Note: Descriptions are shown in the official language in which they were submitted.
PF-0329 PCT
TITLE
CA 02484583 2000-02-24
Glucose Monitor Calibration Methods
FIELD OF THE INVENTION
This invention relates to glucose monitor systems and, in particular
embodiments, to calibration methods for glucose monitoring systems.
BACKGROUND OF THE INVENTION
Over the years, body characteristics have been determined by obtaining a
so sample of bodily fluid. For example, diabetics often test for blood glucose
levels.
Traditional blood glucose determinations have utilized a painful finger prick
using a lancet to withdraw a small blood sample. This. results in discomfort
from
the lancet as it contacts nerves in the subcutaneous tissue. The pain of
lancing
and the cumulative discomfort from multiple needle pricks is a strong reason
why
~5 patients fail to comply with a medical testing regimen used to determine a
change
in a body characteristic over a period of time. Although non-invasive systems
have been proposed, or are in development, none to date have been
commercialized that are effective and provide accurate; results. In addition,
all of
these systems are designed to provide data at discrete points and do not
provide
a o continuous data to show the variations in the characteristic between
testing times.
A variety of implantable electrochemical sensors have been developed for
detecting and/or quantifying specific agents or compositions in a patient's
blood.
For instance, glucose sensors are being developed for use in obtaining an
indication of blood glucose levels in a diabetic patient. Such readings are
useful
25 in monitoring andlor adjusting a treatment regimen which typically includes
the
regular administration of insulin to the patient. Thus, flood glucose readings
improve medical therapies with semi-automated medication infusion pumps of
the external type, as generally described in U.S. Patent Nos. 4,562,751;
4,678,408; and 4,685,903; or automated implantable rr~edication infusion
pumps,
3o as generally described in U.S. Patent No. 4,573,994. Typical thin film
sensors are
described in commonly assigned U.S. Patent Nos. 5,390,671; 5,391,250;
-1-
CA 02484583 2000-02-24
PF-0329 PCT
5,482,473; and 5,586,553. See also U.S. Patent No. 5,299,571.
SUMMARY OF THE DISCLOSURE
It is an object of an embodiment of the present invention to provide an
s improved glucose monitor system and method, which obviates for practical
purposes, the above mentioned limitations.
According to an embodiment of the invention, a method of calibrating
glucose monitor data includes obtaining glucose monitor data at predetermined
intervals over a period of time. It also includes obtaining at least two
reference
so glucose values from a reference source that correspond with the glucose
monitor
data obtained at the predetermined intervals. Additionally, calculating
calibration
characteristics using the at least two reference values and the corresponding
glucose monitor data to regress the obtained glucose monitor data is included.
And calibrating the obtained glucose monitor data using the calibration
15 characteristics is included. In preferred embodiments, the reference source
is a
blood glucose meter, and the at least two reference glucose values are
obtained
from blood tests. In additional embodiments, the calculation of the
calibration
characteristics is obtained using linear regression, and in particular
embodiments,
using least squares linear regression. Alternatively, the calculation of the
2 o calibration characteristics is obtained using non-linear regression or a
non-
regression technique.
In particular embodiments, the predetermined period of time is a 24 hour
period, and the predetermined intervals are 5 minute intervals. Further
embodiments may include the step of shifting the data by a predetermined time
as factor, such as for example, ten minutes. Preferably, the calibration is
performed
while obtaining glucose monitor data. However, alternative embodiments may
perform the calibration on glucose monitor data that has been collected for
post
processing by another processing device.
According to an embodiment of the invention, a method of calibrating
3 o glucose monitor data includes obtaining glucose monitor data at a
predetermined
memory storage rate. Also included is obtaining at least one blood glucose
-2-
w, .. ._ro . a~. _~-a . _ :n ~;..~~:~ :~:,:a= _ = -. - ,~.r~.
CA 02484583 2000-02-24
PF-0329 PCT
reference reading from a blood glucose measuring device that corresponds with
at
least one glucose monitor data point obtained at the predetermined memory
storage rate. Calculating a calibration factor using the at least one blood
glucose
reference reading and the corresponding at least one glucose monitor data
point is
included. And calibrating the obtained glucose monitor data using the
calibration
factor is included. Tn preferred embodiments, after a first calibration factor
is
calculated, at least one previous calibration factor is used with at least one
blood
glucose reference reading from a blood glucose measuring device and its at
least
one corresponding glucose monitor data point to calculate a calibration
factor. In
1 o additional embodiments, at least two blood glucose reference readings are
used
for calibration. In further embodiments, the calculation of the calibration
factor is
obtained using linear regression, and in particular least squares linear
regression.
Alternatively, calculation of the calibration factor uses non-linear
regression or a
non-regression technique
In particular embodiments, the calibration factor is applied to glucose
monitor data obtained before a last blood glucose reference reading from a
blood
glucose measuring device that corresponds with at least one glucose monitor
data
point obtained at a predetermined memory storage rate is used to calculate the
calibration factor. Alternatively, the calibration factor is applied to
glucose
2 o monitor data obtained after the last blood glucose reference reading from
a blood
glucose measuring device that is used to calculate the calibration factor.
In particular embodiments, the predetermined memory storage rate is once
every 5 minutes. And the glucose monitor data that is obtained at a
predetermined memory storage rate is the result of utilizing at least 2 sample
2 5 values sampled from a glucose sensor at a rate faster than the memory
storage
rate.
In preferred embodiments, at least one blood ,glucose reference reading
from a blood glucose measuring device is obtained during a predetermined
calibration period, and a calibration factor is calculated using those
readings after
3 o every predetermined calibration period. In particular embodiments, the
predetermined calibration period is 24 hours. In further preferred
embodiments, a
-3-
CA 02484583 2000-02-24
PF-0329 PCT
predetermined time shift is used to temporally correlate the at least one
blood
glucose reference reading from a blood glucose measuring device with the at
least
one glucose monitor data point obtained at the predetermined memory storage
rate. In particular embodiments; the predetermined time shift is ten minutes.
In particular embodiments, one or more calculations for calculating a first
calibration factor is different from the one or more calculations for
calculating
subsequent calibration factors. In other particular embodiments, the
calculation
for calculating a first calibration factor uses a single-point calibration
equation.
In further particular embodiments, the single-point calibration equation
includes
io an offset value. In other particular embodiments, the one or more
calculations for
calculating a calibration factor other than the first calibration factor uses
a linear
regression calibration equation, a non-linear regression calibration equation,
or a
non-regression technique.
According to an embodiment of the invention, a method of calibrating
15 glucose monitor data includes obtaining glucose monitor data. It also
includes
obtaining from another blood glucose measuring device at least one blood
glucose reference reading that is temporally associated with at least one
glucose
monitor data reading. Determining a calibration equation using the at least
one
blood glucose reference reading and the corresponding at least one glucose
2 o monitor data reading is also included. And calibrating the glucose monitor
data
using the calibration equation is included.
According to another embodiment of the invention, a method of
calibrating body characteristic monitor data includes obtaining body
characteristic
monitor data. It also includes obtaining from another characteristic measuring
25 device at least one characteristic reference reading that is temporally
associated
with at least one characteristic monitor data point. Calculating calibration
characteristics using the at least one characteristic reference reading and
the
corresponding at least one characteristic monitor data point is included. And
calibrating the obtained characteristic monitor data using the calibration
s o characteristics is included. In particular embodiments, at least two body
characteristic reference readings are used for calculating the calibration
-4-
CA 02484583 2000-02-24
PF-0329 PCT
characteristics. In particular embodiments, the calculation for calculating
the
calibration characteristics is a linear regression calculation.
According to additional embodiments of the invention, an apparatus for
calibrating glucose monitor data includes a glucose monitor, glucose sensor, a
blood glucose meter and a processor. 'The glucose monitor includes a glucose
monitor memory for storing glucose monitor data. The glucose sensor is
electronically coupled to the glucose monitor to supply the glucose monitor
data.
The blood glucose measuring device provides at least one blood glucose
reference
reading that is temporally associated with at least one. glucose monitor data
point.
to And the processor includes software to calculate calibration
characteristics using
the at.least one blood glucose reference reading that is temporally associated
with
at least one glucose monitor data point, and the processor applies the
calibration
characteristics to the glucose monitor data. In particular embodiments, the at
least one blood glucose reading is entered into the glucose monitor. Tn
particular
embodiments, the glucose monitor includes the processor, or alternatively, the
processor is in a separate device that receives glucose monitor data from the
glucose monitor.
In other embodiments of the invention, an apparatus for calibrating
glucose monitor data includes means for obtaining glucose monitor data. It
also
2 0 includes means for obtaining from another blood glucose measuring device
at
least one blood glucose reference reading that is temporally associated with
at
least one glucose monitor data reading. Means for calculating a calibration
equation using the at least one blood glucose reference reading and the
corresponding at least one glucose monitor data reading is included. And means
for calibrating the glucose monitor data using the calibration equation is
also
included.
Other features and advantages of the invention will become apparent from
the following detailed description, taken in conjunction with the accompanying
drawings which illustrate, by way of example, various features of embodiments
of
3 o the invention.
-5-
. .bre . _M., ~ ., .~ . _ .~ ~~~.~~~~.~~~~. ;~ ~~~:~,~~.. ~_~~~~yW~~~".
~.,.9,~, a ~.~~.~~~ 1_.~a.~, .m . ~.m.~". ,~,.~,~.~~.... .~ mo._m~
CA 02484583 2000-02-24
PF-0329 PCT
BRIEF DESCRIPTION OF THE DRAWTNGS
A detailed description of embodiments of the invention will be made with
reference to the accompanying drawings, wherein like numerals designate
corresponding parts in the several figures.
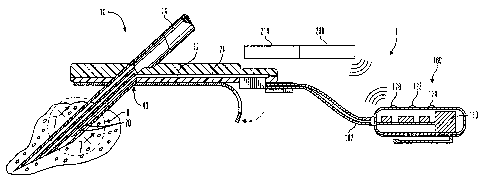
Fig. 1 is a is a perspective view illustrating a subcutaneous glucose sensor
insertion set and glucose monitor device in accordance with an embodiment of
the present invention;
Fig. 2 is a cross-sectional view of the sensor set and glucose monitor
device a~ shown along the line 2-2 of Fig. l;
i o Fig. 3 is a cross-sectional view of a slotted insertion needle used in the
insertion set of Figs. l and 2;
Fig. 4 is a cross-sectional view as shown along line 4-4 of Fig. 3;
Fig. 5 is a cross-sectional view as shown along line 5-5 of Fig. 3;
Fig. 6 is a partial cross-sectional view corresponding generally with the
zs encircled region 6 of Fig. 2;
Fig. 7 is a cross- sectional view as shown along line 7-7 of Fig. 2.
Figs.8(a-c) are diagrams showing a relationship between sampled values,
interval values and memory storage values.
Fig. 9 is a chart showing clipping limits.
2 o Fig. 10 is a sample computer screen image of a post processor analysis of
glucose monitor data.
Fig. 11 is a chart illustrating the pairing of a blood glucose reference
reading with glucose monitor data.
Fig. 12 is a chart illustrating an example of a single-point calibration.
25 Fig. 13 is a block diagram of a single-point calibration technique.
Fig. 14 is a chart illustrating an example of a linear regression calibration.
Fig. 15 is a block diagram of a linear regression calibration technique.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
3 o As shown in the drawings for purposes of illustration, the invention is
embodied in calibration methods for a glucose monitor that is coupled to a
sensor
-6-
CA 02484583 2000-02-24
PF-0329 PCT
set to provide continuous data recording of readings of glucose levels from a
sensor for a period of time. In preferred embodiment s of the present
invention,
the sensor and monitor are a glucose sensor and a glucose monitor for
determining glucose levels in the blood and/or bodily fluids of a user.
However,
it will be recognized that further embodiments of the invention may be used to
determine the levels of other body characteristics including analytes or
agents,
compounds or compositions, such as hormones, cholesterol, medications
concentrations, viral loads (e.g., HIV), bacterial levels, or the like. The
glucose
sensor is primarily adapted for use in subcutaneous human tissue. However, in
so still further embodiments, one or more sensors may be placed in other
tissue
types, such as muscle, lymph, organ tissue, veins, arteries or the like, and
used in
animal tissue to measure body characteristics . Embodiments may record
readings from the sensor on an intermittent, periodic, on-demand, continuous,
or
analog basis.
Figs. 1-7 illustrate a glucose monitor system 1 for use with the calibration
methods. The glucose monitor system 1, in accordance with a preferred
embodiments of the present invention, includes a subcutaneous glucose sensor
set
10 and a glucose monitor 100.
Preferably, the glucose monitor 100 is worn by the user and is connected
2 o to a surface mounted glucose sensor set 10 that is attached to a user's
body by an
electrically conductive cable 102. In preferred embodiments, the sensor
interface
may be configured in the form of a jack to accept different types of cables
that
provide adaptability of the glucose monitor 100 to work with different types
of
subcutaneous glucose sensors and/or glucose sensors placed in different
locations
z 5 of the user's body. However, in alternative embodiments, the sensor
interface is
permanently connected to the cable 102. In additional alternative embodiments,
a
characteristic monitor is connected to one or more sensor sets to record data
of
one or more body characteristics from one or more locations on or in the
user's
body.
3 o The glucose sensor I2, of the type described in commonly assigned U.S.
Patent Nos. 5,390,671; 5,391,250; 5,482,473; and 5,S86,SS3; extends from the
n ,'w -'..~1...~.wv~,.. 7~d.... ,per-,~emk7Nk~~'~S3.~FB7 . a'Fh'F3E;
CT~~,.x.._.g~.fih~~uAARS..we~~:A"~t.wq."...~.mmmmaae-.-
,~....,..,...~..,..,...,.~,.,.,.~...ee,
CA 02484583 2000-02-24
PF-0329 PCT
glucose sensor set 10 into the user's body with electrodes 20 of the glucose
sensor
12 terminating in the user's subcutaneous tissue. See also U.S. Patent No.
5,299,571. However, in alternative embodiments, the glucose sensor 12 may use
other types of sensors, such as chemical based, optical based, or the like. In
further alternative embodiments, the sensors may be of a type that is used on
the
external surface of the skin or placed below the skin layer of the user for
detecting
body characteristics.
The glucose monitor 100 generally includes the capability to record and
store data as it is received from the glucose sensor 12, and includes either a
data
i o port (not shown) or wireless transmitter and/or receiver (also not shown)
for
transferring data to andlor from a data processor 200 such as a computer,
communicatian station, a dedicated processor designed specifically to work
with
the glucose monitor, or the like.
Preferably, the glucose monitor system 1 minimizes inconvenience by
is separating complicated monitoring process electronics into two separate
devices;
the glucose monitor 100, which attaches to the glucose sensor set 10; and the
data
processor 200, which contains the software and programming instructions to
download and evaluate data recorded by the glucose monitor 100. Tn addition,
the
use of multiple components (e.g., glucose monitor 100 and data processor 200)
a o facilitates upgrades or replacements, since one module, or the other, can
be
modified, re-programmed, or replaced without requiring complete replacement of
the monitor system 1. Further, the use of multiple components can improve the
economics of manufacturing, since some components may require replacement on
a more frequent basis, sizing requirements may be different for each module,
25 different assembly environment requirements, and modifications can be made
without affecting the other components.
The glucose monitor 100 takes raw glucose sensor data from the glucose
sensor 12 and assesses it during real-time andlor stores it for later
processing or
downloading to the data processor 200, which in turn analyzes, displays, and
logs
3 o the received data. The data processor 200 utilizes the recorded data from
the
glucose monitor 100 to analyze and review the blood glucose history. In
_g_
. ,oM ., ....,a ay .;yfiyn e;y.".,:.vum., . xm~. :~a,~m, . nrC~ ~.~.rt, anR
nqvpa>.. et~pMme ..a.rr.,aom ,.aaw~:n.~, ,
o- a.~..~-a!,~vz'r.~;.~;za~raF~~fap» :vsaamr..wca. .v~". r
q.:.,s,,~,wy~~!:wsa.,v~zaxeas..~au~"sesame-~raw.~~-wm.:a:..
arr.",;~,,m.~,.,.n.
CA 02484583 2000-02-24
PF-0329 PCT
particular embodiments, the glucose monitor 100 is placed into a com-station
which facilitates downloading data to a personal computer for presentation to
a
physician. A software is used to download the data, create a data file,
calibrate
the data, and display the data in various formats including charts, forms,
reports,
graphs, tables, lists, and the like. In further embodiments, the glucose
monitor
system I may be used in a hospital environment or the like.
In alternative embodiments, the glucose monitor includes at least portions
of the software described as contained within the data processor 200 above.
The
glucose monitor might contain the necessary software to calibrate glucose
sensor
to signals, display a real-time blood glucose value, show blood glucose
trends,
activate alarms and the like. A glucose monitor with these added capabilities
is
useful for patients that might benefit from real-time observations of their
blood
glucose characteristics even while they're not in close proximity to a
computer,
communication device or dedicated independent data processor.
As shown in Fig. 2, the data processor 200, may include a display 214 that
is used to display the calculated results of the raw glucose sensor data
received
via a download from the glucose monitor 100. The results and information
displayed includes, but is not limited to, trending information of the
characteristic
(e.g., rate of change of glucose), graphs of historical data, average
characteristic
levels (e.g., glucose), stabilization and calibration information, raw data,
tables
(showing raw data correlated with the date, time, sample number, corresponding
blood glucose level, alarm messages, and mare), and the like. Alternative
embodiments include the ability to scroll through the data. The display 214
may
also be used with buttons (not shown) on the data processor 200, computer,
a s communication station, characteristic monitor, or the like, to program or
update
data. In preferred embodiments, the glucose monitor 100 includes a display 132
to assist the user in programming the glucose monii;or I00, entering data,
stabilizing, calibrating, downloading data, or the like.
Still further embodiments of the present invention may include one or
3 o more buttons I 22, 124, I 26 and 128 on the glucose monitor I 00 to
program the
monitor 100, to record data, insert flags to correlate data with external
events for
_9_
...., , ..",.., , m. .,nrxa ryo. ..r~:.,.Tn,~x ,~er~sta ,-,us s,m ,*a.~..,
aa,mad,a.h.>,a~bx~q~;t~n,daT. a.~:xaas;~:.;...r~." ~ e.,cc,.o=a~..-,"..,n:--
r,.<*~:.:,~.aa mmr~J.. *:-,. use..«um~,....a m ,
'~' w ,mica: ~~~a~rya~:~m.~, ~.,.",~....~ ..a. ".<.,."",...",
CA 02484583 2000-02-24
PF-0329 PCT
later analysis, input calibration values, or the like. In addition, the
glucose
monitor 100 may include an on/off button 130 for compliance with safety
standards and regulations to temporarily suspend transmissions or recording.
The
glucose monitor 100 may also be combined with other medical devices to accept
other patient data through a common data network and/or telemetry system. The
glucose monitor 100 may be combined with a blood glucose meter to directly
import or correlate glucose calibration reference values. The glucose monitor
100
may also be combined with semi-automated medication infusion pumps of the
external type, as generally described in U.S. Patent Nos. 4,562,751;
4,678,408;
zo and 4,685,903; or automated implantable medication infusion pumps, as
generally
described in U.S. Patent No. 4,573,994. The glucose monitor 100 may record
data from the infusion pumps and/or may process data from both the glucose
sensor 12 and an infusion pump to establish a closed loop system to control
the
infusion pump based on glucose sensor measurements. In other embodiments,
i5 other body characteristics are monitored, and the monitor may be used to
provide
feedback in a closed loop system to control a drug delivery rate. In further
alternative embodiments, the glucose monitor 100 can be combined with a
glucose sensor set 10 as a single unit. .
Glucose sensors are replaced periodically to avoid infection, decaying
2 o enzyme coating and therefore sensor sensitivity, deoxidization of the
electrodes,
and the like. The user will disconnect the glucose sensor set 10 from the
cable
102 and glucose monitor 100. A needle I4 is used t:o install another glucose
sensor set 10 and then the needle 14 is removed. Fi.irther description of the
needle
14 and the sensor set 10 are found in U.S. Patent No. 5,586,SS3, entitled
25 "Transcutaneous Sensor Insertion Set"; and U.S. Patent No. 5,951,521,
entitled
"A Subcutaneous Implantable Sensor Set Having The Capability To Remove Or
Deliver Fluids To An Insertion Site."
The user connects the connection portion 24 of the glucose sensor set 10
through the cable 102 to the glucose monitor 100, so that the glucose sensor
12
3 o can then be used over a prolonged period of time. An initial reading may
be
downloaded from the glucose sensor set 10 and the glucose monitor 100 to the
-I0-
._ .. . r -..p. <.. «a_ ~ -~,g.sa.~0. ~..-~xs~-~.., ~ ~ns~e~cc;~acs .-, ~
~~~,me~vnraW-~ne~:~..~u~es ~ . , v~ maraz~u.sxa~°~~ i.wa...~.~.~a~4m..
~. . , n . ..
~~-~~z.a~ee~~4a-a,~~,~~o~o-~..mm," s»,w.. ..
CA 02484583 2000-02-24
PF-0329 PCT
data processor 200, to verify proper operation of the glucose sensor 10 and
the
glucose monitor 100. In preferred embodiments, the glucose sensor set 10
provides data to the glucose monitor 100 for one to seven days before
replacement. Glucose sensors 12 may last in the user''s body for longer or
shorter
periods of time depending on the quality of the installation, cleanliness, the
durability of the enzyme coating, deoxidization of the sensor, user's comfort,
and
the like.
After installation into the body, the glucose sensor 12 is initialized to
achieve a steady state of operation before starting a calibration process.
Zo Preferably, power supplied by three series silver oxide 357 battery cells I
10 in the
glucose monitor 100 is used to speed the initialization of the glucose sensor
12.
Alternatively, other power supplies may be used such as, different battery
chemistries including lithium, alkaline, or the like, and different numbers of
batteries, solar cells, a DC converter plugged into an AC socket (provided
with
Z5 proper electrical isolation), or the like.
The use of an initialization process can reduce the time for glucose sensor
12 stabilization from several hours to an hour or less. The preferred
initialization
procedure uses a two step process. First, a high voltage (preferably between I
.0-
1.1 volts - although other voltages may be used) is applied between electrodes
20
a o of the sensor 12 for one to two minutes (although different time periods
may be
used) to allow the sensor 12 to stabilize. Then, a lower voltage (preferably
between 0.5-0.6 volts - although other voltages may be used) is applied for
the
remainder of the initialization process {typically 58 minutes or less). Other
stabilization/initialization procedures using differing currents, currents and
z 5 voltages, different numbers of steps, or the like, may be used. Other
embodiments may omit the initializationlstabilization process, if not required
by
the body characteristic sensor or if timing is not a factor. Alternatively,
the
characteristic monitor or the data processor 200 may apply an algorithm to the
sensor data to determine when initial transients are sufficiently diminished
and
3 o the sensor is at a significantly stable state to begin calibration.
In preferred embodiments, data is not considered valid until a sensor
-I I-
CA 02484583 2000-02-24
PF-0329 PCT
initialization event flag (ESI) is set in the data indicating that
stabilization is
complete. Preferably, stabilization is complete after 60 minutes or when a
user
enters a sensor initialization flag using one or more buttons on the glucose
monitor I00. After stabilization/initialization is complete the glucose
monitor
100 is calibrated to accurately interpret readings from the newly installed
glucose
sensor 12.
Beginning with the stabilization process, the glucose monitor 100
measures a continuous electrical current signal (ISIG} generated by the
glucose
sensor 12 relative to a concentration of glucose present in the subcutaneous
tissue
io of the user's body. In preferred embodiments, the glucose monitor 100
samples
the ISIG from the glucose sensor I2 at a sampling rate of once every I O
seconds,
as shown in Fig.Ba-c. Examples of sampled values are labeled A - AD in Fig.
8a.
At an interval rate of once per minute, the highest and lowest of the sampled
values (shown in Fig. 8a as circled sampled values A, E, G, I, M, R, V, W, Y,
and
i5 AB) are ignored, and the remaining 4 sampled values from an interval are
averaged to create interval values (shown in Fig. 8b as values F', L', R', X',
and
AD'). At a glucose monitor memory storage rate of once every S minutes, the
highest and lowest of the interval values (shown in Fig. 8b as values L' and
X')
are ignored and the remaining 3 interval values are averaged and stored in a
2 o glucose monitor memory as memory values (shown i:n Fig. 8c as point AD").
The memory values are retained in memory and may be downloaded to the data
processor 200. The memory values are used to calibrate the glucose monitor 100
and/or the post processor 200 and to analyze blood glucose levels. The
sampling
rate, interval rate and the memory storage rate may be varied as necessary to
25 capture data with sufficient resolution to observe transients or other
changes in
the data depending on the rate at which sensor values can change, which is
affected by the sensor sensitivity, the body characteristic being measured,
the
physical status of the user, and the like. In other embodiments, all of the
sampled
values are included in the average calculations of memory storage values. In
3 o alternative embodiments, more or less sampled values or interval values
are
ignored depending on the signal noise, sensor stability, or ether causes of
-I Z-
,E-.,~. ~ . , ,.. , _ . rn .... , "zm . ~... .xa~. ~..,"~y, ...-~~.az~a,~e
.,u~,..~;-,ns;. ,.ms~.a;~'rauc..".. ,...:~ . 7 .~,.,:~,..~~
< ~ASh MY~K~~ktawrv.. vYVmfifiTiWi. 95UFevaFaFH&d. n,.ncMCPrTW"R"~.s~7dC
,,eebbAW~uW~a, v .i~4'ceZ.zv~HF' s~v2 ~r.mm .a..wnu . p
CA 02484583 2000-02-24
PF-0329 PCT
undesired transient readings. Finally, in still other em.bodirnents, all
sampled
values and/or interval values are stored in memory.
Clipping limits may be used to limit the signal. magnitude variation from
one value to the next thereby reducing the effects of extraneous data,
outlying
data points, or transients. In preferred embodiments, clipping limits are
applied
to the interval values. For instance, interval values that are above a maximum
clipping limit or below a minimum clipping limit are replaced with the nearest
clipping limit value.
In alternative embodiments, interval values that are outside of the clipping
l o limits are ignored and not used to calculate the next memory storage
value. In
particular embodiments, the detection of interval values outside of the
clipping
limits is considered a calibration cancellation event. In further particular
embodiments, more than one value must be deemed outside of clipping limits to
constitute a calibration cancellation event. (Calibration cancellation events
are
i5 discussed below).
In preferred embodiments, the clipping limits are shifted after each data
point. The level that the clipping limits are set to is dependent on an
acceptable
amount of change from the previous interval value to the present interval
value,
which is affected by the sensor sensitivity, signal noise, signal drift, and
the like.
a o In preferred embodiments, the clipping limits are calculated based on the
magnitude of the previous interval value. For example, for a previous interval
value from 0 up to but not including 15 Nano-Amps, the clipping limits are set
at
plus and minus 0.5 Nano-Amps about the previous interval value. For a previous
interval value from 1 S up to but not including 25 Nano-Amps, the clipping
limits
25 are set at plus and minus 3% of the previous interval value, about the
previous
interval value. For a previous interval value from 25 up to but not including
50
Nano-Amps, the clipping limits are set at plus and rr~inus 2% of the previous
interval value, about the previous interval value. Arid for a previous
interval
value of 50 Nano-Amps and greater, the clipping limits are set at plus and
minus
s o 1 % about the previous interval value. In alternative embodiments,
different
clipping limits may be used.
-13-
.,. .r .,.,r, r,...:...~ ".., ,.,. .r, ,-~o;sy sz":-..,..~ ,...F:,n~,a.-, ». .
. ,~rv
~ r. ~ ~., "sG'., ~,t5et...~.;~pnr."~.a~~aw~.'i4xe ,.~,,tmunmu~'~..; .5..,<..
~,p~,,,tr.ccea ,naw,..,ms.:. .~: rcm. "raw,o-., ".>-~,wro.. .,..,.-.">u __
",...~.zr~cmes~. w,~,~.-,.~..
CA 02484583 2000-02-24
PF-0329 PCT
Fig. 9 shows a typical clipping limit example in which a previous interval
value 500, associated with interval N-1, has a magnitude of 13.0 Nano-Amps,
which is less than 15.0 Nano-Amps. Therefore, the rr~aximum clipping limit 502
for the present interval value 506 is set at 13.5 Nano-Amps, which is 0.5 Nano-
Amps greater than the magnitude of the previous interval value.500. And the
minimum clipping limit 504 is set at 12.5 Nano-Amps which is 0.5 Nano-Amps
below the previous interval value 500. The present interval value 506,
associated
with interval N, is between the maximum clipping linnit 502 and the minimum
clipping limit 504 and is therefore acceptable.
zo In another example shown in Fig. 9, the present interval value 508,
associated with interval M, has a value of 25.0 Nana-Amps which is outside of
the clipping limit 514 and will therefore be clipped. The previous interval
value
510, associated with interval M-1, is 26.0 Nano-Amps, which is included in the
range from 25.0 up to but not including 50.0 Nano-Amps as discussed above.
i5 Therefore the clipping limits are ~ 2%. The maximum clipping limit S 12 is
2%
greater than the previous interval value 510,
26.0 + 26.0 * 0.02 = 26.5 Nano-Amps.
Similarly the minimum clipping limit S 14 is 2% less than the previous
interval
value 510,
2 0 26.0 - 26.0 * 0.02 = 25.5 Nano-Amps.
Since the present interval value 508 of 25.0 Nano-Amps is less than the
minimum
clipping limit 514 of 25,5 Nano-Amps, it will be clipped, and 25.5 Nano-Amps
will be used in place of 25.0 Nano-Amps to calculate a memory storage value.
For further illustration, Fig. 8 shows interval value R', which is calculated
by
2 s averaging sampled values N through Q, is outside of the clipping limits
412 and
414, which result from the previous interval value L'. Therefore, the
magnitude
of interval value R' is not used to calculate memory value AD", instead R",
which is the magnitude of the minimum clipping limit 414, is used.
In other embodiments, the clipping limits may be a smaller or larger
3 o number of Nano-Amps or a smaller or larger percentage of the previous
interval
value based on the sensor characteristics mentioned above. Alternatively, the
-14-
CA 02484583 2000-02-24
PF-0329 PCT
clipping limits are calculated as plus or minus the same percent change from
every previous interval value. Other algorithms use several interval values to
extrapolate the next interval value and set the clipping limits to a
percentage
higher and lower than the next anticipated interval value. In further
alternatives,
clipping may be applied to the sampled values, interval values, memory values,
calculated glucose values, estimated values of a measured characteristic, or
any
combination of the values.
In preferred embodiments, all interval values are compared to an out-of
range limit of 200 Nano-Amps. If three consecutive interval values are equal
to
to or exceed the out-of range limit, the sensor sensitivity is deemed to be
too high
and an alarm is activated to notify the user that re-calibration is required
or the
sensor may need replacing. In alternative embodiments, the out-of range limit
is
set at higher or lower values depending on the range of sensor sensitivities,
the
expected working life of the sensor, the range of acceptable measurements, and
is the like. In particular embodiments, the out-of range limit is applied to
the
sampled values. In other embodiments, the out-of range limit is applied to the
memory storage values.
In preferred embodiments, unstable signal alarm limits are set to detect
when memory storage values change too much from. one to another. The signal
2 o alarm limits are established similarly to the clipping limits described
above for
the interval values, but allow for a larger change in value since there is
more time
between memory storage values than between internal values. Re-calibration or
replacement of the glucose sensor 12 is required once an unstable signal alarm
is
activated. In essence, the glucose monitor 100 has detected too much noise in
the
25 ISIG from the glucose sensor 12.
Each memory storage value is considered valid (Valid ISIG value) unless
one of the following calibration cancellation events occurs: an unstable
signal
alarm (as discussed above), a sensor initialization event (as discussed
above), a
sensor disconnect alarm, a power on/off event, an out-of range alarm (as
3o discussed above), or a calibration error alarm. Only Valid ISIG values are
used to
calculate blood glucose levels by the glucose monitor 100 or post processor
200,
-1 S-
w.. , W ".,~. ", d~~ . a rr.~ ,..~ ,~ .. ,~~~.... . . . , ~ ~ ~, .x
CA 02484583 2000-02-24
PF-0329 PCT
as shown in Fig. 10. Once a calibration cancellation event occurs, the
successive
memory storage values are not valid, and therefore are not used to calculate
blood
glucose, until the glucose monitor 100 or post processor 200 is re-calibrated.
Fig.
shows an explanatory computer screen in which cell P3 indicates a sensor
s disconnect alarm with the abbreviation "SeDi". As shown, blood glucose
values
do not appear in column K, titled "Sensor Value", and Valid TSIG values do not
appear in column J until after the sensor is initialized, as indicated by the
"ESI"
flag in cell N17. One exception however, is the power on/off event. If the
glucose monitor 100 is turned off for a short enough period of time, generally
up
so to 30 minutes, the memory storage values are considered Valid ISIG values
as
soon as the power is turned back on. If the power is off for longer than 30
minutes, the glucose monitor must be re-calibrated before ISIG values are
considered valid. Alternatively, the power may be off 30 minutes up to
indefinitely and once the power is restored, the memory storage values are
Valid
i5 ISIG values. The sensor disconnect alarm is activated when the glucose
monitor
100 does not detect a signal. In preferred embodiments, when 2 or more out of
5
interval values collected within a givemnemory storage rate are less than 1.0
Nano-Amp, the disconnect alarm is triggered. In alternative embodiments, more
or less values need be below a particular amperage to trigger the disconnect
alarm
2 o depending of the acceptable range or sensor readings and the stability of
the
sensor signal. The remaining two calibration cancellation events, the
calibration
error and an alternative embodiment for the out-of range alarm, are discussed
in
conjunction with the calibration process below.
Preferred embodiments are directed to calibration techniques that are used
z5 by either glucose monitors 100 during real-time measurements of one or more
signals from the glucose sensor 12, or post processors 200 during post-
processing
of data that has been previously recorded and downloaded (as shown in Fig.
10).
To calibrate the glucose monitor 100, the calibration factor called a
sensitivity ratio (SR) (blood glucose level/Valid ISIG value) is calculated
for a
a o particular glucose sensor 12. The SR is a calibration factor used to
convert the
Valid ISIG value (Nano-Amps) into a blood glucose level (mg/d.~ or mmol/2). In
-16-
n_. "r ~, . ~ n~ ~....~.;~,w,~..w...~.~ .
CA 02484583 2000-02-24
PF-0329 PCT
alternative embodiments, the units for the SR may vary depending on the type
of
signal available from the sensor (frequency, amplitude, phase shift, delta,
current,
voltage, impedance, capacitance, flux, and the like), the magnitude of the
signals,
the units to express the characteristic being monitored, or the like.
In preferred embodiments, the user obtains a blood glucose reference
reading from a common glucose meter, or another blood glucose measuring
device, and immediately enters the bload glucose reference reading into the
glucose monitor 100. The blood glucose reference reading is assumed to be
accurate and is used as a reference for calibration. The glucose monitor 100,
or a
io post processor 200, must temporally correlate the blood glucose reference
reading
with a Valid ISIG value to establish a paired calibration data point. Since
the
glucose level in the interstitial body fluid tends to lag behind the blood
glucose
level, the glucose monitor 100 or post processor 200 applies a delay time and
then
pairs the blood glucose reference reading with a Valid ISIG value as shown in
15 Fig. 1 I . In preferred embodiments, an empirically derived 10 minute delay
is
used. Since Valid ISIG values are averaged and stored every S minutes, the
glucose monitor 100 correlates the blood glucose reference reading with the
third
Valid ISIG stored in memory after the blood glucose reference reading is
entered
(resulting in an effective delay of I O to 15 minutes). Fig. 11 illustrates an
a o example, in which a blood glucose reference reading 600 of 90 mg/d~ is
entered
into the glucose monitor 100 at 127 minutes. The next Valid ISIG value 602 is
stored at 130 minutes. Given a 10 minute delay; the glucose reference reading
600 is paired with the Valid ISIG value 604 which i s stored at 140 minutes
with a
value of 30 Nano-amps. Note that two numbers are needed to establish one
2 5 paired calibration data point, a blood glucose reference reading and a
Valid ISIG.
Other delay times may be used depending on the user's metabolism, the
response time of the sensor, the delay time required for the glucose meter to
calculate a reading and for the reading to be entered into the glucose monitor
100,
the type of analyte being measured, the tissue that the sensor is placed into,
3 o environmental factors, whether the previous glucose Valid ISIG value (or
the
trend of the Valid ISIG values) was higher or lower than current Valid ISIG
-17-
,:~~, ~,~~fw.~~~~~:,~:.~~~~,-,5:~ r ~,,:~a,~~.:~
..Y~...~,wT.~t:.~:~:~k~,~,..~~.,.a.~ .,~.~ A~,~.
CA 02484583 2000-02-24
PF-0329 PCT
value, or the Like. Once paired calibration data is available, the appropriate
calibration process may be applied dependent on how many paired calibration
data points are available since the last calibration, the total period of time
that the
glucose sensor 12 has been in use, and the number of times the glucose sensor
12
s has been calibrated.
In preferred embodiments, blood glucose reference readings are entered
into the glucose monitor 100 periodically through oul: each day of use.
Preferably
calibration is conducted immediately after the initialization/stabilization of
a
glucose sensor I2 and once a day thereafter. However, calibration may be
io conducted more or less often depending on whether a glucose sensor 12 has
been
replaced, whether a calibration cancellation event has occurred, the stability
of the
glucose sensor 12 sensitivity over time, or the Like.
In preferred embodiments, blood glucose reference readings are collected
several times per day but a new calibration factor is calculated only once per
day.
a.5 Therefore, typically more than one paired calibration data point is
collected
between calibrations. In alternative embodiments, the glucose monitor is
calibrated every time a new paired calibration data point is collected.
Preferred embodiments use a single-point calibration technique (shown in
a block diagram of Fig. 13) to calculate the SR when only one paired
calibration
2 o data point is available, such as immediately after
initialization/stabilization. And
a modified linear regression technique (shown in a block diagram in Fig. 15)
is
used when two or more paired calibration data points are available. Particular
embodiments use a single-point calibration technique whether or not more than
one paired calibration data point is available.
2 s A single-point calibration equation is based on the assumption that the
Valid
ISIG will be 0 when the blood glucose is 0. As shown in Fig. 12, a single
paired
calibration point 700 is used with the point (0,0) to establish a line 702.
The slope of
the line from the origin (0,0) and passing through the single paired
calibration point
700 is the single-point sensitivity ratio (SPSR). The single-point calibration
equation
3 o to calculate the calibration factor SPSR is as followse
_ 18-
-e...~. , _i e.r.._ ~ -wa: u. ,~,s~..i'.~'~%S~-'2E~.'i- TdFW cmbe~,x 3u.YRw~'
F'F ~. ~yhL2a~-'"~'o-Yn: " . _ . v "~.A..v. ,.m .., mmn-,.- ,w
CN.A~~N~7~~=i~pgY°'~~F~-v. , a~anwirtm"e.,wvwno-~cnw~,m.,uyn.~nmk-
a..~wavsrermw-.. ,a~.o-w.~...w.w.s
CA 02484583 2000-02-24
PF-0329 PCT
SPSR = Blood Glucose Reference Readinu
Valid. ISIG
Where SPSR = a Single-Point Sensitivity Ratio.
Therefore, the calibrated blood glucose level is,
Blood Glucose Level = Valid ISIG '~ SPSR.
As an example, using the values of 20.1 Nano-Amps and 102 mg/d.~ from the
paired
1 o calibration data point shown in Fig. 12, the calculation of SPSR is:
SPSR = 102 l 20.1 = 5.07 mg/d2 per Nano-amp.
To continue the example, once the calibration is complete, given a glucose
sensor
15 reading of 15.0 Nano-Amps, the calculated blood glucose level is:
Blood Glucose Level = 15.0 * 5.0'1= 76.1 mg/d~.
Additionally, particular embodiments use an offset value in a calibration
a o equation to compensate for the observation that more sensitive glucose
sensors 12
(i.e, glucose sensors 12 that generate higher ISIG values compared to other
glucose
sensors 12 at the same blood glucose level, which result in lower SR values)
often
have a less linear performance at very high blood ghzcose levels when compared
to
glucose sensors 12 with lower sensitivity (and therefore relatively higher SR
values).
2 5 If the SPSR for a particular glucose sensor 12, as calculated above, is
less than a
sensitivity threshold value, then a modified SPSR (MSPSR) is calculated using
an
offset value included in a modified single-point calibration equation. In
preferred
embodiments, the threshold value is 7. When the initial calculation of the
SPSR
(shown above) is less than 7, an offset value of 3 is used to calculate the
MSPSR. If
3 o the initial calculation of SPSR yields a value of 7 or~ greater, then the
offset value is
0. Thus, the calibration factor (MSPSR) is calculated using the offset value
in the
modified single-point calibration equation, as follows:
-19-
."... .,..,.sa,.x .a-",:~-"xm._"~~.;R.W ~W'n'...~.'..c~':,-
~...yW~w~;~.r.S,~.E..,..~~3hw.tvF..a,~.._.x-.~,e,q-.,a'~fta. .. . ... .
..srmm~~aw...,....w~e.~,~r~~,w...-.~..~.
CA 02484583 2000-02-24
PF-0329 PCT
MSPSR = Blood Glucose Reference Reading
(Valid /SIG-offset)
Therefore, the calibrated blood glucose level is,
Blood Glucose Level = (Valid /SIG-offset)* MSPSR.
Continuing the above example since the SPSR is 5.07, which is less than 7, the
sensitivity ratio is recalculated using the MSPSR equation as:
to
MSPSR = 102 / (20.1 - 3) = 5.96 mg/d2 per Nano-amp.
And given a glucose sensor reading of 15.0 Nano-Amps after calibration the
calculated blood glucose level is:
Blood Glucose Level = (15.0 -3}* 5.96 = 71.5 mg/d~.
In another example, given a blood glucose reference reading of 95 from a
typical blood glucose meter and a Valid ISIG value of 22.1, the resulting SPSR
is
a o 95/22.1 = 4.3. Since SR < 7, the offset = 3. Therefore, the MSPSR is 95 /
[22.1 - 3]
= 5Ø Note that when the SPSR is greater than or equal to 7 the offset value
is 0 and
therefore the MSPSR = SPSR.
In alternative embodiments, the offset value is eliminated from the equatian
for calculating the blood glucose value as follows:
Blood Glucose Level = Valid ISIG* MSPSR.
The threshold value of 7 and the associated offset of 3 have been empirically
selected based an the characteristics observed from testing a particular type
of
s o glucose sensors 12, such as those described in U.S. Patent 5,391,250
entitled
"Method of Fabricating Thin Film Sensors." Other threshold values may be used
in
conjunction with other offset values to optimize the accuracy of the
calculated
-20-
CA 02484583 2000-02-24
PF-0329 PCT
MSPSR for various types of glucose sensors 12 and sensors used to detect other
body
characteristics. In fact, many threshold values may be. used to select between
many
offset values. An example using two different threshold values (4 and 7) to
select
between three different offset values (5, 3 and 0) follows:
If the SPSR < 4, then use an offset value of 5, else
if 4 <= SPSR < 7, then use an offset value of 3, else
if SPSR >= 7 use an offset value of 0.
io In preferred embodiments the MSPSR is compared to a valid sensitivity range
to determine if the newly calculated MSPSR is reasonable. In order to identify
potential system problems, a valid MSPSR range of 1..5 to 1 S is employed.
This
range has been determined based upon valid glucose sensor sensitivity
measurements
made in-vitro. MSPSR values outside this range result in a calibration error
alarm
i5 {CAL ERROR) to notify the user of a potential problem. Other valid
sensitivity
ranges may be applied depending on the types of sensors to be calibrated, the
range
of acceptable sensitivity levels for the various sensor types, the
manufacturing
consistency expected for the sensors, environmental conditions, how long the
sensor
has been in use, or the like.
2 o Preferred embodiments augment the single-point calibration technique
using a modified linear regression technique (shown in a block diagram in Fig.
15) when more than one paired calibration data point is available. As shown in
Fig. 14, the paired calibration data points 800 are linearly regressed by a
least
squares method to calculate the best fit straight line 802 correlated with
paired
2 5 calibration data points 800. The slope of the line resulting from the
linear
regression is the linear regression sensitivity ratio (L,RSR) used as the
calibration
factor to calibrate the glucose monitor 100. The linear regression calibration
equation is as follows:
-21-
... , ", n , ~.- , e.x n -, s r A~,r , Fe »~ ..::. ~ ,rp~-s r, nzt .u ...,.
.z. N, .. z, r .. .,".. -. -.,."."....
r -Ar .'.-h"... W 'd7k ~
A%w.SKSOfin',S~M;57,prv,:YZi,"ryvn.~.YFM,N'"'~K3r'A~,~MnA
>n.:~,~tARKe:~zxypypz'i. w ...~~. xmn r_an.er...
xm~e.~~rv..~...,..~,~..,om.~........nw.vm..-m,..a."...""Q,.
CA 02484583 2000-02-24
PF-0329 PCT
N
~cX~Yo
LRSR = ~ N
2
~CX1 J
Where X; is the ith Valid ISIG value of paired calibration data points,
Y,. is the ith Blood Glucose Reference Reading of paired calibration data
points and,
N is the total number of paired calibration data points used for calibration.
i is the identification number of a particular paired calibration data point.
Therefore, the calibrated blood glucose level is,
~.o Blood Glucose Level = Valid ISIG * LRSR.
Note that this linear regression uses a fixed intercept of zero (in other
words,
when the Valid ISIG is 0 the blood glucose value is 0) and therefore the
linear
regression method estimates only one regression parameter, the slope. In
15 alternative embodiments, other linear regression methods may be used that
estimate additional regression parameters such as an offset value.
Additionally, particular embodiments use an offset value in a modified linear
regression calibration equation. The purpose of the offset value, as described
above
for the single-point calibration, is to compensate for the observation that
more
2 o sensitive glucose sensors 12 often have a less linear performance at very
high blood
glucose levels. If the LRSR for a particular glucose sensor 12, as calculated
in the
linear regression calibration equation above, is less than a sensitivity
threshold value,
then a modified linear regression sensitivity ratio (MLRSR) is calculated
using an
offset value included in a modified linear regression. calibration equation.
In
25 preferred embodiments, the threshold value is 7. When the initial
calculation of the
LRSR is less than 7, an offset value of 3 is used to calculate the MLRSR. If
the
initial calculation of LRSR yields a value of 7 or greater, then the offset
value is 0.
Thus, the MLRSR is calculated using the offset value in the modified linear
-22-
~..~ . w.s - .~~ ..~o.~ . .~.. n:n,~.: ,. ~r.:~, ~ .~u.~,, t~. ~~v~.b. ~.~~~F.
,~F~ ~~..~.._ ~.r.m. ~... _.~,~~~~.~m~,~,~~ .~ _.~ ~~M ,..ff.~. _ ~a~~.,",~
~~. ~.
W Mvi'e~u~,R . ~ s.r.K .w~g"lp~~~,~~~" ~
w0.9x~ .
CA 02484583 2000-02-24
PF-0329 PCT
regression calibration equation, thus:
[~XI - offset)Y~
MLRSR = '-N
x. - ~ set)
~ ( a ~f
;_
Therefore, the calibrated blood glucose level is,
Blood Glucose Level = (Valid ISIG-offset)* MLRSR.
Just as in the case of single-point calibration 'techniques described above,
to other threshold values may be used in conjunction with other offset values
in the
modified linear regression calibration equation to optimize the accuracy of
the
calculated MLRSR for various types of glucose sensors 12 and other
characteristic sensors.
In preferred embodiments the MLRSR is compared to a valid sensitivity
15 range to determine if the newly calculated MLRSR is reasonable. In order to
identify potential system problems, a valid MLRSR range of 2.0 to 10.0 is
employed. MLRSR values outside this range result in a calibration error alarm
(CAL ERROR) to notify the user of a potential problem. As described above for
the single-point calibration techniques, other valid sensitivity ranges may be
a o applied.
In preferred embodiments, the glucose monitor data is linearly regressed
over a 24 hour period (or window), and new sensitivity ratios are used for
each 24
hour time period. In alternative embodiments, the time period may be reduced
to
only a few hours or enlarged to cover the entire monitoring period with the
2 s glucose sensor (i.e., several days - or even weeks with implanted
sensors). In
further embodiments, the time window may be fixed at a predetermined size,
such
as 24 hours, 12 hours, 6 hours, or the like, and the window is moved along
over
the operational life of the sensor.
-23-
CA 02484583 2000-02-24
PF-0329 PCT
In particular embodiments, paired calibration data points from
measurements taken before the last calibration may be used to calculate a new
sensitivity ratio. For example, to calibrate the glucose monitor every 6
hours, a
paired calibration data point is established every 6 hours. And the linear
s regression technique described above is executed using 4 paired calibration
data
points, the most recently acquired point and points from 6, 12 and 18 hours
before. Alternatively, the number of paired calibration data points used in
the
calibration may be as few as one or as large as the total number of paired
calibration data points collected since the glucose sensor was installed. In
Zo alternative embodiments, the number of paired calibration data points used
in a
calibration equation may grow or shrink during the life of the glucose sensor
due
to glucose sensor anomalies.
In still other embodiments, the decay characteristics of the glucose sensor
12 over time may be factored into the equation to account for typical
degradation
i5 characteristics of the glucose sensor 12 due to site characteristics,
enzyme
depletion, body movement, or the like. Considering these additional parameters
in the calibration equation will more accurately tailor the calibration
equation
used by the glucose monitor 100 or post processor 200. In particular
embodiments, other parameters may be measured along with the blood glucose
ao such as, temperature, pH, salinity, and the like. And these other
parameters are
used to calibrate the glucose sensor using non-linear techniques.
In alternative embodiments, the calibration factor may be calculated by
first using a single-point technique to calculate the MSPSR for each paired
calibration data point and then averaging them together, either unweighted or
2 s weighted by temporal order of by elapsed time. In other alternative
embodiments,
other straight line curve fitting techniques may be used to calculate a slope
to be
used as the SR. In additional alternative embodiments, other non-regressive
curve fitting techniques may be used to generate equations that express the
blood
glucose level relative to the Valid ISIG. The equations may be polynomial,
3 o parabolic, hyperbolic, asymptotic, logarithmic, exponential, or the like.
In these
embodiments, the SR is not a single value (such as .a slops) but rather an
equation
-24-
CA 02484583 2000-02-24
PF-0329 PCT
representing a curve that is used to convert the Valid ISIG from the glucose
sensor 12 to a blood glucose value in the glucose monitor 100 or a post
processor
200. This method is especially effective when using sensors that produce
signals
that are not linearly proportional to the characteristic being measured across
the
entire range of measurement.
As discussed, preferred embodiments utilize a least squares linear
regression equation to calibrate the glucose monitor 100 or post-processor 200
to
analyze the sensor data. However, alternative embodiments may utilize a
multiple component linear regression, or equations with more variables than
just
so the paired calibration data points, to account for additional calibration
effecting
parameters, such as environment, the individual user's characteristics, sensor
lifetime, manufacturing characteristics (such as lot characteristics),
deoxidization,
enzyme concentration fluctuation or degradation, power supply variations, or
the
like. Still other alternative embodiments may utilize singular and multiple,
non-
1 s linear regression techniques.
In preferred embodiments, after the first calibration is performed on a
particular glucose sensor 12, subsequent calibrations employ a weighted
average
using a sensitivity ratio (SPSR, MSPSR, LRSR, or MLRSR) calculated from data
collected since the last calibration, and previous sensitivity ratios
calculated for
a o previous calibrations. So the initial sensitivity ratio (SRl) is
calculated
immediately after initializationlstabilization using a paired calibration data
point
and is used by the glucose monitor 100 or the post processor 200 until the
second
sensitivity ratio (SR2) is calculated. The second sensitivity ratio (SR2) is
an
average of SRl and the sensitivity ratio as calculated using the paired
calibration
25 data points since the initial calibration (SRdayl). The equation is as
follows:
SRZ = SR1 + SRda 1
2
The third sensitivity ratio (SR3) is an average of SR,2 and the sensitivity
ratio as
3 o calculated using the paired calibration data points since the second
calibration
(SRday2). The equation is as follows:
-25-
CA 02484583 2000-02-24
PF-0329 PCT
SR3 = (SR2 + SRda~2)
2
The sensitivity ratios for successive days use the same format, which is
expressed
below in generic terms:
(SRtn_t) + SRday~n_,~
SRn =
2
Where SRn is the new sensitivity ratio calculated at the beginning of time
period,
n, using data from time period (n-1), to be used by a real time glucose
monitor 100, to convert Valid ISIGs to blood glucose readings throughout
to time period, n.
SR~n_,~ is the previous sensitivity ratio calculated at the beginning of time
period, n-1, using data from time period (n-2).
1 s SRdayt"_,~ is the sensitivity ratio calculated using paired calibration
data
points collected since the last calibration.
Alternatively, the previous sensitivity ratios may be ignored and the SR is
calculated using only the paired calibration data points since the last
calibration.
2 o Another alternative is to equally average all previous SRs with the latest
SR
calculated using only the paired calibration data points since the Iast
calibration.
In alternative embodiments, the paired calibration data points are used to
establish
an equation for a curve representing SR over time. The curve is then used to
extrapolate SR to be used until the next paired calibration data point is
entered.
2 5 In embodiments that use a post processor 200 to evaluate the sensitivity
ratio, the sensitivity ratio is calculated using paired calibration data
points over a
period of time since the last calibration and is not averaged with previous
SRs.
The sensitivity ratio for a period of time can then be applied to the same
period of
time over which the paired calibration data points vvere collected. This is
more
3 o accurate than the real-time case described above for the glucose monitor
100
because, in the real-time case, sensitivity ratios from a previous time period
must
be used to calculate the blood glucose level in the present time period. If
the
sensitivity ratio has changed over time, the calculation of blood glucose
using an
old sensitivity ratio introduces an error.
-26-
_ , , _ . ~ . . ~,. ~ ~ w ~..~ra , W ~~, ~ a . ~:~, w~~, . ~;:..: .H. r
'.~p-n3Sk...,9P.~.E'Mae~MF1' eS~G.
.nnu.~rc~#,u~.3,F~'ire'r~S",..øh;Y:.~.W.W;ahRalt2R~'..P#P:"~CdF.an#y~Yl7F.,N,f~
fi.~ sA.'m.xufr..r~aC.~cmnm .wn..xpw.~.:mw._..wp,~r...w."~,~
CA 02484583 2000-02-24
PF-0329 PCT
In particular embodiments, once calibration is complete, Valid ISIG
values are converted to blood glucose readings based on a particular version
of
the sensitivity ratio, and the resulting blood glucose readings are compared
to an
out-of range limit. If the resulting calculated blood glucose level is greater
than a
maximum out-of range limit of 200 mg/d.~ (or equivalently 3600 mmol/2), the
out-of range alarm is activated. This is a calibration cancellation event,
therefore,
ISIG values are no longer valid once this alarm is activated. The blood
glucose
readings are either not calculated, or at least not considered reliable, until
the
glucose monitor 100 or post processor 200 is re-calibrated. The user is
notified of
~. o the alarm and that re-calibration is needed. In alternative embodiments,
higher
or lower maximum out-of range limits may be used depending on the sensor
characteristics, the characteristic being measured, the user's body
characteristics,
and the Iike. In particular embodiments, a minimum out-of range limit may be
used or both a maximum and a minimum out-of range limits may be used. In
1 s other particular embodiments, the out-of range limits do not cause the
blood
glucose readings to become invalid and/or re-calibration is not required;
however,
an alarm could still be provided. In additional particular embodiments, more
than
one ISIG value must exceed an out-of range limit before an alarm is activated
of a
calibration cancellation event is triggered. The ISIG values that are out-of
range
2 o are not used to display a blood glucose value.
In alternative embodiments, calibration is conducted by injecting a fluid
containing a known value of glucose into the site around the glucose sensor
set
10, and then one or more glucose sensor readings are sent to the glucose
monitor
100. The readings are processed (filtered, smoothed, clipped, averaged, and
the
25 like) and used along with the known glucose value to calculate the SR for
the
glucose sensor 12. Particular alternative embodiments, use a glucose sensor
set
of the type described in U.S. Patent 5,951,521 entitled "A Subcutaneous
Implantable Sensor Set Having the Capability To Remove Or Deliver Fluids To
An Insertion Site".
3 o In other alternative embodiments, the glucose sensor 12 is supplied with a
vessel containing a solution with a known glucose concentration to be used as
a
-27-
_ _ ~ ~, .~~.~ .a.~.~~;::. -. -.:.~ . - .~_ ....r , . _. ._._. .~ _. ._..~.~ .
~s..~y a frLT'.-~a~~:~ .r. W9:..:~ae~s,.._ sC=.~r.~~,~~~ .e." "wn~e.~waw~2,.~
~~.--_.n.,.. .a,~ ..,-m-...,."w.~.w~.,
CA 02484583 2000-02-24
PF-0329 PCT
reference, and the glucose sensor 12 is immersed into the reference glucose
solution during calibration. The glucose sensor 12 may be shipped in the
reference glucose solution. As described above, the glucose sensor readings
are
used to calculate a sensitivity ratio given the known glucose concentration of
the
solution.
In another alternative embodiment, the glucose sensors 12 are calibrated
during the manufacturing process. Sensors from the same manufacturing lot,
that
have similar properties, are calibrated using a sampling of glucose sensors 12
from the population and a solution with a known glucose concentration. The
Zo sensitivity ratio is provided with the glucose sensor 1.2 and is entered
into the
glucose monitor 100 or the post processor 200 by the user or another
individual.
While the description above refers to particular embodiments of the
present invention, it will be understood that many modifications may be made
without departing from the spirit thereof. The accompanying claims are
intended
i5 to cover such modi~eations as would fall within the true scope and spirit
of the
present invention.
The presently disclosed embodiments are therefore to be considered in all
respects as illustrative and not restrictive, the scope of the invention being
indicated by the appended claims, rather than the foregoing description, and
alI
2 o changes which come within the meaning and range of equivalency of the
claims
are therefore intended to be embraced therein.
_28_
,. rvn~~-,rte ~ .. ,,~;~~ .,~. « ~.ry,.,:,~,-~._~~..~ ~.:~~~~,.~~x4..:,~,
f~~,.-~~-~;~ ~..a ,~ ~~_.~:~~.. ~ <~x m.~.~.-,..~~ m _ ..~.. ~,..~ ~ ..~