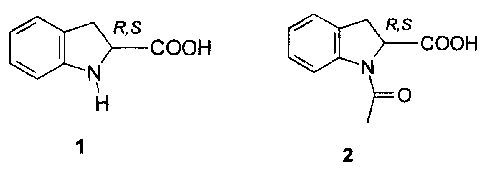Note: Descriptions are shown in the official language in which they were submitted.
CA 02488324 2004-11-22
TITLE OF THE INVENTION
NEW PROCESSES FOR THE PREPARATION OF OPTICALLY PURE INDOLINE-2-
CARBOXYLIC ACID AND N-ACETYL- INDOLINE-2-CARBOXYLIC ACID
FIELD OF THE INVENTION
This invention relates to the production of optically pure indoline-2-
carboxylic acid and
N-acetyl-indoline-2-carboxylic acid.
BACKGROUND OF THE INVENTION
Optically pure indoline-2-carboxylic acid and N-acetyl-indoline-2-carboxylic
acid are
important intermediates in the synthesis of active pharmaceutical compounds.
For example, (S)-
indoline-2-carboxylic acid (1) and (S)-N-acetyl-indoline-2-carboxylic acid (2)
are key
intermediates in the synthesis of the ACE inhibitors perindopril 3 and
pentopril 4.
i
I COOH 1.R=H
N 2.R=Ac
R
i
Et02C H..." ".H ~ ( COOH
N
N ~~~ C02Et
N O
O COOH
3. Perindopril 4. Pentopril
Optically pure indoline-2-carboxylic acid and N-acetyl-indoline-2-carboxylic
acid were
prepared via the prior art (M. Vincent, et al. Tetrahedron Letters, 1982, 23,
1677-1680,
US4954640) by the optical resolution of their racemates mixtures. For example,
(S)-indoline-2-
carboxylic acid was prepared by the resolution of racemic indoline-2-
carboxylic acid using D-a-
methylbenzylamine (M. Vincent, et al. Tetrahedron Letters, 1982, 23, 1677-
1680, US4954640)
CA 02488324 2004-11-22
-2-
or ephedrine (US4520205). Likewise, (S)-N-acetyl-indoline-2-carboxylic acid
was prepared by
the resolution of racemic acid with (-)-cinchonidine (US 4665087), a-amino-s-
caprolactam (EP
0171616) or N-substituted phenylalaninol (JP 61030572). Some of these
resolving agents are
expensive and not widely commercially available and the undesired enantiomers
of indoline-2-
carboxylic acid and N-acetyl-indoline-2-carboxylic acid produced in each case
were not
recovered. Optically pure indoline-2-carboxylic acid and N-acetyl-indoline-2-
carboxylic acid
also could be prepared by the cyclization of optically pure 2-
bromophenylalanine derivatives (T.
Ooi et al., J. Amer. Chem. Soc., 2003, 125, 5139, S. Wagaw et al., J. Amer.
Chem. Soc., 1997,
119, 8451 ). However, the starting material, optically pure 2-
bromophenylalanine derivatives, are
not commercially available and are very difficult to synthesize. Also, the
type of palladium
catalyst used in the cyclization step is expensive and is not commercially
available. Therefore, a
new and efficient process for the preparation of optically pure indoline-2-
carboxylic acid and
derivatives overcoming the deficiencies of the prior art is required.
SUMMARY OF THE INVENTION
It is therefore one object of this invention to provide a new and efficient
process for the
resolution of (R, S)-indoline-2-carboxylic acid of formula 1 using a chiral
acid and for producing
new intermediate compounds.
i I R,S COOH CHZS03H
O
H03SCH2 O
1 (1 S~5 (1 R)-5
CA 02488324 2004-11-22
-3-
We have discovered that (1ST- and (1R)-10-camphorsulfonic acids (5) are
efficient
resolving agents for 1. Thus, (S') and (R)-1 can be isolated as their
diastereomeric salts with
optically pure 10-camphorsulfonic acid.
It is another object of this invention to provide a process for reprocessing
of the undesired
enantiomer and then use of the resolution procedure. In this way, we are able
to obtain almost
complete conversion of the (R, S~-1 to the desired enantiomer (S~ or (R)-1.
We have also found that treatment of the undesired enantiomer or enantiomeric
mixture
(enriched by one enantiomer) of 1 with an acid anhydride, preferably acetic
anhydride, propionic
anhydride or butyric anhydride, with or without a solvent, followed by
hydrolysis furnished the
(R, ,S~-1, which can be optically resolved by the procedure disclosed in the
present invention as
shown in Scheme 1.
Scheme 1.
R,S chiral 5 Desired (S)- or (R~1
--COOH
N
Resolution
Undesired (Rr or (Sr1
(R, S)-1
1. Acid anhydride
2. Hydrolysis
Reprocessing
It is also an object of the present invention to provide a commercial process
for the
resolution of (R, ,S'~-N-acetyl-indoline-2-carboxylic acid of formula 2 using
optically pure
phenylglycinol (b) as a resolving agent and for producing new intermediate
compounds. The
optically pure phenylglycinol (6) is commercially available and inexpensive
and can be easily
produced by the reduction of optically pure phenylglycine.
CA 02488324 2004-11-22
_ L1 _
/ I~S
COOH NH2
N / * _ OH
~O
2 8
It is another object of the present invention to provide a commercial process
for
racemization of the undesired enantiomer of 2 and then use of the resolution
procedure. In this
way, we are able to obtain almost complete conversion of the (R, ,S~-2 to the
desired enantiomer
(S~ or (R)-2. The undesired enantiomer or enantiomeric mixture (enriched by
one enantiomer) of
2 is treated with an acid anhydride to furnish a mixture of (R, S~-2. The
following are exemplary
equations (scheme 2) according to the teachings of the present invention.
Scheme 2.
/ R,S chiral 6 Desired (S)- or (R)-2
-C OOH
N
Resolution
Undesired (R)- or (S)-2
(R,S)-2
Acid anhydride
Racemization
The instant invention has the following advantages:
1. The resolving reagents, optically pure 5 and 6, are inexpensive and
commercially
available.
2. The racemization reaction conditions are mild, the acid anhydride used in
the
racemization steps is inexpensive and commercially available, and the recovery
yields are
good.
CA 02488324 2004-11-22
-
3. The potential recovery yield of desired enantiomer of 1 and 2 are more than
50% of
starting material (R, ,5'~-1 and (R, S~-2, respectively.
DETAILED DESCRIPTION OF ASPECTS OF THE INVENTION
According to an aspect of the present invention, a process for separating the
enantiomers
of indoline-2-carboxylic acid of formula 1 is provided. The process comprises:
(i) combining the (R, ,S~-1 with (1ST- or (1R)-10-camphorsulfonic acid (5) as
the
resolving agent in a resolution solvent and crystallizing from the said
mixture the
diastereomeric salt of (S~- or (R)-1 with optically pure 5;
(ii) regenerating the (,5'~- or (R)-1 from the crystallized diastereomeric
salt by using a
suitable base or basic ion-exchange resin.
Scheme 3
R,S
C\v/~--COOH
N
H
(R,S)-1
(Resolution) ~ chiral5
step i
Undesired Desired
(R~ or (S)-1 ' chiral 5 salt (S)- or (R)-1 ' chiral 5 salt
(mother liquor) (crystal)
step ii ~ Base or basic
ion-exchange resin
(S~ or (R)-1
Suitable resolution solvents include water, C 1 to C7 alcohols such as
methanol, ethanol,
isopropanol and butanols, C3 to C7 ketones such as acetone, methyl ethyl
ketone and methyl
CA 02488324 2004-11-22
-6-
isobutyl ketone, C2 to C7 nitriles such as acetonitrile, C3 to C7 esters such
as ethyl acetate and
methyl acetate, and their mixtures, of which ethanol, isopropanol, water and
their mixture are
preferred.
The amount of resolving agent ranges from 0.5 to 1.1 equivalents relative to
(R, S~-1.
The crystallization can be carried out in the presence or absence of seed
crystals of the
diastereomeric salt of (S~- or (R)-1 with optically pure 5. The amount of seed
is about 0.1 to 10
wt. % relative to the (R, ,5'~-1, preferably the amount is 0.5 to 2.0 wt.%.
Regeneration of the resolved (,S~- or (R)-1 from the crystallized
diastereomeric salts may
be effected by treatment of the salt with a base or by use of an ion-exchange
resin. Suitable
bases include organic and inorganic bases, of which sodium hydroxide,
potassium hydroxide,
sodium carbonate, potassium carbonate and triethylamine are preferred. The pH
of the aqueous
solution during regeneration of the resolved indoline-2-carboxylic acid is
between 1.5 to 4.0,
preferably 2.0 to 3.0, the (,S~- or (R)-1 precipitates from the mixture and
may be isolated by
filtration.
1 S , Further, according to another aspect of the invention, a process is
provided for the
conversion of the enantiomer or enantiomeric mixture (enriched by one
enantiomer) of indoline-
2-carboxylic acid of formula 1 to the mixture of (R, ,S')-1 by treating with
an acid anhydride
followed by hydrolysis and neutralization. The (R, 5')-1 can be subjected to
the resolution process
disclosed above.
Scheme 4
(R}- or (S~1 ~ . Acid anhydride (R,S~1
2. Hydrolysis
CA 02488324 2004-11-22
_7_
The acid anhydride used in racemization process includes C2-C 16 acid
anhydrides, of
which acetic anhydride, propionic anhydride, butyric anhydride and benzoic
anhydride are
preferred. The racemization reaction is carried out in neat acid anhydride or
with a co-solvent.
The suitable solvents include alkylcarboxylic acids such as acetic acid,
propionic acid and
butyric acid, aromatic solvents such as toluene and xylenes, N,N-dialkylamides
such as N,N-
dimethylformamide, N,N-dimethylacetamide and 1-methyl-2-pyrrolidinone, and
alkyl sulfoxides
and sulfones such as dimethyl sulfoxide and sulfolane. The most preferred
solvents are acetic
acid and toluene. The amount of acid anhydride is about 1.0 to 5.0 equivalents
relative to the
indoline-2-carboxylic acid, more preferably the amount is about 2.0 to 3.0
equivalents. The
reaction temperature is between 30 to 150 °C and the preferred
temperature is 70-120 °C.
Hydrolysis may be carried out in the presence of an aqueous acid. The
preferred acids
are hydrochloric acid and sulfuric acid. The reaction temperature is between
30 to 150 °C, and
the preferred temperature is 70-120 °C. After the hydrolysis, the
reaction mixture is neutralized
by the addition of a base. Suitable base includes organic and inorganic bases,
of which sodium
hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and
triethylamine are
preferred. The pH of aqueous for neutralization is between 1.5 to 3.0, and the
preferred pH
range is 2.0 to 2.5. The mixture of (R, ,S~-1 precipitates from the reaction
mixture and may be
isolated by filtration.
Further, according to another aspect of the invention, a process is provided
for the
preparation of (,S~- or (R)-1 via resolution of (R, ~-1 and the recycling of
the undesired
enantiomer of 1. The process comprises:
CA 02488324 2004-11-22
_8-
(i) combining the (R, ,S~-1 with (1,5~- or (1R)-10-camphorsulfonic acid (5) as
the
resolving agent in a resolution solvent and crystallizing from the said
mixture the
diastereomeric salt of (S~- or (R)-1 with optically pure 5;
(ii) regenerating the (,S'~- or (R)-1 ftom the crystallized salt by using a
suitable base or
basic ion-exchange resin;
(iii) optionally regenerating undesired (R)- or (,S~-1 or their mixture
(enriched by one
enantiomer) from the crystallization mother liquors; and
(iv) optionally recovering (R, S)-1 via racemization of the undesired (R)- or
(.S'~-1 or
their mixture (enriched by one enantiomer) with an acid anhydride followed by
hydrolysis and neutralization and converting (R, S~-1 to the desired (S~- or
(R)-1
through steps i) and ii).
Scheme 5
1. Acid ar~ydride i R,S
2. Hydrolysis ~ ~ ~--COON
N
(Reprocessing)
step iv (R,Sr1
(Resolution) chiral5
step i
Undesired Desired
(R}- or (Sr1 ' chiral 5 salt (S~ or (S~1 ' chiral 5 salt
(mother liquor) (crystal)
step iii ~ Base or basic step ii ~ Base or basic
ion-exchange resin iorrexchange resin
Undesired (R)- or (Sr1 ~ ~ (S~ or (R)-1
CA 02488324 2004-11-22
-9-
Suitable resolution solvents include water, C 1 to C7 alcohols such as
methanol, ethanol,
isopropanol and butanols, C3 to C7 ketones such as acetone, methyl ethyl
ketone and methyl
isobutyl ketone, C2 to C7 nitrites such as acetonitrile, C3 to C7 esters such
as ethyl acetate and
methyl acetate, and their mixtures, of which ethanol, isopropanol, water and
their mixture are
S preferred.
The amount of resolving agent ranges from 0.5 to 1.1 equivalents relative to
(R, S~-1.
The crystallization can be carried out in the presence or absence of seed
crystals of the
diastereomeric salt of (,S'~- or (R)-1 with optically pure 5. The amount of
seed is about 0.1 to 10
wt. % relative to the (R, S~-1, preferably the amount is 0.5 to 2.0 wt.%.
Regeneration of the resolved (.S')- or (R)-1 from the crystallized
diastereomeric salts may
be effected by treatment of the salt with a base or by use of an ion-exchange
resin. Suitable
bases include organic and inorganic bases, of which sodium hydroxide,
potassium hydroxide,
sodium carbonate, potassium carbonate and triethylamine are preferred. The pH
of the aqueous
solution during regeneration of the resolved indoline-2-carboxylic acid is
between 1.5 to 4.0,
preferably 2.0 to 3.0, the (S~- or (R)-1 precipitates from the mixture and is
isolated by filtration.
Regeneration of the undesired (R)- or (f)-1 or their mixture (enriched by one
enantiomer)
from the crystallization mother liquors can be carned out using the same
procedure as
regeneration of the desired (,5~- or (R)-1 from the crystallized salt.
The acid anhydride used in the racemization process includes C2-C 16 acid
anhydrides, of
which acetic anhydride, propionic anhydride, butyric anhydride and benzoic
anhydride are
preferred. The racemization reaction is carried out in neat acid anhydride or
with a co-solvent.
The suitable solvents include C1 to CS alkylcarboxylic acids such as acetic
acid, propionic acid
and butyric acid, aromatic solvents such as toluene and xylenes, N,N-
dialkylamides such as N,N-
CA 02488324 2004-11-22
- 10-
dimethylformamide, N,N-dimethylacetamide and 1-methyl-2-pyrrolidinone, and
alkyl sulfoxides
and sulfones such as dimethyl sulfoxide and sulfolane. The most preferred
solvents are acetic
acid and toluene. The amount of acid anhydride is about 1.0 to 5.0 equivalents
relative to the
indoline-2-carboxylic acid, more preferably the amount is about 2.0 to 3.0
equivalents. The
reaction temperature is between 30 to 150 °C and the preferred
temperature is 70-120 °C.
Hydrolysis may be carried out in the presence of an aqueous acid. The
preferred acids
are hydrochloric acid and sulfuric acid. The reaction temperature is between
30 to 150 °C, and
the preferred temperature is 70-120 °C. After the hydrolysis, the
reaction mixture is neutralized
by the addition of a base. Suitable bases include organic and inorganic bases,
of which sodium
hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate and
triethylamine are
preferred. The pH of aqueous phase during neutralization is between 1.5 to 3.0
and the
preferably 2.0 to 2.5. The mixture of (R, S~-1 precipitates from the reaction
mixture and may be
isolated by filtration. The (R, S')-1 can be resolved as disclosed above.
Further, according to another aspect of the invention, a process is provided
for the
resolution of N-acetyl-indoline-2-carboxylic acid of formula 2 using optically
pure
phenylglycinol 6 as a resolving agent. The process comprises:
(i) combining (R, S~-2 with an optically pure (R)- or (,S~-b as the resolving
agent in a
resolution solvent in the presence of or absence of a base and crystallizing
from the
said mixture the diastereomeric salt of (S~- or (R)-2 with optically pure 6;
(ii) regenerating the (f~- or (R)-2 from the crystallized diastereomeric salt
by using a
suitable acid or acidic ion-exchange resin.
CA 02488324 2004-11-22
-11-
Scheme 6
R~ COOH
N
(R,S)-2
(Resolution) ( chiral6
step i
Undesired Desired
(Rr or (S)-2 ' chiral 8 salt (S)- or (R~2 ' chiral 6 salt
(mother liquor) (crystal)
step ii ~ Acid or acidic
ion-exchange resin
(S)- or (R)-2
Suitable resolution solvents include water, C 1 to C7 alcohols such as
methanol, ethanol,
isopropanol and butanols, C3 to C7 ketones such as acetone, methyl ethyl
ketone and methyl
isobutyl ketone, C2 to C7 nitriles such as acetonitrile, C3 to C7 esters such
as ethyl acetate and
methyl acetate, and their mixtures, of which ethanol, isopropanol, water and
their mixture are
preferred.
The resolution may be carried out in the presence of or absence of a base. The
base is
selected from triethylamine, diisopropylethylamine, pyridine and the like. The
amount of base
ranges from 0.0 to 0.8 equivalents, preferable 0.0 to 0.5 equivalents relative
to the (R, ,S'~-2.
The crystallization can be carried out in the presence or absence of seed
crystals of
crystallized diastereomeric salt. The amount of seed is about 0.1 to 10 wt. %
relative to the (R,
S~-2, preferably the amount is 0.5 to 2.0 wt.%.
CA 02488324 2004-11-22
-12-
The amount of resolving reagent is between 0.5 to to 1.2 equivalents relative
to the (R,
S~-2.
Regeneration of the resolved (,S~- or (R)-2 from the crystallized
diastereomeric salts may
be effected by treatment of the salt with an acid or by use of an ion-exchange
resin. Suitable
acids include organic and inorganic acids, of which hydrochloric acid and
sulfuric acid are
preferred. The pH of the aqueous phase during regeneration of the resolved 2
is between 1.0 to
5.0 and preferably 1.0 to 3.0, the (f~- or (R)-2 precipitates from the mixture
and is isolated by
filtration.
Further, according to another aspect of the invention, a process is provided
for the
conversion of the enantiomer or enantiomeric mixture (enriched by one
enantiomer) of 2 or to
mixture of (R, ,S~-2 by treating with an acid anhydride. The (R, S~-2 can be
subjected to the
resolution process disclosed in the present invention.
Scheme 7
(R)- or (S)_2 Acid anhydride (R,S)-2
Acid anhydride includes C2-C 16 acid anhydride, of which acetic anhydride,
propionic
anhydride, butyric anhydride and benzoic anhydride are preferred and acetic
anhydride is the
most preferred. The racemization reaction is carried out in neat acid
anhydride or with a co-
solvent. The suitable solvents include alkylcarboxylic acids such as acetic
acid, propionic acid
and butyric acid, aromatic solvents such as toluene and xylenes, N,N-
dialkylamides such as N,N-
dimethylformamide, N,N-dimethylacetamide and 1-methyl-2-pyrrolidinone, and
alkyl sulfoxides
and sulfones such as dimethyl sulfoxide and sulfolane. The most preferred
solvents are acetic
acid and toluene. The amount of acid anhydride is about 1.0 to 5.0 equivalents
relative to the N-
CA 02488324 2004-11-22
-13-
acetyl-indoline-2-carboxylic acid, more preferably the amount is about 1.0 to
2.0 equivalents.
The reaction temperature is between 30 to 1 _S0 °C, and the preferred
temperature is 70-120 °C.
Further, according to another aspect of the invention, a process is provided
for the
resolution of N-acetyl-indoline-2-carboxylic acid of formula 2 and recycling
the undesired
enantiomer via a racemization process. The process comprises:
(i) combining (R, ,S~-2 with optically pure (R)- or (S~-6 as the resolving
agent in a
resolution solvent in the presence of or absence of a base and crystallizing
from the
said mixture the diastereomeric salt of (,S~- or (R)-2 with optically pure 6;
(ii) regenerating the (S~- or (R)-2 from the crystallized diastereomeric salt
by using a
suitable acid or acidic ion-exchange resin;
(iii) optionally regenerating undesired (R)- or (,S~-2 or their mixture
(enriched by one
enantiomer) from the crystallization mother liquors by using a suitable acid
or
acidic ion-exchange resin; and
(iv) optionally recovering and recycling (R, ,S~-2 by racemization of
undesired (R)- or
l 5 (,S~-2 or their mixture obtained from step (iii) with an acid anhydride.
CA 02488324 2004-11-22
-14-
Scheme 8
Acid anhydride / ~ R~ COOH
N
(Racemization) O
step iv (R,S)-2
(Resolution) ~ chiral6
step i
Undesired Desired
(R)- or (Sr2 ' chiral 6 salt (S~ or (R)-2 ' chiral 6 salt
(mother liquor) (crystal)
step iii ~ Acid or acidic step ii ~ Acid or acidic
ion-exchange resin ion-exchange resin
Undesired (R~ or (S)-2 ~ ~ (S)- or (R)-2
Suitable resolution solvents include water, C1 to C7 alcohols such as
methanol, ethanol,
isopropanol and butanols, C3 to C7 ketones such as acetone, methyl ethyl
ketone and methyl
isobutyl ketone, C2 to C7 nitriles such as acetonitrile, C3 to C7 esters such
as ethyl acetate and
methyl acetate, and their mixtures, of which ethanol, isopropanol, water and
their mixture are
preferred.
The resolution is carried out in the presence of or absence of a base. The
base is selected
from triethylamine, diisopropylethylamine, pyridine and the like. The amount
of base ranges
from 0.0 to 0.8 equivalents, preferable 0.0 to 0.5 equivalents relative to the
(R, ,S~-2.
The crystallization can be carried out in the presence or absence of seed
crystals of
crystallized diastereomeric salt. The amount of seed is about 0.1 to 10 wt. %
relative to the (R,
,S~-2, preferably the amount is 0.5 to 2.0 wt.%.
CA 02488324 2004-11-22
-15-
The amount of resolving reagent is between 0.5 to to 1.2 equivalents relative
to the (R,
f~-2.
Regeneration of the resolved (,S~- or (R)-2 from the crystallized
diastereomeric salts may
be effected by treatment of the salt with an acid or by use of an ion-exchange
resin. Suitable
acids include organic and inorganic acids, of which hydrochloric acid and
sulfuric acid are
preferred. The pH of the aqueous phase during regeneration of the resolved N-
acetyl-indoline-2-
carboxylic acid is between 1.0 to 5.0 and preferably 1.0 to 3.0, the (S~- or
(R)-2 precipitates from
the mixture and is isolated by filtration.
Regeneration of the undesired (R)- or (f~-2 or their mixture (enriched by one
enantiomer)
from the crystallization mother liquors can be carried out using the same
procedure used for
regeneration of (,S'~- or (R)-2 from the crystallized salt.
The acid anhydride used in the racemization reaction includes C2-C16 acid
anhydride, of
which acetic anhydride, propionic anhydride, butyric anhydride and benzoic
anhydride are
preferred and acetic anhydride is the most preferred. The racemization
reaction is carned out in
neat acid anhydride or with a co-solvent. The suitable solvents include
alkylcarboxylic acids
such as acetic acid, propionic acid and butyric acid, aromatic solvents such
as toluene and
xylenes, N,N-dialkylamides such as N,N-dimethylformamide, N,N-
dimethylacetamide and 1-
methyl-2-pyrrolidinone, and alkyl sulfoxides and sulfones such as dimethyl
sulfoxide and
sulfolane. The most preferred solvents are acetic acid and toluene. The amount
of acid
anhydride is about 1.0 to 5.0 equivalents relative to the N-acetyl-indoline-2-
carboxylic acid,
more preferably the amount is about 1.0 to 2.0 equivalents. The reaction
temperature is between
to 150 °C, and the preferred temperature is 70-120 °C.
CA 02488324 2004-11-22
-16-
The following non-limiting embodiments of the invention further illustrate the
manner of
carrying out the inventive processes described herein and the inventive
intermediate compounds
made thereby.
EXAMPLE 1
The mixture of (R, S)-indoline-2-carboxylic acid (8.2 g), (1S)-10-
camphorsulfonic acid
(11.2g) in isopropanol-ethanol (1:1 v/v) (80 mL) was heated to 40 °C to
give a clear solution.
(S)-Indoline-2-carboxylic acid (1S)-10-camphorsulfonic acid salt (0.1 g) was
added as seeds and
the mixture was cooled slowly to 20 °C to give a white suspension.
After stirred at 20 °C for 2 h,
the mixture was filtered and washed with isopropanol to give white solid (6.9
g). The solid was
pulped from isopropanol to give (S)-indoline-2-carboxylic acid (1S)-10-
camphorsulfonic acid
salt as a white solid (5.7 g), [a]Dao = _5,2 (c 0.5, methanol).
The suspension of the above salt (5.15 g) in water (25 mL) was cooled to 0-5
°C and treated
with 15% NaOH solution to pH 2.9. The resulting suspension was filtered and
rinsed with water.
The solid was dried under vacuum to give 1.9 g of (S)-indoline-2-carboxylic
acid, [a]DZO _ -
113.2 (c 1, 1N HCl). A sample was converted to N-acetyl-indoline-2-carboxylic
acid and
analysis by chiral HPLC and showed 99.8% S enantiomer.
EXAMPLE 2
The mixture of (S)-indoline-2-carboxylic acid ( 16.4 g), acetic anhydride
(26.0g) in acetic
acid (33 mL) was heated to 100 °C and stirred for 3 h. Water (80 mL)
and 32% hydrochloric acid
(17.1 g) was added and the mixture was stirred at 100 °C for 5 h. The
solution was concentrated
under vacuum to 30 mL, diluted with water (50 mL) and cooled to 0-5 °C.
The pH of the
CA 02488324 2004-11-22
-17-
solution was adjusted to 2.0-2.5 by the addition of 25% sodium hydroxide
solution. After being
stirred at 0-5 °C for 1 h, the resulting suspension was filtered and
washed with water. The solid
was dried under vacuum to give 14.9 g of (RS)-indoline-2-carboxylic acid, [a]p
° = 0 (c 1, 1N
HCl).
EXAMPLE 3
Prepared as Example 2, starting from (S)-indoline-2-carboxylic acid ( 16.4 g)
and
propionic anhydride (30.0g) to give 15.1 g of (RS)-indoline-2-carboxylic acid,
[a]p2° = 0 (c 1,
1 N HCl).
EXAMPLE 4
The mixture of (R, S)-N-Acetyl-indoline-2-carboxylic acid (50 g), (R)-
phenylglycinol (20
g) in isopropanol (500 mL) and water (45 mL) was heated to 75-80 °C to
give a clear solution.
The solution was slowly cooled to 15-20 °C and stirred for 2 h. The
resulting suspension was
filtered and washed with isopropanol. The solid was blended with isopropanol
(250 mL) and
water (25 mL) to give 25 g (S)-N-Acetyl-indoline-2-carboxylic acid (R)-
phenylglycinol salt as a
white solid. [a]p2o = -106.8 (c 0.5, methanol), Chiral HPLC 99.4% S
enantiomer.
The salt (20 g) was mixed with water (80 mL) and cooled to 0-5 °C. pH
of the mixture
was adjusted to 1.5 to 2.0 by the addition of hydrochloric acid. After being
stirred at 0-5 °C for 1
h, the suspension was filtered and rinsed with water. The solid was dried
under vacuum to give
11.7 g (S)-N-Acetyl-indoline-2-carboxylic acid as a white solid. [a]DZ°
_ -128.4 (c 1.0, ethanol),
Chiral HPLC 99.9% S enantiomer.
CA 02488324 2004-11-22
-18-
The combined resolution and crystallization mother liquor were evaporated
under
vacuum and the residue suspended in water (150 mL). pH of the mixture was
adjusted to 1.5 to
2.0 by the addition of hydrochloric acid. After being stirred at 0-5 °C
for 1 h, the mixture was
filtered, washed with water and dried to give 31 g light yellow solid. Chiral
HPLC showed 70%
R enantiomer. 30 g of this solid was stirred with acetic acid (90 mL) and
acetic anhydride (16.54
g) at 100 °C for 3 h. After cooled to 20 °C, the mixture was
diluted with water ( 180 mL) and the
mixture was filtered, washed with water and dried to give 29 g (R, S~- N-
Acetyl-indoline-2-
carboxylic acid. [a.]DZ° = 0 (c 1, methanol).
EXAMPLE 5
The mixture of (R)-N-acetyl-indoline-2-carboxylic acid (50 g), acetic
anhydride (27.36 g)
in acetic acid (150 mL) was heated to 100 °C and stirred for 3 h. It
was then cooled to 20 °C,
water (250 mL) was added and the mixture was stirred at 0-5 °C for 2 h.
The suspension was
filtered and washed with water. The solid was dried under vacuum to give 46.7
g of (R,
S~-N-acetyl-indoline-2-carboxylic acid, [aJo2° = 0 (c 1, 1N HCl).
While the foregoing embodiments provide detailed description of preferred
embodiments
of the invention, it is to be understood that these are illustrative only of
the principles of the
invention and not limiting. Furthermore, as many changes can be made to the
invention without
departing from the scope of the invention, it is intended that all material
contained herein be
interpreted as illustrative of the invention and not in a limiting sense.