Note: Descriptions are shown in the official language in which they were submitted.
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
- 1 -
MINIMISING CARBON TRANSFER IN AN ELECTROLYTIC CELL
The present invention relates to reduction of
metal oxides in a solid state in an electrolytic cell.
The present invention was made during the course
of an on-going research project on solid state reduction
of titania (Ti02) carried out by the applicant.
During the course of the research project the
applicant carried out experimental work on the reduction
of titania using an electrolytic cell that included a
graphite crucible that formed an anode of the cell, a pool
of molten CaCl2-based electrolyte in the crucible, and a
range of cathodes that included solid titania.
The CaCl2-based electrolyte was a commercially
available source of CaCl2, namely calcium chloride
dihydrate, that partially decomposed on heating and
produced CaO.
The applicant operated the electrolytic cell at a
potential above the decomposition potential of Ca0 and
below the decomposition potential of CaCl2.
The applicant found that the cell could
electrochemically reduce titania to titanium with very low
concentrations of oxygen.
The applicant does not have a clear understanding
of the electrolytic cell mechanism at this stage.
Nevertheless, whilst not wishing to be bound by the
comments in this and the following paragraphs, the
applicant offers the following comments by way of an
outline of a possible cell mechanism.
The experimental work carried out by the
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
- 2 -
applicant produced evidence of Ca metal dissolved in the
electrolyte. The applicant. believes that, at least during
the early stages of operation of the cell, the Ca metal
was the result of electrodeposition of Ca~+ cations as Ca
metal on electrically conductive sections of the cathode.
The experimental work was carried out using a
CaCl2-based electrolyte at a cell potential below the
decomposition potential of CaCl2. The applicant believes
that the initial deposition of Ca metal on the cathode was
due to the presence of Ca++-cations and O-- anions derived
from CaO in the electrolyte. The decomposition potential
of Ca0 is less 'than the decomposition potential of CaClz.
In this cell mechanism the-.cell operation is dependent, at
least during the early stages of cell operation, on
decomposition of CaO, with Ca++ cations migrating to the
cathode and depositing as Ca metal and O-- anions migrating
to the anode and forming CO and/or COz (in a situation, in
which the anode is a graphite anode).
The applicant believes that the Ca metal that
deposited on electrically conductive sections of the
cathode was deposited predominantly as a separate phase in
the early stages of cell operation and thereafter
dissolved in the electrolyte and migrated to the vicinity
of the titania in the cathode and participated in chemical
reduction of titania.
The applicant also believes that at later stages
of the cell operation part of the Ca metal that deposited
on the cathode was deposited directly on partially
deoxidised titanium and thereafter participated in
chemical reduction of titanium.
The applicant also believes that the O'- anions,
once extracted from the titania, migrated to the anode and
reacted with anode carbon and produced CO and/or C02 (and
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
- 3 -
in some instances Ca0) and released electrons that
facilitated electrolytic deposition of Ga metal on the
cathode.
However, notwithstanding that the cell could
electrochemically reduce titania to titanium with very low
concentrations of oxygen, the applicant also found that
there were relatively significant amounts of carbon
transferred from the anode to the electrolyte and to the
titanium produced at the cathode under a wide range of
cell operating conditions.
Carbon in the titanium is an undesirable
contaminant. In addition, carbon transfer was partially
responsible for low energy efficiency of the cell. Both
problems are significant barriers to commercialisation of
electrolytic reduction technology.
The applicant carried out experimental work to
identify the mechanism for carbon transfer and to
determine how to minimise carbon transfer and/or to
minimise the adverse effects of carbon transfer.
The experimental work indicated that the
mechanism of carbon transfer is electrochemical rather
than erosion and that one way of minimising carbon
transfer and therefore contamination of titanium produced
at the cathode by electrochemical reduction of titania at
the cathode is to position a membrane that is permeable to
oxygen anions and is impermeable to carbon in ionic and
non-ionic forms between the cathode and the anode anal
thereby prevent migration of carbon to the cathode.
Accordingly, the present invention provides an
electrolytic cell for reducing a metal oxide in a solid
state, which electrolytic cell includes an anode formed
from carbon, a cathode formed at least in part from the
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
g _
metal oxide, and a membrane that is permeable to oxygen
anions and is impermeable to carbon in ionic and non-ionic
forms positioned between the cathode and the anode to
thereby prevent migration of carbon to the cathode.
material.
Preferably, the anode is formed from graphite.
The membrane may be formed from any suitable
Preferably, the membrane is formed from a solid
electrolyte.
One suitable solid electrolyte tested by the
applicant is yttria stabilised zirconia.
Preferably, the cathode also includes an
electrical conductor.
The present invention also provides a method of
reducing a metal oxide in a solid state using the above-
described electrolytic cell.
Preferably, the method includes a step of
operating the cell at a potential that is above a
decomposition potential of at least one of the
constituents of the electrolyte so that there are rations
of a metal other than that of the metal oxide in the
electrolyte.
In a situation in which the metal oxide is a
titanium oxide, such as titania, it is preferred that the
electrolyte be a CaClz-based electrolyte that includes Ca0
as one of constituents.
In such a situation it is preferred that the cell
potential be above the decomposition potential for CaO.
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
- 5 -
It is also preferred that the cell potential be
below the decomposition potential for CaCl2.
It is preferred that the cell potential be less
than or equal to 3.0 V.
It is preferred particularly that the cell
potential be below 2.5 V,
It is preferred more particularly that the cell
potential be below 2.0 V.
It is preferred that the cell potential be above
1.5 V.
The CaCl2-based electrolyte may be a commercially
available source of CaCl2, such as calcium chloride
dehydrate, that partially decomposes on heating and
produces Ca0 or otherwise includes CaO.
Alternatively, or in addition, the CaClz-based
electrolyte may include CaClz and Ca0 that are added
separately or pre-mixed to form the electrolyte.
The present invention is described further with
reference to the following Example that relates to
experimental work on the above-described electrolytic
cell.
As indicated above, the cell included a high
density graphite crucible that formed the anode of the
cell, a pool of molten CaCl2 electrolyte in the crucible,
and a cathode that included solid titanic. In the initial
experimental set-up the solid titanic was in the form of
titanic pellets connected to a lower end of a Kanthal or
stainless steel electrically conductive wire.
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
- 6 -
As indicated above, experimental work on the cell
identified carbon transfer as a significant issue in terms
of contamination of cathode titanium and causing low
energy efficiency of the cell. Tn addition, as indicated
above, the experimental work established that carbon
transfer was caused by an electrochemical reaction at the
anode.
Thereafter the applicant carried out experimental
work to investigate whether it was possible to prevent
migration of carbon from the anode to the cathode.
One experiment investigated the impact of a solid
ionic barrier on carbon migration.
The ionic barrier was in the form of a yttria
stabilised zirconia membrane positioned between the anode
and the cathode, thereby dividing the cell into an outer
anode chamber and an inner cathode chamber.
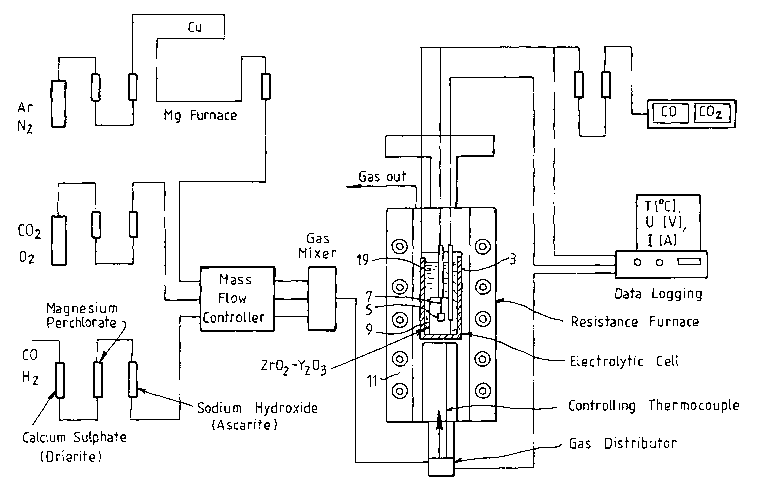
Figure 1 is a schematic of the cell set-up for
the experiment. With reference to the Figure, the cell
included a graphite crucible 3 that formed the anode, a
pool 19 of molten CaCl2 electrolyte in the crucible,
titania pellets 5 and an electrically conductive wire 7
that formed the cathode immersed in the electrolyte, and a
yttria stabilised zirconia membrane 9 immersed in the
electrolyte between the anode and the cathode. The cell
was located in a resistance furnace 11 heated to a
temperature to maintain the electrolyte in a molten state.
The experimental set-up also included gas monitoring,
cleaning, and analysis equipment. The cell was operated at
an applied potential of 3V for a period of 35 hours,
during which time there was continuous monitoring of the
off-gas from the furnace. At the conclusion of the
experiment, the cell was cooled and the solidified
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
7
electrolyte, the membrane, the anode and the cathode were
analysed.
Figure 2 is a summary of the results of the
experiment.
Figure 2 shows measured voltage, current, CO and
C02 composition of the off-gas for the experiment.
Visual and analytical examination of the cathode
and the cathode chamber indicated that there was no carbon
on the cathode and in the cathode chamber.
In addition, the visual and analytical
examination of the cathode indicated that titania was
reduced to titanium. It follows from this finding that
the yttria stabilised zirconia membrane did not restrict
migration of O-- anions from the cathode to the anode.
Many modifications may be made to the present
invention as described above without departing from the
spirit and scope of the invention.
By way of example, whilst the above description
of the invention focuses on reduction of titania, the
invention is not so limited and extends to electrolytic
reduction of other titanium oxides and to oxides of other
metals and alloys.
Examples of other potentially important meals are
aluminium, silicon, germanium, hafnium, magnesium, and
molybdenum.
Furthermore, whilst the above description focuses
on CaCl2-based electrolyte, the invention is not so limited
and extends to any other suitable electrolytes.
CA 02479050 2004-09-13
WO 03/076692 PCT/AU03/00305
_ g _
Generally, suitable electrolytes will be salts
and oxides that are soluble in salts. One example of a
potentially suitable electrolyte is BaCl2.