Note: Descriptions are shown in the official language in which they were submitted.
CA 02485472 2004-10-20
1
GH SECRETAGOGUES AND USES THEREOF
FIELD OF THE INVENTION
The invention relates to growth hormone (GH)
secretagogues, such as GH releasing factor (GRE') and analogs
thereof, and uses thereof.
BACKGROUND OF THE INVENTION
Syndromes associated with fat accumulation
Physiological systems include a number of lipid
compounds /molecules such as triglycerides and sterols (e.g.
cholesterol), and associated carriers/complexes such as HDL,
LDL, etc. Adverse levels or metabolism of such molecules is
associated with related conditions such as fat accumulation,
and associated disease.
Fat accumulation is observed in a range of
conditions or syndromes such as obesity, metabolic syndrome,
and the recently described HIV-related lipodystrophy
syndrome. All these conditions include features which are
known to increase the risk of diabetes and/or cardiovascular
diseases.
The metabolic syndrome, also known as syndrome X,
affect persons with frank obesity as well as those with an
increased amount of abdominal fat, and is characterized by
insulin resistance, dyslipidemia (hypertriglyceridemia, low
serum HDL cholesterol levels, and increased LDL cholesterol
levels) and hypertension.
HIV-infected patients treated with highly active
antiretroviral therapy (HAART) commonly experience changes
in fat distribution that include increased visceral and
central fat accumulation (1), as well as loss of extremity
CA 02485472 2004-10-20
2
and subcutaneous fat (especially in the facial fat pads,
limbs and buttocks) in association with insulin resistance
and dyslipidemia (2, 3). Recent data suggest increased
cardiovascular disease and myocardial infarction rates in
patients treated with prolonged antiretroviral therapy (ART)
(4). In non HIV-infected patients (5) and among HIV-infected
patients with changes in fat distribution (6), increased
waist to hip ratio (WHR) and central fat accumulation is
related to increased metabolic risk indices.
Growth Hormone is known for its lipolytic
properties, and its potential role in reversing several of
the body fat and associated metabolic abnormalities has been
actively studied. Beneficial effects have been shown in GH-
deficient individuals (Gotherstrom G. et al., J din
Endocrinol Metab 2001 86(10):4657-4665) and non-HIV patients
with abdominal obesity (Johannsson G, et al., J Clin
Endocrinol Metab 1997, 82(3):727-734). Recent studies also
suggest that that GH levels are reduced in HIV-infected
patients, and correlate inversely with excess visceral fat
accumulation (7, 8). Studies using higher dose,
pharmacologic GH administration have resulted in reduced
visceral adiposity in this population, but are associated
with increased insulin resistance and side effects (9-12).
Cognitive function
Cognitive abilities are impaired in a number of
conditions including advancing age. Deleterious changes
observed with aging affect particularly fluid intelligence,
or abilities involving concept formation, rule discovery,
planning behavior, and non-verbal reasoning. Conversely,
crystallized intelligence, or abilities dependent upon
accumulated experience and education is relatively resistant
to age-related decline. It has been suggested that the
CA 02485472 2004-10-20
3
decline in GH and IGF-1 observed with aging contribute to
the impaired cognitive function.
Evidence exists from both animal and human studies
that administration of GRF, GH or IGF-1 has significant
effect on cognitive functions in conditions where these
functions are impaired. For example, this has been
demonstrated with GH therapy in GH-deficient adults (Deijen
JB, et al., Psychoneuroendocrinology 1998 23(1):45-55), and
with administration of IGF-l or GRF in the healthy elderly
(Aleman A et al., J Clin Endocrinol Metab 1999 84(2):471-
475; Vitiello M.V., et al., Gerontologist 2002 40(Special
Issue 1):39).
Immune Function
Aging is accompanied by diminished circulating GH
and IGF-1 levels observed in parallel with a declined
function of the immune system, particularly affecting the T-
cell mediated immunity. The age-related T-cell immune
deficiency has been partly attributed to a progressive
atrophy of the thymus gland and is considered to be causally
related to the increased risk and severity of acquired
infections observed in the elderly.
GH and IGF-1 are known to play an integrating role
in the development and function of the immune system, as
endocrine and/or autocrine/paracrine factors, and their
administration has been shown to reverse age-related immune
changes. Immune enhancing effects of these factors have been
investigated in other immune deficiency states and
encouraging results have been observed in HIV-positive
patients (Napolitano LA, et al., AIDS 2002 16(8):1103-1111)
and in animal models of radiotherapy preceding bone marrow
transplantation (Sun R, et al., BMT Meetings, Feb 22-26
Orlando, FL, Abstract 27 2002:68-69).
CA 02485472 2004-10-20
4
Catabolism or Muscle Wasting
Muscle protein catabolism, or muscle wasting,
accompanies many diseases including all critical illness,
regardless of the primary cause of disease. It is an
important factor for the long-term prognosis and the length
of hospital stay and recovery, and may also be a limiting
factor for survival. Although many therapeutic tools have
been investigated including specific nutritional treatment,
there is still a strong need for more effective strategies
to counteract protein catabolism.
Previous studies have reported that GH treatment
increases muscle mass in older patients. The anabolic
effects or abilities of GH to reverse or attenuate muscle
wasting have been investigated in several patient groups. GH
has been shown to improve nitrogen balance, an index of net
whole-body protein balance, after major gastro-intestinal
surgery, burn injury, or major trauma. Anabolic effects have
been translated into clinical benefits in COPD patients
(improvement of the maximal inspiratory pressure) (Papte GS,
et al., Chest 1991 99(6):1495-1500) and elderly patient
undergoing surgery following hip fracture (improvement of
functional recovery defined as return to independence) (Van
der Lely AJ, et al., Eur J Endocrinol 2000 143(5):585-592).
Finally, rGH has been recently approved for management of
AIDS-wasting based on results showing increased body weight,
lean body mass and functional performance following 12 weeks
of treatment (Schambelan M, et al., Ann Intern Med 1996
125(11):873-882).
CA 02485472 2004-10-20
However, the use of GH to treat conditions such as
those noted above, has been associated with adverse side
effects in some cases.
5 SUMMARY OF THE INVENTION
The invention relates to GH secretagogues (e.g.
GRF and analogs thereof) and uses thereof.
Therefore, in a first aspect, the invention
provides a method of altering a lipid parameter in a
subject, said method comprising administering to said
subject an agent selected from the group consisting of: (a)
a growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier.
In a further aspect, the invention provides a
package comprising an agent selected from the group
consisting of (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for altering a lipid parameter in a subject.
In a further aspect, the invention provides a use
of an agent selected from the group consisting of (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier; for altering a lipid parameter in a
subject.
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for altering a lipid parameter in a subject.
In a further aspect, the invention provides a
method of altering a first body composition parameter of a
subject, the method comprising administering to said subject
CA 02485472 2004-10-20
6
an agent selected from the group consisting of (a) a growth
hormone (GH) secretagogue; and (b) a composition comprising
a GH secretagogue and a pharmaceutically acceptable carrier.
In a further aspect, the invention provides a
package comprising an agent selected from the group
consisting of: (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for altering a first body composition parameter
of a subject.
In a further aspect, the invention provides a use
of an agent selected from the group consisting of: (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier; for altering a first body composition
parameter of a subject.
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for altering a first body composition parameter
of a subject.
In a further aspect, the invention provides a
method of treating a condition characterized by deficient or
decreased bone formation in a subject, said method
comprising administering to said subject an agent selected
from the group consisting of: (a) a growth hormone (GH)
secretagogue; and (b) a composition comprising a GH
secretagogue and a pharmaceutically acceptable carrier.
In a further aspect, the invention provides a
package comprising an agent selected from the group
consisting of: (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
CA 02485472 2004-10-20
7
instructions for treating a condition characterized by
deficient or decreased bone formation in a subject.
In a further aspect, the invention provides a use
of an agent selected from the group consisting of: (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier; for treating a condition characterized
by deficient or decreased bone formation in a subject.
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for treating a condition characterized by
deficient or decreased bone formation in a subject.
In further aspects, the invention relates to a
method for (1) stimulating day-time vigilance and/or
cognitive functions e.g. in conditions related to aging or
mild cognitive impairment, (2) improving metabolic
conditions associated with fat accumulation and/or
hypercholesterolemia, (e.g. metabolic conditions including
obesity, HIV-related lipodystrophy, metabolic syndrome or
syndrome X), (3) improving anabolism in catabolic/wasting
conditions (such as those observed in Chronic Renal Failure,
congestive heart failure AIDS, following hip fracture,
trauma, or major surgery, particularly in elderly subjects),
and/or (4) improving immune function or reconstitution of
immunodeficient states such as that associated aging, HIV or
following high-dose chemotherapy and/or radiotherapy; the
method comprising admininistering a GH secretagogue (e.g.
GRF and analogs thereof) or a composition comprising a GH
secretagogue and a pharmaceutically acceptable carrier; to a
subject.
In further aspects, the invention relates to uses
of a GH secretagogue (e.g. GRF and analogs thereof) or a
composition comprising a GH secretagogue and a
CA 02485472 2004-10-20
8
pharmaceutically acceptable carrier, for (1) stimulating
day-time vigilance and/or cognitive functions e.g. in
conditions related to aging or mild cognitive impairment,
(2) improving metabolic conditions associated with fat
accumulation and/or hypercholesterolemia, (e.g. metabolic
conditions including obesity, HIV-related lipodystrophy,
metabolic syndrome or syndrome X), (3) improving anabolism
in catabolic/wasting conditions (such as those observed in
Chronic Renal Failure, congestive heart failure AIDS,
following hip fracture, trauma, or major surgery,
particularly in elderly subjects), and/or (4) improving
immune function or reconstitution of immunodeficient states
such as that associated aging, HIV or following high-dose
chemotherapy and/or radiotherapy.
In further aspects, the invention similarly
relates to a package comprising an agent selected from the
group consisting of: (a) a growth hormone (GH) secretagogue;
and (b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for (1) stimulating day-time vigilance and/or
cognitive functions (2) improving metabolic conditions
associated with fat accumulation and/or
hypercholesterolemia, (3) improving anabolism in
catabolic/wasting conditions, and/or (4) improving immune
function or reconstitution of immunodeficient states.
In further aspects, the invention similarly
relates to a use of a GH secretagogue for the preparation of
a medicament for (1) stimulating day-time vigilance and/or
cognitive functions (2) improving metabolic conditions
associated with fat accumulation and/or
hypercholesterolemia, (3) improving anabolism in
catabolic/wasting conditions, and/or (4) improving immune
function or reconstitution of immunodeficient states.
CA 02485472 2011-06-28
9
In accordance with the present invention, there is
provided a composition for (1) altering a lipid parameter in
a subject; (2) altering a body composition parameter in a
subject, (3) treating a condition characterized by deficient
or decreased bone formation in a subject (4) improving
daytime vigilance and/or cognitive function in a subject,
(5) improving a metabolic condition in a subject, (6)
improving anabolism in a catabolic condition in a subject,
and/or (7) improving and/or reconstituting immune function
in a subject, the composition comprising an effective amount
of a GH secretagogue (e.g. GRF compound or analog thereof)
in association with a pharmaceutically acceptable carrier,
excipient or diluent.
In embodiments, the metabolic condition is
associated with fat accumulation and/or
hypercholesterolemia, e.g. obesity, HIV-related
lipodystrophy, metabolic syndrome and syndrome X.
In embodiments the catabolic condition is related
to one selected from the group consisting of chronic renal
failure, AIDS, hip fracture, trauma or major surgery in a
subject.
In another aspect, the present invention provides
a use of (hexenoyl trans-3)hGRF(1-44)NH2 for altering a
first body composition parameter of a subject with no or
substantially no decrease in a second body composition
parameter of said subject and without significantly
impairing glucose control in said subject, wherein said
alteration of a first body composition parameter is:
(a) an increase in lean body mass;
(b) a decrease in trunk fat;
CA 02485472 2011-06-28
9a
(c) a decrease in visceral fat;
(d) a decrease in abdominal girth;
(e) a decrease in visceral abdominal tissue (VAT);
(f) a decrease in VAT:SAT ratio; or
(g) any combination of (a) to (f);
wherein said second body composition parameter is:
(i) limb fat;
(ii) subcutaneous fat;
(iii)subcutaneous abdominal tissue (SAT); or
(iv) any combination of (i) to (iii);
wherein said use is associated with an alteration of one or
more of the following lipid parameters:
(I) a decrease in cholesterol;
(II) a decrease in non-HDL cholesterol;
(III) a decrease in triglyceride; or
(IV) a decrease in the ratio of total cholesterol:HDL
cholesterol.
In another aspect, the present invention provides
a use of (hexenoyl trans-3)hGRF(1-44)NH2 for the preparation
of a medicament for altering a first body composition
parameter of a subject with no or substantially no decrease
in a second body composition parameter of said subject and
without significantly impairing glucose control in said
subject, wherein said alteration of a first body composition
parameter is:
(a) an increase in lean body mass;
(b) a decrease in trunk fat;
(c) a decrease in visceral fat;
(d) a decrease in abdominal girth;
(e) a decrease in visceral abdominal tissue (VAT);
CA 02485472 2011-06-28
9b
(f) a decrease in VAT:SAT ratio; or
(g) any combination of (a) to (f);
wherein said second body composition parameter is:
(i) limb fat;
(ii) subcutaneous fat;
(iii)subcutaneous abdominal tissue (SAT); or
(iv) any combination of (i) to (iii);
wherein said use is associated with an alteration of one or
more of the following lipid parameters:
(I) a decrease in cholesterol;
(II) a decrease in non-HDL cholesterol;
(III) a decrease in triglyceride; or
(IV) a decrease in the ratio of total cholesterol:HDL
cholesterol.
In another aspect, the present invention provides a
composition for altering a first body composition parameter
of a subject with no or substantially no decrease in a
second body composition parameter of said subject and
without significantly impairing glucose control in said
subject, wherein said alteration of a first body composition
parameter is:
(a) an increase in lean body mass;
(b) a decrease in trunk fat;
(c) a decrease in visceral fat;
(d) a decrease in abdominal girth;
(e) a decrease in visceral abdominal tissue (VAT);
(f) a decrease in VAT:SAT ratio; or
(g) any combination of (a) to (f);
wherein said second body composition parameter is:
(i) limb fat;
(ii) subcutaneous fat;
CA 02485472 2011-06-28
9c
(iii)subcutaneous abdominal tissue (SAT); or
(iv) any combination of (i) to (iii);
wherein said alteration of a first body composition is
associated with an alteration of one or more of the
following lipid parameters:
(I) a decrease in cholesterol;
(II) a decrease in non-HDL cholesterol;
(III) a decrease in triglyceride; or
(IV) a decrease in the ratio of total cholesterol:HDL
cholesterol; and
said composition comprising (hexenoyl trans-3)hGRF(1-44)NH2
and a pharmaceutically acceptable carrier.
In another aspect, the present invention provides a use
of (hexenoyl trans-3)hGRF(1-44)NH2 for altering a lipid
parameter associated with HIV-related lipodystrophy in a
subject without significantly impairing glucose control in
said subject, wherein said alteration of a lipid parameter
is:
(a) a decrease in cholesterol;
(b) a decrease in non-HDL cholesterol;
(c) a decrease in triglyceride;
(d) a decrease in the ratio of total
cholesterol:HDL cholesterol; or
(e) any combination of (a) to (d).
In another aspect, the present invention provides a use
of (hexenoyl trans-3)hGRF(1-44)NH2 for the preparation of a
medicament for altering a lipid parameter associated with
HIV-related lipodystrophy in a subject without significantly
impairing glucose control in said subject, wherein said
alteration of a lipid parameter is:
(a) a decrease in cholesterol;
(b) a decrease in non-HDL cholesterol;
(c) a decrease in triglyceride;
CA 02485472 2011-06-28
9d
(d) a decrease in the ratio of total
cholesterol:HDL cholesterol; or
(e) any combination of (a) to (d).
In another aspect, the present invention provides
a composition for altering a lipid parameter associated with
HIV-related lipodystrophy in a subject without significantly
impairing glucose control in said subject, wherein said
alteration of a lipid parameter is:
(a) a decrease in cholesterol;
(b) a decrease in non-HDL cholesterol;
(c) a decrease in triglyceride;
(d) a decrease in the ratio of total cholesterol:HDL
cholesterol; or
(e) any combination of (a) to (d),
said composition comprising (hexenoyl trans-3)hGRF(1-44)NH2
and a pharmaceutically acceptable carrier.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 illustrates the differences between treatment groups
in changes from baseline to week 2 in the mean reaction time
of the Continuous Performance Test (CPT);
Fig. 2 illustrates changes from baseline to day 9 in Pz
amplitude of evoked related potential (P300) during
wakefulness;
CA 02485472 2004-10-20
Fig. 3 illustrates mean AUC of antigen-specific
proliferative T cell response;
Fig. 4 illustrates the percentage of subjects with
5 protective antibody titers (>1/40) for B/Victoria; and
Fig. 5 illustrates the variation of mean IGF-l levels during
time with placebo, 2mg/day TH9507, 0.5 mg/day TH9507 and
1mg/day TH9507.
Fig. 6 illustrates a flow diagram of patient disposition in
respect of the studies described in Example 6.
Fig. 7 illustrates results of dose response of TH9507 on A)
IGF-I and B) truncal fat, as measured by DEXA, as described
in Example 6. 7A: "ng/ml" represents ng/ml of IGF-1 as
measured. 7B: "kg" represents change in truncal fat, with
the negative values indicating a decrease in truncal fat.
7A and 7B: "placebo", "1 mg" and "2 mg" correspond to
administration of a placebo, 1 mg of Th9507 and 2 mg of
Th9507, respectively. Results are mean (SD) . *=P<0.01 vs.
placebo by ANOVA.
DETAILED DESCRIPTION OF THE INVENTION
In the studies described herein, the effect of
(hexenoyl trans-3)hGRF(1-44)NH2 (also referred to as TH9507
herein), a growth hormone releasing factor (GRF) analog,
was assessed over 12 weeks in HIV-infected men and women
with evidence of fat redistribution and increased truncal
adiposity. The data herein demonstrate significant effects
of TH9507 to increase lean body mass and reduce truncal fat
and visceral fat and improve lipid parameters, sparing
CA 02485472 2004-10-20
11
extremity and subcutaneous fat (Example 6) . These effects
were achieved with physiological increases in GH secretion
and without adverse effects on blood glucose levels.
The studies described herein further demonstrate
beneficial effects on bone markers.
The studies described herein further demonstrate a
beneficial effect of Th9507 on daytime vigilance and
cognitive function (Example 2), immune response (Example 3),
wasting/catabolic conditions (Example 4) and non-HDL
cholesterol (Example 5).
Accordingly, in a first aspect, the invention
provides a method of altering a lipid parameter in a
subject, said method comprising administering to said
subject an agent selected from the group consisting of: (a)
a growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier. In an embodiment, the method results in
no or substantially no increase in blood glucose levels in
said subject.
In a further aspect, the invention provides a
package comprising an agent selected from the group
consisting of (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for altering a lipid parameter in a subject.
In an embodiment, the above-mentioned alteration of a lipid
parameter results in no or substantially no increase in
blood glucose levels in said subject.
In a further aspect, the invention provides a use
of an agent selected from the group consisting of (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier; for altering a lipid parameter in a
CA 02485472 2004-10-20
12
subject. In an embodiment, the above-mentioned alteration
of a lipid parameter results in no or substantially no
increase in blood glucose levels in said subject.
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for altering a lipid parameter in a subject.
In an embodiment, the above-mentioned alteration of a lipid
parameter results in no or substantially no increase in
blood glucose levels in said subject.
In an embodiment, the above-noted the alteration
of a lipid parameter is selected from the group consisting
of: (a) a decrease in cholesterol; (b) a decrease in non-HDL
cholesterol; (c) a decrease in triglyceride; (d) a decrease
in the ratio of total cholesterol:HDL cholesterol; and (e)
any combination of (a) to (d).
In an embodiment, the above-noted lipid parameter
is associated with a condition selected from the group
consisting of lipodystrophy, lipohypertrophy, obesity,
dyslipidemia, hypertriglyceridemia and syndrome X.
In a further aspect, the invention provides a
method of altering a first body composition parameter of a
subject, the method comprising administering to said subject
an agent selected from the group consisting of (a) a growth
hormone (GH) secretagogue; and (b) a composition comprising
a GH secretagogue and a pharmaceutically acceptable carrier.
In an embodiment, the method results in no or substantially
no decrease in a second body composition parameter of the
subject, wherein the second body composition parameter is
selected from the group consisting of: (i) limb fat; (ii)
subcutaneous fat; (iii) subcutaneous abdominal tissue (SAT);
and (iv) any combination of (i) to (iii).
In a further aspect, the invention provides a
package comprising an agent selected from the group
CA 02485472 2004-10-20
13
consisting of: (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for altering a first body composition parameter
of a subject. In an embodiment, the above-mentioned
alteration of a first body composition parameter results in
no or substantially no decrease in a second body composition
parameter of the subject, wherein the second body
composition parameter is selected from the group consisting
of (i) limb fat; (ii) subcutaneous fat; (iii) subcutaneous
abdominal tissue (SAT); and (iv) any combination of (i) to
(iii).
In a further aspect, the invention provides a use
of an agent selected from the group consisting of: (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
acceptable carrier; for altering a first body composition
parameter of a subject. In an embodiment, the above-
mentioned alteration of a first body composition parameter
results in no or substantially no decrease in a second body
composition parameter of the subject, wherein the second
body composition parameter is selected from the group
consisting of (i) limb fat; (ii) subcutaneous fat; (iii)
subcutaneous abdominal tissue (SAT); and (iv) any
combination of (i) to (iii).
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for altering a first body composition parameter
of a subject. In an embodiment, the above-mentioned
alteration of a first body composition parameter results in
no or substantially no decrease in a second body composition
parameter of the subject, wherein the second body
composition parameter is selected from the group consisting
CA 02485472 2004-10-20
14
of (i) limb fat; (ii) subcutaneous fat; (iii) subcutaneous
abdominal tissue (SAT); and (iv) any combination of (i) to
(iii).
In an embodiment, the above-mentioned alteration
of a first body composition parameter is selected from the
group consisting of: (a) an increase in lean body mass; (b)
a decrease in trunk fat; (c) a decrease in visceral fat; (d)
a decrease in abdominal girth; (e) a decrease in visceral
abdominal tissue (VAT); (f) a decrease in VAT:SAT ratio; and
(g) any combination of (a) to (f).
In an embodiment, the first body composition
parameter is associated with a condition selected from the
group consisting of lipodystrophy, lipohypertrophy, obesity,
dyslipidemia, hypertriglyceridemia and syndrome X.
In an embodiment, the above-mentioned alteration
of a first body composition parameter results in an
improvement of quality of life of the subject.
In a further aspect, the invention provides a
method of treating a condition characterized by deficient or
decreased bone formation or for treating bone dysfunction or
defect, in a subject, said method comprising administering
to said subject an agent selected from the group consisting
of: (a) a growth hormone (GH) secretagogue; and (b) a
composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier.
In a further aspect, the invention provides a
package comprising an agent selected from the group
consisting of: (a) a growth hormone (GH) secretagogue; and
(b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for treating a condition characterized by
deficient or decreased bone formation in a subject.
CA 02485472 2004-10-20
In a further aspect, the invention provides a use
of an agent selected from the group consisting of: (a) a
growth hormone (GH) secretagogue; and (b) a composition
comprising a GH secretagogue and a pharmaceutically
5 acceptable carrier; for treating a condition characterized
by deficient or decreased bone formation in a subject.
In a further aspect, the invention provides a use
of a growth hormone (GH) secretagogue for the preparation of
a medicament for treating a condition characterized by
10 deficient or decreased bone formation in a subject.
In an embodiment, the above-noted condition
characterized by deficient or decreased bone formation is
selected from the group consisting of osteopenia and
osteoporosis.
15 In further aspects, the invention relates to uses
of a GH secretagogue (e.g. GRF and analogs thereof) or a
composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier, for (1) stimulating
day-time vigilance and/or cognitive functions e.g. in
conditions related to aging or mild cognitive impairment,
(2) improving metabolic conditions associated with fat
accumulation and/or hypercholesterolemia, (e.g. metabolic
conditions including obesity, HIV-related lipodystrophy,
metabolic syndrome or syndrome X), (3) improving anabolism
in catabolic/wasting conditions (such as those observed in
Chronic Renal Failure, congestive heart failure AIDS,
following hip fracture, trauma, or major surgery,
particularly in elderly subjects), and/or (4) improving
immune function or reconstitution of immunodeficient states
such as that associated aging, HIV or following high-dose
chemotherapy and/or radiotherapy.
In further aspects, the invention similarly
relates to a package comprising an agent selected from the
CA 02485472 2004-10-20
16
group consisting of: (a) a growth hormone (GH) secretagogue;
and (b) a composition comprising a GH secretagogue and a
pharmaceutically acceptable carrier; together with
instructions for (1) stimulating day-time vigilance and/or
cognitive functions (2) improving metabolic conditions
associated with fat accumulation and/or
hypercholesterolemia, (3) improving anabolism in
catabolic/wasting conditions, and/or (4) improving immune
function or reconstitution of immunodeficient states.
In further aspects, the invention similarly
relates to a use of a GH secretagogue for the preparation of
a medicament for (1) stimulating day-time vigilance and/or
cognitive functions (2) improving metabolic conditions
associated with fat accumulation and/or
hypercholesterolemia, (3) improving anabolism in
catabolic/wasting conditions, and/or (4) improving immune
function or reconstitution of immunodeficient states.
In an embodiment, the above-mentioned
lipodystrophy is HIV-related lipodystrophy.
In an embodiment, the above-mentioned subject is
HIV positive.
In an embodiment, the above-mentioned subject is
receiving antiviral therapy.
In an embodiment, the above-mentioned subject
suffers from a condition selected from the group consisting
of diabetes, glucose intolerance and insulin resistance.
In an embodiment the above-mentioned GH
secretagogue is administered at a dose of about 0.0001 to
about 4 mg, in a further embodiment, about 0.0001 to about 2
mg, in a further embodiment, about 1 mg to about 2mg, in a
further embodiment, about 1 mg and in a further embodiment,
about 2 mg.
CA 02485472 2004-10-20
17
In an embodiment, the agent is administered by a
route selected from the group consisting of intravenous,
oral, transdermal, subcutaneous, mucosal, intramuscular,
intranasal, intrapulmonary, parenteral, intrarectal and
topical. in an embodiment, the agent is administered by a
subcutaneous route.
In an embodiment, the subject is a mammal, in a
further embodiment, a human.
For the purpose of the present invention the
following terms are defined bellow.
The term "analog" is intended to mean a molecule
of different structure but having a biological function
similar to the structures of the GRF or to a biologically
functional fragment thereof which may include
peptidomimetics. Peptidomimetics may be conveniently
prepared by direct chemical synthesis using methods well
known in the art.
The term "subject" is intended to mean any mammal
including, but not limited to, human, canine, feline,
equine, caprine, bovine, porcine and ovine.
The term "cognitive function" is intended to mean
functions including, but not limited to thinking, reasoning,
memory and problem solving.
The term "catabolic/wasting conditions" is
intended to mean condition including, but not limited to,
frail bones, low muscular mass and muscle wasting.
In the present application, the compound
identified as TH9507 is [hexenoyl-trans-3-Tyrl]hGRF(1-44)NH2.
In various aspects, the methods and corresponding
uses and packages described herein comprise administering to
a subject a growth hormone (GH) secretagogue. "GH
secretagogue" as used herein refers to any compound or
molecule, natural or synthetic, which may result in, either
CA 02485472 2004-10-20
18
directly or indirectly, GH secretion and/or an increase in
GH secretion.
In embodiments, the GH secretagogue is a growth
hormone-releasing factor (GRF; also referred to as growth
hormone releasing hormone [GHRHJ) or GRF analog.
In an embodiment, the GRF is human GRF (hGRF).
Human growth hormone-releasing factor (hGRF) is a
peptide of 44 amino acids with a C-terminal NH2 modification,
referred to herein as hGRF(1-44)NH2, and has the following
structure:
Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-
Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-
Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu-NH2
(SEQ ID NO: 2)
Therefore, the amino acid sequence of the just-
noted 44 amino acid form is as follows:
Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-
Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-
Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu (SEQ
ID NO: 3)
The minimum active core comprises the first 29
amino acids of the above sequence, which is referred to
herein as hGRF(1-29)NH2, and has the following structure:
Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-
Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH2
(SEQ ID NO: 4)
Therefore, the amino acid sequence of the just-
noted 29 amino acid form is as follows:
CA 02485472 2004-10-20
19
Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-
Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg (SEQ
ID NO: 5)
The 1-44 and 1-29 forms differ in that the 1-44
form contains the following additional amino acids, which
correspond to positions 30-44 of the 1-44 form:
Gln-Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu
(SEQ ID NO: 6)
In an embodiment, the above-mentioned GRF analog is a
GRF analog of formula A:
X-GRF Peptide (A)
wherein;
the GRF peptide is a peptide of formula B;
Al-A2-Asp-Ala-Ile-Phe-Thr-A8-Ser-Tyr-Arg-Lys-A13-Leu-
A15-Gln-Leu-A18-Ala-Arg-Lys-Leu-Leu-A24-A25-Ile-A27-
A28-Arg-A30-RO (B) (SEQ ID NO: 1)
wherein,
Al is Tyr or" His;
A2 is Val or Ala;
A8 is Asn or Ser;
A13 is Val or Ile;
A15 is Ala or Gly;
A18 is Ser or Tyr;
A24 is Gln or His;
A25 is Asp or Glu;
CA 02485472 2004-10-20
A27 is Met, Ile or Nle;
A28 is Ser or Asn;
A30 is a bond or amino acid sequence of 1 up to 15
residues; and
5
RO is NH2 or NH- (CH2) n-CONH2i with n=1 to 12; and
X is a hydrophobic tail anchored via an amide bond to
the N-terminus of the peptide and the hydrophobic tail
10 defining a backbone of 5 to 7 atoms;
wherein the backbone can be substituted by C1-6 alkyl,
C3-6 cycloalkyl, or C6-12 aryl and the backbone
comprises at least one rigidifying moiety connected to
15 at least two atoms of the backbone;
said moiety selected from the group consisting of
double bond, triple bond, saturated or unsaturated C3-9
cycloalkyl, and C6-12 aryl.
In embodiments, X noted above is selected from the
group consisting of:
CA 02485472 2004-10-20
21
R H C"
H
1 (R=H or CH. or CH2CH,3)
GIs or Iran:
2 (R=H or CHI or CH6CH3)
R
3 (R=H or C1f or C H 13)
co or trans, both as racemic mixtures
or pure a nautiomcric pairs
R
4 :fit: H, tai, or t i~
cis or trams, both as t is mixtt
or p 0nomic pairs
.
it oil
(R or CU3 or CHrCH;
ens or tom= (when R -A H)
/-Oil
R
6 QtFff or CHI or CHrCH,)
cis or tram, both as tr + ntixam
or p e do erk Paks
CA 02485472 2004-10-20
22
7 (R-7H or CH, or CH2CH)
cis or trans, (when R 4" H)
both as racemic mixtures
or pure enantiomeric pairs
8 (R-H or (Hr or CH,CCH)
cis or trans. both as mcemic mixtures
or pure enantiomeric pairs
R
9 or CH,3 or H2 R3)
cis or ,(when R A H)
both as mcemic mixtures
or pum a pirs
R
1D (R orC, 3orc.co vws& R, IQ
R
it (RrHorC i3orCH )
CA 02485472 2004-10-20
23
1
R
12 (R=U or CH. or CH2CH3)
R
13 ( or C11, or C14CH3)
14
In embodiments, A30 noted above is selected from the group
consisting of:
(a) a bond;
(b) an amino acid sequence corresponding to positions
30-44 of a natural GRF peptide, and
(c) said amino acid sequence of (b) having a 1-14
amino acid deletion from its C-terminus.
In embodiments, the above-noted GRF peptide is
selected from the group consisting of:
(a) a polypeptide comprising the amino acid sequence
of SEQ ID NO: 3;
(b) a polypeptide comprising the amino acid sequence
of SEQ ID NO: 5; and
(c) the polypeptide of (a) having a 1 to 14 amino acid
deletion from its C-terminus.
CA 02485472 2004-10-20
24
In an embodiment, the above-noted GRF analog is
(hexenoyl trans-3)hGRF(1-44)NH2.
Methods of preparing the above-described GRF
analogs are described in US patents No. 5,861,379 (Ibea et
al., January 19, 1999); No. 6,020,311 (Brazeau et al.,
February 1, 2000), No. 6,458,764 (Gravel et al., October 1,
2002) and published US application No. 2004/0171534 Al
(Gravel et al., published September 2, 2004).
As noted above, in various embodiments, the above-
mentioned GH secretagogue may be used therapeutically in
formulations or medicaments to effect the above-noted
alterations and to prevent or treat the above-noted
conditions. The invention provides corresponding methods of
medical treatment, in which a therapeutic dose of a GH
secretagogue is administered in a pharmacologically
acceptable formulation, e.g. to a patient or subject in need
thereof. Accordingly, the invention also provides
therapeutic compositions comprising a GH secretagogue and a
pharmacologically acceptable excipient or carrier. In one
embodiment, such compositions include GH secretagogue in a
therapeutically or prophylactically effective amount
sufficient to effect the above-noted alterations and to
treat the above-noted conditions. The therapeutic
composition may be soluble in an aqueous solution at a
physiologically acceptable pH.
A "therapeutically effective amount" refers to an
amount effective, at dosages and for periods of time
necessary, to achieve the desired therapeutic result, such
as to effect the above-noted alterations and to reduce the
progression of the above-noted conditions. A therapeutically
effective amount of a GH secretagogue may vary according to
factors such as the disease state, age, sex, and weight of
the individual, and the ability of the compound to elicit a
CA 02485472 2004-10-20
desired response in the individual. Dosage regimens may be
adjusted to provide the optimum therapeutic response. A
therapeutically effective amount is also one in which any
toxic or detrimental effects of the compound are outweighed
5 by the therapeutically beneficial effects. A
"prophylactically effective amount" refers to an amount
effective, at dosages and for periods of time necessary, to
achieve the desired prophylactic result, such as preventing
or inhibiting the rate of onset or progression of the above-
10 noted conditions. A prophylactically effective amount can be
determined as described above for the therapeutically
effective amount. For any particular, subject, specific
dosage regimens may be adjusted over time according to the
individual need and the professional judgement of the person
15 administering or supervising the administration of the
compositions.
As used herein "pharmaceutically acceptable
carrier" or "excipient" includes any and all solvents,
dispersion media, coatings, antibacterial and antifungal
20 agents, isotonic and absorption delaying agents, and the
like that are physiologically compatible. In one
embodiment, the carrier is suitable for parenteral
administration. Alternatively, the carrier can be suitable
for intravenous, intraperitoneal, intramuscular,
25 subcutaneous, sublingual or oral administration.
Pharmaceutically acceptable carriers include sterile aqueous
solutions or dispersions and sterile powders for the
extemporaneous preparation of sterile injectable solutions
or dispersion. The use of such media and agents for
pharmaceutically active substances is well known in the art.
Except insofar as any conventional media or agent is
incompatible with the active compound, use thereof in the
pharmaceutical compositions of the invention is
CA 02485472 2004-10-20
26
contemplated. Supplementary active compounds can also be
incorporated into the compositions.
Therapeutic compositions typically must be sterile
and stable under the conditions of manufacture and storage.
The composition can be formulated as a solution,
microemulsion, liposome, or other ordered structure suitable
to high drug concentration. The carrier can be a solvent or
dispersion medium containing, for example, water, ethanol,
polyol (for example, glycerol, propylene glycol, and liquid
polyethylene glycol, and the like), and suitable mixtures
thereof. The proper fluidity can be maintained, for example,
by the use of a coating such as lecithin, by the maintenance
of the required particle size in the case of dispersion and
by the use of surfactants. In many cases, it will be
preferable to include isotonic agents, for example, sugars,
polyalcohols such as mannitol, sorbitol, or sodium chloride
in the composition. Prolonged absorption of the injectable
compositions can be brought about by including in the
composition an agent which delays absorption, for example,
monostearate salts and gelatin. Moreover, a GH secretagogue
can be administered in a time release formulation, for
example in a composition which includes a slow release
polymer. The active compounds can be prepared with carriers
that will protect the compound against rapid release, such
as a controlled release formulation, including implants and
microencapsulated delivery systems. Biodegradable,
biocompatible polymers can be used, such as ethylene vinyl
acetate, polyanhydrides, polyglycolic acid, collagen,
polyorthoesters, polylactic acid and polylactic,
polyglycolic copolymers (PLG). Many methods for the
preparation of such formulations are patented or generally
known to those skilled in the art.
CA 02485472 2004-10-20
27
Sterile injectable solutions can be prepared by
incorporating the active compound (e.g. a GH secretagogue )
in the required amount in an appropriate solvent with one or
a combination of ingredients enumerated above, as required,
followed by filtered sterilization. Generally, dispersions
are prepared by incorporating the active compound into a
sterile vehicle which contains a basic dispersion medium and
the required other ingredients from those enumerated above.
In the case of sterile powders for the preparation of
sterile injectable solutions, the preferred methods of
preparation are vacuum drying and freeze-drying which yields
a powder of the active ingredient plus any additional
desired ingredient from a previously sterile-filtered
solution thereof. In accordance with an alternative aspect
of the invention, a GH secretagogue may be formulated with
one or more additional compounds that enhance its
solubility.
In accordance with another aspect of the
invention, therapeutic compositions of the present
invention, comprising a GH secretagogue, may be provided in
containers, kits or packages (e.g. commercial packages)
which further comprise instructions for its use to effect
the above-noted alterations and to prevent or treat the
above-noted conditions.
Accordingly, the invention further provides a
package comprising a GH secretagogue or the above-mentioned
composition together with instructions to effect the above-
noted alterations and to prevent or treat the above-noted
conditions.
The invention further provides a use of a GH
secretagogue to effect the above-noted alterations and to
prevent or treat the above-noted conditions. The invention
further provides a use of a GH secretagogue for the
CA 02485472 2004-10-20
28
preparation of a medicament to effect the above-noted
alterations and to prevent or treat the above-noted
conditions.
Although various embodiments of the invention are
disclosed herein, many adaptations and modifications may be
made within the scope of the invention in accordance with
the common general knowledge of those skilled in this art.
Such modifications include the substitution of known
equivalents for any aspect of the invention in order to
achieve the same result in substantially the same way.
Numeric ranges are inclusive of the numbers defining the
range. In the claims, the word "comprising" is used as an
open-ended term, substantially equivalent to the phrase
"including, but not limited to". The following examples are
illustrative of various aspects of the invention, and do not
limit the broad aspects of the invention as disclosed
herein.
EXAMPLES
Example 1: Study drug
The compound used in the studies below is
(hexenoyl trans-3)hGRF(1-44)NH2 (also referred to as TH9507
herein), which is a synthetic human growth hormone releasing
factor analog that comprises the 44-amino acid sequence of
human growth hormone releasing factor (hGRF) on which a
hexenoyl moiety, a C6 side chain has been anchored on Tyr 1
at the n-terminal. (hexenoyl trans-3)hGRF(1-44)NH2 or Th9507
has the following structure:
(trans)
Gly-Ala-Arg-Ala-Arg-Leu-NH2 (SEQ ID NO: 7)
CA 02485472 2004-10-20
29
(hexenoyl trans-3)hGRF(1-44)NH2 was synthesized via the
methods set forth in US 5,861,379 (Ibea et al.; January 19,
1999).
The in vitro half-life of Th9507 is 3-8 hours
compared to 0.56 hours for hGRF. Daily 1 and 2 mg doses have
been shown to increase IGF-I to the physiological range seen
in younger adults (14). The safety profile of TH9507 is
generally good. Th9507 has been studied in patients with
Type II DM, and was not shown to aggravate overall glycemic
control when administered at a daily dose up to 2 mg (15).
Example 2: Administration of TH9507 for improving daytime
vigilance in subjects with sleep maintenance insomnia
The present example shows the effect of a 14 day-
administration of 2 doses of TH9507 (0.1 mg and 1 mg) on
vigilance parameters in subjects of 35 to 50 years of age
exhibiting sleep maintenance insomnia.
Material and Methods
The study involved 82 patients exhibiting sleep
maintenance insomnia (20 females, 62 males; mean age 43.2
5.4 years). Patients were selected based on the Pittsburgh
Sleep Quality Index (Score ?5), the Walters Criteria for
Sleep Maintenance Insomnia, and the Beck Questionnaire
(Score -<17). The primary exclusion criteria were other
primary sleep disorders and the use of any products
affecting sleep or vigilance in the 30 days prior to
entering the study.
The study was a randomized, double-blind, placebo-
controlled, parallel group and multicenter evaluation of two
CA 02485472 2004-10-20
doses of TH9507 (0.1 mg and 1 mg) administered daily by
subcutaneous injection at bedtime for 14 consecutive days.
To evaluate vigilance and the performance in the morning,
patients underwent a battery of cognitive tests including
5 the Continuous Performance Test (CPT) at baseline and at the
end of treatment period.
The CPT has been described in the literature as a
measure of consistency in responding and ability to sustain
attention over time (Aleman A, et al., J Clin Endocrinol
10 Metab 1999 84(2):471-475). This test required subjects to
press space bar each time the letter "A" was followed by
"X". Omission and commission errors, and Mean Reaction Time
of correct responses were analyzed.
15 Results
Demographic characteristics by treatment group are
displayed in the following table:
Table 1
20 Demographic (screening) data by treatment groups
Placebo 0,lmg N=26 1 mg N=27 P value
N=29
Age
(Mean SD) 44.0 5.8 43.2 5.8 42.3 4.5 0.53
(range) (35-56) (34-60) (34-50) (F-test)
Gender
Males 21 19 22 0.68
Females 8 7 5 chi-square
test)
Weight(kg) 0.94
(Mean SD) 78.0 14.3 78.2 12.4 79.3 15.3 (F-test)
CA 02485472 2004-10-20
31
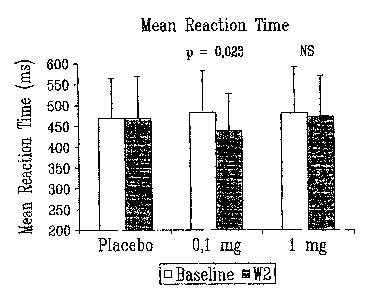
As illustrated in Fig. 1, the Mean Reaction Time
of the CPT was significantly and markedly decreased in the
0.1 mg group when compared to placebo. The decrease from
baseline to day 9 was 45.85 ms (P=0.023 when compared to
placebo as analyzed by an ANOVA model). No significant
effect was observed in the 1 mg group. Circulating IGF-1 and
IGFBP-3 levels were significantly increased at week 2 in the
1 mg group when compared to placebo (P<0.0001, ANOVA on
changes from baseline) As expected the 0.1 mg did not
affect these parameters (P=0.07 and P=0.99) for IGF-l and
IGF-BP3 respectively, ANOVA model on changes from baseline).
Additional data on effects of TH9507 on vigilance
obtained in a previous study are presented in Fig. 2. In
this study, TH9507 was administered daily for 7 days in a
cross-over design. This study involved 12 healthy subjects
aged between 50 to 65 years, exhibiting age-related sleep
impairment (Pittsburgh Sleep Quality Index Score from 3 to
7). At the end of the treatment period, daytime vigilance
was significantly enhanced when compared to placebo in
subjects receiving 1 mg of TH9507, as assessed by P300, an
event-related potential test. Changes from Baseline to day 9
in the Pz amplitude of the Evoked Related Potential (P300)
observed in the placebo and 1 mg group were as follows:
Placebo, -15%; 1 mg, +55%, P=0.0114, as analyzed by an ANOVA
model.
In both studies, the safety profile of TH9507 was
comparable to that of the placebo, except for a higher
incidence of reactions at the site of injection observed at
1 mg in the insomnia study.
In summary, these results provide evidence that
TH9507 improves daytime vigilance in sleep maintenance
insomnia in subjects and would favor a direct mechanism of
action of TH9507, not mediated by IGF-1. Data are supported
CA 02485472 2004-10-20
32
by those obtained by Vitiello et al (Vitiello M.V., et al.,
Gerontologist 2002 40(Special Issue 1):39-N/A. using hGRF in
cognitive tests involving psychomotor and perceptual
processing speed (Deijin JB et al, Psychoneuroendocrinology
1998 23(1):45-55) and may support further clinical
investigations in subjects with impaired cognitive
functions.
Example 3: Effects of TH9507 on the immune response to
influenza vaccination in elderly subjects
The present example describes immune findings
following an influenza vaccination challenge in elderly
subjects.
Material and Methods
Eighty seven (87) subjects aged 75 years in
average were included in a double-bind, randomized, placebo-
controlled study. TH9507 or a placebo was administered at a
daily dose of 1 or 2 mg by subcutaneous injection for 8
weeks. Follow-up assessments were conducted for 12 weeks
after the end of the treatment period. At week 4, in the
middle of the treatment period, subjects received the
commercial Canadian influenza vaccine (Vaccine Fluviral
S/F, Shire, Montreal, Canada) containing 15 pg each of A/New
Caledonia/20/99, A/Panama/2007/99, B/Victoria/504/2000
antigens.
Influenza-specific proliferative T cell response
and antibody titers were evaluated for each of the 3 strains
contained in the vaccine. The proliferative T cell response
was assessed by a mitogen assay using tritiated thymidine
(3H) incorporation and results were log-base 10 transformed
prior to analysis. The antibody titers were determined by
CA 02485472 2004-10-20
33
standard hemaglutinaion inhibition assay and results were
log-base 2 transformed prior to analysis.
Eighty one (81) subjects completed the study as
per protocol. Subject demographics are shown in the
following table:
Table 2
Subject demographics
Placebo 1 mg 2 mg All P-value
TH9507 TH9507
Age 75.9 74.9 73.2 74.6 0.21
(years) 6.5 6.1 4.4 5.8
Total N 29 29 29 187 0.96
Female 13 13 14 40
Male 16 16 15 47
BMI 27.4 26.9 29.2 27.8 0.26
(kg/m2) 5.8 4.2 6.0 5.4
Data for age and BMI are presented as mean SD. Baseline
comparability among treatment groups was tested by ANOVA
(age, BMI) or Pearson's chi-square test (gender).
Results
As shown in Fig. 3 the mean AUC calculated for the
whole study period including both the treatment and follow-
up period (week 0 to week 20) was statistically higher in
the 2 mg group when compared with placebo (Panama, P=0.03;
New Caledonia, P=0.001; Victoria, P=0.02, (Pairwise
comparisons for difference among treatment groups and ANCOVA
analysis for overall treatment significance).
As illustrated in Fig. 4, administration of TH9507
increased the proportion of patients achieving a protective
CA 02485472 2004-10-20
34
antibody level for the Victoria antigen when compared to
placebo. This observation reached statistical significance
at the 2 mg dose and was noted during both the treatment and
the follow-up periods (week 6 : P=0.02; Week 8 : P=0.01;
week 12 : P=0.02; week 16 ; P=0.004, week 20 : P=0.01,
pairwise comparisons for difference among treatment groups
and Pearson chi-square test for overall treatment
difference) indicating a sustained effect for up to 16 weeks
after cessation of treatment. No statistical difference in
the percentage of subjects was observed in the Panama and
New Caledonia strains.
A dose-related increase in the mean IGF-1 values
was observed during the whole treatment period in both
Th9507 groups when compared to baseline. Values returned to
baseline following cessation of treatment.
No major difference in the incidence of adverse
events was observed between treatment groups except for a
dose-related trend in the incidence of reactions at the site
of injection.
In summary, the findings observed in this study
strongly indicate that TH9507 has a therapeutic potential in
immune indications. In particular, its effect of the T-
lymphocyte proliferation response following vaccination
makes it attractive to develop in clinical situations where
the cell-mediated immune'system is depressed, such as viral
infections in the elderly and immune-deficient states
following HIV infection, high-dose chemotherapy or
radiotherapy.
Example 4: ThGRF's benefits in wasting/catabolic conditions
CA 02485472 2004-10-20
The present example shows the effect of a 7-day
administration of TH9507 on circulating IGF-l levels in
healthy middle-aged men.
5 Material and Methods
The study used a randomized, double-blind,
placebo-controlled design and was conducted in healthy men,
aged 50 to 60 years old. Subjects (8 per group) were
injected S.C. once a day for 7 consecutive days with
10 placebo, 0.5, 1 or 2 mg of TH9507. Circulating IGF-l levels
were measured on Days 1 to V. The 12 hour GH response and
TH9507 PK profile were determined on Day 1 and 7.
Results
15 As shown in Fig. 5, IGF-1 increased over
baseline values by 80 (placebo), 370 (0.5 mg), 890 (lmg)
and 106% (2 mg); these increases were statistically
significant for all 3 doses of TH9507. The 1 mg and 2 mg
doses were equally potent and induced a doubling of IGF-1
20 levels up to levels expected for young adults (286 25
and 284 55 ng/ml, respectively), none of the subjects
exhibited levels greater than 400 ng/ml. A plateau was
reached at Day 4 and 6 for the 1 mg and 2 mg doses,
respectively.
25 GH response to TH9507 increased rapidly both on
Days 1 and 2. The increase was dose dependent between the
0.5 and 1 mg dose (P<0.01), and was similar at the 1 mg
and 2 mg doses. No significant modification in prolactin,
ACTH, cortisol, TSH, LH or FSH was observed following
30 single or repeated treatment with TH9507.
PK analysis indicated that Cmax and AUC
parameters increased in function of the dose administered.
The half-life of the TH9507 ranged between 2 and 5 hours.
CA 02485472 2004-10-20
36
These results clearly indicate that TH9507 is
highly specific on GH= secretion and a powerful IGF-1
inducer, suggesting potential clinical benefits in
wasting/catabolic conditions.
Example 5: Effects of GRF on non-HDL cholesterol in
patients with type 2 Diabetes
The present example illustrates beneficial
effects of TH9507 on non-HDL cholesterol levels in a
diabetic population.
Material and Methods
A double-blind placebo-controlled study was
conducted in 53 type II diabetic patients (age = 61 7
[SD]; 34% female) on stable antidiabetic medication (26%
on insulin). Patients were randomized to parallel groups
to receive daily subcutaneous administration of a placebo,
1 mg or 2 mg TH9507, respectively.
Results
A statistically significant difference was
observed at Week 12 between the 3 treatment groups in the
mean total cholesterol change from baseline (P=0.04).
Values decreased in the 2 mg group (-11.1 21.9 mg/dl; -
6%), as compared to increases in the Placebo (+9.7 22.6
mg/dl; +5%) and 1 mg group (+6.1 16.2 mg/dl; +3%). This
effect was accompanied by a decrease in the mean non-HDL
cholesterol values in the 2 mg group (-10.1 19.0 mg/dl;
-7%) and increases in the placebo (+6.8 17.8 mg/dl; +5%)
and 1 mg group (+3.8 15.5 mg/dl; +3%).
No statistically significant differences were
observed between the three groups during the treatment
CA 02485472 2004-10-20
37
period in terms of insulin relative response to an oral
glucose tolerance test. At Week 12, glycosylated
hemoglobin (HbAlc) levels displayed a trend for a decrease
in the placebo group, a decrease in the 1 mg group, and no
change in the 2 mg group. Clinically relevant changes in
antidiabetic medications occurred with a similar incidence
in the three treatment groups.
A dose-related increase in the IGF-l levels was
observed at the end of the treatment period.
In summary, this study indicates that the
repeated administration of TH9507 for 12 weeks decreases
the total and non-HDL cholesterol fraction in diabetic
subjects and can be safely administered to this population
without impairing glucose control. The effects observed on
blood lipids and the known lipolytic properties of GH
warrant the investigation of TH9507 for the treatment of
syndromes associated with visceral fat accumulation.
Example 6: Effect of GRF analog on lipid profile, body
composition and quality of life in HIV-infected patients
This study investigated the effects of TH9507, on
abdominal fat accumulation associated with HIV
lipodystrophy.
Design: Randomized, placebo-controlled, multi-center, dose
ranging (placebo, lmg or 2 mg SC QD), parallel group, 12-
week study
Patients: 61 HIV-infected patients (BMI 28 3 [SD] kg/mz,
28% diabetic or glucose intolerant) with increased waist
circumference (102 cm 8 [SD]) and waist/hip (1.0 0.1
[SD])
CA 02485472 2004-10-20
38
Measurements: Body composition was assessed by DXA and
abdominal CT scan; IGF-I, insulin, glucose, lipid, bone
turnover, immunologic, and safety parameters.
Results: Treatment with TH9507 resulted in a dose-related
increase in serum IGF-1 levels within the physiological
range [18 (32) ng/mL, 87 (67) ng/mL, 123 (79) ng/mL, mean
(SD) for placebo, 1mg, 2 mg, respectively, P<0.01 for change
in each active group vs. placebo]. Lean body mass increased
significantly in both treatment groups compared to placebo
[-0.5 (1.6) kg, 0.7 (2.0) kg, and 1.7 (2.3) kg; placebo,
lmg, 2 mg, respectively; P<0.01 for 2 mg dose vs. placebo,
P<0.05 for the 1 mg group vs. placebo]. Trunk fat decreased
in the 2 mg group compared to placebo (0.8%, -4.6% and -
9.2%; placebo, lmg, 2 mg, respectively, P=0.01 for 2 mg vs.
placebo), with no significant change in limb fat. Visceral
fat decreased most in the 2 mg group (-5.4%, -3.6% and -
15.7%; placebo, 1mg, 2 mg, respectively; P=0.03 for change
within 2 mg group but P=NS vs. placebo) whereas subcutaneous
fat was preserved and did not change between or within
groups. The ratio of VAT: SAT improved significantly in both
treatment. groups compared to placebo (-0.01 (0.10), -
0.23(0.47) and -0.14(0.18), P<0.01 for the 2 mg group vs.
placebo and P<0.05 for the 1 mg group vs. placebo.
Triglyceride and the cholesterol to HDL ratio decreased
significantly in the 2 mg treatment group compared to
placebo. Treatment was generally well-tolerated without
changes in either fasting glucose or 2-hour OGTT.
Conclusion: TH9507 effectively improved body composition and
reduced truncal fat by preferentially decreasing visceral
fat while preserving subcutaneous fat in patients with HIV
lipodystrophy. TH9507 did not increase glucose levels, even
among those with impaired glucose tolerance or diabetes
mellitus at baseline, and improved the lipid profile. These
CA 02485472 2004-10-20
39
data suggest that TH9507, a GRF analog, may be a beneficial
treatment strategy for HIV lipodystrophy.
Methods
Subjects
Eighty-eight subjects were screened between May
2003 and November 2003. Sixty-one subjects were enrolled.
HIV infected males age 18-65 years and non-menopausal
females age > 18 years with visceral fat accumulation
considered to be part of the HIV lipodystrophy syndrome,
waist circumference ? 95 cm for men and ? 94 cm for women,
and a waist to hip ratio ? 0.94 for men and >_ 0.88 for
women were enrolled. We excluded, subjects with a BMI <-20
kg/m2, CD4 cell count <--100 cells/mm3, viral load >_ 10,000
copies/mL, history of opportunistic infection or HIV-related
disease within 3 months of the study, history of prostrate
cancer or PSA > 5 ng/ml in male subjects, history of breast
cancer or abnormal mammography within 6 months of the study
(for these patients without a mammogram within this time
frame, one was performed as part of the study) in female
subjects, known hypopituitarism or history of pituitary
surgery, radiation or significant head trauma, untreated
hypothyroidism, prior history of Type I diabetes mellitus
(DM), any prior use of GH or GH related products,
experimental or marketed, within 6 months of the study,
systemic steroid administration or megestrol acetate within
60 days of the study, fasting glucose > 150 mg/dL, SGOT or
SGPT > 3x upper limit of normal, creatinine > 1.5x upper
limit of normal, or hemoglobin < 9 g/dL. Subjects receiving
testosterone or estrogen within the prior six months, with
known drug or alcohol dependence or who had participated in
another clinical trial with an investigational agent within
30 days were also excluded from the study. All subjects
CA 02485472 2004-10-20
were required to be on a stable antiretroviral regimen for 8
weeks prior to enrollment. Subjects receiving lipid lowering
medications were required to be on a stable regimen for 3
months prior to enrollment. Subjects were not permitted to
5 begin antidiabetic medication, use systemic corticosteroids
for > 10 days, or begin estrogen or testosterone
preparations during the study.
Study Procedures
10 Subjects underwent a screening visit to determine
eligibility. All subjects gave written consent to
participate in the study, and the study was approved by the
Institutional Review Board at each participating site. BMI,
anthropometic measurements, TSH and prolactin were
15 performed. Viral load and CD4 count were performed if not
previously done within 8 weeks of the study. A PSA was
performed in male subjects and a pregnancy test was
performed in female subjects. Mammography was also performed
in female subjects if not previously done within 6 months of
20 the study.
At the baseline visit, anthropometric measurements were
obtained. Total body lean and fat mass as well as regional
fat mass in the trunk and extremities were assessed by DEXA.
Abdominal subcutaneous and visceral fat area and the ratio
25 of VAT to SAT were determined by cross-sectional CT at L4-
L5. Biochemical parameters including insulin-like growth
factor-I, glucose, insulin, lipid profile (cholesterol, HDL,
LDL, triglyceride, non HDL and the ratio of
cholesterol:HDL), free fatty acids, HbAlC, IGFBP-3, and bone
30 markers (serum osteocalcin and NTX-1), CD4, viral load, and
a pregnancy test for female subjects were determined in the
fasting state. In addition, insulin and glucose response to
CA 02485472 2004-10-20
41
a 75 gram standard glucose challenge were assessed and a
quality of life questionnaire was administered.
After baseline evaluations were complete subjects were
randomized equally to one of three groups, placebo, 1 mg
TH9507 or 2 mg TH9507. Randomization was performed by an
independent consulting firm, using a permuted block
algorithm, with block size equal to 3 and kept confidential
in the Theratechnologies Quality Assurance Department until
unblinding. Subjects were stratified for gender at the time
of treatment assignment. Treatment assignment was blinded to
investigators and patients. Active drug and placebo
(mannitol) were provided as lyophilized powder in vials of
identical appearance, each containing 1.1 mg of material.
Each vial was reconstituted with sterile water immediately
prior to injection. Patients were instructed to inject each
AM at approximately 0900 h.
Subjects returned for a safety visit at Week 1 to
assess adverse events and compliance. Subjects returned at 2
weeks to assess weight, anthropometric measures, lipid
profile, free fatty acid, IGFB-1, IGFBP-3, glucose, adverse
events and compliance. Subjects returned for a visit at week
6 identical to baseline and a visit at week 9 to assess
compliance and adverse events. Subjects returned for a last
study visit at week 12 - or, whenever possible, at the time
of early termination - identical to the baseline visit.
Study Drug
TH9507 was used in this study, as noted above.
Methods
Biochemical Indices
Clinical laboratory testing was performed by a central
lab. Serum IGF-1 was measured after acid-ethanol extraction
CA 02485472 2004-10-20
42
using the Esoterix RIA kit (Esoterix Inc., Calabasas Hill,
CA), with a sensitivity of 10 ng/mL. Intra- and inter-assay
coefficients of variation were 4.6-20% and 9-10%,
respectively. Serum IGFBP-3 was measured using the Esoterix
RIA kit (Esoterix Inc., Calabasas Hill, CA) with a
sensitivity of 0.3 mg/mL. Intra- and inter-assay
coefficients of variation were 5.1-13% and 5.5-17%,
respectively. HDL, LDL, total cholesterol, triglycerides and
FFA were determined by enzymatic colorimetric assay. Glucose
was measured by enzymatric test (Gluco-quant , Roche
diagnostics, Indianapolis, IN). HbAlc was determined by
chromatography. PSA was determined using the Hybritech PSA
test (Beckman Coulter, Mississauga, Canada). TSH was
measured by microparticle enzyme immunoassay (AxSYM hTSH II,
Abbott Laboratories, Abbott Park, IL). NTX (N-terminal
telopeptide of type I collagen; a bone resorption marker)
was measured by ELISA using a commercially available kit
(Osteomark't, Ostex International Inc., Seattle, WA).
Osteocalcin was measured using a commercially available
enzymatic immunoassay kit (MetraTM osteocalcin, Quidel
Corporation, San Diego, CA). CD4 and viral load were
performed by the individual site labs by routine
methodology.
HOMA was calculated as:
OInsulin (30min-O min)
Insulin (0min) .
A Glucose (30 min- 0min)
Body Composition
Whole body and regional DEXA (dual energy X-ray
absorptiometry) and cross-sectional abdominal CT scans were
performed at the individual study sites based on previously
established protocols and were standardized across sites.
CA 02485472 2004-10-20
43
Digitized images were sent to a central reading center, St.
Lukes Roosevelt Hospital, for analysis by independent
experts without knowledge of treatment assignment. Each
site was given a procedure manual to follow in obtaining the
body composition data and was certified in the proper
technique. For the CT scans, parameters were standardized as
follows: Tube Voltage = 120 kV, Tube Current = 250 mA,
Exposure = 375 mAs, matrix 512 x 512. DFOV was set to
include the entire slice into the field of view. For the
DEXA scans total body scans were performed with analysis of
lean body mass, total fat mass, and regional fat mass in the
trunk and extremities as previously described (16) . After
analysis the data was transferred to the data management
center and incorporated in the study database.
Statistics
Body composition endpoints included trunk fat and
trunk to limb fat ratio by DEXA and VAT and VAT: SAT by CT
scan. Other endpoints included IGF-I, lipid parameters,
glucose and insulin, CD4, viral load, bone markers, and
quality of life. A sample size of 20 patients/group was
planned to assess a statistically significant change of 10%
or more in intra-group analyses. The planned dropout rate
was 25% and therefore 60 patients were enrolled into the
study. Baseline data are compared between the groups by F-
test based on ANOVA for continuous variables and Chi-Square
test for categorical variables. Change over time between
groups is compared by ANOVA or the Chi-Square test. Where
appropriate, data were rank transformed prior to analysis.
Changes within each group are determined by t-test. The ITT
(intent to treat) population is defined as all subjects who
received at least one dose of the study treatment.
Descriptive statistics and analyses for all efficacy and
CA 02485472 2004-10-20
44
safety analyses are performed on the ITT population. End of
study, 12 week, data are used to calculate changes in body
composition. For biochemical indices, the last observation
available is used to calculate change. Imputation for
missing data is not performed. For body composition, end of
study data are used to calculate change. Interim analyses
were not performed. Results are mean (SD) unless otherwise
noted. All statistical tests were performed with a two-sided
Type I error level of 0.05.
Results
Sixty-one subjects were randomized, 21 to placebo,
19 to TH9507 1 mg and 21 to TH9507 2 mg. Five subjects
discontinued in the placebo group, 2 in the 1 mg group and 6
in the 2 mg group, for a 79% completion rate. See Figure 6
for patient disposition and flow diagram.
Baseline demographic for the three study groups
are shown in Table 3. At baseline no significant differences
were seen between the groups except, including use of
antiretroviral therapy. The percentage of patients with
diabetes (fasting glucose >- 7.0 mmol/L or 2h-glucose -> 11.1
mmol/L) and impaired glucose tolerance (6.1 mmol/L <- fasting
glucose < 6.9 mmol/L or 7.8 mmol/L < 2h-glucose <11.1
mmol/L) was not different between the groups. Among the
entire study group, 23% of subjects demonstrated IGT and 5%
demonstrated diabetes mellitus at baseline.
Table 3: Baseline Characteristics
Placebo 1mg 2mg P
Age 46.1(7.4) 46.5(6.4) 44.6(7.1) 0.64
Gender
Males/Females 18/3 18/1 18/3 NA
CA 02485472 2004-10-20
Ethnic origin
Cauc./Other 18/3 15/4 17/4 0.84
BMI 28.9(2.7) 28.0(3.2) 27.3(4.1) 0.30
WHR 1.0(0.1) 1.0(0.0) 1.0(0.1) 0.65
WC 103.4(8.0) 100.3(6.1) 100.7(9.1) 0.40
IGT-Diabetes 7 4 6 NA
Data are mean (SD). BMI, body-mass index; WHR, waist/hip
ratio; WC, waist circumference
Body Composition
5 Lean body mass increased in both treatment groups
compared to placebo [-0.5 (1.6) kg, 0.7 (2.0) kg, and 1.7
(2.3) kg, mean (SD) for placebo, lmg, 2 mg, respectively,
P<0.01 for change in 2 mg group vs. placebo, P<0.05 for the
change in lmg group vs. placebo, Table 3). Total fat
10 decreased in the 2 mg group compared to placebo [0.3 (1.7)
kg, -0.4 (1.8) kg and -1.4 (2.0) kg, placebo, 1mg, 2 mg,
respectively, P=0.01 for 2 mg group vs. placebo). Trunk fat
decreased in the 2 mg group compared to placebo (0.8%, -4.6%
and -9.2%, placebo, lmg, 2 mg, respectively, P=0.01 for 2 mg
15 group vs. placebo) (Figure 2). VAT tended to decrease more
in the 2 mg group (-5.4%, -3.6% and -15.7%, placebo, 1 mg, 2
mg, respectively), (P=NS for comparison of 2 mg vs. placebo,
P=0.03 for change within 2 mg group). SAT did not change
significantly between the groups and the ratio of VAT:SAT
20 decreased more in the treatment groups compared to placebo
[0.01 (0.10), -0.23 (0.47), -0.14 (0.18)] (Table II) (P<0.01
for 2 mg vs. placebo and P=0.04 for 1 mg vs. placebo).
Biochemical Indices
25 IGF-I increased significantly with the 1 and 2 mg
dose compared to placebo [18 (32) ng/mL, 87 (67) ng/mL, 123
(79) ng/mL, placebo, 1 mg 2mg, respectively, P<0.01 for each
CA 02485472 2004-10-20
46
active group vs. placebo] (Figure 3). Triglyceride levels
decreased in the 2 mg group compared to placebo (-0.2 (1.3),
-0.9 (4.2), -0.9 (1.2), last observation values for placebo,
1 mg, 2 mg, respectively, P<0.05) and the ratio of
cholesterol to HDL improved in both treatment groups
compared to placebo (0.3 (1.1), -0.3 (0.7), -0.3 (0.6) ,
last observation values for placebo, 1 mg, 2 mg,
respectively, P<0.05).
Changes in fasting and 120 minute glucose were not
significant between or within the treatment groups.
Bone Markers
Osteocalcin increased significantly within the 2
mg group, whereas no changes with NTX were seen (Table 4).
CA 02485472 2004-10-20
ti
M
O
II
N r- Ln Ln C) 1-0 N 0
tll W N N C) O O N' N' O C !~ Ol O N' O O
110 110 co O O m M O O O r1 l0 O ri (D
u) N` 0) C- O O fn N' O O M (M (`') O Q0 O O O
C) N ri C) 0 M C)
J O O O O O r1 C) O r-i
04 O O O ri O O 00
O
'H IQ
N N^ (N M O rf co p
3 a '-i Ln N' l0 oo rn `r O M O N M (D M N
N IT C- N .--I Co 0 0 I 61 O O C) p Ov
a) O .--1 r-i (f) N' 0) O p r-
U) O O O 'H L l0 O C
O O O O rl O CD, -1
6 0 0 0 0 0 0 (D' C-) -oo
* i 0
N is ^ .~ ^ r1 ^ NQ
(
-~ O M CO !` co ri O 0 ri O O 0 0
M N p 61 M 'H C
= N ri O 61 Ol rl r1 N p
N r i" r o 0 o U
41 N'rlM = 'H O o
= -i N' Q0 N' I I 0 O 1 I M
= ri rl O N O
rl 1 1 I I N' I N (DO
N
r1 0) O
M
(N Ln - N' N O
N' N' = k I
01 l0 l0 - M N f 'H -~ -~ ^ ^ ^ !- ^ ^
O Ln rl N' O N O M 00 C- l0 l0
M N m O O rl rl rl 0 ri O O O
N r1 N N
rl O 61 'H C- l0 O N rl N 00 M V' N
(N
b Ln 00i N l0 I
l0r ri (N ri 'H rl ri O N' M M ri N N' Ln Ln
m
E ^ N 6 ^ k
~' -- co "r co N Ln r ., N' a)
(0 O = (N N = O \0 Ol = a) N Ln N'
a rt ~--~ rl rl rl N. p
() N - O ' M O O O O O
Ln -- = -- N LO - rl N
O I- -i ri co . = 6l rl C) -I N
= 0 0 = --i = O C` C) O r= O
Q (r O I I O I I co N I I O O 1 I O O
C E
E
Q0 0 M
E Q) o ^ ^ Ln
= C^ N' -- l0 Ol N N
O ,0 Ln co l0 r= M Ol M lfl M co
N'
C r1 Ln M
t6 N co a) M N N O O N' rl O O
d O ri = r
a = rl 1` co r 1 M ao 6l N' r= 6l Ln M N
N' dl (n Ln co
a)
'C l0 1-1 1-1 Ln ri -i -I .-i N' Ln M r~ M (f) Ln Ln
G1
.C
0
Ln a) o
m . = ri r-- i<
l0 ^ ^ N rl ^ ^ ^
C r rl C` M M O r1 N' 00 (0 00 ri (0 ^ ^ (f)
0 41 'H'H O O Ln -i m 0 O 0 0 0 'H = o
=y LO -- .~ . p M ' O p
p = M ri rl N O = rl M N' Ln O N - -' M
Q. p = ri r1 O W . N N
0 I C) O O I I I ri O O O O O O = O
E . I O O 1
O a)
U u
is ^ ^
W ^ ^ - p
0 w o0 o N= ^ (n Ln Ln N ^ ^ ^ ^ ^
. co r co N' C- O O CO M Ln N' 110 (n
C 6l l0 M p N
(N m N' O N rf rl CD* (D'
N ri O O
C Q0 O C- CO N C- o N (n N r1 rl m M ri
M N N' = 0) M M
l0 N rl l0 r= N O rl N (D N' Ln M ri M N' (n Ln
E tP 'H
0 --I
0 ~-i 0
Q) L N
tb O ^ rn ^ Xi a) U)
m C m -- cr -- v ^ a co ,) w 0
C o(a rn x sT U a - a) m -- a) U
(U x =b E: 0 rt a) a s U 0
.~ .~ ~F'i \ X-. .O' O -i '- \ -H rl
U O 1-) ^ ^ .~ 0,- v -c 0 r-1 ri 7-1 as CJ'
"0 U) (0 J-) N c N U O O a) a
o U) G, M E: E- . v M a u a U^ Q p a ~o
O W ro W U U cC v I a >, a x y o- --
u x ri a Q - .-I i - ri == -
c C ..4 = = F = ' py I ! Y 1 - x O M O tP 0 " rl 4 J0
F v N 1) 1 Ei Ei E' E~ o f"J z E 1-) a s -ri w 0
o ro ~i- xC c5 o o ra ra ro oa
awE, 4>M > A I-it-3 z-El axE-I u C7w--x
Ln O L17 0 Lf) O Ln
-i N N (Y) (N
CA 02485472 2004-10-20
0
O co O O M
O m 0 0 O O
O 0 \0 0 0 0
C) ~oo0 00 Q
o o + m
a`)
0
z
O O O O 0 ,=4,
O O 0 =-4 O (n
O O O ri O
O O O M O
0
H H ri O ri U
ao o
r
{~ N l0 0
N N
H O ri N
O Ln =
r- N O M
CD r0 N M 0 i' ro
ro
-d
0
N 0
0
Lr) r I r` -H
-~ -N 41
ri N Ln OD M CO Ud
N M M - C) N N
=O = O M C) U)
= N = C) C)
l0 H M OD M Ln 0
H 4-J
U)
ri I-- c CC)
= N r-I
N ma'r` d' N v 0
H OD Ln Ln 4a
N
O I (D H N N -O
ro 0
oo --
C) 0) - -
o 0
V O l0 61 M M
LI
cr = 0) O 0
N r` N 'C3
(N Ln OJ M Ln -~ N
+)
U)
O U)
P N
0 S4
co N 0a
co - - [)
61 O C) - U)
= Ln = N H a)
4 r-I Ln M (D ci Cl)
OD 0) H H -P
~o H H LO ,q ro .-I
o l0 ro 5 ~.
O Ln N O I M f Q
rn p H
ro
O 0 N 4J
Ln U)
m co l0 IT r` N
r4 H. = r-4 -- ro
N V' v' 0 4J
N M U)
N O
Ln = ~o IT 0
l0 r1 M 0 M Ln Z ro
(-
a 04 '0
o s~~
H U)
o
0) 0 ; =r4 w 04
H > U U U H ro ro m
=0 ri -rl - ~I H O - N =-i
O +-) H I N N ro ff)
O
E~ Ga W O 2 Q if 'J w
Lr) O Ln
r-A H
CA 02485472 2004-10-20
49
Quality of Life
A health-related quality of life questionnaire
(PLC, Quality of Life Profile for the Chronocally Ill) was
self-administered to 61 patients randomized to receive
placebo or TH9507 at 1 or 2 mg s.c. daily. The PLC
questionnaire included a general, non-specific part
assessing 6 dimensions of global health as well as a disease
specific part capturing impact of enlarged abdominal girth,
abdominal bloating, tenseness and pain, as well as diarrhea,
visible facial changes, visible changes in physical
appearance, and the feeling of being recognized as an HIV
positive person.
Study population included 54 men and 7 women.
Baseline mean age was 46 7 [SD] , BMI 28 3 [SD] kg/m2, WC
102 cm 8 [SD] and WHR 1.0 0.1 [SD]. No significant
difference between groups was noted for subscales of the
main portion of the PLC. Slight changes were observed within
the treated groups in the positive mood and social well-
being scores but were not considered clinically significant.
Clinically significant improvements were noted in the
enlarged abdominal girth (placebo :-0.13; 1 mg :-0.93,
P=0.06 vs baseline; 2 mg :-1.19, P<0.05 vs baseline, NS vs
placebo) and bloating scores (placebo : 0.56; 1 mg : -0.50;
2 mg : -0.69, P<0.05 vs. placebo). Improvement in abdominal
pain was observed at 1 mg only (P<0.05 vs baseline, NS vs
placebo) along with a trend for improvement in tenseness
(P=0.07 vs baseline, NS vs placebo). No significant changes
were observed in the other disease-specific items.
These data suggest that administration of TH9507,
a GRF analog, in HIV patients with abdominal fat
accumulation improved QOL with regard to enlarged abdominal
girth and related items, consistent with the decrease in
truncal and visceral fat observed in this population.
CA 02485472 2004-10-20
AE's and Discontinuation
Discontinuation rates were not different between
the groups (24%, 11%, 29%, placebo, 1 mg, 2 mg
5 respectively) . One subject in the placebo group (arthritis),
none in the 1 mg group and 3 in the 2 mg group (rash,
arhtralgia, paresthesia) experienced AE's leading to
treatment discontinuation. Severe AE's were reported in 6%
of the placebo group, 13% of the 1 mg group and 10% of the 2
10 mg group. Musculoskeletal AE's e.g. pain and arthralgias
were noted in 24%, 26% and 29% of subjects in the placebo, 1
mg and 2 mg groups respectively. Carpal tunnel symptoms were
not noted in any patient. Edema and/or peripheral swelling
were noted in 1 patient in the 2 mg group only. Headache
15 and/or paresthesias were noted in 19%, 32% and 52 % of the
subjects in the placebo, lmg and 2 mg groups, respectively.
Blood pressure and heart rate did not change between or
within the groups. One patient in the placebo group compared
to three in the 2 mg group withdrew from the study related
20 to adverse events. Safety laboratory values, including
hemoglobin, WBC, LFT's, creatinine did not differ between
the groups (data not shown). CPK increased in a greater
percentage of the 1 (47%) and 2 mg treated subjects (38%)
compared to placebo (19%), but these changes were small and
25 did not result in a greater proportion of abnormal CPK
values between the groups. Anti-TH9507 antibodies were not
detected after 12 weeks in any patient. CD4 count and viral
load did not change.
Some of the results using a GRF analog in this
30 study are comparable to that seen in response to GHRH 1-29
in a recently published study in men with HIV lipodystrophy,
in which truncal fat, but not extremity or subcutaneous fat
decreased in response to physiologic increases in GH (13).
The current study extends the findings of Koutkia et al in a
CA 02485472 2004-10-20
51
larger group of patients, including men and women, using
graded doses of a novel 1-44 amino acid GRF analog that is
dosed once rather than twice a day. Although the lower of
the 2 doses increased IGF-I significantly, this dose did not
result in a significant decrease in trunk fat, suggesting
that there may be a threshold increase in IGF-I necessary to
reduce truncal fat. Visceral fat also decreased
significantly by more than 15% over 3 months within the 2 mg
group. Of note, the magnitude of this change on a percentage
basis is equivalent to that seen with pharmacologic doses of
GH (9), suggesting that this strategy is highly effective
and potentially very useful because of the general lack of
side effects associated with physiologic increases in GH.
In addition to reducing truncal fat, the 2mg GRF
dose significantly improved triglyceride levels and the
cholesterol to HDL ratio. This is a significant advantage of
a GRF analog, not seen with other treatment strategies for
HIV lipodystrophy (17, 18) . Similar beneficial effects on
triglyceride were seen with lower, alternating day, but not
higher doses of GH in a study reported by Kotler et al.
Growth hormone has been shown to decrease cholesterol and
triglycceride levels in GH deficient patients and among
otherwise healthy men chosen for abdominal obesity (19, 20).
Taken together, our data suggest that treatment with TH9507
resulted in an improved lipid profile in dyslipidemic,
abdominally obese patients with HIV lipodystrophy.
An important issue regarding the use of GH or
related strategies in HIV lipodystrophy is glucose control.
Patients with HIV lipodystrophy are often insulin resistant,
and a significant percentage, more than a third, may have
impaired glucose tolerance (3). In this study, even with the
higher dose of 2 mg, there were no significant differences
CA 02485472 2004-10-20
52
in fasting glucose or 2-hour glucose in response to a
standard GTT and no increase in HbAIc.
TH9507 was also associated with other benefits in
this study. Osteocalcin, a marker of bone formation
increased within the 2 mg group, whereas NTX, a marker of
bone resorption did not suggesting a net positive effect on
bone turnover. Reduced bone density has been described among
patients with HIV disease and among those with
lipodystrophy, in inverse association with visceral and
truncal adiposity (25, 26). Growth hormone is well known to
stimulate bone formation (27). Relative reductions in GH
secretion may therefore contribute to reduced bone density
in some patients with lipodystrophy and a positive effect on
bone formation, with physiologic increases in GH is an
additional benefit of TH9507.
While the invention has been described in
connection with specific embodiments thereof, it will be
understood that it is capable of further modifications and
this application is intended to cover any variations, uses,
or adaptations of the invention following, in general, the
principals of the invention and including such departures
from the present disclosure as come within known or
customary practice within the art to which the invention
pertains and as may be applied to the essential features
hereinbefore set forth, and as follows in the scope of the
appended claims.
CA 02485472 2004-10-20
53
REFERENCES
1. Miller KD, Jones E, Yanovski JA, Shankar R,
Feuerstein I, Falloon J. Visceral abdominal-fat accumulation
associated with use of indinavir. Lancet 1998;351(9106):871-
5.
2. Carr A, Samaras K, Burton S, et al. A syndrome of
peripheral lipodystrophy, hyperlipidaemia and insulin
resistance in patients receiving HIV protease inhibitors.
AIDS 1998;12(7):F51-8.
3. Hadigan C, Meigs JB, Corcoran C, et al. Metabolic
Abnormalities and Cardiovascular Disease Risk Factors in
Adults with Human Immunodeficiency Virus Infection and
Lipodystrophy. Clin Infect Dis 2001;32(1):130-9.
4. Friis-Moller N, Sabin CA, Weber R, et al.
Combination antiretroviral therapy and the risk of
myocardial infarction. N Engl J Med 2003;349(21):1993-2003.
5. Pouliot MC, Despres JP, Nadeau A. Visceral Obesity
in Men: Associations with Glucose Tolerance, Plasma Insulin
and Lipoprotein Levels. Diabetes 1992;41:826-34.
6. Meininger G, Hadigan C, Rietschel P, Grinspoon S.
Body-composition measurements as predictors of glucose and
insulin abnormalities in HIV-positive men. Am J Clin Nutr
2002;76(2):460-5.
7. Rietschel P, Hadigan C, Corcoran C, et al.
Assessment of Growth Hormone Dynamics in Human
Immunodeficiency Virus- Related Lipodystrophy. J Clin
Endocrinol Metab 2001;86(2):504-10.
8. Koutkia P, Meininger G, Canavan B, Breu J,
Grinspoon S. Metabolic regulation of growth hormone by free
fatty acids, somatostatin, and ghrelin in HIV-lipodystrophy.
Am J Physiol Endocrinol Metab 2004;286(2):E296-303.
9. Kotler DP, Muurahainen N, Grunfeld C, et al.
Effects of growth hormone on abnormal visceral adipose
CA 02485472 2004-10-20
54
tissue accumulation and dyslipidemia in HIV-infected
patients. J Acquir Immune Def Syndr 2004;35:239-52.
10. Engelson ES, Glesby MJ, Mendez D, et al. Effect of
recombinant human growth hormone in the treatment of
visceral fat accumulation in HIV infection. J Acquir Immune
Defic Syndr 2002;30(4):379-91.
11. Wanke C, Gerrior J, Kantaros J, Coakley E,
Albrecht M. Recombinant human growth hormone improves the
fat redistribution syndrome (lipodystrophy) in patients with
HIV. AIDS 1999;13(15):2099-103.
12. Lo JC, Mulligan K, Noor MA, et al. The effects of
recombinant human growth hormone on body composition and
glucose metabolism in HIV-infected patients with fat
accumulation. J Clin Endocrinol Metab 2001;86(8):3480-7.
13. Koutkia P, Canavan B, Breu J, Toriani M, Kissko J,
Grinspoon S. Growth Hormone-releasing Hormone in HIV-
infected Men with Lipodystrophy: A Randomized, Controlled
Trial. JAMA 2004;292:210-8.
14. Abribat T, Gravel D, Brazeau P. TH9507 A new
Growth Hormone-Releasing Factor (GRF) analogue is a powerful
Insulin-like Growth Factor-1 (IGF-1) inducer in 50-60 years
old healthy subjects : A 7-Day, Randomizeed Multidose Study.
In: The Endocrine Society's 84 rd Annual Meeting; 2001;
Denver; 2001. p. P2-292.
15. Clemmons D, Miller S, De Villers A, et al. Safety
assessment and metabolic effects of TH9507, a Growth Hormone
Releasing Factor analog(GRF) in patients with Type 2
diabetes mellitus. In: The Endocrine Society's 86 Annual
Meeting; 2003; Philadelphia; 2003. p. P2-354.
16. Park YW, Heymsfield SB, Gallagher D. Are dual-
energy X-ray absorptiometry regional estimates associated
with visceral adipose tissue mass? International Journal of
Obesity & Related Metabolic Disorders: Journal of the
CA 02485472 2004-10-20
International Association for the Study of Obesity
2002;26(7):978-83.
17. Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE,
Grinspoon S. Metabolic effects of rosiglitazone in HIV
5 lipodystrophy: A randomized controlled trial. Annals of
Internal Medicine 2004;140(10):786-94.
18. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P,
Grinspoon S. Metformin in the treatment of HIV lipodystrophy
syndrome: A randomized controlled trial. JAMA
10 2000;284(4):472-7.
19. Johannsson G, Marin P, Lonn L, et al. Growth
hormone treatment of abdominally obese men reduces abdominal
fat mass, improves glucose and lipoprotein metabolism, and
reduces diastolic blood pressure. J Clin Endocrinol Metab
15 1997;82(3):727-34.
20. Colao A, di Somma C, Cuocolo A, et al. Improved
cardiovascular risk factors and cardiac performance after 12
months of growth hormone (GH) replacement in young adult
patients with GH deficiency. J Clin Endocrinol Metab
20 2001;86(5):1874-81.
CA 02485472 2005-07-26
1
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: THERATECHNOLOGIES INC.
(ii) TITLE OF INVENTION: GH SECRETAGOGUES AND USES THEREOF
(iii) NUMBER OF SEQUENCES: 7
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: SMART & BIGGAR
(B) STREET: 1000 de la Gauchetiere ouest, Suite 3300
(C) CITY: Montreal
(D) STATE: Quebec
(E) COUNTRY: CANADA
(F) ZIP: H3B 4W5
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: ASCII (text)
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: CA
(B) FILING DATE:
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: SMART & BIGGAR
(B) REGISTRATION NUMBER: 85795-75
(C) REFERENCE/DOCKET NUMBER:
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (514)-954-1500
(B) TELEFAX: (514)-954-1396
(2) INFORMATION FOR SEQ ID NO.: 1:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 44
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial sequence
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (1)..(1)
(C) OTHER INFORMATION: Xaa = Tyr or His
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (2) .. (2)
(C) OTHER INFORMATION: Xaa = Val or Ala
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (8)..(8)
(C) OTHER INFORMATION: Xaa = Asn or Ser
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (13)..(13)
(C) OTHER INFORMATION: Xaa = Val or Ile
CA 02485472 2005-07-26
2
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (15)..(15)
(C) OTHER INFORMATION: Xaa = Ala or Gly
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (18) .. (18)
(C) OTHER INFORMATION: Xaa = Ser or Tyr
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (24)..(24)
(C) OTHER INFORMATION: Xaa = Gln or His
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (25)..(25)
(C) OTHER INFORMATION: Xaa = Asp or Glu
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (27) .. (27)
(C) OTHER INFORMATION: Xaa = Met or Ile or Nle
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (28) .. (28)
(C) OTHER INFORMATION: Xaa = Ser or Asn
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (30)..(30)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (31) .. (31)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (32)..(32)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (33) .. (33)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (34)..(34)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (35)..(35)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (36)..(36)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (37)..(37)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (38)..(38)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
CA 02485472 2005-07-26
3
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (39) .. (39)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (40)..(40)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (41)..(41)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (42) .. (42)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (43)..(43)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(ix) FEATURE
(A) NAME/KEY: VARIANT
(B) LOCATION: (44)..(44)
(C) OTHER INFORMATION: Xaa = any amino acid or is absent
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 1:
Xaa Xaa Asp Ala Ile Phe Tyr Xaa Ser Tyr Arg Lys Xaa Leu Xaa Gln
1 5 10 15
Leu Xaa Ala Arg Lys Leu Leu Xaa Xaa Ile Xaa Xaa Arg Xaa Xaa Xaa
20 25 30
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
40
(2) INFORMATION FOR SEQ ID NO.: 2:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 44
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
(B) LOCATION: (44)_.(44)
(C) OTHER INFORMATION: Leu residue is capped with an unsubstituted amide
moiety
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 2:
Tyr Ala Asp Ala Ile Phe Thr Asn Ser Tyr Arg Lys Val Leu Gly Gln
1 5 10 15
Leu Ser Ala Arg Lys Leu Leu Gln Asp Ile Met Ser Arg Gln Gln Gly
20 25 30
Glu Ser Asn Gln Glu Arg Gly Ala Arg Ala Arg Leu
35 40
CA 02485472 2005-07-26
4
(2) INFORMATION FOR SEQ ID NO.: 3:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 44
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial sequence
(ix) FEATURE
(C) OTHER INFORMATION: Amino acid sequence of human GRF
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 3:
Tyr Ala Asp Ala Ile Phe Thr Asn Ser Tyr Arg Lys Val Leu Gly Gln
1 5 10 15
Leu Ser Ala Arg Lys Leu Leu Gln Asp Ile Met Ser Arg Gln Gln Gly
25 30
Glu Ser Asn Gln Glu Arg Gly Ala Arg Ala Arg Leu
20 35 40
(2) INFORMATION FOR SEQ ID NO.: 4:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 29
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
(B) LOCATION: (29)_.(29)
(C) OTHER INFORMATION: Arg residue is capped with an unsubstituted amide
moiety
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 4:
Tyr Ala Asp Ala Ile Phe Thr Asn Ser Tyr Arg Lys Val Leu Gly Gln
1 5 10 15
Leu Ser Ala Arg Lys Leu Leu Gln Asp Ile Met Ser Arg
20 25
(2) INFORMATION FOR SEQ ID NO.: 5:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 29
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial sequence
(ix) FEATURE
(C) OTHER INFORMATION: Amino acid sequence of the minimum active core of
human GRF
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 5:
Tyr Ala Asp Ala Ile Phe Thr Asn Ser Tyr Arg Lys Val Leu Gly Gln
1 5 10 15
CA 02485472 2005-07-26
Leu Ser Ala Arg Lys Leu Leu Gln Asp Ile Met Ser Arg
20 25
(2) INFORMATION FOR SEQ ID NO.: 6:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 15
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial sequence
(ix) FEATURE
(C) OTHER INFORMATION: Amino acid sequence corresponding to positions 30
to 44 of human GRF
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 6:
Gln Gln Gly Glu Ser Asn Gln Glu Arg Gly Ala Arg Ala Arg Leu
1 5 10 15
(2) INFORMATION FOR SEQ ID NO.: 7:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 44
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
(ii) MOLECULE TYPE: polypeptide
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Artificial sequence
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
(B) LOCATION: (1).-(1)
(C) OTHER INFORMATION: Tyr residue is linked to an hexenoyl-trans-3
moeity
(ix) FEATURE
(A) NAME/KEY: MISC FEATURE
(B) LOCATION: (44)_.(44)
(C) OTHER INFORMATION: Leu residue is capped with an unsubstituted amide
moiety
(xi) SEQUENCE DESCRIPTION: SEQ ID NO.: 7:
Tyr Ala Asp Ala Ile Phe Thr Asn Ser Tyr Arg Lys Val Leu Gly Gln
1 5 10 15
Leu Ser Ala Arg Lys Leu Leu Gln Asp Ile Met Ser Arg Gln Gln Gly
20 25 30
Glu Ser Asn Gln Glu Arg Gly Ala Arg Ala Arg Leu
35 40