Note: Descriptions are shown in the official language in which they were submitted.
CA 02488789 2010-07-07
77513-36
TITLE OF THE INVENTION
MODEL ANIMALS NON-RESPONSIVE TO MYCOBACTERIA-ORIGIN
LIPOPROTEIN/LIPOPEPTIDE
Technical Field
The presentinvention relates to an non-human animal model
non-responsive to mycobacterial lipoproteins/lipopeptides,
wherein the function of the gene encoding proteins such as TLR1,
specifically recognizing mycobacterial
lipoproteins/lipopeptides is deleted on its chromosome, or to
a method for screening substances promoting or suppressing
,response to mycobacterial lipoproteins/lipopeptides, or the
like.
Background Art
Toll genes are associated with the determination of
dorsoventral axis in the embryogenesis of Drosophilia (Cell 52,
269-279, 1988; Annu. Rev. Cell Dev. Biol. 12, 393-416, 1996),
and with innate immunity detecting invading pathogens in adult
body (Nature 406, 782, 2000; Nat. Immunol. 2, 675, 2001; Annu.
Rev. Immunol. 20, 197, 2002). It has been clarified the Toll
is a type I-transmembrane receptor having Leucine-rich repeat
(LRR) in the extracellular domain, and that the intracytoplasmic
domain is highly homologous with the intracytoplasmic domain
of mammal-Interleukin-1 receptor (IL-1R) (Nature 351, 355-356,
1991; Annu. Rev. Cell Dev. Biol. , 12, 393-416, 1996; J. Leukoc.
Biol. 63, 650-657, 1998).
Recently, mammal homologue of Toll has been identified,
that is the Toll Like Receptor (TLR) (Nature 388, 394-397, 1997;
Proc. Natl. Acad. Sci. USA95, 588-593, 1998; Blood 91, 4020-4027,
1
CA 02488789 2004-12-07
1998; Gene 231, 59-65, 1999) and 10 members of human TLR family
such as TLR2 and TLR4 have been reported so far. The role of
TLR family is to recognize discrete pathogen-associated
molecular patterns (PAMPs) as pattern recognition receptor (PRR)
recognizing common bacterial structure, to trigger the
activation of similar intracellular signaling pathway leading
to the nuclear translocation of a transcription factor, NF-KB.
The signaling pathway ultimately culminates in the production
of inflammatory cytokines to evoke host defense responses and
further evoke host defense responses to acquired immunity.
Moreover, various TLR ligands are reported recently.
TLR2 recognizes a variety of bacterial components, such
as peptidoglycan (PGN), bacterial tri-acylated lipoproteins,
mycoplasmal di-acylated lipoproteins, and GPI anchor of
Trypanosoma cruzi (Science 285, 732, 1999; Science 285, 736,
1999; J. Biol. Chem. 274, 33419, 1999; Immunity 11, 443, 1999;
J. Immunol. 164. 554, 2000; Nature 401, 811, 1999; J. Immunol.
167, 416, 2001). TLR4 is essential for responses to LPS, a
glycolipid specific to Gram-negative bacteria cell wall. TLR5
is reported to recognize flagellin, a protein component of
bacterial flagella. Furthermore, nucleotides specific to
pathogens and nucleotide analogues are also detected by TLRs.
In other words, TLR 3, TLR 7 and TLR 9 participate in the
recognizition of viral double stranded RNA, imidazoquinolines
and bacterial DNA with unmethylated CpG motif, respectively
(Nature 406, 782, 2000; Nat. Immunol. 2, 675, 2001; Annu. Rev.
Immunol. 20, 197, 2002; Nat. Immunol. 3, 196, 2002).
As TLRs can form heterodimers, their ligand specificity
can be further defined. Notably, TLR6 has a unique property
to recognize a mycoplasmal lipoprotein by interacting with TLR2
2
CA 02488789 2004-12-07
(Proc. Natl. Acad. Sci. USA 97, 13766, 2000; Int. Innmunol. 13,
933, 2001). TLR6-deficient mice (TLR6'-) do not respond to
di-acylated mycoplasmal lipopeptides, termed
macrophage-activating lipopeptide 2-kD (MALP-2), and do not
produce inflammatory cytokines. On the other hand,
TLR6-deficient mice respond normally to atri-acylated bacterial
lipopeptide. TLR2-1- macrophages do not respond to neither of
these lipopeptides (Int. Immuno. 13, 933, 2001). That is, it
becomes clear that TLR6 discriminates a subtle difference in
the acylization of lipopept ides derived from microbial pathogens.
Furthermore, these findings raise the possibility that TLR2 forms
a heterodimer with a different TLR to recognize other PAMPs in
the tri-acylated lipopeptides.
On the other hand, lipoproteins are produced by a variety
of pathogens including mycobacteria, Gram-negative bacteria and
mycoplasma species (Microbial. Rev. 60, 316, 1996). The
N-terminus acylated lipopeptide region is responsible for the
immunostimulatory activity of bacterial and mycoplasmal
lipoproteins. Bacterial and mycoplasmal lipoproteins differ
in the degree of acylation of N-terminus cysteine. Lipoproteins
of bacteria are tri-acylated, whereas those of mycoplasma are
di-acylated (Trends Microbiol. 7, 493, 1999). Synthetic
lipoprotein analogue consisting of a palmitoyled version of
N-acyl-S-diacyl cysteine and S-diacyl cysteine mimic the
immunostimulatory activity of bacterial and mycoplasmal
lipoprotein, respectively (Immunobiology 177, 158, 1988; J. Exp.
Med. 185, 1951, 1997).
TLR1 shows high similarity with TLR6 (Gene 231, 59, 1999).
It was reported that overexpression of TLR1 inhibited the
TLR2-mediated responses to modulin which are phenol-soluble
3
CA 02488789 2004-12-07
proteins secreted from Staphylococcus epidermidis (J. Immunol.
166, 15, 2001). On the other hand, another report showed that
TLR1 participates in the recognition of soluble factors from
Neisseria meningitides (J. Immunol. 165, 7125, 2000). However,
the ligand of TLR1 in vivo is yet to be clarified.
The response to bacterial components in-vivo is estimated
to vary upon the difference of the expression level of each TLR
on the surface of the cells, but the involvement of each member
of TLR family to the signaling by the stimulation of bacterial
components in vivo is not yet clarified. Moreover, it was known
that water-insoluble lipoprotein/lipopeptide existing in
biomembranes and the like activates immunocytes. However, no
protein specifically recognizing mycobacterial
lipoproteins/lipopeptides was known. The object of the present
invention is to provide non-human animal model non-responsive
to mycobacterial lipoproteins/lipopeptides wherein the
function of the gene encoding specifically mycobacterial
lipoproteins/lipopeptides, useful to clarify the involvement
of each member of TLR family to the signaling by stimulation
of mycobacterial lipoproteins/lipopeptides, especially the in
vivo function of TLR1, is deleted on its chromosome, especially
a non-human animal wherein the function of TLR1 gene is deleted
on its chromosome, and a method for screening substances
promoting or suppressing response to mycobacterial
lipoproteins/lipopeptides by using these.
Disclosure of the Invention
The present inventors isolated TLR1 genes already
identified from murine genomic library, substituted the gene
site including the intracellular and transmembrane domain of
4
CA 02488789 2004-12-07
the TLR1 gene to a neomycin-resistant gene, introduced HSV-tk
gene, a gene encoding thymidine kinase, on each of 3' end, screened
ES cells clones having double resistance to both G418 and
Ganciclovir. The ES cell clones were injected into C57BL/6 mice
blastocysts, and TLR1 knockout mice wherein the TLR1 gene
function is deleted on its chromosome were obtained according
to the Mendel law through the germline. By comparing and
analyzing the TLR1 knockout mice with wild-type and TLR2 knockout
mice, they confirmed that TLR1 is a receptor protein specifically
recognizing mycobacterial lipoproteins/lipopeptides. The
present invention has been thus completed.
In other words, the present invention relates to a
non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides, wherein the function of the gene
encoding a protein specifically recognizing mycobacterial
lipoproteins/lipopeptides is deleted on its chromosome ("1");
the non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides according to "1", wherein the
function of the gene encoding a protein specifically recognizing
synthesized tri-acylated lipopeptides is deleted on its
chromosome ("2"); the non-human animal model non-responsive to
mycobacterial lipoproteins/lipopeptides according to "2",
wherein the synthetic tri-acylated lipopeptide is a
N-palmitoyl-S-dilaurylglyceryl ("3"); the non-human animal
model non-responsive to mycobacterial
lipoproteins/lipopeptides according to any one of "1" to "3",
wherein the protein specifically recognizing mycobacterial
lipoproteins/lipopeptides is TLR1 ("4"); the non-human animal
model non-responsive to mycobacterial
lipoproteins/lipopeptides according to any one of "1" to "4",
CA 02488789 2004-12-07
wherein the non-human animal is a rodent ("5") ; the non-human
animal model non-responsive to mycobacterial
lipoproteins/lipopeptides according to "5", wherein the rodent
is a mouse ("6") ; the non-human animal model non-responsive to
mycobacterial lipoproteins/lipopeptides according to "6",
wherein the mouse is a TLR1 knockout mouse generated by
constructing a targeting vector by substituting a whole or a
part of the gene fragment of the gene site including the
intracellular and transmenbrane domain of the TLR1 gene, obtained
from screening TLR1 genes from murine genomic library by using
a probe from mouse EST clones; by linearizing the targeting vector
and injecting it into embryonic stem cells, by microinjecting
the targeted stem cells wherein the TLR1 gene function is deleted
into the mouse blastocysts to generate chimeric mice; by breeding
the chimeric mice and wild-type mice to generate heterozygous
mice; and by intercrossing the heterozygous mice ("7") ; and a
method for screening substances promoting or suppressing
response to mycobacterial lipoproteins/lipopeptides, wherein
the response to mycobacterial lipoproteins/lipopeptides in the
immunocytes derived from non-human animal non responsive to
mycobacterial lipoproteins/lipopeptides according to any one
of "1" to "7" is measured/estimated, by using the immunocytes,
a est substance and a mycobacterial lipoprotein/lipopeptide
('8').
Furthermore, the present invention relates to a method
for screening substances promoting or suppressing the response
to mycobacterial lipoproteins/lipopeptides, wherein the
response to mycobacterial lipoproteins/lipopeptides of the
non-human animal non-responsive to mycobacterial
lipoproteins/lipopeptides according to any one of "1" to "7"
6
CA 02488789 2004-12-07
is measured/estimated by using the non-human animal, a test
substance and a mycobacterial lipoprotein/lipopeptide ("9");
the method for screening substances promoting or suppressing
the response to mycobacterial lipoproteins/lipopeptides
according to "S" or "9", wherein the comparison/estimation with
a wild-type non-human animal of its littermate is performed as
a control when measuring/estimating response to mycobacterial
lipoproteins/lipopeptides ("10"); the method for screening
substances promoting or suppressing the response to
mycobacterial lipoproteins/lipopeptides according to any one
of "8" to "10", wherein the substance promoting or suppressing
the response to mycobacterial lipoproteins/lipopeptides is an
agonist or an antogonist to TLR1 ("11") ; the method for screening
substances promoting or suppressing the response to
mycobacterial lipoproteins/lipopeptides according to any one
of "8" to "11", wherein the substance promoting response to
mycobacterial lipoproteins/lipopeptides is a
therapeutic/preventive agent for mycobacterial infection
("12"); the method for screening substances promoting or
suppressing the response to mycobacterial
lipoproteins/lipopeptides according to "12", wherein the
mycobacterial infection is tuberculous or a mycobacterial
infection other than tuberculous ("13"); a substance promoting
or suppressing the response to mycobacterial
lipoproteins /lipopeptides, obtained by the method for screening
a substance promoting or suppressing the response to
mycobacterial lipoproteins/lipopeptides according to any one
of "8" to "13" ("14"); the substance promoting or suppressing
the response to mycobacterial lipoproteins/lipopeptides
according to "14", wherein the substance promoting or suppressing
7
CA 02488789 2010-07-07
77513-36
the response to mycobacterial lipoproteins/lipopeptides is
an agonist or antagonist to TLR1 ("15"); the substance
promoting or suppressing the response to mycobacterial
lipoproteins/lipopeptides according to "14" or "15", wherein
the substance promoting the response to mycobacterial
lipoproteins/lipopeptides is a therapeutic/preventive agent
for mycobacterial infection ("16"); the substance promoting
or suppressing the response to mycobacterial
lipoproteins/lipopeptides according to "15", wherein the
mycobacterial infection is tuberculous or a mycobacterial
infection other than tuberculous ("17").
Furthermore, the present invention relates to a
therapeutic/preventive agent for mycobacterial infection
containing TLR1 and TLR2 expression systems ("18"); the
therapeutic/preventive agent for mycobacterial infection
according to "18", wherein the mycobacterial infection is
tuberculous or a mycobacterial infection other than
tuberculous ("19").
One aspect of the invention relates to a mouse
cell which is non-responsive to mycobacterial lipoproteins
or lipopeptides, wherein the cell comprises a homozygous
deletion mutation of Toll-like receptor-1 (TLR1) gene
encoding a protein that specifically recognizes synthetic
triacylated lipopeptides.
Another aspect of the invention relates to use of
a mouse comprising the cell as described herein, as a model
to assess whether Toll-like receptor-1 (TLR1) is required
for the recognition of a pathogen-associated molecular
pattern (PAMP) protein.
Another aspect of the invention relates to a
method to screen for a pathogen-associated molecular pattern
8
CA 02488789 2010-07-07
77513-36
(PAMP) that is recognized by Toll-like receptor-1 (TLR1),
the method comprising contacting the immunocyte as described
herein with a mycobacterial lipopeptide or a synthetic
lipopeptide, wherein a decreased responsiveness of the
immunocyte that comprises a homozygous deletion mutation of
TLR1 compared to wild-type control immunocyte that does not
carry a homozygous deletion mutation of TLR1, indicates that
the mycobacterial lipopeptide or synthetic lipopeptide is a
PAMP that is recognized by TLR1.
Another aspect of the invention relates to a
method to screen for a substance that promotes or suppresses
a response to mycobacterial lipoproteins or lipopeptides,
the method comprising contacting the immunocyte as described
herein with a mycobacterial triacylated lipoprotein or a
synthetic triacylated lipopeptide in the presence of a test
substance; wherein an increase in responsiveness of the
immunocyte that comprises a homozygous deletion mutation of
TLR1 compared to wild-type control immunocyte that does not
carry a homozygous deletion mutation of TLR1, indicates that
the substance promotes a TLR1-independent response; and
wherein a decrease in responsiveness of the immunocyte that
comprises a homozygous deletion mutation of TLR1 compared to
wild-type control immunocyte that does not carry a
homozygous deletion mutation of TLR1, indicates that the
substance suppresses a TLR1-independent response.
Brief Description of Drawings
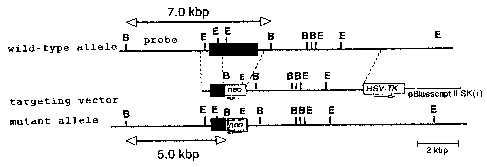
Fig. 1 is a figure that shows a genomic locus of
the TLR1 knockout mouse of the present invention, of a wild-
type mouse and of a targeting vector.
Fig. 2 is a picture that shows the result of
Southern blot analysis of the TLR1 knockout mouse of the
present invention.
8a
CA 02488789 2008-12-16
77513-36
Fig. 3 is a picture that shows the result of
Northern blot analysis of the TLR1 knockout mouse of the
present invention.
Fig. 4 is a figure that shows the result of TNFu
production by 19kD lipoprotein stimulation in TLR1 knockout
mouse of the present invention and in a wild-type mouse.
8b
CA 02488789 2004-12-07
Fig. 5 is a figure that shows the result of TNFa production
by LPS and PGN stimulation in the TLR1 knockout mouse of the
present invention and in a wild-type mouse.
Fig. 6 is a figure that shows the result of IL-6 production
responding to lipoprotein, LPS stimulation in the TLR1 knockout
mouse of the present invention and in a wild-type mouse.
Fig. 7 is a figure that shows the result of TNFa production
by BCG stimulation in the TLR1 knockout mouse of the present
invention and in a wild-type mouse.
Fig. 8 is a figure that shows the result of TNFa production
by Pam3CSK4 stimulation in the TLR1 knockout mouse of the present
invention, in a TLR2 knockout mouse and in a wild-type mouse.
Fig. 9 is a figure that shows the result of TNFa production
by MALP-2 stimulation in the TLR1 knockout mouse of the present
invention, in a TLR2 knockout mouse and in a wild-type mouse.
Fig. 10 is a figure that shows the result of NF-KB activation
induced by Pam3CSK4 stimulation from co-expression of TLRl of
the present invention, TLR2 and TLR6.
Fig. 11 is a figure that shows the result of the
immunoprecipitation of HA- labeled TLRlof the present invention.
Fig. 12 is a figure that shows the result of TNFa production
by Myr3CSK4 stimulation in the TLR1 knockout mouse of the present
invention, in a TLR2 knockout mouse and in a wild-type mouse.
Fig. 13 is a figure that shows the result of TNFa production
by Lau3CSK4 stimulation in the TLR1 knockout mouse of the present
invention, in a TLR2 knockout mouse and in a wild-type mouse.
Fig. 14 is a figure that shows the result of TNFa production
of Lau2N-PamCSK4 stimulation in the TLR 1 knockout mouse of the
present invention, in a TLR2 knockout mouse and in a wild-type
mouse.
9
CA 02488789 2004-12-07
Fig. 15 is a figure that shows the result of TNFa production
by JBT3002 stimulation in the TLR1 knockout mouse of the present
invention, in a TLR2 knockout mouse and in a wild-type mouse.
Best Mode of Carrying Out the Invention
As for a non-human animal model non-responsive to
mycobacterial lipoproteins/lipopeptides of the present
invention, there is no specific limitation as long as it is an
animal model other than human, wherein the function of the gene
encoding a protein specifically recognizing mycobacterial
lipoproteins/lipopeptides is deleted on its chromosome, but it
is preferable that it is an animal model other than human, wherein
the function of the gene encoding a protein specifically
recognizing not only mycobacterial lipoproteins/lipopeptides
but also synthetic tri-acylated lipopeptides such as
N-palmitoyl-S-dilaurylglyceryl, is deleted on its chromosome.
For example, by a gene mutation such as destruction, deletion
or substitution of a whole or a part of an endogenous gene from
a non-human animal encoding a protein specifically recognizing
mycobacterial lipoproteins/lipopeptides to inactivate its
function, the function of the gene encoding a protein
specifically recognizing mycobacterial
lipoproteins/lipopeptides can be deleted on its chromosome.
Moreover, as for the above-described protein specifically
recognizing mycobacterial lipoproteins/lipopeptides, there is
no specific limitation as long as it is a protein that can
specifically recognize mycobacterial
lipoproteins/lipopeptides, and examples include TLR1 or a part
thereof having TLR1 activation. The protein specifically
recognizing mycobacterial lipoprotein/lipopeptide can be
CA 02488789 2004-12-07
prepared by a known method according to its DNA sequence
information and the like. Furthermore, mycobacterial
lipoproteins/lipopeptides of the present invention include, for
convenience, lipoproteins/lipopeptides derived from
Mycobacteria as well as mycobacterial cells itself, or their
disposal, synthetic mycobacterial lipopeptides such as MALP-2.
The non-human animal model non-responsive to
mycobacterial lipoproteins/lipopeptides of the present
invention refers to non-human animals, wherein the
responsiveness of the living body or of the cells, tissues or
organs composing the living body against mycobacterial
lipoproteins/lipopeptidestimulation, is specifically reduced
or deleted, compared to that of wild-type mouse. In other words,
it is related to non-human animals such as mice, rats and rabbits,
wherein the responsiveness of the living body or of the cells,
tissues or organs composing the living body is normal to
lipoproteins/lipopeptides other than Mycobacteria such as
spirochete or Gram-negative bacteria, whereas the
responsiveness of the living body or of the cells, tissues or
organs composing the living body to mycobacterial
lipoproteins/lipopeptides is reduced or deleted. For instance,
non-human animals such as TLR1 knockout mouse, wherein the
function of TLR1 gene is deleted on its chromosome can be
exemplified. Moreover, as for the above-mentioned stimulation
by mycobacterial lipoproteins/lipopeptides, in vivo
stimulation administering mycobacterial
lipoproteins/lipopeptides in the living body, or in vitro
stimulation contacting mycobacterial
lipoproteins/lipopeptides to cells separated from the living
body can be exemplified.
11
CA 02488789 2004-12-07
Next, the method for preparing non-human animal model
non-responsive to mycobacterial lipoproteins jlipopeptides of
the present invention will be explained by taking TLR1 knockout
mouse as an example. With the use of a gene fragment obtained
by PCR method or the like from murine genomic library, genes
encoding TLR1 are screened, and the screened genes encoding TLR1
are subcloned by using viral vectors and the like and are
identified by DNA sequencing. A whole or a part of the gene
encoding TLR1 is replaced with pMC1 neo-gene cassette and the
like, and targeting vectors are prepared by introducing genes
such as Dyphtheria-toxin A fragment (DT-A) or herpes simplex
virus thymidine kinase (HSV-tk) genes to 3'end.
The prepared targeting vector is linearized and introduced
into ES cells by electroporation and the like for homologous
recombination, and ES cells having performed homologous
recombination by antibiotics such as G418 or Gancyclovir (GANC)
are selected from homologous recombinants. Moreover, it is
preferable to confirm if the selected ES cells as the desired
recombinants by Southern blot method or the like. Clones of
the confirmed ES cells are microinjected into mouse blastocysts
which are returned to recepientmice,to generate chimeric mice.
The chimeric mice are bred to wild-type mice to obtain
heterozygous mice (F1 mice: +j-), and by breeding the
heterozygotes, TLR1 knockout mice of the present invention are
generated. Moreover, as for a method for confirming whether
TLR1 has emerged in TLR1 knockout mice, a method by isolating
RNA from a mouse obtained by the above-mentioned method and
examining by Northern Blot method or the like, or a method by
investigating TLR1 expression of the mice can be investigated
by Western Blot method or the like, can be exemplified.
12
CA 02488789 2004-12-07
Furthermore, it can be confirmed that the generated TLR1
knockout mice are non-responsive to mycobacterial
lipoproteins/lipopeptides, by for example contacting
mycobacterial lipoproteins /lipopeptides to immunocytes such as
macrophages, mononuclear cells, dendritic cells, in vitro or
in vivo, and by measuring the production levels of TNF-a, IL-6,
IL-12, IFN-y and the like in said cells, the proliferative
response of splenic B cells, the expression level of antigens
such as CD 40, CD80, CD86, MHC class I I on the surface of splenic
B cells, or the activation of molecules in the signaling pathway
such as NF-KB, JNK, IRAK. Therefore, the TLR1 knockout mice
of the present invention can be a useful model to elucidate the
effect mechanism of mycobacterial lipoproteins/lipopeptides or
to investigate a treatment strategy to mycobacterial infection.
In the meantime, among homozygous non-human animal
generated upon Mendel's law, animals wherein the protein
specifically recognizing mycobacterial
lipoproteins/lipopeptides is deleted and wild-types animals
being its littermates are included. By using at the same time
the deficient-types and wild-types being its littermate of the
homologous non-human animals, it is possible to conduct accurate
comparative examples at individual levels. Therefore, for
example, when screening substances promoting or suppressing the
response to mycobacterial lipoproteins/lipopeptides of the
present invention as described in the following, it is preferable
to use wild-type non-human animals, preferably wild-type
non-human animals of the same species than that of non-human
animals wherein the function of the gene encoding a protein
specifically recognizing Mycobacteria
lipoproteins/lipopeptides is deleted on its chromosome, and its
13
CA 02488789 2004-12-07
littermates at the same time.
Non-human animal models non-responsive to mycobacterial
lipoprotein/lipopetide of the present invention, or immunocytes
such as macrophages, splenic cells, dendritic cells derived from
said non-human model animals can be used for example for screening
substances promoting or suppressing the response to
mycobacterial lipoproteins/lipopeptides of the present
invention such as agonists or antagonists to TLR1, or for
screening preventive/treating agents for mycobacterial
infections such as pulmonary tuberculosis, beside for
elucidating the effect mechanism of mycobacterial
lipoproteins/lipopeptides. The method for screening
substances promoting or suppressing the response to
mycobacterial lipoproteins/lipopeptides such as agonists or
antagonists to TLR1 will be explained in the following by
referring to the examples.
As for the method for screening substances promoting or
suppressing the response to mycobacterial
lipoproteins/lipopeptides of the present invention, it can be
exemplified by a method for measuring/ estimating the response
to mycobacterial lipoproteins/lipopeptides in immunocytes such
as macrophages, splenic cells or dendritic cells derived from
non-human animal models non-responsive to mycobacterial
lipoproteins/lipopeptides, by using the immunocytes, a test
substance and mycobacterial lipoproteins/lipopeptides; or a
method for measuring/estimating the response to mycobacterial
lipoproteins/lipopeptides in non-human model animal
non-responsive to mycobacterial lipoproteins/lipopeptides, by
using the non-human animal model, a test substance and
mycobacterial lipoproteins/lipopeptides.
14
CA 02488789 2004-12-07
As for a method for screening by using immunocytes derived
from non-human animal models non-responsive to mycobacterial
lipoproteins/lipopeptides described above, a method comprising
the steps to contact previously the immunocytes obtained from
non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides with a test substance in vitro, to
culture the immunocytes in the presence of mycobacterial
lipoproteins /lipopeptides and to measure/estimate the response
to mycobacterial lipoproteins/lipopeptides in the immunocytes;
or a method comprising the steps to contact previously the
immunocytes obtained from non-human model animal non-responsive
to mycobacterial lipoproteins/lipopeptides with mycobacterial
lipoproteins/lipopeptides in vitro, to culture the immunocytes
in the presence of a test substance, and to measure/estimate
the response to mycobacterial lipoproteins/lipopeptides in the
immunocytes can be exemplified.
Furthermore, a method comprising the steps to administer
previously a test substance to a non-human model animal
non-responsive to mycobacterial lipoproteins/lipopeptides, to
culture the immunocytes obtained from the non-human model animals
in the presence of mycobacterial lipoproteins/lipopeptides, and
to measure/estimate the response to mycobacterial
lipoproteins/lipopeptides in the immunocytes; or a method
comprising the steps to administer previously a test substance
to a non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides of the present invention, to
administer mycobacterial lipoproteins/lipopeptides to the
non-human animal, and to measure/ estimate the response to
mycobacterial lipoproteins/lipopeptides in the immunocytes
obtained from the non-human animals, can be exemplified.
CA 02488789 2004-12-07
Moreover, a method comprising the steps to administer
previously mycobacterial lipoproteins/lipopeptides to a
non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides of the present invention, to culture
the immunocytes obtained from the non-human animal in the
presence of a test substance, and to measure/estimate the
response to mycobacterial lipoproteins/lipopeptides in the
immunocytes; or a method comprising the steps to administer
previously mycobacterial lipoproteins/lipopeptides to a
non-human animal model non-responsive to mycobacterial
lipoproteins/lipopeptides of the present invention, to
administer a test substance to the non-human animal, and to
measure/estimate the response to mycobacterial
lipoproteins/lipopeptides in the immunocytes obtained from the
non-human animal can be exemplified.
Further, as for a method for measuring/estimating the
response to mycobacterial lipoproteins/lipopeptides in
non-human model animals non-responsive to mycobacterial
lipoproteins/lipopeptides of the present invention, by using
the non-human animal model, a test substance, and a mycobacteria,
a method comprising the steps to administer previously a test
substance to a non-human animal model non-responsive to
mycobacterial lipoproteins/lipopeptides, to infect the
non-human animal model with Mycobacteria, and to
measure/estimate the response to mycobacterial
lipoproteins/lipopeptides in the non-human model animals; or
a method comprising the steps to infect previously the non-human
animal model non-responsive to mycobacterial
lipoproteins/lipopeptides with Mycobacteria, to administer a
test substance to the non-human animal model, and to
16
CA 02488789 2004-12-07
measure/estimate the response to mycobacterial
lipoproteins/lipopeptides in the non-human animal model, can
be exemplified.
In the present invention, measurement /estimation of the
response to mycobacterial lipoproteins/lipopeptides refers to
a measurement/estimation of the function of reacting
specifically with mycobacterial lipoproteins/lipopeptides and
transmitting the signal to the cells, and as for the function
for transmitting the signals, examples include the function
generating cytokines such as TNF-a, IL-6, IL-12, IFN-y; the
function of generating nitrite ion; the function of proliferating
cells; the function of expressing antigens such as CD40, CD80,
CD86, MHC Class I I on the surface of the cells; or the function
of activating molecules in the signaling pathway of TLR9 such
as NF-KB, JNK, IRAK, but it is not limited to these examples.
Moreover, as described above, when measuring/estimating the
response to mycobacterial lipoproteins/lipopeptides, it is
preferable to compare/estimate with the wild-type non-human
animals being its littermates as control, especially with the
estimation levels of wild-type non-human animals being its
littermates, to eliminate individual variety.
As it has been elucidated that TLR1 is specifically related
to the recognition of mycobacterial lipoproteins/lipopeptides,
by the non-human animal models wherein the responsiveness to
mycobacterial lipoproteins/lipopeptides of the present
invention is specifically deleted, it can be assumed that these
non-human animal models can be very useful animal models to
investigate treatment strategy to pulmonary or renal
tuberculosis caused by Mycobacterium tuberculosis.
Furthermore, agonist to TLR1 can possibly be a useful substance
17
CA 02488789 2004-12-07
for diagnosis /treatment of diseases caused by deletion or
abnormality of TLR1 activation such as various mycobacterial
infections mentioned above.
TLR1 and TLR2 interact in mammalian cells, and enhance
further the responsiveness to mycobacterial
lipoproteins/lipopeptides. Therefore, their co-expression in
the host cells may have an effect as preventive/therapeutic
agents for mycobacterial infections. As for the expression
system of preventive/ therapeutic agents for mycobacterial
infections including the expression systems of TLR1 of the
present invention and TLR2, there is no specific limitation as
long as it is an expression system that can express the
above-mentioned TLR1 and TLR2 in the host cells, and examples
include an expression system wherein genes encoding TLR1or TLR2
are individually integrated to viral vectors derived from
papovavirus such as SV40, vaccinia virus, adenovirus, fowl
poxvirus, pseudorabies virus; an expression systemwherein genes
encoding TLR1 or TLR2 are integrated individually; or an
expression system wherein genes encoding TLR1 and TLR2 are
co-integrated. The expression system may include controlling
sequences not only inducing but also controlling the expression.
When a preventive and therapeutic agent is used as drugs in the
present invention, various prescribed compounds such as
pharmaceutically acceptable normal carrier, bonding agent,
stabilizing agent, excipient, diluent, pH buffer agent,
disintegrator, solubilizer, dissolving adjuvant, isotonic
agent can be added. As for a method for preventing or treating
by using these drugs, preventive or therapeutic agents mentioned
above with appropriate dose according to the patient's sex, body
weight, symptoms can be administered orally or parenterally.
18
CA 02488789 2004-12-07
In other words, it can be administered orally in a dosage form
used generally, for example in form of powder, granule, capsules,
syrup, suspension and the like, or parenterally for example in
form of solution, emulsion, suspension and the like by injection,
and moreover, it can be administered in nostril in form of spray.
The present invention will be explained in detail in the
following by referring to the examples, but the technical scope
of the present invention will not be limited to these.
Reference Example (Preparation of TLR2 knockout mice)
TLR2 genes were screened from 129/SvJ murine genomic
library (Stratagene) by using a probe from mouse EST clones,
similar to human-TLR2 genes, subcloned in pBluescript vector
(Stratagene), and were identified by restriction enzyme mapping
and determination of DNA sequence. A targeting vector was
constructed by replacing 1.3 kB of the gene fragment a portion
of an exon containing the intracellular domain of TLR2 with
pMCl-neo having poly A signal (Stratagene). The targeting
vector has 4.8kB if the 5' genomic fragment and 1.0kb of the
3'genomic fragment as franking-sequence, and includes HSV-tk
cassette at 5' end. The targeting vector was linearized with
Sall and electroporated into E14.1 embryonic cells (ES cells).
From the electroporated ES cells mentioned above, cells showing
resistance to G418 and Gancyclovir and containing mutant TLR2
allele were screened. The ES cells were microinjected into
C57BL/6 mouse blastocysts to generate chimeric mice. The male
chimeric mice were bred to C57BL/6 female, and TLR2 knockout
mice were obtained(Immunity 11, 443-451, 1999).
Example 1 (preparation of TLRl knockout mice)
19
CA 02488789 2004-12-07
TLR1 genes were screened from 129Sv murine genomic library
(Clontech) by using a probe derived from mouse TLR1 gene,
subcloned in pBluescript II SK(+) vector and were identified
by restriction enzyme mapping and DNA sequence determination.
A targeting vector was constructed by replacing a gene site
encoding mouse TLR1 intracellular domain and transmembrane
domain (1.0kB of the 5'end and 10kb of the 3'end of a portion
of an exon containing amino acid 575-795 of the mouse TLR1) with
a neomycin cassette (Stratagene), and by introducing herpes
simplex virus thymidine kinase (HSV-TK) as a negative selection
marker (Fig. 1). The targeting vector was linealized with SalI,
electroporated into E14. 1 embryonic stem cells (ES cells). 125
clones showing resistance to G418 and gancyclovir were selected
and three clones were screened by PCR and Southern blot methods.
Three targeted ES clones containing mutant TLR1 allele
were microinjected into C57BL/6 blastocysts to generate chimeric
mice. The male chimeric mice were bred with C57BL/6 female mice
to generate heterozygotes Fl mice. By intercrossing the
heterozygous F1 mice, homozygous mice (TLR1 knockout mice: TLR-I- )
were obtained. Homozygous mice were confirmed by digesting each
genomic DNA extracted from murine tail with EcoRI, by Southern
blot method by using a probe as shown in Fig. 1 (Fig. 2).
Peritoneal macrophages of TLR1-'- mice did not express mRNA of
TLR1 (Fig. 3). On the contrast, expression of TLR2 in TLR-1-
macrophages was normal compared with the case of wild-type cells.
TLR1-'- of the present invention were produced according to
Mendel's law, and the mice were healthy, fertile and did not
show any obvious abnormalities for the first six months.
Moreover, there were no changes in thymocytes and splenocytes
of TLR1-'- mice.
CA 02488789 2004-12-07
Example 2 (preparation of peritoneal macrophages and enzyme
linked immunosorbent assay)
2ml of 4% thioglycollate medium (DIFCO) were
intraperitoneally injected into wild-type mice, TLR1 knockout
(TLR1-1-) and TLR2 knockout (TLR2-"-) mice. Three days later,
peritoneal exudate cells were isolated from each peritoneal
cavity. The cells were cultured for 2h at 37QC in RPMI 1640
medium (Nacalai tesque) supplemented with 10%fetal bovine serum,
cultured peritoneal macrophages (5 x 104) and were stimulated
with indicated bacterial components such as lipoprotein f or 24h.
Concentration of TNFa (Genzyme Techne) and IL-6 (R&D) in culture
supernatants were determined by enzyme linked immunosorbent
assay (ELISA).
(Response to PAMPs)
Native 19-kD lipoprotein purified from Mycobacterium
tuberculosis (purified as described in Science 285, 732, 1999) ,
LPS of Salmonella minnesota RE595, and PGN of Staphylococcus
aureus were used for PAMPs. Peritoneal macrophages of wild- type
and TLR1-1- mice stimulated with thioglycollate were cultured
for 24 h in the presence of these PAMPs. Concentration of TNFa
in culture supernatants was determined. The results of the use
of nature 19kD lipoprotein are shown in Fig. 4, and those of
the use of LPA and PGN are shown in Fig. 5. Wild-type macrophages
responded to 19kD lipoprotein and generated TNFa in a
dose-dependent manner, whereas TNFa production by TLR1-/-
macrophages was impaired when stimulated with concentration of
g/ml and 10 g/ml of lipoprotein in the experiments (Fig.
4). On the other hand, when stimulated with LPS and PGN, TLR1-'-
21
CA 02488789 2004-12-07
macrophages produced TNFa in a dose-dependent manner, almost
at the same extent as those from wild-type cells (Fig. 5).
Moreover, cultured peritoneal macrophages (5 x 104) were
stimulated with native 19kD lipoprotein and LPS for 24 h
respectively, and concentration of IL-6 in culture supernatants
was determined. As it is clear from the results shown in Fig.
6, IL-6 production responding to native 19-kD lipoprotein was
lower in TLR1-'- macrophages compared with that of wild-type cells,
whereas IL-6 production responding to LPS did not show
significant difference between these two. Furthermore, to
investigate whether TLR1 is engaged with recognition for all
Mycobacteria, peritoneal macrophages were stimulated with M.
bovis BCG (Kyowa), viable cells whose level were gradually
increased for 24h, and concentration of TNFa in culture
supernatants was determined. The ability to generate TNGa in
response to BCG was partially impaired in TLR1-1- macrophages
as shown in Fig. 7. From these results, it was revealed that
TLR1 is involved also with the recognition of 19kD lipoprotein
purified fromMycobacteria, and not only with viable Mycobacteria
cells.
(Response to synthetic acylated lipopeptide)
Furthermore, the present inventors have previously shown
that TLR2 is essential for both tri- and di-acylated lipopeptide
response, and that TLR6 interacts with TLR2 and specifically
recognizes di-acylated lipopeptide (Int. Immunol. 13, 933, 2001) .
Cytokine production in response tol9kD lipoprotein preparation
was inhibited in TLR2-"- macrophages (Science 291, 1544, 2001).
These results all show that TLR1 interacts also with TLR2, and
recognizes tri-acylated lipoprotein. To elucidate chemical
22
CA 02488789 2004-12-07
structure that TLR1 recognizes, peritoneal macrophages of
wild-type and TLR1-1- mice were stimulated with Pam3CSK4, the
synthetic bacterial tri-acylated peptide, and MALP-2, the
synthetic Mycoplasmal di-acylated peptide, respectively.
TLR1-1- macrophages showed significantly impaired TNFa
production in response to Pam3CSK4 compared to wild-type cells
(Fig. 8), whereas TLR1-1- cells responded normally to MALP- 2 (Fig.
9). These results indicate that TLR1 is involved in the
recognition oftri-acylated bacterial lipoprotein. In addition,
TLR 1 dif f erentally recognizes TLR2 ligands, distinguishing the
degree of acylation of the lipopeptide.
Example 3 (Control of NF-KB activity in response to lipopeptide
stimulation due to co-expression of TLR1, TLR2 and TLR6)
HEK 293 cells were transformed with TLR1, TLR2 and TLR6
expression vectors, and pELAM-luciferase reporter plasmid were
used together. For normalization of transfection efficiency by
lipofectamine 2000 (Invitrogen), the indicated vector was used
with pELAM- lucif erase reporter plasmid (J. Bio. Chem. 274, 10689,
1999) and pRL-TK (Promega) to transfect transiently human fetal
kidney (HFK)-293 cells. 24 h after transfection, the cells were
stimulated with 10 ng/ml of Pam3CSK4 for 8 h. Then, the cells
were lysed, and luciferase activity was measured by using
Dual-luciferase reporter assay system (Promega) according to
the manufacturer's instruction. The results are shown in Fig.
10. The expression of TLR2 conferred the NF-KB activation in
response to Pam3CSK4 stimulation, and co-expression of TLR1
significantly enhanced the activation. In contrast,
co-expression of TLR6 and TLR2 did not augment the NF-KB
activation induced by Pam3CSK4 stimulation. These results
23
CA 02488789 2004-12-07
indicate that TLR1 and TLR2, but not TLR6, are involved in the
cooperative recognition of Pam3CSK4.
Example 4 (Interaction of TLR1 and TLR2 in mammalian cells)
HEK293 cells were co-transfected with 3 g Flag-tagged
TLR2, TLR4 or 6 g HA-tagged TLR1. After 36 h, the cells were
lysed in the lysis buffer containing 1.0% Nonidet P-40, 150 mm
NaCl, 20 mM tris-HC1 (pH 7. 5), 5 mM EDTA and a protease inhibitor
cocktail tablet, Complete (Roche Diagnostics). The solution
was pre-treated for 1 h with protein G-sepharose, and
immunoprecipitated for 12 h by using 2 g anti-Flag M2 antibody
or 2 Rg anti-HA 12 CA5 antibody. The beads were washed four
times in the lysis buffer, and the immunoprecipitated proteins
were eluted in SDS-PAGE sample buffer solution, separated on
SDS-PAGE and transferred onto PVDF membrane. HA-tagged TLR1
was detected with anti-HA antibody (Roche Diagnostics) and
HRP-tagged anti-mouse Ig antibody. Flag-tagged proteins were
identified with the second antibody, HRP-conjugated anti-Flag
M2 antibody. Then, the antibodies were detected by enhanced
chemiluminescence system (Dupont).
Immunoprecipitation of HA-tagged TLR1 resulted in
co-precipitation of Flag-tagged TLR2, but not of TLR4.
Reciprocally, HA-tagged TLR1 also co-precipitated with
Flag-tagged TLR2 (Fig. 11). However, stimulation with Pam3CSK4
did not affect the extent of association between TLR1 and TLR2.
These results suggest that TLR1 and TLR2 associate in a ligand
independent manner.
Example 5 (Lipopeptides recognized by TLR1 and TLR2)
Though the response to Pam3CSK4 was significantly impaired
24
CA 02488789 2008-12-16
77513-36
in TLR1 mice, the present inventors observed TLRl -independent
cytokine production. To further screen the specific ligands~_
recognized by TLR1, lipopeptides bearing different combination
of fatty acids at their N-terminus were synthesized. The method
for synthesizing synthetic N-palmitoyl-S-dipalmitoyl glyceryl.
(Pam3) CSK4 and MALP-2 are described previously (Science 285,
736, 1999; J. Immunol. 164, 554, 2000). JBT3002, a synthetic
lipoprotein analogue, is as described previously (J. Leukoc.
Biol. 63, 766, 1998). Further, other lipopeptides having
different N-terminus acylation function (Peptide Institute
Inc.) such as N-palmitoyl-S-dilauryl glyceryl
(N-Pam-S-Lau2)CSK4, N-lauryl-S-dilauryl glyceryl (Lau3)CSK4,
N-myristyl-S-dimyristyl glyceryl (Myra) CSK4 were used. These
differ in the length of fatty acid substituted on the N-terminus
cysteine of the peptides. The lipid moiety of N-Pam-S-Lau2CSK4
and JBT3002 are the same.
Macrophages from wild-type, TLRl.'-. and TLR2"1- mice were
stimulated with the above-mentioned synthetic peptide compounds,
Myr3CSK4, Lau3CSK4 , Lau2N-PamCSK4 and JBT3002, and the TNFa
production was measured. The results are shown in Figs. 12 to
15, respectively. All of these synthetic peptides activated
wild-type cells to produce TNFa in a dose-dependent manner.
Macrophages from TLR2"1- mice did not produce any detectable TNFa
response to either of these lipopeptides. Macrophages from
TLR1-'- impaired the production ability of TNFa in response to
Myr3CSK4 and Lau3CSK4 (Figs. 12 -and 13). The ability of TLR1-'-
cells to produce TNFa was considerably impaired when stimulated
with Lau2N-PamCSK4 or JBT3002. This indicates that a subtle
difference in lipid moiety of lipoprotein is critical for the
recognition of TLRl (Figs. 14 and 15). These results provided
CA 02488789 2004-12-07
evidence that TLRl is involved in the recognition of tri-acylated
lipoprotein as well as mycobacterial products. TLR1 and TLR2
cooperate to detect Pam3CSK4, by interacting each other,
indicating that TLR2 pairs with TLR1 or TLR6 to recognize
different PAMPs.
Industrial applicability
As the non-human animal models non-responsive to
mycobacterial lipoproteins/lipopeptides such as TLRl of the
present invention are only non-responsive to mycobacterial
lipoproteins/lipopeptides, by using this non-human animal model
it would be possible to screen substances promoting or
suppressing mycobacterial infections such as pulmonary
tuberculosis, or substances promoting or suppressing the
responsiveness tomycobacterial lipoproteins/lipopeptides such
as agonists or antagonists to TLR1, and therefore to obtain new
useful information to elucidate the molecule structure of
bacterial infections such as mycobacterial species.
26