Note: Descriptions are shown in the official language in which they were submitted.
CA 02484240 1998-10-23
DESCRIPTION
PACKAGED OCULAR IRRIGATING SOLUTION BAG
This is a divisional application of Canadian Patent
Application Serial No..2,307,162 filed on October 23 , 1998.
TECHN'[S;AL FIELD
The present invention relates to a packaged
ocular irrigating solution bag. More specifically, the
present invention relates to a packaged bag comprising a
multiple compartment bag wherein components for preparing,
a bicarbonate ion- and glutathione- and/or dextrose-
containing solution are stably stored and mixed together
when used for intraocular or extraocular irrigation or
cleaning in an ophthalmic surgery. Especially, the
present invention relates to an .improved packaged bag
thereof which is provided with a pii indicating device
whose color changes, thereby permitting a visual
inspection of the pH change of the bicarbonate ion-
containing solution due to carbon dioxide generation. It
should be understood that the ex~aression "the invention" and the
like encompasses the subject-matter of both the parent and the
divisional application.
BACKGROUN~p ART
As solutions for intraocular or extraocular
irrigation or cleaning in ophthalmic surgeries such as
cataract surgery; vitreous surgery and glaucoma surgery,
"Opeguard" (produced by Senju Pharmaceutical Co., Ltd. and
sold by Takeda Chemical Industries, Ltd.) and BSS PLUS
(imported and sold by Santen Pharmaceutical Co., Ltd.) are
commercially available at present.
The former "Opeguard" is in a liquid form within
CA 02484240 1998-10-23
-2-
a pH range of 7.2-8.2 and comprises dextrose as an active
ingredient. This product has a disadvantage of lacking
long-term storage stability because dextrose is stable in
an acid pH environment.
The latter "BSS PI,US° is in such a form that a
solution (10 ml or 20 ml) containing oxiglutatione,
dextrose, calcium ion and magnesium ion and a diluent (240
ml or 480 ml) containing bicarbonate ion are separately
stored in glass vials, the solution containing
oxiglutatione, etc. being transferred into the vial
containing the diluent using a syringe having a double
ended needle and mixed together before use. This is
because it is difficult to stably maintain..oxiglutatione
and dextrose at pH 7 or above for a long time and because
there is a disadvantage that calcium ion and magnesium ion
in contact with the di.luent for a long period of time
cause precipitation.
The above "BSS Plus" can be stably stored for a
long period of time but requires transferring procedures
as shown below in (1) to (4) and consequently has the
disadvantages as shown below in (a) to (f).
(1) A cap is removed from the rubber stopper of the glass
bottle containing 10 ml or 20 ml of the oxiglutatione
solution, and a cap is removed from the resin needle
at one end of the syringe. The rubber stopper of the
CA 02484240 1998-10-23
-3-
bottle is pierced by the needle.
(2) Next, a cap is removed from the stainless steel
needle at the other end.
(3) An aluminum cover is removed from the glass bottle
containing the diluent, and the rubber stopper of the
bottle is pierced by the stainless steel needle.
(4) After transferring the oxiglutatione solution into
the bottle of the diluent, the two liquids are well
mixed.
(a} The above procedure itself is complicated.
Especially, the operation of piercing the rubber
stopper of the oxiglutatione solution-containing
bottle with the resin needle is-considerably
difficult .
(b) The transferring and mixing of the two liquids take
too much time to be responsive to urgent use.
(c) The risk of bacterial contamination at the time of
transferring and mixing can not be avoided.
(d) The risk of foreign matter contamination increases if
the resin needle is stuck into the rubber stopper of
the bottle twice or more.
(e) If the mixing procedure is mistaken, the two liquids
are not mixed or the concentration of the resulting
solution changes.
{f) There is a possibility that only the diluent is
CA 02484240 1998-10-23
inadvertently administered without mixing with the
oxiglutatione solution.
TM TM
Moreover, not only "Opeguard" and "BSS Plus" but
also these types of ocular irrigating solutions are
generally in the liquid form, stored in glass vials and
adjusted to wn alkaline pH range near pH 8 using
bicarbonate ion. Therefore, if placed in glass bottles
and then sterilized by heating for preservation, the
conventional products have a fatal defect that the glass
falls in flakes with the lapse of time.
Therefore, there is desired in this industry the
development of a new ocular irrigating solution which
replaces conventional ocular irrigating solutions and ..
solves all the defects of the conventional solutions.
Bicarbonate ion (hydrogencarbonate ion) used in
these types of ocular irrigating solutions is at
equilibrium in solution, as represented by the following
formula (1):
2 H C03 "~ C02 t "E' CQ32 ...E. H2
2 0 ~---
In an open system, the reaction proceeds-to the right as
the carbon dioxide gas on the right-hand side of formula
(1) evaporates, with the result that the bicarbonate ion
decreases and the carbonate ion increases. As a result,
the pH of the aqueous solution rises progressively.
CA 02484240 1998-10-23
-5-
Healthy human lacrimal fluids are within a pH
range of 7.5'x'0.2 in the arousal state, and at about
pH7.25 in the condition that the eyes are closed for hours.
It is necessary to adjust the pH of an ocular irrigating
solution to the above-mentioned range as much as possible
because the human feels discomfort when it is out of the
above pH range, for example, at pH6 or below or at pH8 or
above. The pH rise deviating from this range must be
prevented to the utmost.
Therefore, in order to prevent the time-
dependent pH change, ocular irrigating solutions are
conventionally contained in glass vials or like sealed
containers for preventing evaporation of evolved carbow
dioxide gas to thereby maintain the equilibrium essential
to the stabilization of bicarbonate ion concentration and
solution pH.
However, containers made of glass are easy to be
broken, are very heavy and involve difficulties in
disposal. In addition to these fatal defects, since the
evolution of carbon dioxide gas in the course of
sterilization of the ocular irrigating solutions is
unavoidable, the risk for an elevation of internal
pressure inducing fracture of the glass container is high:
Further, there is another disadvantage. As
stated above, it is preferable for bicarbonate ion-
CA 02484240 1998-10-23
-6-
containing ocular irrigating solutions to be weak alkaline
solutions within a pH range of about 7-8. At such pH,
however, if the solutions are placed in glass containers
and sterilized by heating for preservation, the glass of
the container wall falls in flakes with the lapse of time,
making long-term preservation impossible.
An object of the present invention is to
overcome all the above-mentioned defects of the prior art
and provide a packaged ocular irrigating solution bag
which is capable of providing an oxiglutatione- and/or
dextrose- and bicarbonate ion-containing ocular irrigating
solution in stable condition for a long period of time,
most effectively preventing the pH change of the
bicarbonate ion-containing solution due to evolution of
carbon dioxide gas and providing an unmistakable visual
indication of the pH change of the solution.
The present inventors carried out intensive
research to achieve the above object and found the
following. In a gas-permeable plastic multiple
compartment bag, a bicarbonate ion-containing solution is
enclosed in one of the compartments and an oxiglutatione-
and/or dextrose-containing solution or solid preparation
is enclosed in another compartment, followed by
sterilization thereof according to a general sterilization
method such as autoclave sterilization, hot water
CA 02484240 1998-10-23
-7-
immersion sterilization or hot water shower sterilization,
or the above pharmaceuticals are aseptically enclosed in
the compartments. Then the obtained bag is packed with a
gas-impermeable plastic packaging member, and carbon
dioxide gas is fed into the space between the bag and the
packaging member. In such a case, an ocular irrigating
solution can be prepared by bringing the compartments of
the bag into communication with each other and mixing the
contents of the bag when used, thus enabling stable
storage of the ocular irrigating solution, which is one of
the objects of the invention.
The present inventor further found that when the
multiple compartment plastic bag is packed with a gas-
imp~ermeable plastic packaging member and a specific pH
indicating device is disposed in the space between the bag
and the packaging member, pH change of the bicarbonate
ion-containing solution in one of the compartments of the
bag can be accurately checked with the eye. The present
invention has been accomplished based on these findings.
CA 02484240 1998-10-23
7a
According to an aspect of the present invention there
is provided a packaged ocular irrigating solution bag,
which comprises a gas-permeable plastic multiple
compartment bag and a gas-impermeable plastic packaging
member for packaging the bag, the bag comprising at least
compartment A comprising oxiglutatione or a mixture of
oxiglutatione and dextrose as a solution or solid
preparation, and compartment B comprising a bicarbonate
ion-containing solution, and a space between the bag and
the packaging member having a carbon dioxide gas
atmosphere, wherein compartment A and compartment B are
intercommunicable with each other such that an ocular
irrigation solution is formed when the contents of
compartment A and compartment B are mixed.
According to another aspect of the present invention
there is provided a packaged ocular irrigating solution
bag, which comprises a gas-permeable plastic multiple
compartment bag and a gas-impermeable plastic packaging
member for packaging the bag, the bag comprising at least
compartment A comprising oxiglutatione. or a mixture of
oxiglutatione and dextrose as a solution or solid
preparation, and compartment B comprising a bicarbonate
ion-containing solution, a space between the bag and the
packaging member having a carbon dioxide gas atmosphere and
pH indicating device comprising a gas-permeable plastic
packet containing a bicarbonate ion-containing solution and
a pH indicator having the property to undergo a change in
color in response to a change in the pH of the solution,
wherein compartment A and compartment B are
intercommunicable with each other such that an ocular
irrigation solution is formed when the contents of
compartment A and compartment B are mixed.
CA 02484240 1998-10-23
7b
According to a further aspect of the present invention
there is provided a method of manufacturing a packaged
ocular irrigating solution bag, comprising the steps of:
(1) enclosing in compartment A a solution or solid
preparation comprising at least one species selected from
oxiglutatione and dextrose, compartment A being one
compartment of a gas-permeable plastic multiple compartment
bag comprising at least two compartments, (2) enclosing in
compartment B a bicarbonate ion-containing solution,
compartment B being another compartment of the gas-
permeable plastic multiple compartment bag, (3) packaging
the gas-permeable plastic multiple compartment bag in a
gas-impermeable plastic packaging member, and (4) in the
above packaging step (3), feeding a mixed gas containing
carbon dioxide gas into a space between the bag and the
packaging member to thereby establish a carbon dioxide gas
atmosphere in the space.
According to a still further aspect of the present
invention there is provided a method of manufacturing a
package ocular irrigating solution bag, comprising the
steps of: (1) enclosing in compartment A a solution or
solid preparation comprising at least one species selected
from oxiglutatione and dextrose, compartment A being one
compartment of a gas-permeable plastic multiple compartment
bag comprising at least two compartments, (2)enclosing in
compartment B a bicarbonate ion-containing solution,
compartment B being another compartment of the gas-
permeable plastic multiple compartment bag, and (3)
packaging the gas-permeable plastic multiple compartment
bag and a carbon dioxide-generating oxygen scavenger in a
gas-impermeable plastic packaging member to thereby
establish a carbon dioxide gas atmosphere inside the
packaging member.
CA 02484240 1998-10-23
7C
According to yet another aspect of the present
invention there is provided a method of manufacturing a
packaged ocular irrigating solution bag, comprising the
steps of: (1) enclosing in compartment A a solution or
solid preparation comprising at least one species selected
from oxiglutatione and dextrose, compartment A being one
compartment of a gas-permeable plastic multiple compartment
bag comprising at least two compartments, (2) enclosing in
compartment B a bicarbonate ion-containing solution,
compartment B being another compartment of the gas-
permeable plastic multiple compartment bag, (3) packaging
the gas-permeable plastic multiple compartment bag and a pH
indicating device in a gas-impermeable plastic packaging
member, the pH indicating device comprising a gas-permeable
plastic packet containing a bicarbonate ion-containing
solution and a pH indicator having the property of
undergoing a change in color in response to a change in the
pH of the solution, and (9) in the above packaging step
(3), feeding a mixed gas containing carbon dioxide gas into
a space between the bag and the packaging member to thereby
establish a carbon dioxide gas atmosphere in the space.
According to yet another aspect of the present
invention there is provided a method of manufacturing a
packaged ocular irrigating solution bag, comprising the
steps of: (1) enclosing in compartment A a solution or
solid preparation comprising at Least one species selected
from oxiglatatione and dextrose, compartment A being one
compartment of a gas-permeable plastic multiple compartment
bag comprising at least two compartments, (2) enclosing in
compartment B a bicarbonate ion-containing solution,
compartment B being another compartment of the gas-
permeable plastic multiple compartment bag, and (3)
CA 02484240 1998-10-23
7d
packaging the gas-permeable plastic multiple compartment
bag, a pH indicating device and a carbon dioxide-generating
oxygen scavenger in a gas-impermeable plastic packaging
member to thereby establish a carbon dioxide gas atmosphere
inside the packaging member, the pH indicating device
comprising a gas-permeable plastic packet containing a
bicarbonate ion-containing solution and a pH indicator
having the property of undergoing a change in color in
response to a change in the pH of the solution.
DIS~LO~LrRE OF INVFNTTnu
The present invention provides a packaged ocular
irrigating solutian bag, which comprises a gas-permeable
plastic multiple compartment bag and a gas-impermeable
plastic packaging member for packaging the bag, the bag
comprising at least compartment A wherein an
CA 02484240 1998-10-23
_8_
oxiglutatione- and/or dextrose-containing solution
(hereinafter referred to as "GSSG/GLU solution") or solid
preparation (hereinafter referred to as "GSSG/GLU solid
preparation") is enclosed, and compartment B wherein a
bicarbonate ion-containing solution (hereinafter referred
to as °bicarbonate ion solution") is enclosed, and the
space between the bag and the packagi.r~g member having a
carbon dioxide gas atmosphere.
The present invention further provides a
packaged ocular irrigating solution bag, which comprises a
gas-permeable plastic multiple compartment bag and a gas-
impermeable plastic packaging member for packaging the bag,
the bag comprising at .least compartment A wherein a .
GSSG/GLU solution or a GSSG/GLU solid preparation is
enclosed, and compartment B wherein a bicarbonate ion
solution is enclosed, the space between the bag and the
packaging member having.a carbon dioxide gas atmosphere
and being provided with a pH indicating device comprising
a gas-permeable plastic packet comprising a bicarbonate
ian-containing solution and a pH indicator whose color
changes in accordance with the pH change of the solution.
Further, the present invention provides 'the
above packaged ocular irrigating solution bags having the
following features
CA 02484240 1998-10-23
_g-
(1) The packaged ocular irrigating solution bag wherein
an oxygen sensor is. disposed in the space between
the multiple compartment bag and the packaging
member.
(2) The packaged ocular irrigating solution bag wherein
a carbon dioxide-generating oxygen scavenger is
disposed in the space between the multiple
compartment bag and the packaging member, thereby
establishing a carbon dioxide gas atmosphere.
(3) The packaged ocular irrigating solution bag wherein
the GSSG/GLU solution enclosed in compartment A
further contains at least one species selected from
calcium ion..and magnesium ion, preferably containing
both ions.
(4) The packaged ocular irrigating solution bag wherein
the solution enclosed in compartment A is at pH 2.5-
6.5, preferably at pH 3.0-6Ø
(5) The packaged ocular irrigating solution bag wherein
the GSSG/GLU solid preparation-enclosed compartment
A further contains at least one species selected
from calcium salts and magnesium salts, preferably
containing both salts.
(6) The packaged ocular irrigating solution bag wherein
the solution enclosed in compartment B further
contains at least one species selected from calcium
CA 02484240 1998-10-23
-10-
ion and magnesium ion, preferably both ions, and
contains citrate ion.
(7) The packaged ocular irrigating solution bag wherein
the solution enclosed in compartment B is at pH 7.0-
9.0, preferably at gH 7.0-8.5.
(8) The packaged ocular irrigating solution bag wherein
the multiple compartment bag further comprises
compartment C wherein a solution containing at least
one species selected from calcium ion and magnesium
ion, preferably both ions, (hereinafter referred to
as °Ca/Mg solution") is enclosed.
(9) The packaged ocular irrigating solution bag wherein
the solution enclosed in.compartment C is at pH 3.5-
5.5, preferably at pH 4.0-5Ø
(IO) The packaged ocular irrigating solution bag wherein
the multiple compartment bag further comprises
compartment G wherein a solid preparation containing
at least one species selected fxom calcium salts and
magnesium salts, preferably both salts, (hereinafter
referred to as "Ca/Mg solid preparation") is
enclosed.
(11) The packaged ocular irrigating solution bag wherein
an ocular irrigating solution is prepared by mixing
the contents of the multiple compartment bag so that
the resulting solution contains the components in
CA 02484240 1998-10-23
-11-
the following permissible range, preferably in the
following optimum range, per 1000 ml:
Component Permissible range,{g) Optimum range (g)
Oxiglutatione 0-0.5 0-0:3
Dextrose 0.4-1.8 0.7-1.65
Sodium bicarbonate 1.5-2.5 1.9-2.3
Calcium chloride
(as an anhydride) 0.09-0.17 0.2-0.15
Magnesium chloride or magnesium sulfate
(as an anhydride) 0.07-0.18 0.08-0.16.
(12) The packaged ocular irrigating solution bag wherein
an ocular irrigating solution is prepared by mixing
the contents of the multiple compartment bag so that
the resulting solution contains the components
within the following permissible range; preferably
within the following optimum range, per 1000 ml:
Component Permissible range (g) Optimum range (g)
Oxiglutatione 0-0.5 0-0.3
Dextrose 0.4-1:8 0.7-1.65
Sodium bicarbonate 1.5-2.5 1.9-2.3
Calcium chloride
{as an anhydride) 0.09-0.17 f.1-0.15
Magnesium chloride or magnesium sulfate
(as an anhydride) 0.07-0.18 0.08-0.16
CA 02484240 1998-10-23
-12-
Sodium citrate
(as an anhydride) 0.4-1.4 0.7-1.1:
(13) The packaged ocular irrigating solution bag wherein
the space between the multiple compartment bag and
the packaging member has a carbon dioxide gas
atmosphere having a carbon dioxide concentration of
0.5-20 v/v~, preferably 1-15 v/v~.
(14) The packaged ocular irrigating solution bag wherein
the bicarbonate ion-containing solution in the pH
indicating device has a concentration of 0.01-2.0
w/v~ and the pH indicator is one selected from the
group consisting of cresol red, m-cresol purple,
._.thymol blue and phenolphthalein and_ha.s a
concentration of 10-2000 ppm.
(15) The packaged ocular irrigating solution bag wherein
the bicarbonate ion in the pH indicating device is
sodium bicarbonate ion.
Based on the utilization of the multiple
compartment bag having at least two compartments A and B,
the packaged bag of the invention achieves long-term
stabilization of an oxiglutatione-, dextrose- and
bicarbonate ion-containing solution.
Based on utilizing a gas-impermeable plastic
packaging member and providing the space with carbon
dioxide gas, for example by disposing a carbon dioxide-
CA 02484240 1998-10-23
-13-
generating oxygen scavenger in the space, the packaged bag
of the invention prevents evaporation of carbon dioxide
evolved from the bicarbonate ion solution into the
atmosphere to thereby securely retain the solution at a
constant pH value.
The packaged bag of the invention may contain an
oxygen sensor in,the space. With this structure, one can
easily detect the leakage of carbon dioxide gas from the
space and invasion of oxygen into the space upon prolonged
storage or due to formation of a pinhole in the packaging
member, the resulting pH change and deterioration in the
quality of the ocular irrigating solution.
. The packaged bag of the invention may contain a ._
specific pH indicating device in the space, in place of
the above oxygen sensor. With this structure also, the pH
change and deterioration of the ocular irrigating solution
upon prolonged storage or due to formation of a pinhole in
the packaging member can be easily detected by the naked
eye.
Based on the utilization of the specific pH
indicating device wherein a bicarbonate ion-containing
solution is used as an internal solution, the pH of this
internal solution alsochanges in proportion to the change
in pH of the bicarbonate ion solution in the bag in
response to the carbon dioxide concentration (C02 partial
CA 02484240 1998-10-23
-14-
pressure) within the space. Therefore, by using a pH
indicator capable of sensing a pH change of the internal
solution, the pH change of the bicarbonate ion solution in
the bag can be visualized tnrith,a change in color of the pH
indicator.
Further, based on the utilization of a plastic
bag, the packaged bag of the invention avoids the risk of
- fracture or generation of glass flakes, is reduced in
weight and can be easily fabricated by the conventional
manufacturing technology.
The packaged ocular irrigating solution bag of
the invention is now described below in detail. The
ocular irrigating solution of the invention is prepared
using a GSSG/GLU solution or a GSSG/GLU solid preparation
and a bicarbonate ion solution and optionally a Ca/Mg
solution or a Ca/Mg solid preparation.
The ion concentrations and salt concentrations
indicated in this specification are concentrations of ions
and salts (calculated as anhydrides) in the ocular
2Q irrigating solution prepared by mixing the contents of the
multiple compartment bag, unless otherwise specified.
The GSSG/GLU solution (solution enclosed in
compartment A) essentially comprises oxiglutatione and/or
dextrose, whereas the bicarbonate ion solution (solution
enclosed in compartment B) essentially comprises
CA 02484240 1998-10-23
-15-
bicarbonate ion. Each salution may further contain at
least one species selected from calcium ion and magnesium
ion. Especially when the bicarbonate ion solution further
contains at least one species selected from calcium ion
and magnesium ion, citrate ion is also added for
preventing the precipitation.
It i.s also possible that at least one species
selected from calcium ion and magnesium ion may be formed
into a Ca/Mg solution and enclosed in another compartment
(compartment C) of the multiple compartment, separately
from the GSSG/GLU solution and bicarbonate ion solution.
The groportions of these components may be
suitably..decided. That is to say, the proportions may be
selected from the range that the solution obtained by
mixing these solutions will have the same composition as
this type of conventional ocular irrigating solution or a
slight modification thereof. A typical example is such
that the ocular irrigating solution obtained by mixing
these solutions has the components within the range
mentioned above.
The GSSG/GLU solution, b3.carbonate ion solution
and Ca/Mg solution may further contain phosphate ion or
ion of trace metals such as copper, zinc; etc.
It is preferable for the GSSG/GLU solution to
contain sodium ion, potassium ion, chlorine ion or the
CA 02484240 1998-10-23
-ls-
like by adding sodium chloride, potassium chloride or the
like. Sodium chloride is usually added in an amount of
about 0.5-0.9 w/v%, preferably about 0.6-0.8 w/v%.
Potassium chloride is usually added in an amount of about
0.02-0.05 w/v%, preferably about 0.025-0.045 w/v%. Sodium
ion and potassium ion may also be added to the bicarbonate
ion solution and the Ca/Mg solution.
The compound added to the bicarbonate ion
solution to generate bicarbonate ion may be any of sodium
bicarbonate, ammonium hydrogencarbonate, potassium
hydrogencarbonate and other hyd=ogencarbonate, and these
are used in the aqueous solution form. Also, sodium
carbonate, potassium carbonate or like carbonate aqueous
solutions forming carbonate ion may be used as the
bicarbonate ion solution, because bicarbonate ion is
generated into the solution in a pH range of the obtained
solution. The bicarbonate ion concentrations of these
aqueous solutions are not limited specifically but usually
selected within a range of about 15-50 mM. This
corresponds to a concentration of about 0.1-0.4 w/v% when
sodium bicarbonate aqueous solution is used. Most
preferably, the sodium bicarbonate aqueous solution has a
concentration of about 0.16-0.24 w/v%.
The calcium ion-generating compound and
magnesium ion-generating compound, which can be added to
CA 02484240 1998-10-23
the GSSG/GLU solution and the bicarbonate ion solution or
constitute a CalMg solution, may be any of those
conventionally used in this type of ocular irrigating
solution, for example, chlorides or sulfates of calcium or
magnesium.
The citrate ion added to the bicarbonate ion
solution together with calcium ion and/or magnesium ion
has the action of preventing the reaction between
bicarbonate ion and calcium ion or magnesium ion
coexisting in the obtained solution, thereby preventing
the precipitation of calcium carbonate and magnesium
carbonate. That is to say, citrate ion forms a chelate
with calcium ion or magnesium ion, thereby preventing
calcium ion or magnesium ion from directly combining with
carbonate ion. For example, citrates such as sodium
citrate can be mentioned as compounds having such action.
The amount of citrate ion is usually selected within the
range of about 0.35-2 w/v$, more preferably about 0.5-1.2
w/v~, calculated as citrates.
In view of the stabilization of bicarbonate ion,
the bicarbonate ion solution is preferably adjusted to a
pH range of about 7.0-9.0, preferably about 7.0-8.5 using
a pH adjusting agent such as sodium hydroxide,
hydrochloric acid or the like: The solution may further
contain a buffer such as disodium hydrogenphosphate,
CA 02484240 1998-10-23
-18-
sodium dihydrogenphosphate, dipotassium hydrogenphosphate,
potassium dihydrogenphosphate, sodium acetate, potassium
acetate or the like having buffer action, which prevents
rapid pH change. The buffer is preferably used at a
concentration at which the buffer demonstrates enough
buffer capacity when the solutions in the compartments of
the multiple compartment bag are mixed. For example, the
concentration of disodium hydrogenphosphate is preferably
selected from the range of about 0.03-0.06 w/v~,
preferably from about 0.035-0.05 w/v~. The concentration
of sodium acetate is preferably selected from the range of
about 0.02-0.06 w/v~, preferably from about 0.03-0.05 w/v~.
On the other hand, the GSSG/GLU solution is
preferably adjusted to a pH range of about 2.5-6.5,
preferably about 3.0-6.0 using a pH adjusting agent as
mentioned above in order to stably preserve oxiglutatione
and/or dextrose in the solution. This solution also may
include a buffer such as sodium acetate, potassium acetate
or the like having buffer action.
The Ca/Mg solution is usually adjusted to a pH
range of about 3.5-5.5; preferably about 4.0-5.0, using a
pH adjusting agent as mentioned above in order to securely
prevent the precipitation of calcium and/or magnesium.
The Ca/Mg solution also may contain a buffer such as
sodium acetate, potassium acetate, sodium dihydrogen-
CA 02484240 1998-10-23
-19-
phosphate, potassium dihydrogenphosphate or the like
having buffer action.
According to the present invention., a GSSG/GLU
solid.preparation may be used in place of the GSSG/GLU
solution. It is also possible to use a Ca/Mg solid
preparation in place of the Ca/Mg solution.
The GSSG/GLU solid preparation comprises
essentially at least one species selected from
oxiglutatione and dextrose and may further contain at
least one species selected from calcium salts and
magnesium salts, preferably both salts. The Ca/Mg solid
preparation may be prepared using at least one species
selected from calcium salts and magnesium salts,
preferably both salts: The calcium salts and the
I5 magnesium salts may be any of those conventionally used in
this type of ocular irrigating solution. For example,
chlorides or sulfates of calcium or magnesium can be
mentioned.
The GSSG/GLU solid preparation and the Ca/Mg
solid preparation may further contain salts of trace
metals such as copper, zinc and the like, sodium salts,
potassium salts and phosphates such as sodium chloride,
potassium chloride, disodium hydrogenphosphate, sodium
dihydragenphosphate, dipotassium hydrogenphosphate,
potassium dihydrogenphosphate and the like. The amounts
CA 02484240 1998-10-23
-20-
of these additives may be selected within the range that
the ion concentration of the ocular irrigating solution
obtained by mixing these compounds, etc. will be within
the range mentioned above.
The solid preparations may be prepared in a
powder form by mixing the component compounds in a powder
form or like generally available form. The mixture of the
components may be shaped into fine grains, granules,
tablets according to the conventional methods.
Alternatively, the components may be dissolved in a
suitable solvent such as water and the solution may be
lyophilized to give a lyophilized powder.
In the packaged bag of the invention,. the gas-
permeable plastic multiple compartment bag for enclosing
(accommodating, filling) the components (GSSG/GLU solution,
GSSG/GLU solid preparation, bicarbonate ion solution,
Ca/Mg solution, Ca/Mg solid preparation) of the ocular
irrigating solution may be selected from bags made of
polyethylene, ethylene-vinyl acetate copolymer,
polypropylene, polyvinyl chloride or the like or a mixture
thereof in a suitable proportion or laminated ones. There
is no particular limitation on the shape, size or
thickness of the bag but rectangular forms are generally
used. The capacity of the bag is generally within the
range of about 20 ml to about 3 litters and the thickness
CA 02484240 1998-10-23
-21-
is preferably within the range of about 100-500 ~u m.
The above-mentioned bag may be a gas-permeable
plastic bag comprising at least two intercommunicable
compartments separated from one another by a partition
wall. Bags of this type are already known in the field of
parenteral infusion. For examgle, a bag equipped with a
closure means for preventing intercommunication of two
compartments (e.g. Japanese Examined Patent Publication No.
20550/1988, Japanese Examined Utility Model Publication No.
1?474/i988) and a bag whose compartments can be simply
brought into intercommunication by pressing the
compartments to open the sealing portion therebetween (e. g.
Japanese Unexamined Patent Publication Nos: 309263/1988
and 4671/1990). According to the present invention, the
bicarbonate ion solution may be enclosed in at least one
of the compartments, and the GSSG/GLU solution or the
GSSG/GLU solid preparation may be enclosed in at least one
of the other compartments.
The term "gas-impermeable" as used in describing
the gas-impermeable plastic packaging member for use in
the invention does not mean that the particular material
is strictly impermeable to gases, but is a relative term
meaning that a.t is less permeable to gases than is the
above-mentioned bag for enclosing the ocular irrigating
solution (solutions for preparation thereof). Thus, even
CA 02484240 1998-10-23
-22-
if the packaging member is made of the same material as
that of the above bag, it can be used as the gas-
impermeable packaging member only provided it is
sufficiently thick. The material that can be used for the
gas-impermeable packaging member includes those
conventionally used, for example, polyethylene
terephthalate (PET), polyethylene naphthalate (PEN),
polyvinyl alcohol (PVA), ethylene-vinyl alcohol copolymer
(EVOH), polyvinylidene chloride (PVDC), and nylon, such
plastic materials carrying a vapor-deposition layer of
inorganic material such as silicon oxide, aluminum oxide,
etc. on the surface, and multiple-layer films (laminate
films) made up of such materials. There is no particular -~
limitation on the shape and size of such packaging members
only provided that the gas-permeable plastic bag can be
suitably accommodated therein. However, it is necessary
that, in shape and size; such a packaging member should
provide a sufficient space for accepting- a carbon dioxide
gas atmosphere after packaging and generally speaking, it
is preferably so large as to provide a volume equal to
about 1.2-3 times the capacity of the gas-permeable
plastic bag.
. According to the invention, it is important to
establish a carbon dioxide gas atmosphere in the space
between the bag and the packaging member.
CA 02484240 1998-10-23
-23-
Referring to the technology for establishing a
carbon dioxide gas atmosphere in the space between the bag
and the packaging member, a typical process comprises
transferring a mixed gas containing carbon dioxide gas,
such as _ a mixture of C02 gas and air or a mixture of C02
gas and nitrogen gas, into said space. The carbon dioxide
concentration of the mixed gas used i.n this process is
selected according to the kind of ocular irrigating
solution to be contained in the plastic bag, particularly
its bicarbonate ion concentration and pH. For example,
when said solution is an aqueous solution prepared by
dissolving 2.1 g of sodium bicarbonate in sterile purified
water to make~a total of 1 litter, the bicarbonate ion w
concentration-of this aqueous solution is 25 mM and the pH
of the solution is 8.2. To maintain these values, the
carbon dioxide concentration of the mixed gas atmosphere
is preferably set to about 0.5-20%.
The bicarbonate ion concentration and pH of the
bicarbonate ion solution for use in the present invention
24 are generally about 15-50 mM and about 7.0-9.0,
respectively. Preferably the carbon dioxide:partial
pressure in said space is generally controlled at about 1
mmHg - 250 mmHg and it is preferable to select the
percentage of carbon dioxide gas in said mixed gas accord-
ingly. More particularly, when the pH of the bicarbonate
CA 02484240 1998-10-23
-z~-
ion solution immediately after preparation i.s within the
predetermined range, the carbon dioxide gas to be enclosed
in the space can be such that its partial pressure will be
substantially equal to the carbon dioxide gas partial
pressure of the solution.
An alternative method for establishing a carbon
dioxide gas atmosphere in the space defined by said bag
and packaging member comprises enclosing a carbon dioxide-
generating oxygen scavenger capable of absorbing the
oxygen gas in the space and releasing a predetermined
proportion relative to the amount of the absorbed oxygen,
by volume, of carbon dioxide. As carbon dioxide-
generating oxygen scavengers, there can be mentioned
"Ageless G" and "Ageless GM", both manufactured by
Mitsubishi Gas Chemical Co., and "Keep Fresh Type C"
manufactured by Toppan Printing Co., Ltd. These products
can be-enclosed in the space as they are.
The procedures for filling the bag with the
ocular irrigating solution, sterilization, packaging with
the packaging member, etc. can all be easily carried out
in accordance with usual,production methods for infections.
According to a preferred embodiment of the
packaged ocular irrigating solution bag of the invention,
the space between the multiple compartment bag and the
packaging member has a carbon dioxide gas atmosphere and
CA 02484240 1998-10-23
-25-
an oxygen sensor is disposed in the space. With the
oxygen sensor disposed therein, one can easily detect the
leakage of carbon dioxide gas from the space and invasion
of.oxygen into the space upon prolonged storage or due to
formation of a pinhole in the packaging member, and
particularly detect the resulting pH change. and
deterioration in quality of the pharmaceutical preparation,
thus securing the safety of the ocular irrigating solution.
It is preferable that the oxygen sensor used
reacts only with oxygen gas and changes color, etc. to
indicate the presence of oxygen, and does not react with
other gas. A typical example of oxygen sensor
commercially available is~"Ageless Eye CS° manufactured by
Mitsubishi Gas Chemical Co., Ltd.
The oxygen sensor can detect the presence of
even a comparatively small amount of oxygen. Therefore,
when it is used in the present invention, it is preferable
to lower the oxygen concentration in the space as much asw
possible. For example, the oxygen concentration is
preferably not higher han about 0.5 v/v%. The means for
reducing the concentration can be selected, for example,
from a method comprising substituting the air in the space
with a mixed gas of carbon dioxide gas and inert gas; and
a method further comprising placing and enclosing a
suitable oxygen scavenger such as the above-mentioned
CA 02484240 1998-10-23
-26-
carbon dioxide-generating oxygen scavenger in the sgace.
According to another preferred embodiment of
the.invention, the space between the multiple compartment
bag and the packaging member has a carbon dioxide gas
atmosphere and is provided with a pH indicating device
comprising a gas-permeable plastic packet enclosing a
bicarbonate-containing solution and a pH-indicator
designed to~undergo a change in color in response to a pH
change of the solution.
Here, only if the bicarbonate ion is contained,
there is no particular limitation on the concentration and
composition of the internal solution of the pH indicating
device but its bicarbonate~ion concentration is preferably
selected usually from the range of 0.01-2.0 w/v~.
The pH indicator to be incorporated in the above
internal solution of the pH indicating device can be
selected from a variety of acid-base indicators which are
capable of indicating a pH change of the device internal
solution as a color change. Since carbon dioxide gas
20~ exists in the space, the pH of the indicating device
internal solution is equal to the pH of the bicarbonate
ion solution enclosed in the bag. Preferred is an
indicator which undergoes a change in color with
particularly high sensitiYity within the critical pH range
of the bicarbonate ion solution. Generally, the critical
CA 02484240 1998-10-23
-2?-
pH of the bicarbonate ion solution is within the neutral
to slightly alkaline range as mentioned above (for example,
the specification upper limit for a '7~ aqueous solution of
sodium bicarbonate is pH 8.6 according to JP XIII and the
corresponding carbon dioxide gas fraction is about 3.9~).
The pH of the indicating device internal,solution which is
proportional to the pH of the bicarbonate ion solution is
also within the neutral to slightly alkaline range (e. g.
the pH of a 0.28 aqueous solution of sodium bicarbonate
is ?.0). Therefore, the above-mentioned pH indicator is
preferably one which undergoes a change in color within
the slightly alkaline range.
The particularly preferred pH indicator is~one
selected from substances having the following
characteristics, viz. (1) a narrow color change interval,
(2) a high intensity of color, (3) a favorable direction
of color change (from an inconspicuous color to a
conspicuous color), (4) high hygieni:city (the substance
should be highly safe and not migratory), (5) high
stability, with the initial color change property being
sustained for'an extended time.
As substances having such characteristics, there
can be mentioned neutral red, aurin, phenol red; o-cresol
red, cx-naphtholphthalein, m-cresol purple, orange I,
thymol blue, phenolphthalein, etc. Among them, more
CA 02484240 1998-10-23
-28-
preferable are phenol red (change from yellow to red at pH
6.8-8.4), o-cresol red (change from yellow to red at pH
7.2-8.8), m-cresol purple (change from yellow to purple at
pH 7.6-9.2), thymol blue (change from yellow to blue at pH
8.0-9.6) and phenolphthalein (change from colorless to red
at pH 8.3-10).
The concentration of the pH indicator should
only be such that its color change can be easily
recognized by the naked eye and is preferably selected,
for example, from the range of about 10-2000 ppm according
to the size of the packet (thickness of the fluid layer)
in which the pH ind~:ca~or is enclosed together with the
w internal solution.
The packet containing said internal solution and
pH indicator can be manufactured by the routine
manufacturing technology, and the raw material for this
gas-permeable plastic packet may be at least equivalent to
the bag described hereinbefore in gas permeability. For
example, said packet can be fabricated in a continuous
20, series of forming, filling, and sealing by means of a
vertical 3-side sealer, a vertical pillow packaging
machine, or a rotary packer. When this manufacturing
method is employed, the raw material for the packet is
preferably a laminated film in consideration of machine
processability. Particularly when the bag is made of
CA 02484240 1998-10-23
-29-
polyethylene, the packet is preferably made of a poly-
propylene (outer layer) - polyethylene (inner layer)
laminated film or a poly-4-methyl-1-pentene (outer layer)
- polyethylene (inner layer) laminated film.
Regarding the size of the packet and the volume
of the internal solution, it should be noted that if the
quantity of the internal solution enclosed in the packet
is too small, the thickness of the indicating device
solution layer will be insufficient to make a visual
IO assessment of the color change difficult. Therefore, the
packet size and internal solution volume should be
selected in consideration of the geomefiric relation of the
bag and the packaging member as well as the ease of
recognition of the color change by the naked eye.
The pH indicating device thus prepared tends to
develop turbidity owing to growth of bacteria in the
internal solution upon prolonged storage. To prevent or
control this clouding problem, it can be sterilized by
autoclaving. As an alternative, an antiseptic such as
benzalkonium chloride, chlorhexidine gluconate or the like,
an antibacterial agent such as nalidixia acid, norfloxacin
or the like, or a preservative such as p-hydroxybenzoic
esters, benzyl alcohol or the like may be added.
Disposition (enclosing) of the packet in said
space can be carried out simply by packaging the bag and
CA 02484240 1998-10-23
-30=
the packet together in the packaging member. The
disposing position is not critical inasmuch as the packet
may be visually recognized from outside the package. In
this manner, there can be provided an improved packaged
bag permitting a visual assessment of the pH.change of the
ocular irrigating solution in accordance with the present
invention.
The preferred embodiment of the packaged ocular
irrigating solution bag of the invention is described with
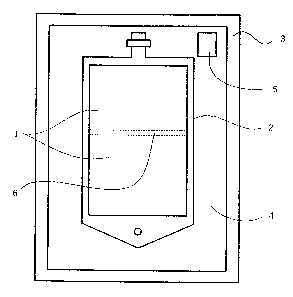
reference to the accompanying drawing (Fig. 1}. The
packaged bag of the invention comprises a gas-permeable
plastic multiple compartment bag 2 separately holding a
bicarbonate ion solution and a GSSG/GLU solut~iori (drug
solutions, 1 and 1~ as divided by a weakly sealed portion
6, a gas-impermeable plastic packaging member 3 for
packaging the bag, and a pH indicating device 5 as
disposed in a space 4 between the bag and the packaging
member, with a carbon dioxide gas atmosphere having been
established within the space. Based on the above
structure, a visual assessment of the pH change of the
bicarbonate ion solution in the bag, which i.s an object of
the invention, is made feasible with the various
advantageous effects mentioned hereinbefore.
Fig. 1 is a schematic view illustrating a
CA 02484240 1998-10-23
-31-
packaged ocular irrigating solution bag according to one
embodiment of the invention.
In Fig. 1, the reference numeral I stands for an
ocular irrigating solution, 2 for a gas-permeable plastic
multiple compartment bag, 3 for a gas-impermeable plastic
packaging member, 4~for a.space between said bag 2 and
packaging member 3, 5 for a gas-permeable plastic packet
(pH indicating device), and 6 for a weakly sealed portion
of the gas-permeable plastic multiple compartment bag.
1~7~S.T~~7E FOR ~A1138~I1~tG 2U~~~~E ~.~ENTIn~1
Production examples of pH indicating devices,
preparation examples of packaged ocular irrigating
~solution~bags and a test example of the obtained packaged
bags are given below to describe the present invention in
more detail.
Production Example 1
In a 0.28 aqueous solution of sodium
bicarbonate was dissolved 10 mg of phenol red to make 500
ml (20 w!v ppm). Using a vertical 3-side sealer, 0.5 ml
of the above solution was packaged with a polypropylene
(outer layer; 20 E,cm thick)-polyethylene (inner layer, 30
,um thick) laminated film to provide a pH indicating device,
mm by 25 mm (inside dimensions). This indicating
device, freshly prepared, was red-purple (color already
25 developed).
CA 02484240 1998-10-23
-32-
Production Example 2
In a 0.28% aqueous solution of. sodium
bicarbonate was dissolved 10 mg of cresol red to make'500
ml (20 w/v ppm). A 0:5 ml portion of this solution was
packaged with a polyethylene film (manufactured by Mitsui
Petrochemical; 250 ~cm thick) to provide a pH indicating
device, 40 mm by 20 mm (inside dimensions). Freshly
prepared, this indicating device was purple (color already
developed).
Production Example 3
In a 0.28 aqueous solution of sodium
bicarbonate was dissolved IO mg of m-cresol purple to make
500 ml (20 w/v ppm):w Using a vertical 3-side sealer, 0.5
ml of the above solution was packaged with a polypropylene
(outer layer, 20 ~Cm thick)-polyethylene (inner layer, 30
,um thick) laminated film to provide a pH indicating device,
30 mm by 15 mm (inside dimensions). Freshly prepared.,
this indicating device was purple (color already
developed).
Production Example 4
In a 0.28% aqueous solution of sodium
bicarbonate was dissolved 1 g of m-cresol purple to make
50 1 (20 w/v ppm). Using Bottlepack 305 (manufactured by
Ro~elag), forming of a low-density polyethylene packet,
filling of a portion of the above solution, and sealing
CA 02484240 1998-10-23
-33-
were continuously carried out to provide a pH indicating
device, about 20 nun b~ about 10 mm and about 0.4 mm in
wall thickness (fluid volume: about 0.4 ml).
Production Example 5
In a 0.28 aqueous solution of sodium
bicarbonate was dissolved 1 g of m-cresol purple to make
50 1 (20 w/v ppm). Using a vertical 3-side sealer, 1 ml
of the solution was packaged with an oriented
polypropylene (outer layer, 20 ,um thick)-linear 1ow-
density polyethylene (inner layer, 60 ~Cm thick) laminated
film to provide a pH indicating device having an external
size of 40 mm by 20 mm and an internal size of 30 mm by 12
mm. Until use; thisW ndicating device was stored as
packed together with a mixed gas of 10~ COZ - 90~ air in a
bag made of nylon (15 ~m thick)-polyvinyl alcohol (18 ~Cm
thick)-low-density polyethylene (60 ~m thick) laminated
film.
Production Examp3e 6
Using a poly-4-methyl-1-pentene (outer layer, 30
E.cm thick)-polyethylene (inner layer, 60 fcm thick)
laminated film as a packaging member, the procedure of
Production Example 5 was otherwise repeated to provide a
pH indicating device. Because of the high heat resistance
of poly-4-methyl-1-pentene , this product showed improved
high-speed sealability for increased productivity. Until
CA 02484240 1998-10-23
-34-
use, this pH indicating device was stored together with a
mixed gas of 10~ C02 - 90% air in a bag made of nylon (15
um thick)-polyvinyl alcohol (18 ,um thick)-low density
polyethylene (60 ~cm thick) laminated film.
Production Example 7
In a 0.28 aqueous solution of sodium
bicarbonate was dissolved 1 g of thymol blue to make 50 1
(20 wJv ppm}. Using a vertical 3-side sealer, a 1 ml
portion of the solution was packaged with an oriented
polypropylene (outer layer, 30 ,um thick)-linear low-
density polyethylene (inner layer, 60 ~.cm thick} laminated
film to provide a pH indicating device having an external
size of 40 mm by 20 mm and an internal size of 30 mm by 12
mm. Until use, this indicating device was stored as
packed together with a mixed gas of 10~ C02 - 90$ air in a
bag made of nylon (15 E.cm thick)-polyvinyl alcohol (18 ,um
thick)-low-density polyethylene (60 ,um thick) laminated
film.
Example 1
A polyethylene bag (wall thickness: about 260
~.m) comprising two intercommunicable compartments
(compartments A and B: Examples 1-10) or three
intercommunicable compartments (compartments A, B and C:
Examples 11 and 12} separated from one another by a
partition wall was prepared. Solutions, or solutions)
CA 02484240 1998-10-23
-35-
and a solid preparation shown below were separately placed
in the compartments and the bag was sealed, followed by
sterilization by the hot-water shower method. The bag was
enclosed together with the pH indicating device prepared
in Production Example 7 and a mixed gas of 10~ C02 - 90~
air in a laminated film bag (secondary packaging member)
made of nylon ( 15 ,um thick) -polyvinyl alcohol ( 18 E,cm
thick)-polyethylene (60 ~cm thick) laminated film {space
volume 400 ml) to provide a packaged ocular irrigating
solution bag.
The packaged bag of Example 1
(Compartment A) A solution having the following
components
Oxiglutatione 0.09 g
Dextrose 0.46 g
Sodium chloride 3.32 g
Potassium chloride 0.19 g
Hydrochloric acid q.s:
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 150 ml
pH 4.5
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.05 g
CA 02484240 1998-10-23
-36-
Sodium acetate trihydrate 0.30 g
Sodium citrate dehydrate 0.50 g
Calcium chloride dehydrate 0.08 g
Magnesium chloride hexahydrate
0.10 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 350 ml
pH 7.8
The packaged bag of Example 2
(Compartment A) A solution having the following
components
Oxiglutatione 0.18 g --
Dextrose 1.5 g
I Sodium chloride 6.7 g
Potassium chloride 0.36 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 500 ml
pH 3.5
(Compartment B) A solution having the following
components
Sodium bicarbonate 2.1 g
Sodium acetate trihydrate 0.5 g
CA 02484240 1998-10-23
-37-
Sodium citrate dehydrate 1.5 g
Calcium chloride dehydrate 0.2 g
Magnesium chloride heptahydrate
0.15 g
Hydrochlorec acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 500 ml
pg ?.8
The packaged bag of Example 3
(Compartment Aj A solution having the following
components
Dextrose . 0.75 g
Sodium chloride 3.32~g ~~
Potassium chloride 0.18 g
Hydrochlorec acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity whech makes a
total of 150 ml
pH 5.0
(Compartment Bj A solution having the following
components
Sodium bicarbonate 1.05 g
Sodium acetate trehydrate 0.3 g
Sodium citrate dehydrate 0.5 g
Calcium chloride dehydrate 0.09 g
CA 02484240 1998-10-23
-38_
Magnesium chloride heptahydrate
0.15 g
Hydrochloric acid q.s.
Sodium hydroxide q'.s.
Sterile purified water the quantity which makes a
total of 350 ml
PH 7.2
The packaged bag of Example 4
(Compartment A) A solution having the following
components
Oxiglutat,ione 0.09 g
Dextrose 0.46 g
Calcium chloride dehydrate 0.08 g
- .Magnesium chloride hexahydr ate 0.1 g w
Hydrochloric acid q.s.
i5 Sodium hydroxide q.s:
Sterile purified water the quantity which makes a
total of 150 ml
pH 4:5
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.05 g
Sodium chloride 3.57 g
Potassium chloride 0.19 g
Disodium hydrogenphosphate dodecahydrate 0.54 g
Hydrochloric acid q.s_
CA 02484240 1998-10-23
-39-
Sodium hydroxide ~ q.s.
Sterile purified water the quantity which makes a
total of 350 ml
pH 7.4
The gackaged bag of Example 5
(Compartment A) A solution having the following
components
Dextrose 0.75 g
Calcium chloride dihydrate 0.09 g
Magnesium sulfate heptahydr ate 0.15 g
Hydrochloric acid q.s. .
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 150 ml
pH 5.0
(Compartment B) A solution having the following
components
Sodium hydrogencarbonate 1.05 g
Sodium chloride 3.55 g
Potassium chloride 0.18 g
Disodium hydrogenphospl~ate dodecahydrate 0.54 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 350 ml
CA 02484240 1998-10-23
-40-
pH 7.5
The packaged bag of Example 6
(Compartment A) A solution having the following
components
Oxiglutatione 0.2 g
Dextrose 1.0 g
Calcium chloride dihydrate 0.16 g
Magnesium chloride hexahydrate 0.2 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 500 ml
pH -- 4.5
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.9 g
Sodium chloride 7.5 g
Potassium chloride 0.3 g
Disodium hydrogenphosphate dodecahydrate 1.0 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 500 ml
PH ?.7
CA 02484240 1998-10-23
-41-
The packaged bag of Example 7
(Compartment A) A solution having the following
components
Dextrose 0.35 g
Calcium chloride dihydrate 0.05 g
Magnesium sulfate heptahydrate 0.09 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 125 ml
pH 4.5
(Compartment B) .A solution having the following
components
Sodium bicarbonate 0.47 g
Sodium chloride 1.95 g
Potassium chloride 0.08 g
Disodium hydrogenphosphate dodecahydrate 0.29 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 125 ml
pH 7.5
The packaged bag of Example 8
(Compartment A) A solution having the following
components
CA 02484240 1998-10-23
-42-
Oxiglutatione 0.09 g
Dextrose 0.46 g
Sodium chloride 3.57 g
Potassium chloride 0.19 g
Calcium chloride dehydrate 0.08 g
Magnesium.chloride hexahydr ate O.l g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 150 ml
pH 4.5
(Compartment B) A solution having the following
components w
Sodium bicarbonate 1.05 g
Disodium hydrogenphosphate dodecahydrate 0.54 g
Hydrochloric acid q.s.
Sodium hydroxide g.s.
Sterile purified water the quantity which makes a
total of 350 ml
pH 7.4
The packaged bag of Example 9
(Compartment A) A solution having the following
components
Dextrose 0.75 g
Potassium chloride 0.18 g
CA 02484240 1998-10-23
-43-
Calcium chloride dihydrate 0_09 g
Magnesium sulfate heptahydrate 0.15 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 100 ml
pH 4.6
(Compartment B} A solution having the following
components
Sodium bicarbonate 1.05 g
Sodium chloride 3.55 g
Disodium hydrogenphosphate dodecahydrate 0.54 g
Hydrochloric acid w q:s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 400 ml
pH 7.5
The packaged bag of Example 10
(Compartment A} A solid obtained by dissolving the
components shown below in 20 m1 of sterile purified water,
followed by filtration through a membrane filter having a
pore size of 0.22 ~ m and lyophilization (the solid being
antiseptically enclosed).
Oxiglutatione 0.09 g
Dextrose 0.46 g
CA 02484240 1998-10-23
-44-
Calcium chloride dihydrate 0.08 g
Magnesium sulfate hexahydra te 0.10 g
Sodium chloride 3.57 g
Potassium chloride 0.19 g
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.05 g
Disodium hydrogenphosphate dodecahydrate 0.52 g
Hydrochloric acid q.s.
Sterile purified water the quantity which makes a
total of 500 ml
pH 7.4
- The packaged bag of Example 11 --
(Compartment A) A solution having the following
components
Oxiglutatione 0.09 g
Dextrose 0.46 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 100 ml
pH 4.5
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.05 g
CA 02484240 1998-10-23
-45-
Disodium hydrogenphosphate dodecahydrate 0.52 g
Potassium chloride 0.19 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes a
total of 400 ml
pH 7.6
(Compartment C) A solid obtained by subjecting the
components shown below to hot air sterilization (the solid
being antiseptically enclosed).
Calcium chloride dihydrate 0.08 g
Magnesium sulfate hexahydrate 0.10 g
Sodium chloride 3:~7 g
The packaged bag of Example 12
(Compartment A) A solid obtained by dissolving the
components shown below in 20 ml of sterile purified water;
followed by filtration through a membrane filter having a
pore size of 0.22 um and Iyophilization (the solid being
antiseptically enclosed).
Oxiglutatione 0.09 g
Dextrose 0.46 g
(Compartment B) A solution having the following
components
Sodium bicarbonate 1.05 g
Disodium hydrogenphosphate dodecahydrate 0.52 g
CA 02484240 1998-10-23
-46-
Sodium chloride 3.57 g
Potassium chloride 0.19 g
Hydrochloric acid q.s.
Sodium hydroxide q.s.
Sterile purified water the quantity which makes
a
total of 450 ml
pH 7.4
(Compartment C) A solution having
the following
components
Calcium chloride dehydrate 0.08 g
Magnesium sulfate hexahydrate 0.10 g
Hydrochloric acid q.s.
-Sodium hydroxide q.s.~
Sterile purified water the quantity which makes
a
total of 50 ml
pH 4.6
Experiment Example 1
Using an injection needle (27G,
Terumo, Neolus),
a pinhole (about 500 ,um in major
diameter and about 50 E.cm
in minor diameter) was made in the packaging members of
the packaged ocular irrigating solution bags of the
invention prepared in Examples 1 and 4.: The color changes
of the pH indicating devices
were monitored.
Three days later, both of the
pH indicating
devices turned green.
CA 02484240 1998-10-23
-47-
According to the gresent invention, there can be
provided a packaged ocular irrigating solution bag wherein
an ocular irrigating solution is stably stored in a
plastic multiple compartment bag, thus avoiding the
fracture of the container or generation of the glass
flakes .