Note: Descriptions are shown in the official language in which they were submitted.
CA 02490018 2010-07-13
WO 2004/002559 PCT/GB2003/002558
-1-
Method and Packaging for Pressurized Containers
Field of the Invention
This invention relates to a method and a package for packaging pressurized
containers suitable for relatively long-term storage. More particularly, it
relates to a
package and packaging method that utilizes an HFA adsorbent material, such as
a
molecular sieve, to absorb or adsorb propellant gases gradually leaked out
from a
pressurized containers, whereby preventing the propellant gas from inflating
the
package.
Background of the Invention
Pressurized containers such as inhalers may need to be packed in impermeable
packages to prevent atmospheric moisture ingress. The use of such impermeable
packages can cause accumulation of propellant gases that gradually leak from
the
pressurized container and may eventually lead to failure of the seals of the
package.
is This problem becomes more prominent when traditional propellants
chlorofluorocarbons
(CFCs) are replaced by hydrofluoroalkane propellants (such as HFA-134a and HFA-
227)
for environmental reasons.
US patent Nos. 6,179,118 BI, 6,119,853 and 6,352,152 address this problem by
using a flexible package that is "impermeable to moisture and permeable to the
propellant." While this appears to be a good approach, applicants had much
difficulty in
fabricating a flexible wrapping material which is impermeable to moisture and
permeable
to the propellant so that the resulting package would operate similar to "a
virtual one-way
valve". Presumably, fabricating such flexible wrapping materials is much more
technically involved and more costly than it appears from reading the
aforementioned
patents. Therefore, there is a need for a simpler and more understandable way
to solve
the inflation problem in packing pressurized containers.
Furthermore, the ability of the packages disclosed in US patent Nos. 6,179,118
131, 6,119,853 and 6,352,152, to prevent gas build up in the packages, would
appear to
be limited by the permeability of the wrapping material to the propellant and
the rate at
which the propellant is released from the container.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-2-
Therefore, there is a need for an enhanced drug product comprising a package
that is impermeable, or substantially impermeable, to the egress of HFA gas
from within
the package, and still is capable of maintaining the enclosed volume of the
sealed
package at about ambient pressure when any leakage of HFA gas propellant
occurs.
Summary of the Invention
A primary object of the present invention is to provide a new package for
pressurized inhalers, which will reduce or eliminate the inflation problems
normally
associated with conventional packaging methods. Another object of the present
invention is to provide simpler method for solving the inflation problem than
the prior art
approaches. Another object of the present invention is to provide a new
package for
pressurized inhalers, which will reduce or eliminate the egress of HFA gas
propellant
from within the package, normally associated with conventional packaging
methods. A
further object of the present invention is to provide a method for maintaining
the
enclosed volume of a sealed package at about ambient pressure, wherein the
package
is contains leakage from a pressurized container comprising an HFA
(hydrofluoroalkane)
propellant.
It is believed that the mechanism by which the HFA adsorbent material prevents
package from inflating is by entrapping the propellant gases gradually leaked
from the
pressurized container.
The various features of novelty which characterize the invention are pointed
out
with particularity in the claims annexed to and forming a part of this
disclosure. For a
better understanding of the invention, its operating advantages, and specific
objects
attained by its use, reference should be made to the drawings and the
following
description in which there are illustrated, and described, preferred
embodiments of the
invention.
Brief Description of the Drawings
Figure 1 is a graph summarizing a study that shows that the molecular sieve is
an
effective HFA adsorbent for entrapping a propellant gas from the air, whereby
preventing
inflation of the package.
Figure 2 shows the rate of moisture absorption by the molecular sieves during
the
first hour of exposure to the atmosphere.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-3-
Figure 3 shows the rate of moisture absorption by the same molecular sieves
used in Figure 2 during an exposure period of 12 hours.
Figure 4 and 5 shows that the molecular sieves' capacity for adsorbing
propellant
gases is reduced if the sieves are pre-exposed to moisture for different time
intervals.
Figure 6 depicts a typical metered dose (pressurized container) inhaler
package
according to the present invention.
Detailed Description of the Preferred Embodiments
(1) In a first embodiment, the invention provides, a method for maintaining
the
io enclosed volume of a sealed package at about ambient pressure, wherein the
package
contains a pressurized MDI (metered dose inhaler) container comprising a drug,
and an
HFA (hydrofluoroalkane) propellant selected from the group consisting of HFA
134a and
HFA p227, or a mixture thereof; wherein the method comprises the steps of:
(i) positioning an effective amount of a HFA adsorbent material, and said
pressurized container, within a sealable package;
(ii) sealing the package so that the pressurized container and adsorbent are
in
an enclosed volume within the package at a pressure equal to about ambient
pressure;
and
(iii) adsorbing any leakage of the HFA propellant into the HFA adsorbent
material so as to maintain the enclosed volume at about ambient pressure.
(2) In another embodiment, the invention provides a method according to
embodiment (1), wherein the drug is selected from the group consisting of
bronchodilators, antihistamines, lung surfactants, antiviral agents,
corticosteroids, ant-
inflammatory agents, anti-cholinergics, and antibacterial agents.
(3) In another embodiment, the invention provides method according to
embodiment (1) or (2), wherein the pressurized MDI (metered dose inhaler)
container
further comprises one or more excipients selected from the group consisting of
surfactants, preservatives, flavorings, antioxidants, anti-aggregating agents
and co-
solvents.
(4) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (3), wherein the HFA propellant is HFA 134a.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-4-
(5) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (3), wherein the HFA propellant is HFA p227.
(6) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (5), wherein the HFA adsorbent material is capable
of
adsorbing the HFA propellant up to about 25% of the weight of the adsorbent.
(7) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (5), wherein the HFA gas adsorbent material is
capable of
adsorbing the HFA propellant up about 20% of the weight of the adsorbent.
(8) In another embodiment, the invention provides a method according to any
io one of embodiments (1) to (7), wherein the HFA adsorbent material comprises
material
selected from the group consisting of molecular sieves, activated clays,
activated
alumina, silica, zeolites, bauxites, and mixtures thereof.
(9) In another embodiment, the invention provides a method according to
embodiment (8), wherein the HFA adsorbent material is 10 A (Angstrom)
molecular
sieves.
(10) In another embodiment, the invention provides a method according to
embodiment (9), wherein the molecular sieves, in an amount of about 4 grams,
absorbs
about 230 ml of HFA p227.
(11) In another embodiment, the invention provides a method according to
embodiment (9), wherein.the molecular sieves, in an amount of about 4 grams,
absorbs
about 230 ml of HFA 134a.
(12) In another embodiment, the invention provides a method according to
anyone of embodiments (1) to (11), wherein the package is impermeable to HFA
134a.
(13) In another embodiment, the invention provides a method according to
anyone of embodiments (1) to (12), wherein the package is impermeable to HFA
p227.
(14) In another embodiment, the invention provides a method according to
anyone of embodiments (1) to (12), wherein the package is permeable to HFA
p227.
(15) In another embodiment, the invention provides a method according to
embodiment (14), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.25 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-5-
(16) In another embodiment, the invention provides a method according to
embodiment (14), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.15 cc of HFA p227 per square meter of package per day at
about I
bar pressure and about room temperature.
(17) In another embodiment, the invention provides a method according to
embodiment (14), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.10 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(18) In another embodiment, the invention provides a method according to
embodiment (14), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.05 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(19) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (11) or (14), wherein the package is permeable to
HFA 134a.
(20) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 4.1 cc of HFA 134a per square meter of package per day at
about I
bar pressure and about room temperature.
(21) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 3.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(22) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 2.5 cc of HFA 134a per square meter of package per day at
about I
bar pressure and about room temperature.
(23) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 1.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-6-
(24) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 1.0 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(25) In another embodiment, the invention provides a method according to
embodiment (19), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 0.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(26) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (25), wherein the package is made of metal, glass,
or plastic,
and is selected from the group consisting of bottles, bags, drum boxes, and
irregularly
shaped containers.
(27) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (26), wherein the package is made of plastic.
(28) In another embodiment, the invention provides a method according to
embodiment (27) wherein the plastic is a flexible laminate having a barrier
layer
providing said package with permeability to HFA 134a and/or HFA p227.
(29) In another embodiment, the invention provides a method according to
embodiment (27), wherein the plastic is a flexible laminate having a barrier
layer
providing said package with impermeability to HFA 134a and/or HFA p227.
(30) In another embodiment, the invention provides a method according to
embodiment (28) or (29), wherein said flexible laminate has three layers:
polyester/
aluminum / polyethylene, wherein the aluminum layer is between the polyester
and
polyethylene layers.
(31) In another embodiment, the invention provides a method according to
embodiment (28) or (29), wherein said barrier layer is made of aluminum foil.
(32) In another embodiment, the invention provides a method according to any
one of embodiments (1) to (31), wherein the sealed package is hermetically
sealed by
heat-sealing, gluing, welding, brazing, mechanical closures or clamps, or
compression.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-7-
(33) In another embodiment, the invention provides a use of an HFA adsorbent
to maintain the pressure of an enclosed volume within a sealed package at
about
ambient pressure, wherein the sealed package comprises:
(i) a pressurized MIDI (metered dose inhaler) container comprising a drug, a
HFA (hydrofluoroalkane) propellant selected from the group consisting of HFA
134a and
HFA p227, or a mixture thereof;
(ii) an effective amount of an HFA adsorbent material;
wherein the pressurized MDI container and HFA adsorbent material are within
the
enclosed volume of the sealed package.
to (34) In another embodiment, the invention provides a use according to
embodiment (33), wherein the drug is selected from the group consisting of
bronchodilators, antihistamines, lung surfactants, antiviral agents,
corticosteroids, ant-
inflammatory agents, anti-cholinergics, and antibiotics.
(35) In another embodiment, the invention provides a use according to
embodiment (33) or (34), wherein the pressurized MDI (metered dose inhaler)
container
further comprises one or more excipients selected from the group consisting of
surfactants, preservatives, flavorings, antioxidants, anti-aggregating agents
and co-
solvents.
(36) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (35), wherein the HFA propellant is HFA 134a.
(37) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (35), wherein the HFA propellant is HFA p227.
(38) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (37), wherein the HFA adsorbent material is capable of
adsorbing the HFA propellant up to about 25% of the weight of the adsorbent.
(39) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (37), wherein the HFA gas adsorbent material is capable
of
adsorbing the HFA propellant up about 20% of the weight of the adsorbent.
(40) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (39), wherein the HFA adsorbent material comprises
material
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-8-
selected from the group consisting of molecular sieves, activated clays,
activated
alumina, silica, zeolites, bauxites, and mixtures thereof.
(41) In another embodiment, the invention provides a use according to
embodiment (40) wherein the HFA adsorbent material is 10 A (Angstrom)
molecular
s sieves.
(42) In another embodiment, the invention provides a use according to
embodiment (41), wherein the molecular sieves, in an amount of about 4 grams,
absorbs
about 230 ml of HFA p227.
(43) In another embodiment, the invention provides a use according to
embodiment (41), wherein the molecular sieves, in an amount of about 4 grams,
absorbs
about 230 ml of HFA 134a.
(44) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (43), wherein the package is impermeable to HFA 134a.
(45) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (42), wherein the package is impermeable to HFA p227.
(46) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (42), wherein the package is permeable to HFA p227.
(47) In another embodiment, the invention provides a use according to
embodiment (46), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.25 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(48) In another embodiment, the invention provides a use according to
embodiment (46), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.15 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(49) In another embodiment, the invention provides a use according to
embodiment (46), wherein the package has a permeability to HFA p227 that is
less than
or equal to about 0.10 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(50) In another embodiment, the invention provides a use according to
embodiment (46), wherein the package has a permeability to HFA p227 that is
less than
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-9-
or equal to about 0.05 cc of HFA p227 per square meter of package per day at
about 1
bar pressure and about room temperature.
(51) In another embodiment, the invention provides a use according to anyone
of embodiments (33) to (43), wherein the package is permeable to HFA 134a.
(52) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 4.1 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(53) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 3.5 cc of HFA 134a per square meter of package per day at
about I
bar pressure and about room temperature.
(54) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 2.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(55) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 1.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(56) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 1.0 cc of HFA 134a per square meter of package per day at
about I
bar pressure and about room temperature.
(57) In another embodiment, the invention provides a use according to
embodiment (51), wherein the package has a permeability to HFA 134a that is
less than
or equal to about 0.5 cc of HFA 134a per square meter of package per day at
about 1
bar pressure and about room temperature.
(58) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (57), wherein the package is made of metal, glass, or
plastic,
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-10-
and is selected from the group consisting of bottles, bags, drum boxes, and
irregularly
shaped containers.
(59) In another embodiment, the invention provides a use according to
embodiment (58), wherein the package is made of plastic.
(60) In another embodiment, the invention provides a use according to
embodiment (59), wherein the plastic is a flexible laminate having a barrier
layer
providing said package with impermeability to HFA 134a and/or HFA p227.
(61) In another embodiment, the invention provides a use according to
embodiment (59) or (60), wherein the plastic is a flexible laminate having a
barrier layer
providing said package with permeability to HFA 134a and/or HFA p227.
(62) In another embodiment, the invention provides a use according to
embodiment (60) or (61), wherein said flexible laminate has three layers:
polyester/
aluminum / polyethylene, wherein the aluminum layer is between the polyester
and
polyethylene layers.
(63) In another embodiment, the invention provides a use according to
embodiment (60) or (61), wherein said barrier layer is made of aluminum foil.
(64) In another embodiment, the invention provides a use according to any one
of embodiments (33) to (63), wherein the sealed package is hermetically sealed
by heat-
sealing, gluing, welding, brazing, mechanical closures or clamps, or
compression.
(65) In another embodiment, the invention provides a pharmaceutical product
comprising:
(i) a pressurized MDI (metered dose inhaler) container comprising a drug, and
an HFA (hydrofluoroalkane) propellant selected from the group consisting of
HFA 134a
and HFA p227, or a mixture thereof;
(ii) an effective amount of an HFA adsorbent material; and
(iii) a sealed package having an enclosed volume within which the pressurized
container and the HFA adsorbent material are situated,
wherein the sealed package is impermeable to the HFA propellant and the
pressure within the enclosed volume of the package is equal to about ambient
pressure;
and
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-11-
wherein the HFA adsorbent material is capable of adsorbing the HFA propellant
so as to maintain a constant pressure within said enclosed volume, when any
leakage of
the HFA propellant occurs from the pressurized container.
(66) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (65), wherein the drug is selected from the group
consisting of
bronchodilators, antihistamines, lung surfactants, antiviral agents,
corticosteroids, ant-
inflammatory agents, anti-cholinergics, and antibiotics.
(67) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (65) or (66), wherein the pressurized MDI (metered
dose
inhaler) container further comprises one or more excipients selected from the
group
consisting of surfactants, preservatives, flavorings, antioxidants, anti-
aggregating agents
and co-solvents.
(68) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (67), wherein the HFA propellant
is HFA
134a.
(69) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (67), wherein the HFA propellant
is HFA
p227.
(70) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (69), wherein the HFA adsorbent
material
is capable of adsorbing the HFA propellant up to about 25% of the weight of
the
adsorbent.
(71) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (69), wherein the HFA gas
adsorbent
material is capable of adsorbing the HFA propellant up about 20% of the weight
of the
adsorbent.
(72) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (71), wherein the HFA adsorbent
material
comprises material selected from the group consisting of molecular sieves,
activated
clays, activated alumina, silica, zeolites, bauxites, and mixtures thereof.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-12-
(73) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (72), wherein the HFA adsorbent material is 10 A
(Angstrom)
molecular sieves.
(74) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (73), wherein the molecular sieves, in an amount of
about 4
grams, absorbs about 230 ml of HFA p227.
(75) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (73), wherein the molecular sieves, in an amount of
about 4
grams, absorbs about 230 ml of HFA 134a.
(76) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (75), wherein the package is
impermeable
to HFA 134a.
(77) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (76), wherein the package is
impermeable
to HFA p227.
(78) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (77), wherein the package is made
of
metal, glass, or plastic, and is selected from the group consisting of
bottles, bags, drum
boxes, and irregularly shaped containers.
(79) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (71), wherein the package is made of plastic.
(80) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (79), wherein the plastic is a flexible laminate
having a barrier
layer providing said package with impermeability to HFA 134a and/or HFA p227.
(81) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (80), wherein said flexible laminate has three layers:
polyester
/ aluminum / polyethylene, wherein the aluminum layer is between the polyester
and
polyethylene layers.
(82) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (80), wherein said barrier layer is made of aluminum
foil.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-13-
(83) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (65) to (82), wherein the sealed package
is
hermetically sealed by heat-sealing, gluing, welding, brazing, mechanical
closures or
clamps, or compression.
(84) A pharmaceutical product comprising:
(i) a pressurized MDI (metered dose inhaler) container comprising a drug, and
an HFA (hydrofluoroalkane) propellant selected from the group consisting of
HFA 134a
and HFA p227, or a mixture thereof;
(ii) an effective amount of an HFA adsorbent material; and
(iii) a sealed package having an enclosed volume within which the pressurized
container and the HFA adsorbent material are situated,
wherein the pressure within the enclosed volume of the package is equal to
about
ambient pressure;
wherein the HFA adsorbent material is capable of adsorbing the HFA propellant
so as to maintain a constant pressure within said enclosed volume, when any
leakage of
the HFA propellant occurs from the pressurized container; and
wherein the package has a permeability to HFA p227 that is less than or equal
to
about 0.25 cc of HFA p227 per square meter of package per day at about 1 bar
pressure
and about room temperature, or a permeability to HFA 134a that is less than or
equal to
about 4.1 cc of HFA 134a per square meter of package per day at about 1 bar
pressure
and about room temperature.
(85) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA p227 that is less than or equal to about
0.15 cc of
HFA p227 per square meter of package per day at about 1 bar pressure and about
room
temperature.
(86) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA p227 that is less than or equal to about
0.10 cc of
HFA p227 per square meter of package per day at about 1 bar pressure and about
room
temperature.
(87) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA p227 that is less than or equal to about
0.05 cc of
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-14-
HFA p227 per square meter of package per day at about 1 bar pressure and about
room
temperature.
(88) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA 134a that is less than or equal to about 3.5
cc of
HFA 134a per square meter of package per day at about 1 bar pressure and about
room
temperature.
(89) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA 134a that is less than or equal to about 2.5
cc of
HFA 134a per square meter of package per day at about 1 bar pressure and about
room
io temperature.
(90) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA 134a that is less than or equal to about 1.5
cc of
HFA 134a per square meter of package per day at about 1 bar pressure and about
room
temperature.
(91) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA 134a that is less than or equal to about 1.0
cc of
HFA 134a per square meter of package per day at about I bar pressure and about
room
temperature.
(92) A pharmaceutical product according to embodiment (84), wherein the
package has a permeability to HFA 134a that is less than or equal to about 0.5
cc of
HFA 134a per square meter of package per day at about I bar pressure and about
room
temperature.
(93) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (92), wherein the drug is selected
from the
group consisting of bronchodilators, antihistamines, lung surfactants,
antiviral agents,
corticosteroids, ant-inflammatory agents, anti-cholinergics, and antibiotics.
(94) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (93), wherein the pressurized MDI
(metered dose inhaler) container further comprises one or more excipients
selected from
the group consisting of surfactants, preservatives, flavorings, antioxidants,
anti-
aggregating agents and co-solvents.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-15-
(95) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (94), wherein the HFA propellant
is HFA
134a.
(96) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (94), wherein the HFA propellant
is HFA
p227.
(97) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (96), wherein the HFA adsorbent
material
is capable of adsorbing the HFA propellant up to about 25% of the weight of
the
adsorbent.
(98) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (96), wherein the HFA gas
adsorbent
material is capable of adsorbing the HFA propellant up about 20% of the weight
of the
adsorbent.
(99) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (98), wherein the HFA adsorbent
material
comprises material selected from the group consisting of molecular sieves,
activated
clays, activated alumina, silica, zeolites, bauxites, and mixtures thereof.
(100) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (99), wherein the HFA adsorbent material is 10 A
(Angstrom)
molecular sieves.
(101) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (100), wherein the molecular sieves, in an amount of
about 4
grams, absorbs about 230 ml of HFA p227.
(102) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (100), wherein the molecular sieves, in an amount of
about 4
grams, absorbs about 230 ml of HFA 134a.
(103) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (102), wherein the package is made
of
metal, glass, or plastic, and is selected from the group consisting of
bottles, bags, drum
boxes, and irregularly shaped containers.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-16-
(104) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (103), wherein the package is made of plastic.
(105) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (104), wherein the plastic is a flexible laminate
having a barrier
layer providing said package with permeability to HFA 134a and/or HFA p227.
(106) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (105), wherein said flexible laminate has three
layers:
polyester / aluminum / polyethylene, wherein the aluminum layer is between the
polyester and polyethylene layers.
(107) In another embodiment, the invention provides a pharmaceutical product
according to embodiment (105), wherein said barrier layer is made of aluminum
foil.
(108) In another embodiment, the invention provides a pharmaceutical product
according to any one of embodiments (84) to (107), wherein the sealed package
is
hermetically sealed by heat-sealing, gluing, welding, brazing, mechanical
closures or
clamps, or compression.
(109) In another embodiment, the invention provides a flexible laminate
according to any one of embodiments (30), (62), (81), and (106) comprising 12
micron
polyester / 9 micron aluminum foil / 50 micron polyethylene.
It is appreciated that certain features of the invention, which are, for
clarity,
described in the context of separate embodiments, may also be provided in
combination
in a single embodiment. Also, various features of the invention which are, for
brevity,
described in the context of a single embodiment, may also be provided
separately or in
any suitable subcombination.
The Ability of HFA adsorbents to Entrap Propellants
It is discovered that HFA adsorbent materials, especially molecular sieves,
are
capable of removing (by entrapping) propellant gases from local environment.
The
present invention takes advantage of this property of the HFA adsorbent
materials and
enclose them in an impermeable, or substantially impermeable, flexible package
as a
means to preventing the leaked out propellant from inflating the package. By
enclosing
one or more HFA adsorbent materials in the package to absorb or adsorb any
leaked-out
propellant, applicants can make the flexible wrapping material as impermeable
as
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-17-
possible to prevent moisture ingress without worrying about the leaked-out
propellant
inflating and causing failure of the seals in the flexible package. To
determine the proper
type and amount of HFA adsorbent material to be used in each package for a
pressurized inhaler containing a specific propellant, applicants conducted the
following
s measurement and determined that about 4 grams of a sachet of 10 Angstrom
molecular
sieves can remove (adsorb) approximately 230 ml of HFA-227 propellant.
Two methods are used to measure the absorption capability of the sieves. The
Initial Measurement method uses flowrap packs containing active product to
obtain an
approximate data on the amount of propellant that would be absorbed. The
Precise
Measurement method builds on the results obtained from the Initial Measurement
method but uses containers filled only with propellant for the purpose of
eliminating any
possible effect from the active compounds (i.e., medicaments).
For the Initial Measurement method, a number of sample packs (flexible package
enclosing a pressurized inhaler containing HFA-227 propellant and the
molecular sieve
to be tested) are obtained and checked for seal integrity by testing on the
Qualitek leak
tester. The packs were orientated with the valve of the pressurized inhaler on
the top.
With minimum disturbance, the orientation of the packs is reversed (with the
valve
pointing downwards) and the aerosol fired for a predetermined number of shots
and the
time taken to deflate each of the packs was recorded. The reason for these
precautions
is to minimize active product expelled with propellant, which may coat the
sieves and
possibly reduce their absorption capacity. The packs are then opened and the
sieves are
examined for the presence of the active product on their surfaces. The
presence of the
active product would indicate that inverting has not prevented the active
product from
being expelled and consequently it could have effected the absorption rate.
The results
of the Initial Measurement are as follows:
All packs with up to 15 shots fired return to original size within 10 minutes,
while
packs with 20 shorts fired show slight inflation after 15 minutes. Examination
of the
sieves used showed evidence of product deposition on the inside of the pouch
and on
the outside of the adsorbent sachet although none could be seen on the surface
of the
adsorbent itself. As such this was considered a good guide to the adsorbent
capacity
prior to the more precise method being undertaken.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-18-
For the Precise Measurement method, the following steps are followed:
1. A number of pressurized inhalers (aerosol cans) filled only with HFA-227
Propellant are obtained. They are numbered and their weights are recorded.
2. A number of flexible packages with a open end are obtained. They are also
numbered.
3. Each aerosol can in turn is placed into an actuator and inserted into the
flexible
package.
4. A predetermined amount of molecular sieves is transferred from an unused
polyethylene bag to a smaller minigrip bag. Using tweezers to avoid transfer
of
moisture, the sieves are weighed and inserted into each of the packages in
turn.
5. Each of the packages, now containing an aerosol can and molecular sieves,
is
immediately heat sealed using a AstraPack Heatsealer that has been set up to
produce effective seals with this particular package material. This step is
repeated
for all the packages.
6. The first five packages remain as sealed. This is for the purpose of
assessing the
effect of moisture pickup from the actuator and/or the air in the package,
which
can serve as the base line for all other measurements.
7. The remaining packages are divided into sets of five. The cans of a set are
fired
for a predetermined number of shots. The maximum number of shorts is
determined based on the information obtained from the Initial Measurement
method. Sets with more than 10 shots are given time to deflate before
continuing
with the next shot. All the sets of packages are stored for a minimum of 24
hours
to allow the maximum propellant absorption to occur.
8. The packages are then leak tested using a Qualitek leak tester to ensure
all
packages have been adequately sealed and hence the data obtained are
relevant. Results from packages failing the leak test are discarded.
9. Each package is opened in turn and the sieve and the can are re-weighed.
The
sieve is weighed first to avoid weight increase due to atmospheric moisture
absorption.
10. The weight loss from each can and the weight gain of the sieve are
obtained and
the average for each set is calculated. The data are then plotted on a graph
to
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-19-
show the rate of weight gain by the sieves and the number of shots (and hence
the volume of gas) required to reach maximum absorption level. Similarly, the
data on the average weight loss from the cans are plotted to show the
equivalent
transfer of propellant from the cans into the sieves until final absorption
for sieves
reached (see Figure 1).
As shown in Figure 1, comparison of weight gain by the molecular sieve against
gas volume indicates a steady rise in weight until about 25 shots (equate to
230 ml) of
propellant has been absorbed. This is matched by the weight loss from the cans
climbed
io while the weight of the sieves remained steady. Thus, it is concluded that
about a 4
grams sachet of a 10 Angstrom molecular sieve can remove (adsorb)
approximately 230
ml of HFA-227 propellant.
Of course, the HFA adsorbent material's capacity to absorb the propellant may
vary under actual production line conditions because the HFA adsorbent
material may
be pre-exposed to the atmosphere for a certain period of time and absorb
atmospheric
moisture. Absorption of moisture limits the eventual capacity of the HFA
adsorbent
material to absorb propellant gases. Therefore, it should be taken into
consideration in
practicing the present invention. In the specific embodiment disclosed herein,
applicants
first determine the rate at which an HFA adsorbent material absorbs
atmospheric
moisture under conditions close to actual production line conditions (see
Figure 2 and 3),
then examine the effect of the length of atmospheric exposure on the eventual
capacity
of propellant adsorption under typical production conditions (see Figure 4 and
5). The
data from this study are used to determine a time allowance for normal
production
processes, whilst still always ensuring that the original targeted amount of
propellant can
be adsorbed.
As indicated in Figures 2 and 3, the moisture absorption during the first hour
of
exposure can reach 20% of the maximum moisture absorption at 20 C/45% RH
(relative
humility) and 34% at 25 C/60% RH. Applicants also examined the difference in
moisture
absorption between the sieves in top and bottom positions of a bulk container
found that
molecular sieves directly exposed to the atmosphere will absorb moisture much
more
rapidly than those protected by virtue of being in a lower position in the
container. This
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-20-
supports the view that sieves in a reel format will maintain their
effectiveness longer.
These data will help determine proper procedures of handling molecular sieves
in the
production environment.
In Figures 4 and 5, the molecular sieves are exposed to the production
conditions
for the prescribed period of time and then immediately packaged using an
Astrapack
heatsealer. The resulting packs are left for 10 minutes to allow the seals to
cool and then
the aerosols (filled with propellant only) are actuated 5 times so that the
packages
expend. The packs are left for another 10 minutes to allow to adsorb the
propellant. The
actuating procedure is repeated until each sieve reached its maximum
adsorption
capacity. At the end of a 24-hour period (a period to ensure maximum
adsorption of the
propellant), each pack is opened and the sieves are weighed immediately.
Figure 4
shows that the amount (in grams) of propellant adsorbed by 4 grams of the
molecular
sieves is reduced following exposure to moisture for the different time
periods indicated.
Figure 5 shows the percentage propellant adsorption per gram of molecular
sieves over
the same time periods as in Figure 4. The goal in this particular case is to
adsorb 100 ml
of HFA-227 propellant (equivalent to 0.76 g) by using a pouch containing 4
grams of
molecular sieves. The data showing in Figure 4 and 5 indicate this goal can be
achieved
even if the sieves are exposed to the normal atmospheric moisture in the
production line
for 30 minutes.
The above study results demonstrate that inclusion of an HFA adsorbent inside
the impermeable, or substantially impermeable, package is a simple, practical
and
effective solution to the inflation problem of packages for pressurized
containers.
Particularly, molecular sieves are very effective HFA adsorbent materials
against
package inflation when used in practicing the present invention.
Although there are various types of HFA adsorbent materials available and
their
effectiveness against any given propellant may vary considerably, it is
understood that
people of ordinary skill in the art can easily adopt some conventional assay
methods,
such as the above-described study, to determine the type and amount of HFA
adsorbent
material that is effective in reducing package inflation caused by a
particular propellant
leaked from the pressurized container enclosed in the package.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-21-
The Propellants
Propellants for use in the invention mean pharmacologically inert liquids with
boiling points from about room temperature (25 C.) to about -25 C. which
singly or in
combination exert a high vapor pressure at room temperature. Upon activation
of the
MDI system, the high vapor pressure of the propellant in the MDI forces a
metered
amount of drug formulation out through the metering valve then the propellant
very
rapidly vaporizes dispersing the drug particles. The propellants used in the
present
invention are preferably hydrofluorocarbons or hydrofluoroalkanes such as HFA-
134a
and HFA-227.
Drugs
The term "drug" as used herein is intended to encompass the presently
available
pharmaceutically active drugs used therapeutically and further encompasses
future
developed therapeutically effective drugs that can be administered by the
intrapulmonary
route. Drugs may be selected from, for example, analgesics, e.g. codeine,
dihydromorphine, ergotamine, fentanyl or morphine, anginal preparations, e.g.
diltiazem;
antiallergics, e.g. cromoglycate, ketotifen or nedocromil; antiinfectives e.g.
cephalosporins, penicillins, streptomycin, sulphonamides, tetracyclines
pentamidine,
and Neuraminidase Inhibitors, such as zanamivir (Relenza ) available from
GlaxoSmithkline; and Ribavirin (Virazole ) manufactured by ICN
Pharmaceuticals, Inc.;
antihistamines, e.g. mnethapyfilene; antitussives, e.g. noscapine; beta-
adrenergics that
include bronchodilators such as salbutamol, salmeterol, ephedrine, adrenaline,
fenoterol,
forinoterol, isoprenaline, phenylephrine, phenylpropanolamine, reproterol,
rimiterol,
terbutaline, isoetharine, tulobuterol, orciprenaline, or (-)4-amino-3,5-
dichloro-.alpha.-[[[6-
[2-(2-pyridinyl)ethoxy]hexyl]-amino]m ethyl]benzenemethanol, epinephrine
(Primatene),
formoterol (Foradil), isoproterenol (Isuprel), isoetharine (Bronkosol),
metaproterenol
(Alupent, Metaprel), albuterol (Proventil, Ventolin), terbutaline (Bricanyl,
Brethine),
bitolterol (Tornalate), pirbuterol (Maxair), salmeterol (Serevent), salmeterol
+ fluticasone
combination (Advair Diskus), and albuterol + atrovent combination (Combivent);
sodium
channel blockers such as amiloride, anticholinergics e.g. ipratropium,
atropine or
oxftropium; hormones, e.g. cortisone, hydrocordisone or prednisolone; and
therapeutic
proteins and peptides, e.g. insulin or glucagon; anti-inflammatory drugs used
in
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-22-
connection with the treatment of respiratory diseases include steroids such as
NASACORT AQ (triamcinolone acetonide), AZMACORT AQ (triamcinolone
acetonide) flunisolide, fluticasone, budesonide, triamcinolone acetonide,
beclomethasone (Vanceril, Beclovent), budesonide (Pulmicort) dexamethasone,
flunisolide (Aerobid), fluticasone (Flovent), salmeterol + fluticasone
combination (Advair
Diskus), and triamcinolone (Azmacort), and Mediator-release inhibitors such as
Intal
(cromolyn sodium), and nedocromil sodium (Tilade); leukotrine (LT) inhibitors,
vasoactive intestinal peptide (VIP), tachykinin antagonists, bradykinin
antagonists,
endothelin antagonists, heparin furosemide, anti-adhesion molecules, cytokine
modulators, biologically active endonucleases, recombinant human (rh) DNase
compounds, alpha-antitrypsin and disodium cromoglycate (DSCG); and lung
surfactants
such as lipid-containing compositions as described in TONGE et. Al, WO
99/09955;
Pulmonary surfactants as decribed in Devendra et. Al, Respir Res 2002, 3:19;
Infasurf available from ONY; Curosurf available from Dey Laboratories; Exosurf
by
Glaxo Wellcome; Survanta available from Abbot; Surfaxin lung surfactant
available
from Discovery Laboratories.
The present invention is intended to encompass the free acids, free bases,
salts,
amines and various hydrate forms including semi-hydrate forms of such drugs
and is
particularly directed towards pharmaceutically acceptable formulations of such
drugs
which are formulated in combination with pharmaceutically acceptable excipient
materials generally known to those skilled in the art, preferably without
other additives
such as preservatives.
Preferred drug formulations do not include additional components such as
preservatives which have a significant effect on the overall formulation. Thus
preferred
formulations consist essentially of pharmaceutically active drug and a
pharmaceutically
acceptable carrier (e.g., water and/or ethanol). However, if a drug is liquid
without an
excipient the formulation may consist essentially of the drug which has a
sufficiently low
viscosity that it can be aerosolized using a dispenser of the present
invention.
Drug Formulations
Drug formulations for use in the invention may be free or substantially free
of
formulation excipients, e.g., surfactants and cosolvents, etc. Such drug
formulations are
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-23-
advantageous since they may be substantially tasteless and odorless, less
irritant and
less toxic than excipient-containing formulations. Thus, a preferred drug
formulation
consists essentially of a drug, or a physiologically acceptable salt or
solvate thereof,
optionally in combination with one or more other pharmacologically active
agent, and a
hydrofluorocarbon propellant.
Optionally, the aerosol formulations according to the invention may further
comprise one or more cosolvent. A polar cosolvent such as C2_6 aliphatic
alcohols and
polyols, e.g., glycerol, ethanol, isopropanol and propylene glycol, preferably
ethanol,
may be included in the drug formulation in the desired amount, either as the
only
excipient or in addition to other excipients, such as surfactants. Suitably,
the drug
formulation may contain 0.01 to 5% w/w based on the propellant of a polar
cosolvent,
e.g., ethanol, preferably 0.1 to 5% w/w, e.g., about 0.1 to I% w/w.
Optionally, the aerosol formulations according to the invention may further
comprise one or more surfactants. The surfactants must be physiologically
acceptable
upon administration by inhalation. Within this category are included
surfactants such as
oleic acid, sorbitan trioleate, sorbitan mono-oleate, sorbitan monolaurate,
polyoxyethylene (20) sorbitan monolaurate, polyoxyethylene (20) sorbitan
monooleate,
natural lecithin, oleyl polyoxyethylene (2) ether, stearyl polyoxyethylene (2)
ether, lauryl
polyoxyethylene (4) ether, block copolymers of oxyethylene and oxypropylene,
synthetic
lecithin, diethylene glycol dioleate, tetrahydrofurfuryl oleate, ethyl oleate,
isopropyl
myristate, glyceryl monooleate, glyceyl monostearate, glyceryl
monoricinoleate, cetyl
alcohol, stearyl alcohol, polyethylene glycol 400, cetyl pyridinium chloride,
benzalkonium
chloride, olive oil, glyceryl monolaurate, corn oil, cotton seed oil and
sunflower seed oil.
Preferred surfactants are lecithin, oleic acid and sorbitan trioleate. The
amount of
surfactant employed is desirably in the range of 0.0001 % to 50% w/w ratio
relative to the
drug, in particular 0.05 to 5% w/w ratio.
Optionally, the aerosol formulations according to the invention may further
comprise one or more stabilizers. The stabilizer is selected from the group
consisting of
glycin, glycine, alanine, valine, leucine, isoleucine, methionine, threonine,
isovaline,
phenylalanine, tyrosine, serine, histidine, tryptophan, proline,
hydroxyproline, arginine,
ornithine, asparagine, citrulline, aspartic acid, cysteine, glutamic acid,
glutamine, lysine,
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-24-
hydroxylysine, N-acetyl-L-cysteine, phenylalanine, trans-4-hydroxy-L-proline,
tyrosine, L-
aspartyl-L-phenylalanine methylester and a mixture of any of the foregoing.
Optionally, the aerosol formulations according to the invention may further
comprise one or more antioxidants. The antioxidant may be selected from the
group
consisting of tocopherol, deteroxime mesylate, methyl paraben, ethyl paraben
and
ascorbic acid and mixtures thereof. A preferred antioxidant is tocopherol.
The Package
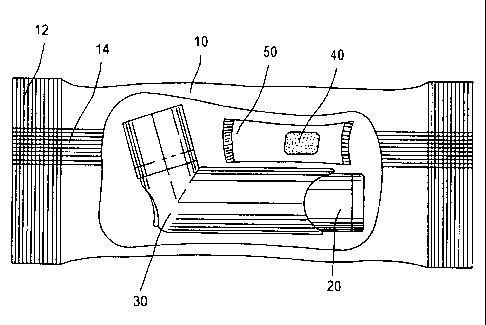
According to one embodiment of the present invention, shown in figure 6, the
pharmaceutical product has an impermeable, or substantially impermeable,
flexible
io package 10, in which a metered dose pressurized container 20, inhalation
device 30 and
a molecular sieve 40 enclosed in a sachet 50, are sealed in an enclosed volume
60.
The flexible package is conventional and its manufacturing is well within the
knowledge of the people skilled in the art. In general, the package is
constructed from
flat reels of laminate which are folded or otherwise formed according to the
packaging
equipment technology into a package by means of sealing and cutting. In this
embodiment the package is constructed from a flat reel of flexible material
which is
curled around into a long tube and a seal 14 is formed by heating (welding)
the edges of
the tube together. The cross seals 12 are formed by a straight heater bar
which clamps
the laminate tube before and after the package contents (i.e., the inhaler and
the
adsorbent sachet). It also cuts the continuous tube into individual packs. As
a result,
there is a long continuous seal 14 down the middle of the pack and the cross
seals 12 at
both ends.
Other package types may include more or less seals according to the desired
shape of the container, which may be flat seals or crimped, and may include
gussets.
The seals may be formed by heating (welding) or by the use of pressure
sensitive
materials. In a further embodiment the flexible laminates may be formed using
heat,
pressure and/or vacuum into blisters or pockets to contain the product and
which are then
sealed by heating.
Although a flexible package is preferred, other types of enclosures or
containers
may be suitable, whether flexible or inflexible, provided that the enclosure
chosen is
impermeable, or substantially impermeable, to moisture ingress. In general,
when the
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-25-
package or enclosure is impermeable, or substantially impermeable, to
moisture, it is
also impermeable, or substantially impermeable, to the propellant that
gradually leaks
out from the enclosed pressurized container. This may gradually build-up a
pressure
within the package or enclosure, which is undesirable. In this context,
"substantially
impermeable" to the propellant means that the level of the propellant in the
enclosed
volume of the package or enclosure will elevate if no measure, such as
inclusion of an
HFA adsorbent material, is taken to reduce it. Or in other words, the egress
rate of the
propellant gas allowed by the package or enclosure is lower than the rate by
which it is
leaked into the enclosed volume of the package or enclosure from the
pressurized
container. Preferably, a substantially impermeable package of the present
invention has
a permeability to HFA p227 that is less than or equal to about 0.25 cc of HFA
p227 per
square meter of package per day at about I bar pressure and about room
temperature,
or a permeability to HFA 134a that is less than or equal to about 4.1 cc of
HFA 134a per
square meter of package per day at about 1 bar pressure and about room
temperature.
Also, in this context, "impermeable" to the propellant means impenetrable by
the HFA
propellant gas used in the invention.
Flexible Material for Making Packages
A preferred flexible material for making the package is a laminate, although
other
materials may also be satisfactorily employed. The main limitations are is
that the
package material must be substantially impermeable to atmosphere moisture, and
impermeable or substantially impermeable, to the HFS propellant used.
The laminate used in making packages generally consists of several layers of
materials either co-extruded or bonded together to form an apparently single
film of
"laminate". As an example, a suitable laminate may have three layers
adhesively
laminated to each other: an inner layer, a barrier layer and an outer layer.
For example,
Pharmaflex Ltd., part of Alcan inc. (Cramlington, Northumberland, England)
supplies a
laminate film having three layers: 12 micron polyester / 9 micron aluminum
foil / 50
micron polyethylene (product catalog LMP-F BRI/72/H1). Also, another laminate
that
could be used in the invention comprises polyester (16.9 gsm/12 micron,
orientated and
acrylic coated)/low density polyethylene (20 gsm, coloured white using
Titanium
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-26-
dioxide)/aluminium foil (24.3 gsm/9 micron)/polyethylene copolymer (5gsm)/low
density
polyethylene (13 gsm)/linear low density polyethylene (37 gsm/40 micron).
The inner layer is disposed on the inner surface of the package (i.e. the side
in
contact with the inhaler device) and is normally a thermoplastic layer and
heat-sealable.
s A common material for the inner layer is polyethylene, but other
polyolefinic or cyclo-
olefinic materials may also be used. In addition, specialist materials such as
ionomers
are also frequently used for making the inner layer, for example, the ionomer
under the
tradename Surlyn. Properties which distinguish these ionomers resins from
other
polyolefin heat-sealed polymers are high clarity, high impact resistance, low
haze in
lamination, tear resistance, abrasion resistance, solid state toughness, and
moisture
imperviousness.
The barrier layer is disposed between the inner and outer layers (i.e. it is
sandwiched between the inner and outer layer) and provides impermeability, or
substantial impermeability, to the package. Aluminum foil is commonly used for
the
barrier layer, although any other metals capable of being rolled into thin
sheets can also
be satisfactorily used. A typical thickness for the aluminum foil layer is
about 8 or 9
microns. Alternatively, the barrier layer may be metalized films, made up of
tin, iron,
zinc, magnesium or other metals coated by vacuum deposition or sputtering onto
a
polymeric sheet.
The outer layer is disposed on the surface of the barrier layer, on the
opposite
side to the inner layer. The outer layer normally provides support, impact
resistance,
protection for the barrier layer and general robustness to the pack. A
commonly used
material for the outer layer is polyester, although other material, such as
paper, may also
be used.
Adhesives may be used to join the respective layers of materials together. The
adhesive layers are typically substantially smaller in thickness relative to
the thickness of
the substrate, heat sealable and/or protective layers which they bond.
The number, size, and shape of the layers are not limited to those layers
shown in
the drawings. Any number of layers with relative areas of any size and
predetermined
thickness may be used so long as the flexible package forms an enclosed volume
which
substantially prevents ingression of water vapor and particulate matter into
the enclosed
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-27-
volume while being impermeable, or substantially impermeable to any HFA
leakage from
the MDI device. The size, shape, and number of layers of the package is
typically a
function of the size and contents of the pressurized container which includes
a drug and
HFA propellant.
Preferred exemplary thicknesses of the three layers include an outer layer 1
to 40,
preferably 4 to 30, more preferably 10 to 23 microns, and most preferably 12
microns; a
barrier layer of 1 to 100, preferably 3 to 70, more preferably 5 to 50
microns, more
preferably 6 to 20 microns and most preferably 9 microns. For the inner layer,
preferred
exemplary thicknesses include thicknesses of I to 100, preferably 5 to 70,
more
io preferably 10 to 60, more preferably 20 to 55 microns, and most preferably
50 microns.
Preferred exemplary embodiments include a polyester film as the outer layer
having a thickness ranging from 12 to 23 microns. The polyester film is
laminated to an
aluminum foil as the substrate layer having a thickness ranging from 6 to 20
microns.
The aluminum foil is laminated to am inner film such as a polyethylene film
having a
thickness ranging from 20 to 50 microns.
Alternative preferred embodiments include aluminum metalized polyester film
laminated to an inner layer as outlined above. Another embodiment includes a
silicon
oxide coplated polyester film laminated to an inner layer as outlined above.
Yet, in
another embodiment, a polyester film as an outer layer having a thickness
ranging from
12 to 30 microns is laminated to an aluminum foil substrate layer having a
thickness
ranging from 6 to 20 microns, the aluminum foil being laminated to a polyester
film of 12
to 30 microns which is laminated to an inner layer as outlined above. In
another
embodiment, a polypropylene film as an outer layer having a thickness ranging
from 15
to 30 microns is laminated to an aluminum foil barrier layer having a
thickness ranging
from 6 to 20 microns, and the aluminum foil is laminated to an inner layer as
outlined
above. The laminates of the present invention can be adhesively laminated or
extrusion
laminated.
The laminate can be formed of any material described above and of any
thickness
as described above, as long as the final laminate is impermeable, or
substantially
impermeable, to HFA 134a or HFA p227.
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-28-
The permeability, or substantial impermeability, of the laminate may be tested
by
a variety of techniques known to the skilled person. For example, three pieces
of 75 mm
diameter discs are die stamped from laminate material. The thickness of the
laminate
disc are then measured and recorded. The samples are then placed into test
chambers
s and vacuumed down to 23 C for at least three hours. Once total vacuum has
stabilized
approximately 50psi of HFA p227 propellant is applied to the top half of the
disc sample,
this being the outlet pressure for the cylinder at laboratory temperature,
whist the bottom
side is still under vacuum. A similar test can be carried out using 30psi of
HFA 134a
propellant applied to the top half of the disc sample.
HFA Adsorbent and Gaseous Substances
"HFA adsorbent" means a substance which has the ability to condense or hold
HFA molecules on its surface or in its inner structure, an activity often
referred as
"adsorbing" or "absorbing". Examples of HFA adsorbents material selected from
the
group consisting of molecular sieves, activated clays (including,
montmorillonite and
bentonite clay, and other known activated clays e.g. those clays supplied by
Colin
Stewart Minchem Ltd, Cheshire, UK), activated alumina, silica, zeolites,
bauxites, and
mixtures thereof. Preferably, 10 A (Angstrom) molecular sieves.
The present invention is not limited to any specific HFA adsorbents or
specific
gaseous substances. Although there are many different HFA adsorbent and there
are
various types of propellant gases, it is believed that any propellant gas can
be in
principle entrapped by a properly-chosen HFA adsorbent. By following the
information
disclosed herein, it is well within the ordinary skill of the artisans in the
field to choose a
proper HFA adsorbent for a given propellant gas. Practitioners can make an
initial choice
based on their knowledge and experience (for example, weighing the factors
such as the
molecular size of the gaseous substance and the pore size of an HFA adsorbent
as well
as electronic charges it carries) and then conduct tests (such as those
disclosed herein
or some other methods) to determine the actual effectiveness of the chosen HFA
adsorbent against a given propellant gas. They may need to repeat the process
until a
proper HFA adsorbent is found.
As described in the foregoing, applicants have found that molecular sieves
with a
pore size of about 10 Angstroms is an effective HFA adsorbent material.
Inclusion of
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-29-
about 4 grams of a sachet of the molecular sieve supplied by AtoFina
(Solihull, England)
under the trade name Siliporite, for example, is found sufficient per package
to prevent
inflation. More detailed technical information about molecular sieves and
their other
industrial uses can be found in the Hajdu article --"Molecular Seives: Unique
Moisture
and Odor-Taste Control Material", D. Hajdu, T.J. Dangieri and S.R. Dunne,
TAPPI
Polym., Laminations Coat. Conf. (1999), Vol. 2, p. 655-662, which is
incorporated herein
by reference.
The HFA Adsorbent Sachet
Although it is not necessary to have a sachet to contain the HFA adsorbent
within
the package, it is usually preferred. The HFA adsorbent sachets are
commercially
available from many suppliers including Sud-Chemie (Middlewich, England). The
sachet,
with a "tea-bag" like appearance, is generally manufactured from synthetic
fibers, such
as polyamide or polyester fibers or blends thereof. Commercially available
materials
suitable for making HFA adsorbent sachets include, for example, GDT-II from
San-ei
is Corporation (Osaka, Japan) and Tyvek from Perfecseal (Londonderry N.
Ireland U.K.).
However, a suitable sachet may be in other convenient shapes or appearances
and
made from other permeable materials. The molecular sieve material, contained
within
the sachet is commercially available from several manufacturers. For example
AtoFina
(Solihull, England) market a molecular sieve under the trade name of
Siliporite.
The Pressurized Container
The pressurized container is preferably an MDI container. The term "MDI" or
"metered dose inhaler" means a unit comprising a can and a drug metering
device.
Exemplary pressurized containers for use in MDIs are disclosed in WO 96/32151,
WO
96/32345, WO 96/32150, WO 96/32099, and United States Patents 6,293,279,
6,253,762, and 6,149,892.
Most often the MDI can and cap are made of aluminum or an alloy of aluminum,
although other metals not affected by the drug formulation, such as stainless
steel, an
alloy of copper or tin plate, may be used. An MDI can may also be fabricated
from glass
or plastic. Preferably, however, the MDI cans employed in the present
invention are
made of aluminum or an alloy thereof. Advantageously, strengthened aluminum or
aluminum alloy MDI cans may be employed. Such strengthened MDI cans are
capable
CA 02490018 2004-12-17
WO 2004/002559 PCT/GB2003/002558
-30-
of withstanding particularly stressful coating and curing conditions, e.g.,
particularly high
temperatures, which may be required for certain fluorocarbon polymers.
Strengthened MDI cans which have a reduced tendency to malform under high
temperatures include MDI cans comprising side walls and a base of increased
thickness
and MDI cans comprising a substantially ellipsoidal base (which increases the
angle
between the side walls and the base of the can), rather than the hemispherical
base of
standard MDI cans. MDI cans having an ellipsoidal base offer the further
advantage of
facilitating the coating process.
The MDI cans of the present invention include MDI cans supplied by Presspart
of
Blackburn, Lancashire, U.K. , or by Neotechnic of Clitheroe, Lancashire U.K.
The MDI
cans typically have a neck diameter of 20 millimeters, although any suitable
neck
diameter may be used and can vary in height from 30 millimeters to 60
millimeters.
While there have been described and pointed out fundamental novel features of
the invention as applied to a preferred embodiment thereof, it will be
understood that
various omissions and substitutions and changes, in the form and details of
the
packages and methods illustrated, may be made by those skilled in the art
without
departing from the spirit of the invention. For example, it is expressly
intended that all
combinations of those elements and/or method steps which perform substantially
the
same function in substantially the same way to achieve the same results are
within the
scope of the invention.
The invention is not limited by the embodiments described above which are
presented as examples only but can be modified in various ways within the
scope of
protection defined by the appended patent claims.