Note: Descriptions are shown in the official language in which they were submitted.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-1-
INHIBITORS OF CHECKPOINT KINASES (Weel and Chkl)
FIELD OF THE INVENTION
This invention relates to small chemical molecules that specifically inhibit
one or both
of the checkpoint kinases Weel and Chkl.
BACKGROUND OF THE INVENTION
The proper orchestration of the steps required for orderly progression of the
cell
through the cell cycle requires a number of signaling pathways within cells.
Many of these
pathways utilize protein kinases to effect the transmission of crucial signals
at the appropriate
time and intracellular location. Cell cycle kinases are naturally occurring
enzymes involved
in regulation of the cell cycle which is generally divided into four segments:
Gl (gapl), S
(DNA systhesis), G2 (gap 2) and M (mitosis). Some of these kinases are
responsible for
inhibiting the cell's normal progression through cell division, while others
are normally
active in promoting the progression of cells through the cell cycle leading to
cell division.
Increased activity or temporally abnormal activation of these kinases has been
shown to
result in development of tumors and other proliferative disorders.
One of the protein kinases involved is a tyrosine specific kinase, Weel, that
has as its
substrate another kinase complex called Cdc2/cyclinB. Weel kinase is a
regulatory kinase
that has Cdc2/cyclinB as its substrate and when Weel is active, it
phosphorylates a specific
tyrosine (TyrlS) on Cdc2 that causes an inactivation of the Cde2/cyclinB
complex which in
turn results in a pause or checkpoint in the cell cycle at the G2 and M
transition. The kinase
activity of Cdc2/cyclinB is absolutely required for cells to progress through
the G2 stage of
the cell division cycle to the M (or mitotic) phase where two daughter cells
are formed from
the division of the parent cell. Under normal circumstances, as cells are
progressing through
the cell cycle, the Cdc2/cyclinB complex is assembled in late S phase and
through G2.
Normally, Weel is active and thus phosphorylates the Cdc2/cyclinB complex
until the end of
G2 when all of the necessary components have been synthesized for the entry of
cells into M
phase. Weel activity then diminishes, a phosphatase removes the inhibitory
phosphorylation
from TyrlS of Cdc2/cyclinB, the complex becomes activated and cells move into
M phase
where the replicated DNA is divided and the daughter cells are formed.
Inhibition of Weel
CONFIRMATION COPY
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-2-
results in no inhibitory phosphorylation of TyrlS on Cdc2/cyclinB and the
potentially
inappropriate and premature entry of the cell into mitosis.
In addition to regulation of the transition of cells between the different
phases of the
cell cycle under normal conditions, the cell cycle transitions are regulated
in response to
damage to DNA presumably giving cells opportunities either to repair
potentially genotoxic
DNA damage before replication using a damaged DNA template or to permanently
exit the
cell cycle and die.
Another kinase of interest named Chkl participates in this DNA damage
dependent
signaling pathway by phosphorylating a phosphatase called Cdc25C which when
itself is
active and co-localized with Cdc2/cyclinB in the nucleus, dephosphorylates
TyrlS and causes
the activation of the Cdc2/cyclinB complex. The Chkl mediated phosphorylation
of Cdc25C
causes Cdc25C to be exported from the nucleus at which point it is no longer
able to
dephosphorylate and thus activate Cdc2/cyclinB. Therefore, if Chkl is active
(in response to
DNA damage) it will indirectly contribute to the inactivation of Cdc2/cyclinB
(whose activity
is required for progression into M phase) through the preservation of the
inactivating
phosphorylation of TyrlS on Cdc2/cyclinB. Conversely, inhibition of Chkl would
result in
the dephosphorylation of Cdc2/cyclinB by the phosphatase Cdc25C in the nucleus
(not
exported to the cytoplasm since it is not phosphorylated by Chkl) and the
consequent
activation of Cdc2/cyclinB with the accompanying entry of the cells in
mitosis.
Inhibition of Weel or Chkl or both kinases in the presence of DNA damaged by
conventional DNA-directed chemotherapeutic agents or by radiation presents an
opportunity
to utilize cellular regulatory pathways to inappropriately and prematurely
cause cells to
progress into M phase. Such cells may be less likely to survive and further
divide since the
commitment to M phase was made in the presence of potentially catastrophically
damaged
DNA ( Alan J. Kraker and Robert N. Booher, "New Cell Cycle Targets,", Ann.
Rep. Med.
Chem., 1999;34:247-256).
Small molecule inhibitors of Weel kinase have been reported, WO 0119825 and
Cancer Res. (2001), 61(22), 8211-8217. Small molecule inhibitors of Chkl
kinase have
also been reported W00016781, Cancer Res. (2000), 60(3), 566-572.
Pyrrolocarbazole derivatives are known to have inhibitory activity against
Protein
kinase c and anti tumor activity (US Patent No. 4,912,107) but compared to the
compounds
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-3-
of the present invention, the compounds disclosed in US Patent No. 4,912,107
have very low
checkpoint kinase abrogator activity. Pyrrolocarbazole derivatives are also
known to
stimulate platelet production (W096/28447) and to promote thrombopoiesis
(W09809967).
EP 0695755 discloses another pyrrolocarbazole derivative having Protein kinase
c activity.
US Patent No. 5,166,204 discloses antitumor isoindole derivatives having a
linkage or lower
alkylene group bonded to the 2 and 3 or 3 and 4 of a carbazole skeleton. US
Patent No.
5,728,709 discloses pyrrolocarbazole derivatives that stimulate platelet
production. WO
01/85686 also discloses pyrrolocarbazole derivatives.
However, there are no reports that any type of pyrrolocarbazole inhibits
either Weel
kinase or Chkl kinase. Nor have there been any reports that
any type of pyrrolocarbazole inhibits both Weel kinase and Chkl kinase.
SUMMARY OF THE INVENTION
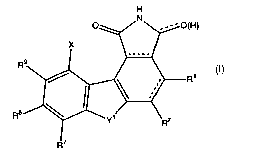
Compounds claimed are of the general structure described by Formula I
H
R
R8
Wherein
Each dashed line represents an optional bond;
R1 is hydrogen, halogen, Cl -Cg alkyl, NRSR6 or
an aryl or heteroaryl ring optionally substituted with up to five substituents
selected
from halogen, alkyl, haloalkyl, hydroxyl, nitro, cyano, C(O)R3, ORS, S(O)mR3,
NR3R4,
OC(O)R3, NR3(CO)OR4, CH2NR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4, NR3CONHR4; or
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-4-
a cycloalkyl or cycloalkenyl ring optionally substituted with up to five
substituents
selected from, halogen, alkyl, haloalkyl, hydroxyl, vitro, cyano, C(O)R3, ORS,
S(O)mR3,
NR3R4, OC(O)R3, NR3(CO)OR4, CH2NR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4, NR3CONHR4; or
a heterocyclic ring optionally substituted with up to five substituents
selected from,
halogen, alkyl, haloalkyl, hydroxyl, vitro, cyano, C(O)R3, ORS, S(O)mR3,
NR3R4,
OC(O)R3, NR3(CO)OR4, CHZNR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4, NR3CONHR4;
m is 0-2;
X is hydrogen or halogen;
Y1 is O, S(O)m, or NR10;
R9 is hydrogen, hydroxyl, halogen, NR3C(O)R4, NHCONR3R4, (C=NR3)NHR4,
NH(C=NR3)NHR4, NH(C=NH)NR3R4, NH(C=O)O R3, NRSR6, (CRSR6)r-Z;
r is 0-6;
R2, R~, R8 and R10 are in each instance independently selected from ((CRS
R6)nT)a(CR11
R12)b)-Z wherein the sum of n, a and b is in each instance less than 10;
T may be absent, or, when present, is in each instance independently selected
from O,
CONR3, CONHS02~ S(O)m, NR3, NR30, OS(O)m, S(O)m0, NR3S(O)2, or
S(O)2NR3;
n is in each instance independently 0-6;
a is in each instance independently 0-6;
b is in each instance independently 0-6;
Z is selected from hydrogen, halogen, alkyl, haloalkyl, cycloalkyl,
cycloalkenyl, heterocyclyl,
aryl, heteroaryl, cyano, vitro, hydroxy, C(O)R3, CONHS02R3, ORS, S(O)mR3,
OS02R3, NR3R4, C02R3, CONR3 R4, NR3COR4, S02NR3R4, OPO(OR3)(OR4),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-5-
CH=CR3R4, CCR3, (C=NR3)NH R4, NH(C=NR3)NHR4, NH(C=NH)NR3R4,
wherein the alkyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl or heteroaryl
group
may be substituted with up to four groups independently selected from halogen,
alkyl,
hydroxyl, nitro, cyano, ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4, C(O)R3,
COORS, CONR3R4, NR3COR4, S02NR3R4, CONHS02R3~ NR3S(O)mR4,
CH2NR3R4, CH20R3 NHCONR3R4, NR3CONHR4;
R5, R6, R11 and R12 are in each instance independently selected from hydrogen,
hydroxyl,
alkyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl, heteroaryl, heterocyclyl,
halogen,
cyano, nitro, CH2NR3R4, CH20R3, C(O)R3, ORS, S(O)mR3, NR3R4, COORS,
CONR3R4, S02NR3R4 , NHCONR3R4, NR3CONHR4;
wherein the alkyl, haloalkyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl or
heteroaryl
group may be substituted with up to four groups independently selected from
halogen,
alkyl, hydroxyl, nitro, cyano, ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4,
C(O)R3, COORS, CONR3R4, NR3COR4, S02NR3R4, CONHS02R3~
NR3S(O)mR4, NHCONR3R4, NR3CONHR4;
RS and R6 or R11 and R12 together with the carbon atom to which they are
attached
may form a carbonyl group; or together with the carbon or heteratom to which
they
are attached may form a cycloalkyl or heterocyclyl group, said carbonyl,
cycloalkyl or
heterocyclyl group may be substituted with up to four groups independently
selected
from halogen, hydroxyl, nitro, cyano, alkyl, haloalkyl, halogen, alkyl, nitro,
cyano,
ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4, C(O)R3, COORS, CONR3R4,
NR3COR4, S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4,
NR3CONHR4;
R3, R4 are independently selected from hydrogen, alkyl, haloalkyl or a
substituted or
unsubstituted carbocyclic group selected from cycloalkyl, cycloalkenyl,
heterocyclyl,
aryl, and heteroaryl, wherein the said alkyl, or a substituted carbocyclic
group may be
substituted with up to 4 groups selected from halogen, hydroxyl, nitro, cyano,
alkyl,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-6-
haloalkyl, alkyloxy, carboxy, COOH, CONH2, NHCOCH3, N(CH3)2, NHCH3,
thiomethyl, thioethyl, SOCH3, S02CH3;
R3 and R4 together with the carbon atom or heteroatom to which they are
attached
may form a cycloalkyl or heterocyclyl group substituted with up to four groups
independently selected from halogen, hydroxyl, nitro, cyano, alkyl, haloalkyl,
alkyloxy, formyl, carboxy, acetyl, CH2NH2, CH20H, COOH, CONH2, NHCOCH3,
N(CH3)2> thiomethyl, thioethyl, SOCH3, S02CH3, alkoxycarbonyl, alkylcarbonyl,
alkynylamino, aminoalkyl, aminoalkylcarbonyl, amino, mono- or dialkylamino,
or
R3 and R4 together with the nitrogen to which they are attached may form a
heterocyclic ring containing 3-8 members, up to four of which members are
optionally carbonyl groups or heteroatoms independently selected from oxygen,
sulfur, S(O), S(O)2, and nitrogen, wherein the carbocyclic group is
unsubstituted or
substituted with up to four groups independently selected from halogen,
hydroxy,
hydroxyalkyl, alkyl, haloalkyl, alkoxy, alkoxycarbonyl, alkylcarbonyl,
alkynylamino,
aminoalkyl, aminoalkylcarbonyl, amino, mono- or dialkylamino.
Unless the context clearly requires otherwise, throughout the description and
the
claims, the words "comprise", "comprising", and the like are to be construed
in an inclusive
sense as opposed to an exclusive or exhaustive sense; that is to say, in the
sense of
"including, but not limited to".
DESCRIPTION OF TIC FIGURES
Figure 1 represents a Western blot of tumor cells treated in vivo with
cisplatin and the
Compound of Example 80.
Figure 2 represent a Western Blot of cells treated in vitro with Adriamycin
and the
Compound of Example 80
Figure 3 represents a Western blot of tumor cells treated in vivo with cpt-11
and the
Compound of Example 362.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
Figure 4 represents a graph of life span enhancement in animals treated with
the Compound
of Example 80
DETAILED DESCRIPTION OF THE INVENTION
Compounds of the present invention are of the general structure described by
Formula
I
H
R
Ra
R' I
Wherein
Each dashed line represents an optional bond;
R1 is hydrogen, halogen, C~ - C$ alkyl; NRSR6 or
an aryl or heteroaryl ring optionally substituted with up to five substituents
selected
from halogen, alkyl, haloalkyl, hydroxyl, nitro, cyano, C(O)R3, ORS, S(O)mR3,
NR3R4,
OC(O)R3, NR3(CO)OR4, CH2NR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4, NR3CONHR4; or
a cycloalkyl or cycloalkenyl ring optionally substituted with up to five
substituents
selected from, halogen, alkyl, haloalkyl, hydroxyl, nitro, cyano, C(O)R3, ORS,
S(O)mR3,
NR3R4, OC(O)R3, NR3(CO)OR4, CH2NR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4, NR3CONHR4; or
a heterocyclic ring optionally substituted with up to five substituents
selected from,
halogen, alkyl, haloalkyl, hydroxyl, nitro, cyano, C(O)R3, ORS, S(O)mR3,
NR3R4,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
_g_
OC(O)R3, NR3(CO)OR4, CH2NR3R4, CH20R3, COORS, CONR3R4, NR3COR4,
S02NR3R4, CONHS02R3~ NR3S(O)mR4 NHCONR3R4, NR3CONHR4;
m is 0-2;
X is hydrogen or halogen;
Y1 is O, S(O)m, or NR10;
R9 is hydrogen, hydroxyl, halogen, NR3C(O)R4, NHCONR3R4, (C=NR3)NHR4, NH(C=N
R3)NH R4, NH(C=NH)N R3 R4, NH(C=O)O R3, NRSR6, (CRSR6)r-Z;
r is 0-6;
R2, R~, Rg and R10 are in each instance independently selected from ((CRS
R6)nT)a(CR11
R12)b)-Z wherein the sum of n, a and b is in each instance less than 10;
T may be absent, or, when present, is in each instance independently selected
from O,
CONR3, CONHS02~ S(O)m, NR3, NR3-O, O-S(O)m, S(O)m-O, NR3-S(O)2, or
S(O)2-NR3;
n is in each instance independently 0-6;
a is in each instance independently 0-6;
b is in each instance independently 0-6;
Z is selected from hydrogen, halogen, alkyl, haloalkyl, cycloalkyl,
cycloalkenyl, heterocyclyl,
aryl, heteroaryl, cyano, nitro, hydroxy, C(O)R3, CONHS02R3, ORS, S(O)mR3
,OS02R3, NR3R4, C02R3, CONR3R4, NR3COR4, S02NR3R4, OPO(OR3)(OR4),
CH=CR3R4, CCR3, (C=NR3)NHR4, NH(C=NR3)NHR4, NH(C=NH)NR3R4, wherein
the alkyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl or heteroaryl group
may be
substituted with up to four groups independently selected from halogen, alkyl,
hydroxyl, nitro, cyano, ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4, C(O)R3,
COORS, CONR3R4, NR3COR4, S02NR3R4, CONHSOZR3~ NR3S(O)mR4,
CH2NR3R4, CH20R3 NHCONR3R4, NR3CONHR4;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-9-
R5, R6, R11 and R12 are in each instance independently selected from hydrogen,
hydroxyl,
alkyl, haloalkyl, cycloalkyl, cycloalkenyl, aryl, heteroaryl, heterocyclyl,
halogen,
cyano, vitro, CH2NR3R4, CH20R3, C(O)R3, ORS, S(O)mR3, NR3R4, COORS,
CONR3R4, S02NR3R4 , NHCONR3R4, NR3CONHR4;
wherein the alkyl, haloalkyl, cycloalkyl, cycloalkenyl, heterocyclyl, aryl or
heteroaryl
group may be substituted with up to four groups independently selected from
halogen,
alkyl, hydroxyl, vitro, cyano, ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4,
C(O)R3, COORS, CONR3R4, NR3COR4, S02NR3R4, CONHS02R3~
NR3S(O)mR4, NHCONR3R4, NR3CONHR4;
RS and R6 or R11 and R12 together with the carbon atom to which they are
attached
may form a carbonyl group; or together with the carbon or heteratom to which
they
are attached may form a cycloalkyl or heterocyclyl group, said carbonyl,
cycloalkyl or
heterocyclyl group may be substituted with up to four groups independently
selected
from halogen, hydroxyl, vitro, cyano, alkyl, haloalkyl, halogen, alkyl, vitro,
cyano,
ORS, S(O)mR3, NR3R4, OC(O)R3, NR3(CO)OR4, C(O)R3, COORS, CONR3R4,
NR3COR4, S02NR3R4, CONHS02R3~ NR3S(O)mR4, NHCONR3R4,
NR3CONHR4;
R3, R4 are independently selected from hydrogen, alkyl, haloalkyl or a
substituted or
unsubstituted carbocyclic group selected from cycloalkyl, cycloalkenyl,
heterocyclyl,
aryl, and heteroaryl, wherein the said alkyl, or a substituted carbocyclic
group may be
substituted with up to 4 groups selected from halogen, hydroxyl, vitro, cyano,
alkyl,
haloalkyl, alkyloxy, carboxy, COOH, CONH2, NHCOCH3, N(CH3)2, NHCH3,
thiomethyl, thioethyl, SOCH3, S02CH3;
R3 and R4 together with the carbon atom or heteroatom to which they are
attached
may form a cycloalkyl or heterocyclyl group substituted with up to four groups
independently selected from halogen, hydroxyl, vitro, cyano, alkyl, haloalkyl,
alkyloxy, formyl, carboxy, acetyl, CH2NH2, CH20H, COOH, CONH2, NHCOCH3,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-10-
N(CH3)2, thiomethyl, thioethyl, SOCH3, S02CH3, alkoxycarbonyl, alkylcarbonyl,
alkynylamino, aminoalkyl, aminoalkylcarbonyl, amino, mono- or dialkylamino,
or
R3 and R4 together with the nitrogen to which they are attached may form a
heterocyclic ring containing 3-8 members, up to four of which members are
optionally carbonyl groups or heteroatoms independently selected from oxygen,
sulfur, S(O), S(O)2, and nitrogen, wherein the carbocyclic group is
unsubstituted or
substituted .with up to four groups independently selected from halogen,
hydroxy,
hydroxyalkyl, alkyl, haloalkyl, alkoxy, alkoxycarbonyl, alkylcarbonyl,
alkynylamino,
aminoalkyl, aminoalkylcarbonyl, amino, mono- or dialkylamino.
In one preferred embodiment of the invention the compound of Formula I is
selected
from the group consisting of:
4-(2-Chlorophenyl)-6-(3-hydroxypropyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(5-Amino-2-methoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(2-hydroxyphenyl)-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-[2-(4-morpholinyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-Benzyl-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-6-(3-hydroxypropyl)-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-Hydroxy-6-(6-hydroxyhexyl)-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(5-Amino-2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
3-{ [3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(1H)-yl)propyl]amino}benzoic acid;
2-{ (3-(4-(2-Chlorophenyl)-9-hydroxy-I,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(1H)-yl)propyl]amino}benzoic acid;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-11-
4-{ [3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(1H)-yl)propyl]amino}benzoic acid;
4-(2-Chlorophenyl)-6-{ 3-[(cis)-3,5-dimethylpiperazinyl]propyl }-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dichlorophenyl)-6-{ 3-[(cis)-3,5-dimethylpiperazinyl]propyl }-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-(2-aminoethyl)-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-hydroxy-6-[2-(methylamino)ethyl]-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
6-(3-aminopropyl)-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-hydroxy-4-phenyl-6-[3-(1-pyrrolidinyl)propyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
6-[3-(diethylamino)propyl]-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
9-hydroxy-4-phenyl-6-[3-( 1-piperidinyl)propyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
9-hydroxy-6-[3-(4-methyl-1-piperazinyl)propyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
6-[6-(dimethylamino)hexyl]-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
9-hydroxy-6-[6-(4-methyl-1-piperazinyl)hexyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
6-(2-aminoethyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-[3-(dimethylamino)ethyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-[2-(1H-imidazol-1-yl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-12-
4-(2-chlorophenyl)-9-hydroxy-6-[2-(4-morpholinyl)ethyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-[2-(4-methyl-1-piperazinyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-(2-anilinoethyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[3-(dimethylamino)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[2-(4-morpholinyl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[2-(4-methyl-1-
piperazinyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-(2-anilinoethyl)-4-(2,6-dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-[3-(methylamino)propyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-[3-(dimethylamino)propyl]-9-hydroxypyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-[3-(4-morpholinyl)propyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-[3-(4-methyl-1-piperazinyl)propyl]pyrrolo[3,4-
c] carbazol e-1, 3 (2H,6H)-di one;
6-(3-anilinopropyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[3-(methylamino)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-6-(3-(dimethylamino)propyl]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-13-
4-(2,6-dichlorophenyl)-9-hydroxy-6-[3-( 1 H-imidazol-1-yl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[3-(4-morpholinyl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-[3-(4-methyl-1-
piperazinyl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-(3-anilinopropyl)-4-(2,6-dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-6-[3-(4-morpholinyl)propyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-6-[2-(4-morpholinyl)ethyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
9-Hydroxy-6-[3-(1H-imidazol-1-yl)propyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-6-[2-(IH-imidazol-1-yl)ethyl]-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-6-[3-(methylamino)propyl]-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-Hydroxy-4-phenyl-6-[3-(1-piperazinyl)propyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
6-[3-(Benzylamino)propyl]-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1;3(2H,6H)-
dione;
6-(3-Anilinopropyl)-9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-Chloro-6-methoxyphenyl)-6-{ 3-[cis-3,5-dimethylpiperazinyl]propyl ~ -9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-(phenylsulfanyl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-14-
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)-N-[2-(dimethylamino)ethyl]-N-methylpropanamide;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)-N-(2,2,6,6-tetramethyl-4-piperidinyl)propanamide (XIII; Ar=2-
chlorophenyl, n=2,
R3=H, R4=2,2,6,6-tetramethyl-4-piperidinyl);
4-(2-Chlorophenyl)-6-{ 3-[cis-3,5-dimethylpiperazinyl]-3-oxopropyl }-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)-N-[2-(1H-imidazol-5-yl)ethyl]propanamide (XIII; Ar=2-chlorophenyl,
n=2, R3=H,
R4=( 1 H-imidazol-5-yl)ethyl);
3-(4-(2,6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(IH)-yl)propanoic acid;
N-[2-(Dimethylamino)ethyl]-3-(9-hydroxy-1,3-dioxo-4-phenyl-2,3-
dihydropyrrolo[3,4-c]carbazol-6( I H)-yl)propanamide;
N-[2-(Dimethylamino)ethyl]-3-(9-hydroxy-4-(2-methoxyphenyl)-1,3-dioxo-2,3-
dihydropyrrolo[3,4-c]carbazol-6(1H)-yl)propanamide;
3,9-dihydroxy-4-phenyl-3,6-dihydropyrrolo[3,4-c]carbazol-1 (2H)-one;
4-(2-Chlorophenyl)-1,9-dihydroxy-6-(3-hydroxypropyl)-1,6-dihydropyrrolo[3,4-
c]carbazol-3(2H)-one;
4-(2-chlorophenyl)-3,9-dihydroxy-6-(3-hydroxypropyl)-3,6-dihydropyrrolo[3,4-
c]carbazol-1 (2H)-one;
4-(2-Chlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)-4,5,6, l Oc-
tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione;
8-Hydroxy-4-phenylcyclopenta[c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-hydroxy-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-6-[3-(cis-3,5-dimethyl-1-piperazinyl)-2-hydroxypropyl]-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-15-
4-(2-Chlorophenyl)-6-[3-(dimethylamino)-2-hydroxypropyl]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-propylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-pentylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-allyl-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(2-phenylethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-(2-propynyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-isopentylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-(2,2,2-trifluoroethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(4-pentenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
2-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-6(
1H)-
yl)acetamide;
6-(3-butenyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-isobutylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-hydroxy-6-(4,4,4-trifluorobutyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
6-sec-butyl-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-6-cyclopentyl-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-Chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-8-[4-(1-pyrrolidinyl)butyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-16-
4-(2-Chlorophenyl)-9-hydroxy-6,8-bis(2-hydroxyethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-(2-hydroxyethyl)-8-(3-(4-
morpholinyl)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-8-(3-hydroxypropyl)-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
8-Ethyl-9-hydroxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
8-(2,3-Dihydroxypropyl)-9-hydroxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-ethyl-9-hydroxy-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
9-Hydroxy-8-(2-hydroxyethyl)-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-8-(2-hydroxyethyl)-6-methylpyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
8-[3-(Dimethylamino)propyl]-9-hydroxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
8-[2-(Dimethylamino)ethyl]-9-hydroxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-5-methyl-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4,5-diphenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-9-methoxy-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-methoxy-6-methylpyrrolo(3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-17-
8-[3-(Dimethylamino)propoxy]-9-hydroxy-6-methyl-4-phenylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-methyl-8-[3-(4-moipholinyl)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-methyl-8-[3-(1-pyrrolidinyl)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-(2-hydroxyethyl)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2-Chlorophenyl)-8-(1,2-dihydroxyethyl)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2- Chlorophenyl)-8-(hydroxymethyl)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxy]-6-methylpyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-6-methyl-8-[3-(methylamino)propoxy]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-[3-(cis-3,5-dimethyl-1-piperazinyl)propoxy]-6-
methylpymolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-(2-hydroxyethoxy)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-8-(2,3-dihydroxypropoxy)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Hydroxy-4-(2-methoxyphenyl)-6-[2-(4-morpholinyl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
1,3-Dioxo-4-phenyl-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-9-yl dihydrogen
phosphate;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-18-
4-(2-Chlorophenyl)-8-(3-dimethylamino-propoxy)-9-hydroxy-6-(3-hydroxy-propyl)-
6H-pyrrolo[3,4-c]carbazole-1,3-dione;
6-(3-Bromo-propyl)-4-(2-chlorophenyl)-8-(3-dimethylamino-propoxy)-9-hydroxy-
6H-pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chlorophenyl)-8-(4-dimethylamino-3-hydroxy-butoxy)-6-methyl-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chlorophenyl)-8-hydroxy-6-(3-hydroxy-propyl)-6H-pyrrolo[3,4-c]carbazole-
1,3-
dione;
4-(2-Chlorophenyl)-8-(4-dimethylamino-butyl)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-
1,3-dione;
4-(2-Chlorophenyl)-6-methyl-8-(4-pyrrolidin-1-yl-butyl)-6H-pyrrolo(3,4-
c]carbazole-
1,3-dione;
4-(2-Chlorophenyl)-6-methyl-8-(4-methylamino-butyl)-6H-pyrrolo[3,4-c]carbazole-
1,3-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-(3-hydroxy-propyl)-8-(3-pyrrolidin-1-yl-
propoxy)-
6H-pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chlorophenyl)-9-hydroxy-8-(4-hydroxy-butyl)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
8-{ 3-[Bis-(2-hydroxy-ethyl)-amino]-propoxy }-4-(2-chlorophenyl)-9-hydroxy-6-
methyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-6-(3-hydroxy-propyl)-8-[3-(4-methyl-piperazin-1-
yl)-
propoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Amino-4-(2-chloro-phenyl)-6-(3-hydroxy-propyl)-6H-pyrrolo[3,4-c]carbazole-
1,3-
dione;
3-[4-(2-Chloro-phenyl)-9-vitro-1,3-dioxo-2,3-dihydro-1 H-pyrrolo(3,4-
c]carbazol-6-
yl]-propionic acid;
4-(2-Chloro-phenyl)-9-hydroxy-8-(4-hydroxy-butoxy)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-19-
4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-methoxy-6H-pyrrolo[3,4-c]carbazole-
1,3-dione;
4-(2-Chloro-phenyl)-9-fluoro-8-methoxy-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-
dione;
4-(2-Chloro-phenyl)-9-fluoro-8-hydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-
dione;
4-(2-Chloro-phenyl)-8-(3-dimethylamino-propoxy)-6-(3-hydroxy-propyl)-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-(3-pyrrolidin-1-yl-propoxy)-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-fluoro-8-(3-hydroxy-propoxy)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-fluoro-6-methyl-8-(3-methylamino-propoxy)-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-morpholin-4-yl-butyl)-6H-
pyrrolo[3,4-
c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-fluoro-6-methyl-8-(3-pyrrolidin-1-yl-propoxy)-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-ethyl)-8-[3-(4-methyl-piperazin-1-
yl)-
propoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-8-(3-diethylamino-propoxy)-9-hydroxy-6-(2-hydroxy-ethyl)-
6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-8-(3-dimethylamino-propoxy)-9-fluoro-6-methyl-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
N-[4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-1,3-dioxo-1,2,3,6-tetrahydro-
pyrrolo[3,4-c]carbazol-9-yl]-formamide;
4-(2-Chloro-phenyl)-9-hydroxy-6-(3-hydroxy-propyl)-8-piperidin-4-yl-6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-20-
4-(2-Chloro-phenyl)-6-methyl-8-piperidin-4-yl-6H-pyrrolo[3,4-c]carbazole-1,3-
dione;
7-(2-Amino-1-hydroxy-ethyl)-4-(2-chlorophenyl)-9-hydroxy-6-oxa-2-aza-
cyclopenta[c]fluorene-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-7-(1-hydroxy-2-methylamino-ethyl)-6-oxa-2-aza-
cyclopenta[c]fluorene-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-7-(1-hydroxy-2-piperazin-1-yl-ethyl)-6-oxa-2-aza-
cyclopenta[c]fluorene-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-7-(1-hydroxy-2-morpholin-4-yl-ethyl)-6-oxa-2-aza-
cyclopenta[c]fluorene-1,3-dione;
4-(2-Chloro-phenyl)-9-hydroxy-7-[ 1-hydroxy-2-(2-methoxy-ethoxy)-ethyl]-6-oxa-
2-
aza-cyclopenta[c]fluorene-1,3-dione;
7-(2-Amino-1-hydroxy-ethyl)-4-(2-Chlorophenyl)-6-oxa-2-aza-cyclopenta
[c]fluorene-1,3-dione;
4-(2-Chlorophenyl)-6-methyl-8-piperidin-3-yl-6H-pyrrolo[3,4-c]carbazole-1,3-
dione;
4-(2-Chlorophenyl)-6-methyl-8-piperidin-4-yl-6H-pyrrolo[3,4-c]carbazole-1,3-
dione;
4-(2-Chlorophenyl)-8-(4-hydroxy-piperidin-3-yl)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
8-( 1-Aminomethyl-2-hydroxy-ethyl)-4-(2-Chlorophenyl)-6-methyl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
4-(2-Chlorophenyl)-6-(3-hydroxy-propyl)-8-piperidin-3-yl-6H-pyrrolo[3,4-
c]carbazole-1,3-dione;
4-(2-Chlorophenyl)-8-(4-hydroxy-piperidin-3-yl)-6-(3-hydroxy-propyl)- 6H-
pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-chlorophenyl)-9-hydroxy-8-[4-(methylamino)butyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-8-[3-(methylamino)propoxy]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-21-
4-(2-chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-6-(2-
hydroxyethyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(2-hydroxyethyl)-8-[3-
(methylamino)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(2-hydroxyethyl)-8-[4-
(methylamino)butyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-(2-
hydroxyethyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-(3-
hydroxypropyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-(3-
hydroxypropyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)-8-[3-
(methylamino)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-6-(3-
hydroxypropyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)-8-[3-(1-
pyrrolidinyl)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-methyl-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-6-methyl-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-{ 3-[(3,5-dimethylpiperazinyl]propoxy }-9-hydroxy-6-
methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-22-
6-butyl-4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-8-[4-(methylamino)butyl]-6-propylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chlorophenyl)-9-hydroxy-8-[3-(methylamino)propoxy]pynolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chlorophenyl)-9-hydroxy-8-[4-(1-pyrrolidinyl)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-9-hydroxy-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-bromophenyl)-9-hydroxy-8-[4-(methylamino)butyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-bromophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-8-[4-(dimethylamino)butyl]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-bromophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-bromophenyl)-9-hydroxy-8-[3-(methylamino)propoxy]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-23-
4-(2-chloro-6-methoxyphenyl)-9-hydroxy-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-9-hydroxy-6-methyl-8-[3-
(methylamino)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-9-hydroxy-6-methyl-8-[3-
(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-bromophenyl)-9-hydroxy-6-methyl-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-bromophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-dichlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-
methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-8-[4-(dimethylamino)butyl]-9-hydroxy-6-
methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-6-methoxyphenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-6-
methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chloro-6-methoxyphenyl)-8-[3-(dimethylamino)propoxy]-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chloro-6-methoxyphenyl)-9-hydroxy-8-[3-
(methylamino)propoxy]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2-chloro-6-methoxyphenyl)-8-[4-(dimethylamino)butyl]-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2,6-dichlorophenyl)-8-[4-(dimethylamino)butyl]-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2,6-dichlorophenyl)-8-[3-(dimethylamino)propoxy]-9-
hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-butyl-4-(2,6-dichlorophenyl)-9-hydroxy-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-24-
6-butyl-4-(2,6-dichlorophenyl)-9-hydroxy-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-hydroxy-8-[4-( 1-pyrrolidinyl)butyl]-1 H-[ 1
]benzofuro[3,2-
e]isoindole-1,3(2H)-dione;
8-(4-aminobutyl)-4-(2-chlorophenyl)-9-hydroxy-1 H-[ 1 ]benzofuro[3,2-
e]isoindole-
1,3(2H)-dione;
8-(4-aminobutyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
8-(4-aminobutyl)-4-(2-chlorophenyl)-9-hydroxy-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-(hydroxymethyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-(trifluoromethyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-(2-amino-1-hydroxyethyl)-4-(2-chlorophenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-9-[ 1-hydroxy-2-(methylamino)ethyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-[2-(dimethylamino)-1-hydroxyethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-[ 1-hydroxy-2-( 1-piperidinyl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-[ 1-hydroxy-2-(4-morpholinyl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-(4-hydroxybutoxy)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-(3,4-dihydroxybutoxy)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-methyl-8-[4-(methylamino)butoxy]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-25-
4-(2-chlorophenyl)-8-[4-(dimethylamino)-3-hydroxybutoxy]-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[3-hydroxy-4-(methylamino)butoxy]-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[3-hydroxy-4-(1-pyrrolidinyl)butoxy]-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[( lE~-4-(dimethylamino)-1-butenyl]-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-meth 1-8-[( 1 E~-4-(methylamino)-1-butenyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-methyl-8-[4-(methylamino)butyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butyl]-6-methylpyrrolo[3,4-c)carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[(lE~-4-hydroxy-1-butenyl]-6-methylpyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[(lE~-4-hydroxy-1-butenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)~~dione;
4-(2-chlorophenyl)-8-(4-hydroxybutyl)pyrrolo[3,4-c)carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(methylamino)butyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione:
4-(2-chlorophenyl)-8-[(lE~-4-(methylamino)-1-butenyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-(3-hydroxypropyl)-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,61~-dione;
4-(2-chlorophenyl)-8-[3-(methylamino)propoxy]-6-[3-
(methylamino)propyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-26-
4-(2-chlorophenyl)-8-(3-hydroxypropoxy)-6-[3-(methylamino)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-8-[3-(methylamino)propoxy]-6-[3-
(methylamino)propyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-8-(3-hydroxypropoxy)-6-[3-
(methylamino)propyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-6-(3-hydroxypropyl)-8-[4-
(methylamino)butyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-8-(4-hydroxybutoxy)-6-methylpyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-6-methyl-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoro-8=(3-hydroxypropoxy)-6-methylpyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-chloro-3-hydroxyphenyl)-8-[4-(methylamino)butyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chloro-3-hydroxyphenyl)-9-fluoro-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-3-hydroxyphenyl)-9-fluoro-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chloro-3-hydroxyphenyl)-9-fluoro-6-(3-hydroxypropyl)-8-[4-
(methylamino)butyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(3-amino-2-chlorophenyl)-9-fluoro-6-(3-hydroxypropyl)-8-[4-
(methylamino)butyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(3-amino-2-chlorophenyl)-9-fluoro-8-[4-(methylamino)butyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(3-amino-2-chlorophenyl)-9-fluoro-8-[3-(methylamino)propoxy]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-27-
4-(2-chlorophenyl)-9-fluoro-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
3-(4-(2-chlorophenyl)-9-(formylamino)-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6( 1H)-yl)-N-[2-(dimethylamino)ethyl]propanamide;
3-(9-amino-4-(2-chlorophenyl)-1,3-dioxo-2,3-di hydropyrrolo[3,4-c]carbazol-6(
1H)-
yl)-N-[2-(dimethylamino)ethyl]propanamide;
4-(2-chlorophenyl)-6-methyl-9-{ [3-(1-piperidinyl)propyl]amino}pymolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-fluoropyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-amino-4-(2-chlorophenyl)-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-(3-hydroxypropyl)-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-
c]carbazol-9-ylformamide;
4-(2-chlorophenyl)-9-{ [4-(dimethylamino)butyl]amino}-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-6-methyl-9-{ [4-(methylamino)butyl]amino}pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-9-{ [3-(1-piperidinyl)propyl]amino }pyrrolo[3,4-c]carbazole-
1, 3 (2H,6H)-dione;
4-(2-methoxyphenyl)-6-methyl-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-
c]carbazol-9-
ylformamide;
4-(2-methoxyphenyl)-6-methyl-9-(methylamino)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-chlorophenyl)-6-methyl-8-[3-(methylamino)propoxy]-1,3-dioxo-1,2,3,6-
tetrahydropyrrolo[3,4-c]carbazol-9-ylformamide;
4-(2-chlorophenyl)-9-hydroxy-6-methyl-8-[4-(methylamino)butanoyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-chlorophenyl)-8-[4-(dimethylamino)butanoyl]-9-hydroxy-6-methylpyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-28-
4-(2-chlorophenyl)-8-[3-(dimethylamino)propoxy]-6-methyl-1,3-dioxo-1,2,3,6-
tetrahydropyrrolo[3,4-c]carbazol-9-ylformamide;
4-(2-chlorophenyl)-8-{ [3-(dimethylamino)propyl]sulfinyl }-9-hydroxy-6-
methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione; and
4-(2-chlorophenyl)-9-hydroxy-6-methyl-8-{ [3-
(methylamino)propyl]sulfonyl }pyrrolo[3,4-c]carbazole-I,3(2H,6H)-dione.
In another preferred embodiment of the present invention, R1 in compounds
according to Formula I is aryl.
In one preferred embodiment are compounds according to Formula I wherein RI is
selected from an unsubstituted aryl ring or an aryl ring substituted with up
to 3 substituents
selected from the group consisting of halogen, haloalkyl, alkoxy, hydroxyl,
nitro or NR3R4.
In a more preferred embodiment are compounds according to Formula I wherein RI
is
an aryl ring substituted with up to 2 halogens or alkoxy groups.
Another preferred embodiment of the present invention comprises compounds
according to Formula I in which R1 is selected from Me and I.
In one preferred embodiment of the present invention, R9 in compounds
according to
Formula I is a hydrogen, hydroxyl, halogen or NHCHO group.
In another embodiment are compounds according to Formula I wherein at least
one of
X, R~, R8, and R9 is not hydrogen. In another embodiment, when three of X, R~,
R8, and R9
are hydrogen and R1 is lower alkyl, then R2 must be hydrogen.
In a more preferred embodiment of the present invention, R9 in compounds
according
to Formula I is a hydroxyl group.
In a more preferred embodiment of the present invention, R9 in compounds
according
to Formula I is a fluorine group.
In a more preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and RI is aryl, such as but not limited to:
4-(2-Chloro-3-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-29-
9-Hydroxy-4-(2-iodophenyl)pyrrolo[3,4-c]carbazole-1,3(2H;6H)-dione;
4-(2-Chloro-6-methoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3 (2H,6H)-
dione;
4-(2,6-Dichloro-3-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(4-Amino-2-bromophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H, 6H)-dione);
4-(3-Amino-2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chloro-4-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-Bromophenyl)-9-hydroxypyrrolo(3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(2-methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3 (2H,6H)-dione;
4-(4-Amino-2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dimethoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,3-Dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chloro-5-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-Hydroxy-4-[2-(methylsulfanyl)phenyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2,6-Dibromophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(3-thienyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chloro-6-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-Hydroxy-4-(2-nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dichloro-4-hydroxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(3-Chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(2-hydroxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(4-hydroxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(3-Aminophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dimethylphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-30-
9-Hydroxy-4-(3-hydroxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(5-Amino-2-methoxyphenyl)-9-hydroxypymolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(3-hydroxy-4-methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
9-Hydroxy-4-(2-thienyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(4-Aminophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
2-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-4-
yl)benzonitrile;
4-(2-Aminophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Bromo-4-nitrophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-[2-(methylsulfinyl)phenyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-Ethoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(3-nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Ethylphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-[2-(hydroxymethyl)phenyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chloro-4-nitrophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(2-trifluoromethylphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-[ 1,1'-Biphenyl]-2-yl-9-hydroxypyrrolo[3,4-c]carbazole-1,3 (2H,6H)-dione;
4-(4-Chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-(4-hydroxymethylphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-4-o-tolyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
3-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-4-yl)-
benzonitrile;
4-Furan-2-yl-9-hydroxy-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Hydroxy-4-m-tolyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Hydroxy-4-(2-methylsulfanyl-phenyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Hydroxy-4-(3-trifluoromethoxy-phenyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Hydroxy-4-(3-hydroxymethyl-phenyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-31-
9-Hydroxy-4-(4-trifluoromethoxy-phenyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione;
9-Hydroxy-4-(3-hydroxy-phenyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione; and
4-(2-Acetyl-phenyl)-9-hydroxy-6H-pyrrolo[3,4-c]carbazole-1,3-dione.
In yet another preferred embodiment of the present invention are compounds
according to Formula I in which Y1 is oxygen , such as but not limited to:
4-(2-Chlorophenyl)-7-( 1,2-dihydroxyethyl)-9-hydroxy-1 H-[ 1 ]benzofuro[3,2-
a]isoindole-1,3(2H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-1H-[1]benzofuro[3,2-a]isoindole-1,3(2H)-dione;
4-(2-Chlorophenyl)-8-ethyl-9-hydroxy-1 H-[ 1 ]benzofuro[3,2-a]isoindole-
1,3(2H)-
dione;
4-(2-Chlorophenyl)-7-ethyl-9-hydroxy-1 H-[ 1 ]benzofuro[3,2-e]isoindole-
1,3(2H)-
dione;
9-Hydroxy-4-phenyl-1H-[ 1 ]benzofuro[3,2-a]isoindole-1,3(2H)-dione;
4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxy]-9-hydroxy-1 H-[ 1
]benzofuro[3,2-
a]isoindole-1,3(2H)-dione; and
4-(2-Chlorophenyl)-9-hydroxy-8-(4-hydroxybutyl)-1H-[ 1 ]benzofuro[3,2-
a]isoindole-
1,3(2H)-dione.
In another preferred embodiment of the present invention are compounds
according to
Formula I in which Y1 is sulfur, such as but not limited to:
4-(2-Chlorophenyl)-9-hydroxy-1 H-[ 1 ]benzothieno[3,2-a]isoindole-1,3(2H)-
dione.
In another preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and Y1 is NR10
In another preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is NRSR6, R1 is aryl and Y1 is NR1~ such as, but not
limited to,
compounds selected from the group consisting of:
9-Amino-4-(2-methoxyphenyl)-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
9-Amino-4-(2-chlorophenyl)-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-32-
9-(Dimethylamino)-4-(2-methoxyphenyl)-6-methylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
9-Amino-4-(2-chlorophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-6-methyl-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-
9-
ylformamide;
4-(2-Chlorophenyl)-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-9-
ylformamide;
4-(2-Chlorophenyl)-6-methyl-9-(methylamino)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
N-[4-(2-Chlorophenyl)-6-methyl-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-
c]carbazol-
9-yl]acetamide;
4-(2-Chlorophenyl)-9-(ethylamino)-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione; and
N-[4-(2-Chlorophenyl)-6-methyl-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-
c]carbazol-
9-yl]-3-(1-piperidinyl)propanamide.
In another preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and Y1 is NR10
In another preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and Y1 is NR10, and T may be absent, O,
CONR3, or
CONHS02. Such compounds are exemplified below:
9-Methoxy-4-(2-methoxy-5-nitrophenyl)-4,5,6, lOc-tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione;
N-[3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)propanoyl]benzenesulfonamide;
4-(2,6-Dichlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2,6-Dichlorophenyl)-9-hydroxy-6-(2-hydroxyethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-33-
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6( 1 H)-yl )-N-( 1H-tetraazol-5-yl)propanamide;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-( 1 H-1,2,4-triazol-5-
ylsulfinyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-(3-Bromopropyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c)carbazole-
1,3(2H,6H)-dione;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)propanoic acid;
N-[3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)propanoyl]-2-(dimethylamino)ethanesulfonamide;
4-(2-Chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
N-[3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)propanoyl]methanesulfonamide;
4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)butanoic acid;
N-[4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)butanoyl]methanesulfonamide;
6-Acetyl-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
3-(4-(2,6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(1H)-yl)-N-[2-(dimethylamino)ethyl]propanamide;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)propanenitrile;
4-(2-Chlorophenyl)-9-hydroxy-6-[3-(1H-tetraazol-5-yl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-(1H-imidazol-2-ylsulfanyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
N-[4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)butanoyl]benzenesulfonamide;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-34-
4-(2-Chlorophenyl)-9-hydroxy-6-[2-(1H-1,2,4-triazol-5-
ylsulfonyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-( 1 H-imidazol-2-
ylsulfinyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-( 1 H-tetraazol-5-yl)ethyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
3-(9-Hydroxy-1,3-dioxo-4-phenyl-2,3-dihydropyrrolo[3,4-c]carbazol-6( 1 H)-
yl)propanamide;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-(1H-imidazol-2-ylsulfonyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
3-(9-Hydroxy-1,3-dioxo-4-phenyl-2,3-dihydropyrrolo[3,4-c]carbazol-6( 1H)-
yl)propanoic acid;
4-(2-Chlorophenyl)-6-(2,3-dihydroxypropyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
2-(4-(2,6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-
6(1H)-yl)ethyl methanesulfonate;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-(4H-1,2,4-triazol-3-
ylsulfanyl)ethyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-6-(2-hydroxyethyl)-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
6-(3-Bromopropyl)-4-(2,6-dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-(3-methoxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2- Chlorophenyl)-9-hydroxy-6-(2-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[(2S)-3-hydroxy-2-methylpropyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-35-
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6( 1 H)-yl)-N-[2-( 1 H-imidazol-5-yl)ethyl]propanamide;
9-Hydroxy-6-(3-hydroxypropyl)-4-(2-methoxyphenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
Methyl 3-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-6(1H)-yl)propanoate;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)-N-[2-(4-morpholinyl)ethyl]propanamide;
4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)butanenitrile;
4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(2-hydroxyethyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6( 1 H)-yl)-N-[2-(dimethylamino)ethyl]propanamide;
4-(2-Chlorophenyl)-6-(3,4-dihydroxybutyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
3-(4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-
c]carbazol-6(1H)-yl)-N-[2-(dimethylamino)ethyl]propanamide;
4-(2-Chlorophenyl)-9-hydroxy-6-[(2R)-3-hydroxy-2-methylpropyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
2-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolo[3,4-c]carbazol-
6(1H)-yl)ethyl methanesulfonate;
4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
9-Hydroxy-6-(2-hydroxyethyl)-4-(2-methoxyphenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-6-(2-hydroxyethyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1, 3 (2H,6H)-di one;
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-36-
4-(2-Chlorophenyl)-9-hydroxy-6-[3-(methylsulfanyl)propyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-6-ethyl-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(VI;
Ar=2-chlorophenyl, R=CH2CH3);
4-(2-Chlorophenyl)-9-hydroxy-6-isopropylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[3-(1H-imidazol-1-yl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-[3-(4-morpholinyl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
6-(2-Chloroethyl)-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione;
6-(3-Bromopropyl)-4-(2-chloro-6-methoxyphenyl)-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-hydroxy-3-(methylamino)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione;
4-(2-Chlorophenyl)-9-hydroxy-6-[2-hydroxy-3-(4-morpholinyl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione;
4-(2,6-Dichlorophenyl)-9-hydroxy-6-[3-( 1 H-imidazol-1-yl)propyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione; and
6-Butyl-4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione.
In a more preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and Y1 is NR1~ and the bond represented by
the dashed
line (C-O) is absent.
In an especially preferred embodiment of the present invention are compounds
according to Formula I in which R9 is hydroxyl and Rg is not hydrogen.
In another preferred embodiment of the present invention are compounds
according to
Formula I in which R9 is hydroxyl and Yl is NR10 and R1 is aryl.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-37-
In another especially preferred embodiment of the present invention are
compounds
according to Formula I in which Rg is not hydrogen, and R9 is hydrogen.
In another especially preferred embodiment of the present invention are
compounds
according to Formula I in which R8 is not hydrogen, R9 is hydrogen and YI is
NRIO.
Another especially preferred embodiment of the present invention comprises
compounds according to Formula I in which R9 is hydrogen and R8 is ((CRS
R6)nT)a(CRI l
RI2)b)-Z; wherein T may be absent or O and Z is NR3R4.
Another especially preferred embodiment of the present invention comprises
compounds according to Formula I in which R~ is selected from halogen or
hydroxyl and R8
is ((CRSR6)nT)a(CRI IR12)b)Z wherein T is absent and Z is hydrogen.
The following are definitions of terms used in this specification. The initial
definition
provided for a-group or term herein applies-to that group or term throughout
the present
specification, individually or as part of another group, unless otherwise
indicated.
Where stereoisomers or enantiomers exist all possible combinations are
claimed.
The terms "halogen" and "halo" refer to fluorine, chlorine, bromine and
iodine.
The term "unsaturated ring" includes partially unsaturated and aromatic rings.
The term "alkyl" in the present invention means a straight or branched
hydrocarbon
radical having from 1 to 8 carbon atoms and includes, for example, methyl,
ethyl, n-propyl,
isopropyl, n-butyl, sec-butyl, isobutyl, tent-butyl, n-pentyl, iso-pentyl, n-
hexyl, and the like.
The term "aryl" means an aromatic carbocyclic group having a single ring
(e.g., phenyl),
multiple rings (e.g., biphenyl), or multiple condensed rings in which at least
one is aromatic,
(e.g., 1,2,3,4-tetrahydronaphthyl, naphthyl, anthryl, or phenanthryl).
The term "heteroaryl" means an aromatic "heterocycle," "heterocyclic,"
"heterocyclyl," or "heterocyclo" group as defined below that comprises at
least one
heteroatom.
The aryl or heteroaryl ring may be optionally substituted with up to five
substituents
selected from NH(CI-C6 alkyl), N(CI-C6 alkyl)2, thio CI-C6 alkyl, CI-C6
alkoxy, hydroxy,
carboxy, CI-C6 alkoxycarbonyl, halo, nitrite, and cycloalkyl.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-38-
By "alkoxy" is meant straight or branched chain alkoxy groups having 1 to 10
carbon
atoms, such as, for example, methoxy, ethoxy, propoxy, isopropoxy, n-butoxy,
sec-butoxy,
tert-butoxy, pentoxy, 2-pentyloxy, isopentoxy, neopentoxy, hexoxy, 2-hexoxy, 3-
hexoxy, and
3-methylpentoxy.
"Alkynyl" means straight and branched hydrocarbon radicals having from 2 to
8 carbon atoms and one triple bond and includes ethynyl, 3-butyn-1-yl,
propynyl, 2-butyn-
1-yl, 3-pentyn-1-yl, and the like.
The terms "cycloalkyl" and "cycloalkenyl" refer to cyclic hydrocarbon groups
of 3 to
8 carbon atoms and includes cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl,
cyclooctyl and the like.
"Acyl" means an alkyl, aryl, cycloalkyl, heterocycle, heterocyclic,
heterocyclyl, or
heterocyclo group bonded through a carbonyl group, i.e., R-C(O)-. Typical acyl
groups
include acetyl, benzoyl, and the like having from 1-10 carbon atoms,
preferably 1-6 carbon
atoms.
The term "haloalkyl" means an akyl group substituted with 1 to 6 halogen atoms
and
include trifluoromethyl, trichloromethyl, tribromomethyl, trifluoroethyl,
trifluoropropyl,
trifluorobutyl , pentafluoroethyl and the like.
The alkyl, alkenyl, alkoxy, and alkynyl groups described above are optionally
substituted, preferably by 1 to 3 groups selected from NH(C1-C6 alkyl), N(C1-
C6 alkyl)2,
phenyl, substituted phenyl, thio C1-C6 alkyl, C1-C6 alkoxy, hydroxy, carboxy,
C1-C6
alkoxycarbonyl, halo, nitrite, cycloalkyl, and a 5- or 6-membered carbocyclic
ring or
heterocyclic ring having 1 or 2 heteroatoms selected from nitrogen,
substituted nitrogen,
oxygen, and sulfur. "Substituted nitrogen" means nitrogen bearing C1-C6 alkyl
or (CH2)pPh
where p is 1, 2, or 3. Perhalo and polyhalo substitution is also included.
The term "heteroatom" means an oxygen, nitrogen, sulfur, or phosphorous atom.
The terms "heterocycle," "heterocyclic," "heterocyclyl," or "heterocyclo"
refer to
fully saturated or unsaturated, including aromatic (heteroaryl) or nonaromatic
cyclic groups,
for example, 4- to 7-membered monocyclic, 7- to 11-membered bicyclic, or 10-
to
15-membered tricyclic ring systems, which have at least one heteroatom in at
least one
carbon atom-containing ring. Each ring of the heterocyclic group containing a
heteroatom
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-39-
may have 1, 2, 3, or 4 heteroatoms selected from nitrogen atoms, oxygen atoms
andlor sulfur
atoms, where the nitrogen and sulfur heteroatoms may optionally be oxidized
and the
nitrogen heteroatoms may optionally be quaternized. The heterocyclic group may
be
attached at any heteroatom or carbon atom of the ring or ring system.
Monocyclic heterocyclic groups include, but are not limited to, piperidine,
2,6-
dimethylpiperazine, piperazine, n-methylpiperazine, pyrrolidinyl, pyrrolyl,
pyrazolyl,
oxetanyl, pyrazolinyl, imidazolyl, imidazolinyl, S(O)-imidazoles, S(O)2-
imidazoles oxazolyl,
oxazolidinyl, isoxazolinyl, isoxazolyl, thiazolyl, thiadiazolyl,
thiazolidinyl, isothiazolyl,
isothiazolidinyl, morpholine and dimethylmorpoline, 2-thiophene, thiophene, 1-
imidazole, 2-
imidazole, furyl, tetrahydrofuryl, thienyl, oxadiazolyl, piperidinyl,
piperazinyl,
2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolodinyl, 2-oxoazepinyl, 2-
azepinyl,
4-piperidonyl, pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl,
tetrahydropyranyl, morpholinyl,
thiamorpholinyl, thiamorpholinyl sulfoxide, thiamorpholinyl sulfone, 1,3-
dioxolane and
tetrahydro-1,1-dioxothianyl, tetrazole, SO-triazole, S02-triazole and the
like.
Bicyclic heterocyclic groups include , but are not limited to, indolyl,
benzothiazolyl,
benzoxazolyl, benzodioxolyl, benzothienyl, quinuclidinyl, quinolinyl,
tetra-hydroisoquinolinyl, isoquinolinyl, benzimidazolyl, benzopyranyl,
indolizinyl,
benzofaryl, chromonyl, coumarinyl, benzopyranyl, cinnolinyl, quinoxalinyl,
indazolyl,
pyrrolopyridyl,furopyridinyl (such as furo[2,3-c]pyridinyl, furo[3,2-
b]pyridinyl or
furo[2,3-blpyridinyl), dihydroisoindolyl, dihydroquinazolinyl (such as 3,4-
dihydro-4-oxo-quinazolinyl), tetrahydroquinolinyl and the like. tricyclic
heterocyclic groups
include , but are not limited to, carbazolyl, benzindolyl, phenanthrolinyl,
acridinyl,
phenanthridinyl, xanthenyl and the like.
The term "cancer" includes, but is not limited to, the following cancers:
cancers of the
breast, ovary, cervix, prostate, testis, esophagus, stomach, skin, lung, bone,
colon, pancreas,
thyroid, biliary passages, buccal cavity and pharynx (oral), lip, tongue,
mouth, pharynx, small
intestine, colon-rectum, large intestine, rectum, brain and central nervous
system,
glioblastoma, neuroblastoma, keratoacanthoma, epidermoid carcinoma, large cell
carcinoma,
adenocarcinoma, adenoma, follicular carcinoma, undifferentiated carcinoma,
papillary
carcinoma, seminoma, melanoma, sarcoma, bladder carcinoma, liver carcinoma ,
kidney
carcinoma, myeloid disorders, lymphoid disorders, Hodgkin's, hairy cells, and
leukemia.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-40-
The term "pharmaceutically acceptable salts, esters, amides, and prodrugs" as
used
herein refers to those carboxylate salts, amino acid addition salts, esters,
amides, and
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgment, suitable for use in contact with the tissues of patients
without undue
toxicity, irritation, allergic response, and the like, commensurate with a
reasonable
benefit/risk ratio, and effective for their intended use, as well as the
zwitterionic forms, where
possible, of the compounds of the invention. The term "salts" refers to the
relatively non-
toxic, inorganic and organic acid addition salts of compounds of the present
invention. These
salts can be prepared in situ during the final isolation and purification of
the compounds or by
separately reacting the purified compound in its free base form with a
suitable organic or
inorganic acid and isolating the salt thus formed. Representative salts
include the
hydrobromide, hydrochloride, sulfate, bisulfate, nitrate, acetate, oxalate,
valerate, oleate,
palmitate, stearate, laurate, borate, benzoate, lactate, phosphate, tosylate,
citrate, maleate,
fumarate, succinate, tartrate, naphthylate mesylate, glucoheptonate,
lactobionate and
laurylsulphonate salts, and the like. These may include canons based on the
alkali and
alkaline earth metals, such as sodium, lithium, potassium, calcium, magnesium
and the like,
as well as non-toxic ammonium, quaternary ammonium, and amine cations
including, but not
limited to ammonium, tetramethylammonium, tetraethylammonium, methylamine,
dimethylamine, trimethylamine, triethylamine, ethylamine, and the like. (See,
for example,
Berge S.M. et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977;66:1-19 which
is incorporated
herein by reference.)
The compounds of Formula I are capable of further forming pharmaceutically
acceptable formulations comprising salts, including but not limited to acid
addition and/or
base salts, solvates and N-oxides of a compound of Formula I. This invention
also provides
pharmaceutical formulations comprising a compound of Formula I together with a
pharmaceutically acceptable carrier, diluent, or excipient therefor. All of
these forms are
within the present invention.
Pharmaceutically acceptable acid addition salts of the compounds of Formula I
include salts derived form inorganic acids such as hydrochloric, nitric,
phosphoric, sulfuric,
hydrobromic, hydriodic, phosphorus, and the like, as well as the salts derived
from organic
acids, such as aliphatic mono- and dicarboxylic acids, phenyl-substituted
alkanoic acids,
hydroxy alkanoic acids, alkanedioic acids, aromatic acids, aliphatic and
aromatic sulfonic
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-41-
acids, etc. Such salts thus include sulfate, pyrosulfate, bisulfate, sulfite,
bisulfate, nitrate,
phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate,
pyrophosphate,
chloride, bromide, iodide, acetate, propionate, caprylate, isobutyrate,
oxalate, malonate,
succinate, suberate, sebacate, fumarate, maleate, mandelate, benzoate,
chlorobenzoate,
methylbenzoate, dinitrobenzoate, phthalate, benzenesulfonate,
toluenesulfonate,
phenylacetate, citrate, lactate, maleate, tartrate, methanesulfonate, and the
like. Also
contemplated are the salts of amino acids such as arginate, gluconate,
galacturonate, and the
like; see, for example, Berge et al., "Pharmaceutical Salts," J. of
Pharmaceutical Science,
1977;66:1-19.
The acid addition salts of the basic compounds are prepared by contacting the
free
base form with a sufficient amount of the desired acid to produce the salt in
the conventional
manner. The free base form may be regenerated by contacting the salt form with
a base and
isolating the free base in the conventional manner. The free base forms differ
from their
respective salt forms somewhat in certain physical properties such as
solubility in polar
solvents, but otherwise the salts are equivalent to their respective free base
for purposes of the
present invention.
Pharmaceutically acceptable base addition salts are formed with metals or
amines,
such as alkali and alkaline earth metal hydroxides, or of organic amines.
Examples of metals
used as canons are sodium, potassium, magnesium, calcium, and the like.
Examples of
suitable amines are N,N'-dibenzylethylenediamine, chloroprocaine, choline,
diethanolamine,
ethylenediamine, N-methylglucamine, and procaine; see, for example, Berge et
al., supra.,
1977.
The base addition salts of acidic compounds are prepared by contacting the
free acid
form with a sufficient amount of the desired base to produce the salt in the
conventional
manner. The free acid form may be regenerated by contacting the salt form with
an acid and
isolating the free acid in a conventional manner. The free acid forms differ
from their
respective salt forms somewhat in certain physical properties such as
solubility in polar
solvents, but otherwise the salts are equivalent to their respective free acid
for purposes of the
present invention.
Examples of pharmaceutically acceptable, non-toxic esters of the compounds of
this
invention include C1-C6 alkyl esters wherein the alkyl group is a straight or
branched chain.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-42-
Acceptable esters also include CS-C~ cycloalkyl esters as well as arylalkyl
esters such as, but
not limited to benzyl. C1-C4 alkyl esters are preferred. Esters of the
compounds of the
present invention may be prepared according to conventional methods "March's
Advanced
Organic Chemistry, 5th Edition". M. B. Smith & J. March, John Wiley & Sons,
2001.
Examples of pharmaceutically acceptable, non-toxic amides of the compounds of
this
invention include amides derived from ammonia, primary C1-C6 alkyl amines and
secondary
C1-C6 dialkyl amines wherein the alkyl groups are straight or branched chain.
In the case of
secondary amines the amine may also be in the form of a 5- or 6-membered
heterocycle
containing one nitrogen atom. Amides derived from ammonia, C1-C3 alkyl primary
amines
and C1-C2 dialkyl secondary amines are preferred. Amides of the compounds of
the
invention may be prepared according to conventional methods such as "March's
Advanced
Organic Chemistry, 5th Edition". M. B. Smith & J. March, John Wiley & Sons,
2001.
The term "prodrug" refers to compounds that are rapidly transformed in vivo to
yield
the parent compound of the above formulae, for example, by hydrolysis in
blood. A thorough
discussion is provided in T. Higuchi and V Stella, "Pro-drugs as Novel
Delivery Systems,"
Vol. 14 of the A.C.S. Symposium Series, and in Bioreversible Carriers in Drug
Design, ed.
Edward B. Roche, American Pharmaceutical Association and Pergamon Press, 1987,
both of
which are hereby incorporated by reference.
The present invention also includes isotopically labelled compounds, which are
identical to those recited in Formula I, but for the fact that one or more
atoms are replaced by
an atom having an atomic mass or mass number different from the atomic mass or
mass
number usually found in nature. Examples of isotopes that can be incorporated
into
compounds of the present invention include isotopes of hydrogen, carbon,
nitrogen, oxygen,
phosphorous, sulfur, fluorine and chlorine, such as ZH, 3H, 13C,1'C, ~aC, ~sN,
1s0, n0, 3~P,
32P, 3sS,'gF, and 36CI, respectively. Compounds of the present invention,
prodrugs thereof,
and pharmaceutically acceptable salts of said compounds or of said prodrugs
which contain
the aforementioned isotopes and/or other isotopes of other atoms are within
the scope of this
invention. Certain isotopically labelled compounds of the present invention,
for example
those into which radioactive isotopes such as 3H and'4C are incorporated, are
useful in drug
andlor substrate tissue distribution assays. Tritiated, i.e., 3H, and carbon-
14, i.e.,'4C, isotopes
are particularly preferred for their ease of preparation and detectability.
Further, substitution
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-43-
with heavier isotopes such as deuterium, i.e., zH, can afford certain
therapeutic advantages
resulting from greater metabolic stability, for example increased in vivo half-
life or reduced
dosage requirements and, hence, may be preferred in some circumstances.
Isotopically
labelled compounds of Formula I of this invention and prodrugs thereof can
generally be
prepared by carrying out the procedures disclosed in the Schemes and/or in the
Examples and
Preparations below, by substituting a readily available isotopically labelled
reagent for a non-
isotopically labelled reagent.
The compounds of Formula I and their pharmaceutically acceptable salts can be
administered to mammals via either the oral, parenteral (such as subcutaneous,
intraveneous,
intramuscular, intrasternal and infusion techniques), rectal, intranasal or
topical routes. In
general, these compounds are most desirably administered in doses ranging from
about 10 to
about 10,000 mg per day, in single or divided doses (i.e., from 1 to 4 doses
per day), although
variations will necessarily occur depending upon the species, weight and
condition of the
subject being treated and the particular route of administration chosen.
However, a dosage
level that is in the range of about 0.15 mg to about 150 mg per kg of body
weight per day is
most desirably employed. Nevertheless, variations may occur depending upon the
species of
animal being treated and its individual response to said medicament, as well
as on the type of
pharmaceutical formulation chosen and the time period and interval at which
such
administration is carried out. In some instances, dosage levels below the
lower limit of the
aforesaid range may be more than adequate, while in other cases still larger
doses may be
employed without causing any harmful side effects, provided that such higher
dose levels are
first divided into several small doses for administration throughout the day.
The compounds of the present invention may be administered alone or in
combination
with pharmaceutically acceptable carriers or diluents by either of the routes
previously
indicated, and such administration may be carried out in single or multiple
doses. More
particularly, the novel therapeutic agents of this invention can be
administered in a wide variety
of different dosage forms, i.e., they may be combined with various
pharmaceutically acceptable
inert carriers in the form of tablets, capsules, lozenges, troches, hard
candies, powders, sprays,
creams, salves, suppositories, jellies, gels, pastes, lotions, ointments,
aqueous suspensions,
injectable solutions, elixirs, syrups, and the like. Such carriers include
solid diluents or fillers,
sterile aqueous media and various non-toxic organic solvents, etc. Moreover,
oral
pharmaceutical compositions can be suitably sweetened and/or flavored. In
general, the
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
_c~_
therapeutically-effective compounds of this invention are present in such
dosage forms at
concentration levels ranging from about 5.0% to about 70% by weight.
For oral administration, tablets containing various excipients such as
microcrystalline
cellulose, sodium citrate, calcium carbonate, dicalcium phosphate and glycine
may be
employed along with various disintegrants such as starch (and preferably corn,
potato or
tapioca starch), alginic acid and certain complex silicates, together with
granulation binders
like polyvinylpyrrolidone, sucrose, gelatin and acacia. Additionally,
lubricating agents such
as magnesium stearate, sodium lauryl sulfate and talc are often very useful
for tabletting
purposes. Solid compositions of a similar type may also be employed as fillers
in gelatin
capsules; preferred materials in this connection also include lactose or milk
sugar as well as
high molecular weight polyethylene glycols. When aqueous suspensions andlor
elixirs are
desired for oral administration, the active ingredient may be combined with
various
sweetening or flavoring agents, coloring matter or dyes, and, if so desired,
emulsifying and/or
suspending agents as well, together with such diluents as water, ethanol,
propylene glycol,
glycerin and various like combinations thereof.
For parenteral administration, solutions of a compound of the present
invention in
either sesame or peanut oil or in aqueous propylene glycol may be employed.
The aqueous
solutions should be suitably buffered (preferably pH from about 3 to about 8)
if necessary
and the liquid diluent first rendered isotonic. These aqueous solutions are
suitable for
intravenous injection purposes. The oily solutions are suitable for infra-
articular, intra-
muscular and subcutaneous injection purposes. The preparation of all these
solutions under
sterile conditions is readily accomplished by standard pharmaceutical
techniques well known
to those skilled in the art.
Additionally, it is also possible to administer the compounds of the present
invention
topically when treating proliferative cell conditions of the skin and this may
be done by way of
creams, jellies, gels, pastes, patches, ointments and the like, in accordance
with standard
pharmaceutical practice.
The activity of the compounds of the present invention is determined by their
ability to
act as check point abrogators. Checkpoint abrogators inhibit kinases involved
in the regulation
of the G2/M checkpoint resulting in the reversal of the imposed checkpoint.
Weel or Chkl are
examples of such kinases. Utilization in cells either having damaged DNA, for
instance, but
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-45-
not limited to, DNA damaged by conventional DNA-directed chemotherapeutic
agents or by
radiation of cells with undamaged DNA presents an opportunity to utilize
cellular regulatory
pathways to inappropriately and prematurely cause cells to progress into M
phase. Such cells
may be less likely to survive and further divide since the commitment to M
phase was made
in the presence of potentially catastrophically damaged DNA. In the case of
undamaged cells
having no detectable DNA damage, treatment of these cells with the checkpoint
abrogators of
the present invention may be forced into M phase prematurely with similar
cytotoxic effects.
( Alan J. Kraker and Robert N. Booher, "New Cell Cycle Targets,", Ann. Rep.
Med. Chem.,
1999;34:247-256).
One can identify the checkpoint abrogators of the present invention by
measuring the
activity in the assays described in Examples 483 (Weel) and 484 (Chkl) and 486
(PKC) and
selecting those compounds that have at least 10- fold less activity in the PKC
assay than they
have in the Weel assay or at least 5-fold less activity in the PKC assay than
they have in the
Chkl assay.
The present invention is illustrated by the following examples. It will be
understood,
however, that the invention is not limited to the specific details of these
examples. Melting
points are uncorrected. Proton nuclear magnetic resonance spectra (1H NMR) and
13C
nuclear magnetic resonance spectra were measured for solutions in
deuterochloroform
(CDC13) or in CD30D or CD3SOCD3 and peak positions are expressed in parts per
million
(ppm) downfield from tetramethylsilane (TMS). The peak shapes are denoted as
follows: s,
singlet; d, doublet; t, triplet; q, quartet; m, multiplet; b, broad.
The compounds of the present invention can exist in unsolvated forms as well
as
solvated forms, including hydrated forms. In general, the solvated forms,
including hydrated
forms, are equivalent to unsolvated forms and are intended to be encompassed
within the
scope of the present invention.
The term "animal" refers to mammals, including rodents, bovine, equine,
canine,
feline, and human.
The compounds of the present invention are useful for treating cancer (for
example,
leukemia and cancer of the lung, breast, prostate, and skin such as melanoma)
and other
proliferative diseases including but not limited to psoriasis, HSV, HIV,
restenosis, and
atherosclerosis in combination with other conventional therapies. To utilize a
compound of
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-46-
the present invention to treat cancer, a patient in need of such treatment,
such as one having
cancer or another cell proliferative diseases is administered an effective
amount of the
compound of the invention.
Furthermore, the compounds of the present invention are useful for treating
cancers
when combined as adjuvant therapy with other clinical treatment agents and
modalities such
as, but not limited to, X-irradiation, beam therapy, conventional
chemotherapeutic agents
such as gemcitabine, paclitaxel, docetaxel, cisplatin, carboplatin, etoposide,
adriamycin,
topotecan, CPT-1 l, capecitabine, or ionizing radiation alkylating agents,
antimetabolites,
antibodies, DNA intercalators, or other such anti-proliferative agents
ultimately leading to
DNA damage. As new antineoplastic agents ~r modalities are discovered, the use
of the
Weel and /or Chkl inhibitors in combination with these other therapeutic
agents or
modalities is contemplated.
While the in vivo tests described herein teach administering the compounds
according
to Formula I simultaneously with or subsequent to the administration of the
conventional
agent, it is contemplated that the compound of the present invention may also
be
administered prior to the conventional chemotherapeutic agent or agents.
The following examples illustrate particular embodiments of the invention and
are not
intended to limit the specification, including the claims, in any way. Those
skilled in the art
will appreciate that numerous changes and modifications can be made to the
preferred
embodiments of the invention and that such changes and modifications can be
made without
departing from the spirit of the invention. It is, therefore, intended that
the cover all such
equivalent variations as fall within the true spirit and scope of the
invention.
The chemical synthesis schemes provided below exemplify the best mode for
preparing the compounds of the present invention. This patent describes a
number of
synthetic transformations known to those skilled in the art. In each case
alternative conditions
known to one skilled in the art and described in the literature, for instance
in Advanced
Organic Chemistry 5'h Edition, Author: Jerry March,m. b. smith & J. March,
John Wiley &
Sons 2001; Comprehensive Organic Transformation, Author: Richard C. Larock,
The Journal
of Organic chemistry, published by the American Chemical Society and the
references cited
therein) may also lead to the desired product.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-47-
Known transformations include, but are not limited to; alkylation,
dealkylation,
oxidation, reduction, Curtus rearrangement, Suzuki reaction, esterification,
Wittig reaction,
amide formation, hydrogenation, protection, deprotection, hydrolysis,
dihydroxylation.,
ozonolysis, acetylation, and hydroboration.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-48-
Scheme 1 - Preparation of substituted 4-aryl pyrrolo[3,4-cJcarbazoles
MeO~~\\~ Ph3P=CHCOOCH2Ph Me0 ~
CHO
/ H DCM, rt / H \
O
(1) (2)
N O O N O
neat1e175 C ~ Me0 \ COOCHZPh MnO~/~ Me0 \ / COOCHzPh
reflux
/ N / N
H H
(3) (4)
H H
N O N O
H~/Pd-C - DPPA
DMF-MeOH Me0 \ \ / COOH E~ Me0 \ ~ / NHCOO-t-Bu
3
t-BuOH/reflux
_ _ H
(5)
N O
O
TFA Me0 NH2 NaNO~/KI/Cul Me0 \
CH2CI2 ' \ \ ~ 0 °C, then 80 °C~
/ H H
(6) (7)
H H
N O N O
200 CI - I ArB(OR~)CI2 HO Ar
HO ~ \ ~ ~ Na2C03/100°C ~ \
/ N METHOD 1 / N
H H
(8) (I)
SCHEME1 EX
AMPLE 1
The preparation of Benzyl (2E)-3-(5-methoxy-1H-indol-2-yl)-2-propenoate (2)
Benzyl (triphenylphosphoranylidene)acetate (49.2 g, 0.120 mol) was added to a
/ \
O
stirred solution of 5-methoxy-1H-indole-2-carbaldehyde (1) (20.0 g, 0.114 mol)
in CHzCl2
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-49-
(500 mL) and the solution was stirred at room temperature for 4 h. The solvent
was removed
in vacuo and the residue slurried with methanol (200 mL), whereupon
crystallisation of the
product occurred. The mixture was filtered, washed with several portions of
cold methanol,
and dried to give benzyl (2E)-3-(5-methoxy-1H-indol-2-yl)-2-propenoate (2) in
a 30.46 g, 87
% yield as pale yellow plates; mp 155-157 °C.'H NMR 8 (CDC13) 8.28 (s,
1H), 7.69 (d,
J=16.1 Hz, 1H), 7.43-7.32 (m, 5H), 7.23 (d, J=9.1 Hz, 1H), 7.03 (d, J=2.3 Hz,
1H), 6.92 (dd,
J=9.1, 2.3 Hz, 1H), 6.74 (d, J=1.8 Hz, 1H), 6.24 (d, J=16.1 Hz, 1H), 5.26 (s,
2H), 3.84 (s,
3H).Found: C, 74.45; H, 5.62; N, 4.58. C19H»N03 requires C, 74.25; H, 5.57; N,
4.56.
EXAMPLE 2
The preparation of Benzyl 9-methoxy-1 3-dioxo-1 2 3,3a,4,5,6,1Oc-
octahydropyrrolof3,4-
clcarbazole-4-carboxylate (3)
Maleimide (4.82 g, 0.050 mol) was added to a solution of benzyl (2E)-3-(5-
methoxy-
1H-indol-2-yl)-2-propenoate, (12.71 g, 0.041 mol) prepared as in example 1 in
THF (150
mL) in a 250 mL flat-bottomed flask and the mixture was stirred until
homogeneous. The
THF was removed in vacuo and the residue dried under high vacuum for 30 min.
The flask
was immersed in a 175 °C oil bath and the mixture was stirred at this
temperature for 3 h. The
solid melt was cooled to room temperature and ethyl acetate (100 mL) was
added. The solid
mass was partially broken up with a spatula and the mixture was stirred
vigorously overnight,
after which time the product (3) was present as a cream precipitate.
Filtration followed by
washing-with diethyl ether gave benzyl 9-methoxy-1,3-dioxo-1,2,3,3a,4,5,6,1Oc-
octahydropyrrolo[3,4-c]carbazole-4-carboxylate (13.76 g, 83%), mp 179-
181°C. 1H NMR b
[(CD3)ZSO] 10.98 (br s, 1H), 10.87 (s, 1H), 7.45-7.32 (m, 5H), 7.20 (d, J=2.4
Hz, 1H), 7.17
(d, J=8.7 Hz, 1H), 6.69 (dd, J=8.7, 2.4 Hz, 1H), 5.21 (s, 1H), 4.22 (br d,
J=7.8 Hz, 1H), 4.14
(dd, J=7.8, 4.2 Hz, 1H), 3.75 (s, 3H), 3.19-3.13 (m, 1H), 3.00 (dd, J=16.5,
4.8 Hz, 1H), 2.79-
2.70 (m, 1 H).
EXAMPLE 3
The preparation of Benzyl 9-methoxy-1 3-dioxo-1,2,3,6-tetrahydropyrrolo(3,4-
clcarbazole-4-
carboxylate (4)
Activated manganese dioxide (69 g) was added to a solution of benzyl 9-methoxy-
1,3-dioxo-1,2,3,3a,4,5,6,1Oc-octahydropyrrolo[3,4-c]carbazole-4-carboxylate
(13.76 g, 0.034
mol), prepared as in example 2, in p-dioxane (300 mL) and the mixture was
refluxed with
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-50-
vigorous stirring for 0.5-2 h. The mixture was filtered while hot through a
plug of Celite,
which was washed exhaustively with a MeOH/p-dioxane (1:1) mixture until the
washings
were colorless. The combined washings and filtrate were concentrated to
dryness and the
residue triturated several times with diethyl ether to give benzyl 9-methoxy-
1,3-dioxo-
1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-carboxylate (4) as a yellow/orange
powder
(12.47 g, 91.5 %), mp 245 °C. A small sample was recrystallised from
EtOAc to give benzyl
9-methoxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-carboxylate as
orange
cubes; mp 289-292 °C. 1H NMR 8 [(CD3)ZSO] 12.11 (br s, 1H), 11.27 (br
s, 1H), 8.45 (d,
J=2.6 Hz, 1H), 7.98 (s, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.55-7.51 (m, 2H), 7.44-
7.34 (m, 3H),
7.27 (dd, J=8.9, 2.6 Hz, 1H), 5.41 (s, 2H), 3.88 (s, 3H). Found: C, 68.99; H,
4.09; 6.91.
CZ3H~6Nz05 requires C, 69.00; H, 4.03; N, 6.99.
EXAMPLE 4
The Qreparation of 9-Methoxy-1 3-dioxo-1 2 3 6-tetrah~rop~rrolof3 4-
clcarbazole-4-
carboxylic acid (5)
A solution of benzyl 9-methoxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-
c]carbazole-
4-carboxylate (2.00 g), prepared as in example 3, in DMF/MeOH (4:1) (50 mL)
containing
5% Pd-C (0.50 g) was hydrogenated at 60 psi for 2 h (Parr apparatus). The
solution was
filtered through a plug of Celite, which was washed 6 times with neat DMF
followed by
MeOH (several cycles). The combined filtrate and washings were concentrated to
dryness in
vacuo and the residue was slurried with diethyl ether to give the 9-methoxy-
1,3-dioxo-
1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-carboxylic acid (5) as a greenish
solid. The
reaction was repeated 5 times on this scale to and the products were combined
to give 9-
methoxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-carboxylic acid,
(8.1 g,
84%). A portion of the product was purified by adsorption onto silica,
followed by
chromatography on silica, eluting with EtOAc. The product was triturated with
diethyl ether
to give 9-methoxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-
carboxylic acid as
an orange solid; mp >300°C. IH NMR 8 [(CD3)ZSO] 12.15 (s, 1H), 8.42 (d,
J=2.5 Hz, 1H),
7.97 (s, 1H), 7.57 (d, J=8.8 Hz, 1H0, 7.25 (dd, J=8.8, 2.5 Hz, 1H), 3.82 (s,
3H), 3.40 (br, 2H).
Found: C, 58.69; H, 3.55; N, 8.40. C~6HIONZO5.H20 requires C, 58.54; H, 3.68;
N, 8.53.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-51-
EXAMPLE 5
The preparation of 4-Amino-9-methoxypyrrolof3 4-clcarbazole-1,3(2H,6H)-dione
(6)
Diphenylphosphoryl azide (1.81 mL, 8.38 mmol) was added to a mixture of the 9-
methoxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazole-4-carboxylic acid
(2.55 g, 8.22
mmol), prepared as in example 4 , and Et3N (1.17 mL, 8.38 mmol) in anhydrous t-
butanol
(300 mL) and the mixture was refluxed under an atmosphere of nitrogen for 16
h. The
solution was concentrated in vacuo and the residue partitioned between EtOAc
and saturated
aqueous NaHC03. Insoluble material was removed by filtration of the two layers
through
Celite, washing through with more EtOAc. The organic phase was dried, the
drying agent
was removed and the solution was concentrated to dryness and gave a yellow
solid. This
material was dissolved in CHzCl2/trifluoroacetic acid (1:1) (200 mL) and the
solution was
held at room temperature for 1 h. After concentration in vacuo the residue was
partitioned
between EtOAc and saturated aqueous NaHC03 solution. The EtOAc solution was
dried, the
drying agent was removed and the solution was concentrated to dryness to give
an orange
solid which was adsorbed onto silica and chromatographed. Elution with ethyl
acetate/petroleum ether (1:1) followed by ethyl acetate and then
methanol/ethyl acetate (1:9)
gave 4-amino-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (6) as an
orange powder
(2.16 g, 93%), mp 342-345 °C.'H NMR 8 [(CD3)ZSO] 11.18 (s, 1H), 10.78
(br s, 1H), 8.18
(d, J=2.5 Hz, 1H), 7.29 (d, J=8.7 Hz, 1H), 7.01 (dd, J=8.7, 2.5 Hz, 1H), 6.83
(s, 1H), 6.28 (br
s, 2H), 3.82 (s, 3H). Found: C, 63.05; H, 3.99; N, 14.05. C~5H1~N303.1/2H20
requires C,
63.04; H, 4.06; N, 14.7.
EXAMPLE 6
The preparation of 4-Iodo-9-methoxypyrrolof3 4-clcarbazole-1,3(2H,6H)-dione
(7)
Concentrated HZS04 (10 mL) was added at room temperature to powdered 4-amino-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (0.50 g, 1.78 mmol), prepared
as in
example S, and the mixture was stirred for 5 min and then cooled in an ice
bath. An ice-water
slurry (ca 40 mL) was added in one portion with vigorous stirring and the
mixture was stirred
for a further 15 min to give a tan precipitate. When the internal temperature
had reached 3 °C
a solution of NaN02 (0.18 g, 2.65 mmol) in cold water (1 mL) was added
dropwise over 30
seconds and the mixture was stirred for an additional 3 minutes. Powdered urea
(74 mg, 1.23
mmol) was added and the mixture was stirred for another 3 min. Finally a
suspension of KI
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-52-
(1.46 g, 8.79 mmol) and CuI (1.46 g, 7.66 mmol) in cold water (10 mL) was
added and the
mixture was stirred vigourously for 5 min, and then warmed slowly to 70
°C and held at this
temperature for 1 h. Ethyl acetate was added and the two-phase mixture was
filtered through
a plug of Celite, washing through with more EtOAc. The combined organic
portions were
washed with 0.5 N aqueous sodium sulfite solution and were dried, the drying
agent was
removed and the solution was concentrated to dryness to give an oil which was
chromatographed on silica. Elution with EtOAc, then crystallisation of the
product from
THF/petroleum ether as a gave the 4-Iodo-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (7) (0.32 g, 46 %) as a yellow powder; mp 322-325 °C. 1H NMR 8
[(CD3)ZSO] 11.89
(s, 1H), 11.29 (s, 1H), 8.40 (d, J=2.6 Hz, 1H), 8.18 (s, 1H), 7.54 (d, J=8.9
Hz, 1H), 7.24 (dd,
J=8.9, 2.6 Hz, 1H), 3.87 (s, 3H). Found: C, 45.49; H, 2.36; N, 6.92.
CISH9IN20~.1/4Hz0
requires C, 45.42; H, 2.41; N, 7.06.
EXAMPLE 7
The preparation of 9-Hydroxy-4-iodopyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(8)
Powdered 4-Iodo-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (0.50 g,
1.27
mmol) prepared as in example 6 was added in one portion to dry, freshly-
prepared pyridine
hydrochloride melt at 200 °C under a CaCl2 drying tube and the mixture
was stirred at this
temperature for 15 min. Water was added and the mixture was extracted with
EtOAc. The
EtOAc extracts were dried, the drying agent was removed and the solution was
concentrated
to dryness to give a solid which was chromatographed on silica. Elution with
EtOAc gave 9-
Hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (8) (0.43 g, 89 %) as
an orange
powder; mp >350 °C.'H NMR 8 [(CD3)ZSO] 11.76 (br s, 1H), 11.24 (br s,
1H), 9.29 (br s,
1H), 8.27 (d, J=2.4 Hz, 1H), 8.13 (s, 1H), 7.44 (d, J=8.7 Hz, 1H), 7.08 (dd,
J=8.7, 2.4 Hz,
1H). Found: C, 44.90; H, 1.79; N, 7.14. C,4H~IN203 requires C, 44.47; H, 1.87;
N, 7.40.
EXAMPLE 8
The preparation of 4-(2-chloroQhenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
~9) (I Ar=2-chloronhenyl)
A mixture of the 9-Hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(41.8
mg, 0.110 mmol) prepared as in example 7 and 2-chlorobenzeneboronic acid (52
mg, 0.332
mmol) in p-dioxane (3 mL) and 2N Na2C03 (0.5 mL) was purged with nitrogen.
Pd(dppf)C12
(35 mg, 0.011 mmol) was added and the mixture was refluxed under NZ for 4 h
and then
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-53-
partitioned between EtOAc and water. The organic layer was dried, the drying
agent was
removed and the solution was concentrated to dryness, adsorbed onto silica and
chromatographed. Elution with EtOAc/petroleum ether (1:4) followed by
EtOAc/petroleum
ether (2:3) gave a solid the carbazole (50) which crystallised from
EtOAc/petroleum ether to
yield 4-(2-chlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (9)
(I, Ar=2-
chlorophenyl) (33.1 mg, 83 %) as a yellow powder, mp 215-220 °C (dec).
IH NMR b
[(CD3)2S0] 11.83 (br s, 1H), 11.01 (br s, 1H), 9.27 (br s, 1H), 8.32 (d, J=2.4
Hz, 1H), 7.65-
7.40 m, SH), 7.08 (dd, J=8.7, 2.4 Hz), 1H). Found: C, 65.72; H, 3.50, N, 6.97.
CZ°H»C1N203.1/4EtOAc requires C, 65.54; H, 3.40; N, 7.28.
EXAMPLE 9
The preparation of 9-Hydroxy-4-(3-h dy roxy-4-methoxyphenyl)pyrrolo(3,4-
clcarbazole-
1 3(2H 6H)-dione (10) (I, Ar=3-hydroxy-4-methoxyphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared as in example 7, with 3-hydroxy-4-methoxybenzeneboronic acid
according to the
procedure described in example 8 gave 9-hydroxy-4-(3-hydroxy-4-
methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (10) (I, Ar=3-hydroxy-4-
methoxyphenyl~ (51 %), mp 265 °C (dec).'H NMR S [(CD3)2S0] 11.67 (s,
1H), 10.96 (s,
1H), 9.22 (s, 1H), 9.15 (s, 1H), 8.33 (d, J=2.4 Hz, 1H), 7.57 (s, 1H), 7.41
(d, J=8.7 Hz, 1H),
7.22 (d, J=2.0 Hz, 1H), 7.06-6.99 (m, 2H), 6.85 (d, J=8.1 Hz, 1H), 3.81 (s,
3H). EIMS found
M+: 374.0900. C2,H14N205 requires 374.0903.
EXAMPLE 10
The preparation of 9-Hydroxy-4-(3-thienyl)pyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (12)
(I, Ar=3-thienyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared as in example 7,with 3-thienylboronic acid according to the procedure
described in
example 8 gave 9-hydroxy-4-(3-thienyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(12) (I,
Ar=3-thienyl) in a 74 % yield; mp 247 °C (dec).'H NMR S [(CD3)zS0]
11.73 (br s, 1H),
11.05 (br s, 1 H), 9.24 (s, 1 H), 9.15 (s, 1 H), 8.34 (d, J=2.4 Hz, 1 H), 7.92
(dd, J=5.0, 2.7 Hz,
1H), 7.70 (s, 1H), 7.61 (dd, J=5.0, 2.7 Hz, 1H), 7.51 (dd, J=5.0, 0.9 Hz, 1H),
7.42 (d, J=8.7
llz, 1H), 7.06 (dd, J=8.7, 2.4 Hz, 1H). Found: C, 61.05; H, 2.96; N, 7.60.
ClgH1°NZS03.H20
requires C, 61.36; H, 3.43; N, 7.95.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-54-
EXAMPLE 11
The preparation of 4-(3-Aminophenyl)-9-hydroxypyrrolo(3,4-clcarbazole-
1,3(2H,6H)-dione
(15) (I Ar=3-aminophenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 3-aminobenzeneboronic acid according to the procedure
described in
example 8 gave 4-(3-aminophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione (15)
(I, Ar=3-aminophenyl) in a 62 % yield; mp 279-284 °C .'H NMR S
[(CD3)ZSO] 11.70 (s,
1H), 10.96 (s, 1H), 9.22 (s, 1H), 8.33 (d, J=2.4 Hz, 1H), 7.50 (s, 1H), 7.42
(d, J=8.7 Hz, 1H),
7.10-7.03 (m, 2H), 6.77-6.74 (m, 1H), 6.72-6.68 (m, 1H), 6.61 (dd, J=8.1, 2.3
Hz), 1H), 5.11
(br s, 2H). Found: C, 69.41; H, 4.12; N, 10.73. CZOH13N303 requires C, 69.96;
H, 3.82; N,
12.24.
EXAMPLE 12
The preparation of 4-(4-Aminophenyl)-9-hydroxypyrrolol3,4-clcarbazole-
1,3(2H,6H)-dione
(16) (I, Ar=4-aminophenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
prepared
as in example 7 with 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)phenylamine according
to the procedure described in example 8 gave 4-(4-Aminophenyl)-9-
hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (16) (I, Ar=4-aminophenyl) in a 57 % yield; mp
256-258 °C .
'H NMR 8 [(CD3)ZSO] 11.60 (s, 1H), 10.92 (s, 1H), 9.19 (s, 1H), 8.31 (d, J=2.3
Hz, 1H),
7.48 (s, 1H), 7.39 (d, J=8.6 Hz, 1H), 7.30 (d, J=8.4 Hz, 2H), 7.02 (dd, J=8.6,
2.3 Hz, 1H),
6.62 (d, J=8.4 Hz, 2H), 5.29 (br s, 2H). Found: C, 68.09; H, 4.34; N, 11.03.
CZOH~3N303.1/2H20 requires C, 68.17; H, 4.00; N, 11.92.
EXAMPLE 13
The reparation of 4-(2 6-Dimethylphenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (17) (I, Ar=2,6-dimethylnhenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2,6-dimethylbenzeneboronic acid according to the
procedure described
in example 8 gave 4-(2,6-dimethylphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (17) (I, Ar=2,6-dimethylphenyl) in a 62 % yield; mp 230-238 °C
(dec).'H NMR 8
[(CD3)ZSO] 11.72 (s, 1H), 10.95 (s, 1H), 9.25 (s, 1H), 8.32 (d, J=2.3 Hz, 1H),
7.44 (d, J=8.7
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-55-
Hz, 1H), 7.35 (s, IH), 7.20 (t, J=7.5 Hz, 1H), 7.12 (d, J=7.5 Hz, 2H), 7.06
((dd, J=8.7, 2.3 Hz,
1H), 1.93 (s, 6H). EIMS found M+: 356.1159. C22H~6N203 requires 356.1161.
EXAMPLE 14
The preparation of 4-(2 3-Dichlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (18) (I, Ar=2,3-dichlorophenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2,3-dichlorobenzeneboronic acid according to the
procedure described
in example 8 gave 4-(2,3-dichlorophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (18) (I, Ar=2,3-dichlorophenyl) in a yield 27 %; mp 233-235 °C.'H
NMR 8
[(CD3)ZSO] 11.87 (br, 1H), 11.05 (br, 1H), 9.29 (br, 1H), 8.31 (d, J=2.5 Hz,
1H), 7.74-7.70
(m, 1H), 7.55 (s, 1H), 7.48-7.45 (m, 3H), 7.09 (dd, J=8.7, 2.SHz, 1H). Found:
C, 59.19; H,
3.04; N, 6.56. CZOHloC12Nz03.1/2H20 requires C, 59.13; H, 2.73; N, 6.89.
EXAMPLE 15
The preparation of 4-(2 6-Dimethoxyphenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (19) (I, Ar=2,6-dimethoxyphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2,6-dimethoxybenzeneboronic acid according to the
procedure
described in example 8 gave 4-(2,6-dimethoxyphenyl)-9-hydroxypyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (19) (I, Ar=2,6-dimethoxyphenyl) in a 43 % yield; mp 275-277
°C (dec).
1H NMR 8 [(CD3)ZSO] 11.62 (br s, 1H), 10.83 (br s, 1H), 9.21 (br s, 1H), 8.29
(d, J=2.4 Hz,
1H), 7.42 (s, 1H), 7.41 (d, J=8.4 Hz, 1H), 7.35 (t, J=8.4 Hz, 1H), 7.04 (dd,
J=8.4, 2.4 Hz,
1H), 6.75 (d, J=8.4 Hz, 2H), 3.63 (s, 6H). Found: C, 66.31; H, 4.50, N, 6.70.
CZZH~6N205.1/2H20 requires C, 66.49; H, 4.31; N, 7.04.
EXAMPLE 16
The preparation of 9-HydroxL-4-(2-methoxyphenyl)pyrrolo~3,4-clcarbazole-
1,3(2H,6H)-
dione (20) (I, Ar=2-methoxyphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-methoxybenzeneboronic acid according to the procedure
described
in example 8 gave 9-hydroxy-4-(2-methoxyphenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(20) (I, Ar=2-methoxyphenyl) in a 75 % yield, mp 216 °C. IH NMR 8
[(CD3)ZSO] 11.70 (s,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-56-
1H), 10.90 (s, 1H), 9.23 (s, 1H), 8.31 (d, J=2.4 Hz, 1H), 7.50 (s, 1H), 7.43
(d, J=8.0 Hz, 1H),
7.43-7.38 (m, 1H), 7.31 (dd, J=8.0, 2.4 Hz, 1H), 7.11-7.01 (m, 3H), 3.68 (s,
3H). Found: C,
69.21; H, 3.80; N, 7.58. Cz~H~4N204.1/4H20 requires C, 69.51; H, 4.03; N,
7.72.
EXAMPLE 17
The preparation of 9-Hydrox~-4-(4-hydroxymethylphenyl)pyrrolof3,4-clcarbazole-
1 3(2H 6H)-dione (21) (I, Ar=4-h d~Ymethylphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 4-hydroxymethylbenzeneboronic acid according to the
procedure
described in example 8 gave 9-hydroxy-4-(4-hydroxymethylphenyl)pymolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (21) (I, Ar=4-hydroxymethylphenyl) in a 34 % yield ; mp 266-
271 °C. 1H
NMR 8 [(CD3)zS0] 11.74 (br s, 1H), 11.00 (br s, 1H), 9.25 (br s, 1H), 8.34 (d,
J=2.4 Hz,
1H), 7.56 (d, J=8.3 Hz, 2H), 7.56 (s, 1H), 7.43 (d, J=8.7 Hz, 1H), 7.40 (d,
J=8.3 Hz, 1H), 7.06
(dd, J=8.7, 2.4 Hz, 1H), 5.27 (t, J=5.7 Hz, 1H), 4.59 (d, J=5.7 Hz, 2H).
Found: C, 69.74; H,
4.04; N, 7.57. CzlH,4N204.1/4H20 requires C, 69.51; H, 4.02; N, 7.72.
EXAMPLE 18
The preparation of 9-H~droxy-4-(2-trifluoromethylphenyl)pyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (22) (I, Ar=2-trifluoromethylphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-trifluoromethylbenzeneboronic acid according to the
proce3ure
described in example 8 gave 9-Hydroxy-4-(2-trifluoromethylphenyl)pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (22) (I, Ar=2-trifluoromethylphenyl) in a 47 % yield, mp 210
°C (dec).'H
NMR 8 [(CD3)zS0] 11.81 (br s, 1H), 11.01 (br s, 1H), 9.30 (br s, 1H), 8.31 (d,
J=2.4 Hz,
1H), 7.86 (br d, J=7.2 Hz, 1H), 7.75-7.64 (m, 2H), 7.51 (s, 1H), 7.49 (br d,
J=7.6 Hz, 1H),
7.46 (d, J=8.7 Hz, 1H), 7.09 (dd, J=8.7, 2.4 Hz, 1H). EIMS found M+: 396.0721.
Cz~HI,F3N203 requires 396.0722.
EXAMPLE 19
The preparation of 9-Hydroxy-4-(4-hydroxy-3-methoxyphenyl)pyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (23) (I, Ar=4-hydroxy-3-methoxyphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-methoxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yl)phenol
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-57-
according to the procedure described in example 8 gave 9-hydroxy-4-(4-hydroxy-
3-
methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (23) (I, Ar=4-hydroxy-3-
methoxyphenyl) in a 58 % yield; mp 290-295 °C (dec).'H NMR 8 [(CD3)ZSO]
11.67 (br s,
1H), 10.97 (br s, 1H), 9.22 (br s, 1H), 9.15 (s, 1H), 8.33 (d, J=2.4 Hz, 1H),
7.57 (s, 1H), 7.41
(d, J=8.6 Hz, 1H), 7.23 (d, J=2.0 Hz, 1H), 7.04 (m, 2H), 6.86 (d, J=8.1 Hz,
1H), 3.82 (s, 3H).
FABMS found [M+H]+: 375.0973. CZ~H15N205 requires 375.0981.
EXAMPLE 20
The preparation of 4-(2-Ethyl~henyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(SO1) (I; Ar=2-ethylnhenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-ethylbenzeneboronic acid according to the procedure
described in
example 8 gave 4-(2-Ethylphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione .
(501) (I; Ar=2-ethylphenyl) in a 69 % yield; mp (MeOH/CHZCl2/hexane) 273-275
°C. 1H
NMR [(CD3)zS0] b 11.73 (br s, 1H), 10.95 (br s, 1H), 9.25 (s, 1H), 8.32 (d,
J=2.4 Hz, 1H), 7.44
(s, 1H), 7.44 (d, J=8.6 Hz, 1H), 7.36 (td, J=7.2, 1.5 Hz, 1H), 7.33 (dd,
J=8.1, 2.2 Hz, 1H), 7.25
(td, J=7.0, 1.8 Hz, 1 H), 7.20 (dd, J=7.5, 1.2 Hz, 1 H), 7.07 (dd, J=8.7, 2.5
Hz, 1 H), 2.42 (m, 2H),
0.96 (t, J=7.6 Hz, 3H). Found: C, 73.83; H, 4.46; N, 7.82. CZZHisN20s requires
C, 74.15; H,
4.53; N, 7.86.
EXAMPLE 21
The preparation of 9-Hydroxy-4-f2-(hydroxymethyl)phenyllpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (503) (I; Ar=2-(hydroxymethyl)phenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-(hydroxymethyl)benzeneboronic acid cyclic monoester
according
to the procedure described in example 8 gave 9-Hydroxy-4-[2-
(hydroxymethyl)phenyl]pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (503) (I; Ar=2-
(hydroxymethyl)phenyl) in a 57 % yield , mp (THF/CHZC12/hexane) 270-280
°C (dec).'H
NMR [(CD3)ZSO] 811.76 (br s, 1H), 10.96 (br s, 1H), 9.25 (br s, 1H), 8.32 (d,
J=2.4 Hz, 1H),
7.57 (d, J=7.6 Hz, 1H), 7.47 (s, 1H), 7.44 (d, J=8.5 Hz, 1H), 7.43 (td, J=7.6,
1.2 Hz, 1H), 7.31
(td, J=7.4, 0.9 Hz, 1 H), 7.23 (dd, J=7.5, 1.0 Hz, 1 H), 7.06 (dd, J=8.7, 2.5
Hz, 1 H), 5.00 (t, J=5.4
Hz, 1H), 4.33 (dd, J=13.6, 5.2 Hz, 1H), 4.24 (dd, J=13.5, 5.3 Hz, 1H). Found:
C, 68.41; H, 4.29;
N, 7.59. CZ~H,4N204.1/2 H20 requires C, 68.66; H, 4.12; N, 7.63.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-5 8-
EXAMPLE 22
The preparation of 4-(2-Ethoxyphenyl)-9-h dy roxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(504) (I~ Ar=2-ethoxyphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-ethoxybenzeneboronic acid according to the procedure
described in
example 8 gave 4-(2-ethoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dione
(504) (I; Ar=2-ethoxyphenyl) in a 74 % yield ; mp (THF/CHzCl2/hexane) 190-193
°C (dec).
'H NMR [(CD3)zS0] b 11.71 (br s, 1H), 10.91 (br s, 1H), 9.23 (s, 1H), 8.31 (d,
J=2.4 Hz, 1H),
7.51 (s, 1H), 7.43 (d, J=8.7 Hz, 1H), 7.38 (td, J=7.8, 1.7 Hz, 1H), 7.33 (dd,
J=7.5, 1.7 Hz, 1H),
7.07 (br d, J=6.7 Hz, 1H), 7.05 (dd, J=8.7, 2.3 Hz, 1H), 7.02 (dd, J=7.3, 6.6
Hz, 1H), 3.97 (q,
J=6.7 Hz, 2H), 1.11 (t, J=6.9 Hz, 3H). Found: C, 70.63; H, 4.74; N, 6.88.
CZZH16N2O4~1/4 THF
requires C, 70.76; H, 4.65; N, 7.18.
EXAMPLE 23
The preparation of 9-Hydroxy=4-(2-thienyl)pmTOlo(3,4-clcarbazole-1,3(2H,6H)-
dione (505)
(I~ Ar=2-thienyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
prepared
as in example 7 with 2-thiopheneboronic acid according to the procedure
described in
example 8 gave 9-hydroxy-4-(2-thienyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(505) (I;
Ar=2-thienyl) in a 69 % yield; mp (THF/CHZCIZ/hexane) 179-184 °C (dec).
1H NMR
[(CD3)ZSO] S 11.75 (br s, 1H), 11.11 (br s, 1H), 9.26 (br s, 1H), 8.34 (d,
J=2.4 Hz, 1H), 7.75 (s,
1H), 7.73 (dd, J=3.6, 1.0 Hz, 1H), 7.68 (dd, J=5.1, 1.3 Hz, 1H), 7.43 (d,
J=8.7 Hz, 1H), 7.20 (dd,
J=5.1, 3.6 Hz, 1H), 7.07 (dd, J=8.7, 2.5 Hz, 1H). Found: C, 62.38; H, 3.11; N,
7.74.
CIBH~oNz03S.3/4 HBO requires C, 62.15; H, 3.33; N, 8.05.
EXAMPLE 24
The preparation of 3-(9-Hydroxy-1 3-dioxo-1 2 3 6-tetrahydropyrrolof3,4-
clcarbazol-4-
r~l)benzaldehyde (507) (I; Ar=3-formylphenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared as in
example 7, with 3-formylbenzeneboronic acid according to the procedure
described in
example 8 gave 3-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-
4-
yl)benzaldehyde (507) (I; Ar=3-formylphenyl) in a 69 % yield; mp (TI-
IFYCH2C12/pentane)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-59-
280-286 °C (dec).'H NMR [(CD3)ZSO] 8 11.82 (br s, 1H), 11.07 (br s,
1H), 10.11 (s, 1H), 9.27
(br s, 1H), 8.35 (d, J=2.4 Hz, 1H), 8.14 (t, J=1.4 Hz, 1H), 7.97 (m, 2H), 7.70
(t, J=7.7 Hz, 2H),
7.65 (s, 1H), 7.46 (d, J=8.7 Hz, 1H), 7.08 (dd, J=8.7, 2.5 Hz, 1H). FABMS
found [M+H]+:
357.0857. CZIH~3N204 requires 357.0875.
EXAMPLE 25
The preparation of 9-Hydroxy-4-(2-(methylsulfanyl)phenyllpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (508) (I; Ar=2-(methylsulfanyl)phenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 2-(methylthio)benzeneboronic acid according to the
procedure
described in example 8 gave 9-hydroxy-4-[2-(methylsulfanyl)phenyl]pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (508) (I; Ar=2-(methylsulfanyl)phenyl) in a 72 %
yield, mp
(MeOH/CHZCIz/hexane) 208-216 °C.'H NMR [(CD3)ZSO] 8 11.76 (br s, 1H),
10.95 (br s, 1H),
9.25 (br s, 1H), 8.31 (d, J=2.5 Hz, 1H), 7.47 (s, 1H), 7.44 (d, J=8.6 Hz, 1H),
7.43 (td, J=7.3, 2.2
Hz, 1 H), 7.38 (br d, J=7.5 Hz, 1 H), 7.27 (dd, J=7.8, 2.2 Hz, 1 H), 7.24 (td,
J=7.3, 1.3 Hz, 1 H),
7.07 (dd, J=8.7, 2.5 Hz, 1H), 2.32 (s, 3H). Found: C, 66.55; H, 3.88; N, 7.38.
CZIHiaN203S.1/4
H20 requires C, 66.57; H, 3.86; N, 7.39.
EXAMPLE 26
The preparation of 4-(9-Hydroxy-1 3-dioxo-1,2,3,6-tetrahydropyrrolof3,4-
clcarbazol-4-
yl)benzaldehyde (509) (I; Ar=4-formylnhenyl)
The reaction of 9-hydroxy-4-iodopyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione,
prepared
as in example 7, with 4-formylbenzeneboronic acid according to the procedure
described in
example 8 gave 4-(9-hydroxy-1,3-dioxo-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-
4-
yl)benzaldehyde (509) (I; Ar=4-formylphenyl) in a 52 % yield; mp
(THF/CH2C12/pentane)
276-280 °C.'H NMR [(CD3)ZSO] 8 11.84 (br s, 1H), 11.07 (br s, 1H),
10.11 (s, 1H), 9.28 (br s,
1 H), 8.34 (d, J=2.3 Hz, 1 H), 8.00 (d, J=8.2 Hz, 2H), 7.85 (d, J=8.1 Hz, 2H),
7.65 (s, 1 H), 7.46
(d, J=8.7 Hz, 1H), 7.08 (dd, J=8.7, 2.4 Hz, 1H). Found: C, 68.43; H, 4.16; N,
7.26.
CZ~H12N~04.3/4 H20 requires C, 68.20; H, 3.68; N, 7.57. FABMS found [M+H]+:
357.0841.
CZ~H~3NZOa requires 357.0875.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-60-
EXAMPLE 27
The preparation of 9-Hydroxy-4-(2-(methylsulfinyl)phenyllnyrrolo(3,4-
clcarbazole-
1 3(2H 6H)-dione (510) (I; Ar=2-(methylsulfinyl)phenyl)
A mixture of the 9-hydroxy-4-[2-(methylsulfanyl)phenyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (508) (34.5 mg, 0.092 mmol), prepared according to example
25, and 2-
phenylsulfonyl-3-phenyloxaziridine (Davis reagent) (26.5 mg, 0.102 mmol) in
THF (10 mL)
was stirred at 20 °C for 2.5 h, then adsorbed directly onto silica gel
and chromatographed.
Elution with 0-3% MeOH/CHZC12 then 3-4% MeOH/CHZC12 gave (after
crystallisation from
THF/CH2C12/pentane) the 9-Hydroxy-4-[2-(methylsulfinyl)phenyl]pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (510) (I; Ar=2-(methylsulfinyl)phenyl) in a 86 % yield as a
yellow solid;
mp 321-323 °C.'H NMR [(CD3)ZSO]-8 11.86, 11.85 (2br s, 1H), 11.09 (br
s, 1H), 9.30 (br s,
1H), 8.32 (d, J=2.4 Hz, 1H), 8.04, 7.97 (2d, J=7.6 Hz, 1H), 7.74 (t, J=7.6 Hz,
1H), 7.65, 7.62 (2t,
J=7.4 Hz, 1H), 7.62, 7.54 (2s, 1H), 7.47 (d, J=9.1 Hz, 1H), 7.44 (d, J=8.4 Hz,
1H), 7.09 (dd,
J=8.7, 2.5 Hz, 1H), 2.43, 2.31 (2s, 3H). Found: C, 64.60; H, 3.88; N, 6.87.
CZ~H14NZOpS requires
C,64.61;H,3.61;N,7.18.
EXAMPLE 28
The preparation of 9-HYdro~-4-(4-hydroxyphenyl)pyrrolo(3,4-clcarbazole-
1,3(2H,6H)-
dione (24) (I Ar=4-hydroxyphenyl)
The reaction of 9-hydroxy-4-(4-methoxyphenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione prepared as decribed in example 34 with BBr3 using the procedure
described in
example 80 of Scheme 2 gave 9-Hydroxy-4-(4-hydroxyphenyl)pyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (24) (I, Ar=4-hydroxyphenyl) in a 92 % yield; mp 230
°C (dec).'H NMR
b [(CD3)ZSO] 11.67 (s, 1H), 10.96 (s, 1H), 9.59 (s, 1H), 9.22 (s, 1H), 8.32
(d, J=2.4 Hz, 1H),
7.51 (s, 1 H), 7.43 (d, J=8.7 Hz, 1 H), 7.41 (d, J=8.6 Hz, 2H), 7.04 (dd,
J=8.7, 2.4 Hz, 1 H),
6.84 (d, J=8.6 Hz, 2H). FABMS found [M+H]+: 345.0875. CZOH13N2O4 requires
345.0875.
EXAMPLE 29
The preparation of 9-Hydroxy-4-(3-hydroxyphenyl)pyrrolo(3,4-clcarbazole-
1,3(2H,6H)-
dione (25) (I, Ar=3-hydroxyphenyl)
9-I-Iydroxy-4-(3-methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(prepared
as decribed in example 35) was reacted with BBr3 using the procedure described
in the
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-61-
proceedure described in example 80 of Scheme 2 to give 9-hydroxy-4-(3-
hydroxyphenyl)pyrrolo(3,4-c]carbazole-1,3(2H,6H)-dione (25) (I, Ar=3-
hydroxyphenyl) in a
yield of 88 %; mp 282-285 °C (dec). 1H NMR 8 [(CD3)ZSO) 11.73 (br s,
1H), 11.00 (br s,
1 H), 9.48 (br s, 1 H), 9.24 (s, 1 H), 8.34 (d, J=2.4 Hz, 1 H), 7.54 (s, 1 H),
7.43 (d, J=8.7 Hz,
1H), 7.24 (dd, J=7.7, 7.7 Hz, 1H), 7.05 (dd, J=8.7, 2.4 Hz, 1H), 7.01-6.96 (m,
2H), 6.82 (dd,
J=8.2, 2.1 Hz, 1H). FABMS found [M+H]+: 345.0865. CZOH~3Nz04 requires
345.0875.
EXAMPLE 30
The preparation of 4-(2-Chloro-6-h droxyphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (26) (I, Ar=2-chloro-6-hydroxyphenyl)
4-(2-Chloro-6-methoxyphenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-
dioneprepared as in example 33 was reacted with BBr3 using the procedure
described in
example 80 of Scheme 2 to give 4-(2-Chloro-6-hydroxyphenyl)-9-
hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (26) (I, Ar=2-chloro-6-hydroxyphenyl) in a yield
of 70 %;
m.p. 228-235 °C.'H NMR 8 [(CD3)ZSO] 11.73 (s, 1H), 10.94 (s, 1H), 9.75
(br s, 1H), 9.25
(s, 1H), 8.30 (d, J=2.4 Hz, 1H), 7.45 (s, 1H), 7.44 (d, J=8.7 Hz, 1H), 7.23
(dd, J=8.1, 8.1 Hz,
1 H), 7.07 (dd, J=8.7, 2.4 Hz, 1 H), 6.99 (d, J=8.1 Hz, 1 H), 6.90 (d, J=8.1
Hz, 1 H). FABMS
found [M+H]+: 381.0461, 379.0479. CZOH12C1Nz04 requires 381.0456, 379.0486.
Compounds described in examples 31-35 were prepared in an array manner by
reaction of the iodide (8) prepared as described in Example 7 with the
appropriate substituted
arylboronic acid using the procedure described in Example 8.
EXAMPLE 31
The preparation of 4-(3-Chlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(I; Ar=3-chlorophenyl) (866) by reaction with 3-chlorobenzeneboronic acid.
Found [M+H]+:
363.
EXAMPLE 32
The preparation of 4-(4-Chlorophenyl)-9-hydroxynyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(I; Ar=4-chlorophenylj (867) by reaction with 4-chlorobenzeneboronic acid.
Found [M+H]+:
363.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-62-
EXAMPLE 33
The preparation of 4-(2-Chloro-6-methoxyphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
3(2H,6H)-dione (I; Ar=2-chloro-6-methoxyphenyl) (868) by reaction with 2-
chloro-6-
methoxybenzeneboronic acid. Found [M+H]+: 393.
EXAMPLE 34
'The preparation of 9-Hydroxy-4-(4-methoxyphenyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (I; Ar=4-methoxyphenyl) (870) by reaction with 4-methoxybenzeneboronic
acid.
Found [M+H]+: 359
EXAMPLE 35
The preparation of 9-Hydroxy-4-(3-methoxyphenyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (I; Ar=4-methoxyphenyl) by reaction with 4-methoxybenzeneboronic acid.
Found
[M+H]+: 359
EXAMPLE 36
The preparation of a series of compounds usin multiple parallel synthetic
techguires from
9-h~droxy-4-iodopyrrolof3 4-clcarbazole-1,3(2H,6H)-dione prepared as in
Example 7
Combichem Procedure 1
In a 8 ml screw cap vial was added a solution of 9-Hydroxy-4-iodo-6H-
pyrrolo[3,4-
c]carbazole-1,3-dione, ( 0.1 mmol) prepared as in example 7 in dioxane (1 ml),
a solution of
Reagent I (see table) ( 0.1 mmol) in 1:1 dioxane/2.5 M KZC03 (I ml) and
[I,1'Bis(diphenylphosphino)ferrocene]-dichloropalladium(II) complex with
dichloromethane
(0.003 g, 0.0037 mmol). The vial was capped and the reaction mixture was
shaken for 4
hours at 90 °C. After cooling to room temperature, the solution was was
removed under
vacuum. Purification was carried out via reverse-phase HPLC (3% n-propanol in
acetonitrile
and 3% n-propanol in water as the eluent; C-18 column). The products were
characterised by
mass spectral analysis ( See Table 1).
Combichem Procedure 2
In a 8 ml screw cap vial was added a solution of 9-Hydroxy-4-iodo-6H-
pyrrolo[3,4-
c]carbazole-1,3-dione, (0.1 mmol) prepared as in example 7 in anhydrous
toluene (1 ml), a
solution of Reagent I (see table) in 1:1 anhydrous toluene/dimethylformamide
(1 ml) and a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-63-
solution of 0.05 M palladium(II) diacetate plus 0.2 M o-
dicyclohexylphosphinobiphenyl in
anhydrous toluene (20 ~tl), and 40 mg of K3P04~2H20 under Nz. The vial was
capped and the
reaction mixture was shaken for 20 hours at 100°C. After cooling to
room temperature, the
solution was was removed under vacuum. Purification was carried out via
reverse-phase
HPLC (3% n-propanol in acetonitrile and 3% n-propanol in water as the eluent;
C-18
column). The products were characterised by mass spectral analysis ( See Table
1).
nalytical
ata
rocedure eagentl roduct
S-APCI
[M+H]'"
ombichem -acetylphenyl-(2-Acetylphenyl)-9-hydroxy-6H-pyrrolo[3,4-
71.2
rocedure oronic acid]carbazole-1,3-dione
1
ombichem -Fluorophenyl-(4-Fluorophenyl)-9-hydroxy-6H-pyrrolo[3,4-
47.2
rocedure oronic acid]carbazole-1,3-dione
1
,4-Methylene
ombichem -( 1,3-Benzodioxol-5-yl-9-hydroxy-6H-
ioxybenzene 73.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
oronic acid
ombichem -Naphthalene-Hydroxy-4-naphthalen-2-yl-6H-pyrrolo[3,4-
79.2
rocedure oronic acid]carbazole-1,3-dione
1
-Methylthio
ombichem -Hydroxy-4-(4-methylsulfanylphenyl)-6H-
henyl boronic 75.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
cid
ombichem - biphenylboronic-Biphenyl-4-yl-9-hydroxy-6H-pyrrolo[3,4-
05.2
rocedure cid ]carbazole-1,3-dione
1
-(trifluoro
ombichem -Hydroxy-4-(3-trifluoromethoxy-phenyl)-6H-
ethoxy) 13.2
benzene
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
oronic acid
ombichem -Methoxyphenyl-Hydroxy-4-(3-methoxyphenyl)-6H-
59.2
rocedure oronic acidyrrolo[3,4-c]carbazole-1,3-dione
1
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-64-
ombichem -Cyanophenyl-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-
354.2
rocedure oronic acidyrrolo[3,4-c]carbazol-4-yl)-benzonitrile
1
ombichem ,5-diehlorophenyl-(2,5-Dichlorophenyl)-9-hydroxy-6H-
g8.2
rocedure oronic acidyrrolo[3,4-c]carbazole-1,3-dione
1
-trifluoromethoxy
ombichem -Hydroxy-4-(4-trifluoromethoxy-phenyl)-6H-
henyl boronie 13.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
cid
-Hydroxymethyl
ombichem -Hydroxy-4-(3-hydroxymethylphenyl)-6H-
henyl boronic 59.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
cid
ombichem uran-3- -Furan-2-yl-9-hydroxy-6H-pyrrolo[3,4-
boronic
19.2
rocedure cid ]carbazole-1,3-dione
1
ombichem yridine-3-boronic-Hydroxy-4-pyridin-3-yl-6H-pyrrolo[3,4-
30.2
rocedure cid ]carbazole-1,3-dione
1
,4-dimethoxy
ombichem -(2,4-Dimethoxy-pyrimidin-5-yl)-9-hydroxy-
yrimidin-5- 91.2
rocedure H-pyrrolo[3,4-c]carbazole-1,3-dione
1
oronic acid
ombichem -Cyanophenyl-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-
54.2
rocedure oronic acidyrrolo[3,4-c]carbazol-4-yl)-benzonitrile
1
-(trifluoromethyl)
ombichem -Hydroxy-4-(4-trifluoromethylphenyl)-6H-
l
henyl boronic 97.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
cid
-(t~fluoromethyl)
ombichem -Hydroxy-4-(3-trifluoromethylphenyl)-6H-
l
henyl boronic 97.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
1
cid
-(methylsulfonyl)
ombichem -Hydroxy-4-(4-methanesulfonylphenyl)-6H-
l
henyl boronic 07.2
rocedure yrrolo[3,4-c]earbazole-1,3-dione
1
cid
ombichem -acetamidophenyl-[3-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-
86.2
rocedure oronic acidyrrolo[3,4-c]carbazol-4-yl)-phenyl]acetamide
1
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-65-
-dimethylamino
ombichem 4-(4-Dimethylaminophenyl)-9-hydroxy-6H-
henyl boronic 72.2
rocedure yrrolo[3,4-c)carbazole-1,3-dione
2
cid
ombichem -acetylphenyl4-(3-Acetylphenyl)-9-hydroxy-6H-
71.2
rocedure oronic acidyrrolo[3,4-c]carbazole-1,3-dione
2
n
hem -hydroxyphenyl9-Hydroxy-4-(3-hydroxyphenyl)-6H-
omu
45.2
rocedure oronic acidyrrolo[3,4-c]carbazole-1,3-dione
2
ombichem -methylbenzene9-Hydroxy-4-m-tolyl-6H-pyrrolo[3,4-
43.2
rocedure oronic acid]carbazole-1,3-dione
2
ombichem 9-Hydroxy-4-o-tolyl-6H-pyrrolo[3,4-
-tolylboronic 43.2
acid
rocedure ]carbazole-1,3-dione
2
rans-2-
ombichem 9-Hydroxy-4-((E)-styryl)-6H-pyrrolo[3,4-
henylvinyl 55.2
rocedure ]carbazole-1,3-dione
2
oronic acid
ombichem -formylphenyl2-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-
57.2
rocedure oronic acidyrrolo[3,4-c]carbazol-4-yl)-benzaldehyde
2
'
ombichem 4-(2,5-Dimethylphenyl)-9-hydroxy-6H-
imethylphenyl 57.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
2
oronic acid
-Methylsulfanyl
ombichem 9-Hydroxy-4-(2-methylsulfanylphenyl)-6H-
enzeneboronic 75.2
rocedure yrrolo[3,4-c]carbazole-1,3-dione
2
cid
ombichem -Methylbenzene9-Hydroxy-4-p-tolyl-6H-pyrrolo[3,4-
43.2
rocedure oronic acid]carbazole-1,3-dione
2
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-66-
Me0 ~ \ ArCHzPPh3X/l_DA Me0
I / CHO 20-65 °C ~ \
METHOD2 ( / \
N Ar
(1) H
(II)
H
O N O
1. NaH Me0
2. R'°X I ~ \ Malei~ Me0 ~ Ar
METHOD 3 / N \ Ar toluene/SnCh/reflux METHOD 4 \
or toluene/reflux METHOD 4a ( / N
(III) or neat/1 SO °C METHOD 5
R
H (IV)
O N O N O
DDO/dioxane/reflux O
METHOD 6 Me0 Ar BBr3/CHzCl2 METHOD 8
or MnO~/dioxane/reflux ~ \ ~ HO ~ \ / Ar
METHOD 7 I / or Py.HCU200-220 °C METHOD 9 I
N /
R'° N ,o
R
(V)
(VI)
1. NBS pph3gr 1. HBr/HOAc
2. PPh3 ~ ~ 2. PPh3 /OH
Ar METHOD 10a Ar METHOD 10b
Ar
SCHEME 2
Scheme 2 Procedures
Representative Procedure for Method 2 of Scheme 2
EXAMPLE 37
The ~renaration of 2-f2-(2-Chlorophenyl)ethenvll-5-methoxv-1H-indole (II; Ar=2-
chlorophen 1)~ (27)
Lithium diisopropyl amide (34.4 mL of a 1.5 N solution, 0.052 mol) was added
dropwise under nitrogen to a suspension of benzyl(triphenyl)phosphonium
chloride (20.17 g,
0.048 mol) in dry THF (200 mL) and the solution was stirred at room
temperature for 15 min.
A solution of the 5-methoxy-1H-indole-2-carbaldehyde (1) (6.99 g, 0.040 mol)
in THF (30
mL) was added and stirring was continued at room temperature for 15 min and
then the
reaction mixture was heated at reflux for 6 h. The cooled solution was diluted
with water,
extracted with EtOAc and the organic phase was dried, the drying agent was
removed and the
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-67-
solution was concentrated to dryness to give an oil which was adsorbed onto
silica and
chromatographed on silica. Elution with ethyl acetate/petroleum ether (1:1)
gave 2-[2-(2-
Chlorophenyl)ethenyl]-5-methoxy-1H-indole (27) as a mixture of E/Z isomers in
a yield of
9.76 g, 87%. Crystallisation of a small samplefrom methanol afforded pure E-
isomer, mp
135-137°C.'H NMR 8 (CDCl3) 11.39 (s, 1H), 7.86 (dd, J=7.8, 1.5 Hz, 1H),
7.49 (dd, J=8.0,
1.2 Hz, 1H), 7.43 (d, J=16.4 Hz, 1H), 7.37 (m, 1H), 7.31-7.23 (m, 3H), 7.01
(d, J=2.4 Hz,
1 H), 6.77 (dd, J=8.7, 2.4 Hz, 1 H), 6.56 (s, 1 H), 3.75 (s, 3H). Found: C,
72.01; H, 5.03; N,
4.98. C1~H14C1N0 requires C, 71.96; H, 4.79; N, 4.94.
Representative Procedure for Method 3 of Scheme 2
EXAMPLE 38
The preparation of 1-(3-1 ftert-Butyl(dimethyl)silylloxy)propyl)-2-f2-(2-
chlorophenyl)
ethenyll-5-methoxy-1H-indole (III; Ar=2-chlorophenyl,
R'°=CHZCH~CH~OSiM~t-Bu) (28)
Sodium hydride (1.05 g of a 50% dispersion in mineral oil, 0.022 mol) was
added to a
solution of the 2-[2-(2-Chlorophenyl)ethenyl]-5-methoxy-1H-indole prepared
according to
example 37 (4.13 g, 0.014 mol) in DMF (30 mL) and the solution was stirred at
room
temperature for 5 min. 3-Bromopropyl tent-butyl(dimethyl)silyl ether (4.04 g,
0.016 mol) was
added and stirring was continued for 2 h. The solution was diluted with water,
extracted with
EtOAc which was washed well with brine and the organic phase was dried, the
drying agent
was removed and the solution was concentrated to dryness to give 1-(3-{ [tert-
Butyl(dimethyl)silyl]oxy }propyl)-2-[2-(2-chlorophenyl)ethenyl]-5-methoxy-1H-
indole_(28)
as an oily solid (4.98 g) (as a mixture of E/Z isomers) which was used without
further
purification.
EXAMPLE 39
The preparation of 6-(3-( ftert-Butyl(dimethyl)silylloxy ~propyl)-4-(2-
chlorophenyl)-9-
methoxvnvrrolo(3,4-clcarbazole-1,3(2H.6H)-dione (V; Ar=2-chlorophenyl,
R1°=CH~CH~CH~OSiMe~t-Bu 29
1-(3-{ [tert-Butyl(dimethyl)silyl]oxy }propyl)-2-[2-(2-chlorophenyl)ethenyl]-5-
methoxy-1H-indole prepared according to example 38 was reacted with maleimide
according to the procedure described in example 68 except that the reaction
time was 16 h.
The resultant product was reacted with MnOz according to the procedure
described in
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-68-
example 81 except that the reaction time was 8 h. Following chromatography on
silica the
product was triturated with diethyl ether to give 6-(3-{ [tert-
Butyl(dimethyl)silyl]oxy}propyl)-
4-(2-chlorophenyl)-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (29) in a
4.87 g, 61
% yield, as a yellow solid; mp 199-201 °C.'H NMR 8 [(CD3)ZSO 11.12 (br,
1H), 8.52 (d,
J=2.6 Hz, 1H), 7.75 (s, 1H), 7.66 (d, J=8.9 Hz, 1H), 7.57 (dd, J=8.3 Hz, 1H),
7.5-7.4 (m, 3H),
7.30 (dd, J=8.9, 2.6 Hz, 1H), 4.53 (t, J=6.4 Hz, 2H), 3.90 (s, 3H), 3.55 (t,
J=5.8 Hz, 2H), 1.94
(m, 2H), 0.77 (s, 9H), -0.06 (s, 3H). Found: C, 65.72; H, 6.09; N, 5.19.
C3°H32C1SiN204
requires C, 65.74; H, 5.88; N, 5.11.
EXAMPLE 40
The preparation of 4-(2-Chlorophenyl)-6-(3-hydroxypropyl)-9-methoxypyrrolof3,4
clcarbazole-1,3(2H,6H)-dione (V; Ar=2-chlorophenvl, R'°=CH~CH~CH20H)
(31)
3N HCl (50 mL) was added to a solution of 6-(3-{ [tert-Butyl(dimethyl)
silyl]oxy }propyl)-4-(2-chlorophenyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(4.87 g, 8.85 mmol) prepared according to example 39 in 1:1 THF/methanol (200
mL). After
stirring at room temperature for 2 h most of the solvents were removed in
vacuo, the residue
was extracted with ethyl acetate, washed well with water and the organic
portion was
concentrated to a volume of 60 mL. Petroleum ether was added to precipitate
the product,
which was filtered off and triturated several times with diethyl ether. The
solid was
crystallised from THF/petroleum ether and gave 4-(2-Chlorophenyl)-6-(3-
hydroxypropyl)-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chlorophenyl,
R'°=CHZCHZCHZOH) (31)in a 3.77 g, 88 % yield as a yellow powder; mp 228-
230 °C.'H
NMR 8 [(CD3)zS0] 11.12 (br s, 1H), 8.52 (d, J=2.5 Hz, 1H), 7.80 (s, 1H), 7.70
(d, J=8.9 Hz,
1H), 7.58 (dd, J=8.1, 2 2 Hz, 1H), 7.53-7.42 (m, 3H), 7.31 (dd, J=8.9, 2.5 Hz,
1H), 4.63 (br,
1H), 4.53 (t, J=6.9 Hz, 2H), 3.91 (s, 3H), 3.40 (m, 2H), 1.91 (m, 2H). Found:
C, 66.00; H,
4.23; N, 6.55. CZQH»C1N204 requires C, 66.29; H, 4.40; N, 6.44.
EXAMPLE 41
The ureparation of 2-(2-f2-(2-Chlorophenyl)ethenyll-5-methoxy-1H-indol-1-
yl~ethanol (III;
Ar=2-chlorophenyl, R'°=CH~CH~OH) (44)
Alkylation of 2-[2-(2-Chlorophenyl)ethenyl]-5-methoxy-1H-indole (27) prepared
according to example 37 with 2-bromoethyl tetrahydro-2H-pyran-2-yl ether using
the
procedure described in example 38 followed by reaction of the crude product
with 2N HCl in
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-69-
methanol gave 2-{2-[2-(2-Chlorophenyl)ethenyl]-5-methoxy-1H-indol-1-yl}ethanol
(III;
Ar=2-chlorophenyl, R'°=CHzCHzOH) (44) in a yield of 86 % as a mixture
of E/Z isomers,
which was used without further purification.
EXAMPLE 42
The preparation of 4-(2-Chlorophenyl)-6-(2-hydroxyethyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (IV; Ar=2-chlorophenyl,
R'°=CH~CH~OH) (45)
Reaction of 2-{2-(2-(2-Chlorophenyl)ethenyl]-5-methoxy-1H-indol-1-yl}ethanol
(III;
Ar=2-chlorophenyl, R'°=CHZCHzOH) (44) prepared according to example 41
with maleimide
according to the procedure described in example 68 gave 4-(2-Chlorophenyl)-6-
(2-
hydroxyethyl)-9-methoxy-4,5,6, l Oc-tetrahydropyrrolo[3,4-c]carbazole-
1,3(2H,3aH)-dione
(IV; Ar=2-chlorophenyl, R'°=CHzCHzOH) (45) in a 79% yield as an oily
solid, which was
used without further purification.
EXAMPLE 43
The preparation of 4-(2-Ghlorophen~ -) 6-(2-hydroxyethyl)-9-methoxypyrrolof
3,4-
clcarbazole-1,3(2H,6H)-dione (V; Ar=2-chlorophenyl, R'°= CH?CH~OH 46
4-(2-Chlorophenyl)-6-(2-hydroxyethyl)-9-methoxy-4,5,6, l Oc-
tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-chlorophenyl, Rl°=CHZCHZOH)
(45) prepared
according to example 42 was reacted with Mn02 using the procedure described in
example
79 except that the reaction time was 18 h gave 4-(2-Chlorophenyl)-6-(2-
hydroxyethyl)-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chlorophenyl, Rlo=
CH2CHZOH) (46~in a 72 % yield as a yellow powder, mp 255-257 °C.
1H NMR 8
[(CD3)2S0] 11.10 (br s, 1H), 8.52 (d, J=2.6 Hz, 1H), 7.80 (s, 1H), 7.71 (d,
J=9.0 Hz, 1H),
7.59-7.56 (m, 1H), 7.52-7.43 (m, 4H), 7.29 (dd, J=9.0, 2.2.6 Hz, 1H), 4.84 (t,
J=5.0 Hz, 1H),
4.53 (t, J=5.2 Hz, 2H), 3.90 (s, 3H), 3.77 (m, 2H). Found: C, 65.50; H, 4.07;
N, 6.59.
C23H»CIN204 requires C, 65.64; H, 4.07; N, 6.66.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-70-
EXAMPLE 44
The preparation of 4-(2-Chlorophenyl)-6-(2-h d~yethyl)-9-hydroxypyrrolo(3 4-
~arbazole-1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R'°=C~CH~OH) (47)
The reaction of 4-(2-Chlorophenyl)-6-(2-hydroxyethyl)-9-methoxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chlorophenyl, Rl°= CHZCHZOH) (46)
prepared
according to example 43 with BBr3 using the procedure described in example 80
gave the 4-
(2-Chlorophenyl)-6-(2-hydroxyethyl)-9-hydroxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(VI; Ar=2-chlorophenyl, R'°= CHZCHZOH) (47) in a yield of 87 °lo
as a yellow/orange
powder; mp 265 °C (dec).'H NMR 8 [(CD3)ZSO] 11.04 (br s, 1H), 9.33 (br
s, 1H), 8.38 (d,
J=2.4 Hz, 1H), 7.75 (s, 1H), 7.60-7.56 (m, 2H), 7.52-7.43 (m, 4H), 7.12 (dd,
J=8.8, 2.4 Hz,
1H), 4.83 (t, J=5.5 Hz, 1H), 4.49 (t, J=5.2 Hz, 2H), 3.77 (dt, J=5.5, 5.2 Hz,
2H). Found: C,
63.41; H, 4.11; N, 6.32. CZZHisC1N204.1/2Hz0 requires C, 63.54; H, 3.87; N,
6.73.
EXAMPLE 45
The preparation of 5-Methoxy-2-(2-(2-methoxy-5-nitrophenyl)ethenyll-1H-indole
(II; Ar=2-
methoxy-5-nitrophen 1)
Reaction of 5-methoxy-1H-indole-2-carbaldehyde (1) with 2-methoxy-5-
nrtrobenzyltriphenylphosphonium chloride using the procedure described in
example 37 gave 5-
methoxy-2-[2-(2-methoxy-5-nitrophenyl)ethenyl]-1H-indole (II; Ar=2-methoxy-5-
nitrophenyl)
(35) in a 76°lo yield as an orange solid (a mixture of E/Z isomers)
which was used without further
purification.
EXAMPLE 46
T'hepreparation of 5-Methoxy-2-(2-(3,5-dinitrophenyl)ethenyll-1H-indole (II;
Ar=3,5-
dinitrophen 1)~ (40)
Reaction of S-methoxy-1H-indole-2-carbaldehyde(1) with 3,5-
dinitrobenzyltriphenylphosphonium bromide using the procedure described in
example 37
gave 5-Methoxy-2-[2-(3,5-dinitrophenyl)ethenyl]-1H-indole (II; Ar=3,5-
dinitrophenyl) (40)
in a 26% yield as an orange solid (a mixture of E/Z isomers) which was used
without further
purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-71-
EXAMPLE 47
The preparation of 4-(3 5-Dinitrophenyl)-9-methoxy-4 5 6 lOc-
tetrah~pyrrolof3,4-
~carbazole-1 3(2H 3aH)-dione (IV; Ar=3,5-dinitrophenyl, R'°=H) (41)
The reaction of 5-Methoxy-2-[2-(3,5-dinitrophenyl)ethenyl]-1H-indole (II;
Ar=3,5-
dinitrophenyl) (40) prepared as described in example 46 with maleimide using
the procedure
described in example 69gave 4-(3,5-Dinitrophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=3,5-dinitrophenyl,
Rl°=H)
(41) in a 89% yield as a glassy solid which was used without further
purification.
EXAMPLE 48
The preparation of 4-(3 5-Dinitrophenyl)-9-methoxypyrrolof3 4-clcarbazole-
1,3(2H,6H)-
dione (V; Ar=3,5-dinitrophenyl, R1°=H) (42)
The reaction of 4-(3,5-Dinitrophenyl)-9-methoxy-4,5,6, lOc-
tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione (IV; Ar=3,5-dinitrophenyl, R=H) (41) prepared as
described
in example 47 with DDQ according to the procedure described in example 70 gave
4-(3,5-
dinitrophenyl)-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=3,5-
dinitrophenyl, RI°=H) (42) in a 42% yield as a reddish solid which was
used without further
purification.
EXAMPLE 49
The preparation of 4-(3 5-Dinitrophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (V~ Ar=3,5-dinitrophenyl, Rl°=H) (43)
The reaction of 4-(3,5-dinitrophenyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (V; Ar=3,5-dinitrophenyl, R'°=H) (42) prepared as described in
example 48 with
pyridinium hydrochloride according to the proceedure described in example 81
gave 4-(3,5-
Dinitrophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=3,5-
dinitrophenyl, Rl°=H) (43) in a 57% yield as an orange powder, mp >330
°C.'H NMR 8
[(CD3)ZSO] 11.94 (br s, 1H), 11.18 (br s, 1H), 9.32 (br s, 1H), 8.91 (s, 3H),
8.35 (d, J=2.3 Hz,
1H), 7.83 (s, 1H), 7.49 (d, J=8.7 Hz, 1H), 7.11 (dd, J=8.7, 2.3 Hz, 1H).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-72-
EXAMPLE 50
The preparation of 5-Methoxy-2-f2-(2-methoxyphenyl)ethenyll-IH-indole (II;
Ar=2-
methoxyphenyl) (97)
The reaction of 5-methoxy-1H-indole-2-carbaldehyde (1) with 2-
methoxybenzyltriphenylphosphonium chloride using the procedure described in
example 37
gave 5-methoxy-2-[2-(2-methoxyphenyl)ethenyl]-1H-indole (II; Ar=2-
methoxyphenyl) (97)
in a 98 % yield as a yellow solid (a mixture of E/Z isomers) which was used
without further
purification.
EXAMPLE 51
The preparation of I-(3-1(tert-Butyl(dimethyl)silylloxy)propyl)-5-methoxy-2-f2-
(2-
methoxyphen~)ethenyll-IH-indole (III; Ar=2-methoxyphenyl,
R'°=CHzCH2CH~OSiMe~t-
Bu 98
Reaction of 5-Methoxy-2-[2-(2-methoxyphenyl)ethenyl]-1H-indole (II; Ar=2-
methoxyphenyl) (97) prepared as in example 50 with 3-bromopropyl tert-
butyl(dimethyl)silyl ether using the procedure described in example 38 gave 1-
(3-{ [tert-
Butyl(dimethyl)silyl]oxy }propyl)-5-methoxy-2-[2-(2-methoxyphenyl)ethenyl]-1H-
indole
(III; Ar=2-methoxyphenyl, R'°=CHZCH2CHZOSiMe2t-Bu) (98) in a 97% yield
which was
used without further purification.
EXAMPLE 52
The Qreparation of 6-(3-( (tert-Butyl(dimethyl)silylloxy lpropyl)-9-methoxy-4-
(2-
methoxyphenyl)-4,5,6,1Oc-tetrahydropyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione
(IV; Ar=2-
methoxyphenyl; R'°=CH~CHzCH~OSiMe~t-Bu 99
The reaction of 1-(3-{ [tert-Butyl(dimethyl)silyl]oxy}propyl)-5-methoxy-2-[2-
(2-
methoxyphenyl)ethenyl]-1H-indole (III; Ar=2-methoxyphenyl,
RI°=CH2CH2CHZOSiMe2t-
Bu) (98) prepared as described in example 51 with maleimide using the
procedure described
in example 68 gave 6-(3-{ [tert-Butyl(dimethyl)silyl)oxy }propyl)-9-methoxy-4-
(2-
methoxyphenyl)-4,5,6,1Oc-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione
(N; Ar=2-
methoxyphenyl; R'°=CHzCHzCHZOSiMe2t-Bu) (99 ) in a 89% yield as a tan
powder, which
was used without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-73-
EXAMPLE 53
The preparation of 6-(3-HydroxyproRyl)-9-methoxy-4-(2-
methoxyphenyl)pyrrolof3,4
c~carbazole-1 3(2H 6H)-dione (V' Ar=2-methoxyphenyl, R1°=CH2CH2CH~OH)
(100).
The reaction of 6-(3-{ [tert-Butyl(dimethyl)silyl]oxy]propyl)-9-methoxy-4-(2
methoxyphenyl)-4,5,6,1Oc-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione
(IV; Ar=2-
methoxyphenyl; R'°=CHZCHZCHZOSiMe2t-Bu) prepared as described in
example 52 with
DDQ using the procedure described in example 70 followed reaction with 2N HCl
in
THF/methanol gave 6-(3-Hydroxypropyl)-9-methoxy-4-(2-methoxyphenyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (V; Ar=2-methoxyphenyl, Rl°=CHZCHZCH20H)
(100) in a
72% yield as an orange powder; mp 223-225 °C. 1H NMR 8 [(CD3)ZSO] 10.99
(br s, 1H),
8.53 (d, 2.6 Hz, 1H), 7.74 (s, 1H), 7.67 (d, J=8.9 Hz, 1H), 7.45-7.40 (m, 1H),
7.36 (dd, J=7.4,
1.7 Hz, 1 H), 7.29 (dd, J=8.9, 2.7 Hz, 1 H), 7.11 (d, J=8.1 Hz, 1 H), 7.06
(dd, J=7.2, 7.2 Hz,
1H), 4.63 (t, J=4.8 Hz, 1H), 4.52 (t, J=6.9 Hz, 2H), 3.90 (s, 3H), 3.69 (s,
3H), 3.40 (m, 2H),
1.91 (m, 2H). Found: C, 69.55; H, 5.31; N, 6.30. CZSHzzN20s requires C69.76;
H, 5.15; N,
6.51.
EXAMPLE 54
The preparation of 9-Hydroxy-4-(2-h d~roxyphenyl)-6-(3-
hydroxypropyl)pyrrolof3,4
clcarbazole-1 3(2H 6H)-dione (VI; Ar=2-hydroxyphenyl, R'°=CH?CHZCH~OH)
(101).
The reaction of 6-(3-Hydroxypropy.l)-9-methoxy-4-(2-methoxyphenyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (V; Ar=2-methoxyphenyl, R'°=CHZCHZCHZOH)
(100)
prepared as described in example 53 with BBr3 using the procedure described in
example 80
gave 9-Hydroxy-4-(2-hydroxyphenyl)-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (VI; Ar=2-hydroxyphenyl, Rl°=CHzCH2CH20H) (101) in an
82% yield as
an orange powder, mp 306-309 °C.'H NMR 8 [(CD3)ZSO] 10.90 (br, 1H),
9.40 (br, 1H), 8.38
(d, J=2.4 Hz, IH), 7.67 (s, 1H), 7.56 (d, J=8.8 Hz, 1H), 7.30-7.23 (m, 2H),
7.13 (dd, J=8.8,
2.4 Hz, 1H), 6.94-6.88 (m, 2H), 4.78 (br, 2H), 4.47 (t, J=6.8 Hz, 2H), 3.41
(t, J=6.0 Hz, 2H),
1.91 (m, 2H). Found: C, 66.85; H, 4.61; N, 6.63. C23H18N205.1/2H20 requires C,
67.14; H,
4.65; N, 6.80.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-74-
EXAMPLE 55
The~reparation of 2-f2-(2-Chloro-6-methoxyphenyl)ethenyll-5-methoxy-1H-indole
(II;
Ar=2-chloro-6-methoxyphenyl) (102)
The reaction of 5-methoxy-1H-indole-2-carbaldehyde (1) with 2-chloro-6-
methoxybenzyltriphenylphosphonium bromide using the procedure described in
example 37
gave 2-[2-(2-Chloro-6-methoxyphenyl)ethenyl]-5-methoxy-1H-indole (II; Ar=2-
chloro-6-
methoxyphenyl) (102) in a 92% yield as a yellow solid (a mixture of E/Z
isomers) which was
used without further purification.
EXAMPLE 56
The preparation of 1-(3-(ftert-Butyl(dimethyl)silylloxy}propel)-2-f2-(2-chloro-
6-
methoxyphenyl ethenyll-5-methoxy-1H-indole (III; Ar=2-chloro-6-methoxynhenyl,
R'°=CHZCH~CH~OSiMe~t-Bu) (103)
The reaction of 2-[2-(2-Chloro-6-methoxyphenyl)ethenyl]-5-methoxy-1H-indole
(II; Ar=2-
chloro-6-methoxyphenyl) (102) prepared as described in example 55 with 3-
bromopropyl
tert-butyl(dimethyl)silyl ether using the procedure described in example 38
gave 1-(3-{ [tert-
Butyl(dimethyl)silyl]oxy }propyl)-2-[2-(2-chloro-6-methoxyphenyl)ethenyl]-5-
methoxy-1H-
indole (III; Ar=2-chloro-6-methoxyphenyl, R'°=CHZCHZCHZOSiMezt-Bu)
(103) in a 91 %
yield which was used without further purification.
EXAMPLE 57
The preparation of 6-(3-( (tert-Butyl(dimethyl)sil l~y }propel)-4-(2-chloro-6-
methoxyphenyl)-9-methoxy-4 5 6 lOc-tetrahydropyrrolof3,4-clcarbazole-
1,3(2H,3aH)-dione
~IV~ Ar=2-chloro-6-methoxyphenyl, R'°=CHzCH~CH?OSiMe~t-Bu) (104).
The reaction of 1-(3-{ [tert-Butyl(dimethyl)silyl]oxy}propyl)-2-[2-(2-chloro-6-
methoxyphenyl)ethenyl]-5-methoxy-1H-indole (III; Ar=2-chloro-6-methoxyphenyl,
R'°=CHZCHZCHZOSiMe2t-Bu) (103)~repared as described in example 56 with
maleimide
using the procedure described in example 68 gave 6-(3-{ [tert-
Butyl(dimethyl)silyl]oxy }propyl)-4-(2-chloro-6-methoxyphenyl)-9-methoxy-
4,5,6, lOc-
tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-chloro-6-
methoxyphenyl;
R'°=CH2CHZCHZOSiMe2t-Bu) (104) in a 77% yield as a cream powder, which
was used
without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-75-
EXAMPLE 58
The Dreparation of 4-(2-Chloro-6-methoxyphenyl)-6-(3-hydroxypropyl)-9-
methoxypyrrolof3 4-clcarbazole-1 3(2H 6H)-dione (V; Ar=2-chloro-6-
methoxyphenyl,
R'°=CH2CH~CH~OH) (105).
The reaction of 6-(3-{ [tert-Butyl(dimethyl)silyl]oxy}propyl)-4-(2-chloro-6-
methoxyphenyl)-9-methoxy-4,5,6, l Oc-tetrahydropyrrolo[3,4-c]carbazole-
1,3(2H,3aH)-dione
(IV; Ar=2-chloro-6-methoxyphenyl, R'°=CHZCHZCHZOSiMeZt-Bu) (104)
prepared as
described in example 57 with DDQ using the procedure described in example 70
followed
by reaction with 2N HCI in THF/methanol gave 4-(2-Chloro-6-methoxyphenyl)-6-(3-
hydroxypropyl)-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-
chloro-6-
methoxyphenyl, R1°=CHzCH2CH20H) (105) in a 76% yield as an orange
powder; mp 224-
227 °C.'H NMR 8 [(CD3)2S0] 11.05 (br, 1H), 8.51 (d, J=2.6 Hz, 1H), 7.75
(s, 1H), 7.69 (d,
J=8.9 Hz, 1 H), 7.44 (dd, J=8.2, 8.2 Hz, 1 H), 7.31 (dd, J=8.9, 2.6 Hz, 1 H),
7.18 (d, J=8.2 Hz,
1H), 7.13 (d, J-8.2 Hz, 1H), 4.63 (t, J=4.9 Hz, 1H), 4.51 (t, J=6.8 Hz, 2H),
3.91 (s, 3H), 3.68
(s, 3H), 3.41 (m, 2H), 1.89 (m, 2H). Found: C, 69.71; H, 4.53; N, 6.43.
CZSH22NzOs requires
C, 69.76; H, 5.15; N, 6.51.
EXAMPLE 59
The preparation of 4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(3-
hydroxypropyl)pyrrolo[3 4-clcarbazole-1 3(2H 6H)-dione (VI' Ar=2-chloro-6-
methoxyphenyl, R'°=CH~CH~CH~OH) (106).
The reaction of 4-(2-Chloro-6-methoxyphenyl)-6-(3-hydroxypropyl)-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chloro-6-
methoxyphenyl,
R'°=CH2CHZCHzOH) (105) prepared as described in example 58 with BBr3
using the
procedure described in example 80 and with a reaction time of 90 minutes gave
4-(2-Chloro-
6-methoxyphenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(VI; Ar=2-chloro-6-methoxyphenyl, R'°=CHZCH2CHzOH) (106) in a 64 %
yield as an orange
powder, mp 270-273 °C.'H NMR 8 [(CD3)ZSO] 11.01 (br s, 1H), 9.35 (br s,
1H), 8.37 (d,
J=2.4 Hz, 1H), 7.70 (s, 1H), 7.58 (d, J=8.7 Hz, 1H), 7.44 (dd, J=8.3, 8.3 Hz,
1H), 7.20-7.11
(m, 3H), 4.61 (br t, 1H), 4.47 (t, J=6.9 Hz, 2H), 3.68 (s, 3H), 3.42 (m, 2H),
1.88 (m, 2H).
Found: C, 64.16; H, 4.55; N, 6.01. C24H,9C1N205 requires C, 63.93; H, 4.25; N,
6.21.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-76-
EXAMPLE 60
The preparation of 9-Hydroxy-6-(3-hydroxypropyl)-4-(2-methoxyphen~pyrrolof 3 4-
lcarbazole-1,3(2H,6H)-dione (VI; Ar=2-methoxyphenyl, R1°=CH~CH2CH~OH)
(107).
4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (VI; Ar=2-chloro-6-methoxyphenyl,
R'°=CHZCHZCHzOH)
(106) (0.20 g, 0.44 mmol) prepared as described in example 59, potassium
acetate (0.20 g)
and S % Pd/C in a solution of ethyl acetate (25m1) and methanol 1:1 (25 mL)
under a
atmosphere of hydrogen at 60 psi was reacted for 7 h. The catalyst was
filtered off and the
filtrate concentrated to dryness. The residue was partitioned between ethyl
acetate and water
and the organic solution was dried, the drying agent was removed and the
solution was
concentrated to dryness and chromatographed on silica. Elution with ethyl
acetate/petroleum
ether (2:1) gave 9-Hydroxy-6-(3-hydroxypropyl)-4-(2-methoxyphenyl)pyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (VI; Ar=2-methoxyphenyl, R'°=CHZCHZCHZOH)
(107) in a
yield of 51 % as an orange powder (from THF/petroleum ether), mp 296-300
°C. 1H NMR 8
[(CD3)zS0] 10.93 (br s, 1H), 9.31 (br s, 1H), 8.38 (d, J=2.4 Hz, 1H), 7.69 (s,
1H), 7.55 (d,
J=8.8 Hz, 1 H), 7.42 (m, 1 H), 7.35 (dd, J=7.5, 1.7 Hz, 1 H), 7.13-7.08 (m,
2H), 7.05 (dd,
J=7.2, 7.2 Hz, 1H), 4.62 (t, J=4.8 Hz, 1H), 4.48 (t, J=6.8 Hz, 2H), 3.68 (s,
3H), 3.40 (m, 2H),
1.90 (m, 2H). Found: C, 69.29; H, 4.90; N; 6.54. Cz4HzoNzOs requires C, 69.22;
H, 4.84; N,
6.73.
EXAMPLE 61
The preparation of 2-(2-(2-(2-Chloro-6-methoxyphenyl)ethenyll-5-methoxy-1H-
indol-1-
vllethanol (III Ar=2-chloro-6-methoxvphenyl, RI°=CH~CH~OH) (109)
The reaction of 2-[2-(2-Chloro-6-methoxyphenyl)ethenyl]-5-methoxy-1H-indole
(II;
.~.r=2-chloro-6-methoxyphenyl) (102) prepared as described in example 55 with
2-
bromoethyl tetrahydro-2H-pyran-2-yl ether using the procedure described in
example 38
followed by reaction of the crude product with 2N HCl in methanol gave 2-( 2-
[2-(2-Chloro-
6-methoxyphenyl)ethenyl]-5-methoxy-1H-indol-1-yl}ethanol (III; Ar=2-chloro-6-
methoxyphenyl, R'°=CHZCHZOH) (109) in a 91 % yield as a mixture of E/Z
isomers, which
was used without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
_77_
EXAMPLE 62
The preparation of 4-(2-Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-methoxy-
4,5,6,1Oc-
tetrahydropyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (IV; Ar=2-chloro-6-
methoxyphenyl,
R'°=CH~CH~OH) (110).
Reaction of the 2-{2-[2-(2-Chloro-6-methoxyphenyl)ethenyl]-5-methoxy-1H-indol-
1-
yl}ethanol (III; Ar=2-chloro-6-methoxyphenyl, R=CHZCHZOH) (109) prepared as
described
in example 61 with maleimide using the procedure described in example 68 gave
4-(2-
Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-methoxy-4,5,6, l Oc-
tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-chloro-6-methoxyphenyl,
R'°=CHZCHZOH) (110)
in a 72% yield as a cream powder, which was used without further purification.
EXAMPLE 63
The preparation of 4-(2-Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-
methoxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (V; Ar=2-chloro-6-
methoxyphenyl,
R 1 °=CH2CH~0 111
The reaction of 4-(2-Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-methoxy-
4,5,6,1Oc-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-chloro-
6-
methoxyphenyl, R'°=CHZCHZOH) (110) prepared as described in example 62
with Mn02
using the procedure described in example 79 except that the reaction time was
8 h 4-(2-
Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (V; Ar=2-chloro-6-methoxyphenyl, R'°=CH2CHZOH) (111)
in a 75 %
yield as a yellow/orange powder; mp 264-268 °C. 1H NMR 8 [(CD3)ZSO]
11.04 (br s, 1H),
8.50 (d, J=2.6 Hz, 1H), 7.75 (s, 1H), 7.69 (d, J=9.0 Hz, 1H), 7.44 (dd, J=8.3,
8.3 Hz, 1H),
7.28 (dd, J=9.0, 2.6 Hz, 1 H), 7.18 (d, J=8.3 Hz, 1 H), 7.13 (d, J=8.3 Hz, 1
H), 4.83 (t, J=5.5
Hz, 1H), 4.51 (t, J=5.3 Hz, 2H), 3.90 )s, 3H), 3.75 (m, 2H), 3.67 (s, 3H).
Found: C, 63.84; H,
4.32; N, 5.97. C24H~9C1N205 requires C, 63.93; H, 4.25; N, 6.21.
EXAMPLE 64
The preparation of 4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(2-
hydroxyethyl)pyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VI; Ar-2-chloro-6-methoxyphenyl,
R'°=CH2CH~OH) (112)
The reaction of 4-(2-Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chloro-6-
methoxyphenyl,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
_78_
R1°=CHZCHzOH) (111) prepared as described in example 63 with BBr3 using
the procedure
described in example 80 except that the reaction conditions were 2 h at 0
°C gave 4-(2-
Chloro-6-methoxyphenyl)-9-hydroxy-6-(2-hydroxyethyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (VI; Ar-2-chloro-6-methoxyphenyl, R'°=CHZCHZOH) (112)
in a 56% yield
as a yellow powder (56 %); mp 275-278 °C.'H NMR 8 [(CD3)zS0] 10.98 (br
s, 1H), 9.30 (br
s, 1H), 8.36 (d, J=2.4 Hz, 1H), 7.70 (s, 1H), 7.57 (d, J=8.7 Hz, 1H), 7.43
(dd, J=8.3, 8.3 Hz,
1 H), 7.17 (d, J=7.9 Hz, 1 H), 7.14-7.09 (m, 2H), 4.81 (t, J=5.5 Hz, 1 H),
4.46 (t, J=5.3 Hz,
2H), 3.74 (dt, J=5.5, 5.3 Hz, 2H), 3.66 (s, 3H). FABMS found [M+H]+: 439.0882,
437.0889.
C23H~8C1N205 requires 439.0875, 437.0904.
EXAMPLE 65
The preparation of 9-Hydroxy-6-(2-hydroxyethyl)-4-(2-methoxyphenyl)pyrrolo(3,4-
~carbazole-1,3(2H,6H)-dione (VI; Ar=2-methoxyphenyl, R'°=CH~CH~OH)
(113)
Reaction of 4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-(2-
hydroxyethyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (VI; Ar-2-chloro-6-
methoxyphenyl,
R1°=CHZCHZOH) (112) prepared as described in example 64 with hydrogen
gas, using a
Pd/C catalyst, according to the procedure for example 60 gave 9-Hydroxy-6-(2-
hydroxyethyl)-4-(2-methoxyphenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (VI;
Ar=2-
methoxyphenyl, R'°=CHZCHZOH) (113) in a 77 % yield as a yellow solid;
mp 285-289 °C.
'H NMR 8 [(CD3)ZSO] 10.91 (br s, 1H), 9.29 (br s, 1H), 8.37 (d, J=2.4 Hz, 1H),
7.70 (s, 1H),
7.55 (d, J=8.8 Hz, 1H), 7.42 (m, 1H), 7.33 (dd, J=8.8, 2.4 Hz, 1H), 7.12-7.02
(m, 3H), 4.84 (t,
J=5.5 Hz, 1H), 4.73 (t, J=5.3 Hz, 2H), 3.76 (dt, J=5.5, 5.3 Hz, 2H), 3.68 (s,
3H). Found: C,
67.30; H, 4.47; N, 6.79. C23H~$N205.1/2H20 requires C, 67.15; H, 4.65; N,
6.81.
EXAMPLE 66
The preparation of 2-(4-(2-Chloro-6-methoxyphen 1~)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-clcarbazol-6(1H)-yl)ethyl methanesulfonate (VII; Ar=2-
chloro-6-
metho~phen~, n=2, mesylate) (114).
Reaction of 4-(2-Chloro-6-methoxyphenyl)-6-(2-hydroxyethyl)-9-
methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-chloro-6-
methoxyphenyl,
R'°=CHZCH20H) (111) prepared as described in example 63 with
methanesulphonyl
chloride, followed by reaction with BBr3 using the procedure described in
example 170 of
Scheme 3 gave 2-(4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-1,3-dioxo-2,3-
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-79-
dihydropyrrolo[3,4-c]carbazol-6(1H)-yl)ethyl methanesulfonate (VII; Ar=2-
chloro-6-
methoxyphenyl, n=2, mesylate) (114). as a yellow solid, which was used without
further
purification.
EXAMPLE 67
The preparation of 4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-6-f2-(4-
morpholinyl)ethyllpyrrolof3 4-clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2-chloro-
6-
methoxyphenyl, n=2, Z=4-morpholinyl) (115).
Reaction of 2-(4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolo[3,4-c]carbazol-6(1H)-yl)ethyl methanesulfonate (VII; Ar=2-
chloro-6-
methoxyphenyl, n=2, mesylate) (114) prepared as described in example 66 with
morpholine
using the procedure described in example 179 of Scheme 3 gave 4-(2-Chloro-6-
methoxyphenyl)-9-hydroxy-6-[2-(4-moipholinyl)ethyl]pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (VIII; Ar=2-chloro-6-methoxyphenyl, n=2, Z=4-morpholinyl) (115) in an
81% yield as
a yellow powder; mp 185 °C.'H NMR 8 ((CD3)ZSO] 10.99 (br s, 1H), 9.34
(br s, 1H), 8.37
(d, J=2.4 Hz, 1H), 7.69 (s, 1H), 7.57 (d, J=8.8 Hz, 1H), 7.44 (dd, J=8.3, 8.3
Hz, 1H), 7.17 (d,
J=8.3 Hz, 1H), 7.14-7.10 (m, 2H), 4.53 (t, J=6.1 Hz, 2H), 3.66 (s, 3H), 3.41
(t, J=4.5 Hz, 4H),
2.64 (t, J=6.1 Hz, 2H), 2.37 (m, 4H). Found: C, 63.57; H, 4.71; N, 8.06.
CZ~H24C1N305.1/4H20 requires C, 63.53; H, 4.84; N, 8.23.
Representative Procedure for Method 4 of Scheme 2
EXAMPLE 68
The preparation of 4-(2-Chlorophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3,4-
clcarbazole-1 3(2H 3aH)-dione (N; Ar=2-chlorophenyl, R'°=H) (32)
A solution of 2-(2-(2-Chlorophenyl)ethenyl]-5-methoxy-1H-indole (II; Ar=2-
chlorophenyl) (27) (1.5 g, 5.29 mmol) prepared according to example 37,
maleimide (0.61 g,
6.34 mmol) and SnCl2 (0.20 g, 1.05 mmol) in toluene (25 mL) was refluxed for 6
h. After
dilution with ethyl acetate the solution was washed with water and The organic
phase was
dried, the drying agent was removed and the solution was concentrated to
dryness to give 4-
(2-Chlorophenyl)-9-methoxy-4,5,6, l Oc-tetrahydropyrrolo(3,4-c]carbazole-
1,3(2H,3aH)-dione
(IV; Ar=2-chlorophenyl, R'°=H) (32) in a yield of 1.98 g , 98% as a
yellow solid (as a
mixture of diastereomers), which was used without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-80-
Representative Procedure for Method 5 of Scheme 2
EXAMPLE 69
The nrenaration of 9-Methoxv- 4-(2-methoxy-5-nitrophenyl)-4,5,6,IOc-
tetrahydronyrrolo[3 4-
~carbazole-1 3(2H 3aH)-dione (N; Ar=2-methoxy-5-nitrophenyl, R'°=H)
(185)
A solution of 5-methoxy-2-[2-(2-methoxy-5-nitrophenyl)ethenyl]-1H-indole (II;
Ar=2-methoxy-5-nitrophenyl) (0.437 g, 1.35 mmol) prepared as described in
example 45 and
maleimide (0.156 g, 1.62 mmol) in THF (10 mL) was concentrated to dryness in a
25 mL
flask. The resulting solid was placed in an oil bath at 180 °C and the
resulting melt was kept
at this temperature for 4 h. The residue was cooled and triturated with
diethyl ether to give 9-
methoxy-4-(2-methoxy-5-nitrophenyl)-4,5,6, l Oc-tetrahydropyrrolo[3,4-
c]carbazole-
1,3(2H,3aH)-dione (IV; Ar=2-methoxy-5-nitrophenyl, R'°=H) (185) in a
yield of 0.51 g, 90%
as a tan powder, which was used without further purification in the next step.
Representative Procedure for Method 6 of Scheme 2
EXAMPLE 70
The preparation of 9-Methoxy-4-(2-methox~5-nitrophenyl)pyrrolo(3,4-clcarbazole-
1 3(2H 6H)-dione (V; Ar=2-methoxy-5-nitrophenyl, R'°=H) (36)
A solution of 9-methoxy-4-(2-methoxy-5-nitrophenyl)-4,5,6,1Oc-
tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-methoxy-5-
nitrophenyl,
R'°=H) (185) (0.51 g, 1.21 mmol) prepared according to example 69 and
DDQ (0.82 g, 3.63
mmol) in dioxane (30 mL) was refluxed for 2 h. After dilution with water the
solution was
extracted with dichloromethane. The organic extracts were washed with
saturated aqueous
NaHC03 solution (3x). The organic phase was dried, the drying agent was
removed and the
solution was concentrated to dryness to give a solid which was chromatographed
on silica.
Elution with ethyl acetate/petroleum ether (3:7) followed by ethyl
acetate/petroleum ether
(1:1) gave a solid which when triturated with diethyl ether gave 9-Methoxy-4-
(2-methoxy-5-
nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-methoxy-5-
nitrophenyl,
R'°=H) (36) in a 0.42 g, 82% yield; mp 302-304 °C.'H NMR 8
[(CD3)ZSO] 11.95 (br s, 1H),
11.06 (br s, 1 H), 8.46 (d, J=2.6 Hz, 1 H), 8.37 (dd, J=9.2, 3.0 Hz, 1 H),
8.22 (d, J=3.0 Hz, 1 H),
7.69 (s, 1H), 7.57 (d, J=8.8 Hz, 1H), 7.36 (d, J=9.2 Hz, 1H), 7.24 (dd, J=8.8,
2.6 Hz, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-81-
3.89 (s, 3H), 3.85 (s, 3H). Found: C, 63.90; H, 3.79; N, 9.52. CzzH~5N306
requires C, 63.61;
H, 3.62; N, 10.07.
EXAMPLE 71
The nrenaration of 9-Hvdroxv-4-(2-hvdroxy-5-nitrophenvl)pvrrolo 3 4-c
carbazole-
1 3(2H 6H)-dione (VI; Ar=2-hydrox~5-nitrophenyl, R'°=H) (37)
9-Methoxy-4-(2-methoxy-5-nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
{V; Ar=2-methoxy-5-nitrophenyl, R=H) (36)~repared according to example 70 was
reacted
using the procedure described in example 81 by reaction at 210 °C for
30 min, to give 9-
hydroxy-4-(2-hydroxy-5-nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(VI; Ar=2-
hydroxy-5-nitrophenyl, R'°=H) (37) in a 82 % yield as a yellow/orange
powder; mp >330 °C.
'H NMR ~ [(CD3)ZSO] 11.78 (s, 1H), 10.99 (s, 1H), 9.26 (s, 1H), 8.32 (d, J=2.4
Hz, 1H),
8.21-8.16 (m, 2H), 7.63 (s, 1H), 7.45 (d, J=8.7 Hz, 1H), 7.08 (m, 2H).
EXAMPLE 72
The preparation of 9-Hydroxy-4-(2-methoxy-5-nitrophenyl)pyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (VI; Ar=2-methoxy-5-nitrophenyl, R'°=H) (38)
9-Methoxy-4-(2-methoxy-5-nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(V; Ar=2-methoxy-5-nitrophenyl, R'°=H) (36) prepared according to
example 70 was
reacted using the procedure described in example 80 to give 9-hydroxy-4-(2-
methoxy-5-
nitrophenyl)pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (VI; Ar=2-methoxy-5-
nitrophenyl,
R'°=H) (38) in a 76% yield as a yellow powder; mp 265-273 °C
(dec).'H NMR 8
[(CD3)zS0] 11.86 (br, 1H), 11.01 (br, 1H), 8.38 (dd, J=9.3, 2.8 Hz, 1H), 8.31
(d, J=2.4 Hz,
iH), 8.20 (d, J=2.8 Hz, 1H), 7.65 (s, lh), 7.48 (d, J=8.7 Hz, 1H), 7.36 (d,
J=9.3 Hz, 1H), 7.10
(dd, J=8.7, 2.4 Hz, 1H), 3.84 (s, 3H). EIMS found M+: 403.0794. CZ~H,~N306
requires
403.0804.
EXAMPLE 73
The preparation of 4-(5-Amino-2-methoxvphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (VI; Ar=5-amino-2-methoxyphenyl, R'°=H) (39)
To a solution of 9-hydroxy-4-(2-methoxy-5-nitrophenyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (VI; Ar=2-methoxy-5-nitrophenyl, R'°=H) (38) (64 mg,
0.159 mmol)
prepared according to example 72 in methanol (10 mL) and THF (1 mL) was added
2N HCl
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-82-
(1 mL) Freshly prepared nickel boride 00.50 g) was added and the mixture was
warmed at
60 °C. After 1 h HCl (1 mL) and NiZB 00.50 g) were added and the
reaction mixture was
maintained at 60°C a total of 4 h. The solvents were removed in vacuo
and the residue was
partitioned between ethyl acetate and 2N HCI. The aqueous layer was basified
with conc.
ammonia solution and extracted with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solvent concentrated to dryness gave a solid which
when
crystallised from THF/petroleum ether gave 4-(5-Amino-2-methoxyphenyl)-9-
hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (VI; Ar=5-amino-2-
methoxyphenyl,
R'°=H) (39) in a yield of 49.7 mg, 84% as a yellow powder; mp 246-250
°C.'H NMR 8
[(CD3)ZSO] 11.67 (s, 1H), 10.87 (s, 1H), 9.21 (s, 1H), 8.30 )d, J=2.4 Hz, 1H),
7.44 (s, 1H),
7.42 (d, J=8.7 Hz, 1H), 7.05 (dd, J=8.6, 2.5 Hz, 1H), 6.80 (d, J=8.7 Hz, 1H),
6.61 (dd, J=8.6,
2.7 Hz, 1H), 6.55 (d, J=2.8 Hz, 1H), 4.68 (br, 2H), 3.53 (s, 3H). FABMS found
[M+H]+:
373.1056. C2~H~SN304 requires 373.1062.
EXAMPLE 74
'The preparation of 2-f(E)-2-(2-Bromo-4-nitrophenyl)ethenyll-S-methoxy-1H-
indole (II,
Ar=2-bromo-4-nitrophenyp (49)
Reaction of 5-methoxy-1H-indole-2-carbaldehyde (1) with 2-bromo-4-
nitrobenzyltriphenylphosphonium bromide using the procedure described in
example 37
gave the dime 2-[(E)-2-(2-Bromo-4-nitrophenyl)ethenyl]-5-methoxy-1H-indole
(II, Ar=2-
bromo-4-nitrophenyl) (49) in a 98 % yield as a red solid which was used
without further
purification.
EXAMPLE 75
The ureparation of 4-(2-Bromo-4-nitrophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3,4-
clcarbazole-1,3(2H,3aH)-dione (IV; Ar=2,bromo-4-nitrophenyl, R'°=H)
(50)
Reaction of 2-[(E)-2-(2-Bromo-4-nitrophenyl)ethenyl]-5-methoxy-1H-indole (II,
Ar=2-bromo-4-nitrophenyl) (49) prepared as described in example 74 with
maleimide using
the procedure described in example 69 gave 4-(2-Bromo-4-nitrophenyl)-9-methoxy-
4,5,6,1Oc-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2,bromo-
4-
nitrophenyl, R1°=H) (50) in a 54% yield as a tan powder; mp 272
°C (dec), which was used
without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-83-
EXAMPLE 76
The preQaration of 4-(2-Bromo-4-nitrophenyl)-9-methoxypyrrolof3,4-clcarbazole-
1,3(2H 6H)-dione (V; Ar=2-bromo-4-nitrophenyl, R'°=H) (51).
Reaction of 4-(2-bromo-4-nitrophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolo[3,4-
c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2,bromo-4-nitrophenyl, R'°=H)
(50) prepared as
described in example 75 with DDQ using the procedure described in example 70
gave 4-(2-
bromo-4-nitrophenyl)-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V;
Ar=2-bromo-
4-nitrophenyl, R'°=H) (51). in a 67% yield as a yellow powder, mp 288-
292 °C (dec).'H
NMR 8 [(CD3)ZSO] 12.07 (br, 1H), 11.18 (br, 1H), 8.56 (d, J=2.3 Hz, 1H), 8.46
(d, J=2.6 Hz,
1H), 8.33 (dd, J=8.5, 2.6 Hz, 1H), 7.80 (d, J=8.5 Hz, 1H), 7.63 (s, 1H), 7.59
(d, J=8.8 Hz,
-- 1H), 7:27 (dd, J=8.8, 2.3 Hz, 1H), 3.90 (s, 3H). Found: C, 54.06; H, 2.59;
N, 8.72.
CZ~Hi2BrN305 requires C, 54.10; H, 2.59; N, 9.01.
EXAMPLE 77
The preparation of 4-(2-Bromo-4-nitrophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1 3(2H 6H)-dione (VI; Ar=2-bromo-4-nitrophenyl, Rl°=H) (52).
Reaction of 4-(2-bromo-4-nitrophenyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (V; Ar=2-bromo-4-nitrophenyl, R'°=H) (51) prepared as
described in
example 76 with pyridinium hydrochloride using the procedure described in the
proceedure
described in example 81 gave 4-(2-bromo-4-nitrophenyl)-9-hydroxypyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (VI; Ar=2-bromo-4-nitrophenyl, Rl°=H) (52) in a 92 %
yield as a yellow
powder; mp >330 °C.'H NMR 8 [(CD3)ZSO] 11.94 (s, 1H), 11.13 (s, 1H),
9.32 (br s, 1H),
8.55 (d, J=2.3 Hz, 1H), 8.34 (d, J=2.4 Hz, 1H), 8.31 (m, 2H), 7.79 (d, J=8.5
Hz, 1H), 7.58 (s,
1H), 7.48 (d, J=8.7 Hz, 1H), 7.11 (dd, J=8.7, 2.4 Hz, 1H).
EXAMPLE 78
The preparation of 4-(4-Amino-2-bromophenyl)-9-h d~ypyrrolof3,4-clcarbazole-
1.3(2H,6H)-dione (VI; Ar=4-aamino-2-bromophenyl, R'°=H) (53).
Freshly prepared nickel boride (5.00 g, 0.039 mol) was added in portions to a
solution
of 4-(2-Bromo-4-nitrophenyl)-9-hydroxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(VI;
Ar=2-bromo-4-nitrophenyl, R'°=H) (52) (0.50 g, 1.10 mmol) prepared as
described in
example 77 in methanol (80 mL) and 2N HCI (5.0 mL) at 60 °C. After 2 h
the mixture was
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-84-
cooled, diluted with water, basified with conc. aqueous ammonia and extracted
with ethyl
acetate. The extract was worked up to give a solid which was chromatographed
on silica.
Elution with ethyl acetate gave 4-(4-amino-2-bromophenyl)-9-hydroxypyrrolo[3,4-
c]carbazole-1,3(2H,6H)-dione (VI; Ar=4-amino-2-bromophenyl, R'°=H) (53)
in a 0.47 g, 97
% yield which crystallised from ethyl acetate/petroleum ether as a yellow
powder, mp 324-
326 °C.'H NMR 8 [(CD3)ZSO] 11.71 (br s, 1H), 10.93 (br s, 1H), 9.23 (br
s, 1H), 8.30 (d,
J=2.4 Hz, 1H), 7.43 (d, J=8.6 Hz, 1H), 7.42 (s, 1H), 7.09-7.03 (m, 2H), 6.89
(d, J=2.1 Hz,
1 H), 6.61 (dd, J=8.6, 2.4 Hz, 1 H), 5.48 (br s, 2H). Found: C, 56.79; H,
3.26; N, 8.77.
CZ°H~zBrN303.1/2EtOAc requires C, 56.67; H, 3.46; N, 9.01.
Representative Procedure for Method 7 of Scheme 2
EXAMPLE 79
The preparation of 4-(2-Chlorophenyl)-9-methoxyRyrrolo(3,4-clcarbazole-
1,3(2H,6H)-dione
(V: Ar=2-chloronhenvl, R'°=H) (33)
Manganese dioxide (12.0 g) was added to a solution of 4-(2-Chlorophenyl)-9-
methoxy-4,5,6,1Oc-tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV;
Ar=2-
chlorophenyl, Rl°=H) (32) prepared as described in example 68 (2.1 g,
5.51 mmol) in
dioxane (100 mL) and the mixture was refluxed with stirring for 16 h. The
mixture was
filtered hot through a plug of Celite, washing through with more dioxane and
then 10%
methanol/dioxane. The combined filtrate and washings were concentrated to
dryness and the
residue was adsorbed onto silica and chromatographed. Elution with ethyl
acetate/petroleum
ether (3:7) gave 4-(2-Chlorophenyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(V; Ar=2-chlorophenyl, R1°=H) (33) in a yield of 1.66 g, 79 % which
crystallised from
THF/petroleum ether as a yellow powder, mp 170-175 °C (dec). 1H NMR 8
[(CD3)ZSO 11.96
(br s, 1H), 11.08 (br s, 1H), 8.46 (d, J=2.6 Hz, 1H), 7.60-7.55 (m, 3H), 7.51-
7.42 (m, 3H),
7.24 (dd, J=8.9, 2.6 Hz, 1H), 3.89 (s, 3H). Found: C, 65.64; H, 3.63; N, 7.12.
CzlH~3C1N203.1/2Hz0 requires C, 65.37; H, 3.66; N, 7.26.
Representative procedure for Method 8 of Scheme 2
EXAMPLE 80
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo~3,4-
clcarbazole-1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R'°~CH~CH~CH~OH)
(34)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-85-
A solution of 1N BBr3 in CHZCIz (11.5 mL, 0.011 mol) was added to a solution
of 4-
(2-Chlorophenyl)-6-(3-hydroxypropyl)-9-methoxypyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione
(V; Ar=2-chlorophenyl, Rl°=CHZCHZCHzOH) (31) prepared as described in
example 40
(1.00 g, 2.30 mmol) in CHZCIz under nitrogen and the reaction mixture was
stirred at room
temperature for 3 h. Saturated aqueous NaHC03 solution was added and the
solution was
diluted with water and extracted with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solution was concentrated to dryness, adsorbed onto
silica and
chromatographed. Elution with ethyl acetate/petroleum ether (1:1) followed by
ethyl acetate
gave 4-(2-Chlorophenyl)-9-hydroxy-6-(3-hydroxypropyl)pyrrolo[3,4-c]carbazole-
1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R1°=CHZCHZCHZOH) (34) in a
yield of 0.90 g,
93%, which crystallised from THF/petroleum ether as a yellow/orange powder, mp
291-294
°C.'H NMR 8 [(CD3)ZSO 11.05 (br s, 1H), 9.36 (s, 1H), 8.38 (d, J=2.5
Hz, 1H), 7.74 (s, 1H),
7.59-7.54 (m, 2H), 7.52-7.42 (m, 3H), 7.14 (dd, J=8.7, 2.5 Hz, 1H), 4.62 (br
t, 1H), 4.49 (t,
J=6.8 Hz, 2H), 3.41 (m, 2H), 1.90 (m, 2H). Found: C, 64.99; H, 4.13; N, 6.43.
CZ~H1~C1N204.1/4H20 requires C, 64.94; H, 4.15; N, 6.58.
Representative Procedure for Method 9 of Scheme 2
EXAMPLE 81
The preparation of 4-(2-Chlorophenyl)-9-hydroxypyrrolo[3,4-clcarbazole-
1,3(2H,6H)-dione
(VI' Ar=2-chlorophenyl, Rl°=H) (9)
4-(2-Chlorophenyl)-9-methoxypyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (V; Ar=2-
chlorophenyl, RI°=H) (33) prepared as described in example 79 (0.512 g,
1.36 mmol) was
added to a pyridinium hydrochloride melt at 200 °C and the mixture was
stirred at this
temperature for 20 min. Water was added and the resultant precipitate was
filtered off,
washed well with water, adsorbed onto silica and chromatographed. Elution with
ethyl
acetate/petroleum ether (1:1) gave 4-(2-Chlorophenyl)-9-hydroxypyrrolo[3,4-
c]carbazole-
1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R'°=H) (9) in a 0.42 g,
85°lo yield; mp 215-220
°C (dec).'H NMR 8 [(CD3)ZSO] 11.83 (br s, 1H), 11.01 (br s, 1H), 9.27
(br s, 1H), 8.32 (d,
J=2.4 Hz, 1H), 7.65-7.40 m, SH), 7.08 (dd, J=8.7, 2.4 Hz), 1H). Found: C,
65.72; H, 3.50; N,
6.97. C~°H,~CIN203.1/4CH3COOCHZCH3 requires C, 65.54; H, 3.40; N, 7.28.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-86-
EXAMPLE 82
The preparation of 6-(2-Hydroxyethyl)-9-methoxy-4-phenylpyrrolof3,4-
clcarbazole-
1~3(2H 6H)-dione (V; Ar=phenyl, R'°=(CH~~OH) (201)
5-methoxy-2-[(E,Z)-2-phenylethenyl]-1H-indole (II; Ar=phenyl) (1.93 g, 7.74
mmolwas reacted with 2-(2-bromoethoxy)tetrahydro-2H-pyran using the procedure
described
in example 38. This material was reacted directly with maleimide (0.79 g)
using the
procedure described in method 4. The product obtained was reacted using the
procedure
described in example 70 gave crude material that was then dissolved in
methanol (100 mL)
to which p-toluenesulfonic acid (30 mg) was added before the solution was
warmed to 50 °C
for 3h. The solution was then diluted with water and extracted with ethyl
acetate. The organic
phase was dried, the drying agent was removed and the solution was
concentrated to dryness
before being adsorbed onto silica and chromatographed eluting with ethyl
acetate/hexane (1:2
to 1:1) to give 6-(2-Hydroxyethyl)-9-methoxy-4-phenylpyrrolo[3,4-c]carbazole-
1,3(2H,6H)-
dione (V; Ar=phenyl, R'°=(CHz)ZOH) (201) in a yield of 0.51 g, 17% as a
yellow powder;
mp 262-264 °C.'H NMR S [(CD3)ZSO] 11.09 (br s, 1H), 8.56 (d, J=2.6 Hz,
1H), 7.83 (s, 1H),
7.66 (m, 3H), 7.47 (m, 3H), 7.28 (dd, J=9.0, 2.6 Hz, 1H), 4.86 (t, J=5.5 Hz,
1H), 4.55 (t,
J=5.3 Hz, 2H), 3.90 (s, 3H), 3.78 (m, 2H). Found: C, 71.47; H, 4.77; N, 7.32.
C23H,8N204
requires: C, 71.49; H, 4.70; N, 7.25.
EXAMPLE 83
The preparation of 6-(3-Hydroxypropyl)-9-methoxy-4-phenylpyrrolof3,4-
clcarbazole-
1.3(2H,6H)-dione (V; Ar=phenyl, R'°=(CH~~OH) (202)
5-Methoxy-2-[(E,Z)-2-phenylethenyl]-1H-indole (II; Ar=phenyl) (6.85 g, 27.5
mmol)
was reacted with with 3-bromopropyl tert-butyl(dimethyl)silyl ether using the
procedure
described in example 38. The product isolated was reacted directly with
maleimide (5.2 g)
using the procedure described in example 68. Aromatisation of the crude Diels-
Alder adduct
using the procedure described in example 79 gave crude material that was then
dissolved in
methanol (300 mL) to which 1N hydrochloric acid (50 mL) was added. This
solution was
stirred at rt for 3h before being diluted with water and extracted with ethyl
acetate. The
organic layer was dried, the drying agent was removed and the solution was
concentrated to
dryness. The residue was adsorbed onto silica and chromatographed eluting with
dichloromethane to ethyl acetate/dichloromethane (7:3). Trituration with
diethyl ether gave
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-87-
alcohol (202) as a yellow powder (2.55 g, 23%), mp 241-243 °C.'H NMR 8
[(CD3)zS0]
11.10 (br s, 1H), 8.56 (d, J=2.6 Hz, 1H), 7.82 (s, 1H), 7.67 (m, 3H), 7.47 (m,
3H), 7.30 (dd,
J=9.0, 2.6 Hz, 1H), 4.66 (t, J=4.9 Hz, 1H), 4.55 (t, J=6.9 Hz, 2H), 3.90 (s,
3H), 3.39 (m, 2H),
1.93 (m, 2H). Found: C, 71.95; H, 5.09; N, 6.93. Cz4HzoNz4a requires: C,
71.99; H, 5.03; N,
6.99.
EXAMPLE 84
Thepreparation of 6-(6-Hydroxyhexyl)-9-methoxy-4-phenylpyrrolof3,4-clcarbazole-
1.3(2H,6H)-dione (V; Ar=phenyl, Rl°=(CHz~60H) (203)
Alkylation of 5-methoxy-2-[(E,Z)-2-phenylethenyl]-1H-indole (II; Ar=phenyl)
(0.30
g, 0.71 mmol) ) with 6-bromohexyl-tert-butyl(dimethyl)silyl ether according to
procedure
described in example 38 gave crude material that was reacted directly with
maleimide (0.14
g) following Method 4a.
Aromatisation of the crude Diels-Alder adduct using the procedure described in
example 79
gave crude material that was then dissolved in methanol (100 mL) to which 1N
hydrochloric
acid (15 mL) was added. This solution was stirred at rt for 2h before being
diluted with water
and extracted with ethyl acetate. The organic layer was dried, the drying
agent was removed
and the solution was concentrated to dryness. Chromatography on silica eluting
with
dichloromethane to ethyl acetate/hexane (4:1) followed by trituration with
diethyl ether gave
alcohol (203) as a yellow powder (0.31 g, 98%), mp 170-173 °C.'H NMR 8
[(CD3)zS0]
11.10 (br s, -1H), 8.56 (d, J=2.6 Hz, 1H); 7.82 (s, 1H), 7.67 (m, 3H), 7.47
(m, 3H), 7.29 (dd,
J=8.9, 2.6 Hz, 1H), 4.50 (t, J=7.0 Hz, 2H), 4.28 (t, J=5.1 Hz, 1H), 3.90 (s,
3H), 3.32 (t, J=6.4
Hz, 2H), 1.76 (m, 2H), 1.36-1.26 (m, 6H). Found: C, 73.57; H, 6.17; N, 6.39.
Cz~Hz6NZOa
requires: C, 73.28; H, 5.92; N, 6.33.
EXAMPLE 85
The preparation of 4-(2 6-Dichlorophenyl)-6-(2-hydroxyethyl)-9-
methoxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione~ Ar=2,6-dichlorophenyl, R'°=(CH2)zOH)
(230)
Alkylation of pure trans-dime (512) (3.0 g, 10.6 mmol) prepared as described
in
example 103 with 2-(2-bromoethoxy)tetrahydro-2H-pyran according to the
procedure
described in example 38 gave crude material that was dissolved in
methanol/tetrahydrofuran
(4:1, 250 mL) to which 2N hydrochloric acid (20 mL) was added before the
solution was
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-88-
stirred at room temperature for 2h. The solution was then diluted with
saturated sodium
bicarbonate and concentrated under reduced pressure to precipitate the crude
product which
was collected by filtration and dried in vacuo. This solid was reacted
directly with maleimide
(1.26 g) following The procedure described in example 68. Aromatisation of the
chromatographed Diels-Alder adduct using the procedure described in example 70
gave
material that was then triturated from methanol to give alcohol (230) as a
yellow powder
(2.14 g, 44%), mp 229-231 °C.'H NMR S [(CD3)zS0) 11.17 (br s, 1H), 8.50
(d, J=2.6 Hz,
1H), 7.86 (s, 1H), 7.72 (d, J=9.0 Hz, 1H), 7.62 (m, 2H), 7.51 (dd, J=8.9, 7.4
Hz, 1H), 7.31
(dd, J=9.0, 2.6 Hz, 1H), 4.83 (t, J=5.3 Hz, 1H), 4.54 (t, J=5.2 Hz, 2H), 3.91
(s, 3H), 3.77 (m,
2H). Found: C, 60.61; H, 3.85; N, 5.88. C23H~6C12N204 requires: C, 60.66; H,
3.54; N, 6.15.
EXAMPLE 86
The preparation of 4-(2,6-Dichlorophenyl)-6-(3-hydroxypropyl)-9-
methoxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (V; Ar=2,6-dichlorophenyl, R'°=(CH2~OH 232
Alkylation of dime (512) (3.0 g, 9.43 mmol) prepared as described in example
103
with 3-bromopropyl tent-butyl(dimethyl)silyl ether according to the procedure
described in
example 38 gave crude material that was dissolved in methanol/dichloromethane
(3:1, 300
mL) to which 1N hydrochloric acid (60 mL) was added. This solution was stirred
at rt for 2h
before being diluted with saturated sodium bicarbonate and concentrated under
reduced
pressure to precipitate the crude product which was collected by filtration
and dried in vacuo.
This solid was reacted with maleimide (1.20 g) following The procedure
described in
example 68. Aromatisation of the crude Diels-Alder adduct using the procedure
described in
example 70 gave material that was then chromatographed on silica eluting with
methanol/dichloromethane (2:98 to 5:95) to give alcohol (232) (2.4 g, 54%) as
a yellow
powder, mp 254-256 °C.'H NMR 8 [(CD3)ZSO] 11.18 (br s, 1H), 8.51 (d,
J=2.6 Hz, 1H),
7.86 (s, 1H), 7.72 (d, J=9.0 Hz, 1H), 7.63 (m, 2H), 7.51 (dd, J=8.8, 7.4 Hz,
1H), 7.34 (dd,
J=9.0, 2.6 Hz, 1H), 4.62 (t, J=5.0 Hz, 1H), 4.54 (t, J=6.9 Hz, 2H), 3.91 (s,
3H), 3.42 (m, 2H),
1.90 (m, 2H). FABMS found M+: 468.0630, 470.0628, 472.0626. C24H~8C12N204
requires
468.0644, 470.0614, 472.0585.
EXAMPLE 87
The preparation of 4-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)butanoic acid (V; Ar=2-chlorophenyl, R'°=(CH2~COOH)
(262)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-89-
Alkylation of dime (27) (0.40 g, 1.41 mmol) prepared as described in example
37
with ethyl bromobutyrate according to the procedure described in example 38
gave crude
material that was reacted with maleimide (0.27 g) following the procedure
described in
example 68. Aromatisation of the Diels-Alder adduct using the procedure
described in
example 70 gave crude material that was then dissolved in methanol (100 mL) to
which 2N
potassium hydroxide (2 mL) was added. This solution was stirred at rt for 2h
before being
diluted with water and acidified by the addition of 1N hydrochloric acid to
precipitate the
product as a yellow solid, which was collected by filtration and washed with
water before
being dried in vacuo. Chromatography on silica eluting with ethyl acetate
followed by
trituration with diethyl ether/hexane, gave acid (262) as a yellow powder
(0.10 g, 16%), mp
251-254 °C.'H NMR 8 [(CD3)ZSO] 12.12 (br s, 1H), 11.12 (br s, 1H), 8.53
(d, J=2.6 Hz, 1H),
7.85 (s, 1H), 7.72 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.53-7.45 (m, 3H), 7.32
(dd, J=9.0, 2.6
Hz, 1H), 4.50 (t, J=7.3 Hz, 2H), 3.91 (s, 3H), 2.30 (m, 2H), 1.97 (m, 2H).
FABMS found
[M+H]+: 463.1025, 465.1034. CZSH~9C1N205 requires 463.1061, 465.1031.
EXAMPLE 88
The preparation of 4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)butanoic acid (VI; Ar=2-chlorophenyl,
R'°=(CHs~COOH) (263)
Demethylation of acid (262) (32 mg, 0.07 mmol) prepared as described in
example 87
employing the procedure described in example 80 gave phenol (263) (6 mg, 19%)
as an
orange powder, mp 274-277 °C.'H NMR 8 [(CD3)ZSO] 12 (v br s, 1H), 11.06
(br s, 1H), 9.38
(br s, 1H), 8.38 (d, J=2.4 Hz, 1H), 7.80 (s, 1H), 7.60 (d, J=8.9 Hz, 1H), 7.58
(m, 1H), 7.52-
7.43 (m, 3H), 7.14 (dd, J=8.9, 2.4 Hz, 1H), 4.45 (t, J=7.5 Hz, 2H), 2.27 (m,
2H), 1.95 (m,
2H). FABMS found [M+H]+: 449.0874, 451.0879. CZQH,~C1Nz05 requires 449.0904,
451.0875.
EXAMPLE 89
The preparation of 9-Hydroxy-6-(2-hydroxyethyl)-4-phenylpyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (VI; Ar=phenyl, R'°=(CHZ)~OH) (220)
Demethylation of alcohol (201) (135 mg, 0.35 mmol) prepared as described in
example 82 employing The procedure described in example 81 gave alcohol (220)
(90 mg,
69%) as an orange powder, mp 298-301 °C. 1H NMR S [(CD3)ZSO] 11.04 (br
s, 1H), 9.31 (br
s, 1H), 8.40 (d, J=2.5 Hz, 1H), 7.79 (s, 1H), 7.64 (m, 2H), 7.55 (d, J=8.8 Hz,
1H), 7.47 (m,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-90-
3H), 7.10 (dd, J=8.8, 2.5 Hz, 1H), 4.85 (t, J=5.3 Hz, 1H), 4.51 (t, J=5.4 Hz,
2H), 3.77 (m,
2H). Found: C, 68.88; H, 4.43; N, 6.76. CzzH~6N204.3/4H20 requires: C, 68.48;
H, 4.57; N,
7.26.
EXAMPLE 90
The preparation of 9-Hydroxy-6-(3-hydroxypropyl)-4-phenylpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (Vh Ar=phenyl, R1°=(CH~)~OH) (221)
Demethylation of alcohol (202) prepared as described in example 83 (60 mg,
0.15
mmol) employing the procedure described in example 80 gave alcohol (221) (45
mg, 78%)
as an orange powder, mp 274-276 °C.'H NMR 8 [(CD3)zS0] 11.05 (br s,
1H), 9.33 (s, 1H),
8.41 (d, J=2.4 Hz, 1H), 7.77 (s, 1H), 7.64 (m,,2H), 7.56 (d, J=8.8 Hz, 1H),
7.46 (m, 3H), 7.12
(dd, J=8.8, 2.4 Hz, 1H), 4.64 (t, J=4.9 Hz, 1H), 4.51 (t, J=6.9 Hz, 2H), 3.39
(m, 2H), 1.92 (m,
2H). Found: C, 71.59; H, 4.74; N, 7.57. Cz3H~$Nz04 requires: C, 71.49; H,
4.70; N, 7.25.
EXAMPLE 91
'The preparation of 9-Hydroxy-6-(6-hydroxyhexyl)-4-phenylpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (VI; Ar=phenyl, Rl°=(CHz)~OH) (222)
Demethylation of alcohol (203) prepared as described in example 84 (55 mg,
0.12
mmol) employing the procedure described in example 80 gave alcohol (222) (12
mg, 23%)
as an orange powder, mp 132-140 °C. 'H NMR 8 [(CD3)zS0] 11.04 (br s,
1H), 9.33 (s, 1H),
8.41 (d, J=2.5 Hz, 1H), 7.77 (s, 1H), 7.64 (m, 2H), 7.54 (d, J=8.8 Hz, 1H),
7.46 (m, 3H), 7.12
(dd, J=8.8, 2.5 Hz, 1H), 4.46 (t, J=7.1 Hz, 2H), 4.29 (t, J=5.1 Hz, 1H), 3.31
(m, 2H), 1.75 (m,
2H), 1.36-1.26 (m, 6H). FABMS found M+: 428.1730. Cz6HzaNzOa requires
428.1736.
EXAMPLE 92
The preparation of 9-Methoxy-6-methyl-4-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(V' Ar=phenyl, R'°=CHI) (223)
Alkylation of pure trans 5-methoxy-2-[(E)-2-phenylethenyl]-1H-indole (II;
Ar=phenyl) (0.20 g, 0.80 mmol) with methyl iodide according to the procedure
described in
example 38 gave crude material that was reacted with maleimide (92 mg)
following Method
4a, except that the reaction time was 24 h. Aromatisation of the crude Diels-
Alder adduct
using the procedure described in example 79 gave crude material that was
chromatographed
on silica eluting with ethyl acetate/dichloromethane (1:3). Trituration from
methanol gave
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-91-
carbazole (223) (0.20 g, 70%) as a yellow powder, mp 286-291 °C.'H NMR
8 [(CD3)ZSO]
11.11 (br s, 1 H), 8.53 (d, J=2.6 Hz, 1 H), 7.80 (s, 1 H), 7.66 (m, 3H), 7.47
(m, 3H), 7.30 (dd,
J=9.0, 2.6 Hz, 1H), 3.96 (s, 3H), 3.90 (s, 3H). Found: C, 74.29; H, 4.67; N,
7.86. Cz2H,6NZOs
requires: C, 74.15; H, 4.53; N, 7.86.
EXAMPLE 93
The preparation of 9-Hydroxy-6-methyl-4-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
~VI~ Ar=phenyl, R'°=CH3) (224)
Demethylation of alcohol (223) prepared as described in example 92 (180 mg,
0.51
mmol) employing the procedure described in example 81, was followed by
chromatography
on silica eluting with ethyl acetate/dichloromethane (1:2). Trituration from
dichloromethane/hexane gave alcohol (224) (155 mg, 90%) as an orange powder,
mp 297-
300 °C.'H NMR 8 [(CD3)ZSO] 11.05 (br s, 1H), 9.34 (s, 1H), 8.39 (d,
J=2.5 Hz, 1H), 7.76 (s,
1H), 7.65 (m, 2H), 7.54 (d, J=8.8 Hz, 1H), 7.46 (m, 3H), 7.13 (dd, J=8.8, 2.5
Hz, 1H), 3.93
(s, 3H). Found: C, 69.96; H, 4.58; N, 7.70. CZ~H~4NZO3.HZO requires: C, 69.99;
H, 4.48; N,
7.77.
EXAMPLE 94
The preparation of 4-(2,6-Dichloropheriyl)-9-hydroxy-6-(2-
hydroxyethyl)pyrrolof3;4-
clcarbazole-1,3(2H,6H)-dione (VI; Ar=2,6-dichlorophenyl, R'°=(CH2)ZOH)
(234)
Demethylation of alcohol (230) (0.10 g, 0.22 mmol) prepared as described in
example
85 employing the procedure described in example 80 gave phenol (234) (49 mg,
50%) as an
orange/yellow powder, mp 254-257 °C.'H NMR b [(CD3)ZSO] 11.10 (br s,
1H), 9.35 (s, 1H),
8.36 (d, J=2.4 Hz, 1H), 7.80 (s, 1H), 7.61 (m, 3H), 7.50 (dd, J=8.7, 7.3 Hz,
1H), 7.13 (dd,
J=8.8, 2.4 Hz, 1H), 4.82 (t, J=5.5 Hz, 1H), 4.49 (t, J=5.2 Hz, 2H), 3.75 (m,
2H). Found: C,
58.76; H, 3.34; N, 6.25. CZZH~4C12N204.1/2Hz0 requires: C, 58.67; H, 3.36; N,
6.22.
EXAMPLE 95
The preparation of 4-(2,6-Dichlorophenyl)-9-hydroxy-6-(3-
hydroxypropyl)pyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VI; Ar=2,6-dichloronhenyl, R1°=(CH~OH)
(235)
Demethylation of alcohol (232) (0.36 g, 0.77 mmol) prepared as described in
example
86 employing the procedure described in example 80 gave phenol (235) (0.32 g,
92%) as an
orange/yellow powder, mp 215-218 °C.'H NMR 8 [(CD3)ZSO] 11.12 (br s,
1H), 9.39 (s, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-92-
8.37 (d, J=2.5 Hz, 1H), 7.80 (s, 1H), 7.61 (m, 3H), 7.51 (dd, J=8.7, 7.2 Hz,
1H), 7.16 (dd,
J=8.8, 2.5 Hz, 1H), 4.61 (t, J=5.0 Hz, 1H), 4.49 (t, J=6.9 Hz, 2H), 3.42 (m,
2H), 1.88 (m, 2H).
Found: C, 60.36; H, 3.47; N, 6.08. C23Hi6C12NzOa requires: C, 60.66; H, 3.54;
N, 6.15.
EXAMPLE 96
The preparation of 3-~2-f(E)-2-(2-Chlorophenyl)ethenyll-5-methoxy-1H-indol-1-
yllpropanenitrile (III; Ar=2-chlorophenyl, R'°=CH~CH~CN) (236)
To a solution of trans-dime (27) prepared as described in example 37 (1.5 g,
5.25
mmol) in acetonitrile (30 mL) was added acrylonitrile (2.42 mL., 36.8 mmol)
and 1,8-
diazabicyclo[5.4.0]undec-7-ene (20 drops). The resulting solution was stirred
at room
temperature under nitrogen for 18 h before being diluted with water and
extracted with ethyl
acetate. The organic layer was dried, the drying agent was removed and the
solution was
concentrated to dryness. Chromatography on silica eluting with ethyl
acetate/hexane (1:1),
followed by trituration from methanol gave the diene (236) (1.36 g, 77%) as an
off-white
solid, mp 136-138 °C. 1H NMR 8 [(CD3)ZSO] 8.05 (dd, J=7.9, 1.5 Hz, 1H),
7.50 (m, 4H),
7.41 (m, 1H), 7.33 (m, 1H), 7.04 (d, J=2.4 Hz, 1H), 6.90 (s, 1H), 6.81 (dd,
J=9.0, 2.4 Hz,
1H), 4.68 (t, J=6.6 Hz, 2H), 3.77 (s, 3H), 2.93 (t, J=6.6 Hz, 2H). Found: C,
71.32; H, 5.14; N,
8.51. CZ°H1~C1N20 requires: C, 71.31; H, 5.09; N, 8.31.
EXAMPLE 97
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)propanenitrile (V; Ar=2-chlorophenyl, Rl°=CH~CH~CN)
(237)
Diene (236) (1.30 g, 3.86 mmol) prepared as described in example 96 was
reacted
with maleimide (0.49 g) following The procedure described in example 68 and
then
aromatized according to the procedure described in example 70 to give crude
material that
was chromatographed on silica eluting with ethyl acetate/hexane (1:1).
Trituration from
methanol gave nitrile (237) (1.33 g, 80%) as a yellow powder, mp 287-288
°C.'H NMR 8
[(CD3)ZSO] 11.16 (br s, 1H), 8.53 (d, J=2.6 Hz, 1H), 8.02 (s, 1H), 7.83 (d,
J=9.0 Hz, 1H),
7.59 (m, 1H), 7.49 (m, 3H), 7.33 (dd, J=9.0, 2.6 Hz, 1H), 4.86 (t, J=6.7 Hz,
2H), 3.91 (s, 3H),
3.04 (t, J=6.7 Hz, 2H). Found: C, 65.32; H, 4.15; N, 9.60. C24H~6C1N303.3/4H20
requires: C,
65.01; H, 3.98; N, 9.47.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-93-
EXAMPLE 98
The preparation of 3-(4-(2-Chlorophenyl)-9-h~droxy-1 3-dioxo-2,3-
dihydropyrrolof3,4-
~carbazol-6(1H)-yl)propanenitrile (VI' Ar=2-chlorophenyl, R'°=CH2CH~CN)
(238)
Demethylation of nitrite (237) (0.15 g, 0.35 mmol) prepared as described in
example
97 employing The procedure described in example 80, followed by trituration
from
methanol, gave phenol (238) (0.13 g, 89%) as an orange/yellow powder, mp 332-
336 °C.'H
NMR 8 [(CD3)ZSO] 11.10 (br s, 1H), 9.42 (br s, 1H), 8.39 (d, J=2.4 Hz, 1H),
7.96 (s, 1H),
7.69 (d, J=8.8 Hz, 1H), 7.58 (m, 1H), 7.48 (m, 3H), 7.15 (dd, J=8.8, 2.4 Hz,
1H), 4.82 (t,
J=6.7 Hz, 2H), 3.02 (t, J=6.7 Hz, 2H). Found: C, 66.24; H, 3.66; N, 9.93.
Cz3H,4CIN3O3
requires: C> 66.43; H, 3.39; N, 10.10.
EXAMPLE 99
The preparation of 6-Benzyl-4-(2-chlorophenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (V; Ar=2-chlorophenyl, R1°=CHZPh) (239)
Alkylation of pure trans-dime (27) (0.20 g, 0.70 mmol) prepared as described
in
example 37 with benzyl bromide according to the procedure described in example
38 gave
crude material that was reacted directly with maleimide (103 mg) following the
procedure
described in example 68, except that the reaction time was 24 h. Aromatisation
of the crude
Diets-Alder adduct using the procedure described in example 70 gave crude
material that
was chromatographed on silica eluting with ethyl acetate/hexane (1:2).
Trituration from ethyl
acetate/hexane gave carbazole (239) (0.15 g, 46%) as a yellow powder, mp 234-
237 °C. 'H
NMR 8 [(CD3)aS0] 11.15 (br s, 1H), 8.55 (d, J=2.6 Hz, 1H), 7.91 (s, 1H), 7.69
(d, J=9.0 Hz,
1H), .7.56 (m, 1H), 7.46 (m, 3H), 7.25 (m, 4H), 7.16 (m, 2H), 5.79 (s, 2H),
3.90 (s, 3H).
Found: C, 71.78; H, 4.39; N, 6.18. CZ$H19C1NZ03 requires: C, 72.02; H, 4.10;
N, 6.00.
EXAMPLE 100
The preparation of 6-Benzyl-4-(2-chlorophenyl)-9-hydroxypyrrolo13,4-
clcarbazole-
1 3(2H 6H)-dione (VI; Ar=2-chlorophenyl, Rl°=CH~Ph) (240)
Demethylation of carbazole (239) (0.10 g, 0.21 mmol) prepared as described in
example 99 employing the procedure described in example 80, followed by
chromatography
on silica eluting with ethyl acetate/hexane (1:2) and trituration from ethyl
acetate/hexane,
gave carbazole (240) (87 mg, 91 %) as an orange/yellow powder, mp 269-271
°C. 'H NMR 8
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-94-
[(CD3)2S0] 11.09 (br s, 1H), 9.40 (s, 1H), 8.40 (d, J=2.5 Hz, 1H), 7.86 (s,
1H), 7.56 (m, 2H),
7.45 (m, 3H), 7.25 (m, 3H), 7.16 (m, 2H), 7.11 (dd, J=8.9, 2.5 Hz, 1H), 5.75
(s, 2H). Found:
C, 72.06; H, 4.18; N, 6.20. CZ~H»C1Nz03 requires: C, 71.60; H, 3.78; N, 6.18.
EXAMPLE 101
The preparation of Methyl 3-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3 4-clcarbazol-6(1H)-yl~propanoate (VI, Ar=2-chlorophenyl,
R'°(CH~~,COOCH~) (264)
Gaseous hydrochloric acid was bubbled through a stirred solution of acid (117)
(35
mg, 0.08 mmol) prepared as described in example 230 in methanol (10 mL) for 30
seconds.
The resulting solution was stirred for 30 minutes at room temperature before
being diluted
with water and extracted with ethyl acetate. The organic layer was dried, the
drying agent
was removed and the solution was concentrated to dryness. Chromatography on
silica eluting
with ethyl acetate/hexane (l:l) followed by crystallisation from ethyl
acetate/hexane gave
ester (264) (25 mg, 70%) as an orange powder, mp 218-220 °C. 1H NMR 8
[(CD3)ZSO] 11.07
(br s, 1H), 9.39 (br s, 1H), 8.38 (d, J=2.5 Hz, 1H), 7.79 (s, 1H), 7.57 (m,
2H), 7.51-7.44 (m,
3H), 7.14 (dd, J=8.8, 2.5 Hz, 1H), 4.71 (t, J=6.8 Hz, 2H), 3.48 (s, 3H), 2.83
(t, J=6.8 Hz, 2H).
Found: C, 62.18; H, 4.04; N, 5.99. C24H»C1N205.3/4H20 requires: C, 62.34; H,
4.03; N,
6.06.
Representative Procedure for Method l0a of Scheme 2
EXAMPLE 102
The preparation of (2,6-Dichlorobenz ly )(triphenyl)phosphonium bromide (511)
A mixture of 2,6-dichlorotoluene (20.1 g, 0.125 mol), N-bromosuccinimide (24.6
g,
0.138 mol) and 2,2'-azobisisobutyronitrile (0.41 g, 2.50 mmol) in dry benzene
(300 mL)
under NZ was stirred at reflux for 6 h with continuous irradiation from a 100W
lamp. The
resulting reaction mixture was concentrated under vacuum (to 50 mL), cooled
and filtered,
washing with dry benzene. The filtrate (containing the crude benzyl bromide)
was treated
rJirectly with triphenylphosphine (49.3 g, 0.188 mol), stirring at reflux for
17 h. After cooling,
the precipitate was collected by filtration, washing thoroughly with dry
benzene, then
pentane, and dried under vacuum at 50 °C to give the phosphonium salt
(511) as a cream
powder (62.1 g, 99 %), mp (benzene) 247-250 °C.'H NMR (CDC13) 8 7.76
(m, 9H), 7.64 (m,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-95-
6H), 7.18 (s, 3H), 5.50 (d, J=14.3 Hz, 2H). Found: C, 60.03; H, 3.76.
C~H2°BrCl2P requires C,
59.79; H, 4.01.
EXAMPLE 103
The preparation of 2-f(E)-2-(2 6-Dichlorophenyl)ethenyll-5-methoxy-1H-indole
(512) (II,
Ar=2,6-dichlorophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2,6-
dichlorobenzyl)(triphenyl)phosphonium bromide (511) prepared as described in
example 102
using the procedure described in example 37, except that the LDA and aldehyde
were
(sequentially) added at 0 °C, the ratio of LDA:aldehyde was 1.37:1 and
the reaction time was
h, to give (after crystallisation from CHZC12/hexane) the dime (512) as a
yellow solid (the
pure E isomer) (97 %), mp 144-147 °C.'H NMR (CDC13) b 8.20 (br s, 1H),
7.36 (d, J=8.1 Hz,
1H), 7.27 (m, 1H), 7.25 (d, J=16.8 Hz, 1H), 7.11 (t, J=8.0 Hz, 1H), 7.03 (d,
J=2.4 Hz, 1H), 6.93
(d, J=16.8 Hz, 1H), 6.88 (dd, J=8.7, 2.5 Hz, 1H), 6.61 (d, J=1.8 Hz, 1H), 3.86
(s, 3H). Found: C,
64.04; H, 4.13; N, 4.39. C1~H~3C1zN0 requires C, 64.17; H, 4.12; N, 4.40.
EXAMPLE 104
The preparation of 4-(2 6-Dichlorophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3,4-
clcarbazole-1 3(2H 3aH)-dione (513) (IV, R'°=H, Ar=2,6-dichlorophenyl)
The pure E dime (512) prepared as described in example 103 was reacted with
maleimide according to the procedure described in example 68 in a foil-covered
sealed vial,
except that the ratio of diene:maleimide:SnCl2 was 1:4:0.075 and the reaction
time was 3 h,
to give a crude solid containing the adduct (513), which was used without
further
purification.
EXAMPLE 105
The preparation of 4-(2 6-Dichlorophenyl)-9-methoxyp. rrolo~3,4-clcarbazole-
1,3(2H,6H)-
dione (514) (V, R'°=H, Ar=2,6-dichlorophenyl)
The crude adduct (513) prepared as described in example 104 was aromatised
with
DDQ (S equiv.) using the procedure described in example 70, except that the
solvent was 1:1
toluene/dioxane and the reaction time was 48 h, to give (after crystallisation
from
MeOH/CHZC12/hexane) the product (514) (78 %) as a yellow crystalline solid, mp
299-301
°C.'H NMR [(CD3)ZSO] 8 12.02 (br s, 1H), 11.15 (br s, 1H), 8.45 (d,
J=2.6 Hz, 1H), 7.61 (d,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-96-
J=7.7 Hz, 2H), 7.61 (s, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.50 (dd, J=8.8, 7.4 Hz,
1H), 7.26 (dd,
J=8.9, 2.7 Hz, 1H), 3.90 (s, 3H). Found: C, 61.26; H, 2.92; N, 6.65.
CZ,H,zCI2N203 requires C,
61.33; H, 2.94; N, 6.81.
EXAMPLE 106
The preparation of 4-(2,6-Dichlorophenyl)-9-hydroxypyrrolo(3,4-clcarbazole-
1,3(2H,6H)-
dione (515) (VI, R'°=H, Ar=2,6-dichlorophenyl)
The methyl ether (514) prepared as described in example 105 was demethylated
with
BBr3 using the procedure described in example 80 to give (after
crystallisation from
MeOH/CHZCl2/hexane) the phenol (515) (98 %) as an orange solid, mp 205-215
°C (dec).'H
NMR [(CD3)zS0] 8 11.89 (br s, 1H), 11.08 (br s, 1H), 9.32 (br s, 1H), 8.30 (d,
J=2.4 Hz, 1H),
7.61 (d, J=7.9 Hz, 2H), 7.55 (s, 1H), 7.50 (dd, J=8.7, 7.4 Hz, 1H), 7.47 (d,
J=8.7 Hz, 1H), 7.10
(dd, J=8.7, 2.4 Hz, 1H). Found: C, 58.98; H, 2.99; N, 6.68.
CZ°Hl°CI2N203.1/2 HZO requires C,
59.13; H, 2.73; N, 6.90.
EXAMPLE 107
(2 6-Dibromobenzyl)(triphenylZphosphonium bromide (516)
Bromination of 2,6-dibromotoluene with NBS/AIBN, followed by reaction of the
crude bromide with triphenylphosphine, using the procedure described in
example 102,
except that the reaction time for the bromination was 3 h, gave the
phosphonium salt (516)
(95 %) as a cream powder, mp (CHzCl2/benzene) 241-243 °C.'H NMR (CDC13)
8 7.78 (m,
9H), 7.64 (m, 6H), 7.40 (br d, J=7.9 Hz, 2H), 6.99 (td, J=8.0, 2.5 Hz, 1H),
5.58 (d, J=14.1 Hz,
2H). Found: C, 47.06; H, 3.37. CZSHZ°Br3P.3/4 CHZCIZ requires C, 47.23;
H, 3.31.
EXAMPLE 108
The preparation of 2-((E)-2-(2,6-Dibromophenyl)ethenyll-5-methoxy-1H-indole
(517) (II,
Ar=2,6-dibromophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2,6-
dibromobenzyl)(triphenyl)phosphonium bromide (516) prepared as described in
example 107
using the procedure described in example 37, except that the ratio of
LDA:aldehyde was
1.3?:1 and the reaction time was 5 h, to give (after crystallisation from
CHZCl2/hexane) the
dime (517) as a yellow solid (the pure E isomer) (80 %), mp 140-141
°C.'H NMR (CDC13) 8
8.20 (br s, 1H), 7.60 (d, J=8.1 Hz, 1H), 7.27 (d, J=8.2 Hz, 1H), 7.05 (br s,
1H), 7.03 (d, J=16.7
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-97-
Hz, 1H), 6.96 (t, J=8.0 Hz, 1H), 6.88 (dd, J=8.7, 2.5 Hz, 1H), 6.82 (d, J=16.7
Hz, 1H), 6.61 (d,
J=1.9 Hz, 1H), 3.86 (s, 3H). Found: C, 50.37; H, 3.24; N, 3.37. C»H~3Br2N0
requires C, 50.16;
H, 3.22; N, 3.44.
EXAMPLE 109
The nrenaration of 4-(2,6-Dibromophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropynolo[3,4-
clcarbazole-1,3(2H,3aH)-dione (518) (IV, R1°=H, Ar=2,6-dibromophenyl)
The pure E dime (517) prepared as described in example 108 was reacted with
maleimide according to the procedure described in example 68 in a foil-covered
sealed vial,
except that the ratio of diene:maleimide:SnCl2 was 1:5:0.075 and the reaction
time was 3.5 h,
to give a crude solid containing the adduct (518), which was used without
further
purification.
EXAMPLE 110
The preparation of 4-(2,6-Dibromophen~)-9-methoxypyrrolo[3,4-clcarbazole-
1,3(2H,6H)-
dione (519) (V, RI°=H, Ar=2,6-dibromophenyl)
The crude adduct (518) prepared as described in example 109 was aromatised
with
DDQ (5 equiv.) using the procedure described in example 70, except that the
solvent was 1:1
toluene/dioxane and the reaction time was 24 h, to give (after crystallisation
from
MeOH/CHZC12/hexane) the product (519) (68 %) as an orange crystalline solid,
mp 329-331
°C. 1H NMR [(CD3)ZSO] b 12.02 (br s, 1H), 11.13 (br s, 1H), 8.45 (d,
J=2.6 Hz, 1H), 7.80 (d,
J=8.1 Hz, 2H), 7.59 (d, J=8.3 Hz, 1H), 7.54 (s, 1H), 7.33 (t, J=8.0 Hz, 1H),
7.26 (dd, J=8.8, 2.6
Hz, 1H), 3.90 (s, 3H). Found: C, 50.29; H, 2.32; N, 5.69. Cz~H12Br2N203
requires C, 50.43; H,
2..42; N, 5.60.
EXAMPLE 111
The preparation of 4-(2,6-Dibromophenyl)-9-hydroxypyrrolo[3,4-clcarbazole-
1,3(2H,6H)-
dione (520) (VI, R1°=H, Ar=2,6-dibromophenyl)
The methyl ether (519) prepared as described in example 110 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 1.5 h, to
give (after crystallisation from MeOH/CHZC121EtOAc/hexane) the phenol (520)
(98 %) as a
yellow-orange solid, mp 180-190 °C (dec).'H NMR [(CD3)zS0] 8 11.90 (br
s, 1H), 11.08 (br s,
1H), 9.35 (br s, 1H), 8.30 (d, J=2.3 Hz, 1H), 7.80 (d, J=8.f Hz, 2H), 7.49 (s,
1H), 7.48 (d, J=8.9
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-98-
Hz, 1H), 7.33 (t, J=8.1 Hz, 1H), 7.11 (dd, J=8.7, 2.5 Hz, 1H). Found: C,
49.13; H, 2.29; N, 6.05.
CZOH,oBrZN203 requires C, 49.42; H, 2.07; N, 5.76.
Representative Procedure for Method l Ob of Scheme 2
EXAMPLE 112
The preparation of (2 6-Dichloro-4-methoxybenzyl)(triphenyl)phosphonium
bromide (521)
A solution of (2,6-dichloro-4-methoxyphenyl)methanol (3.00 g, 14.5 mmol) in
30%
HBr in acetic acid (45 mL) was stirred at 20 °C for 3 h, then poured
onto ice-water (120 mL)
and extracted with pentane (6 x 100 mL). The extracts were washed with water
(3 x 200 mL),
backextracting with pentane (100 mL). Removal of the solvent gave the crude
bromide as white
crystals, which was immediately redissolved in benzene (80 mL) and treated
with
triphenylphosphine (5.70 g, 21.7 mmol), stirring at reflux for 15 h. After
cooling, the
precipitate was collected by filtration, washing thoroughly with dry benzene,
then pentane,
and dried under vacuum at 50 °C to give the phosphonium salt (521) as a
white solid (7.75 g,
100 %), mp (CHZCl2/benzene) 188-190 °C.'H NMR (CDC13) 8 7.83-7.60 (m,
15H), 6.74 (s,
2H), 5.37 (d, J=13.5 Hz, 2H), 3.76 (s, 3I~. Found: C, 58.40; H, 4.35.
CZ6Ha2BrC120P requires C,
58.67; H, 4:17.
EXAMPLE 113
The preparation of 2-f (E)-2-(2 6-Dichloro-4-methoxyphenyl)ethenyll-5-methoxy-
1H-indole
(522) (II, Ar=2,6-dichloro-4-methoxyphenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2,6-dichloro-4-
methoxybenzyl)(triphenyl)phosphonium bromide (521), prepared as described in
example
112, using the procedure described in example 37, except that the aldehyde was
added at 0
°C, the ratio of LDA:aldehyde was 1.37:1 and the reaction time was 5 h,
to give (after
crystallisation from CH2C12/hexane) the dime (522) as a cream solid (the pure
E isomer) (70
%), mp 128-129 °C.'H NMR (CDCI3) 8 8.17 (br s, 1H), 7.25 (d, J=8.7 Hz,
1H), 7.17 (d, J=16.8
Hz, 1H), 7.04 (d, J=2.4 Hz, 1H), 6.93 (s, 2H), 6.88 (d, J=16.8 Hz, 1H), 6.86
(dd, J=8.7, 2.5 Hz,
1H), 6.57 (br d, J=1.7 Hz, 1H), 3.85 (s, 3H), 3.82 (s, 3H). Found: C, 62.01;
H, 4.23; N; 4.21.
C~sH~5C12N0~ requires C, 62.09; H, 4.34; N, 4.02.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-99-
EXAMPLE 114
The~reparation of 4-(2 6-Dichloro-4-methoxyphenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3 4-clcarbazole-1 3(2H,3aH)-dione (523) (IV, R'°=H.
Ar=2.6-dichloro-4-
methoxyphenyl)
The pure E dime (522) prepared as described in example 113 was reacted with
maleimide according to the procedure described in example 68 in a foil-covered
sealed vial,
except that the ratio of diene:maleimide:SnCl2 was 1:6:0.05 and the reaction
time was 1.5 h,
to give a crude solid containing the adduct (523), which was used without
further
purification.
EXAMPLE 115
The preparation of 4-(2 6-Dichloro-4-methoxyphenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (524) (V R'°=H, Ar=2,6-dichloro-4-methoxyphenyl)
The crude adduct (523) prepared as described in example 114 was aromatised
with
DDQ (6 equiv.) using the procedure described in example 70, except that the
solvent was 1:1
toluene/dioxane and the reaction time was 24 h, to give (after crystallisation
from
MeOH/CHzCl2/hexane) the product (524) (74 %) as a yellow-orange crystalline
solid, mp
272-274 °C.'H NMR [(CD3)ZSO] 8 11.98 (br s, 1H), 11.12 (br s, 1H), 8.44
(d, J=2.6 Hz, 1H),
7.58 (d, J=8.7 Hz, 1H), 7.57 (s, 1H), 7.26 (dd, J=8.8, 2.6 Hz, 1H), 7.23 (s,
2H), 3.89 (2s, 2x3H).
Found: C, 56.89; H, 3.18; N, 5.94. C22H~4C12NZO4.S/4 HZO requires C, 56.97; H,
3.59; N, 6.04.
EXAMPLE 116
The preparation of 4-~2,6-Dichloro-4-hydroxyphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (525) (VI, R'°=H, Ar=2,6-dichloro-4-hydroxyphenyl)
The methyl ether (524) prepared as described in example 115 was bis-
demethylated
with BBr3 using the procedure described in example 80, except that the
reaction time was 4 h
with 10 equiv. BBr3, then a further 9 h with an extra 10 equiv. BBr3, to give
(after
crystallisation from MeOH/CHZC12/hexane) the phenol (525) (95 %) as a yellow-
orange
solid, mp 300-308 °C.'H NMR [(CD3)ZSO] 8 11.82 (br s, 1H), 11.03 (br s,
1H), 10.47 (br s,
1 H), 9.28 (br s, 1 H), 8.29 (d, J=2.4 Hz, 1 H), 7.50 (s, 1 H), 7.46 (d, J=8.7
Hz, 1 H), 7.08 (dd,
J=8.7, 2.5 Hz, 1H), 6.96 (s, 2H). Found: C, 56.28; H, 2.94; N, 6.16.
C2°H,°C12N204.3/4 H20
requires C, 56.29; H, 2.72; N, 6.56.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-100-
EXAMPLE 117
The preparation of (2 6-Dichloro-3-methoxybenzyl)(triphenyl)phosphonium
bromide (526)
Bromination of (2,6-dichloro-3-methoxyphenyl)methanol with 30% HBr in acetic
acid, followed by reaction of the crude bromide with triphenylphosphine, using
the procedure
described in example 112, except that the reaction time for the displacement
was 36 h, gave
the phosphonium salt (526) (97 %) as a white solid, mp (CHzCIz/benzene) 242-
244 °C.'H
NMR (CDCl3) 8 7.83-7.60 (m, 15H), 7.15 (dd, J=9.1, 0.8 Hz, 1H), 6.88 (dd,
J=9.0, 2.4 Hz, 1H),
5.41 (d, J=14.3 Hz, 2H), 3.84 (s, 3H). Found: C, 58.68; H, 4.16. Cz6HzzBrCIzOP
requires C,
58.67; H, 4.17.
EXAMPLE 118
The preparation of 2-f(E)-2-(2 6-Dichloro-3-methoxyphenyl)ethenyll-5-methoxy-
1H-indole
(527) (II, Ar=2,6-dichloro-3-methoxyphenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2,6-dichloro-3-
methoxybenzyl)(triphenyl)phosphonium bromide (526), prepared as described in
example
117, using the procedure described in example 37, except that the aldehyde was
added at 0
°C, the ratio of LDA:aldehyde was 1.55:1 and the reaction time was S h,
to give (after
crystallisation from CHZCIz/hexane) the dime (527) as a cream solid (the pure
E isomer) (76
%), mp 138-140 °C. 1H NMR (CDC13) 8 8.21 (br s, 1H), 7.32 (d, J=8.9 Hz,
1H), 7.26 (d, J=8.7
Hz, 1H), 7.22 (d, J=16.8 Hz, 1H), 7.04 (d, J=2.4 Hz, 1H), 6.92 (d, J=16.8 Hz,
1H), 6.87 (dd,
J=8.8, 2.5 Hz, 1H), 6.80 (d, J=8.9 Hz, 1H), 6.61 (d, J=1.7 Hz, 1H), 3.92 (s,
3H), 3.85 (s, 3H).
Found: C, 61.92; H, 4.52; N, 3.91. C18H15C1zNOz requires C, 62..09; H, 4.34;
N, 4.02.
EXAMPLE 119
The preparation of 4-(2,6-Dichloro-3-methoxyphenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (528) (N, R1°=H.
Ar=2,6-dichloro-3-
methoxyphenyl)
The pure E dime (527) prepared as described in example 118 was reacted with
maleimide
according to the procedure described in example 68 in a foil-covered sealed
vial, except that
the ratio of diene:maleimide:SnClz was 1:5:0.03 and the reaction time was 2.5
h, to give a
crude solid containing the adduct (528), which was used without further
purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-101-
EXAMPLE 120
The preparation of 4-(2 6-Dichloro-3-methoxyphenyl)-9-methoxypyrrolo~3,4-
clcarbazole-
1 3(2H 6H)-dione (529) (V R1°=H Ar=2 6-dichloro-3-methoxyphenyl)
The crude adduct (528) prepared as described in example 119 was aromatised
with
DDQ (5 equiv.) using the procedure described in example 70, except that the
solvent was 1:1
toluene/dioxane and the reaction time was 24 h, to give (after crystallisation
from
CHZCIz/hexane) the product (529) (70 %) as an orange crystalline solid, mp 240-
242 °C.'H
NMR [(CD3)ZSO) 8 12.00 (br s, 1H), 11.13 (br s, 1H), 8.45 (d, J=2.6 Hz, 1H),
7.58 (d, J=8.2 Hz,
1H), 7.56 (s, 1H), 7.56 (d, J=8.7 Hz, 1H), 7.28 (d, J=9.3 Hz, 1H), 7.26 (dd,
J=9.0, 2.4 Hz, 1H),
3.94 (s, 3H), 3.90 (s, 3H). Found: C, 60.11; H, 3.16; N, 6.30. CZZH~4C12NZOa
requires C, 59.88;
H, 3.20; N, 6.35.
EXAMPLE 121
The ureparation of 4-(2 6-Dichloro-3-hydroxyphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (530) (VI R'°=H, Ar=2,6-dichloro-3-hydroxyphenyl)
The methyl ether (529) prepared as described in example 120 was bis-
demethylated
with BBr3 using the procedure described in example 80, except that the
reaction time was 6 h
with 10 equiv. BBr3, to give (after crystallisation from MeOH/CHZC12/hexane)
the phenol
(530) (91 %) as an orange crystalline solid, mp 313-318 °C.'H NMR
[(CD3)ZSO] S 11.84 (br
s, 1H), 11.05 (br s, 1H), 10.56 (br s, 1H), 9.29 (br s, 1H), 8.30 (d, J=2.4
Hz, 1H), 7.48 (s, 1H),
7.46 (d, J=8.7 Hz, 1H), 7.36 (d, J=8.8 Hz, 1H), 7.09 (dd, J=8.8, 2.6 Hz, 1H),
7.06 (d, J=8.9 Hz,
1H). Found: C, 57.21; H, 2.65; N, 6.58. CZ°H~°C12NZOa.1/2 MeOH
requires C, 57.36; H, 2.82;
N, 6.53.
EXAMPLE 122
The preQaration of 2-[(E)-2-(2-Bromophenyl)ethenyll-5-methoxy-1H-indole (531)
(II, Ar=2-
bromophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-
bromobenzyl)(triphenyl)phosphonium bromide using the procedure described in
example 37,
except that the aldehyde was added at 0 °C, the ratio of LDA:aldehyde
was 1.46:1 and the
reaction time was 5 h, to give (after crystallisation from CHZC12/hexane) the
dime (531) as a
yellow solid (the pure E isomer) (88 %), mp 120-123 °C.'H NMR (CDCI~) 8
8.21 (br s, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-102-
7.66 (dd, J=7.9, 1.5 Hz, 1H), 7.59 (dd, J=8.1, 1.0 Hz, 1H), 7.32 (br t, J=7.1
Hz, 1H), 7.27 (d,
J=8.3 Hz, 1H), 7.22 (d, J=16.4 Hz, 1H), 7.12 (td, J=7.6, 1.5 Hz, 1H), 7.05 (d,
J=16.4 Hz, 1H),
7.04 (d, J=2.4 Hz, 1H), 6.88 (dd, J=8.8, 2.4 Hz, 1H), 6.59 (d, J=1.7 Hz, 1H),
3.86 (s, 3H).
Found: C, 61.97; H, 4.30; N, 4.17. C»H~4BrN0 requires C, 62.21; H, 4.30; N,
4.27.
Representative Procedure for Combining Method 4a and Method 6 of Scheme 2
EXAMPLE 123
The preparation of 4-(2-bromophenyl)-9-methoxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(532) (V Rl°=H, Ar=2-bromophenyl)
A foil-covered mixture of the pure E dime (531) (1.00 g, 3.05 mmol) prepared
as
described in example 122 and maleimide (1.48 g, 15.2 mmol) in dry toluene (10
mL) was
stirred in a sealed vial at reflux for 24 h. The resulting thick suspension
was transferred to a
flask using further toluene (30 mL) and dioxane (40 mL), then treated with DDQ
(3.49 g,
15.4 mmol), stirring under reflux for 24 h. The resulting mixture was treated
with saturated
aqueous NaHC03 solution (250 mL) and the product extracted with 15%
MeOH/CHZCl2.
These extracts were washed with aqueous NaHC03 solution and water. The aqueous
NaHC03 solution and water was extracting several times with 15% MeOH/CHZC12,
then the
combined extracts were concentrated to dryness. The residue was adsorbed onto
silica gel and
chromatographed. Elution with 0-0.75% MeOH/CH2ClZ, then with 0.75% MeOH/CHZCl2
gave the crude product (532) which crystallised pure from MeOH/CHZC12/hexane
as an
orange crystalline solid (1.23 g, 96 %), mp 273-275 °C.'H NMR
[(CD3)ZSO] 8 11.96 (br s,
1H), 11.08 (br s, 1H), 8.46 (d, J=2.6 Hz, 1H), 7.74 (d, J=7.8 Hz, 1H), 7.58
(d, J=8.9 Hz, 1H),
7.55 (s, 1H), 7.48 (m, 2H), 7.39 (m, 1H), 7.25 (dd, J=8.9, 2.7 Hz, 1H), 3.89
(s, 3H). Found: C,
60.08; H, 3.02; N, 6.68. CZ1H~3BrN203 requires C, 59.88; H, 3.11; N, 6.65.
EXAMPLE 124
The preparation of 4-(2-Bromophe~l)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(533) (VI, Rl°=H, Ar=2-bromonhenyl)
The methyl ether (532) prepared as described in example 123 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 4 h.
Crystallisation from MeOH/CHZC12/hexane gave the phenol (533) in a yield of 89
% as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-103-
yellow-orange solid, mp 244-246 °C. 1H NMR [(CD3)ZSO] 8 11.83 (br s,
1H), 11.01 (br s, 1H),
9.29 (br s, 1 H), 8.31 (d, J=2.4 Hz, 1 H), 7.73 (br d, J=7.9 Hz, 1 H), 7.47
(m, 4H), 7.38 (m, 1 H),
7.08 (dd, J=8.7, 2.5 Hz, 1H). Found: C, 57.82; H, 2.90; N, 6.72.
CZ°H~,BrN2O3.1I2 HZO requires
C,57.71;H,2.91;N,6.73.
EXAMPLE 125
The preparation of 4-f 1 1'-Biphenyll-2-yl-9-methoxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (534) (V, R'°=H, Ar=2-biphenyl)
A mixture of the bromo derivative (532) (85.5 mg, 0.203 mmol) prepared as
described
in example 123, [1,1'-
bis(diphenylphosphino)ferrocene]dichloropalladium(II).CH2C12 (85
mg, 0.104 mmol) and tetraphenyltin (0.451, 1.06 mmol) in dry DMF (2.5 mL) was
stirred in a
sealed vial at 130 °C for 4 d. The resulting mixture was added to
aqueous NaHC03 (100 mL)
then extracted with 2:1 CHzCl2/EtOAc (5 x 80 mL). The extracts were washed
with water.
The water was extracting with 2:1 CHzCl2/EtOAc, then the combined organic
extracts were
concentrated to dryness. The residue was chromatographed several times on
silica gel
(eluting with CHZC12) to give the crude product (44.5 mg, 54%).,This was
combined with
material from similar reactions and purified by preparative reversed phase C-
18 HPLC (using a
gradient of 65-99% CH3CN/aqueous HCOZNH4 buffer, pH 3.45) to give (after
crystallisation
from MeOH/CHZCIZ/hexane) the pure product (534) (21 % overall) as a yellow
solid, mp
220-223 °C.'H NMR [(CD3)ZSO] 8 11.76 (br s, 1H), 10.87 (br s, 1H), 8.39
(d, J=2.6 Hz, 1H),
7.50 (m, SH), 7.38 (s, 1H), 7.20 (dd, J=8.9, 2.6 Hz, 1H), 7.10 (m, SH), 3.86
(s, 3H). Found: C,
77.37; H, 4.21; N, 6.88. CZ~H~8N203 requires C, 77.50; H, 4.34; N, 6.69.
EXAMPLE 126
The preparation of 4-fl 1'-Biphenyll-2-yl-9-h d~pyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (535) (VI, R'°=H, Ar=2-biphenyl)
The methyl ether (534) prepared as described in example 125 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 4 h, to
give (after crystallisation from MeOH/CHZC12/hexane) the phenol (535) (98 %)
as a yellow
solid, mp 198-203 °C (dec).'H NMR [(CD3)ZSO] 8 11.61 (br s, 1H), 10.82
(br s, 1H), 9.22 (br
s, 1H), 8.24 (d, J=2.4 Hz, 1H), 7.52 (m, 1H), 7.45 (m, 3H), 7.37 (d, J=8.7 Hz,
1H), 7.31 (s, 1H),
7.11 (m, SH), 7.03 (dd, J=8.7, 2.5 Hz, 1H). Found: C, 76.38; H, 3.94; N, 6.99.
CZ6H~6Nz03.1/4
H20 requires C, 76.37; H, 4.07; N, 6.85.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-104-
EXAMPLE 127
The preparation of 5-Methoxy-2-f(E)-2-(2-nitrophenyl)ethenyll-1H-indole (536)
(II, Ar=2-
nitrophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-
nitrobenzyl)(triphenyl)phosphonium chloride using the procedure described in
example 37,
except that the ratio of LDA:aldehyde was 1.37:1 and the reaction time was 5
h, to give (after
crystallisation from CHZCIz/hexane) the dime (536) as red-orange crystals (the
pure E
isomer) (47 %), mp 136-138 °C. 1H NMR (CDC13) 8 8.25 (br s, 1H), 7.98
(dd, J=8.2, 1.2 Hz,
1H), 7.78 (dd, J=7.9, 1.0 Hz, 1H), 7.61 (td, J=7.6, 0.9 Hz, 1H), 7.41 (d,
J=16.3 Hz, 1H), 7.39 (td,
J=7.8, 1.3 Hz, 1 H), 7.27 (m, 1 H), 7.13 (d, J=16.3 Hz, 1 H), 7.04 (d, J=2.4
Hz, 1 H), 6.90 (dd,
J=8.8, 2.5 Hz, 1H), 6.62 (d, J=1.8 Hz, 1H), 3.86 (s, 3H). Found: C, 69.44; H,
4.49; N, 9.70.
Cl~HlaNz03 requires C, 69.38; H, 4.79; N, 9.52.
EXAMPLE 128
The preparation of 9-Methoxy-4-(2-nitrophenyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(537) (V, R'°=H, Ar=2-nitrophenyl)
The pure E dime (536) prepared as described in example 127 was subjected to
successive reactions with maleimide and then DDQ according to the above
representative
procedure described in example 123 , to give (after crystallisation from
MeOH/EtOAc/CHZCIz/hexane) the product (537) (97 %) as a yellow-orange solid,
mp 294-
298 °C. 1H NMR [(CD3)zS0] 8 12.01 (br s, 1H), 11.10 (br s, 1H), 8.44
(d, J=2.5 Hz, 1H), 8.21
(dd, J=8.1, 1.0 Hz, 1H), 7.86 (td, J=7.5, 1.4 Hz, 1H), 7.74 (td, J=7.8, 1.3
Hz, 1H), 7.67 (s, 1H),
7.65 (dd, J=7.6, 1.4 Hz, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.25 (dd, J=8.7, 2.6~Hz,
1H), 3.90 (s, 3H).
Found: C, 64.99; H, 3.42; N, 10.79. CZ1H~3N305 requires C, 65.12; H, 3.38; N,
10.85.
EXAMPLE 129
The preparation of 9-H dery-4-(2-nitrophen~pyrrolol3,4-clcarbazole-1,3(2H,6H)-
dione
(538) (VI, R'°=H, Ar=2-nitrophen 1
The methyl ether (537) prepared as described in example 128 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 5 h with
equiv. BBr3, then a further 21 h with an extra 5 equiv. BBr3, to give (after
crystallisation
from MeOH/CHZCIz/hexane) the phenol (538) (53 %) as an orange solid, mp 322-
330 °C.'H
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-105-
NMR [(CD3)zS0] 8 11.88 (br s, 1H), 11.05 (br s, 1H), 9.29 (br s, 1H), 8.30 (d,
J=2.4 Hz, 1H),
8.20 (dd, J=8.2, 1.1 Hz, 1H), 7.85 (td, J=7.5, 1.2 Hz, 1H), 7.73 (td, J=7.9,
1.4 Hz, 1H), 7.63 (dd,
J=7.6, 1.4 Hz, 1H), 7.61 (s, 1H), 7.47 (d, J=8.7 Hz, 1H), 7.09 (dd, J=8.7, 2.5
Hz, 1H). Found: C,
63.03; H, 3.27; N, 10.59. CZ°II»N305.1/2 MeOH requires C, 63.24; H,
3.37; N, 10.79.
EXAMPLE 130
The preparation of 4-(2-Aminophenyl)-9-methoxyp~rrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(539) (V, R'°=H, Ar=2-aminophenyl)
A solution of the nitro derivative (537) (71 mg, 0.183 mmol) prepared as
described in
example 128 in 2:1 THF/MeOH (45 mL) containing wet 10% Pd-C (71 mg) was
hydrogenated at 60 psi for 7 h. The solution was filtered throught celite, the
celite and
catalyst were washed thoroughly with MeOH and THF, then the filtrate was
concentrated to
dryness. Crystallisation of the residue from MeOH/CHZCIz/hexane gave the amine
(539) (67
mg, 97%) as a yellow solid, mp >320 °C. 1H NMR [(CD3)2S0] S 11.80 (br
s, 1H), 10.94 (br s,
1H), 8:47 (d, J=2.6 Hz, 1H), 7.54 (d, J=8.8 Hz, 1H), 7.51 (s, 1H), 7.21 (dd,
J=8.8, 2.6 Hz, 1H),
7.08 (td,J=7.7, 1.5 Hz, 1H), 7.01 (dd, J=7.6, 1.5 Hz, 1H), 6.73 (dd, J=8.1,
0.6 Hz, 1H), 6.61 (td,
J=7.4, 0.9 Hz, 1H), 4.70 (s, 2H), 3.89 (s, 3H). Found: C, 67.22; H, 4.50; N,
11.03.
Cz~HisN303.Hz0 requires C, 67.19; H, 4.56; N, 11.19.
EXAMPLE 131
The preparation of 4-(2-Aminophenyl)-9-hydroxypyrrolof3.4-clcarbazole-
1,3(2H,6H)-dione
(5401 (VI, R'°=H, Ar=2-aminophenvl)
A solution of the nitro derivative (538) (81 mg, 0.217 mmol) prepared as
described in
example 129 in 2:1 THF/MeOH (60 mL) containing wet 10% Pd-C (81 mg) was
hydrogenated at 60 psi for 12 h. The solution was filtered throught celite,
the celite and
catalyst were washed thoroughly with MeOH and THF, then the filtrate was
concentrated to
dryness. Crystallisation of the residue from MeOH/CHZC12/hexane gave the amine
(540) (60
mg, 81%) as a brown solid, mp >330 °C. 1H NMR [(CD3)ZSO] b 11.67 (br s,
1H), 10.88 (br s,
1H), 9.21 (s, 1H), 8.32 (d, J=2.4 Hz, 1H), 7.46 (s, 1H), 7.42 (d, J=8.6 Hz,
1H), 7.08 (td, J=7.9,
1.5 Hz, 1 H), 7.05 (dd, J=8.7, 2.4 Hz, 1 H), 7.00 (dd, J=7.6, 1.4 Hz, 1 H),
6.72 (br d, J=8.1 Hz,
1H), 6.60 (td, J=7.4, 0.9 Hz, 1H), 4.69 (s, 2H). Found: C, 66.35; H, 4.06; N,
11.76.
Cz°H~3N3O3.HZO requires C, 66.48; H, 4.18; N, 11.63.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-106-
EXAMPLE 132
The pr~aration of 4-(2-Hydroxyphenyl)-9-methoxypyrrolof3,4-clcarbazole-1,3(2H
6H)-
dione (541) (V, R'°=H, Ar=2-hydroxyphenyl)
A solution of the amine (539) (129 mg, 0.361 mmol) prepared as described in
example 130 in 90% HZS04 (7.5 mL) was cooled to -10 °C and diluted with
ice-water (20 mL).
After stirring at -10 °C for 10 min, the resulting suspension was
treated dropwise with a solution
of NaN02 (38 mg, 0.551 mmol) in cold water (2x 1 mL), and stirred at -10 to -5
°C for 30 min.
A solution of urea (13 mg, 0.216 mmol) in cold water (2x 0.75 mL) was added
and the mixture
was stirred at -5 °C for 5 min. Finally, a suspension of KI (400 mg,
2.41 mmol) and CuI (402
mg, 2.11 mmol) in cold water (2x 2.5 mL) was added, then the cooling bath was
removed and
the mixture was stirred for 10 min, then at 60-65 °C for 2 h. An
aqueous solution of sodium
metabisulfite (100 mL of 5%) was added, then the pH was adjusted to pH = 3
with NaHC03 and
the mixture extracted with EtOAc (Sx 100 mL). The extracts were washed with
water (200 mL),
the water was extracting with EtOAc, the combined organic extracts were
concentrated to
dryness. The residue was adsorbed onto silica gel and chromatographed. Elution
with 0-0.5%
MeOH/CHzCl2 followed but 0.5% MeOH/CHZCIz gave a mixture of crude iodides
(543,544)
(24 mg, see experiment below for analytical data). Elution with 1% MeOH/CHZCIz
gave
(after crystallisation from THF/CHzCl2/pentane) the phenol (541) (31 mg, 24 %)
as a bright
yellow solid, mp 264-267 °C.'H NMR [(CD3)ZSO] 8 11.82 (br s, 1H), 10.95
(br s, 1H), 9.41 (br
s, 1 H), 8.47 (d, J=2.6 Hz, 1 H), 7.56 (s, 1 H), 7.54 (d, J=8.9 Hz, 1 H), 7.25
(dd, J=7.4, 1.6 Hz, 1 H),
7.23 (td, J=7.9, 1.7 Hz, 1H), 7.22 (dd, J=8.7, 2.6 Hz, 1H), 6.91 (br d, J=7.5
Hz, 1H), 6.87 (td,
J=7.4, 0.9 Hz, 1H), 3.89 (s, 3H). Found: C, 70.40; H, 4.06; N, 7.95.
CZ~H~4N204 requires C,
70.39; H, 3.94; N, 7.82.
EXAMPLE 133
The preparation of 9-Hydroxy-4-(2-hydroxyphenyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (542) (VI, R'°=H, Ar=2-hvdroxyphenyl)
The methyl ether (541) prepared as described in example 132 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 4 h, to
give (after crystallisation from MeOH/THF/CHZC12/hexane) the phenol (542) (73
%) as a
yellow-orange solid, mp 291-296 °C.'H NMR [(CD3)ZSO] 8 11.67 (br s,
1H), 10.88 (br s, 1H),
9.37 (br s, 1H), 9.22 (br s, 1H), 8.31 (d, J=2.4 Hz, 1H), 7.51 (s, IH), 7.42
(d, J--8.7 Hz, IH), 7.24
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-107-
(dd, J=7.5, 1.5 Hz, 1H), 7.22 (td, J=7.7, 1.8 Hz, 1H), 7.05 (dd, J=8.7, 2.5
Hz, 1H), 6.91 (br d,
J=8.0 Hz, 1H), 6.86 (td, J=7.4, 0.9 Hz, 1H). Found: C, 66.61; H, 3.67; N,
7.70. CZ°H~ZNz04.H20
requires C, 66.30; H, 3.89; N, 7.73. .
EXAMPLE 134
The preparation of 4-(2-lodophenyl)-9-methoxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(543) (V R1°=H, Ar=2-iodophenyl)
A solution of the amine (539) (46 mg, 0.129 mmol) prepared as described in
example
130 in 90% HZS04 (2.5 mL) was cooled to -5 °C, diluted with ice-water
(2.5 mL), then allowed
to warm to 7 °C. The resulting suspension was treated dropwise with a
solution of NaN02 (13.6
mg, 0.197 mmol) in cold water (2x 0.5 mL), and stirred at 7 °C for 8
min. A solution of urea (5
mg, 0.083 mmol) in cold water (2x 0.4 mL) was added and the mixture was
stirred at 7 °C for 4
min. Finally, a suspension of HI (140 mg, 0.843 mmol) and CuI (140 mg, 0.735
mmol) in cold
water (2x 1 mL) was added, then the cooling bath was removed and the mixture
was stirred for
min, then at 60-65 °C for 2 h. An aqueous solution of sodium
metabisulfite (100 mL of 0.5%)
was added, then the mixture was extracted with 20% THF/EtOAc (Sx 100 mL). The
extracts
were washed with water (200 mL), the water was extracting with EtOAc, the the
combined
organic extracts were concentrated to dryness. The residue was adsorbed onto
silica gel and
chromatographed. Elution with 0-0.5% MeOH/CHZC12 followed by 0.5% MeOH/CHZCIz
gave
the crude product (22 mg). This was purified by further chromatography on
silica gel (eluting
with 1% EtOH/CHC13) to yield the crude major iodide (543) (18 mg, 30%), which
was
combined with material from similar reactions, rechromatographed (eluting with
20%
EtOAc/petroleum ether) and crystallised from MeOHlTHF/CHZCIz/hexane as yellow-
orange
crystals, mp 311-315 °C.'H NMR [(CD3)ZSO] 8 11.95 (br s, 1H), 11.07 (br
s, 1H), 8.46 (d,
J=2.6 Hz, 1 H), 7.97 (dd, J=7.7, 0.9 Hz, 1 H), 7.5 8 (d, J=8.8 Hz, 1 H), 7.50
(td, J=7.6, 0.9 Hz, 1 H),
7.48 (s, 1H), 7.44 (dd, J=7.6, 1.8 Hz, 1H), 7.25 (dd, J=8.9, 2.7 Hz, 1H), 7.19
(td, J=7.6, 1.8 Hz,
1H), 3.89 (s, 3H). Found: C, 53.87; H, 2.76; N, 5.98. CZ,H~3IN203 requires C,
53.87; H, 2.80; N,
5.98. FABMS found [M+H]+: 469.0046. CZ~H~4IN203 requires 469.0049.
EXAMPLE 135
The preparation of 9-Hydroxy-4-(2-iodo~henyl)pyrrolof3 4-clcarbazole-
1,3(2H,6H)-dione
(545) (VI, Rl°=H, Ar=2-iodophenyl)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-108-
The methyl ether (543) prepared as described in example 134 was demethylated
with
BBr3 using the procedure described in example 80, to give (after
crystallisation from
MeOH/CHZCIz/hexane) the phenol (545) (88 %) as a yellow-orange solid, mp 217-
223 °C.'H
NMR [(CD3)zS0] 811.82 (br s, 1H), 11.01 (br s, 1H), 9.28 (br s, 1H), 8.32 (d,
J=2.4 Hz, 1H),
7.96 (br d, J=7.8 Hz, 1 H), 7.49 (br t, J=7.7 Hz, 1 H), 7.46 (d, J=8.9 Hz, 1
H), 7.42 (s, 1 H), 7.42
(dd, J=7.4, 1.7 Hz, 1H), 7.19 (td, J=7.6, 1.7 Hz, 1H), 7.08 (dd, J=8.7, 2.5
Hz, 1H). Found: C,
52.93; H, 2.55; N, 6.07. CzoII»IN203 requires C, 52.89; H, 2.44; N, 6.17.
FABMS found
[M+HJ+: 454.9889. CzoIIIZINzO3 requires 454.9893.
EXAMPLE 136
The preparation of (2-Cyanobenz l~phenyl)phosphonium bromide (547)
Bromination of o-tolunitrile with NBS/AIBN, followed by reaction of the crude
bromide with triphenylphosphine (1.2 equiv.), using the procedure described in
example 102,
except that the reaction time for the bromination was 2 h, gave the
phosphonium salt (547)
(70 %) as a light brown powder, mp (CHzCIz/benzene) 237-241 °C.'H NMR
(CDC13) 8 7.90
(dd, J=7.8, 2.3 Hz, 1H), 7.85-7.63 (m, 15H), 7.52 (br t, J=7.7 Hz, 1H), 7.44
(br d, J=7.5 Hz, 1H),
7.38 (tdd, J=7.6, 2.1, 1.0 Hz, 1H), 5.86 (d, J=14.7 Hz, 2H). Found: C, 67.88;
H, 4.42; N, 3.09.
Cz6Hz~BrNP requires C, 68.13; H, 4.62; N, 3.06.
EXAMPLE 137
The preparation of 2-f(E)-2-(5-Methoxy-1H-indol-2-yl)ethenyllbenzonitrile
(548) (II, Ar=2-
cyanophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-
cyanobenzyl)(triphenyl)phosphonium bromide (547) prepared as described in
example 136
using the procedure described in example 37, except that the LDA and aldehyde
were
(sequentially) added at 0 °C, the ratio of LDA:aldehyde was 1.55:1 and
the reaction time was
h, to give (after crystallisation from CHZCIz/hexane) the dime (548) as a
yellow solid (the
pure E isomer) (60 %), mp 192-196 °C.'H NMR (CDC13) 8 8.40 (br s, 1H),
7.79 (d, J=8.1 Hz,
1H), 7.66 (dd, J=8.1, 0.9 Hz, 1H), 7.58 (td, J=7.9, 1.3 Hz, 1H), 7.32 (td,
J=8.0, 0.9 Hz, 1H), 7.29
(d, J=8.5 Hz, 1H), 7.28 (d, J=16.9 Hz, 1H), 7.18 (d, J=16.4 Hz, 1H), 7.04 (d,
J=2.4 Hz, 1H), 6.91
(dd, J=8.9, 2.4 Hz, 1H), 6.64 (d, J=1.8 Hz, 1H), 3.86 (s, 3H). Found: C,
79.05; H, 5.16; N, 10.31.
ClgH~4N20 requires C, 78.81; H, 5.14; N, 10.21.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-109-
EXAMPLE 138
The nrebaration of 2-(9-Methoxy-1 3-dioxo-1,2,3,6-tetrahvdropyrrolo(3,4-
clcarbazol-4-
yl)benzonitrile (549) (V, R1°=H, Ar=2-cyanophenyl)
The E dime (548) prepared as described in example 137 was subjected to
successive
reactions with maleimide and then DDQ according to the above representative
procedure
described in example 123 , except that most of the poorly soluble final
product was obtained
by filtration of the diluted product solution, then washing thoroughly with
aqueous NaHC03
solution, 15% MeOH/CHZCIz and water, with the reminder being obtained by the
extraction
of the water phase with ethyl acetate. The organic layer was dried, the drying
agent was
removed and the solution was concentrated to dryness. Crystallization from
THF/CHZCIZ/hexane, to give the product (549) (96 %) as a yellow solid, mp 348-
350 °C.'H
NMR [(CD3)ZSO] 8 12.06 (br s, 1H), 11.19 (br s, 1H), 8.48 (d, J=2.6 Hz, 1H),
7.96 (dd, J=7.7,
1.0 Hz, 1H), 7.82 (td, J=7.7, 1.4 Hz, 1H), 7.71 (br d, J=7.4 Hz, 1H), 7.71 (s,
1H), 7.66 (td, J=7.6,
1.1 Hz, 1H), 7.60 (d, J=8.9 Hz, 1H), 7.27 (dd, J=8.8, 2.6 Hz, 1H), 3.90 (s,
3H). EIMS found M+:
367.0956. C22HI3N3O3 requires 367.0957.
EXAMPLE 139
The preparation of 2-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydropyl'rolof3,4-
clcarbazol-4-
1 benzonitrile (550) (VI, R'°=H, Ar=2-cyanophenyl)
The methyl ether (549) prepared as described in example 138 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 5 h with
equiv. BBr3, then a further 4 h with an extra 5 equiv. BBr3, to give (after
crystallisation
from THF/CHZC12/hexane) the phenol (550) (83 %) as a yellow solid, mp 199-204
°C (dec).
'H NMR [(CD3)zS0] b 11.93 (br s, 1H), 11.13 (br s, 1H), 9.31 (br s, 1H), 8.34
(d, J=2.4 Hz,
1H), 7.96 (dd, J=7.6, 0.9 Hz, 1H), 7.82 (td, J=7.7, 1.4 Hz, 1H), 7.70 (br d,
J=7.5 Hz, 1H), 7.65
(s, 1H), 7.65 (td, J=7.7, 1.0 Hz, 1H), 7.48 (d, J=8.7 Hz, 1H), 7.10 (dd,
J=8.7, 2.5 Hz, 1H).
Found: C, 70.17; H, 3.46; N, 11.49. CzIHI,N303.1/4 HZO requires C, 70.49; H,
3.24; N, 11.74.
EXAMPLE 140
The preparation of 5-Methoxy-2-f(E)-2-(3-nitrophenyl)ethenyll-1H-indole (551)
(II, Ar=3-
nitrophenyl)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-110-
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (3-
nitrobenzyl)(triphenyl)phosphonium bromide using the procedure described in
example 37,
except that the LDA and aldehyde were sequentially added at 0 °C, the
ratio of LDA:aldehyde
was 1.5:1 and the reaction time was 5 h, to give (after crystallisation from
MeOH/CHZC12/hexane) the dime (551 ) as an orange-red solid (the pure E isomer)
(S 1 %),
mp 178-182 °C.'H NMR [(CD3)ZSO] 8 11.27 (br s, 1H), 8.34 (t, J=1.9 Hz,
1H), 8.09 (dd, J=8.1,
1.8 Hz, 1 H), 7.99 (d, J=7.9 Hz, 1 H), 7.67 (t, J=8.0 Hz, 1 H), 7.47 (d,
J=16.5 Hz, 1 H), 7.26 (d,
J=8.6 Hz, 1H), 7.25 (d, J=16.6 Hz, 1H), 7.03 (d, J=2.4 Hz, 1H), 6.77 (dd,
J=8.8, 2.5 Hz, 1H),
6.61 (d, J=1.6 Hz, 1H), 3.76 (s, 3H). Found: C, 69.36; H, 4.77; N, 9.78.
C»H141V203 requires C,
69.38; H, 4.79; N, 9.52.
EXAMPLE 141
The preparation of 9-Methoxy-4-(3-nitrophenyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(552) (V, R'°=H, Ar=3-nitrophenyl)
The pure E dime (551) prepared as described in example 140 was subjected to
successive reactions with maleimide and then DDQ according to the above
representative
procedure described in example 123, except that the poorly soluble final
product was
obtained by filtration of the diluted product solution, then washing
thoroughly with aqueous
NaHC03 solution, 15% MeOH/CHZCIz and water, followed by crystallization from
MeOH/T'HF, to give the product (552) (81 %) as a brown solid, mp >340
°C. 1H NMR
[(CD3)ZSO] 8 11.99 (br s, 1H), 11.18 (br s, 1H), 8.50 (d, J=2.5 Hz, 1H), 8.48
(t, J=1.9 Hz, 1H),
8.31 (dd, J=8.1, 1.7 Hz, 1H), 8.11 (br d, J=7.8 Hz, 1H), 7.78 (t, J=8.0 Hz,
1H), 7.75 (s, 1H), 7.58
(d, J=8.8 Hz, 1H), 7.25 (dd, J=8.8, 2.6 Hz, 1H), 3.90 (s, 3H). Found: C,
62.17; H, 3.52; N, 10.33.
CZ1H~3N305.Hz0 requires C, 62.22; H, 3.73; N, 10.37.
Example 142
The preparation of 9-Hydroxy-4-(3-nitrophenyl)pyrrolof3,4-c~lcarbazole-
1,3(2H,6H)-dione
(553) (VI, RI°=H, Ar=3-nitrophenyl)
The methyl ether (552) prepared as described in example 142 was demethylated
with
pyridinium hydrochloride using the procedure described in example 81, to give
(after
crystallisation from THF/hexane) the phenol (553) (74 %) as an orange-brown
solid, mp 300-
310 °C (dec). IH NMR [(CD3)ZSO] S 11.86 (br s, lI-~, 11.11 (br s, 1H),
9.29 (br s, 1H), 8.46 (t,
J=1.9 Hz, 1H), 8.35 (d, J=2.4 Hz, 1H), 8.30 (ddd, J=8.2, 2.5, 0.8 Hz, 1H),
8.10 (dt, J=7.8, 1.2
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-111-
Hz, 1 H), 7.77 (t, J=8.0 Hz, 1 H), 7.70 (s, 1 H), 7.47 (d, J=8.7 Hz, 1 H),
7.09 (dd, J=8.7, 2.5 Hz,
1H). FABMS found M+: 373.0692. CZ°H11N305 requires 373.0699.
EXAMPLE 143
The ~reDaration of (2-Chloro-3-nitrobenzyl)(triphenyl)phosphonium bromide
(554)
Bromination of 2-chloro-3-nitrotoluene with NBS/AIBN, followed by reaction of
the
crude bromide with triphenylphosphine, using the procedure described in
example 102,
except that the reaction time for the bromination was 2 h and the displacement
reaction was
performed at 70 °C for 1 d, gave the phosphonium salt (554) (44 %) as
alight brown solid,
mp (CHZCIz/benzene) 224-228 °C.'H NMR (CDC13) 8 8.09 (br d, J=8.3 Hz,
1H), 7.86-7.63 (m,
16H), 7.34 (t, J=8.1 Hz, 1H), 5.95 (d, J=14.6 Hz, 2H). Found: C, 58.68; H,
4.12; N, 2.79.
CZSH2°BrCIlVOzP requires C, 58.56; H, 3.93; N, 2.73.
EXAMPLE 144
The-preparation of 2-f(E)-2-(2-Chloro-3-nitrophenyl)ethenyll-5-methoxy-1H-
indole (555) (II,
Ar=2-chloro-3-nitrophen~
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-chloro-3-
nitrobenzyl)(triphenyl)phosphonium bromide (554) preared as described in
example 143
using the procedure described in example 37, except that the LDA and aldehyde
were
(sequentially) added at 0 °C, the ratio of LDA:aldehyde was 1.5:1 and
the reaction time was 5
h, to give (after crystallisation from CHZC12/pentane) the dime (555) as an
orange crystalline
solid (the pure E isomer) (50 %), mp 131-133 °C.'H NMR (CDCl3) 8 8.24
(br s, 1H), 7.86 (dd,
J=8.0, 1.4 Hz, 1H), 7.63 (dd, J=7.9, 1.4 Hz, 1H), 7.40 (t, J=8.0 Hz, 1H), 7.28
(d, J=8.5 Hz, 1H),
'7.25 (d, J=15.8 Hz, 1H), 7.15 (d, J=16.4 Hz, 1H), 7.05 (d, J=2.4 Hz, 1H),
6.91 (dd, J=8.9, 2.4
Hz, 1H), 6.65 (d, J=1.7 Hz, 1H), 3.86 (s, 3H). Found: C, 61.97; H, 3.92; N,
8.50. C1~H13C1NZO3
requires C, 62.11; H, 3.99; N, 8.52.
EXAMPLE 145
The preparation of 4-(2-Chloro-3-nitrophenyl)-9-methoxypyrrolof3,4-clcarbazole-
1.3(2H 6H)-dione (556) (V, R'°=H, Ar=2-chloro-3-nitrophenyl)
The pure E dime (555) prepared as described in example 144 was subjected to
successive reactions with maleimide and then DDQ according to the procedure
described in
example 123 , to give (after crystallisation from THF/pentane) the product
(556) (93 %) as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-112-
yellow-brown solid, mp 315-320 °C (dec).'H NMR [(CD3)ZSO] b 12.07 (br
s, 1H), 11.16 (br s,
1H), 8.46 (d, J=2.6 Hz, 1H), 8.11 (dd, J=8.1, 1.5 Hz, 1H), 7.82 (dd, J=7.7,
1.6 Hz, 1H), 7.69 (t,
J=7.9 Hz, IH), 7.69 (s, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.26 (dd, J=8.8, 2.6 Hz,
1H), 3.90 (s, 3H).
Found: C, 60.47; H, 3.17; N, 9.78. CZ~H,ZC1N305.1/4 THF requires C, 60.08; H,
3.21; N, 9.55.
EXAMPLE 146
The preparation of 4-(2-Chloro-3-nitrophenyl)-9-hydroxypymolo(3,4-clcarbazole-
1,3(2H,6H)-dione (557) (VI, RI°=H, Ar=2-chloro-3-nitrophenyl)
The methyl ether (556) prepared as described in example 145 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 5 h with
equiv. BBr3, to give (after crystallisation from THF/CHZCIz/pentane) the
phenol (557) (84
%) as a yellow solid, mp 304-308 °C.'H NMR [(CD3)ZSO] b 11.92 (br s,
1H), 11.11 (br s, 1H),
9.30 (s, 1 H), 8.31 (d, J=2.4 Hz, 1 H), 8.1 I (dd, J=8.2, 1.5 Hz, 1 H), 7.81
(dd, J=7.6, 1.5 Hz, 1 H),
7.68 (t, J=7.9 Hz, 1H), 7.63 (s, 1H), 7.47 (d, J=8.7 Hz, 1H), 7.10 (dd, J=8.7,
2.5 Hz, 1H). Found:
C, 58.70; H, 2.72; N, 10.10. CZ°I-h°C1N305 requires C, 58.91; H,
2.47; N, 10.30.
EXAMPLE 147
The preparation of 4-(3-Amino-2-chlorophenyl)-9-hydroxypyrrolo(3,4-clcarbazole-
1 3(2H 6H)-dione (558) (VI, R1°= H, Ar=3-amino-2-chlorophenyl)
A mixture of the nitro derivative (557) (66 mg, 0.162 mmol) prepared as
described in
example 146 and freshly prepared (wet) nickel boride (202 mg) in MeOH (4.8 mL)
and 1M
HCl (1.2 mL) was stirred at reflux for 100 min. Conc. aqueous ammonia and
aqueous
NaHC03 (100 mL) were added and the mixture extracted with EtOAc (8x 100 mL).
The
extracts were concentrated, adsorbed onto silica gel and chromatographed.
Elution, with 0-1%
MeOH/CHZC12 then 1.5% MeOH/CHZCIZ and THF gave (after crystallisation from
'I'I~YCHZCIz/pentane) the amine (558) (83 %) as a yellow-orange solid, mp 348-
352 °C.'H
NMR [(CD3)2S0] 811.76 (br s, 1H), 10.96 (br s, 1H), 9.25 (br s, 1H), 8.31 (d,
J=2.4 Hz, 1H),
7.45 (s, 1H), 7.44 (d, J=8.7 Hz, 1H), 7.08 (t, J=7.8 Hz, 1H), 7.07 (dd, J=8.7,
2.5 Hz, 1H), 6.86
(dd, J=8.1, 1.5 Hz, 1H), 6.60 (dd, J=7.4, 1.3 Hz, IH), 5.39 (s, 2H). Found: C,
63.79; H, 3.47; N,
10.37. Cz°H12CIN303.1/2 THF requires C, 63.85; H, 3.90; N, 10.15.
EXAMPLE 148
The preparation of (2-Chloro-4-nitrobenz 1~)(triphenyl)phosphonium bromide
(559)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-113-
Bromination of 2-chloro-4-nitrotoluene with NBS/AIBN, followed by reaction of
the
crude bromide with triphenylphosphine, using the procedure described in
example 102,
except that the reaction time for the bromination was 24 h and the
displacement reaction was
performed at 20 °C for 7 d, gave the crude phosphonium salt (559) (34
%) as a light brown
solid, mp (benzene) 70-75 °C (dec).'H NMR [(CD3)zS0] S 8.21 (d, J=2.5
Hz, 1H), 8.13 (dd,
J=8.7, 2.5 Hz, 1H), 7.94 (td, J=7.5, 1.6 Hz, 3H), 7.80-7.66 (m, 12H), 7.43
(dd, J=8.5, 2.6 Hz,
1H), 5.33 (d, J=15.7 Hz, 2H). FABMS found M+=432.0916, 434.0904. CzSHzoCINOzP
requires
432.0921, 434.0891.
EXAMPLE 149
The preparation of 2-f(E)-2-(2-Chloro-4-nitrophenyl)ethenyll-5-methoxy-1H-
indole (560) (II,
Ar=2-chloro-4-nitrophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-chloro-4-
nitrobenzyl)(triphenyl)phosphonium bromide (559) prepared as described in
example 148
using the procedure described in example 37, except that the LDA and aldehyde
were
(sequentially) added at 0 °C, the ratio of LDA:aldehyde was 1.5:1 and
the reaction time was 5
h, to give (after crystallisation from CHZCIz/hexane) the dime (560) as a dark
red-brown
solid (the pure E isomer) (46 %), mp 239-241 °C.'H NMR [(CD3)zS0] 8
11.57 (br s, 1H), 8.32
(d, J=2.0 Hz, 1H), 8.18 (dd, J=8.8, 2.1 Hz, 1H), 8.15 (d, J=8.9 Hz, 1H), 7.59
(d, J=16.3 Hz, 1H),
7.47 (d, J=16.3 Hz, 1H), 7.29 (d, J=8.9 Hz, 1H), 7.05 (d, J=2.4 Hz, 1H), 6.82
(dd, J=8.7, 2.5 Hz,
1H), 6.70 (s, 1H), 3.76 (s, 3H). Found: C, 62.14; H, 3.91; N, 8.52.
C»H13CINz03 requires C,
62.11; H, 3.99; N, 8.52.
Example 150
The preparation of 4-(2-Chloro-4-nitrouhenyl)-9-methoxypyrrolof3,4-clcarbazole-
1.3(2H.6H)-dione (561) (V, R'°=H, Ar=2-chloro-4-nitronhenyl)
'The pure E dime (560) prepared as described in example 149 was subjected to
successive reactions with maleimide and then DDQ according to the procedure
described in
example 123 , to give (after crystallisation from THF/hexane) the product
(561) (98 %) as an
orange solid, mp 279-282 °C.'H NMR [(CD3)zS0] 8 12.06 (br s, 1H),
1.1.20 (br s, 1H), 8.46 (d,
J=2.6 Hz, 1H), 8.43 (d, J=2.2 Hz, 1H), 8.29 (dd, J=8.4, 2.4 Hz, 1H), 7.83 (d,
J=8.4 Hz, 1H), 7.66
(s, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.27 (dd, J=8.8, 2.6 Hz, 1H), 3.90 (s, 3H).
Found: C, 59.83; H,
3.61; N, 9.57. Cz~H~zC1N305.1/4 THF requires C, 60.08; H, 3.21; N, 9.55.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-114-
EXAMPLE 151
The preparation of 4-(2-Chloro-4-nitrophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H 6H)-dione (562) (VI, RI°=H, Ar=2-chloro-4-nitropheny~
The methyl ether (561) prepared as described in example 150 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 5 h with
equiv. BBr3, then a further 5 h with an extra 5 equiv. BBr3, to give (after
crystallisation
from THF/CHZC12/pentane) the phenol (562) (85 %) as a red-orange solid, mp 301-
305 °C.'H
NMR [(CD3)ZSO] 811.94 (br s, 1H), 11.13 (br s, 1H), 9.32 (br s, 1H), 8.43 (d,
J=2.3 Hz, 1H),
8.32 (d, J=2.4 Hz, 1H), 8.29 (dd, J=8.5, 2.3 Hz, 1H), 7.82 (d, J=8.4 Hz, 1H),
7.61 (s, 1H), 7.48
(d, J=8.7 Hz, 1H), 7.10 (dd, J=8.7, 2.5 Hz, 1H). Found: C, 58.81; H, 2.35; N,
10.05.
CZ°H~°C1N305 requires C, 58.-9-1; H, 2.47; N, 10.30.
EXAMPLE 152
The preparation of 4-(4-Amino-2-chlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1.3~2H.6H1-dione (563) (VI, R'°= H, Ar=4-amino-2-chlorophenyl)
A mixture of the nitro derivative (562) (90 mg, 0.221 mmol) prepared as
described in
example 151 and freshly prepared (wet) nickel boride (277 mg) in MeOH (7.2 mL)
and 1M
HCl (1.8 mL) was stirred at reflux for 1 h. Conc. aqueous ammonia and aqueous
NaHC03
(120 mL) were added and the mixture extracted with EtOAc (6 x 80 mL). The
extracts were
concentrated, adsorbed onto silica gel and chromatographed. Elution with 0-2%
MeOH/CHZC12 then elution with THF gave (after crystallisation from
THF/CHZC12/pentane)
the amine (563) (100 %) as an orange solid, mp 293-303 °C (dec). 1H NMR
[(CD3~S0] 8
11.70 (br s, 1H), 10.92 (br s, 1H), 9.23 (br s, 1H), 8.30 (d, J=2.4 Hz, 1H),
7.44 (s, 1H), 7.43 (d,
J =8.7 Hz, 1 H), 7.09 (d, J=8.2 Hz, 1 H), 7.05 (dd, J=8.7, 2.5 Hz, 1 H), 6.71
(d, J=2.2 Hz, 1 H), 6.58
(dd, J=8.1, 2.2 Hz, 1H), 5.51 (s, 2H). Found: C, 60.42; H, 3.64; N, 10.05.
CZ°H~ZC1N303.5/4
H20 requires C, 60.01; H, 3.65; N, 10.50.
EXAMPLE 153
The preparation of (2-Chloro-S-nitrobenzyl)(tr~henyl)phos~honium bromide (564)
Bromination of (2-chloro-5-nitrophenyl)methanol with 30% HBr in acetic acid,
followed by reaction of the crude bromide with triphenylphosphine, using the
procedure
described in example 112, gave the phosphonium salt (564) (63 %) as a white
solid, mp
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-115-
(CHzCl2/benzene) 239-243 °C.'H NMR (CDC13) b 8.22 (br s, 1H), 8.07 (br
d, J=8.7 Hz, 1H),
7.87-7.65 (m, 15H), 7.41 (d, J=8.9 Hz, IH), 5.80 (d, J=14.8 Hz, 2H). Found: C,
58.56; H, 3.93;
N, 2.73. C25H2°BrCINOZP requires C, 58.47; H, 3.98; N, 2.66.
EXAMPLE 154
The preparation of 2-f(E)-2-(2-Chloro-5-nitrophenyl)ethenyll-5-methoxy-IH-
indole (565) (II,
Ar=2-chloro-5-nitrophenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-chloro-5-
nitrobenzyl)(triphenyl)phosphonium bromide (564) prepared as described in
example 153
using the procedure described in example 37, except that the LDA and aldehyde
were
sequentially added at 0 °C; the ratio of LDA:aldehyde was 1.5:1 and the
reaction time was 5
h, to give (after crystallisation from CHZC12/pentane) the dime (565) as an
orange solid (the
pure E isomer) (57 %), mp 191-193 °C.'H NMR (CDCl3) 8 8.54~(d, J=2.6
Hz, 1H), 8.25 (br s,
1H), 8.02 (dd, J=8.8, 2.6 Hz, 1H), 7.56 (d, J=8.9 Hz, 1H), 7.28 (d, J=8.7 Hz,
1H), 7.26 (d,
J=16.4 Hz, 1H), 7.20 (d, J=16.5 Hz, 1H), 7.06 (d, J=2.4 Hz, 1H), 6.91 (dd,
J=8.8, 2.5 Hz, 1H),
6.70 (br s, 1H), 3.86 (s, 3H). Found: C, 61.44; H, 3.92; N, 8.55.
C1~H13C1Nz03.1/4 HZO requires
C, 61.27; H, 4.08; N, 8.41.
EXAMPLE 155
The preparation of 4-(2-Chloro-5-nitrophenyl)-9-methoxypyrrolof3,4-clcarbazole-
1.3f2H.6H)-dione (566) (V, R'°=H, Ar=2-chloro-S-nitrophenvl)
The pure E dime (565) prepared as described in example 154 was subjected to
successive reactions with maleimide and then DDQ according to the above
representative
procedure described in example 123, to give (after crystallisation from
THF/CHZC12/pentane)
the product (566) (95 %) as a yellow-orange solid, mp 285-287 °C.'H NMR
[(CD3)zS0] S
12.06 (br s, I H), 11.16 (br s, 1 H), 8.46 (d, J=2.6 Hz, 1 H), 8.36 (d, J=2.8
Hz, 1 H), 8.33 (dd,
J=8.7, 2.7 Hz, 1H), 7.91 (d, J=8.7 Hz, 1H), 7.71 (s, 1H), 7.60 (d, J=8.9 Hz,
IH), 7.27 (dd,
J=8.9, 2.6 Hz, 1H), 3.90 (s, 3H). Found: C, 57.34; H, 2.68; N, 9.38.
C2~H~ZC1N305.1/4
CHzCl2 requires C, 57.61; H, 2.84; N, 9.48.
EXAMPLE 156
The preparation of 4-(2-Chloro-5-nitrophenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1~H,6H)-dione (567) (VI, R'°=H, Ar=2-chloro-5-nitrophen~
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-116-
The methyl ether (566) prepared as described in example 155 was demethylated
with
BBr3 using the procedure described in example 80, except that the reaction
time was 6 h with
equiv. BBr3, then a further 4 h with an extra 10 equiv. BBr3, to give (after
crystallisation
from THF/CHZCIz/pentane) the phenol (567) (88 %) as a yellow-orange solid, mp
268 °C
(dec).'H NMR [(CD3)zS0] 8 11.94 (br s, 1H), 11.11 (br s, 1H), 9.31 (br s, 1H),
8.35 (d, J=2.6
Hz, 1 H), 8.32 (dd, J=8.6, 2.9 Hz, 1 H), 8.32 (d, J=2.4 Hz, 1 H), 7.91 (d,
J=8.7 Hz, 1 H), 7.66 (s,
1H), 7.48 (d, J=8.7 Hz, 1H), 7.10 (dd, J=8.7, 2.5 Hz, 1H). Found: C, 58.31; H,
2.45; N, 9.98.
CZ°H~°C1N305.1/4HZ0 requires C, 58.27; H, 2.57; N, 10.19.
EXAMPLE 157
The preparation of 4-(5-Amino-2-chlorophen l~ydroxypyrrolo(3,4-clcarbazole-
1 3(2H 6H)-dione (568) (VI, R'°=H, Ar=5-amino-2-chlorophenyl)
A mixture of the vitro derivative (567) (70 mg, 0.172 mmol) prepared as
described in
example 156 and freshly prepared (wet) nickel boride (266 mg) in MeOH (5.6 mL)
and 1M
HCI (1.4 mL) was stirred at reflux for 3 h. Conc. aqueous ammonia and aqueous
NaHC03
(100 mL) were added and the mixture extracted with EtOAc (Sx 70 mL). The
extracts were
washed with water, concentrated, adsorbed onto silica gel and chromatographed.
Elution with
0-2% MeOH/CHZC12 then 3% MeOH/CHZCIZ gave (after crystallisation from
THF/CHZCIz/pentane) the amine (568) (97 %) as an orange solid, mp 301-306
°C (dec).'H
NMR [(CD3)ZSO] 811.78 (br s, 1H), 10.97 (br s, 1H), 9.26 (br s, 1H), 8.31 (d,
J=2.4 Hz, 1H),
7.45 (s, 1H), 7.45 (d, J=8.7 Hz, 1H), 7.13 (d, J=8.3 Hz, 1H), 7.07 (dd, J=8.7,
2.5 Hz, 1H), 6.62
(dd, J=8.2, 2.8 Hz, 1H), 6.60 (d, J=2.3 Hz, 1H), 5.29 (s, 2H). Found: C,
63.09; H, 3.17; N, 10.91.
Cz°H~ZC1N303.1/4H20 requires C, 62.84; H, 3.30; N, 10.99.
EXAMPLE 158
The yreparation of (2-Chloro-3-methoxybenzyl)(triphenyl)phosphonium bromide
(569)
Bromination of 2-chloro-I-methoxy-3-methylbenzene with NBS/AIBN, followed by
reaction of the crude bromide with triphenylphosphine, using the procedure
described in
example 102, except that the reaction time for the bromination was 5 h, gave a
mixture of
phosphonium salts containing (569) (20 %) as a light brown solid which was
used without
further purification. A small amount of the pure salt (569) was obtained by
crystallisation of
the mother liquors as white needles, mp (CHZC12/benzene) 228-231 °C. tH
NMR (CDCl3) 8
7.81-7.60 (m, 15H), 7.17 (dt, J=7.8, 2.0 Hz, 1H), 7.11 (t, J=8.0 Hz, 1H), 6.87
(dt, J=8.2, 1.8 Hz,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-117-
1H), 5.65 (d, J=14.4 Hz, 2H), 3.81 (s, 3H). Found: C, 62.88; H, 4.64.
C~6H23BrC10P requires C,
62.73; H, 4.66.
EXAMPLE 159
The~renaration of 2-f(E)-2-(2-Chloro-3-methoxvphenyl)ethenyll-5-methoxy-1H-
indole
(570) (II Ar=2-chloro-3-methoxyphenyl) and 2-chloro-3-f(E)-2-(5-methoxy-1H-
indol-2-
yl)ethenyllQhenol (571) (II, Ar=2-chloro-3-hydroxyphenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with very crude (2-
chloro-
3-methoxybenzyl)(triphenyl)phosphonium bromide (569) prepared as described in
example
158 using the procedure described in example 37, except that the LDA and
aldehyde were
sequepfially added at 0 °C,-the ratio of LDA-:aldehyde was 1.5:1 and
the reaction time was 5
h, to give a crude mixture of dimes, which were chromatographed on silica gel.
Elution with
0-50% CHZCIz/petroleum ether then elution with 60% CHzCIZ/petroleum ether gave
(after
crystallisation from CHZC12/pentane) the major product dime (570) as a pale
yellow solid
(the pure E isomer) (31 %), mp 179-183 °C. 1H NMR (CDC13) 8 8.22 (br s,
1H), 7.32 (d,
J=16.4 Hz, 1H), 7.31 (dd, J=8.2, 1.4 Hz, 1H), 7.26 (d, J=8.7 Hz, 1H), 7.23 (t,
J=8.0 Hz, 1H),
7.09 (d, J=16.5 Hz, 1H), 7.04 (d, J=2.4 Hz, 1H), 6.87 (dd, J=8.7, 2.5 Hz, 1H),
6.85 (dd, J=7.8,
1..3 Hz, 1H), 6.58 (d, J=1.6 Hz, 1H), 3.93 (s, 3H), 3.85 (s, 3H). Found: C,
68.79; H, 5.22; N,
4.51. C~$H~6C1N02 requires C, 68.90; H, 5.14; N, 4.46. Further elution of the
column with
60% CHZC12/petroleum ether and CHZCIZ gave (after crystallisation from
CHZC12/pentane)
the minor product dime (571) as a pale yellow solid (the pure E isomer) (25
%), mp 172-175
°C.'H NMR (CDCl3) 8 8.20 (br s, 1H), 7.27 (m, 1H), 7.26 (d, J=8.7 Hz,
1H), 7.19 (t, J=7.9 Hz,
1H), 7.18 (d, J=16.4 Hz, 1H), 7.10 (d, J=16.4 Hz, 1H), 7.04 (d, J=2.4 Hz, 1H),
6.95 (dd, J=8.1,
1.5 Hz, 1H), 6.88 (dd, J=8.8, 2.5 Hz, 1H), 6.60 (d, J=1.7 Hz, 1H), 5.65 (s,
1H), 3.86 (s, 3H).
Found: C, 67.23; H, 4.72; N, 4.72. C»HI4C1N02.1/4 H20 requires C, 67.11; H,
4.80; N, 4.60.
EXAMPLE 160
The preparation of 4-(2-Chloro-3-hydroxyphenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1.3(2H,6H)-dione (572) (V, R'°=H Ar=2-chloro-3-hvdroxypheny~
A foil-covered mixture of the pure E dime (571) (110 mg, 0.367 mmol) prepared
as
described in example 159 and maleimide (180 mg, 1.85 mmol) in dry toluene (2
mL) was
stirred in a sealed vial at reflux for 24 h (Method 4a). The resulting thick
suspension was
transferred to a flask using dioxane (5 mL), then treated with manganese
dioxide (738 mg,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-118-
8.49 mmol), stirnng at reflux under Nz for 22 h (as decribed in the procedure
for example
79), after addition of water and extraction with ethyl acetate. The organic
phase was dried,
the drying agent was removed and the solvent concentrated to dryness.
Chromatography and
crystallisation from TI-IF/CHZC12/pentane the product (572) (34 %) as an
orange-brown solid,
mp 280-290 °C (dec).'H NMR [(CD3)ZSO) 8 11.92 (br s, 1H), 11.04 (br s,
1H), 10.23 (br s,
1H), 8.46 (d, J=2.6 Hz, 1H), 7.57 (d, J=8.8 Hz, 1H), 7.52 (s, 1H), 7.24 (dd,
J=8.9, 2.6 Hz, 1H),
7.21 (t, J=7.8 Hz, 1H), 7.05 (dd, J=8.2, 1.5 Hz, 1H), 6.88 (dd, J=7.5, 1.4 Hz,
1H), 3.89 (s, 3H).
Found: C, 64.09; H, 3.48; N, 7.25. CZiHi3C1N2O4 requires C, 64.21; H, 3.34; N,
7.13.
EXAMPLE 161
The preparation of 4-(2-Chloro-3-hydroxyphenyl)-9-hydroxypyrrolo(3,4-
clcarbazole-
1,3(2H,6H)-dione (573) (VI, R'°=H, Ar=2-chloro-3-hvdroxvnhenvl)
The methyl ether (572) prepared as described in example 160 was demethylated
with
BBr3 using the procedure described in example 80, to give (after
crystallisation from
THF/CHZCh/pentane) the phenol (573) (91 %) as a yellow-orange solid, mp 197-
203 °C
(dec).'H NMR [(CD3)zS0) 8 11.78 (br s, 1H), 10.98 (br s, 1H), 10.17 (br s,
1H), 9.26 (s, 1H),
8.31 (d, J=2.4 Hz, 1H), 7.47 (s, 1H), 7.45 (d, J=8.7 Hz, 1H), 7.20 (t, J=7.8
Hz, 1H), 7.07 (dd,
J=8.7, 2.5 Hz, 1H), 7.04 (dd, J=8.3, 1.5 Hz, 1H), 6.87 (dd, J=7.5, 1.4 Hz,
1H). Found: C, 62.06;
H, 3.29; N, 7.36. CZ°HI~C1N204.1/2 H20 requires C, 61.95; H, 3.12;
N, 7.22.
EXAMPLE 162
The preparation of (2-Chloro-4-methoxybenzyl)(triphenyl)phosphonium bromide
(574)
Bromination of (2-chloro-4-methoxyphenyl)methanol with 30% HBr in acetic acid,
followed by reaction of the crude bromide with triphenylphosphine, using the
procedure
described in example 112, except that the reaction time for the bromination
was 4 h and the
reaction time for the displacement was 28 h, gave the phosphonium salt (574)
(100 %) as a
white solid, mp (CHZC12/benzene) 223-226 °C. 1H NMR (CDC13) 8 7.82-7.61
(m, 15H), 7.53
(dd, J=8.4, 2.7 Hz, 1 H), 6.72 (d, J=2.8 Hz, 1 H), 6.70 (dd, J=8.9, 2.5 Hz, 1
H), 5.57 (d, J=13.6 Hz,
2H), 3.74 (s, 3H). Found: C, 63.05; H, 4.74. Cz6Hz3BrC10P requires C, 62.73;
H, 4.66.
EXAMPLE 163
The preparation of 2-((E)-2-(2-Chloro-4-methoxyphenyl)ethenyll-5-methoxy-1H-
indole
(575) (II, Ar=2-chloro-4-methoxyphen~
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-119-
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-chloro-4-
methoxybenzyl)(triphenyl)phosphonium bromide (574) prepared as described in
example 162
using the procedure described in example 37, except that the LDA and aldehyde
were
sequentially added at 0 °C, the ratio of LDA:aldehyde was 1.5:1 and the
reaction time was 5
h, to give (after crystallisation from CHZC12/pentane) the dime (575) as a
pale yellow solid
(the pure E isomer) (64 %), mp 129-132 °C.'H NMR (CDC13) 8 8.17 (br s,
1H), 7.60 (d, J=8.8
Hz, 1H), 7.25 (d, J=8.3 Hz, 1H), 7.19 (d, J=16.5 Hz, 1H), 7.03 (d, J=2.4 Hz,
1H), 6.96 (d, J=17.3
Hz, 1H), 6.94 (d, J=2.4 Hz, 1H), 6.86 (dd, J=8.7, 2.6 Hz, 1H), 6.85 (dd,
J=8.7, 2.8 Hz, 1H), 6.54
(d, J=1.7 Hz, 1H), 3.85 (s, 3H), 3.83 (s, 3H). Found: C, 69.05; H, 5.41; N,
4.68. C~8H16C1N0z
requires C, 68.90;. H, 5.14; N, 4.46.
EXAMPLE 164
The preparation of 4-(2-Chloro-4-methoxyphenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (576) (V, R'°=H, Ar=2-chloro-4-methoxvnhenvll
The pure E dime (575) prepared as described in example 163 was subjected to
successive reactions with maleimide and then DDQ according to the above
representative
procedure described in example 123 , to give (after crystallisation from
THF/pentane) the
product (576) (86 %) as a yellow-orange solid, mp 284-286 °C. 1H NMR
[(CD3)ZSO] 811.93
(br s, 1H), 11.06 (br s, 1H), 8.46 (d, J=2.6 Hz, 1H), 7.56 (d, J=8.9 Hz, 1H),
7.54 (s, 1H), 7.41 (d,
J=8.5 Hz, 1H), 7.24 (dd, J=8.9, 2.6 Hz, 1H), 7.15 (d, J=2.6 Hz, 1H), 7.01 (dd,
J=8.5, 2.6 Hz,
1H), 3.89 (s, 3H), 3.86 (s, 3H). Found: C, 65.30; H, 4.79; N, 5.82.
CZZHisC1N20a.THF requires
C, 65.20; H, 4.84; N, 5.85.
EXAMPLE 165
The preparation of 4-(2-Chloro-4-hydroxyphenyl)-9-h~~pyrrolof3,4-clcarbazole-
1,3.(2H,6H)-dione (577) (VI, R'°=H, Ar=2-chloro-4-hydroxyphenyl)
The methyl ether (576) prepared as described in example 164 was bis-
demethylated
with BBr3 using the procedure described in example 80, except that the
reaction time was S h
with lO equiv. BBr3, to give (after crystallisation from THF/CHZC12/pentane)
the phenol
(577) (90 %) as a yellow-orange solid, mp 330-340 °C (dec). 1H NMR
[(CD3~S0] 8 11.75 (br
s, 1 H), 10.97 (br s, 1 H), 10.00 (br s, 1 H), 9.25 (s, 1 H), 8.31 (d, J=2.4
Hz, 1 H), 7.47 (s, 1 H), 7.44
(d, J=8.7 Hz, 1H), 7.27 (d, J=8.4 Hz, 1H), 7.06 (dd, J=8.7, 2.5 Hz, 1H), 6.93
(d, J=2.5 Hz, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-120-
6.82 (dd, J=8.3, 2.4 Hz, 1H). Found: C, 62.63; H, 3.44; N, 7.10.
CZOHl~C1N204.1/4 H20 requires
C, 62.68; H, 3.02; N, 7.31.
EXAMPLE 166
The preparation of (2-Chloro-5-methoxybenzyl)(triphenyl)phosphonium bromide
(578)
Bromination of 1-chloro-4-methoxy-2-methylbenzene with NBS/AIBN, followed by
reaction of the crude bromide with triphenylphosphine, using the procedure
described in
example 102, except that the reaction time for the bromination was 4 h, gave
the
phosphonium salt (578) (76 %) as a light brown solid, mp (CHZCl2/benzene) 189-
190.5 °C.
'H NMR (CDC13) S 7.82-7.61 (m, 15H), 7.21 (t, J=2.8 Hz, 1H), 7.04 (dd, J=8.8,
0.8 Hz, 1H),
6.76 (df; J=8.9, 2.7 Hz, 1H), 5.59 (d, J=14.4 Hz, 2H), 3.58 (s, 3H). Found: C,
62.75; H, 4.70.
Cz6H23BrCIOP requires C, 62.73; H, 4.66.
EXAMPLE 167
-The preparation of 2-f(E)-2-(2-Chloro-5-methoxyphenyl)ethenyll-5-methoxy-1H-
indole
(579) (II, Ar=2-chloro-5-methoxyphenyl)
The 5-methoxy-1H-indole-2-carbaldehyde (1) was reacted with (2-chloro-5-
methoxybenzyl)(triphenyl)phosphonium bromide (578) prepared as described in
example 166
using the procedure described in example 37, except that the LDA and aldehyde
were
(sequentially) added at 0 °C, the ratio of LDA:aldehyde was 1.5:1 and
the reaction time was 5
h, to give (after crystallisation from CHZC12/hexane) the dime (579) as a pale
yellow solid
(the pure E isomer) (85 %), mp 119-121 °C.'H NMR (CDC13) b 8.22 (br s,
1H), 7.29 (d, J=8.9
Hz, 1H), 7.26 (br d, J=8.7 Hz, 1H), 7.22 (d, J=16.5 Hz, 1H), 7.18 (d, J=3.0
Hz, 1H), 7.06 (d,
J=16.7 Hz, 1H), 7.04 (br s, 1 H), 6.88 (dd, J=8.7, 2.5 Hz, 1H), 6.77 (dd,
J=8.8, 3.0 Hz, 1H), 6.59
(d, J=1.8 Hz, 1H), 3.86 (s, 3H), 3.85 (s, 3H). Found: C, 68.70; H, 5.11; N,
4.37. ClsH,6C1N02
requires C, 68.90; H, 5.14; N, 4.46.
EXAMPLE 168
The preparation of 4-(2-Chloro-5-methoxyphenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (580) (V, R1°=H, Ar=2-chloro-5-methox py__ henyl)
The pure E dime (579) prepared as described in example 167 was subjected to
successive reactions with maleimide and then DDQ according to the above
representative
procedure described in example 123 , to give (after crystallisation from
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-121-
MeOH/CHZCIz/hexane) the product (580) (84 %) as a bright orange solid, mp 284-
286 °C.'H
NMR [(CD3)zS0] 811.96 (br s, 1H), 11.08 (br s, 1H), 8.46 (d, J=2.6 Hz, 1H),
7.58 (s, 1H), 7.57
(m, 1H), 7.46 (d, J=8.7 Hz, 1H), 7.25 (dd, J=8.8, 2.6 Hz, 1H), 7.07 (d, J=2.9
Hz, 1H), 7.04 (dd,
J=8.7, 3.1 Hz, 1H), 3.89 (s, 3H), 3.80 (s, 3H). Found: C, 62.50; H, 3.77; N,
6.54.
CzzHisC1N20a.H20 requires C, 62.20; H, 4.03; N, 6.59.
EXAMPLE 169
The preparation of 4-(2-Chloro-5-hydroxyphenyl)-9-hydroxypyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (581) (VI, Rl°=H, Ar=2-chloro-5-hvrdroxyphenyl)
The methyl ether (580) prepared as described in example 168 was bis-
demethylated
with BBr3 using the procedure described in example 80, except that the
reaction time was 6.5
h with 10 equiv. BBr3, to give (after crystallisation from MeOH/CHZCIz/hexane)
the phenol
(581) (90 %) as an orange-red crystalline solid, mp 335-340 °C. 1H NMR
[(CD3)zS0] b 11.80
(br s, 1H), 11.00 (br s, 1H), 9.78 (br s, 1H), 9.27 (br s, 1H), 8.31 (d, J=2.4
Hz, 1H), 7.49 (s, 1H),
7.45 (d, J=8.7 Hz, 1H), 7.32 (d, J=8.5 Hz, 1H), 7.07 (dd, J=8.7, 2.5 Hz, 1H),
6.84 (dd, J=8.5, 2.9
Hz, 1H), 6.82 (d, J=2.8 Hz, 1H). Found: C, 63.25; H, 3.63; N, 6.73.
Cz°I-I»ClNz04.1/2 H20.1/4
Hexane requires C, 63.09; H, 3.82; N, 6.84.
H H
O N O 1 ) MsCI/Et3N O N O
METHOD 11
Me0 ~ ~ ~ Ar 2) Bars HO ~ ~ ~ Ar OR
METHOD 8
N ~ N
R~° (CHz)~OMs
(V). R1o=(CH2)nOH (VII)
(VII)
ZH/80 °C
METHOD 12
or
NaCN
(VIII)
SCHEME 3
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-122-
Scheme 3 Procedures
Representative Procedure for Method 11 of Scheme 3
EXAMPLE 170
The preparation of 2-(4-(2-Chlorophenyl)-9-methoxy-1 3-dioxo-2 3-
dihydropyrrolof3 4-
clcarbazol-6(1H)-yl)ethyl methanesulfonate (V' Ar=2-chlorophenyl
R'°=CH~CH~OSO~CH~
(228)
Alcohol (46) prepared as described in example 43 (1.10 g, 2.61 mmol) was
dissolved
in dry tetrahydrofuran (80 mL) under nitrogen. The resulting solution was
cooled to 0 °C and
triethylamine (2.0 mL) was added followed by methanesulfonyl chloride (263 mL,
3.40
mmol) dropwise. After 30 minutes a further portion of methanesulfonyl chloride
(50 mL) was
added and then after another 30 minutes the reaction was diluted with
saturated sodium
bicarbonate and extracted with ethyl acetate. The organic layer was dried, the
drying agent
was removed and the solution was concentrated to drynessCrystallisation from
ethyl
acetate/hexane gave mesylate (228) (0.96 g, 74%) as a yellow solid, mp 254
°C (dec).'H
IJMR 8 [(CD3)ZSO] 11.15 (s, 1H), 8.53 (d, J=2.6 Hz, 1H), 7.90 (s, 1H), 7.74
(d, J=9.0 Hz,
1H), 7.58 (m, 1H), 7.48 (m, 3H), 7.33 (dd, J=9.0, 2.6 Hz, 1H), 4.89 (t, J=5.0
Hz, 2H), 4.56
(m, 2H), 3.91 (s, 3H), 2.94 (s, 3H). FABMS found [M+H]+: 499.0694, 501.0696.
C24H~9C1NZO6S requires 499.0731, 501.0701.
EXAMPLE 171
The re aration of 2-(4-(2-Chlorophenyl)-9-hydroxy-1 3-dioxo-2 3-
dihydropyrrolof3 4-
;.lcarbazol-6(1H)-yl)ethyl methanesulfonate (VIh Ar=2-chlorophenyl n=2
mesylate) (229)
Mesylate (228) (1.23 g, 2.48 mmol) prepared as described in example 170 was
reacted according to the procedure described in example 80, except that the
reaction time
was 7 hours, after which chromatography on silica eluting with ethyl
acetate/hexane (1:1 to
3:1) and trituration from ethyl acetate/hexane gave phenol (229) (1.05 g, 87%)
as a yellow
solid, mp 266 °C (dec).'H NMR b ((CD3)ZSO] 11.09 (br s, 1H), 9.40 (s,
1H), 8.39 (d, J=2.5
Hz, 1H), 7.84 (s, 1H), 7.62 (d, J=8.9 Hz, 1H), 7.58 (m, 1H), 7.48 (m, 3H),
7.14 (dd, J=8.9,
2.5 Hz, 1H), 4.85 (t, J=5.0 Hz, 2H), 4.54 (m, 2H), 2.93 (s, 3H). Found: C,
58.13; H, 3.74; N,
5.72. C23H,»C1NZO6S.1/4hexane requires C, 58.10; H, 4.08; N, 5.53.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-123-
EXAMPLE 172
The preparation of 6-(3-Bromopropyl)-4-(2-chlorophenyl)-9-hydroxypyrrolof3 4-
~carbazole-1,3(2H,6H)-dione (VII , Ar=2-chlorophenyl, n=3, bromide) (58)
Reaction of alcohol (31 ) prepared as described in example 40 with
methanesulfonyl
chloride, followed by demethylation with BBr3 following the proceedure
described in
example 170 gave the bromide (58) (81%), orange powder, mp 278 ° C
(dec).'H NMR 8
[(CD3)ZSO] 11.08 (s, 1H), 9.39 (s, 1H), 8.39 (d, J=2.4 Hz, 1H), 7.81 (s, 1H),
7.60 (d, J=8.7
Hz, 1H), 7.60-7.58 (m, 1H), 7.53-7.43 (m, 3H), 7.16 (dd, J=8.7, 2.4 Hz, 1H),
4.55 (t, J=6.9
Hz, 2H), 3.56-3.49 (m, 2H), 2.34-2.24 (m, 2H). Found: C, 56.94; H, 3.45, N,
5.69.
Cz3Hi6BrC1N203 requires C, 57.10; H, 3.33; N, 5.79.
Example 173
The preparation of 6-(3-Bromopropyl)-9-hydroxy-4-phen~pyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (VII; Ar=phenyl, n=3, bromide) (204)
Alcohol (202) prepared as described in example 83 (0.50 g, 1.25 mmol) was
reacted
according to the proceedure described in example 170 followed by the procedure
described in
example 80. Chromatography on silica eluting with ethyl acetate/hexane (1:1 to
4:1) followed
by crystallisation from ethyl acetate/hexane gave bromide (204) (0.54 g, 97%)
as an orange
powder, mp 280-282 °C.'H NMR 8 [(CD3)ZSO] 11.07 (s, 1H), 9.36 (s, 1H),
8.41 (d, J=2.2
Hz, 1H), 7.83 (s, 1H), 7.66 (m, 2H), 7.58 (d, J=8.8 Hz, 1H), 7.47 (m, 3H),
7.14 (dd, J=8.8,
2.2 Hz, 1H), 4.57 (t, J=6.9 Hz, 2H), 3.53 (t, J=6.5 Hz, 2H), 2.32 (m, 2H).
Found: C, 61.87; H,
3.69; N, 6.59. C23HI~BrNz03 requires: C, 61.48; H, 3.81; N, 6.23.
EXAMPLE 174
The preparation of 2-(9-Hydroxy-1,3-dioxo-4=phenyl-2,3-dihydropyrrolof3 4-
clcarbazol-
6(1H)-vl)ethyl methanesulfonate (VII; Ar=nhenvl, n=2, mesvlate) (205)
Alcohol (201) (0.21 g, 0.53 mmol) prepared as described in example 82 was
reacted
according ~to the proceedure described in example 170 followed by the
procedure described in
example 80. Chromatography on silica eluting with ethyl acetate/hexane (1:1 to
4:1) followed
by crystallisation from ethyl acetate/hexane gave mesylate (205) (0.19 g, 80%)
as a yellow
solid, mp 271-276 °C. 'H NMR 8 [(CD3)ZSO] 11.08 (s, 1H), 9.37 (br s,
1H), 8.40 (d, J=2.4
Hz, 1H), 7.86 (s, 1H), 7.67 (m, 2H), 7.60 (d, J=8.8 Hz, 1H), 7.46 (m, 3H),
7.13 (dd, J=8.8,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-124-
2.4 Hz, 1H), 4.86 (t, J=4.8 Hz, 2H), 4.57 (t, J=4.8 Hz, 2H), 2.97 (s, 3H).
FABMS found
[M+H]+: 451.0958. Cz3H~$NZO6S requires 451.0964.
EXAMPLE 175
The preparation of 6-(6-Bromohexyl)-9-hydroxy-4-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (VII; Ar=phenyl, n=6, bromide) (206)
Alcohol (203) (0.30 g, 0.67 mmol) prepared as described in example 84 was
reacted
according to the proceedure described in example 170 followed using the
procedure
described in example 80. Chromatography on silica eluting with ethyl
acetate/hexane (1:1 to
4:1) followed by crystallisation from ethyl acetate/hexane gave bromide (206)
(0.29 g, 88%)
as a orange solid, mp 214-216 °C. 1H NMR ~ [(CD3)ZSO] 11.04 (s, 1H),
9.33 (s, 1H), 8.41 (d,
J=2.4 Hz, 1H), 7.78 (s, 1H), 7.65 (m, 2H), 7.56 (d, J=8.8 Hz, 1H), 7.46 (m,
3H), 7.13 (dd,
J=8.8, 2.4 Hz, 1H), 4.46 (t, J=7.1 Hz, 2H), 3.46 (t, J=6.7 Hz, 2H), 1.74 (m,
4H), 1.41-1.29 (m,
4H). Found: C, 63.75; H, 4.72; N, 5.94. Cz6H23BrN203 requires: C, 63.66; H,
4.73; N, 5.71.
EXAMPLE 176
The preparation of 6-(3-Bromopropyl)-4-(2-chloro-6-methoxyphe~l)-9-
hydroxypyrrolof3 4-
clcarbazole-1,3(2H,6H)-dione (VII; Ar=2-chloro-6-methoxvphenvl, n=3, bromide)
(2071
Alcohol (105) (0.20 g, 0.43 mmol) prepared as described in example 58 was
reacted
according to the proceedure described in example 170 followed using the
procedure
described in example 80, except that the boron tribromide reaction was
performed at 0 °C for
2 h and the subsequent worked-up crude was then dissolved in ethyl acetate
(100 mL) to
which lithium bromide (1.0 g) was added. This solution was warmed to 50
°C for 2 h before
being absorbed onto silica and chromatographed eluting with ethyl
acetate/hexane (1:3 to 1:1)
to give bromide (207) (132 mg, 60%) as a yellow solid, mp 286-288 °C.'H
NMR 8
[(CD3)ZSO] 11.03 (s, 1H), 9.38 (s, 1H), 8.38 (d, J=2.4 Hz, 1H), 7.77 (s, 1H),
7.58 (d, J=8.8
Hz, 1H), 7.44 (t, J=8.8 Hz, 1H), 7.18 (d, J=8.8 Hz, 1H), 7.14 (m, 2H), 4.54
(t, J=7.0 Hz, 2H),
3.52 (t, J=6.8 Hz, 2H), 2.27 (m, 2H). FABMS found [M+H]+: 513.0182, 515.0192,
517.0169.
C24H18BrC1N204 requires 513.0217, 515.0196, 515.0187, 517.0167.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-125-
EXAMPLE 177
The preparation of 2-(4-(2,6-Dichloro~henyl)-9-hydroxy-1,3-dioxo-2,3-
dihydronyrrolof3 4-
clcarbazol-6(1H)-yl)ethyl methanesulfonate (VII; Ar=2,6-dichlorophenyl, n=2,
mesylate)
231
Alcohol (230) (1.0 g, 2.2 mmol) prepared as described in example 85 was
reacted
according to the proceedure described in example 170 followed by the procedure
described in
example 80, except that the reaction time with boron tribromide was 30 h.
Chromatography
on silica eluting with ethyl acetate/hexane (1:1 to 4:1) followed by
crystallisation from ethyl
acetate/hexane gave mesylate (231) (0.95 g, 83%) as a yellow solid, mp 255-260
°C.'H
NMR 8 [(CD3)zS0] 11.16 (br s, 1H), 9.43 (br s, 1H), 8.38 (d, J=2.5 Hz, 1H),
7.91 (s, 1H),
7.63 (m, 3H), 7.51 (dd, J=8.7, 7.3 Hz, 1H), 7.16 (dd, J=8.9, 2.5 Hz, 1H), 4.85
(t, J=4.9 Hz,
2H), 4.53 (t, J=4.9 Hz, 2H), 2.88 (s, 3H). Found: C, 53.48; H, 3.22; N, 5.23.
Cz3H~6C12N2O6S
requires: C, 53.18; H, 3.10; N, 5.39.
EXAMPLE 178
-The~re~aration of 6-(3-Bromopropyl)-4-(2,6-dichlorophenyl)-9-
hydroxypyrrolof3,4-
c~carbazole-1,3(2H,6H)-dione (VII; Ar=2,6-dichlorophenyl, n=3, bromide) (233)
Alcohol (232) (0.77 g, 1.68 mmol) prepared as described in example 86 was
reacted
according to the proceedure described in example 170 followed by the procedure
described in
example 80, except that the reaction time with boron tribromide was 18 h.
Chromatography
on silica eluting with ethyl acetate/hexane (1:1 to 4:1) followed by
crystallisation from ethyl
acetate/hexane gave bromide (233) (0.70 g, 80%) as an orange solid, mp 273-276
°C.'H
NMR 8 [(CD3)zS0] 11.15 (br s, 1H), 9.42 (s, 1H), 8.38 (d, J=2.4 Hz, 1H), 7.87
(s, 1H), 7.62
(m, 3H), 7.51 (dd, J=8.9, 7.6 Hz, 1H), 7.18 (dd, J=8.8, 2.4 Hz, 1H), 4.56 (t,
J=6.9 Hz, 2H),
3.51 (t, J=6.7 Hz, 2H), 2.27 (m, 2H). Found: C, 53.44; H, 2.96; N, 5.23.
C23HisBrC12N203
requires: C, 53.30; H, 2.92; N, 5.40.
Representative Procedure for Method 12 of Scheme 3
EXAMPLE 179
The preparation of 6-f3-(Dimethylamino Qropyll-9-hvdroxy-4-phen,~lpyrrolof3,4-
ccarbazole-1,3(2H,6H)-dione (VIII; Ar phenyl, n=3, ~NMe~) (208)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-126-
To a solution of bromide (204) prepared as described in example 173 (0.12 g,
0.27
mmol) in dimethylacetamide (4 mL) was added the amine , dimethylamine (25 mol
eq., 0.85
mL in this case as an 40% aqueous solution). The reaction vessel was sealed
and heated at 80
°C with stirring for 18 h, before being diluted with water. The
resulting solution was then
acidified by the dropwise addition of concentrated hydrochloric acid and the
pH was then
adjusted to approximately pH=9 by the addition of solid potassium carbonate.
The
precipitated product was either collected by filtration and washed with water
and diisopropyl
ether before being dried or extracted with ethyl acetate. The organic layer
was dried, the
drying agent was removed and the solution was concentrated to dryness.
Chromatography on
silica eluting with methanol/dichloromethane (1:9 to 1:4), followed by
trituration in ethyl
acetate/hexane gave amine (208) (52 mg, 47%) as a yellow powder, mp 185-189
°C. 1H NMR
8 [(CD3)zS0] 11.05 (s, 1H), 9.34 (s, 1H), 8.41 (d, J=2.4 Hz, 1H), 7.78 (s,
1H), 7.64 (m, 2H),
7.56 (d, J=8.9 Hz, 1H), 7.47 (m, 3H), 7.14 (dd, J=8.9, 2.4 Hz, 1H), 4.48 (t,
J=6.6 Hz, 2H),
2.16 (t, J=6.5 Hz, 2H), 2.08 (s, 6H), 1.90 (m, 2H). Found: C, 71.98; H, 5.67;
N, 10.14.
Cz5H23N3O3.1/4HZO requires: C, 71.84; H, 5.67; N, 10.05.
EXAMPLE 180
The preparation of 6-f2-(Dimethylamino)ethyll-9-hydroxy-4-phenylpyrrolo(3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=2, Z=NMe2_) (209)
Mesylate (205) prepared as described in example 174 (70 mg, 0.16 mmol) was
reacted with aqueous dimethylamine solution according to the procedure
described in
example 179 to give amine (209) (30 mg, 48%) as a yellow powder, mp 283-286
°C.'H
NMR 8 [(CD3)ZSO] 11.05 (br s, 1H), 9.33 (s, 1H), 8.41 (d, J=2.4 Hz, 1H), 7.77
(s, 1H), 7.65
(m, 2H), 7.54 (d, J=8.8 Hz, 1H), 7.46 (m, 3H), 7.12 (dd, J=8.8, 2.4 Hz, 1H),
4.55 (t, J=6.5 Hz,
2H), 2.62 (t, J=6.5 Hz, 2H), 2.19 (s, 6H). Found: C, 71.92; H, 5.16; N, 10.55.
Cz4H2~N3O3
requires: C, 72.17; H, 5.30; N, 10.52.
EXAMPLE 181
The preparation of 9-Hydroxy-6-f3-(4-morpholinyllpropyll-4-uhenylpymolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=4-morpholinyl) (210)
Bromide (204) (0.10 g, 0.22 mmol) prepared as described im example 173 was
reacted
with morpholine according to the procedure described in example 179to give
amine (210) (73
mg, 72%) as a yellow powder, mp 252-255 °C. 'H NMR 8 [(CD3)zS0] 11.04
(br s, 1H), 9.33
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-127-
(s, 1H), 8.41 (d, J=2.5 Hz, 1H), 7.81 (s, 1H), 7.62 (m, 2H), 7.57 (d, J=8.9
Hz, 1H), 7.47 (m,
3H), 7.12 (dd, J=8.9, 2.5 Hz, 1H), 4.51 (t, J=6.3 Hz, 2H),_3.38 (t, J=4.0 Hz,
4H), 2.18 (br s,
4H), 2.14 (t, J=6.3 Hz, 2H), 1.93 (m, 2H). Found: C, 71.11; H, 5.46; N, 9.29.
C27HZSN3O4
requires: C, 71.19; H, 5.53; N, 9.22.
EXAMPLE 182
The preparation of 9-Hydroxy-6-f2-(4-morpholinyl)ethyll-4-phenylpyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=2, Z=4-morpholinyl) (211)
Mesylate (205) (70 mg, 0.16 mmol) prepared as described in example 174 was
reacted with morpholine according to the procedure described in example 179 to
give amine
(211) (53 mg, 75%) as a yellow powder, mp 260-262 °C.'H NMR ~
[(CD3)ZSO]'H NMR b
[(CD3)ZSO] 11.04 (br s, 1H), 9.33 (s, 1H), 8.40 (d, J=2.5 Hz, 1H), 7.78 (s,
1H), 7.64 (m, 2H),
7.54 (d, J=8.9 Hz, 1H), 7.47 (m, 3H), 7.12 (dd, J=8.9, 2.5 Hz, 1H), 4.57 (t,
J=6.4 Hz, 2H),
3.45 (t, J=4.5 Hz, 4H), 2.67 (t, J=6.4 Hz, 2H), 2.18 (br t, J=4 Hz, 4H).
Found: C, 70.55; H,
5.25; N, 9.22. CZ6Hz3N30a requires: C, 70.74; H, 5.25; N, 9.51.
__EXA-MPLE 183 _-___ ____ _ _ _ - _ _ _ -.. _ _ _
The preparation of 9-H dy roxy-6-f3-(1H-imidazol-1-yl)propyll-4-
phen~pyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=1-imidazolyl) (212)
Bromide (204) (80 mg, 0.18 mmol) prepared as described in example 173 was
reacted
with imidazole according to the procedure described in example 179to give
amine (212) (41
mg, 53%) as a yellow powder, mp 309-311 °C. 'H NMR 8 [(CD3)zS0] 11.06
(s, 1H), 9.36 (s,
1H), 8.40 (d, J=2.3 Hz, 1H), 7.69 (s, 1H), 7.64 (m, 3H), 7.47 (m, 4H), 7.22
(s, 1H), 7.14 (dd,
J=8.8, 2.3 Hz, 1H), 6.89 (s, 1H), 4.44 (t, J=7.4 Hz, 2H), 4.07 (t, J=7.3 Hz,
2H), 2.24 (m, 2H).
Found: C, 70.31; H, 4.78; N, 12.43. CZ6HZON403.1/2H20 requires: C, 70.10; H,
4.75; N,
12.57.
EXAMPLE 184
The preparation of 9-Hydroxy-6-f2-(1H-imidazol-1-yl)ethyll-4-phen~pyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=phen~, n=2, Z=1-imidazolyl) (213)
Mesylate (205) (70 mg, 0.16 mmol) prepared as described in example 174 was
reacted with imidazole according to the procedure described in example 179 to
give amine
(213) (34 mg, 50%) as a yellow powder, mp > 345 °C.'H NMR b [(CD3)ZSO]
11.05 (br s,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-128-
1H), 9.34 (s, 1H), 8.39 (d, J=2.4 Hz, 1H), 7.59 (m, 2H), 7.45 (m, 5H), 7.27
(s, 1H), 7.06 (dd,
J=8.8, 2.4 Hz, 1 H), 7.02 (s, 1 H), 6.75 (s, 1 H), 4.80 (t, J=5.7 Hz, 2H),
4.41 (t, J=5.7 Hz, 2H).
Found: C, 70.36; H, 4.38; N, 12.68. Cz5H18N403.1/4H20 requires: C, 70.33; H,
4.37; N,
13.12.
EXAMPLE 185
The preparation of 9-Hydroxy-6-f3-(methylamino)prowll-4-phenywrrolof3 4-
clcarbazole-
1 3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=NHMe) (214)
Bromide (204) (40 mg, 0.09 mmol) prepared as described in example 173 was
reacted
with aqueous methylamine solution (10 mol equiv.) according to the procedure
described in
example 179 except that the reaction was performed in dimethylsulfoxide at
room
temperature for 3 h, to give amine (214) (22 mg, 61%) as an orange/yellow
powder, mp 265-
268 °C. 1H NMR 8 [(CD3)zS0] 9.33 (br s, 1H), 8.41 (d, J=2.5 Hz, 1H),
7.80 (s, 1H), 7.64 (m,
2H), 7.57 (d, J=8.8 Hz, 1H), 7.47 (m, 3H), 7.14 (dd, J=8.8, 2.5 Hz, 1H), 4.51
(t, J=6.8 Hz,
2H), 2.40 (t, J=6.5 Hz, 2H), 2.20 (s, 3H), 1.88 (m, 2H). FABMS found [M+H]+:
400.1659.
CZaHa2N303 requires 400.1661.
EXAMPLE 186
The preparation of 9-Hydroxy-4-phenyl-6-f3-(1-piperazinyl)proQyllpyrrolo(3 4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=uhenvl, n=3, Z=1-niuerazinvl) (215)
Bromide (204) (70 mg, 0.16 mmol) prepared as described in example 173 was
reacted
with piperazine according to The procedure described in example 179except that
the reaction
was performed at room temperature for 20 h, to give amine (215) (40 mg, 55%)
as a yellow
powder, mp 178-183 °C.'H NMR 8 [(CD3)ZSO] 11.0 (br s, 1H), 9.35 (br s,
1H), 8.40 (d,
J=2.4 Hz, 1H), 7.78 (s, 1H), 7.62 (m, 2H), 7.56 (d, J=8.8 Hz, 1H), 7.45 (m,
3H), 7.14 (dd,
J=8.8, 2.4 Hz, 1H), 4.49 (t, J=6.3 Hz, 2H), 2.52 (m, 4H), 2.12 (m, 6H), 1.91
(m, 2H). Found:
C, 69.31; H, 5.97; N, 11.49. CZ~HZ6N403.4/SH20 requires: C, 69.16; H, 5.93; N,
11.94.
EXAMPLE 187
The preparation of 6-f3-(Benzylamino)prop l~hydroxy-4-phenylQyrrolof3 4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar~henyl, n=3, Z=NHBn) (216)
Bromide (204) (75 mg, 0.17 mmol) prepared as described in example 173 was
reacted
with benzylamine according to the procedure described in example 179 except
that the
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-129-
reaction was performed at room temperature for 20 h, to give amine (216) (36
mg, 45%) as a
yellow powder, mp 139-144 °C.'H NMR 8 [(CD3)ZSO] 11.04 (br s, 1H), 9.33
(br s, 1H), 8.41
(d, J=2.4 Hz, 1H), 7.80 (s, 1H), 7.62 (m, 2H), 7.57 (d, J=8.8 Hz, 1H), 7.44
(m, 3H), 7.24-7.19
(m, SH), 7.11 (dd, J=8.8, 2.4 Hz, IH), 4.53 (t, J=6.7 Hz, 2H), 3.57 (s, 2H),
2.46 (t, J=6.4 Hz,
2H), 1.92 (m, 2H). Found: C, 74.84; H, 5.39; N, 8.80. C3oHZ5N3O3.1/4Hz0
requires: C, 75.06;
H, 5.35; N, 8.75.
EXAMPLE 188
The preparation of 6-(3-Anilinopropyl)-9-hydroxy-4-phenylpyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=NHPh) (217)
Bromide (204) (75 mg, 0.17 mmol) prepared as described in example 173 was
reacted
with aniline according to the procedure described in example 179 except that
the reaction was
performed at room temperature for 20 h, to give aniline (217) (30 mg, 38%) as
a yellow
powder, mp 240 °C (dec).'H NMR ~ [(CD3)zS0] 11.04 (br s, 1H), 9.33 (s,
1H), 8.40 (d,
J=2.4 Hz, 1H), 7.78 (s, 1H), 7.59 (d, J=8.9 Hz, 1H), 7.48 (m, 2H), 7.42 (m,
3H), 7.10 (dd,
J=8.8, 2.4 Hz, 1H), 7.05 (m, 2H), 6.52 (m, 3H), 5.70 (t, J=5.3 Hz, 1H), 4.59
(t, J=6.8 Hz, 2H),
2.98 (t, J=6.3 Hz, 2H), 2.07 (m, 2H). Found: C, 74.00; H, 5.22; N, 8.76.
CZgH23N3O3.1~2H2O
requires: C, 74.03; H, 5.14; N, 8.93.
EXAMPLE 189
The~reparation of 4-(2-Chloro-6-methoxyphenyl)-6-( 3-(cis-3,5-
dimeth~piperazinYllpropyl l-9-hydroxypyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione
(VIII;
Ar=2-chloro-6-methoxyphenyl, n=3, Z=1-(cis-3,5-dimethylpiperazinyp) (218)
Bromide (207) (60 mg, 0.12 mmol), prepared as described in example 176, was
reacted with cis-2,6-dimethylpiperazine according to the procedure described
in example 179
except that the reaction was performed at room temperature for 20 h, to give
amine (218) (63
mg, 98%) as a yellow powder, mp 199-202 °C.'H NMR 8 [(CD3)ZSO] 10.99
(br s, 1H), 9.37
(br s, 1H), 8.36 (d, J=2.4 Hz, 1H), 7.75 (s, 1H), 7.57 (d, J=8.8 Hz, 1H), 7.43
(t, J=8.2 Hz,
1H), 7.15 (m, 3H), 4.45 (t, J=6.1 Hz, 2H), 3.67 (s, 3H), 2.49 (m, 4H), 2.11
(m, 2H), 1.90 (m,
2H), 1.27 (m, 2H), 0.79 (m, 6H). Found: C, 61.35; H, 5.68; N, 9.14.
C3pH31C1N4Oq.2~~4HZO
requires: C, 61.32; H, 6.09; N, 9.54.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-130-
EXAMPLE 190
The preparation of 4-(2-Chloro-6-methox py henyl)-9-hydroxy-6-[3-(4-
morpholinyl)propyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2-chloro-
6-
methoxyphenyl, n=3, Z=4-momholinyl) (219)
Bromide (207) (60 mg, 0.12 mmol), prepared as described in example 176, was
reacted with morpholine according to the procedure described in example 179
except that the
reaction was performed at 50 °C for 6 h, to give amine (219) (40 mg,
66°10) as a yellow
powder, mp 169-174 °C.'H NMR b [(CD3)ZSO] 10.99 (br s, 1H), 9.34 (br s,
1H), 8.36 (d,
J=2.4 Hz, 1H), 7.77 (s, 1H), 7.58 (d, J=8.8 Hz, 1H), 7.44 (t, J=8.2 Hz, 1H),
7.18 (d, J=8.2 Hz,
1H), 7.12 (m, 2H), 4.47 (t, J=6.1 Hz, 2H), 3.66 (s, 3H), 3.35 (t, J=4.4 Hz,
4H), 2.22-2.11 (m,
6H),1.91 (m,_2H). Found: C, 63.09; H, 5.26; N, 7.97. CZ8H26C1N305.3/4Hz0
requires: C,
63.04; H, 5.20; N, 7.88.
EXAMPLE 191
The preparation of 4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3 4-
clcarbazol-6(1H)-yl)butanenitrile (VIII; Ar=2-chlorophenyl, n=3, Z=CN) (241)
To a solution of bromide 0 (0.13 g, 0.27 mmol) prepared as described in
example 172
in dimethylsulfoxide (2 mL) was added a solution of sodium cyanide (15 mg,
0.30 mmol) in
dimethylsulfoxide (2 mL) dropwise over 15 minutes. After 30 minutes the
reaction was
diluted with water and extracted with ethyl acetate. The organic layer was
dried, the drying
agent was removed and the solution was concentrated to dryness. Chromatography
on silica
eluting with ethyl acetate/hexane (1:4 to 1:1), followed by crystallization
from ethyl
acetate/hexane gave nitrite (241) (68 mg, 59°10) as an orange powder,
mp 262-266 °C. IH
NMR 8 [(CD3)ZSO] 11.08 (br s, 1H), 9.39 (s, 1H), 8.39 (d, J=2.4 Hz, 1H), 7.83
(s, 1H), 7.59
(m, 2H), 7.49 (m, 3H), 7.15 (dd, J=8.8, 2.4 Hz, 1H), 4.50 (t, J=7.3 Hz, 2H),
2.57 (m, 2H),
2.07 (m, 2H). FABMS found [M+H]+: 430.0927, 432.0916. C24H~6C1N303 requires
430.0958, 432.0929.
EXAMPLE 192
'The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-
(phenylsulfanyl)ethyllpyrrolof3 4-
clcarbazole-1,3(2H,6H)-dione (VII; Ar=2-chlorophenyl n=2 Z=SPh) (242)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-131-
Mesylate (229) (55 mg, 0.11 mmol) prepared as decribed in example 171 was
reacted
with excess thiophenol (0.10 mL) according to the procedure described in
example 179 ,
except that triethylamine (2.0 mL) was added and the reaction was heated at
110 °C for 4
days. Addition of water was followed by extracted of the compound with with
ethyl acetate.
The organic layer was dried, the drying agent was removed and the solution was
concentrated
to dryness. The organic layer was dried, the drying agent was removed and the
solution was
concentrated to dryness followed by chromatography on silica eluting with
ethyl
acetate/hexane (1:4 to 1:1) to give carbazole (242) (16 mg, 27%) as an orange
powder, mp
262-264 °C.'H NMR b [(CD3)ZSO] 11.04 (br s, 1H), 9.37 (br s, 1H), 8.34
(d, J=2.5 Hz, 1H),
7.58 (m, 1H), 7.46 (m, SH), 7.19 (m, 2H), 7.12 (m, 3H), 7.05 (m, 1H), 4.66 (t,
J=6.4 Hz, 2H),
3.46 (t, J=6.4 Hz, 2H). Found: C, 63.64; H, 4.09; N, 4.98.
CZgH~9C1N2O3S.13I4H2O requires:
C, 63.39; H, 4.28; N, 5.28.
EXAMPLE 193
The ureparation of 2-lf3-(4-(2-Chlorophenyl)-9-hydroxv-1 3-dioxo-2 3-
dihydropyrrolof3 4-
clcarbazol-6(1H)-yl)propyllamino}benzoic acid (VlIh Ar=2-chlorophenyl n=3 Z=2-
carboxyanilino) (60).
Reaction of the bromide (58) prepared as described in example 172 with o-
anthranilic
acid using the procedure described in example 179 gave the aniline (60) (88%),
mp 261-263
°C.'H NMR 8 [(CD3)ZSO] 11.05 (s, 1H), 9.38 (s, 1H), 8.39 (d, J=2.4 Hz,
1H), 7.76 (m, 1H),
7.75 (s, 1H), 7.59 (d, J=8.7 Hz, 1H), 7.55 (m, 1H), 7.46 (m, 1H), 7.40 (m,
1H), 7.33 (m, 1H),
7.27 (m, 1H), 7.12 (dd, J=8.7, 2.4 Hz, 1H), 6.59-6.50 (m, 2H), 4.57 (t, J=6.7
Hz, 2H), 3.22-
3.10 (m, 2H), 2.15-2.05 (m, 2H). FABMS found M+: 541.1230, 539.1234.
C3oH22C1N3O5
requires 541.1218, 539.1248.
EXAMPLE 194
The preparation of 3-; f3-(4-(2-Chlorophenyl)-9-hydroxy-1 3-dioxo-2 3-
dihYdropyrrolof3 4-
clcarbazol-6(1H)-yl)propyllamino}benzoic acid (VIII' Ar=2-chlorophenyl n=3 Z=3-
carbox~anilino) (61).
Reaction of the bromide (58) prepared as described in example 172 with m-
anthranilic
acid using the procedure described in example 179 gave the aniline (61) (82%),
mp 160-166
°C (dec). 'H NMR S [(CD3)ZSO] 11.04 (s, 1H), 9.38 (s, 1H), 8.39 (d,
J=2.4 Hz, 1H), 7.77 (s,
1 H), 7.61 (d, J=8.7 Hz, 1 H), 7.54 (m, 1 H), 7.45 (m, 1 H), 7.27 (m, 1 H),
7.14-7.08 (m, 4H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-132-
6.67 (m, 1H), 5.92 (br s, 1H), 4.58 (m, 2H), 3.09-2.92 (m, 2H), 2.12-2.02 (m,
2H). FABMS
found M+: 541.1223, 539.1237. C3oH22CIN305 requires 541.1218, 539.1248.
EXAMPLE 195
The preparation of 4-(f3-(4-(2-Chlorophen l~ydroxy-1,3-dioxo-2,3-
dihydropyrrolof3 4-
clcarbazol-6(IH)-yl)propyllamino}benzoic acid (VIII; Ar=2-chlorophenyl n=3 Z=4-
carboxyanilino) (62).
Reaction of the bromide (58) prepared as described in example 172 with p-
anthranilic
acid using the procedure described in example 179 gave the aniline (62) (77
%), mp 160-
165 °C (dec). IH NMR S [(CD3)ZSO] 11.06 (br s, 1H), 9.43 (br s, 1H),
8.39 (d, J=2.4 Hz,
1 H), 7.75 (s, 1 H), 7.65-7.59 (m, 3H), 7.54 (m, 1 H), 7.45 (m, 1 H), 7.37 (m,
1 H), 7.26 (m, 1 H),
7.13 (dd, J=8.7, 2.4 Hz, 1H), 6.49 (d, J=8.7 Hz, 2H), 4.58 (m, 2H), 3.11-2.99
(m, 2H), 2.12-
2.04 (m, 2H). Found: C, 63.24; H, 4.66; N, 7.95. C3oH22C1N3O5.H20 requires C,
63.55; H,
4.23; N, 7.41.
EXAMPLE 196
The preparation of 4=(2-Chlorbphenyl)=f-(-3=f(cis)-3;5-
dimethylpiperazinyllpropyll-9-
hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2-chlorophenyl n=3
Z=cis-
3,5-dimethylpiperazinyl) (63).
Reaction of the bromide (58) prepared as described in example 172 with cis-1,3-
dimethylpiperazine using the procedure described in example 179 gave (63) (65
%), mp
165-172 °C.'H NMR 8 [(CD3)ZSO] 11.04 (br s, 1H), 9.36 (br s, 1H), 8.38
(d, J=2.4 Hz, 1H),
7.80 (s, IH), 7.59-7.54 (m, 2H), 7.51-7.42 (m, 3H), 7.13 (dd, J=8.7, 2.4 Hz,
1H), 4.47 (m,
2H), 2.58-2.38 (m), 2.09 (t, J=6.2 Hz, 2H), 1.96-1.87 (m, 2H), 1.32-1.23 (m,
2H), 0.81 (d,
J=6.1 Hz, 3H), 0.77 (d, J=6.1 Hz, 3H). Found: C, 64.09; H, 5.43; N, 10.08.
C29HasC1N4O3.l.SH2O requires C, 64.14; H, 5.75; N, 10.31.
EXAMPLE 197
The preparation of 4-(2 6-Dichlorophenyl)-6-~ 3-f (cis)-3 5-
dimethylpiperazinyllpropyl )-9-
hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2 6-dichlorophenyl
n=3 Z=cis-
3,5-dimethylpiperazinyl) (64).
Reaction of the bromide (233) prepared as described in example 178 with cis-
1,3-
dimethylpiperazine using the procedure described in example 179 gave (64) (68
%), mp
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-133-
160-165 °C. 'H NMR 8 [(CD3)ZSO] 11.11 (br, IH), 9.39 (br s, 1H), 8.37
(d, J=2.4 Hz, 1H),
7.85 (s, 1H), 7.62 (d, J=8.1 Hz, 2H), 7.59 (d, J=8.7 Hz, IH), 7.51 (m, 1H),
7.16 (dd, J=8.7,
2.4 Hz, 1H), 4.46 (t, J=5.7 Hz, 2H), 2.55-2.42 (m), 2.10 (t, J=6.4 Hz, 2H),
1.96-1.89 (m,
2H),1.27 (t, J=10 Hz, 2H), 0.80 (d, J=6.2 Hz, 6H). Found: C, 61.34; H, 5.53;
N, 9.56.
Cz9Hz~CI2N403.Hz0 requires C, 61.27; H, 5.14; N, 9.85.
EXAMPLE 198
The following amino-compounds of general structure VIII were prepared in an
array
manner by reaction of the appropriate mesylate or bromide with the appropriate
amine using
the procedure described in example 179:
The preparation of 6-(2-aminoethyl)-9-hydroxy-4~henylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=2, Z=NHZ) (65) by reaction of (205)
prepared as
described in example 174 with aqueous ammonia. Found: M+H=372
The preparation of 9-h droxy-6-f2-(methylamino)ethyll-4-phen~pyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=2, Z=NHCH3) (66) by reaction of (205)
prepared as
described in example 174 with aqueous methylamine. Found: M+H=386
The preparation of 6-(3-aminopropyl)-9-hydroxy-4-phenylnyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (VIII, Ar=phenyl, n=3, Z=NHZ) (67) by reaction of (204)
prepared as
described in example 173 with aqueous ammonia . Found: M+H=386
The preparation of 9-hydrox~phenyl-6-f3-(1-pyrrolidinyllpr~yllRyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=1-pyrrolidinyl) (68) by reaction of
(204)
prepared as described in example 173 with pyrrolidine. Found: M+H=440
The preparation of 6-f3-(diethylamino)propyll-9-hydroxy-4-phen~pyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=N(CHZCH3)Z) (69) by reaction of
(204) prepared
as described in example 173 with diethylamine. Found: M+H=442.
The preparation of 9-hydroxy-4-phenyl-6-f3-(1-piperidinyl)propyllpyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=I-piperidinyl) (70) by reaction of
(204) prepared
as described in example 173 with piperidine. Found: M+H=454.
The preparation of 9-hydroxy-6-f3-(4-meth 1-~piperazinyl)propyll-4-
phenylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=phenyl, n=3, Z=4-methyl-1-piperazinyl)
(71) by
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-134-
reaction of (204) prepared as described in example 173 with 1-
methylpiperazine. Found:
M+H=469
The preparation of 6-f6-(dimethylamino)hexyll-9-hydroxy-4-phenylpyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII; Ar=phenyl, n=6, Z=N(CH3)Z) (72) by reaction of (206)
prepared as
described in example 175 with aqueous dimethylamine. Found: M+H=456
The preparation of 9-hydroxy-6-f6-(4-meth~piperazinyl)hexyll-4-
phenylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=phenyl, n=6, Z=4-methyl-1-piperazinyl)
(73) by
reaction of (206) prepared as described in example 175 with 1-
methylpiperazine. Found:
M+H=512.
The preparation of 6-(2-aminoethyl)-4-(2-chloro~hen ly )-9-h dy
roxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione-(VIII; Ar=2-chlorophenyl, n=2, R=NHZ) (74) by reaction of
(229) prepared
as decribed in example 171 with aqueous ammonia. Found: M-H=404
The preparation of 4-(2-chlorophen l~ydroxy-6-f3-
(dimethylamino)ethyllnyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=2, Z=N(CH3)2) (75) by
reaction
-_ of (229 prepared as decribed in example 171 with aqueous dimethylamine.
Found:
M+H=434.
The preparation of 4-(2-chlorophenyl)-9-hydroxy-6-f2-(1H-imidazol-1-
yl)ethyllpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=2, Z=1H-imidazol-1-
yl) (76) by
reaction of (229) prepared as decribed in example 171 with imidazole. Found:
M+H=457.
The preparation of 4-(2-chloroQhenyl)-9-hydroxy-6-f2-(4-
mor~holinyl)ethyllpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=2, Z=4-morpholinyl)
(77) by
reaction of (229) prepared as decribed in example 171 with morpholine. Found:
M+H=476.
The preparation of 4-(2-chlorophenyl)-9-hydro~-6-f2-(4-methyl-1-
piperazinyl)ethyllQyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-
chlorophenyl, n=2,
Z=4-methyl-1-piperazinyl) (78) by reaction of (229 prepared as decribed in
example 171
with 1-methylpiperazine. Found: M+H=489.
The preparation of 6-(2-anilinoethyl)-4-(2-chlorophenyl)-9-hydroxywrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=2, 2=-anilino) (79) by reaction
of (229)
prepared as decribed in example 171 with aniline. Found: M+H=481
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-135-
The preparation of 4-(2,6-dichlorophenyl)-9-h dery-6-f3-
(dimethylamino)ethyh[Qyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-
dichlorophenyl, n=2, Z=N(CH3)2) (80) by reaction of (231) prepared as decribed
in example
177 with aqueous dimethylamine. Found: M+H=468.
The preQaration of 4-(2,6-dichlorophen ly )9-hydroxy-6-f2-(1H-imidazol-1-
yl ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-
dichlorophenyl, n=2,
Z=1H-imidazol-1-yl) (81) by reaction of (231) prepared as decribed in example
177 with
imidazole. Found: M+H=491.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f2-(4-
morpholinyl)ethyllpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-dichlorophenyl, n=2, Z=4-
morpholinyl) (82) by
-reaction of (231) prepared as decribed in example 177 with morpholine. Found:
M+H=510.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f2-(4-methyl-1-
piperazinyl)ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-
dichlorophenyl,
n=2, Z=4-methyl-1-piperazinyl) (83) by reaction of (231) prepared as decribed
in example
177 with 1-methylpiperazine. Found: M+H=523.
The preparation of 6-(2-anilinoethyl)-4-(2,6-dichlorophenyl)-9-hydroxypyrrolof
3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-dichlorophenyl, n=2, Z=anilino)
(84) by
reaction of (231) prepared as decribed in example 177 with aniline. Found:
M+H=516
The preparation of 4-(2-chlorophen l~ydroxy-6-f3-
(methylamino)propyllpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (V)rtI, Ar=2-chlorophenyl, n=3, Z=NHMe) (85) by
reaction of
(58) prepared as described in example 172 with aqueous methylamine. Found:
M+H=434.
The preparation of 4-(2-chlorophenyl)-6-f3-(dimethylamino)propyll-9-
hydroxypymolof3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=3, 2=N(CH3)2) (86) by
reaction
of (58) prepared as described in example 172 with aqueous dimethylamine.
Found:
M+H=448.
The preparation of 4-(2-chlorophenyl)-9-hydroxy-6-f3-(1H-imidazol-1-
yl rop ~~Ilpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl,
n=3, Z=1H-
imidazol-1-yl) (87) by reaction of bromo prepared as described in example 172
with
imidazole. Found: M+H=471.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-136-
The preparation of 4-(2-chlorophenyl)-9-hydroxy-6-f3-(4-
morpholin~propyllpyrrolof3 4-
~carbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=3, Z=4-morpholinyl)
(88) by
reaction of (58) prepared as described in example 172 with morpholine. Found:
M+H=490.
The preparation of 4-(2-chlorophenyl)-9-~droxy-6-f3-(4-methyl-1-
piperazinyl)propyllpyrrolof3 4-clcarbazole-1 3(2H 6H)-dione (VIII, Ar=2-
chlorophenyl, n=3,
Z=4-methyl-1-piperazinyl) (89) by reaction of (58) prepared as described in
example 172
with 1-methylpiperazine. Found M+H=503.
The preparation of 6-(3-anilinopropyl)-4-(2-chlorophenyl)-9-hydroxyyrrolof3 4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2-chlorophenyl, n=3, Z=anilino) (90) by
reaction of
(58) prepared as described in example 172 with aniline. Found: M+H=495.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f3-
methylamino)propellpyrrolof3,4-clcarbazole-1 3(2H 6H)-dione (VIII, Ar=2,6-
dichlorophenyl, n=3, LrNHMe) (91) by reaction of (233) prepared as decribed in
example
178 with aqueous methylamine. Found: M+H=468.
The preparation of 4-(2,6-dichlorophenyl)-6-f3-(dimethylamino)prop l
hydroxypyrrolof3,4-clcarbazole-1,3(2H 6H)-dione (VIII, Ar=2,6-dichlorophenyl,
n=3,
Z=N(CH3)2) (92) by reaction of (233) prepared as decribed in example 178 with
aqueous
dimethylamine. Found: M+H=482.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f3-(1H-imidazol-1-
yl)propyllpyrrolof3,4-clcarbazole-1 3(2H 6H)-dione (VIII, Ar=2,6-
dichlorophenyl, n=3,
~1H-imidazol-1-yl) (93) by reaction of (233) prepared as decribed in example
178 with
imidazole. Found: M+H=505.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f3-(4-
morpholinyl)propyllpyrrolof3,4-clcarbazole-1 3(2H 6H)-dione (VIII, Ar=2,6-
dichlorophenyl,
n=3, Z=4-morpholinyl) (94) by reaction of (233) prepared as decribed in
example 178 with
morpholine. Found: M+H=524.
The preparation of 4-(2,6-dichlorophenyl)-9-hydroxy-6-f3-(4-methyl-1-
piperazinyl)propyllpyrrolof3,4-clcarbazole-1 3(2H 6H)-dione (VIII, Ar=2,6-
dichlorophenyl,
n=3, Z=4-methyl-1-piperazinyl) (95) by reaction of (233) prepared as decribed
in example
178 with 1-methylpiperazine. Found M+H=537.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-137-
The preparation of 6-(3-anilinopropyl)-4-(2,6-dichlorophenyl)-9-
hydroxypyrrolo[3,4-
clcarbazole-1,3(2H,6H)-dione (VIII, Ar=2,6-chlorophenyl, n=3, Z=anilino) (96)
by reaction
of (233) prepared as decribed in example 178 with aniline. Found: M+H=530.
EXAMPLE 199
Combinatorial Procedure for Aniline Displacements of Br and OMs
To a 8 ml screw cap vial was added a solution of 6-(3-Bromopropyl)-4-(chloro-
phenyl)-9-hydroxy-6H-pyrrolo[3,4-c]carbazole-1,3-dione (reagent A), (0.048 g,
0.1 mmol)
prepared as described in example 83 or 2-(9-Hydroxy-1,3-dioxo-4-phenyl-2,3-
dihydropyrrolo[3,4-c]carbazol-6(1H)-yl)ethyl methanesulfonate (205) prepared
as described
in example 174 (reagent B) (See table 2) in anhydrous dimethylacetamide (1
ml), a solution
of the appropriate aniline (0.012 g, 0.1. mmol) in anhydrous dimethylacetamide
(0.150 ml).
The vial was capped and the reaction mixture was shaken for 18 hours at
100°C. After
cooling to room temperature, the solvent was removed under vacuum.
Purification was
carned out via reverse-phase HPLC (3% n-propanol in acetonitrile and 3% n-
propanol in
water as the eluent; C-18 column). The compounds were analysed by mass
spectral analysis.
Table 2 Compounds made combinatorially by reaction of the appropariate
commercial
anilines
Reagent Product Analytical
Data
MS-APCI
[M+H]+
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(3-
Reagent methoxy-phenylamino)-propyl]-6H-526.2
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(4-
Reagent hydroxy-phenylamino)-propyl]-6H-512.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(2-
Reagent trifluoromethyl-phenylamino)-propyl]-564.3
A
6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-6-[3-(4-ethyl-
Reagent 524.3
A
phenylamino)-propyl]-9-hydroxy-6H-
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-138-
pyrrolo [3,4-c]carbazole-1,3-dione
6-[3-(4-Bromo-phenylamino)-propyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-576.2
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(3-
Reagent methylsulfanyl-phenylamino)-propyl]-542.3
A
6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(3-
Reagent hydroxy-phenylamino)-propyl]-6H-512.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
6-[3-(3-Bromo-phenylamino)-propyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-574.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
( { 3-[4-(2-Chloro-phenyl)-9-hydroxy-1,3-
dioxo-2,3-dihydro-1 H-pyrrolo[3,4-
Reagent 554.3
A
c]carbazol-6-yl]-propyl
}-phenyl-amino)-
acetic acid
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-
Reagent (1H-indazol-6-ylamino)-propyl]-6H-536.3
A
pyrrolo [3,4-c]carbazole-1,3-dione
3-{ 3-[4-(2-Chloro-phenyl)-9-hydroxy-
1,3-dioxo-2,3-dihydro-1H-pyrrolo[3,4-
Reagent 575.3
A
c]carbazol-6-yl]-propylamino
}-
benzenesulfonamide
4-(2-Chloro-phenyl)-6-[3-(3-ethyl-
Reagent phenylamino)-propyl]-9-hydroxy-6H-524.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
6-[3-( 1,3-Benzodioxol-5-ylamino)-
Reagent propyl]-4-(2-chloro-phenyl)-9-hydroxy-540.3
A
6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-
Reagent 536.3
A
(1H-indazol-5-ylamino)-propyl]-6H-
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-139-
pyrrolo[3,4-c)carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-
Reagent (1H-indol-5-ylamino)-propyl]-6H-535.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-6-[3-(
1,1-dioxo-1 H-
1$1>6_-benzo[b]thiophen-6-ylamino)-
Reagent 584.3
A
propyl]-9-hydroxy-6H-pyrrolo[3,4-
c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-
Reagent (indan-5-ylamino)-propyl]-6H-536.2
A
pyrrolo[3,4-c]carbazole-1,3-dione
(4-{3-[4-(2-Chloro-phenyl)-9-hydroxy-
1,3-dioxo-2,3-dihydro-1
H-pyrrolo(3,4-
Reagent 597.3
A
c]carbazol-6-yl]-propylamino
}-
benzoylamino)-acetic acid
4-{ 3-[4-(2-Chloro-phenyl)-9-hydroxy-
1,3-dioxo-2,3-dihydro-1
H-pyrrolo[3,4-
Reagent 638.4
A
c]carbazol-6-yl]-propylamino
}-N-(2-
diethylamino-ethyl)-benzamide
6-[3-(Benzothiazol-6-ylamino)-propyl]-
Reagent 4-(2-chloro-phenyl)-9-hydroxy-6H-553.2
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-{3-
[(2-hydroxy-ethyl)-phenyl-amino]-
Reagent 540.3
A
propyl }-6H-pyrrolo[3,4-c]carbazole-1,3-
dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(3-
Reagent trifluoromethyl-phenylamino)-propyl]-564.2
A
6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-{
3-[3-
Reagent (1,1,2,2-tetrafluoro-ethoxy)-612.3
A
phenylamino]-propyl }-6H-pyrrolo[3,4-
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-140-
c]carbazole-1,3-dione
3-(3-{ 3-[4-(2-Chloro-phenyl)-9-
hydroxy-1,3-dioxo-2,3-dihydro-1
H-
Reagent 568.3
A
pyrrolo[3,4-c]carbazol-6-yl]-
propylamino}-phenyl)-propionic
acid
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-
Reagent (methyl-phenyl-amino)-propyl]-6H-510.3
A
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(3-
Reagent methoxy-phenylamino)-ethyl]-6H-512.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-6-[2-(3-ethyl-
Reagent phenylamino)-ethyl]-9-hydroxy-6H-510.3
B
pyrrolo[3,4-c)carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(3-
Reagent trifluoromethyl-phenylamino)-ethyl]-6H-550.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(2-
Reagent hydroxy-phenylamino)-ethyl]-6H-498.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
6-[2-(3-Acetyl-phenylamino)-ethyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-524.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(4-
Reagent methoxy-phenylamino)-ethyl]-6H-512.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(2-
Reagent methylsulfanyl-phenylamino)-ethyl]-6H-528.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
6-[2-(2-Bromo-phenylamino)-ethyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-562.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-141-
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(4-
Reagent hydroxy-phenylamino)-ethyl]-6H-498.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-6-[2-(4-ethyl-
Reagent phenylamino)-ethyl]-9-hydroxy-6H-510.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
6-[2-(4-Bromo-phenylamino)-ethyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-562.1
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(3-
Reagent methylsulfanyl-phenylamino)-ethyl]-6H-528.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[2-(3-
Reagent hydroxy-phenylamino)-ethyl]-6H-498.3
B
pyrrolo(3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(2-
Reagent hydroxy-phenylamino)-propyl]-6H-512.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
6-[2-(3-Bromo-phenylamino)-ethyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-562.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-{ 2-[4-(2-Chloro-phenyl)-9-hydroxy-
1,3-dioxo-2,3-dihydro-1H-pyrrolo[3,4-
Reagent 507.3
B
c]carbazol-6-yl]-ethylamino
}-
benzonitrile
(3-{ 2-[4-(2-Chloro-phenyl)-9-hydroxy-
1,3-dioxo-2,3-dihydro-1H-pyrrolo[3,4-
Reagent 540.3
B
c]carbazol-6-yl]-ethylamino
}-phenyl)-
acetic acid
6-[3-(3-Acetyl-phenylamino)-propyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-538.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-142-
4-(2-Chloro-phenyl)-9-hydroxy-6-(3-(4-
Reagent methoxy-phenylamino)-propyl]-6H-526.3
B
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-[3-(2-
Reagent methylsulfanyl-phenylamino)-propyl]-542.2
B
6H-pyrrolo [3,4-c]carbazole-1,3-dione
6-[3-(2-Bromo-phenylamino)-propyl]-4-
Reagent (2-chloro-phenyl)-9-hydroxy-6H-576.2
B
pyrrolo[3,4-c]carbazole-1,3-dione
EXAMPLE 200
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-(3-
(methvlsulfanyl)propyllnyrrolo(3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2-chlorophenyl, n=3, Z=SCH~) (130)
A mixture of the bromide (58) (60.0 mg, 0.124 mmol) prepared as described in
example 172 and lithium thiomercaptide (13 mg, 0.2481 mmol) in p-dioxane (5
mL) was
refluxed for 16 h. A further 10 mg of LiSMe was added and refluxing was
continued for a
further 9 h. The mixture was diluted with water, extracted with ethyl acetate.
The organic
layer was dried, the drying agent was removed and the solution was
concentrated to drynessto
give the sulfide (130 directly (47.1 mg, 84%) which crystallised from ethyl
acetate/petroleum
ether as an orange powder, mp 218-220 °C.'H NMR 8 [(CD3)ZSO] 11.07 (br
s, 1H), 9.39 (br
s, 1H), 8.39 (d, J=2.4 Hz, 1H), 7.80 (s, 1H), 7.61-7.56 (m, 2H), 7.53-7.43 (m,
3H), 7.15 (dd,
J=8.8, 2.4 Hz, 1H), 4.52 (t, J=6.9 Hz, 2H), 2.45 (t, J=7.0 Hz, 2H), 2.01 (m,
2H), 2.00 (s, 3H).
FABMS found [M+H]+: 453.0840, 451.0859. C24H2oC1Nz03S requires 453.0854,
451.0883.
EXAMPLE 201
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-[3-
(phenylsulfanyl)propyllpyrrolo(3,4-
clcarbazole-1,3(2H,6H)-dione (VIII; Ar=2-chlorophenyl, n=3, Z=SPh) (131).
A solution of the bromide (58) (41 mg, 0.085 mmol) prepared as described in
example
172, thiophenol (9.5 ~tL, 0.093 mmol) and triethylamine (0.25 mL, 3.40 mmol)
in p-dioxane
(1.5 mL) was refluxed for 16 h. Water was added and the mixture was extracted
with ethyl
acetate. The organic layer was dried, the drying agent was removed and the
solution was
concentrated to dryness the sulfide (131) directly (37.2 mg, 85 %), which
crystallised from
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-143-
ethyl acetate/petroleum ether as an orange powder, mp 229-231 °C. 1H
NMR S [(CD3)2S0]
11.06 (br s, 1H), 9.37 (s, 1H), 8.37 (d, J=2.4 Hz, 1H), 7.76 (s, 1H), 7.60-
7.42 (m, SH), 7.25-
7.19 (m, 4H), 7.16-7.10 m, 2H), 4.57 (t, J=6.8 Hz, 2H), 2.98 (m, 2H), 2.01 (m,
2H). Found:
C, 67.36; H, 4.29; N, 5.36. C29HZ~C1NZSO3.lI4H2O requires C, 67.31; H, 4.19;
N, 5.41.
EXAMPLE 202
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-(3-methoxypropyl)pyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (VIII; Ar=2-chlorophenyl, n=3, Z=OCH~ (132).
A solution of the bromide (58) (50 mg, 0.103 mmol) prepared as described in
example
172 and sodium methoxide (35.2 mg, 0.65 mmol) in methanol (0.5 mL) and p-
dioxane (8
mL) was refluxed for 3 h. The solution was acidified with 2N HCI, extracted
with ethyl
acetate and the organic layer was dried, the drying agent was removed and the
solution was
concentrated to dryness and chromatographed on silica. Elution with ethyl
acetate gave the
anhydride, 4-(2-chlorophenyl)-9-hydroxy-6-(3-methoxypropyl)-1H-furo[3,4-
c]carbazole-
1,3(6H)-dione as an orange powder.'H NMR S [(CD3)ZSO] 9.57 (br, 1H), 8.24 (d,
J=2.3 Hz,
1H), 8.00 (s, 1H), 7.66-7.61 (m, 2H), 7.58-7.48 (m, 3H), 7.22 (dd, J=9.0, 2.3
Hz, 1H), 4.56 (t,
J=6.7 Hz, 2H), 3.22 (t, J=6.0 Hz, 2H), 3.13 (s, 3H), 2.02 (m, 2H). The product
was added to
molten ammonium acetate (10 g) at 140 °C and the mixture was warmed at
this temperature
for 3 h. Water was added and the resultant precipitate was filtered off,
adsorbed onto silica
from a THF solution, and chromatographed. Elution with ethyl acetate/petroleum
ether (1:1)
gave (132) (32 mg, 71 %) an orange powder, mp 260-262 °C.'H NMR 8
[(CD3)ZSO] 11.07
(br s, 1 H), 9.42 (br s, 1 H), 8.39 (d, J=2.4 Hz, 1 H), 7.69 (s, 1 H), 7.60-
7.44 (m, SH), 7.15 (dd,
J=8.8, 2.4 Hz, 1H), 4.49 (t, J=6.6 Hz, 2H), 3.22 (t, J=6.0 Hz, 2H), 3.13 (s,
3H), 1.99 (m, 2H).
FABMS found [M+H]+: 437.1088, 435.1090. C24HZOC1N20a requires 437.1082,
435.1112.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-144-
H H H
O N O 1) Dess-Martin O N O O N O
2) NaC102 _ BBr -
Me0 ~ ~ ~ Ar METHOD 13 Me0 ~ ~ / Ar METHOD 8 HO I y ~ ~ Ar
N I ~ N Py.HCU200-220 °C
Rio (CHZ)nCOOH METHOD 9 (CHZ)nCOOH
(V); R'°=(CHz)"OH
(IX) (X)
1) (CICO)20/DMF
2) R3R4NH R SOzNH~/EDCLHCI/DMAP
METHOD 14 METHOD 15
H H
O N O O N O
Me0 ~ ~ ~ Ar Me0 I ~ ~ ~ Ar
i
I ~ N, O BBr3. ggr3- N' O
(CHZ)n --C' METHOD 8 METHOD 8 (CH2)n -~!
N R4 or or f1 S02R3
Py.HCI/200-220 °C Py.HCI/200-220 °C H
(XI) \METHOD 9 METHOD 9 (XII)
H H
O N O O N O
HO I ~ ~ / Ar HO ~ ~ ~ Ar
N, O I ~ N
O
(CHz)n ~' (CHz)n -f(
R /N ~ Ra H SOZR3
3
(X111) (XIV)
SCHEME4
Scheme 4 Procedures
Representative Procedure for Method 13 of Scheme 4
EXAMPLE 203
The preparation of 3-(9-Methoxy-1,3-dioxo-4~henyl-2,3-dihydropyrrolo(3,4-
clcarbazol-
6(1H)-yl)proQanoic acid (IX; Ar=phenyl, n=2) (243)
Dess-Martin periodinane (1.91 g, 4.5 mmol) was added to a stirred solution of
alcohol
(202) (1.20 g, 3.0 mmol) prepared as decribed in example 83 in dry
tetrahydrofuran (80 mL)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-145-
under nitrogen. After 1 hour at room temperature a solution of saturated
sodium thiosulphate
and saturated sodium bicarbonate (1:1, 100 mL) was added and the reaction
mixture was
stirred vigorously for 15 minutes before being given extracted with ethyl
acetate. The
organic layer was dried, the drying agent was removed and the solution was
concentrated to
dryness. The resulting crude aldehyde was then dissolved in tert-
butanol/tetrahydrofuran
(9:1, 230 mL) and 2-methyl-2-butene was added (12.0 mmol, 6.0 mL of a 2M
solution in
tetrahydrofuran). To this solution was added a solution of sodium chlorite
(1.09 g, 12.0
mmol) and sodium dihydrogen phosphate (2.5 g, 18.0 mmol) in water (100 mL)
containing
tent-butanol (4 mL). The resulting solution was stirred at room temperature
for 18 hours
before being diluted with brine and extracted with ethyl acetate. The organic
layer was dried,
the drying agent was removed and the solution was concentrated to dryness.
Chromatography on silica eluting with ethyl acetate/methanol (1:0 to 9:1),
followed by
crystallisation from ethyl acetate/hexane gave acid (243) (0.86 g, 69%) as a
yellow powder,
mp 252-255 °C.'H NMR 8 [(CD3)ZSO] 12.2 (v br s, 1H), 11.13 (br s, 1H),
8.55 (d, J=2.6 Hz,
1H), 7.89 (s, 1H), 7.68 (m, 3H), 7.47 (m, 3H), 7.28 (dd, J=9.0, 2.6 Hz, 1H),
4.73 (t, J=6.6 Hz,
2H), 3.90 (s, 3H), 2.76 (t, J=6.6 Hz, 2H). Found: C, 67.95; H, 4.37; N, 6.47.
C24H1sNz05.1/2H2) requires C, 68.08; H, 4.52; N, 6.62.
EXAMPLE 204
The preparation of 3-(4-(2,6-Dichlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-vl)propanoic acid (IX; Ar=2,6-dichlorophenyl, n=2) (244)
Oxidation of alcohol (232) prepared as decribed in example 86 (0.50 g, 1.1
mmol)
according to the procedure decrsibed in example 203 , followed by
chromatography on silica
eluting with methanol/dichloromethane (3:97 to 1:9) and trituration from ethyl
acetate/hexane
gave acid (244) (0.35 g, 66%) as a yellow powder, mp 203-209 °C. 'H NMR
8 [(CD3)ZSO]
12.3 (v br s, 1H), 11.19 (br s, 1H), 8.50 (d, J=2.6 Hz, 1H), 7.94 (s, 1H),
7.74 (d, J=9.0 Hz,
1H), 7.62 (m, 2H), 7.51 (dd, J=13.3, 6.1 Hz, 1H), 7.33 (dd, J=9.0, 2.6 Hz,
1H), 4.71 (t, J=6.9
Hz, 2H), 3.91 (s, 3H), 2.75 (t, J=6.9 Hz, 2H). Found: C, 59.74; H, 3.62; N,
5.65.
C24H~6C12N205 requires: C, 59.63; H, 3.34; N, 5.79.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-146-
EXAMPLE 205
The nre~aration of 3-(4-(2-Chloro-6-methoxyphenyl)-9-methoxy-1,3-dioxo-2,3-
dihydrop~rrolo(3clcarbazol-6(1H)-yl)propanoic acid (IX' Ar=2-chloro-6-
methoxyphenyl,
n=2 245
Oxidation of alcohol (105) (2.1 g, 4.5 mmol) prepared as described in example
58
according to the procedure decrsibed in example 203 gave acid (245) (0.81 g,
38%) as a
yellow powder, mp 241-243 °C.'H NMR 8 [(CD3)ZSO] 12.5 (v br s, 1H),
11.07 (br s, 1H),
8.50 (d, J=2.6 Hz, 1H), 7.82 (s, 1H), 7.70 (d, J=9.0 Hz, 1H), 7.44 (t, J=8.3
Hz, 1H), 7.30 (dd,
J=9.0, 2.6 Hz, 1H), 7.18 (d, J=8.3 Hz, 1H), 7.12 (d, J=8.3 Hz, 1H), 4.68 (t,
J=6.8 Hz, 2H),
3.90 (s, 3H), 3.68 (s, 3H), 2.71 (t, J=6.8 Hz, 2H). Found: C, 62.71; H, 4.09;
N, 5.62.
Cz5H~9C1N206 requires C, 62.70; H, 4.00; N, 5.85.
EXAMPLE 206
The preparation of 3-(4-(2 6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)propanoic acid (X; Ar=2,6-dichlorophenyl, n=2) (246)
Demethylation of acid (244) (90 mg, 0.19 mmol) prepared as described in
example
204 employing the procedure described in example 80 gave phenol (246) (63 mg,
71%) as an
orange/yellow powder, mp 245-253 °C (dec).'H NMR 8 [(CD3)ZSO] 12.2 (v
br s, 1H), 11.13
(br s, 1H), 9.40 (br s, 1H), 8.36 (d, J=2.5 Hz, 1H), 7.88 (s, 1H), 7.62 (m,
3H), 7.50 (dd, J=8.7,
7.3 Hz, 1H), 7.15 (dd, J=8.9, 2.5 Hz, 1H), 4.66 (t, J=6.9 Hz, 1H), 2.73 (t,
J=6.9 Hz, 2H).
Found: C, 58.98; H, 3.31; N, 5.81. C23H~4C12N205 requires: C, 58.85; H, 3.01;
N, 5.97.
Representative Procedure for Method 14 of Scheme 4
EXAMPLE 207
The preparation of N-f2-(Dimethylamino)ethyll-3-(9-methoxy-1,3-dioxo-4-phenyl-
2,3-
dihydropyrrolo(3,4-clcarbazol-6(1H)-yl)propanamide (XI; Ar=phenyl, n=2, R3=H,
R4=CH~CH~,N(CH~)2) (247)
To a solution of acid (243) (100 mg, 0.24 mmol) prepared as described in
example
203 in dry tetrahydrofuran (20 mL) under nitrogen was added 1 drop of
dimethylformamide,
followed by oxalyl chloride (84 uL, 0.96 mmol) dropwise. The resulting
solution was stirred
at room temperature for 2 hours before being reduced to dryness in vacuo. Dry
benzene (20
mL) was added to the residue and the suspension was again reduced to dryness
in vacuo,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-147-
before being dissolved in dry tetrahydrofuran (20 uL) and flushed with
nitrogen. To this
solution was added dimethylethylenediamine (lOSmL, 0.96 mmol) via syringe. The
reaction
mixture was stirred at room temperature for 2 hours and then diluted with
water, basified by
the addition of solid potassium carbonate and extracted with ethyl acetate.
The organic layer
was dried, the drying agent was removed and the solution was concentrated to
dryness.
Chromatography on silica eluting with ethyl acetate/methanol/triethylamine
(1:0:0 to
3: l Grace) followed by crystallization from ethyl acetate/hexane, gave amide
(247) (85 mg,
73%) as a yellow powder, mp 206-210 °C.'H NMR 8 [(CD3)ZSO] 11.11 (br s,
1H), 8.54 (d,
J=2.6 Hz, 1H), 7.81 (s, 1H), 7.73 (t, J=5.6 Hz, 1H), 7.67 (m, 3H), 7.48 (m,
3H), 7.29 (dd,
J=8.9, 2.6 Hz, 1H), 4.73 (t, J=6.3 Hz, 2H), 3.89 (s, 3H), 2.93 (dd, J=6.8, 5.6
Hz, 2H), 2.59 (t,
J=6.3 Hz, 2H), 1.90 (s, 6H), 1.84 (t, J=6.8 Hz, 2H). Found: C, 67.02; H, 5.80;
N, 11.17.
CZ8H28N404.H20 requires: C, 66.92; H, 6.02; N, 11.14.
EXAMPLE 208
The-preparation of --3-(9-Methoxy-1,3-dioxo-4-phenyl-2;3-dihydropyrrolof3,4-
clcarbazol-
6(1Hl-vl)nronanamide (XI; Ar=phenyl, n=2, R3=H, R4=H) (248)
Reaction of acid (243) (100 mg, 0.24 mmol) prepared as described in example
203
according to the procedure described in example 207 , except that a saturated
solution of
ammonia gas in tetrahydrofuran (20 mL) was added as the amine, gave amide
(248) (61 mg,
62%) as a yellow powder, mp 266-270 °C.'H NMR b [(CD3)ZSO] 11.11 (br s,
1H), 8.55 (d,
J=2.6 Hz, 1H), 7.87 (s, 1H), 7.68 (m, 3H), 7.48 (m, 3H), 7.33 (br s, 1H), 7.29
(dd, J=9.0, 2.6
Hz, 1H), 6.85 (br s, 1H), 4.71 (t, J=6.5 Hz, 2H), 3.90 (s, 3H), 2.60 (t, J=6.5
Hz, 2H). Found:
C, 68.36; H, 4.70; N, 9.99. Cz4H1~N304.1/2H20 requires: C, 68.24; H, 4.78; N,
9.94.
EXAMPLE 209
The ~reQaration of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)-N-f2-(dimethylamino)ethyllpropanamide (XI; Ar=2-
chlorophenyl, n=2,
R3=H R4=CHZCH~N(CH3)Z) (249)
Reaction of acid (116) (95 mg, 0.21 mmol) prepared as described in example 229
according to the procedure described in example 207 , followed by trituration
in diethyl ether,
gave amide (249) (76 mg, 70%) as a yellow powder, mp 250-252 °C. 'H NMR
8 [(CD3)zS0]
11.12 (br s, 1H), 8.51 (d, J=2.6 Hz, 1H), 7.77 (s, 1H), 7.76 (partially
obscured t, J=5.9 Hz,
1H), 7.68 (d, J=9.0 Hz, 1H), 7.58 (m, 2H), 7.48 (m, 3H), 7.32 (dd, J=9.0, 2.6
Hz, 1H), 4.71 (t,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-148-
J=6.4 Hz, 2H), 3.90 (s, 3H), 2.95 (m, 2H), 2.58 (t, J=6.4 Hz, 2H),1.94 (s,
6H), 1.93 (partially
obscured t, J=6.8 Hz, 2H). Found: C, 64.88; H, 5.56; N, 10.70. CZ$HZ~C1N404
requires: C,
64.79; H, 5.24; N, 10.79.
EXAMPLE 210
The nrenaration of 3-(4-(2,6-Dichlorophenvl)-9-methoxv-1,3-dioxo-2,3-
dihvdropyrrolof3,4-
clcarbazol-6(1H)-yl)propanamide (XI; Ar=2,6-dichlorophenyl, n=2, R3=H, R4=H)
(250)
Reaction of acid (244) (0.23 g, 0.48 mmol) prepared as described in example
204
according to the procedure described in example 207 , except that concentrated
ammonia
(-30%, 20 mL) was added as the amine, gave amide (250) (0.17 g, 73%) as a
yellow powder,
mp 261-264 °C.'H NMR S ((CD3)zS0] 11.19 (br s, 1H), 8.50 (d, J=2.6 Hz,
1H), 7.87 (s, 1H),
7.73 (d, J=9.0 Hz, 1H), 7.63 (m, 2H), 7.52 (dd, J=8.8, 7.3 Hz, 1H), 7.38 (br
s, 1H), 7.33 (dd,
J=9.0, 2.6 Hz, 1H), 6.87 (br s, 1H), 4.69 (t, J=6.7 Hz, 2H), 3.91 (s, 3H),
2.57 (t, J=6.7 Hz,
2H). Found: C, 59.55; H, 3.79; N, 8.73. Cz4H1~C12N304 requires: C, 59.75; H,
3.55; N, 8.71.
EXAMPLE 211
The preparation of 3-(4-(2,6-Dichlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolo~3,4-
clcarbazol-6(1H)-yl)-N-f2-(dimethylamino)ethyllpropanamide (XI; Ar=2,6-
dichlorophenyl,
n=2, R3=H, R4=CHZCH~N_ (CHI)? 251
Reaction of acid (244) (150 mg, 0.30 mmol) prepared as described in example
204
according to the procedure described in example 207 , gave amide (251) (91 mg,
55%) as a
yellow powder, mp 141-146 °C.'H NMR 8 [(CD3)ZSO] 11.19 (br s, 1H), 8.50
(d, J=2.6 Hz,
1H), 7.82 (s, 1H), 7.81 (partially obscured t, J=5.6 Hz, 1H), 7.69 (d, J=9.0
Hz, 1H), 7.62 (m,
2H), 7.52 (dd, J=8.8, 7.4 Hz, 1H), 7.34 (dd, J=9.0, 2.6 Hz, 1H), 4.71 (t,
J=6.4 Hz, 2H), 3.90
(s, 3H), 2.95 (q, J=6.2 Hz, 2H), 2.58 (t, J=6.4 Hz, 2H), 2.07 (m, 2H), 2.02
(s, 6H). Found: C,
59.13; H, 4.73; N, 9.87. CZ$HZ6C12N404.H20 requires C, 58.85; H, 4.94; N,
9.80.
EXAMPLE 212
The preparation of 3-(4-(2-Chloro-6-methoxyphenyl)-9-methoxY 1,3-dioxo-2,3-
dihydroQyrrolof3,4-clcarbazol-6(1H)-yl)-N-f2-(dimethylamino)ethyllpropanamide
(XI;
Ar=2-chloro-6-methoxyphenyl, n=2, R3=H, R4=CH~CH?N(CH~~~) (252)
Reaction of acid (245) prepared as described in example 205 (160 mg, 0.33
mmol)
according to the procedure described in example 207 , gave amide (252) (93 mg,
51 %) as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-149-
yellow powder, mp 164-169 °C. 1H NMR b [(CD3)ZSO] 11.06 (br s, 1H),
8.50 (d, J=2.6 Hz,
1H), 7.80 (t, J=5.6 Hz, 1H), 7.72 (s, 1H), 7.66 (d, J=9.0 Hz, 1H), 7.45 (t,
J=8.3 Hz, 1H), 7.30
(dd, J=9.0, 2.6 Hz, 1 H), 7.18 (d, J=8.3 Hz, 1 H), 7.13 (d, J=8.3 Hz, 1 H),
4.68 (t, J=6.4 Hz,
2H), 3.90 (s, 3H), 3.68 (s, 3H), 2.98 (dd, J=6.6, 5.6 Hz, 2H), 2.56 (t, J=6.4
Hz, 2H), 2.05 (t,
J=6.6 Hz, 2H), 1.90 (s, 6H). Found: C, 61.32; H, 5.28; N, 9.91.
Cz9H29C1N4O5.H2O requires:
C,61.43;H,5.51;N,9.88.
EXAMPLE 213
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydronyrrolo(3,4-
c]carbazol-6(1H)-yl)-N-(1H-tetraazol-5-~propanamide (XI; Ar=2-chlorophenyl
n=2, R3=H
R4=5-tetrazolvl) (253)
Reaction of acid (116) (70 mg, 0.16 mmol) prepared as described in example 229
according to the procedure described in example 207 , except that solid 5-
aminotetrazole (27
mg, 0.31 mmol) was added as the amine and the reaction was heated at reflux
for 2 hours
before work-up, gave amide (253) (52 mg, 63%) as a yellow powder, mp 232
°C (dec). 1H
NMR 8 [(CD3)ZSO] 15.8 (v br s, 1H), 11.90 (br s, 1H), 11.13 (br s, 1H), 8.52
(d, J=2.6 Hz,
1H), 7.84 (s, 1H), 7.75 (d, J=9.0 Hz, 1H), 7.57 (d, J=7.4 Hz, 1H), 7.50-7.40
(m, 3H), 7.30
(dd, J=9.0, 2.6 Hz, 1H), 4.83 (t, J=6.8 Hz, 2H), 3.90 (s, 3H), 2.97 (t, J=6.8
Hz, 2H). FABMS
found [M=H]+=516.1174, 518.1154. Cz5H18C1N~04 requires 516.1187, 518.1158.
EXAMPLE 214
The preparation of N-f2-(Dimethylamino)ethyll-3-(9-hey-1,3-dioxo-4-phenyl-2,3-
dihydropyrrolof3,4-clcarbazol-6(1H)-yl)propanamide (XIII; Ar=phenyl, n=2,
R3=H,
R4=CHcCH~,N(CH3)2) (254)
Reaction of methyl ether (247) (70 mg, 0.14 mmol) ) prepared as described in
example 207 according to the procedure described in example 81, except that
the reaction
mixture was diluted with water, basified by the addition of concentrated
ammonia and
extracted with ethyl acetate. The organic layer was dried, the drying agent
was removed and
the solution was concentrated to dryness. Chromatography on silica eluting
with ethyl
ace.tate/methanol/triethylamine (1:0:0 to 3: l Grace). Crystallization from
ethyl acetate/hexane
then gave amide (254) (35 mg, 53%) as an orange powder, mp 176-180
°C.'H NMR 8
[(CD3)ZSO] 11.06 (br s, 1H), 9.35 (br s, 1H), 8.39 (d, J=2.5 Hz, 1H), 7.77 (s,
1H), 7.74 (t,
J=5.6 Hz, 1H), 7.66 (m, 2H), 7.53 (d, J=8.8 Hz, 1H), 7.48 (m, 3H), 7.12 (dd,
J=8.8, 2.5 Hz,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-150-
1H), 4.69 (t, J=6.4 Hz, 2H), 2.94 (m, 2H), 2.58 (t, J=6.4 Hz, 2H), 1.91 (s,
6H), 1.87 (m, 2H).
Found: C, 66.87; H, 5.83; N, 11.47. CZ~HZ6N404.3/4Hz0 requires: C, 67.00; H,
5.73; N,
11.57.
EXAMPLE 215
The preparation of 3-(9-Hydroxy-1,3-dioxo-4-phenyl-2,3-dihydropyrrolo(3,4-
clcarbazol-
6(1H)-yl)propanamide (XIII; Ar=Qhenyl, n=2, R~=H, R~=H) (255) and 3-(9-l~rdrox
dioxo-4-phenyl-2,3-dihydropyrrolo(3,4-clcarbazol-6(1H)-yl)propanoic acid (X;
Ar=phenyl
n=2) (256)
Reaction of methyl ether (248) (56 mg, 0.14 mmol) ) prepared as described in
example 208 according to the procedure described in example 81, followed by
chromatography on silica eluting with ethyl acetate/hexane (4:1) to ethyl
acetate/methanol
(9:1) gave initially, amide (255) (11 mg, 20%) as an orange powder, mp 284-288
°C. 1H
NMR 8 [(CD3)ZSO] 11.05 (br s, 1H), 9.33 (br s, 1H), 8.40 (d, J=2.5 Hz, 1H),
7.82 (s, 1H),
7.67 (m, 2H), 7.56 (d, J=8.8 Hz, 1H), 7.47 (m, 3H), 7.34 (br s, 1H), 7.12 (dd,
J=8.8, 2.5 Hz,
1H), 6.86 (br s, 1H), 4.67 (t, J=6.5 Hz, 2H), 2.58 (t, J=6.5 Hz, 2H). FABMS
found [M+H]+:
400.1307. C23H1~N304 requires 400.1297. This was followed at lower Rf by the
acid (256)
(34 mg, 63%) as an orange powder, mp 300-310 °C (dec). 1H NMR 8
[(CD3)ZSO] 12.1 (v br
s, 1H), 11.06 (br s, 1H), 9.34 (br s, 1H), 8.40 (d, J=2.4 Hz, 1H), 7.84 (s,
1H), 7.67 (m, 2H),
7.57 (d, J=8.8 Hz, 1H), 7.46 (m, 3H), 7.12 (dd, J=8.8, 2.4 Hz, 1H), 4.69 (t,
J=6.7 Hz, 2H),
2.75 (t, J=6.7 Hz, 2H). Found: C, 67.64; H, 4.29; N, 6.65. C23Hi6NzOs.1/2Hz0
requires: C,
67.48; H, 4.19; N, 6.84.
EXAMPLE 216
The preparation of 3-(4-(2-Chloro~henyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolo(3,4-
clcarbazol-6(1H)-yl)-N-(2-(dimeth~amino)ethyllpropanamide (XIII; Ar=2-
chlorophenyl,
n=2, R3=H, R4=CH~CH~N. (CHI 257
Reaction of methyl ether (249) (65 mg, 0.13 mmol) prepared as described in
example
209 according to the proceedure described in example 81, except that the
reaction mixture
was diluted with water, basified by the addition of concentrated ammonia and
extracted with
ethyl acetate, The organic layer was dried, the drying agent was removed and
the solution
was concentrated to dryness. Chromatography on silica eluting with ethyl
acetate/methanol/triethylamine (1:0:0 to 3:1 aace). Crystallization from ethyl
acetate/hexane
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-151-
then gave amide (257) (28 mg, 44Qlo) as an orange powder, mp 205-215
°C. 1H NMR 8
[(CD3)ZSO] 11.06 (br s, 1H), 9.35 (s, 1H), 8.37 (d, J=2.3 Hz, 1H), 7.81 (br s,
1H), 7.72 (s,
1H), 7.57 (m, 2H), 7.48 (m, 3H), 7.14 (dd, J=8.9, 2.3 Hz, 1H), 4.67 (t, J=6.4
Hz, 2H), 2.99
(m, 2H), 2.57 (t, J=6.4 Hz, 2H), 2.03 (m, 8H). Found: C, 61.32; H, 5.49; N,
10.72.
CZ~HZSC1N4O4.11/4HZO requires: C, 61.47; H, 5.26; N, 10.62.
EXAMPLE 217
The preparation of 3-(4-(2,6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydronyrrolo(3 4
c carbazol-6 1H)-yl)pronanamide (XIII; Ar=2,6-dichlorophenyl, n=2, R3=H, R4=Hl
(2581
Reaction of methyl ether (250) (80 mg, 0.17 mmol) ) prepared as described in
example 210 according to The procedure described in example 80, followed by
chromatography on silica eluting with ethyl acetate then tetrahydrofuran and
trituration from
ethyl acetate, gave amide (258) (47 mg, 59%) as an orange powder, mp 327-329
°C.'H NMR
8 [(CD3)ZSO] 11.12 (br s, 1H), 9.39 (s, 1H), 8.36 (d, J=2.4 Hz, 1H), 7.82 (s,
1H), 7.61 (m,
3H), 7.51 (dd, J=8.8, 7.4 Hz, 1H), 7.38 (br s, 1H), 7.15 (dd, J=8.9, 2.4 Hz,
1H), 6.87 (br s,
1H), 4.65 (t, J=6.7 Hz, 2H), 2.55 (t, J=6.7 Hz, 2H). Found: C, 57.75; H, 3.83;
N, 8.50.
Cz3H~5C12N304.3/4H20 requires: C, 57.33; H, 3.45; N, 8.72.
EXAMPLE 218
The preparation of 3-(4-(2,6-Dichlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydroQyrrolo[3 4-
clcarbazol-6(1H)-yl)-N-(2-(dimethylamino)ethyllpropanamide (XIII; Ar=2,6-
dichlorophenyl
n=2, R3=H, R4=CH~CH~N(CH~hI (2591
Reaction of methyl ether (251 ) (70 mg, 0.13 mmol) ) prepared as described in
example 211 according to The procedure described in example 80, except that
the reaction
time was 6 hours and chromatography was performed eluting with ethyl
acetate/methanol/triethylamine (1:0:0 to 3:1 Grace), gave amide (259) (31 mg,
45%) as a
yellow powder, mp 220-226 °C.'H NMR 8 [(CD3)ZSO] 11.13 (br s, 1H), 9.39
(s, 1H), 8.36
(d, J=2.5 Hz, 1H), 7.84 (t, J=5.4 Hz, 1H), 7.76 (s, 1H), 7.62 (m, 2H), 7.58
(d, J=8.9 Hz, 1H),
7.51 (dd, J=8.9, 7.4 Hz, 1H), 7.16 (dd, J=8.9, 2.5 Hz, 1H), 4.67 (t, J=6.5 Hz,
2H), 3.03 (m,
2H), 2.57 (t, J=6.5 Hz, 2H), 2.16 (br s, 2H), 2.08 (m, 6H). Found: C, 58.09;
H, 4.44; N, 9.72.
CZ~HzaC12N404.Hz0 requires: C, 58.18; H, 4.70; N, 10.05.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-152-
EXAMPLE 219
The preparation of 3-(4-(2-Chloro-6-methoxyphenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-clcarbazol-6(1H)-yl)-N-f2-(dimeth~amino)ethyllpropanamide
(XIII;
Ar=2-chloro-6-methoxyphenyl, n=2, R3=H, R4=CH2CH~N(CH~)~
Reaction of methyl ether (252) prepared as described in example 212 (80 mg,
0.15
mmol) according to The procedure described in example 80, except that the
reaction was
performed at 0 °C for 2 hours and chromatography was performed eluting
with ethyl
acetate/methanol/triethylamine (1:0:0 to 3:larace), gave amide (260) (65 mg,
83%) as a
yellow/orange powder, mp 200-205 °C.'H NMR 8 [(CD3)ZSO] 11.00 (br s,
1H), 9.34 (s, 1H),
8.36 (d; J=2.4 Hz, 1 H), 7.87 (br s, 1 H), 7.66 (s, 1 H), 7.54 (d, J=8.9 Hz, 1
H), 7.44 (t, J=8.2
Hz, 1H), 7.18 (dd, J=8.2, 0.7 Hz, 1H), 7.12 (m, 2H), 4.64 (t, J=6.6 Hz, 2H),
3.68 (s, 3H), 3.05
(m, 2H), 2.56 (t, J=6.6 Hz, 2H), 2.23 (v br s, 2H), 2.15 (br s, 6H). Found: C,
58.98; H, 5.12;
N, 9.59. Cz8H2~C1N405.2H20 requires: C, 58.90; H, 5.47; N, 9.81.
EXAMPLE 220
The preparation of N-f2-(Dimethylamino)ethyll-3-(9-hydroxy-4-(2-methoxyphen~)-
1,3-
dioxo-2,3-dihydropyrrolof3,4-clcarbazol-6(1H)-yl)propanamide (XIII; Ar=2-
methoxyphenyl,
n=2, R3=H, R°=CH~CH~N(CH~z) (330)
To a solution of amide (260) (40 mg, 0.07 mmol) ) prepared as described in
example
219 in ethyl acetate/methanol (1:1, 80 mL) was added 5% Pd-C (catalytic). The
resulting
suspension was hydrogenated at 60 psi with stirring for 4 days, with
additional portions of
Pd-C being added every 24 hours. The reaction mixture was then filtered
through celite and
concentrated under reduced pressure before being chromatographed on silica
eluting with
methanol/ethyl acetate/triethylamine (l:4arace), to give amide (330) (6 mg,
16%) as a yellow
powder, mp 232-238 °C.'H NMR 8 [(CD3)zS0] 10.95 (br s, 1H), 9.33 (br s,
1H), 8.37 (d,
J=2.4 Hz, 1 H), 8.01 (br s, 1 H), 7.69 (s, 1 H), 7.54 (d, J=8.9 Hz, 1 H), 7.43
(m, 1 H), 7.34 (dd,
J=7.5, 1.8 Hz, 1H), 7.13-7.05 (m, 3H), 4.67 (t, J=6.2 Hz, 2H), 3.69 (s, 3H),
~3.2 (obscured m,
2H), 2.60 (t, J=6.2 Hz, 2H), 2.54-2.49 (obscured m, 8H). FABMS found [M+H]+:
501.2142.
CZBHZgN405 requires 501.2138.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-153-
EXAMPLE 221
The nrenaration of 3-(4-(2-Chlorophenvl)-9-hvdroxv-1,3-dioxo-2,3-
dihvdrovvrrolof3.4-
clcarbazol-6(1H)-yl)-N-(IH-tetraazol-5-yl)propanamide (XIII; Ar=2-chlorophen
1,~ n-2,
R3=H, R4=5-tetrazolyl) (261)
Reaction of methyl ether (253) (40 mg, 0.08 mmol) ) prepared as described in
example 213 according to the procedure described in example 80, except that
the
chromatography was performed eluting with ethyl acetate/methanol (1:0 to 9:1),
gave amide
(261) (11 mg, 27%) as an orange powder, mp 283 °C (dec). 1H NMR 8
[(CD3)ZSO] 15.82 (br
s, 1H), 12.03 (br s, 1H), 11.08 (s, 1H), 9.38 (s, 1H), 8.37 (d, J=2.3 Hz, 1H),
7.79 (s, 1H), 7.62
(d, J=8.8 Hz, 1H), 7.56 (d, J=7.6 Hz, 1H), 7.49-7.38 (m, 3H), 7.13 (dd, J=8.8,
2.3 Hz, 1H),
4.80 (t, J=6.7 Hz, 2H), 2.96 (t, J=6.7 Hz, 2H). FABMS found M+: 501.0955,
503.0956.
CZaH16C1N~04 requires 501.0952, 503.0923.
Representative Procedure for Method 15 of Scheme 4
EXAMPLE 222
The pr~aration of N-f3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4
clcarbazol-6(1H)-yl)propanoyllmethanesulfonamide (XII; Ar=2-chlorophenyl, n=2,
R3=CHI
To a stirred solution of acid (116) (0.20 g, 0.45 mmol) prepared as described
in
example 229, 4-dimethylaminopyridine (DMAP) (165 mg, 1.35 mmol) and
methanesulfonamide (86 mg, 0.90 mmol) in dimethylformamide (10 mL) under
nitrogen was
added 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCLHCI)
(259 mg,
1.35 mmol). The resulting mixture was stirred at room temperature for 18 hours
and then
diluted with water, acidified by the addition of 1N hydrochloric acid and
extracted with ethyl
acetate. The organic phase was dried, the drying agent was removed and the
solution was
concentrated to dryness. Chromatography on silica eluting with ethyl
acetate/hexane (4:1) to
ethyl acetate/methanol (1:0 to 9:1), gave acylsulfonamide (265) (151 mg, 64%)
as a yellow
powder, mp 293-295 °C.'H NMR 8 [(CD3)zS0] 11.76 (br s, 1H), 11.14 (br
s, 1H), 8.52 (d,
J=2.6 Hz, 1H), 7.87 (s, 1H), 7.73 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.53-7.46
(m, 3H), 7.32
(dd, J=9.0, 2.6 Hz, 1 H), 4.74 (t, J=6.9 Hz, 2H), 3.91 (s, 3H), 3.08 (s, 3H),
2.81 (t, J=6.9 Hz,
2H). Found: C, 57.30; H, 3.87; N, 7.72. CZSHZOC1N3O6S requires: C, 57.08; H,
3.83; N, 7.99.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-154-
EXAMPLE 223
The preparation of N-f3-(4-(2-Chlorophen l~ydroxy-1,3-dioxo-2,3-
dih~pyrrolof3,4-
clcarbazol-6(IH)-~propanoyllbenzenesulfonamide (XIV; Ar=2-chlorophenyl, n=2,
R3=Ph)
266
Reaction of acid (116) (70 mg, 0.16 mmol) prepared as described in example 229
with
benzenesulfonamide according to the procedure described in example 222
followed by
demethylation employing the procedure described in example 80 and
chromatography on
silica eluting with ethyl acetate/hexane (1:1 to 3:1), gave acylsulfonamide
(266) (63 mg,
70%) as an orange powder, mp 291-293 °C.'H NMR 8 [(CD3)zS0] 12.15 (br
s, 1H), 11.07
(br s, 1H), 9.36 (s, 1H), 8.35 (d; J=2.4 Hz, 1H), 7.81 (m; 2H), 7.76 (s, 1H),
7.65 (m, 1H),
7.57-7.46 (m, 7H), 7.06 (dd, J=8.9, 2.4 Hz, 1H), 4.59 (m, 2H), 2.75 (t, J=7.1
Hz, 2H). Found:
C, 60.62; H, 3.80; N, 7.20. C29HZOC1N3O6S requires: C, 60.68; H, 3.51; N,
7.32.
EXAMPLE 224
The preparation of N-f3-(4-(2-chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)propanoyll 2-(di-methylamino)ethanesulfonamide (XII; Ar=2-
chlorophenyl, n=2, R3=CH~CH~N(CH~)~ 267
Reaction of acid (116) (70 mg, 0.16 mmol) prepared as described in example 229
with
2-(dimethylamino)ethanesulfonamide according to the procedure described in
example 222,
except that the reaction mixture was diluted with brine before being extracted
with ethyl
acetate. The organic phase was dried, the drying agent was removed and the
solution was
concentrated to dryness. and the chromatography was performed eluting with
ethyl
acetate/methanol (1:0 to 4:1),gave acylsulfonamide (267) (43 mg, 47%) as a
yellow powder,
mp 208-213 °C.'H NMR S [(CD3)2S0] 11.11 (br s, 1H), 8.52 (d, J=2.6 Hz,
1H), 7.80 (s, 1H),
7.72 (d, J=9.0 Hz, 1H), 7.57 (m, 2H), 7.47 (m, 2H), 7.31 (dd, J=9.0, 2.6 Hz,
1H), 4.68 (m,
2H), 3.90 (s, 3H), 3.23 (partially obscured m, 2H), 2.87 (t, J=6.9 Hz, 2H),
2.62 (t, J=6.8 Hz,
2H), 2.43 (s, 6H). Found: C, 59.44; H, 5.57; N, 8.52. CZBHZ~C1N406S.1/2C6I314
requires: C,
59.47; H, 5.47; N, 8.95.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-155-
EXAMPLE 225
The preparation of N-f4-(4-(2-Chlorophenyl)-9-h~xy-1,3-dioxo-2,3-
dihydropyrrolo(3 4-
clcarbazol-6(1H)-yl)butanoyllmethanesulfonamide (XIV; Ar=2-chlorophenyl, n=3,
R3=CHI
(268)
Reaction of acid (262) (50 mg, 0.11 mmol) prepared as described in example 87
according to The procedure described in example 222 followed by demethylation
employing
the procedure described in example 80 and chromatography on silica eluting
with ethyl
acetate/hexane (1:1 to 3:1), gave acylsulfonamide (268) (29 mg, 51%) as an
orange powder,
mp 257-260 °C.'H NMR 8 [(CD3)zS0] 11.66 (br s, 1H), 11.06 (br s, 1H),
9.37 (s, 1H), 8.38
(d, J=2.4 Hz, 1H), 7.79 (s, 1H), 7.61-7:45 (m, SH), 7.15 (dd, J=8.8, 2.4 Hz,
1H), 4.45 (t, J=7.2
Hz, 2H), 3.10 (s, 3H), 2.32 (m, 2H), 1.97 (m, 2H). Found: C, 57.40; H, 3.94;
N, 7.73.
CzsHzoC1N306S requires: C, 57.09; H, 3.83; N, 7.99.
EXAMPLE 226
The preparation of N-f4-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
di)~dropyrrolof3,4-
clcarbazol-6(1H)-yl)butanoyllbenzenesulfonamide (XIV; Ar=2-chlorophenyl, n=3,
R3=Ph)
2( 69)
Reaction of acid (262) (45 mg, 0.10 mmol) prepared as described in example 87
with
benzenesulfonamide according to the procedure described in example 222
followed by
demethylation employing the procedure described in example 80 and
chromatography on
silica eluting with ethyl acetate/hexane (1:1 to 3:1), gave acylsulfonamide
(269) (39 mg,
68%) as an orange powder, mp 224-230 °C.'H NMR 8 [(CD3)zS0] 12.09 (br
s, 1H), 11.05
(br s, 1H), 9.36 (s, 1H), 8.36 (d, J=2.4 Hz, 1H), 7.88 (m, 2H), 7.73 (s, 1H),
7.67 (m, 1H), 7.57
(m, 3H), 7.52-7.43 (m, 4H), 7.09 (dd, J=8.8, 2.4 Hz, 1H), 4.33 (t, J=7.3 Hz;
2H), 2.29 (t,
J=7.2 Hz, 2H), 1.86 (m, 2H). Found: C, 61.10; H, 3.81; N, 6.91. C3oHzzC1N306S
requires: C,
61.28; H, 3.77; N, 7.15.
EXAMPLE 227
The preparation of N-f3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)propanoyllmethanesulfonamide (XIV; Ar=2-chlorophen I,
R3=CHI 270
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-156-
Reaction of methyl ether (265) (130 mg, 0.25 mmol) prepared according to
example
222 according to the procedure described in example 80, except that the
chromatography
was performed eluting with methanol/dichloromethane (5:95 to 1:9), gave
acylsulfonamide
(270) (83 mg, 66%) as an orange powder, mp 290-296 °C. 'H NMR 8
[(CD3)ZSO] 11.75 (br s, .
1H), 11.08 (br s, 1H), 9.38 (s, 1H), 8.38 (d, J=2.4 Hz, 1H), 7.80 (s, 1H),
7.59 (m, 2H), 7.53-
7.46 (m, 3H), 7.14 (dd, J=8.9, 2.4 Hz, 1H), 4.69 (t, J=6.9 Hz, 2H), 3.04 (s,
3H), 2.76 (t, J=6.9
Hz, 2H). Found: C, 54.70; H, 3.81; N, 7.67. C24HI8C1N306S.3/4H20 requires: C,
54.85; H,
3.74; N, 7.99.
EXAMPLE 228
-The preparation of N-f3-(4-(2-Chlorophen l~hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
c]carbazol-6(1H)-~propanoyll-2-(dimethylamino)ethanesulfonamide (XIV; Ar=2-
chlorophenyl, n=2, R3=CH~CHZN(CH3)~) (271)
Reaction of methyl ether (267) (35 mg, 0.06 mmol) prepared as described in
example
224 according to the proceedure described in example 81, except that the
reaction mixture
was diluted with saturated sodium bicarbonate and extracted with ethyl
acetate. The organic
phase was dried, the drying agent was removed and the solution was
concentrated to dryness.
Chromatography on silica eluting with methanol/dichloromethane (1:9 to 1:3),
gave
acylsulfonamide (271) (8 mg, 23%) as an orange powder, mp 229-233 °C.
IH NMR 8
[(CD3)zS0] 11.05 (br s, 1H), 9.37 (br s, 1H), 8.37 (d, J=2.5 Hz, 1H), 7.74 (s,
1H), 7.61-7.52
(m, 3H), 7.50-7.43 (m, 2H), 7.14 (dd, J=8.7, 2.5 Hz, 1H), 4.63 (m, 2H), 3.21
(partially
obscured m, 2H), 2.81 (m, 2H), 2.59 (t, J=6.8 Hz, 2H), 2.38 (s, 6H). FABMS
found [M+H]+:
569.1255, 571.1204. C27HZSC1N4O6S requires 569.1262, 571.1232.
EXAMPLE 229
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyriolof3,4-
clcarbazol-6(1H)-yl)propanoic acid (IX; Ar=2-chlorophenyl, n=2) (116)
Oxidation of the alcohol (31) prepared as described in example 40 using the
procedure described in example 203 gave the acid (116) (62 %) as a yellow
solid, mp 277 °C.
'H NMR 8 [(CD3)ZSO] 12.61 (br, 1H), 11.12 (s, 1H), 8.52 (d, J=2.6 Hz, 1H),
7.86 (s, 1H),
7.73 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.54-7.44 (m, 3H), 7.31 (dd, J=9.0, 2.6
Hz, 1H), 4.71
(t, J=6.8 Hz, 2H), 3.93 (s, 3H), 2.80 (t, J=6.8 Hz, 2H). Found: C, 62.90; H,
3.79; N, 5.93.
C24H»CINz05.1/2Hz0 requires C, 62.96; H, 3.96; N, 6.12.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-157-
EXAMPLE 230
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)proganoic acid (X; Ar=2-chlorophenyl, n=2) (117)
Demethylation of (116) prepared as described in example 229 with pyridinium
hydrochloride using the procedure described in example 81 gave (117) (50 %) as
a yellow
powder, mp 286 °C.'H NMR 8 [(CD3)zS0] 12.40 (br, 1H), 11.06 (br s, 1H),
9.37 (br s, 1H),
8.37 (d, J=2.4 Hz, 1H), 7.81 (s, 1H), 7.59 (d, J=8.7 Hz, 1H), 7.57 (m, 1H),
7.53-7.43 (m, 3H),
7.13 (dd, J=8.7, 2.4 Hz, 1H), 4.67 (t, J=6.8 Hz, 2H), 2.74 (t, J=6.8 Hz, 2H).
Found: C, 60.00;
H, 3.65; N, 5.52. Cz3H15C1NzO5.1.5Hz0 requires C, 59.81; H, 3.92; N, 6.06.
EXAMPLE 231
The pr~aration of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydrop_yrtolof3,4-
clcarbazol-6(1H)-vl)pronanamide (XI; Ar=2-chlorophenvl. n=2, R3=R4=Hl (1181
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by ammonia using the procedure described in example 207 gave the
amide (118)
(78 %) as a yellow powder, mp 283-286 °C. 1H NMR 8 [(CD3)zS0] 11.12 (br
s, 1H), 8.52 (d,
J=2.6 Hz, 1H), 7.82 (s, 1H), 7.71 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.54-7.44
(m, 3H), 7.35
(br, 1H), 7.31 (dd, J=9.0, 2.6 Hz, 1H), 6.86 (br, 1H), 4.69 (t, J=6.6 Hz, 2H),
3.90 (s, 3H), 2.58
(t, J=6.6 Hz, 2H). Found: C, 63.52; H, 4.22; N, 9.04. Cz4H,$C1N30a.1/4H20
requires C,
63.72; H, 4.12; N, 9.29.
EXAMPLE 232
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dih~pyrrolof3,4-
clcarbazol-6(1H)-vl)nronanamide (XIII; Ar=2-chloroohenvl, n=2. R3=R4=H7 (1191.
Demethylation of (118) prepared as described in example 231 with BBr3 using
the
procedure described in example 80 gave (119) as a yellow/orange powder (77 %),
mp 319-
322 °C. 1H NMR 8 [(CD3)zS0] 11.06 (br s, 1H), 9.36 (br s, 1H), 8.37 (d,
J=2.4 Hz, 1H), 7.77
(s, 1H), 7.60-7.55 (m, 2H), 7.52-7.43 (m, 3H), 7.35 (br, 1H), 7.13 (dd, J=8.8,
2.4 Hz, 1H),
6.87 (br, 1H), 4.65 (t, J=6.7 Hz, 2H), 2.57 (t, J=6.7 Hz, 2H). FABMS found M+:
435.0833,
433.0828. Cz3H~6C1N304 requires 435.0800, 433.0829.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-158-
EXAMPLE 233
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolo(3,4-
clcarbazol-6(1H)-yl)-N-f2-(4-morpholinyl)ethyllpropanamide (XI; Ar=2-
chlorophenyl, n=2,
R3=H R4=CH~CHzN(CH~,CH~)~O) (120)
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by N-(2-aminoethyl)morpholine using the procedure described in
example 207
gave the amide (120) (73 %) as a yellow powder, mp 174-176 °C.'H NMR b
[(CD3)ZSO]
11.12 (br s, 1 H), 8.51 (d, J=2.6 Hz, 1 H), 7.77 (s, 1 H), 7.74 (t, J=5.6 Hz,
1 H), 7.68 (d, J=9.0
Hz, 1H), 7.58 (m, 1H), 7.53-7.44 (m, 3H), 7.31 (dd, J=9.0, 2.6 Hz, 1H), 4.71
(t, J=6.3 Hz,
-2H), 3.90 (s, 3H), 3.40 (t,-J=4:6-Hz; 4H); 2:97 (m, -1H),-2.58 (t~ J=6.3 Hz;
2H), 2.11 (br t,
J=4~6 Hz, 4H); 2.00 (t, J=6.7 Hz; 2H-). Found: C, 60.46; H, 5.26; N, 9.27.
CsoHz9C1N4O5.2H2O requires C, 60.35; H, 5.94; N, 9.38.
EXAMPLE 234
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)-N-(2-(4-morpholin ly )ethyllpropanamide (XIII; Ar=2-
chlorophenyl,
n=2 R3=H, R4=CH~CH~N(CH~CH~20 121
Demethylation of (120) prepared as described in example 233 with BBr3 using
the
procedure described in example 80 except that the reaction time was 16 h gave
(121) as a
yellow powder (64 %), mp 143-148 °C (dec).'H NMR 8 [(CD3)ZSO] 11.05 (s,
1H), 9.35 (s,
1H), 8.36 (d, J=2.4 Hz, 1H), 7.74 (t, J=5.6 Hz, 1H), 7.71 (s, 1H), 7.60-7.55
(m, 2H), 7.52-
7.43 (m, 3H), 7.13 (dd, J=8.8, 2.4 Hz, 1H), 4.67 (t, J=6.3 Hz, 2H), 3.40 (t,
J=4.7 Hz, 4H),
2.98 (m, 2H), 2.57 (t, J=6.3 Hz, 2H), 2.12 (t, J=4.7 Hz, 4H), 2.02 (t, J=6.7
Hz, 1H). Found: C,
62.37; H, 4.99; N, 9.82. C29HZ~C1N405.1/2Hz0 requires C, 62.64; H, 5.08; N,
10.08.
EXAMPLE 235
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
cLcarbazol-6(1H)-yl)-N-f2-(dimethylamino)ethyll-N-methylpropanamide (XI; Ar=2-
chlorophenyl, n=2. R3=CHI, R4=CH~CH~N(CH~ 122
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by N,N,N'-trimethylethylenediamine using the procedure described in
example 207
gave the amide (122) (77 %) as a yellow powder, mp 146-152 °C (dec).'H
NMR 8
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-159-
[(CD3)ZSO] 11.10 (br, 1H), 8.51 (d, J=2.6 Hz, 1H), 7.83 (s, O.SH), 7.79 (s,
O.SH), 7.72 (m,
1H), 7.58 (m, 1H), 7.54-7.43 (m, 3H), 7.31 (m, 1H), 4.72 (m, 2H), 3.90 (s,
1H), 3.25 (m, 1H),
3.09 (m, 1H), 2.83 (m, 2H), 2.72 (s, 1.SH), 2.71 (s, 1.SH), 2.13 (t, J=4.8 Hz,
1H), 2.05 (s,
3H), 2.00 (t, J=4.8 Hz, 1H), 1.88 (s, 3H). Found: C, 63.42; H, 5.46; N, 10.15.
Cz9Hz9C1N4O4.HzO requires C, 63.21; H, 5.67; N, 10.17.
EXAMPLE 236
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1Hl-yl)-N-f2-(dimethylamino)ethyll-N-methylpropanamide (XIII;
Ar=2-
chlorophenyl, n=2, R3=CHI, R4=CH~CH2N(CH~2 123
Demethylation of (122) prepared as described in example 236 with BBr3 using
the
procedure described in example 80 except that the reaction time was 48 h gave
(123) as a
yellow powder (58 %), mp 184-188 °C.'H NMR S [(CD3)ZSO] 11.06 (br s,
1H), 9.36 (br s,
1H), 8.37 (d, J=2.4 Hz, 1H), 7.78 (s, O.SH), 7.74 (s, O.SH), 7.62-7.54 (m,
2H), 7.53-7.42 (m,
3H), 7.14 (dd, J=8.8, 2.4 Hz, 1H), 4.69 (m, 2H), 3.22 (m, 1H), 3.09 (m, 1H),
2.82 (m, 2H),
2.72 (s, 1.5 H), 2.71 (s, 1.SH), 2.14 (t, J=4.8 Hz, 1H), 2.06 (s, 3H), 2.00
(t, J=4.8 Hz, 1H),
1.88 (s, 3H). Found: C, 62.23; H, 5.23; N, 10.24. CZ$HZ~C1N404.1.25H20
requires C, 62.10;
H, 5.49; N, 10.35.
EXAMPLE 237
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dih~pyrrolo[3,4-
clcarbazol-6(1H)-yl)-N-(2,2,6,6-tetramethyl-4-piperidinyl)propanamide (XI;
Ar=2-
chloronhenvl, n=2, R3=H, R4=2,2,6,6-tetramethvl-4-nineridinvl) (124)
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by 4-amino-(2,2,6,6-tetramethyl)piperidine using the procedure
described in
example 207 gave the amide (124) (72 %) as a yellow powder, mp 155-160
°C (dec).'H
NMR b [(CD3)ZSO] 11.12 (br, 1H), 8.51 (d, J=2.6 Hz, 1H), 7.78 (s, 1H), 7.67
(d, J=9.0 Hz,
1H), 7.59-7.44 (m, 6H), 7.31 (dd, J=9.0, 2.6 Hz, 1H), 4.71 (m, 2H), 3.90 (s,
3H), 3.82 (m,
1H), 2.52 (t, J=6.2 Hz, 2H), 1.26 (m, 1H), 1.10 (m, 1H), 1.03 (s, 3H), 1.02
(s, 3H), 0.91 (s,
3H), 0.96 (s, 3H), 0.64 (t, J=12.3 Hz, 1H), 0.53 (t, J=12.3 Hz, 1H). Found: C,
64.37; H, 5.97;
N, 9.20. C33H35C1N4O4.1.SHZO requires C, 64.54; H, 6.24; N, 9.12.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-160-
EXAMPLE 238
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1 3-dioxo-2,3-
dihydropyrrolof3,4-
~carbazol-6(1H)-yl)-N-(2,2,6,6-tetramethyl-4-piperidinyl)propanamide (XIII;
Ar=2-
chlorophenyl n=2 R3=H R4=2 2 6 6-tetramethyl-4-piperidinyl) (125)
Demethylation of (124) prepared as described in example 237 with BBr3 using
the
procedure described in example 80 except that the reaction time was 48 h gave
(125) as a
yellow powder (47 %), mp 293-299 °C (dec).'H NMR 8 [(CD3)ZSO] 11.08 (br
s, 1H), 9.38
(br s, 1 H), 8.72 (br, 1 H), 8.37 (d, J=2.4 Hz, 1 H), 7.83 (d, J=7.1 Hz, l H),
7.75 (s, 1 H), 7.60-
7.44 (m, SH), 7.14 (dd, J=8.8, 2.4 Hz, 1H), 4.70 (t, J=5.8 Hz, 2H), 3.90 (m,
1H), 2.54 (t,
J=5.8 Hz, 2H), 1.45-1.31 (m; 2H), 1.30 (s, 3H), 1.29 (s, 3H), 1.25 (s, 3H),
1.22 (s, 3H), 1.09-
9.93 (m, 2H). Found: C, 58.67; H, 5.37; N, 8.41. C3ZH33C1N404.1.25CHZC12
requires C,
58.79; H, 5.27; N, 8.25.
EXAMPLE 239
The preparation of 4-(2-Chlorophenyl)-6-( 3-fcis-3 5-dimethylpiperazinyll-3-
oxopropyll-9-
-methoxypyrrolof3 4-clcarbazole-1 3(2H 6H)-dione-(XI= Ar=2-chlorophenyl, n=2,
R3 R4=cis-
3 5-dimethylpiperazinyl) (126)
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by cis-2,6-dimethylpiperazine using the procedure described in
example 207 gave
the amide (126) (76 %) as a yellow powder, mp 168-173 °C (dec).'H NMR 8
[(CD3)ZSO]
11.13 (br, 1H), 8.52 (d, J=2.6 Hz, 1H), 7.80 (s, O.SH), 7.76 (s, O.SH), 7.71
(m, 1H), 7.58 (m,
1H), 7.54-7.43 (m, 3H), 7.31 (dd, J=9.0, 2.6 Hz, 1H), 4.71 (m, 2H), 4.17 (m,
1H), 3.89 (s,
3H), 2.94 (m, 1H), 2.75 (m, 1H), 2.23 (m, 1H), 2.08 (m, 1H), 1.87 (m, 2H),
0.88, 0.86 (2d,
J=5.9 Hz, 3H), 0.67 (d, J=6.1 Hz, 3H). Found: C, 64.03; H, 5.47, N, 9.72.
C3oH29C1N404.H20
requires C, 64.00; H, 5.55; N, 9.95.
EXAMPLE 240
The preparation of 4-(2-Chlorophenyl)-6-(3-f(3R,SS)-3,5-dimethylpiperazinyll-3-
oxopropyl~-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XIII; Ar=2-
chlorophenyl,
n=2, R3 R4=cis-3.5-dimethylpiperazinyl) (127)
Demethylation of (126) prepared as described in example 239 (with BBr3 using
the
procedure described in example 80 except that the reaction time was 48 h gave
(127) as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-161-
yellow powder (38 %), mp 220-224 °C (dec).'H NMR 8 [(CD3)ZSO] 11.07 (br
s, 1H), 9.36
(br s, 1H), 8.72 (br, 1H), 8.34 br, 1H), 7.72 (s, O.SH), 7.71 (s, O.SH), 7.60-
7.54 (m, 2H), 7.52-
7.43 (m, 3H), 7.13 (dd, J=8.8, 2.4 Hz, 1H), 4.68 (m, 3H), 4.15, 4.12 (2s, 2H),
2.94 (m, 1H),
2.72 (m, 1 H), 2.19 (m, 1 H), 2.00 (m, 1 H), 1.84 (m, 1 H), 0.86, 0.84 (2d,
J=5.8 Hz, 3H), 0.64
(2d, J=6.1 Hz, 3H). Found: C, 63.38; H, 5.38; N, 9.96. C29HZ~C1N4O4.HZO
requires C, 63.44;
H, 5.32; N, 10.20.
EXAMPLE 241
The preparation of 3-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)-N-f2-(1H-imidazol-5-yl)ethyllpropanamide (XI; Ar=2-
chlorophenyl,
n=2. R3=H, R4=(1H-imidazol-5-vl)ethvl) (128)
Reaction of acid (117) prepared as described in example 230 with oxalyl
chloride
followed by histamine using the procedure described in example 207 gave the
amide (128)
(81 %) as a yellow powder, mp 144-149 °C (dec).'H NMR 8 [(CD3)ZSO]
11.12 (br, 1H),
8.52 (d, J=2.6 Hz, 1H), 7.91 (t, J=5.5 Hz, 1H), 7.76 (s, 1H), 7.69 (d, J=9.0
Hz, 1H), 7.58 (m,
1H), 7.52-7.42 (m, 4H), 7.32 (dd, J=9.0, 2.6 Hz, 1H), 6.56 (s, 1H), 4.69 (t,
J=6.4 Hz, 2H),
3.90 (s, 3H), 3.12 (m, 2H), 2.58 (t, J=6.4 Hz, 2H), 2.39 (t, J=7.4 Hz, 2H).
Found: C, 60.73; H,
4.49; N, 11.49. CZ~H24C1N504.1/2CHZCIz requires C, 60.62; H, 4.31; N, 11.98.
EXAMPLE 242
The preparation of 3-(4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-
dihydropyrrolof3,4-
clcarbazol-6(1H)-yl)-N-f2-(1H-imidazol-5-yl)ethyllpropanamide (XIII; Ar=2-
chlorophenyl,
n=2. R3=H, R4=(1H-imidazol-5-vl)ethvl) (129)
Demethylation of (128) prepared as described in example 241 with BBr3 using
the
procedure described in example 80 except that the reaction time was 48 h gave
(129) as a
yellow powder (46 %), mp 158-162 °C (dec).'H NMR S [(CD3)ZSO] 11.75
(br, 1H), 11.05
(br s, 1H), 9.36 (br s, 1H), 8.37 (d, J=2.4 Hz, 1H), 7.91 (t, J=5.3 Hz, 1H),
7.71 (s, 1H), 7.59-
7.54 (m, 2H), 7.50-7.42 (m, 4H), 7.14 (dd, J=8.8, 2.4 Hz, 1H), 6.57 (br, 1H),
4.65 (t, J=6.5
Hz, 2H), 3.13 (m, 2H), 2.55 (t, J=6.5 Hz, 1H), 2.40 (t, J=6.5 Hz, 1H). FABMS
found
[M+H]+: 530.143 i, 528.1440. CZgH23C1N504 requires 530.1409, 528.1439.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-162-
NaBH4/MeOH
M ET
(VI) MeOW (XV) (XVI)
p-TsOH / PhSeH/p-TsOH
(XVII) (XVIII)
SCHEME 5
Scheme 5 Procedures
Representative Procedure for Method 16 of Scheme 5
EXAMPLE 243
The nrenaration of 1,9-Dihvdroxv-4-phenyl-1,6-dihvdropvrrolof3,4-clcarbazol-
3(2H)-one
(XV: Ar=nhenvl, R'°=H) (133) and 3,9-dihvdroxv-4-phenyl-3,6-
dihvdropvrrolo13,4-
clcarbazol-1(2H)-one (XVI; Ar=phenyl, Rl°=H) (134).
Sodium borohydride (4 portions of 0.24 g, 0.025 mol total) was added over 5 h
to a
solution of 9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (I;
Ar=phenyl)
(0.50 g, 0.015 mmol) in ethanol (80 mL) and the solution was left overnight. A
further four
portions of sodium borohydride were added at 1 h intervals and the solution
was diluted with
water, extracted with ethyl acetate. The organic phase was dried, the drying
agent was
removed and the solution was concentrated to dryness, which was
chromatographed on silica.
Ethyl acetate/petroleum ether (2:1) eluted starting material (12 mg), followed
by the 3-
hydroxy compound (134) (0.07 g, 14 %) as a cream powder, mp 300-310 °C
(dec).'H NMR
8 [(CD3)ZSO] 11.26 (br s, 1H), 8.98 (s, 1H), 8.83 (s, 1H), 8.50 (d, J=2.3 Hz,
1H), 7.70 (br d,
J==7.0 Hz, 2H), 7.53 (s, 1H), 7.49-7.31 (m, 4H), 6.96 (dd, J=8.7, 2.3 Hz, 1H),
6.25 (d, J=9.7
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-163-
Hz, 1H), 5.87 (d, J=9.7 Hz, 1H). Found: C, 71.77; H, 4.47; N, 8.14.
CZ°H~3N203.1/4H20
requires C, 71.95; H, 4.08; N, 8.39.
Elution with ethyl acetate gave a mixed fraction of (134) and (133) (0.014 g),
followed by pure 1-hydroxy compound (133) (0.32 g, 64 %) as a white powder, mp
300-310
°C (dec).'H NMR 8 [(CD3)2S0] 11.38 (br s, 1H), 9.06 (s, 1H), 8.55 (br
s, 1H), 7.72 (d, J=2.2
Hz, 1H), 7.55 (dd, J=7.8, 2.1 Hz, 2H), 7.47-7.35 (m, 4H), 7.32 (s, 1H), 6.98
(dd, J=8.6, 2.2
Hz, 1H), 6.38 (d, J=10.3 Hz, 1H), 6.20 (d, J=10.3 Hz, 1H). Found: C, 68.77; H,
4.66; N, 7.87.
C2°H~3NZ03.H20 requires C, 69.15; H, 4.35; N, 8.06.
EXAMPLE 244
The Qeparation of 9-HydroXy-1-methoxy-4phenyl-1,6-dihydropyrrolo(3,4-
clcarbazol-
3 2H -one (XVII; Ar=phenyl, R'°=H) (135).
A solution of (133) prepared as described in example 243 (0.050 g, 0.16 mmol)
and
p-toluenesulphonic acid (15 mg) on methanol (5 mL) was stirred at room
temperature for 30
min, then poured into saturated aqueous NaHC03 solution. The mixture was
extracted with
ethyl acetate. The organic phase was dried, the drying agent was removed and
the solution
was concentrated to dryness to give (135), which crystallised from ethyl
acetate/petroleum
ether as a white solid (0.041 g, 74 %), mp 290-300 °C (dec). 1H NMR 8
[(CD3)2S0] 11.47 (br
s, 1H), 9.15 (br s, 1H), 8.75 (s, 1H), 7.57-7.53 (m, 3H), 7.44-7.37 (m, 4H),
7.36 (s, 1H), 7.09
(dd, J=8.7, 2.4 Hz, 1H), 6.26 (s, 1H), 3.25 (s, 3H). Found: C, 71.34; H, 4.79,
N, 7.84.
CZ~H15N203.1/2H20 requires C, 71.58; H, 4.57; N, 7.95.
EXAMPLE 245
The preparation of 9-H droxy-4-phenyl-1,6-dihydropyrrolo(3,4-clcarbazol-3(2H)-
one
IXVIII. Ar=nhenvl, Rl°=H) (136).
To a solution of (133) prepared as described in example 243 (0.20 g, 0.605
mmol) in
tetrahydrofuran (30 mL) was added p-toluenesulfonic acid (23 mg, 0.121 mmol),
followed be
PhSeH (1.48 mL, 4.24 mmol) and the solution was stirred at room temperature
for 1 h. After
diluting with water the mixture was extracted with ethyl acetate and the
extract was washed
with aqueous NaHC03 solution. The organic phase was dried, the drying agent
was removed
and the solution was concentrated to dryness and chromatographed on silica.
Elution with
ethyl acetate/petroleum ether (1:1) then ethyl acetate followed by ethyl
acetate/methanol
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-164-
(95:5) gave (136) as a white powder (0.174 g, 91 %), mp 270-280 °C
(dec).'H NMR 8
[(CD3)ZSO] 11.41 (br s, 1H), 9.13 (br s, 1H), 8.27 (br s, 1H), 7.60-7.52 (m,
2H), 7.47-7.36
(m, 4H), 7.34-7.31 (m, 1H), 7.30 (s, 1H), 6.99 (dd, J=8.6, 2,2 Hz, 1H), 4.78
(br s, 2H).
Found: C, 75.19; H, 4.88, N, 8.37. CZ°H~4N202.1/4H20 requires C, 75.34;
H, 4.58; N, 8.78.
EXAMPLE 246
The~reparation of 4-(2-Chlorophenyl)-1,9-dih dery-6-(3-hydroxypropyl)-1,6-
dih~dropyrrolof3,4-clcarbazol-3(2H)-one (XV; Ar=2-chlorophenyl,
R'°=CHzCH?CH~OH)
(137) and 4-(2-chlorophenyl)-3,9-dihydroxy-6-(3-hydroxypro~Jrl)-3,6-
dihydropyrrolof3,4-
clcarbazol-1(2H)-one (XVI; Ar=2-chlorophenyl, R'°=CH~CH2CH20H) (138).
Reduction of alcohol (34) prepared as described in example 243 with sodium
borohydride using the procedure described in the procedure described in
example 243 of
Scheme 5, followed by chromatography of the product on silica gave firstly the
3-hydroxy
compound (138) (11 %) as a cream powder, mp 160-164 °C (dec). 'H NMR 8
[(CD3)ZSO]
9.08 (br s, 1H), 8.84 (s, 1H), 8.54 (d, J=2.5 Hz, 1H), 7.62-7.36 (m, 6H), 7.03
(dd, J=8.7, 2.5
Hz, 1H), 5.92 (br, 1H), 5.81 (br, 1H), 4.58 (t, J=5.0 Hz, 1H), 4.44 (t, J=6.8
Hz, 2H), 3.38 (m,
2H), 1.88 (m, 2H). Found: C, 64.60; H, 4.82; N, 6.32. C23Hi9C1Nz04.1/4H20
requires C,
64.64; H, 4.60; N, 6.55; followed by (137) (67 %) as a cream powder, mp 240
°C (dec).'H
NMR S [(CD3)ZSO] 9.18 (br s, 1H), 8.53 (br s, 1H), 7.76 (m, 1H), 7.50 (m, 2H),
7.43-7.35
(m, 3H), 7.40 (s, 1H), 7.04 (dd, J-8.7, 2.3 Hz, 1H), 6.52 (d, J=10.0 Hz,
O.SH), 6.40 (d, J=10.0
Hz, O.SH), 6.25 (d, J=10.0 Hz, O.SH), 6.21 (d, J=10.0 Hz, O.SH), 4.61 (t,
J=4.7 Hz, 1H), 4.44
(t, J=6.8 Hz, 2H), 3.39 (m, 2H), 1.88 (m, 2H). Found: C, 65.17; H, 4.80; N,
6.46.
C23H1~C1Nz04 requires C, 65.33; H, 4.53; N, 6.62.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-165-
Me0' ~ 'CHO N'CHzCOOMe Me0 \ \ COOMe feat M~ \
-t ~ ~ ~ OOMe
/ NaOMe ~ / N xylene /
Bn0 Bn0 ' B~O H
(54) (55) (56)
lhMeO \ ~ HO A~HiPPh3X ~ e0 \ ~ 1. NaH Me0
2. MnOz ~ / N NaOH ~ / ~ 2. R'
Bn0 H METHOD 16 gn0 H Ar METHOD 3 Bn0 / N
R'o
(5~ N O (XX) N (XX1) H
O O N O
maleimide _ Me0 Ar MnOx Me0 ~ P HCU220 ~C
neat, 160 °C \ ~ -' \ ~ ~ ~ HO
METHOD 5 ~ / METHOD 7 I / METHOD 9
Bn0 R o Bn0 ~ ~o HO ~ N
R R~ o
(XXII) (XXIII) (XXIV)
Pd-C/NH,OCHO METHOD 17
or c. HCUHOAG100 ~C METHOD 18
or EtSH/BF3 etherate METHOD 19
H
N O
Me0 \ ~ ~ Ar
HO
Rio
(XXV)
SCHEME 6
Scheme 6 Procedures
EXAMPLE 247
The nrenaration of Methyl-2-azido-3-f4'-(benzvloxv)-3'-methoxvnhenvllprop-2-
enoate (55)
A solution of the 4-Benzyloxy-3-methoxy-benzaldehyde (12.0 g, 49.6 mmol) and
methyl azidoacetate (23 g, 0.200 mol) in methanol (50 mL) was added over 30
min to a
cooled (ice-salt bath) solution of sodium methoxide which had been prepared
from addition
of methanol (80 mL) to sodium (3.4 g, 0.149 mol). The reaction mixture was
stirred at 0° C
for 45 min, during this time a thick cream precipitate formed, and then stood
in the freezer
overnight. Ice-cold water was added and the precipitate was removed by
filtration and dried
to give methyl-2-azido-3-[4'-(benzyloxy)-3'-methoxyphenyl)prop-2-enoate (55)
(12.2 g,
73°~0) which was used in the next step without purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-166-
EXAMPLE 248
The~reparation of methyl-6-(benzyloxy)-5-methoxyindole-2-carboxylate (56)
A solution of the azidocinnamate (55) (12.2 g, 36.0 mmol) prepared as
described in
example 247 in xylene (300 mL) was added dropwise to refluxing xylene (100 mL)
over
1.5 h, the reaction mixture was heated at reflex for a further 15 min and then
most of the
xylene was removed by distillation. The residue, on cooling to room
temperature, formed a
fine cream precipitate of methyl-6-(benzyloxy)-5-methoxyindole-2-carboxylate
which was
collected by filtration. The remaining xylene was removed from the mother
liquor
azeotropically with ethanol, the residue was recrystallized from ethanol to
give further
methyl-6-(benzyloxy)-5-methoxyindole-2-carboxylate. The mother liquor was
again
concentrated and the residue recrystallized. The methyl-6-(benzyloxy)-5-
methoxyindole-2-
carboxylate (56) (9.9 g total, 88%) was used in the next step without
purification. Found: C,
69.38; H, 5.54; N, 4.55. C,$H»N04 requires: C, 69.44; H, 5.50; N, 4.50.
EXAMPLE 249
The preparation of 6-(Benzyloxy)-5-methoxy-1H-indole-2-carbaldehyde (57)
To a solution of the ester (56) (1.0 g, 3.22 mmol) prepared as described in
example
248 in tetrahydrofuran (10 mL) was added a suspension of lithium aluminum
hydride
(0.150 g, 3.86 mmol) in tetrahydrofuran (10 mL) dropwise, the reaction mixture
was stirred at
room temperature for 20 min. Water (1 mL) was added dropwise and then dilute
sodium
hydroxide (1 mL) was added, the solution was stirred for 10 min and then
filtered through
Celite and concentrated. The resulting yellow oil was dissolved in chloroform
(20 mL) and
then manganese dioxide (2.2 g, 26.0 mmol) was added, the reaction mixture was
heated at
50° C for 1 h and then filtered through Celite and concentrated. The
residue was
recrystallized from dichloromethane to give 6-(benzyloxy)-5-methoxy-1H-indole-
2-
carbaldehyde (57) (0.74 g, 82%) as pale yellow needles. Found: C, 72.53; H,
5.42; N, 4.92.
C»H15N03 requires: C, 72.58; H, 5.37; N, 4.98.
Representative Procedure for Method 16 of Scheme 6
EXAMPLE 250
The preparation of 6-(Benzyloxy)-5-methoxy-2-f(E)-2-phenylethenyll-1H-indole
(XX;
Ar=nhenyl) (150)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-167-
To a solution of (57) (0.60 g, 2.14 mmol) prepared as described in example 249
and
benzyltriphenylphosphonium bromide (1.0 g, 2.35 mmol) in dichloromethane (10
mL) was
added a 17 M solution of sodium hydroxide (1.1 mL, 18 mmol) dropwise. The
reaction
mixture was stirred at room temperature until all the starting material was
consumed
(30 min), and then it was diluted with water and extracted with
dichloromethane (2 x 40 mL).
The combined extracts were dried and concentrated to give an approximately 1:1
mixture of
6-(benzyloxy)-5-methoxy-2-[(E)-2-phenylethenyl]-1H-indole and 6-(benzyloxy)-5-
methoxy-
2-[(Z)-2-phenylethenyl]-1H-indole (0.75 g, 99%). Recrystallization from
dichloromethane
cleanly gave the E-isomer (150), mp 140-145°C (softens), 158-162
°C (melts). 'H NMR 8
[(CD3)zS0] 11.08 (s, 1H), 7.52-7.47 (m, 4H), 7.42-7.31 (m, SH), 7.25-7.15 (m,
2H),
7.05-7.01 (m, 2H), 6.91 (s, 1H), 6.45 (s, 1H), 5.12 (s, 2H), 3.78 (s, 3H).
Found: C, 80.84; H,
6.11; N, 3.94. C24HZ~N02 requires: C, 81.10; H, 5.96; N, 3.94.
EXAMPLE 251
The preparation of 6-(Benz~y)-5-methoxy-1-methyl-2-f(E)-2-phenylethenyll-1H-
indole
(XXI; Ar=phenyl, R1°=Me) (151)
Reaction of (150) prepared as described in example 250 with sodium hydride
followed by iodomethane using the procedure described in method 3 gave (151)
(100%), mp
142-145°C. 1H NMR 8 [(CD3)ZSO] 7.51-7.46 (m, 4H), 7.40-7.34 (m, 6H),
7.31-7.23 (m,
2H), 7.09-7.05 (m, 2H), 7.30 (s, 1H), 5.21 (s, 2H), 3.93 (s, 3H), 3.70 (s,
3H). Found: C,
81.01; H, 6.31; N, 3.79. CZSH23N02 requires: C, 81.27; H, 6.27; N, 3.79.
EXAMPLE 252
The preparation of 8-(Benzyloxy)-9-methoxy-6-methyl-4-phenyl-4,5,6,1Oc-
tetrahydropyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (XXII; Ar=phenyl,
Rl°=Me) (152)
Reaction of (151) prepared as described in example 251 with maleimide using
the procedure
described in example 69 gave the adduct (152) as a yellow solid, which was
used without
further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-168-
EXAMPLE 253
The preparation of 8-(Benzyloxy)-9-methoxy-6-methyl-4-Qhenylpyrrolof3 4-
clcarbazole-
1 3(2H,6H)-dione (XXIII; Ar=phenyl, R1°=Me) (153)
Aromatisation of crude (152) prepared as described in example 252 with
manganese
dioxide using the procedure described in example 79 gave (153) as a yellow
solid (69%
overall from (151), mp 263-265°C.'H NMR 8 [(CD3)zS0] 11.04 (br s, 1H),
8.48 (s, 1H),
7.73 (s, 1H), 7.66-7.63 (m, 2H), 7.57-7.55 (m, 2H), 7.49-7.36 (m, 7H), 5.28
(s, 2H), 3.95 (s,
3H), 3.90 (s, 3H). Found: C, 74.99; H, 4.67; N, 5.95. C29HzzNzOa requires: C,
75.31; H, 4.79;
N, 6.06.
Representative Procedure for Method 17 of Scheme 6
EXAMPLE 254
The preparation of 8-Hydroxy-9-methoxy-6-methyl-4-phenylpyrrolof3,4-
clcarbazole-
1.3(2H,6H)-dione (XXV; Ar=phenyl, R'°=Me) (154)
To a solution of (153) (1.0 g, 2.16 mmol) prepared as described in example 253
in a
1:1 mixture of tetrahydrofuran and methanol (100 mL) was added 5% Pd/C (200
mg) and
then ammonium formate (1.71 g, 22.0 mmol). The reaction mixture was stirred at
room
temperature for 2 hr and then filtered through Celite, washing well with
tetrahydrofuran. The
filtrate was concentrated and then water was added; the resulting yellow
precipitate was
collected by filtration and dried to give (154) (0.71 g, 88%), mp 298-
301°C. 1H NMR 8
[(CD3)ZSO] 11.01 (s, 1H), 9.70 (s, 1H), 8.46 (s, 1H), 7.71 (s, 1H), 7.65-7.63
(m, 2H), 7.49-
7.41 (m, 3H), 3.92 (s, 3H), 3.87 (s, 3H). Found: C, 69.85; H, 4.35; N, 7.15.
CzzHi6N20c.1/3 Hz0 requires: C, 69.83; H, 4.44; N, 7.40.
EXAMPLE 255
The preparation of 6-(Benzyloxy)-2-f2-(2-chlorophenyl)ethenyll-5-methoxy-1H-
indole (XX;
Ar=2-chlorophenyl) (155)
Reaction of the aldehyde (57) prepared as described in example 249 with 2'-
chlorobenzyltriphenylphosphonium chloride using the procedure described in the
procedure
described in example 243 gave the dime (155) as an E/Z mixture of isomers (78
%), green
solid, mp 192-194 °C. 'H NMR 8 [(CD3)ZSO] 11.25 (s, 1H), 10.70 (s,
minor isomer), 7.83
(dd, J=8.0, 1.4 Hz, 1H), 7.68-7.20 (m), 7.05 (s; 1H), 6.91 (s, 1H), 6.88 (d,
J=2.9 Hz, minor
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-169-
isomer), 6.64 (d, J=12.1 Hz, minor isomer), 6.51 (d, J=1..4 Hz, 1H), 6.46 (d,
J=12.1 Hz,
minor isomer), 5.89 (br s, minor isomer), 5.13 (s, 2H), 5.06 (s, minor
isomer), 3.78 (s, 3H),
3.71 (s, minor isomer). Found: C, 73.92; H, 5.23; N, 3.63. Cz4H»CINOz requires
C, 74.13; H,
4.92; N, 3.60.
EXAMPLE 256
The preparation of 6-(Benzyloxy)-2-f2-(2-chlorophenyl)ethenyll-5-methoxy-I-
methyl-1H-
indole (XXh Ar=2-chloroQhenyl, R1°=CHI) (156).
Alkylation of (155) prepared as described in example 255 with sodium hydride
and
iodomethane using the procedure described in method 3 gave (156) (100 %)
(mixture of E/Z
isomers) as a yellow solid, mp 143-145 °C.'H NMR 8 [(CD3)zS0] 7.98 (dd,
J=7.9, 1.5 Hz,
1H), 7.55-7.22 (m), 7.19 (s), 7.12 (s), 6.89 (s), 6.85 (d, J=12.2 Hz), 6.80
(s), 6.63 (d, J=12.2
Hz), 5.14 (s), 5.11 (s), 3.81 (s), 3.78 (s), 3.70 (s), 3.64 (s). Found: C,
74.04; H, 5.71; N, 3.49.
CzsHzzCINOz requires C, 74.34; H, 5.49; N, 3.47.
EXAMPLE 257
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-6-methyl-
4,5,6,1Oc-
tetrahydrowrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (XXII; Ar=2-chlorophenyl,
R'°=CHI)
1,57).
Reaction of the dime (156) prepared as described in example 256 with maleimide
using the procedure described in example 69 gave the adduct (157) (98 %) as a
cream
powder, mp 174 °C (dec), which was used without further purification.
Found: C, 68.32; H,
4.94; N, 5.51. Cz9Hz4C1N2O4.1/2H20 requires C, 68.43; H, 4.95; N, 5.50.
EXAMPLE 258
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-6-
methylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (XXIII) (158).
Aromatisation of (157) prepared as described in example 257 with manganese
dioxide
using the procedure described in example 79 gave the carbazole (158) (75 %) as
a yellow
powder, mp 282-285 °C.'H NMR 8 [(CD3)zS0] 11.05 (s, 1H), 8.48 (s, 1H),
7.74 (s, 1H),
'7.59-7.54 (m, 3H), 7.52-7.36 (m, 7H), 5.30 (s, 2H), 3.96 (s, 3H), 3.91 (s,
3H). Found: C,
69.89; H, 4.44; N, 5.61. Cz9Hz,C1Nz04 requires C, 70.09; H, 4.26; N, 5.64.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-170-
EXAMPLE 259
The preparation of 4-(2-Chlorophenyl)-8,9-dihydroxy-6-methylpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (XXIV; Ar=2-chlorophenyl, R'°=CH~~(159).
Reaction of (158) prepared as described in example 258 with pyridinium
hydrochloride at 220 °C using the procedure described in example 81
gave (159) (76 %) as a
yellow powder, mp 310-313 °C.'H NMR 8 [(CD3)ZSO] 10.95 (s, 1H), 9.57
(br, 1H), 9.18 (br,
1H), 8.32 (s, 1H), 7.64 (s, 1H), 7.56 (dd, J=8.1, 2.2 Hz, 1H), 7.50-7.41 (m,
4H), 7.02 (s, 1H),
3.84 (s, 3H). Found: C, 64.11; H, 3.96; N, 6.54. CZ~H~3C1N204.1/4CH30H
requires C, 63.67;
H, 3.52; N, 6.98.
Representative Procedure for Method 18 of Scheme 6
EXAMPLE 260
The preparation of 4-(2-Chlorophenyl)-8-hydroxy-9-methoxy-6-methylpyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (XXV; Ar=2-chlorophenyl, R'°=CHI 160 .
A mixture of (158) (1.30 g, 2.62 mmol) prepared as described in example 258,
cone.
HCl (40 mL) and acetic acid (65 mL) was warmed at 100 °C for 2 h. Water
was added and
the precipitated product was filtered off and dried. The solid was adsorbed
onto silica from a
tetrahydrofuran solution and chromatographed. Elution with ethyl
acetate/petroleum ether
(3:2) gave (160) (1.07 g, 85 %), which crystallised from
terahydrofuran/petroleum ether as a
green solid, mp 280-285 °C (dec).'H NMR 8 [(CD3)ZSO) 11.01 (s, 1H),
9.73 (s, 1H), 8.44 (s,
1H), 7.69 (s, 1H), 7.57 (dd, J=8.0, 2.2 Hz, 1H), 7.51-7.41 (m, 4H), 7.09 (s,
1H), 3.93 (s, 3H),
3.87 (s, 3H). Found: C, 64.53; H, 3.66; N, 6.27. CZZH~5C1N204.1/4Hz0 requires
C, 64.24; H,
3.80; N, 6.81.
EXAMPLE 261
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolo(3,4-clcarbazole-1,3(2H,3aH)-dione (XXII, Ar=2-chlorophenyl,
R'°=H)
161 .
Reaction of the dime (155) prepared as described in example 255 with maleimide
using the procedure described in example 69 gave the adduct (161) as a tan
powder (87 %),
which was used without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-171-
EXAMPLE 262
The preparation of 8-(Benzyloxy)-4-(2-chloro~henyl)-9-methoxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (XXIII; Ar=2-chlorophenyl, Rl°=H) (162).
Aromatisation of (161) prepared as described in example 261with manganese
dioxide
using the procedure described in example 79 gave the carbazole (162) (93 %) as
an orange
powder, mp 260-264 °C. 'H NMR 8 [(CD3)ZSO] 11.86 (s, 1H), 11.01 (s,
1H), 8.43 (s, 1H),
7.58-7.34 (m, lOH), 7.26 (s, 1H), 5.26 (s, 2H), 3.91 (s, 3H). Found: C, 69.65;
H, 3.84; N,
5.61. Cz8H1~C1N20a requires C, 69.64; H, 3.97; N, 5.80.
EXAMPLE 263
The~reQaration of 4-(2-Chlorophenyl)-8-hydroxy-9-methoxypyrrolof3 4-
clcarbazole-
1 3(2H 6H)-dione (XXV; Ar=2-chlorophenyl, Rl°=H) (163).
Debenzylation of (162) prepared as described in example 262 with HCI in acetic
acid
using the procedure described in example 260 have (163) (77 %) as a yellow
powder, mp
194-196 °C. 1H NMR 8 [(CD3)ZSO] 11.71 (s, 1H), 10.97 (br s, 1H), 9.68
(br s, 1H), 8.38 (s,
1H), 7.56 (dd, J=8.0, 2.2 Hz, 1H), 7.49-7.39 (m, 4H), 7.02 (s, 1H), 3.90 (s,
3H). Found: C,
63.38; H, 3.38; N, 6.20. CZIHi3C1N204.1/4 H20 requires C, 63.49; H, 3.42; N,
7.05.
EXAMPLE 264
The preparation of 6-(Benzyloxy)-2-f2-(2-chlorophenyl)ethenyll-5-methox -
methox~thyl)-1H-indole (XXI; Ar=2-chlorophenyl, R'°=CH~CH20CH~ () 164).
Alkylation of dime (155) prepared as described in example 255 with sodium
hydride
and 1-bromo2-methoxyethane using the procedure described in method3 gave (164)
(91 %)
(E/Z mixture of isomers) as an orange solid, which was used without further
purification.
EXAMPLE 265
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-6-(2-
methoxyethyl)-
4 5 6 lOc-tetrahydrop. rrolof3,4-clcarbazole-1,3(2H,3aH)-dione (XXII; Ar=2-
chlorophenyl,
R'°=CH~CH?OCH~) (165)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-172-
Reaction of dime (164) prepared as described in example 264 with maleimide
using the
procedure described in example 69 gave the adduct (165) as a tan solid (74 %),
which was
used without further purification.
EXAMPLE 266
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-6-(2-
methoxyethyl)pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXIII; Ar=2-
chlorophenyl,
R'°=CH~CH~OCH~) (166).
Aromatisation of (165) prepared as described in example 265 with manganese
dioxide
using the procedure described in example 79 gave the carbazole (166) (89 %) as
an orange
powder, mp 252-254 °C.'H NMR 8 [(CD3)ZSO] 11.04 (br s, 1H), 8.49 (s,
1H), 7.75 (s, 1H),
7.59-7.35 (m, lOH), 5.29 (s, 2H), 4.66 (t, J=5.0 Hz, 2H), 3.91 (s, 3H), 3.68
(t, J=5.0 Hz, 2H),
3.14 (s, 3H). Found: C, 68.72; H, 4.73; N, 5.28. C3~HZSC1N205 requires C,
68.82; H, 4.66; N,
5.18.
Representative Procedure for Method 19 of Scheme 6
EXAMPLE 267
Thepreparation of 4-(2-Chloronhenyl)-8-hydroxy-9-methoxy-6-(2-
methoxyethyl)pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXV; Ar=2-chlorophenyls
R'°=CH~,CH~OCH~) (167).
Ethane thiol (S mL) and boron trifluoride etherate (2.5 mL) were added to a
solution
of the benzyl ether (155) (0.840 g, 1.55 mmol) prepared as described in
example 255 in
dichloromethane (100 mL) and the solution was stirred at room temperature for
16 h.
Petroleum ether (500 mL) was added and the mixture was chilled at -20
°C for 3 h. The solid
was isolated by decantation of the liquid layer and partitioned between ethyl
acetate and
saturated aqueous NaHC03. The organic phase was dried, the drying agent was
removed and
the solution was concentrated to dryness gave an oily solid which was
chromatographed on
silica. Elution with ethyl acetate/petroleum ether (1:2) gave foreruns, while
ethyl
acetate/petroleum ether (1:1) eluted (167) (80 5) which crystallised from
ethyl
acetate/petroleum ether as a yellow powder, mp 249-252 °C.'H NMR 8
[(CD3)ZSO] 10.98
(br, 1H), 9.70 (br, 1H), 8.44 (s, 1H), 7.70 (s, 1H), 7.57 (dd, J=8.0, 2.2 Hz,
1H), '7.50-7.41 (m,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-173-
3H), 7.12 (s, 1H), 4.56 (t, J=5.1 Hz, 2H), 3.92 (s, 3H), 3.68 (t, J=5.1 Hz,
2H), 3.16 (s, 3H).
Found: C, 64.19; H, 4.43; N, 5.92. CzaHmC1N205 requires C, 63.93; H, 4.25; N,
6.21.
BBr3/CH2CI2
METHOD 8
(IV) XIX
SCHEME 7
Scheme 7 Procedures
EXAMPLE 268
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-(3-h d~ypropyl)-4,5,6,1Oc-
tetrahydroQyrrolof3,4-clcarbazole-1,3(2H,3aH)-dione (XIX; Ar=2-chlorophenyl,
Rl°=CH-
?CH2CH~OH 139.
Demethylation of 4-(2-Chlorophenyl)-6-(3-hydroxypropyl)-9-methoxy-4,5,6,1Oc-
tetrahydropyrrolo[3,4-c]carbazole-1,3(2H,3aH)-dione (IV; Ar=2-chlorophenyl,
R'°=CH-
2CHZCHZOH) (30) prepared by the first step in the procedure described in
example 39 with
BBr3 using the procedure described in example 80 followed by an acidic workup
gave (139)
(77%) as a pink powder, mp 190-196 °C (dec). 'H NMR S [(CD3)ZSO] 10.82
(br s, 1H), 8.72
(br s, 1H), 7.71 (dd, J=7.7, 1.0 Hz, 1H), 7.46 (dd, J=8.0, 1.4 Hz, 1H), 7.39
(m, 1H), 7.31 (m,
1H), 7.21 (d, J=8.5 Hz, 1H), 7.15 (d, J=2.3 Hz, 1H), 6.62 (dd, J=8.5, 2.3 Hz,
1H), 4.57 (t,
J=4.7 Hz, 1 H), 4.25 (d, J=7.5 Hz, 1 H), 4.11 (t, J=6.9 Hz, 2H), 3.94 (dd,
J=7.5, 3.6 Hz, 1 H),
3.53 (dt, J=12.6, 3.7 Hz, 1H), 3.22 (m, 2H), 3.03 (dd, J=15.6, 3.6 Hz, 1H),
1.77 (m, 2H).
Found: C, 63.57; H, 5.13; N, 6.31. Cz3H2~C1NZOa.1/2Hz0 requires C, 63.66; H,
5.11; N, 6.45.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-174-
Me Me Me
I \ ~ ArCHzPPh3X \ t. NaH
HO LDA I \ io '~ \
METHOD 2 ~ H \ Ar METHOD 3 I ~ N \ Ar
(140) (XXVI) R~o
(XXVII)
O N O O N O O N O
Me Me _ H
Ar DDO/dioxane -
Maleimide _ \ ~ _reflux \ ~ ~ Ar B~ \ ~ / Ar
toluene/SnCh/reflux I METHOD 6 I / METHOD 8 I /
METHOD 4
o Rio yo
(xxvnl) (xxvix) (xxx)
HO ArCHzPPh' \ ~ 1. NaH ~ \
MeO~~~'~ METHOD 2 Me0 I ~ N \ pr 2~ R'oX I /
H METHOD 3 Me0 N 'o Ar
(145) (XXXI)
(XXXII)
O N O N O N O
DDQ/dioxane
Malehide _ \ ~ Ar ref \ ~ / Ar BBr~ \ \ / Ar
toluene/SnCh/reflux I Y METHOD B I Y METHOD 8 I
METHOD 4 Me0 / ~ Me0 / N HO
Rio Rio
(XXXIV) (XXXV)
(XXXIII)
SCHEME 8
Procedures for Scheme 8
EXAMPLE 269
The preuaration of 4-Methoxy-2-f2-phenylethenyll-1H-indole (XXVI; Ar=phenyl)
(141).
Reaction of 4-methoxyindole-2-carboxaldehyde with benzyltriphenylphosphonium
bromide using the procedure described in example 37 gave (141) (89°l0)
as a fluorescent oil,
(mixture of E/Z isomers) which was used without further purification.
EXAMPLE 270
The nre~aration of 10-Methoxy-4-phenyl-4,5,6,1Oc-
tetrahydrocyclopentafclcarbazole-
1 3(2H,3aH)-dione (XXVIII; Ar=phenyl, R'°=H) (142).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-175-
Reaction of the dime (141) prepared by the procedure described in example 269
with
maleimide using the procedure described in method4 gave the adduct (142) as a
yellow solid
(86 %), which was used without further purification.
EXAMPLE 271
The~reparation of 10-Methoxy-4-phenylcyclopenta(clcarbazole-1,3(2H,6H)-dione
(XXVIX;
Ar--phenyl, R'°=H) (143).
Aromatisation of (142) prepared by the procedure described in example 270 with
DDQ using the procedure described in example 70 gave the carbazole (143) (78%)
as yellow
needles, mp 273-276 °C.'H NMR S [(CD3)ZSO] 12.10 (s, 1H), 10.78 (s,
1H), 7.59 (s, 1H),
7.57 (dd, J=8.0, 1.8 Hz, 2H), 7.49 (t, J=7.9 Hz, 1H), 7.47-7.39 (m, 3H), 7.16
(d, J=7.9 Hz,
1H), 6.80 (d, J=7.9 Hz, 1H), 3.99 (s, 3H). Found: C, 73.55; H, 4.16; N, 7.93.
CZ1H~4Nz03
requires C, 73.67; H, 4.12; N, 8.18.
EXAMPLE 272
The preparation of 10-Hydroxy-4-phenylcyclopentafclcarbazole-1,3(2H,6H)-dione
(XXX;
Ar--phenyl, R1°=H) (144)
Demethylation of (143) prepared by the procedure described in example 271 with
BBr3 using the procedure described in example 80 gave (144) as a yellow/orange
solid (92
%), mp >300 °C.'H NMR 8 [(CD3)ZSO] 12.33 (s, 1H), 12.16 (s, 1H), 11.65
(br, 1H), 7.62 (s,
1H), 7.60 (dd, J=7.9, 1.8 Hz, 2H), 7.51-7.45 (m, 3H), 7.40 (dd, J=7.6 Hz, 1H),
7.03 (d, J=7.6
Hz, 1H), 6.63 (d, J=7.6 Hz, 1H). Found: C, 72.87; H, 3.76; N, 8.44.
CZ°H12N203 requires C,
73.16; H, 3.68; N, 8.53.
EXAMPLE 273
The preparation of 6-Methoxy-2-(2-phenYlethenyll-1H-indole (XXXI; Ar=phenyl)
(146).
Reaction of 6-methoxyindole-2-carboxaldehyde (145) with
benzyltriphenylphosphonium bromide using the procedure described in example 37
gave
(146) (94%) as a white solid (mixture of E1Z isomers) which was used without
further
purification.
EXAMPLE 274
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-176-
The preparation of 8-Methoxy-4-Qhenyl-4 5 6 lOc-
tetrahydrocyclopenta(clcarbazole-
1,3(2H,3aH)-dione (XXXIII; Ar=phenyl, R'°=H) (147).
Reaction of the dime (146) prepared by the procedure described in example 273
with
maleimide using the procedure described in method4 gave the adduct (147) as a
tan solid (61
%), which was used without further purification.
EXAMPLE 275
The ~renaration of 8-Methoxv-4-nhenvlcvclopentafclcarbazole-1,3(2H,6H)-dione
(XXXIV;
Ar=phenyl, R'°=H) (148).
Aromatisation of (147) prepared by the procedure described in example 274 with
DDQ using the procedure described in example 70 gave the carbazole (148) (65
%) as a
yellow powder, mp 154-156 °C.'H NMR S [(CD3)ZSO] 11.87 (br, 1H), 11.02
(br, 1H), 8.75
(d, J=8.8 Hz, 1H), 7.61 (dd, J=8.0, 1.8 Hz, 2H), 7.60 (s, 1H), 7.49-7.40 (m,
3H), 7.10 (d,
J=2.2 Hz, 1H), 6.94 (dd, J=8.8, 2.2 Hz, 1H), 3.90 (s, 3H). Found: C, 72.49; H,
4.14; N, 8.22.
CuHiaN203.1/4Hz0 requires C, 72.71; H, 4.21; N, 8.07.
EXAMPLE 276
The preparation of 8-Hydroxy-4-phenylcyclopentafclcarbazole-1,3(2H,6H)-dione
(XXXV;
Ar=phenyl, Rl°=H) (149)
Demethylation of (148) prepared by the procedure described in example 276 with
BBr3 using the procedure described in example 80 gave (149) as a yellow powder
(74 %), mp
>330 °C.'H NMR b [(CD3)ZSO] 11.75 (br, 1H), 10.96 (br, 1H), 10.56 (br,
1H), 8.66 (d, J=8.6
Hz, 1H), 7.60 (dd, J=8.0, 1.8 Hz, 2H), 7.53 (s, 1H), 7.49-7.39 (m, 3H), 6.93
(d, J=2.1 Hz,
1H), 6.79 (dd, J=8.6, 2.1 Hz, 1H). Anal. Found: C, 72.36; H, 3.82; N, 8.18.
CZ°H,ZNz03.1/4H20 requires C, 72.17; H, 3.78; N, 8.41.
EXAMPLE 277
The preparation of 2-f(E,Z)-2-(2-Chlorophenyl)ethenyll-6-methoxy-1H-indole
(XXXI; Ar=2-
chlorophenyl) (841)
Reaction of 6-methoxyindole-2-carboxaldehyde (145) with 2-
chlorobenzyltriphenylphosphonium chloride using the procedure described in
example 37
gave (841) as a mixture of E/Z isomers as a yellow solid (93 %), which was
used without
further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-177-
EXAMPLE 278
The preparation of 2-f(E Z)-2-(2-Chlorophenyl)ethenyll-6-methoxy-1-methyl-1H-
indole
~XXXII; Ar=2-chlorophenyl, R'°=CHI) (842)
Alkylation of (841) with iodomethane using the procedure described in method3
gave
(842) as a yellow solid (77 %), which was used without further purification.
EXAMPLE 279
The preparation of 4-(2-Chlorophenyl)-8-methoxZ-6-methylpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (XXXIV; Ar=2-chlorophenyl, R1°=CHI (844)
Reaction of (842) prepared as described in example 278 with maleimide using
the
procedure described in method4 example 68 gave the adduct (XXXIII; Ar=2-
chlorophenyl,
R'°=CH3) (843) as a tan powder, which was used without further
purification. Aromatisation
of (843) with Mn02 using the procedure described in example 79 gave (844) as a
yellow
powder (78 % overall), mp >300 °C. 1H NMR 8 [(CD3)ZSO] 11.07 (br s,
1H), 8.77 (d, J=8.7
Hz, 1H), 7.76 (s, 1H), 7.59 (dd, J=8.0, 2.2 Hz, 1H), 7.52-7.43 (m, 3H), 7.27
(d, J=2.2 Hz,
1H), 7.00 (dd, J=8.7, 2.2 Hz, 1H), 3.95 (s, 3H), 3.94 (s, 3H). Found: C,
66.86; H, 4.04; N,
6.90; Cl, 9.26. CZZH~SC1N203 Ø2H20 requires: C, 66.99; H, 3.94; N, 7.10; Cl,
8.99 .
EXAMPLE 280
The preparation of 4-(2-Chlorophenyl)-8-hydroxy-6-methylpyrrolo[3,4-
clcarbazole-
1 3(2H 6H)-dione (XXXV; Ar=2-chlorophenyl, R'°=CH3) (845)
Demethylation of (844) prepared as described in example 279 with BBr3 using
the
procedure described in example 80 gave (845) (54 %) as a yellow solid, mp 304-
306 °C. 'H
NMR 8 [(CD3)ZSO] 11.02 (br, 1H), 10.08 (br, 1H), 8.69 (d, J=8.6 Hz, 1H), 7.71
(s, 1H), 7.58
(m, 1H), 7.51-7.42 (m, 3H), 6.99 (d, J=2.0 Hz, 1H), 6.88 (dd, J=8.6, 2.0 Hz,
1H), 3.87 (s,
3H). Found: C, 63.93; H, 3.95; N, 7.08; Cl, 9.12. CZ~H13C1N203 .H20 requires:
C, 63.89; H,
3.83; N, 7.10; Cl, 8.98.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-178-
H
H O N O
0 N 0
HO ~ ~ ~ Ar MesSnNs/0 HO I ~ ~ ~ Ar
--/ ~ N
N (CH2)n H
Rto 11
(V~)- Rto=(CH2)nCN N ~ ~j'
(XXXVI)
H H
N
HS ~.N~ OR HS W ~ N
O N O NJ N O O
EtsN/0
CH30 ~ ~ ~ Ar CH30 ~ ~ ~ Ar
i N ~ N
R'° S ~ Rs
(V); Rt°=(CH2)20SOZCHs
(XXXVII)
35% Hz02 N
Bars O 0 THF/AcOH O O
METHOD 8 r.t. - 50 °C
HO I ~ ~ ~ Ar HO I ~ ~ ~ Ar
N~ ~ N
S ~ Rs SOmRs
(xxxVlll) (xxxix)
SCHEME 9
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-179-
Scheme 9 Procedures
EXAMPLE 281
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-(1H-tetraazol-5-
l~yllpyrrolof3 4-
clcarbazole-1.3(2H,6H)-dione (XXXVI: Ar=2-chlorophenvl, n=2) (272)
To a solution of nitrite (238) (0.68 g, 1.63 mmol) prepared as described in
example 98
in toluene/dimethylformamide (5:1, 120 mL) was added azidotrimethyl tin (0.67
g, 3.26
mmol). The resulting solution was heated at reflux for 24 hours before a
further portion of
azidotrimethyl tin (0.34 g, 1.63 mmol) was added. After a further 24 hours at
reflux the
reaction was diluted with water and extraction with ethyl acetate. The organic
phase was
dried, the drying agent was removed and the solution was concentrated to
dryness.
Chromatography on silica eluting with ethyl acetate/hexane (3:1) to ethyl
acetate/methanol
(9:1), followed by crystallization from ethyl acetate/hexane, gave tetrazole
(272) (0.54 g,
72%) as an orange powder, mp 230-234 °C (dec).'H NMR 8 [(CD3)ZSO] 11.06
(br s, 1H),
9.37 (br s, 1H), 8.36 (d, J=2.4 Hz, 1H), 7.61 (s, 1H), 7.56 (m, 1H), 7.48 (m,
4H), 7.10 (dd,
J=8.8, 2.4 Hz, 1H), 4.82 (t, J=6.9 Hz, 2H), 3.32 (obscured t, J=6.9 Hz, 2H).
Found: C, 52.69;
H, 4.05; N, 15.91. Cz3H~5C1N603.3'/ZHZO requires: C, 52.93; H, 4.25; N, 16.09.
EXAMPLE 282
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f3-(1H-tetraazol-5-
yl)propyllpyrrolof3 4-clcarbazole-1 3(2H 6H)-dione (XXXVI~ Ar=2-chlorophen 1
2( 73)
To a solution of nitrite (241) (60 mg, 0.14 mmol) prepared as described in
example
191 in toluene/dimethylformamide (5:1, 35 mL) was added azidotrimethyl tin (57
mg, 0.28
mmol). The resulting solution was heated at reflux for 72 hours before being
diluted with
water and extraction with ethyl acetate. The organic phase was dried, the
drying agent was
removed and the solution was concentrated to dryness. Chromatography on silica
eluting with
ethyl acetate/hexane (3:1) to ethyl acetate/methanol (9:1), followed by
crystallization from
tetrahydrofuran/hexane, gave tetrazole (273) (29 mg, 44%) as an orange powder,
mp 269-272
°C.'H NMR 8 [(CD3)ZSO] ~16 (v br s, 1H), 11.07 (s, 1H), 9.38 (br s,
1H), 8.39 (d, J=2.5 Hz,
iH), 7.84 (s, 1H), 7.61 (d, J=8.9 Hz, 1H), 7.57 (m, 1H), 7.53-7.43 (m, 3H),
7.16 (dd, J=8.9,
2.5 Hz, 1H), 4.59 (t, J=7.1 Hz, 2H), 2.93 (m, 2H), 2.20 (m, 2H). Found: C,
59.71; H, 4.27; N,
16.72. C24H,~C1N603.3/4H~0 requires: C, 59.26; H, 3.83; N, 17.27.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-180-
EXAMPLE 283
The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-(2-(1H-1,2,4-triazol-5-
ylsulfanyl)ethyllpyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (XXXVII; Ar=2-
chlorophenyl,
R3=5-1H-1,2,4-triazolyl) (274)
A solution of mesylate (228) (0.25 g, 0.50 mmol) prepared as described in
example
170 , 1H-1,2,4-triazole-5-thiol (76 mg, 0.75 mmol) and triethylamine (2.0 mL)
in p-dioxane
(50 mL) under nitrogen was heated at reflux for 48 hours, before being diluted
with water and
extraction with ethyl acetate. The organic phase was dried, the drying agent
was removed and
the solution was concentrated to dryness. Chromatography on silica eluting
with ethyl
acetate/hexane (l:l to 1:0), followed by crystallization from ethyl
acetate/hexane, gave
carbazole (274) (0.24 g, 95%) as a yellow powder, mp 211-213 °C.'H NMR
b [(CD3)zS0]
14.09 (br s, 1H), 11.13 (br s, 1H), 8.52 (d, J=2.6 Hz, 1H), 8.43 (v br s, 1H),
7.88 (s, 1H), 7.80
(d, J=9.0 Hz, 1H), 7.57 (m, 1H), 7.48 (m, 3H), 7.31 (dd, J=9.0, 2.6 Hz, 1H),
4.83 (t, J=7.4 Hz,
2H), 3.91 (s, 3H), 3.52 (t, J=7.4 Hz, 2H). Found: C, 59.29; H, 3.77; N, 13.63.
C25H~8CIN503S.1/4H20 requires: C, 59.29; H, 3.68; N, 13.82.
EXAMPLE 284
The preparation of 4-(2-Chlorophenyl)-6-(2-(1H-imidazol-2-ylsulfanyl)ethyll-9-
methoxypyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (XXXVII; Ar=2-chlorophenyl,
R3=2-
imidazolyl) (275)
A solution of mesylate (228) (0.30 g, 0.60 mmol) prepared as described in
example
170 , 2-mercaptoimidazole (0.12 g, 1.20 mmol) and triethylamine (2.0 mL) in p-
dioxane (25
mL) under nitrogen was heated at reflux for 24 hours, before being diluted
with water to
precipitate the crude product, which was collected by filtration, washed with
water and dried
in vacuo. Chromatography on silica eluting with ethyl acetate/hexane (1:1),
followed by
trituration from ethyl acetate, gave carbazole (275) (0.29 g, 96%) as a yellow
powder, mp
245-247 °C.'H NMR 8 [(CD3)ZSO] 12.24 (br s, 1H), 11.13 (br s, 1H), 8.51
(d, J=2.6 Hz,
1H), 7.92 (s, 1H), 7.75 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.48 (m, 3H), 7.29
(dd, J=9.0, 2.6
Hz, 1H), 7.00 (v br s, 2H), 4.78 (t, J=7.5 Hz, 2H), 3.91 (s, 3H), 3.44 (t,
J=7.5 Hz, 2H). Found:
C, 61.57; H, 4.14; N, 10.85. CZ6Hi9C1N4O3S.1/4H20 requires: C, 61.53; H, 3.87;
N, 11.03.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-181-
EXAMPLE 285
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-(4H-1,2,4-triazol-3-
ylsulfanyl)ethyllRyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXXVIII; Ar=2-
chlor~he~rl,
R3=5-1H-1,2,4-triazolyl) (276)
Reaction of methyl ether (274) (0.25 g, 0.50 mmol) prepared as described in
example
283 according to the procedure described in example 80, followed by
chromatography on
silica eluting with ethyl acetate/tetrahydrofuran (1:0 to 9:1 to 1:1) and
trituration from
methanol, gave phenol (276) (0.22 g, 90%) as an orange powder, mp 314-319
°C. 1H NMR 8
[(CD3)ZSO] 14.10 (br s, 1H), 11.07 (br s, 1H), 9.39 (s, 1H), 8.42 (v br s,
1H), 8.38 (d, J=2.3
Hz, 1H), 7.83 (s, 1H), 7.68 (d, J=8.8 Hz, 1H), 7.57 (m, 1H), 7.48 (m, 3H),
7.13 (dd, J=8.8,
2.3 Hz, 1H), 4.79 (t, J=7.4 Hz, 2H), 3.50 (t, J=7.4 Hz, 2H). Found: C, 58.56;
H, 3.82; N,
13.58. C24H~6C1N503S.2/SCH40 requires: C, 58.29; H, 3.53; N, 13.93.
EXAMPLE 286
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-(1H-imidazol-2-
ylsulfanyl)ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXXVIII; Ar=2-
chlorophenyl,
R3=2-imidazolyl) (277)
Reaction of methyl ether (275) (130 mg, 0.24 mmol) prepared as described in
example 284 according to The procedure described in example 80, except that
the reaction
time was 7 hours and the chromatography was performed eluting with ethyl
acetate/hexane
(1:1 to 3:1), gave phenol (277) (97 mg, 83°10) as an orange powder, mp
270-275 °C.'H NMR
S [(CD3)ZSO] 12.23 (br s, 1H), 11.07 (br s, 1H), 9.39 (br s, 1H), 8.37 (d,
J=2.4 Hz, 1H), 7.86
(s, 1H), 7.61 (d, J=8.9 Hz, 1H), 7.57 (m, 1H), 7.48 (m, 3H), 7.12 (dd, J=8.9,
2.4 Hz, 1H),
6.98 (v br s, 2H), 4.74 (t, J=7.7 Hz, 2H), 3.42 (partially obscured t, J=7.7
Hz, 2H). Found: C,
60.87; H, 3.72; N, 11.00. CZSH»C1Na03S.1/4H20 requires: C, 60.85; H, 3.57; N,
11.35.
EXAMPLE 287
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-(1H-1,2,4-triazol-5-
ylsulfinyl)eth ~Lllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXXIX; Ar=2-
chlorophenyl,
m=1, R3=5-1H-1,2,4-triazolyl) (278)
To a solution of carbazole (276) (70 mg, 0.14 mmol) prepared as described in
example 285 in tetrahydrofuran (30 mL) containing glacial acetic acid (8 mL),
was added
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-182-
35% hydrogen peroxide (2 mL). The resulting solution was stirred at room
temperature for 10
hours before being poured onto solid sodium bicarbonate, diluted with water
and extraction
with ethyl acetate. The organic phase was dried, the drying agent was removed
and the
solution was concentrated to dryness. The combined organic layers were
partially
concentrated under reduced pressure, then adsorbed directly onto silica and
chromatographed
eluting with methanol/dichloromethane (5:95 to 1:9). Trituration from diethyl
ether gave
sulfoxide (278) (29 mg, 41 %) as an orange powder, mp 237-242 °C. 1H
NMR 8 [(CD3)ZSO]
14.7 (v br s, 1 H), 11.09 (br s, 1 H), 9.41 (br s, 1 H), 8.69 (br s, 1 H),
8.38 (d, J=2.3 Hz, 1 H),
7.79 (d, J=9.6 Hz, 1H), 7.60-7.46 (m, 5H), 7.15 (dd, J=8.8, 2.3 Hz, 1H), 4.89
(m, 2H), 3.71
(m, 2H). Found: C, 56.85; H, 3.79; N, 13.74. C24H1~CINSO4S.1/SCqH~pO requires:
C, 57.20;
H> 3.48; N, 13.45.
EXAMPLE 288
Th~reparation of 4-(2-Chlorophenyl)-9-h dery-6-(2-(1H-1,2,4-triazol-5-
ylsulfonyl)ethyllpyrrolo~3,4-clcarbazole-1,3(2H,6H)-dione (XXXIX; Ar=2-
chlorophenyl,
m=2, R3=5-1H-1,2,4-triazol I 279
To a solution of carbazole (276) (60 mg, 0.12 mmol) prepared as described in
example 285 in tetrahydrofuran (30 mL) containing glacial acetic acid (10 mL),
was added
35% hydrogen peroxide (2 mL). The resulting solution was stirred at room
temperature for 30
hours before additional 35% hydrogen peroxide (2 mL) was added and the
temperature was
increased to 50 °C for 18 hours. The reaction was then poured onto
solid sodium bicarbonate,
diluted with water and extracted with ethyl acetate. The combined organic
layers were
partially concentrated under reduced pressure, then adsorbed directly onto
silica and
chromatographed eluting with ethyl acetate/hexane (l:l to 1:0).
Crystallisation from ethyl
acetate/hexane gave sulfone (279) (45 mg, 72%) as an orange powder, mp 301-304
°C. IH
NMR 8 [(CD3)ZSO) 14.9 (v br s, 1H), 11.07 (br s, 1H), 9.38 (br s, 1H), 8.60
(s, 1H), 8.34 (d,
J=2.4 Hz, 1H), 7.71 (s, 1H), 7.56 (m, 2H), 7.48 (m, 3H), 7.12 (dd, J=8.8, 2.4
Hz, 1H), 4.89
(m, 2H), 4.03 (m, 2H). Found: C, 55.12; H, 3.52; N, 12.87.
C24H~6CINSOSS.lI4C4HgO2
requires: C, 55.20; H, 3.34; N, 12.88.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-183-
EXAMPLE 289
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-(1H-imidazol-2-
ls~yl)ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXXIX; Ar=2-chlorophen
~Ll,
m=2, R3=2-imidazolyl) (280) and 4-(2-chlorophenyl)-9-hydroxy-6-f2-(1H-imidazol-
2-
ls~yl)ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XXXIX; Ar=2-chlorophen~
m=1, R3=2-imidazolyl) (281)
To a solution of carbazole (277) (66 mg, 0.14 mmol) prepared as described in
example 286 in tetrahydrofuran (30 mL) containing glacial acetic acid (10 mL),
was added
35% hydrogen peroxide (2 mL). The resulting solution was stirred at room
temperature for 20
hours before being poured onto solid sodium bicarbonate, diluted with water
and extracted
with ethyl acetate. The combined organic layers were partially concentrated
under reduced
pressure, then adsorbed directly onto silica and chromatographed eluting with
ethyl
acetate/hexane (1:1 to 4:1) to ethyl acetate/methanol (99:1) to give at
highest Rf, sulfone
(280) (36 mg, 51 %) as an orange powder, mp 263-265 °C. 'H NMR 8
[(CD3)ZSO] 11.09 (br s,
1H),.9.44 (br s, 1H), 8.35 (d, J=2.4 Hz, 1H), 7.63 (s, 1H), 7.58 (m, 1H), 7.52-
7.45 (m, 4H),
7.19 (br s, 2H), 7.12 (dd, J=8.8, 2.4 Hz, 1H), 4.85 (t, J=6.6 Hz, 2H), 3.96
(m, 2H). Found: C,
57.44; H, 3.87; N, 9.80. CZSH»C1N4OSS.1/2C4H802 requires: C, 57.40; H, 3.75;
N, 9.92. This
was followed at lower Rfby the sulfoxide (281) (16 mg, 23%) as an orange
powder, mp 254-
259 °C.'H NMR 8 [(CD3)ZSO] 13.35 (br s, 1H), 11.10 (br s, 1H), 9.42 (s,
1H), 8.39 (d, J=2.4
Hz, 1H), 7.77 (d, J=12.7 Hz, 1H), 7.58 (m, 2H), 7.53-7.44 (m, 3H), 7.24 (br s,
2H), 7.15 (dd,
J=8.8, 2.4 Hz, 1H), 4.85 (m, 2H), 3.69 (m, 2H). FABMS found [M+H]+: 505.0725,
507.0666.
CZSH1~C1N404S requires 505.0737, 507.0708.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-184-
MeOH/H20 Me0 Ar
Me0 ~ ~ ~ Ar epi-bromohydrin Me0 ~ ~ ~ Ar HCIO / D
NaH a
N METHOD 3 ~ N 0~ ~ N
ZH/60 °C HO
O
QI)
Z
(XL)
(XLI)
H H
N O p N O
O
1) Maleimide/SnCh/A - BBrs -
METHOD 4 Me0 ~ ~ ~ Ar METHOD 8 HO ~ ~ ~ ~ Ar
i
N or N
2) DDQ/A ~ Py.HCU200-220 °C
METHOD 6 HO METHOD 9 HO
Z Z
(XLII) (XLIII)
SCHEME 10
Scheme 10 Procedures
EXAMPLE 290
The preparation of 6-(2-Hydroxy-3-methoxypropyl)-9-methoxy-4-phenylnyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (XLII; Ar=phenyl, Z=OCH~) (225)
Alkylation of 5-methoxy-2-[(E)-2-phenylethenyl]-1H-indole (II; Ar=phenyl)
(0.20 g,
0.80 mmol) with epibromohydrin according to the procedure described in example
38
example 38 gave crude epoxide (XL; Ar=phenyl), which was used without further
purification. The epoxide was dissolved in methanol (100 mL) to which 35%
perchloric acid
(10 mL) was added. The resulting solution was heated at reflux for 3 h before
being diluted
with water and extraction with ethyl acetate. The organic phase was dried, the
drying agent
was removed and the solution was concentrated to dryness to give dime (XLI;
Ar=phenyl,
Z=OCH3). This crude material was reacted directly with maleimide (92 mg)
following
Method 4a, except that the reaction time was 18 h. Aromatisation of the crude
Diels-Alder
adduct using the procedure described in example 79 gave material that was
chromatographed
on silica eluting with ethyl acetate/hexane (1:1). Crystallisation from ethyl
acetate/hexane
gave carbazole (225) (0.10 g, 30%) as a yellow powder, mp 128-136 °C.
IH NMR 8
[(CD3)ZSO] 11.10 (br s, 1H), 8.55 (d, J=2.6 Hz, 1H), 7.81 (s, 1H), 7.64 (m,
3H), 7.47 (m,
3H), 7.29 (dd, J=9.1, 2.6 Hz, 1H), 5.17 (d, J=5.5 Hz, 1H), 4.52 (dd, J=4.2,
15.0 Hz, 1H), 4.43
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-185-
(dd, J=7.1, 15.0 Hz, 1H), 4.04 (m, 1H), 3.90 (s, 3H), 3.32 (m, 2H), 3.26 (s,
3H). Found: C,
69.94; H, 5.45; N, 6.36. CZSH22NzOs requires: C, 69.76; H, 5.15; N, 6.51.
EXAMPLE 291
The preparation of 6-(2,3-Dihydroxypropyl)-9-hydroxy-4-phenylpyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (XLIII; Ar=phenyl, Z=OH) (226)
Demethylation of alcohol (225) (96 mg, 0.22 mmol) prepared as described in
example
290 employing the procedure described in example 80 was followed by
chromatography on
silica eluting with ethyl acetate/hexane (4:1). Crystallisation from ethyl
acetate/hexane gave
phenol (226) (28 mg, 31 %) as an orange/yellow powder, mp 290-295 °C.
'H NMR 8
[(CD3)ZSO] 11.04 (br s, 1H), 9.30 (s, 1H), 8.40 (d, J=2.4 Hz, 1H), 7.79 (s,
1H), 7.63 (m, 2H),
7.55 (d, J=8.8 Hz, 1H), 7.47 (m, 3H), 7.11 (dd, J=8.8, 2.4 Hz, 1H), 5.00 (d,
J=5.4 Hz, 1H),
4.86 (t, J=5.4 Hz, 1H), 4.51 (dd, J=3.8, 14.9 Hz, 1H), 4.35 (dd, J=7.5, 14.9
Hz, 1H), 3.89 (m,
1H), 3.41 (m, 2H). Found: C, 67.94; H, 4.69; N, 6.50. C23H~gN2O5.1/4H20
requires: C, 67.89;
H,4.58;N,6.88.
EXAMPLE 292
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-hydroxy-3-(4-
morpholinyl)pronyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XLIII; Ar=2-
chlorophenyl,
Z=4-morpholinvl) (282)
Alkylation of pure trans-dime (27) (0.25 g, 0.88 mmol) prepared as described
in
example 37 with epibromohydrin according to the procedure described in example
38 gave
crude epoxide (XL; Ar=2-chlorophenyl), which was used without further
purification. The
epoxide was dissolved in tetrahydrofuran (2 mL) to which moipholine (0.5 mL)
was added.
The resulting solution was heated at 60 °C for 48 hours before being
diluted with water and
extraction with ethyl acetate. The organic phase was dried, the drying agent
was removed and
the solution was concentrated to dryness to give dime (XLI; Ar=2-chlorophenyl,
Z=4-
morpholinyl). This crude material was triturated in diethyl ether/hexane and
then reacted
directly with maleimide (128 mg) following The procedure described in example
68, except
that the reaction time was 18 h and p-dioxane was added as a co-solvent.
Aromatisation of
the crude Diels-Alder adduct using the procedure described in example 70 gave
carbazole
(XLII; Ar=2-chlorophenyl, Z=4-morpholinyl) which was demethylated employing
the
procedure described in example 81, to give crude material that was
chromatographed on
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-186-
silica eluting with ethyl acetate. Crystallisation from diethyl ether/hexane
gave carbazole
(282) (0.10 g, 22%) as a yellow powder, mp 275-278 °C.'H NMR 8
[(CD3)zS0] 11.05 (br s,
1H), 9.34 (br s, 1H), 8.37 (d, J=2.4 Hz, 1H), 7.79 (s, 1H), 7.63-7.56 (m, 2H),
7.47 (m, 3H),
7.12 (m, 1H), 5.01 (t, J=5.7 Hz, 1H), 4.53 (m, 1H), 4.37 (m, 1H), 4.04 (br s,
1H), 3.38 (m,
4H), 2.39-2.24 (m, 6H). Found: C, 63.90; H, 4.85; N, 8.55. Cz~HzaC1N305
requires: C, 64.10;
H, 4.78; N, 8.31.
EXAMPLE 293
The preparation of 4-(2-Chlorophenyl)-6-f3-(cis-3,5-dimethyl-1-piperazinyl)-2-
hydroxypropyll-9-hydroxypyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (XLIII; Ar=2-
chloronhenyl, Z= cis-3,5-dimethyl-1-piperazinyl) (283)
Alkylation of pure trans-dime (27) (0.25 g, 0.88 mmol) prepared as described
in
example 37 with epibromohydrin according to the procedure described in example
38 gave
crude epoxide (XL; Ar=2-chlorophenyl), which was used without further
purification. The
epoxide was dissolved in tetrahydrofuran (2 mL) to which cis-2,6-
dimethylpiperazine (0.50 g,
4.4 mmol) was added. The resulting solution was heated at 60 °C for 48
hours before being
diluted with water and extraction with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solution was concentrated to dryness to give dime
(XLI; Ar=2-
chlorophenyl, Z=cis-3,5-dimethyl-1-piperazinyl). This crude material was
triturated in diethyl
ether/hexane and then reacted directly with maleimide (0.13 g, 1.5 mmol)
following The
procedure described in example 68, except that the reaction time was 18 h and
p-dioxane was
added as a co-solvent. Aromatisation of the crude Diels-Alder adduct using the
procedure
described in example 70 gave carbazole (XLII; Ar=2-chlorophenyl, Z=cis-3,5-
dimethyl-1-
piperazinyl) which was demethylated employing The procedure described in
example 81 to
give crude material that was chromatographed on silica eluting with ethyl
acetate/methanol/triethylamine (4:1 Grace). Crystallisation from ethyl
acetate/hexane gave
carbazole (283) (65 mg, 14%) as a yellow powder, mp 206-210 °C. 1H NMR
b [(CD3)zS0]
11.02 (br s, 1H), 9.33 (br s, 1H), 8.37 (d, J=2.5 Hz, 1H), 7.76 (d, J=9.1,
1H), 7.58 (m, 2H),
7.47 (m, 3H), 7.11 (m, 1H), 4.95 (br s, 1H), 4.52 (m, 1H), 4.34 (m, 1H), 4.04
(m, 1H), 2.71-
2.53 (m, 4H), 2.33 (m, 1H), 2.20 (m, 1H), 1.46 (m, 2H), 0.81 (m, 6H). Found:
C, 63.76; H,
5.30; N, 10.02. Cz9Hz9C1N404.3/4H20 requires: C, 63.73; H, 5.63; N, 10.25.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-187-
EXAMPLE 294
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-f2-hydroxy-3-
(methylaminoZpropyllp_Yrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XLIII; Ar=2-
chlorophenyl,
Z=NHCH~) (284)
Alkylation of pure trans-dime (27) (0.25 g, 0.88 mmol) prepared as described
in
example 37 with epibromohydrin according to proceedure described in example 38
gave
crude epoxide (XL; Ar=2-chlorophenyl), which was used without further
purification. The
epoxide was dissolved in tetrahydrofuran (2 mL) to which aqueous methylamine
(0.5 mL,
40% solution) was added. The resulting solution was heated at 60 °C for
6 hours before being
diluted with water and extraction with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solution was concentrated to dryness to give dime
(XLI; Ar=2-
chlorophenyl, Z=NHCH3). This crude material was triturated in diethyl
ether/hexane and then
reacted directly with maleimide (0.13 g, 1.5 mmol) following The procedure
described in
example 68, except that the reaction time was 18 h and p-dioxane was added as
a co-solvent.
Aromatisation of the crude Diels-Alder adduct using the procedure described in
example 70
gave carbazole (XLII; Ar=2-chlorophenyl, Z=NHCH3) which was demethylated
employing
The procedure described in example 81 to give crude material that was
chromatographed on
silica eluting with ethyl acetate/methanol/triethylamine (4:1 Grace).
Crystallisation from ethyl
acetate/hexane gave carbazole (284) (61 mg, 15%) as a yellow powder, mp 188-
191 °C. 1H
NMR 8 [(CD3)ZSO] 10.9 (v br s, 1H), 9.36 (br s, 1H), 8.37 (d, J=2.4 Hz, 1H),
7.77 (s, 1H),
7.58 (m, 2H), 7.47 (m, 3H), 7.13 (dd, J=8.8, 2.4 Hz, 1H), 5.09 (br s, 1H),
4.50 (m, 1H), 4.34
(m, 1H), 3.97 (m, 1H), 2.77-2.63 (m, 2H), 2.28 (d, J=2.lHz, 3H). Found: C,
62.66; H, 4.64;
N, 8.69. C24H2oC1N304.2/3H20 requires: C, 62.40; H, 4.66; N, 9.09.
EXAMPLE 295
The preparation of 4-(2-Chlorophenyl)-6-f3-(dimethylamino)-2-hydroxypropyll-9-
hydroxypyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (XLIII; Ar=2-chlorophenyl,
Z=N(CH~,)~)
285
Alkylation of pure trans-dime (27) (0.25 g, 0.88 mmol) prepared as described
in
example 37 with epibromohydrin according to proceedure described in example 38
gave
crude epoxide (XL; Ar=2-chlorophenyl), which was used without further
purification. The
epoxide was dissolved in tetrahydrofuran (2 mL) to which aqueous dimethylamine
(0.5 mL,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-188-
40% solution) was added. The resulting solution was heated at 60 °C for
6 hours before being
diluted with water and extraction with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solution was concentrated to dryness to give dime
(XLI; Ar=2-
chlorophenyl, Z=N(CH3)2). This crude material was triturated in diethyl
ether/hexane and
then reacted directly with maleimide (0.13 g, 1.5 mmol) following the
procedure described in
example 68; except that the reaction time was 18 h and p-dioxane was added as
a co-solvent.
Aromatisation of the crude Diels-Alder adduct using the procedure described in
example 70
gave carbazole (XLII; Ar=2-chlorophenyl, Z=N(CH3)2) which was demethylated
employing
the procedure described in example 81 to give crude material that was
chromatographed on
silica eluting with ethyl acetate/methanol/triethylamine (4:1 Grace).
Crystallisation from ethyl
acetate/hexane gave carbazole (285) (165 mg, 40%) as a yellow powder, mp 225-
228 °C.'H
NMR 8 [(CD3)ZSO) 11.04 (br s, 1H), 9.34 (br s, 1H), 8.38 (d, J=2.4 Hz, 1H),
7.75 (s, 1H),
7.57 (m, 2H), 7.47 (m, 3H), 7.13 (dd, J=8.8, 2.4 Hz, 1H), 4.91 (br s, 1H),
4.50 (m, 1H), 4.34
(m, 1H), 4.00 (m, 1H), 2.35 (m, 1H), 2.24 (m, 1H), 2.17 (s, 6H). Found: C,
63.54; H, 4.60; N,
8.83. CZSHz2C1N3O4.1/2H20 requires: C, 63.49; H, 4.90; N, 8.88.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-189-
0 0 0
Malefic 1) 2,4-dimethoxy
Anhydride
Me0 I ~ ~ ~ Ar Q Me0 I ~ ~ Ar benzylamine/A
NH 2) Mn02/~
METHOD 7
(II) (XLIV)
(XLV)
~(CHZ)nBr 0504
KpCO3l90 °C NMMNO
METHOD 20 AETHOD 21
.\
(CHp)n
HO~
(XLVI) H ~O
H (XLVII)
O N O
1 ) TFA/anisole/90 °C _
2) BBr3 (METHOD 8) HO I ~ ~ ~ Ar
METHOD 22 ~ N
(CHp)n
HO~
HO
(XLVIII)
MeO~OMe
I H
N O
O
O N
O 1) R~oX/K2C05/90 °C HO ~ ~ Ar
Me0 I ~ ~ ~ Ar METHOD 20 I ~ N ~o
r N~ ~R
'H 2) TFA/anisole/90 °C (VI)
(XLV) 3) Bars (METHOD 8)
METHOD 22
SCHEME 11
Scheme 11 Procedures
EXAMPLE 296
The preparation of 4-(2-Chlorophenyl)-9-methoxy-4,5,6,1Oc-tetrahydro-1H-
furof3,4-
clcarbazole-1,3(3aH)-dione (XLIV; Ar=2-chloronhenyl) (286)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-190-
A solution of trans-dime (27) (0.30 g, 1.06 mmol) prepared as described in
example
37 and malefic anhydride (0.16 g, 1.59 mmol) in xylene (30 mL) were heated at
reflux for 18
hours, before being concentrated in vacuo and chromatographed on silica
eluting with ethyl
acetate/hexane (1:2). Crystallisation from ethyl acetate/hexane then gave
anhydride (286)
(0.29 g, 72%) as ~a pale brown powder, mp 189-191 °C. 'H NMR S
[(CD3)ZSO] 11.16 (br s,
1H), 7.69 (dd, J=7.7, 1.4 Hz, 1H), 7.51 (dd, J=7.7, 1.4 Hz, 1H), 7.43 (ddd,
J=7.7, 7.7, 1.4 Hz,
1H), 7.36 (ddd, J=7.7, 7.7, 1.4 Hz, 1H), 7.25 (d, J=8.6 Hz, 1H), 7.15 (d,
J=2.4 Hz, 1H), 6.77
(dd, J=8.6, 2.4 Hz, 1H), 4.70 (d, J=7.7 Hz, 1H), 4.47 (dd, J=7.7, 3.5 Hz, 1H),
3.78 (s, 3H),
3.71-3.66 (m, 1H), 3.36 (dd, J=15.9, 13.0 Hz, 1H), 2.99 (dd, J=15.9, 4.1 Hz,
1H). Found: C,
65.85; H, 3.95; N, 3.70. Cz~H,6C1N04 requires: C, 66.06; H, 4.22; N, 3.67.
EXAMPLE 297
The preparation of 4-(2-Chlorophenyl)-2-(2,4-dimethoxybenzyl)-9-
methoxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (XLV; Ar=2-chlorophenyl) (287)
To a solution of anhydride (286) (2.80 g, 7.42 mmol) prepared as described in
example 296 in glacial acetic acid (70 mL) was added 2,4-dimethoxybenzylamine
(1.67 mL,
11.1 mmol). The resulting solution was heated at reflux for 6 hours before
being partially
concentrated under reduced pressure and diluted with water to precipitate an
orange solid,
which was collected by filtration, washed with water and dried in vacuo. This
crude material
was then dissolved in p-dioxane and aromatized according to the procedure for
example 79,
before being chromatographed on silica eluting with ethyl acetate/hexane
(1:1).
Crystallisation from methanol then gave carbazole (287) (1.46 g, 37%) as a
yellow powder,
mp 224-226 °C.'H NMR 8 [(CD3)ZSO] 12.02 (br s, 1H), 8.45 (d, J=2.6 Hz,
1H), 7.62 (s, 1H),
7.58 (m, 2H), 7.53-7.42 (m, 3H), 7.25 (dd, J=8.8, 2.6 Hz, 1H), 6.95 (d, J=8.4
Hz, 1H), 6.57
(d, J=2.3 Hz, 1H), 6.45 (dd, J=8.4, 2.3 Hz, 1H), 4.68 (s, 2H), 3.88 (s, 3H),
3.80 (s, 3H), 3.72
(s, 3H). Found: C, 68.2.2; H, 4.37; N, 5.29. C3oH23C1N205 requires: C, 68.38;
H, 4.40; N,
5.32.
Representative Procedure for Method 20 of Scheme 11
EXAMPLE 298
The preparation of 6-Allvl-4-(2-chlorophenyl)-2-(2,4-dimethoxybenzyl)-9-
methoxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XLVI; Ar=2-chlorophenyl, n=1)
(288)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-191-
To a solution of carbazole (287) (150 mg, 0.28 mmol) prepared as described in
example 297 in dimethylformamide (10 mL) under nitrogen was added potassium
carbonate
(0.39 g, 2.80 mmol) and allyl bromide (72 ~L, 0.84 mmol). The resulting
suspension was
warmed to 90 °C with stirring for 3 hours before being diluted with
water and extraction with
ethyl acetate. The organic phase was dried, the drying agent was removed and
the solution
was concentrated to dryness. Chromatography on silica eluting with ethyl
acetate/hexane
(1:2), followed by crystallization from diethyl ether/hexane gave carbazole
(288) (90 mg,
57%) as a yellow powder, mp 171-173 °C.'H NMR S [(CD3)ZSO] 8.51 (d,
J=2.6 Hz, 1H),
7.84 (s, 1H), 7.67 (d, J=8.9 Hz, 1H), 7.58 (m, 1H), 7.53-7.46 (m, 3H), 7.32
(dd, J=8.9, 2.6
Hz, 1H), 6.97 (d, J=8.4 Hz, 1H), 6.57 (d, J=2.4 Hz, 1H), 6.45 (dd, J=8.4, 2.4
Hz, 1H), 5.99
(m, 1H), 5.18 (d, J=4.9 Hz, 2H), 5.14 (dd, J=10.3, 1.3 Hz, 1H), 4.99 (dd,
J=17.2, 1.3 Hz, 1H),
4.69 (s, 2H), 3.90 (s, 3H), 3.80 (s, 3H), 3.72 (s, 3H). Found: C, 69.97; H,
4.75; N, 5.12.
C33HZ~C1Nz05 requires: C, 69.90; H, 4.80; N, 4.94.
EXAMPLE 299
The preparation of 6-(3-Butenyl)-4-(2-chlorophenyl)-2-(2,4-dimethoxybenz l
methoxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XLVI; Ar=2-chlorophenyl, n=2)
(289)
Reaction of carbazole (287) (260 mg, 0.51 mmol) prepared as described in
example
297 with 4-bromo-1-butene (155 OL, 1.53 mmol) according to procedure described
in
example 298 gave carbazole (289) (173 mg, 58%) as a yellow powder, mp 161-164
°C.'H
NMR 8 [(CD3)ZSO~ 8.50 (d, J=2.6 Hz, 1H), 7.89 (s, 1H), 7.73 (d, J=9.0 Hz, 1H),
7.60-7.46
(m, 4H), 7.31 (dd, J=9.0, 2.6 Hz, 1H), 6.96 (d, J=8.5 Hz, 1H), 6.57 (d, J=2.3
Hz, 1H), 6.44
(dd, J=8.4, 2.3 Hz, 1H), 5.84 (m, 1H), 4.96-4.86 (m, 2H), 4.68 (s, 2H), 4.60
(t, J=6.9 Hz, 2H),
3.89 (s, 3H), 3.80 (s, 3H), 3.72 (s, 3H), 2.54 (partially obscured m, 2H).
Found: C, 70.23; H,
5.15; N, 4.91. C34H29C1N205 requires: C, 70.28; H, 5.03; N, 4.82.
Representative Procedure for Method 21 of Scheme 11
EXAMPLE 300
The preparation of 4-(2-Chlorophenyl)-6-(2,3-dihydroxypropyl)-2-(2,4-
dimethoxybenzyl)-9-
methoxypymolof3,4-clcarbazole-1,3(2H,6H)-dione (XLVII; Ar=2-chlorophenyl, n=1)
(290)
To a solution of carbazole (288) (80 mg, 0.14 mmol) procedure described in
example
2 98 in acetone/water (4: l, 20 mL) was added N-methylmorpholine N-oxide (33
mg, 0.28
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-192-
mmol) and osmium tetroxide (176 uL, 4% solution in water, 0.028 mmol). The
reaction
mixture was stirred at room temperature for 18 hours before being diluted with
1N
hydrochloric acid and extraction with ethyl acetate. The organic phase was
dried, the drying
agent was removed and the solution was concentrated to dryness. Chromatography
on silica
eluting with ethyl acetate/hexane (2:1), followed by crystallization from
diethyl ether/hexane
gave diol (290) (80 mg, 94%) as a yellow powder, mp 151-156 °C. IH NMR
D [(CD3)zS0]
8.50 (d, J=2.6 Hz, 1H), 7.82 (s, 1H), 7.69 (br d, J=9.0 Hz, 1H), 7.58 (m, 1H),
7.52-7.46 (m,
3H), 7.30 (dd, J=9.0, 2.6 Hz, 1H), 6.95 (d, J=8.4 Hz, 1H), 6.57 (d, J=2.4 Hz,
1H), 6.44 (dd,
J=8.4, 2.4 Hz, 1H), 5.02 (d, J=5.0 Hz, 1H), 4.87 (br s, 1H), 4.69 (s, 2H),
4.55 (m, 1H), 4.38
(m, 1H), 3.89 (m, 4H), 3.80 (s, 3H), 3.72 (s, 3H), 3.41 (partially obscured m,
2H). Found: C,
65.32; H, 5.00; N, 4.83. C33Hz9C1N20~.1/4H20 requires: C, 65.45; H, 4.91; N,
4.63.
EXAMPLE 301
The preparation of 4-(2-Chlorophenyl)-6-(3,4-dihydroxybutyl)-2-(2,4-
dimethoxybenz 1
methoxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (XLVII; Ar=2-chlorophenyl,
n=2) (291)
Reaction of carbazole (289) (100 mg, 0.17 mmol) procedure described in example
299 according to the procedure described in example 300, except that the
reaction time was
48 hours, gave diol (291) (74 mg, 71%) as a yellow powder, mp 126-131
°C.'H NMR 8
[(CD3)ZSO] 8.51 (d, J=2.5 Hz, 1H), 7.83 (s, 1H), 7.72 (d, J=9.0 Hz, 1H), 7.56
(m, 2H), 7.48
(m, 2H), 7.33 (dd, J=9.0, 2.5 Hz, 1H), 6.94 (d, J=8.4 Hz, 1H), 6.57 (d, J=2.3
Hz, 1H), 6.44
(dd, J=8.4, 2.3 Hz, 1H), 4.76 (t, J=5.0 Hz, 1H), 4.69 (s, 2H), 4.60-4.52 (m,
3H), 3.90 (s, 3H),
3.80 (s, 3H), 3.72 (s, 3H), 3.45-3.20 (partially obscured m, 3H), 1.99 (m,
1H), 1.69 (m, 1H).
Found: C, 66.39; H, 5.02; N, 4.44. C34H31C1Nz0~ requires: C, 66.39; H, 5.08;
N, 4.55.
Representative Procedure for Method 22 of Scheme 11
EXAMPLE 302
The preparation of 4-(2-Chlorophenyl)-6-(2,3-dihydroxYpropyl)-9-
hydroxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (XLVIII; Ar=2-chlorophenyl, n=1) (292)
To a solution of diol (290) (75 mg, 0.13 mmol) prepared as described in
example 300
in anisole (0.5 mL) was added trifluoroacetic acid (2.0 mL). The reaction
vessel was sealed
and heated to 90 °C in an oil bath for 18 h before the trifluoroacetic
acid was removed under
reduced pressure. The residue was then diluted with water and extracted with
diethyl ether (3
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-193-
times). The combined organic extracts were washed thoroughly with 1N potassium
hydroxide
and then brine before being dried over anhydrous sodium sulphate and
concentrated in vacuo.
The aqueous layer was then acidified by the addition of concentrated
hydrochloric acid,
extracted with diethyl ether and worked-up as above. The combined crude
material was
triturated with diethyl ether/hexane and then dissolved in dichloromethane (20
mL) and
demethylated according to The procedure described in example 80 to give, after
chromatography on silica eluting with ethyl acetate/hexane (l:l to 1:0),
phenol (292) (31 mg,
57%) as an orange powder, mp 305-309 °C.'H NMR 8 [(CD3)ZSO] 11.04 (br
s, 1H), 9.33 (s,
1H), 8.38 (d, J=2.4 Hz, 1H), 7.73 (s, 1H), 7.57 (m, 2H), 7.47 (m, 3H), 7.13
(dd, J=8.8, 2.4
Hz, 1H), 4.99 (d, J=5.5 Hz, 1H), 4.83 (t, J=5.4 Hz, 1H), 4.50 (m, 1H), 4.32
(m, 1H), 3.87 (br
s, 1H), 3.45-3.36 (m, 2H). Found: C, 63.17; H, 3.94; N, 6.18. C23H»C1N205
requires: C,
63.24; H, 3.92; N, 6.41.
EXAMPLE 303
The preparation of 4-(2-Chlorophenyl)-6-(3,4-dihydroxybutyl)-9-
hydroxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (XLVIII; Ar=2-chlorophenyl, n=2) (293)
Reaction of diol (291) (35 mg, 0.06 mmol) prepared as described in example 301
according to the procedure described in example 302 gave phenol (293) (25 mg,
92%) as an
orange powder, mp 263-267 °C.'H NMR 8 [(CD3)zS0] 11.05 (br s, 1H), 9.35
(s, 1H), 8.38
(d, J=2.4 Hz, 1H), 7.73 (s, 1H), 7.58 (m, 2H), 7.49 (m, 3H), 7.15 (dd, J=8.8,
2.4 Hz, 1H),
4.73 (t, J=4.9 Hz, 1H), 4.52 (m, 3H), 3.48-3.20 (m, 3H), 1.98 (br s, 1H), 1.67
(br s, 1H).
FABMS found [M+H]+=451.1024, 453.1021. C24H~9CIN205 requires 451.1061,
453.1031.
EXAMPLE 304
Reaction of carbazole (287) (50 mg, 0.09 mmol per reaction) prepared as
described in
example 297 with iodomethane, iodoethane, 1-bromopropane, 1-bromobutane, 1-
bromopentane, allyl bromide, (2-bromoethyl)benzene, 3-bromopropyne, 1-bromo-3-
methylbutane, 2-bromopropane, 2-iodo-l,l,l-trifluoroethane, 5-bromo-1-pentene,
iodoacetamide, 4-bromo-1-butene, 1-bromo-2-methylpropane and 1-bromo-4,4,4-
trifluorobutane in an array manner according to the procedure described in
example 298 ,
except that the reaction time wasl8 hours, followed by deprotection according
to the
procedure described in example 302, except that ethyl acetate was used as the
work-up
solvent and the 1N potassium hydroxide wash was omitted, gave respectively:
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-194-
4-(2-chlorophenyl)-9-hydroxy-6-meth~pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI;
Ar=2-chlorophenyl, R'°=CH3) (294). Found: M+H=377.
4-(2-chlorophenyl)-6-ethyl-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI; Ar=2-
chlorophenyl, R'°=CHzCH3) (295). Found: M+H=391.
4-(2-chloroQhenyl)-9-hydroxy-6-propylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI; Ar=2-
chlorophenyl, R'°=(CHz)ZCH3) (296). Found: M+H=405.
6-butyl-4-(2-chlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI; Ar=2-
chlorophenyl, R'°=(CHz)3CH3) (297). Found: M+H=419.
4-(2-chlorophenyl)-9-h dy roxy-6-pentylpyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI; Ar=2-
chlorophenyl, R'°=(CHZ)4CH3) (298). Found: M+H=433.
6-allyl-4-(2-chlorophenyl)-9-hydroxyQyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI; Ar=2-
chlorophenyl, R'°=CHZCH=CHZ) (299). Found: M+H=403.
4-(2-chlorophenyl)-9-h day-6-(2-phen l~ethyl)pyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(VI; Ar=2-chlorophenyl, R'°=CHZCHZPh) (300). Found: M+H=467.
4-(2-chlorophenyl)-9-hydrox -~propyn~p;rrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(VI;
Ar=2-chlorophenyl, R'°=CHIC=CH) (301). Found: M+H=401.
4-(2-chlorophenyl)-9-h d~ -~pent~pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (VI;
Ar=2-chlorophenyl, R'°=CHZCHZCH(CH3)2) (302). Found: M+H=433.
4-(2-chlorophenyl)-9-hydrox -6-isoproQylpyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, R'°=CH(CH3)z) (303). Found: M+H=405.
4-(2-chlorophenyl)-9-hydroxy-6-(2,2,2-trifluoroethyl)pyrrolof 3,4-clcarbazole-
1,3(2H,6H)-
dione (VI; Ar=2-chlorophenyl, R'°=CHZCF3) (304). Found: M+H=445.
4-(2-chloronhenyl)-9-hydroxy-6-(4-pentenyl)pyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, R'°=(CH2)3CH=CHZ) (305). Found: M+H=431.
2-(4-(2-chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydropyrrolof 3,4-clcarbazol-
6( 1 H)-
xl)acetamide (VI; Ar=2-chlorophenyl, R'°=CHZCONHZ) (306). Found:
M+H=420.
6-(3-butenyl)-4-(2-chlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, R'°=(CHZ)ZCH=CHZ) (307). Found: M+H=417.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-195-
4-(2-chloro~hen ly )-9-hydroxy-6-isobutylpyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, Rl°=CHZCH(CH3)z) (308). Found: M+H=419.
4-(2-chloroQhenyl)-9-~drox~6-(4,4,4-trifluorobutyl)pyrrolof 3,4-clcarbazole-
1,3(2H,6H)-
dione (VI; Ar=2-chlorophenyl, R'°=(CH2)3CF3) (309). Found: M+H=473.
EXAMPLE 305
Reaction of carbazole (287) (SO mg, 0.09 mmol per reaction) prepared as
described in
example 297 with 2-iodobutane and cyclopentyl bromide in an array manner
according to the
procedure described in example 298 , except that the reaction time was 48
hours, followed by
deprotection according to the procedure described in example 302, except that
ethyl acetate
was used as the work-up solvent and the 1N potassium hydroxide wash was
omitted, gave
respectively:
6-sec-butyl-4-(2-chlorophenyl)-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, R1°=CH(CH3)CH2CH3) (310). Found: M+H=419.
4-(2-chlorophenyl)-6-cyclopentyl-9-hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione (VI;
Ar=2-chlorophenyl, R1°=cyclopentyl) (311). Found: M+H=431.
EXAMPLE 306
Reaction of carbazole (287) (50 mg, 0.09 mmol) prepared as described in
example
297 with 2-bromoethyl methyl ether in an array manner according to the
procedure described
in example 298 , except that the reaction time was 18 hours, followed by
deprotection
according tothe procedure described in example 302 , except that ethyl acetate
was used as
the work-up solvent, the 1N potassium hydroxide wash was omitted and the
demethylation
was according to the proceedure described in example 81, gave:
6-(2-chloroethyl)-4-(2-chlorophen l~-9-hydroxypyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(VI; Ar=2-chlorophenyl, R1°=CHZCHZCI) (312). Found: M+H=425.
Reaction of carbazole (287) (50 mg, 0.09 mmol per reaction) prepared as
described in
example 297 with 1-chloro-2-propanol, (S)-3-bromo-2-methylpropanol and (R)-3-
bromo-2-
methylpropanol in an array manner according to the procedure described in
example 298 ,
except that the reaction time was 18 hours, followed by deprotection according
to the
procedure described in example 302 gave:
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-196-
4-(2-chlor~henyl)-9-hydroxy-6-(2-hydroxyprop~pyrrolof 3,4-clcarbazole-
1,3(2H,6H)-
dione (VI; Ar=2-chlorophenyl, R'°=CHZCH(OH)CH3) (313). Found: M+H=421.
4-(2-chlor~henyl)-9-hydroxy-6-f (2S)-3-hydroxy-2-methylpropyllpyrrolo f 3,4-
clcarbazole-
1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R'°=CHzCH(CH3)CHZOH) (314).
Found:
M+H=435.
4-(2-chlorophenyl)-9-hydroxy-6-f(2R)-3-h dy roxy-2-methYlprop.~~pyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (VI; Ar=2-chlorophenyl, R'°=CHZCH(CH3)CHZOH) (315).
Found:
M+H=435.
H
H N O N O
N
BBr
METHODS M ~ pr ~ M~'~~\~~./~~
HO \ ~ ~ ~ OR Py.HCI I \ ~ ~ METHOD 12 II /'j[~t~\~/
METHODS / Z(CH~n
/ Z(CH~n ~ 70
Z(OH~jn R10 Rt0 (LIII) R
(uh 1
1. CH~SOxC1/EIJJ METHOD 11
2.NaUEIOPC METHOD27
N O N O N O H
O N O
n~Bu,SnCH=CHI % BBrx O
METHODS
Me0 \ ~ I ~ Pd(FPh~IxG~LICI ~ \ \ / ~ --i Me0 \ Ar --1 H
I METHOD25 ~ METHOD28 ~ ~ ORPy.HGY
CFySOxO / ~ Z \ / ~ Z I / MEfHOD9 I /
70 Z
R70 R
R70 R70
(xux) (uq
(CF SOx)0/Pyddine~ n-B~,sncH,cr~cH, (L) (u)
Pd(PPh,) CI~LICI BBr,
METHOD 24 ~ METHOD d f
METHOD 25
b o o N D b o
N O 1.SNKOH O
2. tN HCI _ t. e-BBN
Me0 ' ~ ~ 3. NH ONrJ740~C Map Ar 2' Hz~ Pc Me0
I / \ ' M~ I \ ~ ~ ~ METHOD2! ~/ I / \ / MEfHOH29 I
HO
R10 ~ / R10 R70
(xxy) ~0 (LVII) (LVIII)
(LVI)
OsOJN-Manorpnoline NoxiEa
METHOD21
N p O N O
BBy _
HO ' \ M MET HOp6 Me0
H I ~ ~ H I
/ /
(CHxjn ~0 ~ (CH~1~ t0
H H
0.x)
(ux)
SCHEME 12
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-197-
Procedures for Scheme 12
Representative Procedure for Method 24 of Scheme 12
EXAMPLE 307
The~reparation of 4-(2-Chlorophenyl)-9-methoxy-6-(2-methoxyethyl)-1,3-dioxo-
1,2,3,6-
tetrahydropyrrolof3,4-clcarbazol-8-yl trifluoromethanesulfonate (XLIX; Ar=2-
chlorophenyl,
R' °=CH~CH~OCH~( 168)
Trifluoromethanesulfonic anhydride (1.30 mL, 7.77 mmol) was added dropwise at
0
°C to a solution of the phenol (167) (0.50 g, 1.11. mmol) prepared as
described in example
267 and pyridine (6.29 mL, 0.077 mol) in tetrahydrofuran (50 mL). The solution
was allowed
to warm to room temperature over lh before dilution with 1N HCl and extraction
with ethyl
acetate. The extraction was dried, the drying agent was removed and the
solution was
concentrated to dryness to give a solid which was adsorbed onto silica and
chromatographed.
Elution with ethyl acetate/petroleum ether (l:l) gave the triflate (168) (0.53
g, 82 %) as a
pale yellow powder, mp 229-231 °C. 'H NMR 8 [(CD3)ZSO] 11.23 (br s,
1H), 8.78 (s, 1H),
8.01 (s, 1H), 7.93 (s, 1H), 7.59 (dd, J=8.0, 2.2 Hz, 1H), 7.54-7.44 (m, 3H),
4.73 (t, J=4.8 Hz,
2H), 4.04 (s, 3H), 3.68 (t, J=4.8 Hz, 2H), 3.14 (s, 3H). Found: C, 49.26; H,
2.91; N, 4.39.
CZSH18C1F3SN20~.1/2CHZCIz requires C, 48.97 ; H, 3.06; N, 4.48.
EXAMPLE 308
The preparation of 4-(2-Chlorophenyl)-9-methoxY-1,3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-
clcarbazol-8-~trifluoromethanesulfonate (XLIX; Ar=2-chlorophenyl,
RI°=H) (169)
Reaction of (163) with trifluoromethanesulfonic anhydride using the procedure
described in example 307 gave the triflate (169) (87 %) as a yellow solid, mp
248-252 °C.'H
NMR cS [(CD3)ZSO] 12.16 (br, 1H), 11.21 (br, 1H), 8.73 (s, 1H), 7.80 (s, 1H),
7.71 (s, 1H),
7.58 (dd, J=8.0, 2.2 Hz, 1H), 7.52-7.43 (m, 3H), 4.03 (s, 3H). Found: C,
52.32; H, 2.87; N,
4.87. CZZH~2C1F3NZSO6.THF requires C, 52.31; H, 3.38; N, 4.69.
Representative Procedure for Method 25 of Scheme 12
EXAMPLE 309
The preparation of 4-(2-Chlorophenyl)-8-f(lE)-4-hydroxy-1-butenyll-9-
methoxypyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (L; Ar=2-chlorophenYl, R'°=H, Z=CH~CH-
_~OH) 170 .
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-198-
A solution of the triflate (169) (0.20 g, 0.35 mmol) prepared as described in
example
308 , (3E)-4-(tributylstannyl)-3-buten-1-of (0.19 g, 0.53 mmol) and lithium
chloride (29 mg,
0.70 mmol) in DMF (10 mL) was purged by bubbling nitrogen through the liquid
for 10 min.
Bis(triphenylphosphine)palladium dichloride (12 mg, 0.017 mmol) was added last
and the
solution was flushed with nitrogen for 2 min more, then warmed under an
atmosphere of
nitrogen for 3 h. The mixture was diluted with brine, extracted with ethyl
acetate and worked
up to give an oil which was chromatographed on silica. Elution with ethyl
acetate/petroleum
ether (1:5) gave foreruns, while ethyl acetate/petroleum ether (1:1)
increasing to pure ethyl
acetate gave (170) as a yellow solid (0.15 g, 95 %), mp 271-275 °C.'H
NMR 8 [(CD3)2S0]
11.86 (br s, 1H), 11.07 (br s, 1H), 8.45 (s, 1H), 7.70 (s, 1H), 7.58 (dd,
J=8.1, 2.2 Hz, 1H),
7.55 (s, 1H), 7.51-7.41 (m, 3H), 6.87 (d, J=16.0 Hz, 1H), 6.44 (dt, J=16.0,
7.1 Hz, 1H), 4.62
(t, J=5.3 Hz, 1H), 3.94 (s, 3H), 3.58 (m, 2H), 2.42 (m, 2H). Found: C, 67.09;
H, 4.36; N,
5.98. C25HI9C1N204 requires C, 67.19; H, 4.28; N, 6.27.
R~resentative Procedure for Method 26 of Scheme 12
EXAMPLE 310
The preparation of 4-(2-Chlorophenyl)-8-(4-hydroxybutyl)-9-methoxypyrrolo(3,4-
~carbazole-1,3(2H,6H)-dione (LI; Ar=2-chlorophenyl, R'°=H, Z=CH2CH~0-
H) (171)
A mixture of (170) (0.15 g, 0.33 mmol) prepared as described in example 309
and
Pt02 (20 mg) in 1:1 tetrahydrofuran/methanol (30 mL) was shaken under an
atmosphere of
hydrogen gas at 40 psi pressure for 30 min. The catalyst was removed by
filtration through
Celite, washing through with more solvent. The combined filtrates were worked
up and
chromatographed on silica. Elution with ethyl acetate/petroleum ether (1:1)
gave (171) as an
orange solid (0.20 g, 100 %), mp 256-260 °C (dec). 1H NMR 8 [(CD3)ZSO]
11.90 (br, 1H),
11.10 (br, 1H), 8.34 (s, 1H), 7.64-7.40 (m, 6H), 4.37 (br, 1H), 3.93 (s, 3H),
3.43 (t, J=6.3 Hz,
2H), 2.76 (t, J=7.6 Hz, 2H), 1.66 (m, 1H), 1.50 (m, 2H).
EXAMPLE 311
The preparation of 4-(4-(2-Chlorophenyl)-9-methoxy-1,3-dioxo-1,2,3,6-
tetrahydropyrrolo(3,4-clcarbazol-8-yllbutyl trifluoromethanesulfonate (LIII;
Ar=2-
chloroQhenyl, R'°=H, n=4, Z=OMs) (172).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-199-
Mesylation of (171) prepared as described in example 310 with methanesulfonyl
chloride and triethylamine using the procedure described in example 170 of
Scheme 3 gave
the mesylate (172) as a yellow solid (98 %), which was used without further
purification.
Representative Procedure for Method 27 of Scheme 12
EXAMPLE 312
The preparation of 4-(2-Chlorophenyl)-8-(4-iodobutyl)-9-methoxypyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (LIII; Ar=2-chlorophenyl, R'°=H, n=4, Z=I) (173).
A mixture of the mesylate (172) 0.12 g, 0.21 mmol) prepared as described in
example
311 and sodium iodide (1 g) in ethyl acetate (50 mL) was refluxed for 4 h. The
cooled
solution was washed with water and worked up to give the iodide (173) which
crystallised
from tetrahydrofuran/petroleum ether as an orange powder (0.10 g, 85 %), mp
142-144 °C.
'H NMR 8 [(CD3)zS0] 11.86 (s, 1H), 11.05 (s, 1H), 8.43 (s, 1H), 7.58 (m, 1H),
7.55 (s, 1H),
7.52-7.43 (m, 3H), 7.42 (s, 1H), 3.94 (s, 3H). EIMS found M+=558.0212,
560.0178.
CzsHzoC1~z03 requires 558.0207, 560.0178.
EXAMPLE 313
The preparation of 4-(2-Chlorophenyl)-8-f4-(dimethylamino)butyll-9-
methoxypyrrolof3,4-
clcarbazole-1 3(2H,6H)-dione (LIV; Ar=2-chlor~henyl, R1°=H, n=4,
Z=N(CH3~~ (174)
Reaction of the iodide (173) prepared as described in example 312 with aqueous
dimethylamine using the procedure described in example 179 of Scheme 3 except
that the
reaction conditions were 6 h at room temperature gave (174) (64 %) as an
orange powder, mp
162-164 °C.'H NMR 8 [(CD3)zS0] 11.85 (s, 1H), 11.04 (br s, 1H), 8.42
(s, 1H), 7.57 (dd,
J=8.0, 2.2 Hz, 1H), 7.55 (s, 1H), 7.51-7.43 (m, 3H), 7.42 (s, 1H), 3.93 (s,
3H), 2.77 (t, J=7.4
Hz, 2H), 2.24 (t, J=7.3 Hz, 2H), 2.11 (s, 6H), 1.63 (m, 2H), 1.47 (m, 2H).
EIMS found
M+=475.1657, 477.1650. Cz~Hz6C1N303 requires 475.1663, 477.1633.
EXAMPLE 314
The preparation of 4-(2-Chlorophenyl)-8-f4-(dimethylamino)butyll-9-
hydroxypyrrolof3,4-
clcarbazole-1,3(2H,6Hidione (LV; Ar=2-chlorophenyl, R1°=H, n=4,
~N(CH3)?) (175)
Demethylation of (174 ???) prepared as described in example 313 with pyridine
hydrochloride using the procedure described in example 81 gave (176) (67 %) as
a yellow
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-200-
powder, mp 220-226 °C (dec).'H NMR 8 [(CD3)zS0] 11.72 (s, 1H), 10.98
(s, 1H), 9.33 (br,
1H), 8.32 (s, 1H), 7.56 (dd, J=8.0, 2.2 Hz, 1H), 7.49 (s, 1H), 7.49-7.40 (m,
3H), 7.33 (s, 1H),
2.74 (t, J=7.33 Hz, 2H), 2.54 (m, 2H), 2.34 (br s, 6H), 1.64 (m, 2H), 1.54 (m,
2H). FABMS
found [M+H)+: 464.1569, 462.1578. Cz6HzsC1N303 requires 464.1555, 462.1584.
EXAMPLE 315
The preparation of 4-(2-Chlorophenyl)-9-methoxy-8-f4-(1-
pyrrolidinyl)butyllQyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LIV; Ar=2-chloro~henyl, Rl°=H, n=4, ~1-
pyrrolidinyl)
176
Reaction of the iodide (173) prepared as described in example 312 with
pyrrolidine
using the procedure described in example 179 except that the reaction
conditions were 2 h at
room temperature gave (176) (75 %) as an orange powder, which was used without
further
purification, mp 173-178 °C. IH NMR 8 [(CD3)zS0] 11.85 (s, 1H), 11.04
(br s, 1H), 8.42 (s,
1H), 7.57 (dd, J=8.0, 2.2 Hz, 1H), 7.55 (s, 1H), 7.51-7.43 (m, 3H), 7.42 (s,
1H), 3.93 (s, 3H),
2.77 (t, J=7.4 Hz, 2H), 2.24 (t, J=7.3 Hz, 2H), 2.11 (s, 6H), 1.63 (m, 2H),
1.47 (m, 2H).
EXAMPLE 316
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-8-f4-(1-pyrrolidinyl)but
~Lllpyrrolof3,4
clcarbazole-1,3(2H,6H)-dione (LV; Ar=2-chlorophenyl, R'°=H, n=4, ~1-
pyrrolidinyl)
1~ 77)..
Demethylation of (173) prepared as described in example 312 with pyridine
hydrochloride using the procedure described in example 81 gave (177) (72 %).'H
NMR 8
[(CD3)zS0] 11.70 (s, 1H), 10.98 (br s, 1H), 9.30 (br, 1H), 8.31 (s, 1H), 7.57
(dd, J=8.0, 2.2
Hz, 1H), 7.49 (s, 1H), 7.49-7.40 (m, 3H), 7.32 (s, 1H), 2.73 (t, J=7.5 Hz,
2H), 2.44-2.36 (m,
6H), 1.69-1.61 (m, 6H), 1.51 (m, 2H). The hydrochloride salt had a mp of 173-
178 °C (dec).
Found: C, 62.27; H, 5.22; N, 7.74. Cz$Hz6C1N303.HC1.Hz0 requires C, 62.00; H,
5.39; N,
7.75.
EXAMPLE 317
The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-(2-methox~yl)-8-
vinylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (L: Ar=2-chlorophenyl, R1°=CHzCH?OCH~, ~H)
(178)
Reaction of the triflate (168) prepared as described in example 307 with
tetravinyl tin
using the procedure described in example 309 gave the vinyl compound (178) (64
%) as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-201-
yellow solid, which was used without further purification.'H NMR 8 [(CD3)ZSO]
11.11 (s,
1H), 8.53 (s, 1H), 7.95 (s, 1H), 7.80 (s, 1H), 7.58 (dd, J=8.1, 2.2 Hz, 1H),
7.52-7.43 (m, 3H),
7.18 (dd, J=17.6, 11.2 Hz, 1H), 6.06 (dd, J=17.6, 1.3 Hz, 1H), 5.42 (dd,
J=11.2, 1.3 Hz, 1H),
4.71 (t, J=5.0 Hz, 2H), 3.97 (s, 3H), 3.71 (t, J=5.0 Hz, 2H), 3.16 (s, 3H).
EXAMPLE 318
The preparation of 4-(2-Chlorophenyl)-8-(2-hydroxyethyl)-9-methoxy-6-(2-
methoxyethyl)pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LI; Ar=2-chlorophenyl.
R'°=H,
Z=OH) (179) .
Hydroboration of (178) prepared as described in example 317 with 9-BBN using
the
procedure described in example 344 gave (179) (86 %) as a yellow solid, mp 271-
273 °C. 1H
NMR b [(CD3)ZSO] 11.08 (br, 1H), 8.49 (s, 1H), 7.78 (s, 1H), 7.60 (s, 1H),
7.57 (dd, J=8.0,
2.2 Hz, 1H), 7.52-7.42 (m, 3H), 4.68 (t, J=5.2 Hz, 1H), 4.65 (t, J=5.1 Hz,
2H), 3.94 (s, 3H),
3.70 (m, 4H), 3.15 (s, 3H), 2.96 (t, J=7.2 Hz, 2H).
EXAMPLE 319
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6,8-bis(2-
hydroxyethyl)pyrrolof3,4-
clcarbazole-1 3(2H,6H)-dione (LII; Ar=2-chlorophenyl, R1°=CH~CH20H,
Z=OH) (180).
Demethylation of (179) prepared as described in example 318 with BBr3 using
the
procedure described in example 80 except that the reaction conditions were 12
equiv. of
reagent at 0 °C for 6 h gave (180) as a yellow powder (35 %), mp 278-
280 °C. IH NMR 8
[(CD3)ZSO] 11.00 (br s, 1H), 9.39 (br, 1H), 8.37 (s, 1H), 7.72 (s, 1H), 7.56
(dd, J=8.0, 2.2 Hz,
1H), 7.51-7.42 (m, 4H), 4.82 (t, J=S.S Hz, 1H), 4.72 (br, 1H), 4.47 (t, J=5.4
Hz, 2H), 3.76 (dt,
J=5.5, 5.4 Hz, 2H), 3.69 (t, J=7.2 Hz, 2H), 2.93 (t, J=7.2 Hz, 2H). FABMS
found M+:
452.0993, 450.0984. C24I-I,9CIN205 requires 452.0953, 450.0982.
EXAMPLE 320
The preparation of 9-Methoxy-6-methyl-1,3-dioxo-4-phenyl-1,2,3,6-
tetrahydropyrrolof3,4-clcarbazol-8-yl trifluoromethanesulfonate (XLIX;
Ar=phenyl,
R'°=CH,) (185)
Reaction of (154) prepared as described in example 254 with
trifluoromethanesulfonic
anhydride using the procedure described in example 307 gave 9-methoxy-6-methyl-
1,3-
dioxo-4-phenyl-1,2,3,6-tetrahydropyrrolo[3,4-c]carbazol-8-yl
trifluoromethanesulfonate as a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-202-
yellow solid (85%), m.p. 244-246°C; 1H NMR 8 [(CD3)zS0] 11.22 (br s,
1H), 8.76 (s, 1H),
7.96 (s, 1H), 7.90 (s, 1H), 7.68-7.66 (m, 2H), 7.52-7.46 (m, 3H), 4.02 (s,
3H), 3.99 (s, 3H).
EXAMPLE 321
The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-methyl-1,3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-clcarbazol-8-yl trifluoromethanesulfonate (XLIX; Ar=2-
chlorophenyl,
R'°=Me) (700).
Reaction of (160) prepared as described in example 260 with
trifluoromethanesulfonic
anhydride using the procedure described in example 307 gave the triflate (700)
(89%) as a
yellow solid, mp 251-253 °C. 'H NMR 8 [(CD3)zS0] 11.26 (br s, 1H), 8.80
(s, 1H), 8.02 (s,
1H), 7.87 (s, 1H), 7.61-7.44 (m, 4H). Found: C, 52.51; H, 3.14; N, 4.97.
Cz3HiaNzC1F3O6S.1/4 Hexane requires: C, 52.51; H, 3.15; N, 5.00. FABMS found
[M+H]+:
539.0262, 541.0240. Cz3H15N2C1F3O6S requires 539.0292, 541.0262.
EXAMPLE 322
The preparation of 2,8-Diallyl-4-(phenyl)-9-methoxy-6-methylpyrrolof3,4-
clcarbazole-
1,3(2H,6H)-dione (LVI; Ar=phenyl, R=Me)(186) and 8-all~phenyl)-9-methoxy-6-
meth~pynolof3,4-clcarbazole-1,3(2H,6H)-dione (LVII; Ar=phenyl, RI°=Me)
(187).
Reaction of triflate (185) prepared as decribed in example 320 and allyltri-n-
butyltin
at 100 °C for 1 h using the procedure described in example 309 gave a
crude product which
was chromatographed on silica gel (ethyl acetate/petroleum ether (1:4) to
afford (i) pure bis-
allyl compound (186) (12%) as an orange solid, mp 188-189 °C. 'H NMR b
[(CD3)zS0]
8.52 (s, 1H), 7.81 (s, 1H), 7.66 (dd, J=8.0, 1.7 Hz, 2H), 7.53-7.43 (m, 4H),
6.10 (m, 1H),
5.91(m, 1H), 5.21-5.06 (m, 4H), 4.22 (d, J=5.1 Hz, 2H), 3.95 (s, 6H), 3.56 (d,
J=6.6 Hz, 2H).
Found: C, 76.43; H, 5.59; N, 6.48. Cz8Hz4NzO3 .1/10 HZO requires: C, 76.73; H,
5.56; N,
6.39; followed by (ii) pure mono-allyl compound (187) (42%) mp 204-208
°C. 'H NMR b
(CDC13) 11.08 (s, 1H), 8.52 (s, 1H), 7.78 (s, 1H), 7.67-7.64 (m, 2H), 7.50 (s,
1H), 7.49-7.43
(m, 3H), 6.09 (ddt, J' = 6.6 Hz, 1 H), 5.15-5.07 (m, 2H), 3.94 (s, 6H), 3.56
(d, J = 6.6 Hz, 2H);
Found: C, 73.75; H, 5.39; N, 6.76. CzSHzoNzOs.3/2 HZO requires C, 73.51; H,
5.26; N, 6.86.
EXAMPLE 323
The preparation of 4-(Phenyl)-9-methoxy-6-methyl-8-vinylpyrrolof3,4-
clcarbazole-
.1 3(2H,6H)-dione (L; Ar=phenyl, Rl°=Me, Z=H) (701).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-203-
Reaction of triflate (185) prepared as decribed in example 320 and
tetravinyltin at
100 °C for 1 h using the procedure described in example 309gave a crude
product which was
chromatographed on silica gel (ethyl acetate/petroleum ether (1:2) to afford
pure 8-vinyl
compound (701) (68%) as an orange solid, mp 220 °C (dec).'H NMR 8
[(CD3)ZSO] 11.12
(br, 1H), 8.57 (s, 1H), 7.92 (s, 1H), 7.79 (s, 1H), 7.69-7.64 (m, 2H), 7.52-
7.43 (m, 3H), 7.20
(dd, J=17.7, 11.2 Hz, 1H), 6.09 (dd, J=17.7, 1.2 Hz, 1H), 5.42 (dd, J=11.2,
1.2 Hz, 1H), 4.00
(s, 3H), 3.96 (s, 3H). Found: C, 74.45; H, 4.78; N, 6.94. Cz4H~gN2O3 .1/4 H20
requires C,
74.50; H, 4.82; N, 7.24.
EXAMPLE 324
EXAMPLE 325
The preparation of 2,8-Diallyl-4-(2-chlorophenyl)-9-methoxy-6-
methylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LVI; Ar=2-chlorophenyl, R=Me) (702) and 8-allyl-
4-(2-
chlorophenyl)-9-methoxy-6-methylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
(LVII; Ar=2-
chloro~henyl, R'°=Me) (703).
Reaction of triflate (700) prepared as in example 321 and allyltri-n-butyltin
at 100 °C
for 1 h using the procedure described in example 309 gave a crude product
which was
chromatographed on silica gel (ethyl acetate/petroleum ether (1:3) to afford
(i) pure bis-allyl
compound (702) (49%) as an orange solid, mp 183-185 °C.'H NMR 8
[(CD3)ZSO] 8.51 (s,
1H), 7.82 (s, 1H), 7.61-7.44 (m, SH), 6.16-6.05 (m, 1H), 5.95-5.85 (m, 1H),
5.17-5.07 (m,
4H), 4.21 (br d, J=5.0 Hz, 2H), 3.96 (s, 6H), 3.57 (d, J=6.5 Hz, 2H). Found:
C, 71.53; H,
5.14; N, 6.20. Cz8H23N2C103 requires: C, 71.41; H, 4.92; N, 5.95; followed by
(ii) mono-
allyl compound (703) (31 %) as a yellow solid, mp 269-272 °C. 1H NMR 8
[(CD3)zS0] 11.10
(br, 1H), 8.52 (s, 1H), 7.77 (s, 1H), 7.58-7.43 (m, 4H), 6.16-6.07 (m, 1H),
5.17-5.07 (m,
2H), 3.95 (s, 6H), 3.57 (d, J=6.5 Hz, 2H). Found: C, 69.58; H, 4.55; N, 6.46.
C25H~9NZC1O3
requires C, 69.69; H, 4.44; N, 6.50.
EXAMPLE 326
The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-methyl-8-vinylpyrrolof3,4-
clcarbazole-
1.3(2H,6H)-dione (L; Ar=2-chlorophenvl, R'°=Me, Z=H) (704).
Reaction of triflate (700) prepared as in example 321 and tetravinyltin at 100
°C for 1
h using the procedure described in example 309 followed by treatment with
excess
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-204-
ammonium acetate at 100 °C for another lh gave a crude product which
was
chromatographed on silica gel (ethyl acetate/petroleum ether) (1:2) to afford
pure 8-vinyl
compound (704) (78%) as an orange solid, mp 330 °C (dec). 'H NMR 8
[(CD3)ZSO] 11.12
(br, 1H), 8.53 (s, 1H), 7.94 (s, 1H), 7.78 (s, 1H), 7.60-7.43 (m, 4H), 7.20
(dd, J=17.7, 11.2
Hz, 1H), 6.10 (dd, J=17.7, 1.2 Hz, 1H), 5.43 (dd, J=11.2, 1.2 Hz, 1H), 3.99
(s, 3H), 3.97 (s,
3H). ). Found: C, 58.54; H, 4.08; N, 6.65. C24H1~NZCI03.CH2ClZ.1/2 NH3
requires C, 58.84;
H, 4.05; N, 6.86. FABMS found [M+H]+: 417.0982, 419.0955. C24HI~NZC103
requires
417.1006, 419.0977.
EXAMPLE 327
The preparation of 4-(2-Chlorophenyl)-8-f(lE)-3-hydroxy-1-propenyll-9-methoxy-
6-
methylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (L; Ar=2-chlorophenyl,
RI°=Me,
Z=CH~,OH) (710).
Reaction of triflate (700) prepared as in example 321 and 3-hydroxyallyltri-n-
butyltin
using the procedure described in example 309 at 100 °C for 1 h to gave
a crude product
which was chromatographed on silica gel (ethyl acetate/petroleum ether) (1:1)
to afford pure
(710) (42%) as an orange solid, mp 283-285 °C.'H NMR 8 [(CD3)ZSO] ]
11.11 (s, 1H), 8.51
(s, 1H), 7.89 (s, 1H), 7.76 (s, 1H), 7.59-7.43 (m, 4H), 7.05 (br d, J=16.0 Hz,
1H), 6.67 (dt,
J=16.0, 5.0 Hz, 1H), 4.94 (t, J=5.4 Hz, 1H), 4.22 (br t, J=5.0 Hz, 2H), 3.99
(s, 3H), 3.97 (s,
3H). FABMS found M+ : 446.1031, 448.1037. CZQH1~NZC103 requires 446.1033,
448.1004.
Representative Procedure for Method 28 of Scheme 12
EXAMPLE 328
The preparation of De-alkylation of bisall I~ compound (LVI; Ar=2-
chlorophenyl, R=Me)
(702) to give monoallyl compound (LVII; Ar=2-chlorophenYl, R1°=Me) 703
.
Bisallyl compound (702) (72 mg, 0.153 mmol) prepared as described in example
325
was dissolved in a mixture of acetonitrile (10 mL) and water (1 mL). To this
homogeneous
solution was added 5M KOH (1 mL) and the mixture was stirred at room
temperature for 64
h. Most of the acetonitrile was evaporated in vacuo and the residue was
diluted with water (3
mL). After acidification with 1N HCl to pH< 1 the acidic solution was stirred
at 50-60 °C
(bath) for 3 h. After partitioning between ethyl acetate and water the ethyl
acetate solution
was evaporated and co-evaporated with toluene to give an orange solid which
was treated
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-205-
with a large excess of molten ammonium acetate at 140-145 oC (bath) for 3 h.
After cooling
to room temperature and partitioning between ethyl acetate and water the ethyl
acetate
solution was evaporated to give a crude product which was chromatographed on
silica gel
(ethyl acetate-petroleum ether) (1:3) to give pure monoallyl compound (703)
(52 mg, 79%).
The Rf, mp and'H NMR spectrum were identical with the authentic sample
described earlier.
EXAMPLE 329
The preparation of De-alkylation of Bisallyl compound (LVI; Ar=phenyl, R=Me)
(186) to
give monoallyl compound (LVII; Ar=phenyl, R'°=Me) 187 .
Using the procedure described in example 328 bisallyl compound (186) prepared
as
described in example 322 was converted into monoallyl compound (187) (84%).
The Rf, mp
and'H NMR spectrum were identical with an authentic sample described earlier.
EXAMPLE 330
The preparation of 8-(2,3-Dih d~ypropyl)-9-methoxy-6-meth~phenylpyrrolo(3,4-
clcarbazole-1,3(2H,6H)-dione (LIX; n=1, Ar=phenyl, R'°=Me) (705).
Reaction of (187) prepared in example 329, N-methylmorpholine N-oxide and
osmium tetroxide (at room temperature for 5 h) using the procedure described
in example 300
followed by chromatography on silica gel (ethyl acetate) gave (705) (81%) as
an orange
solid, mp 243-245 °C.'H NMR 8 [(CD3)ZSO~ 11.06 (br, 1H), 8.51 (s, 1H),
7.78 (s, 1H),
7.68-7.63(m, 2H), 7.55 (s, 1H), 7.50-7.42 (m, 3H), 4.58-4.52 (m, 2H), 3.95 (s,
3H), 3.93 (s,
3H), 3.40 (d, J=5.3 Hz, 2H), 3.30 (dd, J=13.5, 5.2 Hz, 1H), 2.77 (dd, J=13.5,
7.8 Hz, 1H).
Found: C, 69.02; H, 5.48; N, 6.44. C25HZZNaOs.1/5 H20 requires: C, 69.18; H,
5.20; N, 6.45.
EXAMPLE 331
The preparation of 8-(1,2-Dihydroxyethyl)-9-methoxy-6-methyl-4-
phenylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LIX; n=0, Ar=phenyl, R'°=Me) (706).
Reaction of (701) prepared as described in example 321, N-methylmorpholine N
oxide and osmium tetroxide (at room temperature for 5 h) using the procedure
described in
example 300 followed by chromatography on silica gel (chloroform/methanol)
(10:1) gave
(706) (65%) as a yellow solid, mp 242-245 °C.'H NMR 8 [(CD3)ZSO] 11.09
(s, 1H), 8.53 (s,
1H), 7.81 (s, 1H), 7.74(s, 1H), 7.68-7.64 (m, 2H), 7.50-7.41(m, 3H), 5.35(d,
J=4.4 Hz, 1H),
5.12-5.06 (m, 1H), 4.75 (t, J=5.3 Hz, 1H), 3.97 (s, 3H), 3.95 (s, 3H), 3.66-
3.60 (m, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-206-
3.39-3.33 (m, 1H). Found: C, 69.25; H, 5.14; N, 6.43. C24H2°NZOs
requires: C, 69.22; H,
4.84; N, 6.73.
EXAMPLE 332
The Qreuaration of 8-(2,3-Dihydroxypropyl)-9-hydroxy-6-methyl-4-
nhenylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LX; n=1, Ar=phenyl, R'°=Me) (707).
Reaction of (705) prepared as described in example 330 and BBr3 (at 0
°C for 4 h)
using the procedure described in example 80 gave the phenol (707) (53%) as an
orange solid,
mp 283-285 °C. 'H NMR 8 [(CD3)zS0] 11.01 (br, 1H), 9.41 (br, 1H), 8.38
(s, 1H), 7.74 (s,
1H), 7.67-7.62 (m, 2H), 7.50-7.40 (m, 4H), 3.92 (s, 3H), 3.90-3.84 (m, 1H),
3.36 (d, J=5.2
Hz, 2H), 2.98 (dd, J=13.5, 5.0 Hz, 1H), 2.74 (dd, J=13.5, 7.7 Hz, 1H). FABMS
found M+:
416.1365. CZaHaoN2Os requires 416.1372.
EXAMPLE 333
The preparation of 9-Hydroxy-8-(2-hydroxyethyl)-6-methyl-4-phenylpyrrolof3,4-
clcarbazole-1.3(2H,6H)-dione (LII; Ar=phenyl, R'°=Me, Z=OH) (714).
Reaction of (712) prepared as described in example 340 and BBr3 (at 0
°C for 3 h)
using the procedure described in example 80 followed by chromatography on
silica gel
(chloroform/methanol) (20:1) gave the phenol (714) (31%) as a yellow solid, mp
233-235 °C.
'H NMR 8 [(CD3)ZSO] 11.01 (br, 1H), 9.39 (br, 1H), 8.39 (s, 1H), 7.74 (s, 1H),
7.64 (dd,
J=7.6, 1.6 Hz, 2H), 7.50-7.42 (m, 4H), 4.73 (br,lH), 3.92 (s, 3H), 3.70 (t,
J=7.1 Hz, 2H),
2.94 (t, J=7.1 Hz, 2H). FABMS found M+: 386.1263. C23H,8Nz04 requires
386.1267.
EXAMPLE 334
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-8-(2-hydroxyethyl)-6-
methylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LII; Ar=2-chlorophenvl, Rl°=Me, Z=OH)
(715).
Reaction of (713) prepared as described in example 341 and BBr3 (at 0
°C for 3 h)
using the procedure described in example 80 followed by chromatography on
silica gel
(chloroform/methanol) (20:1) gave the phenol (715) (81%) as an orange solid,
mp 304-306
°C: 'H NMR b [(CD3)zS0] 11.00 (br, 1H), 9.30 (br, 1H), 8.37 (s, 1H),
7.71 (s, 1H), 7.57 (dd,
J=7.0, 2.0 Hz, IH), 7.52-7.42 (m, 4H), 4.74 (br, 1H), 3.91 (s, 3H), 3.71 (t,
J=7.0 Hz, 2H),
2.94 (t, J=', .0 Hz, 2H). FABMS found M+: 420.0866, 422.0875. CZ3H»C1N204
requires
420.0877, 422.0847.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-207-
EXAMPLE 335
The preparation of 4-(2-Chlor~henyl)-8-ethyl-9-methoxy-6-methylpyrrolo(3,4-
clcarbazole-
(1 3(2H 6H)-dione (LI; Ar=2-chlor~henyl, R'°=Me, Z=H) (708).
Hydrogenation of alkene (704) prepared as in example 326 over Pt02 (45 min
reaction time) using the procedure described in example 310 gave 8-ethyl
derivative (708)
(97%) as an orange solid, mp 262-264 °C. 'H NMR 8 [(CD3)zS0] 11.08 (br,
1H), 8.48 (s,
1H), 7.77 (s, 1H), 7.60-7.42 (m, SH), 3.95 (s, 6H), 3.90-3.84 (m, 1H), 2.82
(q, J=7.5 Hz, 2H),
1.28 (t, J=7.5 Hz, 3H). Found: C, 68.21; H, 4.38; N, 6.48. C2aHi9N2C103 .1/10
Hz0 requires
C, 68.52; H, 4.60; N, 6.66.
EXAMPLE 336
The preparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropyl)-9-methoxy-6-
methylpyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (LI; Ar=2-chlorophenJ~l, R'°=Me, Z=CH~OH)
(711).
Hydrogenation of alkene (710) prepared as in example 321 over Pt02 using the
procedure described in method26 followed by chromatography on silica gel
(ethyl
acetate/petroleum ether) (1:1) gave pure 8-(3-hydroxypropyl) compound (711)
(44 %) as an
orange solid, mp 260-263 °C.'H NMR b [(CD3)ZSO] 11.08 (s, 1H), 8.48 (s,
1H), 7.77 (s, 1H),
7.59-7.43 (m, SH), 4.50 (t, J=5.2 Hz, 1H), 3.95 (s, 6H), 3.49 (m, 2H), 2.83
(t, J=7.7 Hz, 2H),
1.82 (m, 2H). FABMS found M+: 448.1194, 450.1200. CZSHziC1N204 requires
448.1190,
450.1160.
EXAMPLE 337
'The preparation of 4-(2-Chlorophenyl)-8-eth~-9-hydroxy-6-methylpyrrolo(3,4-
clcarbazole-
1 3(2H 6H)-dione (LII; Ar=2-chlorophenyl, R'°=Me, Z=H) (709).
Reaction of (708) prepared as in example 335 with pyridinium hydrochloride
using
the procedure described in example 81 followed by chromatography on silica gel
(ethyl
acetate/petroleum ether) (1:2) gave phenol (709) (78 %) as an orange solid, mp
265-268 °C.
'H NMR 8 [(CD3)zS0] 11.01 (s, 1H), 9.39 (s, 1H), 8.37 (s, 1H), 7.71 (s, 1H),
7.59-7.55 (m,
1H), 7.52-7.42 (m, 4H), 3.93 (s, 3H), 2.79 (q, J=7.5 Hz, 2H), 1.27 (t, J=7.5
Hz, 3H). EIMS
found M+: 404.0925, 406.0905. Cz3HI~CINz03 requires 404.0928, 406.0898.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-208-
EXAMPLE 338
The preparation of 8-f3-(Dimethylamino)proRyll-9-hydroxL-6-methyl-4-
phenylpyrrolof3 4-
~carbazole-1 3(2H 6H)-dione (LV; n=3, Ar=phenyl, R1°=Me, Z=NMe~) (720).
Reaction of (717) prepared as described in example 342 with pyridinium
hydrochloride using the procedure described in example 81 gave phenol (720)
(83%) as an
orange solid, mp 315 °C.'H NMR b[(CD3)zS0] 11.01 (br, 1H), 9.79 (br,
1H), 8.38 (s, 1H),
7.72 (s, 1H), 7.64 (dd, J=7.9, 1.4 Hz, 2H), 7.50-7.41 (m, 4H), 3.92 (s, 3H),
2.7.7 (t, J=7.3 Hz,
2H), 2.25 (t, J=7.0 Hz, 2H) 2.18 (s, 6H), 1.81 (m, 2H). FABMS found [M+H]+:
428.1972.
Cz6Ha6N303 requires 428.1974.
EXAMPLE 339
The preparation of 8-f2-(Dimethylamino)ethyll-9-hydroxy-6-methyl-4-
phenylpyrrolol3,4-
clcarbazole-1 3(2H 6H)-dione (LV; n=2, Ar=phenyl, R'°=Me, Z=NMe2 721 .
Reaction of (719) prepared as described in example 343 with pyridinium
hydrochloride using the procedure described in example 81 followed by
chromatography on
silica gel (chloroform/methanol) (10:1) gave phenol (721) (97%) as an orange
solid, mp 278
°C (dec).'H NMR b[(CD3)ZSO] 11.01 (br, 2H), 8.36 (s, 1H), 7.73 (s, 1H),
7.64 (dd, J=7.9, 1.4
Hz, 2H), 7.50-7.41 (m, 4H), 3.92 (s, 3H), 2.94 (t, J=6.9 Hz, 2H), 2.64 (t,
J=6.9 Hz, 2H) 2.31
(s, 6H). FABMS found [M+H]+: 414.1821. CZSH2aNsOs requires 414.1818.
EXAMPLE 340
The preparation of 8-(2-Hydroxyethyl)-9-methoxy-6-methyl-4-phenylpyrrolof3,4-
clcarbazole-1,3(2H,6H)-dione (LI; Ar=phenyl, Rl°=Me, Z=OH) (712).
Reaction of alkene (701) prepared as decribed in example 321 with 9-BBN using
the
procedure described in method29 gave after chromatography on silica gel
(chloroform/methanol) (10:1), the 8-(2-hydroxyethyl) compound (712) (51 %) as
a yellow
solid, mp 284-286 °C.'H NMR 8 [(CD3)zS0] 11.09 (s, 1H), 8.52 (s, 1H),
7.79 (s, 1H), 7.66
(br d, J=6.4 Hz, 2H), 7.56 (s, 1H), 7.51-7.41 (m, 3H), 4.68 (t, J=5.2 Hz, 1H),
3.96 (s, 3H),
3.94 (s, 3H), 3.69 (m, 2H), 2.98 (t, J=7.2 Hz, 2H). ). Found: C, 71.77; H,
5.24; N, 6.81.
C24H2°N204 requires C, 71.99; H, 5.03; N, 7.00.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-209-
EXAMPLE 341
The preparation of 4-(2-Chlorophenyl)-8-(2-hydroxyethyl)-9-methoxy-6-
methylpyrrolof3,4-
c~carbazole-1,3(2H,6H)-dione (LI; Ar=2-chlorophenyl, R'°=Me, Z=OH)
(713).
Reaction of alkene (704) prepared as in example 326 with 9-BBN using the
procedure
described in method29 gave after chromatography on silica gel (ethyl
acetate/petroleum
ether) (2:1), the 8-(2-hydroxyethyl) compound (713) (50 %) as an orange solid,
mp 270-272
°C.'H NMR 8 [(CD3)ZSO] 11.08 (s, 1H), 8.49 (s, 1H), 7.77 (s, 1H), 7.59-
7.55 (m, 2H), 7.53-
7.43 (m, 3H), 4.69 (t, J=5.3 Hz, 1H), 3.94 (s, 6H), 3.69 (td, J=7.1, 5.3 Hz,
2H), 2.98 (t, J=7.1
Hz, 2H). Found: C, 66.14; H, 4.69; N, 6.51. C24H~9NZC104 requires: C, 66.29;
H, 4.40; N,
6.44.
EXAMPLE 342
The preparation of 8-f3-(Dimethylamino)propyll-9-methoxy-6-methyl-4-
phenylpyrrolof3,4-
clcarbazole=1-,3(2H,6H)-dione (LIV; n=3; Ar=phenyl, Rl°=Me, Z=NMes)
(717).
Reaction of (188) prepared as described in example 344 with methanesulfonyl
chloride using the procedure described in example 170 gave intermediate LIII
(n=3,
Ar=phenyl, Rl°=Me, Z=OSOzCH3) which was further reacted with aq. 40%
dimethylamine
(using the procedure described in example 179 ) at room temperature for 5 h.
Usual workup
gave a crude product which was refluxed in toluene in the presence of excess
ammonium
acetate for 23h. After chromatography on silica gel (chloroform/methanol)
(10:1) pure
dimethylaminopropyl compound (717) (60 %) was obtained, mp 175-178
°C.'H NMR
b[(CD3)ZSO] 11.07 (s, 1H), 8.51 (s, 1H), 7.78 (s, 1H), 7.68-7.63 (m, 2H), 7.55
(s, 1H), 7.51-
7.42 (m, 3H), 3.96(s, 3H), 3.94 (s, 3H), 2.80 (t, J=7.8 Hz, 2H), 2.34 (t,
J=7.8 Hz, 2H) 2.20 (s,
6H), 1.80 (m, 2H). Found: C, 69.28; H, 6.64; N, 9.05. Cz~H27N3O3, 3/2 H20
requires C,
69.21; H, 6.45; N, 8.97.
EXAMPLE 343
The preparation of 8-f2-(Dimethylamino)ethyll-9-methoxy-6-methyl-4-
phenylpyrrolof3,4-
clarbazole-1,3(2H,6H)-dione (LIV; Ar=phenyl, R'°=Me; Z=NMe~, n=2)
(719).
Reaction of (712) prepared as described in example 340 with methanesulfonyl
chloride using the procedure described in example 170 gave intermediate LIII
(Ar=phenyl,
n=2, R'°=Me, 2=OSOZCH3) which was further reacted with aq. 40%
dimethylamine (using
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-210-
the procedure described in example 179 ) at room temperature for 25 h. Usual
work up gave
a crude product which was refluxed in toluene in the presence of excess
ammonium acetate
for 18 h. After chromatography on silica gel (chloroform/methanol) (10:1) pure
dimethylaminoethyl compound (719) (15 %) was obtained, mp 233-236 °C.
1H NMR
S[(CD3)zS0] 11.08 (s, 1H), 8.51 (s, 1H), 7.78 (s, 1H), 7.68-7.63 (m, 2H), 7.58
(s, 1H), 7.50-
7.41 (m, 3H), 3.96 (s, 3H), 3.94 (s, 3H), 2.93 (t, J=8.0 Hz, 2H), 2.54 (t,
J=8.0 Hz, 2H) 2.23
(s, 6H). EIMS found M+: 427.1890. C26HasN30s requires 427.1896.
Representative Procedure for Method 29 of Scheme 12
EXAMPLE 344
The preparation of 8-(3-Hydroxypropyl)-9-methoxy-6-methyl-4-phenylpyrrolof 3,4-
clcarbazole-1 3(2H 6H)-dione (LVIII; Ar=phenyl, R1°=CHI) (188).
A solution of 9-borabicyclo[3.3.1]nonane (1 mL of a 0.5 M solution in
tetrahydrofuran, 0.49 mmol) was added to a solution of (187) (65 mg, 0.16
mmol) prepared
as descrined in example 329 in tetrahydrofuran (5 mL). The reaction mixture
was stirred at
room temperature for 2 hr 20 min and then 3 M sodium acetate (1.5 mL) and 35%
hydrogen
peroxide (1.0 mL) were added. The mixture was stirred at room temperature for
1 hr 30 min
and then diluted with brine and extracted with ethyl acetate (3 x 30 mL). The
combined
extracts were dried and concentrated. The residue was purified by column
chromatography
on silica eluting with ethyl acetate/ dichloromethane 1:5 to give 8-(3-
hydroxypropyl)-9-
methoxy-6-methyl-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione (58 mg,
85%), mp
105-110 °C softens, 130-134 °C melts.'H NMR 8 [(CD3)ZSO] 11.06
(s, 1H), 8.51 (s, 1H),
7.78 (s, 1H), 7.66-7.64 (m, 2H), 7.53 (s, 1H), 7.50-7.44 (m, 3H), 4.52 (t, J =
5.1 Hz, 1H), 3.96
(s, 3H), 3.94 (s, 3H), 3.52-3.45 (m, 2H), 2.82 (br t, 7.8 Hz, 2H), 1.82 (br
dt, J = 7.8 Hz, 2H).
Found: C, 70.93; H, 5.40; N, 6.54. Cz5H2zN20a~1/2 H2O requires: C, 70.91; H,
5.47; N, 6.62.
EXAMPLE 345
The preparation of 9-H~rdroxy-8-(3-hydroxypropyl)-6-methyl-4-phenylpytrolof
3,4-
c]carbazole-1 3(2H 6H)-dione (LIh Ar=phenyl, R'°=CHI, Z=CH~OH) (189)
Demethylation of (188) prepared as described in example 344 with BBr3 using
the
procedure described in example 80 gave (189) as a yellow solid. IH NMR 8
[(CD3)zSOJ
11.00 (br s, 1H), 9.46 (br s, 1H), 8.39 (s, 1H), 7.73 (s, 1H), 7.46-7.63 (m,
2H), 7.49-7.42 (m,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-211-
4H), 4.50 (br s, 1H), 3.92 (s, 3H), 3.49-3.44 (m, 2H), 2.78 (br t, J = 7.9 Hz,
2H), 1.82 (br dt,
J = 7.8 Hz, 2H).
EXAMPLE 346
The preparation of 8-Ethyl-9-methoxy-6-methyl-4-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (LI; Ar=phenyl, RI°=CHI, Z=H) (190)
Reaction of the triflate (185) prepared as decribed in example 320 with
tetraethyl tin
using the procedure described in example 309 gave (190) as a yellow solid
(83%), mp
251-253 °C.'H NMR 8 [(CD3)ZSO] 11.07 (s, 1H), 8.53 (s, 1H), 7.77 (s,
1H), 7.67-7.64 (m,
2H), 7.53 (s, 1H), 7.50-7.42 (m, 3H),3.95 (s, 3H), 3.94 (s, 3H), 2.81 (q, J =
7.5 Hz, 2H), 1.27
(t, J = 7.5 Hz, 3H). Found: C, 74.48; H, 5.20; N, 7.24. CZaHzoNz03.1/6 HZO
requires: C,
74.40; H, 5.29; N, 7.23.
EXAMPLE 347
- The preparation of 8-Eth~ydroxy-6-methyl-4-phenylpyrrolo~3,4-clcarbazole-
1,3(2H,6H)-dione (LII; Ar~henyl, R'°=CHI, Z=H) (191)
Demethylation of (190) prepared as decried in example 346 with BBr3 using the
procedure described in example 80 gave (191) as a yellow solid (92%), mp 278-
283 °C. 1H
NMR 8 [(CD3)zS0] 11.00 (br s, 1H), 9.36 (s, 1H), 8.40 (s, 1H), 7.73 (s, 1H),
7.66-7.63 (m,
2H), 7.49-7.41 (m, 4H), 3.93 (s, 3H), 2.78 (q, J = 7.5 Hz, 2H), 1.27 (t, J =
7.5 Hz, 3H).
Found: C, 74.36; H, 5.10; N, 7.45. C23H~8N203 requires: C, 74.58; H, 4.90; N,
7.56.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-212-
O N O
O
Me0 ~ ~ Ar
HO ~ ~ I ~ _ 88r°
I / METHOD 8 ~ ~ N
Hz)n1 R1o (CHz)n1 R10
(LXIV) (LXIII)
1. 5N KOH
2.1NHCI
R,(CH~°CLHCVKzCOz 3. NH,OAcJ160~170 °C
METHOD 28
METHOD 20
SCHz)nBr SCHz)nR,
N O N O O N O
Me0 Ar Br(CHz~ ° Me0 ~ ~ ~ Ar ZH.HCU~~N _ Me0 ~ \ / Ar
METHOD20 I / METHOD30 I /
HO I / NR\0 ~ Hz)nBr R1o ~ Hz)n1 NRIo
(XXV) (LXI) (LXII)
Br(CHz)n0 W KZCO~
METHOD 20
(CHz)nOH
H
N O N O
1. 5N KOH
_ 2.1NHCI
,qr 3. NH,OAC/160-170 °C 1. CH°SOzCVEhN
Me0 ~ ~ ~ ~ Me0 ~ \ / Ar METHOD 11
I METHOD 28 I
N / 2. ZH
METHOD 12
Hz)nOH
Hz)nOH
(u~~n
SCHEME 13
Procedures for Scheme 13
EXAMPLE 348
The preparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-2-(3-hydroxypropyl)-
9-
methoxy-6-(2-methoxyethyl)pyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXV; Ar=2-
chlorophenyl, R'°=CH~CH~OCH~, n=3) (181)
Alkylation of (167) prepared as described in example 267 with 3-bromopropanol
using the procedure described in example 298 gave (181) (92 %) as a yellow
oil, which was
used without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-213-
EXAMPLE 349
The preparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-9-methox -
methoxveth~l)nyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXVI; Ar=2-
chlorophenyl,
R1°=CH~CH~OCH~, n=3) (182)
Treatment of (182) with the sequence of reactions outlined in the procedure
described
in example 328 gave (182) (58 %) as an orange powder, mp 251-257 °C.'H
NMR 8
[(CD3)ZSO] 11.03 (br s, 1H), 8.46 (s, 1H), 7.74 (s, 1H), 7.57 (dd, J=8.0, 2.1
Hz, 1H), 7.51-
7.42 (m, 3H), 7.36 (s, 1H), 4.67 (t, J=4.9 Hz, 2H), 4.61 (br, IH), 4.23 (t,
J=6.3 Hz, 2H), 3.90
(s, 3H), 3.70 (t, J=4.9 Hz, 2H), 3.64 (m, 2H), 3.16 (s, 3H), 1.98 (m, 2H).
EIMS found:
M+=508.1398, 510.1358. CZ~H25C1N206 requires 508.1401, 510.1371.
EXAMPLE 350
The~reparation of 4-(2-Chlorophenyl)-9-methoxy-6-(2-methoxyethyl)-8-(3-(4-
-morpholinyl)propox~pyrrolo(3,4-clcarbazole-1;3(2H,6H)-dione (LXIII; Ar=2-
chlorophenyl,
R'°=CH~CH~OCH3, ~4-momholinvl) (183
Conversion of (182) prepared as described in example 349 to the corresponding
mesylate by reaction with methanesulfonyl chloride using the procedure
described in
example 170 followed by reaction with morpholine using the procedure described
in
example 179 gave (183) (71 %) as a yellow powder, mp 271-275 °C, which
was immediately
demethylated.
EXAMPLE 351
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-(2-hydroxyethyl)-8-(3-(4-
mornholin~ propoxylp~rrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXIV; Ar=2-
chlorophenyl,
R1°=CH~CH~OCH~, ~4-morpholinyl) (184)
Demethylation of (183) prepared as decribed in example 350 with BBr3 using the
procedure described in example 80 except that the reaction conditions were 0
°C for 2 h gave
(184) (36 %) as a yellow solid, mp 253-255 °C.'H NMR 8 [(CD3)2S0] 10.97
(br s, IH), 8.94
(br s, 1H), 8.34 (s, 1H), 7.69 (s, 1H), 7.56 (dd, J=8.1, 2.2 Hz, 1H), 7.49-
7.42 (m, 3H), 7.29 (s,
1H), 4.83 (br, IH), 4.49 (t, J=5.2 Hz, 2H), 4.18 (t, J=6.2 Hz, 2H), 3.76 (m,
2H), 3.60 (t, J=4.9
Hz, 4H), 2.52 (t, J=7.1 Hz, 2H), 2.41 (m, 4H), 1.99 (m, 2H). FARMS found
[M+H]+:
552.1732, 550.1740. C29H29C1N306 requires 552.1715, 550.1745.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-214-
EXAMPLE 352
The preparation of 8-(3-Bromopropoxy)-2-(3-bromopropyl)-9-methoxy-6-methyl-4-
phenylnyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXI; Ar=phenyl, R'°=Me,
n=3) (722).
Alkylation of (154) prepared as described in example 254 with 1,3-
dibromopropane
(3.0 equiv.) using the procedure described in example 298 (at room temperature
for 4 d) and
after chromatography on silica gel (dichloromethane/petroleum ether) (3:1)
gave (722) (47
%) as an orange solid, mp 158-160 °C.'H NMR 8 [(CD3)2S0] 8.50 (s, 1H),
7.76 (s, 1H),
7.69-7.64 (m, 2H), 7.51-7.43 (m, 3H), 7.39 (s, 1H), 4.31(t, J=6.0 Hz, 2H),
3.98 (s, 3H), 3.92
(s, 3H), 3.77-3.71(m, 4H), 3.59 (t, J=6.5 Hz, 2H), 2.41-2.34 (m, 2H), 2.23-
2.15 (m, 2H).
Found: C, 57.16; H, 4.58; N, 4.73. Cz8H26N2Br2O4. 1/2 hexane requires: C,
56.64; H, 5.06; N,
4.26. FABMS found M+: 612.0255, 614.0249, 616.0252. CZ8HZ6Br2Nz04 requires
612.0259.
614.0239, 616.0218.
EXAMPLE 353
The~reparation of 8-(3-Bromopropoxy)-2-(3-bromopropyl)-4-(2-chloronhenyl)-9-
methoxy-
6-meth~pyrrolo[3,4-clcarbazole-1,3(2H,6H)-dione (LXI; Ar=2-chlorophenyl,
Rl°=Me, n=3)
7( 23).
Alkylation of (160) prepared as described in example 260 with 1,3-
dibromopropane
(15 equiv.) using the procedure described in example 298 (at room temperature
for 5 d) and
after chromatography on silica gel (dichloromethane/petroleum ether) (3:1)
gave (723) (65
%) as an orange solid, mp 165-167 °C.'H NMR 8 [(CD3)ZSO] 8.47 (s, 1H),
7.76 (s, 1H),
7.60-7.57 (m, 1H), 7.53-7.43 (m, 3H), 7.41 (s, 1H), 4.31(t, J=6.0 Hz, 2H),
3.97(s, 3H), 3.93
(s, 3H), 3.77-3.68 (m, 4H), 3.56 (t, J=6.5 Hz, 2H), 2.42-2.34 (m, 2H), 2.20-
2.12 (m, 2H).
Found: C, 53.54; H, 4.28; N, 4.20. CZ$HZSNZC1Br204. 1/2 hexane requires: C,
53.82; H, 4.66;
N, 4.05. FABMS found M+: 645.9866, 647.9868, 649.9834. Cz8Hz5N2CIBr~04
requires
645.9870, 647.9849, 647.9840, 649.9829, 649.9820.
EXAMPLE 354
The preparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-2-(3-hydroxypropyl)-
9-
methoxy-6-methylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXV; Ar=2-
chlorophenyl,
R1°=Me, n=3) (724).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-215-
Alkylation of (160) prepared as described in example 260 with 3-bromopropanol
(2.2
equiv.) using the procedure described in example 298 (at room temperature for
31 h) and
after chromatography on silica gel (chloroform/methanol) (20:1) gave (724) (98
%) as an
orange solid, mp 180-183 °C.'H NMR 8 [(CD3)ZSO] 8.47 (s, 1H), 7.74 (s,
1H), 7.60-7.57 (m,
1H), 7.53-7.43 (m, 3H), 7.36 (s, 1H), 4.60 (t, J=5.1 Hz, 1H), 4.50(t, J=5.0
Hz, 1H), 4.25 (t,
J=6.3 Hz, 2H), 3.96 (s, 3H), 3.92 (s, 3H), 3.67-3.60 (m, 4H), 3.47-3.41 (m,
1H), 2.02-1.95
(m, 2H), 1.79-1.71 (m, 2H). Found: C, 64.03; H, 5.21; N, 5.25. CZBHZ~NZC106
requires C,
64.31; H, 5.20; N, 5.36.
EXAMPLE 355
The~reparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-9-methoxy-6- .
meth~pyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXVI; Ar=2-chlorophenyl,
R'°=Me, n=3)
7( 25).
Treatment of (724) prepared as described in example 354 with the sequence of
reactions (SM KOH/MeOH/reflux/3h; 1N HCl/100 °C/3 h; NH40Ac/150
°C/20 min) as
described in The procedure described in example 328 gave (725) (85%) as an
orange solid,
mp 285-287 °C. 1H NMR 8 [(CD3)ZSO] 11.02 (br, 1H), 8.46 (s, 1H), 7.72
(s, 1H), 7.59-7.55
(m, 1H), 7.51-7.42 (m, 3H), 7.34 (s, 1H), 4.60 (t, J=S.1 Hz, 1H), 4.25 (t,
J=6.3 Hz, 2H),
3.95(s, 3H), 3.91(s, 3H), 3.67-3.61 (m, 2H), 2.02-1.95 (m, 2H). Found: C,
64.69; H, 4.60; N,
6.11. CZSH2iNaC105 requires: C, 64.31; H, 5.20; N, 5.36.
EXAMPLE 356
Thepreparation of 8-f3-(Dimethylamino)propoxyl-2-f3-(dimethylamino)propyll-9-
methox~
6-methyl-4-phenylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXII; Ar=phenyl,
R'°=Me,
Z=NMe~~n=3) (726).
Reaction of (722) prepared as described in example 352 with excess
dimethylamine.HCl in DMF in the presence of triethylamine and 4A molecular
sieves.at
room temperature (Method 30 ?? ) for 66 h gave (726) (35%) as the
dihydrochloride salt, mp
278-281 °C (dec). 1H NMR 8 [(CD3)zS0] ] 9.95 (br, 2H), 8.51 (s, 1H),
7.81 (s, 1H), 7.70-7.65
(m; 2H), 7.52-7.43 (m, 3H), 7.40 (s, 1H), 4.28 (t, J=6.1 Hz, 2H), 3.99 (s,
3H), 3.93 (s, 3H),
3.67 (t, J=6.4 Hz, 2H), 3.28 (t, J=7.6 Hz, 2H), 3.10 (t, J=7.6 Hz, 2H), 2.83
(s, 6H), 2.73 (s,
6H), 2.29-2.20 (m, 2H), 2.26-1.97 (m, 2H). Found: C, 59.07; H, 6.68; N, 8.44.
Cs2HaoNaClzOa requires: C, 58.98; H, 6.81; N, 8.60.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-216-
EXAMPLE 357
The orenaration of 4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxyl-2-[3-
(dimethylamino)prop. 11-~~-6-methylpyrrolof 3,4-clcarbazole-1,3(2H,6H)-dione
(LXII~ Ar=2-chlorophenyl, R'°=Me, ~NMe~,, n=3) (727).
Reaction of (723) prepared as described in example 353 with excess
dimethylamine.HCl in DMF in the presence of triethylamine and 4A molecular
sieves at
room temperature (Method 30 ??) for 6 d followed by chromatograpy on silica
gel
(chloroform/methanol/triethylamine) (10:1:0.1) gave (727) (63 %) as the
dihydrochloride
salt, mp 207-210 °C.'H NMR b [(CD3)ZSO] 9.89 (br, 2H), 8.48 (s, 1H),
7.79 (s, 1H), 7.61-
7.56 (m, 1H), 7.53-7.44 (m, 3H), 7.41 (s, 1H), 4.28 (t, J=6.0 Hz, 2H), 3.97
(s, 3H), 3.94 (s,
3H), 3.65(t, J=6.6 Hz, 2H), 3.23 (br, 2H), 3.34 (br, 2H), 2.80 (s, 6H), 2.69
(s, 6H), 2.28-2.19
(m, 2H), 2.03-1.92 (m, 2H). Found: C, 58.09; H, 6.13; N, 8.44. C32H37N4C1O4
.2.2 HCl
requires: C, 58.47; H, 6.01; N, 8.52. Found: C, 59.07; H, 6.68; N, 8.44.
C32H4°N4C12O4
requires: C, 58.98; H, 6.81; N, 8.60.
EXAMPLE 358
The preparation of 4-(2-Chlor~henyl)-8-f3-(dimethylamino)propoxyl-2-f3-
(dimethylamino)propyll-9-methoxy-6-methylpyrrolo(3,4-clcarbazole-1,3(2H,6H)-
dione
ILXII; Ar=2-chlorophenyl, R'°=Me Z=NMe~in=3) (727).
On a larger scale (727)was best prepared from (160) prepared as described in
example
260 by reaction with 3-dimethylaminopropylchloride hydrochloride (7.3 equiv.)
in the
presence of excess anhydrous potassium carbonate and 4A molecular sieves at 60-
70 °C (bath
temperature) for 3 h. The yield of crude product was 96 %, which was used for
the next step
without further purification.
EXAMPLE 359
The preparation of 4-(2-Chlorophenyl)-8-f3-(dimethylamino)propoxyl-9-methoxy-6-
methylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXIII; Ar=2-chlorophenyl,
R'°=Me,
Z=NMe2, n=3) (728).
Treatment of (727) prepared as described in example 358 with the sequence of
reactions (SM KOH/MeOH/reflux/3 h; 1N HCl/100 °C/3 h; NH40Ac/170
°C/10 h) as
described in Example 328 , with the modification that after acidic treatment
the reaction
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-217-
mixture was evaporated to dryness and fused with NHdOAc, followed by
chromatography on
silica (dichloromethane/MeOH=5:1) gave (728) (97 %) as an orange solid, mp 288-
290 °C.
1H NMR 8 [(CD3)zS0] 11.04 (br, 1H), 8.46 (s, 1H), 7.72 (s, 1H), 7.59-7.55 (m,
1H), 7.51-
7.42 (m, 3H), 7.33 (s, 1H), 4.21(t, J=6.5 Hz, 2H), 3.95(s, 3H), 3.91(s, 3H),
2.43(t, J=7.0 Hz,
2H), 2.18 (s, 6H), 2.01-1.93 (m, 2H). ). Found: C, 68.22; H, 6.57; N, 7.43.
CZ~H26N3CIO4.
hexane requires: C, 68.58; H, 6.97; N, 7.27. FABMS found [M+H]+: 492.1689,
494.1675.
CZ~HZ~N3CIOa requires 492.1690, 494.1661.
EXAMPLE 360
The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-methyl-8-f3-(4-
mor-pholinyl)propox~pyrrolof3,4-clcarbazole-1,3(2H;6H)-dione (LXIII; Ar=2-
chlorophenyl,
R'°=Me.-Z=4-momholinvl, n=3) (729).
Conversion of (725) prepared as described in example 355 to the corresponding
mesylate by reaction with methanesulfonyl chloride using the procedure
described in
example 170 example 170 followed by reaction with morpholine using the
procedure
described in example 179, and treatment of the crude product with NH40Ac at
160-170 °C
for 2 h, gave (after a silica column; chloroform/MeOH=10:1) (729) (76 %) as an
orange
powder, mp 293-295 °C. 1H NMR 8 [(CD3)ZSO] 11.04 (br, 1H), 8.47 (s,
1H), 7.72 (s, 1H),
7.59-7.55 (m, 1H), 7.52-7.42 (m, 3H), 7.34 (s, 1H), 4.22 (t, J=6.4 Hz, 2H),
3.96 (s, 3H), 3.91
(s, 3H), 3.59 (br t, J=4.6 Hz, 4H), 2.60-2.36 (m, 6H), 2.03-1.95 (m, 2H).
FABMS found
[M+H]+: 534.1792, 536.1769. Cz9Ha9N3C105 requires 534.1796, 536.1766.
EXAMPLE 361
"The preparation of 4-(2-Chlorophenyl)-9-methoxy-6-methyl-8-f3-(1-
Qyrrolidinyl)propoxylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (LXIII; Ar=2-
chloro~hen ~Ll,
R'°=Me, Z=1-nvrrolidinvl, n=3) (730).
Conversion of (725) prepared as described in example 355 to the corresponding
mesylate by reaction with methanesulfonyl chloride using the procedure
described in
example 170 followed by reaction with pyrrolidine using the procedure
described in example
179 of Scheme 3, and treatment of the crude product with NH40Ac at 160-170
°C for 4 h,
gave (after a silica column; chloroform/MeOH=10:1) (730) (78 %) as an orange
solid, 273-
275 °C.'H NMR 8 [(CD3)2S0] 11.03 (br, 1H), 8.45 (s, 1H), 7.73 (s, 1H),
7.59-7.55 (m, 1H),
7.52-7.42 (m, 3H), 7.34 (s, 1H), 4.22(t, J=6.4 Hz, 2H), 3.95(s, 3H), 3.90 (s,
3H), 2.60 (t,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-218-
J=7.0 Hz, 2H), 2.42 (m, 4H), 2.03-1.96 (m, 2H), 1.73-1.67 (m, 4H). Found: C,
66.40; H, 5.47;
N, 7.85. C29Hz$N3C104.1/2H20 requires C, 66.09; H, 5.55; N, 7.97.
EXAMPLE 362
The~reparation of 8-f3-(Dimethylamino)propoxyl-9-methoxy-6-methyl-4-
phenylpyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXIII; Ar=2-phenyl,
R'°=Me, Z=NMe~s
n=3) (731 ).
Treatment of (726) prepared as described in example 356 with the sequence of
reactions (SM KOH/MeOH/reflux/3 h; 1N HCl/100 °C/3 h; NH40Ac/170
°C/10 h) in the
proceedure described example 328 with the modification that after acidic
treatment the
reaction mixture was evaporated to dryness and fused with NH40Ac, gave (731)
(37%), after
a silica column (dichloromethane/MeOH=5:1) as a hygroscopic solid,'H NMR 8
[(CD3)ZSO]
11.04 (br, 1H), 8.48 (s, 1H), 7.75 (s, 1H), 7.68-7.62 (m, 2H), 7.51-7.42 (m,
3H), 7.33 (s, 1H),
4.21(t, J=6.9 Hz, 2H), 3.97 (s, 3H), 3.90 (s, 3H), 2.43 (t, J=7.1 Hz, 2H),
2.19 (s, 6H), 2.01-
1.93 (m, 2H). LCMS(APCI) m/z: 458.101 [M+H]+.
EXAMPLE 363
The preparation of 4-(2-Chlorophenyl)-8-[3-(dimethylamino)propoxyl-9-hydroxy-6-
meth~pyrrolo~3,4-clcarbazole-1,3(2H,6H)-dione (LXIV; Ar=2-chlorophenyl,
R1°=Me,
Z=NMe~, n=3) (732).
Demethylation of (728) prepared as described in example 359 with BBr3 using
the
procedure described in example 80 except that the reaction conditions were 0
°C for 2 h gave
(732) (83%) as an orange solid, mp 259-262 °C. 'H NMR 8 [(CD3)ZSO]
10.99 (s, 1H), 9.22
(br, 1H), 8.35 (s, 1H), 7.70 (s, 1H), 7.58-7.54 (m, 1H), 7.51-7.42 (m, 3H),
7.29 (s, 1H), 4.22
(t, J=6.0 Hz, 2H), 3.92 (s, 3H), 2.89 (br, 2H), 2.50 (br, 6H), 2.13-2.03 (m,
2H). FABMS
found [M+H]+: 478.1532, 480.1519. CZ6H2sN3C104 requires 478.1534, 480.1504.
EXAMPLE 364
The preparation of 8-(3-(Dimethylamino)propoxyl-9-hydroxy-6-meth ~~l-4-
phenylpyrrolof3,4-
c~,carbazole-1,3(2H,6H)-dione (LXIV; Ar=phenyl, RI°=Me, Z=NMe~, n=3)
(733).
Demethylation of (731) prepared as described in example 362 with BBr3 using
the
procedure described in example 80 except that the reaction conditions were 0
°C for 2 h gave
(733) (56 %), mp 303-304 °C . 'H NMR 8 [(CD3)ZSO) 11.00 (s, 1H), 9.00
(br, 2H), 8.39 (s,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-219-
1H), 7.73 (s, 1H), 7.66-7.61 (m, 2H), 7.50-7.41 (m, 3H), 7.29 (s, 1H), 4.26
(t, J=5.7 Hz, 2H),
3.94 (s, 3H), 3.24 (t, J=7.1 Hz, 2H), 2.80 (s, 6H), 2.23-2.14 (m, 2H). Found:
C, 60.61; H,
5.05; N, 7.82. CZ6HasNsOa requires: C, 60.48; H, 5.06; N, 8.14.
EXAMPLE 365
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-methyl-8-(3-(4-
morpholinyl)propoxylpyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXIV; Ar=2-
chlorophenyl,
R'°=Me, Z=4-morpholinyl, n=3) (734).
Demethylation of (729) prepared as described in example 360 with BBr3 using
the
procedure described in example 80 except that the reaction conditions were 0
°C for 2 h gave
(734) (60 %) as an orange solid; mp 235-237 °C .'H NMR 8 [(CD3)ZSO]
10.98 (s, 1H), 8.97
(br, 1H), 8.33-(s, 1H), 7.69 (s, 1H), 7.59-7.54 (m, 1H), 7.51-7.42 (m, 3H),
7.28 (s, 1H), 4.21
(t, J=6.1 Hz, 2H), 3.92 (s, 3H), 3.61 (br, 4H), 2.58-2.37 (m, 6H), 2.05-1.95
(m, 2H). FABMS
found [M+H]+: 520.1648, 522.1635. C28HZ~N3C105 requires 520.1639, 522.1610.
EXAMPLE 366
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-6-methyl-8-(3-(1-
pyrrolidinyl)propoxylpyrrolo(3,4-clcarbazole-1,3(2H,6H)-dione (LXIV; Ar=2-
chlorophenyl,
Rl°=Me, Z=1-pyrrolidinyl, n=3) (735).
Demethylation of (730) prepared as described in example 361 with BBr3 using
the
procedure described in example 80 except that the reaction conditions were 0
°C for 2 h gave
(735) (60%) as an orange solid, mp 234-236 °C.'H NMR 8 [(CD3)ZSO] 10.98
(br, 1H), 9.27
(br, 1H), 8.33 (s, 1H), 7.68 (s, 1H), 7.58-7.55 (m, 1H), 7.51-7.42 (m, 3H),
7.30 (s, 1H), 4.20
(t, J=6.2 Hz, 2H), 3.92 (s, 3H), 2.66 (t, J=6.8 Hz, 2H), 2.54-2.35 (m, 4H),
2.04-1.96 (m, 2H),
1.75-1.68 (m, 4H). FABMS found [M+H]+: 504.1687, 506.1661. CZ$Hz~N3C104
requires
504.1690, 506.1661.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-220-
H
O N O
Mep ArCH2PPh3X/LiN(TMS)2 Maleimide neat 180~C
\ METHOD 31 M~ ~ Me0 ~ Ar
CHO \ METHOD 5
RB / Y, or ArCH2PPh3X/LDA I / y \ Ar or I \
METHOD 2 RB ' Maleimide/toluene R / y,
(LXVII) (LXVIII) METHOD 4a 8
(LXIX)
N
O O O N O
DDO
METHOD 6 M~ ~ ~ \ ~ Ar BBra HO ~ ~ \ / Ar
or / ~ METHOD 8 / i
Mn02 Re Y' Re Y,
METHOD 7
(LXX) (LXXI)
SCHEME 14
Procedures for Scheme 14
Representative Procedure for Method 31 of Scheme 14
EXAMPLE 367
The preparation of 5-Methoxv-2-f (E,Z)-2-phenvlethenvll-1-benzofuran (LXVIII:
Ar=nhenvl
R8=H, Y'=O) (828)
To a suspension of benzyltriphenylphosphonium bromide (1.85 g, 4.26 mmol) in
tetrahydrofuran (30 mL) was added a solution of lithium
bis(timethylsilyl)amide (4 mL of a
1 M solution in tetrahydrofuran, 3.98 mmol), the solution turned a bright
orange/red color.
The reaction mixture was stirred at room temperature for 30 min and then a
solution of 5-
methoxy-1-benzofuran-2-carbaldehyde (827) (0.50 g, 2.84 mmol) in
tetrahydrofuran (10 mL)
was added. After 20 min water was then added and the tetrahydrofuran removed
at reduced
pressure. The residue was extracted with ethyl acetate (2 x 50 mL), the
combined extracts
were dried and concentrated. The residue was purified by column chromatography
on silica
eluting with dichloromethane to give (828) as a 1:2 mixture of Z- and E-
isomers, (0.63 g,
89°10), m.p. 124-128°C. Found: C, 81.60; H, 5.61. C»H~402
requires: C, 81.58, H, 5.64.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-221-
EXAMPLE 368
The preparation of 9-Methoxy-4-phenyl-IH-fllbenzofurof3,2-elisoindole-1,3(2H)-
dione
(LXX; Ar=phenyl, Rg=H, Y'=O) (830)
The dime mixture (828) prepared as described in example 367 was reacted with
maleimide using the procedure described in example 69 to give the adduct
(LXIX;
Ar=phenyl, R8=H, Y'=O) (829), which was used without further purification. The
crude
Diels-Alder adduct was aromatised with Mn02 using the procedure described in
example 79
of Scheme 2 to give the dibenzofuran (830) as a yellow solid (53%), mp 271-
275°C. 1H
NMR 8 [(CD3)ZSO] 11.41 (br s, 1H), 8.24 (d, J = 2.7 Hz, 1H), 7.98 (s, 1H),
7.77 (d, J = 9.0
Hz, 1H), 7.67-7.65 (m, 2H), 7.51-7.45 (m, 3H), 7.30 (dd, J = 9.0, 2.7 Hz, 1H),
3.91 (s, 3H).
Found: C, 72.09; H, 3.84; N, 4.04. CZ~H13N04.1/3 H20 requires: C, 72.20; H,
3.94; N, 4.01.
EXAMPLE 369
The preparation of 9-Hydroxy-4-phenyl-IH-(llbenzofuro13,2-elisoindole-1,3(2H)-
dione
(LXXI; Ar=phenyl, R$=H, Y'=O) (831)
Demethylation of (830) prepared as described in example 368 with BBr3 using
the
procedure described in example 80 gave (831) as a yellow solid (100%), mp 288-
290°C.'H
NMR 8 [(CD3)ZSO] 11.37 (br s, 1H), 9.74 (s, 1H), 8.13 (d, J = 2.6 Hz, 1H),
7.94 (s, 1H),
7.66-7.63 (m, 3H), 7.51-7.44 (m, 3H), 7.11 (dd, J = 8.9, 2.6 Hz, 1H). Found:
C, 71.17; H,
3.48; N, 4.07. CZOH~1N04.1/2 Hz0 requires: C, 71.00; H, 3.58; N, 4.14.
EXAMPLE 370
The preparation of 2-f(E,Z)-2-(2-Chlorophenyl)ethenyll-5-methoxy-1-benzofuran
(LXVIII;
Ar=2-chlorophenyl, R$=H, Yl=O 832
Reaction of 5-methoxy-1-benzofuran-2-carbaldehyde (827) prepared as described
in
example with 2-chlorobenzyltriphenylphosphonium bromide using the procedure
described in
method gave (832) as a 1:2 mixture of Z:E isomers (24%), mp 90-92 °C,
which was used
without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-222-
EXAMPLE 371
The preparation of 4-(2-Chlorophenyl)-9-methoxy-1H-(l lbenzofuro(3,2-
elisoindole-1,3(2H)-
dione (LXX~ Ar=2-chlorophenyl, R$=H, Y'=O) (834)
The dime mixture (832) prepared as described in example 370 was reacted with
maleimide using the procedure described in example 69 to give the adduct
(LXIX; Ar=2-
chlorophenyl, R8=H, YI=O) (833), which was used without further purification.
The crude
Diels-Alder adduct was aromatised with MnOz using the procedure described in
example 79
of Scheme 2 to give the dibenzofuran (834) as a yellow solid (49 %), mp 246-
248°C. 1H
NMR 8 [(CD3)2S0] 11.46 (br s, 1H), 8.22 (d, J = 2.7 Hz, 1H), 7.99 (s, 1H),
7.80 (d, J = 9.0
Hz, 1H), 7.61-7.58 (m, 1H), 7.53-7.44 (m, 3H), 7.33 (dd, J = 9.0, 2.7 Hz, 1H),
3.92 (s, 3H).
Found: C, 66.53; H, 3.41; N, 3.54. CZ,H~ZCIN04 requires: C, 66.77; H, 3.20; N,
3.71.
EXAMPLE 372
The Qreparation of 4-(2-Chlorophenyl)-9-hydroxy-1H-(llbenzofuro(3,2-
elisoindole-1,3(2H)-
dione (LXXI; Ar=2-chlorophenyl, R8=H, Yl=O) (835)
Demethylation of (834) prepared as described in example 371 with BBr3 using
the
procedure described in example 80 gave (835) as a yellow solid (89%), mp 140-
145°C. 1H
NMR b [(CD3)aS0] 11.39 (br s, 1H), 9.78 (s, 1H), 8.12 (d, J = 2.6 Hz, 1H),
7.93 (s, 1H), 7.66
(d, J = 8.9 Hz, 1H), 7.60-7.58 (m, 1H), 7.52-7.43 (m, 3H), 7.13 (dd, J = 8.9,
2.6 Hz, 1H);
EIMS found M+: 363.0294, 365.0269. C2oH1oC1N04 requires: 363.0298, 365.0289.
EXAMPLE 373
The preparation of 2-((E,Z)-2-(2-Chlorophenyl)ethenyll-5-methoxy-1-
benzothiophene
øXVIII; Ar=2-chlorophenyl, R8=H, Y1=S) (837)
Reaction of 5-methoxy-1-benzothiophene-2-carbaldehyde (836) with 2-
chlorobenzyltriphenylphosphonium chloride using the procedure described in
example 37
gave the dime (837) as an E/Z mixture (63 %).'H NMR b (CDC13) Minor isomer:
7.6 (d,
1H), 7.4 (m, 5H), 7.15 (s, 1H), 7.05 (s, 1H), 6.9 (d, 1H), 6.75 (m, 2H), 3.85
(s, 3H). Major
isomer: 7.75 (d, J=9 Hz, 1H), 7.55 (m, 2H), 7.25-7.4 (m, 3H), 7.18 (m, 2H),
7.05 (s, 1H), 6.95
(m, 2H).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-223-
EXAMPLE 374
The preparation of 4-(2-Chlorophenyl)-9-methoxy-1H-(llbenzothienof3,2-
elisoindole-
1,3(2H)-dione.(LXX; Ar=2-chloroRhenyl, R$=H, Y'=S) (839)
Reaction of (837) prepared as described in example 373 with maleimide using
the
procedure described in method4 except that the reaction time was 6 days gave
the adduct
(LXIX; Ar=2-chlorophenyl, R$=H, Y'=S) (838) which was used without further
purification.
Aromatisation of (838) with DDQ using the procedure described in example 70 of
Scheme 2
except that the solvent was chloroform, and the reaction conditions were 5
days at 40 °C gave
(839) as a yellow solid (44 %).'H NMR 8 [(CD3)ZSO] 11.46 (s, 1H), 9.37 (d, J=
2.5 Hz, 1H),
8.38 (s, 1H), 8.2 (d, J=9 Hz, 1H), 7.62 (m, 2H), 7.48 (m, 3H), 7.31 (dd, J=
2.5, 9.0 Hz, 1H),
3.94 (s, 3H).
EXAMPLE 375
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-1H-(llbenzothieno(3,2-
elisoindole-
1,3(2H)-dione (LXXI; Ar=2-chlorophenyl, Rs=H, Y'=S) (840).
Demethylation of (839) prepared as described in example 374 with BBr3 using
the
procedure described in example 80 except that the reaction time was 48 h gave
(840) (65 %)
a.s a yellow solid, mp 311-313 °C.'H NMR 8 [(CD3)ZSO] 11.42 (s, 1H),
9.85 (s, 1H), 9.17 (d,
,1= 2.5 Hz, 1H), 8.32 (s, 1H), 7.9 (d, J= 9 Hz, 1H), 7.62 (m, 2H), 7.5 (m,
3H), 7.17 (dd, J=2.5,
9 Hz, 1H). MH+ 346. Found C, 68.75; H, 3.43; N, 3.89; S, 9.35. CzoH1~N03SØ2
Hz0
requires: C, 68.83; H, 3.29; N, 4.01; S, 9.19.
EXAMPLE 376
The preparation of 6-(Benzyloxy)-2-((E,Z)-2-(chlorophenyl)ethenyll-5-methoxy-1-
benzofuran (LXVIII; R8 =OCH~Ph, Y'=O, Ar = 2-chlorophenyl) (601)
Reaction of 6-(benzyloxy)-5-methoxy-1-benzofuran-2-carbaldehyde (LXVIII; Y'=O,
R$=OCHZPh) with 2-chlorobenzyltriphenylphosphonium chloride using the
procedure
described in method 2 with a reaction time of 4 hours gave (601) as a pale
yellow solid
(83%), mp 130-135 °C. 'H NMR 8 [(CD3)ZSO] 7.88 (d, J= 6.4 Hz, 1H), 7.51-
7.31 (m, 12H),
7.18 (s, 1H), 6.93 (s, 1H), 5.17 (s, 2H), 3.78 (s, 3H). EIMS found:
M+=390.1021. C24H19C1O3
requires 390.1023.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-224-
EXAMPLE 377
The preparation of 8-(Benzyloxy)-4-(2-chlorophenyl)-9-methoxy-1H-
fllbenzofurof3,2-
elisoindole-1,3(2H)-dione (LXX; R8 = OCH~Ph, Y)=O, Ar = 2-chlorophen 1~3)
Compound (602) (LXIX; R$=OCHZPh, Y)=O, Ar=2-chlorophenyl) was prepared from
(601) using the procedure described in method 4a using xylene as the solvent
to give a brown
solid which was used without further purification. The crude Diels-Alder
adduct was
aromatised with Mn02 using the procedure described in example 79, to give
(603) as a bright
yellow solid (24%), mp 296-300 °C. )H NMR 8 [(CD3)ZSO] 11.38 (s, 1H),
8.17 (s, 1H), 7.94
(s, 1H), 7.67 (s, 1H), 7.58-7.36 (m, 13H), 5.28 (s, 2H), 3.93 (s, 3H). EIMS
found:
M+=483.0871. CZBH)gC1N05 requires 483.0873.
H
O N O
H O H N O
0 0 N 0 O 1. CH'SOzCI/Et'N Me0 ~ \ / Ar
HO Ar BBr~ _ ~ ZH ~ METHOD 11 I
w \ / ~ ETHOD 8 Me0 ~ Ar ~ -Me0 ~ Ar ~
\ / METHOD 12 I \ / 2. Nal/65 °C Z O
Z(CH )n I ~ O or Py.HCU200 ~C I , 0 Z(CH )n ~ 0 METHOD 27
z METHOD Z(CHz)n z (LXXV)
(LXXVII) (LXXVI)
(LXXVIII) Hz/PtOz
METHOD 26~
H
N O 0 N 0 N O O N O
0 n-Bu~SnCH=CHZ -
Ar -c' HC~ cMeO ~ ~ / ArTIzO/pyridine Me0 ~ \ / Ar Pd(PPh ) LiCI Me0 I ~ \ /
Ar
Me0 ~ \ / 100 °C I METHOD 24 ~ METHOD 25 ~ i O
I , METHOD 18 HO i O CFzSOzO ~ O Z
PhCHpO O
(LXX; R=OCH=Ph) (LXXII) (LXXIII) (LXXIV)
Br(CHz)nBr/KZCO9
METHOD 20
(CHzn)Br (CHz)nR N H
0 O 0 N 0
0 0 O O 1. SN KOH _ _
_ 2. 1N HCI Me0 Ar BBr3 HO Ar
Me0 ~ \ / Ar Z~MeO ~ \ / Ar 3.NH,OAC/.0°C I ~ \ / MET I
METHOD12 I , METHOD28 ~ O ~ O
Q Hz)~ Q Hz)~
O
(CHz)nZ
(CHz)n8r 0
(LXXIX) (LXXX) (LXXXI) (LXXXII)
SCHEME 15
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-225-
Scheme 15 Procedures
EXAMPLE 378
The preparation of 4-(2-Chlorophenyl)-8-hydroxy-9-methoxy-1H-f llbenzofurof3,-
elisoindole-1,3(H)-dione (LXXII; Ar = 2-chlor~henyl) (604)
Removal of the benzyl ether group of (603) prepared as described in example
377
using the procedure described in example 260 gave (604) as a yellow solid
(86%), mp 294-
298 °C.'H NMR 8 [(CD3)ZSO] 11.34 (s, 1H), 10.40 (s, 1H), 8.12 (s, 1H),
7.87 (s, 1H), 7.59-
7.30 (m, 4H), 7.23 (s, 1H), 3.96 (s, 3H). EIMS found: M+=393.0400. CZIH~zC1N05
requires
393.0404.
EXAMPLE 379
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-1,3-dioxo-2,3-dihydro-1H-
jllbenzofurof3,2-elisoindol-8-yl trifluoromethanesulfonate (LXXIII; Ar = 2-
chlorophenyl)
6( O5) _ ___ _ _
Compound (605) was prepared from (604) using the procedure described in
example
307 as a pale brown solid (88%), mp 237-240 °C.'H NMR 8 [(CD3)ZSO]
11.55 (s, 1H), 8.47
(s, 1H), 8.29 (s, 1H), 8.11 (s, 1H), 7.62-7.45 (m, 4H), 4.07 (s, 3H). FABMS
found:
[M+H]+=525.9958, 527.9940. Cz2H12C1F3NS0~ requires 525.9975, 527.9946.
EXAMPLE 380
The preparation of 4-(2-Chlorophenyl)-8-ethyl-9-methoxy-1H-fllbenzofurof3,2-
elisoindole-
1,3(2H)-dione (LXXV; Z=H, Ar = 2-chlorophenyl) (606)
Compound 606 was prepared from (605) prepared as described in example 379
using
the procedure described in example 309 and tetraethyl tin as the stannane, as
a yellow solid
(87%), mp 252-257 °C.'H NMR 8 [(CD3)ZSO] 11.45-11.15 (br, 1H), 8.16 (s,
1H), 7.91 (s,
1H), 7.60-7.56 (m, 1H), 7.54 (s, 1H), 7.52-7.41 (m, 3H), 4.23 (s, 3H), 3.92
(m, 2H), 2.16-2.09
(m, 3H). EIMS found: M+=405.0766. C23H16C1N04 requires 405.0768.
EXAMPLE 381
The preparation of 4-(2-Chlorophenyl)-8-ethyl-9-hydroxy-1H-f llbenzofurof3,2-
elisoindole-
1,3(2H)-dione (LXXVIII; n = 2, Z=H, Ar = 2-chloronhenyl) (607)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-226-
Demethylation of (606) prepared as described in example 380 via The procedure
described in
example 80 gave (607) as a yellow solid (32%), mp 265-268 °C.'H NMR 8
[(CD3)zS0] 8.13
(s, 1H), 7.68 (s, 1H), 7.57-7.52 (m, 3H), 7.44-7.31 (m, 4H), 2.85 (q, J=7.6
Hz, 2H), 1.35 (t,
J=7.6 Hz, 3H). EIMS found: M+=391.0612. CZZHiaC1N04 requires 391.0611.
EXAMPLE 382
The preparation of 4-(2-Chlorophenyl)-8-((lE)-4-hydroxy-1-butenyll-9-methoxy-
1H-
f llbenzofurof3 2-elisoindole-1 3(2H)-dione (LXXIV; Z = CH2CH~OH, Ar = 2-
chlorophenyl)
(608)
Compound 608 was prepared from triflate (605) prepared as described in example
379
using the procedure described in example 309 and (3E)-4-(tributylstannyl)-3-
buten-1-of as
the stannane as a yellow solid (84%), mp 247-250 °C.'H NMR 8 [(CD3)ZSO]
11.43 (s, 1H),
8.23 (s 1H), 7.92 (s, 1H), 7.86 (s, 1H), 7.58 (d, J=6.5 Hz, 1H), 7.48-7.42 (m,
3H), 6.85 (d,
J=16.0 Hz, 1H), 6.53 (m, 1H), 4.35 (br, 1H), 3.97 (s, 3H), 3.57 (t, J=6.6 Hz,
2H), 2.41 (m,
2H). EIMS found: M+=447.0886. CZSH~8C1N05 requires 447.0873.
EXAMPLE 383
The preparation of 4-(2-Chloroghenyl)-8-(4-hydroxybutyl)-9-methoxy-1H-
(llbenzofuro[3,2-
elisoindole-1 3(2H)-dione (LXXV; Z = CH~CHiOH, Ar = 2-chlorophenyl) (609)
Hydrogenation of (608) prepared as described in example 382 using the
procedure
described in example 310gave (609) as a yellow solid (96%), mp 158-162
°C.'H NMR 8
[(CD3)ZSO] 11.40 (s, 1H), 8.29 (s, 1H), 7.71 (s, 1H), 7.60 (s, 1H), 7.56-7.39
(m, 4H), 4.36
(br, 1H), 3.95 (s, 3H), 3.39 (m, 2H), 2.75 (t, J=7.6 Hz, 2H), 1.65 (m, 2H),
1.51 (m, 2H).
FABMS found: [M+H]+=450.1083, 452.1078. CZSHziC1N05 requires 450.1108,
452.1079.
EXAMPLE 384
The preparation of 4-(2-Chlorophenyl)-9-h d~oxy-8-(4-hydroxybutyl)-1H-f
llbenzofurof3,2-
elisoindole-1 3(2H)-dione (LXXVIII~ n = 4 Z = OH Ar =2-chlorophenyl) (610)
Demethylation of (609) prepared as described in example 383 using the
procedure
described in example 80 gave (610) as a yellow solid (38%), mp 256-259
°C.'H NMR 8
[(CD3)ZSO] 11.40 (br, 1H), 9.80 (s, 1H), 8.13 (s, 1H), 7.90 (s, 1H), 7.60-7.56
(m, 1H), 7.54
(s, 1H), 7.52-7.42 (m, 3H), 4.38 (s, 1H), 3.44 (m, 2H), 2.72 (t, J=7.4 Hz,
2H), 1.66 (m, 2H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-227-
1.50 (m, 2H). FABMS found: [M+H]+=436.0942, 483.0922. Cz4H~9C1N05 requires
436.0952,
438.0915.
EXAMPLE 385
The Qreparation of 8-(3-Bromopropoxy)-2-(3-bromopropyl)-4-(2-chlorophenyl)-9-
methoxy-
1H-(llbenzofurof3 2-elisoindole-1 3(2H)-dione (LXXIX; Ar=2-chlorophenyl, n=3)
(331)
Reaction of phenol (604) (245 mg, 0.62 mmol) prepared as described in example
378
with 1,3-dibromopropane (excess, 3.0 mL) according to the procedure described
in example
298, except that the reaction was performed in refluxing acetone (80 mL), gave
dibromide
(331) (300 mg, 76%) as a yellow powder, mp 180-182 °C.'H NMR S
[(CD3)zS0] 8.16 (s,
1H), 7.96 (s, 1H), 7.60 (m, 2H), 7.53-7.44 (m, 3H), 4.26 (t, J=6.0 Hz, 2H),
3.95 (s, 3H), 3.72
(t, J=6.4 Hz, 4H), 3.57 (t, J=6.6 Hz, 2H), 2.35 (m, 2H), 2.17 (m, 2H). Found:
C, 51.25; H,
3.52; N, 2.37. Cz~HzzBrzC1N05 requires: C, 51.01; H, 3.49; N, 2.20.
EXAMPLE 386
The preparation of 4-(2-Chlorophenyl)-8-f3-(dimethylamino)propoxyl-9-methoxy-
1H-
(llbenzofurof3 2-elisoindole-1 3(2H)-dione (LXXXh Ar=2-chlorophenyl, n=3,
Z=N(CH~)?)
3~
Reaction of dibromide (331) (100 mg, 0.16 mmol) prepared as described in
example
385 with aqueous dimethylamine solution (40%, 5.0 mL) according to The
procedure
described in example 179, except that the reaction was performed in
tetrahydrofuran (50 mL)
at room temperature for 30 hours, gave the crude diamine (LXXX; Ar=2-
chlorophenyl, n=3,
Z=N(CH3)z), which was used without further purification as a tetrahydrofuran
solution. To
this solution was added 5N potassium hydroxide (2.5 mL), then the procedure
outlined in
example 328 was followed except that the HCl treatment was for 24 hours and
the
chromatography was performed eluting with
methanol/dichloromethane/concentrated
ammonia (15:85arace). Trituration from ethyl acetate gave amine (332) (64 mg,
84%) as a
yellow powder, mp 251-253 °C. IH NMR 8 [(CD3)zS0] 11.37 (br s, 1H),
8.15 (s, 1H), 7.93
(s, 1H), 7.59 (m, 1H), 7.55 (s, 1H), 7.52-7.43 (m, 3H), 4.18 (t, J=6.4 Hz,
2H), 3.93 (s, 3H),
~2.5 (obscured m, 2H), 2.23 (br s, 6H), 1.96 (m, 2H). Found: C, 65.03; H,
4.79; N, 6.00.
CzsHz3C1NzO5 requires: C, 65.21; H, 4.84; N, 5.85.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-228-
EXAMPLE 387
The preparation of 4-(2-Chloronhenyp-9-methoxy-8-f3-(1-pyrrolidinyl)propoxyl-
1H-
jl lbenzofurof 3 2-elisoindole-1 3(2H)-dione (LXXXI~ Ar=2-chlorophenyl, n=3,
Z=1-
pyrrolidinyl) (333)
Reaction of dibromide (331) (110 mg, 0.17 mmol) prepared as described in
example
385 with pyrrolidine (361 uL, 4.33 mmol) according to The procedure described
in example
179 , except that the reaction was performed in tetrahydrofuran (50 mL) at
room temperature
for 3 days, gave the crude diamine (LXXX; Ar=2-chlorophenyl, n=3, ~1-
pyrrolidinyl),
which was used without further purification as a tetrahydrofuran solution. To
this solution
was added SN potassium hydroxide (2.5 mL), then the procedure outlined in
example 328
was followed except that the HCl treatment was for 24 hours and the
chromatography was
performed eluting with methanol/dichloromethane/concentrated ammonia
(15:85arace).
Crystallisation from ethyl acetate/hexane, gave amine (333) (51 mg, 59%) as a
yellow
powder, mp 252-255 °C.'H NMR S [(CD3)ZSO] 11.35 (br s, 1H), 8.15 (s,
1H), 7.91 (s, 1H),
7.58 (m, 1H), 7.54 (s, 1H), 7.52-7.43 (m, 3H), 4.19 (t, J=6.4 Hz, 2H), 3.93
(s, 3H), 2.57 (t,
J=7.1 Hz, 2H), 2.46 (m, 4H), 1.97 (m, 2H), 1.69 (m, 4H). Found: C, 66.46; H,
5.15; N, 5.43.
- - -CZ8H25C1N205 requires C, 66.60; H, 4.99; N, 5.55.
EXAMPLE 388
The preparation of 4-(2-Chlorophenyl)-8-f3-(dimethylamino)propoxyl-9-hydroxy-
1H-
jllbenzofuro(3 2-elisoindole-1 3(2H)-dione (LXXXII~ Ar=2-chlorophenyl, n=3,
Z=N(CH~)
334
Demethylation of amine (332) (70 mg, 0.15 mmol) prepared as described in
example
386 according to the procedure described in example 80, except that the
reaction was
performed at 0 °C for 8 hours and the chromatography was performed
eluting with
methanol/dichloromethane/triethylamine (15:85arace), gave amine (334) (48 mg,
71%) as a
yellow powder, which was converted to the hydrochloride salt, mp 255-258
°C.'H NMR 8
[(CD3)ZSO] 11.34 (br s, 1H), 9.96 (br s, 1H), 9.44 (br s, 1H), 8.10 (s, 1H),
7.91 (s, 1H), 7.58
(m, 1H), 7.51-7.43 (m, 4H), 4.23 (t, J=5.9 Hz, 2H), ~3.3 (obscured m, 2H),
2.81 (s, 6H), 2.21
(m, 2H). Found: C, 58.86; H, 4.68; N, 5.35. C25HZ~C1NZOS.HC1.1/2Hz0 requires:
C, 58.84; H,
4.54; N, 5.49.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-229-
EXAMPLE 389
The preparation of 4-(2-Chlorophenyl)-9-hydroxy-8-f3-(1-pyrrolidinyl)propoxyl-
1H-
f l lbenzofuro(3 2-elisoindole-1 3(2H)-dione (LXXXII~ Ar=2-chlorophenyl, n=3,
Z=1-
pyrrolidinyl) (335)
Demethylation of amine (333) (50 mg, 0.10 mmol) prepared as described in
example
387 according to the procedure described in example 80, except that the
reaction was
performed at 0 °C for 6 hours and the chromatography was performed
eluting with
methanol/dichloromethane/triethylamine (15:85arace), gave amine (335) ( mg, %)
as a
yellow powder, which was converted to the hydrochloride salt, mp 302-304
°C. 'H NMR 8
[(CD3)ZSO] 11.34 (s, 1H), 9.99 (br, 1H), 9.47 (br, 1H), 8.10 (s, 1H), 7.90 (s,
1H), 7.58 (m,
1H), 7.51 (s, 1H), 7.52-7.42 (m, 3H), 4.24 (t, J=5.7 Hz, 2H), 3.6 (br, 2H),
3.03 (br, 2H), 2.22
(m, 2H), 2.08-1.81 (m, 6H). Found: C, 61.18; H, 4.56; N, 5.16.
C27H23C1NZOS.HC1 requires
C, 61.49; H, 4.59; N, 5.31.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-230-
p N O
Ar
1) KOH 1) O~/-78 °C HO
2) HCI H 2) NaBH4 f O
p N O 3) NH40Ac/140 °C p N O ~ Rio
_ METHOD 28 (XCIV)
Ar ~ Ar Os04
NMMNO
p ~ ~ ~ O ~ ly METHOD 21 p N O
o Rio
(XCII) (XCIII) I ~ \ ~ Ar
ally) bromide HO O ~
K2COs/90 °C p Rio
METHOD 20 (XCV)
H H tetravinyltin
N Tf20 N O Pd(PPh~zCIZ O N O
p O pyridine p LiCI/100 °C
METHOD 24 - METHOD 25 Ar
~ ~ Ar I ~ \ ~ Ar
HO I ~ ~ Tf0 ~ ~ Rio
R'° (LXXXIII) (LXXXIV)
(XXXV)
1) 9-BBN Os04 1)O~/-78°C
2) NaOAc/H202 NMMNO 2) NaBH4
METHOD 29 METHOD 21
- - ~ H H H
O N O O N 0 O N O
I ~ ~ ~ Ar I ~ \ ~ Ar I ~ \ ~ Ar
bromopropanol HO ~ ~ HO ~ ty HO i
K2C0°l90 °C Rio OH R~o Rio
METHOD 20 (LXXXV) (LXXXVI) (LXXXVII)
OH
1 ) KOH H 1 ) MsCI/Et3N H
N 2) HCI N O METHOD 11 p N p
O O 3) NH40Ac/140 °C O 2) Nal
_ METHOD 27 Ar
Ar METHOD 28 ~ \ / Ar
HO~O I ~ K HO~O I ~ ~ I~O / R
Rio Rio (XC)
(LXXXVIII) (LXXXIX)
ZH
METHOD 12
p N O
Ar
SCHEME 16 Z ~ O I ~,
~~o
(XCI)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-231-
Scheme 16 Procedures
EXAMPLE 390
The preparation of 4-(2-Chlorophenyl)-6-methyl-1 3-dioxo-1,2,3,6-
tetrahydropyrrolol3,4-
clcarbazol-8-yl trifluoromethanesulfonate (LXXXIII~ Ar=2-chlorophenyl,
R'°=CHI) (316)
Reaction of phenol (845) (0.25 g, 0.66 mmol) prepared as described in example
280
according to the procedure described in example 307 gave triflate (316) (323
mg, 96%) as a
pale yellow solid, mp 230-233 °C. 1H NMR S [(CD3)zS0] 11.26 (br s, 1H),
9.04 (d, J=8.7 Hz,
1H), 8.03 (d, J=2.3 Hz, 1H), 7.94 (s, 1H), 7.59 (m, 1H), 7.50 (m, 4H), 4.04
(s, 3H). Found: C,
51.98; H, 2.29; N, 5.41. CzzHizClF3NaS05 requires C, 51.93; H, 2.38; N, 5.51.
EXAMPLE 391
The preparation of 4-(2-ChloroQhen~-6-methyl-8-vinylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (LXXXIV~ Ar=2-chlorophenyl, R'°=CHI) (317)
Reaction of triflate (316) (0.32 g, 0.63 mmol) prepared as described in
example 390
with tetravinyltin (172 OL, 0.94 mmol) according to the procedure described in
example 309
gave alkene (317) (193 mg, 79%) as a pale yellow solid, mp 276-282
°C.'H NMR 8
[(CD3)zS0] 11.13 (br s, 1H), 8.86 (d, J=8.2 Hz, 1H), 7.84 (m, 2H), 7.60-7.46
(m, 5H), 6.98
(dd, J=17.6, 10.9 Hz, 1H), 6.09 (d, J=17.6 Hz, 1H), 5.42 (d, J=10.9 Hz, 1H),
4.00 (s, 3H).
Found: C, 69.64; H, 4.03; N, 6.88. Cz3H~5C1N20z.1/2H20 requires: C, 69.79; H,
4.07; N,
7.08.
EXAMPLE 392
T_he preparation of 4-(2-Chlorophenyl)-8-(2-hydroxyethyl)-6-methylnyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (LXXXV~ Ar=2-chlorophenyl, R'°=CHI) (318)
Reaction of alkene (317) (60 mg, 0.16 mmol) prepared as described in example
391
according to to the procedure described in example 344 gave alcohol (318) (36
mg, 57%) as a
pale yellow solid, mp 289-292 °C. 1H NMR 8 [(CD3)zS0] 11.10 (br s, 1H),
8.81 (d, J=8.1 Hz,
1H), 7.82 (s, 1H), 7.58 (m, 2H), 7.49 (m, 3H), 7.27 (d, J=8.1 Hz, 1H), 4.73
(t, J=5.2 Hz, 1H),
3.97 (s, 3H), 3.75 (m, 2H), 2.99 (t, J=7.0 Hz, 2H). Found: C, 67.47; H, 4.37;
N, 6.51.
Cz3H1~C1NZO3.lI4H2O requires: C, 67.49; H, 4.31; N, 6.84.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-232-
EXAMPLE 393
The~reparation of 4-(2-Chlorophenyl)-8-(I 2-dihydroxyethyl)-6-
methylpyrrolof3,4-
clcarbazole-I 3(2H 6H)-dione (LXXXVI~ Ar=2-chlorophenyl, R'°=CHI 319
Reaction of alkene'(317) (50 mg, 0.13 mmol) prepared as described in example
391
according to the procedure described in example 300 gave diol (319) (40 mg,
74%) as a pale
yellow solid, mp 252-255 °C.'H NMR 8 [(CD3)zS0] 11.11 (br s, 1H), 8.84
(d, J=8.2 Hz,
IH), 7.84 (s, 1H), 7.71 (br s, 1H), 7.58 (m, IH), 7.49 (m, 3H), 7.39 (br d,
J=8.2 Hz, 1H), 5.46
(d, J=4.2 Hz, 1H), 4.79 (m, 2H), 3.98 (s, 3H), 3.57 (m, 2H). Found: C, 65.72;
H, 4.50; N,
6.32. C23H1~C1N~04 requires: C, 65.64; H, 4.07; N, 6.66.
EXAMPLE 394
The preparation of 4-(2-Chlorophenyl)-8-(hydroxymethyl)-6-methylpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (LXXXVIh Ar=2-chlorophenyl, R'°=CHI) (320)
Ozone was bubbled through a solution of alkene (317) (60 mg, 0.16 mmol)
prepared
as described in example 391 in methanol/dichloromethane (l :l, 40 mL) at -78
°C for 10
minutes, by which time the solution had gone from yellow to yellow/green.
Excess ozone was
purged from the solution by bubbling nitrogen through it for 2 minutes and
then a solution of
sodium borohydride (180 mg, 4.76 mmol) in methanol (20 mL) was added. The
resulting
solution was allowed to warm to room temperature over 20 minutes and then it
was diluted
with water and extraction with ethyl acetate. The organic phase was dried, the
drying agent
was removed and the solution was concentrated to dryness. Chromatography on
silica eluting
with ethyl acetate/hexane (3:1 to 1:0), followed by crystallization from ethyl
acetate/hexane,
gave alcohol (320) (39 mg, 62°10) as a pale yellow solid, mp 291-294
°C.'H NMR 8
[(CD~)ZSO] 11.11 (br s, IH), 8.85 (d, J=8.1 Hz, 1H), 7.84 (s, IH), 7.70 (br s,
1H), 7.58 (m,
1H), 7.49 (m, 3H), 7.35 (br d, J=8.1 Hz, 1H), 5.42 (t, J=5.6 Hz, IH), 4.77 (d,
J=5.6 Hz, 2H),
3.98 (s, 3H). Found: C, 67.74; H, 4.11; N, 7.13. CZZH~SC1N203 requires: C,
67.61; H, 3.87; N,
7.17.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-233-
EXAMPLE 395
The preparation of 4-(2-Chloro~henyl)-8-(3-hydroxypropoxy)-2-(3-hydroxypropyl)-
6-
methylpyrrolof3 4-clcarbazole-1 3(2H 6H)-dione (LXXXVIII: Ar=2-chlorophenyl,
R'°=CHI)
321
Reaction of phenol (845) (0.53 g, 1.41 mmol) prepared as described in example
280
with 3-bromopropan-1-of (280 ~L, 3.10 mmol) according to the procedure
described in
example 298 gave diol (321) (0.29 g, 42%) as a yellow powder, mp 136-140
°C. 'H NMR ~
[(CD3)ZSO] 8.77 (d, J=8.7 Hz, 1H), 7.76 (s, 1H), 7.58 (m, 1H), 7.49 (m, 3H),
7.28 (d, J=2.0
Hz, 1H), 7.00 (dd, J=8.7, 2.0 Hz, 1H), 4.61 (t, J=5.1 Hz, 1H), 4.49 (t, J=5.0
Hz, 1H), 4.24 (t,
J=6.3 Hz, 2H), 3.94 (s, 3H), 3.63 (m, 4H), 3.43 (m, 2H), 1.96 (m, 2H), 1.75
(m, 2H). Found:
C, 64.91; H, 5.13; N, 5.63. CZ~H25C1N204.1/4H20 requires C, 65.19; H, 5.17; N,
5.63.
EXAMPLE 396
The preparation of 4-(2-Chlorophenyl)-8-(3-hydroxypropoxy)-6-methylpyrrolo~3,4-
clcarbazole-1 3(2H,6H)-dione (LXXXIX: Ar=2-chlorophenyl, R'°=CHI) (322)
Reaction of diol (321) (270 mg, 0.55 mmol) prepared as described in example
395
according to the proceedure described example 328 , except that ethanol was
used instead of
acetonitrile and the HCl treatment was for 18 hours, gave crude material that
was dissolved in
methanol/dichloromethane (4:1, 80 mL), to which 1M potassium carbonate (2.0
mL) was
added (to hydrolyse a small amount of acetate present by tlc). The resulting
solution was
stirred at room temperature for 2 hours before being diluted with water and
extraction with
ethyl acetate. The organic phase was dried, the drying agent was removed and
the solution
was concentrated to dryness. Chromatography on silica eluting with ethyl
acetate/hexane
(2:1), followed by crystallization from ethyl acetate/hexane, gave alcohol
(322) (136 mg,
57%) as a yellow solid, mp 274-276 °C. 1H NMR 8 [(CD3)ZSO] 11.06 (br s,
1H), 8.75 (d,
:I=8.7 Hz, 1H), 7.75 (s, 1H), 7.57 (m, 1H), 7.48 (m, 3H), 7.27 (d, J=2.1 Hz,
1H), 7.00 (dd,
J=8.7, 2.1 Hz, 1H), 4.61 (t, J=4.9 Hz, 1H), 4.24 (t, J=6.3 Hz, 2H), 3.94 (s,
3H), 3.63 (m, 2H),
1.96 (m, 2H). Found: C, 66.01; H, 4.41; N, 6.41. C24H19C1Nz04 requires: C,
66.29; H, 4.40;
N, 6.44.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-234-
EXAMPLE 397
The preparation of 4-(2-Chlorophenyl)-8-(3-iodopropoxy)-6-methylpyrrolo(3,4-
clcarbazole-
1 3(2H 6H)-dione (XC~ Ar=2-chlorophenyl, Rl°=CHI) (323)
Reaction of alcohol (322) (82 mg, 0.19 mmol) prepared as described in example
396
according to the proceedure described in example 170followed using the
procedure described
in method27, gave after chromatography on silica eluting with ethyl
acetate/hexane (2:1),
iodide (323) (92 mg, 89%) as a yellow solid, mp 264-266 °C. 1H NMR 8
[(CD3)ZSO] 11.07
(br s, 1H), 8.77 (d, J=8.7 Hz, 1H), 7.76 (s, 1H), 7.58 (m, 1H), 7.47 (m, 3H),
7.31 (d, J=2.1
Hz, 1H), 7.02 (dd, J=8.7, 2.1 Hz, 1H), 4.23 (t, J=6.0 Hz, 2H), 3.95 (s, 3H),
3.46 (t, J=6.7 Hz,
2H), 2.30 (m, 2H). Found: C, 53:30; H, 3.27; N, 5.08. C24H,gC1INz03 requires:
C, 52.91; H,
3.33; N, 5.14.
EXAMPLE 398
The preparation of 4-(2-Chlorophenyl)-8-f3-(dimethylamino)propoxyl-6-
methylpyrrolo(3,4-
clcarbazole-1 3(2H 6H)-dione (XCI~ Ar=2-chlorophenyl R'°=CHI,
Z=N(CH~)2) (324)
Reaction of iodide (323) (50 mg, 0.09 mmol) prepared as described in example
397
with dimethylamine according to the procedure described in example 179 ,
except that the
reaction was performed at room temperature for 2 hours and the chromatography
was
performed on alumina (grade II-III) eluting with ethyl acetate/methanol (1:0
to 9:1) gave
amine (324) as a yellow powder, which was crystallized as the hydrochloride
salt (43 mg,
96%) from methanol/diethyl ether/hexane, mp 262-265 °C. 1H NMR 0
[(CD3)ZSO] 11.08 (br
s, 1H), 10.0 (br s, 1H), 8.78 (d, J=8.7 Hz, 1H), 7.78 (s, 1H), 7.58 (m, 1H),
7.48 (m, 3H), 7.29
(d, J=2.1 Hz, 1H), 7.02 (dd, J=8.7, 2.1 Hz, 1H), 4.26 (t, J=6.0 Hz, 2H), 3.96
(s, 3H), 3.28
(partially obscured m, 2H), 2.82 (s, 6H), 2.22 (m, 2H). Found: C, 59.62; H,
5.01; N, 8.02.
Ca6H2aC1NsOs~HC1.1.SH20 requires: C, 59.43; H, 5.37; N, 8.00.
EXAMPLE 399
_The preparation of 4-(2-Chlorophenyl)-6-methyl-8-f3-
(methylamino)propoxylpyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (XCI~ Ar=2-chlorophenyl, R'°=CHI, Z=NHCH~)
(325)
Reaction of iodide (323) (17 mg, 0.03 mmol) prepared as described in example
397
with aqueous methylamine solution (40%, 54 uL) according to The procedure
described in
example 179 , except that the reaction was performed at room temperature in
tetrahydrofuran
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-235-
for 20 hours, gave amine (325) (6 mg, 43%) as a yellow powder, mp 269-271
°C.'H NMR 8
[(CD3)ZSO] 8.75 (d, J=8.7 Hz, 1H), 7.76 (s, 1H), 7.57 (m, 1H), 7.47 (m, 3H),
7.27 (d, J=2.1
Hz, 1H), 8.99 (dd, J=8.7, 2.1 Hz, 1H), 4.22 (t, J=6.4 Hz, 2H), 3.94 (s, 3H),
2.67 (t, J=6.7 Hz,
2H), 2.32 (s, 3H), 1.94 (m, 2H). FABMS found: [M+H]+=448.1447, 450.1428.
CZSHZZC1N3O3
requires 448.1428, 450.1398.
EXAMPLE 400
The preparation of 4-(2-Chlorophenyl)-8-(3-(cis-3,5-dimethyl-1-
piperazinyl)propoxyl-6-
meth~pyrrolo(3 4-clcarbazole-1 3(2H 6H)-dione (XCI~ Ar=2-chlorophenyl,
R'°=CHI, Z=cis-
3 5-dimethyl-1-niperazinyl) (326)
Reaction of iodide (323) (17 mg, 0.03 mmol) prepared as described in example
397
with cis-2,6-dimethylpiperazine (71 mg, 0.62 mmol) according to The procedure
described in
example 179 , except that the reaction was performed at room temperature in
tetrahydrofuran
for 20 hours and the chromatography was performed on alumina (grade II-III)
eluting with
ethyl acetate/methanol (1:0 to 9:1), gave amine (326) (15 mg, 91%) as a yellow
powder, mp
225-227 °C.'H NMR S [(CD3)ZSO] 11.06 (br s, 1H), 8.75 (d, J=8.7 Hz,
1H), 7.76 (s, 1H),
7.57 (m, 1H), 7.47 (m, 3H), 7.25 (d, J=2.1 Hz, 1H), 8.99 (dd, J=8.7, 2.1 I-Iz,
1H), 4.19 (t,
J=6.3 Hz, 2H), 3.94 (s, 3H), 2.73 (m, 4H), 2.44 (t, J=7.1 Hz, 2H), 1.96 (m,
2H), 1.46 (t,
J=10.6 Hz, 2H), 0.92 (d, J=6.2 Hz, 6H). FABMS found: [M+H]+=531.2166,
533.2162.
C3°H3~C1N403 requires 531.2163, 533.2133.
EXAMPLE 401
The preparation of 8-(Allyloxy)-4-(2-chlorophenyl)-6-methylpyrrolof3,4-
clcarbazole-
1~3(2H 6H)-dione (XCIII~ Ar=2-chlorophenyl, R'°=CHI) (327)
Reaction of phenol (845) (250 mg, 0.66 mmol) prepared as described in example
280
with allyl bromide according to the procedure described in example 298 gave
the bis-allyl
derivative (XCII; Ar=2-chlorophenyl, R=CH3), which was used without further
purification.
Reaction of the crude material according to the proceedure described example
328 gave
alkene (327) (216 mg, 79%) as a yellow powder, mp 253-256 °C. 'H NMR D
[(CD3)ZSO]
11.07 (br s, 1H), 8.77 (d, J=8.7 Hz, 1H), 7.76 (s, 1H), 7.57 (m, 1H), 7.47 (m,
3H), 7.30 (d,
J=2.2 Hz, 1 H), 7.03 (dd, J=8.7, 2.2 Hz, 1 H), 6.20-6.11 (m, 1 H), 5.51 (m, 1
H), 5.33 (m, 1 H),
4.7'7 (m, 2H), 3.92 (s, 3H). Found: C, 69.42; H, 4.27; N, 6.50. C24H~7C1N203
requires: C,
69.15; H, 4.11; N, 6.72.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-236-
EXAMPLE 402
The preparation of 4-(2-Chlorophenyl)-8-(2-hydroxyethoxy)-6-methylpyrrolo(3,4-
clcarbazole-1 3(2H 6H)-dione (XCIV~ Ar=2-chlorophenyl, Rl°=CH~_) (328)
Ozone was bubbled through a solution of alkene (327) (SO mg, 0.12 mmol)
prepared
as described in example 401 in methanol/dichloromethane (l:l, 40 mL) at -78
°C for 10
minutes, by which time the solution had gone from yellow to yellow/green.
Excess ozone was
purged from the solution by bubbling nitrogen through it for 2 minutes and
then a solution of
sodium borohydride (136 mg, 3.60 mmol) in methanol (20 mL) was added. The
resulting
solution was allowed to warm to room temperature over 45 minutes and then it
was diluted
with water and extraction with ethyl acetate. The organic phase was dried, the
drying agent
was removed and the solution was concentrated to dryness. Chromatography on
silica eluting
with ethyl acetate/hexane (1:1 to 3:1), followed by trituration from diethyl
ether, gave alcohol
(328) (11 mg, 22%) as a yellow solid, mp 309-312 °C. 1H NMR 8
[(CD3)zSO] 11.06 (br s,
1H), 8.77 (d, J=8.7 Hz, 1H), 7.75 (s, 1H), 7.57 (m, 1H), 7.47 (m, 3H), 7.28
(d, J=2.0 Hz, 1H),
7.01 (dd, J=8.7, 2.0 Hz, 1H), 4.94 (t, J=5.4 Hz, 1H), 4.19 (t, J=5.1 Hz, 2H),
3.95 (s, 3H), 3.81
(m, 2H). FABMS found [M+H]+: 421.0932, 423.0912. Cz3H1~C1N204 requires
421.0955,
423.0926.
EXAMPLE 403
The preparation of 4-(2-Chlorophen~)-8-(2 3-dihydroxypropoxy)-6-
methylpyrrolo(3,4-
~carbazole-1 3(2H 6H)-dione (XCV~ Ar=2-chlorophenyl, Rl°=CHI) (329)
Reaction of alkene (327) (30 mg, 0.07 mmol) prepared as described in example
401
according to the procedure described in example 300 gave diol (329) (19 mg,
60%) as a
yellow solid, mp 287-290 °C.'H NMR S [(CD3)zS0] 11.06 (br s, 1H), 8.77
(d, J=8.7 Hz,
1H), 7.76 (s, 1H), 7.57 (m, 1H), 7.47 (m, 3H), 7.27 (d, J=2.2 Hz, 1H), 7.00
(dd, J=8.7, 2.2
Hz, 1H), 5.04 (d, J=5.1 Hz, 1H), 4.73 (t, J=5.6 Hz, 1H), 4.20 (dd, J=8.8, 4.5
Hz, 1H), 4.07
(dd, J=9.9, 6.1 Hz, 1H), 3.95 (s, 3H), 3.89 (m, 2H), 3.52 (t, J=5.6 Hz, 2H).
FABMS found
[M+H]+: 451.1052, 453.1039. Cz4H~9C1N205 requires 451.1061, 453.1031.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-237-
Me0
I \ \ CHO R_zM9X ~ Me0 \ \ O R~CHZPPh3X/LD~eO I \ \ / R,
/ N METHOD 32 ~ / reflux /
H H Rz METHOD 33 H Rz
(XCVI) (XCVII)
N O O N O N O
O O
Me0 R, Mn0 dioxane Me0 \ R~ BBr~DCM
Maleimide ~ \ ~ I ~ / MET O HO \ \ ~ R,
neaUT80~C I re lux
METHOD 5 ~ N Rz METHOD 7 ~ N Rz I
H H H Rz
(XCVIII) (XCIX) (C)
SCHEME 17
Procedures for Scheme 17
Representative Procedure for Method 32 of Scheme 17
EXAMPLE 404
The nrenaration of 1-(5-Methoxy-1H-indol-2-yl)ethanone (XCVI; R~=CHI 800
To a solution of the 5-methoxy-1H-indole-2-carbaldehyde (1) (2.0 g, 11.0 mmol)
in
tetrahydrofuran (30 mL) at 0°C was added a solution of methyl magnesium
bromide (11 mL
of a 3 M solution in ether, 34.0 mmol) dropwise. The cold-bath was removed and
the
reaction mixture allowed to warm to room temperature over 50 min. Saturated
ammonium
chloride was added and then the tetrahydrofuran was removed at reduced
pressure. The
residue was extracted with ethyl acetate (2 x 60 mL), the combined extracts
were washed,
dried, and concentrated. The crude alcohol was dissolved in chloroform (40 mL)
and
manganese dioxide (15 g, 0.171 mol) was added, the reaction mixture was heated
at reflux for
40 min. The mixture was filtered through Celite and then concentrated to give
an off-white
solid. The solid was purified by recrystallization from dichloromethane to
give (800) (1.80 g,
83°l0), mp 170-172 °C.'H NMR 8 [(CD3)ZSO] 11.58 (br s, 1H), 7.34
(d, J = 9.0 Hz, 1H), 7.23
(s, 1H), 7.12 (d, J = 2.4 Hz, 1H), 6.94 (dd, J = 9.0, 2.4 Hz, 1H), 3.77 (s,
3H), 2.52 (s, 3H).
Found: C, 69.77; H, 5.95; N, 7.54. C11H1,N02 requires: C, 69.83; H, 5.86; N,
7.40.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-238-
Representative Procedure for Method 33 of Scheme 17
EXAMPLE 405
The preparation of Methyl 2-~(E Z)-1-methyl-2-phenylethenyll-1H-indol-5-yl
ether (XCVII;
RZ=CH3;~R1=phenyl) (801)
To a suspension of benzyltriphenylphosphonium bromide (3.4 g, 7.9 mmol) in
tetrahydrofuran (30 mL) was added a solution of LDA (4.9 mI, of a 1.5 M
solution in
cyclohexane, 7.4 mmol). The red/orange reaction mixture was stirred for 10 min
and then a
solution of the ketone (1.0 g, 5.3 mmol) in tetrahydrofuran (15 mL) was added.
The mixture
was heated at reflux overnight and then water was added and the solvent was
removed at
reduced pressure. The organic material was extracted with ethyl acetate (3 x
50 mL). The
combined extracts were dried and concentrated. The residue was purified by
column
chromatography on silica eluting with dichloromethane to give methyl (801)
(0.26 g, 19%) as
a mixture of E- and Z-isomers, which was used without further purification.
EXAMPLE 406
The preparation of 9-Methoxy-5-methyl-4-phenylpyrrolo~3,4-clcarbazole-
1,3(2H,6H)-dione
~XCIX; R2=CHI, Rl=phenyl) (803).
Reaction of (801) prepared as described in example 405 with maleimide at 180
°C for
40 min using the procedure described in example 69 gave the adduct (XCVIII;
RZ=CH3,
Rl=phenyl) (802), which was used without further purification. The crude Diels-
Alder adduct
was aromatised with Mn02 using the procedure described in example 79 of Scheme
2 to give
(803) (69%).
EXAMPLE 407
The preparation of 9-Hydroxy-5-methyl-4~henylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
~C~ RZ=CHI, R'=phenyl) (804)
Demethylation of (803) prepared as described in example 406 with BBr3 using
the
procedure described in example 69 gave (804) (90%), mp 270-280°C.'H NMR
8
[(CD3)ZSOJ 11.60 (s, 1H), 10.82 (s, 1H), 9.22 (s, 1H), 8.31 (d, J = 2.0 Hz,
1H), 7.47-7.42 (m,
4H), 7.36-7.23 (m, 2H), 7.06 (dd, J = 8.7, 2.0 Hz, 1H), 2.32 (s, 3H). EIMS
found M+:
342.1005. Cz~H14Nz03 requires: 342.1004.
EXAMPLE 408
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-239-
The preparation of (5-Methoxy-1H-indol-2-yl)(phenyl)methanone (XCVI;
R2=phenyl) (805)
Reaction of 5-methoxy-1H-indole-2-carbaldehyde with phenylmagnesium bromide
using the procedure described in example 404 gave (805) (91%), mp 159-
161°C.'H NMR 8
[(CD3)ZSO] 11.86 (s, 1H), 7.94-7.91 (m, 2H), 7.71-7.66 (m, 1H), 7.61-7.57 (m,
2H), 7.42 (d,
J = 8.9 Hz, 1H), 7.16 (d, J = 2.4 Hz, 1H), 7.03 (s, 1H), 6.99 (dd, J = 8.9,
2.4 Hz, 1H), 3.77 (s,
3H). Found: C, 76.28; H, 5.21; N, 5.42. C~6H~3N0z requires: C, 76.48; H, 5.21;
N, 5.57.
EXAMPLE 409
The preparation of 2-f(E)-1,2-Diphenylethenyll-5-methoxy-1H-indole (XCVII;
R1=RZ=phen~ () 806)
To freshly washed magnesium turnings (0.29 g, 12 mmol) and an iodine crystal
in
ether (20 mL) was added benzyl chloride (1.4 mL, 12 mmol) at such a rate as to
maintain the
reaction mixture at reflux. After addition of the benzyl chloride was complete
the reaction
mixture was heated at reflux for a further 3 h, it was then added to a
solution of the ketone
(1.0 g, 3.98 mmol) in tetrahydrofuran (20 mL). The reaction mixture was
stirred at room
temperature for 1 h and then 2 M hydrochloric acid (2 mL) was added. The
mixture was
filtered and then the solvent was removed at reduced pressure. The crude oily
yellow alcohol
was dissolved in ethanol/tetrahydrofuran/2 M HCl (2:2:1) and stirred at room
temperature for
15 min, water was added and the organic solvents removed at reduced pressure.
The organic
material was extracted with ethyl acetate (3 x 50 mL), the combined extracts
were dried and
concentrated. The residue was purified by recrystallization from
dichloromethane (0.95 g,
73%), mp 144-147°C. 1H NMR 8 [(CD3)ZSO] 11.24 (s, 1H), 7.49-7.42 (m,
3H), 7.29-7.26
(m, 4H), 7.17-7.08 (m, 3H), 6.97-6.95 (m, 2H), 6.92 (d, J = 2.4 Hz, 1H), 6.75
(dd, J = 8.7, 2.4
Hz, 1H), 5.81 (s, 1H), 3.70 (s, 3H). Found: C, 84.30; H, 5.85; N, 4.36.
Cz3Hi9N0.1/10 HZO
requires: C, 84.43; H, 5.91; N, 4.28.
EXAMPLE 410
The preparation of 9-Methox -~phenylpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione
~XCIX; R'=RZ=phenyl) (808).
Reaction of (806) prepared as described in example 409 with maleimide at 180
°C
using the procedure described in example 69 gave the adduct (XCVIII;
Rl=RZ=phenyl)
(807), which was used without further purification. The crude Diels-Alder
adduct was
aromatised with MnOz using the procedure described in example 79 of Scheme 2
to give
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-240-
(808) (77%).'H NMR 1H NMR 8 [(CD3)zS0] 11'.08 (s, 1H), 11.02 (br s, 1H), 8.52
(d, J = 2.6
Hz, 1H), 7.50 (d, J = 8.9 Hz, 1H), 7.36-7.28 (m, 3H), 7.21-7.11 (m, 8H), 3.89
(s, 3H).
EXAMPLE 411
The preparation of 9-Hydroxy-4 5-diphenylpyrrolo(3,4-clcarbazole-1,3(2H,6H)-
dione (C;
R'=Rz=phenXl) (809)
Demethylation of (807) prepared as described in example 410 with BBr3 using
the
procedure described in example 80 gave (809) as a yellow powder (87%), mp
>300°C.'H
NMR 8 [(CD3)zS0] 10.96 (s, 1H), 10.94 (s, 1H), 9.23 (s, 1H), 8.37 (d, J = 2.4
Hz, 1H), 7.39
(d, J = 8.7 Hz, 1H), 7.35-7.29 (m, 3H), 7.22-7.10 (m, 7H), 7.03 (dd, J = 8.7,
2.4 Hz, 1H).
Found: C, 77.16; H, 4.00; N, 6.83. Cz6H~6N203 requires: C, 77.22; H, 3.99; N,
6.93.
EXAMPLE 412
The preparation of S-Methoxy-2-f(lE)-1-phenyl-1-propenyll-1H-indole (XCVII;
Rz=phenyl,
R'=CHI) (810)
Reaction of (805) prepared as described in example 408 with
ethyltriphenylphosphonium bromide using the procedure described in example 405
gave
(810) (72%), mp 128-130°C.'H NMR 8 [(CD3)zS0] 10.97 (s, 1H), 7.47-7.39
(m, 3H),
7.28-7.19 (m, 3H), 6.88 (d, J = 2.4 Hz, 1H), 6.69 (dd, J = 8.7, 2.4 Hz, 1H),
6.38 (q, J = 6.9
Hz, 1H), 5.69 (s, 1H), 3.69 (s, 3H), 1.67 (d, J = 6.9 Hz, 3H) Found: C, 81.78;
H, 6.26; N,
5.33. ClgHI~NO requires: C, 82.10; H, 6.51; N, 5.32.
EXAMPLE 413
The preQaration of 9-Methoxy-4-methyl-5 phenylpyrrolo[3,4-clcarbazole-
1,3(2H,6H)-dione
(XCVIII~ Rz=nhenyl, R'=CHI) (812)
Reaction of (810) prepared as described in example 412 with maleimide at 180
°C
using the procedure described in example 69 gave the adduct (XCIV; Rz=phenyl,
R'=CH3)
(811), which was used without further purification. The crude Diels-Alder
adduct was
aromatised with MnOz using the procedure described in example 79 of Scheme 2
to give
(812) (74%), mp 284-286°C.'H NMR 8 [(CD3)zS0] 11.06 (br s, 1H), 10.93
(s, 1H), 8.43 (d,
J = 2.6 Hz, 1H), 7.64-7.54 (m, 3H), 7.45-7.42 (m, 3H), 7.14 (dd, J = 8.9, 2.6
Hz, 1H), 3.32 (s,
3H), 2.48 (s, 3H). Found: C, 73.13; H, 4.32; N, 7.72. CzzHi6Nz03.1/3 Hz0
requires: C,
72.92; H, 4.64; N, 7.73.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-241-
EXAMPLE 414
Thepreparation of 9-Hydroxy-4-methyl-5-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(C~ Rz=phenyl, R'=CHI)
Demethylation of (812) prepared as described in example 413 with BBr3 using
the
procedure described in example 80 gave (813) (85%), mp >300°C. 1H NMR 8
((CD3)ZSO]
11.01 (s, 1H), 10.79 (s, 1H), 9.17 (s, 1H), 8.28 (d, J = 2.4 Hz, 1H), 7.34-
7.60 (m, 2H),
7.57-7.52 (m, 1H), 7.43-7.41 (m, 2H), 7.34 (d, J = 8.7 Hz, 1H), 6.98 (dd, J =
8.7, 2.4 Hz, 1H),
2.26 (s, 3H). EIMS found M+: 342.1003. CZ~H14N203 requires: 342.1004.
EXAMPLE 415
The preparation of 5-Methoxy-2-vinyl-1H-indole (XCVII; R1=RZ=H) (814)
Reaction of 5-methoxy-1H-indole-2-carbaldehyde with methyltriphenylphosphonium
bromide using the procedure described in method33 gave (814) (87%), mp 80-
81°C.'H
NMR 8 [(CD3)zS0] 11.09 (s, 1H), 7.21 (d, J = 8.7 Hz, 1H), 6.97 (d, J = 2.3 Hz,
1H),
6.75-6.66 (m, 2H), 6.37 (s, 1H), 5.76 (d, J = 17.3 Hz, 1H), 5.21 (d, J = 11.6,
1H), 3.73 (s,
3H). Found: C, 76.34; H, 6.24; N, 8.11. C~ 1H~ 1N0 requires: C, 76.28; H,
6.40; N, 8.09.
EXAMPLE 416
The preparation of 9-Methoxypyrrolof3 4-clcarbazole-1,3(2H,6H)-dione (XCIX;
R'=RZ=H)
16)
Reaction of (814) prepared as described in example 415 with maleimide at 180
°C
using the procedure described in example 69 gave the adduct (XCVIII; R=R'=H)
(814),
which was used without further purification. The crude Diels-Alder adduct was
aromatised
with MnOa using the procedure described in example 79 to give (815 ?? ) (76%),
mp
260-270°C.'H NMR 8 [(CD3)ZSO] 11.91 (s, 1H), 11.10 (s, 1H), 8.38 (d, J
= 2.6 Hz, 1H),
7.81 (d, J = 8.2 Hz, 1 H), 7.76 (d, J = 8.2 Hz, 1 H), 7.54 (d, J = 8.8 Hz, 1
H), 7.22 (dd, J = 8.8,
2.6 Hz, 1H), 3.88 (s, 3H). Found: C, 65.44; H, 3.96; N, 10.30. ClSH,oN203.1/2
H20 requires:
C, 65.45; H, 4.03; N, 10.18.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-242-
EXAMPLE 417
The preparation of 9-Hydroxypyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (C;
R'=RZ=H)
817
Demethylation of (816) prepared as described in example 416 with BBr3 using
the
procedure described in example 80 gave (817) (79%), mp 335-345°C.'H NMR
8 [(CD3)ZSO]
11.77 (s, 1H), 11.04 (s, 1H), 9.23 (s, 1H), 8.24 (d, J = 2.3 Hz, 1H), 7.75
(2d, J = 8.2 Hz, 2H),
7.43 (d, J = 8.7 Hz, 1H), 7.05 (dd, J = 8.7, 2.3 Hz, 1H). Found: C, 64.44; H,
3.45; N, 10.41.
C~4HgN203.1/2 H20 requires: C, 64.37; H, 3.47; N, 10.72.
EXAMPLE 418
The preparation of Methyl 2-(1-phen~vinyl)-1H-indol-5-yl ether (XCVII;
RZ=phenyl, R1=H)
8( 18)
Reaction of (805) prepared as described in example 408 with
methyltriphenylphosphonium bromide using the procedure described in example
405 gave
(818) (95%), mp 119-121°C. 1H NMR 8 [(CD3)zS0] 11.13 (s, 1H), 7.47-7.39
(m, 5H), 7.26
(d, J = 8.8 Hz, 1 H), 6.97 (d, J = 2.4 Hz, 1 H), 6.76 (dd, J = 8.8, 2.4 Hz, 1
H), 6.12 (s, 1 H), 5.77
(s, 1H), 5.30 (s, 1H), 3.72 (s, 3H). Found: C, 81.83; H, 6.22; N, 5.59.
C1~H15N0 requires: C,
81.90; H, 6.06; N, 5.62.
EXAMPLE 419
The preparation of 9-Methoxy-5-phen~pyrrolo(3 4-clcarbazole-1,3(2H,6H)-dione
(XCIX;
RZ=phenyl, R'=H) (820)
Reaction of (818) prepared as described in example 418 with maleimide at 180
°C
using the procedure described in example 69 gave the adduct (XCVIII; R=phenyl,
R'=H)
(819), which was used without further purification. The crude Diels-Alder
adduct was
aromatised with Mn02 using the procedure described in example 79 to give (820)
(73%), mp
281-285°C. 1H NMR 8 [(CD3)ZSO] 11.63 (br s, 1H), 11.15 (br s, 1H), 8.45
(d, J = 2.5 Hz,
1H), 7.78-7.76 (m, 2H), 7.68 (s, 1H), 7.65-7.62 (m, 2H), 7.57-7.53 (m, 2H),
7.22 (dd, J = 8.8,
2.5 Hz, 1H), 3.89 (s, 3H). Found: C, 72.06; H, 4.57; N, 7.69. CZ~H14N203.1/3
HZO requires:
C, 72.41; H, 4.24; N, 8.04.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-243-
EXAMPLE 420
The preparation of 9-Hydroxy-5-phenylpyrrolo(3 4-clcarbazole-1,3(2H,6H)-dione
(C'
RZ=phenyl, R'=H) (821)
Demethylation of 820 prepared as described in example 419 with BBr3 using the
procedure described in example 80 gave (821) (89%), mp 335-345°C.'H NMR
8 [(CD3)zS0]
11.50 (s, 1H), 11.10 (s, 1H), 9.26 (d, J = 2.4 Hz, 1H), 8.30 (d, J = 2.4 Hz,
1H), 7.77-7.75 (m,
2H), 7.65-7.61 (m, 3H), 7.56-7.52 (m, 1H), 7.46 (d, J = 8.7 Hz, 1H), 7.06 (dd,
J = 8.7, 2.4 Hz,
1H). Found: C, 72.71; H, 3.55; N, 8.16. CzoH12N203 requires: C, 73.16; H,
3.68; N, 8.53.
EXAMPLE 421
The preparation of 1-(5-Methoxy-1H-indol-2-yl)-1-propanone (XCVI; RZ=CH~CH~)
(822)
Reaction of 5-methoxy-1H-indole-2-carbaldehyde with ethylmagnesium bromide
using the procedure described in example 404 gave (822) (82%), mp 170-
171.5°C.'H NMR
S [(CD3)ZSO] 11.56 (s, 1H), 7.34 (d, J = 9.1 Hz, 1H), 7.22 (d, J = 1.7 Hz,
1H), 7.11 (d, J = 2.4
Hz, 1H), 6.93 (d, J = 9.1, 2.4 Hz, 1H), 3.77 (s, 3H), 2.96 (q, J = 7.3 Hz,
2H), 1.13 (t, J = 7.3
Hz, 3H). Found: C, 71.15; H, 6.45; N, 7.07. C12H~3N0z requires: C, 70.92; H,
6.45; N, 6.89.
EXAMPLE 422
The preQaration of 2-((E,Z)-1-Ethyl-2-phenylethenyll-5-methoxy-1H-indole
(XCVII;
Rz=CH?CH3, R'=phenyl) (823)
Reaction of (822) prepared as described in example 421 with
benzyltriphenylphosphonium bromide using the procedure described in example
404 gave
(823) (38%), mp 95-97°C. Found: C, 82.28; H, 6.98; N, 5.07. C19H~9N0
requires: C, 82.28;
H, 6.90; N, 5.05.
EXAMPLE 423
The preparation of 5-Ethyl-9-methoxv-4-phenylpyrrol~,4-clcarbazole-1,3(2H,6H)-
dione
(XCIX; RZ=CH~CH~, R'=phenyl) (825)
Reaction of (823) prepared as described in example 422 with maleimide at 180
°C
using the procedure described in example 69 gave the adduct (XCVIII; R=CH2CH3,
R'=phenyl) (824), which was used without further purification. The crude Diels-
Alder adduct
was aromatised with Mn02 using the procedure described in example 79 to give
(825) (68%),
mp 301-303°C.'H NMR b [(CD3)zS0] 11.78 (s, 1H), 10.87 (s, 1H), 8.47 (d,
J = 2.6 Hz, 1H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-244-
7.56 (d, J = 8.8 Hz, 1H), 7.48-7.41 (m, 3H), 7.32-7.30 (m, 2H), 7.23 (dd, J =
8.8, 2.6 Hz, 1H),
3.89 (s, 3H), 2.76 (q, J = 7.4 Hz, 2H), 1.07 (t, J = 7.4 Hz, 3H).
EXAMPLE 424
The preparation of 5-Ethyl-9-hydroxy-4-phenylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione (C;
RZ=CH~CH~, R'=phenyl) (826)
Demethylation of (825) prepared as described in example 423 with BBr3 using
the
procedure described in example 80 gave (826) (97%), mp 190-196°C. fH
NMR 8 [(CD3)ZSO]
11.63 (s, 1H), 10.81 (s, 1H), 9.21 (s, 1H), 8.31 (d, J = 2.4 Hz, 1H), 7.48-
7.40 (m, 4H),
7.31-7.29 (m, 2H), 7.06 (dd, J = 8.7, 2.4 Hz, 1H), 2.74 (q, J = 7.4 Hz, 2H),
1.06 (t, J = 7.4 Hz,
3H). Found: C, 72.43; H, 4.54; N, 7.54. CZZHf6N203.1/2 H20 requires: C, 72.32;
H, 4.69; N,
7.67.
PhCH20 \ \ ArCH2PPh3X/LDA ~ phCH O
CHO pp_65 ~C Z \
METHOD 2
a N Ar
{848) H
(CI)
H
N O
1. NaH PhCH20 \
2. F~~x ~ \ ~ Maleimide Ar
PhCH O \
METHOD 3 ~ N Ar toluene/reflux
(CII) Rio METHOD 4a
R, o
(CIII)
O H
O N O
DDQ/dioxane/reflux Pd-C/NHQOCHO
METHOD 6 -
PhCHzO \ \ / Ar METHOD 17 Ar
or MnO~/dioxane/reflux HO \
or
METHOD 7 ~ c. HCI/HOAG100 °C
METHOD 18
(CIV) (VI)
SCHEME 18
Procedures for Scheme 18
EXAMPLE 425
The preparation of 5-(Benzyloxy)-2-f(E,Z)-2-(2-methoxyphenyl)ethenyll-1H-
indole (CI;
Ar=2-methoxyphenyl) (847)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-245-
Reaction of 5-(benzyloxy)-1H-indole-2-carbaldehyde (846) W th 2-
methoxybenzyltriphenylphosphonium bromide using the procedure described in
example 37
gave (847) as a cream-colored solid (mixture of E/Z isomers) (89 %), which was
used
without further purification.'H NMR 8 [(CD3)ZSO] (major isomer) 11.26 (s; 1H),
7.62 (d,
J=6.8 Hz, 1H), 7.48 (m), 7.41-7.15 (m), 7.08-7.02 (m), 6.97 (m, 1H), 6.81 (dd,
J=8.7, 2.4 Hz,
1 H), 6.45 (s, l H), 5.08 (s, 2H), 3.87 (s, 3H).
EXAMPLE 426
1'he preparation of 5-(Benzyloxy)-2-f(E Z)-2-(2-methoxyphenyl)ethenyll-1-f2-(4-
morpholin ly )eth,yll-1H-indole (CII~ Ar=2-methoxyphenyl,
R'°=CH~CH2N(CH~CH~~O) (848)
Alkylation of (847) prepared as described in example 425 with 4-(2-
chloroethyl)morpholine using the procedure described in method 3 gave (848)
(87 %) as a
pale yellow solid, which was used without further purification.
EXAMPLE 427
The preparation of 9-(Benzyloxy)-4-(2-methoxyphenyl)-6-f2-(4-
morpholinyl)ethyllpyrrolof3,4-clcarbazole-1,3(2H,6H)-dione (CIV; Ar=2-
methoxyphenyl,
R'°=CH~CH2N(CH~CH~20) (850)
Reaction of (848) prepared as described in example 426 with maleimide using
the
procedure described in method 4a gave the adduct (CIII; Ar=2-methoxyphenyl,
Rl°=CH2CHzN(CHZCHZ)20) (849), which was used without further
purification.
Aromatisation of (849) with Mn02 using the procedure described in example 79
gave (850)
(36%) as a yellow solid, mp 170-172 °C.
EXAMPLE 428
The preparation of 9-Hydroxy-4-(2-methoxyphenyl)-6-[2-(4-
morpholinyl)ethyllpyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (VI' Ar=2-methoxyphenyl,
R1°=CH~CH~N(CH~CH~~O (851)
Removal of the benzyl ether group of (850) prepared as described in example
427 by
hydrogenolysis using the procedure described in example 254 gave (851) (66%)
as a yellow
powder, mp 262-264 °C.'H NMR 8 [(CD3)ZSO] 10.92 (br s, 1H), 9.31 (br s,
1H), 8.37 (d,
I=2.5 Hz, 1H), 7.69 (s, 1H), 7.54 (d, J=8.8 Hz, 1H), 7.42 (m, 1H), 7.33 (dd,
J=7.4, 1.7 Hz,
1H), 7.12-7.08 (m, 2H), 7.05 (m, 1H), 4.54 (t, J=6.3 Hz, 2H), 3.68 (s, 3H),
3.45 (t, J=4.4 Hz,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-246-
4H), 2..65 (t, J=6.3 Hz, 2H), 2.41 (br t, 4H). Found EIMS M+: 471.1789.
CZ~HZSN305
requires 471.1794.
EXAMPLE 429
The preparation of 5-(Benzyloxy)-2-((E)-2-f2-(2-methoxyethoxy)phenyllethenyl?-
1H-indole
(CI' Ar=2-(2-methoxyethoxy)phenyl) (852)
Reaction of 5-(benzyloxy)-1H-indole-2-carbaldehyde (846) with 2-(2-
methoxyethoxy)benzyltriphenylphosphonium bromide using the procedure described
in
example 37 gave (847) as a cream-colored solid (mixture of E/Z isomers) (86
%), which was
used without further purification.
EXAMPLE 430
The preparation of 9-(Benzyloxy)-4-f2-(2-methoxyethoxy)phenyllpyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (CIV; Ar=2-(2-methoxyethox~phenyl, R'°=H) (854)
Reaction of (852) with maleimide using the procedure described in method 4a
gave
the adduct (CIII; Ar=2-(2-methoxyethoxy)phenyl, R'°=H) (853), which was
used without
further purification. Aromatisation of (853) with Mn02 using the procedure
described in
example 79 gave (854) (46%) as an orange solid, mp 157-159 °C, which
was used without
further purification.
EXAMPLE 431
The preparation of 9-Hydroxy-4-f2-(2-methoxyethoxy)phenyllpyrrolof3 4-
clcarbazole-
1 3(2H 6H)-dione (VI; Ar=2-(2-methoxyethoxy)phenyl, R1°=H) (855).
Removal of the benzyl ether group of (854) prepared as described in example
430 by
hydrogenolysis using the procedure described in example 254 gave (855) (53 %)
as a yellow
powder, mp 275-279 °C.'H NMR 8 [(CD3)ZSO] 11.71 (br s, 1H), 10.89 (br
s, 1H), 9.22 (br s,
1H), 8.31 (d, J=2.2 Hz, 1H), 7.51 (s, 1H), 7.43 (d, J=8.5 Hz, 1H), 7.39 (m,
1H), 7.34 (dd,
J=7.5 Hz, 1H), 1.7 Hz, 1H), 7.10 (d, J=7.7 Hz, 1H), 7.05 (m, 2H), 4.04 (m,
2H), 3.45 (t, J=4.7
Hz, 2H), 3.10 (s, 3H). EIMS Found M+: 402.1213. C23H~$N205 requires 402.1215.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-247-
EXAMPLE 432
The preparation of (2-Methoxy-4-nitrobenzyl)(triphenyl)phosphonium bromide
(582)
Bromination of (2-methoxy-4-nitrophenyl)methanol with 30% HBr in acetic acid,
followed by reaction of the crude bromide with triphenylphosphine, using the
procedure
described in example 112, except that the conditions for the displacement were
3 days at 20
°C followed by 1 day at 55 °C, gave the phosphonium salt (582)
(84 %) as a yellow solid, mp
(CHZC12/benzene) 194-196 °C.'H NMR (CDC13) 8 7.83-7.63 (m, 17H), 7.45
(br s, 1H), 5.52 (d,
J=15.0 Hz, 2H). Found: C, 61.10; H, 4.73; N, 3.05. CZ6HzsBrNO3P requires C,
61.43; H, 4.56;
N, 2.76.
EXAMPLE 433
The preQaration of 5-(Benzyloxy)-2-f(E)-2-(2-methoxy-4-nitrophenyl)ethenyll-1H-
indole
(583) (CI, Ar=2-methoxy-4-nitrophenyl~
The aldehyde (846) was reacted with (2-methoxy-4-nitrobenzyl)
(triphenyl)phosphonium bromide (582) prepared as described in example 432
using the
procedure described in method 2, except that the LDA and aldehyde were
(sequentially)
added at 0 °C, the ratio of LDA:aldehyde was 1.5:1 and the reaction
time was 5 h, to give
(after crystallisation from CHZC12/pentane) the dime (583) as an orange solid
(the pure E
isomer) (63 %), mp 156-159 °C.'H NMR (CDC13) 8 8.23 (br s, 1H), 7.87
(dd, J=8.5, 2.1 Hz,
1H), 7.76 (d, J=2.2 Hz, 1H), 7.68 (d, J=8.6 Hz, 1H), 7.48 (d, J=7.3 Hz, 2H),
7.39 (t, J=7.3 Hz,
2H), 7.32 (t, J=7.2 Hz, 1H), 7.28 (d, J=17.1 Hz, 1H), 7.26 (d, J=8.7 Hz, 1H),
7.20 (d, J=16.7 Hz,
1H), 7.12 (d, J=2.4 Hz, 1H), 6.97 (dd, J=8.7, 2.5 Hz, 1H), 6.63 (br s, 1H),
5.11 (s, 2H), 4.01 (s,
3H). Found: C, 71.69; H, 4.94; N, 7.11. C24H2°Nz04 requires C, 71.99;
H, 5.03; N, 7.00.
EXAMPLE 434
The preparation of 9-(Benzyloxy)-4-(2-methoxy-4-nitrophenyl)-4,5,6,1Oc-
tetrahydropyrrolof3 4-clcarbazole-1 3(2H 3aH)-dione (584) (CIII, R'°=H,
Ar=2-methoxy-4-
nitrophenyl) and 9-benzyloxy-4-(2-methoxy-4-nitrophenyl)pyrrolof3,4-
clcarbazole-
1 3(2H 6H)-dione (585) (CIV, R'°=H, Ar=2-methoxy-4-nitrophenyl)
A foil-covered mixture of the pure E dime (583) (185 mg, 0.463 mmol) prepared
as
described in example 433 and maleimide (260 mg, 2.68 mmol) in dry toluene (3
mL) was
stirred in a sealed vial at reflux for 22 h (Method 4a). The resulting thick
suspension was
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-248-
transferred to a flask using dioxane (7 mL), then treated with manganese
dioxide (900 mg,
10.4 mmol), stirring at reflux for 24 h ( according to the procedure for
example 79), to give
(after workup) a mixture, which was adsorbed onto silica gel and
chromatographed. Elution
with 0-0.25% MeOH/CHZC12 gave foreruns, then further elution with 0.25-33%
MeOH/CHZC12 gave (after crystallisation from THF/CHZC12/pentane) the crude
product (585)
(64 mg). This was further purified by chromatography on silica gel (eluting
with 20%
EtOAc/petroleum ether) to give (after crystallisation from THF/CH2Clz/pentane)
the pure
material (585) (58 mg, 25%) as a yellow solid, mp 301-303 °C.'H NMR
[(CD3)ZSO] 8 11.97
(br s, 1 H), 11.08 (br s, 1 H), 8.54 (d, J=2.5 Hz, 1 H), 7.94 (dd, J=8.3, 2.2
Hz, 1 H), 7.88 (d, J=2.2
Hz, 1H), 7.64 (d, J=8.2 Hz, 1H), 7.63 (s, 1H), 7.58 (d, J=8.8 Hz, 1H), 7.56
(br d, J=7.1 Hz, 2H),
7.42 (t, J=7.4 Hz, 2H), 7.35 (t, J=7.4 Hz, 1H), 7.32 (dd, J=8.9, 2.7 Hz, IH),
5.23 (s, 2H), 3.83 (s,
3H). Found: C, 68.14; H, 3.89; N, 8.41. CZ8HI9N306 requires C, 68.15; H, 3.88;
N, 8.52.
Further elution with 1 % MeOH/CHZC12 gave (after crystallisation from
MeOH/CHZC12/petroleum ether) recovered adduct (584) (72 mg, 31%) as a pale
yellow solid
(which was aromatised directly with 5 equiv. DDQ using the procedure described
in example
70 to give further product in 79% yield),'H NMR ((CD3)zS0] 8 10.96 (br s, 1H),
10.86 (br s,
I H), 7.90 (dd, J=8.5, 2.3 Hz, 1 H), 7.78 (d, J=2.2 Hz, I H), 7.72 (d, J=8.6
Hz, 1 H), 7.50 (d, J=7.1
Hz, 2H), 7.40 (t, J=7.3 Hz, 2H), 7.34 (d, J=2.4 Hz, 1H), 7.33 (t, J=7.4 Hz,
1H), 7.21 (d, J=8.7
Hz, 1H), 6.80 (dd, J=8.7, 2.5 Hz, 1H), 5.09 (s, 2H), 4.27 (d, J=7.6 Hz, 1H),
3.98 (dd, J=7.6, 3.6
Hz, 1H), 3.97 (s, 3H), 3.54 (dt, J=12.9, 3.8 Hz, 1H), 3.24 (br dd, J=15.7,
13.5 Hz, 1H), 2.89 (dd,
J=15.4, 3.6 Hz, 1H)-. FABMS found: M+=497.1601. CZ8H23N306 requires 497.1587.
EXAMPLE 435
The preparation of 9-Hydroxy-4-(2-methoxy-4-nitrophenyl)pyrrolof3,4-
clcarbazole-
1.3(2H.6H1-dione (586) (VI, R'°=H, Ar=2-methoxv-4-nitronhenvll
A suspension of the benzyl ether (585) (55 mg, 0.112 mmol) prepared as
described in
example 434 in glacial AcOH (11.2 mL) was treated with concentrated HCl (6.7
mL of
36%), stirring at 100 °C for 50 min (Using the procedure described in
example 260 of Scheme
18). The cooled solution was added slowly to ice/aqueous NaHC03 (150 mL), then
extracted
with EtOAc (Sx 100 mL). The extracts were washed with water, then adsorbed
onto silica gel
and chromatographed. Elution with 0-1 % MeOH/CHZCl2 gave foreruns, then
elution with
1.5% MeOH/CHZC12 gave (after crystallisation from THF/CHzCIz/pentane) the
phenol (586)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-249-
(86 %) as an orange solid, mp 242-244 °C.'H NMR [(CD3)2S0] b 11.84 (br
s, 1H), 11.02 (br s,
1H), 9.28 (br s, 1H), 8.31 (d, J=2.4 Hz, 1H), 7.93 (dd, J=8.2, 2.1 Hz, 1H),
7.87 (d, J=2.1 Hz,
1 H), 7.63 (d, J=8.2 Hz, 1 H), 7.5 8 (s, 1 H), 7.45 (d, J=8.7 Hz, 1 H), 7.08
(dd, J=8.7, 2.4 Hz, 1 H),
3.83 (s, 3H). Found: C, 62.55; H, 3.22; N, 10.13. CZ~H~3N30~ requires C,
62.53; H, 3.25; N,
10.42.
EXAMPLE 436
The~reparation of 4-(4-Amino-2-methoxyphenyl)-9-hydroxypyrrolof3,4-clcarbazole-
1.3(2H.6H)-dione (587) (VI. R'°=H. Ar=4-amino-2-methoxvnhenvl)
A mixture of the nitro derivative (586) (31 mg, 0.0769 mmol) prepared as
described
in example 435 and freshly prepared (wet) nickel boride (242 mg) in MeOH (2.4
mL) and
1M HCl (0.6 mL) was stirred at reflux for 1 h. Six drops of conc. HCl were
added, then the
mixture was stirred at reflux for a further 1 h. Conc. aqueous ammonia and
aqueous NaHC03
(100 mL) were added and the mixture extracted with EtOAc (8x 100 mL). The
extracts were
concentrated, adsorbed onto silica gel and chromatographed. Elution with 0-2%
MeOH/CHZCIZ gave foreruns, then further elution with 2-3% MeOH/CHZC12 gave
(after
crystallisation from MeOH/THF/CHZC12/pentane) the amine (587) (77 %) as a
yellow-orange
solid, mp 308-316 °C.'H NMR [(CD3)ZSO] b 11.57 (br s, 1H), 10.79 (br s,
1H), 9.17 (br s, 1H),
8.29 (d, J=2.4 Hz, 1H), 7.43 (s, 1H), 7.39 (d, J=8.7 Hz, 1H), 7.02 (dd, J=8.7,
2.5 Hz, 1H), 6.95
(d, J=7.9 Hz, 1H), 6.30 (d, J=2.0 Hz, 1H), 6.22 (dd, J=8.0, 1.9 Hz, 1H), 5.26
(br s, 2H), 3.59 (s,
3H). Found: C, 67.65; H, 4.06; N, 11.01. CzlH~5N30a requires C, 67.56; H,
4.05; N, 11.25.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-250-
Me0
Me0 ~ CHO HBr(COOMe)Me0 ~ 1. LiAIH4 I \ \ HO
\ COOMe --
OH ~C03 I ~ O 2. Mn02 ~ O
Br Br Br
(856) (857) (858)
H H
~oec O N p O N O
rH oE~ Me0 ~ Maleimide
nr ~ \ xylene/SnCl2
MnOZ
NaH ~ ~ O~Ar METHOD 4 Me0 ~ ~ \ Ar ME O 7Me0 ~ ~ \ / Ar
METHOD 34 gr ~ O ~ O
(CV) Br Br
(CVI) (CVII)
H
N O
BBr,, HO Ar n-Bu3SnCH=CHZ
METHOD 8 ' ( \ \ / Pd(PPh3)ZCh/LiCI
METHOD 25
Br
(CVIII) , ,
Z
Os04/Nmemorpholine N-oxide
METHOD 21
H~/Raney nickel
Z
Z ~"~, (GXI)
SCHEME 19
Scheme 19 Procedures
EXAMPLE 437
~'he preparation of Methyl 7-bromo-5-methoxy-1-benzofuran-2-carboxylate (856)
To a solution containing 3-Bromo-2-hydroxy-5-methoxy-benzaldehyde (94.7 g,
0.410
mol j and dimethyl bromomalonate (103 g, 0.492 mol) in toluene (1.2 L) was
added freshly
powdered potassium carbonate (85 g, 0.615 mol) and tetra-n-butylammonium
bromide (13.2
g, 0.041 mol). The reaction was heated at reflux under a Dean-Stark trap for
48 h. The
reaction was concentrated and the residue dissolved with dichloromethane (~0.5
L) and
filtered through Celite, washing with dichloromethane (~0.5 L). The filtrate
was washed with
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-251-
1 L of 1M sodium hydroxide and water (2 x 1 L). The organic phase was then
passed
through a 400 g pad of silica gel washing with dichloromethane (~1 L) and the
filtrate was
concentrated to give the desired benzofuran (64 g, 56%) as an orange solid, mp
107-111 °C.
'H NMR 8 (CDC13) 7.50 (s, 1H), 7.23 (d, 1H), 7.03 (d, 1H), 3.98 (s, 3H), 3.82
(s, 3H).
EXAMPLE 438
The preparation of 7-Bromo-2-hydroxymethyl-5-methoxybenzofuran and 7-bromo-5-
methoxybenzofuran-2-carbaldehyde (858).
A solution containing ester (857) (69 g, 242 mmol) prepared as described in
example
856 in anhydrous tetrahydrofuran (2.5 L) was chilled to -78 °C. A 1M
lithium aluminum
hydride solution in tetrahydrofuran (242 mL, 242 mmol) was added over a 30 min
period.
The reaction was stirred at -78 °C for 1.25 h and was carefully
quenched with 50 mL of
water. The mixture was poured into 1 L of an ice-cold 1M hydrochloric acid
solution and
extracted with ethyl acetate (4 x 500 mL). The combined organic phases were
dried
(Na2S04) and concentrated in vacuo to give 67 g of an orange solid. This
residue was
adsorbed onto 200 g of silica gel and added to a column of silica gel (1 kg,
70-230 mesh) and
eluted with 1:9, 1:4, and 1:3 ethyl acetate-heptanes. This gave firstly 25 g
(41 %) of the final
aldehyde (858) as a pale yellow solid, mp 119-121 °C. 1H NMR 8 (CDC13)
9.90 (s, 1H), 7.55
(s, 1H), 7.38 (d, 1H), 7.10 (s, 1H), 3.85 (s, 3H). Found: C, 47.06; H, 2.66;
Br, 31.41.
CIOH~Br03 requires C, 47.09; H, 2.77; N, 31.33; followed by 20 g (33%) of the
expected
alcohol.'H NMR 8 (CDC13) 7.08(m,lH), 6.9(m,lH), 6.68(s,lH), 4.78(s, 2H),
3.85(s, 3H).
EXAMPLE 439
The preparation of 7-Bromo-5-methox~rbenzofuran-2-carbaldehyde (858)
A solution of the alcohol isolated above (560 mg, 2.18 mmol) prepared as
described
in example 858, tetrahydrofuran (20 mL) and ether (60 mL) was added manganese
dioxide
(1.9 g, 21.8 mmol). The reaction was stirred for 2 days and filtered through
Celite. The
filtrate was concentrated in vacuo and chromatographed on silica give 200 mg
(~50%) of the
desired aldehyde (858) (spectra as above).
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-252-
Representative Procedure for Method 34 of Scheme 19
EXAMPLE 440
Thepreparation of 7-Bromo-2-((E)-2-(2-chlorophenyl)ethenyll-S-methoxy-1-
benzofuran
(CV; Ar=2-chlorophenyl) (859)
To a solution of diethyl 2-chlorobenzylphosphonate (4.91 g, 0.018 mol) and 7-
bromo-
5-methoxybenzofuran-2-carbaldehyde (11.27 g, 0.016 mol) in tetrahydrofuran (80
mL) was
added sodium hydride (60% dispersion in mineral oil, 0.74 g, 0.0185 mol).
After 5 min. the
reaction mixture was warmed to 60 °C for 1 h. The reaction mixture was
cooled to room
temperature, water (10 mL) was carefully added and the tetrahydrofuran was
removed. Water
(50 mL) was added and the mixture was extracted with ethyl acetate (3 x
100mL). The
combined ethyl acetate extracts were washed with brine (50 mL), dried over
magnesium
sulphate, filtered and concentrated to dryness. Trituration with diethyl ether
(20 mL) gave
solid material which was further washed with diethyl ether (20m1). The liquors
were
concentrated and triturated with diethyl ether (10 mL), the solid being
collected. The
combined solids gave (859) (4.89 g, 84 %). 1H NMR 8 (CDC13) 7.70 (d, J= 16 Hz,
1H), 7.65
(dd, J= 7.7,1.5 Hz, 1 H), 7.4(dd, J= 6.0, 1 Hz, 1 H), 7.23 (m, 2H), 7.05 (d,
J= 2.3 Hz, 1 H),
6.94 (d, J= 16 Hz, 1H), 6.92 (d, J= 2.3 Hz, 1H), 6.72 (s, 1H), 3.82 (s, 3H);
MH+: 364.9,
362.9 MH-: 362.9, 360.9.
EXAMPLE 441
The preparation of 7-Bromo-4-(2-chlorophenyl)-9-methoxy-3a,4,5,1Oc-tetrahydro-
1H-
fllbenzofurof3,2-elisoindole-1,3(2H)-dione (CVI; Ar=2-chlorophenyl) (860)
A solution of (859) (4.89 g, 0.0185 mol) prepared as described in example 441,
maleimide (1.44 g, 0.02 mol) and tin(II) chloride (2.8 g, 0.02 mol) in xylenes
(40 mL) was
heated to 150 °C overnight. After the addition of more maleimide (1.44
g, 0.02 mol) and
tin(II) chloride (1.44 g, 0.01 mol) the reaction was again heated to 150
°C overnight. The
solid which formed was collected and washed with xylenes (20 mL) before
dissolving in
ethyl acetate (200 mL). Water (50 mL) was added and the mixture was filtered
through a pad
of Celite. The resulting layers were separated, the organic phase was washed
with brine (50
mL) dried over magnesium sulphate, filtered and concentrated to dryness. The
product (860)
(6.1g, 71 %) was used without further purification.'H NMR 8 (CDCI3) 7.60 (d,
J=6.5 Hz,
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-253-
1H), 7.40 (m, 2H), 7.25 (m, 1H), 7.1 (m, 2H), 4.36 (dd, J=7.3, 1 Hz, 1H), 4.0
(m, 1H), 3.86
(s, 3H), 3.81 (m, 1H), 3.45 (m, 1H), 3.10 (dd, J= 4.7, 17 Hz, 1H); MH+:
462,460.
EXAMPLE 442
The preparation of 7-Bromo-4-(2-chlorophenyl)-9-methoxy-1H-f llbenzofurof3,2-
elisoindole-1,3(2H)-dione (CVII; Ar=2-chlorophenyl) (861).
Aromatisation of (860) prepared as described in example442 with Mn02 using the
procedure described in example 79 gave the dibenzofuran (861) as a yellow
solid (67%).'H
NMR b [(CD3)ZSO] 8.22 (d, J=2.6 Hz, 1H), 8.14 (s,lH), 7.62 (m, 2H), 7.48 (m,
3H), 3.98 (s,
3H). MH-: 458,457,456,454.
EXAMPLE 443
The preparation of 7-Bromo-4-(2-chlorophenyl)-9-hydroxy-1H-f llbenzofuro(3,2-
elisoindole-
1,3(2H)-dione (CVIII; Ar=2-chlorophenyl) (862)
Demethylation of (861) prepared as described in example 442 with BBr3 using
the
procedure described in example 80 gave (862) (98 %) which was used without
further
purification.'H NMR S [(CD3)ZSO] 8.04 (d, J=2.3 Hz, 1H), 7.91 (s, 1H), 7.56
(d, J=8 Hz,
1H), 7.45 (m, 3H), 7.2 (d, J=2.5 Hz, 1H). MH-: 443.9, 441.9, 439.9.
EXAMPLE 444
The~re~aration of 4-(2-Chlorophenyl)-9-hydrox -~yl-1H-f llbenzofurof3,2-
elisoindole-
1 3(2H)-dione (CIX; Ar=2-chlorophe~rl, Z=H) (863).
Reaction of (862) prepared as described in example 443 with tributylvinyltin
using the
procedure described in example 309 gave (863) (79 %). 'H NMR 8 [(CD3)ZSO] 9.80
(s,
1H), 8.04 (d, J=2.7 Hz, 1H), 7.91(s,lH), 7.56 (d, J= 8Hz, 1H), 7.45 (m, 3H),
7.2 (d, J=2.5 Hz,
1H), 6.96 (dd, J=18,11 Hz, 1H), 6.25 (d, J=18 Hz, 1H), 5.52 (d, J=11 Hz, 1H).
MH~: 390, 388.
EXAMPLE 445
The preparation of 4-(2-Chlorophenyl)-7-ethyl-9-hydroxy-1H-f llbenzofurof3,2-
elisoindole-
1 3(2H)-dione (CX; Ar=2-chlorophenyl, Z=H) (864).
A solution of (863) (0.054 g, 0. 167 mmol) prepared as described in example
444 in a
methanolaetrahydrofuran mixture (1:1, 4 mL) was hydrogenated over Raney nickel
(100 mg)
and hydrogen. The product was purified by column chromatography using a
gradient of 0-
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-254-
100 % ethyl acetate in dichloromethane to give (864) (0.032 g, 59%). 1H NMR 8
[(CD3)2SOJ
9.65 (s, 1 H), 7.92 (m, 1 H), 7.56 (d, J=3.0 Hz, 1 H), 7.43 (m, 3H), 6.96 (d,
J=2.5 Hz, 1 H), 2.90
(q, 2H), 1.5 (t, 3H). MH-: 392, 390.
EXAMPLE 446
The~reparation of 4-(2-Chlorophenyl)-7-(1,2-dihydroxyethyl)-9-hydroxy-1H-
fllbenzofurof3,2-elisoindole-1,3(2H)-dione (CXI; Ar=2-chlorophenyl, Z=H)
(865).
Reaction of (863) prepared as described in example 444 with Os04 using the
procedure described in example 300 gave (865) (35 %). IH NMR 8 [(CD3)ZSOJ 9.68
(s, 1H),
7.96 (d, J=2.5 Hz, 1H), 7.9 (s,lH), 7.55 (d, J=7.8 Hz, 1H), 7.45, m, 3H), 7.17
(d, J=2.5 Hz,
1 H), 5.55 (d, J=4.6 Hz, 1 H), 5.06 (m, l H), 4.87 (t, J=5.7 Hz, 1 H), 3.65
(m, l H), 3.55 (m, l H).
MH~: 424, 422.
H H H
O N O O N O p N O
_ 1) POCl3lpyridine _ _
HO I ~ \ / Ar 2) BnOH BnO; P,O \ \ / Ar H~/Pd C Ha ,0 \ \ / Ar
Bn0 ~ HO~..
i N 0 / N O i N
H H H
(I) (CXII) (CXIII)
H H H
O N O O N O O N O
_ AczOHCl04 _ BBr3 _
Me0 I ~ \ / Ar Me0 I ~ \ / Ar METHOD 8 HO ( ~ \ / Ar
N ~ N ~ N
H O ~O
(V)
(CXIV) (CXV)
SCHEME 20
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-255-
Scheme 20 Procedures
EXAMPLE 447
The Qreparation of Dibenzyl 1 3-dioxo-4-phenyl-1 2 3,6-tetrahydropyrrolof3,4-
clcarbazol-9-
yl phosphate (CXII; Ar=phen~) (336)
To a solution of 9-hydroxy-4-phenylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione
(I;
Ar=phenyl) (0.50 g, 1.52 mmol) in pyridine (30 mL) under nitrogen was added
phosphorous
oxychloride (140 OL, 1.52 mmol) dropwise. After 40 minutes stirring at room
temperature a
further portion of phosphorous oxychloride (40 OL) was added and then after
another 20
minutes, benzyl alcohol (0.66 mL, 6.38 mmol) was added. After 3 hours the
reaction mixture
was diluted with 1N hydrochloric acid and extraction with ethyl acetate. The
organic phase
was dried, the drying agent was removed and the solution was concentrated to
dryness, before
being chromatographed on silica eluting with methanol/dichloromethane (3:97)
to give the
dibenzyl phosphate (336) (284 mg, 32%) as a pale yellow powder, mp 193-194
°C. 'H NMR
b [(CD3)ZSO] 12.12 (br s, 1H), 11.15 (br s, 1H), 8.81 (br s, 1H), 7.69 (s,
1H), 7.63 (m, 3H),
7.48-7.34 (m, 14H), 5.22 (d, JH_F= 8.3 Hz, 4H). Found: C, 69.65; H, 4.37; N,
4.88; P, 5.26.
C3aHzsN206P requires: C, 69.38; H, 4.28; N, 4.76; P, 5.26.
EXAMPLE 448
The preparation of 1 3-Dioxo-4-phenyl-1 2 3 6-tetrahydropyrrolof3,4-clcarbazol-
9-yl
dihydro~en~hosphate (CXIII; Ar=phen~) (337)
A solution of the dibenzyl phosphate (336) (100 mg, 0.17 mmol) prepared as
described in example 447 in methanol/tetrahydrofuran (3:1, 70 mL) was
hydrogenated at 60
psi over Pd-C (5%, catalytic) with stirring for 3 hours. The reaction mixture
was then filtered
through celite and concentrated in vacuo. Trituration from ethyl
acetate/hexane gave the
phosphate (337) (49 mg, 71 %) as a yellow solid, mp 285-295 °C. 'H NMR
8 [(CD3)ZSO]
12.01 (br s, 1H), 11.09 (br s, 1H), 8.66 (br s, 1H), 7.63 (m, 3H), 7.57 (br d,
J=8.6 Hz, 1H),
7.50-7.44 (m, 4H). Found: C, 55.19; H, 3.29; N, 6.34; P, 6.84.
CZOH~3N206P.1~/zHzO
requires: C, 55.18; H, 3.71; N, 6.43; P, 7.12.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-256-
EXAMPLE 449
The preparation of 6-Acetyl-4-(2-chlorophenyl)-9-methoxypyrrolof3,4-
clcarbazole-
1 3(2H,6H)-dione (CXIV; Ar=2-chloronhenyl) (338)
To a solution of carbazole (33) (45 mg, 0.12 mmol) prepared as described in
example
79 in acetic anhydride (4.0 mL) was added 35% perchloric acid (2 drops). The
resulting
solution was stirred at room temperature for 30 minutes before being poured
onto ice water,
basified by the addition of solid potassium bicarbonate and extraction with
ethyl acetate. The
organic phase was dried, the drying agent was removed and the solution was
concentrated to
dryness. Chromatography on silica eluting with ethyl acetate/hexane (2:3),
followed by
trituration from ethyl acetate gave acetamide (338) (40 mg, 80%) as a pale
yellow powder,
mp 277-280 °C. 'H NMR 8 [(CD3)ZSO] 11.46 (br s, 1H), 8.74 (d, J=2.8 Hz,
1H), 8.53 (s, 1H),
8.17 (d, J=9.2 Hz, 1H), 7.60 (m, 1H), 7.54-7.47 (m, 3H), 7.32 (dd, J=9.2, 2.8
Hz, 1H), 3.93
(s, 3H), 2.91 (s, 3H). Found: C, 65.50; H, 3.49; N, 6.61. C23H~5C1NZO4.1~4HZO
requires: C,
65.26; H, 3.69; N, 6.62.
EXAMPLE 450
The preparation of 6-Acetyl-4-(2-chlorophenyl)-9-hydroxypyrrolol3,4-
clcarbazole-
1,312H,6H)-dione (CXV: Ar=2-chloronhenvl) (339)
Demethylation of acetamide (338) (35 mg, 0.08 mmol) prepared as described in
example 449 according to the procedure described in example 80, except that
the reaction
time was 24 hours and the chromatography was performed eluting with ethyl
acetate/hexane
(1:2 to 2:1), gave phenol (339) (22 mg, 65%) as a pale yellow powder, mp 258-
261 °C.'H
NMR 8 [(CD3)ZSO] 11.41 (br s, 1H), 9.80 (br s, 1H), 8.59 (d, J=2.6 Hz, 1H),
8.53 (s, 1H),
8.07 (d, J=9.1 Hz, 1H), 7.59 (m, 1H), 7.53-7.46 (m, 3H), 7.15 (dd, J=9.1, 2.6
Hz, 1H), 2.89
(s> 3H). Found: C, 65.03; H, 3.14; N, 6.68. CzzH,3C1Nz04 requires: C, 65.28;
H, 3.24; N,
6.92.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-257-
NOz (i) HCI, NaN02
\ ( ) O CO Et OzN \ ~ C02Et pzN
I / Nfapp,~c--~ I / . N P~ I / ~ COZEt
N N
H H
NHz (iii) H30~
(901 ) (902)
(900)
ArCH PPh X
OzN ~ ~ OzN \ z 3 O N
(ii) CDI I , N CHZOH M~ I / ~ CHO LDA z
(iii) NaBH4 H H METHOD 2 ~ H ~ Ar
(903)
(904)
(CXVI)
H H
O N O N O
O
NaHIR~oX OzN I \ ~ maleimide Ar MnOz
METHOD 3 \~~~~~ O N
N ~ Ar z
R o METHOD 5 ~ / N METHOD 6 zN I \ ~ / Ar
N
(CXVII) R,o
R, o
(CXVIII)
(CXIX)
O N O N O H
_ R'COOH/AczO O O O N O
Fe/HOAc HzN Ar METHOD 3 ~ BH .Me S H -
\ ~ / ~4H ~ ~ / Ar s z N \ Ar
METHOD 34 I / N R~CO~H/EDCI I / B(OMe)3 R4CHz~ I
METHOD 36 N METHOD 37 /
N
Rio
(CXX) R,o
(CXXI)
1. R3CH0 (CXXII)
2. Na(CN)BH3
METHOD 38
H
O N O
R3CHz ~
N ~ / Ar
R3CH2 I
N
Rio
(CXXiII) SCHEME 21
Procedures for Scheme 21
EXAMPLE 451
The preparation of Ethyl (2E)-2-f(4-nitrophenyl)hydrazonolpronanoate (901)
A mixture of para-nitroaniline (900) (10.0 g, 72.0 mmol), ice (72 g), and 15%
hydrochloric acid (72 mL) was treated with a solution of sodium nitrite (5.4
g, 78.0 mmol) in
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-258-
water (15 mL) at such a rate that the temperature did not exceed 7° C.
The reaction mixture
was stirred for a further 5 min and then filtered through Celite to give a
clear pale
yellow/brown solution which was added rapidly to a slurry of sodium acetate
(59.0 g,
0.72 mol), ethanol (72 mL), ethyl 2-methylacetoacetate (11.4 mL, 80.0 mmol),
and ice (72 g).
The resulting red mixture was stirred for 2.5 h and then it was extracted with
dichloromethane (3 x 100 mL). The combined extracts were dried and
concentrated to give a
red oil (18 g). The red oil was dissolved in ethanol (40 mL) and concentrated
hydrochloric
acid (15 mL) was added, a fine orange/yellow precipitate formed instantly. The
reaction
mixture was heated at reflux for 15 min and then it was poured onto ice. The
orange/yellow
precipitate was collected by filtration and dried to give ethyl (2E)-2-[(4-
nitrophenyl)hydrazono]propanoate (901) (15.2 g, 84°Io).'H NMR 8
[(CD3)ZSO] 10.50 (s,
1H), 8.20 (m, 2H), 7.40 (m, 2H), 4.23 (q, J=7.2 Hz, 2H), 2.13 (s, 3H), 1.29
(t, J=7 Hz, 3H).
EXAMPLE 452
Ethyl 5-vitro-1H-indole-2-carboxvlate (902)
Ethyl (2E)-2-[(4-nitrophenyl)hydrazono]propanoate (901) (10.6 g, 42 mmol)
prepared
as described in example 451 was heated in polyphosphoric acid (70 g) at
110° C until no
starting material remained by tlc (40 min). Ice (500 g) was added with
vigorous stirring, a
thick brown precipitate formed which was removed by filtration and dried to
give ethyl 5-
nitro-1H-indole-2-carboxylate (902) (6.04 g, 61%). This material was used
without
purification in the next step. 'H NMR 8 [(CD3)ZSO] 12.59 (br, 1H), 8.73 (d,
J=2.4 Hz, 1H),
8.13 (dd, J=9.2, 2.4 Hz, 1H), 7.61.(d, J=9.2 Hz, 1H), 7.44 (s, 1H), 4.38 (q,
J=7.0 Hz, 2H),
1.36 (t, J=7.0 Hz, 3H).
EXAMPLE 453
The pr~aration of (5-Nitro-1H-indol-2-yl)methanol (903)
To a solution of ethyl 5-vitro-1H-indole-2-carboxylate (9.8 g, 42.0 mmol) in
methanol
(300 mL) was added a solution of 2 M potassium hydroxide (31 mL, 63.0 mmol).
The
reaction mixture was heated at reflux for 2 h and then the hot solution was
filtered, poured
onto ice, and acidified. The resultant fine pale brown precipitate was
collected by filtration
and dried. The crude acid was dissolved in tetrahydrofuran (250 mL) and
stirred with 1,1'-
carbonyldiimidazole (10.2 g, 63.0 mmol) with gentle warming for 2 h. Water
(100 mL) was
added and then solid sodium borohydride (7.9 g, 0.21 mol) was added
portionwise over
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-259-
30 min, the reaction mixture was stirred for a further 40 min and then it was
quenched with
1 M hydrochloric acid. The tetrahydrofuran was removed at reduced pressure and
the residue
was dissolved in ethyl acetate, washed, dried, and concentrated to give (5-
nitro-1H-indol-2-
yl)methanol (903). This material was used without purification in the next
step.
EXAMPLE 454
The preparation of 5-Nitro-1H-indole-2-carbaldehyde (904)
A solution of crude (5-vitro-1H-indol-2-yl)methanol (3.60 g, 18.7 mmol) in
chloroform (200 mL) was heated at 50° C with manganese dioxide (20 g,
0.23 mol) for 3 h.
The reaction mixture was then filtered through Celite and concentrated to give
5-vitro-1H-
indole-2-carbaldehyde (904) (2.04 g, 57% through two steps).'H NMR 8
[(CD3)ZSO] 12.64
(br, 1H), 9.96 (s, 1H), 8.83 (d, J=2.3 HZ, 1H), 8.18 (dd, J=9.0 Hz, 2.3 Hz,
1H), 7.69 (d, J=0.7
Hz, 1H), 7.62 (d, J=9.0 Hz, 1H).
EXAMPLE 455
The~reparation of 2-f2-(2-Chlorophenyl)ethenyll-5-vitro-1H-indole (CXVI; Ar=2-
chlorophenyl) (905)
Reaction of the aldehyde (904) prepared as described in example 454 with 2-
chlorobenzyltriphenylphosphonium chloride using the procedure described in
example 37(at
room temperature) gave the diene (905) as a mixture of E/Z isomers as a yellow
solid (95%).
Crystallisation from aqueous methanol yielded the pure E-isomer as yellow
needles (80%),
mp 230-232 °C. 'H NMR 8 [(CD3)ZSO] 12.28 (br s, 1H), 8.55 (d, J=2.3 Hz,
1H), 8.03 (dd,
J=9.1, 2.3 Hz, 1H), 7.91 (dd, J=7.8, 1.5 Hz, 1H), 7.32-7.61 (m, 6H), 6.92 (s,
1H). Found: C,
64.59; H, 5.68; N, 9.49. C~6H»CINZOzrequires C, 64.33; H, 5.68; N, 9.49.
EXAMPLE 456
The preparation of Methyl 2-f2-(5-vitro-1H-indol-2-yl)ethenyllphenyl ether
(CXVI; Ar=2-
methoxyphenyl) (906)
Reaction of the aldehyde (904) prepared as described in example 454 with 2-
methoxybenzyltriphenylphosphonium bromide using the procedure described in
example 37
gave the dime (905) as a mixture of E/Z isomers as a yellow solid (95%) which
was used
without further purification.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-260-
EXAMPLE 457
The preparation of Methyl 2-(2-(1-methyl-5-vitro-1H-indol-2-yl)ethenyllphenyl
ether
~XVII; Ar=2-methoxyphenyl, R1°=Me) (907)
Alkylation of dime (906) prepared as described in example 456 with methyl
iodide
using the procedure described in method3 gave the corresponding dime (908)
(94%) as a
yellow solid (a mixture of E/Z isomers), which was used without further
purification.
EXAMPLE 458
The preparation of 2-(2-(2-Chlorophenyl)ethenyll-1-methyl-5-vitro-1H-indole
(CXVII;
Ar=2-chloropherryl, Rl°=Me) (908)
Alkylation of dime (905) prepared as described in example 455 with methyl
iodide
using the procedure described in example 38 gave the corresponding dime (908)
(99%) as a
yellow solid. A sample was crystallised from methanol as orange needles, mp
161-163 °C.
'-H NMR b [(CD3)zS0] 8.53 (d, J=2.3 Hz, 1H), 8.05 (dd, J=7.7, 1.6 Hz, 1H),
8.02 (dd, J=9.1,
2.3 Hz, 1H), 7.67 (d, J=9.1 Hz, 1H), 7.46-7.64 (m, 3H), 7.43 (dt, J=7.7, 1.6
Hz, 1H), 7.36 (dt,
J=7.7, 1.6 Hz, 1H), 7.22 (s, 1H), 3.95 (s, 3H). Found: C, 65.55; H, 4.19; N,
8.90.
C»H~3C1N202 requires C, 65.28; H, 4.19; N, 8.96.
EXAMPLE 459
The geparation of 4-(2-Methoxyphenyl)-6-methyl-9-vitro-4,5,6,1Oc-
tetrahydropyrrolo(3,4-
clcarbazole-1,3(2H,3aH)-dione (CXVIII; Ar=2-methoxyphenyl, Rl°=Me)
(909)
Reaction of the dime (907) prepared as described in example 457 with maleimide
using the procedure described in example 68 gave the adduct (909) (84%) as a
glassy solid
which was immediately aromatised.
EXAMPLE 460
The preparation of 4-(2-Chlorophenyl)-6-methyl-9-vitro-4,5,6,1Oc-tetrah
d~yrrolo(3,4-
clcarbazole-1,3(2H,3aH)-dione (CXVIII; Ar=2-chlorophenyl, R'°=Me) (910)
Reaction of the dime (908) prepared as described in example 458 with maleimide
using the procedure described in example 68 gave the adduct (910) (88%) as a
glassy dark
solid which was immediately aromatised.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-261-
EXAMPLE 461
The preQaration of 4-(2-Chlorophenyl)-9-nitro-4,5,6,1Oc-tetrahYdropyrrolof3,4-
clcarbazole-
1,3(2H 3aH)-dione (CXVIII; Ar=2-chlorophenyl, R'°=H) (917)
Reaction of the dime (905) prepared as described in example 455 with maleimide
using the procedure described in example 68 gave the adduct (917) (74%) as a
dark solid
which was immediately aromatised.
EXAMPLE 462
The preQaration of 4-(2-Methoxyphenyl)-6-methyl-9-nitropyrrolof3,4-clcarbazole-
1 3(2H,6H)-dione (CXIX; Ar=2-methoxyphenyl, R1°=Me) (911)
Aromatisation of the adduct (909) prepared as described in example 459 with
manganese dioxide using the procedure described in example 79 except that the
reaction time
was only 4 h gave the nitrocarbazole (911) (50%) as a brown solid mp 320-330
°C (dec.). 1H
NMR b [(CD3)ZSO] 11.24 (s, 1H), 9.76 (d, J=2 Hz, 1H), 8.50 (dd, J=9.1, 2.2 Hz,
1H), 7.93 (s,
1H), 7.91 (d, J=9.1 Hz, 1H), 7.46 (t, J=7.3 Hz, 1H), 7.38 (d, J=7.3 Hz, 1H),
7.13 (d, J=7.3 Hz,
1H), 7.08 (t, J=7.3 Hz, 1H), 4.05 (s, 3H), 3.70 (s, 3H). Found: C, 65.76; H,
3.83; N, 9.30.
Ca2HisN30s requires: C, 65.83; H, 3.77; N, 10.47.
EXAMPLE 463
The preparation of 4-(2-Chlorophenyl)-6-methyl-9-nitropyrrolof3,4-clcarbazole-
1,3(2H,6H)-
dione (CXIX; Ar=2-chlorophenvl, R1°=Me) (912)
Aromatisation of the adduct (910) prepared as described in example 460 with
manganese dioxide using the procedure described in example 79 except that the
reaction time
was only 4 h gave the nitrocarbazole (912) (78%) as an orange solid, mp >320
°C. 'H NMR
8 [(CD3)ZSO] 11.35 (s, 1H) 9.68 (d, J=2.3 Hz, 1H), 8.46 (dd, J=9.1, 2.3 Hz,
1H), 8.00 (s, 1H),
7.88 (d, J=9.1 Hz, 1H) 7.47-7.62 (m, 4H) 4.04 (s, 3H). Found: C, 58.90; H,
3.28; N, 10.30.
C2~H~ZC1 N304.Hz0 requires: C, 59.57; H, 3.30; N, 9.93.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-262-
EXAMPLE 464
The preparation of 4-(2-Chlorophenyl)-9-nitropyrrolof 3 4-clcarbazole-
1,3(2H,6H)-dione
(CXIX~ Ar=2-chlorophenyl, R'°=H) (918)
Aromatisation of the adduct (917) prepared as described in example 463 with
manganese dioxide using the procedure described in example 79 except that the
reaction time
was only 4 h gave the nitrocarbazole (918) (84%) as a yellow solid, mp 253-258
°C. 1H
NMR 8 [(CD3)zS0] 12.78 (s, 1H) 11.33 (s, 1H), 9.74 (d, J=2.5 Hz, 1H), 8.45
(dd, J=9.1, 2.5
Hz; 1H), 7.80 (d, J=9.1 Hz, 1H), 7.77 (s, 1H), 7.60 (dd, J=7.2, 1.5 Hz, 1H),
7.45-7.54 (m,
3H). Found: C, 62.39; H, 3.03; N, 10.10. Cz°Hl°Cl N304.1/6
hexane requires: C, 62.22; H,
3.04; N, 10.37
Representative Procedure for Method 34 of Scheme 21
EXAMPLE 465
The preparation of 9-Amino-4-(2-chlorophenyl)-6-methylpyrrolof3,4-clcarbazole-
1.312H,6H)-dione (CXX; Ar=2-chlorophenvl, Rl°=Me) (914)
The nitrocarbazole (912) (0.055 g, 0.14 mmol) prepared as described in example
463
was suspended in a mixture of ethanol:water (4:1, 20 mL) and set at reflux.
After 15 min iron
powder (0.076 g, 1.4 mmol) was added, followed by acetic acid (0.041 g, 0.70
mmol). The
red solution was refluxed for 1 hour, turning a dark colour. The cooled
solution was filtered
through Celite, washing with ethyl acetate (2 x 100 mL). The solvent was
evaporated under
reduced pressure, leaving a brown residue, which was dissolved in ethyl
acetate (200 mL).
The solution was washed with saturated aqueous sodium bicarbonate and
saturated aqueous
sodium chloride, then dried and worked up to give the amine (914) as a red
solid, mp 208-
216 °C.'H NMR 8 [(CD3)zS0] 11.00 (s, 1H), 8.15 (d, J=2.2 Hz, 1H), 7.68
(s, 1H), 7.56 (dd,
J=7.0, 1.9 Hz, 1H), 7.43-7.49 (m, 4H), 7.02 (dd, J=8.7, 2.2 Hz, 1H), 5.03 (br
s, 2H,
exchanges with DZO), 3.88 (s, 3H). Found: C, 64.48; H, 3.82; N, 10.32.
CzlH~4Cl N30z.H20
requires: C, 64.12; H, 4.07; N, 10.68.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-263-
EXAMPLE 466
The preparation of 9-Amino-4-(2-methoxyphenyl)-6-methylpyrrolof3,4-clcarbazole-
1,3(2H 6H)-dione (CXX; Ar=2-methoxyphenyl, RI°=Me) (913)
The nitrocarbazole (911) prepared as described in example 462 was reduced with
iron
powder using the procedure described in method 34, to give the amine (913) as
a brown solid
(74%), mp 188-192 °C. 1H NMR 8 [(CD3)zS0] 10.87 (s, 1H), 8.14 (d, J=1.7
Hz, 1H), 7.61 (s,
1H), 7.40-7.42 (m, 2H), 7.33 (dd, J=7.3, 1.6 Hz, 1H), 6.98-7.10 (m, 3H), 5.00
(broad s, 2H,
exchanges with D20), 3.86 (s, 3H), 3.68 (s, 3H). Found: C, 69.61; H, 4.66; N,
9.32.
CZZH~7N303.1/2C4H80 requires: C, 69.40; H, 5.06; N, 10.12.
EXAMPLE 467
The preparation of 9-Amino-4-(2-chlorophen~pyrrolof3,4-clcarbazole-1,3(2H,6H)-
dione
(CXX; Ar=2-chloronhenyl, R'°=H) (919)
The nitrocarbazole (918) prepared as described in example 464was reduced with
iron
powder using the procedure described in example 465 with a reaction time of 45
min. and the
product was precipitated from THF: petroleum ether to give amine (919) as a
brown solid
(91%), mp 216-222 °C. 'H NMR 8 [(CD3)ZSO] 11.66 (s, 1H), 10.96 (s, 1H),
8.09 (d, J=2.3
Hz, 1H), 7.57 (dd, J=8.1, 1.8 Hz, 1H), 7.40-7.50 (m, 4H), 7.35 (d, J=8.6 Hz,
1H), 6.95 (dd,
J=8.6, 2.3 Hz, 1H) 4.95 (br s, 2H). EIMS found: M+=361.0611, 363.0587.
CZ°HlzC1N302
requires 361.0818, 363.0589.
Representative Procedure for Method 35 of Scheme 21
EXAMPLE 468
The preparation of N-f4-(2-Methoxyphenyl)-6-methyl-1,3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-clcarbazol-9-yllacetamide (CXXI; Ar=2-methoxyphenyl,
RI°=Me,
R4=Me) (916)
Acetic acid (1 mL) and acetic anhydride (1 mL) were mixed under nitrogen at 0
°C
and the solution was stirred at 0 °C for 1 hour. A solution of the
amine (913) (0.06 g, 0.16
mmol) prepared as described in example 466 in dry dichloromethane was added
and the
mixture was allowed to warm to room temperature over 2 h and then the solvent
evaporated
under reduced pressure. The resulting yellow solid was purified by column
chromatography
on silica gel eluting with petroleum ether:ethyl acetate (100:0 to 0:100). The
product (916)
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-264-
was then precipitated from tetrahydrofuran with petroleum ether as a yellow
powder (0.044
g, 67%), mp 300-304 °C.'H NMR 8 [(CD3)ZSO] 10.98 (s, 1H), 10.12 (s,
1H), 8.99 (d, J=2.0
Hz, 1H), 7.97 (dd, J=8.8, 2.0 Hz, 1H) 7.74 (s, 1H), 7.66 (d, J=8.8 Hz, 1H),
7.43 (dt, J=7.4, 1.7
Hz, 1H), 7.34 (dd, J=7.4, 1.7 Hz, 1H), 7.12 (d, J=8.1 Hz, 1H), 7.05 (t, J=8.1
Hz, 1H), 3.95 (s,
3H), 3.68 (s, 3H), 2.10 (s, 3H). Found: C, 66.10; H, 4.60; N, 9.44.
Cz4H»N3O4.HZO requires:
C, 66.82; H, 4.41; N, 9.74.
EXAMPLE 469
The preparation of 4-(2-Chlorophenyl)-6-methyl-1.3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-
clcarbazol-9-ylformamide (CXXI; Ar=2-chlorophe~l, RI°=Me, R4=H) (920)
Amine (914) prepared as described in example 465 and formic acid were reacted
using the procedure described in method35 with a reaction time of 16 h to give
the
formamide (920) (92%) as a yellow powder, mp 345-348 °C.'H NMR 8
[(CD3)ZSO] 11.16 (s,
O.SH), 11.12 (s, 1H), 10.36 (m, 1.SH), 9.08 (d, J=2.0 Hz, 1H), 8.72 (m, O.SH),
8.32 (d, J=1.8
Hz, 1H), 7.99 (dd, J=8.8, 2.0 Hz, 1H), 7.85 (s, O.SH), 7.83 (s, 1H), 7.70-7.73
(m, 1.SH), 7.46-
7.60 (m, 7H), 3.98 (s, 1.SH), 3.98 (s, 3H). FABMS found: [M+H]+=404.0790,
406.0779.
CZZH~SC1N303 requires 404.0802, 406.0772.
EXAMPLE 470
The preparation of 4-(2-Chlorophenyl)-1 3-dioxo-1,2,3,6-tetrahydropyrrolof3,4-
clcarbazol-9-
ylformamide (CXXI; Ar=2-chlorophenyl, R'°=H, R4=H) (921)
Amine (919) prepared as described in example 467 and formic acid were reacted
using the procedure described in example 468 with a reaction time of 4 h to
give the
formamide (921) (54%) as a yellow powder, mp 293-297 °C.'H NMR 8
[(CD3)ZSO] 12.11 (s,
O.SH), 12.08 (s, 1H), 11.12 (s, 0.5 H), 11.08 (s, 1H), 10.34 (s, 1H), 10.30
(s, O.SH), 9.03 (d,
J=1.9 Hz, 1H), 8.69 (m, O.SH), 8.31 (d, J=1.8 Hz, 1.SH) 7.92 (dd, J=8.8, 1.9
Hz, 1H) 7.42-
7.65 (m, 9.SH). Found: C, 64.78; H, 3.53; N, 9.96. CZ~H~zC1N303.'/a THF
requires: C, 64.86;
H, 3.44; N, 10.32.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-265-
EXAMPLE 471
The nre~aration of N-f4-(2-Chlorophenvl)-6-methyl-1,3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-
clcarbazol-9-vllacetamide (CXXI; Ar=2-chlorophenyl, R'°=Me, R4=Me)
(923)
Amine (914) prepared as described in example 465 and acetic acid were reacted
using
the procedure described in example 468 with a reaction time of 14 h to give
the acetamide
(923) (85%) as a yellow powder, mp 338-340 °C.'H NMR 8 [(CD3)ZSO] 11.10
(br s,
exchanges with D20, 1H), 10.14 (s, exchanges with D20, 1H), 9.01 (d, J=2.0 Hz,
1H), 7.99
(dd, J=8.9, 2.0 Hz, 1H), 7.82 (s, 1H), 7.68 (d, J=8.9 Hz, 1H), 7.57 (dd,
J=7.2, 1.9 Hz, 1H),
7.44-7.53 (m, 3H), 3.93 (s, 3H), 2.10 (s, 3H). Found: C, 64.44; H, 4.08; N,
8.98.
CZ3H~6C1N3O3.1/2CH3COOH requires C, 64.43; H, 4.03; N, 9.39.
EXAMPLE 472
The preparation of N-f4-(2-Chlorophenyl)-1,3-dioxo-1,2,3,6-
tetrahydropyrrolol3,4-
clcarbazol-9-yllacetamide (CXXh Ar=2-chlorophenYl, R'°=H, R4=Me) (926)
Amine (919) prepared as described in example 467 and acetic acid were reacted
using
the procedure described in example 468 with a reaction time of 4 h to give the
acetamide
(926) (43%) as a yellow powder, mp 240-245 °C.'H NMR b [(CD3)2S0] 12.04
(br s, 1H),
11.07 (br s, 1H), 10.10 (s, 1H), 8.95 (d, J=2.1 Hz, 1H), 7.90 (dd, J=8.8, 2.1
Hz, 1H), 7.58 (m,
2H), 7.42-7.51 (m, 4H), 2.10 (s, 3H). FABMS found: [M+H]+ =404.0787, 406.0776.
Cz2H~5C1N303 requires 404.0802, 406.0772.
Representative Procedure for Method 36 of Scheme 21
EXAMPLE 473
The preparation of N-f4-(2-Chlorophenyl)-6-methyl-1 3-dioxo-1,2,3,6-
tetrahydropyrrolof3,4-
clcarbazol-9-yll-3-(1-Qperidinyl)pro~anamide (CXXI; Ar=2-chlorophenyl,
R'°=Me, R4=3-
(,1-piperidin ly )propel) (925)
3-Piperidinylpropanoic acid (0.036 g, 0.23 mmol) and 1-[3-
(dimethylamino)propyl]-
3-ethylcarbodiimide hydrochloride (0.044g, 0.23 mmol) were combined in dry
dimethylformamide (2 mL) at 0 °C under an atmosphere of nitrogen and
left to stir for 1 h. A
solution of the amine (914) (0.085 g, 0.23 mmol) in dry dimethylformamide (3
mL) was
added to the reaction mixture. The solution was allowed to warm to room
temperature over
16 h. The solvent was removed under reduced pressure and the residue adsorbed
onto silica.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-266-
Purification by column chromatography eluting with ethyl
acetate:methanolariethylamine
(100:0:0 to 75:24:1) yielded (925) as a yellow solid (0.036 g, 30%), mp 241-
246 °C. 1H
NMR 8 [(CD3)zS0] 11.12 (s, 1H), 10.46 (s, 1H), 9.92 (broad s, 1H), 9.09 (d,
J=2.0 Hz, 1H),
7.98 (dd, J=8.9, 2.0 Hz, 1H) 7.83 (s, 1H), 7.71 (d, J=8.9 Hz, 1H), 7.45-7.60
(m, 4 H), 3.98 (s,
3H), 3.38 (m, 2H), 3.10 (m, 2H), 2.95 (m, 2H), 1.80 (m, 4H), 1.42 (m, 2H).
FABMS found:
[M+H]+=515.1842, 517.1833. C29HZ8C1Na03 requires 515.1850, 517.1820.
EXAMPLE 474
The preparation of N-f4-(2-Chlorophenyl)-6-methyl-1,3-dioxo-1,2,3,6-
tetrahydropyrrolo(3,4-
clcarbazol-9-~l-4-(dimethylamino)butanamide hydrochloride (CXXI; Ar=2-
chlorophenyl,
R'°=Me. R4=4-dimethvlaminobutvl) (928)
Amine (914) prepared as described in example 465 was reacted with EDCI and 4
dimethylaminobutyric acid hydrochloride using the procedure described in
example 473 to
give the amide (928) (44%) as an orange powder, mp 331-333 °C. 'H NMR 8
[(CD3)ZSO]
11.11 (s, 1H), 10.25 (s, 1H), 9.82 (br s, 1H), 9.07 (d, J=2.0 Hz, 1H), 7.98
(dd, J=9.0, 2.0 Hz,
1H), 7.83 (s, 1H), 7.70 (d, J=9.0 Hz, 1H), 7.58 (m, 1H), 7.44-7.52 (m, 3H),
3.97 (s, 3H), 2.99-
3.11 (m, 4H), 2.77 (s, 6H), 2.00 (m, 2H). FABMS found: [M+H]+=489.1689,
491.1681.
CZ~HZ6C1N403 requires 489.1693, 491.1664.
Representative Procedure for Method 37 of Scheme 21
EXAMPLE 475
The preparation of 4-(2-Chlorophenyl)-6-methyl-9-(methylamino)pyrrolof3,4-
clcarbazole-
1.3(2H,6H)-dione (CXXII; Ar=chlorophenvl, Rl°=Me, R4--H) (922
Borane methylsulphide complex (0.074 mL, 0.78 mmol) and trimethylborate (0.089
mL, 0.78 mmol) were added at 0 °C under an atmosphere of nitrogen to a
solution of amide
(920) (0.105 g, 0.26 mmol) prepared as described in example 469 dry
tetrahydrofuran (10
mL) After stirring at 0 °C for 30 min. the solution was allowed to warm
to room temperature
over 16 h. Methanol (200 mL) was added and the reaction mixture was stirred
for 12 h. The
solvent was removed under reduced pressure and the yellow residue was
purificatied by
column chromatography on silica, eluting with petroleum ether:ethyl acetate
(100:0 to 0:100)
to give (922) as a red solid (0.053 g, 45%), mp 293-296 °C. 1H NMR S
[(CD3)2S0] 11.01 (br
s, 1H), 8.10 (d, J=2.3 Hz, 1H), 7.69 (s, 1H), 7.57 (dd, J=7.1, 2.0 Hz, 1H),
7.42-7.52 (m, 4H),
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-267-
7.02 (dd, J=8.8, 2.3 Hz, 1H), 5.61 (q, J=5.2 Hz, 1H), 3.90 (s, 3H), 2.81 (d,
J=5.2 Hz, 3H).
EIMS found: M+=389.0929, 391.0905. CZZH~6C1N302 requires 389.0931, 391.0902.
EXAMPLE 476
The preparation of 4-(2-Chlorophenyl)-9-(ethylamino)-6-methylpyrrolo(3,4-
clcarbazole-
1 3(2H 6H)-dione (CXXII~ Ar=chloronhenyl, R'°=Me, R4=Me) (924)
Amide (923) prepared as described in example 471 was reduced with borane
methylsulphide complex using the procedure described in method37 to give amine
(924) as a
red powder, mp 232-236 °C.'H NMR 8 [(CD3)ZSO] 11.00 (s, exchanges with
D20, 1H), 8.13
(d, J=2.2 Hz, 1H), 7.69 (s, 1H), 7.57 (dd, J=7.0, 2.0 Hz, 1H), 7.42-7.51 (m,
4H), 7.05 (dd,
J=8.8, 2.2 Hz, 1H), 5.51 (t, J=5.4 Hz, exchanges with D20, 1H), 3.15 (m, 2H),
1.27 (t, J=7.1
Hz, 3H). EIMS found: M+=403.1080, 405.1061. C23H~$C1N302 requires 403.1087,
405.1058.
EXAMPLE 477
The preparation of 4-(2-chlorophenyl)-9-(methylamino)pyrrolof3 4-clcarbazole-
1,3(2H,6H)-
dione (GXXII~ Ar=chlorophenyl, R'°=H, R4=H) (927)
Amide (921) (0.051 g, 0.13 mmol) prepared as described in example 470 was
reacted
with borane methylsulphide complex using the procedure described in method 37
to give
amine (927) (0.021 g, 43%) as an orange powder; m.p. 182-184°C.'H NMR 8
[(CD3)ZSO]
11.69 (broad s, 1H), 10.97 (broad s, 1H), 8.05 (d, J=2.1 Hz, 1H), 7.40-7.57
(m, SH), 6.97 (dd,
J=8.7, 2.4 Hz, 1H), 5.55 (q, J=4.9 Hz, 1H), 2.80 (d, J=5.2 Hz, 3H). EIMS
found:
M+=375.0768, 377.0749. CZ~H~4C1N302 requires 375.0774, 377.0745.
Representative Procedure for Method 38 of Scheme 21
EXAMPLE 478
The preparation of 9-(Dimethylamino)-4-(2-methoxynhenyl)-6-methylpyrrolof3,4-
clcarbazole-1 3(2H 6H)-dione (CXXIII~ Ar=methoxyphenyl, Rl°=Me, R3=H)
(915)
Amine (913) (0.049 g, 0.13 mmol) prepared as described in example 466 was
suspended in methanol (15 mL) and stirred at 0 °C. Formaldehyde (0.01
mL of a 37 %
aqueous solution, 0.13 mmol) was added followed immediately by sodium
cyanoborohydride
(0.022 g, 3.5 mmol) portionwise and the mixture was stirred at room
temperature for 14 h.
The solvent was removed under reduced pressure and the residue dissolved in
ethyl acetate
(100 mL). The solution was washed with water (2 x 100 mL), sat. aq. sodium
chloride (2 x
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-268-
100 mL), then dried over anhydrous sodium sulphate and the solvent evaporated.
The residue
was chromatographed on silica, eluting with petroleum ether:diethyl ether
(100:0 to 0:100) to
give (915) as a red solid (0.017 g, 33%), mp 246-251 °C. )H NMR 8
[(CD3)ZSO] 10.92 (s,
IH), 8.39 (d, J=2.SHz, 1H), 7.67 (s, 1H), 7.58 (d, J=9.0 Hz, 1H), 7.41 (dt,
J=7.4, 1.7 Hz, 1H),
7.37 (dd, J=7.4, 1.7 Hz, 1H), 7.25 (dd, J=9.0, 2.5 Hz, 1H), 7.10 (d, J=7.4 Hz,
1H), 7.04 (t,
J=7.4 Hz, 1H) 3.91 (s, 3H), 3.68 (s, 3H), 2.99 (s, 6H). EIMS found M+:
399.1580.
C24H2)N303 requires 399.1583.
H
O N O H H
O N O O N O
HO \ / ~ TtzO/pyridine
F3CS020 ~ tetravinyl tin Ar
/ N METHOD 24 I ~ ~ ~ pd(PPh~yCI~LiCI
\ / N METHOD25 / N
H \ \
H H
(I)
(CX7fIV) (ClU(V)
OsO,/NMNO
METHOD 21 1. 9-BBN
H 2. H20~/NaOAc
METHOD 29
N O
O
OH _ H
i
HO ~ \ ~ Ar O N O
I / N -
\ HO ~ \ / Ar
H I
/ N
(CXXVI) 1
H
SCHEME 22
Scheme 22 Procedures
EXAMPLE 479
(CX7(VII)
The preparation of 4-(2-Chlor~hen~l)-1 3-dioxo-1 2 3 6-tetrahydropyrrolof3 4-
clcarbazol-9-
yl trifluoromethanesulfonate (CXXIV; Ar = 2-chlorophenyl) (615)
Compound (615) was prepared from (9) (I; (Ar =2-chlorophenyl) prepared as
described in example 8 using the procedure described in example 307 to give a
yellow oil.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-269-
'H NMR 8 [(CD3)ZSO] 11.83 (s, 1H), 11.03 (s,.1H), 8.28 (s, 1H), 8.32 (~ 1H),
7.6-7.4 (m,
SH), 7.07 (s, 1H). EIMS found: M+=493.9946. CZ~H,oCIF3NzS05 requires~93.9951.
EXAMPLE 480
The preparation of 4-(2-Chlorophenyl)-9-vinylpyrrolof3,4-clcarbazole-
1,3(2H,6H)-dione
(CXXV~ Ar =2-chlorophenyl) (616)
Compound (616) was prepared from (615) prepared as described in example 479
(using the procedure described in example 309 , with tetraethyl tin as the
stannane to give a
yellow oil.'H NMR 8 [(CD3)ZSO] 11.71 (s, 1H), 11.48 (br, 1H), 8.23 (s, 1H),
8.78, (s, 1H),
7.99 (s, 1H), 7.62-7.43 (m, SH), 7.2-7.1 (m, 1H), 6.10 (d, J= 17.6 Hz, 1H),
5.46 (d, J=11.3
Hz, 1H). EIMS found: M+=372.0661. CZZHi3C1N202 requires 372.0666.
EXAMPLE 481
The preparation of 4-(2-Chlorophenyl)-9-(1 2-dih dy roxyethyl)pyrrolo~3 4-
clcarbazole-
1 3(2H 6H)-dione (CXXVI; Ar =2=chlorophenyl)-(617)--- - --
The diol (617) was prepared from (616) prepared as described in example 480
using
the procedure described in example 300 to give a yellow solid, mp 265-268
°C.'H NMR 8
[(CD3)ZSO] 12.06 (br, 1H), 11.08 (br, 1H), 8.87 (s, 1H), 7.60-7.40 (m, 7H),
5.32 (d, J=3.8
Hz, 1H), 4.74 (m, 2H), 3.55 (m, 2H). EIMS found: M+=406.0718. C22H~SC1N204
requires
406.0720.
EXAMPLE 482
The preparation of 4-(2-Chlorophen~)-9-(2-hydroxyethyl)pyrrolof3,4-clcarbazole-
1 3(2H 6H)-dione (CXXVII; Ar =2-chlorophenyl) (618)
Compound (618) was prepared from (616) prepared as described in example 480
using the procedure described in example 344 to give a yellow solid, mp 253-
257 °C.'H
NMR 8 [(CD3)zS0] 12.03 (br, 1H), 11.08 (br, 1H), 8.73 (s, 1H), 7.60-7.40 (m,
6H), 4.69 (t,
J=5.2 Hz, 1H), 3.71 (m, 2H), 2.93 (t, J=7.2 Hz, 2H). EIMS found: M+=390.0769.
C22H15C1N203 requires 390.0771.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-270-
EXAMPLE 483
Other novel checkpoint inhibitors
Listed below are other checkpoint inhibitors of the present invention which
may be
prepared by process known in the art, processes described herein-above, or a
combination of
said processes. One of skill in the art will understand how each compound is
prepared by
reference to the disclosure herein.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-271-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(4-Amino-2-methoxy-phenyl)-9-hydroxy-6H-
308-316
yrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-1,3-dioxo-1,2,3,6-
240-245
etrahydro-pyrrolo[3,4-c]carbazol-9-yl]-acetamide
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-
1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9-ylJ-4- 488/450
imethylamino-butyramide
-(2-Chloro-phenyl)-9-methylamino-6H-
182-184
yrrolo[3,4-c]carbazole-1,3-dione
-Hydroxy-4-(2-methoxy-4-nitro-phenyl)-6H-
' 242-244
yrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-8-(4-morpholin-4-
1-butyl)-6H-pyrrolo[3,4-cJcarbazole-1,3-dione;191-193
ydrochloride salt
-(2-Chloro-phenyl)-8-[3-(3,5-dimethyl-piperazin-
1-yl)-propoxy]-9-hydroxy-6-methyl-6H-252-254
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-(3-hydroxy-
ropoxy)-6-methyl-6H-pyrrolo[3,4-cJcarbazole-285-288
1,3-dione
-(2-Chloro-phenyl)-8-(4-hydroxy-butoxy)-6-
221-223
ethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3,4-dihydroxy-butoxy)-6-
240-243
ethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-8-(4-methylamino-
204-208
utoxy)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
cetic acid 4-(2-chloro-phenyl)-1,3-dioxo-1,2,3,6-
293-296
etrahydro-pyrrolo[3,4-c]carbazol-9-yl
ester
-(2-Chloro-phenyl)-9-hydroxy-8-(4-methylamino-
212-214
utyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(9-Hydroxy-1,3-dioxo-1,2,3,6-tetrahydro-
330-334
yrrolo[3,4-c]carbazol-4-yl)-benzamide
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-272-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-6-methyl-8-piperidin-3-yl-6H-
444.1 /446.1
yrrolo[3,4-c]carbazole-1,3-dione
8-(3-Amino-pyrrolidine-1-carbonyl)-4-(2-chloro-
henyl)-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3- 4731475
ione;hydrochloride salt
-(2-Chloro-phenyl)-9-pyridin-2-yl-6H-
295-297
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-pyridin-4-yl-6H-
266-269
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-9-hydroxy-6-(2-hydroxy-ethyl)-6H-243-245
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-9-pyridin-2-yl-6H-
257-259
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-(3-pyrrolidin-1-yl-propoxy)-6H-244-247
yrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazol-9-yl]-3-300-310
iperidin-1-yl-propionamide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(3-
ethylamino-propoxy)-6H-pyrrolo[3,4-255-258(dec)
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(4-hydroxy-but-1-enyl)-6-
280-283
ethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(4-hydroxy-butyl)-6-methyl-
269-272
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-9-hydroxy-6-(3-hydroxy-propyl)-6H-180-183
yrrolo[3,4-c]carbazole-1,3-dione
-(3-Bromo-propyl)-4-(2-chloro-phenyl)-8-(3-
imethylamino-propoxy)-9-hydroxy-6H-296-300
(dec)
yarolo[3,4-c]cwbazole-1,3-dione
-(2-Chloro-phenyl)-8-(4-dimethylamino-3-
ydroxy-butoxy)-6-methyl-6H-pyrrolo[3,4-228-233
]carbazole-1,3-dione;hydrochloride
salt
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-273-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-8-hydroxy-6-(3-hydroxy-
283-288
ropyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(4-dimethylamino-butyl)-6-
258-262
ethyl-6H-pyrrolo[3,4-c)carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-8-(4-pyrrolidin-1-yl-
231-234
utyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-8-(4-methylamino-
232-236
utyl)-6H-pyrrolo[3,4-c)carbazole-1,3-dione
~4-(2-Chloro-phenyl)-9-hydroxy-6-(3-hydroxy-
Ipropyl)-8-(3-pyrrolidin-1-yl-propoxy)-6H-252-254
pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[3-(3,5-dimethyl-piperazin-
1-yl)-propoxy]-9-hydroxy-6-(3-hydroxy-propyl)-210-215
(dec)
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-(4-hydroxy-
utyl)-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-287-289
ione
8-{ 3-[Bis-(2-hydroxy-ethyl)-amino]-propoxy
}-4-
(2-chloro-phenyl)-9-hydroxy-6-methyl-6H-129-132
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(3-hydroxy-
ropyl)-8-[3-(4-methyl-piperazin-1-yl)-propoxy]-186-191
(dec)
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-9-nitro-
268-272
H-pyrrolo[3,4-c]carbazole-1,3-dione
-Amino-4-(2-chloro-phenyl)-6-(3-hydroxy-
230 (dec)
ropyl)-6H-pyrrolo[3,4-c)carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-9-nitro-1,3-dioxo-2,3-
ihydro-1H-pyrrolo[3,4-c]carbazol-6-yl]-propionic256-258
cid
-(2-Chloro-phenyl)-9-hydroxy-8-(4-hydroxy-
utoxy)-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-225-227
Tone
-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-
255-257
pethoxy-6H-pyrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-274-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-fluoro-8-methoxy-6-methyl-
347-351
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-fluoro-8-hydroxy-6-methyl-
305-310
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-6-(3-hydroxy-propyl)-6H-pyrrolo[3,4-208-211
]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-(3-
yrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4-208-210
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-fluoro-8-(3-hydroxy-
ropoxy)-6-methyl-6H-pyrrolo[3,4-c]carbazole-311-315
1,3-dione
-(2-Chloro-phenyl)-9-fluoro-6-methyl-8-(3-
ethylamino-propoxy)-6H-pyrrolo[3,4-317-321
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-
orpholin-4-yl-butyl)-6H-pyrrolo[3,4-c]carbazole-257-260
1,3-dione
-(2-Chloro-phenyl)-9-fluoro-6-methyl-8-(3-
yrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4-270-273
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(4-methyl-piperazin-1-yl)-propoxy]-6H-177-183
(dec)
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-diethylamino-propoxy)-
-hydroxy-6-(2-hydroxy-ethyl)-6H-pyrrolo[3,4-165-167
(dec)
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-9-fluoro-6-methyl-6H-pyrrolo[3,4-272-275
]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-1-
ethylene-3-oxo-1,2,3,6-tetrahydro-pyrrolo[3,4-193-196
Jcarbazol-9-yl]-formamide
-(2-Chloro-phenyl)-6-methyl-8-pyridin-4-yl-6H-
438.1
yrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-275-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-(3-hydroxy-
ropyl)-8-piperidin-4-yl-6H-pyrrolo[3,4- 504/506
)carbazole-1,3-dione
8-(3-(R)-Amino-pyrrolidine-1-carbonyl)-4-(2-
hloro-phenyl)-6-methyl-6H-pyrrolo[3,4- 473.1
]carbazole-1,3-dione; hydrochloride
salt
8-(3-(S) Amino-pyrrolidine-1-carbonyl)-4-(2-
chloro-phenyl)-6-methyl-6H-pyrrolo[3,4- 473.1
c]carbazole-1,3-dione; hydrochloride
salt
4-(2-Chloro-phenyl)-6-methyl-8-piperidin-4-yl-
444.1
6H-pyrrolo[3,4-c]carbazole-1,3-dione;
4-(2-Chloro-phenyl)-6-methyl-8-(piperazine-1-
ICarbonyl)-6H-pyrrolo[3,4-c]carbazole-1,3-dione; 473/475
I
ydrochloride salt
-(2-Chloro-phenyl)-8-(4-dimethylamino-butyl)-9-
ydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-246-250
Tone
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-
yrrolidin-1-yl-butyl)-6N-pyrrolo[3,4-c]carbazole-250-254
1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-
ethylamino-butyl)-6H-pyrrolo[3,4-c]carbazole-254 (dec)
1,3-dione
[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
300 (dec)
etrahydro-pyrrolo[3,4-c]carbazol-9-yl]-urea
-(2-Chloro-phenyl)-9-hydroxy-8-(3-hydroxy-2-
ethyl-propoxy)-6-methyl-6H-pyrrolo[3,4-264-266
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(4-dimethylamino-butoxy)-
-hydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole-224-226
1,3-dione
4-(2-Chloro-phenyl)-6-(3-methoxy-propyl)-8-
iperidin-3-yl-6H-pyrrolo[3,4-c)carbazole-1,3- 502.2
Tone; hydrochloride salt
-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-(3-
ethylamino-propoxy)-6H-pyrrolo[3,4-208-214
]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-276-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-Bromo-4-(2-chloro-phenyl)-6-(3-hydroxy-
ropyl)-8-(3-methylamino-propoxy)-6H-190-196
yrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-
1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-8-
221-224
Imethyl]-piperidine-1-carboxylic
acid tert-butyl
ster
-(2-Chloro-phenyl)-6-methyl-8-piperidin-4-
265-270
lmethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-6-(3-methoxy-propyl)-8-
iperidin-4-yl-6H-pyrrolo[3,4-c]carbazole-1,3- 502.1
ione;hydrochloride salt
4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-
iperidin-3-yl-6H-pyrrolo[3,4-c]carbazole-1,3- 488.2
Tone; hydrochloride salt
4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-
iperidin-4-yl-6H-pyrrolo[3,4-c]carbazole-1,3- 488.1
Tone; hydrochloride salt
4-(2-Chloro-phenyl)-6-methyl-8-(perhydro-1,4-
iazepine-1-carbonyl)-6H-pyrrolo[3,4-c]carbazole- 487/489
1,3-dione; hydrochloride
salt
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[4-(4-
ethyl-piperazin-1-yl)-butyl]-6H-pyrrolo[3,4-247-250
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-
yrrolidin-1-yl-butoxy)-6H-pyrrolo[3,4-243-245(dec)
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[4-(3,5-dimethyl-piperazin-
1-yl)-butoxy]-9-hydroxy-6-methyl-6H-pyrrolo[3,4-223-225
]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic
488/490
cid (2-dimethylamino-ethyl)-methyl-amide;
ompound with trifluoro-acetic
acid
-((S)-3-Amino-pyrrolidine-1-carbonyl)-4-(2-
hloro-phenyl)-6-(2-.hydroxy-ethyl)-6H-pyrrolo 503.1
[3,4-c]carbazole-1,3-dione;hydrobromide
salt
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-277-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
4-(2-Chloro-phenyl)-6-(2-hydroxy-ethyl)-8-
iperidin-4-yl-6H-pyrrolo[3,4-c]carbazole-1,3- 474.2
Tone; hydrobromide salt
-Amino-4-(2-chloro-phenyl)-6-(2-hydroxy-ethyl)-
221-224
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[3-(4-
ethyl-piperazin-1-yl)-propoxy]-6H-pyrrolo[3,4-209-212
]carbazole-1,3-dione
-[9-Amino-4-(2-chloro-phenyl)-1,3-dioxo-2,3-
ihydro-1H-pyrrolo[3,4-c]carbazol-6-yl]-N-(2->350
imethylamino-ethyl)-propionamide
-[4-(2-Chloro-phenyl)-9-formylamino-1,3-dioxo-
3-dihydro-1H-pyrrolo[3,4-c]carbazol-6-yl]-N-(2-240-244
imethylamino-ethyl)-propionamide
-Amino-4-(2-chloro-phenyl)-7-methoxy-6-methyl-
318-323
H-pyrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-9-hydroxy-1,3-dioxo-8-(3-
yrrolidin-1-yl-propoxy)-2,3-dihydro-1H-246-248
yrrolo(3,4-c]carbazol-6-yl]-propionamide
-(2-Chloro-phenyl)-6-methyl-8-pyrrolidin-3-yl-
H-pyrrolo[3,4-c]carbazole-1,3-dione; 428/430
rifluoroacetic acid salt
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic 403/405 NB.
apcl(neg)
cid
-[4-(2-Chloro-phenyl)-7-methoxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9-313-317
1]-formamide
-(2-Chloro-phenyl)-7-hydroxy-6-methyl-9-nitro-
>300
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[2-(1-
ethyl-pyrrolidin-2-yl)-ethoxy]-6H-pyrrolo[3,4-232-235
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[4-(4-
ethyl-piperazin-1-yl)-butoxy]-6H-pyrrolo[3,4-210-212
]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-278-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(4-
orpholin-4-yl-butoxy)-6H-pyrrolo[3,4-212
]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-(2-hydroxy-ethyl)-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9-273-275
1]-formamide
-(2-Fluoro-6-methoxy-phenyl)-9-hydroxy-6H-
377
yrrolo(3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-
ethoxy-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4- 492/494
]carbazol-9-yl]-acetamide
-[4-(2-Chloro-phenyl)-6-(3-hydroxy-propyl)-8-
ethoxy-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4- 478/480
]carbazol-9-yl]-formamide
-[4-(2-Chloro-phenyl)-8-hydroxy-6-(3-hydroxy-
ropyl)-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4- 464/466
]carbazol-9-yl]-formamide
-Butyl-4-(2-chloro-phenyl)-9-hydroxy-8-(3-
yrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4-222-225
.]carbazole-1,3-dione
8-(3-(S)-Amino-pyrrolidine-1-carbonyl)-4-(2-
hloro-phenyl)-6-(3-hydroxy-propyl)-6H- 517
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-8-pyrrolidin-2-yl-
430/432
H-pyrrolo[3,4-c]carbazole-1,3-dione
8-(4-Amino-butyl)-4-(2-chloro-phenyl)-6-methyl-
432/434
6H-pyrrolo[3,4-c]carbazole-1,3-dione
I
~,2-Dimethylamino-ethanesulfonic
acid {3-[9-amino-
4-(2-chloro-phenyl)-1,3-dioxo-2,3-dihydro-1H->350
Ipyrrolo[3,4-c]carbazol-6-yl]-propionyl}-amide
2-Dimethylamino-ethanesulfonic
acid {3-[4-(2-
~chloro-phenyl)-9-formylamino-1,3-dioxo-2,3-
>370
dihydro-1 H-pyrrolo[3,4-c]carbazol-6-yl]-
propionyl }-amide
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-279-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(2-
ethyl-3-pyrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4-242-244
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-(3-hydroxy-2,2-
imethyl-propoxy)-6-methyl-6H-pyrrolo[3,4-290-293
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-methoxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 435/437
arboxylic acid
-(2-Chloro-phenyl)-9-methoxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 434/436
arboxylic acid amide
-(2-Chloro-phenyl)-9-methoxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 531/533
arboxylic acid (2-pyrrolidin-1-yl-ethyl)-amide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-cJcarbazole-8- 419/421 (APCIneg)
arboxylic acid
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 420/422
arboxylic acid amide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 517/519
arboxylic acid (2-pyrrolidin-1-yl-ethyl)-amide
-[4-(2-Chloro-phenyl)-7-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9->350
1]-formamide
-(2-Chloro-phenyl)-6-(2-hydroxy-ethyl)-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo(3,4-c]carbazole-8- 449.1
arboxylic acid methyl ester
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic 501/503
cid (2-pyrrolidin-1-yl-ethyl)-amide
-(4-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-9-hydroxy-1,3-dioxo-2,3-dihydro-1H-235-237(dec)
yrrolo[3,4-c]carbazol-6-yl]-propionamide
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-280-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-8-[3-(ethyl-propyl-amino)-
ropylsulfanyl]-9-hydroxy-6-methyl-6H- 520/522
yrrolo[3,4-c]carbazole-1,3-dione
-Hydroxy-6-methyl-4-phenyl-8-(3-pyrrolidin-I-yl-
248-250
ropoxy)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-Amino-4-(2-chloro-phenyl)-6-methyl-7-vinyl-
>350
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-(R)dimethylamino-
yrrolidine-1-carbonyl)-6-(2-hydroxy-ethyl)-6H- 530.2
yrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-7-
inyl-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9-248-254
1]-formamide
-[4-(2-Chloro-phenyl)-7-(
1,2-dihydroxy-ethyl)-6-
ethyl-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4->340
] carbazol-9-yl]-formamide
-[4-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8-310-315
arbonyl]-methanesulfonamide
-Dimethylamino-ethanesulfonic
acid [4-(2-chloro-
henyl)-9-hydroxy-6-methyl-1,3-dioxo-1,2,3,6-
285-293
etrahydro-pynolo[3,4-c]carbazole-8-carbonyl]-
mide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 448/450
arboxylic acid dimethylamide
-(2-Chloro-phenyl)-9-hydroxy-8-(3-morpholin-4-
178-184
I-propoxy)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(2-
yrrolidin-1-yl-ethoxy)-6H-pyrrolo[3,4-242-244
]carbazole-1,3-dione
8-(3-Amino-propoxy)-4-(2-chloro-phenyl)-9-
ydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-293-296
Tone; hydrochloride salt
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-281-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(2-
ethyl-3-pyrrolidin-I-yl-propoxy)-6H-pyrrolo(3,4-245-248
]carbazole-1,3-dione
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic 503/505
cid (2-diethylamino-ethyl)-amide
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo(3,4-c]carbazole-8-carboxylic 544/546
cid [3-(4-methyl-piperazin-1-yl)-propyl]-amide
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic 533/535
cid (3-diethylamino-2-hydroxy-propyl)-amide
-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-1,2,3,6-
etrahydro-pyrrolo[3,4-c]carbazole-8-carboxylic 515/517
cid (3-pyrrolidin-I-yl-propyl)-amide
-(2-Chloro-phenyl)-8-(3-(S)-dimethylamino-
yrrolidine-1-carbonyl)-6-(2-hydroxy-ethyl)-6H-
531.2
yrrolo[3,4-c]carbazole-1,3-dione;
Trifluro acetic
cid salt
-(2-Chloro-phenyl)-6-(2-hydroxy-ethyl)-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo(3,4-c]carbazole-8- 531.3
arboxylic acid (2-pyrrolidin-1-yl-ethyl)-amide
-(2-Chloro-phenyl)-6-(2-hydroxy-ethyl)-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 432.1
arboxylic acid amide
-(2-Chloro-phenyl)-8-(3-dimethylamino-
ropoxy)-9-hydroxy-6H-pyrrolo[3,4-c]carbazole-155-160
(dec)
1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-[3-(4-methyl-
iperazin-1-yl)-propoxy]-6H-pyrrolo[3,4-238-244
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-(3-pyrrolidin-I-
170-175
(dec)
1-propoxy)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(2-
orpholin-4-yl-ethoxy)-6H-pyrrolo(3,4-292-294
]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-282-
COMPOUND NAME MP (deg APCI (pos)Found
I C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[2-(4-
ethyl-piperazin-1-yl)-ethoxy]-6H-pyrrolo[3,4-272-274
]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-8-methoxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazol-9-338-342
1]-formamide
-(2-Chloro-phenyl)-9-hydroxy-8-(3-hydroxy-
235-239
ropoxy)-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8-
505/507
arboxylic acid (2-dimethylamino-ethyl)-methyl
amide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
6-tetrahydro-pyrrolo[3,4-c]carbazole-8-
ioxo-1
2
3
, 531/533
,
,
arboxylic acid (1-ethyl-pyrrolidin-2-ylmethyl)-
mide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 533/535
arboxylic acid (3-diethylamino-propyl)-amide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(
1 H- 445/447
etrazol-5-yl)-6H-pyn'olo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8-
531/533
arboxylic acid [2-(1-methyl-pyrrolidin-2-yl)-
thyl]-amide
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-{ 3-[(2-hydroxy-ethyl)-methyl-amino]- 538/540
ropoxy}-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-{
3-[(2-hydroxy-
thyl)-methyl-amino]-propoxy 508/510
}-6-methyl-6H-
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-(3-piperi.din-1-yl-propoxy)-6H-
48/550
yrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-283-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(3-
iperidin-1-yl-propoxy)-6H-pyrrolo[3,4- 518/520
]carbazole-1,3-dione
8-[3-(Benzyl-methyl-amino)-propoxy]-4-(2-chloro-
henyl)-9-hydroxy-6-(2-hydroxy-ethyl)-6H- 584/586
yrrolo[3,4-c]carbazole-1,3-dione
~8-[3-(Benzyl-methyl-amino)-propoxy]-4-(2-chloro-
phenyl)-9-hydroxy-6-methyl-6H-pyrrolo[3,4- 554/556
c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
ethyl)-8-[3-(4-pyridin-2-yl-piperazin-1-yl)- 626/628
propoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(3-(4-
pyridin-2-yl-piperazin-1-yl)-propoxy]-6H- 596/598
pyrrolo[3,4-c]carbazole-1,3-dione
I
-(2-Chloro-phenyl)-8-(3-dipentylamino-propoxy)-
-hydroxy-6-(2-hydroxy-ethyl)-6H-pyrrolo[3,4- 620/622
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dipentylamino-propoxy)-
-hydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole- 590/592
1,3-dione
-(2-Chloro-phenyl)-8- {
3-[(2-dimethylamino-
thyl)-methyl-amino]-propoxy
}-9-hydroxy-6-(2-
565/567
ydroxy-ethyl)-6H-pyrrolo[3,4-c]carbazole-1,3-
ione
-(2-Chloro-phenyl)-8-{ 3-[(2-dimethylamino-
thyl)-methyl-amino]-propoxy 535/537
}-9-hydroxy-6-
ethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(3-hydroxy-pyrrolidin-1-yl)-propoxy]- 550/552
H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-[3-(3-hydroxy-
yrrolidin-1-yl)-propoxy]-6-methyl-6H- 520/522
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[3-(cyclohexyl-methyl-
mino)-propoxy]-9-hydroxy-6-(2-hydroxy-ethyl)- 576/579
H-pyrrolo[3,4-c]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-284-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-8-[3-(cyclohexyl-methyl-
mino)-propoxy]-9-hydroxy-6-methyl-6H- 546/548
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(2-methyl-piperidin-1-yl)-propoxy]-6H- 562/564
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[3-(2-
ethyl-piperidin-1-yl)-propoxy]-6H-pyrrolo[3,4- 532/534
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(2-hydroxymethyl-piperidin-1-yl)- 578/580
ropoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-[3-(2-
ydroxymethyl-piperidin-1-yl)-propoxy]-6-methyl- 548/550
H-pyrrolo(3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(methyl-pyridin-3-ylmethyl-amino)- 585/587
ropoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-[3-
(methyl-pyridin-3-ylmethyl-amino)-propoxy]-6H- 555/557
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[3-(2-hydroxymethyl-pyrrolidin-1-yl)- 564/566
ropoxy]-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-8-(3-(2-
ydroxymethyl-pyrrolidin-1-yl)-propoxy]-6- 534/536
ethyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[3-(ethyl-methyl-amino)-
ropoxy]-9-hydroxy-6-(2-hydroxy-ethyl)-6H- 522/524
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[3-(ethyl-methyl-amino)-
ropoxy]-9-hydroxy-6-methyl-6H-pyrrolo[3,4- 492/494
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dipropylamino-propoxy)-
-hydroxy-6-(2-hydroxy-ethyl)-6H-pyrrolo[3,4- 564/566
]carbazole-1,3-dione
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-285-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-(2-Chloro-phenyl)-8-(3-dipropylamino-propoxy)-
-hydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole- 534/536
1,3-dione
-(2-Chloro-phenyl)-8-(3-diethylamino-propoxy)-
-hydroxy-6-methyl-6H-pyrrolo[3,4-c]carbazole- 506/508
1,3-dione
~8-{ 3-[Bis-(3-methyl-butyl)-amino]-propoxy}-4-(2-
chloro-phenyl)-9-hydroxy-6-methyl-6H- 590/592
i
pyrrolo[3,4-c]carbazole-1,3-dione
4-(2-Chloro-phenyl)-8-[3-(2,6-dimethyl-piperidin-
1-yl)-propoxy]-9-hydroxy-6-(2-hydroxy-ethyl)-6H- 576/578
pyrrolo[3,4-c]carbazole-1,3-dione
~4-(2-Chloro-phenyl)-8-[3-(2,6-dimethyl-piperidin-
~~1-yl)-propoxy]-9-hydroxy-6-methyl-6H- 546/548
yrrolo[3,4-c]carbazole-1,3-dione
-Hydroxy-6-(2-hydroxy-ethyl)-4-phenyl-8-(3-
yrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4- 500.2
]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-(3-dicyclohexylamino-
ropoxy)-9-hydroxy-6-(2-hydroxy-ethyl)-6H-
644/646
yrrolo[3,4-c]carbazole-1,3-dione;
trifluoro-acetic
cid salt
-(2-Chloro-phenyl)-8-(3-diisopropylamino-
ropoxy)-9-hydroxy-6-(2-hydroxy-ethyl)-6H-
564/566
yrrolo[3,4-c]carbazole-1,3-dione;trifluoro-acetic
cid salt
-Amino-4-(2-chloro-phenyl)-6-methyl-8-(3-
yrrolidin-1-yl-propoxy)-6H-pyrrolo[3,4-285-288
]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-8-(3-
yrrolidin-1-yl-propoxy)-1,2,3,6-tetrahydro-262-264
yrrolo[3,4-c]carbazol-9-yl)-formamide
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-1,3-
ioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-c]carbazole-8- 553/555
ulfonic acid (2-pyrrolidin-1-yl-ethyl)-amide
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-286-
COMPOUND NAME MP (deg APCl (pos)Found
C) Mass
-(2-Chloro-phenyl)-8-(3-cyclohexylamino-
ropoxy)-9-hydroxy-6-methyl-6H-pyrrolo[3,4- 532/534
)carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(3-
yrrolidin-1-yl-propane-1-sulfinyl)-6H-pyrrolo[3,4-305-312
(dec)
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(3-
yrrolidin-1-yl-propane-1-sulfonyl)-6H-252-258
(dec)
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-(4-pyrrolidin-1-yl-butyl)-6H-pyrrolo[3,4-198-203
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-(4-morpholin-4-yl-butyl)-6H-pyrrolo[3,4-269-272
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-(2-hydroxy-
thyl)-8-[4-(4-methyl-piperazin-I-yl)-butyl]-6H-196-201
yrrolo[3,4-c]carbazole-1,3-dione
-(2-Chloro-phenyl)-8-[3-(ethyl-propyl-amino)-
utoxy]-9-hydroxy-6-methyl-6H-pyrrolo(3,4-238-240
]carbazole-1,3-dione
-(2-Chloro-phenyl)-9-hydroxy-6-methyl-8-(
1-
m
ethyl-3-pyrrolidin-i-yl-propoxy)-6H-pyrrolo(3,4-225-227
is]carbazole-1,3-dione
~9-Amino-4-(2-chloro-phenyl)-8-(4-hydroxy-butyl)-
224-227
-methyl-pyrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-8-(4-
yrrolidin-1-yl-butyl)-1,2,3,6-tetrahydro-252-257
yrrolo[3,4-c]carbazol-9-yl]-formamide
-{ 4-(2-Chloro-phenyl)-6-methyl-8-[4-(4-methyl-
iperazin-1-yl)-butyl]-1,3-dioxo-1,2,3,6-tetrahydro-270-273
yrrolo[3,4-c]carbazol-9-yl
}-formamide
-[4-(2-Chloro-phenyl)-6-methyl-8-(4-morpholin-
-yl-butyl)-1,3-dioxo-1,2,3,6-tetrahydro-205-209
yrrolo[3,4-c]carbazol-9-yl]-formamide
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-287-
COMPOUND NAME MP (deg APCI (pos)Found
C) Mass
-Amino-4-(2-chloro-phenyl)-7-(4-hydroxy-butyl)-
202-208
-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-dione
-[4-(2-Chloro-phenyl)-7-(4-hydroxy-butyl)-6-
ethyl-1,3-dioxo-1,2,3,6-tetrahydro-pyrrolo[3,4-230-243
]carbazol-9-yl]-formamide
-[4-(2-Chloro-phenyl)-6-methyl-7-(4-morpholin-
-yl-butyl)-1,3-dioxo-1,2,3,6-tetrahydro-256-260
yrrolo[3,4-c]carbazol-9-yl]-formamide
-( 4-(2-Chloro-phenyl)-6-methyl-7-[4-(4-methyl-
iperazin-1-yl)-butyl]-1,3-dioxo-1,2,3,6-tetrahydro-205-209
yrrolo[3,4-c]carbazol-9-yl
l-formamide
-[4-(2-Chloro-phenyl)-7-(4-dimethylamino-
utyl)-6-methyl-1,3-dioxo-1,2,3,6-tetrahydro-257-259
yrrolo[3,4-c]carbazol-9-yl]-formamide
-[4-(2-Chloro-phenyl)-6-methyl-1,3-dioxo-7-(4-
yrrolidin-1-yl-butyl)-1,2,3,6-tetrahydro-238-240
yrrolo[3,4-c]carbazol-9-yl]-formamide
-Amino-4-(2-chloro-phenyl)-6-methyl-7-(4-
orpholin-4-yl-butyl)-6H-pyrrolo[3,4-c]carbazole-158-162
(dec)
1,3-dione
-Amino-4-(2-chloro-phenyl)-6-methyl-7-(4-(4-
ethyl-piperazin-1-yl)-butyl]-6H-pyrrolo[3,4-162-166
(dec)
]carbazole-1,3-dione
-Amino-4-(2-chloro-phenyl)-7-(4-dimethylamino-
utyl)-6-methyl-6H-pyrrolo[3,4-c]carbazole-1,3-211-215
Tone
-Amino-4-(2-chloro-phenyl)-6-methyl-7-(4-
yrrolidin-1-yl-butyl)-6H-pyrrolo[3,4-c]carbazole-204-206
1,3-dione
EXAMPLE 484
Weel inhibition filter binding? assay (OY) to test for Weel inhibition
activity
This assay provides a measure of inhibitory ability of the test compounds
against
isolated Weel kinase.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-288-
The Weel kinase assay measures enzyme mediated phosphorylation of tyrosine on
a
synthetic peptide substrate in the presence of compounds being tested. The
assay was carried
out in 96-well filter microtiter plates (Millipore #MADP NOB 10). Compounds
were
dissolved and diluted in DMSO. 10 ~l 3 x EDB buffer (150 mM Tris, pH 8.0, 30
mM NaCI,
30 mM MgCl2~ 3 mM DTT), 18 ~1 water, and 2 ~1 of drug dilution were added to
the test
wells and mixed thoroughly. 10 ~l of enzyme-substrate mixture was added to the
wells. The
Weel enzyme (human Weel kinase aa215-647, Onyx Pharmaceuticals, expressed in
and
purified from a baculovirus protein expression system) concentration was 0.01
~.g/~.l and the
substrate (Poly Ornithine:Tyrosine (4:1), Sigma Chemical Co.) was 0.6 pg/pl in
1 x EDB
buffer. The plates were-mixed thoroughly for 5 minutes at room temperature.
The reaction
was started by adding 10 ~1 of 1 x EDB buffer containing 47.5 ~.M ATP (Sigma)
and
0.026 p.Ci/~1 y-32P-ATP (ICN Biomedicals, Inc.) The plates were mixed at room
temperature
for 20 minutes. The reaction was stopped by adding 50 ~.1 of ice cold 20% TCA
with 0.1 M
tetrasodium pyro-phosphate. Plates were incubated on ice or refrigerated at
4°C for 1 hour.
Liquid reaction mixture was removed on a vacuum manifold, and the precipitated
phosphorylated substrate was rinsed 5 times with 200 ~1 ice cold 10% TCA with
0.1 M
tetrasodium pyrophosphate. 25 pl liquid scintillation cocktail were added to
the membrane
bound substrate and the plate read in a Microbeta (Perkin-Elmer) plate reader.
Activity of
compounds was calculated in comparison to uninhibited control determinations
in each assay.
The data shown in Table 3 demonstrates activities (ICgpl of Weel less than 0.1
p.M
ranging up to 1.65 ~M. However, Compound X with the highest Weel activity on
this table
is a representative compound from US Patent No. 4,912,107. Compound X does not
fall
within the ambit of this invention either structurally or as a checkpoint
abrogator of the
present invention. The activity of Compound X against Weel kinase in this
assay is less
potent than the upper limit for the preferred compounds. The data in Table 3
shows that the
modification of the core structure leads to unexpected potency in the
inhibition of Weel
kinase and a selectivity of at least 10-fold over the inhibition of the PKC
enzyme.
The compounds of the present invention are considered to be suitable for use
in an
animal to treat cell proliferative diseases either alone or in conjunction
with one or more
other antineoplastic modalities if the Weel activity has an ICSoof less than 1
~tM in the assay
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-289-
described above. Preferably the compounds have an ICSO of less than 0.5 ~M and
most
preferably an ICSO of less than 0.1 ~,M
TABLE 3. Weel,
Chkl and PKC
Activity
WEElOY CHK1A PKC
IC50(1xM)IC50 (~M)
ICgO
(wM)
Compound X 1.65 0.297 3.6
Compound of Example0.263 0.056 1.8
94
Compound of Example0.006 0.167 1.49
80
Compound of Example0.01 0.435 0.48
68 I
___ Compound of Example0.428 >10 >4
369
Compound of Example0.009 4.46 I.5
230
EXAMPLE 485
Chkl Enzyme Inhibition Asst
In order to determine the inhibitory activity of the compounds of the present
invention
against Chkl, the following assay was performed to measure the inhibition of
isolated Chkl
enzyme.
The assay was carried out in round bottom polypropylene 96-well plates
(Costar).
Compounds were tested in serial dilutions beginning with a high concentration
of 50 l.tM
followed by up to nine 3-fold dilutions. Compounds were dissolved and diluted
in DMSO.
2 ~tl of drug were spotted on the bottom of the assay plates, then diluted
with 58 ~1 of Chkl
buffer (20 mM Tris, pH 8.0, 50 mM NaCI, 10% Glycerol, 10 mM MgCl2, 5 mM
dithiothreitol), and mixed at room temperature for 1 minute. 20 ~.1 of buffer
containing
250 rlg/well Chkl enzyme (Onyx Pharmaceuticals) and 1 ~g/well GST-Cdc25
substrate
(Onyx) were added. Contents of the wells were mixed for 1 minute and incubated
at room
temperature for 10 minutes. 20 ~.1 of buffer containing 20 ~M ATP and 0.4 ~tCi
ATP [y-33p~
were added. The contents were mixed for 1 minute and incubated at 30°C
for 30 minutes. The
reaction was stopped by adding 50 ~1 of 120 mM EDTA to each well, except the
control
wells already containing EDTA. 140 ~1 of the contents of the wells were
transferred to the
wells of Reacti-Bind Glutathione Coated White 96-Well Plates (Pierce).
Contents were mixed
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-290-
for 1 minute and incubated at room temperature for 1 hour. All of the wells
were rinsed three
times each with 300 ~tl PBS and air dried. 200 ~l MicroScint 20 (Packard) was
put in the
wells. Plates were sealed with an adhesive cover and counted in a Top Count
Microplate
Scintillation counter (Packard). Activity of compounds was calculated in
comparison to
uninhibited control determinations in each assay.
The data shown in Table 4 demonstrates activities (IC50) of Chkl less than
0.002 ~,M
ranging up to 0.297 pM. However, Compound X with the highest Chkl activity on
this table
is a representative compound from US Patent No. 4,912,107. Compound X does not
fall
within the ambit of this invention either structurally or as a checkpoint
abrogator of the
present invention. The activity of Compound X against Chkl kinase in this
assay is less
-potent than the upper limit for the preferred compounds. The data in Table 4
shows that the
modification of the core structure leads to unexpected potency in the
inhibition of Chkl
kinase and a selectivity of at least 10-fold over the inhibition of the PKC
enzyme.
The compounds of the present invention are considered to be suitable for use
in an
animal to treat cell proliferative diseases in conjunction with another
antineoplastic modality
if the Chkl activity has an ICSO of less than 0.275 ~,M in the assay described
above.
Preferably the compounds have an ICSO of less than 0.2 p.M and most preferably
an ICSO of
less than 0.1 ~M
TABLE 4. Weel, Chkl and PKC Activity
WEElOY CHK1A PKC
IC50(ttM) IC50 (ttM) IC50 (~M)
Compound X 1.65 0.297 3.6
Compound of
Example 198 0.193 0.013 0.132
Compound of
Example 214 0.295 0.002 0.171
Some of the compounds of the present invention are dual inhibitors, being
selective
for inhibiting both Weel and Chkl activity. These compounds may be equal in
efficacy to
the compounds that selectively inhibit either Weel or Chkl even if the
activity against either
enzyme is higher than that desired for the single inhibitors. Preferably the
dual inhibitors
have an IC 50 of less than 1 ~M, more preferably less than S00 nM and most
preferably less
than 100 nM.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-291
TABLE 5. Dual Inhibitors
WEElOY CHK1A
IC50(pM) IC50 (~M)
Compound of Example 363 0.057 0.02
Compound of Example 366 0.075 0.014
EXAMPLE 486
Mvt-1 Inhibition Scintillation Proximity Assay to Test for Myt-1 Inhibition
Activity
This assay provides a measure of inhibitory activity of the test compounds
against
isolated Myt-1 kinase.
The assay was carried out in 96-well SPA (Scintillation Proximity Assay)
plates from
Wallac. The compounds were tested at 501rM followed by up to nine 3-fold
dilutions (i.e.,
50, 16.67, 5.56, 1.85, 0.617, etc.). Drugs were dissolved and diluted in DMSO.
2 ~1 of drug
were spotted on the bottom surface of the wells of the SPA plates. 30 ~1 of MB
buffer
(50 mM Tris, pH 8.0, 100 mM NACI, 0.1% Tween 20, 1 mM MgCl2~ 100 ~M DTT)
containing 27 ~.M cold ATP were added to each well and then 9 ~1 of MB buffer
with
177.8 r1M Myt-1 enzyme (MW = 54.6 kDa). Plates were mixed at room temperature
for
1 minute, then incubated at room temperature for 5-15 minutes. 9 ~1 MHKB
buffer (50 mM
Tris, pH 8.0, 1 mM MgCl2, 100 ~M DTT, 0.1 mM Na3V04) with 88.9 r1M Cdc2B
(MW = 84.5 kDa) were added, mixed at room temperature for 1 minute. The plates
were
centrifuged up to 2400 RPM, and incubated for 30 minutes at 30°C. 30 ~1
MHKB buffer
containing 1 ~tM biotinylated histone H1 (Amersham) peptide substrate (stock:
1 mM) and
~tCi/~1 ATP [y-33P]) were added. The plates were mixed at room temperature for
1 minute, centrifuged up to 2400 RPM, and incubated for 30 minutes at
30°C. The reaction
was stopped with 200 ~1 stop buffer, 5 mM EDTA and 0.1 % Triton X-100 (from
lOX stock
of 50 mM EDTA and 1.0% Triton X-100 diluted with PBS) with at least 20 ~g/ml
Streptavidin SPA beads (Amersham) and 50 ~,M ATP. Plates were sealed with an
adhesive
cover, mixed by inverting 10 times, and incubated for 10 minutes at room
temperature. The
plates were centrifuged at 2400 RPM for 15 minutes at room temperature and
counted in
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-292-
Wallac Microbeta Trilux counter. Activity of compounds was calculated in
comparison to
uninhibited control determinations in each assay.
None of the compounds of the present invention had IC 5o forMyt-1 activity in
this
assay of less than 10 pM. Since phosphorylation of thr 14 on cdc2 (catalyzed
by Myt-1
kinase) is also a checkpoint establishing event, a compound that inhibits both
Weel kinase
and Myt-1 kinase may be a checkpoint abrogator due to its dual inhibitory
activity. Lack of
Myt-1 inhibition, therefore, rules out the contribnution of Myt-1 to check
point abrogation.
'The absence of significant Myt-1 inhibitory activity of the compounds of the
present
invention demonstrates the selectivity of theses compounds as Weel kinase and
Chkl kinase
inhibitors. _ _ _ ___ .
-EXAMPLE 487- __ _ _ _ _ _ _ _ _ _ _ .
Protein Kinase C (PKC) Assay
The PKC assay provides a measure of inhibitory activity of the test compounds
against PKC contained in a rat brain preparation.
Enzyme was prepared from a preparation of rat brain (Promega) 0.5 pg diluted
with
1.6 ml of 10 mM Hepes buffer pH 7.5. Reaction buffer was comprised of 150 mM
Hepes
buffer, 4 mM CaCl2, 15 mM MgCl2, 3 mM EDTA, 3.75 mM EGTA at pH 7.5. Histone
substrate was made by dissolving histone Hl (Sigma) in water at 1.5 mg/ml.
Phosphatidylserine/diolein liposomes were made by mixing 75 pl of 10 mg/ml
phosphatidylserine (Sigma) in CHCI3 with 60 ~tl of 2.5 mg/ml diolein in CHCI3
(Sigma) in a
glass vial and evaporating the CHCI3 under N2. The film was suspended in 1 ml
water and
sonicated 6 x 15 sec with a microtip probe at room temperature.
To carry out the assay, 50 p,l buffer, 20 pl histone, 20 ~l liposomes, and
inhibitor or
solvent control were added to wells of a 96-well filter plate (Millipore) with
enough water, if
necessary, to make the final volume 110 p.l. 20 pl of enzyme preparation
solution were added
and incubated for 10 minutes at room temperature. This was followed by the
addition of 20 p,l
of 32P ATP (75 pM ATP in water, labeled ATP at 25 ~Ci/ml) which was incubated
for
15 minutes at room temperature. The reaction was terminated by the addition of
50 ~1 of 40%
(w/v) ri-ichloroacetic acid. The filters were washed by vacuum filtration with
S x 125 pl of ice
cold 10% (w/v) trichloroacetic acid. The filters were placed in scintillation
fluor and counted
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-293-
to determine precipitated labeled phosphate incorporated into substrate. The
per cent
inhibition was calculated in comparison to the uninhibited controls.
EXAMPLE 488
Cell-Based 96-Well Cdc2 Histone H1 Kinase Assay for G2 Checkpoint Abrogation
This cellular assay is a measure of the effect of the test compounds on the
activity of
the Cdc2/cyclin B complex on one of its physiological substrates, Histone H1.
HT-29 cells 20,000 per well (NL1NCLONT"' cat no. 163320 96-well plate) were
plated
in 171 ~1 media [Dulbecco's Modified Eagle's Medium 4500 mg/L Glucose (DME
High
Glucose), 1% penicillin and streptomycin, 2% L-Glutamate, 10%FBS]. The plate
was
incubated at 37°C for 24 hours. 9 p.l of a 5 ~.M Adriamycin solution
was added to each well
(250-nM final concentration) and incubated at 37°C for an additional 16
hours. Next, 20 ~,1
of 500 ng/mL nocodazole was added per well immediately followed by addition of
5 ~,1 test
compound. The plate was incubated at 37°C for 4 hrs. The plate was
removed from the
incubator and spun in a Beckman GS-6R Centrifuge for 10 minutes, 800 rpm,
4°C. The
media was removed and the plate surface dried by blotting. 100 ~I. of PBS was
added to
each well. The plate was spun as above. The PBS was removed from plate and the
plate
surface was dried. 20 pl of lysis buffer (50 mM Hepes pH 7.5, 250 mM NaCI,
0.1% NP 40,
mM ~3-Glycerophosphate, l mM NaF, l mM EDTA, l mM Pefabloc, l mM DTT, 0.1 mM
sodium orthovanadate,l0 pg/ml Aprotinin, 20 ~M Leupeptin) was then added to
each well
followed by medium-speed rocking at 4°C for 45 minutes. After lysis, 30
pT. of kinase assay
buffer (50 mM Hepes, 22 mM MgCl2, 1 mM DTT, 166.7 ng/pl Histone H1, 83 p.M
ATP,
C.U33 ~tCi/~1 ['y-33P]ATP) was added. The plate was incubated on a 32°C
plate warmer for
25 minutes. The kinase reaction was stopped by adding 80 pl of 100 mM EDTA pH
7.8 to
each well. The lysate was harvested onto a pre-wetted Wallac P-30 filtermat
(Wallac
1450-523, glass fiber filter with negatively charged P30 active groups size 90
x 120 mm)
using 75 mM H3P04 for 10 seconds, followed by a 10 second aspiration step. The
filtermat
v,~as placed in a 75 mM H3P04 bath and shaken gently for 10 minutes at room
temperature,
then placed within the fold of a single sheet of paper towel, and subjected to
microwaves on
high power for 2 to 3 minutes, or until filtermat is dry. The filtermat was
placed in a sample
bag (Wallac 1450-432), 5-ml nonaqueous scintillation fluid was added to the
sample bag and
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-294-
the bag was sealed. The samples were read in a Wallac 1450 MicroBeta Liquid
Scintilation
Counter. The data shown in Table 6 demonstrate the cellular effect of a Weel
and/or Chkl
inhibitor on a physiological substrate of cdc2/cyclinB which complex is itself
a substrate of
Weel kinase.
TABLE 6. Histone Kinase Assay
WEEIOY CHK1A Histone Kinase
ICsn(ul~ ICsn (u~ ICSn (wM)
Compound of 0.006 0.166 0.646
Example 80
Compound of 0.1933 0.013 0.798
Example 198
Compound of 0.057 0.023 0.505
Example 363
EXAMPLE 489
Procedure for Clonogenic Assays in HT-29 Cells ~ Adriamycin
This cellular assay is a measure of the toxicity of the test compounds in the
absence
and presence of DNA damage induced by a conventional chemotherapeutic agent.
HT-29 cells were grown in Dulbecco's Modified Eagle Medium with high glucose,
supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 16 mM HEPES, 8 mM
MOPS, and 10% fetal bovine serum. The cells were incubated at 37°C in
an atmosphere of
5% C02 and 100% relative humidity.
Two or three T-75 tissue culture flasks were seeded at about 50% confluency in
30 ml
media and incubated for approximately 24 hours. Adriamycin (ADR) (dissolved at
5 mM in
distilled water (dH20)) was added to the flasks to a final concentration of 1
uM or 500 nM.
One flask received no ADR. The cells were allowed to incubate with the ADR for
1 hour. All
flasks were then washed twice with 20 ml media allowed to incubate a further
16 hours in
30 ml media.
The stock agar (3.2% Seaplaque GTG Agarose (BioWhittaker Molecular
hpplications)) was suspended in distilled water and autoclaved for 20 minutes.
The agar was
melted prior to use in a microwave oven. The bottom agar was a 1:4 dilution of
the stock agar
in media with enough fetal bovine serum added to bring the solution to 10%.
One microliter
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-295-
was plated in each well of 6-well tissue culture plates and the plates allowed
to harden. The
cloning agar (a 1:8 dilution of stock agar in media with fetal bovine serum
added to 10%)
was prepared and held at 40°C until used.
The cells were trypsinized using Trypsin-EDTA and their concentration adjusted
to
75,000 cells per microliter with media. One hundred microliters of each cell
suspension were
placed into sterile 15 ml plastic centrifuge tubes. Twenty-five microliters of
each test
compound was added to appropriate tubes, followed by addition of 5 ml of warm
cloning
agar. The tubes were mixed well and 2 ml of the agar/cell suspension were
added to duplicate
wells of the 6-well plates that were coated with agar earlier. The plates were
swirled and
placed in the refrigerator for 5 minutes. After the plates returned to room
temperature they
were incubated for 10 to 14 days, until colonies were visible. The colonies
were stained with
INT (p-Iodonitrotetrazolium Violet) (dissolved in dH20 at 1 mg/ml and filter
sterilized). One
milliliter INT was added to each well and the plates incubated overnight at
37°C, 5% C02,
and-1-00% relative humidity. The colonies were counted using a Hamamatsu video
imaging
system and ImageQuant software.
TABLE 7. Clonogenic Assay Data
% of Control Colonies % of Control Colonies
With No DNA Damage With DNA Damage
Compound of Example 366 38% 2.8%
(250 nM)
EXAMPLE 490
Procedures for Western Blot determination of phosphotyrosine 15 on Cdc-2 and
mobility
shift of Myt-1.
These western blot assays measure the phosphorylation state of the physiologic
substrate of Weel: tyrosine 15 on Cdc2 kinase. This is accomplished by means
of a
phosphospecific antibody whose signal is normalized by comparison to the total
amount of
Cdc2 detected in the samples. The shift of the Myt-1 protein to a lower
mobility on a Western
blot is used as a measure of progression into M phase of the cell cycle.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-296-
A. Procedure for detecting phosphotyrosine 15, Myt-1 shift and total Cdc-2
from cultured
HT-29 cells in response to potential check point abrogators ~ Adriamycin
and/or
Nocodazole
HT-29 cells were grown in Dulbecco's Modified Eagle Medium with high glucose,
supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 16 mM HEPES, 8 mM
MOPS, and 10% fetal bovine serum. The cells were incubated at 37°C, in
5% C02, and
100% relative humidity.
Cells were grown and treated in 6-well tissue culture plates. Cells were
seeded in
3 mL media at a concentration of 200,000 per mL. Once seeded the cells were
allowed to
attach 24 hours.
All treatments were done in duplicate wells. The wells that were treated with
Adriamycin (ADR) were exposed to 1 ~M ADR for 1 hour. ADR was dissolved in
sterile
distilled water. After the 1 hour incubation the cells were washed twice with
2 ml media and
then incubated in 3 ml media for 16 hours. After the 16-hour incubation, the
cells were
treated with various concentrations of abrogator ~ Nocodazole (NOC) at 50
ng/ml.
Abrogators were dissolved in dimethylsulfoxide (DMSO) at a concentration of 10
mM and
diluted with growth medium before being added to the cells and NOC was
dissolved at
1 mg/mL in DMSO and diluted with growth medium before administration to the
cells. The
cells were incubated 6 hours with the abrogator and NOC. The duplicate wells
were scraped,
on ice, and combined in a 15 ml centrifuge tube. The wells were rinsed with
Dulbecco's
phosphate buffered saline (DPBS) without calcium and magnesium and the rinse
combined
with the scraped cells. The cells were centrifuged at 200 x g at 4°C
for 5 minutes. The
supernatant was discarded and the pellets resuspended in 100 ~L. DPBS. The
cell suspension
was then transferred to 1.5 ml eppendorf centrifuge tubes and centrifuged at
4°C for
4 minutes at 4000 rpm. After the supernatant was removed, the pellet was
frozen on dry ice
and stored at -80°C.
The pellets were thawed on ice prior to lysis. The lysis buffer, ELB (2.5 mM
HEPES (7.5), 150 mM NaCI, 25 ~t.M NaF and 0.5% NP40 supplemented with I mM
AEBSF,
1 mM sodium orthovanadate, and 1 mM dithiothretol, and complete protease
inhibitor
cocktail tablets (Roche Biochemicals). The tablets were dissolved in 2 mL
distilled water and
diluted 1:25 in the lysis buffer. The pellets were suspended in 100 ~I
complete lysis buffer
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-297-
and incubated on ice for 30 minutes. Following lysis, the suspension was
centrifuged at
14,000 rpm for 15 minutes at 4°C. The supernatant liquidwas collected
and the protein
concentration determined using the Pierce BCA protein Assay Kit per
manufacturers
instructions. The protein concentration was adjusted to 3 mg/mL with DPBS. The
samples
were then diluted 1:1 with Invitrogen 2 x tris-glycine sample buffer
supplemented with
50 ~1/ml 2-mercaptoethanol, boiled for 3 minutes, and stored frozen at -
20°C.
Thirty micrograms of protein per lane were run on Novex pre-cast 12%, 1.5 mm,
10-well, tris-glycine polyacrylimide gels using Novex running buffer and
Invitrogen (See
Blue Plus 2 molecular weight standards). The gels weare run at 100 volts for
30 minutes then
125 volts for 1.5 hours. The proteins were transferred to 0.45 pm pore
nitrocellulose
membranes using Novex transfer buffer and the Novex X-Cell II blot module. The
nitrocellulose membranes were blocked overnight at room temperature. The
blocking buffer
was 5 mM Tris (8.0), 150 mM NaCI, 0.1% Tween 20, 1 mM NaF, 10 mM
glycerolphosphate,
100 ~.tM sodium-orthovanadate, and 3% bovine serum albumen.
After blocking, the gels were cut with a razor blade and the top portion
treated with
anti Myt-1 antibody diluted 1:5000 with blocking buffer. The lower portion of
the gel was
treated with biotinylated antiphosphotyrosine 15, also diluted 1:5000 in
blocking buffer. The
membranes were incubated for 2 hours at room temperature with constant
rocking. The
antibody solutions were removed and the membranes were washed 3 times for 20
minutes
each with TNT buffer. TNT buffer consisted of 50 mM Tris (8.0), 150 mM NaCI,
and 0.1%
Tween 20. Secondary antibody was then added in blocking buffer. Neutravidin
HRP at
1:40,000 was used for the biotinylated phosphotyrosine 15 blots and Bio Rad
goat anti rabbit
at 1:10,000 was used for the Myt-1 blots. The blots remained in secondary
antibody for
1 hour at room temperature followed by three 20-minute washes with TNT buffer.
Protein
bands were detected using the Amersham Pharmacia ECL detection kit and Kodak
Bio Max
film per manufacturer's instructions.
The phosphotyrosine 15 membranes were stripped using the Chemicon
International
Re-Blot kit per manufacturer's instructions. The blots were then washed twice
with TNT
buffer and once with blocking buffer for 20 minutes each. Anti Cdc-2 (cdkl;
Labvision
Corporation) were diluted 150 ~1 per 50 ml blocking buffer and incubated with
the blots for
2 hours at room temperature followed by three 20-minute washes in TNT buffer.
The
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-298-
secondary antibody was Bio Rad goat antimouse HRP and was diluted 1:10,000 in
blocking
buffer before a 1 hour incubation with the blots at room temperature. Three 20-
minute
washes preceded ECL detection.
B. Procedure for detecting MPM_2 in cultured HT-29 cells +/- potential
checkpoint
abrogators, adriamycin,and nocodazole
This assay uses polyclonal antibody to quantitate the M-phase specific
histological
markers in the determination of a mitotic index (fraction of cells found in
mitosis).
HT-29 cells were grown in Dulbecco's Modified Eagle Medium with high glucose,
supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 16 mM HEPES, 8 mM
MOPS, and 10% fetal bovine serum. They were incubated at 37°C in an
atmosphere of 5%
C02 and 100% relative humidity.
Cells were seeded in 96-well tissue culture plates at 100 ~l per well at a
concentration
of 40,000 per ml. The cells were allowed to attach and begin growing for 24
hours. 100 ~tl of
adriamycin (ADR) at 2 pM (final = 1 ltM) was added to test cells and the cells
incubated for
1 hour as above. Following the incubation, the plates were washed 2 times with
growth
media. The media was replaced with 100 ~tl fresh media and incubate a further
16 hours.
Serial 2-fold dilutions of potential abrogators were added to the test wells.
The rows that
received nocodazole (NOC) have 2 p,l NOC added at 2.5 ~g/ml. The plates were
then
incubated an additional 6 hours. Following the incubation, the plates were
centrifuged for
minutes at 4°C at 200 g. One hundred microliters of Carnoy'.s fixative,
3 parts methanol to
1 part acetic acid, was added directly to each well and left at room
temperature for
minutes. The media/fixative mixture was removed by suction and replaced with
ice cold
ethanol:acetic acid in a 20:1 ratio. The plates were stored at 4°C
until stained.
The fixed cells were stained for MPM-2 using the Upstate Biotechnology MPM-2
rhodamine detection kit per manufacturer's instructions. Briefly, the cells
were washed with
Dulbecco's phosphate buffered saline (DPBS) with calcium and magnesium
followed by
incubation with blocking buffer consisting of 8% bovine serum albumen (BSA)
for a
minimum of 1 hour. The cells were then washed with PBS one time and treated
overnight at
4°C with primary antibody at 5 ~tg/ml (100 ~1 per well) diluted with
DPBS with 1% BSA.
The cells were then washed twice for 15 minutes each with DPBS and then
incubated with a
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-299-
1:1000 dilution of rhodamine conjugated goat antimouse IgG in DPBS with 1 %
BSA for
1 hour. The secondary antibody was washed off in three 30-minute washes with
DPBS. The
cells were then counterstained using the Molecular Probes Mycoflour kit to
stain nuclei
following the instructions for the kit.
The stained cells were viewed under fluorescence microscopy using fluorescence
filters suitable for detecting rhodamine and DAPI stains. Images were captured
using a Spot
digital camera and images analyzed using ImageQuant to quantitate total nuclei
and to count
MPM-2 positive cells.
MPM-2 Assay
- ---- (Values are % of cells in M phase) -
+ADR+
+ Media + ADR + NOC NOC
Control 8.4 7.0 26.4 25.9
Compound of Example
26.4 18.9 37.2 86.2
363 @ 312.5 nM
EXAMPLE 491
Procedure for in vivo testing of Weel, Weel/Chkl or Chkl inhibitors in
combination with
adriamycin or cisplatin
These experiments measured the in vivo modulation of PylS on Cdc2 in a tumor
and
assessed the therapeutic effect of Weel, Weel/Chkl or Chkl inhibitors of the
present
invention combined with a conventional agent. The therapeutic effect is
measured by an
increase in life span of treated animals.
In vivo Experiments
In vivo methods
The initial in vivo experiments demonstrate biochemical and physiological
evidence
of Weel, Weel/Chkl or Chkl inhibition by examining modulation of the
downstream target
(i.e., the phosphorylation status of Cdc2 tyrosine 15) and cell cycle effects
in L1210 murine
leukemia tumor cells. L1210 was chosen for its lack of p53 function,
pronounced G2
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-300-
accumulation in response to DNA damage, ease of propagation in mice, and rapid
cycle time.
CD2F1 mice weighing 25 to 26 g were inoculated intraperitoneally (ip) with 105
or 5 x 105
L1210 cells and randomized into treatment groups of 3 mice each. On Day 4, 5,
or 6 after
tumor implantation, the mice were treated with a single injection of DNA
damaging agent
(cisplatin, 8 or 6 mg/kg iv), one or more injections of the compound of
Example 80
(20 mg/kg ip, sc, or iv), or both agents. The injections of the compound of
Example 80 were
spaced 3 to 8 hours apart and were started simultaneously with the DNA
damaging agent or
after a delay of 8 or 16 hours. Control animals received only 0.2 ml saline
iv. The DNA
damaging agents were dissolved in saline and the compound of Example 80 was
dissolved in
1 of 2 vehicles: (1) 10% cremaphor, 10% ethanol, and 80% H20 or (2) 5%
dimethylacetamide, 25% propylene glycol, and 70% polyvinylpyrrolidine (30% w/v
in H20).
The mice were sacrificed at several time points ranging from 8 to 40 hours
after
commencement of treatment and the tumor cells were harvested in phosphate
buffered saline
_containing 1 mM sodium orthovanadate and 5mM EDTA. The L1210 cells were
counted on
a Coulter Counter and for each group an aliquot of 2.5 x 107 cells were
centrifuged and
frozen as pellets and an aliquot of 2.5 x 106 was frozen in Vindelov's citrate
buffed at
-80°C.
The larger pellets were homogenized for 30 seconds with a sonicator in a cold
lysis
buffer (RIPA) containing 25 mM Tris (base) pH 7.4, 350 mM sodium chloride, 1 %
nonidet
P40, 5 mM EDTA (tetrasodium), 0.5% sodium deoxycholate, 0.1% sodium dodecyl
sulfate,
1 mM AEBSF, 1 mM sodium fluoride, 0.5 mM sodium orthovanadate, 15 ~g/ml
aprotinin,
20 ~M leupeptin, 10 mM (3-glycerophosphate, and 1 mM DTT in H20. The lysates
were
diluted 1:10 with a mixture of 4 parts RIPA and 5 parts 2 times Novex
electrophoresis sample
buffer which, when diluted to working concentration contained 2% sodium
dodecyl sulfate,
0.0025% bromphenol blue, 10% glycerol, and 60 mM dithiothreitol in H20. The
samples
were heated at 98° for 5 minutes. Duplicate Novex 4% to 20%
polyacrylamide Tris-glycine
15-well 1 mm mini-gels were loaded with 15 ~l/well and run at 200 V (constant)
for
45 minutes. The proteins were transferred to nitrocellulose membranes in Novex
transfer
buffer at 25 V (constant) for 2 hours. One of each membrane was blotted for
total Cdc2
protein with LabVision MS-110P antibodies at 1:200, followed by BioRad 172-
1011 goat
antimouse IgG-HRP at 1:10,000. The other membrane was blotted for Cdc2
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-301-
phosphotyrosine 15 (PtyrlS) with biotinylated rabbit polyclonal antibodies
supplied by Onyx
Pharmaceuticals at 1:5000, followed by Pierce 31001 neutravidin-HRP at
1:20,000
(50 ng/ml). The antibodies were detected with Amersham ECL reagents. The blot
images
were captured on Kodak BioMax MR film and imaged on a Molecular Dynamics
Personal
Densitometer SI. Densitometry was performed with Molecular Dynamics Image
QuaNT
software. The densitometric values for Cdc2 PtyrlS were normalized for total
Cdc2 protein
(divided by the values for total Cdc2 to obtain a ratio of phosphoaotal Cdc2)
and divided by
the ratio of the control samples to obtain percent of control phosphorylation
values for each
tumor sample.
The smaller pellets were stained for DNA content by the method of Vindelov
Vindel~v LL, Christensen IJ, and Nissen NI. A detergent-trypsin method for the
preparation
of nuclei for flow cytometric DNA analysis. Cytometry 1983;3: 323-327). They
were
analyzed for propidium iodide fluorescence on a Beckman-Coulter Elite flow
cytometer. Cell
cycle distributions were estimated by the broadened trapezoid model of Bagwell
(Bagwell,
C.B. Theoretical aspects of flow cytometry data analysis. In: K.D. Bauer, R.E.
Duque, T.V.
Shankey, (eds.), Clinical Flow Cytometry: Principles and Application. pp. 41-
61. Baltimore:
Williams & Wilkins, 1993) as implemented by the Verity Software House program
ModFit
LT.
Subsequent in vivo experiments were designed to determine if the addition of a
Weel,
Weel/Chkl or Chkl inhibitor to standard chemotherapy with DNA damaging agents
results
in a therapeutic gain. CD2F1 mice weighing 24 to 26 g were inoculated with 104
L1210 cells
ip and randomized into treatment groups of 6 mice each. On Days 3, 7, and 11
after implant,
the mice were treated with a single iv injection of 1 of 3 dose levels of a
DNA damaging
agent (cisplatin or doxorubicin) alone or in combination with 2 ip injections
of 1 of 2 dose
levels of the compound of Example 80. Control mice received appropriate
vehicle in place of
active agents. Dose levels were based on mean group body weights. Mortality
data were
collected over a 3-week span and the median survival times of each group were
calculated.
Efficacy data (%T/C) are reported as the median life span of the treated
animals divided by
the median life span of the control animals times 100. The maximal %T/C of the
combination
groups was then compared with the maximal %T/C of the DNA damage. As shown on
Table
9, the only groups to derive a measure of the therapeutic gain were associated
with addition
of a Weel, Weel/Chkl or Chkl inhibitor of the present invention.
CA 02483496 2004-10-25
WO 03/091255 PCT/IB03/01417
-302-
Table 9
Cell cycle distribution of L1210 cells
G~ S GZM
Saline control44.3 44.2 11.5
Cisplatin 6mg/kg14.0 26.4 59.6
Compound of 60.0 27.6 12.4
Example 80
20mg/kg
Cisplatin + 82.5 13.3 4.2
Compound of
Example 80
Cell cycle distribution of L1210 cells treated in vivo with cisplatin at
6mg/kg and with the
compound of Example 80 at 20 mg/kg. Cells were examined 24 hours after
administration of
drug and compound. Values are per cent of cells examined.
All of the compositions and/or methods disclosed and claimed herein can be
made
and executed without undue experimentation in light of the present disclosure.
While the
compositions and methods of this invention have been described in terms of
preferred
embodiments, it will be apparent to those of skill in the art that variations
may be applied to
the compositions and/or methods and in the steps or in the sequences of steps
of the methods
for preparing these compounds.