Note: Descriptions are shown in the official language in which they were submitted.
CA 02483570 2004-10-01
TITLE OF THE INVENTION
REAGENT SYSTEM AND PROCESS FOR
ADENOSINE TRIPHOSPHATE MONITORING
FIELD OF THE INVENTION
= The invention relates to reagent systems and processes for adenosine
triphosphate (ATP) monitoring.
BACKGROUND OF THE INVENTION
Microorganisms have been harnessed for a variety of beneficial processes
such as the production of foods, beverages and pharmaceuticals, and the
remediation of air, soil and water.
The factors that influence these processes may include the presence and
quality of food and nutrients, the presence and quantity of toxic chemicals,
the
degree of aeration, pH, and temperature. These factors may interact in an
additive, synergistic, or antagonistic manner. In bioremediation processes,
the
situation is further complicated because the medium to be treated is often
highly
complex and variable.
Effective monitoring technologies are an important component of a
successful biological process. They form the tools to build a stable and
efficient
biological process. Most powerful is a technology that provides information of
the effects of operating environment on the microorganisms. Statistical
process
analyses on the data generated from such a technology can be used to solve
problems and enable continual process improvement.
ATP is the keystone of metabolic activity. Most of the energy for microbial
processes microorganisms is stored and transmitted via ATP. ATP is produced as
= 30 microbial food is consumed and is utilized for cell maintenance
and the synthesis
of new cells and biochemicals.
CA 02483570 2004-10-01
1.
- 2 -
ATP is most easily measured by the firefly luciferase assay. The reaction
is as follows:
Mg++
ATP + 02 + luciferin luciferase > AMP + PPi + oxyluciferin 4- light
in which,
ATP = Adenosine triphosphate
AMP = Adenosine monophosphate
PPi = pyrophosphate
The chemical energy produced from the breakdown of ATP is converted
into light energy. Each molecule of ATP consumed in the reaction produces one
photon of light. This light output can be quantified in a luminometer.
ATP measurement has been proposed as a tool to assist in the process
control of biological waste treatment systems for over 3 decades (1-3).
Generally, the concept has been to substitute ATP as a more accurate estimate
of
the amount of viable biomass in the reactor of a biological wastewater
treatment
facility. This has been commonly estimated by measuring the volatile suspended
solids (VSS).
However, the concepts in these references have never included
measurements of dissolved ATP (d-ATP) or extracellular ATP. All analyses have
been performed measuring total ATP (t-ATP), which is the combination of
intracellular ATP plus extracellular ATP. Dissolved ATP analyses have been
used
in sanitation monitoring, but only as a measure of non-microbial ATP from food
sources. Similarly, in microbial growth control, dissolved ATP has been
monitored, but only in the presence of conditions that are highly lethal to
microorganisms.
In addition, reagents for simple but accurate measurement of dissolved
and total ATP have not been optimized for biological process monitoring.
Biological remediation and production processes differ from the mainstream
applications of ATP analyses - sanitation monitoring and microbial growth
control
- in that samples contain up to 1000 times higher levels of biomass or
greater.
CA 02483570 2004-10-01
- 3 -
Furthermore, biological wastewater samples contain many substances that
interfere with the firefly luciferase assay for measuring ATP.
Reagent formulations containing luciferase and luciferin for assaying ATP
in a sample are known in the art. For example, US patent No. 6,004,767 of
Crouch et al. discloses a bioluminescent reagent as a freeze dried powder, to
be
reconstituted prior to use. US patent No. 5,558,986 of Lundin discloses such
reagent formulation for use in combination with a cyclodextrin. US patent
application No. 2001/0046687 of DiCesare also discloses the use of such
formulation, wherein trehalose is used to enhance the emission of light
intensity.
Published international patent application, WO 94/11528 of Foote and Grant
discloses an aqueous composition including polyols for use in a bioluminescent
assay by adding a strong buffer to give a pH which is close to the optimum pH
for the luciferase reaction.
SUMMARY OF THE INVENTION
The inventors have now discovered a reagent system for the measurement
of total and dissolved ATP from samples containing microorganisms. The reagent
system comprises a luciferase formulation and a buffer formulation. The buffer
formulation is used to prepare stable ATP solutions from the samples, and is
later
mixed with the luciferase formulation to provide an efficient and reliable
quantification of sample ATP concentration.
Total and dissolved ATP analyses then provide the basis for the control of
biological remediation and production processes. The reagent can also be used
in kits.
Accordingly, the invention provides in one aspect, a reagent system
comprising: a first reagent which includes a high pH phosphate buffer; and a
second reagent which includes luciferase, luciferin, a magnesium salt and an
enzyme stabilizer. The second reagent has a low pH and a buffer with a pK
which is near the optimum pH for activity of luciferase. Preferably, the high
pH
phosphate buffer may comprise a tribasic monovalent phosphate salt. This salt
can be selected from the group consisting of trisodium phosphate, tripotassium
CA 02483570 2004-10-01
- 4 -
phosphate, trilithium phosphate and combinations thereof. Other suitable salts
may also be used.
In further embodiments of the reagent system according to the invention,
the first reagent may further comprise a surfactant and/or a base. The
surfactant can be a cationic surfactant or a non-ionic surfactant. A preferred
cationic surfactant may be a quaternary ammonium salt such as benzalkonium
chloride. Other suitable quaternary ammonium salts can also be used. A
preferred non-ionic surfactant can be an octylphenol. The base may be a
monovalent base selected from the group consisting of sodium hydroxide,
potassium hydroxide, lithium hydroxide and combinations thereof.
In other embodiments of the reagent system according to the invention,
the second reagent may further comprise a chelating agent. The enzyme
stabilizer can be selected from the group consisting of an enzyme hydrogen-
bond
stabilizer, a protein enzyme stabilizer, a reducing agent and combinations
thereof. A preferred hydrogen-bond stabilizer can be a polyol. The polyol can
be
selected from the group consisting of trehalose, glycerol, glycol and
combinations
thereof. Other suitable polyols may also be used. A preferred protein enzyme
stabilizer can be selected from the group consisting of bovine serum albumin,
casein and combinations thereof.
A preferred chelating agent can be
ethylenediamine tetra acetic acid (EDTA). The reducing agent can be selected
from the group consisting of dithiothreitol, mercaptoethanol and combinations
thereof.
In yet other embodiments of the reagent system according to the
invention, the first reagent may have a pH which is greater than about 9.9.
More
particularly, the first reagent may have a pH of about 11.1 to 11.7. The pH of
the second reagent may be adjusted to about pH 6 to 8.5, and its pK can be
about 7.5 to 8.5. More particularly, the pH of the second reagent can be
adjusted to about pH7.0, and its pK can be 7.5 to 7.75.
In yet other embodiments of the reagent system according to the
invention, luciferase can be a native luciferase or a recombinant luciferase.
Optionally, the native luciferase can be obtained directly from fireflies.
Luciferase can also be immobilized on a support substrate which can be a
transparent material or a translucent material. These materials may be glass
or
CA 02483570 2004-10-01
A
- 5 --
silica. When luciferase is immobilized on a support substrate, it can be re-
usable. The buffer of the second reagent can be selected from the group
consisting of N[2-hydroxyethyl]piperazine-N'42-ethanesulfonic add] (HEPES),
N-[tris(hydroxymethyl)methyl]glycine (TRICINE),
N-[2-hydroxy-1,1-
bis(hydroxymethyl)ethyl]glycine and combinations thereof.
Other suitable
buffers can also be used. Optionally, the second reagent can be reconstituted
from a freeze-dried formulation.
In other embodiments of the reagent system according to the invention,
the tribasic monovalent phosphate salt can be at a concentration of about 0.5
to
500 mM.
More preferably, the monovalent phosphate salt can be at a
concentration of about 5 mM. The chelating agent may be in an amount of about
0.03 to 1.3% by weight of the second reagent. The surfactant may be at a
concentration of about 100 to 10,000 mg/L. More preferably, the surfactant can
be at a concentration of about 1,000 to 4,000 mg/L. The base can be at a
concentration of about 100 to 10,000 mg/L. More preferably the base can at a
concentration of about 600 mg/L.
The invention provides, in a second aspect, a kit comprising: a first
container having a first reagent which includes a high pH phosphate buffer;
and
a second container having a second reagent which includes luciferase,
luciferin, a
magnesium salt and an enzyme stabilizer. The second reagent has a low pH and
a buffer with a pK which is near the optimum pH for activity of luciferase. In
embodiments of the kit according to the invention, the first and second
containers may have a low gas permeability. The reagents in the first and
second containers may be in a single dose and ready for use.
Further, the first and second reagents in the kit of the invention may have
the characteristics outlined above in relation to the first aspect of the
invention.
According to a third aspect, the invention provides a process for measuring
at least one of total ATP and dissolved extracellular ATP, in a fluid
containing
microorganisms, the process comprising the steps of: (a) obtaining a sample of
the fluid; (b) mixing the sample with a first reagent that includes a high pH
phosphate buffer to obtain a first mixture; (c) mixing the first mixture with
a
second reagent to obtain a second mixture, the second reagent including
luciferase, luciferin, a magnesium salt and an enzyme stabilizer, and having a
CA 02483570 2004-10-01
- 6 --
low pH and a buffer with a pK which is near the optimum pH for activity of
luciferase; and (d) measuring the light produced in the second mixture using a
luminometer.
In embodiments of the process according to the invention, a sub-sample
of the first mixture may be obtained prior to step (c). More particularly, the
sub-
sample can be diluted prior to step (c). The fluid can be agitated, and/or the
microorganisms from the fluid separated prior to step (a). Optionally, the
separation step may further comprise a step selected from the group consisting
of settling, filtration, flocculation, centrifugation and combinations
thereof. Other
suitable separation techniques may also be used.
The first and second reagents of the process of the invention may have the
characteristics outlined above in relation to the first aspect of the
invention.
According to a fourth aspect, the invention provides a method of
controlling a microbiological remediation or production process, the method
comprising the steps of: (a) obtaining a sample from a location in the process
where it is desired to monitor microbiological characteristics; (b) analyzing
the
sample to obtain dissolved extracellular ATP concentration and total ATP
concentration; (c) comparing the concentrations obtained in step (b) with at
least one of the operational variables and outputs of the process; and (d)
adjusting at least one process operational variable responsive to the result
of
step (c).
In embodiments of the method according to the invention, the location
may be selected from the group consisting of process influent, anaerobic
reactor,
aerobic reactor, anaerobic reactor effluent, aerobic reactor effluent,
anaerobic
return, aerobic return, waste streams, clarifier effluent, settling basin and
plant
effluent. The operational variables may be selected from the group consisting
of
biodegradable substrate concentration, biological oxygen demand (BOD),
chemical oxygen demand (COD), pH, acidity, alkalinity, temperature,
conductivity, nutrients, chemical additions, dissolved oxygen concentration
(DO),
pressure, food to microorganism ratio, flow rate, toxic .substance
concentration
and combinations thereof. The outputs may be selected from the group
consisting of BOD removal, COD removal, nutrient consumption, heat, biomass
yield, and concentration or production rate of carbon dioxide, methane,
CA 02483570 2004-10-01
- 7 -
hydrogen, hydrogen sulfide, volatile fatty acids, organic acids, alcohols,
suspended solids and volatile suspended solids.
The step of analyzing the sample to obtain dissolved and extracellular ATP
concentration and total ATP concentration (step (b)) in the method of the
invention, may further comprise the steps of: (i) measuring the dissolved
extracellular ATP concentration; (ii) measuring the total ATP concentration;
and
(iii) subtracting the dissolved extracellular ATP concentration from the total
ATP
concentration to obtain the cellular ATP concentration. Optionally, the
analyzing
step may comprise the steps of: (i) measuring the dissolved extracellular ATP
concentration; (ii) measuring the total ATP concentration; and (iii)
calculating a
ratio of dissolved extracellular ATP concentration to total ATP concentration
to
obtain a first stress index. The analyzing step may also comprise the steps
of:
(i) measuring the dissolved extracellular ATP concentration; (ii) measuring
the
total ATP concentration; (iii) subtracting the dissolved extracellular ATP
concentration from= the total ATP concentration to obtain the cellular ATP
concentration; and (iv) calculating a ratio of dissolved extracellular ATP
concentration to cellular ATP concentration to obtain a second stress index.
Further, the analyzing step may comprise the step of pre-treating the sample
prior to step (b) by: (i) adding a spike of dissolved ATP to the sample to
obtain a
mixture; and (ii) incubating the mixture.
In other embodiments of the method according to the invention, the
mixture may be incubated for about 5 to 120 minutes, at a temperature which is
within about 20 C of the temperature range of the process environment from
which the sample is obtained. The mixture can be incubated for about 10
minutes, at a temperature of about 20 to 300 C.
In the method of the invention, when the sample is pre-treated prior to
the analyzing step, by adding a spike of dissolved ATP to the sample, the
spike
can be about 1 to 100 times higher than the total ATP concentration. More
preferably, the dissolved ATP spike can be about 10 times higher than the
total
ATP concentration. The dissolved ATP spike can also be about 1 to 100 times
higher than the cellular ATP concentration. More particularly the dissolved
ATP
spike can be about 10 times higher than the cellular ATP concentration.
CA 02483570 2004-10-01
- 8 -
In yet other embodiments of the method according to the invention, the
pre-treatment step may comprise allowing the sample to settle by gravity, or
subjecting the sample to centrifugation. More preferably, a flocculating agent
can be added to the sample prior to settling. The flocculating agent can be
selected from the group consisting of an aluminum salt, a ferric salt, a
ferrous
salt, an organic polymer and mixtures thereof. Other suitable flocculating
agents
may also be used. The organic polymer used as flocculating agent can be a high
molecular weight cationic polymer selected from the group consisting of
polyacrylamides, polydiallyldimethylammonium chlorides, polyethylenimines,
polyamines and mixtures thereof.
Other suitable high molecular organic
polymers may also be used. The settling period may range from about 1 to 120
minutes. More particularly, the settling period can be about 10 minutes.
The pre-treatment step of the method according to the invention may
comprise the step of pre-treating the sample prior to step (b) by passing the
sample through at least one sieve to produce a filtrate, the sieve having a
mesh
with openings of about 2 to 1,000 microns. The sieve may have a mesh with
openings of about 500 to 1,000 microns. Optionally, the sieve may have a mesh
with openings of about 300 to 500 microns. The openings of the mesh can be
about 20 to 80 microns or about 2 to 5 microns.
In other embodiments of the method according to the invention, the pre-
treatment step may comprise: (i) sieving or treating the sample to obtain a
floc
containing excessive quantities of bulking filamentous bacteria; and (ii) re-
suspending the floc in a liquid medium. Optionally, step (i) can be performed
using a sieve which has a mesh with openings of about 300 to 600 microns. The
liquid medium may include an influent water or culture medium that supports
the
growth or maintenance of filamentous bacteria.
In yet other embodiments of the method according to the invention, the
= sample may be obtained from a biological process reactor and the method
may
further comprise the step of pre-treating the sample prior to step (b) by: (i)
diluting the sample in a process influent or effluent water to obtain a dilute
sample; and (ii) incubating = the dilute sample for at least one time interval
period. The dilution range of the sample may be about 1 to 50%. The time
interval period may range between about 10 minutes to 30 days. More
CA 02483570 2009-07-06
- 9 -
particularly, the time interval period may range between about 10 minutes to 8
hours. The time interval period may also range between about 1 day to 30
days. The process influent or effluent water may be diluted with water prior
to
adding to the sample. Optionally, additional biodegradable matter and
nutrients
may be added to the process influent or effluent water prior to adding to the
sample.
In yet other embodiments of the method according to the invention, the
pre-treatment step may comprise: (i) adding a suspension of planktonic or free-
floating bacteria to the sample to obtain a mixture; and (ii) incubating the
mixture for at least 30 minutes. The incubation may be performed for about 5
to 120 minutes, at a temperature which may be within about 20 C of the
temperature range of the process environment from which the sample is
obtained. More particularly, the mixture may be incubated for about 30 minutes
to 3 days, and the temperature may be about 20 to 30 C.
Optionally, the above comparison step of the method according to the
invention (step (c)) and/or the adjustment step (step (d)) may be accomplished
through human interface or computer logic.
In other embodiments of the method according to the invention, the step
of analyzing the sample to obtain dissolved extracellular ATP concentration
and
total ATP concentration may comprise the steps of: (i) mixing the sample with
a
first reagent that includes a high pH phosphate buffer to obtain a first
mixture;
(ii) mixing the first mixture with a second reagent to obtain a second
mixture,
the second reagent including luciferase, luciferin, a magnesium salt and an
enzyme stabilizer, and having a low pH and a pK which is near the optimum pH
for activity of luciferase; and (iii) measuring the light produced in the
second
mixture using a luminometer.
More particularly, the first and second reagents of the method of the
invention may have the characteristics outlined above in relation to the first
aspect of the invention.
Other embodiments of the invention are outlined below.
A first embodiment provides for a reagent system for estimating cellular
adenosine triphosphate (ATP) based on the actual measurement of both
extracellular ATP and total ATP in a sample, the reagent system comprising: a
CA 02483570 2009-07-06
- 9a -
first reagent which includes a high pH phosphate buffer with a pH range of
about 9.9 to 12; and a second reagent which includes luciferase, luciferin, a
magnesium salt and an enzyme stabilizer, the second reagent having a low pH
with a pH range of about 6.0 to 7.1 and a buffer with a pK which is near the
optimum pH for activity of luciferase, wherein the application of the first
and
second reagents to the sample is operable for measuring extracellular ATP in
the
sample, and wherein the application to the sample of the first reagent
together
with a cationic surfactant and the second reagent is operable for measuring
total
ATP in the sample.
A second embodiment provides for a kit for use in estimating cellular
adenosine triphosphate (ATP) based on the actual measurement of both
extracellular ATP and total ATP in a sample, the kit comprising: a first
container
having a first reagent which includes a high pH phosphate buffer with a pH
range of about 9.9 to 12; and a second container having a second reagent
which includes luciferase, luciferin, a magnesium salt and an enzyme
stabilizer,
the second reagent having a low pH with a pH range of about 6.0 to 7.1 and a
buffer with a pK which is near the optimum pH for activity of luciferase,
wherein
the application of the first and second reagents to the sample is operable for
measuring extracellular ATP in the sample, and wherein the application to the
sample of the first reagent together with a cationic surfactant and the second
reagent is operable for measuring total ATP in the sample.
A third embodiment provides for a process for measuring at least one of
total ATP and extracellular ATP, in a fluid containing microorganisms, the
process comprising the steps of: (a) obtaining a sample of the fluid; (b)
adding
a spike of dissolved ATP to the sample to obtain a mixture; (c) incubating the
mixture; (d) mixing the mixture with a first reagent which includes a high pH
phosphate buffer with a pH range of about 9.9 to 12, to obtain a first
mixture;
(e) mixing the first mixture with a second reagent together with a cationic
surfactant to obtain a second mixture, the second reagent including
luciferase,
luciferin, a magnesium salt and an enzyme stabilizer, and having a low pH with
a pH range of about 6.0 to 7.1 and a buffer with a pK which is near the
optimum pH activity of luciferase; and (f) measuring the light produced in the
second mixture using a luminometer, wherein the application of the first and
=
CA 02483570 2009-07-06
- 9b -
second reagents to the sample is operable for measuring extracellular ATP in
the
sample, and wherein the application to the sample of the first reagent
together
with a cationic surfactant and the second reagent is operable for measuring
total
ATP in the sample.
A fourth embodiment provides for a method of controlling microbiological
remediation or production in a process, the method comprising the steps of:
(a)
obtaining a sample from a location in the process; (b) adding a spike of
dissolved ATP to the sample to obtain a mixture; (c) incubating the mixture;
(d)
measuring the extracellular ATP concentration; (e) measuring the total ATP
concentration; (f) comparing the concentrations obtained in the previous steps
with at least one process operational variable or process operational output;
and
(g) adjusting the at least one process operational variable or process
operational output, responsive to the result of the previous steps.
In the above fourth embodiment of the invention, steps (d) and (e) may
comprise the following steps: (i) mixing the sample with a first reagent which
includes a high pH phosphate buffer with a pH range of about 9.9 to 12, to
obtain a first mixture; (ii) mixing the first mixture with a second reagent
together with a cationic surfactant to obtain a second mixture, the second
reagent including luciferase, luciferin, a magnesium salt and an enzyme
stabilizer, and having a low pH with a pH range of about 6.0 to 7.1 and a pK
which is near the optimum pH activity of luciferase; and (iii) measuring the
light
produced in the second mixture using a luminometer, wherein the application of
the first and second reagents to the sample is operable for measuring the
extracellular ATP concentration, and wherein the application to the sample of
the first reagent together with a cationic surfactant and the second reagent
is
operable for measuring total ATP concentration.
A fifth embodiment provides for a method of estimating cellular adenosine
triphosphate (ATP) based on the actual measurement of both extracellular ATP
and total ATP in a sample, which comprises the step of applying a reagent
system to the sample, the reagent system comprising: a first reagent which
includes a high pH having a range of about PH 9.9 to 12 phosphate buffer; and
a second reagent which includes luciferase, luciferin, a magnesium salt and an
enzyme stabilizer, the second reagent having a low PH having a range of about
CA 02483570 2009-07-06
- 9c -
6.0 to about 7.1 and a buffer with a pK which is near the optimum pH for
activity of luciferase, wherein the application of the first and second
reagents to
the sample is operable for measuring extracellular ATP in the sample, and
wherein the application to the sample of the first reagent together with a
cationic surfactant and the second reagent is operable for measuring total ATP
in the sample.
The term "about" as used herein expresses a variation of which a person
skilled in the art would know that the benefit of the invention will still be
enabled.
CA 02483570 2013-05-31
. .
µ X
- 10 -
BRIEF DESCRIPTION OF THE FIGURES
These and other advantages of the invention will become apparent upon
reading the following detailed description and upon referring to the drawings
in
which:
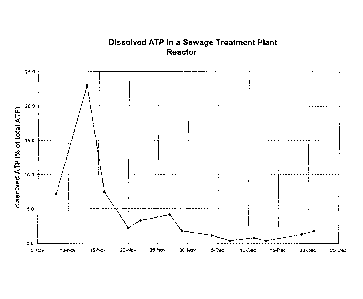
Figure 1 illustrates the change over time of the amount of dissolved ATP in
mixed liquor suspended solids samples from a sewage treatment plant reactor;
Figure 2 illustrates the change in ATP parameters relative to the pH;
Figure 3 illustrates the effect of a 7-day addition of glucose and nutrient
feed on dissolved ATP;
Figure 4 illustrates the effect of stressful anoxic conditions on biomass
stress index;
Figure 5 illustrates the effects of stressful anoxic conditions on dissolved
ATP removal rate;
Figure 6 illustrates the change over time of planktonic ATP;
Figure 7 illustrates a comparison of the performance of the reagent system
according to the invention with several commercially available ATP releasing
agents; and
Figure 8 illustrates the stability of luciferase reagent formulations
according to the invention.
While the invention will be described in conjunction with illustrated
embodiments, it will be understood that it is not intended to limit the
invention to
such embodiments.
DETAILED DESCRIPTION OF THE INVENTION
It has long been assumed that dissolved ATP would not be present under
environmental conditions where life is supported. A publication by Holm-Hansen
and Booth (4) is frequently cited by authors that make this assumption.
However, because the samples in this publication were filtered prior to ATP
analysis, much of the dissolved ATP would not have been measured.
CA 02483570 2004-10-01
- 11 -
In addition, other researchers have anticipated that dissolved ATP
introduced into the reactor of a biological wastewater system would be rapidly
degraded, and therefore have assumed that the dissolved ATP content of a
reactor sample would be undetectable except under conditions that would kill
the
majority of microbial population.
However, our experience has determined that this assumption is not
correct, especially in biological wastewater treatment systems. Figure 1 is a
graph that shows the relative amount of dissolved ATP in mixed liquor
suspended
solids (MLSS) samples analyzed from the main reactor of an operating municipal
sewage treatment system over a period of approximately two months. Dissolved
ATP was measured by settling the biosolids, then measuring the ATP in the
supernatant. Total ATP was measured by suspending the biosolids, adding an
ATP releasing agent to the suspension, then measuring the ATP in the resulting
solution. The details for the methods used to measure ATP are described later
in
this description.
It can be seen from figure 1 that during the first week of this test period,
the dissolved ATP was greater than 20% of the total ATP. The dissolved ATP
reached a minimum of 0.4% of the total ATP several weeks later, but was always
at a measurable concentration.
We have discovered that measuring dissolved ATP in addition to total ATP
not only provides a more accurate estimate of the viable biomass (because
cellular ATP actually consists of total ATP minus dissolved ATP), but also can
be
applied in a variety of new ways to provide an indication of biomass health
and
settling properties.
For example, a pH that is too acidic or too alkaline is detrimental to a
biological process. The laboratory experiment described below as Example 1
demonstrates how the stress of an adverse pH can be detected by monitoring
the dissolved ATP together with total ATP.
Example 1
The pH of the reactor MLSS sample was adjusted to near neutral, then
dissolved ATP and total ATP were measured. Next, the sample pH was raised to
pH 8.0 and the ATP tests were conducted again. This procedure was repeated
several times, raising the pH by increments of approximately 0.5 units each
CA 02483570 2004-10-01
a
- 12 -
time. During the entire experimental period, the sample was aerated to ensure
an adequate supply of oxygen.
Figure 2 shows the changes in three ATP parameters (total ATP, cellular
ATP, and biomass stress index) relative to their values at the beginning of
the
experiment. The biomass stress index is the dissolved ATP calculated as a
percentage of the total ATP.
For example, by the time the sample had been adjusted to pH 10.0, the
total ATP of the sample had decreased by a factor 4.5, while the cellular ATP
(total ATP minus dissolved ATP) had decreased by a factor of 6.2, and the
biomass stress index increased by a factor of 9Ø The use of dissolved ATP to
calculate cellular ATP as done in applications for microbial growth control is
not
as valuable for detecting stress to microbial population as the index
developed
here. For example, as indicated above, the change in biomass stress index is
more dynamic than cellular ATP. In addition, even minor changes from the more
favorable neutral pH could be rapidly detected by using the biomass stress
index,
whereas there was no difference in using the cellular ATP measurement until
the
pH exceeded pH 9.5, a condition known to be lethal to many microorganisms.
While it is important to detect environments that are adverse or hostile to
microbial growth, detection of favorable environments is equally important in
process optimization. An experiment described below as Example 2
demonstrates the importance of monitoring total ATP together with dissolved
ATP
for this purpose.
Example 2
Two bench-scale reactors were filled with samples that had recently been
obtained from the reactor of a municipal sewage plant. The reactors were
maintained at ambient temperature, continuously aerated, and the pH was
adjusted daily to pH 7.0- 7.1. One of the reactors was fed daily with 2,000
mg/L
glucose, 100 mg/L nitrogen (from an NH4CI solution), and 20 mg/L phosphorus
(from a Na3PO4 solution), while the other reactor was not fed. Furthermore,
water losses due to evaporation were replaced daily in both reactors.
The sample from the sewage plant was analyzed for total and dissolved
ATP concentration immediately prior to initiating the experiment. One week
later, the contents of each reactor were also analyzed. The biomass stress
index
CA 02483570 2004-10-01
7
- 13 -
was calculated as a percentage of the total ATP for each. By reviewing the
results, shown in figure 3, it can be seen that the biomass stress index of
the
sample was relatively high (greater than 30%) in the sample before it was
incubated in the reactors. When the sample was incubated under starvation
conditions, the biomass stress index increased to 47%. However, under
favorable conditions, the biomass stress index decreased almost 10 fold to
3.9%.
During the incubation under feeding conditions, the total ATP content
increased about five times. Although simply monitoring the increase of total
ATP
alone also demonstrates the favorable environment, determining dissolved ATP
provides additional advantages. It confirms the indications of the total ATP
monitoring, and it provides information on cellular health without the
necessity of
historical trend data.
It is thus demonstrated that the biomass stress index is useful for
monitoring short-term or 'acute' stress. However, to measure chronic stresses
such as nitrogen, phosphorus, and oxygen deficiency, another method that uses
dissolved ATP and total ATP analyses is required.
This test is a measurement of the effect of enzymes in a sample that
degrades ATP. It appears that they increase during periods of chronic stress.
The method for measuring the activity of these enzymes is performed by
increasing the dissolved ATP levels in the sample. This is done by adding a
small
quantity of a high concentration of ATP solution prepared from crystalline
ATP,
then measuring its removal following an incubation period after the addition.
To
facilitate reporting results of this test, we have named this test, dissolved
ATP
removal rate (DARR).
Alternatively, as discussed below, a dissolved ATP test can be performed
to measure DARR.
If total ATP measurements are used, the sDARR index, which is the DARR
index normalized to the amount of viable biomass in the sample, measured by
determining cellular ATP, is calculated as follows:
DARR - DARR, /
sDARR(tiglmLlminInglmL) =
/ATP
CA 02483570 2004-10-01
- 14 -
wherein,
DARR = total ATP concentration in the sample after spike addition
= (total ATP concentration sample before spike addition + ATP
concentration added from spike);
DARR t (ng/mL) = amount of ATP remaining at time t; and
cATP = cellular ATP
The same results are obtained if only dissolved ATP measurements are
made for DARRo or DARR, because in the above equation, the contribution of
cellular ATP is subtracted out.
An experiment showing the significance of DARR is described below as
Example 3 for monitoring the stress of oxygen deficiency in an aerobic
population.
Example 3
An environment of oxygen deprivation was produced by spiking a reactor
sewage plant sample with 2,000 mg/L glucose plus nitrogen and phosphate
nutrients and distributing the mixture into a series of test tubes. The test
tubes
were capped, sealed with Parafilmm laboratory wax, then placed horizontally in
a
rotary shaker to provide constant mixing at 190 rpm within the tubes. After
various periods of incubation, one of the tubes was removed from the shaker
and
subjected to an ATP test battery consisting of total ATP, dissolved ATP, and
DARR
tests. The spike of ATP delivered to the sub-sample used for the DARR test
raised the dissolved ATP concentration by 10,000 ng/mL. Ten minutes after the
addition of the spike, a total ATP test was conducted on the sample.
It was assumed that as glucose was degraded by the microorganisms in
the test tubes, the oxygen would be depleted. To ensure that this would occur,
a
dissolved oxygen uptake rate (DOUR) test was conducted prior to starting the
experiment. The DOUR test showed that only 0.12 mg/L remained in the MLSS
sample 20 minutes after addition of the glucose solution.
As shown in figure 4, the biomass stress index rises as anticipated on the
application of stressful conditions. However, after 24 hours of continued
stress,
the index drops to approximately 1/3 of the peak value. Without the knowledge
that the anoxic stress was still being applied and basing judgment only on the
CA 02483570 2004-10-01
- 15 -
biomass stress index, one would have predicted that the environment had
become less stressful.
However, as shown in figure 5, it appears that the decrease at the 24 hour
incubation interval in biomass stress index was caused by an increase in DARR
activity. Over the 24 hour period, DARR activity increased more than 9 times
causing a corresponding 10 fold decrease in dissolved ATP.
This experiment demonstrates the importance of using a battery of ATP-
based tests to accurately estimate the physiological status of the microbial
population. The results also indicate that the dissolved ATP concentration of
a
sample at a given time represents the equilibrium value between movement of
ATP from the cellular domain to the aqueous surroundings and the effect of
enzymes and other sample constituents that can degrade it.
In biological processes such as activated sludge wastewater treatment,
settling characteristics of the biomass are also very important. Another test
was
developed to monitor this aspect of the process again using dissolved and
total
ATP analyses.
The experiment described below as Example 4 demonstrates that
monitoring settling characteristics of the biomass can reveal anoxic
conditions
that can lead to deterioration of settling. The experimental design was the
same
used for the DARR test described above. An aerated flask supplied with the
same food and nutrient supplement served as a control. At various intervals
over the 24 hour incubation, samples were tested for poor settling.
Example 4
A total ATP was performed on a suspended sample. The sample was then
allowed to settle. The supernatant was analyzed for dissolved ATP and an
additional portion of the supernatant was analyzed for total ATP. The
difference
of the total ATP and dissolved ATP of the suspended sample provides the
concentration of cellular ATP. The difference of the total ATP and dissolved
ATP
of the settled sample provides the concentration of unsettled cellular ATP.
The
percentage of the unsettled cellular ATP of the suspended cellular ATP is
defined
as planktonic ATP index.
The results of the experiment are shown in figure 6. It can be seen from
the graph that microbial floc of the reaction began to deteriorate and release
CA 02483570 2004-10-01
- 16 -
free-floating cells between 2 and 4 hours as indicated by the rise in
planktonic
ATP. The deterioration continued for the duration of the test. In contrast,
the
planktonic ATP slowly but steadily declined under the favorable aerobic
conditions.
ATP analyses can also be performed on floc from a sample that has been
passed through a series of sieves or other, fractionating devices based on
variations in size, density, and shape to gain further insights into settling
properties.
Therefore by performing a battery of analyses based on both dissolved and
total ATP, fundamental information about the biomass characteristics, health,
and quantity can be gained. When this information is compared with operational
variables together with the process outputs and statistically analyzed, plans
to
improve the process are likely to be more effective. These might include, but
are
not limited to, flow equalization, optimization of sludge return rate in
activated
sludge processes, nutrient optimization (which may or may not include the type
of nutrients added, from nitrogen to phosphorous to micronutrients), addition
of
settling agents and other chemical additives, temperature, aeration and mixing
optimization.
Successful and practical implementation of the above test protocols
required the development of new ATP assay processes to overcome the following
obstacles:
1. Unstable ATP standards
2. Unstable dissolved ATP
3. Unstable sample ATP extracts
4.
Commercially available ATP releasing agents providing poor
extraction efficiency
5. Wastewater being inhibitory to luciferase
6. Unstable luciferase
The first 4 difficulties were overcome by developing reagents based on a
high pH (pH 10-12) trisodium phosphate (TSP) buffer. The fifth difficulty was
partially overcome by the TSP buffer, but also by improvements to the
luciferase
reagent formulation, and sample dilution. However, the high pH of the TSP
buffer was incompatible with luciferase which has a pH optimum ranging
CA 02483570 2009-07-06
- 17 -
between pH 7.5 to 7.75. To overcome this difficulty, a luciferase formulation
was
developed for measurement process that is strongly buffered in the optimum pH
range, but the pH was lowered below the buffered region to pH 7Ø When
combined with an ATP solution in a high pH trisodium phosphate buffer, the
final
pH is raised to the optimum region of the reaction.
Because ATP standard solutions are used to calibrate the luciferase
reagent and luminometer, reliable standards play an essential role in ATP
monitoring. This has presented an obstacle for making the ATP assay a routine
=
laboratory test that relatively unskilled or inexperienced workers can use.
A typical approach has been to store ATP standard solutions at -20 C or
lower and thaw them on the day of use or to prepare standards fresh daily from
crystalline ATP (4,5,6). A method to solve the ATP standard stability problem
was described by Tobin et al. (7) who reported improved stability of ATP
solutions using a high pH (pH 11.7) trisodium phosphate buffer.
However, a problem can occur if an unsuitable container is used for the
ATP solution. At this pH, the walls of glass containers may start to
solubilize and
produce undesirable turbidity to the standard solution. Furthermore, if
plastic
containers are used, a problem of gas diffusion can occur. Some plastic
containers readily allow diffusion of atmospheric gases into the container.
High
pH solutions strongly absorb carbon dioxide. As a consequence, the pH of the
solution is lowered. In the case of ATP standards, lowering of the pH is
unfavorable for stability due to potential for microbial growth, increase in
activity
of ATP-degrading enzymes, and increase of metal-catalyzed ATP hydrolysis.
Table 1 shows that stability of very dilute ATP standard solutions stored in
ATP solutions in 5 mM trisodium phosphate (pH 11.6) in containers with
apparently low gas permeability are stable for 20 weeks at room temperature.
ATP was measured during the test period by mixing 50 microliters of a sub-
sample of the ATP solution with 50 microliters of luciferase reagent. The
resulting light production was measured in a Tuner Designs Model 20e
luminometer.
CA 02483570 2004-10-01
4
- 18 -
Table 1 - Room temperature Stability of ATP standards.
Percentage of light output compared to freshly
prepared standard
ATP standard 4 weeks 12 weeks 20 weeks
mL 111.0 97.5 97.3
1,000 ng/mL 105.4 97.5 96.0
It was also discovered that the trisodium phosphate buffer similar to that
described by Tobin et al. (6) is useful in the measurement of dissolved ATP.
The
first step in the measurement of dissolved ATP is to separate the majority of
the
biomass from the liquid phase of the sample. This is necessary because the
turbidity of a fully suspended sample quenches the light of the bioluminescent
luciferase reaction. This can be accomplished by filtering the sample or
simply
allowing it to settle. The settling could be assisted by addition of a
flocculating
agent.
We also discovered that the liquor of a municipal sewage treatment
reactor is inhibitory to the luciferase enzyme. Therefore, it is necessary to
dilute
it before mixing with luciferase for ATP measurement.
An experiment described below as Example 5 was conducted to determine
the most suitable buffer for the dilution. Two buffers were compared: 5 rnM
trisodium phosphate buffer (pH 11.6) and N-[2-Hydroxyethyl]piperazine-N'-[2-
ethanesulfonic acid] (HEPES), a buffer used in luciferase formulations
adjusted to
the enzyme's pH optimum (pH 7.5).
Example 5
A sample, from the reactor of a municipal sewage treatment plant was
allowed to settle for 10 minutes. The supernatant was filtered in two
instances
then diluted in either HEPES or TSP buffer. In another instance, the
supernatant
was not filtered and diluted in HEPES buffer. At various time intervals, the
dissolved ATP was measured by mixing 50 microliters of diluted supernatant
with
50 microliters of luciferase reagent. The resulting light production was
measured
in a Tuner Designs Model 20e luminometer. The results shown in Table 2 below,
demonstrate that without filtration, dissolved ATP decays very rapidly when
HEPES is used as the diluent. The decay in HEPES is likely the effect of
microbial
and extracellular ATP'ases. These enzymes are inhibited in trisodium phosphate
CA 02483570 2009-07-06
- 19 -
buffer. In contrast, the stability of the filtered supernatant diluted with
trisodium
phosphate was excellent over a 24 hour period.
Table 2 - Stability of Dissolved ATP Diluted in HEPES and
Trisodium Phosphate Buffers.
Time after dilution
0 hr 1 hr '24 hr
RLU* RLU* RLU*
Filtered, 1/10 HEPES 20.6 6.3 0.3
Unfiltered, 1/10 HEPES 2.0 0.4 0.7
Filtered, 1/10 TSP 23.5 23.3 22.8
* Relative Light Units (i.e. luminometer instrument display after ATP is
assayed.)
Another experiment was conducted in a similar manner except that ATP
was measured by mixing 100 microliters of diluted supernatant with 100
microliters of luciferase reagent and the resulting light production was
measured
in a Kikkoman LumiTester C-100.
Table 3 - Stability of Dissolved ATP in
Trisodium Phosphate Buffer.
Minutes after dilution Filtered Unfiltered
in TSP buffer (RLU) (RLU)
1 8515 8849
5 7940 9549
10 8269 9203
15 8269 9457
30 8390 8923
A TSP-based buffer for ATP extraction was also found very useful for
extraction of ATP from microorganisms. For biological wastewater treatment
process samples, significant improvements were made over the process
described by Tobin et al. (7), including the elimination of using a
homogenizer,
= increasing the TSP concentration by five fold, adding NaOH to increase
the
alkalinity, and substituting chloroform with the more user-friendly
benzalkonium
chloride.
The benefit of these modifications compared to other commercial ATP
releasing agent is shown in Example 6 described below and the results are
CA 02483570 2004-10-01
- 20 -
illustrated in figure 7. The formulation outlined in the following Table 4 was
used.
Table 4 - Formulation Containing Trisodium Phosphate
Suffer for ATP Extraction.
Component (CAS #) Concentration (0/0 w/w)
Deionized Water 96.08
Sodium Phosphate, Tribasic Dodecahydrate 3.66
(10101-89-0)
Benzalkonium Chloride (8001-54-5) 0.19
Sodium Hydroxide (1310-73-2) 0.06
Figure 7 shows a comparison of the performance of our formulation with
several commercially available ATP releasing agents.
Example 6
The various commercial releasing agents and our releasing agent
formulation were added to a series of sub-samples taken from the reactor of a
municipal sewage plant and mixed to extract the ATP from the microorganisms.
In order to eliminate problems of reaction quenching, they were then diluted
in
deionized water and measured immediately. The final dilution of all ATP
extracts
was 1/306. Fifty microliters of the diluted ATP extract were mixed with 50
microliters of luciferase reagent and the resulting light was measured in a
Turner
Designs Model 20e luminometer. The ATP concentration in the samples was
calculated from the light measurement of both the sample and a similar
measurement of an ATP standard solution of known concentration.
The results demonstrate the superior efficiency of our TSP-based releasing
agent, UltraLyseTM 30 compared to other commercial reagents. An efficient
extraction reagent is not only important with respect to total ATP
measurement.
Incomplete extraction will also interfere with the accuracy of the biomass
stress
index and cellular ATP measurements because total ATP is a component of these
parameters.
In addition to providing superior extraction efficiency, the use of the TSP-
based releasing agent produces extracts that are relatively stable. This
permits
the analyst to delay measurement of the extract until it is most convenient.
It
also could facilitate automated or mechanized analytical processes.
CA 02483570 2004-10-01
- 21 -
Table 5 - Stability of ATP extracts in the Trisodium
Phosphate Buffer Formulation.
Days of refrigerated storage ATP recovery (0/0 of initial)
In UtIraLyse 30
1 98
3 96
7 91
Despite the many advantages provided by the high pH TSP buffer, it has
one problem. The pH of the buffer is much higher than the optimum for the
activity of luciferase which is approximately pH 7.4 to 7.8.
We discovered that this problem can be overcome by using a luciferase
buffer that has a pK near the optimum of the luciferase enzyme and then
lowering the pH to approximately pH 7Ø Thus, when a solution of ATP in high
pH TSP buffer is added to the luciferase reagent the pH is raised to the
optimum
region. Using a pH of 7.0 for the luciferase preparation has a second benefit.
Luciferase is more stable at pH levels lower than its optimum for activity
(8).
The stability of the luciferase can be further improved by the addition of
other stabilizing agents such as a chelating agent like ethylenediamine tetra
acetic acid (EDTA), protein-based stabilizers such as bovine serum albumin
(BSA), a reducing agent to protect sulfhydryl groups such as dithiothreitol
and a
hydrogen bond stabilizer such as trehalose (8-10).
Foote and Grant (11) also teach the stabilization of luciferase in the
presence of a polyol such as trehalose and glycerol when adjusted to a pH
lower
than the optimum for activity. However, the formulations described by Foote
and Grant use weak buffers, with a pK at the storage pH. Mixing such
formulation with ATP in a high pH TSP buffer would be problematic because
there
would be a tendency for the reaction pH to fall outside the optimum range for
activity.
In addition to contributing to the stabilization of luciferase, EDTA provides
protection of the enzyme from inhibition of heavy metals that are frequently
encountered in wastewater. samples. Therefore, it is advantageous to maximize
the EDTA concentration in the luciferase reagent.
CA 02483570 2004-10-01
- 22 -
Table 6 shows the results of an experiment described below as Example 7
used to determine the maximum EDTA concentration that can be used in a
luciferase formulation without inhibiting luciferase activity. The magnesium
salt
of EDTA was used to ensure that magnesium would be available as a cofactor for
the luciferase. The test was conducted by spiking aliquots of luciferase with
a
concentrated solution of Mg/EDTA and testing luciferase activity by adding ATP
standard (1,000 ng/mL). Fifty microliters of ATP standard were mixed with 50
microliters of luciferase reagent and the resulting light was measured in a
Turner
Designs Model 20e luminometer.
Table 6 - Effect of Increasing the Magnesium-EDTA
Concentration on Luciferase Activity.
Final Mg/EDTA in Instrument Display After
Luciferase Reagent (g/L) Mixing Luciferase with ATP
1.7 292
3.4 280
5.1 206
6.8 169
8.4 119
10.1 115
11.8 75
13.5 88
15.2 70
16.9 58
The results indicate that 3.4 g/L of the chelating agent can be added
without serious loss of enzyme activity.
Example 7
Two formulations having the composition outlined below, in Table 6 were
tested for their stability at room temperature and under refrigerated
conditions.
One formulation was prepared using a luciferase from Kikkornan (LUC-T) that
had been purified from cultures of E. coli into which the genes of the firefly
luciferase had been inserted and altered to produce a more heat-stable enzyme,
while the other formulation contained luciferase from Sigma-Aldrich that had
been purified directly from fireflies. At various time intervals 50 microliter
sub-
samples of each formulation were removed, mixed with 50 microliters of a
1 ng/mL ATP standard and the resulting light was measured in a Turner Designs
CA 02483570 2004-10-01
- 23 -
Model 20e luminometer. The results were expressed as a percentage of the
reading obtained at the beginning of the experiment.
Both formulations in the experiment contained 40 micrograms luciferase
per mL and 100 micrograms luciferin per mL and were adjusted to pH 7Ø The
formulations consisted of the components outlined below in Table 7.
Table 7- Example of Luciferase Formulation
Component Quantity
Deionized water mL 100
HEPES disodium g 0.6508
Mg/EDTA 9 0.346
Trehalose 9 10
Dithiothreitol 9 0.0133
= Bovine serum albumin 9
0.1
= Magnesium
sulphate.7H20 g 0.949
D-luciferin tg 100
Luciferase g 40
Figure 8 shows that the activity of both formulations are similar at room
temperature, illustrating an excellent stability over a three week period at
which
time approximately 50% of the enzyme activity still remains. By comparison,
Sigma-Aldrich has their own luciferin/luciferase ATP assay formulation that
they
claim is stable for 2 weeks when rehydrated and stored under refrigerated
conditions, and do not suggest to leave the enzyme reagent at room
temperature.
The use of refrigerated storage conditions appears to be advantageous for
the recombinant luciferase. Figure 8 shows that the half-life of the activity
is
almost doubled.
Accordingly, the invention provides a method for optimizing biological
processes performed by correlating operational variables of a process together
with the process outputs and a battery of analyses based on the measurement of
dissolved or extracellular ATP in conjunction with the measurement of total
ATP
(dissolved plus cellular ATP). The operational variables may include but are
not
limited to food (biological oxygen demand (BOD), biodegradable substrate
analytical profiles), pH, acidity, alkalinity, temperature, conductivity,
nutrients,
other chemical addition, dissolved oxygen, pressure, and toxic substance
CA 02483570 2004-10-01
- 24 -
concentration. The process outputs may include but are not limited to BOD
removal, nutrient consumption, heat, concentration and production rate of
carbon dioxide, methane, hydrogen, hydrogen sulfide, volatile fatty acids and
other organic acids, alcohols, suspended solids, and volatile suspended
solids.
The invention also provides for a method in which total ATP in a sample
from a biological process may be measured by adding an ATP releasing agent to
= a completely mixed sub-sample, then assaying for ATP. Preferably, the
resulting
mixture can be diluted prior to assaying for ATP.
The dissolved ATP in a sample from a biological process may be measured
by separating the biological solids from the sampe, taking a sub-sample of the
= liquor, and assaying for ATP. The sub-sample can be diluted prior to
assaying for
ATP.
The cellular ATP in a sample from a biological process may be determined
by measuring total and dissolved ATP and subtracting the dissolved ATP value
from the total ATP value.
The biomass stress index of microorganisms in a sample from a biological
process may be determined by measuring total ATP and dissolved ATP and
calculating the ratio of the dissolved ATP to the total ATP. The biomass
stress
index of microorganisms in a sample from a biological process also may be
determined by measuring and calculating the dissolved ATP and the cellular ATP
and calculating the ratio of the dissolved ATP to the cellular ATP.
The dissolved ATP removal rate in a sample from a biological process may
be determined by measuring the disappearance of a dissolved ATP spike added
to the biomass suspension. The dissolved ATP spike can be about 1 to 100 times
higher than the total ATP of the sample. The preferred dissolved ATP spike can
be about 10 times higher than the total ATP of the sample. DARR may be
normalized to the total ATP or cellular ATP content of the sample prior to the
addition of the ATP spike.
The quantity of free-floating or planktonic biomass may be estimated by
measuring the cellular ATP in a sample from a biological process that has been
allowed to settle. The planktonic index may be determined by measuring the
total and/or cellular ATP in a sample from a biological process that has been
CA 02483570 2004-10-01
t
=
- 25 -
allowed to settle and calculating the fraction that this ATP represents of the
total
and/or cellular ATP of the completely mixed sample.
The biomass floc dispersion index may be determined by measuring the
total and/or cellular ATP contained in pin-sized floc and free-floating or
planktonic cells by using a sieve to separate them from the other
microorganisms
in the sample and then measuring the total and/or cellular ATP.
The bulking floc may be measured by sieving or treating a sample to
separate floc containing excessive quantities of bulking filamentous bacteria
and
assaying the total and/or cellular ATP of this component of the sample.
The biomass growth index may be determined by adding nutrients and
adding biomass from a biological process to the process influent or effluent
water
of the biological process and measuring the growth rate of the microorganisms
by measuring total and/or cellular ATP.
The toxicity index may be determined by adding nutrients and adding
biomass from a biological process to the process influent or effluent water of
the
biological process and measuring total and/or cellular ATP to determine the
growth rate or decrease in total and/or cellular ATP or increase in biomass
stress
index of the microorganisms.
The activity of the grazing population of a floc may be measured by adding
a suspension of planktonic or free-floating their bacteria to the sample and
measuring the rate of their disappearance by periodically measuring the
planktonic ATP over timed intervals.
The biomass may be separated from the sample by allowing the sample to
settle by gravity or centrifuging, flocculating, or filtering it.
In the method according to the invention, ATP may be measured in a
luminometer by a bioluminescent reaction produced by mixing an ATP solution
containing a high pH (about pH 10-12) phosphate buffer with a reagent
containing luciferin, luciferase, and magnesium cofactor dissolved in a buffer
with
a pK of about 7.5 to 8.5 but adjusted to about pH 6-7.2. The ATP solution used
may be an ATP standard solution stored in a container with low gas
permeability.
The preferred phosphate can be trisodium phosphate at a concentration of about
5 mM.
CA 02483570 2004-10-01
- 26 -
The luciferase may be immobilized on a support substrate and may be re-
used for more than one analysis. The luciferase may be a recombinant
luciferase. The luciferase reagent may contain at least one of the following
components: an enzyme hydrogen-bond stabilizer such as trehalose, glycerol, or
glycol; a chelating agent such as EDTA, a protein-based enzyme stabilizer e.g.
BSA, casein; and a reducing agent to maintain luciferase sulfhydryl groups in
a
=reduced form, e.g. dithiothreitol, mercaptoethanol. EDTA may be present in an
amount of between about 0.03% to 1.3% by weight of the luciferase reagent.
The source of the ATP solution may be the extracellular ATP in a
suspension containing microorganisms. The sample may be centrifuged or
allowed to settle with or without the assistance of a flocculating agent and
the
supernatant may be mixed with a high pH phosphate buffer. The preferred
phosphate can be trisodium phosphate at a concentration of about 5 mM. The
sample may be filtered and the filtrate can be mixed with a high pH phosphate
buffer. The preferred phosphate can be trisodium phosphate at a concentration
of 5 mM.
The source of the ATP solution may be a sample containing
microorganisms that have been treated with an ATP releasing agent containing a
high pH phosphate buffer. The releasing agent may be composed of phosphate
buffer and a cationic or non-ionic surfactant. The pH of the releasing agent
can
be greater than about pH 9.9 and preferably about pH 11.1 to 11.7. The
preferred phosphate can be trisodium phosphate at a concentration of between
about 0.5 mM and 500 mM. Preferably for biological wastewater treatment
applications, the concentration can be between about 5-100 mM. The
concentration of the surfactant can be about 100 to 10,000 mg/L. In the case
where benzalkonium chloride is the cationic surfactant and the application is
a
biological wastewater treatment plant, the preferred concentration can be
about
1,000-4,000 mg/L.
The releasing agent may also be composed of trisodium phosphate, a
cationic or non-ionic surfactant, and sodium hydroxide. The pH of releasing
agent can be greater than about pH 9.9 and preferably about pH 11.1 to 11.7.
The preferred phosphate can be trisodium phosphate at a concentration of
between about 0.5 mM and 500 mM. Preferably for biological wastewater
CA 02483570 2013-05-31
- 27 -
treatment applications, the concentration can be between about 5-100 mM. The
concentration of the surfactant may be about 100 to 10,000 mg/L. In the case
where benzalkonium chloride is the cationic surfactant and the application is
a
biological wastewater treatment plant, the preferred concentration can be
about
1,000-4,000 mg/L. The concentration of sodium hydroxide may range from
about 100 to 10,000 mg/L, but the preferred concentration for samples from
biological wastewater treatment applications can be about 600 mg/L.
The sample may be filtered and the filter can be treated with a releasing
agent containing a high pH phosphate buffer. The preferred phosphate buffer
can be trisodium phosphate at a concentration between about 0.5 mM and
500 mM.
Preferably for biological wastewater treatment applications, the
concentration can be between about 5-100 mM.
The sample may also be filtered, and the filtrate may be treated with
organic solvent, then treated with high pH phosphate buffer or a releasing
agent
containing a high pH phosphate buffer. The preferred phosphate buffer can be
trisodium phosphate at a concentration of between about 0.5 mM and 500 mM.
The dissolved ATP from microorganisms may be enzymatically or chemically
removed prior to addition of the releasing agent.
According to the invention, the reagents for the process may be used in
test kits.
Data from the luminometer may be statistically analyzed using a computer
program and the analysis may be used to automatically adjust the process
operations.
Process operations may include but are not limited to flow equalization,
aeration, pH adjustment, mixing, sludge return rate, effluent return rate,
nutrient and other chemical addition.
Preferred buffers for the luciferase reagent may be N-[2-
Hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid] (HEPES);
N-
[tris(hydroxymethy)methyl]glycine; and
N-[2-hydroxy-1,1-
bis(hydroxymethyl)ethyl]glycine (TRICINE). The preferred concentration may be
about 0.025 to 0.075 M and the preferred pH can be about pH 6.5 to 7Ø
CA 02483570 2013-05-31
-
- 28 -
The scope of the claims should not be limited by the preferred embodiments set
forth in the examples, but should be given the broadest interpretation
consistent
with the description as a whole. The claims are not to be limited to the
preferred
or exemplified embodiments of the invention.
References
1. Patterson, 3.W., Brezonik, P.L., and Putnam, H.D. (1970) Measurement
and significance of adenosine triphosphate in activated sludge. Environ.
Sci. Technol. 4(7) 569-575.
2. Roe, P.C. Jr. and Bhagat, S.K. (1982) Adenosine triphosphate as a
control
parameter for activated sludge processes. J. Wat. Pollut. Cont. Fed. 54,
244-254.
3.
Archibald, F., Methot, M, Young, F., and Paice, M.G. (2001) A Simple
System To Rapidly Monitor Activated Sludge Health And Performance. Wat.
Res. 35 (10) 2543 -2553.
4. Annual Book of ASTM Standards (1989). Standard Method for Adenosine
Triphosphate (ATP) Content of Microorganism in Water. Volume 11.02
Water (II) 455-458.
5. Sigma Chemicals (1988). Adenosine 5'-Triphosphate (ATP) Bioluminescent
Assay Kit. Technical Bulletin # BAAB-1.
6. Promega Corporation (2001). rLuciferase/Luciferin
Reagent
Bioluminescence Detection Reagent for ATP Measurement. Technical
Bulletin #268.
7.
Tobin R.S., Ryan J.F. and Afghan B.K. (1978) An improved method for the
determination of adenosine triphosphate in environmental samples. Water
Res. 12, 783 -792.
CA 02483570 2004-10-01
-29-
8. Deluca, M. (1975) ATP Methodology Seminar, SAI Technology Co., San
Diego.
9. Lundin, A. (1993) Optimised Assay of Firefly Luciferase with Stable
Light
Emission in Bioluminescence and Chemiluminescence Status Report.
A.A. Szaly, L.J. Kricka, and P. Stanley ed., Wiley, pp. 291-295.
10. Wang, C.Y. and Andrale, 3. D. (1993) Interfacial Behavior of Firefly
Luciferase in Bioluminescence and Chemiluminescence Status Report.
A.A. Szaly, L.J. Kricka, and P. Stanley ed., Wiley, pp.99-103.
11. Foote, N.P.M. and Leonard, G.P. (1994) Bioluminescence Reagent
Formulation. Published international patent application number
W09411528.