Note: Descriptions are shown in the official language in which they were submitted.
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
METHOD FOR CONVERTING METHANE-CONTAINING GASEOUS HYDROCARBON
MIXTURES TO LIQUID HYDROCARBONS
Field of the Invention
This invention relates to an improved method of converting mixtures comprising
major amounts of methane and minor amounts of normally gaseous higher
hydrocarbons
to normally liquid hydrocarbons. More particularly, the invention relates to
an improved
method of converting at least a substantial portion of methane-containing
gaseous
hydrocarbon mixtures to liquid hydrocarbons.
Background of the Invention
Natural gas is typically a mixture of about 70 mole percent to about 98 mole
percent
of methane mixed with heavier hydrocarbons. These heavier hydrocarbons are
mostly
alkanes of two or more carbon atoms present in decreasing amounts according to
increasing carbon number. Although these heavier hydrocarbons are typically
present in
small quantities relative to the methane and other normal gaseous materials
such as
carbon dioxide and even hydrogen sulfide are often present, as used herein,
the term
"natural gas" refers to a mixture which is predominately methane with from
about 2 mole
percent to about 30 mole percent of other normally gaseous hydrocarbons, e.g.,
ethane,
propane and butane. Removal of any other materials, such as carbon dioxide or
water,
if necessary, is accomplished by conventional methods.
Natural gas is an important energy source throughout much of the developed
world
including the United States.--Particularly important is-the-use of-nature-gas
as an energy
source in its property of clean burning without the co-production of
environmentally
-1-
CA 02489383 2010-08-27
damaging oxides of nitrogen and sulfur. Unfortunately, the location of
natural gas usage is often distant from the location of natural gas
production so that transportation of the natural gas as by pipeline, or
electrical energy produced therefrom, is often difficult and/or prohibitive
because of the cost of such transportation.
It has long been desirable to convert natural gas to a liquid product
which would facilitate transportation. Conventional methods for such
conversion include liquefaction of the natural gas, partial oxidation of the
natural gas to produce methanol and utilization of Fisher-Tropsch
technology to convert methane to mixtures of carbon monoxide and
hydrogen which are then converted to light olefins and paraffinic
hydrocarbons. The process of U.S. Patent 3,156,733 includes the
pyrolysis of methane to produce acetylene and hydrogen followed by rapid
quenching.
In copending Canadian Patent File No. 2,352,243, filed November
23, 1999, of Hall, et al., a thermal process is disclosed in which the natural
gas is heated to a temperature where a portion of the methane component
of the natural gas is cracked or pyrolyzed to produce hydrogen and reactive
hydrocarbons such as ethylene and acetylene. This stream, also containing
methane, is reacted in the presence of an acidic catalyst to produce a
transportable liquid product which is predominately pentane illustrative of
the pentane production are the simplified equations which follow.
3 CH4 + C2 H2 -4 C5 H12 + H2
3 CH4 + C2H4 -> C5H12 + 2 H2
Unfortunately, the elevated temperatures required to crack the
methane component is sufficiently high to destroy a portion of the
higher hydrocarbons present in the natural
-2-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
gas as well as a portion of the reactive hydrocarbons produced by methane
cracking
through the formation of coke. This loss of potential liquid products of the
overall process
as by coke formation results in loss of economy of the process.
It would therefore be of advantage to provide an improved thermal process for
the
conversion of natural gas, but also of other methane-containing gaseous
hydrocarbon
mixtures, to normally liquid, more easily transportable, hydrocarbons in which
the loss of
hydrocarbons of two or more carbon atoms is minimized and the efficiency of
the
production of transportable liquid hydrocarbon is increased.
Summary of the Invention
The present invention provides an improved method for the conversion of
methane-
containing gaseous hydrocarbon mixtures into normally liquid hydrocarbons of
increased
ease of transportation. The process includes the separation of the methane
component
of the methane-containing gaseous hydrocarbon mixture from the heavier
hydrocarbon
component. In the process of the invention, this heavier hydrocarbon component
is
subjected to cracking at a relatively low cracking temperature sufficient to
crack the
heavier hydrocarbons, but not crack an appreciable quantity of methane. This
low
temperature cracking process minimizes the loss of heavier hydrocarbons as by
coking
with a resulting overall efficiency of liquid product formation.
The methane-containing gaseous hydrocarbon mixtures to which the invention
relates include natural gas but also the light gases that are produced in
petroleum refinery
operations such as catalytic cracking and delayed coking. Gaseous hydrocarbon
mixtures
-3-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
resulting from these operations are typically separated to recover the
heavier, more
valuable components with the light ends being used for fuel. Such streams
often contain
a significant portion of ethane, propane, alkanes and alkynes and hydrogen in
addition to
methane. The process of the invention is useful to convert these "waste gas"
streams to
more valuable liquid products.
The methane-containing gaseous hydrocarbon mixtures to which the process of
the
invention applies are those normally gaseous hydrocarbon mixtures containing
at least
about 40 mole percent methane and preferably at least about 55 mole percent
methane
with the remainder comprising saturated and unsaturated hydrocarbons as well
as
hydrogen. Of primary interest in the methane-containing gaseous hydrocarbon
mixture is
natural gas. Natural gas, as obtained from the ground, often contains non-
hydrocarbon
materials such as water, hydrogen sulfide and carbon dioxide. As used herein,
however,
"natural gas" is used to describe a mixture of about 70 mole percent to about
98 mole
percent of methane with the remainder being heavier hydrocarbons, mostly
alkanes, of two
or more carbon atoms present in decreasing amounts according to increasing
carbon
number.
In certain embodiments of the process of the invention, at least a major
proportion
of the separated methane is separately cracked in the substantial absence of
the heavier
hydrocarbon component of the natural gas feed. Remaining portions of the
separated
methane are reacted with unsaturated hydrocarbons in the presence of an acid
catalyst
to produce the normally liquid hydrocarbon product as illustrated by the above
equations.
-4-
CA 02489383 2010-03-03
In another embodiment of the process of the present
invention, the separated methane is not cracked but is in part
used for the acid-catalyzed reaction of methane with cracked
heavier hydrocarbons with any methane not so employed being
removed from the process system and sent to disposal or used in
the production of thermal or electrical energy as by conventional
methods. Such energy is useful in portions of the overall process
of the invention or in other applications.
In a specific embodiment of the invention, a novel method
of separating the methane portion of the natural gas feed from the
heavier hydrocarbon component is employed.
Brief Description of the Drawings
Figure 1 illustrates one embodiment of the process
described and claimed in Canadian Patent File No. 2,352,243,
filed November 23, 1999 entitled "Method for Converting Natural
Gas to Liquid Hydrocarbons". This figure is for illustration and
comparison purposes only and does not represent the present
invention. A natural gas stream is cracked at a relatively high
temperature. The effluent from the cracking zone is pressurized
and separated into a hydrogen product and a hydrocarbon
product. The hydrocarbon product is mixed with inlet gas and
reacted in the presence of an acidic catalyst. The resulting
product is separated into an overhead product of hydrogen and
light hydrocarbons, e.g., C, - C41 which is recycled. The bottoms
product of this latter separation is the desired normally liquid
hydrocarbon product, typically ranging from C4 to C12 and higher.
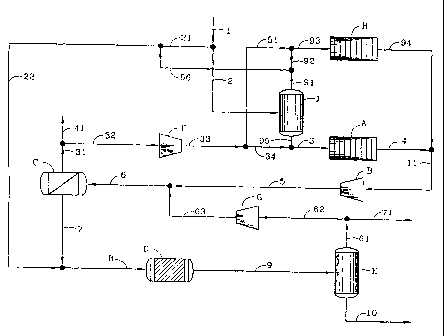
Figure 2 illustrates one embodiment of the present
invention. In this embodiment, the major portion of the methane-
containing gaseous hydrocarbon feed is initially
-5-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
separated into a predominately methane stream and a heavier hydrocarbon
stream.
These streams are separately cracked under differing cracking conditions. The
resulting
cracked streams are combined, pressurized, and separated into a hydrogen
product
stream and a hydrocarbon stream. The hydrocarbon stream is mixed with a
portion of the
gaseous feed and contacted in the presence of an acid catalyst. The resulting
product
stream is separated into a light fraction comprising hydrogen and light
hydrocarbons, e.g.,
C1 - C4, which is recycled. The heavier fraction comprises the desired
normally liquid
product.
Figure 3 depicts an alternate embodiment of the invention wherein a major
portion
of the methane-containing gaseous hydrocarbon feed is cracked in a cracking
zone at
relatively low temperatures. In this zone, the C2 and heavier hydrocarbon
portion of the
feed is cracked. At the cracking temperatures employed, the methane portion of
the
gaseous feed is not reactive and by passing through the low-temperature
cracking zone
essentially unchanged is separated from the heavier hydrocarbon component of
the
gaseous feed as well as the cracking products thereof. Methane recycled from a
downstream portion of the process is cracked at a relatively high temperature
in the
substantial absence of higher hydrocarbon. The two cracking product streams
are
combined, pressurized and then separated into a hydrogen overhead product and
a
hydrocarbon bottoms product. This latter product is mixed with a portion of
the gaseous
feed and reacted in the presence of an acid catalyst. The product from
reaction is
separated into a light fraction of primarily hydrogen and methane which is in
part recycled
-6-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
to the high-temperature cracking process described above. The heavier product
of the
reaction is the desired normally liquid product.
In a somewhat different embodiment modification of the invention illustrated
by
Figure 4, a methane-containing gaseous hydrocarbon feed is separated into a
predominately methane portion and a predominately heavier hydrocarbon portion.
The
higher hydrocarbon portion is cracked at a relatively low cracking
temperature, pressurized
and separated into a hydrogen product and a hydrocarbon product. The
hydrocarbon
product is combined with the methane separated from the natural gas feed and
the mixture
is reacted in the presence of an acidic catalyst. The resulting reaction
product is
separated into a lighter stream of predominately hydrogen and methane, a
portion of which
is vented with the remainder being recycled. The heavier portion of the
reaction product
is the desired normally liquid product.
A specific embodiment of the invention is illustrated by the novel separation
procedure of Figure 5. This procedure serves to separate the methane
components of a
methane-containing gaseous hydrocarbon feed from the heavier hydrocarbon
component
and is particularly, but not exclusively, employed in the processes of Figures
2 and 4. In
this separation process, a methane-containing gaseous hydrocarbon feed is
cooled by
recycle streams and optionally by refrigeration before passing to a high
pressure
separator. The resulting high pressure separation produces an overhead vapor
mixture
of predominately methane and a bottoms liquid stream of heavier hydrocarbons.
The
overhead vapor stream is depressurized to cool the stream and condense at
least a
portion of any heavier hydrocarbon present. This condensed hydrocarbon portion
of the
-7-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
high pressure separator overhead is separated from methane present by means of
a low
pressure separator and then is combined with the liquid effluent of the high
pressure
separatorwhich has undergone depressurization. The cool liquid hydrocarbon
stream and
the cool gaseous methane stream are used to cool the incoming natural gas feed
as by
cross-exchange.
Description of the Invention
The present invention provides an improved process for the conversion of
normally
gaseous methane-containing hydrocarbons such as natural gas to normally liquid
hydrocarbon product which is of particular value because of the ease of its
transportation.
The cracking of natural gas, in a pyrolysis unit or other conventional
apparatus, followed
by processing operations to increase the production of normally liquid product
is known
but has some inherent difficulties. The cracking of methane-containing gaseous
hydrocarbons such as natural gas taken as a whole, requires the use of
relatively high
temperatures in order to crack the methane of the gaseous mixture. Under these
relatively
high-temperature conditions, a significant percentage of the higher
hydrocarbon portion
of the methane-containing gaseous hydrocarbons is effectively lost through
formation of
coke or other undesirable reactions. This loss of the heavier hydrocarbons
results in the
decrease of the desired normally liquid hydrocarbon product of the overall
process. Yet,
the presence of methane and the cracking products thereof, i.e., hydrogen and
unsaturated hydrocarbons of two or more carbon atoms, are necessary in the
overall
process scheme. The hydrogen produced by methane cracking serves as a reaction
diluent in various portions of the overall process with any hydrogen in excess
of that
-8-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
required for diluent purposes being useful as a source of thermal or
electrical energy in
portions of this or other reaction schemes. The methane which is not cracked
is also
useful as a diluent or energy source and is also a reactant in the conversion
of alkenes
and alkynes resulting from cracking of methane as well as from cracking the
heavier
hydrocarbon portion of the gaseous feed.
Accordingly, there is a need for methane and higher hydrocarbon reactants.
However, when mixtures of methane and higher hydrocarbons are cracked at the
elevated
temperatures of methane cracking, potential liquid product is lost through
formation of coke
or through other undesirable reactions. It has now been found to be useful to
separate the
methane component of the methane-containing gaseous hydrocarbon feed from the
heavier hydrocarbon component and to crack the higher hydrocarbon component
under
conditions of temperature and pressure sufficient to cause cracking of the
higher
hydrocarbons but insufficient to cause substantial coke formation. The methane
component, in some embodiments of the invention is separately cracked at
relatively high
temperatures. In such embodiments, the products of the two crackers are
combined for
further processing. In a different embodiment of this process of the
invention, the methane
component of the natural gas feed is not separately cracked but is in part
used as a
reactant in a subsequent portion of the overall process with an excess methane
being
available for energy production or other purposes.
In the embodiment wherein the methane is cracked, the conditions of the
cracking
zone include a temperature above about 1450 K, preferably from about 1500 K to
about
1900 K and a pressure from about 0.5 bar to about 10 bars, preferably from
about 1 bar
-9-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
to about 3 bars. The residence time in the cracking zone is from about 1
millisecond to
about 100 milliseconds. In contrast, where the heavier hydrocarbon portion of
the
methane-containing gaseous hydrocarbon feed is cracked in the substantial
absence of
methane, the cracking zone operates at a temperature from about 900 K to
about 1400'K,
preferably from about 1100 K to about 12500K, and a pressure from about 0.5
bar to about
bars, preferably from about I bar to about 3 bars. The residence time within
this low-
temperature cracking zone is from about 1 millisecond to about 500
milliseconds,
preferably from about 10 milliseconds to about 100 milliseconds.
The effluent from the heavier hydrocarbon cracking zone, with or without being
mixed with the effluent of a methane cracking zone is compressed by a
compressor or
other conventional method to facilitate removal of non-hydrocarbons such as
hydrogen
upon passage to a cracked gas separation zone wherein the hydrocarbons are
separated
into an overhead fraction comprising primarily hydrogen and a bottoms fraction
comprising
primarily methane and unsaturated hydrocarbons, e.g. alkenes and alkynes. This
separation is conducted by conventional methods such as cryogenic
distillation, pressure
swing adsorption, or a selectively permeable membrane. The operating
conditions of the
separation zone are dictated by the chosen technique. The overhead product is
principally
hydrogen and is recycled to a cracking zone or is removed from the system for
disposal
or energy use. The heavier or bottoms product from the cracked gas separation
zone is
mixed with a portion of the methane-containing gaseous hydrocarbon feed or
methane
obtained by separation of the components of that feed.
-10-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
This mixture is passed to an alkylatioh zone where the mixture is contacted
with an
acid alkylation catalyst. Such catalysts are conventional and well-known and
include
hydrofluoric acid and sulfuric acid. Preferred acid catalysts are acidic
zeolite catalysts,
also conventional, and particularly preferred is the acidic zeolite catalyst H-
ZSM-5. The
alkylation zone is operated at a reaction temperature of from about 300 Kto
about 800 K,
preferably from about 500 K to about 700 K, and a pressure from about 2 bars
to about
30 bars, preferably from about 5 bars to about 15 bars.
Subsequent to reaction in the alkylation zone, the liquid product is separated
in a
conventional liquid product separation zone by conventional methods such as
distillation
or membrane separation. The heavier or bottoms product is the desired normally
liquid
product comprising saturated or aromatic hydrocarbons of 4 or more carbon
atoms, most
frequently from 4 to 12 carbon atoms inclusive. The lighter product of the
liquid product
zone comprises hydrogen, methane and small portions of C2 - C4 hydrocarbons.
Depending upon the particular embodiment of the invention, the light product
is principally
recycled to the cracked products separation zone with lesser portions being
recycled to
a methane high-temperature cracking zone or removed from the system for
disposal or
energy use.
Detailed Description of the Drawings
In Figure 1, the process depicted is shown for comparison purposes and is not
of
the invention. In this process, natural gas is introduced via line 101, with a
minor portion
being sent by line 121 to serve as make-up for the alkylation reactor
described below.
The major portion of the natural gas feed passes by line 102 to where it is
combined with
-11-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
recycled hydrogen from line 133 and then by line 103 to the cracking zone II.
In this
cracking zone, operated at a temperature from 1600 K to 2500 K and a
pressure of from
1 bar to 50 bars, a portion of the methane and substantially all of the
heavier hydrocarbon
component of the natural gas are cracked to produce a product mixture of
hydrogen,
methane and a mixture of unsaturated hydrocarbons such as alkenes, alkynes and
aromatic compounds. Also produced from the heavier hydrocarbon component is
coke.
The product of cracking zone 11 is passed by line 104 to a compression zone
III,
typically a compressor, which, if necessary, increases the pressure of the
product stream.
If the cracking zone product is at a suitable pressure, the compression step
can be
omitted. The product stream is then passed by line 105 to mix with a recycle
stream of
hydrogen and hydrocarbons in the C1 - C4 range shown as line 163. The combined
streams 105 and 163, now stream 106, are passed to a separation zone IV shown
as a
membrane system but which also could use other methods such as refrigeration,
distillation, or pressure swing adsorption. The hydrogen overhead from the
separation
zone IV passes by line 131 to where it is split with a portion, line 141,
going to disposal or
use as an energy source and the remainder going by line 132 to a compression
zone VII,
typically a compressor, where the pressure is increased to approximately that
of the inlet
gas feed with which it is mixed. The mixture returns to the cracking zone 11
by lines 133
and 103.
The bottom product of the separation zone IV is a mixture of methane,
hydrogen,
recycled alkanes, and unsaturated hydrocarbons of two or more carbon atoms.
This
product leaves the zone by line 107, is combined with the minor portion of the
natural gas
-12-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
feed, line 121, and the mixture passes by line 108 to a alkylation zone V
where the
methane present reacts with the unsaturated hydrocarbons in the presence of an
acidic
alkylation catalyst to produce a mixture of hydrogen and a range of
hydrocarbon products
of up to about 20 carbon atoms. This mixture passes by line 109 to a
separation zone VI
where it is separated into an overhead product of hydrogen and C, - C4
hydrocarbons
exiting by line 161. A portion of this overhead is removed by line 171 for
disposal or
energy use. The major portion of the overhead product of separation zone VI
passes by
line 162 to a compression zone VIII, typically a compressor, where the
pressure is raised
to one compatible with separation zone IV. The light gas leaves the compressor
by line
163 to where it is combined with gas from the cracking zone II and the mixture
is sent by
line 106 to separation zone IV.
The bottom product of separation zone VI, line 110, is the desired normally
liquid
product comprising a range of hydrocarbons from C4 to C12 and higher.
In the embodiment of the invention shown as Fig. 2, methane-containing gaseous
hydrocarbon feed is introduced by line I where a split sends a minor portion
by line 21 for
use in an alkylation zone D. The remainder of the inlet gaseous hydrocarbon
feed is sent
by line 2 to an inlet gas separation zone J where the methane is substantially
separated
from the higher hydrocarbon portion of the feed. This separator can be a
conventional unit
such as a cryogenic demethanizer, but also is suitably a unit such as that
illustrated by Fig.
5. The overhead of the inlet gas separation zone J, exits by line 91, where it
is split, a
portion of which, line 96, is mixed with some of the inlet gas, line 21, and
fed to the
alkylation zone D by lines 22 and 8. The remainder of the light gas from the
inlet
-13-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
separation zone J, line 92, combines with a recycle stream, line 51, and then
passes by
line 93 to a relatively high temperature cracking zone H where the methane is
cracked to
produce hydrogen and some unsaturated hydrocarbons. The bottom product of
inlet gas
separation zone J exits by line 95 and is mixed with the recycle gas of line
34. The
combined streams are passed by line 3 to a relatively low temperature cracking
zone A
where the heavier hydrocarbon component of the gaseous hydrocarbon feed is
cracked
at the relatively low cracking temperature with relatively little coke
formation. The effluent
from the low temperature cracking zone exits by line 4 to where it is combined
with the
effluent of the high temperature cracking zone H, line 94, and the mixture
passes by line
11 to a compression zone B, typically a compressor. The compressed mixture,
line 5, is
mixed with recycled gas, line 63, and the mixture is sent through line 6 to
cracked products
separation zone C. This separation zone is conventional and is.,suitably a
membrane
system, a cryogenic distillation unit or a pressure swing adsorption system.
The overhead
from the separation zone C, primarily hydrogen with some methane, exits by
line 31. This
mixture is split with a portion removed through line 41 for disposal or energy
use. The
remainder of the separation zone C overhead passes by line 32 to a compression
zone F,
typically a compressor, where the pressure is raised to approximately that of
the inlet gas.
The exiting mixture, passes by line 33 to where it is split, with one portion
being sent by
line 51, mixed with inlet gas separation zone J overhead, line 92, and then
going by line
93 to the relatively high temperature cracking zone H. The remainder of stream
33 passes
by line 34 to where it is mixed with the bottom product of inlet gas
separation zone J,
-14-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
introduced by line 95, and the mixture is sent to the relatively low
temperature cracking
zone A by line 3.
The bottom product of separation zone C exits by line 7, is mixed with a
portion of
the gaseous hydrocarbon feed and a portion of the light product of inlet
separation zone
J, line 22, and the resulting mixture passes by line 8 to the cracked products
alkylation
zone D wherein the mixture is contacted with an acidic alkylation catalyst at
a temperature
of from about 300 K to about 800 K, preferably from about 500 K to about 700
K, and a
pressure of from about 2 bars to about 30 bars, preferably from about 5 bars
to about 15
bars. The resulting product mixture of hydrogen, methane and saturated,
unsaturated, and
aromatic hydrocarbons by line 9, enters alkylation zone product separator E
which serves
to produce an overhead stream 61 comprising hydrogen and C1- C4 hydrocarbons
and a
liquid bottom product exiting by line 10. This bottom product is the desired
liquid product
containing saturated and aromatic hydrocarbons of 4 or more carbon atoms, most
typically
from 4 to 12 carbon atoms inclusive. The overhead product of the alkylatoin
zone
separator E exits by line 61 to where it is split, with a portion passing by
line 71 to disposal
or energy use and the remainder, line 62, is compressed at compression zone G,
typically
a compressor, to a pressure approximating that of cracked gas separation zone
C. The
compressed product, line 63, is mixed with combined cracking zone effluent,
line 5, and
the mixture is transferred to the cracked gas separation zone C by line 6.
An alternate embodiment of the process of the invention is illustrated by
Figure 3
wherein the methane component of the gaseous hydrocarbon feed is separated
from the
heavier hydrocarbon component by selectively cracking the heavier hydrocarbon
-15-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
component in a relatively low temperature cracking zone wherein the methane
passes
through essentially unchanged. The methane is subsequently cracked in a
relatively high
temperature cracking zone.
In Figure 3, methane-containing gaseous hydrocarbon feed enters the process by
line 1 and is split. A portion of the inlet gas feed passes by line 21 for
subsequent use in
the alkylation zone D as discussed below. The major portion of the gaseous
hydrocarbon
feed is combined with recycle gas stream 34 and the mixture passes to a
relatively low
temperature cracking zone A operated at a temperature of from about 900 K to
about
1400 K and a pressure of from about 0.5 bar to about 10 bars. The effluent
from the low
temperature cracking zone passes by line 4 and is mixed with effluent from a
relatively
high temperature cracking zone H, line 93, which operates after one cycle of
the overall
process has taken place. This mixture, line 11, is introduced to compression
zone B,
typically a compressor, where the pressure is elevated to that approximately
equal to the
pressure of cracked gas separation zone C. The compressed gas of stream 5 is
mixed
with a recycle gas, line 63, described below and the mixture enters cracked
gas separation
zone C by way of line 6. The overhead of cracked gas separator C, principally
hydrogen
and methane as stream 31, is split with a portion going to disposal or energy
use, line 41,
and the remainder, line 32, going to compression zone F, typically a
compressor, where
the pressure of the mixture is raised to a pressure approximately equivalent
to that of the
cracking zones. The pressurized mixture, line 33, is split with a first
portion going by lines
34 and 3 to the low temperature cracking zone and a second portion going by
lines 51 and
92 to the high temperature cracking zone. The bottoms product of cracked gas
separation
-16-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
zone C exits by line 7 and is combined with the portion of inlet gas feed,
line 21. The
combined gas stream is sent by line 8 to cracked products alkylation zone D
wherein the
unsaturated hydrocarbons from the cracked gas separation zone C react with
methane in
the presence of an acidic alkylation catalyst. The effluent from alkylation
zone D passes
by line 9 to alkylation zone product separator E which produces an overhead
product
comprising hydrogen, methane and C1 - C4 hydrocarbons and a bottoms product of
a
hydrocarbon mixture. This bottoms product, exiting by line 10, is the desired
normally
liquid hydrocarbon product of saturated, unsaturated, and aromatic
hydrocarbons of 4 or
more carbon atoms, most typically from 4 to 12 carbon atoms inclusive. The
overhead
product of alkylation zone product separator E exits by line 61 to where it is
split into three
portions. A first portion, stream 91, is combined with the recycle gas of line
51 and is sent
to high temperature cracking zone H. A second portion, line 62, is compressed
in
compression zone G, typically a compressor, to a pressure approximating that
of cracked
gas separation zone C. The compressed portion, line 63, is mixed with mixed
cracking
zone effluent lines and sent by line 6 to the cracked gas separation zone C.
The
remaining portion of overhead is removed by line 71 for disposal or energy
use.
Fig. 4 represents a somewhat different embodiment of the invention. In this
embodiment, the methane component of the methane-containing gaseous
hydrocarbon
feed is separated from the heavier hydrocarbon component. This heavier
hydrocarbon
component is cracked in a relatively low temperature cracking zone as in other
embodiments but the methane component is not cracked. Instead, a portion of
the
methane is employed to react in the alkylation zone D with unsaturated
hydrocarbons from
-17-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
cracked gas separation zone C. The remainder of the methane is utilized as a
source of
thermal or electrical energy in this or other processes.
In Fig. 4, a methane-containing gaseous hydrocarbon feed is separated in inlet
gas
separation zone J by conventional means or by the novel procedure of Fig. 5.
The
overhead of the inlet gas separation zone is primarily methane which is
transferred by line
21 for subsequent reaction with unsaturated hydrocarbons of cracked gas
separation zone
C in the cracked products alkylation zone D. The heavier product from inlet
gas
separation zone J comprises the heavier hydrocarbon portion of its gaseous
feed. This
product exits the separation zone by line 2, is combined with recycle gas from
stream 33
and passes by line 3 to a low temperature cracking zone A wherein the heavier
hydrocarbon component is converted largely to alkenes and alkynes. The
unsaturated
hydrocarbon mixture exits, line 4, and is elevated in pressure by compression
zone B,
typically a compressor, to a pressure approximating that of cracked gas
separation zone
C. The compressed gas is sent by line 5 to where it combines with recycle gas,
line 63,
and is transferred via line 6 to cracked products separation zone C. The
overhead product
of this separation zone, line 31, is largely hydrogen with a lesser amount of
methane. A
portion of this overhead product is removed by line 41 for disposal or energy
use and the
remainder, stream 32, is increased in pressure by compression zone F,
typically a
compressor, to that approximating the pressure of the low temperature cracking
zone and
then is transferred by lines 33 and 3 to the low temperature cracking zone A.
The bottoms
product of cracked products separation zone C, exiting by line 7, comprises a
mixture of
unsaturated hydrocarbons. This mixture is mixed with gaseous feed, stream 21,
and is
-18-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
transported by line 8 to alkylation zone D wherein the unsaturated
hydrocarbons react with
methane in the presence of an acidic catalyst. The resulting product mixture
is sent to
alkylation product separation zone E by line 9. The light overhead of
alkylation product
separation zone E exits by line 61. This overhead, largely methane and
hydrogen with
minor amounts of C2 - C4 hydrocarbon, is then split. One portion is removed as
a stream
71 for disposal or energy use. The other portion, stream 62, is pressurized at
compression
zone G, typically a compressor, to a pressure approximately that of cracked
gas separation
zone C and passes by line 63 to where it is mixed with effluent from low
temperature
cracking zone A and returned by line 6 to separation zone C.
The bottoms product of alkylation product separation zone E, exiting by line
10, is
the desired normally liquid hydrocarbon mixture of saturated and aromatic
hydrocarbons
of 4 or more carbon atoms, more typically 4 to 12 carbon atoms inclusive.
Figure 5 represents a novel and preferred method of separating the methane
component of a methane-containing gaseous hydrocarbon mixture inlet feed from
the
heavier hydrocarbon component. One application of this separation system
comprises its
use as an inlet feed separator in the process of Figures 2 and 4. However, the
use of the
separation system of Figure 5 is not limited to such processes and it is
broadly applicable
to other separations of methane from heavier hydrocarbons such as ethane.
The high pressure gaseous hydrocarbon feed, typically at a pressure of from
about
20 bars to about 100 bars, passes by lines 201, 202, 203 and 204 in which it
is cooled, as
described below, into a high pressure separation zone K operated at a
temperature of from
about 200 Kto about 270 K. Within zone K, the feed is separated into an
overhead vapor
-19-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
stream 205 comprising predominately methane, and a bottoms liquid stream 211
which
comprises the large majority of the ethane and the other heavier hydrocarbons
of the
gaseous hydrocarbon feed. The vapor stream 205 is depressurized typically
across a
valve or turboexpander to a pressure of from about 5 bars to about 20 bars
with the
temperature being cooled from about 100 K to about 200 K due to the expansion
of the
vapor. The pressure drop causes additional hydrocarbons of two or more carbon
atoms
to condense as the mixture passes by line 206 to a low pressure separation
zone M. A
cold vapor, stream 207, is obtained as overhead from the high pressure
separation zone
and is routed to high pressure/low pressure cross-exchangers C-2 and A-2 to
provide
cooling and thereby reduce the temperature of the feed. The liquid bottoms
product of
high pressure separation zone K passes by line 211 to a depressurization zone
N, typically
an expansion valve, to reduce the pressure on the liquid. The reduced pressure
fluid
passes by line 212 to where it is mixed with the bottoms product of the low
pressure
separation zone M, line 213, and the resulting mixture is sent by line 214 to
heat
exchanger A-3 where it, together with the vapor of line 208 from cross-
exchanger C-2 is
used to reduce the temperature of the inlet gas feed. The heat exchangers at A-
1
(together with A-2 and A-3) and at C-1 (taken with C-2) are often sufficient
to effect the
desired reduction of gaseous hydrocarbon inlet feed. If desired, however,
additional
temperature reduction is obtained by employing optional refrigeration at
exchanger B. The
outlet lines from the overall separation process, lines 209 and 215, comprise
the methane
component and the stream containing the heavier hydrocarbon component of the
gaseous
hydrocarbon inlet feed, respectively.
-20-
CA 02489383 2004-12-13
WO 03/106593 PCT/US02/19026
While preferred embodiments of the present invention have been illustrated in
detail, it is apparent that modifications and adaptations of the preferred
embodiments will
occur to those skilled in the art. However, it is to be expressly understood
that such
modifications and adaptations are within the spirit and scope of the present
invention as
set forth in the following claims.
-21-