Note: Descriptions are shown in the official language in which they were submitted.
CA 02484994 2010-12-07
Peptide antagonists of TGF-beta family members and therapeutic
uses thereof
GOVERNMENTAL SUPPORT
[002] This work was supported by the U.S. Department of Health and Human
Services/ National
Institutes of Health grant number CA38808. *The U.S. Government has certain
rights in this
invention.
SEQUENCE LISTING
[003] A paper copy of the sequence listing and a computer readable form of the
same sequence
listing are appended below. The information recorded in
computer readable form is identical to the written sequence listing, according
to 37 C.F.R. 1.821 (f).
BACKGROUND OF THE INVENTION
1. Field of the invention
[004] The invention relates generally to antagonists of TGF¨f3 activity,
particularly to peptide
antagonists of TGF-13 activity. The invention also relates to methods of
accelerating wound healing
and preventing scarring by administering peptide antagonists of TGF-13
activity to vertebrates.
2. Description of related art
[005] Transforming growth factor I (TGF--(3) is a family of 25-kDa
structurally homologous
dimeric proteins containing one interchain disulfide bond and four intrachain
disulfide bonds. The
CA 02484994 2010-12-07
TGF¨I3 family is composed of three known members (TGF¨I31, TGF¨I32, and
TGF¨I33) in mammalian
species. TGF-13 is a bifunctional growth regulator: it is a growth inhibitor
for epithelial cells,
endothelial cells, T-cells, and other cell types and a mitogen for mesenchymal
cells. TGF¨I3 also has
other biological activities, including stimulation of collagen, fibronectin,
and plasminogen activator
inhibitor -1 (PAI-1) synthesis, stimulation of angiogenesis, and induction of
differentiation in several
cell lineages.
[006] TGF¨P has been implicated in the pathogenesis of various diseases such
as cancer, macular
degeneration, intimal hyperplasia following angioplasty, tissue fibrosis
(which includes integument
scar tissue formation, liver cirrhosis, kidney fibrosis, lung fibrosis, heart
fibrosis and others) and
glomerulonephritis. It is known in the art that TGF-I3 plays an important role
in scarring of the skin or
organ fibrosis, which occurs as a result of injury or other fibrogenic
stimulus. TGF¨P's role in wound
healing and scarring revolves around its activity as an important regulator of
the extracellular matrix
stimulating fibroplasia and collagen deposition and inhibiting extracellular
matrix degradation by
up-regulating the syntheses of protease inhibitors (see Roberts, 1995; Roberts
and Sporn, 1996;
and O'Kane and Ferguson ,1997). Neutralizing antibodies to TGF¨I3 have been
used experimentally
to reduce scarring of wounds, to prevent lung injury in adult respiratory
distress syndrome (ARDS),
and to block restenosis following angioplasty in animal models. These
promising results warrant the
development of TGF¨P antagonists (inhibitor) that might be useful in
inhibiting, ameliorating or
reversing the effects of TGF¨P and treating diseases. However, practical
applications have been
limited by the large molecular size of the antibodies with resulting
instability and poor tissue
penetration (O'Kane and Ferguson, ibid;, Shah et al., 1994; Shah et al,,
1995).
[007] TGF¨p peptide antagonists that block TGF¨p binding to cell surface
receptors and inhibit
TGF-P-induced growth and transcriptional activation are described in U.S.
Patent
No. 6,500,920 and Huang et al., J. Biol. Chem. 272:27155-27160 (1997). The
effective
concentrations (EC50) of these peptide antagonists, with amino acid sequences
corresponding to the
41st to 65th of TGF-01 and TGF¨I32, range from ¨ 60 nM to 1 M, depending on
the targeted TGF¨p
isoform. In contrast to TGF¨I3 neutralizing antibodies, the peptide
antagonists are relatively stable,
exert rapid inhibitory actions, and can be applied topically. These properties
suggest that they are
useful for treating hypertrophic scarring in cutaneous wounds.
- 2 -
CA 02484994 2010-12-07
3. Related art citations
[008] Throughout the instant specification, numerical citations in parentheses
are used to cite specific references; those references appear below. No
admission to the status of these references as prior art are made.
1. Derynck, R., Jarrett, J.A., Chen, E.Y., Eaton, D.H., Bell, J.R.,
Assoian, R.K.,
Roberts, A.B., Sporn, M.B., and Goeddel, D.V. (1985) Nature 316, 701-705.
2. Laiho, M., Weis, F.M.B., and Massague, J. (1990) J. Biol. Chem.
265:18518-
18524.
3. Madison, L., Webb, N.R., Rose, T.M., Marquardt, H., Ikeda, T., Twardzik,
D.,
Seyedin, S., and Purchio, A.F. (1988) DNA and Cell Biol. 7:18.
4. Schlunegger, M.P., and Gruner, M.G. (1992) Nature 353:430-434.
5. Hinck, A.P., Archer, S.J., Qian, S.W., Roberts, A.B., Sporn, M.B.,
Weatherbee,
J.A., Tsang, M.L.-S., Lucas, R., Zhang, B.-L., Wenker, J., and Torchia, D.A.
(1996) Biochem. 35:8517-8534.
6. Liu, Q., Huang, S.S., and Huang, J.S. (1997) J. Biol. Chem. 1997 272:
18891-
18895.
7. O'Grady, P., Kuo, M.-D., Baldassare, J.J., Huang, S.S., and Huang, J.S.
(1991) J.
Biol. Chem. 288:8583-8589.
8. Roberts, A.B. (1995) Transforming growth factor-13: activity and
efficacy in
animal models of wound healing. Wound Rep. Reg. 3,408-418.
9. Roberts, A.B., and Sporn, M.B. (1996) Transforming growth factor-13. In:
Clark,
R.A.F., ed. The Molecular and Cellular Biology of Wound Repair, 2nd ed. New
York, NY, Plenum Publishing Corp., 275-308.
10. 0' Kane, S. and Ferguson, M.W. (1997) Transforming growth factor Ps and
wound healing. Internat. J. Biochem. Cell Biol. 29, 63-78.
11. Shah, M., Foreman, D.M., and Ferguson, M.W.J. (1994) Neutralising
antibody to
TGF-131,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 107, 1137-
1157.
12. Shah, M., Foreman, D.M., and Ferguson, M.W.J. (1995) Neutralization of
TGF-131 and TGF-02 or exogenous addition of TGF-03 to cutaneous rat wounds
reduces scarring. J. Cell Sci. 108, 985-1002.
13. Huang, S.S., Liu, Q., Johnson, F.E., Konish, Y., and Huang, J.S. (1997)
Transforming growth factor p peptide antagonists and their conversion to
partial
agonists. J. Biol. Chem. 272, 27155-27160.
- 3 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
14. Kaufman, t., Levin, M., and Hurwitz, D.J. (1984) The effect of topical
hyperalimentation on wound healing rate and granulation tissue formation of
experimental deep second degree bums in guinea pigs. Burns 10, 252-256.
15. Knabl, J.S., Bayer, G.S., Bauer, W.A., Schwendenwein, I., Dado, P.F.,
Kucher,
C., Horvat, R., Turkof, E., Schossmann, B., and Meissl, G. (1999) Controlled
partial skin thickness burns: an animal model for studies of burn wound
progression. Bums 25, 229-235.
16. Kitamura, M., Shimizu, M., Ino, H., Okeie, K., Yamaguchi, M., Funjno,
N., and
Nakanishi, I. (2001) Collagen remodeling and cardiac dysfunction in patients
with hypertrophic cardiomyopathy: the significance of type IV and VI
collagens.
Clin. Cardiol. 24, 325-329.
17. Winter, G.D. (1974) Histological aspects of bum wound healing. Bums 1,
191-
196.
18. Mutoe, T.A., Pierce, G.F., Morishima, C., and Deuel, T.F. (1991) Growth
factor-
induced acceleration of tissue repair through direct and inductive activities
in a
rabbit dermal ulcer model. J. Clin. Invest. 87, 694-703.
19. Ashcroft, G.S., Yang, X., Glick, A.B., Weinstein, M., Letterio, J.J.,
Mizel, D.E.,
Anzano, M., Greenwell-Wild, T., Wahl, S.M., Deng, C., and Roberts, A.B.
(1999) Mice lacking Smad3 show accelerated wound healing and an impaired
local inflammatory response. Nature Cell Biology 1,260-266.
20. Zambruno, G., Marchisio, P.C., Marconi, A., Vaschieri, C., Melchiori,
A.,
Giannetti, A., and DeLuca, M. (1995) Transforming growth factor-0 modulates Pi
and 05 integrin receptors and induces the de novo expression of the av136
heterodimer in normal human keratinocytes: implications for wound healing. J.
Cell Biol. 129, 853-865.
21. Xia, Y.-P., Zhao, Y., Marcus, J., Jimenez, P.A., Ruben, S.M., Moore,
P.A., Khan,
F., and Mustoe, T.A. (1999) Effects of keratinocyte growth factor-2 (KGF-2) on
wound healing in an ischemia-impaired rabbit ear model and on scar formation.
J. Pathol. 188, 431-438.
22. Liu, Q., Ling. T.-Y., Shieh, H.-S., Johnson, F.E., Huang, J.S., and
Huang, S.S.
(2001) Identification of the high affinity binding site in transforming growth
factor-13 involved in complex formation with a2-macroglobulin: Implications
regarding the molecular mechanisms of complex formation between ar
macroglobulin and growth factors, cytokines and hormones. J. Biol. Chem. 276,
46212-46218.
- 4 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
SUMMARY OF THE INVENTION
[009] The inventor has discovered that specific peptide-based TGF¨P
antagonists are effective in
accelerating wound healing and reducing scarring due to wounds, such as burns,
scrapes, puncture
wounds and lacerations. The TGF¨P antagonist peptides may comprise any one of
amino acid
sequences as set forth in SEQ ID NO:4-11. The advantages to using the TGF¨P
antagonist peptides
in the treatment of skin wounds and diseases mediated by TGF¨P activity are
the chemical stability of
the peptides, ease of manufacturing the peptides, and small size of the
peptides, which allows for
rapid penetration into the wound relative to anti- TGF¨p antibodies.
=
[010] The invention is drawn to a non-naturally occurring peptide that
comprises an amino acid
sequence derived from TGF¨P1, TGF-132 or TGF¨P3, wherein the peptide is
capable of binding to a
TGF¨p receptor, thereby rendering the TGF¨P receptor unavailable for the
binding of TGF¨p
molecules. The peptide comprises a core stretch of amino acids as set forth in
SEQ ID NO:10 or SEQ
ID NO: ii. The preferred peptide comprises an amino acid sequence according to
SEQ ID NO:4,
SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8 or SEQ ID NO:9. The peptide
may also
comprise an amino acid sequence that is at least 68% identical to any one of
SEQ ID NO:4, SEQ ID
NO:5 and SEQ ID NO:6.
[011] The invention is also drawn to methods of treating diseases in a
vertebrate that are mediated
by TGF¨p or TGF¨P receptor activity, comprising the step of administering to
the vertebrate a
peptide that is a TGF¨P antagonist. Diseases that are mediated by TGF¨p or TGF-
13 receptor activity
include cancer (via reduced immune function or increased angiogenesis), morbid
angiogenesis (which
includes e.g. macular degeneration and tumor growth), intimal hyperplasia,
cancer, scarring, fibrosis
(e.g., liver cirrhosis, kidney fibrosis lung fibrosis, cystic fibrosis, heart
fibrosis), diseases of reduced
immune function, glomerulonephritis, and respiratory distress syndrome. The
peptide comprises a
core stretch of amino acids as set forth in SEQ ID NO:10 or SEQ ID NO:11. The
preferred peptide
comprises an amino acid sequence according to SEQ ID NO:4, SEQ ID NO:5, SEQ ID
NO:6, SEQ ID
NO:7, SEQ ID NO:8 or SEQ ID NO:9. The peptide may also comprise an amino acid
sequence that
is at least 68% identical to any one of SEQ ID NO:4, SEQ ID NO:5 and SEQ ID
NO:6.
[012] The invention is also drawn to methods of inhibiting the activity of
TGF¨p in a vertebrate,
comprising the step of administering to the vertebrate a peptide that is a
TGF¨P antagonist.
=
"Inhibiting the activity of TGF¨P" means inhibiting, ameliorating or reversing
the physiological
effects mediated by TGF¨p in biological systems. Those physiological effects
include scar formation,
deposition of collagen or other extracellular matrix proteins during wound
healing, wound
contraction, inhibition or slowing of re-epithelialization (the proliferation
of epithelial cells, .usually
- 5 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
epidermal cells) during the process of healing, restenosis of a blood vessel
after angioplasty and the
development of some types of cancers. The peptide comprises a core stretch of
amino acids as set
forth in SEQ ID NO:10 or SEQ ID NO:11. The preferred peptide comprises an
amino acid sequence
according to SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8
or SEQ ID
NO:9. The peptide may also comprise an amino acid sequence that is at least
68% identical to any
one of SEQ ID NO:4, SEQ ID NO:5 and SEQ ID NO:6.
[013] The invention is further drawn to methods of treating wounds comprising
the step of topically
administering to a wound a composition comprising a vehicle and a peptide that
is a TGF¨P
antagonist. The method of wound treatment may have any of the following
outcomes, which are
relative to wounds that have not been treated with the composition: the
reduction of scarring, the
reduction of wound contraction, the reduction of the deposition of
extracellular matrix components,
such as adhesion proteins (fibronectin, laminin, and vitronectin are examples
of adhesion proteins)
and collagens (collagens are of several types, including type I, type II, type
III, type IV, type V. type
VI and type IX collagen), and the promotion of re-epithelialization during
wound healing. The
peptide comprises a core stretch of amino acids as set forth in SEQ ID NO:10
or SEQ ID NO:11. The
preferred peptide comprises an amino acid sequence according to SEQ ID NO:4,
SEQ ID NO:5, SEQ
ID NO:6, SEQ ID NO:7, SEQ ID NO:8 or SEQ ID NO:9. The peptide may also
comprise an amino
acid sequence that is at least 68% identical to any one of SEQ ID NO:4, SEQ ID
NO:5 and SEQ ID
NO:6. A preferred vehicle comprises a physiological buffer, such as phosphate
buffered saline and a
gel, which contains a modified carboxymethyl-cellulose polymer and propylene
glycol, such as
IntraSitell) Gel Hydrogel Wound Dressing (Smith & Nephew, plc, London UK).
Wounds include
puncture wounds, pressure wounds, abrasions, lacerations and burns. Wounds may
be in any
vertebrate, including humans.
[014] The invention is further drawn to pharmaceutical compositions comprising
a peptide that is a
TGF-13 antagonist in a pharmaceutically acceptable excipient.
BRIEF DESCRIPTION OF THE DRAWINGS
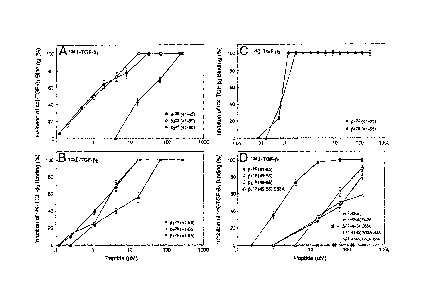
[015] FIGURE 1: Effect of various concentrations of pentacosapeptides,
decapeptides, and their
structural variants on TGF¨fl binding to TGF¨fl receptors in mink lung
epithelial cells. Cells were
incubated with 125I-TGF-131, (Panels A and D), 125I-TGF-132 (Panel B), and
1251-TGF-133 (Panel C)
both with and without 100-fold excess of unlabeled TGF-13 isoforms and various
concentrations of
peptides 13,25 (41-65), 13225 (41-65), and 3325 (41-65) (Panels A, B, and C)
or of /3110 (49_58), 13210(49
58), p310 (49_58), plio (49-58) W52A, 132io (49-58)
S53A, 1321 (49-58) D55A, 13125 (41-65)
W52A/D55A and 13325 (41-65) R52A/D55A (Panel D). The specific binding of 125I-
labeled TGF-131
isoforms was then determined. The specific binding obtained in the absence of
peptide antagonists
- 6 -
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
was taken as 0% inhibition. The specific binding (0% inhibition) of 1251-
TGF131, '251-TGFP2, and
1251-TGF133, were 3930 540 cpm/well, 4512 131 cpm/well, and 4219 125
cpm/well, respectively.
The error bars are means S.D. of triplicate cultures.
[016] FIGURE 2: 1251-TGF¨A-affinity labeling of cell-surface TGF¨I3 receptors
after incubation
of mink lung epithelial cells with 1251-TGFfl in the presence of various
concentrations of peptides fil25
(41-65) and fl325 (41-65). Cells were incubated with '25I-TGF-131 in the
presence of 100-fold excess of
unlabeled TGF-131 (lane 1) and of various concentrations of Pi' (41-65) (lanes
8-13) and 13325 (41-65)
(lanes 2-7). The '25I-TGF-131 affinity labeling was carried out in the
presence of DSS. The
1251-TGF-131 affinity-labeled TGF¨p receptors were analyzed by 5% SDS-
polyacrylamide gel
electrophoresis and autoradiography. The arrow indicates the location of the
1251-TGF-131 affinity-
labeled type V TGF¨p receptor (TI3R-V). The brackets indicate the locations of
the 1251-TGF-01
affinity-labeled type I, type II, and type III TGF¨P receptors (Tf3R-I, TPR-
II, and TPR-III).
[017] FIGURE 3: Effect of peptide 16125 (41-65) on TGF¨fl I induced growth
inhibition as measured
by DNA synthesis, and TGF¨A, induced PAI-1 expression in mink lung epithelial
cells. (Panel A)
Cells were incubated with various concentrations of TGF-131 in the presence of
18 M peptide 0125
(41-65). [Methyl-3H]thymidine incorporation into cellular DNA was then
determined. The [methyl-
3H]thymidine incorporation into cellular DNA in cells treated with and without
10 pM TGF¨f31, were
taken as 100 and 0% inhibition. The error bars are means S.D. of triplicate
cultures. (Panel B)
Cells were incubated with 0.25 pM TGF¨p, in the presence of various
concentrations of peptide p,25
(41-65). The [methyl-311]thymidine incorporation into cellular DNA in cells
treated with and without
pM TGF-131 were taken as 100 and 0% inhibition, respectively. The error bars
are means S.D. of
triplicate cultures. (Panel C) Cells were treated with 0.25 and 2.5 pM TGF¨I31
and various
concentrations of peptide 13125 (41-65) for 3 hr. The transcriptional
expressions of PAI-1 and
glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were determined by Northern
blot analysis.
[018] FIGURE 4: Effect of j3125 (41-65)-CA and )6125 (41-65)-BSA peptide
conjugates on
1251-TGF¨fi1 binding to TGF¨I3 receptors in mink lung epithelial cells and on
mink lung epithelial cell
growth as measured by DNA synthesis. (Panel A) Cells were incubated with 1251-
TGF-131 in the
presence and absence of 100-fold excess of unlabeled TGF¨I31 and various
concentrations of 13i25 (41-
65)-CA peptide conjugate. The specific binding of 125I-TGF-131 was then
determined. The specific
binding of 1251-TGF-131 obtained in the absence of the conjugates was taken as
0% inhibition. The
error bars are means S.D. of triplicate cultures. (Panel B) Cells were
treated with various
concentrations of 13125 (41-65)-CA or 13125 (41-65)-BSA peptide conjugate.
[Methyl-3H]thymidine
incorporation into cellular DNA was determined. The [methyl-3H]thymidine
incorporation into
- 7 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
cellular DNA in cell treated with and without 10 pM TGF-131 were taken as 100
and 0% inhibition,
respectively. The error bars are means S.D. of triplicate cultures.
[019] FIGURE 5: Panel A shows the amino acid sequences of three TGF¨P
molecules and Panel B
shows three peptides derived from the TGF¨P molecules, extending from amino
acid residue number
41 to 65.
[020] FIGURE 6: Kinetics of re-epithelialization and contraction in pig burn
wounds treated with
a TGF¨fl peptantagonist (peptide TGF-beta antagonist). The rates of wound re-
epithelization and
contraction were measured as a percent of the original wound (panels A and B,
respectively). The
burns treated with the TGF¨P peptantagonist (peptide TGF-beta antagonist)
healed faster than the
control wounds after post-burn day 10 (p < 0.005). The burns treated with the
TGF¨P peptantagonist
(peptide TGF-beta antagonist) contracted significantly after post-burn day 10
when compared with the
control treated with vehicle only (p < 0.005).
[021] FIGURE 7: Acceleration of wound healing and reduction of scarring by
application of a
TGF¨fl peptantagonist (peptide TGF-beta antagonist) to burn wounds of pigs.
Burn wounds treated
with a TGF¨p peptantagonist (peptide TGF-beta antagonist) or vehicle (gel
without peptide) only in
two animals (left and right) were photographed immediately after burn injury
(left, panel A and B and
right, panel A and B), post-burn day 23 (left, panel C and D), post-burn day
34 (right, panel C and D),
post-burn day 35 (left, panel E and F) and post-burn day 41 (right, panel E
and F). After burn injury,
necrosis was present (white color) (left and right, panel A and B). The
control wounds exhibited a
large open wound on post-burn day 23 (left, panel D). In contrast, the wound
treated with the TGF-11
peptantagonist (peptide TGF-beta antagonist) showed very little open wound
(left, panel C). On post-
burn day 35 and 41, less scar formation was observed in the wound treated with
a TGF¨p
peptantagonist (peptide TGF-beta antagonist) when compared with the control
wound (left, panel E
versus left, panel F and right, panel F versus panel E, respectively).
[022] FIGURE 8: Kinetics of contraction in pig excision wounds treated with
TGF.--fi.
peptantagonist(peptide TGF-beta antagonist). Excision wounds (3 x 3 cm) were
treated with a
TGF-0 peptantagonist (peptide TGF-beta antagonist) and vehicle-only every two
days for the first 10
days and twice a week for the next 30 days. The rate of wound contraction was
determined as a
percent of the original wound. Both the wounds treated with TGF¨P
peptantagonist (peptide TGF-
beta antagonist) and vehicle only almost contracted completely in a horizontal
direction (width of the
healed wound) on post-excision day 41. The TGF¨P peptantagonist-treated wound
contracted
vertically less than the control wound.
- 8 -
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
[023] FIGURE 9: Reduction of contraction in pig excision wounds treated with a
TGF¨fl
peptantagonist (peptide TGF-beta antagonist). Excision wounds (3 x 3 cm) on
the back of pig skin
were treated with a TGF¨P peptantagonist (peptide TGF-beta antagonist) (panels
A and C) and
vehicle-only (panels B and D) every two days for the first 10 days and twice a
week for the next 30
days. The wounds were photographed immediately after excision injury (panels A
and B) and at post-
wound day 34 (panels C and D). The TGF¨P peptantagonist (peptide TGF-beta
antagonist)-treated
wound exhibited less vertical (length of the healed wound) contraction when
compared with the
control wound.
[024] FIGURE 10: Immunostaining for type I collagen and fibronectin of
excision wounds in pigs.
Sections of pig excision wounds treated with a TGF¨P peptantagonist (peptide
TGF-beta antagonist)
(panels A, C and E), which were harvested on post-excision day 28, were
histologically evaluated
using hematoxylin/eosin staining (panels A and B) and were immunostained for
type I collagen and
fibronectin (panels C, D and E, F, respectively). The wound treated with a
TGF¨P peptantagonist
(peptide TGF-beta antagonist) showed less intensity of staining for type I
collagen and fibronectin
than the control wound.
[025] FIGURE 11: Reduction of scarring in rabbit ear excision wounds treated
with TGF¨fl
peptantagonist (peptide TGF-beta antagonist). Excision wounds (0.5 x 1 cm) in
rabbit ears were
treated with a TGF¨P peptantagonist (peptide TGF-beta antagonist) (TGF-
blocker), vehicle only
(sham) and nothing (negative). These wounds were photographed immediately
after excision injury
(panel A) and at post-excision day 10. The TGF¨P-peptantagonist (peptide TGF-
beta antagonist)
treated wounds showed reduced scarring relative to the control wounds.
DETAILED DESCRIPTION OF THE INVENTION
[026] TGF¨p antagonists or inhibitors that specifically bind to TGF¨P
receptors, which include
type I, type II, type III and type V receptors, are disclosed. It was
discovered that three chemically
synthesized peptides, which correspond in sequence to amino acid numbers 41-65
of TGF-131 (SEQ
ID NO:4), TGF-112 (SEQ ID NO:5), and TGF¨P3 (SEQ ID NO:6), and which comprise
a core amino
acid sequence as set forth in SEQ ID NO:10 or SEQ ID NO:11, inhibit the
binding of TGF-01,
TGF-132, and TGF-03, to TGF¨f3 receptors in epithelial cells. The peptides
also block TGF¨p-
induced growth inhibition and TGF¨P-induced expression of PAT-1 in epithelial
cells. It was also
discovered that the W/RXXD motif found within the peptide sequences determines
the specificity of
activity of the antagonist peptide. In view of these discoveries, peptides
that comprise amino acid
sequences corresponding to SEQ ID NO:10 or SEQ ID NO:11 are considered to be
antagonists of
TGF¨p activity. It was also discovered that these TGF¨I3 peptide antagonists
can be converted to
- 9 -
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
partial agonists (i.e., agent which mimics the effects of TGF¨I3) by
conjugation to carriers such as
proteins or synthetic polymers.
[027] A stepwise sequence comparison between SEQ ID NO:4 ( amino acids 41-65
of TGF¨I31),
SEQ ID NO:5 (amino acids 41-65 of TGF-132), and SEQ ID NO:6 (amino acids 41-65
of TGF-133),
has revealed that SEQ ID NO:4 and SEQ ID NO:6 are 68% identical; SEQ ID NO:4
and SEQ ID
NO:5 are 80% identical; and SEQ ID NO:5 and SEQ ID NO:6 are 72% identical.
Thus non-naturally
occurring TGF¨P peptide agonists may comprise an amino acid sequence that is
at least 68% identical
to any one of SEQ ID NO:4, SEQ ID NO:5, and SEQ ID NO:6; and including the
decapeptides of
SEQ ID NO:7, SEQ ID NO:8, and SEQ ID NO:9.
[028] Percent identity is intended to mean the percentage of the same amino
acid residues between
two sequences. To determine the percent identity of any given peptide, the
reference sequence may
be SEQ ID NO:4, SEQ ID NO:5 or SEQ ID NO:6. The two sequences being compared
are aligned
using the Clustal method (Higgins et al, Cabios 8:189-191, 1992) of multiple
sequence alignment in
the Lasergene biocomputing software (DNASTAR, INC, Madison, WI). In this
method, multiple
alignments are carried out in a progressive manner, in which larger and larger
alignment groups are
assembled using similarity scores calculated from a series of pairwise
alignments. Optimal sequence
alignments are obtained by finding the maximum alignment score, which is the
average of all scores
between the separate amino acid residues in the alignment, determined from a
residue weight table
representing the probability of a given amino acid change occurring in two
related peptides over a
given evolutionary interval. Penalties for opening and lengthening gaps in the
alignment contribute to
the score. The default parameters used with this program are as follows: gap
penalty for multiple
alignment = 10; gap length penalty for multiple alignment = 10; k-tuple value
in pairwise alignment =
1; gap penalty in pairwise alignment = 3; window value in pairwise alignment =
5; diagonals saved in
pairwise alignment = 5. The residue weight table used for the alignment
program is PAM250
(Dayhoff et al., in Atlas of Protein Sequence and Structure, Dayhoff, Ed.,
NBRF, Washington, Vol. 5,
suppl. 3, p. 345, 1978).
[029] The invention is thus drawn to non-naturally occurring peptides, and
modifications thereof,
that antagonize TGF¨r3 activity, and compositions comprising peptides that
antagonize of TGF¨f3
activity. By "non-naturally occurring", it is meant that the peptide is
artificially produced by chemical
synthesis, genetic recombinant methods or enzymatic digestion of isolated
polypeptides, and that the
peptide does not comprise a full length mature TGF¨I3 polypeptide. The non-
naturally occurring
peptide may be modified, wherein such modifications include glycosylation,
lipidation, amidation,
phosphorylation, acetylation, PEGylation (the addition of polyethylene glycol
to stabilize the peptide)
and albumination (the conjugation of an albumin moiety to increase the
biological half-life of the
- 10-
CA 02484994 2010-12-07
peptide). By "antagonize", it is meant that the non-naturally occurring
peptide of the instant invention
binds to a TGF-P receptor and prevents the activation of that TGF-13 receptor.
Antagonization may
be complete or it may be partial, whereby some TGF-13 receptor activation may
occur in the
vertebrate after administration of the non-naturally occurring peptide. As
used herein, "TGF-I3
receptors" are intergral membrane proteins that bind TGF-I3 molecules. TGF-13
receptors generally
comprise a type I receptor component and a type II receptor component.
Presently, there are seven
known mammalian members of type I receptors, including activin receptor-like
kinases 1 to 6 (ALK I-
ALK6), and five known members of type II receptors, including activin type II
and type JIB receptor
(ActRII/IIB), TGF-B type II receptor (TBRII), BMP type II receptor (BMPRII),
and MIS type II
receptor (MISRII). TGF-I3 receptors, in addition to type I and type II types,
also include type III and
type V receptors (Ref. 6). However, in the practice of this invention, yet to
be identified TGF-P
receptors are covered by the term "TGF-13 receptor". For a brief review of TGF-
13 receptor biology,
see Moustakas, etal., .1 Cell Sc!. 114:4359-4369.
[030] The non-naturally occurring peptides bind to TGF-P receptors, thereby
blocking the binding
of active TGF-0 receptor agonists to TGF-P receptors and "inhibiting the
activity of TGF-p". The
activities of TGF-I3, mimetics of TGF-13 or TGF-I3 receptor agonists, which
are well known in the
art, include (a) both proliferation and anti-proliferation effects on certain
cells and tissues, depending
on the state and type of cell, (b) cell differentiation, cell death, cell
migration, embryonic
development, tumor growth and wound healing, and (c) promoting the production
of cell-adhesion
molecules, extracellular matrix molecules and other growth factors. For a
review on TGF-13 structure
and function, see Lodish et al., "Molecular Cell Biology," Third Edition,
Scientific American Books
(1995), Gilbert, Scott F., "Developmental Biology," Fifth Edition, Sinauer
Associates, Inc., (1997),
and Alberts et al., "Molecular Biology of the Cell," Third Edition, Garland
Publishing, Inc. (1994).
[031] The non-naturally occurring peptide TGF-13 antagonists of the present
invention are useful in
treating individuals suffering from diseases or conditions that are modulated
at least in part by
TGF-P. Diseases and conditions which may be ameliorated by the administration
of peptide TGF-P
antagonists include carcinomas, such as breast cancer and pancreatic cancer
(see Gold, L.I., [1999]
"The role of transforming growth factor-13 (TGF-0) in human cancer", Crit.
Rev. Oncol. 10:303-360),
developmental defects, such as neural tube defects,
wounds, such as cutaneous burns, lacerations, punctures and abrasions, intimal
hyperplasia (which
results in blood vessel blockage by the thickening of arterial lining) and
restenosis of blood vessels
after angioplasty, angiogenesis that allows tumor growth, insufficient immune
system function,
angiogenesis (which is involved in e.g. tumor growth and macular
degeneration), tumor metastasis
(through the activity of proteases on the extracellular matrix), fibrosis
(e.g., integument scarring,
- 11 -
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
cystic fibrosis, liver cirrhosis, kidney fibrosis, lung fibrosis, and heart
fibrosis) glomerulonephritis,
and respiratory distress syndrome. The invention is therefore also drawn to
therapeutic or
pharmaceutical compositions, which comprise a peptide TGF-13 antagonist,
useful in the treatment of
diseases or conditions that are modulated at least in part by TGF-13.
[032] It has been discovered by the inventor that a peptide TGF¨p antagonist,
which comprises a
sequence of SEQ ID NO:10 or SEQ ID NO:11, (a) accelerates re-epithelialization
of skin and reduces
wound contraction and scarring during the healing of a burn injury and
diminishes wound contraction
and scarring, relative to untreated control wounds, in both the pig and rabbit
excision injury models.
"Re-epithelialization" is the growth of the outer layer of skin or epidermis
over the wound during the
healing process. "Hypertrophic scarring", "scarring", or "fibrosis" is the
process whereby fibrous
connective tissue replaces dermis or any other connective tissue that lies
subjacent to an epithelium
during tissue repair. "Wound contraction" is the process whereby scar tissue
or granulation tissue
contracts. The discovery that re-epithelialization is accelerated by a peptide
TGF-13 antagonist was
surprising and unexpected. Burn wound healing consists of epithelialization,
contraction and
formation of granulation and scar tissue (Refs. 8-12). TGF-13 is believed to
be involved in most of
these events. The peptide TGF-13 antagonist of the present invention is
thought to block or slow
down the occurrence of these events. However, the data shown in the examples
that follow are
consistent with a report that Smad3-null mice have accelerated cutaneous wound
healing compared
with wild-type mice (Ref. 18). Wounds in these animals have an increased rate
of re-epithelialization
and significantly reduced local infiltration of monocytes. The Smad3 signaling
plays an important
role in TGF-13-stimulated expression of collagen, chemotaxis of monocytes and
growth inhibition of
keratinocytes. The mechanism of enhanced re-epithelialization in wounds
treated with the peptide
TGF¨p antagonist of the present invention may involve increased keratinocyte
proliferation (transient
inhibition of keratinocyte proliferation by TGF-13 may be an integral
component in the complex
process of wound healing) coupled with a migration response stimulated by
growth factors other than
TGF¨(3 (Refs. 18-21). The peptide TGF¨p antagonist of the present invention
has been shown to
block complex formation between a2-macroglobulin and growth factors, cytokines
and hormones (see
reference 15) and thus, may enhance activation of these substances or agents
by blocking inhibition of
their activities mediated by a2-macroglobulin.
[033] Peptide TGF-13 antagonists of the present invention comprise the amino
acid motif W/RSXD,
wherein X is any amino acid (SEQ ID NO:10 and SEQ ID NO:11). The W/RXXD motif
was
demonstrated to be an important site involved in the interaction of peptides
with TGF-13 receptors.
This conclusion is supported by several lines of evidence presented in
Examples, including: 1) among
the seven pentacosapeptides (peptides consisting of 25 amino acids), whose
amino acid sequences
cover most of the TGF-131 molecule, only peptide 13125 (41-65), which contains
the W/RXXD motif in
- 12-
CA 02484994 2010-12-07
)
the middle of the peptide amino acid sequence, has TGF¨p antagonist activity;
2) pentacosapeptides
and decapeptides (peptides consisting of 10 amino acids) containing this
WfRXXD motif are potent
TGF-13 antagonists; 3) replacement of W-52/R-52 and D-55 by alanine residues
abolishes the
antagonist activities of these decapeptides and pentacosapeptides; 4)
conjugation of the peptide 13125
(41-65) antagonist to carrier proteins creates a partial TGF-13 agonist; and
5) several proteins that
possess W/RXXD motifs have TGF-13 agonist and antagonist activities. Preferred
peptide TGF-13
antagonists comprise any one of SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6, SEQ ID
NO:7, SEQ ID
NO:8 or SEQ ID NO:9; or peptides that are at least 68% identical to these
sequences.
[034] The therapeutic or pharmaceutical compositions of the present
invention may be
administered by any , suitable route known in the art including for example
via intraarterial
catheterization, intravenous, subcutaneous, intramuscular, transdermal,
intrathecal, intracerebral, oral
or topical. Administration may be either rapid as by injection or over a
period of time as by slow
infusion or administration of slow release formulation. For treating tissues
in the central nervous
system, administration may be by injection or infusion into the cerebrospinal
fluid (CSF). When it is
intended that a peptide TGF-13 antagonist be administered to cells in the
central nervous system,
administration may be with one or more agents capable of promoting penetration
of a peptide TGF-13
antagonist across the blood-brain barrier. For treating intimal hyperplasia or
restenosis, the peptide
antagonist may be administered via intraarterial catheterization during
angioplasty procedures. The
peptide may also be applied on the surface of the stent that is left in place
during angioplasty. When it
is intended that the peptide TGF¨I3 antagonist be used to reduce scar tissue
(fibrosis) formation during
the healing of surgical incisions, especially incisions made during plastic
surgery procedures, the
peptide TGF-13 antagonist peptide may be subcutaneously injected into the area
of the incision or
healing wound.
[035] A peptide TGF-13 antagonist may also be linked or conjugated with
agents that provide
desirable pharmaceutical or pharmacodynamic properties. For example, a peptide
TGF¨p antagonist
may be coupled to any substance known in the art to promote penetration or
transport across the
blood-brain barrier such as an antibody to the transferrin receptor, and
administered by intravenous
injection. (See for example, Friden et al., Science 259:373-377, 1993).
Furthermore, a peptide TGF-13 antagonist may be stably linked to a polymer
such as
polyethylene glycol or albumin to obtain desirable properties of solubility,
stability, half-life and other
pharmaceutically advantageous properties. (See for example Davis et al. Enzyme
Eng 4:169-73, 1978;
Burnham, Am J Hosp Pharm 5/:210-218, 1994).
[036] The compositions comprising peptide TGF-13 antagonists are usually
employed in the form
of pharmaceutical preparations. Such preparations are made in a manner well
known in the
pharmaceutical art. One preferred preparation utilizes a vehicle of
physiological saline solution, but it
- 13 -
CA 02484994 2010-12-07
is contemplated that other pharmaceutically acceptable carriers such as
physiological concentrations
of other non-toxic salts, five percent aqueous glucose solution, sterile water
or the like may also be
used. It may also be desirable that a suitable buffer be present in the
composition. Such solutions
may, if desired, be lyophilized and stored in a sterile ampoule ready for
reconstitution by the addition
of sterile water for ready injection. The primary solvent may be aqueous or
alternatively non-
aqueous. A peptide TGF-13 antagonist may also be incorporated into a solid or
semi-solid
biologically compatible matrix which may be implanted into tissues requiring
treatment. A peptide
TGF-13 antagonist may also be incorporated into a hydrogel wound dressing,
such as an IntraSite
Gel Hydrogel Wound Dressing (Smith & Nephew, plc, London UK), which comprises
a modified
carboxymethyl-cellulose polymer and propylene glycol.
[037] The carrier may also contain other pharmaceutically-acceptable
excipients for modifying or
maintaining the pH, osmolarity, viscosity, clarity, color, sterility,
stability, rate of dissolution, or odor
of the formulation. Similarly, the carrier may contain still other
pharmaceutically-acceptable
excipients for modifying or maintaining release or absorption or penetration
across membranes or
other barriers, such as the epidermis, the lining of the alimentary canal, the
endothelium or the blood-
brain barrier.
[038] It is also contemplated that certain formulations containing a
peptide TGF-13 antagonist are
to be administered orally. Such formulations are preferably encapsulated and
formulated with
suitable carriers in solid dosage forms. Some examples of suitable carriers,
excipients, and diluents
include lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia,
calcium phosphate,
alginates, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone,
cellulose, gelatin, syrup,
methyl cellulose, methyl- and propylhydroxybenzoates, talc, magnesium,
stearate, water, mineral oil,
and the like. The formulations may additionally include lubricating agents,
wetting agents,
emulsifying and suspending agents, preserving agents, sweetening agents or
flavoring agents. The
compositions may be formulated so as to provide rapid, sustained, or delayed
release of the active
ingredients after administration to the patient by employing procedures well
known in the art. The
formulations may also contain substances that diminish proteolytic degradation
and promote
absorption such as, for example, surface active agents.
[039] The specific dose is calculated according to the approximate body
weight or body surface
area of the patient or the volume of body space to be occupied. The dose will
also be calculated
dependent upon the particular route of administration selected. Further
refinement of the calculations
necessary to determine the appropriate dosage for treatment is routinely made
by those of ordinary
skill in the art. Such calculations may be made without undue experimentation
by one skilled in the
art in light of the activity of a peptide TGF-13 antagonist. The data showing
activity of a peptide
TGF-13 antagonist are herein disclosed in the Examples and in U.S. Patent No.
- 14 -
CA 02484994 2010-12-07
=
6,500,920. Furthermore, the activity of a peptide TGF-13
antagonist on a particular target cell type may be determined by routine
experimentation. Exact
dosages are determined in conjunction with standard dose-response studies. It
will be understood that
the amount of the composition actually administered will be determined by a
practitioner, in the light
of the relevant circumstances including the condition or conditions to be
treated, the choice of
composition to be administered, the age, weight, and response of the
individual patient, the severity of
the patient's symptoms, and the chosen route of administration.
[040] The above disclosure describes several preferred embodiments of the
invention. The skilled
artisan will recognize that other embodiments of this invention, which are not
overtly disclosed
herein, may be employed in the practice of this invention. The invention is
further illustrated by the
examples described below, which are not meant to limit the invention.
EXAMPLES
Example 1: Development of peptide antagonists of TGF¨p
Experimental Procedures
[041] Materials. Na125 (17 Ci/mg) and [methyl-3H]thymidine (67 Ci/mmole) were
purchased from
ICN Radiochemicals (Irvine, CA). High molecular-weight protein standards
(myosin, 205 kDa; 13-
galactosidase, 116 kDa; phosphorylase, 97 kDa; bovine serum albumin, 66 kDa),
chloramine T,
bovine serum albumin (BSA), and human carbonic anhydrase I (CA) were purchased
from Sigma
Company (St. Louis, MO). Disuccinimidyl suberate (DSS) was obtained from
Pierce (Rockford, IL).
TGF-01 was purchased from Austral Biologicals (San Ramon, CA). TGF-132 and TGF-
133 were
purchased from R&D Systems (Minneapolis, MN).
[042] Preparation of peptides. The amino acid sequences of all peptides were
derived from those of
TGF-13i, TGF¨I32, and TGF¨I33. For peptides p125 (41-65), 13225 (41-65), and
13325 (41-65), other
versions in which cysteine-44 and cysteine-48 were replaced by serine residues
were also synthesized.
These C44S/C48S versions of peptides p,25 (41-65) (SEQ ID NO:4), 13225 (41-65)
(SEQ ID NO:5), and
13325 (41-65) (SEQ ID NO:6) had the same TGF¨I3 antagonist activity. The
C44S/C48S versions had
better stability in solution during storage, so they were used in most of the
experiments. The peptides
were synthesized using tert-butoxycarbonyl chemistry on an Applied Biosystems
Model 431A peptide
synthesizer and purified using Sephadex G-25 column chromatography and
reverse-phase HPLC (C-8
column). The purity of the synthesized peptides were verified by automated
Edman degradation on
an Applied Biosystems Model 477A gas/liquid phase protein sequenator with an
on-line Applied
Biosystems Model 120A phenylthiohydantoin amino acid analyzer. The purity of
all peptides was
estimated to be 95%.
- 15 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
[043] Preparation of peptide fli25 (41-65)-carbonic anhydrase (CA) and peptide
18125 (41-65)-bovine
serum albumin (BSA) conjugates. 150 pl of 3 mM peptide 13125 (41-65) (SEQ ID
NO:4) in phosphate
buffer saline (pH adjusted to ¨9.0) was mixed with 300 p.1 of 0.1 M NaHCO3 (pH
¨9.0) containing
BSA or CA (0.5 mg) and 10 ill of 27 mM DSS in dimethyl sulfoxide. After 18 hr
at 4 C, the reaction
mixture was mixed with 50 u.1 of 1 M ethanolamine HCI in 0.1 M NaHCO3 (¨pH
9.0). After 2 hr at
room temperature, the reaction mixture was dialyzed against 2 liters of 0.1 M
NaHCO3 (¨pH 9.0).
After four changes of the dialysis solution, the sample was stored at 4 C
prior to use. The molar ratio
of peptide IV' (41-65)/carrier protein in the conjugate was determined by
amino acid composition
analysis.
[044] Specific binding of 125I-labeled TGF-I 31, TGF-P2, and TGF-P3 (125 I-TGF-
fil, 125 1-TGF-,32,
and 125 1-TGF-P3) to TGF-11 receptors in mink lung epithelial cells. 1251-TGF-
131, 1251-TGF-132, and
125I-TGF¨f33 were prepared by iodination of TGF-131, TGF-132, and TGF-133 with
Na'25I as described
previously (Ref. 7). The specific radioactivities of 1251-TGF¨P1, '25I-TGF¨P2,
and 1251-TGF-133 were
1-3 x 105 cpining. Mink lung epithelial cells were grown on 24-well clustered
dishes to near
confluence in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal
calf serum. The
epithelial cells were incubated with 0.1 nM 125I-TGF¨I31, 1251-TGF-132, or
'25I-TGF¨P3 both with and
without 100-fold excess of unlabeled TGF-131, TGF¨I32, or TGF-133 in binding
buffer (Ref. 7). After
2.5 hr at 0 C, the cells were washed two times with binding buffer, and the
cell-associated
radioactivity was determined. The specific binding of 1251-labeled TGF¨p
isoforms to TGF¨P
receptors in the cells was calculated by subtracting non-specific binding (in
the presence of 100-fold
excess of the unlabeled TGF¨I3 isoforms) from total binding. All experiments
were carried out in
triplicate cell cultures.
[045] 125 1-TGF¨firaffinity labeling of cell-surface TGF¨,8 receptors in mink
lung epithelial cells.
Mink lung epithelial cells grown on 60-mm Petri dishes were incubated with 0.1
nM 1251 in the
presence of various concentrations of peptide (3125 (41-65) or peptide 13325
(41-65) in binding buffer.
After 2.5 hr at 0 C, 125I-TGF¨f3raffinity labeling was carried out in the
presence of DSS as described.
The 125I-TGF-131-affinity-labeled TGF¨P receptors were analyzed by 5% SDS-
polyacrylamide gel
electrophoresis under reducing conditions and autoradiography.
[046] [Methy1-3H]thymidine Incorporation- Mink lung epithelial cells grown on
24-well clustered
dishes were incubated with various concentrations of TGF¨p1 in the presence
and absence of peptide
13,25 (41-65) or with various concentrations of peptide P125 (41-65)-CA, and
peptide 13125 (41-65)-BSA
in DMEM containing 0.1% fetal calf serum. After 16 hr at 37 C, the cells were
pulsed with 1 i.tCi/m1
- 16 -
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
of [methyl-3H]thymidine for 4 hr. The cells were then washed twice with 1 ml
of 10% trichloroacetic
acid and once with 0.5 ml of ethanol:ether (2:1, v/v). The cells were then
dissolved in 0.4 ml of 0.2 N
NaOH and counted with a liquid scintillation counter.
[047] RNA Analysis- Mink lung epithelial cells were grown overnight in 12-well
clustered dishes in
DMEM containing 10% fetal calf serum. The medium was then changed to DMEM
containing 0.1%
fetal calf serum and the cells were incubated with 0.25 and 2.5 pM TGF¨P1 in
the presence of various
concentrations of peptide 13125 (41-65) for 2.5 hr. Total cellular RNA was
extracted using RNAzol B
(Tel-Test Inc.) according to the manufacturer's protocol. RNA was
electrophoresed in 1.2 % agarose-
formaldehyde gel and transferred to Duralon-UV membranes using 10 x SSC. The
membranes were
probed at 42 C with a random-primed, radiolabeled 1-kb fragment from the Hind
III and NeoI digests
of PAI-1 cDNA and glyceraldehyde-3-phosphate dehydrogenase ("GAPDH") cDNA. The
blots were
washed with 0.1 x SSC containing 0.1 % SDS at room temperature.
Experimental Results
[048] To develop peptide antagonists of TGF-13, seven pentacosapeptides
(peptides containing 25
amino acids) were synthesized: peptide 13125 (21-45), peptide 13125 (31-55),
peptide 13125 (41-65) (SEQ
ID NO:4), peptide 13125 ( 51-75), peptide p,25 (61-85), peptide pi25 (71-95),
and peptide 13i25 (81-105),
whose amino acid sequences overlap one another and cover most of the human TGF-
131 molecule, the
monomer of which has 112 amino acid residues (SEQ ID NO:1) (ref. 1). The
antagonist activities of
these peptides were first tested for their abilities to inhibit 1251-labeled
TGF-131 (1251-TGF-131) binding
to cell-surface TGF¨P receptors in mink lung epithelial cells, an art
recognized model system for
investigating TGF-13 receptor types and TGF¨p-induced cellular responses (ref.
2). Peptide 13125 (41-
65) (SEQ ID NO:4), completely inhibited the 1251-TGF-131 binding (specific
binding without peptides
= 3672 524 cpm/well) to TGF-13 receptors in mink lung epithelial cells at 34
M. The other six
pentacosapeptides did not show any effect on '25I-TGF¨P1 binding to TGF-13
receptors in these
epithelial cells, even at a concentration of 136 M. This demonstrates that
peptide 13)25 (41-65) (SEQ
ID NO:4) is a TGF-13 inhibitor or antagonist.
[049] TGF-13 isoforms (TGF-131, TGF-132, and TGF-133) have been shown to
exhibit different
potencies in inducing cellular responses in certain cell types or systems.
There is ¨70% amino acid
sequence homology at the 41st to 65th amino acid residues among these three
TGF-13 isoforms (Refs.
1-3) (Figure 5A). To determine the potencies of peptide 13125 (41-65) (SEQ ID
NO:4), peptide 13225
(41-65) (SEQ ID NO:5), and peptide 13325 (41-65) (SEQ ID NO:6) in terms of TGF-
13 antagonist
activity, the effects of these peptides on the binding of '251-labeled TGF-
131, TGF-132, and TGF-133 to
TGF¨p receptors in mink lung epithelial cells were measured. As shown in
Figure 1, both peptide
13125 (41-65) and peptide 13225 (41-65) inhibited 1251-TGF-131 and 125I-TGF-
132 binding to TGF-13
- 17-
CA 02484994 2010-12-07
receptors in a concentration-dependent manner with an IC50 of ¨1 - 2 M
(Figure 1, A and B).
Peptide 0325 (41-65) was weaker with an IC50 of ¨20 M for inhibiting '25I-TGF-
p, and '25I-TGF¨P2
binding to TGF¨p receptors (Figure 1, A and B). In contrast, peptides 13,25
(41-65) and P325 (41-65)
showed equal potency (IC50 = ¨0.06 - 0.08 M) when '25I-TGF-33 was used as
ligand for testing the
inhibitory activity (Figure 1, C). Peptide P225 (41-65) also had an IC50 of
¨0.08 M for inhibiting
'25I-TGF-33 binding to TGF¨p receptors in these epithelial cells. These
results show that both
peptides 13125 (41-65) and 13225 (41-65) are more potent antagonists than
peptide 13325 (41-65) for
'25I-TGF-p1 and 1251-TGF-02, and that all three pentacosapeptides are potent
antagonists for
'251-TGF-03 with equal IC50.
[050] The region spanning residues 41-65 comprises a loop in the three-
dimensional structure of
TGF-01 and TGF-132 (Ref. 4, 5). This loop is accessible to solvent according
to X-ray and NMR
analyses (Ref. 4, 5). There are two reasons why a WSXD (for TGF¨Pi and TGF¨P2
; SEQ ID NO:10)
or RSXD (for TGF¨P3; SEQ ID NO:11) motif in the loop is a good candidate site
whereby these
antagonist peptides and their parent molecules could interact with TGF¨P
receptors. The W/RSXD
(52n
55th amino acid residues) motif is located on the exposed surface of the loop,
and the side
d
chains of the amino acid residues in the motif orient toward the solvent (Ref.
4, 5). Also, this motif
may determine the affinities of peptides P125 (41-65), 13225 (41-65), and P325
(41-65), and their parent
molecules for binding to TGF-0 receptors. Both peptide 13,25 (41-65) and
peptide P2' (41-65) share
the same motif (WSXD; SEQ ID NO:10) and have equal potencies (IC50 = ¨1-2 M)
for the inhibition
of '251-TGF¨P1 binding to TGF¨P receptors. Peptide 0,25 (41-65) possesses a
distinct motif of RSXD
(SEQ ID NO:11) and is a weaker inhibitor (IC50 of ¨20 M). The Ks for TGF¨P1
and TGF¨I32
binding to the type V TGF¨P receptor are identical (-0.4 nM), whereas the Kd
of TGF-433 binding to
the type V receptor is higher (-5 nM) (Ref. 6).
[0511 To test the possibility that the W/RSXD motif is the active site of
these peptides, three
decapeptides designated 0,' (49-58), IV (49-58), and 031 (49-58), which
respectively correspond to
SEQ ID NO:7, SEQ ID NO:8 and SEQ ID NO:9, were designed. The W/RSXD variants
of these
decapeptides, in which the W-52, S-53, or D-55 residue was replaced by an
alanine residue, were also
synthesized and designated peptide N1 (49-58) W52A, peptide 132' (49-58)
S53A, and peptide p,'
(49-58) D55A, respectively. The ability of each of these decapeptides to
inhibit '251-TGF-131 binding
to TGF¨P receptors in mink lung epithelial cells were then examined. As shown
in Figure 1D, both
peptide p,' (49-58) and peptide IV (49-58) inhibited the '25I-TGF-131
binding to TGF¨P receptors in
a concentration-dependent manner with an IC50 of ¨40-70 M. Peptide NH)
9 8) did not show any
inhibitory activity at concentrations up to ¨300 M. Peptide 1321 (49-58)
S53A was equipotent with
an 1C30 of ¨40 M. The other variants, Peptide 13210 (49-58) W52A and peptide
IV (49-58) D55A,
failed to inhibit '251-TGF¨Pi binding to TGF¨p receptors in these epithelial
cells. Identical
- 18-
CA 02484994 2010-12-07
experiments with peptides Pit (49-58) W52A, p,' (49-58) S53A, and 1311 (49-
58) D55A were also
carried out, and the results were similar to those shown in Figure ID with the
13210 (49-58) peptide
variants. These results suggest that the WXXD motif is important for the
inhibitory activity of the
decapeptides 13I'0 (49-58) and 1321 (49-58).
[052] To demonstrate that the W/RYXD motif is also important for the
inhibitory activities of the
pentacosapeptides 13125 (41-65) and P325 (41-65), variants of peptides 13125
(41-65) and 13325 (41-65), in
which both W- or R-52 and D-55 were replaced by alanine residues, were
prepared. These peptide
variants were designated 13125 (41-65) W52A/D55A and 13325 (41-65) R52AJD55A,
respectively, and
tested for their inhibitory activities. Figure 1D shows that peptide 13125 (41-
65) W52A/D55A and
peptide 13325 (41-65) R52A1D55A did not inhibit '251-TGF-131 binding to TGF-13
receptors, thereby
supporting the conclusion that the motif W/RXXD is involved in the
interactions of the instant peptide
antagonists with TGF-13 receptors.
[053] Mink lung epithelial cells express all of the known TGF-13 receptors
(type I, type H, type III,
and type V receptors) (see Ref. 6). To determine the relative sensitivities of
TGF-0 receptor types to
inhibition by peptides 13125 (41-65) and 13325 (41-65) with respect to ligand
binding, mink lung
epithelial cell-surface TGF¨P receptors were labeled with 1251-TGF-131 in the
presence of various
concentrations of peptides 13125 (41-65) and 13325 (41-65). As shown in Figure
2, all cell-surface
TGF-13 receptors (type I, type II, type III, and type V receptors) were
affinity-labeled with
'251-TGF-131 in the absence of the antagonists (lanes 7 and 13). Peptide 13125
(41-65) appeared to
inhibit the '25I-TGF-131-affinity labeling of all TGF¨P receptor types in a
concentration-dependent
manner (lanes 8-12). However, 13125 (41-65) inhibition of the '251-TGF-131-
affinity labeling of the type
V TGF¨P receptor was greater than its inhibition of other TGF-13 receptor
types. The '25I-TGF-131-
affinity labeling of the type V TGF-13 receptor was almost completely
abolished by peptide 13125 (41-
65) at 2.3 M, whereas the '25I-TGF-131-affinity labeling of other TGF-13
receptor types was only
partially inhibited (30-40%) (Figure 2, lane 10). This result is consistent
with the observation that the
affinity for TGF-131 binding to the type V TGF-13 receptor is ¨20-40-fold
lower than those for
TGF-131 binding to other TGF¨I3 receptor types (Ref. 6). Peptide 13325 (41-65)
showed weak activity in
blocking the 125I-TGF-131 -affinity labeling of the type V TGF¨I3 receptor
(Figure 2, lanes 2-5).
[054] It has been demonstrated that peptides 13125 (41-65), 13225 (41-65), and
13325 (41-65) are potent
inhibitors for 125I-TGF-131 binding to TGF¨I3 receptors. To further establish
the role of the instant
peptides as TGF¨p antagonists or inhibitors, these peptides are shown to block
a TGF-13-induced
cellular response, i.e., growth inhibition. The effect of peptide 13125 (41-
65) on TGF-131induced
growth inhibition was investigated by exposing mink lung epithelial cells to
various concentrations of
TGF-131 in the presence of 18 1.1.M peptide 13125 (41-65) and measuring
cellular DNA synthesis. As
-19-
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
shown in Figure 3A, DNA synthesis inhibition induced by 0.025 pM and 0.25 pM
TGF-31 was
completely blocked by peptide Pi25 (41-65). In the presence of peptide Pi25
(41-65), the dose-response
curve of TGF¨Pi shifted to the right. Peptide 13125 (41-65) blocked TGF-131-
induced growth inhibition
in a concentration-dependent manner (Figure 3B). It is important to note that
peptide 0125 (41-65) (0.1
M to 36 NI) did not have an effect on DNA synthesis in the absence of TGF-
131. These results
suggest that peptide P125 (41-65) is a TGF¨I3 antagonist, which blocks TGF¨P-
induced growth
inhibition.
[055] The other prominent biological activity of TGF¨P is transcriptional
activation of collagen,
adhesion protein (i.e., fibronectin), and PAI-1. To see if peptide 13i25 (41-
65) is able to block this
activity, the effect of peptide 13125 (41-65) on PAT-1 expression in mink lung
epithelial cells stimulated
by 0.25 pM and 2.5 pM TGF-131 was investigated. As shown in Figure 3C, peptide
1325 (41-65)
completely blocked the PAT-1 expression stimulated by TGF-131 (lane 7 versus
lanes 3 and 5). These
results further support the conclusion that peptide Pi' (41-65) is a potent
TGF¨p antagonist.
[056] The .climeric structure of TGF¨P has been shown to be required for its
biological activities.
The hetero-oligomerization of TGF¨P receptors induced by the TGF¨P dimer
appears to trigger
signaling. If peptide Pi' (41-65) contains the active site sequence involved
in the interaction of
TGF-131 with TGF¨P receptors, one may be able to convert its antagonist
activity to agonist activity
by conjugating peptide 0125 (41-65) to carrier proteins, such that the 13i25
(41-65)-protein conjugates
would carry multiple valences of the putative active site. To test this
possibility, peptide Pi25 (41-65)
was conjugated to carrier proteins CA (carbonic anhydrase) and BSA (bovine
serum albumin) using
the cross-linking agent DSS. DSS mainly cross-links the a-amino group of
peptide 13125 (41-65) to the
C-amino groups of the carrier proteins. The 0125 (41-65)-BSA and P125 (41-65)-
CA conjugates
contained ¨5-10 molecules of peptide 13125 (41-65) per molecule of carrier
protein. As shown in
Figure 4A, the 13,25 (41-65)-CA conjugate inhibited '251-TGF-131 binding to
TGF¨P receptors in mink
lung epithelial cells with an IC50 of ¨0.05 M. The P125 (41-65)-BSA conjugate
had a similar IC50 of -
¨0.06 M. These IC50 are ¨20-fold lower than that of peptide Pi25 (41-65)
prior to conjugation. In the
control experiments, both BSA and CA conjugated without peptides did not have
inhibitory activity.
These results demonstrate that the multiple valences of the active site in the
protein conjugates
enhance its affinity for binding to TGF¨P receptors.
[057] Potential agonist activities of the 13i25 (41-65)-protein conjugates was
also examined. As
shown in Figure 4B, both 13125 (41-65)-CA and 13125 (41-65)-BSA conjugates
induced a small but
significant growth inhibition as measured by DNA synthesis with an ED50 of
¨0.1 M, although
neither showed significant effects on the expression of PAI-1 in mink lung
epithelial cells (data not
shown). The growth inhibition (-30-40%) induced by 0.2 M 13125 (41-65)-CA
could be abolished in
-20-
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
the presence of 10 tiM 0125 (41-65) (data not shown). These results suggest
that these 13125 (41-65)-
protein conjugates are partial TGF-0 agonists.
Example 2: Peptide TGF¨I3 antagonist reduces scarring and promotes healing
Experimental Procedures
[058] Materials ¨ Peptide 0125 (41-65) (SEQ ID NO:4) was synthesized and
purified as described
previously (Ref. 13). Sterile IntraSitee gel was obtained from Smith and
Nephew Medical, Limited
(England). Ketamine was obtained from Yung-Shin Pharmaceutical Co. (Taoyuan,
Taiwan).
Strenil (azaperonum) and atropine were purchased from Janssen Animal Health
BVBA, Belgium
and China Chemical and Pharmaceutical Co. (Taipei, Taiwan), respectively.
Monoclonal antibodies
to type I collagen and fibronectin were purchased from Sigma (St. Louis, MO).
[059] Animals - Female pigs (yorkshire strain and house inbred) weighing 20-25
kg and six rabbits
weighing 3 kg were used. The pigs were housed in individual rooms, whereas
female rabbits were
kept in individual cages. Animals were fed standard laboratory chow and water
ad libitum. All study
protocols were reviewed and approved by the respective institutional animal
care committees.
[060] Preparation of IntraSitee gel containing TGF¨fl peptantagonist (peptide
TGF-beta
antagonist). I ml of sterile 6 mM peptide 0125 (41-65) in phosphate buffered
saline or 1 ml of sterile
phosphate buffered saline was vigorously mixed with 3 ml of IntraSiteo gel
using two 10 ml syringes
connected with a three-way connector. The Intrasite gel containing peptide
0125 (41-65) and
Intrasitee) gel containing buffer without peptide were stable at least for
several weeks. The
concentrations of peptide 0125 (41-65) (0.75 and 1.5 mM) were found to be
effective in accelerating
wound healing and reducing scarring under the experimental conditions.
However, 1.5 mM of
peptide 0125 (41-65) was used throughout the experiments described below.
[061] Burn wound model. Four pigs weighing 20-25 kg were anesthetized by
intramuscular
injection of ketamine (5 mg/kg), strenil (cazaporonum) (20 mg/kg) and
atropine (5 mg/kg). Six
uniform burn wounds (110 C, 30 sec) were then made symmetrically on the back
of each pig using a
modified soldering iron (Ref 15) with a flat contact area of 20 cm2. The bum
injury was equivalent
to a full-thickness burn injury in humans and uniformly caused coagulation and
necrosis of dermis.
After wounding, a thin layer of IntrasiteiD gel containing either peptide 0125
(41-65), buffer or nothing
else was applied to the wounds. All wounds were dressed with paraffin gauze.
The dressing was
changed every two days for the first 10 days and twice a week for the next 30
days. Gel comprising
peptide 0125 (41-65) and control gel were applied and wound measurements were
made at each
dressing change.
-21-
CA 02484994 2004-10-28
WO 03/093293 PCT/US03/11437
[062] Excision wound model. Four pigs received intramuscular injection of
ketamine, streni10 and
atropine as described above. Six excision injuries were generated by removing
full-thickness sections
of skin (3 x 3 cm) from standardized sites on the back of each animal using a
scapel. Three rabbits,
were anesthetized by intramuscular injection of ketamine (5 mg/kg). Three
excision injuries were
produced in each by removing full-thickness sections of skin (0.5 x 1 cm) from
each ear. After
wounds, a thin layer of InfraSite gel containing peptide j3125 (41-65) was
applied to alternating
wounds on each animal and IntraSite gel + buffer was applied to the other
half. The excision
wounds were then dressed with a paraffin gauze. For pig experiments, the
dressing was changed
every two days for the first 10 days and twice a week for the next 30 days.
For rabbit experiments,
the dressing was changed for the first 3 days. Gel containing peptide 13125
(41-65) or control gel was
applied at each dressing change.
[063] Assessment of wound healing. Wound healing was assessed by evaluating
the rates of wound
re-epithelization and contraction. The open wound area and the area enclosed
by the normal hair
bearing skin were measured using the macrophotography technique (Ref. 14). The
healing rate was
monitored every two days for the first 10 days and twice a week for 30 more
days. Wound re-
epithelialization as a percent of the original wound size was calculated using
the following formula:
E= An-Ao x100
An
where E = rate of re-epithelialization in percent; An = area enclosed by the
normal hair bearing skin
on a given post-burn day; Ao = area of open wound on the same day as was
measured. Wound
contraction was calculated using the following formula:
C= Al-An x100
Al
where C = rate of wound contraction in percent; Al = wound area as measured
immediately following
the burn or excision injury; An = area enclosed by normal hair bearing skin.
[064] Immunohistochemistty. The wounds were frozen immediately after being
removed from
animals on post-excision day 30. Serial sections were placed on polylysine-
coated slides. The
sections containing wound areas were stained with hematoxylin/eosin and
monoclonal antibodies to
type I collagen and fibronectin and biotin-conjugated rabbit anti-mouse
IgG/streptavidin-conjugated
-22 -
CA 02484994 2010-12-07
horseradish peroxidase (Ref. 16). The stained sections were examined and
photographed by light
microscopy.
[065] Measurement of scar. The volumes of scar tissue were estimated by
multiplying their
thickness by the size of the scar on post-burn day 41 in pigs and post-
excision day 10 in rabbits.
Experimental Results
[066] The pig model is an art recognized model used in burn experiments
because porcine skin is
anatomically very similar to human skin (Ref. 15, 17). Pigs weighing about 20-
25 kg were
anesthetized by intramuscular injection of ketamine (5 mg/kg). A soldering
iron with a flat contact
area of ¨20 cm2 was used to generate a full-thickness burn injury (110 C, 30
sec) on the skin of the
back in four pigs. Six thermal burns (three on each side) were created on each
pig. After wounding,
two lesions were treated with a thin layer of a sterile IntraSitee gel
containing peptide 0,25 (41-65)
(1.5 mM); two received gel alone and two received topical applications. All
wounds were then
bandaged and protected from potential contact irritation with a fixed frame.
Peptide 0125 (41-65) and
vehicle were applied every two days for the first 10 days and twice a week for
the next 30 days, at
which time the re-epithelialization and contraction of the wounds were
measured and photographed as
well. As used herein, the term "vehicle" refers generally to any solvent,
buffer, gel or carrier in which
the active peptide may be dispersed or dissolved. In the topical
administration of peptide TGF-beta
antagonists, the preferred vehicle is a gel, such as the IntraSite gel
comprising modified
carboxymethyl-cellulose polymer and propylene glycol. Each animal served as
its own control. As
shown in Figure 6A, skin burn wounds treated with peptide 0125 (41-65)
exhibited rapid re-
epithelialization and less contraction. The wounds showed significant re-
epithelization and
contraction after post-burn day 10. The re-epithelialization, which progressed
from the surrounding
wound margins toward center, appeared to be complete on post-bum day 26 2
(n=4) in wounds
treated with peptide 13125 (41-65) whereas the wounds treated with vehicle
showed 70 10% (n=4) re-
epithelialization by this time (Fig. 6A). Healing of wounds treated without
peptide 0125 (41-65) or
vehicle was similar to that of the vehicle-only group. Wounds treated with
peptide 0125 (41-65)
exhibited less contraction than those treated with vehicle only (Fig. 6B). On
post-burn day 33,
cutaneous burns treated with peptide 0125 (41-65) and vehicle only exhibited
50 4 (n=4) and 70
2% (n=4) contraction, respectively (Fig. 6B). On post-bum day 34, the wounds
treated with vehicle
only exhibited a large area of open Nound, whereas the wound treated with
peptide 0125 (41-65)
showed very little open wound (Fig. 7 right C and D). On post-burn day 35,
less scarring was
seen in wounds treated with Peptide 0125 (41-65) than in the vehicle-only
control wounds
(Fig. 7 left E and F). The volumes of the scar tissue (on post-burn day 41) in
wounds treated
-23-
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
with peptide 13125 (41-65) and vehicle were 0.07 0.02 and 0.40 0.05 cm3,
respectively. As before,
the non-treated controls were indistinguishable from the vehicle-only group
(data not shown). These
results indicate that Peptide 13125 (41-65) treatment accelerates re-
epithelialization and reduces scarring
in the pig burn injury model.
[067] To test the effect of synthetic peptide 13125 (41-65) on scar formation
after a different type of
standardized injury in pigs, six full-thickness of skin (3 x 3 cm) were
removed from the back of pigs.
A thin layer of sterile gel containing peptide 13125 (41-65) (1.5 mM) or
buffer was applied onto the
wound immediately after the excision injury and every two days for the first
10 days and twice a week
for the remaining experimental days. The dimensions of each wound were
measured each time prior
to the application of TGF1 peptantagonist or vehicle. The peptide 13125 (41-
65) treatment attenuated
contraction of the wound (Fig. 8). In contrast to the burn injury, the
excision injury wound exhibited
near complete horizontal (width of the healed wound) contraction by post-burn
day 30 (Fig. 9). The
wound treated with peptide 13125 (41-65) showed less vertical (length of the
healed wound) contraction
compared with that treated with vehicle only (Fig. 9C and D). On post-incision
day 41, less scar
formation was observed in the wound treated with peptide 13125 (41-65) (Fig.
9C and D).
[068] Accumulation of extracellular matrix proteins such as type I collagen
and fibronectin is
responsible for wound contraction and scar formation (Refs. 8-12). TGF¨f3 is
known to mediate the
deposition of such extracellular matrix proteins by stimulating their
biosynthesis and attenuating their
degradation. Therefore, the content of type I collagen and fibronectin in
excision-injury wounds (on
post-excision day 30) in pigs was determined using immunohistochemistry. As
shown in Fig. 10,
peptide 0125 (41-65) treatment diminished the deposition of type I collagen
and fibronectin (Fig. 10C
vs 10D and 10E vs 10F, respectively).
[069] The effect of the peptide 13125 (41-65) was examined on scar formation
after excision injury in
the rabbit, which is another art-standard model of wound healing (Ref. 18). As
shown in Fig. 11, the
peptide p,25 (41-65) treatment attenuated scar formation after rabbit ear
excision injury on post-
excision day 10, whereas the wounds treated with vehicle only controls
exhibited significant
formation of scars. The volumes of scar tissue on post-excision day 10 in
wounds treated with
peptide 13125 (41-65), vehicle only, and without peptide Pi25 (41-65) or
vehicle were estimated to be
0.005 0.01 (n = 6), 0.05 0.01 (n = 6) and 0.04 0.02 (n = 6) cm3,
respectively. There were no
apparent deleterious effects of peptide [3125 (41-65) or gel in any animal.
[070] Thus it has been demonstrated that a specific synthetic peptide 13125
(41-65) accelerates re-
epithelialization and reduces wound contraction and scarring in the pig burn
injury model and
diminishes wound contraction and scarring in both the pig and rabbit excision
injury models. The
-24 -
CA 02484994 2004-10-28
WO 03/093293
PCT/US03/11437
finding that re-epithelialization is accelerated by the peptide (3125 (41-65)
is somewhat unexpected.
Burn wound healing consists of epithelialization, contraction and formation of
granulation and scar
tissue (Refs. 8-12). TGF¨P is believed to be involved in most of these events.
The antagonist peptide
pi 25 (41-65) is thought to block or slow down the occurrence of these events.
The mechanism of
enhanced re-epithelialization in wounds treated with the peptide 13125 (41-65)
remains to be
determined, but may involve increased keratinocyte proliferation (transient
inhibition of keratinocyte
proliferation by TGF--13 may be an integral component in the complex process
of wound healing)
coupled with a migration response stimulated by growth factors other than
TGF¨P (Refs. 18-21). The
peptide pi25 (41-65), which was recently shown to block complex formation
between a2-
macroglobulin and growth factors, cytokines and hormones (Ref. 22), may
enhance activation of these
substances or agents by blocking inhibition of their activities mediated by a2-
macroglobulin.
- 25 -
CA 02484994 2015-08-28
SEQUENCE LISTING
<110> ST. LOUIS UNIVERSITY
<120> PEPTIDE ANTAGONISTS OF TGF-BETA FAMILY MEMBERS AND
THERAPEUTIC USES THEREOF
<130> PCA23100
<140> 2,484,994
<141> 15-04-2003
<150> PCT/US03/11437
<151> 15-04-2003
<150> US 10/135,946
<151> 29-04-2002
<160> 11
<170> PatentIn Vet. 2.0
<210> 1
<211> 112
<212> PRT
<213> Homo sapiens
<400> 1
Ala Leu Asp Thr An Tyr Cys Phe Ser Ser Thr Glu Lys Asn Cys Cys
1 5 10 15
Val Arg Gin Leu Tyr Ile Asp Phe Arg Lys Asp Leu Gly Trp Lys Trp
20 25 30
Ile His Glu Pro Lys Gly Tyr His Ala Asn Phe Cys Leu Gly Pro Cys
35 40 45
Pro Tyr Ile Trp Ser Leu Asp Thr Gin Tyr Ser Lys Val Leu Ala Leu
50 55 60
Tyr Asn Gin His Asn Pro Gly Ala Ser Ala Ala Pro Cys Cys Val Pro
65 70 75 80
Gin Ala Leu Glu Pro Leu Pro Ile Val Tyr Tyr Val Gly Arg Lys Pro
85 90 95
Lys Val Glu Gin Leu Ser Asn Met Ile Val Arg Ser Cys Lys Cys Ser
100 105 110
<210> 2
<211> 112
<212> PRT
<213> Homo sapiens
<400> 2
Ala Leu Asp Ala Ala Tyr Cys Phe Arg Asn Val Gin Asp Asn Cys Cys
1 5 10 15
26-11
CA 02484994 2015-08-28
Leu Arg Pro Leu Tyr Ile Asp Phe Lys Arg Asp Leu Gly Trp Lys Trp
20 25 30
Ile His Glu Pro Lys Gly Tyr Asn Ala Asn Phe Cys Ala Gly Ala Cys
35 40 45
Pro Tyr Leu Trp Ser Ser Asp Thr Gin His Ser Arg Val Leu Ser Leu
50 55 60
Tyr Asn Thr Ile Asn Pro Glu Ala Ser Ala Ser Pro Cys Cys Val Ser
65 70 75 80
Gin Asp Leu Glu Pro Leu Thr Ile Leu Tyr Tyr Ile Gly Lys Thr Pro
85 90 95
Lys Ile Glu Gin Leu Ser Asn Met Ile Val Lys Ser Cys Lys Cys Ser
100 105 110
<210> 3
<211> 112
<212> PRT
<213> Homo sapiens
<400> 3
Ala Leu Asp Thr Asn Tyr Cys Phe Arg Asn Leu Glu Glu Asn Cys Cys
1 5 10 15
Val Arg Pro Leu Tyr Ile Asp Phe Arg Gin Asp Leu Gly Trp Lys Trp
20 25 30
Val His Glu Pro Lys Gly Tyr Tyr Ala Asn Phe Cys Ser Gly Pro Cys
35 40 45
Pro Tyr Leu Arg Ser Ala Asp Thr Thr His Ser Thr Val Leu Gly Leu
50 55 60
Tyr Asn Thr Leu Asn Pro Glu Ala Ser Ala Ser Pro Cys Cys Val Pro
65 70 75 80
Gin Asp Leu Glu Pro Leu Thr Ile Leu Tyr Tyr Val Gly Arg Thr Pro
85 90 95
Lys Val Glu Gin Leu Ser Asn Met Val Val Lys Ser Cys Lys Cys Ser
100 105 110
<210> 4
<211> 25
<212> PRT
<213> Homo sapiens
<400> 4
Ala Asn Phe Cys Leu Gly Pro Cys Pro Tyr Ile Trp Ser Leu Asp Thr
1 5 10 15
26-/2
CA 02484994 2015-08-28
Gin Tyr Ser Lys Val Leu Ala Leu Tyr
20 25
<210> 5
<211> 25
<212> PRT
<213> Human
<400> 5
Ala Asn Phe Cys Ala Gly Ala Cys Pro Tyr Leu Trp Ser Ser Asp Thr
1 5 10 15
Gin His Ser Arg Val Leu Ser Leu Tyr
20 25
<210> 6
<211> 25
<212> PRT
<213> Homo sapiens
<400> 6
Ala Asn Phe Cys Ser Gly Pro Cys Pro Tyr Leu Arg Ser Ala Asp Thr
1 5 10 15
Thr His Ser Thr Val Leu Gly Leu Tyr
20 25
<210> 7
<211> 10
<212> PRT
<213> Homo sapiens
<400> 7
Pro Tyr Ile Trp Ser Leu Asp Thr Gin Tyr
1 5 10
<210> 8
<211> 10
<212> PRT
<213> Homo sapiens
<400> 8
Pro Tyr Leu Trp Ser Ser Asp Thr Gin His
1 5 10
<210> 9
<211> 10
<212> PRT
<213> Homo sapiens
<400> 9
26-/3
CA 02484994 2015-08-28
Pro Tyr Leu Arg Ser Ala Asp Thr Thr His
1 5 10
<210> 10
<211> 4
<212> PRT
<213> Homo sapiens
<220>
<221> PEPTIDE
<222> (1)..(4)
<223> Residue 3 is any amino acid
<400> 10
Trp Ser Xaa Asp
1
<210> 11
<211> 4
<212> PRT
<213> Homo sapiens
<220>
<221> PEPTIDE
<222> (1)..(4)
<223> Residue 3 is any amino acid
<400> 11
Arg Ser Xaa Asp
1
26-/4