Note: Descriptions are shown in the official language in which they were submitted.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
ALLERGEN MUTANTS
FIELD OF THE INVENTION
The present invention relates to diagnosis and treatment of allergy. More
specifically the invention provides ways of obtaining mutated allergen
molecules suitable for these purposes. The invention furthermore relates to
novel recombinant allergens, which are mutants of naturally occurring
allergens as well as their use. Also, the invention relates to a composition
comprising a mixture of novel recombinant mutant allergens. Further, the
invention relates to a method of preparing such recombinant mutant
allergens as well as to pharmaceutical compositions, including vaccines,
comprising the recombinant mutant allergens. In further embodiments, the
present invention relates to methods of generating immune responses in a
subject, vaccination or treatment of a subject as well as processes for
preparing the compositions of the invention.
BACKGROUND OF THE INVENTION
Genetically predisposed individuals become sensitised (allergic) to antigens
originating from a variety of environmental sources, to the allergens of which
the individuals are exposed. The allergic reaction occurs when a previously
sensitised individual is re-exposed to the same or a homologous allergen.
Allergic responses range from hay fever, rhinoconductivitis, rhinitis and
asthma to systemic anaphylaxis and death in response to e.g. bee or hornet
sting or insect bite. The reaction is immediate and can be caused by a variety
of atopic allergens such as compounds originating from grasses, trees,
weeds, insects, food, drugs, chemicals and perfumes.
However, the responses do not occur when an individual is exposed to an
allergen for the first time. The initial adaptive response takes time and does
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
2
usually not cause any symptoms. But when antibodies and T cells capable of
reacting with the allergen have been produced, any subsequent exposure
may provoke symptoms. Thus, allergic responses demonstrate that the
immune response itself can cause significant pathological states, which may
be life threatening.
The antibodies involved in atopic allergy belong primarily to immunoglobulins
of the IgE class. IgE binds to specific receptors on the surface of mast cells
and basophils. Following complex formation of a specific allergen with IgE
bound to mast cells, receptor cross-linking on the cell surface results in
signalling through the receptors and the physiological response of the target
cells. Degranulation of a mast cell results in the release of i.a. histamine,
heparin, a chemotactic factor for eosinophilic leukocytes, leukotrienes C4, D4
and E4, which cause prolonged constriction of the bronchial smooth muscle
cells. The resulting effects may be systemic or local in nature.
The antibody-mediated hypersensitivity reactions can be divided into four
classes, namely type I, type II, type III and type IV. Type I allergic
reactions is
the classic immediate hypersensitivity reaction occurring within seconds or
minutes following antigen exposure. These symptoms are mediated by
allergen specific IgE.
Commonly, allergic reactions are observed as a response to protein
allergens present e.g. in pollens, house dust mites, animal hair and dandruff,
venoms, and food products.
In order to reduce or eliminate allergic reactions, carefully controlled and
repeated administration of allergy vaccines is commonly used. Allergy
vaccination is traditionally performed by parenteral, intranasal, or
sublingual
administration in increasing doses over a fairly long period of time, and
results in desensitisation of the patient. The exact immunological mechanism
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
3
is not known, but induced differences in the phenotype ofi allergen specific T
cells is thought to be of particular importance.
Allergy vaccination
The concept of vaccination is based on two fundamental characteristics of
the immune system, namely specificity and memory. Vaccination will prime
the immune system of the recipient, and upon repeated exposure to similar
proteins the immune system will be in a position to respond more rigorously
to the challenge of for example a microbial infection. Vaccines are mixtures
of proteins intended to be used in vaccination for the purpose of generating
such a protective immune response in the recipient. The protection will
comprise only components present in the vaccine and homologous antigens.
Compared to other types of vaccination allergy vaccination is complicated by
the existence of an ongoing immune response in allergic patients. This
immune response is characterised by the presence of allergen specific IgE
mediating the release of allergic symptoms upon exposure to allergens.
Thus, allergy vaccination using allergens from natural sources has an
inherent risk of side effects being in the utmost consequence life threatening
to the patient.
Approaches to circumvent this problem may be divided in three categories. In
practise measures from more than one category are often combined. First
category of measures includes the administration of several small doses over
prolonged time to reach a substantial accumulated dose. Second category of
measures includes physical modification of the allergens by incorporation of
the allergens into gel substances such as aluminium hydroxide. Aluminium
hydroxide formulation has an adjuvant effect and a depot effect of slow
allergen release reducing the tissue concentration of active allergen
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
4
components. Third category of measures include chemical modification of the
allergens for the purpose of reducing allergenicity, i.e. IgE binding.
The detailed mechanism behind successful allergy vaccination remains
controversial. It is, however, agreed that T cells play a key role in the
overall
regulation of immune responses. According to current consensus the relation
between two extremes of T cell phenotypes, Th1 and Th2, determine the
allergic status of an individual. Upon stimulation with allergen Th1 cells
secrete interleukines dominated by interferon~y leading to protective immunity
and the individual is healthy. Th2 cells on the other hand secrete
predominantly interleukin 4 and 5 leading to IgE synthesis and eosinophilia
and the individual is allergic. In vitro studies have indicated the
possibility of
altering the responses of allergen specific T cells by challenge with allergen
derived peptides containing relevant T cell epitopes. Current approaches to
new allergy vaccines are therefore largely based on addressing the T cells,
the aim being to silence the T cells (anergy induction) or to shift the
response
from the Th2 phenotype to the Th1 phenotype.
Antibody-binding e~pitopes tB-cell epitopes)
X-ray crystallographic analyses of Fab-antigen complexes has increased the
understanding of antibody-binding epitopes. According to this type of analysis
antibody-binding epitopes can be defined as a section of the surtace of the
antigen comprising atoms from 15-25 amino acid residues, which are Within a
distance from the atoms of the antibody enabling direct interaction. The
affinity of the antigen-antibody interaction can not be predicted from the
enthalpy contributed by van der Waals interactions, hydrogen bonds or ionic
bonds, alone. The entropy associated with the almost complete expulsion of
water molecules from the intertace represent an energy contribution similar in
size. This means that perfect fit between the contours of the interacting
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
molecules is a principal factor underlying antigen-antibody high affinity
interactions.
In WO 97!30150 (ref. 1 ), a population of protein molecules is claimed, which
5 protein molecules have a distribution of specific mutations in the amino
acid
sequence as compared to a parent protein. From the description, it appears
that the invention is concerned with producing analogues which are modified
as compared to the parent protein, but which are taken up, digested and
presented to T cells in the same manner as the parent protein (naturally
occurring allergens). Thereby, a modified T cell response is obtained.
Libraries of modified proteins are prepared using a technique denoted PM
(Parsimonious Mutagenesis).
In WO 92/02621 (ref. 2), recombinant DNA molecules are described, which
molecules comprise a DNA coding for a polypeptide having at least one
epitope of an allergen of trees of the order Fagales, the allergen being
selected from Aln g 1, Cor a 1 and Bet v 1. The recombinant molecules
described herein do all have an amino acid sequence or part of an amino
acid sequence that corresponds to the sequence of a naturally occurring
allergen.
WO 90!11293 (ref. 3) relates i.a. to isolated allergenic peptides of ragweed
pollen and to modified ragweed pollen peptides. The peptides disclosed
therein have an amino acid sequence corresponding either to the sequence
of the naturally occurring allergen or to naturally occurring isoforms
thereof.
Chemical modification of alter. ens
Several approaches to chemical modification of allergens have been taken.
Approaches of the early seventies include chemical coupling of allergens to
polymers, and chemical cross-linking of allergens using formaldehyde, etc.,
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
6
producing the so-called 'allergoids'. The rationale behind these approaches
was random destruction of IgE binding epitopes by attachment of the
chemical ligand thereby reducing IgE-binding while retaining immunogenicity
by the increased molecular weight of the complexes. Inherent disadvantages
of 'allergoid' production are linked to difficulties in controlling the
process of
chemical cross-linking and difficulties in analysis and standardisation of the
resulting high molecular weight complexes. 'Allergoids' are currently in
clinical use and due to the random destruction of IgE binding epitopes higher
doses can be administered as compared to conventional vaccines, but the
safety and efficacy parameters are not improved over use of conventional
vaccines.
More recent approaches to chemical modification of allergens aim at a total
disruption of the tertiary structure of the allergen thus eliminating IgE
binding
assuming that the essential therapeutic target is the allergen specific T
cell.
Such vaccines contain allergen sequence derived synthetic peptides
representing minimal T cells epitopes, longer peptides representing linked T
cells epitopes, longer allergen sequence derived synthetic peptides
representing regions of immunodominant T cell epitopes, or allergen
molecules cut in two halves by recombinant technique. Another approach
based on this rationale has been the proposal of the use of "low IgE binding"
recombinant isoforms. In recent years it has become clear that natural
allergens are heterogeneous containing isoallergens and variants having up
to approximately 25% of their amino acids substituted. Some recombinant
isoallergens have been found to be less efficient in IgE binding possibly due
to irreversible denaturation and hence total disruption of tertiary structure.
In vitro mutaaenesis and alleray vaccination
Attempts to reduce allergenicity by in vitro site directed mutagenesis have
been performed using several allergens including Der f 2 (Takai et al, ref.
4),
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
7
Der p 2 (Smith et al, ref. 5), a 39 kDa Dermatophagoides farinae allergen (Aki
et al, ref. 6), bee venom phospholipase A2 (Forster et al, ref. 7), Ara h 1
(Burks et al, ref. 8), Ara h 2 (Stanley et al, ref. 9), Bet v 1 (Ferreira et
al, ref.
and 11 ), birch profilin (Wiedemann et al, ref. 12), and Ory s 1 (Alvarez et
5 al, ref. 13).
The rationale behind these approaches, again, is addressing allergen specific
T cells while at the same time reducing the risk of IgE mediated side effects
by reduction or elimination of IgE binding by disruption of the tertiary
10 structure of the recombinant mutant allergen.
The article by Ferreira et al (ref. 11 ) discloses the use of site directed
mutagenesis for the purpose of reducing IgE binding. Although the three-
dimensional structure of Bet v 1 is mentioned in the article the authors do
not
use the structure for prediction of solvent exposed amino acid residues for
mutation, half of which have a low degree of solvent exposure. Rather they
use a method developed for prediction of functional residues in proteins.
Although the authors do discuss conservation of a-carbon backbone tertiary
structure this concept is not a part of the therapeutic strategy but merely
included to assess in vitro IgE binding. Furthermore, the evidence presented
is not adequate since normalisation of CD-spectra prevents the evaluation of
denaturation of a proportion of the sample, which is a common problem. The
therapeutic strategy described aim at inducing tolerance in allergen specific
T
cells and initiation of a new immune response is not mentioned.
The article by Wiedemann et al. (ref. 12) describes the use of site directed
mutagenesis and peptide synthesis for the purpose of monoclonal antibody
epitope characterisation. The study demonstrates that substitution of a
surface exposed amino acid has the capacity to modify the binding
characteristics of a monoclonal antibody, which is not surprising considering
common knowledge. The experiments described are not designed to assess
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
modulation in the binding of polyclonal antibodies such as allergic patients'
serum IgE. One of the experiments does apply serum IgE and although this
experiment is not suitable for quantitative assessment, lgE binding does not
seem to be affected by the mutations performed.
The article by Smith et al. (ref. 5) describes the use of site directed
mutagenesis for the purpose of monoclonal antibody epitope mapping and
reduction of IgE binding. The authors have no knowledge of the tertiary
structure and make no attempt to assess the conservation of a-carbon
backbone tertiary structure. The algorithm used does not ensure that amino
acids selected for mutation are actually exposed to the molecular surface.
Only one of the mutants described lead to a substantial reduction in IgE
binding. This mutant is deficient in binding of all antibodies tested
indicating
that the tertiary structure is disrupted. The authors do not define a
therapeutic
strategy and initiation of a new immune response is not mentioned.
The article by Colombo et al. (ref. 14) describes the study of an IgE binding
epitope by use of site directed mutagenesis and peptide synthesis. The
authors use a three dimensional computer model structure based on the
crystal structure of a homologous protein to illustrate the presence of the
epitope on the molecular surtace. The further presence of an epitope on a
different allergen showing primary structure homology is addressed using
synthetic peptides representing the epitope. The therapeutic strategy is
based on treatment using this synthetic peptide representing a monovalent
IgE binding epitope.
The article by Spangfort et al. (ref. 15) describes the three-dimensional
structure and conserved surface exposed areas of the major birch allergen.
The article does not disclose site directed mutagenesis, neither is
therapeutic
application addressed.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
9
In none of the studies described above is IgE binding being reduced by
substitution of surface exposed amino acids while conserving a,-carbon
backbone tertiary structure. Neither is the concept of initiating a new
protective immune response mentioned.
WO 01183559 discloses a method of selecting a protein variant with modified
immunogenicity by using antibody binding peptide sequences to localise
epitope sequences on the 3-dimensional structure of the parent protein. An
epitope area is subsequently defined and one or more of the amino acids
defining the epitope area are mutated. The invention is exemplified by
industrial enzymes that function as allergens.
WO 99/47680 discloses the introduction of artificial amino acid substitutions
into defined critical positions while retaining the a-carbon backbone tertiary
structure of the allergen. In particular, WO 99/47680 discloses a recombinant
allergen, which is a non-naturally occurring mutant derived from a naturally
occurring allergen, wherein at least one surface-exposed, conserved amino
acid residue of a E cell epitope is substituted by another residue which does
not occur in the same position in the amino acid sequence of any known
homologous protein within the taxonomic order from which said naturally
occurring allergen originates, said mutant allergen having essentially the
same a-carbon backbone tertiary structure as said naturally occurring
allergen, and the specific IgE binding to the mutated allergen being reduced
as compared to the binding to said naturally occurring allergen.
The recombinant allergen disclosed in WO 99147680 is obtainable by a)
identifying amino acid residues in a naturally occurring allergen which are
conserved with more than 70% identity in all known homologous proteins
within the taxonomic order from which said naturally occurring allergen
originates, b) defining at least one patch of conserved amino acid residues
being coherently connected over at least 400 r42 of the surface of the three-
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
dimensional structure of the allergen molecule as defined by having a solvent
accessibility of at least 20%, said at least one patch comprising at least one
B cell epitope, and c) substituting at least one amino acid residue in said at
least one patch by another amino acid being non-conservative in the
5 particular position while essentially preserving the overall a-carbon
backbone
tertiary structure of the allergen molecule.
Patent application PCT/DK 01/00764 relates to mutants of naturally occurring
allergens. The following specific Bet v 1 mutants are disclosed therein:
10 Mutant A: Asn28Thr, Lys32Gln, Asn78Lys, Lys103Va1, Arg145G1u,
Asp156His, +160Asn.
Mutant B: TyrSVal, GIu42Ser, GIu45Ser, Asn78Lys, Lys103Va1, Lys12311e,
Lys134G1u, Asp156His.
Mutant 2628: TyrSVal, GIu45Ser, Lys65Asn, Lys97Ser, Lys134G1u.
Mutant 2637: AIa16Pro, Asn28Thr, Lys32Gln, Lys103Thr, Pro108G1y,
Leu152Lys, AIa153G1y, Ser155Pro.
Mutant 2724: N28T, K32Q, N78K, K103V, P108G, R145E, D156H, +160N.
Mutant 2733: TyrSVal, Lys134G1u, Asn28Thr, Lys32Gln, GIu45Ser,
Lys65Asn, Asn78Lys, Lys103Va1, Lys97Ser, Pro108G1y, Arg145G1u,
Asp156His, +160Asn.
Mutant 2744: TyrSVal, Lys134G1u, GIu42Ser, GIu45Ser, Asn78Lys,
Lys103Va1, Lys12311e, Asp156His, +160Asn.
Mutant 2753: Asn28Thr, Lys32Gln, Lys65Asn, GIu96Leu, Lys97Ser,
Pro108G1y, Asp109Asn, Asp125Tyr, GIu127Ser, Arg145G1u.
Mutant 2744 + 2595: YSV, N28T, K32Q, E42S, E45S, N78K, K103V, P108G,
K1231, K134E, D156H, +160N.
Mutant 2744 + 2628: YSV, E42S, E45S, K65N, N78K, K97S, K103V, K1231,
K134E, D156H, +160N.
Mutant 2744 + 2595 + 2628: YSV, N28T, K32Q, E42S, E45S, K65N, N78K,
K97S, K103V, P108G, K1231, K134E, D156H, +160N.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
11
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows a theoretical model of the reaction between an allergen and
mast cells by IgE cross-linking.
Figure 2. (Left) The molecular surface of Bet v 1 with the location of group 1
to 10 shown in black and grey tones. (Right) View of the amino acid residues
constituting group 1 to 10. Groups are marked 1 to 10.
Figure 3 shows mutant-specific oligonucleotide primers used for mutation of
Bet v 1. Mutated nucleotides are underlined.
Figure 4 shows two generally applicable primers (denoted "all-sense" and "all
non-sense"), which were synthesised and used for all mutants.
Figure 5 shows the DNA and amino acid sequence of the naturally occurring
allergen Bet v 1 as well as a number of Bef v 1 mutations.
Figure 6 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 GIu45Ser mutant.
Figure 7 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 mutant Asn28Thr+Lys32Gln.
Figure 8 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 Pro108G1y mutant.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
12
Figure 9 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bef v 1
and
by Bef v 1 GIu60Ser mutant.
Figure 10 shows the CD spectra of recombinant and the (Asn28Thr,
Lys32Gln, GIu45Ser, Pro108G1y) mutant, recorded at close to equal
concentrations.
Figure 11 shows the inhibition of the binding of biotinylated recombinant Bet
v 1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and by the (Asn28Thr, Lys32Gln, GIu45Ser, Pro108G1y) mutant.
Figure 12 shows a graphical illustration of the 2-step PCR mutation technique
used for generating mutated Bet v 1 allergens.
Figure 13 shows a graphical illustration of the PCR mutation events leading
to the cloning of Bet v 1 (3004), (3005), (3007) and (3009). Primers used for
introducing point mutations are listed.
Figure 14 shows a graphical illustration of the PCR mutation events leading
to the cloning of Bet v 1 (3031 ) to (3045). Degenerated primers used for
introducing random mutations in position 10, 20, 36, 73, 87, 129 and 149 are
listed. The possible outcome of mutation for each position is shown at the
top.
Figure 15 shows schematically the primers used to create the mutations. (I)
shows the sense and antisense primers. (II) shows the final recombinant
protein harbouring mutations at the indicated positions.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
13
Figure 16 shows an illustration of the construction of Bet v 1 mutants and a
listing of the primers used. The mutants contain from five to nine amino
acids.
Figure 17 shows introduced point mutations at the surface of Bet v 1 (2628)
and Bet v 1 (2637). In mutant Bet v 1 (2628), five primary mutations were
introduced in one half of Bet v 1 leaving the other half unaltered. In mutant
Bet v 1 (2637), five primary and three secondary mutations were introduced
in the other half, leaving the first half unaltered.
Figure 18 shows the circular dichroism (CD) spectra of recombinant Bet v
1.2801 (wild type) and the Bet v 1 (2637) mutant recorded at nearly identical
concentrations.
Figure 19 shows the inhibition of the binding of biotinylated recombinant Bet
v 1.2801 (wild type) to serum IgE from a pool of allergic patients by non-
biotinylated Bet v 1.2801 and by Bet v 1 (2628), Bet v 1 (2637), and a 1:1 mix
of Bet v 1 (2628) and Bet v1 (2637).
Figure 20 shows histamine release in human basophil cells of Bet v 1.2801
(wild type), Bet v 1 (2628), and Bet v 1 (2637)
Figure 21 shows histamine release in human basophil cells of Bet v 1.2801
(wild type), Bet v 1 (2628), and Bet v 1 (2637).
Figure 22 shows point mutations at the surface of Bet v 1 (2744).
Figure 23 shows point mutations at the surface of Bet v 1 (2753).
Figure 24 shows point mutations at the surface of Bet v 1 (2744) and Bet v 1
(2753).
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
14
Figure 25 shows circular dichroism (CD) spectra of Bet v 1.2801 (wild type)
and Bet v 1 (2744), recorded at nearly equal concentrations.
Figure 26 shows histamine release in human basophil cells of Bet v 1.2801
(wild type), and mutant Bet v 1 (2744).
Figure 27 shows histamine release in human basophil cells of Bet v 1.2801
(wild type), and mutant Bet v 1 (2744).
Figure 28 shows point mutations at the surface of Bet v 1 (2733).
Figure 29 shows the proliferation of Peripheral Blood Lymphocytes
expressed as Stimulation Index (SI) for various Bet v 1 preparations.
Figures 30-32 show the cytokine profile of T cells stimulated with various Bet
v 1 preparations. Figure 30 shows a patient with a Th0 profile, Figure 31 a
Th1 profile and Figure 32 a Th2 profile.
Figure 33 shows Circular dichroism (CD) spectroscopy of rBet v 1.2801 (~)
(wildtype) and the rBet v 1 3007) mutant [O] with 12 mutations, recorded at
equal concentrations. Overlay of circular dichroism (CD) spectra obtained at
15°C are shown.
Figure 34 shows the inhibition of the binding of biotinylated rBet v 1.2801 to
pooled IgE serum from birch allergic patients by rBet v 1.2801 (~) (wildtype)
or mutated rBet v 1 (3007) [O] with 12 mutations.
OBJECT OF THE INVENTION
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
5
The object of the invention is to provide improved recombinant mutant
allergen proteins,
Rationale behind the~resent invention
The current invention is based on a unique rationale. According to this
rationale the mechanism of successful allergy vaccination is not an alteration
of the ongoing Th2-type immune response, but rather a parallel initiation of a
new immune response involving tertiary epitope recognition by B-cells and
10 antibody formation. It is believed that this new immune response is. partly
a
Th1-type immune response. When the vaccine (or pharmaceutical
compositions) is administered through another route than the airways, it is
hypothesised, that the new immune response evolves in a location physically
separated from the ongoing Th2 response thereby enabling the two
responses to exist in parallel.
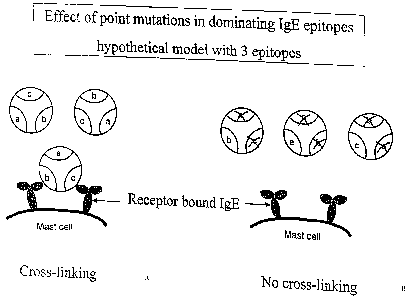
Furthermore, the invention is based on the finding that allergic symptoms are
triggered by the cross-linking of allergen with at least two specific IgE's
bound to the surface of effector cells, i.e. mast cells and basophils, via the
high affinity IgE receptor, Fc~RI. For illustration, we refer to Fig. 1, which
depicts a theoretical model of an allergen with three IgE binding epitopes.
Induction of mediator release from the mast cell and hence allergic
symptoms is effected by allergen-mediated cross-linking of IgE bound to the
surface of the mast cell, cf. Fig 1 A. In the situation shown in Fig. 1 B two
of
the epitopes have been mutated so as to reduce their IgE binding ability, and
hence the allergen-mediated cross-linking is prevented. In this connection it
should be noted that allergens usually comprise more than three B cell
epitopes.
In order for a mutant allergen to be able to raise the new immune response,
including an IgG response, the mutant allergen must comprise at least one
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
16
intact epitope or an epitope, which has been altered only moderately. The
surface topography of a moderately altered epitope preferably resembles the
original epitope, allowing new more numerous IgG antibodies to be raised.
These new IgG antibodies have specificities which can compete and to some
degree oust IgE binding to the natural occurring allergen. Further, it may be
assumed that the more epitopes, which have been mutated so as to
eliminate or reduce their IgE binding ability, the lower the risk of allergen-
mediated cross-linking and resulting allergic symptoms upon administration
of an allergen vaccine.
According to this rationale it is essential that the mutant allergen has an a-
carbon backbone tertiary structure which is essentially the same as that of
the natural allergen.
It has previously been assumed that positions suitable for mutation are
located exclusively in areas consisting of conserved amino acid residues
believed to harbour dominant IgE binding epitope. However, according to the
present invention it appears that surface exposed amino acid residues
suitable for mutation comprise both highly conserved residues and residues
that are not conserved or only conserved to a smaller degree. Such amino
acid residues appear to be distributed over the entire molecular surface with
a tendency to form small groupings covering a defined area on the molecular
surface.
Thus, according to the present invention, surface exposed amino acids
suitable for mutation can be divided into groups as illustrated in Fig. 2. The
groupings rely on the tendency of these amino acid residues to form separate
areas and these groupings are furthermore independent of the degree of
conservation of the amino acid residues. Each group represents a number of
surface exposed amino acid residues that are found within a limited area on
the surface of the allergen. Each individual group most likely comprises part
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
17
of at least one epitope or at least one intact epitope. Each separate group
may comprise as well amino acids positions that will result in a moderately
altered epitope upon mutation as well as amino acid positions that will result
in a more drastic alteration of the epitope upon mutation. A single amino acid
residue typically results in a moderate alteration of an epitope if the
original
amino acid residue is substituted with an amino acid that posseses similar
chemical features (E.g. exchanging a hydrophobic amino acid with another
hydrophobic amino acid residue). In conclusion, by selecting mutations
among amino acid residues from at least four of the defined groups provides
a tool for rendering it very likely that a mutant allergen according to the
present invention is mutated in several B-cell epitopes and has a a-carbon
backbone structure that is similar to the naturally occurring allergen.
It is furthermore an important aspect of the present invention that the
mutated
allergen retains a continous surface region with an area of about 400-800 A2
that contains either no mutations or only moderate mutations. It is believed
that an allergen comprises a number of potential binding regions for specific
IgE's, wherein each region has an area of approximately 800 A2.
The inventive idea of the present invention is based on the recognition that a
mutated allergen having IgE binding reducing mutations in at least 4 defined
groups, each group comprising surface exposed amino acids suitable for
mutation, but retaining at least one intact or moderately altered epitope,
would on the one hand reduce the allergen-mediated cross-linking and on the
other hand allow the raising of an IgG response with a binding ability
competitive with that of IgE. Thus, the said mutated allergen constitutes a
highly advantageous allergen in that the risk of anaphylactic reactions is
being strongly reduced. The mutant allergen has the potential to be
administered in relatively higher doses improving its efficacy in generating a
protective immune response without compromising safety.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
18
Also, the present invention is based on the recognition that a vaccine
comprising a mixture of different such mutated allergens, wherein ideally
many or all epitopes are represented as intact epitopes or epitopes that are
only moderately altered on different mutated allergens, would be equally
efficient in its ability to induce protection against allergic symptoms as the
natural occurring allergen from which the mutated allergens are derived.
SUMMARY OF THE INVENTION
The present invention relates to the introduction of amino acid substitutions
into allergens. The amino acid substitutions are chosen from at least four
groups of amino acids suitable for amino acid substitution. The object being
to reduce the specific IgE binding capability of each mutated epitope while
retaining at least one intact or only moderately altered epitope on the
mutated allergen.
In particular the present invention relates to a recombinant Bet v 1 allergen,
characterised in that it is a mutant of a naturally occurring Bet v 1 allergen
where;
the mutant retains essentially the same oc-carbon backbone structure as the
naturally occurring allergen,
the mutant comprises at least four primary mutations, which each reduce the
specific IgE binding capability of the mutated allergen as compared to the IgE
binding capabiVity of the naturally occurring Bet v 1 allergen,
each primary mutation is a substitution of one surface-exposed amino acid
residue with another residue,
the mutations are placed in such a manner that at least one area of 400-800
A2 comprises either no mutations or one or more moderate mutations,
the primary mutations are selected from at least 4 of the following 10 groups,
each group comprising surface exposed amino acid positions suitable for
amino acid substitution:
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
19
group 1: A130, E131, K134, A135, K137, E138, E141, T142, 8145;
group 2: V2, F3, N4, Y5, E6, T7, K119;
group 3: D27, S39, S40, Y41, E42, N43, 144, E45, G46, N47, P50, G51,
K55, D72, E73;
group 4: E8, T10, V12, P14, V105, A106, T107, P108, D109, 6110, 1113,
K115;
group 5: A16, K20, S149, Y150, L152, A153, H154, S155, D156, Y158,
N159, +160, wherein +160 represents addition of an N-terminal amino acid;
group 6: L24, D25, N28, K32;
group 7: H76, T77, N78, F79, K80, E101, K103;
group 8: K68, R70, 186, E87, E96, K97;
group 9: G1, G92, D93, T94, K123, 6124, D125, H126, E127, K129;
group 10: P35, Q36, E60, G61, P63, F64, K65, Y66;
with the proviso that the recombinant Bet v 1 allergen is not one of the
following specific mutants: (Asn28Thr, Lys32Gln, Asn78Lys, Lys103Va1,
Arg145G1u, Asp156His, +160Asn); (TyrSVal, GIu42Ser, GIu45Ser, Asn78Lys,
Lys103Va1, Lys12311e, Lys134G1u, Asp156His); (TyrSVal, GIu45Ser,
Lys65Asn, Lys97Ser, Lys134G1u); (AIa16Pro, Asn28Thr, Lys32Gln,
Lys103Thr, Pro108G1y, Leu152Lys, AIa153G1y, Ser155Pro); (N28T, K32Q,
N78K, K103V, P108G, R145E, D156H, +160N); (TyrSVal, Lys134G1u,
Asn28Thr, Lys32Gln, GIu45Ser, Lys65Asn, Asn78Lys, Lys103Va1, Lys97Ser,
Pro108G1y, Arg145G1u, Asp156His, +160Asn); (TyrSVal, Lys134G1u,
GIu42Ser, GIu45Ser, Asn78Lys, Lys103Va1, Lys12311e, Asp156His,
+160Asn); (Asn28Thr, Lys32Gln, Lys65Asn, GIu96Leu, Lys97Ser,
Pro108G1y, Asp109Asn, Asp125Tyr, GIu127Ser, Arg145G1u); (YSV, N28T,
K32Q, E42S, E45S, N78K, K103V, P108G, K1231, K134E, D156H, +160N);
(YSV, E42S, E45S, K65N, N78K, K97S, K103V, K1231, K134E, D156H,
+160N); and (YSV, N28T, K32Q, E42S, E45S, K65N, N78K, K97S, K103V,
P108G, K1231, K134E, D156H, +160N).
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
More specifically, the present invention relates to a recombinant Bet v 1
allergen where the primary mutations are selected from at least 4 of the
following 10 groups, each group comprising surface exposed amino acid
positions suitable for the following amino acid substitutions:
5 group 1: A130: A130V, A130G, A1301, A130L, A130S, A130H, A130T; E131:
E131 D, E131 H, E131 K, E131 R, E131 S; K134: K134R, K134H, K134S,
K134Q, K1341, K134E; A135: A135V, A135G, A1351, A135L, A135S, A135H,
A135T; K137: K137R, K137H, K137S, K137Q, K1371, K137E; E138: E138D,
E138H, E138K, E138R, E138S, E138N; E141: E141 D, E141 H, E141 K,
10 E141 R, E141 S; T142: T142A, T142S, T142L, T142V, T142D, T142K, T142N;
8145: R145K, R145H, R145T, R145D, R145E;
group 2: V2: V2A, V21, V2K, V2L, V2R, V2T; F3: F3H, F3W, F3S, F3D; N4:
N4H, N4K, N4M, N4Q, N4R; Y5: YSD, YSG, YSH, Y51, YSK, YSV; E6: E6D,
E6H, E6K, E6R, E6S; T7: T7P, T7S, T7L, T7V, T7D, T7K, T7N; K119:
15 K119R, K119H, K119S, K119Q, K1191, K119E, K119N;
group 3: D27: D27E, D27H, D27K, D27R, D27S; S39: S39T, S39L, S39V,
S39D, S39K; S40: S40T, S40L, S40V, S40D, S40K; Y41: Y41 D, Y41 G,
Y41 H, Y41 I, Y41 K, Y41 V; E42: E42S, E42D, E42H, E42K, E42R; N43:
N43H, N43K, N43M, N43Q, N43R; 144: 144L, 144K, 1448, 144D; E45: E45S,
20 E45D, E45H, E45K, E45R; G46: G46N, G46H, G46K, G46M, G46Q, G46R;
N47: N47H, N47K, N47M, N47Q, N47R; P50: P50G; G51: G51 N, G51 H,
G51 K, G51 M, G51 Q, G51 R; K55: K55R, K55H, K55S, K55Q, K551, K55E,
K55N; D72: D72E, D72S, D72H, D72R, D72K; E73: E73D, E73S, E73H,
E73R, E73K;
group 4: E8: EBD, EBH, EBK, E8R, EBS; T10: T10P, T10S, T10L, T10V,
T10D, T10K, T10N; V12: V12A, V121, V12K, V12L, V12R, V12T; P14: P14G;
V105: V105A, V1051, V105K, V105L, V105R, V105T; A106: A106V, A106G,
A1061, A106L, A106S, A106H, A106T; T107: T107A, T107S, T107L, T107V,
T107D, T107K, T107N; P108: P108G; D109: D109N D109E, D109S" D109H,
D109R, D109K; 6110: G110N, G110H, G110K, G110M, G110Q, G110R;
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
21
1113: 1113L, 1113K, 11138, 1113D, K115: K115R, K115H, K115S, K115Q,
K1151, K115E, K115N;
group 5: A16: A16V, A16G, A161, A16L, A16S, A16H, A16T; K20: K20R,
K20H, K20S, K20Q, K201, K20E, K20N; S149: S149T, S149L, S149V,
S149D, S149K; Y150: Y150T, Y150L, Y150V, Y150D, Y150K; L152: L152A,
L152V, L152G, L1521, L152S, L152H, L152T; A153: A153V, A153G, A1531,
A153L, A153S, A153H, A153T; H154: H154W, H154F, H154S, H154D;
S155: S155T, S155L, S155V, S155D, S155K; D156: D156H, D156E, D156S,
D156R, D156K; Y158: Y158D, Y158G, Y158H, Y1581, Y158K, Y158V; N159:
N159H, N159K, N159M, N159Q, N159R, N159G, +160N;
group 6: L24: L24A, L24V, L24G, L241, L24S, L24H, L24T; D25: D25E,
D25H, D25K, D25R, D25S; N28: N28H, N28K, N28M, N28Q, N28R, N28T;
K32: K32Q, K32R, K32N, K32H, K32S, K321, K32E;
group 7: H76: H76W, H76F, H76S, H76D; T77: T77A, T77S, T77L, T77V,
T77D, T77K, T77N; N78: N78H, N78K, N78M, N78Q, N78R; F79: F79H,
F79W, F79S, F79D; K80: K80R, K80H, K80S, K80Q, K801, K80E, K80N;
E101: E101 D, E101 H, E101 K, E101 R, E101 S; K103: K103R, K103H, K103S,
K103Q, K1031, K103E, K103V;
group 8: K68: K68R, K68H, K68S, K68Q, K681, K68E, K68N; 870: R70K,
R70H, R70T, R70D, R70E, R70N; 186: 186L, 186K, 1868, 186D; E87: E87D,
E87H, E87K, E87R, E87S, E87A; E96: E96D, E96H, E96K, E96R, E96S,
E96L; K97: K97R, K97H, K97S, K97Q, K971, K97E;
group 9: G1: G1 N, G1 H, G1 K, G1 M, G1 Q, G1 R; G92: G92N, G92H, G92K,
G92M, G92Q, G92R; D93: D93N, D93E, D93S, D93H, D93R, D93K; T94:
T94A, T94S, T94L, T94V, T94D, T94K, T94N; K123: K123R, K123H, K123S,
K123Q, K1231, K123E; 6124: G124N, G124H, G124K, G124M, G124Q,
G124R; D125: D125E, D125H, D125K, D125R, D125S, D125Y; H126:
H126W, H126F, H126S, H126D; E127: E127D, E127H, E127K, E127R,
E127S; K129: K129R, K129H, K129S, K129Q, K1291, K129E, K129N;
group 10: P35: P35G; Q36: Q36K, Q36R, Q36N, Q36H, Q36S, Q361, Q36E;
E60: E60H, E60K, E60M, E60Q, E60R; G61: G61 N, G61 H, G61 K, G61 M,
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
22
G61 Q, G61 R; P63: P63G; F64: F64H, F64W, F64S, F64D; K65: K65R,
K65H, K65S, K65Q, K651, K65E, K65N; Y66: Y66D, Y66G, Y66H, Y661,
Y66K, Y66V.
The present invention further relates to a recombinant Bet v 1 mutant
allergen comprising substitutions that are selected from at least four of the
following 10 groups:
Group 1: A130V, K134E, E141 N,
Group 2: V2L, YSV, E6S, K119N,
Group 3: E42S, E45S, N47K, K55N, E73S, E73T, E73S, ,
Group 4: EBS, T10P, P14G, P108G, D109N, K115N,
Group 5: A16G, K20S, S149T L152A A153V, S155T, N159G, +160N,
Group 6: L24A, D25E, N28T, K32Q,
Group 7: T77A, T77N, N78K, K103V,
Group 8: R70N, E87A, E96S, K97S,
Group 9: D93S, K1231, D125Y, K129N,
Group 10: Q36N, E60S, G61 S, P63G.
The present invention further relates to a recombinant Bet v 1 mutant
allergen comprising substitutions that are selected from at least four of the
following 10 groups:
Group 1: K134E,
Group 2: YSV, K119N, V2L,
Group 3: E45S, E42S, K55N, N47K, E73S,
Group 4: E96S, K97S, P108G, D109N, T10N, K115N, P14G,
Group 5: N159G, +160N, S149T, A153V, L152A, A16G, K20S,
Group 6: N28T, K32Q, L24A,
Group 7: K103V, T77N, N78K,
Group 8: E96S, K97S, E87A,
Group 9: K129N, D125Y, K1231, D93S,
Group 10: E60S, Q36N, G61 S, P63G.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
23
The present invention further relates to the following:
Recombinant Bet v 1 allergens variants that can be used as a
pharmaceutical and for preparing a pharmaceutical for preventing and/or
treating birch pollen allergy.
A composition comprising two or more different recombinant mutant Bet v 1
allergen variants according to the present invention wherein each variant has
at least one primary mutation, which is absent in at least one of the other
variants. The composition comprises 2-12, preferably 3-10, more preferably
4-9 and most preferably 5-8 variants. A composition according to the present
invention can be used as a pharmaceutical and for preparing a
pharmaceutical for preventing andlor treating birch pollen allergy. The
pharmaceutical composition preferably comprises a pharmaceutically
acceptable carrier, andlor excipient, and optionally an adjuvant.
A pharmaceutical composition in the form of a vaccine against allergic
reactions elicited by a naturally occurring Bet v 1 allergen in patients
suffering
from birch pollen allergy.
Methods of generating an immune response in a subject comprising
administering to a subject a recombinant allergen, a composition, or a
pharmaceutical composition.
Vaccination or treatment of a subject comprises administering to the subject
a recombinant allergen, a composition, or a pharmaceutical composition.
A method for preparing a pharmaceutical composition comprising mixing a
recombinant allergen, or a composition with pharmaceutically acceptable
substances, and/or excipients.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
24
A method for the treatment, prevention or alleviation of allergic reactions in
a
subject that comprises administering to a subject a recombinant Bet v 1
allergen, a composition, or a pharmaceutical composition.
A method of preparing a recombinant Bet v 1 allergen characterised in that
the substitution of amino acids is carried out by site-directed mutagenesis,
or
DNA shuffling (molecular breeding) (Punnonen et al., ref. 25).
A DNA sequence encoding a recombinant Bet v 1 allergen, a derivative
thereof, a partial sequence thereof, a degenerated sequence thereof or a
sequence which hybridises thereto under stringent conditions, wherein said
derivative, partial sequence, degenerated sequence or hybridising sequence
encodes a peptide having at least one B cell epitope.
A DNA sequence which is a derivative of the DNA sequence encoding the
naturally occurring allergen. The DNA seqeucne encoding the derivative is
obtained by site-directed mutagenesis of the DNA encoding the naturally
occurring Bet v 1 allergen.
An expression vector comprising DNA encoding a recombinant Bet v 1
variant, a host cell comprising the expression vector, and a method of
producing a recombinant mutant Bet v 1 allergen comprising cultivating the
host cell.
A recombinant Bet v 1 allergen or a recombinant Bet v 1 allergen that is
encoded by the DNA sequence comprises at least one T celle epitope
capable of stimulating a T cell clone or T cell line specific for the
naturally
occurring Bet v 1 allergen.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
A diagnostic assay for assessing relevance, safety, or outcome of therapy of
a subject using a recombinant mutant Bet v 1 allergen or a composition,
wherein an IgE containing sample of a subject is mixed with said mutant or
said composition and assessed for the level of reactivity between the IgE in
5 said sample and said mutant.
DETAILED DESCRIPTION OF THE INVENTION
10 In connection with the present invention the expression "reduce the
specific
IaE binding capability as compared to the IgE binding capability of the
naturally occurring allergen" means that the reduction is measurable in a
statistically significant manner (p <0.05) in at least one immunoassay using
serum from a subject allergic to the natural-occurring allergen. Preferably,
the
15 IgE binding capability is reduced by at least 10%, more preferably at least
30%, more preferably at least 50%, and most preferably at least 70%.
The expression "surface-exposed amino acid" means that the amino acid
residue is located at the surface of the three-dimensional structure in such a
20 manner that when the allergen is in solution at least a part of at least
one
atom of the amino acid residue is accessible for contact with the surrounding
solvent. Preferably, the amino acid residue in the three-dimensional structure
has a solvent (water) accessibility of at least 20%, suitably at least 30%,
more suitably at least 40% and most preferably at least 50%.
The expression "solvent accessibility" is defined as the area of the molecule
accessible to a sphere with a radius comparable to a solvent (water, r = 1.4
A) molecule. The expressions "surtace-exposed" and "solvent-exposed" are
used interchangeably.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
26
"Group of amino acids" should be understood as division of surface exposed
amino acids suitable for mutation into groups. Each group represents a
number of surface exposed amino acid residues that are found within a
limited area on the surface of the allergen. An individual group comprises a
number of amino acids that are part of at least one epitope. An individual
group may also cover an area that comprises an entire epitope. One or more
mutations within a single group is defined as one primary mutation. A
mutated allergen with at least four primary mutations thus ensures that
several epitopes will have a lowered IgE binding affinity. Mutation of amino
acids from at least four groups may furthermore ensure an approximately
even distribution of mutations on the molecular surface and ensure that
several epitopes are mutated and thus resulting in a lowered IgE binding
affinity of several epitopes compared to mutants with less than four
mutations.
The expression "the taxonomic species from which said naturally occurring
allergen originates" means species within the taxonomic genus, preferably
the subfamily, more preferably the family, more preferably the superfamily,
more preferably the legion, more preferably the suborder and most preferably
the order from which said naturally occurring allergen originates.
The expression "moderately altered ~oitopes" means epitopes that retain
essentially the same tertiary structure and surface topography as the
corresponding unmutated epitopes. Moderate alterations are, generally
speaking, achieved by exchanging an amino acid with another amino acid
with similar chemical characteristics as the original amino acid. One way of
achieving this is by exchanging one or more surface exposed amino acids
with amino acids that might be found within the taxonomic order wherein the
naturally occurring allergen is found. A moderately altered epitope might also
contain amino acid substitutions where one or more of the substituted amino
acid is not found within the taxonomic order wherein the naturally occurring
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
27
allergen is found, as long as the substitution only slightly affects the
tertiary
structure of the epitope and/or the IgE binding affinity. The mutated allergen
can be evaluated with respect to e.g. structure and IgE binding affinity
subsequently. As opposed to the moderately altered epitopes are epitopes
that are altered in a more drastic manner, e.g. mutations that significantly
reduce the IgE binding affinity. Typically, drastic alterations of epitopes
comprise amino acid substitutions where one or more amino acids have been
exchanged with amio acids with different chemical properties.
Furthermore, the expression "the mutant allergen having essentially the
same a-carbon backbone tertiary structure as the naturally occurring
allergen" means that when comparing the structures of the mutant and the
naturally occurring allergen, the average root mean square deviation of the
atomic coordinates is preferably below 2 A. Conservation of a-carbon
backbone tertiary structure is best determined by obtaining identical
structures by x-ray crystallography or NMR before and after mutagenesis. In
absence of structural data describing the mutant indistinguishable CD-
spectra or immunochemical data, e.g. antibody reactivity, may render
conservation of a-carbon backbone tertiary structure probable, if compared
to the data obtained by analysis of a structurally determined molecule.
In connection with the present invention the expression "mutation" means the
deletion, substitution or addition of an amino acid in comparison to the amino
acid sequence of the naturally occurring allergen. The terms "mutation" and
"substitution" are used interchangeably. A recombinant mutated Bet v 1
allergen according to the invention may furthermore comprise amino acid
insertions or amino acid deletion in particular surface exposed regions of the
molecules e.g. "loop regions". Loop regions connect secondary structure
elements e.g. f3-sheet, a-helixes and random coil structures. Loop regions in
Bet v 1 are: Va112 to a1a16, va133 to ser40, g1u45 to Thr52, pro54 to tyr66,
his76 to asn78, g1y89 to g1u96, va1105 to g1y111, thr122 to g1u131. Mutant
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
28
variants may comprise 1-5, more preferable 1-3 most preferably 1-2
substitutions in a loop region.
A primary mutation is defined as one or more mutations within a single group
of surface exposed amino acids suitable for substitution. Each group of at
least one mutated amino acids will have reduced IgE binding affinity as
compared to the same group without mutations. Preferably, the recombinant
allergen according to the invention comprises from 5 to 10, preferably from 6
to 10, more preferably from 7 to 10, and most preferably from 8 to 10 primary
mutations.
Secondary mutations are defined as additional mutations within a single
group. The recombinant allergen preferably comprises a number of
secondary mutations, which each reduce the specific IgE binding capability of
the mutated allergen as compared to the binding capability of the said
naturally occurring allergen. Thus, a primary mutation that comprises several
secondary mutations will in many cases have a more reduced IgE binding
affinity than a primary mutation that has only one mutation. The recombinant
allergen according to the invention comprises from 1 to 15, preferably 1-10
and most preferably 1-5 secondary mutations per primary mutation.
Conserved residues: Conserved residues in the naturally occurring allergen
are conserved with more than 70 %, preferably 80 % and most preferably 90
identity in all known homologous proteins within the species from which
said allergen originates. Amino acid residues that are highly solvent exposed
and conserved constitute targets for substitution.
Another way of assessing the reduced IgE binding and the reduced ability of
mediating cross-linking of the mutant are the capability of the mutant to
initiate Histamine Release (HR). The release of Histamine can be measured
in several Histamine releasing assays. The reduced Histamine release of the
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
29
mutants originates from reduced affinity toward the specific IgE bound to the
cell surface as well as their reduced ability to facilitate cross-linking. HR
is
preferably reduced by 5-100%, more preferably 25-100%, more preferably
50-100% and most preferably 75-100% for the mutants of the invention in
comparison to the naturally occurring allergens.
In a preferred embodiment of the invention, a surface region comprising no
mutation or only moderate mutations has an area of 800 A~, preferably 600
A~, more preferably 500 A2 and most preferably 400 A2. Typically, a surface
region with an area of 800 A2 comprising no mutation or only moderate
mutations comprises atoms of 15-25 amino acid residues.
In another embodiment, at least one of the amino acid residues to be
incorporated into the mutant allergen does not occur in the same position in
the amino acid sequence of any known homologous protein within the
taxonomic genus, preferably the subfamily, more preferably the family, more
preferably the superfamily, more preferably the legion, more preferably the
suborder and most preferably the order from which said naturally occurring
allergen originates.
According to the invention, the surface-exposed amino acid residues are
ranked with respect to solvent accessibility, and at least four amino acids
among the more solvent accessible ones are substituted.
In a further embodiment, a recombinant allergen is characterised in that the
surface-exposed amino acid residues are ranked with respect to degree of
conversation in all known homologous proteins within the species from which
said naturally occurring allergen originates, and that one or more surface
exposed amino acids among the more conserved ones are substituted. .
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
The principle disclosed in the present invention comprises mutation of
surface exposed amino acid residues selected from at least four groups of
amino acids, wherein each group represents separate areas on the surface
on the molecule. This principle may also be applied to allergens other than
5 Bet v 1. A recombinant allergen according to the invention may suitably be a
mutant of an inhalation allergen originating i.a. from trees, grasses, herbs,
fungi, house dust mites, cockroaches and animal hair and dandruff. Important
pollen allergens from trees, grasses and herbs are such originating from the
taxonomic orders of Fagales, Oleales and Pinales including i.a. birch
10 (Betula), alder (Alnus), hazel (Corylus), hornbeam (Carpinus) and olive
(Olea), the order of Poales including i.a. grasses of the genera Lolium,
Phleum, Poa, Cynodon, Dactyiis and Secale, the orders of Asterales and
Urticales including i.a. herbs of the genera Ambrosia and Artemisia.
Important inhalation allergens from fungi are i.a. such originating from the
15 genera Alternaria and Cladosporium. Other important inhalation allergens
are
those from house dust mites of the genus Dermatophagoides, those from
cockroaches and those from mammals such as cat, dog and horse. Further,
recombinant allergens according to the invention may be mutants of venom
allergens including such originating from stinging or biting insects such as
20 those from the taxonomic order of Hymenoptera including bees (superfamily
Apidae), wasps (superfamily Vespidea), and ants (superfamily Formicoidae).
Specific allergen components include e.g. Bet v 1 (8. verrucosa, birch), Aln g
1 (Alnus glutinosa, alder), Cor a 1 (Corylus avelana, hazel) and Car b 1
25 (Carpinus betulus, hornbeam) of the Fagales order. Others are Cry j 1
(finales), Amb a 1 and 2, Art v 1 (Asterales), Par j 1 (Urticales), Ole a 1
(Olea/es), Ave a 1, Cyn d 1, Dac g 1, Fes p 1, Hol I 1, Lol p 1 and 5, Pas n
1,
Phl p 1 and 5, Poa p 1, 2 and 5, Sec c 1 and 5, and Sor h 1 (various grass
pollens), Alt a 1 and Cla h 1 (fungi), Der f 1 and 2, Der p 1 and 2 (house
dust
30 mites, D. farinae and D, pteronyssinus, respectively), Eur m 1 (mite,
Euroglyphus maynei), (Lep d 1 and 2 (Lepidoglyphus destructor; storage
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
31
mite), Bla g 1 and 2, Per a 1 (cockroaches, Blatella germanica and
Periplaneta americana, respectively), Fel d 1 (cat), Can f 1 (dog), Equ c 1, 2
and 3 (horse), Apis m 1 and 2 (honeybee), Ves v 1, 2 and 5, Pol a 1, 2 and 5
(all wasps) and Sol i 1, 2, 3 and 4 (fire ant).
Some examples of adding further substitutions to a given mutant
In one embodiment of the invention further substitutions are added to mutant
allergens in such a way that it is ensured that the substitutions of the final
mutant allergen are essentially evenly distributed on the molecular surface
and that the groups contain essentially the same number of introduced
mutations. This is illustrated in the following examples where mutants
comprising specific substitutions preferably should have added further
substitutions from a list where the succession of amino acids reflects the
preferred order of adding more substitutions. Without limiting the present
invention, these examples represent one application of how to design
mutants and the man skilled in the art might very well choose a somewhat
different approach in order to ensure an even distribution of substitutions.
Mutants may thus be designed comprising one or more substitutions from the
lists given below.
Bet v 1 mutant ("3004A") allergens comprising the following substitutions:
YSV, E45S, N78K, K97S, K103V, K134E, +160N. Further substitutions may
comprise one or more of the following: E8 or K115, D125 or H126, E138 or
K137 or E141, D25 or N28, E87 or K55, S155 or H154 or N159, N47 or P50
or H76 or N43 or 144 or R70, E73 or P50 or D72, A130, N28 or D25, P 108,
V2 orK119orN4orE6orE96.
Bet v 1 mutant ("30048") allergens comprising the following substitutions:
YSV, E45S, L62F, N78K, K97S, K103V, K134E, +160N. Further substitutions
may comprise one or more of the following: T10P, K65N, N28 or D25 or
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
32
K32Q or E141X or K137X or E138X, D125X or K1231 or H126, P108X or
D109N, E42S or K55X or 144X or N43X, E73X or D72X, E87X, E96X or
K119, A130X, V2X or E6X, E8X or K115, N47X or P50X or R70X or H76X or
T77A, S 155X or D 156H or N 159X, E6X or V2X.
Bet v 1 allergen mutants ("3005A") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, N78K, K97S, K103V, K134E, +160N.
Further substitutions may comprise one or more of the following: E8X or
K115X, D125 or H126, E138X or K137X or E141X, E87X or K55X, S155X or
H154X or N159X, N47X or P50X or H76X or N43X or 144X or 870X, E73X or
P50X or D72X, A130X, D25X, P108X, V2X or K119X or N4X or E6X or
E96X.
Bet v 1 allergen mutants ("3005B") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, N78K, K97S, K103V, K134E, +160N.
Further substitutions may comprise one or more of the following: T10P,
K65N, E141X or K137X or E138X, D125X or K1231 or H126X, P108X or
D109N, E42S or K55X or 144X or N43X, E73X or D72X, E87X, E96X or
K119X, A130X, V2X or E6X, E8X or K115X, N47X or P50X or R70X or H76X
or T77A, S155X or D156H or N159X, E6X or V2X.
Bet v 1 allergen mutants ("3006A") comprising the following substitutions:
YSV, N28T, K32Q, E45S, N78K, E87S, K97S, K103V, K134E, N159G,
+160N. Further substitutions may comprise one or more of the following:
K55, A138 or K137 or E141, D125 or H126, P108, V2 or N4 or K119 or E6,
S155 or H154, N47 or P50 or H76, E73, R70, A130, E8 or K115, E96.
Bet v 1 allergen mutants ("3006B") comprising the following substitutions:
YSV, N28T, K32Q, E45S, N78K, E87S, K97S, K103V, K134E, N159G,
+160N. Further substitutions may comprise one or more of the following:
K65N, T10P, D125, K1231, P108, D109N, N47 or P50 or H76, E138 or K137
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
33
or E141, E42S or K55 or 144 or N43, S155 or D156H, E73 or D72, E6 or V2,
E96.
Bet v 1 allergen mutants ("3007A") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, N78K, K97S, K103V, P108G, D125Y,
K134E, +160N. Further substitutions may comprise one or more of the
following: E87, E141, E138, K55, N47 or N43X or 144 or H76, S155 or H154,
A130, E8, E73, V2 or K119, D25.
Bet v 1 allergen mutants ("3007B") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, N78K, K97S, K103V, P108G, D125Y,
K134E, +160N. Further substitutions may comprise one or more of the
following: K65N, T10P or E8, E87, S155 or D156H, E138, E141, E42S,
A130, E8 or T1 OP, N47, H76, R70, E96.
Bet v 1 allergen mutants ("3008A") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, E73S, E96S, P108G, D125Y, N159G,
+160N. Further substitutions may comprise one or more of the following:
E134, N78, E87, K119, E8, K55, E138, E141, S155, N47, E6, K103, D25,
A130, V2, R70.
Bet v 1 allergen mutants ("3008B") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, E73S, E96S, P108G, D125Y, N159G,
+160N. Further substitutions may comprise one or more of the following:
K65N or K55, T10P or E8 or E141, E138 or K134, E87, E42S or K55 or 144,
S155 or D156H, N78, K119 or V2 or N4, N47 or P50, H76 or T77A, A130,
D25, E6 or K115 or K103.
Bet v 1 allergen mutants ("3009A") comprising the following substitutions:
YSV, N28T, K32Q, E45S, L62F, E96S, P108G, +160N. Further substitutions
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
34
may comprise one or more of the following: E134, N78, E87, K119, E8, K55,
E 138, E 141, S 155, N47, E6, K103, D25, A130, V2, R70.
Bet v 1 allergen mutants ("3009B") comprising the following substitutions:
Y5V, N28T, K32Q, E45S, L62F, E96S, P108G, +160N. Further substitutions
may comprise one or more of the following: N78 or T77A, K103, E134 or
E138, K65N or K55, T10P, D125 or H126, S155 or D156H or HIS154, K119
or V2, E87, N47 or P50 or H76, E42S or K55, 144 or N43, A130.
Loop mutations:
In another embodiment of the invention mutant allergens according to the
invention furthermore comprise amino acid insertions or amino acid deletion
in particular surface exposed regions of the molecules e.g. loop regions.
Loop regions connect secondary structure elements e.g. f3-sheet, a-helixes
and random coil structures. Loop regions in Bet v 1 are: va112 to a1a16, va133
to ser40, g1u45 to Thr52, pro54 to tyr66, his76 to asn78, g1y89 to g1u96,
va1105 to g1y111, thr122 to g1u131. Mutant variants according to this
embodiment comprise 1-5, more preferable 1-3 most preferably 1-2
substitutions in a loop region. In a preferred embodiment, mutant allergens
comprise at least four mutations selected from the 10 groups as well as a
number of additional "loop-mutations". Examples of such "loop mutations",
wherein x represents an added amino acid residue, are:
Bet v 1 (3007-L1 ) with an amino acid insertion between residue E60 and
residue G61:
GVFNVETETTSVIPAARLFKAFILDGDTLFPQVAPQAISSVENISGNGGPGTI
KKISFPExGFPFKYVKDRVDEVDHTKFKYNYSVIEGGPIGDTLESISNEIVIVA
TGDGGSILKISNKYHTKGYHEVKAEQVEASKEMGETLLRAVESYLLAHSDA
YNN
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
Bet v 1 (3007-L2) with amino acid insertion between residue D93 and residue
T94:
GVFNVETETTSVIPAARLFKAFILDGDTLFPQVAPQAISSVENISGNGGPGTI
KKISFPEGFPFKYVKDRVDEVDHTKFKYNYSVIEGGPIGDxTLESISNEIVIVA
5 TGDGGSILKISNKYHTKGYHEVKAEQVEASKEMGETLLRAVESYLLAHSDA
YNN
Bet v 1 (3007-L3) with amino acid insertion between residue V12 and residue
I 13:
10 GVFNVETETTSVxIPAARLFKAFILDGDTLFPQVAPQAISSVENISGNGGPGT
IKKISFPEGFPFKYVKDRVDEVDHTKFKYNYSVIEGGPIGDTLESISNEIVIVA
TGDGGSILKISNKYHTKGYHEVKAEQVEASKEMGETLLRAVESYLLAHSDA
YNN
15 Bet v 1 (3007-L4) with amino acid insertions between residue 156 and
residue
S57 and between residue K65 and residue T66
GVFNVETETTSVIPAARLFKAFILDGDTLFPQVAPQAISSVENISGNGGPGTI
KKIxSFPExGFPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLESISNEIVIV
ATGDGGS I LKISNKYHTKGYH EVKAEQVEAS KEMG ETLLRAVESYLLAHSD
20 AYNN
Bet v 1 (3007-L5) with amino acid deletion of residue 6111
GVFNVETETTSVIPAARLFKAFILDGDTLFPQVAPQAISSVENISGNGGPGTI
KKISFPEGFPFKYVKDRVDEVDHTKFKYNYSVIEGGPIGDTLESISNEIVIVAT
25 GDGSILKISNKYHTKGYHEVKAEQVEASKEMGETLLRAVESYLLAHSDAYN
N
Method of preparing a recombinant allergen according to the invention
30 The surface-exposed amino acids suitable for substitution in accordance
with
the present invention may be identified on the basis of information of their
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
36
solvent (water) accessibility, which expresses the extent of surface exposure.
A preferred embodiment of the method of the invention is characterised in
ranking the said identified amino acid residues with respect to solvent
accessibility and substituting one or more amino acids among the more
solvent accessible ones.
Furthermore, another embodiment of the method of the invention is
characterised in ranking the identified amino acid residues with respect to
degree of conversation in all known homologous proteins within the species
from which said naturally occurring allergen originates and substituting one
or
more amino acids among the more conserved ones.
A further preferred embodiment of the method of the invention comprises
selecting the identified amino acids so as to form a mutant allergen, which
has essentially the same a-carbon backbone tertiary structure as said
naturally occurring allergen.
Another preferred embodiment of the method of the invention is
characterised in that the substitution of amino acid residues is carried out
by
site-directed mutagenesis.
An alternative preferred embodiment of the method of the invention is
characterised in that the substitution of amino acid residues is carried out
by
DNA shuffling or by setting up a library comprising suitable positions and
their preferred substitutents.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
37
Criteria for substitution
For molecules for which the tertiary structure has been determined (e.g. by x-
ray crystallography, or NMR electron microscopy), the mutant carrying the
substituted amino acids) should preferably fulfil the following criteria:
1. The overall a-carbon backbone tertiary structure of the
recombinant mutant is preferably conserved. Conserved is defined as an
average root mean square deviation of the atomic coordinates below 2A
when comparing the structures of the mutated allergen and the naturally
occurring allergen. This is important for two reasons: a) It is anticipated
that
the entire surface of the natural allergen constitutes an overlapping
continuum of potential antibody-binding epitopes. The majority of the surtace
of the molecule is not affected by the substitution(s), and thus retain its
antibody-binding inducing properties, which is important for the generation of
new protective antibody specificities being directed at epitopes present also
on the natural allergen. b) Stability, both concerning shelf-life and upon
injection into body fluids.
Conservation of a-carbon backbone tertiary structure is best determined by
obtaining identical structures by x-ray crystallography or NMR before and
after mutagenesis. In absence of structural data describing the mutant
indistinguishable CD-spectra or immunochemical data, e.a. antibodv
reactivity, may render conservation of a-carbon backbone tertiary structure
probable, if compared to the data obtained by analysis of a structurally
determined molecule.
2. The amino acids to be substituted are preferably located at the
surface, and thus accessible for antibody-binding. Amino acids located on the
surface in the three-dimensional structure usually have a solvent (water)
accessibility of at least 20%, suitably 20-80%, more suitably 30-80%. Solvent
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
38
accessibility is defined as the area of the molecule accessible to a sphere
with a radius comparable to a solvent (water, r = 1.4 A) molecule.
3. The substituted amino acids are selected from at least four groups. Each
group represents a number of preferred surface exposed amino acid
residues that are found within a limited area on the surface of the allergen.
One or more mutations within a single group is defined as one primary
mutation. An individual group comprises a number of amino acids that are
part of at least one epitope. An individual group may also comprise an entire
epitope. A mutated allergen with at least four primary mutations thus ensures
that several epitopes will have a lowered IgE binding affinity. Mutation of
amino acids from at least four groups furthermore ensures an approximately
even distribution of mutations on the molecular surface and it ensures that
several epitopes will become mutated and thus obtaining a lowered IgE
binding affinity of several epitopes.
With an object of essentially retaining the three-dimensional structure of the
allergen, the amino acid to be incorporated may be selected on the basis of a
comparison with a protein, which is a structural homologue to the allergen,
e.g. a protein, which belongs to the same taxonomic order as the allergen,
and which does not have any cross-reactivity with the allergen.
Vaccines:
Preparation of vaccines is generally well known in the art. Vaccines are
typically prepared as injectables either as liquid solutions or suspensions.
Such vaccine may also be emulsified or formulated so as to enable nasal
administration as well as oral, including buccal and sublingual,
administration. The immunogenic component in question (the recombinant
allergen as defined herein) may suitably be mixed with excipients which are
pharmaceutically acceptable and further compatible with the active
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
39
ingredient. Examples of suitable excipients are water, saline, dextrose,
glycerol, ethanol and the like as well as combinations thereof. The vaccine
may additionally contain other substances such as wetting agents,
emulsifying agents, buffering agents or adjuvants enhancing the
effectiveness of the vaccine.
Vaccines are most frequently administered parenterally by subcutaneous or
intramuscular injection. Formulations which are suitable for administration by
another route include oral formulations and suppositories. Vaccines for oral
administration may suitably be formulated with excipients normally employed
for such formulations, e.g. pharmaceutical grades of mannitol, lactose,
starch, magnesium stearate, sodium saccharine, cellulose, magnesium
carbonate and the like. The composition can be formulated as solutions,
suspensions, emulsions, tablets, pills, capsules, sustained release
formulations, aerosols, powders, or granulates.
The vaccines are administered in a way so as to be compatible with the
dosage formulation and in such amount as will be therapeutically effective
and immunogenic. The quantity of active component contained within the
vaccine depends on the subject to be treated, i.a. the capability of the
subject's immune system to respond to the treatment, the route of
administration and the age and weight of the subject. Suitable dosage ranges
can vary within the range from about 0.0001 pg to 1000 pg.
As mentioned above, an increased effect may be obtained by adding
adjuvants to the formulation. Examples of such adjuvants are aluminum
hydroxide and phosphate (alum) or calcium phosphate as a 0.05 to 0.1
percent solution in phosphate buffered saline, synthetic polymers of sugars
or polylactid glycolid (PLG) used as 0.25 percent solution. Mixture with
bacterial cells such as C. parvum, endotoxins or lipopolysaccharide
components of gram-negative bacteria, emulsion in physiologically
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
acceptable oil vehicles such as mannide monoaleate (Aracel A) or emulsion
with 20 percent solution of a perfluorocarbon (e.g. Fluosol-DA) used as a
block substitute may also be employed. Oil emulsions, such as MF-59 may
also be used. Other adjuvants such as Freund's complete and incomplete
5 adjuvants as well as saponins, such as QuilA, Qs-21 and ISGOM, and RIBI
may also be used.
Most often, multiple administrations of the vaccine will be necessary to
ensure an effect. Frequently, the vaccine is administered as an initial
10 administration followed by subsequent inoculations or other
administrations.
The number of vaccinations will typically be in the range of from 1 to 50,
usually not exceeding 35 vaccinations. Vaccination will normally be
performed from biweekly to bimonthly for a period of 3 months to 5 years.
This is contemplated to give desired level of prophylactic or therapeutic
15 effect.
The recombinant allergen may be used as a pharmaceutical preparation,
which is suitable for providing a certain protection against allergic
responses
during the period of the year where symptoms occur (prophylaxis). Usually,
20 the treatment will have to be repeated every year to maintain the
protective
effect. Preparations formulated for nasal, oral and sublingual application are
particular suited for this purpose.
DNA according to the invention
The DNA sequence of the invention is a mutant of a DNA sequence encoding
a naturally occurring Bet v 1 allergen. Examples of naturally occurring Bet v
1
molecules are SEQ ID NO 1 (data base accession number 280104) and SEQ
ID NO 2 (data base accession number P15494). Other Bet v 1 variants
include Bet v 1 sequences with the following data base accession numbers:
P15494=X15877=280106, 280101, AJ002107, 272429, AJ002108, 280105,
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
41
280100, 280103, AJ001555, 280102, AJ002110, 272436, P43183=X77271,
272430, AJ002106, P43178=X77267, P43179=X77268, P43177=X77266,
272438, P43180=X77269, AJ001551, P43185=X77273, AJ001557, 272434,
AJ001556, 272433=P43186, AJ001554, X81972, 272431, P45431=X77200,
P43184=X77272, P43176=X77265, S47250, S47251, 272435, 272439,
272437, and S47249.
Preferably, the DNA derivative is obtained by site-directed or random or semi
random mutagenesis of the DNA encoding the naturally occurring allergen.
A "mutant library" is a library of mutant allergens. This library is
constructed
using degenerated DNA oligonucleotide primers that allow introduction of
none, a single or several different amino acid residues in each position. Such
a library approach allows amino acid residues to be either conservatively or
non-conservatively substituted. As structural integrity may be less affected
by
conserved mutations introduction of such "soft" or moderate mutations in
certain positions may increase the changes of generating stable mutants.
Construction of mutant libraries may be one way to overcome problems with
protein stability of mutated allergens caused by a single or a certain
combination of mutations. A "semi-random library" means that positions to be
mutated are confined to amino acid residues, which are surface exposed.
This approach further enhances the probability of obtaining stable mutant
allergens. "Semi-random" can also mean that the primers designed allow for
a selected number of amino acid residues to be substituted in the chosen
position. The two semi-random approaches can be used independently or in
combination. Theoretically, a library according to the invention comprises a
number of rBet v 1 mutant allergens each having at least 4 amino acid
substitutions compared to non-mutated Bet v 1.
In one embodiment a semi-random library based on rBet v 1 (2744) (mutated
in positions Y5, E42, E45, N78, K103, K123, K134, D156, +160) and rBet v1
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
42
(2628) (mutated in positions Y5, E45, K65, K97, K134) was constructed
where an additional 7 target positions on the allergen surface were targeted:
T10, K20, Q36, E73, E87, K129 and S149. These seven positions were
selected from surface areas that are outside coherent surface areas that are
common among Fagales allergens. The library was based on the use of
degenerated DNA oligonucleotide primers allowing introduction of several
different amino acid residues in each position. In addition, several mutated
amino acid residue positions in rBet v 1 (2744) and rBet v1 (2628) could
either be maintained or mutated back to the residues found in WT rBet v
1.2801.
I another embodiment a semi-random library based on rBet v 1 (2744) and
rBet v1 (2628) and rBet v 1 (2595) i.e. N28, K32, E45, P108 was constructed
where an additional 7 target positions on the allergen surface were targeted:
T10, K20, Q36, E73, E87, K129 and S149.
Mutants:
Examples of specific Bet v 1 allergen mutants according to the present
invention are listed below. Mutated amino acid positions are indicated in bold
small print:
Bet v 1 ("3004") (SEQ ID NO 3):
GVFNvETETTSVIPAARLFKAFILDGDNLFPKVAPQAISSVsNIEGNGGPGTIK
KISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPD
GGSILKISNKYHTKGDHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 ("3005") (SEQ ID NO 4):
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPDG
GSILKISNKYHTKGDH EVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
43
Bet v 1 ("3007") (SEQ ID NO 5):
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATgDG
GSILKISNKYHTKGyHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 ("3009") (SEQ ID NO 6):
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTNFKYNYSVIEGGPIGDTLsKISNEIKIVATgD
GGSILKISNKYHTKGDHEVKAEQVKASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 ("3006") (SEQ ID NO 7):
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPDG
GSILKISNKYHTKGDHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYgn
Bet v 1 ("3008") (SEQ ID NO 8):
GVFNvETETTSVIPAARLFKAFILDGDtLFPkVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDsVDHTNFKYNYSVIEGGPIGDTLsKISNEIKIVATgDG
GSILKISNKYHTKGyHEVKAEQVKASKEMGETLLRAVESYLLAHSDAYgn
The present invention furthermore comprises the following specific mutants:
Bet v 1 ("3005-7") (SEQ ID NO 9):
YSV, N28T, K32Q, E45S, N78K, K97S, K103V, K134E, +160N, EBS, D125Y,
E141 S, D25T, E87A, S155T, N47K, K55N.
GVFNvETsTTSVIPAARLFKAFILtGDtLFPqVAPQAISSVENIsGkGGPGTIKnIS
FPEGLPFKYVKDRVDEVDHTkFKYNYSVIaGGPIGDTLEsISNEIvIVATPDGG
SILKISNKYHTKGyHEVKAEQVeASKEMGsTLLRAVESYLLAHtDAYNn
Bet v 1 ("3005-12") (SEQ ID NO 10):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
44
YSV, N28T, K32Q, E45S, N78K, K97S, K103V, K134E, +160N, EBS, D125Y,
E141 S, D25T, E87A, S155T, N47K, K55N, E73T, A130V, P108G, V2L.
GIFNvETsTTSVIPAARLFKAFILtGDtLFPqVAPQAISSVENIsGkGGPGTIKnIS
FPEGLPFKYVKDRVDtVDHTkFKYNYSVIaGGPIGDTLEsISNEIvIVATgDGGS
ILKISNKYHTKGyHEVKvEQVeASKEMGsTLLRAVESYLLAHtDAYNn
Bet v 1 ("3005-22") (SEQ ID NO 11 ):
YSV, N28T, K32Q, E45S, N78K, K97S, K103V, K134E, +160N, T10K, K65N,
E141 N, K1231, D109N, E42S, E73T, E87A, V2L, N47K.
GIFNvETETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGkGGPGTIKKI
SFPEGLPFnYVKDRVDtVDHTtFKYNYSVIaGGPIGDTLEsISNEIvIVATPnGG
SILKISNKYHTiGDHEVKAEQVeASKEMGnTLLRAVESYLLAHSDAYNn
Bet v 1 ("3005-27") (SEQ ID NO 12):
YSV, N28T, K320, E45S, N78K, K97S, K103V, K134E, +160N, T10K, K65N,
E141 N, K1231, D109N, E42S, E73T, E87A, K119N, A130V, V2L, EBS, N47K,
D156H, E6S.
GIFNvsTsTpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGkGGPGTIKKIS
FPEGLPFnYVKDRVDtVDHTtFKYNYSVIaGGPIGDTLEsISNEIvIVATPnGGSI
LKISNKYHTiGDHEVKAEQVeASKEMGnTLLRAVESYLLAHShAYNn
Bet v 1 ("3007-6") (SEQ ID NO 13):
YSV, N28T, K32S, E45S, N78K, K97S K103V, P108G, D125Y, K134E,
+160N, E87A, E141 N, K55N, N47K, S155T.
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnI
SFPEGLPFKYVKDRVDEVDHTkFKYNYSVIaGGPIGDTLEsISNEIvIVATgDG
GSILKISNKYHTKGyHEVKAEQVeASKEMGnTLLRAVESYLLAHtDAYNn
Bet v 1 ("3007-10") (SEQ ID NO 14):
YSV, N28T, K32S, E45S, N78K, K97S K103V, P108G, D125Y, K134E,
+160N, E87A, E141 N, K55N, N47K, S155T, A130V, EBS, E73T, V2L.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
GIFNvETsTTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnIS
FPEGLPFKYVKDRVDtVDHTkFKYNYSVIaGGPIGDTLEsISNEIvIVATgDGGS
ILKISNKYHTKGyHEVKvEQVeASKEMGnTLLRAVESYLLAHtDAYNn
5 Bet v 1 ("3007-17") (SEQ ID NO 15):
YSV, N28T, K32Q, E45S, N78K, K97S, K103V, P108G, D125Y, K134E,
+160N, K65N, T10P E87A, D156H, E141 N, E42S.
GVFNvETETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGNGGPGTIKK
ISFPEGLPFnYVKDRVDEVDHTkFKYNYSVIaGGPIGDTLEsISNEIvIVATgDG
10 GSILKISNKYHTKGyHEVKAEQVeASKEMGnTLLRAVESYLLAHShAYNn
Bet v 1 ("3007-22") (SEQ ID NO 16):
YSV, N28T, K32Q, E45S, N78K, K97S, K103V, P108G, D125Y, K134E,
+160N, K65N, T10P E87A, D156H, E141 N, E42S, A130V, EBS, N47K,
15 H76T, V2L.
GIFNvETsTpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGkGGPGTIKKIS
FPEGLPFnYVKDRVDEVDtTkFKYNYSVIaGGPIGDTLEsISNEIvIVATgDGGS
ILKISNKYHTKGyHEVKvEQVeASKEMGnTLLRAVESYLLAHShAYNn
20 Bet v 1 ("3008-8") (SEQ ID NO 17):
YSV, N28T, K32Q, E45S, E73S, E96S, P108G, D125Y, N159G, +160N,
K134E, N78K, E87A, K119N, EBS, K55N, E141 N, N47K.
GVFNvETsTTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnI
SFPEGLPFKYVKDRVDsVDHTkFKYNYSVIaGGPIGDTLsKISNEIKIVATgDG
25 GSILKISNnYHTKGyHEVKAEQVeASKEMGnTLLRAVESYLLAHSDAYgn
Bet v 1 ("3008-13") (SEQ ID NO 18):
YSV, N28T, K32Q, E45S, E73S, E96S, P108G, D125Y, N159G, +160N,
K134E, N78K, E87A, K119N, EBS, K55N, E141 N, N47K, S155T, E6S,
30 K103V, A130V, V2L.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
46
GIFNvsTsTTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnIS
FPEGLPFKYVKDRVDsVDHTkFKYNYSVIaGGPIGDTLsKISNEIvIVATgDGG
SILKISNnYHTKGyHEVKvEQVeASKEMGnTLLRAVESYLLAHtDAYgn
Bet v 1 ("3008-20") (SEQ ID NO 19):
YSV, N28T, K32Q, E45S, E73S, E96S, P108G, D125Y, N159G, +160N,
K65N, T10P, E138N, E87A, E42S, D156H, N78K.
GVFNvETETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGNGGPGTIKK
ISFPEGLPFnYVKDRVDsVDHTkFKYNYSVIaGGPIGDTLsKISNEIKIVATgDG
GSILKISNKYHTKGyHEVKAEQVKASKnMGETLLRAVESYLLAHShAYgn
Bet v 1 "3008-25") (SEQ ID NO 20):
YSV, N28T, K32Q, E45S, E73S, E96S, P108G, D125Y, N159G, +160N,
K65N, T10P, E138N, E87A, E42S, D156H, N78K, K119N, N47K, T77A,
E130V, K115N.
GIFNvsTETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGNGGPGTIKKI
SFPEGLPFnYVKDRVDsVDHTkFKYNYSVIaGGPIGDTLsKISNEIvIVATgDG
GSILKISNKYHTKGyHEVKvEQVKASKnMGETLLRAVESYLLAHthAYgn
Bet v 1 ("3009-9") (SEQ ID NO 21 ):
YSV, N28T, K32Q, E45S, E96S, P108G, +160N, K134E, N78K, E87A,
K119N, EBS, K55N, E138N, E141 N, S155T, N47K, E6S, K103V, A130V,
V2L, R70N, D125Y.
GVFNvETsTTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnI
SFPEGLPFKYVKDRVDEVDHTkFKYNYSVIaGGPIGDTLsKISNEIKIVATgDG
GSILKISNnYHTKGDHEVKAEQVeASKnMGnTLLRAVESYLLAHtDAYNn
Bet v 1 ("3009-15") (SEQ ID NO 22):
YSV, N28T, K32Q, E45S, E96S, P108G, +160N, K134E, N78K, E87A,
K119N, EBS, K55N, E138N, E141 N, S155T, N47K, E6S, K103V, A130V,
V2L, R70N, D125Y.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
47
GIFNvsTsTTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGkGGPGTIKnIS
FPEGLPFKYVKDnVDEVDHTkFKYNYSVIaGGPIGDTLsKISNEIvIVATgDGG
SILKISNnYHTKGyHEVKvEQVeASKnMGnTLLRAVESYLLAHtDAYNn
Bet v 1 ("3009-22") (SEQ ID NO 23):
YSV, N28T, K32Q, E45S, E96S, P108G, +160N, T77A, K103V, E138N,
K65N, T10P, D125Y, E42S.
GVFNvETETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsN IsGNGGPGTIKK
ISFPEGLPFnYVKDRVDEVDHaNFKYNYSVIEGGPIGDTLsKISNEIvIVATgD
GGSILKISNKYHTKGyHEVKAEQVKASKnMGETLLRAVESYLLAHSDAYNn
Bet v 1 ("3009-28") (SEQ ID NO 24):
YSV, N28T, K32Q, E45S, E96S, P108G, +160N, T77A, K103V, E138N,
K65N, T10P, D125Y, D156H, K119N E87A, E42S, A130V.
GVFNvETETpSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGNGGPGTIKK
ISFPEGLPFnYVKDRVDEVDHaNFKYNYSVIaGGPIGDTLsKISNEIvIVATgDG
GSILKISNnYHTKGyHEVKvEQVKASKnMGETLLRAVESYLLAHShAYNn
Bet v 1 clone ("3031 ") (SEQ ID NO 25):
GVFNVETETASVIPAARLFNAFILDGDTLFPQVAPQAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIEGGPIGDTLESISN EIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
Bet v 1 clone ("3032") (SEQ ID NO 26):
GVFNVETETASVIPAARLFLAFILDGDTLFPQVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDPVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
Bet v 1 clone ("3033") (SEQ ID NO 27):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
48
GVFNVETETPSVIPAARLFHAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIEGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
Bet v 1 clone ("3034") (SEQ ID NO 28):
GVFNVETETTSVIPAARLFHAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3035") (SEQ ID NO 29):
GVFNVETETPSVIPAARLFMAFILDGDTLFPQVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEAYLLAHSHAYN
N
Bet v 1 clone ("3036") (SEQ ID NO 30):
GVFNVETETPSVIPAARLFLAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDTVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3037") (SEQ ID NO 31 ):
GVFNVETETPSVIPAARLFQAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
Bet v 1 clone ("3038") (SEQ ID NO 32):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
49
GVFNVETETASVIPAARLFLAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3039") (SEQ ID NO 33):
GVFNVETETASVIPAARLFLAFILDGDTLFPQVAPEAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVA
TPDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEAYLLAHSHAY
NN
Bet v 1 clone ("3040") (SEQ ID NO 34):
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVETYLLAHSHAYN
N
Bet v 1 clone "3041") (SEQ ID NO 35):
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3042") (SEQ ID NO 36):
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3043") (SEQ ID NO 37):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
GVFNVETETPSVIPAARLFLAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
5
Bet v 1 clone ("3044") (SEQ ID NO 38):
GVFNVETETPSVIPAARLFLAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVA
TPDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVETYLLAHSHAY
10 NN
Bet v 1 clone ("3045") (SEQ ID NO 39):
GVFNVETETPSVIPAARLFMAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
15 PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
Bet v 1 (3010) (SEQ ID NO 40)
YSV, N28T, K32Q, E45S, K97S, P108G, +160N, E60S, T10N, K103V, K65N,
20 K129N, D125Y, E42S, S149T.
GVFNvETETnSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGNGGPGTIKK
ISFPsGLPFnYVKDRVDEVDHTNFKYNYSVIEGGPIGDTLEsISNEIvIVATgDG
GSILKISNKYHTKGyHEVnAEQVKASKEMGETLLRAVEtYLLAHSDAYNn
25 Bet v 1 (3011 ) (SEQ ID NO 41 )
YSV, N28T, K32Q, E45S, K97S, P108G, +160N, E60S, T10N, K103V, K65N,
K129N, D125Y, E42S, S149T, K134E, N47K, T77N, V2L.
GIFNvETETnSVIPAARLFKAFILDGDtLFPqVAPQAISSVsNIsGkGGPGTIKKI
SFPsGLPFnYVKDRVDEVDHnNFKYNYSVIEGGPIGDTLEsISNEIvIVATgDG
30 GSILKISNKYHTKGyHEVnAEQVeASKEMGETLLRAVEtYLLAHSDAYNn
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
51
Bet v 1 (3012) (SEQ ID NO 42)
YSV, N28T, K32Q, E45S, K97S, P108G, +160N, E60S, T10N, K103V, K65N,
K129N, D125Y, E42S, S149T, K134E, N47K, T77N, V2L, E87A, A16G,
Q36N, E73S, D93S.
GIFNvETETnSVIPAgRLFKAFILDGDtLFPqVAPnAISSVsNIsGkGGPGTIKKIS
FPsGLPFnYVKDRVDsVDHnNFKYNYSVIaGGPIGsTLEsISNEIvIVATgDGGS
ILKISNKYHTKGyHEVnAEQVeASKEMGETLLRAVEtYLLAHSDAYNn
Diagnostic assay
Furthermore, the recombinant mutant allergens according to the invention
have diagnostic possibilities and advantages. Prior art allergy vaccines are
based on extracts of the naturally occurring allergen source, and thus
represent a wide variety of isoforms. The allergic individual has initially
been
sensitised and has IgE to one or some of the isoforms present. Some of the
isoforms may be relevant with respect to the allergic reactions of the
allergic
individual due to homology and subsequent cross-reactivity with the isoform
to which the individual is allergic, whereas other isoforms may be irrelevant
as they do not harbour any of the IgE binding epitopes to which the allergic
individual has specific IgE. Due to this heterogeneity of the specificities of
the
IgE population, some isoforms may therefore be safe to administer, i.e. they
do not result in an allergic response via IgE, whereas other isoforms may be
harmful causing undesirable side-effects.
Thus, the mutants of the invention and the compositions of the invention
intended to be administered therapeutically may also be used for an in vivo
or in vitro diagnostic assay to monitor the relevance, safety or outcome of a
treatment with such mutants or compositions. Diagnostic samples to be
applied include body samples, such as blood or sera.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
52
Thus, the invention also relates to a diagnostic assay for assessing
relevance, safety or outcome of therapy of a subject using a recombinant
mutant allergen according to the invention or a composition according to the
invention, wherein an IgE containing sample of the subject is mixed with said
mutant or said composition and assessed for the level of reactivity between
the IgE in said sample and said mutant. The assessing of the level of
reactivity between the IgE in the sample and the mutant may be carried out
using any known immunoassay.
The present invention is further illustrated by the following non-limiting
examples.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
53
EXAMPLES
EXAMPLE 1
This Example describes characterisation of recombinant mutant Bet v 1
mutant allergens with diminished IgE-binding affinity. The specific mutant
allergens are also disclosed in PCTIDK 01!00764. The following represents
an illustrating example of how to prepare mutants according to the present
invention.
Identification of common epitopes within Fatales pollen allergens
The major birch pollen allergen Bet v 1 shows about 90% amino acid
sequence identity with major allergens from pollens of taxonomically related
trees, i.e Fagales (for instance hazel and hornbeam) and birch pollen allergic
patients often show clinical symptoms of allergic cross-reactivity towards
these Bet v 1 homologous proteins.
Bet v 1 also shows about 50-60% sequence identity with allergic proteins
present in certain fruits (for instance apple and cherry) and vegetables (for
instance celery and carrot) and there are clinical evidence for allergic cross-
reactivity between Bet v 1 and these food related proteins.
In addition, Bet v 1 shares significant sequence identity (20-40%) with a
group of plant proteins called pathogenesis-related proteins (PR-10),
however there are no reports of allergic cross-reactivity towards these PR-10
proteins.
Molecular modelling suggests that the structures of Fagales and food
allergens and PR-10 proteins are close to being identical with the Bet v 1
structure.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
54
The structural basis for allergic Bet v 1 cross-reactivity was reported in
(Gajhede et al 1996, ref. 17). Thus, any IgE recognising epitopes on Bet v 1
would be able to cross-react and bind to other Fagales major pollen allergens
and give rise to allergic symptoms.
Selection of amino acid residues for site-directed mutaaenesis
Amino acid residues for site-directed mutagenesis were selected among
surface exposed residues present in Bet v 1. The relative orientation and
percentage of solvent-exposure of each amino acid residue was calculated
based on their atomic coordinates. Residues having a low degree of solvent
exposure (<20%) were not regarded relevant for mutagenesis due to the
possible disruption of the structure or lack of antibody interaction. The
remaining residues were ranked according to their degree of solvent-
exposure.
Sequence alignment
Sequences homologous to the query sequence (bet v 1 No. 2801, WHO IUIS
Nomenclature Subcommittee on Allergens) were derived from GenBank and
EMBL sequence databases by a BLAST search (Altschul et al., ref. 18). All
sequences with BLAST reported probabilities less than 0.1 were taken into
consideration and one list were constructed containing a non-redundant list
of homologous sequences. These were aligned by CLUSTAL W (Higgins et
al., ref. 19) and the percentage identity were calculated for each position in
the sequence considering the complete list or taxonomically related species
only. A total of 122 sequences were homologous to Bet v 7 No. 2807 of
which 57 sequences originates from taxonomically related species.
Cloning of the Gene encoding Bet v 1
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
RNA was prepared from Betula verrucosa pollen (Allergon, Sweden) by
phenol extraction and LiCI precipitation. Oligo(dT)-cellulose affinity
chromatography was performed batch-wise in Eppendorph tubes, and
5 double-stranded cDNA was synthesised using a commercially available kit
(Amersham). DNA encoding Bet v 1 was amplified by PCR and cloned. In
brief, PCR was performed using cDNA as template, and primers designed to
match the sequence of the cDNA in positions corresponding to the amino
terminus of Bet v 1 and the 3'-untranslated region, respectively. The primers
10 were extended in the 5'-ends to accommodate restriction sites (Ncol and
Hindlll) for directional cloning into pKK233-2.
Subclonina into pMAL-c
15 The gene encoding Bet v 1 was subsequently subcloned into the maltose
binding protein fusion vector pMAL-c (New England Biolabs). The gene was
amplified by PCR and subcloned in frame with malE to generate maltose
binding protein (MBP)-Bet v 1 protein fusion operons in which MBP and Bet v
1 were separated by a factor Xa protease clevage site positioned to restore
20 the authentic aminoterminal sequence of Bet v 1 upon cleavage, as
described in ref. 15. In brief, PCR was performed using pKK233-3 with Bet v
7 inserted as template and primers corresponding to the amino- and
carboxyterminus of the protein, respectively. The promoter proximal primer
was extended in the 5'-end to accommodate 4 codons encoding an in frame
25 factor Xa protease cleavage site. Both primers were furthermore extended in
the 5'-ends to accommodate restriction sites (Kpnl) for cloning. The Bet v 1
encoding genes were subcloned using 20 cycles of PCR to reduce the
frequency of PCR artefacts.
30 In vitro mutaaenesis
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
56
In vitro mutagenesis was performed by PCR using recombinant pMAL-c with
Bet v 7 inserted as template. Each mutant Bet v 7 gene was generated by 3
PCR reactions using 4 primers. The following examples of mutants are
according to prior art PCT/DK 01!00764. Mutants according to the invention
can be prepared and assayed in a similar fashion.
Two mutation-specific oligonucleotide primers were synthesised
accommodating each mutation, one for each DNA strand, see Figs. 3 and 4
Using the mutated nucleotides) as starting point both primers were extended
7 nucleotides in the 5'-end and 15 nucleotides in the 3'-end. The extending
nucleotides were identical in sequence to the Bet v 7 gene in the actual
region.
Two generally applicable primers (denoted "all-sense" and "all non-sense" in
Figure 4) were furthermore synthesised and used for all mutants. These
primers were 15 nucleotides in length and correspond in sequence to regions
of the pMAL-c vector approximately 1 kilobase upstream and downstream
from the Bet v 7. The sequence of the upstream primer is derived from the
sense strand and the sequence of the downstream primer is derived from the
non-sense strand, see Fig. 4
Two independent PCR reactions were performed essentially according to
standard procedures (Saiki et al 1988, ref. 20) with the exception that only
20
temperature cycles were performed in order to reduce the frequency of PCR
artefacts. Each PCR reaction used pMAL-c with Bet v 7 inserted as template
and one mutation-specific and one generally applicable primer in meaningful
combinations.
Introduction of the four amino acid substitutions (Asn28Thr, Lys32Gln,
GIu45Ser, Pro108G1y) in the mutant were performed like described above in
a step by step process. First the GIu45Ser mutation then the Pro108G1y
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
57
mutation and last the Asn28Thr, and Lys32Gln mutations were introduced
using pMAL-c with inserted Bet v 1 No. 2807, Bet v 1 (GIu45Ser), Bet v 1
(GIu45Ser, Pro108G1y) as templates, respectively.
The PCR products were purified by agarose gel electrophoresis and electro-
elution followed by ethanol precipitation. A third PCR reaction was performed
using the combined PCR products from the first two PCR reactions as
template and both generally applicable primers. Again, 20 cycles of standard
PCR were used. The PCR product was purified by agarose gel
electrophoresis and electro-elution followed by ethanol precipitation, cut
with
restriction enzymes (8siWIlEcoRl), and ligated directionally into pMAL-c with
Bet v 1 inserted restricted with the same enzymes.
Figure 5 shows an overview of all 9 Bet v 1 mutations, which are as follows
Thr10Pro, Asp25Gly, Asn28Thr + Lys32Gln, GIu45Ser, Asn47Ser,
Lys55Asn, GIu60Ser, Thr77Ala and Pro108G1y. An additional mutant with
four mutations was also prepared (Asn28Thr, Lys32Gln, GIu45Ser,
Pro108G1y). Of these, five mutants were selected for further testing:
Asn28Thr + Lys32Gln, GIu45Ser, GIu60Ser, Pro108G1y and the Asn28Thr,
Lys32Gln, GIu45Ser, Pro108G1y mutant.
Nucleotide seguencing
Determination of the nucleotide sequence of the Bet v 1 encoding gene was
performed before and after subcloning, and following in vitro mutagenesis,
respectively.
Plasmid DNA's from 10 ml of bacterial culture grown to saturation overnight
in LB medium supplemented with 0.1 g/I ampicillin were purified on Qiagen-
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
58
tip 20 columns and sequenced using the Sequenase version 2.0 DNA
sequencing kit (USB) following the recommendations of the suppliers.
Expression and purification of recombinant Bet v 1 and mutants
Recombinant Bet v 1 (Bet v 1 No. 2801 and mutants) were over-expressed in
Escherichia coli DH 5a fused to maltose-binding protein and purified as
described in ref. 15. Briefly, recombinant E.coli cells were grown at
37°C to
an optical density of 1.0 at 436 nm, whereupon expression of the Bet v 1
fusion protein was induced by addition of IPTG. Cells were harvested by
centrifugation 3 hours post-induction, re-suspended in lysis buffer and broken
by sonication. After sonication and additional centrifugation, recombinant
fusion protein was isolated by amylose affinity chromatography and
subsequently cleaved by incubation with Faotor Xa (ref. 15). After F Xa
cleavage, recombinant Bet v 1 was isolated by gelfiltration and if found
necessary, subjected to another round of amylose affinity chromatography in
order to remove trace amounts of maltose-binding protein.
Purified recombinant Bet v 1 was concentrated by ultrafiltration to about 5
mglml and stored at 4 °C. The final yields of the purified recombinant
Bet v 1
preparations were between 2-5 mg per litre E. coli cell culture.
The purified recombinant Bet v 1 preparations appeared as single bands
after silver-stained SDS-polyacrylamide electrophoresis with an apparent
molecular weight of 17.5 kDa. N-terminal sequencing showed the expected
sequences as derived from the cDNA nucleotide sequences and quantitative
amino acid analysis showed the expected amino acid compositions.
We have previously shown (ref. 15) that recombinant Bet v 1 No. 2801 is
imrnunochemically indistinguishable from naturally occurring Bet v 1.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
59
Immunoelectrophoresis using rabbit polyclonal antibodies
The seven mutant Bet v 1 were produced as recombinant Bet v 1 proteins
and purified as described above and tested for their reactivity towards
polyclonal rabbit antibodies raised against Bet v 1 isolated from birch
pollen.
When analysed by immunoelectrophoresis (rocket-line
immunoelectrophoresis) under native conditions, the rabbit antibodies were
able to precipitate all mutants, indicating that the mutants had conserved a-
carbon backbone tertiary structure.
In order to analyse the effect on human polyclonal IgE-response, the
mutants GIu45Ser, Pro108G1y, Asn28Thr+Lys32Gln and GIu60Ser were
selected for further analysis.
Bet v 1 GIu45Ser mutant
Glutamic acid in position 45 show a high degree of solvent-exposure (40%).
A serine residue was found to occupy position 45 in some of the Bet v 1
homologous PR-10 proteins arguing for that glutamic acid can be replaced by
serine without distortion of the a-carbon backbone tertiary structure. In
addition, as none of the known Fagales allergen sequences have serine in
position 45, the substitution of glutamic acid with serine gives rise to a non-
naturally occurring Bet v 1 molecule.
T cell proliferation assay using recombinant GIu45Ser Bet v 1 mutant
The analysis was carried out as described in Spangfort et al 1996x. It was
found that recombinant Bet v 1 GIu45Ser mutant was able to induce
proliferation in T cell lines from three different birch pollen allergic
patients
with stimulation indices similar to recombinant and naturally occurring.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
Crystallisation and structural determination of recombinant GIu45Ser Bet v 1
Crystals of recombinant GIu45Ser Bet v 1 were grown by vapour diffusion at
25°C, essentially as described in (Spangfort et al 1996b, ref. 21 ).
GIu45Ser
5 Bet v 1, at a concentration of 5 mg/ml, was mixed with an equal volume of
2.0 M ammonium sulphate, 0.1 M sodium citrate, 1 % (v/v) dioxane, pH 6.0
and equilibrated against 100x volume of 2.0 M ammonium sulfate, 0.1 M
sodium citrate, 1 % (v/v) dioxane, pH 6Ø After 24 hours of equilibration,
crystal growth was induced by applying the seeding technique described in
10 ref. 21, using crystals of recombinant wild-type Bet v 1 as a source of
seeds.
After about 2 months, crystals were harvested and analysed using X-rays
generated from a Rigaku rotating anode as described in ref. 21 and the
structure was solved using molecular replacement.
Structure of Bet v 1 GIu45Ser mutant
The structural effect of the mutation was addressed by growing three-
dimensional Bet v 1 GIu45Ser protein crystals diffracting to 3.0 A resolution
when analysed by X-rays generated from a rotating anode. The substitution
of glutamic acid to serine in position 45 was verified by the Bet v 1 GIu45Ser
structure electron density map which also showed that the overall a-carbon
backbone tertiary structure is preserved.
IgE-binding properties of Bet v 1 GIu45Ser mutant
The IgE-binding properties of Bet v 1 GIu45Ser mutant was compared with
recombinant Bet v 1 in a fluid-phase IgE-inhibition assay using a pool of
serum IgE derived from birch allergic patients.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
61
Recombinant Bet v 1 no. 2801 was biotinylated at a molar ratio of 1:5 (Bet v
1 no. 2801:biotin). The inhibition assay was performed as follows: a serum
sample (25 pl) was incubated with solid phase anti IgE, washed, re-
suspended and further incubated with a mixture of biotinylated Bet v 1 no.
2801 (3.4 nM) and a given mutant (0-28.6 nM). The amount of biotinylated
Bet v 1 no. 2801 bound to the solid phase was estimated from the measured
RLU after incubation with acridinium ester labelled streptavidin. The degree
of inhibition was calculated as the ratio between the RLU's obtained using
buffer and mutant as inhibitor.
Figure 6 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 GIu45Ser mutant.
There is a clear difference in the amount of respective recombinant proteins
necessary to reach 50% inhibition of the binding to serum IgE present in the
serum pool. Recombinant Bet v 1 reaches 50% inhibition at about 6.5 ng
whereas the corresponding concentration for Bet v 7 GIu45Ser mutant is
about 12 ng. This show that the point mutation introduced in Bet v 1
GIu45Ser mutant lowers the affinity for specific serum IgE by a factor of
about 2.
The maximum level of inhibition reached by the Bet v 7 GIu45Ser mutant is
clearly lower compared to recombinant Bet v 1. This may indicate that after
the GIu45Ser substitution, some of the specific IgE present in the serum pool
are unable to recognise the Bet v 7 GIu45Ser mutant.
Bet v 1 mutant Asn28Thr+Lys32Gln
Aspartate and lysine in positions 28 and 32, respectively show a high degree
of solvent-exposure (35% and 50%, respectively). In the structure, aspartate
28 and lysine 32 are located close to each other on the molecular surface
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
62
and most likely interact via hydrogen bonds. A threonine and a gluatamate
residue were found to occupy positions 28 and 32, respectively in some of
the Bet v 1 homologous PR-10 proteins arguing for that aspartate and lysine
can be replaced with threonine and glutamate, respectively without distortion
of the a-carbon backbone tertiary structure. In addition, as none of the
naturally occurring isoallergen sequences have threonine and glutamate in
positions 28 and 32, respectively, the substitutions gives rise to a non-
naturally occurring Bet v 1 molecule.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
63
IgE-binding properties of Bet v 1 mutant Asn28Thr+Lys32Gln
The IgE-binding properties of mutant Asn28Thr+Lys32Gln was compared
with recombinant Bet v 1 in a fluid-phase IgE-inhibition assay using the pool
of serum IgE derived from birch allergic patients described above.
15
Figure 7 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 mutant Asn28Thr+Lys32Gln.
There is a clear difference in the amount of respective recombinant proteins
necessary to reach 50% inhibition of the binding to serum IgE present in the
serum pool. Recombinant Bet v 1 reaches 50% inhibition at about 6.5 ng
whereas the corresponding concentration for Bet v 7 mutant
Asn28Thr+Lys32Gln is about 12 ng. This show that the point mutations
introduced in Bet v 1 mutant Asn28Thr+Lys32Gln lowers the affinity for
specific serum IgE by a factor of about 2.
The maximum level of inhibition reached by the Bet v 7 mutant
Asn28Thr+Lys32Gln mutant is clearly lower compared to recombinant Bet v
1. This may indicate that after the Asn28Thr+Lys32Gln substitutions, some of
the specific IgE present in the serum pool are unable to recognise the Bet v 1
mutant Asn28Thr+Lys32Gln.
Bet v 1 mutant Pro108G1y
Proline in position 108 shows a high degree of solvent-exposure (60%). A
glycine residue was found to occupy position 108 in some of the Bet v 1
homologous PR-10 proteins arguing for that proline can be replaced with
glycine without distortion of the a-carbon backbone tertiary structure. In
addition, as none of the naturally occurring isoallergen sequences have
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
64
glycine in position 108, the substitution of proline with glycine gives rise
to a
non-naturally occurring Bet v 1 molecule.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
IcLE-binding properties of Bet v 1 Pro108G1y mutant
The IgE-binding properties of Bet v 1 Pro108G1y mutant was compared with
recombinant Bet v 1 in a fluid-phase IgE-inhibition assay using the pool of
5 serum IgE derived from birch allergic patients described above.
Figure 8 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 Pro108G1y mutant.
There is a clear difference in the amount of respective recombinant proteins
necessary to reach 50% inhibition of the binding to serum IgE present in the
serum pool. Recombinant Bet v 1 reaches 50% inhibition at about 6.5 ng
whereas the corresponding concentration for Bet v 1 Pro108G1y is 15 ng.
This show that the single point mutation introduced in Bet v 1 Pro108G1y
lowers the affinity for specific serum IgE by a factor of about 2.
The maximum level of inhibition reached by the Bet v 1 Pro108G1y mutant is
somewhat lower compared to recombinant Bet v 1. This may indicate that
after the Pro108G1y substitution, some of the specific IgE present in the
serum pool are unable to recognise the Bet v 1 Pro108G1y mutant.
Bet v 1 mutant GIu60Ser mutant
Glutamic acid in position 60 show a high degree of solvent-exposure (60%).
A serine residue was found to occupy position 60 in some of the Bet v 1
homologous PR-10 proteins arguing for that glutamic acid can be replaced
with serine without distortion of the a-carbon backbone tertiary structure. In
addition, as none of the naturally occurring isoallergen sequences have
serine in position 60, the substitution of glutamic acid with serine gives
rise to
a non-naturally occurring Bet v 1 molecule.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
66
IgE-binding properties of Bet v 1 GIu60Ser mutant
The IgE-binding properties of Bet v 1 GIu60Ser mutant was compared with
recombinant Bet v 1 in a fluid-phase IgE-inhibition assay using the pool of
serum IgE derived from birch allergic patients described above.
Figure 9 shows the inhibition of the binding of biotinylated recombinant Bet v
1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and
by Bet v 1 GIu60Ser mutant. In contrast to the GIu45Ser, Pro108G1y and
Asn28Thr+Lys32Gln mutants, the substitution glutamic acid 60 to serine,
does not shown any significant effect on the IgE-binding properties of.
Structural analysis of Bet v 1 GIu45Ser Asn28Thr+Lys32Gln and Pro108G1y
mutant
The structural integrity of the purified recombinant protein was analysed by
circular dichroism (CD) spectroscopy. Figure 10 shows the CD spectra of
recombinant mutant and recombinant naturally occurring protein, recorded at
close to equal concentrations. The overlap in peak amplitudes and positions
in the CD spectra from the two recombinant proteins shows that the two
preparations contain equal amounts of secondary structures strongly
suggesting that the a-carbon backbone tertiary structure is not affected by
the introduced amino acid substitutions.
IgE-binding properties of Bet v 1 GIu45Ser Asn28Thr+Lys32Gln and
Pro108G1y mutant
The IgE-binding properties of the mutant was compared with recombinant
Bet v 1 in a fluid-phase IgE-inhibition assay using the pool of serum IgE
derived from birch allergic patients described above.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
67
Figure 11 shows the inhibition of the binding of biotinylated recombinant Bet
v 1 to serum IgE from a pool of allergic patients by non-biotinylated Bet v 1
and by the Bet v 1 mutant. In contrast to the single mutants described above,
the inhibition curve of the mutant is no longer parallel relative to
recombinant.
This shows that the substitutions introduced in the mutant have changed the
IgE-binding properties and epitope profile compared to recombinant. The lack
of parallellity makes it difficult to quantify the decrease of the mutants
affinity
for specific serum IgE.
Recombinant Bet v 1 reaches 50% inhibition at about 6 ng whereas the
corresponding concentration for Bet v 1 (Asn28Thr, Lys32Gln, GIu45Ser,
Pro108G1y) mutant is 30 ng, i.e a decrease in affinity by a factor 5. However,
in order to reach 80% inhibition the corresponding values are 20 ng and 400
ng, respectively, i.e a decrease by a factor 20.
T cell proliferation assay using the recombinant Bet v 1 GIu45Ser
Asn28Thr+Lys32Gln and Pro108G1y mutant
The analysis was carried out as described in ref. 15. It was found that
recombinant Bet v 1 mutant was able to induce proliferation in T cell lines
from three different birch pollen allergic patients with stimulation indices
similar to recombinant and naturally occurring. This suggests that the mutant
can initiate the cellular immune response necessary for antibody production.
EXAMPLE 2
In vitro mutaaenesis of mutants according to the present invention
In vitro mutagenesis was performed by PCR using recombinant pMAL-c with
Bet v 7 inserted as template. Preparation of recombinant mutant allergens
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
68
included two PCR steps; step I and II. First, each single mutation (or several
mutations if located closely together in the DNA sequence) was introduced
into sequential DNA sequences of Bet v 1.2801 derivatives i.e. 8et v 1 (2595)
or Bet v 1 (2628) or Bet v 1 (2733) using sense and anti-sense mutation-
s specific oligonucleotide primers accommodating each mutations) along with
sense and anti-sense oligonucleotide primers accommodating either
upstream or downstream neighbour mutations or the N-terminus/C-terminus
of Bet v 1, respectively as schematically illustrated in Figure 12 (I).
Secondly,
PCR products from PCR reaction I were purified, mixed and used as
templates for an additional PCR reaction (II) with oligonucleotide primers
accommodating the N-terminus and C-terminus of Bet v 1 as schematically
illustrated in Figure 13 (II). The PCR products were purified by agarose gel
electrophoresis and PCR gel purification (Life Techhnologies) followed by
ethanol precipitation, cut with restriction enzymes (SacllEcoRl) or (Sacll
~Cbal), and ligated directionally into pMAL-c restricted with the same
enzymes.
Figure 13 shows synthesised oligonucleotide primers and schematically
illustrations for the construction of Bet v 1 mutants. The following Bet v 1
mutants were cloned and sequenced (sequencing of nucleic acid molecules
is described in Example 1 ):
Bet v 1 (3004)
GVFNvETETTSVIPAARLFKAFILDGDNLFPKVAPQAISSVsNIEGNGGPGTIK
KISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPD
GGSILKISNKYHTKGDHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 (3005)
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPDG
GSILKISNKYHTKGDHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
69
Bet v 1 (3007)
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsiSNEIvIVATgDG
GSILKISNKYHTKGyHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 (3009)
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTNFKYNYSVIEGGPIGDTLsKISNEIKIVATgD
GGSILKISNKYHTKGDHEVKAEQVKASKEMGETLLRAVESYLLAHSDAYNn
Bet v 1 (3006)
GVFNvETETTSVIPAARLFKAFILDGDtLFPqVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDEVDHTkFKYNYSVIEGGPIGDTLEsISNEIvIVATPDG
GSILKISNKYHTKGDHEVKAEQVeASKEMGETLLRAVESYLLAHSDAYgn
Bet v 1 (3008)
GVFNvETETTSVIPAARLFKAFILDGDtLFPkVAPQAISSVENIsGNGGPGTIKK
ISFPEGfPFKYVKDRVDsVDHTNFKYNYSVIEGGPIGDTLsKISNEIKIVATgDG
GSILKISNKYHTKGyHEVKAEQVKASKEMGETLLRAVESYLLAHSDAYgn
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
Further mutants prepared according to the present invention:
Introduction of multiple point mutations into Bet v 1 may potentially
destabilize the a-carbon backbone folding-pattern of the molecule.
5 Introduction of random amino acid substitutions increases the chances of
generating stable mutant Bet v 1 molecules. We therefore generated a Bet v
1 mutant library containing Bet v 1 mutants with 17-20 point mutations of
which amino acid substitutions were randomly substituted in 7 positions. The
library contained hundreds of different clones. Fifteen Bet v 1 mutants named
10 Bet v 1 (3031 ) to (3045) were obtained from this Bet v 1 mutant library
generated using degenerated oligonucleotide primers. These primers
accommodated random substitution of amino acid residues in the positions
T10, K20, Q36, E73, E87, K129 and S149 of Bet v 1 (figure 14 and 15).
These positions were non-overlapping with point mutations already
15 introduced into Bet v 1 (3002) and Bet v 1 (2595) that were used as DNA
templates for the site directed mutagenesis PCR reactions illustrated in
figure
15.
The cloning procedure was the same as illustrated in figure 12 except that
20 the primers used in the first PCR round were degenerated in certain
positions
as indicated in figure 15 by letters other than G, C, T or A. Use of other
letters than G, C, T or A indicates that the primers contain several different
nucleotides in these positions. Eight PCR products spanning the Bet v 1
gene were produced and purified in the first PCR round and then assembled
25 using end-primers (3076s and 3067a) in a second PCR reaction where the
eight PCR products from the first PCR round were used as a template.
The Bet v 1 mutants 3031 to 3045 were DNA sequenced as described for the
Bet v 1 3004, 3005, 3007 and 3007 mutants in order to verify the number and
30 nature of the introduced point mutations:
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
71
Bet v 1 clone ("3031 ") (SEQ ID NO 25):
GVFNVETETASVIPAARLFNAFILDGDTLFPQVAPQAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIEGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
Bet v 1 clone ("3032") (SEQ ID NO 26):
GVFNVETETASVIPAARLFLAFILDGDTLFPQVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDPVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
Bet v 1 clone ("3033") (SEQ ID NO 27):
GVFNVETETPSVIPAARLFHAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIEGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
Bet v 1 clone ("3034") (SEQ ID NO 28):
GVFNVETETTSVIPAARLFHAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3035") (SEQ ID NO 29):
GVFNVETETPSVIPAARLFMAFILDGDTLFPQVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEAYLLAHSHAYN
N
Bet v 1 clone ("3036") (SEQ ID NO 30):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
72
GVFNVETETPSVIPAARLFLAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDTVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3037") (SEQ ID NO 31 ):
GVFNVETETPSVIPAARLFQAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
Bet v 1 clone ("3038") (SEQ ID NO 32):
GVFNVETETASVIPAARLFLAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3039") (SEQ ID NO 33):
GVFNVETETASVIPAARLFLAFILDGDTLFPQVAPEAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTNFKYNYSVIGGGPIGDTLESISNEIVIVA
TPDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEAYLLAHSHAY
NN
Bet v 1 clone ("3040") (SEQ ID NO 34):
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDSVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVETYLLAHSHAYN
N
Bet v 1 clone "3041") (SEQ ID NO 35):
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
73
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3042") (SEQ ID NO 36):
GVFNVETETPSVIPAARLFKAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVERYLLAHSHAYN
N
Bet v 1 clone ("3043") (SEQ ID NO 37):
GVFNVETETPSVIPAARLFLAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDRVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEPYLLAHSHAYN
N
Bet v 1 clone ("3044") (SEQ ID NO 3B):
GVFNVETETPSVIPAARLFLAFILDGDTLFPQVAPKAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIGGGPIGDTLESISNEIVIVA
TPDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVETYLLAHSHAY
NN
Bet v 1 clone ("3045") (SEQ ID NO 39):
GVFNVETETPSVIPAARLFMAFILDGDNLFPKVAPPAISSVSNISGNGGPGTI
KKISFPEGLPFNYVKDRVDGVDHTKFKYNYSVIDGGPIGDTLESISNEIVIVAT
PDGGSILKISNKYHTIGDHEVEAEQVEASKEMGETLLRAVEGYLLAHSHAYN
N
EXAMPLE 3
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
74
Identification and selection of amino acids for substitution
The parameters of solvent accessibility and conservation degree were used
to identify and select surface-exposed amino acids suitable for substitution
for the allergens Bet v 1, Der p 2 and Ves v 5.
Solvent accessibility
Solvent accessibility was calculated using the software Insightll, version
97.0
(MSI) and a probe radius of 1.4 A (Connolly surface).
Internal cavities were excluded from the analyses by filling with probes using
the software PASS (Putative Active Sites with Spheres). Probes on the
surface were subsequently removed manually.
Conservation
Bet v 1:
3-D structure is based on accession number 280104 (1 bv1.pdb).
38 other Bet v 1 sequences included in the analysis of conserved residues
comprise accession numbers:
P15494=X15877=280106, 280101, AJ002107, 272429, AJ002108, 280105,
280100, 280103, AJ001555, 280102, AJ002110, 272436, P43183=X77271,
272430, AJ002106, P43178=X77267, P43179=X77268, P43177=X77266,
272438, P43180=X77269, AJ001551, P43185=X77273, AJ001557, 272434,
AJ001556, 272433=P43186, AJ001554, X81972, 272431, P45431=X77200,
P43184=X77272, P43176=X77265, S47250, S47251, 272435, 272439,
272437, S47249.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
Bet v 1
59 amino acids highly solvent exposed:
K-129, E-60, N-47, K-65, P-108, N-159, D-93, K-123, K-32, D-125, R-145, D
5 109, T-77, E-127, Q-36, E-131, L-152, E-6, E-96, D-156, P-63, H-76, E-8, K
134, E-45, T-10, V-12, K-20, L-62, S-155, H-126, P-50, N-78, K-119, V-2, L
24, E-42, N-4, A-153, I-44, E-138, G-61, A-130, R-70, N-28, P-35, S-149, K
103, Y-150, H-154, N-43, A-106, K-115, P-14, Y-5, K-137, E-141, E-87, E-73.
10 57 amino acids highly solvent exposed and conserved (>70%):
K-129, E-60, N-47, K-65, P-108, N-159, D-93, K-123, K-32, D-125, R-145, D-
109, E-127, Q-36, E-131, L-152, E-6, E-96, D-156, P-63, H-76, E-8, K-134,
E-45, T-10, V-12, K-20, S-155, H-126, P-50, N-78, K-119, V-2, L-24, E-42, N-
4, A-153, I-44, E-138, G-61, A-130, R-70, N-28, P-35, S-149, K-103, Y-150,
15 H-154, N-43, A-106, K-115, P-14, Y-5, K-137, E-141, E-87, E-73.
Table 1 shows a listing in descending order of solvent exposure of Bet v 1
amino acids. Column 1 lists the amino acid number starting from the amino-
terminal, column 2 lists the amino acid in one letter abbreviation, column 3
20 lists the normalised solvent exposure index, column 4 lists the percent of
known sequences having the concerned amino acid in this position.
Table 1: Bet v 1
NO AA Solv
ex Cons
p
129 K 1, 000 90
60 E 0, 986 97
47 N 0, 979 100
65 K 0,978 100.
108 P 0, 929 100
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
76
159 N 0, 869 100
93 D 0, 866 100
123 K 0, 855 100
32 K 0, 855 100
125 D 0, 821 74
145 R 0, 801 90
109 D 0,778 82
77 T 0,775 56
127 E 0, 760 100
36 Q 0, 749 95
131 E 0, 725 100
152 L 0, 718 97
6 E 0,712 100
96 E 0, 696 100
156 D 0, 693 97
63 P 0, 692 97
76 H 0,683 90
8 E 0, 638 97
134 K 0,630 100
45 E 0,623 100
T 0, 613 97
12 V 0, 592 100
K 0, 584 100
62 L 0, 575 5
155 S 0, 568 97
126 H 0, 551 95
50 P 0, 541 100
78 N 0,538 100
119 K 0, 529 100
2 V 0, 528 100
24 L 0, 528 100
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
77
42 E 0, 519 100
4 N 0, 517 95
153 A 0, 513 100
44 I 0, 508 97
138 E 0, 496 100
61 G 0, 488 100
130 A 0, 479 97
70 R 0,474 100
28 N 0,469 90
35 P 0,467 100
149 S 0,455 92
103 K 0, 447 100
150 Y 0,438 100
154 H 0,436 100
43 N 0, 412 100
106 A 0,411 95
115 K 0, 411 100
14 P 0, 410 97
Y 0, 410 100
137 K 0, 396 100
141 E 0, 387 95
87 E 0, 385 100
73 E 0, 384 100
16 A 0, 367 100
79 F 0,362 100
3 F 0, 355 100
158 Y 0, 346 100
105 V 0, 336 100
101 E 0, 326 100
64 F 0, 325 100
86 I 0, 322 100
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
78
39 S 0, 314 100
124 G 0, 310 100
72 D 0, 308 97
142 T 0, 293 67
66 Y 0,289 100
55 K 0, 288 100
7 T 0,279 67
40 S 0,274 95
25 D 0,271 87
135 A 0, 267 92
68 K 0,262 100
97 K 0, 247 100
46 G 0, 235 100
27 D 0,232 97
1 G 0, 227 100
113 I 0, 225 77
51 G 0,220 100
92 G 0, 218 100
80 K 0,212 100
110 G 0,211 100
107 T 0,203 85
94 T 0,202 92
41 V 0,201 97
48 G 0,198 100
91 I 0,192 18
31 P 0,188 100
75 D 0,188 97
33 V 0,183 100
49 G 0,176 100
17 R 0,172 100
99 S 0,158 64
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
79
89 G 0,154 100
53 I 0,154 100
121 H 0,153 100
9 T 0,150 72
74 V 0,148 97
132 Q 0,146 72
57 S 0,137 49
148 E 0,135 100
82 N 0,133 41
128 V 0,125 64
117 S 0,124 87
90 P 0,117 67
116 I 0,112 100
122 T 0,107 100
139 M 0,104 62
95 L 0,104 97
54 K 0, 096 100
146 A 0, 095 100
59 P 0, 088 97
157 A 0, 088 100
133 V 0, 077 44
88 G 0, 068 100
140 G 0, 053 85
37 A 0, 042 95
81 Y 0, 041 100
23 I 0, 036 95
104 I 0, 036 92
15 A 0, 036 97
58 F 0, 029 100
29 L 0, 028 100
19 F 0, 027 100
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
100 N 0, 022 97
22 F 0, 021 97
71 V 0,014 100
111 G 0,014 100
13 I 0,014 100
18 L 0, 014 97
114 L 0,014 100
11 S 0, 007 100
151 L 0, 007 97
144 L 0, 007 90
52 T 0, 007 100
84 S 0, 007 97
118 N 0, 007 97
102 I 0, 007 100
21 A 0, 000 97
26 G 0, 000 97
30 F 0, 000 44
34 A 0, 000 100
38 I 0, 000 87
56 I 0, 000 100
67 V 0, 000 97
69 D 0,000 62
83 Y 0, 000 95
V 0, 000 72
98 I 0, 000 95
112 S 0,000 77
120 Y 0, 000 95
136 S 0, 000 67
143 L 0, 000 100
147 V 0, 000 100
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
81
EXAMPLE 4
This Example describes preparation and characterisation of recombinant
mutant Bet v 1 allergens with more than four mutations and diminished IgE-
binding affinity according to prior art PCTIDK 01/00764. Mutants according to
the present invention are prepared and assayed accordingly.
Selection of amino acid residues for site-directed mutaaenesis of Bet v 1
Amino acid residues were selected as described in Example 1.
In vitro mutagenesis
In vitro mutagenesis was performed by PCR using recombinant pMAL-c with
Bet v 7 inserted as template. Preparation of recombinant mutant allergens
comprising five to nine primary mutations included two PCR steps; step I and
II. First, each single mutation (or several mutations if located closely
together
in the DNA sequence) was introduced into sequential DNA sequences of Bet
v 9.2809 or Bet v 7.2807 derivatives using sense and anti-sense mutation-
specific oligonucleotide primers accommodating each mutations) along with
sense and anti-sense oligonucleotide primers accommodating either
upstream or downstream neighbour mutations or the N-terminus/C-terminus
of Bet v 1, respectively as schematically illustrated in Figure 15 (I).
Secondly,
PCR products from PCR reaction I were purified, mixed and used as
templates for an additional PCR reaction (II) with oligonucleotide primers
accommodating the N-terminus and C-terminus of Bet v 1 as schematically
illustrated in Figure 15 (II). The PCR products were purified by agarose gel
electrophoresis and PCR gel purification (Life Techhnologies) followed by
ethanol precipitation, cut with restriction enzymes (SacllEcoRl) or (Sacll
Xbal), and ligated directionally into pMAL-c restricted with the same
enzymes.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
82
Figure 16 shows synthesised oligonucleotide primers and schematically
illustrations for the construction of Bet v 1 mutants with more than four
primary mutations. The mutated amino acids were preferably selected from
the group consisting of amino acids that are characterised by being highly
solvent exposed and conserved as described in Example 3. The Bet v 1
mutants are as follows:
Mutant Bet v 1 (2628): TyrSVal, GIu45Ser, Lys65Asn, Lys97Ser, Lys134G1u.
Mutant Bet v 1 (2637): AIa16Pro, Asn28Thr, Lys32Gln, Lys103Thr,
Pro108G1y, Leu152Lys, AIa153G1y, Ser155Pro.
Mutant Bet v 1 (2733): TyrSVal, Lys134G1u, Asn28Thr, Lys32Gln, GIu45Ser,
Lys65Asn, Asn78Lys, Lys103Va1, Lys97Ser, Pro108G1y, Arg145G1u,
Asp156His, +160Asn.
Mutant Bet v 1 (2744): TyrSVal, Lys134G1u, GIu42Ser, GIu45Ser, Asn78Lys,
Lys103Va1, Lys12311e, Asp156His, +160Asn.
Mutant Bet v 1 (2753): Asn28Thr, Lys32Gln, Lys65Asn, GIu96Leu, Lys97Ser,
Pro108G1y, Asp109Asn, Asp125Tyr, GIu127Ser, Arg145G1u.
Nucleotide seauencina and Expression and purification of recombinant Bet v
1 and mutants
Sequencing and expression of recombinant protein was performed as
described in Example 1.
Bet v 1 02628) and Bet v 1 02637) mutants
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
83
Figure 17 shows introduced point mutations at the molecular surface of Bet v
1 (2628) and Bet v 1 (2637).
Crystallisation and structural determination of recombinant Bet v 1 (2628)
mutant protein.
Structural determination was performed as described in Example 1.
Structure of Bet v 1 (2628) mutant
The structural effect of the mutations was addressed by growing three-
dimensional Bet v 1 (2628) protein crystals diffracting to 2.0 A resolution
when analysed by X-rays generated from a rotating anode. The substitutions
TyrSVal, GIu45Ser, Lys65Asn, Lys97Ser, Lys134G1u were verified by the Bet
v 1 (2628) structure electron density map which also showed that the overall
a-carbon backbone tertiary structure is preserved.
Structural analysis of Bet v 1 (2637) mutant
The structural integrity of the purified Bet v 1 (2637) mutant was analysed by
circular dichroism (CD) spectroscopy. Figure 18 shows the CD spectra of
recombinant Bet v 1.2801 (wildtype) and Bet v 1 (2637) mutant, recorded at
close to equal concentrations. The overlap in peak amplitudes and positions
in the CD spectra from the two recombinant proteins shows that the two
preparations contain equal amounts of secondary structures strongly
suggesting that the a-carbon backbone tertiary structure is not affected by
the introduced amino acid substitutions.
IaE-bindinq~aroperties of Bet v 1 (2628) and Bet v 1 (2637) mutants.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
84
The IgE-binding properties of Bet v 1 (2628) and Bet v 1 (2637) as well as a
1:1 mix of Bet v 1 (2628) and Bet v 1 (2637) was compared with recombinant
wild type Bet v 1.2801 in a fluid-phase IgE-inhibition assay using a pool of
serum IgE derived from birch allergic patients.
As described in Example 1, recombinant Bet v 1.2801 was biotinylated at a
molar ratio of 1:5 (Bet v 1 no. 2801:biotin). The inhibition assay was
performed as follows: a serum sample (25 pl) was incubated with solid phase
anti IgE, washed, re-suspended and further incubated with a mixture of
biotinylated Bet v 1.2801 and a given mutant or 1:1 mix of the two mutants.
The amount of biotinylated Bet v 1.2801 bound to the solid phase was
estimated from the measured RLU after incubation with acridinium ester
labelled streptavidin. The degree of inhibition was calculated as the ratio
between the RLU's obtained using buffer and mutant as inhibitor.
Figure 19 shows the inhibition of the binding of biotinylated recombinant Bet
v 1.2801 to serum IgE from a pool of allergic patients by non-biotinylated Bet
v 1.2801 and by Bet v 1 (2628), Bet v 1 (2637) and a 1:1 mix of Bet v 1
(2628) and Bet v 1 (2637).
There is a clear difference in the amount of respective recombinant proteins
necessary to reach 50% inhibition of the binding to serum IgE present in the
serum pool. Recombinant Bet v 1.2801 reaches 50% inhibition at about 5 ng
whereas the corresponding concentration for Bet v 1 (2628) mutant is about
15-20 ng. This show that the point mutation introduced in the Bet v 1 (2628)
mutant lowers the affinity for specific serum IgE by a factor of about 3-4.
The maximum level of inhibition reached by the Bet v 1 (2628) mutant protein
is clearly lower compared to recombinant Bet v 1.2801. This may indicate
that some of the specific IgE present in the serum pool are unable to
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
recognise the Bet v 1 (2628) mutant protein due to the introduced point
mutations.
Bet v 1 (2637) reaches 50% inhibition at about 400-500 ng showing that the
5 point mutation introduced in the Bet v 1 (2637) mutant lowers the affinity
for
specific serum ~IgE by 80 to 100-fold compared to Bet v 1.2801. The large
difference in IgE-binding is further supported by a clear difference in
inclination of the inhibition curve obtained with Bet v 1 (2637) mutant
protein
compared to the inhibition curve for Bet v 1.2801. The different inclination
10 provide evidence that the reduction in IgE-binding is due to a distinctly
different epitope pattern of the mutant compared to Bet v 1.2801.
In addition to the inhibition assays with single modified allergens a 1:1 mix
of
Bet 1 (2628) and Bet v 1 (2637) having same molar concentration of Bet v 1
15 as each of the samples with Bet 1 (2628) or Bet v 1 (2637), respectively
was
tested and showed full (100%) capacity to inhibit IgE-binding to rBet v
1.2801. The capacity to fully inhibit IgE-binding is a clear indication that
all
reactive epitopes present on Bet v 1.2801 were present in the 1:1 allergen
mix. Further support comes from the comparable inclination of the two
20 inhibition curves for Bet v 1.2801 and the allergen mix. Reduced IgE-
reactivity of the mixed allergen sample is demonstrated by the need of a four-
fold higher concentration of the allergen mix, when compared to Bet v
1.2801, for obtaining 50% inhibition of IgE-binding.
25 T cell proliferation assay using mutated recombinant Bet v 1 allergens.
The analysis was carried out as described in ref. 15. Bet v 1 (2628) and Bet v
1 (2637) mutant protein were both able to induce proliferation in T cell lines
from birch pollen allergic patients with stimulation indices similar to
30 recombinant and naturally occurring. This suggests that both of Bet v 1
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
86
(2628) and Bet v 1 (2637) mutant protein can each initiate the cellular
immune response necessary for antibody production.
Histamine release assays with human basophil.
Histamine release from basophil leucocytes was performed as follows.
Heparinized blood (20 ml) was drawn from each birch pollen patient, stored
at room temperature, and used within 24 hours. Twenty-five microlitres of
heparinized whole blood was applied to glass fibre coated microtitre wells
(Reference Laboratory, Copenhagen, Denmark) and incubated with 25
microlitres of allergen or anti-IgE for 1 hour at 37°C. Thereafter the
plates
were rinsed and interfering substances were removed. Finally, histamine
bound to the microfibres was measured spectrophotofluometrically.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
87
Histamine release properties of Bet v 1 (2628) and Bet v 1 (2637) mutant
rotein.
Histamine release data is shown in Figure 20 and Figure 21. The potency of
Bet v 1 (2628) and Bet v 1 (2637) mutant protein to induce histamine release
in human basophil from two birch pollen allergic patients has been tested. In
both cases the release curve of the mutated allergens to induce histamine
release is clearly shifted to the right compared to the release curve of Bet v
1.2801. The shift indicate that the potency of Bet v 1 (2628) and Bet v 1
(2637) is reduced 3 to 10-fold,
Mutant Bet v 1 (2744) and mutant Bet v 1 (2753)
Bet v 1 (2744) and Bet v 1 (2753) was likewise constructed for use as a
mixed allergen vaccine. In these mutated allergens point mutations were
distributed in an all surface arranged fashion as shown in Figure 22 and
Figure 23 and was again designed to affect different surface areas in the two
molecules, respectively, as shown in Figure 24. However these modified
allergens might individually be used as single allergen vaccines as well.
Structural analysis of Bet v 1 (2744) mutant protein
The structural integrity of the purified Bet v 1 (2744) mutant was analysed by
circular dichroism (CD) spectroscopy. Figure 25 shows the CD spectra of
recombinant Bet v 1.2801 (wildtype) and Bet v 1 (2744) mutant, recorded at
close to equal concentrations. The overlap in peak amplitudes and positions
in the CD spectra from the two recombinant proteins shows that the two
preparations contain equal amounts of secondary structures strongly
suggesting that the a-carbon backbone tertiary structure is not affected by
the introduced amino acid substitutions.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
88
Histamine release properties of Bet v 1 (2744)
Histamine release data from five experiments with basophil leucocytes from
five different birch pollen allergic patients is shown in Figure 26 and Figure
27A-D. The potency of Bet v 1 (2744) mutant protein to induce histamine
release in human basophil has been tested. The release curves of the
mutated allergens are clearly shifted to the right compared to the release
curve of Bet v 1.2801 indicating that the potency of Bet v 1 (2744) to release
histamine is reduced 3 to 5-fold.
Mutant Bet v 1 X2733)
A Mutant Bet v 1 (2733) has been constructed and recombinantly expressed.
The distribution of point mutations in Bet v 1 (2733) leave several surface
areas constituting >400A~ unaltered. Figure 28 show introduced point
mutations at the molecular surface of Bet v 1 (2733).
EXAMPLE 5
This Example describes characterisation of recombinant mutant Bet v 1
allergens with more than four mutations and diminished IgE-binding affinity
according to prior art PCTIDIC 01/00764. Mutants according to the present
invention are prepared and assayed accordingly.
T-cell reactivity of recombinant and mutant Bet v 1:
Purpose:
To investigate an in vitro T-cell response to the mutated allergens in terms
of
proliferation and cytokine production.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
89
Methods:
PBL (Peripheral blood lymphocytes) from allergic patients were used in the
following investigation.
Eight bet v 1 specific T-cell lines were established from the PBL with
naturally
purified bet v 1 in order to sustain the variety of bet v 1 isoforms the T-
cells
are presented to, as described in a previously published protocol (26).
Ten PBL and eight T-cell lines were stimulated with birch extract (Bet v),
naturally purified bet v 1 (nBet v 1 ), recombinant Bet v 1 (rBet v 1 or wt;
27)
and four different mutated forms of rBet v 1 (described elsewhere): 2595,
2628, 2637, 2744, 2773. The 2637 mutant was later found to be partly
unfolded and will not be discussed.
In brief: In a round-bottomed 96 well plate PBL were added in 2 x 105 per
well. The different birch samples were added in three different concentrations
in quadroplicates and allowed to grow for 6 days. At day 6 cell half of volume
(100 pl) from each well with the highest concentration of birch were
harvested for cytokine production. Radioactive labelled thymidine was added
to the wells. Next day (day 7) the cells were harvested on a filter.
Scintilation
fluid was added to the filter and the radioactivity was measured in a
scintillation counter.
Likewise in a 96 well round-bottomed 96 well plate T-cells were added in
3x104 T-cells per well and stimulated with irradiated autologous PBL (1x105
cells/well) and 3 different concentrations of the different birch samples.
After
1 day cells from each well with the highest concentration birch were
harvested for cytokine production. Radioactive labelled tnymiame were
added to the wells. At day 2 the cells were harvested onto a filter and
counted as described for PBL.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
Supernatant from the quadroplicates were pooled and cytokines were
measured using a CBA (cytokine bead array) kit from Becton Dickinson.
5 Results:
Ten PBL cultures showed specific stimulation to birch. In general
proliferation
of the PBL to the different birch samples were similar, although variations
could be seen. In 3 PBL, nBet v 1 stimulated proliferation better than rBet v
1
10 and the mutants. The mutant birch samples stimulated PBL almost identical
to rBet v 1 (Fig. 29). Fig. 29 shows the Stimulation Index for the above-
mentioned Bet v 1 preparations. The Stimulation Index (SI) is calculated as
proliferation (cpm: count per minute) of the stimulated sample (highest
concentration) divided with the proliferation (cpm) of the medium control.
15 PPD designates purified protein derivative from mucobacterium tuberculosis,
which serves as a positive control.
Cytokine production was dominated by IFN-gamma and increased
proportionally with PBL proliferation. No signs of a Th1/Th2 shift were
20 apparent (Fig. 30-32). Figure 30 shows a patient with a Th0 profile, Figure
31
a Th1 profile and Figure 32 a Th2 profile. Cytokine production is measured in
pg/ml indicated as the bars and the ratio between IL-SIIFN-gamma is the
lower dashed line (Y-axis to the right). Proliferation is measured in cpm seen
on the Y-axis to the right as a solid line measured in cpm. Medium and MBP
25 (maltose bindig protein) are included as background controls.
Eight T-cell lines established on nBet v 1 and all, except one, proliferated
equally well to all birch samples. Four T-cell lines were secreting Th0 like
cytokines based on the IL-5 and IFN-gamma ratio (Th2 > 5, 5 > Th0 > 0.2,
30 0.2 > Th1 ). Three T-cell lines were secreting Th1 cytokines and one T-cell
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
91
line was secreting Th2 cytokines. The IL-5/IFN-gamma ratio was not affected
by the different birch samples.
Conclusion:
All PBL cultures and 7l8 T-cell lines that showed specific stimulation to nBet
v 1 did also respond to rBet v 1 and the mutants. These data suggests that
for T-cell stimulation a single isoform of Bet v 1 or these 4 mutants can
substitute for the mixture of individual isoforms found in the natural
allergen
preparations. Thus, vaccines based on recombinant allergens or these 4
mutants will address the existing Bet v 1 specific T-cell population.
EXAMPLE 6
This Example describes characterisation of recombinant mutant Bet v 1
allergens with more than four mutations and diminished IgE-binding affinity
according to prior art PCT/DK 01!00764. Mutants according to the present
invention are be prepared and assayed accordingly.
Induction of Bet v 1 specific IgG antibodies and blocking antibodies following
immunization with recombinant and mutant Bet v 1 proteins:
In this section the term "blocking antibodies" is defined as antibodies,
different from human IgE antibodies, that are able to bind to an antigen and
prevent the binding of human IgE antibodies to that antigen.
The ability of recombinant Bet v1 2227 wild type protein (rBet v 1 ) and Bet v
1 2595, 2628, 2744 and 2773 mutant proteins to induce Bet v 1 specific IgG
antibodies and blocking antibodies was tested in immunization experiments
in mice.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
92
BALB/cA mice (8 in each group) were immunized by intraperitoneal injections
with recombinant Bet v1 2227 wild type protein or the four mutant proteins.
The mice were immunized four times with a dose interval of 14 days. The
different proteins were conjugated to 1,25 mg/ml Alhydrogel, (Aluminium
Hydroxide gel, 1,3 % pH 8.0 - 8.4, Superfos Biosector). The mice were
immunized with either 1 ug protein/dose or 10 ug protein/dose. Blood
samples were drawn by orbital bleed at day 0,14,35, 21, 49 and 63.
Specific IgG antibody levels was analyzed by direct ELISA using rBet v 1
coated microtiterplates and biotinylated rabbit anti mouse IgG antibodies
(Jackson) as detection antibody. Immunization with recombinant Bet v1 2227
wild type protein or the four mutant proteins induced a strong r Bet v 1
specific IgG response. This finding demonstrates that the four mutated
proteins are able to induce antibodies that are highly cross reactive to the
Bet
v 1 2227 wild type protein
To assess the induction of blocking antibodies, serum samples from birch
pollen allergic patients were incubated with paramagnetic beads coated with
a monoclonal mouse anti-human IgE antibody. After incubation, the beads
were washed and resuspended in buffer or diluted samples (1:100) of mouse
serum from un-immunized mice (control) or mice immunized as described
above. Biotinylated r Bet v 1 was then added to this mixture of beads and
mouse serum antibodies. After incubation, the beads were washed and
bound biotinylated rBet v 1 was detected using acridinium labeled
streptavidine. Incubation of beads with serum from un-immunized mice did
not change the binding of r Bet v 1 to the beads. In contrast, incubation of
the
beads with serum from mice immunized with the recombinant Bet v1 2227
wild type protein or the four mutant proteins significantly reduced binding of
r
Bet v 1 to the beads demonstrating the presence of Bet v 1 specific blocking
antibodies in the serum samples. Thus, at day 63 one or more serum
samples from all high dose (10 ug/dose) immunization groups were able to
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
93
reduce binding of r Bet v1 to the beads with more than 80%. These findings
demonstrate that the four mutated proteins are able to induce antibodies that
can act as Bet v 1 specific blocking antibodies.
EXAMPLE 7
This example describes the structural characterization and IgE-binding
properties of a mutant according to the invention having 12 point mutation.
The mutations introduced in mutant 3007 are described in example 2.
Structural analysis of Bet v 1 (3007 mutant protein
The structural integrity of the purified Bet v 1 (3007) mutant was analysed by
circular dichroism spectroscopy as described in example 1. Figure 33 shows
the CD spectra of recombinant Bet v 1.2801 (wildtype) and Bet v 1 (3007)
mutant, recorded at equal concentrations as previously described in example
1. The overlap in amplitude-positions in the CD spectra from the two
recombinant proteins indicates that the two preparations contain roughly
equal amounts of secondary structures, strongly suggesting that the a-
carbon backbone tertiary structure is not or affected by the introduced amino
acid substitutions.
IgE-binding analysis of Bet v 1 (3007) mutant protein
Figure 34 shows the inhibition of the binding of biotinylated recombinant Bet
v 1.2801 to serum IgE from a pool of allergic patients by non-biotinylated Bet
v 1.2801 (wildtype) and the Bet v 1 (3007) mutant according to methods
described in example 4. There is a clear difference in the amount of the
respective recombinant proteins necessary to reach 50% inhibition of the
binding to serum IgE present in the serum pool. Recombinant Bet v 1.2801
reaches 50% inhibition at about 5 ng whereas the corresponding
concentration for Bet v 1 (3007) mutant is about 200 ng. The level of
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
94
inhibition reached by the Bet v 1 (3007) mutant protein is clearly lower
compared to recombinant Bet v 1.2801. This show that the 12 point
mutations introduced in the Bet v 1 (3007) mutant lowers the affinity for
specific serum IgE.
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
REFERENCES
1. W0 97/30150 (Pangenetics B.V., Molecules for the induction of
immunological tolerance)
5
2. WO 92!02621 (Biomay Biotechnik Produktions- and Handelsgesellschaft
mbH, Allergens of Alder pollen and applications thereof)
3. WO 90111293 (Immunologic Pharmaceutical Corporation, The University
10 of North Carolina at Chapel Hill, Allergenic proteins from ragweed and uses
thereof)
4. Takai T, Yokota T, Yasue M, Nishiyama C, Yuuki T, Mori A, Okudaira H,
Okumura Y: "Engineering of the major house dust mite allergen Der f 2 for
15 allergen-specific immunotherapy". Nat Biotechnol 15, 754-758 (1997).
5. Smith AM, Chapman MD: "Localization of antigenic sites on Der p 2 using
oligonucleotide-directed mutagenesis targeted to predicted surface residues".
Clin Exp Allergy 27, 593-599 (1997).
6. Aki T, Ono K, Hidaka Y, Shimonishi Y, Jyo T, Wada T, Yamashita M,
Shigeta S, Murooka Y, Oka S: "Structure of IgE epitopes on a new 39-kD
allergen molecule from the house dust mite, Dermatophagoides farinae". Int
Arch Allergy Immunol 103, 357-364 (1994).
7. Forster E, Dudler T, Gmachl M, Aberer W, Urbanek R, Suter M: "Natural
and recombinant enzymatically active or inactive bee venom phospholipase
A2 has the same potency to release histamine from basophils in patients with
Hymenoptera allergy". J Allergy Clin Immunol 95, 1229-1235 (1995).
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
96
8. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA:
"Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a
legume vicilin protein and a major allergen in peanut hypersensitivity". Eur J
Biochem 245, 334-339 (1997).
9. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm
RM, West CM, Bannon GA: "Identification and mutational analysis of the
immunodominant IgE binding epitopes of the major peanut allergen Ara h 2".
Arch Biochem Biophys 342, 244-253 (1997).
10. Ferreira F, Rohlfs A, Hoffmann-Sommergruber K, Schenk S, Ebner C,
Briza P, Jilek A, Kraft D, Breitenbach M, Scheiner 0: "Modulation of IgE-
binding properties of tree pollen allergens by site-directed mutagenesis". Adv
Exp Med Biol 409, 127-135 (1996).
11. Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, Grimm R,
Jah-Schmid B, Breiteneder H, Kraft D, Breitenbach M, Rheinberger H-J,
Scheiner O, "Modulation of IgE reactivity of allergens by site-directed
mutagenesis: Potential use of hypeallergenic variants for immunotherapy",
FASEB Journal for Experimental Biology Vol. 12, No. 2, February 1998, 231-
242 (1998).
12. Wiedemann P, Giehl K, Almo SC, Fedorov AA, Girvin M, Steinberger P,
Rudiger M, Ortner M, Sippl M, Dolecek C, Kraft D, Jockusch B, Valenta R:
"Molecular and structural analysis of a continuous birch profilin epitope
defined by a monoclonal antibody". J Biol Chem 271, 29915-29921 (1996).
13. Alvarez AM, Fukuhara E, Nakase M, Adachi T, Aoki N, Nakamura R,
Matsuda T: "Four rice seed cDNA clones belonging to the alpha-
amylase/trypsin inhibitor gene family encode potential rice allergens". Biosci
Biotechnol Biochem 59, 1304-1308 (1995).
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
97
14. Colombo P, Kennedy D, Ramsdale T, Costa MA, Djro G, Izzo V,
Salvadori S, Guerrini R, Cocchiara R, Mirisola MG, Wood S, Geraci D,
Journal of Immunology Vol. 160, No. 6, 15 March 1998, 2780-2875.
15. Spangfort MD, Ipsen H, Sparholt SH, Aasmul-Olsen S, Larsen MR, Mwrtz
E, Roepstorff P, Larsen JN: "Characterization of Purified Recombinant Bet v
1 with Authentic N-terminus, Cloned in Fusion with Maltose-Binding Protein".
Prot Exp Purification 8, 365-373 (1996x).
16. Ipsen H, Wihl J-,~, Petersen BN, L~wenstein H: "Specificity mapping of
patients IgE response towards the tree pollen major allergens Aln g l, Bet v I
and Cor a I." Clin. Exp. Allergy 22, 391-9, (1992)
17. Gajhede M, Osmark P, Poulsen FM, Ipsen H, Larsen JN, Joost van
Neerven RJ, Schou C, Lmwnstein H, and Spangfort MD: "X-ray and NMR
structure of Bet v 1, the origin of birch pollen allergy". Nature structural
biology 3, 1040-1045 (1996).
18. Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ: "Basic local
alignment search tool". J. Mol. Biol. 215, 403-410 (1990).
19. Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, and
Gibson TJ: "CLUSTAL W: improving the sensitivity of progressive multiple
sequence alignment through sequence weighting, position-specific gap
penalties and weight matrix choice". Nucleic Acids Res. 22, 4673-4680
( 1994).
20. Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis
KB, Erlich HA: "Primer-directed enzymatic amplification of DNA with a
thermostable DNA polymerise". Science 239, 487-491 (1988).
CA 02486112 2004-11-16
WO 03/096869 PCT/DK03/00322
98
21. Spangfort MD, Larsen JN, Gajhede M: "Crystallization and Preliminary X
ray Investigation at 2.0 A Resolution of Bet v 1, a Birch Pollen Protein
Causing IgE-Mediated Allergy". PROTEINS, Struc Func Genet 26, 358-360
( 1996b).
22. Monsalve RI, Lu G, and King TP: "Recombinant venom allergen, antigen
5 of yellowjacket (Vespula vulgaris) and paper wasp (Polistes annularis) by
expression in bacteria or yeast" (1999) Submitted.
23. Fang KSF, Vitale M, Fehlner P and King TP: "cDNA cloning and primary
structure of a white-face hornet venom allergen, antigen 5". Proc. Natl. Acad.
Sci. USA 85, 895 (1988).
24. Lu G, Villalba M, Coscia MR, Hoffman DR and King TP: "Sequence
Analysis and Antigenic Cross-reactivity of a Venom Allergen, Antigen 5, from
Hornets, Wasps, and Yellow Jackets". Journal of Immunology 150, 2823-
2830 (1993).
25. Punnonen J: "Molecular Breeding of Allergy Vaccines and Antiallergic
Cytokines". Int Arch Allergy Immunol 2000; 121:173-182.
26. P.A. Wurtzen, M. Wissenbach, H. Ipsen, A. Bufe, J. Arnved, and R. J. J.
van Neerven. J Allergy Clin Immunol, 1999; 104: 115-23.
27. Sparholt SH, Larsen JN, Ipsen H, Schou C, van Neerven RJ. Clin Exp
Allergy 1997 Aug;27(8):932-41.