Note: Descriptions are shown in the official language in which they were submitted.
CA 02478199 2004-09-O1
1
POLYETHYLENE TEREPHTHALATE RESIN CONTAINER
Technical Field
This invention relates to a container comprising a polyethylene
terephthalate-based resin (hereinafter referred to as PET-based resin) and
having an oxygen-capturing function and an oxygen barrier property, which
have been improved by treating the container with radiation.
In the past, the PET-based resin containers have been utilized in
various applications, including the fields of foods, drinks, and medicines,
due
to easy moldability and distinguished properties, such as transparency,
chemical resistance, heat resistance, and mechanical strength, and have been
used as the containers in which to fill those contents that have to be kept
away
from the contact with oxygen, including beer, fruit drinks, tea, coffee, and
dips.
If the oxygen barrier property falls short in the containers using a PET-
based resin alone, then the PET-based resin is blended with an oxygen barrier
resin, such as an ethylene vinyl alcohol copolymer or a nylon resin, or an
oxygen barrier layer is laminated with the PET-based resin layers.
Even if an oxygen barrier resin is laminated, as described above, to
make the container usable, there still is oxygen in the air on and above the
contents inside the container after it has been filled with the contents.
Since
this oxygen cannot be removed, there was no way to prevent the contents
completely from coming in contact with oxygen and to avoid the oxidation of
the contents. To cope with this problem, Official Gazette of Patent
Application
No. 1989-278344 discloses a multi-layered plastic container that enables
oxygen to be captured by an intermediate layer, which is molded by a resinous
composition containing a transition metal complex in an oxygen barrier resin.
However, the method disclosed in the above patent application 1989-
278344 has some problems. Firstly, a high production cost is derived from the
process for mixing a metal complex with the oxygen barrier resin. Secondly,
moldability gets worse. Thirdly, the container has to be mufti-layered because
the metal complexes leach out and cannot be used for the surface with which
the contents come in direct contact. Lastly, oxygen within the container
cannot be fully captured because the layer having the oxygen-capturing
function cannot be used as the inner layer.
CA 02478199 2004-09-O1
2
This invention has been made to solve the above-described problems
found in conventional art. The technical problem of this invention is to
create
an effective oxygen-capturing function even in the container made of a single
PET-based resin layer, without adding to the resin such an ingredient as a
metal complex. The object of this invention is to provide a PET-based resin
container having the effective oxygen-capturing function and an improved
level of oxygen barrier property.
Disclosure of the Invention
The means of carrying out the invention of Claim 1 to solve the above-
described problems exists in the configuration that the container comprises a
polyethylene terephthalate resin and has an oxygen-capturing property and an
oxygen barrier property that have been improved by treating the container
with radiation after the molding operation.
The polyethylene terephthalate resin(hereinafter referred to as PET
resin)is mainly used as the PET-based resin in the container of this
invention.
But as far as the nature of the PET-based resin is not impaired,
copolymerized polyesters containing other polyester units can be used in
addition to a major portion of ethylene terephthalate units. As the components
that can be used in this invention to form a polyester copolymer, there may be
mentioned dicarboxylic acid components, such as isophthalic acid,
naphthalene-2,6-dicarboxylic acid, and adipic acid; and glycolic components,
such as propylene glycol, 1,4-butane-diol, tetramethylene glycol, neopentyl
glycol, cyclohexane dimethanol, and diethylene glycol.
The PET-based resin blended with another resin can be used within a
limit in which the nature of the PET-based resin is not impaired. For example,
polyethylene naphthalate (PEN) can be blended with the PET-based resin to
improve heat resistance and chemical resistance, or a nylon resin can be
blended to improve heat resistance and gas barrier property.
Those resins having the gas barrier property can also be used by
laminating them with the PET-based resin within the limit in which the
nature of the PET-based resin container is not impaired.
Amorphous PET-based resin can also be used as a PET-based resin.
The amorphous PET-based resin has no melting peak when it is measured the
melting temperature (Tm) using a differential scanning calorimeter (DSC). An
CA 02478199 2004-09-O1
3
example of amorphous PET-based resin is PETG of Eastman Chemical
Company, which is obtained by copolymerizing PET-based with a glycol
component, such as cyclohexane dimethanol.
Radiation, such as alpha ray, beta ray, gamma ray, X-ray, neutron
radiation, and electron beam, can be used in this invention.
Irradiation causes free radicals to be generated inside the PET-based
resin. These radicals react with oxygen existing dissolved in the resin. As a
result, the oxygen-capturing function is fulfilled.
The above-described oxygen-capturing function serves to capture oxygen
that dissolves into the container wall from outside and moves to the inside of
the container. During the period in which this oxygen-capturing function is
active, the transmission of oxygen from outside to the inside of the container
is
controlled. Thus, the irradiation improves the oxygen barrier property of the
exposed container much more than that of the container to which no radiation
has been applied.
On the other hand, since there is no need of adding such substances as a
metal complex, an oxidation catalyst, or an oxidation initiator, to obtain the
oxygen-capturing function, the layer having the oxygen-capturing function can
be brought into direct contact with the contents. After the container has been
filled with the contents and sealed, oxygen existing in the air above the
contents inside the container and oxygen dissolved in the contents move into
the container wall and react there with free radicals. In this manner, oxygen
inside the container can be reduced effectively within a short period.
The means of carrying out the invention of Claim 2 exists in the
configuration that the container comprises a single layer of the PET-based
resin specified in the invention of Claim 1.
In the above configuration of Claim 2, even an ordinary single-layered
PET-based resin container can be provided with the oxygen-capturing function
by irradiating the container after it has been molded, without adding thereto
such substances as a metal complex, an oxidation catalyst, and an oxygen
initiator. For at least the period in which this oxygen-capturing function is
maintained, the container keeps an improved level of oxygen barrier property.
CA 02478199 2004-09-O1
4
The means of carrying out the invention of Claim 3 exists in the
configuration that the container specified in the invention of Claim 1 has at
least an inner layer and an outer layer, with both layers comprising the PET-
based resin.
Multi-layered containers having an intermediate layer of a gas barrier
resin are used to improve the gas barrier property. By the configuration of
Claim 3, the inner and outer layers are made of the PET-based resin.
Therefore, it becomes possible to retain distinguished properties of the PET-
based resin, such as moldability, transparency, heat resistance, chemical
resistance, and mechanical strength.
The means of carrying out the invention of Claim 4 exists in the
configuration that the PET-based resin specified in the invention of Claim 1,
2,
or 3 is blended with an oxygen barrier resin at a rate in the range of 1.0 to
30
wt.%.
Any oxygen barrier resin, which is well known in the art, can be used,
including nylon resins, such as nylon 6, nylon 66, and polyamide containing
xylylene radicals; and an ethylene vinyl alcohol polymer.
By the above-described configuration of Claim 4, the PET-based resin in
use is blended with a resin having an oxygen barrier property. Since oxygen
creeping in from outside can be inhibited to a lower level than when the PET-
based resin is used alone, the free radicals generated by irradiation have
fewer
opportunities in which these radicals are diminished by the oxygen creeping in
from outside. Therefore, the oxygen-capturing function continues to work for a
longer period, and the oxygen barrier property is maintained at an improved
level for a longer period, than the usual.
The oxygen barrier resin is blended with the PET-based resin in a
relatively small amount. Therefore, most of the oxygen barrier resin is not
exposed to the outer or inner surface of the container, but is scattered
inside
the wall, and has little chance of coming in direct contact with oxygen
existing
inside and outside of the container. The oxygen-capturing function of the
scattered oxygen barrier resin is maintained for an extended period, and thus
the oxygen barrier property can be maintained at an improved level during
that period.
CA 02478199 2004-09-O1
The oxygen barrier resin has usually an active radical of some kind,
such as a double bond or a carbonyl radical. In many cases, irradiation tends
to increase the frequency of radical generation, and therefore, improves the
oxygen-capturing function of the container.
5
In this configuration, it is necessary for the oxygen barrier resin to be
blended in an amount in the range of 1 to 30 wt.%. At a level less than 1%,
the
oxygen-capturing function would show only a minor level of improvement.
Above 30%, the PET-based resin would lose its original moldability,
transparency, and mechanical strength.
The means of carrying out the invention of Claim 5 exists in the
configuration that the oxygen barrier resin specified in the invention of
Claim
4 is a polyxylylene diamine adipamide resin (Nylon-MXD6).
Due to the above-described configuration of Claim 5, Nylon-MXD6 resin
has outstanding oxygen barrier property. Since Nylon-MXD6 has xylylene
radicals on the main chain, this resin has a high oxygen-absorbing ability by
nature. Furthermore, since Nylon-MXD6 tends to generate free radicals when
it is exposed to radiation, the blend with the PET-based resin fully
demonstrates the oxygen-capturing function.
The means of carrying out the invention of Claim 6 exists in the
configuration that the container specified in the invention of Claim 1, 2, 3,
4, or
5 is treated with a radiation dose of 20 kGy or more.
Radiation of 20 kGy or more applied to the container can be remarkably
effective in improving the oxygen barrier property. The larger the extent of
irradiation, the more improved oxygen-capturing function is derived, but
coloration may be caused disadvantageously. The upper limit to irradiation
can be set suitably, depending on the color development of the resins, the
purpose intended for the container, and the necessity.
The means of carrying out the invention of Claim 7 exists in the
configuration that the container specified in the invention of Claim 1, 3, 4,
or 5
has at least an intermediate layer comprising an oxygen barrier resin.
In the above-described configuration of Claim 7, the oxygen barrier
property is greatly improved by the existence of an intermediate layer having
the oxygen barrier property. Therefore, the invasion of the container by
CA 02478199 2004-09-O1
6
outside oxygen can be inhibited dramatically. There are much fewer
opportunities for the free radicals generated by irradiation to be consumed by
the incoming outside oxygen. Even at a low dose of radiation, the oxygen-
capturing function can be persistent for an extended period, and the oxygen
barrier property is maintained at an improved level during that period.
The oxygen-capturing function of the oxygen barrier resin is maintained
for an extended period because the intermediate layer comprising the oxygen
barrier resin does not come in direct contact with the oxygen inside or
outside
of the container. This detached condition allows the oxygen barrier property
to
be maintained at an improved level during that period.
The means of carrying out the invention of Claim 8 exists in the
configuration that the oxygen barrier resin of the container specified in the
invention of Claim 7 is a polyxylylene diamine adipamide resin (Nylon-MXD6).
Nylon-MXD6 has an outstanding oxygen barrier property. Because this
nylon has xylylene radicals on the main chain, free radicals tend to be
generated by irradiation. If this nylon is laminated with a PET-based resin,
then it is possible for the container to perform fully the oxygen-capturing
function.
Nylon-MXD6 can be blended easily with the PET-based resin, or the
container of a mufti-layered structure can also be molded. In either way, a
high level of productivity can be maintained for the container.
The means of carrying out the invention of Claim 9 exists in the
35
configuration that a radiation dose of 6 kGy or more is applied to the
container
specified in the invention of Claim 7 or 8.
The oxygen-capturing function is effectively performed by the container
having an intermediate layer comprising an oxygen barrier resin. Even at a
dose as small as 6 kGy, an improved effect of the oxygen barrier property is
demonstrated.
The means of carrying out the invention of Claim 10 exists in the
configuration that an electron beam is used as the source of radiation in the
invention of Claim 1, 2, 3, 4, 5, 6, 7, 8, or 9.
CA 02478199 2004-09-O1
7
Any well-known electron irradiation equipment can be used industrially
with relative ease.
Brief Description of the Drawings
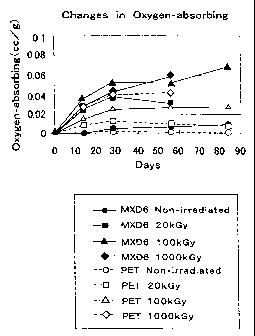
Fig. 1 is a graph showing time-lapsed changes in the oxygen-absorbing
rates for the PET and Nylon-MXD6 resins after exposure to electron beam
radiation.
Fig. 2 is a graph showing time-lapsed changes in the oxygen
transmission rates for the PET-based resin containers of Examples 1 to 3.
Preferred Embodiments of the Invention
This invention comprises applying radiation to the PET resin container
from outside to generate free radicals, which imparts the oxygen-capturing
function to the container and improves the oxygen barrier property. The
action and effect and the actual configuration of this invention are further
described below in examples.
Table 1 shows the results of the measurements using a PET resin and
Nylon-MXD6 (T-600, Toyobo) as the oxygen barrier resin to evaluate the
improvement in the oxygen-capturing function, which should be given by the
exposure to radiation.
~ Method of measurement:
Electron irradiation equipment was used to apply doses of 20, 100, and
1,000 kGy to each type of the resin samples. The irradiated samples
comprising 50 g of PET resin and 45 g of Nylon-MXD6 pellets were sealed in
50-ml sample bottles and kept at 22~C. The oxygen content of air inside the
bottles was measured over time, and was expressed in the amount of oxygen
that was absorbed in 1 g of resin (cc/g). Table 1 shows the values measured
after 56 days, and also shows the results from unexposed resin samples.
Table 1. Oxygen Absorption Rates (cc/g)
Unexposed 20 kGy 100 kGy 1,000 kGy
PET 0.0018 0.0090 0.0282 0.0421
MXD6 0.0067 0.0306 0.0514 0.0596
Fig. 1 shows the graph of variations over time in the oxygen absorption
rates.
CA 02478199 2004-09-O1
g
From Table 1 it is found that after electron irradiation, Nylon-MXD6
resin has a larger oxygen-absorbing ability than the PET resin has. It is also
found that both resins show greatly improved oxygen absorption rates
achieved solely by means of the electron irradiation without adding thereto a
metal complex, an oxidation catalyst, or an oxidation initiator, or without
modifying the resins.
Fig. 1 shows that the oxygen-absorbing function remains active for
about 30 days in the unexposed samples of the PET resin and the PET resine
samples irradiated at doses of 20, 100, and 1,000 kGy and the unexposed
samples of Nylon-MXD6 and the Nylon-MXD6 samples irradiated at a dose of
kGy. Furthermore, it is also found that oxygen absorption continues to
work after 50 days in the samples of Nylon-MXD6 irradiated at doses of 100
15 and 1,000 kGy.
This invention is now described by the examples using 3 types of
containers. In Example 1, the PET resine bottle used in the measurement was
obtained by the biaxial drawing and blow-molding method. In Example 2, the
20 bottle was obtained by using a biaxial extruder to blend 4 wt.°/
Nylon-MXD6
with the PET resin, and then biaxially drawing and blow-molding the blended
material. In Example 3, the multi-layered bottle was obtained by biaxially
drawing and blow-molding the multi-layers comprising PET resin (inner layer)
- Nylon-MXD6 resin - PET resin (outer layer). These bottles were fixed on the
rotary irradiation jigs, and were rotated while electron beams were
irradiated,
using well-known electron irradiation equipment. The Nylon-MXD6 resin
laminated in Example 3 had a proportion of 5 wt.%.
After irradiation, each bottle was measured for its oxygen gas
transmission rate over time. Table 2 shows the transmission rates measured
after certain periods of days. Fig. 2 is a graph showing the changes in the
transmission rates over time.
~ Test conditions:
1) Bottle: A capacity of 300 ml; an average body wall thickness of 0.35 mm
2) Method of transmission rate measurement:
Measuring device: MOCON Ox-Tran, 22°C - 55°/RH, unit in
cc/(day-bottle)
CA 02478199 2004-09-O1
9
Table 2. Oxygen Transmission Rate (cc/(day-bottle))
Lapsed Electron
days irradiation
dose
No exposure20 kGy 100 1,000
kGy kGy
Example Single PET After 20 0.021 0.021 0.019 0.020
1 days
Example MXD6 blend After 40 0.013 0.012 0.012 0.0032
2 days
Example MXD6 laminateAfter 40 0.004 - 0.0008 -
3 days
Fig. 2 shows that concerning the bottle of Example 1 comprising a single
layer of PET resin, the larger the irradiation is, the lower oxygen
transmission
rate is derived in the initial period of the test. In about 20 days,
transmission
was saturated, and the transmission rate came up to the same level as
unexposed bottle. This behavior corresponded to the changes over time in the
oxygen absorption rate of the PET resin. During the initial period, it is
suspected that the oxygen transmission rate stays at a low level because of
the
oxygen absorption.
Thus, even the single-layered PET resin bottle is found to demonstrate
the oxygen-capturing effect in- and outside of the container, managing the
oxygen transmission rate to be kept at a low level for at least 20 days.
Therefore, the exposed single-layered bottle can be effectively utilized in
applications having relatively short shell lives.
The unexposed bottle of Example 2 comprising a single layer of PET
blended with Nylon-MXD6 shows a considerably lower oxygen transmission
rate than the bottle of single-layered PET alone because of the blending
effect.
As found from Fig. 2, the electron beam irradiation gave far lower rates. With
a radiation dose of 1,000 kGy, the low transmission rate is fully demonstrated
even after 50 days. This result can be understood reasonably from the time-
lapsed changes in the oxygen absorption rates shown in Fig. 1.
Due to the lamination effect, the multi-layered bottle of Example 3
comprising also Nylon-MXD6 shows an oxygen transmission rate about 1/5 as
low as that of the single-layered bottle of PET alone under the unexposed
condition. As found from Fig. 2, the electron beam irradiation improved the
oxygen barrier property so as to extend the effective duration for about 10
days
at a dose as low as 6 kGy. A dose of 100 kGy allowed the container to have a
transmission rate about 1/2 as low as found in the unexposed bottle after 50
days. In addition, since the Nylon-MXD6 layer serves as a large barrier and
stands against oxygen that invades from outside the bottle, it is believed
that
the effect of lamination continues for an extended period even when the bottle
has been irradiated at a low dose.
CA 02478199 2004-09-O1
It was thus found that with no addition of a metal complex, an oxidation
catalyst, or an oxidation initiator, the electron beam irradiation onto the
PET
resin container simply gave the container the oxygen-capturing function and
5 greatly improved the oxygen barrier property. The duration of improved
oxygen barrier may differ, depending on whether the body wall consists of a
single layer of PET resin, a blended layer comprising an oxygen barrier resin,
or laminated layers.
With considering the intended use and the shelf lives the most suitable
10 container wall can be selected from among these types.
In Example 3, this invention was described by taking up a multi-layered
bottle having a structure comprising PET resin (inner layer) - Nylon-MXD6 -
PET resin (outer layer). It should be understood that the laminate structure
of
the multi-layered container is not limited to this Example. In Example 3, for
instance, use can be made of a container having an adhesive layer to adhere
the PET resin to the Nylon-MXD6 resin, or a container having an intermediate
layer comprising the PET resin blended with a small amount of Nylon-MXD6.
Indeed, any combination of various layers can be used for any purpose as far
as the nature of the PET resin container is retained. The oxygen barrier
resine to be selected for this invention is also not limited to the Nylon-MXD6
resin that has been taken up in Examples 2 and 3.
Effect of Invention
This invention having the above-described configuration has the
following effects: In the invention of Claim 1, the radiation applied to the
container simply creates free radicals within the PET-based resin, thus giving
the oxygen-capturing function to the container. As a result, oxygen is
prevented from transmitting from outside to the inside of the container for
the
period in which this oxygen-capturing function remains active. So irradiation
improves the oxygen barrier property of the container better than that of the
unexposed container.
There is no need to add, among others, any metal complex, oxidation
catalyst, or oxidation initiator. The contents can be brought to direct
contact
with the inner wall of the container. Oxygen existing inside the container can
be reduced effectively in a short period of time.
CA 02478199 2004-09-O1
11
In the invention of Claim 2, the ordinary single-layered PET-based resin
container can be given an oxygen-capturing function and an improved oxygen
barrier property and can be used in a wide range of applications, simply when
radiation is applied to the container without adding any metal complex,
oxidation catalyst, and oxidation initiator.
In the invention of Claim 3, both the inner and outer layers are made of
a PET-based resin. Thus, the container retains outstanding properties, such
as moldability, transparency, heat resistance, chemical resistance, and
mechanical strength, which the PET-based resin has.
In the invention of Claim 4, a small amount of a resin having the oxygen
barrier property is blended with the PET-based resin. The use of such a blend
allows the container to keep oxygen transmission from outside at a low level.
As a result, free radicals generated by irradiation have fewer opportunities
to
disappear. The oxygen-capturing function continues to work for a further
extended period, and the oxygen barrier property remains in the improved
state during that period.
In many cases, oxygen barrier resins generally tend to generate free
radicals when the resins are exposed to radiation. These resins themselves
come to have an improved oxygen-capturing function, due to irradiation. On
the whole, the container is led to acquire further improved levels of oxygen-
capturing function and oxygen barrier property.
In the invention of Claim 5, the Nylon-MXD6 resin has an excellent
oxygen barrier property. Since this resin has xylylene radicals on the main
chain, free radicals tend to be generated when the resin is exposed to
radiation.
The container can fully demonstrate a high oxygen-capturing function if the
PET-based resin is blended with Nylon-MXD6.
In the invention of Claim 6, the oxygen barrier property is greatly
improved when the container is exposed to a radiation dose of 20 kGy or more.
In the invention of Claim 7, the container acquires an improved oxygen
barrier property because this container has an intermediate layer comprising
an oxygen barrier resin. Thus, it is possible to control the penetration of
outside oxygen at a strikingly low level. As a result, free radicals generated
by
irradiation have far fewer opportunities in which the radicals are diminished
by oxygen creeping in from outside. In that case, even a small radiation dose
CA 02478199 2004-09-O1
12
is enough to keep the oxygen-capturing function working for a longer period
than the usual, and the oxygen barrier property is maintained at an improved
level during that period,.
The intermediate layer comprising an oxygen barrier resin does not
come in direct contact with oxygen inside or outside of the container. Owing
to
this lack of contact, the oxygen-capturing function continues to work for a
more extended period than usual, and the oxygen barrier property is
maintained at an improved level during that period.
In the invention of Claim 8, Nylon-MXD6 has an outstanding oxygen
barrier property. Because this nylon has xylylene radicals on the main chain,
free radicals tend to be generated by irradiation. If this nylon is laminated
with a PET-based resin, it is possible for the container to perform the full
oxygen-capturing function.
Nylon-MXD6 can be blended easily with the PET-based resin, and the
container of a multi-layered structure can also be molded. In either way,
highly productive containers can be obtained.
In the invention of Claim 9, the oxygen-capturing function is effectively
performed by the container having an intermediate layer comprising an
oxygen barrier resin. Even at a dose as small as 6 kGy, an improved effect of
the oxygen barrier property is demonstrated.
In the invention of Claim 10, any well-known electron irradiation
equipment can be used industrially with relative ease.