Note: Descriptions are shown in the official language in which they were submitted.
CA 02493373 2005-O1-11
WO 20041007437 PCTlEP20031006934
Description
Urea-substituted and urethane-substituted acylureas, methods for the
production
s thereof and their use
The invention relates to urea- and urethane-substituted acylureas and to their
physiologically tolerated salts and physiologically functional derivatives.
to EP 0 221 847 describes compounds of similar structure for controlling
pests.
The invention was based on the object of providing compounds with which
prevention
and treatment of type 2 diabetes is possible. The compounds are intended for
this
purpose to bring about a marked reduction in the blood glucose level.
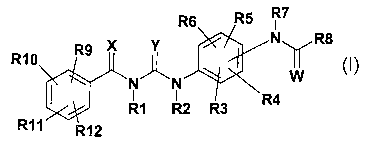
The invention therefore relates to compounds of the formula I
R7
N\~/R8
R10 II
N N
I I .;4
R1 R2 R3
R1 '~ R12
2o in which
W, X, Y are, independently of one another, O or S;
R9, R10, R11, R12 are, independently of one another, H, F, CI, Br, OH, CF3,
2s N02, CN, OCFa, O-(C~-C6)-alkyl, O-{C2-C6)-alkenyl, O-(CZ-Cs)-alkynyl,
O-S02-(C~-C~)-alkyl, O-S02-phenyl, where the phenyl ring may be
substituted up to twice by F, Cl, Br, CN, OR13, R13, CF3, OCF3,
COOR13 or CON(R14)(R15), or S-(C~-Cs)-alkyl, S-(C2-Cs)-alkenyl,
CA 02493373 2005-O1-11
2
S-(C2-Cs)-alkynyl, SO-(C~-Cs)-alkyl, S02-(C~-Cs)-alkyl, SOZ-NH2, (C~-Cs)-
alkyl, (C2-Cs)-alkenyl, (C2-Cs)-alkynyl, (C3-C~)-cycloalkyl, (C3-C7)-
cycloalkyl-(C~-C4)-alkylene, (Co-Cs)-alkylene-COOR13, CON(R14)(R15),
(Co-Cs)-alkylene-N(R14)(R15), NH-COR13, NH-CO-phenyl, NH-S02-
s phenyl or phenyl, where the phenyl ring may be substituted up to twice by
F, CI, Br, CN, OR13, R13, CF3, OCF3, COOR13 or CON(R14)(R15);
R13 is H, (C~-Cs)-alkyl, (C2-Cs)-alkenyl, (C2-Cs)-alkynyl, (C3-C7)-cycloalkyl
or
(C3-C~)-cycloalkyl-(C~-C4)-alkylene;
to
R1, R2 are, independently of one another, H, (C~-Cs)-alkyl, where alkyl may be
substituted by OH, O-(C~-C4)-alkyl or N(R14)(R15), or O-(C~-Cs)-alkyl,
O-(C2-Cs)-alkenyl, O-(C2-Cs)-alkynyl, CO-(C~-Cs)-alkyl, CO-(C2-Cs)-
alkenyl, CO-(C2-Cs)-alkynyl, COOR13 or (Co-Cs)-alkylene-COOR13;
R3, R4, R5, R6 are, independently of one another, H, F, CI, Br, OH, CF3,
NO2, CN, OCF3, (C~-Cs)-alkyl, (C2-Cs)-alkenyl, (C2-Cs)-alkynyl,
O-(C~-Coo)-alkyl, O-(C2-C,o)-alkenyl, O-(C2-Coo)-alkynyl, S-(C~-Cs)-alkyl,
S-(CZ-Cs)-alkenyl, S-(CZ-Cs)-alkynyl, (C3-C7)-cycloalkyl, (C3-C~)-
2o cycloalkyl-(C~-C4)-alkyl, where alkyl, alkenyl, alkynyl and cycloalkyl may
be substituted more than once by F, CI, Br, SO-phenyl, S02-phenyl,
where the phenyl ring may be substituted by F, CI, Br or R13, or OR13,
COOR13, CON(R14)(R15), N(R14))(R15) or CO-heteroalkyl, or are
O-SO-(C~-Cs)-alkyl, O-S02-(C~-Cs)-alkyl, O-SOZ-(Cs-Coo)-aryl,
2s O-(Cs-Coo)-aryl, where aryl may be substituted up to twice by F, Cf, CN,
OR13, R13, CF3 or OCF3, or are SO-(C1-Cs)-alkyl, S02-(C~-Cs)-alkyl,
SOz-(Cs-Coo)-aryl, where the phenyl ring may be substituted up to twice
by F, CI, Br, CN, OR13, R13, CF3, OCF3, COOR13 or CON(R14)(R15),
or are S02-N(R14)(R15), COOR13, CO-heteroalkyl, N(R14)(R15) or
3o heteroalkyl;
R14, R15 are, independently of one another, H, (C~-Cs)-alkyl, where alkyl may
be
substituted by N(R13)2, or are (CZ-Cs)-alkenyl, (C2-Cs)-alkynyl, (C3-C7)-
cycloalkyl, (Cs-C~)-cycloalkyl-(C~-C4)-alkylene, CO-(C~-Cs)-alkyl, COO-
CA 02493373 2005-O1-11
3
(C~-C6}-alkyl, COO-(C~-C6}-alkylene-OCO-(C~-C6)-alkyl, CO-phenyl,
COO-phenyl, COO-(C~-C6)-alkenyl-phenyl, OH, O-(C~-C6)-alkyl,
O-(C~-C6)-alkenyl-phenyl or NH2;
s or the radicals R14 and R15 form with the nitrogen atom to which they are
bonded a
3-7-membered, saturated heterocyclic ring which may comprise up to 2
further heteroatoms from the group of N, O or S, where the heterocyclic
ring may be substituted up to three times by F, CI, Br, OH, oxo,
N(R16)(R17) or (C1-C4)-alkyl;
io
R16, R17 are, independently of one another, H, (C~-C6)-alkyl, where alkyl may
be
substituted by N(R13)2, or are (C2-C6)-alkenyl, (C2-C6)-alkynyl, (C3-C~)-
cycloalkyl, (C3-C~)-cycloalkyl-(C~-C4)-alkylene, CO-(C~-C6)-alkyl, COO-
(C~-C6)-alkyl, COO-(C~-C6)=alkylene-OCO-(C~-C6)-alkyl, CO-phenyl,
is COO-phenyl, COO-(C~-C6)-alkenyl-phenyl, OH, O-(C~-C6)-alkyl,
O-(C~-C6)-alkenyl-phenyl or NHZ;
heteroalkyl is a 3-7-membered, saturated or up to triunsaturated heterocyclic
ring
which may comprise up to 4 heteroatoms which correspond to N, O or S,
2o where the heterocyclic ring may be substituted at alf sensible positions
up to three times by F, CI, Br, CN, oxo, (C~-C4)-alkyl, (Co-C4)-alkylene-
COOR13, CON(R14)(R15), OR13, N(R14)(R15) or phenyl, where
phenyl may be substituted by COOR13;
2s R7 is H, (C,-C6)-alkyl, where alkyl may be substituted by OR13 or
N(R14)(R15), or is O-(C~-C6)-alkyl, CO-(C~-C6)-alkyl or (Co-C6)-alkylene-
COOR13;
R8 is N(R18)(R19) or OR20;
30 or R8 and R4 together form the group -NH-CO-;
R18, R19 are, independently of one another, H, (C~-Coo)-alkyl, (CZ-Coo)-
alkenyl,
(C2-Coo)-alkynyl, (C3-C~)-cycloalkyl, (C3-C7)-cycloalkyl-(C~-C6)-alkyl,
(Cs-C~o)-arYl, (Cs-C1o)-arYl-(C~-C4)-alkyl, (C6-C1o)-aryl-(C2-C4)-alkenyl,
CA 02493373 2005-O1-11
4
(C6-Coo)-aryl-(CZ-C4)-alkynyl, heteroaryl, heteroaryl-(C,-C4)-alkyl,
heteroaryl-(Cz-C4)-alkenyl, heteroaryl-(C2-C4)-alkynyl, where alkyl,
alkenyl, alkynyl and cycloalkyl may be substituted more than once by F,
CI, CN, OR13, R13, CF3, OCF3, (Cs-C,o)-aryl, NH-C(=NR14)-
s N(R14)(R15), N(R14)(R15), C(=NR14)-N(R14)(R15), COOR13 or
CON(R14)(R15), and where aryl may be substituted more than once by
F, CI, CN, O-(C,-C6)-alkyl, O-(C2-C6)-alkenyl, (C~-C6)-alkyl, (C2-C6)-
alkenyl, CO-(C~-C6)-alkyl, CO-(C2-C6)-alkenyl, where alkyl and alkenyl
may be substituted more than once by F, CI, CH3, OCH3 or CN, or
io NH-C(=NR14)-N(R14)(R15), N(R14)(R15), C(=NR14)-N(R14)(R15),
COOR13, CON(R14)(R15), O-phenyl, phenyl or pyridyl;
COOR13, CON-(R14)(R15), CO-heteroalkyl, CO-(C6-Coo)-aryl or
S02-(C6-Coo)-aryl, where aryl may be substituted up to twice by F, CI,
CN, OH, (C~-C6)-alkyl, O-(C~-C6)-alkyl, CF3, OCF3, COOR13 or
is CON(R14)(R15);
or the radicals R18 and R19 form with the nitrogen atom to which they are
bonded a
3-7-membered, saturated heterocyclic ring which may comprise up to 2
further heteroatoms from the group of N, O or S, where the heterocyclic
2o ring may be substituted up to three times by F, CI, Br, OH, oxo,
N(R16)(R17) or (C~-C4)-alkyl;
R20 is (C~-Coo)-alkyl, (C2-Coo)-alkenyl, (C2-Coo)-alkynyl, (C3-C~)-cycloalkyl,
(C3-C7)-cycloalkyl-(C~-C6)-alkyl, (C6-Coo)-aryl, (Cs-Coo)-aryl-(C~-C4)-alkyl,
2s (Cs-Coo)-aryl-(C2-C4)-alkenyl or (C6-Coo)-aryl-(C2-C4)-alkynyl, where aryl
may be substituted more than once by F, CI, CN, O-(C~-Cs)-alkyl,
O-(C2-C6)-alkenyl, (C~-Cs)-alkyl, (C2-C6)-alkenyl, CO-(C~-C6)-alkyl, CO-
(C2-C6)-alkenyl, where alkyl and alkenyl may be substituted more than
once by F, CI, CH3, OCH3 or CN, or NH-C(=NR14)-N(R14)(R15),
3o N(R14)(R15), C(=NR14)-N(R14)(R15), COOR13, CON(R14)(R15),
0-phenyl, phenyl or pyridyl, where phenyl may be substituted by F, CI,
CN or (C~-Cs)-alkyl;
and their physiologically tolerated salts,
CA 02493373 2005-O1-11
s
excluding compounds of the formula I in which the radicals R6, R7, X and R8
have
the following meanings at the same time:
s R6 H, CI, CF3, CH3;
R7 H;
X O;
to
Y O, S;
R8 substituted or unsubstituted NH-phenyl.
is Preference is given to compounds of the formula la,
R5
OR6 / R4
R10 I H
N N \ N'~'R8
H H R3 II
R1n R12 O
la
in which one or more radicals has or have the following meanings:
R9 F, CI, Br, OH, CF3, NOZ, CN, OCF3, O-(C~-C6)-alkyl, O-(C2-C6)-alkenyl,
O-(C2-C6)-alkynyl, O-S02-(C~-C4)-alkyl, O-S02-phenyl, where the phenyl
ring may be substituted up to twice by F, CI, Br, CN, OR13, R13, CF3,
OCF3, COOR13 or CON(R14)(R15), or S-(C~-C6)-alkyl, S-(C2-C6)-
2s alkenyl, S-{C2-Cs)-alkynyl, SO-(C~-C6)-alkyl, SOZ-(C~-C6)-alkyl, SOZ-NH2,
(C~-C6)-alkyl, (C2-C6)-alkenyl, (C2-C6)-alkynyl, (C3-C7)-cycloalkyl, (C3-C7}-
cycloalkyl-(C~-C4)-alkylene, (Co-C6)-alkylene-COOR13, CON(R14)(R15),
CA 02493373 2005-O1-11
6
(Co-C6)-alkylene-N(R14)(R15), NH-COR13, NH-CO-phenyl, NH-S02-
phenyl or phenyl, where the phenyl ring may be substituted up to twice by
F, CI, Br, CN, OR13, R13, CF3, OCF3, COOR13 or CON(R14)(R15);
s R10, R11, R12 independently of one another H, F, CI, Br, OH, CF3, N02, CN,
OCF3, O-(C~-C6)-alkyl, O-(CZ-C6)-alkenyl, O-(C2-C6)-alkynyl, O-SOZ-
(C~-C4}-alkyl, 0-S02-phenyl, where the phenyl ring may be substituted up
to twice by F, CI, Br, CN, OR13, R13, CF3, OCF3, COOR13 or
CON(R14)(R15), or S-(C~-C6)-alkyl, S-(C2-C6)-alkenyl, S-(C2-C6)-alkynyl,
io SO-(C~-Cs)-alkyl, S02-(C~-C6)-alkyl, S02-NH2, (C~-C6)-alkyl, (C2-C6)-
alkenyl, (C2-C6)-alkynyl, (C3-C7}-cycloalkyl, (C3-C~)-cycloalkyl-(C~-C4)-
alkylene, (Co-Cs}-alkylene-COOR13, CON(R14)(R15), (Co-C6)-alkylene-
N(R14)(R15), NH-COR13, NH-CO-phenyl, NH-S02-phenyl or phenyl,
where the phenyl ring may be substituted up to twice by F, CI, Br, CN,
is OR13, R13, CF3, OCF3, COOR13 or CON(R14)(R15);
R13 H, (C~-C6)-alkyl, (C2-C6)-alkenyl, (C2-C6}-alkynyl, (C3-C~)-cycloalkyl or
(C3-C~)-cycloalkyl-(C~-C4)-alkylene;
20 R3, R4, R5 independently of one another H, F, CI, Br, OH, CF3, N02, CN,
OCF3, (C~-C6)-alkyl, (C2-C6)-alkenyl, (C2-C6)-alkynyl, O-(C~-Coo)-alkyl,
O-(C2-Coo)-alkenyl, O-(C2-Coo)-alkynyl, S-(C~-C6}-alkyl, S-(C2-C6}-
alkenyl, S-(C2-C6)-alkynyl, (C3-C~)-cycloalkyl, (C3-C7)-cycloalkyl-(C~-C4)-
alkyl, where alkyl, aikenyl, alkynyl and cycloalkyl may be substituted
2s more than once by F, CI, Br, SO-phenyl, S02-phenyl, where the phenyl
ring may be substituted by F, CI, Br or R13, or OR13, COOR13,
CON(R14)(R15), N(R14))(R15) or CO-heteroalkyl, or O-SO-(C~-C6)-
alkyl, O-S02-(C~-C6)-alkyl, O-S02-(C6-C,o)-aryl, O-(C6-C,o)-aryl, where
aryl may be substituted up to twice by F, CI, CN, OR13, R13, CF3 or
3o OCF3, or SO-(C,-C6)-alkyl, S02-(C~-C6)-alkyl, S02-(C6-C,o)-aryl, where
the phenyl ring may be substituted up to twice by F, CI, Br, CN, OR13,
R13, CF3, OCF3, COOR13 or CON(R14)(R15), or S02-N(R14)(R15),
COOR13, CO-heteroalkyl, N(R14)(R15) or heteroalkyl;
CA 02493373 2005-O1-11
7
R6 F, CI, Br, OH, CF3, N02, CN, OCF3, (C~-C6)-alkyl, (C2-C6)-alkenyl,
(C2-Cs)-alkynyl, 0-(C~-Coo)-alkyl, O-(C2-C,o)-alkenyl, O-(C2-C,o)-alkynyl,
S-(C~-Cs)-alkyl, S-(C2-C6)-alkenyl, S-(CZ-C6)-alkynyl, (Cs-C7)-cycloalkyl,
(C3-C~)-cycioalkyl-(C~-C4)-alkyl, where alkyl, aikenyl, alkynyl and
cycloalkyl may be substituted more than once by F, CI, Br, SO-phenyl,
S02-phenyl, where the phenyl ring may be substituted by F, CI, Br or
R13, or OR13, COOR13, CON(R14)(R15), N(R14))(R15) or CO-hetero-
alkyl, or O-SO-(C~-Cs)-alkyl, O-SOZ-(C~-Cs)-alkyl, O-S02-(C6-Coo)-aryl,
O-(Cs-Coo)-aryl, where aryl may be substituted up to twice by F, CI, CN,
io OR13, R13, CF3 or OCF3, or SO-(C~-C6)-alkyl, S02-(C~-C6)-alkyl, S02-
(Cs-Coo)-aryl, where the phenyl ring may be substituted up to twice by F,
Cl, Br, CN, OR13, R13, CF3, OCF3, COOR13 or CON(R14)(R15), or
S02-N(R14)(R15), COOR13, CO-heteroalkyl, N(R14)(R15) or
heteroalkyl;
is
R14, R15 independently of one another H, (C~-C6)-alkyl, where alkyl may be
substituted by N(R13)2, or {C2-C6)-alkenyl, {C2-C6)-alkynyl, (C3-Cr)-
cycloalkyl, (C3-C7)-cycloalkyl-(C~-C4)-alkylene, CO-(C,-C6)-alkyl, COO-
(C~-C6)-alkyl, COO-(C1-C6)-alkylene-OCO-(C~-C6)-alkyl, CO-phenyl,
2o COO-phenyl, COO-(C~-C6)-alkenyl-phenyl, OH, O-(C~-C6)-alkyl,
O-(C~-Cs)-alkenyl-phenyl or NH2;
or the radicals R14 and R15 form with the nitrogen atom to which they are
bonded a
3-7-membered, saturated heterocyciic ring which may comprise up to 2
2s further heteroatoms from the group of N, O or S, where the heterocyclic
ring may be substituted up to three times by F, CI, Br, OH, oxo,
N(R16)(R17) or (C~-C4)-alkyl;
R16, R17 independently of one another H, (C~-C6)-alkyl, where alkyl may be
3o substituted by N(R13)2, or (CZ-Cs)-alkenyl, (C2-C6)-alkynyl, (C3-C~)-
cycloalkyl, (C3-C7)-cycloalkyl-(C~-C4)-alkylene, CO-(C~-C6)-alkyl, COO-
(C,-Cs)-alkyl, COO-(C~-C6)-alkylene-OCO-(C~-C6)-alkyl, CO-phenyl,
COO-phenyl, COO-(C~-C6)-alkenyl-phenyl, OH, O-(C,-C6)-alkyl,
O-(C~-C6)-alkenyl-phenyl or NH2;
CA 02493373 2005-O1-11
g
heteroalkyl a 3-7-membered, saturated or up to triunsaturated heterocyclic
ring
which may comprise up to 4 heteroatoms which correspond to N, O or S,
where the heterocyclic ring may be substituted at all sensible positions
s up to three times by F, Cl, Br, CN, oxo, (C~-C4)-alkyl, (Co-C~)-alkylene-
COOR13, CON(R14)(R15), OR13 or N(R14)(R15) or phenyl, where
phenyl may be substituted by COOR13;
R8 N{R18)(R19) or OR20;
io or R8 and R4 together form the group -NH-CO-;
R18, R19 independently of one another H, (C~-Coo)-alkyl, (C2-Coo)-alkenyl,
(C2-Coo)-alkynyl, (C3-C~)-cycloalkyl, (C3-C7)-cycloalkyl-(C~-Cs)-alkyl,
(Gs-C,o)-aryl, (Cs-G~o)-ar'YI-{C~-Ca)-alkyl, (Cs-C,o)-aryl-(C2-C4)-alkenyl,
is (Cs-Coo)-aryl-(C2-C4)-alkynyl, heteroaryl, heteroaryl-(C2-C~)-alkyl,
heteroaryl-(C2-Ca)-alkenyl, heteroaryl-(C2-C4)-alkynyl, where alkyl,
alkenyl, alkynyl and cycloalkyl may be substituted more than once by F,
CI, CN, OR13, R13, CFA, OCF3, (Cs-Coo)-aryl, NH-C(=NR14)-
N(R14)(R15), N(R14)(R15), C(=NR14)-N(R14)(R15), COOR13 or
2o CON(R14)(R15), and where aryl may be substituted more than once by
F, CI, CN, O-(C~-Cs)-alkyl, O-(C2-Cs)-alkenyl, (C~-Cs)-alkyl, (CZ-Cs)-
alkenyl, CO-(C~-Cs)-alkyl, CO-(C2-Cs)-alkenyl, where alkyl and alkenyl
may be substituted more than once by F, CI, CH3, OCH3 or CN, or
NH-C(=NR14)-N{R14){R15), N(R14)(R15), C(=NR14)-N(R14)(R15),
2s COOR13, CON(R14)(R15), O-phenyl, phenyl or pyridyl;
COOR13, CON-(R14)(R15), CO-heteroalkyl, CO-(Cs-Coo)-aryl or
SOZ-(Cs-Coo)-aryl, where aryl may be substituted up to twice by F, CI,
CN, OH, (C,-Cs)-alkyl, O-(C~-Cs)-alkyl, CF3, OCF3, COOR13 or
CON(R14)(R15);
or the radicals R18 and R19 form together with the nitrogen atom to which they
are
bonded a 3-7-membered, saturated heterocyclic ring which may
comprise up to 2 further heteroatoms from the group of N, O or S, where
the heterocyclic ring may be substituted up to three times by F, GI, Br,
CA 02493373 2005-O1-11
9
OH, oxo, N(R16)(R17) or (C~-C4)-alkyl;
R20 (C,-Coo)-alkyl, (C2-Coo)-alkenyl, (C2-Coo)-alkynyl, (C3-C7)-cycloalkyl,
(C3-C~)-cycloalkyl-(C~-C6)-alkyl, (C6-C,o)-aryl, (Cs-Coo}-aryl-(C~-C4)-alkyl,
s (Cs-Coo)-aryl-(CZ-C4)-alkenyl or (Cs-Coo)-aryl-(C2-C4)-alkynyl, where aryl
may be substituted more than once by F, CI, CN, O-(C,-C6)-alkyl,
O-(C2-C6)-alkenyl, (C~-C6)-alkyl, (CZ-C6)-alkenyl, CO-(C,-Cs)-alkyl,
CO-(CZ-Cs)-alkenyl, where alkyl and alkenyl may be substituted more
than once by F, CI, CH3, OCH3 or CN, or NH-C(=NR14)-N(R14)(R15),
to N(R14)(R15), C(=NR14)-N(R14)(R15), COOR13, CON(R14)(R15),
O-phenyl, phenyl or pyridyl, where phenyl may be substituted by F, CI,
CN or (C~-Cs)-alkyl;
and their physiologically tolerated salts,
is
excluding compounds of the formula la in which the radicals R6 and R8 have at
the
same time the following meanings:
R6 H, CI, CF3, CH3;
R8 substituted or unsubstituted NH-phenyl.
Particular preference is given to compounds of the formula la in which one or
more
radicals) has or have the following meaning:
2s
R9, R10, R11 independently of one another F, CI;
R12 H;
R13 H, (C~-C6)-alkyl, (CZ-Cs)-alkenyl, (C2-C6)-alkynyl, (C3-C7)-cycloalkyl or
(C3-C~}-cycloalkyl-(C~-C4)-alkylene;
R3, R4, R5 independently of one another H, COOR13;
CA 02493373 2005-O1-11
lU
R6 F, CI, CF3, OCF3, (C~-C6)-alkyl, (CZ-G6)-alkenyl, (CZ-C6)-alkynyl, O-
(C,-Coo)-alkyl, O-(CZ-C,o)-alkenyl, O-(G2-G,o)-alkynyl, (C3-C7)-cycloalkyl,
(C3-C7)-cycloalkyl-(C~-C4)-alkyl, N(R14)(R15) or heteroalkyl, where alkyl,
alkenyl, alkynyl and cycloalkyl may be more than once by F, COOR13,
CON(R14)(R15), N(R14)(R15);
R14, R15 independently of one another H, (C~-C6)-alkyl, where alkyl may be
substituted by N(R13)2;
io heteroalkyl a 3-7-membered, saturated or up to triunsaturated heterocyclic
ring
which may comprise up to 4 heteroatoms which correspond to N, O or S,
where the heterocyclic ring may be substituted at all sensible positions
up to three times by F, Cl, Br, CN, oxo, (C~-C4)-alkyl, (Co-C~)-alkylene-
COOR13, CON(R14)(R15), OR13 or N(R14)(R15) or phenyl, where
is phenyl may be substituted by COOR13;
R8 N(R18)(R19) or OR20;
or R8 and R4 together form the group -NH-CO-;
2o R18, R19 independently of one another H, (C~-Coo)-alkyl, (C2-Coo)-alkenyl,
(CZ-Coo)-alkynyl, (C3-Cr)-cycloalkyl, (C3-C7)-cycloalkyl-(C~-C6)-alkyl,
(Cs-C,o)-aryl, (Gs-G~o)-aryl-(C1-C4)-alkyl, (Cs-C~o)-arYl-(C2-C4)-alkenyl,
(C6-Coo)-aryl-(C2-C4)-alkynyl, heteroaryl, heteroaryl-(C2-C4)-alkyl,
heteroaryl-(C2-C4)-alkenyl, heteroaryl-(C2-C4)-alkynyl, where alkyl,
25 alkenyl, alkynyl and cycloalkyl may be substituted more than once by F,
CI, CN, OR13, R13, CF3, OCF3, (C6-Coo)-aryl, NH-C(=NR14)-
N(R14)(R15), N(R14)(R15), C(=NR14)-N(R14)(R15), COOR13 or
CON(R14)(R15), and where aryl may be substituted more than once by
F, CI, CN, O-(C,-C6)-alkyl, O-(C2-C6)-alkenyl, (C~-Cs)-alkyl, (CZ-C6)-
alkenyl, CO-(C~-Cs)-alkyl, CO-(C2-C6)-alkenyl, where alkyl and alkenyl
may be substituted more than once by F, CI, CH3, OCH3 or CN, or
NH-C(=NR14)-N(R14)(R15), N(R14)(R15), C(=NR14)-N(R14)(R15),
COOR13, CON(R14)(R15), O-phenyl, phenyl or pyridyl;
COOR13, CON-(R14)(R15), CO-heteroalkyl, CO-(Cs-Coo)-aryl or
CA 02493373 2005-O1-11
I1
S02-(C6-Coo)-aryl, where aryl may be substituted up to twice by F, CI,
CN, OH, (C~-Cs)-alkyl, O-(C~-C6)-alkyl, CF3, OCF3, COOR13 or
CON(R14)(R15);
s or the radicals R18 and R19 form together with the nitrogen atom to which
they are
bonded a 3-7-membered, saturated heterocyclic ring which may
comprise up to 2 further heteroatoms from the group of N, O or S, where
the heterocyclic ring may be substituted up to three times by F, Cl, Br,
OH, oxo, N(R16)(R17) or (C~-C4}-alkyl;
io
R20 (C~-C,o)-alkyl, (C2-Coo)-alkenyl, (C2-C,o)-alkynyl, (C3-C7)-cycloalkyl,
(Cs-C~)-cycloalkyl-(C~-Cs)-alkyl, (Cs-Coo)-aryl, (C6-Coo)-aryl-(C,-C4)-alkyl,
(Cs-C,o)-aryl-(CZ-C4)-alkenyl or (Cs-Coo)-aryl-(C2-C4)-alkynyl, where aryl
may be substituted more than once by F, CI, CN, O-(C~-C6)-alkyl,
is O-(C2-C6)-alkenyl, (C~-C6)-alkyl, (C2-C6)-alkenyl, CO-(C~-Cs)-alkyl,
CO-(C2-C6)-alkenyl; where alkyl and alkenyl -may be substituted more
than once by F, CI, CH3, OCH3 or CN, or NH-C(=NR14)-N(R14)(R15),
N(R14)(R15), C(=NR14)-N(R14}(R15}, COOR13, CON(R14}(R15},
O-phenyl, phenyl or pyridyl, where phenyl may be substituted by F, CI,
2o CN or (C,-Cs)-alkyl;
and their physiologically tolerated salts,
excluding compounds of the formula is in which the radicals R6 and R8 have at
the
zs same time the following meanings:
R6 H, CI, CF3, CH3;
R8 substituted or unsubstituted NH-phenyl.
R9, R10, R11 independently of one another F or CI;
R12 H;
- CA 02493373 2005-O1-11
12
R13 independently of one another H or (C~-C6)-alkyl;
R1, R2 H;
s R3, R4, R5 independently of one another H, COOR13;
R6 CI, OCF3, COOR13, N(R14)(R15), (CZ-C6)-alkenyl, O-(C,-Coo)-alkyl,
where alkyl, alkenyl may be substituted more than once by F, COOR13
or CON(R14)(R15);
to
R14, R15 (C,-C6)-alkyl, where alkyl may be substituted by N(R13)2;
or the radicals R14 and R15 form with the nitrogen atom to which they ace
bonded a
5-membered, saturated heterocyclic ring;
is
R8 N(R18)(R19) or OR20;
R18, R19 independently of one another H, (C~-C,o)-alkyl, (C2-Coo)-alkenyl,
where
alkyl may be substituted by COOR13, N(R13)2 or phenyl, or (C3-C7)-
2o cycloalkyl or (Cs-Coo)-aryl, where aryl may be substituted more than
once by F, Cl, CN, (C~-C6)-alkyl, O-(C,-C6)-alkyl, CO-(C~-C6)-alkyl,
where alkyl may be substituted more than once by F, or O-phenyl,
phenyl, pyridyl or COOR13;
2s R20 (C~-Coo)-alkyl, (CZ-Coo)-alkenyl, (C2-Coo)-alkynyl or phenyl, where
phenyl
may be substituted by CI or (C~-C6)-alkyl;
and their physiologically tolerated salts.
3o The alkyl radicals in the substituents R1, R2, R3, R4, R5, Rfi, R7, R8, R9,
R10, R11
R12, R13, R14, R15, R16, R17, R18, R19 or R20 may be both straight-chain and
branched.
CA 02493373 2005-O1-11
13
If radicals or substituents may occur more than once in the compounds of the
formula I, such as, for example, COOR13, they may all, independently of one
another, have the stated meanings and be identical or different.
The invention relates to compounds of the formula I in the form of their
racemates,
racemic mixtures and pure enantiomers, and to their diastereomers and mixtures
thereof.
Pharmaceutically acceptable salts are, because their solubility in water is
greater
to than that of the initial or basic compounds, particularly suitable for
medical
applications. These salts must have a pharmaceutically acceptable anion or
cation.
Suitable pharmaceutically acceptable acid addition salts of the compounds of
the
invention are salts of inorganic acids such as hydrochloric acid, hydrobromic,
phosphoric, metaphosphoric, nitric and sulfuric acid, and of organic acids
such as, for
is example, acetic acid, benzenesulfonic, benzoic, citric, ethanesulfonic,
fumaric,
gluconic, glycolic, isethionic, lactic, lactobionic, malefic, malic,
methanesulfonic,
succinic, p-toluenesulfonic and tartaric acid. Suitable pharmaceutically
acceptable
basic salts are ammonium salts, alkali metal salts (such as sodium and
potassium
salts), alkaline earth metal salts (such as magnesium and calcium salts), and
salts of
2o trometamol (2-amino-2-hydroxymethyl-1,3-propanediol), diethanolamine,
lysine or
ethylenediamine.
Salts with a pharmaceutically unacceptable anion such as, for example,
trifluoroacetate likewise belong within the framework of the invention as
useful
2s intermediates for the preparation or purification of pharmaceutically
acceptable salts
andlor for use in nontherapeutic, for example in vitro, applications.
The term "physiologically functional derivative" used herein refers to any
physiologically tolerated derivative of a compound of the formula 1 of the
invention, for
3o example an ester, which on administration to a mammal such as, for example,
a
human is able to form (directly or indirectly) a compound of the formula 1 or
an active
metabolite thereof.
CA 02493373 2005-O1-11
14
Physiologically functional derivatives include prodrugs of the compounds of
the
invention, as described, for example, in H. Okada et al., Chem. Pharm. Bull.
1994,
42, 57-61. Such prodrugs can be metabolized in vivo to a compound of the
invention.
These prodrugs may themselves be active or not.
s
The compounds of the invention may also exist in various polymorphous forms,
for
example as amorphous and crystalline polymorphous forms. All polymorphous
forms
of the compounds of the invention belong within the framework of the invention
and
are a further aspect of the invention.
to
All references to "compound(s) of formula I" hereinafter refer to compounds)
of the
formula I as described above, and their salts, solvates and physiologically
functional
derivatives as described herein.
is The compounds) of formula (I) may also be administered in combination with
further
active ingredients.
The amount of a compound of formula I necessary to achieve the desired
biological
effect depends on a number of factors, for example the specific compound
chosen,
2o the intended use, the mode of administration and the clinical condition of
the patient.
The daily dose is generally in the range from 0.3 mg to 100 mg (typically from
3 mg
and 50 mg) per day and per kilogram of bodyweight, for example 3-10 mglkglday.
An
intravenous dose may be, for example, in the range from 0.3 mg to 1.0 mglkg,
which
can suitably be administered as infusion of 10 ng to 100 ng per kilogram and
per
2s minute. Suitable infusion solutions for these purposes may contain, for
example, from
0.1 ng to 10 mg, typically from 1 ng to 10 mg, per milliliter. Single doses
may contain,
for example, from 1 mg to 10 g of the active ingredient. Thus, ampoules for
injections
may contain, for example, from 1 mg to 100 mg, and single-dose formulations
which
can be administered orally, such as, for example, capsules or tablets, may
contain,
3o for example, from 1.0 to 1000 mg, typically from 10 to 600 mg. For the
therapy of the
abovementioned conditions, the compounds of formula I may be used as the
compound itself, but they are preferably in the form of a pharmaceutical
composition
with an acceptable carrier. The carrier must, of course, be acceptable in the
sense
that it is compatible with the other ingredients of the composition and is not
harmful
CA 02493373 2005-O1-11
for the patient's health. The carrier may be a solid or a liquid or both and
is preferably
formulated with the compound as a single dose, for example as a tablet, which
may
contain from 0.05% to 95% by weight of the active ingredient. Other
pharmaceutically
active substances may likewise be present, including other compounds of
formula I.
The pharmaceutical compositions of the invention can be produced by one of the
known pharmaceutical methods, which essentially consist of mixing the
ingredients
with pharmacologically acceptable carriers andlor excipients.
Pharmaceutical compositions of the invention are those suitable for oral,
rectal,
to topical, peroral (for example sublingual) and parenteral (for example
subcutaneous,
intramuscular, intradermal or intravenous) administration, although the most
suitable
mode of administration depends in each individual case on the nature and
severity of
the condition to be treated and on the nature of the compound of formula I
used in
each case. Coated formulations and coated slow-release formulations also
belong
is within the framework of the invention. Preference is given to acid- and
gastric juice-
resistant formulations. Suitable coatings resistant to gastric juice comprise
cellulose
acetate phthalate, polyvinyl acetate phthalate, hydroxypropylmethylcellulose
phthalate and anionic polymers of methacrylic acid and methyl methacrylate.
2o Suitable pharmaceutical compounds for oral administration may be in the
form of
separate units such as, for example, capsules, wafers, suckable tablets or
tablets,
each of which contain a defined amount of the compound of formula I; as
powders or
granules, as solution or suspension in an aqueous or nonaqueous liquid; or as
an oil-
in-water or water-in-oil emulsion. These compositions may, as already
mentioned, be
2s prepared by any suitable pharmaceutical method which includes a step in
which the
active ingredient and the carrier (which may consist of one or more additional
ingredients) are brought into contact. The compositions are generally produced
by
uniform and homogeneous mixing of the active ingredient with a liquid andlor
finely
divided solid carrier, after which the product is shaped if necessary. Thus,
for
3o example, a tablet can be produced by compressing or molding a powder or
granules
of the compound, where appropriate with one or more additional ingredients.
Compressed tablets can be produced by tableting the compound in free-flowing
form
such as, for example, a powder or granules, where appropriate mixed with a
binder,
glidant, inert diluent andlor one or more surface-activeldispersing agents) in
a
CA 02493373 2005-O1-11
16
suitable machine. Molded tablets can be produced by molding the compound,
which
is in powder form and is moistened with an inert liquid diluent, in a suitable
machine.
Pharmaceutical compositions which are suitable for peroral (sublingual)
administration comprise suckable tablets which contain a compound of formula I
with
a flavoring, normally sucrose and gum arabic or tragacanth, and pastilles
which
comprise the compound in an inert base such as gelatin and glycerol or sucrose
and
gum arabic.
to Pharmaceutical compositions suitable for parenteral administration comprise
preferably sterile aqueous preparations of a compound of formula I, which are
preferably isotonic with the blood of the intended recipient. These
preparations are
preferably administered intravenously, although administration may also take
place
by subcutaneous, intramuscular or intradermal injection. These preparations
can
is preferably be produced by mixing the compound with water and making the
resulting
solution sterile and isotonic with blood. Injectable compositions of the
invention
generally contain from 0.1 to 5% by weight of the active compound.
Pharmaceutical compositions suitable for rectal administration are preferably
in the
2o form of single-dose suppositories. These can be produced by mixing a
compound of
the formula I with one or more conventional solid carriers, for example cocoa
butter,
and shaping the resulting mixture.
Pharmaceutical compositions suitable for topical use on the skin are
preferably in the
2s form of ointment, cream, lotion, paste, spray, aerosol or oil. Carriers
which can be
used are petrolatum, lanolin, polyethylene glycols, alcohols and combinations
of two
or more of these substances. The active ingredient is generally present in a
concentration of from 0.1 to 15% by weight of the composition, for example
from 0.5
to 2%.
Transdermal administration is also possible. Pharmaceutical compositions
suitable
for transdermal uses can be in the form of single plasters which are suitable
for long-
term close contact with the patient's epidermis. Such plasters suitably
contain the
active ingredient in an aqueous solution which is buffered where appropriate,
CA 02493373 2005-O1-11
17
dissolved and/or dispersed in an adhesive or dispersed in a polymer. A
suitable
active ingredient concentration is about 1 % to 35%, preferably about 3% to
15%. A
particular possibility is for the active ingredient to be released by
electrotransport or
iontophoresis as described, for example, in Pharmaceutical Research, 2(6): 318
s (1986).
Further active ingredients suitable for combination products are:
all antidiabetics mentioned in.the Rote Liste 2001, chapter 12. They may be
combined with the compounds of the formula I of the invention in particular
for a
io synergistic improvement of the effect. Administration of the active
ingredient
combination may take place either by separate administration of the active
ingredients to the patient or in the form of combination products in which a
plurality of
active ingredients are present in one pharmaceutical preparation. Most of the
active
ingredients listed below are disclosed in the USP Dictionary of USAN and
is International Drug Names, US Pharmacopeia, Rockville 2001.
Antidiabetics include insulin and insulin derivatives such as, for example,
Lantus~
(see www.lantus.com) or HMR 1964, fast-acting insulins (see US 6,221,633), GLP-
1
derivatives such as, for example, those disclosed in WO 98108871 of Novo
Nordisk
AIS, and orally effective hypoglycemic active ingredients.
2o The orally effective hypoglycemic active ingredients include, preferably,
sulfonylureas, biguanides, meglitinides, oxadiazolidinediones,
thiazolidinediones,
glucosidase inhibitors, glucagon antagonists, GLP-1 agonists, potassium
channel
openers such as, for example; those disclosed in WO 97/26265 and WO 99!03861
of
Novo Nordisk AIS, insulin sensitizers, inhibitors of liver enzymes involved in
the
2s stimulation of gluconeogenesis andlor glycogenolysis, modulators of glucose
uptake,
compounds which alter lipid metabolism, such as antihyperlipidemic active
ingredients and antilipidemic active ingredients, compounds which reduce food
intake, PPAR and PXR agonists and active ingredients which act on the ATP-
dependent potassium channel of the beta cells.
In one embodiment of the invention, the compounds of the formula 1 are
administered
in combination with an HMG-CoA reductase inhibitor such as simvastatin,
fluvastatin,
pravastatin, lovastatin, atorvastatin, cerivastatin, rosuvastatin.
CA 02493373 2005-O1-11
I8
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a cholesterol absorption inhibitor such as, for example,
ezetimibe,
tiqueside, pamaqueside.
s In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a PPAR gamma agonist, such as, for example, rosiglitazone,
pioglitazone, JTT-501, GI 262570.
In one embodiment of the invention, the compounds of the formula I are
administered
to in combination with a PPAR alpha agonist, such as, for example, GW 9578,
GW 7647.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a mixed PPAR alphalgamma agonist, such as, for example,
is GW 1536, AVE 8042, AVE 8134, AVE 0847, or as described in PCTIUS00111833,
PCTlUS00/11490, DE10142734.4.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a fibrate such as, for example, fenofibrate, clofibrate,
bezafibrate.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with an MTP inhibitor such as, for example, implitapide, BMS-
201038,
R-103757.
2s In one embodiment of the invention; the compounds of the formula I are
administered
in combination with a bile acid absorption inhibitor (see, for example, US
6,245,744 or
US 6,221,897), such as, for example, HMR 1741.
In one embodiment of the invention, the compounds of the formula I are
administered
3o in combination with a CETP inhibitor, such as, for example, JTT-705.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a polymeric bile acid adsorbent such as, for example,
cholestyramine, colesevelam.
CA 02493373 2005-O1-11
19
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with an LDL receptor inducer (see US 6,342,512), such as, for
example, HMR1171, HMR1586.
s
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with an ACAT inhibitor, such as, for example, avasimibe.
In one embodiment of the invention, the compounds of the formula I are
administered
to in combination with an antioxidant, such as, for example, OPC-14117.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a lipoprotein lipase inhibitor, such as, for example, NO-
1886.
is In one embodiment of the invention, the compounds of the formula I are
administered
in combination with an ATP-citrate lyase inhibitor, such as, for example, SB-
204990.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a squalene synthetase inhibitor, such as, for example,
2o BMS-188494.
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a lipoprotein(a) antagonist, such as, for example, CI-1027
or
nicotinic acid.
2s
In one embodiment of the invention, the compounds of the formula I are
administered
in combination with a lipase inhibitor, such as, for example, orlistat.
In one embodiment of the invention, the compounds of the formula I are
administered
3o in combination with insulin.
In one embodiment, the compounds of the formula I are administered in
combination
with a sulfonylurea such as, for example, tolbutamide, glibenclamide,
glipizide or
glimepiride.
CA 02493373 2005-O1-11
In one embodiment, the compounds of the formula I are administered in
combination
with a biguanide, such as, for example, metformin.
In one further embodiment, the compounds of the formula I are administered in
combination with a meglitinide, such as, for example, repaglinide.
In one embodiment, the compounds of the formula I are administered in
combination
with a thiazolidinedione; such as, for example, troglitazone, ciglitazone,
pioglitazone,
rosiglitazone or the compounds disclosed in WO 97141097 of Dr. Reddy's
Research
Foundation, in particular 5-[[4-[(3,4-dihydro-3-methyl-4-oxo-2-
quinazolinylmethoxy]-
to phenyl]methyl]-2,4-thiazolidinedione.
In one embodiment, the compounds of the formula I are administered in
combination
with an a-glucosidase inhibitor, such as, for example, miglitol or acarbose.
In one embodiment, the compounds of the formula 1 are administered in
combination
with an active ingredient which acts on the ATP-dependent potassium channel of
the
is beta cells, such as, for example, tolbutamide, glibenclamide, glipizide,
glimepiride or
repaglinide.
In one embodiment, the compounds of the formula I are administered in
combination
with more than one of the aforementioned compounds, e.g. in combination with a
sulfonylurea and metformin, with a sulfonylurea and acarbose, repaglinide and
2o metformin, insulin and a sulfonylurea, insulin and metformin, insulin and
troglitazone,
insulin and lovastatin, etc.
In a further embodiment, the compounds of the formula I are administered in
combination with CART modulators (see "Cocaine-amphetamine-regulated
transcript
2s influences energy metabolism, anxiety and gastric emptying in mice"
Asakawa, A,
et al. in: Hormone and Metabolic Research (2001 ), 33(9), 554-558), NPY
antagonists,
e.g. naphthalene-1-sulfonic acid {4-[(4-aminoquinazolin-2-ylamino)methyl]
cyclohexylmethyl}amide hydrochloride (CGP 71683A)), MC4 agonists (e.g. 1-amino-
1,2,3,4-tetrahydronaphthalene-2-carboxylic acid [2-(3a-benzyl-2-methyl-3-oxo-
30 2,3,3a,4,fi,7-hexahydropyrazolo[4,3-c]pyridin-5-yl)-1-(4-chlorophenyl)-2-
oxoethyl]-
amide; (WO 01191752)) , orexin antagonists (e.g. 1-(2-methylbenzoxazol-6-yl)-3-
[1,5]naphthyridin-4-ylurea hydrochloride (SB-334867-A)), H3 agonists (3-
cyclohexyl-
1-(4,4-dimethyl-1,4,6,7-tetrahydroimidazo[4,5-c]pyridin-5-yl)propan-1-one
oxalic acid
salt (WO 00163208)); TNF agonists, CRF antagonists (e.g. [2-methyl-9-(2,4,6-
tri-
CA 02493373 2005-O1-11
21
methylphenyl)-9H-1,3,9-triazafluoren-4-yl]dipropylamine (WO 00166585)), CRF BP
antagonists (e.g. urocortin), urocortin agonists, [i3 agonists (e.g. 1-(4-
chloro-3-
methanesulfonylmethylphenyl)-2-[2-(2,3-dimethyl-1 H-indol-6-yloxy)ethylamino)-
ethanol hydrochloride (WO 01/83451)), MSH (melanocyte-stimulating hormone)
s agonists, CCK-A agonists (e.g. {2-[4-(4-chloro-2,5-dimethoxyphenyl)-5-(2-
cyclohexyl-
ethyl)thiazol-2-ylcarbamoyl]-5,7-dimethylindol-1-yl}acetic acid
trifluoroacetic acid salt
(WO 99115525)), serotonin reuptake inhibitors (e.g. dexfenfluramine), mixed
serotoninergic and noradrenergic compounds (e.g. WO 00171549), 5HT agonists
e.g.
1-(3-ethylbenzofuran-7-yl)piperazine oxalic acid salt (WO 01109111 ), bombesin
to agonists, galanin antagonists, growth hormone (e.g. human growth hormone),
growth
hormone-releasing compounds (6-benzyloxy-1-(2-diisopropylaminoethylcarbamoyl)-
3,4-dihydro-1H-isoquinoline-2-carboxylic acid tertiary butyl ester (WO
01/85695)),
TRH agonists (see, for example, EP 0 462 884), uncoupling protein 2 or 3
modulators, leptin agonists (see, for example, Lee, Daniel W.; Leinung,
Matthew C.;
is Rozhavskaya-Arena, Marina; Grasso, Patricia. Leptin agonists as a potential
approach to the treatment of obesity. Drugs of the Future (2001 ), 26(9), 873-
881 ),
DA agonists (bromocriptine, Doprexin), lipaselamylase inhibitors (e.g. WO
00140569),
PPAR modulators (e.g. WO 00/78312), RXR modulators or TR-~i agonists.
2o In one embodiment of the invention, the other active ingredient is leptin;
see, for
example, "Perspectives in the therapeutic use of leptin", Salvador, Javier;
Gomez-
Ambrosi, Javier; Fruhbeck, Gema, Expert Opinion on Pharmacotherapy (2001 ),
2(10), 1615-1622.
2s In one embodiment, the other active ingredient is dexamphetamine or
amphetamine.
In one embodiment, the other active ingredient is fenflurarnine or
dexfenfluramine.
In another embodiment, the other active ingredient is sibutramine.
In one embodiment, the other active ingredient is orlistat.
In one embodiment, the other active ingredient is mazindol or phentermine.
In one embodiment, the compounds of the formula I are administered in
combination
with bulking agents, preferably insoluble bulking agents (see, for example,
caroblCaromax° (Zunft H J; et al., Carob pulp preparation for treatment
of
hypercholesterolemia, ADVANCES IN THERAPY (2001 Sep-Oct), 18(5), 230-6.)
' CA 02493373 2005-O1-11
22
Caromax is a carob-containing product from Nutrinova, Nutrition Specialties 8~
Food
Ingredients GmbH, Industriepark Hochst, 65926 FrankfurtlMain)). Combination
with
Caromax° is possible in one preparation or by separate administration
of compounds
of the formula L and Caramax~. Caromax~ can in this connection also be
s administered in the form of food products such as, for example, in bakery
products or
muesli bars.
It will be appreciated that every suitable combination of the compounds of the
invention with one or more of the aforementioned compounds and optionally one
or
io more other pharmacologically active substances is regarded as falling
within the
protection conferred by the present invention.
CA 02493373 2005-O1-11
23
CH-
CHs O~CH3
CHs H3C
CHs
17
0 CHs
JTT-705 CI \
I ~ .,,, O
v v v OO
Br \ O CI OH
SB-204990 HO
/ H \ O\ ~O~CHs
I \
/ ~O~CHs
I I P
NO-1886 O OH
HsC OH O CHs
HC v v O v v CH
3 3
CI-1027
O
HO~ //
I \ / I S\ HsC CHs
O O O
/ O \ ~ P/ \/ CHs
O HsC O CHs
O,~O
BMS-188494 CHs
O O
CHs
O
\ ~ \ ~ H OH
~. N O I \
O /
61262570
O \
CHs
O
I / No
N O O H
JTT-501
CA 02493373 2005-O1-11
24
The examples detailed below serve to illustrate the invention, but without
restricting it.
CA 02493373 2005-O1-11
s
N
~ 0 0 0 0 0 0 0 0 0
00
O
Z I Z Z Z Z
=-Z d' U U Z U U U U U U
~ Z Z O Z Z Z Z Z Z
et M
C c"7 c"7 CO l'~ M c'~ M ch M
J
N z ~ O
O O O
U U U ~ I U
O O I O Z I U
( ~ co ca ~n cfl co cn ca co co
N
v
Z
O O
O O
v v c%~ v v v v ~r v
Z Z Z Z I Z Z Z Z
N N N N N N N N N
N
v- r
r
r
~n ~ LL LL LL LL LL LL LL LL
C tf7 tf~ LL lf~ !I7 ~ tl~ Ln tn
~ v v v v ~ ~' v~ c
U U U U U U U U U
x
l1J ~ N N N N N N N N N
r
r N M ~ ~ tD h 00 0~
f- LIJ
CA 02493373 2005-O1-11
O O O O O O O O O
Z
n
O ti ~ O Z
U U O U U
/ ~ / ~ / ~ /
N
Z Z
N N
xz zz zz n a
Z Z u.
c'~ c'~ c'~ C'~ f'~ c'~ f~ M M
N
Z Z = Z Z Z 2 I =
p Cp CO CO (O CO CO CD (~
Z Z Z = I Z Z Z Z
Z
N
U
O
Z ~ Z Z Z Z Z Z Z
c v v v v v c
S Z Z Z Z Z I Z Z
N N N N N N N N N
tL LL LL LL 11. LL L~ Li l.~
tI~ L~ tn lO tf7 ~ LO ~7
~ V ~ '~ '~ V'
U U U U U U U U U
N N N N N N N N N
O r N M ~ Ilk ~O t~ 00
r r r r r r r r r
CA 02493373 2005-O1-11
O O O O O O O
v ~ ~ ~ o 0
f ~
xz xz xz n xz xz xz
.
f~7 c'~ c~ c'~ M M M
n
N
c~ cW 7 ~ e~ ef e~
U U U U U U U
O O O O O O O
ca co cp cn ca co co
Z I = Z 2 Z Z
Z Z = Z Z 2 Z
c ~ ~ v v v
I Z Z Z = I I
N N N N N N N
l~ lL LL LL LL LL LL
C ~' ~ d'
U U U U U U U
N N N N N N N
r N N N N N N
CA 02493373 2005-O1-11
O O O O O O O O O O
U ~, U ,~ O u. ~,
Z ~ Z 2
I ~ ~ I ~ I ~ ~ N I ~ I ~ I ~
U V U U
xZ 2 xZ xZ Z Z tZ xZ xz Z
' Z ' ' Z Z ' ' ' Z
M M M M M M M M M M
00
N
_ _ _ _ _ _ _ = Z =
0 ~ ~ ~ ~ ~ ~ 0
C~ (fl (D CO CO ~D (D (O (fl (D
_ _ = Z = _ = S = _
= I = = Z Z = _ _ _
d' d'
N N N N N N N N N N
i ~ i i i i i
L(~ tf~ tL7 l~ lI7 ~ tn t1~ tn l~
N N N N N N N N N N
N N N N C~"7 M c'7 M M M
CA 02493373 2005-O1-11
x .Y x x .~c x x
0 0 0 0 0 0 0
x z
U U
O O
O Z O
Z U O U U
U
I ~ l ~ I ~ l ~ l ~ I ~ l ~ I ~
ZZ ~Z tZ n ZZ =Z =Z
c~ M M C'~ C~ ('~7 ch
N
M co 07 to M n
2 = Z 2 2 Z =
U U U U U U U
O O O O O O O
cfl c~ ca ca co co cfl
z z z z z z z
z z z z z z =
v v ~r ~ v ~ c
x z z z x z z
N N N N N N N
LL Ll. LL LL LL LL LL
tn LW f7 lf7 tf~ tf~ tn
'~ '
U U U U U U U
N N N N N N N
M M th M ~ "Q
CA 02493373 2005-O1-11
O O O O O O O O YO
n ~n
Z
V U
O - - O
I ~ I ~ ~ I ~ ~ I
Z Z
U U
I =Z =Z =Z ZZ = ZZ ZZ ~Z
c'~ f~ c'~ f'~ c'~ f'~ c"~ frJ M
O
M
O O O O O O O O O
tD ~ (D CO (fl f0 (fl tD (D
d'
N N N N N N N N N
LL LL LL LL L1.. LL LL LL LL
d' V ,~' '~ ~ 'ct
U U U U U U U U U
N N N N N N N N N
CA 02493373 2005-O1-11
O O O O O O O O
n
t
N
V
V ~ O ~ Z
O O ~j
N
I
xz xz a xz xz xz n =
\ \ \ \ 1 \ \
M M M M M M M M
O O O O O O
O O O O O O
U U / I / / /
O O
cn co ca co cfl cfl cfl co
I Z Z Z I Z S
Z Z Z Z Z Z Z Z
~t v c v ~ v ~ v
Z Z I 2 Z Z Z S
N N N N N N N N
LL LL LL LL LL ll. LL t1
~7
~' V~
U U U U U U U U
N N N N N N N N
N
CA 02493373 2005-O1-11
O O O O O O p
/
/ ~ ~ O O
/ ~ ~ /
a a a a a a a
.
M M C~ e'~ M ch M
'Y' O O O O O O O
O O O O O O O
/ / / / / /
(p CD tD (D c0 CO CD
Z Z Z Z Z Z 2
i i ~ i
I Z Z I Z Z I
Z Z Z Z I 2 Z
N N N N N N N
LL LL LL li
V 'C
U U U U U U U
N N N N N N N
~O t0 ~C to t~D t0 ~D
CA 02493373 2005-O1-11
O O O O O O O O O
V
Z ~ Z 2
I ~ ~ I ~ I ~ ~ ~ I ~ I
U V U U
xz Z xz xz Z Z n xz Z
' Z ' ' Z Z ' ' Z
M M M M M M M M M
0
CO ~ CO CO Cfl (fl (D CO (D
1 ~ I 1 1 1 1 1 1
1 1 1 1 1 n 1 1 1
d' '~ ~' ~ ~1
1 1 1 1 1 1 1 1 1
N N N N N N N N N
N N N N N N N N N
I 1 1 1 1 1 1 1 1
v ~ ~ v v v v ~ v
U U U U U U U U U
1 1 1 I 1 1 1
N N N N N N N N N
Iw 00 01 O ~ N M ~ 1n
t0 CO t0 h t~ t~ h h. 1~
CA 02493373 2005-O1-11
O O O O O O O
a
Z Z
U U
O O
O U u. u.
V
n xz xz xz =z tz tZ
v v v v v v v
M M M M M M M
M
O O O O O O O
O O O O O
cD CO (D (Q tD CD (D
i ~ ~ i i
i ~ ~ ~ i i
N N N N N N N
LL LL l1 LL LL LL LL
i i i ~ i m
V~ C' ~ C' V~
U U U U U U U
N N N N N N N
~O 1' 00 07 O ~ N
N ~ ~ O O O
CA 02493373 2005-O1-11
O O O O O O O O O
V ~ Z
M O O
/ \. \ / \ / / O / \ / \
N N
Z Z
U U
Z xz xz xz xz I xz xz xZ
v v v v Z ~ v v
M M M M M M M M M
O O O O O O O O O
O O O O O O
(O f0 (D t0 t0 (fl (~ cfl (D
1 I 1 1 1 1 1 I 1
1 1 1 1 1 1 1 1 1
1 1 1 1 1 1 1 I 1
N N N N N N N N N
N N N N N N N N N
1 1 1 1 1 1 I 1 1
v v v q ~ ~ v v v
U U U U U U U U U
1 1 1 1 I 1 1 I 1
N N N N N N N N N
M tT ~ t0 1~ 00 01 O r
00 00 00 00 00 00 00 C1 C1
CA 02493373 2005-O1-11
Y Y Y Y Y Y Y Y Y Y Y Y Y Y
O O O O O O O O O O O O O O
N
_ n S
~ I
I S I U V I Z I
I ~ N U ~ U = ~~i I ~ U U U
U ,~
= V 2 Z = Z = Z Z Z
xz xz U S V O V U U U O U U U_
' ' O Z O ' O O O O ' O O O
ch M ch c~ M C~1 c~? c~7 M ch ch ch M M
M
O O O
O N N O
.~('J0lt~f~lMNf~ N(~
S S S S S S S I S S S I
I I U U U U U U U U U U U
O O O O O O O O O O O
I , 1 I 1 1 1 I 1 1 1
co c~ ca c~ cfl c~ ca co co cfl cfl cfl ca co
S S S S S S S S S S S S S S
1 , I 1 I I I I 1 1 1 1 I I
mmn w wm ~n um wn u»
S S S S S S S S S S S S S S
I 1 1 1 I I , 1 1 1 I 1 I I
v v v ~ v ~r v v v v v v v
S S Z S S S S S S Z S S S S
, , , , , 1 1 , , , 1 1 I 1
N N N N N N N N N N N N N N
N N N N N N N N N N N N N N
1 1 I 1 I 1 1 I 1 I 1 1 1 1
~t ~ ~' ~ ~ rt d' d' ~? ~ ~T ~
1 ~ 1 = = _ ~ ~ = ' 1
U U U U U U U U U U U U U U
I 1 1 1 1 , I I 1 1 1 1 1
N N N N N N N N N N N N N N
O O O O O O
!" t~ T T !~ l"
CA 02493373 2005-O1-11
x x x x x x x x x x x
O O O O O O O O O O O
N
n Z
,~ U
Z U U Z
U = I ~ V / ~ U U
N U N U N N I
Z = 2 = Z = Z U
U_ _ U_ U_
O O O ~ O ~ V O O O Z
M M M M M M M M M M M
O O O O O O O O O
O O O O O O O
/ / / / / / / / p O
cfl co cfl cfl co cfl cfl cfl co co co
x x z z z z z z z z z
1 1 1 I 1 I I 1 1 1
z z s z z z ~ x s z z
1 , I I I 1 1 ~ I I 1
v c v v~ v ~ v ~ v v v
z z z z z z z r x z x
I , 1 I I 1 I 1 ~ I 1
N N N N N N N N N N N
N N N N N N N N N N N
1 I I 1 1 1 I 1 1 I 1
C '~ ~ ~ ~ <t ~'
U U U U U U U U U U U
1 I I 1 1 1 1 I 1 I I
N N N N N N N N N N N
O O O O r r r r r r e-
r r r r r r r r r r !'
'~ I~ I~ I°~ I~ Ir IN 1'~ n
CA 02493373 2005-O1-11
Y ~G ~G.Y ~L Y ~C Y .Y Y Y Y Y Y ~C
O O O O O O O O O O O O . O O
O
N Q
I
U x
O Z
U Z
N P7
N N
M ~ M ~ ~ M
N Z I Z Z Z
U U
U Z U Z U Z U Z
O Z O Z O Z O Z Z Z Z Z Z Z Z
M M M M M M M M M M M M M ~ s~
S Z Z Z Z- Z-
N U U U
U U U U
Z Z
O O O O -Z -Z -Z -Z U
= O O U Z Z
co co c~co ca ca c~ co co c~ cD ca cn c~ cD
Z 2 Z Z I Z I Z Z I Z Z Z I Z
, , , , , , , ,
, , , , , , ,
m m n u m n u~ u m n m ~n
Z Z Z Z Z Z Z Z Z Z Z Z Z Z Z
, , , , , , , ~
, , , , , , ,
'~f~ ~ W ? ~!' ~ tt '~ ~' V '~ M M
Z Z Z I Z Z Z Z I Z Z Z Z = Z
, , , , , , , i
, , , , , , ,
N N N N N N N N N N N N N N N
LL LL LLLL ll. LL LL LL N
N N _N _N
't ~ U U U U ~ U U
U U U U U U U U ~ ~ .~ ~ U
N N N N N N N N N N N N N N N
h 00 C1O r N M ~ 1~'7t0 h Op p1 O r
r r r N N N N N N N N N N M M
r r r r r r r r r r r r r r r
CA 02493373 2005-O1-11
N
f~
w
_(a
U
_O
O
O
N
a
L
U
_N
O
O ~
t
Z~ .~
.O
Q~ Z= C
~
~ ~
N
L
O
U
Q
L
N
t6
o,
M
U
J
O O
L
wr
a
1
C
N O
O
.O
N
U
N
N ~
N
T
CA 02493373 2005-O1-11
The compounds of the formula I are distinguished by beneficial effects on
glucose
metabolism; in particular they lower the blood glucose level and are suitable
for the
treatment of type 2 diabetes. The compounds can therefore be employed alone or
in
combination with other blood glucose-lowering active ingredients
(antidiabetics).
s The compounds of the formula I are further suitable for the treatment of
late
complications of diabetes such as, for example, nephropathy, retinopathy,
neuropathy and myocardial infarction, myocardial infarction, peripheral
arterial
occlusive diseases, thromboses, arteriosclerosis, syndrome X, obesity,
inflammations, immune diseases, autoimmune diseases such as, for example,
AIDS,
to asthma, osteoporosis, cancer, psoriasis, Alzheimer's, schizophrenia and
infectious
diseases.
The activity of the compounds was assayed as follows:
Glycogen phosphorylase a activity assay
The effect of compounds on the activity of the active form of glycogen
phosphorylase
(GPa) was measured in the reverse direction by following the synthesis of
glycogen
2o from glucose 1-phosphate by determining the liberation of inorganic
phosphate. All
the reactions were carried out as duplicate determinations in microtiter
plates with
96 wells (Half Area Plates, Costar No 3696), measuring the change in
absorption
owing to the formation of the reaction product at the wavelength specified
hereinafter
in a Multiskan Ascent Elise Reader (Lab Systems, Finland). In order to measure
the
2s GPa enzymic activity in the reverse direction, the general method of Engers
et al.
(Engers HD, Shechosky S, Madsen NB, Can J Biochem 1970 Ju1;48(7):746-754) was
used to measure the conversion of glucose 1-phosphate into glycogen and
inorganic
phosphate, with the following modifications: human glycogen phosphorylase a
(for
example with 0.76 mg of proteinlml (Aventis Pharma Deutschland GmbH),
dissolved
3o in buffer solution E (25 mM (i-giycerophosphate, pH 7.0, 1 mM EDTA and 1 mM
dithiotreitol) was diluted with buffer T (50 mM Hepes, pH 7.0, 100 mM KCI, 2.5
mM
EDTA, 2.5 mM MgCl2 6H20) and addition of 5 mglml glycogen to a concentration
of
10 Ng of proteinlml. Test substances were prepared as 10 mM solution in DMSO
and
diluted to 50 NM with buffer solution T. To 10 NI of this solution were added
10 NI of
CA 02493373 2005-O1-11
41
37.5 mM glucose, dissolved in buffer solution T, and 5 mglml glycogen, plus 10
NI of
a solution of human glycogen phosphorylase a (10 Ng of proteinlml) and 20 NI
of
glucose 1-phosphate, 2.5 mM. The baseline glycogen phosphorylase a activity in
the
absence of test substance was determined by adding 10 Nf of buffer solution T
(0.1
s DMSO). The mixture was incubated at room temperature for 40 minutes, and the
liberated inorganic phosphate was measured by the general method of Drueckes
et
al. (Drueckes P, Schinzel R, Palm D, AnalBiochem 1995 Sep 1;230(1):173-177)
with
the following modifications: 50 NI of a stop solution of 7.3 mM ammonium
molybdate,
10.9 mM zinc acetate, 3.6% ascorbic acid, 0.9% SDS are added to 50 NI of the
to enzyme mixture. After incubation at 45°C for 60 minutes, the
absorption at 820 nm
was measured. To determine the background absorption, in a separate mixture
the
stop solution was added immediately after addition of the glucose 1-phosphate
solution. This test was carried out with a concentration of 10 NM of the test
substance
in order to determine the particular inhibition of glycogen phosphorylase a in
vitro by
is the test substance.
Table 2: Biological activity
Ex. l inhibition Ex. l inhibition
at10pM at10NM
2 96 91 99
3 53 92 78
4 89 104 66
7 100 108 52
13 103 110 73
14 70 113 83
21 75 114 48
26 61 121 99
42 55 125 74
44 40 127 102
65 60 130 28
76 73 132 97
90 89
CA 02493373 2005-O1-11
42
It is evident from the table that the compounds of the formula I inhibit the
activity of
glycogen phosphorylase a and thus are very suitable for lowering the blood
glucose
level. They are therefore particularly suitable for the prevention and
treatment of
type 2 diabetes.
s
The preparation of some examples is described in detail below, and the other
compounds of the formula I were obtained analogously:
Experimental part:
to
Example 1:
1-{3-[3-(2-Chloro-4,5-difluorobenzoyl)ureido]-4-methoxyphenyl}-3-methylurea
a) 2-Chloro-4,5-difluorobenzoyl isocyanate
is
2-Chloro-4,5-difluorobenzamide was dissolved in dichloromethane, mixed with
1.5 eq. of oxalyl chloride and heated to reflux for 16 hours. The reaction
mixture was
concentrated under high vacuum and employed in stage b without further
purification.
2o b) 1-(2-Chloro-4,5-difluorobenzoyl)-3-(2-methoxy-5-nitrophenyl)urea
4.0 g (23.8 mmol) of 2-methoxy-5-nitroaniline were dissolved in 10 ml of N-
methyl-2-
pyrrolidinone, and 5.2 g (23.8 mmol) of 2-chloro-4,5-difluorobenzoyl
isocyanate were
added. Slight warming occurred. After 15 minutes at room temperature, diethyl
ether
2s was added, and the resulting precipitate was filtered off with suction. 6.6
g (79%) of
the desired product were obtained.
c) 1-(5-Amino-2-methoxyphenyl)-3-(2-chloro-4,5-difluorobenzoyl)urea
30 5.8 g (25.9 mmol) of tin dichloride hydrate were added to 2.0 g (5.2 mmol)
of
1-(2-chloro-4,5-difluorobenzoyl)-3-(2-methoxy-5-nitrophenyl)urea in 20 ml of
ethyl
acetatelmethanol mixture at 70°C. After 1 hour, 30 ml of N-methyl-2-
pyrrolidinone
were added, and the mixture was stirred for a further 2 hours. After cooling,
the
reaction mixture was made basic and the resulting precipitate was filtered off
with
CA 02493373 2005-O1-11
43
suction. The phases were separated. The organic phase was then washed three
times with water, dried and concentrated under high vacuum. 1.2 g (67%) of the
desired product were obtained.
s
d) 1-{3-[3-(2-Chloro-4,5-difluorobenzoyl)ureido]-4-methoxyphenyl}-3-methylurea
600 mg (1.7 mmol) of 1-(5-amino-2-methoxyphenyl)-3-(2-chloro-4,5-difluoro-
benzoyl)urea were dissolved in 5 ml of acetonitrile, and 69 mg (1.7 mmol) of
methyl
to isocyanate were added. After stirring at room temperature for one hour, the
resulting
precipitate was filtered off with suction. fi38 mg (91 %) of the desired
product were
obtained.
Example 3:
is 3-[3-(2-Chloro-4-fluorobenzoyl)ureido]-4-methoxycarbonylaminobenzoic acid
a) 2-Chloro-4-fluorobenzoyl isocyanate
1.64 g (6 mmol) of 2-chloro-4-fluorobenzamide were dissolved in 3 ml of
2o dichloromethane and, at 0°C and under a nitrogen atmosphere, 0.8 ml
(9.3 mmol) of
oxalyl chloride was added, and the mixture was heated to reflux for 9 hours.
The
reaction mixture was concentrated under high vacuum and afforded 1.17 g
(5.8 mmol) of the desired product, which was employed as solution in
dichloromethane (1 mmol in 1.7 ml of solution) in stage b.
b) 4-Amino-3-[3-(2-chloro-4-fluorobenzoyl)ureidoJbenzoic acid
150 mg (1 mmol) of 3,4-diaminobenzoic acid were dissolved in 2 ml of N-methyl-
2-
pyrrolidinone and, at 0°C, 1 ml (1.2 mmol) of the 2-chloro-4-
fluorobenzoyl
3o isocyanateldichloromethane solution prepared in stage a was added. The
resulting
precipitate was filtered oft with suction. The crude mixture (500 mg) was
purified by
column chromatography (dichloromethanelmethanol = 9812 to 9317). 80 mg (25%)
of
the desired product were obtained.
CA 02493373 2005-O1-11
44
c) 3-[3-(2-Chloro-4-fluorobenzoyl)ureido]-4-methoxycarbonylaminobenzoic acid
28 mg (0.08 mmol) of 4-amino-3-[3-(2-chloro-4-fluorobenzoyl)ureido]benzoic
acid
were dissolved in 0.5 ml of N-methyl-2-pyrrolidinone and stirred with 0.02 ml
(0.24 mmol) of pyridine and 0.007 ml of methyl chloroformate at room
temperature for
4 hours. Water and acetic acid were added, and the resulting precipitate was
filtered
off with suction. 18 mg (55°t°) of the desired product were
obtained.
Melting point: decomposition >400°C
io Example 54:
Methyl 3-{2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]-4-[3-(2-
trifluoromethylphenyl)-
ureido]phenyl}acrylate
a) Methyl 3-{2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]-4-nitrophenyl}acrylate
is
4.5 g (20.3 mmol) of methyl 3-(2-amino-4-nitrophenyl)acrylate were stirred
with 4.41 g
(20.3 mmol) of 2-chloro-4,5-difluorobenzoyl isocyanate (Example 1 a) in 50 ml
acetonitrile at 50°C for one hour. The reaction mixture was then
concentrated, the
residue was stirred with diethyl ether, and the resulting solid was filtered
off with
2o suction. 8.5 g (95%) of the desired product were obtained.
b) Methyl 3-{4-amino-2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]phenyl}acrylate
8.5 g (19.3 mmol) of methyl 3-{2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]-4-
nitro-
2s phenyl}acrylate were suspended in 60 ml of a mixture of glacial acetic acid
and
concentrated hydrochloric acid (10:1 ) and heated to 70°C. Then 8.85 g
(135.3 mmol)
of zinc powder were added. After 30 minutes, the mixture was cooled, the solid
was
filtered off with suction, and the filtrate was concentrated. The residue was
taken up
in ethyl acetate and washed with a 10% strength sodium bicarbonate solution.
The
30 organic phase was dried and concentrated. 7.9 g (100%) of the desired
product were
obtained.
c) Methyl 3-{2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]-4-[3-(2-
trifluoromethyl-
phenyl)ureido]phenyl}acrylate
CA 02493373 2005-O1-11
100 mg (0.24 mmol) of methyl 3-{4-amino-2-[3-(2-chloro-4,5-difluorobenzoyl)-
ureido]phenyl}acrylate were dissolved in 1 ml of acetonitrile and mixed with
2-trifluoromethylphenyl isocyanate and shaken at 60°C for 14 hours. The
resulting
s precipitate was filtered off with suction, and 16 mg (11%) of the desired
product were
obtained.
Example 110:
Methyl (E)-3-[2-[3-(2-chloro-4,5-difluorobenzoyl)ureido]-4-(4-chloro-
io phenoxycarbonylamino)phenyl]acrylate
100 mg (0.24 mmol) of methyl 3-{4-amino-2-[3-(2-chloro-4,5-difluorobenzoyl)-
ureido]phenyl}acrylate (example 54 b) were reacted in 2 ml of
dimethylformamide
with potassium carbonate and 4-chlorophenyl chloroformate. The precipitate
resulting
1s after 4 hours was filtered off with suction. Preparative HPLC (column:
Waters
XterraT""MS CAB, 5 Nm, 30x100 mm, mobile phases: A: HZO + 0.2% trifluoroacetic
acid, B: acetonitrile, gradient: 2.5 minutes 90% A I 10% B to 17.5 minutes 10%
A
90% B) resulted in 12 mg (9%) of the desired product.
2o Example 132:
1-(2-Chloro-4,5-difluorobenzoyl)-3-(6-methoxy-2,4-dioxo-1,2,3,4-tetrahydro-
quinazolin-7-yl)urea
a) N-(4-Methoxy-2-methylphenyl)acetamide
41.1 g (0.3 mol) of 4-methoxy-2-methylphenylamine and 37 g (0.5 mol) of
dimethyl-
ethylamine were dissolved in 50 ml of tetrahydrofuran, and 35.7 g (0.35 mol)
of acetic
anhydride were added while stirring. The solution heated to boling during
this. It was
stirred at room temperature for 1 hour and cooled to 0°C. The resulting
precipitate
3o was filtered off with suction and washed several times with a little cold
tetrahydrofuran and dried. 40 g (75%) of colorless crystals of the desired
product
were obtained.
b) N-(4-Methoxy-2-methyl-5-nitrophenyl)acetamide
CA 02493373 2005-O1-11
46
34 g (0.19 mol) of N-(4-methoxy-2-methylphenyl)acetamide were added in small
portions to a mixture of 40 ml of glacial acetic acid and 70 ml of fuming
nitric acid at
-10 to -15°C. These portions were such that the temperature did not
rise above
-10°C. The reaction mixture was then poured onto ice. The resulting
precipitate was
filtered off with suction and washed with water, ethanol and diethyl ether.
22.5 g
(53%) of the desired product were obtained.
c) 2-Acetylamino-5-methoxy-4-nitrobenzoic acid
io
11.2 g (50 mmol) of N-(4-methoxy-2-methyl-5-nitrophenyl)acetamide and 8.5 g
(62.5 mmol) of anhydrous magnesium sulfate were suspended in 500 ml of water
and
heated to 85°C. Over the course of 30 minutes, a solution of 21.8 g
(138 mmol) of
potassium permanganate in 250 ml of water was added dropwise. The reaction
is mixture was stirred at 85°C for 3 hours and then filtered hot to
remove manganese
dioxide. The latter was extracted by boiling three times with 100 ml of water
each
time. The combined aqueous phases were again filtered hot and concentrated to
about 150 ml in vacuo. The residue was acidified to pH 1-2 with concentrated
hydrochloric acid and cooled to 0°C. The resulting product was filtered
off with
zo suction, washed with water and diethyl ether, dried and reacted without
further
purification in the next stage.
d) 2-Amino-5-methoxy-4-nitrobenzoic acid
2s 7.5 g of 2-acety!amino-5-methoxy-4-nitrobenzoic acid (crude mixture from
stage c)
were heated to reflex in 50 ml of water and 20 m4 of concentrated hydrochloric
acid
for 3 hours. The reaction mixture was concentrated in vacuo and purified by
column
chromatography (silica gel, dichloromethanelisopropanol = 9I1 ). 3.1 g (50%)
of the
desired product were obtained.
e) 6-Methoxy-7-nitro-1H-benzo[d][1,3]oxazine-2,4-dione
2.0 g (9.4 mmol) of 2-amino-5-methoxy-4-nitrobenzoic acid were dissolved in 20
ml of
chloroform and 10 ml of tetrahydrofuran, and 20 ml of 20% strength phosgene
CA 02493373 2005-O1-11
47
solution (1.8 M in toluene) were added. After 3 hours under reflux, a further
10 ml of
phosgene solution were added at 60°C, and the mixture was stirred at
60°C for a
further 12 hours. The phosgene was distilled off and the residue was
concentrated in
vacuo after addition of toluene several times. 2.2 g (100%) of the desired
product
were obtained.
f) 2-Amino-5-methoxy-4-nitrobenzamide
476 mg (2 mmol) of 6-methoxy-7-nitro-1 H-benzo[d][1,3]oxazine-2,4-dione and
1.5 g
to (20 mmol) of ammonium acetate were dissolved in 20 ml of acetic acid and
heated at
105°C for 3 hours. The reaction mixture was then poured into ice-water
and slowly
brought to pH 7 with solid sodium bicarbonate. The aqueous phase was extracted
with ethyl acetate. The organic phase was washed with water, dried and
concentrated in vacuo. The crude product was triturated with diethyl ether,
and the
is resulting precipitate was filtered off with suction. 285 mg (68%) of the
desired product
were obtained.
g) 6-Methoxy-7-nitro-1 H-quinazoline-2,4-dione
20 108 mg (0.5 rnmol) of 2-amino-5-methoxy-4-nitrobenzamide were dissolved in
5 ml of
tetrahydrofuran and 5 ml of chloroform, and oxalyl chloride (solution in
toluene) was
added. The mixture was stirred at 60°C for 5 hours and then
concentrated in vacuo.
Addition of toluene was followed by renewed concentration. 120 mg (100%) of
the
desired product were obtained.
h) 7-Amino-6-methoxy-1 H-quinazoline-2,4-dione
120 mg (0.5 mmol) of 6-methoxy-7-nitro-1 H-quinazoline-2,4-dione were taken up
in a
mixture of 5 ml of tetrahydrofuran, 5 ml of methanol and 5 ml of acetic acid
and
3o hydrogenated with palladium catalysis at room temperature for 3 hours. The
reaction
solution was heated to dissolve precipitated product and was filtered hot to
remove
the catalyst. The filtrate was concentrated and, after addition of toluene,
again
concentrated. 100 mg (100%) of the desired product were obtained.
CA 02493373 2005-O1-11
48
i) 1-(2-Chloro-4,5-difluorobenzoyl)-3-(6-methoxy-2,4-dioxo-1,2,3,4-tetrahydro-
quinazolin-7-yl)urea
100 mg (0.53 mmol) of 7-amino-6-methoxy-1 H-quinazoline-2,4-dione were
suspended in 20 ml of acetonitrile and 2 ml of N-methyl-2-pyrrolidinone, and
240 mg
(0.97 mmol) of 2-chloro-4,5-difluorobenzoyl isocyanate (example 1 a) were
added.
The reaction mixture was heated to boiling and then quenched with methanol and
concentrated in vacuo. The residue was stirred with acetonitrile, and the
resulting
precipitate was filtered off. 64 mg {30%) of the desired product were
obtained.