated
monomers, amino-group-bearing ethylenic unsaturated monomers, thiol-group
bearing unsaturated ethylenic unsaturated monomers, unsaturated carboxylic
acids
such as acrylic acid, methacrylic acid, itaconic acid and malefic acid and
salts
thereof, alkyl esters of unsaturated carboxylic acids such as various
acrylates, for
example methyl acrylate and butyl acrylate; alkoxyalkyl esters of unsaturated
carboxylic acids such as methoxy acrylate, ethoxyethyl acrylate, methoxyethyl
acrylate, acrylamide, and methacrylamide.
[0081 ] Also suitable as functional comonomers are various classes of monomers
such as N,N-disubstituted-aminoalkyl acrylates; N,N-disubstituted-aminoalkyl
methacrylates; N,N-disubstituted-aminoalkyl acrylamides; N,N-disubstituted-
aminoalkyl methacrylamides; hydroxyl-substituted-alkyl acrylates and hydroxyl-
substituted-alkyl methacrylates, N-alkylol substituted acrylamides such as N-
methylolacrylamide, N,N'-dimethylolacrylamide and N-ethoxymethylolacrylamide;
N-
substituted methacrylamides such as N-methylolmethacrylamide, N,N'-
dimethylolmethacrylamide and N-ethoxymethylmethacrylamide especially where
free
radical initiated copolymerization occurs in the presence of an alkylthiol
compound
having 12 to 16 carbon atoms three tertiary carbon atoms.
[0082] As specific examples of the hydroxy-substituted-alkyl acrylate and
hydroxy-
substituted-alkyl methacrylate comonomers, there can be mentioned
hydroxymethyl
acrylate, 2-hydroxyethyl acrylate, 2-hydroxypropyl acrylate, 3-hydroxypropyl
acrylate,
3-chloro-2-hydroxypropyl acrylate, 3-phnoxy-2-hydroxypropyl acrylate,
hydroxymethyl methacrylate, 2-hydroxyethyl methacrylate, 2-hydroxypropyl
methacrylate, 3-hydroxypropyl methacrylate, 3-chloro-2-hydroxypropyl
methacrylate
and 3-phnoxy-2-hydroxypropyl methacrylate. Of these, hydroxymethyl acrylate, 2-
hydroxyethyl acrylate, hydroxymethyl methacrylate and 2-hydroxyethyl
methacrylate
are preferable.
[0083] The NBR copolymers are polymerized by reaction of the any of the
aforementioned exemplary conjugated dienes, unsaturated nitrite, and
unsaturated
functional-group containing comonomers in the presence of a free radical
initiator by
31
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
methods well known to those skilled in the art. Suitable free radical
initiators are
beyond the scope of this disclosure, and are typically organic oxides,
peroxides,
hydroperoxides, and azo compounds, etc., such as hydrogen peroxide, benzoyl
peroxide, cumene hydroperoxide, di-tert-butyl peroxide, ascaridole, acetyl
peroxide,
tert-butyl hydroperoxide, trimethylamine oxide, dimethylaniline oxide,
isopropylperoxydicarbonate, diisobutylene ozonide, peracetic acid, nitrates,
chlorates, perchlorates, azobisisobutyronitrile, etc.
[0084] Hydrogenation of nitrite rubber is known to the art and to the
literature. For
example, a preferred commercially available X-HNBR (carboxylated-HNBR) is made
from a carboxylated nitrite-diene copolymer that is hydrogenated in two steps.
It is
known that the C-C double bonds of the 1,2-vinyl-configured butadiene units in
NBR
are hydrogenated very rapidly, followed by the 1,4-cis configured units. The
1,4-traps
configured butadiene units are hydrogenated comparatively slowly. The NBR
products used for hydrogenation are distinguished by a predominant proportion
of
the 1,4-traps configured double bonds.
[0085] In the known 2-stage hydrogenation method, carbon-carbon double bonds
are first reduced, followed by reduction of the carbon-to-nitrogen bond. Other
techniques for hydrogenating acrylonitrile-butadiene copolymers are disclosed
in, for
example, U.S. Pat. Nos. 4,581,417; 4,631,315; and 4,795,788; the disclosures
of
which are incorporated herein by reference.
[0086] A partly or completely hydrogenated nitrite rubber (HNBR) is also
described
in several specifications (for example DE-OS No. (German Published
Specification)
2,539,132; DE-OS No. (German Published Specification) 3,329,974; DE-OS No.
(German Published Specification) 3,046,008 and 3,046,251; and European Patent
No. A-111,412).
[0087] Hydrogenation of X-HNBR latex can be carried out by known conventional
techniques. A carboxylated NBR polymer latex made conventionally using anionic
surfactants is combined with (1 ) an oxidant selected from the group
consisting of
oxygen, air and hydroperoxides; (2) a reducing agent selected from hydrazine
and
hydrates thereof; and (3) a metal ion activator; (b) and heating the mixture
to a
32
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
temperature from 0 °C. to the reflux temperature of the reaction
mixture. This
technique is taught in U.S. Patent No. 4,452,950, assigned to Goodyear Tire
and
Rubber Co., herein incorporated by reference.
[0088] The most preferred acrylonitrile-butadiene copolymers are hydrogenated
to
an extent such that the final product has an unsaturation level of from about
1 to
about 10 mole percent, and preferably from about 1 to about 5 mole percent.
[0089] A suitable carboxylated hydrogenated nitrite rubber (X-HNBR) is
manufactured by Bayer under a trade name of "Therban~", for example Therban KA
8889. X-HNBR may have an iodine value of preferably about 50% or less, more
preferably about 3 to 40%, most preferably from about 8 to 30%.
[0090] The crosslinker-reactive functional groups of the film former provided
by the
aforementioned methods can be done either before or after hydrogenation. As
examples of the unsaturated compound having a functional group, may be
mentioned vinyl compounds having a functional group, and cycloolefins having a
functional group. The introduction of the functional group by the graft-
modifying
method can be carried out by reacting the HNBR with a functional group-
containing
unsaturated compound in the presence of an organic peroxide. No particular
limitation is imposed on the functional group-containing unsaturated compound.
However, epoxy group-containing unsaturated compounds, carboxyl group-
containing unsaturated compounds, hydroxyl group-containing unsaturated
compounds, silyl group-containing unsaturated compounds, unsaturated
organosilicon compounds, etc. are mentioned for reasons of improvements of
crosslinking density and adhesion to substrates at a low modification rate.
[0091 ] Examples of the epoxy group-containing unsaturated compounds or epoxy
group-containing cycloolefins include glycidyl esters of unsaturated
carboxylic acids
such as glycidyl acrylate, glycidyl methacrylate and glycidyl p-styryl-
carboxylate;
mono- or polyglycidyl esters of unsaturated polycarboxylic acids such as endo-
cis-
bicyclo[2,2,1]hept-5-ene-2,3-dicarboxylic acid and endo-cis-bicyclo[2,2,1]kept-
5-ene-
2-methyl-2,3-dicarboxylic acid; unsaturated glycidyl ethers such as allyl
glycidyl
33
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
ether, 2-methyl-allyl glycidyl ether, glycidyl ether of o-allylphenol,
glycidyl ether of m-
allylphenol and glycidyl ether of p-allylphenol; and 2-(o-vinylphenyl)ethylene
oxide, 2-
(p-vinylphenyl)ethylene oxide, 2-(o-allylphenyl)-ethylene oxide, 2-(p-
allylphenyl)ethylene oxide, 2-(o-vinylphenyl)propylene oxide, 2-(p-
vinylphenyl)propylene oxide, 2-(o-allylphenyl)propylene oxide, 2-(p-
allylphenyl)
propylene oxide, p-glycidylstyrene, 3,4-epoxy-1-butane, 3,4-epoxy-3-methyl-1-
butene, 3,4-epoxy-1-pentane, 3,4-epoxy-3-methyl-1-pentane, 5,6-epoxy-1-hexane,
vinylcyclohexene monoxide and allyl-2,3-epoxycyclopentyl ether. These epoxy
group-containing unsaturated compounds may be used either singly or in any
combination thereof.
[0092] As examples of the carboxyl group-containing unsaturated compounds,
there
may be mentioned compounds described in Japanese Patent Application Laid-Open
No. 27135611993, for example, unsaturated carboxylic acids such as acrylic
acid,
methacrylic acid and oc-ethylacrylic acid; and unsaturated dicarboxylic acid
such as
malefic acid, fumaric acid, itaconic acid, endo-cis-bicyclo-[2.2.1 ]hept-5-ene-
2,3-
dicarboxylic acid and methyl-endo-cis-bicyclo[2.2.1 ]hept-5-ene-2,3-
dicarboxylic acid.
As further examples of unsaturated carboxylic acid derivatives, may be
mentioned
anhydrides, esters, halides, amides and imides of unsaturated carboxylic
acids.
Specific examples thereof include acid anhydrides such as malefic anhydride,
chloromaleic anhydride, butenylsuccinic anhydride, tetrahydrophthalic
anhydride and
citraconic anhydride; esters such as monomethyl maleate, dimethyl maleate and
glycidyl maleate; and malenyl chloride and maleimide. Of the aforementioned,
the
unsaturated dicarboxylic acids and anhydrides thereof are preferred for
reasons of
easy introduction of the functional group by a graft reaction, and the like,
with acid
anhydrides such as malefic anhydride and itaconic anhydride being particularly
preferred.
[0093] Examples of the hydroxyl group-containing unsaturated compounds for
incorporation into the film forming polymer include allyl alcohol, 2-allyl-6-
methoxyphenol, 4-allyloxy-2-hydroxybenzophenone, 3-allyloxy-1,2-propanediol, 2-
allyldiphenol, 3-buten-1-ol, 4-penten-1-of and 5-hexen-1-ol.
34
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0094] Examples of the silyl group-containing unsaturated compounds for
incorporation into the film former include chlorodimethylvinylsilane,
trimethylsilylacetylene, 5-trimethylsilyl-1,3-cyclopentadiene, 3-
trimethylsilylallyl
alcohol, trimethylsilyl methacrylate, 1-trimethylsilyloxy-1,3-butadiene, 1-
trimethylsilyloxycyclopentene, 2-trimethylsilyloxyethyl methacrylate, 2-
trimethylsilyloxyfuran, 2-trimethylsilyloxypropene, allyloxy-t-
butyldimethylsilane and
allyloxytrimethylsilane.
[0095] Examples of the unsaturated organosilicon compounds for incorporation
include trisalkoxyvinylsilanes such as trimethoxyvinylsilane,
triethoxyvinylsilane,
tris(methoxyethoxy)vinylsilane. The alkoxy groups in such an unsaturated
organosilicon compounds can be hydrolyzed into silanol groups.
[0096] Examples of unsaturated sulfonic acid or phosphorus ester groups
include 2-
(meth)acrylamido-2-methyl-1-propanesulfonic acid, 3-sulfopropyl
(meth)acrylate, 2-
sulfoethyl (meth)acrylate, and 2-phosphoethyl (meth)acrylate. These comonomers
incorporated into a variety of vinyl-acrylate, acrylate or other flexible
polymers having
a Tg of below 0°C as the film former polymer will cure in the presence
of epoxy
resins, isocyanates, carbodiimides, amino resins, aminosilanes, and other
crosslinking agents reactive with acidic groups. Flexible, low Tg copolymers
which
bear at least abut 2 mol % of sulfur and/or phosphorus-containing acid groups
and
exhibiting an acid number of from 5 to 100, preferably from 10 to 85, and most
preferably from 10 to 30 are useful film-formers in accordance with the
invention.
[0097] A graft-modified HNBR according to the present invention can be
obtained by
graft-reacting one of the aforementioned ethylenic unsaturated compounds
having a
functional group with the HNBR under generation of a radical. As methods for
generating the radical, may be mentioned (i) a method making use of an organic
peroxide, (ii) a method making use of a photo-induced radical generator, (iii)
a
method by irradiation of energy rays, and (iv) a method by heating.
[0098] (i) Method making use of an organic peroxide: As the organic peroxide,
for
example, organic peroxides, organic peresters, etc. may be preferably used. As
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
specific examples of such an organic peroxide, may be mentioned benzoyl
peroxide,
dichlorobenzoyl peroxide, dicumyl peroxide, di-tart-butyl peroxide, 2,5-
dimethyl-2,5-
di(peroxide benzoate)hexyne-3, 1,4-bis(tert-butyl peroxyisopropyl)benzene,
lauroyl
peroxide, tart-butyl peracetate, 2,5-dimethyl-2,5-di(tert-butyl peroxy)hexyne-
3, 2,5-
dimethyl-2,5-di(tert-butyl peroxy)hexane, tent-butyl perbenzoate, tart-butyl
perphenylacetate, tart-butyl perisobutyrate, tart-butyl per-sec-octoate, tart-
butyl
perpivalate, cumyl perpivalate and tart-butyl perdiethylacetate. In the
present
invention, azo compounds may also be used as the organic peroxides. As
specific
examples of the azo compounds, may be mentioned azobisisobutyronitrile and
dimethyl azoisobutyrate. Of these, benzoyl peroxide, and dialkyl peroxides
such as
dicumyl peroxide, di-tart-butyl peroxide, 2,5-dimethyl-2,5-di(tert-butyl
peroxide)hexyne-3, 2,5-dimethyl-2,5-di(tert-butyl peroxy)hexane and 1,4-
bis(tert-
butyl peroxyisopropyl)benzene are preferably used.
[0099] These organic peroxides may be used either singly or in any combination
thereof. A proportion of the organic peroxide used is generally within a range
of
0.001 to about 10 parts by weight, preferably about 0.01 to about 5 parts by
weight,
more preferably about 0.1 to about 2.5 parts by weight per 100 parts by weight
of the
unmodified HNBR. When the proportion of the organic peroxide used falls within
this
range, the rate of reaction of the functional group-containing unsaturated
compound,
and various properties of the resulting functional group-containing polymer,
are
balanced with one another at a high level. It is hence preferable to use the
organic
peroxide within such a range.
[0100] No particular limitation is imposed on the graft-modifying reaction,
and the
reaction may be carried out in accordance with any of the methods known per se
in
the art. The graft reaction can be conducted at a temperature of generally 0
to
400°C, preferably 60 to 350°C. The reaction time is generally
within a range of 1
minute to 24 hours, preferably 30 minutes to 10 hours. After completion of the
reaction, a solvent such as methanol is added in a great amount to the
reaction
system to deposit a polymer formed, and the polymer can be collected by
filtration,
washed and then dried under reduced pressure.
36
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0101 ] (ii) Method making use of a photo-induced radical generator: The
method
making use of the photo-induced radical generator is a method in which after
the
photo-induced radical generator is added, the resultant mixture is exposed to
ultraviolet light to generate a radical, and any conventionally known method
may be
used. The photo-induced radical generator may be any substance so far as it is
activated by irradiation of ultraviolet light. Specific examples thereof
include carbonyl
compounds such as benzoin, benzoin methyl ether, benzoin isopropyl ether,
benzoin
isobutyl ether, acetoin, butyroin, toluoin, benzyl, benzophenone, 2,2-
dimethoxy-2-
phenylacetophenone, alpha-hydroxycyclohexyl phenyl ketone, p-isopropyl-.alpha.-
hydroxyisibutylphenone, alpha, alpha-dichloro-4-phenoxyacetophenone,
methylphenyl glyoxylate, ethylphenyl glyoxylate, 4,4-bis(dimethylaminophenone)
and
1-phenyl-1,2-propandione-2-(o-ethoxycarbonyl).oxime; sulfur compounds such as
tetramethylthiuram monosulfide and tetramethylthiuram disulfide; azo compounds
such as azobisisobutyronitrile and azobis-2,4-dimethylvaleronitrile; peroxide
compounds such as benzoyl peroxide and di(t-butyl) peroxide; acylphosphine
oxides
such as 2,4,6-trimethylbenzoyldiphenylphosphine oxide. A proportion of the
photo-
induced radical generator used is generally within a range of 0.001 to about
10 parts
by weight, preferably about 0.01 to about 5 parts by weight, more preferably
about
0.1 to about 2.5 parts by weight.
[0102] Method by irradiation: The method by irradiation of energy rays is a
publicly
known method in which active energy rays such as alpha-rays, beta-rays and
gamma-rays are irradiated to generate a radical. In particular, it is desired
that
ultraviolet light be used from the viewpoints of efficiency, practicability
and
profitability.
[0103] Method by heating: The radical generating method by heating is carried
out
by heating in a temperature range of 100 to 390°C. Both publicly known
solution
method, and melting and kneading method may be used. Of these, the melting and
kneading method using an extruder or the like by which shear stress is applied
upon
heating is preferred from the viewpoint of reaction efficiency.
[0104] No particular limitation is imposed on the method for introducing the
functional
group on the film former polymer. Examples thereof include (a) a method by
37
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
oxidation of unsaturated bonds, (b) the afore mentioned method by an addition
reaction of a compound containing at least one functional group in its
molecule to
unsaturated bonds, (c) the methods mentioned herein of introducing an epoxy
group,
carboxyl group, hydroxyl group, or aforementioned reaction of an olefinic bond
of the
NBR or HNBR polymer with an unsaturated, preferably a monounsaturated,
carboxylic reactant, and the end group addition to living cationic initiated
polymer.
Alternatively, the polymer can be halogenated using chlorine or bromine-
containing
compounds. The halogenated polymer can then be reacted with the
monounsaturated carboxylic acid. The polymer and the monounsaturated
carboxylic
reactant can also be contacted at elevated temperatures to cause the
aforementioned thermal "ene" reaction to take place. Alternatively, the
monounsaturated carboxylic acid can be reacted with the polymer by free
radical
induced grafting. The functionalized elastomer of the present invention can be
functionalized by contact with a hydroxy aromatic compound in the presence of
a
catalytically effective amount of at least one acidic alkylation catalyst. The
alkylated
hydroxy aromatic compound can then be further reacted to form a derivative by
Mannich Base condensation with an aldehyde and an amine reagent to yield a
Mannich Base condensate. In yet another means to functionalize the polymer,
the
polymer may be contacted with carbon monoxide in the presence of an acid
catalyst
under Koch reaction conditions to yield the polymer substituted with
carboxylic acid
groups. In addition to the above methods of functionalization, the polymer of
the
present invention can be functionalized by methods of air oxidation,
ozonolysis,
hydroformylation, epoxidation and chloroamination, or the like by any other
method
(for example, Japanese Patent Application Laid-Open No. 172423/1994).
Fluoroelastomer Film Former
[0105] Fluorocarbon elastomers (fluoroelastomers) as film forming polymers
useful
herein are derived from hydrocarbons, including vinylidene fluoride,
hexafluoropropylene and are commercially available from a number of suppliers.
A
detailed discussion of the various types of fluoroelastomers is contained in
an article
by R. G. Arnold, A. L. Barney and D. C. Thompson that appeared in the July,
1973
issue of a journal entitled "Rubber Chemistry and Technology" (Volume 46, pp.
619-
652). A fluoroelastomer is distinguished from a thermoplastic fluoropolymer
38
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
principally by whether plastic deformation occurs upon stressing the
fluoroelastomer
to 100% elongation. Fluoroplastics undergo deformation at 100% elongation and
are
unsuitable coating materials for elastomeric substrates according to the
present
invention.
[0106] The representative fluoroelastomers used herein include polymers
derived
from one or more fluorinated monomers including 1,1-dihydroperfluorobutyl
acrylate;
copolymers of vinylidene fluoride and chlorotrifluoroethylene; vinylidene
fluoride and
hexafluoropropylene; vinylidene fluoride and hydropentafluoropropylene;
tetrafluoroethylene and propylene; and terpolymers of vinylidene fluoride,
hexafluoropropylene, and tetrafluoroethylene; vinylidene fluoride,
tetrafluoroethylene
and perfluorovinyl ether; vinylidene fluoride, tetrafluoroethylene, and
propylene;
vinylidene fluoride and hydropentafluoropropylene and tetrafluoroethylene. The
most preferred fluoroelastomer modified according to the invention is
commercially
available under the Viton ~ designation, such as a copolymer of
vinylidenefluoride
and hexafluoropropylene, or a terpolymer of vinylidenefluoride,
tetrafluoroethylene,
and hexafluoropropylene. Other suitable fluoroelastomers are available from
Dyneon under the FLOREL~ mark, and from Ausimont under the TECHNIFLON~
mark.
[0107] A graft-functionalized fluoroelastomer embodiment film former utilized
herein is the reaction product of a fluoroelastomer polymer and a grafting
agent
which contains a graft linking group which covalently bonds to the
fluoroelastomer,
and at least one active hydrogen-containing group, e.g., hydroxyl, thiol, or
carboxyl group that undergoes bond formation to one of the reactive groups of
the
curing agent. The graft-modified fluoroelastomer is combined with the curing
agent in admixture, within the time of the pot life (prior to gelation) of the
admixture, at the time of coating the elastomer substrate.
[0108] The grafting agent for the fluoroelastomer contains one graft-linking
group
and one active hydrogen-bearing group. The preferred grafting agent contains a
primary amine group and one active hydrogen-containing group. Examples
include hydroxyamines, aminoisocyanate, such as (R2 )2 NCH2 CH2 NCO, wherein
39
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
R2 is, for example, hydrogen or a hydrocarbyl group, hydroxyalkylamines,
aminocarboxylates, aminosilane, amino silanol, aminothiols, and the like.
Other
suitable grafting agents that do not contain a primary amine as the graft-
linking
group are mercapto hydroxy, like mercaptoalcohols and mercaptosilanols,
mercaptothiols, and the like. The preferred grafting agents will graft to the
fluoroelastomer at relatively mild temperatures (<60°C) and can be
monomeric,
oligomeric or polymeric, and contains at least one active hydrogen-containing
group and no more than one primary amine group, but can contain optionally
secondary or tertiary amine groups, or other groups not capable of graft-
linking
and crosslinking the fluoroelastomer. An optional secondary amine is believed
to
increase the rate of the graft reaction of the primary amine graft-linking
groups to
the fluoroelastomer. Specific examples of grafting agents include the various
hydroxyalkyl amines, e.g. 3-amino-1-propanol, aminoalkyl silanols, e.g.,
aminoalkyl silane triol or precursor aminoalkyl-alkoxysilanes which include
within
each molecule at least one basic nitrogen capable of catalyzing the hydrolysis
of
the alkoxysilane groups to produce the reactive silane triol; amine-N-oxides,
amino(hydroxy) carboxylic acids, amido(hydroxy)amines, polyoxyalkylene
polyether mono(primary)amines, and amine-terminated polyols. Such amine-
terminal polyols can be made by the known aminating methods for the
polyaddition of alkylene oxides, such as for example ethylene oxide, propylene
oxide, butylene oxide, dodecyl oxide or styrene oxide onto amino-starter
compounds. Generally, the polyol, such as a polyether polyol is aminated with
ammonia in the presence of a catalyst such as a nickel containing catalyst,
e.g., a
Ni/Cu/Cr catalyst. The known methods are taught in U.S. Pat. No. 4,960,942;
U.S. Pat. No. 4,973,761; U.S. Pat. No. 5,003,107; U.S. Pat. No. 5,352,835;
U.S.
Pat. No. 5,422,042; and U.S. Pat. No. 5,457,147, all incorporated herein by
reference. The starter compounds used are ammonia or compounds containing
amine groups and will provide in the reaction product no more than one primary
amino group, such as for example aliphatic polyamines such as ethylenediamine,
ethylenediamine oligomers (for example diethylenetriamine,
triethylenetetramine
or pentaethylenehexamine), ethanolamine, 1,3-propylenediamine, N-(2-
Hydroxyethyl)ethylenediamine , 1,3- or 1,4-butylenediamine, 1,2-, 1,3-, 1,4-,
1,5-,
1,6-hexamethylenediamine, and the like. Suitable polyether blocks for the
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
polyether-monoamines include polyethylene glycol, polypropylene glycol,
copolymers of polyethylene glycol and polypropylene glycol, poly(1,2-butylene
glycol), and poly(tetramethylene glycol).
[0109] The preferred amino-hydroxy grafting agent compounds are compounds
having a molecular weight of less than about 1000, preferably 500, more
preferably less than 250. More preferable amino-hydroxy grafting agents
contain
from 2 to 16 carbon atoms. With grafting agents having a molecular weight
above
about 1000, the degree of flexibility and solvent resistance of the coating is
reduced. Examples of more preferred grafting agents include 3-amino-1-
propanol, 2-(2-aminoethylamino)ethanol and aminoalkyl silanol, e.g.,
aminopropyl
silane triol. The effective amount of grafting agent used in relation to the
weight of
fluoroelastomer is from 1-20%, preferably from 2-10% by weight, more
preferably
3 to 7% by wt.
[0110] Other exemplary grafting agents which provide hydroxyl-functionalized
fluoroelastomers, although less preferred, include grafting hydroxyl-
functional
ethylenic unsaturated compounds via a graft-addition reaction. Aforementioned
mercaptohydroxy and mercaptocarboxy compounds are suitable. Hydroxy or
carboxy group-containing ethylenic unsaturated monomers are suitable and
include,
but are not limited to 2-hydroxyethyl (meth)acrylate, 1-hydroxypropyl
(meth)acrylate,
2-hydroxypropyl (meth)acrylate, 2-hydroxyethyl vinyl ether, N-
methylol(meth)acrylamide, methacrylic acid, and malefic anhydride, and can be
grafted to the fluoroelastomer in the presence of a free radical initiator by
techniques
known in the art of reactive processing of polymers, widely practiced in
thermoplastics such as polyolefins.
[0111 ] In another embodiment, a fluorocarbon elastomer is graft-
functionalized by an
addition reaction with a hydroxy(alkyl)rnercaptan, aminothiol, or
mercaptocarboxylic
acid optionally containing hydroxy group(s). Suitable mercaptans which yield
bound
hydroxyl groups for addition to fluoroelastomers include hydroxymercaptans
like
mercaptoethanol, hydroxyalkylmercaptans, such as 1-mercapto-3-propanol,
mercaptoethanolamine, 1-mercapto-4-butanol, a-mercapto- c~-
hydroxyoligoethylene
41
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
oxides, e,g., a-mercapto, e~-hydroxyoctaethylene glycol, or the corresponding
ethylene oxide/propylene oxide copolyethers. Mercaptoalkoxy compounds which
yield hydroxy groups upon hydrolysis include y-mercaptopropyltrimethoxysilane,
y-
mercaptopropyltriethoxysilane, y-mercaptopropylmethyldimethoxysilane, and y-
mercaptopropylmethyldiethoxysilane, to name a few. Suitable mercaptocarboxylic
acids and corresponding esters are the aforementioned mercaptoacetic acid, and
esters of mercaptoacetic acid, mercaptopropionic acid and esters,
mercaptobutyric
acid and esters. Esterifying compounds containing hydroxy groups include
ethylene
glycol, propylene glycol, butylene glycol, diethylene glycol, triethylene
glycol,
tetraethylene glycol, octaethylene glycol, dipropylene glycol, tripropylene
glycol,
tetrapropylene glycol and N-methyldiethanolamine.
[0112] Mercapto-compounds, especially mercapto alcohols can be graft-linked in
effective amounts for subsequent curing to any hydrocarbon elastomer suitable
herein. Especially useful in the preparation of functionalized
fluoroelastomer,
mercapto compounds can be incorporated under mild temperatures or at ambient
temperatures. The addition of the mercapto-compounds to graft to the
fluoroelastomer can be carried out optionally with a tree radical initiator in
solution at
a temperature above the decomposition temperature of the initiator, using for
instance, an azo initiator such as azobisisobutyronitrile and
azobiscyclohexanenitrile,
a peroxide such as dilauroyl peroxide, benzpinacol silyl ether, or
photoinitiators in the
presence of UV or visible light. Diacyl peroxides, especially dilauroyl
peroxide,
didecanoyl peroxide, di(3,3,5-trimethylhexanoyl) peroxide, disuccinoyl
peroxide and
dibenzoyl peroxide, are suitable. An effective amount of free radical
initiator is 0.5 to
wt. %, based on wt. of mercapto-compound. A preferred marcapto compound is
mercapto alcohol, such as mercaptoethanol. An effective amount of starting
mercapto-compound is from 3% to 10% on wt, of fluoroelastomer, and is
sufficient to
bond at a level of 1 % to 5 % by wt. of bound hydroxyl groups to the
fluoroelastomer.
[0113] The more preferred fiuoroelastomer grafting agents are those that will
graft to
the flu~roelastomer at room temperature, such as 2-(2-aminoethylamino)ethanol
(NH2 -CH2 -CH2 -NH-CH2 -CH2 -OH)(CAS # 111-41-1) and aminopropylsilanetriol,
42
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
such as supplied in a 22-25% solution in water by Gelest, Inc. as SIA0608.0
(CAS #
29159-37-3).
Crosslinkable a-olefin copolymer elastomers
[0114] Poly(olefin/acrylic ester/carboxylate) copolymer film forming
elastomers are
copolymers produced by polymerizing at least one a-olefin with at least one C1
-C1$
alkyl (meth)acrylate and, a minor amount of an unsaturated functional group-
bearing
comonomer that is accessible to form crosslinks with such materials as
polyisocyanates, carbodiimides, and other agents. Functional group bearing
comonomers can comprise an ethylenic unsaturated group and a group bearing an
acid, hydroxy, epoxy, isocyanate, amine, oxazoline, diene or other reactive
groups.
In the absence of such functionalized monomer, crosslinking sites can be
generated
in an a-olefin-ester copolymer, e.g. by partial hydrolysis of pendant ester
groups.
Suitable cc-olefins for polymerization of such olefin copolymer film-forming
elastomers include ethylene, propylene, butene-1, isobutylene, pentenes,
heptenes,
octenes, and the like including combinations. C, -C4 a-olefins are preferred
and
ethylene is most preferred.
[0115] The functionalized comonomer provides copolymerized a-olefin polymers
bearing an active hydrogen, halogen, or a group which can be converted, such
as by
transamidation or hydrolysis to an active hydrogen-bearing group, or
conversely, the
functionalized commoner contains a group that is reactive with crosslinking
agents
bearing an active hydrogen group. The alkyl or alkoxy(meth)acrylate acids and
esters are exemplary functionalized comonomers. Concrete examples of alkyl
groups are a methyl group, ethyl group, n-propyl group, isopropyl group, n-
butyl
group, isobutyl group, sec-butyl group, t-butyl group, pentyl group, hexyl
group, octyl
group, 2-ethylhexyl group and decyl group; cycloalkyl group such as
cyclopentyl
group and cyclohexyl group; aryl group such as phenyl group and tolyl group;
and
aralkyl group such as benzyl group and neophyl group.
43
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0116] Examples of alkoxy groups include methoxy group, ethoxy group, n-
propoxy
group, isopropoxy group, n-butoxy group, isobutoxy group, sec-butoxy group, t-
butoxy group, pentoxy group, hexoxy group and octoxy group.
[0017] Suitable alkyl or alkoxy (meth)acrylates optionally incorporated with a-
olefin
include methyl acrylate, ethyl acrylate, t-butyl acrylate, n-butyl acrylate, 2-
ethylhexyl
acrylate, methyl methacrylate, ethyl methacrylate, n-butyl methacrylate, 2-
ethyle-
hexy acrylate, methoxy acrylate, ethoxyethyl acrylate, methoxyethyl acrylate,
acrylamide, and methacrylamide, and the like or a mixture thereof. Specific
examples of functional ethylenically unsaturated monomers which are
copolymerizable with the a-olefin monomers are: unsaturated carboxylic acids
such
as acrylic acid, methacrylic acid, itaconic acid and malefic acid and salts
thereof, alkyl
esters of unsaturated carboxylic acids such as methyl acrylate and butyl
acrylate.
[0118] A preferred a-olefin-acrylic ester copolymer rubber comprises
unsaturated
carboxylic acid monomer unit, such as acid units, e.g. derived from
(meth)acrylic
acid or malefic acid, or anhydride units, e.g. derived from malefic anhydride
or partial
ester units, e.g. derived from mono ethyl maleate. In a preferred embodiment
the
polymer is a terpolymer of ethylene, C1 -C4 alkyl acrylate and an carboxylic
monomer unit; more preferably such terpolymer comprises at least about 30 mole
percent of ethylene, about 10 to about 69.5 mole percent mono ethyl maleate.
In all
cases it is preferred that the c~-olefin acrylate rubber be essentially non-
crystalline
and have a glass transition temperature (Tg) below room temperature, i.e.
below
about 20°C.
[0119] Other comonomers which contain a reactive group for adding functional
acid,
hydroxy, epoxy, isocyanate, amine, oxazoline, diene or other reactive
functional
groups include the diene monomers, such as non-conjugated dienes such as
alkylidenenorbornene, alkenylnorbornene, dicyclopentadiene,
methylcyclopentadiene and a dimer thereof and conjugated dienes such as
butadiene and isoprene. Examples of the dihydrodicyclopentadienyl group-
containing (meth)acrylate include dihydrodicyclopentadienyl (meth)acrylate and
dihydrodicyclopentadienyloxyethyl (meth)acrylate.
44
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0120] Further examples of functional comonomers include the N-alkylol and N-
alkoxy amides of a,a-olefinically unsaturated carboxylic acids having from 4
to 10
carbon atoms such as N-methylol acrylamide, N-ethanol acrylamide, N-propanol
acrylamide, N-methylol methacrylamide, N-ethanol methacrylamide, n-butoxy
acrylamide and isobutoxy acrylamide, N-methylol maleimide, N-methylol
maleamide,
N-methylol maleamic acid, N-methylol maleamic acid esters, the N-alkylol
amides of
the vinyl aromatic acids such as N-methylol-p-vinyl benzamide, and the like
and
others.
[0121 ] Other examples of functional comonomers bearing groups which are
either
reactive with active hydrogens or themselves contain active hydrogen groups
are
epoxy group-containing ethylenically unsaturated compounds including allyl
glycidyl
ether, glycidyl methacrylate, and glycidyl acrylate. Specific examples of the
active
halogen-containing ethylenically unsaturated compounds include vinylbenzyl
chloride, vinylbenzyl bromide, 2-chloroethyl vinyl ether, vinyl chloroacetate,
vinyl
chloropropionate, allyl chloroacetate, allyl chloropropionate, 2-chloroethyl
acrylate, 2-
chloroethyl methacrylate, chloromethyl vinyl ketone and 2-chloroacetoxymethyl-
5-
norbornene. Specific examples of common carboxyl group-containing
ethylenically
unsaturated compounds include acrylic acid, methacrylic acid, crotonic acid, 2-
pentenoic acid, malefic acid, fumaric acid and itaconic acid.
[0122] Examples of the other ethylenically unsaturated (meth)acrylic esters
comonomers include octyl methacrylate; cyano-substituted alkyl (meth)acrylates
such as 2-cyanoethyl acrylate, 3-cyanopropyl acrylate, and 4-cyanobutyl
acrylate;
amino-substituted alkyl (meth)acrylates such as diethylaminoethyl acrylate;
fluorine-
containing acrylates such as 1,1,1-trifluoroethyl acrylate; hydroxyl group-
substituted
alkyl (meth)acrylates such as hydroxyethyl acrylate; alkyl vinyl ketones such
as
methyl vinyl ketone; vinyl or allyl ethers such as vinyl ethyl ether and ally
methyl
ether; vinyl aromatic compounds such as styrene, a-methylstyrene,
chlorostyrene ad
vinyltoluene; vinylamides such as acrylamide, methacrylamide and N-
methylolacrylamide; and ethylene, propylene, vinyl chloride, vinylidene
chloride, vinyl
fluoride, vinylidene fluoride, vinyl acetate, alkyl fumarate, etc.
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Acrylic Elastomers
[0123] Functionalized acrylate elastomers are suitable if the glass transition
temperature is below -10°C, and are defined as addition polymers
derived from a
major amount (greater than 50 wt. % on total polymer weight) of one or more
copolymerizable a,(3-ethylenic unsaturated ester monomers having the general
structure
H2C=C C-~ R2 (I)
where R1 is hydrogen or methyl; R2 represents C1-C2o alkyl , C2-C7 alkyl, C2-
C7
alkoxyalkyl, C2-C7 alkylthioalkyl, C2-C7 cyanoalkyl, and a minor amount of
active
hydrogen-group bearing comonomer or active bearing group graft-linked
functional
site. The acrylates are available in solid bale, and as emulsions or latexes
from a
variety of commercial sources. Minor amounts of up to about 35% on total
acrylate
rubber weight, of hardening or Tg increasing comonomers , e.g. methyl
methacrylate, acrylonitrile, vinyl acetate, vinylidene chloride and/or
styrene, to name
a few, can be included. Desirably, the functional group bearing comonomer
having
active hydrogen or a group reactive with active hydrogen containing curing
agent is
an unsaturated monocarboxylic acid (e.g, acrylic or methacrylic acid) or
polycarboxylic acid (e.g. itaconic, citraconic acid, etc.) or anhydrides of
polycarboxylic acids.
[0124] Specific examples of suitable acrylic or methacrylic monomers alone and
in
combinations include methyl acrylate, ethyl acrylate, butyl acrylate, butyl
methacrylate, ethylhexyl acrylate, and the like. A preferred copolymer
comprises
one or two different copolymerizable monomers each having structure (I) in
which R1
is hydrogen; and, R2 is C4 -C$ alkyl, or C2 -C$ alkoxyalkyl, either of which
may
contain a primary, secondary or tertiary C atom. Examples of more preferred C4
-C$
alkyl acrylates are n-butyl acrylate, isobutyl acrylate, n-pentyl acrylate,
isoamyl
acrylate, hexyl acrylate, 2-methylpentyl acrylate, n-octyl acrylate, and 2-
ethylhexyl
acrylate; of preferred C4 -C$ alkoxyalkyl acrylates are methoxy acrylate, and
ethoxyethyl acrylate; of a preferred alkylthioalkyl acrylate is
methylthioethyl acrylate;
46
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
of preferred C2 -C7 cyanoalkyl acrylates are cyanoethyl acrylate and
cyanoproyl
acrylate; and mixtures of two or more of the foregoing may be used.
[0125] Preferred active hydrogen bearing comonomers for acrylic elastomers
include many of the above mentioned functional comonomers bearing active
hydrogens, some of which are repeated here include comonomers containing
carboxylic anhydride, carbonamide, N-substituted carbonamide, aldehyde, alkyl
and
aryl keto, hydroxyl radicals, allylic chlorine radicals, methylol, maleimide,
bis-
maliimide, alkyl N-methylol, phenolic methylol, thiol radicals, amino
radicals,
isocyanate radicals, alkoxyalkyl radicals, oxirane radicals, and the like. The
a,(3-
unsturated hydroxy carboxylic acids or anhydrides of dicarboxylic acids are
preferred. If the polymers are only copolymers of acrylate ester and
carboxylic acid
or anhydride comonomers, they desirably have from about 90 to about 98 mole
percent repeat units from acrylate ester, more desirably from about 92 to
about 97 or
98 mole percent of the ester and from 2 to 10% of carboxylic acid or
anhydride, more
preferably 3 to 8% of carboxylic acid or anhydride.
[0126] Exemplary functional comonomers incorporated randomly during addition
polymerization of film former polymer include glycidyl methacrylate, acrylic
and
methacrylic acids, malefic anhydride, N-alkyl maleimide, acrylamide, N-
alkoxyalkyl
acrylamides such as N-isobutoxymethyl acrylamide, N-hydroxymethyl acrylamide
and the like, methyl vinyl ketone, acrolein, vinyl isocyanate, hydroxyalkyl
acrylates
such as 2-hydroxyethyl acrylate, 2-hydroxyethyl methacrylate and the like.
Also
included are mixtures of two or more such functional monomers.
[0127] Included in acrylic elastomers are the so-called core-shell polymers.
The rubbery copolymers useful in soft-shell copolymers include copolymeric
compositions of at least one acrylic monomer whose homopolymer T9 is below -
10°C, and a second copolymerizable functional monomer. These monomers
can be
polymerized in the presence of minor proportions of monovinyl or vinylidene
monomers set forth above such as for example styrene, acrylonitrile, methyl
methacrylate and the tike, in a proportion with the low Tg acrylic
comonomer(s)
selected so as to not raise the Tg of the resulting acrylic copolymer above
about
47
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
-10°C.
[0128] The shell copolymer is an addition polymer and may be varied over a
wide
composition range, however, for most purposes the copolymer will comprise from
about 99.9 to about 95 wt% of at least one rubbery monomer and from about 0.1
to
about 5 wt.% of second copolymerizable functional monomer. The preferred shell
copolymers are copolymers of an alkyl acrylate and 2-hydroxyethyl
methacrylate.
[0129] The elastomeric coatings of this invention based on sequential
polymerized
functionalized addition polymers may exhibit two glass transition
temperatures, one
of which is below 0°C, and one above 0°C. The amount of rubbery
shell copolymer
component as well as the proportion of hard component and rubbery component
may be varied however, for most purposes the ratio of rigid copolymer
component to
rubbery shell copolymer component is less than 1, meaning the amount of
rubbery
component is in a major proportion of greater than 50 wt. %, and preferably
from 60
wt% to 80 wt%.
[0130] Dual (halo, carboxy) functionalized acrylic addition polymers are also
useful
as the film-former for organic solvent-borne embodiments of the invention and
comprise repeating units from acrylic ester monomers or monomer mixtures and
which exhibit a glass transition temperature in the elastomer less than -20
°C. The
functional group is provided from a combination of from about 0.1 % to about
30%,
preferably from 0.2% to about 15% by weight of an active halogen-containing
comonomer and from about 0.1 % to about 20% by weight of a carboxyl-group
containing comonomer. In the preferred level of halogen-containing comonomer,
the
halogen content is from about 0.1 % to about 5% by weight of the
functionalized
acrylic rubber. The halogen groups of the halogen-containing comonomer can be
chlorine, bromine, or iodine. Chlorine containing comonomers are preferred
from an
economic, availability and safety basis.
[0131 ] Examples of halogen containing comonomers are vinyl chloroacetate,
vinyl
bromoacetate, allyl chloroacetate, vinyl chloropropionate, vinyl
chlorobutyrate, vinyl
bromobutyrate, 2-chloroethyl acrylate, 3-chloropropyl acrylate, 4-chlorobutyl
acrylate,
4$
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
2-chloroethyl methacrylate, 2-bromoethyl acrylate, 2-iodoethyl acrylate, 2-
chloroethyl
vinyl ether, chloromethyl vinyl ketone, 4-chloro-2-butenyl acrylate, vinyl
benzyl
chloride, 5-chloromethyl-2-norbornene, 5-a-chloroacetoxymethyl)-2-norbornene,
5-
(a,[i-dichloropropionylmethyl)-2-norbornene, and the like. The preferred
monomers
are vinyl chloroacetate, allyl chloroacetate, 2-chloroethyl acrylate, 2-
chloroethyl vinyl
ether, vinyl benzyl chloride, 5-chloromethyl-2-norbornene, and 5-
chloroacetoxymethyl-2-norbornene.
[0132] A preferred active hydrogen bearing comonomer for acrylic rubber is
present
from about 0.1 % to about 20% by wt., preferably from 0.2% to about 10%, more
preferably from 2% to about 6% by weight of at least one carboxyl group-
containing
comonomer. The carboxyl comonomer is preferably monocarboxylic, but can be
polycarboxylic. Preferred carboxyl comonomers contain from 3 to about 8 carbon
atoms. Examples of such preferred comonomers are acrylic acid, methacrylic
acid,
ethacrylic acid, [i, ~i-dimethylacrylic acid, crotonic acid, 2-pentenoic acid,
2-hexenoic
acid, malefic acid, fumaric acid, citraconic acid, mesaconic acid, itaconic
acid, 3-
butene-1,2,3-tricarboxylic acid, and the like. The most preferred carboxyl
comonomers are the monocarboxylic acid monomers such as acrylic acid,
methacrylic acid, itaconic acid, and the like.
[0133] The functional group-containing comonomers are incorporated as
introduced
above most conveniently during the addition polymerization of acrylate
elastomers.
Polymerization by way of suspension, emulsion, solution, and bulk methods are
suitable. These polymerizations are initiated using free radical initiators.
The
emulsion polymerization method is preferred. Various conventional soaps,
emulsifiers, and surfactants, known to the art and to the literature can be
utilized in
emulsion polymerized functional acrylate rubber synthesis. The weight average
molecular weight of the dual- functionalized acrylate elastomer is generally
in excess
of 100,000. Commercial grades of functionalized acrylic rubber are available
from
Zeon Chemicals under the HYTEMP~ mark.
[0134] A variety of a,~-unsaturated C2-C$ alkyl ester copolymer latexes
containing
active hydrogen functional groups are known and available from a variety of
49
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
commercial sources. A preferred acrylic rubbery latexes are available from
Noveon~ under the HYCAR mark, and Rhoplex~ ex. Rohm and Haas. An emulsion
polymerized copolymer of n-butylacrylate, acrylonitrile, N-methylol acrylamide
and
itaconic acid, exhibiting a Tg of less than 20°C is a preferred acrylic
film former for
use in aqueous coating embodiments.
Crosslinkable a-Olefin Copolymers
[0135] Poly(olefin/acrylic esterlcarboxylate) copolymer are thermoplastic in
the
uncured state and are suitably flexible for use herein. These are principally
copolymers produced by polymerizing at least one a-olefin with at least one C1
-C1$
alkyl (meth)acrylate and a minor amount of an unsaturated protic functional
group-
bearing comonomer that is accessible to form crosslinks with such materials as
polyisocyanates, carbodiimides, and other curing agents. Functional group
bearing
comonomers can comprise an ethylenic unsaturated group and a group bearing an
acid, hydroxy, epoxy, isocyanate, amine, oxazoline, diene or other reactive
groups.
In the absence of such functionalized monomer, crosslinking sites can be
generated
in an a-olefin-ester copolymer, e.g., by partial hydrolysis of pendant ester
groups.
Suitable a-olefins for polymerization of such olefin copolymer film-forming
elastomers
include ethylene, propylene, butene-1, isobutylene, pentenes, heptenes,
octenes,
and the like including combinations. C2 -C~ a-olefins are preferred, and
ethylene is
most preferred.
[0136] Other examples of functional comonomers bearing active hydrogen groups
are epoxy group-containing ethylenically unsaturated compounds including allyl
glycidyl ether, glycidyl methacrylate, and glycidyl acrylate. Specific
examples of the
active halogen-containing ethylenically unsaturated compounds include
vinylbenzyl
chloride, vinylbenzyl bromide, 2-chloroethyl vinyl ether, vinyl chloroacetate,
vinyl
chloropropionate, allyl chloroacetate, allyl chloropropionate, 2-chloroethyl
acrylate, 2-
chloroethyl methacrylate, chloromethyl vinyl ketone and 2-chloroacetoxymethyl-
5-
norbornene. Specific examples of the carboxyl group-containing ethylenically
unsaturated compound include acrylic acid, methacrylic acid, crotonic acid, 2-
pentenoic acid, malefic acid, fumaric acid and itaconic acid.
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0137] Examples of ethylenically unsaturated (meth)acrylic ester comonomers
include octyl methacrylate; cyano-substituted alkyl (meth)acrylates such as 2-
cyanoethyl acrylate, 3-cyanopropyl acrylate, and 4-cyanobutyl acrylate; amino-
substituted alkyl (meth)acrylates such as diethylaminoethyl acrylate; fluorine-
containing acrylates such as 1,1,1-trifluoroethyl acrylate; hydroxyl group-
substituted
alkyl (meth)acrylates such as hydroxyethyl acrylate; alkyl vinyl ketones such
as
methyl vinyl ketone; vinyl or allyl ethers such as vinyl ethyl ether and ally
methyl
ether; vinyl aromatic compounds such as styrene, a-methylstyrene,
chlorostyrene ad
vinyltoluene; vinylamides such as acrylamide, methacrylamide and N-
methylolacrylamide; and ethylene, propylene, vinyl chloride, vinylidene
chloride, vinyl
fluoride, vinylidene fluoride, vinyl acetate, alkyl fumarate, etc.
[0138] A preferred olefin/acrylic ester copolymer rubber comprises unsaturated
carboxylic acid monomer units, such as acid units, e.g. derived from
(meth)acrylic
acid or malefic acid, anhydride units, e.g. derived from malefic anhydride or
partial
ester units, e.g. derived from mono ethyl maleate. In a preferred embodiment
the
polymer is a terpolymer of ethylene, C1 -C4 alkyl acrylate and an carboxylic
monomer unit; more preferably such terpolymer comprises at least about 30 mole
percent of ethylene, about 10 to about 69.5 mole percent mono ethyl maleate.
In all
cases it is preferred that the a-olefin acrylate rubber be essentially non-
crystalline
and have a glass transition temperature (Tg) below about 20°C. Ethylene-
carboxylate copolymers are available commercially under the VAMAC~ mark.
[0139] When the acrylic acids and acrylates are part of the a-olefin copolymer
backbone, transamidation reactions may be made in melt processing techniques
which are known to produce pendant hydroxyl functionality such as by employing
an
aminoalcohol, e.g., 2-amino-1-ethanol. A further reaction by the pendant
hydroxyls
may occur, i.e., transesterification with another acrylate linkage, resulting
in
crosslinking and an increase in product viscosity:
Polyurethanes
51
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0140] A castable film former comprising a curable urethane can be utilized as
the
film former component. The active hydrogen functionalized polymer is a
saturated
prepolymer and is cured with an aliphatic polyisocyanate. The cured glass
transition
temperature of the polyurethane is limited to below 0°C and is lightly
crosslinked by
inclusion of a triol, tetraol or higher OH functionality. Therefore the chain
extending
polyols are limited to those such as hydroxy terminated hydrogenated
polybutadiene
polyol homopolymers and copolymers exhibiting a glass transition temperature
of
0°C or less, polyTHF, polyester diols, polypropylene glycols and the
like, of which
are familiar to those skilled in the art and commercially available.
Conventional
curing agent and catalyst is employed. U.S. Pat. No. 4,669,517 discloses a
suitable
method to apply emissive polyurethane to a prepared post-vulcanized rubber
surface
for obtaining excellent bonding of the polyurethane. The method for preparing
a
post-vulcanized surface is applicable for applying a castable polyurethane
emissive
coating. Cyanuric acid is applied to the rubber surface which contains
incorporated
therein a polybutadiene polyol, prior to application of the polyurethane
reaction
mixture which contains the thermally conductive metal particles. The
polyurethane
reaction mixture cures at ambient temperatures.
Acrylourethanes.
[0141 ] Urethane modified acrylic materials conforming to the requirements of
the
film former as set forth herein are also contemplated. These may be adapted to
be
cure activated by moisture, heat or light. The glass transition temperature of
such
urethan modified acrylates must be °C or less and comprised of a major
amount of
C2-C$ acrylic or methacrylic esters. An example of preferred urethane-modified
acrylic resins usable in the present invention is, in the case of the urethane-
modified
acrylic resin represented by formula (I), an acrylic copolymer produced by
copolymerizing 60 to 70 moles of methyl-, ethyl-, or butyl- acrylate with 10
to 50
moles of methacrylic acid and 30 to 80 moles of 2-hydroxymethyl methacrylate.
Some or all of the hydroxyl and carboxyl groups are capped in a reaction with
~c,a-
ethylenic unsaturated isocyanate, for example, methacryloyloxyethyl isocyanate
(2-
isocyanate ethyl methacrylate). This material is moisture curable, and curable
by UV
by incorporation of a conventional photoinitiator. In mosture curable
acrylourethane
52
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
embodiments, it is preferred that at least 10 mole%, preferably at least 50
mole% of
the hydroxyl groups from the 2-hydroxyethyl methacrylate units have been
reacted
with the methacryloyloxyethyl isocyanate. The a,~-ethylenic unsaturated
isocyanate
is preferably based upon the reaction product of an isocyanate and hydroxyl-
containing monomers, such as N-methylolacrylamide, N-methylolmethacrylamide, 2-
hydroxyethyl acrylate, 2-hydroxyethyl methacrylate, 2-hydroxypropyl acrylate,
2-
hydroxypropyl methacrylate, 4-hydroxybutyl acrylate, and 4-hydroxybutyl
methacrylate, may be used optionally with 3-aminopropyl triethoxy silane,3-
aminopropyl trimethoxy silane, 3-aminopropyl methyl dimethoxysilane or 3-
aminopropyl methyl diethoxy silane, primary secondary amines such as N-(2-
aminoethyl)-3-aminopropyl trimethoxy silane, secondary amines such as N-methyl-
or N-phenyl-3-aminopropyl trimethoxy silane, condensed aminoalkyl silanes such
as
bis(3-aminopropyl) tetramethoxy or tetraethoxy disiloxane NH2 (CH2 )3--
Si(OCH3)2 --
O--(CH3 O)2 Si-(CH2 )3 NH2, polyglycolether-modified aminosilanes such as that
sold
under the Trademark "Dynasylan 121" and triamino functional propyl trimethoxy
silanes such as "Dynasylan TRIAMO" available from Huls AG. Similar silanes
having
two or three silicon atoms can also be used.
Maleated Elastomeric Materials
[0142] Various polymer blends, alloys and dynamically vulcanized composites of
maleated addition polymers based on polyethylenes, such as maleated
polypropylenes, maleated styrene-ethylene-butene-styrene-block copolymers,
maleated styrene-butadiene-styrene block copolymers, maleated ethylene-
propylene
rubbers, and blends thereof can be utilized as the functionalized film-forming
elastomer in accordance with the invention. The maleated elastomers are
dissolved
in an appropriate organic solvent system and mixed with the thermally
conductive
metal particles which are preferably predispersed in a portion of the solvent
used.
Ethylene Vinyl Ester Copolymers
[0143] Film forming, solvent soluble, OH-functional ethylene copolymers are
available in various grades which contain carboxyl or hydroxyl functional
groups and
53
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
are also suitable as the film former used herein. Conventionally, some of
these
polymers are used as cross-linkable hot melt adhesives, however these polymers
are readily adaptable for ambient temperature cured emissive coating films
herein
even though the elevated temperature cohesiveness is relatively low. The
ethylene
vinyl ester polymers containing hydroxyl functionality can be adapted for use
in the
emissive coating composition and cured with unblocked isocyanates and provide
sufficient properties for certain environmental temperatures not exceeding the
temperature at which the cured coating will flow. An ethylene vinyl acetate
copolymer containing OH groups is based on a polymer having monomeric units
ethylene and of vinyl alcohol, and optionally vinyl acetate, the melt
viscosity being
preferably from 4 to 40 Pa.s at 180°C. Ethylene vinyl alcohol
copolymers have
preferably at least 5 wt % of vinyl alcohol units. One example is a terpolymer
(viscosity 20 Pa.s at 180° C., MFR at 125 °C. under 325 gm load
of 6.4 gm/10 min)
with 10% vinyl alcohol, 88.75% ethylene and 1.2 wt % vinyl acetate. The m.p.
is
101.5 °C. (by DSC). Another terpolymer contains 13.7 wt % vinyl
alcohol, 82.3%
ethylene and 4.0 wt % vinyl acetate (viscosity 5.8 Pa.s at 180 °C, MFR
at 125°C
under 325 gm (cf. 30.4 gm/10 min, DSC m.p. 91° C.).
[0144] Film formers of a mixture or interpenetrating network containing partly
functionalized polymer, and partly non-functionalized polymer types are
suitable for
use herein. Blendable with functionalized polymers are olefinic rubber polymer
as
random or block copolymers, e.g., SBS, EBS, EPM and EPDM, hydrogenated
polydiene copolymer, acrylic rubber, and others of the aforementioned film
formers.
As an example, a non-functionalized polymer film former can be blended with a
partially hydrolyzed ethylene vinyl acetate polymer in a proportion of from 10-
90
wt.% to 90-10 wt.%, respectively, and cured with any of the suitable curing
agents
disclosed herein, and equivalents thereof.
Functionalized EPM and EPDM Elastomers
[0145] Functionalized EPM and EPDM elastomers are suitable film forming
elastomers used as the film former in the emissive coating. These comprise two
or
more a-monoolefins, copolymerized with a polyene, usually a non-conjugated
diene
54
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
comonomer. Useful polyenes include 5-ethylidene-2-norbornene; 1,4-hexadiene; 5-
methylene-2-norbornene; 1,6-octadiene; 5-methyl-1,4-hexadiene; 3,7-dimethyl-
1,6-
octadiene; 1,3-cyclopentadiene; 1,4-cyclohexadiene; dicyclopentadiene; 5-vinyl-
2-
norbornene, etc.; or a combination thereof. Preferred polyenes for the EPM and
EPDM functionalized elastomers are 5-vinyl-2-norbornene, 5-ethylidene-2-
norbornene and 1,4-hexadiene. Functional groups can be incorporated by the
aforementioned conventional routes, and by the metathesis route disclosed
herein.
[0146] In one aspect of the methods disclosed in this invention a particularly
useful
scheme for the production of polymers containing organic acid functionality
such as
carboxyl functionality, aliphatic or aromatic hydroxyl functionality, and the
like and
inorganic acid functionality such as sulfonic acid functionality, phosphoric
acid
functionality and the like is provided.
[0147] One such scheme is illustrated below for EPM and EPDM rubber, for
incorporating pendant carboxyl, hydroxyl or non-sterically hindered pendant
olefinic
functionality.
-~-CHZCHz-~- f CH2CH -~CHZCHZ-~-ECHZCH
n ~ m~ ' o Olefin Metathesis Catalyst n ~ in'~' o
CH3 CH \~/3
M
EPDM R
M = Transition Metal
R = H, CH3
P ~ ,O OH
I PL~~\y~C~O P
O
P
Modified EPDM
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
wherein n represents a conventional number of repeating ethylene units for
EPDM
sold commercially, m represents a conventional number of propylene repeating
units, o represents a number of conventional diene monomer repeating units,
and p
represents the number of repeating units of maleated dicyclopentadiene ranging
from 1 to 100.
[00148] The same approach as illustrated above for modifying EPDM can be
utilized
for incorporating a functional group in a conjugated diene polymer, such as a
butadiene-acrylonitrile copolymer containing vinyl unsaturation.
(B) Curing Agent Component
[0149] The ambient temperature curing agent is a multifunctional curing
component
containing either (1 ) at least one group bearing active hydrogen and a
crosslinking
group which is the same active hydrogen group or a different corsslinking
group, or
(2) at least one groups that is reactive with an active hydrogen group and a
crosslinking group which is a group reactive with an active hydrogen group or
a
different crosslinking group. In the case of castable polyurethane or urethane
acrylate (acrylo-urethane), the curing interaction is between a polyol
optionally with
co-curing polyamine and a polyisocyanate or polyisocyanate prepolymer and or
ethylenic unsaturated groups on the acrylated portion. The curing component is
selected from polyisocyanate, chain extended polyisocyanate, polymeric
isocyanate-
polyol adduct, a polycarbodiimide, multifunctional oxazoline, multifunctional
oxazine, multifunctional imidazoline, phenolic novolak, phenolic resole, amino
resin,
and amino(alkoxy)silane. The preferred curing component contains at least one
isocyanate group, or a group bearing an isocyanate group, or a functional
group
reactive crosslinking group, or combinations thereof. The curing component is
used
at a level generally of from about 3 to about 30 wt. parts, desirably from
about 5 to
about 25 wt. parts, and preferably from about 10 to about 20 wt. parts per 100
wt.
parts of a functionalized addition polymer, or in the case of a castable
polyurethane,
in a stoichiometric amount based upon the equivalent weight of the polyol
components.
56
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0150] Suitable curing agents include monomeric polyisocyanates such as
aliphatic
or aromatic diisocyanates containing from 2 to 40 carbons. Exemplary
polyisocyanates include ethylene diisocyanate, trimethylene diisocyanate,
hexamethylene diisocyanate, propylene-1, 2-diisocyanate, ethylidene
diisocyanate,
cyclopentylene-1, 3-diisocyanate, the 1,2-, 1,3- and 1,4-cyclohexylene
diisocyanates,
the 1,3- and 1,4-phenylene diisocyanates, diphenylmethane diisocyanates,
polymethyleneisocyanates, the 2,4- and 2,8-toluene diisocyanates, the 1,3- and
1,4-
xylylene diisocyanates, bis(4-isocyanatoethyl) carbonate, 1,8-diisocyanato-p-
methane, 1-methyl-2, 4-diisocyanatocyclohexane, the chlorophenylene
diisocyanates, naphthalene-1,5-diisocyanate triphenylmethane-4,4',
triisocyanate,
isopropylbenzene-alpha-4-diisocyanate, 5,8-bicyclo[2.2.1 ] hept-2-ene
diisocyanate,
5,8-diisocyanatobutylbicyclo [2.2.1 ] hept-2-ene. Exemplary commercial
products are
trimethylhexamethylene diisocyanate available from VEBA, heptadecyl (C17)
diisocyanate, DDI 1410 an aliphatic C-36 diisocyanate available from the
Henkel
Corporation of Minneapolis, Minn and Isonate~ 143L diisocyanate, a modified
diphenylmethane diisocyanate (MDI) available from Upjohn Corp. Further
urethane
components are isophorone diisocyanate available from VEBA and Desmodur~ N
an aliphatic triisocyanate available from Mobay. Desmodur~ N is more
particularly
defined as the reaction product of 3 moles of hexamethylene diisocyanate and
water
having an isocyanate equivalent weight as later defined of 191. Other adducts
or
prepolymers of the polyisocyanate include Desmodur~ L and Mondur~ CB which
are the adducts of tolylene diisocyanate (TDI).
[0151 ] Examples of alicyclic polyisocyanates include 1,3-cyclopentene
diisocyanate,
1,4-cyclohexane diisocyanate, 1,3-cyclohexane diisocyanate, 1-isocyanato-3,3,5-
trimethyl-5-isocyanatomethyl-cyclohexane (isophorone diisocyanate, IPDI), 4,4'-
methylenebis(cyclohexyl isocyanate), methyl-2,4-cyclohexane diisocyanate,
methyl-
2,6-cyclohexane diisocyanate and 1,3- or 1,4-bis(isocyanatomethyl)cyclohexane)
and polyisocyanates (e.g., 1,3,5-triisocyanatocyclohexane. Polymeric
isocyanates
are preferred crosslinking agents used for curing the emissive coating. Liquid
polymeric isocyanates are more preferred and are also widely available. The
term
"liquid" is defined as a liquid at ambient temperature, or at elevated
temperature, or a
s~
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
solution of polyisocyanate in a solvent for the polyisocyanate.
Polyisocyanates
containing from 10 to 50% reactive NCO groups which are liquid at ambient
temperature, or are liquefied at up to about 70°C, or soluble in
carriers or diluents
are readily adapted for use in the present invention. Numerous types of liquid
isocyanates are described in, for example, U.S. Pat. Nos. 3,644,457,
3,883,571,
4,229,347, 4,055,548, 4,102,833, 4,332,742, 4,448,904, and 4,490,301.
[0152] A useful liquid polyisocyanate is prepared through the reaction with
various
hydroxyl functional materials. These reactions can be catalyzed using an
organometallic or tertiary amine. Useful hydroxy compounds are aliphatic
alcohols
containing about 1 to 36 and preferably 4 to 16 carbon atoms. Non-limiting
examples
of aliphatic alcohols are cycloaliphatic alcohols, aliphatic alcohols
containing
aromatic groups, aliphatic alcohols containing groups that do not react with
isocyanates e.g., ether groups and halogens such as bromine and chlorine.
Specific
non-limiting examples of aliphatic alcohols are 2-methyl-1-propanol,
cetylalcohol,
cyclohexanol, 2-methoxy-ethanol, and 2-bromoethanol. Branched aliphatic
alcohols
having relatively molecular weights up to 150, are most preferred.
[0153] Exemplary liquid adducts of isocyanates compounds include a reaction
product of solid 4,4'- and/or 2,4'-diphenylmethane diisocyanate with a
branched
aliphatic dihydroxy compound in a molar ratio of 0.1 to 0.3 mol of dihydroxy
compound per mol of diisocyanate. Another exemplary liquid MDI-based compound
is a reaction product of MDI with mixtures of monoalcohol, poly-1,2-propylene
ether
glycols and a triol. Another exemplary liquid polyisocyanate is the reaction
product
of an alcohol or thiol having an average functionality of from about 1.5 to
about 4 and
an average equivalent weight of at least about 500 with at least 2 equivalents
per
hydroxyl and/or thiol equivalent of an organic polyisocyanate wherein about
20% of
the initially formed urethane or thiourethane groups are converted to
allophanate
and/or thioallophanate groups.
[0154] Blocked isocyanates, which are known, can be adapted in the practice of
forming the coatings where a heating step is used for curing the coating.
Suitable
blocking agents for reaction with the organic mono- or polyisocyanates are
those
s8
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
isocyanate-reactive compounds, for example, phenols, lactams, oximes, imides,
alcohols, pyrazoles, and the like. The reaction of the organic polyisocyanate
and
the blocking agent can be carried out by any of the methods known in the art.
The
reaction can be carried out in bulk or in inert solvent at temperatures of,
for example,
about 50-120° C. For completely-blocked isocyanates, equivalent ratios
of
isocyanate-reactive groups to isocyanate groups of 1/1-2/1 or higher can be
utilized.
Completely blocked isocyanates are preferredly used herein, but the ratio can
be
adjusted if only a partially-blocked polyisocyanate is desired.
[0155] The aqueous coating containing functionalized elastomer and crosslinker
dispersed therein is utilized shortly after preparation. In the aqueous based
coating
embodiments employing polyisocyanate curing agents such as by the use of an
aqueous dispersed polyisocyanate these materials are known and disclosed, for
example, in U.S. Pat. No. 5,202,377. Exemplary emulsifiable polyisocyanates
taught in the '377 patent comprise a hydrophilic tertiary isocyanate
functional
oligomer rendered hydrophilic by partially reacting with a hydrophilic
polyether.
Other water dispersible isocyanates suitable for aqueous-based embodiments
according to the invention are known. U.S. Pat. No. 4,663,377, teaches an
emulsifiable polyisocyanate mixture comprising (a) a hydrophilic isocyanate-
functional oligomer and (b) a polyisocyanate. A non-limiting example is the
reaction
product of an aliphatic polyisocyanate with a mono- or polyhydric, nonionic
polyalkylene ether alcohol having at least one polyether chain containing at
least 10
ethylene oxide units. Water dispersible isocyanates which are preferred are
based
upon aliphatic and alicyclic isocyanates.
[0156] Coating compositions can be formed by combining (i) the water
dispersible
crosslinkers, such as carbodiimide or polyisocyanate with (ii) the separate
aqueous
solutions, emulsions or dispersions of the functionalized elastomer polymer
containing reactive functionality. Alternatively, the aqueous composition
containing
the functionalized elastomer can be combined with a separate aqueous
dispersion
containing the crosslinker such as is taught in U.S. Pat. No. 5,466,745 for
the
diisocyanate embodiment. The coating can be prepared by admixing the elastomer
in aqueous medium with a non-aqueous, emulsifiable composition comprising an
59
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
unblocked polyisocyanate crosslinking agent and a surface active isocyanate-
reactive material. This alternative will introduce some volatile organic
components
when selecting solvents known as VOC, however there are other solvent diluents
that can be used that are not considered VOC. A known procedure can be
followed
by (i) admixing an unblocked hydrophobic isocyanate and diluent with a mixture
of a
surface active isocyanate-reactive material and water to form a water-in-oil
emulsion,
then (ii) adding this emulsion to the aqueous medium containing the elastomer
in
proportions and under conditions to invert the isocyanate emulsion into an oil-
in-
water emulsion.
[0157] Specific examples of commercial diisocyanates that may be mentioned,
are
1,6-hexane diisocyanate (commercially available, for example, under the trade
designation HMDI from Bayer), isophorone diisocyanate (commercially available,
for
example, under the trade designation IPDI from Huls), tetramethylxylene
diisocyanate (commercially available, for example, under the trade designation
m-
TMXDI from Cytec), 2-methyl-1,5-pentane diisocyanate, 2,2,4-trimethyl-1,6-
hexane
diisocyanate, 1,12-dodecane diisocyanate and methylene bis(4-cyclohexyl
isocyanate) (commercially available, for example, Desmodur~ W from Bayer), and
higher functional isocyanates such as a biuret of 1,6-hexane diisocyanate
(commercially available, for example, as Desmodur~ N from Bayer), an
isocyanurate
of 1,6-hexane diisocyanate (commercially available, for example, as Desmodur~
N-
3390 from Bayer), an isocyanurate of isophorone diisocyanate (commercially
available, for example, as Desmodur~ Z-4370 from Bayer), a reaction product of
tetramethylxylene diisocyanate and trimethylol propane (commercially
available, for
example, as Cythane~ 3160 from Cytec), and a reaction product of one mole of
trimethylol propane and 3 moles of toluene diisocyante (commercially
available, for
example, as Desmodur~ L from Bayer). The amount of di- or polyisocyanate
included should be from 3 to 30 phr. Preferably the amount is from 8 to 15
phr.
[0158] Another class of crosslinking component which can be employed to cure
the
functionalized film former and form siloxane crosslinking, are the various
known
organosilanes. A preferred organosilane is an isocyanatosilane which contain
an
isocyanate group and one or more groups capable of forming crosslinks with the
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
silane and/or film former, such as a hydrolyzable group, hydrazidyl, thio,
halogen,
hydroxy, alkoxy, and other co-reactive substituents on the group bonded to
silicon
through a carbon atom, such as acyloxy, mercapto, amino, phenolic, and
glycido.
The silanes may contain a vinyl group; a vinyl-containing group; another
isocyanate
group; another isocyanate-containing group; an ureido group; an ureido-
containing
group; an imidazole group; or an imidazole-containing group. Such compounds
are
known in the art.
[0159] The reactive silane curing agents used herein will provide ambient
curable
emissive coatings in amounts on a weight basis of from 25 to 150 parts of
silane
curing agent per 100 wt. parts of film former and wherein the film former
contains
no more than 10 wt. % of functional groups which cure with the curing agent.
The
silane curing agents can be monomeric, tetravalent silanes or bis, or oligo-
derivatives containing at least two silicone bonded groups, of the same or
different
coreactive groups depending upon the chosen functional groups on the film
forming
polymer. One such type of curing group is a hydrolyzable group, or group that
interacts with the acidic or basic functional groups on the film former
polymer. The
silicone bonded group is an active hydrogen bearing group coreactive with the
functional group on the film former polymer, or the silicone bonded group is
coreactive with active hydrogen bearing groups on the film former polymer.
These
organosilane compounds are known and available from a number of commercial
sources.
[0160] Representative preferred hydroxyalkyl group-containing silanes have the
general structure:
(R1)a
I A
HO R Si (OR2) s-a ( )
wherein R is a divalent aliphatic, cycloaliphatic or aromatic radical having
from 1 to
20 carbon atoms, and is preferably an alkylene radical having from 1 to 9,
most
preferably 2 to 4 carbon atoms; R1 is a monovalent aliphatic, cycloaliphatic
or
aromatic radical having from 1 to 20 carbon atoms, and is preferably selected
from
the group consisting of alkyl radicals having from 1 to 4 carbon atoms,
cycloalkyl
61
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
radicals having from 4 to 7 ring carbon atoms, and aryl radicals having 6, 10,
or 14
nuclear carbon atoms and optionally one or more substituent alkyl groups
having
from 1 to 4 carbon atoms; R2 is a monovalent aliphatic, cycloaliphatic or
aromatic
organic radical containing from 1 to 8 carbon atoms, and is preferably
selected from
the group consisting of methyl, ethyl, propyl and butyl, and R3-O-R4, and
where
R3 is an alkylene group having from 1 to 4 carbon atoms (methyl, ethyl,
propyl, butyl)
-C=(O)-R, and R4 is an alkyl group having from 1 to 4 carbon atoms; and a is
zero
or 1, preferably zero.
[0161 ] Aminofunctional silanes are preferred for curing carboxy-functional
film
formers and include those having the structure (B)
H (R1 )a
R5 ~ R Si OR2 _ (B)
( )3 a
wherein R, R1, R2 and a are as previously defined for (A); and R5 is selected
from
the group consisting of hydrogen, monovalent aliphatic radicals having from 1
to 8
carbon atoms, monovalent cycloaliphatic radicals having from 4 to 7 ring
carbon
atoms, phenyl, alkaryl radicals having 6 nuclear carbon atoms and containing
one or
more substituent alkyl groups having from 1 to 4 carbon atoms, and the group
R~-
NH-R6- , wherein R6 is selected from the group consisting of divalent
aliphatic,
cycloaliphatic and aromatic radicals having from 1 to 20 carbons, there being
preferably at least two carbon atoms separating any pair of nitrogen atoms,
with R6
being preferably an alkylene group of 2 to 9 carbon atoms; and R~ being the
same
as R5 and preferably is hydrogen.
[0162] Mercaptofunctional silanes include those having the structure ( C)
(R1 )a
HS R Si (OR2)s-a (C)
62
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
wherein R, R1, R2 and a are as previously defined for (A);
[0163] Organosilane compounds useful herein include those contain as a
substituent on the Si atom an organic chain having from 1 to 20 carbon atoms,
at
least one extractable hydrogen atom which is preferably attached to a
functional
group separated from the silicon atom by a chain of at least 3 interconnected
carbon
atoms.
[0164] The preferred organosilane is an isocyanatosilane. Examples of
commercially available isocyanato-alkoxy silanes which are suitable herein
include
gamma-isocyanatopropyltrimethoxysilane, available as Silquest ~ Y-5187 from
OSi
Specialties Group, a Witco company (OSi), and gamma-
isocyanatopropyltriethoxysilane, available as Silquest~ A-1310, also from OSi.
[0165] Representative names and pseudonyms for organosilanes containing active
hydrogen groups are hydroxypropyltrimethoxysilane,
hydroxypropyltriethoxysilane,
hydroxybutyltrimethoxysilane, 'y-aminopropyltrimethoxysilane ~-
aminopropyltriethoxysilane, methylaminopropyltrimethoxysilane, y-
aminopropyltripropoxysilane, y-aminoisobutyltriethoxysilane, 'y-
aminopropylmethyldiethoxysilane, y-aminopropylethyldiethoxysilane, 'y-
aminopropylphenyldiethoxysilane, methyltrimethoxysilane,
ethyltrimethoxysilane,
propyltrimethoxysilane, butyltrimethoxysilane, isobutyltrimethoxysilane,
hexyltrimethoxysilane, octyltrimethoxysilane, decyltrimethoxysilane,
cyclohexyltrimethoxysilane, cyclohexylmethyltrimethoxysilane, and the like.
[0166] Also suitable as the curing agent are hydroxy silanes having an (Si-OH
bond), optionally as either partially neutralized silanediols or silanetriols.
The
silanols preferably contain at least one nucleophile connected to silicon
through a
first connecting group. As used herein, the term "partially neutralized" means
that at
least some of the silanol groups are in the form of mono-, di-, or tribasic
alkali metal
salts, more particularly lithium, sodium, or potassium salts. The extent of
63
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
neutralization is that amount sufficient to inhibit no more than 50% of the
condensation of condensable groups of the silanol, but provide enough
interaction
between the silane with the film forming polymer to form linking bridges but
not gel
the film forming polymer when part A and part B are combined. The curing agent
can be a partially neutralized silanol represented by the structure D:
( ~ H)m
Y R Si (O- M+)n (D)
(R~)P
where n is 1, 2, or 3; m is 0, 1, or 2; p is 0 or 1, preferably 0, with the
proviso that
m+n+p=3; R is the first connecting group; M+ is an alkali salt forming metal;
Y is a
group that contains a nucleophilic moiety; and R' is a linear, branched, or
cyclic C1 -
C$ -alkyl group, preferably methyl or ethyl, more preferably methyl.
Connecting
group R in D is preferably a linear, branched, or cyclic alkylene group, or
arylene
group, or a combination thereof, and may contain one or more heteroatoms,
which
may themselves be nucleophilic. More preferably, X is a C2 -C6 -alkylene group
or --
R'--NH--R'--, where each R' is independently a C2 -C4 -alkylene group.
[0167] Examples of suitable nucleophile groups include amines, phenols,
mercaptans, and carboxylates, with primary and secondary amines and mercaptans
being preferred, primary and secondary amines being more preferred, and
primary
amine being most preferred. A specific example of partially neutralized
aminosilanetriols are typically potassium or sodium salts of 3-aminopropyl-
silane triol
and N-(2-aminoethyl)-3-aminopropyl-silanetriol.
[0168] The more preferred organosilane curing agent will have at least one
silicone
bonded group that contains a substituted or unsubstituted alkylamino group and
alkoxy groups bonded to silicone capable of forming network crosslinks on
condensation of the organosilane. The amine group may be in the free unblocked
form or as a blocked amino group. Blocking of the amine group can be provided
by
reaction with methyl isobutyl ketone or methyl amyl ketone. The preferred
groups
64
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
reactive with the silane compound are preferably a C1-C4 alkoxy groups.
Examples
of curing components include but are not limited within the class of
aminosilanes are
aminopropyltriethoxy or -methoxy silane and aminoethylaminopropyltriethoxy or -
methoxy silane, 3-aminopropyl triethoxy silane, 3-aminopropyl trimethoxy
silane, 3-
aminopropyl methyl dimethoxysilane or 3-aminopropyl methyl diethoxy silane, a
silane containing primary secondary amines such as N-(2-aminoethyl)-3-
aminopropyl
trimethoxy silane, secondary amines such as N-methyl- or N-phenyl-3-
aminopropyl
trimethoxy silane, condensed aminoalkyl silanes such as bis(3-aminopropyl)
tetramethoxy or tetraethoxy disiloxane, NH2 (CH2)3 -Si(OCH3)2 -O-(CH3 O)2 Si-
(CH2)s
NH2, polyglycolether-modified aminosilanes such as that sold under the
Trademark
"Dynasylan 121 " and triamino functional propyl trimethoxy silanes such as
"Dynasylan TRIAMO" available from Huls AG. Similar silanes having two or three
silicon atoms can be used.
[0169] A preferred combination of an aminoalkyl trialkoxy silane and a
fluoroalkyl
trialkoxy silane exhibits improved color stability (non-yellowing) on heat
aging of the
cured coating.
[0170] Fluoroalkyl silanes useful in admixture with another silane containing
active
hydrogens, and most preferably in mixture with an aminosilane curing agent in
the
invention generally have a formula E:
y(R2)
(E)
R Ym (CH2)2 Si (OR)3_v
where R' is a monofluoridated, oligofluoridated, or perfluoridated alkyl group
with 1
to 20 C atoms or a monofluoridated, oligofluoridated, or perfluoridated aryl
group, Y
is a CH2 , O, or S group, R2 is a linear, branched, or cyclic alkyl group with
1 to 8 C
atoms or an aryl group, and R is a linear, branched, or cyclic alkyl group
with 1 to 8
C atoms or an aryl group, y is 0 or 1, and m is 0 or 1,
Specific examples of some of the fluoroalkyl silanes as representative include
3,3,3-
trifluoropropyl trimethoxy silane, 3,3,3-trifluoropropyl methyl dimethoxy
silane, 3,3,3-
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
trifluoropropyl methyl dimethoxy silane, 3,3,3-trifluoropropyl cyclohexyl
dimethoxy
silane, 3,3,3-trifluoropropyl phenyl diethoxy silane, and
heptadecatrifluorodecyl trimethoxysilane CF3(CF2) ~CH2CH2Si(OCH3) .
[0171 ] Amino resins utilized in amounts of less than 10 wt.% on weight of the
film
former can be used as curing components where acid catalyzed heated conditions
can be used. The amino resins refer to any material in the broad class of
materials
based on the reaction of formaldehyde with urea, melamine, benzoguanamine, or
acetylguanamine, and the like. Such compounds are well known and described in,
for example, "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd Ed.,
Volume
2, pages 440-469, Wiley-Interscience, 1978.
[0172] Curing agents containing at least two ethylenically unsaturated double
bonds
each activated by an adjacent electron-withdrawing groups and capable of
Michael
addition when the functional groups on the film forming polymer are suitable
and
known, e.g. malefic dianhydrides and fumaric dianhydrides.
[0173] Examples of other suitable curing components are the carbodiimides.
The polyfunctional carbodiimides exhibit suitable reactivity with functional
group-
containing elastomers used in the present invention. N-acylurea groups form
between carboxylic sites. Carbodiimide linkages can also be formed between a
carboxyl group and other functional groups contained in the functionalized
elastomer, such as hydrazidyl, amino and/or thiol groups. Polyfunctional
carbodiimides can be obtained from polyisocyanates using phospholine oxide as
catalyst as is described, for example, in U.S. Pat. No. 2,941,966. Water
dispersible
carbodiimides can be formed by the addition of hydrophilic polyamines or
polyols
and carbodiimides containing isocyanate groups, by reacting the reactants in
the
presence of from 0.01 to 3% by weight, based on the reaction mixture, of a Sn
catalyst as is taught in U.S. Pat. No. 4,321,394. The re-arrangement products
can
be produced at temperatures as low as 25-150° C, using such catalysts
as
tin(II)acetate or dibutyl tin diacetate. The hydroxyl-bearing compounds are
preferred
hydrophilic groups and include polyols containing from 2 to 8 hydroxyl groups,
and
especially those having a molecular weight in the range from 800 to 10,000.
66
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Exemplary polymeric polyols include for example, polyesters, polyethers,
polythioethers, polyacetals. Hydrophilic polyfunctional carbodiimides
containing
hydrolyzable silane groups with polyfunctional carbodiimides, are also
suitable,
especially for aqueous coating embodiments in accordance with the invention as
are
taught in U. S. Patent 5,258,481.
[0174] Examples of suitable carbodiimide compounds used in the present
invention
are N,N'-dicyclohexylcarbodiimide, 1-ethyl-3-(3'-
dimethylaminopropyl)carbodiimide,
N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide, N'-diisopropyl-carbodiimide,
N'N'-
di-tart-butylcarbodiimide 1-cyclo-hexyl-3-(4-
diethylaminocyclohexyl)carbodiimide,
1,3-di-(4-diethylaminocyclo-hexyl)carbodiimide, 1-cyclohexyl-3-
(diethylaminoethyl)carbodiimide, 1-cyclohexyl-1-cyclohexyl-3-(2-morphonlinyl-
(4)-
ethyl)carbodiimide 1-cyclohexyl-3-(4-diethyl-aminocyclohexyl)carbodiimide, and
the
like. There are a variety of commercially available solvent soluble and water
dispersible carbodiimides. Carbodiimide compounds are commercially available
from Union Carbide Corp., USA under the UCARLNK~ designation.
(C) CARRIER LIQUID
[0175] The coatings are applied in a carrier liquid. A carrier liquid can be
either one
or more organic solvents, or water, predominantly, although minor amounts of
one
can be contained in the other for introducing materials, co-solvating,
dispersing, such
that, the carrier can comprise a minor proportion of solvent, or co-solvent
along with
a major proportion of water, as an example. The coating compositions of the
present
invention are preferably applied to an elastomeric substrate in the form of a
solution
using one or more organic solvent carriers. For the purposes of the present
invention, the term solvent can broadly be defined as a carrier for the other
components of the composition, wherein the solvent is capable of dissolving or
maintaining the component in a substantially dispersed state or mixture.
Preferred
solvents include water based latexes and/or non-HAP (Hazardous Air Pollutant)
or
non-VOC, or non-HAP, non-V~C organic solvents.
[0176] Non-HAP solvents include methyl acetate, n-butyl acetate, t-butyl
acetate,
acetone, ethyl acetate, isopropyl acetate, isobutyl acetate, tetrahydrofuran,
n-methyl
67
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
pyrrolidone, aliphatic hydrocarbons such as heptane, dimethylformamide,
diisobutyl
ketone (DIBK), methyl isoamyl ketone, monochlorotoluene, para-
chlorobenzotrifluoride (PCBTF), and vm&p naphtha. A combination of acetone and
DIBK is the preferred non-HAP solvent mixture. Acetone, methyl acetate, and
para-
chlorobenzotrifluoride (PCBTF) alone or in any combination are the preferred
solvents for HAP, and VOC compliant coatings. Among the HAP solvents which are
photochemically reactive in the atmosphere are hexane, xylene, toluene, MEK,
and
MIBK. Toluene, xylene, MEK and MIBK are the preferred solvents when HAP and
VOC compliance is not critical.
[0177] One such category of solvent useful as the carrier vehicle for the
coating
composition of the present invention can essentially be any organic solvent or
other
material known to dissolve acrylonitrile-butadiene copolymers. Examples of
organic
solvents useful in the present invention include ketones such as methylethyl
ketone,
methylisobutyl ketone, and diisobutyl ketone; acetates such as butyl acetate;
toluene, xylene and their derivatives; nitropropane; and ethylene dichloride.
[0178] The organic solvent of solvent-based embodiments according to the
invention is typically utilized at about 70% to about 97% by weight of the
total coating
composition (solvent, functionalized HNBR, curing component, thermal
conductive
particles and optional components. Preferably solvent comprises from about 85%
by
weight to 95% by weight. Accordingly the coating composition has a total
nonvolatile
solids content ranging from about 3 to about 30% percent, and preferably from
about
to about 15%.
[0179] Often, it is highly desirable and environmentally advantageous to
utilize water
as the carrier. The invention is enabled by utilization of latex polymers
prepared by
emulsion polymerization as well as apueous converted dispersions of polymer
solids,
as follows. A solid bulk elastomer film former can be converted to a
dispersion by
dissolving in a suitable organic solvent or mixture of organic solvents.
Examples of
organic solvents include, but are not limited to, any of the organic solvents
listed
above, and preferably methyl ethyl ketone, methyl isobutyl ketone, and methyl
isopropyl ketone. The solvent, which can be a solvent mixture, preferably has
a low
water-solubility and optionally forms an azeotrope with water at a solvent
content of
68
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
more than about 50%, or a boiling point below about 95°-C, and at least
below the
boiling point of water. The polymer solution as continuous phase is treated by
introducing a surfactant, followed by addition of water. Mixing techniques
known in
the art can employ anionic, cationic, nonionic, or amphoteric emulsifiers,
including
mixtures. The aqueous organic solvent mixture is mixed under high shear and a
phase inversion takes place wherein water become the continuous phase. The
solvent is stripped off, typically by heating below the boiling point of
water, and
generally below 95°-C. The curing component and additional components,
if any, are
added to the latex, preferably shortly before coating.
[0180] An example of a further suitable procedure for preparing an aqueous
based
latex of a X-HNBR rubber is described in U. S. Patent No. 4,826,721, herein
incorporated by reference. The rubber component is dissolved in a solvent such
as
3-chloro-toluene. An emulsifier such as abietic (rosin type) acid derivatives
and
dehydro abietic acid derivatives is also added. Water was also added to the
composition. The composition was emulsified and subsequently the solvent is
freed
utilizing rotary evaporation, preferably under reduced pressure. X-HNBR latex
is
also available from Nippon Zeon of Japan. The aqueous latex coating
compositions
employed according to the present invention generally have solids content 30
to 50
percent by weight.
[0181 ] The emmissive coating compositions of the present invention cure to
form
substantially clear or transparent matrix elastomer. The transparency is
essential in
order to provide transmission of incident radiant heat to the underlying
thermally
conductive metallic particles, which emit heat back through the coating
surface.
Rather than conducting heat into the coated substrate, a surprising level of
heat
reflectance was observed in monitoring the temperature below the surface of
the
article. This emissive property is observed even for low surface area shaped
substrates, although the reduction in substrate temperature is expected to be
also
directly proportional to the ratio of surface area to volume of the underlying
shaped
article.
[0182] At a low level, optional tinting compounds such as dyes or organic
pigments
can be incorporated. Colored coatings provided in accordance with the
invention
69
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
provide outstanding color and coating physical properties for long-term
weathering
uses. An extensive list of organic pigments suitable for adding to emissive
for
tinting can be found in the current volume of the Rubber Blue Book, published
by
Lippincott & Peto Publications and well known to those versed in the art of
formulating elastomers. Organic colors as typically used, can be incorporated
for
different coloring effects. The non-pigmented organic colorants leave the
coating
transparent but with a color or shade.
[0183] Inorganic metal oxide pigments, especially micronized (diameters of 0.5
microns or less) can be included at up to 2.0 weight parts per 100 parts by
weight
of elastomer film former, e.g., titanium is possible without interfering
substantially
with the emissive properties of the coating can be used. Pigments can be mixed
into the solid polymer using a Banbury mixer or a two-roll mill. The rubber
containing
the pigment is then dissolved in the solvent. Alternatively, the pigment may
be
dispersed in the liquid solvent and then added to the solvated polymer blend.
This is
the preferred method for adding aluminum flakes. An exemplary solvent
dispersion
of aluminum flake comprises 50 parts of aluminum flake and a blend of 55 parts
ethylene glycol and 45 parts ethylene glycol monobutyl ether.
Metal Conductor Particles
[0184] In the embodiment coatings which further contain heat emissive
properties,
a minimum surface coverage in the coating is essential in order to provide
effective
emissive properties. The term "particles" is inclusive of irregular shapes,
granular
shapes, leafy shapes or complex assorted shapes. Heat reflective pigments are
available in many forms, as fine-grain solids, or leafs, in dry powder form or
dispersion or as pastes in solvent or plasticizer, e.g., mineral spirit.
Flakes derived
from finely divided vapor deposited films are suitable. Thermally conductive
metal
particles include finely divided irregular particles, or leafy particles of
brass, titanium,
silver, or aluminum. Included are metal-coated particles/metal coated films
which
are preferably introduced as leafing or non-leafing aluminum flakes. Leafing
flakes
such as leafing aluminum particles or flakes are available commercially with a
coating, e.g., stearic acid, and when applied to a surface, the particles
orient in an
interleaved structure parallel to the surface of the finished emissive
coating. Metallic
~o
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
particles of a particle size average of 5 to 25 p,m employed at a level of at
10 to 100
parts by weight per 100 parts by weight of film forming elastomer when cast in
a thin
film of 5 mils (0.01 cm.) provide effective radiant energy emmissivity and yet
provide
sufficient flex-fatigue resistance in the coating so as to not undergo stress-
cracking.
Stress cracking causes loss in emissive performance. Metal particles having an
average particle size of 25 to 100 microns must be employed at a level of at
least 20
parts and up to 150 weight parts per 100 parts by weight of film former to
provide
sufficient radiant heat emissivity without stress cracking. Aluminum flakes
are
typically available in an average particle size of less than about 300 microns
in
diameter. The maximum diameter of the metallic particles with high aspect
ratio is
rather indeterminate with two major dimensions (width and length) and one
minor
dimension (thickness) which may be multiples or orders of magnitude smaller
than
the two major dimensions. Reliance is on supplier specifications to
characterize the
average particle size. Preferably, aluminum flakes have a number average
particle
size of about 1 to about 100 microns, more preferably between 5 and 60
microns,
and still more preferably between 10 and 45 microns. Preferred aluminum
particles
are flakes of a size such that 99.9% pass through 325 mesh screen, i.e., a
diameter
of less than about 45 microns, most preferably from 8 and 35 and especially
from 10
and 20 microns in average particle size.
[0185] The leafing metal flakes can be introduced as a dry flake rather than
the
paste of aluminum and solvents having at least about 40 wt-% aluminum flake
and
more preferably about 60 to 70 wt-% aluminum flake as described in U.S. Pat.
No.
5,045,114. The metal particles are employed in the aforementioned quantity in
relation to the film forming polymer in order to exhibit emissive performance.
The
preferred amount of metal particles is in a range of from 15 to 30 parts by
weight per
100 parts by weight of film former. This proportion of includes consideration
of
surface additives, e.g., surfactants, or adhesion promoter, e.g., silanes.
[0186] The coating composition of the present invention may contain other
optional
ingredients such as, a nitroso compound, ZnO, and QDO, maleimides,
antioxidants
and sub-micron sized particulate reinforcements. The total amount of optional
additive shoud not exceed about 15 parts per 100 parts of the functionalized
film
forming polymer. Specific examples of particulate reinforcements useful in the
71
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
invention include precipitated silica, and fumed silica. Flatting agents,
which are well
known to the art, can be utilized in effective amounts to control the gloss of
the cured
coating and include, but are not limited to, silicates. Optional silica having
a particle
size less than 700 nanometers, more typically from 20 to 200 nanometers. Sub-
micron-sized particulate reinforcement does not affect the transparency of the
film
former to any noticeable effect on reducing the emissive properties of the
coating
and may be utilized in various amounts not to exceed 20 parts per 100 parts by
weight of the functionalized elastomer film forming polymer.
[0187] The coating composition may be prepared by simply mixing the
ingredients
by hand with a spatula or the like or by mechanical mixing or shaking. The
coating
composition is typically applied to an elastomeric material and/or other
substrate by
dipping, spraying, wiping, brushing or the like, after which the coating is
allowed to
dry for a period of time typically ranging from about 30 minutes to 2 hours,
preferably
from about 45 minutes to 1 hour. The coating composition is typically applied
to form
a dry layer on the substrate having a thickness ranging from about 0.1 to 5
mils (2.54
p,m - 127 ~,m), preferably from about 0.5 to 1.5 mils (12.7 - 38.1 p,m). In
the cured
state unsupported or supported coating films can elongate at least 100% of the
original length, and preferably can elongate up to 200%, more preferably up to
300%
without cracking.
[0188] The coating compositions can be applied to substrates which have been
vulcanized or to un-vulcanized or uncured substrates and co-cured therewith,
at
elevated temperatures if necessary.
[0189] The gloss of the cured coated substrate which does not significantly
reduce
transparency therefore can be manipulated at least by utilizing different
amounts of
solvent, controlling the evaporation rate andlor incorporating various known
pigments and/or flatting agents., It has been found that with respect to
organic
carrier-based coatings, a relatively quick or rapid evaporation produces a
flatter or
less glossy surface than a more prolonged cure rate. The cured coatings of the
present invention can impart to a substrate a gloss generally from about 3% to
about
70% at a 60 degree angle when measured using a Byk-Gardner Micro TRI
Glossmeter per ASTM D-523 and D-2457. The desirability on the gloss will vary
72
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
according to the use, with camouflage colors being desirable at low gloss
levels and
decorative coatings being desired at medium to high gloss levels. For example,
the
coating compositions can be beneficially utilized to impart an aesthetically
pleasing
appearance to a tire sidewall, such as a "metallic wet" look. The resulting
gloss of
the cured coating can be effectively controlled to produce a desired surface,
finish, or
appearance on a substrate.
[0190] The coating composition will cure within about 2 to 24 hours in ambient
air
conditions, including room temperature. The cure can be accelerated by
exposing
the coating to elevated temperatures, but this is not required.
(D) Flexible Substrates
[0191 ] Coating compositions of the present invention are able to coat
flexible
substrates, such as the myriad molded elastomeric materials in pre-cured or
post-
cured condition. The coating is applied to the entire exterior surface
thereof. The
coating compositions can be applied to shaped or molded articles such as those
made from thermoplastic vulcanizates or thermosettable rubber. The coating
composition of the present invention is particularly suitable for coating
cured rubber
engine mounting devices which are comprised of vulcanized elastomeric parts
that
have been bonded to metal parts.
[0192] An engine mount structure, comprises a base layer formed from natural
rubber, optionally bonded to and/or formed around one or more metal mounting
members such as for securing with bolts to the vehicle structure and the
engine
housing. The base layer is susceptible to degradation caused by heat,
oxidation,
ozone attack or ultraviolet radiation. The emissive coating is sprayed or
dipped and
conforms to the contours of the mount where applied and allowed to fully cured
after
being applied to said base layer, wherein the emissive coating is applied to
the base
layer such that the operating or equilibrium temperature internal to the
rubber portion
of the mount, when placed in service, is reduced by at least 30 °F
(16°C), more
preferably at least 50°F (27 °C), and most preferably at least
75°F (41.6 °C).
[0193] The preferred emissive coating compositions are particularly effective
as
coatings on cured elastomers that have limited oil and solvent resistance.
Such
elastomers include natural rubber, styrene butadiene rubber, polybutadiene
rubber,
73
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
ethylene propylene and ethylene propylene diene rubber, polyisobutylene-
isoprene
rubber, polychloroprene, low acrylonitrile content (< 35 wt.%) nitrite-
butadiene
rubbers; and the like. The coating composition may also be used over rigid
substrates such as metals, plastics, ceramics, and composites. Examples of
thermoplastic and/or thermosetting substrates include, but are not limited to,
flexible
polyvinyl chloride, PVC-elastomer alloys, like PVC-Nitrite; adhesion promoted
or
modified polyolefins such as compounded polyethylene and polypropylene;
flexible
polyesters like PBT, flexible or rubbery polyurethane-, polyurea-, polyurea-
rim; fiber
reinforced flexible plastics, and cellular vinyl and polyurethane. The
coatings are
particularly useful for bonded rubber mounts which contain both elastomeric
and
rigid components. A substrate is considered flexible if the elongation of the
substrate
material is greater than 25%.
[0194] Further examples of commonly available flexible substrates which can be
coated with the compositions of the present invention include, but are not
limited to,
tires, bumpers, wiper blades, vibration isolators, rubber mounts, rail track
pad
fasteners, helicopter rotor bearings, chassis mounts, wiper frames, gaskets,
heels,
shoe soles, printing rolls, belts, hoses, fuel tanks, rubber moldings, TPO or
TPE
molding, facias, and flexible engineered rubber products. In addition to
emissive
properties the coatings provide improved resistance to oils, solvents, oxygen,
ozone
and UV light.
[0195] The coating composition of the present invention can be applied to one
or all
sides of a substrate. It is to be understood that occasionally it may be
effective for
heat dissipation to only coat one side or surface of a substrate which is
oriented to a
heat source. As stated above, it is advantageous to coat the surfaces of a
substrate
which are exposed to light, air, oils, and solvents. Obviously, surfaces of a
substrate
which are not in contact with the same do not necessarily have to be coated.
The
coating preferably is a continuous coating in film form which completely
covers the
intended surface of a substrate. The coating is of the aforementioned
thickness to
cover the desired surface to be protected, but not overly thick to materially
alter the
mechanical properties of the substrate.
[0196] Tires) can be coated with a composition of the present invention. It is
to be
understood that the coating compositions can be utilized to cover the entire
outside
74
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
and/or inside surfaces of a tire. Furthermore, it may also be desired to only
coat
certain portions of a tire such as the sidewall, tread or the like. Tires
generally
comprise a tread, a pair of sidewalls which abut the tread in the shoulder
regions, a
fabric reinforced rubber carcass of generally toroidal shape and one or more
plies for
supporting the tread and sidewalls, and a circumferential fabric reinforced
belt of one
or more plies, positioned between the carcass and the tread. Tires generally
also
include a pair of circumferentially extending bundled wire beads which are
substantially inextensible, wherein the carcass extends from one bead to the
other
and the side edges may be wrapped around the beads as shown. Tires may also
include a pair of apex components, preferably of a stiff construction and
having a
triangular cross section in the region of the beads, and a pair of stiff
chaffer
components which are positioned in the bead region. The above listed
components
of the tire are conventional, but it is to be understood that additional parts
not listed
may be included and parts listed above may be omitted. Tires may also include
an
inner liner which can be applied to the inner surface of the tire to improve
air
impermeability. Any tire component or components can be coated with the
compositions of the present invention. Preferably, the tread and/or sidewall
regions
are coated.
PREPARATION OF ELASTOMER SUBSTRATE FOR COATING
[0197] The elastomeric surface or substrate to be coated may optionally be
pretreated with a chlorinating agent such as sodium hypochlorite and
hydrochloric
acid. The use of various chlorinating agents to prepare elastomeric materials
for
application of a coating composition is well known in the art. One example of
a
chlorinating agent is commercially available from Lord Corporation under the
CHEMLOK~ mark such as 7701. The chlorinating agent may be applied to the
surface of the elastomeric material by brushing, dipping, spraying, wiping, or
the like,
after which the chlorinating agent is allowed to dry. Chlorinating agents tend
to be
very volatile and typically dry within a matter of seconds or minutes.
[0198] The coating compositions of the present invention have the surprising
ability
to form a tenacious bond to flexible elastomeric parts alone, and also to
metal
components where these are affixed adjacent to the elastomeric part. It is
desirable
to provide the elastomeric coating over both elastomer and metal so that the
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
boundary between the elastomer and metal can be adequately protected by the
coating composition. The present invention is therefore distinguished from
many
traditional protective coating compositions which only have the ability to
bond to one
type of substrate to be protected.
[0199] The following examples are provided for purposes of illustrating the
present
invention and shall not be constructed to limit the scope of the invention
which is
defined by the claims.
[0200] Example 1
[0200] The following example was prepared using Zetpol 2220, an X-HNBR polymer
produced by Zeon Chemical having a 36% acrylonitrile content with 5 mot
percent
unsaturation. A suitable commercial substitute is Therban~ KA 8889.
[0201 ] An elastomer coating solution was prepared as follows:
Ingredient Description PHR
X-HNBR carboxylated hydrogenated nitrite-butadiene 100.0
[0202] This formulation was dissolved in Methyl Isobutyl Ketone (MIBK, CAS No.
108-10-1 ) to a solids content of 12.0% by weight.
[0203] To 40 grams of solution, of bis-[isocyanatopheny] methane
(diisocyanate),
53% in xylene was added at 0.1 g, 0.5 g and 1.0 g levels. At 0.1 g.
diisocyanate
level, the solution cured at room temperature in less than 16 hours. At 0.5 g,
the
solution cured in 30 minutes.
[0204] To 40 grams of solution, 3-isocyanatopropyltriethoxysilane, CAS # 24801-
88-
5, was added at 0.3, 0.7, 1.0, and 1.3 gram quantities. At all levels, the
coating
composition starts to cure within 45 minutes to one hour and was fully cured
in less
than 16 hours.
Fuel Resistance Testing
76
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0205] The coating were tested on a 55 durometer natural rubber compound
(A135Q) which had been treated with Chemlok~ 7701. The coating was then
compared against commercial fluorocarbon coating PLV-2100, and a commercial
HNBR SPE XV coating taught according to US patent 5,314,955 and an uncoated
control.
[0206] When immersed in Jet A fuel for 24 hours at room temperature, the
following
volume % swell results obtained are:
Control Uncoated 192.9%
Control PLV 2100 0.1
Control HNBR SPE XV 33.6%
Example Coating with bis-[isocyanatopheny] methane 2.2%
Example Coating with 3-isocyanatopropyltriethoxysilane 2.3%
ADHESION TESTING
[0207] Rubber adhesion was tested by bonding two one-inch-wide strips
together,
and by pulling in a 180° peel. The rubber strips were made from a 55
durometer
commercial natural rubber compound (A135Q) which had been treated with
Chemlok~ 7701. An approximate two-inch-long section was coated; each strip was
placed in contact with each other and a 472g weight applied to ensure intimate
contact. The weight was left in place for ten minutes. After 8 days drying
time, each
strip was pulled apart in the Tinius Olsen~ tensile tester. The following
table records
the results.
Coating Type Peel Results Lbf
Control PLV 2100 2.03
Control HNBR SPE XV 8.52
Example Coating with bis-[isocyanatopheny] methane 15.5
Example Coating with 3-isocyanatopropyltriethoxysilane 21.1
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0208] Metal adhesion was tested in shear by bonding a one-inch wide rubber
strip
to a one-inch metal coupon with one square inch of overlap. The rubber strips
were
made from a 55 Durometer natural rubber compound (A135Q) which had been
treated with Chemlok~ 7701. The metal coupons were 304 stainless steel.
Stainless was chosen because it is known to be a difficult substrate to bond
to. After
coating, each was placed in contact with each other and a 472g weight applied
to
ensure intimate contact. The weight was left in place for ten minutes. After 8
days
drying time, each specimen was pulled apart in the Tinius Olsen tensile
tester.
Coating Type Adhesion Results, psi
Control PLV 2100 16.78
Control HNBR SPE XV 19.23
Example Coating with bis-[isocyanatopheny] methane 18.2
Example Coating with 3-isocyanatopropyltriethoxysilane 18.5
Ozone Resistance
[0209] Ozone testing was done using a dynamic ozone test (ASTM-D3395) at 50
pphm ozone at 104 °F (40 °C).
[0210] Specimens were based on a 55 durometer commercial sulfur-cured natural
rubber/polybutadiene blend protected with antiozonant wax and an alkyl-aryl
phenylene-diamine antiozonant (M122N). Under dynamic conditions, it appears
that
the carboxylated hydrogenated coating is more effective as an ozone barrier
than the
HNBR coating SPE XV.
Elapsed time until initial cracking:
Control Uncoated 6.5 hrs.
Control HNBR SPE XV 6.5 hrs.
Example 1 Coating with bis-[isocyanatopheny] methane was untracked at 28 hrs.
Example 1 Coating with 3-isocyanatopropyltriethoxysilane was untracked at 28
hrs.
[0211 ] Besides having low adhesion values, the PLV 2100 coating cracks and
delaminates from the rubber surface after flexing. Unpierced DeMattia flex
~s
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
specimens (made from a 55 durometer natural rubber compound) were coated with
these same coatings and flexed in accordance with ASTM D-813. The PLV-2100
coating was severely cracked and delaminated, exposing the substrate in less
than
4000 cycles. Both the baked HNBR SPE XV and Example 1 ran 80,000 cycles at
which point the natural rubber substrate was cracked. There was no sign of
delamination in either of the Example coatings. This base formulation when
provided with the effective amount of thermal conducting metallic exhibits as
good
performance as tested above and further provides emissive properties.
[0212] Example 2
The following example was prepared using an X-HNBR polymer available from
Bayer AG under the Therban~ mark as Therban~ KA 8889.
An elastomer coating solution was prepared as follows:
Ingredient Description PHR
X-HNBR carboxylated hydrogenated nitrite-butadiene 100.0
[0213] This formulation was dissolved in Methyl Isobutyl Ketone (MIBK, CAS No.
108-10-1 ) to a solids content of 15.0% by weight.
33 phr of aluminum flake having an average particle diameter of 16 microns
were
added to the coating solution.
[0214] To 97.5 wet wt. parts of solution, 2.5 wet wt. parts of bis-
[isocyanatopheny]
methane (diisocyanate)(Casabond~ TX, 53% in xylene) was added.
[0215] A cured block of natural rubber 3" x 3" x 0.5" (7.6 cm.x 7.6 cm x 1.2
cm)
having a Durometer A of 65 was coated to a dry film thickness of about 1 mil.
[0216] A hole was drilled 1.5 in. (3.8 cm.) and a thermocouple inserted for
monitoring temperature in the center of the block. The block was placed under
a 250
watt infrared lamp, suspended 8" (20 cm. From the rubber block. The control
block
was uncoated. Temperature recordings were made using a Cole-Parmer Dual J-T-
E-K Thermocouple Thermometer Model 91100-40 at the time intervals below.
79
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Uncoated Rubber Block Coated Rubber Block
Time (minutes) Temperature (°F/°C) Temperature
(°F/°C)
Initial 0' 73.8/ 23.2 73.6/ 23.1
10' 162./ 172.2 97.3/ 36.2
20' 214.9/ 101.6 118.7/ 48.1
30' 238.5/ 114.7 130.5/ 54.7
[0217] The uncoated specimen began smoking within the first 10 minutes of
exposure to the heat source.
[0218] DeMattia Flex specimens were coated with the coating material used in
example 2 in accordance with ASTM D-813. After 77,000 cycles with no signs of
cracking or delamination were observed in the coating. Cracks occurred in the
rubber substrate and coating was split where the substrate crack occurred.
Adhesion was excellent, and failure only observed in the underlying substrate
indicates that the maximum level of coating integrity is obtained.
[0219] The results illustrated in FIG. 1 represent a repeat of Example 2
coated
specimen with a 16 inch, 3 speed fan running at low speed, blowing across the
specimens from 9.5 feet away and the infra-red lamp positioned 4 inches from
specimens. Under air movement simulating actual automotive
Uncoated Rubber Block Coated Rubber Block
Time (minutes)Temperature Temperature
(F) (F)
Initial 0' 73 73
4' 95 78
10' 131 g4
20' 172 92
35' 181 gg
50' 189 99
120' 189 gg
[0220] Example 3 - Functionalized HNBR Water Based Latex
Water based functionalized HNBR latexes were prepared according to the present
invention. A 41 % solids carboxylated-HNBR latex, 404EXPLTX005 also sold as
Latex B from Zeon Chemical was utilized. The following compositions were
prepared.
so
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Components FormulaFormula FormulaFormula FormulaFormula
1 2 3 4 5 6
41 % SOIIdS 100 100 100 100 100 100
X-
HNBR latex grams grams grams grams grams grams
Diphenylmethane1.25 2.5 5.0 -- -- --
Diisocyanategrams grams grams
~prepolymer
aromatic
1 6 - __ __ __ 2.5 5.0 7.5
Hexamethylene grams grams grams
Diisocyanate
based
polyisocyanate2
ali hatic
1
Desmodur~
XO
672
2 Bayhydur~ 302 (1,6-HDI) available from Bayer Corporation
[0221 ] DeMattia Flex specimens were sprayed with the latex/isocyanate
combination
as listed above. The DeMattia specimens were wiped with MIBK and treated with
Chemlok~ 7701, and the coating was applied to the specimens by spraying. All
specimens ran 80,000 cycles with no signs of cracking or delamination.
Adhesion is
excellent.
[0222] Ozone testing was done using a dynamic ozone test (ASTM-D3395) at 50
pphm ozone at 104 °F.
[0223] Specimens were based on a 55 durometer commercial sulfur-cured natural
rubber/polybutadiene blend protected with antiozonant wax and an alkyl-aryl
phenylene-diamine antiozonant (M122N). Observations were made at 2 hour
intervals.
Time to observed edge cracking
A. uncoated control 4.0 hrs.
B. coated with Chemisat~ LCH7302X, a non-functionalized HNBR
2 hours
C. coated with Chemisat~ LCH7302X non-functionalized HNBR with 5.0 parts per
hundred by weight of Bayhydur~ 302 (1,6-HDI))
4.0 hours
81
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
D. coated with Carboxylated HNBR 404EXPLTX005
hours
E. coated with carboxylated Latex 404EXPLTX005 with 5.0 parts per hundred by
weight of 1,1,6-HDI
22.0 hours
Chemisat~ LCH7302X is an HNBR Latex currently produced by Zeon Chemical,
formerly produced by Goodyear Chemical Company.
[0224] Example 4
4E 4F 4G 4A
Silverl Silver 2 Silver 3 Green
Therban~ KA-8889* 100 100 100 100
Akrochem~ E2557 green --- --- --- 2.5
Alglo~ 400 aluminum (AI) paste**10.0 --- ---
AI Paste 586 --- 12.5 ---
Stapa~ Metallux 214 AI paste --- --- 10.0 ---
*carboxylated HNBR from Bayer Ag.
** avg. diameter. 45 microns
[0225] Alglo~ 400 and the aluminum paste 586 are supplied by Toyal America,
Inc.
and the Stapa~ Metallux 214 is supplied by Eckart America L. P. Aluminum Paste
565 and Stapa~ Metallux 2156 were also used. Both leafing and non-leafing
aluminum pigments of varying particle sizes may can be used to obtain
different
visual effects. The compounded elastomers were each dissolved in solvent to
10%
solids content. They were readily blended with tinting colorants to different
tinted
shades conventionally according to the known art of color matching. On the
other
hand, a mixture of 90% Silver 3 and 10% green gives a silver color with a hint
of
pastel green.
[0226] A blend of copper conductive powder from Caswell with silver2 (Example
4F)
gave a metallic gold color.
82
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0227] Example 5 - CONTROL
A control example using a coating cured according to U.S. Pat. No. 5,314,741
of
hydrogenated copolymer of acrylonitrile and butadiene in organic solvent using
zinc-
sulfur curing as taught therein was applied to a peroxide cured natural rubber
substrate.
Coating Composition
Ingredient Parts by Weight
HNBR 100
Zinc Oxide 4.00
Sulfur 1.75
ZMBT (2) 2.00
Zinc dibutyl dithiocarbamate0.75
Total 108.50
* Zinc 2-mercaptobenzothiazole
accelerator
[0228] The ingredients except HNBR were mill mixed and then dissolved to a.10%
solution in MIBK solvent. The coating composition was prepared by mixing the
solid
rubber on a two roll mill followed by dissolving HNBR in solvent. One inch
wide
specimens of sulfur-cured natural rubber sheet were washed with isopropyl
alcohol
prior to applying the coating composition.
[0229] The coating composition was applied to the surfaces of the natural
rubber
substrate specimens. The coating thickness was approximately 1 mil dry. Two
coated, uncured strips were placed together with the coated sides against each
other. The coatings were dried for 24 hours at room temperature. Some of the
specimens were baked in an oven for fifteen (15) minutes at 307° F
(152°C) to cure
the coatings. This gave as the product coated natural rubber tensile sheets
having
thereon coatings, approximately 2 mil thick and bonded together. The bonded
specimens were pulled apart in peel and the force required to separate them
was
recorded.
Uncured coating (dried but not baked) 0.6 Ibs peel strength
Cured coating (baked 15 minutes at 307F) 1.9 Ibs peel strength
83
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0230] These adhesion levels to the rubber substrate as cured and uncured
coatings
are unacceptably low and result in flex fatigue and cracking on elastomer
substrates
subjected to flexing.
[0231 ] Examples 6
A clear base coating was made by dissolving X-HNBR elastomer (Therban KA-8889
from Bayer AG) in MIBK to a solids content of 5% by weight. To 99.25 wet wt.
parts
of solution, 0.75 wet wt. parts of bis-[isocyanatopheny] methane
(diisocyanate), 53%
in xylene (Casabond TX,) was added. Thermal conductive aluminum pigments
were added to the clear coating solution in various weight percents based on
the
weight of the polymer.
[0232] Cured blocks of natural rubber 3" x 3" x 0.5" (7.6 cm.x 7.6 cm x 1.2
cm)
having a Durometer A of 65 were coated to dry film thickness of about 1 mil
(0.0004
cm).
[0233] Holes were drilled 1.5 in. (3.8 cm.) into the center of the block and
thermocouples were inserted for monitoring temperature in the center of the
block.
The blocks were placed under a 250-watt infrared lamp, suspended 4" (10 cm.)
from
the rubber block. The control block was uncoated. Temperature recordings were
made against time using a Cole-Parmer Dual~ J-T-E-K Thermocouple Thermometer
Model 91100-40. No fan was used in this experiment.
Uncoated Rubber Block
Time (minutes) Temperature (F)
Initial 0' 72
5' 96
10' 115
15' 130
20' 145
[0234] Example 6A
STAPA~ Metallux~ 2156 (Eckart America L.P.) ) 70% solids, non-leafing, 16
micron avg. dia.
Coated Rubber Block using STAPA Metallux 2156
phr 20 phr
84
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Time (minutes) Temperature (F) Temperature (F)
I nitial 0' 72 72
5' 87 79
10' 106 90
15' 120 100
20' 130 108
The results are graphically illustrated in FIG. 2
[0235] Example 6B
Aluminum Paste 565 (ex. Toyal America) 65% solids, leafing, 13 micron avg.
dia.
Coated Rubber Block using Aluminum Paste 565
10 phr 20 phr
Time (minutes)Temperature (F) Temperature (F)
Initial 0' 72 72
5' 84 81
10' 97 93
15' 106 101
20' 116 110
The results
are graphically
represented
in FIG. 3
[0236] Example 6C
Alglo~ 400 Aluminum Paste (ex. Toyal America) 70% solids, non-leafing, 45
micron
av. dia.
Coated Rubber Block
using Alglo 400
20 phr 50 phr
Time (minutes)Temperature (F) Temperature
(F)
Initial 0' 72 72
5' 83 81
10' 100 93
15' 112 101
20' 116 110
The results . 4.
are graphically
represented
in FIG
[0237] Example 6D
Sparkle~ Silvex~ 760-20-A (ex. Silberline) 80% solids, non-leafing, 54 micron
Coated Rubber Block using Sparkle Silvex 760-20-A
20 phr 50 phr
Time (minutes) Temperature (F) Temperature (F)
Initial 0' 73 73
5' 86 82
10' 101 92
15' 116 102
20' 124 108
The results are graphically illustrated in FIG. 5.
ss
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0238] Example 7
Three similar coatings were made using a fluoroelastomer, a water based XHNBR
latex, and a polyurethane, respectively. The fluoroelastomer base coating was
made
by mixing the following formulation and then dissolving it in MIBK to a
solution having
a solids content of 30%.
[0239] Example 7A
Viton~ A-100 (DuPont) 100.0 PHR
Magnesium Oxide (Maglite D) 1.0
Calcium Hydroxide Technical Grade 2.0
Metallux~ 2156 (Eckart America L.P.) 10.0
Aluminum Paste 586 (Toyal America) 5.0
To 120.0 grams of the dissolved solution, 1.8 grams of N-(2-
hydroxyethyl)ethylenediamine was added. After 4 hours, 5.0 grams of 3-
isocyanatopropyltrierthoxysilane was added along with an additional 25 grams
of
MIBK.
[0240] Example 7B
The XHNBR Latex was made by starting with Latex B from Zeon Chemical (41
solids content). To 100.0 grams of Latex B, 20.0 grams of Sparkle Silvex~ 760-
20-A
(Silberline~) and 5.O grams of the water dispersible polyisocyanate Bayhydur~
302
(Bayer) were added.
86
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0241 ] Example 7C
The polyurethane was made by adding 7.0 grams (21.8 phr on urethane solids) of
Aluminum Paste 586 (ex. Toyal America) to 100.0 grams of Chemglaze~ V021
clear, moisture curable polyurethane at 32% solids by weight, having a
viscosity of
115 cps, a cured Tg of below 0°C, and cured tensile strength of approx.
3000 p.s.i.
with 350% ultimate elongation.
[0242] Cured blocks of natural rubber 3" x 3" x 0.5" (7.6 cm.x 7.6 cm x 1.2
cm)
having a Durometer A of 65 were coated to dry film thicknesses of about 1 mil
using
the coatings of examples A, B and C.
[0243] Holes were drilled 1.5 in. (3.8 cm.) into the center of the tested
blocks and
thermocouples were inserted for monitoring temperature in the center of the
block.
The blocks were placed under a 250-watt infrared lamp, suspended 3" (7.5 cm.)
from
the top surface of the rubber block. The control block was uncoated.
Temperature
recordings were made at against time. The surface temperature was monitored
using an Omegascope~ Model OS530 Series non-contact infrared thermometer.
The internal temperature was monitored using a Cole-Parmer Dual J-T-E-K
Thermocouple Thermometer Model 91100-40. No fan was used in this experiment
Uncoated Rubber Block
Internal Surface
Time (minutes)Temperature (F) Temperature (F)
Initial 0' 69.5 69
1' 74.0 182
2' 91.8 242 (smoking)
3' 113.6 268
4' ~ 135.0 299
5' 156.0 328
6' 176.0 333
8' 209.1 353
10' 238.0 375
s7
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Fluoroelastomer(Example
7A) coated Rubber
Block
Internal Surface
Time (minutes)Temperature (F) Temperature (F)
Initial 0' 69.1 69
1' 73.4 146
2' 88.2 185
3' 104.6 207
4' 120.9 224
5' 136.7 237
6' 151.5 257
8' 178.8 268 (smoking)
10' 202.1 291
XHNBR Latex (Example7B) covered Rubber Block
Internal Surface
Time (minutes)Temperature (F) Temperature (F)
Initial 0' 69.8 69
1' 72.2 1157
2' 82.8 176
3' 96.1 195
4' 110.3 203
5' 124.5 212
6' 139.8 236
8' 163.0 254 (smoking)
10' 186.1 264
Polyurethane (Example
7C) coated Rubber
Block
Internal Surface
Time (minutes)Temperature (F) Temperature (F)
Initial 0' 72.0 69
1' 75.9 127
2' 90.4 145
3' 105.2 174
4' 120.7 182
5' i 35.0 189
6' 148.5 198
8' 172.5 210
10' 194.4 223 (no smoke)
[0244] The results comparing the surface temperature of the uncoated control
and
coated specimens based on Example 7A, 7B and 7C are graphically illustrated in
FIG. 6
ss
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
[0245] Example 8
Room temperature curable reflective coating formulations were made as follows:
Ex. 8A Ex 8B
Ingredient parts by weight
MIBK 90.0 90.0
DIBK 5.0 5.0
Therban~ KA-8889 (X-HNBR) 5.0 5.0
After the polymer was dissolved, ollowing was added:
the f
Aminopropyltriethoxysilane 5.0 5.0
Aluminum Paste 586 2.5 2.5
KBM-7803 ----- 5.0
[0246] KBM 7803 is Heptadecatrifluorodecyl trimethoxysilane CF3(CF2)
7CH2CH2Si(OCH3)3 and is commercially available from Shinetsu Silicones.
A 6"x6"x0.75" natural rubber pad (65 durometer) was coated with each of the
coatings. After the coatings were cured, they were exposed to an infrared lamp
suspended 6" above the coatings. The surface temperature was monitored using a
Cole-Parmer~ Dual J-T-E-K Thermocouple Model 91100-40 at the time intervals
indicated below. Immediately after exposure, the pads were subjected to
heating in
an oven at 350°F for 7 more minutes to accelerate discoloration.
[0247] Surface Temperature Measurements
Uncoated Coated 93-6 Coated 93-7
Time (minutes)Temperature F/C Temperature F/C Temperature FC
Initial 0' 82/27.7 81 /27.2 81 /27.2
1' 176/80 129/53.8 120/48.8
2' 235/112 159/70 146/63
3' 280/137 190/87.7 170/76
4' 305/151 195/90 185/85
5' 330/165 204/95 196/91
6' 340/171 211 /99.4 204/95
T 345/173.8 216/102 207/97
89
CA 02477740 2004-08-30
WO 03/076537 PCT/US03/07258
Discoloration- aging at 350°F/175 °C: Severe Minimal
(0248] Emissive coatings based on hydrolyzable mixture of aminoalkyl
trialkoxysilane and fluoroalkyl trialkoxysilane demonstrate rapid cure and
reduced
discoloration after heat aging.
[0249] While in accordance with the patent statutes the best mode and
preferred
embodiment have been set forth, the scope of the invention is not limited
thereto, but
rather by the scope of the attached claims.